User login
New study offers details on post-COVID pediatric illness
Multisystem inflammatory syndrome in children (MIS-C) is more common than previously thought. This pediatric illness occurs 2-6 weeks after being infected with COVID-19.
a new study found. The illness is rare, but it causes dangerous multiorgan dysfunction and frequently requires a stay in the ICU. According to the Centers for Disease Control and Prevention, there have been at least 9,333 cases nationwide and 76 deaths from MIS-C.
Researchers said their findings were in such contrast to previous MIS-C research that it may render the old research “misleading.”
The analysis was powered by improved data extracted from hospital billing systems. Previous analyses of MIS-C were limited to voluntarily reported cases, which is likely the reason for the undercount.
The study reported a mortality rate for people with the most severe cases (affecting six to eight organs) of 5.8%. The authors of a companion editorial to the study said the mortality rate was low when considering the widespread impacts, “reflecting the rapid reversibility of MIS-C” with treatment.
Differences in MIS-C cases were also found based on children’s race and ethnicity. Black patients were more likely to have severe cases affecting more organs, compared to white patients.
The study included 4,107 MIS-C cases, using data from 2021 for patients younger than 21 years old. The median age was 9 years old.
The findings provide direction for further research, the editorial writers suggested.
Questions that need to be answered include asking why Black children with MIS-C are more likely to have a higher number of organ systems affected.
“Identifying patient biological or socioeconomic factors that can be targeted for treatment or prevention should be pursued,” they wrote.
The CDC says symptoms of MIS-C are an ongoing fever plus more than one of the following: stomach pain, bloodshot eyes, diarrhea, dizziness or lightheadedness (signs of low blood pressure), skin rash, or vomiting.
A version of this article first appeared on WebMD.com.
Multisystem inflammatory syndrome in children (MIS-C) is more common than previously thought. This pediatric illness occurs 2-6 weeks after being infected with COVID-19.
a new study found. The illness is rare, but it causes dangerous multiorgan dysfunction and frequently requires a stay in the ICU. According to the Centers for Disease Control and Prevention, there have been at least 9,333 cases nationwide and 76 deaths from MIS-C.
Researchers said their findings were in such contrast to previous MIS-C research that it may render the old research “misleading.”
The analysis was powered by improved data extracted from hospital billing systems. Previous analyses of MIS-C were limited to voluntarily reported cases, which is likely the reason for the undercount.
The study reported a mortality rate for people with the most severe cases (affecting six to eight organs) of 5.8%. The authors of a companion editorial to the study said the mortality rate was low when considering the widespread impacts, “reflecting the rapid reversibility of MIS-C” with treatment.
Differences in MIS-C cases were also found based on children’s race and ethnicity. Black patients were more likely to have severe cases affecting more organs, compared to white patients.
The study included 4,107 MIS-C cases, using data from 2021 for patients younger than 21 years old. The median age was 9 years old.
The findings provide direction for further research, the editorial writers suggested.
Questions that need to be answered include asking why Black children with MIS-C are more likely to have a higher number of organ systems affected.
“Identifying patient biological or socioeconomic factors that can be targeted for treatment or prevention should be pursued,” they wrote.
The CDC says symptoms of MIS-C are an ongoing fever plus more than one of the following: stomach pain, bloodshot eyes, diarrhea, dizziness or lightheadedness (signs of low blood pressure), skin rash, or vomiting.
A version of this article first appeared on WebMD.com.
Multisystem inflammatory syndrome in children (MIS-C) is more common than previously thought. This pediatric illness occurs 2-6 weeks after being infected with COVID-19.
a new study found. The illness is rare, but it causes dangerous multiorgan dysfunction and frequently requires a stay in the ICU. According to the Centers for Disease Control and Prevention, there have been at least 9,333 cases nationwide and 76 deaths from MIS-C.
Researchers said their findings were in such contrast to previous MIS-C research that it may render the old research “misleading.”
The analysis was powered by improved data extracted from hospital billing systems. Previous analyses of MIS-C were limited to voluntarily reported cases, which is likely the reason for the undercount.
The study reported a mortality rate for people with the most severe cases (affecting six to eight organs) of 5.8%. The authors of a companion editorial to the study said the mortality rate was low when considering the widespread impacts, “reflecting the rapid reversibility of MIS-C” with treatment.
Differences in MIS-C cases were also found based on children’s race and ethnicity. Black patients were more likely to have severe cases affecting more organs, compared to white patients.
The study included 4,107 MIS-C cases, using data from 2021 for patients younger than 21 years old. The median age was 9 years old.
The findings provide direction for further research, the editorial writers suggested.
Questions that need to be answered include asking why Black children with MIS-C are more likely to have a higher number of organ systems affected.
“Identifying patient biological or socioeconomic factors that can be targeted for treatment or prevention should be pursued,” they wrote.
The CDC says symptoms of MIS-C are an ongoing fever plus more than one of the following: stomach pain, bloodshot eyes, diarrhea, dizziness or lightheadedness (signs of low blood pressure), skin rash, or vomiting.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Expert panel forms strategy for eosinophilic esophagitis monitoring
“Follow-up should comprise symptom assessment and periodic or repeated endoscopy with histological assessment in specific EoE settings,” wrote Ulrike von Arnim, MD, from University Hospital Magdeburg (Germany), and an international team of colleagues in Clinical Gastroenterology and Hepatology.
Although medicine and diet can control EoE, there is presently no cure, and long-term management is needed to prevent recurrence and long-term effects such as esophageal remodeling, fibrosis, and stricture, the researchers said. Yet they could find no evidence-based recommendations for clinical monitoring of the condition.
With the participation of The International Gastrointestinal Eosinophil Researchers (TIGER) and the European Consortium for Eosinophilic Diseases of the GI Tract (EUREOS), they assembled a team of 18 gastroenterologists, pathologists, and allergists from the United States and Western Europe with expertise in the condition.
Almost all panelists had more than 10 years of subspecialty EoE care and more than five relevant research publications. All were members of TIGER or EUREOS. The panel met by video conferencing and responded to surveys to develop a consensus about why, by what means, and when to monitor patients with EoE.
The group reached 75% or greater agreement on 11 statements on these subjects.
Regular follow-ups are needed because they enable clinicians to detect whether treatments have stopped working, improve therapy adherence, and introduce patients to any new treatments that become available, while preventing gaps in care that can worsen outcomes, the group wrote.
Symptoms don’t give a precise indication of esophageal healing and shouldn’t be the sole measure for disease activity, the experts wrote. They recommended other approaches to monitoring, including biopsies. They also endorse the Endoscopic Reference Score as an outcome measure.
The panel recommended noninvasive tissue sampling, mentioning the esophageal string test and the Cytosponge as examples, but called for more research on these two techniques.
Blood markers, oral swabs, breath condensates, and stool and urine samples are not recommended as approaches for monitoring EoE, they wrote.
The optimal interval to measure the efficacy of a therapy is more difficult to decide, the panel noted.
“The clinician’s decision should take into account the clinical severity of the disease, estimated risk of imminent subsequent food impaction, presence of stenosis, as well as mode of action and reported outcome of the chosen medical, dietary, or mechanical treatment,” they wrote. Intervals from 6 to 24 weeks may be appropriate.
For diets and topical corticosteroids, they agreed on an interval of 8-12 weeks to confirm remission but say a longer time might be preferred for slower-acting therapies, such as monoclonal antibodies.
The panel had the most trouble reaching a consensus on how often to follow up on patients whose disease is in remission or is stable. They settled on 12 to 24 months after the last endoscopy. Any longer than 2 years risks missing increased disease activity, they wrote.
This follow-up should include assessment of symptoms and a gastrointestinal endoscopy in cases of relapse or suspected stricture, as well as when treatment modification is being considered or when assessment of histological activity is desired, the panel recommended.
Almost all the panelists disclosed financial relationships with pharmaceutical or medical device companies.
A version of this article first appeared on Medscape.com.
“Follow-up should comprise symptom assessment and periodic or repeated endoscopy with histological assessment in specific EoE settings,” wrote Ulrike von Arnim, MD, from University Hospital Magdeburg (Germany), and an international team of colleagues in Clinical Gastroenterology and Hepatology.
Although medicine and diet can control EoE, there is presently no cure, and long-term management is needed to prevent recurrence and long-term effects such as esophageal remodeling, fibrosis, and stricture, the researchers said. Yet they could find no evidence-based recommendations for clinical monitoring of the condition.
With the participation of The International Gastrointestinal Eosinophil Researchers (TIGER) and the European Consortium for Eosinophilic Diseases of the GI Tract (EUREOS), they assembled a team of 18 gastroenterologists, pathologists, and allergists from the United States and Western Europe with expertise in the condition.
Almost all panelists had more than 10 years of subspecialty EoE care and more than five relevant research publications. All were members of TIGER or EUREOS. The panel met by video conferencing and responded to surveys to develop a consensus about why, by what means, and when to monitor patients with EoE.
The group reached 75% or greater agreement on 11 statements on these subjects.
Regular follow-ups are needed because they enable clinicians to detect whether treatments have stopped working, improve therapy adherence, and introduce patients to any new treatments that become available, while preventing gaps in care that can worsen outcomes, the group wrote.
Symptoms don’t give a precise indication of esophageal healing and shouldn’t be the sole measure for disease activity, the experts wrote. They recommended other approaches to monitoring, including biopsies. They also endorse the Endoscopic Reference Score as an outcome measure.
The panel recommended noninvasive tissue sampling, mentioning the esophageal string test and the Cytosponge as examples, but called for more research on these two techniques.
Blood markers, oral swabs, breath condensates, and stool and urine samples are not recommended as approaches for monitoring EoE, they wrote.
The optimal interval to measure the efficacy of a therapy is more difficult to decide, the panel noted.
“The clinician’s decision should take into account the clinical severity of the disease, estimated risk of imminent subsequent food impaction, presence of stenosis, as well as mode of action and reported outcome of the chosen medical, dietary, or mechanical treatment,” they wrote. Intervals from 6 to 24 weeks may be appropriate.
For diets and topical corticosteroids, they agreed on an interval of 8-12 weeks to confirm remission but say a longer time might be preferred for slower-acting therapies, such as monoclonal antibodies.
The panel had the most trouble reaching a consensus on how often to follow up on patients whose disease is in remission or is stable. They settled on 12 to 24 months after the last endoscopy. Any longer than 2 years risks missing increased disease activity, they wrote.
This follow-up should include assessment of symptoms and a gastrointestinal endoscopy in cases of relapse or suspected stricture, as well as when treatment modification is being considered or when assessment of histological activity is desired, the panel recommended.
Almost all the panelists disclosed financial relationships with pharmaceutical or medical device companies.
A version of this article first appeared on Medscape.com.
“Follow-up should comprise symptom assessment and periodic or repeated endoscopy with histological assessment in specific EoE settings,” wrote Ulrike von Arnim, MD, from University Hospital Magdeburg (Germany), and an international team of colleagues in Clinical Gastroenterology and Hepatology.
Although medicine and diet can control EoE, there is presently no cure, and long-term management is needed to prevent recurrence and long-term effects such as esophageal remodeling, fibrosis, and stricture, the researchers said. Yet they could find no evidence-based recommendations for clinical monitoring of the condition.
With the participation of The International Gastrointestinal Eosinophil Researchers (TIGER) and the European Consortium for Eosinophilic Diseases of the GI Tract (EUREOS), they assembled a team of 18 gastroenterologists, pathologists, and allergists from the United States and Western Europe with expertise in the condition.
Almost all panelists had more than 10 years of subspecialty EoE care and more than five relevant research publications. All were members of TIGER or EUREOS. The panel met by video conferencing and responded to surveys to develop a consensus about why, by what means, and when to monitor patients with EoE.
The group reached 75% or greater agreement on 11 statements on these subjects.
Regular follow-ups are needed because they enable clinicians to detect whether treatments have stopped working, improve therapy adherence, and introduce patients to any new treatments that become available, while preventing gaps in care that can worsen outcomes, the group wrote.
Symptoms don’t give a precise indication of esophageal healing and shouldn’t be the sole measure for disease activity, the experts wrote. They recommended other approaches to monitoring, including biopsies. They also endorse the Endoscopic Reference Score as an outcome measure.
The panel recommended noninvasive tissue sampling, mentioning the esophageal string test and the Cytosponge as examples, but called for more research on these two techniques.
Blood markers, oral swabs, breath condensates, and stool and urine samples are not recommended as approaches for monitoring EoE, they wrote.
The optimal interval to measure the efficacy of a therapy is more difficult to decide, the panel noted.
“The clinician’s decision should take into account the clinical severity of the disease, estimated risk of imminent subsequent food impaction, presence of stenosis, as well as mode of action and reported outcome of the chosen medical, dietary, or mechanical treatment,” they wrote. Intervals from 6 to 24 weeks may be appropriate.
For diets and topical corticosteroids, they agreed on an interval of 8-12 weeks to confirm remission but say a longer time might be preferred for slower-acting therapies, such as monoclonal antibodies.
The panel had the most trouble reaching a consensus on how often to follow up on patients whose disease is in remission or is stable. They settled on 12 to 24 months after the last endoscopy. Any longer than 2 years risks missing increased disease activity, they wrote.
This follow-up should include assessment of symptoms and a gastrointestinal endoscopy in cases of relapse or suspected stricture, as well as when treatment modification is being considered or when assessment of histological activity is desired, the panel recommended.
Almost all the panelists disclosed financial relationships with pharmaceutical or medical device companies.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Modified Atkins diet beneficial in drug-resistant epilepsy
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
In a randomized prospective study, the number of seizures per month dropped by more than half in one-quarter of patients following the high-fat, low-carb diet; and 5% of the group were free from all seizure activity after 6 months.
Both adults and adolescents reported benefits from the diet, which is a less strict version of a traditional ketogenic diet that many patients find difficult to follow. The modified Atkins diet includes foods such as leafy green vegetables and eggs, chicken, fish, bacon, and other animal proteins.
“The use of an exchange list and recipe booklet with local recipes and spices helped in the initiation of modified Atkins diet with the flexibility of meal choices and ease of administration,” said coinvestigator Manjari Tripathi, MD, DM, department of neurology, All India Institute of Medical Science, New Delhi.
“As items were everyday household ingredients in proportion to the requirement of the modified Atkins diet, this diet is possible in low-income countries also,” Dr. Tripathi added.
The findings were published online in the journal Neurology.
Low carbs, high benefit
The modified Atkins diet includes around 65% fat, 25% protein, and 10% carbohydrates. Unlike a traditional ketogenic diet, the modified Atkins diet includes no restrictions on protein, calories, or fluids.
Researchers have long known that ketogenic and Atkins diets are associated with reduced seizure activity in adolescents with epilepsy. But previous studies were small, and many were retrospective analyses.
The current investigators enrolled 160 patients (80 adults, 80 adolescents) aged 10-55 years whose epilepsy was not controlled despite using at least three antiseizure medications at maximum tolerated doses.
The intervention group received training in the modified Atkins diet and were given a food exchange list, sample menu, and recipe booklet. Carbohydrate intake was restricted to 20 grams per day.
Participants took supplemental multivitamins and minerals, kept a food diary, logged seizure activity, and measured urine ketone levels three times a day. They also received weekly check-up phone calls to ensure diet adherence.
The control group received a normal diet with no carbohydrate restrictions. All participants continued their prescribed antiseizure therapy throughout the trial.
Primary outcome met
The primary study outcome was a reduction in seizures of more than 50%. At 6 months, 26.2% of the intervention group had reached that goal, compared with just 2.5% of the control group (P < .001).
When the median number of seizures in the modified Atkins diet group was analyzed, the frequency dropped in the intervention group from 37.5 per month at baseline to 27.5 per month after 3 months of the modified Atkins diet and to 21.5 per month after 6 months.
Adding the modified Atkins diet had a larger effect on seizure activity in adults than in adolescents. At the end of 6 months, 36% of adolescents on the modified Atkins diet had more than a 50% reduction in seizures, while 57.1% of adults on the diet reached that level.
Quality-of-life scores were also higher in the intervention group.
By the end of the trial, 5% of patients on the modified Atkins diet had no seizure activity at all versus none of the control group. In fact, the median number of seizures increased in the control group during the study.
The mean morning and evening levels of urine ketosis in the intervention group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, suggesting satisfactory diet adherence. There was no significant difference between groups in weight loss.
Dr. Tripathi noted that 33% of participants did not complete the study because of poor tolerance of the diet, lack of benefit, or the inability to follow up – in part due to COVID-19. However, she said tolerance of the modified Atkins diet was better than what has been reported with the ketogenic diet.
“Though the exact mechanism by which such a diet protects against seizures is unknown, there is evidence that it causes effects on intermediary metabolism that influences the dynamics of the major inhibitory and excitatory neurotransmitter systems in the brain,” Dr. Tripathi said.
Benefits outweigh cost
Commenting on the research findings, Mackenzie Cervenka, MD, professor of neurology and director of the Adult Epilepsy Diet Center at Johns Hopkins University, Baltimore, noted that the study is the first randomized controlled trial of this size to demonstrate a benefit from adding the modified Atkins diet to standard antiseizure therapy in treatment-resistant epilepsy.
“Importantly, the study also showed improvement in quality of life and behavior over standard-of-care therapies without significant adverse effects,” said Dr. Cervenka, who was not part of the research.
The investigators noted that the flexibility of the modified Atkins diet allows more variation in menu options and a greater intake of protein, making it easier to follow than a traditional ketogenic diet.
One area of debate, however, is whether these diets are manageable for individuals with low income. Poultry, meat, and fish, all of which are staples of a modified Atkins diet, can be more expensive than other high-carb options such as pasta and rice.
“While some of the foods such as protein sources that patients purchase when they are on a ketogenic diet therapy can be more expensive, if you take into account the cost of antiseizure medications and other antiseizure treatments, hospital visits, and missed work related to seizures, et cetera, the overall financial benefits of seizure reduction with incorporating a ketogenic diet therapy may outweigh these costs,” Dr. Cervenka said.
“There are also low-cost foods that can be used since there is a great deal of flexibility with a modified Atkins diet,” she added.
The study was funded by the Centre of Excellence for Epilepsy, which is funded by the Department of Biotechnology, Government of India. Dr. Tripathi and Dr. Cervenka report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Infantile hemangioma: Analysis underscores importance of early propranolol treatment
, results from a post-hoc analysis of phase 2 and 3 clinical trial data showed.
“It is widely accepted that oral propranolol should be started early to improve the success rate, but proposed thresholds have lacked supportive data,” researchers led by Christine Léauté-Labrèze, MD, of the department of dermatology at Pellegrin Children’s Hospital, Bordeaux, France, wrote in the study, which was published online in Pediatric Dermatology. In the pivotal phase 2/3 trial of propranolol of 460 infants, published in 2015, the mean initiation of treatment was 104 days, they added, but “in real-life studies, most infants are referred later than this.”
In addition, a European expert consensus panel set the ideal age for a patient to be seen by a specialist at between 3 and 5 weeks of age, while an American Academy of Pediatrics Clinical Practice Guideline set the ideal age at 1 month.
To determine factors associated with a higher success rate with oral propranolol treatment, such as age at treatment initiation, the researchers analyzed data from the pivotal phase 2-3 clinical trial of oral propranolol in IH. They used Generalized Additive Model (GAM) charts with Generalized Linear Models (GLM), then a rule discovery algorithm, to identify subgroups presenting a high probability of occurrence of the predefined outcome: success at 6 months of treatment (defined as complete or nearly complete resolution of the target hemangioma). Study coauthors were Ilona J. Frieden, MD, of the department of dermatology at the University of California, San Francisco, and director of the UCSF Birthmarks & Vascular Anomalies Center; and Alain Delarue, MD, of medical affairs at Pierre Fabre Dermatologie, Lavaur, France, which markets the pediatric formulation of propranolol approved by the Food and Drug Administration in 2014 for treating IH.
They found that patients who started oral propranolol 3 mg/kg/day before the age of 10 weeks had a success rate of 86%, while those who started treatment after 10 weeks of age had a success rate of 60%. “Our clinical experience suggested that starting early propranolol gave better results on infantile hemangiomas; however, we were surprised” by the significance of the difference, the three study authors stated in an e-mail reply to this news organization.
“It therefore seemed essential to communicate the importance of early treatment to maximize the possibilities of recovery for children. Our findings support early treatment of at-risk infantile hemangiomas, without waiting for complications such as ulceration and/or functional consequences,” they added.
In their e-mail reply, the authors stated that treatment of high-risk IH should be initiated whenever possible before 10 weeks of age. Ideally, infants should be examined by a practitioner between 2 and 5 weeks of age and referred to a specialized center if they have features of an at-risk IH. Tools such as the Infantile Hemangioma Referral Score (IHReS) and consensus guidelines such as the AAP Clinical Practice Guideline “can help guide clinicians seeing newborns and young infants to recognize which IH may need early intervention,” they stated.
For rural-based providers whose patients and their families may not live close to an expert center, the study authors especially recommend using the IHReS scoring tool, which is readily available online and “will be very helpful in assessing whether patients need referral.” For those who do, they added, “triage using photographs is an excellent way to reach out to a referral center for advice and possible urgent referral.” In addition, a recent study emphasized that telemedicine using either live interactive portals or store-and-forward can be helpful in evaluation and management of patients with IH.
Dr. Léauté-Labrèze and colleagues acknowledged certain limitations of the analysis, including the fact that it was performed post-hoc on an existing study and the challenge of translating its findings into clinical practice.
The three study authors were also authors of the 2015 NEJM study; Dr. Léauté-Labrèze was the lead author.
Dr. Léauté-Labrèze disclosed that she has served as a speaker and consultant for Pierre Fabre. Dr. Delarue is an employee of the company. Dr. Frieden reported having no disclosures relevant to the analysis.
, results from a post-hoc analysis of phase 2 and 3 clinical trial data showed.
“It is widely accepted that oral propranolol should be started early to improve the success rate, but proposed thresholds have lacked supportive data,” researchers led by Christine Léauté-Labrèze, MD, of the department of dermatology at Pellegrin Children’s Hospital, Bordeaux, France, wrote in the study, which was published online in Pediatric Dermatology. In the pivotal phase 2/3 trial of propranolol of 460 infants, published in 2015, the mean initiation of treatment was 104 days, they added, but “in real-life studies, most infants are referred later than this.”
In addition, a European expert consensus panel set the ideal age for a patient to be seen by a specialist at between 3 and 5 weeks of age, while an American Academy of Pediatrics Clinical Practice Guideline set the ideal age at 1 month.
To determine factors associated with a higher success rate with oral propranolol treatment, such as age at treatment initiation, the researchers analyzed data from the pivotal phase 2-3 clinical trial of oral propranolol in IH. They used Generalized Additive Model (GAM) charts with Generalized Linear Models (GLM), then a rule discovery algorithm, to identify subgroups presenting a high probability of occurrence of the predefined outcome: success at 6 months of treatment (defined as complete or nearly complete resolution of the target hemangioma). Study coauthors were Ilona J. Frieden, MD, of the department of dermatology at the University of California, San Francisco, and director of the UCSF Birthmarks & Vascular Anomalies Center; and Alain Delarue, MD, of medical affairs at Pierre Fabre Dermatologie, Lavaur, France, which markets the pediatric formulation of propranolol approved by the Food and Drug Administration in 2014 for treating IH.
They found that patients who started oral propranolol 3 mg/kg/day before the age of 10 weeks had a success rate of 86%, while those who started treatment after 10 weeks of age had a success rate of 60%. “Our clinical experience suggested that starting early propranolol gave better results on infantile hemangiomas; however, we were surprised” by the significance of the difference, the three study authors stated in an e-mail reply to this news organization.
“It therefore seemed essential to communicate the importance of early treatment to maximize the possibilities of recovery for children. Our findings support early treatment of at-risk infantile hemangiomas, without waiting for complications such as ulceration and/or functional consequences,” they added.
In their e-mail reply, the authors stated that treatment of high-risk IH should be initiated whenever possible before 10 weeks of age. Ideally, infants should be examined by a practitioner between 2 and 5 weeks of age and referred to a specialized center if they have features of an at-risk IH. Tools such as the Infantile Hemangioma Referral Score (IHReS) and consensus guidelines such as the AAP Clinical Practice Guideline “can help guide clinicians seeing newborns and young infants to recognize which IH may need early intervention,” they stated.
For rural-based providers whose patients and their families may not live close to an expert center, the study authors especially recommend using the IHReS scoring tool, which is readily available online and “will be very helpful in assessing whether patients need referral.” For those who do, they added, “triage using photographs is an excellent way to reach out to a referral center for advice and possible urgent referral.” In addition, a recent study emphasized that telemedicine using either live interactive portals or store-and-forward can be helpful in evaluation and management of patients with IH.
Dr. Léauté-Labrèze and colleagues acknowledged certain limitations of the analysis, including the fact that it was performed post-hoc on an existing study and the challenge of translating its findings into clinical practice.
The three study authors were also authors of the 2015 NEJM study; Dr. Léauté-Labrèze was the lead author.
Dr. Léauté-Labrèze disclosed that she has served as a speaker and consultant for Pierre Fabre. Dr. Delarue is an employee of the company. Dr. Frieden reported having no disclosures relevant to the analysis.
, results from a post-hoc analysis of phase 2 and 3 clinical trial data showed.
“It is widely accepted that oral propranolol should be started early to improve the success rate, but proposed thresholds have lacked supportive data,” researchers led by Christine Léauté-Labrèze, MD, of the department of dermatology at Pellegrin Children’s Hospital, Bordeaux, France, wrote in the study, which was published online in Pediatric Dermatology. In the pivotal phase 2/3 trial of propranolol of 460 infants, published in 2015, the mean initiation of treatment was 104 days, they added, but “in real-life studies, most infants are referred later than this.”
In addition, a European expert consensus panel set the ideal age for a patient to be seen by a specialist at between 3 and 5 weeks of age, while an American Academy of Pediatrics Clinical Practice Guideline set the ideal age at 1 month.
To determine factors associated with a higher success rate with oral propranolol treatment, such as age at treatment initiation, the researchers analyzed data from the pivotal phase 2-3 clinical trial of oral propranolol in IH. They used Generalized Additive Model (GAM) charts with Generalized Linear Models (GLM), then a rule discovery algorithm, to identify subgroups presenting a high probability of occurrence of the predefined outcome: success at 6 months of treatment (defined as complete or nearly complete resolution of the target hemangioma). Study coauthors were Ilona J. Frieden, MD, of the department of dermatology at the University of California, San Francisco, and director of the UCSF Birthmarks & Vascular Anomalies Center; and Alain Delarue, MD, of medical affairs at Pierre Fabre Dermatologie, Lavaur, France, which markets the pediatric formulation of propranolol approved by the Food and Drug Administration in 2014 for treating IH.
They found that patients who started oral propranolol 3 mg/kg/day before the age of 10 weeks had a success rate of 86%, while those who started treatment after 10 weeks of age had a success rate of 60%. “Our clinical experience suggested that starting early propranolol gave better results on infantile hemangiomas; however, we were surprised” by the significance of the difference, the three study authors stated in an e-mail reply to this news organization.
“It therefore seemed essential to communicate the importance of early treatment to maximize the possibilities of recovery for children. Our findings support early treatment of at-risk infantile hemangiomas, without waiting for complications such as ulceration and/or functional consequences,” they added.
In their e-mail reply, the authors stated that treatment of high-risk IH should be initiated whenever possible before 10 weeks of age. Ideally, infants should be examined by a practitioner between 2 and 5 weeks of age and referred to a specialized center if they have features of an at-risk IH. Tools such as the Infantile Hemangioma Referral Score (IHReS) and consensus guidelines such as the AAP Clinical Practice Guideline “can help guide clinicians seeing newborns and young infants to recognize which IH may need early intervention,” they stated.
For rural-based providers whose patients and their families may not live close to an expert center, the study authors especially recommend using the IHReS scoring tool, which is readily available online and “will be very helpful in assessing whether patients need referral.” For those who do, they added, “triage using photographs is an excellent way to reach out to a referral center for advice and possible urgent referral.” In addition, a recent study emphasized that telemedicine using either live interactive portals or store-and-forward can be helpful in evaluation and management of patients with IH.
Dr. Léauté-Labrèze and colleagues acknowledged certain limitations of the analysis, including the fact that it was performed post-hoc on an existing study and the challenge of translating its findings into clinical practice.
The three study authors were also authors of the 2015 NEJM study; Dr. Léauté-Labrèze was the lead author.
Dr. Léauté-Labrèze disclosed that she has served as a speaker and consultant for Pierre Fabre. Dr. Delarue is an employee of the company. Dr. Frieden reported having no disclosures relevant to the analysis.
FROM PEDIATRIC DERMATOLOGY
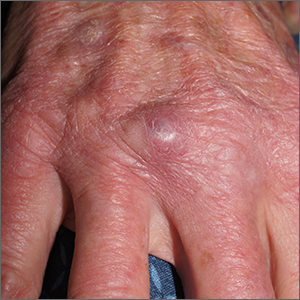
Nodule on gardener’s hand
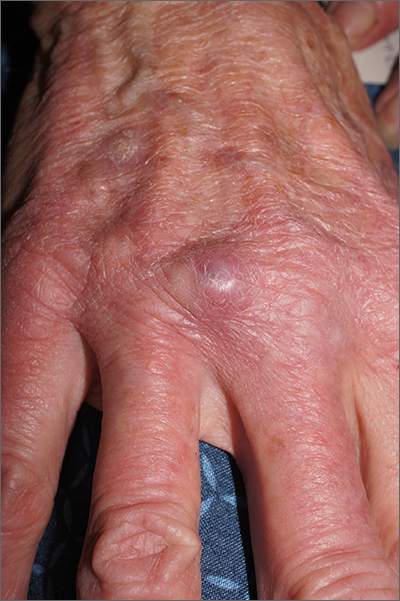
Using an 18-gauge needle, a simple incision and drainage was performed, and copious turbid and bloody material was expressed and cultured for aerobic and acid-fast bacteria, as well as fungus. The patient was started on trimethoprim sulfamethoxazole DS twice daily while cultures and sensitivities were pending. Cultures grew Staphylococcus lugdunensis, a coagulase-negative staph species known to cause a range of infections from simple skin infections to bacteremia and endocarditis.1 If the drainage had been viscous and clear to blood-tinged, that would have been more consistent with a ganglion cyst. Lack of drainage would have prompted a small punch biopsy to exclude a tumor.
Fortunately, S lugdunensis is often broadly sensitive to antibiotics, although treatment choices should follow antibiotic sensitivity testing. Any signs of systemic illness should be worked up with blood cultures and consideration of endocarditis or involvement of an implant. Penicillin is recommended as a first line systemic agent if sensitivities support this, and for abscesses, incision and drainage is recommended. The length of treatment for skin infections is generally 1 to 2 weeks, guided by response to therapy.
This patient’s nodule resolved following the incision and drainage and 7 days of therapy with trimethoprim sulfamethoxazole DS.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Kleiner E, Monk AB, Archer GL, et al. Clinical significance of Staphylococcus lugdunensis isolated from routine cultures. Clin Infect Dis. 2010;51:801-803. doi: 10.1086/656280

Using an 18-gauge needle, a simple incision and drainage was performed, and copious turbid and bloody material was expressed and cultured for aerobic and acid-fast bacteria, as well as fungus. The patient was started on trimethoprim sulfamethoxazole DS twice daily while cultures and sensitivities were pending. Cultures grew Staphylococcus lugdunensis, a coagulase-negative staph species known to cause a range of infections from simple skin infections to bacteremia and endocarditis.1 If the drainage had been viscous and clear to blood-tinged, that would have been more consistent with a ganglion cyst. Lack of drainage would have prompted a small punch biopsy to exclude a tumor.
Fortunately, S lugdunensis is often broadly sensitive to antibiotics, although treatment choices should follow antibiotic sensitivity testing. Any signs of systemic illness should be worked up with blood cultures and consideration of endocarditis or involvement of an implant. Penicillin is recommended as a first line systemic agent if sensitivities support this, and for abscesses, incision and drainage is recommended. The length of treatment for skin infections is generally 1 to 2 weeks, guided by response to therapy.
This patient’s nodule resolved following the incision and drainage and 7 days of therapy with trimethoprim sulfamethoxazole DS.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Using an 18-gauge needle, a simple incision and drainage was performed, and copious turbid and bloody material was expressed and cultured for aerobic and acid-fast bacteria, as well as fungus. The patient was started on trimethoprim sulfamethoxazole DS twice daily while cultures and sensitivities were pending. Cultures grew Staphylococcus lugdunensis, a coagulase-negative staph species known to cause a range of infections from simple skin infections to bacteremia and endocarditis.1 If the drainage had been viscous and clear to blood-tinged, that would have been more consistent with a ganglion cyst. Lack of drainage would have prompted a small punch biopsy to exclude a tumor.
Fortunately, S lugdunensis is often broadly sensitive to antibiotics, although treatment choices should follow antibiotic sensitivity testing. Any signs of systemic illness should be worked up with blood cultures and consideration of endocarditis or involvement of an implant. Penicillin is recommended as a first line systemic agent if sensitivities support this, and for abscesses, incision and drainage is recommended. The length of treatment for skin infections is generally 1 to 2 weeks, guided by response to therapy.
This patient’s nodule resolved following the incision and drainage and 7 days of therapy with trimethoprim sulfamethoxazole DS.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Kleiner E, Monk AB, Archer GL, et al. Clinical significance of Staphylococcus lugdunensis isolated from routine cultures. Clin Infect Dis. 2010;51:801-803. doi: 10.1086/656280
1. Kleiner E, Monk AB, Archer GL, et al. Clinical significance of Staphylococcus lugdunensis isolated from routine cultures. Clin Infect Dis. 2010;51:801-803. doi: 10.1086/656280
Is the limit of viability shifting again?
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.
Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.

Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.

Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
What to know about newly approved Alzheimer’s drug
, offering hope where there has been little for patients and their families affected by the devastating disease.
More than 6 million people in the United States live with Alzheimer’s.
It’s not a cure, but the drug, given intravenously every 2 weeks, has shown moderate positive effects in clinical trials in slowing early-stage disease.
But many are wary. As explained in an editorial in the journal The Lancet, “The Alzheimer’s disease community has become accustomed to false hope, disappointment, and controversy.”
Some worry about lecanemab’s safety as some people in clinical trials experienced serious side effects of bleeding and swelling in the brain. Scientists recently attributed a third death to lecanemab, brand name Leqembi, though the drugmaker disputed the medication was the cause.
So what should patients and their families make of this news? Here we answer some of the top questions surrounding the drug.
What does the FDA action mean?
The FDA granted accelerated approval to Leqembi after it showed positive trial results in slowing the progression of early-stage disease.
The FDA can grant accelerated approval for drugs that treat serious conditions and fill an unmet medical need while drugs continue to be studied in larger trials.
With the FDA approval in hand, doctors can now prescribe the medication.
Rebecca Edelmayer, PhD, the Alzheimer’s Association senior director of scientific engagement, says that with the FDA’s move, ramping up manufacturing – and eventually nationwide distribution and implementation – will take some time.
“Ask your doctor about availability,” she says. “The main issue is that, without insurance and Medicare coverage of this class of treatments, access for those who could benefit from the newly approved treatment will only be available to those who can pay out-of-pocket. Without coverage, people simply won’t be able to get the treatment.”
The Washington Post reports that with accelerated approval, drugmaker Eisai is expected to immediately apply for full FDA approval, which wouldn’t be likely to come before later this year. Full approval could help clear the path for Medicare coverage of the drug.
Potential benefit?
Those who got Leqembi in a clinical trial for 18 months experienced 27% less decline in memory and thinking relative to the group who got a placebo. It also reduced amyloid in the brain, the sticky protein that builds up in the brains of people with Alzheimer’s and is considered a hallmark of the disease.
Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, says, “It’s the first phase 3 study in our field of a disease-modifying drug where the clinical efficacy was very clear.”
Concerns about side effects
The drug has raised safety concerns as it has been linked with certain serious adverse events, including brain swelling and bleeding. In the trial, 14% of patients who received the drug experienced side effects that included brain swelling and bleeding, compared with about 11% in the placebo group.
Scientists have reportedly linked three deaths during the clinical trial to lecanemab, though it is unclear whether it caused the deaths.
Dr. Fillit notes that the first two people who died were on blood thinners when they received lecanemab.
“There are things about the use of the drug in the real world that we need to work out, especially in the context of people with comorbidities,” he says.
The third death is a little different, Dr. Fillit says. The patient, who had a stroke, showed signs of vasculitis, or inflammation of the blood vessels.
“We don’t know exactly what happened, but we do know it was very, very rare” among the people involved in the trials, he says.
Dr. Edelmayer says that the most common reported side effects during the trials were infusion-related reactions, headache, and amyloid-related imaging abnormalities (ARIA). According to the FDA, these abnormalities “are known to occur with antibodies of this class. ARIA usually does not have symptoms, although serious and life-threatening events rarely may occur.”
The FDA has added these as warnings to the drug’s label, describing the possible infusion-related reactions as flu-like symptoms, nausea, vomiting, and changes in blood pressure.
How much will it cost?
Eisai says that lecanemab will cost $26,500 a year.
In a draft report released in December, the Institute for Clinical and Economic Review said a price ranging from $8,500 to $20,600 a year would make the drug cost-effective. While the group has no authority to set prices, many large health insurers consider its reports when they negotiate prices and some drugmakers take into account ICER’s recommendations when setting prices.
An editorial in The Lancet last month warns that the cost will likely be “prohibitive” for low- and middle-income countries and many health systems don’t have the infrastructure for a widespread rollout.
Will Medicare cover it?
The Centers for Medicare & Medicaid Services, which runs Medicare, which covers most people with Alzheimer’s, has indicated it won’t broadly cover amyloid-lowering drugs until the drug gets full U.S. approval based on clinical benefits, as opposed to accelerated approval.
That means people would have to pay thousands out of pocket at first to get it.
The CMS decision effectively denies Medicare coverage of fast-tracked FDA-approved medications for Alzheimer’s disease unless the person is enrolled in an approved clinical trial.
On Dec. 19, the Alzheimer’s Association filed a formal request asking CMS to remove the trial-only requirement and provide full and unrestricted coverage for FDA-approved Alzheimer’s treatments.
CMS says in a statement issued after the announcement: “Because Eisai’s product, lecanemab, was granted accelerated approval by the FDA, it falls under CMS’s existing national coverage determination. CMS is examining available information and may reconsider its current coverage based on this review.”
“If lecanemab subsequently receives traditional FDA approval, CMS would provide broader coverage,” the statement says.
Who benefits most from this drug?
Lecanemab is a treatment for people with early-stage Alzheimer’s disease who have amyloid in their brain. This means people with other types of dementia, or those in the later stages of Alzheimer’s disease, are not likely to improve with this drug.
Who makes lecanemab?
Japan-based Eisai is developing the drug, a monoclonal antibody, in collaboration with the U.S. company Biogen.
What’s the Alzheimer’s Association’s view?
The association urged accelerated FDA approval. In a statement, it says it “welcomes and is further encouraged” by the clinical trial results.
It says data published in the New England Journal of Medicine confirms lecanemab “can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease.”
“We are energized at the progress we are seeing in the research pipeline. The science is telling us that although antiamyloid treatments are not a cure – they are not going to be the end of treating Alzheimer’s – they are certainly the beginning,” Dr. Edelmayer says.
Are there alternatives?
The FDA gave accelerated approval to Biogen to produce another drug for Alzheimer’s, Aduhelm (aducanemab), in 2021, but the move was controversial as the drug’s effectiveness was widely questioned. It has since largely been pulled from the market.
Aduhelm had been the first approved early-stage Alzheimer’s treatment since 2003.
A version of this article first appeared on WebMD.com.
, offering hope where there has been little for patients and their families affected by the devastating disease.
More than 6 million people in the United States live with Alzheimer’s.
It’s not a cure, but the drug, given intravenously every 2 weeks, has shown moderate positive effects in clinical trials in slowing early-stage disease.
But many are wary. As explained in an editorial in the journal The Lancet, “The Alzheimer’s disease community has become accustomed to false hope, disappointment, and controversy.”
Some worry about lecanemab’s safety as some people in clinical trials experienced serious side effects of bleeding and swelling in the brain. Scientists recently attributed a third death to lecanemab, brand name Leqembi, though the drugmaker disputed the medication was the cause.
So what should patients and their families make of this news? Here we answer some of the top questions surrounding the drug.
What does the FDA action mean?
The FDA granted accelerated approval to Leqembi after it showed positive trial results in slowing the progression of early-stage disease.
The FDA can grant accelerated approval for drugs that treat serious conditions and fill an unmet medical need while drugs continue to be studied in larger trials.
With the FDA approval in hand, doctors can now prescribe the medication.
Rebecca Edelmayer, PhD, the Alzheimer’s Association senior director of scientific engagement, says that with the FDA’s move, ramping up manufacturing – and eventually nationwide distribution and implementation – will take some time.
“Ask your doctor about availability,” she says. “The main issue is that, without insurance and Medicare coverage of this class of treatments, access for those who could benefit from the newly approved treatment will only be available to those who can pay out-of-pocket. Without coverage, people simply won’t be able to get the treatment.”
The Washington Post reports that with accelerated approval, drugmaker Eisai is expected to immediately apply for full FDA approval, which wouldn’t be likely to come before later this year. Full approval could help clear the path for Medicare coverage of the drug.
Potential benefit?
Those who got Leqembi in a clinical trial for 18 months experienced 27% less decline in memory and thinking relative to the group who got a placebo. It also reduced amyloid in the brain, the sticky protein that builds up in the brains of people with Alzheimer’s and is considered a hallmark of the disease.
Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, says, “It’s the first phase 3 study in our field of a disease-modifying drug where the clinical efficacy was very clear.”
Concerns about side effects
The drug has raised safety concerns as it has been linked with certain serious adverse events, including brain swelling and bleeding. In the trial, 14% of patients who received the drug experienced side effects that included brain swelling and bleeding, compared with about 11% in the placebo group.
Scientists have reportedly linked three deaths during the clinical trial to lecanemab, though it is unclear whether it caused the deaths.
Dr. Fillit notes that the first two people who died were on blood thinners when they received lecanemab.
“There are things about the use of the drug in the real world that we need to work out, especially in the context of people with comorbidities,” he says.
The third death is a little different, Dr. Fillit says. The patient, who had a stroke, showed signs of vasculitis, or inflammation of the blood vessels.
“We don’t know exactly what happened, but we do know it was very, very rare” among the people involved in the trials, he says.
Dr. Edelmayer says that the most common reported side effects during the trials were infusion-related reactions, headache, and amyloid-related imaging abnormalities (ARIA). According to the FDA, these abnormalities “are known to occur with antibodies of this class. ARIA usually does not have symptoms, although serious and life-threatening events rarely may occur.”
The FDA has added these as warnings to the drug’s label, describing the possible infusion-related reactions as flu-like symptoms, nausea, vomiting, and changes in blood pressure.
How much will it cost?
Eisai says that lecanemab will cost $26,500 a year.
In a draft report released in December, the Institute for Clinical and Economic Review said a price ranging from $8,500 to $20,600 a year would make the drug cost-effective. While the group has no authority to set prices, many large health insurers consider its reports when they negotiate prices and some drugmakers take into account ICER’s recommendations when setting prices.
An editorial in The Lancet last month warns that the cost will likely be “prohibitive” for low- and middle-income countries and many health systems don’t have the infrastructure for a widespread rollout.
Will Medicare cover it?
The Centers for Medicare & Medicaid Services, which runs Medicare, which covers most people with Alzheimer’s, has indicated it won’t broadly cover amyloid-lowering drugs until the drug gets full U.S. approval based on clinical benefits, as opposed to accelerated approval.
That means people would have to pay thousands out of pocket at first to get it.
The CMS decision effectively denies Medicare coverage of fast-tracked FDA-approved medications for Alzheimer’s disease unless the person is enrolled in an approved clinical trial.
On Dec. 19, the Alzheimer’s Association filed a formal request asking CMS to remove the trial-only requirement and provide full and unrestricted coverage for FDA-approved Alzheimer’s treatments.
CMS says in a statement issued after the announcement: “Because Eisai’s product, lecanemab, was granted accelerated approval by the FDA, it falls under CMS’s existing national coverage determination. CMS is examining available information and may reconsider its current coverage based on this review.”
“If lecanemab subsequently receives traditional FDA approval, CMS would provide broader coverage,” the statement says.
Who benefits most from this drug?
Lecanemab is a treatment for people with early-stage Alzheimer’s disease who have amyloid in their brain. This means people with other types of dementia, or those in the later stages of Alzheimer’s disease, are not likely to improve with this drug.
Who makes lecanemab?
Japan-based Eisai is developing the drug, a monoclonal antibody, in collaboration with the U.S. company Biogen.
What’s the Alzheimer’s Association’s view?
The association urged accelerated FDA approval. In a statement, it says it “welcomes and is further encouraged” by the clinical trial results.
It says data published in the New England Journal of Medicine confirms lecanemab “can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease.”
“We are energized at the progress we are seeing in the research pipeline. The science is telling us that although antiamyloid treatments are not a cure – they are not going to be the end of treating Alzheimer’s – they are certainly the beginning,” Dr. Edelmayer says.
Are there alternatives?
The FDA gave accelerated approval to Biogen to produce another drug for Alzheimer’s, Aduhelm (aducanemab), in 2021, but the move was controversial as the drug’s effectiveness was widely questioned. It has since largely been pulled from the market.
Aduhelm had been the first approved early-stage Alzheimer’s treatment since 2003.
A version of this article first appeared on WebMD.com.
, offering hope where there has been little for patients and their families affected by the devastating disease.
More than 6 million people in the United States live with Alzheimer’s.
It’s not a cure, but the drug, given intravenously every 2 weeks, has shown moderate positive effects in clinical trials in slowing early-stage disease.
But many are wary. As explained in an editorial in the journal The Lancet, “The Alzheimer’s disease community has become accustomed to false hope, disappointment, and controversy.”
Some worry about lecanemab’s safety as some people in clinical trials experienced serious side effects of bleeding and swelling in the brain. Scientists recently attributed a third death to lecanemab, brand name Leqembi, though the drugmaker disputed the medication was the cause.
So what should patients and their families make of this news? Here we answer some of the top questions surrounding the drug.
What does the FDA action mean?
The FDA granted accelerated approval to Leqembi after it showed positive trial results in slowing the progression of early-stage disease.
The FDA can grant accelerated approval for drugs that treat serious conditions and fill an unmet medical need while drugs continue to be studied in larger trials.
With the FDA approval in hand, doctors can now prescribe the medication.
Rebecca Edelmayer, PhD, the Alzheimer’s Association senior director of scientific engagement, says that with the FDA’s move, ramping up manufacturing – and eventually nationwide distribution and implementation – will take some time.
“Ask your doctor about availability,” she says. “The main issue is that, without insurance and Medicare coverage of this class of treatments, access for those who could benefit from the newly approved treatment will only be available to those who can pay out-of-pocket. Without coverage, people simply won’t be able to get the treatment.”
The Washington Post reports that with accelerated approval, drugmaker Eisai is expected to immediately apply for full FDA approval, which wouldn’t be likely to come before later this year. Full approval could help clear the path for Medicare coverage of the drug.
Potential benefit?
Those who got Leqembi in a clinical trial for 18 months experienced 27% less decline in memory and thinking relative to the group who got a placebo. It also reduced amyloid in the brain, the sticky protein that builds up in the brains of people with Alzheimer’s and is considered a hallmark of the disease.
Howard Fillit, MD, cofounder and chief science officer of the Alzheimer’s Drug Discovery Foundation, says, “It’s the first phase 3 study in our field of a disease-modifying drug where the clinical efficacy was very clear.”
Concerns about side effects
The drug has raised safety concerns as it has been linked with certain serious adverse events, including brain swelling and bleeding. In the trial, 14% of patients who received the drug experienced side effects that included brain swelling and bleeding, compared with about 11% in the placebo group.
Scientists have reportedly linked three deaths during the clinical trial to lecanemab, though it is unclear whether it caused the deaths.
Dr. Fillit notes that the first two people who died were on blood thinners when they received lecanemab.
“There are things about the use of the drug in the real world that we need to work out, especially in the context of people with comorbidities,” he says.
The third death is a little different, Dr. Fillit says. The patient, who had a stroke, showed signs of vasculitis, or inflammation of the blood vessels.
“We don’t know exactly what happened, but we do know it was very, very rare” among the people involved in the trials, he says.
Dr. Edelmayer says that the most common reported side effects during the trials were infusion-related reactions, headache, and amyloid-related imaging abnormalities (ARIA). According to the FDA, these abnormalities “are known to occur with antibodies of this class. ARIA usually does not have symptoms, although serious and life-threatening events rarely may occur.”
The FDA has added these as warnings to the drug’s label, describing the possible infusion-related reactions as flu-like symptoms, nausea, vomiting, and changes in blood pressure.
How much will it cost?
Eisai says that lecanemab will cost $26,500 a year.
In a draft report released in December, the Institute for Clinical and Economic Review said a price ranging from $8,500 to $20,600 a year would make the drug cost-effective. While the group has no authority to set prices, many large health insurers consider its reports when they negotiate prices and some drugmakers take into account ICER’s recommendations when setting prices.
An editorial in The Lancet last month warns that the cost will likely be “prohibitive” for low- and middle-income countries and many health systems don’t have the infrastructure for a widespread rollout.
Will Medicare cover it?
The Centers for Medicare & Medicaid Services, which runs Medicare, which covers most people with Alzheimer’s, has indicated it won’t broadly cover amyloid-lowering drugs until the drug gets full U.S. approval based on clinical benefits, as opposed to accelerated approval.
That means people would have to pay thousands out of pocket at first to get it.
The CMS decision effectively denies Medicare coverage of fast-tracked FDA-approved medications for Alzheimer’s disease unless the person is enrolled in an approved clinical trial.
On Dec. 19, the Alzheimer’s Association filed a formal request asking CMS to remove the trial-only requirement and provide full and unrestricted coverage for FDA-approved Alzheimer’s treatments.
CMS says in a statement issued after the announcement: “Because Eisai’s product, lecanemab, was granted accelerated approval by the FDA, it falls under CMS’s existing national coverage determination. CMS is examining available information and may reconsider its current coverage based on this review.”
“If lecanemab subsequently receives traditional FDA approval, CMS would provide broader coverage,” the statement says.
Who benefits most from this drug?
Lecanemab is a treatment for people with early-stage Alzheimer’s disease who have amyloid in their brain. This means people with other types of dementia, or those in the later stages of Alzheimer’s disease, are not likely to improve with this drug.
Who makes lecanemab?
Japan-based Eisai is developing the drug, a monoclonal antibody, in collaboration with the U.S. company Biogen.
What’s the Alzheimer’s Association’s view?
The association urged accelerated FDA approval. In a statement, it says it “welcomes and is further encouraged” by the clinical trial results.
It says data published in the New England Journal of Medicine confirms lecanemab “can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s disease.”
“We are energized at the progress we are seeing in the research pipeline. The science is telling us that although antiamyloid treatments are not a cure – they are not going to be the end of treating Alzheimer’s – they are certainly the beginning,” Dr. Edelmayer says.
Are there alternatives?
The FDA gave accelerated approval to Biogen to produce another drug for Alzheimer’s, Aduhelm (aducanemab), in 2021, but the move was controversial as the drug’s effectiveness was widely questioned. It has since largely been pulled from the market.
Aduhelm had been the first approved early-stage Alzheimer’s treatment since 2003.
A version of this article first appeared on WebMD.com.
Atrial fibrillation: Sex differences and modifiable risk factors
This transcript has been edited for clarity.
Hello. This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital.
We looked at these questions in our vitamin D and omega-3 trial VITAL in an ancillary study called VITAL Rhythm, led by Dr. Christine Albert at Cedars-Sinai. And this particular project was led by Dr. Hasan Siddiqi at Vanderbilt.
As you know, AF is the most common arrhythmia in the world, and it’s burgeoning in numbers, primarily because of the aging of the population. It’s also a major cause of stroke, heart failure, and cardiovascular mortality. Although women are known to have lower rates of AF than men, they’re also known to have a higher risk for cardiovascular complications and sequelae, such as higher risk for stroke and CVD mortality. Therefore, we thought that understanding sex differences in risk and modifiable risk factors for AF that could reduce the burden of disease would be important.
It’s known that greater height is a risk factor for AF, but the extent to which it explains the differences in AF risk between men and women isn’t really known. So we looked at these questions in the VITAL cohort. VITAL has more than 25,000 participants. It’s a large, diverse, nationwide cohort. About 51% are women, and all are aged 50 years or older, with a mean age of 67. All were free of known clinical cardiovascular disease at the start of the study.
AF reports were confirmed by medical records and also supplemented by Medicare CMS linkage for fuller ascertainment of outcomes. We had 900 incident cases of AF in the study, and we did see that women were less likely to be diagnosed with AF. They had a 32% lower risk – strongly statistically significant compared with men, with a P < .001. Women were also more likely to be symptomatic: About 77% of women vs. 63% of men had symptoms prior to or at diagnosis.
It was very interesting that adjustment for height eliminated the lower risk for AF in women compared with men. After accounting for height, there was not only no reduction in risk for AF among the women, there was actually a reversal of the association so that there was a slightly higher risk for AF in the women. Other risk factors for AF in the cohort included older age, higher body mass index, hypertension, and higher consumption of alcohol. We did not see an association between diabetes and higher risk for AF. We also saw no clear association with physical activity, although very strenuous physical activity has been linked to AF in some other studies.
We looked at the interventions of vitamin D (2,000 IU/day) and omega-3 fatty acids (460 mg/day of EPA and 380 mg/day of DHA) and found no association with AF, although some other studies have seen increased risk for AF with higher doses of the marine omega-3s > 1 g/day and certainly at doses of 4 g/day. So overall, the findings highlight the fact that many of the risk factors for AF do seem to be modifiable, and it is really important to identify and try to reduce these risk factors in order to reduce the burden of AF. This may be particularly important in women because women are more likely to have stroke and cardiovascular mortality in these adverse cardiovascular outcomes.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital.
We looked at these questions in our vitamin D and omega-3 trial VITAL in an ancillary study called VITAL Rhythm, led by Dr. Christine Albert at Cedars-Sinai. And this particular project was led by Dr. Hasan Siddiqi at Vanderbilt.
As you know, AF is the most common arrhythmia in the world, and it’s burgeoning in numbers, primarily because of the aging of the population. It’s also a major cause of stroke, heart failure, and cardiovascular mortality. Although women are known to have lower rates of AF than men, they’re also known to have a higher risk for cardiovascular complications and sequelae, such as higher risk for stroke and CVD mortality. Therefore, we thought that understanding sex differences in risk and modifiable risk factors for AF that could reduce the burden of disease would be important.
It’s known that greater height is a risk factor for AF, but the extent to which it explains the differences in AF risk between men and women isn’t really known. So we looked at these questions in the VITAL cohort. VITAL has more than 25,000 participants. It’s a large, diverse, nationwide cohort. About 51% are women, and all are aged 50 years or older, with a mean age of 67. All were free of known clinical cardiovascular disease at the start of the study.
AF reports were confirmed by medical records and also supplemented by Medicare CMS linkage for fuller ascertainment of outcomes. We had 900 incident cases of AF in the study, and we did see that women were less likely to be diagnosed with AF. They had a 32% lower risk – strongly statistically significant compared with men, with a P < .001. Women were also more likely to be symptomatic: About 77% of women vs. 63% of men had symptoms prior to or at diagnosis.
It was very interesting that adjustment for height eliminated the lower risk for AF in women compared with men. After accounting for height, there was not only no reduction in risk for AF among the women, there was actually a reversal of the association so that there was a slightly higher risk for AF in the women. Other risk factors for AF in the cohort included older age, higher body mass index, hypertension, and higher consumption of alcohol. We did not see an association between diabetes and higher risk for AF. We also saw no clear association with physical activity, although very strenuous physical activity has been linked to AF in some other studies.
We looked at the interventions of vitamin D (2,000 IU/day) and omega-3 fatty acids (460 mg/day of EPA and 380 mg/day of DHA) and found no association with AF, although some other studies have seen increased risk for AF with higher doses of the marine omega-3s > 1 g/day and certainly at doses of 4 g/day. So overall, the findings highlight the fact that many of the risk factors for AF do seem to be modifiable, and it is really important to identify and try to reduce these risk factors in order to reduce the burden of AF. This may be particularly important in women because women are more likely to have stroke and cardiovascular mortality in these adverse cardiovascular outcomes.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital.
We looked at these questions in our vitamin D and omega-3 trial VITAL in an ancillary study called VITAL Rhythm, led by Dr. Christine Albert at Cedars-Sinai. And this particular project was led by Dr. Hasan Siddiqi at Vanderbilt.
As you know, AF is the most common arrhythmia in the world, and it’s burgeoning in numbers, primarily because of the aging of the population. It’s also a major cause of stroke, heart failure, and cardiovascular mortality. Although women are known to have lower rates of AF than men, they’re also known to have a higher risk for cardiovascular complications and sequelae, such as higher risk for stroke and CVD mortality. Therefore, we thought that understanding sex differences in risk and modifiable risk factors for AF that could reduce the burden of disease would be important.
It’s known that greater height is a risk factor for AF, but the extent to which it explains the differences in AF risk between men and women isn’t really known. So we looked at these questions in the VITAL cohort. VITAL has more than 25,000 participants. It’s a large, diverse, nationwide cohort. About 51% are women, and all are aged 50 years or older, with a mean age of 67. All were free of known clinical cardiovascular disease at the start of the study.
AF reports were confirmed by medical records and also supplemented by Medicare CMS linkage for fuller ascertainment of outcomes. We had 900 incident cases of AF in the study, and we did see that women were less likely to be diagnosed with AF. They had a 32% lower risk – strongly statistically significant compared with men, with a P < .001. Women were also more likely to be symptomatic: About 77% of women vs. 63% of men had symptoms prior to or at diagnosis.
It was very interesting that adjustment for height eliminated the lower risk for AF in women compared with men. After accounting for height, there was not only no reduction in risk for AF among the women, there was actually a reversal of the association so that there was a slightly higher risk for AF in the women. Other risk factors for AF in the cohort included older age, higher body mass index, hypertension, and higher consumption of alcohol. We did not see an association between diabetes and higher risk for AF. We also saw no clear association with physical activity, although very strenuous physical activity has been linked to AF in some other studies.
We looked at the interventions of vitamin D (2,000 IU/day) and omega-3 fatty acids (460 mg/day of EPA and 380 mg/day of DHA) and found no association with AF, although some other studies have seen increased risk for AF with higher doses of the marine omega-3s > 1 g/day and certainly at doses of 4 g/day. So overall, the findings highlight the fact that many of the risk factors for AF do seem to be modifiable, and it is really important to identify and try to reduce these risk factors in order to reduce the burden of AF. This may be particularly important in women because women are more likely to have stroke and cardiovascular mortality in these adverse cardiovascular outcomes.
A version of this article first appeared on Medscape.com.
Medical student well-being during the COVID-19 pandemic
During the initial stage of the COVID-19 pandemic U.S. medical students were suspended from in-person clinical interaction. This decision was based on specific guidance from the Association of American Medical Colleges and subsequently implemented in medical schools across the United States.1 Our research project addressed students’ stress level before and after clinical in-person suspension and assessed medical students perceived COVID-19–related risk level. We were particularly curious to learn about students’ emotional struggles as they navigated the initial pedagogical uncertainty associated with the pandemic.
It is likely that heightened stress was greater than before and the rationale was likely multifactorial in nature.2
One key stressor U.S. medical students faced was the negative impacts of COVID-19 on medical education. U.S. Medical Licensing Examination exam-taking was severely impacted, and some students needed to reschedule their test dates because of increased restrictions at testing centers. Third-year medical students in particular were worried about how COVID-19 would influence their residency application; for example, in-person residency interviews and away rotations as fourth-year medical students. Another concern was not being able to be involved in clinical work during the direst stage of this public health emergency because of personal protective equipment shortages and attempts to reduce community spread of COVID-19.
Our study also showed that students had a relatively lower perceived risk level when it comes to COVID-19 than health care workers in the 2003 SARS epidemic, which we suspect is mostly attributable to the suspension of clinical in-person interaction. We also found that female gender and self-reported mental illness diagnosis were two risk factors for perceived stress level, consistent with our current literature.
The reality of clinical in-person inaction caused by PPE shortage and limited telehealth options, together with social isolation and uncertainty regarding future education opportunities, appear to have had a detrimental effect on medical students’ psychological wellbeing. This did not have to be the case. Some medical students found innovative ways to stay involved.
For example, in 2020 some of Dr. Zhang’s classmates helped proctor virtual group therapy sessions held by the local National Alliance on Mental Illness chapter. Medical students at the Icahn School of Medicine at Mount Sinai, New York were not only able to engage in telehealth but also join other task forces, such as PPE supply, distribution, and coordination, morale promotion, and administrative services.3 Finally, many medical students in New York volunteered in providing child care for frontline doctors to help relieve their burden.4 These actions, if implemented more widely, may have had a protective effect on the stress and well-being of medical students at that time.
While our study focused on the academic side of things, the personal impacts from COVID-19 need to be acknowledged – sickness from COVID-19 and its sequelae, family loss fromCOVID-19, financial struggle, and racial targeting of Asians to name a few. COVID-19 has influenced many families’ livelihood and changed our understanding of ourselves, others, and the world in unprecedented ways.
Fast forward to today – medical students are used to learning and living in a world with an ongoing pandemic, and medical education and residency application process have adapted to this new normal. The once-crippling uncertainty surrounding COVID-19 and disastrous PPE shortages have passed. Yet, COVID-19 continues to be a stressor. In fact, burnout related to “COVID-19 fatigue” has been on the rise and one recent national survey shows one in five physicians intends to leave practice within 2 years.5
Meanwhile, uncertainty continued to persist, as in August 2022 monkeypox was declared a public health emergency in the United States.6 What Dr. Zhang learned as a medical student during the initial months of COVID-19 continues to be relevant: connect with loved ones, understand the changing reality, process the emotions, recognize what is under one’s control, have a solution-oriented mindset, and be forgiving and patient with oneself and others.
Dr. Zhang is a second-year psychiatry resident physician at Saint Elizabeth’s Hospital/DC DBH, Washington. Dr. Himelhoch serves as professor and chair of the department of psychiatry at the University of Kentucky, Lexington. His research focuses on developing and studying the efficacy of innovative strategies aimed at improving the health and welfare among people with co-occurring psychiatric and substance use disorders.
References
1. Association of American Medical Colleges. Important Guidance for Medical Students on Clinical Rotations During the Coronavirus (COVID-19) Outbreak. 2020 Mar 17.
2. Zhang Y et al. Psychiatry Res. 2022;313:114595. doi: 10.1016/j.psychres.2022.114595.
3. Bahethi RR et al. Acad Med. 2021 Jun 1;96(6):859-63. doi: 10.1097/ACM.0000000000003863.
4. Krieger P and Goodnough A. Medical Students, Sidelined for Now, Find New Ways to Fight Coronavirus. The New York Times. 2020 Mar 23.
5. Abbasi J. JAMA. 2022 Apr 19;327(15):1435-7. doi: 10.1001/jama.2022.5074.
6. Department of Health & Human Services. Biden-Harris Administration Bolsters Monkeypox Response; HHS Secretary Becerra Declares Public Health Emergency. 2022 Aug 4.
During the initial stage of the COVID-19 pandemic U.S. medical students were suspended from in-person clinical interaction. This decision was based on specific guidance from the Association of American Medical Colleges and subsequently implemented in medical schools across the United States.1 Our research project addressed students’ stress level before and after clinical in-person suspension and assessed medical students perceived COVID-19–related risk level. We were particularly curious to learn about students’ emotional struggles as they navigated the initial pedagogical uncertainty associated with the pandemic.
It is likely that heightened stress was greater than before and the rationale was likely multifactorial in nature.2
One key stressor U.S. medical students faced was the negative impacts of COVID-19 on medical education. U.S. Medical Licensing Examination exam-taking was severely impacted, and some students needed to reschedule their test dates because of increased restrictions at testing centers. Third-year medical students in particular were worried about how COVID-19 would influence their residency application; for example, in-person residency interviews and away rotations as fourth-year medical students. Another concern was not being able to be involved in clinical work during the direst stage of this public health emergency because of personal protective equipment shortages and attempts to reduce community spread of COVID-19.
Our study also showed that students had a relatively lower perceived risk level when it comes to COVID-19 than health care workers in the 2003 SARS epidemic, which we suspect is mostly attributable to the suspension of clinical in-person interaction. We also found that female gender and self-reported mental illness diagnosis were two risk factors for perceived stress level, consistent with our current literature.
The reality of clinical in-person inaction caused by PPE shortage and limited telehealth options, together with social isolation and uncertainty regarding future education opportunities, appear to have had a detrimental effect on medical students’ psychological wellbeing. This did not have to be the case. Some medical students found innovative ways to stay involved.
For example, in 2020 some of Dr. Zhang’s classmates helped proctor virtual group therapy sessions held by the local National Alliance on Mental Illness chapter. Medical students at the Icahn School of Medicine at Mount Sinai, New York were not only able to engage in telehealth but also join other task forces, such as PPE supply, distribution, and coordination, morale promotion, and administrative services.3 Finally, many medical students in New York volunteered in providing child care for frontline doctors to help relieve their burden.4 These actions, if implemented more widely, may have had a protective effect on the stress and well-being of medical students at that time.
While our study focused on the academic side of things, the personal impacts from COVID-19 need to be acknowledged – sickness from COVID-19 and its sequelae, family loss fromCOVID-19, financial struggle, and racial targeting of Asians to name a few. COVID-19 has influenced many families’ livelihood and changed our understanding of ourselves, others, and the world in unprecedented ways.
Fast forward to today – medical students are used to learning and living in a world with an ongoing pandemic, and medical education and residency application process have adapted to this new normal. The once-crippling uncertainty surrounding COVID-19 and disastrous PPE shortages have passed. Yet, COVID-19 continues to be a stressor. In fact, burnout related to “COVID-19 fatigue” has been on the rise and one recent national survey shows one in five physicians intends to leave practice within 2 years.5
Meanwhile, uncertainty continued to persist, as in August 2022 monkeypox was declared a public health emergency in the United States.6 What Dr. Zhang learned as a medical student during the initial months of COVID-19 continues to be relevant: connect with loved ones, understand the changing reality, process the emotions, recognize what is under one’s control, have a solution-oriented mindset, and be forgiving and patient with oneself and others.
Dr. Zhang is a second-year psychiatry resident physician at Saint Elizabeth’s Hospital/DC DBH, Washington. Dr. Himelhoch serves as professor and chair of the department of psychiatry at the University of Kentucky, Lexington. His research focuses on developing and studying the efficacy of innovative strategies aimed at improving the health and welfare among people with co-occurring psychiatric and substance use disorders.
References
1. Association of American Medical Colleges. Important Guidance for Medical Students on Clinical Rotations During the Coronavirus (COVID-19) Outbreak. 2020 Mar 17.
2. Zhang Y et al. Psychiatry Res. 2022;313:114595. doi: 10.1016/j.psychres.2022.114595.
3. Bahethi RR et al. Acad Med. 2021 Jun 1;96(6):859-63. doi: 10.1097/ACM.0000000000003863.
4. Krieger P and Goodnough A. Medical Students, Sidelined for Now, Find New Ways to Fight Coronavirus. The New York Times. 2020 Mar 23.
5. Abbasi J. JAMA. 2022 Apr 19;327(15):1435-7. doi: 10.1001/jama.2022.5074.
6. Department of Health & Human Services. Biden-Harris Administration Bolsters Monkeypox Response; HHS Secretary Becerra Declares Public Health Emergency. 2022 Aug 4.
During the initial stage of the COVID-19 pandemic U.S. medical students were suspended from in-person clinical interaction. This decision was based on specific guidance from the Association of American Medical Colleges and subsequently implemented in medical schools across the United States.1 Our research project addressed students’ stress level before and after clinical in-person suspension and assessed medical students perceived COVID-19–related risk level. We were particularly curious to learn about students’ emotional struggles as they navigated the initial pedagogical uncertainty associated with the pandemic.
It is likely that heightened stress was greater than before and the rationale was likely multifactorial in nature.2
One key stressor U.S. medical students faced was the negative impacts of COVID-19 on medical education. U.S. Medical Licensing Examination exam-taking was severely impacted, and some students needed to reschedule their test dates because of increased restrictions at testing centers. Third-year medical students in particular were worried about how COVID-19 would influence their residency application; for example, in-person residency interviews and away rotations as fourth-year medical students. Another concern was not being able to be involved in clinical work during the direst stage of this public health emergency because of personal protective equipment shortages and attempts to reduce community spread of COVID-19.
Our study also showed that students had a relatively lower perceived risk level when it comes to COVID-19 than health care workers in the 2003 SARS epidemic, which we suspect is mostly attributable to the suspension of clinical in-person interaction. We also found that female gender and self-reported mental illness diagnosis were two risk factors for perceived stress level, consistent with our current literature.
The reality of clinical in-person inaction caused by PPE shortage and limited telehealth options, together with social isolation and uncertainty regarding future education opportunities, appear to have had a detrimental effect on medical students’ psychological wellbeing. This did not have to be the case. Some medical students found innovative ways to stay involved.
For example, in 2020 some of Dr. Zhang’s classmates helped proctor virtual group therapy sessions held by the local National Alliance on Mental Illness chapter. Medical students at the Icahn School of Medicine at Mount Sinai, New York were not only able to engage in telehealth but also join other task forces, such as PPE supply, distribution, and coordination, morale promotion, and administrative services.3 Finally, many medical students in New York volunteered in providing child care for frontline doctors to help relieve their burden.4 These actions, if implemented more widely, may have had a protective effect on the stress and well-being of medical students at that time.
While our study focused on the academic side of things, the personal impacts from COVID-19 need to be acknowledged – sickness from COVID-19 and its sequelae, family loss fromCOVID-19, financial struggle, and racial targeting of Asians to name a few. COVID-19 has influenced many families’ livelihood and changed our understanding of ourselves, others, and the world in unprecedented ways.
Fast forward to today – medical students are used to learning and living in a world with an ongoing pandemic, and medical education and residency application process have adapted to this new normal. The once-crippling uncertainty surrounding COVID-19 and disastrous PPE shortages have passed. Yet, COVID-19 continues to be a stressor. In fact, burnout related to “COVID-19 fatigue” has been on the rise and one recent national survey shows one in five physicians intends to leave practice within 2 years.5
Meanwhile, uncertainty continued to persist, as in August 2022 monkeypox was declared a public health emergency in the United States.6 What Dr. Zhang learned as a medical student during the initial months of COVID-19 continues to be relevant: connect with loved ones, understand the changing reality, process the emotions, recognize what is under one’s control, have a solution-oriented mindset, and be forgiving and patient with oneself and others.
Dr. Zhang is a second-year psychiatry resident physician at Saint Elizabeth’s Hospital/DC DBH, Washington. Dr. Himelhoch serves as professor and chair of the department of psychiatry at the University of Kentucky, Lexington. His research focuses on developing and studying the efficacy of innovative strategies aimed at improving the health and welfare among people with co-occurring psychiatric and substance use disorders.
References
1. Association of American Medical Colleges. Important Guidance for Medical Students on Clinical Rotations During the Coronavirus (COVID-19) Outbreak. 2020 Mar 17.
2. Zhang Y et al. Psychiatry Res. 2022;313:114595. doi: 10.1016/j.psychres.2022.114595.
3. Bahethi RR et al. Acad Med. 2021 Jun 1;96(6):859-63. doi: 10.1097/ACM.0000000000003863.
4. Krieger P and Goodnough A. Medical Students, Sidelined for Now, Find New Ways to Fight Coronavirus. The New York Times. 2020 Mar 23.
5. Abbasi J. JAMA. 2022 Apr 19;327(15):1435-7. doi: 10.1001/jama.2022.5074.
6. Department of Health & Human Services. Biden-Harris Administration Bolsters Monkeypox Response; HHS Secretary Becerra Declares Public Health Emergency. 2022 Aug 4.
Obesity impacts peripheral airway reactivity, asthma
Peripheral airway response to methacholine was similar among obese adults with and without asthma, although forced expiratory volume was lower for those with asthma, based on data from 53 individuals.
Obesity remains a risk factor for asthma, and obese individuals with asthma tend to have worse control and more severe disease, compared with nonobese asthma patients, wrote Anne E. Dixon, BM, BCh, of the University of Vermont, Burlington, and colleagues.
Previous studies have shown that airway reactivity can occur in obese individuals without airway inflammation, but studies characterizing obese asthma based on lung function are lacking, they said. “Combining spirometry and oscillometry might reveal abnormalities in lung mechanics particularly pertinent to people with obesity and asthma,” the researchers noted.
In a cross-sectional study published in the journal CHEST, the researchers reviewed data from 31 obese adults with asthma and 22 obese adults without asthma. The participants were aged 18 years and older, with forced expiratory volume (FEV1) of at least 60% of predicted. All had class III obesity, with an average BMI of 47.2 kg/m2 for those with asthma and 46.7 kg/m2 for nonasthma controls. Demographic characteristics were similar between the groups.
Airway reactivity was defined as a 20% decrease in FEV1 and/or a 50% change in resistance or reactance at 5 Hz (R5 and X5), at a concentration of 16 mg/mL or less of methacholine. Patients were assessed using spirometry and oscillometry.
For those with asthma, the resistance at 5 Hz, measured by oscillometry, increased by 52% in response to the PC20 methacholine challenge, with an area under the reactance curve (AX) of 361%. For controls without asthma, the resistance at 5 Hz increased by 45%, with an AX of 268% in response to 16 mg/mL of methacholine.
This finding suggests that obesity predisposes individuals to peripheral airway reactivity regardless of asthma status, the researchers wrote in their discussion.
The researchers also identified two distinct groups of asthma patients categorized by respiratory system impedance based on more concordant vs. discordant bronchoconstriction in the central and peripheral airways. The baseline AX for these two groups was 11.8 and 46.7, respectively, with interquartile ranges of 9.9-23.4 and 23.2-53.7, respectively.
The discordant group included only women, and these patients reported significantly more gastroesophageal reflux, increased chest tightness, and more wheezing and asthma exacerbations than the concordant group, which may be related to air trapping, shown on previous studies of obese individuals with asthma, the researchers wrote.
The findings were limited by several factors, including the measurement of airway impedance only at the peak methacholine dose and the measurement of oscillometry after spirometry, the researchers noted. Other limitations included the relatively small study population at a single center, and the focus on obese individuals only.
More research is needed in larger and more diverse patient populations, but the results support the characterization of a subgroup of obese asthma patients with significant peripheral airway dysfunction, the researchers wrote.
“Oscillometry testing can reveal a physiologic phenotype of asthma in obesity that may be related to worse symptoms and more severe disease, and also reveal subclinical abnormalities in people with obesity, but without clinically diagnosed lung disease,” they concluded.
The study was supported in part by the National Institutes of Health. The researchers declared no financial conflicts.
A version of this article first appeared on Medscape.com.
Peripheral airway response to methacholine was similar among obese adults with and without asthma, although forced expiratory volume was lower for those with asthma, based on data from 53 individuals.
Obesity remains a risk factor for asthma, and obese individuals with asthma tend to have worse control and more severe disease, compared with nonobese asthma patients, wrote Anne E. Dixon, BM, BCh, of the University of Vermont, Burlington, and colleagues.
Previous studies have shown that airway reactivity can occur in obese individuals without airway inflammation, but studies characterizing obese asthma based on lung function are lacking, they said. “Combining spirometry and oscillometry might reveal abnormalities in lung mechanics particularly pertinent to people with obesity and asthma,” the researchers noted.
In a cross-sectional study published in the journal CHEST, the researchers reviewed data from 31 obese adults with asthma and 22 obese adults without asthma. The participants were aged 18 years and older, with forced expiratory volume (FEV1) of at least 60% of predicted. All had class III obesity, with an average BMI of 47.2 kg/m2 for those with asthma and 46.7 kg/m2 for nonasthma controls. Demographic characteristics were similar between the groups.
Airway reactivity was defined as a 20% decrease in FEV1 and/or a 50% change in resistance or reactance at 5 Hz (R5 and X5), at a concentration of 16 mg/mL or less of methacholine. Patients were assessed using spirometry and oscillometry.
For those with asthma, the resistance at 5 Hz, measured by oscillometry, increased by 52% in response to the PC20 methacholine challenge, with an area under the reactance curve (AX) of 361%. For controls without asthma, the resistance at 5 Hz increased by 45%, with an AX of 268% in response to 16 mg/mL of methacholine.
This finding suggests that obesity predisposes individuals to peripheral airway reactivity regardless of asthma status, the researchers wrote in their discussion.
The researchers also identified two distinct groups of asthma patients categorized by respiratory system impedance based on more concordant vs. discordant bronchoconstriction in the central and peripheral airways. The baseline AX for these two groups was 11.8 and 46.7, respectively, with interquartile ranges of 9.9-23.4 and 23.2-53.7, respectively.
The discordant group included only women, and these patients reported significantly more gastroesophageal reflux, increased chest tightness, and more wheezing and asthma exacerbations than the concordant group, which may be related to air trapping, shown on previous studies of obese individuals with asthma, the researchers wrote.
The findings were limited by several factors, including the measurement of airway impedance only at the peak methacholine dose and the measurement of oscillometry after spirometry, the researchers noted. Other limitations included the relatively small study population at a single center, and the focus on obese individuals only.
More research is needed in larger and more diverse patient populations, but the results support the characterization of a subgroup of obese asthma patients with significant peripheral airway dysfunction, the researchers wrote.
“Oscillometry testing can reveal a physiologic phenotype of asthma in obesity that may be related to worse symptoms and more severe disease, and also reveal subclinical abnormalities in people with obesity, but without clinically diagnosed lung disease,” they concluded.
The study was supported in part by the National Institutes of Health. The researchers declared no financial conflicts.
A version of this article first appeared on Medscape.com.
Peripheral airway response to methacholine was similar among obese adults with and without asthma, although forced expiratory volume was lower for those with asthma, based on data from 53 individuals.
Obesity remains a risk factor for asthma, and obese individuals with asthma tend to have worse control and more severe disease, compared with nonobese asthma patients, wrote Anne E. Dixon, BM, BCh, of the University of Vermont, Burlington, and colleagues.
Previous studies have shown that airway reactivity can occur in obese individuals without airway inflammation, but studies characterizing obese asthma based on lung function are lacking, they said. “Combining spirometry and oscillometry might reveal abnormalities in lung mechanics particularly pertinent to people with obesity and asthma,” the researchers noted.
In a cross-sectional study published in the journal CHEST, the researchers reviewed data from 31 obese adults with asthma and 22 obese adults without asthma. The participants were aged 18 years and older, with forced expiratory volume (FEV1) of at least 60% of predicted. All had class III obesity, with an average BMI of 47.2 kg/m2 for those with asthma and 46.7 kg/m2 for nonasthma controls. Demographic characteristics were similar between the groups.
Airway reactivity was defined as a 20% decrease in FEV1 and/or a 50% change in resistance or reactance at 5 Hz (R5 and X5), at a concentration of 16 mg/mL or less of methacholine. Patients were assessed using spirometry and oscillometry.
For those with asthma, the resistance at 5 Hz, measured by oscillometry, increased by 52% in response to the PC20 methacholine challenge, with an area under the reactance curve (AX) of 361%. For controls without asthma, the resistance at 5 Hz increased by 45%, with an AX of 268% in response to 16 mg/mL of methacholine.
This finding suggests that obesity predisposes individuals to peripheral airway reactivity regardless of asthma status, the researchers wrote in their discussion.
The researchers also identified two distinct groups of asthma patients categorized by respiratory system impedance based on more concordant vs. discordant bronchoconstriction in the central and peripheral airways. The baseline AX for these two groups was 11.8 and 46.7, respectively, with interquartile ranges of 9.9-23.4 and 23.2-53.7, respectively.
The discordant group included only women, and these patients reported significantly more gastroesophageal reflux, increased chest tightness, and more wheezing and asthma exacerbations than the concordant group, which may be related to air trapping, shown on previous studies of obese individuals with asthma, the researchers wrote.
The findings were limited by several factors, including the measurement of airway impedance only at the peak methacholine dose and the measurement of oscillometry after spirometry, the researchers noted. Other limitations included the relatively small study population at a single center, and the focus on obese individuals only.
More research is needed in larger and more diverse patient populations, but the results support the characterization of a subgroup of obese asthma patients with significant peripheral airway dysfunction, the researchers wrote.
“Oscillometry testing can reveal a physiologic phenotype of asthma in obesity that may be related to worse symptoms and more severe disease, and also reveal subclinical abnormalities in people with obesity, but without clinically diagnosed lung disease,” they concluded.
The study was supported in part by the National Institutes of Health. The researchers declared no financial conflicts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL CHEST