User login
AHA/ACC issues first comprehensive guidance on chest pain
Clinicians should use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain (angina), advises a joint clinical practice guideline released by American Heart Association and American College of Cardiology.
While evaluation of chest pain has been covered in previous guidelines, this is the first comprehensive guideline from the AHA and ACC focused exclusively on the evaluation and diagnosis of chest pain.
“As our imaging technologies have evolved, we needed a contemporary approach to which patients need further testing, and which do not, in addition to what testing is effective,” Martha Gulati, MD, University of Arizona, Phoenix, and chair of the guideline writing group, said in an interview.
“Our hope is that we have provided an evidence-based approach to evaluating patients that will assist all of us who manage, diagnose, and treat patients who experience chest pain,” said Dr. Gulati, who is also president-elect of the American Society for Preventive Cardiology.
The guideline was simultaneously published online Oct. 28, 2021, in Circulation and the Journal of the American College of Cardiology.
‘Atypical’ is out, ‘noncardiac’ is in
Each year, chest pain sends more than 6.5 million adults to the ED and more than 4 million to outpatient clinics in the United States.
Yet, among all patients who come to the ED, only 5% will have acute coronary syndrome (ACS). More than half will ultimately have a noncardiac reason for their chest pain, including respiratory, musculoskeletal, gastrointestinal, psychological, or other causes.
The guideline says evaluating the severity and the cause of chest pain is essential and advises using standard risk assessments to determine if a patient is at low, intermediate, or high risk for having a cardiac event.
“I hope clinicians take from our guidelines the understanding that low-risk patients often do not need additional testing. And if we communicate this effectively with our patients – incorporating shared decision-making into our practice – we can reduce ‘overtesting’ in low-risk patients,” Dr. Gulati said in an interview.
The guideline notes that women are unique when presenting with ACS symptoms. While chest pain is the dominant and most common symptom for both men and women, women may be more likely to also have symptoms such as nausea and shortness of breath.
The guideline also encourages using the term “noncardiac” if heart disease is not suspected in a patient with angina and says the term “atypical” is a “misleading” descriptor of chest pain and should not be used.
“Words matter, and we need to move away from describing chest pain as ‘atypical’ because it has resulted in confusion when these words are used,” Dr. Gulati stressed.
“Rather than meaning a different way of presenting, it has taken on a meaning to imply it is not cardiac. It is more useful to talk about the probability of the pain being cardiac vs noncardiac,” Dr. Gulati explained.
No one best test for everyone
There is also a focus on evaluation of patients with chest pain who present to the ED. The initial goals of ED physicians should be to identify if there are life-threatening causes and to determine if there is a need for hospital admission or testing, the guideline states.
Thorough screening in the ED may help determine who is at high risk versus intermediate or low risk for a cardiac event. An individual deemed to be at low risk may be referred for additional evaluation in an outpatient setting rather than being admitted to the hospital, the authors wrote.
High-sensitivity cardiac troponins are the “preferred standard” for establishing a biomarker diagnosis of acute myocardial infarction, allowing for more accurate detection and exclusion of myocardial injury, they added.
“While there is no one ‘best test’ for every patient, the guideline emphasizes the tests that may be most appropriate, depending on the individual situation, and which ones won’t provide additional information; therefore, these tests should not be done just for the sake of doing them,” Dr. Gulati said in a news release.
“Appropriate testing is also dependent upon the technology and screening devices that are available at the hospital or healthcare center where the patient is receiving care. All imaging modalities highlighted in the guideline have an important role in the assessment of chest pain to help determine the underlying cause, with the goal of preventing a serious cardiac event,” Dr. Gulati added.
The guideline was prepared on behalf of and approved by the AHA and ACC Joint Committee on Clinical Practice Guidelines.
Five other partnering organizations participated in and approved the guideline: the American Society of Echocardiography, the American College of Chest Physicians, the Society for Academic Emergency Medicine, the Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance.
The writing group included representatives from each of the partnering organizations and experts in the field (cardiac intensivists, cardiac interventionalists, cardiac surgeons, cardiologists, emergency physicians, and epidemiologists), as well as a lay/patient representative.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
Clinicians should use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain (angina), advises a joint clinical practice guideline released by American Heart Association and American College of Cardiology.
While evaluation of chest pain has been covered in previous guidelines, this is the first comprehensive guideline from the AHA and ACC focused exclusively on the evaluation and diagnosis of chest pain.
“As our imaging technologies have evolved, we needed a contemporary approach to which patients need further testing, and which do not, in addition to what testing is effective,” Martha Gulati, MD, University of Arizona, Phoenix, and chair of the guideline writing group, said in an interview.
“Our hope is that we have provided an evidence-based approach to evaluating patients that will assist all of us who manage, diagnose, and treat patients who experience chest pain,” said Dr. Gulati, who is also president-elect of the American Society for Preventive Cardiology.
The guideline was simultaneously published online Oct. 28, 2021, in Circulation and the Journal of the American College of Cardiology.
‘Atypical’ is out, ‘noncardiac’ is in
Each year, chest pain sends more than 6.5 million adults to the ED and more than 4 million to outpatient clinics in the United States.
Yet, among all patients who come to the ED, only 5% will have acute coronary syndrome (ACS). More than half will ultimately have a noncardiac reason for their chest pain, including respiratory, musculoskeletal, gastrointestinal, psychological, or other causes.
The guideline says evaluating the severity and the cause of chest pain is essential and advises using standard risk assessments to determine if a patient is at low, intermediate, or high risk for having a cardiac event.
“I hope clinicians take from our guidelines the understanding that low-risk patients often do not need additional testing. And if we communicate this effectively with our patients – incorporating shared decision-making into our practice – we can reduce ‘overtesting’ in low-risk patients,” Dr. Gulati said in an interview.
The guideline notes that women are unique when presenting with ACS symptoms. While chest pain is the dominant and most common symptom for both men and women, women may be more likely to also have symptoms such as nausea and shortness of breath.
The guideline also encourages using the term “noncardiac” if heart disease is not suspected in a patient with angina and says the term “atypical” is a “misleading” descriptor of chest pain and should not be used.
“Words matter, and we need to move away from describing chest pain as ‘atypical’ because it has resulted in confusion when these words are used,” Dr. Gulati stressed.
“Rather than meaning a different way of presenting, it has taken on a meaning to imply it is not cardiac. It is more useful to talk about the probability of the pain being cardiac vs noncardiac,” Dr. Gulati explained.
No one best test for everyone
There is also a focus on evaluation of patients with chest pain who present to the ED. The initial goals of ED physicians should be to identify if there are life-threatening causes and to determine if there is a need for hospital admission or testing, the guideline states.
Thorough screening in the ED may help determine who is at high risk versus intermediate or low risk for a cardiac event. An individual deemed to be at low risk may be referred for additional evaluation in an outpatient setting rather than being admitted to the hospital, the authors wrote.
High-sensitivity cardiac troponins are the “preferred standard” for establishing a biomarker diagnosis of acute myocardial infarction, allowing for more accurate detection and exclusion of myocardial injury, they added.
“While there is no one ‘best test’ for every patient, the guideline emphasizes the tests that may be most appropriate, depending on the individual situation, and which ones won’t provide additional information; therefore, these tests should not be done just for the sake of doing them,” Dr. Gulati said in a news release.
“Appropriate testing is also dependent upon the technology and screening devices that are available at the hospital or healthcare center where the patient is receiving care. All imaging modalities highlighted in the guideline have an important role in the assessment of chest pain to help determine the underlying cause, with the goal of preventing a serious cardiac event,” Dr. Gulati added.
The guideline was prepared on behalf of and approved by the AHA and ACC Joint Committee on Clinical Practice Guidelines.
Five other partnering organizations participated in and approved the guideline: the American Society of Echocardiography, the American College of Chest Physicians, the Society for Academic Emergency Medicine, the Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance.
The writing group included representatives from each of the partnering organizations and experts in the field (cardiac intensivists, cardiac interventionalists, cardiac surgeons, cardiologists, emergency physicians, and epidemiologists), as well as a lay/patient representative.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
Clinicians should use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain (angina), advises a joint clinical practice guideline released by American Heart Association and American College of Cardiology.
While evaluation of chest pain has been covered in previous guidelines, this is the first comprehensive guideline from the AHA and ACC focused exclusively on the evaluation and diagnosis of chest pain.
“As our imaging technologies have evolved, we needed a contemporary approach to which patients need further testing, and which do not, in addition to what testing is effective,” Martha Gulati, MD, University of Arizona, Phoenix, and chair of the guideline writing group, said in an interview.
“Our hope is that we have provided an evidence-based approach to evaluating patients that will assist all of us who manage, diagnose, and treat patients who experience chest pain,” said Dr. Gulati, who is also president-elect of the American Society for Preventive Cardiology.
The guideline was simultaneously published online Oct. 28, 2021, in Circulation and the Journal of the American College of Cardiology.
‘Atypical’ is out, ‘noncardiac’ is in
Each year, chest pain sends more than 6.5 million adults to the ED and more than 4 million to outpatient clinics in the United States.
Yet, among all patients who come to the ED, only 5% will have acute coronary syndrome (ACS). More than half will ultimately have a noncardiac reason for their chest pain, including respiratory, musculoskeletal, gastrointestinal, psychological, or other causes.
The guideline says evaluating the severity and the cause of chest pain is essential and advises using standard risk assessments to determine if a patient is at low, intermediate, or high risk for having a cardiac event.
“I hope clinicians take from our guidelines the understanding that low-risk patients often do not need additional testing. And if we communicate this effectively with our patients – incorporating shared decision-making into our practice – we can reduce ‘overtesting’ in low-risk patients,” Dr. Gulati said in an interview.
The guideline notes that women are unique when presenting with ACS symptoms. While chest pain is the dominant and most common symptom for both men and women, women may be more likely to also have symptoms such as nausea and shortness of breath.
The guideline also encourages using the term “noncardiac” if heart disease is not suspected in a patient with angina and says the term “atypical” is a “misleading” descriptor of chest pain and should not be used.
“Words matter, and we need to move away from describing chest pain as ‘atypical’ because it has resulted in confusion when these words are used,” Dr. Gulati stressed.
“Rather than meaning a different way of presenting, it has taken on a meaning to imply it is not cardiac. It is more useful to talk about the probability of the pain being cardiac vs noncardiac,” Dr. Gulati explained.
No one best test for everyone
There is also a focus on evaluation of patients with chest pain who present to the ED. The initial goals of ED physicians should be to identify if there are life-threatening causes and to determine if there is a need for hospital admission or testing, the guideline states.
Thorough screening in the ED may help determine who is at high risk versus intermediate or low risk for a cardiac event. An individual deemed to be at low risk may be referred for additional evaluation in an outpatient setting rather than being admitted to the hospital, the authors wrote.
High-sensitivity cardiac troponins are the “preferred standard” for establishing a biomarker diagnosis of acute myocardial infarction, allowing for more accurate detection and exclusion of myocardial injury, they added.
“While there is no one ‘best test’ for every patient, the guideline emphasizes the tests that may be most appropriate, depending on the individual situation, and which ones won’t provide additional information; therefore, these tests should not be done just for the sake of doing them,” Dr. Gulati said in a news release.
“Appropriate testing is also dependent upon the technology and screening devices that are available at the hospital or healthcare center where the patient is receiving care. All imaging modalities highlighted in the guideline have an important role in the assessment of chest pain to help determine the underlying cause, with the goal of preventing a serious cardiac event,” Dr. Gulati added.
The guideline was prepared on behalf of and approved by the AHA and ACC Joint Committee on Clinical Practice Guidelines.
Five other partnering organizations participated in and approved the guideline: the American Society of Echocardiography, the American College of Chest Physicians, the Society for Academic Emergency Medicine, the Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance.
The writing group included representatives from each of the partnering organizations and experts in the field (cardiac intensivists, cardiac interventionalists, cardiac surgeons, cardiologists, emergency physicians, and epidemiologists), as well as a lay/patient representative.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
Borderline personality disorder: 6 studies of biological interventions
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
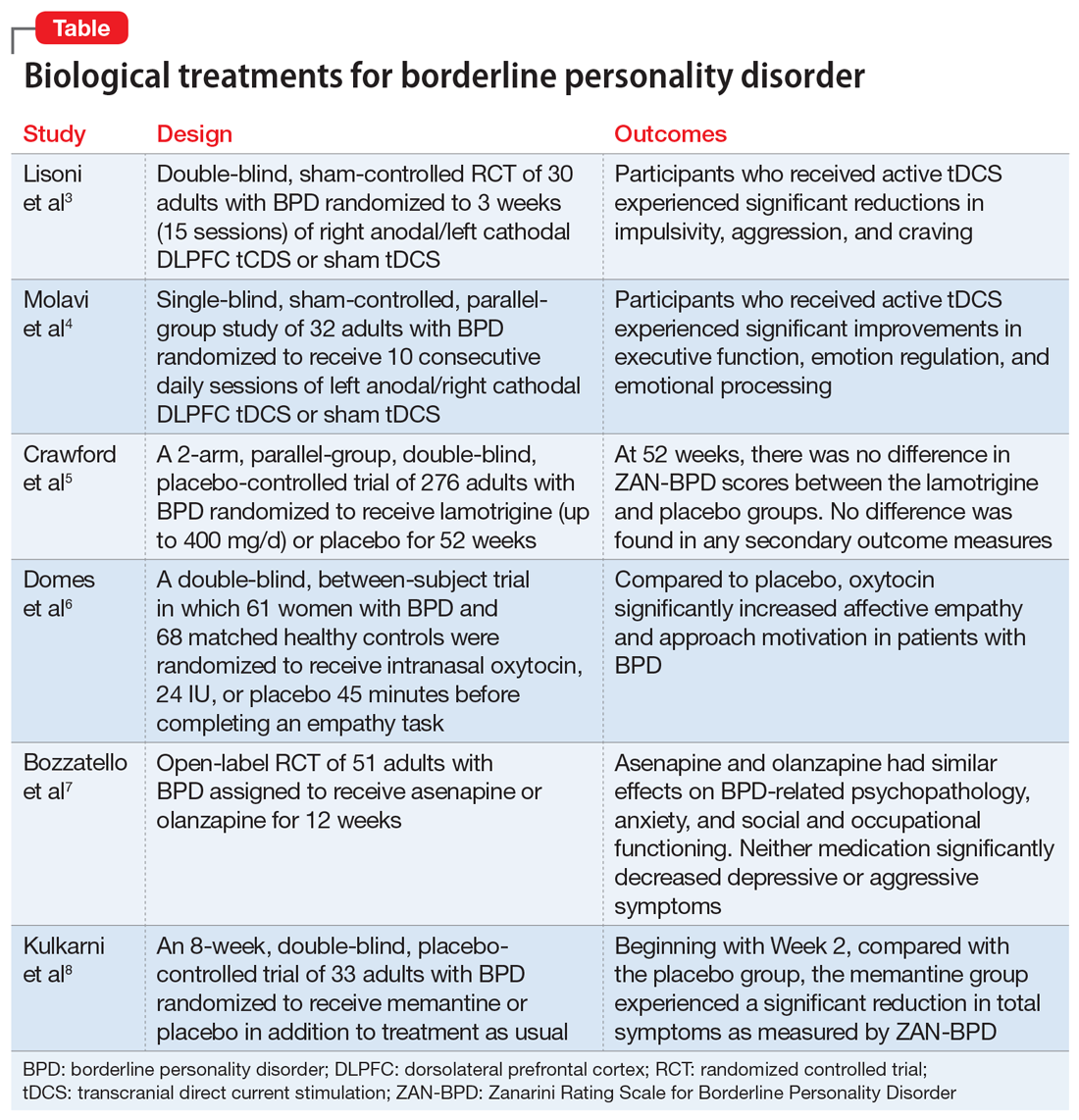
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.
1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.

1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.

1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
Service animals and emotional support animals: Should you write that letter?
For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf

For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals

For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf
I have a dream … for psychiatry
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
Maria A. Oquendo, MD, PhD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
Calcineurin inhibitor–induced psychosis
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.
Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Catching the runner’s high: Anxiety and the endocannabinoid system
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
Higher odds for preterm, C-section births seen in women with PsA
Disease-modifying antirheumatic drugs (DMARDs) such as biologics may carry an increased risk for preterm birth or cesarean delivery for pregnant women with psoriatic arthritis (PsA), according to a recent study published in Arthritis & Rheumatology.
The risk was particularly high for women with PsA who received biologic disease-modifying antirheumatic drugs (bDMARDs), according to Katarina Remaeus, PhD, of the Karolinska Institute in Stockholm and colleagues.
“The results may indicate that a more severe or active PsA disease that requires antirheumatic treatment during pregnancy, especially bDMARDs, is associated with increased risks of adverse pregnancy outcomes compared to non-PsA pregnancies,” Dr. Remaeus and colleagues write in their study. “The risk of preterm birth in PsA pregnancies is further influenced by parity with the most increased risks observed in first pregnancies.”
In a nationwide, register-based cohort study, the researchers evaluated 921 pregnancies of women with PsA between 2007 and 2017, comparing them to the pregnancies of 9,210 women without PsA over the same time frame. The pregnancies for women with PsA were further categorized based on whether the women had not received antirheumatic treatment in the year prior to and/or during pregnancy (495 pregnancies) or had received antirheumatic treatment at any point in the year before and/or during pregnancy (426 pregnancies).
Of the women in the PsA group who were treated in the year prior to pregnancy (170 women), 39.4% received monotherapy with a conventional synthetic DMARD (csDMARD) such as an antimalarial, methotrexate, or sulfasalazine; 24.1% received oral corticosteroids, and 15.9% received a tumor necrosis factor inhibitor (TNFi), whereas about 20% of women received two or more antirheumatic drugs.
In the group of women treated during pregnancy (256 women), 153 did not receive bDMARDs; of these, 41.8% had monotherapy with either a csDMARD or corticosteroids, whereas the group treated with bDMARDs received TNFi monotherapy (43.7%) or TNFi with corticosteroids (35.9%), TNFi with csDMARD (9.7%), or TNFi with csDMARD plus corticosteroids (9.7%).
A majority of women in both groups (70.1%) were between ages 30 and 34 years (37.1%) or older than age 35 years (33%) and had delivered more than one child (63.2%). Women in the PsA group were more likely to be born in a Nordic country (91.8% vs. 82.8%), to have a body mass index between 30.0 and 60.0 kg/m2 (19.9% vs. 12.6%), to be a smoker (9.2% vs. 5.3%), to have hypertension (1.4% vs. 0.8%) or diabetes (1.3% vs. 0.5%) prior to pregnancy, and to have a higher level of education (>12 years; 50.1% vs. 43.3%), compared with women in the non-PsA group.
The results showed women in the PsA group were more likely to experience preterm birth (adjusted odds ratio, 1.69; 95% confidence interval, 1.27-2.24) and undergo an elective (aOR, 1.77; 95% CI, 1.43-2.20) or emergency C-section (aOR, 1.42; 95% CI, 1.10-1.84). The group at highest risk for preterm birth with regard to parity was women with PsA having their first child (aOR, 3.95; 95% CI, 1.43-10.95).
Women who received antirheumatic treatment were at greater risk for experiencing preterm birth (aOR, 2.30; 95% CI, 1.49-3.56), and this risk was even higher for treatment with bDMARDs, compared with women without PsA (aOR, 4.49; 95% CI, 2.60-7.79). Use of bDMARDs also was associated with higher risks for spontaneous preterm birth (aOR, 4.73; 95% CI, 2.53-8.87), preterm birth between 32 and 36 weeks’ gestation (aOR, 5.06; 95% CI, 2.91-8.79), elective C-section (aOR, 2.72; 95% CI, 1.61-4.59), emergency C-section (aOR, 2.06; 95% CI, 1.04-4.07), and preeclampsia (aOR, 2.88, 95% CI, 1.35-6.17).
The researchers note that women with PsA should be evaluated for preterm birth particularly if they are having their first child, and “from a clinical point of view, all women with PsA, regardless of antirheumatic treatment, should be counseled about pregnancy outcomes and receive individualized monitoring during pregnancy.”
Are adverse outcomes linked to disease activity or treatment?
Patients in the study had a higher risk of adverse outcomes when they had a PsA diagnosis, and when they received antirheumatic treatment – but were the adverse outcomes associated with a patient’s high disease activity or need for antirheumatic treatment?
“Our interpretation is that a PsA disease that requires continued antirheumatic treatment during pregnancy is more severe than PsA that does not require treatment,” Dr. Remaeus and colleagues write. “Thus, the increased risk of adverse outcomes in pregnancies with maternal antirheumatic treatment is probably attributed to disease severity rather than an effect of the medication itself.”
Anja Strangfeld, MD, PhD, of the German Rheumatism Research Centre in Berlin, told this news organization that the results of the study are important because it is one of the first to report differences in risk in pregnancy outcomes for women with and without PsA.
“The information is relevant to guide rheumatologists in advising patients with PsA when planning the first or subsequent pregnancies,” she said. “The results are reassuring in reporting that the elevated risk for PsA patients for adverse pregnancy outcomes is low in patients not in need of antirheumatic medication, presumably in low-disease activity.”
However, the study is still unclear on whether the association with adverse pregnancy outcomes in patients is the result of higher disease activity or the need for antirheumatic treatment, she explained.
“It was only hypothesized that those patients under bDMARD treatment are/were in high disease activity. There [is] no information on disease activity in the data sources, which limits the results,” she said. “The investigation still does not solve the important question – if adverse pregnancy outcomes are rather related to high disease activity or the medication to treat this situation.”
There was no specific funding for this study. The study authors and Dr. Strangfeld have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Disease-modifying antirheumatic drugs (DMARDs) such as biologics may carry an increased risk for preterm birth or cesarean delivery for pregnant women with psoriatic arthritis (PsA), according to a recent study published in Arthritis & Rheumatology.
The risk was particularly high for women with PsA who received biologic disease-modifying antirheumatic drugs (bDMARDs), according to Katarina Remaeus, PhD, of the Karolinska Institute in Stockholm and colleagues.
“The results may indicate that a more severe or active PsA disease that requires antirheumatic treatment during pregnancy, especially bDMARDs, is associated with increased risks of adverse pregnancy outcomes compared to non-PsA pregnancies,” Dr. Remaeus and colleagues write in their study. “The risk of preterm birth in PsA pregnancies is further influenced by parity with the most increased risks observed in first pregnancies.”
In a nationwide, register-based cohort study, the researchers evaluated 921 pregnancies of women with PsA between 2007 and 2017, comparing them to the pregnancies of 9,210 women without PsA over the same time frame. The pregnancies for women with PsA were further categorized based on whether the women had not received antirheumatic treatment in the year prior to and/or during pregnancy (495 pregnancies) or had received antirheumatic treatment at any point in the year before and/or during pregnancy (426 pregnancies).
Of the women in the PsA group who were treated in the year prior to pregnancy (170 women), 39.4% received monotherapy with a conventional synthetic DMARD (csDMARD) such as an antimalarial, methotrexate, or sulfasalazine; 24.1% received oral corticosteroids, and 15.9% received a tumor necrosis factor inhibitor (TNFi), whereas about 20% of women received two or more antirheumatic drugs.
In the group of women treated during pregnancy (256 women), 153 did not receive bDMARDs; of these, 41.8% had monotherapy with either a csDMARD or corticosteroids, whereas the group treated with bDMARDs received TNFi monotherapy (43.7%) or TNFi with corticosteroids (35.9%), TNFi with csDMARD (9.7%), or TNFi with csDMARD plus corticosteroids (9.7%).
A majority of women in both groups (70.1%) were between ages 30 and 34 years (37.1%) or older than age 35 years (33%) and had delivered more than one child (63.2%). Women in the PsA group were more likely to be born in a Nordic country (91.8% vs. 82.8%), to have a body mass index between 30.0 and 60.0 kg/m2 (19.9% vs. 12.6%), to be a smoker (9.2% vs. 5.3%), to have hypertension (1.4% vs. 0.8%) or diabetes (1.3% vs. 0.5%) prior to pregnancy, and to have a higher level of education (>12 years; 50.1% vs. 43.3%), compared with women in the non-PsA group.
The results showed women in the PsA group were more likely to experience preterm birth (adjusted odds ratio, 1.69; 95% confidence interval, 1.27-2.24) and undergo an elective (aOR, 1.77; 95% CI, 1.43-2.20) or emergency C-section (aOR, 1.42; 95% CI, 1.10-1.84). The group at highest risk for preterm birth with regard to parity was women with PsA having their first child (aOR, 3.95; 95% CI, 1.43-10.95).
Women who received antirheumatic treatment were at greater risk for experiencing preterm birth (aOR, 2.30; 95% CI, 1.49-3.56), and this risk was even higher for treatment with bDMARDs, compared with women without PsA (aOR, 4.49; 95% CI, 2.60-7.79). Use of bDMARDs also was associated with higher risks for spontaneous preterm birth (aOR, 4.73; 95% CI, 2.53-8.87), preterm birth between 32 and 36 weeks’ gestation (aOR, 5.06; 95% CI, 2.91-8.79), elective C-section (aOR, 2.72; 95% CI, 1.61-4.59), emergency C-section (aOR, 2.06; 95% CI, 1.04-4.07), and preeclampsia (aOR, 2.88, 95% CI, 1.35-6.17).
The researchers note that women with PsA should be evaluated for preterm birth particularly if they are having their first child, and “from a clinical point of view, all women with PsA, regardless of antirheumatic treatment, should be counseled about pregnancy outcomes and receive individualized monitoring during pregnancy.”
Are adverse outcomes linked to disease activity or treatment?
Patients in the study had a higher risk of adverse outcomes when they had a PsA diagnosis, and when they received antirheumatic treatment – but were the adverse outcomes associated with a patient’s high disease activity or need for antirheumatic treatment?
“Our interpretation is that a PsA disease that requires continued antirheumatic treatment during pregnancy is more severe than PsA that does not require treatment,” Dr. Remaeus and colleagues write. “Thus, the increased risk of adverse outcomes in pregnancies with maternal antirheumatic treatment is probably attributed to disease severity rather than an effect of the medication itself.”
Anja Strangfeld, MD, PhD, of the German Rheumatism Research Centre in Berlin, told this news organization that the results of the study are important because it is one of the first to report differences in risk in pregnancy outcomes for women with and without PsA.
“The information is relevant to guide rheumatologists in advising patients with PsA when planning the first or subsequent pregnancies,” she said. “The results are reassuring in reporting that the elevated risk for PsA patients for adverse pregnancy outcomes is low in patients not in need of antirheumatic medication, presumably in low-disease activity.”
However, the study is still unclear on whether the association with adverse pregnancy outcomes in patients is the result of higher disease activity or the need for antirheumatic treatment, she explained.
“It was only hypothesized that those patients under bDMARD treatment are/were in high disease activity. There [is] no information on disease activity in the data sources, which limits the results,” she said. “The investigation still does not solve the important question – if adverse pregnancy outcomes are rather related to high disease activity or the medication to treat this situation.”
There was no specific funding for this study. The study authors and Dr. Strangfeld have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Disease-modifying antirheumatic drugs (DMARDs) such as biologics may carry an increased risk for preterm birth or cesarean delivery for pregnant women with psoriatic arthritis (PsA), according to a recent study published in Arthritis & Rheumatology.
The risk was particularly high for women with PsA who received biologic disease-modifying antirheumatic drugs (bDMARDs), according to Katarina Remaeus, PhD, of the Karolinska Institute in Stockholm and colleagues.
“The results may indicate that a more severe or active PsA disease that requires antirheumatic treatment during pregnancy, especially bDMARDs, is associated with increased risks of adverse pregnancy outcomes compared to non-PsA pregnancies,” Dr. Remaeus and colleagues write in their study. “The risk of preterm birth in PsA pregnancies is further influenced by parity with the most increased risks observed in first pregnancies.”
In a nationwide, register-based cohort study, the researchers evaluated 921 pregnancies of women with PsA between 2007 and 2017, comparing them to the pregnancies of 9,210 women without PsA over the same time frame. The pregnancies for women with PsA were further categorized based on whether the women had not received antirheumatic treatment in the year prior to and/or during pregnancy (495 pregnancies) or had received antirheumatic treatment at any point in the year before and/or during pregnancy (426 pregnancies).
Of the women in the PsA group who were treated in the year prior to pregnancy (170 women), 39.4% received monotherapy with a conventional synthetic DMARD (csDMARD) such as an antimalarial, methotrexate, or sulfasalazine; 24.1% received oral corticosteroids, and 15.9% received a tumor necrosis factor inhibitor (TNFi), whereas about 20% of women received two or more antirheumatic drugs.
In the group of women treated during pregnancy (256 women), 153 did not receive bDMARDs; of these, 41.8% had monotherapy with either a csDMARD or corticosteroids, whereas the group treated with bDMARDs received TNFi monotherapy (43.7%) or TNFi with corticosteroids (35.9%), TNFi with csDMARD (9.7%), or TNFi with csDMARD plus corticosteroids (9.7%).
A majority of women in both groups (70.1%) were between ages 30 and 34 years (37.1%) or older than age 35 years (33%) and had delivered more than one child (63.2%). Women in the PsA group were more likely to be born in a Nordic country (91.8% vs. 82.8%), to have a body mass index between 30.0 and 60.0 kg/m2 (19.9% vs. 12.6%), to be a smoker (9.2% vs. 5.3%), to have hypertension (1.4% vs. 0.8%) or diabetes (1.3% vs. 0.5%) prior to pregnancy, and to have a higher level of education (>12 years; 50.1% vs. 43.3%), compared with women in the non-PsA group.
The results showed women in the PsA group were more likely to experience preterm birth (adjusted odds ratio, 1.69; 95% confidence interval, 1.27-2.24) and undergo an elective (aOR, 1.77; 95% CI, 1.43-2.20) or emergency C-section (aOR, 1.42; 95% CI, 1.10-1.84). The group at highest risk for preterm birth with regard to parity was women with PsA having their first child (aOR, 3.95; 95% CI, 1.43-10.95).
Women who received antirheumatic treatment were at greater risk for experiencing preterm birth (aOR, 2.30; 95% CI, 1.49-3.56), and this risk was even higher for treatment with bDMARDs, compared with women without PsA (aOR, 4.49; 95% CI, 2.60-7.79). Use of bDMARDs also was associated with higher risks for spontaneous preterm birth (aOR, 4.73; 95% CI, 2.53-8.87), preterm birth between 32 and 36 weeks’ gestation (aOR, 5.06; 95% CI, 2.91-8.79), elective C-section (aOR, 2.72; 95% CI, 1.61-4.59), emergency C-section (aOR, 2.06; 95% CI, 1.04-4.07), and preeclampsia (aOR, 2.88, 95% CI, 1.35-6.17).
The researchers note that women with PsA should be evaluated for preterm birth particularly if they are having their first child, and “from a clinical point of view, all women with PsA, regardless of antirheumatic treatment, should be counseled about pregnancy outcomes and receive individualized monitoring during pregnancy.”
Are adverse outcomes linked to disease activity or treatment?
Patients in the study had a higher risk of adverse outcomes when they had a PsA diagnosis, and when they received antirheumatic treatment – but were the adverse outcomes associated with a patient’s high disease activity or need for antirheumatic treatment?
“Our interpretation is that a PsA disease that requires continued antirheumatic treatment during pregnancy is more severe than PsA that does not require treatment,” Dr. Remaeus and colleagues write. “Thus, the increased risk of adverse outcomes in pregnancies with maternal antirheumatic treatment is probably attributed to disease severity rather than an effect of the medication itself.”
Anja Strangfeld, MD, PhD, of the German Rheumatism Research Centre in Berlin, told this news organization that the results of the study are important because it is one of the first to report differences in risk in pregnancy outcomes for women with and without PsA.
“The information is relevant to guide rheumatologists in advising patients with PsA when planning the first or subsequent pregnancies,” she said. “The results are reassuring in reporting that the elevated risk for PsA patients for adverse pregnancy outcomes is low in patients not in need of antirheumatic medication, presumably in low-disease activity.”
However, the study is still unclear on whether the association with adverse pregnancy outcomes in patients is the result of higher disease activity or the need for antirheumatic treatment, she explained.
“It was only hypothesized that those patients under bDMARD treatment are/were in high disease activity. There [is] no information on disease activity in the data sources, which limits the results,” she said. “The investigation still does not solve the important question – if adverse pregnancy outcomes are rather related to high disease activity or the medication to treat this situation.”
There was no specific funding for this study. The study authors and Dr. Strangfeld have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vonoprazan beats PPIs in H. pylori eradication
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
AT ACG 2021
MS fundraising during a pandemic
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.