User login
What's your diagnosis?
Sigmoid colon perforation secondary to transcutaneous epicardial pacer wires.
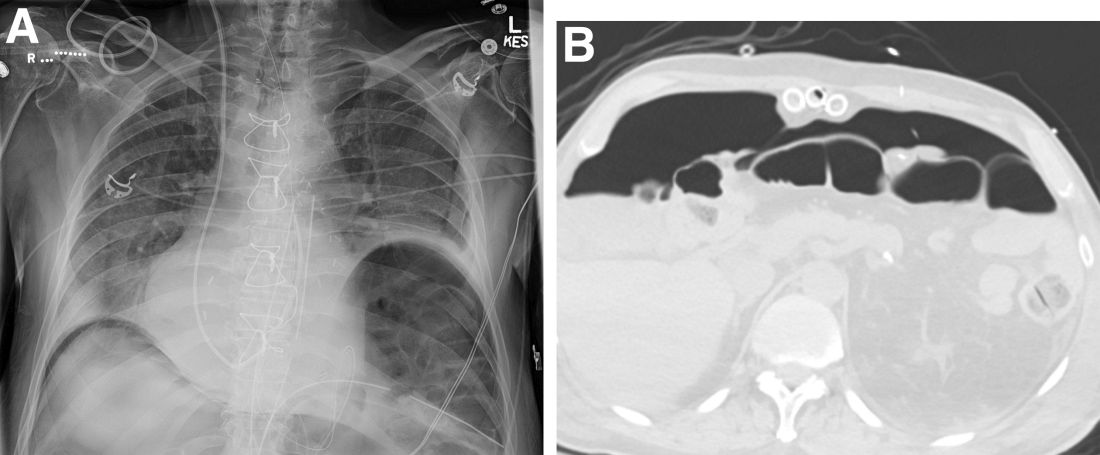
A plain film image (Figure A) shows diffusely dilated loops of bowel with subdiaphragmatic air concerning for GI viscous perforation. Dedicated cross-sectional imaging confirms intra-abdominal free air, and in representative cross section, the epicardial pacing wires can be visualized within the gastrointestinal lumen (Figure D, arrows). At the time of surgical consultation, the radiology report was notable for concern regarding possible disruption of peritoneum secondary to the difficult surgical chest tube placement in a patient with a high-riding left hemidiaphragm. Urgent laparoscopic exploration secondary to these findings unexpectedly revealed that the transcutaneous epicardial pacing wires had been inadvertently placed through the sigmoid colon (Figure C). The pacer wires were cut and removed intraoperatively. Unfortunately, 4 days after removal of pacer wires, the patient continued to have ongoing distension and was found to have sigmoid volvulus necessitating endoscopic decompression. After a prolonged hospitalization and recovery, he was discharged with a normal bowel pattern and tolerating oral intake to a skilled nursing facility.
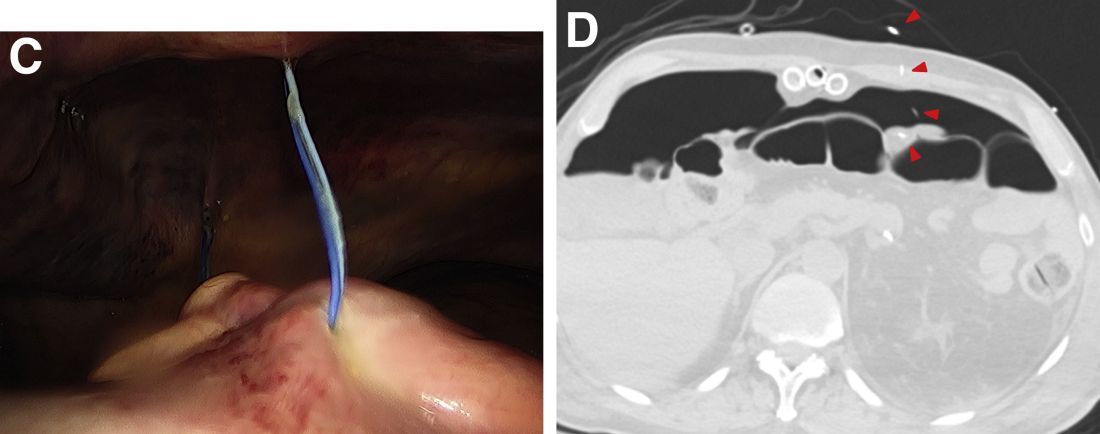
Temporary transcutaneous epicardial pacing wires are often placed after complex cardiovascular surgical procedures. Complications from wire placement are thought to be relatively rare and are typically associated with migration into local structures after wire placement and infectious complications secondary to retained wires.1,2 Perforation of local structures during placement is less common, and GI viscous perforation in particular is not a well-characterized cause of associated morbidity.3
Our case demonstrates that, in patients with hemidiaphragm elevation, epicardial wire placement risks GI viscous perforation. Furthermore, given the frequency of concomitant surgical hardware in this patient population, identification of malpositioned epicardial wires on plain film and even cross-sectional imaging can be difficult and can delay diagnosis.
References
1. Del Nido P, Goldman BS. J Card Surg. 1989 Mar;4(1):99-103.
2. Kapoor A et al. Interact Cardiovasc Thorac Surg. 2011 Jul;13(1):104-6.
3. Haba J et al. Can Assoc Radiol J. 2013 Feb;64(1):77-80.
Sigmoid colon perforation secondary to transcutaneous epicardial pacer wires.
A plain film image (Figure A) shows diffusely dilated loops of bowel with subdiaphragmatic air concerning for GI viscous perforation. Dedicated cross-sectional imaging confirms intra-abdominal free air, and in representative cross section, the epicardial pacing wires can be visualized within the gastrointestinal lumen (Figure D, arrows). At the time of surgical consultation, the radiology report was notable for concern regarding possible disruption of peritoneum secondary to the difficult surgical chest tube placement in a patient with a high-riding left hemidiaphragm. Urgent laparoscopic exploration secondary to these findings unexpectedly revealed that the transcutaneous epicardial pacing wires had been inadvertently placed through the sigmoid colon (Figure C). The pacer wires were cut and removed intraoperatively. Unfortunately, 4 days after removal of pacer wires, the patient continued to have ongoing distension and was found to have sigmoid volvulus necessitating endoscopic decompression. After a prolonged hospitalization and recovery, he was discharged with a normal bowel pattern and tolerating oral intake to a skilled nursing facility.

Temporary transcutaneous epicardial pacing wires are often placed after complex cardiovascular surgical procedures. Complications from wire placement are thought to be relatively rare and are typically associated with migration into local structures after wire placement and infectious complications secondary to retained wires.1,2 Perforation of local structures during placement is less common, and GI viscous perforation in particular is not a well-characterized cause of associated morbidity.3
Our case demonstrates that, in patients with hemidiaphragm elevation, epicardial wire placement risks GI viscous perforation. Furthermore, given the frequency of concomitant surgical hardware in this patient population, identification of malpositioned epicardial wires on plain film and even cross-sectional imaging can be difficult and can delay diagnosis.
References
1. Del Nido P, Goldman BS. J Card Surg. 1989 Mar;4(1):99-103.
2. Kapoor A et al. Interact Cardiovasc Thorac Surg. 2011 Jul;13(1):104-6.
3. Haba J et al. Can Assoc Radiol J. 2013 Feb;64(1):77-80.
Sigmoid colon perforation secondary to transcutaneous epicardial pacer wires.
A plain film image (Figure A) shows diffusely dilated loops of bowel with subdiaphragmatic air concerning for GI viscous perforation. Dedicated cross-sectional imaging confirms intra-abdominal free air, and in representative cross section, the epicardial pacing wires can be visualized within the gastrointestinal lumen (Figure D, arrows). At the time of surgical consultation, the radiology report was notable for concern regarding possible disruption of peritoneum secondary to the difficult surgical chest tube placement in a patient with a high-riding left hemidiaphragm. Urgent laparoscopic exploration secondary to these findings unexpectedly revealed that the transcutaneous epicardial pacing wires had been inadvertently placed through the sigmoid colon (Figure C). The pacer wires were cut and removed intraoperatively. Unfortunately, 4 days after removal of pacer wires, the patient continued to have ongoing distension and was found to have sigmoid volvulus necessitating endoscopic decompression. After a prolonged hospitalization and recovery, he was discharged with a normal bowel pattern and tolerating oral intake to a skilled nursing facility.

Temporary transcutaneous epicardial pacing wires are often placed after complex cardiovascular surgical procedures. Complications from wire placement are thought to be relatively rare and are typically associated with migration into local structures after wire placement and infectious complications secondary to retained wires.1,2 Perforation of local structures during placement is less common, and GI viscous perforation in particular is not a well-characterized cause of associated morbidity.3
Our case demonstrates that, in patients with hemidiaphragm elevation, epicardial wire placement risks GI viscous perforation. Furthermore, given the frequency of concomitant surgical hardware in this patient population, identification of malpositioned epicardial wires on plain film and even cross-sectional imaging can be difficult and can delay diagnosis.
References
1. Del Nido P, Goldman BS. J Card Surg. 1989 Mar;4(1):99-103.
2. Kapoor A et al. Interact Cardiovasc Thorac Surg. 2011 Jul;13(1):104-6.
3. Haba J et al. Can Assoc Radiol J. 2013 Feb;64(1):77-80.
Question: An 82-year-old man was admitted for urgent coronary artery bypass and concurrent mitral valve repair. Intraoperatively, he underwent cardiopulmonary bypass, epicardial pacing, and placement of two anterior mediastinal and one pleural chest tubes. After a relatively unremarkable initial postoperative course and nonnarcotic pain control, concern for ileus developed on postoperative day 4. A nasogastric tube was placed out of concern for worsening somnolence, nausea, and the inability to safely tolerate oral intake. The patient had been passing flatus but had yet to have a bowel movement since the operation. Physical examination at the time was notable for a soft abdomen with diffuse tenderness and voluntary guarding. Subsequent plain film imaging to confirm nasogastric tube placement (Figure A) and follow-up computed tomography imaging (Figure B) are shown.
What's the diagnosis?

A box of memories
My office’s storage room has an old bankers box, which has been there since I moved 8 years ago. Before that it was at my other office, behind an old desk. I had no idea what was in it, I always assumed office supplies, surplus drug company pens and sticky notes, who-knows-whats.
Last week I had one of those days where everyone cancels, so I decided to investigate the box.
It was packed with 10 years worth (2000-2010) of my secretary’s MRI scheduling sheets that had somehow escaped occasional shredding purges. So I sat down next to the office shredder to get rid of them.
As I fed the sheets in, the names jumped out at me. Some I have absolutely no recollection of. Others I still see today.
There were names of the long-deceased, bringing them back to me for the first time in years. There were others that I have no idea what happened to – they must have just stopped seeing me at some point, though for the life of me I can’t remember when, or why. Yet, in my mind, there they were, as if I’d just seen them yesterday. A few times I got curious enough to turn back to my computer and look up their charts, trying to remember their stories.
Then there were those I still remember clearly, every single detail of, in spite of the elapsed time. Something about their case or personality had indelibly etched them in my memory. A valuable lesson learned from them that had something or nothing to do with medicine that’s still with me.
Looking back, I’d guess I’ve seen roughly 15,000-20,000 patients over my career. Not nearly as many as my colleagues in general practice, but still quite a few. A decent sized basketball arena full.
The majority don’t stick with you. That’s the way it is in life.
The ones we didn’t know long – but who are still clearly remembered – are also valuable. In their own way, perhaps unknowingly, they made an impact that hopefully makes us better.
For that I’ll always be grateful to them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My office’s storage room has an old bankers box, which has been there since I moved 8 years ago. Before that it was at my other office, behind an old desk. I had no idea what was in it, I always assumed office supplies, surplus drug company pens and sticky notes, who-knows-whats.
Last week I had one of those days where everyone cancels, so I decided to investigate the box.
It was packed with 10 years worth (2000-2010) of my secretary’s MRI scheduling sheets that had somehow escaped occasional shredding purges. So I sat down next to the office shredder to get rid of them.
As I fed the sheets in, the names jumped out at me. Some I have absolutely no recollection of. Others I still see today.
There were names of the long-deceased, bringing them back to me for the first time in years. There were others that I have no idea what happened to – they must have just stopped seeing me at some point, though for the life of me I can’t remember when, or why. Yet, in my mind, there they were, as if I’d just seen them yesterday. A few times I got curious enough to turn back to my computer and look up their charts, trying to remember their stories.
Then there were those I still remember clearly, every single detail of, in spite of the elapsed time. Something about their case or personality had indelibly etched them in my memory. A valuable lesson learned from them that had something or nothing to do with medicine that’s still with me.
Looking back, I’d guess I’ve seen roughly 15,000-20,000 patients over my career. Not nearly as many as my colleagues in general practice, but still quite a few. A decent sized basketball arena full.
The majority don’t stick with you. That’s the way it is in life.
The ones we didn’t know long – but who are still clearly remembered – are also valuable. In their own way, perhaps unknowingly, they made an impact that hopefully makes us better.
For that I’ll always be grateful to them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My office’s storage room has an old bankers box, which has been there since I moved 8 years ago. Before that it was at my other office, behind an old desk. I had no idea what was in it, I always assumed office supplies, surplus drug company pens and sticky notes, who-knows-whats.
Last week I had one of those days where everyone cancels, so I decided to investigate the box.
It was packed with 10 years worth (2000-2010) of my secretary’s MRI scheduling sheets that had somehow escaped occasional shredding purges. So I sat down next to the office shredder to get rid of them.
As I fed the sheets in, the names jumped out at me. Some I have absolutely no recollection of. Others I still see today.
There were names of the long-deceased, bringing them back to me for the first time in years. There were others that I have no idea what happened to – they must have just stopped seeing me at some point, though for the life of me I can’t remember when, or why. Yet, in my mind, there they were, as if I’d just seen them yesterday. A few times I got curious enough to turn back to my computer and look up their charts, trying to remember their stories.
Then there were those I still remember clearly, every single detail of, in spite of the elapsed time. Something about their case or personality had indelibly etched them in my memory. A valuable lesson learned from them that had something or nothing to do with medicine that’s still with me.
Looking back, I’d guess I’ve seen roughly 15,000-20,000 patients over my career. Not nearly as many as my colleagues in general practice, but still quite a few. A decent sized basketball arena full.
The majority don’t stick with you. That’s the way it is in life.
The ones we didn’t know long – but who are still clearly remembered – are also valuable. In their own way, perhaps unknowingly, they made an impact that hopefully makes us better.
For that I’ll always be grateful to them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Without PrEP, a third of new HIV cases occur in MSM at low risk
Nearly one in three gay and bisexual men who were diagnosed with HIV at U.K. sexual health clinics didn’t meet the criteria for “high risk” that would signal to a clinician that they would be good candidates for pre-exposure prophylaxis (PrEP).
And that means that people who appear lower risk may still be good candidates for the HIV prevention pills, said Ann Sullivan, MD, consulting physician at Chelsea and Westminster Hospital, London.
“If people are coming forward for PrEP, they have self-identified that they need PrEP, [and] we should be allowing them to take PrEP,” said Dr. Sullivan at the 18th European AIDS Society Conference (EACS 2021). “We just need to trust patients. People know their risk, and we just have to accept that they know what they need best.”
And while this trial was made up of 95% gay and bisexual men, that ethos applies to every other group that could benefit from PrEP, including cisgender and transgender women and other gender-diverse people, Latinos, and Black Americans. In the United States, these groups make up nearly half of those who could benefit from PrEP under older guidelines but account for just 8% of people currently taking PrEP.
The finding also reinforces growing calls from health care providers to reduce gatekeeping around PrEP. For instance, there’s a move underway by the U.S. Centers for Disease Control and Prevention, where drafts of updated PrEP guidelines call for clinicians to talk to any sexually active teenager and adult about PrEP.
For the PrEP Impact trial, gay and bisexual men who received sexual health care at UK National Health Service sexual health clinics were invited to enroll in the study based on national PrEP guidelines. Those guidelines included being a cisgender man who had had sex with men not currently living with HIV and reporting condomless anal sex in the last 3 months; having a male partner whose HIV status they don’t know or who doesn’t have an undetectable viral load and with whom they’ve had condomless anal sex; or someone who doesn’t reach those criteria but whom the clinician thinks would be a good candidate.
Between Oct. 2017 and Feb. 2020, a total of 17,770 gay and bisexual men and 503 transgender or nonbinary people enrolled in the trial and were paired with 97,098 gay and bisexual men who didn’t use PrEP. (Data from the transgender participants were reported in a separate presentation.) The median age was 27 years, with 14.4% of the cisgender gay men between the ages of 16 and 24. Three out of four cis men were White, most lived in London, and more than half came from very-low-income neighborhoods.
Participants and controls were assessed for whether they were at particularly high risk for acquiring HIV, such as having used PrEP, having had two or more HIV tests, having had a rectal bacterial sexually transmitted infection (STI), or having had contact with someone with HIV or syphilis.
At the end of Feb. 2020, 24 cisgender men on PrEP had acquired HIV compared with 670 in the control group – an 87% reduction in HIV acquisition. Only one of those 24 cis men had lab-confirmed high adherence to PrEP. However, because the hair samples used to judge drug concentration weren’t long enough, Dr. Sullivan and colleagues were unable to assess whether the person really was fully adherent to treatment for the length of the trial.
But when they looked at the assessed behavior of people who acquired HIV, the two groups diverged. While a full 92% of people using PrEP had had STI diagnoses and other markers of increased risk, that was true for only 71% of people not taking PrEP. That meant, Dr. Sullivan said in an interview, that screening guidelines for PrEP were missing 29% of people with low assessed risk for HIV who nevertheless acquired the virus.
The findings led Antonio Urbina, MD, who both prescribes PrEP and manages Mount Sinai Medical Center’s PrEP program in New York, to the same conclusion that Dr. Sullivan and her team came to: that no screener is going to account for everything, and that there may be things that patients don’t want to tell their clinicians about their risk, either because of their own internalized stigma or their calculation that they aren’t comfortable enough with their providers to be honest.
“It reinforces to me that I need to ask more open-ended questions regarding risk and then just talk more about PrEP,” said Dr. Urbina, professor of medicine at Icahn School of Medicine. “Risk is dynamic and changes. And the great thing about PrEP is that if the risk goes up or down, if you have PrEP on board, you maintain this protection against HIV.”
An accompanying presentation on the transgender and nonbinary participants in the Impact Trial found that just one of 503 PrEP users acquired HIV. But here, too, there were people who could have benefited from PrEP but didn’t take it: Of the 477 trans and nonbinary participants who acted as controls, 97 were eligible by current guidelines but didn’t take PrEP. One in four of those declined the offer to take PrEP; the rest weren’t able to take it because they lived outside the treatment area. That, combined with a significantly lower likelihood that Black trans and nonbinary people took PrEP, indicated that work needs to be done to address the needs of people geographically and ethnically.
The data on gay men also raised the “who’s left out” issue for Gina Simoncini, MD, medical director for the Philadelphia AIDS Healthcare Foundation Healthcare Center. Dr. Simoncini previously taught attending physicians at Temple University how to prescribe PrEP and has done many grand rounds for primary care providers on how to manage PrEP.
“My biggest issue with this data is: What about the people who aren’t going to sexual health clinics?” she said. “What about the kid who’s 16 and maybe just barely putting his feet into the waters of sex and doesn’t feel quite comfortable going to a sexual health clinic? What about the trans Indian girl who can’t get to sexual health clinics because of family stigma and cultural stigma? The more we move toward primary care, the more people need to get on board with this.”
Dr. Sullivan reports no relevant financial relationships. Dr. Simoncini is an employee of AIDS Healthcare Foundation and has received advisory board fees from ViiV Healthcare. Dr. Urbina sits on the scientific advisory councils for Gilead Sciences, ViiV Healthcare, and Merck.
A version of this article first appeared on Medscape.com.
Nearly one in three gay and bisexual men who were diagnosed with HIV at U.K. sexual health clinics didn’t meet the criteria for “high risk” that would signal to a clinician that they would be good candidates for pre-exposure prophylaxis (PrEP).
And that means that people who appear lower risk may still be good candidates for the HIV prevention pills, said Ann Sullivan, MD, consulting physician at Chelsea and Westminster Hospital, London.
“If people are coming forward for PrEP, they have self-identified that they need PrEP, [and] we should be allowing them to take PrEP,” said Dr. Sullivan at the 18th European AIDS Society Conference (EACS 2021). “We just need to trust patients. People know their risk, and we just have to accept that they know what they need best.”
And while this trial was made up of 95% gay and bisexual men, that ethos applies to every other group that could benefit from PrEP, including cisgender and transgender women and other gender-diverse people, Latinos, and Black Americans. In the United States, these groups make up nearly half of those who could benefit from PrEP under older guidelines but account for just 8% of people currently taking PrEP.
The finding also reinforces growing calls from health care providers to reduce gatekeeping around PrEP. For instance, there’s a move underway by the U.S. Centers for Disease Control and Prevention, where drafts of updated PrEP guidelines call for clinicians to talk to any sexually active teenager and adult about PrEP.
For the PrEP Impact trial, gay and bisexual men who received sexual health care at UK National Health Service sexual health clinics were invited to enroll in the study based on national PrEP guidelines. Those guidelines included being a cisgender man who had had sex with men not currently living with HIV and reporting condomless anal sex in the last 3 months; having a male partner whose HIV status they don’t know or who doesn’t have an undetectable viral load and with whom they’ve had condomless anal sex; or someone who doesn’t reach those criteria but whom the clinician thinks would be a good candidate.
Between Oct. 2017 and Feb. 2020, a total of 17,770 gay and bisexual men and 503 transgender or nonbinary people enrolled in the trial and were paired with 97,098 gay and bisexual men who didn’t use PrEP. (Data from the transgender participants were reported in a separate presentation.) The median age was 27 years, with 14.4% of the cisgender gay men between the ages of 16 and 24. Three out of four cis men were White, most lived in London, and more than half came from very-low-income neighborhoods.
Participants and controls were assessed for whether they were at particularly high risk for acquiring HIV, such as having used PrEP, having had two or more HIV tests, having had a rectal bacterial sexually transmitted infection (STI), or having had contact with someone with HIV or syphilis.
At the end of Feb. 2020, 24 cisgender men on PrEP had acquired HIV compared with 670 in the control group – an 87% reduction in HIV acquisition. Only one of those 24 cis men had lab-confirmed high adherence to PrEP. However, because the hair samples used to judge drug concentration weren’t long enough, Dr. Sullivan and colleagues were unable to assess whether the person really was fully adherent to treatment for the length of the trial.
But when they looked at the assessed behavior of people who acquired HIV, the two groups diverged. While a full 92% of people using PrEP had had STI diagnoses and other markers of increased risk, that was true for only 71% of people not taking PrEP. That meant, Dr. Sullivan said in an interview, that screening guidelines for PrEP were missing 29% of people with low assessed risk for HIV who nevertheless acquired the virus.
The findings led Antonio Urbina, MD, who both prescribes PrEP and manages Mount Sinai Medical Center’s PrEP program in New York, to the same conclusion that Dr. Sullivan and her team came to: that no screener is going to account for everything, and that there may be things that patients don’t want to tell their clinicians about their risk, either because of their own internalized stigma or their calculation that they aren’t comfortable enough with their providers to be honest.
“It reinforces to me that I need to ask more open-ended questions regarding risk and then just talk more about PrEP,” said Dr. Urbina, professor of medicine at Icahn School of Medicine. “Risk is dynamic and changes. And the great thing about PrEP is that if the risk goes up or down, if you have PrEP on board, you maintain this protection against HIV.”
An accompanying presentation on the transgender and nonbinary participants in the Impact Trial found that just one of 503 PrEP users acquired HIV. But here, too, there were people who could have benefited from PrEP but didn’t take it: Of the 477 trans and nonbinary participants who acted as controls, 97 were eligible by current guidelines but didn’t take PrEP. One in four of those declined the offer to take PrEP; the rest weren’t able to take it because they lived outside the treatment area. That, combined with a significantly lower likelihood that Black trans and nonbinary people took PrEP, indicated that work needs to be done to address the needs of people geographically and ethnically.
The data on gay men also raised the “who’s left out” issue for Gina Simoncini, MD, medical director for the Philadelphia AIDS Healthcare Foundation Healthcare Center. Dr. Simoncini previously taught attending physicians at Temple University how to prescribe PrEP and has done many grand rounds for primary care providers on how to manage PrEP.
“My biggest issue with this data is: What about the people who aren’t going to sexual health clinics?” she said. “What about the kid who’s 16 and maybe just barely putting his feet into the waters of sex and doesn’t feel quite comfortable going to a sexual health clinic? What about the trans Indian girl who can’t get to sexual health clinics because of family stigma and cultural stigma? The more we move toward primary care, the more people need to get on board with this.”
Dr. Sullivan reports no relevant financial relationships. Dr. Simoncini is an employee of AIDS Healthcare Foundation and has received advisory board fees from ViiV Healthcare. Dr. Urbina sits on the scientific advisory councils for Gilead Sciences, ViiV Healthcare, and Merck.
A version of this article first appeared on Medscape.com.
Nearly one in three gay and bisexual men who were diagnosed with HIV at U.K. sexual health clinics didn’t meet the criteria for “high risk” that would signal to a clinician that they would be good candidates for pre-exposure prophylaxis (PrEP).
And that means that people who appear lower risk may still be good candidates for the HIV prevention pills, said Ann Sullivan, MD, consulting physician at Chelsea and Westminster Hospital, London.
“If people are coming forward for PrEP, they have self-identified that they need PrEP, [and] we should be allowing them to take PrEP,” said Dr. Sullivan at the 18th European AIDS Society Conference (EACS 2021). “We just need to trust patients. People know their risk, and we just have to accept that they know what they need best.”
And while this trial was made up of 95% gay and bisexual men, that ethos applies to every other group that could benefit from PrEP, including cisgender and transgender women and other gender-diverse people, Latinos, and Black Americans. In the United States, these groups make up nearly half of those who could benefit from PrEP under older guidelines but account for just 8% of people currently taking PrEP.
The finding also reinforces growing calls from health care providers to reduce gatekeeping around PrEP. For instance, there’s a move underway by the U.S. Centers for Disease Control and Prevention, where drafts of updated PrEP guidelines call for clinicians to talk to any sexually active teenager and adult about PrEP.
For the PrEP Impact trial, gay and bisexual men who received sexual health care at UK National Health Service sexual health clinics were invited to enroll in the study based on national PrEP guidelines. Those guidelines included being a cisgender man who had had sex with men not currently living with HIV and reporting condomless anal sex in the last 3 months; having a male partner whose HIV status they don’t know or who doesn’t have an undetectable viral load and with whom they’ve had condomless anal sex; or someone who doesn’t reach those criteria but whom the clinician thinks would be a good candidate.
Between Oct. 2017 and Feb. 2020, a total of 17,770 gay and bisexual men and 503 transgender or nonbinary people enrolled in the trial and were paired with 97,098 gay and bisexual men who didn’t use PrEP. (Data from the transgender participants were reported in a separate presentation.) The median age was 27 years, with 14.4% of the cisgender gay men between the ages of 16 and 24. Three out of four cis men were White, most lived in London, and more than half came from very-low-income neighborhoods.
Participants and controls were assessed for whether they were at particularly high risk for acquiring HIV, such as having used PrEP, having had two or more HIV tests, having had a rectal bacterial sexually transmitted infection (STI), or having had contact with someone with HIV or syphilis.
At the end of Feb. 2020, 24 cisgender men on PrEP had acquired HIV compared with 670 in the control group – an 87% reduction in HIV acquisition. Only one of those 24 cis men had lab-confirmed high adherence to PrEP. However, because the hair samples used to judge drug concentration weren’t long enough, Dr. Sullivan and colleagues were unable to assess whether the person really was fully adherent to treatment for the length of the trial.
But when they looked at the assessed behavior of people who acquired HIV, the two groups diverged. While a full 92% of people using PrEP had had STI diagnoses and other markers of increased risk, that was true for only 71% of people not taking PrEP. That meant, Dr. Sullivan said in an interview, that screening guidelines for PrEP were missing 29% of people with low assessed risk for HIV who nevertheless acquired the virus.
The findings led Antonio Urbina, MD, who both prescribes PrEP and manages Mount Sinai Medical Center’s PrEP program in New York, to the same conclusion that Dr. Sullivan and her team came to: that no screener is going to account for everything, and that there may be things that patients don’t want to tell their clinicians about their risk, either because of their own internalized stigma or their calculation that they aren’t comfortable enough with their providers to be honest.
“It reinforces to me that I need to ask more open-ended questions regarding risk and then just talk more about PrEP,” said Dr. Urbina, professor of medicine at Icahn School of Medicine. “Risk is dynamic and changes. And the great thing about PrEP is that if the risk goes up or down, if you have PrEP on board, you maintain this protection against HIV.”
An accompanying presentation on the transgender and nonbinary participants in the Impact Trial found that just one of 503 PrEP users acquired HIV. But here, too, there were people who could have benefited from PrEP but didn’t take it: Of the 477 trans and nonbinary participants who acted as controls, 97 were eligible by current guidelines but didn’t take PrEP. One in four of those declined the offer to take PrEP; the rest weren’t able to take it because they lived outside the treatment area. That, combined with a significantly lower likelihood that Black trans and nonbinary people took PrEP, indicated that work needs to be done to address the needs of people geographically and ethnically.
The data on gay men also raised the “who’s left out” issue for Gina Simoncini, MD, medical director for the Philadelphia AIDS Healthcare Foundation Healthcare Center. Dr. Simoncini previously taught attending physicians at Temple University how to prescribe PrEP and has done many grand rounds for primary care providers on how to manage PrEP.
“My biggest issue with this data is: What about the people who aren’t going to sexual health clinics?” she said. “What about the kid who’s 16 and maybe just barely putting his feet into the waters of sex and doesn’t feel quite comfortable going to a sexual health clinic? What about the trans Indian girl who can’t get to sexual health clinics because of family stigma and cultural stigma? The more we move toward primary care, the more people need to get on board with this.”
Dr. Sullivan reports no relevant financial relationships. Dr. Simoncini is an employee of AIDS Healthcare Foundation and has received advisory board fees from ViiV Healthcare. Dr. Urbina sits on the scientific advisory councils for Gilead Sciences, ViiV Healthcare, and Merck.
A version of this article first appeared on Medscape.com.
Long-acting HIV ART: Lessons from a year of Cabenuva
One year into offering the first long-acting injectable HIV treatment to his patients, Jonathan Angel, MD, head of the division of infectious diseases at the University of Ottawa, reported that 15 of the 21 of patients who started on the regimen are still taking it, all with viral suppression. Those who weren’t cited a combination of inconvenience, injection site pain, and “injection fatigue.”
These are just a few things HIV providers are learning as they begin what Chloe Orkin, MD, professor of HIV medicine at Queen Mary University of London, called a paradigm shift to long-acting treatment, which may soon include not just shots but rings, implants, and microarray patches.
“It’s a paradigm shift, and we are at the very beginning of this paradigm shift,” said Dr. Orkin, commenting during the discussion session of the European AIDS Clinical Society 2021 annual meeting. “We’re having to change our model, and it’s challenging.”
In the United States, the Food and Drug Administration approved the first long-acting injectable, a combination of cabotegravir and rilpivirine (CAB/RIL; Cabenuva, ViiV Healthcare) in January 2021. But it has been approved in Canada since March 2020 and available at Dr. Angel’s clinic since November 2020. It’s also available in Canada as an every-other-month shot. Injected into the buttocks, the shot was found to be noninferior to standard daily oral treatment in many studies, including the ATLAS, the ATLAS-2M – which tested the every-other-month approach – and FLAIR trials.
Dr. Angel’s clinic was part of all three of those trials, so his clinic has had 5 years’ experience preparing for the change in workflow and the new approach the shots require.
Of the 21 people Dr. Angel has treated, 11 were white Canadians, nine were Black African, and one was Indigenous Canadian, with women making up a third of the participants. Median age was 51 years, and all patients had had undetectable viral loads before beginning the regimen. (Studies of the drug’s effectiveness in people who struggle to take daily pills are still ongoing.)
Most of those 21 patients had had undetectable viral loads for more than 5 years, but a few had been undetectable for only 6 months before beginning the shots. Their immune systems were also healthy, with a median CD4 count of 618 cells/mcL. As in the clinical trials, none of the participants had experienced antiretroviral treatment failure. Because public health insurers in Canada have yet to approve the shots, Dr. Angel’s patients receiving Cabenuva also have private health insurance. Up to 90% of people in Canada receive pharmaceutical coverage through public insurance; therefore, the shot is not yet widely available.
Twenty patients switched from integrase-inhibitor regimens, and one had been receiving a nonnucleoside reverse transcriptase inhibitor–based regimen before starting Cabenuva.
And although the drug has not been approved for shot initiation this way, two patients requested – and Dr. Angel agreed – to start them on the shots without first doing a month of daily pills to check for safety.
“This is my conclusion from these data: the oral lead-in period is not necessary,” Dr. Angel said in his presentation at the meeting. “It can provide some comfort to either a physician or a patient, but it does not seem to be medically necessary.”
That approach is not without data to back it up. Research presented at HIV Glasgow 2020 showed that people who switched from daily oral dolutegravir/abacavir/lamivudine straight to the injections did so without problems.
At last clinic visit, 15 of those 21 were still receiving the shots. None have experienced treatment failure, and all were still virally suppressed. Four participants left the trials and one more person opted to return to daily pills, citing some level of what Dr. Angel called “injection fatigue.”
“Just as we use the term ‘pill fatigue’ for patients who are tired of taking pills, patients do get tired of coming in monthly for their visits and injections,” he said. They find the trip to the clinic for the intramuscular injections “inconvenient,” he said.
Unlike in the United States, where Cabenuva is approved for only monthly injections, Health Canada has already approved the shot for every-other-month injections, which Dr. Angel said may reduce the odds of injection fatigue.
Dr. Angel’s presentation drew comments, questions, and excitement from the crowd. Annemarie Wensing, MD, assistant professor of medicine at University Medical Center Utrecht (the Netherlands), asked whether dispensing with the oral lead-in period could mean that these shots could be useful for people going on longer trips, people having surgeries where they can’t swallow pills, or in other scenarios.
“These are not hypothetical conversations,” Dr. Angel said. “I’m having these conversations with patients now – temporary use, they travel for 3 months and come back, can they go from injectable to oral to injectable.”
For now, he said, the answer is, “We’ll figure it out.”
Meanwhile, there’s another big question when it comes to injectables, said Marta Vas ylyev, MD, from Lviv (Ukraine) Regional AIDS Center: When will they be available to the people who might benefit most from them – people in resource-limited settings, people who so far have struggled to remember to take their pills every day?
For now, Dr. Angel replied, injectables continue to be a treatment only for those who are already doing well while receiving HIV treatment: those with already suppressed viral load, who are good at taking daily pills, and who are being treated at well-resourced clinics.
“There are huge obstacles to overcome if this is ever to be available [in resource-limited settings], and way more obstacles than there are with any oral therapies,” he said. “There’s not been much discussion here about the necessity of cold-chain requirements of pharmacies either centrally or locally, [or] the requirements of additional nurses or health care staff to administer the medication. So you’re looking at a very resource-intensive therapy, which now is fairly restrictive [as to] who will have access to it.”
Dr. Angel reports serving on advisory boards for ViiV Healthcare and Gilead Sciences and has done contract research for ViiV Healthcare, Gilead, and Merck. Dr. Orkin has received research grants, fees as a consultant, travel sponsorship, and speaker fees from ViiV, Merck, and GlaxoSmithKline. Dr. Vasylyev reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One year into offering the first long-acting injectable HIV treatment to his patients, Jonathan Angel, MD, head of the division of infectious diseases at the University of Ottawa, reported that 15 of the 21 of patients who started on the regimen are still taking it, all with viral suppression. Those who weren’t cited a combination of inconvenience, injection site pain, and “injection fatigue.”
These are just a few things HIV providers are learning as they begin what Chloe Orkin, MD, professor of HIV medicine at Queen Mary University of London, called a paradigm shift to long-acting treatment, which may soon include not just shots but rings, implants, and microarray patches.
“It’s a paradigm shift, and we are at the very beginning of this paradigm shift,” said Dr. Orkin, commenting during the discussion session of the European AIDS Clinical Society 2021 annual meeting. “We’re having to change our model, and it’s challenging.”
In the United States, the Food and Drug Administration approved the first long-acting injectable, a combination of cabotegravir and rilpivirine (CAB/RIL; Cabenuva, ViiV Healthcare) in January 2021. But it has been approved in Canada since March 2020 and available at Dr. Angel’s clinic since November 2020. It’s also available in Canada as an every-other-month shot. Injected into the buttocks, the shot was found to be noninferior to standard daily oral treatment in many studies, including the ATLAS, the ATLAS-2M – which tested the every-other-month approach – and FLAIR trials.
Dr. Angel’s clinic was part of all three of those trials, so his clinic has had 5 years’ experience preparing for the change in workflow and the new approach the shots require.
Of the 21 people Dr. Angel has treated, 11 were white Canadians, nine were Black African, and one was Indigenous Canadian, with women making up a third of the participants. Median age was 51 years, and all patients had had undetectable viral loads before beginning the regimen. (Studies of the drug’s effectiveness in people who struggle to take daily pills are still ongoing.)
Most of those 21 patients had had undetectable viral loads for more than 5 years, but a few had been undetectable for only 6 months before beginning the shots. Their immune systems were also healthy, with a median CD4 count of 618 cells/mcL. As in the clinical trials, none of the participants had experienced antiretroviral treatment failure. Because public health insurers in Canada have yet to approve the shots, Dr. Angel’s patients receiving Cabenuva also have private health insurance. Up to 90% of people in Canada receive pharmaceutical coverage through public insurance; therefore, the shot is not yet widely available.
Twenty patients switched from integrase-inhibitor regimens, and one had been receiving a nonnucleoside reverse transcriptase inhibitor–based regimen before starting Cabenuva.
And although the drug has not been approved for shot initiation this way, two patients requested – and Dr. Angel agreed – to start them on the shots without first doing a month of daily pills to check for safety.
“This is my conclusion from these data: the oral lead-in period is not necessary,” Dr. Angel said in his presentation at the meeting. “It can provide some comfort to either a physician or a patient, but it does not seem to be medically necessary.”
That approach is not without data to back it up. Research presented at HIV Glasgow 2020 showed that people who switched from daily oral dolutegravir/abacavir/lamivudine straight to the injections did so without problems.
At last clinic visit, 15 of those 21 were still receiving the shots. None have experienced treatment failure, and all were still virally suppressed. Four participants left the trials and one more person opted to return to daily pills, citing some level of what Dr. Angel called “injection fatigue.”
“Just as we use the term ‘pill fatigue’ for patients who are tired of taking pills, patients do get tired of coming in monthly for their visits and injections,” he said. They find the trip to the clinic for the intramuscular injections “inconvenient,” he said.
Unlike in the United States, where Cabenuva is approved for only monthly injections, Health Canada has already approved the shot for every-other-month injections, which Dr. Angel said may reduce the odds of injection fatigue.
Dr. Angel’s presentation drew comments, questions, and excitement from the crowd. Annemarie Wensing, MD, assistant professor of medicine at University Medical Center Utrecht (the Netherlands), asked whether dispensing with the oral lead-in period could mean that these shots could be useful for people going on longer trips, people having surgeries where they can’t swallow pills, or in other scenarios.
“These are not hypothetical conversations,” Dr. Angel said. “I’m having these conversations with patients now – temporary use, they travel for 3 months and come back, can they go from injectable to oral to injectable.”
For now, he said, the answer is, “We’ll figure it out.”
Meanwhile, there’s another big question when it comes to injectables, said Marta Vas ylyev, MD, from Lviv (Ukraine) Regional AIDS Center: When will they be available to the people who might benefit most from them – people in resource-limited settings, people who so far have struggled to remember to take their pills every day?
For now, Dr. Angel replied, injectables continue to be a treatment only for those who are already doing well while receiving HIV treatment: those with already suppressed viral load, who are good at taking daily pills, and who are being treated at well-resourced clinics.
“There are huge obstacles to overcome if this is ever to be available [in resource-limited settings], and way more obstacles than there are with any oral therapies,” he said. “There’s not been much discussion here about the necessity of cold-chain requirements of pharmacies either centrally or locally, [or] the requirements of additional nurses or health care staff to administer the medication. So you’re looking at a very resource-intensive therapy, which now is fairly restrictive [as to] who will have access to it.”
Dr. Angel reports serving on advisory boards for ViiV Healthcare and Gilead Sciences and has done contract research for ViiV Healthcare, Gilead, and Merck. Dr. Orkin has received research grants, fees as a consultant, travel sponsorship, and speaker fees from ViiV, Merck, and GlaxoSmithKline. Dr. Vasylyev reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One year into offering the first long-acting injectable HIV treatment to his patients, Jonathan Angel, MD, head of the division of infectious diseases at the University of Ottawa, reported that 15 of the 21 of patients who started on the regimen are still taking it, all with viral suppression. Those who weren’t cited a combination of inconvenience, injection site pain, and “injection fatigue.”
These are just a few things HIV providers are learning as they begin what Chloe Orkin, MD, professor of HIV medicine at Queen Mary University of London, called a paradigm shift to long-acting treatment, which may soon include not just shots but rings, implants, and microarray patches.
“It’s a paradigm shift, and we are at the very beginning of this paradigm shift,” said Dr. Orkin, commenting during the discussion session of the European AIDS Clinical Society 2021 annual meeting. “We’re having to change our model, and it’s challenging.”
In the United States, the Food and Drug Administration approved the first long-acting injectable, a combination of cabotegravir and rilpivirine (CAB/RIL; Cabenuva, ViiV Healthcare) in January 2021. But it has been approved in Canada since March 2020 and available at Dr. Angel’s clinic since November 2020. It’s also available in Canada as an every-other-month shot. Injected into the buttocks, the shot was found to be noninferior to standard daily oral treatment in many studies, including the ATLAS, the ATLAS-2M – which tested the every-other-month approach – and FLAIR trials.
Dr. Angel’s clinic was part of all three of those trials, so his clinic has had 5 years’ experience preparing for the change in workflow and the new approach the shots require.
Of the 21 people Dr. Angel has treated, 11 were white Canadians, nine were Black African, and one was Indigenous Canadian, with women making up a third of the participants. Median age was 51 years, and all patients had had undetectable viral loads before beginning the regimen. (Studies of the drug’s effectiveness in people who struggle to take daily pills are still ongoing.)
Most of those 21 patients had had undetectable viral loads for more than 5 years, but a few had been undetectable for only 6 months before beginning the shots. Their immune systems were also healthy, with a median CD4 count of 618 cells/mcL. As in the clinical trials, none of the participants had experienced antiretroviral treatment failure. Because public health insurers in Canada have yet to approve the shots, Dr. Angel’s patients receiving Cabenuva also have private health insurance. Up to 90% of people in Canada receive pharmaceutical coverage through public insurance; therefore, the shot is not yet widely available.
Twenty patients switched from integrase-inhibitor regimens, and one had been receiving a nonnucleoside reverse transcriptase inhibitor–based regimen before starting Cabenuva.
And although the drug has not been approved for shot initiation this way, two patients requested – and Dr. Angel agreed – to start them on the shots without first doing a month of daily pills to check for safety.
“This is my conclusion from these data: the oral lead-in period is not necessary,” Dr. Angel said in his presentation at the meeting. “It can provide some comfort to either a physician or a patient, but it does not seem to be medically necessary.”
That approach is not without data to back it up. Research presented at HIV Glasgow 2020 showed that people who switched from daily oral dolutegravir/abacavir/lamivudine straight to the injections did so without problems.
At last clinic visit, 15 of those 21 were still receiving the shots. None have experienced treatment failure, and all were still virally suppressed. Four participants left the trials and one more person opted to return to daily pills, citing some level of what Dr. Angel called “injection fatigue.”
“Just as we use the term ‘pill fatigue’ for patients who are tired of taking pills, patients do get tired of coming in monthly for their visits and injections,” he said. They find the trip to the clinic for the intramuscular injections “inconvenient,” he said.
Unlike in the United States, where Cabenuva is approved for only monthly injections, Health Canada has already approved the shot for every-other-month injections, which Dr. Angel said may reduce the odds of injection fatigue.
Dr. Angel’s presentation drew comments, questions, and excitement from the crowd. Annemarie Wensing, MD, assistant professor of medicine at University Medical Center Utrecht (the Netherlands), asked whether dispensing with the oral lead-in period could mean that these shots could be useful for people going on longer trips, people having surgeries where they can’t swallow pills, or in other scenarios.
“These are not hypothetical conversations,” Dr. Angel said. “I’m having these conversations with patients now – temporary use, they travel for 3 months and come back, can they go from injectable to oral to injectable.”
For now, he said, the answer is, “We’ll figure it out.”
Meanwhile, there’s another big question when it comes to injectables, said Marta Vas ylyev, MD, from Lviv (Ukraine) Regional AIDS Center: When will they be available to the people who might benefit most from them – people in resource-limited settings, people who so far have struggled to remember to take their pills every day?
For now, Dr. Angel replied, injectables continue to be a treatment only for those who are already doing well while receiving HIV treatment: those with already suppressed viral load, who are good at taking daily pills, and who are being treated at well-resourced clinics.
“There are huge obstacles to overcome if this is ever to be available [in resource-limited settings], and way more obstacles than there are with any oral therapies,” he said. “There’s not been much discussion here about the necessity of cold-chain requirements of pharmacies either centrally or locally, [or] the requirements of additional nurses or health care staff to administer the medication. So you’re looking at a very resource-intensive therapy, which now is fairly restrictive [as to] who will have access to it.”
Dr. Angel reports serving on advisory boards for ViiV Healthcare and Gilead Sciences and has done contract research for ViiV Healthcare, Gilead, and Merck. Dr. Orkin has received research grants, fees as a consultant, travel sponsorship, and speaker fees from ViiV, Merck, and GlaxoSmithKline. Dr. Vasylyev reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neighborhood fast food restaurants linked to type 2 diabetes
new research indicates.
The national study of more than 4 million U.S. veterans also found the opposite association with supermarkets in suburban and rural communities but not others.
“Neighborhood food environment was associated with type 2 diabetes risk among U.S. veterans in multiple community types, suggesting potential avenues for action to address the burden of type 2 diabetes,” say Rania Kanchi, MPH, of the department of population health, New York University Langone Health, and colleagues.
Restriction of fast food establishments could benefit all types of communities, while interventions to increase supermarket availability could help minimize diabetes risk in suburban and rural communities, they stress.
“These actions, combined with increasing awareness of the risk of type 2 diabetes and the importance of healthy diet intake, might be associated with a decrease in the burden of type 2 diabetes among adults in the U.S.,” the researchers add.
The data were published online Oct. 29 in JAMA Network Open.
“The more we learn about the relationship between the food environment and chronic diseases like type 2 diabetes, the more policymakers can act by improving the mix of healthy food options sold in restaurants and food outlets, or by creating better zoning laws that promote optimal food options for residents,” commented Lorna Thorpe, PhD, MPH, professor in the department of population health at NYU Langone and senior author of the study in a press release.
In an accompanying editorial, Elham Hatef, MD, MPH, of the Center for Population Health IT at Johns Hopkins Bloomberg School of Public Health, Baltimore, calls the study “a great example of the capabilities of [health information technology] to provide a comprehensive assessment of a person’s health, which goes beyond just documenting clinical diseases and medical interventions.”
Research has large geographic breadth
The study is notable for its large geographic breadth, say the researchers.
“Most studies that examine the built food environment and its relationship to chronic diseases have been much smaller or conducted in localized areas,” Ms. Kanchi said in the press statement.
“Our study design is national in scope and allowed us to identify the types of communities that people are living in, characterize their food environment, and observe what happens to them over time. The size of our cohort allows for geographic generalizability in a way that other studies do not,” Ms. Kanchi continued.
The research included data for 4,100,650 individuals from the Veterans Affairs electronic health records (EHRs) who didn’t have type 2 diabetes at baseline, between 2008 and 2016. After a median follow-up of 5.5 person-years, 13.2% developed type 2 diabetes. Cumulative incidence was greater among those who were older, those who were non-Hispanic Black compared with other races, and those with disabilities and lower incomes.
The proportion of adults with type 2 diabetes was highest among those living in high-density urban communities (14.3%), followed by low-density urban (13.1%), rural (13.2%), and suburban (12.6%) communities.
Overall, a 10% increase in the number of fast food restaurants compared with other food establishments in a given neighborhood was associated with a 1% increased risk for incident type 2 diabetes in high-density urban, low-density urban, and rural communities and a 2% increased risk in suburban communities.
In contrast, a 10% increase in supermarket density compared with other food stores was associated with a lower risk for type 2 diabetes in suburban and rural communities, but the association wasn’t significant elsewhere.
“Taken together, our findings suggest that policies specific to fast food restaurants, such as [those] ... restricting the siting of fast food restaurants and healthy beverage default laws, may be effective in reducing type 2 diabetes risk in all community types,” say the authors.
“In urban areas where population and retail density are growing, it will be even more important to focus on these policies,” they emphasize.
Great example of capabilities of health information technology
In the editorial, Dr. Hatef notes that methodological advances, such as natural language processing and machine learning, have enabled health systems to use real-world data such as the free-text notes in the EHR to identify patient-level risk factors for diseases or disease complications.
Such methods could be further used to “evaluate the associations between social needs and place-based [social determinants of health] and type 2 diabetes incidence and management,” Dr. Hatef adds.
And linkage of data from the EHR to such community-level data “would help to comprehensively assess and identify patients likely to experience type 2 diabetes and its complications as a result of their risk factors or characteristics of the neighborhoods where they reside.”
“This approach could foster collaborations between the health systems and at-risk communities they serve and help to reallocate health system resources to those in most need in the community to reduce the burden of type 2 diabetes and other chronic conditions among racial minority groups and socioeconomically disadvantaged patients and to advance population health.”
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Aging, the Commonwealth Universal Research Enhancement program funded by the Pennsylvania Department of Health, the Urban Health Collaborative at Drexel University, and the Built Environment and Health Research Group at Columbia University. Ms. Kanchi and Dr. Hatef have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research indicates.
The national study of more than 4 million U.S. veterans also found the opposite association with supermarkets in suburban and rural communities but not others.
“Neighborhood food environment was associated with type 2 diabetes risk among U.S. veterans in multiple community types, suggesting potential avenues for action to address the burden of type 2 diabetes,” say Rania Kanchi, MPH, of the department of population health, New York University Langone Health, and colleagues.
Restriction of fast food establishments could benefit all types of communities, while interventions to increase supermarket availability could help minimize diabetes risk in suburban and rural communities, they stress.
“These actions, combined with increasing awareness of the risk of type 2 diabetes and the importance of healthy diet intake, might be associated with a decrease in the burden of type 2 diabetes among adults in the U.S.,” the researchers add.
The data were published online Oct. 29 in JAMA Network Open.
“The more we learn about the relationship between the food environment and chronic diseases like type 2 diabetes, the more policymakers can act by improving the mix of healthy food options sold in restaurants and food outlets, or by creating better zoning laws that promote optimal food options for residents,” commented Lorna Thorpe, PhD, MPH, professor in the department of population health at NYU Langone and senior author of the study in a press release.
In an accompanying editorial, Elham Hatef, MD, MPH, of the Center for Population Health IT at Johns Hopkins Bloomberg School of Public Health, Baltimore, calls the study “a great example of the capabilities of [health information technology] to provide a comprehensive assessment of a person’s health, which goes beyond just documenting clinical diseases and medical interventions.”
Research has large geographic breadth
The study is notable for its large geographic breadth, say the researchers.
“Most studies that examine the built food environment and its relationship to chronic diseases have been much smaller or conducted in localized areas,” Ms. Kanchi said in the press statement.
“Our study design is national in scope and allowed us to identify the types of communities that people are living in, characterize their food environment, and observe what happens to them over time. The size of our cohort allows for geographic generalizability in a way that other studies do not,” Ms. Kanchi continued.
The research included data for 4,100,650 individuals from the Veterans Affairs electronic health records (EHRs) who didn’t have type 2 diabetes at baseline, between 2008 and 2016. After a median follow-up of 5.5 person-years, 13.2% developed type 2 diabetes. Cumulative incidence was greater among those who were older, those who were non-Hispanic Black compared with other races, and those with disabilities and lower incomes.
The proportion of adults with type 2 diabetes was highest among those living in high-density urban communities (14.3%), followed by low-density urban (13.1%), rural (13.2%), and suburban (12.6%) communities.
Overall, a 10% increase in the number of fast food restaurants compared with other food establishments in a given neighborhood was associated with a 1% increased risk for incident type 2 diabetes in high-density urban, low-density urban, and rural communities and a 2% increased risk in suburban communities.
In contrast, a 10% increase in supermarket density compared with other food stores was associated with a lower risk for type 2 diabetes in suburban and rural communities, but the association wasn’t significant elsewhere.
“Taken together, our findings suggest that policies specific to fast food restaurants, such as [those] ... restricting the siting of fast food restaurants and healthy beverage default laws, may be effective in reducing type 2 diabetes risk in all community types,” say the authors.
“In urban areas where population and retail density are growing, it will be even more important to focus on these policies,” they emphasize.
Great example of capabilities of health information technology
In the editorial, Dr. Hatef notes that methodological advances, such as natural language processing and machine learning, have enabled health systems to use real-world data such as the free-text notes in the EHR to identify patient-level risk factors for diseases or disease complications.
Such methods could be further used to “evaluate the associations between social needs and place-based [social determinants of health] and type 2 diabetes incidence and management,” Dr. Hatef adds.
And linkage of data from the EHR to such community-level data “would help to comprehensively assess and identify patients likely to experience type 2 diabetes and its complications as a result of their risk factors or characteristics of the neighborhoods where they reside.”
“This approach could foster collaborations between the health systems and at-risk communities they serve and help to reallocate health system resources to those in most need in the community to reduce the burden of type 2 diabetes and other chronic conditions among racial minority groups and socioeconomically disadvantaged patients and to advance population health.”
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Aging, the Commonwealth Universal Research Enhancement program funded by the Pennsylvania Department of Health, the Urban Health Collaborative at Drexel University, and the Built Environment and Health Research Group at Columbia University. Ms. Kanchi and Dr. Hatef have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research indicates.
The national study of more than 4 million U.S. veterans also found the opposite association with supermarkets in suburban and rural communities but not others.
“Neighborhood food environment was associated with type 2 diabetes risk among U.S. veterans in multiple community types, suggesting potential avenues for action to address the burden of type 2 diabetes,” say Rania Kanchi, MPH, of the department of population health, New York University Langone Health, and colleagues.
Restriction of fast food establishments could benefit all types of communities, while interventions to increase supermarket availability could help minimize diabetes risk in suburban and rural communities, they stress.
“These actions, combined with increasing awareness of the risk of type 2 diabetes and the importance of healthy diet intake, might be associated with a decrease in the burden of type 2 diabetes among adults in the U.S.,” the researchers add.
The data were published online Oct. 29 in JAMA Network Open.
“The more we learn about the relationship between the food environment and chronic diseases like type 2 diabetes, the more policymakers can act by improving the mix of healthy food options sold in restaurants and food outlets, or by creating better zoning laws that promote optimal food options for residents,” commented Lorna Thorpe, PhD, MPH, professor in the department of population health at NYU Langone and senior author of the study in a press release.
In an accompanying editorial, Elham Hatef, MD, MPH, of the Center for Population Health IT at Johns Hopkins Bloomberg School of Public Health, Baltimore, calls the study “a great example of the capabilities of [health information technology] to provide a comprehensive assessment of a person’s health, which goes beyond just documenting clinical diseases and medical interventions.”
Research has large geographic breadth
The study is notable for its large geographic breadth, say the researchers.
“Most studies that examine the built food environment and its relationship to chronic diseases have been much smaller or conducted in localized areas,” Ms. Kanchi said in the press statement.
“Our study design is national in scope and allowed us to identify the types of communities that people are living in, characterize their food environment, and observe what happens to them over time. The size of our cohort allows for geographic generalizability in a way that other studies do not,” Ms. Kanchi continued.
The research included data for 4,100,650 individuals from the Veterans Affairs electronic health records (EHRs) who didn’t have type 2 diabetes at baseline, between 2008 and 2016. After a median follow-up of 5.5 person-years, 13.2% developed type 2 diabetes. Cumulative incidence was greater among those who were older, those who were non-Hispanic Black compared with other races, and those with disabilities and lower incomes.
The proportion of adults with type 2 diabetes was highest among those living in high-density urban communities (14.3%), followed by low-density urban (13.1%), rural (13.2%), and suburban (12.6%) communities.
Overall, a 10% increase in the number of fast food restaurants compared with other food establishments in a given neighborhood was associated with a 1% increased risk for incident type 2 diabetes in high-density urban, low-density urban, and rural communities and a 2% increased risk in suburban communities.
In contrast, a 10% increase in supermarket density compared with other food stores was associated with a lower risk for type 2 diabetes in suburban and rural communities, but the association wasn’t significant elsewhere.
“Taken together, our findings suggest that policies specific to fast food restaurants, such as [those] ... restricting the siting of fast food restaurants and healthy beverage default laws, may be effective in reducing type 2 diabetes risk in all community types,” say the authors.
“In urban areas where population and retail density are growing, it will be even more important to focus on these policies,” they emphasize.
Great example of capabilities of health information technology
In the editorial, Dr. Hatef notes that methodological advances, such as natural language processing and machine learning, have enabled health systems to use real-world data such as the free-text notes in the EHR to identify patient-level risk factors for diseases or disease complications.
Such methods could be further used to “evaluate the associations between social needs and place-based [social determinants of health] and type 2 diabetes incidence and management,” Dr. Hatef adds.
And linkage of data from the EHR to such community-level data “would help to comprehensively assess and identify patients likely to experience type 2 diabetes and its complications as a result of their risk factors or characteristics of the neighborhoods where they reside.”
“This approach could foster collaborations between the health systems and at-risk communities they serve and help to reallocate health system resources to those in most need in the community to reduce the burden of type 2 diabetes and other chronic conditions among racial minority groups and socioeconomically disadvantaged patients and to advance population health.”
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Aging, the Commonwealth Universal Research Enhancement program funded by the Pennsylvania Department of Health, the Urban Health Collaborative at Drexel University, and the Built Environment and Health Research Group at Columbia University. Ms. Kanchi and Dr. Hatef have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Multiple DMTs linked to alopecia, especially in women
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
a new study finds.
From 2009 to 2019, the Food and Drug Administration received 7,978 reports of new-onset alopecia in patients taking DMTs, particularly teriflunomide (3,255, 40.8%; 90% female), dimethyl fumarate (1,641, 20.6%; 89% female), natalizumab (955, 12.0%; 92% female), and fingolimod (776, 9.7% of the total reports; 93% female), several researchers reported at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). Of these, only teriflunomide had previously been linked to alopecia, study coauthor Ahmed Obeidat, MD, PhD, a neurologist at the Medical College of Wisconsin, Milwaukee, said in an interview.
“Our finding of frequent reports of alopecia on DMTs studied calls for further investigation into the subject,” Dr. Obeidat said. “Alopecia can cause deep personal impacts and can be a source of significant psychological concern for some patients.”
According to Dr. Obeidat, alopecia has been linked to the only a few DMTs – cladribine and the interferons – in addition to teriflunomide. “To our surprise, we received anecdotal reports of hair thinning from several of our MS patients treated with various other [DMTs]. Upon further investigation, we could not find substantial literature to explain this phenomenon which led us to conduct our investigation.”
Dr. Obeidat and colleagues identified DMT-related alopecia cases (18.3%) among 43,655 reports in the skin and subcutaneous tissue disorder category in the FDA Adverse Event Reporting System. Other DMTs with more than 1 case report were interferon beta-1a (635, 8.0%; 92% female), glatiramer acetate (332, 4.2%; 87% female), ocrelizumab (142, 1.8%; 94% female), interferon beta-1b (126, 1.6%; 95% female), alemtuzumab (86, 1.1%; 88% female), cladribine (17, 0.2%; 65% female), and rituximab (10, 0.1%; 90% female).
The average age for the case reports varied from 42 to 51 years for most of the drugs except alemtuzumab (mean age, 40 years) and cladribine (average age, 38 years), which had low numbers of cases.
Siponimod (three cases) and ozanimod (no cases) were not included in the age and gender analyses.
Why do so many women seem to be affected, well beyond their percentage of MS cases overall? The answer is unclear, said medical student Mokshal H. Porwal, the study’s lead author. “There could be a biological explanation,” Mr. Porwal said, “or women may report cases more often: “Earlier studies suggested that alopecia may affect women more adversely in terms of body image as well as overall psychological well-being, compared to males.”
The researchers also noted that patients – not medical professionals – provided most of the case reports in the FDA database. “We believe this indicates that alopecia is a patient-centered concern that may have a larger impact on their lives than what the health care teams may perceive,” Mr. Porwal said. “Oftentimes, we as health care providers, look for the more acute and apparent adverse events, which can overshadow issues such as hair thinning/alopecia that could have even greater psychological impacts on our patients.”
Dr. Obeidat said there are still multiple mysteries about DMT and alopecia risk: the true incidence of cases per DMT or DMT class, the mechanism(s) behind a link, the permanent or transient nature of the alopecia cases, and the risk factors in individual patients.
Going forward, he said, “we advise clinicians to discuss hair thinning or alopecia as a possible side effect that has been reported in association with all DMTs in the real-world, postmarketing era.”
No study funding was reported. Dr. Obeidat reported various disclosures; the other authors reported no disclosures.
FROM CMSC 2021
Pushing the Boundaries in Multiple Sclerosis (MS): Reimagining Treatment and Care
It’s undeniable that great strides have been made in advancing the science of multiple sclerosis (MS) over the past 20 years. Continued pursuit of research and discovery has revolutionized the understanding, diagnosis, and treatment of this debilitating condition that causes symptoms that may impact nearly every part of the body and mind. Despite these advancements, there is still more work to be done.
As the search for a cure continues, it’s imperative to ask the big questions: How does MS develop and progress? How can we reduce health disparities in care and treatment? How can we improve existing treatments and expedite the discovery of new ones? To continue making progress, we must stretch the boundaries of scientific understanding until we have answers and solutions to these and other important questions.
We know that MS is a leading cause of non-traumatic disability for young people.1,2 Yet, one of the biggest challenges for neurologists is that every person’s experience with MS is unique, making it difficult to predict what symptoms will manifest, how disruptive the symptoms and relapses will be and how a person will respond to treatment.
Twenty years ago, it was widely thought that MS was mainly driven by T cells, but in the late 1990’s, a team of researchers made a groundbreaking discovery: B cells also played a key role in MS. This discovery redefined how the scientific community thought about the underlying biology of MS and the central role B cells can play.
It was this bold thinking that led researchers at the University of California, San Francisco (UCSF) and scientists at Genentech, a member of the Roche Group, to explore whether a medication that depleted B cells would have an impact on MS. This collaboration, in turn, inspired the clinical research behind Ocrevus® (ocrelizumab), the first and only treatment approved by the FDA for both relapsing MS (RMS) and primary progressive MS (PPMS). MS is a progressive disease from the start. An important goal of treating MS is to reduce disease progression as soon as possible to slow how quickly a person’s disability changes and prevent the long-term consequences of disease progression.3 Ocrevus is administered via infusion twice yearly, with the initial dose split into two separate infusions and given two weeks apart. As with any therapy, this treatment has side effects, which can be found in the full Prescribing Information and Medication Guide. Ocrevus is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reactions to Ocrevus. The warnings and precautions for Ocrevus are infusion reactions, and infections, which include respiratory tract infections, herpes, progressive multifocal leukoencephalopathy (PML), and hepatitis B virus (HBV) reactivation. Additional warnings are possible increased risk of immunosuppressant effects with other immunosuppressants, reduction in immunoglobulins, and malignancies.
Ensuring a Continuum of Care
Another significant challenge facing the MS community is ensuring ongoing support and care for people living with the condition, especially throughout the COVID-19 pandemic. “We know that seeking early and appropriate care for MS is critical to slowing disability progression and achieving successful patient outcomes,” said Dr. Ashish Pradhan, Executive Director of Neuroimmunology at Genentech. “The pandemic has created additional barriers to early diagnosis and treatment, but we are committed to continuing to do everything we can to support patients and physicians at this stressful time.” Dr. Pradhan also observed that FDA's approval of a shorter infusion time for Ocrevus in December 2020 could potentially contribute to reducing some of the burden on the healthcare system in the long-term.
Being able to connect with others in the MS community is a vital part of navigating the uncertainties of living with the condition. This past April, to shine a light on the diverse MS community, Genentech hosted an #MSVisibility Virtual Concert that gathered people from across the world with different backgrounds and MS experiences and encouraged people living with MS to continue seeking appropriate support and care during the pandemic.
Continued support of the MS community through advocacy partnerships and grants over the years has played a sustained role in Genentech’s efforts to ensure people with MS receive necessary medical care and treatment. For example, Genentech partnered with an MS advocacy organization to fund a program that helps people get to and from their medical appointments. In addition, for more than 30 years, Genentech has helped more than two million people get the medicine they need through patient assistance programs like Genentech Access Solutions and the Genentech Patient Foundation.
of Neuroimmunology at Genentech
Advancing Inclusive Research in MS
MS has been shown to impact individuals and specific populations in markedly different ways. Those who identify as Black or of African descent and Hispanic/Latinos for example can experience more severe symptoms and faster disease progression than their Caucasian counterparts.4,5 Unfortunately, it’s not clear exactly why, largely because of their vast underrepresentation in clinical trials (less than 5% and 1%, respectively).6 While there are multiple explanations for these disparities, around one-in-three minority patients don’t participate in clinical trials due to lack of trust and 52% feel that the healthcare system is not only flawed, but is actually working against them.7
“Increased diversity in research is paramount to ensuring that the healthcare system is serving every person living with MS and ultimately reducing health inequities while providing more tailored treatment options,” commented Dr. Pradhan. “To advance the care of undertreated and underserved people with MS, we are creating and supporting programs, grants and other initiatives focused on people of color and inclusive and minority research to better understand how MS presents itself and develops in different patient populations. We want to make sure that all generations of people living with MS are equally represented in clinical research and have access to the treatments that will work best for them.”
Refining Existing Approaches and Leading Treatment Innovation
For decades, Genentech and Roche have been conducting neuroscience research and clinical trials to make forward progress in understanding and treating a variety of neurological conditions, including MS. While the treatment landscape for MS has evolved significantly over the past two decades, continued investment in research and development is critical. Continuing to review and refine existing treatment approaches may improve patient outcomes and discovering new treatment approaches may play an important role in advancing the treatment paradigm.
Dr. Pradhan added, “as the scientific community continues to think boldly, and re-imagine treatment and care for MS, we hope to continue to improve our understanding of the condition for all people with the disease, and advance treatment options. Pushing boundaries means refusing to stop until everyone living with MS can be treated effectively and a cure is found. This sentiment reminds me of a Ralph Waldo Emerson quote, ‘Don’t follow where the path may lead. Go instead where there is no path and leave a trail.’ That’s what we are trying to do at Genentech.”
To learn more, please visit Ocrevus.com.
Indications and Important Safety Information
OCREVUS is indicated for the treatment of:
- Relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
- Primary progressive MS, in adults.
Contraindications
OCREVUS is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reaction to OCREVUS.
Warnings and Precautions
Infusion Reactions
OCREVUS can cause infusion reactions, which can include pruritus, rash, urticaria, erythema, bronchospasm, throat irritation, oropharyngeal pain, dyspnea, pharyngeal or laryngeal edema, flushing, hypotension, pyrexia, fatigue, headache, dizziness, nausea, tachycardia, and anaphylaxis. In multiple sclerosis (MS) clinical trials, the incidence of infusion reactions in OCREVUS-treated patients [who received methylprednisolone (or an equivalent steroid) and possibly other pre-medication to reduce the risk of infusion reactions prior to each infusion] was 34-40%, with the highest incidence with the first infusion. There were no fatal infusion reactions, but 0.3% of OCREVUS-treated MS patients experienced infusion reactions that were serious, some requiring hospitalization.
Observe patients treated with OCREVUS for infusion reactions during the infusion and for at least one hour after completion of the infusion. Inform patients that infusion reactions can occur up to 24 hours after the infusion. Administer pre-medication (e.g., methylprednisolone or an equivalent corticosteroid, and an antihistamine) to reduce the frequency and severity of infusion reactions. The addition of an antipyretic (e.g., acetaminophen) may also be considered. For life-threatening infusion reactions, immediately and permanently stop OCREVUS and administer appropriate supportive treatment. For less severe infusion reactions, management may involve temporarily stopping the infusion, reducing the infusion rate, and/or administering symptomatic treatment.
Infections
A higher proportion of OCREVUS-treated patients experienced infections compared to patients taking REBIF or placebo. In RMS trials, 58% of OCREVUS-treated patients experienced one or more infections compared to 52% of REBIF-treated patients. In the PPMS trial, 70% of OCREVUS-treated patients experienced one or more infections compared to 68% of patients on placebo. OCREVUS increased the risk for upper respiratory tract infections, lower respiratory tract infections, skin infections, and herpes-related infections. OCREVUS was not associated with an increased risk of serious infections in MS patients. Delay OCREVUS administration in patients with an active infection until the infection is resolved.
Respiratory Tract Infections
A higher proportion of OCREVUS-treated patients experienced respiratory tract infections compared to patients taking REBIF or placebo. In RMS trials, 40% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 33% of REBIF-treated patients, and 8% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 5% of REBIF-treated patients. In the PPMS trial, 49% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 43% of patients on placebo and 10% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 9% of patients on placebo. The infections were predominantly mild to moderate and consisted mostly of upper respiratory tract infections and bronchitis.
Herpes
In active-controlled (RMS) clinical trials, herpes infections were reported more frequently in OCREVUS-treated patients than in REBIF-treated patients, including herpes zoster (2.1% vs. 1.0%), herpes simplex (0.7% vs. 0.1%), oral herpes (3.0% vs. 2.2%), genital herpes (0.1% vs. 0%), and herpes virus infection (0.1% vs. 0%). Infections were predominantly mild to moderate in severity. In the placebo-controlled (PPMS) clinical trial, oral herpes was reported more frequently in the OCREVUS-treated patients than in the patients on placebo (2.7% vs 0.8%).
Serious cases of infections caused by herpes simplex virus and varicella zoster virus, including central nervous system infections (encephalitis and meningitis), intraocular infections, and disseminated skin and soft tissue infections, have been reported in the postmarketing setting in multiple sclerosis patients receiving OCREVUS. Serious herpes virus infections may occur at any time during treatment with OCREVUS. Some cases were life-threatening.
If serious herpes infections occur, OCREVUS should be discontinued or withheld until the infection has resolved, and appropriate treatment should be administered.
Progressive Multifocal Leukoencephalopathy (PML)
PML is an opportunistic viral infection of the brain caused by the John Cunningham (JC) virus that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Although no cases of PML were identified in OCREVUS clinical trials, JC virus infection resulting in PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants). At the first sign or symptom suggestive of PML, withhold OCREVUS and perform an appropriate diagnostic evaluation. MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes (per USPI).
Hepatitis B Virus (HBV) Reactivation
Hepatitis B reactivation has been reported in MS patients treated with OCREVUS in the postmarketing setting. Fulminant hepatitis, hepatic failure, and death caused by HBV reactivation have occurred in patients treated with anti-CD20 antibodies. Perform HBV screening in all patients before initiation of treatment with OCREVUS. Do not administer OCREVUS to patients with active HBV confirmed by positive results for HBsAg and anti-HB tests. For patients who are negative for surface antigen [HBsAg] and positive for HB core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment.
Possible Increased Risk of Immunosuppressant Effects with Other Immunosuppressants
When initiating OCREVUS after an immunosuppressive therapy or initiating an immunosuppressive therapy after OCREVUS, consider the potential for increased immunosuppressive effect. OCREVUS has not been studied in combination with other MS therapies.
Vaccinations
Administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of OCREVUS for live or live-attenuated vaccines and, whenever possible, at least 2 weeks prior to initiation of OCREVUS for non-live vaccines. OCREVUS may interfere with the effectiveness of non-live vaccines. The safety of immunization with live or live-attenuated vaccines following OCREVUS therapy has not been studied, and vaccination with live-attenuated or live vaccines is not recommended during treatment and until B-cell repletion.
Vaccination of Infants Born to Mothers Treated with OCREVUS During Pregnancy
In infants of mothers exposed to OCREVUS during pregnancy, do not administer live or live-attenuated vaccines before confirming the recovery of B-cell counts as measured by CD19+ B-cells. Depletion of B-cells in these infants may increase the risks from live or live-attenuated vaccines.
You may administer non-live vaccines, as indicated, prior to recovery from B-cell depletion, but should consider assessing vaccine immune responses, including consultation with a qualified specialist, to assess whether a protective immune response was mounted.
Reduction in Immunoglobulins
As expected with any B-cell depleting therapy, decreased immunoglobulin levels are observed with OCREVUS treatment. The pooled data of OCREVUS clinical studies (RMS and PPMS) and their open-label extensions (up to approximately 7 years of exposure) have shown an association between decreased levels of immunoglobulin G (IgG<LLN) and increased rates of serious infections. Monitor the levels of quantitative serum immunoglobulins during OCREVUS treatment and after discontinuation of treatment, until B-cell repletion, and especially in the setting of recurrent serious infections. Consider discontinuing OCREVUS therapy in patients with serious opportunistic or recurrent serious infections, and if prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
Malignancies
An increased risk of malignancy with OCREVUS may exist. In controlled trials, malignancies, including breast cancer, occurred more frequently in OCREVUS-treated patients. Breast cancer occurred in 6 of 781 females treated with OCREVUS and none of 668 females treated with REBIF or placebo. Patients should follow standard breast cancer screening guidelines.
Use in Specific Populations
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy and fetal/neonatal/infant outcomes in women exposed to OCREVUS during pregnancy. Physicians are encouraged to register patients and pregnant women are encouraged to register themselves by calling 1-833-872-4370 or visiting www.ocrevuspregnancyregistry.com.
There are no adequate data on the developmental risk associated with use of OCREVUS in pregnant women. There are no data on B-cell levels in human neonates following maternal exposure to OCREVUS. However, transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 antibodies during pregnancy. OCREVUS is a humanized monoclonal antibody of an immunoglobulin G1 subtype and immunoglobulins are known to cross the placental barrier.
Lactation
There are no data on the presence of ocrelizumab in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Ocrelizumab was excreted in the milk of ocrelizumab-treated monkeys. Human IgG is excreted in human milk, and the potential for absorption of ocrelizumab to lead to B-cell depletion in the infant is unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OCREVUS and any potential adverse effects on the breastfed infant from OCREVUS or from the underlying maternal condition.
Females and Males of Reproductive Potential
Women of childbearing potential should use effective contraception while receiving OCREVUS and for 6 months after the last infusion of OCREVUS.
Most Common Adverse Reactions
RMS: The most common adverse reactions in RMS trials (incidence ≥10% and >REBIF) were upper respiratory tract infections (40%) and infusion reactions (34%).
PPMS: The most common adverse reactions in PPMS trials (incidence ≥10% and >placebo) were upper respiratory tract infections (49%), infusion reactions (40%), skin infections (14%), and lower respiratory tract infections (10%).
For additional safety information, please see the full Prescribing Information and Medication Guide.
1 Murray TJ. (2006). Diagnosis and Treatment of Multiple Sclerosis. BMJ, 322 (7540):525-527.
2 Tullman M. (2013). Overview of the Epidemiology, Diagnosis, and Disease Progression Associated With Multiple Sclerosis. The American Journal of Managed Care. 19 (2): S15-S20.
3 National Institutes of Health-National Institute of Neurological Disorders and Stroke. (2015). Multiple Sclerosis: Hope Through Research. Available at: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Multiple-Sclerosis-Hope-Through-Research.
4 National MS Society. "Who Gets MS? - African Americans." Retrieved November 2020. nationalmssociety.org/What-is-MS/Who-Gets-MS/African-American-Resources.
5 Langer-Gould A, et al. Neurology. 2013;80:1734-1739; 2. Wallin MT, et al. Brain. 2012;135:1778-1785.
6 U.S. Food and Drug Administration. “Clinical Trials Shed Light on Minority Health.” U.S. Food and Drug Administration Website. https://wayback.archive-it.org/7993/20180908114418/https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm349063.htm. Published 2018.
7 Genentech. “A New Perspective on Health Inequity.” https://www.gene.com/stories/a-new-perspective-on-health-inequity.

It’s undeniable that great strides have been made in advancing the science of multiple sclerosis (MS) over the past 20 years. Continued pursuit of research and discovery has revolutionized the understanding, diagnosis, and treatment of this debilitating condition that causes symptoms that may impact nearly every part of the body and mind. Despite these advancements, there is still more work to be done.
As the search for a cure continues, it’s imperative to ask the big questions: How does MS develop and progress? How can we reduce health disparities in care and treatment? How can we improve existing treatments and expedite the discovery of new ones? To continue making progress, we must stretch the boundaries of scientific understanding until we have answers and solutions to these and other important questions.
We know that MS is a leading cause of non-traumatic disability for young people.1,2 Yet, one of the biggest challenges for neurologists is that every person’s experience with MS is unique, making it difficult to predict what symptoms will manifest, how disruptive the symptoms and relapses will be and how a person will respond to treatment.
Twenty years ago, it was widely thought that MS was mainly driven by T cells, but in the late 1990’s, a team of researchers made a groundbreaking discovery: B cells also played a key role in MS. This discovery redefined how the scientific community thought about the underlying biology of MS and the central role B cells can play.
It was this bold thinking that led researchers at the University of California, San Francisco (UCSF) and scientists at Genentech, a member of the Roche Group, to explore whether a medication that depleted B cells would have an impact on MS. This collaboration, in turn, inspired the clinical research behind Ocrevus® (ocrelizumab), the first and only treatment approved by the FDA for both relapsing MS (RMS) and primary progressive MS (PPMS). MS is a progressive disease from the start. An important goal of treating MS is to reduce disease progression as soon as possible to slow how quickly a person’s disability changes and prevent the long-term consequences of disease progression.3 Ocrevus is administered via infusion twice yearly, with the initial dose split into two separate infusions and given two weeks apart. As with any therapy, this treatment has side effects, which can be found in the full Prescribing Information and Medication Guide. Ocrevus is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reactions to Ocrevus. The warnings and precautions for Ocrevus are infusion reactions, and infections, which include respiratory tract infections, herpes, progressive multifocal leukoencephalopathy (PML), and hepatitis B virus (HBV) reactivation. Additional warnings are possible increased risk of immunosuppressant effects with other immunosuppressants, reduction in immunoglobulins, and malignancies.
Ensuring a Continuum of Care
Another significant challenge facing the MS community is ensuring ongoing support and care for people living with the condition, especially throughout the COVID-19 pandemic. “We know that seeking early and appropriate care for MS is critical to slowing disability progression and achieving successful patient outcomes,” said Dr. Ashish Pradhan, Executive Director of Neuroimmunology at Genentech. “The pandemic has created additional barriers to early diagnosis and treatment, but we are committed to continuing to do everything we can to support patients and physicians at this stressful time.” Dr. Pradhan also observed that FDA's approval of a shorter infusion time for Ocrevus in December 2020 could potentially contribute to reducing some of the burden on the healthcare system in the long-term.
Being able to connect with others in the MS community is a vital part of navigating the uncertainties of living with the condition. This past April, to shine a light on the diverse MS community, Genentech hosted an #MSVisibility Virtual Concert that gathered people from across the world with different backgrounds and MS experiences and encouraged people living with MS to continue seeking appropriate support and care during the pandemic.
Continued support of the MS community through advocacy partnerships and grants over the years has played a sustained role in Genentech’s efforts to ensure people with MS receive necessary medical care and treatment. For example, Genentech partnered with an MS advocacy organization to fund a program that helps people get to and from their medical appointments. In addition, for more than 30 years, Genentech has helped more than two million people get the medicine they need through patient assistance programs like Genentech Access Solutions and the Genentech Patient Foundation.
of Neuroimmunology at Genentech
Advancing Inclusive Research in MS
MS has been shown to impact individuals and specific populations in markedly different ways. Those who identify as Black or of African descent and Hispanic/Latinos for example can experience more severe symptoms and faster disease progression than their Caucasian counterparts.4,5 Unfortunately, it’s not clear exactly why, largely because of their vast underrepresentation in clinical trials (less than 5% and 1%, respectively).6 While there are multiple explanations for these disparities, around one-in-three minority patients don’t participate in clinical trials due to lack of trust and 52% feel that the healthcare system is not only flawed, but is actually working against them.7
“Increased diversity in research is paramount to ensuring that the healthcare system is serving every person living with MS and ultimately reducing health inequities while providing more tailored treatment options,” commented Dr. Pradhan. “To advance the care of undertreated and underserved people with MS, we are creating and supporting programs, grants and other initiatives focused on people of color and inclusive and minority research to better understand how MS presents itself and develops in different patient populations. We want to make sure that all generations of people living with MS are equally represented in clinical research and have access to the treatments that will work best for them.”
Refining Existing Approaches and Leading Treatment Innovation
For decades, Genentech and Roche have been conducting neuroscience research and clinical trials to make forward progress in understanding and treating a variety of neurological conditions, including MS. While the treatment landscape for MS has evolved significantly over the past two decades, continued investment in research and development is critical. Continuing to review and refine existing treatment approaches may improve patient outcomes and discovering new treatment approaches may play an important role in advancing the treatment paradigm.
Dr. Pradhan added, “as the scientific community continues to think boldly, and re-imagine treatment and care for MS, we hope to continue to improve our understanding of the condition for all people with the disease, and advance treatment options. Pushing boundaries means refusing to stop until everyone living with MS can be treated effectively and a cure is found. This sentiment reminds me of a Ralph Waldo Emerson quote, ‘Don’t follow where the path may lead. Go instead where there is no path and leave a trail.’ That’s what we are trying to do at Genentech.”
To learn more, please visit Ocrevus.com.
Indications and Important Safety Information
OCREVUS is indicated for the treatment of:
- Relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
- Primary progressive MS, in adults.
Contraindications
OCREVUS is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reaction to OCREVUS.
Warnings and Precautions
Infusion Reactions
OCREVUS can cause infusion reactions, which can include pruritus, rash, urticaria, erythema, bronchospasm, throat irritation, oropharyngeal pain, dyspnea, pharyngeal or laryngeal edema, flushing, hypotension, pyrexia, fatigue, headache, dizziness, nausea, tachycardia, and anaphylaxis. In multiple sclerosis (MS) clinical trials, the incidence of infusion reactions in OCREVUS-treated patients [who received methylprednisolone (or an equivalent steroid) and possibly other pre-medication to reduce the risk of infusion reactions prior to each infusion] was 34-40%, with the highest incidence with the first infusion. There were no fatal infusion reactions, but 0.3% of OCREVUS-treated MS patients experienced infusion reactions that were serious, some requiring hospitalization.
Observe patients treated with OCREVUS for infusion reactions during the infusion and for at least one hour after completion of the infusion. Inform patients that infusion reactions can occur up to 24 hours after the infusion. Administer pre-medication (e.g., methylprednisolone or an equivalent corticosteroid, and an antihistamine) to reduce the frequency and severity of infusion reactions. The addition of an antipyretic (e.g., acetaminophen) may also be considered. For life-threatening infusion reactions, immediately and permanently stop OCREVUS and administer appropriate supportive treatment. For less severe infusion reactions, management may involve temporarily stopping the infusion, reducing the infusion rate, and/or administering symptomatic treatment.
Infections
A higher proportion of OCREVUS-treated patients experienced infections compared to patients taking REBIF or placebo. In RMS trials, 58% of OCREVUS-treated patients experienced one or more infections compared to 52% of REBIF-treated patients. In the PPMS trial, 70% of OCREVUS-treated patients experienced one or more infections compared to 68% of patients on placebo. OCREVUS increased the risk for upper respiratory tract infections, lower respiratory tract infections, skin infections, and herpes-related infections. OCREVUS was not associated with an increased risk of serious infections in MS patients. Delay OCREVUS administration in patients with an active infection until the infection is resolved.
Respiratory Tract Infections
A higher proportion of OCREVUS-treated patients experienced respiratory tract infections compared to patients taking REBIF or placebo. In RMS trials, 40% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 33% of REBIF-treated patients, and 8% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 5% of REBIF-treated patients. In the PPMS trial, 49% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 43% of patients on placebo and 10% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 9% of patients on placebo. The infections were predominantly mild to moderate and consisted mostly of upper respiratory tract infections and bronchitis.
Herpes
In active-controlled (RMS) clinical trials, herpes infections were reported more frequently in OCREVUS-treated patients than in REBIF-treated patients, including herpes zoster (2.1% vs. 1.0%), herpes simplex (0.7% vs. 0.1%), oral herpes (3.0% vs. 2.2%), genital herpes (0.1% vs. 0%), and herpes virus infection (0.1% vs. 0%). Infections were predominantly mild to moderate in severity. In the placebo-controlled (PPMS) clinical trial, oral herpes was reported more frequently in the OCREVUS-treated patients than in the patients on placebo (2.7% vs 0.8%).
Serious cases of infections caused by herpes simplex virus and varicella zoster virus, including central nervous system infections (encephalitis and meningitis), intraocular infections, and disseminated skin and soft tissue infections, have been reported in the postmarketing setting in multiple sclerosis patients receiving OCREVUS. Serious herpes virus infections may occur at any time during treatment with OCREVUS. Some cases were life-threatening.
If serious herpes infections occur, OCREVUS should be discontinued or withheld until the infection has resolved, and appropriate treatment should be administered.
Progressive Multifocal Leukoencephalopathy (PML)
PML is an opportunistic viral infection of the brain caused by the John Cunningham (JC) virus that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Although no cases of PML were identified in OCREVUS clinical trials, JC virus infection resulting in PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants). At the first sign or symptom suggestive of PML, withhold OCREVUS and perform an appropriate diagnostic evaluation. MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes (per USPI).
Hepatitis B Virus (HBV) Reactivation
Hepatitis B reactivation has been reported in MS patients treated with OCREVUS in the postmarketing setting. Fulminant hepatitis, hepatic failure, and death caused by HBV reactivation have occurred in patients treated with anti-CD20 antibodies. Perform HBV screening in all patients before initiation of treatment with OCREVUS. Do not administer OCREVUS to patients with active HBV confirmed by positive results for HBsAg and anti-HB tests. For patients who are negative for surface antigen [HBsAg] and positive for HB core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment.
Possible Increased Risk of Immunosuppressant Effects with Other Immunosuppressants
When initiating OCREVUS after an immunosuppressive therapy or initiating an immunosuppressive therapy after OCREVUS, consider the potential for increased immunosuppressive effect. OCREVUS has not been studied in combination with other MS therapies.
Vaccinations
Administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of OCREVUS for live or live-attenuated vaccines and, whenever possible, at least 2 weeks prior to initiation of OCREVUS for non-live vaccines. OCREVUS may interfere with the effectiveness of non-live vaccines. The safety of immunization with live or live-attenuated vaccines following OCREVUS therapy has not been studied, and vaccination with live-attenuated or live vaccines is not recommended during treatment and until B-cell repletion.
Vaccination of Infants Born to Mothers Treated with OCREVUS During Pregnancy
In infants of mothers exposed to OCREVUS during pregnancy, do not administer live or live-attenuated vaccines before confirming the recovery of B-cell counts as measured by CD19+ B-cells. Depletion of B-cells in these infants may increase the risks from live or live-attenuated vaccines.
You may administer non-live vaccines, as indicated, prior to recovery from B-cell depletion, but should consider assessing vaccine immune responses, including consultation with a qualified specialist, to assess whether a protective immune response was mounted.
Reduction in Immunoglobulins
As expected with any B-cell depleting therapy, decreased immunoglobulin levels are observed with OCREVUS treatment. The pooled data of OCREVUS clinical studies (RMS and PPMS) and their open-label extensions (up to approximately 7 years of exposure) have shown an association between decreased levels of immunoglobulin G (IgG<LLN) and increased rates of serious infections. Monitor the levels of quantitative serum immunoglobulins during OCREVUS treatment and after discontinuation of treatment, until B-cell repletion, and especially in the setting of recurrent serious infections. Consider discontinuing OCREVUS therapy in patients with serious opportunistic or recurrent serious infections, and if prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
Malignancies
An increased risk of malignancy with OCREVUS may exist. In controlled trials, malignancies, including breast cancer, occurred more frequently in OCREVUS-treated patients. Breast cancer occurred in 6 of 781 females treated with OCREVUS and none of 668 females treated with REBIF or placebo. Patients should follow standard breast cancer screening guidelines.
Use in Specific Populations
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy and fetal/neonatal/infant outcomes in women exposed to OCREVUS during pregnancy. Physicians are encouraged to register patients and pregnant women are encouraged to register themselves by calling 1-833-872-4370 or visiting www.ocrevuspregnancyregistry.com.
There are no adequate data on the developmental risk associated with use of OCREVUS in pregnant women. There are no data on B-cell levels in human neonates following maternal exposure to OCREVUS. However, transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 antibodies during pregnancy. OCREVUS is a humanized monoclonal antibody of an immunoglobulin G1 subtype and immunoglobulins are known to cross the placental barrier.
Lactation
There are no data on the presence of ocrelizumab in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Ocrelizumab was excreted in the milk of ocrelizumab-treated monkeys. Human IgG is excreted in human milk, and the potential for absorption of ocrelizumab to lead to B-cell depletion in the infant is unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OCREVUS and any potential adverse effects on the breastfed infant from OCREVUS or from the underlying maternal condition.
Females and Males of Reproductive Potential
Women of childbearing potential should use effective contraception while receiving OCREVUS and for 6 months after the last infusion of OCREVUS.
Most Common Adverse Reactions
RMS: The most common adverse reactions in RMS trials (incidence ≥10% and >REBIF) were upper respiratory tract infections (40%) and infusion reactions (34%).
PPMS: The most common adverse reactions in PPMS trials (incidence ≥10% and >placebo) were upper respiratory tract infections (49%), infusion reactions (40%), skin infections (14%), and lower respiratory tract infections (10%).
For additional safety information, please see the full Prescribing Information and Medication Guide.
1 Murray TJ. (2006). Diagnosis and Treatment of Multiple Sclerosis. BMJ, 322 (7540):525-527.
2 Tullman M. (2013). Overview of the Epidemiology, Diagnosis, and Disease Progression Associated With Multiple Sclerosis. The American Journal of Managed Care. 19 (2): S15-S20.
3 National Institutes of Health-National Institute of Neurological Disorders and Stroke. (2015). Multiple Sclerosis: Hope Through Research. Available at: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Multiple-Sclerosis-Hope-Through-Research.
4 National MS Society. "Who Gets MS? - African Americans." Retrieved November 2020. nationalmssociety.org/What-is-MS/Who-Gets-MS/African-American-Resources.
5 Langer-Gould A, et al. Neurology. 2013;80:1734-1739; 2. Wallin MT, et al. Brain. 2012;135:1778-1785.
6 U.S. Food and Drug Administration. “Clinical Trials Shed Light on Minority Health.” U.S. Food and Drug Administration Website. https://wayback.archive-it.org/7993/20180908114418/https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm349063.htm. Published 2018.
7 Genentech. “A New Perspective on Health Inequity.” https://www.gene.com/stories/a-new-perspective-on-health-inequity.

It’s undeniable that great strides have been made in advancing the science of multiple sclerosis (MS) over the past 20 years. Continued pursuit of research and discovery has revolutionized the understanding, diagnosis, and treatment of this debilitating condition that causes symptoms that may impact nearly every part of the body and mind. Despite these advancements, there is still more work to be done.
As the search for a cure continues, it’s imperative to ask the big questions: How does MS develop and progress? How can we reduce health disparities in care and treatment? How can we improve existing treatments and expedite the discovery of new ones? To continue making progress, we must stretch the boundaries of scientific understanding until we have answers and solutions to these and other important questions.
We know that MS is a leading cause of non-traumatic disability for young people.1,2 Yet, one of the biggest challenges for neurologists is that every person’s experience with MS is unique, making it difficult to predict what symptoms will manifest, how disruptive the symptoms and relapses will be and how a person will respond to treatment.
Twenty years ago, it was widely thought that MS was mainly driven by T cells, but in the late 1990’s, a team of researchers made a groundbreaking discovery: B cells also played a key role in MS. This discovery redefined how the scientific community thought about the underlying biology of MS and the central role B cells can play.
It was this bold thinking that led researchers at the University of California, San Francisco (UCSF) and scientists at Genentech, a member of the Roche Group, to explore whether a medication that depleted B cells would have an impact on MS. This collaboration, in turn, inspired the clinical research behind Ocrevus® (ocrelizumab), the first and only treatment approved by the FDA for both relapsing MS (RMS) and primary progressive MS (PPMS). MS is a progressive disease from the start. An important goal of treating MS is to reduce disease progression as soon as possible to slow how quickly a person’s disability changes and prevent the long-term consequences of disease progression.3 Ocrevus is administered via infusion twice yearly, with the initial dose split into two separate infusions and given two weeks apart. As with any therapy, this treatment has side effects, which can be found in the full Prescribing Information and Medication Guide. Ocrevus is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reactions to Ocrevus. The warnings and precautions for Ocrevus are infusion reactions, and infections, which include respiratory tract infections, herpes, progressive multifocal leukoencephalopathy (PML), and hepatitis B virus (HBV) reactivation. Additional warnings are possible increased risk of immunosuppressant effects with other immunosuppressants, reduction in immunoglobulins, and malignancies.
Ensuring a Continuum of Care
Another significant challenge facing the MS community is ensuring ongoing support and care for people living with the condition, especially throughout the COVID-19 pandemic. “We know that seeking early and appropriate care for MS is critical to slowing disability progression and achieving successful patient outcomes,” said Dr. Ashish Pradhan, Executive Director of Neuroimmunology at Genentech. “The pandemic has created additional barriers to early diagnosis and treatment, but we are committed to continuing to do everything we can to support patients and physicians at this stressful time.” Dr. Pradhan also observed that FDA's approval of a shorter infusion time for Ocrevus in December 2020 could potentially contribute to reducing some of the burden on the healthcare system in the long-term.
Being able to connect with others in the MS community is a vital part of navigating the uncertainties of living with the condition. This past April, to shine a light on the diverse MS community, Genentech hosted an #MSVisibility Virtual Concert that gathered people from across the world with different backgrounds and MS experiences and encouraged people living with MS to continue seeking appropriate support and care during the pandemic.
Continued support of the MS community through advocacy partnerships and grants over the years has played a sustained role in Genentech’s efforts to ensure people with MS receive necessary medical care and treatment. For example, Genentech partnered with an MS advocacy organization to fund a program that helps people get to and from their medical appointments. In addition, for more than 30 years, Genentech has helped more than two million people get the medicine they need through patient assistance programs like Genentech Access Solutions and the Genentech Patient Foundation.
of Neuroimmunology at Genentech
Advancing Inclusive Research in MS
MS has been shown to impact individuals and specific populations in markedly different ways. Those who identify as Black or of African descent and Hispanic/Latinos for example can experience more severe symptoms and faster disease progression than their Caucasian counterparts.4,5 Unfortunately, it’s not clear exactly why, largely because of their vast underrepresentation in clinical trials (less than 5% and 1%, respectively).6 While there are multiple explanations for these disparities, around one-in-three minority patients don’t participate in clinical trials due to lack of trust and 52% feel that the healthcare system is not only flawed, but is actually working against them.7
“Increased diversity in research is paramount to ensuring that the healthcare system is serving every person living with MS and ultimately reducing health inequities while providing more tailored treatment options,” commented Dr. Pradhan. “To advance the care of undertreated and underserved people with MS, we are creating and supporting programs, grants and other initiatives focused on people of color and inclusive and minority research to better understand how MS presents itself and develops in different patient populations. We want to make sure that all generations of people living with MS are equally represented in clinical research and have access to the treatments that will work best for them.”
Refining Existing Approaches and Leading Treatment Innovation
For decades, Genentech and Roche have been conducting neuroscience research and clinical trials to make forward progress in understanding and treating a variety of neurological conditions, including MS. While the treatment landscape for MS has evolved significantly over the past two decades, continued investment in research and development is critical. Continuing to review and refine existing treatment approaches may improve patient outcomes and discovering new treatment approaches may play an important role in advancing the treatment paradigm.
Dr. Pradhan added, “as the scientific community continues to think boldly, and re-imagine treatment and care for MS, we hope to continue to improve our understanding of the condition for all people with the disease, and advance treatment options. Pushing boundaries means refusing to stop until everyone living with MS can be treated effectively and a cure is found. This sentiment reminds me of a Ralph Waldo Emerson quote, ‘Don’t follow where the path may lead. Go instead where there is no path and leave a trail.’ That’s what we are trying to do at Genentech.”
To learn more, please visit Ocrevus.com.
Indications and Important Safety Information
OCREVUS is indicated for the treatment of:
- Relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
- Primary progressive MS, in adults.
Contraindications
OCREVUS is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reaction to OCREVUS.
Warnings and Precautions
Infusion Reactions
OCREVUS can cause infusion reactions, which can include pruritus, rash, urticaria, erythema, bronchospasm, throat irritation, oropharyngeal pain, dyspnea, pharyngeal or laryngeal edema, flushing, hypotension, pyrexia, fatigue, headache, dizziness, nausea, tachycardia, and anaphylaxis. In multiple sclerosis (MS) clinical trials, the incidence of infusion reactions in OCREVUS-treated patients [who received methylprednisolone (or an equivalent steroid) and possibly other pre-medication to reduce the risk of infusion reactions prior to each infusion] was 34-40%, with the highest incidence with the first infusion. There were no fatal infusion reactions, but 0.3% of OCREVUS-treated MS patients experienced infusion reactions that were serious, some requiring hospitalization.
Observe patients treated with OCREVUS for infusion reactions during the infusion and for at least one hour after completion of the infusion. Inform patients that infusion reactions can occur up to 24 hours after the infusion. Administer pre-medication (e.g., methylprednisolone or an equivalent corticosteroid, and an antihistamine) to reduce the frequency and severity of infusion reactions. The addition of an antipyretic (e.g., acetaminophen) may also be considered. For life-threatening infusion reactions, immediately and permanently stop OCREVUS and administer appropriate supportive treatment. For less severe infusion reactions, management may involve temporarily stopping the infusion, reducing the infusion rate, and/or administering symptomatic treatment.
Infections
A higher proportion of OCREVUS-treated patients experienced infections compared to patients taking REBIF or placebo. In RMS trials, 58% of OCREVUS-treated patients experienced one or more infections compared to 52% of REBIF-treated patients. In the PPMS trial, 70% of OCREVUS-treated patients experienced one or more infections compared to 68% of patients on placebo. OCREVUS increased the risk for upper respiratory tract infections, lower respiratory tract infections, skin infections, and herpes-related infections. OCREVUS was not associated with an increased risk of serious infections in MS patients. Delay OCREVUS administration in patients with an active infection until the infection is resolved.
Respiratory Tract Infections
A higher proportion of OCREVUS-treated patients experienced respiratory tract infections compared to patients taking REBIF or placebo. In RMS trials, 40% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 33% of REBIF-treated patients, and 8% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 5% of REBIF-treated patients. In the PPMS trial, 49% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 43% of patients on placebo and 10% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 9% of patients on placebo. The infections were predominantly mild to moderate and consisted mostly of upper respiratory tract infections and bronchitis.
Herpes
In active-controlled (RMS) clinical trials, herpes infections were reported more frequently in OCREVUS-treated patients than in REBIF-treated patients, including herpes zoster (2.1% vs. 1.0%), herpes simplex (0.7% vs. 0.1%), oral herpes (3.0% vs. 2.2%), genital herpes (0.1% vs. 0%), and herpes virus infection (0.1% vs. 0%). Infections were predominantly mild to moderate in severity. In the placebo-controlled (PPMS) clinical trial, oral herpes was reported more frequently in the OCREVUS-treated patients than in the patients on placebo (2.7% vs 0.8%).
Serious cases of infections caused by herpes simplex virus and varicella zoster virus, including central nervous system infections (encephalitis and meningitis), intraocular infections, and disseminated skin and soft tissue infections, have been reported in the postmarketing setting in multiple sclerosis patients receiving OCREVUS. Serious herpes virus infections may occur at any time during treatment with OCREVUS. Some cases were life-threatening.
If serious herpes infections occur, OCREVUS should be discontinued or withheld until the infection has resolved, and appropriate treatment should be administered.
Progressive Multifocal Leukoencephalopathy (PML)
PML is an opportunistic viral infection of the brain caused by the John Cunningham (JC) virus that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Although no cases of PML were identified in OCREVUS clinical trials, JC virus infection resulting in PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants). At the first sign or symptom suggestive of PML, withhold OCREVUS and perform an appropriate diagnostic evaluation. MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes (per USPI).
Hepatitis B Virus (HBV) Reactivation
Hepatitis B reactivation has been reported in MS patients treated with OCREVUS in the postmarketing setting. Fulminant hepatitis, hepatic failure, and death caused by HBV reactivation have occurred in patients treated with anti-CD20 antibodies. Perform HBV screening in all patients before initiation of treatment with OCREVUS. Do not administer OCREVUS to patients with active HBV confirmed by positive results for HBsAg and anti-HB tests. For patients who are negative for surface antigen [HBsAg] and positive for HB core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment.
Possible Increased Risk of Immunosuppressant Effects with Other Immunosuppressants
When initiating OCREVUS after an immunosuppressive therapy or initiating an immunosuppressive therapy after OCREVUS, consider the potential for increased immunosuppressive effect. OCREVUS has not been studied in combination with other MS therapies.
Vaccinations
Administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of OCREVUS for live or live-attenuated vaccines and, whenever possible, at least 2 weeks prior to initiation of OCREVUS for non-live vaccines. OCREVUS may interfere with the effectiveness of non-live vaccines. The safety of immunization with live or live-attenuated vaccines following OCREVUS therapy has not been studied, and vaccination with live-attenuated or live vaccines is not recommended during treatment and until B-cell repletion.
Vaccination of Infants Born to Mothers Treated with OCREVUS During Pregnancy
In infants of mothers exposed to OCREVUS during pregnancy, do not administer live or live-attenuated vaccines before confirming the recovery of B-cell counts as measured by CD19+ B-cells. Depletion of B-cells in these infants may increase the risks from live or live-attenuated vaccines.
You may administer non-live vaccines, as indicated, prior to recovery from B-cell depletion, but should consider assessing vaccine immune responses, including consultation with a qualified specialist, to assess whether a protective immune response was mounted.
Reduction in Immunoglobulins
As expected with any B-cell depleting therapy, decreased immunoglobulin levels are observed with OCREVUS treatment. The pooled data of OCREVUS clinical studies (RMS and PPMS) and their open-label extensions (up to approximately 7 years of exposure) have shown an association between decreased levels of immunoglobulin G (IgG<LLN) and increased rates of serious infections. Monitor the levels of quantitative serum immunoglobulins during OCREVUS treatment and after discontinuation of treatment, until B-cell repletion, and especially in the setting of recurrent serious infections. Consider discontinuing OCREVUS therapy in patients with serious opportunistic or recurrent serious infections, and if prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
Malignancies
An increased risk of malignancy with OCREVUS may exist. In controlled trials, malignancies, including breast cancer, occurred more frequently in OCREVUS-treated patients. Breast cancer occurred in 6 of 781 females treated with OCREVUS and none of 668 females treated with REBIF or placebo. Patients should follow standard breast cancer screening guidelines.
Use in Specific Populations
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy and fetal/neonatal/infant outcomes in women exposed to OCREVUS during pregnancy. Physicians are encouraged to register patients and pregnant women are encouraged to register themselves by calling 1-833-872-4370 or visiting www.ocrevuspregnancyregistry.com.
There are no adequate data on the developmental risk associated with use of OCREVUS in pregnant women. There are no data on B-cell levels in human neonates following maternal exposure to OCREVUS. However, transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 antibodies during pregnancy. OCREVUS is a humanized monoclonal antibody of an immunoglobulin G1 subtype and immunoglobulins are known to cross the placental barrier.
Lactation
There are no data on the presence of ocrelizumab in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Ocrelizumab was excreted in the milk of ocrelizumab-treated monkeys. Human IgG is excreted in human milk, and the potential for absorption of ocrelizumab to lead to B-cell depletion in the infant is unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OCREVUS and any potential adverse effects on the breastfed infant from OCREVUS or from the underlying maternal condition.
Females and Males of Reproductive Potential
Women of childbearing potential should use effective contraception while receiving OCREVUS and for 6 months after the last infusion of OCREVUS.
Most Common Adverse Reactions
RMS: The most common adverse reactions in RMS trials (incidence ≥10% and >REBIF) were upper respiratory tract infections (40%) and infusion reactions (34%).
PPMS: The most common adverse reactions in PPMS trials (incidence ≥10% and >placebo) were upper respiratory tract infections (49%), infusion reactions (40%), skin infections (14%), and lower respiratory tract infections (10%).
For additional safety information, please see the full Prescribing Information and Medication Guide.
1 Murray TJ. (2006). Diagnosis and Treatment of Multiple Sclerosis. BMJ, 322 (7540):525-527.
2 Tullman M. (2013). Overview of the Epidemiology, Diagnosis, and Disease Progression Associated With Multiple Sclerosis. The American Journal of Managed Care. 19 (2): S15-S20.
3 National Institutes of Health-National Institute of Neurological Disorders and Stroke. (2015). Multiple Sclerosis: Hope Through Research. Available at: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Multiple-Sclerosis-Hope-Through-Research.
4 National MS Society. "Who Gets MS? - African Americans." Retrieved November 2020. nationalmssociety.org/What-is-MS/Who-Gets-MS/African-American-Resources.
5 Langer-Gould A, et al. Neurology. 2013;80:1734-1739; 2. Wallin MT, et al. Brain. 2012;135:1778-1785.
6 U.S. Food and Drug Administration. “Clinical Trials Shed Light on Minority Health.” U.S. Food and Drug Administration Website. https://wayback.archive-it.org/7993/20180908114418/https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm349063.htm. Published 2018.
7 Genentech. “A New Perspective on Health Inequity.” https://www.gene.com/stories/a-new-perspective-on-health-inequity.
PT may lower risk of long-term opioid use after knee replacement
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
Clinicians may overprescribe clarithromycin for H. pylori
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Certain DMTs in MS linked to more psoriasis
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
FROM CMSC 2021