User login
Study points to ideal age for CAC testing in young adults
New risk equations can help determine the need for a first coronary artery calcium (CAC) scan in young adults to identify those most at risk for premature atherosclerosis, researchers say.
“To our knowledge this is the first time to derive a clinical risk equation for the initial conversion from CAC 0, which can be used actually to guide the timing of CAC testing in young adults,” Omar Dzaye, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, said in an interview.
CAC is an independent predictor of adverse atherosclerotic cardiovascular disease (ASCVD), but routine screening is not recommended in low-risk groups. U.S. guidelines say CAC testing may be considered (class IIa) for risk stratification in adults 40 to 75 years at intermediate risk (estimated 10-year ASCVD risk 7.5% to 20%) when the decision to start preventive therapies is unclear.
The new sex-specific risk equations were derived from 22,346 adults 30 to 50 years of age who underwent CAC testing between 1991 and 2010 for ASCVD risk prediction at four high-volume centers in the CAC Consortium. The average age was 43.5 years, 25% were women, and 12.3% were non-White.
The participants were free of clinical ASCVD or CV symptoms at the time of scanning but had underlying traditional ASCVD risk factors (dyslipidemia in 49.6%, hypertension in 20.0%, active smokers 11.0%, and diabetes in 4.0%), an intermediate 10-year ASCVD risk (2.6%), and/or a significant family history of CHD (49.3%).
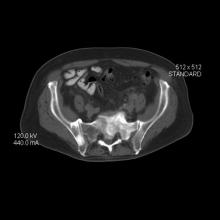
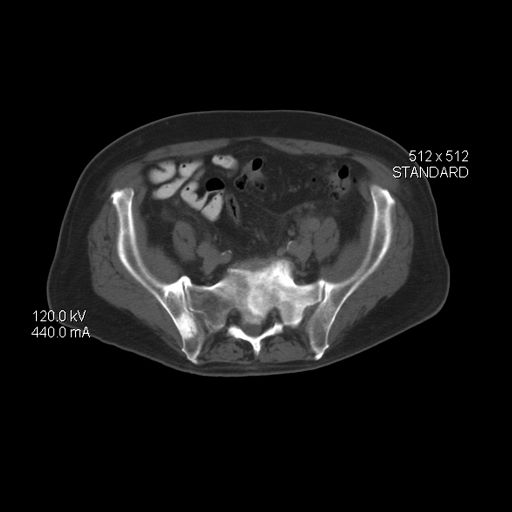
As reported in the Journal of the American College of Cardiology, 92.7% of participants had a low 10-year ASCVD risk below 5%, but 34.4% had CAC scores above 0 (median, 20 Agatston units).
Assuming a 25% testing yield (number needed to scan equals four to detect one CAC score above 0), the optimal age for a first scan in young men without risk factors was 42.3 years, and for women it was 57.6 years.
Young adults with one or more risk factors, however, would convert to CAC above 0 at least 3.3 years earlier on average. Diabetes had the strongest influence on the probability of conversion, with men and women predicted to develop incident CAC a respective 5.5 years and 7.3 years earlier on average.
The findings build on previous observations by the team showing that diabetes confers a 40% reduction in the so-called “warranty period” of a CAC score of 0, Dr. Dzaye noted. The National Lipid Association 2020 statement on CAC scoring also suggests it’s reasonable to obtain a CAC scan in people with diabetes aged 30 to 39 years.
“The predicted utility of CAC for ASCVD outcomes is similar in type 1 and type 2 diabetes; however, individuals with type 1 diabetes may actually develop CAC as young as 17 years of age,” he said. “Therefore, definitely, CAC studies in this population are required.”
In contrast, hypertension, dyslipidemia, active smoking, and a family history of CHD were individually associated with the development of CAC 3.3 to 4.3 years earlier. In general, the time to premature CAC was longer for women than for men with a given risk-factor profile.
The predicted age for a first CAC was 37.5 years for men and 48.9 years for women with an intermediate risk-factor profile (for example, smoking plus hypertension) and 33.8 years and 44.7 years, respectively, for those with a high-risk profile (for example, diabetes plus dyslipidemia).
Asked whether the risk equations can be used to guide CAC scanning in clinical practice, Dr. Dzaye said, “we very much believe that this can be used because for the process we published the internal validation, and we also did an external validation that is not published at the moment in [the] MESA [trial].”
He pointed out that study participants did not have a second CAC scan for true modeling of longitudinal CAC and do not represent the general population but, rather, a general cardiology referral population enriched with ASCVD risk factors. Future studies are needed that incorporate a more diverse population, multiple CAC scans, and genetic risk factors.
“This is helpful from a descriptive, epidemiologic point of view and helps us understand the approximate prevalence of coronary calcium greater than 0 in younger men and women, but I’m not convinced that it will or should change clinical practice,” cardiologist Philip Greenland, MD, a professor of preventive medicine and professor of medicine at Northwestern University in Chicago, said in an interview.
Dr. Greenland, who coauthored a review on CAC testing earlier this month, said CAC is the strongest tool we have to improve risk prediction beyond standard risk scores but does involve radiation exposure and some added costs. CAC testing is especially useful as a tiebreaker in older intermediate-risk patients who may be on the fence about starting primary prevention medications but could fall short among “younger, low-risk patients where, as they show here, the proportion of people who have a positive test is well below half.”
“So that means you’re going to have a very large number of people who are CAC 0, which is what we would expect in relatively younger people, but I wouldn’t be happy to try to explain that to a patient: ‘We’re not seeing coronary atherosclerosis right now, but we still want to treat your risk factors.’ That’s kind of a dissonant message,” Dr. Greenland said.
An accompanying editorial suggests “the study has filled an important clinical gap, providing highly actionable data that could help guide clinical decision making for ASCVD prevention.”
Nevertheless, Tasneem Naqvi, MD, Mayo Clinic, Scottsdale, Arizona, and Tamar Polonsky, MD, University of Chicago, question the generalizability of the results and point out that CAC screening at the authors’ recommended ages “could still miss a substantial number of young women with incident MI.”
Exposure to ionizing radiation with CAC is lower than that used in screening mammography for breast cancer but, they agree, should be considered, particularly in young women.
“Alternatively, ultrasonography avoids radiation altogether and can detect plaque earlier than the development of CAC,” write Dr. Naqvi and Dr. Polonsky. Further, the 2019 European Society of Cardiology guidelines for CV risk give ultrasound assessment of carotid artery and femoral plaque a class IIa recommendation and CAC a class IIb recommendation.
Commenting for this news organization, Roger Blumenthal, MD, director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, said the class IIb recommendation “never really made any sense because the data with coronary calcium is so much stronger than it is with carotid ultrasound.”
“Sometimes smart scientists and researchers differ, but in my strong opinion, the European Society of Cardiology in 2019 did not give it the right classification, while the group I was part of, the American Heart Association/American College of Cardiology [2019 guideline], got it right and emphasized that this is the most cost-effective and useful way to improve risk assessment.”
Dr. Blumenthal, who was not part of the study, noted that U.S. guidelines say CAC measurement is not intended as a screening test for everyone but may be used selectively as a decision aid.
“This study adds to the information about how to use that type of testing. So, I personally think it will be a highly referenced article in the next set of guidelines that the American Heart Association, American College of Cardiology, and other organizations have.”
The study was supported in part by a research grant from the National Institutes of Health National Heart, Lung, and Blood Institute. Dr. Dzaye, Dr. Blumenthal, Dr. Naqvi, and Dr. Polonsky report having no relevant financial relationships.
A version of this article appeared on Medscape.com.
New risk equations can help determine the need for a first coronary artery calcium (CAC) scan in young adults to identify those most at risk for premature atherosclerosis, researchers say.
“To our knowledge this is the first time to derive a clinical risk equation for the initial conversion from CAC 0, which can be used actually to guide the timing of CAC testing in young adults,” Omar Dzaye, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, said in an interview.
CAC is an independent predictor of adverse atherosclerotic cardiovascular disease (ASCVD), but routine screening is not recommended in low-risk groups. U.S. guidelines say CAC testing may be considered (class IIa) for risk stratification in adults 40 to 75 years at intermediate risk (estimated 10-year ASCVD risk 7.5% to 20%) when the decision to start preventive therapies is unclear.
The new sex-specific risk equations were derived from 22,346 adults 30 to 50 years of age who underwent CAC testing between 1991 and 2010 for ASCVD risk prediction at four high-volume centers in the CAC Consortium. The average age was 43.5 years, 25% were women, and 12.3% were non-White.
The participants were free of clinical ASCVD or CV symptoms at the time of scanning but had underlying traditional ASCVD risk factors (dyslipidemia in 49.6%, hypertension in 20.0%, active smokers 11.0%, and diabetes in 4.0%), an intermediate 10-year ASCVD risk (2.6%), and/or a significant family history of CHD (49.3%).
As reported in the Journal of the American College of Cardiology, 92.7% of participants had a low 10-year ASCVD risk below 5%, but 34.4% had CAC scores above 0 (median, 20 Agatston units).
Assuming a 25% testing yield (number needed to scan equals four to detect one CAC score above 0), the optimal age for a first scan in young men without risk factors was 42.3 years, and for women it was 57.6 years.
Young adults with one or more risk factors, however, would convert to CAC above 0 at least 3.3 years earlier on average. Diabetes had the strongest influence on the probability of conversion, with men and women predicted to develop incident CAC a respective 5.5 years and 7.3 years earlier on average.
The findings build on previous observations by the team showing that diabetes confers a 40% reduction in the so-called “warranty period” of a CAC score of 0, Dr. Dzaye noted. The National Lipid Association 2020 statement on CAC scoring also suggests it’s reasonable to obtain a CAC scan in people with diabetes aged 30 to 39 years.
“The predicted utility of CAC for ASCVD outcomes is similar in type 1 and type 2 diabetes; however, individuals with type 1 diabetes may actually develop CAC as young as 17 years of age,” he said. “Therefore, definitely, CAC studies in this population are required.”
In contrast, hypertension, dyslipidemia, active smoking, and a family history of CHD were individually associated with the development of CAC 3.3 to 4.3 years earlier. In general, the time to premature CAC was longer for women than for men with a given risk-factor profile.
The predicted age for a first CAC was 37.5 years for men and 48.9 years for women with an intermediate risk-factor profile (for example, smoking plus hypertension) and 33.8 years and 44.7 years, respectively, for those with a high-risk profile (for example, diabetes plus dyslipidemia).
Asked whether the risk equations can be used to guide CAC scanning in clinical practice, Dr. Dzaye said, “we very much believe that this can be used because for the process we published the internal validation, and we also did an external validation that is not published at the moment in [the] MESA [trial].”
He pointed out that study participants did not have a second CAC scan for true modeling of longitudinal CAC and do not represent the general population but, rather, a general cardiology referral population enriched with ASCVD risk factors. Future studies are needed that incorporate a more diverse population, multiple CAC scans, and genetic risk factors.
“This is helpful from a descriptive, epidemiologic point of view and helps us understand the approximate prevalence of coronary calcium greater than 0 in younger men and women, but I’m not convinced that it will or should change clinical practice,” cardiologist Philip Greenland, MD, a professor of preventive medicine and professor of medicine at Northwestern University in Chicago, said in an interview.
Dr. Greenland, who coauthored a review on CAC testing earlier this month, said CAC is the strongest tool we have to improve risk prediction beyond standard risk scores but does involve radiation exposure and some added costs. CAC testing is especially useful as a tiebreaker in older intermediate-risk patients who may be on the fence about starting primary prevention medications but could fall short among “younger, low-risk patients where, as they show here, the proportion of people who have a positive test is well below half.”
“So that means you’re going to have a very large number of people who are CAC 0, which is what we would expect in relatively younger people, but I wouldn’t be happy to try to explain that to a patient: ‘We’re not seeing coronary atherosclerosis right now, but we still want to treat your risk factors.’ That’s kind of a dissonant message,” Dr. Greenland said.
An accompanying editorial suggests “the study has filled an important clinical gap, providing highly actionable data that could help guide clinical decision making for ASCVD prevention.”
Nevertheless, Tasneem Naqvi, MD, Mayo Clinic, Scottsdale, Arizona, and Tamar Polonsky, MD, University of Chicago, question the generalizability of the results and point out that CAC screening at the authors’ recommended ages “could still miss a substantial number of young women with incident MI.”
Exposure to ionizing radiation with CAC is lower than that used in screening mammography for breast cancer but, they agree, should be considered, particularly in young women.
“Alternatively, ultrasonography avoids radiation altogether and can detect plaque earlier than the development of CAC,” write Dr. Naqvi and Dr. Polonsky. Further, the 2019 European Society of Cardiology guidelines for CV risk give ultrasound assessment of carotid artery and femoral plaque a class IIa recommendation and CAC a class IIb recommendation.
Commenting for this news organization, Roger Blumenthal, MD, director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, said the class IIb recommendation “never really made any sense because the data with coronary calcium is so much stronger than it is with carotid ultrasound.”
“Sometimes smart scientists and researchers differ, but in my strong opinion, the European Society of Cardiology in 2019 did not give it the right classification, while the group I was part of, the American Heart Association/American College of Cardiology [2019 guideline], got it right and emphasized that this is the most cost-effective and useful way to improve risk assessment.”
Dr. Blumenthal, who was not part of the study, noted that U.S. guidelines say CAC measurement is not intended as a screening test for everyone but may be used selectively as a decision aid.
“This study adds to the information about how to use that type of testing. So, I personally think it will be a highly referenced article in the next set of guidelines that the American Heart Association, American College of Cardiology, and other organizations have.”
The study was supported in part by a research grant from the National Institutes of Health National Heart, Lung, and Blood Institute. Dr. Dzaye, Dr. Blumenthal, Dr. Naqvi, and Dr. Polonsky report having no relevant financial relationships.
A version of this article appeared on Medscape.com.
New risk equations can help determine the need for a first coronary artery calcium (CAC) scan in young adults to identify those most at risk for premature atherosclerosis, researchers say.
“To our knowledge this is the first time to derive a clinical risk equation for the initial conversion from CAC 0, which can be used actually to guide the timing of CAC testing in young adults,” Omar Dzaye, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, said in an interview.
CAC is an independent predictor of adverse atherosclerotic cardiovascular disease (ASCVD), but routine screening is not recommended in low-risk groups. U.S. guidelines say CAC testing may be considered (class IIa) for risk stratification in adults 40 to 75 years at intermediate risk (estimated 10-year ASCVD risk 7.5% to 20%) when the decision to start preventive therapies is unclear.
The new sex-specific risk equations were derived from 22,346 adults 30 to 50 years of age who underwent CAC testing between 1991 and 2010 for ASCVD risk prediction at four high-volume centers in the CAC Consortium. The average age was 43.5 years, 25% were women, and 12.3% were non-White.
The participants were free of clinical ASCVD or CV symptoms at the time of scanning but had underlying traditional ASCVD risk factors (dyslipidemia in 49.6%, hypertension in 20.0%, active smokers 11.0%, and diabetes in 4.0%), an intermediate 10-year ASCVD risk (2.6%), and/or a significant family history of CHD (49.3%).
As reported in the Journal of the American College of Cardiology, 92.7% of participants had a low 10-year ASCVD risk below 5%, but 34.4% had CAC scores above 0 (median, 20 Agatston units).
Assuming a 25% testing yield (number needed to scan equals four to detect one CAC score above 0), the optimal age for a first scan in young men without risk factors was 42.3 years, and for women it was 57.6 years.
Young adults with one or more risk factors, however, would convert to CAC above 0 at least 3.3 years earlier on average. Diabetes had the strongest influence on the probability of conversion, with men and women predicted to develop incident CAC a respective 5.5 years and 7.3 years earlier on average.
The findings build on previous observations by the team showing that diabetes confers a 40% reduction in the so-called “warranty period” of a CAC score of 0, Dr. Dzaye noted. The National Lipid Association 2020 statement on CAC scoring also suggests it’s reasonable to obtain a CAC scan in people with diabetes aged 30 to 39 years.
“The predicted utility of CAC for ASCVD outcomes is similar in type 1 and type 2 diabetes; however, individuals with type 1 diabetes may actually develop CAC as young as 17 years of age,” he said. “Therefore, definitely, CAC studies in this population are required.”
In contrast, hypertension, dyslipidemia, active smoking, and a family history of CHD were individually associated with the development of CAC 3.3 to 4.3 years earlier. In general, the time to premature CAC was longer for women than for men with a given risk-factor profile.
The predicted age for a first CAC was 37.5 years for men and 48.9 years for women with an intermediate risk-factor profile (for example, smoking plus hypertension) and 33.8 years and 44.7 years, respectively, for those with a high-risk profile (for example, diabetes plus dyslipidemia).
Asked whether the risk equations can be used to guide CAC scanning in clinical practice, Dr. Dzaye said, “we very much believe that this can be used because for the process we published the internal validation, and we also did an external validation that is not published at the moment in [the] MESA [trial].”
He pointed out that study participants did not have a second CAC scan for true modeling of longitudinal CAC and do not represent the general population but, rather, a general cardiology referral population enriched with ASCVD risk factors. Future studies are needed that incorporate a more diverse population, multiple CAC scans, and genetic risk factors.
“This is helpful from a descriptive, epidemiologic point of view and helps us understand the approximate prevalence of coronary calcium greater than 0 in younger men and women, but I’m not convinced that it will or should change clinical practice,” cardiologist Philip Greenland, MD, a professor of preventive medicine and professor of medicine at Northwestern University in Chicago, said in an interview.
Dr. Greenland, who coauthored a review on CAC testing earlier this month, said CAC is the strongest tool we have to improve risk prediction beyond standard risk scores but does involve radiation exposure and some added costs. CAC testing is especially useful as a tiebreaker in older intermediate-risk patients who may be on the fence about starting primary prevention medications but could fall short among “younger, low-risk patients where, as they show here, the proportion of people who have a positive test is well below half.”
“So that means you’re going to have a very large number of people who are CAC 0, which is what we would expect in relatively younger people, but I wouldn’t be happy to try to explain that to a patient: ‘We’re not seeing coronary atherosclerosis right now, but we still want to treat your risk factors.’ That’s kind of a dissonant message,” Dr. Greenland said.
An accompanying editorial suggests “the study has filled an important clinical gap, providing highly actionable data that could help guide clinical decision making for ASCVD prevention.”
Nevertheless, Tasneem Naqvi, MD, Mayo Clinic, Scottsdale, Arizona, and Tamar Polonsky, MD, University of Chicago, question the generalizability of the results and point out that CAC screening at the authors’ recommended ages “could still miss a substantial number of young women with incident MI.”
Exposure to ionizing radiation with CAC is lower than that used in screening mammography for breast cancer but, they agree, should be considered, particularly in young women.
“Alternatively, ultrasonography avoids radiation altogether and can detect plaque earlier than the development of CAC,” write Dr. Naqvi and Dr. Polonsky. Further, the 2019 European Society of Cardiology guidelines for CV risk give ultrasound assessment of carotid artery and femoral plaque a class IIa recommendation and CAC a class IIb recommendation.
Commenting for this news organization, Roger Blumenthal, MD, director of the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, said the class IIb recommendation “never really made any sense because the data with coronary calcium is so much stronger than it is with carotid ultrasound.”
“Sometimes smart scientists and researchers differ, but in my strong opinion, the European Society of Cardiology in 2019 did not give it the right classification, while the group I was part of, the American Heart Association/American College of Cardiology [2019 guideline], got it right and emphasized that this is the most cost-effective and useful way to improve risk assessment.”
Dr. Blumenthal, who was not part of the study, noted that U.S. guidelines say CAC measurement is not intended as a screening test for everyone but may be used selectively as a decision aid.
“This study adds to the information about how to use that type of testing. So, I personally think it will be a highly referenced article in the next set of guidelines that the American Heart Association, American College of Cardiology, and other organizations have.”
The study was supported in part by a research grant from the National Institutes of Health National Heart, Lung, and Blood Institute. Dr. Dzaye, Dr. Blumenthal, Dr. Naqvi, and Dr. Polonsky report having no relevant financial relationships.
A version of this article appeared on Medscape.com.
AAN blasts ‘runaway’ costs for neurologic and other prescription drugs
This situation is also taking a toll on neurologists’ mental health, who already have the second-highest burnout rate across medical specialties, the statement adds.
The statement was published online Oct. 5, 2021, in Neurology.
Dramatic price increases
Drafted by the Ethics, Law, and Humanities Committee – a joint committee that includes the AAN, the American Neurological Association, and the Child Neurology Society – the statement was prompted by a 2018 report from the AAN Neurology Drug Pricing Task Force to address challenges associated with high drug costs.
It highlights ethical concerns from high drug costs, policy proposals that might temper the problem, and how clinicians can adjust to the current reality of pharmaceutical pricing and better advocate for changes to the healthcare system.
“Runaway drug costs continue to be a pressing problem with recent dramatic price increases not only for specialty drugs, but also generic ones,” said lead author Amy Tsou, MD, MSc, codirector of the ECRI Evidence-Based Practice Center at the Center for Evidence and Guidelines in Plymouth Meeting, Pa.
She noted that one in four Americans has difficulty paying for medication, and many report going without a medication because of cost.
“Ensuring a fair system for drug pricing and coverage rules that balance the goods of individual patients with the needs of broader populations when resources are limited remains more important than ever,” Dr. Tsou said.
Out-of-pocket costs for neurologic medications have risen dramatically over the past decade, with the fastest rise reported among drugs for multiple sclerosis. Results from a study published in 2019 showed that, between 2004 and 2016, patients’ out-of-pocket expenses skyrocketed from $15 a month to $309 a month.
The steep increases have forced some neurology patients to ration their medication or stop taking it altogether, which is one of the ethical concerns cited in the AAN statement.
Patient self-rationing
Commenting on the statement, Ilana Katz Sand, MD, associate director of the Dickinson Center for Multiple Sclerosis at Mount Sinai Medical Center, New York, noted that clinicians are already acutely aware of the effect high drug costs have on their patients’ medical decisions. However, statements such as the current one bring much-needed outside attention to the problem.
“I’ve definitely had more and more people struggling with deductibles and copays, even among people who are insured,” said Dr. Katz Sand, who was not involved with the AAN paper.
She has a number of patients who have rationed their medication or stopped taking it altogether when their copays increased or they lost access to a copay assistance program because their insurance company chose to cover a still-expensive generic drug with no assistance program over a slightly costlier brand-name medication that comes with patient discounts.
Too often, patients don’t tell her they’re not taking their medication as prescribed. At a recent appointment, Dr. Katz Sand learned about a patient’s drug rationing only after a routine MRI showed new brain lesions that regular treatment might have prevented.
Another patient, new to her clinic, questioned the treatment plan Dr. Katz Sand recommended because they could not cover the copay. This sort of self-rationing happens in patients with and without insurance, she added.
“It’s a terrible thing and it’s happening to all patients,” Dr. Katz Sand said, adding that “the old credo of ‘yeah, the drug prices are high, but they are covered by insurance’ is not a sustainable argument anymore.”
What neurologists can do
Some sort of rationing is an unavoidable outcome of steep treatment costs, the authors noted. But what does that mean in clinical practice?
Neurologists should be aware of the costs involved in ordering diagnostic tests, treatment, or medication – and shouldn’t feel compelled to order treatments or tests that they feel are medically inappropriate just because a patient requests them, the authors wrote.
The statement also encourages clinicians to include financial realities in the shared decision-making process with patients.
However, Dr. Katz Sand said that is not always possible. Drug prices aren’t fixed, with different insurance plans offering different pricing, deductibles, and copays. “It’s hard for us to attempt to incorporate discussions about price in our discussions with patients when we can’t even predict what their out-of-pocket cost is going to be,” she said.
“Every single prescription we write requires prior authorization, and that’s directly related to the fact that the cost of these drugs is so high,” she added.
As do many other clinicians, Dr. Katz Sand spends hours each week on preauthorization forms and haggling with insurance companies on behalf of her patients. To get needed medication at a cost they can afford sometimes takes creative problem solving and almost always takes a lot of time. “It all adds to the administrative burden, patients’ stress, and our stress,” she said.
Physician-advocates needed
The AAN paper identifies a number of policy reforms to address drug pricing at a national level, including giving Medicare officials the power to negotiate drug prices, allowing the safe importation of drugs from other countries, and speeding the Food and Drug Administration approval process for generic drugs.
There is also a need to address systemic problems that, the authors noted, help create and perpetuate health care disparities. Lawmakers at the state and federal level are considering a number of these policy ideas and others that could address the kinds of issues Dr. Katz Sand described. However, the chances of their success are slim at best, said Bruce H. Cohen, MD, chair of the AAN advocacy committee and director of the NeuroDevelopmental Science Center at Akron (Ohio) Children’s Hospital.
“On a federal level, we’re watching in real time how the entrenched divisions, even within parties, are resulting in continued stalemate,” said Dr. Cohen, who is not one of the statement authors.
Those ideas need advocates and the AAN paper suggests neurologists should be among the ones championing these changes, he added.
“One of the most effective strategies is to bring attention to the impact high drug costs have on neurology patients and medical practices,” Dr. Cohen said. “It’s so important to make sure policy makers know the significant impact of high drug costs in neurology and within the context of finite resources.”
One way to do that is to share statements such as the current one with members of Congress working on policy reform, Dr. Cohen said. Another is through programs such as the academy’s annual Neurology on the Hill conference.
A third strategy is to encourage individuals such as Dr. Katz Sand to speak out when and where they can, he added.
While she agrees with the idea, finding time for advocacy work amid patient care, administrative work, and research is challenging. Dr. Katz Sand would like to see groups like the AAN work with health care institutions to implement policies that allocate time and resources to train clinicians in advocacy – and then support their efforts on that front.
“I’m really glad they wrote this and think they did a good job of crystallizing the issues,” Dr. Katz Sand said. “It’s good to put it out there as a document that could help serve as the basis for the requests we make collectively. I just hope that people listen.”
The paper received no funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This situation is also taking a toll on neurologists’ mental health, who already have the second-highest burnout rate across medical specialties, the statement adds.
The statement was published online Oct. 5, 2021, in Neurology.
Dramatic price increases
Drafted by the Ethics, Law, and Humanities Committee – a joint committee that includes the AAN, the American Neurological Association, and the Child Neurology Society – the statement was prompted by a 2018 report from the AAN Neurology Drug Pricing Task Force to address challenges associated with high drug costs.
It highlights ethical concerns from high drug costs, policy proposals that might temper the problem, and how clinicians can adjust to the current reality of pharmaceutical pricing and better advocate for changes to the healthcare system.
“Runaway drug costs continue to be a pressing problem with recent dramatic price increases not only for specialty drugs, but also generic ones,” said lead author Amy Tsou, MD, MSc, codirector of the ECRI Evidence-Based Practice Center at the Center for Evidence and Guidelines in Plymouth Meeting, Pa.
She noted that one in four Americans has difficulty paying for medication, and many report going without a medication because of cost.
“Ensuring a fair system for drug pricing and coverage rules that balance the goods of individual patients with the needs of broader populations when resources are limited remains more important than ever,” Dr. Tsou said.
Out-of-pocket costs for neurologic medications have risen dramatically over the past decade, with the fastest rise reported among drugs for multiple sclerosis. Results from a study published in 2019 showed that, between 2004 and 2016, patients’ out-of-pocket expenses skyrocketed from $15 a month to $309 a month.
The steep increases have forced some neurology patients to ration their medication or stop taking it altogether, which is one of the ethical concerns cited in the AAN statement.
Patient self-rationing
Commenting on the statement, Ilana Katz Sand, MD, associate director of the Dickinson Center for Multiple Sclerosis at Mount Sinai Medical Center, New York, noted that clinicians are already acutely aware of the effect high drug costs have on their patients’ medical decisions. However, statements such as the current one bring much-needed outside attention to the problem.
“I’ve definitely had more and more people struggling with deductibles and copays, even among people who are insured,” said Dr. Katz Sand, who was not involved with the AAN paper.
She has a number of patients who have rationed their medication or stopped taking it altogether when their copays increased or they lost access to a copay assistance program because their insurance company chose to cover a still-expensive generic drug with no assistance program over a slightly costlier brand-name medication that comes with patient discounts.
Too often, patients don’t tell her they’re not taking their medication as prescribed. At a recent appointment, Dr. Katz Sand learned about a patient’s drug rationing only after a routine MRI showed new brain lesions that regular treatment might have prevented.
Another patient, new to her clinic, questioned the treatment plan Dr. Katz Sand recommended because they could not cover the copay. This sort of self-rationing happens in patients with and without insurance, she added.
“It’s a terrible thing and it’s happening to all patients,” Dr. Katz Sand said, adding that “the old credo of ‘yeah, the drug prices are high, but they are covered by insurance’ is not a sustainable argument anymore.”
What neurologists can do
Some sort of rationing is an unavoidable outcome of steep treatment costs, the authors noted. But what does that mean in clinical practice?
Neurologists should be aware of the costs involved in ordering diagnostic tests, treatment, or medication – and shouldn’t feel compelled to order treatments or tests that they feel are medically inappropriate just because a patient requests them, the authors wrote.
The statement also encourages clinicians to include financial realities in the shared decision-making process with patients.
However, Dr. Katz Sand said that is not always possible. Drug prices aren’t fixed, with different insurance plans offering different pricing, deductibles, and copays. “It’s hard for us to attempt to incorporate discussions about price in our discussions with patients when we can’t even predict what their out-of-pocket cost is going to be,” she said.
“Every single prescription we write requires prior authorization, and that’s directly related to the fact that the cost of these drugs is so high,” she added.
As do many other clinicians, Dr. Katz Sand spends hours each week on preauthorization forms and haggling with insurance companies on behalf of her patients. To get needed medication at a cost they can afford sometimes takes creative problem solving and almost always takes a lot of time. “It all adds to the administrative burden, patients’ stress, and our stress,” she said.
Physician-advocates needed
The AAN paper identifies a number of policy reforms to address drug pricing at a national level, including giving Medicare officials the power to negotiate drug prices, allowing the safe importation of drugs from other countries, and speeding the Food and Drug Administration approval process for generic drugs.
There is also a need to address systemic problems that, the authors noted, help create and perpetuate health care disparities. Lawmakers at the state and federal level are considering a number of these policy ideas and others that could address the kinds of issues Dr. Katz Sand described. However, the chances of their success are slim at best, said Bruce H. Cohen, MD, chair of the AAN advocacy committee and director of the NeuroDevelopmental Science Center at Akron (Ohio) Children’s Hospital.
“On a federal level, we’re watching in real time how the entrenched divisions, even within parties, are resulting in continued stalemate,” said Dr. Cohen, who is not one of the statement authors.
Those ideas need advocates and the AAN paper suggests neurologists should be among the ones championing these changes, he added.
“One of the most effective strategies is to bring attention to the impact high drug costs have on neurology patients and medical practices,” Dr. Cohen said. “It’s so important to make sure policy makers know the significant impact of high drug costs in neurology and within the context of finite resources.”
One way to do that is to share statements such as the current one with members of Congress working on policy reform, Dr. Cohen said. Another is through programs such as the academy’s annual Neurology on the Hill conference.
A third strategy is to encourage individuals such as Dr. Katz Sand to speak out when and where they can, he added.
While she agrees with the idea, finding time for advocacy work amid patient care, administrative work, and research is challenging. Dr. Katz Sand would like to see groups like the AAN work with health care institutions to implement policies that allocate time and resources to train clinicians in advocacy – and then support their efforts on that front.
“I’m really glad they wrote this and think they did a good job of crystallizing the issues,” Dr. Katz Sand said. “It’s good to put it out there as a document that could help serve as the basis for the requests we make collectively. I just hope that people listen.”
The paper received no funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This situation is also taking a toll on neurologists’ mental health, who already have the second-highest burnout rate across medical specialties, the statement adds.
The statement was published online Oct. 5, 2021, in Neurology.
Dramatic price increases
Drafted by the Ethics, Law, and Humanities Committee – a joint committee that includes the AAN, the American Neurological Association, and the Child Neurology Society – the statement was prompted by a 2018 report from the AAN Neurology Drug Pricing Task Force to address challenges associated with high drug costs.
It highlights ethical concerns from high drug costs, policy proposals that might temper the problem, and how clinicians can adjust to the current reality of pharmaceutical pricing and better advocate for changes to the healthcare system.
“Runaway drug costs continue to be a pressing problem with recent dramatic price increases not only for specialty drugs, but also generic ones,” said lead author Amy Tsou, MD, MSc, codirector of the ECRI Evidence-Based Practice Center at the Center for Evidence and Guidelines in Plymouth Meeting, Pa.
She noted that one in four Americans has difficulty paying for medication, and many report going without a medication because of cost.
“Ensuring a fair system for drug pricing and coverage rules that balance the goods of individual patients with the needs of broader populations when resources are limited remains more important than ever,” Dr. Tsou said.
Out-of-pocket costs for neurologic medications have risen dramatically over the past decade, with the fastest rise reported among drugs for multiple sclerosis. Results from a study published in 2019 showed that, between 2004 and 2016, patients’ out-of-pocket expenses skyrocketed from $15 a month to $309 a month.
The steep increases have forced some neurology patients to ration their medication or stop taking it altogether, which is one of the ethical concerns cited in the AAN statement.
Patient self-rationing
Commenting on the statement, Ilana Katz Sand, MD, associate director of the Dickinson Center for Multiple Sclerosis at Mount Sinai Medical Center, New York, noted that clinicians are already acutely aware of the effect high drug costs have on their patients’ medical decisions. However, statements such as the current one bring much-needed outside attention to the problem.
“I’ve definitely had more and more people struggling with deductibles and copays, even among people who are insured,” said Dr. Katz Sand, who was not involved with the AAN paper.
She has a number of patients who have rationed their medication or stopped taking it altogether when their copays increased or they lost access to a copay assistance program because their insurance company chose to cover a still-expensive generic drug with no assistance program over a slightly costlier brand-name medication that comes with patient discounts.
Too often, patients don’t tell her they’re not taking their medication as prescribed. At a recent appointment, Dr. Katz Sand learned about a patient’s drug rationing only after a routine MRI showed new brain lesions that regular treatment might have prevented.
Another patient, new to her clinic, questioned the treatment plan Dr. Katz Sand recommended because they could not cover the copay. This sort of self-rationing happens in patients with and without insurance, she added.
“It’s a terrible thing and it’s happening to all patients,” Dr. Katz Sand said, adding that “the old credo of ‘yeah, the drug prices are high, but they are covered by insurance’ is not a sustainable argument anymore.”
What neurologists can do
Some sort of rationing is an unavoidable outcome of steep treatment costs, the authors noted. But what does that mean in clinical practice?
Neurologists should be aware of the costs involved in ordering diagnostic tests, treatment, or medication – and shouldn’t feel compelled to order treatments or tests that they feel are medically inappropriate just because a patient requests them, the authors wrote.
The statement also encourages clinicians to include financial realities in the shared decision-making process with patients.
However, Dr. Katz Sand said that is not always possible. Drug prices aren’t fixed, with different insurance plans offering different pricing, deductibles, and copays. “It’s hard for us to attempt to incorporate discussions about price in our discussions with patients when we can’t even predict what their out-of-pocket cost is going to be,” she said.
“Every single prescription we write requires prior authorization, and that’s directly related to the fact that the cost of these drugs is so high,” she added.
As do many other clinicians, Dr. Katz Sand spends hours each week on preauthorization forms and haggling with insurance companies on behalf of her patients. To get needed medication at a cost they can afford sometimes takes creative problem solving and almost always takes a lot of time. “It all adds to the administrative burden, patients’ stress, and our stress,” she said.
Physician-advocates needed
The AAN paper identifies a number of policy reforms to address drug pricing at a national level, including giving Medicare officials the power to negotiate drug prices, allowing the safe importation of drugs from other countries, and speeding the Food and Drug Administration approval process for generic drugs.
There is also a need to address systemic problems that, the authors noted, help create and perpetuate health care disparities. Lawmakers at the state and federal level are considering a number of these policy ideas and others that could address the kinds of issues Dr. Katz Sand described. However, the chances of their success are slim at best, said Bruce H. Cohen, MD, chair of the AAN advocacy committee and director of the NeuroDevelopmental Science Center at Akron (Ohio) Children’s Hospital.
“On a federal level, we’re watching in real time how the entrenched divisions, even within parties, are resulting in continued stalemate,” said Dr. Cohen, who is not one of the statement authors.
Those ideas need advocates and the AAN paper suggests neurologists should be among the ones championing these changes, he added.
“One of the most effective strategies is to bring attention to the impact high drug costs have on neurology patients and medical practices,” Dr. Cohen said. “It’s so important to make sure policy makers know the significant impact of high drug costs in neurology and within the context of finite resources.”
One way to do that is to share statements such as the current one with members of Congress working on policy reform, Dr. Cohen said. Another is through programs such as the academy’s annual Neurology on the Hill conference.
A third strategy is to encourage individuals such as Dr. Katz Sand to speak out when and where they can, he added.
While she agrees with the idea, finding time for advocacy work amid patient care, administrative work, and research is challenging. Dr. Katz Sand would like to see groups like the AAN work with health care institutions to implement policies that allocate time and resources to train clinicians in advocacy – and then support their efforts on that front.
“I’m really glad they wrote this and think they did a good job of crystallizing the issues,” Dr. Katz Sand said. “It’s good to put it out there as a document that could help serve as the basis for the requests we make collectively. I just hope that people listen.”
The paper received no funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gender-affirming care ‘can save lives,’ new research shows
Transgender and nonbinary young people experienced less depression and fewer suicidal thoughts after a year of gender-affirming care with hormones or puberty blockers, according to new research.
“Given the high rates of adverse mental health comorbidities, these data provide critical evidence that expansion of gender-affirming care can save lives,” said David J. Inwards-Breland, MD, MPH, chief of adolescent and young adult medicine and codirector of the Center for Gender-Affirming Care at Rady Children’s Hospital in San Diego, during his presentation.
The findings, presented October 11 at the American Academy of Pediatrics 2021 National Conference, were not at all surprising to Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
“The younger we can provide gender-affirming care, the less likely they’re going to have depression, and then the negative outcomes from untreated depression, which includes suicide intent or even suicide completion,” Dr. Breuner told this news organization. “It’s so obvious that we are saving lives by providing gender-affirming care.”
For their study, Dr. Inwards-Breland and his colleagues tracked depression, anxiety, and suicidality in 104 trans and nonbinary people 13 to 21 years of age who received care at the Seattle Children’s gender clinic between August 2017 and June 2018.
The study population consisted of 63 transgender male or male participants, 27 transgender female or female participants, 10 nonbinary participants, and four participants who had not defined their gender identity. Of this cohort, 62.5% were receiving mental health therapy, and 34.7% reported some substance use.
Participants completed the nine-item Patient Health Questionnaire (PHQ-9) and the seven-item Generalized Anxiety Disorder scale (GAD-7) at baseline and then at 3, 6, and 12 months. The researchers defined severe depression and severe anxiety as a score of 10 or greater on either scale.
At baseline, 56.7% of the participants had moderate to severe depression, 43.3% reported thoughts of self-harm or suicidal in the previous 2 weeks, and 50.0% had moderate to severe anxiety.
After 12 months of care, participants experienced a 60% decrease in depression (adjusted odds ratio, 0.4) and a 73% decrease in suicidality (aOR, 0.27), after adjustment for temporal trends and sex assigned at birth, race/ethnicity, level of parental support, ongoing mental health therapy, substance use, and victimization, including bullying, interpersonal violence, neglect, and abuse.
Although the decline in depression and suicidality after gender-affirming treatment was not a surprise, “those drops are huge,” Dr. Inwards-Breland said in an interview.
He said he attributes the improvement to a health care system that “affirms who these young people are” and enables changes that allow their outward appearance to reflect “who they know they are inside.”
There were no significant changes in anxiety during the study period. “Anxiety, I think, is just a little harder to treat, and it takes a little longer to treat,” he explained. And a lot of factors can trigger anxiety, and those can continue during treatment.
The slow pace of changes to gender identity can have an effect on people’s moods. “Since they’re not happening quickly, these young people are still being misgendered, they’re still seeing the body that they don’t feel like they should have, and they have to go to school and go out in public. I think that continues to fuel anxiety with a lot of these young people.”
Family support is important in reducing depression and suicidal thoughts in this population. Parents will often see positive changes after their child receives gender-affirming care, which can help contribute to positive changes in parents’ attitudes, Dr. Inwards-Breland said.
Such changes reinforce “that protective factor of connectedness with family,” he noted. “Families are crucial for any health care, and if there’s that connectedness with families, we know that, clinically, patients do better.”
Balancing risks
Although there are risks associated with gender-affirming hormones and puberty blockers, the risks of not receiving treatment must also be considered.
“Our young people are killing themselves,” he said. “Our young people are developing severe eating disorders that are killing them. Our young people are increasing their substance abuse, homelessness, depression. The list just goes on.”
For trans-masculine and nonbinary masculine patients, the potential permanent changes of hormone therapy include a deeper voice, hair growth, enlargement of the clitoris, and, in some patients, the development of male pattern baldness. In trans and nonbinary feminine patients, potential long-term effects include breast development and an increased risk for fertility issues.
The consent forms required for young people who want gender-affirming hormones or puberty blockers are extensive, with every possible reversible and irreversible effect described in detail, Dr. Breuner said.
“Parents sign them because they want their child to stay alive,” she explained. “When you compare the cost of someone who has severe debilitating depression and dying by suicide with some of the risks associated with gender-affirming hormone therapy, that’s a no-brainer to me.”
This study is limited by the fact that screening tests, not diagnostic tests, were used to identify depression, anxiety, and suicidality, and the fact that the use of antidepression or antianxiety medications was not taken into account, Dr. Inwards-Breland acknowledged.
“I think future studies should look at a mental health evaluation and diagnosis by a mental health provider,” he added. And mental health, gender dysphoria, suicidality, and self-harm should be tracked over the course of treatment.
He also acknowledged the study’s selection bias. All participants sought care at a multidisciplinary gender clinic, so were likely to be privileged and to have supportive families. “There’s a good chance that if we had more trans and nonbinary youth of color, we may have different findings,” he said.
More qualitative research is needed to assess the effect of gender-affirming therapy on the mental health of these patients, Dr. Breuner said.
“Being able to finally come into who you think you are and enjoy expressing who you are in a gender-affirming way has to be positive in such a way that you’re not depressed anymore,” she added. “It has to be tragic for people who cannot stand the body they’re in and cannot talk about it to anybody or express themselves without fear of recourse, to the point that they would be so devastated that they’d want to die by suicide.”
This research was funded by the Seattle Children’s Center for Diversity and Health Equity and the Pacific Hospital Development and Port Authority. Dr. Inwards-Breland and Dr. Breuner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender and nonbinary young people experienced less depression and fewer suicidal thoughts after a year of gender-affirming care with hormones or puberty blockers, according to new research.
“Given the high rates of adverse mental health comorbidities, these data provide critical evidence that expansion of gender-affirming care can save lives,” said David J. Inwards-Breland, MD, MPH, chief of adolescent and young adult medicine and codirector of the Center for Gender-Affirming Care at Rady Children’s Hospital in San Diego, during his presentation.
The findings, presented October 11 at the American Academy of Pediatrics 2021 National Conference, were not at all surprising to Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
“The younger we can provide gender-affirming care, the less likely they’re going to have depression, and then the negative outcomes from untreated depression, which includes suicide intent or even suicide completion,” Dr. Breuner told this news organization. “It’s so obvious that we are saving lives by providing gender-affirming care.”
For their study, Dr. Inwards-Breland and his colleagues tracked depression, anxiety, and suicidality in 104 trans and nonbinary people 13 to 21 years of age who received care at the Seattle Children’s gender clinic between August 2017 and June 2018.
The study population consisted of 63 transgender male or male participants, 27 transgender female or female participants, 10 nonbinary participants, and four participants who had not defined their gender identity. Of this cohort, 62.5% were receiving mental health therapy, and 34.7% reported some substance use.
Participants completed the nine-item Patient Health Questionnaire (PHQ-9) and the seven-item Generalized Anxiety Disorder scale (GAD-7) at baseline and then at 3, 6, and 12 months. The researchers defined severe depression and severe anxiety as a score of 10 or greater on either scale.
At baseline, 56.7% of the participants had moderate to severe depression, 43.3% reported thoughts of self-harm or suicidal in the previous 2 weeks, and 50.0% had moderate to severe anxiety.
After 12 months of care, participants experienced a 60% decrease in depression (adjusted odds ratio, 0.4) and a 73% decrease in suicidality (aOR, 0.27), after adjustment for temporal trends and sex assigned at birth, race/ethnicity, level of parental support, ongoing mental health therapy, substance use, and victimization, including bullying, interpersonal violence, neglect, and abuse.
Although the decline in depression and suicidality after gender-affirming treatment was not a surprise, “those drops are huge,” Dr. Inwards-Breland said in an interview.
He said he attributes the improvement to a health care system that “affirms who these young people are” and enables changes that allow their outward appearance to reflect “who they know they are inside.”
There were no significant changes in anxiety during the study period. “Anxiety, I think, is just a little harder to treat, and it takes a little longer to treat,” he explained. And a lot of factors can trigger anxiety, and those can continue during treatment.
The slow pace of changes to gender identity can have an effect on people’s moods. “Since they’re not happening quickly, these young people are still being misgendered, they’re still seeing the body that they don’t feel like they should have, and they have to go to school and go out in public. I think that continues to fuel anxiety with a lot of these young people.”
Family support is important in reducing depression and suicidal thoughts in this population. Parents will often see positive changes after their child receives gender-affirming care, which can help contribute to positive changes in parents’ attitudes, Dr. Inwards-Breland said.
Such changes reinforce “that protective factor of connectedness with family,” he noted. “Families are crucial for any health care, and if there’s that connectedness with families, we know that, clinically, patients do better.”
Balancing risks
Although there are risks associated with gender-affirming hormones and puberty blockers, the risks of not receiving treatment must also be considered.
“Our young people are killing themselves,” he said. “Our young people are developing severe eating disorders that are killing them. Our young people are increasing their substance abuse, homelessness, depression. The list just goes on.”
For trans-masculine and nonbinary masculine patients, the potential permanent changes of hormone therapy include a deeper voice, hair growth, enlargement of the clitoris, and, in some patients, the development of male pattern baldness. In trans and nonbinary feminine patients, potential long-term effects include breast development and an increased risk for fertility issues.
The consent forms required for young people who want gender-affirming hormones or puberty blockers are extensive, with every possible reversible and irreversible effect described in detail, Dr. Breuner said.
“Parents sign them because they want their child to stay alive,” she explained. “When you compare the cost of someone who has severe debilitating depression and dying by suicide with some of the risks associated with gender-affirming hormone therapy, that’s a no-brainer to me.”
This study is limited by the fact that screening tests, not diagnostic tests, were used to identify depression, anxiety, and suicidality, and the fact that the use of antidepression or antianxiety medications was not taken into account, Dr. Inwards-Breland acknowledged.
“I think future studies should look at a mental health evaluation and diagnosis by a mental health provider,” he added. And mental health, gender dysphoria, suicidality, and self-harm should be tracked over the course of treatment.
He also acknowledged the study’s selection bias. All participants sought care at a multidisciplinary gender clinic, so were likely to be privileged and to have supportive families. “There’s a good chance that if we had more trans and nonbinary youth of color, we may have different findings,” he said.
More qualitative research is needed to assess the effect of gender-affirming therapy on the mental health of these patients, Dr. Breuner said.
“Being able to finally come into who you think you are and enjoy expressing who you are in a gender-affirming way has to be positive in such a way that you’re not depressed anymore,” she added. “It has to be tragic for people who cannot stand the body they’re in and cannot talk about it to anybody or express themselves without fear of recourse, to the point that they would be so devastated that they’d want to die by suicide.”
This research was funded by the Seattle Children’s Center for Diversity and Health Equity and the Pacific Hospital Development and Port Authority. Dr. Inwards-Breland and Dr. Breuner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender and nonbinary young people experienced less depression and fewer suicidal thoughts after a year of gender-affirming care with hormones or puberty blockers, according to new research.
“Given the high rates of adverse mental health comorbidities, these data provide critical evidence that expansion of gender-affirming care can save lives,” said David J. Inwards-Breland, MD, MPH, chief of adolescent and young adult medicine and codirector of the Center for Gender-Affirming Care at Rady Children’s Hospital in San Diego, during his presentation.
The findings, presented October 11 at the American Academy of Pediatrics 2021 National Conference, were not at all surprising to Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
“The younger we can provide gender-affirming care, the less likely they’re going to have depression, and then the negative outcomes from untreated depression, which includes suicide intent or even suicide completion,” Dr. Breuner told this news organization. “It’s so obvious that we are saving lives by providing gender-affirming care.”
For their study, Dr. Inwards-Breland and his colleagues tracked depression, anxiety, and suicidality in 104 trans and nonbinary people 13 to 21 years of age who received care at the Seattle Children’s gender clinic between August 2017 and June 2018.
The study population consisted of 63 transgender male or male participants, 27 transgender female or female participants, 10 nonbinary participants, and four participants who had not defined their gender identity. Of this cohort, 62.5% were receiving mental health therapy, and 34.7% reported some substance use.
Participants completed the nine-item Patient Health Questionnaire (PHQ-9) and the seven-item Generalized Anxiety Disorder scale (GAD-7) at baseline and then at 3, 6, and 12 months. The researchers defined severe depression and severe anxiety as a score of 10 or greater on either scale.
At baseline, 56.7% of the participants had moderate to severe depression, 43.3% reported thoughts of self-harm or suicidal in the previous 2 weeks, and 50.0% had moderate to severe anxiety.
After 12 months of care, participants experienced a 60% decrease in depression (adjusted odds ratio, 0.4) and a 73% decrease in suicidality (aOR, 0.27), after adjustment for temporal trends and sex assigned at birth, race/ethnicity, level of parental support, ongoing mental health therapy, substance use, and victimization, including bullying, interpersonal violence, neglect, and abuse.
Although the decline in depression and suicidality after gender-affirming treatment was not a surprise, “those drops are huge,” Dr. Inwards-Breland said in an interview.
He said he attributes the improvement to a health care system that “affirms who these young people are” and enables changes that allow their outward appearance to reflect “who they know they are inside.”
There were no significant changes in anxiety during the study period. “Anxiety, I think, is just a little harder to treat, and it takes a little longer to treat,” he explained. And a lot of factors can trigger anxiety, and those can continue during treatment.
The slow pace of changes to gender identity can have an effect on people’s moods. “Since they’re not happening quickly, these young people are still being misgendered, they’re still seeing the body that they don’t feel like they should have, and they have to go to school and go out in public. I think that continues to fuel anxiety with a lot of these young people.”
Family support is important in reducing depression and suicidal thoughts in this population. Parents will often see positive changes after their child receives gender-affirming care, which can help contribute to positive changes in parents’ attitudes, Dr. Inwards-Breland said.
Such changes reinforce “that protective factor of connectedness with family,” he noted. “Families are crucial for any health care, and if there’s that connectedness with families, we know that, clinically, patients do better.”
Balancing risks
Although there are risks associated with gender-affirming hormones and puberty blockers, the risks of not receiving treatment must also be considered.
“Our young people are killing themselves,” he said. “Our young people are developing severe eating disorders that are killing them. Our young people are increasing their substance abuse, homelessness, depression. The list just goes on.”
For trans-masculine and nonbinary masculine patients, the potential permanent changes of hormone therapy include a deeper voice, hair growth, enlargement of the clitoris, and, in some patients, the development of male pattern baldness. In trans and nonbinary feminine patients, potential long-term effects include breast development and an increased risk for fertility issues.
The consent forms required for young people who want gender-affirming hormones or puberty blockers are extensive, with every possible reversible and irreversible effect described in detail, Dr. Breuner said.
“Parents sign them because they want their child to stay alive,” she explained. “When you compare the cost of someone who has severe debilitating depression and dying by suicide with some of the risks associated with gender-affirming hormone therapy, that’s a no-brainer to me.”
This study is limited by the fact that screening tests, not diagnostic tests, were used to identify depression, anxiety, and suicidality, and the fact that the use of antidepression or antianxiety medications was not taken into account, Dr. Inwards-Breland acknowledged.
“I think future studies should look at a mental health evaluation and diagnosis by a mental health provider,” he added. And mental health, gender dysphoria, suicidality, and self-harm should be tracked over the course of treatment.
He also acknowledged the study’s selection bias. All participants sought care at a multidisciplinary gender clinic, so were likely to be privileged and to have supportive families. “There’s a good chance that if we had more trans and nonbinary youth of color, we may have different findings,” he said.
More qualitative research is needed to assess the effect of gender-affirming therapy on the mental health of these patients, Dr. Breuner said.
“Being able to finally come into who you think you are and enjoy expressing who you are in a gender-affirming way has to be positive in such a way that you’re not depressed anymore,” she added. “It has to be tragic for people who cannot stand the body they’re in and cannot talk about it to anybody or express themselves without fear of recourse, to the point that they would be so devastated that they’d want to die by suicide.”
This research was funded by the Seattle Children’s Center for Diversity and Health Equity and the Pacific Hospital Development and Port Authority. Dr. Inwards-Breland and Dr. Breuner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Call them by their names in your office
Given that approximately 9.5% of youth aged 13-17 in the United States identify as lesbian, gay, bisexual, transgender, or queer (LGBTQ),1 it is likely that a general pediatrician or pediatric subspecialist is going to encounter at least one LGBTQ patient during the course of the average workweek. By having an easy way to identify these patients and store this data in a user-friendly manner, you can ensure that your practice is LGBTQ friendly and an affirming environment for all sexual- and gender-minority youth.
One way to do this is to look over any paper or electronic forms your practice uses and make sure that they provide patients and families a range of options to identify themselves. For example, you could provide more options for gender, other than male or female, including a nonbinary or “other” (with a free text line) option. This allows your patients to give you an accurate description of what their affirmed gender is. Instead of having a space for mother’s name and father’s name, you could list these fields as “parent/guardian #1” and “parent/guardian #2.” These labels allow for more inclusivity and to reflect the diverse makeup of modern families. Providing a space for a patient to put the name and pronouns that they use allows your staff to make sure that you are calling a patient by the correct name and using the correct pronouns.
Within your EMR, there may be editable fields that allow for you or your staff to list the patient’s affirmed name and pronouns. Making this small change allows any staff member who accesses the chart to have that information displayed correctly for them and reduces the chances of staff misgendering or dead-naming a patient. Underscoring the importance of this, Sequeira et al. found that in a sample of youth from a gender clinic, only 9% of those adolescents reported that they were asked their name/pronouns outside of the gender clinic.2 If those fields are not there, you may check with your IT staff or your EMR vendor to see if these fields may be added in. However, staff needs to make sure that they check with the child/adolescent first to discern with whom the patient has discussed their gender identity. If you were to put a patient’s affirmed name into the chart and then call the patient by that name in front of the parent/guardian, the parent/guardian may look at you quizzically about why you are calling their child by that name. This could then cause an uncomfortable conversation in the exam room or result in harm to the patient after the visit.
It is not just good clinical practice to ensure that you use a patient’s affirmed name and pronouns. Russell et al. looked at the relationship between depressive symptoms and suicidal ideation and whether an adolescent’s name/pronouns were used in the context of their home, school, work, and/or friend group. They found that use of an adolescent’s affirmed name in at least one of these contexts was associated with a decrease in depressive symptoms and a 29% decrease in suicidal ideation.3 Therefore, the use of an adolescent’s affirmed name and pronouns in your office contributes to the overall mental well-being of your patients.
Fortunately, there are many guides to help you and your practice be successful at implementing some of these changes. The Gay, Lesbian, Bisexual and Transgender Health Access Project put together its “Community Standards of Practice for the Provision of Quality Health Care Services to Lesbian, Gay, Bisexual, and Transgender Clients” to aid practices in developing environments that are LGBTQ affirming. The National LGBTQIA+ Health Education Center, a part of the Fenway Institute, has a series of learning modules that you and your staff can view for interactive training and tips for best practices. These resources offer pediatricians and their practices free resources to improve their policies and procedures. By instituting these small changes, you can ensure that your practice continues to be an affirming environment for your LGBTQ children and adolescents.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Conran KJ. LGBT youth population in the United States, UCLA School of Law, Williams Institute, 2020 Sep.
2. Sequeira GM et al. Affirming transgender youths’ names and pronouns in the electronic medical record. JAMA Pediatr. 2020;174(5):501-3.
3. Russell ST et al. Chosen name use is linked to reduced depressive symptoms, suicidal ideation, and suicidal behavior among transgender youth. J Adolesc Health. 2018;63(4):503-5.
Given that approximately 9.5% of youth aged 13-17 in the United States identify as lesbian, gay, bisexual, transgender, or queer (LGBTQ),1 it is likely that a general pediatrician or pediatric subspecialist is going to encounter at least one LGBTQ patient during the course of the average workweek. By having an easy way to identify these patients and store this data in a user-friendly manner, you can ensure that your practice is LGBTQ friendly and an affirming environment for all sexual- and gender-minority youth.
One way to do this is to look over any paper or electronic forms your practice uses and make sure that they provide patients and families a range of options to identify themselves. For example, you could provide more options for gender, other than male or female, including a nonbinary or “other” (with a free text line) option. This allows your patients to give you an accurate description of what their affirmed gender is. Instead of having a space for mother’s name and father’s name, you could list these fields as “parent/guardian #1” and “parent/guardian #2.” These labels allow for more inclusivity and to reflect the diverse makeup of modern families. Providing a space for a patient to put the name and pronouns that they use allows your staff to make sure that you are calling a patient by the correct name and using the correct pronouns.
Within your EMR, there may be editable fields that allow for you or your staff to list the patient’s affirmed name and pronouns. Making this small change allows any staff member who accesses the chart to have that information displayed correctly for them and reduces the chances of staff misgendering or dead-naming a patient. Underscoring the importance of this, Sequeira et al. found that in a sample of youth from a gender clinic, only 9% of those adolescents reported that they were asked their name/pronouns outside of the gender clinic.2 If those fields are not there, you may check with your IT staff or your EMR vendor to see if these fields may be added in. However, staff needs to make sure that they check with the child/adolescent first to discern with whom the patient has discussed their gender identity. If you were to put a patient’s affirmed name into the chart and then call the patient by that name in front of the parent/guardian, the parent/guardian may look at you quizzically about why you are calling their child by that name. This could then cause an uncomfortable conversation in the exam room or result in harm to the patient after the visit.
It is not just good clinical practice to ensure that you use a patient’s affirmed name and pronouns. Russell et al. looked at the relationship between depressive symptoms and suicidal ideation and whether an adolescent’s name/pronouns were used in the context of their home, school, work, and/or friend group. They found that use of an adolescent’s affirmed name in at least one of these contexts was associated with a decrease in depressive symptoms and a 29% decrease in suicidal ideation.3 Therefore, the use of an adolescent’s affirmed name and pronouns in your office contributes to the overall mental well-being of your patients.
Fortunately, there are many guides to help you and your practice be successful at implementing some of these changes. The Gay, Lesbian, Bisexual and Transgender Health Access Project put together its “Community Standards of Practice for the Provision of Quality Health Care Services to Lesbian, Gay, Bisexual, and Transgender Clients” to aid practices in developing environments that are LGBTQ affirming. The National LGBTQIA+ Health Education Center, a part of the Fenway Institute, has a series of learning modules that you and your staff can view for interactive training and tips for best practices. These resources offer pediatricians and their practices free resources to improve their policies and procedures. By instituting these small changes, you can ensure that your practice continues to be an affirming environment for your LGBTQ children and adolescents.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Conran KJ. LGBT youth population in the United States, UCLA School of Law, Williams Institute, 2020 Sep.
2. Sequeira GM et al. Affirming transgender youths’ names and pronouns in the electronic medical record. JAMA Pediatr. 2020;174(5):501-3.
3. Russell ST et al. Chosen name use is linked to reduced depressive symptoms, suicidal ideation, and suicidal behavior among transgender youth. J Adolesc Health. 2018;63(4):503-5.
Given that approximately 9.5% of youth aged 13-17 in the United States identify as lesbian, gay, bisexual, transgender, or queer (LGBTQ),1 it is likely that a general pediatrician or pediatric subspecialist is going to encounter at least one LGBTQ patient during the course of the average workweek. By having an easy way to identify these patients and store this data in a user-friendly manner, you can ensure that your practice is LGBTQ friendly and an affirming environment for all sexual- and gender-minority youth.
One way to do this is to look over any paper or electronic forms your practice uses and make sure that they provide patients and families a range of options to identify themselves. For example, you could provide more options for gender, other than male or female, including a nonbinary or “other” (with a free text line) option. This allows your patients to give you an accurate description of what their affirmed gender is. Instead of having a space for mother’s name and father’s name, you could list these fields as “parent/guardian #1” and “parent/guardian #2.” These labels allow for more inclusivity and to reflect the diverse makeup of modern families. Providing a space for a patient to put the name and pronouns that they use allows your staff to make sure that you are calling a patient by the correct name and using the correct pronouns.
Within your EMR, there may be editable fields that allow for you or your staff to list the patient’s affirmed name and pronouns. Making this small change allows any staff member who accesses the chart to have that information displayed correctly for them and reduces the chances of staff misgendering or dead-naming a patient. Underscoring the importance of this, Sequeira et al. found that in a sample of youth from a gender clinic, only 9% of those adolescents reported that they were asked their name/pronouns outside of the gender clinic.2 If those fields are not there, you may check with your IT staff or your EMR vendor to see if these fields may be added in. However, staff needs to make sure that they check with the child/adolescent first to discern with whom the patient has discussed their gender identity. If you were to put a patient’s affirmed name into the chart and then call the patient by that name in front of the parent/guardian, the parent/guardian may look at you quizzically about why you are calling their child by that name. This could then cause an uncomfortable conversation in the exam room or result in harm to the patient after the visit.
It is not just good clinical practice to ensure that you use a patient’s affirmed name and pronouns. Russell et al. looked at the relationship between depressive symptoms and suicidal ideation and whether an adolescent’s name/pronouns were used in the context of their home, school, work, and/or friend group. They found that use of an adolescent’s affirmed name in at least one of these contexts was associated with a decrease in depressive symptoms and a 29% decrease in suicidal ideation.3 Therefore, the use of an adolescent’s affirmed name and pronouns in your office contributes to the overall mental well-being of your patients.
Fortunately, there are many guides to help you and your practice be successful at implementing some of these changes. The Gay, Lesbian, Bisexual and Transgender Health Access Project put together its “Community Standards of Practice for the Provision of Quality Health Care Services to Lesbian, Gay, Bisexual, and Transgender Clients” to aid practices in developing environments that are LGBTQ affirming. The National LGBTQIA+ Health Education Center, a part of the Fenway Institute, has a series of learning modules that you and your staff can view for interactive training and tips for best practices. These resources offer pediatricians and their practices free resources to improve their policies and procedures. By instituting these small changes, you can ensure that your practice continues to be an affirming environment for your LGBTQ children and adolescents.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Conran KJ. LGBT youth population in the United States, UCLA School of Law, Williams Institute, 2020 Sep.
2. Sequeira GM et al. Affirming transgender youths’ names and pronouns in the electronic medical record. JAMA Pediatr. 2020;174(5):501-3.
3. Russell ST et al. Chosen name use is linked to reduced depressive symptoms, suicidal ideation, and suicidal behavior among transgender youth. J Adolesc Health. 2018;63(4):503-5.
What’s behind the rise in youth anxiety and depression?
It’s well known that levels of anxiety and depression in youth are on the rise. While some of this increase may be because of other things, such as a lowering of the threshold for what counts as clinically relevant symptoms and decreased stigma when it comes to seeking out mental health services, there seems little debate that the number of children and adolescents who are actually struggling with their mental health is taking a sharp turn for the worse.
What is much less certain are the causes behind this surge. The answer to this important question will likely defy a clear answer from a definitive study. In its place then are a number of different theories that have been circulated and discussed. Each comes with some evidence to support the hypothesis, but none seems able to make a truly compelling argument as the single driving force behind this trend. This column briefly describes and examines some of the factors that may be contributing to the rise in anxiety and depression while providing some explanation for why each factor is unlikely to be the sole culprit.
Some of the biggest suggested causes for the rise in child and adolescent mental health problems include the following:
- COVID. Multiple studies have documented increases in mood and anxiety associated with the pandemic, which in turn, may be because of a number of factors such as social isolation, loss of family members, family financial stressors, and many other contributors.1 Yet, while it certainly makes sense that COVID is a powerful instigator of mood and anxiety problems, there is good evidence that the upward tic in emotional-behavioral problems began well before the COVID pandemic.2
- Smartphones. In 2017, psychologist Jean Twenge penned a provocative essay in the Atlantic with the title “Have Smartphones Destroyed a Generation?” and the basic answer was yes.3 The foundation for this conclusion was the tracking between the rise in mood and anxiety problems and the meteoric rise of smartphone use in youth. None of these associations, however, can be proven as casual, and more experimental data on the link between smartphone usage and mental health have been inconsistent.
- Bullying. The toxic effect of bullying and, in particular, online or “cyberbullying” has frequently been brought up as a potential cause. Yet while the negative effects of bullying have been well documented, there is evidence that overall bullying has actually decreased over recent years.4
These three factors have arguably been the most discussed, but a few others also probably deserve mention.
- Helicopter parenting. Critics of this common and increasingly popular approach to parenting are concerned that all the parental hovering and stepping in convey the message that the world is a very dangerous place while depriving children of opportunities to gain the exposure and competence they need to succeed. The critique is certainly logical and even has been supported in some studies but lacks the needed evidence for a more definitive conclusion.5
- Medications. Of course there will be stories blaming the mental health treatment itself, rather than the reasons people seek treatment, for this disturbing trend. And while it is always important to consider that medications can be part of the problem rather than the solution, the majority of evidence points overall to a lack of treatment rather than too much. A recent important study, for example, found that the peak of suicidal thoughts and behaviors occurred a month before medications were started, rather than after.6
- Cannabis. While there seems to be a lot of geographic variability with regard to whether or not the number of youth using cannabis is increasing or not, it’s clear that the product now being consumed is considerably stronger than what was used in decades past. This high-potency cannabis now being used has been shown to increase the risk for later mental health problems including psychosis and suicidal behavior.7 Unfortunately, these risks are not being heard as a powerful industry fights to increase their market share.
Putting all this together, it seems likely that a tidy and simple explanation for the alarming increase in youth mental health problems will be hard to pin down. It’s also worth pointing out that many of the above factors could work in a synergistic manner. For example, helicopter parenting may be keeping kids more confined to their rooms where they interact more and more on their phones and are exposed to higher amounts of online bullying, all of which has been magnified recently with the COVID pandemic. Obviously, understanding the causes behind this surge is much more than an academic exercise as the amount of stress and suffering rises and treatment resources get overwhelmed. In the meantime, addressing all of the above factors in both primary and specialty care is worthwhile in an effort to reverse this worrying and wide-ranging pattern.
Dr. Rettew is a child and adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Ore. He is the author of the 2021 book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
References
1. Hawes MT et al. Psychol Med. 2021;13:1-9.
2. Twenge JM et al. J Abnorm Psych. 2019;128(3):185-99.
3. Twenge JM. Have Smartphones Destroyed a Generation? The Atlantic. 2017:September.
4. Rettew DC. Bullying: An update. Child Psych Clin North Am. 2021; in press.
5. Van Der Bruggen CO et al. J Child Psychol Psychiatry. 2008;49(12):1257-69.
6. Lagerberg T et al. Selective serotonin reuptake inhibitors and suicidal behaviour: A population-based cohort study. Neuropsychopharmacology 2021 Sep 24.
7. Gobbi G et al. JAMA Psychiatry. 2019;76(4):426-34.
It’s well known that levels of anxiety and depression in youth are on the rise. While some of this increase may be because of other things, such as a lowering of the threshold for what counts as clinically relevant symptoms and decreased stigma when it comes to seeking out mental health services, there seems little debate that the number of children and adolescents who are actually struggling with their mental health is taking a sharp turn for the worse.
What is much less certain are the causes behind this surge. The answer to this important question will likely defy a clear answer from a definitive study. In its place then are a number of different theories that have been circulated and discussed. Each comes with some evidence to support the hypothesis, but none seems able to make a truly compelling argument as the single driving force behind this trend. This column briefly describes and examines some of the factors that may be contributing to the rise in anxiety and depression while providing some explanation for why each factor is unlikely to be the sole culprit.
Some of the biggest suggested causes for the rise in child and adolescent mental health problems include the following:
- COVID. Multiple studies have documented increases in mood and anxiety associated with the pandemic, which in turn, may be because of a number of factors such as social isolation, loss of family members, family financial stressors, and many other contributors.1 Yet, while it certainly makes sense that COVID is a powerful instigator of mood and anxiety problems, there is good evidence that the upward tic in emotional-behavioral problems began well before the COVID pandemic.2
- Smartphones. In 2017, psychologist Jean Twenge penned a provocative essay in the Atlantic with the title “Have Smartphones Destroyed a Generation?” and the basic answer was yes.3 The foundation for this conclusion was the tracking between the rise in mood and anxiety problems and the meteoric rise of smartphone use in youth. None of these associations, however, can be proven as casual, and more experimental data on the link between smartphone usage and mental health have been inconsistent.
- Bullying. The toxic effect of bullying and, in particular, online or “cyberbullying” has frequently been brought up as a potential cause. Yet while the negative effects of bullying have been well documented, there is evidence that overall bullying has actually decreased over recent years.4
These three factors have arguably been the most discussed, but a few others also probably deserve mention.
- Helicopter parenting. Critics of this common and increasingly popular approach to parenting are concerned that all the parental hovering and stepping in convey the message that the world is a very dangerous place while depriving children of opportunities to gain the exposure and competence they need to succeed. The critique is certainly logical and even has been supported in some studies but lacks the needed evidence for a more definitive conclusion.5
- Medications. Of course there will be stories blaming the mental health treatment itself, rather than the reasons people seek treatment, for this disturbing trend. And while it is always important to consider that medications can be part of the problem rather than the solution, the majority of evidence points overall to a lack of treatment rather than too much. A recent important study, for example, found that the peak of suicidal thoughts and behaviors occurred a month before medications were started, rather than after.6
- Cannabis. While there seems to be a lot of geographic variability with regard to whether or not the number of youth using cannabis is increasing or not, it’s clear that the product now being consumed is considerably stronger than what was used in decades past. This high-potency cannabis now being used has been shown to increase the risk for later mental health problems including psychosis and suicidal behavior.7 Unfortunately, these risks are not being heard as a powerful industry fights to increase their market share.
Putting all this together, it seems likely that a tidy and simple explanation for the alarming increase in youth mental health problems will be hard to pin down. It’s also worth pointing out that many of the above factors could work in a synergistic manner. For example, helicopter parenting may be keeping kids more confined to their rooms where they interact more and more on their phones and are exposed to higher amounts of online bullying, all of which has been magnified recently with the COVID pandemic. Obviously, understanding the causes behind this surge is much more than an academic exercise as the amount of stress and suffering rises and treatment resources get overwhelmed. In the meantime, addressing all of the above factors in both primary and specialty care is worthwhile in an effort to reverse this worrying and wide-ranging pattern.
Dr. Rettew is a child and adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Ore. He is the author of the 2021 book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
References
1. Hawes MT et al. Psychol Med. 2021;13:1-9.
2. Twenge JM et al. J Abnorm Psych. 2019;128(3):185-99.
3. Twenge JM. Have Smartphones Destroyed a Generation? The Atlantic. 2017:September.
4. Rettew DC. Bullying: An update. Child Psych Clin North Am. 2021; in press.
5. Van Der Bruggen CO et al. J Child Psychol Psychiatry. 2008;49(12):1257-69.
6. Lagerberg T et al. Selective serotonin reuptake inhibitors and suicidal behaviour: A population-based cohort study. Neuropsychopharmacology 2021 Sep 24.
7. Gobbi G et al. JAMA Psychiatry. 2019;76(4):426-34.
It’s well known that levels of anxiety and depression in youth are on the rise. While some of this increase may be because of other things, such as a lowering of the threshold for what counts as clinically relevant symptoms and decreased stigma when it comes to seeking out mental health services, there seems little debate that the number of children and adolescents who are actually struggling with their mental health is taking a sharp turn for the worse.
What is much less certain are the causes behind this surge. The answer to this important question will likely defy a clear answer from a definitive study. In its place then are a number of different theories that have been circulated and discussed. Each comes with some evidence to support the hypothesis, but none seems able to make a truly compelling argument as the single driving force behind this trend. This column briefly describes and examines some of the factors that may be contributing to the rise in anxiety and depression while providing some explanation for why each factor is unlikely to be the sole culprit.
Some of the biggest suggested causes for the rise in child and adolescent mental health problems include the following:
- COVID. Multiple studies have documented increases in mood and anxiety associated with the pandemic, which in turn, may be because of a number of factors such as social isolation, loss of family members, family financial stressors, and many other contributors.1 Yet, while it certainly makes sense that COVID is a powerful instigator of mood and anxiety problems, there is good evidence that the upward tic in emotional-behavioral problems began well before the COVID pandemic.2
- Smartphones. In 2017, psychologist Jean Twenge penned a provocative essay in the Atlantic with the title “Have Smartphones Destroyed a Generation?” and the basic answer was yes.3 The foundation for this conclusion was the tracking between the rise in mood and anxiety problems and the meteoric rise of smartphone use in youth. None of these associations, however, can be proven as casual, and more experimental data on the link between smartphone usage and mental health have been inconsistent.
- Bullying. The toxic effect of bullying and, in particular, online or “cyberbullying” has frequently been brought up as a potential cause. Yet while the negative effects of bullying have been well documented, there is evidence that overall bullying has actually decreased over recent years.4
These three factors have arguably been the most discussed, but a few others also probably deserve mention.
- Helicopter parenting. Critics of this common and increasingly popular approach to parenting are concerned that all the parental hovering and stepping in convey the message that the world is a very dangerous place while depriving children of opportunities to gain the exposure and competence they need to succeed. The critique is certainly logical and even has been supported in some studies but lacks the needed evidence for a more definitive conclusion.5
- Medications. Of course there will be stories blaming the mental health treatment itself, rather than the reasons people seek treatment, for this disturbing trend. And while it is always important to consider that medications can be part of the problem rather than the solution, the majority of evidence points overall to a lack of treatment rather than too much. A recent important study, for example, found that the peak of suicidal thoughts and behaviors occurred a month before medications were started, rather than after.6
- Cannabis. While there seems to be a lot of geographic variability with regard to whether or not the number of youth using cannabis is increasing or not, it’s clear that the product now being consumed is considerably stronger than what was used in decades past. This high-potency cannabis now being used has been shown to increase the risk for later mental health problems including psychosis and suicidal behavior.7 Unfortunately, these risks are not being heard as a powerful industry fights to increase their market share.
Putting all this together, it seems likely that a tidy and simple explanation for the alarming increase in youth mental health problems will be hard to pin down. It’s also worth pointing out that many of the above factors could work in a synergistic manner. For example, helicopter parenting may be keeping kids more confined to their rooms where they interact more and more on their phones and are exposed to higher amounts of online bullying, all of which has been magnified recently with the COVID pandemic. Obviously, understanding the causes behind this surge is much more than an academic exercise as the amount of stress and suffering rises and treatment resources get overwhelmed. In the meantime, addressing all of the above factors in both primary and specialty care is worthwhile in an effort to reverse this worrying and wide-ranging pattern.
Dr. Rettew is a child and adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Ore. He is the author of the 2021 book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.” You can follow him on Twitter and Facebook @PediPsych.
References
1. Hawes MT et al. Psychol Med. 2021;13:1-9.
2. Twenge JM et al. J Abnorm Psych. 2019;128(3):185-99.
3. Twenge JM. Have Smartphones Destroyed a Generation? The Atlantic. 2017:September.
4. Rettew DC. Bullying: An update. Child Psych Clin North Am. 2021; in press.
5. Van Der Bruggen CO et al. J Child Psychol Psychiatry. 2008;49(12):1257-69.
6. Lagerberg T et al. Selective serotonin reuptake inhibitors and suicidal behaviour: A population-based cohort study. Neuropsychopharmacology 2021 Sep 24.
7. Gobbi G et al. JAMA Psychiatry. 2019;76(4):426-34.
Telehealth a game changer for addiction treatment?
Providing addiction treatment remotely via telehealth has the potential to boost patients’ engagement in treatment by improving access and convenience. However, whether telehealth results in better retention or other outcomes than in-person treatment remains an open question, new research indicates.
“Telehealth really might be a game changer for getting people into addiction treatment, but we still need more research to confirm the benefits of telehealth and to determine under what conditions telehealth is best used,” study investigator Tami L. Mark, PhD, said during a press briefing held by the American Psychiatric Association.
The study was published online October 13 in Psychiatric Services ahead of the organization’s first-ever Mental Health Services Conference, which will be held online October 14-15.
COVID turned on the telehealth light switch
“COVID-19 was like turning on a light switch for telehealth,” said Dr. Mark, with the nonprofit research institute RTI International, in Rockville, Maryland.
“Before the COVID-19 public health emergency and stay-at-home order that the governor of California issued in March of 2020, only about 1 in 4 addiction service providers in California offered any type of telehealth. By July 2020, almost 100% were offering telehealth,” she noted.
This was possible through relaxation of federal and state regulations that had previously constrained use of telehealth for addiction treatment. Policymakers and payers are now considering which of these changes should be maintained.
For the study, investigators used mixed qualitative and quantitative methods to assess the efficacy of telehealth for addiction treatment and to gain insights from practitioners regarding their experiences during the pandemic.
They reviewed eight published studies that compared addiction treatment via telehealth with in-person treatment.
Seven found telehealth treatment to be as effective but not more effective than in-person treatment in terms of retention, satisfaction with treatment, therapeutic alliance, and substance use. Most of the studies were small (less than 150 patients).
However, one large study from Canada showed that telehealth facilitated methadone prescribing and improved retention.
The researchers also conducted an online survey in 2020 of 100 California addiction treatment practitioners and interviewed 30 California addiction professionals and other stakeholders.
Survey respondents indicated that more than 50% of their patients were being treated via telehealth for intensive outpatient treatment, individual counseling, group counseling, and intake assessment.
They were less sure about the relative effectiveness of managing medication via telehealth, group counseling, and intake assessments.
Remote challenges
Many of the practitioners interviewed for the study noted that telehealth reduces the time and cost to patients of participating in treatment and that it offers an opportunity for clinicians to observe patients’ home environment and engage patients’ families.
Others felt strongly that patients with substance use disorders need personal relationships and connectedness, which are hard to establish virtually.
They also noted that it is more difficult to sense how a patient is doing when meeting virtually and that it can be challenging to keep patients focused online.
“Providers seem to be moving to a hybrid approach where telehealth is used for some patients and some services but not others,” Dr. Mark said.
“Additional research is needed to determine how best to tailor telehealth to each patient’s circumstances and the best mix of in-person and telehealth services,” she added.
Speaking at the briefing, Lisa Dixon, MD, MPH, editor of Psychiatric Services, said the research “tackles arguably the most important issue in psychiatry today – telehealth.”
“The pandemic brought it to the forefront more quickly than otherwise, but appreciation of its potential positive and negative impacts, I think, was inevitable,” said Dr. Dixon.
“Research has taught us a lot, as has our experience, but we have a long way to go in understanding telehealth and addiction treatment. I really like this article because it appreciates some of the unique issues with the treatment for substance use as opposed to other mental health challenges,” said Dr. Dixon.
Funding for the study was provided by the Patient-Centered Outcomes Research Institute. Dr. Mark and Dr. Dixon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Providing addiction treatment remotely via telehealth has the potential to boost patients’ engagement in treatment by improving access and convenience. However, whether telehealth results in better retention or other outcomes than in-person treatment remains an open question, new research indicates.
“Telehealth really might be a game changer for getting people into addiction treatment, but we still need more research to confirm the benefits of telehealth and to determine under what conditions telehealth is best used,” study investigator Tami L. Mark, PhD, said during a press briefing held by the American Psychiatric Association.
The study was published online October 13 in Psychiatric Services ahead of the organization’s first-ever Mental Health Services Conference, which will be held online October 14-15.
COVID turned on the telehealth light switch
“COVID-19 was like turning on a light switch for telehealth,” said Dr. Mark, with the nonprofit research institute RTI International, in Rockville, Maryland.
“Before the COVID-19 public health emergency and stay-at-home order that the governor of California issued in March of 2020, only about 1 in 4 addiction service providers in California offered any type of telehealth. By July 2020, almost 100% were offering telehealth,” she noted.
This was possible through relaxation of federal and state regulations that had previously constrained use of telehealth for addiction treatment. Policymakers and payers are now considering which of these changes should be maintained.
For the study, investigators used mixed qualitative and quantitative methods to assess the efficacy of telehealth for addiction treatment and to gain insights from practitioners regarding their experiences during the pandemic.
They reviewed eight published studies that compared addiction treatment via telehealth with in-person treatment.
Seven found telehealth treatment to be as effective but not more effective than in-person treatment in terms of retention, satisfaction with treatment, therapeutic alliance, and substance use. Most of the studies were small (less than 150 patients).
However, one large study from Canada showed that telehealth facilitated methadone prescribing and improved retention.
The researchers also conducted an online survey in 2020 of 100 California addiction treatment practitioners and interviewed 30 California addiction professionals and other stakeholders.
Survey respondents indicated that more than 50% of their patients were being treated via telehealth for intensive outpatient treatment, individual counseling, group counseling, and intake assessment.
They were less sure about the relative effectiveness of managing medication via telehealth, group counseling, and intake assessments.
Remote challenges
Many of the practitioners interviewed for the study noted that telehealth reduces the time and cost to patients of participating in treatment and that it offers an opportunity for clinicians to observe patients’ home environment and engage patients’ families.
Others felt strongly that patients with substance use disorders need personal relationships and connectedness, which are hard to establish virtually.
They also noted that it is more difficult to sense how a patient is doing when meeting virtually and that it can be challenging to keep patients focused online.
“Providers seem to be moving to a hybrid approach where telehealth is used for some patients and some services but not others,” Dr. Mark said.
“Additional research is needed to determine how best to tailor telehealth to each patient’s circumstances and the best mix of in-person and telehealth services,” she added.
Speaking at the briefing, Lisa Dixon, MD, MPH, editor of Psychiatric Services, said the research “tackles arguably the most important issue in psychiatry today – telehealth.”
“The pandemic brought it to the forefront more quickly than otherwise, but appreciation of its potential positive and negative impacts, I think, was inevitable,” said Dr. Dixon.
“Research has taught us a lot, as has our experience, but we have a long way to go in understanding telehealth and addiction treatment. I really like this article because it appreciates some of the unique issues with the treatment for substance use as opposed to other mental health challenges,” said Dr. Dixon.
Funding for the study was provided by the Patient-Centered Outcomes Research Institute. Dr. Mark and Dr. Dixon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Providing addiction treatment remotely via telehealth has the potential to boost patients’ engagement in treatment by improving access and convenience. However, whether telehealth results in better retention or other outcomes than in-person treatment remains an open question, new research indicates.
“Telehealth really might be a game changer for getting people into addiction treatment, but we still need more research to confirm the benefits of telehealth and to determine under what conditions telehealth is best used,” study investigator Tami L. Mark, PhD, said during a press briefing held by the American Psychiatric Association.
The study was published online October 13 in Psychiatric Services ahead of the organization’s first-ever Mental Health Services Conference, which will be held online October 14-15.
COVID turned on the telehealth light switch
“COVID-19 was like turning on a light switch for telehealth,” said Dr. Mark, with the nonprofit research institute RTI International, in Rockville, Maryland.
“Before the COVID-19 public health emergency and stay-at-home order that the governor of California issued in March of 2020, only about 1 in 4 addiction service providers in California offered any type of telehealth. By July 2020, almost 100% were offering telehealth,” she noted.
This was possible through relaxation of federal and state regulations that had previously constrained use of telehealth for addiction treatment. Policymakers and payers are now considering which of these changes should be maintained.
For the study, investigators used mixed qualitative and quantitative methods to assess the efficacy of telehealth for addiction treatment and to gain insights from practitioners regarding their experiences during the pandemic.
They reviewed eight published studies that compared addiction treatment via telehealth with in-person treatment.
Seven found telehealth treatment to be as effective but not more effective than in-person treatment in terms of retention, satisfaction with treatment, therapeutic alliance, and substance use. Most of the studies were small (less than 150 patients).
However, one large study from Canada showed that telehealth facilitated methadone prescribing and improved retention.
The researchers also conducted an online survey in 2020 of 100 California addiction treatment practitioners and interviewed 30 California addiction professionals and other stakeholders.
Survey respondents indicated that more than 50% of their patients were being treated via telehealth for intensive outpatient treatment, individual counseling, group counseling, and intake assessment.
They were less sure about the relative effectiveness of managing medication via telehealth, group counseling, and intake assessments.
Remote challenges
Many of the practitioners interviewed for the study noted that telehealth reduces the time and cost to patients of participating in treatment and that it offers an opportunity for clinicians to observe patients’ home environment and engage patients’ families.
Others felt strongly that patients with substance use disorders need personal relationships and connectedness, which are hard to establish virtually.
They also noted that it is more difficult to sense how a patient is doing when meeting virtually and that it can be challenging to keep patients focused online.
“Providers seem to be moving to a hybrid approach where telehealth is used for some patients and some services but not others,” Dr. Mark said.
“Additional research is needed to determine how best to tailor telehealth to each patient’s circumstances and the best mix of in-person and telehealth services,” she added.
Speaking at the briefing, Lisa Dixon, MD, MPH, editor of Psychiatric Services, said the research “tackles arguably the most important issue in psychiatry today – telehealth.”
“The pandemic brought it to the forefront more quickly than otherwise, but appreciation of its potential positive and negative impacts, I think, was inevitable,” said Dr. Dixon.
“Research has taught us a lot, as has our experience, but we have a long way to go in understanding telehealth and addiction treatment. I really like this article because it appreciates some of the unique issues with the treatment for substance use as opposed to other mental health challenges,” said Dr. Dixon.
Funding for the study was provided by the Patient-Centered Outcomes Research Institute. Dr. Mark and Dr. Dixon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Radiofrequency ablation gains favor for thyroid nodules in U.S.
And in one case, a hospital has taken the unique step of forming a multidisciplinary thyroid nodule RFA tumor board, which helps in the often tricky decision-making process that is involved.
“Our multidisciplinary RFA tumor board has been invaluable in this process, and it is the only one of its kind in the nation that I’m aware of,” James Lim, MD, of the Division of Surgical Oncology, Thyroid, and Parathyroid Center at Oregon Health Sciences University (OHSU), told this news organization.
Dr. Lim reports receiving referrals from “all avenues, some from thyroid specialists and others from nonthyroid specialists such as primary care practitioners or patient self-referrals.”
“Because of this, our centralized process of multidisciplinary review ensures that each patient is evaluated thoroughly through each thyroid specialists’ lens to optimize patient outcomes,” noted Dr. Lim, an assistant professor of endocrine surgery.
The RFA tumor board consists of experts in all specialties involved in thyroid nodule assessment and treatment, including surgeons, interventional radiologists, and endocrinologists.
Just because you can, doesn’t mean you should
However, there should be some caution that although there is enthusiasm regarding this noninvasive alternative to surgery, there is another option, that of mere observation, which is appropriate in many cases of thyroid nodules and should not be overlooked.
“For a number of reasons, the key to keep in mind is that, just because we can do something doesn’t mean we should,” Michael Singer, MD, director of the Division of Thyroid & Parathyroid Surgery, Department of Otolaryngology – Head and Neck Surgery, at the Henry Ford Health System, Detroit, said in an interview.
While emphasizing that he believes RFA to be a promising technology that will likely benefit patients in the future, Dr. Singer voiced concern about the approach becoming an easy choice – particularly if profit is to be had – when observation is a clear alternative. “If RFA becomes seen as an opportunity to create revenue, potential conflicts of interest may arise,” he said.
“As it is not a major procedure with a dramatic risk profile, my concern is that some clinicians [could] adopt the attitude of ‘Why not do it?’ even when the indication is minimal or nonexistent,” he added.
Dr. Lim said he agrees that “any new medical technology requires thoughtful evaluation and appropriate patient selection in order to ensure optimal patient outcomes.”
That’s where the tumor board has been especially beneficial.
“We have found great benefit in reviewing potential RFA cases in a multidisciplinary fashion within our tumor board and would recommend other institutions to consider it,” he noted. In the absence of a tumor board, “at a minimum, a thyroid specialist should be involved in the evaluation of a potential thyroid RFA patient prior to ablation treatment,” he advised.
Tumor board was able to identify a small subset of patients for surgery
In his research presented at the 90th Annual Meeting of the American Thyroid Association (ATA), Dr. Lim and colleagues evaluated the tumor board’s efficacy in altering diagnosis and treatment plans in a retrospective review of cases presented to the board for RFA consideration since its inception in July 2020 through June 2021.
Over the study period, 65 patients with biopsy-proven benign thyroid nodules were newly referred for RFA, with 58 referred for mass effect symptoms and seven for autonomous function.
After the multidisciplinary review, about half of the cases, 37 (56.9%), were approved for RFA.
Of the remainder, 22 (33.8%) were determined to need additional studies, just two (3.0%) were recommended for surgery, and four (6.2%) were recommended to not receive any intervention.
Of the 22 cases recommended for additional studies, 15 were subsequently recommended for RFA and four were recommended to receive surgery due to suspicious clinical findings.
Of those that underwent surgery, two showed thyroid cancer on final pathology.
Among the nodules recommended to RFA, the average nodule volume was 15.1 mL, whereas the average volume for those recommended for surgery was 40.9 mL (P = .08).
No significant complications occurred among patients that underwent RFA or those who had surgery.
“The tumor board’s multidisciplinary review was able to identify high-risk features in some patients with benign biopsies. This led to a change in recommendation from RFA to surgery for possible malignancy in a small subset of patients,” Dr. Lim noted.
In a separate analysis, Dr. Lim and colleagues reported that, among patients treated with RFA (with a mean baseline nodule volume of 11.9 mL), mean nodule volume was 6.4 mL after 1 month, 4.5 mL after 3 months, and 3.8 mL at 6 months, which were all significantly reduced versus baseline (P < .001). Similar improvements were also reported in symptom and cosmetic scores at each timepoint (all P < .001).
There were no cases of postprocedural hypothyroidism or symptomatic thyrotoxicosis.
Underlining that patients can expect noticeable improvement in symptom scores by their 30-day visit, Dr. Lim noted that patients should be warned of some early swelling.
“It is important to inform patients that they may have swelling of their treated nodule immediately after the procedure, but this should subside within a few days,” he said.
Outpatient RFA safe and efficacious
In a separate study also presented at the meeting, three practitioners described their experiences with RFA in their outpatient thyroid practices in San Antonio; Santa Monica, California; and Gettysburg, Pennsylvania.
Overall, there were 68 cases involving benign, class II thyroid nodules, and the authors reported average procedure times of under an hour, with actual RFA time varying from 7 to 22 minutes.
Of note, for nodules larger than 4.5 cm, two procedures were necessary to achieve desired results.
Excluding the larger nodules requiring more than one procedure, there was an average decrease in nodule size of 48% at 1 month and a decrease of 82% after 3 months in more than 80% of cases.
None of the cases required surgery. There were no major complications, and all patients had preserved baseline thyroid function.
“This preliminary study of 68 patients shows how thyroid RFA is safe and efficacious when performed in an endocrine outpatient office practice,” Kathleen Hands, MD, of the Thyroid Center of South Texas, and coauthors concluded.
Insurance coverage an issue in U.S.
Among much larger studies demonstrating the safety and efficacy of RFA for benign nodules, a study of 450 Chinese patients published in January showed RFA to be superior to conventional thyroidectomy in terms of patient satisfaction, postoperative quality of life, and shorter hospital stay, although the caveat was it took longer to achieve nodule volume reduction.
But if RFA use is to become more widespread in the United States, a key obstacle is that insurance companies generally do not cover the procedure. Although patients in Dr. Lim’s analyses did have coverage, it didn’t come easily, he said.
“Thankfully, all of our patients have been approved by insurance, and no one has had to pay by themselves, but this has sometimes required multiple appeals to the insurance company,” Dr. Lim said.
“The American Association of Endocrine Surgeons and Society of Interventional Radiology are both working towards getting this valuable treatment more readily accepted by more insurance companies,” he said.
Dr. Lim and Dr. Singer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
And in one case, a hospital has taken the unique step of forming a multidisciplinary thyroid nodule RFA tumor board, which helps in the often tricky decision-making process that is involved.
“Our multidisciplinary RFA tumor board has been invaluable in this process, and it is the only one of its kind in the nation that I’m aware of,” James Lim, MD, of the Division of Surgical Oncology, Thyroid, and Parathyroid Center at Oregon Health Sciences University (OHSU), told this news organization.
Dr. Lim reports receiving referrals from “all avenues, some from thyroid specialists and others from nonthyroid specialists such as primary care practitioners or patient self-referrals.”
“Because of this, our centralized process of multidisciplinary review ensures that each patient is evaluated thoroughly through each thyroid specialists’ lens to optimize patient outcomes,” noted Dr. Lim, an assistant professor of endocrine surgery.
The RFA tumor board consists of experts in all specialties involved in thyroid nodule assessment and treatment, including surgeons, interventional radiologists, and endocrinologists.
Just because you can, doesn’t mean you should
However, there should be some caution that although there is enthusiasm regarding this noninvasive alternative to surgery, there is another option, that of mere observation, which is appropriate in many cases of thyroid nodules and should not be overlooked.
“For a number of reasons, the key to keep in mind is that, just because we can do something doesn’t mean we should,” Michael Singer, MD, director of the Division of Thyroid & Parathyroid Surgery, Department of Otolaryngology – Head and Neck Surgery, at the Henry Ford Health System, Detroit, said in an interview.
While emphasizing that he believes RFA to be a promising technology that will likely benefit patients in the future, Dr. Singer voiced concern about the approach becoming an easy choice – particularly if profit is to be had – when observation is a clear alternative. “If RFA becomes seen as an opportunity to create revenue, potential conflicts of interest may arise,” he said.
“As it is not a major procedure with a dramatic risk profile, my concern is that some clinicians [could] adopt the attitude of ‘Why not do it?’ even when the indication is minimal or nonexistent,” he added.
Dr. Lim said he agrees that “any new medical technology requires thoughtful evaluation and appropriate patient selection in order to ensure optimal patient outcomes.”
That’s where the tumor board has been especially beneficial.
“We have found great benefit in reviewing potential RFA cases in a multidisciplinary fashion within our tumor board and would recommend other institutions to consider it,” he noted. In the absence of a tumor board, “at a minimum, a thyroid specialist should be involved in the evaluation of a potential thyroid RFA patient prior to ablation treatment,” he advised.
Tumor board was able to identify a small subset of patients for surgery
In his research presented at the 90th Annual Meeting of the American Thyroid Association (ATA), Dr. Lim and colleagues evaluated the tumor board’s efficacy in altering diagnosis and treatment plans in a retrospective review of cases presented to the board for RFA consideration since its inception in July 2020 through June 2021.
Over the study period, 65 patients with biopsy-proven benign thyroid nodules were newly referred for RFA, with 58 referred for mass effect symptoms and seven for autonomous function.
After the multidisciplinary review, about half of the cases, 37 (56.9%), were approved for RFA.
Of the remainder, 22 (33.8%) were determined to need additional studies, just two (3.0%) were recommended for surgery, and four (6.2%) were recommended to not receive any intervention.
Of the 22 cases recommended for additional studies, 15 were subsequently recommended for RFA and four were recommended to receive surgery due to suspicious clinical findings.
Of those that underwent surgery, two showed thyroid cancer on final pathology.
Among the nodules recommended to RFA, the average nodule volume was 15.1 mL, whereas the average volume for those recommended for surgery was 40.9 mL (P = .08).
No significant complications occurred among patients that underwent RFA or those who had surgery.
“The tumor board’s multidisciplinary review was able to identify high-risk features in some patients with benign biopsies. This led to a change in recommendation from RFA to surgery for possible malignancy in a small subset of patients,” Dr. Lim noted.
In a separate analysis, Dr. Lim and colleagues reported that, among patients treated with RFA (with a mean baseline nodule volume of 11.9 mL), mean nodule volume was 6.4 mL after 1 month, 4.5 mL after 3 months, and 3.8 mL at 6 months, which were all significantly reduced versus baseline (P < .001). Similar improvements were also reported in symptom and cosmetic scores at each timepoint (all P < .001).
There were no cases of postprocedural hypothyroidism or symptomatic thyrotoxicosis.
Underlining that patients can expect noticeable improvement in symptom scores by their 30-day visit, Dr. Lim noted that patients should be warned of some early swelling.
“It is important to inform patients that they may have swelling of their treated nodule immediately after the procedure, but this should subside within a few days,” he said.
Outpatient RFA safe and efficacious
In a separate study also presented at the meeting, three practitioners described their experiences with RFA in their outpatient thyroid practices in San Antonio; Santa Monica, California; and Gettysburg, Pennsylvania.
Overall, there were 68 cases involving benign, class II thyroid nodules, and the authors reported average procedure times of under an hour, with actual RFA time varying from 7 to 22 minutes.
Of note, for nodules larger than 4.5 cm, two procedures were necessary to achieve desired results.
Excluding the larger nodules requiring more than one procedure, there was an average decrease in nodule size of 48% at 1 month and a decrease of 82% after 3 months in more than 80% of cases.
None of the cases required surgery. There were no major complications, and all patients had preserved baseline thyroid function.
“This preliminary study of 68 patients shows how thyroid RFA is safe and efficacious when performed in an endocrine outpatient office practice,” Kathleen Hands, MD, of the Thyroid Center of South Texas, and coauthors concluded.
Insurance coverage an issue in U.S.
Among much larger studies demonstrating the safety and efficacy of RFA for benign nodules, a study of 450 Chinese patients published in January showed RFA to be superior to conventional thyroidectomy in terms of patient satisfaction, postoperative quality of life, and shorter hospital stay, although the caveat was it took longer to achieve nodule volume reduction.
But if RFA use is to become more widespread in the United States, a key obstacle is that insurance companies generally do not cover the procedure. Although patients in Dr. Lim’s analyses did have coverage, it didn’t come easily, he said.
“Thankfully, all of our patients have been approved by insurance, and no one has had to pay by themselves, but this has sometimes required multiple appeals to the insurance company,” Dr. Lim said.
“The American Association of Endocrine Surgeons and Society of Interventional Radiology are both working towards getting this valuable treatment more readily accepted by more insurance companies,” he said.
Dr. Lim and Dr. Singer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
And in one case, a hospital has taken the unique step of forming a multidisciplinary thyroid nodule RFA tumor board, which helps in the often tricky decision-making process that is involved.
“Our multidisciplinary RFA tumor board has been invaluable in this process, and it is the only one of its kind in the nation that I’m aware of,” James Lim, MD, of the Division of Surgical Oncology, Thyroid, and Parathyroid Center at Oregon Health Sciences University (OHSU), told this news organization.
Dr. Lim reports receiving referrals from “all avenues, some from thyroid specialists and others from nonthyroid specialists such as primary care practitioners or patient self-referrals.”
“Because of this, our centralized process of multidisciplinary review ensures that each patient is evaluated thoroughly through each thyroid specialists’ lens to optimize patient outcomes,” noted Dr. Lim, an assistant professor of endocrine surgery.
The RFA tumor board consists of experts in all specialties involved in thyroid nodule assessment and treatment, including surgeons, interventional radiologists, and endocrinologists.
Just because you can, doesn’t mean you should
However, there should be some caution that although there is enthusiasm regarding this noninvasive alternative to surgery, there is another option, that of mere observation, which is appropriate in many cases of thyroid nodules and should not be overlooked.
“For a number of reasons, the key to keep in mind is that, just because we can do something doesn’t mean we should,” Michael Singer, MD, director of the Division of Thyroid & Parathyroid Surgery, Department of Otolaryngology – Head and Neck Surgery, at the Henry Ford Health System, Detroit, said in an interview.
While emphasizing that he believes RFA to be a promising technology that will likely benefit patients in the future, Dr. Singer voiced concern about the approach becoming an easy choice – particularly if profit is to be had – when observation is a clear alternative. “If RFA becomes seen as an opportunity to create revenue, potential conflicts of interest may arise,” he said.
“As it is not a major procedure with a dramatic risk profile, my concern is that some clinicians [could] adopt the attitude of ‘Why not do it?’ even when the indication is minimal or nonexistent,” he added.
Dr. Lim said he agrees that “any new medical technology requires thoughtful evaluation and appropriate patient selection in order to ensure optimal patient outcomes.”
That’s where the tumor board has been especially beneficial.
“We have found great benefit in reviewing potential RFA cases in a multidisciplinary fashion within our tumor board and would recommend other institutions to consider it,” he noted. In the absence of a tumor board, “at a minimum, a thyroid specialist should be involved in the evaluation of a potential thyroid RFA patient prior to ablation treatment,” he advised.
Tumor board was able to identify a small subset of patients for surgery
In his research presented at the 90th Annual Meeting of the American Thyroid Association (ATA), Dr. Lim and colleagues evaluated the tumor board’s efficacy in altering diagnosis and treatment plans in a retrospective review of cases presented to the board for RFA consideration since its inception in July 2020 through June 2021.
Over the study period, 65 patients with biopsy-proven benign thyroid nodules were newly referred for RFA, with 58 referred for mass effect symptoms and seven for autonomous function.
After the multidisciplinary review, about half of the cases, 37 (56.9%), were approved for RFA.
Of the remainder, 22 (33.8%) were determined to need additional studies, just two (3.0%) were recommended for surgery, and four (6.2%) were recommended to not receive any intervention.
Of the 22 cases recommended for additional studies, 15 were subsequently recommended for RFA and four were recommended to receive surgery due to suspicious clinical findings.
Of those that underwent surgery, two showed thyroid cancer on final pathology.
Among the nodules recommended to RFA, the average nodule volume was 15.1 mL, whereas the average volume for those recommended for surgery was 40.9 mL (P = .08).
No significant complications occurred among patients that underwent RFA or those who had surgery.
“The tumor board’s multidisciplinary review was able to identify high-risk features in some patients with benign biopsies. This led to a change in recommendation from RFA to surgery for possible malignancy in a small subset of patients,” Dr. Lim noted.
In a separate analysis, Dr. Lim and colleagues reported that, among patients treated with RFA (with a mean baseline nodule volume of 11.9 mL), mean nodule volume was 6.4 mL after 1 month, 4.5 mL after 3 months, and 3.8 mL at 6 months, which were all significantly reduced versus baseline (P < .001). Similar improvements were also reported in symptom and cosmetic scores at each timepoint (all P < .001).
There were no cases of postprocedural hypothyroidism or symptomatic thyrotoxicosis.
Underlining that patients can expect noticeable improvement in symptom scores by their 30-day visit, Dr. Lim noted that patients should be warned of some early swelling.
“It is important to inform patients that they may have swelling of their treated nodule immediately after the procedure, but this should subside within a few days,” he said.
Outpatient RFA safe and efficacious
In a separate study also presented at the meeting, three practitioners described their experiences with RFA in their outpatient thyroid practices in San Antonio; Santa Monica, California; and Gettysburg, Pennsylvania.
Overall, there were 68 cases involving benign, class II thyroid nodules, and the authors reported average procedure times of under an hour, with actual RFA time varying from 7 to 22 minutes.
Of note, for nodules larger than 4.5 cm, two procedures were necessary to achieve desired results.
Excluding the larger nodules requiring more than one procedure, there was an average decrease in nodule size of 48% at 1 month and a decrease of 82% after 3 months in more than 80% of cases.
None of the cases required surgery. There were no major complications, and all patients had preserved baseline thyroid function.
“This preliminary study of 68 patients shows how thyroid RFA is safe and efficacious when performed in an endocrine outpatient office practice,” Kathleen Hands, MD, of the Thyroid Center of South Texas, and coauthors concluded.
Insurance coverage an issue in U.S.
Among much larger studies demonstrating the safety and efficacy of RFA for benign nodules, a study of 450 Chinese patients published in January showed RFA to be superior to conventional thyroidectomy in terms of patient satisfaction, postoperative quality of life, and shorter hospital stay, although the caveat was it took longer to achieve nodule volume reduction.
But if RFA use is to become more widespread in the United States, a key obstacle is that insurance companies generally do not cover the procedure. Although patients in Dr. Lim’s analyses did have coverage, it didn’t come easily, he said.
“Thankfully, all of our patients have been approved by insurance, and no one has had to pay by themselves, but this has sometimes required multiple appeals to the insurance company,” Dr. Lim said.
“The American Association of Endocrine Surgeons and Society of Interventional Radiology are both working towards getting this valuable treatment more readily accepted by more insurance companies,” he said.
Dr. Lim and Dr. Singer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Impressive’ results for novel antidepressant, so why the FDA delay?
A novel antidepressant (AXS-05, Axsome Therapeutics) appears to have a rapid and durable effect in patients with major depressive disorder (MDD), results of an open label, phase 3 trial, show. Yet, its new drug application (NDA) remains in limbo with the U.S. Food and Drug Administration for reasons that are unclear.
In the study, which included 876 patients with MDD, results showed the drug, a combination of dextromethorphan and bupropion, had a clinical response rate of 80% and a remission rate of almost 70%. In addition, functional improvements were “substantial” and AXS-05 was determined to be “generally safe and well-tolerated.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
The study
The COMET trial was a phase 3, multicenter, U.S. trial, in which patients with MDD were treated with AXS-05 twice daily for up to 12 months. Patients had to have a Montgomery-Åsberg Depression Rating Scale (MADRS) score of at least 25. They could have completed a prior AXS-05 study or be newly enrolled.
Of 876 patients included in the study, 611 were newly enrolled. The mean age was 42.4 years, and 62.4% were women. Just over half (58.1%) were White, with 35.6% Black, and 2.0% Asian. The mean body mass index was 31.4 kg/m2.
The mean MADRS total score at baseline was 32.7 and the Sheehan Disability Scale (SDS) score was 20.0.
Presenting efficacy data in 609 newly enrolled patients, the team showed that MADRS total scores fell sharply on starting AXS-05, by 9.1 points at week 1, 14.0 points at week 2, and 21.2 points at week 6.
By 6 months, the reduction over baseline was 23.9 points, which was maintained out to 12 months, at a mean reduction of 23.0 points.
The proportion of patients achieving a clinical response, defined as a greater than or equal to 50% improvement in MADRS scores, was 18.8% at week 1, 39.7% at week 2, and 73.2% at week 6. There was a clinical response in 84.6% of patients at 6 months and in 82.8% at 12 months.
Clinical remission, defined as a MADRS score less than or equal to 10, was achieved in 8.3% of patients at week 1, rising to 21.5% at week 2, and 52.5% at week 6. At 6 months, 68.7% of patients were in clinical remission, reaching 69.0% at 12 months.
These benefits were accompanied by substantial improvements in depressive symptoms on the Clinical Global Impression of Improvement scale, with a marked or moderate improvement seen in 86.7% of patients at 6 months and 93.1% at 12 months.
Moreover, a clinical response in functioning on the SDS was achieved by 80.6% of patients at 6 months and 75.9% at 12 months.
The safety analysis of AXS-05 in the entire cohort suggested it was well-tolerated, with dizziness seen in 12.7% of patients, along with nausea in 11.9%, headache in 8.8%, dry mouth in 7.1%, and decreased appetite in 6.1%.
The rate of discontinuation due to adverse events was 8.4%, and there were no signs of psychotomimetic effects, cognitive impairment, weight gain, or increased sexual dysfunction.
and increased rates of suicidal ideation resolution, and was also effective for treatment-resistant depression.
Results from an analysis of the ASCEND phase 2 and GEMINI phase 3 trials also suggested that AXS-05 was superior to both bupropion and placebo in achieving rapid and sustained improvements in depression symptoms.
FDA delay
Yet despite these seemingly positive findings, the FDA appears to have issues with the agent’s new drug application.
As reported in August, the agency reviewed the NDA for AXS-05 for the treatment of MDD, but at that time the drug’s manufacturer revealed that the agency had identified “deficiencies that preclude labeling discussions at this time.”
With the latest results presented at ECNP 2021, this news organization asked Axsome about the status of the NDA and whether there had been any further discussions and/or movement with the FDA.
Instead of a direct reply from the drug company, this news organization was directed to a statement released by Axsome in August announcing that the FDA had informed the company that its NDA review “would not be completed by the Prescription Drug User Fee Act target action date of August 22, 2021.”
“The FDA did not request additional information from the company, and the review of the application is ongoing,” the statement said. Axsome did not respond to further questions.
‘Impressive’ remission rate
Commenting on the research, Marin Jukic, PhD, department of physiology and pharmacology, Karolinska Institutet, Stockholm, who was not involved in the research, said AXS-05 “looks promising in relation to the efficacy and tolerability results” with a remission rate that is “truly impressive.”
However, Dr. Jukic cautioned that it was an open-label trial and therefore had no placebo or active comparator arms.
He noted that it would be “interesting to compare the efficacy with placebo and escitalopram, for example, to evaluate the potential for the benefits and efficacy better.”
The research was funded by Axsome Therapeutics, and, except for one, the researchers for the four studies are employees of Axsome Therapeutics.
A version of this article first appeared on Medscape.com.
A novel antidepressant (AXS-05, Axsome Therapeutics) appears to have a rapid and durable effect in patients with major depressive disorder (MDD), results of an open label, phase 3 trial, show. Yet, its new drug application (NDA) remains in limbo with the U.S. Food and Drug Administration for reasons that are unclear.
In the study, which included 876 patients with MDD, results showed the drug, a combination of dextromethorphan and bupropion, had a clinical response rate of 80% and a remission rate of almost 70%. In addition, functional improvements were “substantial” and AXS-05 was determined to be “generally safe and well-tolerated.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
The study
The COMET trial was a phase 3, multicenter, U.S. trial, in which patients with MDD were treated with AXS-05 twice daily for up to 12 months. Patients had to have a Montgomery-Åsberg Depression Rating Scale (MADRS) score of at least 25. They could have completed a prior AXS-05 study or be newly enrolled.
Of 876 patients included in the study, 611 were newly enrolled. The mean age was 42.4 years, and 62.4% were women. Just over half (58.1%) were White, with 35.6% Black, and 2.0% Asian. The mean body mass index was 31.4 kg/m2.
The mean MADRS total score at baseline was 32.7 and the Sheehan Disability Scale (SDS) score was 20.0.
Presenting efficacy data in 609 newly enrolled patients, the team showed that MADRS total scores fell sharply on starting AXS-05, by 9.1 points at week 1, 14.0 points at week 2, and 21.2 points at week 6.
By 6 months, the reduction over baseline was 23.9 points, which was maintained out to 12 months, at a mean reduction of 23.0 points.
The proportion of patients achieving a clinical response, defined as a greater than or equal to 50% improvement in MADRS scores, was 18.8% at week 1, 39.7% at week 2, and 73.2% at week 6. There was a clinical response in 84.6% of patients at 6 months and in 82.8% at 12 months.
Clinical remission, defined as a MADRS score less than or equal to 10, was achieved in 8.3% of patients at week 1, rising to 21.5% at week 2, and 52.5% at week 6. At 6 months, 68.7% of patients were in clinical remission, reaching 69.0% at 12 months.
These benefits were accompanied by substantial improvements in depressive symptoms on the Clinical Global Impression of Improvement scale, with a marked or moderate improvement seen in 86.7% of patients at 6 months and 93.1% at 12 months.
Moreover, a clinical response in functioning on the SDS was achieved by 80.6% of patients at 6 months and 75.9% at 12 months.
The safety analysis of AXS-05 in the entire cohort suggested it was well-tolerated, with dizziness seen in 12.7% of patients, along with nausea in 11.9%, headache in 8.8%, dry mouth in 7.1%, and decreased appetite in 6.1%.
The rate of discontinuation due to adverse events was 8.4%, and there were no signs of psychotomimetic effects, cognitive impairment, weight gain, or increased sexual dysfunction.
and increased rates of suicidal ideation resolution, and was also effective for treatment-resistant depression.
Results from an analysis of the ASCEND phase 2 and GEMINI phase 3 trials also suggested that AXS-05 was superior to both bupropion and placebo in achieving rapid and sustained improvements in depression symptoms.
FDA delay
Yet despite these seemingly positive findings, the FDA appears to have issues with the agent’s new drug application.
As reported in August, the agency reviewed the NDA for AXS-05 for the treatment of MDD, but at that time the drug’s manufacturer revealed that the agency had identified “deficiencies that preclude labeling discussions at this time.”
With the latest results presented at ECNP 2021, this news organization asked Axsome about the status of the NDA and whether there had been any further discussions and/or movement with the FDA.
Instead of a direct reply from the drug company, this news organization was directed to a statement released by Axsome in August announcing that the FDA had informed the company that its NDA review “would not be completed by the Prescription Drug User Fee Act target action date of August 22, 2021.”
“The FDA did not request additional information from the company, and the review of the application is ongoing,” the statement said. Axsome did not respond to further questions.
‘Impressive’ remission rate
Commenting on the research, Marin Jukic, PhD, department of physiology and pharmacology, Karolinska Institutet, Stockholm, who was not involved in the research, said AXS-05 “looks promising in relation to the efficacy and tolerability results” with a remission rate that is “truly impressive.”
However, Dr. Jukic cautioned that it was an open-label trial and therefore had no placebo or active comparator arms.
He noted that it would be “interesting to compare the efficacy with placebo and escitalopram, for example, to evaluate the potential for the benefits and efficacy better.”
The research was funded by Axsome Therapeutics, and, except for one, the researchers for the four studies are employees of Axsome Therapeutics.
A version of this article first appeared on Medscape.com.
A novel antidepressant (AXS-05, Axsome Therapeutics) appears to have a rapid and durable effect in patients with major depressive disorder (MDD), results of an open label, phase 3 trial, show. Yet, its new drug application (NDA) remains in limbo with the U.S. Food and Drug Administration for reasons that are unclear.
In the study, which included 876 patients with MDD, results showed the drug, a combination of dextromethorphan and bupropion, had a clinical response rate of 80% and a remission rate of almost 70%. In addition, functional improvements were “substantial” and AXS-05 was determined to be “generally safe and well-tolerated.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
The study
The COMET trial was a phase 3, multicenter, U.S. trial, in which patients with MDD were treated with AXS-05 twice daily for up to 12 months. Patients had to have a Montgomery-Åsberg Depression Rating Scale (MADRS) score of at least 25. They could have completed a prior AXS-05 study or be newly enrolled.
Of 876 patients included in the study, 611 were newly enrolled. The mean age was 42.4 years, and 62.4% were women. Just over half (58.1%) were White, with 35.6% Black, and 2.0% Asian. The mean body mass index was 31.4 kg/m2.
The mean MADRS total score at baseline was 32.7 and the Sheehan Disability Scale (SDS) score was 20.0.
Presenting efficacy data in 609 newly enrolled patients, the team showed that MADRS total scores fell sharply on starting AXS-05, by 9.1 points at week 1, 14.0 points at week 2, and 21.2 points at week 6.
By 6 months, the reduction over baseline was 23.9 points, which was maintained out to 12 months, at a mean reduction of 23.0 points.
The proportion of patients achieving a clinical response, defined as a greater than or equal to 50% improvement in MADRS scores, was 18.8% at week 1, 39.7% at week 2, and 73.2% at week 6. There was a clinical response in 84.6% of patients at 6 months and in 82.8% at 12 months.
Clinical remission, defined as a MADRS score less than or equal to 10, was achieved in 8.3% of patients at week 1, rising to 21.5% at week 2, and 52.5% at week 6. At 6 months, 68.7% of patients were in clinical remission, reaching 69.0% at 12 months.
These benefits were accompanied by substantial improvements in depressive symptoms on the Clinical Global Impression of Improvement scale, with a marked or moderate improvement seen in 86.7% of patients at 6 months and 93.1% at 12 months.
Moreover, a clinical response in functioning on the SDS was achieved by 80.6% of patients at 6 months and 75.9% at 12 months.
The safety analysis of AXS-05 in the entire cohort suggested it was well-tolerated, with dizziness seen in 12.7% of patients, along with nausea in 11.9%, headache in 8.8%, dry mouth in 7.1%, and decreased appetite in 6.1%.
The rate of discontinuation due to adverse events was 8.4%, and there were no signs of psychotomimetic effects, cognitive impairment, weight gain, or increased sexual dysfunction.
and increased rates of suicidal ideation resolution, and was also effective for treatment-resistant depression.
Results from an analysis of the ASCEND phase 2 and GEMINI phase 3 trials also suggested that AXS-05 was superior to both bupropion and placebo in achieving rapid and sustained improvements in depression symptoms.
FDA delay
Yet despite these seemingly positive findings, the FDA appears to have issues with the agent’s new drug application.
As reported in August, the agency reviewed the NDA for AXS-05 for the treatment of MDD, but at that time the drug’s manufacturer revealed that the agency had identified “deficiencies that preclude labeling discussions at this time.”
With the latest results presented at ECNP 2021, this news organization asked Axsome about the status of the NDA and whether there had been any further discussions and/or movement with the FDA.
Instead of a direct reply from the drug company, this news organization was directed to a statement released by Axsome in August announcing that the FDA had informed the company that its NDA review “would not be completed by the Prescription Drug User Fee Act target action date of August 22, 2021.”
“The FDA did not request additional information from the company, and the review of the application is ongoing,” the statement said. Axsome did not respond to further questions.
‘Impressive’ remission rate
Commenting on the research, Marin Jukic, PhD, department of physiology and pharmacology, Karolinska Institutet, Stockholm, who was not involved in the research, said AXS-05 “looks promising in relation to the efficacy and tolerability results” with a remission rate that is “truly impressive.”
However, Dr. Jukic cautioned that it was an open-label trial and therefore had no placebo or active comparator arms.
He noted that it would be “interesting to compare the efficacy with placebo and escitalopram, for example, to evaluate the potential for the benefits and efficacy better.”
The research was funded by Axsome Therapeutics, and, except for one, the researchers for the four studies are employees of Axsome Therapeutics.
A version of this article first appeared on Medscape.com.
FROM ECNP 2021
Weakness in the legs and edema
Carcinomas like prostate cancer possess notable bony tropism and can metastasize to the lumbar‐sacral spine and pelvis, draining through the pelvic plexus of the lumbar region. Approximately 90% of patients with advanced prostate cancer develop bone metastasis, the spine being the most common site. Manifestations of metastatic prostate cancer include weight loss and loss of appetite; bone pain, with or without pathologic fracture; and lower-extremity pain and edema. Urinary symptoms are also common. Other physical examination findings are adenopathy, bony tenderness, and lower-extremity edema, as seen in the present case.
Radiologic findings of bone metastases can mimic Paget disease, and even though bone metastases are blastic, lytic lesions may develop and cause pathologic fractures. Such fractures must be distinguished from osteoporotic fractures that can occur after prolonged luteinizing hormone–releasing hormone therapy. Also included in the differential of the present case are lymphomas, which can manifest as pelvic masses and bone lesions and have been reported with prostate cancer. However, considering the patient's history, physical examination, and lab results, bone metastasis is the most likely diagnosis.
Bone imaging should be performed for any patient with suspected bone metastases; specifically, multiparametric MRI outperforms bone scan and targeted x-rays for detection of bone metastases. Because activity in the bone scan may not be observed until 5 years after micrometastasis has occurred, negative bone scan results cannot be used to definitively exclude metastasis.
The alpha emitter radium-223 is a category 1 option to treat symptomatic bone metastases (but should not be used in patients with visceral metastases). It is not recommended for use in combination with docetaxel or any other systemic therapy but may be used with androgen-deprivation therapy (ADT), as studies have suggested that the addition of ADT improves progression-free survival in patients with castrate-resistant prostate cancer with metastasis. Concomitant use of denosumab or zoledronic acid is also recommended.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Carcinomas like prostate cancer possess notable bony tropism and can metastasize to the lumbar‐sacral spine and pelvis, draining through the pelvic plexus of the lumbar region. Approximately 90% of patients with advanced prostate cancer develop bone metastasis, the spine being the most common site. Manifestations of metastatic prostate cancer include weight loss and loss of appetite; bone pain, with or without pathologic fracture; and lower-extremity pain and edema. Urinary symptoms are also common. Other physical examination findings are adenopathy, bony tenderness, and lower-extremity edema, as seen in the present case.
Radiologic findings of bone metastases can mimic Paget disease, and even though bone metastases are blastic, lytic lesions may develop and cause pathologic fractures. Such fractures must be distinguished from osteoporotic fractures that can occur after prolonged luteinizing hormone–releasing hormone therapy. Also included in the differential of the present case are lymphomas, which can manifest as pelvic masses and bone lesions and have been reported with prostate cancer. However, considering the patient's history, physical examination, and lab results, bone metastasis is the most likely diagnosis.
Bone imaging should be performed for any patient with suspected bone metastases; specifically, multiparametric MRI outperforms bone scan and targeted x-rays for detection of bone metastases. Because activity in the bone scan may not be observed until 5 years after micrometastasis has occurred, negative bone scan results cannot be used to definitively exclude metastasis.
The alpha emitter radium-223 is a category 1 option to treat symptomatic bone metastases (but should not be used in patients with visceral metastases). It is not recommended for use in combination with docetaxel or any other systemic therapy but may be used with androgen-deprivation therapy (ADT), as studies have suggested that the addition of ADT improves progression-free survival in patients with castrate-resistant prostate cancer with metastasis. Concomitant use of denosumab or zoledronic acid is also recommended.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Carcinomas like prostate cancer possess notable bony tropism and can metastasize to the lumbar‐sacral spine and pelvis, draining through the pelvic plexus of the lumbar region. Approximately 90% of patients with advanced prostate cancer develop bone metastasis, the spine being the most common site. Manifestations of metastatic prostate cancer include weight loss and loss of appetite; bone pain, with or without pathologic fracture; and lower-extremity pain and edema. Urinary symptoms are also common. Other physical examination findings are adenopathy, bony tenderness, and lower-extremity edema, as seen in the present case.
Radiologic findings of bone metastases can mimic Paget disease, and even though bone metastases are blastic, lytic lesions may develop and cause pathologic fractures. Such fractures must be distinguished from osteoporotic fractures that can occur after prolonged luteinizing hormone–releasing hormone therapy. Also included in the differential of the present case are lymphomas, which can manifest as pelvic masses and bone lesions and have been reported with prostate cancer. However, considering the patient's history, physical examination, and lab results, bone metastasis is the most likely diagnosis.
Bone imaging should be performed for any patient with suspected bone metastases; specifically, multiparametric MRI outperforms bone scan and targeted x-rays for detection of bone metastases. Because activity in the bone scan may not be observed until 5 years after micrometastasis has occurred, negative bone scan results cannot be used to definitively exclude metastasis.
The alpha emitter radium-223 is a category 1 option to treat symptomatic bone metastases (but should not be used in patients with visceral metastases). It is not recommended for use in combination with docetaxel or any other systemic therapy but may be used with androgen-deprivation therapy (ADT), as studies have suggested that the addition of ADT improves progression-free survival in patients with castrate-resistant prostate cancer with metastasis. Concomitant use of denosumab or zoledronic acid is also recommended.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
A 66-year-old male patient presents with weakness in the legs and edema. He takes medication to control his hypertension. About 8 years ago, he was diagnosed with prostate cancer during screening. Tumor staging was T3 and Gleason score was 8. The patient underwent successful radiation combined with hormone therapy. While he does not have urologic symptoms at this time, he does report that he is easily fatigued. Serum calcium is 10.6 mg/dL and hemoglobin is 10.5 g/dL. There is no evidence of neurologic deficit.
Scientists use 3D printing to create injection-free vaccine patch
Most vaccines are given with hypodermic needle injections. But shots aren’t necessarily the most efficient or effective way to deliver a vaccine. Scientists have been experimenting with microneedle patches to painlessly deliver a vaccine into the outermost layer of the skin with dozens of extremely tiny needles coated in the vaccine solution.
Now, researchers have found a three-dimensional printing method that lets them customize microneedle shapes in the patches for different pathogens, such as flu, measles, hepatitis, or COVID-19. In tests using mice, the patches led to stronger and longer-lasting immune responses than traditional shots under the skin. The research team described their findings in the Proceedings of the National Academy of Sciences.
Tiny needles, big advantages
Previous research has shown delivering vaccines into the skin can cause a stronger immune response because the skin has a high concentration of immune cells. But shots can be painful and require skilled medical providers.
Microneedles painlessly deliver the vaccine into the skin without the need for a trained clinician. In fact, a person can even give the vaccine to themselves.
The needles – made of metal, silicon, or plastic – are so tiny that they puncture only the tough outermost layer of skin. The prospect of a painless vaccination without a hypodermic needle may ease anxiety in people who fear needles.
Scientists also can store dried patches after coating them with the vaccine solution, so there’s no preparation needed before giving the vaccine and the patches may not even require cold storage. This latest study suggests that the patches generate a stronger immune response than standard shots, allowing for a smaller dose than traditional vaccine delivery methods and possibly fewer side effects.
Breaking the mold
Past methods of making microneedle patches often used molds, but that approach limited the ability to customize patches for different diseases. Repeatedly using same mold also can blunt the tiny needles.
For the three-dimensional–printed patches, Cassie Caudill at the University of North Carolina at Chapel Hill and colleagues used a printing technique that allows greater control over and consistency in the shape of the microneedles. The investigators printed two shapes: a slender pyramid microneedle that is similar to previous versions, and one with serrated grooves that resembles a pine tree.
The increased surface area from the grooves let researchers add 36% more of the ingredient that causes an immune response, compared with using only the pyramid shape, yet still less than a conventional shot. At only 1 cm by 1 cm, each patch contains 100 microneedles that are just over 1 mm long. The researchers found that in mice the patch drew a stronger immune response than a conventional shot, despite carrying a much smaller dose of vaccine ingredient.
A version of this article first appeared on WebMD.com.
Most vaccines are given with hypodermic needle injections. But shots aren’t necessarily the most efficient or effective way to deliver a vaccine. Scientists have been experimenting with microneedle patches to painlessly deliver a vaccine into the outermost layer of the skin with dozens of extremely tiny needles coated in the vaccine solution.
Now, researchers have found a three-dimensional printing method that lets them customize microneedle shapes in the patches for different pathogens, such as flu, measles, hepatitis, or COVID-19. In tests using mice, the patches led to stronger and longer-lasting immune responses than traditional shots under the skin. The research team described their findings in the Proceedings of the National Academy of Sciences.
Tiny needles, big advantages
Previous research has shown delivering vaccines into the skin can cause a stronger immune response because the skin has a high concentration of immune cells. But shots can be painful and require skilled medical providers.
Microneedles painlessly deliver the vaccine into the skin without the need for a trained clinician. In fact, a person can even give the vaccine to themselves.
The needles – made of metal, silicon, or plastic – are so tiny that they puncture only the tough outermost layer of skin. The prospect of a painless vaccination without a hypodermic needle may ease anxiety in people who fear needles.
Scientists also can store dried patches after coating them with the vaccine solution, so there’s no preparation needed before giving the vaccine and the patches may not even require cold storage. This latest study suggests that the patches generate a stronger immune response than standard shots, allowing for a smaller dose than traditional vaccine delivery methods and possibly fewer side effects.
Breaking the mold
Past methods of making microneedle patches often used molds, but that approach limited the ability to customize patches for different diseases. Repeatedly using same mold also can blunt the tiny needles.
For the three-dimensional–printed patches, Cassie Caudill at the University of North Carolina at Chapel Hill and colleagues used a printing technique that allows greater control over and consistency in the shape of the microneedles. The investigators printed two shapes: a slender pyramid microneedle that is similar to previous versions, and one with serrated grooves that resembles a pine tree.
The increased surface area from the grooves let researchers add 36% more of the ingredient that causes an immune response, compared with using only the pyramid shape, yet still less than a conventional shot. At only 1 cm by 1 cm, each patch contains 100 microneedles that are just over 1 mm long. The researchers found that in mice the patch drew a stronger immune response than a conventional shot, despite carrying a much smaller dose of vaccine ingredient.
A version of this article first appeared on WebMD.com.
Most vaccines are given with hypodermic needle injections. But shots aren’t necessarily the most efficient or effective way to deliver a vaccine. Scientists have been experimenting with microneedle patches to painlessly deliver a vaccine into the outermost layer of the skin with dozens of extremely tiny needles coated in the vaccine solution.
Now, researchers have found a three-dimensional printing method that lets them customize microneedle shapes in the patches for different pathogens, such as flu, measles, hepatitis, or COVID-19. In tests using mice, the patches led to stronger and longer-lasting immune responses than traditional shots under the skin. The research team described their findings in the Proceedings of the National Academy of Sciences.
Tiny needles, big advantages
Previous research has shown delivering vaccines into the skin can cause a stronger immune response because the skin has a high concentration of immune cells. But shots can be painful and require skilled medical providers.
Microneedles painlessly deliver the vaccine into the skin without the need for a trained clinician. In fact, a person can even give the vaccine to themselves.
The needles – made of metal, silicon, or plastic – are so tiny that they puncture only the tough outermost layer of skin. The prospect of a painless vaccination without a hypodermic needle may ease anxiety in people who fear needles.
Scientists also can store dried patches after coating them with the vaccine solution, so there’s no preparation needed before giving the vaccine and the patches may not even require cold storage. This latest study suggests that the patches generate a stronger immune response than standard shots, allowing for a smaller dose than traditional vaccine delivery methods and possibly fewer side effects.
Breaking the mold
Past methods of making microneedle patches often used molds, but that approach limited the ability to customize patches for different diseases. Repeatedly using same mold also can blunt the tiny needles.
For the three-dimensional–printed patches, Cassie Caudill at the University of North Carolina at Chapel Hill and colleagues used a printing technique that allows greater control over and consistency in the shape of the microneedles. The investigators printed two shapes: a slender pyramid microneedle that is similar to previous versions, and one with serrated grooves that resembles a pine tree.
The increased surface area from the grooves let researchers add 36% more of the ingredient that causes an immune response, compared with using only the pyramid shape, yet still less than a conventional shot. At only 1 cm by 1 cm, each patch contains 100 microneedles that are just over 1 mm long. The researchers found that in mice the patch drew a stronger immune response than a conventional shot, despite carrying a much smaller dose of vaccine ingredient.
A version of this article first appeared on WebMD.com.