User login
VEST: External sheath for CABG vein grafts shows promise
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
FROM AHA 2021
What is the diagnosis?
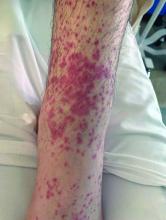
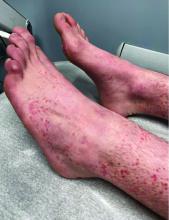
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Striae gravidarum: More than a ‘nuisance,’ say researchers
In the study of healthy pregnant women, “we found that SG can be associated with a host of negative reactions reflecting increased psychological and emotional distress,” reported Kaveri Karhade, MD, from the Berman Skin Institute, Los Altos, Calif., and coauthors from the University of Michigan, Ann Arbor. Dr. Karhade was with the department of dermatology at the University of Michigan at the time the study was conducted.
“We suggest that health care providers should avoid thinking of SG as merely a cosmetic ‘nuisance,’ ” they wrote in an article published in the International Journal of Women’s Dermatology. “Instead, it would be reasonable for providers to approach SG like other dermatologic concerns, and to consider asking patients whether SG cause emotional distress and whether prevention or treatment strategies should be attempted, even if not completely effective and potentially costly.”
The investigators did not evaluate treatments, but Frank Wang, MD, senior author of the study and professor of clinical dermatology at the University of Michigan Medicine, said in an interview that, “while they aren’t completely effective, some treatments can still help.” In addition, “recommending something also shows that you are listening to patients’ concerns – taking their concerns and skin lesions seriously,” he said.
Patient survey
The authors conducted a cross-sectional survey of 116 healthy pregnant women with SG. Participants were asked about the emotional and psychological effects of the lesions and how SG affects quality of life. The survey was modeled on questions from the Dermatology Life Quality Index, which asks about the impact of skin disease on embarrassment/self-consciousness, clothing choice, leisure activities, and interpersonal problems. “Content of questions was also devised from direct discussion with pregnant women attending clinic appointments or participating in other research studies on SG at our institution, and discussion with expert colleagues in obstetrics and dermatology,” the authors explained.
The survey consisted of 35 questions concerning demographics, pregnancy characteristics, personal and family history of SG, specific physical concerns about SG, impact of SG on attitude toward pregnancy, willingness to prevent SG or seek treatment, severity of SG (self-evaluated), the impact of SG on specific life-quality facets, and the location of lesions.
About two-thirds of respondents were aged 25-36 years and were White; the remainder self-identified as Asian, Black, Native American, or “other.” Most women reported “average” weight gain during the current pregnancy. Almost half of participants (45%) reporting a history of SG from prior pregnancies, and 65% reported a family history of SG.
The abdomen was identified most frequently as the location of SG (75%), followed by the breasts (43%), hips (43%), thighs (36%), buttocks (19%), and other areas (6%).
For most women (75%), permanency of the lesions was their top concern. About half (51%) reported that they had attempted to prevent SG, mostly with topical creams or oils. Three-quarters (75%) expressed interest in seeking treatment for SG, but this percentage dropped significantly to 33% (P =.008) if that treatment would not be covered by insurance.
Regarding the psychological impact of SG, embarrassment/self-consciousness correlated most strongly with lesion severity, followed by general quality of life, impact on choice of attire, impact on self-image/self-esteem, feelings of anxiety/depression related to SG, alteration of social/leisure activities related to SG (all P < .0001), and creation of interpersonal problems related to SG (P = .02).
The investigators also found that an increase in the effect of SG on self-image/self-esteem was “moderately associated” with younger age (P < .001) and that increased embarrassment related to SG was “moderately associated” with weight gain during pregnancy (P < .001).
“For years, stretch marks have been a topic to avoid and something many women try to hide,” Timothy Johnson, MD, professor of obstetrics and gynecology at the University of Michigan and coauthor of the study, said in a press release from the university. “Pregnant women talk about stretch marks with me every single week at clinic, and it’s time we break the stigma and start talking about them openly with all patients. ... By doing this study, we have an opportunity to normalize stretch marks in the context of all other dermatological conditions.”
Asked to comment on the findings, Tina Alster, MD, director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington, said her 3 decades of clinical experience support the authors’ findings. “Most patients who have striae are very self-conscious about them and report that their presence has negatively impacted their quality of life and self-confidence,” she said in an interview. “Of course, patients who come to my office are interested in having them treated, so my patient subset is skewed.”
She said treatment strategies that she discusses with patients include topical retinol/retinoids, which she said provide “low clinical response”; microneedling, which provides “marked” clinical response; and nonablative laser treatment, which provides “good” clinical response.
Considering particular patient characteristics, including budget, Dr. Alster said, “For those on a limited budget, I would propose daily use of a topical retinol, despite the low clinical effect. Many retinol-containing products are available over the counter. Prescription-strength retinoic acid tends to be pricey, often costing as much as in-office treatments.” Medical microneedling (not the cosmetic “roller” microneedling performed by aestheticians), she added, “gives the best results for the money and produces clinical results that mirror those achieved with lasers.”
Dr. Wang agreed that even recommending less expensive and less efficacious options such as over-the-counter creams can help alleviate patients’ concerns. “It shows that you are being holistic – not just caring for medical issues around pregnancy, but that you also take the emotional/psychological concerns of pregnant individuals and new parents seriously and that you recognize the impact of skin problems on quality of life. In the end, recommending something – in other words, providing some options, like creams or other therapies, for instance – is still, in my opinion, better than not recommending anything.”
Dr. Wang is involved with a study that is currently enrolling patients and that is evaluating the formation of early SG, which includes performing skin biopsies as soon as lesions appear.
The study had no funding. The study authors and Dr. Alster disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the study of healthy pregnant women, “we found that SG can be associated with a host of negative reactions reflecting increased psychological and emotional distress,” reported Kaveri Karhade, MD, from the Berman Skin Institute, Los Altos, Calif., and coauthors from the University of Michigan, Ann Arbor. Dr. Karhade was with the department of dermatology at the University of Michigan at the time the study was conducted.
“We suggest that health care providers should avoid thinking of SG as merely a cosmetic ‘nuisance,’ ” they wrote in an article published in the International Journal of Women’s Dermatology. “Instead, it would be reasonable for providers to approach SG like other dermatologic concerns, and to consider asking patients whether SG cause emotional distress and whether prevention or treatment strategies should be attempted, even if not completely effective and potentially costly.”
The investigators did not evaluate treatments, but Frank Wang, MD, senior author of the study and professor of clinical dermatology at the University of Michigan Medicine, said in an interview that, “while they aren’t completely effective, some treatments can still help.” In addition, “recommending something also shows that you are listening to patients’ concerns – taking their concerns and skin lesions seriously,” he said.
Patient survey
The authors conducted a cross-sectional survey of 116 healthy pregnant women with SG. Participants were asked about the emotional and psychological effects of the lesions and how SG affects quality of life. The survey was modeled on questions from the Dermatology Life Quality Index, which asks about the impact of skin disease on embarrassment/self-consciousness, clothing choice, leisure activities, and interpersonal problems. “Content of questions was also devised from direct discussion with pregnant women attending clinic appointments or participating in other research studies on SG at our institution, and discussion with expert colleagues in obstetrics and dermatology,” the authors explained.
The survey consisted of 35 questions concerning demographics, pregnancy characteristics, personal and family history of SG, specific physical concerns about SG, impact of SG on attitude toward pregnancy, willingness to prevent SG or seek treatment, severity of SG (self-evaluated), the impact of SG on specific life-quality facets, and the location of lesions.
About two-thirds of respondents were aged 25-36 years and were White; the remainder self-identified as Asian, Black, Native American, or “other.” Most women reported “average” weight gain during the current pregnancy. Almost half of participants (45%) reporting a history of SG from prior pregnancies, and 65% reported a family history of SG.
The abdomen was identified most frequently as the location of SG (75%), followed by the breasts (43%), hips (43%), thighs (36%), buttocks (19%), and other areas (6%).
For most women (75%), permanency of the lesions was their top concern. About half (51%) reported that they had attempted to prevent SG, mostly with topical creams or oils. Three-quarters (75%) expressed interest in seeking treatment for SG, but this percentage dropped significantly to 33% (P =.008) if that treatment would not be covered by insurance.
Regarding the psychological impact of SG, embarrassment/self-consciousness correlated most strongly with lesion severity, followed by general quality of life, impact on choice of attire, impact on self-image/self-esteem, feelings of anxiety/depression related to SG, alteration of social/leisure activities related to SG (all P < .0001), and creation of interpersonal problems related to SG (P = .02).
The investigators also found that an increase in the effect of SG on self-image/self-esteem was “moderately associated” with younger age (P < .001) and that increased embarrassment related to SG was “moderately associated” with weight gain during pregnancy (P < .001).
“For years, stretch marks have been a topic to avoid and something many women try to hide,” Timothy Johnson, MD, professor of obstetrics and gynecology at the University of Michigan and coauthor of the study, said in a press release from the university. “Pregnant women talk about stretch marks with me every single week at clinic, and it’s time we break the stigma and start talking about them openly with all patients. ... By doing this study, we have an opportunity to normalize stretch marks in the context of all other dermatological conditions.”
Asked to comment on the findings, Tina Alster, MD, director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington, said her 3 decades of clinical experience support the authors’ findings. “Most patients who have striae are very self-conscious about them and report that their presence has negatively impacted their quality of life and self-confidence,” she said in an interview. “Of course, patients who come to my office are interested in having them treated, so my patient subset is skewed.”
She said treatment strategies that she discusses with patients include topical retinol/retinoids, which she said provide “low clinical response”; microneedling, which provides “marked” clinical response; and nonablative laser treatment, which provides “good” clinical response.
Considering particular patient characteristics, including budget, Dr. Alster said, “For those on a limited budget, I would propose daily use of a topical retinol, despite the low clinical effect. Many retinol-containing products are available over the counter. Prescription-strength retinoic acid tends to be pricey, often costing as much as in-office treatments.” Medical microneedling (not the cosmetic “roller” microneedling performed by aestheticians), she added, “gives the best results for the money and produces clinical results that mirror those achieved with lasers.”
Dr. Wang agreed that even recommending less expensive and less efficacious options such as over-the-counter creams can help alleviate patients’ concerns. “It shows that you are being holistic – not just caring for medical issues around pregnancy, but that you also take the emotional/psychological concerns of pregnant individuals and new parents seriously and that you recognize the impact of skin problems on quality of life. In the end, recommending something – in other words, providing some options, like creams or other therapies, for instance – is still, in my opinion, better than not recommending anything.”
Dr. Wang is involved with a study that is currently enrolling patients and that is evaluating the formation of early SG, which includes performing skin biopsies as soon as lesions appear.
The study had no funding. The study authors and Dr. Alster disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the study of healthy pregnant women, “we found that SG can be associated with a host of negative reactions reflecting increased psychological and emotional distress,” reported Kaveri Karhade, MD, from the Berman Skin Institute, Los Altos, Calif., and coauthors from the University of Michigan, Ann Arbor. Dr. Karhade was with the department of dermatology at the University of Michigan at the time the study was conducted.
“We suggest that health care providers should avoid thinking of SG as merely a cosmetic ‘nuisance,’ ” they wrote in an article published in the International Journal of Women’s Dermatology. “Instead, it would be reasonable for providers to approach SG like other dermatologic concerns, and to consider asking patients whether SG cause emotional distress and whether prevention or treatment strategies should be attempted, even if not completely effective and potentially costly.”
The investigators did not evaluate treatments, but Frank Wang, MD, senior author of the study and professor of clinical dermatology at the University of Michigan Medicine, said in an interview that, “while they aren’t completely effective, some treatments can still help.” In addition, “recommending something also shows that you are listening to patients’ concerns – taking their concerns and skin lesions seriously,” he said.
Patient survey
The authors conducted a cross-sectional survey of 116 healthy pregnant women with SG. Participants were asked about the emotional and psychological effects of the lesions and how SG affects quality of life. The survey was modeled on questions from the Dermatology Life Quality Index, which asks about the impact of skin disease on embarrassment/self-consciousness, clothing choice, leisure activities, and interpersonal problems. “Content of questions was also devised from direct discussion with pregnant women attending clinic appointments or participating in other research studies on SG at our institution, and discussion with expert colleagues in obstetrics and dermatology,” the authors explained.
The survey consisted of 35 questions concerning demographics, pregnancy characteristics, personal and family history of SG, specific physical concerns about SG, impact of SG on attitude toward pregnancy, willingness to prevent SG or seek treatment, severity of SG (self-evaluated), the impact of SG on specific life-quality facets, and the location of lesions.
About two-thirds of respondents were aged 25-36 years and were White; the remainder self-identified as Asian, Black, Native American, or “other.” Most women reported “average” weight gain during the current pregnancy. Almost half of participants (45%) reporting a history of SG from prior pregnancies, and 65% reported a family history of SG.
The abdomen was identified most frequently as the location of SG (75%), followed by the breasts (43%), hips (43%), thighs (36%), buttocks (19%), and other areas (6%).
For most women (75%), permanency of the lesions was their top concern. About half (51%) reported that they had attempted to prevent SG, mostly with topical creams or oils. Three-quarters (75%) expressed interest in seeking treatment for SG, but this percentage dropped significantly to 33% (P =.008) if that treatment would not be covered by insurance.
Regarding the psychological impact of SG, embarrassment/self-consciousness correlated most strongly with lesion severity, followed by general quality of life, impact on choice of attire, impact on self-image/self-esteem, feelings of anxiety/depression related to SG, alteration of social/leisure activities related to SG (all P < .0001), and creation of interpersonal problems related to SG (P = .02).
The investigators also found that an increase in the effect of SG on self-image/self-esteem was “moderately associated” with younger age (P < .001) and that increased embarrassment related to SG was “moderately associated” with weight gain during pregnancy (P < .001).
“For years, stretch marks have been a topic to avoid and something many women try to hide,” Timothy Johnson, MD, professor of obstetrics and gynecology at the University of Michigan and coauthor of the study, said in a press release from the university. “Pregnant women talk about stretch marks with me every single week at clinic, and it’s time we break the stigma and start talking about them openly with all patients. ... By doing this study, we have an opportunity to normalize stretch marks in the context of all other dermatological conditions.”
Asked to comment on the findings, Tina Alster, MD, director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington, said her 3 decades of clinical experience support the authors’ findings. “Most patients who have striae are very self-conscious about them and report that their presence has negatively impacted their quality of life and self-confidence,” she said in an interview. “Of course, patients who come to my office are interested in having them treated, so my patient subset is skewed.”
She said treatment strategies that she discusses with patients include topical retinol/retinoids, which she said provide “low clinical response”; microneedling, which provides “marked” clinical response; and nonablative laser treatment, which provides “good” clinical response.
Considering particular patient characteristics, including budget, Dr. Alster said, “For those on a limited budget, I would propose daily use of a topical retinol, despite the low clinical effect. Many retinol-containing products are available over the counter. Prescription-strength retinoic acid tends to be pricey, often costing as much as in-office treatments.” Medical microneedling (not the cosmetic “roller” microneedling performed by aestheticians), she added, “gives the best results for the money and produces clinical results that mirror those achieved with lasers.”
Dr. Wang agreed that even recommending less expensive and less efficacious options such as over-the-counter creams can help alleviate patients’ concerns. “It shows that you are being holistic – not just caring for medical issues around pregnancy, but that you also take the emotional/psychological concerns of pregnant individuals and new parents seriously and that you recognize the impact of skin problems on quality of life. In the end, recommending something – in other words, providing some options, like creams or other therapies, for instance – is still, in my opinion, better than not recommending anything.”
Dr. Wang is involved with a study that is currently enrolling patients and that is evaluating the formation of early SG, which includes performing skin biopsies as soon as lesions appear.
The study had no funding. The study authors and Dr. Alster disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Allopurinol proves noninferior to febuxostat for gout relief
Allopurinol may finally start to get the respect that many rheumatologists feel it deserves as a first-line urate-lowering treatment for gout, following results of a randomized trial showing that it was noninferior to febuxostat both in the overall trial population and in patients with stage 3 chronic kidney disease (CKD).
In the multicenter, randomized, double-blinded comparison trial that used a treat-to-target strategy, allopurinol met the primary outcome of noninferiority to febuxostat for preventing gout flare during the observation phase of therapy, reported James O’Dell, MD, chief of the division of rheumatology and vice chair for education in the department of internal medicine at the University of Nebraska Medical Center in Omaha.
“Both agents were well tolerated, with or without CKD. Most importantly, both agents were highly effective when used in a treat-to-target protocol in getting patients to target urate levels,” he said in an oral abstract presentation during the American College of Rheumatology (ACR) 2021 Annual Meeting, which was held online.
And although febuxostat contains a boxed warning about the risks of cardiovascular adverse events with its use, there were no signals for increased cardiovascular toxicity with febuxostat compared with allopurinol, the investigators found.
The trial is the first to compare allopurinol, a decades-old drug, with febuxostat, approved in 2009, in a treat-to-target approach, Dr. O’Dell said.
American College of Physicians’ guideline ‘antiquated’
The results of the study “will hopefully teach doctors how to treat gout better by encouraging them to use higher doses of gout medications safely than they’re actually using at this time,” said Donald Thomas Jr., MD, in private practice in Greenbelt, Md., and associate professor of medicine at the Uniformed Services University of the Health Sciences in Bethesda, Md.
Dr. Thomas, who moderated a media briefing where Dr. O’Dell discussed the results of the trial, said that he had recently read the 2017 gout guideline by the American College of Physicians (ACP), which he called “antiquated.”
The ACP recommends the use of corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), or low-dose colchicine to treat patients with acute gout. The ACP also recommends “against initiating long-term urate-lowering therapy in most patients after a first gout attack or in patients with infrequent attacks.”
The guideline recommends that clinicians discuss potential benefits, risks, costs, and personal preferences before starting patients on urate-lowering therapy in patients with recurrent gout attacks.
The 2017 guidelines also state, however, that “[e]vidence was insufficient to conclude whether the benefits of escalating urate-lowering therapy to reach a serum urate target (‘treat to target’) outweigh the harms associated with repeated monitoring and medication escalation.”
“I’ve been a proud member of the American College of Physicians for years, I’m a master of the ACP, and they do a lot of great things, but this is one case where their insistence that they’re not going to have a guideline that isn’t completely based in evidence from studies is getting in the way of common sense,” Dr. O’Dell said.
“Their contention is that what matters to a gout patient is a gout flare, and how do we know that gout flares are less if you treat to target or not – and that’s a fair question,” he continued, “except for the fact that in uric acid metabolism we know physiologically that there’s a magic number and that’s 6.8 mg/dL, and anything above that, every day uric acid is above 6.8, you are literally putting crystal out into all places in your body.”
In contrast, the ACR’s 2020 guideline for the management of gout strongly recommends starting urate-lowering therapy for all patients with tophaceous gout, radiographic damage because of gout, or frequent gout flares. It also advises using allopurinol as the preferred first-line urate-lowering therapy, including for those with stage 3 or greater CKD, and using a low starting dose of allopurinol of 100 mg/day or less (lower in CKD) or febuxostat at 40 mg/day or less. It endorses a treat-to-target management strategy that aims for serum urate < 6 mg/dL with dose titration of urate-lowering agents guided by serial serum urate measurements.
Dr. Thomas and Dr. O’Dell expressed hope that the results of this clinical trial will put the issue to rest, and that the ACP will update its guideline accordingly.
VA-sponsored trial
The study was conducted at 19 Veterans Affairs medical centers and two non-VA sites. The trial was divided into dose-titration, maintenance, and observation phases, each lasting 24 weeks.
A total of 950 participants with gout and a serum urate concentration 6.8 mg/dL or greater were randomly assigned on a 1:1 basis to receive allopurinol 100-800 mg or febuxostat 40 mg to 80/120 mg daily. In 2019, the Food and Drug Administration requested that the maximum titrated dose of febuxostat in the trial be capped at 80 mg daily. All patients stopped prophylaxis with NSAIDs, colchicine, or prednisone before the observation phase.
Patients with persistent hyperuricemia despite treatment with allopurinol were eligible, and these patients were started in the titration phase at their current dose.
The mean patient age was 62.9 years in the allopurinol arm and 61.3 years in the febuxostat arm. Men comprised 98% of patients in each study arm.
The racial/ethnic distribution of patients was similar between the groups. In all, 38.7% of patients assigned to allopurinol and 36% assigned to febuxostat had CKD stages 1-3. (Patients with stage 4 or 5 CKD were excluded from the study.)
A gout flare occurred if a participants reported three or more symptoms of tender, warm, swollen joints, or gout flare, or if the participant reported use of medication for gout flare in the observation phase during weeks 49-72.
As noted before, the trial met its primary endpoint, with 36.5% of patients on allopurinol reporting gout flare in the observation phase, compared with 43.5% on febuxostat (P for noninferiority < .001).
Among patients with CKD stage 3, the respective percentages of patients reporting at least one gout flare in the observation phase were 31.9% and 45.3% (P for noninferiority < .001).
Approximately 80% of patients in each arm had mean serum urate concentrations less than 6.0 mg/dL during the maintenance phase (weeks 36, 42, and 48).
In each arm, about 20% of patients left the study before completing 72 weeks of follow-up. Serious adverse events occurred in 26.7% of patients assigned to allopurinol and 26.1% of patients assigned to febuxostat.
Cardiovascular adverse events occurred in 8.1% and 6.8%, respectively. There were three cases of cardiovascular death in the allopurinol arm and one in the febuxostat arm. Nonfatal myocardial infarction occurred in two and four patients, respectively, stroke in one and two, and unstable angina requiring urgent revascularization in four and three patients.
In the question-and-answer session of the briefing, this news organization asked Dr. Thomas whether he would use the agents interchangeably in his practice. He replied “no, I start off with allopurinol in all of my patients, even those with chronic kidney disease, because it has been shown to be safe. I start off at a very low dose, go up slowly, [and] if they have a reaction, I change it to febuxostat.”
The study was supported by the U.S. Department of Veterans Affairs. Dr. O’Dell and Dr. Thomas have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Allopurinol may finally start to get the respect that many rheumatologists feel it deserves as a first-line urate-lowering treatment for gout, following results of a randomized trial showing that it was noninferior to febuxostat both in the overall trial population and in patients with stage 3 chronic kidney disease (CKD).
In the multicenter, randomized, double-blinded comparison trial that used a treat-to-target strategy, allopurinol met the primary outcome of noninferiority to febuxostat for preventing gout flare during the observation phase of therapy, reported James O’Dell, MD, chief of the division of rheumatology and vice chair for education in the department of internal medicine at the University of Nebraska Medical Center in Omaha.
“Both agents were well tolerated, with or without CKD. Most importantly, both agents were highly effective when used in a treat-to-target protocol in getting patients to target urate levels,” he said in an oral abstract presentation during the American College of Rheumatology (ACR) 2021 Annual Meeting, which was held online.
And although febuxostat contains a boxed warning about the risks of cardiovascular adverse events with its use, there were no signals for increased cardiovascular toxicity with febuxostat compared with allopurinol, the investigators found.
The trial is the first to compare allopurinol, a decades-old drug, with febuxostat, approved in 2009, in a treat-to-target approach, Dr. O’Dell said.
American College of Physicians’ guideline ‘antiquated’
The results of the study “will hopefully teach doctors how to treat gout better by encouraging them to use higher doses of gout medications safely than they’re actually using at this time,” said Donald Thomas Jr., MD, in private practice in Greenbelt, Md., and associate professor of medicine at the Uniformed Services University of the Health Sciences in Bethesda, Md.
Dr. Thomas, who moderated a media briefing where Dr. O’Dell discussed the results of the trial, said that he had recently read the 2017 gout guideline by the American College of Physicians (ACP), which he called “antiquated.”
The ACP recommends the use of corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), or low-dose colchicine to treat patients with acute gout. The ACP also recommends “against initiating long-term urate-lowering therapy in most patients after a first gout attack or in patients with infrequent attacks.”
The guideline recommends that clinicians discuss potential benefits, risks, costs, and personal preferences before starting patients on urate-lowering therapy in patients with recurrent gout attacks.
The 2017 guidelines also state, however, that “[e]vidence was insufficient to conclude whether the benefits of escalating urate-lowering therapy to reach a serum urate target (‘treat to target’) outweigh the harms associated with repeated monitoring and medication escalation.”
“I’ve been a proud member of the American College of Physicians for years, I’m a master of the ACP, and they do a lot of great things, but this is one case where their insistence that they’re not going to have a guideline that isn’t completely based in evidence from studies is getting in the way of common sense,” Dr. O’Dell said.
“Their contention is that what matters to a gout patient is a gout flare, and how do we know that gout flares are less if you treat to target or not – and that’s a fair question,” he continued, “except for the fact that in uric acid metabolism we know physiologically that there’s a magic number and that’s 6.8 mg/dL, and anything above that, every day uric acid is above 6.8, you are literally putting crystal out into all places in your body.”
In contrast, the ACR’s 2020 guideline for the management of gout strongly recommends starting urate-lowering therapy for all patients with tophaceous gout, radiographic damage because of gout, or frequent gout flares. It also advises using allopurinol as the preferred first-line urate-lowering therapy, including for those with stage 3 or greater CKD, and using a low starting dose of allopurinol of 100 mg/day or less (lower in CKD) or febuxostat at 40 mg/day or less. It endorses a treat-to-target management strategy that aims for serum urate < 6 mg/dL with dose titration of urate-lowering agents guided by serial serum urate measurements.
Dr. Thomas and Dr. O’Dell expressed hope that the results of this clinical trial will put the issue to rest, and that the ACP will update its guideline accordingly.
VA-sponsored trial
The study was conducted at 19 Veterans Affairs medical centers and two non-VA sites. The trial was divided into dose-titration, maintenance, and observation phases, each lasting 24 weeks.
A total of 950 participants with gout and a serum urate concentration 6.8 mg/dL or greater were randomly assigned on a 1:1 basis to receive allopurinol 100-800 mg or febuxostat 40 mg to 80/120 mg daily. In 2019, the Food and Drug Administration requested that the maximum titrated dose of febuxostat in the trial be capped at 80 mg daily. All patients stopped prophylaxis with NSAIDs, colchicine, or prednisone before the observation phase.
Patients with persistent hyperuricemia despite treatment with allopurinol were eligible, and these patients were started in the titration phase at their current dose.
The mean patient age was 62.9 years in the allopurinol arm and 61.3 years in the febuxostat arm. Men comprised 98% of patients in each study arm.
The racial/ethnic distribution of patients was similar between the groups. In all, 38.7% of patients assigned to allopurinol and 36% assigned to febuxostat had CKD stages 1-3. (Patients with stage 4 or 5 CKD were excluded from the study.)
A gout flare occurred if a participants reported three or more symptoms of tender, warm, swollen joints, or gout flare, or if the participant reported use of medication for gout flare in the observation phase during weeks 49-72.
As noted before, the trial met its primary endpoint, with 36.5% of patients on allopurinol reporting gout flare in the observation phase, compared with 43.5% on febuxostat (P for noninferiority < .001).
Among patients with CKD stage 3, the respective percentages of patients reporting at least one gout flare in the observation phase were 31.9% and 45.3% (P for noninferiority < .001).
Approximately 80% of patients in each arm had mean serum urate concentrations less than 6.0 mg/dL during the maintenance phase (weeks 36, 42, and 48).
In each arm, about 20% of patients left the study before completing 72 weeks of follow-up. Serious adverse events occurred in 26.7% of patients assigned to allopurinol and 26.1% of patients assigned to febuxostat.
Cardiovascular adverse events occurred in 8.1% and 6.8%, respectively. There were three cases of cardiovascular death in the allopurinol arm and one in the febuxostat arm. Nonfatal myocardial infarction occurred in two and four patients, respectively, stroke in one and two, and unstable angina requiring urgent revascularization in four and three patients.
In the question-and-answer session of the briefing, this news organization asked Dr. Thomas whether he would use the agents interchangeably in his practice. He replied “no, I start off with allopurinol in all of my patients, even those with chronic kidney disease, because it has been shown to be safe. I start off at a very low dose, go up slowly, [and] if they have a reaction, I change it to febuxostat.”
The study was supported by the U.S. Department of Veterans Affairs. Dr. O’Dell and Dr. Thomas have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Allopurinol may finally start to get the respect that many rheumatologists feel it deserves as a first-line urate-lowering treatment for gout, following results of a randomized trial showing that it was noninferior to febuxostat both in the overall trial population and in patients with stage 3 chronic kidney disease (CKD).
In the multicenter, randomized, double-blinded comparison trial that used a treat-to-target strategy, allopurinol met the primary outcome of noninferiority to febuxostat for preventing gout flare during the observation phase of therapy, reported James O’Dell, MD, chief of the division of rheumatology and vice chair for education in the department of internal medicine at the University of Nebraska Medical Center in Omaha.
“Both agents were well tolerated, with or without CKD. Most importantly, both agents were highly effective when used in a treat-to-target protocol in getting patients to target urate levels,” he said in an oral abstract presentation during the American College of Rheumatology (ACR) 2021 Annual Meeting, which was held online.
And although febuxostat contains a boxed warning about the risks of cardiovascular adverse events with its use, there were no signals for increased cardiovascular toxicity with febuxostat compared with allopurinol, the investigators found.
The trial is the first to compare allopurinol, a decades-old drug, with febuxostat, approved in 2009, in a treat-to-target approach, Dr. O’Dell said.
American College of Physicians’ guideline ‘antiquated’
The results of the study “will hopefully teach doctors how to treat gout better by encouraging them to use higher doses of gout medications safely than they’re actually using at this time,” said Donald Thomas Jr., MD, in private practice in Greenbelt, Md., and associate professor of medicine at the Uniformed Services University of the Health Sciences in Bethesda, Md.
Dr. Thomas, who moderated a media briefing where Dr. O’Dell discussed the results of the trial, said that he had recently read the 2017 gout guideline by the American College of Physicians (ACP), which he called “antiquated.”
The ACP recommends the use of corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), or low-dose colchicine to treat patients with acute gout. The ACP also recommends “against initiating long-term urate-lowering therapy in most patients after a first gout attack or in patients with infrequent attacks.”
The guideline recommends that clinicians discuss potential benefits, risks, costs, and personal preferences before starting patients on urate-lowering therapy in patients with recurrent gout attacks.
The 2017 guidelines also state, however, that “[e]vidence was insufficient to conclude whether the benefits of escalating urate-lowering therapy to reach a serum urate target (‘treat to target’) outweigh the harms associated with repeated monitoring and medication escalation.”
“I’ve been a proud member of the American College of Physicians for years, I’m a master of the ACP, and they do a lot of great things, but this is one case where their insistence that they’re not going to have a guideline that isn’t completely based in evidence from studies is getting in the way of common sense,” Dr. O’Dell said.
“Their contention is that what matters to a gout patient is a gout flare, and how do we know that gout flares are less if you treat to target or not – and that’s a fair question,” he continued, “except for the fact that in uric acid metabolism we know physiologically that there’s a magic number and that’s 6.8 mg/dL, and anything above that, every day uric acid is above 6.8, you are literally putting crystal out into all places in your body.”
In contrast, the ACR’s 2020 guideline for the management of gout strongly recommends starting urate-lowering therapy for all patients with tophaceous gout, radiographic damage because of gout, or frequent gout flares. It also advises using allopurinol as the preferred first-line urate-lowering therapy, including for those with stage 3 or greater CKD, and using a low starting dose of allopurinol of 100 mg/day or less (lower in CKD) or febuxostat at 40 mg/day or less. It endorses a treat-to-target management strategy that aims for serum urate < 6 mg/dL with dose titration of urate-lowering agents guided by serial serum urate measurements.
Dr. Thomas and Dr. O’Dell expressed hope that the results of this clinical trial will put the issue to rest, and that the ACP will update its guideline accordingly.
VA-sponsored trial
The study was conducted at 19 Veterans Affairs medical centers and two non-VA sites. The trial was divided into dose-titration, maintenance, and observation phases, each lasting 24 weeks.
A total of 950 participants with gout and a serum urate concentration 6.8 mg/dL or greater were randomly assigned on a 1:1 basis to receive allopurinol 100-800 mg or febuxostat 40 mg to 80/120 mg daily. In 2019, the Food and Drug Administration requested that the maximum titrated dose of febuxostat in the trial be capped at 80 mg daily. All patients stopped prophylaxis with NSAIDs, colchicine, or prednisone before the observation phase.
Patients with persistent hyperuricemia despite treatment with allopurinol were eligible, and these patients were started in the titration phase at their current dose.
The mean patient age was 62.9 years in the allopurinol arm and 61.3 years in the febuxostat arm. Men comprised 98% of patients in each study arm.
The racial/ethnic distribution of patients was similar between the groups. In all, 38.7% of patients assigned to allopurinol and 36% assigned to febuxostat had CKD stages 1-3. (Patients with stage 4 or 5 CKD were excluded from the study.)
A gout flare occurred if a participants reported three or more symptoms of tender, warm, swollen joints, or gout flare, or if the participant reported use of medication for gout flare in the observation phase during weeks 49-72.
As noted before, the trial met its primary endpoint, with 36.5% of patients on allopurinol reporting gout flare in the observation phase, compared with 43.5% on febuxostat (P for noninferiority < .001).
Among patients with CKD stage 3, the respective percentages of patients reporting at least one gout flare in the observation phase were 31.9% and 45.3% (P for noninferiority < .001).
Approximately 80% of patients in each arm had mean serum urate concentrations less than 6.0 mg/dL during the maintenance phase (weeks 36, 42, and 48).
In each arm, about 20% of patients left the study before completing 72 weeks of follow-up. Serious adverse events occurred in 26.7% of patients assigned to allopurinol and 26.1% of patients assigned to febuxostat.
Cardiovascular adverse events occurred in 8.1% and 6.8%, respectively. There were three cases of cardiovascular death in the allopurinol arm and one in the febuxostat arm. Nonfatal myocardial infarction occurred in two and four patients, respectively, stroke in one and two, and unstable angina requiring urgent revascularization in four and three patients.
In the question-and-answer session of the briefing, this news organization asked Dr. Thomas whether he would use the agents interchangeably in his practice. He replied “no, I start off with allopurinol in all of my patients, even those with chronic kidney disease, because it has been shown to be safe. I start off at a very low dose, go up slowly, [and] if they have a reaction, I change it to febuxostat.”
The study was supported by the U.S. Department of Veterans Affairs. Dr. O’Dell and Dr. Thomas have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
Botulinum toxin for chronic pain: What's on the horizon?
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.
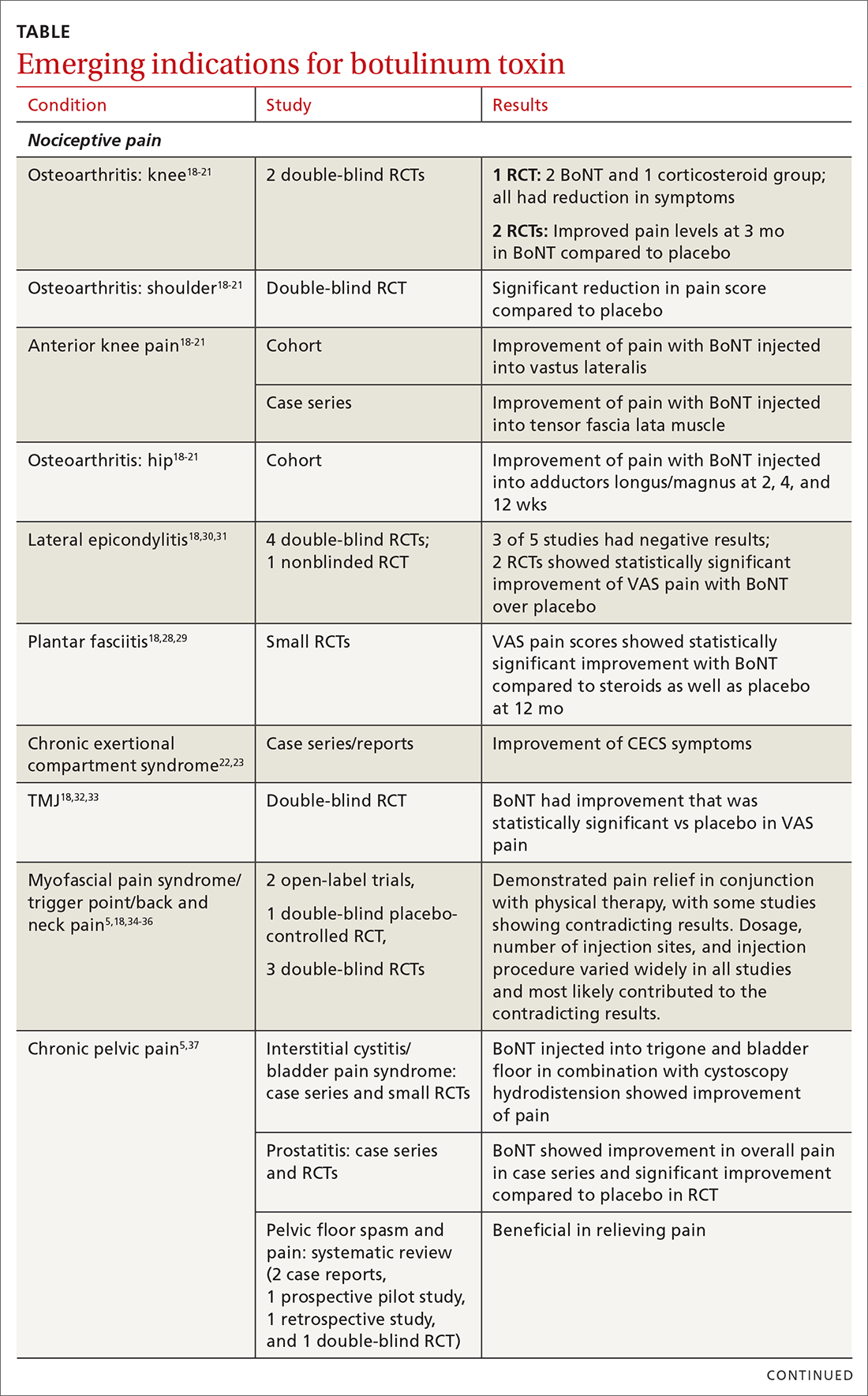
The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.
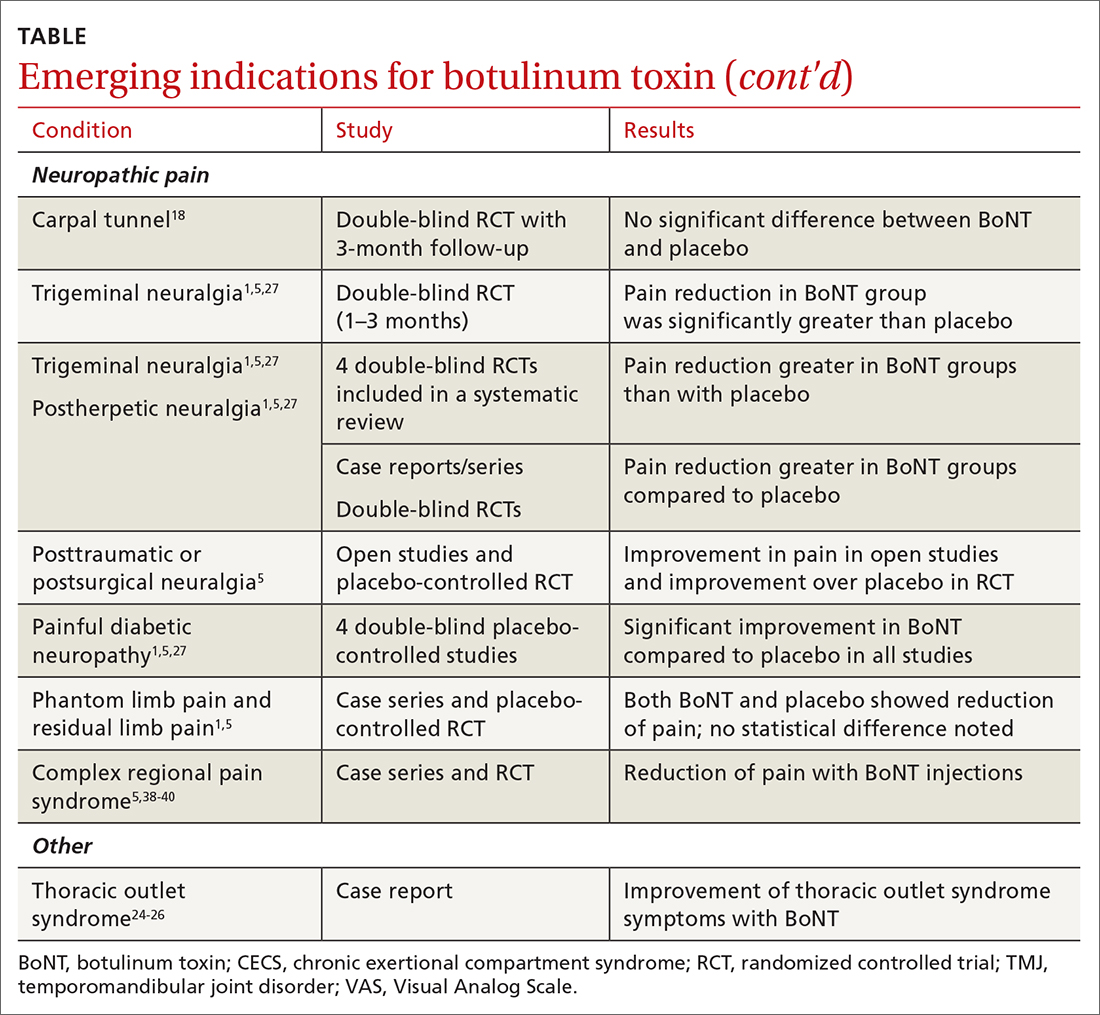
Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
Botulinum toxin (BoNT) was first approved by the US Food and Drug Administration (FDA) for the treatment of strabismus and blepharospasm in 1989. Since then, approved indications have expanded to include spasticity, cervical dystonia, severe axillary hyperhidrosis, bladder dysfunction, and chronic migraine headache, as well as multiple cosmetic uses.1,2 Over the course of 30 years of clinical use, BoNT has proven to be effective and safe.3,4 This has led to the expanded use of BoNT for additional medical conditions.1,2
In the review that follows, we will discuss the utility of BoNT in the treatment of headaches, spasticity, and cervical dystonia. We will then explore the evidence for emerging indications that include chronic joint pain, trigeminal neuralgia, and plantar fasciitis. But first, a brief word about how BoNT works and its safety profile.
Seven toxins, but only 2 are used for medical purposes
BoNT is naturally produced by Clostridium botulinum, an anaerobic, spore-forming bacteria.1 BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junctions, which results in flaccid paralysis in peripheral skeletal musculature and autonomic nerve terminals.1,5 These effects from BoNT can last up to 3 to 6 months.1
Seven different toxins have been identified (A, B, C, D, E, F, and G), but only toxins A and B are currently used for medical purposes.5 Both have similar effects, although there are slight differences in mechanism of action. Toxin B injections are also reported to be slightly more painful. There are also differences in preparation, with some requiring reconstitution, which vary by brand. Certain types of BoNT require refrigeration, and an in-depth review of the manufacturer’s guidelines is recommended before use.
Safety and adverse effects
Although BoNT is 1 of the most lethal toxins known to humans, it has been used in clinical medicine for more than 30 years and has proven to be safe if used properly.3 Adverse effects are rare and are often location and dose dependent (200 U and higher). Immediate or acute adverse effects are usually mild and can include bruising, headache, allergic reactions, edema, skin conditions, infection, or pain at the injection site.4 Delayed adverse effects can include muscle weakness that persists throughout the 3 to 6 months of duration and is usually related to incorrect placement or unintentional spread.4
Serious adverse events are rare: there are reports of the development of botulism, generalized paralysis, dysphagia, respiratory effects, and even death in patients who had received BoNT injections.3 In a majority of cases, a direct relationship with BoNT was never established, and in most incidents reported, there were significant comorbidities that could have contributed to the adverse event.3 These events appear to be related to higher doses of BoNT, as well as possible incorrect injection placement.3
Knowledge of anatomy and correct placement of BoNT are vitally important, as they have a significant impact on the effectiveness of treatment and adverse events.3 In preventing adverse events, those administering BoNT need to be familiar with the BoNT brand being used, verify proper storage consistent with the manufacturer’s recommendations, and confirm correct dosages with proper reconstitution process.3
Continue to: BoNT is contraindicated
BoNT is contraindicated in those with a history of a previous anaphylactic reaction to BoNT. Patients with known hypersensitivity to BoNT, including those with neuromuscular junction diseases and anterior horn disorders, should be considered for other forms of treatment due to the risk of an exaggerated response. No adverse events have been recorded in regard to pregnancy and lactation, although these remain a potential contraindication.3,4,6
Taking a closer look at current indications
Headaches
Chronic migraine (CM) is defined by the International Headache Society as at least 15 days per month with headaches and 8 of those days with migraine features. BoNT has been FDA approved for treatment of CM since 2011. This was based on 2 large, double-blind, randomized, placebo-controlled trials that showed a significant reduction from baseline for headaches and migraine days, total time, and frequency of migraines.7,8
Subsequent studies have continued to show benefit for CM treatment. In a recent Cochrane systematic review and meta-analysis, it was determined that BoNT can decrease frequency of CM by 2 days per month, and it is recommended by several organizations as a treatment option for CM.9
Low-quality evidence has not shown benefit for tension-type headaches. However, further research is warranted, especially for chronic tension-type headache, which is defined as daily tension headaches.10
Spasticity
Spasticity is caused by an insult to the brain or spinal cord and can often occur after a stroke, brain or spinal cord injury, cerebral palsy, or other neurologic condition.11 BoNT was initially FDA approved in 2010 for treatment of upper limb spasticity in adults, although it had been used for treatment for spasticity for more than 20 years prior to that. It currently is approved for upper and lower spasticity in adults and recently was expanded to include pediatrics.12
Continue to: A small case series...
A small case series conducted soon after BoNT was introduced showed promising results, and subsequent meta-analyses and systematic reviews have shown positive results for use of BoNT for the management of spasticity.13 Studies have begun to focus on specific regions of the upper and lower limbs to identify optimal sites for injections.
Cervical dystonia
Cervical dystonia (CD) is the most common form of dystonia and is defined as impairment of activities of daily living due to abnormal postures of the head and neck. BoNT was approved for CD in 1999 after several pivotal randomized placebo-controlled double-blind studies showed improvement of symptoms.14 Several BoNT formulations have been given Level A classification, and can be considered a potential first-line treatment for CD.15,16 The most common adverse effects reported have been dry mouth, dysphagia, muscle weakness, and neck pain.14-16
BoNT is currently being used off-label for management of multiple types of dystonia with reported success, as research on its use for noncervical dystonia (including limb, laryngeal, oromandibular, and truncal) continues. Although there are case series and some randomized trials exploring BoNT for certain types of dystonia, most are lacking high-quality evidence from double-blind, randomized controlled trials.14-16
Exploring the evidence for emerging indications
There has been significant interest in using BoNT for management for both nociceptive and neuropathic pain symptoms.5
Nociceptive pain is the irritation and painful response to actual or potential tissue damage. It is a major component of chronic pain and is difficult to treat, with limited effective options.5,17
Continue to: Neuropathic pain
Neuropathic pain is related to abnormalities that disrupt the normal function of the nervous system. Abnormalities could be related to anatomic or structural changes that cause compression, trauma, scar tissue, or a number of other conditions that affect nerve function. These can be either central or peripheral and can be caused by multiple etiologies.

The following discussion explores the evidence for potential emerging indications for BoNT. The TABLE1,5,18-40 summarizes what we know to date.

Chronic joint pain
Refractory joint pain is difficult to treat and can be debilitating for patients. It can have multiple causes but is most commonly related to arthritic changes. Due to the difficulty with treatment, there have been attempts to use BoNT as an intra-articular treatment for refractory joint pain. Results vary and are related to several factors, including the initial degree of pain, the BoNT dosage, and the formulation used, as well as the joint injected.
There appears to be a potentially significant improvement in short-term pain with BoNT compared to conventional therapies, such as physical therapy, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and hyaluronic acid injections. In studies evaluating long-term benefits, it was noted that after 6 months, there was no significant difference between BoNT and control groups.19-21
The knee joint has been the focus of most research, but BoNT has also been used for shoulder and ankle pain, with success. Recent meta-analyses evaluating knee and shoulder pain have shown BoNT is safe and effective for joint pain.20,21 There has been no significant difference noted in adverse events with BoNT compared to controls. Currently, more long-term data and research are needed, but BoNT is safe and a potentially effective treatment option for short-term relief of refractory joint pain.19-21
Continue to: Chronic exertional compartment sydrome
Chronic exertional compartment syndrome
Chronic exertional compartment syndrome (CECS) is defined subjectively as pain in a specific compartment that develops during exercise and resolves upon stopping, as well as objectively with an increase in intra-muscular pressure.22 It is most common in the lower leg and is a difficult condition to manage. Nonsurgical and surgical options are only successful at returning the patient to full activity 40% to 80% of the time.23
An initial study done in 2013 of BoNT injected into the anterior and lateral compartments of the lower extremity showed that symptoms resolved completely in 94% of patients treated.22 The actual mechanism of benefit is not clearly understood but is potentially related to muscle atrophy and loss of contractile tissue. However, it has not been reported that these changes have affected the strength or performance of patients who receive BoNT for CECS.23
Thoracic outlet syndrome
Thoracic outlet syndrome (TOS) is a compression of neurovascular structures within the thoracic outlet. There are several locations of potential compression, as well as possible neurogenic, vascular, or nonspecific manifestations.24 Compression can be from a structural variant, such as a cervical rib, or due to soft tissue from the scalene or pectoralis musculature. TOS is difficult to diagnose and treat. Physical therapy is the mainstay of treatment, but failure is common and treatment options are otherwise limited. Decompression surgery is an option if conservative management fails, but it has a high recurrence rate.24
In an effort to harness the therapeutic value of muscle atrophy, denervation, and relaxation afforded by BoNT, clinicians have injected the agent into the anterior and middle scalenes and the pectoralis minor to provide patients with relief from TOS.24 This treatment requires advanced imaging with either fluoroscopy or ultrasound guidance for correct placement and knowledge of surrounding anatomy. Small case reports and case series have demonstrated success, but a small double-blind randomized controlled study of 37 individuals with neurogenic TOS in 2011 did not show a reduction in symptoms.25 Multiple subsequent case reports and case series have continued to show positive results.24,25 A recent retrospective study showed that patients with TOS who had positive results with BoNT had better surgical outcomes.26
Trigeminal neuralgia and peripheral nerve pain
A meta-analysis in 2019 reviewed evidence for trigeminal neuralgia as well as other types of peripheral neuropathies, including diabetic neuropathy and postherpetic neuropathy. It showed that BoNT injections are safe, as well as effective, for short-term relief at 3 months. However, overall study sizes were small and long-term data are still lacking; larger high-quality studies are needed for further substantiation.27
Continue to: Plantar fascitis
Plantar fasciitis
BoNT has been used for treatment of plantar fasciitis. Small randomized controlled studies have compared BoNT to both placebo and corticosteroids, showing that BoNT has better long-term outcomes at 3, 6, and 12 months.28,29 BoNT is currently being used when standard treatments have failed; however, larger randomized controlled studies are still needed prior to BoNT being accepted as standard treatment.29
Lateral epicondylitis
A systematic review and meta-analysis done in 2017 showed that BoNT is superior to placebo at 16 weeks. No significant difference was noted between BoNT and corticosteroids at 8 weeks, although corticosteroids did demonstrate better improvement at the short-term interval of 2 to 4 weeks.30 As expected, BoNT was associated with grip-strength weakness compared to placebo and corticosteroids at 12 weeks. Subsequent small randomized controlled studies have continued to show benefit with BoNT, but all studies noted grip weakness (which resolved) and duration of effect was dose dependent.30,31
Temporomandibular joint pain
BoNT has been studied in the treatment of temporomandibular joint (TMJ) pain and dislocations since 1998, and was shown to improve quality of life.32 BoNT has been injected into the musculature surrounding the TMJ, as well as into the joint, and has proven to be effective in these areas.33 There are limited treatment options for TMJ pain and dislocations, and although research is still ongoing, BoNT is considered a potential treatment option.32,33
Myofascial, neck, and back chronic pain
Chronic back pain is common and can be due to multiple conditions. BoNT has been studied for treatment focusing on myofascial pain in the neck and back region. Case series have shown improvement with targeted BoNT injections.34 However, in randomized controlled double-blind studies comparing BoNT to placebo, local anesthetics, and steroids, there were no significant differences in pain scores.35,36 The majority of studies have been landmark based or used the site of maximal tenderness as guidance for injections, but there is some evidence that targeted injections focusing on specific muscle groups may improve benefit.5 This usually requires the use of imaging for guidance.
Chronic pelvic pain
Chronic pelvic pain is common and has been reported to affect 1 in 7 women.37 It is often difficult to diagnose the exact source of the pain, and it can be very difficult to treat. In a 2020 systematic review (including 12 observational studies and 5 randomized controlled trials) of BoNT for treatment of chronic pelvic pain, the quality of evidence varied widely.38 Observational studies showed good benefit, but only 1 randomized trial showed statistical difference with the use of BoNT for pelvic pain. No serious adverse events were reported in any of the studies.38 Chronic pelvic pain can be caused by a number of different conditions, and more high-quality research for BoNT is needed, focusing on specific causes.5,38
Continue to: Complex regional pain
Complex regional pain
Complex regional pain syndrome (CRPS) can be a debilitating condition that causes pain, sympathetic dysregulation, and central nervous system sensitization, often related to a traumatic event. Incidence is reported as 5 to 26 per 100,000, although it most likely is severely underdiagnosed.39 Treatment options are limited, and often patients continue to struggle with pain.
Due to the mechanism of action of BoNT, it has a high potential benefit for treatment of the allodynia and hyperalgesia associated with CRPS. BoNT injections have been used for the treatment of CRPS with limited success.40
There is currently limited evidence on BoNT for CRPS, and uncertainty regarding the best injection location remains. Studies have looked at lumbar sympathetic blocks, intra-articular, and grid-like BoNT injections over the area affected by CRPS.39-41 Case studies/series and observational studies have shown success with minimal adverse reactions, but larger high-quality, randomized controlled double-blind studies are still lacking.39-41
Concluding thoughts
Most chronic pain conditions have very limited treatment options, making the exploration of BoNT as a potential addition to those treatments an appealing possibility. Since it was first introduced in 1989, it has been proven to be safe, with limited adverse events, for the treatment of chronic pain.
However, providers need to be familiar with the type and formulation of BoNT product being used. Extensive knowledge of surrounding anatomy and ability to place BoNT in an exact location (which may require either fluoroscopy or ultrasound guidance) is essential.
Continue to: Adequate research and evidence...
Adequate research and evidence for most of the applications discussed in this article are still lacking; some limitations include small sample size, bias, lower quality, and poor methodology. There is also a lack of standardization, including which BoNT product is used, dosage, and location of BoNT placement. All of these issues will need to be addressed in further research.
CORRESPONDENCE
Caleb Dickison, DO, CAQSM, 36065 Darnall Loop, Fort Hood, TX 76544; [email protected]
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
1. Hehr JD, Schoenbrunner AR, Janis JE. The use of botulinum toxin in pain management: basic science and clinical applications. Plast Reconstr Surg. 2020;145:629e-636e. doi: 10.1097/PRS.0000000000006559
2. Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64-68. doi: 10.1016/j.toxicon.2019.12.005
3. Yiannakopoulou E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology. 2015;95:65-69. doi: 10.1159/000370245
4. Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type A. Am J Clin Dermatol. 2005;6:141-150. https://doi.org/10.2165/00128071-200506030-00001
5. Guzman S, Helander E, Elhassan A. Use of botulinum toxin for chronic pain management. Topics in Pain Management. 2016;31:1-8. doi: 10.1097/01.TPM.0000482997.94909.69
6. Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53:407‐415. doi: 10.1016/j.jaad.2005.06.011
7. Aurora SK, Dodick DW, Turkel CC, et al; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803. doi: 10.1177/0333102410364676
8. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010Jul;30:804-814. doi: 10.1177/0333102410364677
9. Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. doi: 10.1136/bmjopen-2018-027953
10. Freund B, Rao A. Efficacy of botulinum toxin in tension-type headaches: a systematic review of the literature. Pain Pract. 2019;19:541-551. doi: 10.1111/papr.12773
11. Ward A. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607-616. https://doi.org/10.1007/s00702-007-0833-2
12. Ipsen announces FDA approval of Dysport® (abobotulinumtoxinA) for the treatment of upper limb spasticity in children, excluding cerebral palsy [press release]. September 26, 2019. Accessed October 27, 2021. www.businesswire.com/news/home/20190926005480/en/Ipsen-Announces-FDA-Approval-Dysport%C2%AE-abobotulinumtoxinA-Treatment
13. Das TK, Park DM. Effect of treatment with botulinum toxin on spasticity. Postgrad Med J. 1989;65:208-210. doi: 10.1136/pgmj.65.762.208
14. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:332. doi: 10.3390/toxins12050332
15. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3
16. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035
17. Kumar R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018;2:NS20180058. https://doi.org/10.1042/NS20180058
18. Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Research. 2013;2:52. https://doi.org/10.12688/f1000research.2-52.v2
19. Blanshan N, Krug H. The use of botulinum toxin for the treatment of chronic joint pain: clinical and experimental evidence. Toxins (Basel). 2020;12:314. doi: 10.3390/toxins12050314
20. Hsu P-C, Wu W-T, Han D-S, et al. Comparative effectiveness of botulinum toxin injection for chronic shoulder pain: a meta-analysis of randomized controlled trials. Toxins (Basel). 2020;12:251. doi: 10.3390/toxins12040251
21. Zhai S, Huang B, Yu K. The efficacy and safety of botulinum toxin type A in painful knee osteoarthritis: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060519895868. doi: 10.1177/0300060519895868
22. Isner-Horobeti ME, Dufour SP, Blaes C, et al. Intramuscular pressure before and after botulinum toxin in chronic exertional compartment syndrome of the leg: a preliminary study. Am J Sports Med. 2013;41:2558‐2566. doi: 10.1177/0363546513499183
23. Hutto WM, Schroeder PB, Leggit JC. Botulinum toxin as a novel treatment for chronic exertional compartment syndrome in the US Military. Mil Med. 2019;184:e458‐e461. doi: 10.1093/milmed/usy223
24. Rahman A, Hamid A, Inozemtsev K, et al. Thoracic outlet syndrome treated with injecting botulinum toxin into middle scalene muscle and pectoral muscle interfascial planes: a case report. A A Pract. 2019;12:235‐237. doi: 10.1213/XAA.0000000000000894
25. Finlayson HC, O’Connor RJ, Brasher PMA, et al. Botulinum toxin injection for management of thoracic outlet syndrome: a double-blind, randomized, controlled trial. Pain. 2011;152:2023-2028. doi: 10.1016/j.pain.2011.04.027
26. Donahue DM, Godoy IRB, Gupta R, et al. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49:715-722. https://doi.org/10.1007/s00256-019-03331-9
27. Wei J, Zhu X, Yang G, et al. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: a meta‐analysis of randomized controlled trials. Brain Behav. 2019;9:e01409. doi: 10.1002/brb3.1409
28. Samant PD, Kale SY, Ahmed S, et al. Randomized controlled study comparing clinical outcomes after injection botulinum toxin type A versus corticosteroids in chronic plantar fasciitis. Int J Res Orthop. 2018;4:672-675. http://dx.doi.org/10.18203/issn.2455-4510.IntJResOrthop20182744
29. Fry DA. Is botulinum toxin injection effective in reducing pain in patients diagnosed with plantar fasciitis? PCOM Physician Assistant Studies Student Scholarship. 2019;461. https://digitalcommons.pcom.edu/pa_systematic_reviews/461
30. Lin YC, Wu WT, Hsu YC, et al. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131-145. doi: 10.1177/0269215517702517
31. Ruiz AG, Díaz GV, Fernández BR, et al. Effects of ultrasound-guided administration of botulinum toxin (incobotulinumtoxinA) in patients with lateral epicondylitis. Toxins (Basel). 2019;11:46. doi: 10.3390/toxins11010046
32. Villa S, Raoul G, Machuron F, et al. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J Stomatol Oral Maxillofac Surg. 2019;120:2-6. doi: 10.1016/j.jormas.2018.10.00
33. Fu KY, Che, HM, Sun ZP, et al. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br J Oral Maxillofac Surg. 2010;48:281-284. doi: 10.1016/j.bjoms.2009.07.014
34. Machado D, Kumar A, Jabbari B. Abobotulinum toxin A in the treatment of chronic low back pain. Toxins (Basel). 2016;8:374. doi: 10.3390/toxins8120374
35. Cogné M, Petit H, Creuzé A, et al. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. https://doi.org/10.1186/s12891-017-1816-6
36. Ahmed S, Subramaniam S, Sidhu K, et al. Effect of local anesthetic versus botulinum toxin-A injections for myofascial pain disorders. Clin J Pain. 2019;35:353-367. doi: 10.1097/AJP.0000000000000681
37. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327. doi: 10.1016/0029-7844(95)00458-0
38. Luo FY, Nasr-Esfahani M, Jarrell J, et al. Botulinum toxin injection for chronic pelvic pain: a systematic review. Acta Obstet Gynecol Scand. 2020;99:1595-1602. https://doi.org/10.1111/aogs.13946
39. Lessard L, Bartow MJ, Lee J, et al. Botulinum toxin A: a novel therapeutic modality for upper extremity chronic regional pain syndrome. Plast Reconstr Surg Glob Open. 2018;6:e1847. doi: 10.1097/GOX.0000000000001847
40. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins (Basel). 2018;10:164. doi: 10.3390/toxins10040164
41. Kwak H, Koh DJ, Min K. Botulinum toxin treatment for intractable allodynia in a patient with complex regional pain syndrome: a case report. Neurology Asia. 2020;25:215-219.
PRACTICE RECOMMENDATIONS
› Consider botulinum toxin (BoNT) for patients with headache, spasticity, or cervical dystonia, as the FDA has approved BoNT for pain relief in these conditions. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
AGA Clinical Care Pathway: Screening, diagnosis, and treatment of NAFLD and NASH
The American Gastroenterological Association recently published a Clinical Care Pathway for screening, diagnosis, and treatment of patients with nonalcoholic fatty liver disease (NAFLD).
Recommendations are intended for a spectrum of clinical settings, including primary care, obesity medicine, gastroenterology, hepatology, and endocrinology practices, reported lead author Fasiha Kanwal, MD, of Baylor College of Medicine, Houston, and colleagues.
“Most patients with NAFLD and NASH [nonalcoholic steatohepatitis] are seen in primary care or endocrine clinics,” the authors wrote in Gastroenterology. “Although not all patients with NAFLD/NASH require secondary (i.e., hepatology) care, not knowing which patients might benefit from such care and when to refer them results in inconsistent care processes and possibly poor outcomes. Clinical Care Pathways, with careful explication of each step in screening, diagnosis, and treatment, have been shown to improve the quality of health care delivery in other areas of medicine, [and] are crucial to addressing the often inconsistent care processes characterizing current approaches to NAFLD/NASH.”
The guidance was drafted by a group of 15 multidisciplinary experts from around the world representing the AGA, the American Diabetes Association, the American Osteopathic Association, the Obesity Society, and the Endocrine Society. Recommendations were based on available literature and clinical experience.
The authors recommended a four-step screening process for NAFLD/NASH: Check for risk factors predicting clinically relevant fibrosis (stage F2 or higher), review history and perform relevant laboratory tests, conduct noninvasive liver fibrosis testing, and measure liver stiffness.
Patients at greatest risk for clinically significant fibrosis include those with two or more metabolic risk factors, those with type 2 diabetes, and those with incidentally detected steatosis and/or elevated aminotransferases.
“A recent retrospective cohort study found that patients with hepatic steatosis and elevated alanine aminotransferase had a significantly higher risk of progression to cirrhosis or hepatocellular carcinoma than patients with hepatic steatosis and persistently normal alanine aminotransferase,” the authors noted.
When any of the above risk factors are present, the authors recommended checking the patient’s history for excessive alcohol intake, conducting a complete blood count and liver function tests, and screening for other hepatic and biliary diseases, such as chronic hepatitis C virus infection and liver mass lesions.
If other liver diseases have been ruled out, the first step in liver fibrosis risk stratification involves noninvasive testing, with the authors favoring the Fibrosis-4 (FIB-4) score “because it has been shown to have the best diagnostic accuracy for advanced fibrosis, compared with other noninvasive markers of fibrosis in patients with NAFLD.”
The next step in risk stratification involves liver stiffness measurement (LSM) with FibroScan (vibration controlled transient elastography [VCTE]), or newer modalities, such as bidimensional shear wave elastography or point shear wave elastography, which offer “diagnostic performances at least as good as VCTE.”
According to the publication, patients with NAFLD at low risk of advanced fibrosis (FIB-4 less than 1.3 or LSM less than 8 kPa or liver biopsy F0-F1) can be managed by one provider, such as a primary care provider or endocrinologist, whereas indeterminate-risk patients (FIB-4 of 1.3-2.67 and/or LSM 8-12 kPa and liver biopsy unavailable) and high-risk patients (FIB-4 greater than 2.67 or LSM greater than 12 kPa or liver biopsy F2-F4) should be managed by a multidisciplinary team led by a hepatologist.
Lifestyle intervention, weight loss (if overweight or obese), and cardiovascular disease risk reduction are advised for patients of all risk categories.
“There are no large, long-term behavioral modification or pharmacotherapy studies regarding weight loss in individuals with NAFLD,” the authors wrote. “However, weight loss of any magnitude should be encouraged as beneficial.”
For patients with indeterminate and high risk, NASH pharmacotherapy is recommended, and if needed, diabetes care should involve medications with efficacy in NASH, such as pioglitazone.
“Although we recognize that knowledge is continuing to evolve and that recommendations may change accordingly over time, we believe this Pathway provides accessible, standardized, evidence-based, timely, and testable recommendations that will allow clinicians to care for a rapidly growing population of patients, most of whom are managed in primary care or endocrine clinics,” the authors concluded.
The article was supported by the American Gastroenterological Association, Intercept Pharmaceuticals, Pfizer, and others. The authors disclosed relationships with Novo Nordisk, Eli Lilly, Sanofi, and others.
The American Gastroenterological Association recently published a Clinical Care Pathway for screening, diagnosis, and treatment of patients with nonalcoholic fatty liver disease (NAFLD).
Recommendations are intended for a spectrum of clinical settings, including primary care, obesity medicine, gastroenterology, hepatology, and endocrinology practices, reported lead author Fasiha Kanwal, MD, of Baylor College of Medicine, Houston, and colleagues.
“Most patients with NAFLD and NASH [nonalcoholic steatohepatitis] are seen in primary care or endocrine clinics,” the authors wrote in Gastroenterology. “Although not all patients with NAFLD/NASH require secondary (i.e., hepatology) care, not knowing which patients might benefit from such care and when to refer them results in inconsistent care processes and possibly poor outcomes. Clinical Care Pathways, with careful explication of each step in screening, diagnosis, and treatment, have been shown to improve the quality of health care delivery in other areas of medicine, [and] are crucial to addressing the often inconsistent care processes characterizing current approaches to NAFLD/NASH.”
The guidance was drafted by a group of 15 multidisciplinary experts from around the world representing the AGA, the American Diabetes Association, the American Osteopathic Association, the Obesity Society, and the Endocrine Society. Recommendations were based on available literature and clinical experience.
The authors recommended a four-step screening process for NAFLD/NASH: Check for risk factors predicting clinically relevant fibrosis (stage F2 or higher), review history and perform relevant laboratory tests, conduct noninvasive liver fibrosis testing, and measure liver stiffness.
Patients at greatest risk for clinically significant fibrosis include those with two or more metabolic risk factors, those with type 2 diabetes, and those with incidentally detected steatosis and/or elevated aminotransferases.
“A recent retrospective cohort study found that patients with hepatic steatosis and elevated alanine aminotransferase had a significantly higher risk of progression to cirrhosis or hepatocellular carcinoma than patients with hepatic steatosis and persistently normal alanine aminotransferase,” the authors noted.
When any of the above risk factors are present, the authors recommended checking the patient’s history for excessive alcohol intake, conducting a complete blood count and liver function tests, and screening for other hepatic and biliary diseases, such as chronic hepatitis C virus infection and liver mass lesions.
If other liver diseases have been ruled out, the first step in liver fibrosis risk stratification involves noninvasive testing, with the authors favoring the Fibrosis-4 (FIB-4) score “because it has been shown to have the best diagnostic accuracy for advanced fibrosis, compared with other noninvasive markers of fibrosis in patients with NAFLD.”
The next step in risk stratification involves liver stiffness measurement (LSM) with FibroScan (vibration controlled transient elastography [VCTE]), or newer modalities, such as bidimensional shear wave elastography or point shear wave elastography, which offer “diagnostic performances at least as good as VCTE.”
According to the publication, patients with NAFLD at low risk of advanced fibrosis (FIB-4 less than 1.3 or LSM less than 8 kPa or liver biopsy F0-F1) can be managed by one provider, such as a primary care provider or endocrinologist, whereas indeterminate-risk patients (FIB-4 of 1.3-2.67 and/or LSM 8-12 kPa and liver biopsy unavailable) and high-risk patients (FIB-4 greater than 2.67 or LSM greater than 12 kPa or liver biopsy F2-F4) should be managed by a multidisciplinary team led by a hepatologist.
Lifestyle intervention, weight loss (if overweight or obese), and cardiovascular disease risk reduction are advised for patients of all risk categories.
“There are no large, long-term behavioral modification or pharmacotherapy studies regarding weight loss in individuals with NAFLD,” the authors wrote. “However, weight loss of any magnitude should be encouraged as beneficial.”
For patients with indeterminate and high risk, NASH pharmacotherapy is recommended, and if needed, diabetes care should involve medications with efficacy in NASH, such as pioglitazone.
“Although we recognize that knowledge is continuing to evolve and that recommendations may change accordingly over time, we believe this Pathway provides accessible, standardized, evidence-based, timely, and testable recommendations that will allow clinicians to care for a rapidly growing population of patients, most of whom are managed in primary care or endocrine clinics,” the authors concluded.
The article was supported by the American Gastroenterological Association, Intercept Pharmaceuticals, Pfizer, and others. The authors disclosed relationships with Novo Nordisk, Eli Lilly, Sanofi, and others.
The American Gastroenterological Association recently published a Clinical Care Pathway for screening, diagnosis, and treatment of patients with nonalcoholic fatty liver disease (NAFLD).
Recommendations are intended for a spectrum of clinical settings, including primary care, obesity medicine, gastroenterology, hepatology, and endocrinology practices, reported lead author Fasiha Kanwal, MD, of Baylor College of Medicine, Houston, and colleagues.
“Most patients with NAFLD and NASH [nonalcoholic steatohepatitis] are seen in primary care or endocrine clinics,” the authors wrote in Gastroenterology. “Although not all patients with NAFLD/NASH require secondary (i.e., hepatology) care, not knowing which patients might benefit from such care and when to refer them results in inconsistent care processes and possibly poor outcomes. Clinical Care Pathways, with careful explication of each step in screening, diagnosis, and treatment, have been shown to improve the quality of health care delivery in other areas of medicine, [and] are crucial to addressing the often inconsistent care processes characterizing current approaches to NAFLD/NASH.”
The guidance was drafted by a group of 15 multidisciplinary experts from around the world representing the AGA, the American Diabetes Association, the American Osteopathic Association, the Obesity Society, and the Endocrine Society. Recommendations were based on available literature and clinical experience.
The authors recommended a four-step screening process for NAFLD/NASH: Check for risk factors predicting clinically relevant fibrosis (stage F2 or higher), review history and perform relevant laboratory tests, conduct noninvasive liver fibrosis testing, and measure liver stiffness.
Patients at greatest risk for clinically significant fibrosis include those with two or more metabolic risk factors, those with type 2 diabetes, and those with incidentally detected steatosis and/or elevated aminotransferases.
“A recent retrospective cohort study found that patients with hepatic steatosis and elevated alanine aminotransferase had a significantly higher risk of progression to cirrhosis or hepatocellular carcinoma than patients with hepatic steatosis and persistently normal alanine aminotransferase,” the authors noted.
When any of the above risk factors are present, the authors recommended checking the patient’s history for excessive alcohol intake, conducting a complete blood count and liver function tests, and screening for other hepatic and biliary diseases, such as chronic hepatitis C virus infection and liver mass lesions.
If other liver diseases have been ruled out, the first step in liver fibrosis risk stratification involves noninvasive testing, with the authors favoring the Fibrosis-4 (FIB-4) score “because it has been shown to have the best diagnostic accuracy for advanced fibrosis, compared with other noninvasive markers of fibrosis in patients with NAFLD.”
The next step in risk stratification involves liver stiffness measurement (LSM) with FibroScan (vibration controlled transient elastography [VCTE]), or newer modalities, such as bidimensional shear wave elastography or point shear wave elastography, which offer “diagnostic performances at least as good as VCTE.”
According to the publication, patients with NAFLD at low risk of advanced fibrosis (FIB-4 less than 1.3 or LSM less than 8 kPa or liver biopsy F0-F1) can be managed by one provider, such as a primary care provider or endocrinologist, whereas indeterminate-risk patients (FIB-4 of 1.3-2.67 and/or LSM 8-12 kPa and liver biopsy unavailable) and high-risk patients (FIB-4 greater than 2.67 or LSM greater than 12 kPa or liver biopsy F2-F4) should be managed by a multidisciplinary team led by a hepatologist.
Lifestyle intervention, weight loss (if overweight or obese), and cardiovascular disease risk reduction are advised for patients of all risk categories.
“There are no large, long-term behavioral modification or pharmacotherapy studies regarding weight loss in individuals with NAFLD,” the authors wrote. “However, weight loss of any magnitude should be encouraged as beneficial.”
For patients with indeterminate and high risk, NASH pharmacotherapy is recommended, and if needed, diabetes care should involve medications with efficacy in NASH, such as pioglitazone.
“Although we recognize that knowledge is continuing to evolve and that recommendations may change accordingly over time, we believe this Pathway provides accessible, standardized, evidence-based, timely, and testable recommendations that will allow clinicians to care for a rapidly growing population of patients, most of whom are managed in primary care or endocrine clinics,” the authors concluded.
The article was supported by the American Gastroenterological Association, Intercept Pharmaceuticals, Pfizer, and others. The authors disclosed relationships with Novo Nordisk, Eli Lilly, Sanofi, and others.
FROM GASTROENTEROLOGY
Stroke thrombectomy alone fails noninferiority to bridging tPA
In the prospective, multicenter trial, the rate of good functional outcome was 57% for patients who underwent direct thrombectomy and 65% among patients who received IV thrombolysis before undergoing thrombectomy. This result failed to demonstrate noninferiority of direct mechanical thrombectomy compared to combination therapy, the researchers conclude.
“Good outcome was high in both treatment arms, with the point estimate in favor of the bridging cohort,” said lead investigator Urs Fischer, MD, co-chair of the stroke center at Inselspital, Bern University Hospital, Switzerland, during his presentation. “Postinterventional reperfusion was very high in both treatment arms and higher in patients with bridging thrombolysis, compared to direct mechanical thrombectomy.”
The findings were presented at the 13th World Stroke Congress (WSC) 2021.
Two views of thrombolysis
The value of bridging thrombolysis for patients who undergo mechanical thrombectomy is a matter of debate. One argument is that, for patients with large-vessel occlusion, IV thrombolysis may improve reperfusion before and after thrombectomy and yield better clinical outcomes. The opposing argument is that bridging thrombolysis may increase the risk for distal emboli, delay mechanical thrombectomy, and increase the rate of hemorrhage.
The researchers conducted the SWIFT DIRECT trial to investigate this question. They enrolled patients with acute ischemic stroke due to occlusion of the internal carotid artery or the M1 segment of the middle cerebral artery.
The trial was conducted at 48 sites in seven European countries and Canada. The investigators randomly assigned patients to receive IV alteplase (0.9 mg/kg) plus mechanical thrombectomy with the Solitaire device or to receive direct mechanical thrombectomy with the same device. Treatment was open label, but the assessment of endpoints was blinded.
Investigators assigned 423 patients to treatment, and 408 were included in the full analysis set. Of this group, 201 participants received direct mechanical thrombectomy, and 207 received IV thrombolysis plus thrombectomy. There were three crossovers in each treatment arm.
The primary outcome was functional independence, defined as a Modified Rankin Scale (mRS) score of 0-2, at 90 days. Secondary outcomes included mortality at 90 days, mRS shift, change in National Institutes of Health Stroke Scale (NIHSS) score at 24 hours, successful reperfusion, and symptomatic and asymptomatic intracranial hemorrhage (ICH).
Noninferiority not demonstrated
At baseline, patient characteristics were well balanced between the treatment groups. The median age of the patients was 72 years, and about 50% of participants were women. The median NIHSS score was 17 in both arms.
Approximately 57% of patients who underwent direct thrombectomy and 65% of those who received IV thrombolysis plus thrombectomy were functionally independent at 90 days, the primary outcome.
In addition, the researchers found no difference in mRS shift, mortality at 90 days, or change in NIHSS score at 24 hours. Postinterventional reperfusion was very high in both arms and was higher in patients who received IV tissue plasminogen activator, compared with those who received direct mechanical thrombectomy, said Dr. Fischer.
The rate of successful postinterventional reperfusion, however, was higher among patients who received thrombolysis than among those who underwent direct thrombectomy. The rate of symptomatic ICH was 1.5% in the direct thrombectomy group and 4.9% in the thrombolysis-plus-thrombectomy group.
New endpoints needed?
The investigators used noninferiority margins of 12%. “This question about the noninferiority margins, that’s a very tricky and difficult one in randomized clinical trials,” said Dr. Fischer. The investigators defined their margin using the 2015 HERMES data because no trials had yet compared direct mechanical thrombectomy and bridging thrombolysis at the time.
The researchers are performing a pooled analysis of all the trials that compared bridging thrombolysis with direct mechanical thrombectomy. “We are therefore looking at several margins, and I think this is the way we should look at these noninferiority margins,” said Dr. Fischer. “There’s not a clear-cut level which you can define.”
Enrollment in the trial was well balanced with respect to gender, which is not always the case in stroke studies, said Kevin Sheth, MD, professor of neurology and neurosurgery at Yale School of Medicine, New Haven, Conn., who commented on the study for this news organization.
The findings indicate that the likelihood of there being a difference between groups on this question is low, said Dr. Sheth. Both groups had large-vessel occlusion, both received thrombectomy, and both achieved reperfusion. But the higher rate of successful reperfusion in the bridging cohort was not reflected in any of the clinical endpoints that the investigators examined.
Observing a difference in this context will require very large trials or different endpoints that are more responsive to the intervention, said Dr. Sheth. “This is going to be a challenge for not just this but for any neuroprotection trial in the future,” he said.
The study was supported by Medtronic. Dr. Fischer has served as a consultant for Medtronic, Stryker, and CSL Behring. Dr. Sheth has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the prospective, multicenter trial, the rate of good functional outcome was 57% for patients who underwent direct thrombectomy and 65% among patients who received IV thrombolysis before undergoing thrombectomy. This result failed to demonstrate noninferiority of direct mechanical thrombectomy compared to combination therapy, the researchers conclude.
“Good outcome was high in both treatment arms, with the point estimate in favor of the bridging cohort,” said lead investigator Urs Fischer, MD, co-chair of the stroke center at Inselspital, Bern University Hospital, Switzerland, during his presentation. “Postinterventional reperfusion was very high in both treatment arms and higher in patients with bridging thrombolysis, compared to direct mechanical thrombectomy.”
The findings were presented at the 13th World Stroke Congress (WSC) 2021.
Two views of thrombolysis
The value of bridging thrombolysis for patients who undergo mechanical thrombectomy is a matter of debate. One argument is that, for patients with large-vessel occlusion, IV thrombolysis may improve reperfusion before and after thrombectomy and yield better clinical outcomes. The opposing argument is that bridging thrombolysis may increase the risk for distal emboli, delay mechanical thrombectomy, and increase the rate of hemorrhage.
The researchers conducted the SWIFT DIRECT trial to investigate this question. They enrolled patients with acute ischemic stroke due to occlusion of the internal carotid artery or the M1 segment of the middle cerebral artery.
The trial was conducted at 48 sites in seven European countries and Canada. The investigators randomly assigned patients to receive IV alteplase (0.9 mg/kg) plus mechanical thrombectomy with the Solitaire device or to receive direct mechanical thrombectomy with the same device. Treatment was open label, but the assessment of endpoints was blinded.
Investigators assigned 423 patients to treatment, and 408 were included in the full analysis set. Of this group, 201 participants received direct mechanical thrombectomy, and 207 received IV thrombolysis plus thrombectomy. There were three crossovers in each treatment arm.
The primary outcome was functional independence, defined as a Modified Rankin Scale (mRS) score of 0-2, at 90 days. Secondary outcomes included mortality at 90 days, mRS shift, change in National Institutes of Health Stroke Scale (NIHSS) score at 24 hours, successful reperfusion, and symptomatic and asymptomatic intracranial hemorrhage (ICH).
Noninferiority not demonstrated
At baseline, patient characteristics were well balanced between the treatment groups. The median age of the patients was 72 years, and about 50% of participants were women. The median NIHSS score was 17 in both arms.
Approximately 57% of patients who underwent direct thrombectomy and 65% of those who received IV thrombolysis plus thrombectomy were functionally independent at 90 days, the primary outcome.
In addition, the researchers found no difference in mRS shift, mortality at 90 days, or change in NIHSS score at 24 hours. Postinterventional reperfusion was very high in both arms and was higher in patients who received IV tissue plasminogen activator, compared with those who received direct mechanical thrombectomy, said Dr. Fischer.
The rate of successful postinterventional reperfusion, however, was higher among patients who received thrombolysis than among those who underwent direct thrombectomy. The rate of symptomatic ICH was 1.5% in the direct thrombectomy group and 4.9% in the thrombolysis-plus-thrombectomy group.
New endpoints needed?
The investigators used noninferiority margins of 12%. “This question about the noninferiority margins, that’s a very tricky and difficult one in randomized clinical trials,” said Dr. Fischer. The investigators defined their margin using the 2015 HERMES data because no trials had yet compared direct mechanical thrombectomy and bridging thrombolysis at the time.
The researchers are performing a pooled analysis of all the trials that compared bridging thrombolysis with direct mechanical thrombectomy. “We are therefore looking at several margins, and I think this is the way we should look at these noninferiority margins,” said Dr. Fischer. “There’s not a clear-cut level which you can define.”
Enrollment in the trial was well balanced with respect to gender, which is not always the case in stroke studies, said Kevin Sheth, MD, professor of neurology and neurosurgery at Yale School of Medicine, New Haven, Conn., who commented on the study for this news organization.
The findings indicate that the likelihood of there being a difference between groups on this question is low, said Dr. Sheth. Both groups had large-vessel occlusion, both received thrombectomy, and both achieved reperfusion. But the higher rate of successful reperfusion in the bridging cohort was not reflected in any of the clinical endpoints that the investigators examined.
Observing a difference in this context will require very large trials or different endpoints that are more responsive to the intervention, said Dr. Sheth. “This is going to be a challenge for not just this but for any neuroprotection trial in the future,” he said.
The study was supported by Medtronic. Dr. Fischer has served as a consultant for Medtronic, Stryker, and CSL Behring. Dr. Sheth has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the prospective, multicenter trial, the rate of good functional outcome was 57% for patients who underwent direct thrombectomy and 65% among patients who received IV thrombolysis before undergoing thrombectomy. This result failed to demonstrate noninferiority of direct mechanical thrombectomy compared to combination therapy, the researchers conclude.
“Good outcome was high in both treatment arms, with the point estimate in favor of the bridging cohort,” said lead investigator Urs Fischer, MD, co-chair of the stroke center at Inselspital, Bern University Hospital, Switzerland, during his presentation. “Postinterventional reperfusion was very high in both treatment arms and higher in patients with bridging thrombolysis, compared to direct mechanical thrombectomy.”
The findings were presented at the 13th World Stroke Congress (WSC) 2021.
Two views of thrombolysis
The value of bridging thrombolysis for patients who undergo mechanical thrombectomy is a matter of debate. One argument is that, for patients with large-vessel occlusion, IV thrombolysis may improve reperfusion before and after thrombectomy and yield better clinical outcomes. The opposing argument is that bridging thrombolysis may increase the risk for distal emboli, delay mechanical thrombectomy, and increase the rate of hemorrhage.
The researchers conducted the SWIFT DIRECT trial to investigate this question. They enrolled patients with acute ischemic stroke due to occlusion of the internal carotid artery or the M1 segment of the middle cerebral artery.
The trial was conducted at 48 sites in seven European countries and Canada. The investigators randomly assigned patients to receive IV alteplase (0.9 mg/kg) plus mechanical thrombectomy with the Solitaire device or to receive direct mechanical thrombectomy with the same device. Treatment was open label, but the assessment of endpoints was blinded.
Investigators assigned 423 patients to treatment, and 408 were included in the full analysis set. Of this group, 201 participants received direct mechanical thrombectomy, and 207 received IV thrombolysis plus thrombectomy. There were three crossovers in each treatment arm.
The primary outcome was functional independence, defined as a Modified Rankin Scale (mRS) score of 0-2, at 90 days. Secondary outcomes included mortality at 90 days, mRS shift, change in National Institutes of Health Stroke Scale (NIHSS) score at 24 hours, successful reperfusion, and symptomatic and asymptomatic intracranial hemorrhage (ICH).
Noninferiority not demonstrated
At baseline, patient characteristics were well balanced between the treatment groups. The median age of the patients was 72 years, and about 50% of participants were women. The median NIHSS score was 17 in both arms.
Approximately 57% of patients who underwent direct thrombectomy and 65% of those who received IV thrombolysis plus thrombectomy were functionally independent at 90 days, the primary outcome.
In addition, the researchers found no difference in mRS shift, mortality at 90 days, or change in NIHSS score at 24 hours. Postinterventional reperfusion was very high in both arms and was higher in patients who received IV tissue plasminogen activator, compared with those who received direct mechanical thrombectomy, said Dr. Fischer.
The rate of successful postinterventional reperfusion, however, was higher among patients who received thrombolysis than among those who underwent direct thrombectomy. The rate of symptomatic ICH was 1.5% in the direct thrombectomy group and 4.9% in the thrombolysis-plus-thrombectomy group.
New endpoints needed?
The investigators used noninferiority margins of 12%. “This question about the noninferiority margins, that’s a very tricky and difficult one in randomized clinical trials,” said Dr. Fischer. The investigators defined their margin using the 2015 HERMES data because no trials had yet compared direct mechanical thrombectomy and bridging thrombolysis at the time.
The researchers are performing a pooled analysis of all the trials that compared bridging thrombolysis with direct mechanical thrombectomy. “We are therefore looking at several margins, and I think this is the way we should look at these noninferiority margins,” said Dr. Fischer. “There’s not a clear-cut level which you can define.”
Enrollment in the trial was well balanced with respect to gender, which is not always the case in stroke studies, said Kevin Sheth, MD, professor of neurology and neurosurgery at Yale School of Medicine, New Haven, Conn., who commented on the study for this news organization.
The findings indicate that the likelihood of there being a difference between groups on this question is low, said Dr. Sheth. Both groups had large-vessel occlusion, both received thrombectomy, and both achieved reperfusion. But the higher rate of successful reperfusion in the bridging cohort was not reflected in any of the clinical endpoints that the investigators examined.
Observing a difference in this context will require very large trials or different endpoints that are more responsive to the intervention, said Dr. Sheth. “This is going to be a challenge for not just this but for any neuroprotection trial in the future,” he said.
The study was supported by Medtronic. Dr. Fischer has served as a consultant for Medtronic, Stryker, and CSL Behring. Dr. Sheth has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM WSC 2021
Pandemic stresses harder on physician moms than physician dads: Study
COVID-19 has been difficult for parents trying to balance careers, home life, and keeping their loved ones safe. A new study indicates that, not only are physicians not immune to these stressors, but the long-term effects could be devastating for health care overall.
In a study published Nov. 11, 2021, in JAMA Network Open , researchers found that stresses to work/life balance and family life caused by the pandemic have differed among men and women physicians.
Physicians and other health care workers have been at the front lines of the COVID-19 pandemic, and their work lives have been the focus of a lot of attention in the media and by researchers. Their family lives, not so much. But physicians have families, and the pandemic has upended almost everything about their lives, particularly where work life and home life intersect. School and day care closures, working from home, working extra hours, or working less – all of these changes have consequences on family life and the mental health of parents who are also physicians.
Findings from a Medscape survey published in early 2021 indicate that more female physicians than male physicians were either “conflicted” or “very conflicted” as parents because of work demands (42% vs. 23%) nearly 6 months into the pandemic.
In the current study, researchers from the University of Michigan, Harvard University, and the Medical University of South Carolina teamed up to investigate gender differences in how work/family factors affected the mental health of early-career physician parents in the United States during the first year of the COVID-19 pandemic. The results suggest that the pandemic has increased gender disparity and added disproportionately to the burden of female physicians.
Managing the household falls mostly on moms
Participants were physicians enrolled in the Intern Health Study, a longitudinal study that regularly surveys medical interns in the United States to assess stress and mood. When researchers compared survey results from before the onset of the pandemic (2018) with later results (2020), they found a striking gender difference in how the pandemic has changed family and work duties for physicians.
The authors of the study pointed out that previous research had found that female physicians take on a greater share of household and childcare duties than male physicians. The current study found that their share had increased with the pandemic. Physician moms are now 30 times more likely to be in charge of these tasks than physician dads.
In families in which both parents were physicians, none of the men said they took the primary role in managing the extra demands caused by the pandemic. In addition, women were twice as likely as men to work primarily from home and to work reduced hours.
The extra stress seems to be taking a toll on women physicians. In the 2020 survey, physician mothers had higher scores for anxiety and depression symptoms, compared with men. Notably, the 2018 survey did not show a significant difference in depression scores between men and women. Nor were there significant differences in depression and anxiety scores between women and men who were not parents or in reports of work/family conflict before and after the pandemic.
In general, the results indicate that the pandemic has only widened the gender gap between women and men physicians when it comes to managing family life and dealing with the stresses of maintaining a suitable work-life balance.
‘Long-term repercussions’ for gender equity in medicine
Although these are serious problems for women physicians and their families, the effects go beyond the home and beyond individuals. Even before the pandemic, women in medicine struggled for parity in career advancement and opportunities as well as in pay, and this new setback could make those challenges even greater.
“Even short-term adjustments can have serious long-term repercussions as they may lead to lower earnings and negatively impact opportunities for promotion, further exacerbating gender inequalities in compensation and advancement,” the study’s authors wrote.
The potential damage extends to the entire profession and the health care system itself. The profession is already struggling to retain young female physicians, and this situation is likely to make that problem worse and have long-term consequences. Citing data showing that female physicians spend more time with patients and that their patients may have better outcomes, the authors wrote that the consequences of losing more early-career female physicians “could be devastating to the U.S. health care system, particularly in the context of a global pandemic and an impending physician shortage.”
The sample size was small (276 U.S. physicians), and the study relied on self-reported data. The findings suggest that more research on this topic is needed, especially research that includes other demographic factors, such as sexual orientation and ethnicity. The authors recommend that institutional and public policymakers take into account the effects of the pandemic on physician mothers to ensure that recent gains in gender equity for women physicians do not fall victim to COVID-19.
A version of this article first appeared on Medscape.com.
COVID-19 has been difficult for parents trying to balance careers, home life, and keeping their loved ones safe. A new study indicates that, not only are physicians not immune to these stressors, but the long-term effects could be devastating for health care overall.
In a study published Nov. 11, 2021, in JAMA Network Open , researchers found that stresses to work/life balance and family life caused by the pandemic have differed among men and women physicians.
Physicians and other health care workers have been at the front lines of the COVID-19 pandemic, and their work lives have been the focus of a lot of attention in the media and by researchers. Their family lives, not so much. But physicians have families, and the pandemic has upended almost everything about their lives, particularly where work life and home life intersect. School and day care closures, working from home, working extra hours, or working less – all of these changes have consequences on family life and the mental health of parents who are also physicians.
Findings from a Medscape survey published in early 2021 indicate that more female physicians than male physicians were either “conflicted” or “very conflicted” as parents because of work demands (42% vs. 23%) nearly 6 months into the pandemic.
In the current study, researchers from the University of Michigan, Harvard University, and the Medical University of South Carolina teamed up to investigate gender differences in how work/family factors affected the mental health of early-career physician parents in the United States during the first year of the COVID-19 pandemic. The results suggest that the pandemic has increased gender disparity and added disproportionately to the burden of female physicians.
Managing the household falls mostly on moms
Participants were physicians enrolled in the Intern Health Study, a longitudinal study that regularly surveys medical interns in the United States to assess stress and mood. When researchers compared survey results from before the onset of the pandemic (2018) with later results (2020), they found a striking gender difference in how the pandemic has changed family and work duties for physicians.
The authors of the study pointed out that previous research had found that female physicians take on a greater share of household and childcare duties than male physicians. The current study found that their share had increased with the pandemic. Physician moms are now 30 times more likely to be in charge of these tasks than physician dads.
In families in which both parents were physicians, none of the men said they took the primary role in managing the extra demands caused by the pandemic. In addition, women were twice as likely as men to work primarily from home and to work reduced hours.
The extra stress seems to be taking a toll on women physicians. In the 2020 survey, physician mothers had higher scores for anxiety and depression symptoms, compared with men. Notably, the 2018 survey did not show a significant difference in depression scores between men and women. Nor were there significant differences in depression and anxiety scores between women and men who were not parents or in reports of work/family conflict before and after the pandemic.
In general, the results indicate that the pandemic has only widened the gender gap between women and men physicians when it comes to managing family life and dealing with the stresses of maintaining a suitable work-life balance.
‘Long-term repercussions’ for gender equity in medicine
Although these are serious problems for women physicians and their families, the effects go beyond the home and beyond individuals. Even before the pandemic, women in medicine struggled for parity in career advancement and opportunities as well as in pay, and this new setback could make those challenges even greater.
“Even short-term adjustments can have serious long-term repercussions as they may lead to lower earnings and negatively impact opportunities for promotion, further exacerbating gender inequalities in compensation and advancement,” the study’s authors wrote.
The potential damage extends to the entire profession and the health care system itself. The profession is already struggling to retain young female physicians, and this situation is likely to make that problem worse and have long-term consequences. Citing data showing that female physicians spend more time with patients and that their patients may have better outcomes, the authors wrote that the consequences of losing more early-career female physicians “could be devastating to the U.S. health care system, particularly in the context of a global pandemic and an impending physician shortage.”
The sample size was small (276 U.S. physicians), and the study relied on self-reported data. The findings suggest that more research on this topic is needed, especially research that includes other demographic factors, such as sexual orientation and ethnicity. The authors recommend that institutional and public policymakers take into account the effects of the pandemic on physician mothers to ensure that recent gains in gender equity for women physicians do not fall victim to COVID-19.
A version of this article first appeared on Medscape.com.
COVID-19 has been difficult for parents trying to balance careers, home life, and keeping their loved ones safe. A new study indicates that, not only are physicians not immune to these stressors, but the long-term effects could be devastating for health care overall.
In a study published Nov. 11, 2021, in JAMA Network Open , researchers found that stresses to work/life balance and family life caused by the pandemic have differed among men and women physicians.
Physicians and other health care workers have been at the front lines of the COVID-19 pandemic, and their work lives have been the focus of a lot of attention in the media and by researchers. Their family lives, not so much. But physicians have families, and the pandemic has upended almost everything about their lives, particularly where work life and home life intersect. School and day care closures, working from home, working extra hours, or working less – all of these changes have consequences on family life and the mental health of parents who are also physicians.
Findings from a Medscape survey published in early 2021 indicate that more female physicians than male physicians were either “conflicted” or “very conflicted” as parents because of work demands (42% vs. 23%) nearly 6 months into the pandemic.
In the current study, researchers from the University of Michigan, Harvard University, and the Medical University of South Carolina teamed up to investigate gender differences in how work/family factors affected the mental health of early-career physician parents in the United States during the first year of the COVID-19 pandemic. The results suggest that the pandemic has increased gender disparity and added disproportionately to the burden of female physicians.
Managing the household falls mostly on moms
Participants were physicians enrolled in the Intern Health Study, a longitudinal study that regularly surveys medical interns in the United States to assess stress and mood. When researchers compared survey results from before the onset of the pandemic (2018) with later results (2020), they found a striking gender difference in how the pandemic has changed family and work duties for physicians.
The authors of the study pointed out that previous research had found that female physicians take on a greater share of household and childcare duties than male physicians. The current study found that their share had increased with the pandemic. Physician moms are now 30 times more likely to be in charge of these tasks than physician dads.
In families in which both parents were physicians, none of the men said they took the primary role in managing the extra demands caused by the pandemic. In addition, women were twice as likely as men to work primarily from home and to work reduced hours.
The extra stress seems to be taking a toll on women physicians. In the 2020 survey, physician mothers had higher scores for anxiety and depression symptoms, compared with men. Notably, the 2018 survey did not show a significant difference in depression scores between men and women. Nor were there significant differences in depression and anxiety scores between women and men who were not parents or in reports of work/family conflict before and after the pandemic.
In general, the results indicate that the pandemic has only widened the gender gap between women and men physicians when it comes to managing family life and dealing with the stresses of maintaining a suitable work-life balance.
‘Long-term repercussions’ for gender equity in medicine
Although these are serious problems for women physicians and their families, the effects go beyond the home and beyond individuals. Even before the pandemic, women in medicine struggled for parity in career advancement and opportunities as well as in pay, and this new setback could make those challenges even greater.
“Even short-term adjustments can have serious long-term repercussions as they may lead to lower earnings and negatively impact opportunities for promotion, further exacerbating gender inequalities in compensation and advancement,” the study’s authors wrote.
The potential damage extends to the entire profession and the health care system itself. The profession is already struggling to retain young female physicians, and this situation is likely to make that problem worse and have long-term consequences. Citing data showing that female physicians spend more time with patients and that their patients may have better outcomes, the authors wrote that the consequences of losing more early-career female physicians “could be devastating to the U.S. health care system, particularly in the context of a global pandemic and an impending physician shortage.”
The sample size was small (276 U.S. physicians), and the study relied on self-reported data. The findings suggest that more research on this topic is needed, especially research that includes other demographic factors, such as sexual orientation and ethnicity. The authors recommend that institutional and public policymakers take into account the effects of the pandemic on physician mothers to ensure that recent gains in gender equity for women physicians do not fall victim to COVID-19.
A version of this article first appeared on Medscape.com.
Substantial declines in mortality for most cancers
according to a new analysis.
Researchers found that rates for all cancers combined declined by 27% overall between 1971 and 2019 and decreased significantly for 12 of the 15 top cancer sites analyzed.
The data revealed even greater mortality declines for certain cancers in particular years. For example, mortality from lung cancer was 44% lower in 2019, compared with its peak rate in 1993, whereas it was only 13% lower, compared with morality rates in 1971.
“The cancer mortality rate has reduced considerably since 1971 overall and for most cancer sites because of improvements in prevention, early detection, and treatment,” lead author Ahmedin Jemal, DVM, PhD, American Cancer Society, Kennesaw, Ga., and colleagues wrote.
Advances in surgery, radiotherapy, chemotherapy, precision medicine, and combinations therapies over the past 5 decades have contributed to these significant declines in mortality, Dr. Jemal and colleagues explained. The researchers also credit the “expanded investment” in the National Cancer Institute’s annual budget following the 1971 National Cancer Act, which increased the budget 25-fold from $227 million in 1971 to $6 billion in 2019.
The report, published online Nov. 11, 2021, in JAMA Oncology, analyzed mortality rates for all cancers as well as the top 15 sites using the National Center for Health Statistics.
The researchers found that, overall, deaths declined significantly for all cancers over the study period. Some of the biggest headway since 1971 occurred for stomach and cervical cancers – with 72% and 69% lower mortality rates, respectively – as well as colorectal cancer (56%), oral cavity and pharynx cancer (43%), and ovarian cancer (41%). Mortality rates of female breast cancer and prostate cancer also dropped considerably – both by 39%.
“The decline in mortality for female breast, cervical, colorectal, and prostate cancer in part reflects increased detection (and removal) of premalignant lesions and early-stage cancers,” Dr. Jemal and colleagues noted.
Data suggest that screening likely explains about half of the observed decline in mortality from colorectal cancer between 1975 and 2002. A 2018 study also found that the use of adjuvant chemotherapy was responsible for 63% of the decline in mortality from female breast cancer between 2000 and 2012.
In addition, the authors noted, “the decline in lung, oral cavity and bladder cancers largely reflects reductions in smoking because of enhanced public awareness of the health consequences, implementation of increased cigarette excise taxes, and comprehensive smoke-free laws.”
However, mortality did increase in a few categories. For instance, the mortality rate from pancreatic cancer increased by 3% between 1971 and 2019, and by 8% for both esophageal and brain cancers. Mortality rates from cancer were also greater for 29% of the U.S. counties included in the analysis, mostly those in the South.
The increase in mortality from pancreatic cancer likely reflects the growing rates of obesity in the United States, along with no real advances in pancreatic cancer prevention, early detection, or treatment, the authors suggested. In addition, lack of progress in regions of the south may be related to unequal access to improvements in treatment compared with other parts of the country.
“Improving equity through investment in the social determinants of health and implementation research is critical to furthering the national cancer-control agenda,” the authors concluded.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new analysis.
Researchers found that rates for all cancers combined declined by 27% overall between 1971 and 2019 and decreased significantly for 12 of the 15 top cancer sites analyzed.
The data revealed even greater mortality declines for certain cancers in particular years. For example, mortality from lung cancer was 44% lower in 2019, compared with its peak rate in 1993, whereas it was only 13% lower, compared with morality rates in 1971.
“The cancer mortality rate has reduced considerably since 1971 overall and for most cancer sites because of improvements in prevention, early detection, and treatment,” lead author Ahmedin Jemal, DVM, PhD, American Cancer Society, Kennesaw, Ga., and colleagues wrote.
Advances in surgery, radiotherapy, chemotherapy, precision medicine, and combinations therapies over the past 5 decades have contributed to these significant declines in mortality, Dr. Jemal and colleagues explained. The researchers also credit the “expanded investment” in the National Cancer Institute’s annual budget following the 1971 National Cancer Act, which increased the budget 25-fold from $227 million in 1971 to $6 billion in 2019.
The report, published online Nov. 11, 2021, in JAMA Oncology, analyzed mortality rates for all cancers as well as the top 15 sites using the National Center for Health Statistics.
The researchers found that, overall, deaths declined significantly for all cancers over the study period. Some of the biggest headway since 1971 occurred for stomach and cervical cancers – with 72% and 69% lower mortality rates, respectively – as well as colorectal cancer (56%), oral cavity and pharynx cancer (43%), and ovarian cancer (41%). Mortality rates of female breast cancer and prostate cancer also dropped considerably – both by 39%.
“The decline in mortality for female breast, cervical, colorectal, and prostate cancer in part reflects increased detection (and removal) of premalignant lesions and early-stage cancers,” Dr. Jemal and colleagues noted.
Data suggest that screening likely explains about half of the observed decline in mortality from colorectal cancer between 1975 and 2002. A 2018 study also found that the use of adjuvant chemotherapy was responsible for 63% of the decline in mortality from female breast cancer between 2000 and 2012.
In addition, the authors noted, “the decline in lung, oral cavity and bladder cancers largely reflects reductions in smoking because of enhanced public awareness of the health consequences, implementation of increased cigarette excise taxes, and comprehensive smoke-free laws.”
However, mortality did increase in a few categories. For instance, the mortality rate from pancreatic cancer increased by 3% between 1971 and 2019, and by 8% for both esophageal and brain cancers. Mortality rates from cancer were also greater for 29% of the U.S. counties included in the analysis, mostly those in the South.
The increase in mortality from pancreatic cancer likely reflects the growing rates of obesity in the United States, along with no real advances in pancreatic cancer prevention, early detection, or treatment, the authors suggested. In addition, lack of progress in regions of the south may be related to unequal access to improvements in treatment compared with other parts of the country.
“Improving equity through investment in the social determinants of health and implementation research is critical to furthering the national cancer-control agenda,” the authors concluded.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new analysis.
Researchers found that rates for all cancers combined declined by 27% overall between 1971 and 2019 and decreased significantly for 12 of the 15 top cancer sites analyzed.
The data revealed even greater mortality declines for certain cancers in particular years. For example, mortality from lung cancer was 44% lower in 2019, compared with its peak rate in 1993, whereas it was only 13% lower, compared with morality rates in 1971.
“The cancer mortality rate has reduced considerably since 1971 overall and for most cancer sites because of improvements in prevention, early detection, and treatment,” lead author Ahmedin Jemal, DVM, PhD, American Cancer Society, Kennesaw, Ga., and colleagues wrote.
Advances in surgery, radiotherapy, chemotherapy, precision medicine, and combinations therapies over the past 5 decades have contributed to these significant declines in mortality, Dr. Jemal and colleagues explained. The researchers also credit the “expanded investment” in the National Cancer Institute’s annual budget following the 1971 National Cancer Act, which increased the budget 25-fold from $227 million in 1971 to $6 billion in 2019.
The report, published online Nov. 11, 2021, in JAMA Oncology, analyzed mortality rates for all cancers as well as the top 15 sites using the National Center for Health Statistics.
The researchers found that, overall, deaths declined significantly for all cancers over the study period. Some of the biggest headway since 1971 occurred for stomach and cervical cancers – with 72% and 69% lower mortality rates, respectively – as well as colorectal cancer (56%), oral cavity and pharynx cancer (43%), and ovarian cancer (41%). Mortality rates of female breast cancer and prostate cancer also dropped considerably – both by 39%.
“The decline in mortality for female breast, cervical, colorectal, and prostate cancer in part reflects increased detection (and removal) of premalignant lesions and early-stage cancers,” Dr. Jemal and colleagues noted.
Data suggest that screening likely explains about half of the observed decline in mortality from colorectal cancer between 1975 and 2002. A 2018 study also found that the use of adjuvant chemotherapy was responsible for 63% of the decline in mortality from female breast cancer between 2000 and 2012.
In addition, the authors noted, “the decline in lung, oral cavity and bladder cancers largely reflects reductions in smoking because of enhanced public awareness of the health consequences, implementation of increased cigarette excise taxes, and comprehensive smoke-free laws.”
However, mortality did increase in a few categories. For instance, the mortality rate from pancreatic cancer increased by 3% between 1971 and 2019, and by 8% for both esophageal and brain cancers. Mortality rates from cancer were also greater for 29% of the U.S. counties included in the analysis, mostly those in the South.
The increase in mortality from pancreatic cancer likely reflects the growing rates of obesity in the United States, along with no real advances in pancreatic cancer prevention, early detection, or treatment, the authors suggested. In addition, lack of progress in regions of the south may be related to unequal access to improvements in treatment compared with other parts of the country.
“Improving equity through investment in the social determinants of health and implementation research is critical to furthering the national cancer-control agenda,” the authors concluded.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Multivitamins, but not cocoa, tied to slowed brain aging
, with the effects especially pronounced in patients with cardiovascular (CVD) disease, new research suggests.
In addition to testing the effect of a daily multivitamin on cognition, the COSMOS-Mind study examined the effect of cocoa flavanols, but showed no beneficial effect.
The findings “may have important public health implications, particularly for brain health, given the accessibility of multivitamins and minerals, and their low cost and safety,” said study investigator Laura D. Baker, PhD, professor, gerontology and geriatric medicine, Wake Forest University, Winston-Salem, N.C.
The findings were presented at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Placebo-controlled study
The study is a substudy of a large parent trial that compared the effects of cocoa extract (500 mg/day cocoa flavanols) and a standard multivitamin-mineral (MVM) to placebo on cardiovascular and cancer outcomes in more than 21,000 older participants.
COSMOS-Mind included 2,262 adults aged 65 and over without dementia who underwent cognitive testing at baseline and annually for 3 years. The mean age at baseline was 73 years, and 40.4% were men. Most participants (88.7%) were non-Hispanic White and almost half (49.2%) had some post-college education.
All study groups were balanced with respect to demographics, CVD history, diabetes, depression, smoking status, alcohol intake, chocolate intake, and prior multivitamin use. Baseline cognitive scores were similar between study groups. Researchers had complete data on 77% of study participants.
The primary endpoint was the effect of cocoa extract (CE) vs. placebo on Global Cognitive Function composite score. The secondary outcome was the effect of MVM vs. placebo on global cognitive function.
Additional outcomes included the impact of supplements on executive function and memory and the treatment effects for prespecified subgroups, including subjects with a history of CVD.
Using a graph of change over time, Dr. Baker showed there was no effect of cocoa on global cognitive function (effect: 0.03; 95% confidence interval, –0.02 to 0.08; P = .28). “We see the to-be-expected practice effects, but there’s no separation between the active and placebo groups,” she said.
It was a different story for MVM. Here, there was the same practice effect, but the graph showed the lines separated for global cognitive function composite score (effect: 0.07; 95% CI, 0.02-0.12; P = .007).
“We see a positive effect of multivitamins for the active group relative to placebo, peaking at 2 years and then remaining stable over time,” said Dr. Baker.
There were similar findings with MVM for the memory composite score, and the executive function composite score. “We have significance in all three, where the two lines do separate over and above the practice effects,” said Dr. Baker.
New evidence
Investigators found a baseline history of CVD, including transient ischemic attack, heart failure, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, and stent, but not myocardial infarction or stroke as these were excluded in the parent trial because they affected the response to multivitamins.
As expected, those with CVD had lower cognitive scores at baseline. “But after an initial bump due to practice effect, at year 1, the cardiovascular disease history folks continue to benefit from multivitamins, whereas those who got placebo multivitamins continue to decline over time,” said Dr. Baker.
Based on information from a baseline scatter plot of cognitive function scores by age, the study’s modeling estimated the multivitamin treatment effect had a positive benefit of .028 standard deviations (SD) per year.
“Daily multivitamin-mineral supplementation appears to slow cognitive aging by 60% or by 1.8 years,” Dr. Baker added.
To date, the effect of MVM supplementation on cognition has been tested in only one large randomized clinical trial – the Physicians Health Study II. That study did not show an effect, but included only older male physicians – and cognitive testing began 2.5 years after randomization, said Dr. Baker.
“Our study provides new evidence that daily multivitamin supplementation may benefit cognitive function in older women and men, and the multivitamin effects may be more pronounced in participants with cardiovascular disease,” she noted.
For effects of multivitamins on Alzheimer’s disease prevalence and progression, “stay tuned,” Dr. Baker concluded.
Following the presentation, session cochair Suzanne Schindler, MD, PhD, instructor in the department of neurology at Washington University, St. Louis, said she and her colleagues “always check vitamin B12 levels” in patients with memory and cognitive difficulties and wondered if study subjects with a low level or deficiency of vitamin B12 benefited from the intervention.
“We are asking ourselves that as well,” said Dr. Baker.
“Some of this is a work in progress,” Dr. Baker added. “We still need to look at that more in-depth to understand whether it might be a mechanism for improvement. I think the results are still out on that topic.”
The study received support from the National Institute on Aging. Pfizer Consumer Healthcare (now GSK Consumer Healthcare) provided study pills and packaging. Dr. Baker has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, with the effects especially pronounced in patients with cardiovascular (CVD) disease, new research suggests.
In addition to testing the effect of a daily multivitamin on cognition, the COSMOS-Mind study examined the effect of cocoa flavanols, but showed no beneficial effect.
The findings “may have important public health implications, particularly for brain health, given the accessibility of multivitamins and minerals, and their low cost and safety,” said study investigator Laura D. Baker, PhD, professor, gerontology and geriatric medicine, Wake Forest University, Winston-Salem, N.C.
The findings were presented at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Placebo-controlled study
The study is a substudy of a large parent trial that compared the effects of cocoa extract (500 mg/day cocoa flavanols) and a standard multivitamin-mineral (MVM) to placebo on cardiovascular and cancer outcomes in more than 21,000 older participants.
COSMOS-Mind included 2,262 adults aged 65 and over without dementia who underwent cognitive testing at baseline and annually for 3 years. The mean age at baseline was 73 years, and 40.4% were men. Most participants (88.7%) were non-Hispanic White and almost half (49.2%) had some post-college education.
All study groups were balanced with respect to demographics, CVD history, diabetes, depression, smoking status, alcohol intake, chocolate intake, and prior multivitamin use. Baseline cognitive scores were similar between study groups. Researchers had complete data on 77% of study participants.
The primary endpoint was the effect of cocoa extract (CE) vs. placebo on Global Cognitive Function composite score. The secondary outcome was the effect of MVM vs. placebo on global cognitive function.
Additional outcomes included the impact of supplements on executive function and memory and the treatment effects for prespecified subgroups, including subjects with a history of CVD.
Using a graph of change over time, Dr. Baker showed there was no effect of cocoa on global cognitive function (effect: 0.03; 95% confidence interval, –0.02 to 0.08; P = .28). “We see the to-be-expected practice effects, but there’s no separation between the active and placebo groups,” she said.
It was a different story for MVM. Here, there was the same practice effect, but the graph showed the lines separated for global cognitive function composite score (effect: 0.07; 95% CI, 0.02-0.12; P = .007).
“We see a positive effect of multivitamins for the active group relative to placebo, peaking at 2 years and then remaining stable over time,” said Dr. Baker.
There were similar findings with MVM for the memory composite score, and the executive function composite score. “We have significance in all three, where the two lines do separate over and above the practice effects,” said Dr. Baker.
New evidence
Investigators found a baseline history of CVD, including transient ischemic attack, heart failure, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, and stent, but not myocardial infarction or stroke as these were excluded in the parent trial because they affected the response to multivitamins.
As expected, those with CVD had lower cognitive scores at baseline. “But after an initial bump due to practice effect, at year 1, the cardiovascular disease history folks continue to benefit from multivitamins, whereas those who got placebo multivitamins continue to decline over time,” said Dr. Baker.
Based on information from a baseline scatter plot of cognitive function scores by age, the study’s modeling estimated the multivitamin treatment effect had a positive benefit of .028 standard deviations (SD) per year.
“Daily multivitamin-mineral supplementation appears to slow cognitive aging by 60% or by 1.8 years,” Dr. Baker added.
To date, the effect of MVM supplementation on cognition has been tested in only one large randomized clinical trial – the Physicians Health Study II. That study did not show an effect, but included only older male physicians – and cognitive testing began 2.5 years after randomization, said Dr. Baker.
“Our study provides new evidence that daily multivitamin supplementation may benefit cognitive function in older women and men, and the multivitamin effects may be more pronounced in participants with cardiovascular disease,” she noted.
For effects of multivitamins on Alzheimer’s disease prevalence and progression, “stay tuned,” Dr. Baker concluded.
Following the presentation, session cochair Suzanne Schindler, MD, PhD, instructor in the department of neurology at Washington University, St. Louis, said she and her colleagues “always check vitamin B12 levels” in patients with memory and cognitive difficulties and wondered if study subjects with a low level or deficiency of vitamin B12 benefited from the intervention.
“We are asking ourselves that as well,” said Dr. Baker.
“Some of this is a work in progress,” Dr. Baker added. “We still need to look at that more in-depth to understand whether it might be a mechanism for improvement. I think the results are still out on that topic.”
The study received support from the National Institute on Aging. Pfizer Consumer Healthcare (now GSK Consumer Healthcare) provided study pills and packaging. Dr. Baker has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, with the effects especially pronounced in patients with cardiovascular (CVD) disease, new research suggests.
In addition to testing the effect of a daily multivitamin on cognition, the COSMOS-Mind study examined the effect of cocoa flavanols, but showed no beneficial effect.
The findings “may have important public health implications, particularly for brain health, given the accessibility of multivitamins and minerals, and their low cost and safety,” said study investigator Laura D. Baker, PhD, professor, gerontology and geriatric medicine, Wake Forest University, Winston-Salem, N.C.
The findings were presented at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Placebo-controlled study
The study is a substudy of a large parent trial that compared the effects of cocoa extract (500 mg/day cocoa flavanols) and a standard multivitamin-mineral (MVM) to placebo on cardiovascular and cancer outcomes in more than 21,000 older participants.
COSMOS-Mind included 2,262 adults aged 65 and over without dementia who underwent cognitive testing at baseline and annually for 3 years. The mean age at baseline was 73 years, and 40.4% were men. Most participants (88.7%) were non-Hispanic White and almost half (49.2%) had some post-college education.
All study groups were balanced with respect to demographics, CVD history, diabetes, depression, smoking status, alcohol intake, chocolate intake, and prior multivitamin use. Baseline cognitive scores were similar between study groups. Researchers had complete data on 77% of study participants.
The primary endpoint was the effect of cocoa extract (CE) vs. placebo on Global Cognitive Function composite score. The secondary outcome was the effect of MVM vs. placebo on global cognitive function.
Additional outcomes included the impact of supplements on executive function and memory and the treatment effects for prespecified subgroups, including subjects with a history of CVD.
Using a graph of change over time, Dr. Baker showed there was no effect of cocoa on global cognitive function (effect: 0.03; 95% confidence interval, –0.02 to 0.08; P = .28). “We see the to-be-expected practice effects, but there’s no separation between the active and placebo groups,” she said.
It was a different story for MVM. Here, there was the same practice effect, but the graph showed the lines separated for global cognitive function composite score (effect: 0.07; 95% CI, 0.02-0.12; P = .007).
“We see a positive effect of multivitamins for the active group relative to placebo, peaking at 2 years and then remaining stable over time,” said Dr. Baker.
There were similar findings with MVM for the memory composite score, and the executive function composite score. “We have significance in all three, where the two lines do separate over and above the practice effects,” said Dr. Baker.
New evidence
Investigators found a baseline history of CVD, including transient ischemic attack, heart failure, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, and stent, but not myocardial infarction or stroke as these were excluded in the parent trial because they affected the response to multivitamins.
As expected, those with CVD had lower cognitive scores at baseline. “But after an initial bump due to practice effect, at year 1, the cardiovascular disease history folks continue to benefit from multivitamins, whereas those who got placebo multivitamins continue to decline over time,” said Dr. Baker.
Based on information from a baseline scatter plot of cognitive function scores by age, the study’s modeling estimated the multivitamin treatment effect had a positive benefit of .028 standard deviations (SD) per year.
“Daily multivitamin-mineral supplementation appears to slow cognitive aging by 60% or by 1.8 years,” Dr. Baker added.
To date, the effect of MVM supplementation on cognition has been tested in only one large randomized clinical trial – the Physicians Health Study II. That study did not show an effect, but included only older male physicians – and cognitive testing began 2.5 years after randomization, said Dr. Baker.
“Our study provides new evidence that daily multivitamin supplementation may benefit cognitive function in older women and men, and the multivitamin effects may be more pronounced in participants with cardiovascular disease,” she noted.
For effects of multivitamins on Alzheimer’s disease prevalence and progression, “stay tuned,” Dr. Baker concluded.
Following the presentation, session cochair Suzanne Schindler, MD, PhD, instructor in the department of neurology at Washington University, St. Louis, said she and her colleagues “always check vitamin B12 levels” in patients with memory and cognitive difficulties and wondered if study subjects with a low level or deficiency of vitamin B12 benefited from the intervention.
“We are asking ourselves that as well,” said Dr. Baker.
“Some of this is a work in progress,” Dr. Baker added. “We still need to look at that more in-depth to understand whether it might be a mechanism for improvement. I think the results are still out on that topic.”
The study received support from the National Institute on Aging. Pfizer Consumer Healthcare (now GSK Consumer Healthcare) provided study pills and packaging. Dr. Baker has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.