User login
Men die more often than women after bariatric surgery
Men had a much higher rate of death following bariatric surgery performed in Austria during 2010-2018, compared with women in a retrospective analysis of nearly 20,000 patients based on health insurance records.
The reason may be that men undergoing bariatric surgery have “worse overall health at the time of surgery” than women, Hannes Beiglböck, MD, suggested at the annual meeting of the European Association for the Study of Diabetes.
The results also showed that “men tend to be older [at time of surgery] and that might have the biggest impact on outcomes after bariatric surgery,” said Dr. Beiglböck, a researcher in the division of endocrinology and metabolism at the Medical University of Vienna.
The findings confirm those of prior studies in various worldwide locations, he noted; that is, men undergoing bariatric surgery tend to be older than women and have more comorbidities and perioperative mortality.
Dr. Beiglböck also highlighted earlier reports that indicate “profound” sex-specific differences in why patients undergo bariatric surgery, with men often driven by a medical condition and women motivated by appearance.
Hence, for men, it may be important to focus on preoperative counseling to try to get them to think about bariatric surgery earlier, “which may improve their postsurgical mortality rate,” he observed.
Nearly threefold higher mortality among men
Dr. Beiglböck and associates used medical claims data filed with the Austrian health system, which includes nearly all residents. In 2010-2018, 19,901 Austrian patients underwent bariatric surgery, and researchers tracked their outcomes for a median of 5.4 years, through April 2020.
During the 9-year period, 74% of patients who underwent bariatric surgery were women, again, a finding consistent with prior reports from other countries.
The 5,220 men were an average of 41.8 years old, with 65% undergoing gastric bypass and 30% gastric banding. The 14,681 women were an average of 40.1 years old, with 70% undergoing gastric bypass and 22% gastric banding.
During follow-up, 367 patients (1.8%) died. Among men, the overall mortality rate was 2.6-fold higher, compared with women (1.3% vs. 3.4%) and average mortality per year was 2.8-fold higher (0.64% vs. 0.24%).
The rate of death on the day of surgery among men also substantially exceeded that of women (0.29% vs. 0.05%), as did death within 30 days of surgery (0.48% vs. 0.08%). All of these between-sex differences were significant.
Baseline prevalence of four categories of comorbidities and how these differed by sex among patients who died during follow-up was also examined. Underlying cardiovascular disease was prevalent in 299 patients (81% of the deceased group), 200 (54%) had a psychiatric disorder, 138 (38%) had diabetes, and 132 (36%) had a malignancy.
The prevalence of cardiovascular disease and psychiatric disorders was roughly the same in men and women. Men had a significantly higher prevalence of diabetes, and a higher proportion of women had a malignancy.
Consistent with U.S. studies
A U.S. report in 2015 documented a higher prevalence of comorbidities and more severe illness among men undergoing bariatric surgery, compared with women, noted session chair Zhila Semnani-Azad, PhD, a researcher in the department of nutrition at Harvard School of Public Health in Boston.
“I think the [Austrian] data presented have relevance to the U.S. population,” Dr. Semnani-Azad said in an interview.
“The main limitation of these univariate analyses is they don’t account for potential confounding variables that could affect the association, such as lifestyle variables, age, and family history. There is always potential for other variables” to influence apparent sex-specific associations, she commented. Another limitation is the small total number of deaths analyzed, at 367.
“These results are a good starting point for future studies. More work is needed to better understand the impact of comorbidities and sex on postsurgical mortality,” Dr. Semnani-Azad concluded.
Dr. Beiglböck and Dr. Semnani-Azad have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Men had a much higher rate of death following bariatric surgery performed in Austria during 2010-2018, compared with women in a retrospective analysis of nearly 20,000 patients based on health insurance records.
The reason may be that men undergoing bariatric surgery have “worse overall health at the time of surgery” than women, Hannes Beiglböck, MD, suggested at the annual meeting of the European Association for the Study of Diabetes.
The results also showed that “men tend to be older [at time of surgery] and that might have the biggest impact on outcomes after bariatric surgery,” said Dr. Beiglböck, a researcher in the division of endocrinology and metabolism at the Medical University of Vienna.
The findings confirm those of prior studies in various worldwide locations, he noted; that is, men undergoing bariatric surgery tend to be older than women and have more comorbidities and perioperative mortality.
Dr. Beiglböck also highlighted earlier reports that indicate “profound” sex-specific differences in why patients undergo bariatric surgery, with men often driven by a medical condition and women motivated by appearance.
Hence, for men, it may be important to focus on preoperative counseling to try to get them to think about bariatric surgery earlier, “which may improve their postsurgical mortality rate,” he observed.
Nearly threefold higher mortality among men
Dr. Beiglböck and associates used medical claims data filed with the Austrian health system, which includes nearly all residents. In 2010-2018, 19,901 Austrian patients underwent bariatric surgery, and researchers tracked their outcomes for a median of 5.4 years, through April 2020.
During the 9-year period, 74% of patients who underwent bariatric surgery were women, again, a finding consistent with prior reports from other countries.
The 5,220 men were an average of 41.8 years old, with 65% undergoing gastric bypass and 30% gastric banding. The 14,681 women were an average of 40.1 years old, with 70% undergoing gastric bypass and 22% gastric banding.
During follow-up, 367 patients (1.8%) died. Among men, the overall mortality rate was 2.6-fold higher, compared with women (1.3% vs. 3.4%) and average mortality per year was 2.8-fold higher (0.64% vs. 0.24%).
The rate of death on the day of surgery among men also substantially exceeded that of women (0.29% vs. 0.05%), as did death within 30 days of surgery (0.48% vs. 0.08%). All of these between-sex differences were significant.
Baseline prevalence of four categories of comorbidities and how these differed by sex among patients who died during follow-up was also examined. Underlying cardiovascular disease was prevalent in 299 patients (81% of the deceased group), 200 (54%) had a psychiatric disorder, 138 (38%) had diabetes, and 132 (36%) had a malignancy.
The prevalence of cardiovascular disease and psychiatric disorders was roughly the same in men and women. Men had a significantly higher prevalence of diabetes, and a higher proportion of women had a malignancy.
Consistent with U.S. studies
A U.S. report in 2015 documented a higher prevalence of comorbidities and more severe illness among men undergoing bariatric surgery, compared with women, noted session chair Zhila Semnani-Azad, PhD, a researcher in the department of nutrition at Harvard School of Public Health in Boston.
“I think the [Austrian] data presented have relevance to the U.S. population,” Dr. Semnani-Azad said in an interview.
“The main limitation of these univariate analyses is they don’t account for potential confounding variables that could affect the association, such as lifestyle variables, age, and family history. There is always potential for other variables” to influence apparent sex-specific associations, she commented. Another limitation is the small total number of deaths analyzed, at 367.
“These results are a good starting point for future studies. More work is needed to better understand the impact of comorbidities and sex on postsurgical mortality,” Dr. Semnani-Azad concluded.
Dr. Beiglböck and Dr. Semnani-Azad have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Men had a much higher rate of death following bariatric surgery performed in Austria during 2010-2018, compared with women in a retrospective analysis of nearly 20,000 patients based on health insurance records.
The reason may be that men undergoing bariatric surgery have “worse overall health at the time of surgery” than women, Hannes Beiglböck, MD, suggested at the annual meeting of the European Association for the Study of Diabetes.
The results also showed that “men tend to be older [at time of surgery] and that might have the biggest impact on outcomes after bariatric surgery,” said Dr. Beiglböck, a researcher in the division of endocrinology and metabolism at the Medical University of Vienna.
The findings confirm those of prior studies in various worldwide locations, he noted; that is, men undergoing bariatric surgery tend to be older than women and have more comorbidities and perioperative mortality.
Dr. Beiglböck also highlighted earlier reports that indicate “profound” sex-specific differences in why patients undergo bariatric surgery, with men often driven by a medical condition and women motivated by appearance.
Hence, for men, it may be important to focus on preoperative counseling to try to get them to think about bariatric surgery earlier, “which may improve their postsurgical mortality rate,” he observed.
Nearly threefold higher mortality among men
Dr. Beiglböck and associates used medical claims data filed with the Austrian health system, which includes nearly all residents. In 2010-2018, 19,901 Austrian patients underwent bariatric surgery, and researchers tracked their outcomes for a median of 5.4 years, through April 2020.
During the 9-year period, 74% of patients who underwent bariatric surgery were women, again, a finding consistent with prior reports from other countries.
The 5,220 men were an average of 41.8 years old, with 65% undergoing gastric bypass and 30% gastric banding. The 14,681 women were an average of 40.1 years old, with 70% undergoing gastric bypass and 22% gastric banding.
During follow-up, 367 patients (1.8%) died. Among men, the overall mortality rate was 2.6-fold higher, compared with women (1.3% vs. 3.4%) and average mortality per year was 2.8-fold higher (0.64% vs. 0.24%).
The rate of death on the day of surgery among men also substantially exceeded that of women (0.29% vs. 0.05%), as did death within 30 days of surgery (0.48% vs. 0.08%). All of these between-sex differences were significant.
Baseline prevalence of four categories of comorbidities and how these differed by sex among patients who died during follow-up was also examined. Underlying cardiovascular disease was prevalent in 299 patients (81% of the deceased group), 200 (54%) had a psychiatric disorder, 138 (38%) had diabetes, and 132 (36%) had a malignancy.
The prevalence of cardiovascular disease and psychiatric disorders was roughly the same in men and women. Men had a significantly higher prevalence of diabetes, and a higher proportion of women had a malignancy.
Consistent with U.S. studies
A U.S. report in 2015 documented a higher prevalence of comorbidities and more severe illness among men undergoing bariatric surgery, compared with women, noted session chair Zhila Semnani-Azad, PhD, a researcher in the department of nutrition at Harvard School of Public Health in Boston.
“I think the [Austrian] data presented have relevance to the U.S. population,” Dr. Semnani-Azad said in an interview.
“The main limitation of these univariate analyses is they don’t account for potential confounding variables that could affect the association, such as lifestyle variables, age, and family history. There is always potential for other variables” to influence apparent sex-specific associations, she commented. Another limitation is the small total number of deaths analyzed, at 367.
“These results are a good starting point for future studies. More work is needed to better understand the impact of comorbidities and sex on postsurgical mortality,” Dr. Semnani-Azad concluded.
Dr. Beiglböck and Dr. Semnani-Azad have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EASD 2021
Botanical Briefs: Bloodroot (Sanguinaria canadensis)
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
Practice Points
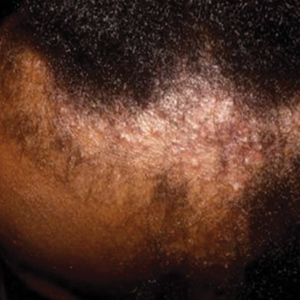
- Bloodroot (Sanguinaria canadensis) is a plant historically used in Mohs micrographic surgery as chemopaste.
- Bloodroot has been shown to have remarkable antimicrobial effects.
- The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging both neoplastic and healthy tissue. They have been shown to cause skin erosions and cellular atypia.
Skin of Color in Preclinical Medical Education: A Cross-Institutional Comparison and A Call to Action
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.
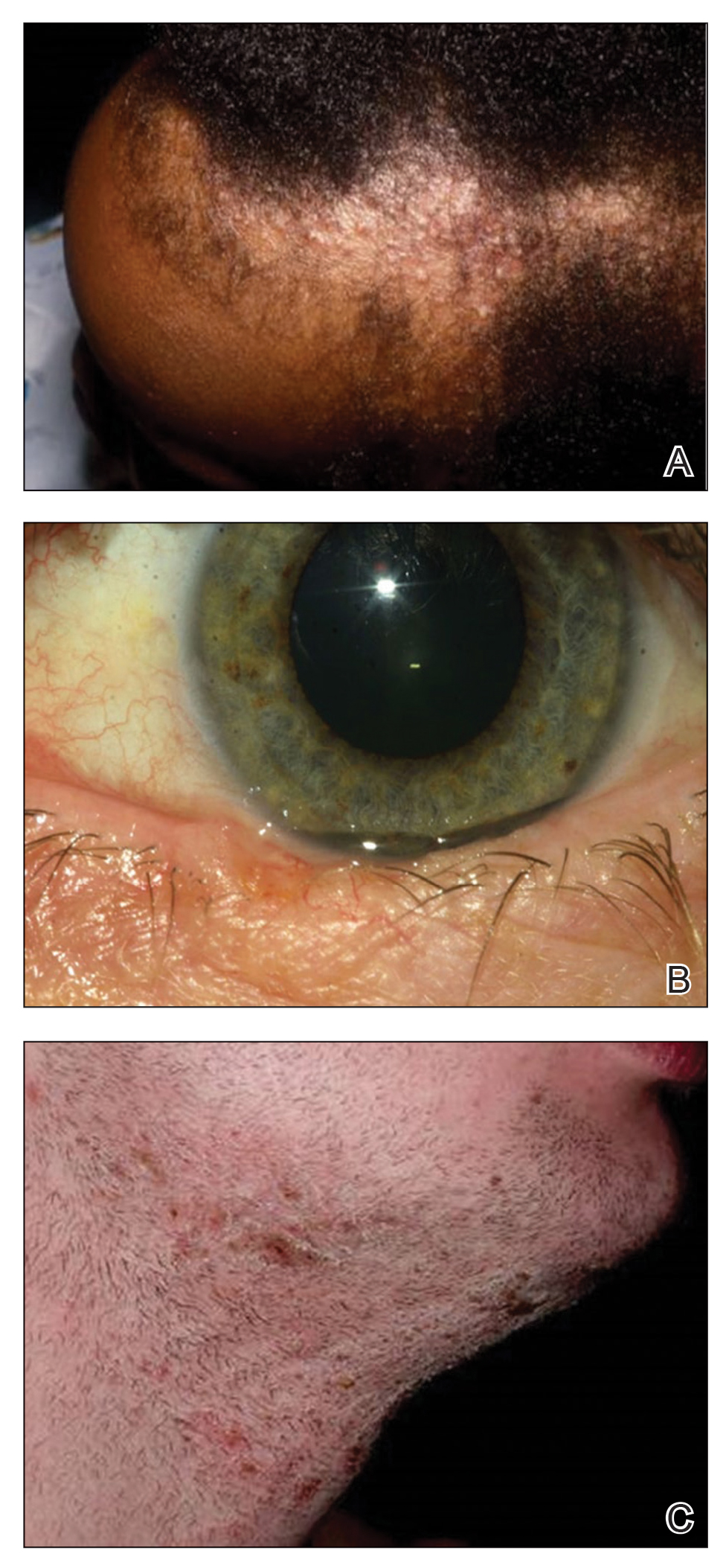
We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.
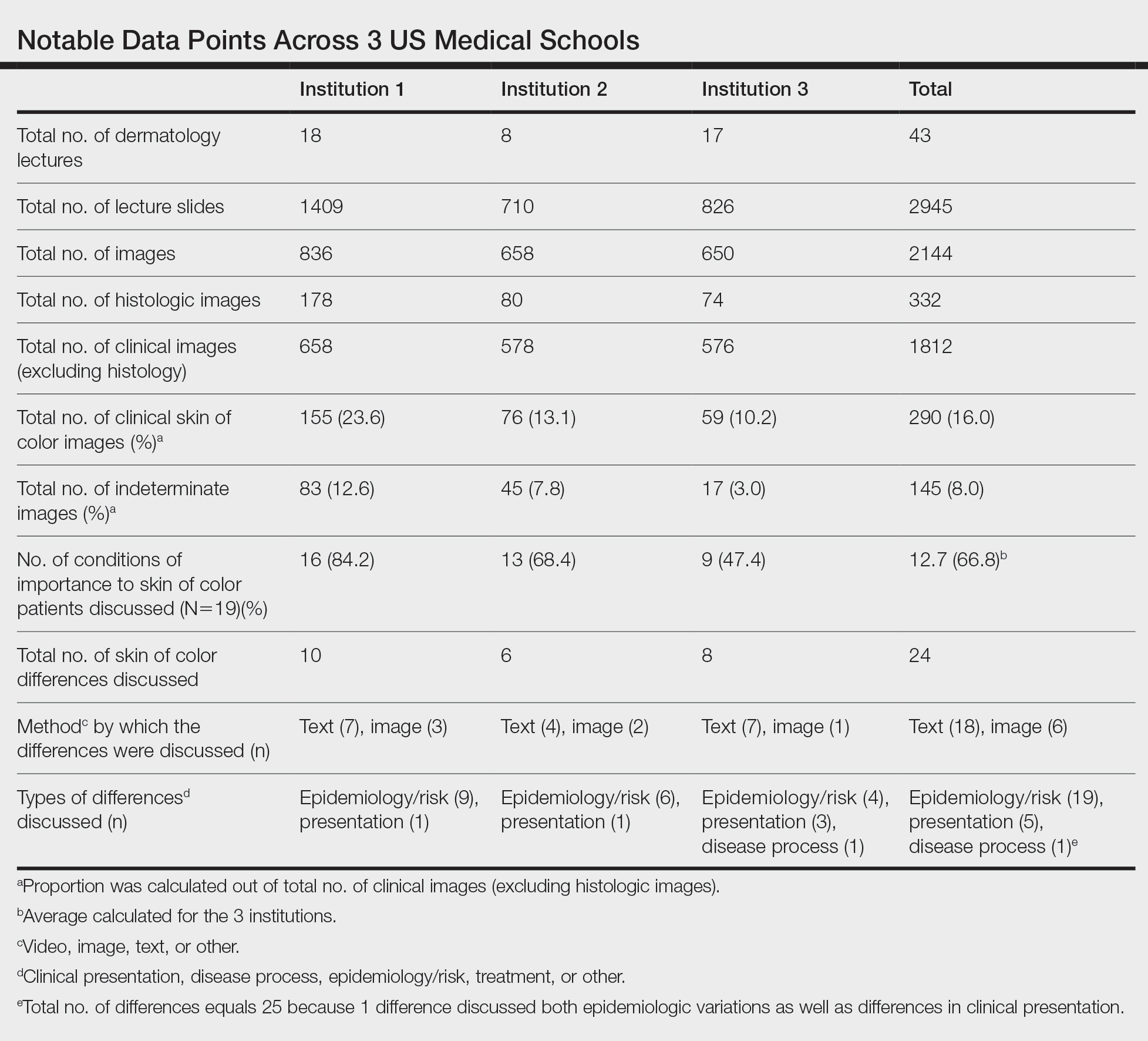
Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

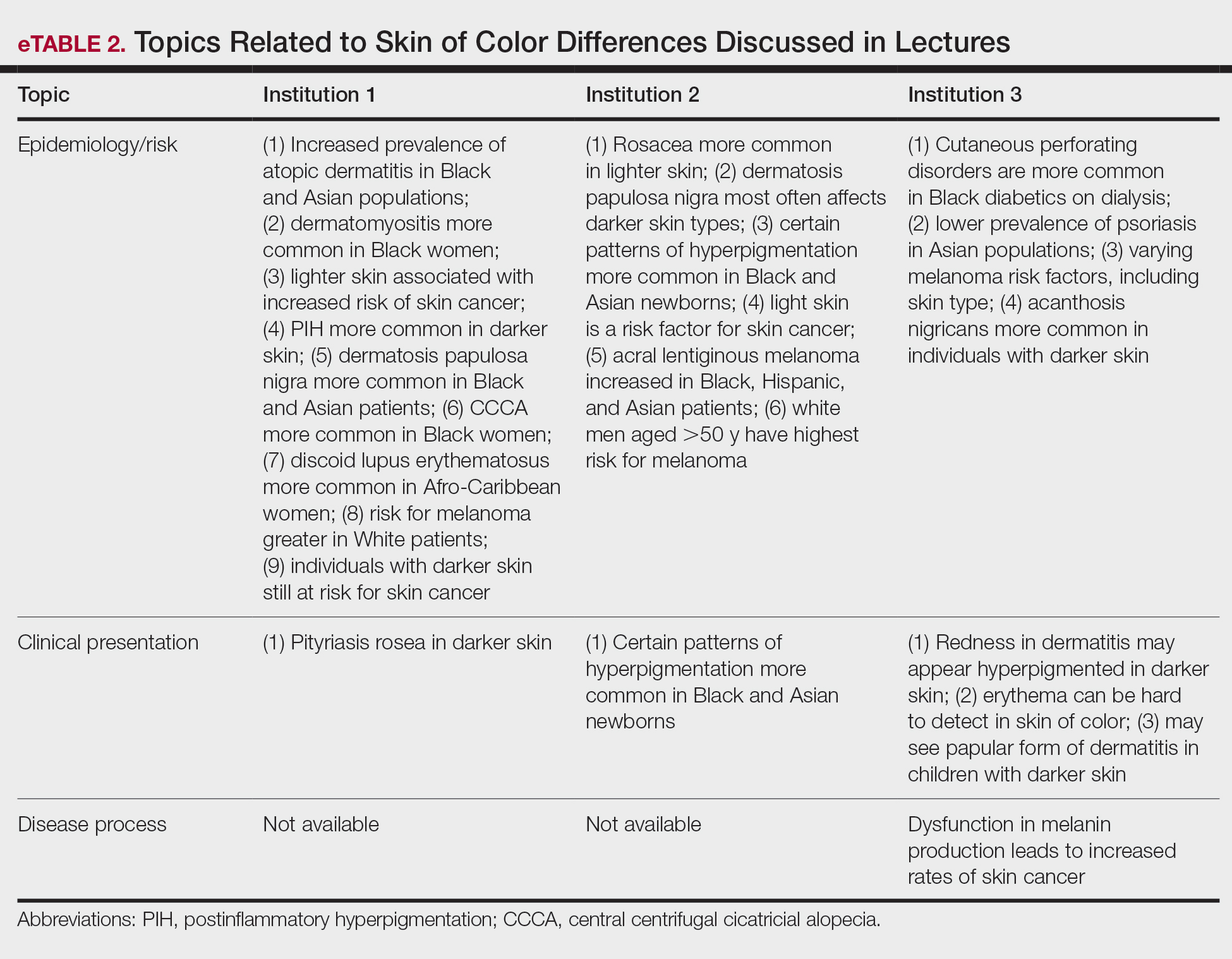
Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).
Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
Practice Points
- The United States rapidly is becoming a country in which the majority of citizens will have skin of color.
- Our study results strongly suggest that skin of color may be seriously underrepresented in medical education and can guide modifications to preclinical dermatology medical education to develop a more comprehensive and inclusive curriculum.
- Efforts should be made to increase images and discussion of skin of color in preclinical didactics.
Hospitals must identify and empower women leaders
Many potential leaders in academic medicine go unidentified, and finding those leaders is key to improving gender equity in academic medicine, said Nancy Spector, MD, in a presentation at the virtual Advance PHM Gender Equity Conference.
“I think it is important to reframe what it means to be a leader, and to empower yourself to think of yourself as a leader,” said Dr. Spector, executive director for executive leadership in academic medicine program at Drexel University, Philadelphia.
“Some of the best leaders I know do not have titles,” she emphasized.
Steps to stimulate the system changes needed to promote gender equity include building policies around the life cycle, revising departmental and division governance, and tracking metrics at the individual, departmental, and organizational level, Dr. Spector said.
Aligning gender-equity efforts with institutional priorities and navigating politics to effect changes in the gender equity landscape are ongoing objectives, she said.
Dr. Spector offered advice to men and women looking to shift the system and promote gender equity. She emphasized the challenge of overcoming psychological associations of men and women in leadership roles. “Men are more often associated with agentic qualities, which convey assertion and control,” she said. Men in leadership are more often described as aggressive, ambitious, dominant, self-confident, forceful, self-reliant, and individualistic.
By contrast, “women are associated with communal qualities, which convey a concern for compassionate treatment of others,” and are more often described as affectionate, helpful, kind, sympathetic, sensitive, gentle, and well spoken, she noted.
Although agentic traits are most often associated with effective leadership, in fact, “the most effective contemporary leaders have both agentic and communal traits,” said Dr. Spector.
However, “if a woman leader is very communal, she may be viewed as not assertive enough, and it she is highly agentic, she is criticized for being too domineering or controlling,” she said.
To help get past these associations, changes are needed at the individual level, leader level, and institutional level, Dr. Spector said.
On the individual level, women seeking to improve the situation for gender equity should engage with male allies and build a pipeline of mentorship and sponsorship to help identify future leaders, she said.
Women and men should obtain leadership training, and “become a student of leadership,” she advised. “Be in a learning mode,” and then think how to apply what you have learned, which may include setting challenging learning goals, experimenting with alternative strategies, learning about different leadership styles, and learning about differences in leaders’ values and attitudes.
For women, being pulled in many directions is the norm. “Are you being strategic with how you serve on committees?” Dr. Spector asked.
Make the most of how you choose to share your time, and “garner the skill of graceful self-promotion, which is often a hard skill for women,” she noted. She also urged women to make the most of professional networking and social capital.
At the leader level, the advice Dr. Spector offered to leaders on building gender equity in their institutions include ensuring a critical mass of women in leadership track positions. “Avoid having a sole woman member of a team,” she said.
Dr. Spector also emphasized the importance of giving employees with family responsibilities more time for promotion, and welcoming back women who step away from the workforce and choose to return. Encourage men to participate in family-friendly benefits. “Standardize processes that support the life cycle of a faculty member or the person you’re hiring,” and ensure inclusive times and venues for major meetings, committee work, and social events, she added.
Dr. Spector’s strategies for institutions include quantifying disparities by using real time dashboards to show both leading and lagging indicators, setting goals, and measuring achievements.
“Create an infrastructure to support women’s leadership,” she said. Such an infrastructure could include not only robust committees for women in science and medicine, but also supporting women to attend leadership training both inside and outside their institutions.
Dr. Spector noted that professional organizations also have a role to play in support of women’s leadership.
“Make a public pledge to gender equity,” she said. She encouraged professional organizations to tie diversity and inclusion metrics to performance reviews, and to prioritize the examination and mitigation of disparities, and report challenges and successes.
When creating policies to promote gender equity, “get out of your silo,” Dr. Spector emphasized. Understand the drivers rather than simply judging the behaviors.
“Even if we disagree on something, we need to work together, and empower everyone to be thoughtful drivers of change,” she concluded.
Dr. Spector disclosed grant funding from the Department of Health & Human Services, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. She also disclosed receiving monetary awards, honoraria, and travel reimbursement from multiple academic and professional organization for teaching and consulting programs. Dr. Spector also cofunded and holds equity interest in the I-PASS Patient Safety Institute, a company created to assist institutions in implementing the I-PASS Handoff Program.
Many potential leaders in academic medicine go unidentified, and finding those leaders is key to improving gender equity in academic medicine, said Nancy Spector, MD, in a presentation at the virtual Advance PHM Gender Equity Conference.
“I think it is important to reframe what it means to be a leader, and to empower yourself to think of yourself as a leader,” said Dr. Spector, executive director for executive leadership in academic medicine program at Drexel University, Philadelphia.
“Some of the best leaders I know do not have titles,” she emphasized.
Steps to stimulate the system changes needed to promote gender equity include building policies around the life cycle, revising departmental and division governance, and tracking metrics at the individual, departmental, and organizational level, Dr. Spector said.
Aligning gender-equity efforts with institutional priorities and navigating politics to effect changes in the gender equity landscape are ongoing objectives, she said.
Dr. Spector offered advice to men and women looking to shift the system and promote gender equity. She emphasized the challenge of overcoming psychological associations of men and women in leadership roles. “Men are more often associated with agentic qualities, which convey assertion and control,” she said. Men in leadership are more often described as aggressive, ambitious, dominant, self-confident, forceful, self-reliant, and individualistic.
By contrast, “women are associated with communal qualities, which convey a concern for compassionate treatment of others,” and are more often described as affectionate, helpful, kind, sympathetic, sensitive, gentle, and well spoken, she noted.
Although agentic traits are most often associated with effective leadership, in fact, “the most effective contemporary leaders have both agentic and communal traits,” said Dr. Spector.
However, “if a woman leader is very communal, she may be viewed as not assertive enough, and it she is highly agentic, she is criticized for being too domineering or controlling,” she said.
To help get past these associations, changes are needed at the individual level, leader level, and institutional level, Dr. Spector said.
On the individual level, women seeking to improve the situation for gender equity should engage with male allies and build a pipeline of mentorship and sponsorship to help identify future leaders, she said.
Women and men should obtain leadership training, and “become a student of leadership,” she advised. “Be in a learning mode,” and then think how to apply what you have learned, which may include setting challenging learning goals, experimenting with alternative strategies, learning about different leadership styles, and learning about differences in leaders’ values and attitudes.
For women, being pulled in many directions is the norm. “Are you being strategic with how you serve on committees?” Dr. Spector asked.
Make the most of how you choose to share your time, and “garner the skill of graceful self-promotion, which is often a hard skill for women,” she noted. She also urged women to make the most of professional networking and social capital.
At the leader level, the advice Dr. Spector offered to leaders on building gender equity in their institutions include ensuring a critical mass of women in leadership track positions. “Avoid having a sole woman member of a team,” she said.
Dr. Spector also emphasized the importance of giving employees with family responsibilities more time for promotion, and welcoming back women who step away from the workforce and choose to return. Encourage men to participate in family-friendly benefits. “Standardize processes that support the life cycle of a faculty member or the person you’re hiring,” and ensure inclusive times and venues for major meetings, committee work, and social events, she added.
Dr. Spector’s strategies for institutions include quantifying disparities by using real time dashboards to show both leading and lagging indicators, setting goals, and measuring achievements.
“Create an infrastructure to support women’s leadership,” she said. Such an infrastructure could include not only robust committees for women in science and medicine, but also supporting women to attend leadership training both inside and outside their institutions.
Dr. Spector noted that professional organizations also have a role to play in support of women’s leadership.
“Make a public pledge to gender equity,” she said. She encouraged professional organizations to tie diversity and inclusion metrics to performance reviews, and to prioritize the examination and mitigation of disparities, and report challenges and successes.
When creating policies to promote gender equity, “get out of your silo,” Dr. Spector emphasized. Understand the drivers rather than simply judging the behaviors.
“Even if we disagree on something, we need to work together, and empower everyone to be thoughtful drivers of change,” she concluded.
Dr. Spector disclosed grant funding from the Department of Health & Human Services, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. She also disclosed receiving monetary awards, honoraria, and travel reimbursement from multiple academic and professional organization for teaching and consulting programs. Dr. Spector also cofunded and holds equity interest in the I-PASS Patient Safety Institute, a company created to assist institutions in implementing the I-PASS Handoff Program.
Many potential leaders in academic medicine go unidentified, and finding those leaders is key to improving gender equity in academic medicine, said Nancy Spector, MD, in a presentation at the virtual Advance PHM Gender Equity Conference.
“I think it is important to reframe what it means to be a leader, and to empower yourself to think of yourself as a leader,” said Dr. Spector, executive director for executive leadership in academic medicine program at Drexel University, Philadelphia.
“Some of the best leaders I know do not have titles,” she emphasized.
Steps to stimulate the system changes needed to promote gender equity include building policies around the life cycle, revising departmental and division governance, and tracking metrics at the individual, departmental, and organizational level, Dr. Spector said.
Aligning gender-equity efforts with institutional priorities and navigating politics to effect changes in the gender equity landscape are ongoing objectives, she said.
Dr. Spector offered advice to men and women looking to shift the system and promote gender equity. She emphasized the challenge of overcoming psychological associations of men and women in leadership roles. “Men are more often associated with agentic qualities, which convey assertion and control,” she said. Men in leadership are more often described as aggressive, ambitious, dominant, self-confident, forceful, self-reliant, and individualistic.
By contrast, “women are associated with communal qualities, which convey a concern for compassionate treatment of others,” and are more often described as affectionate, helpful, kind, sympathetic, sensitive, gentle, and well spoken, she noted.
Although agentic traits are most often associated with effective leadership, in fact, “the most effective contemporary leaders have both agentic and communal traits,” said Dr. Spector.
However, “if a woman leader is very communal, she may be viewed as not assertive enough, and it she is highly agentic, she is criticized for being too domineering or controlling,” she said.
To help get past these associations, changes are needed at the individual level, leader level, and institutional level, Dr. Spector said.
On the individual level, women seeking to improve the situation for gender equity should engage with male allies and build a pipeline of mentorship and sponsorship to help identify future leaders, she said.
Women and men should obtain leadership training, and “become a student of leadership,” she advised. “Be in a learning mode,” and then think how to apply what you have learned, which may include setting challenging learning goals, experimenting with alternative strategies, learning about different leadership styles, and learning about differences in leaders’ values and attitudes.
For women, being pulled in many directions is the norm. “Are you being strategic with how you serve on committees?” Dr. Spector asked.
Make the most of how you choose to share your time, and “garner the skill of graceful self-promotion, which is often a hard skill for women,” she noted. She also urged women to make the most of professional networking and social capital.
At the leader level, the advice Dr. Spector offered to leaders on building gender equity in their institutions include ensuring a critical mass of women in leadership track positions. “Avoid having a sole woman member of a team,” she said.
Dr. Spector also emphasized the importance of giving employees with family responsibilities more time for promotion, and welcoming back women who step away from the workforce and choose to return. Encourage men to participate in family-friendly benefits. “Standardize processes that support the life cycle of a faculty member or the person you’re hiring,” and ensure inclusive times and venues for major meetings, committee work, and social events, she added.
Dr. Spector’s strategies for institutions include quantifying disparities by using real time dashboards to show both leading and lagging indicators, setting goals, and measuring achievements.
“Create an infrastructure to support women’s leadership,” she said. Such an infrastructure could include not only robust committees for women in science and medicine, but also supporting women to attend leadership training both inside and outside their institutions.
Dr. Spector noted that professional organizations also have a role to play in support of women’s leadership.
“Make a public pledge to gender equity,” she said. She encouraged professional organizations to tie diversity and inclusion metrics to performance reviews, and to prioritize the examination and mitigation of disparities, and report challenges and successes.
When creating policies to promote gender equity, “get out of your silo,” Dr. Spector emphasized. Understand the drivers rather than simply judging the behaviors.
“Even if we disagree on something, we need to work together, and empower everyone to be thoughtful drivers of change,” she concluded.
Dr. Spector disclosed grant funding from the Department of Health & Human Services, the Agency for Healthcare Research and Quality, and the Patient-Centered Outcomes Research Institute. She also disclosed receiving monetary awards, honoraria, and travel reimbursement from multiple academic and professional organization for teaching and consulting programs. Dr. Spector also cofunded and holds equity interest in the I-PASS Patient Safety Institute, a company created to assist institutions in implementing the I-PASS Handoff Program.
FROM THE ADVANCE PHM GENDER EQUITY CONFERENCE
The Role of Inpatient Dermatology Consultations
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.
Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.
Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.

Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.

Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.

Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.

Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Practice Points
- Inpatient dermatologists fill knowledge gaps that often alter the diagnosis, management, and hospital course of hospitalized patients.
- Several medical specialties benefit from niche expertise of inpatient dermatologists specific to their patient population.
- Integration of inpatient dermatology consultations can prevent unnecessary hospital admissions and medication administration.
Cutaneous Cold Weather Injuries in the US Military
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
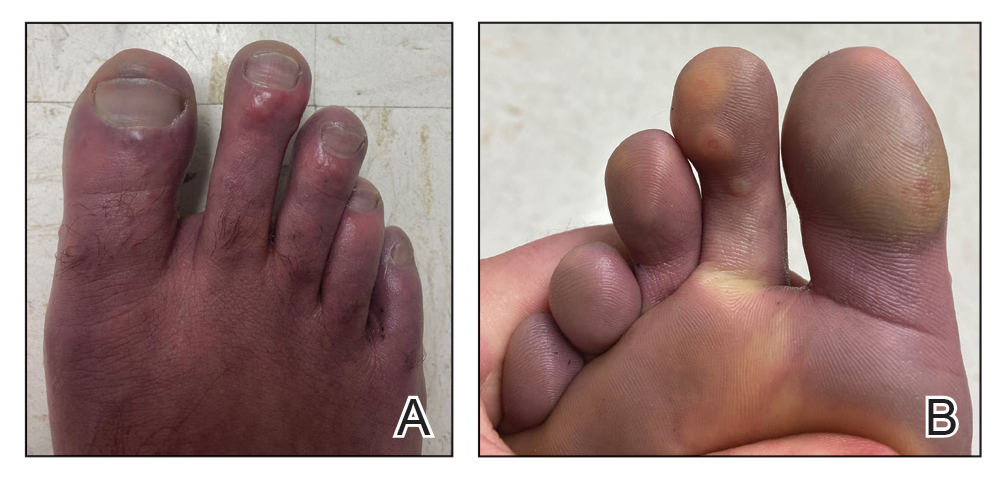
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
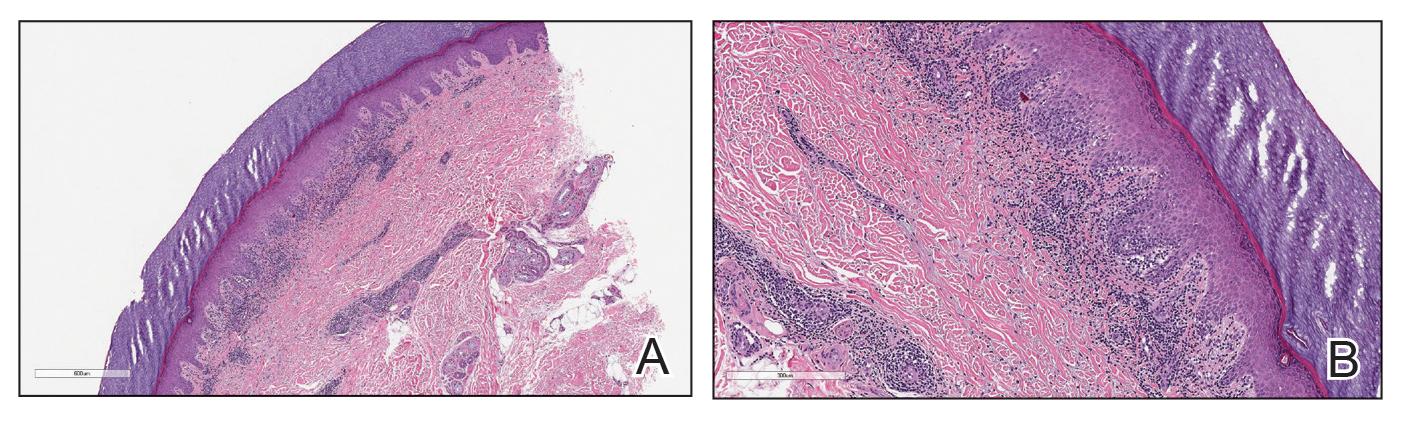
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.

Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.

Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.

Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.

Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
Practice Points
- Military service members are at an increased risk for cutaneous cold weather injuries in certain circumstances due to the demands of military training and combat operations.
- Cold weather may cause injury by directly damaging tissues, leading to neurovascular disruption, and by exacerbating existing medical conditions.
Treatment Stacking: Optimizing Therapeutic Regimens for Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).
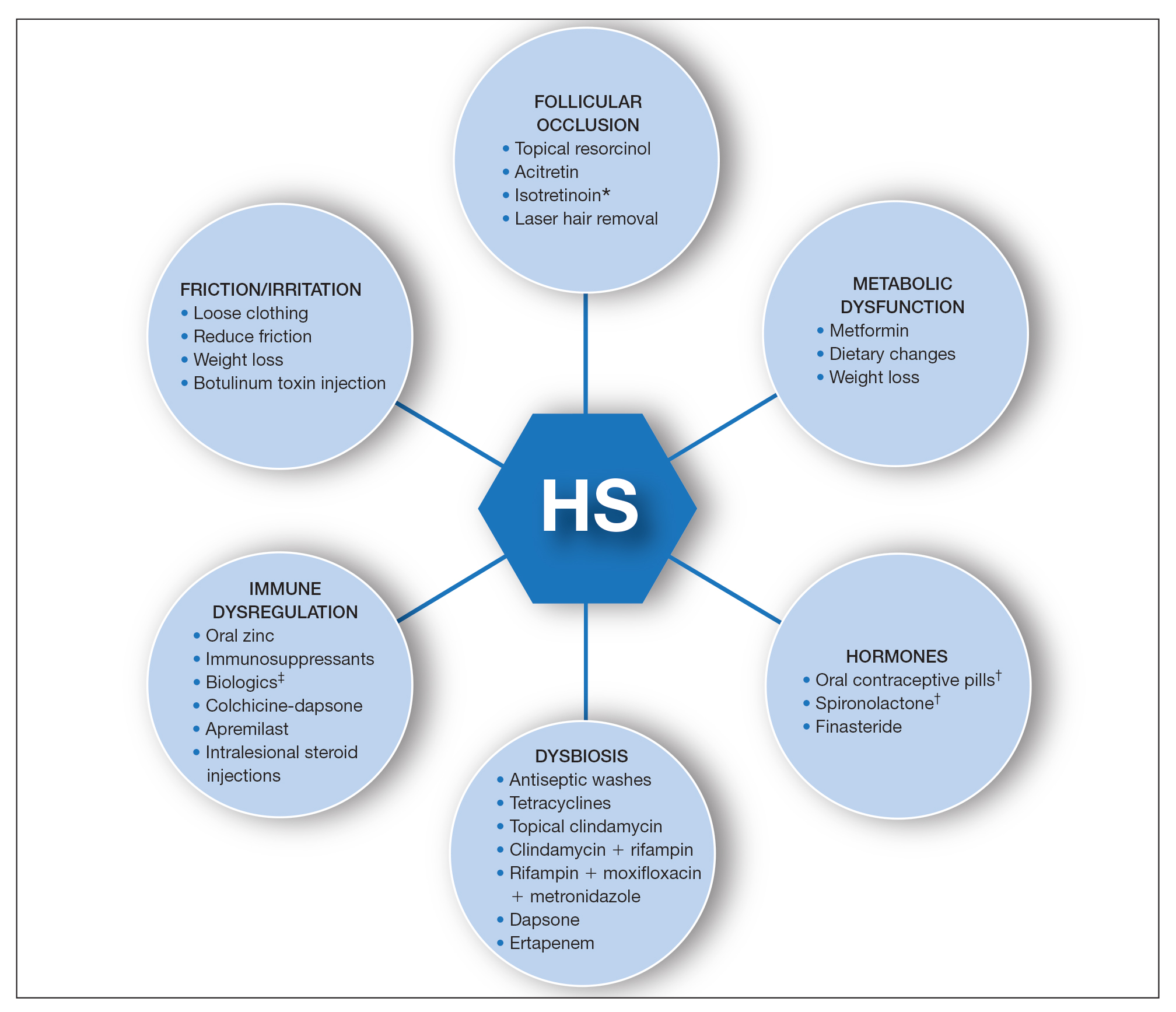
Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).

Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).

Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768
Exercise appears to improve bone structure, not density
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2021
2021 update on contraception
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.
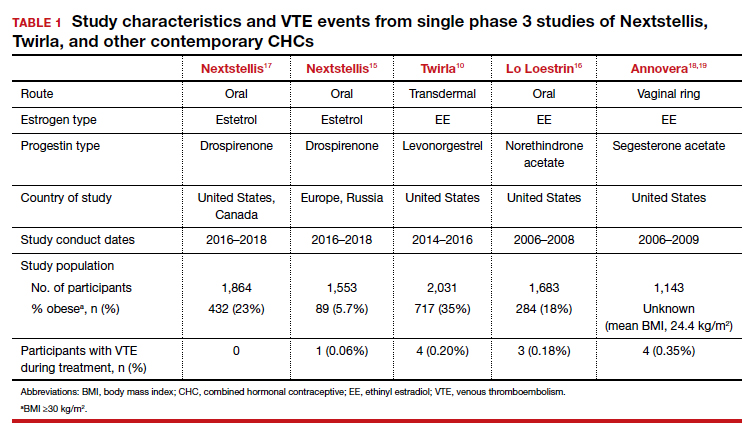
Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%
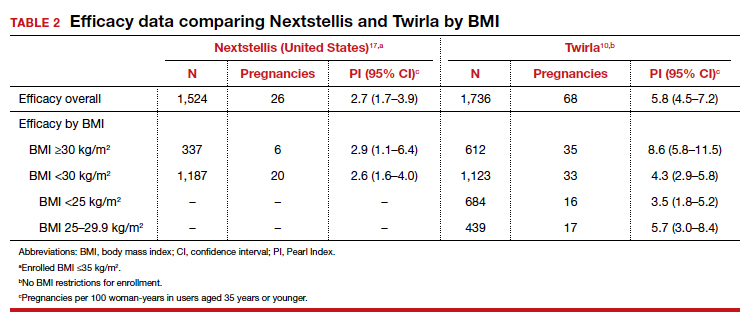
Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.
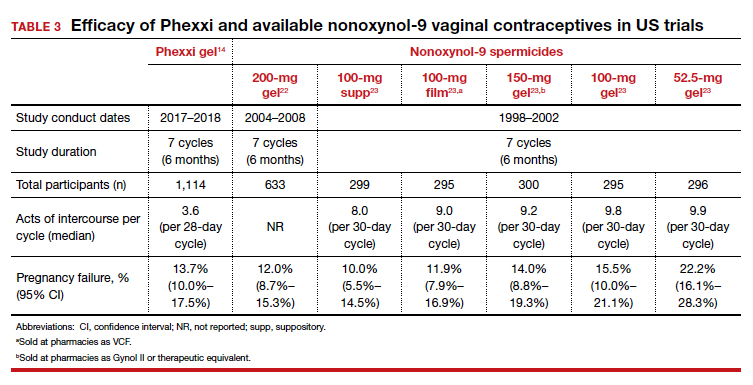
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
Evolving management strategies for patient service excellence: Is your practice up to speed?
Over the past decade, the use of technology with the focus on optimizing the consumer experience has exploded throughout numerous industries, including education, retail, and entertainment. Within health care, we would be naïve to ignore patient expectations for an optimized consumer experience within our offices. Thus, clinicians across all health care disciplines must remain cognizant of and work to optimize the patient experience in the ever-expanding world of health care.
Reengineering one’s practice will continue to be a work in progress. As medicine and technology continuously advance, clinicians must be able to adapt and implement changes. An excellent example of such adaptation is the use of telemedicine during the COVID-19 pandemic.1 We hope that the use of telemedicine remains an integral part of our armamentarium as we move forward.
In this article, we offer perspectives on using telemedicine, improving the patient experience, and implementing the use of social media in your practice. We look for a common denominator when provision of clinical care is the topic of discussion. Knowing the details of your medical practice and addressing its highlights as well as its concerns will benefit patients, staff, and health care providers. We hope that you glean some insights that you can apply in your practice.
Telemedicine: Part of the new normal
The American College of Obstetricians and Gynecologists defines telehealth as a “technology-enhanced health care framework that includes services, such as virtual visits, remote patient monitoring and mobile health care.”2 The American Telemedicine Association and the World Health Organization use the terms telemedicine and telehealth interchangeably.3 We live in a relatively new era since the COVID-19 pandemic necessitated that traditional face-to-face meeting(s) with patients be conducted virtually. The good news is that the outcomes with telehealth visits appear to be on par with those of traditional office visits.4
Telehealth allows clinicians to deliver medical evaluation and management plans right in a patient’s home and to receive appropriate reimbursement for doing so. This is a result of actions by Congress and the Department of Health and Human Services that removed restrictions related to telemedicine.5 The telemedicine approach provides a different perspective on provision of care (FIGURE 1).

For telemedicine practice, prerequisites include having the appropriate hardware, software, and a secure internet connection to maintain quality and patient safety.4 It is wise to check with regulatory laws at the local, state, and federal levels, as some states have separate licensure requirements for delivering this type of health care. Review insurance carrier guidelines as well as medical malpractice coverage for telehealth care provision. Ideally, obtain proof in writing from third-party payers and malpractice insurance carriers. TABLE 1 lists ObGyn-related activities and services that can be provided via telemedicine.3
While in many circumstances the indications for telemedicine are obvious, some remain less apparent. For example, patients may be more receptive to the use of telemedicine for counseling and education for family planning services and termination of pregnancy.6 Psychological counseling lends itself to a telemedicine approach to address levels of anxiety and depression, especially in the postpartum setting.
An initial telemedicine consultation often is complemented by subsequent patient examination when deemed necessary. Pelvic imaging often is ordered to address concerns expressed during the telemedicine visit. Teleradiology is an interesting aspect of telemedicine that is expanding. Telesonography, the use of ultrasonography, is extremely relevant to obstetrics and gynecology. Specifically, the development of self-operated endovaginal telemonitors and 3D as well as 4D imaging incorporates self-operated endovaginal telemonitoring. This technology remains a work in progress.7
Another aspect to telemedicine is telesurgery. Although an operative procedure cannot be performed virtually, pre- and postoperative counseling can be provided via telemedicine, offering tremendous convenience to patients.
Understanding the infrastructure of telemedicine and assuring security, adherence with HIPAA (Health Insurance Portability and Accountability Act), state licensure, reimbursement, and medical malpractice aspects is well worth the effort.
Continue to: Reengineer your office to enhance the patient experience...
Reengineer your office to enhance the patient experience
Create a hospitable environment. One way to do this is by having your front desk staffer standing up to greet patients. The medical management literature has reported an interesting analogy.8 Picture going to a retailer whose job is to sell you the product you are interested in. Where is that person positioned? Standing at the counter, at eye level with you, doing his or her best to convince you to buy a particular product. Having your office front desk personnel standing is analogous to the “atmosphere (when approaching the front desk) that conveys clear energy and a clear tone or readiness,” all of which contribute to a more positive patient experience.8
A hospitable environment at the check-in desk sets the stage for the office visit. When a staffer is sitting at the front desk office entrance point, the concept conveyed to the patient is, “You can wait for us because you need us more than we need you.” Changing the staffer’s posture to a standing position conveys, “Welcome, we are glad to see you and address why you are here.”8
Conduct a flow analysis of your office procedures. It is clear that the front desk serves as an advertisement of what your practice has to offer. A friendly smile from the receptionist goes a long way. In addition, the total time from patient check-in to checkout should be monitored. Having this type of data aids staff evaluation and patient satisfaction.9
Examine your office’s aspects of what the business world calls throughput. In essence, problems related to throughput include that the clinician is chronically late or slow with patients or that inadequate time was allocated per patient visit or per procedure.
It is valuable to allocate staff resources ahead of time, including patient registration and insurance verification details. Staff records review and preparation for the clinician streamlines time with the patient. Having lab tests, other consultations, and so on readily available for the clinician is time well spent by the medical assistants. For procedures, preparation of equipment that is in good working order and having supplies appropriately stocked can help facilitate success and efficiency. Creation of an “electronic on-time board” displays if the clinician is running on time or not.9 These practical tips can result in better patient and staff satisfaction. In addition, periodic surveys help engage patients in the process. TABLE 2 provides sample survey questions to ask patients.10
Taking a careful look at your current office practices and reengineering them as needed is an investment that provides an excellent return.
Continue to: Develop a presence on social media...
Develop a presence on social media
Having a social media presence is becoming one of the most effective strategies for reaching an intended audience. In the United States, more than 70% of the public uses at least one social media platform.11 It can be an effective and efficient tool for clinicians to grow their practice; distribute information about unique areas of the practice; and reach potential patients, referring physicians, and prospective faculty/trainees. Social media also is increasingly being used by clinicians to connect with other health care providers in their own specialty or other specialties. Digital communities have been created where ideas are shared and topics of interest are discussed. Clinicians can listen in on expert opinions, disseminate their research, and discuss practice management challenges or health advocacy. FIGURE 2 provides a snapshot of the social media landscape.
There is a wealth of options when it comes to social media platforms, including but not limited to Facebook, Twitter, LinkedIn, Instagram, YouTube, and blogs (TABLE 3). Facebook has the largest user base of all social media platforms, with approximately 1.7 billion active monthly users; thus, its use creates an opportunity to reach a massive audience.12,13 People use Facebook for both personal and professional reasons. The platform allows for sharing of photos, live videos, posted text, and comments. It can be used as a helpful resource to engage patients and disseminate accurate medical information. Importantly, remember that content posted should comply with the HIPAA Privacy Rule and that information shared should come from a credible source.
The Mayo Clinic is an impressive example of the use of social media for consumer education, research, and expansion of the reach of its brand. They incorporated social media into their strategic marketing plan, and between 2015 and 2016, social media referrals led to a 139% increase in patient appointment requests.13 Of the 20 different social media sites used, Facebook was the top social media referrer, accounting for 81% of social media referrals in 2015 and 88% in 2016. They have expanded their reach through different social media platforms and have more than 1.5 million followers on Twitter. Their videos on YouTube were viewed more than 4.9 million times in 2016 alone. This example illustrates social media’s effectiveness and the potential role it can play in connecting with patients.
Final thoughts
The practice of medicine has undeniably changed over the years and will continue to evolve. Understanding how to implement change to ensure that high-quality, efficient patient care is being delivered is paramount.
We have highlighted various aspects of practice management that you can use to overcome current obstacles and changing standards. The advent of telemedicine has provided easy access to clinicians. Consultation occurs in the comfort of the patient’s home, and the ability to provide local examination telecast to a clinician allows physicians and advanced practice practitioners to reach a wider range of patients. Social media has established an infrastructure for educating patients and providers while at the same time conveying educational tools to patients. This level of communication will continue to expand as time progresses.
Practitioners have a whole new cadre to add to their toolbox to provide patients with state-of-the-art communication and care. ●
- Anifandis G, Tempest H, Oliva R, et al. COVID-19 and human reproduction: a pandemic that packs a serious punch. Systems Biol Reprod Med. 2021;67:3-23.
- American College of Obstetricians and Gynecologists Presidential Task Force on Telehealth. Implementing telehealth in practice: ACOG Committee Opinion No. 798. Obstet Gynecol. 2020;135:e73-e79.
- Lee S, Hitt WC. Clinical applications of telemedicine in gynecology and women’s health. Obstet Gynecol Clin North Am. 2020;47:259-270.
- DeNicola N, Grossman D, Marko K, et al. Telehealth interventions to improve obstetrics and gynecologic health outcomes: a systematic review. Obstet Gynecol. 2020;135:371-382.
- Keesara S, Jonas A, Schulman K. Covid-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82.
- Grossman D, Grindlay K. Safety of medical abortion provided through telemedicine compared with in person. Obstet Gynecol. 2017;130:778-782.
- Pereira I, von Horn K, Depebusch M, et al. Self-operated endovaginal telemonitoring: a prospective clinical validation study. Fertil Steril. 2016;106:306-310e1.
- Massey GG, Hunter DG. Enhancing the patient experience with stand-up check-in. MGMA Connex. 2016;34-36.
- The patient experience, from check-in to check out. MGMA Connex. 2017;17:45-46.
- Swankoski KE, Peikes DN, Morrison N, et al. Patient experience during a large primary care practice transformation initiative. Am J Manag Care. 2018;24:607-613.
- Pew Research Center. Social media fact sheet. https://www .pewinternet.org/fact-sheet/social-media/. April 7. 2021. Accessed September 21, 2021.
- Small Business Trends website. Mansfield M. Social media statistics 2016. https://smallbiztrends.com/2016/11/social -media-statistics-2016.html. Updated June 4, 2021. Accessed September 21, 2021.
- Kotsenas AL, Arce M, Aase L, et al. The strategic imperative for the use of social media in health care. J Am Coll Radiol. 2018;15(1 pt B):155-161.
Over the past decade, the use of technology with the focus on optimizing the consumer experience has exploded throughout numerous industries, including education, retail, and entertainment. Within health care, we would be naïve to ignore patient expectations for an optimized consumer experience within our offices. Thus, clinicians across all health care disciplines must remain cognizant of and work to optimize the patient experience in the ever-expanding world of health care.
Reengineering one’s practice will continue to be a work in progress. As medicine and technology continuously advance, clinicians must be able to adapt and implement changes. An excellent example of such adaptation is the use of telemedicine during the COVID-19 pandemic.1 We hope that the use of telemedicine remains an integral part of our armamentarium as we move forward.
In this article, we offer perspectives on using telemedicine, improving the patient experience, and implementing the use of social media in your practice. We look for a common denominator when provision of clinical care is the topic of discussion. Knowing the details of your medical practice and addressing its highlights as well as its concerns will benefit patients, staff, and health care providers. We hope that you glean some insights that you can apply in your practice.
Telemedicine: Part of the new normal
The American College of Obstetricians and Gynecologists defines telehealth as a “technology-enhanced health care framework that includes services, such as virtual visits, remote patient monitoring and mobile health care.”2 The American Telemedicine Association and the World Health Organization use the terms telemedicine and telehealth interchangeably.3 We live in a relatively new era since the COVID-19 pandemic necessitated that traditional face-to-face meeting(s) with patients be conducted virtually. The good news is that the outcomes with telehealth visits appear to be on par with those of traditional office visits.4
Telehealth allows clinicians to deliver medical evaluation and management plans right in a patient’s home and to receive appropriate reimbursement for doing so. This is a result of actions by Congress and the Department of Health and Human Services that removed restrictions related to telemedicine.5 The telemedicine approach provides a different perspective on provision of care (FIGURE 1).

For telemedicine practice, prerequisites include having the appropriate hardware, software, and a secure internet connection to maintain quality and patient safety.4 It is wise to check with regulatory laws at the local, state, and federal levels, as some states have separate licensure requirements for delivering this type of health care. Review insurance carrier guidelines as well as medical malpractice coverage for telehealth care provision. Ideally, obtain proof in writing from third-party payers and malpractice insurance carriers. TABLE 1 lists ObGyn-related activities and services that can be provided via telemedicine.3
While in many circumstances the indications for telemedicine are obvious, some remain less apparent. For example, patients may be more receptive to the use of telemedicine for counseling and education for family planning services and termination of pregnancy.6 Psychological counseling lends itself to a telemedicine approach to address levels of anxiety and depression, especially in the postpartum setting.
An initial telemedicine consultation often is complemented by subsequent patient examination when deemed necessary. Pelvic imaging often is ordered to address concerns expressed during the telemedicine visit. Teleradiology is an interesting aspect of telemedicine that is expanding. Telesonography, the use of ultrasonography, is extremely relevant to obstetrics and gynecology. Specifically, the development of self-operated endovaginal telemonitors and 3D as well as 4D imaging incorporates self-operated endovaginal telemonitoring. This technology remains a work in progress.7
Another aspect to telemedicine is telesurgery. Although an operative procedure cannot be performed virtually, pre- and postoperative counseling can be provided via telemedicine, offering tremendous convenience to patients.
Understanding the infrastructure of telemedicine and assuring security, adherence with HIPAA (Health Insurance Portability and Accountability Act), state licensure, reimbursement, and medical malpractice aspects is well worth the effort.
Continue to: Reengineer your office to enhance the patient experience...
Reengineer your office to enhance the patient experience
Create a hospitable environment. One way to do this is by having your front desk staffer standing up to greet patients. The medical management literature has reported an interesting analogy.8 Picture going to a retailer whose job is to sell you the product you are interested in. Where is that person positioned? Standing at the counter, at eye level with you, doing his or her best to convince you to buy a particular product. Having your office front desk personnel standing is analogous to the “atmosphere (when approaching the front desk) that conveys clear energy and a clear tone or readiness,” all of which contribute to a more positive patient experience.8
A hospitable environment at the check-in desk sets the stage for the office visit. When a staffer is sitting at the front desk office entrance point, the concept conveyed to the patient is, “You can wait for us because you need us more than we need you.” Changing the staffer’s posture to a standing position conveys, “Welcome, we are glad to see you and address why you are here.”8
Conduct a flow analysis of your office procedures. It is clear that the front desk serves as an advertisement of what your practice has to offer. A friendly smile from the receptionist goes a long way. In addition, the total time from patient check-in to checkout should be monitored. Having this type of data aids staff evaluation and patient satisfaction.9
Examine your office’s aspects of what the business world calls throughput. In essence, problems related to throughput include that the clinician is chronically late or slow with patients or that inadequate time was allocated per patient visit or per procedure.
It is valuable to allocate staff resources ahead of time, including patient registration and insurance verification details. Staff records review and preparation for the clinician streamlines time with the patient. Having lab tests, other consultations, and so on readily available for the clinician is time well spent by the medical assistants. For procedures, preparation of equipment that is in good working order and having supplies appropriately stocked can help facilitate success and efficiency. Creation of an “electronic on-time board” displays if the clinician is running on time or not.9 These practical tips can result in better patient and staff satisfaction. In addition, periodic surveys help engage patients in the process. TABLE 2 provides sample survey questions to ask patients.10
Taking a careful look at your current office practices and reengineering them as needed is an investment that provides an excellent return.
Continue to: Develop a presence on social media...
Develop a presence on social media
Having a social media presence is becoming one of the most effective strategies for reaching an intended audience. In the United States, more than 70% of the public uses at least one social media platform.11 It can be an effective and efficient tool for clinicians to grow their practice; distribute information about unique areas of the practice; and reach potential patients, referring physicians, and prospective faculty/trainees. Social media also is increasingly being used by clinicians to connect with other health care providers in their own specialty or other specialties. Digital communities have been created where ideas are shared and topics of interest are discussed. Clinicians can listen in on expert opinions, disseminate their research, and discuss practice management challenges or health advocacy. FIGURE 2 provides a snapshot of the social media landscape.

There is a wealth of options when it comes to social media platforms, including but not limited to Facebook, Twitter, LinkedIn, Instagram, YouTube, and blogs (TABLE 3). Facebook has the largest user base of all social media platforms, with approximately 1.7 billion active monthly users; thus, its use creates an opportunity to reach a massive audience.12,13 People use Facebook for both personal and professional reasons. The platform allows for sharing of photos, live videos, posted text, and comments. It can be used as a helpful resource to engage patients and disseminate accurate medical information. Importantly, remember that content posted should comply with the HIPAA Privacy Rule and that information shared should come from a credible source.
The Mayo Clinic is an impressive example of the use of social media for consumer education, research, and expansion of the reach of its brand. They incorporated social media into their strategic marketing plan, and between 2015 and 2016, social media referrals led to a 139% increase in patient appointment requests.13 Of the 20 different social media sites used, Facebook was the top social media referrer, accounting for 81% of social media referrals in 2015 and 88% in 2016. They have expanded their reach through different social media platforms and have more than 1.5 million followers on Twitter. Their videos on YouTube were viewed more than 4.9 million times in 2016 alone. This example illustrates social media’s effectiveness and the potential role it can play in connecting with patients.
Final thoughts
The practice of medicine has undeniably changed over the years and will continue to evolve. Understanding how to implement change to ensure that high-quality, efficient patient care is being delivered is paramount.
We have highlighted various aspects of practice management that you can use to overcome current obstacles and changing standards. The advent of telemedicine has provided easy access to clinicians. Consultation occurs in the comfort of the patient’s home, and the ability to provide local examination telecast to a clinician allows physicians and advanced practice practitioners to reach a wider range of patients. Social media has established an infrastructure for educating patients and providers while at the same time conveying educational tools to patients. This level of communication will continue to expand as time progresses.
Practitioners have a whole new cadre to add to their toolbox to provide patients with state-of-the-art communication and care. ●
Over the past decade, the use of technology with the focus on optimizing the consumer experience has exploded throughout numerous industries, including education, retail, and entertainment. Within health care, we would be naïve to ignore patient expectations for an optimized consumer experience within our offices. Thus, clinicians across all health care disciplines must remain cognizant of and work to optimize the patient experience in the ever-expanding world of health care.
Reengineering one’s practice will continue to be a work in progress. As medicine and technology continuously advance, clinicians must be able to adapt and implement changes. An excellent example of such adaptation is the use of telemedicine during the COVID-19 pandemic.1 We hope that the use of telemedicine remains an integral part of our armamentarium as we move forward.
In this article, we offer perspectives on using telemedicine, improving the patient experience, and implementing the use of social media in your practice. We look for a common denominator when provision of clinical care is the topic of discussion. Knowing the details of your medical practice and addressing its highlights as well as its concerns will benefit patients, staff, and health care providers. We hope that you glean some insights that you can apply in your practice.
Telemedicine: Part of the new normal
The American College of Obstetricians and Gynecologists defines telehealth as a “technology-enhanced health care framework that includes services, such as virtual visits, remote patient monitoring and mobile health care.”2 The American Telemedicine Association and the World Health Organization use the terms telemedicine and telehealth interchangeably.3 We live in a relatively new era since the COVID-19 pandemic necessitated that traditional face-to-face meeting(s) with patients be conducted virtually. The good news is that the outcomes with telehealth visits appear to be on par with those of traditional office visits.4
Telehealth allows clinicians to deliver medical evaluation and management plans right in a patient’s home and to receive appropriate reimbursement for doing so. This is a result of actions by Congress and the Department of Health and Human Services that removed restrictions related to telemedicine.5 The telemedicine approach provides a different perspective on provision of care (FIGURE 1).

For telemedicine practice, prerequisites include having the appropriate hardware, software, and a secure internet connection to maintain quality and patient safety.4 It is wise to check with regulatory laws at the local, state, and federal levels, as some states have separate licensure requirements for delivering this type of health care. Review insurance carrier guidelines as well as medical malpractice coverage for telehealth care provision. Ideally, obtain proof in writing from third-party payers and malpractice insurance carriers. TABLE 1 lists ObGyn-related activities and services that can be provided via telemedicine.3
While in many circumstances the indications for telemedicine are obvious, some remain less apparent. For example, patients may be more receptive to the use of telemedicine for counseling and education for family planning services and termination of pregnancy.6 Psychological counseling lends itself to a telemedicine approach to address levels of anxiety and depression, especially in the postpartum setting.
An initial telemedicine consultation often is complemented by subsequent patient examination when deemed necessary. Pelvic imaging often is ordered to address concerns expressed during the telemedicine visit. Teleradiology is an interesting aspect of telemedicine that is expanding. Telesonography, the use of ultrasonography, is extremely relevant to obstetrics and gynecology. Specifically, the development of self-operated endovaginal telemonitors and 3D as well as 4D imaging incorporates self-operated endovaginal telemonitoring. This technology remains a work in progress.7
Another aspect to telemedicine is telesurgery. Although an operative procedure cannot be performed virtually, pre- and postoperative counseling can be provided via telemedicine, offering tremendous convenience to patients.
Understanding the infrastructure of telemedicine and assuring security, adherence with HIPAA (Health Insurance Portability and Accountability Act), state licensure, reimbursement, and medical malpractice aspects is well worth the effort.
Continue to: Reengineer your office to enhance the patient experience...
Reengineer your office to enhance the patient experience
Create a hospitable environment. One way to do this is by having your front desk staffer standing up to greet patients. The medical management literature has reported an interesting analogy.8 Picture going to a retailer whose job is to sell you the product you are interested in. Where is that person positioned? Standing at the counter, at eye level with you, doing his or her best to convince you to buy a particular product. Having your office front desk personnel standing is analogous to the “atmosphere (when approaching the front desk) that conveys clear energy and a clear tone or readiness,” all of which contribute to a more positive patient experience.8
A hospitable environment at the check-in desk sets the stage for the office visit. When a staffer is sitting at the front desk office entrance point, the concept conveyed to the patient is, “You can wait for us because you need us more than we need you.” Changing the staffer’s posture to a standing position conveys, “Welcome, we are glad to see you and address why you are here.”8
Conduct a flow analysis of your office procedures. It is clear that the front desk serves as an advertisement of what your practice has to offer. A friendly smile from the receptionist goes a long way. In addition, the total time from patient check-in to checkout should be monitored. Having this type of data aids staff evaluation and patient satisfaction.9
Examine your office’s aspects of what the business world calls throughput. In essence, problems related to throughput include that the clinician is chronically late or slow with patients or that inadequate time was allocated per patient visit or per procedure.
It is valuable to allocate staff resources ahead of time, including patient registration and insurance verification details. Staff records review and preparation for the clinician streamlines time with the patient. Having lab tests, other consultations, and so on readily available for the clinician is time well spent by the medical assistants. For procedures, preparation of equipment that is in good working order and having supplies appropriately stocked can help facilitate success and efficiency. Creation of an “electronic on-time board” displays if the clinician is running on time or not.9 These practical tips can result in better patient and staff satisfaction. In addition, periodic surveys help engage patients in the process. TABLE 2 provides sample survey questions to ask patients.10
Taking a careful look at your current office practices and reengineering them as needed is an investment that provides an excellent return.
Continue to: Develop a presence on social media...
Develop a presence on social media
Having a social media presence is becoming one of the most effective strategies for reaching an intended audience. In the United States, more than 70% of the public uses at least one social media platform.11 It can be an effective and efficient tool for clinicians to grow their practice; distribute information about unique areas of the practice; and reach potential patients, referring physicians, and prospective faculty/trainees. Social media also is increasingly being used by clinicians to connect with other health care providers in their own specialty or other specialties. Digital communities have been created where ideas are shared and topics of interest are discussed. Clinicians can listen in on expert opinions, disseminate their research, and discuss practice management challenges or health advocacy. FIGURE 2 provides a snapshot of the social media landscape.

There is a wealth of options when it comes to social media platforms, including but not limited to Facebook, Twitter, LinkedIn, Instagram, YouTube, and blogs (TABLE 3). Facebook has the largest user base of all social media platforms, with approximately 1.7 billion active monthly users; thus, its use creates an opportunity to reach a massive audience.12,13 People use Facebook for both personal and professional reasons. The platform allows for sharing of photos, live videos, posted text, and comments. It can be used as a helpful resource to engage patients and disseminate accurate medical information. Importantly, remember that content posted should comply with the HIPAA Privacy Rule and that information shared should come from a credible source.
The Mayo Clinic is an impressive example of the use of social media for consumer education, research, and expansion of the reach of its brand. They incorporated social media into their strategic marketing plan, and between 2015 and 2016, social media referrals led to a 139% increase in patient appointment requests.13 Of the 20 different social media sites used, Facebook was the top social media referrer, accounting for 81% of social media referrals in 2015 and 88% in 2016. They have expanded their reach through different social media platforms and have more than 1.5 million followers on Twitter. Their videos on YouTube were viewed more than 4.9 million times in 2016 alone. This example illustrates social media’s effectiveness and the potential role it can play in connecting with patients.
Final thoughts
The practice of medicine has undeniably changed over the years and will continue to evolve. Understanding how to implement change to ensure that high-quality, efficient patient care is being delivered is paramount.
We have highlighted various aspects of practice management that you can use to overcome current obstacles and changing standards. The advent of telemedicine has provided easy access to clinicians. Consultation occurs in the comfort of the patient’s home, and the ability to provide local examination telecast to a clinician allows physicians and advanced practice practitioners to reach a wider range of patients. Social media has established an infrastructure for educating patients and providers while at the same time conveying educational tools to patients. This level of communication will continue to expand as time progresses.
Practitioners have a whole new cadre to add to their toolbox to provide patients with state-of-the-art communication and care. ●
- Anifandis G, Tempest H, Oliva R, et al. COVID-19 and human reproduction: a pandemic that packs a serious punch. Systems Biol Reprod Med. 2021;67:3-23.
- American College of Obstetricians and Gynecologists Presidential Task Force on Telehealth. Implementing telehealth in practice: ACOG Committee Opinion No. 798. Obstet Gynecol. 2020;135:e73-e79.
- Lee S, Hitt WC. Clinical applications of telemedicine in gynecology and women’s health. Obstet Gynecol Clin North Am. 2020;47:259-270.
- DeNicola N, Grossman D, Marko K, et al. Telehealth interventions to improve obstetrics and gynecologic health outcomes: a systematic review. Obstet Gynecol. 2020;135:371-382.
- Keesara S, Jonas A, Schulman K. Covid-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82.
- Grossman D, Grindlay K. Safety of medical abortion provided through telemedicine compared with in person. Obstet Gynecol. 2017;130:778-782.
- Pereira I, von Horn K, Depebusch M, et al. Self-operated endovaginal telemonitoring: a prospective clinical validation study. Fertil Steril. 2016;106:306-310e1.
- Massey GG, Hunter DG. Enhancing the patient experience with stand-up check-in. MGMA Connex. 2016;34-36.
- The patient experience, from check-in to check out. MGMA Connex. 2017;17:45-46.
- Swankoski KE, Peikes DN, Morrison N, et al. Patient experience during a large primary care practice transformation initiative. Am J Manag Care. 2018;24:607-613.
- Pew Research Center. Social media fact sheet. https://www .pewinternet.org/fact-sheet/social-media/. April 7. 2021. Accessed September 21, 2021.
- Small Business Trends website. Mansfield M. Social media statistics 2016. https://smallbiztrends.com/2016/11/social -media-statistics-2016.html. Updated June 4, 2021. Accessed September 21, 2021.
- Kotsenas AL, Arce M, Aase L, et al. The strategic imperative for the use of social media in health care. J Am Coll Radiol. 2018;15(1 pt B):155-161.
- Anifandis G, Tempest H, Oliva R, et al. COVID-19 and human reproduction: a pandemic that packs a serious punch. Systems Biol Reprod Med. 2021;67:3-23.
- American College of Obstetricians and Gynecologists Presidential Task Force on Telehealth. Implementing telehealth in practice: ACOG Committee Opinion No. 798. Obstet Gynecol. 2020;135:e73-e79.
- Lee S, Hitt WC. Clinical applications of telemedicine in gynecology and women’s health. Obstet Gynecol Clin North Am. 2020;47:259-270.
- DeNicola N, Grossman D, Marko K, et al. Telehealth interventions to improve obstetrics and gynecologic health outcomes: a systematic review. Obstet Gynecol. 2020;135:371-382.
- Keesara S, Jonas A, Schulman K. Covid-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82.
- Grossman D, Grindlay K. Safety of medical abortion provided through telemedicine compared with in person. Obstet Gynecol. 2017;130:778-782.
- Pereira I, von Horn K, Depebusch M, et al. Self-operated endovaginal telemonitoring: a prospective clinical validation study. Fertil Steril. 2016;106:306-310e1.
- Massey GG, Hunter DG. Enhancing the patient experience with stand-up check-in. MGMA Connex. 2016;34-36.
- The patient experience, from check-in to check out. MGMA Connex. 2017;17:45-46.
- Swankoski KE, Peikes DN, Morrison N, et al. Patient experience during a large primary care practice transformation initiative. Am J Manag Care. 2018;24:607-613.
- Pew Research Center. Social media fact sheet. https://www .pewinternet.org/fact-sheet/social-media/. April 7. 2021. Accessed September 21, 2021.
- Small Business Trends website. Mansfield M. Social media statistics 2016. https://smallbiztrends.com/2016/11/social -media-statistics-2016.html. Updated June 4, 2021. Accessed September 21, 2021.
- Kotsenas AL, Arce M, Aase L, et al. The strategic imperative for the use of social media in health care. J Am Coll Radiol. 2018;15(1 pt B):155-161.