User login
Combatting Climate Change: 10 Interventions for Dermatologists to Consider for Sustainability
The impacts of anthropogenic climate change on human health are numerous and growing. The evidence that climate change is occurring due to the burning of fossil fuels is substantial, with a 2019 report elevating the data supporting anthropogenic climate change to a gold standard 5-sigma level of significance.1 In the peer-reviewed scientific literature, the consensus that humans are causing climate change is greater than 99%.2 Both the American Medical Association and the American College of Physicians have acknowledged the health impacts of climate change and importance for action. They encourage physicians to engage in environmentally sustainable practices and to advocate for effective climate change mitigation strategies.3,4 A survey of dermatologists also found that 99.3% (n=148) recognize climate change is occurring, and similarly high numbers are concerned about its health impacts.5
Notably, the health care industry must grapple not only with the health impacts of climate change but with the fact that the health care sector itself is responsible for a large amount of carbon emissions.6 The global health care industry as a whole produces enough carbon emissions to be ranked as the fifth largest emitting nation in the world.7 A quarter of these emissions are attributed to the US health care system.8,9 Climate science has shown we must limit CO2 emissions to avoid catastrophic climate change, with the sixth assessment report of the United Nations’ Intergovernmental Panel on Climate Change and the Paris Agreement targeting large emission reductions within the next decade.10 In August 2021, the US Department of Health and Human Services created the Office of Climate Change and Health Equity. Assistant Secretary for Health ADM Rachel L. Levine, MD, has committed to reducing the carbon emissions from the health care sector by 25% in the next decade, in line with scientific consensus regarding necessary changes.11
The dermatologic impacts of climate change are myriad. Rising temperatures, increasing air and water pollution, and stratospheric ozone depletion will lead to expanded geographic ranges of vector-borne diseases, worsening of chronic skin conditions such as atopic dermatitis/eczema and pemphigus, and increasing rates of skin cancer.12 For instance, warmer temperatures have allowed mosquitoes of the Aedes genus to infest new areas, leading to outbreaks of viral illnesses with cutaneous manifestations such as dengue, chikungunya, and Zika virus in previously nonindigenous regions.13 Rising temperatures also have been associated with an expanding geographic range of tick- and sandfly-borne illnesses such as Lyme disease, Rocky Mountain spotted fever, and cutaneous leishmaniasis.13,14 Additionally, short-term exposure to air pollution from wildfire smoke has been associated with an increased use of health care services by patients with atopic dermatitis.15 Increased levels of air pollutants also have been found to be associated with psoriasis flares as well as hyperpigmentation and wrinkle formation.16,17 Skin cancer incidence is predicted to rise due to increased UV radiation exposure secondary to stratospheric ozone depletion.18
Although the effects of climate change are significant and the magnitude of the climate crisis may feel overwhelming, it is essential to avoid doomerism and focus on meaningful impactful actions. Current CO2 emissions will remain in the atmosphere for hundreds to thousands of years, and the choices we make now commit future generations to live in a world shaped by our decisions. Importantly, there are impactful and low-cost, cost-effective, or cost-saving changes that can be made to mitigate the climate crisis. Herein, we provide 10 practical actionable interventions for dermatologists to help combat climate change.
10 Interventions for Dermatologists to Combat Climate Change
1. Consider switching to renewable sources of energy. Making this switch often is the most impactful decision a dermatologist can make to address climate change. The electricity sector is the largest source of greenhouse gas emissions in the US health care system, and dermatology outpatient practices in particular have been observed to have a higher peak energy consumption than most other specialties studied.19,20 Many dermatology practices—both privately owned and academic—can switch to renewable energy seamlessly through power purchase agreements (PPAs), which are contracts between power providers and private entities to install renewable energy equipment or source renewable energy from offsite sources at a fixed rate. Using PPAs instead of traditional fossil fuel energy can provide cost savings as well as protect buyers from electrical price volatility. Numerous health care systems utilize PPAs such as Kaiser Permanente, Cleveland Clinic, and Rochester Regional Health. Additionally, dermatologists can directly purchase renewable energy equipment and eventually receive a return on investment from substantially lowered electric bills. It is important to note that the cost of commercial solar energy systems has decreased 69% since 2010 with further cost reductions predicted.21,22
2. Reduce standby power consumption. This refers to the use of electricity by a device when it appears to be off or is not in use, which can lead to considerable energy consumption and subsequently a larger carbon footprint for your practice. Ensuring electronics such as phone chargers, light fixtures, television screens, and computers are switched off prior to the end of the workday can make a large difference; for instance, a single radiology department at the University of Maryland (College Park, Maryland) found that if clinical workstations were shut down when not in use after an 8-hour workday, it would save 83,866 kWh of energy and $9225.33 per year.23 Additionally, using power strips with an automatic shutoff feature to shut off power to devices not in use provides a more convenient way to reduce standby power.
3. Optimize thermostat settings. An analysis of energy consumption in 157,000 US health care facilities found that space heating and cooling accounted for 40% of their total energy consumption.24 Thus, ensuring your thermostat and heating/cooling systems are working efficiently can conserve a substantial amount of energy. For maximum efficiency, it is recommended to set air conditioners to 74 °F (24 °C) and heaters to 68 °F (20 °C) or employ smart thermostats to optimally adjust temperatures when the office is not in use.25 In addition, routinely replacing or cleaning air conditioner filters can lower energy consumption by 5% to 15%.26 Similarly, improving insulation and ruggedization of both homes and offices may reduce heating and cooling waste and limit costs and emissions as a result.
4. Offer bicycle racks and charging ports for electric vehicles. In the United States, transportation generates more greenhouse gas emissions than any other source, primarily due to the burning of fossil fuels to power automobiles, trains, and planes. Because bicycles do not consume any fossil fuels and the use of electric vehicles has been found to result in substantial air pollution health benefits, encouraging the use of both can make a considerable positive impact on our climate.27 Providing these resources not only allows those who already travel sustainably to continue to do so but also serves as a reminder to your patients that sustainability is important to you as their health care provider. As electric vehicle sales continue to climb, infrastructure to support their use, including charging stations, will grow in importance. A physician’s office that offers a car-charging station may soon have a competitive advantage over others in the area.
5. Ensure properly regulated medical waste management. Regulated medical waste (also known as infectious medical waste or red bag waste) refers to health care–generated waste unsuitable for disposal in municipal solid waste systems due to concern for the spread of infectious or pathogenic materials. This waste largely is disposed via incineration, which harms the environment in a multitude of ways—both through harmful byproducts and from the CO2 emissions required to ship the waste to special processing facilities.28 Incineration of regulated medical waste emits potent toxins such as dioxins and furans as well as particulate matter, which contribute to air pollution. Ensuring only materials with infectious potential (as defined by each state’s Environmental Protection Agency) are disposed in regulated medical waste containers can dramatically reduce the harmful effects of incineration. Additionally, limiting regulated medical waste can be very cost-effective, as its disposal is 5- to 10-times more expensive than that of unregulated medical waste.29 Simple nudge measures such as educating staff about what waste goes in which receptacle, placing signage over the red bag waste to prompt staff to pause to consider if use of that bin is required before utilizing, using weights or clasps to make opening red bag waste containers slightly harder, and positioning different trash receptacles in different parts of examination rooms may help reduce inappropriate use of red bag waste.
6. Consider virtual platforms when possible. Due to the COVID-19 pandemic, virtual meeting platforms saw a considerable increase in usage by dermatologists. Teledermatology for patient care became much more widely adopted, and traditionally in-person meetings turned virtual.30 The reduction in emissions from these changes was remarkable. A recent study looking at the environmental impact of 3 months of teledermatology visits early during the COVID-19 pandemic found that 1476 teledermatology appointments saved 55,737 miles of car travel, equivalent to 15.37 metric tons of CO2.31 Whether for patient care when appropriate, academic conferences and continuing medical education credit, or for interviews (eg, medical students, residents, other staff), use of virtual platforms can reduce unnecessary travel and therefore substantially reduce travel-related emissions. When travel is unavoidable, consider exploring validated vetted companies that offer carbon offsets to reduce the harmful environmental impact of high-emission flights.
7. Limit use of single-use disposable items. Although single-use items such as examination gloves or needles are necessary in a dermatology practice, there are many opportunities to incorporate reusable items in your workplace. For instance, you can replace plastic cutlery and single-use plates in kitchen or dining areas with reusable alternatives. Additionally, using reusable isolation gowns instead of their single-use counterparts can help reduce waste; a reusable isolation gown system for providers including laundering services was found to consume 28% less energy and emit 30% fewer greenhouse gases than a single-use isolation gown system.32 Similarly, opting for reusable instruments instead of single-use instruments when possible also can help reduce your practice’s carbon footprint. Carefully evaluating each part of your “dermatology visit supply chain” may offer opportunities to utilize additional cost-saving, environmentally friendly options; for example, an individually plastic-wrapped Dermablade vs a bulk-packaged blade for shave biopsies has a higher cost and worse environmental impact. A single gauze often is sufficient for shave biopsies, but many practices open a plastic container of bulk gauze, much of which results in waste that too often is inappropriately disposed of as regulated medical waste despite not being saturated in blood/body fluids.
8. Educate on the effects of climate change. Dermatologists and other physicians have the unique opportunity to teach members of their community every day through patient care. Physicians are trusted messengers, and appropriately counseling patients regarding the risks of climate change and its effects on their dermatologic health is in line with both American Medical Association and American College of Physicians guidelines.3,4 For instance, patients with Lyme disease in Canada or Maine were unheard of a few decades ago, but now they are common; flares of atopic dermatitis in regions adjacent to recent wildfires may be attributable to harmful particulate matter resulting from fossil-fueled climate change and record droughts. Educating medical trainees on the impacts of climate change is just as vital, as it is a topic that often is neglected in medical school and residency curricula.33
9. Install water-efficient toilets and faucets. Anthropogenic climate change has been shown to increase the duration and intensity of droughts throughout the world.34 Much of the western United States also is experiencing record droughts. One way in which dermatology practices can work to combat droughts is through the use of water-conserving toilets, faucets, and urinals. Using water fixtures with the US Environmental Protection Agency’s WaterSense label is a convenient way to do so. The WaterSense label helps identify water fixtures certified to use at least 20% less water as well as save energy and decrease water costs.
10. Advocate through local and national organizations. There are numerous ways in which dermatologists can advocate for action against climate change. Joining professional organizations focused on addressing the climate crisis can help you connect with fellow dermatologists and physicians. The Expert Resource Group on Climate Change and Environmental Issues affiliated with the American Academy of Dermatology (AAD) is one such organization with many opportunities to raise awareness within the field of dermatology. The AAD recently joined the Medical Society Consortium on Climate and Health, an organization providing opportunities for policy and media outreach as well as research on climate change. Advocacy also can mean joining your local chapter of Physicians for Social Responsibility or encouraging divestment from fossil fuel companies within your institution. Voicing support for climate change–focused lectures at events such as grand rounds and society meetings at the local, regional, and state-wide levels can help raise awareness. As the dermatologic effects of climate change grow, being knowledgeable of the views of future leaders in our specialty and country on this issue will become increasingly important.
Final Thoughts
In addition to the climate-friendly decisions one can make as a dermatologist, there are many personal lifestyle choices to consider. Small dietary changes such as limiting consumption of beef and minimizing food waste can have large downstream effects. Opting for transportation via train and limiting air travel are both impactful decisions in reducing CO2 emissions. Similarly, switching to an electric vehicle or vehicle with minimal emissions can work to reduce greenhouse gas accumulation. For additional resources, note the AAD has partnered with My Green Doctor, a nonprofit service for health care practices that includes practical cost-saving suggestions to support sustainability in physician practices.
A recent joint publication in more than 200 medical journals described climate change as the greatest threat to global public health.35 Climate change is having devastating effects on dermatologic health and will only continue to do so if not addressed now. Dermatologists have the opportunity to join with our colleagues in the house of medicine and to take action to fight climate change and mitigate the health impacts on our patients, the population, and future generations.
- Santer BD, Bonfils CJW, Fu Q, et al. Celebrating the anniversary of three key events in climate change science. Nat Clim Chang. 2019;9:180-182.
- Lynas M, Houlton BZ, Perry S. Greater than 99% consensus on human caused climate change in the peer-reviewed scientific literature. Environ Res Lett. 2021;16:114005.
- Crowley RA; Health and Public Policy Committee of the American College of Physicians. Climate change and health: a position paper of the American College of Physicians [published online April 19, 2016]. Ann Intern Med. 2016;164:608-610. doi:10.7326/M15-2766
- Global climate change and human health H-135.398. American Medical Association website. Updated 2019. Accessed July 13, 2022. https://policysearch.ama-assn.org/policyfinder/detail/climate%20change?uri=%2FAMADoc%2FHOD.xml-0-309.xml
- Mieczkowska K, Stringer T, Barbieri JS, et al. Surveying the attitudes of dermatologists regarding climate change. Br J Dermatol. 2022;186:748-750.
- Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. PLoS One. 2016;11:e0157014. doi:10.1371/journal.pone.0157014
- Karliner J, Slotterback S, Boyd R, et al. Health care’s climate footprint: how the health sector contributes to the global climate crisis and opportunities for action. Health Care Without Harm website. Published September 2019. Accessed July 13, 2022. https://noharm-global.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_090619.pdf
- Pichler PP, Jaccard IS, Weisz U, et al. International comparison of health care carbon footprints. Environ Res Lett. 2019;14:064004.
- Solomon CG, LaRocque RC. Climate change—a health emergency. N Engl J Med. 2019;380:209-211. doi:10.1056/NEJMp1817067
- IPCC, 2021: Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, et al, eds. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2021:3-32.
- Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. Health Sector—a call to action [published online October 13, 2021]. N Engl J Med. 2021;385:2117-2119. doi:10.1056/NEJMp2115675
- Silva GS, Rosenbach M. Climate change and dermatology: an introduction to a special topic, for this special issue. Int J Womens Dermatol 2021;7:3-7.
- Coates SJ, Norton SA. The effects of climate change on infectious diseases with cutaneous manifestations. Int J Womens Dermatol. 2021;7:8-16. doi:10.1016/j.ijwd.2020.07.005
- Andersen LK, Davis MD. Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the International Society of Dermatology Climate Change Task Force [published online October 1, 2016]. Int J Dermatol. 2017;56:252-259. doi:10.1111/ijd.13438
- Fadadu RP, Grimes B, Jewell NP, et al. Association of wildfire air pollution and health care use for atopic dermatitis and itch. JAMA Dermatol. 2021;157:658-666. doi:10.1001/jamadermatol.2021.0179
- Bellinato F, Adami G, Vaienti S, et al. Association between short-term exposure to environmental air pollution and psoriasis flare. JAMA Dermatol. 2022;158:375-381. doi:10.1001/jamadermatol.2021.6019
- Krutmann J, Bouloc A, Sore G, et al. The skin aging exposome [published online September 28, 2016]. J Dermatol Sci. 2017;85:152-161.
- Parker ER. The influence of climate change on skin cancer incidence—a review of the evidence. Int J Womens Dermatol. 2020;7:17-27. doi:10.1016/j.ijwd.2020.07.003
- Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
- Sheppy M, Pless S, Kung F. Healthcare energy end-use monitoring. US Department of Energy website. Published August 2014. Accessed July 13, 2022. https://www.energy.gov/sites/prod/files/2014/09/f18/61064.pdf
- Feldman D, Ramasamy V, Fu R, et al. U.S. solar photovoltaic system and energy storage cost benchmark: Q1 2020. Published January 2021. Accessed July 7, 2022. https://www.nrel.gov/docs/fy21osti/77324.pdf
- 22. Apostoleris H, Sgouridis S, Stefancich M, et al. Utility solar prices will continue to drop all over the world even without subsidies. Nat Energy. 2019;4:833-834.
- Prasanna PM, Siegel E, Kunce A. Greening radiology. J Am Coll Radiol. 2011;8:780-784. doi:10.1016/j.jacr.2011.07.017
- Bawaneh K, Nezami FG, Rasheduzzaman MD, et al. Energy consumption analysis and characterization of healthcare facilities in the United States. Energies. 2019;12:1-20. doi:10.3390/en12193775
- Blum S, Buckland M, Sack TL, et al. Greening the office: saving resources, saving money, and educating our patients [published online July 4, 2020]. Int J Womens Dermatol. 2020;7:112-116.
- Maintaining your air conditioner. US Department of Energy website. Accessed July 13, 2022. https://www.energy.gov/energysaver/maintaining-your-air-conditioner
- Choma EF, Evans JS, Hammitt JK, et al. Assessing the health impacts of electric vehicles through air pollution in the United States [published online August 25, 2020]. Environ Int. 2020;144:106015.
- Windfeld ES, Brooks MS. Medical waste management—a review [published online August 22, 2015]. J Environ Manage. 2015;1;163:98-108. doi:10.1016/j.jenvman.2015.08.013
- Fathy R, Nelson CA, Barbieri JS. Combating climate change in the clinic: cost-effective strategies to decrease the carbon footprint of outpatient dermatologic practice. Int J Womens Dermatol. 2020;7:107-111.
- Pulsipher KJ, Presley CL, Rundle CW, et al. Teledermatology application use in the COVID-19 era. Dermatol Online J. 2020;26:13030/qt1fs0m0tp.
- O’Connell G, O’Connor C, Murphy M. Every cloud has a silver lining: the environmental benefit of teledermatology during the COVID-19 pandemic [published online July 9, 2021]. Clin Exp Dermatol. 2021;46:1589-1590. doi:10.1111/ced.14795
- Vozzola E, Overcash M, Griffing E. Environmental considerations in the selection of isolation gowns: a life cycle assessment of reusable and disposable alternatives [published online April 11, 2018]. Am J Infect Control. 2018;46:881-886. doi:10.1016/j.ajic.2018.02.002
- Rabin BM, Laney EB, Philipsborn RP. The unique role of medical students in catalyzing climate change education [published online October 14, 2020]. J Med Educ Curric Dev. doi:10.1177/2382120520957653
- Chiang F, Mazdiyasni O, AghaKouchak A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity [published online May 12, 2021]. Nat Commun. 2021;12:2754. doi:10.1038/s41467-021-22314-w
- Atwoli L, Baqui AH, Benfield T, et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health [published online September 5, 2021]. N Engl J Med. 2021;385:1134-1137. doi:10.1056/NEJMe2113200
The impacts of anthropogenic climate change on human health are numerous and growing. The evidence that climate change is occurring due to the burning of fossil fuels is substantial, with a 2019 report elevating the data supporting anthropogenic climate change to a gold standard 5-sigma level of significance.1 In the peer-reviewed scientific literature, the consensus that humans are causing climate change is greater than 99%.2 Both the American Medical Association and the American College of Physicians have acknowledged the health impacts of climate change and importance for action. They encourage physicians to engage in environmentally sustainable practices and to advocate for effective climate change mitigation strategies.3,4 A survey of dermatologists also found that 99.3% (n=148) recognize climate change is occurring, and similarly high numbers are concerned about its health impacts.5
Notably, the health care industry must grapple not only with the health impacts of climate change but with the fact that the health care sector itself is responsible for a large amount of carbon emissions.6 The global health care industry as a whole produces enough carbon emissions to be ranked as the fifth largest emitting nation in the world.7 A quarter of these emissions are attributed to the US health care system.8,9 Climate science has shown we must limit CO2 emissions to avoid catastrophic climate change, with the sixth assessment report of the United Nations’ Intergovernmental Panel on Climate Change and the Paris Agreement targeting large emission reductions within the next decade.10 In August 2021, the US Department of Health and Human Services created the Office of Climate Change and Health Equity. Assistant Secretary for Health ADM Rachel L. Levine, MD, has committed to reducing the carbon emissions from the health care sector by 25% in the next decade, in line with scientific consensus regarding necessary changes.11
The dermatologic impacts of climate change are myriad. Rising temperatures, increasing air and water pollution, and stratospheric ozone depletion will lead to expanded geographic ranges of vector-borne diseases, worsening of chronic skin conditions such as atopic dermatitis/eczema and pemphigus, and increasing rates of skin cancer.12 For instance, warmer temperatures have allowed mosquitoes of the Aedes genus to infest new areas, leading to outbreaks of viral illnesses with cutaneous manifestations such as dengue, chikungunya, and Zika virus in previously nonindigenous regions.13 Rising temperatures also have been associated with an expanding geographic range of tick- and sandfly-borne illnesses such as Lyme disease, Rocky Mountain spotted fever, and cutaneous leishmaniasis.13,14 Additionally, short-term exposure to air pollution from wildfire smoke has been associated with an increased use of health care services by patients with atopic dermatitis.15 Increased levels of air pollutants also have been found to be associated with psoriasis flares as well as hyperpigmentation and wrinkle formation.16,17 Skin cancer incidence is predicted to rise due to increased UV radiation exposure secondary to stratospheric ozone depletion.18
Although the effects of climate change are significant and the magnitude of the climate crisis may feel overwhelming, it is essential to avoid doomerism and focus on meaningful impactful actions. Current CO2 emissions will remain in the atmosphere for hundreds to thousands of years, and the choices we make now commit future generations to live in a world shaped by our decisions. Importantly, there are impactful and low-cost, cost-effective, or cost-saving changes that can be made to mitigate the climate crisis. Herein, we provide 10 practical actionable interventions for dermatologists to help combat climate change.
10 Interventions for Dermatologists to Combat Climate Change
1. Consider switching to renewable sources of energy. Making this switch often is the most impactful decision a dermatologist can make to address climate change. The electricity sector is the largest source of greenhouse gas emissions in the US health care system, and dermatology outpatient practices in particular have been observed to have a higher peak energy consumption than most other specialties studied.19,20 Many dermatology practices—both privately owned and academic—can switch to renewable energy seamlessly through power purchase agreements (PPAs), which are contracts between power providers and private entities to install renewable energy equipment or source renewable energy from offsite sources at a fixed rate. Using PPAs instead of traditional fossil fuel energy can provide cost savings as well as protect buyers from electrical price volatility. Numerous health care systems utilize PPAs such as Kaiser Permanente, Cleveland Clinic, and Rochester Regional Health. Additionally, dermatologists can directly purchase renewable energy equipment and eventually receive a return on investment from substantially lowered electric bills. It is important to note that the cost of commercial solar energy systems has decreased 69% since 2010 with further cost reductions predicted.21,22
2. Reduce standby power consumption. This refers to the use of electricity by a device when it appears to be off or is not in use, which can lead to considerable energy consumption and subsequently a larger carbon footprint for your practice. Ensuring electronics such as phone chargers, light fixtures, television screens, and computers are switched off prior to the end of the workday can make a large difference; for instance, a single radiology department at the University of Maryland (College Park, Maryland) found that if clinical workstations were shut down when not in use after an 8-hour workday, it would save 83,866 kWh of energy and $9225.33 per year.23 Additionally, using power strips with an automatic shutoff feature to shut off power to devices not in use provides a more convenient way to reduce standby power.
3. Optimize thermostat settings. An analysis of energy consumption in 157,000 US health care facilities found that space heating and cooling accounted for 40% of their total energy consumption.24 Thus, ensuring your thermostat and heating/cooling systems are working efficiently can conserve a substantial amount of energy. For maximum efficiency, it is recommended to set air conditioners to 74 °F (24 °C) and heaters to 68 °F (20 °C) or employ smart thermostats to optimally adjust temperatures when the office is not in use.25 In addition, routinely replacing or cleaning air conditioner filters can lower energy consumption by 5% to 15%.26 Similarly, improving insulation and ruggedization of both homes and offices may reduce heating and cooling waste and limit costs and emissions as a result.
4. Offer bicycle racks and charging ports for electric vehicles. In the United States, transportation generates more greenhouse gas emissions than any other source, primarily due to the burning of fossil fuels to power automobiles, trains, and planes. Because bicycles do not consume any fossil fuels and the use of electric vehicles has been found to result in substantial air pollution health benefits, encouraging the use of both can make a considerable positive impact on our climate.27 Providing these resources not only allows those who already travel sustainably to continue to do so but also serves as a reminder to your patients that sustainability is important to you as their health care provider. As electric vehicle sales continue to climb, infrastructure to support their use, including charging stations, will grow in importance. A physician’s office that offers a car-charging station may soon have a competitive advantage over others in the area.
5. Ensure properly regulated medical waste management. Regulated medical waste (also known as infectious medical waste or red bag waste) refers to health care–generated waste unsuitable for disposal in municipal solid waste systems due to concern for the spread of infectious or pathogenic materials. This waste largely is disposed via incineration, which harms the environment in a multitude of ways—both through harmful byproducts and from the CO2 emissions required to ship the waste to special processing facilities.28 Incineration of regulated medical waste emits potent toxins such as dioxins and furans as well as particulate matter, which contribute to air pollution. Ensuring only materials with infectious potential (as defined by each state’s Environmental Protection Agency) are disposed in regulated medical waste containers can dramatically reduce the harmful effects of incineration. Additionally, limiting regulated medical waste can be very cost-effective, as its disposal is 5- to 10-times more expensive than that of unregulated medical waste.29 Simple nudge measures such as educating staff about what waste goes in which receptacle, placing signage over the red bag waste to prompt staff to pause to consider if use of that bin is required before utilizing, using weights or clasps to make opening red bag waste containers slightly harder, and positioning different trash receptacles in different parts of examination rooms may help reduce inappropriate use of red bag waste.
6. Consider virtual platforms when possible. Due to the COVID-19 pandemic, virtual meeting platforms saw a considerable increase in usage by dermatologists. Teledermatology for patient care became much more widely adopted, and traditionally in-person meetings turned virtual.30 The reduction in emissions from these changes was remarkable. A recent study looking at the environmental impact of 3 months of teledermatology visits early during the COVID-19 pandemic found that 1476 teledermatology appointments saved 55,737 miles of car travel, equivalent to 15.37 metric tons of CO2.31 Whether for patient care when appropriate, academic conferences and continuing medical education credit, or for interviews (eg, medical students, residents, other staff), use of virtual platforms can reduce unnecessary travel and therefore substantially reduce travel-related emissions. When travel is unavoidable, consider exploring validated vetted companies that offer carbon offsets to reduce the harmful environmental impact of high-emission flights.
7. Limit use of single-use disposable items. Although single-use items such as examination gloves or needles are necessary in a dermatology practice, there are many opportunities to incorporate reusable items in your workplace. For instance, you can replace plastic cutlery and single-use plates in kitchen or dining areas with reusable alternatives. Additionally, using reusable isolation gowns instead of their single-use counterparts can help reduce waste; a reusable isolation gown system for providers including laundering services was found to consume 28% less energy and emit 30% fewer greenhouse gases than a single-use isolation gown system.32 Similarly, opting for reusable instruments instead of single-use instruments when possible also can help reduce your practice’s carbon footprint. Carefully evaluating each part of your “dermatology visit supply chain” may offer opportunities to utilize additional cost-saving, environmentally friendly options; for example, an individually plastic-wrapped Dermablade vs a bulk-packaged blade for shave biopsies has a higher cost and worse environmental impact. A single gauze often is sufficient for shave biopsies, but many practices open a plastic container of bulk gauze, much of which results in waste that too often is inappropriately disposed of as regulated medical waste despite not being saturated in blood/body fluids.
8. Educate on the effects of climate change. Dermatologists and other physicians have the unique opportunity to teach members of their community every day through patient care. Physicians are trusted messengers, and appropriately counseling patients regarding the risks of climate change and its effects on their dermatologic health is in line with both American Medical Association and American College of Physicians guidelines.3,4 For instance, patients with Lyme disease in Canada or Maine were unheard of a few decades ago, but now they are common; flares of atopic dermatitis in regions adjacent to recent wildfires may be attributable to harmful particulate matter resulting from fossil-fueled climate change and record droughts. Educating medical trainees on the impacts of climate change is just as vital, as it is a topic that often is neglected in medical school and residency curricula.33
9. Install water-efficient toilets and faucets. Anthropogenic climate change has been shown to increase the duration and intensity of droughts throughout the world.34 Much of the western United States also is experiencing record droughts. One way in which dermatology practices can work to combat droughts is through the use of water-conserving toilets, faucets, and urinals. Using water fixtures with the US Environmental Protection Agency’s WaterSense label is a convenient way to do so. The WaterSense label helps identify water fixtures certified to use at least 20% less water as well as save energy and decrease water costs.
10. Advocate through local and national organizations. There are numerous ways in which dermatologists can advocate for action against climate change. Joining professional organizations focused on addressing the climate crisis can help you connect with fellow dermatologists and physicians. The Expert Resource Group on Climate Change and Environmental Issues affiliated with the American Academy of Dermatology (AAD) is one such organization with many opportunities to raise awareness within the field of dermatology. The AAD recently joined the Medical Society Consortium on Climate and Health, an organization providing opportunities for policy and media outreach as well as research on climate change. Advocacy also can mean joining your local chapter of Physicians for Social Responsibility or encouraging divestment from fossil fuel companies within your institution. Voicing support for climate change–focused lectures at events such as grand rounds and society meetings at the local, regional, and state-wide levels can help raise awareness. As the dermatologic effects of climate change grow, being knowledgeable of the views of future leaders in our specialty and country on this issue will become increasingly important.
Final Thoughts
In addition to the climate-friendly decisions one can make as a dermatologist, there are many personal lifestyle choices to consider. Small dietary changes such as limiting consumption of beef and minimizing food waste can have large downstream effects. Opting for transportation via train and limiting air travel are both impactful decisions in reducing CO2 emissions. Similarly, switching to an electric vehicle or vehicle with minimal emissions can work to reduce greenhouse gas accumulation. For additional resources, note the AAD has partnered with My Green Doctor, a nonprofit service for health care practices that includes practical cost-saving suggestions to support sustainability in physician practices.
A recent joint publication in more than 200 medical journals described climate change as the greatest threat to global public health.35 Climate change is having devastating effects on dermatologic health and will only continue to do so if not addressed now. Dermatologists have the opportunity to join with our colleagues in the house of medicine and to take action to fight climate change and mitigate the health impacts on our patients, the population, and future generations.
The impacts of anthropogenic climate change on human health are numerous and growing. The evidence that climate change is occurring due to the burning of fossil fuels is substantial, with a 2019 report elevating the data supporting anthropogenic climate change to a gold standard 5-sigma level of significance.1 In the peer-reviewed scientific literature, the consensus that humans are causing climate change is greater than 99%.2 Both the American Medical Association and the American College of Physicians have acknowledged the health impacts of climate change and importance for action. They encourage physicians to engage in environmentally sustainable practices and to advocate for effective climate change mitigation strategies.3,4 A survey of dermatologists also found that 99.3% (n=148) recognize climate change is occurring, and similarly high numbers are concerned about its health impacts.5
Notably, the health care industry must grapple not only with the health impacts of climate change but with the fact that the health care sector itself is responsible for a large amount of carbon emissions.6 The global health care industry as a whole produces enough carbon emissions to be ranked as the fifth largest emitting nation in the world.7 A quarter of these emissions are attributed to the US health care system.8,9 Climate science has shown we must limit CO2 emissions to avoid catastrophic climate change, with the sixth assessment report of the United Nations’ Intergovernmental Panel on Climate Change and the Paris Agreement targeting large emission reductions within the next decade.10 In August 2021, the US Department of Health and Human Services created the Office of Climate Change and Health Equity. Assistant Secretary for Health ADM Rachel L. Levine, MD, has committed to reducing the carbon emissions from the health care sector by 25% in the next decade, in line with scientific consensus regarding necessary changes.11
The dermatologic impacts of climate change are myriad. Rising temperatures, increasing air and water pollution, and stratospheric ozone depletion will lead to expanded geographic ranges of vector-borne diseases, worsening of chronic skin conditions such as atopic dermatitis/eczema and pemphigus, and increasing rates of skin cancer.12 For instance, warmer temperatures have allowed mosquitoes of the Aedes genus to infest new areas, leading to outbreaks of viral illnesses with cutaneous manifestations such as dengue, chikungunya, and Zika virus in previously nonindigenous regions.13 Rising temperatures also have been associated with an expanding geographic range of tick- and sandfly-borne illnesses such as Lyme disease, Rocky Mountain spotted fever, and cutaneous leishmaniasis.13,14 Additionally, short-term exposure to air pollution from wildfire smoke has been associated with an increased use of health care services by patients with atopic dermatitis.15 Increased levels of air pollutants also have been found to be associated with psoriasis flares as well as hyperpigmentation and wrinkle formation.16,17 Skin cancer incidence is predicted to rise due to increased UV radiation exposure secondary to stratospheric ozone depletion.18
Although the effects of climate change are significant and the magnitude of the climate crisis may feel overwhelming, it is essential to avoid doomerism and focus on meaningful impactful actions. Current CO2 emissions will remain in the atmosphere for hundreds to thousands of years, and the choices we make now commit future generations to live in a world shaped by our decisions. Importantly, there are impactful and low-cost, cost-effective, or cost-saving changes that can be made to mitigate the climate crisis. Herein, we provide 10 practical actionable interventions for dermatologists to help combat climate change.
10 Interventions for Dermatologists to Combat Climate Change
1. Consider switching to renewable sources of energy. Making this switch often is the most impactful decision a dermatologist can make to address climate change. The electricity sector is the largest source of greenhouse gas emissions in the US health care system, and dermatology outpatient practices in particular have been observed to have a higher peak energy consumption than most other specialties studied.19,20 Many dermatology practices—both privately owned and academic—can switch to renewable energy seamlessly through power purchase agreements (PPAs), which are contracts between power providers and private entities to install renewable energy equipment or source renewable energy from offsite sources at a fixed rate. Using PPAs instead of traditional fossil fuel energy can provide cost savings as well as protect buyers from electrical price volatility. Numerous health care systems utilize PPAs such as Kaiser Permanente, Cleveland Clinic, and Rochester Regional Health. Additionally, dermatologists can directly purchase renewable energy equipment and eventually receive a return on investment from substantially lowered electric bills. It is important to note that the cost of commercial solar energy systems has decreased 69% since 2010 with further cost reductions predicted.21,22
2. Reduce standby power consumption. This refers to the use of electricity by a device when it appears to be off or is not in use, which can lead to considerable energy consumption and subsequently a larger carbon footprint for your practice. Ensuring electronics such as phone chargers, light fixtures, television screens, and computers are switched off prior to the end of the workday can make a large difference; for instance, a single radiology department at the University of Maryland (College Park, Maryland) found that if clinical workstations were shut down when not in use after an 8-hour workday, it would save 83,866 kWh of energy and $9225.33 per year.23 Additionally, using power strips with an automatic shutoff feature to shut off power to devices not in use provides a more convenient way to reduce standby power.
3. Optimize thermostat settings. An analysis of energy consumption in 157,000 US health care facilities found that space heating and cooling accounted for 40% of their total energy consumption.24 Thus, ensuring your thermostat and heating/cooling systems are working efficiently can conserve a substantial amount of energy. For maximum efficiency, it is recommended to set air conditioners to 74 °F (24 °C) and heaters to 68 °F (20 °C) or employ smart thermostats to optimally adjust temperatures when the office is not in use.25 In addition, routinely replacing or cleaning air conditioner filters can lower energy consumption by 5% to 15%.26 Similarly, improving insulation and ruggedization of both homes and offices may reduce heating and cooling waste and limit costs and emissions as a result.
4. Offer bicycle racks and charging ports for electric vehicles. In the United States, transportation generates more greenhouse gas emissions than any other source, primarily due to the burning of fossil fuels to power automobiles, trains, and planes. Because bicycles do not consume any fossil fuels and the use of electric vehicles has been found to result in substantial air pollution health benefits, encouraging the use of both can make a considerable positive impact on our climate.27 Providing these resources not only allows those who already travel sustainably to continue to do so but also serves as a reminder to your patients that sustainability is important to you as their health care provider. As electric vehicle sales continue to climb, infrastructure to support their use, including charging stations, will grow in importance. A physician’s office that offers a car-charging station may soon have a competitive advantage over others in the area.
5. Ensure properly regulated medical waste management. Regulated medical waste (also known as infectious medical waste or red bag waste) refers to health care–generated waste unsuitable for disposal in municipal solid waste systems due to concern for the spread of infectious or pathogenic materials. This waste largely is disposed via incineration, which harms the environment in a multitude of ways—both through harmful byproducts and from the CO2 emissions required to ship the waste to special processing facilities.28 Incineration of regulated medical waste emits potent toxins such as dioxins and furans as well as particulate matter, which contribute to air pollution. Ensuring only materials with infectious potential (as defined by each state’s Environmental Protection Agency) are disposed in regulated medical waste containers can dramatically reduce the harmful effects of incineration. Additionally, limiting regulated medical waste can be very cost-effective, as its disposal is 5- to 10-times more expensive than that of unregulated medical waste.29 Simple nudge measures such as educating staff about what waste goes in which receptacle, placing signage over the red bag waste to prompt staff to pause to consider if use of that bin is required before utilizing, using weights or clasps to make opening red bag waste containers slightly harder, and positioning different trash receptacles in different parts of examination rooms may help reduce inappropriate use of red bag waste.
6. Consider virtual platforms when possible. Due to the COVID-19 pandemic, virtual meeting platforms saw a considerable increase in usage by dermatologists. Teledermatology for patient care became much more widely adopted, and traditionally in-person meetings turned virtual.30 The reduction in emissions from these changes was remarkable. A recent study looking at the environmental impact of 3 months of teledermatology visits early during the COVID-19 pandemic found that 1476 teledermatology appointments saved 55,737 miles of car travel, equivalent to 15.37 metric tons of CO2.31 Whether for patient care when appropriate, academic conferences and continuing medical education credit, or for interviews (eg, medical students, residents, other staff), use of virtual platforms can reduce unnecessary travel and therefore substantially reduce travel-related emissions. When travel is unavoidable, consider exploring validated vetted companies that offer carbon offsets to reduce the harmful environmental impact of high-emission flights.
7. Limit use of single-use disposable items. Although single-use items such as examination gloves or needles are necessary in a dermatology practice, there are many opportunities to incorporate reusable items in your workplace. For instance, you can replace plastic cutlery and single-use plates in kitchen or dining areas with reusable alternatives. Additionally, using reusable isolation gowns instead of their single-use counterparts can help reduce waste; a reusable isolation gown system for providers including laundering services was found to consume 28% less energy and emit 30% fewer greenhouse gases than a single-use isolation gown system.32 Similarly, opting for reusable instruments instead of single-use instruments when possible also can help reduce your practice’s carbon footprint. Carefully evaluating each part of your “dermatology visit supply chain” may offer opportunities to utilize additional cost-saving, environmentally friendly options; for example, an individually plastic-wrapped Dermablade vs a bulk-packaged blade for shave biopsies has a higher cost and worse environmental impact. A single gauze often is sufficient for shave biopsies, but many practices open a plastic container of bulk gauze, much of which results in waste that too often is inappropriately disposed of as regulated medical waste despite not being saturated in blood/body fluids.
8. Educate on the effects of climate change. Dermatologists and other physicians have the unique opportunity to teach members of their community every day through patient care. Physicians are trusted messengers, and appropriately counseling patients regarding the risks of climate change and its effects on their dermatologic health is in line with both American Medical Association and American College of Physicians guidelines.3,4 For instance, patients with Lyme disease in Canada or Maine were unheard of a few decades ago, but now they are common; flares of atopic dermatitis in regions adjacent to recent wildfires may be attributable to harmful particulate matter resulting from fossil-fueled climate change and record droughts. Educating medical trainees on the impacts of climate change is just as vital, as it is a topic that often is neglected in medical school and residency curricula.33
9. Install water-efficient toilets and faucets. Anthropogenic climate change has been shown to increase the duration and intensity of droughts throughout the world.34 Much of the western United States also is experiencing record droughts. One way in which dermatology practices can work to combat droughts is through the use of water-conserving toilets, faucets, and urinals. Using water fixtures with the US Environmental Protection Agency’s WaterSense label is a convenient way to do so. The WaterSense label helps identify water fixtures certified to use at least 20% less water as well as save energy and decrease water costs.
10. Advocate through local and national organizations. There are numerous ways in which dermatologists can advocate for action against climate change. Joining professional organizations focused on addressing the climate crisis can help you connect with fellow dermatologists and physicians. The Expert Resource Group on Climate Change and Environmental Issues affiliated with the American Academy of Dermatology (AAD) is one such organization with many opportunities to raise awareness within the field of dermatology. The AAD recently joined the Medical Society Consortium on Climate and Health, an organization providing opportunities for policy and media outreach as well as research on climate change. Advocacy also can mean joining your local chapter of Physicians for Social Responsibility or encouraging divestment from fossil fuel companies within your institution. Voicing support for climate change–focused lectures at events such as grand rounds and society meetings at the local, regional, and state-wide levels can help raise awareness. As the dermatologic effects of climate change grow, being knowledgeable of the views of future leaders in our specialty and country on this issue will become increasingly important.
Final Thoughts
In addition to the climate-friendly decisions one can make as a dermatologist, there are many personal lifestyle choices to consider. Small dietary changes such as limiting consumption of beef and minimizing food waste can have large downstream effects. Opting for transportation via train and limiting air travel are both impactful decisions in reducing CO2 emissions. Similarly, switching to an electric vehicle or vehicle with minimal emissions can work to reduce greenhouse gas accumulation. For additional resources, note the AAD has partnered with My Green Doctor, a nonprofit service for health care practices that includes practical cost-saving suggestions to support sustainability in physician practices.
A recent joint publication in more than 200 medical journals described climate change as the greatest threat to global public health.35 Climate change is having devastating effects on dermatologic health and will only continue to do so if not addressed now. Dermatologists have the opportunity to join with our colleagues in the house of medicine and to take action to fight climate change and mitigate the health impacts on our patients, the population, and future generations.
- Santer BD, Bonfils CJW, Fu Q, et al. Celebrating the anniversary of three key events in climate change science. Nat Clim Chang. 2019;9:180-182.
- Lynas M, Houlton BZ, Perry S. Greater than 99% consensus on human caused climate change in the peer-reviewed scientific literature. Environ Res Lett. 2021;16:114005.
- Crowley RA; Health and Public Policy Committee of the American College of Physicians. Climate change and health: a position paper of the American College of Physicians [published online April 19, 2016]. Ann Intern Med. 2016;164:608-610. doi:10.7326/M15-2766
- Global climate change and human health H-135.398. American Medical Association website. Updated 2019. Accessed July 13, 2022. https://policysearch.ama-assn.org/policyfinder/detail/climate%20change?uri=%2FAMADoc%2FHOD.xml-0-309.xml
- Mieczkowska K, Stringer T, Barbieri JS, et al. Surveying the attitudes of dermatologists regarding climate change. Br J Dermatol. 2022;186:748-750.
- Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. PLoS One. 2016;11:e0157014. doi:10.1371/journal.pone.0157014
- Karliner J, Slotterback S, Boyd R, et al. Health care’s climate footprint: how the health sector contributes to the global climate crisis and opportunities for action. Health Care Without Harm website. Published September 2019. Accessed July 13, 2022. https://noharm-global.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_090619.pdf
- Pichler PP, Jaccard IS, Weisz U, et al. International comparison of health care carbon footprints. Environ Res Lett. 2019;14:064004.
- Solomon CG, LaRocque RC. Climate change—a health emergency. N Engl J Med. 2019;380:209-211. doi:10.1056/NEJMp1817067
- IPCC, 2021: Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, et al, eds. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2021:3-32.
- Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. Health Sector—a call to action [published online October 13, 2021]. N Engl J Med. 2021;385:2117-2119. doi:10.1056/NEJMp2115675
- Silva GS, Rosenbach M. Climate change and dermatology: an introduction to a special topic, for this special issue. Int J Womens Dermatol 2021;7:3-7.
- Coates SJ, Norton SA. The effects of climate change on infectious diseases with cutaneous manifestations. Int J Womens Dermatol. 2021;7:8-16. doi:10.1016/j.ijwd.2020.07.005
- Andersen LK, Davis MD. Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the International Society of Dermatology Climate Change Task Force [published online October 1, 2016]. Int J Dermatol. 2017;56:252-259. doi:10.1111/ijd.13438
- Fadadu RP, Grimes B, Jewell NP, et al. Association of wildfire air pollution and health care use for atopic dermatitis and itch. JAMA Dermatol. 2021;157:658-666. doi:10.1001/jamadermatol.2021.0179
- Bellinato F, Adami G, Vaienti S, et al. Association between short-term exposure to environmental air pollution and psoriasis flare. JAMA Dermatol. 2022;158:375-381. doi:10.1001/jamadermatol.2021.6019
- Krutmann J, Bouloc A, Sore G, et al. The skin aging exposome [published online September 28, 2016]. J Dermatol Sci. 2017;85:152-161.
- Parker ER. The influence of climate change on skin cancer incidence—a review of the evidence. Int J Womens Dermatol. 2020;7:17-27. doi:10.1016/j.ijwd.2020.07.003
- Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
- Sheppy M, Pless S, Kung F. Healthcare energy end-use monitoring. US Department of Energy website. Published August 2014. Accessed July 13, 2022. https://www.energy.gov/sites/prod/files/2014/09/f18/61064.pdf
- Feldman D, Ramasamy V, Fu R, et al. U.S. solar photovoltaic system and energy storage cost benchmark: Q1 2020. Published January 2021. Accessed July 7, 2022. https://www.nrel.gov/docs/fy21osti/77324.pdf
- 22. Apostoleris H, Sgouridis S, Stefancich M, et al. Utility solar prices will continue to drop all over the world even without subsidies. Nat Energy. 2019;4:833-834.
- Prasanna PM, Siegel E, Kunce A. Greening radiology. J Am Coll Radiol. 2011;8:780-784. doi:10.1016/j.jacr.2011.07.017
- Bawaneh K, Nezami FG, Rasheduzzaman MD, et al. Energy consumption analysis and characterization of healthcare facilities in the United States. Energies. 2019;12:1-20. doi:10.3390/en12193775
- Blum S, Buckland M, Sack TL, et al. Greening the office: saving resources, saving money, and educating our patients [published online July 4, 2020]. Int J Womens Dermatol. 2020;7:112-116.
- Maintaining your air conditioner. US Department of Energy website. Accessed July 13, 2022. https://www.energy.gov/energysaver/maintaining-your-air-conditioner
- Choma EF, Evans JS, Hammitt JK, et al. Assessing the health impacts of electric vehicles through air pollution in the United States [published online August 25, 2020]. Environ Int. 2020;144:106015.
- Windfeld ES, Brooks MS. Medical waste management—a review [published online August 22, 2015]. J Environ Manage. 2015;1;163:98-108. doi:10.1016/j.jenvman.2015.08.013
- Fathy R, Nelson CA, Barbieri JS. Combating climate change in the clinic: cost-effective strategies to decrease the carbon footprint of outpatient dermatologic practice. Int J Womens Dermatol. 2020;7:107-111.
- Pulsipher KJ, Presley CL, Rundle CW, et al. Teledermatology application use in the COVID-19 era. Dermatol Online J. 2020;26:13030/qt1fs0m0tp.
- O’Connell G, O’Connor C, Murphy M. Every cloud has a silver lining: the environmental benefit of teledermatology during the COVID-19 pandemic [published online July 9, 2021]. Clin Exp Dermatol. 2021;46:1589-1590. doi:10.1111/ced.14795
- Vozzola E, Overcash M, Griffing E. Environmental considerations in the selection of isolation gowns: a life cycle assessment of reusable and disposable alternatives [published online April 11, 2018]. Am J Infect Control. 2018;46:881-886. doi:10.1016/j.ajic.2018.02.002
- Rabin BM, Laney EB, Philipsborn RP. The unique role of medical students in catalyzing climate change education [published online October 14, 2020]. J Med Educ Curric Dev. doi:10.1177/2382120520957653
- Chiang F, Mazdiyasni O, AghaKouchak A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity [published online May 12, 2021]. Nat Commun. 2021;12:2754. doi:10.1038/s41467-021-22314-w
- Atwoli L, Baqui AH, Benfield T, et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health [published online September 5, 2021]. N Engl J Med. 2021;385:1134-1137. doi:10.1056/NEJMe2113200
- Santer BD, Bonfils CJW, Fu Q, et al. Celebrating the anniversary of three key events in climate change science. Nat Clim Chang. 2019;9:180-182.
- Lynas M, Houlton BZ, Perry S. Greater than 99% consensus on human caused climate change in the peer-reviewed scientific literature. Environ Res Lett. 2021;16:114005.
- Crowley RA; Health and Public Policy Committee of the American College of Physicians. Climate change and health: a position paper of the American College of Physicians [published online April 19, 2016]. Ann Intern Med. 2016;164:608-610. doi:10.7326/M15-2766
- Global climate change and human health H-135.398. American Medical Association website. Updated 2019. Accessed July 13, 2022. https://policysearch.ama-assn.org/policyfinder/detail/climate%20change?uri=%2FAMADoc%2FHOD.xml-0-309.xml
- Mieczkowska K, Stringer T, Barbieri JS, et al. Surveying the attitudes of dermatologists regarding climate change. Br J Dermatol. 2022;186:748-750.
- Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. PLoS One. 2016;11:e0157014. doi:10.1371/journal.pone.0157014
- Karliner J, Slotterback S, Boyd R, et al. Health care’s climate footprint: how the health sector contributes to the global climate crisis and opportunities for action. Health Care Without Harm website. Published September 2019. Accessed July 13, 2022. https://noharm-global.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_090619.pdf
- Pichler PP, Jaccard IS, Weisz U, et al. International comparison of health care carbon footprints. Environ Res Lett. 2019;14:064004.
- Solomon CG, LaRocque RC. Climate change—a health emergency. N Engl J Med. 2019;380:209-211. doi:10.1056/NEJMp1817067
- IPCC, 2021: Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, et al, eds. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2021:3-32.
- Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. Health Sector—a call to action [published online October 13, 2021]. N Engl J Med. 2021;385:2117-2119. doi:10.1056/NEJMp2115675
- Silva GS, Rosenbach M. Climate change and dermatology: an introduction to a special topic, for this special issue. Int J Womens Dermatol 2021;7:3-7.
- Coates SJ, Norton SA. The effects of climate change on infectious diseases with cutaneous manifestations. Int J Womens Dermatol. 2021;7:8-16. doi:10.1016/j.ijwd.2020.07.005
- Andersen LK, Davis MD. Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the International Society of Dermatology Climate Change Task Force [published online October 1, 2016]. Int J Dermatol. 2017;56:252-259. doi:10.1111/ijd.13438
- Fadadu RP, Grimes B, Jewell NP, et al. Association of wildfire air pollution and health care use for atopic dermatitis and itch. JAMA Dermatol. 2021;157:658-666. doi:10.1001/jamadermatol.2021.0179
- Bellinato F, Adami G, Vaienti S, et al. Association between short-term exposure to environmental air pollution and psoriasis flare. JAMA Dermatol. 2022;158:375-381. doi:10.1001/jamadermatol.2021.6019
- Krutmann J, Bouloc A, Sore G, et al. The skin aging exposome [published online September 28, 2016]. J Dermatol Sci. 2017;85:152-161.
- Parker ER. The influence of climate change on skin cancer incidence—a review of the evidence. Int J Womens Dermatol. 2020;7:17-27. doi:10.1016/j.ijwd.2020.07.003
- Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
- Sheppy M, Pless S, Kung F. Healthcare energy end-use monitoring. US Department of Energy website. Published August 2014. Accessed July 13, 2022. https://www.energy.gov/sites/prod/files/2014/09/f18/61064.pdf
- Feldman D, Ramasamy V, Fu R, et al. U.S. solar photovoltaic system and energy storage cost benchmark: Q1 2020. Published January 2021. Accessed July 7, 2022. https://www.nrel.gov/docs/fy21osti/77324.pdf
- 22. Apostoleris H, Sgouridis S, Stefancich M, et al. Utility solar prices will continue to drop all over the world even without subsidies. Nat Energy. 2019;4:833-834.
- Prasanna PM, Siegel E, Kunce A. Greening radiology. J Am Coll Radiol. 2011;8:780-784. doi:10.1016/j.jacr.2011.07.017
- Bawaneh K, Nezami FG, Rasheduzzaman MD, et al. Energy consumption analysis and characterization of healthcare facilities in the United States. Energies. 2019;12:1-20. doi:10.3390/en12193775
- Blum S, Buckland M, Sack TL, et al. Greening the office: saving resources, saving money, and educating our patients [published online July 4, 2020]. Int J Womens Dermatol. 2020;7:112-116.
- Maintaining your air conditioner. US Department of Energy website. Accessed July 13, 2022. https://www.energy.gov/energysaver/maintaining-your-air-conditioner
- Choma EF, Evans JS, Hammitt JK, et al. Assessing the health impacts of electric vehicles through air pollution in the United States [published online August 25, 2020]. Environ Int. 2020;144:106015.
- Windfeld ES, Brooks MS. Medical waste management—a review [published online August 22, 2015]. J Environ Manage. 2015;1;163:98-108. doi:10.1016/j.jenvman.2015.08.013
- Fathy R, Nelson CA, Barbieri JS. Combating climate change in the clinic: cost-effective strategies to decrease the carbon footprint of outpatient dermatologic practice. Int J Womens Dermatol. 2020;7:107-111.
- Pulsipher KJ, Presley CL, Rundle CW, et al. Teledermatology application use in the COVID-19 era. Dermatol Online J. 2020;26:13030/qt1fs0m0tp.
- O’Connell G, O’Connor C, Murphy M. Every cloud has a silver lining: the environmental benefit of teledermatology during the COVID-19 pandemic [published online July 9, 2021]. Clin Exp Dermatol. 2021;46:1589-1590. doi:10.1111/ced.14795
- Vozzola E, Overcash M, Griffing E. Environmental considerations in the selection of isolation gowns: a life cycle assessment of reusable and disposable alternatives [published online April 11, 2018]. Am J Infect Control. 2018;46:881-886. doi:10.1016/j.ajic.2018.02.002
- Rabin BM, Laney EB, Philipsborn RP. The unique role of medical students in catalyzing climate change education [published online October 14, 2020]. J Med Educ Curric Dev. doi:10.1177/2382120520957653
- Chiang F, Mazdiyasni O, AghaKouchak A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity [published online May 12, 2021]. Nat Commun. 2021;12:2754. doi:10.1038/s41467-021-22314-w
- Atwoli L, Baqui AH, Benfield T, et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health [published online September 5, 2021]. N Engl J Med. 2021;385:1134-1137. doi:10.1056/NEJMe2113200
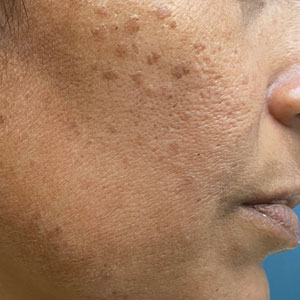
Pigmented Papules on the Face, Neck, and Chest
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.
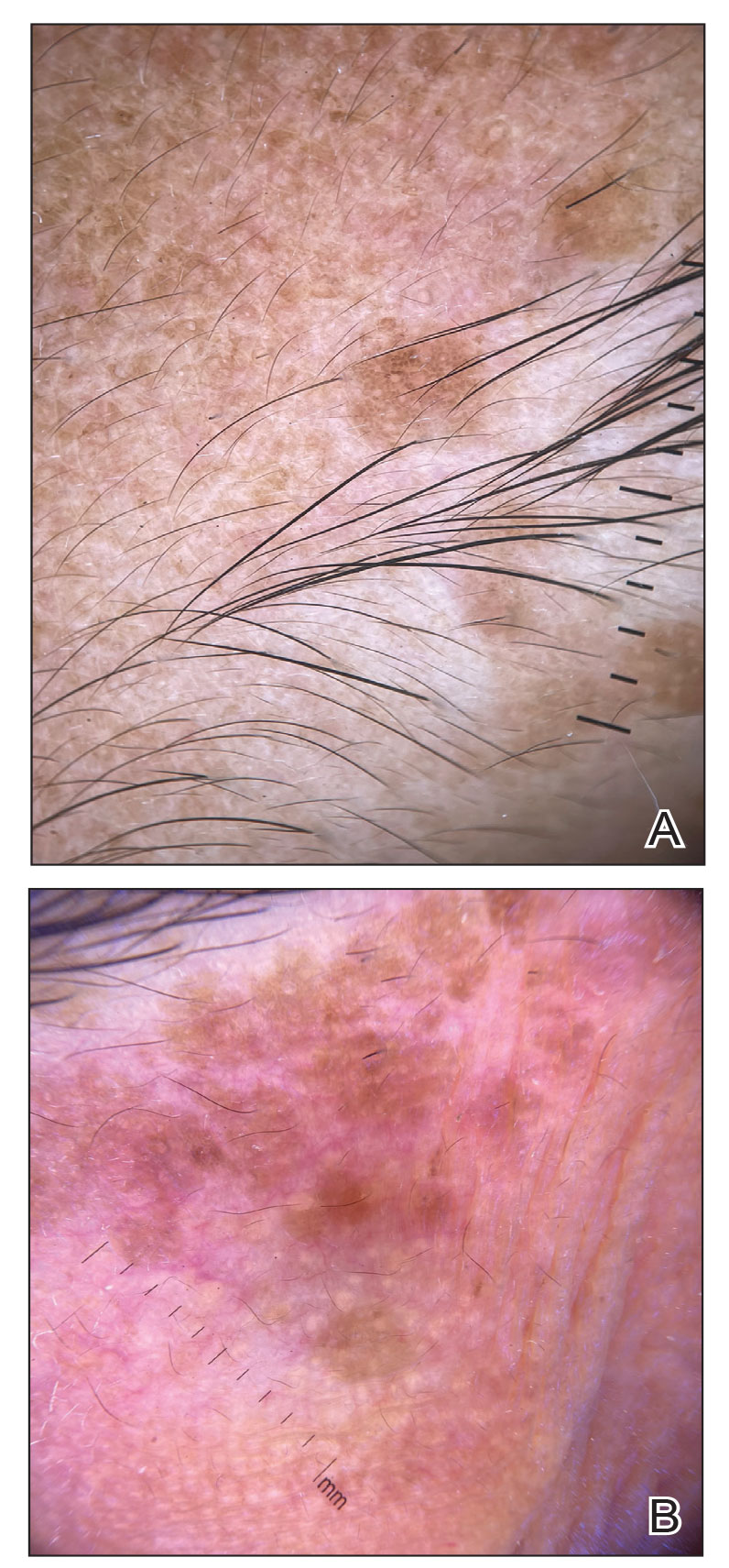
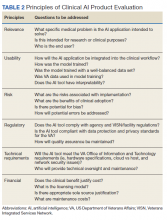
In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).
Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
A 46-year-old woman presented with multiple asymptomatic, flesh-colored, hyperpigmented papules on the face of 5 to 6 months’ duration that were progressively increasing in number. The lesions first appeared near the eyebrows and cheeks (top) and subsequently spread to involve the neck. She had no notable medical history. Cutaneous examination revealed multiple tan to brown papules over the periorbital, malar, and neck regions ranging in size from 1 to 5 mm. The lesions over the periorbital region were arranged in a linear pattern (bottom). Similar lesions also were present on the chest and arms. No other sites were involved, and systemic examination was normal.
Consensus Statement Supporting the Presence of Onsite Radiation Oncology Departments at VHA Medical Centers
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
Agent Orange Exposure, Transformation From MGUS to Multiple Myeloma, and Outcomes in Veterans
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
Chemokine expression predicts severity of major depressive disorder
Chemokines and their receptors “influence neuroendocrine signaling, neurotransmission, and interaction between neurons and microglia and have therefore been suggested to be involved in the pathophysiology of MDD and depression-like behavior,” but their potential as a predictor of disease severity has not been explored, Jana Freff, a PhD candidate at the University of Münster (Germany), and colleagues wrote.
Recent research has identified disease-associated single-nucleotide polymorphisms that can be used to calculate a cumulative polygenic risk score (PRS) for an individual, they said. “While PRS only explain a small proportion of the phenotypic variance, they provide a valuable tool to study the influence of common genetic factors in complex disorders, such as MDD.”
In a study published in the Journal of Affective Disorders , the researchers identified 33 adult inpatients with MDD and 21 healthy controls. Blood samples were collected at the time of inpatient admission and after 6 weeks of treatment. MDD severity was measured using the Hamilton Rating Scale for Depression and Inventory of Depressive Symptomatology. Chemokine receptor 4 (CCR4) was measured using mean fluorescence intensity (MFI).
The MDD patients showed significant decreases in CCR4 expression on CD4+ T cells; these patients also had elevated serum levels of the ligands CLL17 and CLL22, compared with healthy controls.
The researchers examined the relationship between CCR4 expression on T cells and MDD severity. Individuals with severe depression had lower levels of CCR4 on CD4+ T cells, compared with nondepressed or mildly depressed individuals.
CCR4 expression also was significantly associated with several somatic and cognitive-affective items on the Beck Depression Inventory II including loss of pleasure (P < .05), agitation (P < .01), and difficulty concentrating (P < .05). “In addition, the total score of BDI-II correlated negatively with the CCR4 MFI (P < .05) emphasizing that greater MDD severity is associated with lower CCR4 expression on CD4+ T cells,” the researchers said.
The researchers also assessed the predictive value of immune parameters and PRS for MDD severity. They did not find significant correlations between PRS and these parameters; however, “including PRS for a cross-disorder phenotype and chronotype could improve the predictive performance of immune parameters on MDD severity,” and genetic data should be considered in future studies.
The study findings were limited mainly by the small size, the researchers noted. Additional studies with larger patient populations are needed, not only to investigate the functional role of CCR4 in MDD, and the association between CCR4 and remission or treatment resistance, but also the impact of genetic factors on MDD status, they said.
However, the results of the current study show an altered expression of CCR4 in MDD, and “Future augmented strategies to treat depression may therefore target CCR4 specifically on CD4+ T cells,” they concluded.
The study was funded in part by grants to several coauthors from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy. Ms. Freff had no financial conflicts to disclose.
Chemokines and their receptors “influence neuroendocrine signaling, neurotransmission, and interaction between neurons and microglia and have therefore been suggested to be involved in the pathophysiology of MDD and depression-like behavior,” but their potential as a predictor of disease severity has not been explored, Jana Freff, a PhD candidate at the University of Münster (Germany), and colleagues wrote.
Recent research has identified disease-associated single-nucleotide polymorphisms that can be used to calculate a cumulative polygenic risk score (PRS) for an individual, they said. “While PRS only explain a small proportion of the phenotypic variance, they provide a valuable tool to study the influence of common genetic factors in complex disorders, such as MDD.”
In a study published in the Journal of Affective Disorders , the researchers identified 33 adult inpatients with MDD and 21 healthy controls. Blood samples were collected at the time of inpatient admission and after 6 weeks of treatment. MDD severity was measured using the Hamilton Rating Scale for Depression and Inventory of Depressive Symptomatology. Chemokine receptor 4 (CCR4) was measured using mean fluorescence intensity (MFI).
The MDD patients showed significant decreases in CCR4 expression on CD4+ T cells; these patients also had elevated serum levels of the ligands CLL17 and CLL22, compared with healthy controls.
The researchers examined the relationship between CCR4 expression on T cells and MDD severity. Individuals with severe depression had lower levels of CCR4 on CD4+ T cells, compared with nondepressed or mildly depressed individuals.
CCR4 expression also was significantly associated with several somatic and cognitive-affective items on the Beck Depression Inventory II including loss of pleasure (P < .05), agitation (P < .01), and difficulty concentrating (P < .05). “In addition, the total score of BDI-II correlated negatively with the CCR4 MFI (P < .05) emphasizing that greater MDD severity is associated with lower CCR4 expression on CD4+ T cells,” the researchers said.
The researchers also assessed the predictive value of immune parameters and PRS for MDD severity. They did not find significant correlations between PRS and these parameters; however, “including PRS for a cross-disorder phenotype and chronotype could improve the predictive performance of immune parameters on MDD severity,” and genetic data should be considered in future studies.
The study findings were limited mainly by the small size, the researchers noted. Additional studies with larger patient populations are needed, not only to investigate the functional role of CCR4 in MDD, and the association between CCR4 and remission or treatment resistance, but also the impact of genetic factors on MDD status, they said.
However, the results of the current study show an altered expression of CCR4 in MDD, and “Future augmented strategies to treat depression may therefore target CCR4 specifically on CD4+ T cells,” they concluded.
The study was funded in part by grants to several coauthors from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy. Ms. Freff had no financial conflicts to disclose.
Chemokines and their receptors “influence neuroendocrine signaling, neurotransmission, and interaction between neurons and microglia and have therefore been suggested to be involved in the pathophysiology of MDD and depression-like behavior,” but their potential as a predictor of disease severity has not been explored, Jana Freff, a PhD candidate at the University of Münster (Germany), and colleagues wrote.
Recent research has identified disease-associated single-nucleotide polymorphisms that can be used to calculate a cumulative polygenic risk score (PRS) for an individual, they said. “While PRS only explain a small proportion of the phenotypic variance, they provide a valuable tool to study the influence of common genetic factors in complex disorders, such as MDD.”
In a study published in the Journal of Affective Disorders , the researchers identified 33 adult inpatients with MDD and 21 healthy controls. Blood samples were collected at the time of inpatient admission and after 6 weeks of treatment. MDD severity was measured using the Hamilton Rating Scale for Depression and Inventory of Depressive Symptomatology. Chemokine receptor 4 (CCR4) was measured using mean fluorescence intensity (MFI).
The MDD patients showed significant decreases in CCR4 expression on CD4+ T cells; these patients also had elevated serum levels of the ligands CLL17 and CLL22, compared with healthy controls.
The researchers examined the relationship between CCR4 expression on T cells and MDD severity. Individuals with severe depression had lower levels of CCR4 on CD4+ T cells, compared with nondepressed or mildly depressed individuals.
CCR4 expression also was significantly associated with several somatic and cognitive-affective items on the Beck Depression Inventory II including loss of pleasure (P < .05), agitation (P < .01), and difficulty concentrating (P < .05). “In addition, the total score of BDI-II correlated negatively with the CCR4 MFI (P < .05) emphasizing that greater MDD severity is associated with lower CCR4 expression on CD4+ T cells,” the researchers said.
The researchers also assessed the predictive value of immune parameters and PRS for MDD severity. They did not find significant correlations between PRS and these parameters; however, “including PRS for a cross-disorder phenotype and chronotype could improve the predictive performance of immune parameters on MDD severity,” and genetic data should be considered in future studies.
The study findings were limited mainly by the small size, the researchers noted. Additional studies with larger patient populations are needed, not only to investigate the functional role of CCR4 in MDD, and the association between CCR4 and remission or treatment resistance, but also the impact of genetic factors on MDD status, they said.
However, the results of the current study show an altered expression of CCR4 in MDD, and “Future augmented strategies to treat depression may therefore target CCR4 specifically on CD4+ T cells,” they concluded.
The study was funded in part by grants to several coauthors from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy. Ms. Freff had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Antibiotic Stewardship Improvement Initiative at a Veterans Health Administration Ambulatory Care Center
The negative impact of the unnecessary prescribing of antibiotic is well known. Consequences include exposing patients to antibiotic adverse effects, risk of overgrowth of pathogenetic organisms such as clostridial species, unnecessary cost of drugs, and development of selection of antibiotic-resistant organisms in the populace at large. Acute viral respiratory infections are among the leading causes of inappropriate antibiotic usage.1 In a study of 1000 adults with respiratory tract infections in an outpatient setting, 77% of patients were prescribed antibiotics, and the treatment was inappropriate in 64% of those who received prescriptions.2 Patient expectations and clinician perceptions of these expectations play a role. One study showed that 54% of clinicians felt their patients expected to receive antibiotics for a visit due to an acute respiratory infection (ARI), such as a cough or cold; 26% of patients did in fact have this expectation.3
The US Department of Veterans Affairs (VA) Central Ohio Health Care System is a large ambulatory care facility, with 4 associated community-based outpatient clinics, serving more than 43,000 central Ohio veterans and completing more than 500,000 medical appointments annually. An antimicrobial stewardship program has been in place since 2013. In May 2018, the clinical pharmacist assigned to the program alerted medical leadership that, of 67 patients seen in primary care for ARIs between April 16, 2018, and May 15, 2018, 42 (63%) had been prescribed an antibiotic. Based on this finding, clinical leadership designed a process improvement program aimed at reducing inappropriate antibiotic usage for the treatment of uncomplicated ARls likely due to viral pathogens. Key components were clinician and patient education and the substitution of a symptomatic treatment kit in place of an antibiotic prescription.
Methods
Facility clinical leadership, assisted by Volunteer Services, developed a Viral Illness Support Pack to be dispensed by primary care practitioners (PCPs) to patients presenting with symptoms of viral ARIs. The contents of this support pack are shown in the Figure. Patients were provided with tissues, throat lozenges, lip balm, acetaminophen, hand sanitizer, a surgical mask, patient instructions, and the Antibiotics Aren’t Always the Answer pamphlet.4 The contents of the viral support pack were purchased through Volunteer Services using donated funds. In total, 460 packs were distributed to the primary care patient aligned care teams (PACTs), including the community-based outpatient clinics.
Clinicians and care teams received academic detailing prior to distribution of the viral support packs, stressing the importance of avoiding antibiotics to treat viral illnesses. Viral illness support packs were available for distribution from December 1, 2018, through March 31, 2019. The frequency of antibiotic dispensing to patients coded for ARI during this period was compared with that of the same time period in the previous year. All charts were reviewed for coding accuracy. Patients with illnesses requiring antibiotic treatment, such as pneumonia, exacerbations of chronic obstructive pulmonary disease and chronic bronchitis, and streptococcal pharyngitis, were excluded from the study. Statistical significance was determined using the unpaired t test.
Results
From December 1, 2018, to March 31, 2019, 357 viral support packs were distributed to patients (Table). For the historical control period from December 1, 2017, through March 31, 2018, 508 patients were treated for ARIs. Of these, 295 (58%) received clinically inappropriate antibiotics. In contrast, of the 627 patients treated for ARIs during the study period from December 1, 2018, through March 31, 2019, 310 (49%) received clinically inappropriate antibiotics. The 9% decrease during the period when viral support packs were distributed, compared with the prior year, was statistically significant (P = .02).
Discussion
The decrease in antibiotic prescriptions for ARIs was statistically significant. The success of this project can be attributed to 3 factors: clinician education, patient education, and the option for PCPs to provide symptomatic treatment for these patients rather than prescribe an antibiotic.
The importance of antibiotic stewardship has been emphasized to all PCPs at the VA Central Ohio Health Care System. Antibiotic stewardship has been the subject of grand rounds. Prior to distribution of the viral support pack, the chief of specialty medicine, the project’s champion, spoke to all PCPs. Adequate numbers of viral support packs were distributed to all primary care teams.
In addition to direct clinician-to-patient education at the time of the patients’ visits, educational materials were included in the viral support pack. The Antibiotics Aren’t Always the Answer pamphlet is available from the Centers for Disease Control and Prevention. It covers the importance of antibiotic awareness, discusses what antibiotics do and do not treat, how to stay healthy, and causes of antibiotic resistance. The pamphlet contains the clear message that antibiotics are not only ineffective against viral illness, but also can cause significant undesirable outcomes.
The pamphlet Viral Illness Support Pack Traffic Light Card (eAppendix available online at doi:10.12788/fp.0302) provides important clinical information to the patients about their illness. Patients are instructed to contact their primary care team if they are worse after 3 days of illness; symptoms are not improving after 10 days; or they experience blood in respiratory secretions, chills or generalized aching, and localized pain that is one-sided or significantly worsening. Patients are clearly informed to seek further care if not improving with symptomatic treatment.
The ability to provide patients with symptomatic relief, including throat lozenges, lip balm, and acetaminophen, was felt to be important in the success of the project. Furthermore, this eliminated an extra step of the patient needing to visit the pharmacy.
Limitations
Limitations of the study included starting distribution of the support packs somewhat after the onset of the viral illness season, failure to reach all prescribers for academic detailing at the start of the program, and several instances of temporary unavailability of the support packs in some areas.
Conclusions
Patients with ARIs are often significantly symptomatic and frequently believe that they require an antibiotic for treatment. Clinicians may adjust their behavior in response to their patients’ expectations, stated or unstated. The results of this project demonstrate that the combination of patient education and the ready availability of a nonantibiotic symptomatic treatment option can significantly decrease the unnecessary prescribing of antibiotics for viral illnesses.
Acknowledgments
The authors are grateful to Ms. Traci Washington for assistance in sourcing materials; to Karen Corr, PhD, and Anthony Restuccio, MD, for advice on methods; to Mr. Daniel Pignatelli for assistance with data interpretation; and to Mr. Keith Skidmore, Ms. Crystal Conley, and Ms. Megan Harris for assistance with assembling the Viral Illness Support Packs.
1. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
2. Schroeck JL, Ruh CA, Sellick JA Jr, Ott MC, Mattappallil A, Mergenhagen KA. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59(7):3848-3852. doi:10.1128/AAC.00652-15
3. Francois Watkins LK, Sanchez GV, Albert AP, Roberts RM, Hicks LA. Knowledge and attitudes regarding antibiotic use among adult consumers, adult Hispanic consumers, and health care providers—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2015;64(28):767-770. doi:10.15585/mmwr.mm6428a5
4. Centers for Disease Control and Prevention. Antibiotics Aren’t Always the Answer. Accessed June 28, 2022.www.cdc.gov/antibiotic-use/pdfs/AntibioticsArentAlwaystheAnswer-H.pdf
The negative impact of the unnecessary prescribing of antibiotic is well known. Consequences include exposing patients to antibiotic adverse effects, risk of overgrowth of pathogenetic organisms such as clostridial species, unnecessary cost of drugs, and development of selection of antibiotic-resistant organisms in the populace at large. Acute viral respiratory infections are among the leading causes of inappropriate antibiotic usage.1 In a study of 1000 adults with respiratory tract infections in an outpatient setting, 77% of patients were prescribed antibiotics, and the treatment was inappropriate in 64% of those who received prescriptions.2 Patient expectations and clinician perceptions of these expectations play a role. One study showed that 54% of clinicians felt their patients expected to receive antibiotics for a visit due to an acute respiratory infection (ARI), such as a cough or cold; 26% of patients did in fact have this expectation.3
The US Department of Veterans Affairs (VA) Central Ohio Health Care System is a large ambulatory care facility, with 4 associated community-based outpatient clinics, serving more than 43,000 central Ohio veterans and completing more than 500,000 medical appointments annually. An antimicrobial stewardship program has been in place since 2013. In May 2018, the clinical pharmacist assigned to the program alerted medical leadership that, of 67 patients seen in primary care for ARIs between April 16, 2018, and May 15, 2018, 42 (63%) had been prescribed an antibiotic. Based on this finding, clinical leadership designed a process improvement program aimed at reducing inappropriate antibiotic usage for the treatment of uncomplicated ARls likely due to viral pathogens. Key components were clinician and patient education and the substitution of a symptomatic treatment kit in place of an antibiotic prescription.
Methods
Facility clinical leadership, assisted by Volunteer Services, developed a Viral Illness Support Pack to be dispensed by primary care practitioners (PCPs) to patients presenting with symptoms of viral ARIs. The contents of this support pack are shown in the Figure. Patients were provided with tissues, throat lozenges, lip balm, acetaminophen, hand sanitizer, a surgical mask, patient instructions, and the Antibiotics Aren’t Always the Answer pamphlet.4 The contents of the viral support pack were purchased through Volunteer Services using donated funds. In total, 460 packs were distributed to the primary care patient aligned care teams (PACTs), including the community-based outpatient clinics.
Clinicians and care teams received academic detailing prior to distribution of the viral support packs, stressing the importance of avoiding antibiotics to treat viral illnesses. Viral illness support packs were available for distribution from December 1, 2018, through March 31, 2019. The frequency of antibiotic dispensing to patients coded for ARI during this period was compared with that of the same time period in the previous year. All charts were reviewed for coding accuracy. Patients with illnesses requiring antibiotic treatment, such as pneumonia, exacerbations of chronic obstructive pulmonary disease and chronic bronchitis, and streptococcal pharyngitis, were excluded from the study. Statistical significance was determined using the unpaired t test.
Results
From December 1, 2018, to March 31, 2019, 357 viral support packs were distributed to patients (Table). For the historical control period from December 1, 2017, through March 31, 2018, 508 patients were treated for ARIs. Of these, 295 (58%) received clinically inappropriate antibiotics. In contrast, of the 627 patients treated for ARIs during the study period from December 1, 2018, through March 31, 2019, 310 (49%) received clinically inappropriate antibiotics. The 9% decrease during the period when viral support packs were distributed, compared with the prior year, was statistically significant (P = .02).
Discussion
The decrease in antibiotic prescriptions for ARIs was statistically significant. The success of this project can be attributed to 3 factors: clinician education, patient education, and the option for PCPs to provide symptomatic treatment for these patients rather than prescribe an antibiotic.
The importance of antibiotic stewardship has been emphasized to all PCPs at the VA Central Ohio Health Care System. Antibiotic stewardship has been the subject of grand rounds. Prior to distribution of the viral support pack, the chief of specialty medicine, the project’s champion, spoke to all PCPs. Adequate numbers of viral support packs were distributed to all primary care teams.
In addition to direct clinician-to-patient education at the time of the patients’ visits, educational materials were included in the viral support pack. The Antibiotics Aren’t Always the Answer pamphlet is available from the Centers for Disease Control and Prevention. It covers the importance of antibiotic awareness, discusses what antibiotics do and do not treat, how to stay healthy, and causes of antibiotic resistance. The pamphlet contains the clear message that antibiotics are not only ineffective against viral illness, but also can cause significant undesirable outcomes.
The pamphlet Viral Illness Support Pack Traffic Light Card (eAppendix available online at doi:10.12788/fp.0302) provides important clinical information to the patients about their illness. Patients are instructed to contact their primary care team if they are worse after 3 days of illness; symptoms are not improving after 10 days; or they experience blood in respiratory secretions, chills or generalized aching, and localized pain that is one-sided or significantly worsening. Patients are clearly informed to seek further care if not improving with symptomatic treatment.
The ability to provide patients with symptomatic relief, including throat lozenges, lip balm, and acetaminophen, was felt to be important in the success of the project. Furthermore, this eliminated an extra step of the patient needing to visit the pharmacy.
Limitations
Limitations of the study included starting distribution of the support packs somewhat after the onset of the viral illness season, failure to reach all prescribers for academic detailing at the start of the program, and several instances of temporary unavailability of the support packs in some areas.
Conclusions
Patients with ARIs are often significantly symptomatic and frequently believe that they require an antibiotic for treatment. Clinicians may adjust their behavior in response to their patients’ expectations, stated or unstated. The results of this project demonstrate that the combination of patient education and the ready availability of a nonantibiotic symptomatic treatment option can significantly decrease the unnecessary prescribing of antibiotics for viral illnesses.
Acknowledgments
The authors are grateful to Ms. Traci Washington for assistance in sourcing materials; to Karen Corr, PhD, and Anthony Restuccio, MD, for advice on methods; to Mr. Daniel Pignatelli for assistance with data interpretation; and to Mr. Keith Skidmore, Ms. Crystal Conley, and Ms. Megan Harris for assistance with assembling the Viral Illness Support Packs.
The negative impact of the unnecessary prescribing of antibiotic is well known. Consequences include exposing patients to antibiotic adverse effects, risk of overgrowth of pathogenetic organisms such as clostridial species, unnecessary cost of drugs, and development of selection of antibiotic-resistant organisms in the populace at large. Acute viral respiratory infections are among the leading causes of inappropriate antibiotic usage.1 In a study of 1000 adults with respiratory tract infections in an outpatient setting, 77% of patients were prescribed antibiotics, and the treatment was inappropriate in 64% of those who received prescriptions.2 Patient expectations and clinician perceptions of these expectations play a role. One study showed that 54% of clinicians felt their patients expected to receive antibiotics for a visit due to an acute respiratory infection (ARI), such as a cough or cold; 26% of patients did in fact have this expectation.3
The US Department of Veterans Affairs (VA) Central Ohio Health Care System is a large ambulatory care facility, with 4 associated community-based outpatient clinics, serving more than 43,000 central Ohio veterans and completing more than 500,000 medical appointments annually. An antimicrobial stewardship program has been in place since 2013. In May 2018, the clinical pharmacist assigned to the program alerted medical leadership that, of 67 patients seen in primary care for ARIs between April 16, 2018, and May 15, 2018, 42 (63%) had been prescribed an antibiotic. Based on this finding, clinical leadership designed a process improvement program aimed at reducing inappropriate antibiotic usage for the treatment of uncomplicated ARls likely due to viral pathogens. Key components were clinician and patient education and the substitution of a symptomatic treatment kit in place of an antibiotic prescription.
Methods
Facility clinical leadership, assisted by Volunteer Services, developed a Viral Illness Support Pack to be dispensed by primary care practitioners (PCPs) to patients presenting with symptoms of viral ARIs. The contents of this support pack are shown in the Figure. Patients were provided with tissues, throat lozenges, lip balm, acetaminophen, hand sanitizer, a surgical mask, patient instructions, and the Antibiotics Aren’t Always the Answer pamphlet.4 The contents of the viral support pack were purchased through Volunteer Services using donated funds. In total, 460 packs were distributed to the primary care patient aligned care teams (PACTs), including the community-based outpatient clinics.
Clinicians and care teams received academic detailing prior to distribution of the viral support packs, stressing the importance of avoiding antibiotics to treat viral illnesses. Viral illness support packs were available for distribution from December 1, 2018, through March 31, 2019. The frequency of antibiotic dispensing to patients coded for ARI during this period was compared with that of the same time period in the previous year. All charts were reviewed for coding accuracy. Patients with illnesses requiring antibiotic treatment, such as pneumonia, exacerbations of chronic obstructive pulmonary disease and chronic bronchitis, and streptococcal pharyngitis, were excluded from the study. Statistical significance was determined using the unpaired t test.
Results
From December 1, 2018, to March 31, 2019, 357 viral support packs were distributed to patients (Table). For the historical control period from December 1, 2017, through March 31, 2018, 508 patients were treated for ARIs. Of these, 295 (58%) received clinically inappropriate antibiotics. In contrast, of the 627 patients treated for ARIs during the study period from December 1, 2018, through March 31, 2019, 310 (49%) received clinically inappropriate antibiotics. The 9% decrease during the period when viral support packs were distributed, compared with the prior year, was statistically significant (P = .02).
Discussion
The decrease in antibiotic prescriptions for ARIs was statistically significant. The success of this project can be attributed to 3 factors: clinician education, patient education, and the option for PCPs to provide symptomatic treatment for these patients rather than prescribe an antibiotic.
The importance of antibiotic stewardship has been emphasized to all PCPs at the VA Central Ohio Health Care System. Antibiotic stewardship has been the subject of grand rounds. Prior to distribution of the viral support pack, the chief of specialty medicine, the project’s champion, spoke to all PCPs. Adequate numbers of viral support packs were distributed to all primary care teams.
In addition to direct clinician-to-patient education at the time of the patients’ visits, educational materials were included in the viral support pack. The Antibiotics Aren’t Always the Answer pamphlet is available from the Centers for Disease Control and Prevention. It covers the importance of antibiotic awareness, discusses what antibiotics do and do not treat, how to stay healthy, and causes of antibiotic resistance. The pamphlet contains the clear message that antibiotics are not only ineffective against viral illness, but also can cause significant undesirable outcomes.
The pamphlet Viral Illness Support Pack Traffic Light Card (eAppendix available online at doi:10.12788/fp.0302) provides important clinical information to the patients about their illness. Patients are instructed to contact their primary care team if they are worse after 3 days of illness; symptoms are not improving after 10 days; or they experience blood in respiratory secretions, chills or generalized aching, and localized pain that is one-sided or significantly worsening. Patients are clearly informed to seek further care if not improving with symptomatic treatment.
The ability to provide patients with symptomatic relief, including throat lozenges, lip balm, and acetaminophen, was felt to be important in the success of the project. Furthermore, this eliminated an extra step of the patient needing to visit the pharmacy.
Limitations
Limitations of the study included starting distribution of the support packs somewhat after the onset of the viral illness season, failure to reach all prescribers for academic detailing at the start of the program, and several instances of temporary unavailability of the support packs in some areas.
Conclusions
Patients with ARIs are often significantly symptomatic and frequently believe that they require an antibiotic for treatment. Clinicians may adjust their behavior in response to their patients’ expectations, stated or unstated. The results of this project demonstrate that the combination of patient education and the ready availability of a nonantibiotic symptomatic treatment option can significantly decrease the unnecessary prescribing of antibiotics for viral illnesses.
Acknowledgments
The authors are grateful to Ms. Traci Washington for assistance in sourcing materials; to Karen Corr, PhD, and Anthony Restuccio, MD, for advice on methods; to Mr. Daniel Pignatelli for assistance with data interpretation; and to Mr. Keith Skidmore, Ms. Crystal Conley, and Ms. Megan Harris for assistance with assembling the Viral Illness Support Packs.
1. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
2. Schroeck JL, Ruh CA, Sellick JA Jr, Ott MC, Mattappallil A, Mergenhagen KA. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59(7):3848-3852. doi:10.1128/AAC.00652-15
3. Francois Watkins LK, Sanchez GV, Albert AP, Roberts RM, Hicks LA. Knowledge and attitudes regarding antibiotic use among adult consumers, adult Hispanic consumers, and health care providers—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2015;64(28):767-770. doi:10.15585/mmwr.mm6428a5
4. Centers for Disease Control and Prevention. Antibiotics Aren’t Always the Answer. Accessed June 28, 2022.www.cdc.gov/antibiotic-use/pdfs/AntibioticsArentAlwaystheAnswer-H.pdf
1. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
2. Schroeck JL, Ruh CA, Sellick JA Jr, Ott MC, Mattappallil A, Mergenhagen KA. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59(7):3848-3852. doi:10.1128/AAC.00652-15
3. Francois Watkins LK, Sanchez GV, Albert AP, Roberts RM, Hicks LA. Knowledge and attitudes regarding antibiotic use among adult consumers, adult Hispanic consumers, and health care providers—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2015;64(28):767-770. doi:10.15585/mmwr.mm6428a5
4. Centers for Disease Control and Prevention. Antibiotics Aren’t Always the Answer. Accessed June 28, 2022.www.cdc.gov/antibiotic-use/pdfs/AntibioticsArentAlwaystheAnswer-H.pdf
Postdeployment Respiratory Health: The Roles of the Airborne Hazards and Open Burn Pit Registry and the Post-Deployment Cardiopulmonary Evaluation Network
Case Example
A 37-year-old female never smoker presents to your clinic with progressive dyspnea over the past 15 years. She reports dyspnea on exertion, wheezing, chronic nasal congestion, and difficulty sleeping that started a year after she returned from military deployment to Iraq. She has been unable to exercise, even at low intensity, for the past 5 years, despite being previously active. She has experienced some symptom improvement by taking an albuterol inhaler as needed, loratadine (10 mg), and fluticasone nasal spray (50 mcg). She occasionally uses famotidine for reflux (40 mg). She deployed to Southwest Asia for 12 months (2002-2003) and was primarily stationed in Qayyarah West, an Air Force base in the Mosul district in northern Iraq. She reports exposure during deployment to the fire in the Al-Mishraq sulfur mine, located approximately 25 km north of Qayyarah West, as well as dust storms and burn pits. She currently works as a medical assistant. Her examination is remarkable for normal bronchovesicular breath sounds without any wheezing or crackles on pulmonary evaluation. Her body mass index is 31. You obtain a chest radiograph and spirometry, which are both normal.
The veteran reports feeling frustrated as she has had multiple specialty evaluations in community clinics without receiving a diagnosis, despite worsening symptoms. She reports that she added her information to the Airborne Hazards and Open Burn Pit Registry (AHOBPR). She recently received a letter from the US Department of Veterans Affairs (VA) Post-Deployment Cardiopulmonary Evaluation Network (PDCEN) and is asking you whether she should participate in the PDCEN specialty evaluation. You are not familiar with the military experiences she has described or the programs she asks you about; however, you would like to know more to best care for your patient.
Background
The year 2021 marked the 20th anniversary of the September 11 attacks and the launch of the Global War on Terrorism. Almost 3 million US military personnel have been deployed in support of these operations along with about 300,000 US civilian contractors and thousands of troops from more than 40 nations.1-3
Respiratory hazards associated with deployment to Southwest Asia and Afghanistan are unique and varied. These exposures include blast injuries and a variety of particulate matter sources, such as burn pit combustion byproducts, aeroallergens, and dust storms.7,8,15,16 One air sampling study conducted at 15 deployment sites in Southwest Asia and Afghanistan found mean fine particulate matter (PM2.5) levels were as much as 10 times greater than sampling sites in both rural and urban cities in the United States; all sites sampled exceeded military exposure guidelines (65 µg/m3 for 1 year).17,18 Long-term exposure to PM2.5 has been associated with the development of chronic respiratory and cardiovascular disease; therefore, there has been considerable attention to the respiratory (and nonrespiratory) health of deployed military personnel.19
Concerns regarding the association between deployment and lung disease led to the creation of the national VA Airborne Hazards and Open Burn Pit Registry (AHOBPR) in 2014 and consists of (1) an online questionnaire to document deployment and medical history, exposure concerns, and symptoms; and (2) an optional in-person or virtual clinical health evaluation at the individual’s local VA medical center or military treatment facility (MTF). As of March 2022, more than 300,000 individuals have completed the online questionnaire of which about 30% declined the optional clinical health evaluation.
The clinical evaluation available to AHOBPR participants has not yet been described in the literature. Therefore, our objectives are to examine AHOBPR clinical evaluation data and review its application throughout the VA. In addition, we will also describe a parallel effort by the VA PDCEN, which is to provide comprehensive multiday clinical evaluations for unique AHOBPR participants with unexplained dyspnea and self-reported respiratory disease. A secondary aim of this publication is to disseminate information to health care professionals (HCPs) within and outside of the VA to aid in the referral and evaluation of previously deployed veterans who experience unexplained dyspnea.
AHOBPR Overview
The AHOBPR is an online questionnaire and optional in-person health evaluation that includes 7 major categories targeting deployment history, symptoms, medical history, health concerns, residential history, nonmilitary occupational history, nonmilitary environmental exposures, and health care utilization. The VA Defense Information Repository is used to obtain service dates for the service member/veteran, conflict involvement, and primary location during deployment. The questionnaire portion of the AHOBPR is administered online. It currently is open to all veterans who served in the Southwest Asia theater of operations (including Iraq, Kuwait, and Egypt) any time after August 2, 1990, or Afghanistan, Djibouti, Syria, or Uzbekistan after September 11, 2001. Veterans are eligible for completing the AHOBPR and optional health evaluation at no cost to the veteran regardless of VA benefits or whether they are currently enrolled in VA health care. Though the focus of the present manuscript is to profile a VA program, it is important to note that the US Department of Defense (DoD) is an active partner with the VA in the promotion of the AHOBPR to service members and similarly provides health evaluations for active-duty service members (including activating Reserve and Guard) through their local MTF.
We reviewed and analyzed AHOBPR operations and VA data from 2014 to 2020. Our analyses were limited to veterans seeking evaluation as well as their corresponding symptoms and HCP’s clinical impression from the electronic health record. As of September 20, 2021, 267,125 individuals completed the AHOBPR. The mean age was 43 years (range, 19-84), and the majority were male (86%) and served in the Army (58%). Open-air burn pits (91%), engine maintenance (38.8%), and convoy operations (71.7%) were the most common deployment-related exposures.
The optional in-person AHOBPR health evaluation may be requested by the veteran after completing the online questionnaire and is performed at the veteran’s local VA facility. The evaluation is most often completed by an environmental health clinician or primary care practitioner (PCP). A variety of resources are available to providers for training on this topic, including fact sheets, webinars, monthly calls, conferences, and accredited e-learning.20 As part of the clinical evaluation, the veteran’s chief concerns are assessed and evaluated. At the time of our analysis, 24,578 clinical examinations were performed across 126 VA medical facilities, with considerable geographic variation. Veterans receiving evaluations were predominantly male (89%) with a median age of 46.0 years (IQR, 15). Veterans’ major respiratory concerns included dyspnea (45.1%), decreased exercise ability (34.8%), and cough > 3 weeks (30.3%) (Table). After clinical evaluation by a VA or MTF HCP, 47.8% were found to have a respiratory diagnosis, including asthma (30.1%), COPD (12.8%), and bronchitis (11.9%).
Registry participants who opt to receive the clinical evaluation may benefit directly by undergoing a detailed clinical history and physical examination as well as having the opportunity to document their health concerns. For some, clinicians may need to refer veterans for additional specialty testing beyond this standard AHOBPR clinical evaluation. Although these evaluations can help address some of the veterans’ concerns, a substantial number may have unexplained respiratory symptoms that warrant further investigation.
Post-Deployment Cardiopulmonary Evaluation Network Clinical Evaluation
In May 2019, the VA established the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). One of the AHBPCE’s objectives is to deliver specialized care and consultation for veterans with concerns about their postdeployment health, including, but not limited to, unexplained dyspnea. To meet this objective, the AHBPCE developed the PDCEN, a national network consisting of specialty HCPs from 5 VA medical centers—located in San Francisco, California; Denver, Colorado; Baltimore, Maryland; Ann Arbor, Michigan; and East Orange, New Jersey. Collectively, the PDCEN has developed a standardized approach for the comprehensive clinical evaluation of unexplained dyspnea that is implemented uniformly across sites. Staff at the PDCEN screen the AHOBPR to identify veterans with features of respiratory disease and invite them to participate in an in-person evaluation at the nearest PDCEN site. Given the specialty expertise (detailed below) within the Network, the PDCEN focuses on complex cases that are resource intensive. To address complex cases of unexplained dyspnea, the PDCEN has developed a core clinical evaluation approach (Figure).
The first step in a veteran’s PDCEN evaluation entails a set of detailed questionnaires that request information about the veteran’s current respiratory, sleep, and mental health symptoms and any associated medical diagnoses. Questionnaires also identify potential exposures to military burn pits, sulfur mine and oil field fires, diesel exhaust fumes, dust storms, urban pollution, explosions/blasts, and chemical weapons. In addition, the questionnaires include deployment geographic location, which may inform future estimates of particulate matter exposure.21 Prior VA and non-VA evaluations and testing of their respiratory concerns are obtained for review. Exposure and health records from the DoD are also reviewed when available.
The next step in the PDCEN evaluation comprises comprehensive testing, including complete pulmonary function testing, methacholine challenge, cardiopulmonary exercise testing, forced oscillometry and exhaled nitric oxide testing, paired high-resolution inspiratory and expiratory chest computed tomography (CT) imaging, sinus CT imaging, direct flexible laryngoscopy, echocardiography, polysomnography, and laboratory blood testing. The testing process is managed by local site coordinators and varies by institution based on availability of each testing modality and subspecialist appointments.
Once testing is completed, the veteran is evaluated by a team of HCPs, including physicians from the disciplines of pulmonary medicine, environmental and occupational health, sleep medicine, otolaryngology and speech pathology, and mental health (when appropriate). After the clinical evaluation has been completed, this team of expert HCPs at each site convenes to provide a final summary review visit intended to be a comprehensive assessment of the veteran’s primary health concerns. The 3 primary objectives of this final review are to inform the veteran of (1) what respiratory and related conditions they have; (2) whether the conditions is/are deployment related; and (3) what treatments and/or follow-up care may enhance their current state of health in partnership with their local HCPs. The PDCEN does not provide ongoing management of any conditions identified during the veteran’s evaluation but communicates findings and recommendations to the veteran and their PCP for long-term care.
Discussion
The AHOBPR was established in response to mounting concerns that service members and veterans were experiencing adverse health effects that might be attributable to deployment-related exposures. Nearly half of all patients currently enrolled in the AHOBPR report dyspnea, and about one-third have decreased exercise tolerance and/or cough. Of those who completed the questionnaire and the subsequent in-person and generalized AHOBPR examination, our interim analysis showed that about half were assigned a respiratory diagnosis. Yet for many veterans, their breathing symptoms remained unexplained or did not respond to treatment.
While the AHOBPR and related examinations address the needs of many veterans, others may require more comprehensive examination. The PDCEN attends to the latter by providing more detailed and comprehensive clinical evaluations of veterans with deployment-related respiratory health concerns and seeks to learn from these evaluations by analyzing data obtained from veterans across sites. As such, the PDCEN hopes not only to improve the health of individual veterans, but also create standard practices for both VA and non-VA community evaluation of veterans exposed to respiratory hazards during deployment.
One of the major challenges in the field of postdeployment respiratory health is the lack of clear universal language or case definitions that encompass the veteran’s clinical concerns. In an influential case series published in 2011, 38 (77%) of 49 soldiers with history of airborne hazard exposure and unexplained exercise intolerance were reported to have histopathology consistent with constrictive bronchiolitis on surgical lung biopsy.14 Subsequent publications have described other histopathologic features in deployed military personnel, including granulomatous inflammation, interstitial lung disease, emphysema, and pleuritis.12-14 Reconciling these findings from surgical lung biopsy with the clinical presentation and noninvasive studies has proved difficult. Therefore, several groups of investigators have proposed terms, including postdeployment respiratory syndrome, deployment-related distal lung disease, and Iraq/Afghanistan War lung injury to describe the increased respiratory symptoms and variety of histopathologic and imaging findings in this population.9,12,22 At present, there remains a lack of consensus on terminology and case definitions as well as the role of military environmental exposures in exacerbating and/or causing these conditions. As HCPs, it is important to appreciate and acknowledge that the ambiguity and controversy pertaining to terminology, causation, and service connections are a common source of frustration experienced by veterans, which are increasingly reflected among reports in popular media and lay press.
A second and related challenge in the field of postdeployment respiratory health that contributes to veteran and HCP frustration is that many of the aforementioned abnormalities described on surgical lung biopsy are not readily identifiable on noninvasive tests, including traditional interpretation of pulmonary function tests or chest CT imaging.12-14,22 Thus, underlying conditions could be overlooked and veterans’ concerns and symptoms may be dismissed or misattributed to other comorbid conditions. While surgical lung biopsies may offer diagnostic clarity in identifying lung disease, there are significant procedural risks of surgical and anesthetic complications. Furthermore, a definitive diagnosis does not necessarily guarantee a clear treatment plan. For example, there are no current therapies approved by the US Food and Drug Administration for the treatment of constrictive bronchiolitis.
Research efforts are underway, including within the PDCEN, to evaluate a more sensitive and noninvasive assessment of the small airways that may even reduce or eliminate the need for surgical lung biopsy. In contrast to traditional pulmonary function testing, which is helpful for evaluation of the larger airways, forced oscillation technique can be used noninvasively, using pressure oscillations to evaluate for diseases of the smaller airways and has been used in the veteran population and in those exposed to dust from the World Trade Center disaster.23-25 Multiple breath washout technique provides a lung clearance index that is determined by the number of lung turnovers it takes to clear the lungs of an inert gas (eg, sulfur hexafluoride, nitrogen). Elevated lung clearance index values suggest ventilation heterogeneity and have been shown to be higher among deployed veterans with dyspnea.26,27 Finally, advanced CT analytic techniques may help identify functional small airways disease and are higher in deployed service members with constrictive bronchiolitis on surgical lung biopsy.28 These innovative noninvasive techniques are experimental but promising, especially as part of a broader evaluation of small airways disease.
AHOBPR clinical evaluations represent an initial step to better understand postdeployment health conditions available to all AHOBPR participants. The PDCEN clinical evaluation extends the AHOBPR evaluation by providing specialty care for certain veterans requiring more comprehensive evaluation while systematically collecting and analyzing clinical data to advance the field. The VA is committed to leveraging these data and all available expertise to provide a clear description of the spectrum of disease in this population and improve our ability to diagnose, follow, and treat respiratory health conditions occurring after deployment to Southwest Asia and Afghanistan.
Case Conclusion
The veteran was referred to a PDCEN site and underwent a comprehensive multidisciplinary evaluation. Pulmonary function testing showed lung volumes and vital capacity within the predicted normal range, mild air trapping, and a low diffusion capacity for carbon monoxide. Methacholine challenge testing was normal; however, forced oscillometry suggested small airways obstruction. A high-resolution CT showed air trapping without parenchymal changes. Cardiopulmonary exercise testing demonstrated a peak exercise capacity within the predicted normal range but low breathing reserve. Otolaryngology evaluation including laryngoscopy suggested chronic nonallergic rhinitis.
At the end of the veteran’s evaluation, a summary review reported nonallergic rhinitis and distal airway obstruction consistent with small airways disease. Both were reported as most likely related to deployment given her significant environmental exposures and the temporal relationship with her deployment and symptom onset as well as lack of other identifiable causes. A more precise histopathologic diagnosis could be firmly established with a surgical lung biopsy, but after shared decision making with a PDCEN HCP, the patient declined to undergo this invasive procedure. After you review the summary review and recommendations from the PDCEN group, you start the veteran on intranasal steroids and a combined inhaled corticosteroid/long-acting β agonist inhaler as well as refer the veteran to pulmonary rehabilitation. After several weeks, she reports an improvement in sleep and nasal symptoms but continues to experience residual exercise intolerance.
This case serves as an example of the significant limitations that a previously active and healthy patient can develop after deployment to Southwest Asia and Afghanistan. Encouraging this veteran to complete the AHOBPR allowed her to be considered for a PDCEN evaluation that provided the opportunity to undergo a comprehensive noninvasive evaluation of her chronic dyspnea. In doing so, she obtained 2 important diagnoses and data from her evaluation will help establish best practices for standardized evaluations of respiratory concerns following deployment. Through the AHOBPR and PDCEN, the VA seeks to better understand postdeployment health conditions, their relationship to military and environmental exposures, and how best to diagnose and treat these conditions.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) Airborne Hazards and Burn Pits Center of Excellence (Public Law 115-929). The authors acknowledge support and contributions from Dr. Eric Shuping and leadership at VA’s Health Outcomes Military Exposures office as well as the New Jersey War Related Illness and Injury Study Center. In addition, we thank Erin McRoberts and Rajeev Swarup for their contributions to the Post-Deployment Cardiopulmonary Evaluation Network. Post-Deployment Cardiopulmonary Evaluation Network members:
Mehrdad Arjomandi, Caroline Davis, Michelle DeLuca, Nancy Eager, Courtney A. Eberhardt, Michael J. Falvo, Timothy Foley, Fiona A.S. Graff, Deborah Heaney, Stella E. Hines, Rachel E. Howard, Nisha Jani, Sheena Kamineni, Silpa Krefft, Mary L. Langlois, Helen Lozier, Simran K. Matharu, Anisa Moore, Lydia Patrick-DeLuca, Edward Pickering, Alexander Rabin, Michelle Robertson, Samantha L. Rogers, Aaron H. Schneider, Anand Shah, Anays Sotolongo, Jennifer H. Therkorn, Rebecca I. Toczylowski, Matthew Watson, Alison D. Wilczynski, Ian W. Wilson, Romi A. Yount.
1. Wenger J, O’Connell C, Cottrell L. Examination of recent deployment experience across the services and components. Exam. RAND Corporation; 2018. Accessed June 27, 2022. doi:10.7249/rr1928
2. Torreon BS. U.S. periods of war and dates of recent conflicts, RS21405. Congressional Research Service; 2017. June 5, 2020. Accessed June 27, 2022. https://crsreports.congress.gov/product/details?prodcode=RS21405
3. Dunigan M, Farmer CM, Burns RM, Hawks A, Setodji CM. Out of the shadows: the health and well-being of private contractors working in conflict environments. RAND Corporation; 2013. Accessed June 27, 2022. https://www.rand.org/pubs/research_reports/RR420.html
4. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
5. Pugh MJ, Jaramillo CA, Leung KW, et al. Increasing prevalence of chronic lung disease in veterans of the wars in Iraq and Afghanistan. Mil Med. 2016;181(5):476-481. doi:10.7205/MILMED-D-15-00035
6. Falvo MJ, Osinubi OY, Sotolongo AM, Helmer DA. Airborne hazards exposure and respiratory health of Iraq and Afghanistan veterans. Epidemiol Rev. 2015;37:116-130. doi:10.1093/epirev/mxu009
7. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669. doi:10.1097/JOM.0b013e318255ba1b
8. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. doi:10.1093/aje/kwp287
9. Szema AM, Salihi W, Savary K, Chen JJ. Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury. J Occup Environ Med. 2011;53(9):961-965. doi:10.1097/JOM.0b013e31822c9f05
10. Committee on the Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan; Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. Accessed June 27, 2022. doi:10.17226/1320911. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. Accessed June 27, 2022. doi:10.17226/25837
12. Krefft SD, Wolff J, Zell-Baran L, et al. Respiratory diseases in post-9/11 military personnel following Southwest Asia deployment. J Occup Environ Med. 2020;62(5):337-343. doi:10.1097/JOM.0000000000001817
13. Gordetsky J, Kim C, Miller RF, Mehrad M. Non-necrotizing granulomatous pneumonitis and chronic pleuritis in soldiers deployed to Southwest Asia. Histopathology. 2020;77(3):453-459. doi:10.1111/his.14135
14. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. doi:10.1056/NEJMoa1101388
15. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480. doi:10.1097/JOM.0b013e318042d682
16. Kim YH, Warren SH, Kooter I, et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part Fibre Toxicol. 2021;18(1):45. Published 2021 Dec 16. doi:10.1186/s12989-021-00435-w
17. Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—Part 1: ambient sampling. Inhal Toxicol. 2009;21(4):297-326. doi:10.1080/08958370802464273
18. US Army Public Health Command. Technical guide 230: environmental health risk assessment and chemical exposure guidelines for deployed military personnel, 2013 revision. Accessed June 27, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/TG230-DeploymentEHRA-and-MEGs-2013-Revision.pdf
19. Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8(2):166-175. doi:10.1007/s13181-011-0203-1
20. Shuping E, Schneiderman A. Resources on environmental exposures for military veterans. Am Fam Physician. 2020;101(12):709-710.
21. Masri S, Garshick E, Coull BA, Koutrakis P. A novel calibration approach using satellite and visibility observations to estimate fine particulate matter exposures in Southwest Asia and Afghanistan. J Air Waste Manag Assoc. 2017;67(1):86-95. doi:10.1080/10962247.2016.1230079
22. Gutor SS, Richmond BW, Du RH, et al. Postdeployment respiratory syndrome in soldiers with chronic exertional dyspnea. Am J Surg Pathol. 2021;45(12):1587-1596. doi:10.1097/PAS.0000000000001757
23. Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol. 2005;148(1-2):179-194. doi:10.1016/j.resp.2005.05.026
24. Butzko RP, Sotolongo AM, Helmer DA, et al. Forced oscillation technique in veterans with preserved spirometry and chronic respiratory symptoms. Respir Physiol Neurobiol. 2019;260:8-16. doi:10.1016/j.resp.2018.11.012
25. Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132(4):1275-1282. doi:10.1378/chest.07-0913
26. Zell-Baran LM, Krefft SD, Moore CM, Wolff J, Meehan R, Rose CS. Multiple breath washout: a noninvasive tool for identifying lung disease in symptomatic military deployers. Respir Med. 2021;176:106281. doi:10.1016/j.rmed.2020.106281
27. Krefft SD, Strand M, Smith J, Stroup C, Meehan R, Rose C. Utility of lung clearance index testing as a noninvasive marker of deployment-related lung disease. J Occup Environ Med. 2017;59(8):707-711. doi:10.1097/JOM.000000000000105828. Davis CW, Lopez CL, Bell AJ, et al. The severity of functional small airways disease in military personnel with constrictive bronchiolitis as measured by quantitative CT [published online ahead of print, 2022 May 24]. Am J Respir Crit Care Med. 2022;10.1164/rccm.202201-0153LE. doi:10.1164/rccm.202201-0153LE
Case Example
A 37-year-old female never smoker presents to your clinic with progressive dyspnea over the past 15 years. She reports dyspnea on exertion, wheezing, chronic nasal congestion, and difficulty sleeping that started a year after she returned from military deployment to Iraq. She has been unable to exercise, even at low intensity, for the past 5 years, despite being previously active. She has experienced some symptom improvement by taking an albuterol inhaler as needed, loratadine (10 mg), and fluticasone nasal spray (50 mcg). She occasionally uses famotidine for reflux (40 mg). She deployed to Southwest Asia for 12 months (2002-2003) and was primarily stationed in Qayyarah West, an Air Force base in the Mosul district in northern Iraq. She reports exposure during deployment to the fire in the Al-Mishraq sulfur mine, located approximately 25 km north of Qayyarah West, as well as dust storms and burn pits. She currently works as a medical assistant. Her examination is remarkable for normal bronchovesicular breath sounds without any wheezing or crackles on pulmonary evaluation. Her body mass index is 31. You obtain a chest radiograph and spirometry, which are both normal.
The veteran reports feeling frustrated as she has had multiple specialty evaluations in community clinics without receiving a diagnosis, despite worsening symptoms. She reports that she added her information to the Airborne Hazards and Open Burn Pit Registry (AHOBPR). She recently received a letter from the US Department of Veterans Affairs (VA) Post-Deployment Cardiopulmonary Evaluation Network (PDCEN) and is asking you whether she should participate in the PDCEN specialty evaluation. You are not familiar with the military experiences she has described or the programs she asks you about; however, you would like to know more to best care for your patient.
Background
The year 2021 marked the 20th anniversary of the September 11 attacks and the launch of the Global War on Terrorism. Almost 3 million US military personnel have been deployed in support of these operations along with about 300,000 US civilian contractors and thousands of troops from more than 40 nations.1-3
Respiratory hazards associated with deployment to Southwest Asia and Afghanistan are unique and varied. These exposures include blast injuries and a variety of particulate matter sources, such as burn pit combustion byproducts, aeroallergens, and dust storms.7,8,15,16 One air sampling study conducted at 15 deployment sites in Southwest Asia and Afghanistan found mean fine particulate matter (PM2.5) levels were as much as 10 times greater than sampling sites in both rural and urban cities in the United States; all sites sampled exceeded military exposure guidelines (65 µg/m3 for 1 year).17,18 Long-term exposure to PM2.5 has been associated with the development of chronic respiratory and cardiovascular disease; therefore, there has been considerable attention to the respiratory (and nonrespiratory) health of deployed military personnel.19
Concerns regarding the association between deployment and lung disease led to the creation of the national VA Airborne Hazards and Open Burn Pit Registry (AHOBPR) in 2014 and consists of (1) an online questionnaire to document deployment and medical history, exposure concerns, and symptoms; and (2) an optional in-person or virtual clinical health evaluation at the individual’s local VA medical center or military treatment facility (MTF). As of March 2022, more than 300,000 individuals have completed the online questionnaire of which about 30% declined the optional clinical health evaluation.
The clinical evaluation available to AHOBPR participants has not yet been described in the literature. Therefore, our objectives are to examine AHOBPR clinical evaluation data and review its application throughout the VA. In addition, we will also describe a parallel effort by the VA PDCEN, which is to provide comprehensive multiday clinical evaluations for unique AHOBPR participants with unexplained dyspnea and self-reported respiratory disease. A secondary aim of this publication is to disseminate information to health care professionals (HCPs) within and outside of the VA to aid in the referral and evaluation of previously deployed veterans who experience unexplained dyspnea.
AHOBPR Overview
The AHOBPR is an online questionnaire and optional in-person health evaluation that includes 7 major categories targeting deployment history, symptoms, medical history, health concerns, residential history, nonmilitary occupational history, nonmilitary environmental exposures, and health care utilization. The VA Defense Information Repository is used to obtain service dates for the service member/veteran, conflict involvement, and primary location during deployment. The questionnaire portion of the AHOBPR is administered online. It currently is open to all veterans who served in the Southwest Asia theater of operations (including Iraq, Kuwait, and Egypt) any time after August 2, 1990, or Afghanistan, Djibouti, Syria, or Uzbekistan after September 11, 2001. Veterans are eligible for completing the AHOBPR and optional health evaluation at no cost to the veteran regardless of VA benefits or whether they are currently enrolled in VA health care. Though the focus of the present manuscript is to profile a VA program, it is important to note that the US Department of Defense (DoD) is an active partner with the VA in the promotion of the AHOBPR to service members and similarly provides health evaluations for active-duty service members (including activating Reserve and Guard) through their local MTF.
We reviewed and analyzed AHOBPR operations and VA data from 2014 to 2020. Our analyses were limited to veterans seeking evaluation as well as their corresponding symptoms and HCP’s clinical impression from the electronic health record. As of September 20, 2021, 267,125 individuals completed the AHOBPR. The mean age was 43 years (range, 19-84), and the majority were male (86%) and served in the Army (58%). Open-air burn pits (91%), engine maintenance (38.8%), and convoy operations (71.7%) were the most common deployment-related exposures.
The optional in-person AHOBPR health evaluation may be requested by the veteran after completing the online questionnaire and is performed at the veteran’s local VA facility. The evaluation is most often completed by an environmental health clinician or primary care practitioner (PCP). A variety of resources are available to providers for training on this topic, including fact sheets, webinars, monthly calls, conferences, and accredited e-learning.20 As part of the clinical evaluation, the veteran’s chief concerns are assessed and evaluated. At the time of our analysis, 24,578 clinical examinations were performed across 126 VA medical facilities, with considerable geographic variation. Veterans receiving evaluations were predominantly male (89%) with a median age of 46.0 years (IQR, 15). Veterans’ major respiratory concerns included dyspnea (45.1%), decreased exercise ability (34.8%), and cough > 3 weeks (30.3%) (Table). After clinical evaluation by a VA or MTF HCP, 47.8% were found to have a respiratory diagnosis, including asthma (30.1%), COPD (12.8%), and bronchitis (11.9%).
Registry participants who opt to receive the clinical evaluation may benefit directly by undergoing a detailed clinical history and physical examination as well as having the opportunity to document their health concerns. For some, clinicians may need to refer veterans for additional specialty testing beyond this standard AHOBPR clinical evaluation. Although these evaluations can help address some of the veterans’ concerns, a substantial number may have unexplained respiratory symptoms that warrant further investigation.
Post-Deployment Cardiopulmonary Evaluation Network Clinical Evaluation
In May 2019, the VA established the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). One of the AHBPCE’s objectives is to deliver specialized care and consultation for veterans with concerns about their postdeployment health, including, but not limited to, unexplained dyspnea. To meet this objective, the AHBPCE developed the PDCEN, a national network consisting of specialty HCPs from 5 VA medical centers—located in San Francisco, California; Denver, Colorado; Baltimore, Maryland; Ann Arbor, Michigan; and East Orange, New Jersey. Collectively, the PDCEN has developed a standardized approach for the comprehensive clinical evaluation of unexplained dyspnea that is implemented uniformly across sites. Staff at the PDCEN screen the AHOBPR to identify veterans with features of respiratory disease and invite them to participate in an in-person evaluation at the nearest PDCEN site. Given the specialty expertise (detailed below) within the Network, the PDCEN focuses on complex cases that are resource intensive. To address complex cases of unexplained dyspnea, the PDCEN has developed a core clinical evaluation approach (Figure).
The first step in a veteran’s PDCEN evaluation entails a set of detailed questionnaires that request information about the veteran’s current respiratory, sleep, and mental health symptoms and any associated medical diagnoses. Questionnaires also identify potential exposures to military burn pits, sulfur mine and oil field fires, diesel exhaust fumes, dust storms, urban pollution, explosions/blasts, and chemical weapons. In addition, the questionnaires include deployment geographic location, which may inform future estimates of particulate matter exposure.21 Prior VA and non-VA evaluations and testing of their respiratory concerns are obtained for review. Exposure and health records from the DoD are also reviewed when available.
The next step in the PDCEN evaluation comprises comprehensive testing, including complete pulmonary function testing, methacholine challenge, cardiopulmonary exercise testing, forced oscillometry and exhaled nitric oxide testing, paired high-resolution inspiratory and expiratory chest computed tomography (CT) imaging, sinus CT imaging, direct flexible laryngoscopy, echocardiography, polysomnography, and laboratory blood testing. The testing process is managed by local site coordinators and varies by institution based on availability of each testing modality and subspecialist appointments.
Once testing is completed, the veteran is evaluated by a team of HCPs, including physicians from the disciplines of pulmonary medicine, environmental and occupational health, sleep medicine, otolaryngology and speech pathology, and mental health (when appropriate). After the clinical evaluation has been completed, this team of expert HCPs at each site convenes to provide a final summary review visit intended to be a comprehensive assessment of the veteran’s primary health concerns. The 3 primary objectives of this final review are to inform the veteran of (1) what respiratory and related conditions they have; (2) whether the conditions is/are deployment related; and (3) what treatments and/or follow-up care may enhance their current state of health in partnership with their local HCPs. The PDCEN does not provide ongoing management of any conditions identified during the veteran’s evaluation but communicates findings and recommendations to the veteran and their PCP for long-term care.
Discussion
The AHOBPR was established in response to mounting concerns that service members and veterans were experiencing adverse health effects that might be attributable to deployment-related exposures. Nearly half of all patients currently enrolled in the AHOBPR report dyspnea, and about one-third have decreased exercise tolerance and/or cough. Of those who completed the questionnaire and the subsequent in-person and generalized AHOBPR examination, our interim analysis showed that about half were assigned a respiratory diagnosis. Yet for many veterans, their breathing symptoms remained unexplained or did not respond to treatment.
While the AHOBPR and related examinations address the needs of many veterans, others may require more comprehensive examination. The PDCEN attends to the latter by providing more detailed and comprehensive clinical evaluations of veterans with deployment-related respiratory health concerns and seeks to learn from these evaluations by analyzing data obtained from veterans across sites. As such, the PDCEN hopes not only to improve the health of individual veterans, but also create standard practices for both VA and non-VA community evaluation of veterans exposed to respiratory hazards during deployment.
One of the major challenges in the field of postdeployment respiratory health is the lack of clear universal language or case definitions that encompass the veteran’s clinical concerns. In an influential case series published in 2011, 38 (77%) of 49 soldiers with history of airborne hazard exposure and unexplained exercise intolerance were reported to have histopathology consistent with constrictive bronchiolitis on surgical lung biopsy.14 Subsequent publications have described other histopathologic features in deployed military personnel, including granulomatous inflammation, interstitial lung disease, emphysema, and pleuritis.12-14 Reconciling these findings from surgical lung biopsy with the clinical presentation and noninvasive studies has proved difficult. Therefore, several groups of investigators have proposed terms, including postdeployment respiratory syndrome, deployment-related distal lung disease, and Iraq/Afghanistan War lung injury to describe the increased respiratory symptoms and variety of histopathologic and imaging findings in this population.9,12,22 At present, there remains a lack of consensus on terminology and case definitions as well as the role of military environmental exposures in exacerbating and/or causing these conditions. As HCPs, it is important to appreciate and acknowledge that the ambiguity and controversy pertaining to terminology, causation, and service connections are a common source of frustration experienced by veterans, which are increasingly reflected among reports in popular media and lay press.
A second and related challenge in the field of postdeployment respiratory health that contributes to veteran and HCP frustration is that many of the aforementioned abnormalities described on surgical lung biopsy are not readily identifiable on noninvasive tests, including traditional interpretation of pulmonary function tests or chest CT imaging.12-14,22 Thus, underlying conditions could be overlooked and veterans’ concerns and symptoms may be dismissed or misattributed to other comorbid conditions. While surgical lung biopsies may offer diagnostic clarity in identifying lung disease, there are significant procedural risks of surgical and anesthetic complications. Furthermore, a definitive diagnosis does not necessarily guarantee a clear treatment plan. For example, there are no current therapies approved by the US Food and Drug Administration for the treatment of constrictive bronchiolitis.
Research efforts are underway, including within the PDCEN, to evaluate a more sensitive and noninvasive assessment of the small airways that may even reduce or eliminate the need for surgical lung biopsy. In contrast to traditional pulmonary function testing, which is helpful for evaluation of the larger airways, forced oscillation technique can be used noninvasively, using pressure oscillations to evaluate for diseases of the smaller airways and has been used in the veteran population and in those exposed to dust from the World Trade Center disaster.23-25 Multiple breath washout technique provides a lung clearance index that is determined by the number of lung turnovers it takes to clear the lungs of an inert gas (eg, sulfur hexafluoride, nitrogen). Elevated lung clearance index values suggest ventilation heterogeneity and have been shown to be higher among deployed veterans with dyspnea.26,27 Finally, advanced CT analytic techniques may help identify functional small airways disease and are higher in deployed service members with constrictive bronchiolitis on surgical lung biopsy.28 These innovative noninvasive techniques are experimental but promising, especially as part of a broader evaluation of small airways disease.
AHOBPR clinical evaluations represent an initial step to better understand postdeployment health conditions available to all AHOBPR participants. The PDCEN clinical evaluation extends the AHOBPR evaluation by providing specialty care for certain veterans requiring more comprehensive evaluation while systematically collecting and analyzing clinical data to advance the field. The VA is committed to leveraging these data and all available expertise to provide a clear description of the spectrum of disease in this population and improve our ability to diagnose, follow, and treat respiratory health conditions occurring after deployment to Southwest Asia and Afghanistan.
Case Conclusion
The veteran was referred to a PDCEN site and underwent a comprehensive multidisciplinary evaluation. Pulmonary function testing showed lung volumes and vital capacity within the predicted normal range, mild air trapping, and a low diffusion capacity for carbon monoxide. Methacholine challenge testing was normal; however, forced oscillometry suggested small airways obstruction. A high-resolution CT showed air trapping without parenchymal changes. Cardiopulmonary exercise testing demonstrated a peak exercise capacity within the predicted normal range but low breathing reserve. Otolaryngology evaluation including laryngoscopy suggested chronic nonallergic rhinitis.
At the end of the veteran’s evaluation, a summary review reported nonallergic rhinitis and distal airway obstruction consistent with small airways disease. Both were reported as most likely related to deployment given her significant environmental exposures and the temporal relationship with her deployment and symptom onset as well as lack of other identifiable causes. A more precise histopathologic diagnosis could be firmly established with a surgical lung biopsy, but after shared decision making with a PDCEN HCP, the patient declined to undergo this invasive procedure. After you review the summary review and recommendations from the PDCEN group, you start the veteran on intranasal steroids and a combined inhaled corticosteroid/long-acting β agonist inhaler as well as refer the veteran to pulmonary rehabilitation. After several weeks, she reports an improvement in sleep and nasal symptoms but continues to experience residual exercise intolerance.
This case serves as an example of the significant limitations that a previously active and healthy patient can develop after deployment to Southwest Asia and Afghanistan. Encouraging this veteran to complete the AHOBPR allowed her to be considered for a PDCEN evaluation that provided the opportunity to undergo a comprehensive noninvasive evaluation of her chronic dyspnea. In doing so, she obtained 2 important diagnoses and data from her evaluation will help establish best practices for standardized evaluations of respiratory concerns following deployment. Through the AHOBPR and PDCEN, the VA seeks to better understand postdeployment health conditions, their relationship to military and environmental exposures, and how best to diagnose and treat these conditions.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) Airborne Hazards and Burn Pits Center of Excellence (Public Law 115-929). The authors acknowledge support and contributions from Dr. Eric Shuping and leadership at VA’s Health Outcomes Military Exposures office as well as the New Jersey War Related Illness and Injury Study Center. In addition, we thank Erin McRoberts and Rajeev Swarup for their contributions to the Post-Deployment Cardiopulmonary Evaluation Network. Post-Deployment Cardiopulmonary Evaluation Network members:
Mehrdad Arjomandi, Caroline Davis, Michelle DeLuca, Nancy Eager, Courtney A. Eberhardt, Michael J. Falvo, Timothy Foley, Fiona A.S. Graff, Deborah Heaney, Stella E. Hines, Rachel E. Howard, Nisha Jani, Sheena Kamineni, Silpa Krefft, Mary L. Langlois, Helen Lozier, Simran K. Matharu, Anisa Moore, Lydia Patrick-DeLuca, Edward Pickering, Alexander Rabin, Michelle Robertson, Samantha L. Rogers, Aaron H. Schneider, Anand Shah, Anays Sotolongo, Jennifer H. Therkorn, Rebecca I. Toczylowski, Matthew Watson, Alison D. Wilczynski, Ian W. Wilson, Romi A. Yount.
Case Example
A 37-year-old female never smoker presents to your clinic with progressive dyspnea over the past 15 years. She reports dyspnea on exertion, wheezing, chronic nasal congestion, and difficulty sleeping that started a year after she returned from military deployment to Iraq. She has been unable to exercise, even at low intensity, for the past 5 years, despite being previously active. She has experienced some symptom improvement by taking an albuterol inhaler as needed, loratadine (10 mg), and fluticasone nasal spray (50 mcg). She occasionally uses famotidine for reflux (40 mg). She deployed to Southwest Asia for 12 months (2002-2003) and was primarily stationed in Qayyarah West, an Air Force base in the Mosul district in northern Iraq. She reports exposure during deployment to the fire in the Al-Mishraq sulfur mine, located approximately 25 km north of Qayyarah West, as well as dust storms and burn pits. She currently works as a medical assistant. Her examination is remarkable for normal bronchovesicular breath sounds without any wheezing or crackles on pulmonary evaluation. Her body mass index is 31. You obtain a chest radiograph and spirometry, which are both normal.
The veteran reports feeling frustrated as she has had multiple specialty evaluations in community clinics without receiving a diagnosis, despite worsening symptoms. She reports that she added her information to the Airborne Hazards and Open Burn Pit Registry (AHOBPR). She recently received a letter from the US Department of Veterans Affairs (VA) Post-Deployment Cardiopulmonary Evaluation Network (PDCEN) and is asking you whether she should participate in the PDCEN specialty evaluation. You are not familiar with the military experiences she has described or the programs she asks you about; however, you would like to know more to best care for your patient.
Background
The year 2021 marked the 20th anniversary of the September 11 attacks and the launch of the Global War on Terrorism. Almost 3 million US military personnel have been deployed in support of these operations along with about 300,000 US civilian contractors and thousands of troops from more than 40 nations.1-3
Respiratory hazards associated with deployment to Southwest Asia and Afghanistan are unique and varied. These exposures include blast injuries and a variety of particulate matter sources, such as burn pit combustion byproducts, aeroallergens, and dust storms.7,8,15,16 One air sampling study conducted at 15 deployment sites in Southwest Asia and Afghanistan found mean fine particulate matter (PM2.5) levels were as much as 10 times greater than sampling sites in both rural and urban cities in the United States; all sites sampled exceeded military exposure guidelines (65 µg/m3 for 1 year).17,18 Long-term exposure to PM2.5 has been associated with the development of chronic respiratory and cardiovascular disease; therefore, there has been considerable attention to the respiratory (and nonrespiratory) health of deployed military personnel.19
Concerns regarding the association between deployment and lung disease led to the creation of the national VA Airborne Hazards and Open Burn Pit Registry (AHOBPR) in 2014 and consists of (1) an online questionnaire to document deployment and medical history, exposure concerns, and symptoms; and (2) an optional in-person or virtual clinical health evaluation at the individual’s local VA medical center or military treatment facility (MTF). As of March 2022, more than 300,000 individuals have completed the online questionnaire of which about 30% declined the optional clinical health evaluation.
The clinical evaluation available to AHOBPR participants has not yet been described in the literature. Therefore, our objectives are to examine AHOBPR clinical evaluation data and review its application throughout the VA. In addition, we will also describe a parallel effort by the VA PDCEN, which is to provide comprehensive multiday clinical evaluations for unique AHOBPR participants with unexplained dyspnea and self-reported respiratory disease. A secondary aim of this publication is to disseminate information to health care professionals (HCPs) within and outside of the VA to aid in the referral and evaluation of previously deployed veterans who experience unexplained dyspnea.
AHOBPR Overview
The AHOBPR is an online questionnaire and optional in-person health evaluation that includes 7 major categories targeting deployment history, symptoms, medical history, health concerns, residential history, nonmilitary occupational history, nonmilitary environmental exposures, and health care utilization. The VA Defense Information Repository is used to obtain service dates for the service member/veteran, conflict involvement, and primary location during deployment. The questionnaire portion of the AHOBPR is administered online. It currently is open to all veterans who served in the Southwest Asia theater of operations (including Iraq, Kuwait, and Egypt) any time after August 2, 1990, or Afghanistan, Djibouti, Syria, or Uzbekistan after September 11, 2001. Veterans are eligible for completing the AHOBPR and optional health evaluation at no cost to the veteran regardless of VA benefits or whether they are currently enrolled in VA health care. Though the focus of the present manuscript is to profile a VA program, it is important to note that the US Department of Defense (DoD) is an active partner with the VA in the promotion of the AHOBPR to service members and similarly provides health evaluations for active-duty service members (including activating Reserve and Guard) through their local MTF.
We reviewed and analyzed AHOBPR operations and VA data from 2014 to 2020. Our analyses were limited to veterans seeking evaluation as well as their corresponding symptoms and HCP’s clinical impression from the electronic health record. As of September 20, 2021, 267,125 individuals completed the AHOBPR. The mean age was 43 years (range, 19-84), and the majority were male (86%) and served in the Army (58%). Open-air burn pits (91%), engine maintenance (38.8%), and convoy operations (71.7%) were the most common deployment-related exposures.
The optional in-person AHOBPR health evaluation may be requested by the veteran after completing the online questionnaire and is performed at the veteran’s local VA facility. The evaluation is most often completed by an environmental health clinician or primary care practitioner (PCP). A variety of resources are available to providers for training on this topic, including fact sheets, webinars, monthly calls, conferences, and accredited e-learning.20 As part of the clinical evaluation, the veteran’s chief concerns are assessed and evaluated. At the time of our analysis, 24,578 clinical examinations were performed across 126 VA medical facilities, with considerable geographic variation. Veterans receiving evaluations were predominantly male (89%) with a median age of 46.0 years (IQR, 15). Veterans’ major respiratory concerns included dyspnea (45.1%), decreased exercise ability (34.8%), and cough > 3 weeks (30.3%) (Table). After clinical evaluation by a VA or MTF HCP, 47.8% were found to have a respiratory diagnosis, including asthma (30.1%), COPD (12.8%), and bronchitis (11.9%).
Registry participants who opt to receive the clinical evaluation may benefit directly by undergoing a detailed clinical history and physical examination as well as having the opportunity to document their health concerns. For some, clinicians may need to refer veterans for additional specialty testing beyond this standard AHOBPR clinical evaluation. Although these evaluations can help address some of the veterans’ concerns, a substantial number may have unexplained respiratory symptoms that warrant further investigation.
Post-Deployment Cardiopulmonary Evaluation Network Clinical Evaluation
In May 2019, the VA established the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). One of the AHBPCE’s objectives is to deliver specialized care and consultation for veterans with concerns about their postdeployment health, including, but not limited to, unexplained dyspnea. To meet this objective, the AHBPCE developed the PDCEN, a national network consisting of specialty HCPs from 5 VA medical centers—located in San Francisco, California; Denver, Colorado; Baltimore, Maryland; Ann Arbor, Michigan; and East Orange, New Jersey. Collectively, the PDCEN has developed a standardized approach for the comprehensive clinical evaluation of unexplained dyspnea that is implemented uniformly across sites. Staff at the PDCEN screen the AHOBPR to identify veterans with features of respiratory disease and invite them to participate in an in-person evaluation at the nearest PDCEN site. Given the specialty expertise (detailed below) within the Network, the PDCEN focuses on complex cases that are resource intensive. To address complex cases of unexplained dyspnea, the PDCEN has developed a core clinical evaluation approach (Figure).
The first step in a veteran’s PDCEN evaluation entails a set of detailed questionnaires that request information about the veteran’s current respiratory, sleep, and mental health symptoms and any associated medical diagnoses. Questionnaires also identify potential exposures to military burn pits, sulfur mine and oil field fires, diesel exhaust fumes, dust storms, urban pollution, explosions/blasts, and chemical weapons. In addition, the questionnaires include deployment geographic location, which may inform future estimates of particulate matter exposure.21 Prior VA and non-VA evaluations and testing of their respiratory concerns are obtained for review. Exposure and health records from the DoD are also reviewed when available.
The next step in the PDCEN evaluation comprises comprehensive testing, including complete pulmonary function testing, methacholine challenge, cardiopulmonary exercise testing, forced oscillometry and exhaled nitric oxide testing, paired high-resolution inspiratory and expiratory chest computed tomography (CT) imaging, sinus CT imaging, direct flexible laryngoscopy, echocardiography, polysomnography, and laboratory blood testing. The testing process is managed by local site coordinators and varies by institution based on availability of each testing modality and subspecialist appointments.
Once testing is completed, the veteran is evaluated by a team of HCPs, including physicians from the disciplines of pulmonary medicine, environmental and occupational health, sleep medicine, otolaryngology and speech pathology, and mental health (when appropriate). After the clinical evaluation has been completed, this team of expert HCPs at each site convenes to provide a final summary review visit intended to be a comprehensive assessment of the veteran’s primary health concerns. The 3 primary objectives of this final review are to inform the veteran of (1) what respiratory and related conditions they have; (2) whether the conditions is/are deployment related; and (3) what treatments and/or follow-up care may enhance their current state of health in partnership with their local HCPs. The PDCEN does not provide ongoing management of any conditions identified during the veteran’s evaluation but communicates findings and recommendations to the veteran and their PCP for long-term care.
Discussion
The AHOBPR was established in response to mounting concerns that service members and veterans were experiencing adverse health effects that might be attributable to deployment-related exposures. Nearly half of all patients currently enrolled in the AHOBPR report dyspnea, and about one-third have decreased exercise tolerance and/or cough. Of those who completed the questionnaire and the subsequent in-person and generalized AHOBPR examination, our interim analysis showed that about half were assigned a respiratory diagnosis. Yet for many veterans, their breathing symptoms remained unexplained or did not respond to treatment.
While the AHOBPR and related examinations address the needs of many veterans, others may require more comprehensive examination. The PDCEN attends to the latter by providing more detailed and comprehensive clinical evaluations of veterans with deployment-related respiratory health concerns and seeks to learn from these evaluations by analyzing data obtained from veterans across sites. As such, the PDCEN hopes not only to improve the health of individual veterans, but also create standard practices for both VA and non-VA community evaluation of veterans exposed to respiratory hazards during deployment.
One of the major challenges in the field of postdeployment respiratory health is the lack of clear universal language or case definitions that encompass the veteran’s clinical concerns. In an influential case series published in 2011, 38 (77%) of 49 soldiers with history of airborne hazard exposure and unexplained exercise intolerance were reported to have histopathology consistent with constrictive bronchiolitis on surgical lung biopsy.14 Subsequent publications have described other histopathologic features in deployed military personnel, including granulomatous inflammation, interstitial lung disease, emphysema, and pleuritis.12-14 Reconciling these findings from surgical lung biopsy with the clinical presentation and noninvasive studies has proved difficult. Therefore, several groups of investigators have proposed terms, including postdeployment respiratory syndrome, deployment-related distal lung disease, and Iraq/Afghanistan War lung injury to describe the increased respiratory symptoms and variety of histopathologic and imaging findings in this population.9,12,22 At present, there remains a lack of consensus on terminology and case definitions as well as the role of military environmental exposures in exacerbating and/or causing these conditions. As HCPs, it is important to appreciate and acknowledge that the ambiguity and controversy pertaining to terminology, causation, and service connections are a common source of frustration experienced by veterans, which are increasingly reflected among reports in popular media and lay press.
A second and related challenge in the field of postdeployment respiratory health that contributes to veteran and HCP frustration is that many of the aforementioned abnormalities described on surgical lung biopsy are not readily identifiable on noninvasive tests, including traditional interpretation of pulmonary function tests or chest CT imaging.12-14,22 Thus, underlying conditions could be overlooked and veterans’ concerns and symptoms may be dismissed or misattributed to other comorbid conditions. While surgical lung biopsies may offer diagnostic clarity in identifying lung disease, there are significant procedural risks of surgical and anesthetic complications. Furthermore, a definitive diagnosis does not necessarily guarantee a clear treatment plan. For example, there are no current therapies approved by the US Food and Drug Administration for the treatment of constrictive bronchiolitis.
Research efforts are underway, including within the PDCEN, to evaluate a more sensitive and noninvasive assessment of the small airways that may even reduce or eliminate the need for surgical lung biopsy. In contrast to traditional pulmonary function testing, which is helpful for evaluation of the larger airways, forced oscillation technique can be used noninvasively, using pressure oscillations to evaluate for diseases of the smaller airways and has been used in the veteran population and in those exposed to dust from the World Trade Center disaster.23-25 Multiple breath washout technique provides a lung clearance index that is determined by the number of lung turnovers it takes to clear the lungs of an inert gas (eg, sulfur hexafluoride, nitrogen). Elevated lung clearance index values suggest ventilation heterogeneity and have been shown to be higher among deployed veterans with dyspnea.26,27 Finally, advanced CT analytic techniques may help identify functional small airways disease and are higher in deployed service members with constrictive bronchiolitis on surgical lung biopsy.28 These innovative noninvasive techniques are experimental but promising, especially as part of a broader evaluation of small airways disease.
AHOBPR clinical evaluations represent an initial step to better understand postdeployment health conditions available to all AHOBPR participants. The PDCEN clinical evaluation extends the AHOBPR evaluation by providing specialty care for certain veterans requiring more comprehensive evaluation while systematically collecting and analyzing clinical data to advance the field. The VA is committed to leveraging these data and all available expertise to provide a clear description of the spectrum of disease in this population and improve our ability to diagnose, follow, and treat respiratory health conditions occurring after deployment to Southwest Asia and Afghanistan.
Case Conclusion
The veteran was referred to a PDCEN site and underwent a comprehensive multidisciplinary evaluation. Pulmonary function testing showed lung volumes and vital capacity within the predicted normal range, mild air trapping, and a low diffusion capacity for carbon monoxide. Methacholine challenge testing was normal; however, forced oscillometry suggested small airways obstruction. A high-resolution CT showed air trapping without parenchymal changes. Cardiopulmonary exercise testing demonstrated a peak exercise capacity within the predicted normal range but low breathing reserve. Otolaryngology evaluation including laryngoscopy suggested chronic nonallergic rhinitis.
At the end of the veteran’s evaluation, a summary review reported nonallergic rhinitis and distal airway obstruction consistent with small airways disease. Both were reported as most likely related to deployment given her significant environmental exposures and the temporal relationship with her deployment and symptom onset as well as lack of other identifiable causes. A more precise histopathologic diagnosis could be firmly established with a surgical lung biopsy, but after shared decision making with a PDCEN HCP, the patient declined to undergo this invasive procedure. After you review the summary review and recommendations from the PDCEN group, you start the veteran on intranasal steroids and a combined inhaled corticosteroid/long-acting β agonist inhaler as well as refer the veteran to pulmonary rehabilitation. After several weeks, she reports an improvement in sleep and nasal symptoms but continues to experience residual exercise intolerance.
This case serves as an example of the significant limitations that a previously active and healthy patient can develop after deployment to Southwest Asia and Afghanistan. Encouraging this veteran to complete the AHOBPR allowed her to be considered for a PDCEN evaluation that provided the opportunity to undergo a comprehensive noninvasive evaluation of her chronic dyspnea. In doing so, she obtained 2 important diagnoses and data from her evaluation will help establish best practices for standardized evaluations of respiratory concerns following deployment. Through the AHOBPR and PDCEN, the VA seeks to better understand postdeployment health conditions, their relationship to military and environmental exposures, and how best to diagnose and treat these conditions.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) Airborne Hazards and Burn Pits Center of Excellence (Public Law 115-929). The authors acknowledge support and contributions from Dr. Eric Shuping and leadership at VA’s Health Outcomes Military Exposures office as well as the New Jersey War Related Illness and Injury Study Center. In addition, we thank Erin McRoberts and Rajeev Swarup for their contributions to the Post-Deployment Cardiopulmonary Evaluation Network. Post-Deployment Cardiopulmonary Evaluation Network members:
Mehrdad Arjomandi, Caroline Davis, Michelle DeLuca, Nancy Eager, Courtney A. Eberhardt, Michael J. Falvo, Timothy Foley, Fiona A.S. Graff, Deborah Heaney, Stella E. Hines, Rachel E. Howard, Nisha Jani, Sheena Kamineni, Silpa Krefft, Mary L. Langlois, Helen Lozier, Simran K. Matharu, Anisa Moore, Lydia Patrick-DeLuca, Edward Pickering, Alexander Rabin, Michelle Robertson, Samantha L. Rogers, Aaron H. Schneider, Anand Shah, Anays Sotolongo, Jennifer H. Therkorn, Rebecca I. Toczylowski, Matthew Watson, Alison D. Wilczynski, Ian W. Wilson, Romi A. Yount.
1. Wenger J, O’Connell C, Cottrell L. Examination of recent deployment experience across the services and components. Exam. RAND Corporation; 2018. Accessed June 27, 2022. doi:10.7249/rr1928
2. Torreon BS. U.S. periods of war and dates of recent conflicts, RS21405. Congressional Research Service; 2017. June 5, 2020. Accessed June 27, 2022. https://crsreports.congress.gov/product/details?prodcode=RS21405
3. Dunigan M, Farmer CM, Burns RM, Hawks A, Setodji CM. Out of the shadows: the health and well-being of private contractors working in conflict environments. RAND Corporation; 2013. Accessed June 27, 2022. https://www.rand.org/pubs/research_reports/RR420.html
4. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
5. Pugh MJ, Jaramillo CA, Leung KW, et al. Increasing prevalence of chronic lung disease in veterans of the wars in Iraq and Afghanistan. Mil Med. 2016;181(5):476-481. doi:10.7205/MILMED-D-15-00035
6. Falvo MJ, Osinubi OY, Sotolongo AM, Helmer DA. Airborne hazards exposure and respiratory health of Iraq and Afghanistan veterans. Epidemiol Rev. 2015;37:116-130. doi:10.1093/epirev/mxu009
7. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669. doi:10.1097/JOM.0b013e318255ba1b
8. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. doi:10.1093/aje/kwp287
9. Szema AM, Salihi W, Savary K, Chen JJ. Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury. J Occup Environ Med. 2011;53(9):961-965. doi:10.1097/JOM.0b013e31822c9f05
10. Committee on the Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan; Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. Accessed June 27, 2022. doi:10.17226/1320911. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. Accessed June 27, 2022. doi:10.17226/25837
12. Krefft SD, Wolff J, Zell-Baran L, et al. Respiratory diseases in post-9/11 military personnel following Southwest Asia deployment. J Occup Environ Med. 2020;62(5):337-343. doi:10.1097/JOM.0000000000001817
13. Gordetsky J, Kim C, Miller RF, Mehrad M. Non-necrotizing granulomatous pneumonitis and chronic pleuritis in soldiers deployed to Southwest Asia. Histopathology. 2020;77(3):453-459. doi:10.1111/his.14135
14. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. doi:10.1056/NEJMoa1101388
15. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480. doi:10.1097/JOM.0b013e318042d682
16. Kim YH, Warren SH, Kooter I, et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part Fibre Toxicol. 2021;18(1):45. Published 2021 Dec 16. doi:10.1186/s12989-021-00435-w
17. Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—Part 1: ambient sampling. Inhal Toxicol. 2009;21(4):297-326. doi:10.1080/08958370802464273
18. US Army Public Health Command. Technical guide 230: environmental health risk assessment and chemical exposure guidelines for deployed military personnel, 2013 revision. Accessed June 27, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/TG230-DeploymentEHRA-and-MEGs-2013-Revision.pdf
19. Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8(2):166-175. doi:10.1007/s13181-011-0203-1
20. Shuping E, Schneiderman A. Resources on environmental exposures for military veterans. Am Fam Physician. 2020;101(12):709-710.
21. Masri S, Garshick E, Coull BA, Koutrakis P. A novel calibration approach using satellite and visibility observations to estimate fine particulate matter exposures in Southwest Asia and Afghanistan. J Air Waste Manag Assoc. 2017;67(1):86-95. doi:10.1080/10962247.2016.1230079
22. Gutor SS, Richmond BW, Du RH, et al. Postdeployment respiratory syndrome in soldiers with chronic exertional dyspnea. Am J Surg Pathol. 2021;45(12):1587-1596. doi:10.1097/PAS.0000000000001757
23. Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol. 2005;148(1-2):179-194. doi:10.1016/j.resp.2005.05.026
24. Butzko RP, Sotolongo AM, Helmer DA, et al. Forced oscillation technique in veterans with preserved spirometry and chronic respiratory symptoms. Respir Physiol Neurobiol. 2019;260:8-16. doi:10.1016/j.resp.2018.11.012
25. Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132(4):1275-1282. doi:10.1378/chest.07-0913
26. Zell-Baran LM, Krefft SD, Moore CM, Wolff J, Meehan R, Rose CS. Multiple breath washout: a noninvasive tool for identifying lung disease in symptomatic military deployers. Respir Med. 2021;176:106281. doi:10.1016/j.rmed.2020.106281
27. Krefft SD, Strand M, Smith J, Stroup C, Meehan R, Rose C. Utility of lung clearance index testing as a noninvasive marker of deployment-related lung disease. J Occup Environ Med. 2017;59(8):707-711. doi:10.1097/JOM.000000000000105828. Davis CW, Lopez CL, Bell AJ, et al. The severity of functional small airways disease in military personnel with constrictive bronchiolitis as measured by quantitative CT [published online ahead of print, 2022 May 24]. Am J Respir Crit Care Med. 2022;10.1164/rccm.202201-0153LE. doi:10.1164/rccm.202201-0153LE
1. Wenger J, O’Connell C, Cottrell L. Examination of recent deployment experience across the services and components. Exam. RAND Corporation; 2018. Accessed June 27, 2022. doi:10.7249/rr1928
2. Torreon BS. U.S. periods of war and dates of recent conflicts, RS21405. Congressional Research Service; 2017. June 5, 2020. Accessed June 27, 2022. https://crsreports.congress.gov/product/details?prodcode=RS21405
3. Dunigan M, Farmer CM, Burns RM, Hawks A, Setodji CM. Out of the shadows: the health and well-being of private contractors working in conflict environments. RAND Corporation; 2013. Accessed June 27, 2022. https://www.rand.org/pubs/research_reports/RR420.html
4. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
5. Pugh MJ, Jaramillo CA, Leung KW, et al. Increasing prevalence of chronic lung disease in veterans of the wars in Iraq and Afghanistan. Mil Med. 2016;181(5):476-481. doi:10.7205/MILMED-D-15-00035
6. Falvo MJ, Osinubi OY, Sotolongo AM, Helmer DA. Airborne hazards exposure and respiratory health of Iraq and Afghanistan veterans. Epidemiol Rev. 2015;37:116-130. doi:10.1093/epirev/mxu009
7. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669. doi:10.1097/JOM.0b013e318255ba1b
8. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. doi:10.1093/aje/kwp287
9. Szema AM, Salihi W, Savary K, Chen JJ. Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury. J Occup Environ Med. 2011;53(9):961-965. doi:10.1097/JOM.0b013e31822c9f05
10. Committee on the Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan; Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. Accessed June 27, 2022. doi:10.17226/1320911. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. Accessed June 27, 2022. doi:10.17226/25837
12. Krefft SD, Wolff J, Zell-Baran L, et al. Respiratory diseases in post-9/11 military personnel following Southwest Asia deployment. J Occup Environ Med. 2020;62(5):337-343. doi:10.1097/JOM.0000000000001817
13. Gordetsky J, Kim C, Miller RF, Mehrad M. Non-necrotizing granulomatous pneumonitis and chronic pleuritis in soldiers deployed to Southwest Asia. Histopathology. 2020;77(3):453-459. doi:10.1111/his.14135
14. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. doi:10.1056/NEJMoa1101388
15. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480. doi:10.1097/JOM.0b013e318042d682
16. Kim YH, Warren SH, Kooter I, et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part Fibre Toxicol. 2021;18(1):45. Published 2021 Dec 16. doi:10.1186/s12989-021-00435-w
17. Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—Part 1: ambient sampling. Inhal Toxicol. 2009;21(4):297-326. doi:10.1080/08958370802464273
18. US Army Public Health Command. Technical guide 230: environmental health risk assessment and chemical exposure guidelines for deployed military personnel, 2013 revision. Accessed June 27, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/TG230-DeploymentEHRA-and-MEGs-2013-Revision.pdf
19. Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8(2):166-175. doi:10.1007/s13181-011-0203-1
20. Shuping E, Schneiderman A. Resources on environmental exposures for military veterans. Am Fam Physician. 2020;101(12):709-710.
21. Masri S, Garshick E, Coull BA, Koutrakis P. A novel calibration approach using satellite and visibility observations to estimate fine particulate matter exposures in Southwest Asia and Afghanistan. J Air Waste Manag Assoc. 2017;67(1):86-95. doi:10.1080/10962247.2016.1230079
22. Gutor SS, Richmond BW, Du RH, et al. Postdeployment respiratory syndrome in soldiers with chronic exertional dyspnea. Am J Surg Pathol. 2021;45(12):1587-1596. doi:10.1097/PAS.0000000000001757
23. Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol. 2005;148(1-2):179-194. doi:10.1016/j.resp.2005.05.026
24. Butzko RP, Sotolongo AM, Helmer DA, et al. Forced oscillation technique in veterans with preserved spirometry and chronic respiratory symptoms. Respir Physiol Neurobiol. 2019;260:8-16. doi:10.1016/j.resp.2018.11.012
25. Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132(4):1275-1282. doi:10.1378/chest.07-0913
26. Zell-Baran LM, Krefft SD, Moore CM, Wolff J, Meehan R, Rose CS. Multiple breath washout: a noninvasive tool for identifying lung disease in symptomatic military deployers. Respir Med. 2021;176:106281. doi:10.1016/j.rmed.2020.106281
27. Krefft SD, Strand M, Smith J, Stroup C, Meehan R, Rose C. Utility of lung clearance index testing as a noninvasive marker of deployment-related lung disease. J Occup Environ Med. 2017;59(8):707-711. doi:10.1097/JOM.000000000000105828. Davis CW, Lopez CL, Bell AJ, et al. The severity of functional small airways disease in military personnel with constrictive bronchiolitis as measured by quantitative CT [published online ahead of print, 2022 May 24]. Am J Respir Crit Care Med. 2022;10.1164/rccm.202201-0153LE. doi:10.1164/rccm.202201-0153LE
Establishing a Hospital Artificial Intelligence Committee to Improve Patient Care
In the past 10 years, artificial intelligence (AI) applications have exploded in numerous fields, including medicine. Myriad publications report that the use of AI in health care is increasing, and AI has shown utility in many medical specialties, eg, pathology, radiology, and oncology.1,2
In cancer pathology, AI was able not only to detect various cancers, but also to subtype and grade them. In addition, AI could predict survival, the success of therapeutic response, and underlying mutations from histopathologic images.3 In other medical fields, AI applications are as notable. For example, in imaging specialties like radiology, ophthalmology, dermatology, and gastroenterology, AI is being used for image recognition, enhancement, and segmentation. In addition, AI is beneficial for predicting disease progression, survival, and response to therapy in other medical specialties. Finally, AI may help with administrative tasks like scheduling.
However, many obstacles to successfully implementing AI programs in the clinical setting exist, including clinical data limitations and ethical use of data, trust in the AI models, regulatory barriers, and lack of clinical buy-in due to insufficient basic AI understanding.2 To address these barriers to successful clinical AI implementation, we decided to create a formal governing body at James A. Haley Veterans’ Hospital in Tampa, Florida. Accordingly, the hospital AI committee charter was officially approved on July 22, 2021. Our model could be used by both US Department of Veterans Affairs (VA) and non-VA hospitals throughout the country.
AI Committee
The vision of the AI committee is to improve outcomes and experiences for our veterans by developing trustworthy AI capabilities to support the VA mission. The mission is to build robust capacity in AI to create and apply innovative AI solutions and transform the VA by facilitating a learning environment that supports the delivery of world-class benefits and services to our veterans. Our vision and mission are aligned with the VA National AI Institute. 4
The AI Committee comprises 7 subcommittees: ethics, AI clinical product evaluation, education, data sharing and acquisition, research, 3D printing, and improvement and innovation. The role of the ethics subcommittee is to ensure the ethical and equitable implementation of clinical AI. We created the ethics subcommittee guidelines based on the World Health Organization ethics and governance of AI for health documents.5 They include 6 basic principles: protecting human autonomy; promoting human well-being and safety and the public interest; ensuring transparency, explainability, and intelligibility; fostering responsibility and accountability; ensuring inclusiveness and equity; and promoting AI that is responsive and sustainable (Table 1).
As the name indicates, the role of the AI clinical product evaluation subcommittee is to evaluate commercially available clinical AI products. More than 400 US Food and Drug Administration–approved AI medical applications exist, and the list is growing rapidly. Most AI applications are in medical imaging like radiology, dermatology, ophthalmology, and pathology.6,7 Each clinical product is evaluated according to 6 principles: relevance, usability, risks, regulatory, technical requirements, and financial (Table 2).8 We are in the process of evaluating a few commercial AI algorithms for pathology and radiology, using these 6 principles.
Implementations
After a comprehensive evaluation, we implemented 2 ClearRead (Riverain Technologies) AI radiology solutions. ClearRead CT Vessel Suppress produces a secondary series of computed tomography (CT) images, suppressing vessels and other normal structures within the lungs to improve nodule detectability, and ClearRead Xray Bone Suppress, which increases the visibility of soft tissue in standard chest X-rays by suppressing the bone on the digital image without the need for 2 exposures.
The role of the education subcommittee is to educate the staff about AI and how it can improve patient care. Every Friday, we email an AI article of the week to our practitioners. In addition, we publish a newsletter, and we organize an annual AI conference. The first conference in 2022 included speakers from the National AI Institute, Moffitt Cancer Center, the University of South Florida, and our facility.
As the name indicates, the data sharing and acquisition subcommittee oversees preparing data for our clinical and research projects. The role of the research subcommittee is to coordinate and promote AI research with the ultimate goal of improving patient care.
Other Technologies
Although 3D printing does not fall under the umbrella of AI, we have decided to include it in our future-oriented AI committee. We created an online 3D printing course to promote the technology throughout the VA. We 3D print organ models to help surgeons prepare for complicated operations. In addition, together with our colleagues from the University of Florida, we used 3D printing to address the shortage of swabs for COVID-19 testing. The VA Sunshine Healthcare Network (Veterans Integrated Services Network 8) has an active Innovation and Improvement Committee. 9 Our improvement and innovation subcommittee serves as a coordinating body with the network committee .
Conclusions
Through the hospital AI committee, we believe that we may overcome many obstacles to successfully implementing AI applications in the clinical setting, including the ethical use of data, trust in the AI models, regulatory barriers, and lack of clinical buy-in due to insufficient basic AI knowledge.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
In the past 10 years, artificial intelligence (AI) applications have exploded in numerous fields, including medicine. Myriad publications report that the use of AI in health care is increasing, and AI has shown utility in many medical specialties, eg, pathology, radiology, and oncology.1,2
In cancer pathology, AI was able not only to detect various cancers, but also to subtype and grade them. In addition, AI could predict survival, the success of therapeutic response, and underlying mutations from histopathologic images.3 In other medical fields, AI applications are as notable. For example, in imaging specialties like radiology, ophthalmology, dermatology, and gastroenterology, AI is being used for image recognition, enhancement, and segmentation. In addition, AI is beneficial for predicting disease progression, survival, and response to therapy in other medical specialties. Finally, AI may help with administrative tasks like scheduling.
However, many obstacles to successfully implementing AI programs in the clinical setting exist, including clinical data limitations and ethical use of data, trust in the AI models, regulatory barriers, and lack of clinical buy-in due to insufficient basic AI understanding.2 To address these barriers to successful clinical AI implementation, we decided to create a formal governing body at James A. Haley Veterans’ Hospital in Tampa, Florida. Accordingly, the hospital AI committee charter was officially approved on July 22, 2021. Our model could be used by both US Department of Veterans Affairs (VA) and non-VA hospitals throughout the country.
AI Committee
The vision of the AI committee is to improve outcomes and experiences for our veterans by developing trustworthy AI capabilities to support the VA mission. The mission is to build robust capacity in AI to create and apply innovative AI solutions and transform the VA by facilitating a learning environment that supports the delivery of world-class benefits and services to our veterans. Our vision and mission are aligned with the VA National AI Institute. 4
The AI Committee comprises 7 subcommittees: ethics, AI clinical product evaluation, education, data sharing and acquisition, research, 3D printing, and improvement and innovation. The role of the ethics subcommittee is to ensure the ethical and equitable implementation of clinical AI. We created the ethics subcommittee guidelines based on the World Health Organization ethics and governance of AI for health documents.5 They include 6 basic principles: protecting human autonomy; promoting human well-being and safety and the public interest; ensuring transparency, explainability, and intelligibility; fostering responsibility and accountability; ensuring inclusiveness and equity; and promoting AI that is responsive and sustainable (Table 1).
As the name indicates, the role of the AI clinical product evaluation subcommittee is to evaluate commercially available clinical AI products. More than 400 US Food and Drug Administration–approved AI medical applications exist, and the list is growing rapidly. Most AI applications are in medical imaging like radiology, dermatology, ophthalmology, and pathology.6,7 Each clinical product is evaluated according to 6 principles: relevance, usability, risks, regulatory, technical requirements, and financial (Table 2).8 We are in the process of evaluating a few commercial AI algorithms for pathology and radiology, using these 6 principles.
Implementations
After a comprehensive evaluation, we implemented 2 ClearRead (Riverain Technologies) AI radiology solutions. ClearRead CT Vessel Suppress produces a secondary series of computed tomography (CT) images, suppressing vessels and other normal structures within the lungs to improve nodule detectability, and ClearRead Xray Bone Suppress, which increases the visibility of soft tissue in standard chest X-rays by suppressing the bone on the digital image without the need for 2 exposures.
The role of the education subcommittee is to educate the staff about AI and how it can improve patient care. Every Friday, we email an AI article of the week to our practitioners. In addition, we publish a newsletter, and we organize an annual AI conference. The first conference in 2022 included speakers from the National AI Institute, Moffitt Cancer Center, the University of South Florida, and our facility.
As the name indicates, the data sharing and acquisition subcommittee oversees preparing data for our clinical and research projects. The role of the research subcommittee is to coordinate and promote AI research with the ultimate goal of improving patient care.
Other Technologies
Although 3D printing does not fall under the umbrella of AI, we have decided to include it in our future-oriented AI committee. We created an online 3D printing course to promote the technology throughout the VA. We 3D print organ models to help surgeons prepare for complicated operations. In addition, together with our colleagues from the University of Florida, we used 3D printing to address the shortage of swabs for COVID-19 testing. The VA Sunshine Healthcare Network (Veterans Integrated Services Network 8) has an active Innovation and Improvement Committee. 9 Our improvement and innovation subcommittee serves as a coordinating body with the network committee .
Conclusions
Through the hospital AI committee, we believe that we may overcome many obstacles to successfully implementing AI applications in the clinical setting, including the ethical use of data, trust in the AI models, regulatory barriers, and lack of clinical buy-in due to insufficient basic AI knowledge.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
In the past 10 years, artificial intelligence (AI) applications have exploded in numerous fields, including medicine. Myriad publications report that the use of AI in health care is increasing, and AI has shown utility in many medical specialties, eg, pathology, radiology, and oncology.1,2
In cancer pathology, AI was able not only to detect various cancers, but also to subtype and grade them. In addition, AI could predict survival, the success of therapeutic response, and underlying mutations from histopathologic images.3 In other medical fields, AI applications are as notable. For example, in imaging specialties like radiology, ophthalmology, dermatology, and gastroenterology, AI is being used for image recognition, enhancement, and segmentation. In addition, AI is beneficial for predicting disease progression, survival, and response to therapy in other medical specialties. Finally, AI may help with administrative tasks like scheduling.
However, many obstacles to successfully implementing AI programs in the clinical setting exist, including clinical data limitations and ethical use of data, trust in the AI models, regulatory barriers, and lack of clinical buy-in due to insufficient basic AI understanding.2 To address these barriers to successful clinical AI implementation, we decided to create a formal governing body at James A. Haley Veterans’ Hospital in Tampa, Florida. Accordingly, the hospital AI committee charter was officially approved on July 22, 2021. Our model could be used by both US Department of Veterans Affairs (VA) and non-VA hospitals throughout the country.
AI Committee
The vision of the AI committee is to improve outcomes and experiences for our veterans by developing trustworthy AI capabilities to support the VA mission. The mission is to build robust capacity in AI to create and apply innovative AI solutions and transform the VA by facilitating a learning environment that supports the delivery of world-class benefits and services to our veterans. Our vision and mission are aligned with the VA National AI Institute. 4
The AI Committee comprises 7 subcommittees: ethics, AI clinical product evaluation, education, data sharing and acquisition, research, 3D printing, and improvement and innovation. The role of the ethics subcommittee is to ensure the ethical and equitable implementation of clinical AI. We created the ethics subcommittee guidelines based on the World Health Organization ethics and governance of AI for health documents.5 They include 6 basic principles: protecting human autonomy; promoting human well-being and safety and the public interest; ensuring transparency, explainability, and intelligibility; fostering responsibility and accountability; ensuring inclusiveness and equity; and promoting AI that is responsive and sustainable (Table 1).
As the name indicates, the role of the AI clinical product evaluation subcommittee is to evaluate commercially available clinical AI products. More than 400 US Food and Drug Administration–approved AI medical applications exist, and the list is growing rapidly. Most AI applications are in medical imaging like radiology, dermatology, ophthalmology, and pathology.6,7 Each clinical product is evaluated according to 6 principles: relevance, usability, risks, regulatory, technical requirements, and financial (Table 2).8 We are in the process of evaluating a few commercial AI algorithms for pathology and radiology, using these 6 principles.
Implementations
After a comprehensive evaluation, we implemented 2 ClearRead (Riverain Technologies) AI radiology solutions. ClearRead CT Vessel Suppress produces a secondary series of computed tomography (CT) images, suppressing vessels and other normal structures within the lungs to improve nodule detectability, and ClearRead Xray Bone Suppress, which increases the visibility of soft tissue in standard chest X-rays by suppressing the bone on the digital image without the need for 2 exposures.
The role of the education subcommittee is to educate the staff about AI and how it can improve patient care. Every Friday, we email an AI article of the week to our practitioners. In addition, we publish a newsletter, and we organize an annual AI conference. The first conference in 2022 included speakers from the National AI Institute, Moffitt Cancer Center, the University of South Florida, and our facility.
As the name indicates, the data sharing and acquisition subcommittee oversees preparing data for our clinical and research projects. The role of the research subcommittee is to coordinate and promote AI research with the ultimate goal of improving patient care.
Other Technologies
Although 3D printing does not fall under the umbrella of AI, we have decided to include it in our future-oriented AI committee. We created an online 3D printing course to promote the technology throughout the VA. We 3D print organ models to help surgeons prepare for complicated operations. In addition, together with our colleagues from the University of Florida, we used 3D printing to address the shortage of swabs for COVID-19 testing. The VA Sunshine Healthcare Network (Veterans Integrated Services Network 8) has an active Innovation and Improvement Committee. 9 Our improvement and innovation subcommittee serves as a coordinating body with the network committee .
Conclusions
Through the hospital AI committee, we believe that we may overcome many obstacles to successfully implementing AI applications in the clinical setting, including the ethical use of data, trust in the AI models, regulatory barriers, and lack of clinical buy-in due to insufficient basic AI knowledge.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
HCV reinfection uncommon among people who inject drugs
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Underweight in early childhood persists
The association was most pronounced for girls, as well as for children with lower growth rates, write the authors of the prospective Canadian cohort study published in JAMA Network Open.
The findings “highlight the importance of preventing underweight in early life,” because this can have “lasting effects” in later childhood, senior author Jonathon L. Maguire, MD, from St Michael’s Hospital Pediatric Clinic, and the University of Toronto said in an interview.
Methods and results
The study recruited 5,803 healthy children, mean age 4.07 months, between February 2008 and September 2020 during well-child visits at clinics in The Applied Research Group for Kids! (TARGet Kids!) practice-based research network in Canada. The study’s exclusion criteria included a premature birth, or a health condition affecting growth.
The primary outcome of the study was the child’s age- and sex-adjusted weight, also known as the body mass index z score (zBMI), between the ages of 2 and 10 years.
At baseline, a total of 550 children (9.5%) were classified as underweight, based on the World Health Organization definition of zBMI less than –2. Underweight children were more likely to be younger, have lower birth weight, and to report Asian maternal ethnicity, the researchers observed.
The study found that, compared with children with normal weight, those who were underweight in the first 2 years had lower zBMI at ages 5 and 10 years (–0.49 and –0.39 respectively). This meant that at 10 years old, they were a mean of 1.23 kg lighter than 10-year-olds who had been normal weight at age 2 years.
Height-for-age z score (HAZ) was also lower for underweight 2-year-olds (–0.24), making them a mean of 0.68 cm shorter than normal-weight 2-year-olds. This difference was attenuated at age 5 years.
Growth rate modified the association of underweight with both zBMI and HAZ. Among children who were underweight in the first 2 years, those with lower growth rate had lower zBMI at 10 years (–0.64) compared with those with average (–0.38) or high growth rate (0.11). Similarly, children who were underweight and had a lower growth rate at age 2 years also a lower HAZ at age 10 years (–0.12), compared with those with average (0.02) or high growth rates (0.16). These effects were more pronounced in girls.
Increased health risks linked with chronic underweight
This study did not assess the reasons for early underweight, Dr. Maguire commented in an interview. But, he cited challenges with dietary transitions as a possible explanation.
“Considerable dietary changes happen around 2 years of age with increasing diversity of foods as children transition from primarily liquid foods to primarily solid foods,” he noted.
Asked for comment on the study, Colleen Spees, PhD, associate professor in the division of medical dietetics and director of Hope lab at the Ohio State University, Columbus, said that “at age 10, it’s not surprising to see a lower zBMI and height-for-age in those that were underweight at age 2 with poor growth trajectories.”
Although, this is the first study she is aware of to document these findings in a Canadian cohort, “the results align with what we know about low birth weight and underweight infants and children in terms of linear growth trajectories from child stunting studies,” Dr. Spees said.
She said child stunting, which is more common in less developed countries where children have lower birth weights and greater socioeconomic and environmental risk factors, is defined by the WHO as impaired linear growth with adverse functional consequences.
“In short, a chronic underweight status in infants and young children can lead to greater risk of malnutrition, vitamin and mineral deficiencies, decreased immune function, as well as physical growth and development issues,” she said. “Hence, the most recent 2020-2025 Dietary Guidelines for Americans now includes both pregnancy, breastfeeding, and the first 2 years of life (referred to as the “first 1,000 days”) in their recommendations.”
She added that, if caregivers are concerned about their child’s weight, they should consult with their pediatrician to rule out any medical issues. If no medical issues are identified, they should ask for a referral to a pediatric dietitian.
The study was funded by the Canadian Institute of Health Research. Dr Maguire reported receiving grants from the CIHR, Physician Services, Ontario SPOR Support Unit, and Dairy Farmers of Canada during the conduct of the study and nonfinancial support from DDrops outside the submitted work. Other authors of the paper reported receiving grants from various institutions. Dr. Spees reported no relevant disclosures.
The association was most pronounced for girls, as well as for children with lower growth rates, write the authors of the prospective Canadian cohort study published in JAMA Network Open.
The findings “highlight the importance of preventing underweight in early life,” because this can have “lasting effects” in later childhood, senior author Jonathon L. Maguire, MD, from St Michael’s Hospital Pediatric Clinic, and the University of Toronto said in an interview.
Methods and results
The study recruited 5,803 healthy children, mean age 4.07 months, between February 2008 and September 2020 during well-child visits at clinics in The Applied Research Group for Kids! (TARGet Kids!) practice-based research network in Canada. The study’s exclusion criteria included a premature birth, or a health condition affecting growth.
The primary outcome of the study was the child’s age- and sex-adjusted weight, also known as the body mass index z score (zBMI), between the ages of 2 and 10 years.
At baseline, a total of 550 children (9.5%) were classified as underweight, based on the World Health Organization definition of zBMI less than –2. Underweight children were more likely to be younger, have lower birth weight, and to report Asian maternal ethnicity, the researchers observed.
The study found that, compared with children with normal weight, those who were underweight in the first 2 years had lower zBMI at ages 5 and 10 years (–0.49 and –0.39 respectively). This meant that at 10 years old, they were a mean of 1.23 kg lighter than 10-year-olds who had been normal weight at age 2 years.
Height-for-age z score (HAZ) was also lower for underweight 2-year-olds (–0.24), making them a mean of 0.68 cm shorter than normal-weight 2-year-olds. This difference was attenuated at age 5 years.
Growth rate modified the association of underweight with both zBMI and HAZ. Among children who were underweight in the first 2 years, those with lower growth rate had lower zBMI at 10 years (–0.64) compared with those with average (–0.38) or high growth rate (0.11). Similarly, children who were underweight and had a lower growth rate at age 2 years also a lower HAZ at age 10 years (–0.12), compared with those with average (0.02) or high growth rates (0.16). These effects were more pronounced in girls.
Increased health risks linked with chronic underweight
This study did not assess the reasons for early underweight, Dr. Maguire commented in an interview. But, he cited challenges with dietary transitions as a possible explanation.
“Considerable dietary changes happen around 2 years of age with increasing diversity of foods as children transition from primarily liquid foods to primarily solid foods,” he noted.
Asked for comment on the study, Colleen Spees, PhD, associate professor in the division of medical dietetics and director of Hope lab at the Ohio State University, Columbus, said that “at age 10, it’s not surprising to see a lower zBMI and height-for-age in those that were underweight at age 2 with poor growth trajectories.”
Although, this is the first study she is aware of to document these findings in a Canadian cohort, “the results align with what we know about low birth weight and underweight infants and children in terms of linear growth trajectories from child stunting studies,” Dr. Spees said.
She said child stunting, which is more common in less developed countries where children have lower birth weights and greater socioeconomic and environmental risk factors, is defined by the WHO as impaired linear growth with adverse functional consequences.
“In short, a chronic underweight status in infants and young children can lead to greater risk of malnutrition, vitamin and mineral deficiencies, decreased immune function, as well as physical growth and development issues,” she said. “Hence, the most recent 2020-2025 Dietary Guidelines for Americans now includes both pregnancy, breastfeeding, and the first 2 years of life (referred to as the “first 1,000 days”) in their recommendations.”
She added that, if caregivers are concerned about their child’s weight, they should consult with their pediatrician to rule out any medical issues. If no medical issues are identified, they should ask for a referral to a pediatric dietitian.
The study was funded by the Canadian Institute of Health Research. Dr Maguire reported receiving grants from the CIHR, Physician Services, Ontario SPOR Support Unit, and Dairy Farmers of Canada during the conduct of the study and nonfinancial support from DDrops outside the submitted work. Other authors of the paper reported receiving grants from various institutions. Dr. Spees reported no relevant disclosures.
The association was most pronounced for girls, as well as for children with lower growth rates, write the authors of the prospective Canadian cohort study published in JAMA Network Open.
The findings “highlight the importance of preventing underweight in early life,” because this can have “lasting effects” in later childhood, senior author Jonathon L. Maguire, MD, from St Michael’s Hospital Pediatric Clinic, and the University of Toronto said in an interview.
Methods and results
The study recruited 5,803 healthy children, mean age 4.07 months, between February 2008 and September 2020 during well-child visits at clinics in The Applied Research Group for Kids! (TARGet Kids!) practice-based research network in Canada. The study’s exclusion criteria included a premature birth, or a health condition affecting growth.
The primary outcome of the study was the child’s age- and sex-adjusted weight, also known as the body mass index z score (zBMI), between the ages of 2 and 10 years.
At baseline, a total of 550 children (9.5%) were classified as underweight, based on the World Health Organization definition of zBMI less than –2. Underweight children were more likely to be younger, have lower birth weight, and to report Asian maternal ethnicity, the researchers observed.
The study found that, compared with children with normal weight, those who were underweight in the first 2 years had lower zBMI at ages 5 and 10 years (–0.49 and –0.39 respectively). This meant that at 10 years old, they were a mean of 1.23 kg lighter than 10-year-olds who had been normal weight at age 2 years.
Height-for-age z score (HAZ) was also lower for underweight 2-year-olds (–0.24), making them a mean of 0.68 cm shorter than normal-weight 2-year-olds. This difference was attenuated at age 5 years.
Growth rate modified the association of underweight with both zBMI and HAZ. Among children who were underweight in the first 2 years, those with lower growth rate had lower zBMI at 10 years (–0.64) compared with those with average (–0.38) or high growth rate (0.11). Similarly, children who were underweight and had a lower growth rate at age 2 years also a lower HAZ at age 10 years (–0.12), compared with those with average (0.02) or high growth rates (0.16). These effects were more pronounced in girls.
Increased health risks linked with chronic underweight
This study did not assess the reasons for early underweight, Dr. Maguire commented in an interview. But, he cited challenges with dietary transitions as a possible explanation.
“Considerable dietary changes happen around 2 years of age with increasing diversity of foods as children transition from primarily liquid foods to primarily solid foods,” he noted.
Asked for comment on the study, Colleen Spees, PhD, associate professor in the division of medical dietetics and director of Hope lab at the Ohio State University, Columbus, said that “at age 10, it’s not surprising to see a lower zBMI and height-for-age in those that were underweight at age 2 with poor growth trajectories.”
Although, this is the first study she is aware of to document these findings in a Canadian cohort, “the results align with what we know about low birth weight and underweight infants and children in terms of linear growth trajectories from child stunting studies,” Dr. Spees said.
She said child stunting, which is more common in less developed countries where children have lower birth weights and greater socioeconomic and environmental risk factors, is defined by the WHO as impaired linear growth with adverse functional consequences.
“In short, a chronic underweight status in infants and young children can lead to greater risk of malnutrition, vitamin and mineral deficiencies, decreased immune function, as well as physical growth and development issues,” she said. “Hence, the most recent 2020-2025 Dietary Guidelines for Americans now includes both pregnancy, breastfeeding, and the first 2 years of life (referred to as the “first 1,000 days”) in their recommendations.”
She added that, if caregivers are concerned about their child’s weight, they should consult with their pediatrician to rule out any medical issues. If no medical issues are identified, they should ask for a referral to a pediatric dietitian.
The study was funded by the Canadian Institute of Health Research. Dr Maguire reported receiving grants from the CIHR, Physician Services, Ontario SPOR Support Unit, and Dairy Farmers of Canada during the conduct of the study and nonfinancial support from DDrops outside the submitted work. Other authors of the paper reported receiving grants from various institutions. Dr. Spees reported no relevant disclosures.
FROM JAMA NETWORK OPEN