User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
CAR-T in children branching out to solid tumors
Although the only pediatric indication for chimeric antigen receptor T-cell therapy currently approved by the Food and Drug Administration is B-lineage acute lymphoblastic leukemia (ALL) that is refractory to at least two frontline induction attempts or is in second or later relapse, clinical trials of CAR-T therapy for pediatric solid tumors are also currently in progress, said Gregory Yanik, MD, from the CS Mott Children’s Hospital at the University of Michigan, Ann Arbor, at the Transplant & Cellular Therapies Meetings.
In his presentation, Dr. Yanik discussed progress in solid tumor studies as well as some issues involving the current use of CAR-T therapy for ALL.
Solid tumor studies
Malignancies such as sarcomas, brain tumors, and neuroblastomas pose unique challenges, “In contrast to hematologic malignancies, the protein we’re targeting may not be present on the cell surface of all the tumor cells. There are lower-expression profiles, and this is a problem. In fact, many people have postulated that with CAR-T for pediatric solid tumors we’ll have to do repeated cycles, almost like we do with chemotherapy,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
There are currently 14 studies of CAR-T for central nervous system tumors in children, targeting either epidermal growth factor receptor (EGFR) in glioblastoma multiforme and high-grade gliomas, HER2 in a variety of CNS tumors, the GD2 antigen on pontine gliomas, and the checkpoint molecular B7H3 in medulloblastomas and pontine gliomas.
“In sarcomas in kids there are currently 12 trials in progress. Most of the targeting epitopes are targeting either HER2 or GD2. Repetitive CAR-T infusions are being used in several of these trials in sarcomas.
For neuroblastomas there are currently 13 studies in progress, nearly all of which target GD2. Some of the trials include combining CAR-T with immune checkpoint inhibitors or C7R, an engineered cytokine driver designed to prevent T-cell exhaustion.
In addition, several trials of tumor pulsed dendritic cell vaccines are underway for treatment of children with Wilms tumor, Dr. Yanik noted.
Unresolved procedural questions
It’s still early days in CAR-T therapy, and there are several still unanswered questions regarding optimal therapy for and management of patients undergoing CAR-T procedures, Dr. Yanik said.
For example, the optimal time to collect T cells during apheresis is still unclear, he said. Collecting prior to reinduction therapy raises the risk of transducing leukemic cells, while collecting after reinduction may result in inadequate quantity or quality of cells. Regardless of when cells are collected, apheresis should be performed only when the absolute lymphocyte count is above 500/mcL or the CD3 count is above 150/mcL at the time of apheresis.
In the case tisagenlecleucel (Kymriah), his center typically collects 1x109 CD3 cells regardless of age or weight.
The number of CAR T-cells infused also appears to matter, as responses are improved at CAR-T doses above 1.5x106/kg, while risk for higher-grade cytokine release syndrome (CRS) occurs at higher infusion doses.
Blinatumomab or inotuzumab?
Along with CAR-T, two other agents, the bispecific T-cell engager blinatumomab (Blincyto) and the antibody conjugate inotuzumab ozogamicin (Besponsa) are also approved for the treatment of patients with relapsed/refractory B-cell ALL.
Like CAR-T therapy, the primary toxicities associated with blinatumomab are CRS and neurologic adverse events, whereas at inotuzumab is largely associated with hematologic and hepatic toxicities.
The logistics of therapy differ widely, with a 28-day infusion required for blinatumomab, compared with weekly dosing of inotuzumab, and the multiple visits for apheresis and infusion required for CAR-T.
Blinatumomab is approved for both children and adults with relapsed/refractory ALL, but inotuzumab is approved only for adults, and CAR-T with tisagenlecleucel is approved only for children in this indication.
CD-19 expression
There is evidence to suggest that CD19 expression prior to CAR-T has an effect on outcomes, Dr. Yanik said.
“Does blinatumomab pre–CAR-T impact outcome? The answer is probably yes,” he said.
He referred to a study by investigators at the Children’s Hospital of Philadelphia showing that, “if you’re giving blinatumomab prior to CAR-T therapy, you’re potentially reducing the cell-surface expression of CD19 on your leukemic blasts, and now while you’re bringing these patients in for CAR-T therapy, you’re getting a much higher population of dim CD19 expressers, and this is associated with a higher relapse rate and lower remission rate.”
Predicting relapse
Dr. Yanik referred to a study, currently unpublished, which will show that next-generation sequencing (NGS) is more sensitive than flow cytometry for detection of minimal residual disease (MRD), and that MRD analysis of marrow was more sensitive than analysis of peripheral blood.
“Poor outcomes were seen post CAR-T for patients who were in morphologic remission on day 28 or day 100, but had positive MRD. This especially held true if it was next-gen sequencing MRD-positive at day 100, for which relapse rates were over 95%,” he said.
The absence of B-cells is a surrogate marker for the persistence of CAR-T, and conversely, the recovery of CD19-positive B cells may be a predictor for relapse, especially if the B-cell recovery occurs within the first 6 months following CAR-T infusion.
Transplant after CAR-T?
Bone marrow transplant after CAR-T is recommend for patients with high risk of relapse, including those with B-cell recovery within the first 6 months after CAR-T, patients with MRD positivity at days 28 or 100, and patients with mixed lineage leukemia.
“Should we transplant good-risk patients, meaning, if you have NGS-MRD negative patients, is there a role for transplant? You have to look at the risk versus benefit there. These patients may have a cure rate that’s in the 80%-plus range, could we potentially optimize that even more if we consolidate them with an allo[geneic] transplant,” Dr. Yank said.
Move CAR-T up front?
A Children’s Oncology Group study is currently examining whether giving CAR-T therapy to patients with MRD of 0.01% or greater following first consolidation could result in lower tumor burden, fewer relapse, and less CRS with CAR-T.
Dr. Yanik reported that he had no conflicts of interest to disclose.
Although the only pediatric indication for chimeric antigen receptor T-cell therapy currently approved by the Food and Drug Administration is B-lineage acute lymphoblastic leukemia (ALL) that is refractory to at least two frontline induction attempts or is in second or later relapse, clinical trials of CAR-T therapy for pediatric solid tumors are also currently in progress, said Gregory Yanik, MD, from the CS Mott Children’s Hospital at the University of Michigan, Ann Arbor, at the Transplant & Cellular Therapies Meetings.
In his presentation, Dr. Yanik discussed progress in solid tumor studies as well as some issues involving the current use of CAR-T therapy for ALL.
Solid tumor studies
Malignancies such as sarcomas, brain tumors, and neuroblastomas pose unique challenges, “In contrast to hematologic malignancies, the protein we’re targeting may not be present on the cell surface of all the tumor cells. There are lower-expression profiles, and this is a problem. In fact, many people have postulated that with CAR-T for pediatric solid tumors we’ll have to do repeated cycles, almost like we do with chemotherapy,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
There are currently 14 studies of CAR-T for central nervous system tumors in children, targeting either epidermal growth factor receptor (EGFR) in glioblastoma multiforme and high-grade gliomas, HER2 in a variety of CNS tumors, the GD2 antigen on pontine gliomas, and the checkpoint molecular B7H3 in medulloblastomas and pontine gliomas.
“In sarcomas in kids there are currently 12 trials in progress. Most of the targeting epitopes are targeting either HER2 or GD2. Repetitive CAR-T infusions are being used in several of these trials in sarcomas.
For neuroblastomas there are currently 13 studies in progress, nearly all of which target GD2. Some of the trials include combining CAR-T with immune checkpoint inhibitors or C7R, an engineered cytokine driver designed to prevent T-cell exhaustion.
In addition, several trials of tumor pulsed dendritic cell vaccines are underway for treatment of children with Wilms tumor, Dr. Yanik noted.
Unresolved procedural questions
It’s still early days in CAR-T therapy, and there are several still unanswered questions regarding optimal therapy for and management of patients undergoing CAR-T procedures, Dr. Yanik said.
For example, the optimal time to collect T cells during apheresis is still unclear, he said. Collecting prior to reinduction therapy raises the risk of transducing leukemic cells, while collecting after reinduction may result in inadequate quantity or quality of cells. Regardless of when cells are collected, apheresis should be performed only when the absolute lymphocyte count is above 500/mcL or the CD3 count is above 150/mcL at the time of apheresis.
In the case tisagenlecleucel (Kymriah), his center typically collects 1x109 CD3 cells regardless of age or weight.
The number of CAR T-cells infused also appears to matter, as responses are improved at CAR-T doses above 1.5x106/kg, while risk for higher-grade cytokine release syndrome (CRS) occurs at higher infusion doses.
Blinatumomab or inotuzumab?
Along with CAR-T, two other agents, the bispecific T-cell engager blinatumomab (Blincyto) and the antibody conjugate inotuzumab ozogamicin (Besponsa) are also approved for the treatment of patients with relapsed/refractory B-cell ALL.
Like CAR-T therapy, the primary toxicities associated with blinatumomab are CRS and neurologic adverse events, whereas at inotuzumab is largely associated with hematologic and hepatic toxicities.
The logistics of therapy differ widely, with a 28-day infusion required for blinatumomab, compared with weekly dosing of inotuzumab, and the multiple visits for apheresis and infusion required for CAR-T.
Blinatumomab is approved for both children and adults with relapsed/refractory ALL, but inotuzumab is approved only for adults, and CAR-T with tisagenlecleucel is approved only for children in this indication.
CD-19 expression
There is evidence to suggest that CD19 expression prior to CAR-T has an effect on outcomes, Dr. Yanik said.
“Does blinatumomab pre–CAR-T impact outcome? The answer is probably yes,” he said.
He referred to a study by investigators at the Children’s Hospital of Philadelphia showing that, “if you’re giving blinatumomab prior to CAR-T therapy, you’re potentially reducing the cell-surface expression of CD19 on your leukemic blasts, and now while you’re bringing these patients in for CAR-T therapy, you’re getting a much higher population of dim CD19 expressers, and this is associated with a higher relapse rate and lower remission rate.”
Predicting relapse
Dr. Yanik referred to a study, currently unpublished, which will show that next-generation sequencing (NGS) is more sensitive than flow cytometry for detection of minimal residual disease (MRD), and that MRD analysis of marrow was more sensitive than analysis of peripheral blood.
“Poor outcomes were seen post CAR-T for patients who were in morphologic remission on day 28 or day 100, but had positive MRD. This especially held true if it was next-gen sequencing MRD-positive at day 100, for which relapse rates were over 95%,” he said.
The absence of B-cells is a surrogate marker for the persistence of CAR-T, and conversely, the recovery of CD19-positive B cells may be a predictor for relapse, especially if the B-cell recovery occurs within the first 6 months following CAR-T infusion.
Transplant after CAR-T?
Bone marrow transplant after CAR-T is recommend for patients with high risk of relapse, including those with B-cell recovery within the first 6 months after CAR-T, patients with MRD positivity at days 28 or 100, and patients with mixed lineage leukemia.
“Should we transplant good-risk patients, meaning, if you have NGS-MRD negative patients, is there a role for transplant? You have to look at the risk versus benefit there. These patients may have a cure rate that’s in the 80%-plus range, could we potentially optimize that even more if we consolidate them with an allo[geneic] transplant,” Dr. Yank said.
Move CAR-T up front?
A Children’s Oncology Group study is currently examining whether giving CAR-T therapy to patients with MRD of 0.01% or greater following first consolidation could result in lower tumor burden, fewer relapse, and less CRS with CAR-T.
Dr. Yanik reported that he had no conflicts of interest to disclose.
Although the only pediatric indication for chimeric antigen receptor T-cell therapy currently approved by the Food and Drug Administration is B-lineage acute lymphoblastic leukemia (ALL) that is refractory to at least two frontline induction attempts or is in second or later relapse, clinical trials of CAR-T therapy for pediatric solid tumors are also currently in progress, said Gregory Yanik, MD, from the CS Mott Children’s Hospital at the University of Michigan, Ann Arbor, at the Transplant & Cellular Therapies Meetings.
In his presentation, Dr. Yanik discussed progress in solid tumor studies as well as some issues involving the current use of CAR-T therapy for ALL.
Solid tumor studies
Malignancies such as sarcomas, brain tumors, and neuroblastomas pose unique challenges, “In contrast to hematologic malignancies, the protein we’re targeting may not be present on the cell surface of all the tumor cells. There are lower-expression profiles, and this is a problem. In fact, many people have postulated that with CAR-T for pediatric solid tumors we’ll have to do repeated cycles, almost like we do with chemotherapy,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
There are currently 14 studies of CAR-T for central nervous system tumors in children, targeting either epidermal growth factor receptor (EGFR) in glioblastoma multiforme and high-grade gliomas, HER2 in a variety of CNS tumors, the GD2 antigen on pontine gliomas, and the checkpoint molecular B7H3 in medulloblastomas and pontine gliomas.
“In sarcomas in kids there are currently 12 trials in progress. Most of the targeting epitopes are targeting either HER2 or GD2. Repetitive CAR-T infusions are being used in several of these trials in sarcomas.
For neuroblastomas there are currently 13 studies in progress, nearly all of which target GD2. Some of the trials include combining CAR-T with immune checkpoint inhibitors or C7R, an engineered cytokine driver designed to prevent T-cell exhaustion.
In addition, several trials of tumor pulsed dendritic cell vaccines are underway for treatment of children with Wilms tumor, Dr. Yanik noted.
Unresolved procedural questions
It’s still early days in CAR-T therapy, and there are several still unanswered questions regarding optimal therapy for and management of patients undergoing CAR-T procedures, Dr. Yanik said.
For example, the optimal time to collect T cells during apheresis is still unclear, he said. Collecting prior to reinduction therapy raises the risk of transducing leukemic cells, while collecting after reinduction may result in inadequate quantity or quality of cells. Regardless of when cells are collected, apheresis should be performed only when the absolute lymphocyte count is above 500/mcL or the CD3 count is above 150/mcL at the time of apheresis.
In the case tisagenlecleucel (Kymriah), his center typically collects 1x109 CD3 cells regardless of age or weight.
The number of CAR T-cells infused also appears to matter, as responses are improved at CAR-T doses above 1.5x106/kg, while risk for higher-grade cytokine release syndrome (CRS) occurs at higher infusion doses.
Blinatumomab or inotuzumab?
Along with CAR-T, two other agents, the bispecific T-cell engager blinatumomab (Blincyto) and the antibody conjugate inotuzumab ozogamicin (Besponsa) are also approved for the treatment of patients with relapsed/refractory B-cell ALL.
Like CAR-T therapy, the primary toxicities associated with blinatumomab are CRS and neurologic adverse events, whereas at inotuzumab is largely associated with hematologic and hepatic toxicities.
The logistics of therapy differ widely, with a 28-day infusion required for blinatumomab, compared with weekly dosing of inotuzumab, and the multiple visits for apheresis and infusion required for CAR-T.
Blinatumomab is approved for both children and adults with relapsed/refractory ALL, but inotuzumab is approved only for adults, and CAR-T with tisagenlecleucel is approved only for children in this indication.
CD-19 expression
There is evidence to suggest that CD19 expression prior to CAR-T has an effect on outcomes, Dr. Yanik said.
“Does blinatumomab pre–CAR-T impact outcome? The answer is probably yes,” he said.
He referred to a study by investigators at the Children’s Hospital of Philadelphia showing that, “if you’re giving blinatumomab prior to CAR-T therapy, you’re potentially reducing the cell-surface expression of CD19 on your leukemic blasts, and now while you’re bringing these patients in for CAR-T therapy, you’re getting a much higher population of dim CD19 expressers, and this is associated with a higher relapse rate and lower remission rate.”
Predicting relapse
Dr. Yanik referred to a study, currently unpublished, which will show that next-generation sequencing (NGS) is more sensitive than flow cytometry for detection of minimal residual disease (MRD), and that MRD analysis of marrow was more sensitive than analysis of peripheral blood.
“Poor outcomes were seen post CAR-T for patients who were in morphologic remission on day 28 or day 100, but had positive MRD. This especially held true if it was next-gen sequencing MRD-positive at day 100, for which relapse rates were over 95%,” he said.
The absence of B-cells is a surrogate marker for the persistence of CAR-T, and conversely, the recovery of CD19-positive B cells may be a predictor for relapse, especially if the B-cell recovery occurs within the first 6 months following CAR-T infusion.
Transplant after CAR-T?
Bone marrow transplant after CAR-T is recommend for patients with high risk of relapse, including those with B-cell recovery within the first 6 months after CAR-T, patients with MRD positivity at days 28 or 100, and patients with mixed lineage leukemia.
“Should we transplant good-risk patients, meaning, if you have NGS-MRD negative patients, is there a role for transplant? You have to look at the risk versus benefit there. These patients may have a cure rate that’s in the 80%-plus range, could we potentially optimize that even more if we consolidate them with an allo[geneic] transplant,” Dr. Yank said.
Move CAR-T up front?
A Children’s Oncology Group study is currently examining whether giving CAR-T therapy to patients with MRD of 0.01% or greater following first consolidation could result in lower tumor burden, fewer relapse, and less CRS with CAR-T.
Dr. Yanik reported that he had no conflicts of interest to disclose.
FROM TCT 2021
NfL levels linked to worse disability in real-world MS
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS 2021
Vitamin D deficiency linked to early cognitive impairment in MS
according to new research that adds to the known adverse relationship between low vitamin D and MS.
“We confirmed that low vitamin D may affect not only early disability but also cognition in newly MS diagnosed patients,” said lead author Eleonora Virgilio, MD, of the MS Center, neurology unit, at the University of Eastern Piedmont, Novara, Italy.
“The possible effects of vitamin D on both cognition (in particular, information processing speed) and early disability in newly diagnosed MS patients needs to be further investigated because this association might represent a marker of future disability, supporting the need for prompt supplementation,” she said.
The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Low vitamin D and MS
Previous studies have linked insufficient serum vitamin D with everything from the development of MS to activity and disease progression, but less has been reported on a specific link to the impairment of cognitive function, an important complication of MS.
“Cognitive impairment, and, in particular, slowed information processing speed, is very frequent in the MS population from the early stages of disease, and frequently underestimated,” Dr. Virgilio noted. “It has yet to be completely elucidated what the exact underlying mechanisms are.”
To evaluate the relationship, Dr. Virgilio and colleagues enrolled 60 patients in Italy with MS who were newly diagnosed and had serum vitamin D levels collected upon diagnosis. The participants were also tested at diagnosis with the Symbol Digit Modalities Test (SDMT) for information processing speed, which is a hallmark of the cognitive impairment that can occur in MS and is typically the first cognitive domain to show effects of the disease.
Among the patients, 40 were female and the mean age at diagnosis was 39.5 years; 90% had relapsing remitting MS at baseline and 10% had progressive MS. Their median Expanded Disability Status Scale score at diagnosis was 1.5.
At baseline, as many as 85% of the participants (51) had low serum vitamin D levels, defined as below 30 ng/mL, which Dr. Virgilio noted is consistent with other rates reported among people with MS in the Lombardy region of Italy, where the study was conducted.
The patients had a mean vitamin D level of 21.17 ng/mL (± 10.02), with 51.7% considered to have a deficiency (less than 20 ng/mL) and 33.3% with an insufficiency (20-30 ng/mL).
Of the patients, 16 (27%) had cognitive impairment, defined as a z score of 1.5 or less. Their mean raw SDMT score was 46.50 (± 14.73) and mean z score was –0.62 (± 1.29).
Importantly, those with cognitive impairment were significantly more likely to have severe hypovitaminosis D, compared with those with sufficient vitamin D levels, none of whom showed cognitive impairment (P = .02).
Furthermore, vitamin D levels positively correlated with SDMT raw values (P = .001) and z score (P = .008).
Over a mean follow-up of 2 years, a significant correlation was observed between serum vitamin D levels at diagnosis and early disability on the MS severity score (MSSS; P = .02) and a weak correlation with age-related MSSS (ARMSS; P = .08) at the last clinical follow-up.
Dr. Virgilio noted that factors including disease treatment effects or other factors could have played a role in the weaker results. “It is possible that the linear correlation we found was not as strong as expected [because of] an effect of treatment with disease-modifying therapies or vitamin D supplementation, or because of the short follow-up available at the moment for our population – only for a mean period of 2 years after MS diagnosis.”
The mechanisms for vitamin D deficiency in the MS population are likely multifactorial, with genetic as well as environmental links, she noted.
“The immunomodulatory effects of vitamin D are well known,” Dr. Virgilio said.
“Vitamin D was already linked to cognitive function in other neurodegenerative diseases, [including] Alzheimer’s disease, but more importantly, also in other autoimmune diseases, such as systemic lupus erythematosus,” she explained.
Vitamin D also linked to long-term cognitive function
The study adds to recent research showing longer-term effects of vitamin D deficiency and cognitive impairment in MS: In the longitudinal BENEFIT trial published in 2020, researchers following 278 patients with MS over the course of 11 years found that a 50 ng/L higher mean vitamin D level in the first 2 years of the study was associated with a 65% lower odds of a poor performance on Paced Auditory Serial Addition Test scores at the 11-year follow-up.
That study also looked at neurofilament light chain concentrations, which are associated with MS disease activity, and found they were 20% lower among those with higher vitamin D at baseline. Smokers also had lower cognitive scores.
“Lower vitamin D and smoking after clinical onset predicted worse long-term cognitive function and neuronal integrity in patients with MS,” the authors concluded.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new research that adds to the known adverse relationship between low vitamin D and MS.
“We confirmed that low vitamin D may affect not only early disability but also cognition in newly MS diagnosed patients,” said lead author Eleonora Virgilio, MD, of the MS Center, neurology unit, at the University of Eastern Piedmont, Novara, Italy.
“The possible effects of vitamin D on both cognition (in particular, information processing speed) and early disability in newly diagnosed MS patients needs to be further investigated because this association might represent a marker of future disability, supporting the need for prompt supplementation,” she said.
The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Low vitamin D and MS
Previous studies have linked insufficient serum vitamin D with everything from the development of MS to activity and disease progression, but less has been reported on a specific link to the impairment of cognitive function, an important complication of MS.
“Cognitive impairment, and, in particular, slowed information processing speed, is very frequent in the MS population from the early stages of disease, and frequently underestimated,” Dr. Virgilio noted. “It has yet to be completely elucidated what the exact underlying mechanisms are.”
To evaluate the relationship, Dr. Virgilio and colleagues enrolled 60 patients in Italy with MS who were newly diagnosed and had serum vitamin D levels collected upon diagnosis. The participants were also tested at diagnosis with the Symbol Digit Modalities Test (SDMT) for information processing speed, which is a hallmark of the cognitive impairment that can occur in MS and is typically the first cognitive domain to show effects of the disease.
Among the patients, 40 were female and the mean age at diagnosis was 39.5 years; 90% had relapsing remitting MS at baseline and 10% had progressive MS. Their median Expanded Disability Status Scale score at diagnosis was 1.5.
At baseline, as many as 85% of the participants (51) had low serum vitamin D levels, defined as below 30 ng/mL, which Dr. Virgilio noted is consistent with other rates reported among people with MS in the Lombardy region of Italy, where the study was conducted.
The patients had a mean vitamin D level of 21.17 ng/mL (± 10.02), with 51.7% considered to have a deficiency (less than 20 ng/mL) and 33.3% with an insufficiency (20-30 ng/mL).
Of the patients, 16 (27%) had cognitive impairment, defined as a z score of 1.5 or less. Their mean raw SDMT score was 46.50 (± 14.73) and mean z score was –0.62 (± 1.29).
Importantly, those with cognitive impairment were significantly more likely to have severe hypovitaminosis D, compared with those with sufficient vitamin D levels, none of whom showed cognitive impairment (P = .02).
Furthermore, vitamin D levels positively correlated with SDMT raw values (P = .001) and z score (P = .008).
Over a mean follow-up of 2 years, a significant correlation was observed between serum vitamin D levels at diagnosis and early disability on the MS severity score (MSSS; P = .02) and a weak correlation with age-related MSSS (ARMSS; P = .08) at the last clinical follow-up.
Dr. Virgilio noted that factors including disease treatment effects or other factors could have played a role in the weaker results. “It is possible that the linear correlation we found was not as strong as expected [because of] an effect of treatment with disease-modifying therapies or vitamin D supplementation, or because of the short follow-up available at the moment for our population – only for a mean period of 2 years after MS diagnosis.”
The mechanisms for vitamin D deficiency in the MS population are likely multifactorial, with genetic as well as environmental links, she noted.
“The immunomodulatory effects of vitamin D are well known,” Dr. Virgilio said.
“Vitamin D was already linked to cognitive function in other neurodegenerative diseases, [including] Alzheimer’s disease, but more importantly, also in other autoimmune diseases, such as systemic lupus erythematosus,” she explained.
Vitamin D also linked to long-term cognitive function
The study adds to recent research showing longer-term effects of vitamin D deficiency and cognitive impairment in MS: In the longitudinal BENEFIT trial published in 2020, researchers following 278 patients with MS over the course of 11 years found that a 50 ng/L higher mean vitamin D level in the first 2 years of the study was associated with a 65% lower odds of a poor performance on Paced Auditory Serial Addition Test scores at the 11-year follow-up.
That study also looked at neurofilament light chain concentrations, which are associated with MS disease activity, and found they were 20% lower among those with higher vitamin D at baseline. Smokers also had lower cognitive scores.
“Lower vitamin D and smoking after clinical onset predicted worse long-term cognitive function and neuronal integrity in patients with MS,” the authors concluded.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new research that adds to the known adverse relationship between low vitamin D and MS.
“We confirmed that low vitamin D may affect not only early disability but also cognition in newly MS diagnosed patients,” said lead author Eleonora Virgilio, MD, of the MS Center, neurology unit, at the University of Eastern Piedmont, Novara, Italy.
“The possible effects of vitamin D on both cognition (in particular, information processing speed) and early disability in newly diagnosed MS patients needs to be further investigated because this association might represent a marker of future disability, supporting the need for prompt supplementation,” she said.
The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Low vitamin D and MS
Previous studies have linked insufficient serum vitamin D with everything from the development of MS to activity and disease progression, but less has been reported on a specific link to the impairment of cognitive function, an important complication of MS.
“Cognitive impairment, and, in particular, slowed information processing speed, is very frequent in the MS population from the early stages of disease, and frequently underestimated,” Dr. Virgilio noted. “It has yet to be completely elucidated what the exact underlying mechanisms are.”
To evaluate the relationship, Dr. Virgilio and colleagues enrolled 60 patients in Italy with MS who were newly diagnosed and had serum vitamin D levels collected upon diagnosis. The participants were also tested at diagnosis with the Symbol Digit Modalities Test (SDMT) for information processing speed, which is a hallmark of the cognitive impairment that can occur in MS and is typically the first cognitive domain to show effects of the disease.
Among the patients, 40 were female and the mean age at diagnosis was 39.5 years; 90% had relapsing remitting MS at baseline and 10% had progressive MS. Their median Expanded Disability Status Scale score at diagnosis was 1.5.
At baseline, as many as 85% of the participants (51) had low serum vitamin D levels, defined as below 30 ng/mL, which Dr. Virgilio noted is consistent with other rates reported among people with MS in the Lombardy region of Italy, where the study was conducted.
The patients had a mean vitamin D level of 21.17 ng/mL (± 10.02), with 51.7% considered to have a deficiency (less than 20 ng/mL) and 33.3% with an insufficiency (20-30 ng/mL).
Of the patients, 16 (27%) had cognitive impairment, defined as a z score of 1.5 or less. Their mean raw SDMT score was 46.50 (± 14.73) and mean z score was –0.62 (± 1.29).
Importantly, those with cognitive impairment were significantly more likely to have severe hypovitaminosis D, compared with those with sufficient vitamin D levels, none of whom showed cognitive impairment (P = .02).
Furthermore, vitamin D levels positively correlated with SDMT raw values (P = .001) and z score (P = .008).
Over a mean follow-up of 2 years, a significant correlation was observed between serum vitamin D levels at diagnosis and early disability on the MS severity score (MSSS; P = .02) and a weak correlation with age-related MSSS (ARMSS; P = .08) at the last clinical follow-up.
Dr. Virgilio noted that factors including disease treatment effects or other factors could have played a role in the weaker results. “It is possible that the linear correlation we found was not as strong as expected [because of] an effect of treatment with disease-modifying therapies or vitamin D supplementation, or because of the short follow-up available at the moment for our population – only for a mean period of 2 years after MS diagnosis.”
The mechanisms for vitamin D deficiency in the MS population are likely multifactorial, with genetic as well as environmental links, she noted.
“The immunomodulatory effects of vitamin D are well known,” Dr. Virgilio said.
“Vitamin D was already linked to cognitive function in other neurodegenerative diseases, [including] Alzheimer’s disease, but more importantly, also in other autoimmune diseases, such as systemic lupus erythematosus,” she explained.
Vitamin D also linked to long-term cognitive function
The study adds to recent research showing longer-term effects of vitamin D deficiency and cognitive impairment in MS: In the longitudinal BENEFIT trial published in 2020, researchers following 278 patients with MS over the course of 11 years found that a 50 ng/L higher mean vitamin D level in the first 2 years of the study was associated with a 65% lower odds of a poor performance on Paced Auditory Serial Addition Test scores at the 11-year follow-up.
That study also looked at neurofilament light chain concentrations, which are associated with MS disease activity, and found they were 20% lower among those with higher vitamin D at baseline. Smokers also had lower cognitive scores.
“Lower vitamin D and smoking after clinical onset predicted worse long-term cognitive function and neuronal integrity in patients with MS,” the authors concluded.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS FORUM 2021
Study clarifies who gets post–COVID-19 interstitial lung disease
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Opioid use common for pain in multiple sclerosis
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS FORUM 2021
Neurologic disorders ubiquitous and rising in the U.S.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
FROM JAMA NEUROLOGY
Fired for good judgment a sign of physicians’ lost respect
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
FDA grants emergency use authorization to Johnson & Johnson COVID-19 vaccine
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
J&J COVID-19 vaccine wins unanimous backing of FDA panel
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.
Identifying Acute Migraine Triggers
To read a digital version of the print supplement, click here.
To gain a full understanding of the challenges faced by neurologists, headache specialists, and other clinicians when managing triggers that prompt migraine headache attacks, consider the case of Lola H, a 35-year-old woman with a history of recurrent headaches that was diagnosed 12 months earlier as migraine without aura. Lola is taking oral sumatriptan, 100 mg, for acute migraine episodes, which she reports experiencing 3 or 4 times a month. She also has up to 3 headaches a week that are less severe. Lola’s insurance carrier limits the number of sumatriptan tablets covered in a month, forcing her use the medication only when she has severe headaches. However, the rationing strategy adds stress to her life that could be contributing to additional headache episodes.
Lola raised this issue during a recent visit with her neurologist, at which time she also presented a lengthy list of things she felt were triggering her attacks: stress, her menstrual cycle, chocolate, the odor of gasoline, and the weather, among other potential causes. Regarding the weather, she reported experiencing headaches—sometimes severe—whenever barometric pressure drops. Lola told her clinician that she believes she has gained control of 2 triggers—chocolate and gasoline fumes—through avoidance. She no longer consumes chocolate and other sweets and relies on family members and friends to fill her automobile fuel tank. However, Lola said she cannot control other triggers and needs her clinician’s help. She insists on leaving the appointment with answers she feels satisfied with. More than anything, she wants to feel secure that she has enough medication to meet her needs. (We will reveal how Lola fared later in this article.)
Heterogeneity of triggers: A daunting challenge
Lola’s circumstances are not uncommon among the nearly 40 million migraine sufferers in the United States.1 For clinicians seeking to better understand how potential triggers cause migraine attacks, it is important to appreciate the fine lines that exist between (1) perceived triggers, (2) their apparent frequency and strength, (3) patients’ efforts to control these factors, and (4) the distinction between real migraine triggers and premonitory symptoms. Moreover, recognizing how each of these 4 issues influence the patient’s experience enables the clinician to optimally manage individuals who experience migraine headache.
A diverse array of potential stimuli
Perhaps the most daunting challenge when trying to uncover what is triggering Lola’s and other migraine patients’ headaches is the realization that triggers and perceived triggers comprise a diverse array of potential stimuli. A meta-analysis of 85 studies conducted over a 60-year period and involving more than 27,000 participants revealed 420 unique headache triggers categorized to 1 of 15 categories.2 Characterizing these triggers as vastly heterogenous is an understatement, considering that they include factors such as raising the arms overhead, sneezing, abstaining from coffee, using a low pillow, weekend sleep habits, use of shower gels, relaxing after stressful events, bus journeys, road stripes, and more.2 The authors of the meta-analysis note that the variability observed likely is because of methodology differences among the studies included in their analysis. For this reason, the authors advised caution in interpreting the results.2
Nevertheless, the investigators concluded that (1) most of those who experience frequent headaches—including migraine sufferers—believe they have at least 1 trigger, and (2) stress is the trigger most frequently cited by patients, followed by sleep and environmental triggers.2
Perceived differences in trigger frequency and strength
Heterogeneity extends beyond the triggers and perceived triggers; incidence and strength also can vary greatly. In a cross-sectional observational study, 300 headache sufferers were asked to rate 33 triggers by frequency and potency.3 Triggers that were perceived to be encountered most days per month were air conditioning and caffeine (both 30 days). Red wine was perceived to be encountered least frequently (0 days). Importantly, interperson variability for frequency of encounter was significant—most ranges for each trigger spanned
15 days or more.3
Triggers that were perceived to increase the chance of a headache were stress (75%), missing a meal, and dehydration (both 60%). Nuts and salty food were least perceived to increase the likelihood of a headache (both 0%). Similar to frequency, perceptions about trigger strengths varied widely, with most ranges spreading by more than 50% for each trigger.3
The authors noted that the heterogeneity seen in frequency of encounters with triggers remained even when 2 patients had matching perceptions about the importance of the trigger in precipitating a headache. The fact that this was observed for nearly every perceived trigger is an important aspect of understanding trigger frequency at the patient-care level.3 Additionally, most headache sufferers do not believe that triggers are simply present or absent. Rather, they have diverse beliefs about the strength of perceived triggers.3 These broad differences among patients make it difficult to predict how an individual headache sufferer will rate triggers.3
The patient’s trigger belief system
Layered onto this challenge is the observation that many migraine sufferers use the so-called trigger belief system to create a safe and controlled environment to stave off headache attacks. Indeed, perception often dictates how headache triggers are experienced, and this adds to the complexity of managing these patients. This is borne out in 2 studies. One, involving more than 200 individuals, revealed that perceived control over and impact of headaches were related to headache severity.4 Another trial involving more than 300 patients seeking treatment for headache showed that participants who were confident they could avoid and better manage their headaches also believed that they could control factors impacting their headaches. Although this led to the use of positive coping strategies, it also triggered more anxiety.5
Trigger avoidance can also lead to reduced quality of life. Consider, for example, physical activity, which is recommended to prevent and manage migraine: As many as 8 in 10 migraine sufferers avoid physical activity because they fear triggering an episode.6 In a recent study, investigators examined the potential correlation between anxiety sensitivity and physical activity avoidance among 100 women with migraine. Participants completed an anonymous survey about migraine and avoiding physical activity. The survey included a tool that measured anxiety sensitivity. Migraine attacks in the previous 30 days were self-reported.6 Higher anxiety scores were linked with more frequent physical activity avoidance. Participants who cited physical concerns were nearly 8 times more likely to avoid vigorous physical activity. Those who mentioned cognitive concerns were more than 5 times more likely to avoid moderate physical activity.6 The authors noted that even though avoiding triggers often is recommended, in some situations it can lead to more migraines because of increased anxiety. For this reason, although avoidance is probably best for certain triggers that have true biologic onset mechanisms, triggers that work through anticipatory anxiety or fearful expectations might best be handled via gradual exposure and desensitization.6
Distinguishing between real triggers and premonitory symptoms
Once clinicians gain an understanding of these factors, they may consider themselves adequately prepared to optimally manage migraine sufferers. Yet, there is one more factor to consider: premonitory symptoms. Separating premonitory symptoms from actual migraine triggers gets the clinician closer to optimal management. Yet, it is not always easy to make the distinction.
Premonitory symptoms typically present 2 to 48 hours (or more) before migraine headache onset. Symptoms include changes in mood and activity, irritability, fatigue, food cravings, repetitive yawning, stiff neck, and photophobia. Because these symptoms can continue even past the premonitory stage, they often can be misinterpreted as actual migraine triggers.7 Premonitory symptoms have been found to accompany 30% to 80% of migraine attacks. Signs that often are confused with actual migraine triggers include neck pain, photophobia, and food cravings.8
Timing of symptoms: Potential clues
It is critical to understand the interval between a symptom and migraine onset, which can help determine whether it is an actual trigger, premonitory symptom, or other manifestation. For this, it helps to review the timeline of a migraine attack. The 4 phases of a migraine attack—premonitory/prodrome, aura, headache, and postdrome—are well known. According to the American Migraine Foundation, the 4 phases are marked by specific symptoms that typically—but not always—occur along a general timeline (Figure 1).9
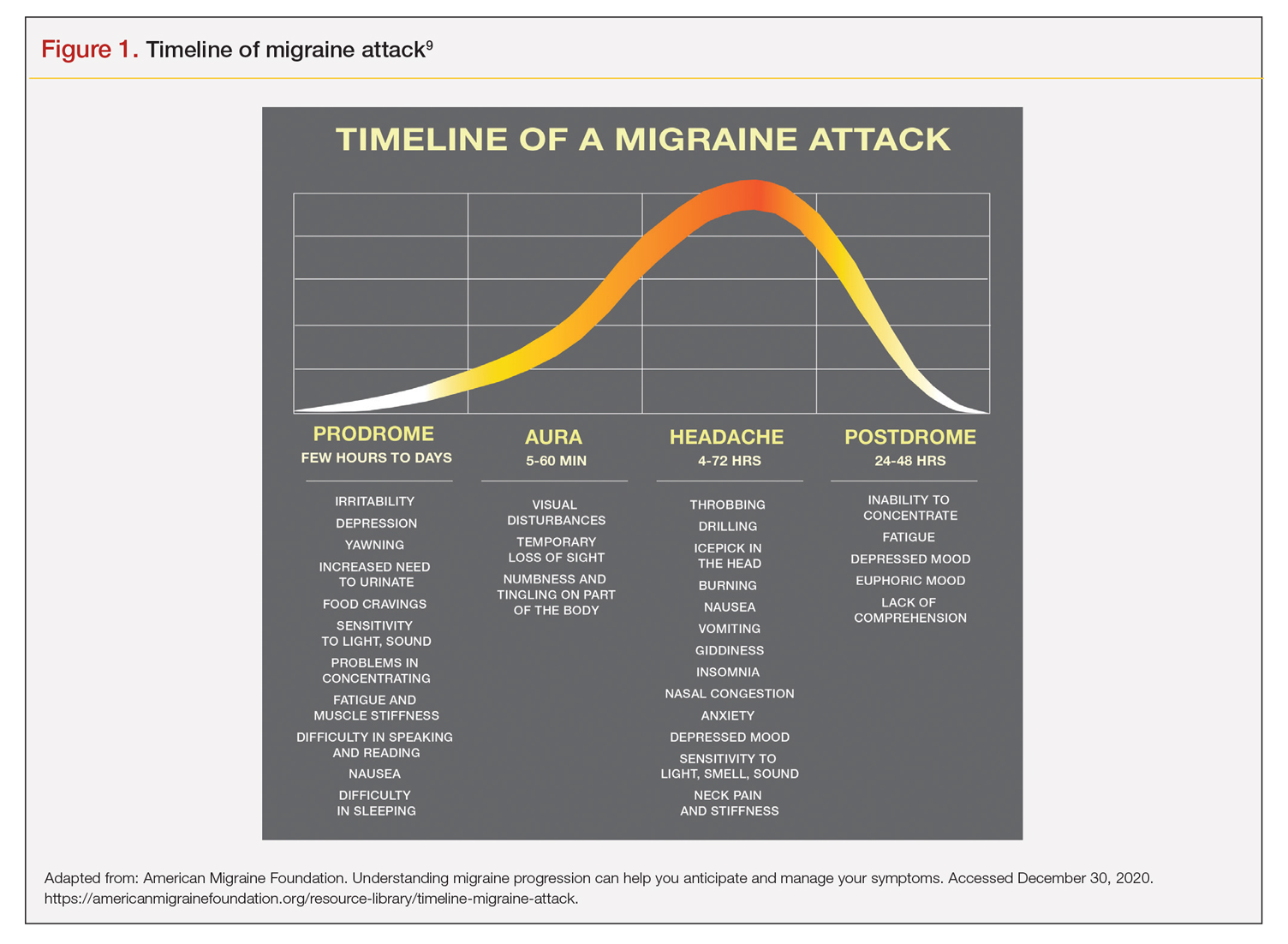
Although this symptom timeline can help guide clinicians and migraine sufferers, it is important to understand that these phases do not always occur linearly, and there often is significant overlap, which can mislead both the clinician and patient.7
Common triggers
Stress: Stress is the most frequently self-reported migraine trigger, and numerous studies show a link between stress and migraine. However, retrospective and prospective diary studies have not been able to confirm a link between stressful days and acute migraine attacks. Moreover, although 1 study demonstrated that migraines were less likely to occur on holidays or days off, relaxation did not play a role. Fatigue was more common premigraine than mental exhaustion or insomnia, leading investigators to surmise it is a premonitory symptom and not a migraine cause.8 Further confounding is the finding that reduced stress from one day to the next is linked with migraine onset the next day, making it a possible predictor of a migraine attack because of the so-called letdown effect.10
Menstruation: Menstruation appears to be the most common migraine trigger in women. A large 3-month study showed an increase of migraine prevalence and persistence up to 96%. Additionally, diary studies demonstrate menstruation as a trigger, and in a population-based trial, more than half of female migraine sufferers reported an increased rate
of migraine. However, only 4% actually had a migraine during menstruation.8,11
Diet: Studies that seek to find a link between specific foods and migraine are open to question because they do not have a placebo component, and it is difficult to tell the difference between premonitory food cravings and actual triggers. Moreover, in most patients food appears to be 1 of several contributors to migraine headache, and not the only factor.8 Fasting is among the most studied and consistent migraine triggers and seems to be more common during longer fasts. Dehydration is not thought to be a cause; rather, it could be because of hypoglycemia.8 A trial involving more than 3000 women revealed that those with migraine had a lower diet quality than their counterparts who did not experience migraine. Other smaller studies show benefit from low-fat vegan and ketogenic diets. Also, patients with irritable bowel syndrome and migraine might benefit from eliminating such foods that cause irritation to improve both migraine frequency and bowel symptoms.8
Weather: A few small studies have sought to evaluate the link between weather and migraines. One involving 77 individuals with migraine showed that slightly more than half were sensitive to at least 1 weather factor (i.e., barometric changes, lightning, temperature, and precipitation). Another involving 238 participants demonstrated a nonstatistically significant trend toward increased frequency of migraine attacks on days with high pressure, low wind speed, and increased sunshine, but they called the correlation questionable. A study of 90 individuals found lightning to be an independent risk factor for migraine.8
Sensory stimuli: Although visual, aural, olfactory, and other sensory stimuli have been shown to cause migraine in susceptible individuals and worsen migraine intensity, it is difficult to determine if these are true triggers or premonitory symptoms. Indeed, the discomfort thresholds of various sensory stimuli are lower in migraine sufferers.8 Odors are the most commonly reported triggers among all sensory stimuli. In 1 study, 7 in every 10 migraine sufferers reported that odors triggered their attack. Common offenders include perfumes, paint, gasoline, bleach, and rancid odors. Smoke and exhaust fumes were also found to be problematic.8
Best evidence on migraine triggers
A large retrospective survey looked at acute migraine trigger factors in 1750 individuals treated in a clinical practice. A detailed headache evaluation was conducted that included neurologic history, structured headache interview, and a neurologic exam.12 More than three-fourths of patients experienced triggers. Approximately 6 in every 10 patients had between 4 and 9 triggers. Nearly one-fourth experienced 1 to 3 triggers.12 As shown in Figure 2, the 5 triggers that occurred occasionally in at least half of patients were stress (80%), hormone (65%), fasting (57%), weather (53%), and sleep disturbance (50%). The most common triggers, occurring very frequently, were hormone (33%) and stress (26%).12
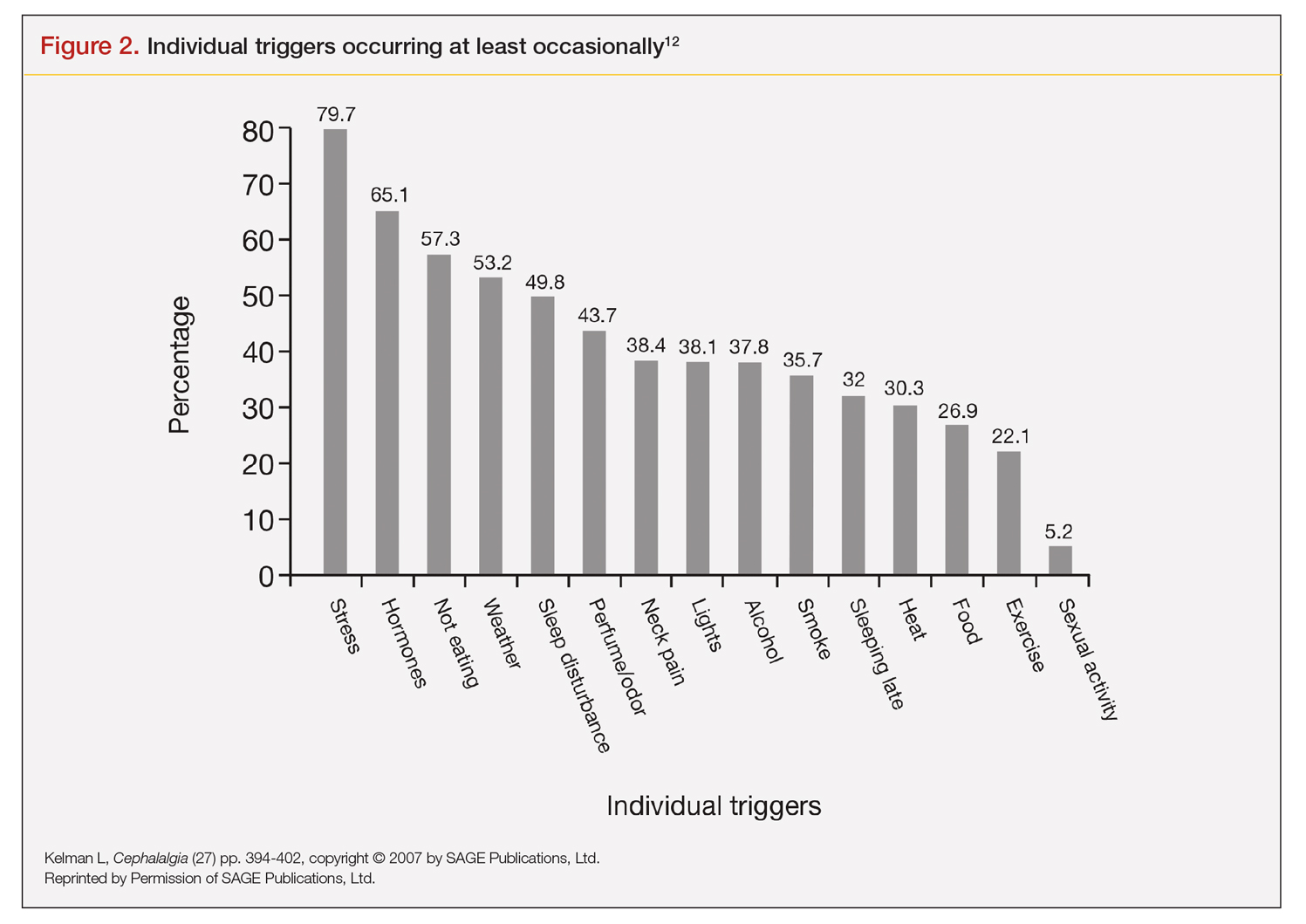
Trials that use randomized and blinded exposure to possible triggers can—at face value—appear most useful. Potent compounds such as glyceryl trinitrite, prostaglandin I2 and E2, calcitonin-gene related peptide, and pituitary adenylate cyclase-activating polypeptide have been shown to be dependable triggers in the experimental setting. However, trial designs that do not closely mimic real-world experience lack usefulness in clinical practice. Moreover, studies of low intensity migraine triggers that are common in the real world—chocolate and aspartame, for instance—have produced mixed results.13 So-called N-of-1 studies—in which a single patient is repeatedly exposed in blinded fashion to a trigger or placebo—might be the best way to uncover actual triggers. However, migraine sufferers are, understandably, not interested in deliberately inducing an attack.13
This leaves results of diary studies as the most reflective of clinical practice. These evaluations limit the chance of bias that retrospective studies could carry and often provide a significant amount of real-world data that can be used to test hypotheses. Moreover, the advent of smartphone technology enables researchers to capture data in real time, which can help distinguish premonitory symptoms from actual triggers.13 A high-quality paper diary study (PAMINA) involving 327 individuals showed strong evidence that a migraine was likely to occur with the following factors: menstruation, muscle tension/neck pain, psychic tension, and sunshine that lasted 3 or more hours per day.14
Diary studies are also able to assess the ability of migraine sufferers to predict a headache. In an evaluation that used an electronic diary design, researchers looked at self-prediction through use of a single question asked daily: “How likely are you to have a headache today?” When participants reported at the beginning of a particular day that headache was “almost certain,” actual headache occurred more than 90% of the time on that day.13
Leveraging best evidence into best practices
The clinician–patient conversation
A clinician’s detective skills are put to the test when evaluating patients with migraine. Avoiding dead-ends and fruitless pursuits can spare providers and patients frustration and help patients avoid needless suffering. Yet, as noted, the list of potential triggers appears endless, duration and frequency are variable, patients often try to control their environment with mixed results, and it is difficult to tell the difference between actual triggers and premonitory symptoms.
Which brings us back to Lola H. She desperately wanted to leave her appointment with confidence that she had enough medication to meet her needs each month. She might have expected to go home with access to more medication, but she did not. Rather, she left her clinician’s office with a plan to reduce the number of monthly migraine episodes she experienced, enabling her to use medication as needed, rather than only when her attacks were severe.
Her clinician confirmed that avoiding gasoline odor would reduce the number of attacks. Eliminating chocolates and sweets might, but it is not known whether chocolate is a trigger. He suggested that she try allowing herself chocolate now and then, which would probably serve as a stress reducer. The net result would likely be no change in number of episodes, while allowing for a small improvement in quality of life.
Perhaps most striking, Lola left the appointment certain that her issue with lower barometric pressure was resolved because her clinician took the time to ask how she knew about the daily barometric pressure levels.
Her smartwatch, she told him. He strongly suggested that she delete the watch’s barometric pressure app, which she did on the spot. At her next appointment, Lola was happy to report that barometric pressure no longer played a significant role in her episodes. Moreover, the other strategies she and her clinician discussed have reduced the number of severe and less severe episodes. She no longer lives with the stress of pill-rationing and looks forward to enjoying a small amount of chocolate ice cream each weekend with her family.
Lola’s case demonstrates that in clinical practice the best way to elicit meaningful responses from migraine sufferers is to ask open-ended questions. Yet, clinicians often fail to do so. In a study evaluating communication strategies between clinicians and 60 long-term migraineurs (average 14 years) who experienced frequent headaches (average 5 per month), 90% of migraine-specific questions asked during an appointment were either close-ended or consisted of a short answer. Postvisit interviews with both clinicians and patients revealed these pairs misaligned on migraine frequency (55% of the time) and impairment (51%). One-third of patients were candidates to receive preventive medication, yet only 20% of them did so. Prevention was not discussed in half of the visits with patients who were candidates to receive preventive therapy but did not get it.10
A panel with extensive experience in migraine reviewed questions from migraine diaries and noted that migraine sufferers find value in capturing wide-ranging information about their condition, whereas clinicians prefer small amounts of data they perceive as relevant to managing migraine.15 It also is reported that the number of questions patients ask is inversely related to the amount of information a clinician gives.16
Recommended strategies
In his review article, Mamura reinforces the concept of open 2-way communication, which involves patients in their care more deeply and reinforces positive habits.8 It is important to be mindful that discussing triggers exclusively can be counterproductive and frustrating for patients, particularly if the conversation is about trigger avoidance. Patients might feel stigmatized by triggers, come away thinking nothing can be done to control them, and become more anxious about impending episodes.8 In fact, a better strategy could be to work with the patient to train their brain to become acclimated to certain triggers or premonitory symptoms, rather than avoiding them.17
Mamura provides a concise summary of 4 best-practice strategies.8 The first 2 should be factored into advice given to patients about migraine triggers and premonitory symptoms; the remaining 2 help frame the treatment approach:
1. Ask patients to keep a headache diary, which eliminates faulty recall and provides a clearer picture of migraine frequency and severity. Include symptoms and other important factors—missed time from work, canceled family activities, nausea and vomiting, etc. Journaling might uncover triggers that can be eliminated or acclimated to via behavior modification. It also can reveal to the patient that certain triggers might not be as useful in predicting migraine as originally thought.
2. Rather than trigger avoidance, suggest that patients concentrate on healthy lifestyle choices, including maintaining healthy weight, getting sufficient sleep, and exercising regularly. The latter has been shown in some studies to work better than preventive medications. Although a behavior change, such as acclimating to certain noises, is not thought to be a healthy lifestyle habit, it ends up being just that if it improves tolerance and reduces migraine episodes.
3. Recognize that many patients have a fear of experiencing a migraine between episodes. If observed in a patient, treat it aggressively. Left unmanaged, this condition could lead to chronic migraine and medication overuse. The right acute medication might be sufficient, but sometimes cognitive-behavioral therapy and biofeedback techniques could be necessary to address behavioral issues that might be interfering with successful treatment.
4. If attacks are frequent and making it difficult to identify distinctive triggers, consider preventive therapy. Patients might resist using preventive medications because they feel eliminating triggers is sufficient, or they might be concerned about medication cost and adverse effects. Eliminating a stressful event alone likely will not help, so it’s important to review with patients—using plain language—how preventive medication treats the underlying cause of migraine and decreases brain excitability, making the patient less prone to attacks.8
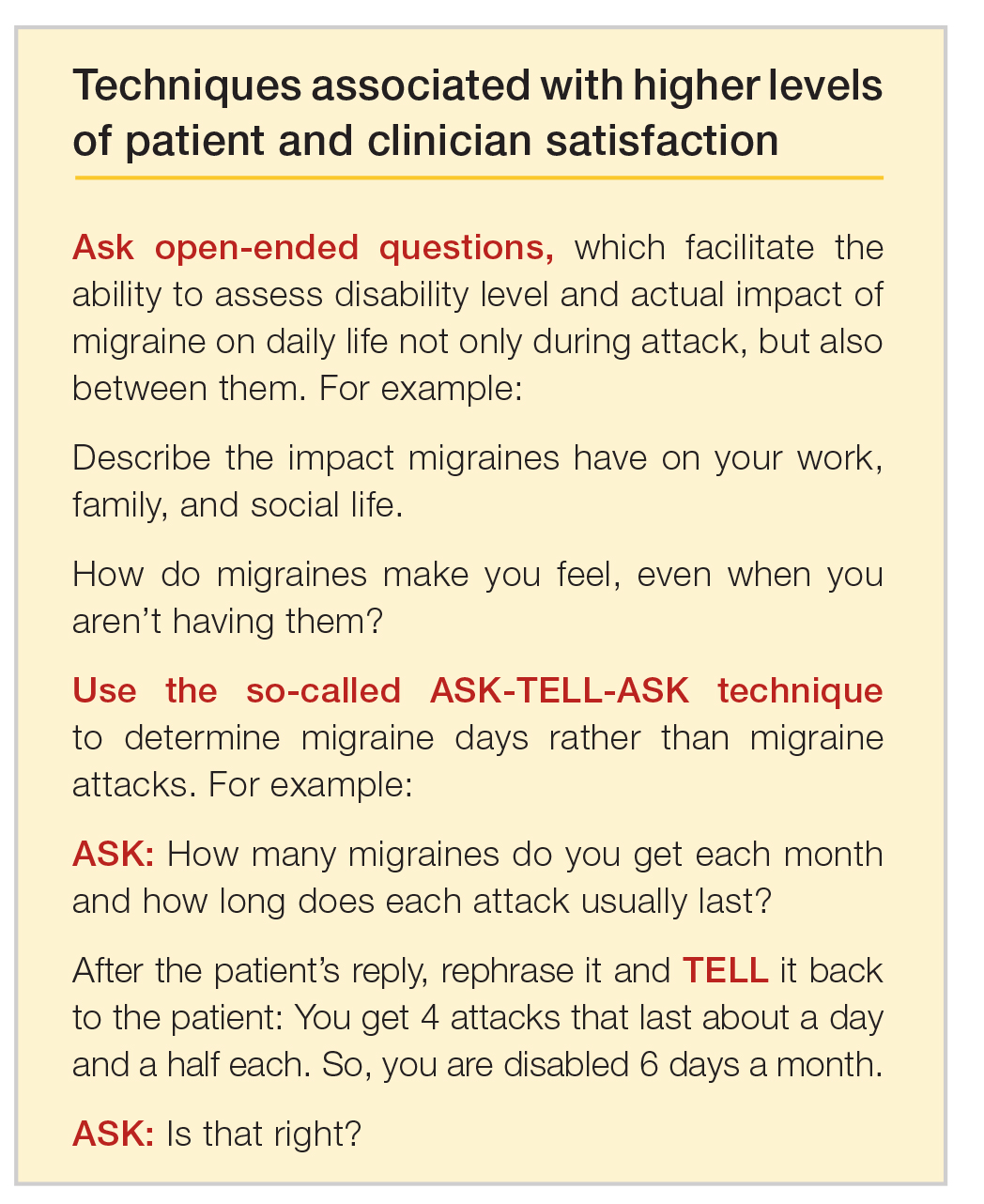
Emerging medications
New treatments offer increased options for the acute and preventive treatment of migraine attacks. New acute pharmacologic treatments include gepants and ditans. The gepants target calcitonin gene-related peptide (CGRP), a potent vasodilator and neurotransmitter. During migraine episodes, serum levels of CGRP increase as the peptide is released, modulating the transmission of nociceptive signals from peripheral trigeminal sensory afferents into the central trigeminal system.19 In clinical trials of acute migraine treatment, the gepants provided pain freedom at 2 hours in approximately 20% of attacks, pain relief at 2 hours in 58%, and freedom from the most bothersome symptom at 2 hours in 36% with significant benefit over placebo.20,21 As these oral formulations became available in 2020, there are not enough data on potential long-term adverse events, particularly cardiovascular effects.18 However, the gepants are not associated with medication overuse headache and there is no contraindication to their use in patients with cardiovascular disease. These agents, currently available for oral administration, also show benefit as preventive agents and parenteral formulations are in development.
Ditans target the serotonin 5HT-1F receptor. Triptans target this receptor as well as the 5HT-1B and 5HT-1D receptors. The 5HT-1B receptor action is responsible for constriction of cerebral and peripheral blood vessels that occurs with triptans. Thus, ditans are also options for treating patients with vascular disease. Lasmiditan, the only compound in this class thus far, yielded pain freedom in 2 hours in 31%, pain relief 2 hours in 64%, and relief of the most bothersome symptom at 2 hours in 41%.22 Lasmiditan crosses the blood-brain barrier. Thus, the most common side effects are dizziness, somnolence, and paresthesias, which are generally mild to moderate in severity. Based on driving simulation studies performed in the absence of migraine, patients should not drive within 8 hours of dosing.
Neuromodulation devices are effective options that avoid potential adverse events of systemic treatments. They include external trigeminal nerve stimulation, noninvasive vagus nerve stimulation, remote electrical neuromodulation, caloric vestibular stimulation, and single-pulse transcranial magnetic stimulation. Other treatments in development for the acute treatment of migraine include improved parenteral delivery systems and combination oral preparations of existing medications.
Monoclonal antibodies that block the CGRP ligand or its receptor were introduced in 2018, with 4 medications currently available. This is the first class of migraine medications specifically designed for migraine prevention. They are administered parenterally (subcutaneously or intravenously) either monthly or quarterly, depending on the medication. The anti-CGRP monoclonal antibodies are indicated for the prevention of episodic and chronic migraine and are eliminated by proteolytic degradation, not involving the liver and reducing the potential for hepatotoxicity and drug-drug interactions.23 A meta-analysis evaluated the subcutaneous formulations studied for chronic migraine, enrolling participants with at least 15 headache days monthly with migraine occurring on at least 8 of those days. In this cohort with a large disease burden, the pooled 50% responder rate 9-12 weeks after administration of the first dose was 37.4% and the 75% responder rate was 12.7%.24 There was also improvement in the number of migraine days, number of days using acute treatment, and total headache hours. The most common side effects were injection site reactions and constipation, with post-marketing reports of hypertension in the compound targeting the CGRP receptor, with or without pre-existing hypertension.25 The safety profile for use during pregnancy was not studied in any of the medications discussed in this section.
Summary
Acute migraine headache is a significant management challenge for neurologists, headache specialists, and other clinicians involved in its treatment. Addressing potential migraine triggers can be a particularly overwhelming task because multiple components and factors play into the disorder. Perceived triggers; their frequency and strength; the effort by patients to control their environment; and the blurred line between real triggers and premonitory symptoms present the clinician with an amalgam of components that must be sorted through, defined, prioritized, and managed. Even though migraine symptoms generally appear along a 4-phase timeline, some symptoms stretch across multiple phases and not all symptoms play out in a linear fashion.
There are specific steps clinicians can take to simplify the process, optimize management, and improve patient outcomes and quality of life. Start by gaining an understanding of common triggers and the best evidence regarding triggers and premonitory symptoms. Use this evidence to modify your approach in practice, if necessary. Specifically:
• Improve the information you gather from patients by asking open-ended questions about their experience during a migraine headache, as well as the period between attacks.
• Rather than having patients tell you how many episodes they experience each month, try to elicit from them how many days per month they feel disabled.
• Ask your patients to keep a headache diary.
• Talk less about trigger avoidance and more about making healthy lifestyle choices.
• Address potential cephalgiaphobia via cognitive-behavioral therapy and biofeedback techniques.
• Consider managing frequent migraine attacks with preventive treatment, which now includes CGRP-targeted medications that are as effective as other agents but have fewer adverse effects and might avoid medication overuse headache.
Dr. Friedman reports being an Advisory Board member for Allergan, Biohaven Pharmaceuticals, Eli Lilly, Impel NeuroPharma, Invex, Lundbeck, Revance Therapeutics, Satsuma Pharmaceuticals, Teva Pharmaceuticals, Theranica, and Zosano Pharma. She has received grant support from Allergan, Eli Lilly, Merck, and Zosano Pharma. She is on the Medical Advisory Board for Spinal CSF Leak Foundation, HealthyWomen, and the Board of Directors of the Southern Headache Society. She currently sits on the Editorial Board of Neurology Reviews and has been a contributing author for MedLink Neurology and Medscape.
1. Migraine Research Foundation. Migraine facts. Accessed January 24, 2021. https://migraineresearchfoundation.org/about-migraine/migraine-facts.
2. Pellegrino A, Davis-Martin R, Houle T, Turner D, Smitherman T. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia. 2018;38(6):1188-1198.
3. Turner D, Houle T. Influences on headache trigger beliefs and perceptions. Cephalalgia. 2018;38(9):1545-1553.
4. Scharff L, Turk D, Marcus D. The relationship of locus of control and psychosocial‐behavioral response in chronic headache. Headache. 1995;35(9):527-533.
5. French D, Holroyd K, Pinell C, Malinoski P, O’Donnell F, Hill K. Perceived self‐efficacy and headache‐related disability. Headache. 2000;40(8):647-656.
6. Farris S, Thomas J, Abrantes A, et al. Anxiety sensitivity and intentional avoidance of physical activity in women with probable migraine. Cephalalgia. 2019;39(11):1465-1469.
7. Goadsby P, Holland P, Martins-Oliveira M, Hoffman J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97(2):553-622.
8. Mamura M. Triggers, protectors, and predictors in episodic migraine. Curr Pain Headache Rep. 2018;22:81.
9. American Migraine Foundation. Understanding migraine progression can help you anticipate and manage your symptoms. Accessed December 30, 2020. https://americanmigrainefoundation.org/resource-library/timeline-migraine-attack.
10. Lipton R, Buse D, Hall C, et al. Reduction in perceived stress as a migraine trigger: Testing the “let-down headache” hypothesis. Neurology. 2014;82:1395-1401.
11. Loder E. Prophylaxis of menstrual migraine with triptans: Problems and possibilities. Neurology. 2002;59(11):1677-1681.
12. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
13. Pavolic J, Buse D, Sollars M, Haut S, Lipton R. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache. 2014;54(10):1670-1679.
14. Wöber C, Brannath W, Schmidt K, et al; PAMINA Study Group. Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia. 2007;27:304-314.
15. Dodick D, Tepper S, Lipton R, et al. Improving medical communication in migraine management: A modified Delphi study to develop a digital migraine tracker. Headache. 2018;58(9):1358-1372.
16. Sheftell F. Communicating the right therapy for the right patient at the right time: Acute therapy. Can J Neurol Sci. 2002;29(suppl 2):S33-S39.
17. Goadsby P, Silberstein S. Migraine triggers: Harnessing the messages of clinical practice. Neurology. 2013;80:434-425.
18. Woo M. Refining a marvel. Nature. 2020;586:s4-s6.
19. Dodick DW. Migraine. Lancet. 2018;10(127):1315-30.
20. Croop R, Goadsby P. J., Stock D. A. et al. Efficacy and safety of rimegepant for the acute treatment of migraine: Evidence from randomized controlled trials. Front Pharmacol. 2020;24 https://doi.org/10.3389/fphar.2019.01577.
21. Yang Y, Chen C, Sun Y et al. Safety and efficacy of ubrogepant for acute treatment of episodic migraine: A meta-analysis of randomized clinical trials. CNS Drugs. 2020;34:463-71.
22. Curto M, Cipolla F, Cisale GY et al. Profiling lasmiditan as a treatment option for migraine. Exp Rev Pharmacother. 2020;21(2):147-53.
23. Raffaelli B, Reuter U. The biology of monoclonal antibodies: Focus on calcitonin gene-related peptide for prophylactic migraine therapy. Neurotherapeutics. 2018;15(2): 324-35.
24. Han L, Lui Y, Xiong H et al. CRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019;9(2):e01215.
25. Saely S, Croteau D, Jawidzik L et al. Hypertension: A new safety risk for patients treated with erenumab. Headache. 2021 (epub ahead of print) https://doi.org/10.1111/head.14051.
To read a digital version of the print supplement, click here.
To gain a full understanding of the challenges faced by neurologists, headache specialists, and other clinicians when managing triggers that prompt migraine headache attacks, consider the case of Lola H, a 35-year-old woman with a history of recurrent headaches that was diagnosed 12 months earlier as migraine without aura. Lola is taking oral sumatriptan, 100 mg, for acute migraine episodes, which she reports experiencing 3 or 4 times a month. She also has up to 3 headaches a week that are less severe. Lola’s insurance carrier limits the number of sumatriptan tablets covered in a month, forcing her use the medication only when she has severe headaches. However, the rationing strategy adds stress to her life that could be contributing to additional headache episodes.
Lola raised this issue during a recent visit with her neurologist, at which time she also presented a lengthy list of things she felt were triggering her attacks: stress, her menstrual cycle, chocolate, the odor of gasoline, and the weather, among other potential causes. Regarding the weather, she reported experiencing headaches—sometimes severe—whenever barometric pressure drops. Lola told her clinician that she believes she has gained control of 2 triggers—chocolate and gasoline fumes—through avoidance. She no longer consumes chocolate and other sweets and relies on family members and friends to fill her automobile fuel tank. However, Lola said she cannot control other triggers and needs her clinician’s help. She insists on leaving the appointment with answers she feels satisfied with. More than anything, she wants to feel secure that she has enough medication to meet her needs. (We will reveal how Lola fared later in this article.)
Heterogeneity of triggers: A daunting challenge
Lola’s circumstances are not uncommon among the nearly 40 million migraine sufferers in the United States.1 For clinicians seeking to better understand how potential triggers cause migraine attacks, it is important to appreciate the fine lines that exist between (1) perceived triggers, (2) their apparent frequency and strength, (3) patients’ efforts to control these factors, and (4) the distinction between real migraine triggers and premonitory symptoms. Moreover, recognizing how each of these 4 issues influence the patient’s experience enables the clinician to optimally manage individuals who experience migraine headache.
A diverse array of potential stimuli
Perhaps the most daunting challenge when trying to uncover what is triggering Lola’s and other migraine patients’ headaches is the realization that triggers and perceived triggers comprise a diverse array of potential stimuli. A meta-analysis of 85 studies conducted over a 60-year period and involving more than 27,000 participants revealed 420 unique headache triggers categorized to 1 of 15 categories.2 Characterizing these triggers as vastly heterogenous is an understatement, considering that they include factors such as raising the arms overhead, sneezing, abstaining from coffee, using a low pillow, weekend sleep habits, use of shower gels, relaxing after stressful events, bus journeys, road stripes, and more.2 The authors of the meta-analysis note that the variability observed likely is because of methodology differences among the studies included in their analysis. For this reason, the authors advised caution in interpreting the results.2
Nevertheless, the investigators concluded that (1) most of those who experience frequent headaches—including migraine sufferers—believe they have at least 1 trigger, and (2) stress is the trigger most frequently cited by patients, followed by sleep and environmental triggers.2
Perceived differences in trigger frequency and strength
Heterogeneity extends beyond the triggers and perceived triggers; incidence and strength also can vary greatly. In a cross-sectional observational study, 300 headache sufferers were asked to rate 33 triggers by frequency and potency.3 Triggers that were perceived to be encountered most days per month were air conditioning and caffeine (both 30 days). Red wine was perceived to be encountered least frequently (0 days). Importantly, interperson variability for frequency of encounter was significant—most ranges for each trigger spanned
15 days or more.3
Triggers that were perceived to increase the chance of a headache were stress (75%), missing a meal, and dehydration (both 60%). Nuts and salty food were least perceived to increase the likelihood of a headache (both 0%). Similar to frequency, perceptions about trigger strengths varied widely, with most ranges spreading by more than 50% for each trigger.3
The authors noted that the heterogeneity seen in frequency of encounters with triggers remained even when 2 patients had matching perceptions about the importance of the trigger in precipitating a headache. The fact that this was observed for nearly every perceived trigger is an important aspect of understanding trigger frequency at the patient-care level.3 Additionally, most headache sufferers do not believe that triggers are simply present or absent. Rather, they have diverse beliefs about the strength of perceived triggers.3 These broad differences among patients make it difficult to predict how an individual headache sufferer will rate triggers.3
The patient’s trigger belief system
Layered onto this challenge is the observation that many migraine sufferers use the so-called trigger belief system to create a safe and controlled environment to stave off headache attacks. Indeed, perception often dictates how headache triggers are experienced, and this adds to the complexity of managing these patients. This is borne out in 2 studies. One, involving more than 200 individuals, revealed that perceived control over and impact of headaches were related to headache severity.4 Another trial involving more than 300 patients seeking treatment for headache showed that participants who were confident they could avoid and better manage their headaches also believed that they could control factors impacting their headaches. Although this led to the use of positive coping strategies, it also triggered more anxiety.5
Trigger avoidance can also lead to reduced quality of life. Consider, for example, physical activity, which is recommended to prevent and manage migraine: As many as 8 in 10 migraine sufferers avoid physical activity because they fear triggering an episode.6 In a recent study, investigators examined the potential correlation between anxiety sensitivity and physical activity avoidance among 100 women with migraine. Participants completed an anonymous survey about migraine and avoiding physical activity. The survey included a tool that measured anxiety sensitivity. Migraine attacks in the previous 30 days were self-reported.6 Higher anxiety scores were linked with more frequent physical activity avoidance. Participants who cited physical concerns were nearly 8 times more likely to avoid vigorous physical activity. Those who mentioned cognitive concerns were more than 5 times more likely to avoid moderate physical activity.6 The authors noted that even though avoiding triggers often is recommended, in some situations it can lead to more migraines because of increased anxiety. For this reason, although avoidance is probably best for certain triggers that have true biologic onset mechanisms, triggers that work through anticipatory anxiety or fearful expectations might best be handled via gradual exposure and desensitization.6
Distinguishing between real triggers and premonitory symptoms
Once clinicians gain an understanding of these factors, they may consider themselves adequately prepared to optimally manage migraine sufferers. Yet, there is one more factor to consider: premonitory symptoms. Separating premonitory symptoms from actual migraine triggers gets the clinician closer to optimal management. Yet, it is not always easy to make the distinction.
Premonitory symptoms typically present 2 to 48 hours (or more) before migraine headache onset. Symptoms include changes in mood and activity, irritability, fatigue, food cravings, repetitive yawning, stiff neck, and photophobia. Because these symptoms can continue even past the premonitory stage, they often can be misinterpreted as actual migraine triggers.7 Premonitory symptoms have been found to accompany 30% to 80% of migraine attacks. Signs that often are confused with actual migraine triggers include neck pain, photophobia, and food cravings.8
Timing of symptoms: Potential clues
It is critical to understand the interval between a symptom and migraine onset, which can help determine whether it is an actual trigger, premonitory symptom, or other manifestation. For this, it helps to review the timeline of a migraine attack. The 4 phases of a migraine attack—premonitory/prodrome, aura, headache, and postdrome—are well known. According to the American Migraine Foundation, the 4 phases are marked by specific symptoms that typically—but not always—occur along a general timeline (Figure 1).9

Although this symptom timeline can help guide clinicians and migraine sufferers, it is important to understand that these phases do not always occur linearly, and there often is significant overlap, which can mislead both the clinician and patient.7
Common triggers
Stress: Stress is the most frequently self-reported migraine trigger, and numerous studies show a link between stress and migraine. However, retrospective and prospective diary studies have not been able to confirm a link between stressful days and acute migraine attacks. Moreover, although 1 study demonstrated that migraines were less likely to occur on holidays or days off, relaxation did not play a role. Fatigue was more common premigraine than mental exhaustion or insomnia, leading investigators to surmise it is a premonitory symptom and not a migraine cause.8 Further confounding is the finding that reduced stress from one day to the next is linked with migraine onset the next day, making it a possible predictor of a migraine attack because of the so-called letdown effect.10
Menstruation: Menstruation appears to be the most common migraine trigger in women. A large 3-month study showed an increase of migraine prevalence and persistence up to 96%. Additionally, diary studies demonstrate menstruation as a trigger, and in a population-based trial, more than half of female migraine sufferers reported an increased rate
of migraine. However, only 4% actually had a migraine during menstruation.8,11
Diet: Studies that seek to find a link between specific foods and migraine are open to question because they do not have a placebo component, and it is difficult to tell the difference between premonitory food cravings and actual triggers. Moreover, in most patients food appears to be 1 of several contributors to migraine headache, and not the only factor.8 Fasting is among the most studied and consistent migraine triggers and seems to be more common during longer fasts. Dehydration is not thought to be a cause; rather, it could be because of hypoglycemia.8 A trial involving more than 3000 women revealed that those with migraine had a lower diet quality than their counterparts who did not experience migraine. Other smaller studies show benefit from low-fat vegan and ketogenic diets. Also, patients with irritable bowel syndrome and migraine might benefit from eliminating such foods that cause irritation to improve both migraine frequency and bowel symptoms.8
Weather: A few small studies have sought to evaluate the link between weather and migraines. One involving 77 individuals with migraine showed that slightly more than half were sensitive to at least 1 weather factor (i.e., barometric changes, lightning, temperature, and precipitation). Another involving 238 participants demonstrated a nonstatistically significant trend toward increased frequency of migraine attacks on days with high pressure, low wind speed, and increased sunshine, but they called the correlation questionable. A study of 90 individuals found lightning to be an independent risk factor for migraine.8
Sensory stimuli: Although visual, aural, olfactory, and other sensory stimuli have been shown to cause migraine in susceptible individuals and worsen migraine intensity, it is difficult to determine if these are true triggers or premonitory symptoms. Indeed, the discomfort thresholds of various sensory stimuli are lower in migraine sufferers.8 Odors are the most commonly reported triggers among all sensory stimuli. In 1 study, 7 in every 10 migraine sufferers reported that odors triggered their attack. Common offenders include perfumes, paint, gasoline, bleach, and rancid odors. Smoke and exhaust fumes were also found to be problematic.8
Best evidence on migraine triggers
A large retrospective survey looked at acute migraine trigger factors in 1750 individuals treated in a clinical practice. A detailed headache evaluation was conducted that included neurologic history, structured headache interview, and a neurologic exam.12 More than three-fourths of patients experienced triggers. Approximately 6 in every 10 patients had between 4 and 9 triggers. Nearly one-fourth experienced 1 to 3 triggers.12 As shown in Figure 2, the 5 triggers that occurred occasionally in at least half of patients were stress (80%), hormone (65%), fasting (57%), weather (53%), and sleep disturbance (50%). The most common triggers, occurring very frequently, were hormone (33%) and stress (26%).12

Trials that use randomized and blinded exposure to possible triggers can—at face value—appear most useful. Potent compounds such as glyceryl trinitrite, prostaglandin I2 and E2, calcitonin-gene related peptide, and pituitary adenylate cyclase-activating polypeptide have been shown to be dependable triggers in the experimental setting. However, trial designs that do not closely mimic real-world experience lack usefulness in clinical practice. Moreover, studies of low intensity migraine triggers that are common in the real world—chocolate and aspartame, for instance—have produced mixed results.13 So-called N-of-1 studies—in which a single patient is repeatedly exposed in blinded fashion to a trigger or placebo—might be the best way to uncover actual triggers. However, migraine sufferers are, understandably, not interested in deliberately inducing an attack.13
This leaves results of diary studies as the most reflective of clinical practice. These evaluations limit the chance of bias that retrospective studies could carry and often provide a significant amount of real-world data that can be used to test hypotheses. Moreover, the advent of smartphone technology enables researchers to capture data in real time, which can help distinguish premonitory symptoms from actual triggers.13 A high-quality paper diary study (PAMINA) involving 327 individuals showed strong evidence that a migraine was likely to occur with the following factors: menstruation, muscle tension/neck pain, psychic tension, and sunshine that lasted 3 or more hours per day.14
Diary studies are also able to assess the ability of migraine sufferers to predict a headache. In an evaluation that used an electronic diary design, researchers looked at self-prediction through use of a single question asked daily: “How likely are you to have a headache today?” When participants reported at the beginning of a particular day that headache was “almost certain,” actual headache occurred more than 90% of the time on that day.13
Leveraging best evidence into best practices
The clinician–patient conversation
A clinician’s detective skills are put to the test when evaluating patients with migraine. Avoiding dead-ends and fruitless pursuits can spare providers and patients frustration and help patients avoid needless suffering. Yet, as noted, the list of potential triggers appears endless, duration and frequency are variable, patients often try to control their environment with mixed results, and it is difficult to tell the difference between actual triggers and premonitory symptoms.
Which brings us back to Lola H. She desperately wanted to leave her appointment with confidence that she had enough medication to meet her needs each month. She might have expected to go home with access to more medication, but she did not. Rather, she left her clinician’s office with a plan to reduce the number of monthly migraine episodes she experienced, enabling her to use medication as needed, rather than only when her attacks were severe.
Her clinician confirmed that avoiding gasoline odor would reduce the number of attacks. Eliminating chocolates and sweets might, but it is not known whether chocolate is a trigger. He suggested that she try allowing herself chocolate now and then, which would probably serve as a stress reducer. The net result would likely be no change in number of episodes, while allowing for a small improvement in quality of life.
Perhaps most striking, Lola left the appointment certain that her issue with lower barometric pressure was resolved because her clinician took the time to ask how she knew about the daily barometric pressure levels.
Her smartwatch, she told him. He strongly suggested that she delete the watch’s barometric pressure app, which she did on the spot. At her next appointment, Lola was happy to report that barometric pressure no longer played a significant role in her episodes. Moreover, the other strategies she and her clinician discussed have reduced the number of severe and less severe episodes. She no longer lives with the stress of pill-rationing and looks forward to enjoying a small amount of chocolate ice cream each weekend with her family.
Lola’s case demonstrates that in clinical practice the best way to elicit meaningful responses from migraine sufferers is to ask open-ended questions. Yet, clinicians often fail to do so. In a study evaluating communication strategies between clinicians and 60 long-term migraineurs (average 14 years) who experienced frequent headaches (average 5 per month), 90% of migraine-specific questions asked during an appointment were either close-ended or consisted of a short answer. Postvisit interviews with both clinicians and patients revealed these pairs misaligned on migraine frequency (55% of the time) and impairment (51%). One-third of patients were candidates to receive preventive medication, yet only 20% of them did so. Prevention was not discussed in half of the visits with patients who were candidates to receive preventive therapy but did not get it.10
A panel with extensive experience in migraine reviewed questions from migraine diaries and noted that migraine sufferers find value in capturing wide-ranging information about their condition, whereas clinicians prefer small amounts of data they perceive as relevant to managing migraine.15 It also is reported that the number of questions patients ask is inversely related to the amount of information a clinician gives.16
Recommended strategies
In his review article, Mamura reinforces the concept of open 2-way communication, which involves patients in their care more deeply and reinforces positive habits.8 It is important to be mindful that discussing triggers exclusively can be counterproductive and frustrating for patients, particularly if the conversation is about trigger avoidance. Patients might feel stigmatized by triggers, come away thinking nothing can be done to control them, and become more anxious about impending episodes.8 In fact, a better strategy could be to work with the patient to train their brain to become acclimated to certain triggers or premonitory symptoms, rather than avoiding them.17
Mamura provides a concise summary of 4 best-practice strategies.8 The first 2 should be factored into advice given to patients about migraine triggers and premonitory symptoms; the remaining 2 help frame the treatment approach:
1. Ask patients to keep a headache diary, which eliminates faulty recall and provides a clearer picture of migraine frequency and severity. Include symptoms and other important factors—missed time from work, canceled family activities, nausea and vomiting, etc. Journaling might uncover triggers that can be eliminated or acclimated to via behavior modification. It also can reveal to the patient that certain triggers might not be as useful in predicting migraine as originally thought.
2. Rather than trigger avoidance, suggest that patients concentrate on healthy lifestyle choices, including maintaining healthy weight, getting sufficient sleep, and exercising regularly. The latter has been shown in some studies to work better than preventive medications. Although a behavior change, such as acclimating to certain noises, is not thought to be a healthy lifestyle habit, it ends up being just that if it improves tolerance and reduces migraine episodes.
3. Recognize that many patients have a fear of experiencing a migraine between episodes. If observed in a patient, treat it aggressively. Left unmanaged, this condition could lead to chronic migraine and medication overuse. The right acute medication might be sufficient, but sometimes cognitive-behavioral therapy and biofeedback techniques could be necessary to address behavioral issues that might be interfering with successful treatment.
4. If attacks are frequent and making it difficult to identify distinctive triggers, consider preventive therapy. Patients might resist using preventive medications because they feel eliminating triggers is sufficient, or they might be concerned about medication cost and adverse effects. Eliminating a stressful event alone likely will not help, so it’s important to review with patients—using plain language—how preventive medication treats the underlying cause of migraine and decreases brain excitability, making the patient less prone to attacks.8

Emerging medications
New treatments offer increased options for the acute and preventive treatment of migraine attacks. New acute pharmacologic treatments include gepants and ditans. The gepants target calcitonin gene-related peptide (CGRP), a potent vasodilator and neurotransmitter. During migraine episodes, serum levels of CGRP increase as the peptide is released, modulating the transmission of nociceptive signals from peripheral trigeminal sensory afferents into the central trigeminal system.19 In clinical trials of acute migraine treatment, the gepants provided pain freedom at 2 hours in approximately 20% of attacks, pain relief at 2 hours in 58%, and freedom from the most bothersome symptom at 2 hours in 36% with significant benefit over placebo.20,21 As these oral formulations became available in 2020, there are not enough data on potential long-term adverse events, particularly cardiovascular effects.18 However, the gepants are not associated with medication overuse headache and there is no contraindication to their use in patients with cardiovascular disease. These agents, currently available for oral administration, also show benefit as preventive agents and parenteral formulations are in development.
Ditans target the serotonin 5HT-1F receptor. Triptans target this receptor as well as the 5HT-1B and 5HT-1D receptors. The 5HT-1B receptor action is responsible for constriction of cerebral and peripheral blood vessels that occurs with triptans. Thus, ditans are also options for treating patients with vascular disease. Lasmiditan, the only compound in this class thus far, yielded pain freedom in 2 hours in 31%, pain relief 2 hours in 64%, and relief of the most bothersome symptom at 2 hours in 41%.22 Lasmiditan crosses the blood-brain barrier. Thus, the most common side effects are dizziness, somnolence, and paresthesias, which are generally mild to moderate in severity. Based on driving simulation studies performed in the absence of migraine, patients should not drive within 8 hours of dosing.
Neuromodulation devices are effective options that avoid potential adverse events of systemic treatments. They include external trigeminal nerve stimulation, noninvasive vagus nerve stimulation, remote electrical neuromodulation, caloric vestibular stimulation, and single-pulse transcranial magnetic stimulation. Other treatments in development for the acute treatment of migraine include improved parenteral delivery systems and combination oral preparations of existing medications.
Monoclonal antibodies that block the CGRP ligand or its receptor were introduced in 2018, with 4 medications currently available. This is the first class of migraine medications specifically designed for migraine prevention. They are administered parenterally (subcutaneously or intravenously) either monthly or quarterly, depending on the medication. The anti-CGRP monoclonal antibodies are indicated for the prevention of episodic and chronic migraine and are eliminated by proteolytic degradation, not involving the liver and reducing the potential for hepatotoxicity and drug-drug interactions.23 A meta-analysis evaluated the subcutaneous formulations studied for chronic migraine, enrolling participants with at least 15 headache days monthly with migraine occurring on at least 8 of those days. In this cohort with a large disease burden, the pooled 50% responder rate 9-12 weeks after administration of the first dose was 37.4% and the 75% responder rate was 12.7%.24 There was also improvement in the number of migraine days, number of days using acute treatment, and total headache hours. The most common side effects were injection site reactions and constipation, with post-marketing reports of hypertension in the compound targeting the CGRP receptor, with or without pre-existing hypertension.25 The safety profile for use during pregnancy was not studied in any of the medications discussed in this section.
Summary
Acute migraine headache is a significant management challenge for neurologists, headache specialists, and other clinicians involved in its treatment. Addressing potential migraine triggers can be a particularly overwhelming task because multiple components and factors play into the disorder. Perceived triggers; their frequency and strength; the effort by patients to control their environment; and the blurred line between real triggers and premonitory symptoms present the clinician with an amalgam of components that must be sorted through, defined, prioritized, and managed. Even though migraine symptoms generally appear along a 4-phase timeline, some symptoms stretch across multiple phases and not all symptoms play out in a linear fashion.
There are specific steps clinicians can take to simplify the process, optimize management, and improve patient outcomes and quality of life. Start by gaining an understanding of common triggers and the best evidence regarding triggers and premonitory symptoms. Use this evidence to modify your approach in practice, if necessary. Specifically:
• Improve the information you gather from patients by asking open-ended questions about their experience during a migraine headache, as well as the period between attacks.
• Rather than having patients tell you how many episodes they experience each month, try to elicit from them how many days per month they feel disabled.
• Ask your patients to keep a headache diary.
• Talk less about trigger avoidance and more about making healthy lifestyle choices.
• Address potential cephalgiaphobia via cognitive-behavioral therapy and biofeedback techniques.
• Consider managing frequent migraine attacks with preventive treatment, which now includes CGRP-targeted medications that are as effective as other agents but have fewer adverse effects and might avoid medication overuse headache.
Dr. Friedman reports being an Advisory Board member for Allergan, Biohaven Pharmaceuticals, Eli Lilly, Impel NeuroPharma, Invex, Lundbeck, Revance Therapeutics, Satsuma Pharmaceuticals, Teva Pharmaceuticals, Theranica, and Zosano Pharma. She has received grant support from Allergan, Eli Lilly, Merck, and Zosano Pharma. She is on the Medical Advisory Board for Spinal CSF Leak Foundation, HealthyWomen, and the Board of Directors of the Southern Headache Society. She currently sits on the Editorial Board of Neurology Reviews and has been a contributing author for MedLink Neurology and Medscape.
To read a digital version of the print supplement, click here.
To gain a full understanding of the challenges faced by neurologists, headache specialists, and other clinicians when managing triggers that prompt migraine headache attacks, consider the case of Lola H, a 35-year-old woman with a history of recurrent headaches that was diagnosed 12 months earlier as migraine without aura. Lola is taking oral sumatriptan, 100 mg, for acute migraine episodes, which she reports experiencing 3 or 4 times a month. She also has up to 3 headaches a week that are less severe. Lola’s insurance carrier limits the number of sumatriptan tablets covered in a month, forcing her use the medication only when she has severe headaches. However, the rationing strategy adds stress to her life that could be contributing to additional headache episodes.
Lola raised this issue during a recent visit with her neurologist, at which time she also presented a lengthy list of things she felt were triggering her attacks: stress, her menstrual cycle, chocolate, the odor of gasoline, and the weather, among other potential causes. Regarding the weather, she reported experiencing headaches—sometimes severe—whenever barometric pressure drops. Lola told her clinician that she believes she has gained control of 2 triggers—chocolate and gasoline fumes—through avoidance. She no longer consumes chocolate and other sweets and relies on family members and friends to fill her automobile fuel tank. However, Lola said she cannot control other triggers and needs her clinician’s help. She insists on leaving the appointment with answers she feels satisfied with. More than anything, she wants to feel secure that she has enough medication to meet her needs. (We will reveal how Lola fared later in this article.)
Heterogeneity of triggers: A daunting challenge
Lola’s circumstances are not uncommon among the nearly 40 million migraine sufferers in the United States.1 For clinicians seeking to better understand how potential triggers cause migraine attacks, it is important to appreciate the fine lines that exist between (1) perceived triggers, (2) their apparent frequency and strength, (3) patients’ efforts to control these factors, and (4) the distinction between real migraine triggers and premonitory symptoms. Moreover, recognizing how each of these 4 issues influence the patient’s experience enables the clinician to optimally manage individuals who experience migraine headache.
A diverse array of potential stimuli
Perhaps the most daunting challenge when trying to uncover what is triggering Lola’s and other migraine patients’ headaches is the realization that triggers and perceived triggers comprise a diverse array of potential stimuli. A meta-analysis of 85 studies conducted over a 60-year period and involving more than 27,000 participants revealed 420 unique headache triggers categorized to 1 of 15 categories.2 Characterizing these triggers as vastly heterogenous is an understatement, considering that they include factors such as raising the arms overhead, sneezing, abstaining from coffee, using a low pillow, weekend sleep habits, use of shower gels, relaxing after stressful events, bus journeys, road stripes, and more.2 The authors of the meta-analysis note that the variability observed likely is because of methodology differences among the studies included in their analysis. For this reason, the authors advised caution in interpreting the results.2
Nevertheless, the investigators concluded that (1) most of those who experience frequent headaches—including migraine sufferers—believe they have at least 1 trigger, and (2) stress is the trigger most frequently cited by patients, followed by sleep and environmental triggers.2
Perceived differences in trigger frequency and strength
Heterogeneity extends beyond the triggers and perceived triggers; incidence and strength also can vary greatly. In a cross-sectional observational study, 300 headache sufferers were asked to rate 33 triggers by frequency and potency.3 Triggers that were perceived to be encountered most days per month were air conditioning and caffeine (both 30 days). Red wine was perceived to be encountered least frequently (0 days). Importantly, interperson variability for frequency of encounter was significant—most ranges for each trigger spanned
15 days or more.3
Triggers that were perceived to increase the chance of a headache were stress (75%), missing a meal, and dehydration (both 60%). Nuts and salty food were least perceived to increase the likelihood of a headache (both 0%). Similar to frequency, perceptions about trigger strengths varied widely, with most ranges spreading by more than 50% for each trigger.3
The authors noted that the heterogeneity seen in frequency of encounters with triggers remained even when 2 patients had matching perceptions about the importance of the trigger in precipitating a headache. The fact that this was observed for nearly every perceived trigger is an important aspect of understanding trigger frequency at the patient-care level.3 Additionally, most headache sufferers do not believe that triggers are simply present or absent. Rather, they have diverse beliefs about the strength of perceived triggers.3 These broad differences among patients make it difficult to predict how an individual headache sufferer will rate triggers.3
The patient’s trigger belief system
Layered onto this challenge is the observation that many migraine sufferers use the so-called trigger belief system to create a safe and controlled environment to stave off headache attacks. Indeed, perception often dictates how headache triggers are experienced, and this adds to the complexity of managing these patients. This is borne out in 2 studies. One, involving more than 200 individuals, revealed that perceived control over and impact of headaches were related to headache severity.4 Another trial involving more than 300 patients seeking treatment for headache showed that participants who were confident they could avoid and better manage their headaches also believed that they could control factors impacting their headaches. Although this led to the use of positive coping strategies, it also triggered more anxiety.5
Trigger avoidance can also lead to reduced quality of life. Consider, for example, physical activity, which is recommended to prevent and manage migraine: As many as 8 in 10 migraine sufferers avoid physical activity because they fear triggering an episode.6 In a recent study, investigators examined the potential correlation between anxiety sensitivity and physical activity avoidance among 100 women with migraine. Participants completed an anonymous survey about migraine and avoiding physical activity. The survey included a tool that measured anxiety sensitivity. Migraine attacks in the previous 30 days were self-reported.6 Higher anxiety scores were linked with more frequent physical activity avoidance. Participants who cited physical concerns were nearly 8 times more likely to avoid vigorous physical activity. Those who mentioned cognitive concerns were more than 5 times more likely to avoid moderate physical activity.6 The authors noted that even though avoiding triggers often is recommended, in some situations it can lead to more migraines because of increased anxiety. For this reason, although avoidance is probably best for certain triggers that have true biologic onset mechanisms, triggers that work through anticipatory anxiety or fearful expectations might best be handled via gradual exposure and desensitization.6
Distinguishing between real triggers and premonitory symptoms
Once clinicians gain an understanding of these factors, they may consider themselves adequately prepared to optimally manage migraine sufferers. Yet, there is one more factor to consider: premonitory symptoms. Separating premonitory symptoms from actual migraine triggers gets the clinician closer to optimal management. Yet, it is not always easy to make the distinction.
Premonitory symptoms typically present 2 to 48 hours (or more) before migraine headache onset. Symptoms include changes in mood and activity, irritability, fatigue, food cravings, repetitive yawning, stiff neck, and photophobia. Because these symptoms can continue even past the premonitory stage, they often can be misinterpreted as actual migraine triggers.7 Premonitory symptoms have been found to accompany 30% to 80% of migraine attacks. Signs that often are confused with actual migraine triggers include neck pain, photophobia, and food cravings.8
Timing of symptoms: Potential clues
It is critical to understand the interval between a symptom and migraine onset, which can help determine whether it is an actual trigger, premonitory symptom, or other manifestation. For this, it helps to review the timeline of a migraine attack. The 4 phases of a migraine attack—premonitory/prodrome, aura, headache, and postdrome—are well known. According to the American Migraine Foundation, the 4 phases are marked by specific symptoms that typically—but not always—occur along a general timeline (Figure 1).9

Although this symptom timeline can help guide clinicians and migraine sufferers, it is important to understand that these phases do not always occur linearly, and there often is significant overlap, which can mislead both the clinician and patient.7
Common triggers
Stress: Stress is the most frequently self-reported migraine trigger, and numerous studies show a link between stress and migraine. However, retrospective and prospective diary studies have not been able to confirm a link between stressful days and acute migraine attacks. Moreover, although 1 study demonstrated that migraines were less likely to occur on holidays or days off, relaxation did not play a role. Fatigue was more common premigraine than mental exhaustion or insomnia, leading investigators to surmise it is a premonitory symptom and not a migraine cause.8 Further confounding is the finding that reduced stress from one day to the next is linked with migraine onset the next day, making it a possible predictor of a migraine attack because of the so-called letdown effect.10
Menstruation: Menstruation appears to be the most common migraine trigger in women. A large 3-month study showed an increase of migraine prevalence and persistence up to 96%. Additionally, diary studies demonstrate menstruation as a trigger, and in a population-based trial, more than half of female migraine sufferers reported an increased rate
of migraine. However, only 4% actually had a migraine during menstruation.8,11
Diet: Studies that seek to find a link between specific foods and migraine are open to question because they do not have a placebo component, and it is difficult to tell the difference between premonitory food cravings and actual triggers. Moreover, in most patients food appears to be 1 of several contributors to migraine headache, and not the only factor.8 Fasting is among the most studied and consistent migraine triggers and seems to be more common during longer fasts. Dehydration is not thought to be a cause; rather, it could be because of hypoglycemia.8 A trial involving more than 3000 women revealed that those with migraine had a lower diet quality than their counterparts who did not experience migraine. Other smaller studies show benefit from low-fat vegan and ketogenic diets. Also, patients with irritable bowel syndrome and migraine might benefit from eliminating such foods that cause irritation to improve both migraine frequency and bowel symptoms.8
Weather: A few small studies have sought to evaluate the link between weather and migraines. One involving 77 individuals with migraine showed that slightly more than half were sensitive to at least 1 weather factor (i.e., barometric changes, lightning, temperature, and precipitation). Another involving 238 participants demonstrated a nonstatistically significant trend toward increased frequency of migraine attacks on days with high pressure, low wind speed, and increased sunshine, but they called the correlation questionable. A study of 90 individuals found lightning to be an independent risk factor for migraine.8
Sensory stimuli: Although visual, aural, olfactory, and other sensory stimuli have been shown to cause migraine in susceptible individuals and worsen migraine intensity, it is difficult to determine if these are true triggers or premonitory symptoms. Indeed, the discomfort thresholds of various sensory stimuli are lower in migraine sufferers.8 Odors are the most commonly reported triggers among all sensory stimuli. In 1 study, 7 in every 10 migraine sufferers reported that odors triggered their attack. Common offenders include perfumes, paint, gasoline, bleach, and rancid odors. Smoke and exhaust fumes were also found to be problematic.8
Best evidence on migraine triggers
A large retrospective survey looked at acute migraine trigger factors in 1750 individuals treated in a clinical practice. A detailed headache evaluation was conducted that included neurologic history, structured headache interview, and a neurologic exam.12 More than three-fourths of patients experienced triggers. Approximately 6 in every 10 patients had between 4 and 9 triggers. Nearly one-fourth experienced 1 to 3 triggers.12 As shown in Figure 2, the 5 triggers that occurred occasionally in at least half of patients were stress (80%), hormone (65%), fasting (57%), weather (53%), and sleep disturbance (50%). The most common triggers, occurring very frequently, were hormone (33%) and stress (26%).12

Trials that use randomized and blinded exposure to possible triggers can—at face value—appear most useful. Potent compounds such as glyceryl trinitrite, prostaglandin I2 and E2, calcitonin-gene related peptide, and pituitary adenylate cyclase-activating polypeptide have been shown to be dependable triggers in the experimental setting. However, trial designs that do not closely mimic real-world experience lack usefulness in clinical practice. Moreover, studies of low intensity migraine triggers that are common in the real world—chocolate and aspartame, for instance—have produced mixed results.13 So-called N-of-1 studies—in which a single patient is repeatedly exposed in blinded fashion to a trigger or placebo—might be the best way to uncover actual triggers. However, migraine sufferers are, understandably, not interested in deliberately inducing an attack.13
This leaves results of diary studies as the most reflective of clinical practice. These evaluations limit the chance of bias that retrospective studies could carry and often provide a significant amount of real-world data that can be used to test hypotheses. Moreover, the advent of smartphone technology enables researchers to capture data in real time, which can help distinguish premonitory symptoms from actual triggers.13 A high-quality paper diary study (PAMINA) involving 327 individuals showed strong evidence that a migraine was likely to occur with the following factors: menstruation, muscle tension/neck pain, psychic tension, and sunshine that lasted 3 or more hours per day.14
Diary studies are also able to assess the ability of migraine sufferers to predict a headache. In an evaluation that used an electronic diary design, researchers looked at self-prediction through use of a single question asked daily: “How likely are you to have a headache today?” When participants reported at the beginning of a particular day that headache was “almost certain,” actual headache occurred more than 90% of the time on that day.13
Leveraging best evidence into best practices
The clinician–patient conversation
A clinician’s detective skills are put to the test when evaluating patients with migraine. Avoiding dead-ends and fruitless pursuits can spare providers and patients frustration and help patients avoid needless suffering. Yet, as noted, the list of potential triggers appears endless, duration and frequency are variable, patients often try to control their environment with mixed results, and it is difficult to tell the difference between actual triggers and premonitory symptoms.
Which brings us back to Lola H. She desperately wanted to leave her appointment with confidence that she had enough medication to meet her needs each month. She might have expected to go home with access to more medication, but she did not. Rather, she left her clinician’s office with a plan to reduce the number of monthly migraine episodes she experienced, enabling her to use medication as needed, rather than only when her attacks were severe.
Her clinician confirmed that avoiding gasoline odor would reduce the number of attacks. Eliminating chocolates and sweets might, but it is not known whether chocolate is a trigger. He suggested that she try allowing herself chocolate now and then, which would probably serve as a stress reducer. The net result would likely be no change in number of episodes, while allowing for a small improvement in quality of life.
Perhaps most striking, Lola left the appointment certain that her issue with lower barometric pressure was resolved because her clinician took the time to ask how she knew about the daily barometric pressure levels.
Her smartwatch, she told him. He strongly suggested that she delete the watch’s barometric pressure app, which she did on the spot. At her next appointment, Lola was happy to report that barometric pressure no longer played a significant role in her episodes. Moreover, the other strategies she and her clinician discussed have reduced the number of severe and less severe episodes. She no longer lives with the stress of pill-rationing and looks forward to enjoying a small amount of chocolate ice cream each weekend with her family.
Lola’s case demonstrates that in clinical practice the best way to elicit meaningful responses from migraine sufferers is to ask open-ended questions. Yet, clinicians often fail to do so. In a study evaluating communication strategies between clinicians and 60 long-term migraineurs (average 14 years) who experienced frequent headaches (average 5 per month), 90% of migraine-specific questions asked during an appointment were either close-ended or consisted of a short answer. Postvisit interviews with both clinicians and patients revealed these pairs misaligned on migraine frequency (55% of the time) and impairment (51%). One-third of patients were candidates to receive preventive medication, yet only 20% of them did so. Prevention was not discussed in half of the visits with patients who were candidates to receive preventive therapy but did not get it.10
A panel with extensive experience in migraine reviewed questions from migraine diaries and noted that migraine sufferers find value in capturing wide-ranging information about their condition, whereas clinicians prefer small amounts of data they perceive as relevant to managing migraine.15 It also is reported that the number of questions patients ask is inversely related to the amount of information a clinician gives.16
Recommended strategies
In his review article, Mamura reinforces the concept of open 2-way communication, which involves patients in their care more deeply and reinforces positive habits.8 It is important to be mindful that discussing triggers exclusively can be counterproductive and frustrating for patients, particularly if the conversation is about trigger avoidance. Patients might feel stigmatized by triggers, come away thinking nothing can be done to control them, and become more anxious about impending episodes.8 In fact, a better strategy could be to work with the patient to train their brain to become acclimated to certain triggers or premonitory symptoms, rather than avoiding them.17
Mamura provides a concise summary of 4 best-practice strategies.8 The first 2 should be factored into advice given to patients about migraine triggers and premonitory symptoms; the remaining 2 help frame the treatment approach:
1. Ask patients to keep a headache diary, which eliminates faulty recall and provides a clearer picture of migraine frequency and severity. Include symptoms and other important factors—missed time from work, canceled family activities, nausea and vomiting, etc. Journaling might uncover triggers that can be eliminated or acclimated to via behavior modification. It also can reveal to the patient that certain triggers might not be as useful in predicting migraine as originally thought.
2. Rather than trigger avoidance, suggest that patients concentrate on healthy lifestyle choices, including maintaining healthy weight, getting sufficient sleep, and exercising regularly. The latter has been shown in some studies to work better than preventive medications. Although a behavior change, such as acclimating to certain noises, is not thought to be a healthy lifestyle habit, it ends up being just that if it improves tolerance and reduces migraine episodes.
3. Recognize that many patients have a fear of experiencing a migraine between episodes. If observed in a patient, treat it aggressively. Left unmanaged, this condition could lead to chronic migraine and medication overuse. The right acute medication might be sufficient, but sometimes cognitive-behavioral therapy and biofeedback techniques could be necessary to address behavioral issues that might be interfering with successful treatment.
4. If attacks are frequent and making it difficult to identify distinctive triggers, consider preventive therapy. Patients might resist using preventive medications because they feel eliminating triggers is sufficient, or they might be concerned about medication cost and adverse effects. Eliminating a stressful event alone likely will not help, so it’s important to review with patients—using plain language—how preventive medication treats the underlying cause of migraine and decreases brain excitability, making the patient less prone to attacks.8

Emerging medications
New treatments offer increased options for the acute and preventive treatment of migraine attacks. New acute pharmacologic treatments include gepants and ditans. The gepants target calcitonin gene-related peptide (CGRP), a potent vasodilator and neurotransmitter. During migraine episodes, serum levels of CGRP increase as the peptide is released, modulating the transmission of nociceptive signals from peripheral trigeminal sensory afferents into the central trigeminal system.19 In clinical trials of acute migraine treatment, the gepants provided pain freedom at 2 hours in approximately 20% of attacks, pain relief at 2 hours in 58%, and freedom from the most bothersome symptom at 2 hours in 36% with significant benefit over placebo.20,21 As these oral formulations became available in 2020, there are not enough data on potential long-term adverse events, particularly cardiovascular effects.18 However, the gepants are not associated with medication overuse headache and there is no contraindication to their use in patients with cardiovascular disease. These agents, currently available for oral administration, also show benefit as preventive agents and parenteral formulations are in development.
Ditans target the serotonin 5HT-1F receptor. Triptans target this receptor as well as the 5HT-1B and 5HT-1D receptors. The 5HT-1B receptor action is responsible for constriction of cerebral and peripheral blood vessels that occurs with triptans. Thus, ditans are also options for treating patients with vascular disease. Lasmiditan, the only compound in this class thus far, yielded pain freedom in 2 hours in 31%, pain relief 2 hours in 64%, and relief of the most bothersome symptom at 2 hours in 41%.22 Lasmiditan crosses the blood-brain barrier. Thus, the most common side effects are dizziness, somnolence, and paresthesias, which are generally mild to moderate in severity. Based on driving simulation studies performed in the absence of migraine, patients should not drive within 8 hours of dosing.
Neuromodulation devices are effective options that avoid potential adverse events of systemic treatments. They include external trigeminal nerve stimulation, noninvasive vagus nerve stimulation, remote electrical neuromodulation, caloric vestibular stimulation, and single-pulse transcranial magnetic stimulation. Other treatments in development for the acute treatment of migraine include improved parenteral delivery systems and combination oral preparations of existing medications.
Monoclonal antibodies that block the CGRP ligand or its receptor were introduced in 2018, with 4 medications currently available. This is the first class of migraine medications specifically designed for migraine prevention. They are administered parenterally (subcutaneously or intravenously) either monthly or quarterly, depending on the medication. The anti-CGRP monoclonal antibodies are indicated for the prevention of episodic and chronic migraine and are eliminated by proteolytic degradation, not involving the liver and reducing the potential for hepatotoxicity and drug-drug interactions.23 A meta-analysis evaluated the subcutaneous formulations studied for chronic migraine, enrolling participants with at least 15 headache days monthly with migraine occurring on at least 8 of those days. In this cohort with a large disease burden, the pooled 50% responder rate 9-12 weeks after administration of the first dose was 37.4% and the 75% responder rate was 12.7%.24 There was also improvement in the number of migraine days, number of days using acute treatment, and total headache hours. The most common side effects were injection site reactions and constipation, with post-marketing reports of hypertension in the compound targeting the CGRP receptor, with or without pre-existing hypertension.25 The safety profile for use during pregnancy was not studied in any of the medications discussed in this section.
Summary
Acute migraine headache is a significant management challenge for neurologists, headache specialists, and other clinicians involved in its treatment. Addressing potential migraine triggers can be a particularly overwhelming task because multiple components and factors play into the disorder. Perceived triggers; their frequency and strength; the effort by patients to control their environment; and the blurred line between real triggers and premonitory symptoms present the clinician with an amalgam of components that must be sorted through, defined, prioritized, and managed. Even though migraine symptoms generally appear along a 4-phase timeline, some symptoms stretch across multiple phases and not all symptoms play out in a linear fashion.
There are specific steps clinicians can take to simplify the process, optimize management, and improve patient outcomes and quality of life. Start by gaining an understanding of common triggers and the best evidence regarding triggers and premonitory symptoms. Use this evidence to modify your approach in practice, if necessary. Specifically:
• Improve the information you gather from patients by asking open-ended questions about their experience during a migraine headache, as well as the period between attacks.
• Rather than having patients tell you how many episodes they experience each month, try to elicit from them how many days per month they feel disabled.
• Ask your patients to keep a headache diary.
• Talk less about trigger avoidance and more about making healthy lifestyle choices.
• Address potential cephalgiaphobia via cognitive-behavioral therapy and biofeedback techniques.
• Consider managing frequent migraine attacks with preventive treatment, which now includes CGRP-targeted medications that are as effective as other agents but have fewer adverse effects and might avoid medication overuse headache.
Dr. Friedman reports being an Advisory Board member for Allergan, Biohaven Pharmaceuticals, Eli Lilly, Impel NeuroPharma, Invex, Lundbeck, Revance Therapeutics, Satsuma Pharmaceuticals, Teva Pharmaceuticals, Theranica, and Zosano Pharma. She has received grant support from Allergan, Eli Lilly, Merck, and Zosano Pharma. She is on the Medical Advisory Board for Spinal CSF Leak Foundation, HealthyWomen, and the Board of Directors of the Southern Headache Society. She currently sits on the Editorial Board of Neurology Reviews and has been a contributing author for MedLink Neurology and Medscape.
1. Migraine Research Foundation. Migraine facts. Accessed January 24, 2021. https://migraineresearchfoundation.org/about-migraine/migraine-facts.
2. Pellegrino A, Davis-Martin R, Houle T, Turner D, Smitherman T. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia. 2018;38(6):1188-1198.
3. Turner D, Houle T. Influences on headache trigger beliefs and perceptions. Cephalalgia. 2018;38(9):1545-1553.
4. Scharff L, Turk D, Marcus D. The relationship of locus of control and psychosocial‐behavioral response in chronic headache. Headache. 1995;35(9):527-533.
5. French D, Holroyd K, Pinell C, Malinoski P, O’Donnell F, Hill K. Perceived self‐efficacy and headache‐related disability. Headache. 2000;40(8):647-656.
6. Farris S, Thomas J, Abrantes A, et al. Anxiety sensitivity and intentional avoidance of physical activity in women with probable migraine. Cephalalgia. 2019;39(11):1465-1469.
7. Goadsby P, Holland P, Martins-Oliveira M, Hoffman J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97(2):553-622.
8. Mamura M. Triggers, protectors, and predictors in episodic migraine. Curr Pain Headache Rep. 2018;22:81.
9. American Migraine Foundation. Understanding migraine progression can help you anticipate and manage your symptoms. Accessed December 30, 2020. https://americanmigrainefoundation.org/resource-library/timeline-migraine-attack.
10. Lipton R, Buse D, Hall C, et al. Reduction in perceived stress as a migraine trigger: Testing the “let-down headache” hypothesis. Neurology. 2014;82:1395-1401.
11. Loder E. Prophylaxis of menstrual migraine with triptans: Problems and possibilities. Neurology. 2002;59(11):1677-1681.
12. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
13. Pavolic J, Buse D, Sollars M, Haut S, Lipton R. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache. 2014;54(10):1670-1679.
14. Wöber C, Brannath W, Schmidt K, et al; PAMINA Study Group. Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia. 2007;27:304-314.
15. Dodick D, Tepper S, Lipton R, et al. Improving medical communication in migraine management: A modified Delphi study to develop a digital migraine tracker. Headache. 2018;58(9):1358-1372.
16. Sheftell F. Communicating the right therapy for the right patient at the right time: Acute therapy. Can J Neurol Sci. 2002;29(suppl 2):S33-S39.
17. Goadsby P, Silberstein S. Migraine triggers: Harnessing the messages of clinical practice. Neurology. 2013;80:434-425.
18. Woo M. Refining a marvel. Nature. 2020;586:s4-s6.
19. Dodick DW. Migraine. Lancet. 2018;10(127):1315-30.
20. Croop R, Goadsby P. J., Stock D. A. et al. Efficacy and safety of rimegepant for the acute treatment of migraine: Evidence from randomized controlled trials. Front Pharmacol. 2020;24 https://doi.org/10.3389/fphar.2019.01577.
21. Yang Y, Chen C, Sun Y et al. Safety and efficacy of ubrogepant for acute treatment of episodic migraine: A meta-analysis of randomized clinical trials. CNS Drugs. 2020;34:463-71.
22. Curto M, Cipolla F, Cisale GY et al. Profiling lasmiditan as a treatment option for migraine. Exp Rev Pharmacother. 2020;21(2):147-53.
23. Raffaelli B, Reuter U. The biology of monoclonal antibodies: Focus on calcitonin gene-related peptide for prophylactic migraine therapy. Neurotherapeutics. 2018;15(2): 324-35.
24. Han L, Lui Y, Xiong H et al. CRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019;9(2):e01215.
25. Saely S, Croteau D, Jawidzik L et al. Hypertension: A new safety risk for patients treated with erenumab. Headache. 2021 (epub ahead of print) https://doi.org/10.1111/head.14051.
1. Migraine Research Foundation. Migraine facts. Accessed January 24, 2021. https://migraineresearchfoundation.org/about-migraine/migraine-facts.
2. Pellegrino A, Davis-Martin R, Houle T, Turner D, Smitherman T. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia. 2018;38(6):1188-1198.
3. Turner D, Houle T. Influences on headache trigger beliefs and perceptions. Cephalalgia. 2018;38(9):1545-1553.
4. Scharff L, Turk D, Marcus D. The relationship of locus of control and psychosocial‐behavioral response in chronic headache. Headache. 1995;35(9):527-533.
5. French D, Holroyd K, Pinell C, Malinoski P, O’Donnell F, Hill K. Perceived self‐efficacy and headache‐related disability. Headache. 2000;40(8):647-656.
6. Farris S, Thomas J, Abrantes A, et al. Anxiety sensitivity and intentional avoidance of physical activity in women with probable migraine. Cephalalgia. 2019;39(11):1465-1469.
7. Goadsby P, Holland P, Martins-Oliveira M, Hoffman J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97(2):553-622.
8. Mamura M. Triggers, protectors, and predictors in episodic migraine. Curr Pain Headache Rep. 2018;22:81.
9. American Migraine Foundation. Understanding migraine progression can help you anticipate and manage your symptoms. Accessed December 30, 2020. https://americanmigrainefoundation.org/resource-library/timeline-migraine-attack.
10. Lipton R, Buse D, Hall C, et al. Reduction in perceived stress as a migraine trigger: Testing the “let-down headache” hypothesis. Neurology. 2014;82:1395-1401.
11. Loder E. Prophylaxis of menstrual migraine with triptans: Problems and possibilities. Neurology. 2002;59(11):1677-1681.
12. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
13. Pavolic J, Buse D, Sollars M, Haut S, Lipton R. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache. 2014;54(10):1670-1679.
14. Wöber C, Brannath W, Schmidt K, et al; PAMINA Study Group. Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia. 2007;27:304-314.
15. Dodick D, Tepper S, Lipton R, et al. Improving medical communication in migraine management: A modified Delphi study to develop a digital migraine tracker. Headache. 2018;58(9):1358-1372.
16. Sheftell F. Communicating the right therapy for the right patient at the right time: Acute therapy. Can J Neurol Sci. 2002;29(suppl 2):S33-S39.
17. Goadsby P, Silberstein S. Migraine triggers: Harnessing the messages of clinical practice. Neurology. 2013;80:434-425.
18. Woo M. Refining a marvel. Nature. 2020;586:s4-s6.
19. Dodick DW. Migraine. Lancet. 2018;10(127):1315-30.
20. Croop R, Goadsby P. J., Stock D. A. et al. Efficacy and safety of rimegepant for the acute treatment of migraine: Evidence from randomized controlled trials. Front Pharmacol. 2020;24 https://doi.org/10.3389/fphar.2019.01577.
21. Yang Y, Chen C, Sun Y et al. Safety and efficacy of ubrogepant for acute treatment of episodic migraine: A meta-analysis of randomized clinical trials. CNS Drugs. 2020;34:463-71.
22. Curto M, Cipolla F, Cisale GY et al. Profiling lasmiditan as a treatment option for migraine. Exp Rev Pharmacother. 2020;21(2):147-53.
23. Raffaelli B, Reuter U. The biology of monoclonal antibodies: Focus on calcitonin gene-related peptide for prophylactic migraine therapy. Neurotherapeutics. 2018;15(2): 324-35.
24. Han L, Lui Y, Xiong H et al. CRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019;9(2):e01215.
25. Saely S, Croteau D, Jawidzik L et al. Hypertension: A new safety risk for patients treated with erenumab. Headache. 2021 (epub ahead of print) https://doi.org/10.1111/head.14051.










