User login
Novel COVID-19 vaccine could fill the void for patients with blood cancers
according to study results presented at the annual meeting of the American Association for Cancer Research.
The phase 1/2 trial included 54 patients with a B-cell deficiency (mean age, 63 years; 28% female): 4 had congenital B-cell deficiency and 50 had a blood cancer (lymphocytic leukemia or lymphoma). T-cell immune responses were observed in 86% of patients 28 days after vaccination with a single CoVac-1 dose. The potency of CoVac-1–induced T-cell responses exceeded those seen typically with B cell–deficient patient responses after mRNA vaccine treatment and were comparable with those seen among nonimmunocompromised COVID-19 patients.
In the majority of individuals, currently approved SARS-CoV-2 vaccines induce a robust immune response, however, their efficacy, has been shown to be decreased among individuals who are immunocompromised. Patients treated for hematologic cancers, in particular, receive treatment regimens that damage healthy immune cells, particularly B cells, said Juliane Walz, MD, the study’s senior author and professor of medicine at University Hospital Tübingen (Germany).
“In the clinic, we see many cancer patients who do not mount sufficient humoral immune responses after vaccination with available SARS-CoV-2 vaccines,” Dr. Walz said. “These patients are at a high risk for a severe course of COVID-19.”
B-cell deficiency, she stated, can be compensated for by enhancing T-cell responses against SARS-CoV-2, which can then combat infections in the absence of neutralizing antibodies.
In a prior study of CoVac-1 among 36 adults without immune deficiency, the vaccine elicited T-cell responses that were still robust 3 months post vaccination, and that included responses against omicron and other key SARS-CoV-2 variants.
While mRNA-based or adenoviral vector-based vaccines are limited to the spike protein and are thus prone to loss of activity because of viral mutations, CoVac-1–induced T-cell immunity is far more intense and broader, Dr. Walz said.
CoVac-1 is a peptide vaccine that is injected directly rather than being encoded via mRNA and targets different viral components. It would not be given, however, to healthy, immunocompetent adults because it is important for them to have both B-cell antibody and T-cell response.
The patients with B-cell deficiency recruited for the study were given a single dose of CoVac-1 and assessed for safety and immunogenicity until day 56. Prior vaccinations with an approved SARS-CoV-2 vaccine had failed to elicit a humoral response in 87% of the subjects.
“Our vaccine does not induce antibody responses,” Dr. Walz said. “However, it could be used to induce broad T-cell responses as a complementary or additive vaccine for elderly adults. In the elderly, antibody responses decline very, very fast after vaccination.”
Dr. Walz said that CoVac-1 could find application in various syndromes associated with congenital B-cell deficiencies, in autoimmune diseases such as rheumatoid arthritis and multiple sclerosis, or diseases treated with rituximab or other B cell–depleting therapies (for example, ofatumumab, blinatumomab, or chimeric antigen receptor T cells), and in transplant patients.
A phase 3 study of CoVac-1 versus placebo is under discussion and would require about 300-500 subjects, Dr. Walz said.
“CoVac-1 is designed to induce broad and long-lasting SARS-CoV-2 T-cell immunity, even in individuals who have impaired ability to mount sufficient immunity from a currently approved vaccine, and thus protect these high-risk patients from a severe course of COVID-19,” Dr. Walz said.
“Having an option for these patients is just critical – so this is significant work,” said Ana Maria Lopez, MD, MPH, of the Sidney Kimmel Cancer Center–Jefferson Health, Philadelphia.
Limitations of this study included the small sample size with low racial and ethnic diversity, Dr. Walz stated.
Funding was provided by the Ministry of Science, Research and the Arts of the state of Baden-Württemberg; the Federal Ministry of Research and Education in Germany; the German Research Foundation under Germany’s Excellence Strategy; and the Clinical Cooperation Unit Translational Immunology at University Hospital Tübingen. Dr. Walz holds the CoVac-1 patent.
according to study results presented at the annual meeting of the American Association for Cancer Research.
The phase 1/2 trial included 54 patients with a B-cell deficiency (mean age, 63 years; 28% female): 4 had congenital B-cell deficiency and 50 had a blood cancer (lymphocytic leukemia or lymphoma). T-cell immune responses were observed in 86% of patients 28 days after vaccination with a single CoVac-1 dose. The potency of CoVac-1–induced T-cell responses exceeded those seen typically with B cell–deficient patient responses after mRNA vaccine treatment and were comparable with those seen among nonimmunocompromised COVID-19 patients.
In the majority of individuals, currently approved SARS-CoV-2 vaccines induce a robust immune response, however, their efficacy, has been shown to be decreased among individuals who are immunocompromised. Patients treated for hematologic cancers, in particular, receive treatment regimens that damage healthy immune cells, particularly B cells, said Juliane Walz, MD, the study’s senior author and professor of medicine at University Hospital Tübingen (Germany).
“In the clinic, we see many cancer patients who do not mount sufficient humoral immune responses after vaccination with available SARS-CoV-2 vaccines,” Dr. Walz said. “These patients are at a high risk for a severe course of COVID-19.”
B-cell deficiency, she stated, can be compensated for by enhancing T-cell responses against SARS-CoV-2, which can then combat infections in the absence of neutralizing antibodies.
In a prior study of CoVac-1 among 36 adults without immune deficiency, the vaccine elicited T-cell responses that were still robust 3 months post vaccination, and that included responses against omicron and other key SARS-CoV-2 variants.
While mRNA-based or adenoviral vector-based vaccines are limited to the spike protein and are thus prone to loss of activity because of viral mutations, CoVac-1–induced T-cell immunity is far more intense and broader, Dr. Walz said.
CoVac-1 is a peptide vaccine that is injected directly rather than being encoded via mRNA and targets different viral components. It would not be given, however, to healthy, immunocompetent adults because it is important for them to have both B-cell antibody and T-cell response.
The patients with B-cell deficiency recruited for the study were given a single dose of CoVac-1 and assessed for safety and immunogenicity until day 56. Prior vaccinations with an approved SARS-CoV-2 vaccine had failed to elicit a humoral response in 87% of the subjects.
“Our vaccine does not induce antibody responses,” Dr. Walz said. “However, it could be used to induce broad T-cell responses as a complementary or additive vaccine for elderly adults. In the elderly, antibody responses decline very, very fast after vaccination.”
Dr. Walz said that CoVac-1 could find application in various syndromes associated with congenital B-cell deficiencies, in autoimmune diseases such as rheumatoid arthritis and multiple sclerosis, or diseases treated with rituximab or other B cell–depleting therapies (for example, ofatumumab, blinatumomab, or chimeric antigen receptor T cells), and in transplant patients.
A phase 3 study of CoVac-1 versus placebo is under discussion and would require about 300-500 subjects, Dr. Walz said.
“CoVac-1 is designed to induce broad and long-lasting SARS-CoV-2 T-cell immunity, even in individuals who have impaired ability to mount sufficient immunity from a currently approved vaccine, and thus protect these high-risk patients from a severe course of COVID-19,” Dr. Walz said.
“Having an option for these patients is just critical – so this is significant work,” said Ana Maria Lopez, MD, MPH, of the Sidney Kimmel Cancer Center–Jefferson Health, Philadelphia.
Limitations of this study included the small sample size with low racial and ethnic diversity, Dr. Walz stated.
Funding was provided by the Ministry of Science, Research and the Arts of the state of Baden-Württemberg; the Federal Ministry of Research and Education in Germany; the German Research Foundation under Germany’s Excellence Strategy; and the Clinical Cooperation Unit Translational Immunology at University Hospital Tübingen. Dr. Walz holds the CoVac-1 patent.
according to study results presented at the annual meeting of the American Association for Cancer Research.
The phase 1/2 trial included 54 patients with a B-cell deficiency (mean age, 63 years; 28% female): 4 had congenital B-cell deficiency and 50 had a blood cancer (lymphocytic leukemia or lymphoma). T-cell immune responses were observed in 86% of patients 28 days after vaccination with a single CoVac-1 dose. The potency of CoVac-1–induced T-cell responses exceeded those seen typically with B cell–deficient patient responses after mRNA vaccine treatment and were comparable with those seen among nonimmunocompromised COVID-19 patients.
In the majority of individuals, currently approved SARS-CoV-2 vaccines induce a robust immune response, however, their efficacy, has been shown to be decreased among individuals who are immunocompromised. Patients treated for hematologic cancers, in particular, receive treatment regimens that damage healthy immune cells, particularly B cells, said Juliane Walz, MD, the study’s senior author and professor of medicine at University Hospital Tübingen (Germany).
“In the clinic, we see many cancer patients who do not mount sufficient humoral immune responses after vaccination with available SARS-CoV-2 vaccines,” Dr. Walz said. “These patients are at a high risk for a severe course of COVID-19.”
B-cell deficiency, she stated, can be compensated for by enhancing T-cell responses against SARS-CoV-2, which can then combat infections in the absence of neutralizing antibodies.
In a prior study of CoVac-1 among 36 adults without immune deficiency, the vaccine elicited T-cell responses that were still robust 3 months post vaccination, and that included responses against omicron and other key SARS-CoV-2 variants.
While mRNA-based or adenoviral vector-based vaccines are limited to the spike protein and are thus prone to loss of activity because of viral mutations, CoVac-1–induced T-cell immunity is far more intense and broader, Dr. Walz said.
CoVac-1 is a peptide vaccine that is injected directly rather than being encoded via mRNA and targets different viral components. It would not be given, however, to healthy, immunocompetent adults because it is important for them to have both B-cell antibody and T-cell response.
The patients with B-cell deficiency recruited for the study were given a single dose of CoVac-1 and assessed for safety and immunogenicity until day 56. Prior vaccinations with an approved SARS-CoV-2 vaccine had failed to elicit a humoral response in 87% of the subjects.
“Our vaccine does not induce antibody responses,” Dr. Walz said. “However, it could be used to induce broad T-cell responses as a complementary or additive vaccine for elderly adults. In the elderly, antibody responses decline very, very fast after vaccination.”
Dr. Walz said that CoVac-1 could find application in various syndromes associated with congenital B-cell deficiencies, in autoimmune diseases such as rheumatoid arthritis and multiple sclerosis, or diseases treated with rituximab or other B cell–depleting therapies (for example, ofatumumab, blinatumomab, or chimeric antigen receptor T cells), and in transplant patients.
A phase 3 study of CoVac-1 versus placebo is under discussion and would require about 300-500 subjects, Dr. Walz said.
“CoVac-1 is designed to induce broad and long-lasting SARS-CoV-2 T-cell immunity, even in individuals who have impaired ability to mount sufficient immunity from a currently approved vaccine, and thus protect these high-risk patients from a severe course of COVID-19,” Dr. Walz said.
“Having an option for these patients is just critical – so this is significant work,” said Ana Maria Lopez, MD, MPH, of the Sidney Kimmel Cancer Center–Jefferson Health, Philadelphia.
Limitations of this study included the small sample size with low racial and ethnic diversity, Dr. Walz stated.
Funding was provided by the Ministry of Science, Research and the Arts of the state of Baden-Württemberg; the Federal Ministry of Research and Education in Germany; the German Research Foundation under Germany’s Excellence Strategy; and the Clinical Cooperation Unit Translational Immunology at University Hospital Tübingen. Dr. Walz holds the CoVac-1 patent.
FROM AACR 2022
1 in 7 breast cancer patients report worsening personal finances
a new study found. Factors like disease severity and treatment type didn’t seem to have an impact on financial status.
The findings, presented at the annual meeting of the American Association for Cancer Research, were unexpected. “We were surprised that we did not find that patients who received more aggressive therapies were more likely to experience worsening financial concerns,” said corresponding author and medical oncologist Kathryn J. Ruddy, MD, of the Mayo Clinici in Rochester, Minn.
The study was undertaken to understand the financial stress facing patients with breast cancer. The question was whether individual or disease factors, or both, were at play.
The study is based on results from the Mayo Clinic Breast Disease Registry, a prospective cohort of patient who were at Mayo Clinic Rochester. Participants answered questions about their finances at baseline and then again at annual follow-ups.
Researchers examined survey findings from 1,957 patients (mean age 58.5, 99.1% female, 95.4% White, 54.9% bachelor degree or higher) who answered questions at least twice from 2015-2020. The average time between diagnosis and the most recent follow-up was 25.6 months.
Of the 1,957 patients, 357 (18.2%) said their finances deteriorated as measured by a 1 point or higher decline on a 10-point scale.
There was no statistically significant link between deteriorating finances and age, race, employment status, stage of cancer at diagnosis, type of cancer, or treatment type. There was a slight link between deteriorating finances and reporting that they were in the category of “pay bills, no money for special things” near diagnosis.
Other research has suggested that breast cancer may not disrupt finances to a large extent, at least early on. Earlier in 2022, Stanford (Calif.) University researchers reported the results of a survey of 273 breast and gynecologic cancer patients who were surveyed about their finances at a mean of 3.4 years after diagnosis. While one-third said their cancer caused career changes, the study described overall financial toxicity as mild.
In regard to limitations, the subject population of the new study is overwhelmingly White, and the finances were self-reported by those who participated in the survey. Also, “because our participants were recruited at a tertiary medical center, there were relatively financially secure at baseline,” Dr. Ruddy said. “More financial hardship would be expected in a more financially diverse population.”
In an interview, Cathy Bradley, PhD, associate dean for research at the University of Colorado at Denver and deputy director of the University of Colorado Cancer Center, both in Aurora, praised the study as “an important start toward assessing financial burden in the clinic. Having more universal assessments in the clinic would remove stigma.”
She cautioned about interpreting a seemingly low number of patients whose financial situation worsened. “This was for a single site where there is a high rate of health insurance either through Medicare or Medicaid. There may be some selection bias as well given that Mayo may attract a wealthier patient population. Most women completed treatment and may not have been on long-term therapies.”
Moving forward, Dr. Ruddy said, “we hope to study cost of oncologic care in more geographically and financially diverse populations with breast cancer and other cancers.”
The study was funded by the Breast Cancer Research Foundation and National Cancer Institute. The study authors and Dr. Ruddy report no relevant disclosures.
a new study found. Factors like disease severity and treatment type didn’t seem to have an impact on financial status.
The findings, presented at the annual meeting of the American Association for Cancer Research, were unexpected. “We were surprised that we did not find that patients who received more aggressive therapies were more likely to experience worsening financial concerns,” said corresponding author and medical oncologist Kathryn J. Ruddy, MD, of the Mayo Clinici in Rochester, Minn.
The study was undertaken to understand the financial stress facing patients with breast cancer. The question was whether individual or disease factors, or both, were at play.
The study is based on results from the Mayo Clinic Breast Disease Registry, a prospective cohort of patient who were at Mayo Clinic Rochester. Participants answered questions about their finances at baseline and then again at annual follow-ups.
Researchers examined survey findings from 1,957 patients (mean age 58.5, 99.1% female, 95.4% White, 54.9% bachelor degree or higher) who answered questions at least twice from 2015-2020. The average time between diagnosis and the most recent follow-up was 25.6 months.
Of the 1,957 patients, 357 (18.2%) said their finances deteriorated as measured by a 1 point or higher decline on a 10-point scale.
There was no statistically significant link between deteriorating finances and age, race, employment status, stage of cancer at diagnosis, type of cancer, or treatment type. There was a slight link between deteriorating finances and reporting that they were in the category of “pay bills, no money for special things” near diagnosis.
Other research has suggested that breast cancer may not disrupt finances to a large extent, at least early on. Earlier in 2022, Stanford (Calif.) University researchers reported the results of a survey of 273 breast and gynecologic cancer patients who were surveyed about their finances at a mean of 3.4 years after diagnosis. While one-third said their cancer caused career changes, the study described overall financial toxicity as mild.
In regard to limitations, the subject population of the new study is overwhelmingly White, and the finances were self-reported by those who participated in the survey. Also, “because our participants were recruited at a tertiary medical center, there were relatively financially secure at baseline,” Dr. Ruddy said. “More financial hardship would be expected in a more financially diverse population.”
In an interview, Cathy Bradley, PhD, associate dean for research at the University of Colorado at Denver and deputy director of the University of Colorado Cancer Center, both in Aurora, praised the study as “an important start toward assessing financial burden in the clinic. Having more universal assessments in the clinic would remove stigma.”
She cautioned about interpreting a seemingly low number of patients whose financial situation worsened. “This was for a single site where there is a high rate of health insurance either through Medicare or Medicaid. There may be some selection bias as well given that Mayo may attract a wealthier patient population. Most women completed treatment and may not have been on long-term therapies.”
Moving forward, Dr. Ruddy said, “we hope to study cost of oncologic care in more geographically and financially diverse populations with breast cancer and other cancers.”
The study was funded by the Breast Cancer Research Foundation and National Cancer Institute. The study authors and Dr. Ruddy report no relevant disclosures.
a new study found. Factors like disease severity and treatment type didn’t seem to have an impact on financial status.
The findings, presented at the annual meeting of the American Association for Cancer Research, were unexpected. “We were surprised that we did not find that patients who received more aggressive therapies were more likely to experience worsening financial concerns,” said corresponding author and medical oncologist Kathryn J. Ruddy, MD, of the Mayo Clinici in Rochester, Minn.
The study was undertaken to understand the financial stress facing patients with breast cancer. The question was whether individual or disease factors, or both, were at play.
The study is based on results from the Mayo Clinic Breast Disease Registry, a prospective cohort of patient who were at Mayo Clinic Rochester. Participants answered questions about their finances at baseline and then again at annual follow-ups.
Researchers examined survey findings from 1,957 patients (mean age 58.5, 99.1% female, 95.4% White, 54.9% bachelor degree or higher) who answered questions at least twice from 2015-2020. The average time between diagnosis and the most recent follow-up was 25.6 months.
Of the 1,957 patients, 357 (18.2%) said their finances deteriorated as measured by a 1 point or higher decline on a 10-point scale.
There was no statistically significant link between deteriorating finances and age, race, employment status, stage of cancer at diagnosis, type of cancer, or treatment type. There was a slight link between deteriorating finances and reporting that they were in the category of “pay bills, no money for special things” near diagnosis.
Other research has suggested that breast cancer may not disrupt finances to a large extent, at least early on. Earlier in 2022, Stanford (Calif.) University researchers reported the results of a survey of 273 breast and gynecologic cancer patients who were surveyed about their finances at a mean of 3.4 years after diagnosis. While one-third said their cancer caused career changes, the study described overall financial toxicity as mild.
In regard to limitations, the subject population of the new study is overwhelmingly White, and the finances were self-reported by those who participated in the survey. Also, “because our participants were recruited at a tertiary medical center, there were relatively financially secure at baseline,” Dr. Ruddy said. “More financial hardship would be expected in a more financially diverse population.”
In an interview, Cathy Bradley, PhD, associate dean for research at the University of Colorado at Denver and deputy director of the University of Colorado Cancer Center, both in Aurora, praised the study as “an important start toward assessing financial burden in the clinic. Having more universal assessments in the clinic would remove stigma.”
She cautioned about interpreting a seemingly low number of patients whose financial situation worsened. “This was for a single site where there is a high rate of health insurance either through Medicare or Medicaid. There may be some selection bias as well given that Mayo may attract a wealthier patient population. Most women completed treatment and may not have been on long-term therapies.”
Moving forward, Dr. Ruddy said, “we hope to study cost of oncologic care in more geographically and financially diverse populations with breast cancer and other cancers.”
The study was funded by the Breast Cancer Research Foundation and National Cancer Institute. The study authors and Dr. Ruddy report no relevant disclosures.
FROM AACR 2022
Aged black garlic supplement may help lower BP
After 6 weeks, consumption of ABG with a high concentration of s-allyl-L-cystine (SAC) was associated with a nearly 6-mm Hg reduction in DBP in men. Other cardiovascular disease (CVD) risk factors were not significantly affected.
“The observed reduction in DBP by ABG extract was similar to the effects of dietary approaches, including the effects of the Dietary Approaches to Stop Hypertension(DASH) diet on BP,” say Rosa M. Valls, PhD, Universitat Rovira i Virgili, Reus, Spain, and colleagues.
“The potential beneficial effects of ABG may contribute to obtaining an optimal DBP” but were “better observed in men and in nonoptimal DBP populations,” they write in the study, published in Nutrients.
Pure SAC and aged garlics have shown healthy effects on multiple targets in in vitro and in vivo tests. However, previous studies in humans have not focused on ABG but rather on other types of aged garlic in patients with some type of CVD risk factor and suffered from methodologic or design weaknesses, the authors note.
To address this gap, Dr. Valls and colleagues randomly assigned 67 individuals with moderate hypercholesterolemia (defined as LDL levels of at least 115 mg/dL) to receive one ABG tablet (250 mg ABG extract/1.25 mg SAC) or placebo daily for 6 weeks. Following a 3-week washout, the groups were reversed and the new intervention continued for another 6 weeks.
Participants received dietary recommendations regarding CVD risk factors and had their dietary habits assessed through a 3-day food record at baseline and after 6 weeks during both treatments.
Individuals receiving lipid-lowering treatment or antihypertensives were excluded, as were those with a body mass index of 35 kg/m2 or higher, those with a fasting blood glucose of at least 126 mg/dL, or active smokers.
There were no differences in baseline characteristics between the two groups. The mean systolic and diastolic pressures at baseline were 124/75 mm Hg in the ABG group and 121/74 mm Hg in the placebo group. Their mean age was 53 years.
Adherence with the protocol was “high” at 96.5% in both groups, and no adverse effects were reported.
Reduced risk of death from stroke, ischemic heart disease
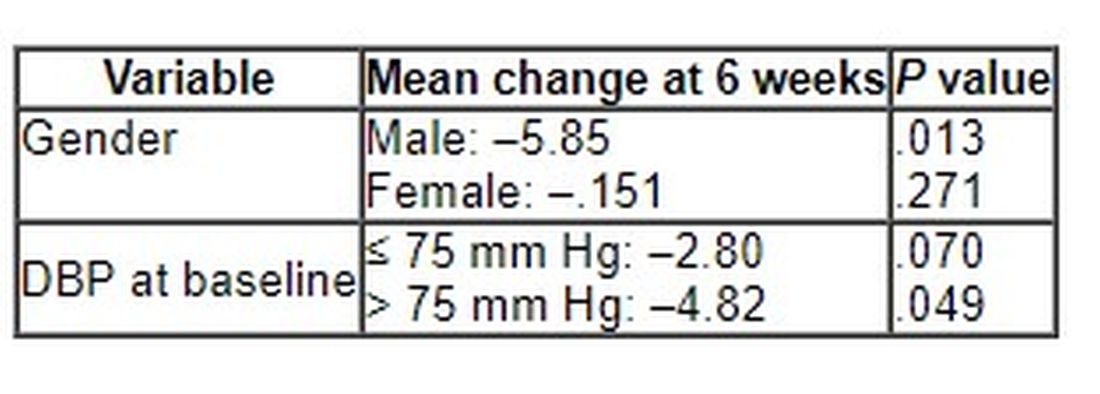
Although no significant differences between ABG and placebo were observed at 3 weeks, the decline in DBP after consumption of the ABG extract became significant at 6 weeks (mean change, –3.7 mm Hg vs. –0.10 mm Hg; P = .007).
When stratified by sex and categories of DBP, the mean change in DBP after 6 weeks of ABG consumption was particularly prominent in men and in those with a baseline DBP of at least 75 mm Hg.
The 6-week change in systolic blood pressure with ABG and placebo was 1.32 mm Hg and 2.84 mm Hg, respectively (P = .694).
At week 6, total cholesterol levels showed a “quadratic decreasing trend” after ABG treatment (P = .047), but no other significant differences between groups were observed for lipid profile, apolipoproteins, or other outcomes of interest, including serum insulin, waist circumference, and body mass index.
The authors note that although systolic BP elevation “has a greater effect on outcomes, both systolic and diastolic hypertension independently influence the risk of adverse cardiovascular events, regardless of the definition of hypertension” and that the risk of death from ischemic heart disease and stroke doubles with every 10 mm Hg increase in DBP in people between the ages of 40 and 89 years.
“Thus, reducing DBP by 5 mm Hg results in a 40% lower risk of death from stroke and a 30% lower risk of death from ischemic heart disease or other vascular death,” they state.
Small study
Commenting for this news organization, Linda Van Horn, PhD, RDN, professor and chief of the department of preventive medicine’s nutrition division, Northwestern University, Chicago, said that for many years, garlic has been “reported to be an adjunct to the benefits of a healthy eating pattern, with inconclusive results.”
She noted that ABG is “literally aged for many months to years, and the resulting concentrate is found higher in many organosulfur compounds and phytochemicals that suggest enhanced response.”
Dr. Van Horn, a member of the American Heart Association’s Nutrition Committee, who was not involved with the study, continued: “The data suggest that ABG that is much more highly concentrated than fresh or processed garlic might be helpful in lowering BP in certain subgroups, in this case men with higher BP.”
However, she cautioned, “these results are limited in a small study, and ... potential other issues, such as sodium, potassium, or other nutrients known to be associated with blood pressure, were not reported, thereby raising questions about the exclusivity of the ABG over other accompanying dietary factors.”
The study was funded by the Center for the Development of Industrial Technology of the Spanish Ministry of Science and Innovation. Two authors are employees of Pharmactive Biotech Products, SL (Madrid), which manufactured the ABG product, but neither played a role in any result or conclusion. The other authors and Dr. Van Horn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After 6 weeks, consumption of ABG with a high concentration of s-allyl-L-cystine (SAC) was associated with a nearly 6-mm Hg reduction in DBP in men. Other cardiovascular disease (CVD) risk factors were not significantly affected.
“The observed reduction in DBP by ABG extract was similar to the effects of dietary approaches, including the effects of the Dietary Approaches to Stop Hypertension(DASH) diet on BP,” say Rosa M. Valls, PhD, Universitat Rovira i Virgili, Reus, Spain, and colleagues.
“The potential beneficial effects of ABG may contribute to obtaining an optimal DBP” but were “better observed in men and in nonoptimal DBP populations,” they write in the study, published in Nutrients.
Pure SAC and aged garlics have shown healthy effects on multiple targets in in vitro and in vivo tests. However, previous studies in humans have not focused on ABG but rather on other types of aged garlic in patients with some type of CVD risk factor and suffered from methodologic or design weaknesses, the authors note.
To address this gap, Dr. Valls and colleagues randomly assigned 67 individuals with moderate hypercholesterolemia (defined as LDL levels of at least 115 mg/dL) to receive one ABG tablet (250 mg ABG extract/1.25 mg SAC) or placebo daily for 6 weeks. Following a 3-week washout, the groups were reversed and the new intervention continued for another 6 weeks.
Participants received dietary recommendations regarding CVD risk factors and had their dietary habits assessed through a 3-day food record at baseline and after 6 weeks during both treatments.
Individuals receiving lipid-lowering treatment or antihypertensives were excluded, as were those with a body mass index of 35 kg/m2 or higher, those with a fasting blood glucose of at least 126 mg/dL, or active smokers.
There were no differences in baseline characteristics between the two groups. The mean systolic and diastolic pressures at baseline were 124/75 mm Hg in the ABG group and 121/74 mm Hg in the placebo group. Their mean age was 53 years.
Adherence with the protocol was “high” at 96.5% in both groups, and no adverse effects were reported.
Reduced risk of death from stroke, ischemic heart disease
Although no significant differences between ABG and placebo were observed at 3 weeks, the decline in DBP after consumption of the ABG extract became significant at 6 weeks (mean change, –3.7 mm Hg vs. –0.10 mm Hg; P = .007).
When stratified by sex and categories of DBP, the mean change in DBP after 6 weeks of ABG consumption was particularly prominent in men and in those with a baseline DBP of at least 75 mm Hg.
The 6-week change in systolic blood pressure with ABG and placebo was 1.32 mm Hg and 2.84 mm Hg, respectively (P = .694).
At week 6, total cholesterol levels showed a “quadratic decreasing trend” after ABG treatment (P = .047), but no other significant differences between groups were observed for lipid profile, apolipoproteins, or other outcomes of interest, including serum insulin, waist circumference, and body mass index.
The authors note that although systolic BP elevation “has a greater effect on outcomes, both systolic and diastolic hypertension independently influence the risk of adverse cardiovascular events, regardless of the definition of hypertension” and that the risk of death from ischemic heart disease and stroke doubles with every 10 mm Hg increase in DBP in people between the ages of 40 and 89 years.
“Thus, reducing DBP by 5 mm Hg results in a 40% lower risk of death from stroke and a 30% lower risk of death from ischemic heart disease or other vascular death,” they state.
Small study
Commenting for this news organization, Linda Van Horn, PhD, RDN, professor and chief of the department of preventive medicine’s nutrition division, Northwestern University, Chicago, said that for many years, garlic has been “reported to be an adjunct to the benefits of a healthy eating pattern, with inconclusive results.”
She noted that ABG is “literally aged for many months to years, and the resulting concentrate is found higher in many organosulfur compounds and phytochemicals that suggest enhanced response.”
Dr. Van Horn, a member of the American Heart Association’s Nutrition Committee, who was not involved with the study, continued: “The data suggest that ABG that is much more highly concentrated than fresh or processed garlic might be helpful in lowering BP in certain subgroups, in this case men with higher BP.”
However, she cautioned, “these results are limited in a small study, and ... potential other issues, such as sodium, potassium, or other nutrients known to be associated with blood pressure, were not reported, thereby raising questions about the exclusivity of the ABG over other accompanying dietary factors.”
The study was funded by the Center for the Development of Industrial Technology of the Spanish Ministry of Science and Innovation. Two authors are employees of Pharmactive Biotech Products, SL (Madrid), which manufactured the ABG product, but neither played a role in any result or conclusion. The other authors and Dr. Van Horn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After 6 weeks, consumption of ABG with a high concentration of s-allyl-L-cystine (SAC) was associated with a nearly 6-mm Hg reduction in DBP in men. Other cardiovascular disease (CVD) risk factors were not significantly affected.
“The observed reduction in DBP by ABG extract was similar to the effects of dietary approaches, including the effects of the Dietary Approaches to Stop Hypertension(DASH) diet on BP,” say Rosa M. Valls, PhD, Universitat Rovira i Virgili, Reus, Spain, and colleagues.
“The potential beneficial effects of ABG may contribute to obtaining an optimal DBP” but were “better observed in men and in nonoptimal DBP populations,” they write in the study, published in Nutrients.
Pure SAC and aged garlics have shown healthy effects on multiple targets in in vitro and in vivo tests. However, previous studies in humans have not focused on ABG but rather on other types of aged garlic in patients with some type of CVD risk factor and suffered from methodologic or design weaknesses, the authors note.
To address this gap, Dr. Valls and colleagues randomly assigned 67 individuals with moderate hypercholesterolemia (defined as LDL levels of at least 115 mg/dL) to receive one ABG tablet (250 mg ABG extract/1.25 mg SAC) or placebo daily for 6 weeks. Following a 3-week washout, the groups were reversed and the new intervention continued for another 6 weeks.
Participants received dietary recommendations regarding CVD risk factors and had their dietary habits assessed through a 3-day food record at baseline and after 6 weeks during both treatments.
Individuals receiving lipid-lowering treatment or antihypertensives were excluded, as were those with a body mass index of 35 kg/m2 or higher, those with a fasting blood glucose of at least 126 mg/dL, or active smokers.
There were no differences in baseline characteristics between the two groups. The mean systolic and diastolic pressures at baseline were 124/75 mm Hg in the ABG group and 121/74 mm Hg in the placebo group. Their mean age was 53 years.
Adherence with the protocol was “high” at 96.5% in both groups, and no adverse effects were reported.
Reduced risk of death from stroke, ischemic heart disease
Although no significant differences between ABG and placebo were observed at 3 weeks, the decline in DBP after consumption of the ABG extract became significant at 6 weeks (mean change, –3.7 mm Hg vs. –0.10 mm Hg; P = .007).
When stratified by sex and categories of DBP, the mean change in DBP after 6 weeks of ABG consumption was particularly prominent in men and in those with a baseline DBP of at least 75 mm Hg.
The 6-week change in systolic blood pressure with ABG and placebo was 1.32 mm Hg and 2.84 mm Hg, respectively (P = .694).
At week 6, total cholesterol levels showed a “quadratic decreasing trend” after ABG treatment (P = .047), but no other significant differences between groups were observed for lipid profile, apolipoproteins, or other outcomes of interest, including serum insulin, waist circumference, and body mass index.
The authors note that although systolic BP elevation “has a greater effect on outcomes, both systolic and diastolic hypertension independently influence the risk of adverse cardiovascular events, regardless of the definition of hypertension” and that the risk of death from ischemic heart disease and stroke doubles with every 10 mm Hg increase in DBP in people between the ages of 40 and 89 years.
“Thus, reducing DBP by 5 mm Hg results in a 40% lower risk of death from stroke and a 30% lower risk of death from ischemic heart disease or other vascular death,” they state.
Small study
Commenting for this news organization, Linda Van Horn, PhD, RDN, professor and chief of the department of preventive medicine’s nutrition division, Northwestern University, Chicago, said that for many years, garlic has been “reported to be an adjunct to the benefits of a healthy eating pattern, with inconclusive results.”
She noted that ABG is “literally aged for many months to years, and the resulting concentrate is found higher in many organosulfur compounds and phytochemicals that suggest enhanced response.”
Dr. Van Horn, a member of the American Heart Association’s Nutrition Committee, who was not involved with the study, continued: “The data suggest that ABG that is much more highly concentrated than fresh or processed garlic might be helpful in lowering BP in certain subgroups, in this case men with higher BP.”
However, she cautioned, “these results are limited in a small study, and ... potential other issues, such as sodium, potassium, or other nutrients known to be associated with blood pressure, were not reported, thereby raising questions about the exclusivity of the ABG over other accompanying dietary factors.”
The study was funded by the Center for the Development of Industrial Technology of the Spanish Ministry of Science and Innovation. Two authors are employees of Pharmactive Biotech Products, SL (Madrid), which manufactured the ABG product, but neither played a role in any result or conclusion. The other authors and Dr. Van Horn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NUTRIENTS
Pediatric hepatitis cases may be linked to adenovirus, CDC says
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.
Who doesn’t text in 2022? Most state Medicaid programs
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Omega-3 fatty acids linked to less FOXA1 in benign breast tissue
in benign breast tissue, potentially pointing the way toward the use of the pioneer transcription factor as a helpful biomarker for breast cancer researchers.
The findings were released at the annual meeting of the American Association for Cancer Research.
In the study, researchers who were led by Bruce F. Kimler, PhD, a radiation biologist and breast cancer researcher at the University of Kansas Medical Center, Kansas City, examined benign breast tissue cells aspirated from 12 women (mean age, 53 years; 7 on low-dose hormone replacement) before and after 6 months of high-dose omega-3 fatty acid supplementation. After the supplementation, FOXA1 positive cells fell in 11 of 12 women (P = .019). “There was a robust linear relationship between stain positivity for FOXA1 and AGR2,” the researchers reported (P < .001).
Increased FOXA1 activity along with GRHL2) transcription factor can boost endocrine resistance, while omega-3 fatty acids can reduce it.
In an interview, Robert S. Chapkin, PhD, the Allen Endowed Chair in Nutrition and Chronic Disease Prevention at Texas A&M University, College Station, said it’s important to examine the value of omega-3 fatty acid supplementation, and the understanding of biomarkers is crucial. “Omega 3 fatty acids are pleiotropic, dose dependent, and likely impact multiple signaling mechanisms in select cells types and cancer contexts. The key is to dissect out the highest impact targets and pursue them in the context of preclinical and clinical studies.”
However, he said, “in many cases, the lack of a mechanistic understanding detracts from the merit of the work.”
Studies like this are useful in the development of clinical trials to test the value of high-dose omega-3 fatty acids in breast cancer prevention trials, said Carol Fabian, MD, a breast medical oncologist with the University of Kansas Medical Center, and the study’s first author.
“They help us understand both what dose will be needed and biomarkers that will likely be helpful in predicting response. Early-phase trials with biomarker modulation as a primary endpoint are generally necessary to make sure you have the right dose for the target population prior to committing to a long-term cancer incidence study involving thousands of women and tens of millions of dollars,” she said.
What’s next? “This work was done on reserved specimens from a prior pilot trial,” Dr. Fabian said. “We need a placebo-controlled study to know for sure that omega-3 FA in a dose of about 3.2g daily, or about 2% of calories, modulates FOXA1 and/or AGR2 in postmenopausal women.”
Previously, she said, the researchers “found that high dose omega-3 administered to overweight peri- and postmenopausal high-risk women undergoing a 6-month weight loss intervention increased the number of systemic risk biomarkers which were favorably modulated compared to placebo despite the same median weight loss in each group [–10%],” Dr. Fabian said. “We want to duplicate that finding in a larger study as well as determine if omega-3 fatty acids can block tamoxifen-induced increases in AGR2 associated with endocrine resistance.”
The study was funded by the Breast Cancer Research Foundation, the Morris Family Foundation, and the University of Kansas Cancer Center. The authors and Chapkin report no relevant disclosures.
in benign breast tissue, potentially pointing the way toward the use of the pioneer transcription factor as a helpful biomarker for breast cancer researchers.
The findings were released at the annual meeting of the American Association for Cancer Research.
In the study, researchers who were led by Bruce F. Kimler, PhD, a radiation biologist and breast cancer researcher at the University of Kansas Medical Center, Kansas City, examined benign breast tissue cells aspirated from 12 women (mean age, 53 years; 7 on low-dose hormone replacement) before and after 6 months of high-dose omega-3 fatty acid supplementation. After the supplementation, FOXA1 positive cells fell in 11 of 12 women (P = .019). “There was a robust linear relationship between stain positivity for FOXA1 and AGR2,” the researchers reported (P < .001).
Increased FOXA1 activity along with GRHL2) transcription factor can boost endocrine resistance, while omega-3 fatty acids can reduce it.
In an interview, Robert S. Chapkin, PhD, the Allen Endowed Chair in Nutrition and Chronic Disease Prevention at Texas A&M University, College Station, said it’s important to examine the value of omega-3 fatty acid supplementation, and the understanding of biomarkers is crucial. “Omega 3 fatty acids are pleiotropic, dose dependent, and likely impact multiple signaling mechanisms in select cells types and cancer contexts. The key is to dissect out the highest impact targets and pursue them in the context of preclinical and clinical studies.”
However, he said, “in many cases, the lack of a mechanistic understanding detracts from the merit of the work.”
Studies like this are useful in the development of clinical trials to test the value of high-dose omega-3 fatty acids in breast cancer prevention trials, said Carol Fabian, MD, a breast medical oncologist with the University of Kansas Medical Center, and the study’s first author.
“They help us understand both what dose will be needed and biomarkers that will likely be helpful in predicting response. Early-phase trials with biomarker modulation as a primary endpoint are generally necessary to make sure you have the right dose for the target population prior to committing to a long-term cancer incidence study involving thousands of women and tens of millions of dollars,” she said.
What’s next? “This work was done on reserved specimens from a prior pilot trial,” Dr. Fabian said. “We need a placebo-controlled study to know for sure that omega-3 FA in a dose of about 3.2g daily, or about 2% of calories, modulates FOXA1 and/or AGR2 in postmenopausal women.”
Previously, she said, the researchers “found that high dose omega-3 administered to overweight peri- and postmenopausal high-risk women undergoing a 6-month weight loss intervention increased the number of systemic risk biomarkers which were favorably modulated compared to placebo despite the same median weight loss in each group [–10%],” Dr. Fabian said. “We want to duplicate that finding in a larger study as well as determine if omega-3 fatty acids can block tamoxifen-induced increases in AGR2 associated with endocrine resistance.”
The study was funded by the Breast Cancer Research Foundation, the Morris Family Foundation, and the University of Kansas Cancer Center. The authors and Chapkin report no relevant disclosures.
in benign breast tissue, potentially pointing the way toward the use of the pioneer transcription factor as a helpful biomarker for breast cancer researchers.
The findings were released at the annual meeting of the American Association for Cancer Research.
In the study, researchers who were led by Bruce F. Kimler, PhD, a radiation biologist and breast cancer researcher at the University of Kansas Medical Center, Kansas City, examined benign breast tissue cells aspirated from 12 women (mean age, 53 years; 7 on low-dose hormone replacement) before and after 6 months of high-dose omega-3 fatty acid supplementation. After the supplementation, FOXA1 positive cells fell in 11 of 12 women (P = .019). “There was a robust linear relationship between stain positivity for FOXA1 and AGR2,” the researchers reported (P < .001).
Increased FOXA1 activity along with GRHL2) transcription factor can boost endocrine resistance, while omega-3 fatty acids can reduce it.
In an interview, Robert S. Chapkin, PhD, the Allen Endowed Chair in Nutrition and Chronic Disease Prevention at Texas A&M University, College Station, said it’s important to examine the value of omega-3 fatty acid supplementation, and the understanding of biomarkers is crucial. “Omega 3 fatty acids are pleiotropic, dose dependent, and likely impact multiple signaling mechanisms in select cells types and cancer contexts. The key is to dissect out the highest impact targets and pursue them in the context of preclinical and clinical studies.”
However, he said, “in many cases, the lack of a mechanistic understanding detracts from the merit of the work.”
Studies like this are useful in the development of clinical trials to test the value of high-dose omega-3 fatty acids in breast cancer prevention trials, said Carol Fabian, MD, a breast medical oncologist with the University of Kansas Medical Center, and the study’s first author.
“They help us understand both what dose will be needed and biomarkers that will likely be helpful in predicting response. Early-phase trials with biomarker modulation as a primary endpoint are generally necessary to make sure you have the right dose for the target population prior to committing to a long-term cancer incidence study involving thousands of women and tens of millions of dollars,” she said.
What’s next? “This work was done on reserved specimens from a prior pilot trial,” Dr. Fabian said. “We need a placebo-controlled study to know for sure that omega-3 FA in a dose of about 3.2g daily, or about 2% of calories, modulates FOXA1 and/or AGR2 in postmenopausal women.”
Previously, she said, the researchers “found that high dose omega-3 administered to overweight peri- and postmenopausal high-risk women undergoing a 6-month weight loss intervention increased the number of systemic risk biomarkers which were favorably modulated compared to placebo despite the same median weight loss in each group [–10%],” Dr. Fabian said. “We want to duplicate that finding in a larger study as well as determine if omega-3 fatty acids can block tamoxifen-induced increases in AGR2 associated with endocrine resistance.”
The study was funded by the Breast Cancer Research Foundation, the Morris Family Foundation, and the University of Kansas Cancer Center. The authors and Chapkin report no relevant disclosures.
FROM AACR 2022
Omicron BA.2: What do we know so far?
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
New York NPs join half of states with full practice authority
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
More medical schools build training in transgender care
Klay Noto wants to be the kind of doctor he never had when he began to question his gender identity.
A second-year student at Tulane University in New Orleans, he wants to listen compassionately to patients’ concerns and recognize the hurt when they question who they are. He will be the kind of doctor who knows that a breast exam can be traumatizing if someone has been breast binding or that instructing a patient to take everything off and put on a gown can be triggering for someone with gender dysphoria.
Being in the room for hard conversations is part of why he pursued med school. “There aren’t many LGBT people in medicine and as I started to understand all the dynamics that go into it, I started to see that I could do it and I could be that different kind of doctor,” he told this news organization.
Mr. Noto, who transitioned after college, wants to see more transgender people like himself teaching gender medicine, and for all medical students to be trained in what it means to be transgender and how to give compassionate and comprehensive care to all patients.
Gains have been made in providing curriculum in transgender care that trains medical students in such concepts as how to approach gender identity with sensitivity and how to manage hormone therapy and surgery for transitioning patients who request that, according to those interviewed for this story.
But they agree there’s a long way to go to having widespread medical school integration of the health care needs of about 1.4 million transgender people in the United States.
According to the Association of American Medical Colleges (AAMC) Curriculum Inventory data collected from 131 U.S. medical schools, more than 65% offered some form of transgender-related education in 2018, and more than 80% of those provided such curriculum in required courses.
Lack of transgender, nonbinary faculty
Jason Klein, MD, is a pediatric endocrinologist and medical director of the Transgender Youth Health Program at New York (N.Y.) University.
He said in an interview that the number of programs nationally that have gender medicine as a structured part of their curriculum has increased over the last 5-10 years, but that education is not standardized from program to program.
The program at NYU includes lecture-style learning, case presentations, real-world conversations with people in the community, group discussions, and patient care, Dr. Klein said. There are formal lectures as part of adolescent medicine where students learn the differences between gender and sexual identity, and education on medical treatment of transgender and nonbinary adolescents, starting with puberty blockers and moving into affirming hormones.
Doctors also learn to know their limits and decide when to refer patients to a specialist.
“The focus is really about empathic and supportive care,” said Dr. Klein, assistant professor in the department of pediatrics at Hassenfeld Children’s Hospital at NYU Langone Health. “It’s about communication and understanding and the language we use and how to deliver affirming care in a health care setting in general.”
Imagine the potential stressors, he said, of a transgender person entering a typical health care setting. The electronic health record may only have room for the legal name of a person and not the name a person may currently be using. The intake form typically asks patients to check either male or female. The bathrooms give the same two choices.
“Every physician should know how to speak with, treat, emote with, and empathize with care for the trans and nonbinary individual,” Dr. Klein said.
Dr. Klein noted there is a glaring shortage of trans and nonbinary physicians to lead efforts to expand education on integrating the medical, psychological, and psychosocial care that patients will receive.
Currently, gender medicine is not included on board exams for adolescent medicine or endocrinology, he said.
“Adding formal training in gender medicine to board exams would really help solidify the importance of this arena of medicine,” he noted.
First AAMC standards
In 2014, the AAMC released the first standards to guide curricula across medical school and residency to support training doctors to be competent in caring for transgender patients.
The standards include recommending that all doctors be able to communicate with patients related to their gender identity and understand how to deliver high-quality care to transgender and gender-diverse patients within their specialty, Kristen L. Eckstrand, MD, a coauthor of the guidelines, told this news organization.
“Many medical schools have developed their own curricula to meet these standards,” said Dr. Eckstrand, medical director for LGBTQIA+ Health at the University of Pittsburgh Medical Center.
Norma Poll-Hunter, PhD, AAMC’s senior director for workforce diversity, noted that the organization recently released its diversity, equity, and inclusion competencies that guide the medical education of students, residents, and faculty.
Dr. Poll-Hunter told this news organization that AAMC partners with the Building the Next Generation of Academic Physicians LGBT Health Workforce Conference “to support safe spaces for scholarly efforts and mentorship to advance this area of work.”
Team approach at Rutgers
Among the medical schools that incorporate comprehensive transgender care into the curriculum is Rutgers University’s Robert Wood Johnson Medical School in New Brunswick, N.J.
Gloria Bachmann, MD, is professor of obstetrics and gynecology at the school and medical director of its partner, the PROUD Gender Center of New Jersey. PROUD stands for “Promoting Respect, Outreach, Understanding, and Dignity,” and the center provides comprehensive care for transgender and nonbinary patients in one location.
Dr. Bachmann said Rutgers takes a team approach with both instructors and learners teaching medical students about transgender care. The teachers are not only professors in traditional classroom lectures, but patient navigators and nurses at the PROUD center, established as part of the medical school in 2020. Students learn from the navigators, for instance, how to help patients through the spectrum of inpatient and outpatient care.
“All of our learners do get to care for individuals who identify as transgender,” said Dr. Bachmann.
Among the improvements in educating students on transgender care over the years, she said, is the emphasis on social determinants of health. In the transgender population, initial questions may include whether the person is able to access care through insurance as laws vary widely on what care and procedures are covered.
As another example, Dr. Bachmann cites: “If they are seen on an emergency basis and are sent home with medication and follow-up, can they afford it?”
Another consideration is whether there is a home to which they can return.
“Many individuals who are transgender may not have a home. Their family may not be accepting of them. Therefore, it’s the social determinants of health as well as their transgender identity that have to be put into the equation of best care,” she said.
Giving back to the trans community
Mr. Noto doesn’t know whether he will specialize in gender medicine, but he is committed to serving the transgender community in whatever physician path he chooses.
He said he realizes he is fortunate to have strong family support and good insurance and that he can afford fees, such as the copay to see transgender care specialists. Many in the community do not have those resources and are likely to get care “only if they have to.”
At Tulane, training in transgender care starts during orientation week and continues on different levels, with different options, throughout medical school and residency, he added.
Mr. Noto said he would like to see more mandatory learning such as a “queer-centered exam, where you have to give an organ inventory and you have to ask patients if it’s OK to talk about X, Y, and Z.” He’d also like more opportunities for clinical interaction with transgender patients, such as queer-centered rotations.
When physicians aren’t well trained in transgender care, you have patients educating the doctors, which, Mr. Noto said, should not be acceptable.
“People come to you on their worst day. And to not be informed about them in my mind is negligent. In what other population can you choose not to learn about someone just because you don’t want to?” he said.
A version of this article first appeared on Medscape.com.
Klay Noto wants to be the kind of doctor he never had when he began to question his gender identity.
A second-year student at Tulane University in New Orleans, he wants to listen compassionately to patients’ concerns and recognize the hurt when they question who they are. He will be the kind of doctor who knows that a breast exam can be traumatizing if someone has been breast binding or that instructing a patient to take everything off and put on a gown can be triggering for someone with gender dysphoria.
Being in the room for hard conversations is part of why he pursued med school. “There aren’t many LGBT people in medicine and as I started to understand all the dynamics that go into it, I started to see that I could do it and I could be that different kind of doctor,” he told this news organization.
Mr. Noto, who transitioned after college, wants to see more transgender people like himself teaching gender medicine, and for all medical students to be trained in what it means to be transgender and how to give compassionate and comprehensive care to all patients.
Gains have been made in providing curriculum in transgender care that trains medical students in such concepts as how to approach gender identity with sensitivity and how to manage hormone therapy and surgery for transitioning patients who request that, according to those interviewed for this story.
But they agree there’s a long way to go to having widespread medical school integration of the health care needs of about 1.4 million transgender people in the United States.
According to the Association of American Medical Colleges (AAMC) Curriculum Inventory data collected from 131 U.S. medical schools, more than 65% offered some form of transgender-related education in 2018, and more than 80% of those provided such curriculum in required courses.
Lack of transgender, nonbinary faculty
Jason Klein, MD, is a pediatric endocrinologist and medical director of the Transgender Youth Health Program at New York (N.Y.) University.
He said in an interview that the number of programs nationally that have gender medicine as a structured part of their curriculum has increased over the last 5-10 years, but that education is not standardized from program to program.
The program at NYU includes lecture-style learning, case presentations, real-world conversations with people in the community, group discussions, and patient care, Dr. Klein said. There are formal lectures as part of adolescent medicine where students learn the differences between gender and sexual identity, and education on medical treatment of transgender and nonbinary adolescents, starting with puberty blockers and moving into affirming hormones.
Doctors also learn to know their limits and decide when to refer patients to a specialist.
“The focus is really about empathic and supportive care,” said Dr. Klein, assistant professor in the department of pediatrics at Hassenfeld Children’s Hospital at NYU Langone Health. “It’s about communication and understanding and the language we use and how to deliver affirming care in a health care setting in general.”
Imagine the potential stressors, he said, of a transgender person entering a typical health care setting. The electronic health record may only have room for the legal name of a person and not the name a person may currently be using. The intake form typically asks patients to check either male or female. The bathrooms give the same two choices.
“Every physician should know how to speak with, treat, emote with, and empathize with care for the trans and nonbinary individual,” Dr. Klein said.
Dr. Klein noted there is a glaring shortage of trans and nonbinary physicians to lead efforts to expand education on integrating the medical, psychological, and psychosocial care that patients will receive.
Currently, gender medicine is not included on board exams for adolescent medicine or endocrinology, he said.
“Adding formal training in gender medicine to board exams would really help solidify the importance of this arena of medicine,” he noted.
First AAMC standards
In 2014, the AAMC released the first standards to guide curricula across medical school and residency to support training doctors to be competent in caring for transgender patients.
The standards include recommending that all doctors be able to communicate with patients related to their gender identity and understand how to deliver high-quality care to transgender and gender-diverse patients within their specialty, Kristen L. Eckstrand, MD, a coauthor of the guidelines, told this news organization.
“Many medical schools have developed their own curricula to meet these standards,” said Dr. Eckstrand, medical director for LGBTQIA+ Health at the University of Pittsburgh Medical Center.
Norma Poll-Hunter, PhD, AAMC’s senior director for workforce diversity, noted that the organization recently released its diversity, equity, and inclusion competencies that guide the medical education of students, residents, and faculty.
Dr. Poll-Hunter told this news organization that AAMC partners with the Building the Next Generation of Academic Physicians LGBT Health Workforce Conference “to support safe spaces for scholarly efforts and mentorship to advance this area of work.”
Team approach at Rutgers
Among the medical schools that incorporate comprehensive transgender care into the curriculum is Rutgers University’s Robert Wood Johnson Medical School in New Brunswick, N.J.
Gloria Bachmann, MD, is professor of obstetrics and gynecology at the school and medical director of its partner, the PROUD Gender Center of New Jersey. PROUD stands for “Promoting Respect, Outreach, Understanding, and Dignity,” and the center provides comprehensive care for transgender and nonbinary patients in one location.
Dr. Bachmann said Rutgers takes a team approach with both instructors and learners teaching medical students about transgender care. The teachers are not only professors in traditional classroom lectures, but patient navigators and nurses at the PROUD center, established as part of the medical school in 2020. Students learn from the navigators, for instance, how to help patients through the spectrum of inpatient and outpatient care.
“All of our learners do get to care for individuals who identify as transgender,” said Dr. Bachmann.
Among the improvements in educating students on transgender care over the years, she said, is the emphasis on social determinants of health. In the transgender population, initial questions may include whether the person is able to access care through insurance as laws vary widely on what care and procedures are covered.
As another example, Dr. Bachmann cites: “If they are seen on an emergency basis and are sent home with medication and follow-up, can they afford it?”
Another consideration is whether there is a home to which they can return.
“Many individuals who are transgender may not have a home. Their family may not be accepting of them. Therefore, it’s the social determinants of health as well as their transgender identity that have to be put into the equation of best care,” she said.
Giving back to the trans community
Mr. Noto doesn’t know whether he will specialize in gender medicine, but he is committed to serving the transgender community in whatever physician path he chooses.
He said he realizes he is fortunate to have strong family support and good insurance and that he can afford fees, such as the copay to see transgender care specialists. Many in the community do not have those resources and are likely to get care “only if they have to.”
At Tulane, training in transgender care starts during orientation week and continues on different levels, with different options, throughout medical school and residency, he added.
Mr. Noto said he would like to see more mandatory learning such as a “queer-centered exam, where you have to give an organ inventory and you have to ask patients if it’s OK to talk about X, Y, and Z.” He’d also like more opportunities for clinical interaction with transgender patients, such as queer-centered rotations.
When physicians aren’t well trained in transgender care, you have patients educating the doctors, which, Mr. Noto said, should not be acceptable.
“People come to you on their worst day. And to not be informed about them in my mind is negligent. In what other population can you choose not to learn about someone just because you don’t want to?” he said.
A version of this article first appeared on Medscape.com.
Klay Noto wants to be the kind of doctor he never had when he began to question his gender identity.
A second-year student at Tulane University in New Orleans, he wants to listen compassionately to patients’ concerns and recognize the hurt when they question who they are. He will be the kind of doctor who knows that a breast exam can be traumatizing if someone has been breast binding or that instructing a patient to take everything off and put on a gown can be triggering for someone with gender dysphoria.
Being in the room for hard conversations is part of why he pursued med school. “There aren’t many LGBT people in medicine and as I started to understand all the dynamics that go into it, I started to see that I could do it and I could be that different kind of doctor,” he told this news organization.
Mr. Noto, who transitioned after college, wants to see more transgender people like himself teaching gender medicine, and for all medical students to be trained in what it means to be transgender and how to give compassionate and comprehensive care to all patients.
Gains have been made in providing curriculum in transgender care that trains medical students in such concepts as how to approach gender identity with sensitivity and how to manage hormone therapy and surgery for transitioning patients who request that, according to those interviewed for this story.
But they agree there’s a long way to go to having widespread medical school integration of the health care needs of about 1.4 million transgender people in the United States.
According to the Association of American Medical Colleges (AAMC) Curriculum Inventory data collected from 131 U.S. medical schools, more than 65% offered some form of transgender-related education in 2018, and more than 80% of those provided such curriculum in required courses.
Lack of transgender, nonbinary faculty
Jason Klein, MD, is a pediatric endocrinologist and medical director of the Transgender Youth Health Program at New York (N.Y.) University.
He said in an interview that the number of programs nationally that have gender medicine as a structured part of their curriculum has increased over the last 5-10 years, but that education is not standardized from program to program.
The program at NYU includes lecture-style learning, case presentations, real-world conversations with people in the community, group discussions, and patient care, Dr. Klein said. There are formal lectures as part of adolescent medicine where students learn the differences between gender and sexual identity, and education on medical treatment of transgender and nonbinary adolescents, starting with puberty blockers and moving into affirming hormones.
Doctors also learn to know their limits and decide when to refer patients to a specialist.
“The focus is really about empathic and supportive care,” said Dr. Klein, assistant professor in the department of pediatrics at Hassenfeld Children’s Hospital at NYU Langone Health. “It’s about communication and understanding and the language we use and how to deliver affirming care in a health care setting in general.”
Imagine the potential stressors, he said, of a transgender person entering a typical health care setting. The electronic health record may only have room for the legal name of a person and not the name a person may currently be using. The intake form typically asks patients to check either male or female. The bathrooms give the same two choices.
“Every physician should know how to speak with, treat, emote with, and empathize with care for the trans and nonbinary individual,” Dr. Klein said.
Dr. Klein noted there is a glaring shortage of trans and nonbinary physicians to lead efforts to expand education on integrating the medical, psychological, and psychosocial care that patients will receive.
Currently, gender medicine is not included on board exams for adolescent medicine or endocrinology, he said.
“Adding formal training in gender medicine to board exams would really help solidify the importance of this arena of medicine,” he noted.
First AAMC standards
In 2014, the AAMC released the first standards to guide curricula across medical school and residency to support training doctors to be competent in caring for transgender patients.
The standards include recommending that all doctors be able to communicate with patients related to their gender identity and understand how to deliver high-quality care to transgender and gender-diverse patients within their specialty, Kristen L. Eckstrand, MD, a coauthor of the guidelines, told this news organization.
“Many medical schools have developed their own curricula to meet these standards,” said Dr. Eckstrand, medical director for LGBTQIA+ Health at the University of Pittsburgh Medical Center.
Norma Poll-Hunter, PhD, AAMC’s senior director for workforce diversity, noted that the organization recently released its diversity, equity, and inclusion competencies that guide the medical education of students, residents, and faculty.
Dr. Poll-Hunter told this news organization that AAMC partners with the Building the Next Generation of Academic Physicians LGBT Health Workforce Conference “to support safe spaces for scholarly efforts and mentorship to advance this area of work.”
Team approach at Rutgers
Among the medical schools that incorporate comprehensive transgender care into the curriculum is Rutgers University’s Robert Wood Johnson Medical School in New Brunswick, N.J.
Gloria Bachmann, MD, is professor of obstetrics and gynecology at the school and medical director of its partner, the PROUD Gender Center of New Jersey. PROUD stands for “Promoting Respect, Outreach, Understanding, and Dignity,” and the center provides comprehensive care for transgender and nonbinary patients in one location.
Dr. Bachmann said Rutgers takes a team approach with both instructors and learners teaching medical students about transgender care. The teachers are not only professors in traditional classroom lectures, but patient navigators and nurses at the PROUD center, established as part of the medical school in 2020. Students learn from the navigators, for instance, how to help patients through the spectrum of inpatient and outpatient care.
“All of our learners do get to care for individuals who identify as transgender,” said Dr. Bachmann.
Among the improvements in educating students on transgender care over the years, she said, is the emphasis on social determinants of health. In the transgender population, initial questions may include whether the person is able to access care through insurance as laws vary widely on what care and procedures are covered.
As another example, Dr. Bachmann cites: “If they are seen on an emergency basis and are sent home with medication and follow-up, can they afford it?”
Another consideration is whether there is a home to which they can return.
“Many individuals who are transgender may not have a home. Their family may not be accepting of them. Therefore, it’s the social determinants of health as well as their transgender identity that have to be put into the equation of best care,” she said.
Giving back to the trans community
Mr. Noto doesn’t know whether he will specialize in gender medicine, but he is committed to serving the transgender community in whatever physician path he chooses.
He said he realizes he is fortunate to have strong family support and good insurance and that he can afford fees, such as the copay to see transgender care specialists. Many in the community do not have those resources and are likely to get care “only if they have to.”
At Tulane, training in transgender care starts during orientation week and continues on different levels, with different options, throughout medical school and residency, he added.
Mr. Noto said he would like to see more mandatory learning such as a “queer-centered exam, where you have to give an organ inventory and you have to ask patients if it’s OK to talk about X, Y, and Z.” He’d also like more opportunities for clinical interaction with transgender patients, such as queer-centered rotations.
When physicians aren’t well trained in transgender care, you have patients educating the doctors, which, Mr. Noto said, should not be acceptable.
“People come to you on their worst day. And to not be informed about them in my mind is negligent. In what other population can you choose not to learn about someone just because you don’t want to?” he said.
A version of this article first appeared on Medscape.com.
Probiotic LGG doesn’t lessen eczema, asthma, or rhinitis risk by age 7
Giving the probiotic supplement Lactobacillus rhamnosus GG (LGG) to high-risk infants in the first 6 months of life is not effective in lessening incidence of eczema, asthma, or rhinitis in later childhood, researchers have found.
The researchers, led by Michael D. Cabana, MD, MPH, with the Children’s Hospital of Montefiore, New York, said they cannot support its use in this population of children at high risk for allergic disease. Findings were published in Pediatrics.
Jonathan Spergel, MD, PhD, chief of the allergy program at Children’s Hospital of Philadelphia, who was not part of the study, said the “small, but very interesting study adds to the literature indicating that allergy prevention needs to be a multifactorial approach and simply adding LGG in a select population makes no difference.”
He noted that the study of probiotics for allergic conditions is complex as it depends on many factors, such as the child’s environment, including exposure to pets and pollution, and whether the child was delivered vaginally or by cesarean section.
Study builds on previous work
The new study builds on the same researchers’ randomized, double-masked, parallel-arm, controlled Trial of Infant Probiotic Supplementation (TIPS). That study investigated whether daily administration of LGG in the first 6 months to children at high risk for allergic disease because of asthma in a parent, could decrease their cumulative incidence of eczema. Investigators found LGG had no effect.
These additional results included participants at least 7 years old and also included physician-diagnosed asthma and physician-diagnosed rhinitis as secondary outcomes.
Retention rate over the 7-year follow-up was 56%; 49 (53%) of 92 in the intervention group and 54 (59%) of 92 in the control group.
The researchers performed modified intention-to-treat analyses with all children who received treatment in the study arm to which they had been randomized.
Eczema was diagnosed in 78 participants, asthma in 32, and rhinitis in 15. Incidence of eczema was high in infancy, but low thereafter. Incidence rates for asthma and rhinitis were constant throughout childhood.
The researchers used modeling to compare the incidence of each outcome between the intervention and control groups, adjusting for mode of delivery and how long a child was breastfed.
Cesarean delivery was linked to a greater incidence of rhinitis, with a hazard ratio of 3.33 (95% confidence interval, 1.21-9.21).
Finding the right strain
Heather Cassell, MD, a pediatric allergist and immunologist at University of Arizona, Tucson, who was not part of the study, said in an interview that many researchers, including those at her institution, are trying to find which strain of probiotic might be beneficial in lowering risk for allergic disease.
Though it appears LGG doesn’t have an effect, she said, another strain might be successful and this helps zero in on the right one.
The TIPS trial showed that there were no significant side effects from giving LGG early, which is good information to have as the search resumes for the right strain, she said.
“We know that there’s probably some immune dysregulation in kids with asthma, eczema, other allergies, but we don’t fully know the extent of it,” she said, adding that it may be that skin flora or respiratory flora and microbiomes in other parts of the body play a role.
“We don’t have bacteria just in our guts,” she noted. “It may be a combination of strains or a combination of bacteria.”
The authors, Dr. Spergel, and Dr. Cassell reported no relevant financial relationships.
Giving the probiotic supplement Lactobacillus rhamnosus GG (LGG) to high-risk infants in the first 6 months of life is not effective in lessening incidence of eczema, asthma, or rhinitis in later childhood, researchers have found.
The researchers, led by Michael D. Cabana, MD, MPH, with the Children’s Hospital of Montefiore, New York, said they cannot support its use in this population of children at high risk for allergic disease. Findings were published in Pediatrics.
Jonathan Spergel, MD, PhD, chief of the allergy program at Children’s Hospital of Philadelphia, who was not part of the study, said the “small, but very interesting study adds to the literature indicating that allergy prevention needs to be a multifactorial approach and simply adding LGG in a select population makes no difference.”
He noted that the study of probiotics for allergic conditions is complex as it depends on many factors, such as the child’s environment, including exposure to pets and pollution, and whether the child was delivered vaginally or by cesarean section.
Study builds on previous work
The new study builds on the same researchers’ randomized, double-masked, parallel-arm, controlled Trial of Infant Probiotic Supplementation (TIPS). That study investigated whether daily administration of LGG in the first 6 months to children at high risk for allergic disease because of asthma in a parent, could decrease their cumulative incidence of eczema. Investigators found LGG had no effect.
These additional results included participants at least 7 years old and also included physician-diagnosed asthma and physician-diagnosed rhinitis as secondary outcomes.
Retention rate over the 7-year follow-up was 56%; 49 (53%) of 92 in the intervention group and 54 (59%) of 92 in the control group.
The researchers performed modified intention-to-treat analyses with all children who received treatment in the study arm to which they had been randomized.
Eczema was diagnosed in 78 participants, asthma in 32, and rhinitis in 15. Incidence of eczema was high in infancy, but low thereafter. Incidence rates for asthma and rhinitis were constant throughout childhood.
The researchers used modeling to compare the incidence of each outcome between the intervention and control groups, adjusting for mode of delivery and how long a child was breastfed.
Cesarean delivery was linked to a greater incidence of rhinitis, with a hazard ratio of 3.33 (95% confidence interval, 1.21-9.21).
Finding the right strain
Heather Cassell, MD, a pediatric allergist and immunologist at University of Arizona, Tucson, who was not part of the study, said in an interview that many researchers, including those at her institution, are trying to find which strain of probiotic might be beneficial in lowering risk for allergic disease.
Though it appears LGG doesn’t have an effect, she said, another strain might be successful and this helps zero in on the right one.
The TIPS trial showed that there were no significant side effects from giving LGG early, which is good information to have as the search resumes for the right strain, she said.
“We know that there’s probably some immune dysregulation in kids with asthma, eczema, other allergies, but we don’t fully know the extent of it,” she said, adding that it may be that skin flora or respiratory flora and microbiomes in other parts of the body play a role.
“We don’t have bacteria just in our guts,” she noted. “It may be a combination of strains or a combination of bacteria.”
The authors, Dr. Spergel, and Dr. Cassell reported no relevant financial relationships.
Giving the probiotic supplement Lactobacillus rhamnosus GG (LGG) to high-risk infants in the first 6 months of life is not effective in lessening incidence of eczema, asthma, or rhinitis in later childhood, researchers have found.
The researchers, led by Michael D. Cabana, MD, MPH, with the Children’s Hospital of Montefiore, New York, said they cannot support its use in this population of children at high risk for allergic disease. Findings were published in Pediatrics.
Jonathan Spergel, MD, PhD, chief of the allergy program at Children’s Hospital of Philadelphia, who was not part of the study, said the “small, but very interesting study adds to the literature indicating that allergy prevention needs to be a multifactorial approach and simply adding LGG in a select population makes no difference.”
He noted that the study of probiotics for allergic conditions is complex as it depends on many factors, such as the child’s environment, including exposure to pets and pollution, and whether the child was delivered vaginally or by cesarean section.
Study builds on previous work
The new study builds on the same researchers’ randomized, double-masked, parallel-arm, controlled Trial of Infant Probiotic Supplementation (TIPS). That study investigated whether daily administration of LGG in the first 6 months to children at high risk for allergic disease because of asthma in a parent, could decrease their cumulative incidence of eczema. Investigators found LGG had no effect.
These additional results included participants at least 7 years old and also included physician-diagnosed asthma and physician-diagnosed rhinitis as secondary outcomes.
Retention rate over the 7-year follow-up was 56%; 49 (53%) of 92 in the intervention group and 54 (59%) of 92 in the control group.
The researchers performed modified intention-to-treat analyses with all children who received treatment in the study arm to which they had been randomized.
Eczema was diagnosed in 78 participants, asthma in 32, and rhinitis in 15. Incidence of eczema was high in infancy, but low thereafter. Incidence rates for asthma and rhinitis were constant throughout childhood.
The researchers used modeling to compare the incidence of each outcome between the intervention and control groups, adjusting for mode of delivery and how long a child was breastfed.
Cesarean delivery was linked to a greater incidence of rhinitis, with a hazard ratio of 3.33 (95% confidence interval, 1.21-9.21).
Finding the right strain
Heather Cassell, MD, a pediatric allergist and immunologist at University of Arizona, Tucson, who was not part of the study, said in an interview that many researchers, including those at her institution, are trying to find which strain of probiotic might be beneficial in lowering risk for allergic disease.
Though it appears LGG doesn’t have an effect, she said, another strain might be successful and this helps zero in on the right one.
The TIPS trial showed that there were no significant side effects from giving LGG early, which is good information to have as the search resumes for the right strain, she said.
“We know that there’s probably some immune dysregulation in kids with asthma, eczema, other allergies, but we don’t fully know the extent of it,” she said, adding that it may be that skin flora or respiratory flora and microbiomes in other parts of the body play a role.
“We don’t have bacteria just in our guts,” she noted. “It may be a combination of strains or a combination of bacteria.”
The authors, Dr. Spergel, and Dr. Cassell reported no relevant financial relationships.
FROM PEDIATRICS