User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


N.Y. hospitals report near-universal CMV screening when newborns fail hearing tests
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
REPORTING FROM PAS 2019
Key clinical point: A metropolitan N.Y. health system provides a model for how to implement cytomegalovirus screening for infants who fail hearing tests.
Major finding: .
Study details: Pre-post quality improvement project.
Disclosures: The lead investigator had no disclosures. No funding source was mentioned.
Source: Chauhan A et al. PAS 2019. Abstract 306.
AFib screening cuts hospitalizations and ED visits
NEW ORLEANS – People diagnosed with atrial fibrillation by screening with a wearable ECG patch had significantly fewer emergency department visits or hospital admissions, compared with similar people diagnosed with atrial fibrillation by usual-care surveillance in an observational study with 5,109 total participants.
People diagnosed with atrial fibrillation (AFib) through screening had a statistically significant 80% relative cut in hospitalizations and a 65% cut in emergency department visits during 12 months of follow-up, compared with controls in the study who had their AFib identified and diagnosed as part of routine practice, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The data also showed no difference between the screened and control patients identified with AFib in the average number of cardiologist consultations during a year of follow-up, and a trend that missed statistical significance for 16% fewer primary care physician visits in Afib patients diagnosed by screening rather than by routine surveillance.
These findings provided some insight into the potential clinical impact of AFib screening in at-risk people. Dr. Steinhubl and his associates plan to report on the incidence of strokes and MIs in the two study subgroups after 3 years of follow-up, but he noted that preliminary findings for these two outcomes after 1 year indicated that active screening for AFib also had reduced these rates, compared with waiting for the arrhythmia to become apparent by emergence of symptoms.
The data came from the mSToPS (mHealth Screening to Prevent Strokes) study, which randomized 2,659 U.S. residents enrolled in a large health plan who had risk factors for AFib to either immediate or delayed arrhythmia assessment by ECG patches. Half the participants used a patch for about 14 days immediately and then a second time 3 months later, while the other half waited 4 months and then wore an ECG patch for 2 weeks and again 3 months later. The primary endpoint, first reported at the ACC annual meeting a year before and subsequently published, was the incidence of newly diagnosed AFib during the first 4 months in the actively monitored cohort, compared with a cohort followed by usual care. The results showed that screening identified AFib in 3.9% of people, while no screening and usual-practice follow-up identified a 0.9% incidence of AFib, showing that screening worked better for AFib case identification (JAMA. 2018 Jul 10;320[2]:146-55).
To examine the clinical impact of screening and an increased incidence of diagnosed AFib cases, Dr. Steinhubl and his associates focused on 1,725 of the original 2,659 patients who underwent ECG patch assessment, either immediate or delayed, and continued through 12 months of follow-up, and compared them with 3,384 matched controls who never underwent ECG patch screening but were also followed for 12 months for incident AFib identified during routine care and surveillance. This resulted in a cumulative incidence of newly diagnosed AFib of 6.3% in those who had worn two ECG patches and 2.3% among the matched controls.
During follow-up, use of various interventions was more common among the screened people than the controls. Initiation of anticoagulation treatment started in 4.0% of the entire screened group, compared with 1.9% of the controls, The screened people also had a 0.9% rate of receiving a pacemaker or defibrillator, a 0.8% rate of starting on treatment with an antiarrhythmic drug, and a 0.3% rate of undergoing catheter ablation, compared with none, 0.3%, and one of the controls, respectively, said Dr. Steinhubl, director of digital medicine at the Scripps Research Translational Institute in La Jolla, Calif.
The mSToPS study was funded by Janssen. Dr. Steinhubl has received research funding from DynoSense, EasyG, Janssen, the Qualcomm Foundation, and Striv.
SOURCE: Steinhubl SR et al. J Am Coll Cardiol. 2019 Mar 12;73(9)suppl 1:296.
NEW ORLEANS – People diagnosed with atrial fibrillation by screening with a wearable ECG patch had significantly fewer emergency department visits or hospital admissions, compared with similar people diagnosed with atrial fibrillation by usual-care surveillance in an observational study with 5,109 total participants.
People diagnosed with atrial fibrillation (AFib) through screening had a statistically significant 80% relative cut in hospitalizations and a 65% cut in emergency department visits during 12 months of follow-up, compared with controls in the study who had their AFib identified and diagnosed as part of routine practice, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The data also showed no difference between the screened and control patients identified with AFib in the average number of cardiologist consultations during a year of follow-up, and a trend that missed statistical significance for 16% fewer primary care physician visits in Afib patients diagnosed by screening rather than by routine surveillance.
These findings provided some insight into the potential clinical impact of AFib screening in at-risk people. Dr. Steinhubl and his associates plan to report on the incidence of strokes and MIs in the two study subgroups after 3 years of follow-up, but he noted that preliminary findings for these two outcomes after 1 year indicated that active screening for AFib also had reduced these rates, compared with waiting for the arrhythmia to become apparent by emergence of symptoms.
The data came from the mSToPS (mHealth Screening to Prevent Strokes) study, which randomized 2,659 U.S. residents enrolled in a large health plan who had risk factors for AFib to either immediate or delayed arrhythmia assessment by ECG patches. Half the participants used a patch for about 14 days immediately and then a second time 3 months later, while the other half waited 4 months and then wore an ECG patch for 2 weeks and again 3 months later. The primary endpoint, first reported at the ACC annual meeting a year before and subsequently published, was the incidence of newly diagnosed AFib during the first 4 months in the actively monitored cohort, compared with a cohort followed by usual care. The results showed that screening identified AFib in 3.9% of people, while no screening and usual-practice follow-up identified a 0.9% incidence of AFib, showing that screening worked better for AFib case identification (JAMA. 2018 Jul 10;320[2]:146-55).
To examine the clinical impact of screening and an increased incidence of diagnosed AFib cases, Dr. Steinhubl and his associates focused on 1,725 of the original 2,659 patients who underwent ECG patch assessment, either immediate or delayed, and continued through 12 months of follow-up, and compared them with 3,384 matched controls who never underwent ECG patch screening but were also followed for 12 months for incident AFib identified during routine care and surveillance. This resulted in a cumulative incidence of newly diagnosed AFib of 6.3% in those who had worn two ECG patches and 2.3% among the matched controls.
During follow-up, use of various interventions was more common among the screened people than the controls. Initiation of anticoagulation treatment started in 4.0% of the entire screened group, compared with 1.9% of the controls, The screened people also had a 0.9% rate of receiving a pacemaker or defibrillator, a 0.8% rate of starting on treatment with an antiarrhythmic drug, and a 0.3% rate of undergoing catheter ablation, compared with none, 0.3%, and one of the controls, respectively, said Dr. Steinhubl, director of digital medicine at the Scripps Research Translational Institute in La Jolla, Calif.
The mSToPS study was funded by Janssen. Dr. Steinhubl has received research funding from DynoSense, EasyG, Janssen, the Qualcomm Foundation, and Striv.
SOURCE: Steinhubl SR et al. J Am Coll Cardiol. 2019 Mar 12;73(9)suppl 1:296.
NEW ORLEANS – People diagnosed with atrial fibrillation by screening with a wearable ECG patch had significantly fewer emergency department visits or hospital admissions, compared with similar people diagnosed with atrial fibrillation by usual-care surveillance in an observational study with 5,109 total participants.
People diagnosed with atrial fibrillation (AFib) through screening had a statistically significant 80% relative cut in hospitalizations and a 65% cut in emergency department visits during 12 months of follow-up, compared with controls in the study who had their AFib identified and diagnosed as part of routine practice, Steven R. Steinhubl, MD, said at the annual meeting of the American College of Cardiology.
The data also showed no difference between the screened and control patients identified with AFib in the average number of cardiologist consultations during a year of follow-up, and a trend that missed statistical significance for 16% fewer primary care physician visits in Afib patients diagnosed by screening rather than by routine surveillance.
These findings provided some insight into the potential clinical impact of AFib screening in at-risk people. Dr. Steinhubl and his associates plan to report on the incidence of strokes and MIs in the two study subgroups after 3 years of follow-up, but he noted that preliminary findings for these two outcomes after 1 year indicated that active screening for AFib also had reduced these rates, compared with waiting for the arrhythmia to become apparent by emergence of symptoms.
The data came from the mSToPS (mHealth Screening to Prevent Strokes) study, which randomized 2,659 U.S. residents enrolled in a large health plan who had risk factors for AFib to either immediate or delayed arrhythmia assessment by ECG patches. Half the participants used a patch for about 14 days immediately and then a second time 3 months later, while the other half waited 4 months and then wore an ECG patch for 2 weeks and again 3 months later. The primary endpoint, first reported at the ACC annual meeting a year before and subsequently published, was the incidence of newly diagnosed AFib during the first 4 months in the actively monitored cohort, compared with a cohort followed by usual care. The results showed that screening identified AFib in 3.9% of people, while no screening and usual-practice follow-up identified a 0.9% incidence of AFib, showing that screening worked better for AFib case identification (JAMA. 2018 Jul 10;320[2]:146-55).
To examine the clinical impact of screening and an increased incidence of diagnosed AFib cases, Dr. Steinhubl and his associates focused on 1,725 of the original 2,659 patients who underwent ECG patch assessment, either immediate or delayed, and continued through 12 months of follow-up, and compared them with 3,384 matched controls who never underwent ECG patch screening but were also followed for 12 months for incident AFib identified during routine care and surveillance. This resulted in a cumulative incidence of newly diagnosed AFib of 6.3% in those who had worn two ECG patches and 2.3% among the matched controls.
During follow-up, use of various interventions was more common among the screened people than the controls. Initiation of anticoagulation treatment started in 4.0% of the entire screened group, compared with 1.9% of the controls, The screened people also had a 0.9% rate of receiving a pacemaker or defibrillator, a 0.8% rate of starting on treatment with an antiarrhythmic drug, and a 0.3% rate of undergoing catheter ablation, compared with none, 0.3%, and one of the controls, respectively, said Dr. Steinhubl, director of digital medicine at the Scripps Research Translational Institute in La Jolla, Calif.
The mSToPS study was funded by Janssen. Dr. Steinhubl has received research funding from DynoSense, EasyG, Janssen, the Qualcomm Foundation, and Striv.
SOURCE: Steinhubl SR et al. J Am Coll Cardiol. 2019 Mar 12;73(9)suppl 1:296.
REPORTING FROM ACC 2019
Biomarker-based score predicts poor outcomes after acute ischemic stroke
PHILADELPHIA – A prognostic score for acute ischemic stroke that incorporates copeptin levels, age, recanalization, and National Institutes of Health Stroke Scale score has been externally validated and accurately predicts unfavorable outcome, according to research presented at the annual meeting of the American Academy of Neurology.
Although the four-item score could not be validated for mortality prediction, it had reasonable accuracy for predicting unfavorable functional outcome, defined as disability or mortality 3 months after ischemic stroke, Gian Marco De Marchis, MD, of the department of neurology and the stroke center at University Hospital Basel (Switzerland), said in a presentation.
“The use of a biomarker increases prognostic accuracy, allowing us to personalize prognosis in the frame of individualized, precision medicine,” Dr. De Marchis said.
Copeptin has been linked to disability and mortality at 3 months in two independent, large cohort studies of patients with ischemic stroke, he said.
The four-item prognostic score devised by Dr. De Marchis and his coinvestigators, which they call the CoRisk score, was developed based on a derivation cohort of 319 acute ischemic stroke patients and a validation cohort including another 783 patients in the Copeptin for Risk Stratification in Acute Stroke Patients (CoRisk) Study.
Diagnostic accuracy was 82% for the endpoint of unfavorable functional outcome at 3 months, according to Dr. De Marchis.
“The observed outcomes matched well with the expected outcomes,” he said in his presentation.
Further analyses demonstrated that the addition of copeptin indeed contributed to the diagnostic accuracy of the score, improving the classification for 46%; in other words, about half of the patients were reclassified based on addition of the biomarker data.
By contrast, the score is not well suited to predict mortality alone at 3 months, the results of the analyses showed.
The algorithm used to calculate the score based on its four variables is somewhat complex, but available as a free app and online calculator, Dr. De Marchis said.
Dr. De Marchis and his coauthors had nothing to disclose related to their study. A full report on the study was published ahead of print on March 1 in Neurology.
SOURCE: De Marchis GM et al. AAN 2019, Abstract S47.001.
PHILADELPHIA – A prognostic score for acute ischemic stroke that incorporates copeptin levels, age, recanalization, and National Institutes of Health Stroke Scale score has been externally validated and accurately predicts unfavorable outcome, according to research presented at the annual meeting of the American Academy of Neurology.
Although the four-item score could not be validated for mortality prediction, it had reasonable accuracy for predicting unfavorable functional outcome, defined as disability or mortality 3 months after ischemic stroke, Gian Marco De Marchis, MD, of the department of neurology and the stroke center at University Hospital Basel (Switzerland), said in a presentation.
“The use of a biomarker increases prognostic accuracy, allowing us to personalize prognosis in the frame of individualized, precision medicine,” Dr. De Marchis said.
Copeptin has been linked to disability and mortality at 3 months in two independent, large cohort studies of patients with ischemic stroke, he said.
The four-item prognostic score devised by Dr. De Marchis and his coinvestigators, which they call the CoRisk score, was developed based on a derivation cohort of 319 acute ischemic stroke patients and a validation cohort including another 783 patients in the Copeptin for Risk Stratification in Acute Stroke Patients (CoRisk) Study.
Diagnostic accuracy was 82% for the endpoint of unfavorable functional outcome at 3 months, according to Dr. De Marchis.
“The observed outcomes matched well with the expected outcomes,” he said in his presentation.
Further analyses demonstrated that the addition of copeptin indeed contributed to the diagnostic accuracy of the score, improving the classification for 46%; in other words, about half of the patients were reclassified based on addition of the biomarker data.
By contrast, the score is not well suited to predict mortality alone at 3 months, the results of the analyses showed.
The algorithm used to calculate the score based on its four variables is somewhat complex, but available as a free app and online calculator, Dr. De Marchis said.
Dr. De Marchis and his coauthors had nothing to disclose related to their study. A full report on the study was published ahead of print on March 1 in Neurology.
SOURCE: De Marchis GM et al. AAN 2019, Abstract S47.001.
PHILADELPHIA – A prognostic score for acute ischemic stroke that incorporates copeptin levels, age, recanalization, and National Institutes of Health Stroke Scale score has been externally validated and accurately predicts unfavorable outcome, according to research presented at the annual meeting of the American Academy of Neurology.
Although the four-item score could not be validated for mortality prediction, it had reasonable accuracy for predicting unfavorable functional outcome, defined as disability or mortality 3 months after ischemic stroke, Gian Marco De Marchis, MD, of the department of neurology and the stroke center at University Hospital Basel (Switzerland), said in a presentation.
“The use of a biomarker increases prognostic accuracy, allowing us to personalize prognosis in the frame of individualized, precision medicine,” Dr. De Marchis said.
Copeptin has been linked to disability and mortality at 3 months in two independent, large cohort studies of patients with ischemic stroke, he said.
The four-item prognostic score devised by Dr. De Marchis and his coinvestigators, which they call the CoRisk score, was developed based on a derivation cohort of 319 acute ischemic stroke patients and a validation cohort including another 783 patients in the Copeptin for Risk Stratification in Acute Stroke Patients (CoRisk) Study.
Diagnostic accuracy was 82% for the endpoint of unfavorable functional outcome at 3 months, according to Dr. De Marchis.
“The observed outcomes matched well with the expected outcomes,” he said in his presentation.
Further analyses demonstrated that the addition of copeptin indeed contributed to the diagnostic accuracy of the score, improving the classification for 46%; in other words, about half of the patients were reclassified based on addition of the biomarker data.
By contrast, the score is not well suited to predict mortality alone at 3 months, the results of the analyses showed.
The algorithm used to calculate the score based on its four variables is somewhat complex, but available as a free app and online calculator, Dr. De Marchis said.
Dr. De Marchis and his coauthors had nothing to disclose related to their study. A full report on the study was published ahead of print on March 1 in Neurology.
SOURCE: De Marchis GM et al. AAN 2019, Abstract S47.001.
REPORTING FROM AAN 2019
Unit-based models of care
A tool for ensuring patient safety
“To me, teamwork is the beauty of our sport, where you have five acting as one. You become selfless.” – Mike Krzyzewski
High-performing teams plan, communicate, reflect, and take action together. Teamwork can transform seemingly impossible tasks into opportunities for people to come together and create value.
The increasing complexity of health care makes team-based care necessary to achieve successful health outcomes for patients. At the Brooklyn (N.Y.) Hospital Center, a 464-bed care center, we transformed the model of care on the medical wards into a geographic, unit-based team model. Here we describe our journey – the successes, the challenges, and the opportunities for growth.
Previous model
In the previous care model on our medical wards, no set structures were in place. Teams would travel to multiple wards throughout the hospital to see the patients they were rounding on. Each floor had its own set of social workers and case managers, therefore a hospital medicine team routinely dealt with more than eight social workers and case managers to address their patients’ needs in a single day.
Multidisciplinary rounds for all medical patients were held at 11 a.m. in a room located a significant distance away from the medical wards. All case managers and social workers would sit in this room from 11 a.m. until noon, and teams would travel to that room to discuss their patients.
Many challenges were identified in this model, including a lack of communication, a de-emphasis on teamwork, and a design that did not take physician workflows into account resulting in low efficiency. Thus, these challenges sparked a desire to create a more effective and team-based methodology of accomplishing excellence in delivery of clinical care. Dr. Pendharkar, having worked primarily in centers with unit-based care, determined that a geographic, unit-based model of care could transform care delivery at the Brooklyn Hospital Center.
Looking ahead
The efforts for transforming the vision of geographic, unit-based teams into a reality started by gathering all stakeholders together to unite for a common mission. Initial meetings were held with all parties including social workers, case managers, residents, nursing staff, bed board and attending physicians in internal medicine, and the emergency department.
The vision of a geographic, unit-based team was shared and explained to all team members. Exercises in LEAN methodology were conducted, including one-piece flow exercises, to highlight the possibilities of what could be accomplished through teamwork. Once support for the vision was in place from all parties, the logistics were addressed.
The biggest challenge to overcome was how to place all of one team’s patients on a singular medical ward. In our hospital, a medical ward holds anywhere from 30 to 33 patients. Each hospital medicine team, of which there are many, typically carries 20-23 patients. We created a blueprint to map out the floor to which each team and attending would be assigned. Next, we partnered with both IT and bed board to design an admission order set that specified the particular geographic location that a team and attending were associated with so that patients could be placed accordingly from the ED.
It was important for the ED doctors, bed board, and the internal medicine residents to understand these changes because all of these parties were involved in the initial admitting process. Dr. Pendharkar and Dr. Malieckal provided all groups with in-person training on how the logistics of the system would unfold. Noon conference lectures were also held to explain the vision to residents.
Over 3 weeks, the first ward we chose to implement our model on slowly accumulated the patients of one team – this was the gradual trickle phase. We then selected a “re-set” date. On the re-set date, it was determined that all patients would go to the team that was assigned to that floor, with the exception of any private attendings’ patients.
On the day before the re-set date, time was spent ensuring that all hand-offs were safe. Dr. Pendharkar and Dr. Malieckal spoke with every intern and team that would be handing off and/or receiving patients as a result of the re-set policy. The goal was to ensure that on that date a ward had close to 100% of its patients belonging to the team/attending that was assigned to that area.
The good
Once we began our geographic, unit-based model, our rounding process was transformed.
Now, our morning rounds were joined by the bedside nurse, case manager, social worker, clinical pharmacy, and nutrition in addition to the core team. The entire team went from room to room on one ward rounding on all 20 to 25 patients back to back, which created an unparalleled level of efficiency and a forum for effective communication lasting throughout the day.
We also added workstations on wheels (WOWS) to the rounding process so that labs, radiology, and more could be reviewed on rounds with the entire team. A standard script was developed so that each patient was introduced to all members of the team, and the care plan was disclosed and highlighted. One patient noted, “I feel so cared for, knowing I have this entire team taking care of me.” We also rounded in the afternoon with the case managers and social workers to follow up tasks that were to be completed that day.
Our first few weeks utilizing the geographic, unit-based model of rounding was largely successful. The residents, now able to round on all of their patients in one location with one case manager and one social worker, noted, “This model of rounding makes my life so much easier, I feel like I can focus on the patient rather than running around. … and I know the social worker and case manager will help me.”
Provider satisfaction had improved, from residents to physicians to nurses, case managers, social workers, and more. Our case manager also noted her satisfaction with the new model, stating that her communication with the medical team was much easier. As the attending, I witnessed firsthand how working together with the team moved care forward much more quickly, compared with the previous model, because of the simple factor of increased ease of communication.
Now all team members were together in the patient room and discussion was much easier. There was less confusion, fewer delays, and better communication – I think unit-based teams can even be described as a lifesaving measure that reduces harm to patients. An additional benefit is the relationship that now developed between doctors, social workers, and case managers – they spent more time together and really got to know one another, creating a feeling of shared success and a deeper drive to help one another succeed.
In our model, 87% of surveyed residents said they felt less burned out in the new geographic, unit-based model of care, and 91% of physicians surveyed said it was easier to talk with team members to coordinate care. Additionally, our HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) scores saw a drastic increase in many domains. Nursing communication improved by more than 42% on domain 7B; doctor communication improved by more than 31%. Additionally, all other domains saw at least 10% improvement. We are now 5 months out from our initial rollout of the model and continue to see sustained improvements in quality measures.
The bad
The biggest challenges that we are working through with this model are hand-offs and transfer of patients from one team to another. Sometimes, it happens that one team’s patient will wind up on a floor that is the designated floor of another team because of bed availability. We continue to work with bed board to address this issue. We want to minimize transfers and hand-offs to promote continuity and have to balance that with the need for geographic location. With clear communication, hospital collaboration from bed board and safe hand-off methods, this problem can be safely addressed.
Conclusions
The experience of implementing the unit-based team model has been an eye-opening journey. One thing that stands out is that, in an increasingly complex health care system, design thinking is critical.
Design thinking takes into consideration the needs of those who are using a system. In this case, patients and health care workers including doctors, nurses, case managers, and social workers are the end users of the health care system. All parties are utilizing the health care system to optimize patient health. Therefore, we must create systems that are easy to navigate and use by patients and health care workers so that they can ensure the success of patients.
Unit-based teams offer a basic framework to optimize the inpatient system to facilitate better workflow. In our system, it allowed us to optimize communications between health care workers and also between health care workers and patients. It allowed team members to work in close proximity to better share ideas with each other.
We spent a significant amount of time upfront earning the support of all of the disciplines for this effort. We had support from all leaders within the organization and continue to make our case for this model by sharing metrics and holding forums to discuss the process.
Initial data show a marked improvement in many domains of HCAHPS scores. Our frontline staff, including attendings, residents, nursing, case managers, and social workers, also continue to support this effort since it has a positive impact on their workflow and improves their workday quality. One nurse mentioned specifically, “in my 30 years at this hospital I have never seen people work together so well.”
To sustain this effort, we continue to have regular meetings, and there are new features that we would like to add to the program. For example, we are working with our IT group to ensure that each unit-based team will have dashboards available to incorporate real time, actionable data into daily workflows.
We are excited by the potential of our high-performing teams to highlight the patient experience, placing the patient at the center for care, decision making, and rounding. Health care is a team sport, and anytime you build something where all teams are playing together and approaching the finish line as a unit, you will never go wrong!
Dr. Pendharkar is division chief of hospital medicine at the Brooklyn (N.Y.) Hospital Center, medical director of inpatient services and director of quality for the department of medicine at the Brooklyn Hospital Center and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York. Dr. Malieckal is chief resident, internal medicine, at the Brooklyn Hospital Center. Dr. Gasperino is chair, department of medicine; vice president for critical care, perioperative, and hospital medicine; and associate chief medical officer at the Brooklyn Hospital Center.
A tool for ensuring patient safety
A tool for ensuring patient safety
“To me, teamwork is the beauty of our sport, where you have five acting as one. You become selfless.” – Mike Krzyzewski
High-performing teams plan, communicate, reflect, and take action together. Teamwork can transform seemingly impossible tasks into opportunities for people to come together and create value.
The increasing complexity of health care makes team-based care necessary to achieve successful health outcomes for patients. At the Brooklyn (N.Y.) Hospital Center, a 464-bed care center, we transformed the model of care on the medical wards into a geographic, unit-based team model. Here we describe our journey – the successes, the challenges, and the opportunities for growth.
Previous model
In the previous care model on our medical wards, no set structures were in place. Teams would travel to multiple wards throughout the hospital to see the patients they were rounding on. Each floor had its own set of social workers and case managers, therefore a hospital medicine team routinely dealt with more than eight social workers and case managers to address their patients’ needs in a single day.
Multidisciplinary rounds for all medical patients were held at 11 a.m. in a room located a significant distance away from the medical wards. All case managers and social workers would sit in this room from 11 a.m. until noon, and teams would travel to that room to discuss their patients.
Many challenges were identified in this model, including a lack of communication, a de-emphasis on teamwork, and a design that did not take physician workflows into account resulting in low efficiency. Thus, these challenges sparked a desire to create a more effective and team-based methodology of accomplishing excellence in delivery of clinical care. Dr. Pendharkar, having worked primarily in centers with unit-based care, determined that a geographic, unit-based model of care could transform care delivery at the Brooklyn Hospital Center.
Looking ahead
The efforts for transforming the vision of geographic, unit-based teams into a reality started by gathering all stakeholders together to unite for a common mission. Initial meetings were held with all parties including social workers, case managers, residents, nursing staff, bed board and attending physicians in internal medicine, and the emergency department.
The vision of a geographic, unit-based team was shared and explained to all team members. Exercises in LEAN methodology were conducted, including one-piece flow exercises, to highlight the possibilities of what could be accomplished through teamwork. Once support for the vision was in place from all parties, the logistics were addressed.
The biggest challenge to overcome was how to place all of one team’s patients on a singular medical ward. In our hospital, a medical ward holds anywhere from 30 to 33 patients. Each hospital medicine team, of which there are many, typically carries 20-23 patients. We created a blueprint to map out the floor to which each team and attending would be assigned. Next, we partnered with both IT and bed board to design an admission order set that specified the particular geographic location that a team and attending were associated with so that patients could be placed accordingly from the ED.
It was important for the ED doctors, bed board, and the internal medicine residents to understand these changes because all of these parties were involved in the initial admitting process. Dr. Pendharkar and Dr. Malieckal provided all groups with in-person training on how the logistics of the system would unfold. Noon conference lectures were also held to explain the vision to residents.
Over 3 weeks, the first ward we chose to implement our model on slowly accumulated the patients of one team – this was the gradual trickle phase. We then selected a “re-set” date. On the re-set date, it was determined that all patients would go to the team that was assigned to that floor, with the exception of any private attendings’ patients.
On the day before the re-set date, time was spent ensuring that all hand-offs were safe. Dr. Pendharkar and Dr. Malieckal spoke with every intern and team that would be handing off and/or receiving patients as a result of the re-set policy. The goal was to ensure that on that date a ward had close to 100% of its patients belonging to the team/attending that was assigned to that area.
The good
Once we began our geographic, unit-based model, our rounding process was transformed.
Now, our morning rounds were joined by the bedside nurse, case manager, social worker, clinical pharmacy, and nutrition in addition to the core team. The entire team went from room to room on one ward rounding on all 20 to 25 patients back to back, which created an unparalleled level of efficiency and a forum for effective communication lasting throughout the day.
We also added workstations on wheels (WOWS) to the rounding process so that labs, radiology, and more could be reviewed on rounds with the entire team. A standard script was developed so that each patient was introduced to all members of the team, and the care plan was disclosed and highlighted. One patient noted, “I feel so cared for, knowing I have this entire team taking care of me.” We also rounded in the afternoon with the case managers and social workers to follow up tasks that were to be completed that day.
Our first few weeks utilizing the geographic, unit-based model of rounding was largely successful. The residents, now able to round on all of their patients in one location with one case manager and one social worker, noted, “This model of rounding makes my life so much easier, I feel like I can focus on the patient rather than running around. … and I know the social worker and case manager will help me.”
Provider satisfaction had improved, from residents to physicians to nurses, case managers, social workers, and more. Our case manager also noted her satisfaction with the new model, stating that her communication with the medical team was much easier. As the attending, I witnessed firsthand how working together with the team moved care forward much more quickly, compared with the previous model, because of the simple factor of increased ease of communication.
Now all team members were together in the patient room and discussion was much easier. There was less confusion, fewer delays, and better communication – I think unit-based teams can even be described as a lifesaving measure that reduces harm to patients. An additional benefit is the relationship that now developed between doctors, social workers, and case managers – they spent more time together and really got to know one another, creating a feeling of shared success and a deeper drive to help one another succeed.
In our model, 87% of surveyed residents said they felt less burned out in the new geographic, unit-based model of care, and 91% of physicians surveyed said it was easier to talk with team members to coordinate care. Additionally, our HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) scores saw a drastic increase in many domains. Nursing communication improved by more than 42% on domain 7B; doctor communication improved by more than 31%. Additionally, all other domains saw at least 10% improvement. We are now 5 months out from our initial rollout of the model and continue to see sustained improvements in quality measures.
The bad
The biggest challenges that we are working through with this model are hand-offs and transfer of patients from one team to another. Sometimes, it happens that one team’s patient will wind up on a floor that is the designated floor of another team because of bed availability. We continue to work with bed board to address this issue. We want to minimize transfers and hand-offs to promote continuity and have to balance that with the need for geographic location. With clear communication, hospital collaboration from bed board and safe hand-off methods, this problem can be safely addressed.
Conclusions
The experience of implementing the unit-based team model has been an eye-opening journey. One thing that stands out is that, in an increasingly complex health care system, design thinking is critical.
Design thinking takes into consideration the needs of those who are using a system. In this case, patients and health care workers including doctors, nurses, case managers, and social workers are the end users of the health care system. All parties are utilizing the health care system to optimize patient health. Therefore, we must create systems that are easy to navigate and use by patients and health care workers so that they can ensure the success of patients.
Unit-based teams offer a basic framework to optimize the inpatient system to facilitate better workflow. In our system, it allowed us to optimize communications between health care workers and also between health care workers and patients. It allowed team members to work in close proximity to better share ideas with each other.
We spent a significant amount of time upfront earning the support of all of the disciplines for this effort. We had support from all leaders within the organization and continue to make our case for this model by sharing metrics and holding forums to discuss the process.
Initial data show a marked improvement in many domains of HCAHPS scores. Our frontline staff, including attendings, residents, nursing, case managers, and social workers, also continue to support this effort since it has a positive impact on their workflow and improves their workday quality. One nurse mentioned specifically, “in my 30 years at this hospital I have never seen people work together so well.”
To sustain this effort, we continue to have regular meetings, and there are new features that we would like to add to the program. For example, we are working with our IT group to ensure that each unit-based team will have dashboards available to incorporate real time, actionable data into daily workflows.
We are excited by the potential of our high-performing teams to highlight the patient experience, placing the patient at the center for care, decision making, and rounding. Health care is a team sport, and anytime you build something where all teams are playing together and approaching the finish line as a unit, you will never go wrong!
Dr. Pendharkar is division chief of hospital medicine at the Brooklyn (N.Y.) Hospital Center, medical director of inpatient services and director of quality for the department of medicine at the Brooklyn Hospital Center and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York. Dr. Malieckal is chief resident, internal medicine, at the Brooklyn Hospital Center. Dr. Gasperino is chair, department of medicine; vice president for critical care, perioperative, and hospital medicine; and associate chief medical officer at the Brooklyn Hospital Center.
“To me, teamwork is the beauty of our sport, where you have five acting as one. You become selfless.” – Mike Krzyzewski
High-performing teams plan, communicate, reflect, and take action together. Teamwork can transform seemingly impossible tasks into opportunities for people to come together and create value.
The increasing complexity of health care makes team-based care necessary to achieve successful health outcomes for patients. At the Brooklyn (N.Y.) Hospital Center, a 464-bed care center, we transformed the model of care on the medical wards into a geographic, unit-based team model. Here we describe our journey – the successes, the challenges, and the opportunities for growth.
Previous model
In the previous care model on our medical wards, no set structures were in place. Teams would travel to multiple wards throughout the hospital to see the patients they were rounding on. Each floor had its own set of social workers and case managers, therefore a hospital medicine team routinely dealt with more than eight social workers and case managers to address their patients’ needs in a single day.
Multidisciplinary rounds for all medical patients were held at 11 a.m. in a room located a significant distance away from the medical wards. All case managers and social workers would sit in this room from 11 a.m. until noon, and teams would travel to that room to discuss their patients.
Many challenges were identified in this model, including a lack of communication, a de-emphasis on teamwork, and a design that did not take physician workflows into account resulting in low efficiency. Thus, these challenges sparked a desire to create a more effective and team-based methodology of accomplishing excellence in delivery of clinical care. Dr. Pendharkar, having worked primarily in centers with unit-based care, determined that a geographic, unit-based model of care could transform care delivery at the Brooklyn Hospital Center.
Looking ahead
The efforts for transforming the vision of geographic, unit-based teams into a reality started by gathering all stakeholders together to unite for a common mission. Initial meetings were held with all parties including social workers, case managers, residents, nursing staff, bed board and attending physicians in internal medicine, and the emergency department.
The vision of a geographic, unit-based team was shared and explained to all team members. Exercises in LEAN methodology were conducted, including one-piece flow exercises, to highlight the possibilities of what could be accomplished through teamwork. Once support for the vision was in place from all parties, the logistics were addressed.
The biggest challenge to overcome was how to place all of one team’s patients on a singular medical ward. In our hospital, a medical ward holds anywhere from 30 to 33 patients. Each hospital medicine team, of which there are many, typically carries 20-23 patients. We created a blueprint to map out the floor to which each team and attending would be assigned. Next, we partnered with both IT and bed board to design an admission order set that specified the particular geographic location that a team and attending were associated with so that patients could be placed accordingly from the ED.
It was important for the ED doctors, bed board, and the internal medicine residents to understand these changes because all of these parties were involved in the initial admitting process. Dr. Pendharkar and Dr. Malieckal provided all groups with in-person training on how the logistics of the system would unfold. Noon conference lectures were also held to explain the vision to residents.
Over 3 weeks, the first ward we chose to implement our model on slowly accumulated the patients of one team – this was the gradual trickle phase. We then selected a “re-set” date. On the re-set date, it was determined that all patients would go to the team that was assigned to that floor, with the exception of any private attendings’ patients.
On the day before the re-set date, time was spent ensuring that all hand-offs were safe. Dr. Pendharkar and Dr. Malieckal spoke with every intern and team that would be handing off and/or receiving patients as a result of the re-set policy. The goal was to ensure that on that date a ward had close to 100% of its patients belonging to the team/attending that was assigned to that area.
The good
Once we began our geographic, unit-based model, our rounding process was transformed.
Now, our morning rounds were joined by the bedside nurse, case manager, social worker, clinical pharmacy, and nutrition in addition to the core team. The entire team went from room to room on one ward rounding on all 20 to 25 patients back to back, which created an unparalleled level of efficiency and a forum for effective communication lasting throughout the day.
We also added workstations on wheels (WOWS) to the rounding process so that labs, radiology, and more could be reviewed on rounds with the entire team. A standard script was developed so that each patient was introduced to all members of the team, and the care plan was disclosed and highlighted. One patient noted, “I feel so cared for, knowing I have this entire team taking care of me.” We also rounded in the afternoon with the case managers and social workers to follow up tasks that were to be completed that day.
Our first few weeks utilizing the geographic, unit-based model of rounding was largely successful. The residents, now able to round on all of their patients in one location with one case manager and one social worker, noted, “This model of rounding makes my life so much easier, I feel like I can focus on the patient rather than running around. … and I know the social worker and case manager will help me.”
Provider satisfaction had improved, from residents to physicians to nurses, case managers, social workers, and more. Our case manager also noted her satisfaction with the new model, stating that her communication with the medical team was much easier. As the attending, I witnessed firsthand how working together with the team moved care forward much more quickly, compared with the previous model, because of the simple factor of increased ease of communication.
Now all team members were together in the patient room and discussion was much easier. There was less confusion, fewer delays, and better communication – I think unit-based teams can even be described as a lifesaving measure that reduces harm to patients. An additional benefit is the relationship that now developed between doctors, social workers, and case managers – they spent more time together and really got to know one another, creating a feeling of shared success and a deeper drive to help one another succeed.
In our model, 87% of surveyed residents said they felt less burned out in the new geographic, unit-based model of care, and 91% of physicians surveyed said it was easier to talk with team members to coordinate care. Additionally, our HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) scores saw a drastic increase in many domains. Nursing communication improved by more than 42% on domain 7B; doctor communication improved by more than 31%. Additionally, all other domains saw at least 10% improvement. We are now 5 months out from our initial rollout of the model and continue to see sustained improvements in quality measures.
The bad
The biggest challenges that we are working through with this model are hand-offs and transfer of patients from one team to another. Sometimes, it happens that one team’s patient will wind up on a floor that is the designated floor of another team because of bed availability. We continue to work with bed board to address this issue. We want to minimize transfers and hand-offs to promote continuity and have to balance that with the need for geographic location. With clear communication, hospital collaboration from bed board and safe hand-off methods, this problem can be safely addressed.
Conclusions
The experience of implementing the unit-based team model has been an eye-opening journey. One thing that stands out is that, in an increasingly complex health care system, design thinking is critical.
Design thinking takes into consideration the needs of those who are using a system. In this case, patients and health care workers including doctors, nurses, case managers, and social workers are the end users of the health care system. All parties are utilizing the health care system to optimize patient health. Therefore, we must create systems that are easy to navigate and use by patients and health care workers so that they can ensure the success of patients.
Unit-based teams offer a basic framework to optimize the inpatient system to facilitate better workflow. In our system, it allowed us to optimize communications between health care workers and also between health care workers and patients. It allowed team members to work in close proximity to better share ideas with each other.
We spent a significant amount of time upfront earning the support of all of the disciplines for this effort. We had support from all leaders within the organization and continue to make our case for this model by sharing metrics and holding forums to discuss the process.
Initial data show a marked improvement in many domains of HCAHPS scores. Our frontline staff, including attendings, residents, nursing, case managers, and social workers, also continue to support this effort since it has a positive impact on their workflow and improves their workday quality. One nurse mentioned specifically, “in my 30 years at this hospital I have never seen people work together so well.”
To sustain this effort, we continue to have regular meetings, and there are new features that we would like to add to the program. For example, we are working with our IT group to ensure that each unit-based team will have dashboards available to incorporate real time, actionable data into daily workflows.
We are excited by the potential of our high-performing teams to highlight the patient experience, placing the patient at the center for care, decision making, and rounding. Health care is a team sport, and anytime you build something where all teams are playing together and approaching the finish line as a unit, you will never go wrong!
Dr. Pendharkar is division chief of hospital medicine at the Brooklyn (N.Y.) Hospital Center, medical director of inpatient services and director of quality for the department of medicine at the Brooklyn Hospital Center and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York. Dr. Malieckal is chief resident, internal medicine, at the Brooklyn Hospital Center. Dr. Gasperino is chair, department of medicine; vice president for critical care, perioperative, and hospital medicine; and associate chief medical officer at the Brooklyn Hospital Center.
Survey: High costs lead to skipped or postponed health care
Half of all Americans with employer-sponsored health benefits say that they or someone in their family has skipped or postponed care because of the cost, according to a survey by the Kaiser Family Foundation and the Los Angeles Times.
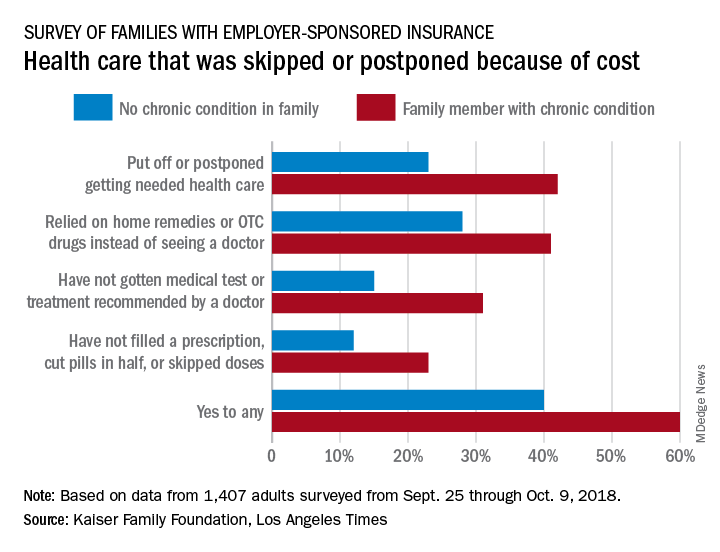
That number changes, however, when chronic conditions are considered. In the survey of Americans covered by employer-sponsored health insurance, 60% of those with a family member who had a chronic condition said that cost had altered the care of someone in the family over the previous 12 months, compared with 40% of those who had no chronic condition in their family, the KFF and L.A. Times noted in their report.
More specifically, families with an individual who had a chronic condition were more likely to put off or postpone needed care (42% vs. 23%) and to rely on home remedies or OTC drugs instead of visiting a physician (41% vs. 28%) than were families without chronic conditions, the report’s authors said.
When asked about the affordability of their health care, 49% of those in families with a chronic health condition said they had a problem paying for their coverage in the past year, compared with 29% of respondents in families with no chronic condition.
“Drilling down into the consequences of these affordability problems reveals more about the financial burden of health care on families with chronic conditions,” compared with those without chronic conditions: cut back spending on food, clothes, household items (35% vs. 16%); used up all or most of their savings (26% vs. 11%); and borrowed money from friends or family (14% vs. 6%), according to the researchers.
Although respondents felt “that the cost of health care for people like them is too high, more say the current U.S. health insurance system works well for people with employer coverage than say it works well for people on Medicare or Medicaid or those who purchase their own insurance. Asked who is to blame for high costs, majorities point the finger at pharmaceutical and insurance companies, while fewer see hospitals, doctors, or employers as deserving of blame,” the KFF and L.A. Times investigators wrote.
The survey involved a sample of 1,407 adults aged 18-64 years and was conducted from Sept. 25 through Oct. 9, 2018. The margin of the sampling error is ±3 percentage points.
Half of all Americans with employer-sponsored health benefits say that they or someone in their family has skipped or postponed care because of the cost, according to a survey by the Kaiser Family Foundation and the Los Angeles Times.

That number changes, however, when chronic conditions are considered. In the survey of Americans covered by employer-sponsored health insurance, 60% of those with a family member who had a chronic condition said that cost had altered the care of someone in the family over the previous 12 months, compared with 40% of those who had no chronic condition in their family, the KFF and L.A. Times noted in their report.
More specifically, families with an individual who had a chronic condition were more likely to put off or postpone needed care (42% vs. 23%) and to rely on home remedies or OTC drugs instead of visiting a physician (41% vs. 28%) than were families without chronic conditions, the report’s authors said.
When asked about the affordability of their health care, 49% of those in families with a chronic health condition said they had a problem paying for their coverage in the past year, compared with 29% of respondents in families with no chronic condition.
“Drilling down into the consequences of these affordability problems reveals more about the financial burden of health care on families with chronic conditions,” compared with those without chronic conditions: cut back spending on food, clothes, household items (35% vs. 16%); used up all or most of their savings (26% vs. 11%); and borrowed money from friends or family (14% vs. 6%), according to the researchers.
Although respondents felt “that the cost of health care for people like them is too high, more say the current U.S. health insurance system works well for people with employer coverage than say it works well for people on Medicare or Medicaid or those who purchase their own insurance. Asked who is to blame for high costs, majorities point the finger at pharmaceutical and insurance companies, while fewer see hospitals, doctors, or employers as deserving of blame,” the KFF and L.A. Times investigators wrote.
The survey involved a sample of 1,407 adults aged 18-64 years and was conducted from Sept. 25 through Oct. 9, 2018. The margin of the sampling error is ±3 percentage points.
Half of all Americans with employer-sponsored health benefits say that they or someone in their family has skipped or postponed care because of the cost, according to a survey by the Kaiser Family Foundation and the Los Angeles Times.

That number changes, however, when chronic conditions are considered. In the survey of Americans covered by employer-sponsored health insurance, 60% of those with a family member who had a chronic condition said that cost had altered the care of someone in the family over the previous 12 months, compared with 40% of those who had no chronic condition in their family, the KFF and L.A. Times noted in their report.
More specifically, families with an individual who had a chronic condition were more likely to put off or postpone needed care (42% vs. 23%) and to rely on home remedies or OTC drugs instead of visiting a physician (41% vs. 28%) than were families without chronic conditions, the report’s authors said.
When asked about the affordability of their health care, 49% of those in families with a chronic health condition said they had a problem paying for their coverage in the past year, compared with 29% of respondents in families with no chronic condition.
“Drilling down into the consequences of these affordability problems reveals more about the financial burden of health care on families with chronic conditions,” compared with those without chronic conditions: cut back spending on food, clothes, household items (35% vs. 16%); used up all or most of their savings (26% vs. 11%); and borrowed money from friends or family (14% vs. 6%), according to the researchers.
Although respondents felt “that the cost of health care for people like them is too high, more say the current U.S. health insurance system works well for people with employer coverage than say it works well for people on Medicare or Medicaid or those who purchase their own insurance. Asked who is to blame for high costs, majorities point the finger at pharmaceutical and insurance companies, while fewer see hospitals, doctors, or employers as deserving of blame,” the KFF and L.A. Times investigators wrote.
The survey involved a sample of 1,407 adults aged 18-64 years and was conducted from Sept. 25 through Oct. 9, 2018. The margin of the sampling error is ±3 percentage points.
CBO predicts more Medicare spending with drug rebate proposal
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.
SGLT2 inhibitors prevent HF hospitalization regardless of baseline LVEF
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
REPORTING FROM ACC 19
HM19: Pediatric clinical conundrums
Atypical symptoms and diagnoses
Presenters
Yemisi Jones, MD; Mirna Giordano, MD
Session title
Pediatric Clinical Conundrums
Session summary
Dr. Mirna Giordano of Columbia University Irving Medical Center, New York, and Dr. Yemisi Jones of Cincinnati Children’s Hospital Medical Center, moderated the Pediatric Clinical Conundrums session at HM19. After reviewing multiple submissions, they invited four trainees to present their interesting cases.
Malignancy or infection? Dr. Jeremy Brown, a resident at the University of Louisville, presented a case of a 15-year-old male with right upper quadrant abdominal pain with associated weight loss and intermittent fevers, over the course of several weeks. CT revealed multiple liver lesions, providing concern for possible malignancy, although liver biopsy proved otherwise, with mostly liquefactive tissue and benign liver parenchyma. After a large infectious work-up ensued, the patient was diagnosed with disseminated Bartonella. He was treated with a 10-day course of azithromycin, and his symptoms resolved.
Leg blisters as an uncommon manifestation of a common childhood disease. Dr. Stefan Mammele, a resident at Kapi’olani Medical Center in Honolulu, and the University of Hawaii, presented a case of an 11-year-old boy with a painful and pruritic rash associated with multiple 5- to 10-mm tense bullae located on the patient’s bilateral lower extremities with extension to the trunk. The patient was also found to have hematuria and proteinuria. The bullae drained both serosanguinous and purulent material. Fluid culture grew group A Streptococcus and skin biopsy confirmed IgA vasculitis. Bullae are a rare characteristic of Henoch Schönlein pupura in children, but are more commonly seen as a disease manifestation in adults. The patient was treated with cefazolin, and his lesions improved over the course of several weeks with resolution of his hematuria by 6 months.
Is she crying blood? Dr. Joshua Price, a resident at Baystate Children’s Hospital in Springfield, Mass., described a 12-year-old female who presented with 7 days of left-sided hemolacria with acute vision loss and unilateral eye pain. This patient did not respond to outpatient topical steroids and antibiotics, as prescribed by ophthalmology. For this reason, she underwent further work-up and imaging. MRI of the head and orbits revealed left maxillary sinus disease. She was treated with antibiotics for acute left maxillary sinusitis and her hemolacria resolved within 24 hours. While the differential diagnosis for hemolacria is broad, rarely acute sinusitis has been reported as a cause in medical literature.
Recurrent bronchiolitis or something more? Dr. Moira Black, a resident at Children’s Memorial Hermann in Houston, presented a case of a 7-month-old female with a history of recurrent admissions for increased work of breathing believed to be secondary to viral bronchiolitis. Her first hospitalization occurred at 7 weeks of age and was complicated by spontaneous pneumothorax requiring chest tube placement. She was again hospitalized at 5 months of age with resolution of her increased work of breathing with high-flow nasal cannula. She presented again at 7 months of age with presumed bronchiolitis, however, she decompensated and required intubation on the 5th day of hospitalization. A bronchoscopy was performed and revealed a significantly narrowed left bronchus at the carina and a blind pouch on the right with notable pulsation of the walls. She underwent further imaging and was ultimately diagnosed with a left pulmonary artery sling. Left pulmonary artery slings are a rare, but potentially fatal anomaly that can present with wheezing, stridor, and recurrent respiratory infections. Patient underwent correction by cardiovascular surgery and has since been doing well.
Key takeaways for HM
• Bartonella is a common cause of fever of unknown origin, and should be considered in unusual presentations of febrile illnesses.
• Bullae in IgA vasculitis are rare in children and do not have prognostic value, but streptococcal infection may be a trigger for IgA vasculitis.
• Hemolacria is an atypical presentation of rare and common diagnoses that should prompt further work-up.
• Acute respiratory distress can be caused by underlying cardiac or vascular anomalies and can be mistaken for common viral illnesses.
Dr. Marsicek is a pediatric hospital medicine fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla. Dr. Wysocka is a pediatric resident at Johns Hopkins All Children’s Hospital.
Atypical symptoms and diagnoses
Atypical symptoms and diagnoses
Presenters
Yemisi Jones, MD; Mirna Giordano, MD
Session title
Pediatric Clinical Conundrums
Session summary
Dr. Mirna Giordano of Columbia University Irving Medical Center, New York, and Dr. Yemisi Jones of Cincinnati Children’s Hospital Medical Center, moderated the Pediatric Clinical Conundrums session at HM19. After reviewing multiple submissions, they invited four trainees to present their interesting cases.
Malignancy or infection? Dr. Jeremy Brown, a resident at the University of Louisville, presented a case of a 15-year-old male with right upper quadrant abdominal pain with associated weight loss and intermittent fevers, over the course of several weeks. CT revealed multiple liver lesions, providing concern for possible malignancy, although liver biopsy proved otherwise, with mostly liquefactive tissue and benign liver parenchyma. After a large infectious work-up ensued, the patient was diagnosed with disseminated Bartonella. He was treated with a 10-day course of azithromycin, and his symptoms resolved.
Leg blisters as an uncommon manifestation of a common childhood disease. Dr. Stefan Mammele, a resident at Kapi’olani Medical Center in Honolulu, and the University of Hawaii, presented a case of an 11-year-old boy with a painful and pruritic rash associated with multiple 5- to 10-mm tense bullae located on the patient’s bilateral lower extremities with extension to the trunk. The patient was also found to have hematuria and proteinuria. The bullae drained both serosanguinous and purulent material. Fluid culture grew group A Streptococcus and skin biopsy confirmed IgA vasculitis. Bullae are a rare characteristic of Henoch Schönlein pupura in children, but are more commonly seen as a disease manifestation in adults. The patient was treated with cefazolin, and his lesions improved over the course of several weeks with resolution of his hematuria by 6 months.
Is she crying blood? Dr. Joshua Price, a resident at Baystate Children’s Hospital in Springfield, Mass., described a 12-year-old female who presented with 7 days of left-sided hemolacria with acute vision loss and unilateral eye pain. This patient did not respond to outpatient topical steroids and antibiotics, as prescribed by ophthalmology. For this reason, she underwent further work-up and imaging. MRI of the head and orbits revealed left maxillary sinus disease. She was treated with antibiotics for acute left maxillary sinusitis and her hemolacria resolved within 24 hours. While the differential diagnosis for hemolacria is broad, rarely acute sinusitis has been reported as a cause in medical literature.
Recurrent bronchiolitis or something more? Dr. Moira Black, a resident at Children’s Memorial Hermann in Houston, presented a case of a 7-month-old female with a history of recurrent admissions for increased work of breathing believed to be secondary to viral bronchiolitis. Her first hospitalization occurred at 7 weeks of age and was complicated by spontaneous pneumothorax requiring chest tube placement. She was again hospitalized at 5 months of age with resolution of her increased work of breathing with high-flow nasal cannula. She presented again at 7 months of age with presumed bronchiolitis, however, she decompensated and required intubation on the 5th day of hospitalization. A bronchoscopy was performed and revealed a significantly narrowed left bronchus at the carina and a blind pouch on the right with notable pulsation of the walls. She underwent further imaging and was ultimately diagnosed with a left pulmonary artery sling. Left pulmonary artery slings are a rare, but potentially fatal anomaly that can present with wheezing, stridor, and recurrent respiratory infections. Patient underwent correction by cardiovascular surgery and has since been doing well.
Key takeaways for HM
• Bartonella is a common cause of fever of unknown origin, and should be considered in unusual presentations of febrile illnesses.
• Bullae in IgA vasculitis are rare in children and do not have prognostic value, but streptococcal infection may be a trigger for IgA vasculitis.
• Hemolacria is an atypical presentation of rare and common diagnoses that should prompt further work-up.
• Acute respiratory distress can be caused by underlying cardiac or vascular anomalies and can be mistaken for common viral illnesses.
Dr. Marsicek is a pediatric hospital medicine fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla. Dr. Wysocka is a pediatric resident at Johns Hopkins All Children’s Hospital.
Presenters
Yemisi Jones, MD; Mirna Giordano, MD
Session title
Pediatric Clinical Conundrums
Session summary
Dr. Mirna Giordano of Columbia University Irving Medical Center, New York, and Dr. Yemisi Jones of Cincinnati Children’s Hospital Medical Center, moderated the Pediatric Clinical Conundrums session at HM19. After reviewing multiple submissions, they invited four trainees to present their interesting cases.
Malignancy or infection? Dr. Jeremy Brown, a resident at the University of Louisville, presented a case of a 15-year-old male with right upper quadrant abdominal pain with associated weight loss and intermittent fevers, over the course of several weeks. CT revealed multiple liver lesions, providing concern for possible malignancy, although liver biopsy proved otherwise, with mostly liquefactive tissue and benign liver parenchyma. After a large infectious work-up ensued, the patient was diagnosed with disseminated Bartonella. He was treated with a 10-day course of azithromycin, and his symptoms resolved.
Leg blisters as an uncommon manifestation of a common childhood disease. Dr. Stefan Mammele, a resident at Kapi’olani Medical Center in Honolulu, and the University of Hawaii, presented a case of an 11-year-old boy with a painful and pruritic rash associated with multiple 5- to 10-mm tense bullae located on the patient’s bilateral lower extremities with extension to the trunk. The patient was also found to have hematuria and proteinuria. The bullae drained both serosanguinous and purulent material. Fluid culture grew group A Streptococcus and skin biopsy confirmed IgA vasculitis. Bullae are a rare characteristic of Henoch Schönlein pupura in children, but are more commonly seen as a disease manifestation in adults. The patient was treated with cefazolin, and his lesions improved over the course of several weeks with resolution of his hematuria by 6 months.
Is she crying blood? Dr. Joshua Price, a resident at Baystate Children’s Hospital in Springfield, Mass., described a 12-year-old female who presented with 7 days of left-sided hemolacria with acute vision loss and unilateral eye pain. This patient did not respond to outpatient topical steroids and antibiotics, as prescribed by ophthalmology. For this reason, she underwent further work-up and imaging. MRI of the head and orbits revealed left maxillary sinus disease. She was treated with antibiotics for acute left maxillary sinusitis and her hemolacria resolved within 24 hours. While the differential diagnosis for hemolacria is broad, rarely acute sinusitis has been reported as a cause in medical literature.
Recurrent bronchiolitis or something more? Dr. Moira Black, a resident at Children’s Memorial Hermann in Houston, presented a case of a 7-month-old female with a history of recurrent admissions for increased work of breathing believed to be secondary to viral bronchiolitis. Her first hospitalization occurred at 7 weeks of age and was complicated by spontaneous pneumothorax requiring chest tube placement. She was again hospitalized at 5 months of age with resolution of her increased work of breathing with high-flow nasal cannula. She presented again at 7 months of age with presumed bronchiolitis, however, she decompensated and required intubation on the 5th day of hospitalization. A bronchoscopy was performed and revealed a significantly narrowed left bronchus at the carina and a blind pouch on the right with notable pulsation of the walls. She underwent further imaging and was ultimately diagnosed with a left pulmonary artery sling. Left pulmonary artery slings are a rare, but potentially fatal anomaly that can present with wheezing, stridor, and recurrent respiratory infections. Patient underwent correction by cardiovascular surgery and has since been doing well.
Key takeaways for HM
• Bartonella is a common cause of fever of unknown origin, and should be considered in unusual presentations of febrile illnesses.
• Bullae in IgA vasculitis are rare in children and do not have prognostic value, but streptococcal infection may be a trigger for IgA vasculitis.
• Hemolacria is an atypical presentation of rare and common diagnoses that should prompt further work-up.
• Acute respiratory distress can be caused by underlying cardiac or vascular anomalies and can be mistaken for common viral illnesses.
Dr. Marsicek is a pediatric hospital medicine fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla. Dr. Wysocka is a pediatric resident at Johns Hopkins All Children’s Hospital.
Hospitalist movers and shakers – May 2019
Christina L. Andrew, DO, a medical director on the hospitalist team at McLeod Regional Medical Center in Florence, S.C., and Zeshan Anwar, MD, medical director of Evangelical Community Hospital’s hospitalist group in Lewisburg, Pa., recently were named Senior Fellows in Hospital Medicine (SFHM) by the Society of Hospital Medicine. SFHMs are dedicated to promoting excellence, innovation and improving the quality of patient care.
Dr. Andrew has been with McLeod since 2008. The board-certified internist received her medical degree from Des Moines (Iowa) University Osteopathic Medical Center and did her residency at the Cleveland Clinic. To earn SFHM status, physicians must have worked as a hospitalist for at least 5 years and be a member of SHM for 5 years, as well.
Dr. Anwar has been in his current position since 2015. He coordinates staff resources and inpatient care for the facility where he has worked since 2013. He has his medical degree from King Edward Medical University, Lahore, Pakistan, and did his residency at Bronx-Lebanon Hospital Center in New York.
Tiffany Egbe, MD, has been named to the board of directors of Refuge International, an organization that builds relationships in Guatemala that allow for medical services to be provided to an underserved population.
Dr. Egbe is a hospitalist in internal medicine at Christus Good Shepherd in Longview and Marshall, Tex. She also serves as program director of internal medicine residency for the University of Texas Health Science Center in Tyler, Tex.
Dr. Egbe earned her medical degree from the University of Alabama at Birmingham.
Il Jun Chon, MD, has been named vice president of medical affairs with WellSpan Ephrata (Pa.) Community Hospital. Dr. Chon, a hospitalist, had previously been the medical director of WellSpan Ephrata’s hospitalist services and president of the facility’s medical staff.
Dr. Chon earned his medical degree from the Medical College of Pennsylvania (now Drexel College of Medicine) and completed his residency at Thomas Jefferson University Hospital, both in Philadelphia.
Megan Hamreus, DO, recently was named chief of staff at Scripps Mercy Hospital in San Diego, Calif. Dr. Hamreus will oversee 1,000 doctors at two facilities.
Dr. Hamreus has been with Scripps Mercy for 10 years, serving as a hospitalist and a faculty member of the family medicine residence training program of Family Health Centers of San Diego.
Chief of staff is a 2-year, elected term. Among her duties, Dr. Hamreus will be Scripps Mercy’s liaison to the facilities’ administrative staff and Scripps Health’s board of trustees.
Jade Brice Roshell, MD, recently was named chief medical officer at Shelby Baptist Medical Center in Alabaster, Ala. Dr. Brice Roshell was promoted from director of the center’s hospitalist program.
In addition to her new position, Dr. Brice Roshell was named as one of 68 honorees on Becker’s 2019 list of African-American Leaders in Health.
Dr. Brice Roshell has been with Shelby Baptist since 2015. Previously, she was an internist at centers in Louisiana, Georgia, and Nebraska. Her medical degree is from Howard University in Washington, and she completed her residency at Tulane University in New Orleans.
Anju Manral, MD, recently was appointed as medical director for the University of New Mexico Student Health and Counseling Center in Albuquerque. The internist has experience as a hospitalist focused on palliative care and most recently has worked at UNM’s Family Health Clinic, providing care to patients of all ages and conditions.
Dr. Manral also serves as an assistant professor in the UNM General Internal Medicine Department and mentors UNM medical students in the health science learning community.
The Hiawatha (Kan.) Community Hospital unveiled its new hospitalist program on Feb. 12.
The program will be led by Dustin Williams, DNP. Dr. Williams will provide hospitalist and emergency medical services to patients every Tuesday through Friday, while an on-call specialist will serve as hospitalist on Saturday, Sunday, and Monday.
Christina L. Andrew, DO, a medical director on the hospitalist team at McLeod Regional Medical Center in Florence, S.C., and Zeshan Anwar, MD, medical director of Evangelical Community Hospital’s hospitalist group in Lewisburg, Pa., recently were named Senior Fellows in Hospital Medicine (SFHM) by the Society of Hospital Medicine. SFHMs are dedicated to promoting excellence, innovation and improving the quality of patient care.
Dr. Andrew has been with McLeod since 2008. The board-certified internist received her medical degree from Des Moines (Iowa) University Osteopathic Medical Center and did her residency at the Cleveland Clinic. To earn SFHM status, physicians must have worked as a hospitalist for at least 5 years and be a member of SHM for 5 years, as well.
Dr. Anwar has been in his current position since 2015. He coordinates staff resources and inpatient care for the facility where he has worked since 2013. He has his medical degree from King Edward Medical University, Lahore, Pakistan, and did his residency at Bronx-Lebanon Hospital Center in New York.
Tiffany Egbe, MD, has been named to the board of directors of Refuge International, an organization that builds relationships in Guatemala that allow for medical services to be provided to an underserved population.
Dr. Egbe is a hospitalist in internal medicine at Christus Good Shepherd in Longview and Marshall, Tex. She also serves as program director of internal medicine residency for the University of Texas Health Science Center in Tyler, Tex.
Dr. Egbe earned her medical degree from the University of Alabama at Birmingham.
Il Jun Chon, MD, has been named vice president of medical affairs with WellSpan Ephrata (Pa.) Community Hospital. Dr. Chon, a hospitalist, had previously been the medical director of WellSpan Ephrata’s hospitalist services and president of the facility’s medical staff.
Dr. Chon earned his medical degree from the Medical College of Pennsylvania (now Drexel College of Medicine) and completed his residency at Thomas Jefferson University Hospital, both in Philadelphia.
Megan Hamreus, DO, recently was named chief of staff at Scripps Mercy Hospital in San Diego, Calif. Dr. Hamreus will oversee 1,000 doctors at two facilities.
Dr. Hamreus has been with Scripps Mercy for 10 years, serving as a hospitalist and a faculty member of the family medicine residence training program of Family Health Centers of San Diego.
Chief of staff is a 2-year, elected term. Among her duties, Dr. Hamreus will be Scripps Mercy’s liaison to the facilities’ administrative staff and Scripps Health’s board of trustees.
Jade Brice Roshell, MD, recently was named chief medical officer at Shelby Baptist Medical Center in Alabaster, Ala. Dr. Brice Roshell was promoted from director of the center’s hospitalist program.
In addition to her new position, Dr. Brice Roshell was named as one of 68 honorees on Becker’s 2019 list of African-American Leaders in Health.
Dr. Brice Roshell has been with Shelby Baptist since 2015. Previously, she was an internist at centers in Louisiana, Georgia, and Nebraska. Her medical degree is from Howard University in Washington, and she completed her residency at Tulane University in New Orleans.
Anju Manral, MD, recently was appointed as medical director for the University of New Mexico Student Health and Counseling Center in Albuquerque. The internist has experience as a hospitalist focused on palliative care and most recently has worked at UNM’s Family Health Clinic, providing care to patients of all ages and conditions.
Dr. Manral also serves as an assistant professor in the UNM General Internal Medicine Department and mentors UNM medical students in the health science learning community.
The Hiawatha (Kan.) Community Hospital unveiled its new hospitalist program on Feb. 12.
The program will be led by Dustin Williams, DNP. Dr. Williams will provide hospitalist and emergency medical services to patients every Tuesday through Friday, while an on-call specialist will serve as hospitalist on Saturday, Sunday, and Monday.
Christina L. Andrew, DO, a medical director on the hospitalist team at McLeod Regional Medical Center in Florence, S.C., and Zeshan Anwar, MD, medical director of Evangelical Community Hospital’s hospitalist group in Lewisburg, Pa., recently were named Senior Fellows in Hospital Medicine (SFHM) by the Society of Hospital Medicine. SFHMs are dedicated to promoting excellence, innovation and improving the quality of patient care.
Dr. Andrew has been with McLeod since 2008. The board-certified internist received her medical degree from Des Moines (Iowa) University Osteopathic Medical Center and did her residency at the Cleveland Clinic. To earn SFHM status, physicians must have worked as a hospitalist for at least 5 years and be a member of SHM for 5 years, as well.
Dr. Anwar has been in his current position since 2015. He coordinates staff resources and inpatient care for the facility where he has worked since 2013. He has his medical degree from King Edward Medical University, Lahore, Pakistan, and did his residency at Bronx-Lebanon Hospital Center in New York.
Tiffany Egbe, MD, has been named to the board of directors of Refuge International, an organization that builds relationships in Guatemala that allow for medical services to be provided to an underserved population.
Dr. Egbe is a hospitalist in internal medicine at Christus Good Shepherd in Longview and Marshall, Tex. She also serves as program director of internal medicine residency for the University of Texas Health Science Center in Tyler, Tex.
Dr. Egbe earned her medical degree from the University of Alabama at Birmingham.
Il Jun Chon, MD, has been named vice president of medical affairs with WellSpan Ephrata (Pa.) Community Hospital. Dr. Chon, a hospitalist, had previously been the medical director of WellSpan Ephrata’s hospitalist services and president of the facility’s medical staff.
Dr. Chon earned his medical degree from the Medical College of Pennsylvania (now Drexel College of Medicine) and completed his residency at Thomas Jefferson University Hospital, both in Philadelphia.
Megan Hamreus, DO, recently was named chief of staff at Scripps Mercy Hospital in San Diego, Calif. Dr. Hamreus will oversee 1,000 doctors at two facilities.
Dr. Hamreus has been with Scripps Mercy for 10 years, serving as a hospitalist and a faculty member of the family medicine residence training program of Family Health Centers of San Diego.
Chief of staff is a 2-year, elected term. Among her duties, Dr. Hamreus will be Scripps Mercy’s liaison to the facilities’ administrative staff and Scripps Health’s board of trustees.
Jade Brice Roshell, MD, recently was named chief medical officer at Shelby Baptist Medical Center in Alabaster, Ala. Dr. Brice Roshell was promoted from director of the center’s hospitalist program.
In addition to her new position, Dr. Brice Roshell was named as one of 68 honorees on Becker’s 2019 list of African-American Leaders in Health.
Dr. Brice Roshell has been with Shelby Baptist since 2015. Previously, she was an internist at centers in Louisiana, Georgia, and Nebraska. Her medical degree is from Howard University in Washington, and she completed her residency at Tulane University in New Orleans.
Anju Manral, MD, recently was appointed as medical director for the University of New Mexico Student Health and Counseling Center in Albuquerque. The internist has experience as a hospitalist focused on palliative care and most recently has worked at UNM’s Family Health Clinic, providing care to patients of all ages and conditions.
Dr. Manral also serves as an assistant professor in the UNM General Internal Medicine Department and mentors UNM medical students in the health science learning community.
The Hiawatha (Kan.) Community Hospital unveiled its new hospitalist program on Feb. 12.
The program will be led by Dustin Williams, DNP. Dr. Williams will provide hospitalist and emergency medical services to patients every Tuesday through Friday, while an on-call specialist will serve as hospitalist on Saturday, Sunday, and Monday.
Racial, economic disparities found in buprenorphine prescriptions
Buprenorphine for opioid use disorder is much less likely to be prescribed to patients who are black or who do not have health insurance, an analysis of two national surveys shows.
Researchers analyzed data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey from 2004 to 2015, including 13.4 million visits in which buprenorphine was prescribed. The analysis was published as a research letter in JAMA Psychiatry.
From 2012 to 2015, the number of ambulatory visits involving buprenorphine rose from 0.04% to 0.36%. Black patients were 77% less likely to receive a prescription for buprenorphine at their visit – even after adjustment for payment method, sex, and age – while the number of prescription received by white patients was considerably higher than for patients of any other ethnicity, wrote Pooja A. Lagisetty, MD, and coauthors.
, and the age group with the highest incidence of buprenorphine prescriptions was 30-50 years.
Self-pay and private health insurance were the most common payment methods, but the number of self-paying patients receiving buprenorphine prescriptions dramatically increased from 585,568 in 2004-2007 to 5.3 million in 2012-2015.
“This finding in nationally representative data builds on a previous study that reported buprenorphine treatment disparities on the basis of race/ethnicity and income in New York City,” said Dr. Lagisetty of the department of medicine at the University of Michigan, Ann Arbor, and coauthors.
However, they acknowledged that it was unclear whether the treatment disparity might in fact reflect a difference in the prevalence of opioid use disorder across ethnicities.
Commenting on the differences in payment methods, the authors noted that, despite the enactment of mental health parity legislation and the expansion of Medicaid, the proportion of self-pay visits remained relatively unchanged across the study period.
“A recent study demonstrated that half of the physicians prescribing buprenorphine in Ohio accepted cash alone, and our findings suggest that this practice may be widespread and may be associated with additional financial barriers for low-income populations,” the researchers wrote. “With rising rates of opioid overdoses, it is imperative that policy and research efforts specifically address racial/ethnic and economic differences in treatment access and engagement.”
No conflicts of interest were declared.
SOURCE: Lagisetty P et al. JAMA Psychiatry. 2019 May 8. doi: 10.1001/jamapsychiatry.2019.0876.
Buprenorphine for opioid use disorder is much less likely to be prescribed to patients who are black or who do not have health insurance, an analysis of two national surveys shows.
Researchers analyzed data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey from 2004 to 2015, including 13.4 million visits in which buprenorphine was prescribed. The analysis was published as a research letter in JAMA Psychiatry.
From 2012 to 2015, the number of ambulatory visits involving buprenorphine rose from 0.04% to 0.36%. Black patients were 77% less likely to receive a prescription for buprenorphine at their visit – even after adjustment for payment method, sex, and age – while the number of prescription received by white patients was considerably higher than for patients of any other ethnicity, wrote Pooja A. Lagisetty, MD, and coauthors.
, and the age group with the highest incidence of buprenorphine prescriptions was 30-50 years.
Self-pay and private health insurance were the most common payment methods, but the number of self-paying patients receiving buprenorphine prescriptions dramatically increased from 585,568 in 2004-2007 to 5.3 million in 2012-2015.
“This finding in nationally representative data builds on a previous study that reported buprenorphine treatment disparities on the basis of race/ethnicity and income in New York City,” said Dr. Lagisetty of the department of medicine at the University of Michigan, Ann Arbor, and coauthors.
However, they acknowledged that it was unclear whether the treatment disparity might in fact reflect a difference in the prevalence of opioid use disorder across ethnicities.
Commenting on the differences in payment methods, the authors noted that, despite the enactment of mental health parity legislation and the expansion of Medicaid, the proportion of self-pay visits remained relatively unchanged across the study period.
“A recent study demonstrated that half of the physicians prescribing buprenorphine in Ohio accepted cash alone, and our findings suggest that this practice may be widespread and may be associated with additional financial barriers for low-income populations,” the researchers wrote. “With rising rates of opioid overdoses, it is imperative that policy and research efforts specifically address racial/ethnic and economic differences in treatment access and engagement.”
No conflicts of interest were declared.
SOURCE: Lagisetty P et al. JAMA Psychiatry. 2019 May 8. doi: 10.1001/jamapsychiatry.2019.0876.
Buprenorphine for opioid use disorder is much less likely to be prescribed to patients who are black or who do not have health insurance, an analysis of two national surveys shows.
Researchers analyzed data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey from 2004 to 2015, including 13.4 million visits in which buprenorphine was prescribed. The analysis was published as a research letter in JAMA Psychiatry.
From 2012 to 2015, the number of ambulatory visits involving buprenorphine rose from 0.04% to 0.36%. Black patients were 77% less likely to receive a prescription for buprenorphine at their visit – even after adjustment for payment method, sex, and age – while the number of prescription received by white patients was considerably higher than for patients of any other ethnicity, wrote Pooja A. Lagisetty, MD, and coauthors.
, and the age group with the highest incidence of buprenorphine prescriptions was 30-50 years.
Self-pay and private health insurance were the most common payment methods, but the number of self-paying patients receiving buprenorphine prescriptions dramatically increased from 585,568 in 2004-2007 to 5.3 million in 2012-2015.
“This finding in nationally representative data builds on a previous study that reported buprenorphine treatment disparities on the basis of race/ethnicity and income in New York City,” said Dr. Lagisetty of the department of medicine at the University of Michigan, Ann Arbor, and coauthors.
However, they acknowledged that it was unclear whether the treatment disparity might in fact reflect a difference in the prevalence of opioid use disorder across ethnicities.
Commenting on the differences in payment methods, the authors noted that, despite the enactment of mental health parity legislation and the expansion of Medicaid, the proportion of self-pay visits remained relatively unchanged across the study period.
“A recent study demonstrated that half of the physicians prescribing buprenorphine in Ohio accepted cash alone, and our findings suggest that this practice may be widespread and may be associated with additional financial barriers for low-income populations,” the researchers wrote. “With rising rates of opioid overdoses, it is imperative that policy and research efforts specifically address racial/ethnic and economic differences in treatment access and engagement.”
No conflicts of interest were declared.
SOURCE: Lagisetty P et al. JAMA Psychiatry. 2019 May 8. doi: 10.1001/jamapsychiatry.2019.0876.
FROM JAMA PSYCHIATRY
















