User login
Is genetic testing valuable in the clinical management of epilepsy?
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.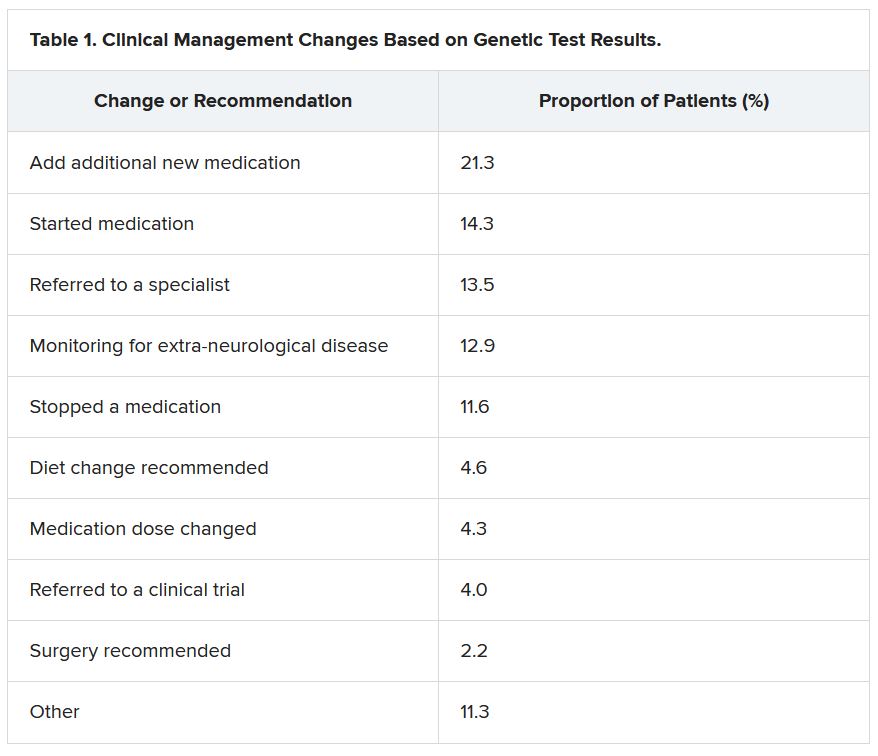
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”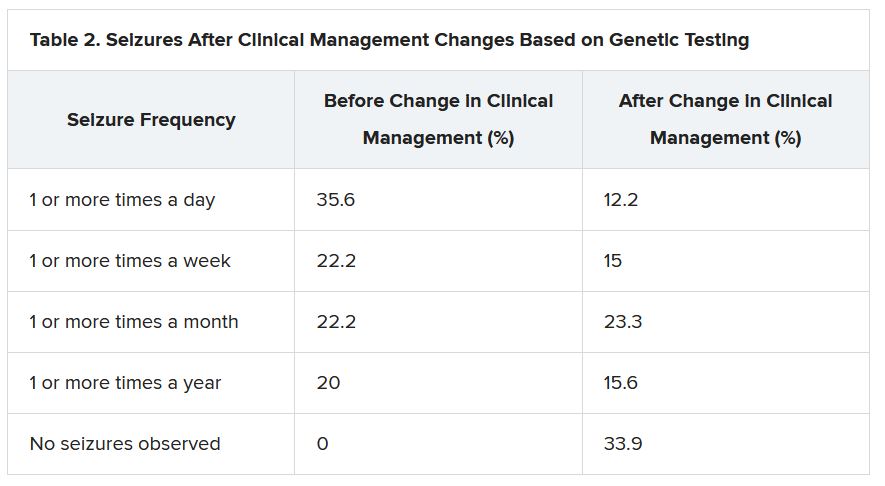
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From WCN 2021
USPSTF rules out aspirin for over 60s in primary CVD prevention
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
Soft Nodule on the Forearm
The Diagnosis: Schwannoma
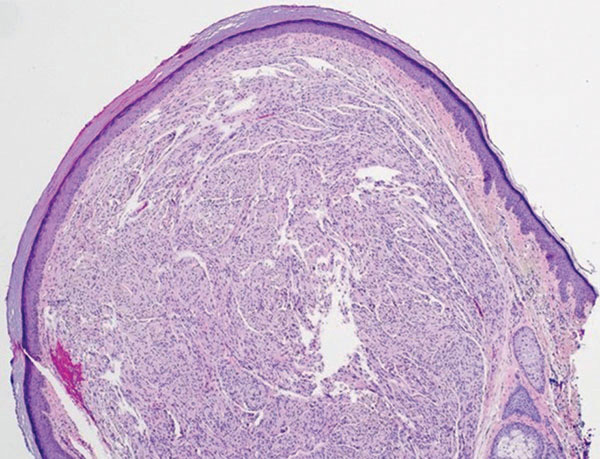
Schwannoma, also known as neurilemmoma, is a benign encapsulated neoplasm of the peripheral nerve sheath that presents as a subcutaneous nodule.1 It also may present in the retroperitoneum, mediastinum, and viscera (eg, gastrointestinal tract, bone, upper respiratory tract, lymph nodes). It may occur as multiple lesions when associated with certain syndromes. It usually is an asymptomatic indolent tumor with neurologic symptoms, such as pain and tenderness, in the lesions that are deeper, larger, or closer in proximity to nearby structures.2,3
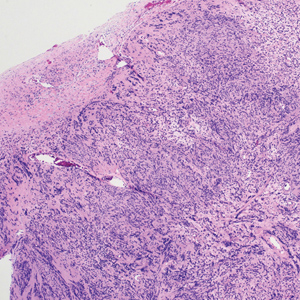
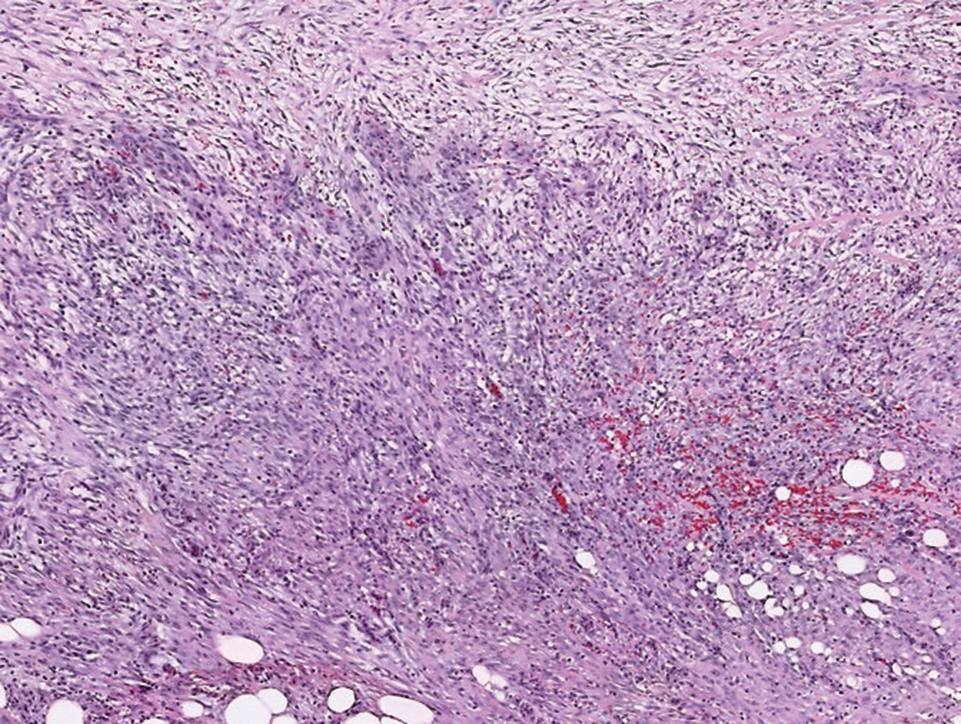
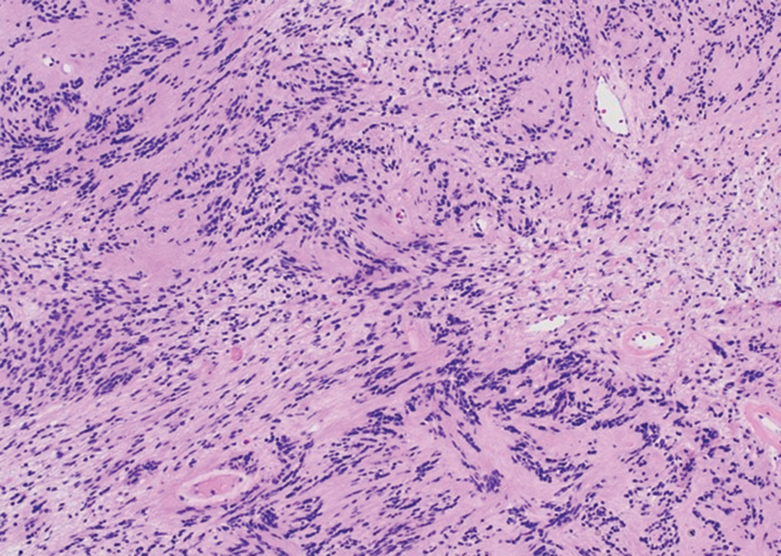
Histologically, a schwannoma is encapsulated by the perineurium of the nerve bundle from which it originates (quiz image [top]). The tumor consists of hypercellular (Antoni type A) and hypocellular (Antoni type B) areas. Antoni type A areas consist of tightly packed, spindleshaped cells with elongated wavy nuclei and indistinct cytoplasmic borders. These nuclei tend to align into parallel rows with intervening anuclear zones forming Verocay bodies (quiz image [bottom]).4 Verocay bodies are not seen in all schwannomas, and similar formations may be seen in other tumors as well. Solitary circumscribed neuromas also have Verocay bodies, whereas dermatofibromas and leiomyomas have Verocay-like bodies. Antoni type B areas have scattered spindled or ovoid cells in an edematous or myxoid matrix interspersed with inflammatory cells such as lymphocytes and histiocytes. Vessels with thick hyalinized walls are a helpful feature in diagnosis.2 Schwann cells of a schwannoma stain diffusely positive with S-100 protein. The capsule stains positively with epithelial membrane antigen due to the presence of perineurial cells.2
The morphologic variants of this entity include conventional (common, solitary), cellular, plexiform, ancient, melanotic, epithelioid, pseudoglandular, neuroblastomalike, and microcystic/reticular schwannomas. There are additional variants that are associated with genetic syndromes, such as multiple cutaneous plexiform schwannomas linked with neurofibromatosis type 2, psammomatous melanotic schwannoma presenting in Carney complex, schwannomatosis, and segmental schwannomatosis (a distinct form of neurofibromatosis characterized by multiple schwannomas localized to one limb). Either presentation may have alteration or deletion of the neurofibromatosis type 2 gene, NF2, on chromosome 22.2,5
Nodular fasciitis is a benign tumor of fibroblasts and myofibroblasts that usually arises in the subcutaneous tissues. It most commonly occurs in the upper extremities, trunk, head, and neck. It presents as a single, often painful, rapidly growing, subcutaneous nodule. Histologically, lesions mostly are well circumscribed yet unencapsulated, in contrast to schwannomas. They may be hypocellular or hypercellular and are composed of uniform spindle cells with a feathery or fascicular (tissue culture–like) appearance in a loose, myxoid to collagenous stroma. There may be foci of hemorrhage and conspicuous mitoses but not atypical figures (Figure 1). Immunohistochemically, the cells stain positively for smooth muscle actin and negatively for S-100 protein, which sets it apart from a schwannoma. Most cases contain fusion genes, with myosin heavy chain 9 ubiquitin-specific peptidase 6, MYH9-USP6, being the most common fusion product.6
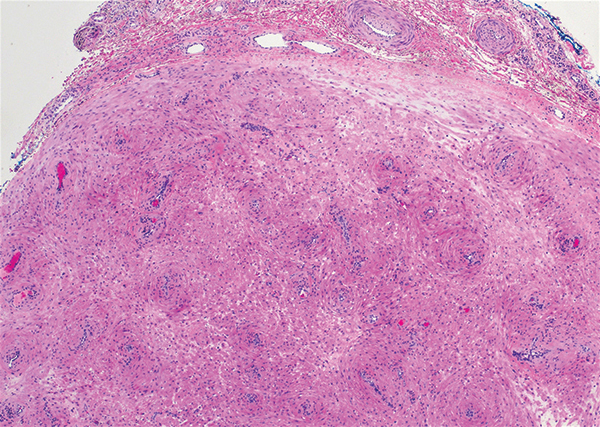
Solitary circumscribed neuroma (palisaded encapsulated neuroma) is a benign, usually solitary dermal lesion. It most commonly occurs in middle-aged to elderly adults as a small (<1 cm), firm, flesh-colored to pink papule on the face (ie, cheeks, nose, nasolabial folds) and less commonly in the oral and acral regions and on the eyelids and penis. The lesion usually is unilobular; however, other growth patterns such as plexiform, multilobular, and fungating variants have been identified. Histologically, it is a well-circumscribed nodule with a thin capsule of perineurium that is composed of interlacing bundles of Schwann cells with a characteristic clefting artifact (Figure 2). Cells have wavy dark nuclei with scant cytoplasm that occasionally form palisades or Verocay bodies causing these lesions to be confused with schwannomas. Immunohistochemically, the Schwann cells stain positively with S-100 protein, and the perineurium stains positively with epithelial membrane antigen, Claudin-1, and Glut-1. Neurofilament protein stains axons throughout neuromas, whereas in schwannoma, the expression often is limited to entrapped axons at the periphery of the tumor.7
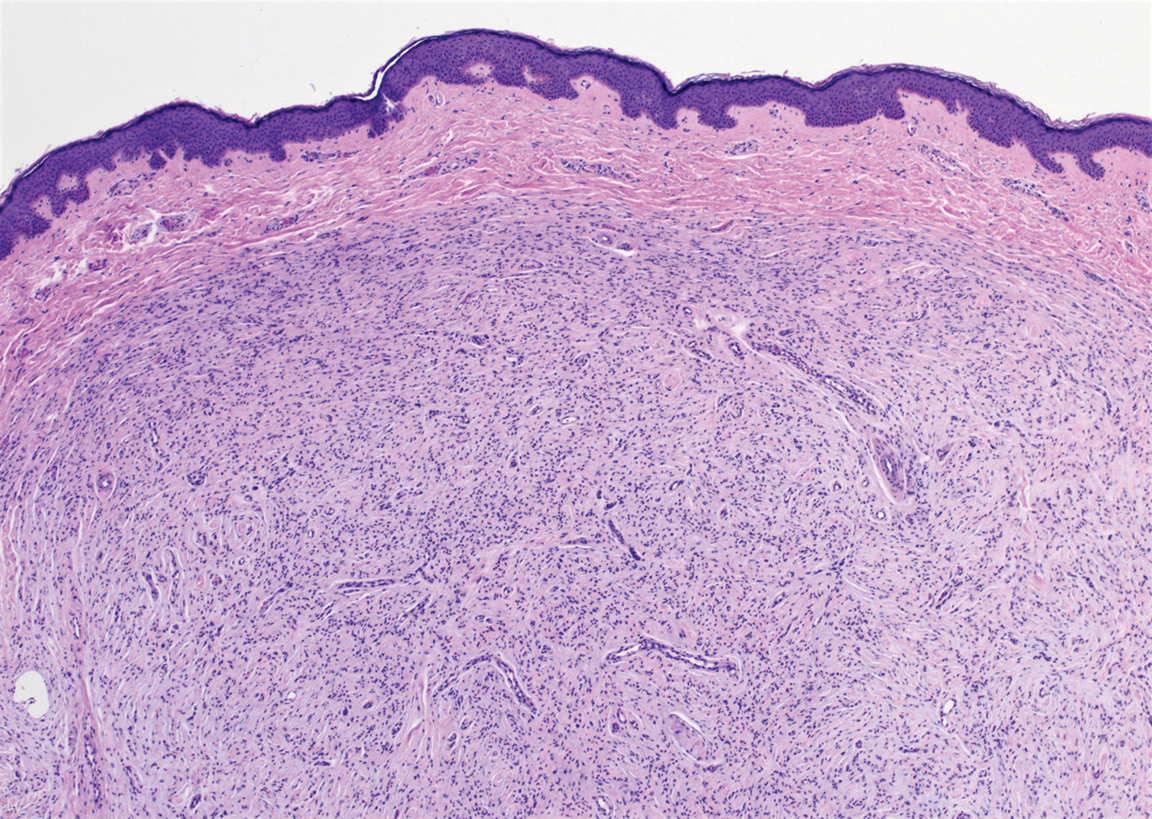
Angioleiomyoma is an uncommon, benign, smooth muscle neoplasm of the skin and subcutaneous tissue that originates from vascular smooth muscle. It most commonly presents in adult females aged 30 to 60 years, with a predilection for the lower limbs. These tumors typically are solitary, slow growing, and less than 2 cm in diameter and may be painful upon compression. Similar to schwannoma, angioleiomyoma is an encapsulated lesion composed of interlacing, uniform, smooth muscle bundles distributed around vessels (Figure 3). Smooth muscle cells have oval- or cigar-shaped nuclei with a small perinuclear vacuole of glycogen. Immunohistochemically, there is strong diffuse staining for smooth muscle actin and h-caldesmon. Recurrence after excision is rare.2,8
Neurofibroma is a common, mostly sporadic, benign tumor of nerve sheath origin. The solitary type may be localized (well circumscribed, unencapsulated) or diffuse. The presence of multiple, deep, and plexiform lesions is associated with neurofibromatosis type 1 (von Recklinghausen disease) that is caused by germline mutations in the NF1 gene. Histologically, the tumor is composed of Schwann cells, fibroblasts, perineurial cells, and nerve axons within an extracellular myxoid to collagenous matrix (Figure 4). The diffuse type is an ill-defined proliferation that entraps adnexal structures. The plexiform type is defined by multinodular serpentine fascicles. Immunohistochemically, the Schwann cells stain positive for S-100 protein and SOX10 (SRY-Box Transcription Factor 10). Epithelial membrane antigen stains admixed perineurial cells. Neurofilament protein highlights intratumoral axons, which generally are not found throughout schwannomas. Transformation to a malignant peripheral nerve sheath tumor occurs in up to 10% of patients with neurofibromatosis type 1, usually in plexiform neurofibromas, and is characterized by increased cellularity, atypia, mitotic activity, and necrosis.9
- Ritter SE, Elston DM. Cutaneous schwannoma of the foot. Cutis. 2001;67:127-129.
- Calonje E, Damaskou V, Lazar AJ. Connective tissue tumors. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Vol 2. Elsevier Saunders; 2020:1698-1894.
- Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382-387.
- Lespi PJ, Smit R. Verocay body—prominent cutaneous leiomyoma. Am J Dermatopathol. 1999;21:110-111.
- Kurtkaya-Yapicier O, Scheithauer B, Woodruff JM. The pathobiologic spectrum of schwannomas. Histol Histopathol. 2003;18:925-934.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Leblebici C, Savli TC, Yeni B, et al. Palisaded encapsulated (solitary circumscribed) neuroma: a review of 30 cases. Int J Surg Pathol. 2019;27:506-514.
- Yeung CM, Moore L, Lans J, et al. Angioleiomyoma of the hand: a case series and review of the literature. Arch Bone Jt Surg. 2020; 8:373-377.
- Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin North Am. 2004;15:157-166.
The Diagnosis: Schwannoma
Schwannoma, also known as neurilemmoma, is a benign encapsulated neoplasm of the peripheral nerve sheath that presents as a subcutaneous nodule.1 It also may present in the retroperitoneum, mediastinum, and viscera (eg, gastrointestinal tract, bone, upper respiratory tract, lymph nodes). It may occur as multiple lesions when associated with certain syndromes. It usually is an asymptomatic indolent tumor with neurologic symptoms, such as pain and tenderness, in the lesions that are deeper, larger, or closer in proximity to nearby structures.2,3
Histologically, a schwannoma is encapsulated by the perineurium of the nerve bundle from which it originates (quiz image [top]). The tumor consists of hypercellular (Antoni type A) and hypocellular (Antoni type B) areas. Antoni type A areas consist of tightly packed, spindleshaped cells with elongated wavy nuclei and indistinct cytoplasmic borders. These nuclei tend to align into parallel rows with intervening anuclear zones forming Verocay bodies (quiz image [bottom]).4 Verocay bodies are not seen in all schwannomas, and similar formations may be seen in other tumors as well. Solitary circumscribed neuromas also have Verocay bodies, whereas dermatofibromas and leiomyomas have Verocay-like bodies. Antoni type B areas have scattered spindled or ovoid cells in an edematous or myxoid matrix interspersed with inflammatory cells such as lymphocytes and histiocytes. Vessels with thick hyalinized walls are a helpful feature in diagnosis.2 Schwann cells of a schwannoma stain diffusely positive with S-100 protein. The capsule stains positively with epithelial membrane antigen due to the presence of perineurial cells.2
The morphologic variants of this entity include conventional (common, solitary), cellular, plexiform, ancient, melanotic, epithelioid, pseudoglandular, neuroblastomalike, and microcystic/reticular schwannomas. There are additional variants that are associated with genetic syndromes, such as multiple cutaneous plexiform schwannomas linked with neurofibromatosis type 2, psammomatous melanotic schwannoma presenting in Carney complex, schwannomatosis, and segmental schwannomatosis (a distinct form of neurofibromatosis characterized by multiple schwannomas localized to one limb). Either presentation may have alteration or deletion of the neurofibromatosis type 2 gene, NF2, on chromosome 22.2,5
Nodular fasciitis is a benign tumor of fibroblasts and myofibroblasts that usually arises in the subcutaneous tissues. It most commonly occurs in the upper extremities, trunk, head, and neck. It presents as a single, often painful, rapidly growing, subcutaneous nodule. Histologically, lesions mostly are well circumscribed yet unencapsulated, in contrast to schwannomas. They may be hypocellular or hypercellular and are composed of uniform spindle cells with a feathery or fascicular (tissue culture–like) appearance in a loose, myxoid to collagenous stroma. There may be foci of hemorrhage and conspicuous mitoses but not atypical figures (Figure 1). Immunohistochemically, the cells stain positively for smooth muscle actin and negatively for S-100 protein, which sets it apart from a schwannoma. Most cases contain fusion genes, with myosin heavy chain 9 ubiquitin-specific peptidase 6, MYH9-USP6, being the most common fusion product.6

Solitary circumscribed neuroma (palisaded encapsulated neuroma) is a benign, usually solitary dermal lesion. It most commonly occurs in middle-aged to elderly adults as a small (<1 cm), firm, flesh-colored to pink papule on the face (ie, cheeks, nose, nasolabial folds) and less commonly in the oral and acral regions and on the eyelids and penis. The lesion usually is unilobular; however, other growth patterns such as plexiform, multilobular, and fungating variants have been identified. Histologically, it is a well-circumscribed nodule with a thin capsule of perineurium that is composed of interlacing bundles of Schwann cells with a characteristic clefting artifact (Figure 2). Cells have wavy dark nuclei with scant cytoplasm that occasionally form palisades or Verocay bodies causing these lesions to be confused with schwannomas. Immunohistochemically, the Schwann cells stain positively with S-100 protein, and the perineurium stains positively with epithelial membrane antigen, Claudin-1, and Glut-1. Neurofilament protein stains axons throughout neuromas, whereas in schwannoma, the expression often is limited to entrapped axons at the periphery of the tumor.7

Angioleiomyoma is an uncommon, benign, smooth muscle neoplasm of the skin and subcutaneous tissue that originates from vascular smooth muscle. It most commonly presents in adult females aged 30 to 60 years, with a predilection for the lower limbs. These tumors typically are solitary, slow growing, and less than 2 cm in diameter and may be painful upon compression. Similar to schwannoma, angioleiomyoma is an encapsulated lesion composed of interlacing, uniform, smooth muscle bundles distributed around vessels (Figure 3). Smooth muscle cells have oval- or cigar-shaped nuclei with a small perinuclear vacuole of glycogen. Immunohistochemically, there is strong diffuse staining for smooth muscle actin and h-caldesmon. Recurrence after excision is rare.2,8

Neurofibroma is a common, mostly sporadic, benign tumor of nerve sheath origin. The solitary type may be localized (well circumscribed, unencapsulated) or diffuse. The presence of multiple, deep, and plexiform lesions is associated with neurofibromatosis type 1 (von Recklinghausen disease) that is caused by germline mutations in the NF1 gene. Histologically, the tumor is composed of Schwann cells, fibroblasts, perineurial cells, and nerve axons within an extracellular myxoid to collagenous matrix (Figure 4). The diffuse type is an ill-defined proliferation that entraps adnexal structures. The plexiform type is defined by multinodular serpentine fascicles. Immunohistochemically, the Schwann cells stain positive for S-100 protein and SOX10 (SRY-Box Transcription Factor 10). Epithelial membrane antigen stains admixed perineurial cells. Neurofilament protein highlights intratumoral axons, which generally are not found throughout schwannomas. Transformation to a malignant peripheral nerve sheath tumor occurs in up to 10% of patients with neurofibromatosis type 1, usually in plexiform neurofibromas, and is characterized by increased cellularity, atypia, mitotic activity, and necrosis.9

The Diagnosis: Schwannoma
Schwannoma, also known as neurilemmoma, is a benign encapsulated neoplasm of the peripheral nerve sheath that presents as a subcutaneous nodule.1 It also may present in the retroperitoneum, mediastinum, and viscera (eg, gastrointestinal tract, bone, upper respiratory tract, lymph nodes). It may occur as multiple lesions when associated with certain syndromes. It usually is an asymptomatic indolent tumor with neurologic symptoms, such as pain and tenderness, in the lesions that are deeper, larger, or closer in proximity to nearby structures.2,3
Histologically, a schwannoma is encapsulated by the perineurium of the nerve bundle from which it originates (quiz image [top]). The tumor consists of hypercellular (Antoni type A) and hypocellular (Antoni type B) areas. Antoni type A areas consist of tightly packed, spindleshaped cells with elongated wavy nuclei and indistinct cytoplasmic borders. These nuclei tend to align into parallel rows with intervening anuclear zones forming Verocay bodies (quiz image [bottom]).4 Verocay bodies are not seen in all schwannomas, and similar formations may be seen in other tumors as well. Solitary circumscribed neuromas also have Verocay bodies, whereas dermatofibromas and leiomyomas have Verocay-like bodies. Antoni type B areas have scattered spindled or ovoid cells in an edematous or myxoid matrix interspersed with inflammatory cells such as lymphocytes and histiocytes. Vessels with thick hyalinized walls are a helpful feature in diagnosis.2 Schwann cells of a schwannoma stain diffusely positive with S-100 protein. The capsule stains positively with epithelial membrane antigen due to the presence of perineurial cells.2
The morphologic variants of this entity include conventional (common, solitary), cellular, plexiform, ancient, melanotic, epithelioid, pseudoglandular, neuroblastomalike, and microcystic/reticular schwannomas. There are additional variants that are associated with genetic syndromes, such as multiple cutaneous plexiform schwannomas linked with neurofibromatosis type 2, psammomatous melanotic schwannoma presenting in Carney complex, schwannomatosis, and segmental schwannomatosis (a distinct form of neurofibromatosis characterized by multiple schwannomas localized to one limb). Either presentation may have alteration or deletion of the neurofibromatosis type 2 gene, NF2, on chromosome 22.2,5
Nodular fasciitis is a benign tumor of fibroblasts and myofibroblasts that usually arises in the subcutaneous tissues. It most commonly occurs in the upper extremities, trunk, head, and neck. It presents as a single, often painful, rapidly growing, subcutaneous nodule. Histologically, lesions mostly are well circumscribed yet unencapsulated, in contrast to schwannomas. They may be hypocellular or hypercellular and are composed of uniform spindle cells with a feathery or fascicular (tissue culture–like) appearance in a loose, myxoid to collagenous stroma. There may be foci of hemorrhage and conspicuous mitoses but not atypical figures (Figure 1). Immunohistochemically, the cells stain positively for smooth muscle actin and negatively for S-100 protein, which sets it apart from a schwannoma. Most cases contain fusion genes, with myosin heavy chain 9 ubiquitin-specific peptidase 6, MYH9-USP6, being the most common fusion product.6

Solitary circumscribed neuroma (palisaded encapsulated neuroma) is a benign, usually solitary dermal lesion. It most commonly occurs in middle-aged to elderly adults as a small (<1 cm), firm, flesh-colored to pink papule on the face (ie, cheeks, nose, nasolabial folds) and less commonly in the oral and acral regions and on the eyelids and penis. The lesion usually is unilobular; however, other growth patterns such as plexiform, multilobular, and fungating variants have been identified. Histologically, it is a well-circumscribed nodule with a thin capsule of perineurium that is composed of interlacing bundles of Schwann cells with a characteristic clefting artifact (Figure 2). Cells have wavy dark nuclei with scant cytoplasm that occasionally form palisades or Verocay bodies causing these lesions to be confused with schwannomas. Immunohistochemically, the Schwann cells stain positively with S-100 protein, and the perineurium stains positively with epithelial membrane antigen, Claudin-1, and Glut-1. Neurofilament protein stains axons throughout neuromas, whereas in schwannoma, the expression often is limited to entrapped axons at the periphery of the tumor.7

Angioleiomyoma is an uncommon, benign, smooth muscle neoplasm of the skin and subcutaneous tissue that originates from vascular smooth muscle. It most commonly presents in adult females aged 30 to 60 years, with a predilection for the lower limbs. These tumors typically are solitary, slow growing, and less than 2 cm in diameter and may be painful upon compression. Similar to schwannoma, angioleiomyoma is an encapsulated lesion composed of interlacing, uniform, smooth muscle bundles distributed around vessels (Figure 3). Smooth muscle cells have oval- or cigar-shaped nuclei with a small perinuclear vacuole of glycogen. Immunohistochemically, there is strong diffuse staining for smooth muscle actin and h-caldesmon. Recurrence after excision is rare.2,8

Neurofibroma is a common, mostly sporadic, benign tumor of nerve sheath origin. The solitary type may be localized (well circumscribed, unencapsulated) or diffuse. The presence of multiple, deep, and plexiform lesions is associated with neurofibromatosis type 1 (von Recklinghausen disease) that is caused by germline mutations in the NF1 gene. Histologically, the tumor is composed of Schwann cells, fibroblasts, perineurial cells, and nerve axons within an extracellular myxoid to collagenous matrix (Figure 4). The diffuse type is an ill-defined proliferation that entraps adnexal structures. The plexiform type is defined by multinodular serpentine fascicles. Immunohistochemically, the Schwann cells stain positive for S-100 protein and SOX10 (SRY-Box Transcription Factor 10). Epithelial membrane antigen stains admixed perineurial cells. Neurofilament protein highlights intratumoral axons, which generally are not found throughout schwannomas. Transformation to a malignant peripheral nerve sheath tumor occurs in up to 10% of patients with neurofibromatosis type 1, usually in plexiform neurofibromas, and is characterized by increased cellularity, atypia, mitotic activity, and necrosis.9

- Ritter SE, Elston DM. Cutaneous schwannoma of the foot. Cutis. 2001;67:127-129.
- Calonje E, Damaskou V, Lazar AJ. Connective tissue tumors. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Vol 2. Elsevier Saunders; 2020:1698-1894.
- Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382-387.
- Lespi PJ, Smit R. Verocay body—prominent cutaneous leiomyoma. Am J Dermatopathol. 1999;21:110-111.
- Kurtkaya-Yapicier O, Scheithauer B, Woodruff JM. The pathobiologic spectrum of schwannomas. Histol Histopathol. 2003;18:925-934.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Leblebici C, Savli TC, Yeni B, et al. Palisaded encapsulated (solitary circumscribed) neuroma: a review of 30 cases. Int J Surg Pathol. 2019;27:506-514.
- Yeung CM, Moore L, Lans J, et al. Angioleiomyoma of the hand: a case series and review of the literature. Arch Bone Jt Surg. 2020; 8:373-377.
- Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin North Am. 2004;15:157-166.
- Ritter SE, Elston DM. Cutaneous schwannoma of the foot. Cutis. 2001;67:127-129.
- Calonje E, Damaskou V, Lazar AJ. Connective tissue tumors. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Vol 2. Elsevier Saunders; 2020:1698-1894.
- Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br. 2007;89:382-387.
- Lespi PJ, Smit R. Verocay body—prominent cutaneous leiomyoma. Am J Dermatopathol. 1999;21:110-111.
- Kurtkaya-Yapicier O, Scheithauer B, Woodruff JM. The pathobiologic spectrum of schwannomas. Histol Histopathol. 2003;18:925-934.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Leblebici C, Savli TC, Yeni B, et al. Palisaded encapsulated (solitary circumscribed) neuroma: a review of 30 cases. Int J Surg Pathol. 2019;27:506-514.
- Yeung CM, Moore L, Lans J, et al. Angioleiomyoma of the hand: a case series and review of the literature. Arch Bone Jt Surg. 2020; 8:373-377.
- Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin North Am. 2004;15:157-166.

A 54-year-old woman presented with an enlarging mass on the right volar forearm. Physical examination revealed a 1-cm, soft, mobile, subcutaneous nodule. Excision revealed tan-pink, indurated, fibrous, nodular tissue.
Product News October 2021
Opzelura FDA Approved for Atopic Dermatitis Incyte
Corporation announces US Food and Drug Administration (FDA) approval of Opzelura (ruxolitinib) cream 1.5% for the short-term and noncontinuous chronic treatment of mild to moderate atopic dermatitis (AD) in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Opzelura is formulated with ruxolitinib, a selective Janus kinase (JAK) 1/JAK2 inhibitor, to target key cytokine signals believed to contribute to itch and inflammation. For more information, visit www.opzelurahcp.com/.
Twyneo FDA Approved for Acne Vulgaris
Sol-Gel Technologies, Ltd, announces US Food and Drug Administration (FDA) approval of Twyneo (tretinoin 0.1% /benzoyl peroxide 3%) cream for the treatment of acne vulgaris in adult and pediatric patients 9 years and older. Tretinoin and benzoyl peroxide are widely prescribed separately for acne vulgaris; however, benzoyl peroxide causes degradation of the tretinoin molecule, thereby potentially reducing its effectiveness if used at the same time or combined in the same formulation. The formulation of Twyneo uses silica (silicon dioxide) core shell structures to separately microencapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the 2 active ingredients in the cream. For more information, visit www.sol-gel.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Opzelura FDA Approved for Atopic Dermatitis Incyte
Corporation announces US Food and Drug Administration (FDA) approval of Opzelura (ruxolitinib) cream 1.5% for the short-term and noncontinuous chronic treatment of mild to moderate atopic dermatitis (AD) in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Opzelura is formulated with ruxolitinib, a selective Janus kinase (JAK) 1/JAK2 inhibitor, to target key cytokine signals believed to contribute to itch and inflammation. For more information, visit www.opzelurahcp.com/.
Twyneo FDA Approved for Acne Vulgaris
Sol-Gel Technologies, Ltd, announces US Food and Drug Administration (FDA) approval of Twyneo (tretinoin 0.1% /benzoyl peroxide 3%) cream for the treatment of acne vulgaris in adult and pediatric patients 9 years and older. Tretinoin and benzoyl peroxide are widely prescribed separately for acne vulgaris; however, benzoyl peroxide causes degradation of the tretinoin molecule, thereby potentially reducing its effectiveness if used at the same time or combined in the same formulation. The formulation of Twyneo uses silica (silicon dioxide) core shell structures to separately microencapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the 2 active ingredients in the cream. For more information, visit www.sol-gel.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Opzelura FDA Approved for Atopic Dermatitis Incyte
Corporation announces US Food and Drug Administration (FDA) approval of Opzelura (ruxolitinib) cream 1.5% for the short-term and noncontinuous chronic treatment of mild to moderate atopic dermatitis (AD) in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Opzelura is formulated with ruxolitinib, a selective Janus kinase (JAK) 1/JAK2 inhibitor, to target key cytokine signals believed to contribute to itch and inflammation. For more information, visit www.opzelurahcp.com/.
Twyneo FDA Approved for Acne Vulgaris
Sol-Gel Technologies, Ltd, announces US Food and Drug Administration (FDA) approval of Twyneo (tretinoin 0.1% /benzoyl peroxide 3%) cream for the treatment of acne vulgaris in adult and pediatric patients 9 years and older. Tretinoin and benzoyl peroxide are widely prescribed separately for acne vulgaris; however, benzoyl peroxide causes degradation of the tretinoin molecule, thereby potentially reducing its effectiveness if used at the same time or combined in the same formulation. The formulation of Twyneo uses silica (silicon dioxide) core shell structures to separately microencapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the 2 active ingredients in the cream. For more information, visit www.sol-gel.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Things We Do for No Reason™: Routine Use of Corticosteroids for the Treatment of Anaphylaxis
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11
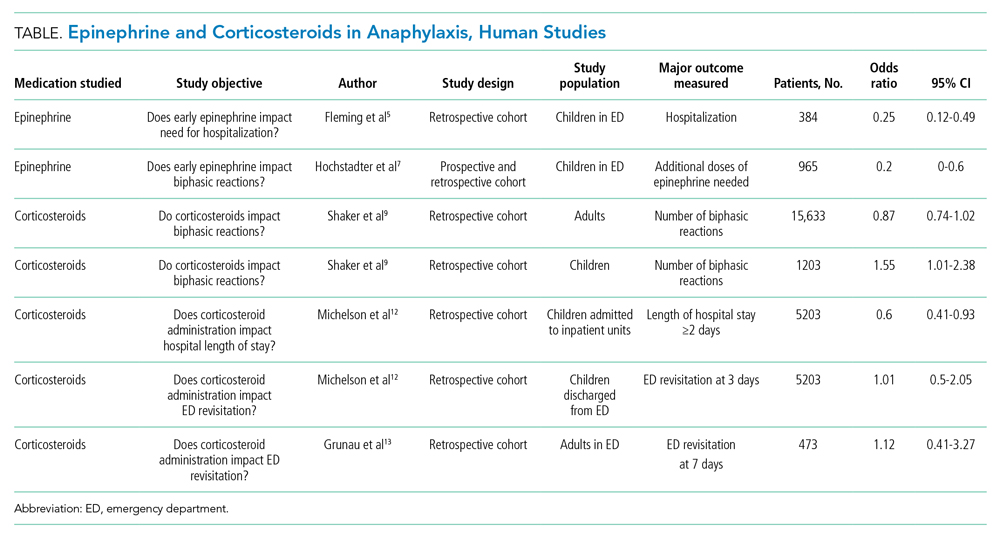
The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11

The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with coronary artery disease (CAD) undergoes hospital treatment for diverticulitis. He receives ketorolac for abdominal pain upon arrival to the medical ward despite his known allergy to nonsteroidal anti-inflammatory drugs. Fifteen minutes after administration, he develops lightheadedness and experiences swelling of his lips. On exam, he has tachycardia and a diffuse urticarial rash across his torso. The admitting physician prescribes methylprednisolone, diphenhydramine, and a liter bolus of normal saline for suspected anaphylaxis. Epinephrine is not administered for fear of precipitating an adverse cardiovascular event given the patient’s history of CAD.
BACKGROUND
Anaphylaxis, a rapid-onset generalized immunoglobulin E (IgE)–mediated hypersensitivity reaction, can lead to significant morbidity and mortality when not managed properly. Patients can present with anaphylaxis in heterogeneous ways. Fulfilling any one of three criteria establishes the diagnosis of anaphylaxis: (1) rapid onset of skin or mucosal symptoms complicated by either respiratory compromise or hypotension; (2) two or more symptoms involving the respiratory, mucosal, cardiovascular, or gastrointestinal systems following exposure to a likely allergen; and (3) reduced blood pressure in response to a known allergen.1 Up to 5% of the population experiences anaphylaxis in a lifetime. Medication and stinging insects account for the majority of anaphylactic reactions in adults, while food and insect stings commonly trigger it in children and adolescents.2
The majority of anaphylactic reactions, known as uniphasic or monophasic, occur rapidly as single episodes following exposure to a specific trigger and resolve within minutes to hours after treatment. Meanwhile, biphasic, or delayed-phase, anaphylaxis occurs when symptoms recur after an apparent resolution and in the absence of reexposure to the trigger. Symptoms restart within 1 to 72 hours after resolution of an initial anaphylaxis episode, with a median time to onset of 11 hours. Biphasic reactions occur in roughly 5% of patients with anaphylaxis.3
Epinephrine is the only recommended first-line medication for the treatment of anaphylaxis in all age groups.4 Epinephrine counteracts the cardiovascular and respiratory compromise induced by anaphylaxis through its α- and β-adrenergic activity and stabilizes mast cells.4 Early administration of intramuscular epinephrine decreases the need for additional interventions, reduces the likelihood of hospitalization, and is associated with reduced biphasic reactions.5-7 Paradoxically, patients receive corticosteroids more often than epinephrine for suspected anaphylaxis, despite no robust evidence for their efficacy.4,8,9
WHY YOU MIGHT THINK STEROIDS aRE HELPFUL FOR ANAPHYLAXIS
Corticosteroids act as potent anti-inflammatory medications that modulate mast-cell maturation, activation, and degranulation. Known to work primarily through downregulation of gene transcription responsible for cytokine, chemokine, and arachidonic acid production, their maximal anti-inflammatory effects manifest 2 to 6 hours after administration. Demonstrated efficacy in treating and preventing relapse of other inflammatory conditions, such as asthma and croup, may, in part, explain the widespread glucocorticoid use in anaphylaxis. Some believe that administration of corticosteroids may also help reduce the risk of biphasic or delayed-phase anaphylaxis.10
WHY THERE IS NO REASON TO PRESCRIBE CORTICOSTEROIDS FOR ANAPHYLAXIS
Based on their mechanism of action, corticosteroids do not exert any anti-inflammatory effects for several hours, regardless of their route of administration.10 In contrast, epinephrine exerts an almost immediate effect to increase cardiac output and vascular resistance, to reverse edema and bronchoconstriction, and to stabilize mast cells, preventing release of harmful chemokines and cytokines.4
The American Academy of Allergy, Asthma & Immunology (AAAAI) recommends early administration of epinephrine as the first-line treatment of anaphylaxis and emphasizes that evidence does not support routine corticosteroid use in the management of acute anaphylaxis or for prevention of biphasic reactions.9 To date, no randomized controlled trials have explored the role of corticosteroids in the treatment of acute anaphylaxis, although one is currently under way looking at whether dexamethasone has an impact on preventing biphasic reactions (Table).11

The AAAAI Joint Task Force on Practice Parameters (JTFPP) conducted a pooled analysis of observational studies that did not find a reduction in biphasic reactions in adult patients receiving corticosteroids (odds ratio [OR], 0.87; 95% CI, 0.74-1.02).9 Further, their analysis suggests an association with administration of corticosteroids and an increased likelihood of biphasic reactions in children (OR, 1.55; 95% CI, 1.01-2.38).9
An observational study in children across 35 hospitals demonstrated an association with corticosteroid administration and a reduced length of hospital stay for anaphylaxis, but the same study found no reduction in repeat emergency department (ED) visits within 72 hours.12 Similarly, a retrospective cohort study in adults did not find that corticosteroid administration reduced the 7-day risk of returning to the hospital.13 These studies highlight the importance of anticipatory guidance in both ED and hospital discharges for anaphylaxis since the literature does not provide data that corticosteroid administration reduces the likelihood of a biphasic course.
Long-term corticosteroids have well-known deleterious health effects. Recent evidence highlights the possible adverse events associated with even short courses of corticosteroids. A large case series from Taiwan containing 2,623,327 adults administered brief courses (<14 days) of corticosteroids demonstrated increased incidence of gastrointestinal bleeding, sepsis, and heart failure beginning 5 to 30 days after starting corticosteroid treatments for common medical conditions, with respective absolute risk increases of 10.3, 0.1, and 1 per 1000 patient-years for each condition.14 The same group of researchers found a nearly two-fold increased risk of sepsis, gastrointestinal bleeding, and pneumonia in a nearly 1 million children who had received corticosteroids within the previous year.15 Other common side effects of short-term corticosteroids include insomnia, agitation, mood disturbances, and hyperglycemia.
A growing body of evidence demonstrates that corticosteroids likely do not alter the natural disease course of anaphylaxis and carry increased risks of significant adverse events. The AAAAI recommends against the use of glucocorticoids as a first-line agent for anaphylaxis and suggests against the use of glucocorticoids to prevent biphasic reactions.9
WHEN TREATING WITH CORTICOSTEROIDS MAY BE INDICATED
The recent JTFPP analysis of observational studies demonstrated reduced hypersensitivity reactions to chemotherapeutics with corticosteroid premedication (OR, 0.49; 95% CI, 0.37-0.66). The AAAAI favors administration of corticosteroids to reduce the risk of anaphylactoid reactions—non–IgE-mediated mast cell activation—for some chemotherapeutic protocols.9
There is robust evidence regarding the benefits of corticosteroids in the treatment of asthma and upper-airway edema.16,17 Allergen exposures can precipitate significant bronchospasm in individuals with asthma and trigger an exacerbation. Although routine corticosteroid use for anaphylaxis in these populations has not been directly studied, their use as an adjunctive therapy may be beneficial if there is clinical evidence of bronchospasm or significant upper-airway edema.
WHAT YOU SHOULD DO INSTEAD
Rapid administration of epinephrine saves lives, reduces need for adjuvant treatments and hospitalization, and is associated with decreased risk of developing biphasic anaphylactic reactions (OR, 0.2; 95% CI, 0-0.6).5-7 Some clinicians are apprehensive about using epinephrine owing to fears related to negative side effects, particularly adverse cardiovascular events. Kawano et al18 performed a retrospective evaluation of 492 ED visits for anaphylaxis and found that epinephrine is administered less often in older patients (age >50 years); however, when administered intramuscularly, there was no significant difference in adverse cardiovascular events in this population compared with younger individuals. The study did demonstrate an increased rate of adverse cardiac events in older patients receiving intravenous epinephrine, an observation that the authors attributed partly to dosing errors that were reported more often with intravenous use.18
RECOMMENDATIONS
- Always promptly administer intramuscular epinephrine when treating anaphylaxis.
- Routine administration of corticosteroids in the treatment of anaphylaxis is not advised owing to insufficient data supporting their efficacy and potential for adverse events. Some patient populations may derive benefit from corticosteroids, including individuals with history of asthma exhibiting bronchospastic symptoms, individuals with significant upper-airway edema, and those undergoing certain chemotherapy regimens.
CONCLUSIONS
In the clinical vignette, the hospitalist withheld the first-line treatment for anaphylaxis, epinephrine. Without the support of evidence in the literature, patients receive corticosteroids and antihistamines more often than epinephrine for suspected anaphylaxis. No evidence supports the routine use of corticosteroids in the management of anaphylaxis or in the prevention of biphasic reactions. Further, recent research demonstrates significant adverse events are associated with even short courses of corticosteroids.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
1. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. https://doi.org/10.1016/j.jaci.2005.12.1303
2. Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461-467. https://doi.org/10.1016/j.jaci.2013.08.016
3. Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e162. https://doi.org/10.1016/j.jaip.2014.12.010
4. Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354-361. https://doi.org/10.1097/ACI.0b013e32833bc670
5. Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract. 2015;3(1):57-62. https://doi.org/10.1016/j.jaip.2014.07.004
6. Sundquist BK, Jose J, Pauze D, Pauze D, Wang H, Järvinen KM. Anaphylaxis risk factors for hospitalization and intensive care: a comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019;40(1):41-47. https://doi.org/10.2500/aap.2019.40.4189
7. Hochstadter E, Clarke A, De Schryver S, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888-1890.e4. https://doi.org/10.1016/j.jaci.2016.02.016
8. Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397-1404. https://doi.org/10.1111/all.12475
9. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systemic review, and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123. https://doi.org/10.1016/j.jaci.2020.01.017
10. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196-207. https://doi.org/10.23822/EurAnnACI.1764-1489.15
11. Use of dexamethasone in prevention of the second phase of a biphasic reaction of anaphylaxis. ClinicalTrials.gov identifier: NCT03523221. Updated July 29, 2020. Accessed July 16, 2021. https://clinicaltrials.gov/ct2/show/NCT03523221
12. Michelson KA, Monuteaux MC, Neuman MI. Glucocorticoids and hospital length of stay for children with anaphylaxis: a retrospective study. J Pediatr. 2015;167(3):719-24.e243. https://doi.org/10.1016/j.jpeds.2015.05.033
13. Grunau BE, Wiens MO, Rowe BH, et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(4):381-389. https://doi.org/10.1016/j.annemergmed.2015.03.003
14. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325-330. https://doi.org/10.7326/M20-0432
15. Yao TC, Wang JY, Chang SM, et al. Association of oral corticosteroid bursts with severe adverse events in children. JAMA Pediatr. 2021;175(7):723-729. https://doi.org/10.1001/jamapediatrics.2021.0433
16. Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000195. https://doi.org/10.1002/14651858.CD000195.pub2
17. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database Syst Rev. 2018;8(8):CD001955. https://doi.org/10.1002/14651858.CD001955.pub4
18. Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017;112:53-58. https://doi.org/10.1016/j.resuscitation.2016.12.020
© 2021 Society of Hospital Medicine
Policy in Clinical Practice: Emergency Medicaid and Access to Allogeneic Stem Cell Transplant for Undocumented Immigrants
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.
Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.

Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
Clinical Scenario
Juan, a 50-year-old man with acute myeloid leukemia (AML), sat on the edge of his bed, dejected. Juan’s leukemia had relapsed for a third time, and he was low on options and optimism. Originally from Mexico, he had made the journey to Colorado to work as a mechanic and care for his disabled son. Like millions of other individuals in the United States, he did not obtain a visa and had no affordable options for health insurance. For nearly a decade, that had seemed not to matter, until he became ill. Initially presenting to the emergency department with fatigue and night sweats, Juan was diagnosed with poor-risk AML and underwent emergent induction chemotherapy reimbursed under Emergency Medicaid (Table). Just when his bone marrow biopsy showed remission, however, Juan was told there was no chance to cure him, as his documentation status precluded him from receiving the next recommended therapy: stem cell transplant (SCT). Without transplant, Juan’s leukemia relapsed within a few months. He decided to undergo all the salvage chemotherapy that was offered, worrying about how his son would survive without his father.

Background and History
For the patient with a new cancer diagnosis, a difference in immigration status may be the difference between life and death. Undocumented immigrants are excluded from federally funded benefits, including those offered under Medicare, most Medicaid programs, and the Patient Protection and Affordable Care Act (Table).1 The nearly 11 million undocumented immigrants residing in the United States are integral to the workforce and economy. Although they pay taxes that fund Medicaid, contributing approximately $11.7 billion nationally in 2017, undocumented immigrants are ineligible to benefit from such programs.2 The inequity of this policy is highlighted by Juan, an undocumented immigrant presenting with a new diagnosis of AML.
The Emergency Medical Treatment and Active Labor Act (EMTALA) is a 1986 federal law which mandates that patients who present to the hospital with an emergency medical condition receive appropriate evaluation and stabilizing treatment. An emergency condition is defined as “manifesting itself by acute symptoms of sufficient severity … such that the absence of immediate medical attention could reasonably be expected to result in (A) placing the patient’s health in serious jeopardy; (B) serious impairment to bodily functions; or (C) serious dysfunction of any bodily organ or part” (Table).3,4 The Centers for Medicare & Medicaid manual restates the EMTALA definition and notes that services for an emergency medical condition cannot include care related to organ transplantation. Most state Emergency Medicaid programs have adopted the federal definition of what constitutes a medical emergency.5 As a result, undocumented individuals who qualify for Medicaid benefits but who do not meet citizenship requirements are eligible to “receive Medical Assistance benefits for emergency medical care only.”3
Similar to our patient Juan, individuals who initially present with an acute leukemia would be eligible for induction chemotherapy, as blast crisis is imminently fatal. Once in remission, however, standard-of-care therapy for patients without disqualifying comorbidities, depending on cytogenetic disease phenotypes, recommends the only current potential cure: allogeneic SCT, a treatment that was far from routine practice at the time EMTALA was enacted.6 When preparing for transplant, a patient is stable and no longer fits EMTALA’s “emergency” criteria, even though their health is still in “serious jeopardy,” as their cancer has been incompletely treated. Because most state Emergency Medicaid programs adopt the federal definition of an emergency medical condition, the cure is out of reach.
Policy in Clinical Practice
This policy requires clinicians to deviate from the usual standard of care and results in inferior outcomes. For AML patients in the poor-risk category, allogeneic SCT is recommended following induction chemotherapy.7 The risk of relapse is 30% to 40% if consolidation therapy includes SCT, vs 70% to 80% if treated with chemotherapeutic consolidation alone.6 AML patients in the intermediate-, and sometimes even favorable- risk categories, have been shown to benefit from allogeneic SCT as well, with risk of relapse half that of a patient who undergoes consolidation without transplant. Undocumented individuals with AML are therefore resigned to inadequate cancer treatment, including lifelong salvage chemotherapy, and have a substantially decreased chance of achieving sustained remission.6 Furthermore, providing inequitable care for undocumented patients with other medical conditions, such as end-stage kidney disease (ESKD), has been associated with inferior patient-reported outcomes, higher mortality and hospital costs, and clinician burnout. In many states, undocumented immigrants with ESKD rely on emergency dialysis (dialysis made available only after presenting critically ill to an emergency department). In 2019, Colorado’s Medicaid agency opted to include ESKD as a qualifying condition for Emergency Medicaid, thereby expanding access to scheduled dialysis. This led to improved patient quality of life, a decreased emotional toll on patients and clinicians, and reduced costs.8,9
Economic Considerations
Policy discussions must consider cost. The average cost of allogeneic SCT in the United States was approximately $226,000 in 2018, which is often compared to the cost of managing a patient with refractory disease who does not receive transplant.10 This study reported a cost of active disease without transplant, including chemotherapy and hospitalizations, of approximately $69,000, plus terminal care costs of nearly $89,000; at a total of $158,000, this comes out to $68,000 less than SCT.10 This cost savings, however, results in a patient’s death rather than an up to 85% chance of long-term, relapse-free survival.6
To more completely capture the relationship between the healthcare value and cost-effectiveness of SCT, a second study calculated the incremental cost-effectiveness ratio (ICER) of transplantation in acute leukemias in the first 100 days post transplant, including management of complications, such as hospitalization, acute graft-versus-host disease (GVHD), infection, and blood product transfusions. ICER represents the economic value of an intervention compared to an alternative, calculated as cost per quality-adjusted life years. The ICER of SCT compared to no transplant is $16,346 to $34,360, depending on type of transplant and conditioning regimen.11 An ICER of less than $50,000 is considered an acceptable expense for the value achieved—in this case, a significant opportunity for cure. This finding supports SCT, including management of complications, as an economically valuable intervention. Furthermore, if a sustained remission is achieved with SCT, this difference in expense buys the individual patient potentially decades of productivity to contribute back into society and the economy. According to the National Bureau of Economic Research, undocumented workers as a whole contribute $5 trillion to the US Gross Domestic Product over a 10-year period, or about $45,000 per worker per year.12 According to the costs cited, curing a single undocumented worker with acute leukemia via SCT and allowing them to return to work would lead to a return on investment in less than 2 years. If the goal is high-quality, high-value, equitable care, it is logical to spend the money upfront and allow all patients the best chance for recovery.
One might suggest that patients instead receive treatment in their country of origin. This proposition, however, is often unrealistic. Latin American countries, for example, lack access to many standard-of-care cancer treatments available domestically. In Mexico, SCT is only available at a single facility in Mexico City, which is unable to track outcomes.13 The mortality-to-incidence ratio for cancer, a marker of availability of effective treatment, for Latin America is 0.48, substantially inferior to that of the United States (0.29).14 Importantly, almost two thirds of undocumented immigrants in the United States have lived in the country for 10 or more years, and 43% are parents of minor children, an increasing proportion of whom are American citizens.15 This highlights the impracticality of these individuals returning to their country of origin for treatment.
Commentary and Recommendations
Medicaid laws in several states have made it possible for undocumented immigrants to receive access to standard-of-care therapies. Washington and California have included provisions that enable undocumented immigrants to receive allogeneic SCT if they are otherwise medically eligible. In the course of this policy change, legal arguments from the California Court of Appeals expressed that the language of the law was not intended to deny lifesaving treatment to an individual.16 California’s Emergency Medicaid policy is comparable to that of other states, but because the courts considered SCT a “continuation of medically necessary inpatient hospital services … directly related to the emergency” for which the patient initially presented, they concluded that it could be covered under California Medicaid. Despite covering SCT for undocumented immigrants, California maintains lower costs for those patients compared to US citizens on Medicaid while providing evidence-based cancer care.17 This exemplifies sustainable and equitable healthcare policy for the rest of the nation.
A proposed change in policy could occur at either the federal or state level. One option would be to follow the example set by the State of Washington. Under Emergency Medicaid, Washington modified qualifying conditions to include “emergency room care, inpatient admission, or outpatient surgery; a cancer treatment plan; dialysis treatment; anti-rejection medication for an organ transplant” and long-term care services.18 Federal policy reform for undocumented immigrants would also improve access to care. The US Citizenship Act of 2021, introduced to the House of Representatives in February 2021, offers a path to citizenship for undocumented immigrants, ultimately allowing for undocumented individuals to be eligible for the same programs as citizens, though after a period of up to 8 years.19 More immediate revisions of qualifying conditions under state Emergency Medicaid programs, coupled with a path to citizenship, would make significant progress towards reducing structural health inequities. Such policy change would also have broader implications. Three quarters of undocumented immigrants in the United States originate from Mexico, Central America, and South America, and the incidence rate of AML for Latinx individuals is 3.6 per 100,000, a figure which can be extrapolated to an estimated 380 cases per year in the US undocumented population.20-22 In addition to benefiting patients with acute leukemias, the proposed policy change would also benefit numerous others who are frequently hospitalized for acute decompensations of chronic conditions, including congestive heart failure, liver disease, ESKD, and chronic lung conditions. Enabling follow-up care for these diseases under Emergency Medicaid would likewise be expected to reduce costs and improve both quality of care and patient-centered and clinical outcomes.
What Should I Tell My Patient?
Hospitalists frequently care for undocumented immigrants with acute leukemias because the hospital can only be reimbursed by Emergency Medicaid when a patient is admitted to the hospital. Patients may ask about what they can expect in the course of their illness and, while details may be left to the oncologist, hospitalists will be faced with responding to many of these questions. Clinicians at our institution hold honest conversations with patients like Juan. We are compelled to provide the care that hospital and state policies allow, and can only offer the best care available to them because of the restrictions of an insurance system to which they contribute financially, yet cannot benefit from, in their time of need. We can tell our undocumented immigrant patients that we find this unacceptable and are actively advocating to change this policy.
Conclusion
The State of Colorado and the nation must amend its healthcare policy to include comprehensive cancer care for everyone. Offering standard-of-care therapy to all patients is not only ethical, but also an economically sound policy benefiting patients, clinicians, and the workforce.
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
1. Skopec L, Holahan J, Elmendorf C. Changes in Health Insurance Coverage in 2013-2016: Medicaid Expansion States Lead the Way. Urban Institute. September 11, 2018. Accessed July 12, 2021. https://www.urban.org/research/publication/changes-health-insurance-coverage-2013-2016-medicaid-expansion-states-lead-way
2. Christensen Gee L, Gardner M, Hill ME, Wiehe M. Undocumented Immigrants’ State & Local Tax Contributions. Institute on Taxation & Economic Policy. Updated March 2017. Accessed July 12, 2021. https://www.immigrationresearch.org/system/files/immigration_taxes_2017.pdf
3. Emergency Medical Treatment and Labor Act (EMTALA), Public Law 42 U.S.C. 1395dd. 2010.
4. Social Security Act. Sec. 1903 [42 U.S.C. 1396b]. Accessed July 12, 2021. https://www.ssa.gov/OP_Home/ssact/title19/1903.htm.
5. Cervantes L, Mundo W, Powe NR. The status of provision of standard outpatient dialysis for US undocumented immigrants with ESKD. Clin J Am Soc Nephrol. 2019;14(8):1258-1260. https://doi.org/10.2215/CJN.03460319
6. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62-70. https://doi.org/10.1182/blood-2015-07-604546
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. 2021.
8. Cervantes L, Richardson S, Raghavan R, et al. Clinicians’ perspectives on providing emergency-only hemodialysis to undocumented immigrants: a qualitative study. Ann Intern Med. 2018;169(2):78-86. https://doi.org/10.7326/M18-0400
9. Cervantes L, Tong A, Camacho C, Collings A, Powe NR. Patient-reported outcomes and experiences in the transition of undocumented patients from emergency to scheduled hemodialysis. Kidney Int. 2021;99(1):198-207. https://doi.org/10.1016/j.kint.2020.07.024
10. Stein E, Xie J, Duchesneau E, et al. Cost effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in the United States. Pharmacoeconomics. 2019;37(2):239-253. https://doi.org/10.1007/s40273-018-0732-4
11. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620-1628. https://doi.org/10.1016/j.bbmt.2012.04.001
12. Edwards R, Ortega F. The Economic Contribution of Unauthorized Workers: An Industry Analysis. National Bureau of Economic Research. November 2016. Accessed July 12, 2021. https://www.nber.org/system/files/working_papers/w22834/w22834.pdf
13. Nunnery SE, Fintel AE, Jackson WC, Chandler JC, Ugwueke MO, Martin MG. Treatment disparities faced by undocumented workers from low- and middle-income countries in the United States with hematologic malignancies. J Natl Compr Canc Netw. 2016;14(4):483-486. https://doi.org/10.6004/jnccn.2016.0053
14. World Cancer Initiative. Cancer Preparedness in Latin America: The Need to Build on Recent Progress. 2019. Accessed July 7, 2021. https://worldcancerinitiative.economist.com/cancer-preparedness-latin-america
15. Taylor P, Lopez MH, Passel JS, Motel S; Pew Research Center. Unauthorized Immigrants: Length of Residency, Patterns of Parenthood. December 1, 2011. Accessed July 12, 2021. https://www.pewresearch.org/hispanic/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
16. California Supreme Court, Records and Briefs: S019427, Dominguez vs. Superior Court of Alameda County. 1990.
17. Wallace SP, Torres J, Sadegh-Nobari T, Pourat N, Brown ER. Undocumented Immigrants and Health Care Reform. UCLA Center for Health Policy Research. August 31, 2012. Accessed July 7, 2021. https://healthpolicy.ucla.edu/publications/Documents/PDF/undocumentedreport-aug2013.pdf
18. Washington State Health Care Authority. Health care services and supports. Noncitizens. Accessed July 12, 2021. https://www.hca.wa.gov/health-care-services-supports/apple-health-medicaid-coverage/non-citizens
19. 117th Congress of the United States. H.R.1177, U.S. Citizenship Act of 2021.
20. National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program. Accessed July 7, 2021. https://seer.cancer.gov/
21. Migration Policy Institute. Profile of the unauthorized population: United States. Accessed July 12, 2021. https://www.migrationpolicy.org/data/unauthorized-immigrant-population/state/US. 2021.
22. Torres L. Latinx? Lat Stud. 2018;16:283-285. https://doi.org/10.1057/s41276-018-0142-y
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Prescribing Appetite Stimulants to Hospitalized Older Adults With Unintentional Weight Loss
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.
Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.

Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An 87-year-old hospitalized man has lost 7% of his body weight in the past year. His family and the inpatient nutritionist ask about a prescription appetite stimulant.
Why You Might Think Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Helpful
Unintentional weight loss—the loss of more than 10 lb or 5% of usual body weight over 6 to 12 months—affects up to 27% of older adults in the community and 50% to 60% of older adults in nursing homes.1,2 Patients who report weight loss on hospital admission have an almost four times greater risk of death in the 12 months following discharge.3 To address unintentional weight loss, clinicians may prescribe appetite stimulants.
Megestrol acetate is approved by the US Food and Drug Administration (FDA) for the treatment of weight loss in patients with AIDS.4 Megestrol acetate promotes weight gain through inhibition of cytokines, interleukin-6, and tumor necrosis factor-alpha, which are increased in older adults. In a randomized, placebo-controlled trial of 69 nursing home residents with ≥6 months’ life expectancy and Karnofsky score of ≥40%, patients treated with megestrol acetate for 12 weeks reported increased appetite and well-being. They achieved significant weight gain (>1.82 kg), but not until 3 months after therapy ended.5 No significant adverse events were reported; however, adverse event monitoring continued only for the 12-week treatment period. This follow-up duration may have been insufficient to identify some adverse events, such as venous thromboembolism.
Mirtazapine, an antidepressant and serotonin receptor antagonist, reduces levels of serotonin, a neurotransmitter that promotes early satiety.6 In a meta-analysis of 11 trials comparing mirtazapine to selective serotonin reuptake inhibitors for depression, patients treated with mirtazapine demonstrated an increase in the composite secondary outcome of weight gain or increased appetite.7 The amount of weight gain was not specified. Weight gain is more common with low-dose mirtazapine, potentially due to increased antihistamine activity at lower doses.8 Overall, mirtazapine is well-tolerated and efficacious in the treatment of depression and may benefit older adults with concomitant weight loss.6
Cyproheptadine is a first-generation antihistamine with appetite-stimulating effects. It has been found to increase weight or appetite in various disease states, particularly in the pediatric population,9 including cystic fibrosis10 and malignancy.11 Given this evidence, there has been interest in its use in the geriatric population with unintentional weight loss.
Dronabinol is an orally active cannabinoid approved for anorexia-associated weight loss in patients with AIDS.12 In a randomized, placebo-controlled trial in patients with AIDS-related anorexia and weight loss, participants receiving dronabinol had a statistically significant increase in appetite but no change in weight. Participants receiving dronabinol also experienced more nervous system-related adverse events, including dizziness, thinking abnormalities, and somnolence.13
Why Prescribing Appetite Stimulants for Unintentional Weight Loss in Older Adults Is Not Helpful
Weight gain may not improve clinically meaningful outcomes. The absence of consistent evidence that prescription appetite stimulants improve patient-centered outcomes, such as quality of life or functional status, and the potential morbidity and mortality of these medications make prescribing appetite stimulants in older adults concerning.
Megestrol Acetate
A 2018 systematic review of randomized controlled trials studying megestrol acetate for treatment of anorexia-cachexia, primarily in adults with AIDS and cancer, found that treatment resulted in a 2.25-kg weight gain, with no improvement in quality of life and an increased risk of adverse events.14
Three prospective trials studied the effect of megestrol acetate in older adults (Appendix Table). One trial randomized 47 patients receiving skilled nursing services following an admission for acute illness to megestrol acetate vs placebo. While the investigators noted increases in appetite at higher doses of megestrol acetate, there was no change in weight or clinically relevant outcomes.15 In a second randomized controlled trial, 29 patients with illness-induced functional decline were enrolled in a strength training program in addition to being assigned to megestrol acetate or placebo. While patients receiving megestrol acetate with the exercise program had significant increases in weight and nutritional intake, they suffered a deterioration in physical function.16 In a pilot study, 17 nursing home residents who consistently ate less than 75% of their meals received megestrol acetate plus standard or optimal feeding assistance. The percentage of meals consumed increased only when patients received optimal feeding assistance in conjunction with megestrol acetate.17
The largest case-control study examining megestrol acetate for unintentional weight loss in older adults compared 709 residents in a multistate nursing home system treated with megestrol acetate to matched untreated controls. After 6 months of treatment, the median weight and change in weight did not differ significantly. Patients receiving megestrol acetate had a significant increase in mortality, surviving an average of 23.9 months, compared to 31.2 months for controls (P < .001).18
Additionally, two retrospective reviews of nursing home patients who were prescribed megestrol acetate showed incidences of venous thrombosis of 5% and 32%.19,20 Other potentially significant adverse effects include adrenal insufficiency and fluid retention.6 In 2019, the American Geriatrics Society’s Beers Criteria included megestrol acetate as a medication to avoid given its “minimal effect on weight; increases [in] risk of thrombotic events and possibly death in older adults.”21
Mirtazapine
No studies have evaluated mirtazapine for weight gain without concomitant depression. In older adults with depression, mirtazapine has minimal impact on promoting weight gain compared to other antidepressants. In two retrospective studies of older patients with depression and weight loss, researchers found no difference in weight gain in those treated with mirtazapine vs sertraline or other nontricyclic antidepressants, excluding fluoxetine.22,23
Cyproheptadine
There have been no controlled trials evaluating the use of cyproheptadine in older adults, in part due to anticholinergic side effects. In a trial of cancer patients, sedation and dizziness were common adverse effects.11 The 2019 American Geriatrics Society’s Beers Criteria include cyproheptadine as a medication to avoid based upon the “risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity.”21
Dronabinol
In a retrospective cohort study of 28 long-term care residents with anorexia and weight loss, participants receiving dronabinol for 12 weeks had no statistically significant weight gain.24 The FDA cautions against prescribing dronabinol for older adults due to neurological side effects.12 A systematic review of randomized controlled trials found that cannabinoid-based medications in patients older than 50 years were associated with a significant increase in dizziness or lightheadedness and thinking or perception disorder.25
What You Should Do Instead
In the Choosing Wisely® initiative, the American Geriatrics Society recommends avoiding prescription appetite stimulants for patients with anorexia or cachexia.26 Instead, hospitalists should evaluate older patients for causes of unintentional weight loss, including malignancy, nonmalignant gastrointestinal disorders, depression, and dementia. Hospitalists can identify most causes based on the history, physical exam, and laboratory studies and initiate treatment for modifiable causes, such as constipation and depression.2
Hospitalists should work with an interprofessional team to develop an individualized plan to optimize caloric intake in the hospital (Table).27 One in five hospitalized older adults has insufficient caloric intake during admission, which is associated with increased risk for in-hospital and 90-day mortality.28 Removing dietary restrictions, increasing the variety of foods offered, and assisted eating may increase food intake.27,29 Hospitalists should also consider discontinuing or changing medications with gastrointestinal side effects, such as metformin, cholinesterase inhibitors, bisphosphonates, and oral iron supplements. Dietitians may recommend oral nutrition supplements; if started, patients should be offered supplements after discharge.27,29 For patients with limited access to food, social workers can help optimize social supports and identify community resources following discharge. Finally, hospitalists should coordinate with outpatient providers to monitor weight long-term.

Recommendations
- Recognize and address unintentional weight loss in older adults in the hospital.
- Do not prescribe appetite stimulants for unintentional weight loss in hospitalized older adults as they have no proven benefit for improving long-term outcomes and, in the case of megestrol acetate, may increase mortality.
- Work with an interprofessional team to address factors contributing to unintentional weight loss using nonpharmacologic options for improving food intake.
Conclusion
After discussing the lack of evidence supporting prescription appetite stimulants and the potential risks, we shifted the focus to optimizing oral intake. The team worked with the patient and the patient’s family to optimize nutrition following discharge and communicated the need for ongoing monitoring to the primary care provider.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Acknowledgment
The authors thank Claire Campbell, MD, for her review of this manuscript.
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
1. Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923-929. https://doi.org/10.4065/76.9.923
2. McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. BMJ. 2011;342:d1732. https://doi.org/10.1136/bmj.d1732
3. Satish S, Winograd CH, Chavez C, Bloch DA. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914-921. https://doi.org/10.1111/j.1532-5415.1996.tb01860.x
4. Megace (megestrol acetate) [package insert]. Par Pharmaceutical Inc. Revised July 2005. Accessed January 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000TOC.cfm
5. Yeh SS, Wu SY, Lee TP, et al. Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double-blind, placebo-controlled study. J Am Geriatr Soc. 2000;48(5):485-492. https://doi.org/10.1111/j.1532-5415.2000.tb04993.x
6. Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Reviews of therapeutics megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29(4):383-397. https://doi.org/10.1592/phco.29.4.383
7. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
8. Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267-285. https://doi.org/10.1016/S0165-0327(98)00224-9
9. Najib K, Moghtaderi M, Karamizadeh Z, Fallahzadeh E. Beneficial effect of cyproheptadine on body mass index in undernourished children: a randomized controlled trial. Iran J Pediatr. 2014;24(6):753-758.
10. Epifanio M, Marostica PC, Mattiello R, et al. A randomized, double-blind, placebo-controlled trial of cyproheptadine for appetite stimulation in cystic fibrosis. J Pediatr (Rio J). 2012;88(2):155-160. https://doi.org/10.2223/JPED.2174
11. Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer. 1990;65(12):2657-2662. https://doi.org/10.1002/1097-0142(19900615)65:12<2657::aid-cncr2820651210>3.0.co;2-s
12. MARINOL (dronabinol) [package insert]. Solvay Pharmaceuticals, Inc. Revised August 2017. Accessed April 27, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
13. Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89-97. https://doi.org/10.1016/0885-3924(94)00117-4
14. Ruiz-García V, López-Briz E, Carbonell-Sanchis R, Bort-Martí S, Gonzálvez-Perales JL. Megestrol acetate for cachexia–anorexia syndrome. A systematic review. J Cachexia Sarcopenia Muscle. 2018;9(3):444-452. https://doi.org/10.1002/jcsm.12292
15. Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. J Am Geriatr Soc. 2005;53(6):970-975. https://doi.org/10.1111/j.1532-5415.2005.53307.x
16. Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20-28. https://doi.org/10.1111/j.1532-5415.2006.01010.x
17. Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. J Am Med Dir Assoc. 2005;6(3):S5-S11. https://doi.org/10.1016/j.jamda.2005.03.014
18. Bodenner D, Spencer T, Riggs AT, Redman C, Strunk B, Hughes T. A retrospective study of the association between megestrol acetate administration and mortality among nursing home residents with clinically significant weight loss. Am J Geriatr Pharmacother. 2007;5(2):137-146. https://doi.org/10.1016/J.AMJOPHARM.2007.06.004
19. Kropsky B, Shi Y, Cherniack EP. Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate. J Am Med Dir Assoc. 2003;4(5):255-256. https://doi.org/10.1097/01.JAM.0000083384.84558.75
20. Bolen JC, Andersen RE, Bennett RG. Deep vein thrombosis as a complication of megestrol acetate therapy among nursing home residents. J Am Med Dir Assoc. 2000;1(6):248-252.
21. Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
22. Mihara IQT, McCombs JS, Williams BR. The impact of mirtazapine compared with non-TCA antidepressants on weight change in nursing facility residents. Consult Pharm. 2005;20(3):217-223. https://doi.org/10.4140/tcp.n.2005.217
23. Goldberg RJ. Weight change in depressed nursing home patients on mirtazapine. J Am Geriatr Soc. 2002;50(8):1461. https://doi.org/10.1046/j.1532-5415.2002.50374.x
24. Wilson MMG, Philpot C, Morley JE. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant?--a pilot study. J Nutr Health Aging. 2007;11(2):195-198.
25. Velayudhan L, McGoohan KL, Bhattacharyya S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: a systematic review and metaregression analysis. JAMA Netw Open. 2021;4(2):e2035913. https://doi.org/10.1001/jamanetworkopen.2020.35913
26. AGS Choosing Wisely Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950-960. https://doi.org/10.1111/jgs.12770
27. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10-47. https://doi.org/10.1016/j.clnu.2018.05.024
28. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013-2019. https://doi.org/10.1001/jama.281.21.2013
29. Feinberg J, Nielsen EE, Korang SK, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;2017(5). https://doi.org/10.1002/14651858.CD011598.pub2
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Focused Updates to Pediatric Asthma Management
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
Asthma is a heterogeneous condition characterized by airway hyperresponsiveness and obstruction, with associated airway inflammation and remodeling.2 Asthma affects 25 million people in the United States and 334 million people worldwide, with significant healthcare disparities across race and ethnicity.2-6 Asthma is the third most common reason for hospitalizations in pediatrics, accounting for 180,000 annual hospitalizations for children and adults.3,7 In 2020, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel provided a focused update to the Asthma Management Guidelines, centered on six topics with sufficient new evidence. The management of status asthmaticus was not included in this update. We spotlight four of the recommendations applicable to the practice of pediatric hospital medicine.
Key Recommendations for the Hospitalist
Recommendation 1. Children 0 to 4 years old with recurrent wheezing triggered by a respiratory tract infection (RTI) and no wheezing between infections should receive a short course of daily inhaled corticosteroids (ICS) at the onset of a RTI, with an as-needed short-acting beta agonist (SABA) for quick-relief therapy compared to SABA alone (evidence quality: high; recommendation strength: conditional).
Recurrent wheezing is defined as clinically significant periods of wheezing that are reversible or consistent with bronchospasm and as ≥3 episodes in a lifetime or 2 episodes in the past year. It is important to adhere to this definition to prevent inappropriate use of ICS for bronchiolitis. This treatment is associated with a reduction of use of systemic steroids (relative risk [RR], 0.67; 95% CI, 0.46-0.98) without a statistical decrease in acute care visits (RR, 0.90; 95% CI, 0.77-1.05) or hospitalizations (RR, 0.77; 95% CI, 0.06-9.68). Improved transition of care is essential between the primary care provider, hospitalist, and family to ensure an understanding of how/when to initiate ICS at the onset of a RTI. Potential harms include effect on growth and overprescribing. Growth should be monitored because data are conflicting.
Recommendation 2. Individuals ages 12 years and older with mild persistent asthma should use as-needed SABA and may use either daily low-dose ICS or as-needed ICS when symptoms flare (evidence quality: moderate; recommendation strength: conditional).
In intermittent therapy, patients take a SABA followed by an ICS as needed for acute asthma symptoms. This recommendation is driven by asthma-control and quality-of-life outcomes, with caregivers reporting that intermittent dosing could “offer flexibility and potentially reduce side effects.” There were no differences between management regimens with respect to systemic steroid use (RR, 0.70; 95% CI, 0.30-1.64) or urgent care visits (RR, 0.25; 95% CI, 0.05-1.16). Differing perception of symptoms by individuals may lead to undertreating or overtreating, and intermittent administration makes it challenging for clinicians to assess the need to adjust therapy.
Recommendation 3. Children 4 years and older with moderate to severe persistent asthma should use ICS-formoterol in a single inhaler used as both daily controller and reliever therapy compared to either (a) higher-dose ICS as daily controller therapy and SABA for quick-relief therapy or (b) a same-dose ICS-long-acting beta agonist (LABA) as daily controller therapy and SABA for quick-relief therapy (evidence quality: high for ages ≥12 years, moderate for ages 4-11 years; recommendation strength: strong).
For children 4 years and older, it is recommended to use “single maintenance and reliever therapy” (SMART) with a single-inhaler containing either low- or medium-dose ICS and formoterol when stepping up from Step 2 (daily low-dose ICS and as-needed SABA) to Step 3 (daily and as-needed low-dose ICS-formoterol) and Step 4 (daily and as-needed medium-dose ICS-formoterol).
Recommendation 4. If individuals with asthma have symptoms related to indoor allergens, confirmed by history or allergy testing, they should use a multicomponent allergen-specific mitigation intervention. Allergen mitigation interventions should not be a part of routine asthma management for individuals with asthma who do not have symptoms related to exposure to specific indoor allergens (evidence quality: low; recommendation strength: conditional).
Providers often emphasize exposure to potential indoor allergens such as carpets and pets when taking an asthma history and counsel removal of these triggers. However, all recommendations related to allergies in the 2020 updates have low-moderate evidence quality and conditional recommendation strength. Hospitalists should instead focus their questions on allergy symptoms and triggers and recommend multicomponent mitigation intervention only if there is a confirmed allergy history. Families should continue routine good practices such as house cleaning and laundering, but other interventions are not evidence-based.
CRITIQUE
Methods
The Expert Panel included a diverse group of clinicians, a pharmacist, and health policy experts. In 2015, a needs assessment identified 6 out of 17 priority topics with sufficient new information for updates. Key questions were drafted, and systematic reviews were published through 2018. The Expert Panel made its recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. The Expert Panel informed its recommendations with input from focus groups, including individuals with asthma and caregivers. The NHLBI posted the draft report for public review, and comments were considered. We believe these methods effectively developed evidence-based recommendations, and the diversity of stakeholders increases the value of this guideline. However, the infrequency of updates limits the utility of the NHLBI guidelines as compared with annual GINA (Global Initiative for Asthma) updates.
There are important considerations in assessing these guidelines. Specifically, the validity of systemic steroid courses as an outcome for children ages 0 to 4 years is controversial. Second, the studies cited in defense of intermittent ICS use in children >12 years of age excluded pediatric patients and did not include readmissions as a primary outcome, which is of particular interest to the hospitalist.
Potential Conflicts for Guideline Authors
The Expert Panel reported all potential conflicts of interest (COIs), which were rated by the Expert Panel Chair and Journal of Allergy and Clinical Immunology editors. Individuals with high COIs were excluded from the Expert Panel. Those with moderate COIs were recused for that topic. Low COIs were not related to the guideline.
Generalizability of the Guideline
These guidelines are based on systematic reviews with large sample sizes and patients of all ages. They are generalizable. However, the authors recognize that variations in asthma require individualized approaches. They identify this as a reason for the lack of strong recommendations for asthma standards of care.
AREAS OF FUTURE STUDY
Biologics have progressed considerably since revision of the guidelines. The 2020 guidelines did not address these to prevent delay of the guideline release, but recommendations should be included in future guidelines. Future studies should address healthcare disparities in asthma, barriers to equitable care, and how to eliminate them, as guided by the President’s Task Force.8 Status asthmaticus should be included in future updates.
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
1. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217-1270. https://doi.org/10.1016/j.jaci.2020.10.003.
2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783-800. https://doi.org/10.1016/S0140-6736(17)33311-1
3. Centers for Disease Control and Prevention. Most recent asthma data. Reviewed March 30 2021. Accessed October 5, 2021. www.cdc.gov/asthma/most_recent_data.htm
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. https://doi.org/10.1016/S0140-6736(12)61729-2
5. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. https://doi.org/10.1513/AnnalsATS.201703-259OC
6. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58.
7. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012: Statistical Brief #187. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality; February 2006.
8. U.S. Environmental Protection Agency. President’s Task Force on Environmental Health Risks and Safety Risks to Children: Coordinated Federal Action Plan to Reduce Racial and Ethnic Asthma Disparities. May 2012. https://19january2017snapshot.epa.gov/sites/production/files/2014-08/documents/federal_asthma_disparities_action_plan.pdf
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Prescribing Thiamine, Folate and Multivitamins on Discharge for Patients With Alcohol Use Disorder
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old man with alcohol use disorder (AUD) is admitted with decompensated heart failure and experiences alcohol withdrawal during the hospitalization. He improves with guideline-directed heart failure therapy and benzodiazepines for alcohol withdrawal. Discharge medications are metoprolol succinate, lisinopril, furosemide, aspirin, atorvastatin, thiamine, folic acid, and a multivitamin. No medications are offered for AUD treatment. At follow-up a week later, he presents with dyspnea and reports poor medication adherence and a return to heavy drinking.
WHY YOU MIGHT THINK IT IS HELPFUL TO PRESCRIBE VITAMIN SUPPLEMENTATION TO PATIENTS WITH AUD AT HOSPITAL DISCHARGE
AUD is common among hospitalized patients.1 AUD increases the risk of vitamin deficiencies due to the toxic effects of alcohol on the gastrointestinal tract and liver, causing impaired digestion, reduced absorption, and increased degradation of key micronutrients.2,3 Other risk factors for AUD-associated vitamin deficiencies include food insecurity and the replacement of nutrient-rich food with alcohol. Since the body does not readily store water-soluble vitamins, including thiamine (vitamin B1) and folate (vitamin B9), people require regular dietary replenishment of these nutrients. Thus, if individuals with AUD eat less fortified food, they risk developing thiamine, folate, niacin, and other vitamin deficiencies. Since AUD puts patients at risk for vitamin deficiencies, hospitalized patients typically receive vitamin supplementation, including thiamine, folic acid, and a multivitamin (most formulations contain water-soluble vitamins B and C and micronutrients).1 Hospitalists often continue these medications at discharge.
Thiamine deficiency may manifest as Wernicke encephalopathy (WE), peripheral neuropathy, or a high-output heart failure state.
Hospitalists empirically treat with thiamine, folate, and other vitamins upon hospital admission with the intent of reducing morbidity associated with nutritional deficiencies.1 Repletion poses few risks to patients since the kidneys eliminate water-soluble vitamins. Multivitamins also have a low potential for direct harm and a low cost. Given the consequences of missing a deficiency, alcohol withdrawal–management order sets commonly embed vitamin repletion orders.6
WHY ROUTINELY PRESCRIBING VITAMIN SUPPLEMENTATION AT HOSPITAL DISCHARGE IN PATIENTS WITH AUD IS A TWDFNR
Hospitalists often reflexively continue vitamin supplementation on discharge. Unfortunately, there is no evidence that prescribing vitamin supplementation leads to clinically significant improvements for people with AUD, and patients can experience harms.
Literature and specialty guidelines lack consensus on rational vitamin supplementation in patients with AUD.2,7,8 Folate testing is not recommended due to inaccuracies.9 In fact, clinical data, such as body mass index, more accurately predict alcohol-related cognitive impairment than blood levels of vitamins.10 In one small study of vitamin deficiencies among patients with acute alcohol intoxication, none had low B12 or folate levels.11 A systematic review among people experiencing homelessness with unhealthy alcohol use showed no clear pattern of vitamin deficiencies across studies, although vitamin C and thiamine deficiencies predominated.12
In the absence of reliable thiamine and folate testing to confirm deficiencies, clinicians must use their clinical assessment skills. Clinicians rarely evaluate patients with AUD for vitamin deficiency risk factors and instead reflexively prescribe vitamin supplementation. An AUD diagnosis may serve as a sensitive, but not specific, risk factor for those in need of vitamin supplementation.
Other limitations make prescribing oral vitamins reflexively at discharge a low-value practice. Thiamine, often prescribed orally in the hospital and on discharge, has poor oral bioavailability.13 Unfortunately, people with AUD have decreased and variable thiamine absorption. To prevent WE, thiamine must cross the blood-brain barrier, and the literature provides insufficient evidence to guide clinicians on an appropriate oral thiamine dose, frequency, or duration of treatment.14 While early high-dose IV thiamine may treat or prevent WE during hospitalization, low-dose oral thiamine may not provide benefit to patients with AUD.5
The literature also provides sparse evidence for folate supplementation and its optimal dose. Since 1998, when the United States mandated fortifying grain products with folic acid, people rarely have low serum folate levels. Though patients with AUD have lower folate levels relative to the general population,15 this difference does not seem clinically significant. While limited data show an association between oral multivitamin supplementation and improved serum nutrient levels among people with AUD, we lack evidence on clinical outcomes.16
Most importantly, for a practice lacking strong evidence, prescribing multiple vitamins at discharge may result in harm from polypharmacy and unnecessary costs for the recently hospitalized patient. Alcohol use is associated with decreased adherence to medications for chronic conditions,17 including HIV, hypertension, hyperlipidemia, and psychiatric diseases. In addition, research shows an association between an increased number of discharge medications and higher risk for hospital readmission. The harm may actually correlate with the number of medications and complexity of the regimen rather than the risk profile of the medications themselves.18 Providers underestimate the impact of adding multiple vitamins at discharge, especially for patients who have several co-occurring medical conditions that require other medications. Furthermore, insurance rarely covers vitamins, leading hospitals or patients to incur the costs at discharge.
WHEN TO CONSIDER VITAMIN SUPPLEMENTATION AT DISCHARGE FOR PATIENTS WITH AUD
When treating patients with AUD, consider the potential benefit of vitamin supplementation for the individual. If a patient with regular, heavy alcohol use is at high risk of vitamin deficiencies due to ongoing risk factors (Table), hospitalists should discuss vitamin therapy via a patient-centered risk-benefit process.

When considering discharge vitamins, make concurrent efforts to enhance patient nutrition via decreased alcohol consumption and improved healthy food intake. While some patients do not have a goal of abstaining from alcohol, providing resources to food access may help decrease the harms of drinking. Education may help patients learn that vitamin deficiencies can result from heavy alcohol use.
Multivitamin formulations have variable doses of vitamins but can contain 100% or more of the daily value of thiamine and folic acid. For patients with AUD at lower risk of vitamin deficiencies (ie, mild alcohol use disorder with a healthy diet), discuss risks and benefits of supplementation. If they desire supplementation, a single thiamine-containing vitamin alone may be highest yield since it is the most morbid vitamin deficiency. Conversely, a patient with heavy alcohol intake and other risk factors for malnutrition may benefit from a higher dose of supplementation, achieved by prescribing a multivitamin alongside additional doses of thiamine and folate. However, the literature lacks evidence to guide clinicians on optimal vitamin dosing and formulations.
WHAT WE SHOULD DO INSTEAD
Instead of reflexively prescribing thiamine, folate, and multivitamin, clinicians can assess patients for AUD, provide motivational interviewing, and offer AUD treatment. Hospitalists should initiate and prescribe evidence-based medications for AUD for patients interested in reducing or stopping their alcohol intake. We can choose from Food and Drug Administration–approved AUD medications, including naltrexone and acamprosate. Unfortunately, less than 3% of patients with AUD receive medication therapy.19 Our healthcare systems can also refer individuals to community psychosocial treatment.
For patients with risk factors, prescribe empiric IV thiamine during hospitalization. Clinicians should then perform a risk-benefit assessment rather than reflexively prescribe vitamins to patients with AUD at discharge. We should also counsel patients to eat food when drinking to decrease alcohol-related harms.20 Patients experiencing food insecurity should be linked to food resources through inpatient nutritional and social work consultations.
Elicit patient preference around vitamin supplementation after discharge. For patients with AUD who desire supplementation without risk factors for malnutrition (Table), consider prescribing a single thiamine-containing vitamin for prevention of thiamine deficiency, which, unlike other vitamin deficiencies, has the potential to be irreversible and life-threatening. Though no evidence currently supports this practice, it stands to reason that prescribing a single tablet could decrease the number of pills for patients who struggle with pill burden.
RECOMMENDATIONS
- Offer evidence-based medication treatment for AUD.
- Connect patients experiencing food insecurity with appropriate resources.
- For patients initiated on a multivitamin, folate, and high-dose IV thiamine at admission, perform vitamin de-escalation during hospitalization.
- Risk-stratify hospitalized patients with AUD for additional risk factors for vitamin deficiencies (Table). In those with additional risk factors, offer supplementation if consistent with patient preference. Balance the benefits of vitamin supplementation with the risks of polypharmacy, particularly if the patient has conditions requiring multiple medications.
CONCLUSION
Returning to our case, the hospitalist initiates IV thiamine, folate, and a multivitamin at admission and assesses the patient’s nutritional status and food insecurity. The hospitalist deems the patient—who eats regular, balanced meals—to be at low risk for vitamin deficiencies. The medical team discontinues folate and multivitamins before discharge and continues IV thiamine throughout the 3-day hospitalization. The patient and clinician agree that unaddressed AUD played a key role in the patient’s heart failure exacerbation. The clinician elicits the patient’s goals around their alcohol use, discusses AUD treatment, and initiates naltrexone for AUD.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
1. Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract. 2013;8(1):11. https://doi.org/10.1186/1940-0640-8-11
2. Lewis MJ. Alcoholism and nutrition: a review of vitamin supplementation and treatment. Curr Opin Clin Nutr Metab Care. 2020;23(2):138-144. https://doi.org/10.1097/mco.0000000000000622
3. Bergmans RS, Coughlin L, Wilson T, Malecki K. Cross-sectional associations of food insecurity with smoking cigarettes and heavy alcohol use in a population-based sample of adults. Drug Alcohol Depend. 2019;205:107646. https://doi.org/10.1016/j.drugalcdep.2019.107646
4. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-915. https://doi.org/10.1111/imj.12522
5. Flannery AH, Adkins DA, Cook AM. Unpeeling the evidence for the banana bag: evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016;44(8):1545-1552. https://doi.org/10.1097/ccm.0000000000001659
6. Wai JM, Aloezos C, Mowrey WB, Baron SW, Cregin R, Forman HL. Using clinical decision support through the electronic medical record to increase prescribing of high-dose parenteral thiamine in hospitalized patients with alcohol use disorder. J Subst Abuse Treat. 2019;99:117-123. https://doi.org/10.1016/j.jsat.2019.01.017
7. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. January 2020. https://www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf?sfvrsn=ba255c2_2
8. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307-328. https://doi.org/10.1002/hep.23258
9. Breu AC, Theisen-Toupal J, Feldman LS. Serum and red blood cell folate testing on hospitalized patients. J Hosp Med. 2015;10(11):753-755. https://doi.org/10.1002/jhm.2385
10. Gautron M-A, Questel F, Lejoyeux M, Bellivier F, Vorspan F. Nutritional status during inpatient alcohol detoxification. Alcohol Alcohol. 2018;53(1):64-70. https://doi.org/10.1093/alcalc/agx086
11. Li SF, Jacob J, Feng J, Kulkarni M. Vitamin deficiencies in acutely intoxicated patients in the ED. Am J Emerg Med. 2008;26(7):792-795. https://doi.org/10.1016/j.ajem.2007.10.003
12. Ijaz S, Jackson J, Thorley H, et al. Nutritional deficiencies in homeless persons with problematic drinking: a systematic review. Int J Equity Health. 2017;16(1):71. https://doi.org/10.1186/s12939-017-0564-4
13. Day GS, Ladak S, Curley K, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246-253. https://doi.org/10.1002/jhm.2324
14. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3
15. Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596-606. https://doi.org/10.1002/mnfr.201200077
16. Ijaz S, Thorley H, Porter K, et al. Interventions for preventing or treating malnutrition in homeless problem-drinkers: a systematic review. Int J Equity Health. 2018;17(1):8. https://doi.org/10.1186/s12939-018-0722-3
17. Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795-803. https://doi.org/10.7326/0003-4819-149-11-200812020-00004
18. Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res. 2015;15:282. https://doi.org/10.1186/s12913-015-0950-9
19. Han B, Jones CM, Einstein EB, Powell PA, Compton WM. Use of medications for alcohol use disorder in the US: results From the 2019 National Survey on Drug Use and Health. JAMA Psychiatry. 2021;78(8):922–4. https://doi.org/10.1001/jamapsychiatry.2021.1271
20. Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomised clinical trial. Lancet Psychiatry. 2021;8(4):287-300. https://doi.org/10.1016/S2215-0366(20)30489-2
© 2021 Society of Hospital Medicine
Things We Do for No Reason:™ Prescribing Tramadol for Inpatients in Pain
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2

Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2

Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits an 80-year-old man for a chronic obstructive pulmonary disease exacerbation. The patient’s history is significant for chronic right knee pain. While hospitalized, the patient reports worsening of his knee pain. Radiographs of the right knee show severe osteoarthritic changes. Since acetaminophen does not relieve the patient’s pain, the hospitalist orders tramadol as needed.
BACKGROUND
Hospitalists, who commonly evaluate and treat acute and chronic pain in the inpatient setting, have a wide selection of interventions from which to choose, including tramadol. Tramadol hydrochloride is a synthetic, central-acting analgesic with multiple mechanisms of action. It is a serotonin-norepinephrine reuptake inhibitor (SNRI) with a structure similar to venlafaxine and produces antineuropathic analgesic effects.1 Tramadol and its primary active metabolite O-desmethyltramadol (also known as the M1 metabolite) mediate its effects by binding at the mu-opioid receptor.2 Phase I metabolism in the liver by cytochrome P450 isoenzyme 2D6 (CYP2D6) facilitates conversion of tramadol to M1 (Figure). Importantly, genetic polymorphisms in CYP2D6 result in individual variations in gene expression, which impacts the metabolism of tramadol.2

Although tramadol is available over the counter in some countries, in the United States it is a Schedule IV controlled substance. Tramadol consistently ranks among the top 50 prescribed medications in the United States.3
WHY YOU MIGHT THINK PRESCRIBING TRAMADOL FOR PAIN MAY BE HELPFUL
Given the growing concerns regarding the use of opioids, the pharmaceutical industry has marketed tramadol as a safer opioid option for pain management. Tramadol binds at the mu-opioid receptor with an affinity that is less than 4000-fold that of morphine; the binding potency of M1, the metabolite of tramadol, is less than 5-fold that of morphine.4 Due to its lower binding affinity at the mu-opioid receptor, tramadol is considered a weak opioid, one believed to have minimal withdrawal symptoms and a lower potential for overdose or misuse compared to other opioids.1,5 Based on this characterization, many clinicians prescribe tramadol for elderly patients or patients otherwise at risk for medication misuse or adverse effects of opioids.6 In addition, hospitalized patients often have contraindications to nonopioid medications (eg, acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs]), limiting their options for pain management.
WHY PRESCRIBING TRAMADOL FOR PAIN SHOULD BE AVOIDED
Despite being marketed as an effective and safe medication, tramadol has an unpredictable metabolism, complex pharmacology, and drug-drug interactions that can cause significant adverse effects. Similar to other opioids, tramadol is associated with a risk of misuse, physiologic dependency, and overdose. In addition, tramadol has a black box warning for addiction, misuse, respiratory depression, ultra-rapid metabolism, neonatal opioid withdrawal syndrome, CYP450 drug interactions, and interactions with other central nervous system depressants.
While tramadol has multiple mechanisms of action, the literature lacks high-quality evidence (eg, large randomized controlled trials) supporting its use, especially in hospitalized medical patients. A recent retrospective study of tramadol looked at the diagnoses of 250 hospitalized patients who received tramadol for pain management. While this study did not examine efficacy, it found mild-to-moderate acute noncancer pain to be the primary reason for prescribing tramadol.7 This study also showed the risk of severe drug-drug interactions increased the longer patients were on tramadol.7
As a result of the limited evidence in hospitalized patients, hospitalists must rely on outpatient studies.8-10 The size and quality of these studies, especially given the magnitude of tramadol prescribing in the United States, make them less useful. A series of Cochrane reviews examining the beneficial effects of tramadol for neuropathic pain, osteoarthritis, and cancer pain show insufficient evidence for tramadol when compared to placebo or active controls such as acetaminophen, NSAIDs, or other opioids.8-10
The side-effect profile of tramadol outweighs its mild analgesic effects. The 2019 American Geriatric Society Beers criteria for potentially inappropriate medication use in older adults strongly recommends clinicians use caution when prescribing tramadol to older adult patients, as tramadol may worsen or cause hyponatremia.11 In one large, population-based study, the use of tramadol doubled patients’ risk of hospitalization for hyponatremia when compared to codeine, though the incidence remains rather low at 4.6 per 10,000 person-months.12 Studies have also demonstrated an increased risk of hospitalization for hypoglycemia in nondiabetic patients receiving tramadol.13 A large propensity-score matched cohort study of patients with osteoarthritis found tramadol to have an associated higher all-cause mortality compared to NSAIDs; however, these differences may be due to confounding variables.1 In addition to hyponatremia and all-cause mortality, patients taking tramadol also have an associated increased risk of falls and hip fractures when compared to codeine or NSAIDs.14
The increased serotonergic activity associated with tramadol can lead to serotonin syndrome (serotonin toxicity), a rare but serious condition. Although serotonin syndrome can develop in patients taking tramadol as a monotherapy, the risk for this toxidrome increases when tramadol is taken in combination with other serotonergic agents or agents that inhibit metabolism of tramadol at CYP2D6.5 Seizures may also occur with tramadol at therapeutic and supratherapeutic doses. Population-based studies estimate seizures occur in 0.15% to 0.86% of patients receiving tramadol, which is two to six times the risk of those not on tramadol.5 Patients concurrently taking tramadol with a tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) are estimated to have seizures five to nine times more often than patients not taking a TCA or SSRI.5 Risk factors for tramadol-induced seizure include tramadol misuse or overdose, tramadol doses >1000 mg daily (maximum recommended dose is 400 mg/day), chronic tramadol use, concurrent use of a serotonergic agent or medications that inhibit CYP2D6, and history of epilepsy, renal disease, stroke, or traumatic brain injury.5
Differences in the genetic polymorphisms of CYP2D6 can produce a range of CYP2D6 activity from “poor metabolizers” (little-to-no analgesic effect) to “ultra-rapid metabolizers” (enhanced analgesia and increased risk of adverse effects), leading to unpredictable pharmacodynamic effects of tramadol.2 In North Africa and the Arabian peninsula, more than 25% of the population rapidly metabolizes tramadol; these pharmacogenomic effects result in higher rates of tramadol addiction and overdose in these regions.5 An estimated 7% to 10% of Caucasians slowly metabolize tramadol, which may place them at risk of adverse effects from tramadol in addition to inadequate analgesia.15 In contrast, Ethiopian populations have the highest rate of ultra-rapid tramadol metabolism at 29%.15
Drugs that induce CYP2D6 (eg, dexamethasone, rifampin) or inhibit CYP2D6 (eg, bupropion, fluoxetine) also impact tramadol efficacy, pharmacokinetics, and pharmacodynamics.16,17 Patients taking strong CYP2D6 inhibitors require significantly higher doses of tramadol to achieve analgesic effects.17 Tramadol undergoes extensive hepatic metabolism, producing several active metabolites, including M1 (Figure). Hepatic impairment increases the elimination half-life of tramadol and its metabolites.18 The majority of tramadol and its metabolites are eliminated through the kidneys. Accumulation of tramadol and its metabolites may occur in patients with renal impairment, placing them at increased risk of adverse effects.2
Finally, although some clinicians assume that tramadol has lower rates of misuse, diversion, or overdose compared to other opioids, rates of nonprescription use have increased with its proliferation.19,20 The US Substance Abuse and Mental Health Services Administration estimates that 1,287,000 persons misused tramadol in 2019.21 Patients may exhibit symptoms of physiologic opioid dependence and withdrawal from chronic tramadol use.2,22 In one study, patients prescribed tramadol monotherapy for acute pain from elective surgery had an increased risk for prolonged opioid use compared to patients prescribed other short-acting opioids.22
WHAT YOU SHOULD DO INSTEAD
Clinicians should determine the nature of the patient’s pain by obtaining a complete medical history, performing a thorough physical examination, and ordering diagnostic tests and imaging studies, as necessary. After consulting with the patient’s primary care physician, the clinician should employ a multimodal approach to pain that includes topical agents, psychotherapy, injections or interventions, and nonopioid medications. Patients with neuropathic pain may benefit from adjuvant analgesics such as gabapentinoids, TCAs, or SNRIs. In patients with evidence-based indications for opioid therapy (eg, pancreatitis, cancer pain, postsurgical pain), the hospitalist should assess the risk for opioid misuse and discuss risks and benefits with the patient before considering a time-limited trial of opioid therapy. If available and when indicated, clinicians should consult with specialists in pain management or palliative care. For cases wherein clinicians have already prescribed tramadol to the patient, they should discuss deprescribing strategies and alternative analgesic options with the patient and the patient’s primary care physician. Finally, before initiating tramadol therapy for hospitalized patients with pain, hospitalists should consider the risks, benefits, and alternative approaches to prescribing tramadol.
RECOMMENDATIONS
- For hospitalized patients reporting pain, complete a pain assessment by history, physical exam, chart review, and diagnostic studies to examine the etiology of the pain.
- Utilize multiple modalities for pain control when possible, including acetaminophen, NSAIDs, topical agents, ice or heat, neuropathic pain medications, and interventions such as injections, psychotherapy, or radiation, if indicated.
- Avoid prescribing tramadol due to unpredictable pharmacodynamics, adverse effects, and lack of quality evidence for efficacy in hospitalized medical patients.
CONCLUSION
Tramadol is a commonly used opioid medication associated with adverse effects and unpredictable analgesia. Regarding this case scenario, the use of tramadol in this patient places him at risk for drug-drug interactions, hyponatremia, hypoglycemia, serotonin syndrome, seizures, and pronounced side effects of opioid medications. Moderate quality evidence in the outpatient setting suggests that tramadol is unlikely to provide significant analgesia for his osteoarthritic pain.9 Instead of prescribing tramadol, the hospitalist should consider alternative treatments for this patient’s pain, such as intraarticular glucocorticoids, a short course of oral NSAIDs (unless contraindicated), topical treatments (eg, menthol, capsaicin, NSAIDs), physical therapy, and close follow-up with an orthopedist after hospital discharge. Further randomized controlled studies of tramadol vs active controls are needed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
1. Zeng C, Dubreuil M, LaRochelle MR, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969-982. https://doi.org/10.1001/jama.2019.1347
2. Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. https://doi.org/10.1097/FPC.0000000000000057
3. The top 200 drugs of 2019. ClinCalc DrugStats Database. Accessed June 10, 2021. https://clincalc.com/DrugStats
4. Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116-121. https://doi.org/10.1007/s002100000266
5. Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131(11):1382.e1-1382.e6. https://doi.org/10.1016/j.amjmed.2018.04.025
6. Shipton EA. Tramadol—present and future. Anaesth Intensive Care. 2000;28(4):363-374. https://doi.org/10.1177/0310057X0002800403
7. Mohan N, Edmonds KP, Ajayi TA, Atayee RS, Clinical tolerability and safety of tramadol in hospitalized patients. J Pain & Palliat Care Pharmacother. 2020:34(4):211-218. https://doi.org/10.1080/15360288.2020.1817227
8. Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. https://doi.org/10.1002/14651858.cd003726.pub4
9. Toupin-April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522. https://doi.org/10.1002/14651858.cd005522.pub3
10. Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. https://doi.org/10.1002/14651858.cd012508.pub2
11. The American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
12. Fournier JP, Yin H, Nessim SJ, Montastruc JL, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med. 2015;128(4):418-425.e5. https://doi.org/10.1016/j.amjmed.2014.10.046
13. Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175(2):186-193. https://doi.org/10.1001/jamainternmed.2014.6512
14. Wei J, Lane NE, Bolster MB, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35(4):631-640. https://doi.org/10.1002/jbmr.3935
15. Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5-6):274-285. https://doi.org/10.1159/000326085
16. Flockhart DA, Thacker D, McDonald C, Desta Z. The Flockhart cytochrome P450 drug-drug interaction table. Division of Clinical Pharmacology, Indiana University School of Medicine. Updated 2021. Accessed April 21, 2021. https://drug-interactions.medicine.iu.edu
17. Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy. 2019;39(6):724-729. https://doi.org/10.1002/phar.2269
18. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. https://doi.org/10.2165/00003088-200443130-00004
19. Bush DM. The CBHSQ report: emergency department visits for drug misuse or abuse involving the pain medication tramadol. Substance Abuse and Mental Health Service Administration. May 14, 2015. Accessed June 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK343535/
20. Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. https://doi.org/10.1007/s11916-019-0777-x
21. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National survey on drug use and health 2019 (NSDUH-2019). Accessed June 16, 2021. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases
22. Thiels CA, Habermann EB, Hooten WM, Jeffery MM. Chronic use of tramadol after acute pain episode: cohort study. BMJ. 2019;365:l1849. https://doi.org/10.1136/bmj.l1849
© 2021 Society of Hospital Medicine