User login
In Case You Missed It: COVID
Adolescent immunizations and protecting our children from COVID-19
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
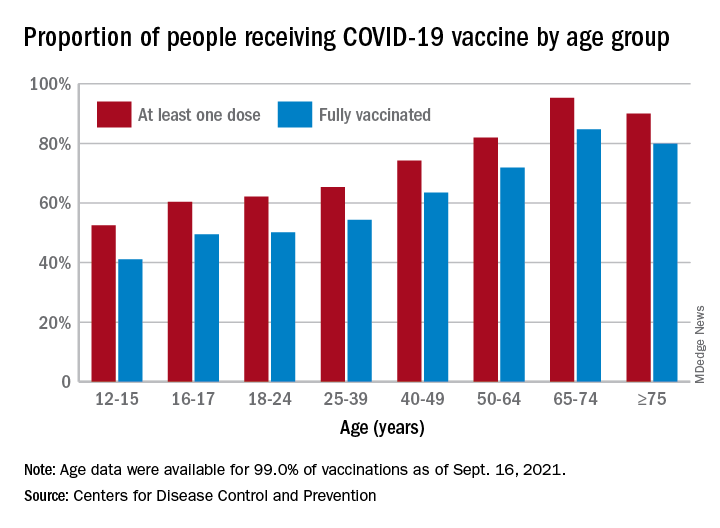
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)
It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)

It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)

It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Moderna vaccine more effective than Pfizer and J&J
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
COVID vaccine is safe, effective for children aged 5-11, Pfizer says
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
U.S. seniors’ pandemic care worst among wealthy nations: Survey
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA panel backs Pfizer's COVID booster for 65 and older, those at high risk
An expert panel that advises the Food and Drug Administration on its regulatory decisions voted Sept. 17 against recommending third doses of Pfizer’s COVID-19 vaccine for younger Americans.
But they didn’t kill the idea of booster shots completely.
In a dramatic, last-minute pivot, the 18 members of the FDA’s Vaccines and Related Biological Products Advisory Committee unanimously voted to recommend the FDA make boosters available for seniors and others at high risk of severe outcomes from COVID-19, including health care workers.
The 16-2 vote was a rebuttal to Pfizer’s initial request. The company had asked the FDA to allow it to offer third doses to all Americans over the age of 16 at least six months after their second shot.
The company requested an amendment to the full approval the FDA granted in August. That is the typical way boosters are authorized in the U.S., but it requires a higher bar of evidence and more regulatory scrutiny than the agency had been able to give since Pfizer filed for the change just days after its vaccine was granted full approval.
The committee’s actions were also a rebuff to the Biden administration, which announced before the FDA approved them that boosters would be rolled out to the general public Sept. 20. The announcement triggered the resignations of two of the agency’s top vaccine reviewers, who both participated in the Sept. 17 meeting.
After initially voting against Pfizer’s request to amend its license, the committee then worked on the fly with FDA officials to craft a strategy that would allow third doses to be offered under an emergency use authorization (EUA).
An EUA requires a lower standard of evidence and is more specific. It will restrict third doses to a more defined population than a change to the license would. It will also require Pfizer to continue to monitor the safety of third doses as they begin to be administered.
“This should demonstrate to the public that the members of this committee are independent of the FDA and that we do, in fact, bring our voices to the table when we are asked to serve on this committee,” said Archana Chattergee, MD, a pediatric infectious disease specialist who is dean of the Chicago Medical School at Rosalind Franklin University in Illinois.
The FDA doesn’t have to follow the committee’s recommendation, but almost certainly will, though regulators said they may still make some changes.
“We are not bound at FDA by your vote, we can tweak this,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA. Dr. Marks participated in the meeting and helped to draft the revised proposal.
If the FDA issues the recommended EUA, a council of independent advisors to the CDC will make specific recommendations about how the third doses should be given. After the CDC director weighs in, boosters will begin rolling out to the public.
Moderna submitted data to the FDA on Sept. 1 in support of adding a booster dose to its regimen. The agency has not yet scheduled a public review of that data.
The Biden administration is prepared to administer shots as soon as they get the green light, Surgeon General Vivek Murthy, MD, said at a White House briefing earlier Sept. 17.
"This process is consistent with what we outlined in August where our goals were to stay ahead of the virus," Dr. Murthy said. "Our goal then and now is to protect the health and well-being of the public. As soon as the FDA and CDC complete their evaluations, we will be ready to move forward accordingly."
He added, "We've used this time since our August announcement to communicate and coordinate with pharmacy partners, nursing homes, states, and localities."
White House COVID-19 Response Coordinator Jeff Zients said vaccine supply is "in good shape for all Americans to get boosters as recommended."
Taking cues from Israel
In considering Pfizer’s original request, the committee overwhelmingly felt that they didn’t have enough information to say that the benefits of an additional dose of vaccine in 16- and 17-year-olds would outweigh its risk. Teens have the highest risk of rare heart inflammation after vaccination, a side effect known as myocarditis. It is not known how the vaccines are causing these cases of heart swelling. Most who have been diagnosed with the condition have recovered, though some have needed hospital care.
Pfizer didn’t include 16- and 17-year-olds in its studies of boosters, which included about 300 people between the ages of 18 and 55. The company acknowledged that gap in its data but pointed to FDA guidance that said evidence from adults could be extrapolated to teens.
“We don’t know that much about risks,” said committee member Eric Rubin, MD, who is editor-in-chief of the New England Journal of Medicine.
Much of the data on the potential benefits and harms of third Pfizer doses comes from Israel, which first began rolling out boosters to older adults in July.
In a highly anticipated presentation, Sharon Alroy-Preis, Israel’s director of public health services, joined the meeting to describe Israel’s experience with boosters.
Israel began to see a third surge of COVID-19 cases in December.
“This was after having two waves and two lockdowns,” Ms. Alroy-Preis said. By the third surge, she said, Israelis were tired.
“We decided on a lockdown, but the compliance of the public wasn’t as it was in the previous two waves,” she said.
Then the vaccine arrived. Israel started vaccinations as soon as the FDA approved it, and they quickly vaccinated a high percentage of their population, about 3 months faster than the rest of the world.
All vaccinations are reported and tracked by the Ministry of Health, so the country is able to keep close tabs on how well the shots are working.
As vaccines rolled out, cases fell dramatically. The pandemic seemed to be behind them. Delta arrived in March. By June, their cases were doubling every 10 days, despite about 80% of their most vulnerable adults being fully vaccinated, she said.
Most concerning was that about 60% of severe cases were breakthrough cases in fully vaccinated individuals.
“We had to stop and figure out, was this a Delta issue,” she said. “Or was this a waning immunity issue.”
“We had some clue that it might not be the Delta variant, at least not alone,” she said.
People who had originally been first in line for the vaccines, seniors and health care workers, were having the highest rates of breakthrough infections. People further away from their second dose were more likely to get a breakthrough infection.
Ms. Alroy-Preis said that if they had not started booster doses in July, their hospitals would have been overwhelmed. They had projected that they would have 2,000 cases in the hospital each day.
Boosters have helped to flatten the curve, though they are still seeing a significant number of infections.
Data from Israel presented at the meeting show boosters are largely safe and effective at reducing severe outcomes in seniors. Israeli experience also showed that third doses, which generate very high levels of neutralizing antibodies—the first and fastest line of the body’s immune defense - -may also slow transmission of the virus.
Key differences in the U.S.
The benefit of slowing down the explosive spread of a highly contagious virus was tantalizing, but many members noted that circumstances in Israel are very different than in the United States. Israel went into its current Delta surge already having high levels of vaccination in its population. They also relied on the Pfizer vaccine almost exclusively for their campaign.
The United States used a different mix of vaccines – Pfizer, Moderna, and Johnson & Johnson -- and doesn’t have the same high level of vaccination coverage of its population.
In the United States, transmission is mainly being driven by unvaccinated people, Dr. Rubin noted.
“That really means the primary benefit is going to be in reducing disease,” he said, “And we know the people who are going to benefit from that … and those are the kinds of people the FDA has already approved a third dose for,” he said, referring to those with underlying health conditions.
But Israel only began vaccinating younger people a few weeks ago. Most are still within a window where rare risks like myocarditis could appear, Rubin noted.
He and other members of the committee said they wished they had more information about the safety of third doses in younger adults.
“We don’t have that right now, and I don’t think I would be comfortable giving it to a 16-year-old,” he said.
At the same time, the primary benefit for third doses would be in preventing severe disease, and overall, data from the United States and other countries show that two doses of the vaccines remain highly effective at preventing hospitalization and death.
Asked why Israel began to see more severe cases in fully vaccinated people, the CDC’s Sara Oliver, MD, a disease detective with the CDC, said it was probably due to a mix of factors including the fact that Israel defines severe cases a little differently.
In the United States, a severe case is generally a person who has to be hospitalized or who has died from the infection. In Israel, a person with a severe case is someone who has an elevated respiratory rate and someone who has a blood oxygen level less than 94%. In the United States, that kind of patient wouldn’t necessarily be hospitalized.
In the end, one of the two committee members who wanted full approval for Pfizer’s third doses said he was satisfied with the outcome.
Mark Sawyer, MD, a professor of pediatrics and infectious disease at the University of California at San Diego, said he voted yes on the first question because he thought full approval was the best way to give doctors the flexibility to prescribe the shots to vulnerable individuals.
“I’m really glad we authorized a vaccine for a third dose, and I plan to go out and get my vaccine this afternoon,” Dr. Sawyer said, noting that he was at high risk as a health care provider.
This article was updated 9/19/21.
A version of this article first appeared on Medscape.com.
An expert panel that advises the Food and Drug Administration on its regulatory decisions voted Sept. 17 against recommending third doses of Pfizer’s COVID-19 vaccine for younger Americans.
But they didn’t kill the idea of booster shots completely.
In a dramatic, last-minute pivot, the 18 members of the FDA’s Vaccines and Related Biological Products Advisory Committee unanimously voted to recommend the FDA make boosters available for seniors and others at high risk of severe outcomes from COVID-19, including health care workers.
The 16-2 vote was a rebuttal to Pfizer’s initial request. The company had asked the FDA to allow it to offer third doses to all Americans over the age of 16 at least six months after their second shot.
The company requested an amendment to the full approval the FDA granted in August. That is the typical way boosters are authorized in the U.S., but it requires a higher bar of evidence and more regulatory scrutiny than the agency had been able to give since Pfizer filed for the change just days after its vaccine was granted full approval.
The committee’s actions were also a rebuff to the Biden administration, which announced before the FDA approved them that boosters would be rolled out to the general public Sept. 20. The announcement triggered the resignations of two of the agency’s top vaccine reviewers, who both participated in the Sept. 17 meeting.
After initially voting against Pfizer’s request to amend its license, the committee then worked on the fly with FDA officials to craft a strategy that would allow third doses to be offered under an emergency use authorization (EUA).
An EUA requires a lower standard of evidence and is more specific. It will restrict third doses to a more defined population than a change to the license would. It will also require Pfizer to continue to monitor the safety of third doses as they begin to be administered.
“This should demonstrate to the public that the members of this committee are independent of the FDA and that we do, in fact, bring our voices to the table when we are asked to serve on this committee,” said Archana Chattergee, MD, a pediatric infectious disease specialist who is dean of the Chicago Medical School at Rosalind Franklin University in Illinois.
The FDA doesn’t have to follow the committee’s recommendation, but almost certainly will, though regulators said they may still make some changes.
“We are not bound at FDA by your vote, we can tweak this,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA. Dr. Marks participated in the meeting and helped to draft the revised proposal.
If the FDA issues the recommended EUA, a council of independent advisors to the CDC will make specific recommendations about how the third doses should be given. After the CDC director weighs in, boosters will begin rolling out to the public.
Moderna submitted data to the FDA on Sept. 1 in support of adding a booster dose to its regimen. The agency has not yet scheduled a public review of that data.
The Biden administration is prepared to administer shots as soon as they get the green light, Surgeon General Vivek Murthy, MD, said at a White House briefing earlier Sept. 17.
"This process is consistent with what we outlined in August where our goals were to stay ahead of the virus," Dr. Murthy said. "Our goal then and now is to protect the health and well-being of the public. As soon as the FDA and CDC complete their evaluations, we will be ready to move forward accordingly."
He added, "We've used this time since our August announcement to communicate and coordinate with pharmacy partners, nursing homes, states, and localities."
White House COVID-19 Response Coordinator Jeff Zients said vaccine supply is "in good shape for all Americans to get boosters as recommended."
Taking cues from Israel
In considering Pfizer’s original request, the committee overwhelmingly felt that they didn’t have enough information to say that the benefits of an additional dose of vaccine in 16- and 17-year-olds would outweigh its risk. Teens have the highest risk of rare heart inflammation after vaccination, a side effect known as myocarditis. It is not known how the vaccines are causing these cases of heart swelling. Most who have been diagnosed with the condition have recovered, though some have needed hospital care.
Pfizer didn’t include 16- and 17-year-olds in its studies of boosters, which included about 300 people between the ages of 18 and 55. The company acknowledged that gap in its data but pointed to FDA guidance that said evidence from adults could be extrapolated to teens.
“We don’t know that much about risks,” said committee member Eric Rubin, MD, who is editor-in-chief of the New England Journal of Medicine.
Much of the data on the potential benefits and harms of third Pfizer doses comes from Israel, which first began rolling out boosters to older adults in July.
In a highly anticipated presentation, Sharon Alroy-Preis, Israel’s director of public health services, joined the meeting to describe Israel’s experience with boosters.
Israel began to see a third surge of COVID-19 cases in December.
“This was after having two waves and two lockdowns,” Ms. Alroy-Preis said. By the third surge, she said, Israelis were tired.
“We decided on a lockdown, but the compliance of the public wasn’t as it was in the previous two waves,” she said.
Then the vaccine arrived. Israel started vaccinations as soon as the FDA approved it, and they quickly vaccinated a high percentage of their population, about 3 months faster than the rest of the world.
All vaccinations are reported and tracked by the Ministry of Health, so the country is able to keep close tabs on how well the shots are working.
As vaccines rolled out, cases fell dramatically. The pandemic seemed to be behind them. Delta arrived in March. By June, their cases were doubling every 10 days, despite about 80% of their most vulnerable adults being fully vaccinated, she said.
Most concerning was that about 60% of severe cases were breakthrough cases in fully vaccinated individuals.
“We had to stop and figure out, was this a Delta issue,” she said. “Or was this a waning immunity issue.”
“We had some clue that it might not be the Delta variant, at least not alone,” she said.
People who had originally been first in line for the vaccines, seniors and health care workers, were having the highest rates of breakthrough infections. People further away from their second dose were more likely to get a breakthrough infection.
Ms. Alroy-Preis said that if they had not started booster doses in July, their hospitals would have been overwhelmed. They had projected that they would have 2,000 cases in the hospital each day.
Boosters have helped to flatten the curve, though they are still seeing a significant number of infections.
Data from Israel presented at the meeting show boosters are largely safe and effective at reducing severe outcomes in seniors. Israeli experience also showed that third doses, which generate very high levels of neutralizing antibodies—the first and fastest line of the body’s immune defense - -may also slow transmission of the virus.
Key differences in the U.S.
The benefit of slowing down the explosive spread of a highly contagious virus was tantalizing, but many members noted that circumstances in Israel are very different than in the United States. Israel went into its current Delta surge already having high levels of vaccination in its population. They also relied on the Pfizer vaccine almost exclusively for their campaign.
The United States used a different mix of vaccines – Pfizer, Moderna, and Johnson & Johnson -- and doesn’t have the same high level of vaccination coverage of its population.
In the United States, transmission is mainly being driven by unvaccinated people, Dr. Rubin noted.
“That really means the primary benefit is going to be in reducing disease,” he said, “And we know the people who are going to benefit from that … and those are the kinds of people the FDA has already approved a third dose for,” he said, referring to those with underlying health conditions.
But Israel only began vaccinating younger people a few weeks ago. Most are still within a window where rare risks like myocarditis could appear, Rubin noted.
He and other members of the committee said they wished they had more information about the safety of third doses in younger adults.
“We don’t have that right now, and I don’t think I would be comfortable giving it to a 16-year-old,” he said.
At the same time, the primary benefit for third doses would be in preventing severe disease, and overall, data from the United States and other countries show that two doses of the vaccines remain highly effective at preventing hospitalization and death.
Asked why Israel began to see more severe cases in fully vaccinated people, the CDC’s Sara Oliver, MD, a disease detective with the CDC, said it was probably due to a mix of factors including the fact that Israel defines severe cases a little differently.
In the United States, a severe case is generally a person who has to be hospitalized or who has died from the infection. In Israel, a person with a severe case is someone who has an elevated respiratory rate and someone who has a blood oxygen level less than 94%. In the United States, that kind of patient wouldn’t necessarily be hospitalized.
In the end, one of the two committee members who wanted full approval for Pfizer’s third doses said he was satisfied with the outcome.
Mark Sawyer, MD, a professor of pediatrics and infectious disease at the University of California at San Diego, said he voted yes on the first question because he thought full approval was the best way to give doctors the flexibility to prescribe the shots to vulnerable individuals.
“I’m really glad we authorized a vaccine for a third dose, and I plan to go out and get my vaccine this afternoon,” Dr. Sawyer said, noting that he was at high risk as a health care provider.
This article was updated 9/19/21.
A version of this article first appeared on Medscape.com.
An expert panel that advises the Food and Drug Administration on its regulatory decisions voted Sept. 17 against recommending third doses of Pfizer’s COVID-19 vaccine for younger Americans.
But they didn’t kill the idea of booster shots completely.
In a dramatic, last-minute pivot, the 18 members of the FDA’s Vaccines and Related Biological Products Advisory Committee unanimously voted to recommend the FDA make boosters available for seniors and others at high risk of severe outcomes from COVID-19, including health care workers.
The 16-2 vote was a rebuttal to Pfizer’s initial request. The company had asked the FDA to allow it to offer third doses to all Americans over the age of 16 at least six months after their second shot.
The company requested an amendment to the full approval the FDA granted in August. That is the typical way boosters are authorized in the U.S., but it requires a higher bar of evidence and more regulatory scrutiny than the agency had been able to give since Pfizer filed for the change just days after its vaccine was granted full approval.
The committee’s actions were also a rebuff to the Biden administration, which announced before the FDA approved them that boosters would be rolled out to the general public Sept. 20. The announcement triggered the resignations of two of the agency’s top vaccine reviewers, who both participated in the Sept. 17 meeting.
After initially voting against Pfizer’s request to amend its license, the committee then worked on the fly with FDA officials to craft a strategy that would allow third doses to be offered under an emergency use authorization (EUA).
An EUA requires a lower standard of evidence and is more specific. It will restrict third doses to a more defined population than a change to the license would. It will also require Pfizer to continue to monitor the safety of third doses as they begin to be administered.
“This should demonstrate to the public that the members of this committee are independent of the FDA and that we do, in fact, bring our voices to the table when we are asked to serve on this committee,” said Archana Chattergee, MD, a pediatric infectious disease specialist who is dean of the Chicago Medical School at Rosalind Franklin University in Illinois.
The FDA doesn’t have to follow the committee’s recommendation, but almost certainly will, though regulators said they may still make some changes.
“We are not bound at FDA by your vote, we can tweak this,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA. Dr. Marks participated in the meeting and helped to draft the revised proposal.
If the FDA issues the recommended EUA, a council of independent advisors to the CDC will make specific recommendations about how the third doses should be given. After the CDC director weighs in, boosters will begin rolling out to the public.
Moderna submitted data to the FDA on Sept. 1 in support of adding a booster dose to its regimen. The agency has not yet scheduled a public review of that data.
The Biden administration is prepared to administer shots as soon as they get the green light, Surgeon General Vivek Murthy, MD, said at a White House briefing earlier Sept. 17.
"This process is consistent with what we outlined in August where our goals were to stay ahead of the virus," Dr. Murthy said. "Our goal then and now is to protect the health and well-being of the public. As soon as the FDA and CDC complete their evaluations, we will be ready to move forward accordingly."
He added, "We've used this time since our August announcement to communicate and coordinate with pharmacy partners, nursing homes, states, and localities."
White House COVID-19 Response Coordinator Jeff Zients said vaccine supply is "in good shape for all Americans to get boosters as recommended."
Taking cues from Israel
In considering Pfizer’s original request, the committee overwhelmingly felt that they didn’t have enough information to say that the benefits of an additional dose of vaccine in 16- and 17-year-olds would outweigh its risk. Teens have the highest risk of rare heart inflammation after vaccination, a side effect known as myocarditis. It is not known how the vaccines are causing these cases of heart swelling. Most who have been diagnosed with the condition have recovered, though some have needed hospital care.
Pfizer didn’t include 16- and 17-year-olds in its studies of boosters, which included about 300 people between the ages of 18 and 55. The company acknowledged that gap in its data but pointed to FDA guidance that said evidence from adults could be extrapolated to teens.
“We don’t know that much about risks,” said committee member Eric Rubin, MD, who is editor-in-chief of the New England Journal of Medicine.
Much of the data on the potential benefits and harms of third Pfizer doses comes from Israel, which first began rolling out boosters to older adults in July.
In a highly anticipated presentation, Sharon Alroy-Preis, Israel’s director of public health services, joined the meeting to describe Israel’s experience with boosters.
Israel began to see a third surge of COVID-19 cases in December.
“This was after having two waves and two lockdowns,” Ms. Alroy-Preis said. By the third surge, she said, Israelis were tired.
“We decided on a lockdown, but the compliance of the public wasn’t as it was in the previous two waves,” she said.
Then the vaccine arrived. Israel started vaccinations as soon as the FDA approved it, and they quickly vaccinated a high percentage of their population, about 3 months faster than the rest of the world.
All vaccinations are reported and tracked by the Ministry of Health, so the country is able to keep close tabs on how well the shots are working.
As vaccines rolled out, cases fell dramatically. The pandemic seemed to be behind them. Delta arrived in March. By June, their cases were doubling every 10 days, despite about 80% of their most vulnerable adults being fully vaccinated, she said.
Most concerning was that about 60% of severe cases were breakthrough cases in fully vaccinated individuals.
“We had to stop and figure out, was this a Delta issue,” she said. “Or was this a waning immunity issue.”
“We had some clue that it might not be the Delta variant, at least not alone,” she said.
People who had originally been first in line for the vaccines, seniors and health care workers, were having the highest rates of breakthrough infections. People further away from their second dose were more likely to get a breakthrough infection.
Ms. Alroy-Preis said that if they had not started booster doses in July, their hospitals would have been overwhelmed. They had projected that they would have 2,000 cases in the hospital each day.
Boosters have helped to flatten the curve, though they are still seeing a significant number of infections.
Data from Israel presented at the meeting show boosters are largely safe and effective at reducing severe outcomes in seniors. Israeli experience also showed that third doses, which generate very high levels of neutralizing antibodies—the first and fastest line of the body’s immune defense - -may also slow transmission of the virus.
Key differences in the U.S.
The benefit of slowing down the explosive spread of a highly contagious virus was tantalizing, but many members noted that circumstances in Israel are very different than in the United States. Israel went into its current Delta surge already having high levels of vaccination in its population. They also relied on the Pfizer vaccine almost exclusively for their campaign.
The United States used a different mix of vaccines – Pfizer, Moderna, and Johnson & Johnson -- and doesn’t have the same high level of vaccination coverage of its population.
In the United States, transmission is mainly being driven by unvaccinated people, Dr. Rubin noted.
“That really means the primary benefit is going to be in reducing disease,” he said, “And we know the people who are going to benefit from that … and those are the kinds of people the FDA has already approved a third dose for,” he said, referring to those with underlying health conditions.
But Israel only began vaccinating younger people a few weeks ago. Most are still within a window where rare risks like myocarditis could appear, Rubin noted.
He and other members of the committee said they wished they had more information about the safety of third doses in younger adults.
“We don’t have that right now, and I don’t think I would be comfortable giving it to a 16-year-old,” he said.
At the same time, the primary benefit for third doses would be in preventing severe disease, and overall, data from the United States and other countries show that two doses of the vaccines remain highly effective at preventing hospitalization and death.
Asked why Israel began to see more severe cases in fully vaccinated people, the CDC’s Sara Oliver, MD, a disease detective with the CDC, said it was probably due to a mix of factors including the fact that Israel defines severe cases a little differently.
In the United States, a severe case is generally a person who has to be hospitalized or who has died from the infection. In Israel, a person with a severe case is someone who has an elevated respiratory rate and someone who has a blood oxygen level less than 94%. In the United States, that kind of patient wouldn’t necessarily be hospitalized.
In the end, one of the two committee members who wanted full approval for Pfizer’s third doses said he was satisfied with the outcome.
Mark Sawyer, MD, a professor of pediatrics and infectious disease at the University of California at San Diego, said he voted yes on the first question because he thought full approval was the best way to give doctors the flexibility to prescribe the shots to vulnerable individuals.
“I’m really glad we authorized a vaccine for a third dose, and I plan to go out and get my vaccine this afternoon,” Dr. Sawyer said, noting that he was at high risk as a health care provider.
This article was updated 9/19/21.
A version of this article first appeared on Medscape.com.
Baylor gets restraining order against COVID-19 vaccine–skeptic doc
in which he agreed to stop mentioning his prior leadership and academic appointments.
Baylor was the first institution to cut ties with Dr. McCullough, who has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines. Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed Dr. McCullough from their faculties.
Granted by the 191st District Court in Dallas County, Tex., the Baylor restraining order – which is in effect at least until a hearing on the case on September 30 – was sought as part of Baylor Scott & White’s breach of contract suit against McCullough, who had previously been known as a well-respected expert in cardiorenal issues. The suit is seeking $1 million in damages, as well as attorneys’ fees.
The suit seeks to “enforce the terms” of the confidential employment separation agreement signed by Dr. McCullough in February and prevent Dr. McCullough from continuing “improper use of titles and claimed affiliations that have already confused the media, the medical community and the public,” it reads.
“This ongoing confusion regarding [Dr.] McCullough’s affiliations, and whether Plaintiffs support his opinions, is exactly what Plaintiffs bargained to avoid in the Separation Agreement,” and is likely to cause “irreparable reputational and business harm that is incapable of remedy by money damages alone,” the suit states.
One of Dr. McCullough’s attorneys, Clinton Mikel, maintains that all the times the physician was identified in the “thousands of hours of media interviews and countless publications since his departure from Baylor” were “said/printed by a third party with no encouragement from Dr. McCullough,” and that the doctor “does not and cannot control third parties.”
Mr. Mikel said in a statement emailed to this news organization by Dr. McCullough that the suit is “a politically motivated attempt to silence Dr. McCullough,” because it was filed on the same day the organization mandated COVID-19 vaccination for employees.
Dr. McCullough “intends to vigorously defend against Baylor’s unfounded lawsuit,” will seek to dissolve the restraining order, and recover “all payments due him from Baylor under the terms of the settlement agreement,” wrote Mr. Mikel.
The cardiologist’s legal team filed a motion to dismiss the suit on Aug. 9, essentially arguing that Baylor Scott & White’s action restricted Dr. McCullough’s right to free speech under the Texas Citizen’s Participation Act.
COVID-19 vaccines = bioterrorism?
Dr. McCullough accumulated a following in 2020 by promoting early at-home multidrug treatment of COVID-19 in interviews with conservative websites and at a U.S. Senate hearing in November.
Although Dr. McCullough does not appear to have any personal social media accounts, his broadcast and podcast interviews are tweeted by thousands daily around the world and featured on Facebook pages like “Pandemic Debate.”
Some Facebook posts with Dr. McCullough’s pronouncements have been labeled as misinformation or removed. Some of his videos remain on YouTube, where they are posted by the Association of American Physicians and Surgeons, a group that believes Dr. McCullough is “under fierce attack for speaking out about COVID-19 early treatment and vaccine safety.”
Dr. McCullough’s March 2021 testimony to the Texas Senate’s Health and Human Services Committee – in which he claimed that COVID-19 patients are being denied what he called proven treatments like hydroxychloroquine – has been viewed more than 3.7 million times on YouTube. The appearance has also been tweeted repeatedly.
Most of Dr. McCullough’s interviews and presentations are aggregated on Rumble, an alternative to YouTube.
In interviews, Dr. McCullough promotes the use of zinc, hydroxychloroquine, azithromycin, doxycycline, favipiravir, prednisone, and ivermectin as COVID-19 treatments – based on an outpatient treatment algorithm published in August 2020 in the American Journal of Medicine. The cardiologist was the lead author of that paper, which proposed treating people with COVID-like symptoms whether or not they had confirmed infection.
Dr. McCullough and colleagues published a follow-up paper that added colchicine to the mix in Reviews in Cardiovascular Medicine. Dr. McCullough is editor-in-chief of the journal, but this was not noted in the disclosures.
Similarly, Dr. McCullough has not disclosed in his COVID-19 publications or any interviews that he has received consulting fees from a host of pharmaceutical manufacturers that produce COVID-19 drugs and vaccines, including AstraZeneca, Eli Lilly, and Regeneron Pharmaceuticals. According to the Centers for Medicare & Medicaid Services’ Open Payments database, Dr. McCullough was paid about $300,000 annually by drug companies from 2014 to 2019, mostly for consulting on cardiovascular and diabetes medications. His payments dropped to $169,406.06 in 2020.
Dr. McCullough appeared on “The Ingraham Angle” on Fox News in December 2020, claiming that sequential, early treatment with “anti-infectives, corticosteroids, and then antithrombotics” could “reduce [COVID-19] hospitalizations by 85% and cut mortality in half.”
He repeated the claim on the Ingraham show in July and agreed with host Laura Ingraham that the vast majority of healthy people would do fine if they got COVID. He also made the claim that 84% of the COVID-19 cases in Israel were in people who had been vaccinated. “So it’s clear, we can’t vaccinate our way out of this,” he said. An Associated Press “fact check” report has pushed back on similar assertions about vaccine data from Israel.
In a separate interview posted in June, Dr. McCullough called the pandemic the first phase of a bioterrorism event, which was “all about keeping the population in fear and in isolation and preparing them to accept the vaccine, which appears to be phase two of a bioterrorism operation.”
In addition, he said, “good doctors are doing unthinkable things like injecting biologically active messenger RNA that produces this pathogenic spike protein into pregnant women.”
According to the Centers for Disease Control and Prevention, the vaccines teach the body to produce the spike protein, which then triggers an immune response that creates antibodies that will attack the virus.
A PolitiFact review debunks the notion that the mRNA vaccines are toxic, cytotoxic, or introduce live, active virus proteins into the body.
FactCheck.org also disputed Dr. McCullough’s claim in a July 13 Ingraham Angle appearance that the mRNA vaccines are ineffective against the Delta variant.
In the FactCheck article, Frederic Bushman, codirector of the University of Pennsylvania’s Center for Research on Coronaviruses and Other Emerging Pathogens, said that people were much better off being vaccinated than not,” adding, “the Delta variant may reduce the effectiveness [of the vaccines] a little, but still, they’re so effective that you get a lot of benefit.”
“The vaccines are failing,” Dr. McCullough asserted in an Aug. 3 video interview posted on Odysee. “As we sit here today, we have 11,000 Americans that the CDC has certified have died after the vaccine,” he said, citing two analyses – one by Jessica Rose, PhD, and another by British researchers.
Similar figures reportedly based on cases reported to the Food and Drug Administration’s Vaccine Adverse Events Reporting System (VAERS) were forwarded to this news organization by Dr. McCullough.
The CDC website notes that the agency has received reports of 7,653 deaths in people who received a vaccine as of Sept. 13 (0.0020% of vaccine doses given since Dec. 14, 2020), but it cautions that those deaths do not mean the vaccine was the cause.
Dr. McCullough repeatedly claimed in the Aug. 3 interview that the government has not been transparent on vaccine safety. Since June 2020, the CDC’s Advisory Committee on Immunization Practices has held 16 public meetings on the COVID-19 vaccines.
To date, the agency has advised clinicians to monitor for rare side effects including Guillain-Barré syndrome and thrombosis with thrombocytopenia syndrome after the Johnson & Johnson vaccine and myocarditis after mRNA (Pfizer-BioNTech and Moderna) vaccines.
Med schools distance themselves
According to the Baylor Scott & White suit, Dr. McCullough agreed on Feb. 24 in a confidential separation agreement that he would no longer use his academic or leadership titles nor hold himself out to be affiliated with Baylor University Medical Center, Baylor Heart and Vascular Institute, the Baylor Research Institute, or any other related institutions.
However, as of August, according to a Baylor spokesperson, McCullough continued to have privileges at Baylor University Medical Center and Baylor Scott & White Heart and Vascular Hospital, Dallas.
The lawsuit points to three interviews posted in June and July where Dr. McCullough is identified as a “vice chief of medicine” or a “vice chief of internal medicine,” both at Baylor University. It also cites a profile at the Cardiometabolic Health Congress website – which this news organization had also viewed – that was still active in late July with a similar title. The profile was later scrubbed from the site.
Social media posts and other media continue to refer to Dr. McCullough’s Baylor credentials. An episode of the Faith and Freedom podcast posted on Aug. 2 identified McCullough as a “professor of medicine at Baylor University Medical Center.”
As of Sept. 16, Dr. McCullough’s bio page at his current practice, Heart Place, lists him as a professor of medicine at Texas A&M College of Medicine. A spokesperson for Texas A&M told this news organization that McCullough is no longer affiliated with the school.
Dr. McCullough acknowledged in the Aug. 3 interview that his Texas A&M title had been “stripped away” at “around the same time this lawsuit was filed.”
He was still a professor of medicine at the TCU and UNTHSC School of Medicine in Fort Worth, but a school spokesperson notified this news organization on Aug. 19 that Dr. McCullough was no longer with the school.
Dr. McCullough has portrayed himself as both a victim and a truth-teller, a “concerned physician” warning the world about the dangers of COVID-19 vaccines. The Baylor Scott & White lawsuit “is really a strong-armed tactic,” he said in the Aug. 3 interview. “I’m just a little guy, so I have to hire my legal teams, and in a sense be drained dry on legal fees,” he said.
But Dr. McCullough apparently has a plan for helping to defray his legal costs. In the Aug. 3 interview, he said a foundation he helped start, Truth for Health, has a “donation side to it,” adding “some of that may be used for legal expense.”
Cheryl Jones, an attorney with PK Law in Towson, Md., said that might draw interest from the Internal Revenue Service. “I would expect IRS scrutiny if contributions to the Medical Censorship Defense Fund are used to defend Dr McCullough in his personal breach of contract lawsuit,” she told this news organization.
The IRS generally recognizes defending “human and civil rights secured by law” as a legitimate charitable purpose for a legal defense fund, she said, adding that such a fund “must serve only public, rather than private, interests.”
Misinformation from a physician more damaging?
Some in the medical field have refuted Dr. McCullough’s pronouncements on how to treat COVID-19, including two infectious disease specialists with Monash University, Melbourne, who responded to the cardiologist’s original paper in the American Journal of Medicine.
Tony Korman, MBBS, a professor at the Centre for Inflammatory Diseases at Monash, told this news organization, “we had concerns that reputable medical journals would accept and publish papers proposing treatment of COVID-19 which was not supported by evidence.”
The website Healthfeedback.org has also challenged McCullough’s and his supporters’ claims, including that the American Journal of Medicine endorsed the use of hydroxychloroquine and that the COVID-19 vaccines have caused thousands of deaths.
David Broniatowski, PhD, associate director for the Institute for Data, Democracy and Politics at George Washington University, Washington, said in an interview that Dr. McCullough’s casting himself as a “rebel doctor” is a well-known trope in the vaccine misinformation universe.
Although he was not familiar with Dr. McCullough, Dr. Broniatowski said the cardiologist’s claims are not unique – they’ve been circulating among antivaccine and conspiracy-oriented groups for months.
For instance, Dr. McCullough has claimed in interviews that a whistleblower within the CDC knows of 50,000 vaccine-related deaths. Using data from the supposed whistleblower, the group America’s Frontline Doctors sued the federal government in July to stop the administration of COVID-19 vaccines to those under 18, people who have already had COVID, and individuals who the group said have not been adequately informed about the risks.
The idea of a whistleblower inside the CDC is recycled from antivaccine claims from decades ago, Dr. Broniatowski said.
But, he added, “somebody who speaks with the credibility of a major institution will be more likely to be listened to by some people.” That vulnerable group is “being taken advantage of by a relatively small number of disinformation purveyors, who, in some cases, profit from that disinformation,” said Dr. Broniatowski.
“We rely on our doctors because we trust them,” he said. “And we trust them because we believe that as physicians, their value system places the patient’s best interests first. That’s why it’s so much of a disappointment when you have a physician that appears to be exercising this sort of bad judgment.”
Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, also said that he was not familiar with Dr. McCullough. But apprised of his claims, Dr. Offit told this news organization, “Peter McCullough is a friend of the virus.”
“The kind of information he promotes allows the virus to continue to spread, continue to do an enormous amount of harm, and continue to mutate and create variants that have become more contagious and more resistant to vaccine-induced immunity,” said Dr. Offit, the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia.
Dr. Offit added that the war should be against SARS-CoV-2, but “because this virus has so many supporters, the war in essence becomes a war against ourselves, which is much harder.”
Dr. McCullough maintains he is doing a service to his patients. “I’m just giving and trying to help America understand the pandemic,” he told Ms. Ingraham on Fox News on July 29.
But he acknowledged concern about the Federation of State Medical Board’s announcement that physicians who spread COVID-19 vaccine misinformation risk suspension or revocation of their license.
“I have to tell you I’m worried – that no matter what I do and how careful I am to cite the scientific studies, I’m still gonna be hunted down for quote, misinformation,” he said in the Aug. 3 interview.
A version of this article first appeared on Medscape.com.
in which he agreed to stop mentioning his prior leadership and academic appointments.
Baylor was the first institution to cut ties with Dr. McCullough, who has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines. Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed Dr. McCullough from their faculties.
Granted by the 191st District Court in Dallas County, Tex., the Baylor restraining order – which is in effect at least until a hearing on the case on September 30 – was sought as part of Baylor Scott & White’s breach of contract suit against McCullough, who had previously been known as a well-respected expert in cardiorenal issues. The suit is seeking $1 million in damages, as well as attorneys’ fees.
The suit seeks to “enforce the terms” of the confidential employment separation agreement signed by Dr. McCullough in February and prevent Dr. McCullough from continuing “improper use of titles and claimed affiliations that have already confused the media, the medical community and the public,” it reads.
“This ongoing confusion regarding [Dr.] McCullough’s affiliations, and whether Plaintiffs support his opinions, is exactly what Plaintiffs bargained to avoid in the Separation Agreement,” and is likely to cause “irreparable reputational and business harm that is incapable of remedy by money damages alone,” the suit states.
One of Dr. McCullough’s attorneys, Clinton Mikel, maintains that all the times the physician was identified in the “thousands of hours of media interviews and countless publications since his departure from Baylor” were “said/printed by a third party with no encouragement from Dr. McCullough,” and that the doctor “does not and cannot control third parties.”
Mr. Mikel said in a statement emailed to this news organization by Dr. McCullough that the suit is “a politically motivated attempt to silence Dr. McCullough,” because it was filed on the same day the organization mandated COVID-19 vaccination for employees.
Dr. McCullough “intends to vigorously defend against Baylor’s unfounded lawsuit,” will seek to dissolve the restraining order, and recover “all payments due him from Baylor under the terms of the settlement agreement,” wrote Mr. Mikel.
The cardiologist’s legal team filed a motion to dismiss the suit on Aug. 9, essentially arguing that Baylor Scott & White’s action restricted Dr. McCullough’s right to free speech under the Texas Citizen’s Participation Act.
COVID-19 vaccines = bioterrorism?
Dr. McCullough accumulated a following in 2020 by promoting early at-home multidrug treatment of COVID-19 in interviews with conservative websites and at a U.S. Senate hearing in November.
Although Dr. McCullough does not appear to have any personal social media accounts, his broadcast and podcast interviews are tweeted by thousands daily around the world and featured on Facebook pages like “Pandemic Debate.”
Some Facebook posts with Dr. McCullough’s pronouncements have been labeled as misinformation or removed. Some of his videos remain on YouTube, where they are posted by the Association of American Physicians and Surgeons, a group that believes Dr. McCullough is “under fierce attack for speaking out about COVID-19 early treatment and vaccine safety.”
Dr. McCullough’s March 2021 testimony to the Texas Senate’s Health and Human Services Committee – in which he claimed that COVID-19 patients are being denied what he called proven treatments like hydroxychloroquine – has been viewed more than 3.7 million times on YouTube. The appearance has also been tweeted repeatedly.
Most of Dr. McCullough’s interviews and presentations are aggregated on Rumble, an alternative to YouTube.
In interviews, Dr. McCullough promotes the use of zinc, hydroxychloroquine, azithromycin, doxycycline, favipiravir, prednisone, and ivermectin as COVID-19 treatments – based on an outpatient treatment algorithm published in August 2020 in the American Journal of Medicine. The cardiologist was the lead author of that paper, which proposed treating people with COVID-like symptoms whether or not they had confirmed infection.
Dr. McCullough and colleagues published a follow-up paper that added colchicine to the mix in Reviews in Cardiovascular Medicine. Dr. McCullough is editor-in-chief of the journal, but this was not noted in the disclosures.
Similarly, Dr. McCullough has not disclosed in his COVID-19 publications or any interviews that he has received consulting fees from a host of pharmaceutical manufacturers that produce COVID-19 drugs and vaccines, including AstraZeneca, Eli Lilly, and Regeneron Pharmaceuticals. According to the Centers for Medicare & Medicaid Services’ Open Payments database, Dr. McCullough was paid about $300,000 annually by drug companies from 2014 to 2019, mostly for consulting on cardiovascular and diabetes medications. His payments dropped to $169,406.06 in 2020.
Dr. McCullough appeared on “The Ingraham Angle” on Fox News in December 2020, claiming that sequential, early treatment with “anti-infectives, corticosteroids, and then antithrombotics” could “reduce [COVID-19] hospitalizations by 85% and cut mortality in half.”
He repeated the claim on the Ingraham show in July and agreed with host Laura Ingraham that the vast majority of healthy people would do fine if they got COVID. He also made the claim that 84% of the COVID-19 cases in Israel were in people who had been vaccinated. “So it’s clear, we can’t vaccinate our way out of this,” he said. An Associated Press “fact check” report has pushed back on similar assertions about vaccine data from Israel.
In a separate interview posted in June, Dr. McCullough called the pandemic the first phase of a bioterrorism event, which was “all about keeping the population in fear and in isolation and preparing them to accept the vaccine, which appears to be phase two of a bioterrorism operation.”
In addition, he said, “good doctors are doing unthinkable things like injecting biologically active messenger RNA that produces this pathogenic spike protein into pregnant women.”
According to the Centers for Disease Control and Prevention, the vaccines teach the body to produce the spike protein, which then triggers an immune response that creates antibodies that will attack the virus.
A PolitiFact review debunks the notion that the mRNA vaccines are toxic, cytotoxic, or introduce live, active virus proteins into the body.
FactCheck.org also disputed Dr. McCullough’s claim in a July 13 Ingraham Angle appearance that the mRNA vaccines are ineffective against the Delta variant.
In the FactCheck article, Frederic Bushman, codirector of the University of Pennsylvania’s Center for Research on Coronaviruses and Other Emerging Pathogens, said that people were much better off being vaccinated than not,” adding, “the Delta variant may reduce the effectiveness [of the vaccines] a little, but still, they’re so effective that you get a lot of benefit.”
“The vaccines are failing,” Dr. McCullough asserted in an Aug. 3 video interview posted on Odysee. “As we sit here today, we have 11,000 Americans that the CDC has certified have died after the vaccine,” he said, citing two analyses – one by Jessica Rose, PhD, and another by British researchers.
Similar figures reportedly based on cases reported to the Food and Drug Administration’s Vaccine Adverse Events Reporting System (VAERS) were forwarded to this news organization by Dr. McCullough.
The CDC website notes that the agency has received reports of 7,653 deaths in people who received a vaccine as of Sept. 13 (0.0020% of vaccine doses given since Dec. 14, 2020), but it cautions that those deaths do not mean the vaccine was the cause.
Dr. McCullough repeatedly claimed in the Aug. 3 interview that the government has not been transparent on vaccine safety. Since June 2020, the CDC’s Advisory Committee on Immunization Practices has held 16 public meetings on the COVID-19 vaccines.
To date, the agency has advised clinicians to monitor for rare side effects including Guillain-Barré syndrome and thrombosis with thrombocytopenia syndrome after the Johnson & Johnson vaccine and myocarditis after mRNA (Pfizer-BioNTech and Moderna) vaccines.
Med schools distance themselves
According to the Baylor Scott & White suit, Dr. McCullough agreed on Feb. 24 in a confidential separation agreement that he would no longer use his academic or leadership titles nor hold himself out to be affiliated with Baylor University Medical Center, Baylor Heart and Vascular Institute, the Baylor Research Institute, or any other related institutions.
However, as of August, according to a Baylor spokesperson, McCullough continued to have privileges at Baylor University Medical Center and Baylor Scott & White Heart and Vascular Hospital, Dallas.
The lawsuit points to three interviews posted in June and July where Dr. McCullough is identified as a “vice chief of medicine” or a “vice chief of internal medicine,” both at Baylor University. It also cites a profile at the Cardiometabolic Health Congress website – which this news organization had also viewed – that was still active in late July with a similar title. The profile was later scrubbed from the site.
Social media posts and other media continue to refer to Dr. McCullough’s Baylor credentials. An episode of the Faith and Freedom podcast posted on Aug. 2 identified McCullough as a “professor of medicine at Baylor University Medical Center.”
As of Sept. 16, Dr. McCullough’s bio page at his current practice, Heart Place, lists him as a professor of medicine at Texas A&M College of Medicine. A spokesperson for Texas A&M told this news organization that McCullough is no longer affiliated with the school.
Dr. McCullough acknowledged in the Aug. 3 interview that his Texas A&M title had been “stripped away” at “around the same time this lawsuit was filed.”
He was still a professor of medicine at the TCU and UNTHSC School of Medicine in Fort Worth, but a school spokesperson notified this news organization on Aug. 19 that Dr. McCullough was no longer with the school.
Dr. McCullough has portrayed himself as both a victim and a truth-teller, a “concerned physician” warning the world about the dangers of COVID-19 vaccines. The Baylor Scott & White lawsuit “is really a strong-armed tactic,” he said in the Aug. 3 interview. “I’m just a little guy, so I have to hire my legal teams, and in a sense be drained dry on legal fees,” he said.
But Dr. McCullough apparently has a plan for helping to defray his legal costs. In the Aug. 3 interview, he said a foundation he helped start, Truth for Health, has a “donation side to it,” adding “some of that may be used for legal expense.”
Cheryl Jones, an attorney with PK Law in Towson, Md., said that might draw interest from the Internal Revenue Service. “I would expect IRS scrutiny if contributions to the Medical Censorship Defense Fund are used to defend Dr McCullough in his personal breach of contract lawsuit,” she told this news organization.
The IRS generally recognizes defending “human and civil rights secured by law” as a legitimate charitable purpose for a legal defense fund, she said, adding that such a fund “must serve only public, rather than private, interests.”
Misinformation from a physician more damaging?
Some in the medical field have refuted Dr. McCullough’s pronouncements on how to treat COVID-19, including two infectious disease specialists with Monash University, Melbourne, who responded to the cardiologist’s original paper in the American Journal of Medicine.
Tony Korman, MBBS, a professor at the Centre for Inflammatory Diseases at Monash, told this news organization, “we had concerns that reputable medical journals would accept and publish papers proposing treatment of COVID-19 which was not supported by evidence.”
The website Healthfeedback.org has also challenged McCullough’s and his supporters’ claims, including that the American Journal of Medicine endorsed the use of hydroxychloroquine and that the COVID-19 vaccines have caused thousands of deaths.
David Broniatowski, PhD, associate director for the Institute for Data, Democracy and Politics at George Washington University, Washington, said in an interview that Dr. McCullough’s casting himself as a “rebel doctor” is a well-known trope in the vaccine misinformation universe.
Although he was not familiar with Dr. McCullough, Dr. Broniatowski said the cardiologist’s claims are not unique – they’ve been circulating among antivaccine and conspiracy-oriented groups for months.
For instance, Dr. McCullough has claimed in interviews that a whistleblower within the CDC knows of 50,000 vaccine-related deaths. Using data from the supposed whistleblower, the group America’s Frontline Doctors sued the federal government in July to stop the administration of COVID-19 vaccines to those under 18, people who have already had COVID, and individuals who the group said have not been adequately informed about the risks.
The idea of a whistleblower inside the CDC is recycled from antivaccine claims from decades ago, Dr. Broniatowski said.
But, he added, “somebody who speaks with the credibility of a major institution will be more likely to be listened to by some people.” That vulnerable group is “being taken advantage of by a relatively small number of disinformation purveyors, who, in some cases, profit from that disinformation,” said Dr. Broniatowski.
“We rely on our doctors because we trust them,” he said. “And we trust them because we believe that as physicians, their value system places the patient’s best interests first. That’s why it’s so much of a disappointment when you have a physician that appears to be exercising this sort of bad judgment.”
Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, also said that he was not familiar with Dr. McCullough. But apprised of his claims, Dr. Offit told this news organization, “Peter McCullough is a friend of the virus.”
“The kind of information he promotes allows the virus to continue to spread, continue to do an enormous amount of harm, and continue to mutate and create variants that have become more contagious and more resistant to vaccine-induced immunity,” said Dr. Offit, the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia.
Dr. Offit added that the war should be against SARS-CoV-2, but “because this virus has so many supporters, the war in essence becomes a war against ourselves, which is much harder.”
Dr. McCullough maintains he is doing a service to his patients. “I’m just giving and trying to help America understand the pandemic,” he told Ms. Ingraham on Fox News on July 29.
But he acknowledged concern about the Federation of State Medical Board’s announcement that physicians who spread COVID-19 vaccine misinformation risk suspension or revocation of their license.
“I have to tell you I’m worried – that no matter what I do and how careful I am to cite the scientific studies, I’m still gonna be hunted down for quote, misinformation,” he said in the Aug. 3 interview.
A version of this article first appeared on Medscape.com.
in which he agreed to stop mentioning his prior leadership and academic appointments.
Baylor was the first institution to cut ties with Dr. McCullough, who has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines. Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed Dr. McCullough from their faculties.
Granted by the 191st District Court in Dallas County, Tex., the Baylor restraining order – which is in effect at least until a hearing on the case on September 30 – was sought as part of Baylor Scott & White’s breach of contract suit against McCullough, who had previously been known as a well-respected expert in cardiorenal issues. The suit is seeking $1 million in damages, as well as attorneys’ fees.
The suit seeks to “enforce the terms” of the confidential employment separation agreement signed by Dr. McCullough in February and prevent Dr. McCullough from continuing “improper use of titles and claimed affiliations that have already confused the media, the medical community and the public,” it reads.
“This ongoing confusion regarding [Dr.] McCullough’s affiliations, and whether Plaintiffs support his opinions, is exactly what Plaintiffs bargained to avoid in the Separation Agreement,” and is likely to cause “irreparable reputational and business harm that is incapable of remedy by money damages alone,” the suit states.
One of Dr. McCullough’s attorneys, Clinton Mikel, maintains that all the times the physician was identified in the “thousands of hours of media interviews and countless publications since his departure from Baylor” were “said/printed by a third party with no encouragement from Dr. McCullough,” and that the doctor “does not and cannot control third parties.”
Mr. Mikel said in a statement emailed to this news organization by Dr. McCullough that the suit is “a politically motivated attempt to silence Dr. McCullough,” because it was filed on the same day the organization mandated COVID-19 vaccination for employees.
Dr. McCullough “intends to vigorously defend against Baylor’s unfounded lawsuit,” will seek to dissolve the restraining order, and recover “all payments due him from Baylor under the terms of the settlement agreement,” wrote Mr. Mikel.
The cardiologist’s legal team filed a motion to dismiss the suit on Aug. 9, essentially arguing that Baylor Scott & White’s action restricted Dr. McCullough’s right to free speech under the Texas Citizen’s Participation Act.
COVID-19 vaccines = bioterrorism?
Dr. McCullough accumulated a following in 2020 by promoting early at-home multidrug treatment of COVID-19 in interviews with conservative websites and at a U.S. Senate hearing in November.
Although Dr. McCullough does not appear to have any personal social media accounts, his broadcast and podcast interviews are tweeted by thousands daily around the world and featured on Facebook pages like “Pandemic Debate.”
Some Facebook posts with Dr. McCullough’s pronouncements have been labeled as misinformation or removed. Some of his videos remain on YouTube, where they are posted by the Association of American Physicians and Surgeons, a group that believes Dr. McCullough is “under fierce attack for speaking out about COVID-19 early treatment and vaccine safety.”
Dr. McCullough’s March 2021 testimony to the Texas Senate’s Health and Human Services Committee – in which he claimed that COVID-19 patients are being denied what he called proven treatments like hydroxychloroquine – has been viewed more than 3.7 million times on YouTube. The appearance has also been tweeted repeatedly.
Most of Dr. McCullough’s interviews and presentations are aggregated on Rumble, an alternative to YouTube.
In interviews, Dr. McCullough promotes the use of zinc, hydroxychloroquine, azithromycin, doxycycline, favipiravir, prednisone, and ivermectin as COVID-19 treatments – based on an outpatient treatment algorithm published in August 2020 in the American Journal of Medicine. The cardiologist was the lead author of that paper, which proposed treating people with COVID-like symptoms whether or not they had confirmed infection.
Dr. McCullough and colleagues published a follow-up paper that added colchicine to the mix in Reviews in Cardiovascular Medicine. Dr. McCullough is editor-in-chief of the journal, but this was not noted in the disclosures.
Similarly, Dr. McCullough has not disclosed in his COVID-19 publications or any interviews that he has received consulting fees from a host of pharmaceutical manufacturers that produce COVID-19 drugs and vaccines, including AstraZeneca, Eli Lilly, and Regeneron Pharmaceuticals. According to the Centers for Medicare & Medicaid Services’ Open Payments database, Dr. McCullough was paid about $300,000 annually by drug companies from 2014 to 2019, mostly for consulting on cardiovascular and diabetes medications. His payments dropped to $169,406.06 in 2020.
Dr. McCullough appeared on “The Ingraham Angle” on Fox News in December 2020, claiming that sequential, early treatment with “anti-infectives, corticosteroids, and then antithrombotics” could “reduce [COVID-19] hospitalizations by 85% and cut mortality in half.”
He repeated the claim on the Ingraham show in July and agreed with host Laura Ingraham that the vast majority of healthy people would do fine if they got COVID. He also made the claim that 84% of the COVID-19 cases in Israel were in people who had been vaccinated. “So it’s clear, we can’t vaccinate our way out of this,” he said. An Associated Press “fact check” report has pushed back on similar assertions about vaccine data from Israel.
In a separate interview posted in June, Dr. McCullough called the pandemic the first phase of a bioterrorism event, which was “all about keeping the population in fear and in isolation and preparing them to accept the vaccine, which appears to be phase two of a bioterrorism operation.”
In addition, he said, “good doctors are doing unthinkable things like injecting biologically active messenger RNA that produces this pathogenic spike protein into pregnant women.”
According to the Centers for Disease Control and Prevention, the vaccines teach the body to produce the spike protein, which then triggers an immune response that creates antibodies that will attack the virus.
A PolitiFact review debunks the notion that the mRNA vaccines are toxic, cytotoxic, or introduce live, active virus proteins into the body.
FactCheck.org also disputed Dr. McCullough’s claim in a July 13 Ingraham Angle appearance that the mRNA vaccines are ineffective against the Delta variant.
In the FactCheck article, Frederic Bushman, codirector of the University of Pennsylvania’s Center for Research on Coronaviruses and Other Emerging Pathogens, said that people were much better off being vaccinated than not,” adding, “the Delta variant may reduce the effectiveness [of the vaccines] a little, but still, they’re so effective that you get a lot of benefit.”
“The vaccines are failing,” Dr. McCullough asserted in an Aug. 3 video interview posted on Odysee. “As we sit here today, we have 11,000 Americans that the CDC has certified have died after the vaccine,” he said, citing two analyses – one by Jessica Rose, PhD, and another by British researchers.
Similar figures reportedly based on cases reported to the Food and Drug Administration’s Vaccine Adverse Events Reporting System (VAERS) were forwarded to this news organization by Dr. McCullough.
The CDC website notes that the agency has received reports of 7,653 deaths in people who received a vaccine as of Sept. 13 (0.0020% of vaccine doses given since Dec. 14, 2020), but it cautions that those deaths do not mean the vaccine was the cause.
Dr. McCullough repeatedly claimed in the Aug. 3 interview that the government has not been transparent on vaccine safety. Since June 2020, the CDC’s Advisory Committee on Immunization Practices has held 16 public meetings on the COVID-19 vaccines.
To date, the agency has advised clinicians to monitor for rare side effects including Guillain-Barré syndrome and thrombosis with thrombocytopenia syndrome after the Johnson & Johnson vaccine and myocarditis after mRNA (Pfizer-BioNTech and Moderna) vaccines.
Med schools distance themselves
According to the Baylor Scott & White suit, Dr. McCullough agreed on Feb. 24 in a confidential separation agreement that he would no longer use his academic or leadership titles nor hold himself out to be affiliated with Baylor University Medical Center, Baylor Heart and Vascular Institute, the Baylor Research Institute, or any other related institutions.
However, as of August, according to a Baylor spokesperson, McCullough continued to have privileges at Baylor University Medical Center and Baylor Scott & White Heart and Vascular Hospital, Dallas.
The lawsuit points to three interviews posted in June and July where Dr. McCullough is identified as a “vice chief of medicine” or a “vice chief of internal medicine,” both at Baylor University. It also cites a profile at the Cardiometabolic Health Congress website – which this news organization had also viewed – that was still active in late July with a similar title. The profile was later scrubbed from the site.
Social media posts and other media continue to refer to Dr. McCullough’s Baylor credentials. An episode of the Faith and Freedom podcast posted on Aug. 2 identified McCullough as a “professor of medicine at Baylor University Medical Center.”
As of Sept. 16, Dr. McCullough’s bio page at his current practice, Heart Place, lists him as a professor of medicine at Texas A&M College of Medicine. A spokesperson for Texas A&M told this news organization that McCullough is no longer affiliated with the school.
Dr. McCullough acknowledged in the Aug. 3 interview that his Texas A&M title had been “stripped away” at “around the same time this lawsuit was filed.”
He was still a professor of medicine at the TCU and UNTHSC School of Medicine in Fort Worth, but a school spokesperson notified this news organization on Aug. 19 that Dr. McCullough was no longer with the school.
Dr. McCullough has portrayed himself as both a victim and a truth-teller, a “concerned physician” warning the world about the dangers of COVID-19 vaccines. The Baylor Scott & White lawsuit “is really a strong-armed tactic,” he said in the Aug. 3 interview. “I’m just a little guy, so I have to hire my legal teams, and in a sense be drained dry on legal fees,” he said.
But Dr. McCullough apparently has a plan for helping to defray his legal costs. In the Aug. 3 interview, he said a foundation he helped start, Truth for Health, has a “donation side to it,” adding “some of that may be used for legal expense.”
Cheryl Jones, an attorney with PK Law in Towson, Md., said that might draw interest from the Internal Revenue Service. “I would expect IRS scrutiny if contributions to the Medical Censorship Defense Fund are used to defend Dr McCullough in his personal breach of contract lawsuit,” she told this news organization.
The IRS generally recognizes defending “human and civil rights secured by law” as a legitimate charitable purpose for a legal defense fund, she said, adding that such a fund “must serve only public, rather than private, interests.”
Misinformation from a physician more damaging?
Some in the medical field have refuted Dr. McCullough’s pronouncements on how to treat COVID-19, including two infectious disease specialists with Monash University, Melbourne, who responded to the cardiologist’s original paper in the American Journal of Medicine.
Tony Korman, MBBS, a professor at the Centre for Inflammatory Diseases at Monash, told this news organization, “we had concerns that reputable medical journals would accept and publish papers proposing treatment of COVID-19 which was not supported by evidence.”
The website Healthfeedback.org has also challenged McCullough’s and his supporters’ claims, including that the American Journal of Medicine endorsed the use of hydroxychloroquine and that the COVID-19 vaccines have caused thousands of deaths.
David Broniatowski, PhD, associate director for the Institute for Data, Democracy and Politics at George Washington University, Washington, said in an interview that Dr. McCullough’s casting himself as a “rebel doctor” is a well-known trope in the vaccine misinformation universe.
Although he was not familiar with Dr. McCullough, Dr. Broniatowski said the cardiologist’s claims are not unique – they’ve been circulating among antivaccine and conspiracy-oriented groups for months.
For instance, Dr. McCullough has claimed in interviews that a whistleblower within the CDC knows of 50,000 vaccine-related deaths. Using data from the supposed whistleblower, the group America’s Frontline Doctors sued the federal government in July to stop the administration of COVID-19 vaccines to those under 18, people who have already had COVID, and individuals who the group said have not been adequately informed about the risks.
The idea of a whistleblower inside the CDC is recycled from antivaccine claims from decades ago, Dr. Broniatowski said.
But, he added, “somebody who speaks with the credibility of a major institution will be more likely to be listened to by some people.” That vulnerable group is “being taken advantage of by a relatively small number of disinformation purveyors, who, in some cases, profit from that disinformation,” said Dr. Broniatowski.
“We rely on our doctors because we trust them,” he said. “And we trust them because we believe that as physicians, their value system places the patient’s best interests first. That’s why it’s so much of a disappointment when you have a physician that appears to be exercising this sort of bad judgment.”
Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, also said that he was not familiar with Dr. McCullough. But apprised of his claims, Dr. Offit told this news organization, “Peter McCullough is a friend of the virus.”
“The kind of information he promotes allows the virus to continue to spread, continue to do an enormous amount of harm, and continue to mutate and create variants that have become more contagious and more resistant to vaccine-induced immunity,” said Dr. Offit, the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia.
Dr. Offit added that the war should be against SARS-CoV-2, but “because this virus has so many supporters, the war in essence becomes a war against ourselves, which is much harder.”
Dr. McCullough maintains he is doing a service to his patients. “I’m just giving and trying to help America understand the pandemic,” he told Ms. Ingraham on Fox News on July 29.
But he acknowledged concern about the Federation of State Medical Board’s announcement that physicians who spread COVID-19 vaccine misinformation risk suspension or revocation of their license.
“I have to tell you I’m worried – that no matter what I do and how careful I am to cite the scientific studies, I’m still gonna be hunted down for quote, misinformation,” he said in the Aug. 3 interview.
A version of this article first appeared on Medscape.com.
‘Empathy fatigue’ in clinicians rises with latest COVID-19 surge
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
How could this happen? Judge forces doctors to give ivermectin
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
New Moderna vaccine data ‘support’ booster shot after 8 months
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
COVID vaccine preprint study prompts Twitter outrage
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.