User login
WATS-3D plus Seattle protocol increases dysplasia detection in Barrett’s esophagus
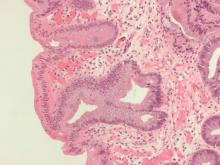
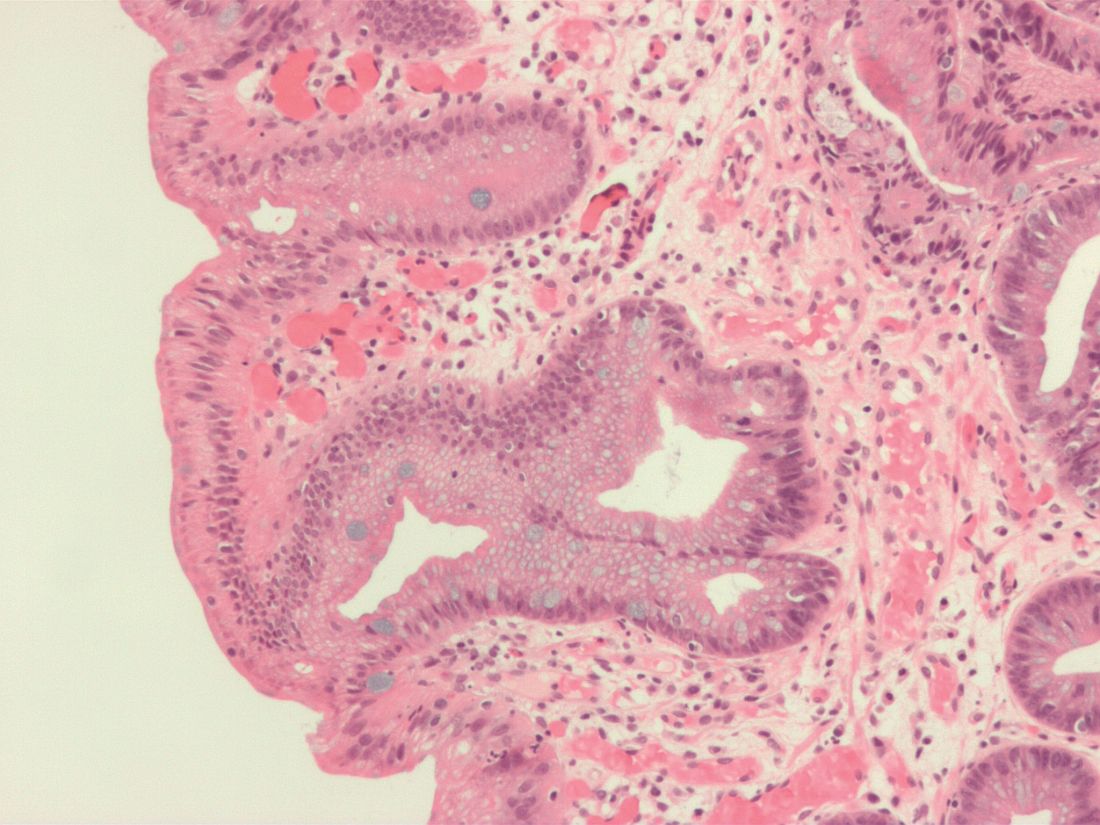
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
Wide-area transepithelial sampling with 3D (WATS-3D) analysis increased detection of dysplasia when used as an adjunct to the Seattle forceps biopsy protocol in patients with Barrett’s esophagus, according to a recent meta-analysis. While the findings demonstrate potential for increased dysplasia detection, the analysis failed to identify the clinical significance of this detection.
Despite its ability to evaluate Barrett’s esophagus segments and examine targeted biopsies of mucosal abnormalities, the Seattle protocol is primarily limited by lack of adherence and increased risk of sampling error. “Moreover, the rates of ‘missed’ dysplasia and EAC [esophageal adenocarcinoma] remain high, with up to a quarter of all EAC being ‘missed,’ ” wrote study authors Don Codipilly, MD, of the Mayo Clinic, and colleagues. The report is in Gastrointestinal Endoscopy.
There are challenges associated with the Seattle protocol, specifically poor protocol adherence and missed identification of subtle abnormalities potentially harboring dysplasia. In contrast, the novel WATS-3D may overcome issues related to sampling error due to its ability to obtain higher proportions of Barrett’s esophagus mucosa through the use of a brush-only technique. According to the researchers, previous studies suggest WATS-3D may increase dysplasia yield by approximately 40% compared with conventional surveillance methods.
To gauge the incremental yield of WATS-3D for dysplasia detection compared with the Seattle forceps biopsy protocol, Dr. Codipilly and colleagues performed a systematic review and meta-analysis of seven studies using the two techniques from 2000 to 2020. The researchers defined “incremental yield” of detected dysplasia as a composite of indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). They also compared the two surveillance techniques in terms of incremental yields of HGD/EAC, as well as the rate of reconfirmation of WATS-3D dysplasia on subsequent forceps biopsies.
The seven studies in the final analysis included a pooled cohort of 3,206 patients. According to the meta-analysis, forceps biopsies diagnosed dysplasia in 15.9% (95% confidence interval, 5.4-30.5) of all cases, while the incremental yield of WATS-3D was 7.2% (95% CI, 3.9-11.5). In the pooled analysis of six studies that reported the secondary outcomes, forceps biopsies diagnosed HGD/EAC in 2.3% (95% CI, 0.6-5.1) of patients, while the incremental yield with WATS-3D was 2.1% (95% CI, 0.4-5.3). The researchers point out that WATS-3D was negative in 62.5% of cases where forceps biopsies detected dysplasia. Reports from two of the studies reconfirmed WATS-3D dysplasia with forceps biopsies histology in 20 patients.
“Based on these findings, it cannot be recommended to replace the Seattle Protocol but instead to use both techniques in conjunction to detect dysplasia most effectively,” Omar Awais, DO, assistant professor of surgery in the Department of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine, said in an email to this news organization.
Dr. Awais, who was not involved in the meta-analysis, suggests further prospective, randomized studies are needed to confirm the results. “Additionally, we will also need studies to show cost-effectiveness for using WATS-3D in addition to Seattle protocol, as these may help verify WATS-3D dysplasia by standard endoscopic protocol and show we are not missing dysplasia using the technique,” he said.
Felice H. Schnoll-Sussman, MD, professor of clinical medicine and director of the Jay Monahan Center for Gastrointestinal Health at New York–Presbyterian Hospital/Weill Medical College, added that the meta-analysis “adds to our understanding” of the place of WATS as an adjunct approach in dysplasia detection. “In spite of the rigid selection of studies, this analysis also leaves us with questions about the overall utility of WATS given the lack of follow-up cases where dysplasia was only identified on the WATS brush as well as the overall cost-effectiveness of this approach,” she said.
Dr. Schnoll-Sussman, who was not involved in the study conducted by Dr. Codipilly and colleagues, told this news organization that one of the issues with the WATS brush is obtaining adequate sampling, which may impede adherence. “Attention has to be paid to sampling all quadrants with the brush, which at times may be challenging, especially in esophagi that are tortuous, angulated, or dilated,” she explained. “Like with any endoscopic technique, care must be taken to obtain high-yield sampling.”
Dr. Schnoll-Sussman noted that the subtle, small areas of denuded mucosa left where the brush has made appropriate contact with the mucosa should be appreciated during sampling. “Taking one’s time to sample the esophageal lining – a major reason for missed lesions in the Seattle protocol – can also become an issue with WATS,” she added.
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study. Dr. Awais and Dr. Schnoll-Sussman had no conflicts to disclose.
FROM GASTROINTESTINAL ENDOSCOPY
Study of biologics’ impact on psoriasis-to-PsA transition contradicts previous findings
Data source likely contributes biases
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
Data source likely contributes biases
Data source likely contributes biases
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
FROM ANNALS OF THE RHEUMATIC DISEASES
Resident physician work-hour regulations associated with improved physician safety and health
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Pandemic data challenges infection link to Guillain-Barré syndrome
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
While pediatric cases of various types of infections fell by 45%-95% during the early months of the pandemic, cases of acute inflammatory demyelinating polyneuropathy (AIDP), an inflammatory neuropathy belonging to the clinical spectrum of Guillain-Barré syndrome, only fell by about 32%-37%, a rate that’s similar to the 35.1% decline in overall hospital admissions over that time period, researchers found. There was also no apparent link between the appearance of COVID-19 and the number of reported AIDP cases.
“There was no clear association between respiratory or gastrointestinal infections and rates of AIDP. Further, we found that AIDP did not have the expected dramatic reduction when community-acquired infections decreased during the pandemic,” Children’s Hospital of Philadelphia neurologist Craig A. Press, MD, PhD, said in an interview.
Dr. Press and colleagues presented their findings in a poster at the 50th annual meeting of the Child Neurology Society.
According to Dr. Press, the cause of AIDP in most patients is unclear, although infections and vaccinations are often linked to cases. “However, the data supporting this link is often weak. Infections with Campylobacter jejuni [bacteria that causes food poisoning] are known to be associated with AIDP, while rates of AIDP in the general population and in those with influenza are similar.”
For the new multicenter, cross-sectional study, researchers tracked AIDP data from the 47 pediatric hospitals that provide statistics to the Pediatric Health Information System. They focused on the period from January 2017 to September 2020, which included the first months of the COVID-19 pandemic in the United States.
“Social distancing, masks, and increased hand hygiene decrease community-acquired infectious rates in a dramatic way,” Dr. Press said. “If these infections were causing AIDP, we hypothesized that the cases of AIDP would drop substantially as a result.”
But this didn’t appear to happen. Researchers found that the numbers of various types of infections declined from April to September 2020: Respiratory infections dipped by 73%-78%, gastrointestinal infections fell by 45%-61%, and influenza infections dipped by 88%-95%. But AIDP cases didn’t fall as precipitously. In fact, their levels were about the same as they were in April 2017, a month when rates of gastrointestinal, respiratory disease and influenza infections were at seasonally low – but not abnormal – ebbs.
“While we must be cautious interpreting the results,” Dr. Press said, “this makes the link between infections as the main driver of pediatric AIDP less likely.”
However, he said, “this study does not exclude the possibility that rare infections cause AIDP – the data supporting that some more rare infections like campylobacter have a connection to AIDP are more robust – or that common infections very rarely lead to AIDP. While we look for triggers causing inflammatory disorders, AIDP maybe an autoinflammatory disorder without a clear trigger.”
Going forward, Dr. Press said, “we hope to look at infectious data in a more granular way to identify if specific viral or bacterial infectious may be associated with this or other inflammatory disorders. We believe that the use of data like this and the natural experiment that COVID-19 provided may help us to explore the impact of infections on disorders thought to be postinfectious.”
No study funding is reported, and the authors report no relevant disclosures.
FROM CNS 2021
Epidiolex plus THC lowers seizures in pediatric epilepsy
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
the component of cannabis that makes people high in larger quantities, researchers reported.
“THC can contribute to seizure control and mitigation some of the side effects of CBD,” said study coauthor and Austin, Tex., child neurologist Karen Keough, MD, in an interview. Dr. Keough and colleagues presented their findings at the 50th annual meeting of the Child Neurology Society.
In a landmark move, the Food and Drug Administration approved Epidiolex in 2018 for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. The agency had never before approved a drug with a purified ingredient derived from marijuana.
CBD, the active ingredient in Epidiolex, is nonpsychoactive. The use in medicine of THC, the main driver of marijuana’s ability to make people stoned, is much more controversial.
Dr. Keough said she had treated 60-70 children with CBD, at the same strength as in Epidiolex (100 mg), and 5 mg of THC before the drug was approved. “I was seeing some very impressive results, and some became seizure free who’d always been refractory,” she said.
When the Epidiolex became available, she said, some patients transitioned to it and stopped taking THC. According to her, some patients fared well. But others immediately experienced worse seizures, she said, and some developed side effects to Epidiolex in the absence of THC, such as agitation and appetite suppression.
Combination therapy
For the new study, a retrospective, unblinded cohort analysis, Dr. Keough and colleagues tracked patients who received various doses of CBD, in some cases as Epidiolex, and various doses of THC prescribed by the Texas Original Compassionate Cultivation dispensary, where she serves as chief medical officer.
The initial number of patients was 212; 135 consented to review and 10 were excluded for various reasons leaving a total of 74 subjects in the study. The subjects, whose median age at the start of the study was 12 years (range, 2-25 years), were tracked from 2018 to2021. Just over half (55%) were male, and they remained on the regimen for a median of 805 days (range, 400-1,141).
Of the 74 subjects, 45.9% had a reduction of seizures of more than 75%, and 20.3% had a reduction of 50%-75%. Only 4.1% saw their seizures worsen.
The THC doses varied from none to more than 12 mg/day; CBD doses varied from none to more than 26 mg/kg per day. O the 74 patients, 18 saw their greatest seizure reduction from baseline when they received no THC; 12 saw their greatest seizure reduction from baseline when they received 0-2 mg/kg per day of CBD.
Still controversial
Did the patients get high? In some cases they did, Dr. Keough said. However, “a lot of these patients are either too young or too cognitively limited to describe whether they’re feeling intoxicated. That’s one of the many reasons why this is so controversial. You have to go into this with eyes wide open. We’re working in an environment with limited information as to what an intoxicating dose is for a small kid.”
However, she said, it seems clear that “THC can enhance the effect of CBD in children with epilepsy” and reduce CBD side effects. It’s not surprising that the substances work differently since they interact with brain cells in different ways, she said.
For neurologists, she said, “the challenge is to find a reliable source of THC that you can count on and verify so you aren’t overdosing the patients.”
University of Saskatchewan, Saskatoon, child neurologist and cannabinoid researcher Richard Huntsman, MD, who’s familiar with the study findings, said in an interview that they “provide another strong signal that the addition of THC provides benefit, at least in some patients.”
But it’s still unclear “why some children respond best in regards to seizure reduction and side effect profile with combination CBD:THC therapy, and others seemed to do better with CBD alone,” he said. Also unknown: “the ideal THC:CBD ratio that allows optimal seizure control while preventing the potential harmful effects of THC.”
As for the future, he said, “as we are just scratching the surface of our knowledge about the use of cannabis-based therapies in children with neurological disorders, I suspect that the use of these therapies will expand over time.”
No study funding is reported. Dr. Keough disclosed serving as chief medical officer of Texas Original Compassionate Cultivation. Dr. Huntsman disclosed serving as lead investigator of the Cannabidiol in Children with Refractory Epileptic Encephalopathy study and serving on the boards of the Cannabinoid Research Initiative of Saskatchewan (University of Saskatchewan) and Canadian Childhood Cannabinoid Clinical Trials Consortium. He is also cochair of Health Canada’s Scientific Advisory Committee on Cannabinoids for Health Purposes.
FROM CNS 2021
True or false: Breast density increases breast cancer risk
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).
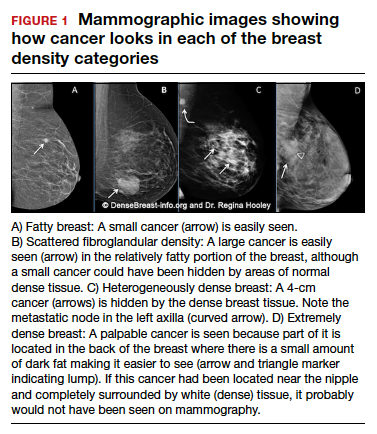
Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8
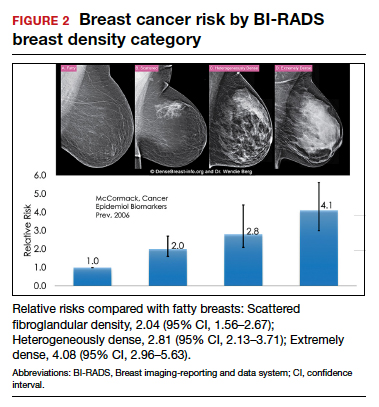
Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Quiz developed in collaboration with 
Lupus may confer higher risk of death from COVID-19
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
GERD: Composite pH impedance monitoring better identifies treatment escalation need
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
The management of gastroesophageal reflux disease (GERD) is the most common referral for a gastroenterologist; however, metrics to determine dose-escalation for persistent symptoms in patients with proven GERD is an unmet need. The Lyon consensus aimed to standardize abnormal pH parameters but used similar thresholds for off– and on–proton pump inhibitor testing; these thresholds for on-PPI testing are likely too high to detect refractory reflux on PPI therapy. The use of pH-impedance testing is an optimal test for patients with persistent symptoms in the setting of proven GERD to determine escalation of antireflux therapy. In this multicenter, international cohort study, Gyawali and colleagues rigorously challenged the definition of abnormal pH-impedance testing with an evaluation of pH impedance parameters comparing controls (n = 66) versus proven GERD (n = 43) on twice-daily PPI dosing to define pH-impedance parameters.
Rishi D. Naik, MD, MSCI, is an assistant professor in the department of medicine in the section of gastroenterology & hepatology at the Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
The management of gastroesophageal reflux disease (GERD) is the most common referral for a gastroenterologist; however, metrics to determine dose-escalation for persistent symptoms in patients with proven GERD is an unmet need. The Lyon consensus aimed to standardize abnormal pH parameters but used similar thresholds for off– and on–proton pump inhibitor testing; these thresholds for on-PPI testing are likely too high to detect refractory reflux on PPI therapy. The use of pH-impedance testing is an optimal test for patients with persistent symptoms in the setting of proven GERD to determine escalation of antireflux therapy. In this multicenter, international cohort study, Gyawali and colleagues rigorously challenged the definition of abnormal pH-impedance testing with an evaluation of pH impedance parameters comparing controls (n = 66) versus proven GERD (n = 43) on twice-daily PPI dosing to define pH-impedance parameters.
Rishi D. Naik, MD, MSCI, is an assistant professor in the department of medicine in the section of gastroenterology & hepatology at the Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
The management of gastroesophageal reflux disease (GERD) is the most common referral for a gastroenterologist; however, metrics to determine dose-escalation for persistent symptoms in patients with proven GERD is an unmet need. The Lyon consensus aimed to standardize abnormal pH parameters but used similar thresholds for off– and on–proton pump inhibitor testing; these thresholds for on-PPI testing are likely too high to detect refractory reflux on PPI therapy. The use of pH-impedance testing is an optimal test for patients with persistent symptoms in the setting of proven GERD to determine escalation of antireflux therapy. In this multicenter, international cohort study, Gyawali and colleagues rigorously challenged the definition of abnormal pH-impedance testing with an evaluation of pH impedance parameters comparing controls (n = 66) versus proven GERD (n = 43) on twice-daily PPI dosing to define pH-impedance parameters.
Rishi D. Naik, MD, MSCI, is an assistant professor in the department of medicine in the section of gastroenterology & hepatology at the Esophageal Center at Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
FROM GASTROENTEROLOGY
Double antiglutamatergic therapy is ‘promising’ for super-refractory status epilepticus
(SRSE), new research suggests.
In a retrospective cohort study of survivors of cardiac arrest with postanoxic sustained SRSE, resolution of the condition was achieved by 81% of those who received intensive treatment of ketamine plus perampanel, versus 41% of those who received standard care.
The novelty of the new treatment approach is the duration of therapy as well as the dual antiglutamate drugs, researchers note.
“So the logic is to continue treatment until resolution of refractory status epilepticus under continuous EEG [electroencephalographic] monitoring,” reported lead investigator Simone Beretta, MD, San Gerardo University Hospital, Monza, Italy.
Therapy was guided by data on brainstem reflexes, N20 cortical responses, neuronal serum enolase levels, and neuroimaging.
If all or most of these indicators are favorable, “we continue to treat without any time limit,” Dr. Beretta said. However, if the indicators become unfavorable, clinicians should consider lowering the intensity of care, he added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
SUPER-CAT trial
In SRSE, epileptic seizures occur one after another without patients recovering consciousness in between. Standard aggressive therapy for the condition does not include antiglutamatergic drugs, the researchers noted.
In the Super-Refractory Status Epilepticus After Cardiac Arrest: Aggressive Treatment Guided by Multimodal Prognostic Indicators (SUPER-CAT) study, researchers assessed the combination of two such medications.
The first was the anti-NMDA receptor drug ketamine, which was given by intravenous bolus and then continuous infusion for 3 days guided by continuous EEG to reach a ketamine EEG pattern, as evidenced by alpha and beta waves. It was combined with the anti-AMPA receptor antiepileptic perampanel via nasogastric tube for 5 days, followed by slow tapering.
Dr. Beretta noted that in the ongoing TELSTAR trial, which involved a similar patient population, a different drug combination is being used. A major difference between the two trials is that in the TELSTAR trial, aggressive therapy continues for only 2 days if there is no response.
“In the SUPER-CAT study, we continue far beyond 2 days in the majority of patients,” he said. In addition, ketamine and perampanel were not assessed in TELSTAR.
In SUPER-CAT, 489 survivors of cardiac arrest were recruited over 10 years. Of these, 101 had refractory status epilepticus. After excluding those with more than two indicators of poor prognosis (n = 31) or whose status epilepticus resolved (n = 14), 56 patients were determined to have SRSE. All had experienced relapse after undergoing one cycle of anesthetic.
The 56 participants received one of three treatment regimens: double antiglutamate (DAG) therapy of ketamine and perampanel (n = 26), single antiglutamate therapy with either agent (n = 8), or aggressive nonantiglutamate (NAG) therapy with antiepilepsy drugs and anesthetics other than ketamine or perampanel (n = 22).
The single-antiglutamate group was not included in the analysis of patient outcomes.
The DAG and NAG groups were well balanced at baseline. There were no significant differences in median age (60 years vs. 66 years), gender, low cerebral blood flow, presence of bilateral pupillary or corneal reflexes, neuron-specific enolase levels, cortical N20 somatosenory evoked potentials, moderate to severe postanoxic brain injury, and hypothermia/targeted temperature management.
Primary outcome met
More patients in the DAG group (42%) had moderate to severe postanoxic brain injury than in the NAG group (28%). However, the difference was not statistically significant (P = .08), possibly because of the small sample sizes. The number of antiepileptic drugs and the number of cycles of anesthetics did not differ between the groups.
Results showed that efficacy and safety outcomes favored DAG therapy.
The primary efficacy outcome was resolution of status epilepticus within 3 days after initiation of treatments. Status epilepticus resolved for 21 of 26 patients in the DAG group (81%), versus 9 of 22 patients in the NAG group (41%; odds ratio, 6.06; 95% confidence interval, 1.66-22.12; P = .005).
For secondary efficacy outcomes, there was a trend in favor of DAG, but differences from the NAG group were not statistically significant. In the groups, 46% versus 32% awakened and responded to commands before discharge from the intensive care unit, and 32% versus 23% showed good neurologic outcome at 6 months.
The primary safety outcome of all-cause mortality risk in the ICU was 90% lower for patients treated with DAG than for those treated with NAG (15% vs. 64%; OR, 0.1; 95% CI, 0.02-0.41; P < .01). Dr. Beretta explained that the high mortality rate in the NAG group was presumably a result of unresolved status epilepticus.
The secondary safety outcome of a transitory rise of gamma-glutamyl transferase greater than three times the upper limit of normal in the DAG group was expected with high-dose perampanel, the investigators noted. This outcome occurred in 77% of the DAG group versus 27% of the NAG group (OR, 9.88; 95% CI, 2.4-32.9; P < .001).
There was no statistically significant difference in incidence of recurrent cardiac arrest during therapy. This occurred in one member of the DAG group and in none in the NAG group.
Dr. Beretta reported that their investigations are still in a retrospective phase, but the researchers plan to move the work into a prospective phase and possibly a randomized trial soon.
Fascinating, promising
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center, Columbus, said the study provides “fascinating, very helpful data” about a condition that has responded well to current treatment options.
He added that his center has used “the innovative treatments” discussed in the study for a few patients.
“More concrete evidence will push us to use it more uniformly across all our patient population [that] has refractory status. So I’m very optimistic, and the data were very promising,” said Dr. Singh, who was not involved with the research.
He cautioned that the study was retrospective, not randomized or controlled, and that it involved a small number of patients but said that the data were “heading in the right direction.”
Although resolution of status epilepticus was better among patients in the DAG group than in the NAG group, the awakenings and neurologic outcomes were “pretty much same as standard medical therapy, which we commonly give to our patients,” said Dr. Singh. “We see this phenomenon all the time in our patients.”
He noted that other factors can determine how patients respond, such as conditions of the heart or kidneys, the presence of sepsis, and multiorgan dysfunction. These factors were not controlled for in the study.
Nonetheless, he said the study achieved its primary endpoint of better resolution of status epilepticus “because that’s the first thing you want to see: whether the treatment is taking care of that.”
The study received no outside funding. Dr. Beretta and Dr. Singh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SRSE), new research suggests.
In a retrospective cohort study of survivors of cardiac arrest with postanoxic sustained SRSE, resolution of the condition was achieved by 81% of those who received intensive treatment of ketamine plus perampanel, versus 41% of those who received standard care.
The novelty of the new treatment approach is the duration of therapy as well as the dual antiglutamate drugs, researchers note.
“So the logic is to continue treatment until resolution of refractory status epilepticus under continuous EEG [electroencephalographic] monitoring,” reported lead investigator Simone Beretta, MD, San Gerardo University Hospital, Monza, Italy.
Therapy was guided by data on brainstem reflexes, N20 cortical responses, neuronal serum enolase levels, and neuroimaging.
If all or most of these indicators are favorable, “we continue to treat without any time limit,” Dr. Beretta said. However, if the indicators become unfavorable, clinicians should consider lowering the intensity of care, he added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
SUPER-CAT trial
In SRSE, epileptic seizures occur one after another without patients recovering consciousness in between. Standard aggressive therapy for the condition does not include antiglutamatergic drugs, the researchers noted.
In the Super-Refractory Status Epilepticus After Cardiac Arrest: Aggressive Treatment Guided by Multimodal Prognostic Indicators (SUPER-CAT) study, researchers assessed the combination of two such medications.
The first was the anti-NMDA receptor drug ketamine, which was given by intravenous bolus and then continuous infusion for 3 days guided by continuous EEG to reach a ketamine EEG pattern, as evidenced by alpha and beta waves. It was combined with the anti-AMPA receptor antiepileptic perampanel via nasogastric tube for 5 days, followed by slow tapering.
Dr. Beretta noted that in the ongoing TELSTAR trial, which involved a similar patient population, a different drug combination is being used. A major difference between the two trials is that in the TELSTAR trial, aggressive therapy continues for only 2 days if there is no response.
“In the SUPER-CAT study, we continue far beyond 2 days in the majority of patients,” he said. In addition, ketamine and perampanel were not assessed in TELSTAR.
In SUPER-CAT, 489 survivors of cardiac arrest were recruited over 10 years. Of these, 101 had refractory status epilepticus. After excluding those with more than two indicators of poor prognosis (n = 31) or whose status epilepticus resolved (n = 14), 56 patients were determined to have SRSE. All had experienced relapse after undergoing one cycle of anesthetic.
The 56 participants received one of three treatment regimens: double antiglutamate (DAG) therapy of ketamine and perampanel (n = 26), single antiglutamate therapy with either agent (n = 8), or aggressive nonantiglutamate (NAG) therapy with antiepilepsy drugs and anesthetics other than ketamine or perampanel (n = 22).
The single-antiglutamate group was not included in the analysis of patient outcomes.
The DAG and NAG groups were well balanced at baseline. There were no significant differences in median age (60 years vs. 66 years), gender, low cerebral blood flow, presence of bilateral pupillary or corneal reflexes, neuron-specific enolase levels, cortical N20 somatosenory evoked potentials, moderate to severe postanoxic brain injury, and hypothermia/targeted temperature management.
Primary outcome met
More patients in the DAG group (42%) had moderate to severe postanoxic brain injury than in the NAG group (28%). However, the difference was not statistically significant (P = .08), possibly because of the small sample sizes. The number of antiepileptic drugs and the number of cycles of anesthetics did not differ between the groups.
Results showed that efficacy and safety outcomes favored DAG therapy.
The primary efficacy outcome was resolution of status epilepticus within 3 days after initiation of treatments. Status epilepticus resolved for 21 of 26 patients in the DAG group (81%), versus 9 of 22 patients in the NAG group (41%; odds ratio, 6.06; 95% confidence interval, 1.66-22.12; P = .005).
For secondary efficacy outcomes, there was a trend in favor of DAG, but differences from the NAG group were not statistically significant. In the groups, 46% versus 32% awakened and responded to commands before discharge from the intensive care unit, and 32% versus 23% showed good neurologic outcome at 6 months.
The primary safety outcome of all-cause mortality risk in the ICU was 90% lower for patients treated with DAG than for those treated with NAG (15% vs. 64%; OR, 0.1; 95% CI, 0.02-0.41; P < .01). Dr. Beretta explained that the high mortality rate in the NAG group was presumably a result of unresolved status epilepticus.
The secondary safety outcome of a transitory rise of gamma-glutamyl transferase greater than three times the upper limit of normal in the DAG group was expected with high-dose perampanel, the investigators noted. This outcome occurred in 77% of the DAG group versus 27% of the NAG group (OR, 9.88; 95% CI, 2.4-32.9; P < .001).
There was no statistically significant difference in incidence of recurrent cardiac arrest during therapy. This occurred in one member of the DAG group and in none in the NAG group.
Dr. Beretta reported that their investigations are still in a retrospective phase, but the researchers plan to move the work into a prospective phase and possibly a randomized trial soon.
Fascinating, promising
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center, Columbus, said the study provides “fascinating, very helpful data” about a condition that has responded well to current treatment options.
He added that his center has used “the innovative treatments” discussed in the study for a few patients.
“More concrete evidence will push us to use it more uniformly across all our patient population [that] has refractory status. So I’m very optimistic, and the data were very promising,” said Dr. Singh, who was not involved with the research.
He cautioned that the study was retrospective, not randomized or controlled, and that it involved a small number of patients but said that the data were “heading in the right direction.”
Although resolution of status epilepticus was better among patients in the DAG group than in the NAG group, the awakenings and neurologic outcomes were “pretty much same as standard medical therapy, which we commonly give to our patients,” said Dr. Singh. “We see this phenomenon all the time in our patients.”
He noted that other factors can determine how patients respond, such as conditions of the heart or kidneys, the presence of sepsis, and multiorgan dysfunction. These factors were not controlled for in the study.
Nonetheless, he said the study achieved its primary endpoint of better resolution of status epilepticus “because that’s the first thing you want to see: whether the treatment is taking care of that.”
The study received no outside funding. Dr. Beretta and Dr. Singh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SRSE), new research suggests.
In a retrospective cohort study of survivors of cardiac arrest with postanoxic sustained SRSE, resolution of the condition was achieved by 81% of those who received intensive treatment of ketamine plus perampanel, versus 41% of those who received standard care.
The novelty of the new treatment approach is the duration of therapy as well as the dual antiglutamate drugs, researchers note.
“So the logic is to continue treatment until resolution of refractory status epilepticus under continuous EEG [electroencephalographic] monitoring,” reported lead investigator Simone Beretta, MD, San Gerardo University Hospital, Monza, Italy.
Therapy was guided by data on brainstem reflexes, N20 cortical responses, neuronal serum enolase levels, and neuroimaging.
If all or most of these indicators are favorable, “we continue to treat without any time limit,” Dr. Beretta said. However, if the indicators become unfavorable, clinicians should consider lowering the intensity of care, he added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
SUPER-CAT trial
In SRSE, epileptic seizures occur one after another without patients recovering consciousness in between. Standard aggressive therapy for the condition does not include antiglutamatergic drugs, the researchers noted.
In the Super-Refractory Status Epilepticus After Cardiac Arrest: Aggressive Treatment Guided by Multimodal Prognostic Indicators (SUPER-CAT) study, researchers assessed the combination of two such medications.
The first was the anti-NMDA receptor drug ketamine, which was given by intravenous bolus and then continuous infusion for 3 days guided by continuous EEG to reach a ketamine EEG pattern, as evidenced by alpha and beta waves. It was combined with the anti-AMPA receptor antiepileptic perampanel via nasogastric tube for 5 days, followed by slow tapering.
Dr. Beretta noted that in the ongoing TELSTAR trial, which involved a similar patient population, a different drug combination is being used. A major difference between the two trials is that in the TELSTAR trial, aggressive therapy continues for only 2 days if there is no response.
“In the SUPER-CAT study, we continue far beyond 2 days in the majority of patients,” he said. In addition, ketamine and perampanel were not assessed in TELSTAR.
In SUPER-CAT, 489 survivors of cardiac arrest were recruited over 10 years. Of these, 101 had refractory status epilepticus. After excluding those with more than two indicators of poor prognosis (n = 31) or whose status epilepticus resolved (n = 14), 56 patients were determined to have SRSE. All had experienced relapse after undergoing one cycle of anesthetic.
The 56 participants received one of three treatment regimens: double antiglutamate (DAG) therapy of ketamine and perampanel (n = 26), single antiglutamate therapy with either agent (n = 8), or aggressive nonantiglutamate (NAG) therapy with antiepilepsy drugs and anesthetics other than ketamine or perampanel (n = 22).
The single-antiglutamate group was not included in the analysis of patient outcomes.
The DAG and NAG groups were well balanced at baseline. There were no significant differences in median age (60 years vs. 66 years), gender, low cerebral blood flow, presence of bilateral pupillary or corneal reflexes, neuron-specific enolase levels, cortical N20 somatosenory evoked potentials, moderate to severe postanoxic brain injury, and hypothermia/targeted temperature management.
Primary outcome met
More patients in the DAG group (42%) had moderate to severe postanoxic brain injury than in the NAG group (28%). However, the difference was not statistically significant (P = .08), possibly because of the small sample sizes. The number of antiepileptic drugs and the number of cycles of anesthetics did not differ between the groups.
Results showed that efficacy and safety outcomes favored DAG therapy.
The primary efficacy outcome was resolution of status epilepticus within 3 days after initiation of treatments. Status epilepticus resolved for 21 of 26 patients in the DAG group (81%), versus 9 of 22 patients in the NAG group (41%; odds ratio, 6.06; 95% confidence interval, 1.66-22.12; P = .005).
For secondary efficacy outcomes, there was a trend in favor of DAG, but differences from the NAG group were not statistically significant. In the groups, 46% versus 32% awakened and responded to commands before discharge from the intensive care unit, and 32% versus 23% showed good neurologic outcome at 6 months.
The primary safety outcome of all-cause mortality risk in the ICU was 90% lower for patients treated with DAG than for those treated with NAG (15% vs. 64%; OR, 0.1; 95% CI, 0.02-0.41; P < .01). Dr. Beretta explained that the high mortality rate in the NAG group was presumably a result of unresolved status epilepticus.
The secondary safety outcome of a transitory rise of gamma-glutamyl transferase greater than three times the upper limit of normal in the DAG group was expected with high-dose perampanel, the investigators noted. This outcome occurred in 77% of the DAG group versus 27% of the NAG group (OR, 9.88; 95% CI, 2.4-32.9; P < .001).
There was no statistically significant difference in incidence of recurrent cardiac arrest during therapy. This occurred in one member of the DAG group and in none in the NAG group.
Dr. Beretta reported that their investigations are still in a retrospective phase, but the researchers plan to move the work into a prospective phase and possibly a randomized trial soon.
Fascinating, promising
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center, Columbus, said the study provides “fascinating, very helpful data” about a condition that has responded well to current treatment options.
He added that his center has used “the innovative treatments” discussed in the study for a few patients.
“More concrete evidence will push us to use it more uniformly across all our patient population [that] has refractory status. So I’m very optimistic, and the data were very promising,” said Dr. Singh, who was not involved with the research.
He cautioned that the study was retrospective, not randomized or controlled, and that it involved a small number of patients but said that the data were “heading in the right direction.”
Although resolution of status epilepticus was better among patients in the DAG group than in the NAG group, the awakenings and neurologic outcomes were “pretty much same as standard medical therapy, which we commonly give to our patients,” said Dr. Singh. “We see this phenomenon all the time in our patients.”
He noted that other factors can determine how patients respond, such as conditions of the heart or kidneys, the presence of sepsis, and multiorgan dysfunction. These factors were not controlled for in the study.
Nonetheless, he said the study achieved its primary endpoint of better resolution of status epilepticus “because that’s the first thing you want to see: whether the treatment is taking care of that.”
The study received no outside funding. Dr. Beretta and Dr. Singh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From WCN 2021
‘Baby-wearing’ poses serious injury risks for infants, ED data show
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
FROM AAP 2021