User login
New combo therapy for breast implant–associated lymphoma
The immediate treatment is surgical removal of the implant, which is sometimes followed with chemotherapy.
New data show that women who develop breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) who require chemotherapy can achieve excellent results with a combination of chemotherapy (cyclophosphamide, doxorubicin, and prednisone) and the antibody–drug conjugate brentuximab vedotin.
The findings were published in Blood.
The authors, led by Fabien Le Bras, MD, from the Henri Mondor Hospital, Créteil, France, note that despite BIA-ALCL being recently recognized as a provisional entity by the World Health Organization, its pathogenesis has yet to be fully elucidated, and a standard of care has not been established.
Results from the ECHELON 2 trial established brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (BV-CHP) as a new standard of care in CD30-positive peripheral T-cell lymphoma.
That trial included 316 patients with ACLC, although none of these cases were associated with breast implants.
The principal investigator on that trial, Steven Horwitz, MD, from Memorial Sloan Kettering Center, New York, told this news organization that although BIA-ALCL is “incredibly rare,” it causes “distress” to patients, as “many of them made a choice for reconstruction ... that they thought was safe.”
He said that the latest data from France is “interesting” and that the application of the ECHELON-2 findings to BIA-ALCL is “very logical.”
“For the people who need systemic therapy,” it appears from the current results that BV-CHP “is a very good option,” he said.
The “difficulty” in interpreting the data, however, is that “perhaps 80% of people with BIA-ALCL don’t need any systemic therapy” and are “cured with surgery alone.”
Dr. Horwitz said that while patients with infiltrative disease have a “higher risk of recurrence ... many of those are still cured with surgery alone.”
The main outstanding question he has is how many of the patients who received BV-CHP “might have been okay with observation.”
Details of the new data from France
For their study, Dr. Le Bras and colleagues analyzed data from the Lymphoma Study Association registry between 2009 and 2021 and identified 85 patients with BIA-ALCL, including 73 in France and 12 in Belgium.
Most of these patients (whose median age was 57 years) had unilateral lymphoma (94.1%), and only a few patients (5.9%) had bilateral disease.
The team notes that 41.2% of these women had received breast implants once, 41.2% received implants twice, and 17.6% received them three times or more.
In 45.9% of cases, the first implant followed mastectomy for breast cancer.
All patients had at least one textured implant. These have been associated with more cases of BIA-ALCL than smooth implants, and in 2019, Allergan recalled all BioCell textured breast implant products from the United States and around the world, due to the risk for BIA-ALCL, as reported, at the time, by this news organization.
For the women in this registry, the median time from the last implant to BIA-ALCL diagnosis was 7 years.
The most common presentation was seroma, which occurred in 75.3% of patients, while 21.2% of had a breast tumor mass with or without seroma.
Stage I-II disease was identified in 76.5% of patients, and 21.2% of cases were stage IV. Infiltrative disease was present in 24.7%.
Implant removal with total capsulectomy was performed in 77.6%; 29.4% of women also received chemotherapy, with 11.8% receiving BV-CHP.
A complete response was achieved in 84% of patients who received chemotherapy, while 8% failed to respond. Among the patients who received BV-CHP, 80% achieved a complete response.
After a median follow-up of 28.6 months, 91.8% patients were alive and progression free. All patients treated with BV-CHP were alive and progression free after a median follow-up of 1 year.
Patients with infiltrative disease had a significantly worse 2-year progression-free survival than those with in situ/mixed disease, at 73.8% versus 96.7%, or a hazard ratio for progression of 5.3 (P = .0039).
They also had worse 2-year overall survival, at 78.7% versus 100%, or a hazard ratio for death of 8.5 (P = .0022).
The authors note that these patients with infiltrative disease had significantly worse survival outcomes and may benefit most from BV-CHP.
No funding for the study was declared. Dr. Le Bras reports relationships with Novartis, Celgene, BMS, Takeda, Kite, and Gilead. Other authors declare numerous relevant financial relationships.
A version of this article first appeared on Medscape.com.
The immediate treatment is surgical removal of the implant, which is sometimes followed with chemotherapy.
New data show that women who develop breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) who require chemotherapy can achieve excellent results with a combination of chemotherapy (cyclophosphamide, doxorubicin, and prednisone) and the antibody–drug conjugate brentuximab vedotin.
The findings were published in Blood.
The authors, led by Fabien Le Bras, MD, from the Henri Mondor Hospital, Créteil, France, note that despite BIA-ALCL being recently recognized as a provisional entity by the World Health Organization, its pathogenesis has yet to be fully elucidated, and a standard of care has not been established.
Results from the ECHELON 2 trial established brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (BV-CHP) as a new standard of care in CD30-positive peripheral T-cell lymphoma.
That trial included 316 patients with ACLC, although none of these cases were associated with breast implants.
The principal investigator on that trial, Steven Horwitz, MD, from Memorial Sloan Kettering Center, New York, told this news organization that although BIA-ALCL is “incredibly rare,” it causes “distress” to patients, as “many of them made a choice for reconstruction ... that they thought was safe.”
He said that the latest data from France is “interesting” and that the application of the ECHELON-2 findings to BIA-ALCL is “very logical.”
“For the people who need systemic therapy,” it appears from the current results that BV-CHP “is a very good option,” he said.
The “difficulty” in interpreting the data, however, is that “perhaps 80% of people with BIA-ALCL don’t need any systemic therapy” and are “cured with surgery alone.”
Dr. Horwitz said that while patients with infiltrative disease have a “higher risk of recurrence ... many of those are still cured with surgery alone.”
The main outstanding question he has is how many of the patients who received BV-CHP “might have been okay with observation.”
Details of the new data from France
For their study, Dr. Le Bras and colleagues analyzed data from the Lymphoma Study Association registry between 2009 and 2021 and identified 85 patients with BIA-ALCL, including 73 in France and 12 in Belgium.
Most of these patients (whose median age was 57 years) had unilateral lymphoma (94.1%), and only a few patients (5.9%) had bilateral disease.
The team notes that 41.2% of these women had received breast implants once, 41.2% received implants twice, and 17.6% received them three times or more.
In 45.9% of cases, the first implant followed mastectomy for breast cancer.
All patients had at least one textured implant. These have been associated with more cases of BIA-ALCL than smooth implants, and in 2019, Allergan recalled all BioCell textured breast implant products from the United States and around the world, due to the risk for BIA-ALCL, as reported, at the time, by this news organization.
For the women in this registry, the median time from the last implant to BIA-ALCL diagnosis was 7 years.
The most common presentation was seroma, which occurred in 75.3% of patients, while 21.2% of had a breast tumor mass with or without seroma.
Stage I-II disease was identified in 76.5% of patients, and 21.2% of cases were stage IV. Infiltrative disease was present in 24.7%.
Implant removal with total capsulectomy was performed in 77.6%; 29.4% of women also received chemotherapy, with 11.8% receiving BV-CHP.
A complete response was achieved in 84% of patients who received chemotherapy, while 8% failed to respond. Among the patients who received BV-CHP, 80% achieved a complete response.
After a median follow-up of 28.6 months, 91.8% patients were alive and progression free. All patients treated with BV-CHP were alive and progression free after a median follow-up of 1 year.
Patients with infiltrative disease had a significantly worse 2-year progression-free survival than those with in situ/mixed disease, at 73.8% versus 96.7%, or a hazard ratio for progression of 5.3 (P = .0039).
They also had worse 2-year overall survival, at 78.7% versus 100%, or a hazard ratio for death of 8.5 (P = .0022).
The authors note that these patients with infiltrative disease had significantly worse survival outcomes and may benefit most from BV-CHP.
No funding for the study was declared. Dr. Le Bras reports relationships with Novartis, Celgene, BMS, Takeda, Kite, and Gilead. Other authors declare numerous relevant financial relationships.
A version of this article first appeared on Medscape.com.
The immediate treatment is surgical removal of the implant, which is sometimes followed with chemotherapy.
New data show that women who develop breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) who require chemotherapy can achieve excellent results with a combination of chemotherapy (cyclophosphamide, doxorubicin, and prednisone) and the antibody–drug conjugate brentuximab vedotin.
The findings were published in Blood.
The authors, led by Fabien Le Bras, MD, from the Henri Mondor Hospital, Créteil, France, note that despite BIA-ALCL being recently recognized as a provisional entity by the World Health Organization, its pathogenesis has yet to be fully elucidated, and a standard of care has not been established.
Results from the ECHELON 2 trial established brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (BV-CHP) as a new standard of care in CD30-positive peripheral T-cell lymphoma.
That trial included 316 patients with ACLC, although none of these cases were associated with breast implants.
The principal investigator on that trial, Steven Horwitz, MD, from Memorial Sloan Kettering Center, New York, told this news organization that although BIA-ALCL is “incredibly rare,” it causes “distress” to patients, as “many of them made a choice for reconstruction ... that they thought was safe.”
He said that the latest data from France is “interesting” and that the application of the ECHELON-2 findings to BIA-ALCL is “very logical.”
“For the people who need systemic therapy,” it appears from the current results that BV-CHP “is a very good option,” he said.
The “difficulty” in interpreting the data, however, is that “perhaps 80% of people with BIA-ALCL don’t need any systemic therapy” and are “cured with surgery alone.”
Dr. Horwitz said that while patients with infiltrative disease have a “higher risk of recurrence ... many of those are still cured with surgery alone.”
The main outstanding question he has is how many of the patients who received BV-CHP “might have been okay with observation.”
Details of the new data from France
For their study, Dr. Le Bras and colleagues analyzed data from the Lymphoma Study Association registry between 2009 and 2021 and identified 85 patients with BIA-ALCL, including 73 in France and 12 in Belgium.
Most of these patients (whose median age was 57 years) had unilateral lymphoma (94.1%), and only a few patients (5.9%) had bilateral disease.
The team notes that 41.2% of these women had received breast implants once, 41.2% received implants twice, and 17.6% received them three times or more.
In 45.9% of cases, the first implant followed mastectomy for breast cancer.
All patients had at least one textured implant. These have been associated with more cases of BIA-ALCL than smooth implants, and in 2019, Allergan recalled all BioCell textured breast implant products from the United States and around the world, due to the risk for BIA-ALCL, as reported, at the time, by this news organization.
For the women in this registry, the median time from the last implant to BIA-ALCL diagnosis was 7 years.
The most common presentation was seroma, which occurred in 75.3% of patients, while 21.2% of had a breast tumor mass with or without seroma.
Stage I-II disease was identified in 76.5% of patients, and 21.2% of cases were stage IV. Infiltrative disease was present in 24.7%.
Implant removal with total capsulectomy was performed in 77.6%; 29.4% of women also received chemotherapy, with 11.8% receiving BV-CHP.
A complete response was achieved in 84% of patients who received chemotherapy, while 8% failed to respond. Among the patients who received BV-CHP, 80% achieved a complete response.
After a median follow-up of 28.6 months, 91.8% patients were alive and progression free. All patients treated with BV-CHP were alive and progression free after a median follow-up of 1 year.
Patients with infiltrative disease had a significantly worse 2-year progression-free survival than those with in situ/mixed disease, at 73.8% versus 96.7%, or a hazard ratio for progression of 5.3 (P = .0039).
They also had worse 2-year overall survival, at 78.7% versus 100%, or a hazard ratio for death of 8.5 (P = .0022).
The authors note that these patients with infiltrative disease had significantly worse survival outcomes and may benefit most from BV-CHP.
No funding for the study was declared. Dr. Le Bras reports relationships with Novartis, Celgene, BMS, Takeda, Kite, and Gilead. Other authors declare numerous relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dapivirine vaginal ring for HIV prevention no longer under consideration by the FDA
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tosha Rogers, MD, is a one-woman HIV prevention evangelist. For nearly a decade now, the Atlanta-based ob/gyn has been on a mission to increase her gynecological colleagues’ awareness and prescribing of the oral HIV prevention pill. At the same time, she’s been tracking the development of a flexible vaginal ring loaded with a month’s worth of the HIV prevention medication dapivirine. That, she thought, would fit easily into women’s lives and into the toolbox of methods women already use to prevent pregnancy.
But now she’s not sure when – or if – the ring will find its way to her patients. In December, the ring’s maker, the International Partnership for Microbicides (IPM), pulled its application for FDA approval for the pre-exposure prophylaxis (PrEP) ring. Now, one year after the World Health Organization recommended the ring for member nations, there appears to be no path forward in the United States for either the dapivirine-only ring or an approach Dr. Rogers said would change the game: a vaginal ring that supplies both contraception and HIV prevention.
“It would take things to a whole other level,” she said. “It sucks that this happened, and I do think it was not anything medical. I think it was everything political.”
That leaves cisgender women – especially the Black and Latinx women who make up the vast majority of women who acquire HIV every year – with two HIV prevention options. One is the daily pill, first approved in 2012. It’s now generic but previously sold as Truvada by Gilead Sciences. The other is monthly injectable cabotegravir long-acting (Apretude). Another HIV prevention pill, tenofovir alafenamide/emtricitabine (Descovy), is approved for gay men and transgender women but not cisgender women.
Vagina-specific protection from HIV
The WHO recommendation for the vaginal ring was followed last July by a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) for women in low- and middle-income countries outside the European Union.
The flexible silicone ring, similar to the hormonal NuvaRing contraceptive, works by slowly releasing the antiretroviral dapivirine directly into the vaginal canal, thereby protecting women who might be exposed to the virus through vaginal sex only. Because the medicine stays where it’s delivered and doesn’t circulate through the body, it has been found to be extremely safe with few adverse events.
However, in initial studies, the ring was found to be just 27% effective overall. Later studies, where scientists divided women by how much drug was missing from the ring – a proxy for use – found that higher use was associated with higher protection (as much as 54%). By comparison, Truvada has been found to be up to 99% effective when used daily, though it can take up to 21 days to be available in the vagina in high enough concentrations to protect women from vaginal exposure. And the HIV prevention shot was found to be 90% more effective than that in a recent trial of the two methods conducted by the HIV Prevention Trials Network.
This, and an orientation away from topical HIV prevention drugs and toward systemic options, led the National Institute of Allergy and Infectious Diseases (NIAID) to discontinue funding for such projects under its Microbicide Trials Network.
“Clearly you want to counsel women to use the highest efficacy method, and that is part of our label,” Zeda Rosenberg, ScD, IPM’s founder and chief executive officer, told this news organization. “Women should not choose the ring if they can and will use oral PrEP, and I would argue it should be the same thing for [cabotegravir shots]. But if they can’t or don’t want to – and we know that especially many young women don’t want to use systemic methods – then the dapivirine ring is a great option.”
Still, Dr. Rosenberg said that the gap in efficacy, the relatively small number of women affected by HIV in the U.S. compared with gay and bisexual men, and the emergence of products like the HIV prevention shot cabotegravir, made it “very unlikely” that FDA regulators would approve the ring. And rather than be “distracted” by the FDA process, Dr. Rosenberg said IPM chose to concentrate on the countries where the ring has already been approved or where women make up the vast majority of people affected by HIV.
Zimbabwe publicly announced it has approved the ring, and three other countries may have approved it, according to Dr. Rosenberg. She declined to name them, saying they had requested silence while they formulate their new HIV prevention guidelines. Aside from Zimbabwe, the other countries where women participated in the ring clinical trials were South Africa, Malawi, and Uganda.
“The U.S. population ... has widespread access to oral PrEP, which is unlike countries in Africa, and which would have widespread access to injectable cabotegravir,” she said. “The U.S. FDA may not see choice in the same way that African women and African activists and advocates see the need for choice.”
But women’s rates of accessing HIV prevention medications in the U.S. continues to be frustratingly low. At the end of 2018, just 7% of women who could benefit from HIV prevention drugs were taking them, according to Centers for Disease Control and Prevention data.
New CDC guidelines recommend clinicians talk to every sexually active adult and adolescent about HIV prevention medications at least once and prescribe it to anyone who asks for it, whether or not they understand their patients’ HIV risks. However, research continues to show that clinicians struggle with willingness to prescribe PrEP to Black women, and the American College of Obstetrics and Gynecology’s committee opinion on managing women using HIV prevention drugs has not been updated to reflect the new guidelines. And while the HIV prevention shot is approved for women and its maker ViiV Healthcare is already initiating postmarket studies of the ring in key populations including women, there are lots of things that need to line up in order for clinicians to be willing to stock it and prescribe it to women.
From where Dázon Dixon Diallo, executive director of the nonprofit SisterLove, sits, the decision to withdraw the ring from FDA consideration and the FDA’s seeming argument that the epidemiology in the U.S. doesn’t warrant the ring’s approval is a slap in the face to the Black women who have led the movement to end HIV in the U.S. for decades.
“No matter how you slice it, we’re talking about Black women, and then we’re talking about brown women,” said Ms. Diallo. “The value [they place on us] from a government standpoint, from a political standpoint, from a public health standpoint is just woeful. It’s woeful and it’s disrespectful and it’s insulting and I’m sick of it.”
‘America sneezes and Africa catches a cold’
When she first heard the decision to pull the ring from FDA consideration, Yvette Raphael, the South Africa-based executive director of Advocates for the Prevention of HIV in Africa, started asking, “What can we do to help our sisters in America get this ring?” And then she started worrying about other women in her own country and those nearby.
“The FDA plays a big role,” she said. “You know, America sneezes and Africa catches a cold.”
She worries that IPM’s decision to withdraw the ring from FDA consideration will signal to regulators in other countries either (a) that they should not approve it or (b) in countries where it’s already been approved but guidelines have not been issued, that they won’t invest money in rolling it out to women in those countries – especially now with the U.S. approval of the prevention shot. In much of Africa, ministries of health prefer to provide injectable contraception, often giving women few or no other options. But women, she said, think about more than administration of the drug. They look at if it’s an easier option for them to manage.
“This is a long journey, an emotional one too, for women in South Africa, because the idea of a microbicide is one of the ideas that came directly from women in South Africa,” she said. “[The jab] can be seen as a solution to all. We can just give jabs to all the women. And after all, we know that women don’t adhere, so we can just grab them.”
Dr. Rosenberg pointed to the positive opinion from the EMA as another “rigorous review” process that she said ought to equally influence ministries of health in countries where women tested the ring. And she pointed to the WHO statement released last month, the same day as IPM’s announcement that it was withdrawing the ring from FDA considerations, recommitting the ring as a good option in sub-Saharan Africa: “The U.S. FDA decision is not based on any new or additional data on efficacy and safety,” it stated. “WHO will continue to support countries as they consider whether to include the [dapivirine vaginal ring]. WHO recognizes that country decisionmaking will vary based on their context and that women’s voices remain central to discussions about their prevention choices.”
Dual action ring on the horizon, but not in U.S.
What this means, though, is that the next step in the ring’s development – the combination dapivirine ring with contraceptive levonorgestrel (used in the Mirena intrauterine device) – may not come to the U.S., at least for a long while.
“It’s not out of the question,” Dr. Rosenberg said of conducting HIV/pregnancy prevention ring trials in the U.S. “But without the approval of the dapivirine-only ring by FDA, I imagine they would want to see new efficacy data on dapivirine. That is a very difficult hill to climb. There would have to be an active control group [using oral PrEP or injectable cabotegravir], and it would be very difficult for the dapivirine ring to be able to go head-to-head for either noninferiority and certainly for superiority.”
The study would need to be quite large to get enough results to prove anything, and IPM is a research organization, not a large pharmaceutical company with deep enough pockets to fund that, she said. Raising those funds “would be difficult.”
In addition to NIAID discontinuing its funding for the Microbicides Trials Network, a new 5-year, $85 million research collaboration through USAID hasn’t slated any money to fund trials of the combination HIV prevention and contraceptive ring, according to Dr. Rosenberg.
But that doesn’t mean avenues for its development are closed. NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is currently funding a phase 1/2 trial of the combination ring, and IPM continues to receive funding from research agencies in Germany, the Netherlands, Denmark, and Ireland. And this means, she said, that the E.U. – not the U.S. – is where they would seek approval for a combination ring first.
That leaves Ms. Rafael and Ms. Diallo debating how to work together to push the FDA – and maybe IPM – to reconsider the ring. For instance, Ms. Diallo suggested that instead of seeking an indication for all women, the FDA might consider the ring for women with very high risk of HIV, such as sex workers or women with HIV positive partners not on treatment. And she said that this has to be bigger than HIV prevention. It has to be about the ways in which women’s health issues in general lag at the FDA. For instance, she pointed to the movement to get contraceptive pills available over the counter, fights against FDA rulings on hormone replacement therapy, and fights for emergency contraception.
In the meantime, ob/gyn Dr. Rogers is expecting access to the ring to follow a similar path as the copper IUD, which migrated to the U.S. from Europe, where it has been among the most popular contraceptive methods for women.
“Contrary to what we may think, we are not innovators, especially for something like this,” she said. “Once we see it is working and doing a good job – that women in Europe love it – then someone here is going to pick it up and make it as if it’s the greatest thing. But for now, I think we’re going to have to take a back seat to Europe.”
Ms. Diallo reports receiving fees from Johnson & Johnson, ViiV Healthcare, and Gilead Sciences. Dr. Rosenberg and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: United States passes 10 million total cases
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.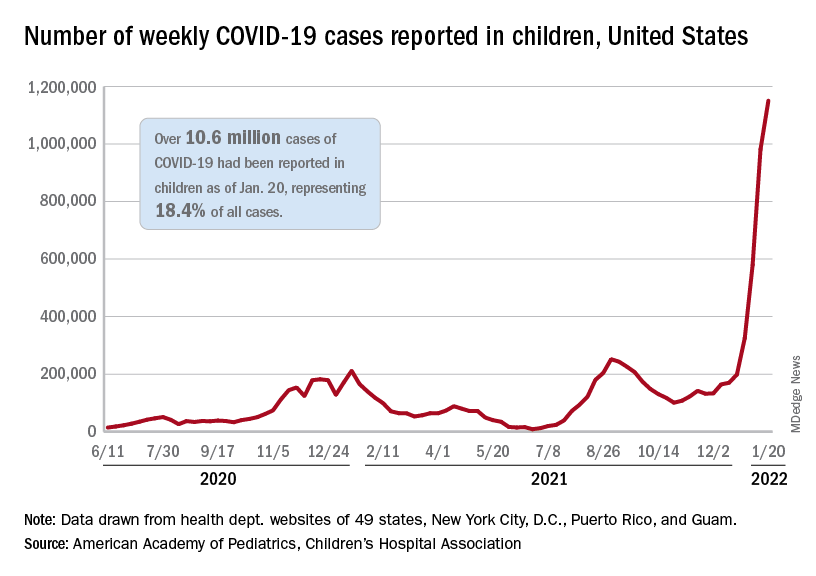
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.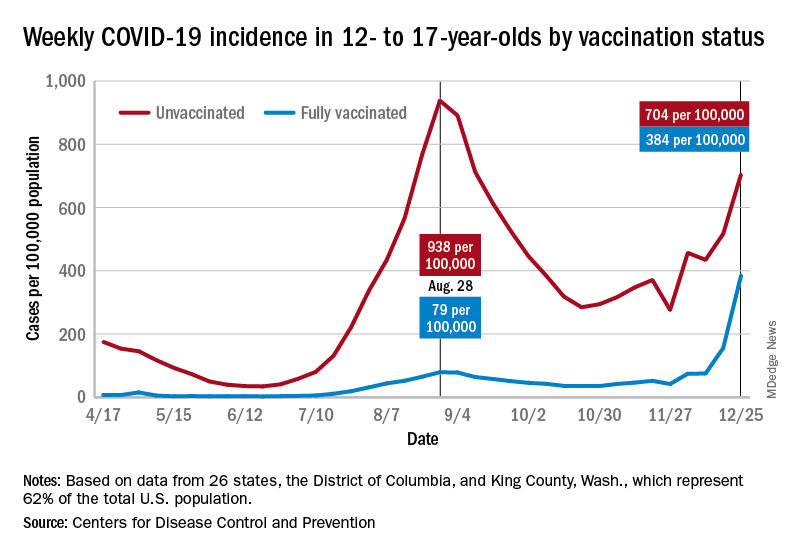
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Oxaliplatin add-on not recommended for elderly with colon cancer
A Japanese study finds that the addition of oxaliplatin (Eloxatin, Sanofi-Aventis) to fluoropyrimidine with bevacizumab, does not extend progression-free survival in elderly patients with metastatic colorectal cancer and in fact, can lead to more severe adverse events.
The results of the trial were presented by Tetsuya Hamaguchi, MD, PhD, at the 2022 Gastrointestinal Cancers Symposium.
Fluoropyrimidine with oxaliplatin and bevacizumab is a standard intensive initial therapy for metastatic colorectal cancer, but how well this combination works in elderly patients isn’t known because few clinical trials include senior-aged patients. It is known that the combination of fluoropyrimidine and bevacizumab can lead to significantly longer progression-free survival in elderly patients with metastatic colorectal cancer.
Dr. Hamaguchi, an oncologist with Saitama Medical University International Medical Center in Hidaka, Japan, included 251 patients (93% were at least 75 years old) with newly diagnosed metastatic colorectal cancer in a randomized, phase 3 trial of modified FOLFOX7 (folinic acid, fluorouracil, and oxaliplatin) or CapeOX (capecitabine and oxaliplatin) plus bevacizumab versus 5-fluorouracil/l-LV or capecitabine plus bevacizumab.
The patients were treated between September 2012 and March 2019. 125 patients received combination therapy and 126 received the addition of oxaliplatin. Researchers found the addition of oxaliplatin to fluoropyrimidine with bevacizumab did not extend progression-free survival and it was associated with more frequent and severe adverse events, including neutropenia, nausea, stomatitis, diarrhea, fatigue, sensory neuropathy, and hypertension.
Triggering sensory neuropathy
At issue is neuropathy caused by oxaliplatin. Grade 3 neuropathy can occur in 25% of patients after 12 cycles of FOLFOX. It has also been recorded in more than 20% of patients at 18 months, according to Gabriel Brooks, MD, an oncologist with the Dartmouth-Hitchcock Norris Cotton Cancer Center, Lebanon, N.H., who served as the discussant for the oral abstract session featuring the study presented by Dr. Hamaguchi.
“Even if we carefully ask our patients about their symptoms, and even if we stop oxaliplatin as soon as they have evidence of grade 2 neuropathy, many of those patients who didn’t have any neuropathy when the oxaliplatin is stopped will go on to develop it. And many of those patients who have grade 2 neuropathy will have worsening of their neuropathy and it will become a grade 3 neuropathy, even if we try to stop conscientiously,” Dr. Brooks said during his presentation.
The MOSAIC trial demonstrated that oxaliplatin improved disease-free and overall survival when added to 5-fluorouracil in the adjuvant treatment of stage II/III colon cancer. However, neuropathy concerns led to studies examining shortened regimens in an effort to reduce neuropathy incidence. In 2018, the IDEA study compared 3 months versus 6 months of oxaliplatin with fluoropyrimidine as adjuvant therapy for stage III colon cancer. The study found that the 6-month regimen was superior with respect to disease-free survival, but the benefit was very small, according to Dr. Brooks.
“What was not small was the difference in the incidence of [grade] 3-4 neuropathy, which was only 2.5% among patients who received 3 months of adjuvant chemotherapy with oxaliplatin, and it was 15.9% among people randomized to 6 months of adjuvant chemotherapy,” he said. The same study suggested that 3 months of the CapeOX regimen is noninferior to 6 months.
The new study included patients aged 70-74 years with Performance Status 2, or 75 or older with PS 0-2. They were randomized to receive fluoropyrimidine plus bevacizumab (arm A) or fluoropyrimidine, bevacizumab, and oxaliplatin (arm B). The study had to be terminated because of poor accrual, and the study was later amended to include a smaller required sample size. After a recruitment of 251 patients and a 2-year minimum follow-up, there was no significant difference in progression-free survival between the two groups (hazard ratio, 0.837; one-sided P = .086), overall survival, or objective response rate.
Adverse events were more common in the oxaliplatin group, including neutropenia (24% vs. 15%), nausea (22% vs. 10%), diarrhea (16% vs. 7%), fatigue (32% vs. 21%), and sensory neuropathy (57% vs. 15%).
Subgroup analyses did identify a subgroup of patients with KRAS wild type benefited from oxaliplatin (HR, 0.578; 95% confidence interval, 0.380-0.879), and Dr. Brooks said that this echoes findings from other studies.
Dr. Brooks advocated for deintensification of oxaliplatin. “The benefits of oxaliplatin are less than are often assumed in multiple settings, and the harms of oxaliplatin are well described. We are probably overly aggressive in our use of oxaliplatin. I submit that we should use oxaliplatin cautiously and sparingly in a couple of situations: We should use it very cautiously and sparingly beyond the third month of adjuvant therapy for colon cancer, and we should use it cautiously and sparingly in elderly or frail patients with metastatic colorectal cancer.”
The study was funded, in part, by the National Cancer Center Research and Development Fund, Grant-in-Aid for Clinical Cancer Research, and the Agency for Medical Research and Development in Japan. Dr. Brooks has consulted for CareCentrix, Ipsen, and UnitedHealthcare and has received research funding from Boston Biomedical and Roche/Genentech. Dr. Hamaguchi has received honoraria from Bayer, Bristol-Myers Squibb Japan, and other pharmaceutical companies. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A Japanese study finds that the addition of oxaliplatin (Eloxatin, Sanofi-Aventis) to fluoropyrimidine with bevacizumab, does not extend progression-free survival in elderly patients with metastatic colorectal cancer and in fact, can lead to more severe adverse events.
The results of the trial were presented by Tetsuya Hamaguchi, MD, PhD, at the 2022 Gastrointestinal Cancers Symposium.
Fluoropyrimidine with oxaliplatin and bevacizumab is a standard intensive initial therapy for metastatic colorectal cancer, but how well this combination works in elderly patients isn’t known because few clinical trials include senior-aged patients. It is known that the combination of fluoropyrimidine and bevacizumab can lead to significantly longer progression-free survival in elderly patients with metastatic colorectal cancer.
Dr. Hamaguchi, an oncologist with Saitama Medical University International Medical Center in Hidaka, Japan, included 251 patients (93% were at least 75 years old) with newly diagnosed metastatic colorectal cancer in a randomized, phase 3 trial of modified FOLFOX7 (folinic acid, fluorouracil, and oxaliplatin) or CapeOX (capecitabine and oxaliplatin) plus bevacizumab versus 5-fluorouracil/l-LV or capecitabine plus bevacizumab.
The patients were treated between September 2012 and March 2019. 125 patients received combination therapy and 126 received the addition of oxaliplatin. Researchers found the addition of oxaliplatin to fluoropyrimidine with bevacizumab did not extend progression-free survival and it was associated with more frequent and severe adverse events, including neutropenia, nausea, stomatitis, diarrhea, fatigue, sensory neuropathy, and hypertension.
Triggering sensory neuropathy
At issue is neuropathy caused by oxaliplatin. Grade 3 neuropathy can occur in 25% of patients after 12 cycles of FOLFOX. It has also been recorded in more than 20% of patients at 18 months, according to Gabriel Brooks, MD, an oncologist with the Dartmouth-Hitchcock Norris Cotton Cancer Center, Lebanon, N.H., who served as the discussant for the oral abstract session featuring the study presented by Dr. Hamaguchi.
“Even if we carefully ask our patients about their symptoms, and even if we stop oxaliplatin as soon as they have evidence of grade 2 neuropathy, many of those patients who didn’t have any neuropathy when the oxaliplatin is stopped will go on to develop it. And many of those patients who have grade 2 neuropathy will have worsening of their neuropathy and it will become a grade 3 neuropathy, even if we try to stop conscientiously,” Dr. Brooks said during his presentation.
The MOSAIC trial demonstrated that oxaliplatin improved disease-free and overall survival when added to 5-fluorouracil in the adjuvant treatment of stage II/III colon cancer. However, neuropathy concerns led to studies examining shortened regimens in an effort to reduce neuropathy incidence. In 2018, the IDEA study compared 3 months versus 6 months of oxaliplatin with fluoropyrimidine as adjuvant therapy for stage III colon cancer. The study found that the 6-month regimen was superior with respect to disease-free survival, but the benefit was very small, according to Dr. Brooks.
“What was not small was the difference in the incidence of [grade] 3-4 neuropathy, which was only 2.5% among patients who received 3 months of adjuvant chemotherapy with oxaliplatin, and it was 15.9% among people randomized to 6 months of adjuvant chemotherapy,” he said. The same study suggested that 3 months of the CapeOX regimen is noninferior to 6 months.
The new study included patients aged 70-74 years with Performance Status 2, or 75 or older with PS 0-2. They were randomized to receive fluoropyrimidine plus bevacizumab (arm A) or fluoropyrimidine, bevacizumab, and oxaliplatin (arm B). The study had to be terminated because of poor accrual, and the study was later amended to include a smaller required sample size. After a recruitment of 251 patients and a 2-year minimum follow-up, there was no significant difference in progression-free survival between the two groups (hazard ratio, 0.837; one-sided P = .086), overall survival, or objective response rate.
Adverse events were more common in the oxaliplatin group, including neutropenia (24% vs. 15%), nausea (22% vs. 10%), diarrhea (16% vs. 7%), fatigue (32% vs. 21%), and sensory neuropathy (57% vs. 15%).
Subgroup analyses did identify a subgroup of patients with KRAS wild type benefited from oxaliplatin (HR, 0.578; 95% confidence interval, 0.380-0.879), and Dr. Brooks said that this echoes findings from other studies.
Dr. Brooks advocated for deintensification of oxaliplatin. “The benefits of oxaliplatin are less than are often assumed in multiple settings, and the harms of oxaliplatin are well described. We are probably overly aggressive in our use of oxaliplatin. I submit that we should use oxaliplatin cautiously and sparingly in a couple of situations: We should use it very cautiously and sparingly beyond the third month of adjuvant therapy for colon cancer, and we should use it cautiously and sparingly in elderly or frail patients with metastatic colorectal cancer.”
The study was funded, in part, by the National Cancer Center Research and Development Fund, Grant-in-Aid for Clinical Cancer Research, and the Agency for Medical Research and Development in Japan. Dr. Brooks has consulted for CareCentrix, Ipsen, and UnitedHealthcare and has received research funding from Boston Biomedical and Roche/Genentech. Dr. Hamaguchi has received honoraria from Bayer, Bristol-Myers Squibb Japan, and other pharmaceutical companies. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A Japanese study finds that the addition of oxaliplatin (Eloxatin, Sanofi-Aventis) to fluoropyrimidine with bevacizumab, does not extend progression-free survival in elderly patients with metastatic colorectal cancer and in fact, can lead to more severe adverse events.
The results of the trial were presented by Tetsuya Hamaguchi, MD, PhD, at the 2022 Gastrointestinal Cancers Symposium.
Fluoropyrimidine with oxaliplatin and bevacizumab is a standard intensive initial therapy for metastatic colorectal cancer, but how well this combination works in elderly patients isn’t known because few clinical trials include senior-aged patients. It is known that the combination of fluoropyrimidine and bevacizumab can lead to significantly longer progression-free survival in elderly patients with metastatic colorectal cancer.
Dr. Hamaguchi, an oncologist with Saitama Medical University International Medical Center in Hidaka, Japan, included 251 patients (93% were at least 75 years old) with newly diagnosed metastatic colorectal cancer in a randomized, phase 3 trial of modified FOLFOX7 (folinic acid, fluorouracil, and oxaliplatin) or CapeOX (capecitabine and oxaliplatin) plus bevacizumab versus 5-fluorouracil/l-LV or capecitabine plus bevacizumab.
The patients were treated between September 2012 and March 2019. 125 patients received combination therapy and 126 received the addition of oxaliplatin. Researchers found the addition of oxaliplatin to fluoropyrimidine with bevacizumab did not extend progression-free survival and it was associated with more frequent and severe adverse events, including neutropenia, nausea, stomatitis, diarrhea, fatigue, sensory neuropathy, and hypertension.
Triggering sensory neuropathy
At issue is neuropathy caused by oxaliplatin. Grade 3 neuropathy can occur in 25% of patients after 12 cycles of FOLFOX. It has also been recorded in more than 20% of patients at 18 months, according to Gabriel Brooks, MD, an oncologist with the Dartmouth-Hitchcock Norris Cotton Cancer Center, Lebanon, N.H., who served as the discussant for the oral abstract session featuring the study presented by Dr. Hamaguchi.
“Even if we carefully ask our patients about their symptoms, and even if we stop oxaliplatin as soon as they have evidence of grade 2 neuropathy, many of those patients who didn’t have any neuropathy when the oxaliplatin is stopped will go on to develop it. And many of those patients who have grade 2 neuropathy will have worsening of their neuropathy and it will become a grade 3 neuropathy, even if we try to stop conscientiously,” Dr. Brooks said during his presentation.
The MOSAIC trial demonstrated that oxaliplatin improved disease-free and overall survival when added to 5-fluorouracil in the adjuvant treatment of stage II/III colon cancer. However, neuropathy concerns led to studies examining shortened regimens in an effort to reduce neuropathy incidence. In 2018, the IDEA study compared 3 months versus 6 months of oxaliplatin with fluoropyrimidine as adjuvant therapy for stage III colon cancer. The study found that the 6-month regimen was superior with respect to disease-free survival, but the benefit was very small, according to Dr. Brooks.
“What was not small was the difference in the incidence of [grade] 3-4 neuropathy, which was only 2.5% among patients who received 3 months of adjuvant chemotherapy with oxaliplatin, and it was 15.9% among people randomized to 6 months of adjuvant chemotherapy,” he said. The same study suggested that 3 months of the CapeOX regimen is noninferior to 6 months.
The new study included patients aged 70-74 years with Performance Status 2, or 75 or older with PS 0-2. They were randomized to receive fluoropyrimidine plus bevacizumab (arm A) or fluoropyrimidine, bevacizumab, and oxaliplatin (arm B). The study had to be terminated because of poor accrual, and the study was later amended to include a smaller required sample size. After a recruitment of 251 patients and a 2-year minimum follow-up, there was no significant difference in progression-free survival between the two groups (hazard ratio, 0.837; one-sided P = .086), overall survival, or objective response rate.
Adverse events were more common in the oxaliplatin group, including neutropenia (24% vs. 15%), nausea (22% vs. 10%), diarrhea (16% vs. 7%), fatigue (32% vs. 21%), and sensory neuropathy (57% vs. 15%).
Subgroup analyses did identify a subgroup of patients with KRAS wild type benefited from oxaliplatin (HR, 0.578; 95% confidence interval, 0.380-0.879), and Dr. Brooks said that this echoes findings from other studies.
Dr. Brooks advocated for deintensification of oxaliplatin. “The benefits of oxaliplatin are less than are often assumed in multiple settings, and the harms of oxaliplatin are well described. We are probably overly aggressive in our use of oxaliplatin. I submit that we should use oxaliplatin cautiously and sparingly in a couple of situations: We should use it very cautiously and sparingly beyond the third month of adjuvant therapy for colon cancer, and we should use it cautiously and sparingly in elderly or frail patients with metastatic colorectal cancer.”
The study was funded, in part, by the National Cancer Center Research and Development Fund, Grant-in-Aid for Clinical Cancer Research, and the Agency for Medical Research and Development in Japan. Dr. Brooks has consulted for CareCentrix, Ipsen, and UnitedHealthcare and has received research funding from Boston Biomedical and Roche/Genentech. Dr. Hamaguchi has received honoraria from Bayer, Bristol-Myers Squibb Japan, and other pharmaceutical companies. The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
FROM GI CANCERS SYMPOSIUM 2022
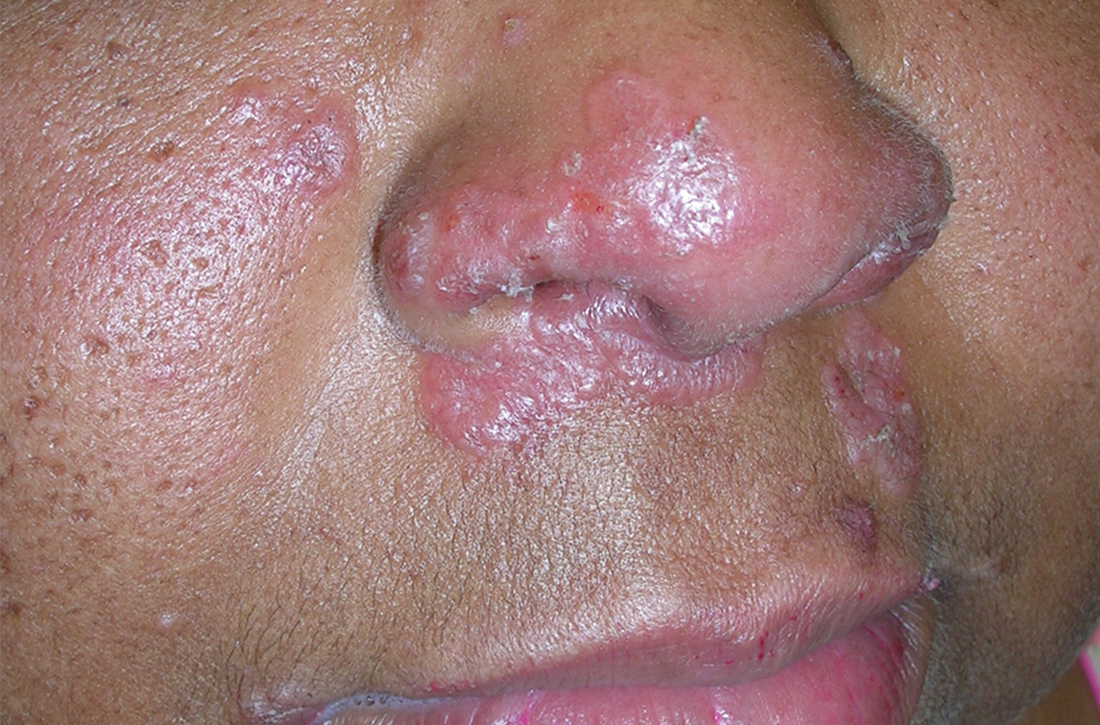
Sarcoidosis
THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
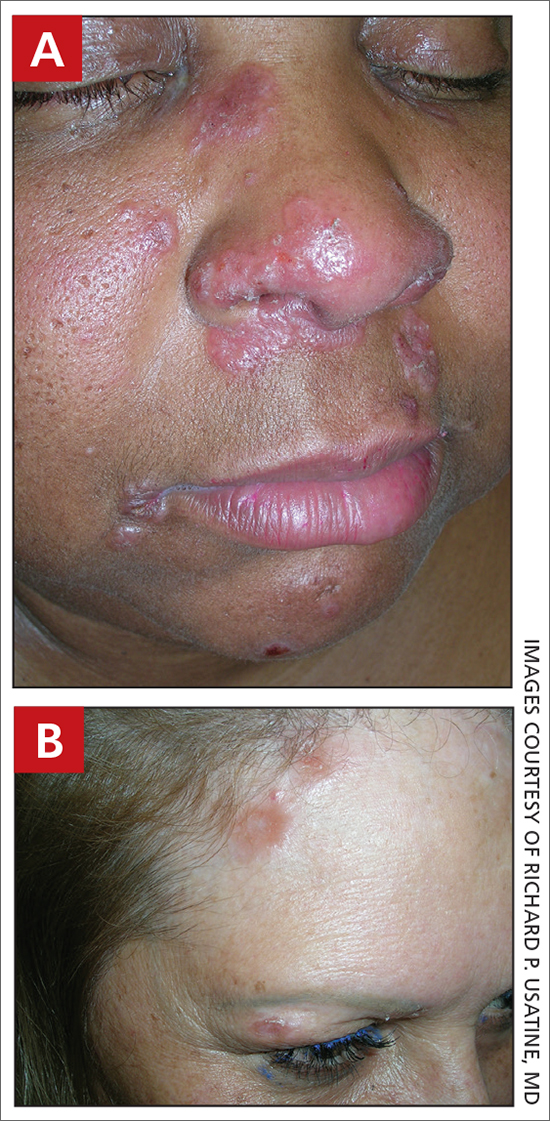
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous.
• In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjustment for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
1. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
2. Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66: 121.e1-121.e14.
3. Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/ cells10040766
4. Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/ oxfordjournals.aje.a009096
5. Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
6. Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of features in 170 patients. Respir Med. 2003;97:978-982.
7. Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
8. Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
9. Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
10. Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
11. James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
12. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
13. Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
14. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
15. Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
16. Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
17. Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
18. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
19. Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
20. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.

Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous.
• In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjustment for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.

Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous.
• In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjustment for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
1. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
2. Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66: 121.e1-121.e14.
3. Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/ cells10040766
4. Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/ oxfordjournals.aje.a009096
5. Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
6. Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of features in 170 patients. Respir Med. 2003;97:978-982.
7. Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
8. Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
9. Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
10. Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
11. James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
12. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
13. Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
14. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
15. Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
16. Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
17. Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
18. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
19. Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
20. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
1. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
2. Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66: 121.e1-121.e14.
3. Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/ cells10040766
4. Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/ oxfordjournals.aje.a009096
5. Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
6. Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of features in 170 patients. Respir Med. 2003;97:978-982.
7. Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
8. Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
9. Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
10. Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
11. James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
12. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
13. Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
14. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
15. Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
16. Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
17. Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
18. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
19. Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
20. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
COVID brain fog is a ‘true neurologic condition’
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF CLINICAL AND TRANSLATIONAL NEUROLOGY
Watch, but don’t worry yet, about new Omicron subvariant
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
Is it time to approach spontaneous pneumothorax more conservatively?
ILLUSTRATIVE CASE
A 26-year-old man presents to the emergency department complaining of sudden-onset left-side chest pain and mild dyspnea that started while he was playing basketball. He denies any medical problems and takes no medications. He is able to speak in complete sentences as he answers your questions. His O2 saturation is 95% and a chest x-ray reveals a left-side, moderate-to-large pneumothorax.
A primary spontaneous pneumothorax is one that occurs in the absence of underlying clinical lung disease and is not associated with an inciting cause, such as a rib fracture.2 In the United States, the estimated incidence of primary spontaneous pneumothorax is 7.4 cases per 100,000 men and 1.2 cases per 100,000 women.3 The etiology is often unknown, but it is associated with several risk factors, including male sex, smoking, and a tall, thin body habitus.2
The management strategy for stable patients with a primary spontaneous pneumothorax largely depends on pneumothorax size and institutional practice. Multiple methods define pneumothorax size; the US standard cutoff for a small or large pneumothorax is 3 cm, between the pleural line and chest wall at the level of the apex,4 compared with 2 cm in Europe, when evaluating the distance at the hilum in an upright chest radiograph.5 The Collins method uses a formula to calculate the percentage of lung area affected based on 3 distinct measurements on a posterior/anterior upright chest radiograph.6
Management options include observation, supplemental oxygen, simple aspiration, and thoracostomy or chest tube placement. British Thoracic Society guidelines published in 2010 state that only a small pneumothorax can be managed conservatively with observation alone; for a large pneumothorax, the guidelines recommend needle aspiration to achieve lung reinflation, followed by chest tube placement if unsuccessful.5
In practice, management of a large primary spontaneous pneumothorax varies, but the most common treatment is chest tube placement.7 This procedure can be painful and may result in complications such as bleeding, infection, injury to internal structures, or the need for surgical intervention.7 In addition, once a chest tube is placed, hospital admission ensues, lasting an average of 4 days.8 Given these consequences, there is a need for safe and feasible treatment options for a large primary spontaneous pneumothorax.
STUDY SUMMARY
Observational management judged noninferior, with multiple advantages
The Primary Spontaneous Pneumothorax (PSP) trial was a prospective noninferiority trial conducted at 39 hospitals in Australia and New Zealand. This randomized controlled trial compared observational (“watch and wait”) vs interventional (chest tube placement) management of uncomplicated, unilateral, primary spontaneous pneumothorax. Patients ages 14 to 50 years with a moderate-to-large pneumothorax—32% or greater, as defined by the Collins method4—were randomly assigned to a study group to examine the primary outcome of lung reexpansion at 8 weeks.
The intervention included chest tube insertion attached to an underwater seal without suction for 1 hour, followed by an x-ray and clamping for 4 hours if there was no air leak, followed by a repeat chest x-ray. If there was no evidence of radiographic resolution, or if during observation the pneumothorax recurred, the underwater seal was recommenced and the patient was admitted to the hospital, with further intervention at the discretion of the inpatient clinicians. If radiographic improvement was seen, the tube was removed and the patient discharged.
Continue to: In contrast...
In contrast, conservative management entailed patient observation for at least 4 hours followed by a repeat chest x-ray. If after the observation period, patients were walking comfortably and without supplemental oxygen, they were discharged. Patients in the observation group underwent an intervention if they met a variety of criteria, including unstable vitals or an enlarging pneumothorax. All patients received standard care with analgesia and supplemental oxygen as needed.
A total of 316 patients were randomized, with 154 assigned to the intervention group and 162 to the observation group. The mean age for all participants was 26. Most patients were male (84.4% in the intervention group and 87.7% in the observation group) and almost half were current smokers (49.3% in the intervention and 42.5% in the observation group). The mean body mass index of participants was 21.4 in the intervention and 21.3 in the observation group. Twenty-five patients (15%) in the observation group underwent interventions for reasons specified in the research protocol (eg, “significant symptoms” such as abnormal physiologic observations and intolerable symptoms, or patient unwillingness to continue in the assigned group), and 10 patients assigned to the intervention group declined treatment.
Using a complete-case analysis, 129 of 131 patients (98.5%) in the intervention group and 118 of 125 patients (94.4%) in the observation group met the primary outcome of radiographic resolution within 8 weeks (risk difference [RD] = –4.1%; 95% CI, –8.6 to 0.5), thereby falling within the prespecified margin for noninferiority of less than 9%.
Per-protocol analysis at 8 weeks also proved observational management noninferior, with 124 of 126 patients (98.4%) in the intervention group and 123 of 130 patients (94.6%) in the observation group achieving lung reexpansion within 8 weeks (RD = –3.8%; 95% CI, –8.3 to 0.7). The time to symptom resolution was similar between groups, with a median time of 15.5 days in the intervention group compared with 14 days in the observation group (hazard ratio = 1.11; 95% CI, 0.88-1.4). A lower risk of serious adverse events (relative risk [RR] = 3.3; 95% CI, 1.37-8.1) and pneumothorax recurrence (absolute RD = 8%; 95% CI, 0.5-15.4) occurred in the observation group vs the intervention group. The average length of hospital stay for patients in the intervention group was 6.1 days, vs 1.6 days in the observation group (RR = 2.8; 95% CI, 1.8-3.6).
Two additional sensitivity analyses were performed because multiple study participants were lost to follow-up or had data collected after 8 weeks. Noninferiority was maintained when data collected after the 8-week visit were included and extended to 63 days (RD = –3.7%: 95% CI, –7.9 to 0.6). However, noninferiority was lost when missing data after 8 weeks were deemed “treatment failure” (RD = –11%; 95% CI, –18.4 to –3.5).
Continue to: WHAT'S NEW
WHAT’S NEW
Conservative management enabled most patients to avoid invasive Tx risks
In this specific patient population, conservative management of primary spontaneous pneumothorax was noninferior to interventional management and had a lower risk of serious adverse events. This management practice spared 85% of the patients from invasive intervention. As a result, they experienced a shortened hospital stay, fewer days missed from school or work, less exposure to radiation from repeat chest x-rays, and a lower rate of adverse events. Additionally, fewer of these patients had early pneumothorax recurrence.
CAVEATS
There were limitations in the trial’s original statistical design
This study had a specific follow-up timetable, and some of the participants were not examined until after the 8-week checkpoint or were lost to follow-up entirely. The authors attempted to address these limitations (and show transparency) by providing additional sensitivity analyses as well as providing the intention-to-treat and per-protocol analyses for the primary outcome at 8 weeks. Noninferiority was maintained in all analyses except for the sensitivity analysis that treated missing data as treatment failure. Therefore, the authors note these approaches result in “statistical fragility” and are exploratory.
CHALLENGES TO IMPLEMENTATION
Pneumothorax is not commonly seen in outpatient settings
Family physicians working in outpatient settings generally do not encounter pneumothorax and, using current guidelines, would refer for emergency or inpatient care. This study opens the possibility of managing selected patients in an outpatient setting; however, this would require at least a 4-hour period of observation, which may be impractical for many outpatient-based physicians. Additionally, the study uses the Collins method to define moderate-to-large pneumothorax, which is likely an uncommon practice and thus not applicable in most primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Brown SGA, Ball EL, Perrin K, et al; PSP Investigators. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405-415. doi: 10.1056/NEJMoa1910775
2. Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the incidence and recurrence of inpatient-treated spontaneous pneumothorax, 1968-2016. JAMA. 2018;320:1471-1480. doi: 10.1001/jama.2018.14299
3. Melton LJ III, Hepper NGG, Offord KP. Incidence of spontaneous pneumothorax in Olmstead County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379-1382. doi: 10.1164/arrd.1979.120.6.1379
4. Baumann MH, Strange C, Heffner JE, et al; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602. doi: 10.1378/chest.119.2.590
5. MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl):ii18-ii31. doi: 10.1136/thx.2010.136986
6. Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. Am J Roentgenol. 1995;165:1127-1130. doi: 10.2214/ajr.165.5.7572489
7. Kwiatt M, Tarbox A, Seamon MJ, et al. Thoracostomy tubes: a comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143-155. doi: 10.4103/2229-5151.134182
8. Maskell NA, Medford A, Gleeson FV. Seldinger chest drain insertion: simpler but not necessarily safer. Thorax. 2010;65:5-6. doi: 10.1136/thx.2009.117200
ILLUSTRATIVE CASE
A 26-year-old man presents to the emergency department complaining of sudden-onset left-side chest pain and mild dyspnea that started while he was playing basketball. He denies any medical problems and takes no medications. He is able to speak in complete sentences as he answers your questions. His O2 saturation is 95% and a chest x-ray reveals a left-side, moderate-to-large pneumothorax.
A primary spontaneous pneumothorax is one that occurs in the absence of underlying clinical lung disease and is not associated with an inciting cause, such as a rib fracture.2 In the United States, the estimated incidence of primary spontaneous pneumothorax is 7.4 cases per 100,000 men and 1.2 cases per 100,000 women.3 The etiology is often unknown, but it is associated with several risk factors, including male sex, smoking, and a tall, thin body habitus.2
The management strategy for stable patients with a primary spontaneous pneumothorax largely depends on pneumothorax size and institutional practice. Multiple methods define pneumothorax size; the US standard cutoff for a small or large pneumothorax is 3 cm, between the pleural line and chest wall at the level of the apex,4 compared with 2 cm in Europe, when evaluating the distance at the hilum in an upright chest radiograph.5 The Collins method uses a formula to calculate the percentage of lung area affected based on 3 distinct measurements on a posterior/anterior upright chest radiograph.6
Management options include observation, supplemental oxygen, simple aspiration, and thoracostomy or chest tube placement. British Thoracic Society guidelines published in 2010 state that only a small pneumothorax can be managed conservatively with observation alone; for a large pneumothorax, the guidelines recommend needle aspiration to achieve lung reinflation, followed by chest tube placement if unsuccessful.5
In practice, management of a large primary spontaneous pneumothorax varies, but the most common treatment is chest tube placement.7 This procedure can be painful and may result in complications such as bleeding, infection, injury to internal structures, or the need for surgical intervention.7 In addition, once a chest tube is placed, hospital admission ensues, lasting an average of 4 days.8 Given these consequences, there is a need for safe and feasible treatment options for a large primary spontaneous pneumothorax.
STUDY SUMMARY
Observational management judged noninferior, with multiple advantages
The Primary Spontaneous Pneumothorax (PSP) trial was a prospective noninferiority trial conducted at 39 hospitals in Australia and New Zealand. This randomized controlled trial compared observational (“watch and wait”) vs interventional (chest tube placement) management of uncomplicated, unilateral, primary spontaneous pneumothorax. Patients ages 14 to 50 years with a moderate-to-large pneumothorax—32% or greater, as defined by the Collins method4—were randomly assigned to a study group to examine the primary outcome of lung reexpansion at 8 weeks.
The intervention included chest tube insertion attached to an underwater seal without suction for 1 hour, followed by an x-ray and clamping for 4 hours if there was no air leak, followed by a repeat chest x-ray. If there was no evidence of radiographic resolution, or if during observation the pneumothorax recurred, the underwater seal was recommenced and the patient was admitted to the hospital, with further intervention at the discretion of the inpatient clinicians. If radiographic improvement was seen, the tube was removed and the patient discharged.
Continue to: In contrast...
In contrast, conservative management entailed patient observation for at least 4 hours followed by a repeat chest x-ray. If after the observation period, patients were walking comfortably and without supplemental oxygen, they were discharged. Patients in the observation group underwent an intervention if they met a variety of criteria, including unstable vitals or an enlarging pneumothorax. All patients received standard care with analgesia and supplemental oxygen as needed.
A total of 316 patients were randomized, with 154 assigned to the intervention group and 162 to the observation group. The mean age for all participants was 26. Most patients were male (84.4% in the intervention group and 87.7% in the observation group) and almost half were current smokers (49.3% in the intervention and 42.5% in the observation group). The mean body mass index of participants was 21.4 in the intervention and 21.3 in the observation group. Twenty-five patients (15%) in the observation group underwent interventions for reasons specified in the research protocol (eg, “significant symptoms” such as abnormal physiologic observations and intolerable symptoms, or patient unwillingness to continue in the assigned group), and 10 patients assigned to the intervention group declined treatment.
Using a complete-case analysis, 129 of 131 patients (98.5%) in the intervention group and 118 of 125 patients (94.4%) in the observation group met the primary outcome of radiographic resolution within 8 weeks (risk difference [RD] = –4.1%; 95% CI, –8.6 to 0.5), thereby falling within the prespecified margin for noninferiority of less than 9%.
Per-protocol analysis at 8 weeks also proved observational management noninferior, with 124 of 126 patients (98.4%) in the intervention group and 123 of 130 patients (94.6%) in the observation group achieving lung reexpansion within 8 weeks (RD = –3.8%; 95% CI, –8.3 to 0.7). The time to symptom resolution was similar between groups, with a median time of 15.5 days in the intervention group compared with 14 days in the observation group (hazard ratio = 1.11; 95% CI, 0.88-1.4). A lower risk of serious adverse events (relative risk [RR] = 3.3; 95% CI, 1.37-8.1) and pneumothorax recurrence (absolute RD = 8%; 95% CI, 0.5-15.4) occurred in the observation group vs the intervention group. The average length of hospital stay for patients in the intervention group was 6.1 days, vs 1.6 days in the observation group (RR = 2.8; 95% CI, 1.8-3.6).
Two additional sensitivity analyses were performed because multiple study participants were lost to follow-up or had data collected after 8 weeks. Noninferiority was maintained when data collected after the 8-week visit were included and extended to 63 days (RD = –3.7%: 95% CI, –7.9 to 0.6). However, noninferiority was lost when missing data after 8 weeks were deemed “treatment failure” (RD = –11%; 95% CI, –18.4 to –3.5).
Continue to: WHAT'S NEW
WHAT’S NEW
Conservative management enabled most patients to avoid invasive Tx risks
In this specific patient population, conservative management of primary spontaneous pneumothorax was noninferior to interventional management and had a lower risk of serious adverse events. This management practice spared 85% of the patients from invasive intervention. As a result, they experienced a shortened hospital stay, fewer days missed from school or work, less exposure to radiation from repeat chest x-rays, and a lower rate of adverse events. Additionally, fewer of these patients had early pneumothorax recurrence.
CAVEATS
There were limitations in the trial’s original statistical design
This study had a specific follow-up timetable, and some of the participants were not examined until after the 8-week checkpoint or were lost to follow-up entirely. The authors attempted to address these limitations (and show transparency) by providing additional sensitivity analyses as well as providing the intention-to-treat and per-protocol analyses for the primary outcome at 8 weeks. Noninferiority was maintained in all analyses except for the sensitivity analysis that treated missing data as treatment failure. Therefore, the authors note these approaches result in “statistical fragility” and are exploratory.
CHALLENGES TO IMPLEMENTATION
Pneumothorax is not commonly seen in outpatient settings
Family physicians working in outpatient settings generally do not encounter pneumothorax and, using current guidelines, would refer for emergency or inpatient care. This study opens the possibility of managing selected patients in an outpatient setting; however, this would require at least a 4-hour period of observation, which may be impractical for many outpatient-based physicians. Additionally, the study uses the Collins method to define moderate-to-large pneumothorax, which is likely an uncommon practice and thus not applicable in most primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 26-year-old man presents to the emergency department complaining of sudden-onset left-side chest pain and mild dyspnea that started while he was playing basketball. He denies any medical problems and takes no medications. He is able to speak in complete sentences as he answers your questions. His O2 saturation is 95% and a chest x-ray reveals a left-side, moderate-to-large pneumothorax.
A primary spontaneous pneumothorax is one that occurs in the absence of underlying clinical lung disease and is not associated with an inciting cause, such as a rib fracture.2 In the United States, the estimated incidence of primary spontaneous pneumothorax is 7.4 cases per 100,000 men and 1.2 cases per 100,000 women.3 The etiology is often unknown, but it is associated with several risk factors, including male sex, smoking, and a tall, thin body habitus.2
The management strategy for stable patients with a primary spontaneous pneumothorax largely depends on pneumothorax size and institutional practice. Multiple methods define pneumothorax size; the US standard cutoff for a small or large pneumothorax is 3 cm, between the pleural line and chest wall at the level of the apex,4 compared with 2 cm in Europe, when evaluating the distance at the hilum in an upright chest radiograph.5 The Collins method uses a formula to calculate the percentage of lung area affected based on 3 distinct measurements on a posterior/anterior upright chest radiograph.6
Management options include observation, supplemental oxygen, simple aspiration, and thoracostomy or chest tube placement. British Thoracic Society guidelines published in 2010 state that only a small pneumothorax can be managed conservatively with observation alone; for a large pneumothorax, the guidelines recommend needle aspiration to achieve lung reinflation, followed by chest tube placement if unsuccessful.5
In practice, management of a large primary spontaneous pneumothorax varies, but the most common treatment is chest tube placement.7 This procedure can be painful and may result in complications such as bleeding, infection, injury to internal structures, or the need for surgical intervention.7 In addition, once a chest tube is placed, hospital admission ensues, lasting an average of 4 days.8 Given these consequences, there is a need for safe and feasible treatment options for a large primary spontaneous pneumothorax.
STUDY SUMMARY
Observational management judged noninferior, with multiple advantages
The Primary Spontaneous Pneumothorax (PSP) trial was a prospective noninferiority trial conducted at 39 hospitals in Australia and New Zealand. This randomized controlled trial compared observational (“watch and wait”) vs interventional (chest tube placement) management of uncomplicated, unilateral, primary spontaneous pneumothorax. Patients ages 14 to 50 years with a moderate-to-large pneumothorax—32% or greater, as defined by the Collins method4—were randomly assigned to a study group to examine the primary outcome of lung reexpansion at 8 weeks.
The intervention included chest tube insertion attached to an underwater seal without suction for 1 hour, followed by an x-ray and clamping for 4 hours if there was no air leak, followed by a repeat chest x-ray. If there was no evidence of radiographic resolution, or if during observation the pneumothorax recurred, the underwater seal was recommenced and the patient was admitted to the hospital, with further intervention at the discretion of the inpatient clinicians. If radiographic improvement was seen, the tube was removed and the patient discharged.
Continue to: In contrast...
In contrast, conservative management entailed patient observation for at least 4 hours followed by a repeat chest x-ray. If after the observation period, patients were walking comfortably and without supplemental oxygen, they were discharged. Patients in the observation group underwent an intervention if they met a variety of criteria, including unstable vitals or an enlarging pneumothorax. All patients received standard care with analgesia and supplemental oxygen as needed.
A total of 316 patients were randomized, with 154 assigned to the intervention group and 162 to the observation group. The mean age for all participants was 26. Most patients were male (84.4% in the intervention group and 87.7% in the observation group) and almost half were current smokers (49.3% in the intervention and 42.5% in the observation group). The mean body mass index of participants was 21.4 in the intervention and 21.3 in the observation group. Twenty-five patients (15%) in the observation group underwent interventions for reasons specified in the research protocol (eg, “significant symptoms” such as abnormal physiologic observations and intolerable symptoms, or patient unwillingness to continue in the assigned group), and 10 patients assigned to the intervention group declined treatment.
Using a complete-case analysis, 129 of 131 patients (98.5%) in the intervention group and 118 of 125 patients (94.4%) in the observation group met the primary outcome of radiographic resolution within 8 weeks (risk difference [RD] = –4.1%; 95% CI, –8.6 to 0.5), thereby falling within the prespecified margin for noninferiority of less than 9%.
Per-protocol analysis at 8 weeks also proved observational management noninferior, with 124 of 126 patients (98.4%) in the intervention group and 123 of 130 patients (94.6%) in the observation group achieving lung reexpansion within 8 weeks (RD = –3.8%; 95% CI, –8.3 to 0.7). The time to symptom resolution was similar between groups, with a median time of 15.5 days in the intervention group compared with 14 days in the observation group (hazard ratio = 1.11; 95% CI, 0.88-1.4). A lower risk of serious adverse events (relative risk [RR] = 3.3; 95% CI, 1.37-8.1) and pneumothorax recurrence (absolute RD = 8%; 95% CI, 0.5-15.4) occurred in the observation group vs the intervention group. The average length of hospital stay for patients in the intervention group was 6.1 days, vs 1.6 days in the observation group (RR = 2.8; 95% CI, 1.8-3.6).
Two additional sensitivity analyses were performed because multiple study participants were lost to follow-up or had data collected after 8 weeks. Noninferiority was maintained when data collected after the 8-week visit were included and extended to 63 days (RD = –3.7%: 95% CI, –7.9 to 0.6). However, noninferiority was lost when missing data after 8 weeks were deemed “treatment failure” (RD = –11%; 95% CI, –18.4 to –3.5).
Continue to: WHAT'S NEW
WHAT’S NEW
Conservative management enabled most patients to avoid invasive Tx risks
In this specific patient population, conservative management of primary spontaneous pneumothorax was noninferior to interventional management and had a lower risk of serious adverse events. This management practice spared 85% of the patients from invasive intervention. As a result, they experienced a shortened hospital stay, fewer days missed from school or work, less exposure to radiation from repeat chest x-rays, and a lower rate of adverse events. Additionally, fewer of these patients had early pneumothorax recurrence.
CAVEATS
There were limitations in the trial’s original statistical design
This study had a specific follow-up timetable, and some of the participants were not examined until after the 8-week checkpoint or were lost to follow-up entirely. The authors attempted to address these limitations (and show transparency) by providing additional sensitivity analyses as well as providing the intention-to-treat and per-protocol analyses for the primary outcome at 8 weeks. Noninferiority was maintained in all analyses except for the sensitivity analysis that treated missing data as treatment failure. Therefore, the authors note these approaches result in “statistical fragility” and are exploratory.
CHALLENGES TO IMPLEMENTATION
Pneumothorax is not commonly seen in outpatient settings
Family physicians working in outpatient settings generally do not encounter pneumothorax and, using current guidelines, would refer for emergency or inpatient care. This study opens the possibility of managing selected patients in an outpatient setting; however, this would require at least a 4-hour period of observation, which may be impractical for many outpatient-based physicians. Additionally, the study uses the Collins method to define moderate-to-large pneumothorax, which is likely an uncommon practice and thus not applicable in most primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Brown SGA, Ball EL, Perrin K, et al; PSP Investigators. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405-415. doi: 10.1056/NEJMoa1910775
2. Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the incidence and recurrence of inpatient-treated spontaneous pneumothorax, 1968-2016. JAMA. 2018;320:1471-1480. doi: 10.1001/jama.2018.14299
3. Melton LJ III, Hepper NGG, Offord KP. Incidence of spontaneous pneumothorax in Olmstead County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379-1382. doi: 10.1164/arrd.1979.120.6.1379
4. Baumann MH, Strange C, Heffner JE, et al; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602. doi: 10.1378/chest.119.2.590
5. MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl):ii18-ii31. doi: 10.1136/thx.2010.136986
6. Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. Am J Roentgenol. 1995;165:1127-1130. doi: 10.2214/ajr.165.5.7572489
7. Kwiatt M, Tarbox A, Seamon MJ, et al. Thoracostomy tubes: a comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143-155. doi: 10.4103/2229-5151.134182
8. Maskell NA, Medford A, Gleeson FV. Seldinger chest drain insertion: simpler but not necessarily safer. Thorax. 2010;65:5-6. doi: 10.1136/thx.2009.117200
1. Brown SGA, Ball EL, Perrin K, et al; PSP Investigators. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405-415. doi: 10.1056/NEJMoa1910775
2. Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the incidence and recurrence of inpatient-treated spontaneous pneumothorax, 1968-2016. JAMA. 2018;320:1471-1480. doi: 10.1001/jama.2018.14299
3. Melton LJ III, Hepper NGG, Offord KP. Incidence of spontaneous pneumothorax in Olmstead County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379-1382. doi: 10.1164/arrd.1979.120.6.1379
4. Baumann MH, Strange C, Heffner JE, et al; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602. doi: 10.1378/chest.119.2.590
5. MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl):ii18-ii31. doi: 10.1136/thx.2010.136986
6. Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. Am J Roentgenol. 1995;165:1127-1130. doi: 10.2214/ajr.165.5.7572489
7. Kwiatt M, Tarbox A, Seamon MJ, et al. Thoracostomy tubes: a comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143-155. doi: 10.4103/2229-5151.134182
8. Maskell NA, Medford A, Gleeson FV. Seldinger chest drain insertion: simpler but not necessarily safer. Thorax. 2010;65:5-6. doi: 10.1136/thx.2009.117200
PRACTICE CHANGER
Consider observation rather than chest tube placement for primary, uncomplicated, unilateral moderate-to-large spontaneous pneumothorax in patients ages 14 to 50.
STRENGTH OF RECOMMENDATION
B: Based on a single, lower-quality randomized controlled trial1
Brown SGA, Ball EL, Perrin K, et al; PSP Investigators. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405-415. doi: 10.1056/NEJMoa1910775
Medicare NCDs hinder access to cancer biomarker testing for minorities
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
of data from patients with advanced non–small cell lung cancer (aNSCLC), metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma. The finding was reported in JAMA Network Open.
Biomarker testing has become an essential tool in cancer care over the last decade. In 2011, for example, less than 1% of patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma underwent NGS testing, but by 2019, 40% of patients with these cancers received the testing.
“Next-generation sequencing testing has become increasingly important because it enables identification of multiple biomarkers simultaneously and efficiently while minimizing the number of biopsies required,” wrote the authors, led by William B. Wong, PharmD, of Genentech.
It has been unknown whether for Medicare beneficiaries and the overall population, if the NCD affected health equity issues, the authors wrote. While increased use of appropriate targeted therapies facilitated by NGS testing is associated with improved survival rates in patients with advanced or metastatic cancer, variability in health care coverage policies has posed a significant barrier to obtaining NGS testing for cancer patients, specifically through policy coverage limitations. It has remained unclear if the NCD has influenced NGS testing coverage in insurance types (for example, Medicaid) encompassing a larger population of minority racial and ethnic groups often experiencing poorer care and outcomes.
The retrospective cohort analysis compared EHR data from 280 U.S. cancer clinics in the (800 sites of care) pre- versus post-NCD period for patients with aNSCLC, metastatic colorectal cancer, metastatic breast cancer, or advanced melanoma (January 2011–March 2020). Nearly 70% of all patients in the study were Medicare recipients who needed NCD approval to cover the cost of testing.
Among 92,687 patients (mean age, 66.6 years; 55.7% women), compared with Medicare beneficiaries, changes in pre- to post-NCD NGS testing trends were similar in commercially insured patients (odds ratio, 1.03; 95% CI, 0.98-1.08; P = .25). Pre- to post-NCD NGS testing trends increased at a slower rate among patients in assistance programs (OR, 0.93; 95% CI, 0.87-0.99; P = .03), compared with Medicare beneficiaries. The rate of increase for patients receiving Medicaid was not significantly different statistically compared with those receiving Medicare (OR, 0.92; 95% CI, 0.84-1.01; P = .07). Also, the NCD was not associated with racial and ethnic groups within Medicare beneficiaries alone or across all insurance types.
Compared with non-Hispanic White individuals, increases in average NGS use from the pre-NCD to post-NCD period were 14% lower (OR, 0.86; 95% CI, 0.74-0.99; P = .04) among African American and 23% lower (OR, 0.77; 95% CI, 0.62-0.96; P = .02) among Hispanic/Latino individuals; increases were similar, however, among Asian individuals and other races and ethnicities.
The authors observed that the post-NCD trend of increasing NGS testing seen in Medicare beneficiaries was similarly observed in those with commercial insurance. Testing rate differences, however, widened or were maintained after versus before the NCD in PAP (personal assistance program) and Medicaid beneficiaries relative to Medicare beneficiaries, suggesting that access to NGS testing did not improve equally across insurance types. Since Medicare coverage is determined at the state level, the authors urged research examining individual state coverage policies to further elucidate factors slowing uptake among Medicaid beneficiaries. “Additional efforts beyond coverage policies,” the authors concluded, “are needed to ensure equitable access to the benefits of precision medicine.”
The study was supported by Genentech.
FROM JAMA NETWORK OPEN
Digital algorithm better predicts risk for postpartum hemorrhage
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.