User login
Erythematous Papules on the Ears
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
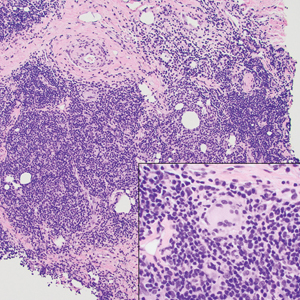
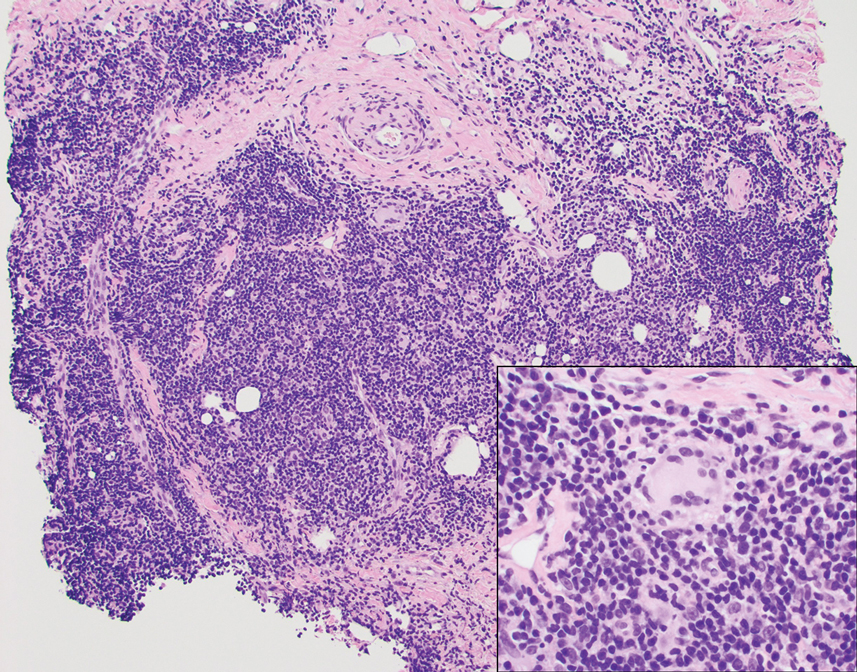
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
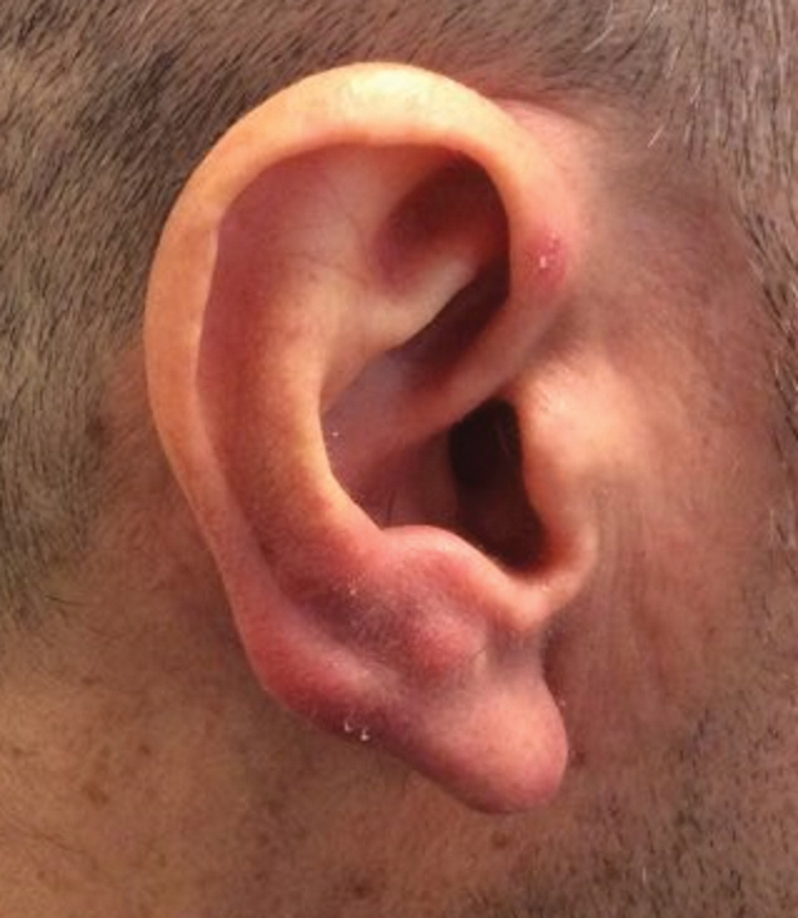
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
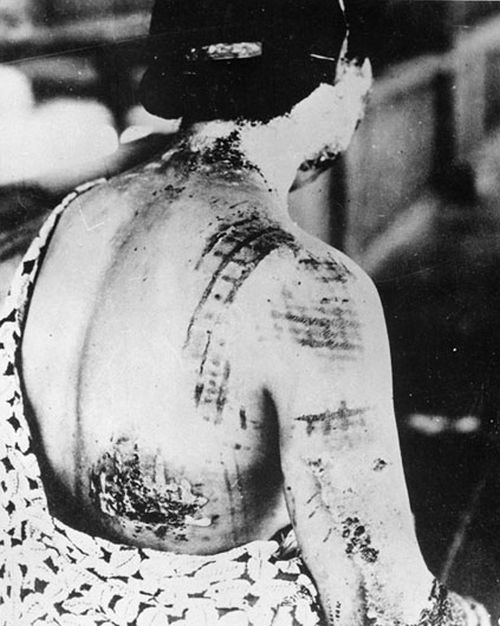
A 53-year-old man with a history of atopic dermatitis presented with pain and redness of the lobules of both ears of 9 months’ duration. He had no known allergies and took no medications. He lived in suburban Virginia and had not recently traveled outside of the region. Physical examination revealed tender erythematous and edematous nodules on the lobules of both ears (top). There was no evidence of arthritis or neurologic deficits. A punch biopsy was performed (bottom).
If nuclear disaster strikes, U.S. hematologists stand ready
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.
“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”
And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
Chest Infections & Disaster Response Network
Disaster Response and Global Health Section
Physician response to Ukraine and beyond
Displaced persons, international refugee crises, gun violence, and other disasters remain prevalent in current news. Recent events highlight the need for continued civilian physician leadership and response to disasters.
Before the Ukraine crisis, the United Nations Refugee Agency estimated displaced persons more than doubled to greater than 82 million persons over the last decade (unhcr.org). Since that analysis, there have been over 6.5 million externally displaced persons, 7.5 million internally displaced persons, and significant numbers of injured patients from the Ukraine crisis alone. The Ukraine Ministry of Health has shown preparedness in its ability to handle significant patient surges with minimal assistance.
However, organizations like the Ukraine Medical Association of North America, Razom for Ukraine, Doctors Without Borders (MSF), MedGlobal, Samaritan’s Purse, Global Response Management, and many more have deployed to assist in Ukraine. These NGOs continue to help with medical care, fulfill critical supply needs, and provide training in cutting-edge medicine (POCUS, trauma updates).
Challenges posed by unstable environments, from wars to active shooter situations, further underscore the need for continued education, advances in technology, and preparedness. Providers responding to these events often treat vulnerable populations suffering from physical and mental violence, requiring physicians to step out of their comfort zone.
Physicians should continue to be leaders in the care of vulnerable displaced persons.
Christopher Miller, DO, MPH
Fellow-in-Training Member
Thomas Marston, MD
Member-at-Large
Disaster Response and Global Health Section
Physician response to Ukraine and beyond
Displaced persons, international refugee crises, gun violence, and other disasters remain prevalent in current news. Recent events highlight the need for continued civilian physician leadership and response to disasters.
Before the Ukraine crisis, the United Nations Refugee Agency estimated displaced persons more than doubled to greater than 82 million persons over the last decade (unhcr.org). Since that analysis, there have been over 6.5 million externally displaced persons, 7.5 million internally displaced persons, and significant numbers of injured patients from the Ukraine crisis alone. The Ukraine Ministry of Health has shown preparedness in its ability to handle significant patient surges with minimal assistance.
However, organizations like the Ukraine Medical Association of North America, Razom for Ukraine, Doctors Without Borders (MSF), MedGlobal, Samaritan’s Purse, Global Response Management, and many more have deployed to assist in Ukraine. These NGOs continue to help with medical care, fulfill critical supply needs, and provide training in cutting-edge medicine (POCUS, trauma updates).
Challenges posed by unstable environments, from wars to active shooter situations, further underscore the need for continued education, advances in technology, and preparedness. Providers responding to these events often treat vulnerable populations suffering from physical and mental violence, requiring physicians to step out of their comfort zone.
Physicians should continue to be leaders in the care of vulnerable displaced persons.
Christopher Miller, DO, MPH
Fellow-in-Training Member
Thomas Marston, MD
Member-at-Large
Disaster Response and Global Health Section
Physician response to Ukraine and beyond
Displaced persons, international refugee crises, gun violence, and other disasters remain prevalent in current news. Recent events highlight the need for continued civilian physician leadership and response to disasters.
Before the Ukraine crisis, the United Nations Refugee Agency estimated displaced persons more than doubled to greater than 82 million persons over the last decade (unhcr.org). Since that analysis, there have been over 6.5 million externally displaced persons, 7.5 million internally displaced persons, and significant numbers of injured patients from the Ukraine crisis alone. The Ukraine Ministry of Health has shown preparedness in its ability to handle significant patient surges with minimal assistance.
However, organizations like the Ukraine Medical Association of North America, Razom for Ukraine, Doctors Without Borders (MSF), MedGlobal, Samaritan’s Purse, Global Response Management, and many more have deployed to assist in Ukraine. These NGOs continue to help with medical care, fulfill critical supply needs, and provide training in cutting-edge medicine (POCUS, trauma updates).
Challenges posed by unstable environments, from wars to active shooter situations, further underscore the need for continued education, advances in technology, and preparedness. Providers responding to these events often treat vulnerable populations suffering from physical and mental violence, requiring physicians to step out of their comfort zone.
Physicians should continue to be leaders in the care of vulnerable displaced persons.
Christopher Miller, DO, MPH
Fellow-in-Training Member
Thomas Marston, MD
Member-at-Large
Early childhood allergies linked with ADHD and ASD
, according to a large retrospective study.
“Our study provides strong evidence for the association between allergic disorders in early childhood and the development of ADHD,” Shay Nemet, MD, of the Kaplan Medical Center, Rehovot, Israel, and colleagues write in Pediatric Allergy and Immunology. “The risk of those children to develop ASD was less significant.”
The researchers analyzed data from 117,022 consecutive children diagnosed with at least one allergic disorder – asthma, conjunctivitis, rhinitis, and drug, food, or skin allergy – and 116,968 children without allergies in the Clalit Health Services pediatric database. The children had been treated from 2000 to 2018; the mean follow-up period was 11 years.
The children who were diagnosed with one or more allergies (mean age, 4.5 years) were significantly more likely to develop ADHD (odds ratio, 2.45; 95% confidence interval, 2.39-2.51), ASD (OR, 1.17; 95% CI, 1.08-1.27), or both ADHD and ASD (OR, 1.56; 95% CI, 1.35-1.79) than were the control children who did not have allergies.
Children diagnosed with rhinitis (OR, 3.96; 95% CI, 3.80-4.12) and conjunctivitis (OR, 3.63; 95% CI, 3.53-3.74) were the most likely to develop ADHD.
Allergy correlation with ADHD and ASD
Cy B. Nadler, PhD, a clinical psychologist and the director of Autism Services at Children’s Mercy Kansas City, Missouri, told this news organization that children and adults with neurodevelopmental differences are also more likely to have other health problems.
“Clinicians practicing in subspecialties such as allergy and immunology may have opportunities to help psychologists identify developmental and behavioral concerns early in childhood,” he added.
“Studies like this can’t be accomplished without large health care databases, but this approach has drawbacks, too,” Dr. Nadler said in an email. “Without more information about these patients’ co-occurring medical and behavioral conditions, we are almost certainly missing important contributors to the observed associations.”
Dr. Nadler, who was not involved in the study, noted that in the multivariable analysis that controlled for age at study entry, gender, and number of annual visits, the link between allergy and ASD diagnosis was not significant.
“It is important to remember not to interpret these study results as causal,” he added.
Desha M. Jordan, MD, FAAP, an assistant professor of pediatrics at UPMC Children’s Hospital of Pittsburgh, called the study “an interesting new area that has been speculated about for some time” and “one of the first I have seen with statistically significant correlations found between ADHD, ASD, and allergic conditions.”
More questions for future studies
Health care providers need to understand the potential sequelae of allergic conditions so that they can manage their patients appropriately, she advised.
Although symptoms and diagnoses were confirmed for all patients, the study’s retrospective design and the possibility of recall bias were limitations, said Dr. Jordan in an email. She also was not involved in the study.
“For example, the family of a child diagnosed with ADHD or ASD may have been more mindful of anything out of the norm in that child’s past, while the family of a child without these conditions may not have recalled allergic symptoms as important,” she explained.
Another question that arises is whether some patients were treated and managed well while others were not and whether this disparity in care affected the development or severity of ADHD or ASD, she added.
“Is a patient with a well-controlled allergic condition less likely to develop ADHD or ASD than a patient with an uncontrolled allergic condition? Does a well-controlled patient ever return to the same probability of getting ADHD or ASD as a nonallergic patient?”
“While this study expands our understanding of these conditions and their interrelationships, it also brings up many additional questions and opens a new segment of research,” Dr. Jordan said. “More studies in this area are necessary to confirm the findings of this paper.”
The study was partially funded by the Israel Ambulatory Pediatric Association. The authors, Dr. Nadler, and Dr. Jordan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a large retrospective study.
“Our study provides strong evidence for the association between allergic disorders in early childhood and the development of ADHD,” Shay Nemet, MD, of the Kaplan Medical Center, Rehovot, Israel, and colleagues write in Pediatric Allergy and Immunology. “The risk of those children to develop ASD was less significant.”
The researchers analyzed data from 117,022 consecutive children diagnosed with at least one allergic disorder – asthma, conjunctivitis, rhinitis, and drug, food, or skin allergy – and 116,968 children without allergies in the Clalit Health Services pediatric database. The children had been treated from 2000 to 2018; the mean follow-up period was 11 years.
The children who were diagnosed with one or more allergies (mean age, 4.5 years) were significantly more likely to develop ADHD (odds ratio, 2.45; 95% confidence interval, 2.39-2.51), ASD (OR, 1.17; 95% CI, 1.08-1.27), or both ADHD and ASD (OR, 1.56; 95% CI, 1.35-1.79) than were the control children who did not have allergies.
Children diagnosed with rhinitis (OR, 3.96; 95% CI, 3.80-4.12) and conjunctivitis (OR, 3.63; 95% CI, 3.53-3.74) were the most likely to develop ADHD.
Allergy correlation with ADHD and ASD
Cy B. Nadler, PhD, a clinical psychologist and the director of Autism Services at Children’s Mercy Kansas City, Missouri, told this news organization that children and adults with neurodevelopmental differences are also more likely to have other health problems.
“Clinicians practicing in subspecialties such as allergy and immunology may have opportunities to help psychologists identify developmental and behavioral concerns early in childhood,” he added.
“Studies like this can’t be accomplished without large health care databases, but this approach has drawbacks, too,” Dr. Nadler said in an email. “Without more information about these patients’ co-occurring medical and behavioral conditions, we are almost certainly missing important contributors to the observed associations.”
Dr. Nadler, who was not involved in the study, noted that in the multivariable analysis that controlled for age at study entry, gender, and number of annual visits, the link between allergy and ASD diagnosis was not significant.
“It is important to remember not to interpret these study results as causal,” he added.
Desha M. Jordan, MD, FAAP, an assistant professor of pediatrics at UPMC Children’s Hospital of Pittsburgh, called the study “an interesting new area that has been speculated about for some time” and “one of the first I have seen with statistically significant correlations found between ADHD, ASD, and allergic conditions.”
More questions for future studies
Health care providers need to understand the potential sequelae of allergic conditions so that they can manage their patients appropriately, she advised.
Although symptoms and diagnoses were confirmed for all patients, the study’s retrospective design and the possibility of recall bias were limitations, said Dr. Jordan in an email. She also was not involved in the study.
“For example, the family of a child diagnosed with ADHD or ASD may have been more mindful of anything out of the norm in that child’s past, while the family of a child without these conditions may not have recalled allergic symptoms as important,” she explained.
Another question that arises is whether some patients were treated and managed well while others were not and whether this disparity in care affected the development or severity of ADHD or ASD, she added.
“Is a patient with a well-controlled allergic condition less likely to develop ADHD or ASD than a patient with an uncontrolled allergic condition? Does a well-controlled patient ever return to the same probability of getting ADHD or ASD as a nonallergic patient?”
“While this study expands our understanding of these conditions and their interrelationships, it also brings up many additional questions and opens a new segment of research,” Dr. Jordan said. “More studies in this area are necessary to confirm the findings of this paper.”
The study was partially funded by the Israel Ambulatory Pediatric Association. The authors, Dr. Nadler, and Dr. Jordan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a large retrospective study.
“Our study provides strong evidence for the association between allergic disorders in early childhood and the development of ADHD,” Shay Nemet, MD, of the Kaplan Medical Center, Rehovot, Israel, and colleagues write in Pediatric Allergy and Immunology. “The risk of those children to develop ASD was less significant.”
The researchers analyzed data from 117,022 consecutive children diagnosed with at least one allergic disorder – asthma, conjunctivitis, rhinitis, and drug, food, or skin allergy – and 116,968 children without allergies in the Clalit Health Services pediatric database. The children had been treated from 2000 to 2018; the mean follow-up period was 11 years.
The children who were diagnosed with one or more allergies (mean age, 4.5 years) were significantly more likely to develop ADHD (odds ratio, 2.45; 95% confidence interval, 2.39-2.51), ASD (OR, 1.17; 95% CI, 1.08-1.27), or both ADHD and ASD (OR, 1.56; 95% CI, 1.35-1.79) than were the control children who did not have allergies.
Children diagnosed with rhinitis (OR, 3.96; 95% CI, 3.80-4.12) and conjunctivitis (OR, 3.63; 95% CI, 3.53-3.74) were the most likely to develop ADHD.
Allergy correlation with ADHD and ASD
Cy B. Nadler, PhD, a clinical psychologist and the director of Autism Services at Children’s Mercy Kansas City, Missouri, told this news organization that children and adults with neurodevelopmental differences are also more likely to have other health problems.
“Clinicians practicing in subspecialties such as allergy and immunology may have opportunities to help psychologists identify developmental and behavioral concerns early in childhood,” he added.
“Studies like this can’t be accomplished without large health care databases, but this approach has drawbacks, too,” Dr. Nadler said in an email. “Without more information about these patients’ co-occurring medical and behavioral conditions, we are almost certainly missing important contributors to the observed associations.”
Dr. Nadler, who was not involved in the study, noted that in the multivariable analysis that controlled for age at study entry, gender, and number of annual visits, the link between allergy and ASD diagnosis was not significant.
“It is important to remember not to interpret these study results as causal,” he added.
Desha M. Jordan, MD, FAAP, an assistant professor of pediatrics at UPMC Children’s Hospital of Pittsburgh, called the study “an interesting new area that has been speculated about for some time” and “one of the first I have seen with statistically significant correlations found between ADHD, ASD, and allergic conditions.”
More questions for future studies
Health care providers need to understand the potential sequelae of allergic conditions so that they can manage their patients appropriately, she advised.
Although symptoms and diagnoses were confirmed for all patients, the study’s retrospective design and the possibility of recall bias were limitations, said Dr. Jordan in an email. She also was not involved in the study.
“For example, the family of a child diagnosed with ADHD or ASD may have been more mindful of anything out of the norm in that child’s past, while the family of a child without these conditions may not have recalled allergic symptoms as important,” she explained.
Another question that arises is whether some patients were treated and managed well while others were not and whether this disparity in care affected the development or severity of ADHD or ASD, she added.
“Is a patient with a well-controlled allergic condition less likely to develop ADHD or ASD than a patient with an uncontrolled allergic condition? Does a well-controlled patient ever return to the same probability of getting ADHD or ASD as a nonallergic patient?”
“While this study expands our understanding of these conditions and their interrelationships, it also brings up many additional questions and opens a new segment of research,” Dr. Jordan said. “More studies in this area are necessary to confirm the findings of this paper.”
The study was partially funded by the Israel Ambulatory Pediatric Association. The authors, Dr. Nadler, and Dr. Jordan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC ALLERGY AND IMMUNOLOGY
Botanical Briefs: Ginkgo (Ginkgo biloba)
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
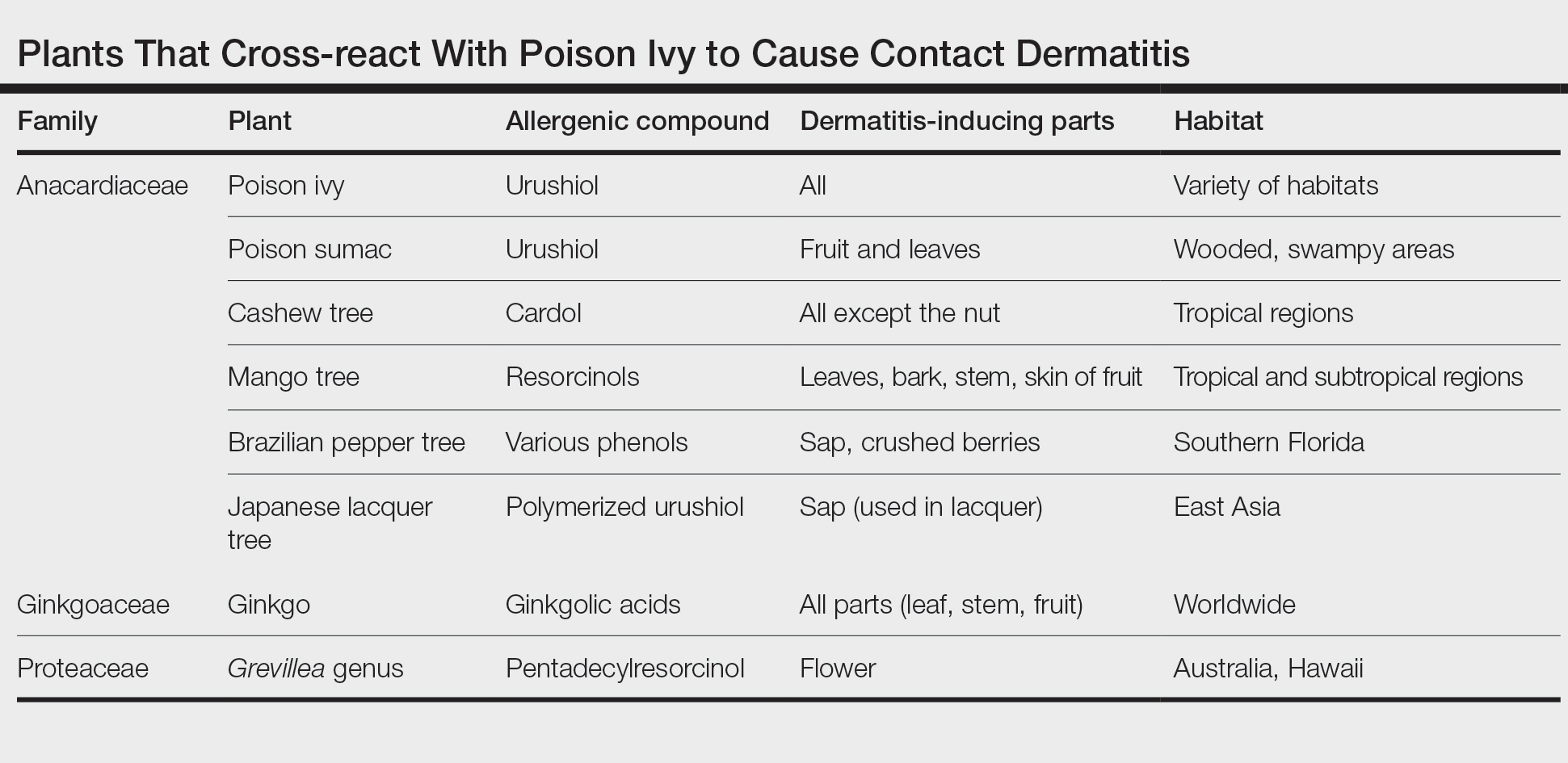
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.
Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.

Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.

Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
PRACTICE POINTS
- Contact with the Ginkgo biloba tree can cause allergic contact dermatitis; ingestion can cause systemic dermatitis in a previously sensitized patient.
- Ginkgo biloba can cross-react with plants of the family Anacardiaceae, such as poison ivy, poison oak, poison sumac, cashew tree, and mango.
- Ginkgo extract is widely considered safe for use; however, dermatologists should be aware that it can cause systemic dermatitis and serious adverse effects, including internal hemorrhage and convulsions.
Aluminum: The 2022 American Contact Dermatitis Society Allergen of the Year
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16
Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16

Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16

Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
Practice Points
- Aluminum is an allergen of significance relating to its use in vaccines, immunotherapies, and antiperspirants.
- There is a greater prevalence of aluminum contact allergy in children than in adults, affecting up to 5% of the pediatric patch-test population.
- The recommended patch test formulation is aluminum chloride hexahydrate 10% in petrolatum, with consideration of reducing the concentration to 2% in children younger than 8 years to avoid strong reactions.
Orf Virus in Humans: Case Series and Clinical Review
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
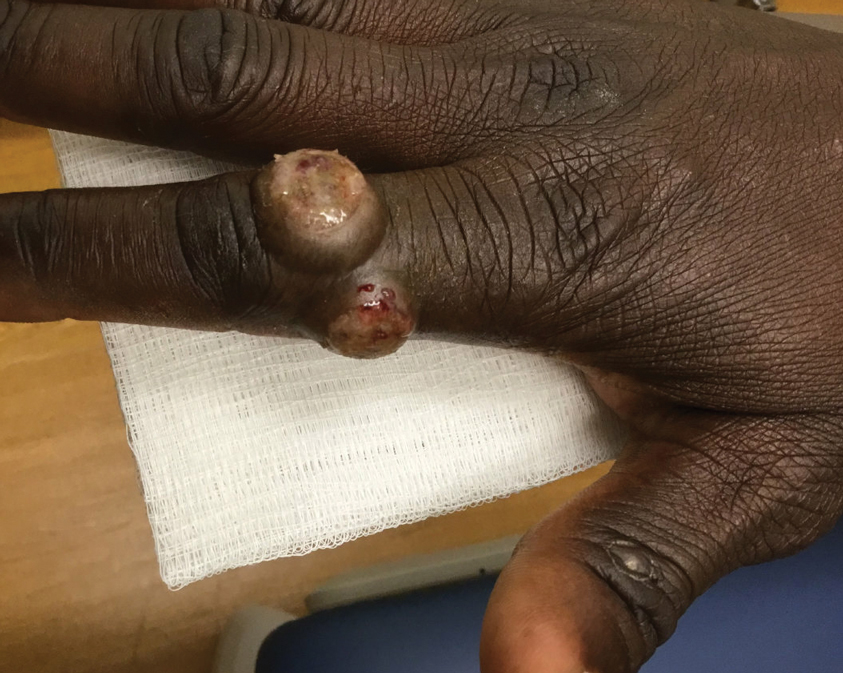
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
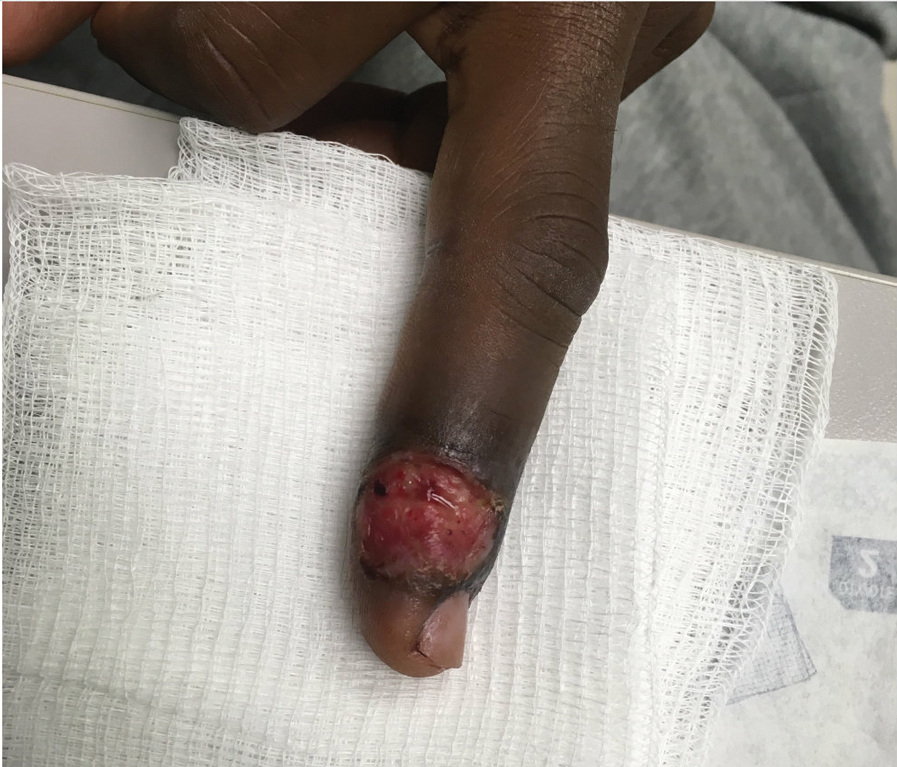
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
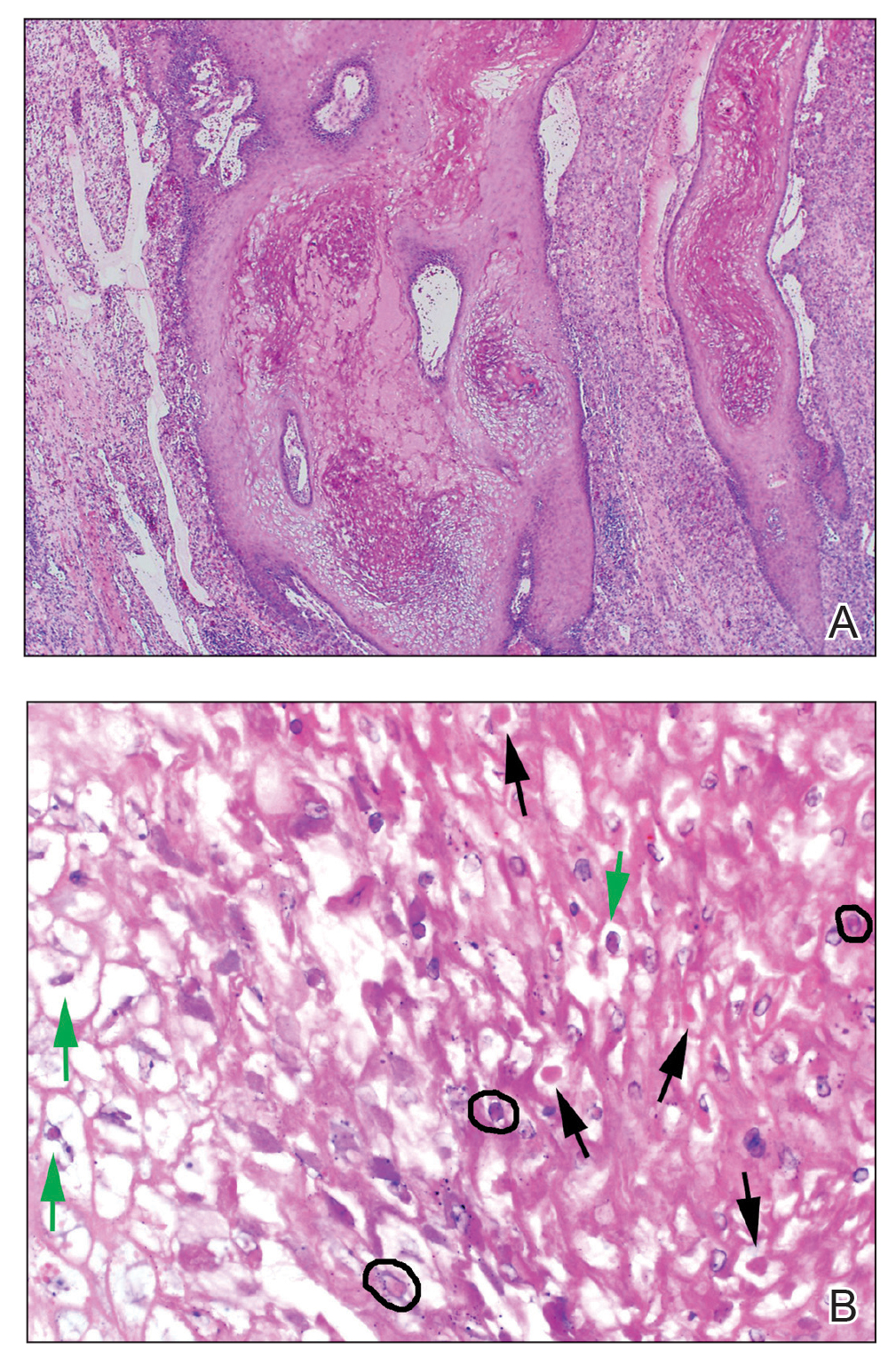
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
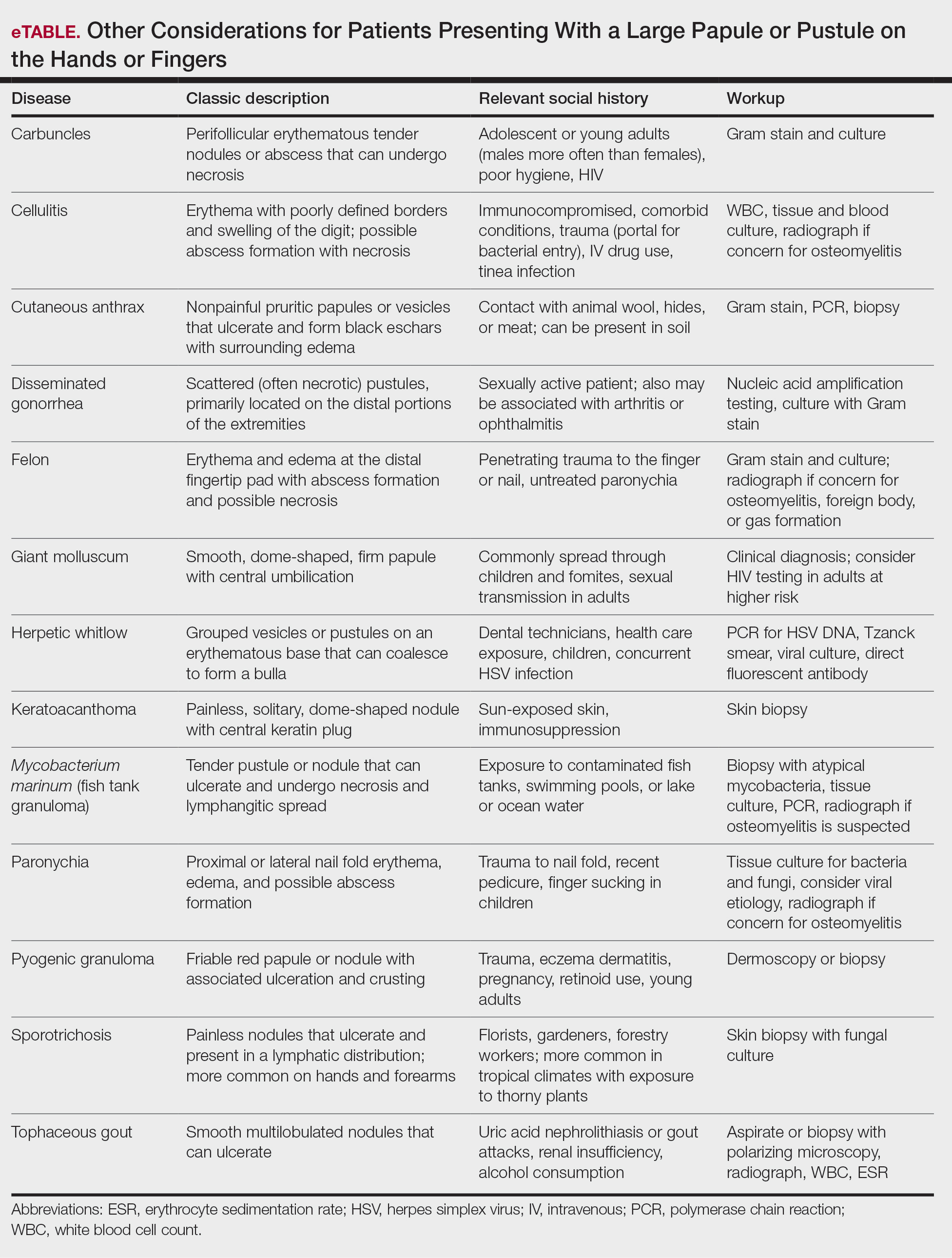
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.

Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.

Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8

Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24

Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26

Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.

Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.

Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8

Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24

Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26

Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
Practice Points
- Ecthyma contagiosum is a discrete clinical entity that occurs worldwide and demands careful attention to clinical course and social history.
- Ecthyma contagiosum is caused by orf virus, an epitheliotropic zoonotic infection that spreads from ruminants to humans.
- Early and rapid diagnosis of this classic condition is critical to prevent unnecessary biopsies or extensive testing, and determination of etiology can be important in preventing reinfection or spread to other humans by the same infected animal.
Telemental health linked with improvements in key outcomes
, new research suggests.
In a nationwide study, researchers drew on Medicare data from nearly 3,000 counties covering the period from 2000 to 2018. Results show that counties in which there was greater use of telemental health services reported higher increases of clinical visits and better follow-up after hospitalization among patients with bipolar 1 disorder and schizophrenia or other psychotic disorders.
In the study, “clinical visits” referred to both in-person and telemental health visits.
“These findings really support the idea that telemental health can be safe and effective and beneficial for in-person care for people with severe mental illness,” coinvestigator Haiden Huskamp, PhD, professor of health care policy at Harvard Medical School, Boston, said in an interview.
The findings were published online in JAMA Network Open.
Continuing trend?
Past studies have pointed to a sharp increase in the use of telepsychiatry services for patients with SMI. As reported by this news organization, this is a trend some clinicians say is likely to continue after the pandemic.
Use of telemedicine during the pandemic received a boost by the temporary suspension of certain Medicare rules that restrict telehealth use. Debate continues at the federal and state levels on whether to make that suspension permanent. Dr. Huskamp said more information is needed about the efficacy and accessibility of telemental health.
To investigate, researchers used Medicare fee-for-service data from 118,170 patients in 2,916 counties. More than two-thirds of the patients were aged 65 years or younger.
During the study period, telemental health service increased from 0.03 visits per patient with SMI in 2010 to 0.19 visits per patient in 2018. This increase was broad, with the number of counties reporting high use of telemental health increasing from 2% in 2010 to 17% in 2018.
Compared with counties in which there was no telemental health services, those with high use were less densely populated and had fewer health care professionals and hospital beds.
The number of overall visits with a mental health professional increased slightly in high-use counties compared to no-use counties, from 4.65 visits in 2010 to 4.79 visits in 2018. The number of in-person visits during that period declined from 4.55 visits in 2010 to 3.73 visits in 2018, which suggests that the overall increase was due to higher use of telemental health.
In the high-use group, the number of patients who had at least four mental health care visits increased 8%, and the number of patients who had a follow-up visit within 30 days of a hospitalization increased 20.4%.
A ‘helpful option’
“Telemedicine doesn’t address the national shortage of providers, but it definitely helps in underserved areas [and] rural areas,” Dr. Huskamp said.
“We need more mental health providers and need to develop new models of care that can leverage the providers we have in the best way possible. This is at least a helpful option, especially when you’re thinking about the maldistribution of providers across the country,” she added.
The study results showed that there was no difference in medication adherence between low- and high-use counties.
There was greater contact with mental health care providers in counties with high use of telemental health, and patients in the high-use group were 7.6% more likely to be hospitalized within a year compared with their peers in counties that had no telemental health use.
“We did see modest increases in inpatient use in counties that shifted the most to telemental health services, but that’s not typically viewed as a measure of quality because it can mean so many different things,” Dr. Huskamp said.
For example, it could mean that counties with greater telemental health use did a better job of identifying and responding to patients’ need for acute care, she noted. It could also be a reflection of the loss of psychiatric inpatient care in low-use communities.
Another tool
Commenting on the findings, Robert Caudill, MD, director of Telemedicine and Information Technology Programs at the University of Louisville (Ky.), called the increase in hospitalization in high-use counties “surprising.” However, he noted it might be a reflection of the need to fine-tune telemental health for patients with SMI.
“I think that more time and experience with telehealth will further normalize the practice and help to narrow, if not close, the gap,” said Dr. Caudill, who was not involved with the research.
“There are so many side benefits to doing things via telehealth,” he added. “It is a simple matter of continuing to learn how to do those things better.”
A multidisciplinary approach that includes psychiatric care and case management is generally considered to be the gold standard in treating patients with the types of mental illness included in this study, Dr. Caudill said.
While some of that care can be delivered effectively via telemedicine, it is possible other aspects, such as case management, are better handled in person, he added.
“I don’t think it is the role of telehealth to make in-person care obsolete. It is simply a tool to be used when appropriate,” said Dr. Caudill, past chair of the American Telemedicine Association’s Telemental Health Special Interest Group.
“Surgeons did not abandon scalpels when laser surgery became possible,” he said.
The study was funded by the National Institutes of Mental Health. Dr. Huskamp and Dr. Caudill report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a nationwide study, researchers drew on Medicare data from nearly 3,000 counties covering the period from 2000 to 2018. Results show that counties in which there was greater use of telemental health services reported higher increases of clinical visits and better follow-up after hospitalization among patients with bipolar 1 disorder and schizophrenia or other psychotic disorders.
In the study, “clinical visits” referred to both in-person and telemental health visits.
“These findings really support the idea that telemental health can be safe and effective and beneficial for in-person care for people with severe mental illness,” coinvestigator Haiden Huskamp, PhD, professor of health care policy at Harvard Medical School, Boston, said in an interview.
The findings were published online in JAMA Network Open.
Continuing trend?
Past studies have pointed to a sharp increase in the use of telepsychiatry services for patients with SMI. As reported by this news organization, this is a trend some clinicians say is likely to continue after the pandemic.
Use of telemedicine during the pandemic received a boost by the temporary suspension of certain Medicare rules that restrict telehealth use. Debate continues at the federal and state levels on whether to make that suspension permanent. Dr. Huskamp said more information is needed about the efficacy and accessibility of telemental health.
To investigate, researchers used Medicare fee-for-service data from 118,170 patients in 2,916 counties. More than two-thirds of the patients were aged 65 years or younger.
During the study period, telemental health service increased from 0.03 visits per patient with SMI in 2010 to 0.19 visits per patient in 2018. This increase was broad, with the number of counties reporting high use of telemental health increasing from 2% in 2010 to 17% in 2018.
Compared with counties in which there was no telemental health services, those with high use were less densely populated and had fewer health care professionals and hospital beds.
The number of overall visits with a mental health professional increased slightly in high-use counties compared to no-use counties, from 4.65 visits in 2010 to 4.79 visits in 2018. The number of in-person visits during that period declined from 4.55 visits in 2010 to 3.73 visits in 2018, which suggests that the overall increase was due to higher use of telemental health.
In the high-use group, the number of patients who had at least four mental health care visits increased 8%, and the number of patients who had a follow-up visit within 30 days of a hospitalization increased 20.4%.
A ‘helpful option’
“Telemedicine doesn’t address the national shortage of providers, but it definitely helps in underserved areas [and] rural areas,” Dr. Huskamp said.
“We need more mental health providers and need to develop new models of care that can leverage the providers we have in the best way possible. This is at least a helpful option, especially when you’re thinking about the maldistribution of providers across the country,” she added.
The study results showed that there was no difference in medication adherence between low- and high-use counties.
There was greater contact with mental health care providers in counties with high use of telemental health, and patients in the high-use group were 7.6% more likely to be hospitalized within a year compared with their peers in counties that had no telemental health use.
“We did see modest increases in inpatient use in counties that shifted the most to telemental health services, but that’s not typically viewed as a measure of quality because it can mean so many different things,” Dr. Huskamp said.
For example, it could mean that counties with greater telemental health use did a better job of identifying and responding to patients’ need for acute care, she noted. It could also be a reflection of the loss of psychiatric inpatient care in low-use communities.
Another tool
Commenting on the findings, Robert Caudill, MD, director of Telemedicine and Information Technology Programs at the University of Louisville (Ky.), called the increase in hospitalization in high-use counties “surprising.” However, he noted it might be a reflection of the need to fine-tune telemental health for patients with SMI.
“I think that more time and experience with telehealth will further normalize the practice and help to narrow, if not close, the gap,” said Dr. Caudill, who was not involved with the research.
“There are so many side benefits to doing things via telehealth,” he added. “It is a simple matter of continuing to learn how to do those things better.”
A multidisciplinary approach that includes psychiatric care and case management is generally considered to be the gold standard in treating patients with the types of mental illness included in this study, Dr. Caudill said.
While some of that care can be delivered effectively via telemedicine, it is possible other aspects, such as case management, are better handled in person, he added.
“I don’t think it is the role of telehealth to make in-person care obsolete. It is simply a tool to be used when appropriate,” said Dr. Caudill, past chair of the American Telemedicine Association’s Telemental Health Special Interest Group.
“Surgeons did not abandon scalpels when laser surgery became possible,” he said.
The study was funded by the National Institutes of Mental Health. Dr. Huskamp and Dr. Caudill report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a nationwide study, researchers drew on Medicare data from nearly 3,000 counties covering the period from 2000 to 2018. Results show that counties in which there was greater use of telemental health services reported higher increases of clinical visits and better follow-up after hospitalization among patients with bipolar 1 disorder and schizophrenia or other psychotic disorders.
In the study, “clinical visits” referred to both in-person and telemental health visits.
“These findings really support the idea that telemental health can be safe and effective and beneficial for in-person care for people with severe mental illness,” coinvestigator Haiden Huskamp, PhD, professor of health care policy at Harvard Medical School, Boston, said in an interview.
The findings were published online in JAMA Network Open.
Continuing trend?
Past studies have pointed to a sharp increase in the use of telepsychiatry services for patients with SMI. As reported by this news organization, this is a trend some clinicians say is likely to continue after the pandemic.
Use of telemedicine during the pandemic received a boost by the temporary suspension of certain Medicare rules that restrict telehealth use. Debate continues at the federal and state levels on whether to make that suspension permanent. Dr. Huskamp said more information is needed about the efficacy and accessibility of telemental health.
To investigate, researchers used Medicare fee-for-service data from 118,170 patients in 2,916 counties. More than two-thirds of the patients were aged 65 years or younger.
During the study period, telemental health service increased from 0.03 visits per patient with SMI in 2010 to 0.19 visits per patient in 2018. This increase was broad, with the number of counties reporting high use of telemental health increasing from 2% in 2010 to 17% in 2018.
Compared with counties in which there was no telemental health services, those with high use were less densely populated and had fewer health care professionals and hospital beds.
The number of overall visits with a mental health professional increased slightly in high-use counties compared to no-use counties, from 4.65 visits in 2010 to 4.79 visits in 2018. The number of in-person visits during that period declined from 4.55 visits in 2010 to 3.73 visits in 2018, which suggests that the overall increase was due to higher use of telemental health.
In the high-use group, the number of patients who had at least four mental health care visits increased 8%, and the number of patients who had a follow-up visit within 30 days of a hospitalization increased 20.4%.
A ‘helpful option’
“Telemedicine doesn’t address the national shortage of providers, but it definitely helps in underserved areas [and] rural areas,” Dr. Huskamp said.
“We need more mental health providers and need to develop new models of care that can leverage the providers we have in the best way possible. This is at least a helpful option, especially when you’re thinking about the maldistribution of providers across the country,” she added.
The study results showed that there was no difference in medication adherence between low- and high-use counties.
There was greater contact with mental health care providers in counties with high use of telemental health, and patients in the high-use group were 7.6% more likely to be hospitalized within a year compared with their peers in counties that had no telemental health use.
“We did see modest increases in inpatient use in counties that shifted the most to telemental health services, but that’s not typically viewed as a measure of quality because it can mean so many different things,” Dr. Huskamp said.
For example, it could mean that counties with greater telemental health use did a better job of identifying and responding to patients’ need for acute care, she noted. It could also be a reflection of the loss of psychiatric inpatient care in low-use communities.
Another tool
Commenting on the findings, Robert Caudill, MD, director of Telemedicine and Information Technology Programs at the University of Louisville (Ky.), called the increase in hospitalization in high-use counties “surprising.” However, he noted it might be a reflection of the need to fine-tune telemental health for patients with SMI.
“I think that more time and experience with telehealth will further normalize the practice and help to narrow, if not close, the gap,” said Dr. Caudill, who was not involved with the research.
“There are so many side benefits to doing things via telehealth,” he added. “It is a simple matter of continuing to learn how to do those things better.”
A multidisciplinary approach that includes psychiatric care and case management is generally considered to be the gold standard in treating patients with the types of mental illness included in this study, Dr. Caudill said.
While some of that care can be delivered effectively via telemedicine, it is possible other aspects, such as case management, are better handled in person, he added.
“I don’t think it is the role of telehealth to make in-person care obsolete. It is simply a tool to be used when appropriate,” said Dr. Caudill, past chair of the American Telemedicine Association’s Telemental Health Special Interest Group.
“Surgeons did not abandon scalpels when laser surgery became possible,” he said.
The study was funded by the National Institutes of Mental Health. Dr. Huskamp and Dr. Caudill report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.K. survey: Dermatologists want training in prescribing antipsychotics for delusional infestation
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT BAD 2022
Sociogenomics may explain race disparities in breast cancer mortality
Racial differences in cancer outcomes are widespread. Studies indicate that Black people face higher rates of mortality for most cancers than their White counterparts. To bridge this racial gap, researchers need to investigate the biological effects of structural racism and discrimination on cancer outcomes, experts say.
“As a physician, I always like to think that I can influence care in that if I just find the right drugs, help patients understand what their options are, it will help them,” said Ruth Carlos, MD, a radiologist with the University of Michigan Hospital, Ann Arbor. But these things alone are often not enough, because a large proportion of the variation in cancer outcomes is attributable to neighborhood social conditions and the physical environment. “It is incredibly important for us to start to understand just how the neighborhood exerts this effect.”
In a commentary published in the Journal of Clinical Oncology, Dr. Carlos and colleagues highlighted the limitations of previous studies aimed at identifying the causes of racial differences in cancer outcomes. They call upon researchers to turn instead to the long-underexamined biological effects of structural racism and discrimination that contribute to these differences.
In the past, studies on the role of race in health outcomes largely looked at race as a proxy for genetic predisposition. But such an interpretation is flawed, because no genes are specific for a racial or ethnic group, Dr. Carlos and coauthors wrote. Researchers have shown that the vast majority of genetic variation occurs within, rather than between groups.
In an analysis published in Science, researchers reported that within-group differences account for more than 90% of genetic variation.
“Using race in these analyses was not necessarily wrong, but the inferences may have been flawed or incomplete,” Dr. Carlos said. On one hand, looking at genetic predisposition has led to important insights, such as the link between mutations in the BRCA gene and increased risk for breast and ovarian cancer.
However, genetic variation alone is not enough to explain the disparities in cancer outcomes between racial and ethnic groups. The fact that breast cancer can be more aggressive in Black women raises several questions, Dr. Carlos said. Is the cancer worse because Black women have a specific genetic predisposition? Is it worse because Black women exist in a society that marginalizes them and exposes them to increased stress, which in turn produces bad outcomes? Or, could it be both?
Despite progress in the screening, diagnosis and treatment of breast cancer, Black women are 40% more likely to die from the disease than White women. At the time of diagnosis, Black women are more likely to have high-grade, more aggressive breast cancer molecular subtypes, and to have had their cancer spread to the lymph nodes. They also tend to be diagnosed at more advanced stages of breast cancer while at the same time, experience higher rates of false-positive screening results.
Although researchers have hypothesized that genetic differences related to African or European ancestry might contribute, studies have not turned up any differences in cancer susceptibility genes by race. Other factors, such as racial differences in the stage of presentation, molecular subtypes, and disparities in treatment, have also emerged as potential culprits.
In her commentary, Dr. Carlos and colleagues wrote that disparities in breast cancer outcomes previously attributed to race need to be examined from multiple angles. This means looking at both the complex interactions between social conditions and policies, which encompass racism both at the individual and structural level, and stressors such as the experience of discrimination in addition to potential biological and genetic contributions.
Many studies now provide evidence for the harmful effects of racism on health. For breast cancer, specifically, studies also suggest that factors such as racial segregation can influence the stage at which Black women get diagnosed and their likelihood of dying from the disease.
However, an important question that remains is what biological changes occur in women exposed to the kind of persistent low-level stress that is associated with structural racism and discrimination, Dr. Carlos said. “We don’t know what stress pathways actually manifest in the body and how they eventually produce the disease.” Studies to address this issue are important, “especially if you would like to develop interventions to prevent or mitigate disease.”
To address this issue, Dr. Carlos and colleagues called upon the research community to conduct both studies that delineate the underlying biology as well as those that test potential interventions – particularly those associated with breast cancer screening outcomes – to try to shed light on why Black women receive more false positives and diagnoses of more aggressive cancer.
Interventions that can target these specific biological pathways could potentially reduce the negative effects of structural racism and discrimination as well as the effects of other social factors that contribute to breast cancer outcomes, “to ultimately help enhance clinical outcomes and close persistent disparities gaps,” the authors wrote.
Racial differences in cancer outcomes are widespread. Studies indicate that Black people face higher rates of mortality for most cancers than their White counterparts. To bridge this racial gap, researchers need to investigate the biological effects of structural racism and discrimination on cancer outcomes, experts say.
“As a physician, I always like to think that I can influence care in that if I just find the right drugs, help patients understand what their options are, it will help them,” said Ruth Carlos, MD, a radiologist with the University of Michigan Hospital, Ann Arbor. But these things alone are often not enough, because a large proportion of the variation in cancer outcomes is attributable to neighborhood social conditions and the physical environment. “It is incredibly important for us to start to understand just how the neighborhood exerts this effect.”
In a commentary published in the Journal of Clinical Oncology, Dr. Carlos and colleagues highlighted the limitations of previous studies aimed at identifying the causes of racial differences in cancer outcomes. They call upon researchers to turn instead to the long-underexamined biological effects of structural racism and discrimination that contribute to these differences.
In the past, studies on the role of race in health outcomes largely looked at race as a proxy for genetic predisposition. But such an interpretation is flawed, because no genes are specific for a racial or ethnic group, Dr. Carlos and coauthors wrote. Researchers have shown that the vast majority of genetic variation occurs within, rather than between groups.
In an analysis published in Science, researchers reported that within-group differences account for more than 90% of genetic variation.
“Using race in these analyses was not necessarily wrong, but the inferences may have been flawed or incomplete,” Dr. Carlos said. On one hand, looking at genetic predisposition has led to important insights, such as the link between mutations in the BRCA gene and increased risk for breast and ovarian cancer.
However, genetic variation alone is not enough to explain the disparities in cancer outcomes between racial and ethnic groups. The fact that breast cancer can be more aggressive in Black women raises several questions, Dr. Carlos said. Is the cancer worse because Black women have a specific genetic predisposition? Is it worse because Black women exist in a society that marginalizes them and exposes them to increased stress, which in turn produces bad outcomes? Or, could it be both?
Despite progress in the screening, diagnosis and treatment of breast cancer, Black women are 40% more likely to die from the disease than White women. At the time of diagnosis, Black women are more likely to have high-grade, more aggressive breast cancer molecular subtypes, and to have had their cancer spread to the lymph nodes. They also tend to be diagnosed at more advanced stages of breast cancer while at the same time, experience higher rates of false-positive screening results.
Although researchers have hypothesized that genetic differences related to African or European ancestry might contribute, studies have not turned up any differences in cancer susceptibility genes by race. Other factors, such as racial differences in the stage of presentation, molecular subtypes, and disparities in treatment, have also emerged as potential culprits.
In her commentary, Dr. Carlos and colleagues wrote that disparities in breast cancer outcomes previously attributed to race need to be examined from multiple angles. This means looking at both the complex interactions between social conditions and policies, which encompass racism both at the individual and structural level, and stressors such as the experience of discrimination in addition to potential biological and genetic contributions.
Many studies now provide evidence for the harmful effects of racism on health. For breast cancer, specifically, studies also suggest that factors such as racial segregation can influence the stage at which Black women get diagnosed and their likelihood of dying from the disease.
However, an important question that remains is what biological changes occur in women exposed to the kind of persistent low-level stress that is associated with structural racism and discrimination, Dr. Carlos said. “We don’t know what stress pathways actually manifest in the body and how they eventually produce the disease.” Studies to address this issue are important, “especially if you would like to develop interventions to prevent or mitigate disease.”
To address this issue, Dr. Carlos and colleagues called upon the research community to conduct both studies that delineate the underlying biology as well as those that test potential interventions – particularly those associated with breast cancer screening outcomes – to try to shed light on why Black women receive more false positives and diagnoses of more aggressive cancer.
Interventions that can target these specific biological pathways could potentially reduce the negative effects of structural racism and discrimination as well as the effects of other social factors that contribute to breast cancer outcomes, “to ultimately help enhance clinical outcomes and close persistent disparities gaps,” the authors wrote.
Racial differences in cancer outcomes are widespread. Studies indicate that Black people face higher rates of mortality for most cancers than their White counterparts. To bridge this racial gap, researchers need to investigate the biological effects of structural racism and discrimination on cancer outcomes, experts say.
“As a physician, I always like to think that I can influence care in that if I just find the right drugs, help patients understand what their options are, it will help them,” said Ruth Carlos, MD, a radiologist with the University of Michigan Hospital, Ann Arbor. But these things alone are often not enough, because a large proportion of the variation in cancer outcomes is attributable to neighborhood social conditions and the physical environment. “It is incredibly important for us to start to understand just how the neighborhood exerts this effect.”
In a commentary published in the Journal of Clinical Oncology, Dr. Carlos and colleagues highlighted the limitations of previous studies aimed at identifying the causes of racial differences in cancer outcomes. They call upon researchers to turn instead to the long-underexamined biological effects of structural racism and discrimination that contribute to these differences.
In the past, studies on the role of race in health outcomes largely looked at race as a proxy for genetic predisposition. But such an interpretation is flawed, because no genes are specific for a racial or ethnic group, Dr. Carlos and coauthors wrote. Researchers have shown that the vast majority of genetic variation occurs within, rather than between groups.
In an analysis published in Science, researchers reported that within-group differences account for more than 90% of genetic variation.
“Using race in these analyses was not necessarily wrong, but the inferences may have been flawed or incomplete,” Dr. Carlos said. On one hand, looking at genetic predisposition has led to important insights, such as the link between mutations in the BRCA gene and increased risk for breast and ovarian cancer.
However, genetic variation alone is not enough to explain the disparities in cancer outcomes between racial and ethnic groups. The fact that breast cancer can be more aggressive in Black women raises several questions, Dr. Carlos said. Is the cancer worse because Black women have a specific genetic predisposition? Is it worse because Black women exist in a society that marginalizes them and exposes them to increased stress, which in turn produces bad outcomes? Or, could it be both?
Despite progress in the screening, diagnosis and treatment of breast cancer, Black women are 40% more likely to die from the disease than White women. At the time of diagnosis, Black women are more likely to have high-grade, more aggressive breast cancer molecular subtypes, and to have had their cancer spread to the lymph nodes. They also tend to be diagnosed at more advanced stages of breast cancer while at the same time, experience higher rates of false-positive screening results.
Although researchers have hypothesized that genetic differences related to African or European ancestry might contribute, studies have not turned up any differences in cancer susceptibility genes by race. Other factors, such as racial differences in the stage of presentation, molecular subtypes, and disparities in treatment, have also emerged as potential culprits.
In her commentary, Dr. Carlos and colleagues wrote that disparities in breast cancer outcomes previously attributed to race need to be examined from multiple angles. This means looking at both the complex interactions between social conditions and policies, which encompass racism both at the individual and structural level, and stressors such as the experience of discrimination in addition to potential biological and genetic contributions.
Many studies now provide evidence for the harmful effects of racism on health. For breast cancer, specifically, studies also suggest that factors such as racial segregation can influence the stage at which Black women get diagnosed and their likelihood of dying from the disease.
However, an important question that remains is what biological changes occur in women exposed to the kind of persistent low-level stress that is associated with structural racism and discrimination, Dr. Carlos said. “We don’t know what stress pathways actually manifest in the body and how they eventually produce the disease.” Studies to address this issue are important, “especially if you would like to develop interventions to prevent or mitigate disease.”
To address this issue, Dr. Carlos and colleagues called upon the research community to conduct both studies that delineate the underlying biology as well as those that test potential interventions – particularly those associated with breast cancer screening outcomes – to try to shed light on why Black women receive more false positives and diagnoses of more aggressive cancer.
Interventions that can target these specific biological pathways could potentially reduce the negative effects of structural racism and discrimination as well as the effects of other social factors that contribute to breast cancer outcomes, “to ultimately help enhance clinical outcomes and close persistent disparities gaps,” the authors wrote.
FROM THE JOURNAL OF CLINICAL ONCOLOGY