User login
Tracking adenomas per colonoscopy shows promise as quality measure
The number of adenomas per colonoscopy (APC) is inversely correlated with postcolonoscopy colorectal cancer (PCCRC), which supports use of APC as a new quality control measure, according to investigators.
Data from 138 endoscopists showed that patients screened by physicians with higher APCs had significantly lower rates of PCCRC, and an APC of 0.6 offered more protection than either an APC of 0.4 or an adenoma detection rate (ADR) of 25%, reported lead author Joseph C. Anderson, MD, of White River Junction VA Medical Center, Hanover, N.H., and colleagues.
“Unfortunately, APC has never been validated as a quality measure by demonstrating a reduction in PCCRC in exams performed by endoscopists with higher rates,” Dr. Anderson said at the annual meeting of the American College of Gastroenterology.
To this end, Dr. Anderson and colleagues reviewed data from the New Hampshire Colonoscopy Registry (NHCR), including 9,023 screening colonoscopies with a follow-up event 6-60 months after the initial exam. Procedures were conducted by 138 endoscopists in New Hampshire, Vermont, Massachusetts, and Maine.
Three quality measures were analyzed for associations with PCCRC: an APC of 0.4, an APC of 0.6, and an ADR of 25%. Hazard ratios were calculated for all PCCRCs, as well as PCCRCs diagnosed at first follow-up event. Rates were reported for two time periods: 6-36 months and 6-60 months.
From 6 to 60 months, 82 cases of PCCRC were diagnosed, among which 50 were diagnosed between 6 and 36 months.
For both periods, all three quality measures were significantly associated with reductions in PCCRC. The higher APC of 0.6, however, offered greater protection, reducing all PCCRCs by 71% and 61% in the shorter and longer period, respectively. In comparison, the lower APC of 0.4 reduced rates by 63% and 53%, while the ADR benchmark reduced rates by 62% and 42%.
These trends were maintained for PCCRCs diagnosed at first follow-up event. An APC of 0.6 was associated with respective reductions of 79% and 65% for the shorter and longer period, compared with 64% and 57% for the lower APC, and 67% and 49% for ADR.
Additional analysis clarified the relationship between APC level and likelihood of developing PCCRC. In terms of absolute risk, patients screened by an endoscopist with an APC greater than 0.6 had a 0.5% chance of developing PCCRC from 6 to 36 months, compared with 0.7% for an APC of 0.4-0.6, and 2.1% for an APC of less than 0.4 (P = .0001). This pattern held through 60 months, during which time an APC greater than 0.6 was associated with an absolute risk of PCCRC of 0.4%, compared with 0.7% for an APC of 0.4-0.6, and 1.6% for an APC less than 0.4 (P = .0001).
“Our novel data support the use of APC as a quality measure by demonstrating a reduction in PCCRC risk in exams performed by endoscopists with higher APCs,” Dr. Anderson concluded, noting that an APC of 0.6 appeared to offer more protection than an APC of 0.4. “I feel that ... APC as a quality measure, now that we’ve validated it, may be accepted because of its ability to differentiate endoscopists on their adenoma detection skills.”
According to Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., “It’s an important study that will probably contribute to where we’re going forward.”
Dr. Lawrence, chair of the division and medical director of the endoscopy units at Kingston General and Hotel Dieu hospitals, said that APC may overcome the main concern with ADR – that endoscopists who find one adenoma may not be motivated to seek out as many as possible.
“The problem with ADR, in general, is that if you find one polyp, and if ADR is the stat you’re living by, then you don’t need to find any other polyps, and that obviously doesn’t do that patient a favor, necessarily,” Dr. Hookey said in an interview. “It does bring them back sooner for surveillance, but it doesn’t help remove the rest of the polyps that they have. And not that someone is going to find one polyp and turn off the light and pull the scope out, but you may not be looking as hard.”
APC mitigates this issue, he explained, because it determines “whether or not you’re truly clearing things out and getting rid of as many [polyps] as possible.”
Dr. Hookey said that APC is “probably the best” quality control measure on the horizon, and he suggested that more work is needed to determine the optimal benchmark figure, which should ideally be investigated through larger studies.
“I just want to see it in bigger groups,” he said.
The investigators and Dr. Hookey reported no conflicts of interest.
The number of adenomas per colonoscopy (APC) is inversely correlated with postcolonoscopy colorectal cancer (PCCRC), which supports use of APC as a new quality control measure, according to investigators.
Data from 138 endoscopists showed that patients screened by physicians with higher APCs had significantly lower rates of PCCRC, and an APC of 0.6 offered more protection than either an APC of 0.4 or an adenoma detection rate (ADR) of 25%, reported lead author Joseph C. Anderson, MD, of White River Junction VA Medical Center, Hanover, N.H., and colleagues.
“Unfortunately, APC has never been validated as a quality measure by demonstrating a reduction in PCCRC in exams performed by endoscopists with higher rates,” Dr. Anderson said at the annual meeting of the American College of Gastroenterology.
To this end, Dr. Anderson and colleagues reviewed data from the New Hampshire Colonoscopy Registry (NHCR), including 9,023 screening colonoscopies with a follow-up event 6-60 months after the initial exam. Procedures were conducted by 138 endoscopists in New Hampshire, Vermont, Massachusetts, and Maine.
Three quality measures were analyzed for associations with PCCRC: an APC of 0.4, an APC of 0.6, and an ADR of 25%. Hazard ratios were calculated for all PCCRCs, as well as PCCRCs diagnosed at first follow-up event. Rates were reported for two time periods: 6-36 months and 6-60 months.
From 6 to 60 months, 82 cases of PCCRC were diagnosed, among which 50 were diagnosed between 6 and 36 months.
For both periods, all three quality measures were significantly associated with reductions in PCCRC. The higher APC of 0.6, however, offered greater protection, reducing all PCCRCs by 71% and 61% in the shorter and longer period, respectively. In comparison, the lower APC of 0.4 reduced rates by 63% and 53%, while the ADR benchmark reduced rates by 62% and 42%.
These trends were maintained for PCCRCs diagnosed at first follow-up event. An APC of 0.6 was associated with respective reductions of 79% and 65% for the shorter and longer period, compared with 64% and 57% for the lower APC, and 67% and 49% for ADR.
Additional analysis clarified the relationship between APC level and likelihood of developing PCCRC. In terms of absolute risk, patients screened by an endoscopist with an APC greater than 0.6 had a 0.5% chance of developing PCCRC from 6 to 36 months, compared with 0.7% for an APC of 0.4-0.6, and 2.1% for an APC of less than 0.4 (P = .0001). This pattern held through 60 months, during which time an APC greater than 0.6 was associated with an absolute risk of PCCRC of 0.4%, compared with 0.7% for an APC of 0.4-0.6, and 1.6% for an APC less than 0.4 (P = .0001).
“Our novel data support the use of APC as a quality measure by demonstrating a reduction in PCCRC risk in exams performed by endoscopists with higher APCs,” Dr. Anderson concluded, noting that an APC of 0.6 appeared to offer more protection than an APC of 0.4. “I feel that ... APC as a quality measure, now that we’ve validated it, may be accepted because of its ability to differentiate endoscopists on their adenoma detection skills.”
According to Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., “It’s an important study that will probably contribute to where we’re going forward.”
Dr. Lawrence, chair of the division and medical director of the endoscopy units at Kingston General and Hotel Dieu hospitals, said that APC may overcome the main concern with ADR – that endoscopists who find one adenoma may not be motivated to seek out as many as possible.
“The problem with ADR, in general, is that if you find one polyp, and if ADR is the stat you’re living by, then you don’t need to find any other polyps, and that obviously doesn’t do that patient a favor, necessarily,” Dr. Hookey said in an interview. “It does bring them back sooner for surveillance, but it doesn’t help remove the rest of the polyps that they have. And not that someone is going to find one polyp and turn off the light and pull the scope out, but you may not be looking as hard.”
APC mitigates this issue, he explained, because it determines “whether or not you’re truly clearing things out and getting rid of as many [polyps] as possible.”
Dr. Hookey said that APC is “probably the best” quality control measure on the horizon, and he suggested that more work is needed to determine the optimal benchmark figure, which should ideally be investigated through larger studies.
“I just want to see it in bigger groups,” he said.
The investigators and Dr. Hookey reported no conflicts of interest.
The number of adenomas per colonoscopy (APC) is inversely correlated with postcolonoscopy colorectal cancer (PCCRC), which supports use of APC as a new quality control measure, according to investigators.
Data from 138 endoscopists showed that patients screened by physicians with higher APCs had significantly lower rates of PCCRC, and an APC of 0.6 offered more protection than either an APC of 0.4 or an adenoma detection rate (ADR) of 25%, reported lead author Joseph C. Anderson, MD, of White River Junction VA Medical Center, Hanover, N.H., and colleagues.
“Unfortunately, APC has never been validated as a quality measure by demonstrating a reduction in PCCRC in exams performed by endoscopists with higher rates,” Dr. Anderson said at the annual meeting of the American College of Gastroenterology.
To this end, Dr. Anderson and colleagues reviewed data from the New Hampshire Colonoscopy Registry (NHCR), including 9,023 screening colonoscopies with a follow-up event 6-60 months after the initial exam. Procedures were conducted by 138 endoscopists in New Hampshire, Vermont, Massachusetts, and Maine.
Three quality measures were analyzed for associations with PCCRC: an APC of 0.4, an APC of 0.6, and an ADR of 25%. Hazard ratios were calculated for all PCCRCs, as well as PCCRCs diagnosed at first follow-up event. Rates were reported for two time periods: 6-36 months and 6-60 months.
From 6 to 60 months, 82 cases of PCCRC were diagnosed, among which 50 were diagnosed between 6 and 36 months.
For both periods, all three quality measures were significantly associated with reductions in PCCRC. The higher APC of 0.6, however, offered greater protection, reducing all PCCRCs by 71% and 61% in the shorter and longer period, respectively. In comparison, the lower APC of 0.4 reduced rates by 63% and 53%, while the ADR benchmark reduced rates by 62% and 42%.
These trends were maintained for PCCRCs diagnosed at first follow-up event. An APC of 0.6 was associated with respective reductions of 79% and 65% for the shorter and longer period, compared with 64% and 57% for the lower APC, and 67% and 49% for ADR.
Additional analysis clarified the relationship between APC level and likelihood of developing PCCRC. In terms of absolute risk, patients screened by an endoscopist with an APC greater than 0.6 had a 0.5% chance of developing PCCRC from 6 to 36 months, compared with 0.7% for an APC of 0.4-0.6, and 2.1% for an APC of less than 0.4 (P = .0001). This pattern held through 60 months, during which time an APC greater than 0.6 was associated with an absolute risk of PCCRC of 0.4%, compared with 0.7% for an APC of 0.4-0.6, and 1.6% for an APC less than 0.4 (P = .0001).
“Our novel data support the use of APC as a quality measure by demonstrating a reduction in PCCRC risk in exams performed by endoscopists with higher APCs,” Dr. Anderson concluded, noting that an APC of 0.6 appeared to offer more protection than an APC of 0.4. “I feel that ... APC as a quality measure, now that we’ve validated it, may be accepted because of its ability to differentiate endoscopists on their adenoma detection skills.”
According to Lawrence Hookey, MD, of Queen’s University, Kingston, Ont., “It’s an important study that will probably contribute to where we’re going forward.”
Dr. Lawrence, chair of the division and medical director of the endoscopy units at Kingston General and Hotel Dieu hospitals, said that APC may overcome the main concern with ADR – that endoscopists who find one adenoma may not be motivated to seek out as many as possible.
“The problem with ADR, in general, is that if you find one polyp, and if ADR is the stat you’re living by, then you don’t need to find any other polyps, and that obviously doesn’t do that patient a favor, necessarily,” Dr. Hookey said in an interview. “It does bring them back sooner for surveillance, but it doesn’t help remove the rest of the polyps that they have. And not that someone is going to find one polyp and turn off the light and pull the scope out, but you may not be looking as hard.”
APC mitigates this issue, he explained, because it determines “whether or not you’re truly clearing things out and getting rid of as many [polyps] as possible.”
Dr. Hookey said that APC is “probably the best” quality control measure on the horizon, and he suggested that more work is needed to determine the optimal benchmark figure, which should ideally be investigated through larger studies.
“I just want to see it in bigger groups,” he said.
The investigators and Dr. Hookey reported no conflicts of interest.
FROM ACG 2021
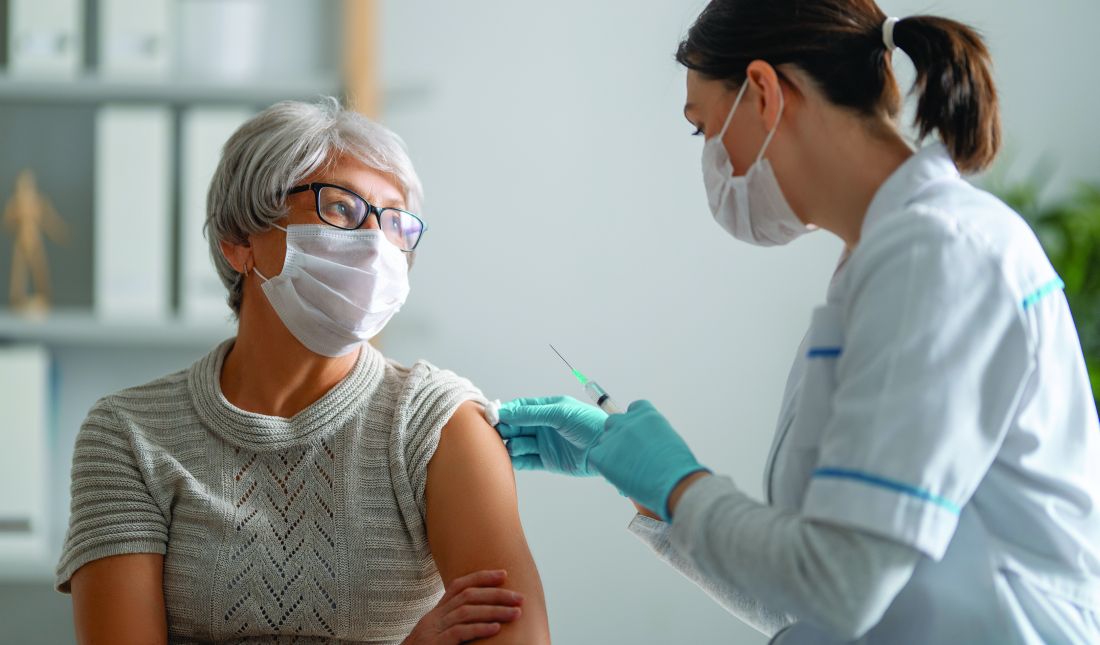
Immune response detected in most IBD patients after COVID vaccines
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
AT ACG 2021
SGLT2 inhibitors for diabetes: No link to fractures in older adults
Use of sodium-glucose cotransporter-2 (SGLT2) inhibitors does not appear to raise the risk for fractures in older adults, new research suggests.
The data come from a nationwide propensity score-matched study of U.S. Medicare recipients with type 2 diabetes who were new users of either an SGLT2 inhibitor, a dipeptidyl peptidase 4 (DPP-4) inhibitor, or a glucagon-like peptide (GLP-1) receptor agonist.
“The use of SGLT2 inhibitors was not associated with an increased risk of nontraumatic fractures compared with DPP-4 inhibitors or GLP-1 agonists. Results were consistent across categories of sex, frailty, age, and insulin use,” say Min Zhuo, MD, of Harvard Medical School, Boston, and colleagues, who published their work online October 27 in JAMA Network Open.
“Our results add to the evidence base evaluating the safety profile of SGLT2 inhibitors in older adults outside of [randomized controlled trials] and further characterize the risk-benefit balance of SGLT2 inhibitors in clinical practice,” they write.
Asked to comment, Simeon I. Taylor, MD, PhD, told this news organization, “This is a high-quality study that is generally reassuring that relatively short, less than 1 year, treatment with an SGLT2 inhibitor does not appear to significantly increase the risk of bone fractures.”
However, Dr. Taylor, of the Division of Endocrinology, Diabetes, and Nutrition, University of Maryland School of Medicine, Baltimore, also noted: “Notwithstanding these reassuring data, the paper also does a good job of pointing out important limitations.”
“Most importantly, these data do not address questions related to the risk of long-term chronic therapy. It is instructive to refer back to the published data demonstrating an approximately 2-year lag before a significant increase in the risk of fracture was observed in rosiglitazone-treated patients in the ADOPT study. The length of the lag is likely related to the baseline bone mineral density at the time drug therapy is initiated. These considerations may contribute to the observed variation in bone-related outcomes in different studies.”
Concern about SGLT2 inhibitors and fractures first arose in 2017 from the CANVAS study, in which the overall fracture risk with canagliflozin was a significant 26% higher than placebo. However, subsequent larger randomized trials of canagliflozin and other SGLT2 inhibitors did not find the same risk.
In addition, previous observational studies in younger adults have also not found use of SGLT2 inhibitors to be associated with increased fracture risk compared with DPP-4 inhibitors or GLP-1 agonists.
Understanding fracture risk with SGLT2 inhibitors is ‘critical’
Older adults with type 2 diabetes may benefit from reductions in atherosclerotic cardiovascular events, hospitalization for heart failure, end-stage kidney disease, and death associated with SGLT2 inhibitors, but the fact that aging may have negative effects on bone metabolism means “understanding the fracture risk associated with SGLT2 inhibitors in older adults with type 2 diabetes is critical,” say Dr. Zhuo and colleagues.
In the current study, they analyzed claims data for Medicare beneficiaries aged 66 years and older (1 year past Medicare eligibility) who were newly prescribed an SGLT2 inhibitor, DPP-4 inhibitor, or GLP-1 agonist between April 1, 2013 and Dec. 31, 2017.
A total of 45,889 patients from each treatment group were propensity-matched using 58 baseline characteristics, for a total of 137,667 patients.
After matching, there were 501 events of the primary composite outcome (nontraumatic pelvic fracture, hip fracture requiring surgery, or humerus, radius, or ulna fracture requiring intervention) within 30 days. By treatment group, fracture rates per 1,000 person-years were 4.69, 5.26, and 4.71 for SGLT2 inhibitors, DPP-4 inhibitors, and GLP-1 agonists respectively.
The differences between patients taking DPP-4 inhibitors or GLP-1 agonists compared with SGLT2 inhibitors were not significant, with hazard ratios of 0.90 and 1.00, respectively.
Results remained consistent in various sensitivity and subgroup analyses, including limiting the data to just the canagliflozin group. Overall, the fracture rate was greater with female sex, frailty, older age, and insulin use, consistent across drug classes.
The risks for falls and hypoglycemia were lower in the SGLT2 inhibitor versus matched DPP-4 inhibitor groups (hazard ratio, 0.82), and there was no difference in syncope. None of those differences were significant for the SGLT2 inhibitor group compared with the GLP-1 agonist group.
Consistent with previous data, the risk for diabetic ketoacidosis was higher with SGLT2 inhibitors versus DPP-4 inhibitors and GLP-1 agonists (HR, 1.29 and 1.58), and the risk for heart failure hospitalization was lower (HR, 0.42 and 0.69).
The study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, Harvard Medical School. Dr. Zhuo was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Use of sodium-glucose cotransporter-2 (SGLT2) inhibitors does not appear to raise the risk for fractures in older adults, new research suggests.
The data come from a nationwide propensity score-matched study of U.S. Medicare recipients with type 2 diabetes who were new users of either an SGLT2 inhibitor, a dipeptidyl peptidase 4 (DPP-4) inhibitor, or a glucagon-like peptide (GLP-1) receptor agonist.
“The use of SGLT2 inhibitors was not associated with an increased risk of nontraumatic fractures compared with DPP-4 inhibitors or GLP-1 agonists. Results were consistent across categories of sex, frailty, age, and insulin use,” say Min Zhuo, MD, of Harvard Medical School, Boston, and colleagues, who published their work online October 27 in JAMA Network Open.
“Our results add to the evidence base evaluating the safety profile of SGLT2 inhibitors in older adults outside of [randomized controlled trials] and further characterize the risk-benefit balance of SGLT2 inhibitors in clinical practice,” they write.
Asked to comment, Simeon I. Taylor, MD, PhD, told this news organization, “This is a high-quality study that is generally reassuring that relatively short, less than 1 year, treatment with an SGLT2 inhibitor does not appear to significantly increase the risk of bone fractures.”
However, Dr. Taylor, of the Division of Endocrinology, Diabetes, and Nutrition, University of Maryland School of Medicine, Baltimore, also noted: “Notwithstanding these reassuring data, the paper also does a good job of pointing out important limitations.”
“Most importantly, these data do not address questions related to the risk of long-term chronic therapy. It is instructive to refer back to the published data demonstrating an approximately 2-year lag before a significant increase in the risk of fracture was observed in rosiglitazone-treated patients in the ADOPT study. The length of the lag is likely related to the baseline bone mineral density at the time drug therapy is initiated. These considerations may contribute to the observed variation in bone-related outcomes in different studies.”
Concern about SGLT2 inhibitors and fractures first arose in 2017 from the CANVAS study, in which the overall fracture risk with canagliflozin was a significant 26% higher than placebo. However, subsequent larger randomized trials of canagliflozin and other SGLT2 inhibitors did not find the same risk.
In addition, previous observational studies in younger adults have also not found use of SGLT2 inhibitors to be associated with increased fracture risk compared with DPP-4 inhibitors or GLP-1 agonists.
Understanding fracture risk with SGLT2 inhibitors is ‘critical’
Older adults with type 2 diabetes may benefit from reductions in atherosclerotic cardiovascular events, hospitalization for heart failure, end-stage kidney disease, and death associated with SGLT2 inhibitors, but the fact that aging may have negative effects on bone metabolism means “understanding the fracture risk associated with SGLT2 inhibitors in older adults with type 2 diabetes is critical,” say Dr. Zhuo and colleagues.
In the current study, they analyzed claims data for Medicare beneficiaries aged 66 years and older (1 year past Medicare eligibility) who were newly prescribed an SGLT2 inhibitor, DPP-4 inhibitor, or GLP-1 agonist between April 1, 2013 and Dec. 31, 2017.
A total of 45,889 patients from each treatment group were propensity-matched using 58 baseline characteristics, for a total of 137,667 patients.
After matching, there were 501 events of the primary composite outcome (nontraumatic pelvic fracture, hip fracture requiring surgery, or humerus, radius, or ulna fracture requiring intervention) within 30 days. By treatment group, fracture rates per 1,000 person-years were 4.69, 5.26, and 4.71 for SGLT2 inhibitors, DPP-4 inhibitors, and GLP-1 agonists respectively.
The differences between patients taking DPP-4 inhibitors or GLP-1 agonists compared with SGLT2 inhibitors were not significant, with hazard ratios of 0.90 and 1.00, respectively.
Results remained consistent in various sensitivity and subgroup analyses, including limiting the data to just the canagliflozin group. Overall, the fracture rate was greater with female sex, frailty, older age, and insulin use, consistent across drug classes.
The risks for falls and hypoglycemia were lower in the SGLT2 inhibitor versus matched DPP-4 inhibitor groups (hazard ratio, 0.82), and there was no difference in syncope. None of those differences were significant for the SGLT2 inhibitor group compared with the GLP-1 agonist group.
Consistent with previous data, the risk for diabetic ketoacidosis was higher with SGLT2 inhibitors versus DPP-4 inhibitors and GLP-1 agonists (HR, 1.29 and 1.58), and the risk for heart failure hospitalization was lower (HR, 0.42 and 0.69).
The study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, Harvard Medical School. Dr. Zhuo was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Use of sodium-glucose cotransporter-2 (SGLT2) inhibitors does not appear to raise the risk for fractures in older adults, new research suggests.
The data come from a nationwide propensity score-matched study of U.S. Medicare recipients with type 2 diabetes who were new users of either an SGLT2 inhibitor, a dipeptidyl peptidase 4 (DPP-4) inhibitor, or a glucagon-like peptide (GLP-1) receptor agonist.
“The use of SGLT2 inhibitors was not associated with an increased risk of nontraumatic fractures compared with DPP-4 inhibitors or GLP-1 agonists. Results were consistent across categories of sex, frailty, age, and insulin use,” say Min Zhuo, MD, of Harvard Medical School, Boston, and colleagues, who published their work online October 27 in JAMA Network Open.
“Our results add to the evidence base evaluating the safety profile of SGLT2 inhibitors in older adults outside of [randomized controlled trials] and further characterize the risk-benefit balance of SGLT2 inhibitors in clinical practice,” they write.
Asked to comment, Simeon I. Taylor, MD, PhD, told this news organization, “This is a high-quality study that is generally reassuring that relatively short, less than 1 year, treatment with an SGLT2 inhibitor does not appear to significantly increase the risk of bone fractures.”
However, Dr. Taylor, of the Division of Endocrinology, Diabetes, and Nutrition, University of Maryland School of Medicine, Baltimore, also noted: “Notwithstanding these reassuring data, the paper also does a good job of pointing out important limitations.”
“Most importantly, these data do not address questions related to the risk of long-term chronic therapy. It is instructive to refer back to the published data demonstrating an approximately 2-year lag before a significant increase in the risk of fracture was observed in rosiglitazone-treated patients in the ADOPT study. The length of the lag is likely related to the baseline bone mineral density at the time drug therapy is initiated. These considerations may contribute to the observed variation in bone-related outcomes in different studies.”
Concern about SGLT2 inhibitors and fractures first arose in 2017 from the CANVAS study, in which the overall fracture risk with canagliflozin was a significant 26% higher than placebo. However, subsequent larger randomized trials of canagliflozin and other SGLT2 inhibitors did not find the same risk.
In addition, previous observational studies in younger adults have also not found use of SGLT2 inhibitors to be associated with increased fracture risk compared with DPP-4 inhibitors or GLP-1 agonists.
Understanding fracture risk with SGLT2 inhibitors is ‘critical’
Older adults with type 2 diabetes may benefit from reductions in atherosclerotic cardiovascular events, hospitalization for heart failure, end-stage kidney disease, and death associated with SGLT2 inhibitors, but the fact that aging may have negative effects on bone metabolism means “understanding the fracture risk associated with SGLT2 inhibitors in older adults with type 2 diabetes is critical,” say Dr. Zhuo and colleagues.
In the current study, they analyzed claims data for Medicare beneficiaries aged 66 years and older (1 year past Medicare eligibility) who were newly prescribed an SGLT2 inhibitor, DPP-4 inhibitor, or GLP-1 agonist between April 1, 2013 and Dec. 31, 2017.
A total of 45,889 patients from each treatment group were propensity-matched using 58 baseline characteristics, for a total of 137,667 patients.
After matching, there were 501 events of the primary composite outcome (nontraumatic pelvic fracture, hip fracture requiring surgery, or humerus, radius, or ulna fracture requiring intervention) within 30 days. By treatment group, fracture rates per 1,000 person-years were 4.69, 5.26, and 4.71 for SGLT2 inhibitors, DPP-4 inhibitors, and GLP-1 agonists respectively.
The differences between patients taking DPP-4 inhibitors or GLP-1 agonists compared with SGLT2 inhibitors were not significant, with hazard ratios of 0.90 and 1.00, respectively.
Results remained consistent in various sensitivity and subgroup analyses, including limiting the data to just the canagliflozin group. Overall, the fracture rate was greater with female sex, frailty, older age, and insulin use, consistent across drug classes.
The risks for falls and hypoglycemia were lower in the SGLT2 inhibitor versus matched DPP-4 inhibitor groups (hazard ratio, 0.82), and there was no difference in syncope. None of those differences were significant for the SGLT2 inhibitor group compared with the GLP-1 agonist group.
Consistent with previous data, the risk for diabetic ketoacidosis was higher with SGLT2 inhibitors versus DPP-4 inhibitors and GLP-1 agonists (HR, 1.29 and 1.58), and the risk for heart failure hospitalization was lower (HR, 0.42 and 0.69).
The study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, Harvard Medical School. Dr. Zhuo was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Nondiabetes hospitalization is wrong time to up diabetes meds
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
FROM JAMA NETWORK OPEN
Good data is lacking on best first-line MS drug strategies
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
FROM CMSC 2021
Free vitamin D no better at predicting death in men than standard testing
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Lung cancer screening rates in U.S. nowhere near goal
according to a review and meta-analysis published in the Journal of Thoracic Oncology.
“Lung cancer screening is effective in reducing mortality, particularly when patients adhere to follow-up recommendations standardized by the Lung CT Screening Reporting & Data System (Lung-RADS),” Yannan Lin, MD, MPH, of the University of California, Los Angeles, and colleagues wrote. ”Patient adherence to Lung-RADS–recommended screening intervals is suboptimal across clinical lung cancer screening programs in the U.S., especially among patients with Lung-RADS category 1-2 results.”
Lung cancer screening can identify tumors at earlier, more treatable stages, but patients with lung cancer diagnoses based on new nodules at incidence screening have shown shortened survivals. The National Lung Screening Trial (NLST) has shown a 20% relative reduction in lung cancer mortality with low-dose chest CT screening relative to chest radiography. The Lung-RADS guidelines to standardize the reporting of lung cancer screening were developed based on findings from the NLST and other screening studies, partly to reduce false-positive rates. Lung-RADS scores are based upon nodule size, characteristics and location, with management guidelines specific to Lung-RADS categories, ranging from low-dose chest CT in 12 months for Lung-RADS 1-2 to chest CT, PET/CT, or tissue sampling for Lung-RADS 4B/X.
The rate of adherence to lung cancer screening based on Lung-RADS guidelines is unclear. This systematic review and meta-analysis looked at patient adherence to Lung-RADS recommended screening intervals in clinical practice.
The meta-analysis included 21 studies. The pooled adherence rate was 57% for defined adherence, which included an annual incidence screen performed within 15 months, among 6,689 patients and 65% for anytime adherence among 5,085 patients. The authors noted that overall rates of adherence to Lung-RADS recommended screening intervals in clinical practices is low as compared with the over 90% adherence seen in the NLST, adversely affecting the mortality benefits of lung cancer screening.
Higher adherence rates were found in patients with Lung-RADS 3 (risk for lung cancer, 1%-2%) and 4 (risk, >5%) than Lung-RADS 1 and 2 (risk, <1%; P < .05), which the authors said suggests that tailored interventions based on Lung-RADS categories may be beneficial.
“It is likely that patients and referrers are more concerned about nodules at a higher risk for lung cancer, prompting greater adherence to recommended screening intervals in Lung-RADS 3-4,” the authors wrote. “It is crucial that patients and referrers alike understand that screening is most effective when performed regularly, including for those with negative baseline screens, as de novo nodules, those detected after a negative screen, are more aggressive than those detected at baseline screen.”
These low adherence rates seen in the clinical practices could be explained by patient characteristics, insurance coverage and interventions to ensure adherence, among other factors.
Further, inconsistent reporting of adherence rates was observed. Standardized reporting of adherence rates to lung cancer screening is needed to identify interventions to improve adherence, the authors wrote.
The authors of this study noted no conflicts of interest.
according to a review and meta-analysis published in the Journal of Thoracic Oncology.
“Lung cancer screening is effective in reducing mortality, particularly when patients adhere to follow-up recommendations standardized by the Lung CT Screening Reporting & Data System (Lung-RADS),” Yannan Lin, MD, MPH, of the University of California, Los Angeles, and colleagues wrote. ”Patient adherence to Lung-RADS–recommended screening intervals is suboptimal across clinical lung cancer screening programs in the U.S., especially among patients with Lung-RADS category 1-2 results.”
Lung cancer screening can identify tumors at earlier, more treatable stages, but patients with lung cancer diagnoses based on new nodules at incidence screening have shown shortened survivals. The National Lung Screening Trial (NLST) has shown a 20% relative reduction in lung cancer mortality with low-dose chest CT screening relative to chest radiography. The Lung-RADS guidelines to standardize the reporting of lung cancer screening were developed based on findings from the NLST and other screening studies, partly to reduce false-positive rates. Lung-RADS scores are based upon nodule size, characteristics and location, with management guidelines specific to Lung-RADS categories, ranging from low-dose chest CT in 12 months for Lung-RADS 1-2 to chest CT, PET/CT, or tissue sampling for Lung-RADS 4B/X.
The rate of adherence to lung cancer screening based on Lung-RADS guidelines is unclear. This systematic review and meta-analysis looked at patient adherence to Lung-RADS recommended screening intervals in clinical practice.
The meta-analysis included 21 studies. The pooled adherence rate was 57% for defined adherence, which included an annual incidence screen performed within 15 months, among 6,689 patients and 65% for anytime adherence among 5,085 patients. The authors noted that overall rates of adherence to Lung-RADS recommended screening intervals in clinical practices is low as compared with the over 90% adherence seen in the NLST, adversely affecting the mortality benefits of lung cancer screening.
Higher adherence rates were found in patients with Lung-RADS 3 (risk for lung cancer, 1%-2%) and 4 (risk, >5%) than Lung-RADS 1 and 2 (risk, <1%; P < .05), which the authors said suggests that tailored interventions based on Lung-RADS categories may be beneficial.
“It is likely that patients and referrers are more concerned about nodules at a higher risk for lung cancer, prompting greater adherence to recommended screening intervals in Lung-RADS 3-4,” the authors wrote. “It is crucial that patients and referrers alike understand that screening is most effective when performed regularly, including for those with negative baseline screens, as de novo nodules, those detected after a negative screen, are more aggressive than those detected at baseline screen.”
These low adherence rates seen in the clinical practices could be explained by patient characteristics, insurance coverage and interventions to ensure adherence, among other factors.
Further, inconsistent reporting of adherence rates was observed. Standardized reporting of adherence rates to lung cancer screening is needed to identify interventions to improve adherence, the authors wrote.
The authors of this study noted no conflicts of interest.
according to a review and meta-analysis published in the Journal of Thoracic Oncology.
“Lung cancer screening is effective in reducing mortality, particularly when patients adhere to follow-up recommendations standardized by the Lung CT Screening Reporting & Data System (Lung-RADS),” Yannan Lin, MD, MPH, of the University of California, Los Angeles, and colleagues wrote. ”Patient adherence to Lung-RADS–recommended screening intervals is suboptimal across clinical lung cancer screening programs in the U.S., especially among patients with Lung-RADS category 1-2 results.”
Lung cancer screening can identify tumors at earlier, more treatable stages, but patients with lung cancer diagnoses based on new nodules at incidence screening have shown shortened survivals. The National Lung Screening Trial (NLST) has shown a 20% relative reduction in lung cancer mortality with low-dose chest CT screening relative to chest radiography. The Lung-RADS guidelines to standardize the reporting of lung cancer screening were developed based on findings from the NLST and other screening studies, partly to reduce false-positive rates. Lung-RADS scores are based upon nodule size, characteristics and location, with management guidelines specific to Lung-RADS categories, ranging from low-dose chest CT in 12 months for Lung-RADS 1-2 to chest CT, PET/CT, or tissue sampling for Lung-RADS 4B/X.
The rate of adherence to lung cancer screening based on Lung-RADS guidelines is unclear. This systematic review and meta-analysis looked at patient adherence to Lung-RADS recommended screening intervals in clinical practice.
The meta-analysis included 21 studies. The pooled adherence rate was 57% for defined adherence, which included an annual incidence screen performed within 15 months, among 6,689 patients and 65% for anytime adherence among 5,085 patients. The authors noted that overall rates of adherence to Lung-RADS recommended screening intervals in clinical practices is low as compared with the over 90% adherence seen in the NLST, adversely affecting the mortality benefits of lung cancer screening.
Higher adherence rates were found in patients with Lung-RADS 3 (risk for lung cancer, 1%-2%) and 4 (risk, >5%) than Lung-RADS 1 and 2 (risk, <1%; P < .05), which the authors said suggests that tailored interventions based on Lung-RADS categories may be beneficial.
“It is likely that patients and referrers are more concerned about nodules at a higher risk for lung cancer, prompting greater adherence to recommended screening intervals in Lung-RADS 3-4,” the authors wrote. “It is crucial that patients and referrers alike understand that screening is most effective when performed regularly, including for those with negative baseline screens, as de novo nodules, those detected after a negative screen, are more aggressive than those detected at baseline screen.”
These low adherence rates seen in the clinical practices could be explained by patient characteristics, insurance coverage and interventions to ensure adherence, among other factors.
Further, inconsistent reporting of adherence rates was observed. Standardized reporting of adherence rates to lung cancer screening is needed to identify interventions to improve adherence, the authors wrote.
The authors of this study noted no conflicts of interest.
FROM THE JOURNAL OF THORACIC ONCOLOGY
Immunocompromised people face highest risk of cutaneous SCC metastasis
However, no study has thoroughly evaluated the prognostic factors associated with metastasis until now.
In the Journal of Otolaryngology – Head & Neck Surgery, researchers wrote that immunocompromised individuals, such as solid organ transplant patients, make up 73.3% of all patients with cutaneous SCC who are at risk of metastasis and decreased overall survival.
Led by Alex M. Mlynarek, MD, a specialist in head and neck oncology and microvascular reconstruction at McGill University, Montreal, the finding is based on a systematic literature review of 40 studies involving 8,535 patients.
“The prognostic factors for head and neck cutaneous squamous cell carcinoma that were most consistently reported as significant in the literature are a state of immunosuppression, tumor depth, margins involved, number of lymph nodes affected by carcinoma, parotideal disease, and age,” Dr. Mlynarek and colleagues wrote.
Cutaneous SCC is the second most common nonmelanoma skin cancer with an increase of 263% between 2000 and 2010, shows research from the Mayo Clinic Rochester Epidemiology Project.
Patients in this study with tumors that are 6 mm or greater, or whose tumor invaded fat tissue, were found to have a poor prognosis followed by patients with perineural and lymphovascular invasion and in particular, patients with a poorer grade of cellular differentiation. The number of lymph nodes was significant at 70%, with more than two nodes involved linked to a worse the prognosis, followed by 66.7% for margins involved with carcinoma and 50% for tumor depth.
“The majority of patients with cutaneous SCC undergoes electrodesiccation and curettage, cryosurgery, or Mohs surgery, and have an excellent prognosis,” the authors wrote. “However, there is a subset of patients in which these therapies are unsuccessful and where cutaneous SCC appears to be far more aggressive, often resulting in metastasis and recurrence.”
Age was shown to be a significant factor in 53.3% of the studies, but the extent of its effect on prognosis was questionable.
Sentinel lymph node biopsy is commonly used to stage melanoma and has been used in oral SCC.
“A patient post biopsy with either two major criteria or one major and two minor criteria should be considered as a candidate for sentinel lymph node biopsy,” the authors wrote, adding that the findings were consistent with those for cutaneous SCC generally, not specified to the head and neck.
Limitations of the systematic review include potential selection bias as the majority of the studies were based in Australia and most studies were not specified to cutaneous SCC of the head and neck region.
“Given the low rate of metastasis from head and neck cutaneous SCC lesions, it can be challenging to identify the patients who are at high risk of having metastatic disease,” the authors wrote. “We believe this review could help identify patients that would require a closer follow-up and those that could possibly profit from a sentinel lymph node biopsy.”
No disclosures were disclosed for the authors.
However, no study has thoroughly evaluated the prognostic factors associated with metastasis until now.
In the Journal of Otolaryngology – Head & Neck Surgery, researchers wrote that immunocompromised individuals, such as solid organ transplant patients, make up 73.3% of all patients with cutaneous SCC who are at risk of metastasis and decreased overall survival.
Led by Alex M. Mlynarek, MD, a specialist in head and neck oncology and microvascular reconstruction at McGill University, Montreal, the finding is based on a systematic literature review of 40 studies involving 8,535 patients.
“The prognostic factors for head and neck cutaneous squamous cell carcinoma that were most consistently reported as significant in the literature are a state of immunosuppression, tumor depth, margins involved, number of lymph nodes affected by carcinoma, parotideal disease, and age,” Dr. Mlynarek and colleagues wrote.
Cutaneous SCC is the second most common nonmelanoma skin cancer with an increase of 263% between 2000 and 2010, shows research from the Mayo Clinic Rochester Epidemiology Project.
Patients in this study with tumors that are 6 mm or greater, or whose tumor invaded fat tissue, were found to have a poor prognosis followed by patients with perineural and lymphovascular invasion and in particular, patients with a poorer grade of cellular differentiation. The number of lymph nodes was significant at 70%, with more than two nodes involved linked to a worse the prognosis, followed by 66.7% for margins involved with carcinoma and 50% for tumor depth.
“The majority of patients with cutaneous SCC undergoes electrodesiccation and curettage, cryosurgery, or Mohs surgery, and have an excellent prognosis,” the authors wrote. “However, there is a subset of patients in which these therapies are unsuccessful and where cutaneous SCC appears to be far more aggressive, often resulting in metastasis and recurrence.”
Age was shown to be a significant factor in 53.3% of the studies, but the extent of its effect on prognosis was questionable.
Sentinel lymph node biopsy is commonly used to stage melanoma and has been used in oral SCC.
“A patient post biopsy with either two major criteria or one major and two minor criteria should be considered as a candidate for sentinel lymph node biopsy,” the authors wrote, adding that the findings were consistent with those for cutaneous SCC generally, not specified to the head and neck.
Limitations of the systematic review include potential selection bias as the majority of the studies were based in Australia and most studies were not specified to cutaneous SCC of the head and neck region.
“Given the low rate of metastasis from head and neck cutaneous SCC lesions, it can be challenging to identify the patients who are at high risk of having metastatic disease,” the authors wrote. “We believe this review could help identify patients that would require a closer follow-up and those that could possibly profit from a sentinel lymph node biopsy.”
No disclosures were disclosed for the authors.
However, no study has thoroughly evaluated the prognostic factors associated with metastasis until now.
In the Journal of Otolaryngology – Head & Neck Surgery, researchers wrote that immunocompromised individuals, such as solid organ transplant patients, make up 73.3% of all patients with cutaneous SCC who are at risk of metastasis and decreased overall survival.
Led by Alex M. Mlynarek, MD, a specialist in head and neck oncology and microvascular reconstruction at McGill University, Montreal, the finding is based on a systematic literature review of 40 studies involving 8,535 patients.
“The prognostic factors for head and neck cutaneous squamous cell carcinoma that were most consistently reported as significant in the literature are a state of immunosuppression, tumor depth, margins involved, number of lymph nodes affected by carcinoma, parotideal disease, and age,” Dr. Mlynarek and colleagues wrote.
Cutaneous SCC is the second most common nonmelanoma skin cancer with an increase of 263% between 2000 and 2010, shows research from the Mayo Clinic Rochester Epidemiology Project.
Patients in this study with tumors that are 6 mm or greater, or whose tumor invaded fat tissue, were found to have a poor prognosis followed by patients with perineural and lymphovascular invasion and in particular, patients with a poorer grade of cellular differentiation. The number of lymph nodes was significant at 70%, with more than two nodes involved linked to a worse the prognosis, followed by 66.7% for margins involved with carcinoma and 50% for tumor depth.
“The majority of patients with cutaneous SCC undergoes electrodesiccation and curettage, cryosurgery, or Mohs surgery, and have an excellent prognosis,” the authors wrote. “However, there is a subset of patients in which these therapies are unsuccessful and where cutaneous SCC appears to be far more aggressive, often resulting in metastasis and recurrence.”
Age was shown to be a significant factor in 53.3% of the studies, but the extent of its effect on prognosis was questionable.
Sentinel lymph node biopsy is commonly used to stage melanoma and has been used in oral SCC.
“A patient post biopsy with either two major criteria or one major and two minor criteria should be considered as a candidate for sentinel lymph node biopsy,” the authors wrote, adding that the findings were consistent with those for cutaneous SCC generally, not specified to the head and neck.
Limitations of the systematic review include potential selection bias as the majority of the studies were based in Australia and most studies were not specified to cutaneous SCC of the head and neck region.
“Given the low rate of metastasis from head and neck cutaneous SCC lesions, it can be challenging to identify the patients who are at high risk of having metastatic disease,” the authors wrote. “We believe this review could help identify patients that would require a closer follow-up and those that could possibly profit from a sentinel lymph node biopsy.”
No disclosures were disclosed for the authors.
FROM THE JOURNAL OF OTOLARYNGOLOGY – HEAD AND NECK SURGERY
Spiders, dogs, and PTSD: A virtual treatment for phobias and fear
At Wayne State University’s Stress, Trauma, and Anxiety Research Clinic (STARC) in Michigan, researchers are developing novel interventions for treating some very ancient phobias hardwired into the human brain. By using augmented reality as means of conducting exposure therapy, STARC researchers – including Shantanu Madaboosi, Rakesh Ramaswamy, and Lana Grasser – and STARC director Arash Javanbakht, MD, have produced compelling evidence that they can free patients of their often debilitating fears of spiders, dogs, and snakes. Yet their work doesn’t stop there, and research into treating anxiety and posttraumatic stress disorder among first responders and others with high-stress occupations is ongoing.

This news organization spoke with Dr. Javanbakht, a psychiatrist, about the technological advances that have made this work possible; the future of remote-based psychiatry; and his tarantula colleague, Tony.
Augmenting exposure therapy
How did you begin using artificial intelligence as a way of delivering exposure therapy?
Exposure therapy is a very effective treatment for phobias, obsessive-compulsive disorder, and PTSD. But the problem we had is that, if someone comes to me and says they’re afraid of dogs, snakes, or spiders, I don’t have those in my office. Or, if its social phobia, I can’t create those scenarios. So, despite being such an effective treatment, it’s not utilized as much as it should be.
Several years ago, I saw a TED talk by the CEO of an augmented reality company who happened to be a neuroscientist. I thought the concept was amazing, because it offered a way to overcome those limitations.
Mixed augmented reality allows us to bring all those feared objects to the clinic. I can bring my Labrador to the office for someone who’s afraid of dogs, and they can get the exposure to that one dog. But we know good exposure therapy needs to be generalizable, with as many different breeds of dogs as possible, and is context dependent. If the patient sees a dog in their neighborhood, their fear response may come back. Doing it in a real-life context, and offering as many contexts as possible, makes it more effective.
Augmented reality allows all of these options because you can have as many different types of virtual objects as you want, and the difference between augmented reality and virtual reality is that augmented reality happens in a real-life context. You wear the goggles and you can walk around the environment and track the object, so the context is more realistic.
When did you begin researching augmented reality as a clinical tool?
I became a faculty member here in 2015, right out of my residency training, and I think it was around 2016 or 2017 that we began this work.
I’m very much involved in exposure therapy, utilize it myself, train others, and research how it works and changes the brain. I knew the ins and outs and what would make a better exposure therapy, based on my knowledge of neuroscience.
We spend time thinking about how we can apply these neuroscientific principles in software that can also be easily used by a not very technologically savvy therapist. Because that has been a big barrier when it comes to technology and human use in medicine.
Initially, we had a company create the software for us, but we’ve since brought all the programming inside.
The cool thing about these augmented reality devices is that they have excellent surface mapping. As soon as the person wears the goggles, it automatically maps the surfaces and provides a 3D view of the patient’s environment on the therapist’s computer. Say you’re treating a patient with a fear of spiders. Through drop-down menus, the therapist can choose what type of spider, its color and size, where it should be placed, and the motion. I can choose to move the spider from 6 feet away on the floor to the walls to the ceiling.
Virtual phobias, real fear
A big question for a lot of people was if the spiders are virtual, will they be scary, because it has to be realistic enough to create a fear response for the therapy to work. We use a couple of wires that you can put on a person’s finger and hook them up to a tablet or a cell phone. This provides an online measure of a person’s autonomic sympathetic response.
Like a lie detector test?
Exactly. We put that on their fingers and exposed them to a real-life tarantula and to our virtual tarantulas, and the fear response was no different. That means these do create an objective fear reaction in the body.
We also had people who said, “I know this is not real. I won’t be scared.” And when we started the therapy, it was with a tiny spider 5 meters away from them, and they’d lift their legs off the floor.
With the treatment, we’d come to one room and start with a very little spider, far from them. Then gradually we move them up to bigger, more diverse types of spiders, which are moving around. The patient comes near and tries to touch them.
Then at some point, I’d put a spiderweb on the door, put a few spiders on that, open the door, and have the patient walk through it. They kept walking through this spiderweb.
When they were desensitized to these spiders in this context – and as I said, context is important – we’d go to another room. This was darker, more like a basement, and we’d continue the same thing. That would actually take much less time because they already had desensitized a lot.
In our field, sense of control is very important, especially for when a patient goes home. So at the end, I’d leave the room and talk to the patient via a baby monitor. The patient was surrounded by 20 tarantulas, without the prompt moving around the environment.
Now that they’re desensitized to my virtual spiders, the question is, how would that apply to a real spider? So, we had a real live tarantula, whose name was Tony Stark, because we’re the STARC lab. We’d put Tony at the end of a long hallway before the treatment and see how close the patients could get to him.
It was only one treatment session; nobody’s was longer than 1 hour, and the average treatment time was 38 minutes.
That’s pretty effective.
It’s pretty good, compared with other studies. And I believe this is because of all the components I mentioned: being able to use your real environment; combining it with the real tarantula; the variety of the types of the feared objects; and, of course, giving the patient a sense of autonomy at the end.
Then we had to see how prolonged the effects are. We had them come in 1 week and 1 month after the treatment. I’d remind them of the principles of good exposure therapy and ask them to keep practicing at home between the sessions, looking at pictures and videos. But we never tested who did or did not do it.
After 1 week and 1 month, the effects were either the same or better. A larger number of people at 1 month were able to touch the tarantula than right after treatment.
Treating PTSD in first responders
Did you start with spiders and dogs because those are common fears?
We started with spiders because that worked with the initial goal of creating a prototype. Spiders’ behavior is simple enough for the programming, which takes a lot of time. Another reason for choosing spiders was that we had a lot of other studies of real and virtual reality exposure therapy to compare against.
I think another reason for our success is that, when you do real exposure therapy, you have just one scared tarantula in the corner of their tank, and they don’t listen to you. But my spiders listen to me and do exactly what I tell them.
After our initial success, we obtained more funds to expand it to other phobias. The cool thing is that we don’t need separate software for different phobias. You can choose dogs or snakes, add it to the person’s environment, and decide their behaviors.
We just started a clinical trial using dogs, and another group in Turkey is running a clinical trial with dogs. Eastern Michigan University is working with spiders. And a clinic at the University of Nebraska Medical Center is going to start using them in real-world clinics, not for research.
We have another project whose goal is helping reduce the impact of trauma and also treating PTSD in first responders, who are exposed to a lot of horrible things. Rates of PTSD are around 20%-30% among cops, firefighters, and EMS personnel.
They commonly find it very painful being in crowds because the fight-or-flight instinct in the brain is constantly screening for any sign of threat in their environment. We’re working on them walking into an empty room wearing the goggles, and then their therapist can scale the stimulus up and down.
There’ll be two people in front of you talking to each other, and then another group comes in, and people get louder. People can look at you and talk to you. There’s kids running, Fourth of July fireworks, and other things that might bother someone who’s been involved in gun- or explosion-related traumas. You gradually scale up when the person is next to their therapist.
Another thing we’re doing is related to cardiopulmonary resuscitation. If a young person dies in a CPR situation, that is really painful and traumatic. So, for exposure therapy to that, we’re creating a difficult CPR scenario when that person may die. The responder wears the goggles and basically watches a group of people doing CPR while standing next to a therapist who can help them navigate it and then scale it off.
Another goal is combining this with telemedicine, where the person can do it in their real-life environment. Imagine a person with military trauma. You can put them back in the barracks, connected with their psychiatrists via telemedicine. Then we would put humans in military fatigues near them and have them interact with them to feel comfortable with that situation.
What else is next for you and your group?
The next biggest challenge that we’re tackling is PTSD, because of course creating human-encounter scenarios is much more complicated than spiders and dogs. We’re in the midst of developing this so we can basically bring it to people’s homes.
We’ve been working with some military personnel to see if we can basically give a device to a veteran with PTSD, so they can go home and practice on their own.
There’s another possibility for training. Let’s take the example of a police force, which can have a lot of difficulties and mistakes because of lack of exposure and training. They can wear these goggles, get fully geared up, and be placed in encounters with people of different backgrounds, of different severity, with people who could be severely mentally ill or present different challenges for the officers.
Those situations can teach them a lot. I’m the creator of this thing, but even I’m often surprised by how realistic this technology can be. I find myself interacting with avatars the same way I would if they were real humans. I actually had one of my colleagues, when we started launching the programming with the dogs, immediately jump back. It’s just like the animal brain reacts to them.
Last question: Do you actually interact with Tony, the tarantula?
Oh, Tony is my friend. Unfortunately, he’s not with our lab at this moment. He’s on a sabbatical at Eastern Michigan University for their clinical trials. But yes, I’ve held him. He’s very friendly.
A version of this article first appeared on Medscape.com.
At Wayne State University’s Stress, Trauma, and Anxiety Research Clinic (STARC) in Michigan, researchers are developing novel interventions for treating some very ancient phobias hardwired into the human brain. By using augmented reality as means of conducting exposure therapy, STARC researchers – including Shantanu Madaboosi, Rakesh Ramaswamy, and Lana Grasser – and STARC director Arash Javanbakht, MD, have produced compelling evidence that they can free patients of their often debilitating fears of spiders, dogs, and snakes. Yet their work doesn’t stop there, and research into treating anxiety and posttraumatic stress disorder among first responders and others with high-stress occupations is ongoing.

This news organization spoke with Dr. Javanbakht, a psychiatrist, about the technological advances that have made this work possible; the future of remote-based psychiatry; and his tarantula colleague, Tony.
Augmenting exposure therapy
How did you begin using artificial intelligence as a way of delivering exposure therapy?
Exposure therapy is a very effective treatment for phobias, obsessive-compulsive disorder, and PTSD. But the problem we had is that, if someone comes to me and says they’re afraid of dogs, snakes, or spiders, I don’t have those in my office. Or, if its social phobia, I can’t create those scenarios. So, despite being such an effective treatment, it’s not utilized as much as it should be.
Several years ago, I saw a TED talk by the CEO of an augmented reality company who happened to be a neuroscientist. I thought the concept was amazing, because it offered a way to overcome those limitations.
Mixed augmented reality allows us to bring all those feared objects to the clinic. I can bring my Labrador to the office for someone who’s afraid of dogs, and they can get the exposure to that one dog. But we know good exposure therapy needs to be generalizable, with as many different breeds of dogs as possible, and is context dependent. If the patient sees a dog in their neighborhood, their fear response may come back. Doing it in a real-life context, and offering as many contexts as possible, makes it more effective.
Augmented reality allows all of these options because you can have as many different types of virtual objects as you want, and the difference between augmented reality and virtual reality is that augmented reality happens in a real-life context. You wear the goggles and you can walk around the environment and track the object, so the context is more realistic.
When did you begin researching augmented reality as a clinical tool?
I became a faculty member here in 2015, right out of my residency training, and I think it was around 2016 or 2017 that we began this work.
I’m very much involved in exposure therapy, utilize it myself, train others, and research how it works and changes the brain. I knew the ins and outs and what would make a better exposure therapy, based on my knowledge of neuroscience.
We spend time thinking about how we can apply these neuroscientific principles in software that can also be easily used by a not very technologically savvy therapist. Because that has been a big barrier when it comes to technology and human use in medicine.
Initially, we had a company create the software for us, but we’ve since brought all the programming inside.
The cool thing about these augmented reality devices is that they have excellent surface mapping. As soon as the person wears the goggles, it automatically maps the surfaces and provides a 3D view of the patient’s environment on the therapist’s computer. Say you’re treating a patient with a fear of spiders. Through drop-down menus, the therapist can choose what type of spider, its color and size, where it should be placed, and the motion. I can choose to move the spider from 6 feet away on the floor to the walls to the ceiling.
Virtual phobias, real fear
A big question for a lot of people was if the spiders are virtual, will they be scary, because it has to be realistic enough to create a fear response for the therapy to work. We use a couple of wires that you can put on a person’s finger and hook them up to a tablet or a cell phone. This provides an online measure of a person’s autonomic sympathetic response.
Like a lie detector test?
Exactly. We put that on their fingers and exposed them to a real-life tarantula and to our virtual tarantulas, and the fear response was no different. That means these do create an objective fear reaction in the body.
We also had people who said, “I know this is not real. I won’t be scared.” And when we started the therapy, it was with a tiny spider 5 meters away from them, and they’d lift their legs off the floor.
With the treatment, we’d come to one room and start with a very little spider, far from them. Then gradually we move them up to bigger, more diverse types of spiders, which are moving around. The patient comes near and tries to touch them.
Then at some point, I’d put a spiderweb on the door, put a few spiders on that, open the door, and have the patient walk through it. They kept walking through this spiderweb.
When they were desensitized to these spiders in this context – and as I said, context is important – we’d go to another room. This was darker, more like a basement, and we’d continue the same thing. That would actually take much less time because they already had desensitized a lot.
In our field, sense of control is very important, especially for when a patient goes home. So at the end, I’d leave the room and talk to the patient via a baby monitor. The patient was surrounded by 20 tarantulas, without the prompt moving around the environment.
Now that they’re desensitized to my virtual spiders, the question is, how would that apply to a real spider? So, we had a real live tarantula, whose name was Tony Stark, because we’re the STARC lab. We’d put Tony at the end of a long hallway before the treatment and see how close the patients could get to him.
It was only one treatment session; nobody’s was longer than 1 hour, and the average treatment time was 38 minutes.
That’s pretty effective.
It’s pretty good, compared with other studies. And I believe this is because of all the components I mentioned: being able to use your real environment; combining it with the real tarantula; the variety of the types of the feared objects; and, of course, giving the patient a sense of autonomy at the end.
Then we had to see how prolonged the effects are. We had them come in 1 week and 1 month after the treatment. I’d remind them of the principles of good exposure therapy and ask them to keep practicing at home between the sessions, looking at pictures and videos. But we never tested who did or did not do it.
After 1 week and 1 month, the effects were either the same or better. A larger number of people at 1 month were able to touch the tarantula than right after treatment.
Treating PTSD in first responders
Did you start with spiders and dogs because those are common fears?
We started with spiders because that worked with the initial goal of creating a prototype. Spiders’ behavior is simple enough for the programming, which takes a lot of time. Another reason for choosing spiders was that we had a lot of other studies of real and virtual reality exposure therapy to compare against.
I think another reason for our success is that, when you do real exposure therapy, you have just one scared tarantula in the corner of their tank, and they don’t listen to you. But my spiders listen to me and do exactly what I tell them.
After our initial success, we obtained more funds to expand it to other phobias. The cool thing is that we don’t need separate software for different phobias. You can choose dogs or snakes, add it to the person’s environment, and decide their behaviors.
We just started a clinical trial using dogs, and another group in Turkey is running a clinical trial with dogs. Eastern Michigan University is working with spiders. And a clinic at the University of Nebraska Medical Center is going to start using them in real-world clinics, not for research.
We have another project whose goal is helping reduce the impact of trauma and also treating PTSD in first responders, who are exposed to a lot of horrible things. Rates of PTSD are around 20%-30% among cops, firefighters, and EMS personnel.
They commonly find it very painful being in crowds because the fight-or-flight instinct in the brain is constantly screening for any sign of threat in their environment. We’re working on them walking into an empty room wearing the goggles, and then their therapist can scale the stimulus up and down.
There’ll be two people in front of you talking to each other, and then another group comes in, and people get louder. People can look at you and talk to you. There’s kids running, Fourth of July fireworks, and other things that might bother someone who’s been involved in gun- or explosion-related traumas. You gradually scale up when the person is next to their therapist.
Another thing we’re doing is related to cardiopulmonary resuscitation. If a young person dies in a CPR situation, that is really painful and traumatic. So, for exposure therapy to that, we’re creating a difficult CPR scenario when that person may die. The responder wears the goggles and basically watches a group of people doing CPR while standing next to a therapist who can help them navigate it and then scale it off.
Another goal is combining this with telemedicine, where the person can do it in their real-life environment. Imagine a person with military trauma. You can put them back in the barracks, connected with their psychiatrists via telemedicine. Then we would put humans in military fatigues near them and have them interact with them to feel comfortable with that situation.
What else is next for you and your group?
The next biggest challenge that we’re tackling is PTSD, because of course creating human-encounter scenarios is much more complicated than spiders and dogs. We’re in the midst of developing this so we can basically bring it to people’s homes.
We’ve been working with some military personnel to see if we can basically give a device to a veteran with PTSD, so they can go home and practice on their own.
There’s another possibility for training. Let’s take the example of a police force, which can have a lot of difficulties and mistakes because of lack of exposure and training. They can wear these goggles, get fully geared up, and be placed in encounters with people of different backgrounds, of different severity, with people who could be severely mentally ill or present different challenges for the officers.
Those situations can teach them a lot. I’m the creator of this thing, but even I’m often surprised by how realistic this technology can be. I find myself interacting with avatars the same way I would if they were real humans. I actually had one of my colleagues, when we started launching the programming with the dogs, immediately jump back. It’s just like the animal brain reacts to them.
Last question: Do you actually interact with Tony, the tarantula?
Oh, Tony is my friend. Unfortunately, he’s not with our lab at this moment. He’s on a sabbatical at Eastern Michigan University for their clinical trials. But yes, I’ve held him. He’s very friendly.
A version of this article first appeared on Medscape.com.
At Wayne State University’s Stress, Trauma, and Anxiety Research Clinic (STARC) in Michigan, researchers are developing novel interventions for treating some very ancient phobias hardwired into the human brain. By using augmented reality as means of conducting exposure therapy, STARC researchers – including Shantanu Madaboosi, Rakesh Ramaswamy, and Lana Grasser – and STARC director Arash Javanbakht, MD, have produced compelling evidence that they can free patients of their often debilitating fears of spiders, dogs, and snakes. Yet their work doesn’t stop there, and research into treating anxiety and posttraumatic stress disorder among first responders and others with high-stress occupations is ongoing.

This news organization spoke with Dr. Javanbakht, a psychiatrist, about the technological advances that have made this work possible; the future of remote-based psychiatry; and his tarantula colleague, Tony.
Augmenting exposure therapy
How did you begin using artificial intelligence as a way of delivering exposure therapy?
Exposure therapy is a very effective treatment for phobias, obsessive-compulsive disorder, and PTSD. But the problem we had is that, if someone comes to me and says they’re afraid of dogs, snakes, or spiders, I don’t have those in my office. Or, if its social phobia, I can’t create those scenarios. So, despite being such an effective treatment, it’s not utilized as much as it should be.
Several years ago, I saw a TED talk by the CEO of an augmented reality company who happened to be a neuroscientist. I thought the concept was amazing, because it offered a way to overcome those limitations.
Mixed augmented reality allows us to bring all those feared objects to the clinic. I can bring my Labrador to the office for someone who’s afraid of dogs, and they can get the exposure to that one dog. But we know good exposure therapy needs to be generalizable, with as many different breeds of dogs as possible, and is context dependent. If the patient sees a dog in their neighborhood, their fear response may come back. Doing it in a real-life context, and offering as many contexts as possible, makes it more effective.
Augmented reality allows all of these options because you can have as many different types of virtual objects as you want, and the difference between augmented reality and virtual reality is that augmented reality happens in a real-life context. You wear the goggles and you can walk around the environment and track the object, so the context is more realistic.
When did you begin researching augmented reality as a clinical tool?
I became a faculty member here in 2015, right out of my residency training, and I think it was around 2016 or 2017 that we began this work.
I’m very much involved in exposure therapy, utilize it myself, train others, and research how it works and changes the brain. I knew the ins and outs and what would make a better exposure therapy, based on my knowledge of neuroscience.
We spend time thinking about how we can apply these neuroscientific principles in software that can also be easily used by a not very technologically savvy therapist. Because that has been a big barrier when it comes to technology and human use in medicine.
Initially, we had a company create the software for us, but we’ve since brought all the programming inside.
The cool thing about these augmented reality devices is that they have excellent surface mapping. As soon as the person wears the goggles, it automatically maps the surfaces and provides a 3D view of the patient’s environment on the therapist’s computer. Say you’re treating a patient with a fear of spiders. Through drop-down menus, the therapist can choose what type of spider, its color and size, where it should be placed, and the motion. I can choose to move the spider from 6 feet away on the floor to the walls to the ceiling.
Virtual phobias, real fear
A big question for a lot of people was if the spiders are virtual, will they be scary, because it has to be realistic enough to create a fear response for the therapy to work. We use a couple of wires that you can put on a person’s finger and hook them up to a tablet or a cell phone. This provides an online measure of a person’s autonomic sympathetic response.
Like a lie detector test?
Exactly. We put that on their fingers and exposed them to a real-life tarantula and to our virtual tarantulas, and the fear response was no different. That means these do create an objective fear reaction in the body.
We also had people who said, “I know this is not real. I won’t be scared.” And when we started the therapy, it was with a tiny spider 5 meters away from them, and they’d lift their legs off the floor.
With the treatment, we’d come to one room and start with a very little spider, far from them. Then gradually we move them up to bigger, more diverse types of spiders, which are moving around. The patient comes near and tries to touch them.
Then at some point, I’d put a spiderweb on the door, put a few spiders on that, open the door, and have the patient walk through it. They kept walking through this spiderweb.
When they were desensitized to these spiders in this context – and as I said, context is important – we’d go to another room. This was darker, more like a basement, and we’d continue the same thing. That would actually take much less time because they already had desensitized a lot.
In our field, sense of control is very important, especially for when a patient goes home. So at the end, I’d leave the room and talk to the patient via a baby monitor. The patient was surrounded by 20 tarantulas, without the prompt moving around the environment.
Now that they’re desensitized to my virtual spiders, the question is, how would that apply to a real spider? So, we had a real live tarantula, whose name was Tony Stark, because we’re the STARC lab. We’d put Tony at the end of a long hallway before the treatment and see how close the patients could get to him.
It was only one treatment session; nobody’s was longer than 1 hour, and the average treatment time was 38 minutes.
That’s pretty effective.
It’s pretty good, compared with other studies. And I believe this is because of all the components I mentioned: being able to use your real environment; combining it with the real tarantula; the variety of the types of the feared objects; and, of course, giving the patient a sense of autonomy at the end.
Then we had to see how prolonged the effects are. We had them come in 1 week and 1 month after the treatment. I’d remind them of the principles of good exposure therapy and ask them to keep practicing at home between the sessions, looking at pictures and videos. But we never tested who did or did not do it.
After 1 week and 1 month, the effects were either the same or better. A larger number of people at 1 month were able to touch the tarantula than right after treatment.
Treating PTSD in first responders
Did you start with spiders and dogs because those are common fears?
We started with spiders because that worked with the initial goal of creating a prototype. Spiders’ behavior is simple enough for the programming, which takes a lot of time. Another reason for choosing spiders was that we had a lot of other studies of real and virtual reality exposure therapy to compare against.
I think another reason for our success is that, when you do real exposure therapy, you have just one scared tarantula in the corner of their tank, and they don’t listen to you. But my spiders listen to me and do exactly what I tell them.
After our initial success, we obtained more funds to expand it to other phobias. The cool thing is that we don’t need separate software for different phobias. You can choose dogs or snakes, add it to the person’s environment, and decide their behaviors.
We just started a clinical trial using dogs, and another group in Turkey is running a clinical trial with dogs. Eastern Michigan University is working with spiders. And a clinic at the University of Nebraska Medical Center is going to start using them in real-world clinics, not for research.
We have another project whose goal is helping reduce the impact of trauma and also treating PTSD in first responders, who are exposed to a lot of horrible things. Rates of PTSD are around 20%-30% among cops, firefighters, and EMS personnel.
They commonly find it very painful being in crowds because the fight-or-flight instinct in the brain is constantly screening for any sign of threat in their environment. We’re working on them walking into an empty room wearing the goggles, and then their therapist can scale the stimulus up and down.
There’ll be two people in front of you talking to each other, and then another group comes in, and people get louder. People can look at you and talk to you. There’s kids running, Fourth of July fireworks, and other things that might bother someone who’s been involved in gun- or explosion-related traumas. You gradually scale up when the person is next to their therapist.
Another thing we’re doing is related to cardiopulmonary resuscitation. If a young person dies in a CPR situation, that is really painful and traumatic. So, for exposure therapy to that, we’re creating a difficult CPR scenario when that person may die. The responder wears the goggles and basically watches a group of people doing CPR while standing next to a therapist who can help them navigate it and then scale it off.
Another goal is combining this with telemedicine, where the person can do it in their real-life environment. Imagine a person with military trauma. You can put them back in the barracks, connected with their psychiatrists via telemedicine. Then we would put humans in military fatigues near them and have them interact with them to feel comfortable with that situation.
What else is next for you and your group?
The next biggest challenge that we’re tackling is PTSD, because of course creating human-encounter scenarios is much more complicated than spiders and dogs. We’re in the midst of developing this so we can basically bring it to people’s homes.
We’ve been working with some military personnel to see if we can basically give a device to a veteran with PTSD, so they can go home and practice on their own.
There’s another possibility for training. Let’s take the example of a police force, which can have a lot of difficulties and mistakes because of lack of exposure and training. They can wear these goggles, get fully geared up, and be placed in encounters with people of different backgrounds, of different severity, with people who could be severely mentally ill or present different challenges for the officers.
Those situations can teach them a lot. I’m the creator of this thing, but even I’m often surprised by how realistic this technology can be. I find myself interacting with avatars the same way I would if they were real humans. I actually had one of my colleagues, when we started launching the programming with the dogs, immediately jump back. It’s just like the animal brain reacts to them.
Last question: Do you actually interact with Tony, the tarantula?
Oh, Tony is my friend. Unfortunately, he’s not with our lab at this moment. He’s on a sabbatical at Eastern Michigan University for their clinical trials. But yes, I’ve held him. He’s very friendly.
A version of this article first appeared on Medscape.com.
The devil in the (masking) details
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.