User login
A valuable learning experience
It was a pleasure to serve as editor-in-chief (EIC) of GI & Hepatology News from 2011 to 2016. As the second EIC of the newspaper, I was preceded by Dr. Charles Lightdale – big shoes to fill! I was fortunate to attract a strong group of associate editors who covered many key areas of interest for the paper’s readership. With the enthusiastic support of American Gastroenterological Association staff members, we published once-monthly and received generally positive feedback from readers – predominantly U.S.-based AGA members.
Serving as EIC was also a learning opportunity for me. A number of potentially newsworthy items were brought to my attention – some of which I would not otherwise have seen. Although not all were of direct relevance to the readership, I believe that most of those we published were of value.
One rewarding aspect of the editorship was the opportunity to liaise with those experts from whom I solicited commentaries on some of our featured items. These busy individuals were consistently generous with their time and expertise, and I believe that their contributions added to the paper’s overall appeal.
I initiated the inclusion of two DDSEP questions per edition, and am pleased that this feature continues. One less successful venture was the attempt at a Correspondence section, which ultimately proved too cumbersome to maintain.
I congratulate the AGA on the 15th anniversary of GIHN, and I wish the current EIC, Dr. Megan Adams, and her editorial colleagues continued success in providing this benefit to AGA members.
Colin W. Howden, MD, AGAF, is professor emeritus in the division of gastroenterology, department of medicine, at the University of Tennessee, Memphis. He is a consultant for Allakos, Ironwood, Phathom, and RedHill Biopharma. He is a member of speakers’ bureaus for Alnylam, RedHill Biopharma, and Sanofi/Genzyme. He owns stock in Antibe Therapeutics.
It was a pleasure to serve as editor-in-chief (EIC) of GI & Hepatology News from 2011 to 2016. As the second EIC of the newspaper, I was preceded by Dr. Charles Lightdale – big shoes to fill! I was fortunate to attract a strong group of associate editors who covered many key areas of interest for the paper’s readership. With the enthusiastic support of American Gastroenterological Association staff members, we published once-monthly and received generally positive feedback from readers – predominantly U.S.-based AGA members.
Serving as EIC was also a learning opportunity for me. A number of potentially newsworthy items were brought to my attention – some of which I would not otherwise have seen. Although not all were of direct relevance to the readership, I believe that most of those we published were of value.
One rewarding aspect of the editorship was the opportunity to liaise with those experts from whom I solicited commentaries on some of our featured items. These busy individuals were consistently generous with their time and expertise, and I believe that their contributions added to the paper’s overall appeal.
I initiated the inclusion of two DDSEP questions per edition, and am pleased that this feature continues. One less successful venture was the attempt at a Correspondence section, which ultimately proved too cumbersome to maintain.
I congratulate the AGA on the 15th anniversary of GIHN, and I wish the current EIC, Dr. Megan Adams, and her editorial colleagues continued success in providing this benefit to AGA members.
Colin W. Howden, MD, AGAF, is professor emeritus in the division of gastroenterology, department of medicine, at the University of Tennessee, Memphis. He is a consultant for Allakos, Ironwood, Phathom, and RedHill Biopharma. He is a member of speakers’ bureaus for Alnylam, RedHill Biopharma, and Sanofi/Genzyme. He owns stock in Antibe Therapeutics.
It was a pleasure to serve as editor-in-chief (EIC) of GI & Hepatology News from 2011 to 2016. As the second EIC of the newspaper, I was preceded by Dr. Charles Lightdale – big shoes to fill! I was fortunate to attract a strong group of associate editors who covered many key areas of interest for the paper’s readership. With the enthusiastic support of American Gastroenterological Association staff members, we published once-monthly and received generally positive feedback from readers – predominantly U.S.-based AGA members.
Serving as EIC was also a learning opportunity for me. A number of potentially newsworthy items were brought to my attention – some of which I would not otherwise have seen. Although not all were of direct relevance to the readership, I believe that most of those we published were of value.
One rewarding aspect of the editorship was the opportunity to liaise with those experts from whom I solicited commentaries on some of our featured items. These busy individuals were consistently generous with their time and expertise, and I believe that their contributions added to the paper’s overall appeal.
I initiated the inclusion of two DDSEP questions per edition, and am pleased that this feature continues. One less successful venture was the attempt at a Correspondence section, which ultimately proved too cumbersome to maintain.
I congratulate the AGA on the 15th anniversary of GIHN, and I wish the current EIC, Dr. Megan Adams, and her editorial colleagues continued success in providing this benefit to AGA members.
Colin W. Howden, MD, AGAF, is professor emeritus in the division of gastroenterology, department of medicine, at the University of Tennessee, Memphis. He is a consultant for Allakos, Ironwood, Phathom, and RedHill Biopharma. He is a member of speakers’ bureaus for Alnylam, RedHill Biopharma, and Sanofi/Genzyme. He owns stock in Antibe Therapeutics.
A bittersweet farewell
Dear colleagues,
It is with bittersweet sentiments that I introduce my last issue of The New Gastroenterologist as its Editor-in-Chief. As I reflect on my time as EIC, I am immensely grateful to the AGA for this opportunity and am proud of the journal’s continuing evolution.
To begin with this issue’s content, our “In Focus” clinical feature reviews nonalcoholic fatty liver disease (NAFLD) and is written by Dr. Naga Chalasani and Dr. Eduardo Vilar-Gomez (Indiana University). This is an excellent, comprehensive piece that details the diagnosis of NAFLD and a multifaceted management approach including dietary and lifestyle modifications, pharmacotherapy, and surgical options.
Learning endoscopy is hard, but so is teaching it. Dr. Navin Kumar (Brigham and Women’s Hospital) lends tangible advice to faculty on how to optimize their endoscopy teaching skills.
Our short clinical review for this quarter, brought to you by Dr. Grace Kim and Dr. Uzma Siddiqui (University of Chicago), offers a helpful discussion on appropriate endoscopic management of duodenal and ampullary adenomas.
Statistical concepts can often be difficult to understand; Dr. Manol Jovani (University of Kentucky) provides a useful, practical explanation of effect modification.
The AGA editorial fellowship is a wonderful opportunity and we are fortunate to have two current fellows share their experience in this issue. Dr. Judy Trieu (Loyola University Chicago) discusses her experience with Clinical Gastroenterology and Hepatology and Dr. Helenie Kefalakes (Hannover Medical School – Germany) reports on her time with Gastroenterology.
Ethics manifests itself in gastroenterology in many ways, and I am happy to have introduced a case-based ethics series to our publication. For my last issue, Dr. Ariel Sims (University of Chicago) and I discuss a case of repeated deliberate foreign body ingestion and the ethical considerations for us as endoscopists.
It can be difficult to navigate the many options that exist within the realm of disability insurance. Dr. Trevor Smith (Advanced EyeCare Professionals) reviews the reasons to obtain disability insurance, how to apply and what to look for in a policy.
Lastly, the DHPA Private Practice Perspectives article for this issue is written by Dr. Marc Sonenshine (Atlanta Gastroenterology Associates) who shares his practice’s innovative approach to implementing a formalized mentorship program for physicians early in their career.
As my editorship comes to a close, I would be remiss not to thank several key people who have been instrumental in the last 3 years. First, Dr. Gautham Reddy, an important mentor of mine and former program director who sent me this opportunity and encouraged me to apply. In addition, I am grateful to the chief of our Section at the University of Chicago, Dr. David Rubin, for his collaboration and support. Ryan Farrell, the managing editor of TNG, has been nothing short of amazing to work with and is the true backbone of our publication. I would also like to thank the staff of our publisher, Frontline Medical Communications, especially Kathy Scarbeck and Christopher Palmer, as well as the EIC and former EIC of our parent publication, GI & Hepatology News, Dr. Megan Adams and Dr. John Allen. Finally, thank you to our readers, whose continued interest has made TNG a success.
Taken together, my experience as EIC of TNG for the last 3 years has been unparalleled. The opportunity to translate the questions, challenges, and experiences of early-career gastroenterologists into written pieces that would be shared with the international community is one I truly never thought I would find within a career in medicine. I am excited for the future and growth of TNG in the hands of a new EIC.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Respectfully,
Vijaya L. Rao, MD
Editor-in-Chief
Dear colleagues,
It is with bittersweet sentiments that I introduce my last issue of The New Gastroenterologist as its Editor-in-Chief. As I reflect on my time as EIC, I am immensely grateful to the AGA for this opportunity and am proud of the journal’s continuing evolution.
To begin with this issue’s content, our “In Focus” clinical feature reviews nonalcoholic fatty liver disease (NAFLD) and is written by Dr. Naga Chalasani and Dr. Eduardo Vilar-Gomez (Indiana University). This is an excellent, comprehensive piece that details the diagnosis of NAFLD and a multifaceted management approach including dietary and lifestyle modifications, pharmacotherapy, and surgical options.
Learning endoscopy is hard, but so is teaching it. Dr. Navin Kumar (Brigham and Women’s Hospital) lends tangible advice to faculty on how to optimize their endoscopy teaching skills.
Our short clinical review for this quarter, brought to you by Dr. Grace Kim and Dr. Uzma Siddiqui (University of Chicago), offers a helpful discussion on appropriate endoscopic management of duodenal and ampullary adenomas.
Statistical concepts can often be difficult to understand; Dr. Manol Jovani (University of Kentucky) provides a useful, practical explanation of effect modification.
The AGA editorial fellowship is a wonderful opportunity and we are fortunate to have two current fellows share their experience in this issue. Dr. Judy Trieu (Loyola University Chicago) discusses her experience with Clinical Gastroenterology and Hepatology and Dr. Helenie Kefalakes (Hannover Medical School – Germany) reports on her time with Gastroenterology.
Ethics manifests itself in gastroenterology in many ways, and I am happy to have introduced a case-based ethics series to our publication. For my last issue, Dr. Ariel Sims (University of Chicago) and I discuss a case of repeated deliberate foreign body ingestion and the ethical considerations for us as endoscopists.
It can be difficult to navigate the many options that exist within the realm of disability insurance. Dr. Trevor Smith (Advanced EyeCare Professionals) reviews the reasons to obtain disability insurance, how to apply and what to look for in a policy.
Lastly, the DHPA Private Practice Perspectives article for this issue is written by Dr. Marc Sonenshine (Atlanta Gastroenterology Associates) who shares his practice’s innovative approach to implementing a formalized mentorship program for physicians early in their career.
As my editorship comes to a close, I would be remiss not to thank several key people who have been instrumental in the last 3 years. First, Dr. Gautham Reddy, an important mentor of mine and former program director who sent me this opportunity and encouraged me to apply. In addition, I am grateful to the chief of our Section at the University of Chicago, Dr. David Rubin, for his collaboration and support. Ryan Farrell, the managing editor of TNG, has been nothing short of amazing to work with and is the true backbone of our publication. I would also like to thank the staff of our publisher, Frontline Medical Communications, especially Kathy Scarbeck and Christopher Palmer, as well as the EIC and former EIC of our parent publication, GI & Hepatology News, Dr. Megan Adams and Dr. John Allen. Finally, thank you to our readers, whose continued interest has made TNG a success.
Taken together, my experience as EIC of TNG for the last 3 years has been unparalleled. The opportunity to translate the questions, challenges, and experiences of early-career gastroenterologists into written pieces that would be shared with the international community is one I truly never thought I would find within a career in medicine. I am excited for the future and growth of TNG in the hands of a new EIC.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Respectfully,
Vijaya L. Rao, MD
Editor-in-Chief
Dear colleagues,
It is with bittersweet sentiments that I introduce my last issue of The New Gastroenterologist as its Editor-in-Chief. As I reflect on my time as EIC, I am immensely grateful to the AGA for this opportunity and am proud of the journal’s continuing evolution.
To begin with this issue’s content, our “In Focus” clinical feature reviews nonalcoholic fatty liver disease (NAFLD) and is written by Dr. Naga Chalasani and Dr. Eduardo Vilar-Gomez (Indiana University). This is an excellent, comprehensive piece that details the diagnosis of NAFLD and a multifaceted management approach including dietary and lifestyle modifications, pharmacotherapy, and surgical options.
Learning endoscopy is hard, but so is teaching it. Dr. Navin Kumar (Brigham and Women’s Hospital) lends tangible advice to faculty on how to optimize their endoscopy teaching skills.
Our short clinical review for this quarter, brought to you by Dr. Grace Kim and Dr. Uzma Siddiqui (University of Chicago), offers a helpful discussion on appropriate endoscopic management of duodenal and ampullary adenomas.
Statistical concepts can often be difficult to understand; Dr. Manol Jovani (University of Kentucky) provides a useful, practical explanation of effect modification.
The AGA editorial fellowship is a wonderful opportunity and we are fortunate to have two current fellows share their experience in this issue. Dr. Judy Trieu (Loyola University Chicago) discusses her experience with Clinical Gastroenterology and Hepatology and Dr. Helenie Kefalakes (Hannover Medical School – Germany) reports on her time with Gastroenterology.
Ethics manifests itself in gastroenterology in many ways, and I am happy to have introduced a case-based ethics series to our publication. For my last issue, Dr. Ariel Sims (University of Chicago) and I discuss a case of repeated deliberate foreign body ingestion and the ethical considerations for us as endoscopists.
It can be difficult to navigate the many options that exist within the realm of disability insurance. Dr. Trevor Smith (Advanced EyeCare Professionals) reviews the reasons to obtain disability insurance, how to apply and what to look for in a policy.
Lastly, the DHPA Private Practice Perspectives article for this issue is written by Dr. Marc Sonenshine (Atlanta Gastroenterology Associates) who shares his practice’s innovative approach to implementing a formalized mentorship program for physicians early in their career.
As my editorship comes to a close, I would be remiss not to thank several key people who have been instrumental in the last 3 years. First, Dr. Gautham Reddy, an important mentor of mine and former program director who sent me this opportunity and encouraged me to apply. In addition, I am grateful to the chief of our Section at the University of Chicago, Dr. David Rubin, for his collaboration and support. Ryan Farrell, the managing editor of TNG, has been nothing short of amazing to work with and is the true backbone of our publication. I would also like to thank the staff of our publisher, Frontline Medical Communications, especially Kathy Scarbeck and Christopher Palmer, as well as the EIC and former EIC of our parent publication, GI & Hepatology News, Dr. Megan Adams and Dr. John Allen. Finally, thank you to our readers, whose continued interest has made TNG a success.
Taken together, my experience as EIC of TNG for the last 3 years has been unparalleled. The opportunity to translate the questions, challenges, and experiences of early-career gastroenterologists into written pieces that would be shared with the international community is one I truly never thought I would find within a career in medicine. I am excited for the future and growth of TNG in the hands of a new EIC.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Respectfully,
Vijaya L. Rao, MD
Editor-in-Chief
Therapeutic management of NAFLD
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
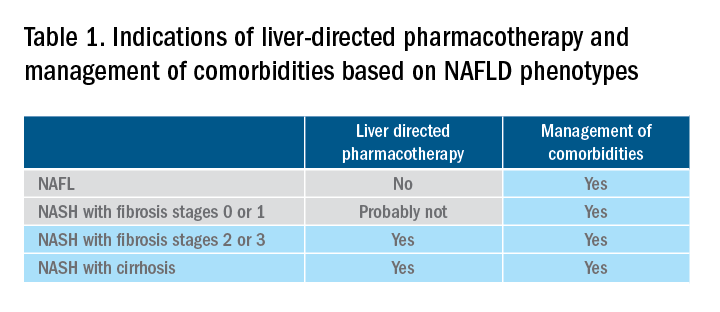
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.
Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45
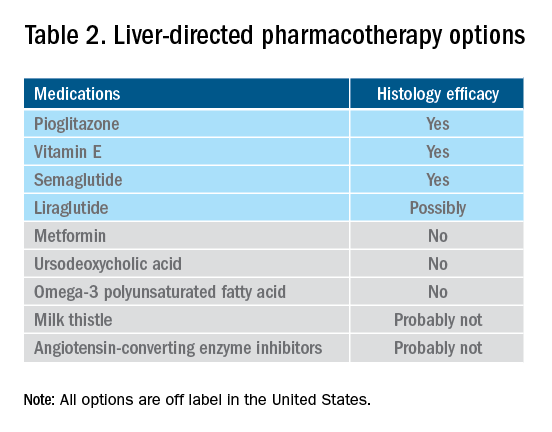
Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.

Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45

Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Nonalcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic steatosis detected on either imaging or histology in the absence of secondary causes of fatty liver (e.g., excessive alcohol consumption) or other chronic liver diseases.1 For practical NAFLD diagnosis purposes, excessive alcohol intake can be defined as an active or recent history of more than 21 standard drinks per week in men and more than14 standard drinks per week in women. For the sake of terminology, NAFLD is characterized by fatty liver infiltration, affecting at least 5% of hepatocytes, with no evidence of hepatocyte injury, whereas nonalcoholic steatohepatitis (NASH) is defined as the presence of necroinflammation with or without fibrosis in a background of fatty liver.1
Natural history
NASH and the degree of fibrosis are the two most important determinants of the natural history of NAFLD. NASH can evolve into fibrosis and cirrhosis, whereas advanced fibrosis and cirrhosis (stages 3 or 4 of fibrosis) significantly increase the risk of liver-related decompensation and mortality. NAFLD, per se, has been associated with an increased risk of overall mortality, compared with that of the general population.2 The three most common causes of mortality for patients with NAFLD are cardiovascular diseases (CVD), extrahepatic malignancies, and liver-related deaths. Mortality and liver-related events, including hepatic decompensation and hepatocellular carcinoma (HCC), may significantly increase in a dose-dependent manner with increasing fibrosis stages, and stages 3 or 4 of fibrosis may display the highest rates of all-cause mortality and liver-related events.3,4 It is important to note, however, that almost 15% of HCCs occur in patients with NAFLD who do not have cirrhosis.5 The presence of commonly associated comorbidities such as obesity, insulin resistance or diabetes, dyslipidemia, hypothyroidism, polycystic ovary syndrome, and sleep apnea may contribute to an increased risk of NASH and advanced fibrosis and, therefore, an accelerated clinical course of NAFLD.
Nonpharmacological interventions
Lifestyle modification
Lifestyle modification to achieve weight loss remains a first-line intervention in patients with NAFLD. Weight loss achieved either by hypocaloric diet alone or in conjunction with increased physical activity can be beneficial for all patients with NAFLD. The benefits extend not only to those who are overweight and obese but also to those within normal body weight (lean NAFLD).1,6,7 Weight loss of approximately 3%-5% is necessary to improve hepatic steatosis, but a greater weight loss (7%-10%) is required to improve other histopathological features like necroinflammatory lesions and fibrosis.8-10 Individuals with higher BMI and/or type 2 diabetes (T2D) will require a larger weight reduction to achieve a similar benefit on NAFLD-related features.7,8 Weight loss via lifestyle changes can also decrease hepatic venous pressure gradient (HVPG), with greater declines reported among those with more than 10% weight loss.11
Weight loss can be achieved through a variety of modalities, but long-term maintenance of lost weight is much more challenging. A combination of a hypocaloric diet with a caloric deficit of 500-1,000 kcal/d, alongside moderate-intensity exercise and intensive on-site behavioral treatment, will likely increase the possibility of a sustained weight loss over time.1,12 A growing body of scientific evidence indicates that a healthy diet that includes a reduction of high-glycemic-index foods and refined carbohydrates; increased consumption of monounsaturated fatty acids, omega-3 fatty acids, and fibers; and high intakes of olive oil, nuts, vegetables, fruits, legumes, whole grains, and fish can have beneficial effects on NAFLD and its severity.13-16 Adherence to these healthy dietary patterns has been associated with a marked reduction in CVD morbidity and mortality and is, thus, a strategic lifestyle recommendation for patients with NAFLD in whom the leading cause of morbidity and death is CVD.1,3
Exercise alone in adults with NAFLD may reduce hepatic steatosis, but its ability to improve inflammation and fibrosis has not been proven in well-designed RCTs.17,18 Physical activity and exercise have been shown to curb both the development and the progression of NAFLD, and beneficial effects could be achieved independent of weight loss.17,19,20 Most importantly, moderate-to-vigorous physical activity is likely associated with lower all-cause and cardiovascular mortality in patients with NAFLD.21
Heavy alcohol intake should be avoided by patients with NAFLD or NASH, and those with cirrhotic NASH should avoid any alcohol consumption given the risk of HCC and hepatic decompensation.1,4,22 Limiting light-to-moderate alcohol intake among patients without cirrhosis is still under debate.1 People with NAFLD may be advised to drink an equivalent of two to three 8-oz cups of regular brewed coffee daily as it has shown certain antifibrotic effects in NAFLD patients.23
Bariatric surgery
Bariatric surgery is an attractive therapeutic option for eligible obese patients with NAFLD. Bariatric surgery has the potential for inducing great weight loss and, therefore, reverses not only the steatosis, inflammation, and fibrosis among NAFLD individuals but also important comorbid conditions like T2D. A recent systematic review and meta-analysis examining data on the effects of bariatric surgery on histologic features of NAFLD from 32 cohort studies (no RCTs included) showed that bariatric surgery was associated with significant improvements in steatosis (66%), lobular inflammation (50%), ballooning degeneration (76%), and fibrosis (40%), and the benefits were significantly higher in those who underwent Roux-en-Y gastric bypass (RYGB). Of note, worsening of liver histology, including fibrosis, could be seen in up to 12% of patients who underwent bariatric surgery.24 The postsurgical weight regained after RYGB could explain partly the lack of fibrosis improvement or even worsening of fibrosis, although further research is needed to clarify these controversial findings.
RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries worldwide. Patients who undergo RYGB achieve higher weight loss when compared with those treated with SG.25 Among all bariatric procedures, RYGB could result in a higher proportion of complete resolution of NAFLD than SG, although evidence is inconclusive on fibrosis improvement rates.24,26 Most recently, a single-center RCT has compared the effects of RYGB vs. SG on liver fat content and fibrosis in patients with severe obesity and T2D.27 Data showed that both surgical procedures were highly and equally effective in reducing fatty liver content (quantified by magnetic resonance imaging), with an almost complete resolution of the fatty liver at 1 year of both surgical interventions. The beneficial effects of both GB and SG on fibrosis (assessed by enhanced liver test [ELF]) were less evident with no substantial difference between the two groups. Importantly, 69% of participants had an increase in their ELF scores during the study, despite the majority of participants achieving significant reductions in their body weights and better glycemic control at the end of the study. These findings might be considered with caution as several factors, such as the duration of the study (only 1 year) and lack of a liver biopsy to confirm fibrosis changes over time, could be influencing the study results.
Among all NAFLD phenotypes, those with cirrhosis and, most importantly, hepatic decompensation appear to be at increased risk of perioperative mortality and inpatient hospital stays than those without cirrhosis.28-29 Bariatric surgery is an absolute contraindication in patients with decompensated cirrhosis (Child B and Child C). Among compensated -Child A- cirrhotics, those with portal hypertension are at increased risk of morbidity and perioperative mortality.30 A recent analysis of National Inpatient Sample data suggested that the rates of complications in those with cirrhosis have decreased with time, which could be due to a better selection process and the use of more restrictive bariatric surgery in those with cirrhosis. Low volume centers (defined as less than 50 procedures per year) and nonrestrictive bariatric surgery were associated with a higher mortality rate. These data may suggest that patients with cirrhosis should undergo bariatric surgery only in high-volume centers after a multidisciplinary evaluation.31 Bariatric endoscopy is emerging as a new treatment for obesity, but the long-term durability of its effects remains to be determined.
A recent retrospective cohort study, including 1,158 adult patients with biopsy-proven NASH, has investigated the benefits of bariatric surgery on the occurrence of major adverse liver and cardiovascular outcomes in 650 patients who underwent bariatric surgery, compared with 508 patients who received nonsurgical usual care. This study showed that bariatric surgery was associated with 88% lower risk of progression of fatty liver to cirrhosis, liver cancer, or liver-related death, and 70% lower risk of serious CVD events during a follow-up period of 10 years.32 Within 1 year after surgery, 0.6% of patients died from surgical complications. The potential benefits of bariatric surgery in patients with NAFLD must be balanced against surgical risk, especially in eligible obese individuals with established cirrhosis. Data from a retrospective cohort study have shown that bariatric surgery in obese cirrhotic patients does not seem to associate with excessive mortality, compared with noncirrhotic obese patients.33 More data on immediate complication rates and long-term outcomes in patients with NAFLD by type of bariatric surgery is also required.
NAFLD as a standalone is not an indication for bariatric surgery. However, it could be considered in NAFLD patients who have a BMI of 40 kg/m2 or more without coexisting comorbidities or with a BMI of 35 kg/m2 or more and one or more severe obesity-related comorbidities, including T2D, hypertension, hyperlipidemia, or obstructive sleep apnea. Bariatric surgery must always be offered in centers with an experienced bariatric surgery program.1
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.

Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43
Currently available but off label medications
Vitamin E, an antioxidant, administered at a daily dose of 800 IU/day improves steatosis, inflammation, and ballooning, but not fibrosis in nondiabetic adults with biopsy-proven NASH.44 Vitamin E for 96 weeks was associated with a significantly higher rate of improvement in NASH (43% vs. 19%, P less than .01), compared with placebo.44 In the Treatment of Nonalcoholic Fatty Liver Disease in Children trial (TONIC), which examined vitamin E (800 IU/day) or metformin (500 mg twice daily) against placebo in children with biopsy-proven NAFLD, resolution of NASH was significantly greater in children treated with vitamin E than in children treated with placebo (58% vs. 28%, P less than .01). Metformin did not significantly improve the NASH resolution rates, compared with placebo (41% vs. 28%, P = .23). Vitamin E could be recommended for nondiabetic adults or children if lifestyle modifications do not produce the expected results as a result of noncompliance or ineffectiveness. Since continued use of vitamin E has been suggested to be associated with a very small increase in the risk for prostate cancer (an absolute increase of 1.6 per 1,000 person-years of vitamin E use) in men, risks and benefits should be discussed with each patient before starting therapy. A meta-analysis of nine placebo-controlled trials including roughly 119,000 patients reported that vitamin E supplementation increases the risk of hemorrhagic stroke by 20% while reducing ischemic stroke by 10%. It was estimated that vitamin E supplementation would prevent one ischemic stroke per 476 treated patients while inducing one hemorrhagic stroke for every 1,250 patients. It is noteworthy that the combination of vitamin E with anticoagulant and/or antiplatelet therapy was not examined in this trial, so we could not determine how combination therapy might affect the risk of ischemic or hemorrhagic stroke.45

Thiazolidinediones drugs have been reported to be effective in improving NAFLD in many human studies. Evidence from RCTs suggests that pioglitazone could significantly improve glucose metabolism, alanine aminotransferase, and liver histology – such as hepatic steatosis, lobular inflammation, and ballooning degeneration – among patients with or without T2D. However, the beneficial effects on improving fibrosis remain to be verified.1,46 Because of safety concerns, the risk/benefit balance of using pioglitazone to treat NASH should be discussed with each patient.47-48 Pioglitazone has been associated with long-term risk of bladder cancer,49 congestive heart failure,50 and bone fractures.51 Data from the Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis (PIVENS) trial showed that pioglitazone was significantly associated with weight gain but with no other serious adverse events. However, this study was not powered to test any safety-related hypotheses.44
Glucagon-like peptide 1 analogs have been reported to induce weight loss and reduce insulin resistance, which may lead to improvements in NAFLD. Phase II RCTs of glucagon-like peptide 1 receptor agonists (liraglutide and semaglutide) for the treatment of biopsy-proven NASH showed significant improvements in serum liver enzymes, steatosis, and inflammation, as well as NASH resolution without worsening liver fibrosis, although no direct benefit was observed in reversing fibrosis.52-53 One of these studies explores the efficacy and safety of different doses of daily subcutaneous semaglutide vs. placebo on the rates of resolution of NASH with no worsening of fibrosis. The highest dose (0.4 mg) showed the greatest difference (59% vs. 17%, P less than .01), compared with the placebo arm. However, there was no difference in improvement in fibrosis stage between the two groups (43% in the 0.4-mg group vs. 33% in the placebo group, P = .48).53 Gastrointestinal adverse events were common in the semaglutide arm.
“Spontaneous” NASH resolution and fibrosis improvement are commonly seen in participants assigned to placebo arms in clinical trials. A recent meta-analysis of 43 RCTs including 2649 placebo-treated patients showed a pooled estimate of NASH resolution without worsening of fibrosis and 1 stage reduction or more in fibrosis of 12% and 19%, respectively. Relevant factors involved in “spontaneous” NASH improvement are unknown but could be related to changes in BMI resulting from lifestyle changes, race and ethnicity, age, and, likely, NAFLD-related genetic variations, although more data is needed to better understand the histologic response in placebo-treated patients.54
Semaglutide injections (2.4 mg once weekly) or (2.0 mg once weekly) have been recently approved by the FDA for chronic weight management in adults with obesity or overweight with at least one weight-related condition or glucose control of T2D, respectively. Of note, the semaglutide dose used in the NASH trial is not currently available for the treatment of patients who are overweight/obese or have T2D, but the beneficial effects on body weight reductions and glucose control are similar overall to the effects seen with currently available doses for management of obesity or diabetes. One may consider using semaglutide in patients who are overweight/obese or have T2D with NASH, but in the senior author’s experience, it has been quite challenging to receive the payer’s approval, as its use is not specifically approved to treat liver disease.1
How to follow patients with NAFLD in the clinic
Once a diagnosis of NAFLD is made, the use of noninvasive testing may aid to identify which patients are at high risk of fibrosis. Easy to use clinical tools, such as the NAFLD Fibrosis Score and the Fib-4 index, and liver stiffness measurements using vibration-controlled transient elastography (FibroScan) or magnetic resonance elastography (MRE) are clinically useful noninvasive tools for identifying patients with NAFLD who have a higher likelihood of progressing to advanced fibrosis.1,55 The use of either NAFLD Fibrosis Score (less than -1.455) or Fib-4 index (less than 1.30) low cutoffs may be particularly useful to rule out advanced fibrosis. People with a NAFLD Fibrosis Score (greater than –1.455) or Fib-4 index (greater than 1.30) should undergo liver stiffness measurement (LSM) via FibroScan. Those with an LSM of 8 kPa or higher should be referred to specialized care, where a decision to perform a liver biopsy and initiate monitoring and therapy will be taken. MRE is the most accurate noninvasive method for the estimation of liver fibrosis. When MRE is available, it can be a diagnostic alternative to accurately rule in and rule out patients with advanced fibrosis. This technique can be preferred in clinical trials, but it is rarely used in clinical practice because it is expensive and not easily available. Reassessment by noninvasive scores at 1-3 years’ follow-up will be considered for those with an LSM less than 8 kPa. Patients with NASH cirrhosis should be screened for both gastroesophageal varix and HCC according to the American Association for the Study of Liver Diseases guidelines.56-57
Dr. Vilar-Gomez is assistant professor in the division of gastroenterology and hepatology at Indiana University, Indianapolis. Dr. Chalasani is vice president for academic affairs at Indiana University Health, Indianapolis, and the David W. Crabb Professor of Gastroenterology and Hepatology and an adjunct professor of anatomy, cell biology, and physiology in the division of gastroenterology and hepatology at Indiana University. Dr. Vilar-Gomez reports no financial conflicts of interest. Dr. Chalasani serves as a paid consultant to AbbVie, Boehringer-Ingelheim, Altimmune, Madrigal, Lilly, Zydus, and Galectin. He receives research support from Galectin and DSM.
References
1. Chalasani N et al. Hepatology 2018;67:328-57.
2. Söderberg C et al. Hepatology 2010;51:595-602.
3. Sanyal AJ et al. N Engl J Med 2021;385:1559-69.
4. Vilar-Gomez E et al. Gastroenterology 2018;155:443-57.e17.
5. Younossi ZM et al. Hepatology 2016;64:73-84.
6. EASL-EASD-EASO. J Hepatol 2016;64:1388-402.
7. Wong VW et al. J Hepatol 2018; 69:1349-56.
8. Vilar-Gomez E et al. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
9. Promrat K et al. Hepatology 2010;51:121-9.
10. Wong VW et al. J Hepatol 2013;59:536-42.
11. Berzigotti A et al. Hepatology 2017;65:1293-1305.
12. Sacks FM et al. N Engl J Med 2009;360:859-73.
13. Vilar-Gomez E et al. Hepatology 2022 Jun;75(6):1491-1506.
14. Zelber-Sagi S et al. Liver Int 2017;37:936-49.
15. Hassani Zadeh S et al. J Gastroenterol Hepatol 2021;36:1470-8.
16. Yaskolka Meir A et al. Gut 2021;70:2085-95.
17. Sung KC et al. J Hepatol 2016;65:791-7.
18. Orci LA et al. Clin Gastroenterol Hepatol 2016;14:1398-411.
19. Ryu S et al. J Hepatol 2015;63:1229-37.
20. Kim D et al. Hepatology 2020;72:1556-68.
21. Kim D et al. Clin Gastroenterol Hepatol 2021;19:1240-7.e5.
22. Ascha MS et al. Hepatology 2010;51:1972-8.
23. Bambha K et al. Liver Int 2014;34:1250-8.
24. Lee Y et al. Clin Gastroenterol Hepatol 2019;17:1040-60.e11.
25. Grönroos S et al. JAMA Surg 2021;156:137-46.
26. Fakhry TK et al. Surg Obes Relat Dis 2019;15:502-11.
27. Seeberg KA et al. Ann Intern Med 2022;175:74-83.
28. Bower G et al. Obes Surg 2015;25:2280-9.
29. Jan A et al. Obes Surg 2015;25:1518-26.
30. Hanipah ZN et al. Obes Surg 2018;28:3431-8.
31. Are VS et al. Am J Gastroenterol 2020;115:1849-56.
32. Aminian A et al. JAMA 2021;326:2031-42.
33. Vuppalanchi R et al. Ann Surg 2022;275:e174-80.
34. Simon TG et al. Gut 2021. doi: 10.1136/gutjnl-2021-325724.
35. Lonardo A et al. J Hepatol 2018;68:335-52.
36. Chalasani N et al. Gastroenterology 2004;126:1287-92.
37. Pastori D et al. Dig Liver Dis 2015;47:4-11.
38. Kim RG et al. Clin Gastroenterol Hepatol 2017;15:1521-30.e8.
39. Ahmad J et al. Dig Dis Sci 2017;62:3596-604.
40. Chalasani NP et al. Am J Gastroenterol 2021;116(5):878-98.
41. Rinella ME et al. Hepatology 2019;70:1424-36.
42. Younossi ZM et al. Lancet 2019;394:2184-96.
43. Ratziu V. Clin Liver Dis (Hoboken) 2021;17:398-400.
44. Sanyal AJ et al. N Engl J Med 2010;341:1675-85.
45. Schürks M et al. BMJ 2010;341:c5702.
46. Cusi K et al. Ann Intern Med 2016;165:305-15.
47. Lewis JD et al. JAMA 2015;314:265-77.
48. Billington EO et al. Diabetologia 2015;58:2238-46.
49. Lewis JD et al. Diabetes Care 2011;34:916-22.
50. Erdmann E et al. Diabetes Care 2007;30:2773-8.
51. Viscoli CM et al. J Clin Endocrinol Metab 2017;102:914-22.
52. Armstong MJ et al. Lancet 2016;387:679-90.
53. Newsome PN et al. N Engl J Med 2021;384:1113-24.
54. Ng CH et al. Hepatology 2022;75:1647-61.
55. Kanwal F et al. Gastroenterology 2021;161:1030-1042.e8.
56. Garcia-Tsao G et al. Hepatology 2017;65:310-35.
57. Heimbach JK et al. Hepatology 2018;67:358-80.
Gastric cancer: Perioperative outcomes with laparoscopic vs open surger
Key clinical point: Compared with the open gastrectomy, laparoscopic gastrectomy is associated with less intraoperative blood loss, shorter hospitalization time, and early postoperative exhaust in patients with gastric cancer.
Major finding: Patients in the laparoscopic vs open gastrectomy group had less intraoperative bleeding (standardized mean difference [SMD] −1.11; P = .0006), early postoperative exhaust (SMD −0.45; P = .0004), first postoperative feeding (SMD −0.45; P = .0004), and shorter postoperative hospital stay (SMD −0.97; P = .008), but had a longer operation time (SMD 0.65; P < .00001). There was no significant difference in the number of lymph nodes dissected (P = .45) and the incidence of complications after 30 days of surgery (P = .23) between the 2 groups.
Study details: These results are from a meta-analysis 11 studies including 1027 patients with gastric cancer who underwent laparoscopic or open gastrectomy.
Disclosures: This study was supported by Key Clinical Subject construction Program of Chongqing Medical University, China. The authors declared no conflicts of interest.
Source: Huang K et al. Effects of laparoscopic versus open surgery for advanced gastric cancer after neoadjuvant chemotherapy: A meta-analysis. J Healthc Eng. 2022;3255403 (Jun 18). Doi: 10.1155/2022/3255403
Key clinical point: Compared with the open gastrectomy, laparoscopic gastrectomy is associated with less intraoperative blood loss, shorter hospitalization time, and early postoperative exhaust in patients with gastric cancer.
Major finding: Patients in the laparoscopic vs open gastrectomy group had less intraoperative bleeding (standardized mean difference [SMD] −1.11; P = .0006), early postoperative exhaust (SMD −0.45; P = .0004), first postoperative feeding (SMD −0.45; P = .0004), and shorter postoperative hospital stay (SMD −0.97; P = .008), but had a longer operation time (SMD 0.65; P < .00001). There was no significant difference in the number of lymph nodes dissected (P = .45) and the incidence of complications after 30 days of surgery (P = .23) between the 2 groups.
Study details: These results are from a meta-analysis 11 studies including 1027 patients with gastric cancer who underwent laparoscopic or open gastrectomy.
Disclosures: This study was supported by Key Clinical Subject construction Program of Chongqing Medical University, China. The authors declared no conflicts of interest.
Source: Huang K et al. Effects of laparoscopic versus open surgery for advanced gastric cancer after neoadjuvant chemotherapy: A meta-analysis. J Healthc Eng. 2022;3255403 (Jun 18). Doi: 10.1155/2022/3255403
Key clinical point: Compared with the open gastrectomy, laparoscopic gastrectomy is associated with less intraoperative blood loss, shorter hospitalization time, and early postoperative exhaust in patients with gastric cancer.
Major finding: Patients in the laparoscopic vs open gastrectomy group had less intraoperative bleeding (standardized mean difference [SMD] −1.11; P = .0006), early postoperative exhaust (SMD −0.45; P = .0004), first postoperative feeding (SMD −0.45; P = .0004), and shorter postoperative hospital stay (SMD −0.97; P = .008), but had a longer operation time (SMD 0.65; P < .00001). There was no significant difference in the number of lymph nodes dissected (P = .45) and the incidence of complications after 30 days of surgery (P = .23) between the 2 groups.
Study details: These results are from a meta-analysis 11 studies including 1027 patients with gastric cancer who underwent laparoscopic or open gastrectomy.
Disclosures: This study was supported by Key Clinical Subject construction Program of Chongqing Medical University, China. The authors declared no conflicts of interest.
Source: Huang K et al. Effects of laparoscopic versus open surgery for advanced gastric cancer after neoadjuvant chemotherapy: A meta-analysis. J Healthc Eng. 2022;3255403 (Jun 18). Doi: 10.1155/2022/3255403
Advanced gastric cancer: Surgical palliation worsens survival outcomes
Key clinical point: In patients with advanced gastric cancer, surgical vs nonsurgical palliation is associated with a significantly shorter overall survival (OS) and a significantly higher hazard of mortality.
Major finding: The use of surgical palliation declined significantly during 2004-2015 (P < .0001). The median OS was significantly longer in patients who received nonsurgical vs surgical palliation (6.0 vs 3.6 months; P < .001). Surgical vs nonsurgical palliation was associated with a significantly higher risk for mortality (adjusted hazard ratio 1.20; P < .001).
Study details: A retrospective study of 6829 patients with clinical stage IV gastric cancer from the National Cancer Database (2004-2015) who received surgical or nonsurgical palliation.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Nohria A et al. Outcomes after surgical palliation of patients with gastric cancer. J Surg Res. 2022;279:304-311 (Jul 6). Doi: 10.1016/j.jss.2022.06.018
Key clinical point: In patients with advanced gastric cancer, surgical vs nonsurgical palliation is associated with a significantly shorter overall survival (OS) and a significantly higher hazard of mortality.
Major finding: The use of surgical palliation declined significantly during 2004-2015 (P < .0001). The median OS was significantly longer in patients who received nonsurgical vs surgical palliation (6.0 vs 3.6 months; P < .001). Surgical vs nonsurgical palliation was associated with a significantly higher risk for mortality (adjusted hazard ratio 1.20; P < .001).
Study details: A retrospective study of 6829 patients with clinical stage IV gastric cancer from the National Cancer Database (2004-2015) who received surgical or nonsurgical palliation.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Nohria A et al. Outcomes after surgical palliation of patients with gastric cancer. J Surg Res. 2022;279:304-311 (Jul 6). Doi: 10.1016/j.jss.2022.06.018
Key clinical point: In patients with advanced gastric cancer, surgical vs nonsurgical palliation is associated with a significantly shorter overall survival (OS) and a significantly higher hazard of mortality.
Major finding: The use of surgical palliation declined significantly during 2004-2015 (P < .0001). The median OS was significantly longer in patients who received nonsurgical vs surgical palliation (6.0 vs 3.6 months; P < .001). Surgical vs nonsurgical palliation was associated with a significantly higher risk for mortality (adjusted hazard ratio 1.20; P < .001).
Study details: A retrospective study of 6829 patients with clinical stage IV gastric cancer from the National Cancer Database (2004-2015) who received surgical or nonsurgical palliation.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Nohria A et al. Outcomes after surgical palliation of patients with gastric cancer. J Surg Res. 2022;279:304-311 (Jul 6). Doi: 10.1016/j.jss.2022.06.018
Mediterranean diet protects against gastric cancer
Key clinical point: Mediterranean diet is associated with a lower risk of developing gastric cancer.
Major finding: The Mediterranean dietary pattern, which includes a diet rich in fresh fruits, vegetables, milk, yogurt, lentils, and olive oil, was associated with a significantly lower risk for gastric cancer in the third (adjusted odds ratio [aOR] 0.394; 95% CI 0.211-0.736) and fourth (aOR 0.212; 95% CI 0.107-0.419) quartiles. The Prudent, Unhealthy, and High-fruit diets did not show any association with the risk for gastric cancer.
Study details: A case-control study of 172 patients with incident gastric cancer and 314 controls. Four dietary patterns were identified and analyzed: Mediterranean, Prudent, Unhealthy, and High-fruit dietary patterns.
Disclosures: This study was funded by Hashemite University, Jordan. The authors declared no competing interests.
Source: Tayyem R et al. Mediterranean dietary pattern is associated with lower odds of gastric cancer: A case–control study. Cancer Manag Res. 2022;14:2017-2029 (Jun 17). Doi: 10.2147/CMAR.S360468
Key clinical point: Mediterranean diet is associated with a lower risk of developing gastric cancer.
Major finding: The Mediterranean dietary pattern, which includes a diet rich in fresh fruits, vegetables, milk, yogurt, lentils, and olive oil, was associated with a significantly lower risk for gastric cancer in the third (adjusted odds ratio [aOR] 0.394; 95% CI 0.211-0.736) and fourth (aOR 0.212; 95% CI 0.107-0.419) quartiles. The Prudent, Unhealthy, and High-fruit diets did not show any association with the risk for gastric cancer.
Study details: A case-control study of 172 patients with incident gastric cancer and 314 controls. Four dietary patterns were identified and analyzed: Mediterranean, Prudent, Unhealthy, and High-fruit dietary patterns.
Disclosures: This study was funded by Hashemite University, Jordan. The authors declared no competing interests.
Source: Tayyem R et al. Mediterranean dietary pattern is associated with lower odds of gastric cancer: A case–control study. Cancer Manag Res. 2022;14:2017-2029 (Jun 17). Doi: 10.2147/CMAR.S360468
Key clinical point: Mediterranean diet is associated with a lower risk of developing gastric cancer.
Major finding: The Mediterranean dietary pattern, which includes a diet rich in fresh fruits, vegetables, milk, yogurt, lentils, and olive oil, was associated with a significantly lower risk for gastric cancer in the third (adjusted odds ratio [aOR] 0.394; 95% CI 0.211-0.736) and fourth (aOR 0.212; 95% CI 0.107-0.419) quartiles. The Prudent, Unhealthy, and High-fruit diets did not show any association with the risk for gastric cancer.
Study details: A case-control study of 172 patients with incident gastric cancer and 314 controls. Four dietary patterns were identified and analyzed: Mediterranean, Prudent, Unhealthy, and High-fruit dietary patterns.
Disclosures: This study was funded by Hashemite University, Jordan. The authors declared no competing interests.
Source: Tayyem R et al. Mediterranean dietary pattern is associated with lower odds of gastric cancer: A case–control study. Cancer Manag Res. 2022;14:2017-2029 (Jun 17). Doi: 10.2147/CMAR.S360468
Dietary iron protects against gastric cancer
Key clinical point: Dietary iron shows an inverse association with the risk for gastric cancer irrespective of cancer subsite and histological type.
Major finding: Dietary iron intake was inversely associated with the risk for gastric cancer (per quartile odds ratio [OR] 0.88; 95% CI 0.83-0.93). The association (per quartile) was significant for cardia (OR 0.85; 95% CI 0.77-0.94) and noncardia (OR 0.87; 95% CI 0.81-0.94) gastric cancer and diffuse (OR,0.79; 95% CI 0.69-0.89) and intestinal (OR 0.88; 95% CI 0.79-0.98) type.
Study details: An analysis of pooled data from 11 case-control studies including 4658 participants with gastric cancer and 12,247 control participants from the Stomach Cancer Pooling Project.
Disclosures: This study was supported by AIRC Foundation for Cancer Research, Italy. The authors declared no conflicts of interest.
Source: Collatuzzo G et al. Inverse association between dietary iron intake and gastric cancer: A pooled analysis of case-control studies of the stop consortium. Nutrients. 2022;14(12):2555 (Jun 20). Doi: 10.3390/nu14122555
Key clinical point: Dietary iron shows an inverse association with the risk for gastric cancer irrespective of cancer subsite and histological type.
Major finding: Dietary iron intake was inversely associated with the risk for gastric cancer (per quartile odds ratio [OR] 0.88; 95% CI 0.83-0.93). The association (per quartile) was significant for cardia (OR 0.85; 95% CI 0.77-0.94) and noncardia (OR 0.87; 95% CI 0.81-0.94) gastric cancer and diffuse (OR,0.79; 95% CI 0.69-0.89) and intestinal (OR 0.88; 95% CI 0.79-0.98) type.
Study details: An analysis of pooled data from 11 case-control studies including 4658 participants with gastric cancer and 12,247 control participants from the Stomach Cancer Pooling Project.
Disclosures: This study was supported by AIRC Foundation for Cancer Research, Italy. The authors declared no conflicts of interest.
Source: Collatuzzo G et al. Inverse association between dietary iron intake and gastric cancer: A pooled analysis of case-control studies of the stop consortium. Nutrients. 2022;14(12):2555 (Jun 20). Doi: 10.3390/nu14122555
Key clinical point: Dietary iron shows an inverse association with the risk for gastric cancer irrespective of cancer subsite and histological type.
Major finding: Dietary iron intake was inversely associated with the risk for gastric cancer (per quartile odds ratio [OR] 0.88; 95% CI 0.83-0.93). The association (per quartile) was significant for cardia (OR 0.85; 95% CI 0.77-0.94) and noncardia (OR 0.87; 95% CI 0.81-0.94) gastric cancer and diffuse (OR,0.79; 95% CI 0.69-0.89) and intestinal (OR 0.88; 95% CI 0.79-0.98) type.
Study details: An analysis of pooled data from 11 case-control studies including 4658 participants with gastric cancer and 12,247 control participants from the Stomach Cancer Pooling Project.
Disclosures: This study was supported by AIRC Foundation for Cancer Research, Italy. The authors declared no conflicts of interest.
Source: Collatuzzo G et al. Inverse association between dietary iron intake and gastric cancer: A pooled analysis of case-control studies of the stop consortium. Nutrients. 2022;14(12):2555 (Jun 20). Doi: 10.3390/nu14122555
Cisplatin not inferior to oxaliplatin for systemic treatment of resectable GC
Key clinical point: Cisplatin and oxaliplatin are both suitable as a part of the systemic therapy for resectable gastric cancer.
Major finding: Patients receiving epirubicin+cisplatin+capecitabine (ECX) and those receiving epirubicin+oxaliplatin+capecitabine (EOX) had comparable 5-year overall survival (42% vs 47%; P = .303), preoperative (67% vs 60%; P = .105) and postoperative (60% vs 51%; P = .266) severe (grades 3-5) toxicity, and complete or near-complete pathological response (21% vs 15%; P = .126) rates.
Study details: This post hoc analysis included 781 adult patients with resectable gastric cancer from the CRITICS trial who received preoperative ECX (n = 632) or EOX (n = 149), of which 636 and 233 received potentially curative surgery and postoperative chemotherapy, respectively.
Disclosures: The CRITICS trial was sponsored by the Dutch Cancer Society, Dutch Colorectal Cancer Group, and Hoffmann-La Roche; this analysis required no additional funding. The authors declared no conflicts of interest.
Source: Slagter AE et al. Triplet chemotherapy with cisplatin versus oxaliplatin in the CRITICS trial: Treatment compliance, toxicity, outcomes and quality of life in patients with resectable gastric cancer. Cancers (Basel). 2022;14(12):2963 (Jun 15). Doi: 10.3390/cancers14122963
Key clinical point: Cisplatin and oxaliplatin are both suitable as a part of the systemic therapy for resectable gastric cancer.
Major finding: Patients receiving epirubicin+cisplatin+capecitabine (ECX) and those receiving epirubicin+oxaliplatin+capecitabine (EOX) had comparable 5-year overall survival (42% vs 47%; P = .303), preoperative (67% vs 60%; P = .105) and postoperative (60% vs 51%; P = .266) severe (grades 3-5) toxicity, and complete or near-complete pathological response (21% vs 15%; P = .126) rates.
Study details: This post hoc analysis included 781 adult patients with resectable gastric cancer from the CRITICS trial who received preoperative ECX (n = 632) or EOX (n = 149), of which 636 and 233 received potentially curative surgery and postoperative chemotherapy, respectively.
Disclosures: The CRITICS trial was sponsored by the Dutch Cancer Society, Dutch Colorectal Cancer Group, and Hoffmann-La Roche; this analysis required no additional funding. The authors declared no conflicts of interest.
Source: Slagter AE et al. Triplet chemotherapy with cisplatin versus oxaliplatin in the CRITICS trial: Treatment compliance, toxicity, outcomes and quality of life in patients with resectable gastric cancer. Cancers (Basel). 2022;14(12):2963 (Jun 15). Doi: 10.3390/cancers14122963
Key clinical point: Cisplatin and oxaliplatin are both suitable as a part of the systemic therapy for resectable gastric cancer.
Major finding: Patients receiving epirubicin+cisplatin+capecitabine (ECX) and those receiving epirubicin+oxaliplatin+capecitabine (EOX) had comparable 5-year overall survival (42% vs 47%; P = .303), preoperative (67% vs 60%; P = .105) and postoperative (60% vs 51%; P = .266) severe (grades 3-5) toxicity, and complete or near-complete pathological response (21% vs 15%; P = .126) rates.
Study details: This post hoc analysis included 781 adult patients with resectable gastric cancer from the CRITICS trial who received preoperative ECX (n = 632) or EOX (n = 149), of which 636 and 233 received potentially curative surgery and postoperative chemotherapy, respectively.
Disclosures: The CRITICS trial was sponsored by the Dutch Cancer Society, Dutch Colorectal Cancer Group, and Hoffmann-La Roche; this analysis required no additional funding. The authors declared no conflicts of interest.
Source: Slagter AE et al. Triplet chemotherapy with cisplatin versus oxaliplatin in the CRITICS trial: Treatment compliance, toxicity, outcomes and quality of life in patients with resectable gastric cancer. Cancers (Basel). 2022;14(12):2963 (Jun 15). Doi: 10.3390/cancers14122963
Gastric cancer: Neoadjuvant chemotherapy improves outcomes
Key clinical point: In patients with locally advanced gastric cancer (LAGC), neoadjuvant chemotherapy (NACT) followed by laparoscopic gastrectomy (LG) vs upfront LG is associated with a lower rate of severe postoperative complications and improved survival.
Major finding: Grade ≥ 3 severe postoperative complication rate was significantly lower in the NACT-LG vs upfront LG group (0% vs 17.1%; P = .001). The postoperative complication-related death rate was 0% in the NACT-LG group vs 2.9% in the upfront LG group. NACT-LG vs upfront LG was associated with improved disease-free survival (14.4% vs 5.7%; P = .0299) and overall survival (34.1% vs 8.6%; P = .0061) at 3 years.
Study details: This was a retrospective study of 76 consecutive patients with LAGC who received either LG following NACT or upfront LG between March 2013 and October 2018.
Disclosures: This study did not receive any funding. The authors declare no competing interests.
Source: Liu L et al. The safety and efficacy of laparoscopic gastrectomy for patients with locally advanced gastric cancer following neoadjuvant chemotherapy. Sci Rep. 2022;12:10384 (Jun 20). Doi: 10.1038/s41598-022-14717-6
Key clinical point: In patients with locally advanced gastric cancer (LAGC), neoadjuvant chemotherapy (NACT) followed by laparoscopic gastrectomy (LG) vs upfront LG is associated with a lower rate of severe postoperative complications and improved survival.
Major finding: Grade ≥ 3 severe postoperative complication rate was significantly lower in the NACT-LG vs upfront LG group (0% vs 17.1%; P = .001). The postoperative complication-related death rate was 0% in the NACT-LG group vs 2.9% in the upfront LG group. NACT-LG vs upfront LG was associated with improved disease-free survival (14.4% vs 5.7%; P = .0299) and overall survival (34.1% vs 8.6%; P = .0061) at 3 years.
Study details: This was a retrospective study of 76 consecutive patients with LAGC who received either LG following NACT or upfront LG between March 2013 and October 2018.
Disclosures: This study did not receive any funding. The authors declare no competing interests.
Source: Liu L et al. The safety and efficacy of laparoscopic gastrectomy for patients with locally advanced gastric cancer following neoadjuvant chemotherapy. Sci Rep. 2022;12:10384 (Jun 20). Doi: 10.1038/s41598-022-14717-6
Key clinical point: In patients with locally advanced gastric cancer (LAGC), neoadjuvant chemotherapy (NACT) followed by laparoscopic gastrectomy (LG) vs upfront LG is associated with a lower rate of severe postoperative complications and improved survival.
Major finding: Grade ≥ 3 severe postoperative complication rate was significantly lower in the NACT-LG vs upfront LG group (0% vs 17.1%; P = .001). The postoperative complication-related death rate was 0% in the NACT-LG group vs 2.9% in the upfront LG group. NACT-LG vs upfront LG was associated with improved disease-free survival (14.4% vs 5.7%; P = .0299) and overall survival (34.1% vs 8.6%; P = .0061) at 3 years.
Study details: This was a retrospective study of 76 consecutive patients with LAGC who received either LG following NACT or upfront LG between March 2013 and October 2018.
Disclosures: This study did not receive any funding. The authors declare no competing interests.
Source: Liu L et al. The safety and efficacy of laparoscopic gastrectomy for patients with locally advanced gastric cancer following neoadjuvant chemotherapy. Sci Rep. 2022;12:10384 (Jun 20). Doi: 10.1038/s41598-022-14717-6
Hyperglycemia and low BMI increase the risk for gastric cancer
Key clinical point: Low body mass index (BMI) increased the risk for gastric cancer in men and women, whereas high fasting glucose levels slightly increased the risk in women but not in men.
Major finding: Low BMI (<18.5 kg/m2) increased the risk for gastric cancer in both men (adjusted odds ratio [aOR] 1.39; P < .001) and women (aOR 1.48; P < .001). High fasting glucose levels (≥126 mg/dL) slightly elevated gastric cancer risk in women (aOR 1.19; P < .001) but not in men.
Study details: This prospective, population-based cohort study included 5174 million individuals (men, 43.1%) who underwent national gastric cancer screening and were followed up for 9 years.
Disclosures: This study was funded by the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea; and others. The authors declared no conflicts of interest.
Source: Nam SY et al. Sex-specific effect of body mass index and fasting glucose on gastric cancer risk and all causes mortality; a cohort study of 5.17 million. Int J Obes. 2022 (Jun 10). Doi: 10.1038/s41366-022-01161-9
Key clinical point: Low body mass index (BMI) increased the risk for gastric cancer in men and women, whereas high fasting glucose levels slightly increased the risk in women but not in men.
Major finding: Low BMI (<18.5 kg/m2) increased the risk for gastric cancer in both men (adjusted odds ratio [aOR] 1.39; P < .001) and women (aOR 1.48; P < .001). High fasting glucose levels (≥126 mg/dL) slightly elevated gastric cancer risk in women (aOR 1.19; P < .001) but not in men.
Study details: This prospective, population-based cohort study included 5174 million individuals (men, 43.1%) who underwent national gastric cancer screening and were followed up for 9 years.
Disclosures: This study was funded by the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea; and others. The authors declared no conflicts of interest.
Source: Nam SY et al. Sex-specific effect of body mass index and fasting glucose on gastric cancer risk and all causes mortality; a cohort study of 5.17 million. Int J Obes. 2022 (Jun 10). Doi: 10.1038/s41366-022-01161-9
Key clinical point: Low body mass index (BMI) increased the risk for gastric cancer in men and women, whereas high fasting glucose levels slightly increased the risk in women but not in men.
Major finding: Low BMI (<18.5 kg/m2) increased the risk for gastric cancer in both men (adjusted odds ratio [aOR] 1.39; P < .001) and women (aOR 1.48; P < .001). High fasting glucose levels (≥126 mg/dL) slightly elevated gastric cancer risk in women (aOR 1.19; P < .001) but not in men.
Study details: This prospective, population-based cohort study included 5174 million individuals (men, 43.1%) who underwent national gastric cancer screening and were followed up for 9 years.
Disclosures: This study was funded by the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea; and others. The authors declared no conflicts of interest.
Source: Nam SY et al. Sex-specific effect of body mass index and fasting glucose on gastric cancer risk and all causes mortality; a cohort study of 5.17 million. Int J Obes. 2022 (Jun 10). Doi: 10.1038/s41366-022-01161-9






