User login
Atopic Dermatitis: Clinical Outcomes
AGA Section: Gastroenterology and hepatology training milestones
Updated milestones for professional development aim to help specialists in gastroenterology and transplant hepatology achieve knowledge, skills, and attitudes that will help them establish their own practices.
The new version, Milestones 2.0, represents the latest milestones created by the Accreditation Council for Graduate Medical Education, including six core competencies developed initially in 1999: Patient care (PC), medical knowledge (MK), interpersonal and communication skills (ICS), professionalism (PROF), systems-based practice (SBP), and practice-based learning and improvement (PBLI).
In 2013, the Oversight Working Network, working with gastroenterology societies, developed a companion document of 13 entrustable professional activities (EPAs) aimed at gastroenterologists: These include management of various individual disorders such as liver or pancreatic diseases, performance of specific diagnostic procedures, and managing patient adverse events and nutritional status.
Milestones 1.0 encountered some resistance from the graduate education community. Too many of the milestones were deemed to be too vague or were described using language that was too complex. Some viewed the milestones as burdensome, and a review suggested hundreds of different ways to describe ICS and PROF, leading to confusion.
In an effort to improve matters, the ACGME made some changes. The first involved standardizing milestones used for ICS, PROF, SBP, and PBLI so that they could be used across disciplines. They also developed PC and MK milestones tailored to each specialty.
In the latest article on the topic, appearing in Gastroenterology, the authors led by Brijen J. Shah, MD, of the Icahn School of Medicine at Mount Sinai, New York, outlined a second group of changes, which included development of specialty-specific milestones aimed at gastroenterology and transplant hepatology.
Development
The new set of milestones includes 17 for gastroenterology and 16 for transplant hepatology.
There are four PC milestones, which include taking a history and conducting patient examinations, patient management, and two more related to cognitive and technical components of procedures. The MK milestones include competency in gastrointestinal and liver diseases (MK1) and medical reasoning (MK2). These milestones are different from the internal medicine milestones met by graduating residents. MK1 includes specialty-specific disorders and diagnostic, therapeutic, and pharmacologic options for treatment or prevention. MK2 encompasses differential diagnoses and how cognitive bias can influence decision-making, a new concept introduced in Milestones 2.0.
Because the skills represented in the four other core milestones (ICS, PROF, SBP, and PBLI) are “common across specialties,” the authors drafted subcompetencies for these four areas with “harmonized” language for use by every specialty. These harmonized milestones were then tailored for each specialty. An important change occurred with SBP because transplant hepatology poses unique challenges in this domain. They ultimately split SBP into two, with SBP1 focusing on unique liver transplant regulatory requirements and SBP2 covering organ allocation and Model for End-Stage Liver Disease (MELD) score exceptions.
Public response
The researchers sought out comment on the updated milestones from program directors and coordinators, and published on the ACGME website, and members of the working group also shared it with faculty, fellows, and specialty societies. Overall, 48 respondents assessed “whether the updated milestone provided a realistic measure of knowledge, skills, and behavior; whether it discriminated between different levels of competency; whether the respondent knew how to assess the milestone effectively; and whether the Supplemental Guide was a useful resource in understanding the milestone.” They rated each on a scale of 1 (strongly disagree) to 4 (strongly agree). They could also provide free-text comments.
Respondents agreed that milestones realistically measure progression (mean, 3.49), could distinguish levels of competency (mean, 3.41), could be used accurately (mean, 3.43), and were explained well by the supplemental guide (mean, 3.42). No trends that suggested a need for additional action were found in the free-text comments.
Role of milestones
The milestones can be used to develop learning objectives, which in turn can be worked into clinical rotations and learning activities. For instance, the inpatient consult rotation could be used to address the SBP2 (organ allocation/MELD score exemptions), SBP3 (the physician’s role in the health care system), PBLI1 (evidence-based medicine), and some of the PC (patient care) milestones.
The milestones should not be used as an assessment method by supervisors, the authors cautioned, but rather should be used by the Clinical Competency Committee to assess trainees at various time points. The committee may combine milestones with direct observation, chart-simulated recall, multiple evaluations, and other factors to determine a trainee’s progress.
An institution’s program directors can use the milestones to adjust curriculum development and ensure that any gaps are filled. Milestones can be used at multiple times throughout training: When trainees repeat rotations, they can be used to determine year-to-year progress. Trainees who are not progressing adequately may be identified earlier on, then offered supplemental learning opportunities. On the other hand, trainees who exceed expectations may be offered additional opportunities.
Trainees can also use milestones in self-directed learning, though they should work with the program director and clinical faculty to identify gaps in their learning as well as any deficiencies.
The authors have no relevant financial disclosures.
Updated milestones for professional development aim to help specialists in gastroenterology and transplant hepatology achieve knowledge, skills, and attitudes that will help them establish their own practices.
The new version, Milestones 2.0, represents the latest milestones created by the Accreditation Council for Graduate Medical Education, including six core competencies developed initially in 1999: Patient care (PC), medical knowledge (MK), interpersonal and communication skills (ICS), professionalism (PROF), systems-based practice (SBP), and practice-based learning and improvement (PBLI).
In 2013, the Oversight Working Network, working with gastroenterology societies, developed a companion document of 13 entrustable professional activities (EPAs) aimed at gastroenterologists: These include management of various individual disorders such as liver or pancreatic diseases, performance of specific diagnostic procedures, and managing patient adverse events and nutritional status.
Milestones 1.0 encountered some resistance from the graduate education community. Too many of the milestones were deemed to be too vague or were described using language that was too complex. Some viewed the milestones as burdensome, and a review suggested hundreds of different ways to describe ICS and PROF, leading to confusion.
In an effort to improve matters, the ACGME made some changes. The first involved standardizing milestones used for ICS, PROF, SBP, and PBLI so that they could be used across disciplines. They also developed PC and MK milestones tailored to each specialty.
In the latest article on the topic, appearing in Gastroenterology, the authors led by Brijen J. Shah, MD, of the Icahn School of Medicine at Mount Sinai, New York, outlined a second group of changes, which included development of specialty-specific milestones aimed at gastroenterology and transplant hepatology.
Development
The new set of milestones includes 17 for gastroenterology and 16 for transplant hepatology.
There are four PC milestones, which include taking a history and conducting patient examinations, patient management, and two more related to cognitive and technical components of procedures. The MK milestones include competency in gastrointestinal and liver diseases (MK1) and medical reasoning (MK2). These milestones are different from the internal medicine milestones met by graduating residents. MK1 includes specialty-specific disorders and diagnostic, therapeutic, and pharmacologic options for treatment or prevention. MK2 encompasses differential diagnoses and how cognitive bias can influence decision-making, a new concept introduced in Milestones 2.0.
Because the skills represented in the four other core milestones (ICS, PROF, SBP, and PBLI) are “common across specialties,” the authors drafted subcompetencies for these four areas with “harmonized” language for use by every specialty. These harmonized milestones were then tailored for each specialty. An important change occurred with SBP because transplant hepatology poses unique challenges in this domain. They ultimately split SBP into two, with SBP1 focusing on unique liver transplant regulatory requirements and SBP2 covering organ allocation and Model for End-Stage Liver Disease (MELD) score exceptions.
Public response
The researchers sought out comment on the updated milestones from program directors and coordinators, and published on the ACGME website, and members of the working group also shared it with faculty, fellows, and specialty societies. Overall, 48 respondents assessed “whether the updated milestone provided a realistic measure of knowledge, skills, and behavior; whether it discriminated between different levels of competency; whether the respondent knew how to assess the milestone effectively; and whether the Supplemental Guide was a useful resource in understanding the milestone.” They rated each on a scale of 1 (strongly disagree) to 4 (strongly agree). They could also provide free-text comments.
Respondents agreed that milestones realistically measure progression (mean, 3.49), could distinguish levels of competency (mean, 3.41), could be used accurately (mean, 3.43), and were explained well by the supplemental guide (mean, 3.42). No trends that suggested a need for additional action were found in the free-text comments.
Role of milestones
The milestones can be used to develop learning objectives, which in turn can be worked into clinical rotations and learning activities. For instance, the inpatient consult rotation could be used to address the SBP2 (organ allocation/MELD score exemptions), SBP3 (the physician’s role in the health care system), PBLI1 (evidence-based medicine), and some of the PC (patient care) milestones.
The milestones should not be used as an assessment method by supervisors, the authors cautioned, but rather should be used by the Clinical Competency Committee to assess trainees at various time points. The committee may combine milestones with direct observation, chart-simulated recall, multiple evaluations, and other factors to determine a trainee’s progress.
An institution’s program directors can use the milestones to adjust curriculum development and ensure that any gaps are filled. Milestones can be used at multiple times throughout training: When trainees repeat rotations, they can be used to determine year-to-year progress. Trainees who are not progressing adequately may be identified earlier on, then offered supplemental learning opportunities. On the other hand, trainees who exceed expectations may be offered additional opportunities.
Trainees can also use milestones in self-directed learning, though they should work with the program director and clinical faculty to identify gaps in their learning as well as any deficiencies.
The authors have no relevant financial disclosures.
Updated milestones for professional development aim to help specialists in gastroenterology and transplant hepatology achieve knowledge, skills, and attitudes that will help them establish their own practices.
The new version, Milestones 2.0, represents the latest milestones created by the Accreditation Council for Graduate Medical Education, including six core competencies developed initially in 1999: Patient care (PC), medical knowledge (MK), interpersonal and communication skills (ICS), professionalism (PROF), systems-based practice (SBP), and practice-based learning and improvement (PBLI).
In 2013, the Oversight Working Network, working with gastroenterology societies, developed a companion document of 13 entrustable professional activities (EPAs) aimed at gastroenterologists: These include management of various individual disorders such as liver or pancreatic diseases, performance of specific diagnostic procedures, and managing patient adverse events and nutritional status.
Milestones 1.0 encountered some resistance from the graduate education community. Too many of the milestones were deemed to be too vague or were described using language that was too complex. Some viewed the milestones as burdensome, and a review suggested hundreds of different ways to describe ICS and PROF, leading to confusion.
In an effort to improve matters, the ACGME made some changes. The first involved standardizing milestones used for ICS, PROF, SBP, and PBLI so that they could be used across disciplines. They also developed PC and MK milestones tailored to each specialty.
In the latest article on the topic, appearing in Gastroenterology, the authors led by Brijen J. Shah, MD, of the Icahn School of Medicine at Mount Sinai, New York, outlined a second group of changes, which included development of specialty-specific milestones aimed at gastroenterology and transplant hepatology.
Development
The new set of milestones includes 17 for gastroenterology and 16 for transplant hepatology.
There are four PC milestones, which include taking a history and conducting patient examinations, patient management, and two more related to cognitive and technical components of procedures. The MK milestones include competency in gastrointestinal and liver diseases (MK1) and medical reasoning (MK2). These milestones are different from the internal medicine milestones met by graduating residents. MK1 includes specialty-specific disorders and diagnostic, therapeutic, and pharmacologic options for treatment or prevention. MK2 encompasses differential diagnoses and how cognitive bias can influence decision-making, a new concept introduced in Milestones 2.0.
Because the skills represented in the four other core milestones (ICS, PROF, SBP, and PBLI) are “common across specialties,” the authors drafted subcompetencies for these four areas with “harmonized” language for use by every specialty. These harmonized milestones were then tailored for each specialty. An important change occurred with SBP because transplant hepatology poses unique challenges in this domain. They ultimately split SBP into two, with SBP1 focusing on unique liver transplant regulatory requirements and SBP2 covering organ allocation and Model for End-Stage Liver Disease (MELD) score exceptions.
Public response
The researchers sought out comment on the updated milestones from program directors and coordinators, and published on the ACGME website, and members of the working group also shared it with faculty, fellows, and specialty societies. Overall, 48 respondents assessed “whether the updated milestone provided a realistic measure of knowledge, skills, and behavior; whether it discriminated between different levels of competency; whether the respondent knew how to assess the milestone effectively; and whether the Supplemental Guide was a useful resource in understanding the milestone.” They rated each on a scale of 1 (strongly disagree) to 4 (strongly agree). They could also provide free-text comments.
Respondents agreed that milestones realistically measure progression (mean, 3.49), could distinguish levels of competency (mean, 3.41), could be used accurately (mean, 3.43), and were explained well by the supplemental guide (mean, 3.42). No trends that suggested a need for additional action were found in the free-text comments.
Role of milestones
The milestones can be used to develop learning objectives, which in turn can be worked into clinical rotations and learning activities. For instance, the inpatient consult rotation could be used to address the SBP2 (organ allocation/MELD score exemptions), SBP3 (the physician’s role in the health care system), PBLI1 (evidence-based medicine), and some of the PC (patient care) milestones.
The milestones should not be used as an assessment method by supervisors, the authors cautioned, but rather should be used by the Clinical Competency Committee to assess trainees at various time points. The committee may combine milestones with direct observation, chart-simulated recall, multiple evaluations, and other factors to determine a trainee’s progress.
An institution’s program directors can use the milestones to adjust curriculum development and ensure that any gaps are filled. Milestones can be used at multiple times throughout training: When trainees repeat rotations, they can be used to determine year-to-year progress. Trainees who are not progressing adequately may be identified earlier on, then offered supplemental learning opportunities. On the other hand, trainees who exceed expectations may be offered additional opportunities.
Trainees can also use milestones in self-directed learning, though they should work with the program director and clinical faculty to identify gaps in their learning as well as any deficiencies.
The authors have no relevant financial disclosures.
FROM GASTROENTEROLOGY
Adalimumab biosimilar Cyltezo gets interchangeability designation
The Food and Drug Administration approved a supplement to the biologics license application of the adalimumab biosimilar drug Cyltezo (adalimumab-adbm) that makes it the first interchangeable biosimilar with Humira (adalimumab), the original branded version of the drug, its manufacturer Boehringer Ingelheim announced Oct. 15.
The FDA originally approved Cyltezo in 2017 for the treatment of multiple chronic inflammatory diseases, including seven of Humira’s nine indications for adults and pediatric patients: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis.
The interchangeability designation means that Cyltezo was tested in an additional clinical trial in which patients were successfully switched back and forth multiple times from Humira to Cyltezo and allows pharmacists to autosubstitute Humira with Cyltezo. In these cases, individual state laws control how and whether physicians will be notified of this switch.
Cyltezo is just the second biosimilar to be designated as interchangeable with its originator biologic product. The first approval, announced July 28, was for the interchangeability of Semglee (insulin glargine-yfgn) with the originator Lantus.
The agency based its decision on positive data from the VOLTAIRE-X study of 238 patients with moderate to severe chronic plaque psoriasis in which Cyltezo had no meaningful clinical differences from Humira in pharmacokinetics, efficacy, immunogenicity, and safety between the switching and continuous treatment groups.
Cyltezo will not be commercially available in the United States until July 1, 2023, according to Boehringer Ingelheim.
The Food and Drug Administration approved a supplement to the biologics license application of the adalimumab biosimilar drug Cyltezo (adalimumab-adbm) that makes it the first interchangeable biosimilar with Humira (adalimumab), the original branded version of the drug, its manufacturer Boehringer Ingelheim announced Oct. 15.
The FDA originally approved Cyltezo in 2017 for the treatment of multiple chronic inflammatory diseases, including seven of Humira’s nine indications for adults and pediatric patients: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis.
The interchangeability designation means that Cyltezo was tested in an additional clinical trial in which patients were successfully switched back and forth multiple times from Humira to Cyltezo and allows pharmacists to autosubstitute Humira with Cyltezo. In these cases, individual state laws control how and whether physicians will be notified of this switch.
Cyltezo is just the second biosimilar to be designated as interchangeable with its originator biologic product. The first approval, announced July 28, was for the interchangeability of Semglee (insulin glargine-yfgn) with the originator Lantus.
The agency based its decision on positive data from the VOLTAIRE-X study of 238 patients with moderate to severe chronic plaque psoriasis in which Cyltezo had no meaningful clinical differences from Humira in pharmacokinetics, efficacy, immunogenicity, and safety between the switching and continuous treatment groups.
Cyltezo will not be commercially available in the United States until July 1, 2023, according to Boehringer Ingelheim.
The Food and Drug Administration approved a supplement to the biologics license application of the adalimumab biosimilar drug Cyltezo (adalimumab-adbm) that makes it the first interchangeable biosimilar with Humira (adalimumab), the original branded version of the drug, its manufacturer Boehringer Ingelheim announced Oct. 15.
The FDA originally approved Cyltezo in 2017 for the treatment of multiple chronic inflammatory diseases, including seven of Humira’s nine indications for adults and pediatric patients: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis.
The interchangeability designation means that Cyltezo was tested in an additional clinical trial in which patients were successfully switched back and forth multiple times from Humira to Cyltezo and allows pharmacists to autosubstitute Humira with Cyltezo. In these cases, individual state laws control how and whether physicians will be notified of this switch.
Cyltezo is just the second biosimilar to be designated as interchangeable with its originator biologic product. The first approval, announced July 28, was for the interchangeability of Semglee (insulin glargine-yfgn) with the originator Lantus.
The agency based its decision on positive data from the VOLTAIRE-X study of 238 patients with moderate to severe chronic plaque psoriasis in which Cyltezo had no meaningful clinical differences from Humira in pharmacokinetics, efficacy, immunogenicity, and safety between the switching and continuous treatment groups.
Cyltezo will not be commercially available in the United States until July 1, 2023, according to Boehringer Ingelheim.
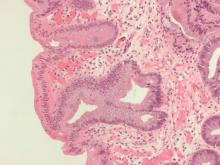
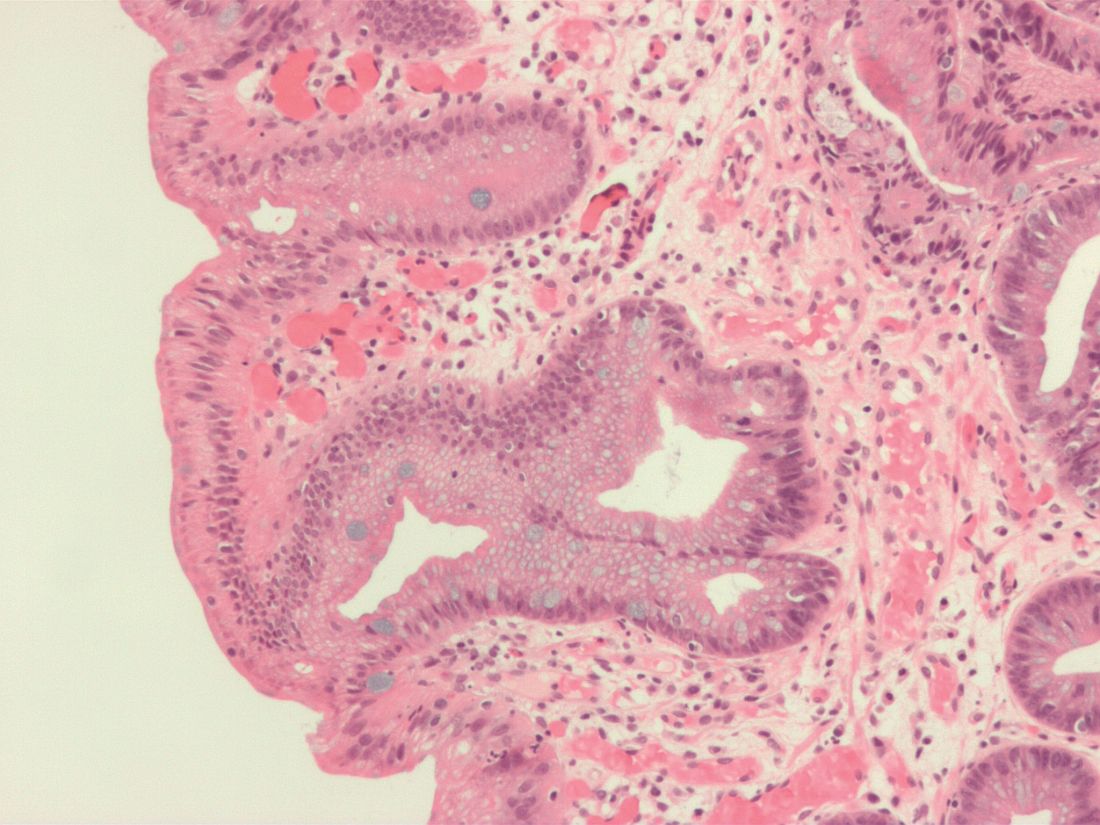
AGA Clinical Practice Update: Expert review on atrophic gastritis
A new clinical practice update expert review for the diagnosis and management of atrophic gastritis (AG) from the American Gastroenterological Association focuses on cases linked to Helicobacter pylori infection or autoimmunity.
This update addresses a sparsity of guidelines for AG in the United States and should be seen as complementary to the AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia, according to the authors led by Shailja C. Shah, MD, MPH, of the gastroenterology section at Veterans Affairs San Diego Healthcare System and the division of gastroenterology at the University of California, San Diego.
The 2020 guidelines didn’t specifically discuss diagnosis and management of AG; however, a diagnosis of intestinal metaplasia based on gastric histopathology indicates the presence AG since metaplasia occurs in atrophic mucosa. Nevertheless, AG often goes unmentioned in histopathology reports. Such omissions are important because AG is an important stage in the potential development of gastric cancer.
AG is believed to result from genetic and environmental factors. The two primary triggers for the condition are H. pylori infection (HpAG) and autoimmunity (AIG). The condition results from chronic inflammation and replacement of normal gastric glandular structures with connective tissue or nonnative epithelium. It can proceed to other precancerous conditions, including gastric intestinal metaplasia and dysplasia. An estimated 15% of the U.S. population has AG, according to the authors, although this prevalence could be higher in populations with higher rates of H. pylori infection. AIG is rare, occurring in 0.5%-2% of the U.S. population.
Among individuals with AG, 0.1%-0.3% per year go on to develop gastric adenocarcinoma, though additional factors could heighten this risk. Furthermore, 0.4%-0.7% per year go on develop type 1 neuroendocrine tumors.
HpAG and AIG have different patterns of mucosal involvement. During diagnosis, the authors advised careful mucosal visualization with air insufflation and mucosal cleansing. High-definition white-light endoscopy is more sensitive than traditional WLE in the identification of premalignant mucosal changes.
AG diagnosis should be confirmed by histopathology. The updated Sydney protocol should be used to obtain biopsies, and serum pepsinogens can be used to identify extensive atrophy, though this testing is not generally available in the United States for clinical use. When histology results are suggestive of AIG, the presence of parietal cell antibodies and intrinsic factor antibodies can contribute to a diagnosis, although the former can be prone to false positives because of H. pylori infection, and the latter has low sensitivity.
Patients identified with AG should be tested for H. pylori and treated for infection, followed by nonserologic testing to confirm treatment success. If H. pylori is present, successful eradication may allow for reversal of AG to normal gastric mucosa; however, patients may have irreversible changes. This could leave them at elevated risk of further progression, though elimination of H. pylori does appear to blunt that risk somewhat.
Neoplastic complications from AG are rare, and the benefits of surveillance among those with AG have not been demonstrated in prospective trials. Observational trials show that severe AG is associated with greater risk of gastric adenocarcinoma, and other factors, such as comorbidities and patient values and priorities, should inform decision-making. When called for, providers should consider surveillance endoscopies every 3 years, though the authors noted that the optimal surveillance interval is unknown. Factors such as the quality of the original endoscopy, family history of gastric cancer, and a history of immigration from regions with high rates of H. pylori infection may impact decisions on surveillance intervals.
AG can lead to iron or vitamin B12 deficiency, so patients with AG, especially those with corpus-predominant AG, should be evaluated for both. AG should also be considered as a differential diagnosis in patients presenting with either deficiency.
A diagnosis of AIG should be accompanied by screening for autoimmune thyroid disease, and type 1 diabetes or Addison’s disease may also be indicated if clinical presentation is consistent.
Because AG is commonly underdiagnosed, the authors advise that gastroenterologists and pathologists should improve coordination to maximize diagnosis of the condition, and they call for comparative clinical trials to improve risk stratification algorithms and surveillance strategies.
The authors disclose no relevant conflicts of interest.
A new clinical practice update expert review for the diagnosis and management of atrophic gastritis (AG) from the American Gastroenterological Association focuses on cases linked to Helicobacter pylori infection or autoimmunity.
This update addresses a sparsity of guidelines for AG in the United States and should be seen as complementary to the AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia, according to the authors led by Shailja C. Shah, MD, MPH, of the gastroenterology section at Veterans Affairs San Diego Healthcare System and the division of gastroenterology at the University of California, San Diego.
The 2020 guidelines didn’t specifically discuss diagnosis and management of AG; however, a diagnosis of intestinal metaplasia based on gastric histopathology indicates the presence AG since metaplasia occurs in atrophic mucosa. Nevertheless, AG often goes unmentioned in histopathology reports. Such omissions are important because AG is an important stage in the potential development of gastric cancer.
AG is believed to result from genetic and environmental factors. The two primary triggers for the condition are H. pylori infection (HpAG) and autoimmunity (AIG). The condition results from chronic inflammation and replacement of normal gastric glandular structures with connective tissue or nonnative epithelium. It can proceed to other precancerous conditions, including gastric intestinal metaplasia and dysplasia. An estimated 15% of the U.S. population has AG, according to the authors, although this prevalence could be higher in populations with higher rates of H. pylori infection. AIG is rare, occurring in 0.5%-2% of the U.S. population.
Among individuals with AG, 0.1%-0.3% per year go on to develop gastric adenocarcinoma, though additional factors could heighten this risk. Furthermore, 0.4%-0.7% per year go on develop type 1 neuroendocrine tumors.
HpAG and AIG have different patterns of mucosal involvement. During diagnosis, the authors advised careful mucosal visualization with air insufflation and mucosal cleansing. High-definition white-light endoscopy is more sensitive than traditional WLE in the identification of premalignant mucosal changes.
AG diagnosis should be confirmed by histopathology. The updated Sydney protocol should be used to obtain biopsies, and serum pepsinogens can be used to identify extensive atrophy, though this testing is not generally available in the United States for clinical use. When histology results are suggestive of AIG, the presence of parietal cell antibodies and intrinsic factor antibodies can contribute to a diagnosis, although the former can be prone to false positives because of H. pylori infection, and the latter has low sensitivity.
Patients identified with AG should be tested for H. pylori and treated for infection, followed by nonserologic testing to confirm treatment success. If H. pylori is present, successful eradication may allow for reversal of AG to normal gastric mucosa; however, patients may have irreversible changes. This could leave them at elevated risk of further progression, though elimination of H. pylori does appear to blunt that risk somewhat.
Neoplastic complications from AG are rare, and the benefits of surveillance among those with AG have not been demonstrated in prospective trials. Observational trials show that severe AG is associated with greater risk of gastric adenocarcinoma, and other factors, such as comorbidities and patient values and priorities, should inform decision-making. When called for, providers should consider surveillance endoscopies every 3 years, though the authors noted that the optimal surveillance interval is unknown. Factors such as the quality of the original endoscopy, family history of gastric cancer, and a history of immigration from regions with high rates of H. pylori infection may impact decisions on surveillance intervals.
AG can lead to iron or vitamin B12 deficiency, so patients with AG, especially those with corpus-predominant AG, should be evaluated for both. AG should also be considered as a differential diagnosis in patients presenting with either deficiency.
A diagnosis of AIG should be accompanied by screening for autoimmune thyroid disease, and type 1 diabetes or Addison’s disease may also be indicated if clinical presentation is consistent.
Because AG is commonly underdiagnosed, the authors advise that gastroenterologists and pathologists should improve coordination to maximize diagnosis of the condition, and they call for comparative clinical trials to improve risk stratification algorithms and surveillance strategies.
The authors disclose no relevant conflicts of interest.
A new clinical practice update expert review for the diagnosis and management of atrophic gastritis (AG) from the American Gastroenterological Association focuses on cases linked to Helicobacter pylori infection or autoimmunity.
This update addresses a sparsity of guidelines for AG in the United States and should be seen as complementary to the AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia, according to the authors led by Shailja C. Shah, MD, MPH, of the gastroenterology section at Veterans Affairs San Diego Healthcare System and the division of gastroenterology at the University of California, San Diego.
The 2020 guidelines didn’t specifically discuss diagnosis and management of AG; however, a diagnosis of intestinal metaplasia based on gastric histopathology indicates the presence AG since metaplasia occurs in atrophic mucosa. Nevertheless, AG often goes unmentioned in histopathology reports. Such omissions are important because AG is an important stage in the potential development of gastric cancer.
AG is believed to result from genetic and environmental factors. The two primary triggers for the condition are H. pylori infection (HpAG) and autoimmunity (AIG). The condition results from chronic inflammation and replacement of normal gastric glandular structures with connective tissue or nonnative epithelium. It can proceed to other precancerous conditions, including gastric intestinal metaplasia and dysplasia. An estimated 15% of the U.S. population has AG, according to the authors, although this prevalence could be higher in populations with higher rates of H. pylori infection. AIG is rare, occurring in 0.5%-2% of the U.S. population.
Among individuals with AG, 0.1%-0.3% per year go on to develop gastric adenocarcinoma, though additional factors could heighten this risk. Furthermore, 0.4%-0.7% per year go on develop type 1 neuroendocrine tumors.
HpAG and AIG have different patterns of mucosal involvement. During diagnosis, the authors advised careful mucosal visualization with air insufflation and mucosal cleansing. High-definition white-light endoscopy is more sensitive than traditional WLE in the identification of premalignant mucosal changes.
AG diagnosis should be confirmed by histopathology. The updated Sydney protocol should be used to obtain biopsies, and serum pepsinogens can be used to identify extensive atrophy, though this testing is not generally available in the United States for clinical use. When histology results are suggestive of AIG, the presence of parietal cell antibodies and intrinsic factor antibodies can contribute to a diagnosis, although the former can be prone to false positives because of H. pylori infection, and the latter has low sensitivity.
Patients identified with AG should be tested for H. pylori and treated for infection, followed by nonserologic testing to confirm treatment success. If H. pylori is present, successful eradication may allow for reversal of AG to normal gastric mucosa; however, patients may have irreversible changes. This could leave them at elevated risk of further progression, though elimination of H. pylori does appear to blunt that risk somewhat.
Neoplastic complications from AG are rare, and the benefits of surveillance among those with AG have not been demonstrated in prospective trials. Observational trials show that severe AG is associated with greater risk of gastric adenocarcinoma, and other factors, such as comorbidities and patient values and priorities, should inform decision-making. When called for, providers should consider surveillance endoscopies every 3 years, though the authors noted that the optimal surveillance interval is unknown. Factors such as the quality of the original endoscopy, family history of gastric cancer, and a history of immigration from regions with high rates of H. pylori infection may impact decisions on surveillance intervals.
AG can lead to iron or vitamin B12 deficiency, so patients with AG, especially those with corpus-predominant AG, should be evaluated for both. AG should also be considered as a differential diagnosis in patients presenting with either deficiency.
A diagnosis of AIG should be accompanied by screening for autoimmune thyroid disease, and type 1 diabetes or Addison’s disease may also be indicated if clinical presentation is consistent.
Because AG is commonly underdiagnosed, the authors advise that gastroenterologists and pathologists should improve coordination to maximize diagnosis of the condition, and they call for comparative clinical trials to improve risk stratification algorithms and surveillance strategies.
The authors disclose no relevant conflicts of interest.
FROM GASTROENTEROLOGY
Lupin recalls irbesartan and hydrochlorothiazide/irbesartan tablets
Lupin Pharmaceuticals is recalling all batches of irbesartan tablets USP 75 mg, 150 mg, and 300 mg and irbesartan and hydrochlorothiazide (HCTZ) tablets USP 150 mg/12.5 mg and 300 mg/12.5 mg because of the potential presence of the N-nitrosoirbesartan impurity.
“As part of Lupin’s ongoing assessment, analysis revealed that certain tested active pharmaceutical ingredient (API) batches (but not finished product batches) were above the specification limit for the impurity, N-nitrosoirbesartan,” the company said in a news release posted on the U.S. Food and Drug Administration’s website. It notes that the impurity is a “probable human carcinogen.”
Lupin discontinued the marketing of irbesartan and irbesartan/HCTZ tablets on Jan. 7, 2021. It says it “has received no reports of illness that appear to relate to this issue” and is issuing the recall out of “an abundance of caution.”
The company, however, goes on to note that from Oct. 8, 2018 (the earliest date of shipment from the manufacturing site of any of the affected batches) to September 30 of this year, Lupin received four reports of illness from irbesartan and 0 reports from irbesartan/HCTZ.
Irbesartan is an angiotensin II receptor blocker indicated for treatment of hypertension in patients with type 2 diabetes, elevated serum creatinine, and proteinuria.
Irbesartan/HCTZ tablets include irbesartan and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension in patients not adequately controlled with monotherapy or as an initial therapy in patients likely to need multiple drugs to achieve blood pressure goals.
Lupin is notifying wholesalers, distributors, and retail outlets to immediately discontinue sales of the affected product lots and return them to the company. Specific lot numbers can be found here.
The company is advising patients to continue taking their medication and to contact their pharmacist, physician, or health care professional for advice regarding an alternative treatment.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the U.S. Food and Drug Administration’s Safety Information and Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
Lupin Pharmaceuticals is recalling all batches of irbesartan tablets USP 75 mg, 150 mg, and 300 mg and irbesartan and hydrochlorothiazide (HCTZ) tablets USP 150 mg/12.5 mg and 300 mg/12.5 mg because of the potential presence of the N-nitrosoirbesartan impurity.
“As part of Lupin’s ongoing assessment, analysis revealed that certain tested active pharmaceutical ingredient (API) batches (but not finished product batches) were above the specification limit for the impurity, N-nitrosoirbesartan,” the company said in a news release posted on the U.S. Food and Drug Administration’s website. It notes that the impurity is a “probable human carcinogen.”
Lupin discontinued the marketing of irbesartan and irbesartan/HCTZ tablets on Jan. 7, 2021. It says it “has received no reports of illness that appear to relate to this issue” and is issuing the recall out of “an abundance of caution.”
The company, however, goes on to note that from Oct. 8, 2018 (the earliest date of shipment from the manufacturing site of any of the affected batches) to September 30 of this year, Lupin received four reports of illness from irbesartan and 0 reports from irbesartan/HCTZ.
Irbesartan is an angiotensin II receptor blocker indicated for treatment of hypertension in patients with type 2 diabetes, elevated serum creatinine, and proteinuria.
Irbesartan/HCTZ tablets include irbesartan and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension in patients not adequately controlled with monotherapy or as an initial therapy in patients likely to need multiple drugs to achieve blood pressure goals.
Lupin is notifying wholesalers, distributors, and retail outlets to immediately discontinue sales of the affected product lots and return them to the company. Specific lot numbers can be found here.
The company is advising patients to continue taking their medication and to contact their pharmacist, physician, or health care professional for advice regarding an alternative treatment.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the U.S. Food and Drug Administration’s Safety Information and Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
Lupin Pharmaceuticals is recalling all batches of irbesartan tablets USP 75 mg, 150 mg, and 300 mg and irbesartan and hydrochlorothiazide (HCTZ) tablets USP 150 mg/12.5 mg and 300 mg/12.5 mg because of the potential presence of the N-nitrosoirbesartan impurity.
“As part of Lupin’s ongoing assessment, analysis revealed that certain tested active pharmaceutical ingredient (API) batches (but not finished product batches) were above the specification limit for the impurity, N-nitrosoirbesartan,” the company said in a news release posted on the U.S. Food and Drug Administration’s website. It notes that the impurity is a “probable human carcinogen.”
Lupin discontinued the marketing of irbesartan and irbesartan/HCTZ tablets on Jan. 7, 2021. It says it “has received no reports of illness that appear to relate to this issue” and is issuing the recall out of “an abundance of caution.”
The company, however, goes on to note that from Oct. 8, 2018 (the earliest date of shipment from the manufacturing site of any of the affected batches) to September 30 of this year, Lupin received four reports of illness from irbesartan and 0 reports from irbesartan/HCTZ.
Irbesartan is an angiotensin II receptor blocker indicated for treatment of hypertension in patients with type 2 diabetes, elevated serum creatinine, and proteinuria.
Irbesartan/HCTZ tablets include irbesartan and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension in patients not adequately controlled with monotherapy or as an initial therapy in patients likely to need multiple drugs to achieve blood pressure goals.
Lupin is notifying wholesalers, distributors, and retail outlets to immediately discontinue sales of the affected product lots and return them to the company. Specific lot numbers can be found here.
The company is advising patients to continue taking their medication and to contact their pharmacist, physician, or health care professional for advice regarding an alternative treatment.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the U.S. Food and Drug Administration’s Safety Information and Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
COVID-19, yes, but so much more
Nearly 2 years into the pandemic, all things COVID-19 will of course be a major focus at the annual meeting of the American College of Chest Physicians, held virtually this year.
“During the pandemic, critical care has been at the forefront of what people are interested in,” said CHEST 2021 program cochair Christopher Carroll, MD, FCCP, from Connecticut Children’s Medical Center, Hartford.
“There is so much volume in critical care units that people have interest in anything related to critical care, including infectious diseases and disaster management in critical care,” he said in an interview.
Sessions at this year’s meeting that have a COVID-19 theme will focus on care of both patients and caregivers. Multiple symposiums, oral abstracts, scientific posters, and case reports will focus on clinical aspects of SARS-CoV-2 infections and COVID-19, including complications such as ventilator-associated and hospital-acquired pneumonia, a session called “Viruses, Variants, Vaccines, and Virulence: The Present and Future of COVID-19,” and support of patients and families.
Other presentations will focus on how COVID-19 has affected lung cancer screening, outpatient practices, pulmonary care, and sleep medicine.
Importantly, the meeting will include sessions focusing on the mental, physical, and social health of clinicians, especially those on the front lines in critical care and intensive care units. There will be sessions on how to recognize and avoid burnout, practice mental health awareness, and take time for self-care.
Presidential Honor Lecturer Curtis N. Sessler, MD, Master FCCP, FCCM, of Virginia Commonwealth University, Richmond, with give a talk titled, “Navigating the Road to Well-Being in the ICU.”
Empathy and diversity
Program cochair David Zielinski, MD, FCCP, of Montreal Children’s Hospital, Quebec, told this news organization that the COVID-19 pandemic has brought into even starker contrast inequities in patient care and in the health care system at large.
“This is a subject people are very keen about right now, whether it’s about how providers are treated, opportunities for improvement, or correcting inequities in outcomes for patients,” he said in an interview.
The program doesn’t shy away from the controversy, either, with a session provocatively titled, “Racism in Health Care: The Fuel That Lit the COVID-19 Fire.”
Keynote speaker Demondes Haynes, MD, FCCP, professor of medicine and associate dean of admissions at the University of Mississippi, Jackson, will speak on the importance of empathy in physician-patient communications and on ways to create a more diverse and inclusive workforce in medicine.
Beyond COVID-19
CHEST 2021 will include important information for sleep medicine physicians, including the latest news regarding an equipment recall affecting one of the two major manufacturers of continuous positive airway pressure devices.
“This has caused a lot of angst among sleep physicians and patients, and there are not enough devices to replace them,” Dr. Zielinski said.
“People who are on home ventilators are also affected by this recall,” Dr. Carroll added. “The population of patients who have sleep apnea is much greater than the population who are on home ventilators, but patients who are on home ventilators need it to save their lives.”
The meeting will include informative sessions summarizing U.S. and European asthma guidelines published over the past 2 years, as well as pending guidelines from the American College of Chest Physicians on venous thromboembolism and lung cancer screening.
Additional topics of importance include chronic obstructive pulmonary disease, interstitial lung disease, asthma management, acute respiratory failure in special populations, new therapies and strategies for treating tuberculosis, and many others.
There will be skill-polishing simulation sessions designed to help clinicians connect with experts in the field, update their knowledge, and sharpen their technique.
Fun and games
In addition to the serious subjects, CHEST 2021 attendees will have the chance to network with colleagues and friends and enjoy online games, including the 20th annual CHEST Challenge, which will pit teams of fellows-in-training from the Interfaith Medical Center, in Brooklyn, New York; the State University of New York, in Buffalo; and the Ohio State University Medical Center, in Columbus, in a Jeopardy!-style battle of wits and medical knowledge.
Close to 7,000 registrants from around the world took part in last year’s CHEST annual meeting, and a similar number is expected for this year’s edition, Dr. Carroll and Dr. Zielinski said.
A version of this article first appeared on Medscape.com.
Nearly 2 years into the pandemic, all things COVID-19 will of course be a major focus at the annual meeting of the American College of Chest Physicians, held virtually this year.
“During the pandemic, critical care has been at the forefront of what people are interested in,” said CHEST 2021 program cochair Christopher Carroll, MD, FCCP, from Connecticut Children’s Medical Center, Hartford.
“There is so much volume in critical care units that people have interest in anything related to critical care, including infectious diseases and disaster management in critical care,” he said in an interview.
Sessions at this year’s meeting that have a COVID-19 theme will focus on care of both patients and caregivers. Multiple symposiums, oral abstracts, scientific posters, and case reports will focus on clinical aspects of SARS-CoV-2 infections and COVID-19, including complications such as ventilator-associated and hospital-acquired pneumonia, a session called “Viruses, Variants, Vaccines, and Virulence: The Present and Future of COVID-19,” and support of patients and families.
Other presentations will focus on how COVID-19 has affected lung cancer screening, outpatient practices, pulmonary care, and sleep medicine.
Importantly, the meeting will include sessions focusing on the mental, physical, and social health of clinicians, especially those on the front lines in critical care and intensive care units. There will be sessions on how to recognize and avoid burnout, practice mental health awareness, and take time for self-care.
Presidential Honor Lecturer Curtis N. Sessler, MD, Master FCCP, FCCM, of Virginia Commonwealth University, Richmond, with give a talk titled, “Navigating the Road to Well-Being in the ICU.”
Empathy and diversity
Program cochair David Zielinski, MD, FCCP, of Montreal Children’s Hospital, Quebec, told this news organization that the COVID-19 pandemic has brought into even starker contrast inequities in patient care and in the health care system at large.
“This is a subject people are very keen about right now, whether it’s about how providers are treated, opportunities for improvement, or correcting inequities in outcomes for patients,” he said in an interview.
The program doesn’t shy away from the controversy, either, with a session provocatively titled, “Racism in Health Care: The Fuel That Lit the COVID-19 Fire.”
Keynote speaker Demondes Haynes, MD, FCCP, professor of medicine and associate dean of admissions at the University of Mississippi, Jackson, will speak on the importance of empathy in physician-patient communications and on ways to create a more diverse and inclusive workforce in medicine.
Beyond COVID-19
CHEST 2021 will include important information for sleep medicine physicians, including the latest news regarding an equipment recall affecting one of the two major manufacturers of continuous positive airway pressure devices.
“This has caused a lot of angst among sleep physicians and patients, and there are not enough devices to replace them,” Dr. Zielinski said.
“People who are on home ventilators are also affected by this recall,” Dr. Carroll added. “The population of patients who have sleep apnea is much greater than the population who are on home ventilators, but patients who are on home ventilators need it to save their lives.”
The meeting will include informative sessions summarizing U.S. and European asthma guidelines published over the past 2 years, as well as pending guidelines from the American College of Chest Physicians on venous thromboembolism and lung cancer screening.
Additional topics of importance include chronic obstructive pulmonary disease, interstitial lung disease, asthma management, acute respiratory failure in special populations, new therapies and strategies for treating tuberculosis, and many others.
There will be skill-polishing simulation sessions designed to help clinicians connect with experts in the field, update their knowledge, and sharpen their technique.
Fun and games
In addition to the serious subjects, CHEST 2021 attendees will have the chance to network with colleagues and friends and enjoy online games, including the 20th annual CHEST Challenge, which will pit teams of fellows-in-training from the Interfaith Medical Center, in Brooklyn, New York; the State University of New York, in Buffalo; and the Ohio State University Medical Center, in Columbus, in a Jeopardy!-style battle of wits and medical knowledge.
Close to 7,000 registrants from around the world took part in last year’s CHEST annual meeting, and a similar number is expected for this year’s edition, Dr. Carroll and Dr. Zielinski said.
A version of this article first appeared on Medscape.com.
Nearly 2 years into the pandemic, all things COVID-19 will of course be a major focus at the annual meeting of the American College of Chest Physicians, held virtually this year.
“During the pandemic, critical care has been at the forefront of what people are interested in,” said CHEST 2021 program cochair Christopher Carroll, MD, FCCP, from Connecticut Children’s Medical Center, Hartford.
“There is so much volume in critical care units that people have interest in anything related to critical care, including infectious diseases and disaster management in critical care,” he said in an interview.
Sessions at this year’s meeting that have a COVID-19 theme will focus on care of both patients and caregivers. Multiple symposiums, oral abstracts, scientific posters, and case reports will focus on clinical aspects of SARS-CoV-2 infections and COVID-19, including complications such as ventilator-associated and hospital-acquired pneumonia, a session called “Viruses, Variants, Vaccines, and Virulence: The Present and Future of COVID-19,” and support of patients and families.
Other presentations will focus on how COVID-19 has affected lung cancer screening, outpatient practices, pulmonary care, and sleep medicine.
Importantly, the meeting will include sessions focusing on the mental, physical, and social health of clinicians, especially those on the front lines in critical care and intensive care units. There will be sessions on how to recognize and avoid burnout, practice mental health awareness, and take time for self-care.
Presidential Honor Lecturer Curtis N. Sessler, MD, Master FCCP, FCCM, of Virginia Commonwealth University, Richmond, with give a talk titled, “Navigating the Road to Well-Being in the ICU.”
Empathy and diversity
Program cochair David Zielinski, MD, FCCP, of Montreal Children’s Hospital, Quebec, told this news organization that the COVID-19 pandemic has brought into even starker contrast inequities in patient care and in the health care system at large.
“This is a subject people are very keen about right now, whether it’s about how providers are treated, opportunities for improvement, or correcting inequities in outcomes for patients,” he said in an interview.
The program doesn’t shy away from the controversy, either, with a session provocatively titled, “Racism in Health Care: The Fuel That Lit the COVID-19 Fire.”
Keynote speaker Demondes Haynes, MD, FCCP, professor of medicine and associate dean of admissions at the University of Mississippi, Jackson, will speak on the importance of empathy in physician-patient communications and on ways to create a more diverse and inclusive workforce in medicine.
Beyond COVID-19
CHEST 2021 will include important information for sleep medicine physicians, including the latest news regarding an equipment recall affecting one of the two major manufacturers of continuous positive airway pressure devices.
“This has caused a lot of angst among sleep physicians and patients, and there are not enough devices to replace them,” Dr. Zielinski said.
“People who are on home ventilators are also affected by this recall,” Dr. Carroll added. “The population of patients who have sleep apnea is much greater than the population who are on home ventilators, but patients who are on home ventilators need it to save their lives.”
The meeting will include informative sessions summarizing U.S. and European asthma guidelines published over the past 2 years, as well as pending guidelines from the American College of Chest Physicians on venous thromboembolism and lung cancer screening.
Additional topics of importance include chronic obstructive pulmonary disease, interstitial lung disease, asthma management, acute respiratory failure in special populations, new therapies and strategies for treating tuberculosis, and many others.
There will be skill-polishing simulation sessions designed to help clinicians connect with experts in the field, update their knowledge, and sharpen their technique.
Fun and games
In addition to the serious subjects, CHEST 2021 attendees will have the chance to network with colleagues and friends and enjoy online games, including the 20th annual CHEST Challenge, which will pit teams of fellows-in-training from the Interfaith Medical Center, in Brooklyn, New York; the State University of New York, in Buffalo; and the Ohio State University Medical Center, in Columbus, in a Jeopardy!-style battle of wits and medical knowledge.
Close to 7,000 registrants from around the world took part in last year’s CHEST annual meeting, and a similar number is expected for this year’s edition, Dr. Carroll and Dr. Zielinski said.
A version of this article first appeared on Medscape.com.
FROM CHEST 2021
Case report: Lung cancer shrinks in patient using CBD oil
A case report describes the dramatic shrinkage of a tumor to a quarter of its original size in a patient with non–small cell lung cancer (NSCLC) who had declined conventional treatment, continued smoking, and who later revealed that she was ingesting cannabidiol (CBD) oil.
The patient was an 80-year-old woman.
At diagnosis, the tumor measured 41 mm, and there was no evidence of local or further spread. Hence, it was suitable for a standard treatment regimen of surgery, chemotherapy, and radiotherapy, note the authors.
The patient declined conventional treatment. She underwent monitoring with regular CT scans every 3–6 months.
After 2.5 years, the CT scans showed that the tumor had shrunk to 10 mm.
It was taken orally about two to three times a day.
Details of the case were published on October 14 in BMJ Case Reports.
“We are aware of the limitations of this case report,” write the authors, led by Kah Ling Liew, MD, of Watford General Hospital, Watford, United Kingdom.
“Although there appears to be a relationship between the intake of ‘CBD oil’ and the observed tumour regression, we are unable to conclusively confirm that the tumour regression is due to the patient taking ‘CBD oil,’ ” they comment.
The team also notes that there are similar case reports in the medical literature.
Both points were emphasized by experts reacting to the publication via the UK Science Media Center.
“This is one of many such promising single case reports of medical cannabis self-treatment for various cancers,” said David Nutt, DM, FRCP, FRCPsych, the Edmond J. Safra Chair in Neuropsychopharmacology, Imperial College London, United Kingdom. “Such case reports are biologically credible given the adaptogenic nature of the endocannabinoid system.”
He noted that a “case report itself is not sufficient to give any form of proof that one thing caused the other -- we need trials for that. There are some controlled trials already started and more are required to properly explore the potential of medical cannabis in a range of cancers.”
Another expert, Edzard Ernst, MD, PhD, professor emeritus of complementary medicine, University of Exeter, United Kingdom, pointed out that in animal models, cannabinoids have reduced the size of prostate cancer tumors. “Previous case reports have yielded encouraging findings also in human cancers,” he noted. He too said that further study is needed.
“Case reports cannot be considered to be reliable evidence, and there are currently no data from rigorous clinical trials to suggest that cannabis products will alter the natural history of any cancer,”Dr. Ernst said.
Patient declined recommended treatment
The patient initially presented with a persistent cough that did not resolve with antibiotic therapy. She has a history of mild chronic obstructive pulmonary disease, osteoarthritis, and hypertension. She is a current smoker with a 68 pack-year history of smoking. She has no history of alcohol consumption and is taking several prescription medications.
After an initial CT scan, she underwent a CT-guided lung biopsy and was diagnosed with NSCLC (TNM stage T2bN0Mx). Further analysis of the tumor tissue showed that it was negative for ALK and EGFR mutations. PDL1 was expressed by <1% of the tumor cells. No distant metastases were detected.
A subsequent CT scan revealed that the main tumor in her right middle lobe had shrunk from 41 mm to 33 mm. There were new bilateral upper lobe nodules, one in the left apex, which measured 4 mm, and one in right apex, which measured 6 mm.
The patient was referred to cardiothoracic surgeons for a possible lobectomy, but the patient declined to have surgery. She was then referred to the oncologists. She underwent repeat CT and positron-emission tomography (PET) scans, which showed that her cancer had continued to shrink. On CT, there was an 11-mm reduction, and on PET, an 18-mm reduction. The left apical nodule had resolved, and the right upper lobe nodule was reduced in size.
The patient was offered stereotactic ablative radiotherapy, but she declined this treatment. Because she had refused all standard therapies, a decision was made to “watch and wait.” The patient underwent regular CT surveillance.
Over the course of 2.5 years, the tumor continued to regress. By February 2021, it had shrunk to 10 mm, which represents an overall reduction of 76% in maximum axial diameter. The average rate of reduction was 2.4% per month throughout the monitoring period.
“This case was brought to the attention of the local lung MDT [multidisciplinary team] in February 2019 when the serial imaging showed a reduction in tumor size despite having received no conventional treatment for her lung cancer,” write the authors.
The patient was contacted to discuss her results. She revealed that she was using CBD oil and that she had started taking it in August 2018. No changes had been made in her prescription medications, diet, and lifestyle, and she continued to smoke a pack of cigarettes every week.
“I was not very interested in traditional cancer treatments,” the patient said, “as I was worried about the risks of surgery, and I saw my late husband suffer through the side effects of radiotherapy. My relative suggested that I should try ‘cannabidiol (CBD) oil’ to treat my cancer, and I have been taking it regularly ever since. I am ‘over the moon’ with my cancer shrinking, which I believe was caused by the ‘CBD oil’. I am tolerating it very well and I intend to take this treatment indefinitely.”
The source of the CBD oil was outside the United Kingdom. The main active ingredients, according to her supplier, were Δ9-tetrahydrocannabinol (THC), at 19.5%, CBD, at 20.05%, and tetrahydrocannabinolic acid, at 23.8%.
“The product used by this patient reportedly contained high levels of THC (the intoxicating component of cannabis) and was sourced from outside the U.K.,” commented Tom Freeman, PhD, senior lecturer and director of the Addiction and Mental Health Group, University of Bath, United Kingdom. “This type of product is very different to most CBD oils, which predominantly contain CBD. Unlike prescribed medicines, CBD wellness products lack assurance of quality, safety, or efficacy and should not be used for medicinal purposes.”
The authors have disclosed no relevant financial relationships. Dr. Nutt chairs the scientific committee of the charity Drug Science, which receives unrestricted educational grants from some medical cannabis companies. Dr. Ernst and Dr. Freeman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A case report describes the dramatic shrinkage of a tumor to a quarter of its original size in a patient with non–small cell lung cancer (NSCLC) who had declined conventional treatment, continued smoking, and who later revealed that she was ingesting cannabidiol (CBD) oil.
The patient was an 80-year-old woman.
At diagnosis, the tumor measured 41 mm, and there was no evidence of local or further spread. Hence, it was suitable for a standard treatment regimen of surgery, chemotherapy, and radiotherapy, note the authors.
The patient declined conventional treatment. She underwent monitoring with regular CT scans every 3–6 months.
After 2.5 years, the CT scans showed that the tumor had shrunk to 10 mm.
It was taken orally about two to three times a day.
Details of the case were published on October 14 in BMJ Case Reports.
“We are aware of the limitations of this case report,” write the authors, led by Kah Ling Liew, MD, of Watford General Hospital, Watford, United Kingdom.
“Although there appears to be a relationship between the intake of ‘CBD oil’ and the observed tumour regression, we are unable to conclusively confirm that the tumour regression is due to the patient taking ‘CBD oil,’ ” they comment.
The team also notes that there are similar case reports in the medical literature.
Both points were emphasized by experts reacting to the publication via the UK Science Media Center.
“This is one of many such promising single case reports of medical cannabis self-treatment for various cancers,” said David Nutt, DM, FRCP, FRCPsych, the Edmond J. Safra Chair in Neuropsychopharmacology, Imperial College London, United Kingdom. “Such case reports are biologically credible given the adaptogenic nature of the endocannabinoid system.”
He noted that a “case report itself is not sufficient to give any form of proof that one thing caused the other -- we need trials for that. There are some controlled trials already started and more are required to properly explore the potential of medical cannabis in a range of cancers.”
Another expert, Edzard Ernst, MD, PhD, professor emeritus of complementary medicine, University of Exeter, United Kingdom, pointed out that in animal models, cannabinoids have reduced the size of prostate cancer tumors. “Previous case reports have yielded encouraging findings also in human cancers,” he noted. He too said that further study is needed.
“Case reports cannot be considered to be reliable evidence, and there are currently no data from rigorous clinical trials to suggest that cannabis products will alter the natural history of any cancer,”Dr. Ernst said.
Patient declined recommended treatment
The patient initially presented with a persistent cough that did not resolve with antibiotic therapy. She has a history of mild chronic obstructive pulmonary disease, osteoarthritis, and hypertension. She is a current smoker with a 68 pack-year history of smoking. She has no history of alcohol consumption and is taking several prescription medications.
After an initial CT scan, she underwent a CT-guided lung biopsy and was diagnosed with NSCLC (TNM stage T2bN0Mx). Further analysis of the tumor tissue showed that it was negative for ALK and EGFR mutations. PDL1 was expressed by <1% of the tumor cells. No distant metastases were detected.
A subsequent CT scan revealed that the main tumor in her right middle lobe had shrunk from 41 mm to 33 mm. There were new bilateral upper lobe nodules, one in the left apex, which measured 4 mm, and one in right apex, which measured 6 mm.
The patient was referred to cardiothoracic surgeons for a possible lobectomy, but the patient declined to have surgery. She was then referred to the oncologists. She underwent repeat CT and positron-emission tomography (PET) scans, which showed that her cancer had continued to shrink. On CT, there was an 11-mm reduction, and on PET, an 18-mm reduction. The left apical nodule had resolved, and the right upper lobe nodule was reduced in size.
The patient was offered stereotactic ablative radiotherapy, but she declined this treatment. Because she had refused all standard therapies, a decision was made to “watch and wait.” The patient underwent regular CT surveillance.
Over the course of 2.5 years, the tumor continued to regress. By February 2021, it had shrunk to 10 mm, which represents an overall reduction of 76% in maximum axial diameter. The average rate of reduction was 2.4% per month throughout the monitoring period.
“This case was brought to the attention of the local lung MDT [multidisciplinary team] in February 2019 when the serial imaging showed a reduction in tumor size despite having received no conventional treatment for her lung cancer,” write the authors.
The patient was contacted to discuss her results. She revealed that she was using CBD oil and that she had started taking it in August 2018. No changes had been made in her prescription medications, diet, and lifestyle, and she continued to smoke a pack of cigarettes every week.
“I was not very interested in traditional cancer treatments,” the patient said, “as I was worried about the risks of surgery, and I saw my late husband suffer through the side effects of radiotherapy. My relative suggested that I should try ‘cannabidiol (CBD) oil’ to treat my cancer, and I have been taking it regularly ever since. I am ‘over the moon’ with my cancer shrinking, which I believe was caused by the ‘CBD oil’. I am tolerating it very well and I intend to take this treatment indefinitely.”
The source of the CBD oil was outside the United Kingdom. The main active ingredients, according to her supplier, were Δ9-tetrahydrocannabinol (THC), at 19.5%, CBD, at 20.05%, and tetrahydrocannabinolic acid, at 23.8%.
“The product used by this patient reportedly contained high levels of THC (the intoxicating component of cannabis) and was sourced from outside the U.K.,” commented Tom Freeman, PhD, senior lecturer and director of the Addiction and Mental Health Group, University of Bath, United Kingdom. “This type of product is very different to most CBD oils, which predominantly contain CBD. Unlike prescribed medicines, CBD wellness products lack assurance of quality, safety, or efficacy and should not be used for medicinal purposes.”
The authors have disclosed no relevant financial relationships. Dr. Nutt chairs the scientific committee of the charity Drug Science, which receives unrestricted educational grants from some medical cannabis companies. Dr. Ernst and Dr. Freeman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A case report describes the dramatic shrinkage of a tumor to a quarter of its original size in a patient with non–small cell lung cancer (NSCLC) who had declined conventional treatment, continued smoking, and who later revealed that she was ingesting cannabidiol (CBD) oil.
The patient was an 80-year-old woman.
At diagnosis, the tumor measured 41 mm, and there was no evidence of local or further spread. Hence, it was suitable for a standard treatment regimen of surgery, chemotherapy, and radiotherapy, note the authors.
The patient declined conventional treatment. She underwent monitoring with regular CT scans every 3–6 months.
After 2.5 years, the CT scans showed that the tumor had shrunk to 10 mm.
It was taken orally about two to three times a day.
Details of the case were published on October 14 in BMJ Case Reports.
“We are aware of the limitations of this case report,” write the authors, led by Kah Ling Liew, MD, of Watford General Hospital, Watford, United Kingdom.
“Although there appears to be a relationship between the intake of ‘CBD oil’ and the observed tumour regression, we are unable to conclusively confirm that the tumour regression is due to the patient taking ‘CBD oil,’ ” they comment.
The team also notes that there are similar case reports in the medical literature.
Both points were emphasized by experts reacting to the publication via the UK Science Media Center.
“This is one of many such promising single case reports of medical cannabis self-treatment for various cancers,” said David Nutt, DM, FRCP, FRCPsych, the Edmond J. Safra Chair in Neuropsychopharmacology, Imperial College London, United Kingdom. “Such case reports are biologically credible given the adaptogenic nature of the endocannabinoid system.”
He noted that a “case report itself is not sufficient to give any form of proof that one thing caused the other -- we need trials for that. There are some controlled trials already started and more are required to properly explore the potential of medical cannabis in a range of cancers.”
Another expert, Edzard Ernst, MD, PhD, professor emeritus of complementary medicine, University of Exeter, United Kingdom, pointed out that in animal models, cannabinoids have reduced the size of prostate cancer tumors. “Previous case reports have yielded encouraging findings also in human cancers,” he noted. He too said that further study is needed.
“Case reports cannot be considered to be reliable evidence, and there are currently no data from rigorous clinical trials to suggest that cannabis products will alter the natural history of any cancer,”Dr. Ernst said.
Patient declined recommended treatment
The patient initially presented with a persistent cough that did not resolve with antibiotic therapy. She has a history of mild chronic obstructive pulmonary disease, osteoarthritis, and hypertension. She is a current smoker with a 68 pack-year history of smoking. She has no history of alcohol consumption and is taking several prescription medications.
After an initial CT scan, she underwent a CT-guided lung biopsy and was diagnosed with NSCLC (TNM stage T2bN0Mx). Further analysis of the tumor tissue showed that it was negative for ALK and EGFR mutations. PDL1 was expressed by <1% of the tumor cells. No distant metastases were detected.
A subsequent CT scan revealed that the main tumor in her right middle lobe had shrunk from 41 mm to 33 mm. There were new bilateral upper lobe nodules, one in the left apex, which measured 4 mm, and one in right apex, which measured 6 mm.
The patient was referred to cardiothoracic surgeons for a possible lobectomy, but the patient declined to have surgery. She was then referred to the oncologists. She underwent repeat CT and positron-emission tomography (PET) scans, which showed that her cancer had continued to shrink. On CT, there was an 11-mm reduction, and on PET, an 18-mm reduction. The left apical nodule had resolved, and the right upper lobe nodule was reduced in size.
The patient was offered stereotactic ablative radiotherapy, but she declined this treatment. Because she had refused all standard therapies, a decision was made to “watch and wait.” The patient underwent regular CT surveillance.
Over the course of 2.5 years, the tumor continued to regress. By February 2021, it had shrunk to 10 mm, which represents an overall reduction of 76% in maximum axial diameter. The average rate of reduction was 2.4% per month throughout the monitoring period.
“This case was brought to the attention of the local lung MDT [multidisciplinary team] in February 2019 when the serial imaging showed a reduction in tumor size despite having received no conventional treatment for her lung cancer,” write the authors.
The patient was contacted to discuss her results. She revealed that she was using CBD oil and that she had started taking it in August 2018. No changes had been made in her prescription medications, diet, and lifestyle, and she continued to smoke a pack of cigarettes every week.
“I was not very interested in traditional cancer treatments,” the patient said, “as I was worried about the risks of surgery, and I saw my late husband suffer through the side effects of radiotherapy. My relative suggested that I should try ‘cannabidiol (CBD) oil’ to treat my cancer, and I have been taking it regularly ever since. I am ‘over the moon’ with my cancer shrinking, which I believe was caused by the ‘CBD oil’. I am tolerating it very well and I intend to take this treatment indefinitely.”
The source of the CBD oil was outside the United Kingdom. The main active ingredients, according to her supplier, were Δ9-tetrahydrocannabinol (THC), at 19.5%, CBD, at 20.05%, and tetrahydrocannabinolic acid, at 23.8%.
“The product used by this patient reportedly contained high levels of THC (the intoxicating component of cannabis) and was sourced from outside the U.K.,” commented Tom Freeman, PhD, senior lecturer and director of the Addiction and Mental Health Group, University of Bath, United Kingdom. “This type of product is very different to most CBD oils, which predominantly contain CBD. Unlike prescribed medicines, CBD wellness products lack assurance of quality, safety, or efficacy and should not be used for medicinal purposes.”
The authors have disclosed no relevant financial relationships. Dr. Nutt chairs the scientific committee of the charity Drug Science, which receives unrestricted educational grants from some medical cannabis companies. Dr. Ernst and Dr. Freeman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WATS-3D plus Seattle protocol increases dysplasia detection in Barrett’s esophagus
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
FROM GASTROINTESTINAL ENDOSCOPY
The Barcelona baseline risk score may predict long-term MS course
The high-risk group had the shortest time to progression to an Expanded Disability Status Score (EDSS) of 3.0, and were also more likely to progress by MRI and quality of life measures.
The ranking is based on sex, age at the first clinically isolated syndrome, CIS topography, the number of T2 lesions, and the presence of infratentorial and spinal cord lesions, contrast-enhancing lesions, and oligoclonal bands.
“What we wanted to do is merge all of the different prognostic variables for one single patient into one single score,” said Mar Tintoré, MD, PhD, in an interview. Dr. Tintoré is a professor of neurology at Vall d’Hebron University Hospital in Barcelona and a senior consultant at the Multiple Sclerosis Centre of Catalonia (Cemcat). Dr. Tintoré presented the results of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The three groups had different outcomes in MRI, clinical factors, and MRI scans, and quality of life outcomes over the course of their disease. “So this is a confirmation that this classification at baseline is really meaningful,” Dr. Tintoré said in an interview.
She attributed the success of the model to its reliance on multiple factors, but it is also designed to be simple to use. “We have been trying to use it with simple factors, [information] that you always have, like age, sex, gender, number of lesions, and topography of the region. Everybody has this information at their desk.”
Proof of concept
The study validates the approach that neurologists already utilize, according to Patricia Coyle, MD, who moderated the session. “I think the prospective study is a really unique and powerful concept,” said Dr. Coyle, who is a professor of neurology, vice chair of neurology, and director of the Stony Brook (N.Y.) Comprehensive Care Center.
The new study “kind of confirmed their concept of the initial rating, judging long-term disability progression measures in subsets. They also looked at brain atrophy, they looked at gray-matter atrophy, and that also traveled with these three different groups of severity. So it kind of gives value to looking at prognostic indicators at a first attack,” said Dr. Coyle.
The results also validate the Barcelona group’s heavy emphasis on MRI, which Dr. Coyle pointed out is common practice. “If the brain MRI looks very bad, if there are a lot of spinal cord lesions, then that’s somebody we’re much more worried about.”
Once the model is confirmed in other cohorts, the researchers plan to release the model as a generally available algorithm that clinicians could use to help manage patients. Dr. Tintoré pointed out the debate over when to begin a patient on high-efficacy disease-modifying therapies. That choice depends on a lot of factors, including patient choice, safety, and comorbidities. “But knowing that your patient is at risk of having a bad prognosis is something that is helpful” in that decision-making process, she said.
Predicting time to EDSS 3.0
The researchers used the Barcelona CIS cohort to build a Weibull survival regression in order to estimate the median time to EDSS 3.0. The model produced three categories with widely divergent predicted times from CIS to EDSS 3.0: low risk, medium risk, and high risk.
In the current report, the researchers compared the model to a “360 degree” measure of measures in 1,308 patients, including clinical milestones (McDonald 2017 MS, confirmed secondary progressive MS, progression independent of relapse activity [PIRA], EDSS disability, number of T2 MRI lesions and brain atrophy, and Patient Reported Outcomes for MS [walking speed, manual dexterity, processing speed, and contrast sensitivity]).
At 30 years after CIS, the risk of reaching EDSS 3.0 was higher in the medium risk (hazard ratio, 3.0 versus low risk) and in the high risk group (HR, 8.3 versus low risk). At 10 years, the low risk group had a 40% risk of fulfilling McDonald 2017 criteria, versus 89% in the intermediate group, and 98% in the high risk group. A similar relationship was seen for SPMS (1%, 8%, 16%) and PIRA (17%, 26%, 35%).
At 10 years, the estimated accumulated T2 lesions was 7 in the low-risk group (95% CI, 5-9), 15 in the medium-risk group (95% CI, 12-17), and 21 in the high-risk group (95% CI, 15-27).
Compared with the low- and medium-risk groups, the high-risk group had lower brain parenchymal fraction and gray-matter fraction at 5 years. They also experienced higher stigma, had worse perception of upper and lower limb function as measured by Neuro QoL, and had worse cognitive performance.
Dr. Tintoré has received compensation for consulting services and speaking honoraria from Aimirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Teva Pharmaceuticals, and Viela Bio. Dr. Coyle has no relevant disclosures.
The high-risk group had the shortest time to progression to an Expanded Disability Status Score (EDSS) of 3.0, and were also more likely to progress by MRI and quality of life measures.
The ranking is based on sex, age at the first clinically isolated syndrome, CIS topography, the number of T2 lesions, and the presence of infratentorial and spinal cord lesions, contrast-enhancing lesions, and oligoclonal bands.
“What we wanted to do is merge all of the different prognostic variables for one single patient into one single score,” said Mar Tintoré, MD, PhD, in an interview. Dr. Tintoré is a professor of neurology at Vall d’Hebron University Hospital in Barcelona and a senior consultant at the Multiple Sclerosis Centre of Catalonia (Cemcat). Dr. Tintoré presented the results of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The three groups had different outcomes in MRI, clinical factors, and MRI scans, and quality of life outcomes over the course of their disease. “So this is a confirmation that this classification at baseline is really meaningful,” Dr. Tintoré said in an interview.
She attributed the success of the model to its reliance on multiple factors, but it is also designed to be simple to use. “We have been trying to use it with simple factors, [information] that you always have, like age, sex, gender, number of lesions, and topography of the region. Everybody has this information at their desk.”
Proof of concept
The study validates the approach that neurologists already utilize, according to Patricia Coyle, MD, who moderated the session. “I think the prospective study is a really unique and powerful concept,” said Dr. Coyle, who is a professor of neurology, vice chair of neurology, and director of the Stony Brook (N.Y.) Comprehensive Care Center.
The new study “kind of confirmed their concept of the initial rating, judging long-term disability progression measures in subsets. They also looked at brain atrophy, they looked at gray-matter atrophy, and that also traveled with these three different groups of severity. So it kind of gives value to looking at prognostic indicators at a first attack,” said Dr. Coyle.
The results also validate the Barcelona group’s heavy emphasis on MRI, which Dr. Coyle pointed out is common practice. “If the brain MRI looks very bad, if there are a lot of spinal cord lesions, then that’s somebody we’re much more worried about.”
Once the model is confirmed in other cohorts, the researchers plan to release the model as a generally available algorithm that clinicians could use to help manage patients. Dr. Tintoré pointed out the debate over when to begin a patient on high-efficacy disease-modifying therapies. That choice depends on a lot of factors, including patient choice, safety, and comorbidities. “But knowing that your patient is at risk of having a bad prognosis is something that is helpful” in that decision-making process, she said.
Predicting time to EDSS 3.0
The researchers used the Barcelona CIS cohort to build a Weibull survival regression in order to estimate the median time to EDSS 3.0. The model produced three categories with widely divergent predicted times from CIS to EDSS 3.0: low risk, medium risk, and high risk.
In the current report, the researchers compared the model to a “360 degree” measure of measures in 1,308 patients, including clinical milestones (McDonald 2017 MS, confirmed secondary progressive MS, progression independent of relapse activity [PIRA], EDSS disability, number of T2 MRI lesions and brain atrophy, and Patient Reported Outcomes for MS [walking speed, manual dexterity, processing speed, and contrast sensitivity]).
At 30 years after CIS, the risk of reaching EDSS 3.0 was higher in the medium risk (hazard ratio, 3.0 versus low risk) and in the high risk group (HR, 8.3 versus low risk). At 10 years, the low risk group had a 40% risk of fulfilling McDonald 2017 criteria, versus 89% in the intermediate group, and 98% in the high risk group. A similar relationship was seen for SPMS (1%, 8%, 16%) and PIRA (17%, 26%, 35%).
At 10 years, the estimated accumulated T2 lesions was 7 in the low-risk group (95% CI, 5-9), 15 in the medium-risk group (95% CI, 12-17), and 21 in the high-risk group (95% CI, 15-27).
Compared with the low- and medium-risk groups, the high-risk group had lower brain parenchymal fraction and gray-matter fraction at 5 years. They also experienced higher stigma, had worse perception of upper and lower limb function as measured by Neuro QoL, and had worse cognitive performance.
Dr. Tintoré has received compensation for consulting services and speaking honoraria from Aimirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Teva Pharmaceuticals, and Viela Bio. Dr. Coyle has no relevant disclosures.
The high-risk group had the shortest time to progression to an Expanded Disability Status Score (EDSS) of 3.0, and were also more likely to progress by MRI and quality of life measures.
The ranking is based on sex, age at the first clinically isolated syndrome, CIS topography, the number of T2 lesions, and the presence of infratentorial and spinal cord lesions, contrast-enhancing lesions, and oligoclonal bands.
“What we wanted to do is merge all of the different prognostic variables for one single patient into one single score,” said Mar Tintoré, MD, PhD, in an interview. Dr. Tintoré is a professor of neurology at Vall d’Hebron University Hospital in Barcelona and a senior consultant at the Multiple Sclerosis Centre of Catalonia (Cemcat). Dr. Tintoré presented the results of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The three groups had different outcomes in MRI, clinical factors, and MRI scans, and quality of life outcomes over the course of their disease. “So this is a confirmation that this classification at baseline is really meaningful,” Dr. Tintoré said in an interview.
She attributed the success of the model to its reliance on multiple factors, but it is also designed to be simple to use. “We have been trying to use it with simple factors, [information] that you always have, like age, sex, gender, number of lesions, and topography of the region. Everybody has this information at their desk.”
Proof of concept
The study validates the approach that neurologists already utilize, according to Patricia Coyle, MD, who moderated the session. “I think the prospective study is a really unique and powerful concept,” said Dr. Coyle, who is a professor of neurology, vice chair of neurology, and director of the Stony Brook (N.Y.) Comprehensive Care Center.
The new study “kind of confirmed their concept of the initial rating, judging long-term disability progression measures in subsets. They also looked at brain atrophy, they looked at gray-matter atrophy, and that also traveled with these three different groups of severity. So it kind of gives value to looking at prognostic indicators at a first attack,” said Dr. Coyle.
The results also validate the Barcelona group’s heavy emphasis on MRI, which Dr. Coyle pointed out is common practice. “If the brain MRI looks very bad, if there are a lot of spinal cord lesions, then that’s somebody we’re much more worried about.”
Once the model is confirmed in other cohorts, the researchers plan to release the model as a generally available algorithm that clinicians could use to help manage patients. Dr. Tintoré pointed out the debate over when to begin a patient on high-efficacy disease-modifying therapies. That choice depends on a lot of factors, including patient choice, safety, and comorbidities. “But knowing that your patient is at risk of having a bad prognosis is something that is helpful” in that decision-making process, she said.
Predicting time to EDSS 3.0
The researchers used the Barcelona CIS cohort to build a Weibull survival regression in order to estimate the median time to EDSS 3.0. The model produced three categories with widely divergent predicted times from CIS to EDSS 3.0: low risk, medium risk, and high risk.
In the current report, the researchers compared the model to a “360 degree” measure of measures in 1,308 patients, including clinical milestones (McDonald 2017 MS, confirmed secondary progressive MS, progression independent of relapse activity [PIRA], EDSS disability, number of T2 MRI lesions and brain atrophy, and Patient Reported Outcomes for MS [walking speed, manual dexterity, processing speed, and contrast sensitivity]).
At 30 years after CIS, the risk of reaching EDSS 3.0 was higher in the medium risk (hazard ratio, 3.0 versus low risk) and in the high risk group (HR, 8.3 versus low risk). At 10 years, the low risk group had a 40% risk of fulfilling McDonald 2017 criteria, versus 89% in the intermediate group, and 98% in the high risk group. A similar relationship was seen for SPMS (1%, 8%, 16%) and PIRA (17%, 26%, 35%).
At 10 years, the estimated accumulated T2 lesions was 7 in the low-risk group (95% CI, 5-9), 15 in the medium-risk group (95% CI, 12-17), and 21 in the high-risk group (95% CI, 15-27).
Compared with the low- and medium-risk groups, the high-risk group had lower brain parenchymal fraction and gray-matter fraction at 5 years. They also experienced higher stigma, had worse perception of upper and lower limb function as measured by Neuro QoL, and had worse cognitive performance.
Dr. Tintoré has received compensation for consulting services and speaking honoraria from Aimirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Teva Pharmaceuticals, and Viela Bio. Dr. Coyle has no relevant disclosures.
FROM ECTRIMS 2021
Dupilumab-improved lung function lasts in children with moderate to severe asthma
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
FROM CHEST 2021