User login
Updates on treatment/prevention of VTE in cancer patients
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
Firm Exophytic Tumor on the Shin
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
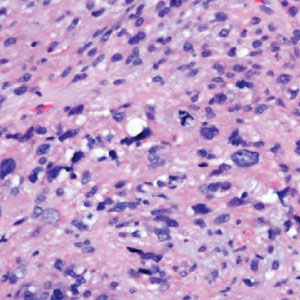
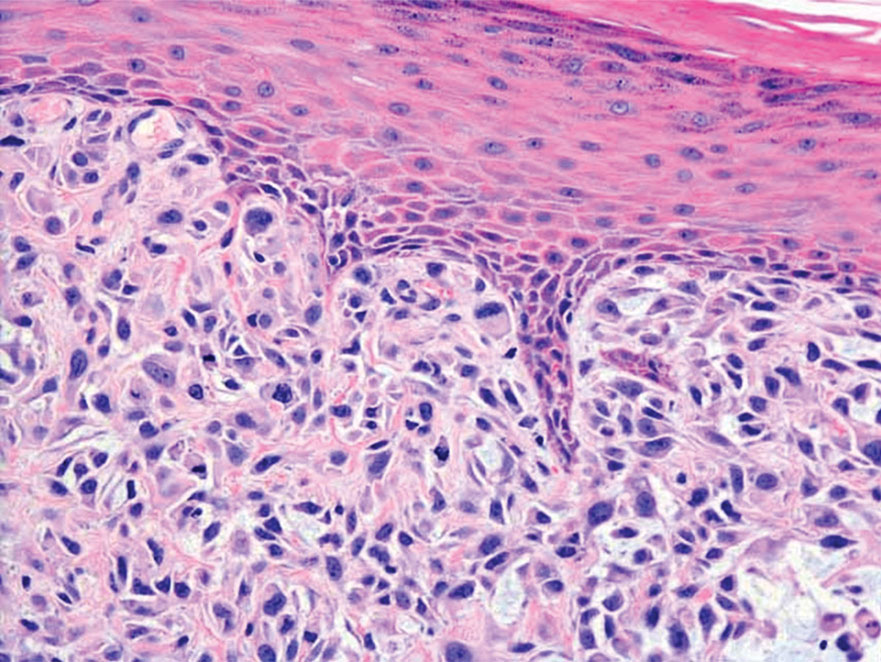
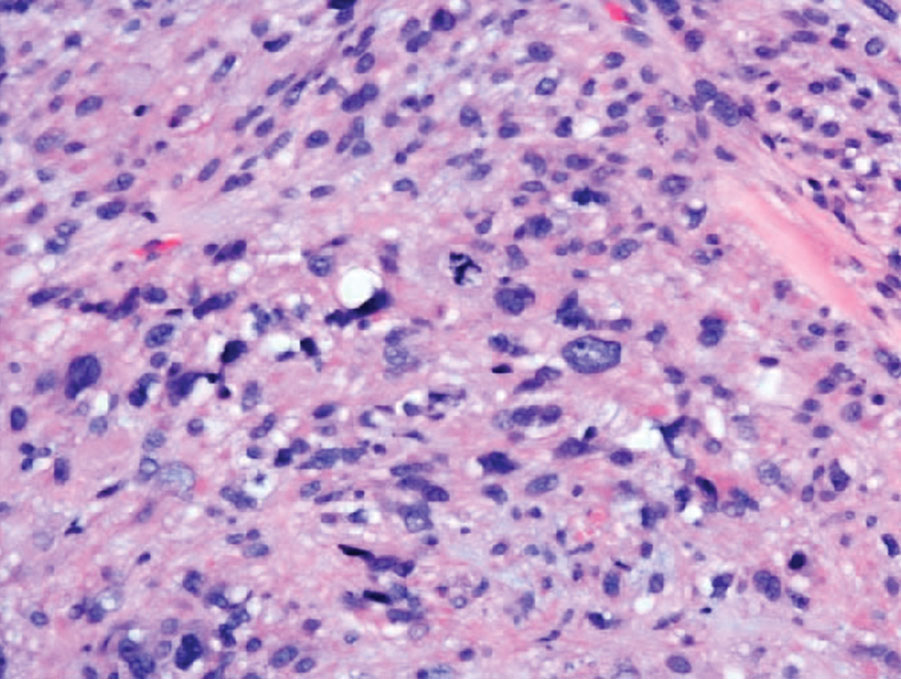
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.
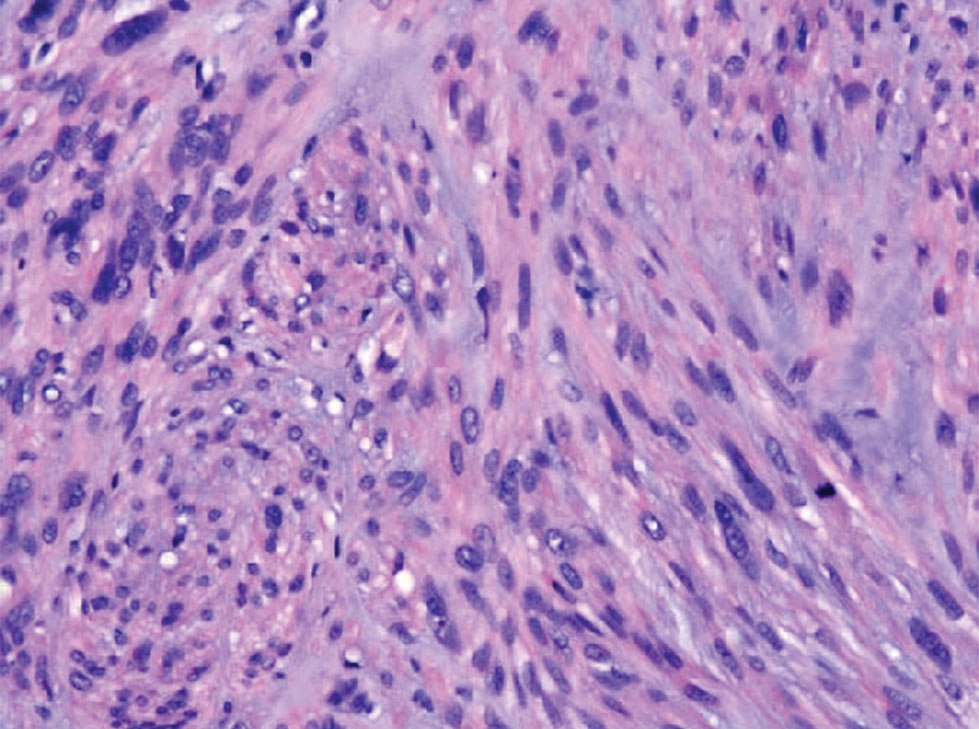
Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11
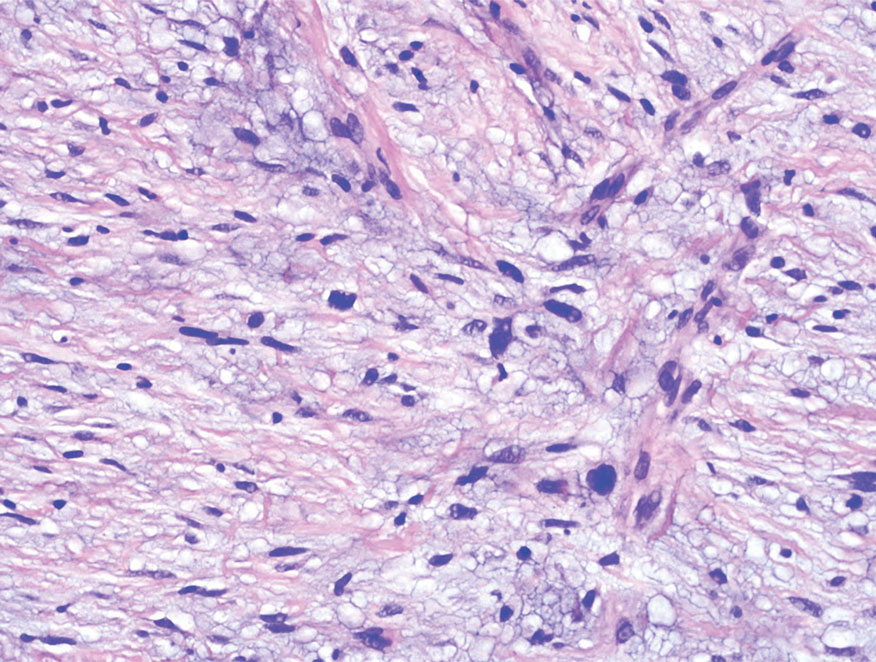
Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6
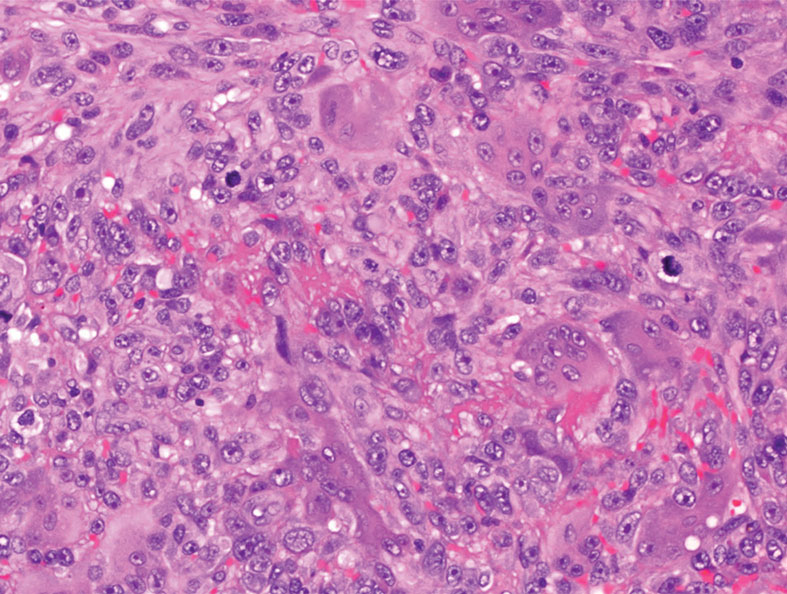
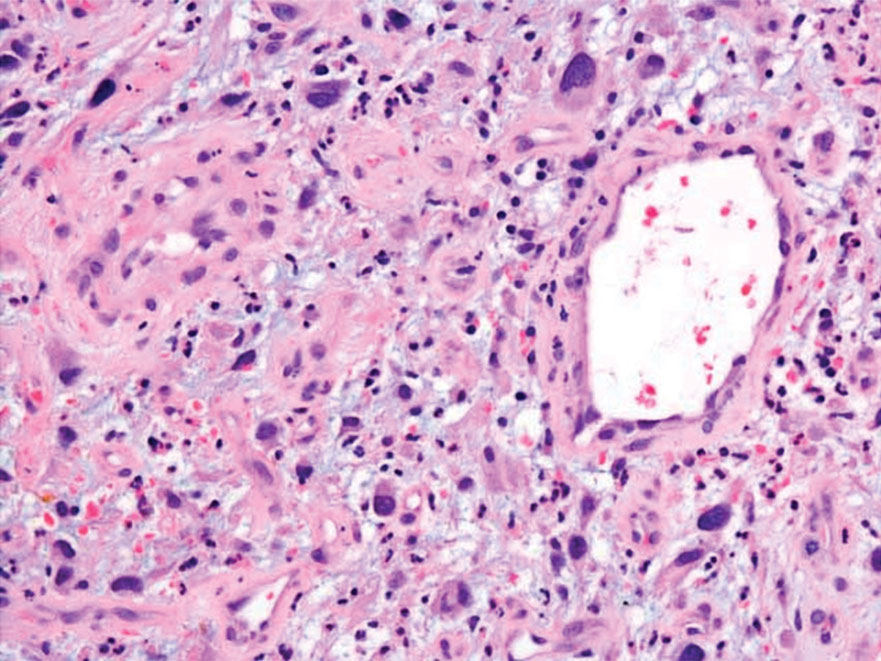
Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10
- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
A 62-year-old man presented with a firm, exophytic, 2.8×1.5-cm tumor on the left shin of 6 to 7 years’ duration. An excisional biopsy was obtained for histopathologic evaluation.
Blood test for cancer available, but is it ready for prime time?
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.
FDA acts against sales of unapproved mole and skin tag products on Amazon, other sites
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
Unique Treatment for Alopecia Areata Combining Epinephrine With an Intralesional Steroid
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.
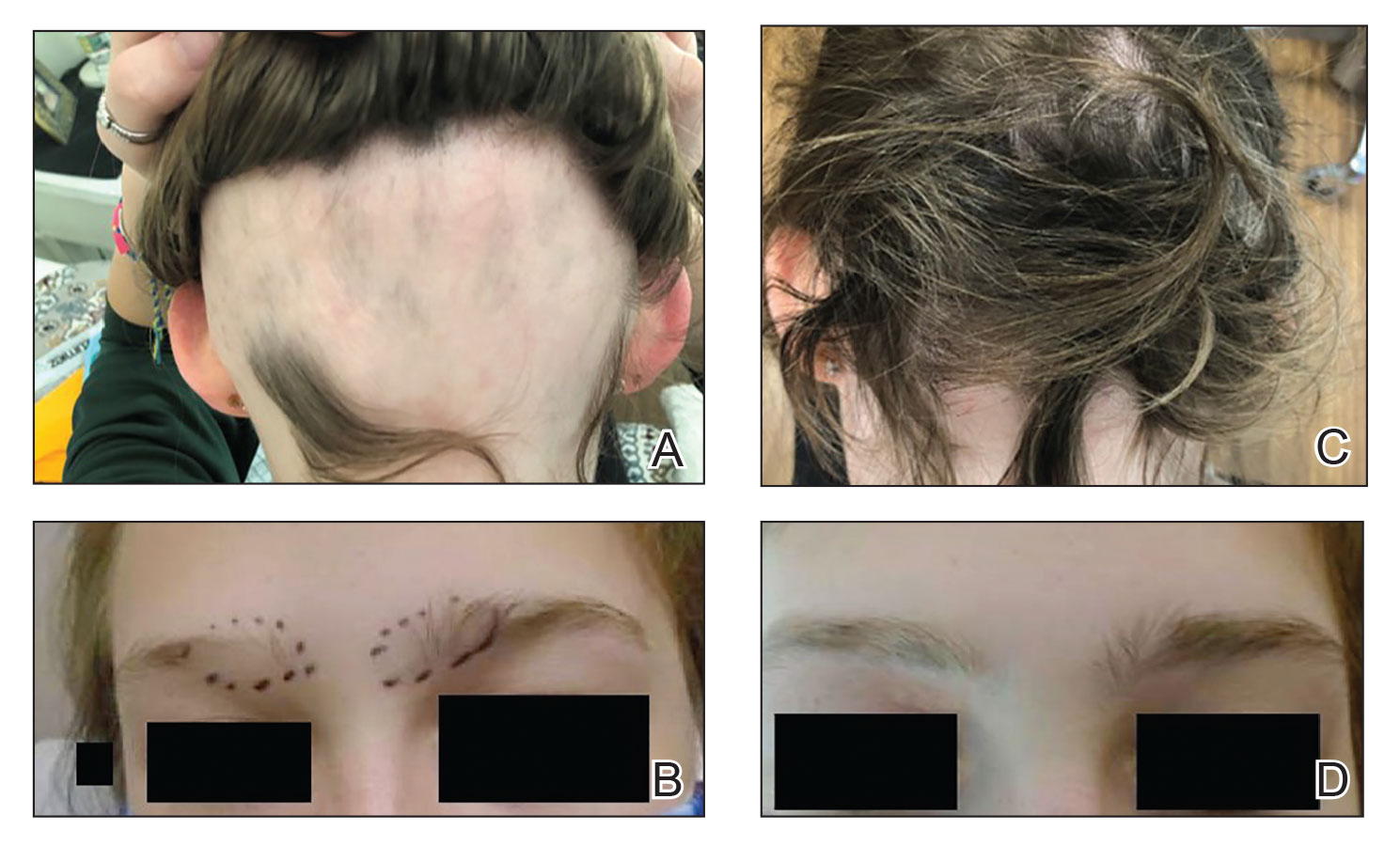
Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.
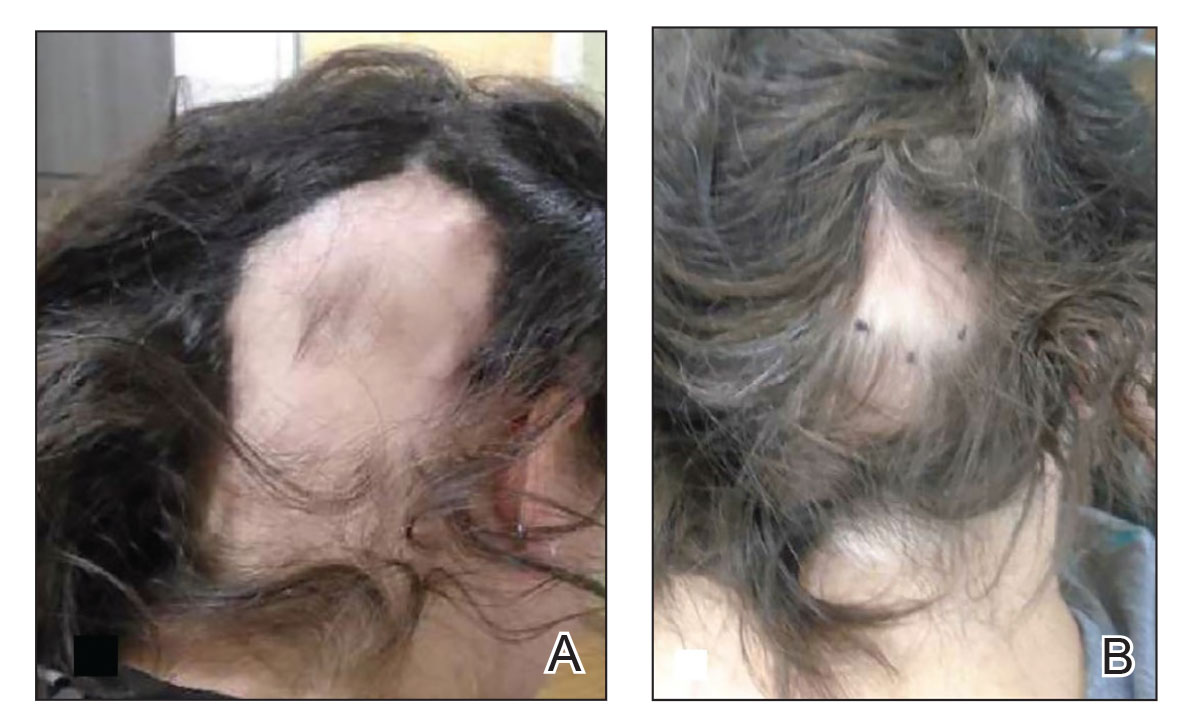
Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.
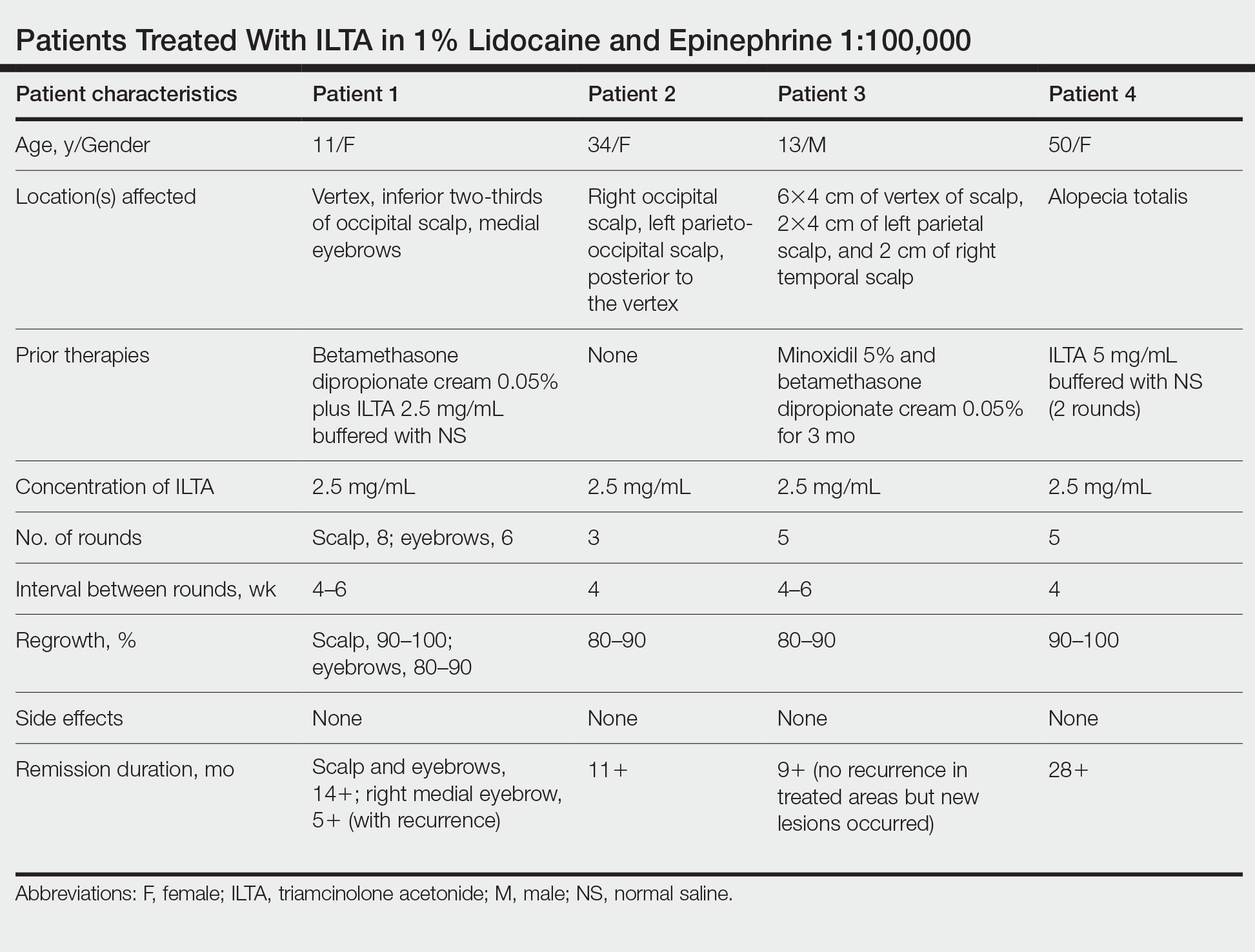
Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Practice Points
- Patients with alopecia areata that is refractory to first-line treatments may benefit from intralesional triamcinolone acetonide (ILTA) diluted to 2.5 mg/mL in 1% lidocaine and epinephrine 1:100,000 in place of normal saline.
- Local vasoconstriction due to epinephrine may potentiate ILTA effects and play an independent role.
Pancreatic cancer screening in new-onset diabetes appears cost effective
A risk-tailored early-detection strategy for pancreatic cancer that targets patients with new-onset diabetes could be cost effective, according to a recent study.
Screening for pancreatic ductal adenocarcinoma in asymptomatic adults is not recommended, but patients with new-onset diabetes have a risk that’s eight times higher than expected. Screening these patients could improve diagnosis and survival rates if the cancer can be identified at earlier stages, researchers led by Louise Wang, MD, a gastroenterology fellow at the University of Pennsylvania, Philadelphia, wrote in Clinical Gastroenterology and Hepatology.
“As we continue to improve therapies for early-stage pancreatic cancers, especially among the local/resectable stage, the case for the targeted early-detection strategy will be stronger,” they wrote. “Policy makers should take into consideration these novel findings when formulating [pancreatic ductal adenocarcinoma] screening policy and making coverage determinations.”
The research team compared early detection strategies for pancreatic ductal adenocarcinoma that target new-onset diabetes patients at age 50 years and older with standard of care, defined as no early detection strategy. They looked at various minimal predicted cancer risk thresholds versus current standard of care in a Markov state-transition decision model. The analysis assumed a health care sector perspective and a lifetime horizon, with two willingness-to-pay thresholds ($100,000 and $150,000) per quality-adjusted life-year gained.
The researchers used data from one of their previously published studies, which included 89,881 patients with new-onset diabetes diagnosed at age 50 or older. The cumulative incidence of pancreatic cancer was 0.42% during the 3 years after diagnosis.
In the early detection strategy, all patients 50 years and older who were newly diagnosed with diabetes mellitus were placed into low-risk and high-risk cohorts based on their predicted 3-year risk of pancreatic ductal adenocarcinoma under a range of assumed minimum-risk thresholds – 0.5%, 1%, 2%, 3%, 4%, and 5%; these thresholds were based on a previously established prediction model.
The research team found that the early detection strategy that targeted patients with a minimum predicted 3-year pancreatic ductal adenocarcinoma risk of 1% was cost effective, based on a willingness-to-pay threshold of $150,000 per quality-adjusted life-year. The incremental cost-effectiveness ratio was $116,911 per quality-adjusted life-year.
At a willingness-to-pay threshold of $100,000 per quality-adjusted life-year, the early detection strategy at the 2% risk threshold was cost effective. The incremental cost-effectiveness ratio was $63,045 per quality-adjusted life-year.
The most influential factors included the proportion of pancreatic ductal adenocarcinomas detected at the local stage, costs of treatment for metastatic cancer, utilities of local and regional cancers, and sensitivity of screening.
A probabilistic sensitivity analysis confirmed that, at a willingness-to-pay threshold of $150,000, early detection at the 1% risk threshold was favored at 30.6%, followed by the 0.5% risk threshold at 20.4%, compared with the standard of care at 1.7%. In addition, at a willingness-to-pay threshold of $100,000, early detection at the 1% risk threshold was favored at 27.3%, followed by the 2% risk threshold at 22.8%, as compared with the standard of care at 2%.
The two early detection strategies were cost effective, capturing 26%-45% of the pancreatic ductal adenocarcinoma cases in patients with new-onset diabetes.
The study authors noted several limitations, including the inability to incorporate out-of-pocket costs for patients, as well as focusing the analysis on the health care perspective.
“We acknowledge that, by incorporating the full consequences of decisions for all stakeholders, a societal perspective would have offered a more complete view on which to base public policy,” they wrote.
At the same time, “given the substantial prevalence of [new-onset diabetes] among [pancreatic ductal adenocarcinoma] cases, this strategy could improve the survival of a substantial proportion of sporadic PDAC cases in the general population,” they concluded.
The study authors reported various disclosures, including grants and research support from Takeda Pharmaceuticals USA, Janssen Pharmaceuticals, the National Institutes of Health, the Crohn’s and Colitis Foundation, Lilly Oncology, GSK, and Clovis Oncology.
Earlier detection of pancreatic ductal adenocarcinoma (PDAC) is essential to improving the survival for the group of patients diagnosed with PDAC each year. New-onset diabetes in adults 50 years or older is recognized as a risk factor for being diagnosed with PDAC within the following 3 years.
This study by Wang et al. uses previously described clinical prediction models to stratify the risk of PDAC in patients with new-onset diabetes. These models include age, body mass index, weight change, smoking, diabetic medications, and laboratory values (hemoglobin A1c, cholesterol, creatinine, alkaline phosphatase). They ran simulation models to determine the cost-effectiveness of screening for pancreatic cancer at various risk cut-offs. At the $150,000 willingness-to-pay threshold per quality-adjusted life-year, the 1% risk threshold was cost-effective. Stage shifting from a higher-stage cancer to a lower-stage cancer was the driving force behind the cost-effectiveness ratios.
Providers need to have a high index of suspicion when an adult over the age of 50 has had a new diagnosis of diabetes. Abnormalities detected in laboratory data, weight trends, symptoms, a history of underlying smoking or pancreatic disease may appropriately prompt an MRI/MRCP or endoscopic ultrasound. Better and more accessible risk progression calculators for these patients could be used in real time. The current study by Wang et al. will be a helpful tool as well for navigating disputes with payers about the utility of covering screening tests in the subgroup of patients that are higher risk.
Mark A. Gromski, MD, is assistant professor of medicine at Indiana University School of Medicine and a pancreatobiliary specialist and advanced endoscopist at IU Health. He reports having no relevant disclosures.
Earlier detection of pancreatic ductal adenocarcinoma (PDAC) is essential to improving the survival for the group of patients diagnosed with PDAC each year. New-onset diabetes in adults 50 years or older is recognized as a risk factor for being diagnosed with PDAC within the following 3 years.
This study by Wang et al. uses previously described clinical prediction models to stratify the risk of PDAC in patients with new-onset diabetes. These models include age, body mass index, weight change, smoking, diabetic medications, and laboratory values (hemoglobin A1c, cholesterol, creatinine, alkaline phosphatase). They ran simulation models to determine the cost-effectiveness of screening for pancreatic cancer at various risk cut-offs. At the $150,000 willingness-to-pay threshold per quality-adjusted life-year, the 1% risk threshold was cost-effective. Stage shifting from a higher-stage cancer to a lower-stage cancer was the driving force behind the cost-effectiveness ratios.
Providers need to have a high index of suspicion when an adult over the age of 50 has had a new diagnosis of diabetes. Abnormalities detected in laboratory data, weight trends, symptoms, a history of underlying smoking or pancreatic disease may appropriately prompt an MRI/MRCP or endoscopic ultrasound. Better and more accessible risk progression calculators for these patients could be used in real time. The current study by Wang et al. will be a helpful tool as well for navigating disputes with payers about the utility of covering screening tests in the subgroup of patients that are higher risk.
Mark A. Gromski, MD, is assistant professor of medicine at Indiana University School of Medicine and a pancreatobiliary specialist and advanced endoscopist at IU Health. He reports having no relevant disclosures.
Earlier detection of pancreatic ductal adenocarcinoma (PDAC) is essential to improving the survival for the group of patients diagnosed with PDAC each year. New-onset diabetes in adults 50 years or older is recognized as a risk factor for being diagnosed with PDAC within the following 3 years.
This study by Wang et al. uses previously described clinical prediction models to stratify the risk of PDAC in patients with new-onset diabetes. These models include age, body mass index, weight change, smoking, diabetic medications, and laboratory values (hemoglobin A1c, cholesterol, creatinine, alkaline phosphatase). They ran simulation models to determine the cost-effectiveness of screening for pancreatic cancer at various risk cut-offs. At the $150,000 willingness-to-pay threshold per quality-adjusted life-year, the 1% risk threshold was cost-effective. Stage shifting from a higher-stage cancer to a lower-stage cancer was the driving force behind the cost-effectiveness ratios.
Providers need to have a high index of suspicion when an adult over the age of 50 has had a new diagnosis of diabetes. Abnormalities detected in laboratory data, weight trends, symptoms, a history of underlying smoking or pancreatic disease may appropriately prompt an MRI/MRCP or endoscopic ultrasound. Better and more accessible risk progression calculators for these patients could be used in real time. The current study by Wang et al. will be a helpful tool as well for navigating disputes with payers about the utility of covering screening tests in the subgroup of patients that are higher risk.
Mark A. Gromski, MD, is assistant professor of medicine at Indiana University School of Medicine and a pancreatobiliary specialist and advanced endoscopist at IU Health. He reports having no relevant disclosures.
A risk-tailored early-detection strategy for pancreatic cancer that targets patients with new-onset diabetes could be cost effective, according to a recent study.
Screening for pancreatic ductal adenocarcinoma in asymptomatic adults is not recommended, but patients with new-onset diabetes have a risk that’s eight times higher than expected. Screening these patients could improve diagnosis and survival rates if the cancer can be identified at earlier stages, researchers led by Louise Wang, MD, a gastroenterology fellow at the University of Pennsylvania, Philadelphia, wrote in Clinical Gastroenterology and Hepatology.
“As we continue to improve therapies for early-stage pancreatic cancers, especially among the local/resectable stage, the case for the targeted early-detection strategy will be stronger,” they wrote. “Policy makers should take into consideration these novel findings when formulating [pancreatic ductal adenocarcinoma] screening policy and making coverage determinations.”
The research team compared early detection strategies for pancreatic ductal adenocarcinoma that target new-onset diabetes patients at age 50 years and older with standard of care, defined as no early detection strategy. They looked at various minimal predicted cancer risk thresholds versus current standard of care in a Markov state-transition decision model. The analysis assumed a health care sector perspective and a lifetime horizon, with two willingness-to-pay thresholds ($100,000 and $150,000) per quality-adjusted life-year gained.
The researchers used data from one of their previously published studies, which included 89,881 patients with new-onset diabetes diagnosed at age 50 or older. The cumulative incidence of pancreatic cancer was 0.42% during the 3 years after diagnosis.
In the early detection strategy, all patients 50 years and older who were newly diagnosed with diabetes mellitus were placed into low-risk and high-risk cohorts based on their predicted 3-year risk of pancreatic ductal adenocarcinoma under a range of assumed minimum-risk thresholds – 0.5%, 1%, 2%, 3%, 4%, and 5%; these thresholds were based on a previously established prediction model.
The research team found that the early detection strategy that targeted patients with a minimum predicted 3-year pancreatic ductal adenocarcinoma risk of 1% was cost effective, based on a willingness-to-pay threshold of $150,000 per quality-adjusted life-year. The incremental cost-effectiveness ratio was $116,911 per quality-adjusted life-year.
At a willingness-to-pay threshold of $100,000 per quality-adjusted life-year, the early detection strategy at the 2% risk threshold was cost effective. The incremental cost-effectiveness ratio was $63,045 per quality-adjusted life-year.
The most influential factors included the proportion of pancreatic ductal adenocarcinomas detected at the local stage, costs of treatment for metastatic cancer, utilities of local and regional cancers, and sensitivity of screening.
A probabilistic sensitivity analysis confirmed that, at a willingness-to-pay threshold of $150,000, early detection at the 1% risk threshold was favored at 30.6%, followed by the 0.5% risk threshold at 20.4%, compared with the standard of care at 1.7%. In addition, at a willingness-to-pay threshold of $100,000, early detection at the 1% risk threshold was favored at 27.3%, followed by the 2% risk threshold at 22.8%, as compared with the standard of care at 2%.
The two early detection strategies were cost effective, capturing 26%-45% of the pancreatic ductal adenocarcinoma cases in patients with new-onset diabetes.
The study authors noted several limitations, including the inability to incorporate out-of-pocket costs for patients, as well as focusing the analysis on the health care perspective.
“We acknowledge that, by incorporating the full consequences of decisions for all stakeholders, a societal perspective would have offered a more complete view on which to base public policy,” they wrote.
At the same time, “given the substantial prevalence of [new-onset diabetes] among [pancreatic ductal adenocarcinoma] cases, this strategy could improve the survival of a substantial proportion of sporadic PDAC cases in the general population,” they concluded.
The study authors reported various disclosures, including grants and research support from Takeda Pharmaceuticals USA, Janssen Pharmaceuticals, the National Institutes of Health, the Crohn’s and Colitis Foundation, Lilly Oncology, GSK, and Clovis Oncology.
A risk-tailored early-detection strategy for pancreatic cancer that targets patients with new-onset diabetes could be cost effective, according to a recent study.
Screening for pancreatic ductal adenocarcinoma in asymptomatic adults is not recommended, but patients with new-onset diabetes have a risk that’s eight times higher than expected. Screening these patients could improve diagnosis and survival rates if the cancer can be identified at earlier stages, researchers led by Louise Wang, MD, a gastroenterology fellow at the University of Pennsylvania, Philadelphia, wrote in Clinical Gastroenterology and Hepatology.
“As we continue to improve therapies for early-stage pancreatic cancers, especially among the local/resectable stage, the case for the targeted early-detection strategy will be stronger,” they wrote. “Policy makers should take into consideration these novel findings when formulating [pancreatic ductal adenocarcinoma] screening policy and making coverage determinations.”
The research team compared early detection strategies for pancreatic ductal adenocarcinoma that target new-onset diabetes patients at age 50 years and older with standard of care, defined as no early detection strategy. They looked at various minimal predicted cancer risk thresholds versus current standard of care in a Markov state-transition decision model. The analysis assumed a health care sector perspective and a lifetime horizon, with two willingness-to-pay thresholds ($100,000 and $150,000) per quality-adjusted life-year gained.
The researchers used data from one of their previously published studies, which included 89,881 patients with new-onset diabetes diagnosed at age 50 or older. The cumulative incidence of pancreatic cancer was 0.42% during the 3 years after diagnosis.
In the early detection strategy, all patients 50 years and older who were newly diagnosed with diabetes mellitus were placed into low-risk and high-risk cohorts based on their predicted 3-year risk of pancreatic ductal adenocarcinoma under a range of assumed minimum-risk thresholds – 0.5%, 1%, 2%, 3%, 4%, and 5%; these thresholds were based on a previously established prediction model.
The research team found that the early detection strategy that targeted patients with a minimum predicted 3-year pancreatic ductal adenocarcinoma risk of 1% was cost effective, based on a willingness-to-pay threshold of $150,000 per quality-adjusted life-year. The incremental cost-effectiveness ratio was $116,911 per quality-adjusted life-year.
At a willingness-to-pay threshold of $100,000 per quality-adjusted life-year, the early detection strategy at the 2% risk threshold was cost effective. The incremental cost-effectiveness ratio was $63,045 per quality-adjusted life-year.
The most influential factors included the proportion of pancreatic ductal adenocarcinomas detected at the local stage, costs of treatment for metastatic cancer, utilities of local and regional cancers, and sensitivity of screening.
A probabilistic sensitivity analysis confirmed that, at a willingness-to-pay threshold of $150,000, early detection at the 1% risk threshold was favored at 30.6%, followed by the 0.5% risk threshold at 20.4%, compared with the standard of care at 1.7%. In addition, at a willingness-to-pay threshold of $100,000, early detection at the 1% risk threshold was favored at 27.3%, followed by the 2% risk threshold at 22.8%, as compared with the standard of care at 2%.
The two early detection strategies were cost effective, capturing 26%-45% of the pancreatic ductal adenocarcinoma cases in patients with new-onset diabetes.
The study authors noted several limitations, including the inability to incorporate out-of-pocket costs for patients, as well as focusing the analysis on the health care perspective.
“We acknowledge that, by incorporating the full consequences of decisions for all stakeholders, a societal perspective would have offered a more complete view on which to base public policy,” they wrote.
At the same time, “given the substantial prevalence of [new-onset diabetes] among [pancreatic ductal adenocarcinoma] cases, this strategy could improve the survival of a substantial proportion of sporadic PDAC cases in the general population,” they concluded.
The study authors reported various disclosures, including grants and research support from Takeda Pharmaceuticals USA, Janssen Pharmaceuticals, the National Institutes of Health, the Crohn’s and Colitis Foundation, Lilly Oncology, GSK, and Clovis Oncology.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Mysterious cases of illness with an unusual cause
So begins the search for evidence.
No relations or common journeys
Between March and July 2021, cases of the bacterial infectious disease sprang up in Georgia, Kansas, Minnesota, and Texas, with the disease being fatal for two of those affected. Usually, cases of melioidosis occur in the United States after traveling to regions where the pathogen is prevalent. However, none of the patients had undertaken any previous international travel.
When the genomes of the bacterial strains (Burkholderia pseudomallei) were sequenced, they showed a high level of concordance, suggesting a common source of infection. The bacterial strain is similar to those that are found in Southeast Asia above all. An imported product from there was taken into consideration as the trigger.
The Centers for Disease Control and Prevention examined blood samples from the patients, as well as samples from the soil, water, food, and household items around their homes.
Aroma spray as a trigger
In October, the cause of the melioidosis was finally identified in the house of the patient from Georgia: an aromatherapy spray. The genetic fingerprint of the bacterial strain matched with that from the other patients. The common trigger was thus discovered.
The contaminated spray, with a lavender-chamomile scent for room fragrancing, was sold between February and October in some branches of Walmart, as well as in their online store. The product was therefore recalled and it was checked whether the ingredients were also being used in other products.
The CDC requested physicians to also take melioidosis into account if they were presented with acute bacterial infections that did not respond to normal antibiotics and to inquire whether the affected room spray had been used.
More information about melioidosis
Melioidosis is an infectious disease affecting humans and animals. The trigger is the bacteria B pseudomallei. The disease appears predominantly in tropical regions, especially in Southeast Asia and northern Australia.
Transmission
The bacteria can be found in contaminated water and soil. It is disseminated between humans and animals through direct contact with the infectious source, such as through inhaling dust particles or water droplets, or through consuming contaminated water or food. Human-to-human transmission is extremely rare. Recently however, tropical saltwater fish were identified as potential carriers.
Symptoms
Melioidosis has a wide range of symptoms, which can lead to its being confused with other diseases such as tuberculosis or other forms of pneumonia. There are different forms of the disease, each with different symptoms.
- Localized infection: localized pain and swelling, fever, ulceration, and abscess.
- Pulmonary infection: cough, chest pain, high fever, headaches, and loss of appetite
- Bacteremia: fever, headaches, breathing problems, stomach discomfort, joint pain, and disorientation.
- Disseminated infection: fever, weight loss, stomach or chest pain, muscle or joint pain, headaches, central nervous system infections, and epileptic seizures.
The incubation time is not clearly defined and can be from 1 day to several years; however, the symptoms mostly emerge 2-4 weeks after exposure. The risk factors include diabetes, high alcohol consumption, chronic pulmonary or kidney disease, and immunodeficiencies.
Diagnosis based on the symptoms is often difficult since the clinical picture is similar to other, more common conditions.
Therapy
If the melioidosis is identified as such, it can be treated with only mildly effective antibiotics, since it has a natural resistance to many commonly used antibiotics. The type of infection and the course of treatment also affects the long-term outcome. Without treatment, 90% of the infections have a fatal outcome. With appropriate treatment, the mortality rate still lies at 40%.
Therapy generally begins with intravenous antibiotic therapy for at least 2-8 weeks (ceftazidime or meropenem). Oral antibiotic therapy then follows for 3-6 months (trimethoprim-sulfamethoxazole or amoxicillin/clavulanic acid). If the patient is allergic to penicillin, alternative antibiotics can be used.
Use as a bioweapon
The CDC classifies B. pseudomallei as a potential pathogen for biological attack (class-B candidate). The agency lists the potential reasons for use as a bioweapon as:
- The pathogen can be found naturally in certain regions.
- The triggered disease can take a serious course and ultimately be fatal without appropriate therapy.
- In the past, the United States has used similar pathogens in wars as bioweapons.
In a potential attack, the pathogen could be spread through air, water, or food, and by doing so, many people would be exposed. Any contact with the bacteria can result in melioidosis. As the bacteria cannot be seen, smelled, or tasted, the biological attack would not be recognized for some time. A certain amount of time can also pass until the pathogen is identified, once fever and respiratory diseases have developed.
In such an emergency, the CDC would collaborate with other federal and local authorities to supply specialized testing laboratories and provide the public with information.
This content was translated from Coliquio. A version appeared on Medscape.com.
So begins the search for evidence.
No relations or common journeys
Between March and July 2021, cases of the bacterial infectious disease sprang up in Georgia, Kansas, Minnesota, and Texas, with the disease being fatal for two of those affected. Usually, cases of melioidosis occur in the United States after traveling to regions where the pathogen is prevalent. However, none of the patients had undertaken any previous international travel.
When the genomes of the bacterial strains (Burkholderia pseudomallei) were sequenced, they showed a high level of concordance, suggesting a common source of infection. The bacterial strain is similar to those that are found in Southeast Asia above all. An imported product from there was taken into consideration as the trigger.
The Centers for Disease Control and Prevention examined blood samples from the patients, as well as samples from the soil, water, food, and household items around their homes.
Aroma spray as a trigger
In October, the cause of the melioidosis was finally identified in the house of the patient from Georgia: an aromatherapy spray. The genetic fingerprint of the bacterial strain matched with that from the other patients. The common trigger was thus discovered.
The contaminated spray, with a lavender-chamomile scent for room fragrancing, was sold between February and October in some branches of Walmart, as well as in their online store. The product was therefore recalled and it was checked whether the ingredients were also being used in other products.
The CDC requested physicians to also take melioidosis into account if they were presented with acute bacterial infections that did not respond to normal antibiotics and to inquire whether the affected room spray had been used.
More information about melioidosis
Melioidosis is an infectious disease affecting humans and animals. The trigger is the bacteria B pseudomallei. The disease appears predominantly in tropical regions, especially in Southeast Asia and northern Australia.
Transmission
The bacteria can be found in contaminated water and soil. It is disseminated between humans and animals through direct contact with the infectious source, such as through inhaling dust particles or water droplets, or through consuming contaminated water or food. Human-to-human transmission is extremely rare. Recently however, tropical saltwater fish were identified as potential carriers.
Symptoms
Melioidosis has a wide range of symptoms, which can lead to its being confused with other diseases such as tuberculosis or other forms of pneumonia. There are different forms of the disease, each with different symptoms.
- Localized infection: localized pain and swelling, fever, ulceration, and abscess.
- Pulmonary infection: cough, chest pain, high fever, headaches, and loss of appetite
- Bacteremia: fever, headaches, breathing problems, stomach discomfort, joint pain, and disorientation.
- Disseminated infection: fever, weight loss, stomach or chest pain, muscle or joint pain, headaches, central nervous system infections, and epileptic seizures.
The incubation time is not clearly defined and can be from 1 day to several years; however, the symptoms mostly emerge 2-4 weeks after exposure. The risk factors include diabetes, high alcohol consumption, chronic pulmonary or kidney disease, and immunodeficiencies.
Diagnosis based on the symptoms is often difficult since the clinical picture is similar to other, more common conditions.
Therapy
If the melioidosis is identified as such, it can be treated with only mildly effective antibiotics, since it has a natural resistance to many commonly used antibiotics. The type of infection and the course of treatment also affects the long-term outcome. Without treatment, 90% of the infections have a fatal outcome. With appropriate treatment, the mortality rate still lies at 40%.
Therapy generally begins with intravenous antibiotic therapy for at least 2-8 weeks (ceftazidime or meropenem). Oral antibiotic therapy then follows for 3-6 months (trimethoprim-sulfamethoxazole or amoxicillin/clavulanic acid). If the patient is allergic to penicillin, alternative antibiotics can be used.
Use as a bioweapon
The CDC classifies B. pseudomallei as a potential pathogen for biological attack (class-B candidate). The agency lists the potential reasons for use as a bioweapon as:
- The pathogen can be found naturally in certain regions.
- The triggered disease can take a serious course and ultimately be fatal without appropriate therapy.
- In the past, the United States has used similar pathogens in wars as bioweapons.
In a potential attack, the pathogen could be spread through air, water, or food, and by doing so, many people would be exposed. Any contact with the bacteria can result in melioidosis. As the bacteria cannot be seen, smelled, or tasted, the biological attack would not be recognized for some time. A certain amount of time can also pass until the pathogen is identified, once fever and respiratory diseases have developed.
In such an emergency, the CDC would collaborate with other federal and local authorities to supply specialized testing laboratories and provide the public with information.
This content was translated from Coliquio. A version appeared on Medscape.com.
So begins the search for evidence.
No relations or common journeys
Between March and July 2021, cases of the bacterial infectious disease sprang up in Georgia, Kansas, Minnesota, and Texas, with the disease being fatal for two of those affected. Usually, cases of melioidosis occur in the United States after traveling to regions where the pathogen is prevalent. However, none of the patients had undertaken any previous international travel.
When the genomes of the bacterial strains (Burkholderia pseudomallei) were sequenced, they showed a high level of concordance, suggesting a common source of infection. The bacterial strain is similar to those that are found in Southeast Asia above all. An imported product from there was taken into consideration as the trigger.
The Centers for Disease Control and Prevention examined blood samples from the patients, as well as samples from the soil, water, food, and household items around their homes.
Aroma spray as a trigger
In October, the cause of the melioidosis was finally identified in the house of the patient from Georgia: an aromatherapy spray. The genetic fingerprint of the bacterial strain matched with that from the other patients. The common trigger was thus discovered.
The contaminated spray, with a lavender-chamomile scent for room fragrancing, was sold between February and October in some branches of Walmart, as well as in their online store. The product was therefore recalled and it was checked whether the ingredients were also being used in other products.
The CDC requested physicians to also take melioidosis into account if they were presented with acute bacterial infections that did not respond to normal antibiotics and to inquire whether the affected room spray had been used.
More information about melioidosis
Melioidosis is an infectious disease affecting humans and animals. The trigger is the bacteria B pseudomallei. The disease appears predominantly in tropical regions, especially in Southeast Asia and northern Australia.
Transmission
The bacteria can be found in contaminated water and soil. It is disseminated between humans and animals through direct contact with the infectious source, such as through inhaling dust particles or water droplets, or through consuming contaminated water or food. Human-to-human transmission is extremely rare. Recently however, tropical saltwater fish were identified as potential carriers.
Symptoms
Melioidosis has a wide range of symptoms, which can lead to its being confused with other diseases such as tuberculosis or other forms of pneumonia. There are different forms of the disease, each with different symptoms.
- Localized infection: localized pain and swelling, fever, ulceration, and abscess.
- Pulmonary infection: cough, chest pain, high fever, headaches, and loss of appetite
- Bacteremia: fever, headaches, breathing problems, stomach discomfort, joint pain, and disorientation.
- Disseminated infection: fever, weight loss, stomach or chest pain, muscle or joint pain, headaches, central nervous system infections, and epileptic seizures.
The incubation time is not clearly defined and can be from 1 day to several years; however, the symptoms mostly emerge 2-4 weeks after exposure. The risk factors include diabetes, high alcohol consumption, chronic pulmonary or kidney disease, and immunodeficiencies.
Diagnosis based on the symptoms is often difficult since the clinical picture is similar to other, more common conditions.
Therapy
If the melioidosis is identified as such, it can be treated with only mildly effective antibiotics, since it has a natural resistance to many commonly used antibiotics. The type of infection and the course of treatment also affects the long-term outcome. Without treatment, 90% of the infections have a fatal outcome. With appropriate treatment, the mortality rate still lies at 40%.
Therapy generally begins with intravenous antibiotic therapy for at least 2-8 weeks (ceftazidime or meropenem). Oral antibiotic therapy then follows for 3-6 months (trimethoprim-sulfamethoxazole or amoxicillin/clavulanic acid). If the patient is allergic to penicillin, alternative antibiotics can be used.
Use as a bioweapon
The CDC classifies B. pseudomallei as a potential pathogen for biological attack (class-B candidate). The agency lists the potential reasons for use as a bioweapon as:
- The pathogen can be found naturally in certain regions.
- The triggered disease can take a serious course and ultimately be fatal without appropriate therapy.
- In the past, the United States has used similar pathogens in wars as bioweapons.
In a potential attack, the pathogen could be spread through air, water, or food, and by doing so, many people would be exposed. Any contact with the bacteria can result in melioidosis. As the bacteria cannot be seen, smelled, or tasted, the biological attack would not be recognized for some time. A certain amount of time can also pass until the pathogen is identified, once fever and respiratory diseases have developed.
In such an emergency, the CDC would collaborate with other federal and local authorities to supply specialized testing laboratories and provide the public with information.
This content was translated from Coliquio. A version appeared on Medscape.com.
CV admissions on the rise in Americans with cancer
Although cardiovascular disease (CVD) is known to often strike the mortal blow in patients with cancer, a national analysis puts in stark relief the burden of CV-related hospitalizations in this vulnerable population.
, whereas admissions fell 10.9% among those without cancer.
Admissions increased steadily across all cancer types, except prostate cancer, with heart failure being the most common reason for admission.
“Hospital admissions is really important because we know that the size of this group is increasing, given that they live longer and many of the treatments that we offer cause cardiovascular disease or increase the risk of having cardiovascular events. So, from a health care planning perspective, I think it’s really important to see what the burden is likely to be in the next few years,” senior author Mamas Mamas, MD, Keele University, England, told this news organization.
For physicians and the wider population, he said, the findings underscore the need to shift the conversation from saying that patients with cancer are at increased CVD risk to asking how to mitigate this risk. “Because I would say that this increase in cardiovascular admissions, that’s a failure from a preventative perspective.”
The study was published in the European Heart Journal: Quality of Care & Clinical Outcomes.
Individual cancer types
The researchers, led by Ofer Kobo, MD, also with Keele University, used the National Inpatient Sample to identify 42.5 million weighted cases of CV admissions for acute myocardial infarction (AMI), pulmonary embolism, ischemic stroke, heart failure, atrial fibrillation (AFib) or atrial flutter, and intracranial hemorrhage from January 2004 to December 2017. Of these, 1.9 million had a record of cancer.
Patients with cancer were older; had a higher prevalence of valvular disease, anemia, and coagulopathy; and had a lower prevalence of hypertension, diabetes mellitus, and obesity than did patients without cancer.
The most common cancer type was hematologic cancers (26.1%), followed by lung (18.7%), gastrointestinal (12.4%), prostate (11.6%), breast (6.7%), and other in 24.4%.
The admission rate increased across all six admission causes – between 7% for AMI and ischemic stroke and 46% for AFib.
Heart failure was the chief reason for admission among all patients. Annual rates per 100,000 U.S. population increased in patients with cancer (from 13.6 to 16.6; P for trend = .02) and declined in those without (from 352.2 to 349.8; P for trend < .001).
“In the past, patients would be started on medications, and perhaps the importance of monitoring [left ventricular] LV function wasn’t as widely known, whereas now we’re much more aggressive in looking at it and much more aggressive at trying to prevent it,” Dr. Mamas said. “But even with this greater identification and attempting to modify regimens, we’re still getting quite substantial increases in heart failure admissions in this population. And what really surprised me is that it wasn’t just in the breast cancer population, but it was nearly across the board.”
He noted that patients are at highest risk from CV events within the first 2 years of cancer diagnosis. “So that’s really the time where you’ve got to be really aggressive in looking and working up their cardiovascular profile.”
Patients with hematologic cancers (9.7-13.5), lung (7.4-8.9), and gastrointestinal cancer (4.6-6.3) had the highest crude admission rates of CV hospitalizations per 100,000 U.S. population.
The CV admission rate went up from 2.5 to 3.7 per 100,000 U.S. population for breast cancer, and in prostate cancer, the rate dropped from 5.8 to 4.8 per 100,000 U.S. population.
Of note, patients with hematologic cancers also had the highest rate of heart failure hospitalization across all cancer types, which, coupled with their increasing admission rates, likely reflects their exposure to a “constellation of cardiotoxic therapies” as well as pathologic processes related to the cancers themselves, the authors suggest.
In-hospital mortality rates were higher among patients with cancer than those without, ranging from 5% for patients with breast cancer to 9.6% for patients with lung cancer versus 4.2% for those without cancer.
Among patients with cancer, the odds ratio for mortality was highest in those admitted with AFib (4.43), followed by pulmonary embolism (2.36), AMI (2.31), ischemic stroke (2.29), and heart failure (2.24).
In line with prior work and general population trends, in-hospital deaths in primary CV admissions trended lower among patients with cancer over the study period.
Mitigating risk
Commenting on the study, Joerg Herrmann, MD, director of the cardio-oncology clinic at Mayo Clinic, Rochester, Minn., said that the data are “extremely important” because they reflect admissions during a new era of cancer therapy. “Targeted therapies all came out about the turn of the millennium, so we’re not really looking at cancer patients treated with only old and ancient strategies.”
This may be one reason for the increased admissions, but because the study lacked information on specific cancer treatments and the date of cancer diagnosis, it’s not possible to tease out whether the uptick is related to cardiotoxicity or because the oncology outcomes have improved so much that this is a growing population, he said.
One clear implication, however, is that whoever is working on the hospital service will see more patients with a cancer diagnosis, Dr. Herrmann observed.
“Though some may have tried to maybe not get involved with this topic as much, it really calls for some broader scope to get familiar with this very entity,” he said. “And that plays out, in particular, in those patients with a diagnosis of active cancer.”
Dr. Herrmann and colleagues previously reported that patients with active leukemia or lymphoma who were hospitalized with acute coronary syndrome were less likely to receive guideline-directed therapies, even at the Mayo Clinic.
Similarly, a 2020 report by Dr. Mamas and colleagues found that patients with a variety of active cancers derived similar benefit from primary percutaneous coronary intervention for ST-segment–elevation MI as those without cancer but received the treatment less commonly.
Although there’s a greater appreciation that patients with cancer benefit equally from aggressive treatment, much more can be done to mitigate CV risk, Dr. Mamas noted. Valuable coronary information captured by MRI and CT done as part of the cancer investigation is often overlooked. For example, “we know that breast calcification and vascular calcification in the breast are very strong predictors of cardiovascular outcomes and yet people aren’t using this information.”
There are numerous shared risk factors in the development of cancer and coronary artery disease, and patients with cancer often have much worse CV risk profiles but aren’t routinely risk stratified from a CV perspective, he said.
Dr. Mamas said that his team is also studying whether CVD risk prediction tools like the Framingham Risk Score, which were derived from noncancer populations, work as well in patients with cancer. “Often, when you look at the performance of these tools in populations that weren’t covered, they’re much worse.”
“A lot of cancer survivors worry about the recurrence of their cancer and will religiously go and have repeated scans, religiously check themselves, and have all these investigations but don’t think about the actual risk that is greater for them, which is cardiovascular risk,” he said.
The authors report no study funding or relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although cardiovascular disease (CVD) is known to often strike the mortal blow in patients with cancer, a national analysis puts in stark relief the burden of CV-related hospitalizations in this vulnerable population.
, whereas admissions fell 10.9% among those without cancer.
Admissions increased steadily across all cancer types, except prostate cancer, with heart failure being the most common reason for admission.
“Hospital admissions is really important because we know that the size of this group is increasing, given that they live longer and many of the treatments that we offer cause cardiovascular disease or increase the risk of having cardiovascular events. So, from a health care planning perspective, I think it’s really important to see what the burden is likely to be in the next few years,” senior author Mamas Mamas, MD, Keele University, England, told this news organization.
For physicians and the wider population, he said, the findings underscore the need to shift the conversation from saying that patients with cancer are at increased CVD risk to asking how to mitigate this risk. “Because I would say that this increase in cardiovascular admissions, that’s a failure from a preventative perspective.”
The study was published in the European Heart Journal: Quality of Care & Clinical Outcomes.
Individual cancer types
The researchers, led by Ofer Kobo, MD, also with Keele University, used the National Inpatient Sample to identify 42.5 million weighted cases of CV admissions for acute myocardial infarction (AMI), pulmonary embolism, ischemic stroke, heart failure, atrial fibrillation (AFib) or atrial flutter, and intracranial hemorrhage from January 2004 to December 2017. Of these, 1.9 million had a record of cancer.
Patients with cancer were older; had a higher prevalence of valvular disease, anemia, and coagulopathy; and had a lower prevalence of hypertension, diabetes mellitus, and obesity than did patients without cancer.
The most common cancer type was hematologic cancers (26.1%), followed by lung (18.7%), gastrointestinal (12.4%), prostate (11.6%), breast (6.7%), and other in 24.4%.
The admission rate increased across all six admission causes – between 7% for AMI and ischemic stroke and 46% for AFib.
Heart failure was the chief reason for admission among all patients. Annual rates per 100,000 U.S. population increased in patients with cancer (from 13.6 to 16.6; P for trend = .02) and declined in those without (from 352.2 to 349.8; P for trend < .001).
“In the past, patients would be started on medications, and perhaps the importance of monitoring [left ventricular] LV function wasn’t as widely known, whereas now we’re much more aggressive in looking at it and much more aggressive at trying to prevent it,” Dr. Mamas said. “But even with this greater identification and attempting to modify regimens, we’re still getting quite substantial increases in heart failure admissions in this population. And what really surprised me is that it wasn’t just in the breast cancer population, but it was nearly across the board.”
He noted that patients are at highest risk from CV events within the first 2 years of cancer diagnosis. “So that’s really the time where you’ve got to be really aggressive in looking and working up their cardiovascular profile.”
Patients with hematologic cancers (9.7-13.5), lung (7.4-8.9), and gastrointestinal cancer (4.6-6.3) had the highest crude admission rates of CV hospitalizations per 100,000 U.S. population.
The CV admission rate went up from 2.5 to 3.7 per 100,000 U.S. population for breast cancer, and in prostate cancer, the rate dropped from 5.8 to 4.8 per 100,000 U.S. population.
Of note, patients with hematologic cancers also had the highest rate of heart failure hospitalization across all cancer types, which, coupled with their increasing admission rates, likely reflects their exposure to a “constellation of cardiotoxic therapies” as well as pathologic processes related to the cancers themselves, the authors suggest.
In-hospital mortality rates were higher among patients with cancer than those without, ranging from 5% for patients with breast cancer to 9.6% for patients with lung cancer versus 4.2% for those without cancer.
Among patients with cancer, the odds ratio for mortality was highest in those admitted with AFib (4.43), followed by pulmonary embolism (2.36), AMI (2.31), ischemic stroke (2.29), and heart failure (2.24).
In line with prior work and general population trends, in-hospital deaths in primary CV admissions trended lower among patients with cancer over the study period.
Mitigating risk
Commenting on the study, Joerg Herrmann, MD, director of the cardio-oncology clinic at Mayo Clinic, Rochester, Minn., said that the data are “extremely important” because they reflect admissions during a new era of cancer therapy. “Targeted therapies all came out about the turn of the millennium, so we’re not really looking at cancer patients treated with only old and ancient strategies.”
This may be one reason for the increased admissions, but because the study lacked information on specific cancer treatments and the date of cancer diagnosis, it’s not possible to tease out whether the uptick is related to cardiotoxicity or because the oncology outcomes have improved so much that this is a growing population, he said.
One clear implication, however, is that whoever is working on the hospital service will see more patients with a cancer diagnosis, Dr. Herrmann observed.
“Though some may have tried to maybe not get involved with this topic as much, it really calls for some broader scope to get familiar with this very entity,” he said. “And that plays out, in particular, in those patients with a diagnosis of active cancer.”
Dr. Herrmann and colleagues previously reported that patients with active leukemia or lymphoma who were hospitalized with acute coronary syndrome were less likely to receive guideline-directed therapies, even at the Mayo Clinic.
Similarly, a 2020 report by Dr. Mamas and colleagues found that patients with a variety of active cancers derived similar benefit from primary percutaneous coronary intervention for ST-segment–elevation MI as those without cancer but received the treatment less commonly.
Although there’s a greater appreciation that patients with cancer benefit equally from aggressive treatment, much more can be done to mitigate CV risk, Dr. Mamas noted. Valuable coronary information captured by MRI and CT done as part of the cancer investigation is often overlooked. For example, “we know that breast calcification and vascular calcification in the breast are very strong predictors of cardiovascular outcomes and yet people aren’t using this information.”
There are numerous shared risk factors in the development of cancer and coronary artery disease, and patients with cancer often have much worse CV risk profiles but aren’t routinely risk stratified from a CV perspective, he said.
Dr. Mamas said that his team is also studying whether CVD risk prediction tools like the Framingham Risk Score, which were derived from noncancer populations, work as well in patients with cancer. “Often, when you look at the performance of these tools in populations that weren’t covered, they’re much worse.”
“A lot of cancer survivors worry about the recurrence of their cancer and will religiously go and have repeated scans, religiously check themselves, and have all these investigations but don’t think about the actual risk that is greater for them, which is cardiovascular risk,” he said.
The authors report no study funding or relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although cardiovascular disease (CVD) is known to often strike the mortal blow in patients with cancer, a national analysis puts in stark relief the burden of CV-related hospitalizations in this vulnerable population.
, whereas admissions fell 10.9% among those without cancer.
Admissions increased steadily across all cancer types, except prostate cancer, with heart failure being the most common reason for admission.
“Hospital admissions is really important because we know that the size of this group is increasing, given that they live longer and many of the treatments that we offer cause cardiovascular disease or increase the risk of having cardiovascular events. So, from a health care planning perspective, I think it’s really important to see what the burden is likely to be in the next few years,” senior author Mamas Mamas, MD, Keele University, England, told this news organization.
For physicians and the wider population, he said, the findings underscore the need to shift the conversation from saying that patients with cancer are at increased CVD risk to asking how to mitigate this risk. “Because I would say that this increase in cardiovascular admissions, that’s a failure from a preventative perspective.”
The study was published in the European Heart Journal: Quality of Care & Clinical Outcomes.
Individual cancer types
The researchers, led by Ofer Kobo, MD, also with Keele University, used the National Inpatient Sample to identify 42.5 million weighted cases of CV admissions for acute myocardial infarction (AMI), pulmonary embolism, ischemic stroke, heart failure, atrial fibrillation (AFib) or atrial flutter, and intracranial hemorrhage from January 2004 to December 2017. Of these, 1.9 million had a record of cancer.
Patients with cancer were older; had a higher prevalence of valvular disease, anemia, and coagulopathy; and had a lower prevalence of hypertension, diabetes mellitus, and obesity than did patients without cancer.
The most common cancer type was hematologic cancers (26.1%), followed by lung (18.7%), gastrointestinal (12.4%), prostate (11.6%), breast (6.7%), and other in 24.4%.
The admission rate increased across all six admission causes – between 7% for AMI and ischemic stroke and 46% for AFib.
Heart failure was the chief reason for admission among all patients. Annual rates per 100,000 U.S. population increased in patients with cancer (from 13.6 to 16.6; P for trend = .02) and declined in those without (from 352.2 to 349.8; P for trend < .001).
“In the past, patients would be started on medications, and perhaps the importance of monitoring [left ventricular] LV function wasn’t as widely known, whereas now we’re much more aggressive in looking at it and much more aggressive at trying to prevent it,” Dr. Mamas said. “But even with this greater identification and attempting to modify regimens, we’re still getting quite substantial increases in heart failure admissions in this population. And what really surprised me is that it wasn’t just in the breast cancer population, but it was nearly across the board.”
He noted that patients are at highest risk from CV events within the first 2 years of cancer diagnosis. “So that’s really the time where you’ve got to be really aggressive in looking and working up their cardiovascular profile.”
Patients with hematologic cancers (9.7-13.5), lung (7.4-8.9), and gastrointestinal cancer (4.6-6.3) had the highest crude admission rates of CV hospitalizations per 100,000 U.S. population.
The CV admission rate went up from 2.5 to 3.7 per 100,000 U.S. population for breast cancer, and in prostate cancer, the rate dropped from 5.8 to 4.8 per 100,000 U.S. population.
Of note, patients with hematologic cancers also had the highest rate of heart failure hospitalization across all cancer types, which, coupled with their increasing admission rates, likely reflects their exposure to a “constellation of cardiotoxic therapies” as well as pathologic processes related to the cancers themselves, the authors suggest.
In-hospital mortality rates were higher among patients with cancer than those without, ranging from 5% for patients with breast cancer to 9.6% for patients with lung cancer versus 4.2% for those without cancer.
Among patients with cancer, the odds ratio for mortality was highest in those admitted with AFib (4.43), followed by pulmonary embolism (2.36), AMI (2.31), ischemic stroke (2.29), and heart failure (2.24).
In line with prior work and general population trends, in-hospital deaths in primary CV admissions trended lower among patients with cancer over the study period.
Mitigating risk
Commenting on the study, Joerg Herrmann, MD, director of the cardio-oncology clinic at Mayo Clinic, Rochester, Minn., said that the data are “extremely important” because they reflect admissions during a new era of cancer therapy. “Targeted therapies all came out about the turn of the millennium, so we’re not really looking at cancer patients treated with only old and ancient strategies.”
This may be one reason for the increased admissions, but because the study lacked information on specific cancer treatments and the date of cancer diagnosis, it’s not possible to tease out whether the uptick is related to cardiotoxicity or because the oncology outcomes have improved so much that this is a growing population, he said.
One clear implication, however, is that whoever is working on the hospital service will see more patients with a cancer diagnosis, Dr. Herrmann observed.
“Though some may have tried to maybe not get involved with this topic as much, it really calls for some broader scope to get familiar with this very entity,” he said. “And that plays out, in particular, in those patients with a diagnosis of active cancer.”
Dr. Herrmann and colleagues previously reported that patients with active leukemia or lymphoma who were hospitalized with acute coronary syndrome were less likely to receive guideline-directed therapies, even at the Mayo Clinic.
Similarly, a 2020 report by Dr. Mamas and colleagues found that patients with a variety of active cancers derived similar benefit from primary percutaneous coronary intervention for ST-segment–elevation MI as those without cancer but received the treatment less commonly.
Although there’s a greater appreciation that patients with cancer benefit equally from aggressive treatment, much more can be done to mitigate CV risk, Dr. Mamas noted. Valuable coronary information captured by MRI and CT done as part of the cancer investigation is often overlooked. For example, “we know that breast calcification and vascular calcification in the breast are very strong predictors of cardiovascular outcomes and yet people aren’t using this information.”
There are numerous shared risk factors in the development of cancer and coronary artery disease, and patients with cancer often have much worse CV risk profiles but aren’t routinely risk stratified from a CV perspective, he said.
Dr. Mamas said that his team is also studying whether CVD risk prediction tools like the Framingham Risk Score, which were derived from noncancer populations, work as well in patients with cancer. “Often, when you look at the performance of these tools in populations that weren’t covered, they’re much worse.”
“A lot of cancer survivors worry about the recurrence of their cancer and will religiously go and have repeated scans, religiously check themselves, and have all these investigations but don’t think about the actual risk that is greater for them, which is cardiovascular risk,” he said.
The authors report no study funding or relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM European Heart Journal: Quality of Care & Clinical Outcomes
How well do vaccines protect against long COVID?
New York City veterinarian Erin Kulick used to be a weekend warrior. Only 2½ years ago, the 38-year-old new mother played ultimate Frisbee and flag football with friends. She went for regular 30-minute runs to burn off stress.
Now, Dr. Kulick is usually so exhausted, she can’t walk nonstop for 15 minutes. She recently tried to take her 4-year-old son, Cooper, to the American Museum of Natural History for his first visit, but ended up on a bench outside the museum, sobbing in the rain, because she couldn’t even get through the first hurdle of standing in line. “I just wanted to be there with my kid,” she said.
Dr. Kulick got sick with COVID-19 at the start of the pandemic in March 2020, 9 months before the first vaccine would be approved. Now she is among the estimated one in five infected Americans, or 19%, whose symptoms developed into long COVID.
Dr. Kulick also is now vaccinated and boosted. Had a vaccine been available sooner, could it have protected her from long COVID?
Evidence is starting to show it’s likely.
“The best way not to have long COVID is not to have COVID at all,” said Leora Horwitz, MD, a professor of population health and medicine at New York University. “To the extent that vaccination can prevent you from getting COVID at all, then it helps to reduce long COVID.”
And People with more serious initial illness appear more likely to have prolonged symptoms, but those with milder disease can certainly get it, too.
“You’re more likely to have long COVID with more severe disease, and we have ample evidence that vaccination reduces the severity of disease,” Dr. Horwitz said. “We also now have quite a lot of evidence that vaccination does reduce your risk of long COVID – probably because it reduces your risk of severe disease.”
There is little consensus about how much vaccines can lower the risk of long-term COVID symptoms, but several studies suggest that number lies anywhere from 15% to more than 80%.
That might seem like a big variation, but infectious disease experts argue that trying to interpret the gap isn’t as important as noticing what’s consistent across all these studies: “Vaccines do offer some protection, but it’s incomplete,” said Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System. Dr. Al-Aly, who has led several large studies on long COVID, said focusing on the fact that vaccines do offer some protection is a much better public health message than looking at the different levels of risk.
“Vaccines do a miraculous job for what they were designed to do,” said Dr. Al-Aly. “Vaccines were designed to reduce the risk of hospitalization ... and for that, vaccines are still holding up, even with all the changes in the virus.”
Still, Elena Azzolini, MD, PhD, head of the Humanitas Research Hospital’s vaccination center in Milan, thinks some studies may have underestimated the level of long COVID protection from vaccines because of limits in the study methods, such as not including enough women, who are more affected by long COVID. Her recent study, which looked at 2,560 health care professionals working in nine Italian centers from March 2020 to April 2022, focused on the risk for healthy women and men in their 20s to their 70s.
In the paper, Dr. Azzolini and associates reported that two or three doses of vaccine reduced the risk of hospitalization from COVID-19 from 42% among those who are unvaccinated to 16%-17%. In other words, they found unvaccinated people in the study were nearly three times as likely to have serious symptoms for longer than 4 weeks.
But Dr. Azzolini and Dr. Al-Aly still say that, even for the vaccinated, as long as COVID is around, masks are necessary. That’s because current vaccines don’t do enough to reduce transmission, said Dr. Al-Aly. “The only way that can really help [stop] transmission is covering our nose and mouth with a mask.”
How vaccinations affect people who already have long COVID
Some long COVID patients have said they got better after they get boosted, while some say they’re getting worse, said Dr. Horwitz, who is also a lead investigator at the National Institutes of Health’s flagship RECOVER program, a 4-year research project to study long COVID across the United States. (The NIH is still recruiting volunteers for these studies, which are also open to people who have never had COVID.)
One study published in the British Medical Journal analyzed survey data of more than 28,000 people infected with COVID in the United Kingdom and found a 13% reduction in long-term symptoms after a first dose of the vaccine, although it was unclear from the data if the improvement was sustained.
A second dose was associated with another 8% improvement over a 2-month period. “It’s reassuring that we see an average modest improvement in symptoms, not an average worsening in symptoms,” said Daniel Ayoubkhani, principal statistician at the U.K. Office for National Statistics and lead author of the study. Of course, the experience will differ among different people.
“It doesn’t appear that vaccination is the silver bullet that’s going to eradicate long COVID,” he said, but evidence from multiple studies suggests vaccines may help people with long-term symptoms.
Akiko Iwasaki, PhD, an immunobiologist at Yale University, New Haven, Conn., told a White House summit in July that one of the best ways to prevent long COVID is to develop the next generation of vaccines that also prevent milder cases by blocking transmission in the first place.
Back in New York, Dr. Kulick is now triple vaccinated. She’s due for a fourth dose soon but admits she’s “terrified every time” that she’s going to get sicker.
In her Facebook support group for long COVID, she reads that most people with prolonged symptoms handle it well. She has also noticed some of her symptoms eased after her first two doses of vaccine.
Since being diagnosed, Dr. Kulick learned she has a genetic condition, Ehlers-Danlos syndrome, which affects connective tissues that support skin, joints, organs, and blood vessels, and which her doctors say may have made her more prone to long COVID. She’s also being screened for autoimmune diseases, but for now, the only relief she has found has come from long COVID physical therapy, changes to her diet, and integrative medicine.
Dr. Kulick is still trying to figure out how she can get better while keeping her long hours at her veterinary job – and her health benefits. She is thankful her husband is a devoted caregiver to their son and a professional jazz musician with a schedule that allows for some flexibility.
“But it’s really hard when every week feels like I’ve run a marathon,” she said. “I can barely make it through.”
A version of this article first appeared on WebMD.com.
New York City veterinarian Erin Kulick used to be a weekend warrior. Only 2½ years ago, the 38-year-old new mother played ultimate Frisbee and flag football with friends. She went for regular 30-minute runs to burn off stress.
Now, Dr. Kulick is usually so exhausted, she can’t walk nonstop for 15 minutes. She recently tried to take her 4-year-old son, Cooper, to the American Museum of Natural History for his first visit, but ended up on a bench outside the museum, sobbing in the rain, because she couldn’t even get through the first hurdle of standing in line. “I just wanted to be there with my kid,” she said.
Dr. Kulick got sick with COVID-19 at the start of the pandemic in March 2020, 9 months before the first vaccine would be approved. Now she is among the estimated one in five infected Americans, or 19%, whose symptoms developed into long COVID.
Dr. Kulick also is now vaccinated and boosted. Had a vaccine been available sooner, could it have protected her from long COVID?
Evidence is starting to show it’s likely.
“The best way not to have long COVID is not to have COVID at all,” said Leora Horwitz, MD, a professor of population health and medicine at New York University. “To the extent that vaccination can prevent you from getting COVID at all, then it helps to reduce long COVID.”
And People with more serious initial illness appear more likely to have prolonged symptoms, but those with milder disease can certainly get it, too.
“You’re more likely to have long COVID with more severe disease, and we have ample evidence that vaccination reduces the severity of disease,” Dr. Horwitz said. “We also now have quite a lot of evidence that vaccination does reduce your risk of long COVID – probably because it reduces your risk of severe disease.”
There is little consensus about how much vaccines can lower the risk of long-term COVID symptoms, but several studies suggest that number lies anywhere from 15% to more than 80%.
That might seem like a big variation, but infectious disease experts argue that trying to interpret the gap isn’t as important as noticing what’s consistent across all these studies: “Vaccines do offer some protection, but it’s incomplete,” said Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System. Dr. Al-Aly, who has led several large studies on long COVID, said focusing on the fact that vaccines do offer some protection is a much better public health message than looking at the different levels of risk.
“Vaccines do a miraculous job for what they were designed to do,” said Dr. Al-Aly. “Vaccines were designed to reduce the risk of hospitalization ... and for that, vaccines are still holding up, even with all the changes in the virus.”
Still, Elena Azzolini, MD, PhD, head of the Humanitas Research Hospital’s vaccination center in Milan, thinks some studies may have underestimated the level of long COVID protection from vaccines because of limits in the study methods, such as not including enough women, who are more affected by long COVID. Her recent study, which looked at 2,560 health care professionals working in nine Italian centers from March 2020 to April 2022, focused on the risk for healthy women and men in their 20s to their 70s.
In the paper, Dr. Azzolini and associates reported that two or three doses of vaccine reduced the risk of hospitalization from COVID-19 from 42% among those who are unvaccinated to 16%-17%. In other words, they found unvaccinated people in the study were nearly three times as likely to have serious symptoms for longer than 4 weeks.
But Dr. Azzolini and Dr. Al-Aly still say that, even for the vaccinated, as long as COVID is around, masks are necessary. That’s because current vaccines don’t do enough to reduce transmission, said Dr. Al-Aly. “The only way that can really help [stop] transmission is covering our nose and mouth with a mask.”
How vaccinations affect people who already have long COVID
Some long COVID patients have said they got better after they get boosted, while some say they’re getting worse, said Dr. Horwitz, who is also a lead investigator at the National Institutes of Health’s flagship RECOVER program, a 4-year research project to study long COVID across the United States. (The NIH is still recruiting volunteers for these studies, which are also open to people who have never had COVID.)
One study published in the British Medical Journal analyzed survey data of more than 28,000 people infected with COVID in the United Kingdom and found a 13% reduction in long-term symptoms after a first dose of the vaccine, although it was unclear from the data if the improvement was sustained.
A second dose was associated with another 8% improvement over a 2-month period. “It’s reassuring that we see an average modest improvement in symptoms, not an average worsening in symptoms,” said Daniel Ayoubkhani, principal statistician at the U.K. Office for National Statistics and lead author of the study. Of course, the experience will differ among different people.
“It doesn’t appear that vaccination is the silver bullet that’s going to eradicate long COVID,” he said, but evidence from multiple studies suggests vaccines may help people with long-term symptoms.
Akiko Iwasaki, PhD, an immunobiologist at Yale University, New Haven, Conn., told a White House summit in July that one of the best ways to prevent long COVID is to develop the next generation of vaccines that also prevent milder cases by blocking transmission in the first place.
Back in New York, Dr. Kulick is now triple vaccinated. She’s due for a fourth dose soon but admits she’s “terrified every time” that she’s going to get sicker.
In her Facebook support group for long COVID, she reads that most people with prolonged symptoms handle it well. She has also noticed some of her symptoms eased after her first two doses of vaccine.
Since being diagnosed, Dr. Kulick learned she has a genetic condition, Ehlers-Danlos syndrome, which affects connective tissues that support skin, joints, organs, and blood vessels, and which her doctors say may have made her more prone to long COVID. She’s also being screened for autoimmune diseases, but for now, the only relief she has found has come from long COVID physical therapy, changes to her diet, and integrative medicine.
Dr. Kulick is still trying to figure out how she can get better while keeping her long hours at her veterinary job – and her health benefits. She is thankful her husband is a devoted caregiver to their son and a professional jazz musician with a schedule that allows for some flexibility.
“But it’s really hard when every week feels like I’ve run a marathon,” she said. “I can barely make it through.”
A version of this article first appeared on WebMD.com.
New York City veterinarian Erin Kulick used to be a weekend warrior. Only 2½ years ago, the 38-year-old new mother played ultimate Frisbee and flag football with friends. She went for regular 30-minute runs to burn off stress.
Now, Dr. Kulick is usually so exhausted, she can’t walk nonstop for 15 minutes. She recently tried to take her 4-year-old son, Cooper, to the American Museum of Natural History for his first visit, but ended up on a bench outside the museum, sobbing in the rain, because she couldn’t even get through the first hurdle of standing in line. “I just wanted to be there with my kid,” she said.
Dr. Kulick got sick with COVID-19 at the start of the pandemic in March 2020, 9 months before the first vaccine would be approved. Now she is among the estimated one in five infected Americans, or 19%, whose symptoms developed into long COVID.
Dr. Kulick also is now vaccinated and boosted. Had a vaccine been available sooner, could it have protected her from long COVID?
Evidence is starting to show it’s likely.
“The best way not to have long COVID is not to have COVID at all,” said Leora Horwitz, MD, a professor of population health and medicine at New York University. “To the extent that vaccination can prevent you from getting COVID at all, then it helps to reduce long COVID.”
And People with more serious initial illness appear more likely to have prolonged symptoms, but those with milder disease can certainly get it, too.
“You’re more likely to have long COVID with more severe disease, and we have ample evidence that vaccination reduces the severity of disease,” Dr. Horwitz said. “We also now have quite a lot of evidence that vaccination does reduce your risk of long COVID – probably because it reduces your risk of severe disease.”
There is little consensus about how much vaccines can lower the risk of long-term COVID symptoms, but several studies suggest that number lies anywhere from 15% to more than 80%.
That might seem like a big variation, but infectious disease experts argue that trying to interpret the gap isn’t as important as noticing what’s consistent across all these studies: “Vaccines do offer some protection, but it’s incomplete,” said Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System. Dr. Al-Aly, who has led several large studies on long COVID, said focusing on the fact that vaccines do offer some protection is a much better public health message than looking at the different levels of risk.
“Vaccines do a miraculous job for what they were designed to do,” said Dr. Al-Aly. “Vaccines were designed to reduce the risk of hospitalization ... and for that, vaccines are still holding up, even with all the changes in the virus.”
Still, Elena Azzolini, MD, PhD, head of the Humanitas Research Hospital’s vaccination center in Milan, thinks some studies may have underestimated the level of long COVID protection from vaccines because of limits in the study methods, such as not including enough women, who are more affected by long COVID. Her recent study, which looked at 2,560 health care professionals working in nine Italian centers from March 2020 to April 2022, focused on the risk for healthy women and men in their 20s to their 70s.
In the paper, Dr. Azzolini and associates reported that two or three doses of vaccine reduced the risk of hospitalization from COVID-19 from 42% among those who are unvaccinated to 16%-17%. In other words, they found unvaccinated people in the study were nearly three times as likely to have serious symptoms for longer than 4 weeks.
But Dr. Azzolini and Dr. Al-Aly still say that, even for the vaccinated, as long as COVID is around, masks are necessary. That’s because current vaccines don’t do enough to reduce transmission, said Dr. Al-Aly. “The only way that can really help [stop] transmission is covering our nose and mouth with a mask.”
How vaccinations affect people who already have long COVID
Some long COVID patients have said they got better after they get boosted, while some say they’re getting worse, said Dr. Horwitz, who is also a lead investigator at the National Institutes of Health’s flagship RECOVER program, a 4-year research project to study long COVID across the United States. (The NIH is still recruiting volunteers for these studies, which are also open to people who have never had COVID.)
One study published in the British Medical Journal analyzed survey data of more than 28,000 people infected with COVID in the United Kingdom and found a 13% reduction in long-term symptoms after a first dose of the vaccine, although it was unclear from the data if the improvement was sustained.
A second dose was associated with another 8% improvement over a 2-month period. “It’s reassuring that we see an average modest improvement in symptoms, not an average worsening in symptoms,” said Daniel Ayoubkhani, principal statistician at the U.K. Office for National Statistics and lead author of the study. Of course, the experience will differ among different people.
“It doesn’t appear that vaccination is the silver bullet that’s going to eradicate long COVID,” he said, but evidence from multiple studies suggests vaccines may help people with long-term symptoms.
Akiko Iwasaki, PhD, an immunobiologist at Yale University, New Haven, Conn., told a White House summit in July that one of the best ways to prevent long COVID is to develop the next generation of vaccines that also prevent milder cases by blocking transmission in the first place.
Back in New York, Dr. Kulick is now triple vaccinated. She’s due for a fourth dose soon but admits she’s “terrified every time” that she’s going to get sicker.
In her Facebook support group for long COVID, she reads that most people with prolonged symptoms handle it well. She has also noticed some of her symptoms eased after her first two doses of vaccine.
Since being diagnosed, Dr. Kulick learned she has a genetic condition, Ehlers-Danlos syndrome, which affects connective tissues that support skin, joints, organs, and blood vessels, and which her doctors say may have made her more prone to long COVID. She’s also being screened for autoimmune diseases, but for now, the only relief she has found has come from long COVID physical therapy, changes to her diet, and integrative medicine.
Dr. Kulick is still trying to figure out how she can get better while keeping her long hours at her veterinary job – and her health benefits. She is thankful her husband is a devoted caregiver to their son and a professional jazz musician with a schedule that allows for some flexibility.
“But it’s really hard when every week feels like I’ve run a marathon,” she said. “I can barely make it through.”
A version of this article first appeared on WebMD.com.
2022 Update on female sexual health
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.