User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Flu now riding on COVID-19’s coattails
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.
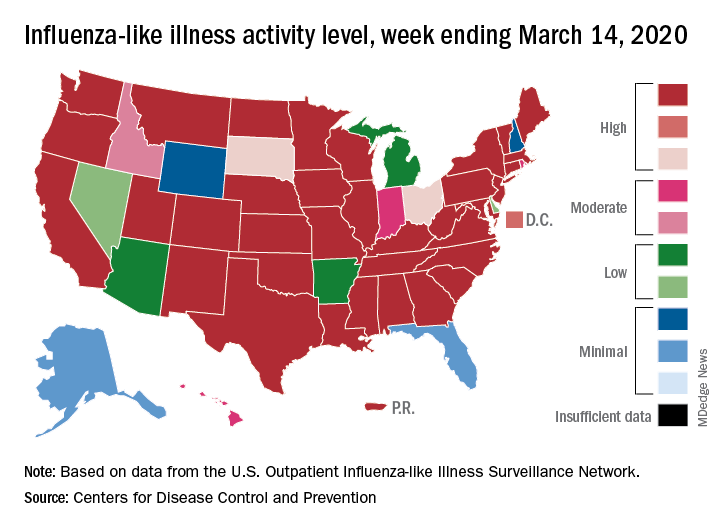
according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
Preventable diseases could gain a foothold because of COVID-19
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
Emergency Rule: Docs can bill for telehealth and COVID-19 tests. Here’s how
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.
Many medical practices have long wanted to use telehealth to perform office visits and other evaluation and management (E/M) services. The technology readily exists and many electronic health records are set up to do telehealth visits. The problem has been getting paid for those visits. Medicare limited telehealth services to patients in underserved areas, and commercial insurances wouldn’t pay. But amid the COVID-19 crisis, things have changed.
On March 17, Congress passed a law allowing Medicare to waive some telehealth restrictions during a government state of emergency only, which we are in now. Specifically, the patient no longer needs to be in a medically underserved area and no longer needs to go to an originating site, such as a hospital. The patient can be located anywhere in the country and be in their own home.
Further, the Centers for Medicare & Medicaid is waiving the requirement that the practitioner use a HIPAA-compliant platform for the telehealth service. The service must still be provided using a real-time audiovisual platform, but that could be via FaceTime or Skype, both of which are readily available via a patient’s smartphone or home computer. Audio alone – that is, phone calls between physician and patient – is still insufficient.
Billing for telemedicine
There are two lists of services that you can bill for telehealth. One of the lists is in Medicare’s telehealth fact sheet and includes both CPT and HCPCS codes. The second is in your CPT book, Appendix P, and lists only CPT codes.
Practices may bill all of the Medicare-covered telehealth services using these new rules. This includes new and established patient visits 99201–99215. It includes inpatient and skilled nursing services, for which CMS uses HCPCS codes in place of CPT codes.
Some notable additional services that you may bill via telehealth are: smoking cessation, transitional care management, advanced care planning, psychiatric diagnostic interviews and psychotherapy, and initial and subsequent Medicare wellness visits. The Welcome to Medicare visit is not on the list.
Report these services to Medicare with the correct CPT code and use place of service 02 (telehealth) on the claim. There is a CPT modifier for telehealth (Modifier -95 Synchronous Telemedicine Service Rendered Via a Real-Time Interactive Audio and Video Telecommunications System) but Medicare does not require it.
If you perform an office visit and also do smoking cessation, document those just as you would if you saw the patient in person. Document the history; observational exam, if relevant; and the assessment and plan. Note the additional time spent in smoking cessation counseling. If it was a level three established patient, code 99213-25 and 99406 (smoking and tobacco use cessation counseling visit, intermediate, 3-10 minutes).
The Office of Inspector General is allowing practices to reduce or waive copays and patient due amounts. However, a practice is not required to waive the copay or patient due amount for a telehealth service.
Medicare Advantage plans are required to cover all services that original Medicare covers. State Medicaid plans and Medicaid managed care organizations can set their own rules.
What about commercial payers?
While CMS has issued its Medicare guidelines, commercial insurance companies can also set their own rules about covering telehealth services. Many of them have rushed to update their policies to allow office visits to be billed via telehealth.
Unfortunately, each payer can set its own rules about whether to cover telehealth and if the place of service 02 and/or modifier -95 is needed. UnitedHealthcare is covering telehealth visits for all of its Medicare Advantage, Medicaid, and commercial accounts.
Humana also is covering telemedicine for urgent care needs. Some private insurers are continuing to offer virtual visits with their contracted telehealth provider, not with the patient’s own physician. It is likely that this will change in the days ahead, but it means practices must check their payer policies and pay attention to the emails they receive from the payers. If patient foot traffic is slow, this may be a good time to call each payer to not only find out their telehealth rules, but to also learn what else is being suspended during the COVID-19 pandemic.
This would also be a good job for an employee to do from home versus coming into the practice.
None of the payers are limiting the diagnosis code for telemedicine services. The patient does not need to have a cough or fever to have telemedicine covered. Any diagnosis or condition is eligible to be billed via telehealth.
The waived restrictions by Medicare are in place only as long as the government state of emergency. Commercial payers are also describing these as temporary. However, it may be hard to put the genie back in the bottle. Medical practices and patients may find that these visits are just what the doctor ordered.
COVID-19 testing
Although testing is still not widely available, the American Medical Association has developed a CPT code for the test:
- 87635: Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique
CMS has also developed codes for testing for this new coronavirus. One (U0001) is specifically for tests done in the CDC lab. The second (U0002) was for other labs, but it seems likely that the CPT code will replace it.
In February, the U.S. Food and Drug Administration issued a new policy for certain labs to develop their own validated COVID-19 diagnostics. This second HCPCS code could be used for such tests when submitting claims to Medicare or other insurers.
The hope by CMS is that having these specific codes will encourage further testing and improve tracking of the virus.
This article first appeared on Medscape.com.
New ASAM guideline released amid COVID-19 concerns
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
Home-based buprenorphine induction deemed safe for OUD
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
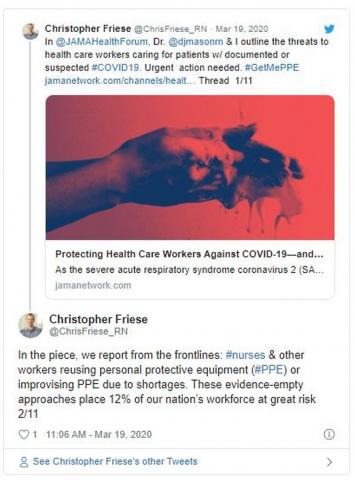
DIY masks: Worth the risk? Researchers are conflicted
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
COVID-19 prompts ‘lifesaving’ policy change for opioid addiction
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
Match Day 2020: Online announcements replace celebrations, champagne
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
Feds tout drug candidates to treat COVID-19
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
20% of U.S. COVID-19 deaths were aged 20-64 years
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
Rheumatologists to share knowledge in COVID-19 patient-centered registry
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.