User login
Stressed about weight gain? Well, stress causes weight gain
Stress, meet weight gain. Weight gain, meet stress
You’re not eating differently and you’re keeping active, but your waistline is expanding. How is that happening? Since eating healthy and exercising shouldn’t make you gain weight, there may be a hidden factor getting in your way. Stress. The one thing that can have a grip on your circadian rhythm stronger than any bodybuilder.
Investigators at Weill Cornell Medicine published two mouse studies that suggest stress and other factors that throw the body’s circadian clocks out of rhythm may contribute to weight gain.
In the first study, the researchers imitated disruptive condition effects like high cortisol exposure and chronic stress by implanting pellets under the skin that released glucocorticoid at a constant rate for 21 days. Mice that received the pellets had twice as much white and brown fat, as well as much higher insulin levels, regardless of their unchanged and still-healthy diet.
In the second study, they used tagged proteins as markers to monitor the daily fluctuations of a protein that regulates fat cell production and circadian gene expression in mouse fat cell precursors. The results showed “that fat cell precursors commit to becoming fat cells only during the circadian cycle phase corresponding to evening in humans,” they said in a written statement.
“Every cell in our body has an intrinsic cell clock, just like the fat cells, and we have a master clock in our brain, which controls hormone secretion,” said senior author Mary Teruel of Cornell University. “A lot of forces are working against a healthy metabolism when we are out of circadian rhythm. The more we understand, the more likely we will be able to do something about it.”
So if you’re stressing out that the scale is or isn’t moving in the direction you want, you could be standing in your own way. Take a chill pill.
Who can smell cancer? The locust nose
If you need to smell some gas, there’s nothing better than a nose. Just ask a scientist: “Noses are still state of the art,” said Debajit Saha, PhD, of Michigan State University. “There’s really nothing like them when it comes to gas sensing.”
And when it comes to noses, dogs are best, right? After all, there’s a reason we don’t have bomb-sniffing wombats and drug-sniffing ostriches. Dogs are better. Better, but not perfect. And if they’re not perfect, then human technology can do better.
Enter the electronic nose. Which is better than dogs … except that it isn’t. “People have been working on ‘electronic noses’ for more than 15 years, but they’re still not close to achieving what biology can do seamlessly,” Dr. Saha explained in a statement from the university.
Which brings us back to dogs. If you want to detect early-stage cancer using smell, you go to the dogs, right? Nope.
Here’s Christopher Contag, PhD, also of Michigan State, who recruited Dr. Saha to the university: “I told him, ‘When you come here, we’ll detect cancer. I’m sure your locusts can do it.’ ”
Yes, locusts. Dr. Contag and his research team were looking at mouth cancers and noticed that different cell lines had different appearances. Then they discovered that those different-looking cell lines produced different metabolites, some of which were volatile.
Enter Dr. Saha’s locusts. They were able to tell the difference between normal cells and cancer cells and could even distinguish between the different cell lines. And how they were able to share this information? Not voluntarily, that’s for sure. The researchers attached electrodes to the insects’ brains and recorded their responses to gas samples from both healthy and cancer cells. Those brain signals were then used to create chemical profiles of the different cells. Piece of cake.
The whole getting-electrodes-attached-to-their-brains thing seemed at least a bit ethically ambiguous, so we contacted the locusts’ PR office, which offered some positive spin: “Humans get their early cancer detection and we get that whole swarms-that-devour-entire-countrysides thing off our backs. Win win.”
Bad news for vampires everywhere
Pop culture has been extraordinarily kind to the vampire. A few hundred years ago, vampires were demon-possessed, often-inhuman monsters. Now? They’re suave, sophisticated, beautiful, and oh-so dramatic and angst-filled about their “curse.” Drink a little human blood, live and look young forever. Such monsters they are.
It does make sense in a morbid sort of way. An old person receiving the blood of the young does seem like a good idea for rejuvenation, right? A team of Ukrainian researchers sought to find out, conducting a study in which older mice were linked with young mice via heterochronic parabiosis. For 3 months, old-young mice pairs were surgically connected and shared blood. After 3 months, the mice were disconnected from each other and the effects of the blood link were studied.
For all the vampire enthusiasts out there, we have bad news and worse news. The bad news first: The older mice received absolutely no benefit from heterochronic parabiosis. No youthfulness, no increased lifespan, nothing. The worse news is that the younger mice were adversely affected by the older blood. They aged more and experienced a shortened lifespan, even after the connection was severed. The old blood, according to the investigators, contains factors capable of inducing aging in younger mice, but the opposite is not true. Further research into aging, they added, should focus on suppressing the aging factors in older blood.
Of note, the paper was written by doctors who are currently refugees, fleeing the war in Ukraine. We don’t want to speculate on the true cause of the war, but we’re onto you, Putin. We know you wanted the vampire research for yourself, but it won’t work. Your dream of becoming Vlad “Dracula” Putin will never come to pass.
Hearing is not always believing
Have you ever heard yourself on a voice mail, or from a recording you did at work? No matter how good you sound, you still might feel like the recording sounds nothing like you. It may even cause low self-esteem for those who don’t like how their voice sounds or don’t recognize it when it’s played back to them.
Since one possible symptom of schizophrenia is not recognizing one’s own speech and having a false sense of control over actions, and those with schizophrenia may hallucinate or hear voices, not being able to recognize their own voices may be alarming.
A recent study on the sense of agency, or sense of control, involved having volunteers speak with different pitches in their voices and then having it played back to them to gauge their reactions.
“Our results demonstrate that hearing one’s own voice is a critical factor to increased self-agency over speech. In other words, we do not strongly feel that ‘I’ am generating the speech if we hear someone else’s voice as an outcome of the speech. Our study provides empirical evidence of the tight link between the sense of agency and self-voice identity,” lead author Ryu Ohata, PhD, of the University of Tokyo, said in a written statement.
As social interaction becomes more digital through platforms such as FaceTime, Zoom, and voicemail, especially since the pandemic has promoted social distancing, it makes sense that people may be more aware and more surprised by how they sound on recordings.
So, if you ever promised someone something that you don’t want to do, and they play it back to you from the recording you made, maybe you can just say you don’t recognize the voice. And if it’s not you, then you don’t have to do it.
Stress, meet weight gain. Weight gain, meet stress
You’re not eating differently and you’re keeping active, but your waistline is expanding. How is that happening? Since eating healthy and exercising shouldn’t make you gain weight, there may be a hidden factor getting in your way. Stress. The one thing that can have a grip on your circadian rhythm stronger than any bodybuilder.
Investigators at Weill Cornell Medicine published two mouse studies that suggest stress and other factors that throw the body’s circadian clocks out of rhythm may contribute to weight gain.
In the first study, the researchers imitated disruptive condition effects like high cortisol exposure and chronic stress by implanting pellets under the skin that released glucocorticoid at a constant rate for 21 days. Mice that received the pellets had twice as much white and brown fat, as well as much higher insulin levels, regardless of their unchanged and still-healthy diet.
In the second study, they used tagged proteins as markers to monitor the daily fluctuations of a protein that regulates fat cell production and circadian gene expression in mouse fat cell precursors. The results showed “that fat cell precursors commit to becoming fat cells only during the circadian cycle phase corresponding to evening in humans,” they said in a written statement.
“Every cell in our body has an intrinsic cell clock, just like the fat cells, and we have a master clock in our brain, which controls hormone secretion,” said senior author Mary Teruel of Cornell University. “A lot of forces are working against a healthy metabolism when we are out of circadian rhythm. The more we understand, the more likely we will be able to do something about it.”
So if you’re stressing out that the scale is or isn’t moving in the direction you want, you could be standing in your own way. Take a chill pill.
Who can smell cancer? The locust nose
If you need to smell some gas, there’s nothing better than a nose. Just ask a scientist: “Noses are still state of the art,” said Debajit Saha, PhD, of Michigan State University. “There’s really nothing like them when it comes to gas sensing.”
And when it comes to noses, dogs are best, right? After all, there’s a reason we don’t have bomb-sniffing wombats and drug-sniffing ostriches. Dogs are better. Better, but not perfect. And if they’re not perfect, then human technology can do better.
Enter the electronic nose. Which is better than dogs … except that it isn’t. “People have been working on ‘electronic noses’ for more than 15 years, but they’re still not close to achieving what biology can do seamlessly,” Dr. Saha explained in a statement from the university.
Which brings us back to dogs. If you want to detect early-stage cancer using smell, you go to the dogs, right? Nope.
Here’s Christopher Contag, PhD, also of Michigan State, who recruited Dr. Saha to the university: “I told him, ‘When you come here, we’ll detect cancer. I’m sure your locusts can do it.’ ”
Yes, locusts. Dr. Contag and his research team were looking at mouth cancers and noticed that different cell lines had different appearances. Then they discovered that those different-looking cell lines produced different metabolites, some of which were volatile.
Enter Dr. Saha’s locusts. They were able to tell the difference between normal cells and cancer cells and could even distinguish between the different cell lines. And how they were able to share this information? Not voluntarily, that’s for sure. The researchers attached electrodes to the insects’ brains and recorded their responses to gas samples from both healthy and cancer cells. Those brain signals were then used to create chemical profiles of the different cells. Piece of cake.
The whole getting-electrodes-attached-to-their-brains thing seemed at least a bit ethically ambiguous, so we contacted the locusts’ PR office, which offered some positive spin: “Humans get their early cancer detection and we get that whole swarms-that-devour-entire-countrysides thing off our backs. Win win.”
Bad news for vampires everywhere
Pop culture has been extraordinarily kind to the vampire. A few hundred years ago, vampires were demon-possessed, often-inhuman monsters. Now? They’re suave, sophisticated, beautiful, and oh-so dramatic and angst-filled about their “curse.” Drink a little human blood, live and look young forever. Such monsters they are.
It does make sense in a morbid sort of way. An old person receiving the blood of the young does seem like a good idea for rejuvenation, right? A team of Ukrainian researchers sought to find out, conducting a study in which older mice were linked with young mice via heterochronic parabiosis. For 3 months, old-young mice pairs were surgically connected and shared blood. After 3 months, the mice were disconnected from each other and the effects of the blood link were studied.
For all the vampire enthusiasts out there, we have bad news and worse news. The bad news first: The older mice received absolutely no benefit from heterochronic parabiosis. No youthfulness, no increased lifespan, nothing. The worse news is that the younger mice were adversely affected by the older blood. They aged more and experienced a shortened lifespan, even after the connection was severed. The old blood, according to the investigators, contains factors capable of inducing aging in younger mice, but the opposite is not true. Further research into aging, they added, should focus on suppressing the aging factors in older blood.
Of note, the paper was written by doctors who are currently refugees, fleeing the war in Ukraine. We don’t want to speculate on the true cause of the war, but we’re onto you, Putin. We know you wanted the vampire research for yourself, but it won’t work. Your dream of becoming Vlad “Dracula” Putin will never come to pass.
Hearing is not always believing
Have you ever heard yourself on a voice mail, or from a recording you did at work? No matter how good you sound, you still might feel like the recording sounds nothing like you. It may even cause low self-esteem for those who don’t like how their voice sounds or don’t recognize it when it’s played back to them.
Since one possible symptom of schizophrenia is not recognizing one’s own speech and having a false sense of control over actions, and those with schizophrenia may hallucinate or hear voices, not being able to recognize their own voices may be alarming.
A recent study on the sense of agency, or sense of control, involved having volunteers speak with different pitches in their voices and then having it played back to them to gauge their reactions.
“Our results demonstrate that hearing one’s own voice is a critical factor to increased self-agency over speech. In other words, we do not strongly feel that ‘I’ am generating the speech if we hear someone else’s voice as an outcome of the speech. Our study provides empirical evidence of the tight link between the sense of agency and self-voice identity,” lead author Ryu Ohata, PhD, of the University of Tokyo, said in a written statement.
As social interaction becomes more digital through platforms such as FaceTime, Zoom, and voicemail, especially since the pandemic has promoted social distancing, it makes sense that people may be more aware and more surprised by how they sound on recordings.
So, if you ever promised someone something that you don’t want to do, and they play it back to you from the recording you made, maybe you can just say you don’t recognize the voice. And if it’s not you, then you don’t have to do it.
Stress, meet weight gain. Weight gain, meet stress
You’re not eating differently and you’re keeping active, but your waistline is expanding. How is that happening? Since eating healthy and exercising shouldn’t make you gain weight, there may be a hidden factor getting in your way. Stress. The one thing that can have a grip on your circadian rhythm stronger than any bodybuilder.
Investigators at Weill Cornell Medicine published two mouse studies that suggest stress and other factors that throw the body’s circadian clocks out of rhythm may contribute to weight gain.
In the first study, the researchers imitated disruptive condition effects like high cortisol exposure and chronic stress by implanting pellets under the skin that released glucocorticoid at a constant rate for 21 days. Mice that received the pellets had twice as much white and brown fat, as well as much higher insulin levels, regardless of their unchanged and still-healthy diet.
In the second study, they used tagged proteins as markers to monitor the daily fluctuations of a protein that regulates fat cell production and circadian gene expression in mouse fat cell precursors. The results showed “that fat cell precursors commit to becoming fat cells only during the circadian cycle phase corresponding to evening in humans,” they said in a written statement.
“Every cell in our body has an intrinsic cell clock, just like the fat cells, and we have a master clock in our brain, which controls hormone secretion,” said senior author Mary Teruel of Cornell University. “A lot of forces are working against a healthy metabolism when we are out of circadian rhythm. The more we understand, the more likely we will be able to do something about it.”
So if you’re stressing out that the scale is or isn’t moving in the direction you want, you could be standing in your own way. Take a chill pill.
Who can smell cancer? The locust nose
If you need to smell some gas, there’s nothing better than a nose. Just ask a scientist: “Noses are still state of the art,” said Debajit Saha, PhD, of Michigan State University. “There’s really nothing like them when it comes to gas sensing.”
And when it comes to noses, dogs are best, right? After all, there’s a reason we don’t have bomb-sniffing wombats and drug-sniffing ostriches. Dogs are better. Better, but not perfect. And if they’re not perfect, then human technology can do better.
Enter the electronic nose. Which is better than dogs … except that it isn’t. “People have been working on ‘electronic noses’ for more than 15 years, but they’re still not close to achieving what biology can do seamlessly,” Dr. Saha explained in a statement from the university.
Which brings us back to dogs. If you want to detect early-stage cancer using smell, you go to the dogs, right? Nope.
Here’s Christopher Contag, PhD, also of Michigan State, who recruited Dr. Saha to the university: “I told him, ‘When you come here, we’ll detect cancer. I’m sure your locusts can do it.’ ”
Yes, locusts. Dr. Contag and his research team were looking at mouth cancers and noticed that different cell lines had different appearances. Then they discovered that those different-looking cell lines produced different metabolites, some of which were volatile.
Enter Dr. Saha’s locusts. They were able to tell the difference between normal cells and cancer cells and could even distinguish between the different cell lines. And how they were able to share this information? Not voluntarily, that’s for sure. The researchers attached electrodes to the insects’ brains and recorded their responses to gas samples from both healthy and cancer cells. Those brain signals were then used to create chemical profiles of the different cells. Piece of cake.
The whole getting-electrodes-attached-to-their-brains thing seemed at least a bit ethically ambiguous, so we contacted the locusts’ PR office, which offered some positive spin: “Humans get their early cancer detection and we get that whole swarms-that-devour-entire-countrysides thing off our backs. Win win.”
Bad news for vampires everywhere
Pop culture has been extraordinarily kind to the vampire. A few hundred years ago, vampires were demon-possessed, often-inhuman monsters. Now? They’re suave, sophisticated, beautiful, and oh-so dramatic and angst-filled about their “curse.” Drink a little human blood, live and look young forever. Such monsters they are.
It does make sense in a morbid sort of way. An old person receiving the blood of the young does seem like a good idea for rejuvenation, right? A team of Ukrainian researchers sought to find out, conducting a study in which older mice were linked with young mice via heterochronic parabiosis. For 3 months, old-young mice pairs were surgically connected and shared blood. After 3 months, the mice were disconnected from each other and the effects of the blood link were studied.
For all the vampire enthusiasts out there, we have bad news and worse news. The bad news first: The older mice received absolutely no benefit from heterochronic parabiosis. No youthfulness, no increased lifespan, nothing. The worse news is that the younger mice were adversely affected by the older blood. They aged more and experienced a shortened lifespan, even after the connection was severed. The old blood, according to the investigators, contains factors capable of inducing aging in younger mice, but the opposite is not true. Further research into aging, they added, should focus on suppressing the aging factors in older blood.
Of note, the paper was written by doctors who are currently refugees, fleeing the war in Ukraine. We don’t want to speculate on the true cause of the war, but we’re onto you, Putin. We know you wanted the vampire research for yourself, but it won’t work. Your dream of becoming Vlad “Dracula” Putin will never come to pass.
Hearing is not always believing
Have you ever heard yourself on a voice mail, or from a recording you did at work? No matter how good you sound, you still might feel like the recording sounds nothing like you. It may even cause low self-esteem for those who don’t like how their voice sounds or don’t recognize it when it’s played back to them.
Since one possible symptom of schizophrenia is not recognizing one’s own speech and having a false sense of control over actions, and those with schizophrenia may hallucinate or hear voices, not being able to recognize their own voices may be alarming.
A recent study on the sense of agency, or sense of control, involved having volunteers speak with different pitches in their voices and then having it played back to them to gauge their reactions.
“Our results demonstrate that hearing one’s own voice is a critical factor to increased self-agency over speech. In other words, we do not strongly feel that ‘I’ am generating the speech if we hear someone else’s voice as an outcome of the speech. Our study provides empirical evidence of the tight link between the sense of agency and self-voice identity,” lead author Ryu Ohata, PhD, of the University of Tokyo, said in a written statement.
As social interaction becomes more digital through platforms such as FaceTime, Zoom, and voicemail, especially since the pandemic has promoted social distancing, it makes sense that people may be more aware and more surprised by how they sound on recordings.
So, if you ever promised someone something that you don’t want to do, and they play it back to you from the recording you made, maybe you can just say you don’t recognize the voice. And if it’s not you, then you don’t have to do it.
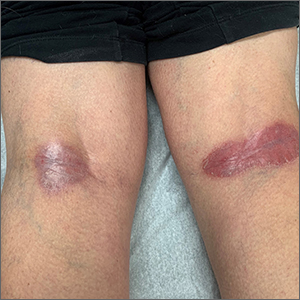
Popliteal plaques
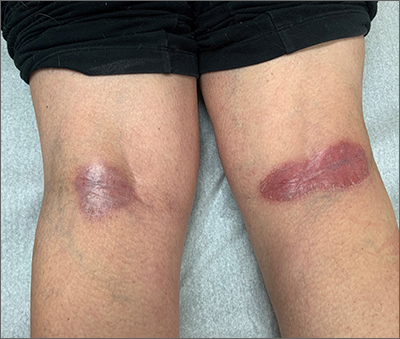
Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733

Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
Plasma biomarkers predict COVID’s neurological sequelae
SAN DIEGO – Even after recovery of an acute COVID-19 infection, some patients experience extended or even long-term symptoms that can range from mild to debilitating. Some of these symptoms are neurological: headaches, brain fog, cognitive impairment, loss of taste or smell, and even cerebrovascular complications such stroke. There are even hints that COVID-19 infection could lead to future neurodegeneration.
Those issues have prompted efforts to identify biomarkers that can help track and monitor neurological complications of COVID-19. “Throughout the course of the pandemic, it has become apparent that COVID-19 can cause various neurological symptoms. Because of this, ,” Jennifer Cooper said during a lecture at the Alzheimer’s Association International Conference. She presented new research suggesting that neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) may prove useful.
Ms. Cooper is a master’s degree student at the University of British Columbia and Canada.
Looking for sensitivity and specificity in plasma biomarkers
The researchers turned to plasma-based markers because they can reflect underlying pathology in the central nervous system. They focused on NfL, which reflects axonal damage, and GFAP, which is a marker of astrocyte activation.
The researchers analyzed data from 209 patients with COVID-19 who were admitted to the Vancouver (B.C.) General Hospital intensive care unit. Sixty-four percent were male, and the median age was 61 years. Sixty percent were ventilated, and 17% died.
The researchers determined if an individual patient’s biomarker level at hospital admission fell within a normal biomarker reference interval. A total of 53% had NfL levels outside the normal range, and 42% had GFAP levels outside the normal range. In addition, 31% of patients had both GFAP and NfL levels outside of the normal range.
Among all patients, 12% experienced ischemia, 4% hemorrhage, 2% seizures, and 10% degeneration.
At admission, NfL predicted a neurological complication with an area under the curve (AUC) of 0.702. GFAP had an AUC of 0.722. In combination, they had an AUC of 0.743. At 1 week, NfL had an AUC of 0.802, GFAP an AUC of 0.733, and the combination an AUC of 0.812.
Using age-specific cutoff values, the researchers found increased risks for neurological complications at admission (NfL odds ratio [OR], 2.9; GFAP OR, 1.6; combined OR, 2.1) and at 1 week (NfL OR, not significant; GFAP OR, 4.8; combined OR, 6.6). “We can see that both NFL and GFAP have utility in detecting neurological complications. And combining both of our markers improves detection at both time points. NfL is a marker that provides more sensitivity, where in this cohort GFAP is a marker that provides a little bit more specificity,” said Ms. Cooper.
Will additional biomarkers help?
The researchers are continuing to follow up patients at 6 months and 18 months post diagnosis, using neuropsychiatric tests and additional biomarker analysis, as well as PET and MRI scans. The patient sample is being expanded to those in the general hospital ward and some who were not hospitalized.
During the Q&A session, Ms. Cooper was asked if the group had collected reference data from patients who were admitted to the ICU with non-COVID disease. She responded that the group has some of that data, but as the pandemic went on they had difficulty finding patients who had never been infected with COVID to serve as reliable controls. To date, they have identified 33 controls who had a respiratory condition when admitted to the ICU. “What we see is the neurological biomarker levels in COVID are slightly lower than those with another respiratory condition in the ICU. But the data has a massive spread and the significance is very small between the two groups,” said Ms. Cooper.
Unanswered questions
The study is interesting, but leaves a lot of unanswered questions, according to Wiesje van der Flier, PhD, who moderated the session where the study was presented. “There are a lot of unknowns still: Will [the biomarkers] become normal again, once the COVID is over? Also, there was an increased risk, but it was not a one-to-one correspondence, so you can also have the increased markers but not have the neurological signs or symptoms. So I thought there were lots of questions as well,” said Dr. van der Flier, professor of neurology at Amsterdam University Medical Center.
She noted that researchers at her institution in Amsterdam have observed similar relationships, and that the associations between neurological complications and plasma biomarkers over time will be an important topic of study.
The work could provide more information on neurological manifestations of long COVID, such as long-haul fatigue. “You might also think that’s some response in their brain. It would be great if we could actually capture that [using biomarkers],” said Dr. van der Flier.
Ms. Cooper and Dr. van der Flier have no relevant financial disclosures.
SAN DIEGO – Even after recovery of an acute COVID-19 infection, some patients experience extended or even long-term symptoms that can range from mild to debilitating. Some of these symptoms are neurological: headaches, brain fog, cognitive impairment, loss of taste or smell, and even cerebrovascular complications such stroke. There are even hints that COVID-19 infection could lead to future neurodegeneration.
Those issues have prompted efforts to identify biomarkers that can help track and monitor neurological complications of COVID-19. “Throughout the course of the pandemic, it has become apparent that COVID-19 can cause various neurological symptoms. Because of this, ,” Jennifer Cooper said during a lecture at the Alzheimer’s Association International Conference. She presented new research suggesting that neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) may prove useful.
Ms. Cooper is a master’s degree student at the University of British Columbia and Canada.
Looking for sensitivity and specificity in plasma biomarkers
The researchers turned to plasma-based markers because they can reflect underlying pathology in the central nervous system. They focused on NfL, which reflects axonal damage, and GFAP, which is a marker of astrocyte activation.
The researchers analyzed data from 209 patients with COVID-19 who were admitted to the Vancouver (B.C.) General Hospital intensive care unit. Sixty-four percent were male, and the median age was 61 years. Sixty percent were ventilated, and 17% died.
The researchers determined if an individual patient’s biomarker level at hospital admission fell within a normal biomarker reference interval. A total of 53% had NfL levels outside the normal range, and 42% had GFAP levels outside the normal range. In addition, 31% of patients had both GFAP and NfL levels outside of the normal range.
Among all patients, 12% experienced ischemia, 4% hemorrhage, 2% seizures, and 10% degeneration.
At admission, NfL predicted a neurological complication with an area under the curve (AUC) of 0.702. GFAP had an AUC of 0.722. In combination, they had an AUC of 0.743. At 1 week, NfL had an AUC of 0.802, GFAP an AUC of 0.733, and the combination an AUC of 0.812.
Using age-specific cutoff values, the researchers found increased risks for neurological complications at admission (NfL odds ratio [OR], 2.9; GFAP OR, 1.6; combined OR, 2.1) and at 1 week (NfL OR, not significant; GFAP OR, 4.8; combined OR, 6.6). “We can see that both NFL and GFAP have utility in detecting neurological complications. And combining both of our markers improves detection at both time points. NfL is a marker that provides more sensitivity, where in this cohort GFAP is a marker that provides a little bit more specificity,” said Ms. Cooper.
Will additional biomarkers help?
The researchers are continuing to follow up patients at 6 months and 18 months post diagnosis, using neuropsychiatric tests and additional biomarker analysis, as well as PET and MRI scans. The patient sample is being expanded to those in the general hospital ward and some who were not hospitalized.
During the Q&A session, Ms. Cooper was asked if the group had collected reference data from patients who were admitted to the ICU with non-COVID disease. She responded that the group has some of that data, but as the pandemic went on they had difficulty finding patients who had never been infected with COVID to serve as reliable controls. To date, they have identified 33 controls who had a respiratory condition when admitted to the ICU. “What we see is the neurological biomarker levels in COVID are slightly lower than those with another respiratory condition in the ICU. But the data has a massive spread and the significance is very small between the two groups,” said Ms. Cooper.
Unanswered questions
The study is interesting, but leaves a lot of unanswered questions, according to Wiesje van der Flier, PhD, who moderated the session where the study was presented. “There are a lot of unknowns still: Will [the biomarkers] become normal again, once the COVID is over? Also, there was an increased risk, but it was not a one-to-one correspondence, so you can also have the increased markers but not have the neurological signs or symptoms. So I thought there were lots of questions as well,” said Dr. van der Flier, professor of neurology at Amsterdam University Medical Center.
She noted that researchers at her institution in Amsterdam have observed similar relationships, and that the associations between neurological complications and plasma biomarkers over time will be an important topic of study.
The work could provide more information on neurological manifestations of long COVID, such as long-haul fatigue. “You might also think that’s some response in their brain. It would be great if we could actually capture that [using biomarkers],” said Dr. van der Flier.
Ms. Cooper and Dr. van der Flier have no relevant financial disclosures.
SAN DIEGO – Even after recovery of an acute COVID-19 infection, some patients experience extended or even long-term symptoms that can range from mild to debilitating. Some of these symptoms are neurological: headaches, brain fog, cognitive impairment, loss of taste or smell, and even cerebrovascular complications such stroke. There are even hints that COVID-19 infection could lead to future neurodegeneration.
Those issues have prompted efforts to identify biomarkers that can help track and monitor neurological complications of COVID-19. “Throughout the course of the pandemic, it has become apparent that COVID-19 can cause various neurological symptoms. Because of this, ,” Jennifer Cooper said during a lecture at the Alzheimer’s Association International Conference. She presented new research suggesting that neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) may prove useful.
Ms. Cooper is a master’s degree student at the University of British Columbia and Canada.
Looking for sensitivity and specificity in plasma biomarkers
The researchers turned to plasma-based markers because they can reflect underlying pathology in the central nervous system. They focused on NfL, which reflects axonal damage, and GFAP, which is a marker of astrocyte activation.
The researchers analyzed data from 209 patients with COVID-19 who were admitted to the Vancouver (B.C.) General Hospital intensive care unit. Sixty-four percent were male, and the median age was 61 years. Sixty percent were ventilated, and 17% died.
The researchers determined if an individual patient’s biomarker level at hospital admission fell within a normal biomarker reference interval. A total of 53% had NfL levels outside the normal range, and 42% had GFAP levels outside the normal range. In addition, 31% of patients had both GFAP and NfL levels outside of the normal range.
Among all patients, 12% experienced ischemia, 4% hemorrhage, 2% seizures, and 10% degeneration.
At admission, NfL predicted a neurological complication with an area under the curve (AUC) of 0.702. GFAP had an AUC of 0.722. In combination, they had an AUC of 0.743. At 1 week, NfL had an AUC of 0.802, GFAP an AUC of 0.733, and the combination an AUC of 0.812.
Using age-specific cutoff values, the researchers found increased risks for neurological complications at admission (NfL odds ratio [OR], 2.9; GFAP OR, 1.6; combined OR, 2.1) and at 1 week (NfL OR, not significant; GFAP OR, 4.8; combined OR, 6.6). “We can see that both NFL and GFAP have utility in detecting neurological complications. And combining both of our markers improves detection at both time points. NfL is a marker that provides more sensitivity, where in this cohort GFAP is a marker that provides a little bit more specificity,” said Ms. Cooper.
Will additional biomarkers help?
The researchers are continuing to follow up patients at 6 months and 18 months post diagnosis, using neuropsychiatric tests and additional biomarker analysis, as well as PET and MRI scans. The patient sample is being expanded to those in the general hospital ward and some who were not hospitalized.
During the Q&A session, Ms. Cooper was asked if the group had collected reference data from patients who were admitted to the ICU with non-COVID disease. She responded that the group has some of that data, but as the pandemic went on they had difficulty finding patients who had never been infected with COVID to serve as reliable controls. To date, they have identified 33 controls who had a respiratory condition when admitted to the ICU. “What we see is the neurological biomarker levels in COVID are slightly lower than those with another respiratory condition in the ICU. But the data has a massive spread and the significance is very small between the two groups,” said Ms. Cooper.
Unanswered questions
The study is interesting, but leaves a lot of unanswered questions, according to Wiesje van der Flier, PhD, who moderated the session where the study was presented. “There are a lot of unknowns still: Will [the biomarkers] become normal again, once the COVID is over? Also, there was an increased risk, but it was not a one-to-one correspondence, so you can also have the increased markers but not have the neurological signs or symptoms. So I thought there were lots of questions as well,” said Dr. van der Flier, professor of neurology at Amsterdam University Medical Center.
She noted that researchers at her institution in Amsterdam have observed similar relationships, and that the associations between neurological complications and plasma biomarkers over time will be an important topic of study.
The work could provide more information on neurological manifestations of long COVID, such as long-haul fatigue. “You might also think that’s some response in their brain. It would be great if we could actually capture that [using biomarkers],” said Dr. van der Flier.
Ms. Cooper and Dr. van der Flier have no relevant financial disclosures.
AT AAIC 2022
Cardiorespiratory fitness key to longevity for all?
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
In MCI, combo training boosts effect
SAN DIEGO – The findings were drawn from an unusual study design that split patients into five groups, one of which included both interventions.
After the study was completed, researchers collapsed the groups into a single analysis to compare the different regimens, according to Manuel Montero-Odasso, MD, PhD, who presented the work at the Alzheimer’s Association International Conference. He is a geriatrician at Parkwood Institute, London, Ont.
Two previous trials looked at whether the combination of exercise plus cognitive training could outperform either intervention alone. In both, the combination improved cognition but not as much as either intervention alone. “So it seemed that when they combine it, they didn’t do as well,” said Dr. Montero-Odasso. Those findings left doubt about whether or not there is synergism between the two approaches.
Sequential, not simultaneous
A possible explanation for the finding is that patients who are doing both cognitive training and physical exercise simultaneously might not be able to focus enough on either task to do get the maximum benefit. “When we try to combine concurrently, participants or patients cannot focus and do enough progression in both at the same time. That’s the reason we designed the trial in a way that the interventions were sequential. You got a very good quality (cognitive) training, and later you got the exercise,” said Dr. Montero-Odasso.
In the new study, patients receiving both interventions conducted the cognitive training first, then did physical exercises 30 minutes later. “The practical message is that you should follow a program. Something I see in my patients, when they do the two things at the same time, they don’t pay enough attention,” said Dr. Montero-Odasso.
The researchers added vitamin D to the regimen as there have been small studies reporting that vitamin D supplementation can lead to greater muscle mass resulting from exercise.
The study included 176 patients aged 60-85 with MCI. The researchers excluded patients already participating in an active exercise program with a personal trainer, as well as those taking vitamin D at doses higher than 1,000 IU/day.
Over 20 weeks, the randomized groups included combination exercise and cognitive training with vitamin D (10,000 IU three times per week), exercise and cognitive training with placebo, exercise with a cognitive control and vitamin D, exercise with a cognitive control and placebo, and an exercise control (balance and toning) with cognitive control and placebo.
The interventions were completed three times per week. Cognitive training employed a tablet with multifunctional tasks and memory components. It was adaptive, becoming more difficult as patients improved or simplifying the task if a patient struggled. The exercise component included 40 minutes of progressive, supervised resistance training, followed by 20 minutes of aerobic exercise.
Compared with the double-placebo group, the double-intervention group had significant improvement in cognitive performance. “Exercise alone without cognitive training shows an effect, but that effect was lower than a combination with cognitive training,” said Dr. Montero-Odasso.
The combined groups had medium effect sizes on cognition when combined with vitamin D (Cohen’s d, 0.65; P = .003) and with vitamin D placebo (Cohen’s d, 0.58; P = .013). There were nonsignificant improvements in the exercise and vitamin D group (Cohen’s d, 0.30; P = .241) and the exercise plus placebo group (Cohen’s d, 0.42; P = .139)
After collapsing the arms, the researchers found that the exercise plus cognitive training regimen had an effect size of 0.62 (P = .002), while exercise alone only trended toward improvement and with a small effect size (Cohen’s d, 0.36; P = .13). There was no apparent effect of vitamin D supplementation, though Dr. Montero-Odasso pointed out that most participants were taking vitamin D supplements before study entry and had normal to high serum levels of vitamin D.
‘Optimistic’ results
The study was limited by an inability to retain patients due to the COVID-19 pandemic, leading to a dropout rate of 17%.
“I think the idea of combining risk reduction strategies together in a population and individuals with MCI is really exciting. These are optimistic results. You certainly need to look into a larger and more diverse population as it goes forward,” said Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, who was asked to comment on the study.
She noted that the study looked at all-cause cognitive impairment. It would be interesting, Dr. Snyder said, to see how individuals with different underlying conditions handle the combination intervention.
The researchers are now in the planning stage of the Synergic 2 trial, which will incorporate exercise and cognitive training, plus diet and sleep counseling. It will be conducted virtually, involving one-to-one interactions with coaches.
Dr. Montero-Odasso and Dr. Snyder have no relevant financial disclosures.
SAN DIEGO – The findings were drawn from an unusual study design that split patients into five groups, one of which included both interventions.
After the study was completed, researchers collapsed the groups into a single analysis to compare the different regimens, according to Manuel Montero-Odasso, MD, PhD, who presented the work at the Alzheimer’s Association International Conference. He is a geriatrician at Parkwood Institute, London, Ont.
Two previous trials looked at whether the combination of exercise plus cognitive training could outperform either intervention alone. In both, the combination improved cognition but not as much as either intervention alone. “So it seemed that when they combine it, they didn’t do as well,” said Dr. Montero-Odasso. Those findings left doubt about whether or not there is synergism between the two approaches.
Sequential, not simultaneous
A possible explanation for the finding is that patients who are doing both cognitive training and physical exercise simultaneously might not be able to focus enough on either task to do get the maximum benefit. “When we try to combine concurrently, participants or patients cannot focus and do enough progression in both at the same time. That’s the reason we designed the trial in a way that the interventions were sequential. You got a very good quality (cognitive) training, and later you got the exercise,” said Dr. Montero-Odasso.
In the new study, patients receiving both interventions conducted the cognitive training first, then did physical exercises 30 minutes later. “The practical message is that you should follow a program. Something I see in my patients, when they do the two things at the same time, they don’t pay enough attention,” said Dr. Montero-Odasso.
The researchers added vitamin D to the regimen as there have been small studies reporting that vitamin D supplementation can lead to greater muscle mass resulting from exercise.
The study included 176 patients aged 60-85 with MCI. The researchers excluded patients already participating in an active exercise program with a personal trainer, as well as those taking vitamin D at doses higher than 1,000 IU/day.
Over 20 weeks, the randomized groups included combination exercise and cognitive training with vitamin D (10,000 IU three times per week), exercise and cognitive training with placebo, exercise with a cognitive control and vitamin D, exercise with a cognitive control and placebo, and an exercise control (balance and toning) with cognitive control and placebo.
The interventions were completed three times per week. Cognitive training employed a tablet with multifunctional tasks and memory components. It was adaptive, becoming more difficult as patients improved or simplifying the task if a patient struggled. The exercise component included 40 minutes of progressive, supervised resistance training, followed by 20 minutes of aerobic exercise.
Compared with the double-placebo group, the double-intervention group had significant improvement in cognitive performance. “Exercise alone without cognitive training shows an effect, but that effect was lower than a combination with cognitive training,” said Dr. Montero-Odasso.
The combined groups had medium effect sizes on cognition when combined with vitamin D (Cohen’s d, 0.65; P = .003) and with vitamin D placebo (Cohen’s d, 0.58; P = .013). There were nonsignificant improvements in the exercise and vitamin D group (Cohen’s d, 0.30; P = .241) and the exercise plus placebo group (Cohen’s d, 0.42; P = .139)
After collapsing the arms, the researchers found that the exercise plus cognitive training regimen had an effect size of 0.62 (P = .002), while exercise alone only trended toward improvement and with a small effect size (Cohen’s d, 0.36; P = .13). There was no apparent effect of vitamin D supplementation, though Dr. Montero-Odasso pointed out that most participants were taking vitamin D supplements before study entry and had normal to high serum levels of vitamin D.
‘Optimistic’ results
The study was limited by an inability to retain patients due to the COVID-19 pandemic, leading to a dropout rate of 17%.
“I think the idea of combining risk reduction strategies together in a population and individuals with MCI is really exciting. These are optimistic results. You certainly need to look into a larger and more diverse population as it goes forward,” said Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, who was asked to comment on the study.
She noted that the study looked at all-cause cognitive impairment. It would be interesting, Dr. Snyder said, to see how individuals with different underlying conditions handle the combination intervention.
The researchers are now in the planning stage of the Synergic 2 trial, which will incorporate exercise and cognitive training, plus diet and sleep counseling. It will be conducted virtually, involving one-to-one interactions with coaches.
Dr. Montero-Odasso and Dr. Snyder have no relevant financial disclosures.
SAN DIEGO – The findings were drawn from an unusual study design that split patients into five groups, one of which included both interventions.
After the study was completed, researchers collapsed the groups into a single analysis to compare the different regimens, according to Manuel Montero-Odasso, MD, PhD, who presented the work at the Alzheimer’s Association International Conference. He is a geriatrician at Parkwood Institute, London, Ont.
Two previous trials looked at whether the combination of exercise plus cognitive training could outperform either intervention alone. In both, the combination improved cognition but not as much as either intervention alone. “So it seemed that when they combine it, they didn’t do as well,” said Dr. Montero-Odasso. Those findings left doubt about whether or not there is synergism between the two approaches.
Sequential, not simultaneous
A possible explanation for the finding is that patients who are doing both cognitive training and physical exercise simultaneously might not be able to focus enough on either task to do get the maximum benefit. “When we try to combine concurrently, participants or patients cannot focus and do enough progression in both at the same time. That’s the reason we designed the trial in a way that the interventions were sequential. You got a very good quality (cognitive) training, and later you got the exercise,” said Dr. Montero-Odasso.
In the new study, patients receiving both interventions conducted the cognitive training first, then did physical exercises 30 minutes later. “The practical message is that you should follow a program. Something I see in my patients, when they do the two things at the same time, they don’t pay enough attention,” said Dr. Montero-Odasso.
The researchers added vitamin D to the regimen as there have been small studies reporting that vitamin D supplementation can lead to greater muscle mass resulting from exercise.
The study included 176 patients aged 60-85 with MCI. The researchers excluded patients already participating in an active exercise program with a personal trainer, as well as those taking vitamin D at doses higher than 1,000 IU/day.
Over 20 weeks, the randomized groups included combination exercise and cognitive training with vitamin D (10,000 IU three times per week), exercise and cognitive training with placebo, exercise with a cognitive control and vitamin D, exercise with a cognitive control and placebo, and an exercise control (balance and toning) with cognitive control and placebo.
The interventions were completed three times per week. Cognitive training employed a tablet with multifunctional tasks and memory components. It was adaptive, becoming more difficult as patients improved or simplifying the task if a patient struggled. The exercise component included 40 minutes of progressive, supervised resistance training, followed by 20 minutes of aerobic exercise.
Compared with the double-placebo group, the double-intervention group had significant improvement in cognitive performance. “Exercise alone without cognitive training shows an effect, but that effect was lower than a combination with cognitive training,” said Dr. Montero-Odasso.
The combined groups had medium effect sizes on cognition when combined with vitamin D (Cohen’s d, 0.65; P = .003) and with vitamin D placebo (Cohen’s d, 0.58; P = .013). There were nonsignificant improvements in the exercise and vitamin D group (Cohen’s d, 0.30; P = .241) and the exercise plus placebo group (Cohen’s d, 0.42; P = .139)
After collapsing the arms, the researchers found that the exercise plus cognitive training regimen had an effect size of 0.62 (P = .002), while exercise alone only trended toward improvement and with a small effect size (Cohen’s d, 0.36; P = .13). There was no apparent effect of vitamin D supplementation, though Dr. Montero-Odasso pointed out that most participants were taking vitamin D supplements before study entry and had normal to high serum levels of vitamin D.
‘Optimistic’ results
The study was limited by an inability to retain patients due to the COVID-19 pandemic, leading to a dropout rate of 17%.
“I think the idea of combining risk reduction strategies together in a population and individuals with MCI is really exciting. These are optimistic results. You certainly need to look into a larger and more diverse population as it goes forward,” said Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, who was asked to comment on the study.
She noted that the study looked at all-cause cognitive impairment. It would be interesting, Dr. Snyder said, to see how individuals with different underlying conditions handle the combination intervention.
The researchers are now in the planning stage of the Synergic 2 trial, which will incorporate exercise and cognitive training, plus diet and sleep counseling. It will be conducted virtually, involving one-to-one interactions with coaches.
Dr. Montero-Odasso and Dr. Snyder have no relevant financial disclosures.
AT AAIC 2022
Stopping JIA drugs? Many can regain control after a flare
About two-thirds of children with juvenile idiopathic arthritis (JIA) were able to return to an inactive disease state within 12 months after a flare occurred when they took a break from medication, and slightly more than half – 55% – reached this state within 6 months, according to findings from registry data examined in a study published in Arthritis Care & Research.
Sarah Ringold, MD, MS, of the Seattle Children’s Hospital, and coauthors used data from participants in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry to track what happened to patients when they took a break from antirheumatic drugs. They described their paper as being the first to use a large multicenter database such as the CARRA Registry to focus on JIA outcomes after medication discontinuation and flare, to describe flare severity after medication discontinuation, and to report patterns of medication use for flares.
“To date, JIA studies have established that flares after medication discontinuation are common but have generated conflicting data regarding flare risk factors,” Dr. Ringold and coauthors wrote. “Since it is not yet possible to predict reliably which children will successfully discontinue medication, families and physicians face uncertainty when deciding to stop medications, and there is significant variation in approach.”
The study will be “very helpful” to physicians working with parents and patients to make decisions about discontinuing medications, said Grant Schulert, MD, PhD, of Cincinnati Children’s Hospital, who was not involved with the study.
“It gives some numbers to help us have those conversations,” he said in an interview.
But interpreting those numbers still will present parents with a challenge, Dr. Schulert said.
“You can say: ‘The glass is half full; 55% of them could go back into remission in 6 months, a little bit higher in a year,’ ” he said. “Or the glass is half empty; some of them, even at a year, are still not back in remission.”
But “patients aren’t a statistic. They’re each one person,” he said. “They’re going to be in one of those two situations.”
There are many challenges in explaining the potential advantages and disadvantages of medication breaks to patients and families, said the study’s senior author, Daniel B. Horton, MD, MSCE, of Rutgers Robert Wood Johnson Medical School and the Rutgers Center for Pharmacoepidemiology and Treatment Science, both in New Brunswick, N.J., and the department of biostatistics and epidemiology at Rutgers School of Public Health, Piscataway, N.J.
“One of the challenges of explaining the pros and cons about stopping medicines is the uncertainty – not knowing if and when a flare will occur, if and when a flare would be well controlled, and, for treatments that are continued, if and when complications of that treatment could occur,” Dr. Horton said in an interview. “Many patients and families are afraid about what the medicines might do long-term and want to stop treatment as soon as possible, despite the risks of stopping. Another challenge is that we do not yet have accurate, widely available tests that help us predict these various outcomes. Still, it is important for clinicians to explain the risks of continuing treatment and of stopping treatment, and to give patients and families time to ask questions and share their own values and preferences. If these conversations don’t happen, patients or families may just stop the medicines even if stopping is not warranted or is likely to lead to a poor outcome.”
Study details
Of the 367 patients studied, 270 (74%) were female. Half of all patients in the study had extended oligoarticular/rheumatoid factor (RF)–negative polyarticular JIA, and the second most common category was persistent oligoarthritis at 25%.The median age at disease onset was 4, with a range of 2-9 years.
The median age at disease flare was 11.3, with a range of 7.5-15.7 years. At the time of flare, children had a median disease duration of 5.1 years and had been off systemic disease-modifying antirheumatic drugs (DMARDs) for a median of 205 days. In addition, at the time of flare, the median active joint count was 1 and the maximum active joint count was 33, and approximately 13% of children had 5 or more active joints.
Conventional synthetic DMARDs were the most commonly stopped medications (48%), and tumor necrosis factor inhibitors (TNFi) were second (42%), Dr. Ringold and coauthors wrote.
Independent predictors of successful recapture of inactive disease included TNFi as recapture medication and history of a non-TNFi biologic use.
Dr. Ringold and coauthors noted limitations of the registry-based study. This is “a convenience sample of patients who are cared for and consented at academic sites, and additional study may be needed to understand how these results generalize to other countries and health systems,” they wrote.
And there may have been misclassification and inclusion of patients who stopped medications for self-perceived well-controlled disease, they wrote.
“Although the intent was to include children who stopped their medications at their physician’s direction due to physician-confirmed inactive disease, patients who had been previously enrolled in the registry were included if inactive disease was listed as the reason for medication discontinuation,” they said.
Still, these results should serve as a “benchmark for future studies of medication discontinuation” in JIA, the researchers wrote.
‘Fortunate challenge’
In an accompanying editorial, Melissa L. Mannion, MD, MSPH, and Randy Q. Cron, MD, PhD, of the University of Alabama at Birmingham noted that pediatric rheumatologists now face what they call the “fortunate challenge” of helping patients and parents decide whether treatments can be stopped in cases where there’s been a sustained period of inactive disease.
“Once a patient has reached the goal of inactive disease, why would patients or providers want to stop medications?” Dr. Mannion and Dr. Cron wrote. “We tell our patients that we want them to be like everyone else and have no limitations on their goals. However, the burden of chronic medication to achieve that goal is a constant reminder that they are different from their peers.”
In their article, Dr. Mannion and Dr. Cron noted what they called “interesting” results observed among children with different forms of JIA in the study.
Children with “systemic JIA had the highest recapture rates at 6 or 12 months, perhaps reflecting the high percentage use of [biologic] DMARDs targeting interleukin-1 and IL-6, or maybe the timeliness of recognition (e.g., fever, rash) of disease flare,” Dr. Mannion and Dr. Cron wrote. “Conversely, children with JIA enthesitis-related arthritis (ERA) had the lowest recapture rate at 6 months (27.6%, even lower than RF-positive polyarticular JIA, 42.9%).”
Still, the editorial authors said that “additional well-controlled studies are needed to move pediatric rheumatology deeper into the realm of precision medicine and the ability to decide whether or not to wean DMARD therapy for those with clinically inactive disease.”
Pamela Weiss, MD, of Children’s Hospital of Philadelphia, said in a comment that the study by Dr. Ringold and colleagues, as well as others that address similar questions, “are critically needed to move our field towards a personalized medicine approach.” But she added that while the paper from Dr. Ringold and colleagues addresses an important question, it “should be interpreted with some caution.”
She noted, for example, that “disease flare,” which prompted reinitiation of treatment and study entry, was not always aligned with a registry visit, which makes determination of the primary exposure less stringent. The rate of recapture across JIA categories differed by as much as 20% depending upon which inactive disease assessment outcome was used – either the study’s novel but unvalidated primary outcome or the validated secondary outcome of using the clinical Juvenile Arthritis Disease Activity Score based on 10 joints. The resulting difference was marked for some JIA categories and minimal for others.
“The flare and recapture rates are likely to be vastly different for JIA categories with distinct pathophysiology – namely systemic JIA, psoriatic arthritis, and enthesitis-related arthritis,” Dr. Weiss said. “While numbers for these categories were too small to make meaningful conclusions, grouping them with the other JIA categories has limitations.”
The research was funded by a Rheumatology Research Foundation Innovative Research Award.
Dr. Ringold’s current employment is through Janssen Research & Development. She changed primary employment from Seattle Children’s to Janssen during completion of the analyses and preparation of the manuscript. She has maintained her affiliation with Seattle Children’s. Dr. Schulert has consulting for Novartis. Dr. Cron reported speaker fees, consulting fees, and grant support from Sobi, consulting fees from Sironax and Novartis, speaker fees from Lilly, and support from Pfizer for working on a committee adjudicating clinical trial side effects.
* This article was updated on 8/11/2022.
About two-thirds of children with juvenile idiopathic arthritis (JIA) were able to return to an inactive disease state within 12 months after a flare occurred when they took a break from medication, and slightly more than half – 55% – reached this state within 6 months, according to findings from registry data examined in a study published in Arthritis Care & Research.
Sarah Ringold, MD, MS, of the Seattle Children’s Hospital, and coauthors used data from participants in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry to track what happened to patients when they took a break from antirheumatic drugs. They described their paper as being the first to use a large multicenter database such as the CARRA Registry to focus on JIA outcomes after medication discontinuation and flare, to describe flare severity after medication discontinuation, and to report patterns of medication use for flares.
“To date, JIA studies have established that flares after medication discontinuation are common but have generated conflicting data regarding flare risk factors,” Dr. Ringold and coauthors wrote. “Since it is not yet possible to predict reliably which children will successfully discontinue medication, families and physicians face uncertainty when deciding to stop medications, and there is significant variation in approach.”
The study will be “very helpful” to physicians working with parents and patients to make decisions about discontinuing medications, said Grant Schulert, MD, PhD, of Cincinnati Children’s Hospital, who was not involved with the study.
“It gives some numbers to help us have those conversations,” he said in an interview.
But interpreting those numbers still will present parents with a challenge, Dr. Schulert said.
“You can say: ‘The glass is half full; 55% of them could go back into remission in 6 months, a little bit higher in a year,’ ” he said. “Or the glass is half empty; some of them, even at a year, are still not back in remission.”
But “patients aren’t a statistic. They’re each one person,” he said. “They’re going to be in one of those two situations.”
There are many challenges in explaining the potential advantages and disadvantages of medication breaks to patients and families, said the study’s senior author, Daniel B. Horton, MD, MSCE, of Rutgers Robert Wood Johnson Medical School and the Rutgers Center for Pharmacoepidemiology and Treatment Science, both in New Brunswick, N.J., and the department of biostatistics and epidemiology at Rutgers School of Public Health, Piscataway, N.J.
“One of the challenges of explaining the pros and cons about stopping medicines is the uncertainty – not knowing if and when a flare will occur, if and when a flare would be well controlled, and, for treatments that are continued, if and when complications of that treatment could occur,” Dr. Horton said in an interview. “Many patients and families are afraid about what the medicines might do long-term and want to stop treatment as soon as possible, despite the risks of stopping. Another challenge is that we do not yet have accurate, widely available tests that help us predict these various outcomes. Still, it is important for clinicians to explain the risks of continuing treatment and of stopping treatment, and to give patients and families time to ask questions and share their own values and preferences. If these conversations don’t happen, patients or families may just stop the medicines even if stopping is not warranted or is likely to lead to a poor outcome.”
Study details
Of the 367 patients studied, 270 (74%) were female. Half of all patients in the study had extended oligoarticular/rheumatoid factor (RF)–negative polyarticular JIA, and the second most common category was persistent oligoarthritis at 25%.The median age at disease onset was 4, with a range of 2-9 years.
The median age at disease flare was 11.3, with a range of 7.5-15.7 years. At the time of flare, children had a median disease duration of 5.1 years and had been off systemic disease-modifying antirheumatic drugs (DMARDs) for a median of 205 days. In addition, at the time of flare, the median active joint count was 1 and the maximum active joint count was 33, and approximately 13% of children had 5 or more active joints.
Conventional synthetic DMARDs were the most commonly stopped medications (48%), and tumor necrosis factor inhibitors (TNFi) were second (42%), Dr. Ringold and coauthors wrote.
Independent predictors of successful recapture of inactive disease included TNFi as recapture medication and history of a non-TNFi biologic use.
Dr. Ringold and coauthors noted limitations of the registry-based study. This is “a convenience sample of patients who are cared for and consented at academic sites, and additional study may be needed to understand how these results generalize to other countries and health systems,” they wrote.
And there may have been misclassification and inclusion of patients who stopped medications for self-perceived well-controlled disease, they wrote.
“Although the intent was to include children who stopped their medications at their physician’s direction due to physician-confirmed inactive disease, patients who had been previously enrolled in the registry were included if inactive disease was listed as the reason for medication discontinuation,” they said.
Still, these results should serve as a “benchmark for future studies of medication discontinuation” in JIA, the researchers wrote.
‘Fortunate challenge’
In an accompanying editorial, Melissa L. Mannion, MD, MSPH, and Randy Q. Cron, MD, PhD, of the University of Alabama at Birmingham noted that pediatric rheumatologists now face what they call the “fortunate challenge” of helping patients and parents decide whether treatments can be stopped in cases where there’s been a sustained period of inactive disease.
“Once a patient has reached the goal of inactive disease, why would patients or providers want to stop medications?” Dr. Mannion and Dr. Cron wrote. “We tell our patients that we want them to be like everyone else and have no limitations on their goals. However, the burden of chronic medication to achieve that goal is a constant reminder that they are different from their peers.”
In their article, Dr. Mannion and Dr. Cron noted what they called “interesting” results observed among children with different forms of JIA in the study.
Children with “systemic JIA had the highest recapture rates at 6 or 12 months, perhaps reflecting the high percentage use of [biologic] DMARDs targeting interleukin-1 and IL-6, or maybe the timeliness of recognition (e.g., fever, rash) of disease flare,” Dr. Mannion and Dr. Cron wrote. “Conversely, children with JIA enthesitis-related arthritis (ERA) had the lowest recapture rate at 6 months (27.6%, even lower than RF-positive polyarticular JIA, 42.9%).”
Still, the editorial authors said that “additional well-controlled studies are needed to move pediatric rheumatology deeper into the realm of precision medicine and the ability to decide whether or not to wean DMARD therapy for those with clinically inactive disease.”
Pamela Weiss, MD, of Children’s Hospital of Philadelphia, said in a comment that the study by Dr. Ringold and colleagues, as well as others that address similar questions, “are critically needed to move our field towards a personalized medicine approach.” But she added that while the paper from Dr. Ringold and colleagues addresses an important question, it “should be interpreted with some caution.”
She noted, for example, that “disease flare,” which prompted reinitiation of treatment and study entry, was not always aligned with a registry visit, which makes determination of the primary exposure less stringent. The rate of recapture across JIA categories differed by as much as 20% depending upon which inactive disease assessment outcome was used – either the study’s novel but unvalidated primary outcome or the validated secondary outcome of using the clinical Juvenile Arthritis Disease Activity Score based on 10 joints. The resulting difference was marked for some JIA categories and minimal for others.
“The flare and recapture rates are likely to be vastly different for JIA categories with distinct pathophysiology – namely systemic JIA, psoriatic arthritis, and enthesitis-related arthritis,” Dr. Weiss said. “While numbers for these categories were too small to make meaningful conclusions, grouping them with the other JIA categories has limitations.”
The research was funded by a Rheumatology Research Foundation Innovative Research Award.
Dr. Ringold’s current employment is through Janssen Research & Development. She changed primary employment from Seattle Children’s to Janssen during completion of the analyses and preparation of the manuscript. She has maintained her affiliation with Seattle Children’s. Dr. Schulert has consulting for Novartis. Dr. Cron reported speaker fees, consulting fees, and grant support from Sobi, consulting fees from Sironax and Novartis, speaker fees from Lilly, and support from Pfizer for working on a committee adjudicating clinical trial side effects.
* This article was updated on 8/11/2022.
About two-thirds of children with juvenile idiopathic arthritis (JIA) were able to return to an inactive disease state within 12 months after a flare occurred when they took a break from medication, and slightly more than half – 55% – reached this state within 6 months, according to findings from registry data examined in a study published in Arthritis Care & Research.
Sarah Ringold, MD, MS, of the Seattle Children’s Hospital, and coauthors used data from participants in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry to track what happened to patients when they took a break from antirheumatic drugs. They described their paper as being the first to use a large multicenter database such as the CARRA Registry to focus on JIA outcomes after medication discontinuation and flare, to describe flare severity after medication discontinuation, and to report patterns of medication use for flares.
“To date, JIA studies have established that flares after medication discontinuation are common but have generated conflicting data regarding flare risk factors,” Dr. Ringold and coauthors wrote. “Since it is not yet possible to predict reliably which children will successfully discontinue medication, families and physicians face uncertainty when deciding to stop medications, and there is significant variation in approach.”
The study will be “very helpful” to physicians working with parents and patients to make decisions about discontinuing medications, said Grant Schulert, MD, PhD, of Cincinnati Children’s Hospital, who was not involved with the study.
“It gives some numbers to help us have those conversations,” he said in an interview.
But interpreting those numbers still will present parents with a challenge, Dr. Schulert said.
“You can say: ‘The glass is half full; 55% of them could go back into remission in 6 months, a little bit higher in a year,’ ” he said. “Or the glass is half empty; some of them, even at a year, are still not back in remission.”
But “patients aren’t a statistic. They’re each one person,” he said. “They’re going to be in one of those two situations.”
There are many challenges in explaining the potential advantages and disadvantages of medication breaks to patients and families, said the study’s senior author, Daniel B. Horton, MD, MSCE, of Rutgers Robert Wood Johnson Medical School and the Rutgers Center for Pharmacoepidemiology and Treatment Science, both in New Brunswick, N.J., and the department of biostatistics and epidemiology at Rutgers School of Public Health, Piscataway, N.J.
“One of the challenges of explaining the pros and cons about stopping medicines is the uncertainty – not knowing if and when a flare will occur, if and when a flare would be well controlled, and, for treatments that are continued, if and when complications of that treatment could occur,” Dr. Horton said in an interview. “Many patients and families are afraid about what the medicines might do long-term and want to stop treatment as soon as possible, despite the risks of stopping. Another challenge is that we do not yet have accurate, widely available tests that help us predict these various outcomes. Still, it is important for clinicians to explain the risks of continuing treatment and of stopping treatment, and to give patients and families time to ask questions and share their own values and preferences. If these conversations don’t happen, patients or families may just stop the medicines even if stopping is not warranted or is likely to lead to a poor outcome.”
Study details
Of the 367 patients studied, 270 (74%) were female. Half of all patients in the study had extended oligoarticular/rheumatoid factor (RF)–negative polyarticular JIA, and the second most common category was persistent oligoarthritis at 25%.The median age at disease onset was 4, with a range of 2-9 years.
The median age at disease flare was 11.3, with a range of 7.5-15.7 years. At the time of flare, children had a median disease duration of 5.1 years and had been off systemic disease-modifying antirheumatic drugs (DMARDs) for a median of 205 days. In addition, at the time of flare, the median active joint count was 1 and the maximum active joint count was 33, and approximately 13% of children had 5 or more active joints.
Conventional synthetic DMARDs were the most commonly stopped medications (48%), and tumor necrosis factor inhibitors (TNFi) were second (42%), Dr. Ringold and coauthors wrote.
Independent predictors of successful recapture of inactive disease included TNFi as recapture medication and history of a non-TNFi biologic use.
Dr. Ringold and coauthors noted limitations of the registry-based study. This is “a convenience sample of patients who are cared for and consented at academic sites, and additional study may be needed to understand how these results generalize to other countries and health systems,” they wrote.
And there may have been misclassification and inclusion of patients who stopped medications for self-perceived well-controlled disease, they wrote.
“Although the intent was to include children who stopped their medications at their physician’s direction due to physician-confirmed inactive disease, patients who had been previously enrolled in the registry were included if inactive disease was listed as the reason for medication discontinuation,” they said.
Still, these results should serve as a “benchmark for future studies of medication discontinuation” in JIA, the researchers wrote.
‘Fortunate challenge’
In an accompanying editorial, Melissa L. Mannion, MD, MSPH, and Randy Q. Cron, MD, PhD, of the University of Alabama at Birmingham noted that pediatric rheumatologists now face what they call the “fortunate challenge” of helping patients and parents decide whether treatments can be stopped in cases where there’s been a sustained period of inactive disease.
“Once a patient has reached the goal of inactive disease, why would patients or providers want to stop medications?” Dr. Mannion and Dr. Cron wrote. “We tell our patients that we want them to be like everyone else and have no limitations on their goals. However, the burden of chronic medication to achieve that goal is a constant reminder that they are different from their peers.”
In their article, Dr. Mannion and Dr. Cron noted what they called “interesting” results observed among children with different forms of JIA in the study.
Children with “systemic JIA had the highest recapture rates at 6 or 12 months, perhaps reflecting the high percentage use of [biologic] DMARDs targeting interleukin-1 and IL-6, or maybe the timeliness of recognition (e.g., fever, rash) of disease flare,” Dr. Mannion and Dr. Cron wrote. “Conversely, children with JIA enthesitis-related arthritis (ERA) had the lowest recapture rate at 6 months (27.6%, even lower than RF-positive polyarticular JIA, 42.9%).”
Still, the editorial authors said that “additional well-controlled studies are needed to move pediatric rheumatology deeper into the realm of precision medicine and the ability to decide whether or not to wean DMARD therapy for those with clinically inactive disease.”
Pamela Weiss, MD, of Children’s Hospital of Philadelphia, said in a comment that the study by Dr. Ringold and colleagues, as well as others that address similar questions, “are critically needed to move our field towards a personalized medicine approach.” But she added that while the paper from Dr. Ringold and colleagues addresses an important question, it “should be interpreted with some caution.”
She noted, for example, that “disease flare,” which prompted reinitiation of treatment and study entry, was not always aligned with a registry visit, which makes determination of the primary exposure less stringent. The rate of recapture across JIA categories differed by as much as 20% depending upon which inactive disease assessment outcome was used – either the study’s novel but unvalidated primary outcome or the validated secondary outcome of using the clinical Juvenile Arthritis Disease Activity Score based on 10 joints. The resulting difference was marked for some JIA categories and minimal for others.
“The flare and recapture rates are likely to be vastly different for JIA categories with distinct pathophysiology – namely systemic JIA, psoriatic arthritis, and enthesitis-related arthritis,” Dr. Weiss said. “While numbers for these categories were too small to make meaningful conclusions, grouping them with the other JIA categories has limitations.”
The research was funded by a Rheumatology Research Foundation Innovative Research Award.
Dr. Ringold’s current employment is through Janssen Research & Development. She changed primary employment from Seattle Children’s to Janssen during completion of the analyses and preparation of the manuscript. She has maintained her affiliation with Seattle Children’s. Dr. Schulert has consulting for Novartis. Dr. Cron reported speaker fees, consulting fees, and grant support from Sobi, consulting fees from Sironax and Novartis, speaker fees from Lilly, and support from Pfizer for working on a committee adjudicating clinical trial side effects.
* This article was updated on 8/11/2022.
FROM ARTHRITIS CARE & RESEARCH
Rethinking histology as treatment target in ulcerative colitis
For patients who experience endoscopic remission of ulcerative colitis (UC), signs of active disease on histology did not affect their risk of clinical relapse, according to a large prospective study that reinforces a low endoscopy score as the treatment target.
In the study of more than 250 patients in endoscopic remission from UC, 19% experienced a clinical relapse within 1 year. The researchers found that a lower baseline endoscopy score was linked to a lower risk of relapse.
While histologic activity, as reflected in the Geboes Score, was not associated with clinical relapse, the presence of basal plasmacytosis independently doubled the risk of relapse.
“Our findings do not support the use of histology as a target for treatment in patients with ulcerative colitis who already achieved clinical and endoscopic remission,” say Talat Bessissow, MD, McGill University Health Center, Montreal, and colleagues.
They add that the results “support the use of the Mayo endoscopic subscore of zero as the optimal target for endoscopic remission.”
Further prospective data are needed to “define the role of histology activity and basal plasmacytosis in the management of ulcerative colitis,” the authors write.
The study was published online in The American Journal of Gastroenterology.
Uncertain role of histology
Dr. Bessissow told this news organization that “some studies have shown that histologic healing is associated with better long-term outcomes and less relapse, but this topic remains controversial because other studies have shown the opposite.”
“Our study does not support histology as a treatment target,” he continued, adding that therapy should not be changed solely on the basis of histology.
Dr. Bessissow clarified that although histology was not associated with less relapse over 1 year of follow-up, the role of histology on other, longer-term outcomes, such as surgery and colorectal cancer, still needs to be studied.
The natural history of UC is characterized by frequent relapse, the authors write, but “treating symptoms alone is not sufficient to prevent long-term complications.”
This led to a shift toward using endoscopic healing as a therapeutic goal, a move that was aided by the advent of novel medical therapies, including biologic agents. Crucially, endoscopic healing is associated with improved long-term outcomes, as well as improved quality of life.
The authors continue, however, that a “significant proportion” of patients experience relapse despite achieving endoscopic healing, which “could be explained in part by the fact that up to 40% of patients in endoscopic healing will have ongoing active histologic disease.”
However, in studies in which histologic activity was an endpoint, results have conflicted, and questions remain as to which parameters to include when assessing histologic activity.
Measuring the predictive values of endoscopy and histology
To investigate further, the researchers conducted a prospective observational study of consecutive adult patients with confirmed UC who presented to an endoscopy unit for colonoscopy for disease assessment or surveillance.
To qualify for the study, the patients’ conditions had to have been in clinical remission for at least 3 months prior to the colonoscopy. They were excluded if they had undergone prior surgical resection, had experienced disease remission for a period of over 10 years, or had used oral or rectal steroids within 90 days, among other criteria.
During an initial colonoscopy, two biopsies were performed, with specimens taken from the rectosigmoid and, when possible, from the right and left colon. Blood and stool samples were taken, and demographic and clinical data were collected.
The study enrolled 253 patients. Almost half (47.4%) were younger than 50 years, and 46.3% were women. They were followed for 12 months, during which 19% developed clinical relapse, defined as a partial Mayo endoscopic score (MES) of greater than 2.
When compared with patients with an MES of 0, the team found that patients with an MES of 1 or greater than or equal to 2 were at higher risk of relapse, with an adjusted hazard ratio of 2.65 and 2.57, respectively.
Interestingly, a lower baseline MES also was associated with a lower risk of relapse, and patients with proctitis were more likely to experience relapse than those with pancolitis.
No impact of histology on relapse risk
Further analysis revealed that there was no association between clinical relapse and age, sex, disease extent, and C-reactive protein, hemoglobin, and albumin levels. However, there was a significant association between relapse and the occurrence of at least one relapse in the 2 years prior to enrollment.
While the mean baseline fecal calprotectin (FC) level was numerically higher in patients who experienced relapse, compared with those who did not (306.9 mcg/g vs. 213.7 mcg/g), the difference was not significant.
FC of greater than 100 mcg/g was, however, significantly associated with relapse, at an odds ratio of 2.26, although the association was no longer significant when using the False Discovery Rate test.
Active histology was no more common among those who experienced relapse than among those who did not. But with regard to histologic factors, the team found that the presence of basal plasmacytosis was associated with clinical relapse, at an adjusted odds ratio of 2.07.
On the other hand, a Geboes Score of greater than or equal to 3.1, indicating the presence of epithelial neutrophils with or without crypt destruction or erosions, was not significantly associated with the risk of relapse, nor with the time to clinical relapse.
Clinical implications
Approached for comment, Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic, said that this is “the largest prospective study assessing histologic activity or remission to predict future disease relapse in ulcerative colitis.”
He told this news organization that what the findings mean for clinical practice is that “patients who achieve an endoscopic and clinical remission are at a low likelihood of clinical relapse,” and added that “these should be the ‘treat-to-target’ endpoints.”
“Patients who have biopsy evidence, [such as] histologic activity based on the Geboes Score, do not require an escalation of therapy or a change in inflammatory bowel disease therapy,” Dr. Regueiro said.
He noted, however, that one primary question remains: Aside from surveillance of dysplasia, is there a role for biopsy in cases of UC in which the Mayo score is 0?
“In my practice, I still take biopsies from a previously involved colitis segment, even if Mayo 0,” he said.
“If there is histologic activity, I would not increase or optimize the current medications, but I also would not deescalate,” Dr. Regueiro added. “I would keep the patient on a regular surveillance colonoscopy regimen, too.”
No funding for the study has been reported. Dr. Bessissow has relationships with AbbVie, Alimentiv (formerly Robarts), Amgen, Bristol-Myers-Squibb, Ferring, Gilead, Janssen, Merck, Pentax, Pfizer, Roche, Sandoz, Takeda, and Viatris. Other authors have disclosed numerous financial relationships. Dr. Regueiro has disclosed no such relationships.
A version of this article first appeared on Medscape.com.
For patients who experience endoscopic remission of ulcerative colitis (UC), signs of active disease on histology did not affect their risk of clinical relapse, according to a large prospective study that reinforces a low endoscopy score as the treatment target.
In the study of more than 250 patients in endoscopic remission from UC, 19% experienced a clinical relapse within 1 year. The researchers found that a lower baseline endoscopy score was linked to a lower risk of relapse.
While histologic activity, as reflected in the Geboes Score, was not associated with clinical relapse, the presence of basal plasmacytosis independently doubled the risk of relapse.
“Our findings do not support the use of histology as a target for treatment in patients with ulcerative colitis who already achieved clinical and endoscopic remission,” say Talat Bessissow, MD, McGill University Health Center, Montreal, and colleagues.
They add that the results “support the use of the Mayo endoscopic subscore of zero as the optimal target for endoscopic remission.”
Further prospective data are needed to “define the role of histology activity and basal plasmacytosis in the management of ulcerative colitis,” the authors write.
The study was published online in The American Journal of Gastroenterology.
Uncertain role of histology
Dr. Bessissow told this news organization that “some studies have shown that histologic healing is associated with better long-term outcomes and less relapse, but this topic remains controversial because other studies have shown the opposite.”
“Our study does not support histology as a treatment target,” he continued, adding that therapy should not be changed solely on the basis of histology.
Dr. Bessissow clarified that although histology was not associated with less relapse over 1 year of follow-up, the role of histology on other, longer-term outcomes, such as surgery and colorectal cancer, still needs to be studied.
The natural history of UC is characterized by frequent relapse, the authors write, but “treating symptoms alone is not sufficient to prevent long-term complications.”
This led to a shift toward using endoscopic healing as a therapeutic goal, a move that was aided by the advent of novel medical therapies, including biologic agents. Crucially, endoscopic healing is associated with improved long-term outcomes, as well as improved quality of life.
The authors continue, however, that a “significant proportion” of patients experience relapse despite achieving endoscopic healing, which “could be explained in part by the fact that up to 40% of patients in endoscopic healing will have ongoing active histologic disease.”
However, in studies in which histologic activity was an endpoint, results have conflicted, and questions remain as to which parameters to include when assessing histologic activity.
Measuring the predictive values of endoscopy and histology
To investigate further, the researchers conducted a prospective observational study of consecutive adult patients with confirmed UC who presented to an endoscopy unit for colonoscopy for disease assessment or surveillance.
To qualify for the study, the patients’ conditions had to have been in clinical remission for at least 3 months prior to the colonoscopy. They were excluded if they had undergone prior surgical resection, had experienced disease remission for a period of over 10 years, or had used oral or rectal steroids within 90 days, among other criteria.
During an initial colonoscopy, two biopsies were performed, with specimens taken from the rectosigmoid and, when possible, from the right and left colon. Blood and stool samples were taken, and demographic and clinical data were collected.
The study enrolled 253 patients. Almost half (47.4%) were younger than 50 years, and 46.3% were women. They were followed for 12 months, during which 19% developed clinical relapse, defined as a partial Mayo endoscopic score (MES) of greater than 2.
When compared with patients with an MES of 0, the team found that patients with an MES of 1 or greater than or equal to 2 were at higher risk of relapse, with an adjusted hazard ratio of 2.65 and 2.57, respectively.
Interestingly, a lower baseline MES also was associated with a lower risk of relapse, and patients with proctitis were more likely to experience relapse than those with pancolitis.
No impact of histology on relapse risk
Further analysis revealed that there was no association between clinical relapse and age, sex, disease extent, and C-reactive protein, hemoglobin, and albumin levels. However, there was a significant association between relapse and the occurrence of at least one relapse in the 2 years prior to enrollment.
While the mean baseline fecal calprotectin (FC) level was numerically higher in patients who experienced relapse, compared with those who did not (306.9 mcg/g vs. 213.7 mcg/g), the difference was not significant.
FC of greater than 100 mcg/g was, however, significantly associated with relapse, at an odds ratio of 2.26, although the association was no longer significant when using the False Discovery Rate test.
Active histology was no more common among those who experienced relapse than among those who did not. But with regard to histologic factors, the team found that the presence of basal plasmacytosis was associated with clinical relapse, at an adjusted odds ratio of 2.07.
On the other hand, a Geboes Score of greater than or equal to 3.1, indicating the presence of epithelial neutrophils with or without crypt destruction or erosions, was not significantly associated with the risk of relapse, nor with the time to clinical relapse.
Clinical implications
Approached for comment, Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic, said that this is “the largest prospective study assessing histologic activity or remission to predict future disease relapse in ulcerative colitis.”
He told this news organization that what the findings mean for clinical practice is that “patients who achieve an endoscopic and clinical remission are at a low likelihood of clinical relapse,” and added that “these should be the ‘treat-to-target’ endpoints.”
“Patients who have biopsy evidence, [such as] histologic activity based on the Geboes Score, do not require an escalation of therapy or a change in inflammatory bowel disease therapy,” Dr. Regueiro said.
He noted, however, that one primary question remains: Aside from surveillance of dysplasia, is there a role for biopsy in cases of UC in which the Mayo score is 0?
“In my practice, I still take biopsies from a previously involved colitis segment, even if Mayo 0,” he said.
“If there is histologic activity, I would not increase or optimize the current medications, but I also would not deescalate,” Dr. Regueiro added. “I would keep the patient on a regular surveillance colonoscopy regimen, too.”
No funding for the study has been reported. Dr. Bessissow has relationships with AbbVie, Alimentiv (formerly Robarts), Amgen, Bristol-Myers-Squibb, Ferring, Gilead, Janssen, Merck, Pentax, Pfizer, Roche, Sandoz, Takeda, and Viatris. Other authors have disclosed numerous financial relationships. Dr. Regueiro has disclosed no such relationships.
A version of this article first appeared on Medscape.com.
For patients who experience endoscopic remission of ulcerative colitis (UC), signs of active disease on histology did not affect their risk of clinical relapse, according to a large prospective study that reinforces a low endoscopy score as the treatment target.
In the study of more than 250 patients in endoscopic remission from UC, 19% experienced a clinical relapse within 1 year. The researchers found that a lower baseline endoscopy score was linked to a lower risk of relapse.
While histologic activity, as reflected in the Geboes Score, was not associated with clinical relapse, the presence of basal plasmacytosis independently doubled the risk of relapse.
“Our findings do not support the use of histology as a target for treatment in patients with ulcerative colitis who already achieved clinical and endoscopic remission,” say Talat Bessissow, MD, McGill University Health Center, Montreal, and colleagues.
They add that the results “support the use of the Mayo endoscopic subscore of zero as the optimal target for endoscopic remission.”
Further prospective data are needed to “define the role of histology activity and basal plasmacytosis in the management of ulcerative colitis,” the authors write.
The study was published online in The American Journal of Gastroenterology.
Uncertain role of histology
Dr. Bessissow told this news organization that “some studies have shown that histologic healing is associated with better long-term outcomes and less relapse, but this topic remains controversial because other studies have shown the opposite.”
“Our study does not support histology as a treatment target,” he continued, adding that therapy should not be changed solely on the basis of histology.
Dr. Bessissow clarified that although histology was not associated with less relapse over 1 year of follow-up, the role of histology on other, longer-term outcomes, such as surgery and colorectal cancer, still needs to be studied.
The natural history of UC is characterized by frequent relapse, the authors write, but “treating symptoms alone is not sufficient to prevent long-term complications.”
This led to a shift toward using endoscopic healing as a therapeutic goal, a move that was aided by the advent of novel medical therapies, including biologic agents. Crucially, endoscopic healing is associated with improved long-term outcomes, as well as improved quality of life.
The authors continue, however, that a “significant proportion” of patients experience relapse despite achieving endoscopic healing, which “could be explained in part by the fact that up to 40% of patients in endoscopic healing will have ongoing active histologic disease.”
However, in studies in which histologic activity was an endpoint, results have conflicted, and questions remain as to which parameters to include when assessing histologic activity.
Measuring the predictive values of endoscopy and histology
To investigate further, the researchers conducted a prospective observational study of consecutive adult patients with confirmed UC who presented to an endoscopy unit for colonoscopy for disease assessment or surveillance.
To qualify for the study, the patients’ conditions had to have been in clinical remission for at least 3 months prior to the colonoscopy. They were excluded if they had undergone prior surgical resection, had experienced disease remission for a period of over 10 years, or had used oral or rectal steroids within 90 days, among other criteria.
During an initial colonoscopy, two biopsies were performed, with specimens taken from the rectosigmoid and, when possible, from the right and left colon. Blood and stool samples were taken, and demographic and clinical data were collected.
The study enrolled 253 patients. Almost half (47.4%) were younger than 50 years, and 46.3% were women. They were followed for 12 months, during which 19% developed clinical relapse, defined as a partial Mayo endoscopic score (MES) of greater than 2.
When compared with patients with an MES of 0, the team found that patients with an MES of 1 or greater than or equal to 2 were at higher risk of relapse, with an adjusted hazard ratio of 2.65 and 2.57, respectively.
Interestingly, a lower baseline MES also was associated with a lower risk of relapse, and patients with proctitis were more likely to experience relapse than those with pancolitis.
No impact of histology on relapse risk
Further analysis revealed that there was no association between clinical relapse and age, sex, disease extent, and C-reactive protein, hemoglobin, and albumin levels. However, there was a significant association between relapse and the occurrence of at least one relapse in the 2 years prior to enrollment.
While the mean baseline fecal calprotectin (FC) level was numerically higher in patients who experienced relapse, compared with those who did not (306.9 mcg/g vs. 213.7 mcg/g), the difference was not significant.
FC of greater than 100 mcg/g was, however, significantly associated with relapse, at an odds ratio of 2.26, although the association was no longer significant when using the False Discovery Rate test.
Active histology was no more common among those who experienced relapse than among those who did not. But with regard to histologic factors, the team found that the presence of basal plasmacytosis was associated with clinical relapse, at an adjusted odds ratio of 2.07.
On the other hand, a Geboes Score of greater than or equal to 3.1, indicating the presence of epithelial neutrophils with or without crypt destruction or erosions, was not significantly associated with the risk of relapse, nor with the time to clinical relapse.
Clinical implications
Approached for comment, Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic, said that this is “the largest prospective study assessing histologic activity or remission to predict future disease relapse in ulcerative colitis.”
He told this news organization that what the findings mean for clinical practice is that “patients who achieve an endoscopic and clinical remission are at a low likelihood of clinical relapse,” and added that “these should be the ‘treat-to-target’ endpoints.”
“Patients who have biopsy evidence, [such as] histologic activity based on the Geboes Score, do not require an escalation of therapy or a change in inflammatory bowel disease therapy,” Dr. Regueiro said.
He noted, however, that one primary question remains: Aside from surveillance of dysplasia, is there a role for biopsy in cases of UC in which the Mayo score is 0?
“In my practice, I still take biopsies from a previously involved colitis segment, even if Mayo 0,” he said.
“If there is histologic activity, I would not increase or optimize the current medications, but I also would not deescalate,” Dr. Regueiro added. “I would keep the patient on a regular surveillance colonoscopy regimen, too.”
No funding for the study has been reported. Dr. Bessissow has relationships with AbbVie, Alimentiv (formerly Robarts), Amgen, Bristol-Myers-Squibb, Ferring, Gilead, Janssen, Merck, Pentax, Pfizer, Roche, Sandoz, Takeda, and Viatris. Other authors have disclosed numerous financial relationships. Dr. Regueiro has disclosed no such relationships.
A version of this article first appeared on Medscape.com.
The role of aspirin today
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the faculty of medicine at the University of Duisburg-Essen in Germany.
Usually in this video series, I report on interesting scientific studies in the field of neurology published in the last month. But I have to admit, June was a lousy month for new science in neurology. Therefore, this month I’d like to take a different approach and tell you about a very interesting, old drug.
We are celebrating the 125th anniversary of aspirin. Aspirin was first synthesized in Wuppertal, Germany, a city which is only 40 km from my location, by Felix Hoffmann. Hoffmann was searching for a new drug for his father who suffered from severe joint pain, and the available drugs at that time had terrible adverse events. This prompted him to work on a new drug, which was later called aspirin acetylsalicylic acid.
Aspirin has been used very successfully to the present day as therapy for joint pain or arthritis. But as you know, it’s also effective in headaches, in particular, tension-type headache. I think it’s one of the most used drugs in the world for the treatment of acute migraine attacks.
It’s also available in some European countries in intravenous form for the treatment of severe migraine attacks or in the emergency room, and it’s as effective as subcutaneous sumatriptan. It’s also an effective migraine preventive drug in a dose of 300 mg/d.
Discovering aspirin’s antiplatelet activity
There was an interesting observation by a dentist in the 1930s, who noted bleeding when he extracted teeth in people who took aspirin for joint pain. When he started to ask his patients about possible bleeding complications and vascular events, he observed that people who took aspirin didn’t have coronary myocardial infarctions.
It took a long time for people to discover that aspirin is not only a pain medication but also an antiplatelet agent. The first randomized study that showed that aspirin is effective in secondary prevention after myocardial infarction was published in 1974 in The New England Journal of Medicine. In 1980, aspirin was approved by the U.S. Food and Drug Administration for the secondary prevention of stroke and in 1984 for secondary prevention after myocardial infarction.
A history of efficacy
Aspirin also has a proven role in the secondary prevention of transient ischemic attack and ischemic stroke. Given early, it reduces the risk for a recurrent vascular event by 50% and long-term, compared with placebo, by 20%.
Interestingly, the doses are different in different areas of the world. In the United States, it’s either 81 mg or 325 mg. In Europe, it’s usually 100 mg. Until a few years ago, there was no single trial which used 100 mg of aspirin, compared with placebo for the secondary prevention of stroke.
If we look at dual antiplatelet therapy, the combination of aspirin and clopidogrel was not superior to aspirin alone or clopidogrel alone for long-term prevention, but the combination of dipyridamole and aspirin and the combination of cilostazol and aspirin were superior to aspirin alone for secondary stroke prevention. Short-term, within the first 30 days, the combination of aspirin and clopidogrel and the combination of ticagrelor and aspirin is superior to monotherapy but also have an increased risk for bleeding.
People with atrial fibrillation or embolic strokes need to be anticoagulated, but the addition of aspirin to anticoagulation does not increase efficacy, it only increases the risk for bleeding.
In people above the age of 75 years who have to take aspirin, there is an increased risk for upper gastrointestinal bleeding. These patients should, in addition, receive proton pump inhibitors.
The use of aspirin for the primary prevention of vascular events was promoted for almost 50 years all over the world, but in the last 5 years, a number of randomized trials clearly showed that aspirin is not effective, compared with placebo, in the primary prevention of vascular event stroke, myocardial infarction, and vascular death. It only increases the risk for bleeding.
So it’s a clear separation. Aspirin should not be used for primary prevention of vascular events, but it should be used in basically everyone who doesn’t have contraindications for secondary prevention of vascular events and vascular death.
Ladies and gentlemen, a drug that is 125 years old is also still one of the most used and affordable drugs all around the world. It’s highly effective and has only a small risk for major bleeding complications. It’s really time to celebrate aspirin for this achievement.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany). A complete list of his financial disclosures is available at the link below.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the faculty of medicine at the University of Duisburg-Essen in Germany.
Usually in this video series, I report on interesting scientific studies in the field of neurology published in the last month. But I have to admit, June was a lousy month for new science in neurology. Therefore, this month I’d like to take a different approach and tell you about a very interesting, old drug.
We are celebrating the 125th anniversary of aspirin. Aspirin was first synthesized in Wuppertal, Germany, a city which is only 40 km from my location, by Felix Hoffmann. Hoffmann was searching for a new drug for his father who suffered from severe joint pain, and the available drugs at that time had terrible adverse events. This prompted him to work on a new drug, which was later called aspirin acetylsalicylic acid.
Aspirin has been used very successfully to the present day as therapy for joint pain or arthritis. But as you know, it’s also effective in headaches, in particular, tension-type headache. I think it’s one of the most used drugs in the world for the treatment of acute migraine attacks.
It’s also available in some European countries in intravenous form for the treatment of severe migraine attacks or in the emergency room, and it’s as effective as subcutaneous sumatriptan. It’s also an effective migraine preventive drug in a dose of 300 mg/d.
Discovering aspirin’s antiplatelet activity
There was an interesting observation by a dentist in the 1930s, who noted bleeding when he extracted teeth in people who took aspirin for joint pain. When he started to ask his patients about possible bleeding complications and vascular events, he observed that people who took aspirin didn’t have coronary myocardial infarctions.
It took a long time for people to discover that aspirin is not only a pain medication but also an antiplatelet agent. The first randomized study that showed that aspirin is effective in secondary prevention after myocardial infarction was published in 1974 in The New England Journal of Medicine. In 1980, aspirin was approved by the U.S. Food and Drug Administration for the secondary prevention of stroke and in 1984 for secondary prevention after myocardial infarction.
A history of efficacy
Aspirin also has a proven role in the secondary prevention of transient ischemic attack and ischemic stroke. Given early, it reduces the risk for a recurrent vascular event by 50% and long-term, compared with placebo, by 20%.
Interestingly, the doses are different in different areas of the world. In the United States, it’s either 81 mg or 325 mg. In Europe, it’s usually 100 mg. Until a few years ago, there was no single trial which used 100 mg of aspirin, compared with placebo for the secondary prevention of stroke.
If we look at dual antiplatelet therapy, the combination of aspirin and clopidogrel was not superior to aspirin alone or clopidogrel alone for long-term prevention, but the combination of dipyridamole and aspirin and the combination of cilostazol and aspirin were superior to aspirin alone for secondary stroke prevention. Short-term, within the first 30 days, the combination of aspirin and clopidogrel and the combination of ticagrelor and aspirin is superior to monotherapy but also have an increased risk for bleeding.
People with atrial fibrillation or embolic strokes need to be anticoagulated, but the addition of aspirin to anticoagulation does not increase efficacy, it only increases the risk for bleeding.
In people above the age of 75 years who have to take aspirin, there is an increased risk for upper gastrointestinal bleeding. These patients should, in addition, receive proton pump inhibitors.
The use of aspirin for the primary prevention of vascular events was promoted for almost 50 years all over the world, but in the last 5 years, a number of randomized trials clearly showed that aspirin is not effective, compared with placebo, in the primary prevention of vascular event stroke, myocardial infarction, and vascular death. It only increases the risk for bleeding.
So it’s a clear separation. Aspirin should not be used for primary prevention of vascular events, but it should be used in basically everyone who doesn’t have contraindications for secondary prevention of vascular events and vascular death.
Ladies and gentlemen, a drug that is 125 years old is also still one of the most used and affordable drugs all around the world. It’s highly effective and has only a small risk for major bleeding complications. It’s really time to celebrate aspirin for this achievement.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany). A complete list of his financial disclosures is available at the link below.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the faculty of medicine at the University of Duisburg-Essen in Germany.
Usually in this video series, I report on interesting scientific studies in the field of neurology published in the last month. But I have to admit, June was a lousy month for new science in neurology. Therefore, this month I’d like to take a different approach and tell you about a very interesting, old drug.
We are celebrating the 125th anniversary of aspirin. Aspirin was first synthesized in Wuppertal, Germany, a city which is only 40 km from my location, by Felix Hoffmann. Hoffmann was searching for a new drug for his father who suffered from severe joint pain, and the available drugs at that time had terrible adverse events. This prompted him to work on a new drug, which was later called aspirin acetylsalicylic acid.
Aspirin has been used very successfully to the present day as therapy for joint pain or arthritis. But as you know, it’s also effective in headaches, in particular, tension-type headache. I think it’s one of the most used drugs in the world for the treatment of acute migraine attacks.
It’s also available in some European countries in intravenous form for the treatment of severe migraine attacks or in the emergency room, and it’s as effective as subcutaneous sumatriptan. It’s also an effective migraine preventive drug in a dose of 300 mg/d.
Discovering aspirin’s antiplatelet activity
There was an interesting observation by a dentist in the 1930s, who noted bleeding when he extracted teeth in people who took aspirin for joint pain. When he started to ask his patients about possible bleeding complications and vascular events, he observed that people who took aspirin didn’t have coronary myocardial infarctions.
It took a long time for people to discover that aspirin is not only a pain medication but also an antiplatelet agent. The first randomized study that showed that aspirin is effective in secondary prevention after myocardial infarction was published in 1974 in The New England Journal of Medicine. In 1980, aspirin was approved by the U.S. Food and Drug Administration for the secondary prevention of stroke and in 1984 for secondary prevention after myocardial infarction.
A history of efficacy
Aspirin also has a proven role in the secondary prevention of transient ischemic attack and ischemic stroke. Given early, it reduces the risk for a recurrent vascular event by 50% and long-term, compared with placebo, by 20%.
Interestingly, the doses are different in different areas of the world. In the United States, it’s either 81 mg or 325 mg. In Europe, it’s usually 100 mg. Until a few years ago, there was no single trial which used 100 mg of aspirin, compared with placebo for the secondary prevention of stroke.
If we look at dual antiplatelet therapy, the combination of aspirin and clopidogrel was not superior to aspirin alone or clopidogrel alone for long-term prevention, but the combination of dipyridamole and aspirin and the combination of cilostazol and aspirin were superior to aspirin alone for secondary stroke prevention. Short-term, within the first 30 days, the combination of aspirin and clopidogrel and the combination of ticagrelor and aspirin is superior to monotherapy but also have an increased risk for bleeding.
People with atrial fibrillation or embolic strokes need to be anticoagulated, but the addition of aspirin to anticoagulation does not increase efficacy, it only increases the risk for bleeding.
In people above the age of 75 years who have to take aspirin, there is an increased risk for upper gastrointestinal bleeding. These patients should, in addition, receive proton pump inhibitors.
The use of aspirin for the primary prevention of vascular events was promoted for almost 50 years all over the world, but in the last 5 years, a number of randomized trials clearly showed that aspirin is not effective, compared with placebo, in the primary prevention of vascular event stroke, myocardial infarction, and vascular death. It only increases the risk for bleeding.
So it’s a clear separation. Aspirin should not be used for primary prevention of vascular events, but it should be used in basically everyone who doesn’t have contraindications for secondary prevention of vascular events and vascular death.
Ladies and gentlemen, a drug that is 125 years old is also still one of the most used and affordable drugs all around the world. It’s highly effective and has only a small risk for major bleeding complications. It’s really time to celebrate aspirin for this achievement.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany). A complete list of his financial disclosures is available at the link below.
A version of this article first appeared on Medscape.com.
Hyperthyroidism rebound in pregnancy boosts adverse outcomes
Discontinuing antithyroid drugs during early pregnancy is linked to a possible rebound of hyperthyroidism and a high risk of adverse pregnancy outcomes, new research shows.
“Our study provides preliminary evidence that the risk of rebound increases in women with subnormal thyroid-stimulating hormone (TSH) and/or positive thyrotropin receptor antibody (TRAb) who stop antithyroid drugs in early pregnancy,” first author Xin Hou told this news organization.
“When discussing the pros and cons of antithyroid drug withdrawal early in pregnancy [clinicians] should consider the level of TSH and TRAb in early pregnancy,” said Hou, of the department of endocrinology and metabolism, Institute of Endocrinology, The First Affiliated Hospital of China Medical University, Shenyang.
Suvi Turunen, MD, of the University of Oulu (Finland), who has also conducted research on the issue, said the study adds important insights.
“I find this study very interesting,” Dr. Turunen said in an interview. “It is well known that medical treatment of hyperthyroidism outweighs the potential harms of antithyroid treatment.”
The new findings add to the evidence, she added. “I think that withdrawal of antithyroid drugs should be carefully considered, especially with autoantibody-positive patients,” Dr. Turunen said.
Hyperthyroidism a risk in pregnancy – with or without treatment
The potential risks of hyperthyroidism in pregnancy are well established and can range from preeclampsia to premature birth or miscarriage.
However, antithyroid drugs, including methimazole and propylthiouracil, carry their own risks. In crossing the placental barrier, the drugs can increase the risk of birth defects, particularly during 6-10 weeks of gestation, yet their discontinuation is linked to as much as a 50%-60% risk of relapse, the authors explain.
Because of the risks, the American Thyroid Association recommends that “women with a stable euthyroid state on 5-10 mg methimazole per day achieved within a few months, and a falling TRAb level, are likely candidates to withdraw from antithyroid drug therapy in early pregnancy,” the authors noted.
However, as the recommendations for women who are already pregnant are largely based on evidence from nonpregnant patients, Hou and colleagues sought to evaluate withdrawal among women who were pregnant.
For the study, published in Thyroid, they enrolled 63 women who were pregnant and part of an outpatient service of the department of endocrinology and metabolism at The First Affiliated Hospital of China Medical University, between September 2014 and March 2017, who had well-controlled hyperthyroidism in early pregnancy and discontinued the drugs.
The women were an average age of 27 years, and 28 were multigravida. Twenty-two had a history of miscarriage.
A follow-up of the patients until the end of their pregnancy showed that, overall, 20 (31.7%) had a rebound of hyperthyroidism during their pregnancy after withdrawing from the drugs.
Key factors associated with the highest risk of a rebound after discontinuation included having subnormal TSH levels (TSH < 0.35 mIU/L; odds ratio, 5.12; P = .03) or having positive TRAb (TRAb > 1.75 IU/L; OR, 3.79; P = .02) at the time of medication withdrawal, compared with those with either normal TSH levels or negative TRAb.
The combination of both subnormal TSH and positive TRAb at the time of antithyroid medication withdrawal further boosted the risk of hyperthyroidism rebound (83.3%, 5 of 6), compared with those who had both normal TSH and negative TRAb (13%, 3 of 23; OR, 33.33; P = .003).
Adverse pregnancy outcomes increased
Importantly, among the 20 patients who had a rebound, 11 (55%) had adverse pregnancy outcomes, including miscarriage, premature birth, induced labor, gestational hypertension, and gestational diabetes, compared with only 4 (9.3%) of the 43 who had no rebound (OR, 11.92; P = .0002).
Neonatal abnormalities were also higher among those experiencing a rebound (20% vs. 4.7%), however, the authors noted that “larger prospective studies are required to conclude whether antithyroid drug withdrawal affects fetal outcome.”
In the rebound group, the mean duration of antithyroid medication use was 24.7 months versus 35.1 months in the nonrebound group, however, the difference was not statistically significant (P = .07). And 40% of the rebound group had a history of miscarriage versus 32.6% in the non-rebound group, but was also not significantly different (P = .56).
The authors noted that half of those in the rebound group developed hyperthyroidism more than 4 weeks after their withdrawal from antithyroid medications, “which seemed to have circumvented the most sensitive period of teratogenesis between 6 and 10 weeks of pregnancy.”
Hou added that restarting antithyroid medication did not increase the risk of adverse outcomes for offspring.
“A low dose of antithyroid medications may be a good choice for women with subnormal TSH and/or positive TRAb in early pregnancy,” Hou concluded. “Because of the small size of our study, a larger prospective study is needed to overcome the potential selection bias and to verify the conclusions.”
Findings consistent with Finnish study
In her own recent study, which included 2,144 women in Finland who experienced hyperthyroidism during pregnancy, Dr. Turunen and colleagues found that having hyperthyroidism, with or without antithyroid drug treatment, was associated with an increased odds of pregnancy and/or prenatal complications, compared with those without thyroid disease.
“In our study, we observed an increased risk of adverse pregnancy outcomes also in mothers with previous diagnosis and/or treatment of hyperthyroidism, not only with overt hyperthyroidism treated with antithyroid drugs,” she told this news organization.
“I think that especially those patients with positive antibodies [TRAbs] are at risk even if they are euthyroid,” she noted. “Withdrawal of antithyroid drugs in these patients is a risk.”
“Probably continuing antithyroid treatment with low dose is a better option,” she said.
The authors and Dr. Turunen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Discontinuing antithyroid drugs during early pregnancy is linked to a possible rebound of hyperthyroidism and a high risk of adverse pregnancy outcomes, new research shows.
“Our study provides preliminary evidence that the risk of rebound increases in women with subnormal thyroid-stimulating hormone (TSH) and/or positive thyrotropin receptor antibody (TRAb) who stop antithyroid drugs in early pregnancy,” first author Xin Hou told this news organization.
“When discussing the pros and cons of antithyroid drug withdrawal early in pregnancy [clinicians] should consider the level of TSH and TRAb in early pregnancy,” said Hou, of the department of endocrinology and metabolism, Institute of Endocrinology, The First Affiliated Hospital of China Medical University, Shenyang.
Suvi Turunen, MD, of the University of Oulu (Finland), who has also conducted research on the issue, said the study adds important insights.
“I find this study very interesting,” Dr. Turunen said in an interview. “It is well known that medical treatment of hyperthyroidism outweighs the potential harms of antithyroid treatment.”
The new findings add to the evidence, she added. “I think that withdrawal of antithyroid drugs should be carefully considered, especially with autoantibody-positive patients,” Dr. Turunen said.
Hyperthyroidism a risk in pregnancy – with or without treatment
The potential risks of hyperthyroidism in pregnancy are well established and can range from preeclampsia to premature birth or miscarriage.
However, antithyroid drugs, including methimazole and propylthiouracil, carry their own risks. In crossing the placental barrier, the drugs can increase the risk of birth defects, particularly during 6-10 weeks of gestation, yet their discontinuation is linked to as much as a 50%-60% risk of relapse, the authors explain.
Because of the risks, the American Thyroid Association recommends that “women with a stable euthyroid state on 5-10 mg methimazole per day achieved within a few months, and a falling TRAb level, are likely candidates to withdraw from antithyroid drug therapy in early pregnancy,” the authors noted.
However, as the recommendations for women who are already pregnant are largely based on evidence from nonpregnant patients, Hou and colleagues sought to evaluate withdrawal among women who were pregnant.
For the study, published in Thyroid, they enrolled 63 women who were pregnant and part of an outpatient service of the department of endocrinology and metabolism at The First Affiliated Hospital of China Medical University, between September 2014 and March 2017, who had well-controlled hyperthyroidism in early pregnancy and discontinued the drugs.
The women were an average age of 27 years, and 28 were multigravida. Twenty-two had a history of miscarriage.
A follow-up of the patients until the end of their pregnancy showed that, overall, 20 (31.7%) had a rebound of hyperthyroidism during their pregnancy after withdrawing from the drugs.
Key factors associated with the highest risk of a rebound after discontinuation included having subnormal TSH levels (TSH < 0.35 mIU/L; odds ratio, 5.12; P = .03) or having positive TRAb (TRAb > 1.75 IU/L; OR, 3.79; P = .02) at the time of medication withdrawal, compared with those with either normal TSH levels or negative TRAb.
The combination of both subnormal TSH and positive TRAb at the time of antithyroid medication withdrawal further boosted the risk of hyperthyroidism rebound (83.3%, 5 of 6), compared with those who had both normal TSH and negative TRAb (13%, 3 of 23; OR, 33.33; P = .003).
Adverse pregnancy outcomes increased
Importantly, among the 20 patients who had a rebound, 11 (55%) had adverse pregnancy outcomes, including miscarriage, premature birth, induced labor, gestational hypertension, and gestational diabetes, compared with only 4 (9.3%) of the 43 who had no rebound (OR, 11.92; P = .0002).
Neonatal abnormalities were also higher among those experiencing a rebound (20% vs. 4.7%), however, the authors noted that “larger prospective studies are required to conclude whether antithyroid drug withdrawal affects fetal outcome.”
In the rebound group, the mean duration of antithyroid medication use was 24.7 months versus 35.1 months in the nonrebound group, however, the difference was not statistically significant (P = .07). And 40% of the rebound group had a history of miscarriage versus 32.6% in the non-rebound group, but was also not significantly different (P = .56).
The authors noted that half of those in the rebound group developed hyperthyroidism more than 4 weeks after their withdrawal from antithyroid medications, “which seemed to have circumvented the most sensitive period of teratogenesis between 6 and 10 weeks of pregnancy.”
Hou added that restarting antithyroid medication did not increase the risk of adverse outcomes for offspring.
“A low dose of antithyroid medications may be a good choice for women with subnormal TSH and/or positive TRAb in early pregnancy,” Hou concluded. “Because of the small size of our study, a larger prospective study is needed to overcome the potential selection bias and to verify the conclusions.”
Findings consistent with Finnish study
In her own recent study, which included 2,144 women in Finland who experienced hyperthyroidism during pregnancy, Dr. Turunen and colleagues found that having hyperthyroidism, with or without antithyroid drug treatment, was associated with an increased odds of pregnancy and/or prenatal complications, compared with those without thyroid disease.
“In our study, we observed an increased risk of adverse pregnancy outcomes also in mothers with previous diagnosis and/or treatment of hyperthyroidism, not only with overt hyperthyroidism treated with antithyroid drugs,” she told this news organization.
“I think that especially those patients with positive antibodies [TRAbs] are at risk even if they are euthyroid,” she noted. “Withdrawal of antithyroid drugs in these patients is a risk.”
“Probably continuing antithyroid treatment with low dose is a better option,” she said.
The authors and Dr. Turunen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Discontinuing antithyroid drugs during early pregnancy is linked to a possible rebound of hyperthyroidism and a high risk of adverse pregnancy outcomes, new research shows.
“Our study provides preliminary evidence that the risk of rebound increases in women with subnormal thyroid-stimulating hormone (TSH) and/or positive thyrotropin receptor antibody (TRAb) who stop antithyroid drugs in early pregnancy,” first author Xin Hou told this news organization.
“When discussing the pros and cons of antithyroid drug withdrawal early in pregnancy [clinicians] should consider the level of TSH and TRAb in early pregnancy,” said Hou, of the department of endocrinology and metabolism, Institute of Endocrinology, The First Affiliated Hospital of China Medical University, Shenyang.
Suvi Turunen, MD, of the University of Oulu (Finland), who has also conducted research on the issue, said the study adds important insights.
“I find this study very interesting,” Dr. Turunen said in an interview. “It is well known that medical treatment of hyperthyroidism outweighs the potential harms of antithyroid treatment.”
The new findings add to the evidence, she added. “I think that withdrawal of antithyroid drugs should be carefully considered, especially with autoantibody-positive patients,” Dr. Turunen said.
Hyperthyroidism a risk in pregnancy – with or without treatment
The potential risks of hyperthyroidism in pregnancy are well established and can range from preeclampsia to premature birth or miscarriage.
However, antithyroid drugs, including methimazole and propylthiouracil, carry their own risks. In crossing the placental barrier, the drugs can increase the risk of birth defects, particularly during 6-10 weeks of gestation, yet their discontinuation is linked to as much as a 50%-60% risk of relapse, the authors explain.
Because of the risks, the American Thyroid Association recommends that “women with a stable euthyroid state on 5-10 mg methimazole per day achieved within a few months, and a falling TRAb level, are likely candidates to withdraw from antithyroid drug therapy in early pregnancy,” the authors noted.
However, as the recommendations for women who are already pregnant are largely based on evidence from nonpregnant patients, Hou and colleagues sought to evaluate withdrawal among women who were pregnant.
For the study, published in Thyroid, they enrolled 63 women who were pregnant and part of an outpatient service of the department of endocrinology and metabolism at The First Affiliated Hospital of China Medical University, between September 2014 and March 2017, who had well-controlled hyperthyroidism in early pregnancy and discontinued the drugs.
The women were an average age of 27 years, and 28 were multigravida. Twenty-two had a history of miscarriage.
A follow-up of the patients until the end of their pregnancy showed that, overall, 20 (31.7%) had a rebound of hyperthyroidism during their pregnancy after withdrawing from the drugs.
Key factors associated with the highest risk of a rebound after discontinuation included having subnormal TSH levels (TSH < 0.35 mIU/L; odds ratio, 5.12; P = .03) or having positive TRAb (TRAb > 1.75 IU/L; OR, 3.79; P = .02) at the time of medication withdrawal, compared with those with either normal TSH levels or negative TRAb.
The combination of both subnormal TSH and positive TRAb at the time of antithyroid medication withdrawal further boosted the risk of hyperthyroidism rebound (83.3%, 5 of 6), compared with those who had both normal TSH and negative TRAb (13%, 3 of 23; OR, 33.33; P = .003).
Adverse pregnancy outcomes increased
Importantly, among the 20 patients who had a rebound, 11 (55%) had adverse pregnancy outcomes, including miscarriage, premature birth, induced labor, gestational hypertension, and gestational diabetes, compared with only 4 (9.3%) of the 43 who had no rebound (OR, 11.92; P = .0002).
Neonatal abnormalities were also higher among those experiencing a rebound (20% vs. 4.7%), however, the authors noted that “larger prospective studies are required to conclude whether antithyroid drug withdrawal affects fetal outcome.”
In the rebound group, the mean duration of antithyroid medication use was 24.7 months versus 35.1 months in the nonrebound group, however, the difference was not statistically significant (P = .07). And 40% of the rebound group had a history of miscarriage versus 32.6% in the non-rebound group, but was also not significantly different (P = .56).
The authors noted that half of those in the rebound group developed hyperthyroidism more than 4 weeks after their withdrawal from antithyroid medications, “which seemed to have circumvented the most sensitive period of teratogenesis between 6 and 10 weeks of pregnancy.”
Hou added that restarting antithyroid medication did not increase the risk of adverse outcomes for offspring.
“A low dose of antithyroid medications may be a good choice for women with subnormal TSH and/or positive TRAb in early pregnancy,” Hou concluded. “Because of the small size of our study, a larger prospective study is needed to overcome the potential selection bias and to verify the conclusions.”
Findings consistent with Finnish study
In her own recent study, which included 2,144 women in Finland who experienced hyperthyroidism during pregnancy, Dr. Turunen and colleagues found that having hyperthyroidism, with or without antithyroid drug treatment, was associated with an increased odds of pregnancy and/or prenatal complications, compared with those without thyroid disease.
“In our study, we observed an increased risk of adverse pregnancy outcomes also in mothers with previous diagnosis and/or treatment of hyperthyroidism, not only with overt hyperthyroidism treated with antithyroid drugs,” she told this news organization.
“I think that especially those patients with positive antibodies [TRAbs] are at risk even if they are euthyroid,” she noted. “Withdrawal of antithyroid drugs in these patients is a risk.”
“Probably continuing antithyroid treatment with low dose is a better option,” she said.
The authors and Dr. Turunen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THYROID
NAFLD linked with increased heart failure risk
The risk of developing incident heart failure is 1.5-times higher in people with nonalcoholic fatty liver disease (NAFLD) during a median follow-up of 10 years, according to a new meta-analysis.
The risk appears to increase with greater liver disease severity and was independent of age, sex, ethnicity, obesity, and the presence of diabetes, hypertension, and other common cardiovascular risk factors.
“Health care professionals should be aware that the risk of new-onset heart failure is moderately higher in patients with NAFLD,” senior author Giovanni Targher, MD, said in an interview.
“Because of the link between the two conditions, more careful surveillance of these patients will be needed,” said Dr. Targher, who is an associate professor of diabetes and endocrinology at the University of Verona (Italy). “In particular, the results of this meta-analysis highlight the need for a patient-centered, multidisciplinary, and holistic approach to manage both liver disease and cardiovascular risk in patients with NAFLD.”
The study was published online in Gut.
Risk calculations
NAFLD has become one of the most common causes of chronic liver disease worldwide (affecting up to about 30% of the world’s adults), and is expected to rise sharply in the next decade, the study authors write. The disease is linked with liver-related conditions, such as nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma, as well as complications in other organs.
Previous meta-analyses have found an association between NAFLD and a higher risk of heart failure, though the analyses included a relatively small number of studies and a relatively modest sample size, Dr. Targher and colleagues write.
Since then, several new cohort studies have examined the association, which inspired a new meta-analysis.
The research team analyzed 11 observational cohort studies with aggregate data on more than 11 million middle-aged people from different countries, including nearly 3 million with NAFLD and nearly 98,000 cases of incident heart failure over a median follow-up of 10 years.
In the studies, NAFLD was diagnosed by serum liver enzyme levels, serum biomarkers or scores, diagnostic codes, imaging techniques, or liver histology. Four studies were conducted in the United States, three were conducted in South Korea, and four were carried out in Europe, including Finland, Sweden, and the United Kingdom.
Dr. Targher and colleagues found that the presence of NAFLD was associated with a moderately higher risk of new-onset heart failure, with a pooled random-effects hazard ratio of 1.5. The risk was independent of age, sex, ethnicity, adiposity measures, diabetes, hypertension, and other typical cardiovascular risk factors.
The association between NAFLD and heart failure risk was consistent even when the comparison was stratified by study country, follow-up length, modality of heart failure diagnosis, and modality of NAFLD diagnosis.
In addition, sensitivity analyses didn’t change the results, and a funnel plot suggested that publication bias was unlikely.
“Accumulating evidence supports that NAFLD is part of a multisystem disease that adversely affects several extrahepatic organs, including the heart,” Dr. Targher said.
“NAFLD not only promotes accelerated coronary atherosclerosis but also confers a higher risk of myocardial abnormalities (cardiac remodeling and hypertrophy) and certain arrhythmias (mostly atrial fibrillation), which may precede and promote the development of new-onset heart failure over time,” he said.
Future research
Dr. Targher and colleagues also found that the risk of incident heart failure appeared to further increase with more advanced liver disease, particularly with higher levels of liver fibrosis, as assessed by noninvasive fibrosis biomarkers or histology. With only two cohort studies that examined the association, the authors judged there was insufficient data available to combine the studies into a meta-analysis.
But the observations are consistent with other recent meta-analyses that reported a significant association between the presence and severity of NAFLD and the risk of developing adverse cardiovascular outcomes, atrial fibrillation, chronic kidney disease, or other non-liver complications.
“It’s reassuring that the observations that have come from single studies hold true when you look at the totality of evidence,” Ambarish Pandey, MD, a cardiologist and assistant professor of internal medicine at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
Dr. Pandey, who wasn’t involved with this study, conducted one of the recent meta-analyses that found a 1.6-times increased risk of heart failure associated with NAFLD, as well as a further increased risk with more advanced liver disease.
Now Dr. Pandey and colleagues are studying the underlying mechanisms for the link between NAFLD and heart failure risk, including cardiac structure and function, biomarkers of injury and stress, and how proportions of liver fat influence risk. Additional studies should investigate whether resolving NAFLD could reduce the risk of heart failure, he said.
“It’s really important to look for patients with NAFLD in primary care and think about cardiovascular disease in our liver patients,” he said. “Early strategies to implement the prevention of heart failure would go a long way in reducing long-term risks for these patients.”
The study authors did not declare a specific grant for this research from any funding agency in the public, commercial, or nonprofit sectors. Dr. Targher and Dr. Pandey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The risk of developing incident heart failure is 1.5-times higher in people with nonalcoholic fatty liver disease (NAFLD) during a median follow-up of 10 years, according to a new meta-analysis.
The risk appears to increase with greater liver disease severity and was independent of age, sex, ethnicity, obesity, and the presence of diabetes, hypertension, and other common cardiovascular risk factors.
“Health care professionals should be aware that the risk of new-onset heart failure is moderately higher in patients with NAFLD,” senior author Giovanni Targher, MD, said in an interview.
“Because of the link between the two conditions, more careful surveillance of these patients will be needed,” said Dr. Targher, who is an associate professor of diabetes and endocrinology at the University of Verona (Italy). “In particular, the results of this meta-analysis highlight the need for a patient-centered, multidisciplinary, and holistic approach to manage both liver disease and cardiovascular risk in patients with NAFLD.”
The study was published online in Gut.
Risk calculations
NAFLD has become one of the most common causes of chronic liver disease worldwide (affecting up to about 30% of the world’s adults), and is expected to rise sharply in the next decade, the study authors write. The disease is linked with liver-related conditions, such as nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma, as well as complications in other organs.
Previous meta-analyses have found an association between NAFLD and a higher risk of heart failure, though the analyses included a relatively small number of studies and a relatively modest sample size, Dr. Targher and colleagues write.
Since then, several new cohort studies have examined the association, which inspired a new meta-analysis.
The research team analyzed 11 observational cohort studies with aggregate data on more than 11 million middle-aged people from different countries, including nearly 3 million with NAFLD and nearly 98,000 cases of incident heart failure over a median follow-up of 10 years.
In the studies, NAFLD was diagnosed by serum liver enzyme levels, serum biomarkers or scores, diagnostic codes, imaging techniques, or liver histology. Four studies were conducted in the United States, three were conducted in South Korea, and four were carried out in Europe, including Finland, Sweden, and the United Kingdom.
Dr. Targher and colleagues found that the presence of NAFLD was associated with a moderately higher risk of new-onset heart failure, with a pooled random-effects hazard ratio of 1.5. The risk was independent of age, sex, ethnicity, adiposity measures, diabetes, hypertension, and other typical cardiovascular risk factors.
The association between NAFLD and heart failure risk was consistent even when the comparison was stratified by study country, follow-up length, modality of heart failure diagnosis, and modality of NAFLD diagnosis.
In addition, sensitivity analyses didn’t change the results, and a funnel plot suggested that publication bias was unlikely.
“Accumulating evidence supports that NAFLD is part of a multisystem disease that adversely affects several extrahepatic organs, including the heart,” Dr. Targher said.
“NAFLD not only promotes accelerated coronary atherosclerosis but also confers a higher risk of myocardial abnormalities (cardiac remodeling and hypertrophy) and certain arrhythmias (mostly atrial fibrillation), which may precede and promote the development of new-onset heart failure over time,” he said.
Future research
Dr. Targher and colleagues also found that the risk of incident heart failure appeared to further increase with more advanced liver disease, particularly with higher levels of liver fibrosis, as assessed by noninvasive fibrosis biomarkers or histology. With only two cohort studies that examined the association, the authors judged there was insufficient data available to combine the studies into a meta-analysis.
But the observations are consistent with other recent meta-analyses that reported a significant association between the presence and severity of NAFLD and the risk of developing adverse cardiovascular outcomes, atrial fibrillation, chronic kidney disease, or other non-liver complications.
“It’s reassuring that the observations that have come from single studies hold true when you look at the totality of evidence,” Ambarish Pandey, MD, a cardiologist and assistant professor of internal medicine at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
Dr. Pandey, who wasn’t involved with this study, conducted one of the recent meta-analyses that found a 1.6-times increased risk of heart failure associated with NAFLD, as well as a further increased risk with more advanced liver disease.
Now Dr. Pandey and colleagues are studying the underlying mechanisms for the link between NAFLD and heart failure risk, including cardiac structure and function, biomarkers of injury and stress, and how proportions of liver fat influence risk. Additional studies should investigate whether resolving NAFLD could reduce the risk of heart failure, he said.
“It’s really important to look for patients with NAFLD in primary care and think about cardiovascular disease in our liver patients,” he said. “Early strategies to implement the prevention of heart failure would go a long way in reducing long-term risks for these patients.”
The study authors did not declare a specific grant for this research from any funding agency in the public, commercial, or nonprofit sectors. Dr. Targher and Dr. Pandey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The risk of developing incident heart failure is 1.5-times higher in people with nonalcoholic fatty liver disease (NAFLD) during a median follow-up of 10 years, according to a new meta-analysis.
The risk appears to increase with greater liver disease severity and was independent of age, sex, ethnicity, obesity, and the presence of diabetes, hypertension, and other common cardiovascular risk factors.
“Health care professionals should be aware that the risk of new-onset heart failure is moderately higher in patients with NAFLD,” senior author Giovanni Targher, MD, said in an interview.
“Because of the link between the two conditions, more careful surveillance of these patients will be needed,” said Dr. Targher, who is an associate professor of diabetes and endocrinology at the University of Verona (Italy). “In particular, the results of this meta-analysis highlight the need for a patient-centered, multidisciplinary, and holistic approach to manage both liver disease and cardiovascular risk in patients with NAFLD.”
The study was published online in Gut.
Risk calculations
NAFLD has become one of the most common causes of chronic liver disease worldwide (affecting up to about 30% of the world’s adults), and is expected to rise sharply in the next decade, the study authors write. The disease is linked with liver-related conditions, such as nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma, as well as complications in other organs.
Previous meta-analyses have found an association between NAFLD and a higher risk of heart failure, though the analyses included a relatively small number of studies and a relatively modest sample size, Dr. Targher and colleagues write.
Since then, several new cohort studies have examined the association, which inspired a new meta-analysis.
The research team analyzed 11 observational cohort studies with aggregate data on more than 11 million middle-aged people from different countries, including nearly 3 million with NAFLD and nearly 98,000 cases of incident heart failure over a median follow-up of 10 years.
In the studies, NAFLD was diagnosed by serum liver enzyme levels, serum biomarkers or scores, diagnostic codes, imaging techniques, or liver histology. Four studies were conducted in the United States, three were conducted in South Korea, and four were carried out in Europe, including Finland, Sweden, and the United Kingdom.
Dr. Targher and colleagues found that the presence of NAFLD was associated with a moderately higher risk of new-onset heart failure, with a pooled random-effects hazard ratio of 1.5. The risk was independent of age, sex, ethnicity, adiposity measures, diabetes, hypertension, and other typical cardiovascular risk factors.
The association between NAFLD and heart failure risk was consistent even when the comparison was stratified by study country, follow-up length, modality of heart failure diagnosis, and modality of NAFLD diagnosis.
In addition, sensitivity analyses didn’t change the results, and a funnel plot suggested that publication bias was unlikely.
“Accumulating evidence supports that NAFLD is part of a multisystem disease that adversely affects several extrahepatic organs, including the heart,” Dr. Targher said.
“NAFLD not only promotes accelerated coronary atherosclerosis but also confers a higher risk of myocardial abnormalities (cardiac remodeling and hypertrophy) and certain arrhythmias (mostly atrial fibrillation), which may precede and promote the development of new-onset heart failure over time,” he said.
Future research
Dr. Targher and colleagues also found that the risk of incident heart failure appeared to further increase with more advanced liver disease, particularly with higher levels of liver fibrosis, as assessed by noninvasive fibrosis biomarkers or histology. With only two cohort studies that examined the association, the authors judged there was insufficient data available to combine the studies into a meta-analysis.
But the observations are consistent with other recent meta-analyses that reported a significant association between the presence and severity of NAFLD and the risk of developing adverse cardiovascular outcomes, atrial fibrillation, chronic kidney disease, or other non-liver complications.
“It’s reassuring that the observations that have come from single studies hold true when you look at the totality of evidence,” Ambarish Pandey, MD, a cardiologist and assistant professor of internal medicine at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
Dr. Pandey, who wasn’t involved with this study, conducted one of the recent meta-analyses that found a 1.6-times increased risk of heart failure associated with NAFLD, as well as a further increased risk with more advanced liver disease.
Now Dr. Pandey and colleagues are studying the underlying mechanisms for the link between NAFLD and heart failure risk, including cardiac structure and function, biomarkers of injury and stress, and how proportions of liver fat influence risk. Additional studies should investigate whether resolving NAFLD could reduce the risk of heart failure, he said.
“It’s really important to look for patients with NAFLD in primary care and think about cardiovascular disease in our liver patients,” he said. “Early strategies to implement the prevention of heart failure would go a long way in reducing long-term risks for these patients.”
The study authors did not declare a specific grant for this research from any funding agency in the public, commercial, or nonprofit sectors. Dr. Targher and Dr. Pandey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GUT