User login
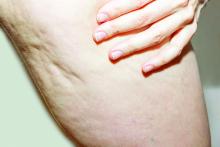
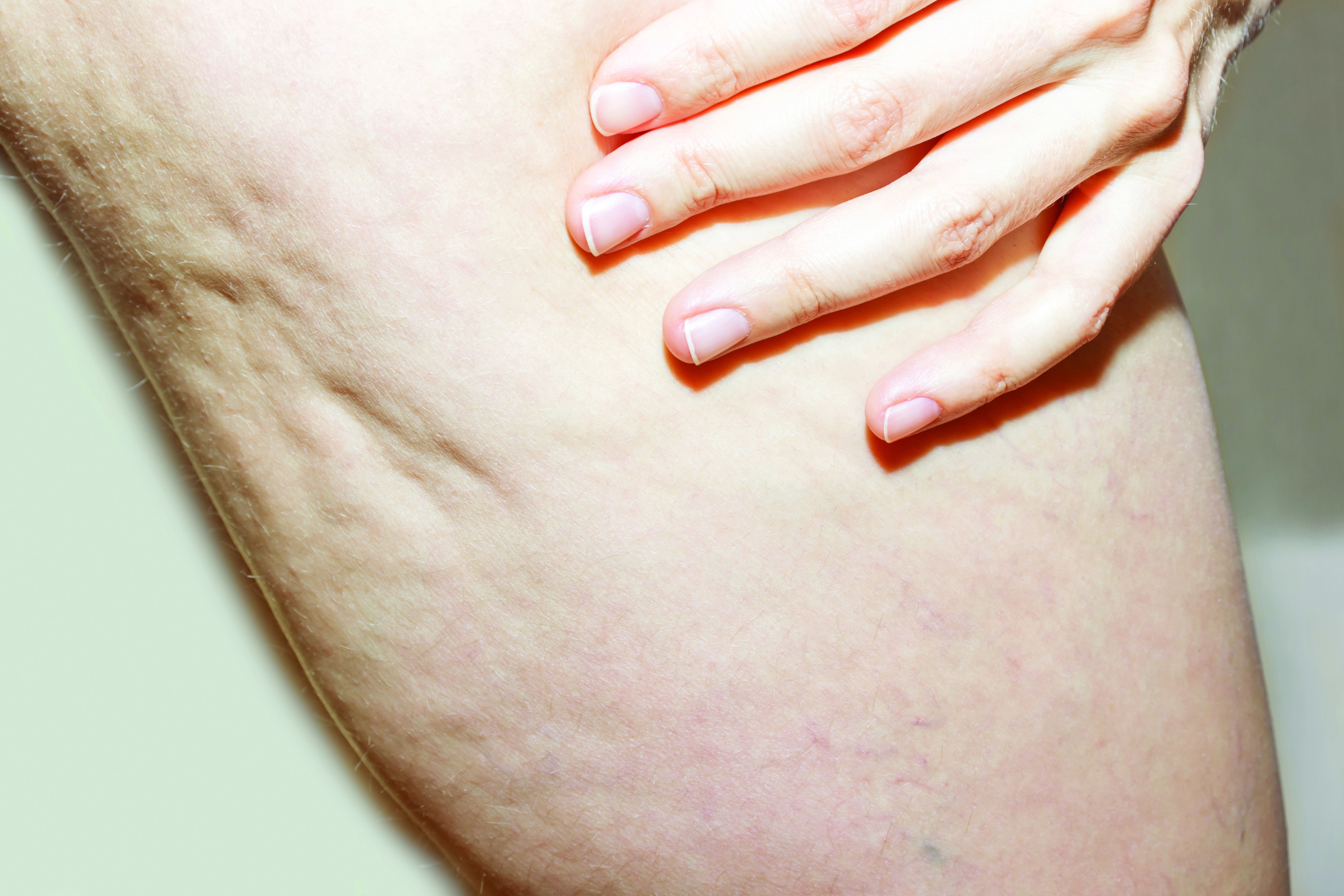
HIFEM procedure helped to improve UI and female sexual function
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
FROM ASLMS 2021
Photobiomodulation: Evaluation in a wide range of medical specialties underway
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
FROM ASLMS 2021
Expert offers 10 ‘tips and tricks’ for everyday cosmetic practice
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
FROM ASLMS 2021
Pilot study: Hybrid laser found effective for treating genitourinary syndrome of menopause
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
FROM ASLMS 2021
Trial yields evidence that laser resurfacing may prevent NMSC in aged skin
A on treated areas, according to the results of a small, randomized trial.
“Previous research suggests a new model to explain why older patients obtain nonmelanoma skin cancer in areas of ongoing sun exposure,” presenting author Jeffrey Wargo, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “Insulinlike growth factor-1 produced by dermal fibroblasts dictates how overlying skin keratinocytes respond to UVB radiation. The skin of a patient aged in their 20s produces normal levels of healthy fibroblasts, normal levels of insulinlike growth factor 1, and appropriate UVB response via activation of nucleotide excision, repair, and DNA damage checkpoint-signaling systems.”
Older patients, meanwhile, have an increase in senescent fibroblasts, decreased insulinlike growth factor-1 (IGF-1), and an inappropriate UVB response to DNA damage, continued Dr. Wargo, a dermatologist at the Ohio State University Wexner Medical Center in Columbus. Previous studies conducted by his mentor, Jeffrey B. Travers, MD, PhD, a dermatologist and pharmacologist at Wright State University, Dayton, showed that fractionated laser resurfacing (FLR) restores UVB response in older patients’ skin by resulting in new fibroblasts and increased levels of IGF 2 years post wounding.
To determine if FLR of aged skin can prevent the development of actinic keratosis (AK) and nonmelanoma skin cancer, Dr. Travers and Dr. Wargo recruited 48 patients at the Dayton VA Medical Center who were 60 years or older and had at least five AKs on each arm that were 3 mm or smaller, with nothing concerning for skin cancer at the screening visit.
Randomization of which arm was treated was based on an odd or even Social Security Number. That arm was treated with the 2,790 nm Erbium:YSSG ablative laser at 120 J/m2 with one pass at 24% coverage from the elbow to hand dorsally. Previously published data reported outcomes for 30 of these patients at 3 and 6 months following treatment. Subsequent to that report, 18 additional subjects have been recruited to the study and follow-up has been extended. Of the 48 patients, 47 were male and their average age was 74, with a range between 61 and 87 years.
At 3 months following FLR, the ratio of AKs on the treated vs. untreated arms was reduced by fourfold, with a P value less than .00001, Dr. Wargo reported. “Throughout the current 30-month follow-up period, this ratio has been maintained,” he said. “In fact, none of the ratios determined at 3, 6, 12, 18, 24, or 30 months post FLR are significantly different. Hence, as described in our first report on this work, these data indicate FLR is an effective treatment for existing AKs. However, our model predicts that FLR treatment will also prevent the occurrence of new AK lesions.”
Among 19 of the study participants who have been followed out to 30 months, untreated arms continued to accumulate increasing number of AKs. In contrast, AKs on treated arms are decreasing with time, indicating the lack of newly initiated lesions.
“A second analysis of the data posits that, if FLR were only removing existing lesions, one would predict the number of AKs that were present at 3 months on both the untreated and FLR-treated [arms] would accumulate at the same rate subsequent to 3 months point in time,” Dr. Wargo said.
He pointed out that 12 patients were removed from the study: two at 12 months, one at 18 months, eight at 24 months, and one at 30 months, as they were found to have 20 or more AKs on their untreated arm and required treatment.
Over the entire study period, “consistent with the notion that FLR was preventing new actinic neoplasia, we noted a dramatic difference in numbers of nonmelanoma skin cancer diagnosed in the untreated areas (22) versus FLR treated areas (2),” Dr. Wargo said. The majority of nonmelanoma skin cancers diagnosed were SCC (17) and 5 basal cell carcinomas on the untreated arms, whereas the 2 diagnosed on the treated arm were SCC. “These studies indicate that a dermal-wounding strategy involving FLR, which upregulates dermal IGF-1 levels, not only treats AKs but prevents nonmelanoma skin cancer,” he said.
The study was funded by the National Institutes of Health. Dr. Travers is the principal investigator. Dr. Wargo reported having no financial disclosures.
A on treated areas, according to the results of a small, randomized trial.
“Previous research suggests a new model to explain why older patients obtain nonmelanoma skin cancer in areas of ongoing sun exposure,” presenting author Jeffrey Wargo, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “Insulinlike growth factor-1 produced by dermal fibroblasts dictates how overlying skin keratinocytes respond to UVB radiation. The skin of a patient aged in their 20s produces normal levels of healthy fibroblasts, normal levels of insulinlike growth factor 1, and appropriate UVB response via activation of nucleotide excision, repair, and DNA damage checkpoint-signaling systems.”
Older patients, meanwhile, have an increase in senescent fibroblasts, decreased insulinlike growth factor-1 (IGF-1), and an inappropriate UVB response to DNA damage, continued Dr. Wargo, a dermatologist at the Ohio State University Wexner Medical Center in Columbus. Previous studies conducted by his mentor, Jeffrey B. Travers, MD, PhD, a dermatologist and pharmacologist at Wright State University, Dayton, showed that fractionated laser resurfacing (FLR) restores UVB response in older patients’ skin by resulting in new fibroblasts and increased levels of IGF 2 years post wounding.
To determine if FLR of aged skin can prevent the development of actinic keratosis (AK) and nonmelanoma skin cancer, Dr. Travers and Dr. Wargo recruited 48 patients at the Dayton VA Medical Center who were 60 years or older and had at least five AKs on each arm that were 3 mm or smaller, with nothing concerning for skin cancer at the screening visit.
Randomization of which arm was treated was based on an odd or even Social Security Number. That arm was treated with the 2,790 nm Erbium:YSSG ablative laser at 120 J/m2 with one pass at 24% coverage from the elbow to hand dorsally. Previously published data reported outcomes for 30 of these patients at 3 and 6 months following treatment. Subsequent to that report, 18 additional subjects have been recruited to the study and follow-up has been extended. Of the 48 patients, 47 were male and their average age was 74, with a range between 61 and 87 years.
At 3 months following FLR, the ratio of AKs on the treated vs. untreated arms was reduced by fourfold, with a P value less than .00001, Dr. Wargo reported. “Throughout the current 30-month follow-up period, this ratio has been maintained,” he said. “In fact, none of the ratios determined at 3, 6, 12, 18, 24, or 30 months post FLR are significantly different. Hence, as described in our first report on this work, these data indicate FLR is an effective treatment for existing AKs. However, our model predicts that FLR treatment will also prevent the occurrence of new AK lesions.”
Among 19 of the study participants who have been followed out to 30 months, untreated arms continued to accumulate increasing number of AKs. In contrast, AKs on treated arms are decreasing with time, indicating the lack of newly initiated lesions.
“A second analysis of the data posits that, if FLR were only removing existing lesions, one would predict the number of AKs that were present at 3 months on both the untreated and FLR-treated [arms] would accumulate at the same rate subsequent to 3 months point in time,” Dr. Wargo said.
He pointed out that 12 patients were removed from the study: two at 12 months, one at 18 months, eight at 24 months, and one at 30 months, as they were found to have 20 or more AKs on their untreated arm and required treatment.
Over the entire study period, “consistent with the notion that FLR was preventing new actinic neoplasia, we noted a dramatic difference in numbers of nonmelanoma skin cancer diagnosed in the untreated areas (22) versus FLR treated areas (2),” Dr. Wargo said. The majority of nonmelanoma skin cancers diagnosed were SCC (17) and 5 basal cell carcinomas on the untreated arms, whereas the 2 diagnosed on the treated arm were SCC. “These studies indicate that a dermal-wounding strategy involving FLR, which upregulates dermal IGF-1 levels, not only treats AKs but prevents nonmelanoma skin cancer,” he said.
The study was funded by the National Institutes of Health. Dr. Travers is the principal investigator. Dr. Wargo reported having no financial disclosures.
A on treated areas, according to the results of a small, randomized trial.
“Previous research suggests a new model to explain why older patients obtain nonmelanoma skin cancer in areas of ongoing sun exposure,” presenting author Jeffrey Wargo, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “Insulinlike growth factor-1 produced by dermal fibroblasts dictates how overlying skin keratinocytes respond to UVB radiation. The skin of a patient aged in their 20s produces normal levels of healthy fibroblasts, normal levels of insulinlike growth factor 1, and appropriate UVB response via activation of nucleotide excision, repair, and DNA damage checkpoint-signaling systems.”
Older patients, meanwhile, have an increase in senescent fibroblasts, decreased insulinlike growth factor-1 (IGF-1), and an inappropriate UVB response to DNA damage, continued Dr. Wargo, a dermatologist at the Ohio State University Wexner Medical Center in Columbus. Previous studies conducted by his mentor, Jeffrey B. Travers, MD, PhD, a dermatologist and pharmacologist at Wright State University, Dayton, showed that fractionated laser resurfacing (FLR) restores UVB response in older patients’ skin by resulting in new fibroblasts and increased levels of IGF 2 years post wounding.
To determine if FLR of aged skin can prevent the development of actinic keratosis (AK) and nonmelanoma skin cancer, Dr. Travers and Dr. Wargo recruited 48 patients at the Dayton VA Medical Center who were 60 years or older and had at least five AKs on each arm that were 3 mm or smaller, with nothing concerning for skin cancer at the screening visit.
Randomization of which arm was treated was based on an odd or even Social Security Number. That arm was treated with the 2,790 nm Erbium:YSSG ablative laser at 120 J/m2 with one pass at 24% coverage from the elbow to hand dorsally. Previously published data reported outcomes for 30 of these patients at 3 and 6 months following treatment. Subsequent to that report, 18 additional subjects have been recruited to the study and follow-up has been extended. Of the 48 patients, 47 were male and their average age was 74, with a range between 61 and 87 years.
At 3 months following FLR, the ratio of AKs on the treated vs. untreated arms was reduced by fourfold, with a P value less than .00001, Dr. Wargo reported. “Throughout the current 30-month follow-up period, this ratio has been maintained,” he said. “In fact, none of the ratios determined at 3, 6, 12, 18, 24, or 30 months post FLR are significantly different. Hence, as described in our first report on this work, these data indicate FLR is an effective treatment for existing AKs. However, our model predicts that FLR treatment will also prevent the occurrence of new AK lesions.”
Among 19 of the study participants who have been followed out to 30 months, untreated arms continued to accumulate increasing number of AKs. In contrast, AKs on treated arms are decreasing with time, indicating the lack of newly initiated lesions.
“A second analysis of the data posits that, if FLR were only removing existing lesions, one would predict the number of AKs that were present at 3 months on both the untreated and FLR-treated [arms] would accumulate at the same rate subsequent to 3 months point in time,” Dr. Wargo said.
He pointed out that 12 patients were removed from the study: two at 12 months, one at 18 months, eight at 24 months, and one at 30 months, as they were found to have 20 or more AKs on their untreated arm and required treatment.
Over the entire study period, “consistent with the notion that FLR was preventing new actinic neoplasia, we noted a dramatic difference in numbers of nonmelanoma skin cancer diagnosed in the untreated areas (22) versus FLR treated areas (2),” Dr. Wargo said. The majority of nonmelanoma skin cancers diagnosed were SCC (17) and 5 basal cell carcinomas on the untreated arms, whereas the 2 diagnosed on the treated arm were SCC. “These studies indicate that a dermal-wounding strategy involving FLR, which upregulates dermal IGF-1 levels, not only treats AKs but prevents nonmelanoma skin cancer,” he said.
The study was funded by the National Institutes of Health. Dr. Travers is the principal investigator. Dr. Wargo reported having no financial disclosures.
FROM ASLMS 2021
One year after a single RAP treatment, patients give the results high marks
One year after a single treatment of cellulite with the Rapid Acoustic Pulse (RAP) device, 42 out of 43 patients said that they felt good about the results, an interim analysis from a multicenter study showed.
“I think this speaks to the duration of improvement” associated with this treatment, lead study author Elizabeth L. Tanzi, MD, said during the annual conference of the American Society for Laser Medicine and Surgery.
The study is an extension of a prospective pivotal clinical trial that Dr. Tanzi first presented during late-breaking abstract session at the 2020 virtual annual meeting of the American Academy of Dermatology. For the trial, she and her colleagues at four sites evaluated the safety and effectiveness of the RAP device in 62 women who were treated with a single, rapid acoustic pulse treatment comprised of 1-2 minutes on each identified dimple or large ridge of cellulite. In February 2021, the Food and Drug Administration cleared the device for the short-term improvement in the appearance of cellulite.
“The high peak pressure and fast repetition rate of this device will exploit the viscoelastic tissue compared to other acoustic wave devices that are on the market,” said Dr. Tanzi, associate clinical professor of dermatology at George Washington University, Washington. “Those compressed pulses from electronic filtering and reflector shape eliminate cavitation, heat, and pain. So, what we see physically is fiber septa disruption, as well as other nonthermal physical effects.”
She and her colleagues used a specific photography and fixed lighting setup to record the treated areas at baseline, 12 weeks, and 52 weeks, and administered a patient satisfaction questionnaire at 12 and 52 weeks. Prior to treatment, the investigators marked the dimples and ridges intended for treatment. After placing a hydrogel dressing, it took 45-60 minutes to treat both buttocks and thighs with the RAP device. “It was completely noninvasive,” said Dr. Tanzi, who practices in Chevy Chase, Md. “There was no anesthesia used: no incisions and no needles.”
Among 57 patients who were evaluated at 12 weeks, the pain score on a scale of 0-10 was 2.4, while 61.5% strongly agreed and 35.9% agreed that their cellulite appeared improved. In addition, three blinded assessors were able to correctly identify the treated thigh 96% of the time and physician-graded Global Aesthetic Improvement Scale assessments showed 90% improvement of cellulite.
Follow-up analysis
For the current subject satisfaction analysis, the investigators evaluated results from 43 patients in the trial who were followed for at least 52 weeks (a mean of 60 weeks). Of the 43 patients, 30 (69.8%) strongly agreed and 13 (30.2%) agreed that their cellulite “appears improved.” In addition, 29 (67.4%) strongly agreed, 13 (30.2%) agreed, and 1 (2.3%) disagreed with the statement “I feel there is good improvement” of their cellulite.
“Currently, we are evaluating the blinded assessors’ view of these patients and not just going with [findings from] the patient satisfaction survey, but I think these are encouraging results,” Dr. Tanzi said. “We found that 42 out of 43 patients said, ‘yes; I feel that there was good improvement of the area at 52 weeks.’ ”
Dr. Tanzi disclosed that she is a member of the speakers bureau for Eucerin. She is a member of the advisory board for Allergan, Endo Pharmaceuticals, Pulse Biosciences, Sciton, and Soliton. Soliton markets the RAP device.
One year after a single treatment of cellulite with the Rapid Acoustic Pulse (RAP) device, 42 out of 43 patients said that they felt good about the results, an interim analysis from a multicenter study showed.
“I think this speaks to the duration of improvement” associated with this treatment, lead study author Elizabeth L. Tanzi, MD, said during the annual conference of the American Society for Laser Medicine and Surgery.
The study is an extension of a prospective pivotal clinical trial that Dr. Tanzi first presented during late-breaking abstract session at the 2020 virtual annual meeting of the American Academy of Dermatology. For the trial, she and her colleagues at four sites evaluated the safety and effectiveness of the RAP device in 62 women who were treated with a single, rapid acoustic pulse treatment comprised of 1-2 minutes on each identified dimple or large ridge of cellulite. In February 2021, the Food and Drug Administration cleared the device for the short-term improvement in the appearance of cellulite.
“The high peak pressure and fast repetition rate of this device will exploit the viscoelastic tissue compared to other acoustic wave devices that are on the market,” said Dr. Tanzi, associate clinical professor of dermatology at George Washington University, Washington. “Those compressed pulses from electronic filtering and reflector shape eliminate cavitation, heat, and pain. So, what we see physically is fiber septa disruption, as well as other nonthermal physical effects.”
She and her colleagues used a specific photography and fixed lighting setup to record the treated areas at baseline, 12 weeks, and 52 weeks, and administered a patient satisfaction questionnaire at 12 and 52 weeks. Prior to treatment, the investigators marked the dimples and ridges intended for treatment. After placing a hydrogel dressing, it took 45-60 minutes to treat both buttocks and thighs with the RAP device. “It was completely noninvasive,” said Dr. Tanzi, who practices in Chevy Chase, Md. “There was no anesthesia used: no incisions and no needles.”
Among 57 patients who were evaluated at 12 weeks, the pain score on a scale of 0-10 was 2.4, while 61.5% strongly agreed and 35.9% agreed that their cellulite appeared improved. In addition, three blinded assessors were able to correctly identify the treated thigh 96% of the time and physician-graded Global Aesthetic Improvement Scale assessments showed 90% improvement of cellulite.
Follow-up analysis
For the current subject satisfaction analysis, the investigators evaluated results from 43 patients in the trial who were followed for at least 52 weeks (a mean of 60 weeks). Of the 43 patients, 30 (69.8%) strongly agreed and 13 (30.2%) agreed that their cellulite “appears improved.” In addition, 29 (67.4%) strongly agreed, 13 (30.2%) agreed, and 1 (2.3%) disagreed with the statement “I feel there is good improvement” of their cellulite.
“Currently, we are evaluating the blinded assessors’ view of these patients and not just going with [findings from] the patient satisfaction survey, but I think these are encouraging results,” Dr. Tanzi said. “We found that 42 out of 43 patients said, ‘yes; I feel that there was good improvement of the area at 52 weeks.’ ”
Dr. Tanzi disclosed that she is a member of the speakers bureau for Eucerin. She is a member of the advisory board for Allergan, Endo Pharmaceuticals, Pulse Biosciences, Sciton, and Soliton. Soliton markets the RAP device.
One year after a single treatment of cellulite with the Rapid Acoustic Pulse (RAP) device, 42 out of 43 patients said that they felt good about the results, an interim analysis from a multicenter study showed.
“I think this speaks to the duration of improvement” associated with this treatment, lead study author Elizabeth L. Tanzi, MD, said during the annual conference of the American Society for Laser Medicine and Surgery.
The study is an extension of a prospective pivotal clinical trial that Dr. Tanzi first presented during late-breaking abstract session at the 2020 virtual annual meeting of the American Academy of Dermatology. For the trial, she and her colleagues at four sites evaluated the safety and effectiveness of the RAP device in 62 women who were treated with a single, rapid acoustic pulse treatment comprised of 1-2 minutes on each identified dimple or large ridge of cellulite. In February 2021, the Food and Drug Administration cleared the device for the short-term improvement in the appearance of cellulite.
“The high peak pressure and fast repetition rate of this device will exploit the viscoelastic tissue compared to other acoustic wave devices that are on the market,” said Dr. Tanzi, associate clinical professor of dermatology at George Washington University, Washington. “Those compressed pulses from electronic filtering and reflector shape eliminate cavitation, heat, and pain. So, what we see physically is fiber septa disruption, as well as other nonthermal physical effects.”
She and her colleagues used a specific photography and fixed lighting setup to record the treated areas at baseline, 12 weeks, and 52 weeks, and administered a patient satisfaction questionnaire at 12 and 52 weeks. Prior to treatment, the investigators marked the dimples and ridges intended for treatment. After placing a hydrogel dressing, it took 45-60 minutes to treat both buttocks and thighs with the RAP device. “It was completely noninvasive,” said Dr. Tanzi, who practices in Chevy Chase, Md. “There was no anesthesia used: no incisions and no needles.”
Among 57 patients who were evaluated at 12 weeks, the pain score on a scale of 0-10 was 2.4, while 61.5% strongly agreed and 35.9% agreed that their cellulite appeared improved. In addition, three blinded assessors were able to correctly identify the treated thigh 96% of the time and physician-graded Global Aesthetic Improvement Scale assessments showed 90% improvement of cellulite.
Follow-up analysis
For the current subject satisfaction analysis, the investigators evaluated results from 43 patients in the trial who were followed for at least 52 weeks (a mean of 60 weeks). Of the 43 patients, 30 (69.8%) strongly agreed and 13 (30.2%) agreed that their cellulite “appears improved.” In addition, 29 (67.4%) strongly agreed, 13 (30.2%) agreed, and 1 (2.3%) disagreed with the statement “I feel there is good improvement” of their cellulite.
“Currently, we are evaluating the blinded assessors’ view of these patients and not just going with [findings from] the patient satisfaction survey, but I think these are encouraging results,” Dr. Tanzi said. “We found that 42 out of 43 patients said, ‘yes; I feel that there was good improvement of the area at 52 weeks.’ ”
Dr. Tanzi disclosed that she is a member of the speakers bureau for Eucerin. She is a member of the advisory board for Allergan, Endo Pharmaceuticals, Pulse Biosciences, Sciton, and Soliton. Soliton markets the RAP device.
FROM ASLMS 2021
How early can laser treatment for port wine stains in infants be initiated?
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
FROM ASLMS 2021
Review finds diverse outcomes in clinical trials of rosacea
according to authors of a new systematic review of rosacea treatment studies.
“Rosacea is a chronic dermatologic condition that affects 16 million Americans,” one of the study authors, Sarah A. Ibrahim, told this news organization after the annual conference of the American Society for Laser Medicine and Surgery. “The features of rosacea, such as inflammatory lesions, redness, burning sensations, and swelling, can have a negative impact on the quality of life for many patients. Additionally, patients with rosacea are at an increased risk for other conditions such as autoimmune diseases, like inflammatory bowel disease.”
In an effort led by principal investigator Murad Alam, MD, vice chair of the department of dermatology at Northwestern University, Chicago, Ms. Ibrahim conducted a systematic review to identify all outcomes that have previously been reported in clinical trials of rosacea, as part of the development of the core outcome set established by the Measurement of Priority Outcome Variables in Dermatologic Surgery (IMPROVED) group. “This has not been done before and is an important first step in understanding what outcomes should be measured in every future clinical study of rosacea,” said Ms. Ibrahim, a medical student at Northwestern University, and predoctoral research fellow in Northwestern’s department of dermatology.
The researchers limited their analysis to randomized, controlled trials of rosacea interventions published between 2010 and 2020 and categorized outcomes into domains based on similar themes.
A total of 58 studies were included in the systematic review, of which 7 (12%) evaluated laser-based interventions. The researchers identified 55 unique outcomes that encompassed eight domains: Quality of life, treatment effects, patient perception of health, clinical assessment, acceptance of care, laboratory assessment, physiological skin assessment, and patient satisfaction. Of the eight domains, clinical assessment-related outcomes were measured in all studies. Nontransient erythema was the most commonly reported outcome (43 studies, 78%), followed by inflammatory lesions (36 studies, 65%) and telangiectasia (22 studies, 40%).
Outcomes pertaining to treatment effects such as adverse events were measured in 49 of the 55 studies (89%), while patient-reported outcomes were measured in 21 (38%). Quality of life and patient satisfaction were reported in 18 (33%) and 13 (24%) studies, respectively.
“There were two main take-home messages of our study,” said Ms. Ibrahim, who presented the results at the meeting. “The first is that there is a wide range of outcomes that are reported in clinical trials of rosacea therapies. Second, that there is a need to standardize the outcomes that are reported in clinical trials of rosacea, in order to be able to combine the results from different studies to better understand which interventions for rosacea are most effective.”
She acknowledged certain limitations of the review, including that other trials related to the topic were not included. “Because of the date range and types of studies that we used to narrow down our search, it is possible that additional outcomes were reported in studies that were not included here,” she said.
“This is a very important study because rosacea is a very common condition and one that I have seen more frequently in clinic since the pandemic started,” said Omar Ibrahimi, PhD, MD, a dermatologist with the Connecticut Skin Institute in Stamford, who was asked to comment on the work. “One of the limitations with rosacea studies is that the studies done are often fairly small and the outcome measures are heterogenous. The current study by Ibrahim and coworkers does a wonderful job of highlighting the various outcomes measures used to measure the success of rosacea treatments with energy-based devices.”
This information, he added, “will be very useful for further research studies because it forms the basis for formulating a set of core outcome measures to judge treatment interventions with consensus input from a variety of key opinion leaders. This will prove to be valuable because if we can have a uniform set of outcome measures to judge rosacea treatments with then we will be able to compare the results from different studies better.”
Ms. Ibrahim and colleagues reported having no relevant financial disclosures. Dr. Ibrahimi disclosed that he has been a speaker for both Candela and Cutera and he is currently on the medical advisory board for Cutera.
according to authors of a new systematic review of rosacea treatment studies.
“Rosacea is a chronic dermatologic condition that affects 16 million Americans,” one of the study authors, Sarah A. Ibrahim, told this news organization after the annual conference of the American Society for Laser Medicine and Surgery. “The features of rosacea, such as inflammatory lesions, redness, burning sensations, and swelling, can have a negative impact on the quality of life for many patients. Additionally, patients with rosacea are at an increased risk for other conditions such as autoimmune diseases, like inflammatory bowel disease.”
In an effort led by principal investigator Murad Alam, MD, vice chair of the department of dermatology at Northwestern University, Chicago, Ms. Ibrahim conducted a systematic review to identify all outcomes that have previously been reported in clinical trials of rosacea, as part of the development of the core outcome set established by the Measurement of Priority Outcome Variables in Dermatologic Surgery (IMPROVED) group. “This has not been done before and is an important first step in understanding what outcomes should be measured in every future clinical study of rosacea,” said Ms. Ibrahim, a medical student at Northwestern University, and predoctoral research fellow in Northwestern’s department of dermatology.
The researchers limited their analysis to randomized, controlled trials of rosacea interventions published between 2010 and 2020 and categorized outcomes into domains based on similar themes.
A total of 58 studies were included in the systematic review, of which 7 (12%) evaluated laser-based interventions. The researchers identified 55 unique outcomes that encompassed eight domains: Quality of life, treatment effects, patient perception of health, clinical assessment, acceptance of care, laboratory assessment, physiological skin assessment, and patient satisfaction. Of the eight domains, clinical assessment-related outcomes were measured in all studies. Nontransient erythema was the most commonly reported outcome (43 studies, 78%), followed by inflammatory lesions (36 studies, 65%) and telangiectasia (22 studies, 40%).
Outcomes pertaining to treatment effects such as adverse events were measured in 49 of the 55 studies (89%), while patient-reported outcomes were measured in 21 (38%). Quality of life and patient satisfaction were reported in 18 (33%) and 13 (24%) studies, respectively.
“There were two main take-home messages of our study,” said Ms. Ibrahim, who presented the results at the meeting. “The first is that there is a wide range of outcomes that are reported in clinical trials of rosacea therapies. Second, that there is a need to standardize the outcomes that are reported in clinical trials of rosacea, in order to be able to combine the results from different studies to better understand which interventions for rosacea are most effective.”
She acknowledged certain limitations of the review, including that other trials related to the topic were not included. “Because of the date range and types of studies that we used to narrow down our search, it is possible that additional outcomes were reported in studies that were not included here,” she said.
“This is a very important study because rosacea is a very common condition and one that I have seen more frequently in clinic since the pandemic started,” said Omar Ibrahimi, PhD, MD, a dermatologist with the Connecticut Skin Institute in Stamford, who was asked to comment on the work. “One of the limitations with rosacea studies is that the studies done are often fairly small and the outcome measures are heterogenous. The current study by Ibrahim and coworkers does a wonderful job of highlighting the various outcomes measures used to measure the success of rosacea treatments with energy-based devices.”
This information, he added, “will be very useful for further research studies because it forms the basis for formulating a set of core outcome measures to judge treatment interventions with consensus input from a variety of key opinion leaders. This will prove to be valuable because if we can have a uniform set of outcome measures to judge rosacea treatments with then we will be able to compare the results from different studies better.”
Ms. Ibrahim and colleagues reported having no relevant financial disclosures. Dr. Ibrahimi disclosed that he has been a speaker for both Candela and Cutera and he is currently on the medical advisory board for Cutera.
according to authors of a new systematic review of rosacea treatment studies.
“Rosacea is a chronic dermatologic condition that affects 16 million Americans,” one of the study authors, Sarah A. Ibrahim, told this news organization after the annual conference of the American Society for Laser Medicine and Surgery. “The features of rosacea, such as inflammatory lesions, redness, burning sensations, and swelling, can have a negative impact on the quality of life for many patients. Additionally, patients with rosacea are at an increased risk for other conditions such as autoimmune diseases, like inflammatory bowel disease.”
In an effort led by principal investigator Murad Alam, MD, vice chair of the department of dermatology at Northwestern University, Chicago, Ms. Ibrahim conducted a systematic review to identify all outcomes that have previously been reported in clinical trials of rosacea, as part of the development of the core outcome set established by the Measurement of Priority Outcome Variables in Dermatologic Surgery (IMPROVED) group. “This has not been done before and is an important first step in understanding what outcomes should be measured in every future clinical study of rosacea,” said Ms. Ibrahim, a medical student at Northwestern University, and predoctoral research fellow in Northwestern’s department of dermatology.
The researchers limited their analysis to randomized, controlled trials of rosacea interventions published between 2010 and 2020 and categorized outcomes into domains based on similar themes.
A total of 58 studies were included in the systematic review, of which 7 (12%) evaluated laser-based interventions. The researchers identified 55 unique outcomes that encompassed eight domains: Quality of life, treatment effects, patient perception of health, clinical assessment, acceptance of care, laboratory assessment, physiological skin assessment, and patient satisfaction. Of the eight domains, clinical assessment-related outcomes were measured in all studies. Nontransient erythema was the most commonly reported outcome (43 studies, 78%), followed by inflammatory lesions (36 studies, 65%) and telangiectasia (22 studies, 40%).
Outcomes pertaining to treatment effects such as adverse events were measured in 49 of the 55 studies (89%), while patient-reported outcomes were measured in 21 (38%). Quality of life and patient satisfaction were reported in 18 (33%) and 13 (24%) studies, respectively.
“There were two main take-home messages of our study,” said Ms. Ibrahim, who presented the results at the meeting. “The first is that there is a wide range of outcomes that are reported in clinical trials of rosacea therapies. Second, that there is a need to standardize the outcomes that are reported in clinical trials of rosacea, in order to be able to combine the results from different studies to better understand which interventions for rosacea are most effective.”
She acknowledged certain limitations of the review, including that other trials related to the topic were not included. “Because of the date range and types of studies that we used to narrow down our search, it is possible that additional outcomes were reported in studies that were not included here,” she said.
“This is a very important study because rosacea is a very common condition and one that I have seen more frequently in clinic since the pandemic started,” said Omar Ibrahimi, PhD, MD, a dermatologist with the Connecticut Skin Institute in Stamford, who was asked to comment on the work. “One of the limitations with rosacea studies is that the studies done are often fairly small and the outcome measures are heterogenous. The current study by Ibrahim and coworkers does a wonderful job of highlighting the various outcomes measures used to measure the success of rosacea treatments with energy-based devices.”
This information, he added, “will be very useful for further research studies because it forms the basis for formulating a set of core outcome measures to judge treatment interventions with consensus input from a variety of key opinion leaders. This will prove to be valuable because if we can have a uniform set of outcome measures to judge rosacea treatments with then we will be able to compare the results from different studies better.”
Ms. Ibrahim and colleagues reported having no relevant financial disclosures. Dr. Ibrahimi disclosed that he has been a speaker for both Candela and Cutera and he is currently on the medical advisory board for Cutera.
FROM ASLMS 2021
One treatment with a 1,060-nm diode laser helped reduce unwanted fat
A a small single-center study showed.
Nonsurgical fat reduction was the third-most common nonsurgical aesthetic procedure in the United States in 2018 and includes lasers, high-intensity focused ultrasound, radiofrequency, photobiomodulation therapy, and cryolipolysis, according to 2018 data from the American Society for Aesthetic Plastic Surgery.
“Our study is unique because we used a 1,060-nm diode laser with integrated skin cooling to evaluate the efficacy and safety of its use for the reduction of unwanted fat of the abdomen and flanks,” lead study author Alison S. Kang, MD, told this news organization following the annual conference of the American Society for Laser Medicine and Surgery, where the data were presented. “A 1,060-nm laser works by delivering controlled thermal energy between 42 °C and 47 °C, temperatures at which adipocytes are permanently destroyed,” she explained.
Dr. Kang and Suzanne Kilmer, MD, both of the Laser & Skin Surgery Center of Northern California, Sacramento, enrolled 28 women and 2 men into the study. Each study participant received a single treatment with Venus Bliss, a 1,060-nm diode laser with four laser applicators and a built-in skin-cooling mechanism. Half received treatment of the flanks delivered at up to 1.4 watts per cm2 on each diode for 25 minutes, while the other 15 received treatment of the abdomen with the same energy settings. Photos and ultrasound images were taken at baseline, 6 weeks, and 12 weeks, and the investigators administered a satisfaction questionnaire upon study exit. The primary endpoint was efficacy, defined as the percentage of correctly identified posttreatment photographs by three blinded reviewers (one plastic surgeon and two dermatologists). Secondary endpoints of interest were change in adipose thickness on ultrasound, subject satisfaction, and adverse events.
After losing 1 patient to follow-up, 29 completed the study. Dr. Kang reported that the blinded evaluators could identify the pretreatment image, compared with the posttreatment image in an average of 67% of patients. Between baseline and 12 weeks, the ultrasound images showed an average reduction in the adipose layer of 9% on the abdomen and 7% on the flank, while the average self-reported pain score based on the Wong-Baker FACES Pain Rating Scale was 2 out of 10 among those in the abdomen treatment group and 2.6 out of 10 among those in the flank treatment group.
In addition, 76% of subjects stated they were “satisfied” to “very satisfied” with the treatment, and 79% stated that they would recommend this treatment to a friend. The most common posttreatment responses in both groups were erythema and trace edema, but no serious or permanent adverse events were observed.
Dr. Kang acknowledged certain limitations of the study, including its small sample size. “Only one treatment was performed in our study, so it is unclear if multiple treatments will improve efficacy or if multiple treatments will have no effect on efficacy,” she said.
The work won a “best of session early career-clinical” abstract award from the ASLMS.
The study was funded by Venus Concept, the manufacturer of the Venus Bliss laser. Dr. Kang reported having no relevant financial disclosures. Dr. Kilmer has received grants and honoraria from Venus Concept.
[email protected]
A a small single-center study showed.
Nonsurgical fat reduction was the third-most common nonsurgical aesthetic procedure in the United States in 2018 and includes lasers, high-intensity focused ultrasound, radiofrequency, photobiomodulation therapy, and cryolipolysis, according to 2018 data from the American Society for Aesthetic Plastic Surgery.
“Our study is unique because we used a 1,060-nm diode laser with integrated skin cooling to evaluate the efficacy and safety of its use for the reduction of unwanted fat of the abdomen and flanks,” lead study author Alison S. Kang, MD, told this news organization following the annual conference of the American Society for Laser Medicine and Surgery, where the data were presented. “A 1,060-nm laser works by delivering controlled thermal energy between 42 °C and 47 °C, temperatures at which adipocytes are permanently destroyed,” she explained.
Dr. Kang and Suzanne Kilmer, MD, both of the Laser & Skin Surgery Center of Northern California, Sacramento, enrolled 28 women and 2 men into the study. Each study participant received a single treatment with Venus Bliss, a 1,060-nm diode laser with four laser applicators and a built-in skin-cooling mechanism. Half received treatment of the flanks delivered at up to 1.4 watts per cm2 on each diode for 25 minutes, while the other 15 received treatment of the abdomen with the same energy settings. Photos and ultrasound images were taken at baseline, 6 weeks, and 12 weeks, and the investigators administered a satisfaction questionnaire upon study exit. The primary endpoint was efficacy, defined as the percentage of correctly identified posttreatment photographs by three blinded reviewers (one plastic surgeon and two dermatologists). Secondary endpoints of interest were change in adipose thickness on ultrasound, subject satisfaction, and adverse events.
After losing 1 patient to follow-up, 29 completed the study. Dr. Kang reported that the blinded evaluators could identify the pretreatment image, compared with the posttreatment image in an average of 67% of patients. Between baseline and 12 weeks, the ultrasound images showed an average reduction in the adipose layer of 9% on the abdomen and 7% on the flank, while the average self-reported pain score based on the Wong-Baker FACES Pain Rating Scale was 2 out of 10 among those in the abdomen treatment group and 2.6 out of 10 among those in the flank treatment group.
In addition, 76% of subjects stated they were “satisfied” to “very satisfied” with the treatment, and 79% stated that they would recommend this treatment to a friend. The most common posttreatment responses in both groups were erythema and trace edema, but no serious or permanent adverse events were observed.
Dr. Kang acknowledged certain limitations of the study, including its small sample size. “Only one treatment was performed in our study, so it is unclear if multiple treatments will improve efficacy or if multiple treatments will have no effect on efficacy,” she said.
The work won a “best of session early career-clinical” abstract award from the ASLMS.
The study was funded by Venus Concept, the manufacturer of the Venus Bliss laser. Dr. Kang reported having no relevant financial disclosures. Dr. Kilmer has received grants and honoraria from Venus Concept.
[email protected]
A a small single-center study showed.
Nonsurgical fat reduction was the third-most common nonsurgical aesthetic procedure in the United States in 2018 and includes lasers, high-intensity focused ultrasound, radiofrequency, photobiomodulation therapy, and cryolipolysis, according to 2018 data from the American Society for Aesthetic Plastic Surgery.
“Our study is unique because we used a 1,060-nm diode laser with integrated skin cooling to evaluate the efficacy and safety of its use for the reduction of unwanted fat of the abdomen and flanks,” lead study author Alison S. Kang, MD, told this news organization following the annual conference of the American Society for Laser Medicine and Surgery, where the data were presented. “A 1,060-nm laser works by delivering controlled thermal energy between 42 °C and 47 °C, temperatures at which adipocytes are permanently destroyed,” she explained.
Dr. Kang and Suzanne Kilmer, MD, both of the Laser & Skin Surgery Center of Northern California, Sacramento, enrolled 28 women and 2 men into the study. Each study participant received a single treatment with Venus Bliss, a 1,060-nm diode laser with four laser applicators and a built-in skin-cooling mechanism. Half received treatment of the flanks delivered at up to 1.4 watts per cm2 on each diode for 25 minutes, while the other 15 received treatment of the abdomen with the same energy settings. Photos and ultrasound images were taken at baseline, 6 weeks, and 12 weeks, and the investigators administered a satisfaction questionnaire upon study exit. The primary endpoint was efficacy, defined as the percentage of correctly identified posttreatment photographs by three blinded reviewers (one plastic surgeon and two dermatologists). Secondary endpoints of interest were change in adipose thickness on ultrasound, subject satisfaction, and adverse events.
After losing 1 patient to follow-up, 29 completed the study. Dr. Kang reported that the blinded evaluators could identify the pretreatment image, compared with the posttreatment image in an average of 67% of patients. Between baseline and 12 weeks, the ultrasound images showed an average reduction in the adipose layer of 9% on the abdomen and 7% on the flank, while the average self-reported pain score based on the Wong-Baker FACES Pain Rating Scale was 2 out of 10 among those in the abdomen treatment group and 2.6 out of 10 among those in the flank treatment group.
In addition, 76% of subjects stated they were “satisfied” to “very satisfied” with the treatment, and 79% stated that they would recommend this treatment to a friend. The most common posttreatment responses in both groups were erythema and trace edema, but no serious or permanent adverse events were observed.
Dr. Kang acknowledged certain limitations of the study, including its small sample size. “Only one treatment was performed in our study, so it is unclear if multiple treatments will improve efficacy or if multiple treatments will have no effect on efficacy,” she said.
The work won a “best of session early career-clinical” abstract award from the ASLMS.
The study was funded by Venus Concept, the manufacturer of the Venus Bliss laser. Dr. Kang reported having no relevant financial disclosures. Dr. Kilmer has received grants and honoraria from Venus Concept.
[email protected]
FROM ASLMS 2021
Combined imaging methods found to enhance detection of squamous cell carcinoma
and distinguishing SCC in-situ and actinic keratosis (AK) from invasive SCC, results from a small prospective study demonstrated.
“A solitary scaly papule or plaque could represent an inflammatory or neoplastic process, and when neoplastic, it could be benign, premalignant, malignant in situ, or invasive malignant,” lead study author Abdullah Aleisa, MD, said in an interview during the annual conference of the American Society for Laser Medicine and Surgery. Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT), “have been used to help in the diagnosis of those clinically suspicious lesions, however each device has its own limitation.”
RCM images are horizontal sections of the skin with high cellular resolution but limited to 250 mcm of depth in skin, he said, while OCT images are vertical sections of the skin with low cellular resolution, but image up to 1,000-2,000 mcm of depth in skin.
“Combined RCM-OCT enables high cellular resolution and deep tissue evaluation,” said Dr. Aleisa, a micrographic surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “The value of combined RCM-OCT has been shown in the detection and depth assessment of basal cell carcinoma, but it has never been studied in SCC. Our objective is to combine RCM and OCT simultaneously to detect SCC and assess the depth of invasion.”
Between September and December 2020, Dr. Aleisa and colleagues prospectively imaged 36 lesions suspicious of SCC, SCC in situ, or AK between September 2020 and December 2020. The mean age of the cohort was 68 years and 63% were male. Using a prototype device from Andover, Mass.–based Caliber I.D., the investigators performed handheld RCM-OCT imaging at the center of clinically suspected lesions before biopsy and to previously diagnosed lesions before Mohs micrographic surgery (to check for residual tumor) and correlated RCM-OCT findings with histopathology results. A total of 36 lesions were treated.
Dr. Aleisa reported that most common RCM-OCT feature for invasive SCC was presence of vertical blood vessels (in 89% of lesions), while for SCC in situ/AK, it was acanthosis and parakeratosis without vertical blood vessels (in 84% of lesions). For the detection of invasive SCC, RCM-OCT had a sensitivity of 82%, a specificity of 92%, a negative predictive value of 92%, and a positive predictive value of 82%. For the detection of SCC in situ/AK, RCM-OCT had a sensitivity of 86%, a specificity of 100%, a negative predictive value of 92%, and a positive predictive value of 100%. The OCT depth measurement correlated well with histopathology with a concordance correlation coefficient of r2 = 0.9.
“Using RCM’s high-resolution pictures allowed us to easily spot the vertical ‘buttonhole’ vessels associated with SCC,” Dr. Aleisa said. “However, given the depth limitation of RCM, the distinction between SCC in situ and invasive SCC could not be accomplished using RCM alone. Therefore, having simultaneous OCT live feedback to the RCM images in the combined RCM-OCT device enabled us to assess the depth of those vertical ‘buttonholes’ and distinguish between SCC in situ and invasive SCC.”
He acknowledged certain limitations of the approach, including that it requires approximately 20 minutes per imaging session, there is a steep learning curve for interpreting images, and certain anatomical sites are challenging to image, especially the nose, periocular area, and lip.
The study won a “best of session” emerging technologies abstract award from the ASLMS.
Milind Rajadhyaksha, PhD, of Memorial Sloan Kettering Cancer Center helped to develop the prototype device. Dr. Aleisa reported having no relevant financial disclosures.
and distinguishing SCC in-situ and actinic keratosis (AK) from invasive SCC, results from a small prospective study demonstrated.
“A solitary scaly papule or plaque could represent an inflammatory or neoplastic process, and when neoplastic, it could be benign, premalignant, malignant in situ, or invasive malignant,” lead study author Abdullah Aleisa, MD, said in an interview during the annual conference of the American Society for Laser Medicine and Surgery. Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT), “have been used to help in the diagnosis of those clinically suspicious lesions, however each device has its own limitation.”
RCM images are horizontal sections of the skin with high cellular resolution but limited to 250 mcm of depth in skin, he said, while OCT images are vertical sections of the skin with low cellular resolution, but image up to 1,000-2,000 mcm of depth in skin.
“Combined RCM-OCT enables high cellular resolution and deep tissue evaluation,” said Dr. Aleisa, a micrographic surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “The value of combined RCM-OCT has been shown in the detection and depth assessment of basal cell carcinoma, but it has never been studied in SCC. Our objective is to combine RCM and OCT simultaneously to detect SCC and assess the depth of invasion.”
Between September and December 2020, Dr. Aleisa and colleagues prospectively imaged 36 lesions suspicious of SCC, SCC in situ, or AK between September 2020 and December 2020. The mean age of the cohort was 68 years and 63% were male. Using a prototype device from Andover, Mass.–based Caliber I.D., the investigators performed handheld RCM-OCT imaging at the center of clinically suspected lesions before biopsy and to previously diagnosed lesions before Mohs micrographic surgery (to check for residual tumor) and correlated RCM-OCT findings with histopathology results. A total of 36 lesions were treated.
Dr. Aleisa reported that most common RCM-OCT feature for invasive SCC was presence of vertical blood vessels (in 89% of lesions), while for SCC in situ/AK, it was acanthosis and parakeratosis without vertical blood vessels (in 84% of lesions). For the detection of invasive SCC, RCM-OCT had a sensitivity of 82%, a specificity of 92%, a negative predictive value of 92%, and a positive predictive value of 82%. For the detection of SCC in situ/AK, RCM-OCT had a sensitivity of 86%, a specificity of 100%, a negative predictive value of 92%, and a positive predictive value of 100%. The OCT depth measurement correlated well with histopathology with a concordance correlation coefficient of r2 = 0.9.
“Using RCM’s high-resolution pictures allowed us to easily spot the vertical ‘buttonhole’ vessels associated with SCC,” Dr. Aleisa said. “However, given the depth limitation of RCM, the distinction between SCC in situ and invasive SCC could not be accomplished using RCM alone. Therefore, having simultaneous OCT live feedback to the RCM images in the combined RCM-OCT device enabled us to assess the depth of those vertical ‘buttonholes’ and distinguish between SCC in situ and invasive SCC.”
He acknowledged certain limitations of the approach, including that it requires approximately 20 minutes per imaging session, there is a steep learning curve for interpreting images, and certain anatomical sites are challenging to image, especially the nose, periocular area, and lip.
The study won a “best of session” emerging technologies abstract award from the ASLMS.
Milind Rajadhyaksha, PhD, of Memorial Sloan Kettering Cancer Center helped to develop the prototype device. Dr. Aleisa reported having no relevant financial disclosures.
and distinguishing SCC in-situ and actinic keratosis (AK) from invasive SCC, results from a small prospective study demonstrated.
“A solitary scaly papule or plaque could represent an inflammatory or neoplastic process, and when neoplastic, it could be benign, premalignant, malignant in situ, or invasive malignant,” lead study author Abdullah Aleisa, MD, said in an interview during the annual conference of the American Society for Laser Medicine and Surgery. Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT), “have been used to help in the diagnosis of those clinically suspicious lesions, however each device has its own limitation.”
RCM images are horizontal sections of the skin with high cellular resolution but limited to 250 mcm of depth in skin, he said, while OCT images are vertical sections of the skin with low cellular resolution, but image up to 1,000-2,000 mcm of depth in skin.
“Combined RCM-OCT enables high cellular resolution and deep tissue evaluation,” said Dr. Aleisa, a micrographic surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “The value of combined RCM-OCT has been shown in the detection and depth assessment of basal cell carcinoma, but it has never been studied in SCC. Our objective is to combine RCM and OCT simultaneously to detect SCC and assess the depth of invasion.”
Between September and December 2020, Dr. Aleisa and colleagues prospectively imaged 36 lesions suspicious of SCC, SCC in situ, or AK between September 2020 and December 2020. The mean age of the cohort was 68 years and 63% were male. Using a prototype device from Andover, Mass.–based Caliber I.D., the investigators performed handheld RCM-OCT imaging at the center of clinically suspected lesions before biopsy and to previously diagnosed lesions before Mohs micrographic surgery (to check for residual tumor) and correlated RCM-OCT findings with histopathology results. A total of 36 lesions were treated.
Dr. Aleisa reported that most common RCM-OCT feature for invasive SCC was presence of vertical blood vessels (in 89% of lesions), while for SCC in situ/AK, it was acanthosis and parakeratosis without vertical blood vessels (in 84% of lesions). For the detection of invasive SCC, RCM-OCT had a sensitivity of 82%, a specificity of 92%, a negative predictive value of 92%, and a positive predictive value of 82%. For the detection of SCC in situ/AK, RCM-OCT had a sensitivity of 86%, a specificity of 100%, a negative predictive value of 92%, and a positive predictive value of 100%. The OCT depth measurement correlated well with histopathology with a concordance correlation coefficient of r2 = 0.9.
“Using RCM’s high-resolution pictures allowed us to easily spot the vertical ‘buttonhole’ vessels associated with SCC,” Dr. Aleisa said. “However, given the depth limitation of RCM, the distinction between SCC in situ and invasive SCC could not be accomplished using RCM alone. Therefore, having simultaneous OCT live feedback to the RCM images in the combined RCM-OCT device enabled us to assess the depth of those vertical ‘buttonholes’ and distinguish between SCC in situ and invasive SCC.”
He acknowledged certain limitations of the approach, including that it requires approximately 20 minutes per imaging session, there is a steep learning curve for interpreting images, and certain anatomical sites are challenging to image, especially the nose, periocular area, and lip.
The study won a “best of session” emerging technologies abstract award from the ASLMS.
Milind Rajadhyaksha, PhD, of Memorial Sloan Kettering Cancer Center helped to develop the prototype device. Dr. Aleisa reported having no relevant financial disclosures.
FROM ASLMS 2021