User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Social determinants of health and the hospitalist
Are access to housing and food as important as therapeutics?
While physicians acknowledge that the social determinants of health can impact outcomes from medical care, some may feel that trying to address factors such as homelessness, food insecurity, or lack of ready access to transportation or pharmacy services is just not part of the doctor’s job. A majority of 621 physicians surveyed in the summer of 2017 by Salt Lake City–based health care intelligence firm Leavitt Partners say they are neither capable of nor responsible for addressing such issues.1
But that view may become unsustainable as the U.S. health care system continues to advance toward value- and population-based models of health care and as evidence mounts that social factors are important contributors to costly outcomes, such as avoidable hospital readmissions or emergency room visits. A recent report from the Robert Wood Johnson Foundation estimates that at least 40% of health outcomes are the result of social and economic factors, while only 20% can be attributed to medical care.2
“This is a hot topic – getting a lot of attention these days,” said hospitalist and care transitions expert Ramon Jacobs-Shaw, MD, MPA, regional medical officer for CareMore Health, a California-based physician-led health delivery organization and subsidiary of Anthem. “If you go around the country, some doctors still see social factors as the realm of the social worker. But large health care organizations are coming to recognize that social determinants are huge contributors to the health of their members and to the outcomes of their care.”
Hospitalists could be the natural providers to delve into the specific psychosocial aspects of their patients’ lives, or try to figure out how those factors contribute to health care needs, Dr. Jacobs-Shaw said. They typically confront such issues while the patient is in the hospital bed, but what are the steps that led to the hospitalization in the first place? What will happen after the patient is discharged?
“For example, if patients lack transportation, how can they get to their follow-up medical appointment in the primary care office in order to manage their diabetes? If you can’t follow up with them, their diabetes could get out of control, with complications as a result, such as an infected wound,” he said. Another big issue is access to affordable medications. “CareMore has pharmacists embedded on our care teams. They try to figure out the best medicine for the patient but at the lowest cost. They meet individually with patients and do medication counseling, particularly for those with polypharmacy issues.”
Making health care more equitable
Dr. Jacobs-Shaw has long held a personal interest in issues of inclusiveness, diversity, and how to make health care more equitable for historically underserved groups. Asking how to have a bigger impact on these issues is what brought him, after 13 years as a hospitalist on the East Coast, to CareMore, a company that has made addressing social needs central to its care model. “In California, where I am based, we are a wrap-around for patients who are covered by Medicare Advantage plans. We are whatever the patient needs us to be.”
He oversees a group of hospitalists, dubbed extensivists, who provide advanced patient care and chronic disease management. In the extensivist model, physicians and advanced practice nurses provide comprehensive and coordinated care to patients with complex medical issues, taking their scope of practice beyond the hospital into homes, post-acute care facilities, and other settings, with a focus on keeping patients healthier and reducing readmission.3
“Our patients get access to extra services and resources, some of which are available at our care centers – which are one-stop outpatient facilities. We also focus on a lot of things physicians didn’t historically think were within their wheelhouse. Hospitalists deal with these kinds of issues every day, but may not label them as social determinants of health,” Dr. Jacobs-Shaw said. He emphasized that hospitalists should realize that they are not powerless to address these issues, working in partnership with other groups in and out of the hospital. They should also know that health care payers increasingly are dedicating resources to these issues.
“We just started trying to address homelessness through a pilot in Orange County, working with nonprofit organizations and philanthropy to offer a transitional site of care for our patients who are being discharged from the hospital and have housing insecurity issues, to get them transitioned into more secure housing,” Dr. Jacobs-Shaw said. CareMore also has a transportation collaborative that offers no-cost, nonemergency transportation to medical appointments. “That’s meeting them where they are at, based on an assessment of their needs and resources.”
What are social determinants?
The social determinants of health – social, environmental, and other nonmedical factors that contribute to overall health status and medical need – have been defined by the World Health Organization as: “conditions in which people are born, grow, live, work, and age.” That is a broad complex of overlapping social and systems issues, but it provides a context for a broader understanding of the patient’s health and response to medical interventions.
Socioeconomic status is a huge determinant. Level of education may be more important than income if the person lacks the health literacy to navigate the system and access needed care. Housing instability may include poor sanitation, substandard dwellings, or unsafe neighborhoods – all of which can affect a person’s well-being. Environmental health may include compromised air quality – which can impact pulmonary health. Other issues include access to employment and child care, utility needs, and interpersonal violence.
A 2014 paper in Annals of Internal Medicine found that residence within a disadvantaged neighborhood was a factor in hospital readmission rates as often as was chronic pulmonary disease.4 A recent report on social determinants of health by the National Institute for Health Care Management notes that patients with food insecurity are 2.4 times more likely to go to the emergency room, while those with transportation needs are 2.6 times more likely.5
What can health care leaders do to better equip their clinicians and teams to help patients deal with this array of complex needs? Intermountain Healthcare, based in Salt Lake City, spearheaded in 2018 the development of the Alliance for the Determinants of Health, starting in the communities of Ogden and St. George, Utah. The Alliance seeks to promote health, improve access to care, and decrease health care costs through a charitable contribution of $12 million over 3 years to seed collaborative demonstration projects.
Lisa Nichols, assistant vice president for community health at Intermountain, said that, while hospitalists were not directly involved in planning the Alliance, hospitalists and ED physicians have become essential to the patient-screening process for health and social needs.
“We met with hospitalists, emergency departments, and hospital administrators, because we wanted their feedback on how to raise awareness of the social needs of patients,” she said. “They have good ideas. They see the patients who come in from the homeless shelters.”
Other hospitals are subsidizing apartments for homeless patients being discharged from the hospital. CommonSpirit Health, the new national Catholic health care organization formed by the 2019 merger of Dignity Health and Catholic Health Initiatives, has explored how to help create and sustain affordable housing in the communities it serves. Investments like this have inspired others, such as Kaiser Permanente, to get involved in supporting housing initiatives.6
Comprehensive community care
David Meltzer, MD, PhD, a hospitalist and professor of medicine at the University of Chicago, said most hospitalists these days believe social determinants of health are part of their job responsibilities.
“That’s not to say we all do it well. We may fail at addressing some of the barriers our patients face. But I don’t know anyone who still says it’s not their job,” he said.
Since 2012, Dr. Meltzer has led a pilot called Comprehensive Care Physicians (CCP), in which the same physician cares for patients with chronic health problems in the clinic and in the hospital, working with a team of nurse practitioners, social workers, care coordinators, and other specialists. A total of 2,000 patients with chronic health problems were enrolled in the study from 2012 to 2016, half assigned to standard care and half assigned to five CCP doctors. The result: The CCP model has shown large improvements in outcomes – particularly among the more vulnerable, less activated patients, is preferred by patients, and has significantly reduced health care utilization.
The next step for the research team is another randomized controlled trial called Comprehensive Care, Community, and Culture, designed to address unmet social needs. Study group patients will also be screened for unmet social needs and have access to a community health worker and to the initiative’s Artful Living Program, which includes community and cultural activities like yoga and dance classes, cooking classes, art classes, and music concerts. To address the complex dimensions and determinants of health, Dr. Meltzer explained, efforts to improve health must extend to sectors far beyond traditional health care.
“I think trying to understand your patients’ social and nonmedical needs starts with getting to know them, and asking about their needs,” he said. “The better you know them, the better you are able to make medical decisions that will promote positive outcomes.”
Sound Physicians, a national hospitalist company based in Tacoma, Wash., and working in 350 hospitals in 41 states, recently published a blog post on its website about the importance of social determinants of health.7 Sound Physicians participates in value-based care through bundled Medicare/Medicaid contracts based on episodes of care for hospitalized patients with certain diagnoses or DRGs, explained John Dickey, MD, the company’s chief medical officer for population health.
“We’ve been heavily involved in trying to improve cost and outcomes of care since 2015. Social determinants absolutely play into trying to lower costs of care and reduce rates of readmissions, which are often multifactorial in cause,” he said. Hospitalists are uniquely equipped to impact post-acute outcomes, Dr. Dickey said, working in partnership with a position Sound Physicians calls the clinical performance nurse.
“We can also partner with primary care providers, provide education for our hospitalist staff, and work with in-home care supports for patients such as these, who otherwise might end up in a skilled nursing facility – even though they’d rather be at home,” he said.
Innovations at Northwell Health
Northwell Health, a multihospital comprehensive health system serving the New York City metro area and Long Island, has shown innovative leadership in addressing social factors. The 23-hospital system initiated in early 2019 a 15-item Self-Reported Social Determinants Screening Tool, which is now used with hospitalized patients to connect them with the support they need to fully recover and avoid readmissions.
Northwell is also providing professional education on social determinants for different constituencies across its system, said Johanna Martinez, MD, MS, a hospitalist and GME Director of Diversity and Health Equity at the Zucker School of Medicine at Hofstra/Northwell. A day-long training retreat was offered to GME faculty, and learning platforms have been developed for physicians, social workers, nurses, and others.
“One of the questions that comes up is that if you find social needs, what do you do about them?” Dr. Martinez explained. That’s more a difficult challenge, she said, so at Northwell, orthopedic surgeons are now asking patients questions like: “What’s going to happen when you go home? What are your social supports? Can you get to the physical therapist’s office?”
Another example of Northwell’s innovations is its Food as Health Program, initially piloted at Long Island Jewish Hospital in Valley Stream, N.Y. Hospitalized patients are asked two questions using a validated screening tool called the Hunger Vital Sign to identify their food insecurities.8 Those who answer yes are referred to a dietitian, and if they have a nutrition-related diagnosis, they enter the multidisciplinary wraparound program.
A key element is the food and health center, located on the hospital campus, where they can get food to take home and referrals to other services, with culturally tailored, disease-specific food education incorporated into the discharge plan. One of the partnering organizations is Island Harvest Food Bank, which helps about 1 in every 10 residents of Long Island with their food insecurity issues.
“When I talk to clinicians, most of us went into medicine to save lives and cure people. Yet the research shows that no matter who we are, we can’t do the best work that our patients need unless we consider their social determinants,” Dr. Martinez said. Ultimately, she noted, there is a need to change the culture of health care. “We have to create system change, reimbursement change, policy change.”
Omolara Uwemedimo, MD, MPH, associate professor of pediatrics and occupational medicine at Northwell and a former nocturnist, said the treatment of illness and health improvement don’t begin in the hospital, they begin in the community. Identifying where people are struggling and what communities they come from requires a broader view of the provider’s role. “Are patients who are readmitted to the hospital generally coming from certain demographics or from certain zip codes?” she asked. “Start there. How can we better connect with those communities?”
Education is key
In 2020 and beyond, hospitalists will hear more about the social determinants of health, Dr. Jacobs-Shaw concluded. “Without addressing those social determinants, we aren’t going to be able to meaningfully impact outcomes or be effective stewards of health care costs – addressing the psychosocial factors and root causes of patients coming in and out of the hospital.”
He added that self-education is key for hospitalists and the teams they work with – to be more aware of the link between health outcomes and social determinants. Guidelines and other resources on social determinants of health are available from the American College of Physicians and the American Association of Family Physicians. ACP issued a position paper on addressing social determinants of health to improve patient care,while AAFP has a research page on its website dedicated to social determinants of health, highlighting a number of initiatives and resources for physicians and others.9
The American Hospital Association has produced fact sheets on ICD-10CM code categories for social determinants of health, including 11 ICD-10 “Z” codes, numbered Z55-Z65, which can be used for coding interventions to address social determinants of health. Other experts are looking at how to adapt the electronic health record to capture sociodemographic and behavioral factors, and then trigger referrals to resources in the hospital and the broader community, and how to mobilize artificial intelligence and machine learning to better identify social needs.
“Our doctors really want to be able to take care of the whole patient, while being stewards of health care resources. But sometimes we feel powerless and wonder how we can have a bigger impact on people, on populations” Dr. Jacobs-Shaw said. “Remember it only takes one voice within an organization to start to elevate this topic.”
References
1. Rappleye E. Physicians say social determinants of health are not their responsibility. Becker’s Hospital Review. 2018 May 15.
2. Robert Wood Johnson Foundation, University of Wisconsin Population Health Institute. County Health Rankings, 2014.
3. Freeman, GA. The extensivist model. Health Leaders Magazine, 2016 Sep 15.
4. Kind AJ et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: A retrospective cohort study. Ann Intern Med. 2014 Dec 2;161(11):765-74.
5. National Institute for Health Care Management. Addressing social determinants of health can improve community health & reduce costs.
6. Vial PB. Boundless collaboration: A philosophy for sustainable and stabilizing housing investment strategies. Health Progress: Journal of the Catholic Health Association of the United States. September-October 2019.
7. Social determinants of health: New solutions for growing complexities. Op-Med, a blog by Sound Physicians. 2019 Aug 1.
8. The hunger vital sign: A new standard of care for preventive health.
9. Daniel H et al. Addressing social determinants to improve patient care and promote health equity: An American College of Physicians position paper. Ann Intern Med. 2018;168:557-578.
Are access to housing and food as important as therapeutics?
Are access to housing and food as important as therapeutics?
While physicians acknowledge that the social determinants of health can impact outcomes from medical care, some may feel that trying to address factors such as homelessness, food insecurity, or lack of ready access to transportation or pharmacy services is just not part of the doctor’s job. A majority of 621 physicians surveyed in the summer of 2017 by Salt Lake City–based health care intelligence firm Leavitt Partners say they are neither capable of nor responsible for addressing such issues.1
But that view may become unsustainable as the U.S. health care system continues to advance toward value- and population-based models of health care and as evidence mounts that social factors are important contributors to costly outcomes, such as avoidable hospital readmissions or emergency room visits. A recent report from the Robert Wood Johnson Foundation estimates that at least 40% of health outcomes are the result of social and economic factors, while only 20% can be attributed to medical care.2
“This is a hot topic – getting a lot of attention these days,” said hospitalist and care transitions expert Ramon Jacobs-Shaw, MD, MPA, regional medical officer for CareMore Health, a California-based physician-led health delivery organization and subsidiary of Anthem. “If you go around the country, some doctors still see social factors as the realm of the social worker. But large health care organizations are coming to recognize that social determinants are huge contributors to the health of their members and to the outcomes of their care.”
Hospitalists could be the natural providers to delve into the specific psychosocial aspects of their patients’ lives, or try to figure out how those factors contribute to health care needs, Dr. Jacobs-Shaw said. They typically confront such issues while the patient is in the hospital bed, but what are the steps that led to the hospitalization in the first place? What will happen after the patient is discharged?
“For example, if patients lack transportation, how can they get to their follow-up medical appointment in the primary care office in order to manage their diabetes? If you can’t follow up with them, their diabetes could get out of control, with complications as a result, such as an infected wound,” he said. Another big issue is access to affordable medications. “CareMore has pharmacists embedded on our care teams. They try to figure out the best medicine for the patient but at the lowest cost. They meet individually with patients and do medication counseling, particularly for those with polypharmacy issues.”
Making health care more equitable
Dr. Jacobs-Shaw has long held a personal interest in issues of inclusiveness, diversity, and how to make health care more equitable for historically underserved groups. Asking how to have a bigger impact on these issues is what brought him, after 13 years as a hospitalist on the East Coast, to CareMore, a company that has made addressing social needs central to its care model. “In California, where I am based, we are a wrap-around for patients who are covered by Medicare Advantage plans. We are whatever the patient needs us to be.”
He oversees a group of hospitalists, dubbed extensivists, who provide advanced patient care and chronic disease management. In the extensivist model, physicians and advanced practice nurses provide comprehensive and coordinated care to patients with complex medical issues, taking their scope of practice beyond the hospital into homes, post-acute care facilities, and other settings, with a focus on keeping patients healthier and reducing readmission.3
“Our patients get access to extra services and resources, some of which are available at our care centers – which are one-stop outpatient facilities. We also focus on a lot of things physicians didn’t historically think were within their wheelhouse. Hospitalists deal with these kinds of issues every day, but may not label them as social determinants of health,” Dr. Jacobs-Shaw said. He emphasized that hospitalists should realize that they are not powerless to address these issues, working in partnership with other groups in and out of the hospital. They should also know that health care payers increasingly are dedicating resources to these issues.
“We just started trying to address homelessness through a pilot in Orange County, working with nonprofit organizations and philanthropy to offer a transitional site of care for our patients who are being discharged from the hospital and have housing insecurity issues, to get them transitioned into more secure housing,” Dr. Jacobs-Shaw said. CareMore also has a transportation collaborative that offers no-cost, nonemergency transportation to medical appointments. “That’s meeting them where they are at, based on an assessment of their needs and resources.”
What are social determinants?
The social determinants of health – social, environmental, and other nonmedical factors that contribute to overall health status and medical need – have been defined by the World Health Organization as: “conditions in which people are born, grow, live, work, and age.” That is a broad complex of overlapping social and systems issues, but it provides a context for a broader understanding of the patient’s health and response to medical interventions.
Socioeconomic status is a huge determinant. Level of education may be more important than income if the person lacks the health literacy to navigate the system and access needed care. Housing instability may include poor sanitation, substandard dwellings, or unsafe neighborhoods – all of which can affect a person’s well-being. Environmental health may include compromised air quality – which can impact pulmonary health. Other issues include access to employment and child care, utility needs, and interpersonal violence.
A 2014 paper in Annals of Internal Medicine found that residence within a disadvantaged neighborhood was a factor in hospital readmission rates as often as was chronic pulmonary disease.4 A recent report on social determinants of health by the National Institute for Health Care Management notes that patients with food insecurity are 2.4 times more likely to go to the emergency room, while those with transportation needs are 2.6 times more likely.5
What can health care leaders do to better equip their clinicians and teams to help patients deal with this array of complex needs? Intermountain Healthcare, based in Salt Lake City, spearheaded in 2018 the development of the Alliance for the Determinants of Health, starting in the communities of Ogden and St. George, Utah. The Alliance seeks to promote health, improve access to care, and decrease health care costs through a charitable contribution of $12 million over 3 years to seed collaborative demonstration projects.
Lisa Nichols, assistant vice president for community health at Intermountain, said that, while hospitalists were not directly involved in planning the Alliance, hospitalists and ED physicians have become essential to the patient-screening process for health and social needs.
“We met with hospitalists, emergency departments, and hospital administrators, because we wanted their feedback on how to raise awareness of the social needs of patients,” she said. “They have good ideas. They see the patients who come in from the homeless shelters.”
Other hospitals are subsidizing apartments for homeless patients being discharged from the hospital. CommonSpirit Health, the new national Catholic health care organization formed by the 2019 merger of Dignity Health and Catholic Health Initiatives, has explored how to help create and sustain affordable housing in the communities it serves. Investments like this have inspired others, such as Kaiser Permanente, to get involved in supporting housing initiatives.6
Comprehensive community care
David Meltzer, MD, PhD, a hospitalist and professor of medicine at the University of Chicago, said most hospitalists these days believe social determinants of health are part of their job responsibilities.
“That’s not to say we all do it well. We may fail at addressing some of the barriers our patients face. But I don’t know anyone who still says it’s not their job,” he said.
Since 2012, Dr. Meltzer has led a pilot called Comprehensive Care Physicians (CCP), in which the same physician cares for patients with chronic health problems in the clinic and in the hospital, working with a team of nurse practitioners, social workers, care coordinators, and other specialists. A total of 2,000 patients with chronic health problems were enrolled in the study from 2012 to 2016, half assigned to standard care and half assigned to five CCP doctors. The result: The CCP model has shown large improvements in outcomes – particularly among the more vulnerable, less activated patients, is preferred by patients, and has significantly reduced health care utilization.
The next step for the research team is another randomized controlled trial called Comprehensive Care, Community, and Culture, designed to address unmet social needs. Study group patients will also be screened for unmet social needs and have access to a community health worker and to the initiative’s Artful Living Program, which includes community and cultural activities like yoga and dance classes, cooking classes, art classes, and music concerts. To address the complex dimensions and determinants of health, Dr. Meltzer explained, efforts to improve health must extend to sectors far beyond traditional health care.
“I think trying to understand your patients’ social and nonmedical needs starts with getting to know them, and asking about their needs,” he said. “The better you know them, the better you are able to make medical decisions that will promote positive outcomes.”
Sound Physicians, a national hospitalist company based in Tacoma, Wash., and working in 350 hospitals in 41 states, recently published a blog post on its website about the importance of social determinants of health.7 Sound Physicians participates in value-based care through bundled Medicare/Medicaid contracts based on episodes of care for hospitalized patients with certain diagnoses or DRGs, explained John Dickey, MD, the company’s chief medical officer for population health.
“We’ve been heavily involved in trying to improve cost and outcomes of care since 2015. Social determinants absolutely play into trying to lower costs of care and reduce rates of readmissions, which are often multifactorial in cause,” he said. Hospitalists are uniquely equipped to impact post-acute outcomes, Dr. Dickey said, working in partnership with a position Sound Physicians calls the clinical performance nurse.
“We can also partner with primary care providers, provide education for our hospitalist staff, and work with in-home care supports for patients such as these, who otherwise might end up in a skilled nursing facility – even though they’d rather be at home,” he said.
Innovations at Northwell Health
Northwell Health, a multihospital comprehensive health system serving the New York City metro area and Long Island, has shown innovative leadership in addressing social factors. The 23-hospital system initiated in early 2019 a 15-item Self-Reported Social Determinants Screening Tool, which is now used with hospitalized patients to connect them with the support they need to fully recover and avoid readmissions.
Northwell is also providing professional education on social determinants for different constituencies across its system, said Johanna Martinez, MD, MS, a hospitalist and GME Director of Diversity and Health Equity at the Zucker School of Medicine at Hofstra/Northwell. A day-long training retreat was offered to GME faculty, and learning platforms have been developed for physicians, social workers, nurses, and others.
“One of the questions that comes up is that if you find social needs, what do you do about them?” Dr. Martinez explained. That’s more a difficult challenge, she said, so at Northwell, orthopedic surgeons are now asking patients questions like: “What’s going to happen when you go home? What are your social supports? Can you get to the physical therapist’s office?”
Another example of Northwell’s innovations is its Food as Health Program, initially piloted at Long Island Jewish Hospital in Valley Stream, N.Y. Hospitalized patients are asked two questions using a validated screening tool called the Hunger Vital Sign to identify their food insecurities.8 Those who answer yes are referred to a dietitian, and if they have a nutrition-related diagnosis, they enter the multidisciplinary wraparound program.
A key element is the food and health center, located on the hospital campus, where they can get food to take home and referrals to other services, with culturally tailored, disease-specific food education incorporated into the discharge plan. One of the partnering organizations is Island Harvest Food Bank, which helps about 1 in every 10 residents of Long Island with their food insecurity issues.
“When I talk to clinicians, most of us went into medicine to save lives and cure people. Yet the research shows that no matter who we are, we can’t do the best work that our patients need unless we consider their social determinants,” Dr. Martinez said. Ultimately, she noted, there is a need to change the culture of health care. “We have to create system change, reimbursement change, policy change.”
Omolara Uwemedimo, MD, MPH, associate professor of pediatrics and occupational medicine at Northwell and a former nocturnist, said the treatment of illness and health improvement don’t begin in the hospital, they begin in the community. Identifying where people are struggling and what communities they come from requires a broader view of the provider’s role. “Are patients who are readmitted to the hospital generally coming from certain demographics or from certain zip codes?” she asked. “Start there. How can we better connect with those communities?”
Education is key
In 2020 and beyond, hospitalists will hear more about the social determinants of health, Dr. Jacobs-Shaw concluded. “Without addressing those social determinants, we aren’t going to be able to meaningfully impact outcomes or be effective stewards of health care costs – addressing the psychosocial factors and root causes of patients coming in and out of the hospital.”
He added that self-education is key for hospitalists and the teams they work with – to be more aware of the link between health outcomes and social determinants. Guidelines and other resources on social determinants of health are available from the American College of Physicians and the American Association of Family Physicians. ACP issued a position paper on addressing social determinants of health to improve patient care,while AAFP has a research page on its website dedicated to social determinants of health, highlighting a number of initiatives and resources for physicians and others.9
The American Hospital Association has produced fact sheets on ICD-10CM code categories for social determinants of health, including 11 ICD-10 “Z” codes, numbered Z55-Z65, which can be used for coding interventions to address social determinants of health. Other experts are looking at how to adapt the electronic health record to capture sociodemographic and behavioral factors, and then trigger referrals to resources in the hospital and the broader community, and how to mobilize artificial intelligence and machine learning to better identify social needs.
“Our doctors really want to be able to take care of the whole patient, while being stewards of health care resources. But sometimes we feel powerless and wonder how we can have a bigger impact on people, on populations” Dr. Jacobs-Shaw said. “Remember it only takes one voice within an organization to start to elevate this topic.”
References
1. Rappleye E. Physicians say social determinants of health are not their responsibility. Becker’s Hospital Review. 2018 May 15.
2. Robert Wood Johnson Foundation, University of Wisconsin Population Health Institute. County Health Rankings, 2014.
3. Freeman, GA. The extensivist model. Health Leaders Magazine, 2016 Sep 15.
4. Kind AJ et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: A retrospective cohort study. Ann Intern Med. 2014 Dec 2;161(11):765-74.
5. National Institute for Health Care Management. Addressing social determinants of health can improve community health & reduce costs.
6. Vial PB. Boundless collaboration: A philosophy for sustainable and stabilizing housing investment strategies. Health Progress: Journal of the Catholic Health Association of the United States. September-October 2019.
7. Social determinants of health: New solutions for growing complexities. Op-Med, a blog by Sound Physicians. 2019 Aug 1.
8. The hunger vital sign: A new standard of care for preventive health.
9. Daniel H et al. Addressing social determinants to improve patient care and promote health equity: An American College of Physicians position paper. Ann Intern Med. 2018;168:557-578.
While physicians acknowledge that the social determinants of health can impact outcomes from medical care, some may feel that trying to address factors such as homelessness, food insecurity, or lack of ready access to transportation or pharmacy services is just not part of the doctor’s job. A majority of 621 physicians surveyed in the summer of 2017 by Salt Lake City–based health care intelligence firm Leavitt Partners say they are neither capable of nor responsible for addressing such issues.1
But that view may become unsustainable as the U.S. health care system continues to advance toward value- and population-based models of health care and as evidence mounts that social factors are important contributors to costly outcomes, such as avoidable hospital readmissions or emergency room visits. A recent report from the Robert Wood Johnson Foundation estimates that at least 40% of health outcomes are the result of social and economic factors, while only 20% can be attributed to medical care.2
“This is a hot topic – getting a lot of attention these days,” said hospitalist and care transitions expert Ramon Jacobs-Shaw, MD, MPA, regional medical officer for CareMore Health, a California-based physician-led health delivery organization and subsidiary of Anthem. “If you go around the country, some doctors still see social factors as the realm of the social worker. But large health care organizations are coming to recognize that social determinants are huge contributors to the health of their members and to the outcomes of their care.”
Hospitalists could be the natural providers to delve into the specific psychosocial aspects of their patients’ lives, or try to figure out how those factors contribute to health care needs, Dr. Jacobs-Shaw said. They typically confront such issues while the patient is in the hospital bed, but what are the steps that led to the hospitalization in the first place? What will happen after the patient is discharged?
“For example, if patients lack transportation, how can they get to their follow-up medical appointment in the primary care office in order to manage their diabetes? If you can’t follow up with them, their diabetes could get out of control, with complications as a result, such as an infected wound,” he said. Another big issue is access to affordable medications. “CareMore has pharmacists embedded on our care teams. They try to figure out the best medicine for the patient but at the lowest cost. They meet individually with patients and do medication counseling, particularly for those with polypharmacy issues.”
Making health care more equitable
Dr. Jacobs-Shaw has long held a personal interest in issues of inclusiveness, diversity, and how to make health care more equitable for historically underserved groups. Asking how to have a bigger impact on these issues is what brought him, after 13 years as a hospitalist on the East Coast, to CareMore, a company that has made addressing social needs central to its care model. “In California, where I am based, we are a wrap-around for patients who are covered by Medicare Advantage plans. We are whatever the patient needs us to be.”
He oversees a group of hospitalists, dubbed extensivists, who provide advanced patient care and chronic disease management. In the extensivist model, physicians and advanced practice nurses provide comprehensive and coordinated care to patients with complex medical issues, taking their scope of practice beyond the hospital into homes, post-acute care facilities, and other settings, with a focus on keeping patients healthier and reducing readmission.3
“Our patients get access to extra services and resources, some of which are available at our care centers – which are one-stop outpatient facilities. We also focus on a lot of things physicians didn’t historically think were within their wheelhouse. Hospitalists deal with these kinds of issues every day, but may not label them as social determinants of health,” Dr. Jacobs-Shaw said. He emphasized that hospitalists should realize that they are not powerless to address these issues, working in partnership with other groups in and out of the hospital. They should also know that health care payers increasingly are dedicating resources to these issues.
“We just started trying to address homelessness through a pilot in Orange County, working with nonprofit organizations and philanthropy to offer a transitional site of care for our patients who are being discharged from the hospital and have housing insecurity issues, to get them transitioned into more secure housing,” Dr. Jacobs-Shaw said. CareMore also has a transportation collaborative that offers no-cost, nonemergency transportation to medical appointments. “That’s meeting them where they are at, based on an assessment of their needs and resources.”
What are social determinants?
The social determinants of health – social, environmental, and other nonmedical factors that contribute to overall health status and medical need – have been defined by the World Health Organization as: “conditions in which people are born, grow, live, work, and age.” That is a broad complex of overlapping social and systems issues, but it provides a context for a broader understanding of the patient’s health and response to medical interventions.
Socioeconomic status is a huge determinant. Level of education may be more important than income if the person lacks the health literacy to navigate the system and access needed care. Housing instability may include poor sanitation, substandard dwellings, or unsafe neighborhoods – all of which can affect a person’s well-being. Environmental health may include compromised air quality – which can impact pulmonary health. Other issues include access to employment and child care, utility needs, and interpersonal violence.
A 2014 paper in Annals of Internal Medicine found that residence within a disadvantaged neighborhood was a factor in hospital readmission rates as often as was chronic pulmonary disease.4 A recent report on social determinants of health by the National Institute for Health Care Management notes that patients with food insecurity are 2.4 times more likely to go to the emergency room, while those with transportation needs are 2.6 times more likely.5
What can health care leaders do to better equip their clinicians and teams to help patients deal with this array of complex needs? Intermountain Healthcare, based in Salt Lake City, spearheaded in 2018 the development of the Alliance for the Determinants of Health, starting in the communities of Ogden and St. George, Utah. The Alliance seeks to promote health, improve access to care, and decrease health care costs through a charitable contribution of $12 million over 3 years to seed collaborative demonstration projects.
Lisa Nichols, assistant vice president for community health at Intermountain, said that, while hospitalists were not directly involved in planning the Alliance, hospitalists and ED physicians have become essential to the patient-screening process for health and social needs.
“We met with hospitalists, emergency departments, and hospital administrators, because we wanted their feedback on how to raise awareness of the social needs of patients,” she said. “They have good ideas. They see the patients who come in from the homeless shelters.”
Other hospitals are subsidizing apartments for homeless patients being discharged from the hospital. CommonSpirit Health, the new national Catholic health care organization formed by the 2019 merger of Dignity Health and Catholic Health Initiatives, has explored how to help create and sustain affordable housing in the communities it serves. Investments like this have inspired others, such as Kaiser Permanente, to get involved in supporting housing initiatives.6
Comprehensive community care
David Meltzer, MD, PhD, a hospitalist and professor of medicine at the University of Chicago, said most hospitalists these days believe social determinants of health are part of their job responsibilities.
“That’s not to say we all do it well. We may fail at addressing some of the barriers our patients face. But I don’t know anyone who still says it’s not their job,” he said.
Since 2012, Dr. Meltzer has led a pilot called Comprehensive Care Physicians (CCP), in which the same physician cares for patients with chronic health problems in the clinic and in the hospital, working with a team of nurse practitioners, social workers, care coordinators, and other specialists. A total of 2,000 patients with chronic health problems were enrolled in the study from 2012 to 2016, half assigned to standard care and half assigned to five CCP doctors. The result: The CCP model has shown large improvements in outcomes – particularly among the more vulnerable, less activated patients, is preferred by patients, and has significantly reduced health care utilization.
The next step for the research team is another randomized controlled trial called Comprehensive Care, Community, and Culture, designed to address unmet social needs. Study group patients will also be screened for unmet social needs and have access to a community health worker and to the initiative’s Artful Living Program, which includes community and cultural activities like yoga and dance classes, cooking classes, art classes, and music concerts. To address the complex dimensions and determinants of health, Dr. Meltzer explained, efforts to improve health must extend to sectors far beyond traditional health care.
“I think trying to understand your patients’ social and nonmedical needs starts with getting to know them, and asking about their needs,” he said. “The better you know them, the better you are able to make medical decisions that will promote positive outcomes.”
Sound Physicians, a national hospitalist company based in Tacoma, Wash., and working in 350 hospitals in 41 states, recently published a blog post on its website about the importance of social determinants of health.7 Sound Physicians participates in value-based care through bundled Medicare/Medicaid contracts based on episodes of care for hospitalized patients with certain diagnoses or DRGs, explained John Dickey, MD, the company’s chief medical officer for population health.
“We’ve been heavily involved in trying to improve cost and outcomes of care since 2015. Social determinants absolutely play into trying to lower costs of care and reduce rates of readmissions, which are often multifactorial in cause,” he said. Hospitalists are uniquely equipped to impact post-acute outcomes, Dr. Dickey said, working in partnership with a position Sound Physicians calls the clinical performance nurse.
“We can also partner with primary care providers, provide education for our hospitalist staff, and work with in-home care supports for patients such as these, who otherwise might end up in a skilled nursing facility – even though they’d rather be at home,” he said.
Innovations at Northwell Health
Northwell Health, a multihospital comprehensive health system serving the New York City metro area and Long Island, has shown innovative leadership in addressing social factors. The 23-hospital system initiated in early 2019 a 15-item Self-Reported Social Determinants Screening Tool, which is now used with hospitalized patients to connect them with the support they need to fully recover and avoid readmissions.
Northwell is also providing professional education on social determinants for different constituencies across its system, said Johanna Martinez, MD, MS, a hospitalist and GME Director of Diversity and Health Equity at the Zucker School of Medicine at Hofstra/Northwell. A day-long training retreat was offered to GME faculty, and learning platforms have been developed for physicians, social workers, nurses, and others.
“One of the questions that comes up is that if you find social needs, what do you do about them?” Dr. Martinez explained. That’s more a difficult challenge, she said, so at Northwell, orthopedic surgeons are now asking patients questions like: “What’s going to happen when you go home? What are your social supports? Can you get to the physical therapist’s office?”
Another example of Northwell’s innovations is its Food as Health Program, initially piloted at Long Island Jewish Hospital in Valley Stream, N.Y. Hospitalized patients are asked two questions using a validated screening tool called the Hunger Vital Sign to identify their food insecurities.8 Those who answer yes are referred to a dietitian, and if they have a nutrition-related diagnosis, they enter the multidisciplinary wraparound program.
A key element is the food and health center, located on the hospital campus, where they can get food to take home and referrals to other services, with culturally tailored, disease-specific food education incorporated into the discharge plan. One of the partnering organizations is Island Harvest Food Bank, which helps about 1 in every 10 residents of Long Island with their food insecurity issues.
“When I talk to clinicians, most of us went into medicine to save lives and cure people. Yet the research shows that no matter who we are, we can’t do the best work that our patients need unless we consider their social determinants,” Dr. Martinez said. Ultimately, she noted, there is a need to change the culture of health care. “We have to create system change, reimbursement change, policy change.”
Omolara Uwemedimo, MD, MPH, associate professor of pediatrics and occupational medicine at Northwell and a former nocturnist, said the treatment of illness and health improvement don’t begin in the hospital, they begin in the community. Identifying where people are struggling and what communities they come from requires a broader view of the provider’s role. “Are patients who are readmitted to the hospital generally coming from certain demographics or from certain zip codes?” she asked. “Start there. How can we better connect with those communities?”
Education is key
In 2020 and beyond, hospitalists will hear more about the social determinants of health, Dr. Jacobs-Shaw concluded. “Without addressing those social determinants, we aren’t going to be able to meaningfully impact outcomes or be effective stewards of health care costs – addressing the psychosocial factors and root causes of patients coming in and out of the hospital.”
He added that self-education is key for hospitalists and the teams they work with – to be more aware of the link between health outcomes and social determinants. Guidelines and other resources on social determinants of health are available from the American College of Physicians and the American Association of Family Physicians. ACP issued a position paper on addressing social determinants of health to improve patient care,while AAFP has a research page on its website dedicated to social determinants of health, highlighting a number of initiatives and resources for physicians and others.9
The American Hospital Association has produced fact sheets on ICD-10CM code categories for social determinants of health, including 11 ICD-10 “Z” codes, numbered Z55-Z65, which can be used for coding interventions to address social determinants of health. Other experts are looking at how to adapt the electronic health record to capture sociodemographic and behavioral factors, and then trigger referrals to resources in the hospital and the broader community, and how to mobilize artificial intelligence and machine learning to better identify social needs.
“Our doctors really want to be able to take care of the whole patient, while being stewards of health care resources. But sometimes we feel powerless and wonder how we can have a bigger impact on people, on populations” Dr. Jacobs-Shaw said. “Remember it only takes one voice within an organization to start to elevate this topic.”
References
1. Rappleye E. Physicians say social determinants of health are not their responsibility. Becker’s Hospital Review. 2018 May 15.
2. Robert Wood Johnson Foundation, University of Wisconsin Population Health Institute. County Health Rankings, 2014.
3. Freeman, GA. The extensivist model. Health Leaders Magazine, 2016 Sep 15.
4. Kind AJ et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: A retrospective cohort study. Ann Intern Med. 2014 Dec 2;161(11):765-74.
5. National Institute for Health Care Management. Addressing social determinants of health can improve community health & reduce costs.
6. Vial PB. Boundless collaboration: A philosophy for sustainable and stabilizing housing investment strategies. Health Progress: Journal of the Catholic Health Association of the United States. September-October 2019.
7. Social determinants of health: New solutions for growing complexities. Op-Med, a blog by Sound Physicians. 2019 Aug 1.
8. The hunger vital sign: A new standard of care for preventive health.
9. Daniel H et al. Addressing social determinants to improve patient care and promote health equity: An American College of Physicians position paper. Ann Intern Med. 2018;168:557-578.
Cardiac arrest: Targeted temperature management a game changer
SNOWMASS, COLO. – Targeted temperature management maintained at 32-36 degrees Celsius is now a strong class I recommendation for all comatose patients who experience return of spontaneous circulation after out-of-hospital cardiac arrest, including those with nonshockable rhythms, Erin A. Bohula, MD, PhD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Our practice is that ,” said Dr. Bohula, a cardiologist and critical care specialist at Brigham and Women’s Hospital and Harvard Medical School, Boston.
The current ACC/AHA guidelines declare: “There are essentially no patients for whom temperature control somewhere in the range between 32 degrees C [89.6 F) and 36 degrees C [96.8 F] is contraindicated.” The writing committee cited “recent clinical trial data enrolling patients with all rhythms, the rarity of adverse effects in trials, the high neurologic morbidity and mortality without any specific interventions, and the preponderance of data suggesting that temperature is an important variable for neurologic recovery” (Circulation. 2015 Nov 3;132[18 Suppl 2]:S465-82).
“That’s a pretty strong statement,” Dr. Bohula observed.
The current guidelines, which date back to 2015, give a class I, level of evidence B recommendation for targeted temperature management (TTM) in patients who are comatose with return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest involving ventricular fibrillation or pulseless ventricular fibrillation. The bedside definition of comatose is lack of meaningful response to verbal commands to squeeze hands, blink, or move toes.
The current recommendation for TTM in patients resuscitated from out-of-hospital cardiac arrest with a nonshockable rhythm is class I, level of evidence C, meaning it’s based on expert consensus. However, that recommendation is now out of date and due for a level-of-evidence upgrade in light of the recent results of the French HYPERION trial, an open-label randomized trial of 584 patients resuscitated from cardiac arrest with a nonshockable rhythm. Although 90-day mortality was similarly high in the TTM and targeted normothermia groups, the rate of favorable neurologic outcome as assessed by a Cerebral Performance Category scale score of 1 or 2 was 10.2% in the TTM group, significantly better than the 5.7% rate in controls (N Engl J Med. 2019 Dec 12;381[24]:2327-37).
The 2010, ACC/AHA guidelines recommended a TTM range of 32-34 degrees C, but on the basis of subsequent persuasive randomized trial data, that range was broadened to 32-36 degrees C in the 2015 guidelines, with a class IB recommendation. Maintenance of TTM for at least 24 hours has a IIa, level of evidence C recommendation in the current guidelines.
The guidelines emphasize that specific features may favor selection of one temperature for TTM over another. For example, patients with seizures or cerebral edema might be better off with TTM at a lower temperature, while a higher temperature may be best for those with bleeding or severe bradycardia. At Brigham and Women’s Hospital, the default temperature is 33 degrees C. However, TTM with a goal of 36 degrees C is seriously considered in patients with recent head trauma, major surgery within the past 2 weeks, refractory hypotension, severe sepsis, pregnancy, or high bleeding risk. Rewarming is done at a rate of 0.25 degrees C per hour, with sedation maintained until the patient has been returned to 98.6 degrees F, according to Dr. Bohula.
Based on several negative studies of TTM using rapid infusion of chilled fluids in the ambulance en route to the hospital, the guidelines rate that practice class IIIA, meaning don’t do it. Avoidance of a systolic blood pressure below 90 mm Hg and a mean arterial pressure of less than 65 mm Hg gets a class IIb level of evidence C recommendation to lessen the risk of cerebral hypoxia.
TTM a major breakthrough
Prior to the introduction of TTM, comatose patients with ROSC after out-of-hospital cardiac arrest had a dreadful prognosis, with survival rates of 1%-10% in registry studies. In contrast, the survival rate in the landmark TTM clinical trials was 50%-60%. And while that’s a dramatic improvement, ROSC after cardiac arrest remains a high-mortality condition. Dr. Bohula was first author of a report by the Critical Care Cardiology Trials Network, composed of 16 tertiary cardiac intensive care units in the United States and Canada. Cardiac arrest was the primary indication for 8.7% of 3,049 consecutive admissions, and its 38% mortality rate was the highest of all cardiac critical care indications (JAMA Cardiol. 2019 Jul 24;4[9]:928-35).
TTM was developed in response to a recognition that two-thirds of deaths in patients who make it to the hospital after out-of-hospital cardiac arrest are neurologic – the result of brain anoxia – rather than being due to the myocardial ischemia that may have initially brought them to medical attention.
“Time is brain cells, the same way we think of time as cardiac muscle,” Dr. Bohula observed.
The main idea behind therapeutic hypothermia is that it lowers the cerebral metabolic rate of oxygen to reduce the consequences of ongoing anoxia. The brain doesn’t require as much perfusion when cooled.
TTM has other beneficial neurologic effects as well: It reduces cerebral blood volume via autoregulation, decreases intracranial pressure, and blunts the inflammatory response involved in the postcardiac arrest syndrome. In addition, TTM has anticonvulsant properties, an important effect because seizures and/or myoclonus occur in up to 15% of adults who achieve ROSC after cardiac arrest – and in even more of those who are comatose after doing so. And seizures increase the brain’s metabolic rate threefold, resulting in more cerebral ischemic injury, she explained.
Seizure activity can be difficult to distinguish from shivering in a patient on TTM. For this reason Dr. Bohula recommends putting patients on continuous EEG monitoring from the time of admission, as is the routine practice at the Brigham.
She reported serving as a consultant to Daiichi Sankyo, Servier, Lexicon, Kowa, Merck, Novartis, Novo Nordisk, and the National Institutes of Health. In addition, she generates institutional research grants provided by a half-dozen pharmaceutical companies.
SNOWMASS, COLO. – Targeted temperature management maintained at 32-36 degrees Celsius is now a strong class I recommendation for all comatose patients who experience return of spontaneous circulation after out-of-hospital cardiac arrest, including those with nonshockable rhythms, Erin A. Bohula, MD, PhD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Our practice is that ,” said Dr. Bohula, a cardiologist and critical care specialist at Brigham and Women’s Hospital and Harvard Medical School, Boston.
The current ACC/AHA guidelines declare: “There are essentially no patients for whom temperature control somewhere in the range between 32 degrees C [89.6 F) and 36 degrees C [96.8 F] is contraindicated.” The writing committee cited “recent clinical trial data enrolling patients with all rhythms, the rarity of adverse effects in trials, the high neurologic morbidity and mortality without any specific interventions, and the preponderance of data suggesting that temperature is an important variable for neurologic recovery” (Circulation. 2015 Nov 3;132[18 Suppl 2]:S465-82).
“That’s a pretty strong statement,” Dr. Bohula observed.
The current guidelines, which date back to 2015, give a class I, level of evidence B recommendation for targeted temperature management (TTM) in patients who are comatose with return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest involving ventricular fibrillation or pulseless ventricular fibrillation. The bedside definition of comatose is lack of meaningful response to verbal commands to squeeze hands, blink, or move toes.
The current recommendation for TTM in patients resuscitated from out-of-hospital cardiac arrest with a nonshockable rhythm is class I, level of evidence C, meaning it’s based on expert consensus. However, that recommendation is now out of date and due for a level-of-evidence upgrade in light of the recent results of the French HYPERION trial, an open-label randomized trial of 584 patients resuscitated from cardiac arrest with a nonshockable rhythm. Although 90-day mortality was similarly high in the TTM and targeted normothermia groups, the rate of favorable neurologic outcome as assessed by a Cerebral Performance Category scale score of 1 or 2 was 10.2% in the TTM group, significantly better than the 5.7% rate in controls (N Engl J Med. 2019 Dec 12;381[24]:2327-37).
The 2010, ACC/AHA guidelines recommended a TTM range of 32-34 degrees C, but on the basis of subsequent persuasive randomized trial data, that range was broadened to 32-36 degrees C in the 2015 guidelines, with a class IB recommendation. Maintenance of TTM for at least 24 hours has a IIa, level of evidence C recommendation in the current guidelines.
The guidelines emphasize that specific features may favor selection of one temperature for TTM over another. For example, patients with seizures or cerebral edema might be better off with TTM at a lower temperature, while a higher temperature may be best for those with bleeding or severe bradycardia. At Brigham and Women’s Hospital, the default temperature is 33 degrees C. However, TTM with a goal of 36 degrees C is seriously considered in patients with recent head trauma, major surgery within the past 2 weeks, refractory hypotension, severe sepsis, pregnancy, or high bleeding risk. Rewarming is done at a rate of 0.25 degrees C per hour, with sedation maintained until the patient has been returned to 98.6 degrees F, according to Dr. Bohula.
Based on several negative studies of TTM using rapid infusion of chilled fluids in the ambulance en route to the hospital, the guidelines rate that practice class IIIA, meaning don’t do it. Avoidance of a systolic blood pressure below 90 mm Hg and a mean arterial pressure of less than 65 mm Hg gets a class IIb level of evidence C recommendation to lessen the risk of cerebral hypoxia.
TTM a major breakthrough
Prior to the introduction of TTM, comatose patients with ROSC after out-of-hospital cardiac arrest had a dreadful prognosis, with survival rates of 1%-10% in registry studies. In contrast, the survival rate in the landmark TTM clinical trials was 50%-60%. And while that’s a dramatic improvement, ROSC after cardiac arrest remains a high-mortality condition. Dr. Bohula was first author of a report by the Critical Care Cardiology Trials Network, composed of 16 tertiary cardiac intensive care units in the United States and Canada. Cardiac arrest was the primary indication for 8.7% of 3,049 consecutive admissions, and its 38% mortality rate was the highest of all cardiac critical care indications (JAMA Cardiol. 2019 Jul 24;4[9]:928-35).
TTM was developed in response to a recognition that two-thirds of deaths in patients who make it to the hospital after out-of-hospital cardiac arrest are neurologic – the result of brain anoxia – rather than being due to the myocardial ischemia that may have initially brought them to medical attention.
“Time is brain cells, the same way we think of time as cardiac muscle,” Dr. Bohula observed.
The main idea behind therapeutic hypothermia is that it lowers the cerebral metabolic rate of oxygen to reduce the consequences of ongoing anoxia. The brain doesn’t require as much perfusion when cooled.
TTM has other beneficial neurologic effects as well: It reduces cerebral blood volume via autoregulation, decreases intracranial pressure, and blunts the inflammatory response involved in the postcardiac arrest syndrome. In addition, TTM has anticonvulsant properties, an important effect because seizures and/or myoclonus occur in up to 15% of adults who achieve ROSC after cardiac arrest – and in even more of those who are comatose after doing so. And seizures increase the brain’s metabolic rate threefold, resulting in more cerebral ischemic injury, she explained.
Seizure activity can be difficult to distinguish from shivering in a patient on TTM. For this reason Dr. Bohula recommends putting patients on continuous EEG monitoring from the time of admission, as is the routine practice at the Brigham.
She reported serving as a consultant to Daiichi Sankyo, Servier, Lexicon, Kowa, Merck, Novartis, Novo Nordisk, and the National Institutes of Health. In addition, she generates institutional research grants provided by a half-dozen pharmaceutical companies.
SNOWMASS, COLO. – Targeted temperature management maintained at 32-36 degrees Celsius is now a strong class I recommendation for all comatose patients who experience return of spontaneous circulation after out-of-hospital cardiac arrest, including those with nonshockable rhythms, Erin A. Bohula, MD, PhD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Our practice is that ,” said Dr. Bohula, a cardiologist and critical care specialist at Brigham and Women’s Hospital and Harvard Medical School, Boston.
The current ACC/AHA guidelines declare: “There are essentially no patients for whom temperature control somewhere in the range between 32 degrees C [89.6 F) and 36 degrees C [96.8 F] is contraindicated.” The writing committee cited “recent clinical trial data enrolling patients with all rhythms, the rarity of adverse effects in trials, the high neurologic morbidity and mortality without any specific interventions, and the preponderance of data suggesting that temperature is an important variable for neurologic recovery” (Circulation. 2015 Nov 3;132[18 Suppl 2]:S465-82).
“That’s a pretty strong statement,” Dr. Bohula observed.
The current guidelines, which date back to 2015, give a class I, level of evidence B recommendation for targeted temperature management (TTM) in patients who are comatose with return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest involving ventricular fibrillation or pulseless ventricular fibrillation. The bedside definition of comatose is lack of meaningful response to verbal commands to squeeze hands, blink, or move toes.
The current recommendation for TTM in patients resuscitated from out-of-hospital cardiac arrest with a nonshockable rhythm is class I, level of evidence C, meaning it’s based on expert consensus. However, that recommendation is now out of date and due for a level-of-evidence upgrade in light of the recent results of the French HYPERION trial, an open-label randomized trial of 584 patients resuscitated from cardiac arrest with a nonshockable rhythm. Although 90-day mortality was similarly high in the TTM and targeted normothermia groups, the rate of favorable neurologic outcome as assessed by a Cerebral Performance Category scale score of 1 or 2 was 10.2% in the TTM group, significantly better than the 5.7% rate in controls (N Engl J Med. 2019 Dec 12;381[24]:2327-37).
The 2010, ACC/AHA guidelines recommended a TTM range of 32-34 degrees C, but on the basis of subsequent persuasive randomized trial data, that range was broadened to 32-36 degrees C in the 2015 guidelines, with a class IB recommendation. Maintenance of TTM for at least 24 hours has a IIa, level of evidence C recommendation in the current guidelines.
The guidelines emphasize that specific features may favor selection of one temperature for TTM over another. For example, patients with seizures or cerebral edema might be better off with TTM at a lower temperature, while a higher temperature may be best for those with bleeding or severe bradycardia. At Brigham and Women’s Hospital, the default temperature is 33 degrees C. However, TTM with a goal of 36 degrees C is seriously considered in patients with recent head trauma, major surgery within the past 2 weeks, refractory hypotension, severe sepsis, pregnancy, or high bleeding risk. Rewarming is done at a rate of 0.25 degrees C per hour, with sedation maintained until the patient has been returned to 98.6 degrees F, according to Dr. Bohula.
Based on several negative studies of TTM using rapid infusion of chilled fluids in the ambulance en route to the hospital, the guidelines rate that practice class IIIA, meaning don’t do it. Avoidance of a systolic blood pressure below 90 mm Hg and a mean arterial pressure of less than 65 mm Hg gets a class IIb level of evidence C recommendation to lessen the risk of cerebral hypoxia.
TTM a major breakthrough
Prior to the introduction of TTM, comatose patients with ROSC after out-of-hospital cardiac arrest had a dreadful prognosis, with survival rates of 1%-10% in registry studies. In contrast, the survival rate in the landmark TTM clinical trials was 50%-60%. And while that’s a dramatic improvement, ROSC after cardiac arrest remains a high-mortality condition. Dr. Bohula was first author of a report by the Critical Care Cardiology Trials Network, composed of 16 tertiary cardiac intensive care units in the United States and Canada. Cardiac arrest was the primary indication for 8.7% of 3,049 consecutive admissions, and its 38% mortality rate was the highest of all cardiac critical care indications (JAMA Cardiol. 2019 Jul 24;4[9]:928-35).
TTM was developed in response to a recognition that two-thirds of deaths in patients who make it to the hospital after out-of-hospital cardiac arrest are neurologic – the result of brain anoxia – rather than being due to the myocardial ischemia that may have initially brought them to medical attention.
“Time is brain cells, the same way we think of time as cardiac muscle,” Dr. Bohula observed.
The main idea behind therapeutic hypothermia is that it lowers the cerebral metabolic rate of oxygen to reduce the consequences of ongoing anoxia. The brain doesn’t require as much perfusion when cooled.
TTM has other beneficial neurologic effects as well: It reduces cerebral blood volume via autoregulation, decreases intracranial pressure, and blunts the inflammatory response involved in the postcardiac arrest syndrome. In addition, TTM has anticonvulsant properties, an important effect because seizures and/or myoclonus occur in up to 15% of adults who achieve ROSC after cardiac arrest – and in even more of those who are comatose after doing so. And seizures increase the brain’s metabolic rate threefold, resulting in more cerebral ischemic injury, she explained.
Seizure activity can be difficult to distinguish from shivering in a patient on TTM. For this reason Dr. Bohula recommends putting patients on continuous EEG monitoring from the time of admission, as is the routine practice at the Brigham.
She reported serving as a consultant to Daiichi Sankyo, Servier, Lexicon, Kowa, Merck, Novartis, Novo Nordisk, and the National Institutes of Health. In addition, she generates institutional research grants provided by a half-dozen pharmaceutical companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
CDC begins coronavirus diagnostic test kit distribution; new case confirmed in Wisconsin
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
The Centers for Disease Control and Prevention and the Wisconsin Department of Health Services confirmed a new case of the 2019 Novel Coronavirus (2019-nCoV) on Feb. 5, 2020, bringing the total number of cases in the United States to 12.*
Earlier in the day, Nancy Messonnier, MD, director of the CDC National Center for Immunization and Respiratory Diseases, told reporters that 206 individuals under investigation had tested negative for infection with the novel virus and that tests were pending on another 76 individuals.
The agency also announced during a press briefing call that diagnostic test kits will begin shipping on Feb. 5, less than 24 hours after receiving an emergency use authorization from the Food and Drug Administration. Full information is available in an article published in the Morbidity and Mortality Weekly Report.
The emergency use authorization will allow for broader use of the CDC’s 2019-nCoV Real Time RT-PCR Diagnostic Panel, which to date has been limited for use at CDC laboratories. Under the emergency use authorization, the diagnostic kit is authorized for patients who meed the CDC criteria for 2019-nCoV testing. The diagnostic test is a reverse transcriptase polymerase chain reaction test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs. A positive test indicates likely infection, although a negative test does not preclude infection and should not be the sole determination for patient management decisions.
“Today, the test kits will start shipping to over 100 U.S. public health labs,” she said. “Each of these labs is required to perform international verification for [Clinical Laboratory Improvement Amendments] compliance prior to reporting out. This process is expected to take a few days.”
Dr. Messonnier said that 200 test kits will be distributed to domestic labs and another 200 test kits will go to select international labs. Each kit can perform diagnostics on 700-800 patient samples.
“What that means is that, by the start of next week, we expect there to be much enhanced capacity for laboratory testing closer to our patients,” she said, adding that additional test kits are being produced and will be available for ordering in the future. Each laboratory that places an order will receive one test kit.
“Distribution of these tests will improve the global capacity to detect and respond to this new virus,” Dr. Messonnier said. “Availability of this test is a starting place for greater commercial availability of diagnostic testing for nCoV.”
The CDC also said that the next batch of passengers arriving from Wuhan, China, will be arriving in one of four locations: Travis Air Force Base, Fairfield, Calif.; Marine Corps Air Station Miramar, San Diego; Lackland Air Force Base, San Antonio; and Eppley Airfield, Omaha, Neb. Passengers will be quarantined for up to 14 days from the day the flight left Wuhan and medical care will be provided if needed.
“We do not believe these people pose a threat to the communities where they are being housed as we are taking measures to minimize any contact,” she said, adding that confirmed infections are expected among these and other returning travelers.
Dr. Messonnier warned that the quarantine measures “may not catch every single returning traveler returning with novel coronavirus, given the nature of this virus and how it is spreading. But if we can catch the majority of them, that will slow the entry of this virus into the United States.”
*This story was updated on 02/05/2020.
The 2019 novel coronavirus: Case review IDs clinical characteristics
A group of physicians in Wuhan, China, who are treating patients with the 2019 novel coronavirus have gone the extra mile to share their clinical experiences with colleagues around the world.
Nanshan Chen, MD, of Jinyintan Hospital, Wuhan, and his team conducted a retrospective study on 99 cases and, in very short order, published their initial findings in the Lancet online on Jan. 29. These findings could guide action in other cases and help clinicians all over the world create treatment plans for patients of the 2019-nCoV.
The findings show that and characteristics of those with fatal infections align with the MuLBSTA score – an early warning model for predicting viral pneumonia–related mortality, according to a case review.
Of 99 patients who presented with 2019-nCoV pneumonia at Jinyintan Hospital between Jan. 1 and Jan. 20, 67 were men, the mean age was 55.5 years, and 50 patients had chronic diseases.
“All the data of included cases have been shared with [the World Health Organization]. The study was approved by Jinyintan Hospital Ethics Committee and written informed consent was obtained from patients involved before enrollment when data were collected retrospectively,” the researchers noted.
Nearly half of the patients (49%) lived or worked near a specific seafood market, suggesting disease clustering.
Clinical manifestations affecting the majority of patients included fever and cough in 83% and 82% of patients, respectively. Other symptoms included shortness of breath in 31%, muscle aches in 11%, confusion in 9%, headache in 8%, sore throat in 5%, and rhinorrhea, chest pain, diarrhea, and nausea and vomiting in 1%-4% of patients, the investigators found.
Imaging showed bilateral pneumonia in 75% of cases, multiple mottling and ground-glass opacity in 14%, and pneumothorax in 1%. Organ function damage was present in a third of patients at admission: 17% had acute respiratory distress syndrome (ARDS) – including 11 patients who worsened quickly and died of multiple organ failure. Eight percent had acute respiratory injury, 3% had acute renal injury, 4% had septic shock, and 1% had ventilator-associated pneumonia, they said, noting that all cases were confirmed by real-time polymerase chain reaction.
A notable laboratory finding was reduced absolute lymphocyte counts in most patients, the investigators said.
All patients were treated in isolation and 76% received antiviral treatment with oseltamivir, ganciclovir, lopinavir, or ritonavir for 3-14 days (median, 3 days). Most patients also received antibiotic treatment, including a single antibiotic in 25% of cases and combination therapy in 45%, with most antibiotics used to cover “common pathogens and some atypical pathogens,” they said, adding that “when secondary bacterial infection occurred, medication was administered according to the results of bacterial culture and drug sensitivity.”
Cephalosporins, quinolones, carbapenems, tigecycline against methicillin-resistant Staphylococcus aureus, linezolid, and antifungal drugs were used, and duration ranged from 3 to 17 days (median, 5 days).
Nineteen patients also received steroid treatments.
As of Jan. 25, 31 patients had been discharged and 57 remained hospitalized. Of the 11 who died, the first 2 were a 61-year-old man and a 69-year-old man, each diagnosed with severe pneumonia and ARDS. The first experienced sudden cardiac arrest and died on admission day 11, and the second died of severe pneumonia, septic shock, and respiratory failure on admission day 9. Neither had underlying disease, but both had a long history of smoking, the investigators noted.
“The deaths of these two patients were consistent with the MuLBSTA score,” they wrote, explaining that the scoring system takes into account multilobular infiltration, lymphopenia, bacterial coinfection, smoking history, hypertension, and age.
Eight of the nine other patients who died had lymphopenia, seven had bilateral pneumonia, five were over age 60 years, three had hypertension, and one was a heavy smoker, they added.
Most coronavirus infections cause mild symptoms and have good prognosis, but some patients with the 2019-nCoV, which was identified Jan. 7 following the development of several cases of pneumonia of unknown etiology in Wuhan, develop fatal disease. The paucity of data regarding epidemiology and clinical features of pneumonia associated with 2019-nCoV prompted the current retrospective study at the center where the first cases were admitted, the investigators explained.
They noted that the sequence of 2019-nCoV “is relatively different from the six other coronavirus subtypes, including the highly pathogenic severe acute respiratory syndrome (SARS)-CoV and Middle East Respiratory Syndrome (MERS)-CoV, as well as the human coronaviruses (HCoV)-OC43, -229E, -NL63, and -HKU1 that induce mild upper respiratory disease, but can be classified as a betacoronavirus with evidence of human-to-human transmission.
Mortality associated with SARS-CoV and MERS-CoV have been reported as more than 10% and more than 35%, respectively; at data cutoff for the current study, mortality among the 99 included cases was 11%, which is similar to that in another recent 2019-nCoV report, they said.
The finding of greater risk among older men also has been seen with SARS-CoV and MERS-CoV, and the high rate among individuals with chronic diseases, mainly cerebrovascular disease, cardiovascular disease, and diabetes, also has been reported with MERS-CoV, they added.
“Our results suggest that 2019-nCoV is more likely to infect older adult males with chronic comorbidities as a result of the weaker immune functions of these patients,” they wrote.
Coinfection with bacteria and fungi occurred in some patients, particularly those with severe illness, and cultures most often showed A. baumannii, K. pneumoniae, A. flavus, C. glabrata, and C. albicans, and the findings of reduced absolute lymphocyte values in most patients suggests that “2019-nCoV might mainly act on lymphocytes, especially T lymphocytes, as does SARS-CoV,” they noted.
Given the rapid progression with ARDS and septic shock in some patients in this review, “early identification and timely treatment of critical cases is of crucial importance,” they said.
“Use of intravenous immunoglobulin is recommended to enhance the ability of anti-infection for severely ill patients, and steroids (methylprednisolone 1-2 mg/kg per day) are recommended for patients with ARDS, for as short a duration of treatment as possible,” they added.
Further, since some studies suggest that a substantial decrease in lymphocyte count indicates consumption of many immune cells by coronavirus, thereby inhibiting cellular immune function, damage to T lymphocytes might be “an important factor leading to exacerbations of patients,” they wrote, adding that “[t]he low absolute value of lymphocytes could be used as a reference index in the diagnosis of new coronavirus infections in the clinic.”
The MuLBSTA score also should be investigated to determine its applicability for predicting mortality risk in patients with 2019-nCoV infection, they added.
The current study is limited by its small sample size; additional studies are needed to include “as many patients as possible in Wuhan, in other cities in China, and even in other countries to get a more comprehensive understanding of 2019-nCoV,” they said.
The National Key R&D Program of China funded the study. The authors reported having no conflicts of interest.
SOURCE: Chen N et al. Lancet. 2020 Jan 29. doi: 10.1016/S0140-6736(20)30211-7.
A group of physicians in Wuhan, China, who are treating patients with the 2019 novel coronavirus have gone the extra mile to share their clinical experiences with colleagues around the world.
Nanshan Chen, MD, of Jinyintan Hospital, Wuhan, and his team conducted a retrospective study on 99 cases and, in very short order, published their initial findings in the Lancet online on Jan. 29. These findings could guide action in other cases and help clinicians all over the world create treatment plans for patients of the 2019-nCoV.
The findings show that and characteristics of those with fatal infections align with the MuLBSTA score – an early warning model for predicting viral pneumonia–related mortality, according to a case review.
Of 99 patients who presented with 2019-nCoV pneumonia at Jinyintan Hospital between Jan. 1 and Jan. 20, 67 were men, the mean age was 55.5 years, and 50 patients had chronic diseases.
“All the data of included cases have been shared with [the World Health Organization]. The study was approved by Jinyintan Hospital Ethics Committee and written informed consent was obtained from patients involved before enrollment when data were collected retrospectively,” the researchers noted.
Nearly half of the patients (49%) lived or worked near a specific seafood market, suggesting disease clustering.
Clinical manifestations affecting the majority of patients included fever and cough in 83% and 82% of patients, respectively. Other symptoms included shortness of breath in 31%, muscle aches in 11%, confusion in 9%, headache in 8%, sore throat in 5%, and rhinorrhea, chest pain, diarrhea, and nausea and vomiting in 1%-4% of patients, the investigators found.
Imaging showed bilateral pneumonia in 75% of cases, multiple mottling and ground-glass opacity in 14%, and pneumothorax in 1%. Organ function damage was present in a third of patients at admission: 17% had acute respiratory distress syndrome (ARDS) – including 11 patients who worsened quickly and died of multiple organ failure. Eight percent had acute respiratory injury, 3% had acute renal injury, 4% had septic shock, and 1% had ventilator-associated pneumonia, they said, noting that all cases were confirmed by real-time polymerase chain reaction.
A notable laboratory finding was reduced absolute lymphocyte counts in most patients, the investigators said.
All patients were treated in isolation and 76% received antiviral treatment with oseltamivir, ganciclovir, lopinavir, or ritonavir for 3-14 days (median, 3 days). Most patients also received antibiotic treatment, including a single antibiotic in 25% of cases and combination therapy in 45%, with most antibiotics used to cover “common pathogens and some atypical pathogens,” they said, adding that “when secondary bacterial infection occurred, medication was administered according to the results of bacterial culture and drug sensitivity.”
Cephalosporins, quinolones, carbapenems, tigecycline against methicillin-resistant Staphylococcus aureus, linezolid, and antifungal drugs were used, and duration ranged from 3 to 17 days (median, 5 days).
Nineteen patients also received steroid treatments.
As of Jan. 25, 31 patients had been discharged and 57 remained hospitalized. Of the 11 who died, the first 2 were a 61-year-old man and a 69-year-old man, each diagnosed with severe pneumonia and ARDS. The first experienced sudden cardiac arrest and died on admission day 11, and the second died of severe pneumonia, septic shock, and respiratory failure on admission day 9. Neither had underlying disease, but both had a long history of smoking, the investigators noted.
“The deaths of these two patients were consistent with the MuLBSTA score,” they wrote, explaining that the scoring system takes into account multilobular infiltration, lymphopenia, bacterial coinfection, smoking history, hypertension, and age.
Eight of the nine other patients who died had lymphopenia, seven had bilateral pneumonia, five were over age 60 years, three had hypertension, and one was a heavy smoker, they added.
Most coronavirus infections cause mild symptoms and have good prognosis, but some patients with the 2019-nCoV, which was identified Jan. 7 following the development of several cases of pneumonia of unknown etiology in Wuhan, develop fatal disease. The paucity of data regarding epidemiology and clinical features of pneumonia associated with 2019-nCoV prompted the current retrospective study at the center where the first cases were admitted, the investigators explained.
They noted that the sequence of 2019-nCoV “is relatively different from the six other coronavirus subtypes, including the highly pathogenic severe acute respiratory syndrome (SARS)-CoV and Middle East Respiratory Syndrome (MERS)-CoV, as well as the human coronaviruses (HCoV)-OC43, -229E, -NL63, and -HKU1 that induce mild upper respiratory disease, but can be classified as a betacoronavirus with evidence of human-to-human transmission.
Mortality associated with SARS-CoV and MERS-CoV have been reported as more than 10% and more than 35%, respectively; at data cutoff for the current study, mortality among the 99 included cases was 11%, which is similar to that in another recent 2019-nCoV report, they said.
The finding of greater risk among older men also has been seen with SARS-CoV and MERS-CoV, and the high rate among individuals with chronic diseases, mainly cerebrovascular disease, cardiovascular disease, and diabetes, also has been reported with MERS-CoV, they added.
“Our results suggest that 2019-nCoV is more likely to infect older adult males with chronic comorbidities as a result of the weaker immune functions of these patients,” they wrote.
Coinfection with bacteria and fungi occurred in some patients, particularly those with severe illness, and cultures most often showed A. baumannii, K. pneumoniae, A. flavus, C. glabrata, and C. albicans, and the findings of reduced absolute lymphocyte values in most patients suggests that “2019-nCoV might mainly act on lymphocytes, especially T lymphocytes, as does SARS-CoV,” they noted.
Given the rapid progression with ARDS and septic shock in some patients in this review, “early identification and timely treatment of critical cases is of crucial importance,” they said.
“Use of intravenous immunoglobulin is recommended to enhance the ability of anti-infection for severely ill patients, and steroids (methylprednisolone 1-2 mg/kg per day) are recommended for patients with ARDS, for as short a duration of treatment as possible,” they added.
Further, since some studies suggest that a substantial decrease in lymphocyte count indicates consumption of many immune cells by coronavirus, thereby inhibiting cellular immune function, damage to T lymphocytes might be “an important factor leading to exacerbations of patients,” they wrote, adding that “[t]he low absolute value of lymphocytes could be used as a reference index in the diagnosis of new coronavirus infections in the clinic.”
The MuLBSTA score also should be investigated to determine its applicability for predicting mortality risk in patients with 2019-nCoV infection, they added.
The current study is limited by its small sample size; additional studies are needed to include “as many patients as possible in Wuhan, in other cities in China, and even in other countries to get a more comprehensive understanding of 2019-nCoV,” they said.
The National Key R&D Program of China funded the study. The authors reported having no conflicts of interest.
SOURCE: Chen N et al. Lancet. 2020 Jan 29. doi: 10.1016/S0140-6736(20)30211-7.
A group of physicians in Wuhan, China, who are treating patients with the 2019 novel coronavirus have gone the extra mile to share their clinical experiences with colleagues around the world.
Nanshan Chen, MD, of Jinyintan Hospital, Wuhan, and his team conducted a retrospective study on 99 cases and, in very short order, published their initial findings in the Lancet online on Jan. 29. These findings could guide action in other cases and help clinicians all over the world create treatment plans for patients of the 2019-nCoV.
The findings show that and characteristics of those with fatal infections align with the MuLBSTA score – an early warning model for predicting viral pneumonia–related mortality, according to a case review.
Of 99 patients who presented with 2019-nCoV pneumonia at Jinyintan Hospital between Jan. 1 and Jan. 20, 67 were men, the mean age was 55.5 years, and 50 patients had chronic diseases.
“All the data of included cases have been shared with [the World Health Organization]. The study was approved by Jinyintan Hospital Ethics Committee and written informed consent was obtained from patients involved before enrollment when data were collected retrospectively,” the researchers noted.
Nearly half of the patients (49%) lived or worked near a specific seafood market, suggesting disease clustering.
Clinical manifestations affecting the majority of patients included fever and cough in 83% and 82% of patients, respectively. Other symptoms included shortness of breath in 31%, muscle aches in 11%, confusion in 9%, headache in 8%, sore throat in 5%, and rhinorrhea, chest pain, diarrhea, and nausea and vomiting in 1%-4% of patients, the investigators found.
Imaging showed bilateral pneumonia in 75% of cases, multiple mottling and ground-glass opacity in 14%, and pneumothorax in 1%. Organ function damage was present in a third of patients at admission: 17% had acute respiratory distress syndrome (ARDS) – including 11 patients who worsened quickly and died of multiple organ failure. Eight percent had acute respiratory injury, 3% had acute renal injury, 4% had septic shock, and 1% had ventilator-associated pneumonia, they said, noting that all cases were confirmed by real-time polymerase chain reaction.
A notable laboratory finding was reduced absolute lymphocyte counts in most patients, the investigators said.
All patients were treated in isolation and 76% received antiviral treatment with oseltamivir, ganciclovir, lopinavir, or ritonavir for 3-14 days (median, 3 days). Most patients also received antibiotic treatment, including a single antibiotic in 25% of cases and combination therapy in 45%, with most antibiotics used to cover “common pathogens and some atypical pathogens,” they said, adding that “when secondary bacterial infection occurred, medication was administered according to the results of bacterial culture and drug sensitivity.”
Cephalosporins, quinolones, carbapenems, tigecycline against methicillin-resistant Staphylococcus aureus, linezolid, and antifungal drugs were used, and duration ranged from 3 to 17 days (median, 5 days).
Nineteen patients also received steroid treatments.
As of Jan. 25, 31 patients had been discharged and 57 remained hospitalized. Of the 11 who died, the first 2 were a 61-year-old man and a 69-year-old man, each diagnosed with severe pneumonia and ARDS. The first experienced sudden cardiac arrest and died on admission day 11, and the second died of severe pneumonia, septic shock, and respiratory failure on admission day 9. Neither had underlying disease, but both had a long history of smoking, the investigators noted.
“The deaths of these two patients were consistent with the MuLBSTA score,” they wrote, explaining that the scoring system takes into account multilobular infiltration, lymphopenia, bacterial coinfection, smoking history, hypertension, and age.
Eight of the nine other patients who died had lymphopenia, seven had bilateral pneumonia, five were over age 60 years, three had hypertension, and one was a heavy smoker, they added.
Most coronavirus infections cause mild symptoms and have good prognosis, but some patients with the 2019-nCoV, which was identified Jan. 7 following the development of several cases of pneumonia of unknown etiology in Wuhan, develop fatal disease. The paucity of data regarding epidemiology and clinical features of pneumonia associated with 2019-nCoV prompted the current retrospective study at the center where the first cases were admitted, the investigators explained.
They noted that the sequence of 2019-nCoV “is relatively different from the six other coronavirus subtypes, including the highly pathogenic severe acute respiratory syndrome (SARS)-CoV and Middle East Respiratory Syndrome (MERS)-CoV, as well as the human coronaviruses (HCoV)-OC43, -229E, -NL63, and -HKU1 that induce mild upper respiratory disease, but can be classified as a betacoronavirus with evidence of human-to-human transmission.
Mortality associated with SARS-CoV and MERS-CoV have been reported as more than 10% and more than 35%, respectively; at data cutoff for the current study, mortality among the 99 included cases was 11%, which is similar to that in another recent 2019-nCoV report, they said.
The finding of greater risk among older men also has been seen with SARS-CoV and MERS-CoV, and the high rate among individuals with chronic diseases, mainly cerebrovascular disease, cardiovascular disease, and diabetes, also has been reported with MERS-CoV, they added.
“Our results suggest that 2019-nCoV is more likely to infect older adult males with chronic comorbidities as a result of the weaker immune functions of these patients,” they wrote.
Coinfection with bacteria and fungi occurred in some patients, particularly those with severe illness, and cultures most often showed A. baumannii, K. pneumoniae, A. flavus, C. glabrata, and C. albicans, and the findings of reduced absolute lymphocyte values in most patients suggests that “2019-nCoV might mainly act on lymphocytes, especially T lymphocytes, as does SARS-CoV,” they noted.
Given the rapid progression with ARDS and septic shock in some patients in this review, “early identification and timely treatment of critical cases is of crucial importance,” they said.
“Use of intravenous immunoglobulin is recommended to enhance the ability of anti-infection for severely ill patients, and steroids (methylprednisolone 1-2 mg/kg per day) are recommended for patients with ARDS, for as short a duration of treatment as possible,” they added.
Further, since some studies suggest that a substantial decrease in lymphocyte count indicates consumption of many immune cells by coronavirus, thereby inhibiting cellular immune function, damage to T lymphocytes might be “an important factor leading to exacerbations of patients,” they wrote, adding that “[t]he low absolute value of lymphocytes could be used as a reference index in the diagnosis of new coronavirus infections in the clinic.”
The MuLBSTA score also should be investigated to determine its applicability for predicting mortality risk in patients with 2019-nCoV infection, they added.
The current study is limited by its small sample size; additional studies are needed to include “as many patients as possible in Wuhan, in other cities in China, and even in other countries to get a more comprehensive understanding of 2019-nCoV,” they said.
The National Key R&D Program of China funded the study. The authors reported having no conflicts of interest.
SOURCE: Chen N et al. Lancet. 2020 Jan 29. doi: 10.1016/S0140-6736(20)30211-7.
FROM THE LANCET
Documentation matters
Quality over quantity
Documentation has always been part of a physician’s job. Historically, in the days of paper records, physicians saw a patient on rounds and immediately following, while still on the unit, wrote a daily note detailing the events, test results, and plans since the last note. Addenda were written over the course of the day and night as needed.
The medical record was a chronological itemization of the encounter. The chart told the patient’s story, hopefully legibly and without excessive rehashing of previous material. The discharge summary then encapsulated the hospitalization in several coherent paragraphs.
In the current electronic records environment, we are inundated with excessive and repetitious information, data without interpretation, differentials without diagnoses. Prepopulation of templated notes, defaults without edit, and dictation without revision have degraded our documentation to the point of unintelligibility. The chronological storytelling and trustworthiness of the medical record has become suspect.
The Centers for Medicare & Medicaid Services is touting its “Patients over Paperwork” initiative. The solution is flawed (that is, future relaxation of documentation requirements for professional billing) because the premise is delusive. Documentation isn’t fundamentally the problem. Having clinicians jump through regulatory hoops which do not advance patients’ care, and providers misunderstanding the requirements for level-of-service billing are the essential issues. Getting no training on how to properly document in medical school/residency and receiving no formative feedback on documentation throughout one’s career compounds the problem. Having clinical documentation serve too many masters, including compliance, quality, medicolegal, utilization review, and reimbursement, is also to blame. The advent of the electronic medical record was just the straw that broke the camel’s back.
Many hospitals now have a clinical documentation integrity (CDI) team which is tasked with querying the provider when the health record documentation is conflicting, imprecise, incomplete, illegible, ambiguous, or inconsistent. They are charged with getting practitioners to associate clinical indicators with diagnoses and to consider removal of diagnoses which do not seem clinically valid from the existing documentation. From this explanation, you might well conclude that the CDI specialist could generate a query on every patient if they were so inclined, and you would be correct. But the goal isn’t to torture the physician – it is to ensure that the medical record is accurately depicting the encounter.
You are not being asked for more documentation by the CDI team; they are entreating you for higher-quality documentation. Let me give you some pointers to ward off queries.
- Tell the story. The most important goal of documentation is to clinically communicate to other caregivers. Think to yourself: “What would a fellow clinician need to know about this patient to understand why I drew those conclusions or to pick up where I left off?” At 2 a.m., that information, or lack thereof, could literally be a matter of life or death.
- Tell the truth. Embellishing the record or including invalid diagnoses with the intent to increase the severity of illness resulting in a more favorable diagnosis-related group – the inpatient risk-adjustment system – is considered fraud.
- You may like the convenience of copy forward, but do you relish reading other people’s copy and paste? Consider doing a documentation time-out. Before you copy and paste yesterday’s assessment and plan, stop and think: “Why is the patient still here? Why are we doing what we are doing?” If you choose to copy and paste, be certain to do mindful editing so the documentation represents the current situation and avoids redundancy. Appropriately editing copy and pasted documentation may prove more time consuming than generating a note de novo.
- Translate findings into diagnoses using your best medical judgment. One man’s hypotension may be another health care provider’s shock. Coders are not clinical and are not permitted to make inferences. A potassium of 6.7 may be hyperkalemia or it may be spurious – only a clinician may make that determination using their clinical expertise and experience. The coder is not allowed to read your mind. You must explicitly draw the conclusion that a febrile patient with bacteremia, encephalopathy, hypoxemia, and a blood pressure of 85/60 is in septic shock.
- Uncertain diagnoses (heralded by words such as: likely, possible, probable, suspected, rule out, etc.) which are not ruled out prior to discharge or demise are coded as if they were definitively present, for the inpatient technical side of hospital billing. This is distinctly different than the professional fee where you can only code definitive diagnoses. If you have a strong suspicion (not wild speculation) that a condition is present, best practice is to offer an uncertain diagnosis. Associate signs and symptoms with your most likely diagnosis: “Shortness of breath, pleuritic chest pain, and hypoxemia in the setting of cancer, probable pulmonary embolism.”
- Evolve, resolve, remove, and recap. If an uncertain diagnosis is ruled in, take away the uncertainty. If it is ruled out, don’t have 4 days of copy and pasted: “Possible eosinophilic pneumonia.” You do not have to maintain a resolved diagnosis ad infinitum. It can drop off the diagnosis list but be sure to have it reappear in the discharge summary.
- I know it can be a hASSLe to do excellent documentation, but it is critical for many reasons, most importantly for superlative patient care. More accurate coding and billing is an intended consequence. A: Acuity; S: Severity; S: Specificity (may affect the coding and the risk-adjustment implications. Acute systolic heart failure does not equal heart failure; type 2 diabetes mellitus with diabetic chronic kidney disease, stage 4 does not equal chronic kidney disease); and L: Linkage (of diagnosis with underlying cause or manifestation [e.g., because of, associated with, as a result of, secondary to, or from diabetic nephropathy, hypertensive encephalopathy]).
- If you have the capability to keep a running summary throughout the hospital stay, do so and keep it updated. A few moments of daily careful editing and composing can save time and effort at the back end creating the discharge summary. The follow-up care provider can reconstruct the hospital course and it is your last chance to spin the narrative for the lawyers.
- Read your documentation over. Ensure that it is clear, accurate, concise, and tells the story and the plans for the patient. Make sure that someone reading the note will know what you were thinking.
- Set up a program to self-audit documentation where monthly or quarterly, you and your partners mutually review a certain number of records and give each other feedback. Design an assessment tool which rates the quality of documentation elements which your hospital/network/service line values (clarity, copy and paste, complete and specific diagnoses, etc.). You know who the best documenters are. Why do you think their documentation is superior? How can you emulate them?
Finally, answer CDI queries. The CDI specialist is your ally, not your enemy. They want you to get credit for taking care of sick and complex patients. They are not permitted to lead the provider, so don’t ask them what they want you to write. But, if you don’t understand the query or issue, have a conversation and get it clarified. It is in everyone’s best interest to get this right.
Documentation improves patient care and demonstrates that you provided excellent patient care. Put mentation back into documentation.
Dr. Remer was a practicing emergency physician for 25 years and a physician advisor for 4 years. She is on the board of directors of the American College of Physician Advisors and the advisory board of the Association of Clinical Documentation Improvement Specialists. She currently provides consulting services for provider education on documentation, CDI, and ICD-10 coding. Dr. Remer can be reached at [email protected]
Quality over quantity
Quality over quantity
Documentation has always been part of a physician’s job. Historically, in the days of paper records, physicians saw a patient on rounds and immediately following, while still on the unit, wrote a daily note detailing the events, test results, and plans since the last note. Addenda were written over the course of the day and night as needed.
The medical record was a chronological itemization of the encounter. The chart told the patient’s story, hopefully legibly and without excessive rehashing of previous material. The discharge summary then encapsulated the hospitalization in several coherent paragraphs.
In the current electronic records environment, we are inundated with excessive and repetitious information, data without interpretation, differentials without diagnoses. Prepopulation of templated notes, defaults without edit, and dictation without revision have degraded our documentation to the point of unintelligibility. The chronological storytelling and trustworthiness of the medical record has become suspect.
The Centers for Medicare & Medicaid Services is touting its “Patients over Paperwork” initiative. The solution is flawed (that is, future relaxation of documentation requirements for professional billing) because the premise is delusive. Documentation isn’t fundamentally the problem. Having clinicians jump through regulatory hoops which do not advance patients’ care, and providers misunderstanding the requirements for level-of-service billing are the essential issues. Getting no training on how to properly document in medical school/residency and receiving no formative feedback on documentation throughout one’s career compounds the problem. Having clinical documentation serve too many masters, including compliance, quality, medicolegal, utilization review, and reimbursement, is also to blame. The advent of the electronic medical record was just the straw that broke the camel’s back.
Many hospitals now have a clinical documentation integrity (CDI) team which is tasked with querying the provider when the health record documentation is conflicting, imprecise, incomplete, illegible, ambiguous, or inconsistent. They are charged with getting practitioners to associate clinical indicators with diagnoses and to consider removal of diagnoses which do not seem clinically valid from the existing documentation. From this explanation, you might well conclude that the CDI specialist could generate a query on every patient if they were so inclined, and you would be correct. But the goal isn’t to torture the physician – it is to ensure that the medical record is accurately depicting the encounter.
You are not being asked for more documentation by the CDI team; they are entreating you for higher-quality documentation. Let me give you some pointers to ward off queries.
- Tell the story. The most important goal of documentation is to clinically communicate to other caregivers. Think to yourself: “What would a fellow clinician need to know about this patient to understand why I drew those conclusions or to pick up where I left off?” At 2 a.m., that information, or lack thereof, could literally be a matter of life or death.
- Tell the truth. Embellishing the record or including invalid diagnoses with the intent to increase the severity of illness resulting in a more favorable diagnosis-related group – the inpatient risk-adjustment system – is considered fraud.
- You may like the convenience of copy forward, but do you relish reading other people’s copy and paste? Consider doing a documentation time-out. Before you copy and paste yesterday’s assessment and plan, stop and think: “Why is the patient still here? Why are we doing what we are doing?” If you choose to copy and paste, be certain to do mindful editing so the documentation represents the current situation and avoids redundancy. Appropriately editing copy and pasted documentation may prove more time consuming than generating a note de novo.
- Translate findings into diagnoses using your best medical judgment. One man’s hypotension may be another health care provider’s shock. Coders are not clinical and are not permitted to make inferences. A potassium of 6.7 may be hyperkalemia or it may be spurious – only a clinician may make that determination using their clinical expertise and experience. The coder is not allowed to read your mind. You must explicitly draw the conclusion that a febrile patient with bacteremia, encephalopathy, hypoxemia, and a blood pressure of 85/60 is in septic shock.
- Uncertain diagnoses (heralded by words such as: likely, possible, probable, suspected, rule out, etc.) which are not ruled out prior to discharge or demise are coded as if they were definitively present, for the inpatient technical side of hospital billing. This is distinctly different than the professional fee where you can only code definitive diagnoses. If you have a strong suspicion (not wild speculation) that a condition is present, best practice is to offer an uncertain diagnosis. Associate signs and symptoms with your most likely diagnosis: “Shortness of breath, pleuritic chest pain, and hypoxemia in the setting of cancer, probable pulmonary embolism.”
- Evolve, resolve, remove, and recap. If an uncertain diagnosis is ruled in, take away the uncertainty. If it is ruled out, don’t have 4 days of copy and pasted: “Possible eosinophilic pneumonia.” You do not have to maintain a resolved diagnosis ad infinitum. It can drop off the diagnosis list but be sure to have it reappear in the discharge summary.
- I know it can be a hASSLe to do excellent documentation, but it is critical for many reasons, most importantly for superlative patient care. More accurate coding and billing is an intended consequence. A: Acuity; S: Severity; S: Specificity (may affect the coding and the risk-adjustment implications. Acute systolic heart failure does not equal heart failure; type 2 diabetes mellitus with diabetic chronic kidney disease, stage 4 does not equal chronic kidney disease); and L: Linkage (of diagnosis with underlying cause or manifestation [e.g., because of, associated with, as a result of, secondary to, or from diabetic nephropathy, hypertensive encephalopathy]).
- If you have the capability to keep a running summary throughout the hospital stay, do so and keep it updated. A few moments of daily careful editing and composing can save time and effort at the back end creating the discharge summary. The follow-up care provider can reconstruct the hospital course and it is your last chance to spin the narrative for the lawyers.
- Read your documentation over. Ensure that it is clear, accurate, concise, and tells the story and the plans for the patient. Make sure that someone reading the note will know what you were thinking.
- Set up a program to self-audit documentation where monthly or quarterly, you and your partners mutually review a certain number of records and give each other feedback. Design an assessment tool which rates the quality of documentation elements which your hospital/network/service line values (clarity, copy and paste, complete and specific diagnoses, etc.). You know who the best documenters are. Why do you think their documentation is superior? How can you emulate them?
Finally, answer CDI queries. The CDI specialist is your ally, not your enemy. They want you to get credit for taking care of sick and complex patients. They are not permitted to lead the provider, so don’t ask them what they want you to write. But, if you don’t understand the query or issue, have a conversation and get it clarified. It is in everyone’s best interest to get this right.
Documentation improves patient care and demonstrates that you provided excellent patient care. Put mentation back into documentation.
Dr. Remer was a practicing emergency physician for 25 years and a physician advisor for 4 years. She is on the board of directors of the American College of Physician Advisors and the advisory board of the Association of Clinical Documentation Improvement Specialists. She currently provides consulting services for provider education on documentation, CDI, and ICD-10 coding. Dr. Remer can be reached at [email protected]
Documentation has always been part of a physician’s job. Historically, in the days of paper records, physicians saw a patient on rounds and immediately following, while still on the unit, wrote a daily note detailing the events, test results, and plans since the last note. Addenda were written over the course of the day and night as needed.
The medical record was a chronological itemization of the encounter. The chart told the patient’s story, hopefully legibly and without excessive rehashing of previous material. The discharge summary then encapsulated the hospitalization in several coherent paragraphs.
In the current electronic records environment, we are inundated with excessive and repetitious information, data without interpretation, differentials without diagnoses. Prepopulation of templated notes, defaults without edit, and dictation without revision have degraded our documentation to the point of unintelligibility. The chronological storytelling and trustworthiness of the medical record has become suspect.
The Centers for Medicare & Medicaid Services is touting its “Patients over Paperwork” initiative. The solution is flawed (that is, future relaxation of documentation requirements for professional billing) because the premise is delusive. Documentation isn’t fundamentally the problem. Having clinicians jump through regulatory hoops which do not advance patients’ care, and providers misunderstanding the requirements for level-of-service billing are the essential issues. Getting no training on how to properly document in medical school/residency and receiving no formative feedback on documentation throughout one’s career compounds the problem. Having clinical documentation serve too many masters, including compliance, quality, medicolegal, utilization review, and reimbursement, is also to blame. The advent of the electronic medical record was just the straw that broke the camel’s back.
Many hospitals now have a clinical documentation integrity (CDI) team which is tasked with querying the provider when the health record documentation is conflicting, imprecise, incomplete, illegible, ambiguous, or inconsistent. They are charged with getting practitioners to associate clinical indicators with diagnoses and to consider removal of diagnoses which do not seem clinically valid from the existing documentation. From this explanation, you might well conclude that the CDI specialist could generate a query on every patient if they were so inclined, and you would be correct. But the goal isn’t to torture the physician – it is to ensure that the medical record is accurately depicting the encounter.
You are not being asked for more documentation by the CDI team; they are entreating you for higher-quality documentation. Let me give you some pointers to ward off queries.
- Tell the story. The most important goal of documentation is to clinically communicate to other caregivers. Think to yourself: “What would a fellow clinician need to know about this patient to understand why I drew those conclusions or to pick up where I left off?” At 2 a.m., that information, or lack thereof, could literally be a matter of life or death.
- Tell the truth. Embellishing the record or including invalid diagnoses with the intent to increase the severity of illness resulting in a more favorable diagnosis-related group – the inpatient risk-adjustment system – is considered fraud.
- You may like the convenience of copy forward, but do you relish reading other people’s copy and paste? Consider doing a documentation time-out. Before you copy and paste yesterday’s assessment and plan, stop and think: “Why is the patient still here? Why are we doing what we are doing?” If you choose to copy and paste, be certain to do mindful editing so the documentation represents the current situation and avoids redundancy. Appropriately editing copy and pasted documentation may prove more time consuming than generating a note de novo.
- Translate findings into diagnoses using your best medical judgment. One man’s hypotension may be another health care provider’s shock. Coders are not clinical and are not permitted to make inferences. A potassium of 6.7 may be hyperkalemia or it may be spurious – only a clinician may make that determination using their clinical expertise and experience. The coder is not allowed to read your mind. You must explicitly draw the conclusion that a febrile patient with bacteremia, encephalopathy, hypoxemia, and a blood pressure of 85/60 is in septic shock.
- Uncertain diagnoses (heralded by words such as: likely, possible, probable, suspected, rule out, etc.) which are not ruled out prior to discharge or demise are coded as if they were definitively present, for the inpatient technical side of hospital billing. This is distinctly different than the professional fee where you can only code definitive diagnoses. If you have a strong suspicion (not wild speculation) that a condition is present, best practice is to offer an uncertain diagnosis. Associate signs and symptoms with your most likely diagnosis: “Shortness of breath, pleuritic chest pain, and hypoxemia in the setting of cancer, probable pulmonary embolism.”
- Evolve, resolve, remove, and recap. If an uncertain diagnosis is ruled in, take away the uncertainty. If it is ruled out, don’t have 4 days of copy and pasted: “Possible eosinophilic pneumonia.” You do not have to maintain a resolved diagnosis ad infinitum. It can drop off the diagnosis list but be sure to have it reappear in the discharge summary.
- I know it can be a hASSLe to do excellent documentation, but it is critical for many reasons, most importantly for superlative patient care. More accurate coding and billing is an intended consequence. A: Acuity; S: Severity; S: Specificity (may affect the coding and the risk-adjustment implications. Acute systolic heart failure does not equal heart failure; type 2 diabetes mellitus with diabetic chronic kidney disease, stage 4 does not equal chronic kidney disease); and L: Linkage (of diagnosis with underlying cause or manifestation [e.g., because of, associated with, as a result of, secondary to, or from diabetic nephropathy, hypertensive encephalopathy]).
- If you have the capability to keep a running summary throughout the hospital stay, do so and keep it updated. A few moments of daily careful editing and composing can save time and effort at the back end creating the discharge summary. The follow-up care provider can reconstruct the hospital course and it is your last chance to spin the narrative for the lawyers.
- Read your documentation over. Ensure that it is clear, accurate, concise, and tells the story and the plans for the patient. Make sure that someone reading the note will know what you were thinking.
- Set up a program to self-audit documentation where monthly or quarterly, you and your partners mutually review a certain number of records and give each other feedback. Design an assessment tool which rates the quality of documentation elements which your hospital/network/service line values (clarity, copy and paste, complete and specific diagnoses, etc.). You know who the best documenters are. Why do you think their documentation is superior? How can you emulate them?
Finally, answer CDI queries. The CDI specialist is your ally, not your enemy. They want you to get credit for taking care of sick and complex patients. They are not permitted to lead the provider, so don’t ask them what they want you to write. But, if you don’t understand the query or issue, have a conversation and get it clarified. It is in everyone’s best interest to get this right.
Documentation improves patient care and demonstrates that you provided excellent patient care. Put mentation back into documentation.
Dr. Remer was a practicing emergency physician for 25 years and a physician advisor for 4 years. She is on the board of directors of the American College of Physician Advisors and the advisory board of the Association of Clinical Documentation Improvement Specialists. She currently provides consulting services for provider education on documentation, CDI, and ICD-10 coding. Dr. Remer can be reached at [email protected]
Novel coronavirus cases now at 11; entry ban and quarantine measures begin
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a Centers for Disease Control and Prevention press briefing.
Four of the new cases are in California, and one in Massachusetts. Although four of the new cases have recent travel history to Wuhan, China, the epicenter of the 2019-nCoV outbreak, the fifth is a close household contact of one of the other California patients, said Dr. Messonnier. This last case is the second instance of person-to-person spread of 2019-nCoV in the United States.
“We expect to find additional cases of the novel coronavirus in the United States,” she said. “We expect to see more cases of person-to-person spread among close contacts. And we continue to expect this will happen given the explosive nature of this outbreak in China.”
As of the morning of Feb. 3, 167 persons under investigation, or PUIs, for possible 2019-nCoV have tested negative for the virus, and an additional 82 PUIs have testing pending – this latter figure includes some tests that are still in transit to the CDC, said Dr. Messonnier.
During the briefing, Dr. Messonnier emphasized both the aggressive nature of the U.S. public health response and the rationale for quick and assertive action. “The goal of our public health response is to protect and contain,” she said. “Strong measures now may blunt the impact of this virus on the United States.”
She cited the intensity of transmission in Hubei Province, the expansion of transmission to other provinces in China, the expansion of cases outside of China, and sporadic ongoing deaths from 2019-nCoV as drivers of the aggressive U.S. public health response.
A presidential proclamation is currently in place that bars U.S. entry to foreign nationals who have visited mainland China within the past 14 days; the ban does not apply to travelers from Hong Kong and Macao. Immediate family members of U.S. citizens and individuals who have U.S. permanent resident status are exempted from the entry ban and will be allowed entry into the United States.
However, explained Dr. Messonnier, those who have traveled to China recently and are permitted entry will be subject to screening. All passengers with such recent travel will be directed to one of 11 U.S. airports set up to perform additional screening.
As of Feb 3, the list of airports includes:
- San Francisco International Airport in California.
- Los Angeles International Airport in California.
- Hartsfield-Jackson Atlanta International Airport in Georgia.
- Daniel K. Inouye International Airport in Hawaii.
- O’Hare International Airport in Illinois.
- Detroit Metropolitan Airport in Michigan.
- Newark Liberty International Airport in New Jersey.
- John F. Kennedy International Airport in New York.
- Dallas/Fort Worth International Airport in Texas.
- Washington Dulles International Airport in Virginia.
- Seattle-Tacoma International Airport in Washington.
Travelers who have been to Hubei Province in the previous 14 days will have an additional health assessment at which they will be screened for fever, cough, or difficulty breathing. Any American citizens or exempt individuals who are symptomatic would then be transferred for further medical evaluation. Asymptomatic travelers in this category will be subject to a mandatory 14-day quarantine near their point of entry, rather than continuing on to their final destinations.
Dr. Messonnier emphasized that the mandatory 14-day quarantine is specifically for Americans or exempt individuals returning from Hubei Province, adding that the CDC is presently working with individual states to determine the exact venues for quarantine.
American citizens and exempt individuals returning from other parts of mainland China will be routed to one of the 11 airports and will also receive additional health screening. Symptomatic individuals in this travel category would be referred for further evaluation before being able to complete their itinerary.
Asymptomatic American citizens and exempt individuals who are returning from mainland China – but not Hubei Province – will be allowed to travel on to their final destinations, but will be asked to stay home as much as possible and to monitor their health during the 14 days after their return.
The U.S. Department of State is bringing back more Americans from Wuhan province this week, and these individuals will also be kept under federal quarantine for 14 days.
“There are likely to be confirmed infections among returning travelers,” said Dr. Messonnier. “It is important to note that this strategy is not meant to catch every single traveler returning from China with novel coronavirus; given the nature of this virus and how it’s spreading, that would be impossible, but working together we can catch the majority of them.
“The goal here is to slow the entry of this virus into the United States,” she said, adding that the nation’s health care and public health systems stand on high alert to detect the virus in community settings. In response to questioning from the press, Dr. Messonnier defended the stringent quarantine measures, noting that they are in line with those taken by some other nations, and with the aggressive action being taken by the Chinese government itself. “These actions are science based and aimed at protecting the health of all Americans,” she said.
The real-time reverse transcription polymerase chain reaction (rRT-PCR) assay that the CDC has developed detects 2019-nCoV in both respiratory and serum specimens. Dr. Messonnier reported that the CDC is today filing an emergency use authorization (EUA) application to the U.S. Food and Drug Administration to expedite access to the assay for public health laboratories across the country. “This will greatly enhance our capacity to test for this virus,” she said, noting that EUA approval may come as soon as the end of this week.
Although the CDC is poised to send an expert team to China, it’s still awaiting favorable results from the international negotiations currently underway. “This is a horrible situation in China,” said Dr. Messonnier. “Our presence on the ground in China would be a help to China. ... Science should trump everything else; that’s what we’re hoping – that the scientific expertise of the global community can be brought to bear on the incredibly complicated, difficult situation that our colleagues in China are dealing with.”
FROM A CDC PRESS BRIEFING
Don’t forget about the flu: 2019-2010 season is not over
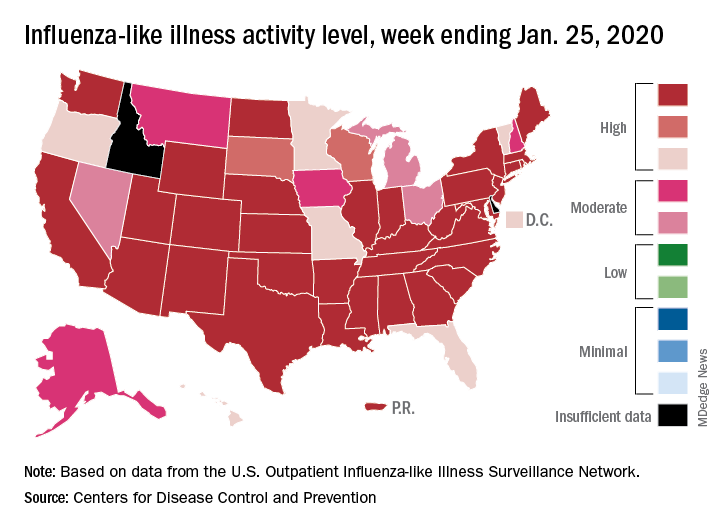
Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.

Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.

Nationally, an estimated 5.7% of all outpatients visiting health care providers had influenza-like illness (ILI) for the week ending Jan. 25, which was up from 5.1% the previous week but still lower than the current seasonal high of 7.1% recorded during the week of Dec. 22-28, the CDC’s influenza division reported.
Another key indicator of influenza activity, the percentage of respiratory specimens testing positive, also remains high as it rose from 25.7% the week before to 27.7% for the week ending Jan. 25, the influenza division said. That is the highest rate of the 2019-2020 season so far, surpassing the 26.8% reached during Dec. 22-28.
Another new seasonal high involves the number of states, 33 plus Puerto Rico, at the highest level of ILI activity on the CDC’s 1-10 scale for the latest reporting week, topping the 32 jurisdictions from the last full week of December. Another eight states and the District of Columbia were in the “high” range with activity levels of 8 and 9, and no state with available data was lower than level 6, the CDC data show.
Going along with the recent 2-week increase in activity is a large increase in the number of ILI-related pediatric deaths, which rose from 39 on Jan. 11 to the current count of 68, the CDC said. At the same point last year, there had been 36 pediatric deaths.
Other indicators of ILI severity, however, “are not high at this point in the season,” the influenza division noted. “Overall, hospitalization rates remain similar to what has been seen at this time during recent seasons, but rates among children and young adults are higher at this time than in recent seasons.” Overall pneumonia and influenza mortality is also low, the CDC added.
Initial ultrasound assessment of appendicitis curbs costs
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
Assessing appendicitis in children with initial ultrasound followed by computed tomography in the absence of appendix visualization and presence of secondary signs was the most cost-effective approach, according to data from a modeling study of 10 strategies.
Ultrasound is safer and less expensive than computed tomography and avoids radiation exposure; however, cost-effectiveness models of various approaches to imaging have not been well studied, wrote Rebecca Jennings, MD, of Seattle Children’s Hospital, Washington, and colleagues.
In a study published in Pediatrics, the researchers simulated a hypothetical patient population using a Markov cohort model and compared 10 different strategies including CT only, MRI only, and ultrasound followed by CT or MRI after ultrasounds that are negative or fail to visualize the appendix.
Overall, the most cost-effective strategy for moderate-risk patients was the use of ultrasound followed by CT or MRI if the ultrasound failed to visualize the appendix and secondary signs of inflammation were present in the right lower quadrant. The cost of this strategy was $4,815, with effectiveness of 0.99694 quality-adjusted life-years. “The most cost-effective strategy is highly dependent on a patient’s risk stratification,” the researchers noted. Based on their model, imaging was not cost effective for patients with a prevalence less than 16% or greater than 95%. However, those with appendicitis risk between 16% and 95% and no secondary signs of inflammation can forgo further imaging, even without visualization of the appendix for maximum cost-effectiveness, the researchers said.
The study was limited by several factors, including the inability to account for all potential costs related to imaging and outcomes, lack of accounting for the use of sedation when assessing costs, and inability to separate imaging costs from total hospital costs, the researchers noted. However, the results suggest that tailored imaging approaches based on patient risk are the most cost-effective strategies to assess appendicitis, they said.
“The diagnosis and exclusion of appendicitis continues to be one of the primary concerns of providers who care for children with abdominal pain,” wrote Rebecca M. Rentea, MD, and Charles L. Snyder, MD, of Children’s Mercy Hospital Kansas City, Mo., in an accompanying editorial (Pediatrics. 2020 Feb;145:e20193349).
“The best diagnostic and imaging approach to appendicitis has been a topic of interest for some time, and improvements such as appendicitis scoring systems, decreased use of ionized radiation, and adoption of clinical algorithms have been incremental but steady,” they said. Despite the potential of missed appendicitis, the use of an algorithm based on an initial ultrasound and previous possibility of appendicitis described in the study was the most cost effective, they said. In addition, “the ability to visualize the appendix did not alter the most cost-effective approach in those with a moderate risk of appendicitis (most patients),” they concluded.
The study was supported by the University of Washington and Seattle Children’s Hospital Quality Improvement Scholars Program. The researchers had no financial conflicts to disclose.
Dr. Rentea and Dr. Snyder had no financial conflicts to disclose.
SOURCE: Jennings R et al. Pediatrics. 2020. doi: 10.1542/peds.2019-1352.
FROM PEDIATRICS
Noninjectable modes of insulin delivery coming of age
LOS ANGELES – Injections may be the most common way for patients with diabetes to take insulin, but other modes of delivery are coming of age.
George Grunberger, MD, chairman of the Grunberger Diabetes Institute in Bloomfield Township, Mich., said that at least seven different agents that are being studied for the oral delivery of biologics for diabetes.
He outlined several at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Oral insulin
ORMD-0801 from Oramed is an oral insulin capsule that prevents enzyme degradation and enhances intestinal absorption. Top-line, unpublished findings from a phase 2 study, which the company announced in November 2019, showed that ORMD-0801 significantly reduced hemoglobin A1c levels in patients with type 2 diabetes who were inadequately controlled on other standard-of-care drugs. ORMD-0801 dosed once daily reduced HbA1c by 0.60%, compared with 0.06% by placebo. “We’ll see when it’s going to wind up in the clinic,” Dr. Grunberger said. Oramed is also developing an oral glucagonlike peptide–1 analogue capsule, ORMD-0901, which has potential to be the first orally ingestible GLP-1 analogue.
Inhaled and absorbed insulin
Technosphere insulin (Affreza) is a novel inhalation powder for the treatment of diabetes that was developed by MannKind and approved by the Food and Drug Administration in 2014. Clinical studies have shown that Technosphere insulin delivers insulin with an ultrarapid pharmacokinetic profile that is different from all other insulin products, but similar to natural insulin release. “The idea was to develop a more patient-friendly device to deliver insulin directly into the lungs,” said Dr. Grunberger, who is also a clinical professor of internal medicine and molecular medicine and genetics at Wayne State University, Detroit. “When you inhale this into the lungs, there is one cell layer between the air sac and the circulation, so it works very quickly. The idea is to try to avoid injecting insulin to see if it helps. This is a prandial insulin – you inhale it before meals. The whole idea is that hopefully, you can reduce any fear of delayed postprandial hyperglycemia.”
In a randomized trial of 353 patients with inadequately controlled type 2 diabetes, those in the Technosphere insulin arm significantly reduced HbA1c by 0.8% from a baseline of 8.3%, compared with the placebo arm, which was reduced by 0.4% (P less than .0001; Diabetes Care. 2015;38[12]:2274-81). A greater number of patients treated with Technosphere insulin achieved an HbA1c of 7.0% or less, compared with placebo (38% vs. 19%; P = .0005). Dr. Grunberger noted that, in clinical trials lasting up to 2 years, patients treated with Technosphere insulin had a 40-mL greater decline from baseline in forced expiratory volume in 1 second (FEV1 ), compared with patients treated with comparator antidiabetes treatments. “But once you stop using the drug, FEV1 reverts to normal,” he said. “So, there does not appear to be lasting damage to your lungs and respiratory ability.”
In another development, Oral-Lyn from Generex Biotechnology, which delivers insulin through the oral mucosa, is being evaluated as a potential treatment option. In 2015, Generex partnered with the University of Toronto’s Center for Molecular Design and Preformulations to increase the bioavailability of insulin in the product and to reduce the number of sprays required to achieve effective prandial glucose control. In 2019, the company formed the NuGenerex Diabetes Research Center, which intended to accelerate the development of the reformulated Oral-Lyn-2, for type 2 diabetes, and Altsulin, for the treatment of type 1 diabetes. The programs are expected to initiate in the first quarter of 2020.
In the meantime, studies of intranasally delivered insulin continue to advance. “It works. It lowers glucose, but there is a whole slew of knowledge now about how it can also improve neurocognitive function,” Dr. Grunberger said.
Oral GLP-1 receptor agonists
Oral versions of glucagonlike peptide–1 (GLP-1) receptor agonists are also emerging as a treatment option. The FDA recently approved the first oral GLP-1 receptor agonist, semaglutide bound in the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC). According to data from manufacturer Novo Nordisk, SNAC facilitates local increase of pH, which leads to a higher solubility. SNAC interacts with cell membranes of gastric mucosa, facilitating absorption within 30 minutes, “so the drug can penetrate the mucosa without lasting damage,” Dr. Grunberger said. The SNAC effect is size dependent and fully reversible.
In PIONEER 3, researchers found that, in adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, oral semaglutide at dosages of 7 and 14 mg/day resulted in significantly greater reductions in HbA1c over 26 weeks, compared with sitagliptin, but there was no significant benefit with the 3-mg/d dosage (JAMA. 2019;321[15]:1466-80). In PIONEER 4, researchers compared the efficacy and safety of oral semaglutide with subcutaneous liraglutide (Lancet. 2019;394[10192]:P39-50). “There was no difference in HbA1c effect between the two groups, but oral semaglutide beat out sitagliptin in terms of weight loss,” Dr. Grunberger said. “It’s going to be interesting to see what’s going to happen in the marketplace as the drug gets widely launched.”
Nasal glucagon
He closed out his presentation by discussing the July 2019 FDA approval of Eli Lilly’s nasal glucagon for severe hypoglycemia – the first such treatment that can be administered without an injection. The nasally administered dry powder, known as Baqsimi, is a welcome alternative to current glucagon kits, “which contain multiple components,” said Dr. Grunberger, who is also a past president of the American Association of Clinical Endocrinologists. An adult pivotal study showed that supraphysiologic levels of glucagon were achieved within 5 minutes with both nasal and intramuscular glucagon (Diabetes Care. 2016;39[2]:264-70). Headache and nasal symptoms occurred more frequently with nasal glucagon, but most were resolved within 1 day. In addition, nausea and vomiting occurred at similar frequencies with nasal and intramuscular glucacon, and most cases were resolved within 1 day.
Similar results were observed in a pediatric study of 48 patients with type 1 diabetes who were older than 4 years, (Diabetes Care. 2016;39[4]:555-62).
Dr. Grunberger disclosed that has research contracts with Medtronic and Eli Lilly, and that he serves on speakers bureaus of Eli Lilly, Janssen, Novo Nordisk, and Sanofi.
LOS ANGELES – Injections may be the most common way for patients with diabetes to take insulin, but other modes of delivery are coming of age.
George Grunberger, MD, chairman of the Grunberger Diabetes Institute in Bloomfield Township, Mich., said that at least seven different agents that are being studied for the oral delivery of biologics for diabetes.
He outlined several at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Oral insulin
ORMD-0801 from Oramed is an oral insulin capsule that prevents enzyme degradation and enhances intestinal absorption. Top-line, unpublished findings from a phase 2 study, which the company announced in November 2019, showed that ORMD-0801 significantly reduced hemoglobin A1c levels in patients with type 2 diabetes who were inadequately controlled on other standard-of-care drugs. ORMD-0801 dosed once daily reduced HbA1c by 0.60%, compared with 0.06% by placebo. “We’ll see when it’s going to wind up in the clinic,” Dr. Grunberger said. Oramed is also developing an oral glucagonlike peptide–1 analogue capsule, ORMD-0901, which has potential to be the first orally ingestible GLP-1 analogue.
Inhaled and absorbed insulin
Technosphere insulin (Affreza) is a novel inhalation powder for the treatment of diabetes that was developed by MannKind and approved by the Food and Drug Administration in 2014. Clinical studies have shown that Technosphere insulin delivers insulin with an ultrarapid pharmacokinetic profile that is different from all other insulin products, but similar to natural insulin release. “The idea was to develop a more patient-friendly device to deliver insulin directly into the lungs,” said Dr. Grunberger, who is also a clinical professor of internal medicine and molecular medicine and genetics at Wayne State University, Detroit. “When you inhale this into the lungs, there is one cell layer between the air sac and the circulation, so it works very quickly. The idea is to try to avoid injecting insulin to see if it helps. This is a prandial insulin – you inhale it before meals. The whole idea is that hopefully, you can reduce any fear of delayed postprandial hyperglycemia.”
In a randomized trial of 353 patients with inadequately controlled type 2 diabetes, those in the Technosphere insulin arm significantly reduced HbA1c by 0.8% from a baseline of 8.3%, compared with the placebo arm, which was reduced by 0.4% (P less than .0001; Diabetes Care. 2015;38[12]:2274-81). A greater number of patients treated with Technosphere insulin achieved an HbA1c of 7.0% or less, compared with placebo (38% vs. 19%; P = .0005). Dr. Grunberger noted that, in clinical trials lasting up to 2 years, patients treated with Technosphere insulin had a 40-mL greater decline from baseline in forced expiratory volume in 1 second (FEV1 ), compared with patients treated with comparator antidiabetes treatments. “But once you stop using the drug, FEV1 reverts to normal,” he said. “So, there does not appear to be lasting damage to your lungs and respiratory ability.”
In another development, Oral-Lyn from Generex Biotechnology, which delivers insulin through the oral mucosa, is being evaluated as a potential treatment option. In 2015, Generex partnered with the University of Toronto’s Center for Molecular Design and Preformulations to increase the bioavailability of insulin in the product and to reduce the number of sprays required to achieve effective prandial glucose control. In 2019, the company formed the NuGenerex Diabetes Research Center, which intended to accelerate the development of the reformulated Oral-Lyn-2, for type 2 diabetes, and Altsulin, for the treatment of type 1 diabetes. The programs are expected to initiate in the first quarter of 2020.
In the meantime, studies of intranasally delivered insulin continue to advance. “It works. It lowers glucose, but there is a whole slew of knowledge now about how it can also improve neurocognitive function,” Dr. Grunberger said.
Oral GLP-1 receptor agonists
Oral versions of glucagonlike peptide–1 (GLP-1) receptor agonists are also emerging as a treatment option. The FDA recently approved the first oral GLP-1 receptor agonist, semaglutide bound in the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC). According to data from manufacturer Novo Nordisk, SNAC facilitates local increase of pH, which leads to a higher solubility. SNAC interacts with cell membranes of gastric mucosa, facilitating absorption within 30 minutes, “so the drug can penetrate the mucosa without lasting damage,” Dr. Grunberger said. The SNAC effect is size dependent and fully reversible.
In PIONEER 3, researchers found that, in adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, oral semaglutide at dosages of 7 and 14 mg/day resulted in significantly greater reductions in HbA1c over 26 weeks, compared with sitagliptin, but there was no significant benefit with the 3-mg/d dosage (JAMA. 2019;321[15]:1466-80). In PIONEER 4, researchers compared the efficacy and safety of oral semaglutide with subcutaneous liraglutide (Lancet. 2019;394[10192]:P39-50). “There was no difference in HbA1c effect between the two groups, but oral semaglutide beat out sitagliptin in terms of weight loss,” Dr. Grunberger said. “It’s going to be interesting to see what’s going to happen in the marketplace as the drug gets widely launched.”
Nasal glucagon
He closed out his presentation by discussing the July 2019 FDA approval of Eli Lilly’s nasal glucagon for severe hypoglycemia – the first such treatment that can be administered without an injection. The nasally administered dry powder, known as Baqsimi, is a welcome alternative to current glucagon kits, “which contain multiple components,” said Dr. Grunberger, who is also a past president of the American Association of Clinical Endocrinologists. An adult pivotal study showed that supraphysiologic levels of glucagon were achieved within 5 minutes with both nasal and intramuscular glucagon (Diabetes Care. 2016;39[2]:264-70). Headache and nasal symptoms occurred more frequently with nasal glucagon, but most were resolved within 1 day. In addition, nausea and vomiting occurred at similar frequencies with nasal and intramuscular glucacon, and most cases were resolved within 1 day.
Similar results were observed in a pediatric study of 48 patients with type 1 diabetes who were older than 4 years, (Diabetes Care. 2016;39[4]:555-62).
Dr. Grunberger disclosed that has research contracts with Medtronic and Eli Lilly, and that he serves on speakers bureaus of Eli Lilly, Janssen, Novo Nordisk, and Sanofi.
LOS ANGELES – Injections may be the most common way for patients with diabetes to take insulin, but other modes of delivery are coming of age.
George Grunberger, MD, chairman of the Grunberger Diabetes Institute in Bloomfield Township, Mich., said that at least seven different agents that are being studied for the oral delivery of biologics for diabetes.
He outlined several at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
Oral insulin
ORMD-0801 from Oramed is an oral insulin capsule that prevents enzyme degradation and enhances intestinal absorption. Top-line, unpublished findings from a phase 2 study, which the company announced in November 2019, showed that ORMD-0801 significantly reduced hemoglobin A1c levels in patients with type 2 diabetes who were inadequately controlled on other standard-of-care drugs. ORMD-0801 dosed once daily reduced HbA1c by 0.60%, compared with 0.06% by placebo. “We’ll see when it’s going to wind up in the clinic,” Dr. Grunberger said. Oramed is also developing an oral glucagonlike peptide–1 analogue capsule, ORMD-0901, which has potential to be the first orally ingestible GLP-1 analogue.
Inhaled and absorbed insulin
Technosphere insulin (Affreza) is a novel inhalation powder for the treatment of diabetes that was developed by MannKind and approved by the Food and Drug Administration in 2014. Clinical studies have shown that Technosphere insulin delivers insulin with an ultrarapid pharmacokinetic profile that is different from all other insulin products, but similar to natural insulin release. “The idea was to develop a more patient-friendly device to deliver insulin directly into the lungs,” said Dr. Grunberger, who is also a clinical professor of internal medicine and molecular medicine and genetics at Wayne State University, Detroit. “When you inhale this into the lungs, there is one cell layer between the air sac and the circulation, so it works very quickly. The idea is to try to avoid injecting insulin to see if it helps. This is a prandial insulin – you inhale it before meals. The whole idea is that hopefully, you can reduce any fear of delayed postprandial hyperglycemia.”
In a randomized trial of 353 patients with inadequately controlled type 2 diabetes, those in the Technosphere insulin arm significantly reduced HbA1c by 0.8% from a baseline of 8.3%, compared with the placebo arm, which was reduced by 0.4% (P less than .0001; Diabetes Care. 2015;38[12]:2274-81). A greater number of patients treated with Technosphere insulin achieved an HbA1c of 7.0% or less, compared with placebo (38% vs. 19%; P = .0005). Dr. Grunberger noted that, in clinical trials lasting up to 2 years, patients treated with Technosphere insulin had a 40-mL greater decline from baseline in forced expiratory volume in 1 second (FEV1 ), compared with patients treated with comparator antidiabetes treatments. “But once you stop using the drug, FEV1 reverts to normal,” he said. “So, there does not appear to be lasting damage to your lungs and respiratory ability.”
In another development, Oral-Lyn from Generex Biotechnology, which delivers insulin through the oral mucosa, is being evaluated as a potential treatment option. In 2015, Generex partnered with the University of Toronto’s Center for Molecular Design and Preformulations to increase the bioavailability of insulin in the product and to reduce the number of sprays required to achieve effective prandial glucose control. In 2019, the company formed the NuGenerex Diabetes Research Center, which intended to accelerate the development of the reformulated Oral-Lyn-2, for type 2 diabetes, and Altsulin, for the treatment of type 1 diabetes. The programs are expected to initiate in the first quarter of 2020.
In the meantime, studies of intranasally delivered insulin continue to advance. “It works. It lowers glucose, but there is a whole slew of knowledge now about how it can also improve neurocognitive function,” Dr. Grunberger said.
Oral GLP-1 receptor agonists
Oral versions of glucagonlike peptide–1 (GLP-1) receptor agonists are also emerging as a treatment option. The FDA recently approved the first oral GLP-1 receptor agonist, semaglutide bound in the absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC). According to data from manufacturer Novo Nordisk, SNAC facilitates local increase of pH, which leads to a higher solubility. SNAC interacts with cell membranes of gastric mucosa, facilitating absorption within 30 minutes, “so the drug can penetrate the mucosa without lasting damage,” Dr. Grunberger said. The SNAC effect is size dependent and fully reversible.
In PIONEER 3, researchers found that, in adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, oral semaglutide at dosages of 7 and 14 mg/day resulted in significantly greater reductions in HbA1c over 26 weeks, compared with sitagliptin, but there was no significant benefit with the 3-mg/d dosage (JAMA. 2019;321[15]:1466-80). In PIONEER 4, researchers compared the efficacy and safety of oral semaglutide with subcutaneous liraglutide (Lancet. 2019;394[10192]:P39-50). “There was no difference in HbA1c effect between the two groups, but oral semaglutide beat out sitagliptin in terms of weight loss,” Dr. Grunberger said. “It’s going to be interesting to see what’s going to happen in the marketplace as the drug gets widely launched.”
Nasal glucagon
He closed out his presentation by discussing the July 2019 FDA approval of Eli Lilly’s nasal glucagon for severe hypoglycemia – the first such treatment that can be administered without an injection. The nasally administered dry powder, known as Baqsimi, is a welcome alternative to current glucagon kits, “which contain multiple components,” said Dr. Grunberger, who is also a past president of the American Association of Clinical Endocrinologists. An adult pivotal study showed that supraphysiologic levels of glucagon were achieved within 5 minutes with both nasal and intramuscular glucagon (Diabetes Care. 2016;39[2]:264-70). Headache and nasal symptoms occurred more frequently with nasal glucagon, but most were resolved within 1 day. In addition, nausea and vomiting occurred at similar frequencies with nasal and intramuscular glucacon, and most cases were resolved within 1 day.
Similar results were observed in a pediatric study of 48 patients with type 1 diabetes who were older than 4 years, (Diabetes Care. 2016;39[4]:555-62).
Dr. Grunberger disclosed that has research contracts with Medtronic and Eli Lilly, and that he serves on speakers bureaus of Eli Lilly, Janssen, Novo Nordisk, and Sanofi.
EXPERT ANALYSIS FROM WCIRDC 2019
HHS declares coronavirus emergency, orders quarantine
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.