User login
Precocious puberty – how early is too soon?
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.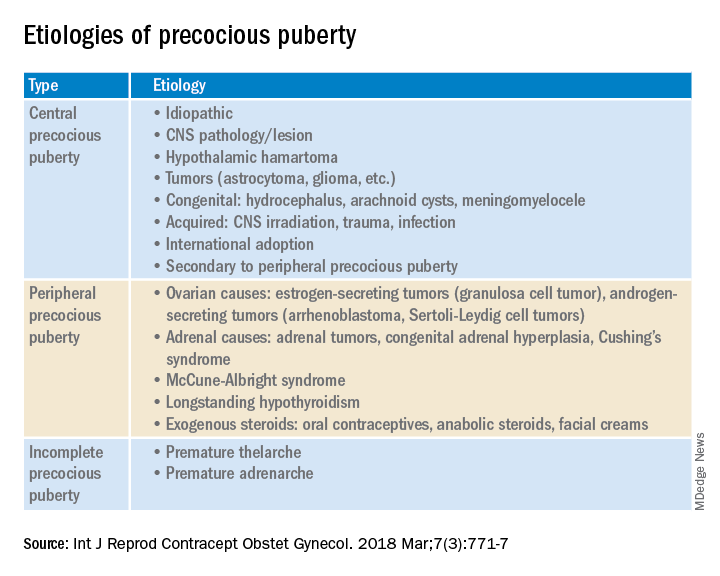
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
How to manage cancer pain when patients misuse opioids
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Head and neck tumor grade may predict response to immunotherapy
Findings from a cohort study highlight a potential role of tumor grade for predicting mucosal head and neck squamous cell carcinoma response to immunotherapy, researchers report in JAMA Otolaryngology–Head & Neck Surgery.
The analysis, which was among patients with either high-grade or low-grade recurrent or metastatic mucosal head and neck squamous cell carcinoma (HNSCC) tumors, revealed that tumor grade was independently associated with immunotherapy response.
“Generally, one would expect high-grade tumors to do worse because they are more aggressive,” said Rajarsi Mandal, MD, the study’s lead author. “But it’s thought that high-grade tumors have higher degrees of chromosomal instability with a lot more mutations generated throughout the genome of these tumors ... and we know that higher mutation rates correlate with higher responses to immunotherapy.”
Researchers found that the proportion of patients having a beneficial response to immunotherapy was larger for patients with high grade tumors (12 of 35; 34.3%) than those with low grade tumors (2 of 25, 8.0%) (difference, 26.3%; 95% confidence interval, 7.3%-45.3%). The odds of having a clinically beneficial response to immunotherapy was increased 5.35-fold (95% CI, 1.04-27.37) in patients with high-grade tumors. Among four patients with low-grade tumors and eight patients with high-grade tumors with available tumor mutational burden data, the mean tumor mutational burden was greater for patients with high-grade tumors (mean [standard deviation], 8.6 [5.4] mut/Mb; n = 8) than for patients with low grade tumors (mean [SD], 3.6 [1.1] mut/Mb; n = 4) (difference, 5.0 mut/Mb; 95% CI, −1.4 to 11.4 mut/Mb; Cohen d, 1.2).
“ said Dr. Mandal who is a head and neck cancer surgeon with the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Previous studies of HNSCC tumors that are refractory to traditional therapies, including surgery, chemotherapy, and radiation therapy, have demonstrated a clinically beneficial response to immune checkpoint inhibitors (ICIs). An association between increased tumor mutational burden and beneficial response to ICIs has been shown in other cancers.
Researchers hypothesized that tumor histological grade may be associated with responses to immune checkpoint blockade, and designed their study to examine the association between tumor grade and immunotherapy response in patients treated with ICIs for recurrent or metastatic mucosal HNSCC.
In a single-center retrospective cohort study, investigators reviewed the medical records of 60 adult patients (mean age, 64.6 years; 85% male) with a primary mucosal HNSCC tumor treated with an immune checkpoint inhibitor (pembrolizumab, nivolumab, ipilimumab, or durvalumab) treated between July 1, 2015, and Jan. 22, 2020. They stratified them into those with low grade tumors (well differentiated and moderately differentiated) and those with high grade tumors (poorly differentiated). A clinically beneficial immunotherapy response, defined as complete response or partial response was the primary outcome.
Thirty-eight patients in the study cohort were current or former smokers. The most common primary tumor sight was at the oropharynx. Outcomes among those with high-grade tumors were improved, compared with those with low-grade tumors, for median progression-free survival (5.9 months vs. 3.3 months), median overall survival (16.6 months vs. 15.0 months, and risk of death (hazard ratio, 0.94).
The study’s main limitation was its small sample size and small number of patients who had a clinically beneficial immunotherapy response.
Dr. Mandal had no conflicts of interest to disclose.
Findings from a cohort study highlight a potential role of tumor grade for predicting mucosal head and neck squamous cell carcinoma response to immunotherapy, researchers report in JAMA Otolaryngology–Head & Neck Surgery.
The analysis, which was among patients with either high-grade or low-grade recurrent or metastatic mucosal head and neck squamous cell carcinoma (HNSCC) tumors, revealed that tumor grade was independently associated with immunotherapy response.
“Generally, one would expect high-grade tumors to do worse because they are more aggressive,” said Rajarsi Mandal, MD, the study’s lead author. “But it’s thought that high-grade tumors have higher degrees of chromosomal instability with a lot more mutations generated throughout the genome of these tumors ... and we know that higher mutation rates correlate with higher responses to immunotherapy.”
Researchers found that the proportion of patients having a beneficial response to immunotherapy was larger for patients with high grade tumors (12 of 35; 34.3%) than those with low grade tumors (2 of 25, 8.0%) (difference, 26.3%; 95% confidence interval, 7.3%-45.3%). The odds of having a clinically beneficial response to immunotherapy was increased 5.35-fold (95% CI, 1.04-27.37) in patients with high-grade tumors. Among four patients with low-grade tumors and eight patients with high-grade tumors with available tumor mutational burden data, the mean tumor mutational burden was greater for patients with high-grade tumors (mean [standard deviation], 8.6 [5.4] mut/Mb; n = 8) than for patients with low grade tumors (mean [SD], 3.6 [1.1] mut/Mb; n = 4) (difference, 5.0 mut/Mb; 95% CI, −1.4 to 11.4 mut/Mb; Cohen d, 1.2).
“ said Dr. Mandal who is a head and neck cancer surgeon with the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Previous studies of HNSCC tumors that are refractory to traditional therapies, including surgery, chemotherapy, and radiation therapy, have demonstrated a clinically beneficial response to immune checkpoint inhibitors (ICIs). An association between increased tumor mutational burden and beneficial response to ICIs has been shown in other cancers.
Researchers hypothesized that tumor histological grade may be associated with responses to immune checkpoint blockade, and designed their study to examine the association between tumor grade and immunotherapy response in patients treated with ICIs for recurrent or metastatic mucosal HNSCC.
In a single-center retrospective cohort study, investigators reviewed the medical records of 60 adult patients (mean age, 64.6 years; 85% male) with a primary mucosal HNSCC tumor treated with an immune checkpoint inhibitor (pembrolizumab, nivolumab, ipilimumab, or durvalumab) treated between July 1, 2015, and Jan. 22, 2020. They stratified them into those with low grade tumors (well differentiated and moderately differentiated) and those with high grade tumors (poorly differentiated). A clinically beneficial immunotherapy response, defined as complete response or partial response was the primary outcome.
Thirty-eight patients in the study cohort were current or former smokers. The most common primary tumor sight was at the oropharynx. Outcomes among those with high-grade tumors were improved, compared with those with low-grade tumors, for median progression-free survival (5.9 months vs. 3.3 months), median overall survival (16.6 months vs. 15.0 months, and risk of death (hazard ratio, 0.94).
The study’s main limitation was its small sample size and small number of patients who had a clinically beneficial immunotherapy response.
Dr. Mandal had no conflicts of interest to disclose.
Findings from a cohort study highlight a potential role of tumor grade for predicting mucosal head and neck squamous cell carcinoma response to immunotherapy, researchers report in JAMA Otolaryngology–Head & Neck Surgery.
The analysis, which was among patients with either high-grade or low-grade recurrent or metastatic mucosal head and neck squamous cell carcinoma (HNSCC) tumors, revealed that tumor grade was independently associated with immunotherapy response.
“Generally, one would expect high-grade tumors to do worse because they are more aggressive,” said Rajarsi Mandal, MD, the study’s lead author. “But it’s thought that high-grade tumors have higher degrees of chromosomal instability with a lot more mutations generated throughout the genome of these tumors ... and we know that higher mutation rates correlate with higher responses to immunotherapy.”
Researchers found that the proportion of patients having a beneficial response to immunotherapy was larger for patients with high grade tumors (12 of 35; 34.3%) than those with low grade tumors (2 of 25, 8.0%) (difference, 26.3%; 95% confidence interval, 7.3%-45.3%). The odds of having a clinically beneficial response to immunotherapy was increased 5.35-fold (95% CI, 1.04-27.37) in patients with high-grade tumors. Among four patients with low-grade tumors and eight patients with high-grade tumors with available tumor mutational burden data, the mean tumor mutational burden was greater for patients with high-grade tumors (mean [standard deviation], 8.6 [5.4] mut/Mb; n = 8) than for patients with low grade tumors (mean [SD], 3.6 [1.1] mut/Mb; n = 4) (difference, 5.0 mut/Mb; 95% CI, −1.4 to 11.4 mut/Mb; Cohen d, 1.2).
“ said Dr. Mandal who is a head and neck cancer surgeon with the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Previous studies of HNSCC tumors that are refractory to traditional therapies, including surgery, chemotherapy, and radiation therapy, have demonstrated a clinically beneficial response to immune checkpoint inhibitors (ICIs). An association between increased tumor mutational burden and beneficial response to ICIs has been shown in other cancers.
Researchers hypothesized that tumor histological grade may be associated with responses to immune checkpoint blockade, and designed their study to examine the association between tumor grade and immunotherapy response in patients treated with ICIs for recurrent or metastatic mucosal HNSCC.
In a single-center retrospective cohort study, investigators reviewed the medical records of 60 adult patients (mean age, 64.6 years; 85% male) with a primary mucosal HNSCC tumor treated with an immune checkpoint inhibitor (pembrolizumab, nivolumab, ipilimumab, or durvalumab) treated between July 1, 2015, and Jan. 22, 2020. They stratified them into those with low grade tumors (well differentiated and moderately differentiated) and those with high grade tumors (poorly differentiated). A clinically beneficial immunotherapy response, defined as complete response or partial response was the primary outcome.
Thirty-eight patients in the study cohort were current or former smokers. The most common primary tumor sight was at the oropharynx. Outcomes among those with high-grade tumors were improved, compared with those with low-grade tumors, for median progression-free survival (5.9 months vs. 3.3 months), median overall survival (16.6 months vs. 15.0 months, and risk of death (hazard ratio, 0.94).
The study’s main limitation was its small sample size and small number of patients who had a clinically beneficial immunotherapy response.
Dr. Mandal had no conflicts of interest to disclose.
FROM JAMA OTOLARYNGOLOGY – HEAD & NECK SURGERY
Experimental cancer drug promising for hospitalized COVID patients
, a new study shows.
The medication, called sabizabulin and given as a pill, reduced by half the risk of death among participants. It could be more effective than other drugs for those severely sick with COVID-19, The New York Times reports.
The manufacturer, Veru, is seeking emergency use authorization from the Food and Drug Administration. Hospitalized COVID-19 patients currently have only a few pharmaceutical options.
Sabizabulin blocks cells from building molecular cables that carry material from one part of a cell to another. It was created to fight cancer, because tumor cells need those cables (called microtubules) to grow quickly.
Researchers tried it against COVID-19 2 years ago, because viral replication also requires microtubules to bring pieces of new viruses together.
To participate in the small trial, patients had to be receiving oxygen or on a ventilator and at a high risk of dying from COVID-19, “with risk factors such as hypertension, advanced age or obesity,” the Times reported.
A total of 134 patients received the medicine; 70 got a placebo. Among those receiving sabizabulin, 20.2% died within 2 months; 45.1% of those who took the placebo died.
One infectious disease expert told the Times that the high mortality rate of those on the placebo could mean the study was too small to offer conclusive results.
“The 45% mortality rate in the control group jumps out at me as rather high,” said David Boulware, MD, of the University of Minnesota.
A version of this article first appeared on WebMD.com.
, a new study shows.
The medication, called sabizabulin and given as a pill, reduced by half the risk of death among participants. It could be more effective than other drugs for those severely sick with COVID-19, The New York Times reports.
The manufacturer, Veru, is seeking emergency use authorization from the Food and Drug Administration. Hospitalized COVID-19 patients currently have only a few pharmaceutical options.
Sabizabulin blocks cells from building molecular cables that carry material from one part of a cell to another. It was created to fight cancer, because tumor cells need those cables (called microtubules) to grow quickly.
Researchers tried it against COVID-19 2 years ago, because viral replication also requires microtubules to bring pieces of new viruses together.
To participate in the small trial, patients had to be receiving oxygen or on a ventilator and at a high risk of dying from COVID-19, “with risk factors such as hypertension, advanced age or obesity,” the Times reported.
A total of 134 patients received the medicine; 70 got a placebo. Among those receiving sabizabulin, 20.2% died within 2 months; 45.1% of those who took the placebo died.
One infectious disease expert told the Times that the high mortality rate of those on the placebo could mean the study was too small to offer conclusive results.
“The 45% mortality rate in the control group jumps out at me as rather high,” said David Boulware, MD, of the University of Minnesota.
A version of this article first appeared on WebMD.com.
, a new study shows.
The medication, called sabizabulin and given as a pill, reduced by half the risk of death among participants. It could be more effective than other drugs for those severely sick with COVID-19, The New York Times reports.
The manufacturer, Veru, is seeking emergency use authorization from the Food and Drug Administration. Hospitalized COVID-19 patients currently have only a few pharmaceutical options.
Sabizabulin blocks cells from building molecular cables that carry material from one part of a cell to another. It was created to fight cancer, because tumor cells need those cables (called microtubules) to grow quickly.
Researchers tried it against COVID-19 2 years ago, because viral replication also requires microtubules to bring pieces of new viruses together.
To participate in the small trial, patients had to be receiving oxygen or on a ventilator and at a high risk of dying from COVID-19, “with risk factors such as hypertension, advanced age or obesity,” the Times reported.
A total of 134 patients received the medicine; 70 got a placebo. Among those receiving sabizabulin, 20.2% died within 2 months; 45.1% of those who took the placebo died.
One infectious disease expert told the Times that the high mortality rate of those on the placebo could mean the study was too small to offer conclusive results.
“The 45% mortality rate in the control group jumps out at me as rather high,” said David Boulware, MD, of the University of Minnesota.
A version of this article first appeared on WebMD.com.
Single-donor fecal transplant trial for IBS shows lasting response
new data show.
Nearly three out of four people in a clinical trial experienced fewer symptoms and fatigue and a greater quality of life at both 2 years and 3 years after FMT in Norway. Those FMT-treated patients who relapsed subsequently responded to FMT upon retransplantation, reported the authors, who also correlated individual microbial profiles with clinical outcomes.
The study, led by Magdy El-Salhy, MD, PhD, department of medicine, Stord (Norway) Hospital, was published online in Gastroenterology.
An expert not involved with the study, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic in Jacksonville, Fla., said that the results of the study are important, but he cautions against treating IBS with FMT outside clinical trials, at least until the protocol is validated, given demonstrated risks.
The new study included 125 patients (104 women, 21 men) in three groups: 38 received a placebo, 42 received 30 g of donor feces, and 45 received 60 g of donor feces. The feces – all from one male donor – was administered to the duodenum.
The response rates for those who received FMT were significantly higher than for those who received placebo. Those receiving 30 g of feces had a response rate of 69.1%, and those in the 60-g group had a response rate of 77.8%, whereas the response rate in the placebo group was 26.3%.
Patients provided a fecal sample and completed five questionnaires at the beginning of the study and at 2 and 3 years after FMT.
Patients in both treatment groups had significantly fewer IBS symptoms – such as abdominal pain, abdominal bloating, dissatisfaction with bowel habits, and quality-of-life interruption – and less fatigue compared with the placebo group, as well as higher quality of life scores at 2 and 3 years.
No long-term adverse effects were reported.
The dysbiosis index decreased only in the treatment group at years 2 and 3.
Microbial modifications correlate with IBS symptoms
In addition, the fluorescent signals of 10 bacteria that had changed after FMT were significantly correlated with improved IBS symptoms and fatigue in both treatment groups.
“Of the bacteria markers whose fluorescence signals changed in the 30-g and 60-g groups, but not in the placebo group, at both 2 and 3 years after FMT, nine were significantly correlated with the total IBS-SSS [IBS–Severity Scoring System] scores,” the authors wrote. One more bacterium with a changed fluorescence signal in the active treatment group also correlated with total fatigue.
Dr. El-Salhy told this news organization that those findings open the door for the select bacteria to be used, for example, in capsule form to treat IBS and fatigue.
The most surprising finding for the team was that the “majority of IBS patients [who] responded to FMT maintained response up to 3 years” or more, said Dr. El-Salhy, alluding to unpublished data up to 5 years.
“Furthermore, 80% of those who relapsed after 3 years responded to a new FMT,” he said.
Women had higher response rates than men at years 2 and 3 after FMT, but there were no differences between complete remission rates of women and men at years 2 and 3.
‘Impressive’ results, but caution warranted
“The results are impressive,” Dr. Lacy said. “I believe that this will help researchers around the world refine their techniques, as we learn more about FMT for the treatment of IBS. It also clearly plays up the importance of the gut microbiome in symptom generation in IBS patients.”
However, he said, until the results are replicated, and the protocol validated, FMT should not be used routinely to treat IBS because there are risks with the procedure.
Dr. Lacy pointed out there have been mixed results in the literature regarding FMT and IBS.
He cited a meta-analysis of four studies (n = 254) in 2019 that did not show a benefit for FMT in patients with IBS. However, a second meta-analysis of five studies (n = 267) did show some benefit, possibly owing to the type of donor and small-bowel infusion.
The authors added that, in the seven randomized, controlled trials investigating FMT for IBS, four concluded that FMT eased symptoms and improved quality of life in patients with IBS, whereas treatment was not effective in the other three.
The authors pointed to differences in protocols, donors, the cohort treated, FMT dose, and route of administration.
The longest response time previously studied in the randomized, controlled trials was 1 year, the authors pointed out. The new study was a 3-year follow-up of these authors’ previous randomly assigned placebo-controlled trial participants.
The ‘super-donor’ concept
The authors of the current study described the single chosen donor as “a healthy male aged 36 years with a normal BMI [body mass index] who was born via vaginal delivery, breastfed, a nonsmoker, was not taking any medication, was treated only a few times with antibiotics, exercised regularly, and consumed a sport-specific diet that was richer in protein, fiber, minerals, and vitamins than the average diet.”
The donor had high microbial diversity, and his fecal bacteria makeup was different from that of 254 healthy subjects for 14 of the 48 bacterial markers investigators tested.
Dr. Lacy said that the “super-donor” concept is noteworthy and an apparent key to success.
“Other studies have not done this,” Dr. Lacy noted.
Among the strengths of the study are that it included a relatively large cohort of patients with IBS, with three IBS subtypes and a single well-defined donor. However, it did not include the fourth IBS subtype, unsubtyped IBS, and it only investigated a part of the intestinal bacterial content, the authors acknowledged.
Most interesting, Dr. Lacy said, is that the IBS subtype did not seem to matter to FMT outcomes at 3 years and that all three subtypes responded better than placebo.
“That’s encouraging,” Dr. Lacy said.
The investigators received a grant from Helse Fonna. The study authors and Dr. Lacy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The treatment of IBS remains a major health care challenge. It is now evident that dysregulation of the microbiota-gut-brain axis is an important contributing factor that may drive and perpetuate the symptoms of IBS.
This study by El-Salhy et al. highlights the safety and sustained benefit of fecal microbiota transplantation (FMT) in patients with IBS for up to 3 years following transplantation. The authors also identified 10 fecal bacterial markers that correlated with IBS symptoms in patients who underwent FMT. The researchers linked their high response rates to donor selection criteria that were based on specific bacterial species that positively affect the gut microbiota in terms of abundance, diversity, and stability over time.
Further larger age- and gender-matched studies should be undertaken to validate these important observations, which also focus on deciphering optimal route of administration for FMT. More extensive research is needed to understand the complex multi-kingdom microbial, metabolic, epigenetic, and immunological correlates of FMT treatment response in IBS patients. It will be critical to support longitudinal human observational FMT-based interventional studies with rigorous experimental approaches for addressing causal relationships between altered microbiome and disease phenotype.
Dr. Tanya M. Monaghan, BSc (Hons), BM, PhD, FRCP, is clinical associate professor and honorary consultant in gastroenterology, Nottingham Digestive Diseases Centre; NIHR Nottingham Biomedical Research Centre, Nottingham University Hospitals NHS Trust, and the University of Nottingham, England. Dr. Monaghan has no relevant conflicts of interest.
The treatment of IBS remains a major health care challenge. It is now evident that dysregulation of the microbiota-gut-brain axis is an important contributing factor that may drive and perpetuate the symptoms of IBS.
This study by El-Salhy et al. highlights the safety and sustained benefit of fecal microbiota transplantation (FMT) in patients with IBS for up to 3 years following transplantation. The authors also identified 10 fecal bacterial markers that correlated with IBS symptoms in patients who underwent FMT. The researchers linked their high response rates to donor selection criteria that were based on specific bacterial species that positively affect the gut microbiota in terms of abundance, diversity, and stability over time.
Further larger age- and gender-matched studies should be undertaken to validate these important observations, which also focus on deciphering optimal route of administration for FMT. More extensive research is needed to understand the complex multi-kingdom microbial, metabolic, epigenetic, and immunological correlates of FMT treatment response in IBS patients. It will be critical to support longitudinal human observational FMT-based interventional studies with rigorous experimental approaches for addressing causal relationships between altered microbiome and disease phenotype.
Dr. Tanya M. Monaghan, BSc (Hons), BM, PhD, FRCP, is clinical associate professor and honorary consultant in gastroenterology, Nottingham Digestive Diseases Centre; NIHR Nottingham Biomedical Research Centre, Nottingham University Hospitals NHS Trust, and the University of Nottingham, England. Dr. Monaghan has no relevant conflicts of interest.
The treatment of IBS remains a major health care challenge. It is now evident that dysregulation of the microbiota-gut-brain axis is an important contributing factor that may drive and perpetuate the symptoms of IBS.
This study by El-Salhy et al. highlights the safety and sustained benefit of fecal microbiota transplantation (FMT) in patients with IBS for up to 3 years following transplantation. The authors also identified 10 fecal bacterial markers that correlated with IBS symptoms in patients who underwent FMT. The researchers linked their high response rates to donor selection criteria that were based on specific bacterial species that positively affect the gut microbiota in terms of abundance, diversity, and stability over time.
Further larger age- and gender-matched studies should be undertaken to validate these important observations, which also focus on deciphering optimal route of administration for FMT. More extensive research is needed to understand the complex multi-kingdom microbial, metabolic, epigenetic, and immunological correlates of FMT treatment response in IBS patients. It will be critical to support longitudinal human observational FMT-based interventional studies with rigorous experimental approaches for addressing causal relationships between altered microbiome and disease phenotype.
Dr. Tanya M. Monaghan, BSc (Hons), BM, PhD, FRCP, is clinical associate professor and honorary consultant in gastroenterology, Nottingham Digestive Diseases Centre; NIHR Nottingham Biomedical Research Centre, Nottingham University Hospitals NHS Trust, and the University of Nottingham, England. Dr. Monaghan has no relevant conflicts of interest.
new data show.
Nearly three out of four people in a clinical trial experienced fewer symptoms and fatigue and a greater quality of life at both 2 years and 3 years after FMT in Norway. Those FMT-treated patients who relapsed subsequently responded to FMT upon retransplantation, reported the authors, who also correlated individual microbial profiles with clinical outcomes.
The study, led by Magdy El-Salhy, MD, PhD, department of medicine, Stord (Norway) Hospital, was published online in Gastroenterology.
An expert not involved with the study, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic in Jacksonville, Fla., said that the results of the study are important, but he cautions against treating IBS with FMT outside clinical trials, at least until the protocol is validated, given demonstrated risks.
The new study included 125 patients (104 women, 21 men) in three groups: 38 received a placebo, 42 received 30 g of donor feces, and 45 received 60 g of donor feces. The feces – all from one male donor – was administered to the duodenum.
The response rates for those who received FMT were significantly higher than for those who received placebo. Those receiving 30 g of feces had a response rate of 69.1%, and those in the 60-g group had a response rate of 77.8%, whereas the response rate in the placebo group was 26.3%.
Patients provided a fecal sample and completed five questionnaires at the beginning of the study and at 2 and 3 years after FMT.
Patients in both treatment groups had significantly fewer IBS symptoms – such as abdominal pain, abdominal bloating, dissatisfaction with bowel habits, and quality-of-life interruption – and less fatigue compared with the placebo group, as well as higher quality of life scores at 2 and 3 years.
No long-term adverse effects were reported.
The dysbiosis index decreased only in the treatment group at years 2 and 3.
Microbial modifications correlate with IBS symptoms
In addition, the fluorescent signals of 10 bacteria that had changed after FMT were significantly correlated with improved IBS symptoms and fatigue in both treatment groups.
“Of the bacteria markers whose fluorescence signals changed in the 30-g and 60-g groups, but not in the placebo group, at both 2 and 3 years after FMT, nine were significantly correlated with the total IBS-SSS [IBS–Severity Scoring System] scores,” the authors wrote. One more bacterium with a changed fluorescence signal in the active treatment group also correlated with total fatigue.
Dr. El-Salhy told this news organization that those findings open the door for the select bacteria to be used, for example, in capsule form to treat IBS and fatigue.
The most surprising finding for the team was that the “majority of IBS patients [who] responded to FMT maintained response up to 3 years” or more, said Dr. El-Salhy, alluding to unpublished data up to 5 years.
“Furthermore, 80% of those who relapsed after 3 years responded to a new FMT,” he said.
Women had higher response rates than men at years 2 and 3 after FMT, but there were no differences between complete remission rates of women and men at years 2 and 3.
‘Impressive’ results, but caution warranted
“The results are impressive,” Dr. Lacy said. “I believe that this will help researchers around the world refine their techniques, as we learn more about FMT for the treatment of IBS. It also clearly plays up the importance of the gut microbiome in symptom generation in IBS patients.”
However, he said, until the results are replicated, and the protocol validated, FMT should not be used routinely to treat IBS because there are risks with the procedure.
Dr. Lacy pointed out there have been mixed results in the literature regarding FMT and IBS.
He cited a meta-analysis of four studies (n = 254) in 2019 that did not show a benefit for FMT in patients with IBS. However, a second meta-analysis of five studies (n = 267) did show some benefit, possibly owing to the type of donor and small-bowel infusion.
The authors added that, in the seven randomized, controlled trials investigating FMT for IBS, four concluded that FMT eased symptoms and improved quality of life in patients with IBS, whereas treatment was not effective in the other three.
The authors pointed to differences in protocols, donors, the cohort treated, FMT dose, and route of administration.
The longest response time previously studied in the randomized, controlled trials was 1 year, the authors pointed out. The new study was a 3-year follow-up of these authors’ previous randomly assigned placebo-controlled trial participants.
The ‘super-donor’ concept
The authors of the current study described the single chosen donor as “a healthy male aged 36 years with a normal BMI [body mass index] who was born via vaginal delivery, breastfed, a nonsmoker, was not taking any medication, was treated only a few times with antibiotics, exercised regularly, and consumed a sport-specific diet that was richer in protein, fiber, minerals, and vitamins than the average diet.”
The donor had high microbial diversity, and his fecal bacteria makeup was different from that of 254 healthy subjects for 14 of the 48 bacterial markers investigators tested.
Dr. Lacy said that the “super-donor” concept is noteworthy and an apparent key to success.
“Other studies have not done this,” Dr. Lacy noted.
Among the strengths of the study are that it included a relatively large cohort of patients with IBS, with three IBS subtypes and a single well-defined donor. However, it did not include the fourth IBS subtype, unsubtyped IBS, and it only investigated a part of the intestinal bacterial content, the authors acknowledged.
Most interesting, Dr. Lacy said, is that the IBS subtype did not seem to matter to FMT outcomes at 3 years and that all three subtypes responded better than placebo.
“That’s encouraging,” Dr. Lacy said.
The investigators received a grant from Helse Fonna. The study authors and Dr. Lacy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new data show.
Nearly three out of four people in a clinical trial experienced fewer symptoms and fatigue and a greater quality of life at both 2 years and 3 years after FMT in Norway. Those FMT-treated patients who relapsed subsequently responded to FMT upon retransplantation, reported the authors, who also correlated individual microbial profiles with clinical outcomes.
The study, led by Magdy El-Salhy, MD, PhD, department of medicine, Stord (Norway) Hospital, was published online in Gastroenterology.
An expert not involved with the study, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic in Jacksonville, Fla., said that the results of the study are important, but he cautions against treating IBS with FMT outside clinical trials, at least until the protocol is validated, given demonstrated risks.
The new study included 125 patients (104 women, 21 men) in three groups: 38 received a placebo, 42 received 30 g of donor feces, and 45 received 60 g of donor feces. The feces – all from one male donor – was administered to the duodenum.
The response rates for those who received FMT were significantly higher than for those who received placebo. Those receiving 30 g of feces had a response rate of 69.1%, and those in the 60-g group had a response rate of 77.8%, whereas the response rate in the placebo group was 26.3%.
Patients provided a fecal sample and completed five questionnaires at the beginning of the study and at 2 and 3 years after FMT.
Patients in both treatment groups had significantly fewer IBS symptoms – such as abdominal pain, abdominal bloating, dissatisfaction with bowel habits, and quality-of-life interruption – and less fatigue compared with the placebo group, as well as higher quality of life scores at 2 and 3 years.
No long-term adverse effects were reported.
The dysbiosis index decreased only in the treatment group at years 2 and 3.
Microbial modifications correlate with IBS symptoms
In addition, the fluorescent signals of 10 bacteria that had changed after FMT were significantly correlated with improved IBS symptoms and fatigue in both treatment groups.
“Of the bacteria markers whose fluorescence signals changed in the 30-g and 60-g groups, but not in the placebo group, at both 2 and 3 years after FMT, nine were significantly correlated with the total IBS-SSS [IBS–Severity Scoring System] scores,” the authors wrote. One more bacterium with a changed fluorescence signal in the active treatment group also correlated with total fatigue.
Dr. El-Salhy told this news organization that those findings open the door for the select bacteria to be used, for example, in capsule form to treat IBS and fatigue.
The most surprising finding for the team was that the “majority of IBS patients [who] responded to FMT maintained response up to 3 years” or more, said Dr. El-Salhy, alluding to unpublished data up to 5 years.
“Furthermore, 80% of those who relapsed after 3 years responded to a new FMT,” he said.
Women had higher response rates than men at years 2 and 3 after FMT, but there were no differences between complete remission rates of women and men at years 2 and 3.
‘Impressive’ results, but caution warranted
“The results are impressive,” Dr. Lacy said. “I believe that this will help researchers around the world refine their techniques, as we learn more about FMT for the treatment of IBS. It also clearly plays up the importance of the gut microbiome in symptom generation in IBS patients.”
However, he said, until the results are replicated, and the protocol validated, FMT should not be used routinely to treat IBS because there are risks with the procedure.
Dr. Lacy pointed out there have been mixed results in the literature regarding FMT and IBS.
He cited a meta-analysis of four studies (n = 254) in 2019 that did not show a benefit for FMT in patients with IBS. However, a second meta-analysis of five studies (n = 267) did show some benefit, possibly owing to the type of donor and small-bowel infusion.
The authors added that, in the seven randomized, controlled trials investigating FMT for IBS, four concluded that FMT eased symptoms and improved quality of life in patients with IBS, whereas treatment was not effective in the other three.
The authors pointed to differences in protocols, donors, the cohort treated, FMT dose, and route of administration.
The longest response time previously studied in the randomized, controlled trials was 1 year, the authors pointed out. The new study was a 3-year follow-up of these authors’ previous randomly assigned placebo-controlled trial participants.
The ‘super-donor’ concept
The authors of the current study described the single chosen donor as “a healthy male aged 36 years with a normal BMI [body mass index] who was born via vaginal delivery, breastfed, a nonsmoker, was not taking any medication, was treated only a few times with antibiotics, exercised regularly, and consumed a sport-specific diet that was richer in protein, fiber, minerals, and vitamins than the average diet.”
The donor had high microbial diversity, and his fecal bacteria makeup was different from that of 254 healthy subjects for 14 of the 48 bacterial markers investigators tested.
Dr. Lacy said that the “super-donor” concept is noteworthy and an apparent key to success.
“Other studies have not done this,” Dr. Lacy noted.
Among the strengths of the study are that it included a relatively large cohort of patients with IBS, with three IBS subtypes and a single well-defined donor. However, it did not include the fourth IBS subtype, unsubtyped IBS, and it only investigated a part of the intestinal bacterial content, the authors acknowledged.
Most interesting, Dr. Lacy said, is that the IBS subtype did not seem to matter to FMT outcomes at 3 years and that all three subtypes responded better than placebo.
“That’s encouraging,” Dr. Lacy said.
The investigators received a grant from Helse Fonna. The study authors and Dr. Lacy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
CDC recommends high-dose flu vaccines for seniors
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
Surgical Specimens and Margins
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.
Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.

Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.

Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
Practice Points
- Margin analysis in simple excisions can provide useful information by proxy about more than the 1% of the margin often quoted in the literature.
- Simple excisions of uncomplicated keratinocytic carcinomas are associated with high cure rates.
Doc releases song after racist massacre in Buffalo
Physician-musician Cleveland Francis, MD, responded to the recent mass shooting in Buffalo, New York, which left 10 dead, in the only way he knew how. He wrote and recorded a song to honor the victims as “a plea to the other side to recognize us as people,” the Black cardiologist told this news organization.
He couldn’t sleep after the shooting, and “this song was just in my head.” In the 1990s, Dr. Francis took a 3-year sabbatical from medicine to perform and tour as a country singer. He leveraged his Nashville connections to get “Buffalo” produced and recorded.
Acclaimed artist James Threalkill created the accompanying art, titled “The Heavenly Escort of the Buffalo 10,” after listening to a scratch demo.
Dr. Francis doesn’t want people to overlook the massacre as just another gun violence incident because this was “overt hate-crime racism,” he said.
According to the affidavit submitted by FBI agent Christopher J. Dlugokinski, the suspect’s “motive for the mass shooting was to prevent Black people from replacing White people and eliminating the White race, and to inspire others to commit similar attacks.”
Dr. Francis views the Buffalo shooting as distinct from cases like the murder of George Floyd that involved crime or police. It immediately made him think of the Mother Emanuel Church shooting in Charleston, South Carolina. “Having a black skin is now a death warrant,” he said.
The song is also an appeal for White people to fight racism. Dr. Francis is concerned about young men caught up in white supremacy and suggests that we be more alert to children or grandchildren who disconnect from their families, spend time on the dark web, and access guns. The lyrics deliberately don’t mention guns because Dr. Francis wanted to stay out of that debate. “I just sang: ‘What else do I have to do to prove to you that I’m human too?’ ”
Despite his country credentials, Dr. Francis wrote “Buffalo” as a Gospel song because that genre “connects with Black people more and because that civil rights movement was through the church with Dr. Martin Luther King,” he explained. Although he sings all styles of music, the song is performed by Nashville-based singer Michael Lusk so that it’s not a “Cleve Francis thing,” he said, referring to his stage name.
Songwriter Norman Kerner collaborated on the song. The music was produced and recorded by David Thein and mixed by Bob Bullock of Nashville, who Dr. Francis had worked with when he was an artist on Capitol Records.
They sent the video and artwork to the Mayor of Buffalo, Byron Brown, but have yet to hear back. Dr. Francis hopes it could be part of their healing, noting that some people used the song in their Juneteenth celebrations.
The Louisiana native grew up during segregation and was one of two Black students in the Medical College of Virginia class of 1973. After completing his cardiology fellowship, no one would hire him, so Dr. Francis set up his own practice in Northern Virginia. He now works at Inova Heart and Vascular Institute in Alexandria, Va. He remains optimistic about race relations in America and would love a Black pop or Gospel star to record “Buffalo” and bring it to a wider audience.
Dr. Francis is a regular blogger for Medscape. His contribution to country music is recognized in the National Museum of African American History and Culture in Washington, DC. You can find more of his music on YouTube.
A version of this article first appeared on Medscape.com.
Physician-musician Cleveland Francis, MD, responded to the recent mass shooting in Buffalo, New York, which left 10 dead, in the only way he knew how. He wrote and recorded a song to honor the victims as “a plea to the other side to recognize us as people,” the Black cardiologist told this news organization.
He couldn’t sleep after the shooting, and “this song was just in my head.” In the 1990s, Dr. Francis took a 3-year sabbatical from medicine to perform and tour as a country singer. He leveraged his Nashville connections to get “Buffalo” produced and recorded.
Acclaimed artist James Threalkill created the accompanying art, titled “The Heavenly Escort of the Buffalo 10,” after listening to a scratch demo.
Dr. Francis doesn’t want people to overlook the massacre as just another gun violence incident because this was “overt hate-crime racism,” he said.
According to the affidavit submitted by FBI agent Christopher J. Dlugokinski, the suspect’s “motive for the mass shooting was to prevent Black people from replacing White people and eliminating the White race, and to inspire others to commit similar attacks.”
Dr. Francis views the Buffalo shooting as distinct from cases like the murder of George Floyd that involved crime or police. It immediately made him think of the Mother Emanuel Church shooting in Charleston, South Carolina. “Having a black skin is now a death warrant,” he said.
The song is also an appeal for White people to fight racism. Dr. Francis is concerned about young men caught up in white supremacy and suggests that we be more alert to children or grandchildren who disconnect from their families, spend time on the dark web, and access guns. The lyrics deliberately don’t mention guns because Dr. Francis wanted to stay out of that debate. “I just sang: ‘What else do I have to do to prove to you that I’m human too?’ ”
Despite his country credentials, Dr. Francis wrote “Buffalo” as a Gospel song because that genre “connects with Black people more and because that civil rights movement was through the church with Dr. Martin Luther King,” he explained. Although he sings all styles of music, the song is performed by Nashville-based singer Michael Lusk so that it’s not a “Cleve Francis thing,” he said, referring to his stage name.
Songwriter Norman Kerner collaborated on the song. The music was produced and recorded by David Thein and mixed by Bob Bullock of Nashville, who Dr. Francis had worked with when he was an artist on Capitol Records.
They sent the video and artwork to the Mayor of Buffalo, Byron Brown, but have yet to hear back. Dr. Francis hopes it could be part of their healing, noting that some people used the song in their Juneteenth celebrations.
The Louisiana native grew up during segregation and was one of two Black students in the Medical College of Virginia class of 1973. After completing his cardiology fellowship, no one would hire him, so Dr. Francis set up his own practice in Northern Virginia. He now works at Inova Heart and Vascular Institute in Alexandria, Va. He remains optimistic about race relations in America and would love a Black pop or Gospel star to record “Buffalo” and bring it to a wider audience.
Dr. Francis is a regular blogger for Medscape. His contribution to country music is recognized in the National Museum of African American History and Culture in Washington, DC. You can find more of his music on YouTube.
A version of this article first appeared on Medscape.com.
Physician-musician Cleveland Francis, MD, responded to the recent mass shooting in Buffalo, New York, which left 10 dead, in the only way he knew how. He wrote and recorded a song to honor the victims as “a plea to the other side to recognize us as people,” the Black cardiologist told this news organization.
He couldn’t sleep after the shooting, and “this song was just in my head.” In the 1990s, Dr. Francis took a 3-year sabbatical from medicine to perform and tour as a country singer. He leveraged his Nashville connections to get “Buffalo” produced and recorded.
Acclaimed artist James Threalkill created the accompanying art, titled “The Heavenly Escort of the Buffalo 10,” after listening to a scratch demo.
Dr. Francis doesn’t want people to overlook the massacre as just another gun violence incident because this was “overt hate-crime racism,” he said.
According to the affidavit submitted by FBI agent Christopher J. Dlugokinski, the suspect’s “motive for the mass shooting was to prevent Black people from replacing White people and eliminating the White race, and to inspire others to commit similar attacks.”
Dr. Francis views the Buffalo shooting as distinct from cases like the murder of George Floyd that involved crime or police. It immediately made him think of the Mother Emanuel Church shooting in Charleston, South Carolina. “Having a black skin is now a death warrant,” he said.
The song is also an appeal for White people to fight racism. Dr. Francis is concerned about young men caught up in white supremacy and suggests that we be more alert to children or grandchildren who disconnect from their families, spend time on the dark web, and access guns. The lyrics deliberately don’t mention guns because Dr. Francis wanted to stay out of that debate. “I just sang: ‘What else do I have to do to prove to you that I’m human too?’ ”
Despite his country credentials, Dr. Francis wrote “Buffalo” as a Gospel song because that genre “connects with Black people more and because that civil rights movement was through the church with Dr. Martin Luther King,” he explained. Although he sings all styles of music, the song is performed by Nashville-based singer Michael Lusk so that it’s not a “Cleve Francis thing,” he said, referring to his stage name.
Songwriter Norman Kerner collaborated on the song. The music was produced and recorded by David Thein and mixed by Bob Bullock of Nashville, who Dr. Francis had worked with when he was an artist on Capitol Records.
They sent the video and artwork to the Mayor of Buffalo, Byron Brown, but have yet to hear back. Dr. Francis hopes it could be part of their healing, noting that some people used the song in their Juneteenth celebrations.
The Louisiana native grew up during segregation and was one of two Black students in the Medical College of Virginia class of 1973. After completing his cardiology fellowship, no one would hire him, so Dr. Francis set up his own practice in Northern Virginia. He now works at Inova Heart and Vascular Institute in Alexandria, Va. He remains optimistic about race relations in America and would love a Black pop or Gospel star to record “Buffalo” and bring it to a wider audience.
Dr. Francis is a regular blogger for Medscape. His contribution to country music is recognized in the National Museum of African American History and Culture in Washington, DC. You can find more of his music on YouTube.
A version of this article first appeared on Medscape.com.
Nevada sees increase in out-of-state abortion patients
Nevada is already seeing more out-of-state patients seeking an abortion, which state officials expected after the Supreme Court overturned Roe v. Wade.
Las Vegas has seen a 200% increase in patients traveling from Texas, compared with the same time last year, according to the Las Vegas Review-Journal.
Patients are also expected from Arizona, Idaho, Oklahoma, and Utah after the ruling. Abortion providers are preparing for a ripple effect as abortion bans begin across the country.
“We haven’t seen the peak yet,” Kristina Tocce, MD, medical director for Planned Parenthood of the Rocky Mountains and an obstetrician-gynecologist in Colorado, told the newspaper.
“I don’t think we’re going to see any decrease anytime in the near future,” she said.
Nevada made the right to abortion part of state law more than 3 decades ago, in 1990, which protects abortions up to 24 weeks. Colorado passed a similar law this year.
In June, before Roe was overturned, Dr. Tocce said the organization expected abortions to rise by 80% – or about 10,000 patients – in the Rocky Mountain region, which includes southern Nevada, Colorado, and New Mexico.
Even before the ruling took place, Planned Parenthood saw higher numbers of patients as abortion bans took effect in Texas and Oklahoma, she said. After the 6-week ban took place in Texas, about 45% of traveling patients went to Oklahoma. Now that a ban is in place in Oklahoma, patients are going elsewhere.
Las Vegas providers have asked patients why they decided to travel to southern Nevada for services rather than Colorado or New Mexico, which are closer to Texas, Dr. Tocce said. Patients cited several reasons, including direct flight paths, cheaper plane tickets, and the presence of family or friends who could support them.
“We’re going to see such a demand on abortion in any state that has secure access,” Dr. Tocce said. “Patients may be forced to travel further away.”
After Roe was overturned, Nevada Gov. Steve Sisolak held an emergency news conference to reaffirm the state’s commitment to protecting abortion rights. He also said he wasn’t sure about Nevada’s capacity to support out-of-state patients but providers were researching and preparing.
Two Planned Parenthood centers in southern Nevada are adding staff and increasing their hours, Dr. Tocce told the newspaper last month, though there weren’t immediate plans to increase the number of locations or add centers near state borders.
Last week, Governor Sisolak signed an executive order that stops Nevada agencies from helping other states investigate patients seeking an abortion in Nevada. The order also protects patients from extradition and health care providers from losing their license for providing abortion services.
As abortion bans continue to roll out across the U.S., patients will likely consider traveling to states that have certain protections and accessible appointments, Dr. Tocce said.
“We’re in such an ambiguous time right now, we just don’t know what each state is going to attempt to enact,” she said. “My head just swims with all of the possibilities. If that’s challenging for me, I can’t even imagine what it’s going to be like for a patient to navigate.”
A version of this article first appeared on WebMD.com.
Nevada is already seeing more out-of-state patients seeking an abortion, which state officials expected after the Supreme Court overturned Roe v. Wade.
Las Vegas has seen a 200% increase in patients traveling from Texas, compared with the same time last year, according to the Las Vegas Review-Journal.
Patients are also expected from Arizona, Idaho, Oklahoma, and Utah after the ruling. Abortion providers are preparing for a ripple effect as abortion bans begin across the country.
“We haven’t seen the peak yet,” Kristina Tocce, MD, medical director for Planned Parenthood of the Rocky Mountains and an obstetrician-gynecologist in Colorado, told the newspaper.
“I don’t think we’re going to see any decrease anytime in the near future,” she said.
Nevada made the right to abortion part of state law more than 3 decades ago, in 1990, which protects abortions up to 24 weeks. Colorado passed a similar law this year.
In June, before Roe was overturned, Dr. Tocce said the organization expected abortions to rise by 80% – or about 10,000 patients – in the Rocky Mountain region, which includes southern Nevada, Colorado, and New Mexico.
Even before the ruling took place, Planned Parenthood saw higher numbers of patients as abortion bans took effect in Texas and Oklahoma, she said. After the 6-week ban took place in Texas, about 45% of traveling patients went to Oklahoma. Now that a ban is in place in Oklahoma, patients are going elsewhere.
Las Vegas providers have asked patients why they decided to travel to southern Nevada for services rather than Colorado or New Mexico, which are closer to Texas, Dr. Tocce said. Patients cited several reasons, including direct flight paths, cheaper plane tickets, and the presence of family or friends who could support them.
“We’re going to see such a demand on abortion in any state that has secure access,” Dr. Tocce said. “Patients may be forced to travel further away.”
After Roe was overturned, Nevada Gov. Steve Sisolak held an emergency news conference to reaffirm the state’s commitment to protecting abortion rights. He also said he wasn’t sure about Nevada’s capacity to support out-of-state patients but providers were researching and preparing.
Two Planned Parenthood centers in southern Nevada are adding staff and increasing their hours, Dr. Tocce told the newspaper last month, though there weren’t immediate plans to increase the number of locations or add centers near state borders.
Last week, Governor Sisolak signed an executive order that stops Nevada agencies from helping other states investigate patients seeking an abortion in Nevada. The order also protects patients from extradition and health care providers from losing their license for providing abortion services.
As abortion bans continue to roll out across the U.S., patients will likely consider traveling to states that have certain protections and accessible appointments, Dr. Tocce said.
“We’re in such an ambiguous time right now, we just don’t know what each state is going to attempt to enact,” she said. “My head just swims with all of the possibilities. If that’s challenging for me, I can’t even imagine what it’s going to be like for a patient to navigate.”
A version of this article first appeared on WebMD.com.
Nevada is already seeing more out-of-state patients seeking an abortion, which state officials expected after the Supreme Court overturned Roe v. Wade.
Las Vegas has seen a 200% increase in patients traveling from Texas, compared with the same time last year, according to the Las Vegas Review-Journal.
Patients are also expected from Arizona, Idaho, Oklahoma, and Utah after the ruling. Abortion providers are preparing for a ripple effect as abortion bans begin across the country.
“We haven’t seen the peak yet,” Kristina Tocce, MD, medical director for Planned Parenthood of the Rocky Mountains and an obstetrician-gynecologist in Colorado, told the newspaper.
“I don’t think we’re going to see any decrease anytime in the near future,” she said.
Nevada made the right to abortion part of state law more than 3 decades ago, in 1990, which protects abortions up to 24 weeks. Colorado passed a similar law this year.
In June, before Roe was overturned, Dr. Tocce said the organization expected abortions to rise by 80% – or about 10,000 patients – in the Rocky Mountain region, which includes southern Nevada, Colorado, and New Mexico.
Even before the ruling took place, Planned Parenthood saw higher numbers of patients as abortion bans took effect in Texas and Oklahoma, she said. After the 6-week ban took place in Texas, about 45% of traveling patients went to Oklahoma. Now that a ban is in place in Oklahoma, patients are going elsewhere.
Las Vegas providers have asked patients why they decided to travel to southern Nevada for services rather than Colorado or New Mexico, which are closer to Texas, Dr. Tocce said. Patients cited several reasons, including direct flight paths, cheaper plane tickets, and the presence of family or friends who could support them.
“We’re going to see such a demand on abortion in any state that has secure access,” Dr. Tocce said. “Patients may be forced to travel further away.”
After Roe was overturned, Nevada Gov. Steve Sisolak held an emergency news conference to reaffirm the state’s commitment to protecting abortion rights. He also said he wasn’t sure about Nevada’s capacity to support out-of-state patients but providers were researching and preparing.
Two Planned Parenthood centers in southern Nevada are adding staff and increasing their hours, Dr. Tocce told the newspaper last month, though there weren’t immediate plans to increase the number of locations or add centers near state borders.
Last week, Governor Sisolak signed an executive order that stops Nevada agencies from helping other states investigate patients seeking an abortion in Nevada. The order also protects patients from extradition and health care providers from losing their license for providing abortion services.
As abortion bans continue to roll out across the U.S., patients will likely consider traveling to states that have certain protections and accessible appointments, Dr. Tocce said.
“We’re in such an ambiguous time right now, we just don’t know what each state is going to attempt to enact,” she said. “My head just swims with all of the possibilities. If that’s challenging for me, I can’t even imagine what it’s going to be like for a patient to navigate.”
A version of this article first appeared on WebMD.com.
Neck floats may not be right for certain babies, FDA warns
The FDA is warning that parents should avoid using neck floats for infants with special needs or developmental delays.
According to the agency, companies have been advertising the products as having health benefits for children with physical and developmental problems, despite a lack of evidence for such claims. The companies, which the FDA did not name, claimed that water therapy with floats could help babies with special needs – like those with spina bifida – to increase muscle tone, boost flexibility and range of motion, and build lung capacity, among other benefits.
But used improperly, neck floats can lead to serious injury and death. At least one baby has died, and one was hospitalized, after using the floats, FDA officials said.
The inflatable plastic rings are worn around a baby’s neck, allowing them to float freely in water. Some of these products are being marketed for infants as young as 2 weeks old, as well as for premature babies. But the FDA said the safety and effectiveness of the products for these children have not been proven.
The floats “have not been evaluated by the FDA, and we are not aware of any demonstrated benefit with the use of neck floats for water therapy interventions,” the agency said in the June 28 statement.
While injuries and deaths from neck floats are rare, the FDA said families and caregivers should be aware that these incidents can and do occur.
People who have problems with the neck floats are encouraged to report them through MedWatch, the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by the FDA are required to file new reports with the FDA.
A version of this article first appeared on WebMD.com.
The FDA is warning that parents should avoid using neck floats for infants with special needs or developmental delays.
According to the agency, companies have been advertising the products as having health benefits for children with physical and developmental problems, despite a lack of evidence for such claims. The companies, which the FDA did not name, claimed that water therapy with floats could help babies with special needs – like those with spina bifida – to increase muscle tone, boost flexibility and range of motion, and build lung capacity, among other benefits.
But used improperly, neck floats can lead to serious injury and death. At least one baby has died, and one was hospitalized, after using the floats, FDA officials said.
The inflatable plastic rings are worn around a baby’s neck, allowing them to float freely in water. Some of these products are being marketed for infants as young as 2 weeks old, as well as for premature babies. But the FDA said the safety and effectiveness of the products for these children have not been proven.
The floats “have not been evaluated by the FDA, and we are not aware of any demonstrated benefit with the use of neck floats for water therapy interventions,” the agency said in the June 28 statement.
While injuries and deaths from neck floats are rare, the FDA said families and caregivers should be aware that these incidents can and do occur.
People who have problems with the neck floats are encouraged to report them through MedWatch, the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by the FDA are required to file new reports with the FDA.
A version of this article first appeared on WebMD.com.
The FDA is warning that parents should avoid using neck floats for infants with special needs or developmental delays.
According to the agency, companies have been advertising the products as having health benefits for children with physical and developmental problems, despite a lack of evidence for such claims. The companies, which the FDA did not name, claimed that water therapy with floats could help babies with special needs – like those with spina bifida – to increase muscle tone, boost flexibility and range of motion, and build lung capacity, among other benefits.
But used improperly, neck floats can lead to serious injury and death. At least one baby has died, and one was hospitalized, after using the floats, FDA officials said.
The inflatable plastic rings are worn around a baby’s neck, allowing them to float freely in water. Some of these products are being marketed for infants as young as 2 weeks old, as well as for premature babies. But the FDA said the safety and effectiveness of the products for these children have not been proven.
The floats “have not been evaluated by the FDA, and we are not aware of any demonstrated benefit with the use of neck floats for water therapy interventions,” the agency said in the June 28 statement.
While injuries and deaths from neck floats are rare, the FDA said families and caregivers should be aware that these incidents can and do occur.
People who have problems with the neck floats are encouraged to report them through MedWatch, the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by the FDA are required to file new reports with the FDA.
A version of this article first appeared on WebMD.com.





