User login
Balloon-enhanced colonoscopy finds more adenomas
LAS VEGAS – The G-EYE colonoscope facilitates detection of adenomas more so than does the Endocuff Vision, researchers say.
In the first head-to-head comparison of two mechanical enhancement colonoscopy devices, “the G-EYE demonstrated a meaningful increase in adenoma detection rate [ADR] over Endocuff, particularly for advanced adenomas,” said Seth Gross, MD, a professor of medicine at New York University.
Previous studies have shown that mechanical enhancements are more effective than optical enhancements, Dr. Gross said. “To take it a step further, when you look at mechanical devices, especially these two, in past studies, the G-EYE has been sort of the leader in adenoma detection,” he told this news organization.
But until now, no studies had compared them head to head, said Dr. Gross, who presented the finding here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
The two devices work differently. The Endocuff Vision fits onto the colonoscope tip. During withdrawal, it expands radially, and its arms flatten the folds within the colon. The G-EYE balloon is deflated at insertion, then is inflated at the cecum, smoothing the colon wall while centering the colonoscopic view.
To compare the two, Dr. Gross and colleagues randomly assigned 363 patients to undergo colonoscopy with G-EYE and 364 patients to undergo colonoscopy with Endocuff Vision. The two groups were similar in demographics.
Withdrawal times were >6 minutes in both groups. The researchers detected adenomas in a higher percentage of patients with the G-EYE than with the Endocuff Vision. The same was true for advanced adenomas.
When using the G-EYE, the researchers also found more adenomas per patient, more sessile serrated adenomas per patient, more large adenomas per patient, and more right colon adenomas per patient.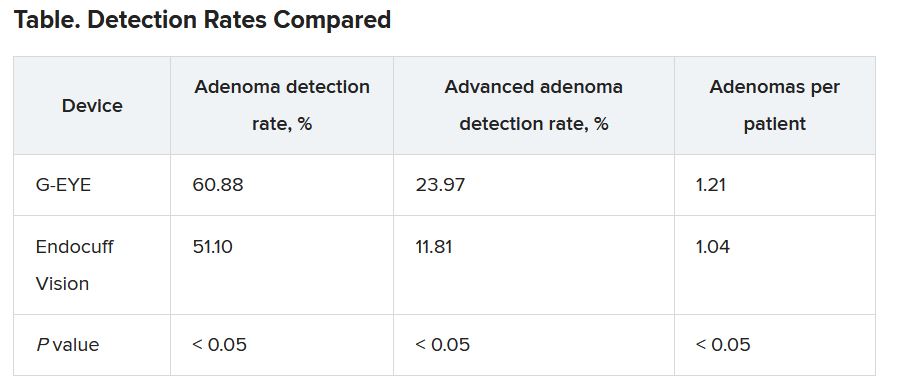
The benchmark for ADR is only 25%, Dr. Gross said, suggesting that both devices are a worthwhile improvement over standard colonoscopes. “It supports the past literature that a mechanical enhancement is something that should be considered during colonoscopy,” he said.
Costs differ as well. The G-EYE requires a permanent modification to the bending rubber of the colonoscope, so the cost is up front. The Endocuff Vision utilizes a single-use cap that is placed on the tip, so costs are spread over time.
The G-EYE gained U.S. Food and Drug Administration clearance in May 2020. In 2016, the Endocuff (an earlier version of the Endocuff Vision) became the first mechanical device the use of which the FDA acknowledged improved ADRs.
Dr. Gross said that it would be interesting to see whether the mechanical devices and artificial intelligence enhancements could complement each other so as to yield even higher detection rates.
Session moderator Brooks Cash, MD, a professor of medicine at the University of Texas Health Science Center, Houston, said the difference in detection rates made an impressive case for the G-EYE.
“I wouldn’t say I’m convinced,” Dr. Cash said in an interview. “I’d like to see more data. But I think that the plurality of the evidence that they presented and the size of the study were certainly compelling.”
He added that he’d like to see evidence that adding the balloon to a colonoscope doesn’t complicate the cleaning of the device.
Dr. Gross has a financial relationship with Olympus, the maker of the Endocuff Vision. Dr. Cash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAS VEGAS – The G-EYE colonoscope facilitates detection of adenomas more so than does the Endocuff Vision, researchers say.
In the first head-to-head comparison of two mechanical enhancement colonoscopy devices, “the G-EYE demonstrated a meaningful increase in adenoma detection rate [ADR] over Endocuff, particularly for advanced adenomas,” said Seth Gross, MD, a professor of medicine at New York University.
Previous studies have shown that mechanical enhancements are more effective than optical enhancements, Dr. Gross said. “To take it a step further, when you look at mechanical devices, especially these two, in past studies, the G-EYE has been sort of the leader in adenoma detection,” he told this news organization.
But until now, no studies had compared them head to head, said Dr. Gross, who presented the finding here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
The two devices work differently. The Endocuff Vision fits onto the colonoscope tip. During withdrawal, it expands radially, and its arms flatten the folds within the colon. The G-EYE balloon is deflated at insertion, then is inflated at the cecum, smoothing the colon wall while centering the colonoscopic view.
To compare the two, Dr. Gross and colleagues randomly assigned 363 patients to undergo colonoscopy with G-EYE and 364 patients to undergo colonoscopy with Endocuff Vision. The two groups were similar in demographics.
Withdrawal times were >6 minutes in both groups. The researchers detected adenomas in a higher percentage of patients with the G-EYE than with the Endocuff Vision. The same was true for advanced adenomas.
When using the G-EYE, the researchers also found more adenomas per patient, more sessile serrated adenomas per patient, more large adenomas per patient, and more right colon adenomas per patient.
The benchmark for ADR is only 25%, Dr. Gross said, suggesting that both devices are a worthwhile improvement over standard colonoscopes. “It supports the past literature that a mechanical enhancement is something that should be considered during colonoscopy,” he said.
Costs differ as well. The G-EYE requires a permanent modification to the bending rubber of the colonoscope, so the cost is up front. The Endocuff Vision utilizes a single-use cap that is placed on the tip, so costs are spread over time.
The G-EYE gained U.S. Food and Drug Administration clearance in May 2020. In 2016, the Endocuff (an earlier version of the Endocuff Vision) became the first mechanical device the use of which the FDA acknowledged improved ADRs.
Dr. Gross said that it would be interesting to see whether the mechanical devices and artificial intelligence enhancements could complement each other so as to yield even higher detection rates.
Session moderator Brooks Cash, MD, a professor of medicine at the University of Texas Health Science Center, Houston, said the difference in detection rates made an impressive case for the G-EYE.
“I wouldn’t say I’m convinced,” Dr. Cash said in an interview. “I’d like to see more data. But I think that the plurality of the evidence that they presented and the size of the study were certainly compelling.”
He added that he’d like to see evidence that adding the balloon to a colonoscope doesn’t complicate the cleaning of the device.
Dr. Gross has a financial relationship with Olympus, the maker of the Endocuff Vision. Dr. Cash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAS VEGAS – The G-EYE colonoscope facilitates detection of adenomas more so than does the Endocuff Vision, researchers say.
In the first head-to-head comparison of two mechanical enhancement colonoscopy devices, “the G-EYE demonstrated a meaningful increase in adenoma detection rate [ADR] over Endocuff, particularly for advanced adenomas,” said Seth Gross, MD, a professor of medicine at New York University.
Previous studies have shown that mechanical enhancements are more effective than optical enhancements, Dr. Gross said. “To take it a step further, when you look at mechanical devices, especially these two, in past studies, the G-EYE has been sort of the leader in adenoma detection,” he told this news organization.
But until now, no studies had compared them head to head, said Dr. Gross, who presented the finding here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
The two devices work differently. The Endocuff Vision fits onto the colonoscope tip. During withdrawal, it expands radially, and its arms flatten the folds within the colon. The G-EYE balloon is deflated at insertion, then is inflated at the cecum, smoothing the colon wall while centering the colonoscopic view.
To compare the two, Dr. Gross and colleagues randomly assigned 363 patients to undergo colonoscopy with G-EYE and 364 patients to undergo colonoscopy with Endocuff Vision. The two groups were similar in demographics.
Withdrawal times were >6 minutes in both groups. The researchers detected adenomas in a higher percentage of patients with the G-EYE than with the Endocuff Vision. The same was true for advanced adenomas.
When using the G-EYE, the researchers also found more adenomas per patient, more sessile serrated adenomas per patient, more large adenomas per patient, and more right colon adenomas per patient.
The benchmark for ADR is only 25%, Dr. Gross said, suggesting that both devices are a worthwhile improvement over standard colonoscopes. “It supports the past literature that a mechanical enhancement is something that should be considered during colonoscopy,” he said.
Costs differ as well. The G-EYE requires a permanent modification to the bending rubber of the colonoscope, so the cost is up front. The Endocuff Vision utilizes a single-use cap that is placed on the tip, so costs are spread over time.
The G-EYE gained U.S. Food and Drug Administration clearance in May 2020. In 2016, the Endocuff (an earlier version of the Endocuff Vision) became the first mechanical device the use of which the FDA acknowledged improved ADRs.
Dr. Gross said that it would be interesting to see whether the mechanical devices and artificial intelligence enhancements could complement each other so as to yield even higher detection rates.
Session moderator Brooks Cash, MD, a professor of medicine at the University of Texas Health Science Center, Houston, said the difference in detection rates made an impressive case for the G-EYE.
“I wouldn’t say I’m convinced,” Dr. Cash said in an interview. “I’d like to see more data. But I think that the plurality of the evidence that they presented and the size of the study were certainly compelling.”
He added that he’d like to see evidence that adding the balloon to a colonoscope doesn’t complicate the cleaning of the device.
Dr. Gross has a financial relationship with Olympus, the maker of the Endocuff Vision. Dr. Cash reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2021
‘Green’ Mediterranean diet benefits may arise from ‘hunger hormone’
A “green” adaptation to the traditional Mediterranean diet could help improve insulin sensitivity and reduce visceral fat by increasing levels of ghrelin, the “hunger hormone,” new research suggests.
The current study is a new analysis of data from the randomized DIRECT-PLUS trial, which showed that the addition of green tea and substitution of red meat for a plant-based (Mankai) protein shake at dinner – dubbed the “green Mediterranean diet” – resulted in further improved cardiometabolic benefits compared with the traditional Mediterranean diet among people with baseline abdominal obesity and/or dyslipidemia, according to the researchers.
They specifically looked at ghrelin, nicknamed the “hunger hormone,” a neuropeptide mainly secreted by the gastric epithelium. It acts on the pituitary gland to release growth hormone. Ghrelin concentrations increase during fasting and decrease after eating. Lower levels are associated with insulin resistance and obesity.
Fasting ghrelin levels were elevated with weight loss, but those increases were associated with improved insulin sensitivity and regression of visceral adipose tissue even beyond weight loss.
Although the caloric restriction and weight loss were comparable with the two Mediterranean diets, the green Mediterranean diet group had double the increase in fasting ghrelin as the traditional Mediterranean diet group, the researchers point out in their report .
‘Hypothesis-generating’ study pushes many hot topic buttons
“This specific study is the first to show that ghrelin levels play an important role in metabolic adaptation to a dietary or lifestyle intervention and that ghrelin is an important player in the axis of adiposity, insulin resistance, and metabolic health,” lead researcher Gal Tsaban, MD, told this news organization.
The data partially explain some of the prior beneficial effects seen with the Green Mediterranean diet, even after adjustment for weight loss, he explained, noting that the revised version of the diet “could be considered as an alternative lifestyle intervention with possible metabolic benefits even beyond the Mediterranean diet, which is what we currently recommend for patients.”
Asked for comment, Christopher Gardner, PhD, was not as enthusiastic.
He took issue with the fact that ghrelin wasn’t a primary or even a prespecified secondary outcome of the DIRECT-PLUS trial and because the specific plant-based ingredients of the green Mediterranean diet used in the study may not be widely available or desirable and therefore limit the study’s generalizability.
Dr. Gardner, who is director of nutrition studies at the Stanford Prevention Research Center, California, also said: “They’re tying lots of interesting things together. The Mediterranean diet is a cool thing, ghrelin is a cool thing, and insulin resistance is hugely important in this day and age, even though we don’t all agree on how to measure it.”
“But it gets tough as you try to link them all together for an exploratory outcome. ... To me it’s an interesting hypothesis-generating study that pushes a lot of interesting buttons that are hot topics in the field.”
Green Mediterranean diet led to higher ghrelin, metabolic benefits
In DIRECT-PLUS, a total of 294 adults (88% men) older than 30 years of age with abdominal obesity (waist circumference >102 cm for men or >88 cm for women), or dyslipidemia (triglycerides >150 mg/dL and HDL-cholesterol ≤40 mg/dL for men or ≤50 mg/dL for women) were included. Half had prediabetes or type 2 diabetes.
They were randomized to one of three diets: a diet based on standard healthy dietary guidelines; a traditional Mediterranean diet low in simple carbohydrates, rich in vegetables, with poultry and fish replacing beef and lamb and 28 g/day of walnuts; or the Green-Mediterranean diet, including 3-4 cups/day of green tea and 100 g/day of a green shake made from the Mankai strain of Wolffia globosa (also known as duckweed) replacing dinner, and 28 g/day of walnuts.
The Green Mediterranean diet included 800 mg more polyphenols than the traditional Mediterranean diet. Both were equally calorie-restricted, at about 1,500-1,800 kcal/day for men and 1,200-1,400 kcal/day for women. All three groups were instructed to engage in regular physical activity and were given free gym memberships.
The retention rate was 98.3% after 6 months and 89.8% after 18 months.
Weight loss was similar between the two Mediterranean diet groups (2.9% and 3.9% for the traditional and green versions, respectively) compared with the standard healthy diet (0.6%) (P < .05 for both Mediterranean diet groups vs. control).
After 6 months, fasting ghrelin increased in the traditional (8.0%; P = .015) and green (10.5%; P = 0.031) Mediterranean groups versus baseline, with no significant change in the control group.
By 18 months, fasting ghrelin was significantly greater compared with baseline only in the green Mediterranean group (P = .012).
Because the differences in fasting ghrelin trajectories were only significant in men – likely due to the small sample size of women – a subsequent 18-month analysis was limited to the men. In a multivariate model adjusted for age, intervention group, baseline biomarker values, and 18-month weight changes, the 18-month change in fasting ghrelin remained a significant predictor for changes in A1c and homeostatic model of insulin resistance (HOMA-IR; P = .022).
Because weight loss remained the most significant predictor of improved insulin resistance, a further analysis examined the association between changes in fasting ghrelin levels with changes in the fraction of insulin resistance marker that were not attributed to weight loss, per se. With the other adjustments, fasting ghrelin was associated with residual reductions in A1c (P = .003), HOMA-IR (P = .021), increased HDL-cholesterol (P = .024), and relative visceral adipose tissue loss (P = .003).
No specific product needed to push Mediterranean diet towards vegan
Dr. Tsaban, a nutritional researcher and cardiologist at Ben-Gurion University and Soroka University Medical Center, Be’er-Sheva, Israel, said the Mankai shake is commonly consumed in Israel but is also available worldwide. The study participants, all employees at an isolated nuclear research facility in the Negev, were particularly motivated. “They didn’t have a satiety problem with the drink. It made them very full,” he said. The manufacturer supplied the shakes but didn’t fund the study, he added.
However, Dr. Tsaban said that the “green Mediterranean diet” doesn’t depend on specific products.
Rather, “the concept is to push the Mediterranean diet a bit further and to replace the animal-based protein with vegetable-based protein, to shift your dietary habits towards a more vegan lifestyle. It’s not completely vegan, but it’s trending there. ... Our main goal was to increase the polyphenol intake, the antioxidant intake from vegetables. ... I think it can be replicated.”
Dr. Gardner said, “At the end of the day, it’s an exploratory study. ... It raises some interesting points that give the rest of us room to follow-up on.”
The study was funded by grants from the German Research Foundation, the Israel Ministry of Health, the Israel Ministry of Science and Technology, and the California Walnut Commission. Dr. Tsaban has reported no further relevant financial relationships. Dr. Gardner has reported receiving study funding from Beyond Meat.
A version of this article first appeared on Medscape.com.
A “green” adaptation to the traditional Mediterranean diet could help improve insulin sensitivity and reduce visceral fat by increasing levels of ghrelin, the “hunger hormone,” new research suggests.
The current study is a new analysis of data from the randomized DIRECT-PLUS trial, which showed that the addition of green tea and substitution of red meat for a plant-based (Mankai) protein shake at dinner – dubbed the “green Mediterranean diet” – resulted in further improved cardiometabolic benefits compared with the traditional Mediterranean diet among people with baseline abdominal obesity and/or dyslipidemia, according to the researchers.
They specifically looked at ghrelin, nicknamed the “hunger hormone,” a neuropeptide mainly secreted by the gastric epithelium. It acts on the pituitary gland to release growth hormone. Ghrelin concentrations increase during fasting and decrease after eating. Lower levels are associated with insulin resistance and obesity.
Fasting ghrelin levels were elevated with weight loss, but those increases were associated with improved insulin sensitivity and regression of visceral adipose tissue even beyond weight loss.
Although the caloric restriction and weight loss were comparable with the two Mediterranean diets, the green Mediterranean diet group had double the increase in fasting ghrelin as the traditional Mediterranean diet group, the researchers point out in their report .
‘Hypothesis-generating’ study pushes many hot topic buttons
“This specific study is the first to show that ghrelin levels play an important role in metabolic adaptation to a dietary or lifestyle intervention and that ghrelin is an important player in the axis of adiposity, insulin resistance, and metabolic health,” lead researcher Gal Tsaban, MD, told this news organization.
The data partially explain some of the prior beneficial effects seen with the Green Mediterranean diet, even after adjustment for weight loss, he explained, noting that the revised version of the diet “could be considered as an alternative lifestyle intervention with possible metabolic benefits even beyond the Mediterranean diet, which is what we currently recommend for patients.”
Asked for comment, Christopher Gardner, PhD, was not as enthusiastic.
He took issue with the fact that ghrelin wasn’t a primary or even a prespecified secondary outcome of the DIRECT-PLUS trial and because the specific plant-based ingredients of the green Mediterranean diet used in the study may not be widely available or desirable and therefore limit the study’s generalizability.
Dr. Gardner, who is director of nutrition studies at the Stanford Prevention Research Center, California, also said: “They’re tying lots of interesting things together. The Mediterranean diet is a cool thing, ghrelin is a cool thing, and insulin resistance is hugely important in this day and age, even though we don’t all agree on how to measure it.”
“But it gets tough as you try to link them all together for an exploratory outcome. ... To me it’s an interesting hypothesis-generating study that pushes a lot of interesting buttons that are hot topics in the field.”
Green Mediterranean diet led to higher ghrelin, metabolic benefits
In DIRECT-PLUS, a total of 294 adults (88% men) older than 30 years of age with abdominal obesity (waist circumference >102 cm for men or >88 cm for women), or dyslipidemia (triglycerides >150 mg/dL and HDL-cholesterol ≤40 mg/dL for men or ≤50 mg/dL for women) were included. Half had prediabetes or type 2 diabetes.
They were randomized to one of three diets: a diet based on standard healthy dietary guidelines; a traditional Mediterranean diet low in simple carbohydrates, rich in vegetables, with poultry and fish replacing beef and lamb and 28 g/day of walnuts; or the Green-Mediterranean diet, including 3-4 cups/day of green tea and 100 g/day of a green shake made from the Mankai strain of Wolffia globosa (also known as duckweed) replacing dinner, and 28 g/day of walnuts.
The Green Mediterranean diet included 800 mg more polyphenols than the traditional Mediterranean diet. Both were equally calorie-restricted, at about 1,500-1,800 kcal/day for men and 1,200-1,400 kcal/day for women. All three groups were instructed to engage in regular physical activity and were given free gym memberships.
The retention rate was 98.3% after 6 months and 89.8% after 18 months.
Weight loss was similar between the two Mediterranean diet groups (2.9% and 3.9% for the traditional and green versions, respectively) compared with the standard healthy diet (0.6%) (P < .05 for both Mediterranean diet groups vs. control).
After 6 months, fasting ghrelin increased in the traditional (8.0%; P = .015) and green (10.5%; P = 0.031) Mediterranean groups versus baseline, with no significant change in the control group.
By 18 months, fasting ghrelin was significantly greater compared with baseline only in the green Mediterranean group (P = .012).
Because the differences in fasting ghrelin trajectories were only significant in men – likely due to the small sample size of women – a subsequent 18-month analysis was limited to the men. In a multivariate model adjusted for age, intervention group, baseline biomarker values, and 18-month weight changes, the 18-month change in fasting ghrelin remained a significant predictor for changes in A1c and homeostatic model of insulin resistance (HOMA-IR; P = .022).
Because weight loss remained the most significant predictor of improved insulin resistance, a further analysis examined the association between changes in fasting ghrelin levels with changes in the fraction of insulin resistance marker that were not attributed to weight loss, per se. With the other adjustments, fasting ghrelin was associated with residual reductions in A1c (P = .003), HOMA-IR (P = .021), increased HDL-cholesterol (P = .024), and relative visceral adipose tissue loss (P = .003).
No specific product needed to push Mediterranean diet towards vegan
Dr. Tsaban, a nutritional researcher and cardiologist at Ben-Gurion University and Soroka University Medical Center, Be’er-Sheva, Israel, said the Mankai shake is commonly consumed in Israel but is also available worldwide. The study participants, all employees at an isolated nuclear research facility in the Negev, were particularly motivated. “They didn’t have a satiety problem with the drink. It made them very full,” he said. The manufacturer supplied the shakes but didn’t fund the study, he added.
However, Dr. Tsaban said that the “green Mediterranean diet” doesn’t depend on specific products.
Rather, “the concept is to push the Mediterranean diet a bit further and to replace the animal-based protein with vegetable-based protein, to shift your dietary habits towards a more vegan lifestyle. It’s not completely vegan, but it’s trending there. ... Our main goal was to increase the polyphenol intake, the antioxidant intake from vegetables. ... I think it can be replicated.”
Dr. Gardner said, “At the end of the day, it’s an exploratory study. ... It raises some interesting points that give the rest of us room to follow-up on.”
The study was funded by grants from the German Research Foundation, the Israel Ministry of Health, the Israel Ministry of Science and Technology, and the California Walnut Commission. Dr. Tsaban has reported no further relevant financial relationships. Dr. Gardner has reported receiving study funding from Beyond Meat.
A version of this article first appeared on Medscape.com.
A “green” adaptation to the traditional Mediterranean diet could help improve insulin sensitivity and reduce visceral fat by increasing levels of ghrelin, the “hunger hormone,” new research suggests.
The current study is a new analysis of data from the randomized DIRECT-PLUS trial, which showed that the addition of green tea and substitution of red meat for a plant-based (Mankai) protein shake at dinner – dubbed the “green Mediterranean diet” – resulted in further improved cardiometabolic benefits compared with the traditional Mediterranean diet among people with baseline abdominal obesity and/or dyslipidemia, according to the researchers.
They specifically looked at ghrelin, nicknamed the “hunger hormone,” a neuropeptide mainly secreted by the gastric epithelium. It acts on the pituitary gland to release growth hormone. Ghrelin concentrations increase during fasting and decrease after eating. Lower levels are associated with insulin resistance and obesity.
Fasting ghrelin levels were elevated with weight loss, but those increases were associated with improved insulin sensitivity and regression of visceral adipose tissue even beyond weight loss.
Although the caloric restriction and weight loss were comparable with the two Mediterranean diets, the green Mediterranean diet group had double the increase in fasting ghrelin as the traditional Mediterranean diet group, the researchers point out in their report .
‘Hypothesis-generating’ study pushes many hot topic buttons
“This specific study is the first to show that ghrelin levels play an important role in metabolic adaptation to a dietary or lifestyle intervention and that ghrelin is an important player in the axis of adiposity, insulin resistance, and metabolic health,” lead researcher Gal Tsaban, MD, told this news organization.
The data partially explain some of the prior beneficial effects seen with the Green Mediterranean diet, even after adjustment for weight loss, he explained, noting that the revised version of the diet “could be considered as an alternative lifestyle intervention with possible metabolic benefits even beyond the Mediterranean diet, which is what we currently recommend for patients.”
Asked for comment, Christopher Gardner, PhD, was not as enthusiastic.
He took issue with the fact that ghrelin wasn’t a primary or even a prespecified secondary outcome of the DIRECT-PLUS trial and because the specific plant-based ingredients of the green Mediterranean diet used in the study may not be widely available or desirable and therefore limit the study’s generalizability.
Dr. Gardner, who is director of nutrition studies at the Stanford Prevention Research Center, California, also said: “They’re tying lots of interesting things together. The Mediterranean diet is a cool thing, ghrelin is a cool thing, and insulin resistance is hugely important in this day and age, even though we don’t all agree on how to measure it.”
“But it gets tough as you try to link them all together for an exploratory outcome. ... To me it’s an interesting hypothesis-generating study that pushes a lot of interesting buttons that are hot topics in the field.”
Green Mediterranean diet led to higher ghrelin, metabolic benefits
In DIRECT-PLUS, a total of 294 adults (88% men) older than 30 years of age with abdominal obesity (waist circumference >102 cm for men or >88 cm for women), or dyslipidemia (triglycerides >150 mg/dL and HDL-cholesterol ≤40 mg/dL for men or ≤50 mg/dL for women) were included. Half had prediabetes or type 2 diabetes.
They were randomized to one of three diets: a diet based on standard healthy dietary guidelines; a traditional Mediterranean diet low in simple carbohydrates, rich in vegetables, with poultry and fish replacing beef and lamb and 28 g/day of walnuts; or the Green-Mediterranean diet, including 3-4 cups/day of green tea and 100 g/day of a green shake made from the Mankai strain of Wolffia globosa (also known as duckweed) replacing dinner, and 28 g/day of walnuts.
The Green Mediterranean diet included 800 mg more polyphenols than the traditional Mediterranean diet. Both were equally calorie-restricted, at about 1,500-1,800 kcal/day for men and 1,200-1,400 kcal/day for women. All three groups were instructed to engage in regular physical activity and were given free gym memberships.
The retention rate was 98.3% after 6 months and 89.8% after 18 months.
Weight loss was similar between the two Mediterranean diet groups (2.9% and 3.9% for the traditional and green versions, respectively) compared with the standard healthy diet (0.6%) (P < .05 for both Mediterranean diet groups vs. control).
After 6 months, fasting ghrelin increased in the traditional (8.0%; P = .015) and green (10.5%; P = 0.031) Mediterranean groups versus baseline, with no significant change in the control group.
By 18 months, fasting ghrelin was significantly greater compared with baseline only in the green Mediterranean group (P = .012).
Because the differences in fasting ghrelin trajectories were only significant in men – likely due to the small sample size of women – a subsequent 18-month analysis was limited to the men. In a multivariate model adjusted for age, intervention group, baseline biomarker values, and 18-month weight changes, the 18-month change in fasting ghrelin remained a significant predictor for changes in A1c and homeostatic model of insulin resistance (HOMA-IR; P = .022).
Because weight loss remained the most significant predictor of improved insulin resistance, a further analysis examined the association between changes in fasting ghrelin levels with changes in the fraction of insulin resistance marker that were not attributed to weight loss, per se. With the other adjustments, fasting ghrelin was associated with residual reductions in A1c (P = .003), HOMA-IR (P = .021), increased HDL-cholesterol (P = .024), and relative visceral adipose tissue loss (P = .003).
No specific product needed to push Mediterranean diet towards vegan
Dr. Tsaban, a nutritional researcher and cardiologist at Ben-Gurion University and Soroka University Medical Center, Be’er-Sheva, Israel, said the Mankai shake is commonly consumed in Israel but is also available worldwide. The study participants, all employees at an isolated nuclear research facility in the Negev, were particularly motivated. “They didn’t have a satiety problem with the drink. It made them very full,” he said. The manufacturer supplied the shakes but didn’t fund the study, he added.
However, Dr. Tsaban said that the “green Mediterranean diet” doesn’t depend on specific products.
Rather, “the concept is to push the Mediterranean diet a bit further and to replace the animal-based protein with vegetable-based protein, to shift your dietary habits towards a more vegan lifestyle. It’s not completely vegan, but it’s trending there. ... Our main goal was to increase the polyphenol intake, the antioxidant intake from vegetables. ... I think it can be replicated.”
Dr. Gardner said, “At the end of the day, it’s an exploratory study. ... It raises some interesting points that give the rest of us room to follow-up on.”
The study was funded by grants from the German Research Foundation, the Israel Ministry of Health, the Israel Ministry of Science and Technology, and the California Walnut Commission. Dr. Tsaban has reported no further relevant financial relationships. Dr. Gardner has reported receiving study funding from Beyond Meat.
A version of this article first appeared on Medscape.com.
CDC: Urgency remains to vaccinate children
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.
Some diuretics tied to increased skin cancer risk
The findings were originally reported in two Danish case-control studies in which physicians reported a fourfold increased risk of squamous cell carcinoma, and a moderate increased risk of basal cell carcinoma and cutaneous malignant melanoma in patients who used hydrochlorothiazide long-term.
And, while the new study did not find an increased risk of basal cell carcinoma and cutaneous malignant melanoma among long-term users of hydrochlorothiazide, they suggest that bendroflumethiazide “may be a safer alternative for patients at increased risk of skin cancer.” The long-term use of indapamide was associated with a moderately increased risk of cutaneous malignant melanoma but did not alter the risk of either squamous cell or basal cell carcinoma
“Our results suggest that bendroflumethiazide may be a safer alternative to hydrochlorothiazide and indapamide, especially for patients at increased risk of skin cancer, but future studies are needed to rule out a causal association between bendroflumethiazide and cutaneous malignant melanoma,” wrote authors who were led by Christoph R. Meier, PhD, a professor in pharmacy with University Hospital Basel (Switzerland) and a contributor to the Boston Collaborative Drug Surveillance Program.
This study adds to existing evidence that there is a dose-dependent increased risk of squamous cell carcinoma in users of high cumulative doses of hydrochlorothiazide, compared with non–hydrochlorothiazide users.
The study, an observational cohort study, was published earlier this year. It is based on data from the U.K.-based Clinical Practice Research Datalink. It included 271,154 new users of thiazides and thiazidelike diuretics, the majority at 87.6% having been prescribed bendroflumethiazide, 5.8% indapamide, and 3.6% hydrochlorothiazide. Outcomes were compared to those observed in 275,263 users of calcium channel blockers.
“The three primary outcomes of interest were a first-time diagnosis of cutaneous malignant melanoma, basal cell carcinoma, or squamous cell carcinoma,” the authors wrote.
Incidence rates and incidence rate ratios were estimated for both short-term and long-term users of thiazidelike diuretics and calcium channel blockers, while a propensity score (PS) analysis was done in order to control for 23 baseline covariates. The mean follow-up after PS weighting was 3.9 years for indapamide users and 5.5 years for hydrochlorothiazide users. Overall, the incidence rate ratios of squamous cell carcinoma were not markedly increased for either short-term or long-term users of thiazidelike diuretics, the authors reported.
In contrast, the incidence rate ratios of squamous cell carcinoma for hydrochlorothiazide users were increased by 29% for short-term users at an IRR of 1.29 while they were increased by almost twofold for long-term hydrochlorothiazide users at an IRR of 1.95.
Long-term use of hydrochlorothiazide was again associated with a 64% increased risk of basal cell carcinoma, compared with users of a renin-angiotensin inhibitor at a weighted IRR of 1.64.
In contrast, weighted incident rate ratios for basal cell carcinoma for both short-term and long-term thiazide users were not significantly different and results were similar for patients who took hydrochlorothiazide, indapamide, or bendroflumethiazide.
Weighted overall incident rate ratios for cutaneous malignant melanoma were not significantly different for either short-term or long-term users of thiazidelike diuretics, compared with calcium channel blocker users.
However, there was a 43% increased risk of cutaneous malignant melanoma among long-term indapamide users at a weighted IRR of 1.43, compared with calcium channel blocker users, the authors reported.
“Given the biological plausibility and the severe clinical implications of cutaneous malignant melanoma, this finding should be considered carefully,” they cautioned.
Limitations to the study include the fact that the database analyzed does not have information on sun exposure, skin characteristics, or socioeconomic status which may affect the amount of sun exposure participants received.
The authors had no conflicts of interest to declare.
The findings were originally reported in two Danish case-control studies in which physicians reported a fourfold increased risk of squamous cell carcinoma, and a moderate increased risk of basal cell carcinoma and cutaneous malignant melanoma in patients who used hydrochlorothiazide long-term.
And, while the new study did not find an increased risk of basal cell carcinoma and cutaneous malignant melanoma among long-term users of hydrochlorothiazide, they suggest that bendroflumethiazide “may be a safer alternative for patients at increased risk of skin cancer.” The long-term use of indapamide was associated with a moderately increased risk of cutaneous malignant melanoma but did not alter the risk of either squamous cell or basal cell carcinoma
“Our results suggest that bendroflumethiazide may be a safer alternative to hydrochlorothiazide and indapamide, especially for patients at increased risk of skin cancer, but future studies are needed to rule out a causal association between bendroflumethiazide and cutaneous malignant melanoma,” wrote authors who were led by Christoph R. Meier, PhD, a professor in pharmacy with University Hospital Basel (Switzerland) and a contributor to the Boston Collaborative Drug Surveillance Program.
This study adds to existing evidence that there is a dose-dependent increased risk of squamous cell carcinoma in users of high cumulative doses of hydrochlorothiazide, compared with non–hydrochlorothiazide users.
The study, an observational cohort study, was published earlier this year. It is based on data from the U.K.-based Clinical Practice Research Datalink. It included 271,154 new users of thiazides and thiazidelike diuretics, the majority at 87.6% having been prescribed bendroflumethiazide, 5.8% indapamide, and 3.6% hydrochlorothiazide. Outcomes were compared to those observed in 275,263 users of calcium channel blockers.
“The three primary outcomes of interest were a first-time diagnosis of cutaneous malignant melanoma, basal cell carcinoma, or squamous cell carcinoma,” the authors wrote.
Incidence rates and incidence rate ratios were estimated for both short-term and long-term users of thiazidelike diuretics and calcium channel blockers, while a propensity score (PS) analysis was done in order to control for 23 baseline covariates. The mean follow-up after PS weighting was 3.9 years for indapamide users and 5.5 years for hydrochlorothiazide users. Overall, the incidence rate ratios of squamous cell carcinoma were not markedly increased for either short-term or long-term users of thiazidelike diuretics, the authors reported.
In contrast, the incidence rate ratios of squamous cell carcinoma for hydrochlorothiazide users were increased by 29% for short-term users at an IRR of 1.29 while they were increased by almost twofold for long-term hydrochlorothiazide users at an IRR of 1.95.
Long-term use of hydrochlorothiazide was again associated with a 64% increased risk of basal cell carcinoma, compared with users of a renin-angiotensin inhibitor at a weighted IRR of 1.64.
In contrast, weighted incident rate ratios for basal cell carcinoma for both short-term and long-term thiazide users were not significantly different and results were similar for patients who took hydrochlorothiazide, indapamide, or bendroflumethiazide.
Weighted overall incident rate ratios for cutaneous malignant melanoma were not significantly different for either short-term or long-term users of thiazidelike diuretics, compared with calcium channel blocker users.
However, there was a 43% increased risk of cutaneous malignant melanoma among long-term indapamide users at a weighted IRR of 1.43, compared with calcium channel blocker users, the authors reported.
“Given the biological plausibility and the severe clinical implications of cutaneous malignant melanoma, this finding should be considered carefully,” they cautioned.
Limitations to the study include the fact that the database analyzed does not have information on sun exposure, skin characteristics, or socioeconomic status which may affect the amount of sun exposure participants received.
The authors had no conflicts of interest to declare.
The findings were originally reported in two Danish case-control studies in which physicians reported a fourfold increased risk of squamous cell carcinoma, and a moderate increased risk of basal cell carcinoma and cutaneous malignant melanoma in patients who used hydrochlorothiazide long-term.
And, while the new study did not find an increased risk of basal cell carcinoma and cutaneous malignant melanoma among long-term users of hydrochlorothiazide, they suggest that bendroflumethiazide “may be a safer alternative for patients at increased risk of skin cancer.” The long-term use of indapamide was associated with a moderately increased risk of cutaneous malignant melanoma but did not alter the risk of either squamous cell or basal cell carcinoma
“Our results suggest that bendroflumethiazide may be a safer alternative to hydrochlorothiazide and indapamide, especially for patients at increased risk of skin cancer, but future studies are needed to rule out a causal association between bendroflumethiazide and cutaneous malignant melanoma,” wrote authors who were led by Christoph R. Meier, PhD, a professor in pharmacy with University Hospital Basel (Switzerland) and a contributor to the Boston Collaborative Drug Surveillance Program.
This study adds to existing evidence that there is a dose-dependent increased risk of squamous cell carcinoma in users of high cumulative doses of hydrochlorothiazide, compared with non–hydrochlorothiazide users.
The study, an observational cohort study, was published earlier this year. It is based on data from the U.K.-based Clinical Practice Research Datalink. It included 271,154 new users of thiazides and thiazidelike diuretics, the majority at 87.6% having been prescribed bendroflumethiazide, 5.8% indapamide, and 3.6% hydrochlorothiazide. Outcomes were compared to those observed in 275,263 users of calcium channel blockers.
“The three primary outcomes of interest were a first-time diagnosis of cutaneous malignant melanoma, basal cell carcinoma, or squamous cell carcinoma,” the authors wrote.
Incidence rates and incidence rate ratios were estimated for both short-term and long-term users of thiazidelike diuretics and calcium channel blockers, while a propensity score (PS) analysis was done in order to control for 23 baseline covariates. The mean follow-up after PS weighting was 3.9 years for indapamide users and 5.5 years for hydrochlorothiazide users. Overall, the incidence rate ratios of squamous cell carcinoma were not markedly increased for either short-term or long-term users of thiazidelike diuretics, the authors reported.
In contrast, the incidence rate ratios of squamous cell carcinoma for hydrochlorothiazide users were increased by 29% for short-term users at an IRR of 1.29 while they were increased by almost twofold for long-term hydrochlorothiazide users at an IRR of 1.95.
Long-term use of hydrochlorothiazide was again associated with a 64% increased risk of basal cell carcinoma, compared with users of a renin-angiotensin inhibitor at a weighted IRR of 1.64.
In contrast, weighted incident rate ratios for basal cell carcinoma for both short-term and long-term thiazide users were not significantly different and results were similar for patients who took hydrochlorothiazide, indapamide, or bendroflumethiazide.
Weighted overall incident rate ratios for cutaneous malignant melanoma were not significantly different for either short-term or long-term users of thiazidelike diuretics, compared with calcium channel blocker users.
However, there was a 43% increased risk of cutaneous malignant melanoma among long-term indapamide users at a weighted IRR of 1.43, compared with calcium channel blocker users, the authors reported.
“Given the biological plausibility and the severe clinical implications of cutaneous malignant melanoma, this finding should be considered carefully,” they cautioned.
Limitations to the study include the fact that the database analyzed does not have information on sun exposure, skin characteristics, or socioeconomic status which may affect the amount of sun exposure participants received.
The authors had no conflicts of interest to declare.
FROM BRITISH JOURNAL OF DERMATOLOGY
Evaluating phantom hCG and low-level hCG elevations in the nonpregnant patient
A human chorionic gonadotropin (hCG) test is commonly ordered by gynecologists prior to surgical procedures, in the workup of bleeding abnormalities, and in the follow-up of ectopic and molar pregnancies, to name a few indications. In doing so, occasionally clinicians will find themselves in the diagnostic dilemma of discovering an inexplicable low-level elevation in hCG, such as in a postmenopausal patient. This clinical picture can be confusing and can be concerning for conditions such as postmolar gestational trophoblastic neoplasia (GTN). However, there can be benign causes of this phenomenon.1 To prevent unnecessary worry, investigation of treatments is important. In fact, misdiagnosis and inappropriate treatment of benign, low-level hCG levels with unnecessary chemotherapy is problematic mismanagement of gestational trophoblastic disease (GTD), and a major cause of litigation.
Human chorionic gonadotropin is a glycoprotein hormone with two subunits (alpha and beta). It can come from multiple sources, including trophoblastic cells, malignant trophoblastic cells, the pituitary gland, and exogenous sources.1 Its alpha-subunit is identical to that of follicle stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH). Its beta-subunit is unique, though very similar to that of LH. The free hCG beta subunit can be produced by nontrophoblastic neoplasms. The gene for the beta subunit of hCG is in close proximity to the beta subunit of LH and increases in gonadotropin-releasing hormone (GnRH) in menopause can result in the stimulation of both genes. Understanding the sources of hCG-like glycoproteins and mechanisms for testing is important when considering possible causes for falsely elevated hCG.
Most commercially available serum hCG assays detect normal intact hCG and free beta subunits. They are typically sandwich assays utilizing antibody binding sites in which a solid-phase anti-hCG antibody to a specific hCG target is then mixed with the patient’s serum, trapping or binding the hCG, which is then treated with an indicator antibody. After being washed with the indicator or “capture” antibody, its relative (quantitative) levels can be measured.1
Urine hCG testing (such as urine pregnancy tests) work through capillary action, drawing the patient’s urine across absorbent pads before reaching a pad which contains anti-hCG antibodies (the detection zone) in the test line. These tests are less sensitive than serum tests, but many can detect hCG levels <15-20 mIU/mL.1
When ob.gyns. are asked to consult on or evaluate persistently low-level elevations of hCG in nonpregnant patients they should consider both malignant and nonmalignant etiologies. Malignant causes include GTN or quiescent GTD (e.g., after treatment of a molar pregnancy or GTN), choriocarcinoma (e.g., ovarian germ cell tumors), and nonchoriocarcinoma malignancies (such as cervical, pancreatic, breast, renal). Nonmalignant causes of hCG elevations in nonpregnant patients include pituitary hCG (in postmenopausal patients), exogenous hCG, and phantom hCG.
The first step in diagnostic workup is to perform a urine pregnancy test. Provided that the serum hCG level is > 20 mIU/mL, the urine HCG should be positive unless the cause of elevated levels is “phantom hCG” from heterophilic antibodies. When patients are exposed to animal antigens (such as in vaccines) they can develop antibodies such as human anti-mouse antibody. These antibodies have affinity to the binding antibodies used in many hCG sandwich assays and form a linkage between the solid phase antibody and the detection antibody creating a false-positive result. This false-positive test is only present in serum testing but not urine tests because the patient’s heterophilic antibodies are not excreted by the kidney and thus not available to create a false-positive result. An alternative method to make the diagnosis of phantom hCG is to request that the hCG testing be run at a different lab with a different assay (which may not react with the same affinity to the patient’s anti-animal heterophile antibodies), or to request that the lab perform serial dilutions. If phantom hCG from heterophile antibodies is at play, serial dilutions will result in a nonlinear dilution response.
If the patient’s urine hCG test is positive, then pregnancy should be ruled out with a transvaginal ultrasound. If negative, an ectopic pregnancy should still be considered (unless not medically plausible, such as in postmenopausal women or women who have undergone hysterectomy). In the absence of an intrauterine or ectopic pregnancy, a positive serum and urine pregnancy test could be from exogenous hCG, from malignancy or pituitary hCG. Use of exogenous hCG can be ruled out by taking a thorough history, with particular focus on asking about weight loss medications and muscle building therapies.
If pregnancy and exogenous hCG are ruled out, clinicians should assess for an occult hCG-secreting malignancy. The lab should be asked to measure the proportion of the free beta subunit of hCG, as this is typically what is secreted by malignancies. CT imaging of the chest, abdomen, and pelvis to search for an occult primary tumor should take place. If the patient has been recently treated for molar pregnancy or GTN, and serum hCG levels reside between 100 and 300 mIU/mL, quiescent GTD should be considered the diagnosis. Determination of the proportion of hyperglycosylated hCG to total hCG can help differentiate active choriocarcinoma from quiescent GTD. After restaging imaging has been done to confirm no measurable metastatic foci, observation can follow with monthly hCG measurements. The majority of these cases will eventually resolve without intervention within a year. Quiescent GTD and persistent low-level HCG in the absence of measurable GTN on imaging or symptoms does not require treatment with chemotherapy or hysterectomy, particularly in women who desire future fertility.2
Once occult malignancy has been ruled out, the remaining potential source of hCG is the pituitary gland. As mentioned earlier, hCG shares its morphology with TSH, LH, and FSH. This can result in cross reactivity and false positives. In the menopausal state, GnRH levels increase and thus so do pituitary LH and hCG levels. To confirm that the pituitary is the source of the low-level hCG levels, the provider should prescribe a course of hormonal treatment such as an oral contraceptive pill for a 2- to 3-month period. This should result in suppression of pituitary hCG, and serum hCG levels, as part of a negative feedback loop. Pituitary source of hCG is a benign condition, and, like quiescent GTD, phantom hCG or exogenous hCG does not require intervention.
Getting to the bottom of persistent low-level hCG elevations can be challenging. By following the step-wise algorithm listed here, clinicians can sequentially test for urine hCG, heterophilic antibodies, elevated free beta-subunit, occult malignancy, and pituitary hCG.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Email her at [email protected].
References
1. Oyatogun O et al. Ther Adv Reprod Health 2021 Jun 13. doi: 10.1177/2F26334941211016412.
2. Soper JT. Obstet Gynecol. 2021 Feb 1;137(2):355-70.
A human chorionic gonadotropin (hCG) test is commonly ordered by gynecologists prior to surgical procedures, in the workup of bleeding abnormalities, and in the follow-up of ectopic and molar pregnancies, to name a few indications. In doing so, occasionally clinicians will find themselves in the diagnostic dilemma of discovering an inexplicable low-level elevation in hCG, such as in a postmenopausal patient. This clinical picture can be confusing and can be concerning for conditions such as postmolar gestational trophoblastic neoplasia (GTN). However, there can be benign causes of this phenomenon.1 To prevent unnecessary worry, investigation of treatments is important. In fact, misdiagnosis and inappropriate treatment of benign, low-level hCG levels with unnecessary chemotherapy is problematic mismanagement of gestational trophoblastic disease (GTD), and a major cause of litigation.
Human chorionic gonadotropin is a glycoprotein hormone with two subunits (alpha and beta). It can come from multiple sources, including trophoblastic cells, malignant trophoblastic cells, the pituitary gland, and exogenous sources.1 Its alpha-subunit is identical to that of follicle stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH). Its beta-subunit is unique, though very similar to that of LH. The free hCG beta subunit can be produced by nontrophoblastic neoplasms. The gene for the beta subunit of hCG is in close proximity to the beta subunit of LH and increases in gonadotropin-releasing hormone (GnRH) in menopause can result in the stimulation of both genes. Understanding the sources of hCG-like glycoproteins and mechanisms for testing is important when considering possible causes for falsely elevated hCG.
Most commercially available serum hCG assays detect normal intact hCG and free beta subunits. They are typically sandwich assays utilizing antibody binding sites in which a solid-phase anti-hCG antibody to a specific hCG target is then mixed with the patient’s serum, trapping or binding the hCG, which is then treated with an indicator antibody. After being washed with the indicator or “capture” antibody, its relative (quantitative) levels can be measured.1
Urine hCG testing (such as urine pregnancy tests) work through capillary action, drawing the patient’s urine across absorbent pads before reaching a pad which contains anti-hCG antibodies (the detection zone) in the test line. These tests are less sensitive than serum tests, but many can detect hCG levels <15-20 mIU/mL.1
When ob.gyns. are asked to consult on or evaluate persistently low-level elevations of hCG in nonpregnant patients they should consider both malignant and nonmalignant etiologies. Malignant causes include GTN or quiescent GTD (e.g., after treatment of a molar pregnancy or GTN), choriocarcinoma (e.g., ovarian germ cell tumors), and nonchoriocarcinoma malignancies (such as cervical, pancreatic, breast, renal). Nonmalignant causes of hCG elevations in nonpregnant patients include pituitary hCG (in postmenopausal patients), exogenous hCG, and phantom hCG.
The first step in diagnostic workup is to perform a urine pregnancy test. Provided that the serum hCG level is > 20 mIU/mL, the urine HCG should be positive unless the cause of elevated levels is “phantom hCG” from heterophilic antibodies. When patients are exposed to animal antigens (such as in vaccines) they can develop antibodies such as human anti-mouse antibody. These antibodies have affinity to the binding antibodies used in many hCG sandwich assays and form a linkage between the solid phase antibody and the detection antibody creating a false-positive result. This false-positive test is only present in serum testing but not urine tests because the patient’s heterophilic antibodies are not excreted by the kidney and thus not available to create a false-positive result. An alternative method to make the diagnosis of phantom hCG is to request that the hCG testing be run at a different lab with a different assay (which may not react with the same affinity to the patient’s anti-animal heterophile antibodies), or to request that the lab perform serial dilutions. If phantom hCG from heterophile antibodies is at play, serial dilutions will result in a nonlinear dilution response.
If the patient’s urine hCG test is positive, then pregnancy should be ruled out with a transvaginal ultrasound. If negative, an ectopic pregnancy should still be considered (unless not medically plausible, such as in postmenopausal women or women who have undergone hysterectomy). In the absence of an intrauterine or ectopic pregnancy, a positive serum and urine pregnancy test could be from exogenous hCG, from malignancy or pituitary hCG. Use of exogenous hCG can be ruled out by taking a thorough history, with particular focus on asking about weight loss medications and muscle building therapies.
If pregnancy and exogenous hCG are ruled out, clinicians should assess for an occult hCG-secreting malignancy. The lab should be asked to measure the proportion of the free beta subunit of hCG, as this is typically what is secreted by malignancies. CT imaging of the chest, abdomen, and pelvis to search for an occult primary tumor should take place. If the patient has been recently treated for molar pregnancy or GTN, and serum hCG levels reside between 100 and 300 mIU/mL, quiescent GTD should be considered the diagnosis. Determination of the proportion of hyperglycosylated hCG to total hCG can help differentiate active choriocarcinoma from quiescent GTD. After restaging imaging has been done to confirm no measurable metastatic foci, observation can follow with monthly hCG measurements. The majority of these cases will eventually resolve without intervention within a year. Quiescent GTD and persistent low-level HCG in the absence of measurable GTN on imaging or symptoms does not require treatment with chemotherapy or hysterectomy, particularly in women who desire future fertility.2
Once occult malignancy has been ruled out, the remaining potential source of hCG is the pituitary gland. As mentioned earlier, hCG shares its morphology with TSH, LH, and FSH. This can result in cross reactivity and false positives. In the menopausal state, GnRH levels increase and thus so do pituitary LH and hCG levels. To confirm that the pituitary is the source of the low-level hCG levels, the provider should prescribe a course of hormonal treatment such as an oral contraceptive pill for a 2- to 3-month period. This should result in suppression of pituitary hCG, and serum hCG levels, as part of a negative feedback loop. Pituitary source of hCG is a benign condition, and, like quiescent GTD, phantom hCG or exogenous hCG does not require intervention.
Getting to the bottom of persistent low-level hCG elevations can be challenging. By following the step-wise algorithm listed here, clinicians can sequentially test for urine hCG, heterophilic antibodies, elevated free beta-subunit, occult malignancy, and pituitary hCG.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Email her at [email protected].
References
1. Oyatogun O et al. Ther Adv Reprod Health 2021 Jun 13. doi: 10.1177/2F26334941211016412.
2. Soper JT. Obstet Gynecol. 2021 Feb 1;137(2):355-70.
A human chorionic gonadotropin (hCG) test is commonly ordered by gynecologists prior to surgical procedures, in the workup of bleeding abnormalities, and in the follow-up of ectopic and molar pregnancies, to name a few indications. In doing so, occasionally clinicians will find themselves in the diagnostic dilemma of discovering an inexplicable low-level elevation in hCG, such as in a postmenopausal patient. This clinical picture can be confusing and can be concerning for conditions such as postmolar gestational trophoblastic neoplasia (GTN). However, there can be benign causes of this phenomenon.1 To prevent unnecessary worry, investigation of treatments is important. In fact, misdiagnosis and inappropriate treatment of benign, low-level hCG levels with unnecessary chemotherapy is problematic mismanagement of gestational trophoblastic disease (GTD), and a major cause of litigation.
Human chorionic gonadotropin is a glycoprotein hormone with two subunits (alpha and beta). It can come from multiple sources, including trophoblastic cells, malignant trophoblastic cells, the pituitary gland, and exogenous sources.1 Its alpha-subunit is identical to that of follicle stimulating hormone (FSH), luteinizing hormone (LH), and thyroid-stimulating hormone (TSH). Its beta-subunit is unique, though very similar to that of LH. The free hCG beta subunit can be produced by nontrophoblastic neoplasms. The gene for the beta subunit of hCG is in close proximity to the beta subunit of LH and increases in gonadotropin-releasing hormone (GnRH) in menopause can result in the stimulation of both genes. Understanding the sources of hCG-like glycoproteins and mechanisms for testing is important when considering possible causes for falsely elevated hCG.
Most commercially available serum hCG assays detect normal intact hCG and free beta subunits. They are typically sandwich assays utilizing antibody binding sites in which a solid-phase anti-hCG antibody to a specific hCG target is then mixed with the patient’s serum, trapping or binding the hCG, which is then treated with an indicator antibody. After being washed with the indicator or “capture” antibody, its relative (quantitative) levels can be measured.1
Urine hCG testing (such as urine pregnancy tests) work through capillary action, drawing the patient’s urine across absorbent pads before reaching a pad which contains anti-hCG antibodies (the detection zone) in the test line. These tests are less sensitive than serum tests, but many can detect hCG levels <15-20 mIU/mL.1
When ob.gyns. are asked to consult on or evaluate persistently low-level elevations of hCG in nonpregnant patients they should consider both malignant and nonmalignant etiologies. Malignant causes include GTN or quiescent GTD (e.g., after treatment of a molar pregnancy or GTN), choriocarcinoma (e.g., ovarian germ cell tumors), and nonchoriocarcinoma malignancies (such as cervical, pancreatic, breast, renal). Nonmalignant causes of hCG elevations in nonpregnant patients include pituitary hCG (in postmenopausal patients), exogenous hCG, and phantom hCG.
The first step in diagnostic workup is to perform a urine pregnancy test. Provided that the serum hCG level is > 20 mIU/mL, the urine HCG should be positive unless the cause of elevated levels is “phantom hCG” from heterophilic antibodies. When patients are exposed to animal antigens (such as in vaccines) they can develop antibodies such as human anti-mouse antibody. These antibodies have affinity to the binding antibodies used in many hCG sandwich assays and form a linkage between the solid phase antibody and the detection antibody creating a false-positive result. This false-positive test is only present in serum testing but not urine tests because the patient’s heterophilic antibodies are not excreted by the kidney and thus not available to create a false-positive result. An alternative method to make the diagnosis of phantom hCG is to request that the hCG testing be run at a different lab with a different assay (which may not react with the same affinity to the patient’s anti-animal heterophile antibodies), or to request that the lab perform serial dilutions. If phantom hCG from heterophile antibodies is at play, serial dilutions will result in a nonlinear dilution response.
If the patient’s urine hCG test is positive, then pregnancy should be ruled out with a transvaginal ultrasound. If negative, an ectopic pregnancy should still be considered (unless not medically plausible, such as in postmenopausal women or women who have undergone hysterectomy). In the absence of an intrauterine or ectopic pregnancy, a positive serum and urine pregnancy test could be from exogenous hCG, from malignancy or pituitary hCG. Use of exogenous hCG can be ruled out by taking a thorough history, with particular focus on asking about weight loss medications and muscle building therapies.
If pregnancy and exogenous hCG are ruled out, clinicians should assess for an occult hCG-secreting malignancy. The lab should be asked to measure the proportion of the free beta subunit of hCG, as this is typically what is secreted by malignancies. CT imaging of the chest, abdomen, and pelvis to search for an occult primary tumor should take place. If the patient has been recently treated for molar pregnancy or GTN, and serum hCG levels reside between 100 and 300 mIU/mL, quiescent GTD should be considered the diagnosis. Determination of the proportion of hyperglycosylated hCG to total hCG can help differentiate active choriocarcinoma from quiescent GTD. After restaging imaging has been done to confirm no measurable metastatic foci, observation can follow with monthly hCG measurements. The majority of these cases will eventually resolve without intervention within a year. Quiescent GTD and persistent low-level HCG in the absence of measurable GTN on imaging or symptoms does not require treatment with chemotherapy or hysterectomy, particularly in women who desire future fertility.2
Once occult malignancy has been ruled out, the remaining potential source of hCG is the pituitary gland. As mentioned earlier, hCG shares its morphology with TSH, LH, and FSH. This can result in cross reactivity and false positives. In the menopausal state, GnRH levels increase and thus so do pituitary LH and hCG levels. To confirm that the pituitary is the source of the low-level hCG levels, the provider should prescribe a course of hormonal treatment such as an oral contraceptive pill for a 2- to 3-month period. This should result in suppression of pituitary hCG, and serum hCG levels, as part of a negative feedback loop. Pituitary source of hCG is a benign condition, and, like quiescent GTD, phantom hCG or exogenous hCG does not require intervention.
Getting to the bottom of persistent low-level hCG elevations can be challenging. By following the step-wise algorithm listed here, clinicians can sequentially test for urine hCG, heterophilic antibodies, elevated free beta-subunit, occult malignancy, and pituitary hCG.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest. Email her at [email protected].
References
1. Oyatogun O et al. Ther Adv Reprod Health 2021 Jun 13. doi: 10.1177/2F26334941211016412.
2. Soper JT. Obstet Gynecol. 2021 Feb 1;137(2):355-70.
Antibiotic and glucocorticoid use before cancer therapy could have detrimental effect on outcomes
“Our results confirm the detrimental impact on oncological outcomes of antibiotics and glucocorticoids at a dosage ≥10 mg/day when given within 1 month before or after ICI onset,” Marie Kostine, MD, of Bordeaux (France) University Hospital, and colleagues wrote in the European Journal of Cancer. “Moreover, we show that other comedications may significantly alter the antitumoral response of ICI, such as proton pump inhibitors, psychotropic drugs, morphine, aspirin, and insulin, whereas others seem to have no impact.”
While immune checkpoint inhibitors are transforming the treatment of advanced cancers, gut microbiota composition is an important determinant of response to ICIs. Antibiotic treatments are known to alter the gut microbiota. Other drugs, such as proton pump inhibitors, antidiabetic agents, aspirin, NSAIDs, glucocorticoids, immunomodulators, psychotropic drugs, and analgesics, have been associated with changes in microbiome composition. Since many patients with advanced cancer are exposed to such drugs, this study looked at the possible influence of these comedications on the antitumor effect and safety of ICIs.
The observational study included 635 patients with advanced cancer treated with ICIs between May 2015 and September 2017. Comedications given within 1 month before or 1 month after the first administration of an ICI were reviewed from medical records. Psychotropic drugs, proton pump inhibitors, ACE inhibitors and/or angiotensin II receptor blockers (ARBs), glucocorticoids, antibiotics, statins, and morphine were the most prescribed comedications.
Baseline use of antibiotics, glucocorticoids greater than 10 mg/day, proton pump inhibitors, psychotropic drugs, morphine, and insulin was associated with decreased overall survival and tumor response. However, the coadministration of statins, ACE inhibitors and/or ARBs, NSAIDs, aspirin, and oral diabetes drugs did not impact patient outcomes. Additionally, treatments that altered the response to ICIs were associated with a decreased incidence of immune-related adverse events.
“These results suggest some practical advice in a patient candidate to ICIs,” the authors wrote. “First, antibiotic treatment should be limited to documented infections,” and “withdrawal of proton pump inhibitors and psychotropic drugs should be considered.
“Regarding baseline glucocorticoids use, the cutoff of 10 mg/day should be respected, considering the deleterious effect of higher dosage. Moreover, because of the lack of impact of inhaled or topical glucocorticoids, local routes should be preferred,” the authors wrote. “Conversely, our study brings reassuring data regarding the use of glucocorticoids for the management of immune-related adverse events, which did not alter ICI efficacy, confirming previous reports.”
The authors noted that the observational nature of the study does not allow any causal conclusion, adding that it remains unknown whether the effect of comedications “on cancer outcomes is thoroughly mediated by changes in microbiota or other immunomodulatory properties.”
Along with the retrospective design, study limitations included reporting bias and missing data on baseline comedications, specific prognostic factors and cancer outcomes.
The authors noted no conflicts of interest.
“Our results confirm the detrimental impact on oncological outcomes of antibiotics and glucocorticoids at a dosage ≥10 mg/day when given within 1 month before or after ICI onset,” Marie Kostine, MD, of Bordeaux (France) University Hospital, and colleagues wrote in the European Journal of Cancer. “Moreover, we show that other comedications may significantly alter the antitumoral response of ICI, such as proton pump inhibitors, psychotropic drugs, morphine, aspirin, and insulin, whereas others seem to have no impact.”
While immune checkpoint inhibitors are transforming the treatment of advanced cancers, gut microbiota composition is an important determinant of response to ICIs. Antibiotic treatments are known to alter the gut microbiota. Other drugs, such as proton pump inhibitors, antidiabetic agents, aspirin, NSAIDs, glucocorticoids, immunomodulators, psychotropic drugs, and analgesics, have been associated with changes in microbiome composition. Since many patients with advanced cancer are exposed to such drugs, this study looked at the possible influence of these comedications on the antitumor effect and safety of ICIs.
The observational study included 635 patients with advanced cancer treated with ICIs between May 2015 and September 2017. Comedications given within 1 month before or 1 month after the first administration of an ICI were reviewed from medical records. Psychotropic drugs, proton pump inhibitors, ACE inhibitors and/or angiotensin II receptor blockers (ARBs), glucocorticoids, antibiotics, statins, and morphine were the most prescribed comedications.
Baseline use of antibiotics, glucocorticoids greater than 10 mg/day, proton pump inhibitors, psychotropic drugs, morphine, and insulin was associated with decreased overall survival and tumor response. However, the coadministration of statins, ACE inhibitors and/or ARBs, NSAIDs, aspirin, and oral diabetes drugs did not impact patient outcomes. Additionally, treatments that altered the response to ICIs were associated with a decreased incidence of immune-related adverse events.
“These results suggest some practical advice in a patient candidate to ICIs,” the authors wrote. “First, antibiotic treatment should be limited to documented infections,” and “withdrawal of proton pump inhibitors and psychotropic drugs should be considered.
“Regarding baseline glucocorticoids use, the cutoff of 10 mg/day should be respected, considering the deleterious effect of higher dosage. Moreover, because of the lack of impact of inhaled or topical glucocorticoids, local routes should be preferred,” the authors wrote. “Conversely, our study brings reassuring data regarding the use of glucocorticoids for the management of immune-related adverse events, which did not alter ICI efficacy, confirming previous reports.”
The authors noted that the observational nature of the study does not allow any causal conclusion, adding that it remains unknown whether the effect of comedications “on cancer outcomes is thoroughly mediated by changes in microbiota or other immunomodulatory properties.”
Along with the retrospective design, study limitations included reporting bias and missing data on baseline comedications, specific prognostic factors and cancer outcomes.
The authors noted no conflicts of interest.
“Our results confirm the detrimental impact on oncological outcomes of antibiotics and glucocorticoids at a dosage ≥10 mg/day when given within 1 month before or after ICI onset,” Marie Kostine, MD, of Bordeaux (France) University Hospital, and colleagues wrote in the European Journal of Cancer. “Moreover, we show that other comedications may significantly alter the antitumoral response of ICI, such as proton pump inhibitors, psychotropic drugs, morphine, aspirin, and insulin, whereas others seem to have no impact.”
While immune checkpoint inhibitors are transforming the treatment of advanced cancers, gut microbiota composition is an important determinant of response to ICIs. Antibiotic treatments are known to alter the gut microbiota. Other drugs, such as proton pump inhibitors, antidiabetic agents, aspirin, NSAIDs, glucocorticoids, immunomodulators, psychotropic drugs, and analgesics, have been associated with changes in microbiome composition. Since many patients with advanced cancer are exposed to such drugs, this study looked at the possible influence of these comedications on the antitumor effect and safety of ICIs.
The observational study included 635 patients with advanced cancer treated with ICIs between May 2015 and September 2017. Comedications given within 1 month before or 1 month after the first administration of an ICI were reviewed from medical records. Psychotropic drugs, proton pump inhibitors, ACE inhibitors and/or angiotensin II receptor blockers (ARBs), glucocorticoids, antibiotics, statins, and morphine were the most prescribed comedications.
Baseline use of antibiotics, glucocorticoids greater than 10 mg/day, proton pump inhibitors, psychotropic drugs, morphine, and insulin was associated with decreased overall survival and tumor response. However, the coadministration of statins, ACE inhibitors and/or ARBs, NSAIDs, aspirin, and oral diabetes drugs did not impact patient outcomes. Additionally, treatments that altered the response to ICIs were associated with a decreased incidence of immune-related adverse events.
“These results suggest some practical advice in a patient candidate to ICIs,” the authors wrote. “First, antibiotic treatment should be limited to documented infections,” and “withdrawal of proton pump inhibitors and psychotropic drugs should be considered.
“Regarding baseline glucocorticoids use, the cutoff of 10 mg/day should be respected, considering the deleterious effect of higher dosage. Moreover, because of the lack of impact of inhaled or topical glucocorticoids, local routes should be preferred,” the authors wrote. “Conversely, our study brings reassuring data regarding the use of glucocorticoids for the management of immune-related adverse events, which did not alter ICI efficacy, confirming previous reports.”
The authors noted that the observational nature of the study does not allow any causal conclusion, adding that it remains unknown whether the effect of comedications “on cancer outcomes is thoroughly mediated by changes in microbiota or other immunomodulatory properties.”
Along with the retrospective design, study limitations included reporting bias and missing data on baseline comedications, specific prognostic factors and cancer outcomes.
The authors noted no conflicts of interest.
FROM THE EUROPEAN JOURNAL OF CANCER
Stress, depression during pregnancy can harm child
New evidence points to the importance of helping mothers with their mental health during pregnancy.
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
New evidence points to the importance of helping mothers with their mental health during pregnancy.
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
New evidence points to the importance of helping mothers with their mental health during pregnancy.
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
Uncertainty looms large in treatment options for high-risk cutaneous SCC
say the authors of a clinical review recently published in the journal Head and Neck.
The review, led by Jason G. Newman, MD, director of head and neck surgery at Penn Medicine, Philadelphia, includes evidence-based research findings from the last 10 years which describe the possible roles for adjuvant radiation, chemotherapy, immunotherapy, and/or targeted therapy in the management of high-risk cSCC.
Dr. Newman and colleagues wrote that more data – and high-quality data – are needed for physicians to determine with more confidence which adjuvant therapies would be best for specific patients with high-risk cSCC. But without that data, uncertainty in treatment decisions will persist.
“The requirements for and efficacy of adjuvant therapies in cutaneous squamous cell carcinoma are unclear, and the gap in evidence for practice decisions regarding adjuvant therapy in patients with high-risk cSCC has been apparent for more than a decade,” they wrote.
While surgical excision with clear margins of the primary cSCC lesion remains the standard of care, certain high-risk factors necessitate adjuvant therapy, the authors wrote. However, since the evidence consists of small retrospective studies with conflicting results, it is unclear which patients might benefit from adjuvant therapy. This review included recent and current trials in cutaneous SCC and the role of immune checkpoint inhibitors.
According to the review, adjuvant radiation therapy is usually considered with high-risk features, such as perineural invasion, lymph node metastasis and extracapsular/extranodal extension, if the patient is otherwise at high risk for metastasis or recurrence, or if further surgery is not possible.
The National Comprehensive Cancer Network, the American College of Radiology, and the American Society for Radiation Oncology do not recommend adjuvant radiation therapy for most patients with cSCC. However, adjuvant radiation therapy with or without systemic therapy may be considered in locally advanced disease, when further surgery is not an option, or if there is regional lymph node metastasis, but multidisciplinary consultation is recommended.
Regarding checkpoint inhibitors, the NCCN, ACR, and ASTRO do not recommend the use of systemic therapy for local disease amenable to surgery. Potential use of a checkpoint inhibitor with radiation therapy in a clinical trial is recommended for residual disease in locally advanced cSCC as palliative care when other options are not available. While the use of cemiplimab or pembrolizumab are preferred in regional recurrence when curative surgery and radiation therapy are not an option, a targeted therapy can be considered if this is not feasible.
“Given the current activity of checkpoint inhibition in this disease, enthusiasm for the addition of cytotoxic chemotherapeutic agents in the adjuvant setting may be on the decline,” the authors wrote. “Multidisciplinary approaches will most likely continue to be recommended in complicated cases, including those involving immunosuppression.”
The authors said that further study is needed on prognostic testing, such as gene expression profile testing or sentinel lymph node biopsy, as such testing early in disease could identify patients who would likely benefit from adjuvant therapy. They added that the need to identify patients at early stages of disease who are at high risk for metastasis continues to remain critical.
say the authors of a clinical review recently published in the journal Head and Neck.
The review, led by Jason G. Newman, MD, director of head and neck surgery at Penn Medicine, Philadelphia, includes evidence-based research findings from the last 10 years which describe the possible roles for adjuvant radiation, chemotherapy, immunotherapy, and/or targeted therapy in the management of high-risk cSCC.
Dr. Newman and colleagues wrote that more data – and high-quality data – are needed for physicians to determine with more confidence which adjuvant therapies would be best for specific patients with high-risk cSCC. But without that data, uncertainty in treatment decisions will persist.
“The requirements for and efficacy of adjuvant therapies in cutaneous squamous cell carcinoma are unclear, and the gap in evidence for practice decisions regarding adjuvant therapy in patients with high-risk cSCC has been apparent for more than a decade,” they wrote.
While surgical excision with clear margins of the primary cSCC lesion remains the standard of care, certain high-risk factors necessitate adjuvant therapy, the authors wrote. However, since the evidence consists of small retrospective studies with conflicting results, it is unclear which patients might benefit from adjuvant therapy. This review included recent and current trials in cutaneous SCC and the role of immune checkpoint inhibitors.
According to the review, adjuvant radiation therapy is usually considered with high-risk features, such as perineural invasion, lymph node metastasis and extracapsular/extranodal extension, if the patient is otherwise at high risk for metastasis or recurrence, or if further surgery is not possible.
The National Comprehensive Cancer Network, the American College of Radiology, and the American Society for Radiation Oncology do not recommend adjuvant radiation therapy for most patients with cSCC. However, adjuvant radiation therapy with or without systemic therapy may be considered in locally advanced disease, when further surgery is not an option, or if there is regional lymph node metastasis, but multidisciplinary consultation is recommended.
Regarding checkpoint inhibitors, the NCCN, ACR, and ASTRO do not recommend the use of systemic therapy for local disease amenable to surgery. Potential use of a checkpoint inhibitor with radiation therapy in a clinical trial is recommended for residual disease in locally advanced cSCC as palliative care when other options are not available. While the use of cemiplimab or pembrolizumab are preferred in regional recurrence when curative surgery and radiation therapy are not an option, a targeted therapy can be considered if this is not feasible.
“Given the current activity of checkpoint inhibition in this disease, enthusiasm for the addition of cytotoxic chemotherapeutic agents in the adjuvant setting may be on the decline,” the authors wrote. “Multidisciplinary approaches will most likely continue to be recommended in complicated cases, including those involving immunosuppression.”
The authors said that further study is needed on prognostic testing, such as gene expression profile testing or sentinel lymph node biopsy, as such testing early in disease could identify patients who would likely benefit from adjuvant therapy. They added that the need to identify patients at early stages of disease who are at high risk for metastasis continues to remain critical.
say the authors of a clinical review recently published in the journal Head and Neck.
The review, led by Jason G. Newman, MD, director of head and neck surgery at Penn Medicine, Philadelphia, includes evidence-based research findings from the last 10 years which describe the possible roles for adjuvant radiation, chemotherapy, immunotherapy, and/or targeted therapy in the management of high-risk cSCC.
Dr. Newman and colleagues wrote that more data – and high-quality data – are needed for physicians to determine with more confidence which adjuvant therapies would be best for specific patients with high-risk cSCC. But without that data, uncertainty in treatment decisions will persist.
“The requirements for and efficacy of adjuvant therapies in cutaneous squamous cell carcinoma are unclear, and the gap in evidence for practice decisions regarding adjuvant therapy in patients with high-risk cSCC has been apparent for more than a decade,” they wrote.
While surgical excision with clear margins of the primary cSCC lesion remains the standard of care, certain high-risk factors necessitate adjuvant therapy, the authors wrote. However, since the evidence consists of small retrospective studies with conflicting results, it is unclear which patients might benefit from adjuvant therapy. This review included recent and current trials in cutaneous SCC and the role of immune checkpoint inhibitors.
According to the review, adjuvant radiation therapy is usually considered with high-risk features, such as perineural invasion, lymph node metastasis and extracapsular/extranodal extension, if the patient is otherwise at high risk for metastasis or recurrence, or if further surgery is not possible.
The National Comprehensive Cancer Network, the American College of Radiology, and the American Society for Radiation Oncology do not recommend adjuvant radiation therapy for most patients with cSCC. However, adjuvant radiation therapy with or without systemic therapy may be considered in locally advanced disease, when further surgery is not an option, or if there is regional lymph node metastasis, but multidisciplinary consultation is recommended.
Regarding checkpoint inhibitors, the NCCN, ACR, and ASTRO do not recommend the use of systemic therapy for local disease amenable to surgery. Potential use of a checkpoint inhibitor with radiation therapy in a clinical trial is recommended for residual disease in locally advanced cSCC as palliative care when other options are not available. While the use of cemiplimab or pembrolizumab are preferred in regional recurrence when curative surgery and radiation therapy are not an option, a targeted therapy can be considered if this is not feasible.
“Given the current activity of checkpoint inhibition in this disease, enthusiasm for the addition of cytotoxic chemotherapeutic agents in the adjuvant setting may be on the decline,” the authors wrote. “Multidisciplinary approaches will most likely continue to be recommended in complicated cases, including those involving immunosuppression.”
The authors said that further study is needed on prognostic testing, such as gene expression profile testing or sentinel lymph node biopsy, as such testing early in disease could identify patients who would likely benefit from adjuvant therapy. They added that the need to identify patients at early stages of disease who are at high risk for metastasis continues to remain critical.
FROM HEAD AND NECK
Warn patients about illicit drugs doctored with fentanyl
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
FROM PSYCHOPHARMACOLOGY UPDATE
Chatbots can improve mental health in vulnerable populations
In this modern age of health care where telemedicine rules, conversational agents (CAs) that use text messaging systems are becoming a major mode of communication.
Many people are familiar with voice-enabled agents, such as Apple’s Siri, Google Now, and Microsoft’s Cortana. However, CAs come in different forms of complexity, ranging from a short message service–based texting platform to an embodied conversational agent (ECA).
ECAs allow participants to interact with a physical or graphical figure that simulates a person in appearance, behavior, and dialect. These are essentially virtual humans, or avatars, who talk with participants. By taking greater advantage of these automated agents, some have projected there may be $11 billion in combined cost savings across a variety of business sectors by 2023.1 The health care field is one sector in which CAs can play an important role. Because of their accessibility, CAs have the potential to improve mental health by combating health care inequities and stigma, encouraging disclosure from participants, and serving as companions during the COVID-19 pandemic.
CAs provide accessible health care for rural, low socioeconomic status (SES), and minority communities in a variety of advantageous ways. For example, one study found that long-term use of a text-based agent that combines motivational interviewing and cognitive-behavioral therapy (CBT) can support smoking cessation in adolescents of low SES.2
CAs can help vulnerable participants advocate for themselves and proactively maintain their mental health through access to health care resources. In specific cases, these agents equalize health care treatment for different populations. Even though some participants live in secluded areas or are blocked by barriers, these text-based agents can still provide self-help intervention for them at any time on an individual basis, regardless of their location or socioeconomic status. Furthermore, they serve as highly cost-effective mental health promotion tools for large populations, some of which might not otherwise be reached by mental health care.
In combating mental illnesses such as depression and anxiety, studies have found that CAs are great treatment tools. For example, participants in an experimental group who received a self-help program based on CBT from a text-based CA named Woebot experienced significantly reduced depression symptoms when compared to the control group of participants, who received only information from a self-help electronic book.3 As a result, CAs might prove successful in treating younger populations who find online tools more feasible and accessible. Often, this population self-identifies depressive and anxiety symptoms without consulting a health care professional. Thus, this tool would prove useful to those who are bothered by the stigma of seeing a mental health professional.
Virtual human–based CAs also encourage participants to disclose more information in a nonjudgmental manner, especially among people with diseases with stigma. CAs use neutral languages, which may be helpful when dealing with stigmatized issues such as HIV, family planning, and abortion care because this heightens confidentiality and privacy. When participants believe that the agent does not “judge” or evaluate their capabilities, this elicits more sensitive information from them. For example, one study found that military service members who believed that they were interacting with a computer rather than a human operator reported lower fear of self-disclosure, displayed more sadness, and were rated by observers as more willing to disclose posttraumatic stress disorder symptoms.4 Additional findings show that participants prefer CAs when topics are highly sensitive and more likely to evoke negative self-admissions.
In what we hope will soon be a post–COVID-19 landscape of medicine, CAs are fast being used on the front lines of health care technology. Empathetic CAs can combat adverse effects of social exclusion during these pressing times. Etsuko Ishii, a researcher affiliated with the Hong Kong University of Science and Technology, and associates demonstrated that a virtual CA was as effective as a COVID-19 companion because it uses natural language processing (NLP) and nonverbal facial expressions to give users the feeling that they are being treated with empathy.5 While minimizing the number of in-person interactions that could potentially spread COVID-19, these agents promote virtual companionship that mirrors natural conversations and provide emotional support with psychological safety as participants express their pent-up thoughts. Not only do these agents help recover mood quickly, but they also have the power to overcome geographic barriers, be constantly available, and alleviate the high demand for mental health care. As a result, CAs have the potential to facilitate better communication and sustain social interactions within the isolated environment the pandemic has created.
CAs can predict, detect, and determine treatment solutions for mental health conditions based on behavioral insights. These agents’ natural language processing also allows them to be powerful therapeutic agents that can serve different communities, particularly for populations with limited access to medical resources. As the use of CAs becomes more integrated into telemedicine, their utility will continue to grow as their proven versatility in many situations expands the boundaries of health care technology.
Ms. Wong, a medical student at New York Institute of Technology College of Osteopathic Medicine in Old Westbury, conducts research related to mental health care services. She disclosed writing a telemental health software platform called Orchid. Dr. Vo, a board-certified psychiatrist, is the medical director of telehealth for the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia. She is a faculty member of the University of Pennsylvania, also in Philadelphia. Dr. Vo conducts digital health research focused on using automation and artificial intelligence for suicide risk screening and connecting patients to mental health care services. She disclosed serving as cofounder of Orchid.
References
1. Chatbots: Vendor opportunities & market forecasts 2020-2024. Juniper Research, 2020.
2. Simon P et al. On using chatbots to promote smoking cessation among adolescents of low socioeconomic status, Artificial Intelligence and Work: Association for the Advancement of Artificial Intelligence (AAAI) 2019 Fall Symposium, 2019.
3. Fitzpatrick KK et al. JMIR Mental Health. 2017;4(2):e19.
4. Lucas GM et al. Front Robot AI. 2017 Oct 12. doi: 10.3389/frobt.2017.00051.
5. Ishii E et al. ERICA: An empathetic android companion for COVID-19 quarantine. arXiv preprint arXiv:2106.02325.
In this modern age of health care where telemedicine rules, conversational agents (CAs) that use text messaging systems are becoming a major mode of communication.
Many people are familiar with voice-enabled agents, such as Apple’s Siri, Google Now, and Microsoft’s Cortana. However, CAs come in different forms of complexity, ranging from a short message service–based texting platform to an embodied conversational agent (ECA).
ECAs allow participants to interact with a physical or graphical figure that simulates a person in appearance, behavior, and dialect. These are essentially virtual humans, or avatars, who talk with participants. By taking greater advantage of these automated agents, some have projected there may be $11 billion in combined cost savings across a variety of business sectors by 2023.1 The health care field is one sector in which CAs can play an important role. Because of their accessibility, CAs have the potential to improve mental health by combating health care inequities and stigma, encouraging disclosure from participants, and serving as companions during the COVID-19 pandemic.
CAs provide accessible health care for rural, low socioeconomic status (SES), and minority communities in a variety of advantageous ways. For example, one study found that long-term use of a text-based agent that combines motivational interviewing and cognitive-behavioral therapy (CBT) can support smoking cessation in adolescents of low SES.2
CAs can help vulnerable participants advocate for themselves and proactively maintain their mental health through access to health care resources. In specific cases, these agents equalize health care treatment for different populations. Even though some participants live in secluded areas or are blocked by barriers, these text-based agents can still provide self-help intervention for them at any time on an individual basis, regardless of their location or socioeconomic status. Furthermore, they serve as highly cost-effective mental health promotion tools for large populations, some of which might not otherwise be reached by mental health care.
In combating mental illnesses such as depression and anxiety, studies have found that CAs are great treatment tools. For example, participants in an experimental group who received a self-help program based on CBT from a text-based CA named Woebot experienced significantly reduced depression symptoms when compared to the control group of participants, who received only information from a self-help electronic book.3 As a result, CAs might prove successful in treating younger populations who find online tools more feasible and accessible. Often, this population self-identifies depressive and anxiety symptoms without consulting a health care professional. Thus, this tool would prove useful to those who are bothered by the stigma of seeing a mental health professional.
Virtual human–based CAs also encourage participants to disclose more information in a nonjudgmental manner, especially among people with diseases with stigma. CAs use neutral languages, which may be helpful when dealing with stigmatized issues such as HIV, family planning, and abortion care because this heightens confidentiality and privacy. When participants believe that the agent does not “judge” or evaluate their capabilities, this elicits more sensitive information from them. For example, one study found that military service members who believed that they were interacting with a computer rather than a human operator reported lower fear of self-disclosure, displayed more sadness, and were rated by observers as more willing to disclose posttraumatic stress disorder symptoms.4 Additional findings show that participants prefer CAs when topics are highly sensitive and more likely to evoke negative self-admissions.
In what we hope will soon be a post–COVID-19 landscape of medicine, CAs are fast being used on the front lines of health care technology. Empathetic CAs can combat adverse effects of social exclusion during these pressing times. Etsuko Ishii, a researcher affiliated with the Hong Kong University of Science and Technology, and associates demonstrated that a virtual CA was as effective as a COVID-19 companion because it uses natural language processing (NLP) and nonverbal facial expressions to give users the feeling that they are being treated with empathy.5 While minimizing the number of in-person interactions that could potentially spread COVID-19, these agents promote virtual companionship that mirrors natural conversations and provide emotional support with psychological safety as participants express their pent-up thoughts. Not only do these agents help recover mood quickly, but they also have the power to overcome geographic barriers, be constantly available, and alleviate the high demand for mental health care. As a result, CAs have the potential to facilitate better communication and sustain social interactions within the isolated environment the pandemic has created.
CAs can predict, detect, and determine treatment solutions for mental health conditions based on behavioral insights. These agents’ natural language processing also allows them to be powerful therapeutic agents that can serve different communities, particularly for populations with limited access to medical resources. As the use of CAs becomes more integrated into telemedicine, their utility will continue to grow as their proven versatility in many situations expands the boundaries of health care technology.
Ms. Wong, a medical student at New York Institute of Technology College of Osteopathic Medicine in Old Westbury, conducts research related to mental health care services. She disclosed writing a telemental health software platform called Orchid. Dr. Vo, a board-certified psychiatrist, is the medical director of telehealth for the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia. She is a faculty member of the University of Pennsylvania, also in Philadelphia. Dr. Vo conducts digital health research focused on using automation and artificial intelligence for suicide risk screening and connecting patients to mental health care services. She disclosed serving as cofounder of Orchid.
References
1. Chatbots: Vendor opportunities & market forecasts 2020-2024. Juniper Research, 2020.
2. Simon P et al. On using chatbots to promote smoking cessation among adolescents of low socioeconomic status, Artificial Intelligence and Work: Association for the Advancement of Artificial Intelligence (AAAI) 2019 Fall Symposium, 2019.
3. Fitzpatrick KK et al. JMIR Mental Health. 2017;4(2):e19.
4. Lucas GM et al. Front Robot AI. 2017 Oct 12. doi: 10.3389/frobt.2017.00051.
5. Ishii E et al. ERICA: An empathetic android companion for COVID-19 quarantine. arXiv preprint arXiv:2106.02325.
In this modern age of health care where telemedicine rules, conversational agents (CAs) that use text messaging systems are becoming a major mode of communication.
Many people are familiar with voice-enabled agents, such as Apple’s Siri, Google Now, and Microsoft’s Cortana. However, CAs come in different forms of complexity, ranging from a short message service–based texting platform to an embodied conversational agent (ECA).
ECAs allow participants to interact with a physical or graphical figure that simulates a person in appearance, behavior, and dialect. These are essentially virtual humans, or avatars, who talk with participants. By taking greater advantage of these automated agents, some have projected there may be $11 billion in combined cost savings across a variety of business sectors by 2023.1 The health care field is one sector in which CAs can play an important role. Because of their accessibility, CAs have the potential to improve mental health by combating health care inequities and stigma, encouraging disclosure from participants, and serving as companions during the COVID-19 pandemic.
CAs provide accessible health care for rural, low socioeconomic status (SES), and minority communities in a variety of advantageous ways. For example, one study found that long-term use of a text-based agent that combines motivational interviewing and cognitive-behavioral therapy (CBT) can support smoking cessation in adolescents of low SES.2
CAs can help vulnerable participants advocate for themselves and proactively maintain their mental health through access to health care resources. In specific cases, these agents equalize health care treatment for different populations. Even though some participants live in secluded areas or are blocked by barriers, these text-based agents can still provide self-help intervention for them at any time on an individual basis, regardless of their location or socioeconomic status. Furthermore, they serve as highly cost-effective mental health promotion tools for large populations, some of which might not otherwise be reached by mental health care.
In combating mental illnesses such as depression and anxiety, studies have found that CAs are great treatment tools. For example, participants in an experimental group who received a self-help program based on CBT from a text-based CA named Woebot experienced significantly reduced depression symptoms when compared to the control group of participants, who received only information from a self-help electronic book.3 As a result, CAs might prove successful in treating younger populations who find online tools more feasible and accessible. Often, this population self-identifies depressive and anxiety symptoms without consulting a health care professional. Thus, this tool would prove useful to those who are bothered by the stigma of seeing a mental health professional.
Virtual human–based CAs also encourage participants to disclose more information in a nonjudgmental manner, especially among people with diseases with stigma. CAs use neutral languages, which may be helpful when dealing with stigmatized issues such as HIV, family planning, and abortion care because this heightens confidentiality and privacy. When participants believe that the agent does not “judge” or evaluate their capabilities, this elicits more sensitive information from them. For example, one study found that military service members who believed that they were interacting with a computer rather than a human operator reported lower fear of self-disclosure, displayed more sadness, and were rated by observers as more willing to disclose posttraumatic stress disorder symptoms.4 Additional findings show that participants prefer CAs when topics are highly sensitive and more likely to evoke negative self-admissions.
In what we hope will soon be a post–COVID-19 landscape of medicine, CAs are fast being used on the front lines of health care technology. Empathetic CAs can combat adverse effects of social exclusion during these pressing times. Etsuko Ishii, a researcher affiliated with the Hong Kong University of Science and Technology, and associates demonstrated that a virtual CA was as effective as a COVID-19 companion because it uses natural language processing (NLP) and nonverbal facial expressions to give users the feeling that they are being treated with empathy.5 While minimizing the number of in-person interactions that could potentially spread COVID-19, these agents promote virtual companionship that mirrors natural conversations and provide emotional support with psychological safety as participants express their pent-up thoughts. Not only do these agents help recover mood quickly, but they also have the power to overcome geographic barriers, be constantly available, and alleviate the high demand for mental health care. As a result, CAs have the potential to facilitate better communication and sustain social interactions within the isolated environment the pandemic has created.
CAs can predict, detect, and determine treatment solutions for mental health conditions based on behavioral insights. These agents’ natural language processing also allows them to be powerful therapeutic agents that can serve different communities, particularly for populations with limited access to medical resources. As the use of CAs becomes more integrated into telemedicine, their utility will continue to grow as their proven versatility in many situations expands the boundaries of health care technology.
Ms. Wong, a medical student at New York Institute of Technology College of Osteopathic Medicine in Old Westbury, conducts research related to mental health care services. She disclosed writing a telemental health software platform called Orchid. Dr. Vo, a board-certified psychiatrist, is the medical director of telehealth for the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia. She is a faculty member of the University of Pennsylvania, also in Philadelphia. Dr. Vo conducts digital health research focused on using automation and artificial intelligence for suicide risk screening and connecting patients to mental health care services. She disclosed serving as cofounder of Orchid.
References
1. Chatbots: Vendor opportunities & market forecasts 2020-2024. Juniper Research, 2020.
2. Simon P et al. On using chatbots to promote smoking cessation among adolescents of low socioeconomic status, Artificial Intelligence and Work: Association for the Advancement of Artificial Intelligence (AAAI) 2019 Fall Symposium, 2019.
3. Fitzpatrick KK et al. JMIR Mental Health. 2017;4(2):e19.
4. Lucas GM et al. Front Robot AI. 2017 Oct 12. doi: 10.3389/frobt.2017.00051.
5. Ishii E et al. ERICA: An empathetic android companion for COVID-19 quarantine. arXiv preprint arXiv:2106.02325.





