User login
Comparison of Adverse Events With Vancomycin Diluted in Normal Saline vs Dextrose 5%
Vancomycin is a widely used IV antibiotic due to its broad-spectrum of activity, bactericidal nature, and low rates of resistance; however, adverse effects (AEs), including nephrotoxicity, are commonly associated with its use.1 The vancomycin therapeutic monitoring guidelines recognize the incidence of nephrotoxicity and suggest strategies for reducing the risk, including area under the curve/mean inhibitory concentration (AUC/MIC) monitoring rather than trough-only monitoring. Vancomycin-associated acute kidney injury (AKI) has been defined as an increase in serum creatinine (SCr) over a 48-hour period of ≥ 0.3 mg/dL or a percentage increase of ≥ 50%, which is consistent with the Acute Kidney Injury Network (AKIN) guidelines.2,3 Vancomycin-associated AKI is a common AE, with its incidence reported in previous studies ranging from 10 to 20%.4,5
The most common crystalloid fluid administered to patients in the United States is 0.9% sodium chloride (NaCl), also known as normal saline (NS), and recent trials have explored its potential to cause AEs.6-8 Balanced crystalloid solutions, such as Plasma-Lyte and lactated Ringer’s solution (LR), contain buffering agents and lower concentrations of sodium and chloride compared with that of NS. Trials in the intensive care unit (ICU) and emergency department, such as the SMART-MED, SMART-SURG, and SALT-ED have reported a significantly lower rate of AKI when using balanced crystalloids compared with NS due to the concentration of sodium and chloride in NS being supraphysiologic to normal serum concentrations.6,7 Alternatively, the SPLIT trial evaluated the use of NS compared with Plasma-Lyte for ICU fluid therapy and did not find a statistically significant difference in AKI.8 Furthermore, some studies have reported increased risk for hyperchloremia when using NS compared with dextrose 5% in water (D5W) or balanced crystalloids, which can result in metabolic acidosis.6,7,9,10 These studies have shown how the choice of fluid can have a large effect on the incidence of AEs; bringing into question whether these effects could be additive when combined with the nephrotoxicity associated with vancomycin.6-9
Vancomycin is physically and chemically stable if diluted in D5W, NS, 5% dextrose in NS, LR, or 5% dextrose in LR.1 It is not known whether the selection of diluent has an effect on nephrotoxicity or other AEs of vancomycin therapy. Furthermore, clinicians may be unaware or unable to specify which diluent to use. There are currently no practice guidelines that favor one diluent over another for vancomycin; however, trials showing higher rates of AKI and hyperchloremia using NS for fluid resuscitation may indicate an increased potential for vancomycin-associated AKI when using NS as a diluent.6,7,9 This study was performed to evaluate whether the type of crystalloid used (D5W vs NS) can influence adverse outcomes for patients. While many factors may contribute to these AEs, the potential to reduce the risk of negative adverse outcomes for hospitalized patients is a significant area of exploration.
The primary outcome of this study was the incidence of AKI, defined using AKIN guidelines where the increase in SCr occurred at least 24 hours after starting vancomycin and within 36 hours of receiving the last vancomycin dose.3 AKI was staged using the AKIN guidelines (stage 1: increase in SCr of ≥ 0.3 mg/dL or by 50 to 99%; stage 2: increase in SCr by 100 to 199%; stage 3: increase in SCr by > 200%) based on changes in SCr from baseline during vancomycin therapy or within 36 hours of stopping vancomycin therapy.3 Secondary outcomes included the incidence of hyperglycemia, hyperchloremia, metabolic acidosis, hypernatremia, mortality in hospital, and mortality within 30 days from hospital discharge.
Methods
This single-center, retrospective study of veterans who received IV vancomycin within the North Florida/South Georgia Veterans Health System (NF/SGVHS) in Gainesville, Florida, from July 1, 2015 to June 30, 2020, compared veterans who received vancomycin diluted in NS with those who received vancomycin diluted in D5W to assess for differences in AEs, including AKI, metabolic acidosis (serum bicarbonate level < 23 mmol/L), hyperchloremia (serum chloride levels > 108 mmol/L), hypernatremia (serum sodium > 145 mmol/L), and hyperglycemia (blood glucose > 180 mg/dL). The endpoint values were defined using the reference ranges determined by the local laboratory. At NF/SGVHS, vancomycin is diluted in D5W or NS based primarily on factors such as product availability and cost.
Study Criteria
Veterans were included if they received IV vancomycin between July 1, 2015 and June 30, 2020. The cohorts were grouped into those receiving vancomycin doses diluted in NS and those receiving vancomycin doses diluted in D5W. Veterans were excluded if they received < 80% of vancomycin doses diluted in their respective fluid, if they were on vancomycin for < 48 hours, or if they did not have laboratory results collected both before and after vancomycin therapy to assess a change. There were more patients receiving vancomycin in D5W, so a random sample was selected to have an equal size comparison group with those receiving NS. A sample size calculation was performed with an anticipated AKI incidence of 14%.5 To detect a 10% difference in the primary outcome with an α of 0.05 and 75% power, 226 patients (113 in each cohort) were needed for inclusion.
Data were collected using the Data Access Request Tracker tool through the US Department of Veterans Affairs (VA) Informatics and Computing Infrastructure. Data collected included demographics, laboratory data at baseline and during vancomycin therapy, characteristics of antibiotic therapy, and mortality data. Of note, all laboratory values assessed in this study were obtained while the veteran was receiving vancomycin or within 36 hours of receiving the last vancomycin dose to appropriately assess any changes.
Statistical analysis of categorical data were analyzed using a χ2 test on the GraphPad online program. This study received institutional review board approval from the University of Florida and was conducted in accordance with protections for human subjects.
Results
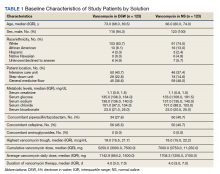
A total of 792 veterans received IV vancomycin NF/SGVHS in the defined study period. Of these, 381 veterans were excluded, including having < 80% of doses in a single solution (213 veterans), receiving IV vancomycin for < 48 hours (149 veterans), and not having necessary laboratory data available to assess a change in kidney function (19 veterans). An additional 165 veterans were randomly excluded from the D5W cohort in order to have an equal comparison group to the NS cohort; therefore, a total of 246 veterans were included in the final assessment (123 veterans in each cohort). The median patient age was 73 years (IQR, 68.0, 80.5) in the D5W group and 66 years (IQR, 60.0, 74.0) in the NS group; 83.7% of veterans in the D5W group and 74% veterans in the NS group were white; 94.3% of the D5W group and 100% of the NS group were male (Table 1).
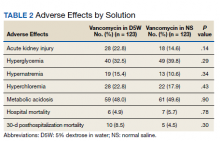
The percentage of AKI in the D5W group was 22.8% compared with 14.6% in the NS group (P = .14), and all cases were classified as stage 1 AKI. Baseline cases of hyperglycemia, hypernatremia, hyperchloremia, or metabolic acidosis were not included in the reported rates of each in order to determine a change during vancomycin therapy (Table 2).
The percentage of patients with hyperglycemia in the D5W group was 32.5% compared with 39.8% in the NS group (P = .29). The percentage of patients with hypernatremia in the D5W group was 15.4% compared with 10.6% in the NS group (P = .34). The percentage of patients with hyperchloremia in the D5W group was 22.8% compared with 17.9% in the NS group (P = .43). The percentage of patients with metabolic acidosis in the D5W group was 48.0% compared with 49.6% in the NS group (P = .90).
There were no significant differences in either in-hospital or posthospital mortality between the D5W and NS groups (in-hospital: 4.9% vs 5.7%, respectively; P = .78; 30-day posthospitalization: 8.5% vs 4.5%, respectively; P = .30).
Discussion
This retrospective cohort study comparing the AEs of vancomycin diluted in NS and vancomycin diluted with D5W showed no statistically significant differences in the incidence of AKI or any metabolic AEs. Although these results did not show an association between the incidence of AEs and the dilution fluid for vancomycin, other factors may contribute to the overall incidence of AEs. Factors such as cumulative vancomycin dose, duration of therapy, and presence of concomitant nephrotoxins have been known to increase the incidence of AKI and may have a greater impact on this incidence than the fluid used in administering the vancomycin.
These results specifically the incidence of AKI were not consistent with previous trials evaluating the AEs of NS. Based on previous trials, we expected the vancomycin in the NS cohort to have a significantly higher incidence of hypernatremia, hyperchloremia, and AKI. Our results may indicate that the volume of crystalloid received played a greater role on the incidence of AEs. Our study assessed the effect of a diluent for one IV medication that may have been only a few hundred milliliters of fluid per day. The total volume of IV fluid received from vancomycin was not assessed; thus, it is not known how the volume of fluid may have impacted the results.
One consideration with this study is the method used for monitoring vancomycin levels. Most of the patients included in this study were admitted prior to the release of the updated vancomycin guidelines, which advocated for the transition from traditional trough-only monitoring to AUC/MIC. In September 2019, NF/SGVHS ICUs made the transition to this new method of monitoring with a hospital-wide transition following the study end date. The D5W group had a slightly higher percentage of patients admitted to the ICU, thus were more likely to be monitored using AUC/MIC during this period. Literature has shown the AUC/MIC method of monitoring can result in a decreased daily dose, decreased trough levels, and decreased incidence of nephrotoxicity.11-14 Although the method for monitoring vancomycin has the potential to affect the incidence of AKI, the majority of patients were monitored using the traditional trough-only method with similar trough levels reported in both groups.
Limitations
This study is limited by its retrospective nature, the potential introduction of biases, and the inability to control for confounders that may have influenced the incidence of AEs. Potential confounders present in this study included the use of concomitant nephrotoxic medications, vancomycin dose, and underlying conditions, as these could have impacted the overall incidence of AEs.
The combination of piperacillin/tazobactam plus vancomycin has commonly been associated with an increased risk of nephrotoxicity. Previous studies have identified this nephrotoxic combination to have a significantly increased risk of AKI compared with vancomycin alone or when used in combination with alternative antibiotics such as cefepime or meropenem.15,16 In our study, there was a higher percentage of patients in the NS group with concomitant piperacillin/tazobactam, so this difference between the groups may have influenced the incidence of AKI. Nephrotoxic medications other than antibiotics were not assessed in this study; however, these also could have impacted our results significantly. While the vancomycin duration of therapy and highest trough levels were similar between groups, the NS group had a larger average daily dose and overall cumulative dose. Studies have identified the risk of nephrotoxicity increases with a vancomycin daily dose of 4 g, troughs > 15 mg/mL, and a duration of therapy > 7 days.15,16 In our study, the daily doses in both groups were < 4 g, so it is likely the average daily vancomycin dose had little impact on the incidence of AKI.
Another potential confounder identified was assessment of underlying conditions in the patients. Due to the limitations associated with the data extraction method, we could not assess for underlying conditions that may have impacted the results. Notably, the potential nephrotoxicity of NS has mostly been shown in critically ill patients. Therefore, the mixed acutely ill patient sample in this study may have been less likely to develop AKI from NS compared with an exclusively critically ill patient sample.
Selection bias and information bias are common with observational studies. In our study, selection bias may have been present since prospective randomization of patient samples was not possible. Since all data were extracted from the medical health record, information bias may have been present with the potential to impact the results. Due to the single-center nature of this study with a predominantly older, white male veteran patient sample, generalizability to other patient populations may be limited. We would expect the results of this study to be similar among other patient populations of a similar age and demographic; however, the external validity of this study may be weak among other populations. Although this study included enough patients based on sample size estimate, a larger sample size could have allowed for detection of smaller differences between groups and decreased the chance for type II error.
Conclusions
Overall, the results of this study do not suggest that the crystalloid used to dilute IV vancomycin is associated with differences in nephrotoxicity or other relevant AEs. Future studies evaluating the potential for AEs from medication diluent are warranted and would benefit from a prospective, randomized design. Further studies are both necessary and crucial for enhancing the quality of care to minimize the rates of AEs of commonly used medications.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Vancomycin hydrochloride intravenous injection, pharmacy bulk package. Package insert. Schaumburg, IL: APP Pharmaceuticals, LLC; 2011.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-System Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
4. Elaysi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations–a literature review. Eur J Clin Pharmacol. 2012;68(9):1243-1255. doi:10.1007/s00228-012-1259-9
5. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-associated acute kidney injury in a large veteran population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
6. Semler MW, Self WH, Wanderer JB, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584
7. Self WH, Semler MW, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(20):819-828. doi:10.1056/NEJMc1804294
8. Young P, Bailey M, Beasley R, et al; SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT Randomized Clinical Trial. JAMA. 2015;314(16):1701-1710. doi:10.1001/jama.2015.12334
9. Magee CA, Bastin ML, Bastin T, et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit Care Med. 2018;46(8):1217-1223. doi:10.1097/CCM.0000000000003191
10. Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal. 2014;2014:627673. doi:10.1155/2014/627673
11. Mcgrady KA, Benton M, Tart S, Bowers R. Evaluation of traditional vancomycin dosing versus utilizing an electronic AUC/MIC dosing program. Pharm Pract (Granada). 2020;18(3):2024. doi:10.18549/PharmPract.2020.3.2024
12. Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther Drug Monit. 2019;41(4):483-488. doi:10.1097/FTD.0000000000000622
13. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. doi:10.1128/AAC.02042-17
14. Aljefri DM, Avedissian SN, Youn G, et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881-1887. doi:10.1128/AAC.02042-17
15. Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The risk of acute kidney injury in critically ill patients receiving concomitant vancomycin with piperacillin-tazobactam or cefepime. J Intensive Care Med. 2019;35(12):1434-1438. doi:10.1177/0885066619828290
16. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014; 34(7):670-676. doi:10.1002/phar.1442
Vancomycin is a widely used IV antibiotic due to its broad-spectrum of activity, bactericidal nature, and low rates of resistance; however, adverse effects (AEs), including nephrotoxicity, are commonly associated with its use.1 The vancomycin therapeutic monitoring guidelines recognize the incidence of nephrotoxicity and suggest strategies for reducing the risk, including area under the curve/mean inhibitory concentration (AUC/MIC) monitoring rather than trough-only monitoring. Vancomycin-associated acute kidney injury (AKI) has been defined as an increase in serum creatinine (SCr) over a 48-hour period of ≥ 0.3 mg/dL or a percentage increase of ≥ 50%, which is consistent with the Acute Kidney Injury Network (AKIN) guidelines.2,3 Vancomycin-associated AKI is a common AE, with its incidence reported in previous studies ranging from 10 to 20%.4,5
The most common crystalloid fluid administered to patients in the United States is 0.9% sodium chloride (NaCl), also known as normal saline (NS), and recent trials have explored its potential to cause AEs.6-8 Balanced crystalloid solutions, such as Plasma-Lyte and lactated Ringer’s solution (LR), contain buffering agents and lower concentrations of sodium and chloride compared with that of NS. Trials in the intensive care unit (ICU) and emergency department, such as the SMART-MED, SMART-SURG, and SALT-ED have reported a significantly lower rate of AKI when using balanced crystalloids compared with NS due to the concentration of sodium and chloride in NS being supraphysiologic to normal serum concentrations.6,7 Alternatively, the SPLIT trial evaluated the use of NS compared with Plasma-Lyte for ICU fluid therapy and did not find a statistically significant difference in AKI.8 Furthermore, some studies have reported increased risk for hyperchloremia when using NS compared with dextrose 5% in water (D5W) or balanced crystalloids, which can result in metabolic acidosis.6,7,9,10 These studies have shown how the choice of fluid can have a large effect on the incidence of AEs; bringing into question whether these effects could be additive when combined with the nephrotoxicity associated with vancomycin.6-9
Vancomycin is physically and chemically stable if diluted in D5W, NS, 5% dextrose in NS, LR, or 5% dextrose in LR.1 It is not known whether the selection of diluent has an effect on nephrotoxicity or other AEs of vancomycin therapy. Furthermore, clinicians may be unaware or unable to specify which diluent to use. There are currently no practice guidelines that favor one diluent over another for vancomycin; however, trials showing higher rates of AKI and hyperchloremia using NS for fluid resuscitation may indicate an increased potential for vancomycin-associated AKI when using NS as a diluent.6,7,9 This study was performed to evaluate whether the type of crystalloid used (D5W vs NS) can influence adverse outcomes for patients. While many factors may contribute to these AEs, the potential to reduce the risk of negative adverse outcomes for hospitalized patients is a significant area of exploration.
The primary outcome of this study was the incidence of AKI, defined using AKIN guidelines where the increase in SCr occurred at least 24 hours after starting vancomycin and within 36 hours of receiving the last vancomycin dose.3 AKI was staged using the AKIN guidelines (stage 1: increase in SCr of ≥ 0.3 mg/dL or by 50 to 99%; stage 2: increase in SCr by 100 to 199%; stage 3: increase in SCr by > 200%) based on changes in SCr from baseline during vancomycin therapy or within 36 hours of stopping vancomycin therapy.3 Secondary outcomes included the incidence of hyperglycemia, hyperchloremia, metabolic acidosis, hypernatremia, mortality in hospital, and mortality within 30 days from hospital discharge.
Methods
This single-center, retrospective study of veterans who received IV vancomycin within the North Florida/South Georgia Veterans Health System (NF/SGVHS) in Gainesville, Florida, from July 1, 2015 to June 30, 2020, compared veterans who received vancomycin diluted in NS with those who received vancomycin diluted in D5W to assess for differences in AEs, including AKI, metabolic acidosis (serum bicarbonate level < 23 mmol/L), hyperchloremia (serum chloride levels > 108 mmol/L), hypernatremia (serum sodium > 145 mmol/L), and hyperglycemia (blood glucose > 180 mg/dL). The endpoint values were defined using the reference ranges determined by the local laboratory. At NF/SGVHS, vancomycin is diluted in D5W or NS based primarily on factors such as product availability and cost.
Study Criteria
Veterans were included if they received IV vancomycin between July 1, 2015 and June 30, 2020. The cohorts were grouped into those receiving vancomycin doses diluted in NS and those receiving vancomycin doses diluted in D5W. Veterans were excluded if they received < 80% of vancomycin doses diluted in their respective fluid, if they were on vancomycin for < 48 hours, or if they did not have laboratory results collected both before and after vancomycin therapy to assess a change. There were more patients receiving vancomycin in D5W, so a random sample was selected to have an equal size comparison group with those receiving NS. A sample size calculation was performed with an anticipated AKI incidence of 14%.5 To detect a 10% difference in the primary outcome with an α of 0.05 and 75% power, 226 patients (113 in each cohort) were needed for inclusion.
Data were collected using the Data Access Request Tracker tool through the US Department of Veterans Affairs (VA) Informatics and Computing Infrastructure. Data collected included demographics, laboratory data at baseline and during vancomycin therapy, characteristics of antibiotic therapy, and mortality data. Of note, all laboratory values assessed in this study were obtained while the veteran was receiving vancomycin or within 36 hours of receiving the last vancomycin dose to appropriately assess any changes.
Statistical analysis of categorical data were analyzed using a χ2 test on the GraphPad online program. This study received institutional review board approval from the University of Florida and was conducted in accordance with protections for human subjects.
Results
A total of 792 veterans received IV vancomycin NF/SGVHS in the defined study period. Of these, 381 veterans were excluded, including having < 80% of doses in a single solution (213 veterans), receiving IV vancomycin for < 48 hours (149 veterans), and not having necessary laboratory data available to assess a change in kidney function (19 veterans). An additional 165 veterans were randomly excluded from the D5W cohort in order to have an equal comparison group to the NS cohort; therefore, a total of 246 veterans were included in the final assessment (123 veterans in each cohort). The median patient age was 73 years (IQR, 68.0, 80.5) in the D5W group and 66 years (IQR, 60.0, 74.0) in the NS group; 83.7% of veterans in the D5W group and 74% veterans in the NS group were white; 94.3% of the D5W group and 100% of the NS group were male (Table 1).
The percentage of AKI in the D5W group was 22.8% compared with 14.6% in the NS group (P = .14), and all cases were classified as stage 1 AKI. Baseline cases of hyperglycemia, hypernatremia, hyperchloremia, or metabolic acidosis were not included in the reported rates of each in order to determine a change during vancomycin therapy (Table 2).
The percentage of patients with hyperglycemia in the D5W group was 32.5% compared with 39.8% in the NS group (P = .29). The percentage of patients with hypernatremia in the D5W group was 15.4% compared with 10.6% in the NS group (P = .34). The percentage of patients with hyperchloremia in the D5W group was 22.8% compared with 17.9% in the NS group (P = .43). The percentage of patients with metabolic acidosis in the D5W group was 48.0% compared with 49.6% in the NS group (P = .90).
There were no significant differences in either in-hospital or posthospital mortality between the D5W and NS groups (in-hospital: 4.9% vs 5.7%, respectively; P = .78; 30-day posthospitalization: 8.5% vs 4.5%, respectively; P = .30).
Discussion
This retrospective cohort study comparing the AEs of vancomycin diluted in NS and vancomycin diluted with D5W showed no statistically significant differences in the incidence of AKI or any metabolic AEs. Although these results did not show an association between the incidence of AEs and the dilution fluid for vancomycin, other factors may contribute to the overall incidence of AEs. Factors such as cumulative vancomycin dose, duration of therapy, and presence of concomitant nephrotoxins have been known to increase the incidence of AKI and may have a greater impact on this incidence than the fluid used in administering the vancomycin.
These results specifically the incidence of AKI were not consistent with previous trials evaluating the AEs of NS. Based on previous trials, we expected the vancomycin in the NS cohort to have a significantly higher incidence of hypernatremia, hyperchloremia, and AKI. Our results may indicate that the volume of crystalloid received played a greater role on the incidence of AEs. Our study assessed the effect of a diluent for one IV medication that may have been only a few hundred milliliters of fluid per day. The total volume of IV fluid received from vancomycin was not assessed; thus, it is not known how the volume of fluid may have impacted the results.
One consideration with this study is the method used for monitoring vancomycin levels. Most of the patients included in this study were admitted prior to the release of the updated vancomycin guidelines, which advocated for the transition from traditional trough-only monitoring to AUC/MIC. In September 2019, NF/SGVHS ICUs made the transition to this new method of monitoring with a hospital-wide transition following the study end date. The D5W group had a slightly higher percentage of patients admitted to the ICU, thus were more likely to be monitored using AUC/MIC during this period. Literature has shown the AUC/MIC method of monitoring can result in a decreased daily dose, decreased trough levels, and decreased incidence of nephrotoxicity.11-14 Although the method for monitoring vancomycin has the potential to affect the incidence of AKI, the majority of patients were monitored using the traditional trough-only method with similar trough levels reported in both groups.
Limitations
This study is limited by its retrospective nature, the potential introduction of biases, and the inability to control for confounders that may have influenced the incidence of AEs. Potential confounders present in this study included the use of concomitant nephrotoxic medications, vancomycin dose, and underlying conditions, as these could have impacted the overall incidence of AEs.
The combination of piperacillin/tazobactam plus vancomycin has commonly been associated with an increased risk of nephrotoxicity. Previous studies have identified this nephrotoxic combination to have a significantly increased risk of AKI compared with vancomycin alone or when used in combination with alternative antibiotics such as cefepime or meropenem.15,16 In our study, there was a higher percentage of patients in the NS group with concomitant piperacillin/tazobactam, so this difference between the groups may have influenced the incidence of AKI. Nephrotoxic medications other than antibiotics were not assessed in this study; however, these also could have impacted our results significantly. While the vancomycin duration of therapy and highest trough levels were similar between groups, the NS group had a larger average daily dose and overall cumulative dose. Studies have identified the risk of nephrotoxicity increases with a vancomycin daily dose of 4 g, troughs > 15 mg/mL, and a duration of therapy > 7 days.15,16 In our study, the daily doses in both groups were < 4 g, so it is likely the average daily vancomycin dose had little impact on the incidence of AKI.
Another potential confounder identified was assessment of underlying conditions in the patients. Due to the limitations associated with the data extraction method, we could not assess for underlying conditions that may have impacted the results. Notably, the potential nephrotoxicity of NS has mostly been shown in critically ill patients. Therefore, the mixed acutely ill patient sample in this study may have been less likely to develop AKI from NS compared with an exclusively critically ill patient sample.
Selection bias and information bias are common with observational studies. In our study, selection bias may have been present since prospective randomization of patient samples was not possible. Since all data were extracted from the medical health record, information bias may have been present with the potential to impact the results. Due to the single-center nature of this study with a predominantly older, white male veteran patient sample, generalizability to other patient populations may be limited. We would expect the results of this study to be similar among other patient populations of a similar age and demographic; however, the external validity of this study may be weak among other populations. Although this study included enough patients based on sample size estimate, a larger sample size could have allowed for detection of smaller differences between groups and decreased the chance for type II error.
Conclusions
Overall, the results of this study do not suggest that the crystalloid used to dilute IV vancomycin is associated with differences in nephrotoxicity or other relevant AEs. Future studies evaluating the potential for AEs from medication diluent are warranted and would benefit from a prospective, randomized design. Further studies are both necessary and crucial for enhancing the quality of care to minimize the rates of AEs of commonly used medications.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Vancomycin is a widely used IV antibiotic due to its broad-spectrum of activity, bactericidal nature, and low rates of resistance; however, adverse effects (AEs), including nephrotoxicity, are commonly associated with its use.1 The vancomycin therapeutic monitoring guidelines recognize the incidence of nephrotoxicity and suggest strategies for reducing the risk, including area under the curve/mean inhibitory concentration (AUC/MIC) monitoring rather than trough-only monitoring. Vancomycin-associated acute kidney injury (AKI) has been defined as an increase in serum creatinine (SCr) over a 48-hour period of ≥ 0.3 mg/dL or a percentage increase of ≥ 50%, which is consistent with the Acute Kidney Injury Network (AKIN) guidelines.2,3 Vancomycin-associated AKI is a common AE, with its incidence reported in previous studies ranging from 10 to 20%.4,5
The most common crystalloid fluid administered to patients in the United States is 0.9% sodium chloride (NaCl), also known as normal saline (NS), and recent trials have explored its potential to cause AEs.6-8 Balanced crystalloid solutions, such as Plasma-Lyte and lactated Ringer’s solution (LR), contain buffering agents and lower concentrations of sodium and chloride compared with that of NS. Trials in the intensive care unit (ICU) and emergency department, such as the SMART-MED, SMART-SURG, and SALT-ED have reported a significantly lower rate of AKI when using balanced crystalloids compared with NS due to the concentration of sodium and chloride in NS being supraphysiologic to normal serum concentrations.6,7 Alternatively, the SPLIT trial evaluated the use of NS compared with Plasma-Lyte for ICU fluid therapy and did not find a statistically significant difference in AKI.8 Furthermore, some studies have reported increased risk for hyperchloremia when using NS compared with dextrose 5% in water (D5W) or balanced crystalloids, which can result in metabolic acidosis.6,7,9,10 These studies have shown how the choice of fluid can have a large effect on the incidence of AEs; bringing into question whether these effects could be additive when combined with the nephrotoxicity associated with vancomycin.6-9
Vancomycin is physically and chemically stable if diluted in D5W, NS, 5% dextrose in NS, LR, or 5% dextrose in LR.1 It is not known whether the selection of diluent has an effect on nephrotoxicity or other AEs of vancomycin therapy. Furthermore, clinicians may be unaware or unable to specify which diluent to use. There are currently no practice guidelines that favor one diluent over another for vancomycin; however, trials showing higher rates of AKI and hyperchloremia using NS for fluid resuscitation may indicate an increased potential for vancomycin-associated AKI when using NS as a diluent.6,7,9 This study was performed to evaluate whether the type of crystalloid used (D5W vs NS) can influence adverse outcomes for patients. While many factors may contribute to these AEs, the potential to reduce the risk of negative adverse outcomes for hospitalized patients is a significant area of exploration.
The primary outcome of this study was the incidence of AKI, defined using AKIN guidelines where the increase in SCr occurred at least 24 hours after starting vancomycin and within 36 hours of receiving the last vancomycin dose.3 AKI was staged using the AKIN guidelines (stage 1: increase in SCr of ≥ 0.3 mg/dL or by 50 to 99%; stage 2: increase in SCr by 100 to 199%; stage 3: increase in SCr by > 200%) based on changes in SCr from baseline during vancomycin therapy or within 36 hours of stopping vancomycin therapy.3 Secondary outcomes included the incidence of hyperglycemia, hyperchloremia, metabolic acidosis, hypernatremia, mortality in hospital, and mortality within 30 days from hospital discharge.
Methods
This single-center, retrospective study of veterans who received IV vancomycin within the North Florida/South Georgia Veterans Health System (NF/SGVHS) in Gainesville, Florida, from July 1, 2015 to June 30, 2020, compared veterans who received vancomycin diluted in NS with those who received vancomycin diluted in D5W to assess for differences in AEs, including AKI, metabolic acidosis (serum bicarbonate level < 23 mmol/L), hyperchloremia (serum chloride levels > 108 mmol/L), hypernatremia (serum sodium > 145 mmol/L), and hyperglycemia (blood glucose > 180 mg/dL). The endpoint values were defined using the reference ranges determined by the local laboratory. At NF/SGVHS, vancomycin is diluted in D5W or NS based primarily on factors such as product availability and cost.
Study Criteria
Veterans were included if they received IV vancomycin between July 1, 2015 and June 30, 2020. The cohorts were grouped into those receiving vancomycin doses diluted in NS and those receiving vancomycin doses diluted in D5W. Veterans were excluded if they received < 80% of vancomycin doses diluted in their respective fluid, if they were on vancomycin for < 48 hours, or if they did not have laboratory results collected both before and after vancomycin therapy to assess a change. There were more patients receiving vancomycin in D5W, so a random sample was selected to have an equal size comparison group with those receiving NS. A sample size calculation was performed with an anticipated AKI incidence of 14%.5 To detect a 10% difference in the primary outcome with an α of 0.05 and 75% power, 226 patients (113 in each cohort) were needed for inclusion.
Data were collected using the Data Access Request Tracker tool through the US Department of Veterans Affairs (VA) Informatics and Computing Infrastructure. Data collected included demographics, laboratory data at baseline and during vancomycin therapy, characteristics of antibiotic therapy, and mortality data. Of note, all laboratory values assessed in this study were obtained while the veteran was receiving vancomycin or within 36 hours of receiving the last vancomycin dose to appropriately assess any changes.
Statistical analysis of categorical data were analyzed using a χ2 test on the GraphPad online program. This study received institutional review board approval from the University of Florida and was conducted in accordance with protections for human subjects.
Results
A total of 792 veterans received IV vancomycin NF/SGVHS in the defined study period. Of these, 381 veterans were excluded, including having < 80% of doses in a single solution (213 veterans), receiving IV vancomycin for < 48 hours (149 veterans), and not having necessary laboratory data available to assess a change in kidney function (19 veterans). An additional 165 veterans were randomly excluded from the D5W cohort in order to have an equal comparison group to the NS cohort; therefore, a total of 246 veterans were included in the final assessment (123 veterans in each cohort). The median patient age was 73 years (IQR, 68.0, 80.5) in the D5W group and 66 years (IQR, 60.0, 74.0) in the NS group; 83.7% of veterans in the D5W group and 74% veterans in the NS group were white; 94.3% of the D5W group and 100% of the NS group were male (Table 1).
The percentage of AKI in the D5W group was 22.8% compared with 14.6% in the NS group (P = .14), and all cases were classified as stage 1 AKI. Baseline cases of hyperglycemia, hypernatremia, hyperchloremia, or metabolic acidosis were not included in the reported rates of each in order to determine a change during vancomycin therapy (Table 2).
The percentage of patients with hyperglycemia in the D5W group was 32.5% compared with 39.8% in the NS group (P = .29). The percentage of patients with hypernatremia in the D5W group was 15.4% compared with 10.6% in the NS group (P = .34). The percentage of patients with hyperchloremia in the D5W group was 22.8% compared with 17.9% in the NS group (P = .43). The percentage of patients with metabolic acidosis in the D5W group was 48.0% compared with 49.6% in the NS group (P = .90).
There were no significant differences in either in-hospital or posthospital mortality between the D5W and NS groups (in-hospital: 4.9% vs 5.7%, respectively; P = .78; 30-day posthospitalization: 8.5% vs 4.5%, respectively; P = .30).
Discussion
This retrospective cohort study comparing the AEs of vancomycin diluted in NS and vancomycin diluted with D5W showed no statistically significant differences in the incidence of AKI or any metabolic AEs. Although these results did not show an association between the incidence of AEs and the dilution fluid for vancomycin, other factors may contribute to the overall incidence of AEs. Factors such as cumulative vancomycin dose, duration of therapy, and presence of concomitant nephrotoxins have been known to increase the incidence of AKI and may have a greater impact on this incidence than the fluid used in administering the vancomycin.
These results specifically the incidence of AKI were not consistent with previous trials evaluating the AEs of NS. Based on previous trials, we expected the vancomycin in the NS cohort to have a significantly higher incidence of hypernatremia, hyperchloremia, and AKI. Our results may indicate that the volume of crystalloid received played a greater role on the incidence of AEs. Our study assessed the effect of a diluent for one IV medication that may have been only a few hundred milliliters of fluid per day. The total volume of IV fluid received from vancomycin was not assessed; thus, it is not known how the volume of fluid may have impacted the results.
One consideration with this study is the method used for monitoring vancomycin levels. Most of the patients included in this study were admitted prior to the release of the updated vancomycin guidelines, which advocated for the transition from traditional trough-only monitoring to AUC/MIC. In September 2019, NF/SGVHS ICUs made the transition to this new method of monitoring with a hospital-wide transition following the study end date. The D5W group had a slightly higher percentage of patients admitted to the ICU, thus were more likely to be monitored using AUC/MIC during this period. Literature has shown the AUC/MIC method of monitoring can result in a decreased daily dose, decreased trough levels, and decreased incidence of nephrotoxicity.11-14 Although the method for monitoring vancomycin has the potential to affect the incidence of AKI, the majority of patients were monitored using the traditional trough-only method with similar trough levels reported in both groups.
Limitations
This study is limited by its retrospective nature, the potential introduction of biases, and the inability to control for confounders that may have influenced the incidence of AEs. Potential confounders present in this study included the use of concomitant nephrotoxic medications, vancomycin dose, and underlying conditions, as these could have impacted the overall incidence of AEs.
The combination of piperacillin/tazobactam plus vancomycin has commonly been associated with an increased risk of nephrotoxicity. Previous studies have identified this nephrotoxic combination to have a significantly increased risk of AKI compared with vancomycin alone or when used in combination with alternative antibiotics such as cefepime or meropenem.15,16 In our study, there was a higher percentage of patients in the NS group with concomitant piperacillin/tazobactam, so this difference between the groups may have influenced the incidence of AKI. Nephrotoxic medications other than antibiotics were not assessed in this study; however, these also could have impacted our results significantly. While the vancomycin duration of therapy and highest trough levels were similar between groups, the NS group had a larger average daily dose and overall cumulative dose. Studies have identified the risk of nephrotoxicity increases with a vancomycin daily dose of 4 g, troughs > 15 mg/mL, and a duration of therapy > 7 days.15,16 In our study, the daily doses in both groups were < 4 g, so it is likely the average daily vancomycin dose had little impact on the incidence of AKI.
Another potential confounder identified was assessment of underlying conditions in the patients. Due to the limitations associated with the data extraction method, we could not assess for underlying conditions that may have impacted the results. Notably, the potential nephrotoxicity of NS has mostly been shown in critically ill patients. Therefore, the mixed acutely ill patient sample in this study may have been less likely to develop AKI from NS compared with an exclusively critically ill patient sample.
Selection bias and information bias are common with observational studies. In our study, selection bias may have been present since prospective randomization of patient samples was not possible. Since all data were extracted from the medical health record, information bias may have been present with the potential to impact the results. Due to the single-center nature of this study with a predominantly older, white male veteran patient sample, generalizability to other patient populations may be limited. We would expect the results of this study to be similar among other patient populations of a similar age and demographic; however, the external validity of this study may be weak among other populations. Although this study included enough patients based on sample size estimate, a larger sample size could have allowed for detection of smaller differences between groups and decreased the chance for type II error.
Conclusions
Overall, the results of this study do not suggest that the crystalloid used to dilute IV vancomycin is associated with differences in nephrotoxicity or other relevant AEs. Future studies evaluating the potential for AEs from medication diluent are warranted and would benefit from a prospective, randomized design. Further studies are both necessary and crucial for enhancing the quality of care to minimize the rates of AEs of commonly used medications.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Vancomycin hydrochloride intravenous injection, pharmacy bulk package. Package insert. Schaumburg, IL: APP Pharmaceuticals, LLC; 2011.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-System Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
4. Elaysi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations–a literature review. Eur J Clin Pharmacol. 2012;68(9):1243-1255. doi:10.1007/s00228-012-1259-9
5. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-associated acute kidney injury in a large veteran population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
6. Semler MW, Self WH, Wanderer JB, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584
7. Self WH, Semler MW, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(20):819-828. doi:10.1056/NEJMc1804294
8. Young P, Bailey M, Beasley R, et al; SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT Randomized Clinical Trial. JAMA. 2015;314(16):1701-1710. doi:10.1001/jama.2015.12334
9. Magee CA, Bastin ML, Bastin T, et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit Care Med. 2018;46(8):1217-1223. doi:10.1097/CCM.0000000000003191
10. Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal. 2014;2014:627673. doi:10.1155/2014/627673
11. Mcgrady KA, Benton M, Tart S, Bowers R. Evaluation of traditional vancomycin dosing versus utilizing an electronic AUC/MIC dosing program. Pharm Pract (Granada). 2020;18(3):2024. doi:10.18549/PharmPract.2020.3.2024
12. Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther Drug Monit. 2019;41(4):483-488. doi:10.1097/FTD.0000000000000622
13. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. doi:10.1128/AAC.02042-17
14. Aljefri DM, Avedissian SN, Youn G, et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881-1887. doi:10.1128/AAC.02042-17
15. Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The risk of acute kidney injury in critically ill patients receiving concomitant vancomycin with piperacillin-tazobactam or cefepime. J Intensive Care Med. 2019;35(12):1434-1438. doi:10.1177/0885066619828290
16. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014; 34(7):670-676. doi:10.1002/phar.1442
1. Vancomycin hydrochloride intravenous injection, pharmacy bulk package. Package insert. Schaumburg, IL: APP Pharmaceuticals, LLC; 2011.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-System Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
4. Elaysi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations–a literature review. Eur J Clin Pharmacol. 2012;68(9):1243-1255. doi:10.1007/s00228-012-1259-9
5. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-associated acute kidney injury in a large veteran population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
6. Semler MW, Self WH, Wanderer JB, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584
7. Self WH, Semler MW, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(20):819-828. doi:10.1056/NEJMc1804294
8. Young P, Bailey M, Beasley R, et al; SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT Randomized Clinical Trial. JAMA. 2015;314(16):1701-1710. doi:10.1001/jama.2015.12334
9. Magee CA, Bastin ML, Bastin T, et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit Care Med. 2018;46(8):1217-1223. doi:10.1097/CCM.0000000000003191
10. Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal. 2014;2014:627673. doi:10.1155/2014/627673
11. Mcgrady KA, Benton M, Tart S, Bowers R. Evaluation of traditional vancomycin dosing versus utilizing an electronic AUC/MIC dosing program. Pharm Pract (Granada). 2020;18(3):2024. doi:10.18549/PharmPract.2020.3.2024
12. Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther Drug Monit. 2019;41(4):483-488. doi:10.1097/FTD.0000000000000622
13. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. doi:10.1128/AAC.02042-17
14. Aljefri DM, Avedissian SN, Youn G, et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881-1887. doi:10.1128/AAC.02042-17
15. Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The risk of acute kidney injury in critically ill patients receiving concomitant vancomycin with piperacillin-tazobactam or cefepime. J Intensive Care Med. 2019;35(12):1434-1438. doi:10.1177/0885066619828290
16. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014; 34(7):670-676. doi:10.1002/phar.1442
Enhancing Access to Yoga for Older Male Veterans After Cancer: Examining Beliefs About Yoga
Yoga is an effective clinical intervention for cancer survivors. Studies indicate a wide range of benefits, including improvements in physical functioning, emotional well-being and overall quality of life.1-7 Two-thirds of National Cancer Institute designated comprehensive cancer centers offer yoga on-site.8 Yoga is endorsed by the National Comprehensive Cancer Network and American Society of Clinical Oncology for managing symptoms, such as cancer-related anxiety and depression and for improving overall quality of life.9,10
Although the positive effects of yoga on cancer patients are well studied, most published research in this area reports on predominantly middle-aged women with breast cancer.11,12 Less is known about the use of yoga in other groups of cancer patients, such as older adults, veterans, and those from diverse racial or ethnic backgrounds. This gap in the literature is concerning considering that the majority of cancer survivors are aged 60 years or older, and veterans face unique risk factors for cancer associated with herbicide exposure (eg, Agent Orange) and other military-related noxious exposures.13,14 Older cancer survivors may have more difficulty recovering from treatment-related adverse effects, making it especially important to target recovery efforts to older adults.15 Yoga can be adapted for older cancer survivors with age-related comorbidities, similar to adaptations made for older adults who are not cancer survivors but require accommodations for physical limitations.16-20 Similarly, yoga programs targeted to racially diverse cancer survivors are associated with improved mood and well-being in racially diverse cancer survivors, but studies suggest community engagement and cultural adaptation may be important to address the needs of culturally diverse cancer survivors.21-23
Yoga has been increasingly studied within the Veterans Health Administration (VHA) for treatment of posttraumatic stress disorder (PTSD) and has been found effective in reducing symptoms through the use of trauma-informed and military-relevant instruction as well as a military veteran yoga teacher.24-26 This work has not targeted older veterans or cancer survivors who may be more difficult to recruit into such programs, but who would nevertheless benefit.
Clinically, the VHA whole health model is providing increased opportunities for veterans to engage in holistic care including yoga.27 Resources include in-person yoga classes (varies by facility), videos, and handouts with practices uniquely designed for veterans or wounded warriors. As clinicians increasingly refer veterans to these programs, it will be important to develop strategies to engage older veterans in these services.
One important strategy to enhancing access to yoga for older veterans is to consider beliefs about yoga. Beliefs about yoga or general expectations about the outcomes of yoga may be critical to consider in expanding access to yoga in underrepresented groups. Beliefs about yoga may include beliefs about yoga improving health, yoga being difficult or producing discomfort, and yoga involving specific social norms.28 For example, confidence in one’s ability to perform yoga despite discomfort predicted class attendance and practice in a sample of 32 breast cancer survivors.29 Relatedly, positive beliefs about the impact of yoga on health were associated with improvements in mood and quality of life in a sample of 66 cancer survivors.30
The aim of this study was to examine avenues to enhance access to yoga for older veterans, including those from diverse backgrounds, with a focus on the role of beliefs. In the first study we investigate the association between beliefs about and barriers to yoga in a group of older cancer survivors, and we consider the role of demographic and clinical variables in such beliefs and how education may alter beliefs. In alignment with the whole health model of holistic health, we posit that yoga educational materials and resources may contribute to yoga beliefs and work to decrease these barriers. We apply these findings in a second study that enrolled older veterans in yoga and examining the impact of program participation on beliefs and the role of beliefs in program outcomes. In the discussion we return to consider how to increase access to yoga to older veterans based on these findings.
Methods
Study 1 participants were identified from VHA tumor registries. Eligible patients had head and neck, esophageal, gastric, or colorectal cancers and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed a face-to-face semistructured interview at 6, 12, and 18 months after their cancer diagnosis with a trained interviewer. Complete protocol methods, including nonresponder information, are described elsewhere.31
Questions about yoga were asked at the 12 month postdiagnosis interview. Participants were read the following: “Here is a list of services some patients use to recover from cancer. Please tell me if you have used any of these.” The list included yoga, physical therapy, occupational therapy, exercise, meditation, or massage therapy. Next participants were provided education about yoga via the following description: “Yoga is a practice of stress reduction and exercise with stretching, holding positions and deep breathing. For some, it may improve your sleep, energy, flexibility, anxiety, and pain. The postures are done standing, sitting, or lying down. If needed, it can be done all from a chair.” We then asked whether they would attend if yoga was offered at the VHA hospital (yes, no, maybe). Participants provided brief responses to 2 open-ended questions: (“If I came to a yoga class, I …”; and “Is there anything that might make you more likely to come to a yoga class?”) Responses were transcribed verbatim and entered into a database for qualitative analysis. Subsequently, participants completed standardized measures of health-related quality of life and beliefs about yoga as described below.
Study 2 participants were identified from VHA tumor registries and a cancer support group. Eligible patients had a diagnosis of cancer (any type except basil cell carcinoma) within the previous 3 years and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed face-to-face semistructured interviews with a trained interviewer before and after participation in an 8-week yoga group that met twice per week. Complete protocol methods are described elsewhere.16 This paper focuses on 28 of the 37 enrolled patients for whom we have complete pre- and postclass interview data. We previously reported on adaptations made to yoga in our pilot group of 14 individuals, who in this small sample did not show statistically significant changes in their quality of life from before to after the class.16 This analysis includes those 14 individuals and 14 who participated in additional classes, focusing on beliefs, which were not previously reported.
Measures
Participants reported their age, gender, ethnicity (Hispanic/Latino or not), race, and level of education. Information about the cancer diagnosis, American Joint Committee on Cancer (AJCC) cancer stage, and treatments was obtained from the medical record. The Physical Function and Anxiety Subscales from the Patient-Reported Outcomes Measurement Information System were used to measure health-related quality of life (HRQoL).32-34 Items are rated on a Likert scale from 1 (not at all) to 5 (very much).
The Beliefs About Yoga Scale (BAYS) was used to measure beliefs about the outcomes of engaging in yoga.28 The 11-item scale has 3 factors: expected health benefits (5 items), expected discomfort (3 items), and expected social norms (3 items). Items from the expected discomfort and expected social norms are reverse scored so that a higher score indicates more positive beliefs. To reduce participant burden, in study 1 we selected 1 item from each factor with high factor loadings in the original cross-validation sample.28 It would improve my overall health (Benefit, factor loading = .89); I would have to be more flexible to take a class (Discomfort, factor loading = .67); I would be embarrassed in a class (Social norms, factor loading = .75). Participants in study 2 completed the entire 11-item scale. Items were summed to create subscales and total scales.
Analysis
Descriptive statistics were used in study 1 to characterize participants’ yoga experience and interest. Changes in interest pre- and posteducation were evaluated with χ2 comparison of distribution. The association of beliefs about yoga with 3 levels of interest (yes, no, maybe) was evaluated through analysis of variance (ANOVA) comparing the mean score on the summed BAYS items among the 3 groups. The association of demographic (age, education, race) and clinical factors (AJCC stage, physical function) with BAYS was determined through multivariate linear regression.
For analytic purposes, due to small subgroup sample sizes we compared those who identified as non-Hispanic White adults to those who identified as African American/Hispanic/other persons. To further evaluate the relationship of age to yoga beliefs, we examined beliefs about yoga in 3 age groups (40-59 years [n = 24]; 60-69 years [n = 58]; 70-89 years [n = 28]) using ANOVA comparing the mean score on the summed BAYS items among the 3 groups. In study 2, changes in interest before and after the yoga program were evaluated with paired t tests and repeated ANOVA, with beliefs about yoga prior to class as a covariate. The association of demographic and clinical factors with BAYS was determined as in the first sample through multivariate linear regression, except the variable of race was not included due to small sample size (ie, only 3 individuals identified as persons of color).
Thematic analysis in which content-related codes were developed and subsequently grouped together was applied to the data of 110 participants who responded to the open-ended survey questions in study 1 to further illuminate responses to closed-ended questions.35 Transcribed responses to the open-ended questions were transferred to a spreadsheet. An initial code book with code names, definitions, and examples was developed based on an inductive method by one team member (EA).35 Initially, coding and tabulation were conducted separately for each question but it was noted that content extended across response prompts (eg, responses to question 2 “What might make you more likely to come?” were spontaneously provided when answering question 1), thus coding was collapsed across questions. Next, 2 team members (EA, KD) coded the same responses, meeting weekly to discuss discrepancies. The code book was revised following each meeting to reflect refinements in code names and definitions, adding newly generated codes as needed. The process continued until consensus and data saturation was obtained, with 90% intercoder agreement. Next, these codes were subjected to thematic analysis by 2 team members (EA, KD) combining codes into 6 overarching themes. The entire team reviewed the codes and identified 2 supra themes: positive beliefs or facilitators and negative beliefs or barriers.
Consistent with the concept of reflexivity in qualitative research, we acknowledge the influence of the research team members on the qualitative process.36 The primary coding team (EA, KD) are both researchers and employees of Veterans Affairs Boston Healthcare System who have participated in other research projects involving veterans and qualitative analyses but are not yoga instructors or yoga researchers.
Results
Study 1
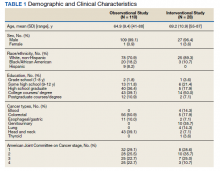
The sample of 110 military veterans was mostly male (99.1%) with a mean (SD) age of 64.9 (9.4) years (range, 41-88)(Table 1). The majority (70.9%) described their race/ethnicity as White, non-Hispanic followed by Black/African American (18.2%) and Hispanic (8.2%) persons; 50.0% had no more than a high school education. The most common cancer diagnoses were colorectal (50.9%), head and neck (39.1%), and esophageal and gastric (10.0%) and ranged from AJCC stages I to IV.
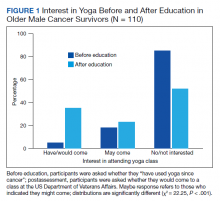
When first asked, the majority of participants (78.2%) reported that they were not interested in yoga, 16.4% reported they might be interested, and 5.5% reported they had tried a yoga class since their cancer diagnosis. In contrast, 40.9% used exercise, 32.7% used meditation, 14.5% used physical or occupational therapy, and 11.8% used massage therapy since their cancer diagnosis.
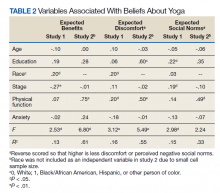
After participants were provided the brief scripted education about yoga, the level of interest shifted: 46.4% not interested, 21.8% interested, and 31.8% definitely interested, demonstrating a statistically significant shift in interest following education (χ2 = 22.25, P < .001) (Figure 1). Those with the most positive beliefs about yoga were most likely to indicate interest. Using the BAYS 3-item survey, the mean (SD) for the definitely interested, might be interested, and not interested groups was 15.1 (3.2), 14.1 (3.2), and 12.3 (2.5), respectively (F = 10.63, P < .001).
A multivariable regression was run to examine possible associations between participants’ demographic characteristics, clinical characteristics, and beliefs about yoga as measured by the 3 BAYS items (Table 2). Higher expected health benefits of yoga was associated with identifying as
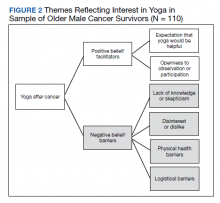
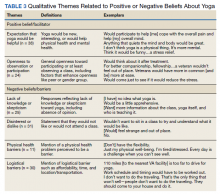
Six themes were identified in qualitative analysis of semistructured interviews reflecting older veterans’ beliefs about yoga, which were grouped into the following suprathemes of positive vs negative beliefs (Figure 2). Exemplar responses appear in Table 3.
Study 2 Intervention Sample
This sample of 28 veterans was mostly male (96.4%) with a mean (SD) age of 69.2 (10.9) years (range, 57-87). The majority (89.3%) described their race as White, followed by Black/African American (10.7%); no participants self-identified in other categories for race/ethnicity. Twelve veterans (42.9%) had no more than a high school education. The most common cancer diagnosis was genitourinary (35.7%) and the AJCC stage ranged from I to IV.
We employed information learned in study 1 to enhance access in study 2. We mailed letters to 278 veterans diagnosed with cancer in the previous 3 years that provided education about yoga based on study 1 findings. Of 207 veterans reached by phone, 133 (64%) stated they were not interested in coming to a yoga class; 74 (36%) were interested, but 30 felt they were unable to attend due to obstacles such as illness or travel. Ultimately 37 (18%) veterans agreed and consented to the class, and 28 (14%) completed postclass surveys.
In multivariate regression, higher expected health benefits of yoga were associated with higher physical function, lower concern about expected discomfort was also associated with higher physical function as well as higher education; similarly, lower concern about expected social norms was associated with higher physical function. Age was not associated with any of the BAYS factors.
Beliefs about yoga improved from before to after class for all 3 domains with greater expected benefit and lower concerns about discomfort or social norms:
Discussion
Yoga is an effective clinical intervention for addressing some long-term adverse effects in cancer survivors, although the body of research focuses predominantly on middle aged, female, White, college-educated breast cancer survivors. There is no evidence to suggest yoga would be less effective in other groups, but it has not been extensively studied in survivors from diverse subgroups. Beliefs about yoga are a factor that may enhance interest in yoga interventions and research, and measures aimed at addressing potential beliefs and fears may capture information that can be used to support older cancer survivors in holistic health. The aims of this study were to examine beliefs about yoga in 2 samples of older cancer survivors who received VHA care. The main findings are (1) interest in yoga was initially low and lower than that of other complementary or exercise-based interventions, but increased when participants were provided brief education about yoga; (2) interest in yoga was associated with beliefs about yoga with qualitative comments illuminating these beliefs; (3) demographic characteristics (education, race) and physical function were associated with beliefs about yoga; and (4) positive beliefs about yoga increased following a brief yoga intervention and was associated with improvements in physical function.
Willingness to consider a class appeared to shift for some older veterans when they were presented brief information about yoga that explained what is involved, how it might help, and that it could be done from a chair if needed. These findings clearly indicated that when trying to enhance participation in yoga in clinical or research programs, it will be important that recruitment materials provide such information. This finding is consistent with the qualitative findings that reflected a lack of knowledge or skepticism about benefits of yoga among some participants. Given the finding that physical function was associated with beliefs about yoga and was also a prominent theme in qualitative analyses,
Age was not associated with beliefs about yoga in either study. Importantly, in a more detailed study 1 follow-up analysis, beliefs about yoga were equivalent for aged > 70 years compared with those aged 40 to 69 years. It is not entirely clear why older adults have been underrepresented in studies of yoga in cancer survivors. However, older adults are vastly underrepresented in clinical trials for many health conditions, even though they are more likely to experience many diseases, including cancer.37 A new National Institutes of Health policy requires that individuals of all ages, including older adults, must be included in all human subjects research unless there are scientific reasons not to include them.38 It is therefore imperative to consider strategies to address underrepresentation of older adults.
Qualitative findings here suggest it will be important to consider logistical barriers including transportation and affordability as well as adaptations requested by older adults (eg, preferences for older teachers).18
Although our sample was small, we also found that adults from diverse racial and ethnic backgrounds had more positive beliefs about yoga, such that this finding should be interpreted with caution. Similar to older adults, individuals from diverse racial and ethnic groups are also underrepresented in clinical trials and may have lower access to complementary treatments. Cultural and linguistic adaptations and building community partnerships should be considered in both recruitment and intervention delivery strategies.40We learned that education about yoga may increase interest and that it is possible to recruit older veterans to yoga class. Nevertheless, in study 2, our rate of full participation was low, with only about 1 in 10 participating. Additional efforts to enhance beliefs about yoga and to addresslogistical barriers (offering telehealth yoga) are needed to best reach older veterans.
Limitations
These findings have several limitations. First, participants were homogeneous in age, gender, race/ethnicity and veteran status, which provides a window into this understudied population but limits generalizability and our ability to control across populations. Second, the sample size limited the ability to conduct subgroup and interaction analyses, such as examining potential differential effects of cancer type, treatment, and PTSD on yoga beliefs or to consider the relationship of yoga beliefs with changes in quality of life before and after the yoga intervention in study 2. Additionally, age was not associated with beliefs about yoga in these samples that of mostly older adults. We were able to compare middle-aged and older adults but could not compare beliefs about yoga to adults aged in their 20s and 30s. Last, our study excluded people with dementia and psychotic disorders. Further research is needed to examine yoga for older cancer survivors who have these conditions.
Conclusions
Education that specifically informs potential participants about yoga practice, potential modifications, and potential benefits, as well as adaptations to programs that address physical and logistical barriers may be useful in increasing access to and participation in yoga for older Veterans who are cancer survivors.
Acknowledgments/Funding
The authors have no financial or personal relationships to disclose. This work was supported by the US Department of Veterans Affairs (VA) Rehabilitation Research and Development Service. This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System, Bedford VA Medical Center, and Michael E. DeBakey VA Medical Center in Houston, Texas. We thank the members of the Veterans Cancer Rehabilitation Study (Vetcares) Research teams in Boston and in Houston and the veterans who have participated in our research studies and allow us to contribute to their health care.
1. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233-3241. doi:10.1200/JCO.2012.43.7707
2. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8(2):43-55.
3. Erratum: Primary follicular lymphoma of disguised as multiple miliary like lesions: A case report and review of literature. Indian J Pathol Microbiol. 2018;61(4):643. doi:10.4103/0377-4929.243009
4. Eyigor S, Uslu R, Apaydın S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Complement Ther Clin Pract. 2018;32:40-45. doi:10.1016/j.ctcp.2018.04.010
5. Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214. Published 2014 Jul 1. doi:10.1186/1472-6882-14-214
6. Browning KK, Kue J, Lyons F, Overcash J. Feasibility of mind-body movement programs for cancer survivors. Oncol Nurs Forum. 2017;44(4):446-456. doi:10.1188/17.ONF.446-456
7. Rosenbaum MS, Velde J. The effects of yoga, massage, and reiki on patient well-being at a cancer resource center. Clin J Oncol Nurs. 2016;20(3):E77-E81. doi:10.1188/16.CJON.E77-E81
8. Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-designated comprehensive cancer center websites. J Natl Cancer Inst Monogr. 2017;2017(52):lgx004. doi:10.1093/jncimonographs/lgx004
9. Sanft T, Denlinger CS, Armenian S, et al. NCCN guidelines insights: survivorship, version 2.2019. J Natl Compr Canc Netw. 2019;17(7):784-794. doi:10.6004/jnccn.2019.0034
10. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36(25):2647-2655. doi:10.1200/JCO.2018.79.2721
11. Culos-Reed SN, Mackenzie MJ, Sohl SJ, Jesse MT, Zahavich AN, Danhauer SC. Yoga & cancer interventions: a review of the clinical significance of patient reported outcomes for cancer survivors. Evid Based Complement Alternat Med. 2012;2012:642576. doi:10.1155/2012/642576
12. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer. 2019;125(12):1979-1989. doi:10.1002/cncr.31979
13. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
14. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed September 22, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions
15. Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57(suppl 2):S289-S292. doi:10.1111/j.1532-5415.2009.02515.x
16. King K, Gosian J, Doherty K, et al. Implementing yoga therapy adapted for older veterans who are cancer survivors. Int J Yoga Therap. 2014;24:87-96.
17. Wertman A, Wister AV, Mitchell BA. On and off the mat: yoga experiences of middle-aged and older adults. Can J Aging. 2016;35(2):190-205. doi:10.1017/S0714980816000155
18. Chen KM, Wang HH, Li CH, Chen MH. Community vs. institutional elders’ evaluations of and preferences for yoga exercises. J Clin Nurs. 2011;20(7-8):1000-1007. doi:10.1111/j.1365-2702.2010.03337.x
19. Saravanakumar P, Higgins IJ, Van Der Riet PJ, Sibbritt D. Tai chi and yoga in residential aged care: perspectives of participants: A qualitative study. J Clin Nurs. 2018;27(23-24):4390-4399. doi:10.1111/jocn.14590
20. Fan JT, Chen KM. Using silver yoga exercises to promote physical and mental health of elders with dementia in long-term care facilities. Int Psychogeriatr. 2011;23(8):1222-1230. doi:10.1017/S1041610211000287
21. Taylor TR, Barrow J, Makambi K, et al. A restorative yoga intervention for African-American breast cancer survivors: a pilot study. J Racial Ethn Health Disparities. 2018;5(1):62-72. doi:10.1007/s40615-017-0342-4
22. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387-4395. doi:10.1200/JCO.2006.06.6027
23. Smith SA, Whitehead MS, Sheats JQ, Chubb B, Alema-Mensah E, Ansa BE. Community engagement to address socio-ecological barriers to physical activity among African American breast cancer survivors. J Ga Public Health Assoc. 2017;6(3):393-397. doi:10.21633/jgpha.6.312
24. Cushing RE, Braun KL, Alden C-Iayt SW, Katz AR. Military-Tailored Yoga for Veterans with Post-traumatic Stress Disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
25. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. US Department of Veterans Affairs. Whole health. Updated September 13, 2021. Accessed September 22, 2021. https://www.va.gov/wholehealth
28. Sohl SJ, Schnur JB, Daly L, Suslov K, Montgomery GH. Development of the beliefs about yoga scale. Int J Yoga Therap. 2011;(21):85-91.
29. Cadmus-Bertram L, Littman AJ, Ulrich CM, et al. Predictors of adherence to a 26-week viniyoga intervention among post-treatment breast cancer survivors. J Altern Complement Med. 2013;19(9):751-758. doi:10.1089/acm.2012.0118
30. Mackenzie MJ, Carlson LE, Ekkekakis P, Paskevich DM, Culos-Reed SN. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496. doi:10.1155/2013/419496
31. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93. Published 2013 Mar 11. doi:10.1186/1472-6963-13-93
32. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v2.0 - Physical Function 6b. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=793&Itemid=992
33. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v1.0 - Anxiety 6a. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=145&Itemid=992
34. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS-43 Profile v2.1. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=858&Itemid=992
35. Todd NJ, Jones SH, Lobban FA. “Recovery” in bipolar disorder: how can service users be supported through a self-management intervention? A qualitative focus group study. J Ment Health. 2012;21(2):114-126. doi:10.3109/09638237.2011.621471
36. Finlay L. “Outing” the researcher: the provenance, process, and practice of reflexivity. Qual Health Res. 2002;12(4):531-545. doi:10.1177/104973202129120052
37. Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;10(suppl 1):S105-S112. doi:10.2105/AJPH.2009.162982
38. National Institutes of Health. Revision: NIH policy and guidelines on the inclusion of individuals across the lifespan as participants in research involving human subjects. Published December 19, 2017. Accessed September 22, 2021. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html
39. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112-3124. doi:10.1200/JCO.2005.00.141
40. Vuong I, Wright J, Nolan MB, et al. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials-a narrative review. J Cancer Educ. 2020;35(5):841-849. doi:10.1007/s13187-019-01650-y
Yoga is an effective clinical intervention for cancer survivors. Studies indicate a wide range of benefits, including improvements in physical functioning, emotional well-being and overall quality of life.1-7 Two-thirds of National Cancer Institute designated comprehensive cancer centers offer yoga on-site.8 Yoga is endorsed by the National Comprehensive Cancer Network and American Society of Clinical Oncology for managing symptoms, such as cancer-related anxiety and depression and for improving overall quality of life.9,10
Although the positive effects of yoga on cancer patients are well studied, most published research in this area reports on predominantly middle-aged women with breast cancer.11,12 Less is known about the use of yoga in other groups of cancer patients, such as older adults, veterans, and those from diverse racial or ethnic backgrounds. This gap in the literature is concerning considering that the majority of cancer survivors are aged 60 years or older, and veterans face unique risk factors for cancer associated with herbicide exposure (eg, Agent Orange) and other military-related noxious exposures.13,14 Older cancer survivors may have more difficulty recovering from treatment-related adverse effects, making it especially important to target recovery efforts to older adults.15 Yoga can be adapted for older cancer survivors with age-related comorbidities, similar to adaptations made for older adults who are not cancer survivors but require accommodations for physical limitations.16-20 Similarly, yoga programs targeted to racially diverse cancer survivors are associated with improved mood and well-being in racially diverse cancer survivors, but studies suggest community engagement and cultural adaptation may be important to address the needs of culturally diverse cancer survivors.21-23
Yoga has been increasingly studied within the Veterans Health Administration (VHA) for treatment of posttraumatic stress disorder (PTSD) and has been found effective in reducing symptoms through the use of trauma-informed and military-relevant instruction as well as a military veteran yoga teacher.24-26 This work has not targeted older veterans or cancer survivors who may be more difficult to recruit into such programs, but who would nevertheless benefit.
Clinically, the VHA whole health model is providing increased opportunities for veterans to engage in holistic care including yoga.27 Resources include in-person yoga classes (varies by facility), videos, and handouts with practices uniquely designed for veterans or wounded warriors. As clinicians increasingly refer veterans to these programs, it will be important to develop strategies to engage older veterans in these services.
One important strategy to enhancing access to yoga for older veterans is to consider beliefs about yoga. Beliefs about yoga or general expectations about the outcomes of yoga may be critical to consider in expanding access to yoga in underrepresented groups. Beliefs about yoga may include beliefs about yoga improving health, yoga being difficult or producing discomfort, and yoga involving specific social norms.28 For example, confidence in one’s ability to perform yoga despite discomfort predicted class attendance and practice in a sample of 32 breast cancer survivors.29 Relatedly, positive beliefs about the impact of yoga on health were associated with improvements in mood and quality of life in a sample of 66 cancer survivors.30
The aim of this study was to examine avenues to enhance access to yoga for older veterans, including those from diverse backgrounds, with a focus on the role of beliefs. In the first study we investigate the association between beliefs about and barriers to yoga in a group of older cancer survivors, and we consider the role of demographic and clinical variables in such beliefs and how education may alter beliefs. In alignment with the whole health model of holistic health, we posit that yoga educational materials and resources may contribute to yoga beliefs and work to decrease these barriers. We apply these findings in a second study that enrolled older veterans in yoga and examining the impact of program participation on beliefs and the role of beliefs in program outcomes. In the discussion we return to consider how to increase access to yoga to older veterans based on these findings.
Methods
Study 1 participants were identified from VHA tumor registries. Eligible patients had head and neck, esophageal, gastric, or colorectal cancers and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed a face-to-face semistructured interview at 6, 12, and 18 months after their cancer diagnosis with a trained interviewer. Complete protocol methods, including nonresponder information, are described elsewhere.31
Questions about yoga were asked at the 12 month postdiagnosis interview. Participants were read the following: “Here is a list of services some patients use to recover from cancer. Please tell me if you have used any of these.” The list included yoga, physical therapy, occupational therapy, exercise, meditation, or massage therapy. Next participants were provided education about yoga via the following description: “Yoga is a practice of stress reduction and exercise with stretching, holding positions and deep breathing. For some, it may improve your sleep, energy, flexibility, anxiety, and pain. The postures are done standing, sitting, or lying down. If needed, it can be done all from a chair.” We then asked whether they would attend if yoga was offered at the VHA hospital (yes, no, maybe). Participants provided brief responses to 2 open-ended questions: (“If I came to a yoga class, I …”; and “Is there anything that might make you more likely to come to a yoga class?”) Responses were transcribed verbatim and entered into a database for qualitative analysis. Subsequently, participants completed standardized measures of health-related quality of life and beliefs about yoga as described below.
Study 2 participants were identified from VHA tumor registries and a cancer support group. Eligible patients had a diagnosis of cancer (any type except basil cell carcinoma) within the previous 3 years and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed face-to-face semistructured interviews with a trained interviewer before and after participation in an 8-week yoga group that met twice per week. Complete protocol methods are described elsewhere.16 This paper focuses on 28 of the 37 enrolled patients for whom we have complete pre- and postclass interview data. We previously reported on adaptations made to yoga in our pilot group of 14 individuals, who in this small sample did not show statistically significant changes in their quality of life from before to after the class.16 This analysis includes those 14 individuals and 14 who participated in additional classes, focusing on beliefs, which were not previously reported.
Measures
Participants reported their age, gender, ethnicity (Hispanic/Latino or not), race, and level of education. Information about the cancer diagnosis, American Joint Committee on Cancer (AJCC) cancer stage, and treatments was obtained from the medical record. The Physical Function and Anxiety Subscales from the Patient-Reported Outcomes Measurement Information System were used to measure health-related quality of life (HRQoL).32-34 Items are rated on a Likert scale from 1 (not at all) to 5 (very much).
The Beliefs About Yoga Scale (BAYS) was used to measure beliefs about the outcomes of engaging in yoga.28 The 11-item scale has 3 factors: expected health benefits (5 items), expected discomfort (3 items), and expected social norms (3 items). Items from the expected discomfort and expected social norms are reverse scored so that a higher score indicates more positive beliefs. To reduce participant burden, in study 1 we selected 1 item from each factor with high factor loadings in the original cross-validation sample.28 It would improve my overall health (Benefit, factor loading = .89); I would have to be more flexible to take a class (Discomfort, factor loading = .67); I would be embarrassed in a class (Social norms, factor loading = .75). Participants in study 2 completed the entire 11-item scale. Items were summed to create subscales and total scales.
Analysis
Descriptive statistics were used in study 1 to characterize participants’ yoga experience and interest. Changes in interest pre- and posteducation were evaluated with χ2 comparison of distribution. The association of beliefs about yoga with 3 levels of interest (yes, no, maybe) was evaluated through analysis of variance (ANOVA) comparing the mean score on the summed BAYS items among the 3 groups. The association of demographic (age, education, race) and clinical factors (AJCC stage, physical function) with BAYS was determined through multivariate linear regression.
For analytic purposes, due to small subgroup sample sizes we compared those who identified as non-Hispanic White adults to those who identified as African American/Hispanic/other persons. To further evaluate the relationship of age to yoga beliefs, we examined beliefs about yoga in 3 age groups (40-59 years [n = 24]; 60-69 years [n = 58]; 70-89 years [n = 28]) using ANOVA comparing the mean score on the summed BAYS items among the 3 groups. In study 2, changes in interest before and after the yoga program were evaluated with paired t tests and repeated ANOVA, with beliefs about yoga prior to class as a covariate. The association of demographic and clinical factors with BAYS was determined as in the first sample through multivariate linear regression, except the variable of race was not included due to small sample size (ie, only 3 individuals identified as persons of color).
Thematic analysis in which content-related codes were developed and subsequently grouped together was applied to the data of 110 participants who responded to the open-ended survey questions in study 1 to further illuminate responses to closed-ended questions.35 Transcribed responses to the open-ended questions were transferred to a spreadsheet. An initial code book with code names, definitions, and examples was developed based on an inductive method by one team member (EA).35 Initially, coding and tabulation were conducted separately for each question but it was noted that content extended across response prompts (eg, responses to question 2 “What might make you more likely to come?” were spontaneously provided when answering question 1), thus coding was collapsed across questions. Next, 2 team members (EA, KD) coded the same responses, meeting weekly to discuss discrepancies. The code book was revised following each meeting to reflect refinements in code names and definitions, adding newly generated codes as needed. The process continued until consensus and data saturation was obtained, with 90% intercoder agreement. Next, these codes were subjected to thematic analysis by 2 team members (EA, KD) combining codes into 6 overarching themes. The entire team reviewed the codes and identified 2 supra themes: positive beliefs or facilitators and negative beliefs or barriers.
Consistent with the concept of reflexivity in qualitative research, we acknowledge the influence of the research team members on the qualitative process.36 The primary coding team (EA, KD) are both researchers and employees of Veterans Affairs Boston Healthcare System who have participated in other research projects involving veterans and qualitative analyses but are not yoga instructors or yoga researchers.
Results
Study 1
The sample of 110 military veterans was mostly male (99.1%) with a mean (SD) age of 64.9 (9.4) years (range, 41-88)(Table 1). The majority (70.9%) described their race/ethnicity as White, non-Hispanic followed by Black/African American (18.2%) and Hispanic (8.2%) persons; 50.0% had no more than a high school education. The most common cancer diagnoses were colorectal (50.9%), head and neck (39.1%), and esophageal and gastric (10.0%) and ranged from AJCC stages I to IV.
When first asked, the majority of participants (78.2%) reported that they were not interested in yoga, 16.4% reported they might be interested, and 5.5% reported they had tried a yoga class since their cancer diagnosis. In contrast, 40.9% used exercise, 32.7% used meditation, 14.5% used physical or occupational therapy, and 11.8% used massage therapy since their cancer diagnosis.
After participants were provided the brief scripted education about yoga, the level of interest shifted: 46.4% not interested, 21.8% interested, and 31.8% definitely interested, demonstrating a statistically significant shift in interest following education (χ2 = 22.25, P < .001) (Figure 1). Those with the most positive beliefs about yoga were most likely to indicate interest. Using the BAYS 3-item survey, the mean (SD) for the definitely interested, might be interested, and not interested groups was 15.1 (3.2), 14.1 (3.2), and 12.3 (2.5), respectively (F = 10.63, P < .001).
A multivariable regression was run to examine possible associations between participants’ demographic characteristics, clinical characteristics, and beliefs about yoga as measured by the 3 BAYS items (Table 2). Higher expected health benefits of yoga was associated with identifying as
Six themes were identified in qualitative analysis of semistructured interviews reflecting older veterans’ beliefs about yoga, which were grouped into the following suprathemes of positive vs negative beliefs (Figure 2). Exemplar responses appear in Table 3.
Study 2 Intervention Sample
This sample of 28 veterans was mostly male (96.4%) with a mean (SD) age of 69.2 (10.9) years (range, 57-87). The majority (89.3%) described their race as White, followed by Black/African American (10.7%); no participants self-identified in other categories for race/ethnicity. Twelve veterans (42.9%) had no more than a high school education. The most common cancer diagnosis was genitourinary (35.7%) and the AJCC stage ranged from I to IV.
We employed information learned in study 1 to enhance access in study 2. We mailed letters to 278 veterans diagnosed with cancer in the previous 3 years that provided education about yoga based on study 1 findings. Of 207 veterans reached by phone, 133 (64%) stated they were not interested in coming to a yoga class; 74 (36%) were interested, but 30 felt they were unable to attend due to obstacles such as illness or travel. Ultimately 37 (18%) veterans agreed and consented to the class, and 28 (14%) completed postclass surveys.
In multivariate regression, higher expected health benefits of yoga were associated with higher physical function, lower concern about expected discomfort was also associated with higher physical function as well as higher education; similarly, lower concern about expected social norms was associated with higher physical function. Age was not associated with any of the BAYS factors.
Beliefs about yoga improved from before to after class for all 3 domains with greater expected benefit and lower concerns about discomfort or social norms:
Discussion
Yoga is an effective clinical intervention for addressing some long-term adverse effects in cancer survivors, although the body of research focuses predominantly on middle aged, female, White, college-educated breast cancer survivors. There is no evidence to suggest yoga would be less effective in other groups, but it has not been extensively studied in survivors from diverse subgroups. Beliefs about yoga are a factor that may enhance interest in yoga interventions and research, and measures aimed at addressing potential beliefs and fears may capture information that can be used to support older cancer survivors in holistic health. The aims of this study were to examine beliefs about yoga in 2 samples of older cancer survivors who received VHA care. The main findings are (1) interest in yoga was initially low and lower than that of other complementary or exercise-based interventions, but increased when participants were provided brief education about yoga; (2) interest in yoga was associated with beliefs about yoga with qualitative comments illuminating these beliefs; (3) demographic characteristics (education, race) and physical function were associated with beliefs about yoga; and (4) positive beliefs about yoga increased following a brief yoga intervention and was associated with improvements in physical function.
Willingness to consider a class appeared to shift for some older veterans when they were presented brief information about yoga that explained what is involved, how it might help, and that it could be done from a chair if needed. These findings clearly indicated that when trying to enhance participation in yoga in clinical or research programs, it will be important that recruitment materials provide such information. This finding is consistent with the qualitative findings that reflected a lack of knowledge or skepticism about benefits of yoga among some participants. Given the finding that physical function was associated with beliefs about yoga and was also a prominent theme in qualitative analyses,
Age was not associated with beliefs about yoga in either study. Importantly, in a more detailed study 1 follow-up analysis, beliefs about yoga were equivalent for aged > 70 years compared with those aged 40 to 69 years. It is not entirely clear why older adults have been underrepresented in studies of yoga in cancer survivors. However, older adults are vastly underrepresented in clinical trials for many health conditions, even though they are more likely to experience many diseases, including cancer.37 A new National Institutes of Health policy requires that individuals of all ages, including older adults, must be included in all human subjects research unless there are scientific reasons not to include them.38 It is therefore imperative to consider strategies to address underrepresentation of older adults.
Qualitative findings here suggest it will be important to consider logistical barriers including transportation and affordability as well as adaptations requested by older adults (eg, preferences for older teachers).18
Although our sample was small, we also found that adults from diverse racial and ethnic backgrounds had more positive beliefs about yoga, such that this finding should be interpreted with caution. Similar to older adults, individuals from diverse racial and ethnic groups are also underrepresented in clinical trials and may have lower access to complementary treatments. Cultural and linguistic adaptations and building community partnerships should be considered in both recruitment and intervention delivery strategies.40We learned that education about yoga may increase interest and that it is possible to recruit older veterans to yoga class. Nevertheless, in study 2, our rate of full participation was low, with only about 1 in 10 participating. Additional efforts to enhance beliefs about yoga and to addresslogistical barriers (offering telehealth yoga) are needed to best reach older veterans.
Limitations
These findings have several limitations. First, participants were homogeneous in age, gender, race/ethnicity and veteran status, which provides a window into this understudied population but limits generalizability and our ability to control across populations. Second, the sample size limited the ability to conduct subgroup and interaction analyses, such as examining potential differential effects of cancer type, treatment, and PTSD on yoga beliefs or to consider the relationship of yoga beliefs with changes in quality of life before and after the yoga intervention in study 2. Additionally, age was not associated with beliefs about yoga in these samples that of mostly older adults. We were able to compare middle-aged and older adults but could not compare beliefs about yoga to adults aged in their 20s and 30s. Last, our study excluded people with dementia and psychotic disorders. Further research is needed to examine yoga for older cancer survivors who have these conditions.
Conclusions
Education that specifically informs potential participants about yoga practice, potential modifications, and potential benefits, as well as adaptations to programs that address physical and logistical barriers may be useful in increasing access to and participation in yoga for older Veterans who are cancer survivors.
Acknowledgments/Funding
The authors have no financial or personal relationships to disclose. This work was supported by the US Department of Veterans Affairs (VA) Rehabilitation Research and Development Service. This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System, Bedford VA Medical Center, and Michael E. DeBakey VA Medical Center in Houston, Texas. We thank the members of the Veterans Cancer Rehabilitation Study (Vetcares) Research teams in Boston and in Houston and the veterans who have participated in our research studies and allow us to contribute to their health care.
Yoga is an effective clinical intervention for cancer survivors. Studies indicate a wide range of benefits, including improvements in physical functioning, emotional well-being and overall quality of life.1-7 Two-thirds of National Cancer Institute designated comprehensive cancer centers offer yoga on-site.8 Yoga is endorsed by the National Comprehensive Cancer Network and American Society of Clinical Oncology for managing symptoms, such as cancer-related anxiety and depression and for improving overall quality of life.9,10
Although the positive effects of yoga on cancer patients are well studied, most published research in this area reports on predominantly middle-aged women with breast cancer.11,12 Less is known about the use of yoga in other groups of cancer patients, such as older adults, veterans, and those from diverse racial or ethnic backgrounds. This gap in the literature is concerning considering that the majority of cancer survivors are aged 60 years or older, and veterans face unique risk factors for cancer associated with herbicide exposure (eg, Agent Orange) and other military-related noxious exposures.13,14 Older cancer survivors may have more difficulty recovering from treatment-related adverse effects, making it especially important to target recovery efforts to older adults.15 Yoga can be adapted for older cancer survivors with age-related comorbidities, similar to adaptations made for older adults who are not cancer survivors but require accommodations for physical limitations.16-20 Similarly, yoga programs targeted to racially diverse cancer survivors are associated with improved mood and well-being in racially diverse cancer survivors, but studies suggest community engagement and cultural adaptation may be important to address the needs of culturally diverse cancer survivors.21-23
Yoga has been increasingly studied within the Veterans Health Administration (VHA) for treatment of posttraumatic stress disorder (PTSD) and has been found effective in reducing symptoms through the use of trauma-informed and military-relevant instruction as well as a military veteran yoga teacher.24-26 This work has not targeted older veterans or cancer survivors who may be more difficult to recruit into such programs, but who would nevertheless benefit.
Clinically, the VHA whole health model is providing increased opportunities for veterans to engage in holistic care including yoga.27 Resources include in-person yoga classes (varies by facility), videos, and handouts with practices uniquely designed for veterans or wounded warriors. As clinicians increasingly refer veterans to these programs, it will be important to develop strategies to engage older veterans in these services.
One important strategy to enhancing access to yoga for older veterans is to consider beliefs about yoga. Beliefs about yoga or general expectations about the outcomes of yoga may be critical to consider in expanding access to yoga in underrepresented groups. Beliefs about yoga may include beliefs about yoga improving health, yoga being difficult or producing discomfort, and yoga involving specific social norms.28 For example, confidence in one’s ability to perform yoga despite discomfort predicted class attendance and practice in a sample of 32 breast cancer survivors.29 Relatedly, positive beliefs about the impact of yoga on health were associated with improvements in mood and quality of life in a sample of 66 cancer survivors.30
The aim of this study was to examine avenues to enhance access to yoga for older veterans, including those from diverse backgrounds, with a focus on the role of beliefs. In the first study we investigate the association between beliefs about and barriers to yoga in a group of older cancer survivors, and we consider the role of demographic and clinical variables in such beliefs and how education may alter beliefs. In alignment with the whole health model of holistic health, we posit that yoga educational materials and resources may contribute to yoga beliefs and work to decrease these barriers. We apply these findings in a second study that enrolled older veterans in yoga and examining the impact of program participation on beliefs and the role of beliefs in program outcomes. In the discussion we return to consider how to increase access to yoga to older veterans based on these findings.
Methods
Study 1 participants were identified from VHA tumor registries. Eligible patients had head and neck, esophageal, gastric, or colorectal cancers and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed a face-to-face semistructured interview at 6, 12, and 18 months after their cancer diagnosis with a trained interviewer. Complete protocol methods, including nonresponder information, are described elsewhere.31
Questions about yoga were asked at the 12 month postdiagnosis interview. Participants were read the following: “Here is a list of services some patients use to recover from cancer. Please tell me if you have used any of these.” The list included yoga, physical therapy, occupational therapy, exercise, meditation, or massage therapy. Next participants were provided education about yoga via the following description: “Yoga is a practice of stress reduction and exercise with stretching, holding positions and deep breathing. For some, it may improve your sleep, energy, flexibility, anxiety, and pain. The postures are done standing, sitting, or lying down. If needed, it can be done all from a chair.” We then asked whether they would attend if yoga was offered at the VHA hospital (yes, no, maybe). Participants provided brief responses to 2 open-ended questions: (“If I came to a yoga class, I …”; and “Is there anything that might make you more likely to come to a yoga class?”) Responses were transcribed verbatim and entered into a database for qualitative analysis. Subsequently, participants completed standardized measures of health-related quality of life and beliefs about yoga as described below.
Study 2 participants were identified from VHA tumor registries and a cancer support group. Eligible patients had a diagnosis of cancer (any type except basil cell carcinoma) within the previous 3 years and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed face-to-face semistructured interviews with a trained interviewer before and after participation in an 8-week yoga group that met twice per week. Complete protocol methods are described elsewhere.16 This paper focuses on 28 of the 37 enrolled patients for whom we have complete pre- and postclass interview data. We previously reported on adaptations made to yoga in our pilot group of 14 individuals, who in this small sample did not show statistically significant changes in their quality of life from before to after the class.16 This analysis includes those 14 individuals and 14 who participated in additional classes, focusing on beliefs, which were not previously reported.
Measures
Participants reported their age, gender, ethnicity (Hispanic/Latino or not), race, and level of education. Information about the cancer diagnosis, American Joint Committee on Cancer (AJCC) cancer stage, and treatments was obtained from the medical record. The Physical Function and Anxiety Subscales from the Patient-Reported Outcomes Measurement Information System were used to measure health-related quality of life (HRQoL).32-34 Items are rated on a Likert scale from 1 (not at all) to 5 (very much).
The Beliefs About Yoga Scale (BAYS) was used to measure beliefs about the outcomes of engaging in yoga.28 The 11-item scale has 3 factors: expected health benefits (5 items), expected discomfort (3 items), and expected social norms (3 items). Items from the expected discomfort and expected social norms are reverse scored so that a higher score indicates more positive beliefs. To reduce participant burden, in study 1 we selected 1 item from each factor with high factor loadings in the original cross-validation sample.28 It would improve my overall health (Benefit, factor loading = .89); I would have to be more flexible to take a class (Discomfort, factor loading = .67); I would be embarrassed in a class (Social norms, factor loading = .75). Participants in study 2 completed the entire 11-item scale. Items were summed to create subscales and total scales.
Analysis
Descriptive statistics were used in study 1 to characterize participants’ yoga experience and interest. Changes in interest pre- and posteducation were evaluated with χ2 comparison of distribution. The association of beliefs about yoga with 3 levels of interest (yes, no, maybe) was evaluated through analysis of variance (ANOVA) comparing the mean score on the summed BAYS items among the 3 groups. The association of demographic (age, education, race) and clinical factors (AJCC stage, physical function) with BAYS was determined through multivariate linear regression.
For analytic purposes, due to small subgroup sample sizes we compared those who identified as non-Hispanic White adults to those who identified as African American/Hispanic/other persons. To further evaluate the relationship of age to yoga beliefs, we examined beliefs about yoga in 3 age groups (40-59 years [n = 24]; 60-69 years [n = 58]; 70-89 years [n = 28]) using ANOVA comparing the mean score on the summed BAYS items among the 3 groups. In study 2, changes in interest before and after the yoga program were evaluated with paired t tests and repeated ANOVA, with beliefs about yoga prior to class as a covariate. The association of demographic and clinical factors with BAYS was determined as in the first sample through multivariate linear regression, except the variable of race was not included due to small sample size (ie, only 3 individuals identified as persons of color).
Thematic analysis in which content-related codes were developed and subsequently grouped together was applied to the data of 110 participants who responded to the open-ended survey questions in study 1 to further illuminate responses to closed-ended questions.35 Transcribed responses to the open-ended questions were transferred to a spreadsheet. An initial code book with code names, definitions, and examples was developed based on an inductive method by one team member (EA).35 Initially, coding and tabulation were conducted separately for each question but it was noted that content extended across response prompts (eg, responses to question 2 “What might make you more likely to come?” were spontaneously provided when answering question 1), thus coding was collapsed across questions. Next, 2 team members (EA, KD) coded the same responses, meeting weekly to discuss discrepancies. The code book was revised following each meeting to reflect refinements in code names and definitions, adding newly generated codes as needed. The process continued until consensus and data saturation was obtained, with 90% intercoder agreement. Next, these codes were subjected to thematic analysis by 2 team members (EA, KD) combining codes into 6 overarching themes. The entire team reviewed the codes and identified 2 supra themes: positive beliefs or facilitators and negative beliefs or barriers.
Consistent with the concept of reflexivity in qualitative research, we acknowledge the influence of the research team members on the qualitative process.36 The primary coding team (EA, KD) are both researchers and employees of Veterans Affairs Boston Healthcare System who have participated in other research projects involving veterans and qualitative analyses but are not yoga instructors or yoga researchers.
Results
Study 1
The sample of 110 military veterans was mostly male (99.1%) with a mean (SD) age of 64.9 (9.4) years (range, 41-88)(Table 1). The majority (70.9%) described their race/ethnicity as White, non-Hispanic followed by Black/African American (18.2%) and Hispanic (8.2%) persons; 50.0% had no more than a high school education. The most common cancer diagnoses were colorectal (50.9%), head and neck (39.1%), and esophageal and gastric (10.0%) and ranged from AJCC stages I to IV.
When first asked, the majority of participants (78.2%) reported that they were not interested in yoga, 16.4% reported they might be interested, and 5.5% reported they had tried a yoga class since their cancer diagnosis. In contrast, 40.9% used exercise, 32.7% used meditation, 14.5% used physical or occupational therapy, and 11.8% used massage therapy since their cancer diagnosis.
After participants were provided the brief scripted education about yoga, the level of interest shifted: 46.4% not interested, 21.8% interested, and 31.8% definitely interested, demonstrating a statistically significant shift in interest following education (χ2 = 22.25, P < .001) (Figure 1). Those with the most positive beliefs about yoga were most likely to indicate interest. Using the BAYS 3-item survey, the mean (SD) for the definitely interested, might be interested, and not interested groups was 15.1 (3.2), 14.1 (3.2), and 12.3 (2.5), respectively (F = 10.63, P < .001).
A multivariable regression was run to examine possible associations between participants’ demographic characteristics, clinical characteristics, and beliefs about yoga as measured by the 3 BAYS items (Table 2). Higher expected health benefits of yoga was associated with identifying as
Six themes were identified in qualitative analysis of semistructured interviews reflecting older veterans’ beliefs about yoga, which were grouped into the following suprathemes of positive vs negative beliefs (Figure 2). Exemplar responses appear in Table 3.
Study 2 Intervention Sample
This sample of 28 veterans was mostly male (96.4%) with a mean (SD) age of 69.2 (10.9) years (range, 57-87). The majority (89.3%) described their race as White, followed by Black/African American (10.7%); no participants self-identified in other categories for race/ethnicity. Twelve veterans (42.9%) had no more than a high school education. The most common cancer diagnosis was genitourinary (35.7%) and the AJCC stage ranged from I to IV.
We employed information learned in study 1 to enhance access in study 2. We mailed letters to 278 veterans diagnosed with cancer in the previous 3 years that provided education about yoga based on study 1 findings. Of 207 veterans reached by phone, 133 (64%) stated they were not interested in coming to a yoga class; 74 (36%) were interested, but 30 felt they were unable to attend due to obstacles such as illness or travel. Ultimately 37 (18%) veterans agreed and consented to the class, and 28 (14%) completed postclass surveys.
In multivariate regression, higher expected health benefits of yoga were associated with higher physical function, lower concern about expected discomfort was also associated with higher physical function as well as higher education; similarly, lower concern about expected social norms was associated with higher physical function. Age was not associated with any of the BAYS factors.
Beliefs about yoga improved from before to after class for all 3 domains with greater expected benefit and lower concerns about discomfort or social norms:
Discussion
Yoga is an effective clinical intervention for addressing some long-term adverse effects in cancer survivors, although the body of research focuses predominantly on middle aged, female, White, college-educated breast cancer survivors. There is no evidence to suggest yoga would be less effective in other groups, but it has not been extensively studied in survivors from diverse subgroups. Beliefs about yoga are a factor that may enhance interest in yoga interventions and research, and measures aimed at addressing potential beliefs and fears may capture information that can be used to support older cancer survivors in holistic health. The aims of this study were to examine beliefs about yoga in 2 samples of older cancer survivors who received VHA care. The main findings are (1) interest in yoga was initially low and lower than that of other complementary or exercise-based interventions, but increased when participants were provided brief education about yoga; (2) interest in yoga was associated with beliefs about yoga with qualitative comments illuminating these beliefs; (3) demographic characteristics (education, race) and physical function were associated with beliefs about yoga; and (4) positive beliefs about yoga increased following a brief yoga intervention and was associated with improvements in physical function.
Willingness to consider a class appeared to shift for some older veterans when they were presented brief information about yoga that explained what is involved, how it might help, and that it could be done from a chair if needed. These findings clearly indicated that when trying to enhance participation in yoga in clinical or research programs, it will be important that recruitment materials provide such information. This finding is consistent with the qualitative findings that reflected a lack of knowledge or skepticism about benefits of yoga among some participants. Given the finding that physical function was associated with beliefs about yoga and was also a prominent theme in qualitative analyses,
Age was not associated with beliefs about yoga in either study. Importantly, in a more detailed study 1 follow-up analysis, beliefs about yoga were equivalent for aged > 70 years compared with those aged 40 to 69 years. It is not entirely clear why older adults have been underrepresented in studies of yoga in cancer survivors. However, older adults are vastly underrepresented in clinical trials for many health conditions, even though they are more likely to experience many diseases, including cancer.37 A new National Institutes of Health policy requires that individuals of all ages, including older adults, must be included in all human subjects research unless there are scientific reasons not to include them.38 It is therefore imperative to consider strategies to address underrepresentation of older adults.
Qualitative findings here suggest it will be important to consider logistical barriers including transportation and affordability as well as adaptations requested by older adults (eg, preferences for older teachers).18
Although our sample was small, we also found that adults from diverse racial and ethnic backgrounds had more positive beliefs about yoga, such that this finding should be interpreted with caution. Similar to older adults, individuals from diverse racial and ethnic groups are also underrepresented in clinical trials and may have lower access to complementary treatments. Cultural and linguistic adaptations and building community partnerships should be considered in both recruitment and intervention delivery strategies.40We learned that education about yoga may increase interest and that it is possible to recruit older veterans to yoga class. Nevertheless, in study 2, our rate of full participation was low, with only about 1 in 10 participating. Additional efforts to enhance beliefs about yoga and to addresslogistical barriers (offering telehealth yoga) are needed to best reach older veterans.
Limitations
These findings have several limitations. First, participants were homogeneous in age, gender, race/ethnicity and veteran status, which provides a window into this understudied population but limits generalizability and our ability to control across populations. Second, the sample size limited the ability to conduct subgroup and interaction analyses, such as examining potential differential effects of cancer type, treatment, and PTSD on yoga beliefs or to consider the relationship of yoga beliefs with changes in quality of life before and after the yoga intervention in study 2. Additionally, age was not associated with beliefs about yoga in these samples that of mostly older adults. We were able to compare middle-aged and older adults but could not compare beliefs about yoga to adults aged in their 20s and 30s. Last, our study excluded people with dementia and psychotic disorders. Further research is needed to examine yoga for older cancer survivors who have these conditions.
Conclusions
Education that specifically informs potential participants about yoga practice, potential modifications, and potential benefits, as well as adaptations to programs that address physical and logistical barriers may be useful in increasing access to and participation in yoga for older Veterans who are cancer survivors.
Acknowledgments/Funding
The authors have no financial or personal relationships to disclose. This work was supported by the US Department of Veterans Affairs (VA) Rehabilitation Research and Development Service. This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System, Bedford VA Medical Center, and Michael E. DeBakey VA Medical Center in Houston, Texas. We thank the members of the Veterans Cancer Rehabilitation Study (Vetcares) Research teams in Boston and in Houston and the veterans who have participated in our research studies and allow us to contribute to their health care.
1. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233-3241. doi:10.1200/JCO.2012.43.7707
2. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8(2):43-55.
3. Erratum: Primary follicular lymphoma of disguised as multiple miliary like lesions: A case report and review of literature. Indian J Pathol Microbiol. 2018;61(4):643. doi:10.4103/0377-4929.243009
4. Eyigor S, Uslu R, Apaydın S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Complement Ther Clin Pract. 2018;32:40-45. doi:10.1016/j.ctcp.2018.04.010
5. Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214. Published 2014 Jul 1. doi:10.1186/1472-6882-14-214
6. Browning KK, Kue J, Lyons F, Overcash J. Feasibility of mind-body movement programs for cancer survivors. Oncol Nurs Forum. 2017;44(4):446-456. doi:10.1188/17.ONF.446-456
7. Rosenbaum MS, Velde J. The effects of yoga, massage, and reiki on patient well-being at a cancer resource center. Clin J Oncol Nurs. 2016;20(3):E77-E81. doi:10.1188/16.CJON.E77-E81
8. Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-designated comprehensive cancer center websites. J Natl Cancer Inst Monogr. 2017;2017(52):lgx004. doi:10.1093/jncimonographs/lgx004
9. Sanft T, Denlinger CS, Armenian S, et al. NCCN guidelines insights: survivorship, version 2.2019. J Natl Compr Canc Netw. 2019;17(7):784-794. doi:10.6004/jnccn.2019.0034
10. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36(25):2647-2655. doi:10.1200/JCO.2018.79.2721
11. Culos-Reed SN, Mackenzie MJ, Sohl SJ, Jesse MT, Zahavich AN, Danhauer SC. Yoga & cancer interventions: a review of the clinical significance of patient reported outcomes for cancer survivors. Evid Based Complement Alternat Med. 2012;2012:642576. doi:10.1155/2012/642576
12. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer. 2019;125(12):1979-1989. doi:10.1002/cncr.31979
13. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
14. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed September 22, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions
15. Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57(suppl 2):S289-S292. doi:10.1111/j.1532-5415.2009.02515.x
16. King K, Gosian J, Doherty K, et al. Implementing yoga therapy adapted for older veterans who are cancer survivors. Int J Yoga Therap. 2014;24:87-96.
17. Wertman A, Wister AV, Mitchell BA. On and off the mat: yoga experiences of middle-aged and older adults. Can J Aging. 2016;35(2):190-205. doi:10.1017/S0714980816000155
18. Chen KM, Wang HH, Li CH, Chen MH. Community vs. institutional elders’ evaluations of and preferences for yoga exercises. J Clin Nurs. 2011;20(7-8):1000-1007. doi:10.1111/j.1365-2702.2010.03337.x
19. Saravanakumar P, Higgins IJ, Van Der Riet PJ, Sibbritt D. Tai chi and yoga in residential aged care: perspectives of participants: A qualitative study. J Clin Nurs. 2018;27(23-24):4390-4399. doi:10.1111/jocn.14590
20. Fan JT, Chen KM. Using silver yoga exercises to promote physical and mental health of elders with dementia in long-term care facilities. Int Psychogeriatr. 2011;23(8):1222-1230. doi:10.1017/S1041610211000287
21. Taylor TR, Barrow J, Makambi K, et al. A restorative yoga intervention for African-American breast cancer survivors: a pilot study. J Racial Ethn Health Disparities. 2018;5(1):62-72. doi:10.1007/s40615-017-0342-4
22. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387-4395. doi:10.1200/JCO.2006.06.6027
23. Smith SA, Whitehead MS, Sheats JQ, Chubb B, Alema-Mensah E, Ansa BE. Community engagement to address socio-ecological barriers to physical activity among African American breast cancer survivors. J Ga Public Health Assoc. 2017;6(3):393-397. doi:10.21633/jgpha.6.312
24. Cushing RE, Braun KL, Alden C-Iayt SW, Katz AR. Military-Tailored Yoga for Veterans with Post-traumatic Stress Disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
25. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. US Department of Veterans Affairs. Whole health. Updated September 13, 2021. Accessed September 22, 2021. https://www.va.gov/wholehealth
28. Sohl SJ, Schnur JB, Daly L, Suslov K, Montgomery GH. Development of the beliefs about yoga scale. Int J Yoga Therap. 2011;(21):85-91.
29. Cadmus-Bertram L, Littman AJ, Ulrich CM, et al. Predictors of adherence to a 26-week viniyoga intervention among post-treatment breast cancer survivors. J Altern Complement Med. 2013;19(9):751-758. doi:10.1089/acm.2012.0118
30. Mackenzie MJ, Carlson LE, Ekkekakis P, Paskevich DM, Culos-Reed SN. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496. doi:10.1155/2013/419496
31. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93. Published 2013 Mar 11. doi:10.1186/1472-6963-13-93
32. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v2.0 - Physical Function 6b. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=793&Itemid=992
33. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v1.0 - Anxiety 6a. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=145&Itemid=992
34. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS-43 Profile v2.1. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=858&Itemid=992
35. Todd NJ, Jones SH, Lobban FA. “Recovery” in bipolar disorder: how can service users be supported through a self-management intervention? A qualitative focus group study. J Ment Health. 2012;21(2):114-126. doi:10.3109/09638237.2011.621471
36. Finlay L. “Outing” the researcher: the provenance, process, and practice of reflexivity. Qual Health Res. 2002;12(4):531-545. doi:10.1177/104973202129120052
37. Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;10(suppl 1):S105-S112. doi:10.2105/AJPH.2009.162982
38. National Institutes of Health. Revision: NIH policy and guidelines on the inclusion of individuals across the lifespan as participants in research involving human subjects. Published December 19, 2017. Accessed September 22, 2021. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html
39. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112-3124. doi:10.1200/JCO.2005.00.141
40. Vuong I, Wright J, Nolan MB, et al. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials-a narrative review. J Cancer Educ. 2020;35(5):841-849. doi:10.1007/s13187-019-01650-y
1. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233-3241. doi:10.1200/JCO.2012.43.7707
2. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8(2):43-55.
3. Erratum: Primary follicular lymphoma of disguised as multiple miliary like lesions: A case report and review of literature. Indian J Pathol Microbiol. 2018;61(4):643. doi:10.4103/0377-4929.243009
4. Eyigor S, Uslu R, Apaydın S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Complement Ther Clin Pract. 2018;32:40-45. doi:10.1016/j.ctcp.2018.04.010
5. Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214. Published 2014 Jul 1. doi:10.1186/1472-6882-14-214
6. Browning KK, Kue J, Lyons F, Overcash J. Feasibility of mind-body movement programs for cancer survivors. Oncol Nurs Forum. 2017;44(4):446-456. doi:10.1188/17.ONF.446-456
7. Rosenbaum MS, Velde J. The effects of yoga, massage, and reiki on patient well-being at a cancer resource center. Clin J Oncol Nurs. 2016;20(3):E77-E81. doi:10.1188/16.CJON.E77-E81
8. Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-designated comprehensive cancer center websites. J Natl Cancer Inst Monogr. 2017;2017(52):lgx004. doi:10.1093/jncimonographs/lgx004
9. Sanft T, Denlinger CS, Armenian S, et al. NCCN guidelines insights: survivorship, version 2.2019. J Natl Compr Canc Netw. 2019;17(7):784-794. doi:10.6004/jnccn.2019.0034
10. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36(25):2647-2655. doi:10.1200/JCO.2018.79.2721
11. Culos-Reed SN, Mackenzie MJ, Sohl SJ, Jesse MT, Zahavich AN, Danhauer SC. Yoga & cancer interventions: a review of the clinical significance of patient reported outcomes for cancer survivors. Evid Based Complement Alternat Med. 2012;2012:642576. doi:10.1155/2012/642576
12. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer. 2019;125(12):1979-1989. doi:10.1002/cncr.31979
13. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
14. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed September 22, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions
15. Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57(suppl 2):S289-S292. doi:10.1111/j.1532-5415.2009.02515.x
16. King K, Gosian J, Doherty K, et al. Implementing yoga therapy adapted for older veterans who are cancer survivors. Int J Yoga Therap. 2014;24:87-96.
17. Wertman A, Wister AV, Mitchell BA. On and off the mat: yoga experiences of middle-aged and older adults. Can J Aging. 2016;35(2):190-205. doi:10.1017/S0714980816000155
18. Chen KM, Wang HH, Li CH, Chen MH. Community vs. institutional elders’ evaluations of and preferences for yoga exercises. J Clin Nurs. 2011;20(7-8):1000-1007. doi:10.1111/j.1365-2702.2010.03337.x
19. Saravanakumar P, Higgins IJ, Van Der Riet PJ, Sibbritt D. Tai chi and yoga in residential aged care: perspectives of participants: A qualitative study. J Clin Nurs. 2018;27(23-24):4390-4399. doi:10.1111/jocn.14590
20. Fan JT, Chen KM. Using silver yoga exercises to promote physical and mental health of elders with dementia in long-term care facilities. Int Psychogeriatr. 2011;23(8):1222-1230. doi:10.1017/S1041610211000287
21. Taylor TR, Barrow J, Makambi K, et al. A restorative yoga intervention for African-American breast cancer survivors: a pilot study. J Racial Ethn Health Disparities. 2018;5(1):62-72. doi:10.1007/s40615-017-0342-4
22. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387-4395. doi:10.1200/JCO.2006.06.6027
23. Smith SA, Whitehead MS, Sheats JQ, Chubb B, Alema-Mensah E, Ansa BE. Community engagement to address socio-ecological barriers to physical activity among African American breast cancer survivors. J Ga Public Health Assoc. 2017;6(3):393-397. doi:10.21633/jgpha.6.312
24. Cushing RE, Braun KL, Alden C-Iayt SW, Katz AR. Military-Tailored Yoga for Veterans with Post-traumatic Stress Disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
25. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. US Department of Veterans Affairs. Whole health. Updated September 13, 2021. Accessed September 22, 2021. https://www.va.gov/wholehealth
28. Sohl SJ, Schnur JB, Daly L, Suslov K, Montgomery GH. Development of the beliefs about yoga scale. Int J Yoga Therap. 2011;(21):85-91.
29. Cadmus-Bertram L, Littman AJ, Ulrich CM, et al. Predictors of adherence to a 26-week viniyoga intervention among post-treatment breast cancer survivors. J Altern Complement Med. 2013;19(9):751-758. doi:10.1089/acm.2012.0118
30. Mackenzie MJ, Carlson LE, Ekkekakis P, Paskevich DM, Culos-Reed SN. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496. doi:10.1155/2013/419496
31. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93. Published 2013 Mar 11. doi:10.1186/1472-6963-13-93
32. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v2.0 - Physical Function 6b. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=793&Itemid=992
33. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v1.0 - Anxiety 6a. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=145&Itemid=992
34. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS-43 Profile v2.1. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=858&Itemid=992
35. Todd NJ, Jones SH, Lobban FA. “Recovery” in bipolar disorder: how can service users be supported through a self-management intervention? A qualitative focus group study. J Ment Health. 2012;21(2):114-126. doi:10.3109/09638237.2011.621471
36. Finlay L. “Outing” the researcher: the provenance, process, and practice of reflexivity. Qual Health Res. 2002;12(4):531-545. doi:10.1177/104973202129120052
37. Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;10(suppl 1):S105-S112. doi:10.2105/AJPH.2009.162982
38. National Institutes of Health. Revision: NIH policy and guidelines on the inclusion of individuals across the lifespan as participants in research involving human subjects. Published December 19, 2017. Accessed September 22, 2021. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html
39. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112-3124. doi:10.1200/JCO.2005.00.141
40. Vuong I, Wright J, Nolan MB, et al. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials-a narrative review. J Cancer Educ. 2020;35(5):841-849. doi:10.1007/s13187-019-01650-y
Cavitary Lung Lesion in a Tuberculosis-Negative Patient
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326
What’s in a Name? The Problematic Term “Provider”
Health care has been dramatically transformed and influenced by medical and technological advances, insurance companies, state and federal legislation, and medical ethics. Amid these changes, including crises such as the ongoing coronavirus pandemic, earning the trust of patients to care for their mental and physical health remains a priority and a privilege.
It is troubling that federal health care agencies, in addition to hospitals, clinics, pharmacies, insurance companies, and administrators, often use the term provider when referring to clinicians on the multidisciplinary health care treatment team, which has become the predominant model for health care delivery. The word provider does not originate in the health care arena but from the world of commerce and contains no reference to professionalism or therapeutic relationships.1 Therefore, it should be replaced with more appropriate terminology that acknowledges clinicians’ roles and expertise and values our unique relationship with patients.
Why Is Provider a Problem?
First, the origin of the term provider is deplorable. During its ascent to power in the 1930s, the Nazi Party promoted the devaluation and exclusion of Jews in German society, including the medical community. Due to its eugenics campaign, the Nazi Party first targeted pediatrics, a specialty in which nearly half of its practitioners were Jewish.2 Beginning with female pediatricians, all Jewish physicians were redesignated as Behandler (provider) instead of Arzt (doctor).2 This is the first documented demeaning of physicians as providers in modern history. Jewish doctors were soon restricted to treating only Jewish patients and were further persecuted during the Holocaust. Knowing this background, what health care organization would use a term once associated with Nazi ideology?3
Second, using provider changes the treatment relationship. The nomenclature shift in the United States also seems to have originated in political and legislative circles. Although the reasons for this shift are unclear, the terminology became more pervasive after the government first used the term provider in Title XIX of the 1965 Social Security Amendments that established Medicare and Medicaid. Paydarfar and Schwartz noted it was used “in the sense of a contractor being paid for delivering any health-related products and services.”4 Ironically, a 1967 medical student health organization grant proposal discussed the role of a patient advocate in facilitating communication between “health care provider and patient.”5 A journalist for the New York Times used the word to describe a 1970 New York Senate debate surrounding the sale of Medicaid bills to collection agencies, but it is unclear whether the senators themselves used the term.6 Provider was later used in the National Health Planning and Resource Development Act of 1974.7
Ultimately, the adaptation of this terminology led to medicine being thought of only as a business, a commoditization of care, and reinforced by referring to patients as consumers, clients, or customers.3 This terminology suggests that the clinician-patient relationship is a commercial transaction based on a market concept where patients are consumers to be serviced.1,8 Emphasis is placed on following algorithms and treating symptoms rather than patients.9 Despite a goal of minimizing cost, a mismatched referral to a provider may actually compromise patient safety and cost-effectiveness due to missed diagnoses or excessive diagnostic testing.10
In addition to government, other nonclinical entities (eg, insurance companies, advocacy groups) and some clinicians may prefer the generic term provider. Besides health care commoditization, reasons may include convenience, simplifying health care nomenclature, or removing distinctions among health care professionals to reduce costs and/or increase autonomy.
However, our value as health care professionals is not simply what we can “provide.”11 We seek to know patients as people, putting their needs ahead of ours.1 We serve as confidants and advocates and not merely providers of medications, tests, or procedures.11 This personalized nature of health care depends on trust and professionalism rather than dispassionate delivery of commoditized services.1 Using traditional terminology acknowledges the true nature of the treatment relationship—one that is established not on market concepts but on medical ethics of autonomy, justice, beneficence, and nonmaleficence.
Third, provider is inaccurate and potentially disrespectful and harmful. The word doctor is derived from Latin doctus or docere, meaning to teach or instruct—a valued function in our interactions with patients, families, students, and colleagues.12,13 In contrast, provider refers to commercial transactions or the provision of shelter, food, and love within families and communities.1,14
Although there are no studies assessing the impact of this terminology on individual clinicians, the term provider may have a negative impact on both individual clinicians and on the health care system. Health care professionals may feel they are being disrespected by being portrayed as dispensers of services rather than as individuals.13,15 Furthermore, provider does not acknowledge the specialized training and qualifications of multidisciplinary treatment team members. The historical and theoretical foundation, degrees awarded, and scopes of practice for physicians, physician assistants, nurse practitioners, dentists, psychologists, optometrists, physical therapists, or social workers are different yet valuable, and their expertise and accomplishment should be recognized.
The use of this term has potential for causing moral injury and reduced self-worth, sense of purpose, and meaning in our daily work; this could threaten satisfaction and commitment and lead to demoralization and burnout.1,16 It may impair effective team dynamics, as it makes no reference to professional values and may lead patients and clinicians to place lower value on professionalism and conduct.10 It may negatively impact primary care specialties by propagating the connotation that primary care is simple care and promoting low compensation, lagging recruitment, and diminished respect.10 Finally, it is detrimental to patients by changing the nature of the relationship and failing to evoke the compassion and support that sick people (that is, patients) need and deserve.3
Last, use of this term can mislead patients. By law, a health care provider is defined as “a doctor of medicine or osteopathy who is authorized to practice medicine or surgery… or any other person determined by the Secretary [of Labor] to be capable of providing health care services,” which includes podiatrists, dentists, clinical psychologists, optometrists, chiropractors, nurse practitioners, nurse-midwives, clinical social workers, and physician assistants.17
When clinicians are categorized as providers rather than by their degrees and roles/responsibilities, patients may assume that all team members have equal training, interchangeable skills, and uniform expertise and knowledge and may conclude they can receive the same level of care from anyone.8,10 Potential for confusion is increased by the nearly ubiquitous white laboratory coat in clinical settings and doctoral degrees attainable in different health care disciplines (eg, medicine, nursing, psychology, pharmacy, physical therapy). Patients deserve to know who does what on the team of professionals who care for them and may not be fully informed when requesting or receiving treatment if they are not provided important information, such as a clinician’s title, training, and scope of practice.8,16
Reversing the Trend
Increasing awareness among patients, their families, health professions students, and health care colleagues and administrators of the importance of traditional nomenclature is a first step in reversing this trend or mitigating its impact. If an overarching generic term is required, then health care professional, clinician, or practitioner are preferred.10,12 Fifteen years ago, the Southern California Permanente Medical Group prohibited the use of the word provider to describe physicians, and its editorial style deemed it cold and institutional.16 Many, but not all, state, regional, or national medical associations and journals avoid provider in their names or titles.
I am encouraged that this journal—drawing its audience from several government health care agencies—is named Federal Practitioner rather than Federal Provider. This is reasonable and accurate, as practitioner refers to the practice of a profession, usually associated with health care.
I hope other professions can resist this trend. Lawyers are not considered legal aid providers, and teachers are not called knowledge providers.3 We do not refer to airline pilots as air transportation providers or musicians as instrument-playing melody providers. Many veterans likely would be offended if they were referred to as Constitution support and defense providers rather than by the military branch-specific titles that they earned through dedication, training, and sacrifice. The individuals in these examples demonstrate commitment to representing clients, educating students, flying passengers, playing instruments, or ensuring national defense. As health care professionals, our commitment to treating patients is equally important.4
Language matters when it comes to people feeling respected and achieving their full potential.1 I encourage government health care agencies to stop referring to us as providers and resume using traditional nomenclature. This will demonstrate genuine respect for us, transparency for the patients we serve, and recognition that caring for the sick is a calling, not a commodity.
Dedication
The author dedicates this article to his father John E. Scarff, Jr, a physician and United States Army veteran.
1. Beasley JW, Roberts RG, Goroll AH. Promoting trust and morale by changing how the word provider is used: encouraging specificity and transparency. JAMA. 2021;325(23):2343-2344. doi:10.1001/jama.2021.6046
2. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
3. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Curr Psychiatr. 2020;19(2):5-7,29.
4. Paydarfar D, Schwartz WJ. A piece of my mind. Dear provider. JAMA. 2011;305(20):2046-2047. doi:10.1001/jama.2011.702
5. Student Health Organization. Grant Proposal of Student Health Organization. Summer Project of the South Bronx, 1967. Albert Einstein College of Medicine, unpublished.
6. Clines FX. Doctors face ban on sale of bills. New York Times. February 25, 1970:51
7. The National Health Planning and Resource Development Act of 1974. 42 USC § 300 (1975).
8. American Academy of Family Physicians. Provider, use of term (Position paper). Published 2018. Accessed September 22, 2021. https://www.aafp.org/about/policies/all/provider.html
9. Sanniec K, Gellis M. I am not a medical provider; I am a doctor. Aesthet Surg J. 2013;33(5):749-750. doi:10.1177/1090820X13487017
10. Goroll AH. Eliminating the term primary care “provider”: consequences of language for the future of primary care. JAMA. 2016;315(17):1833-1834. doi:10.1001/jama.2016.2329
11. Lee BY. Time to stop labeling physicians as providers. Published May 5, 2019. Accessed September 22, 2021. https://www.forbes.com/sites/brucelee/2019/05/05/time-to-stop-labeling-physicians-as-providers/?sh=7edfc865118e
12. Dhand S, Carbone WJ. Physicians are not providers: An open letter to the AMA and medical boards. Published November 30, 2015. Accessed September 22, 2021. https://www.kevinmd.com/blog/2015/11/physicians-are-not-providers-an-open-letter-to-the-ama-and-medical-boards.html
13. Al-Agba N. If you call me a provider, I will assume you are a Nazi. Published February 8, 2019. Accessed September 22, 2021. https://thedeductible.com/2019/02/08/if-you-call-me-a-provider-i-will-assume-you-are-a-nazi
14. Weiss JM. Physician or surgeon, but not “provider.” Published February 5, 2020. Accessed September 22, 2021. https://www.psychologytoday.com/us/blog/women-in-medicine/202002/physician-or-surgeon-not-provider
15. Liao L. Providers or professionals: how our conceptions of physician as machine or person lead to burnout. Med Teach. 2021;43(2):234-236. doi:10.1080/0142159X.2020.1769049
16. Weiss J. ‘Physician’ not ‘provider’ is better for doctor and patient. Published December 16, 2019. Accessed September 22, 2021. https://permanente.org/physician-not-provider-is-better-for-doctor-and-patient
17. Definition of Health Care Provider. 29 CFR § 825.125 (1993).
Health care has been dramatically transformed and influenced by medical and technological advances, insurance companies, state and federal legislation, and medical ethics. Amid these changes, including crises such as the ongoing coronavirus pandemic, earning the trust of patients to care for their mental and physical health remains a priority and a privilege.
It is troubling that federal health care agencies, in addition to hospitals, clinics, pharmacies, insurance companies, and administrators, often use the term provider when referring to clinicians on the multidisciplinary health care treatment team, which has become the predominant model for health care delivery. The word provider does not originate in the health care arena but from the world of commerce and contains no reference to professionalism or therapeutic relationships.1 Therefore, it should be replaced with more appropriate terminology that acknowledges clinicians’ roles and expertise and values our unique relationship with patients.
Why Is Provider a Problem?
First, the origin of the term provider is deplorable. During its ascent to power in the 1930s, the Nazi Party promoted the devaluation and exclusion of Jews in German society, including the medical community. Due to its eugenics campaign, the Nazi Party first targeted pediatrics, a specialty in which nearly half of its practitioners were Jewish.2 Beginning with female pediatricians, all Jewish physicians were redesignated as Behandler (provider) instead of Arzt (doctor).2 This is the first documented demeaning of physicians as providers in modern history. Jewish doctors were soon restricted to treating only Jewish patients and were further persecuted during the Holocaust. Knowing this background, what health care organization would use a term once associated with Nazi ideology?3
Second, using provider changes the treatment relationship. The nomenclature shift in the United States also seems to have originated in political and legislative circles. Although the reasons for this shift are unclear, the terminology became more pervasive after the government first used the term provider in Title XIX of the 1965 Social Security Amendments that established Medicare and Medicaid. Paydarfar and Schwartz noted it was used “in the sense of a contractor being paid for delivering any health-related products and services.”4 Ironically, a 1967 medical student health organization grant proposal discussed the role of a patient advocate in facilitating communication between “health care provider and patient.”5 A journalist for the New York Times used the word to describe a 1970 New York Senate debate surrounding the sale of Medicaid bills to collection agencies, but it is unclear whether the senators themselves used the term.6 Provider was later used in the National Health Planning and Resource Development Act of 1974.7
Ultimately, the adaptation of this terminology led to medicine being thought of only as a business, a commoditization of care, and reinforced by referring to patients as consumers, clients, or customers.3 This terminology suggests that the clinician-patient relationship is a commercial transaction based on a market concept where patients are consumers to be serviced.1,8 Emphasis is placed on following algorithms and treating symptoms rather than patients.9 Despite a goal of minimizing cost, a mismatched referral to a provider may actually compromise patient safety and cost-effectiveness due to missed diagnoses or excessive diagnostic testing.10
In addition to government, other nonclinical entities (eg, insurance companies, advocacy groups) and some clinicians may prefer the generic term provider. Besides health care commoditization, reasons may include convenience, simplifying health care nomenclature, or removing distinctions among health care professionals to reduce costs and/or increase autonomy.
However, our value as health care professionals is not simply what we can “provide.”11 We seek to know patients as people, putting their needs ahead of ours.1 We serve as confidants and advocates and not merely providers of medications, tests, or procedures.11 This personalized nature of health care depends on trust and professionalism rather than dispassionate delivery of commoditized services.1 Using traditional terminology acknowledges the true nature of the treatment relationship—one that is established not on market concepts but on medical ethics of autonomy, justice, beneficence, and nonmaleficence.
Third, provider is inaccurate and potentially disrespectful and harmful. The word doctor is derived from Latin doctus or docere, meaning to teach or instruct—a valued function in our interactions with patients, families, students, and colleagues.12,13 In contrast, provider refers to commercial transactions or the provision of shelter, food, and love within families and communities.1,14
Although there are no studies assessing the impact of this terminology on individual clinicians, the term provider may have a negative impact on both individual clinicians and on the health care system. Health care professionals may feel they are being disrespected by being portrayed as dispensers of services rather than as individuals.13,15 Furthermore, provider does not acknowledge the specialized training and qualifications of multidisciplinary treatment team members. The historical and theoretical foundation, degrees awarded, and scopes of practice for physicians, physician assistants, nurse practitioners, dentists, psychologists, optometrists, physical therapists, or social workers are different yet valuable, and their expertise and accomplishment should be recognized.
The use of this term has potential for causing moral injury and reduced self-worth, sense of purpose, and meaning in our daily work; this could threaten satisfaction and commitment and lead to demoralization and burnout.1,16 It may impair effective team dynamics, as it makes no reference to professional values and may lead patients and clinicians to place lower value on professionalism and conduct.10 It may negatively impact primary care specialties by propagating the connotation that primary care is simple care and promoting low compensation, lagging recruitment, and diminished respect.10 Finally, it is detrimental to patients by changing the nature of the relationship and failing to evoke the compassion and support that sick people (that is, patients) need and deserve.3
Last, use of this term can mislead patients. By law, a health care provider is defined as “a doctor of medicine or osteopathy who is authorized to practice medicine or surgery… or any other person determined by the Secretary [of Labor] to be capable of providing health care services,” which includes podiatrists, dentists, clinical psychologists, optometrists, chiropractors, nurse practitioners, nurse-midwives, clinical social workers, and physician assistants.17
When clinicians are categorized as providers rather than by their degrees and roles/responsibilities, patients may assume that all team members have equal training, interchangeable skills, and uniform expertise and knowledge and may conclude they can receive the same level of care from anyone.8,10 Potential for confusion is increased by the nearly ubiquitous white laboratory coat in clinical settings and doctoral degrees attainable in different health care disciplines (eg, medicine, nursing, psychology, pharmacy, physical therapy). Patients deserve to know who does what on the team of professionals who care for them and may not be fully informed when requesting or receiving treatment if they are not provided important information, such as a clinician’s title, training, and scope of practice.8,16
Reversing the Trend
Increasing awareness among patients, their families, health professions students, and health care colleagues and administrators of the importance of traditional nomenclature is a first step in reversing this trend or mitigating its impact. If an overarching generic term is required, then health care professional, clinician, or practitioner are preferred.10,12 Fifteen years ago, the Southern California Permanente Medical Group prohibited the use of the word provider to describe physicians, and its editorial style deemed it cold and institutional.16 Many, but not all, state, regional, or national medical associations and journals avoid provider in their names or titles.
I am encouraged that this journal—drawing its audience from several government health care agencies—is named Federal Practitioner rather than Federal Provider. This is reasonable and accurate, as practitioner refers to the practice of a profession, usually associated with health care.
I hope other professions can resist this trend. Lawyers are not considered legal aid providers, and teachers are not called knowledge providers.3 We do not refer to airline pilots as air transportation providers or musicians as instrument-playing melody providers. Many veterans likely would be offended if they were referred to as Constitution support and defense providers rather than by the military branch-specific titles that they earned through dedication, training, and sacrifice. The individuals in these examples demonstrate commitment to representing clients, educating students, flying passengers, playing instruments, or ensuring national defense. As health care professionals, our commitment to treating patients is equally important.4
Language matters when it comes to people feeling respected and achieving their full potential.1 I encourage government health care agencies to stop referring to us as providers and resume using traditional nomenclature. This will demonstrate genuine respect for us, transparency for the patients we serve, and recognition that caring for the sick is a calling, not a commodity.
Dedication
The author dedicates this article to his father John E. Scarff, Jr, a physician and United States Army veteran.
Health care has been dramatically transformed and influenced by medical and technological advances, insurance companies, state and federal legislation, and medical ethics. Amid these changes, including crises such as the ongoing coronavirus pandemic, earning the trust of patients to care for their mental and physical health remains a priority and a privilege.
It is troubling that federal health care agencies, in addition to hospitals, clinics, pharmacies, insurance companies, and administrators, often use the term provider when referring to clinicians on the multidisciplinary health care treatment team, which has become the predominant model for health care delivery. The word provider does not originate in the health care arena but from the world of commerce and contains no reference to professionalism or therapeutic relationships.1 Therefore, it should be replaced with more appropriate terminology that acknowledges clinicians’ roles and expertise and values our unique relationship with patients.
Why Is Provider a Problem?
First, the origin of the term provider is deplorable. During its ascent to power in the 1930s, the Nazi Party promoted the devaluation and exclusion of Jews in German society, including the medical community. Due to its eugenics campaign, the Nazi Party first targeted pediatrics, a specialty in which nearly half of its practitioners were Jewish.2 Beginning with female pediatricians, all Jewish physicians were redesignated as Behandler (provider) instead of Arzt (doctor).2 This is the first documented demeaning of physicians as providers in modern history. Jewish doctors were soon restricted to treating only Jewish patients and were further persecuted during the Holocaust. Knowing this background, what health care organization would use a term once associated with Nazi ideology?3
Second, using provider changes the treatment relationship. The nomenclature shift in the United States also seems to have originated in political and legislative circles. Although the reasons for this shift are unclear, the terminology became more pervasive after the government first used the term provider in Title XIX of the 1965 Social Security Amendments that established Medicare and Medicaid. Paydarfar and Schwartz noted it was used “in the sense of a contractor being paid for delivering any health-related products and services.”4 Ironically, a 1967 medical student health organization grant proposal discussed the role of a patient advocate in facilitating communication between “health care provider and patient.”5 A journalist for the New York Times used the word to describe a 1970 New York Senate debate surrounding the sale of Medicaid bills to collection agencies, but it is unclear whether the senators themselves used the term.6 Provider was later used in the National Health Planning and Resource Development Act of 1974.7
Ultimately, the adaptation of this terminology led to medicine being thought of only as a business, a commoditization of care, and reinforced by referring to patients as consumers, clients, or customers.3 This terminology suggests that the clinician-patient relationship is a commercial transaction based on a market concept where patients are consumers to be serviced.1,8 Emphasis is placed on following algorithms and treating symptoms rather than patients.9 Despite a goal of minimizing cost, a mismatched referral to a provider may actually compromise patient safety and cost-effectiveness due to missed diagnoses or excessive diagnostic testing.10
In addition to government, other nonclinical entities (eg, insurance companies, advocacy groups) and some clinicians may prefer the generic term provider. Besides health care commoditization, reasons may include convenience, simplifying health care nomenclature, or removing distinctions among health care professionals to reduce costs and/or increase autonomy.
However, our value as health care professionals is not simply what we can “provide.”11 We seek to know patients as people, putting their needs ahead of ours.1 We serve as confidants and advocates and not merely providers of medications, tests, or procedures.11 This personalized nature of health care depends on trust and professionalism rather than dispassionate delivery of commoditized services.1 Using traditional terminology acknowledges the true nature of the treatment relationship—one that is established not on market concepts but on medical ethics of autonomy, justice, beneficence, and nonmaleficence.
Third, provider is inaccurate and potentially disrespectful and harmful. The word doctor is derived from Latin doctus or docere, meaning to teach or instruct—a valued function in our interactions with patients, families, students, and colleagues.12,13 In contrast, provider refers to commercial transactions or the provision of shelter, food, and love within families and communities.1,14
Although there are no studies assessing the impact of this terminology on individual clinicians, the term provider may have a negative impact on both individual clinicians and on the health care system. Health care professionals may feel they are being disrespected by being portrayed as dispensers of services rather than as individuals.13,15 Furthermore, provider does not acknowledge the specialized training and qualifications of multidisciplinary treatment team members. The historical and theoretical foundation, degrees awarded, and scopes of practice for physicians, physician assistants, nurse practitioners, dentists, psychologists, optometrists, physical therapists, or social workers are different yet valuable, and their expertise and accomplishment should be recognized.
The use of this term has potential for causing moral injury and reduced self-worth, sense of purpose, and meaning in our daily work; this could threaten satisfaction and commitment and lead to demoralization and burnout.1,16 It may impair effective team dynamics, as it makes no reference to professional values and may lead patients and clinicians to place lower value on professionalism and conduct.10 It may negatively impact primary care specialties by propagating the connotation that primary care is simple care and promoting low compensation, lagging recruitment, and diminished respect.10 Finally, it is detrimental to patients by changing the nature of the relationship and failing to evoke the compassion and support that sick people (that is, patients) need and deserve.3
Last, use of this term can mislead patients. By law, a health care provider is defined as “a doctor of medicine or osteopathy who is authorized to practice medicine or surgery… or any other person determined by the Secretary [of Labor] to be capable of providing health care services,” which includes podiatrists, dentists, clinical psychologists, optometrists, chiropractors, nurse practitioners, nurse-midwives, clinical social workers, and physician assistants.17
When clinicians are categorized as providers rather than by their degrees and roles/responsibilities, patients may assume that all team members have equal training, interchangeable skills, and uniform expertise and knowledge and may conclude they can receive the same level of care from anyone.8,10 Potential for confusion is increased by the nearly ubiquitous white laboratory coat in clinical settings and doctoral degrees attainable in different health care disciplines (eg, medicine, nursing, psychology, pharmacy, physical therapy). Patients deserve to know who does what on the team of professionals who care for them and may not be fully informed when requesting or receiving treatment if they are not provided important information, such as a clinician’s title, training, and scope of practice.8,16
Reversing the Trend
Increasing awareness among patients, their families, health professions students, and health care colleagues and administrators of the importance of traditional nomenclature is a first step in reversing this trend or mitigating its impact. If an overarching generic term is required, then health care professional, clinician, or practitioner are preferred.10,12 Fifteen years ago, the Southern California Permanente Medical Group prohibited the use of the word provider to describe physicians, and its editorial style deemed it cold and institutional.16 Many, but not all, state, regional, or national medical associations and journals avoid provider in their names or titles.
I am encouraged that this journal—drawing its audience from several government health care agencies—is named Federal Practitioner rather than Federal Provider. This is reasonable and accurate, as practitioner refers to the practice of a profession, usually associated with health care.
I hope other professions can resist this trend. Lawyers are not considered legal aid providers, and teachers are not called knowledge providers.3 We do not refer to airline pilots as air transportation providers or musicians as instrument-playing melody providers. Many veterans likely would be offended if they were referred to as Constitution support and defense providers rather than by the military branch-specific titles that they earned through dedication, training, and sacrifice. The individuals in these examples demonstrate commitment to representing clients, educating students, flying passengers, playing instruments, or ensuring national defense. As health care professionals, our commitment to treating patients is equally important.4
Language matters when it comes to people feeling respected and achieving their full potential.1 I encourage government health care agencies to stop referring to us as providers and resume using traditional nomenclature. This will demonstrate genuine respect for us, transparency for the patients we serve, and recognition that caring for the sick is a calling, not a commodity.
Dedication
The author dedicates this article to his father John E. Scarff, Jr, a physician and United States Army veteran.
1. Beasley JW, Roberts RG, Goroll AH. Promoting trust and morale by changing how the word provider is used: encouraging specificity and transparency. JAMA. 2021;325(23):2343-2344. doi:10.1001/jama.2021.6046
2. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
3. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Curr Psychiatr. 2020;19(2):5-7,29.
4. Paydarfar D, Schwartz WJ. A piece of my mind. Dear provider. JAMA. 2011;305(20):2046-2047. doi:10.1001/jama.2011.702
5. Student Health Organization. Grant Proposal of Student Health Organization. Summer Project of the South Bronx, 1967. Albert Einstein College of Medicine, unpublished.
6. Clines FX. Doctors face ban on sale of bills. New York Times. February 25, 1970:51
7. The National Health Planning and Resource Development Act of 1974. 42 USC § 300 (1975).
8. American Academy of Family Physicians. Provider, use of term (Position paper). Published 2018. Accessed September 22, 2021. https://www.aafp.org/about/policies/all/provider.html
9. Sanniec K, Gellis M. I am not a medical provider; I am a doctor. Aesthet Surg J. 2013;33(5):749-750. doi:10.1177/1090820X13487017
10. Goroll AH. Eliminating the term primary care “provider”: consequences of language for the future of primary care. JAMA. 2016;315(17):1833-1834. doi:10.1001/jama.2016.2329
11. Lee BY. Time to stop labeling physicians as providers. Published May 5, 2019. Accessed September 22, 2021. https://www.forbes.com/sites/brucelee/2019/05/05/time-to-stop-labeling-physicians-as-providers/?sh=7edfc865118e
12. Dhand S, Carbone WJ. Physicians are not providers: An open letter to the AMA and medical boards. Published November 30, 2015. Accessed September 22, 2021. https://www.kevinmd.com/blog/2015/11/physicians-are-not-providers-an-open-letter-to-the-ama-and-medical-boards.html
13. Al-Agba N. If you call me a provider, I will assume you are a Nazi. Published February 8, 2019. Accessed September 22, 2021. https://thedeductible.com/2019/02/08/if-you-call-me-a-provider-i-will-assume-you-are-a-nazi
14. Weiss JM. Physician or surgeon, but not “provider.” Published February 5, 2020. Accessed September 22, 2021. https://www.psychologytoday.com/us/blog/women-in-medicine/202002/physician-or-surgeon-not-provider
15. Liao L. Providers or professionals: how our conceptions of physician as machine or person lead to burnout. Med Teach. 2021;43(2):234-236. doi:10.1080/0142159X.2020.1769049
16. Weiss J. ‘Physician’ not ‘provider’ is better for doctor and patient. Published December 16, 2019. Accessed September 22, 2021. https://permanente.org/physician-not-provider-is-better-for-doctor-and-patient
17. Definition of Health Care Provider. 29 CFR § 825.125 (1993).
1. Beasley JW, Roberts RG, Goroll AH. Promoting trust and morale by changing how the word provider is used: encouraging specificity and transparency. JAMA. 2021;325(23):2343-2344. doi:10.1001/jama.2021.6046
2. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
3. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Curr Psychiatr. 2020;19(2):5-7,29.
4. Paydarfar D, Schwartz WJ. A piece of my mind. Dear provider. JAMA. 2011;305(20):2046-2047. doi:10.1001/jama.2011.702
5. Student Health Organization. Grant Proposal of Student Health Organization. Summer Project of the South Bronx, 1967. Albert Einstein College of Medicine, unpublished.
6. Clines FX. Doctors face ban on sale of bills. New York Times. February 25, 1970:51
7. The National Health Planning and Resource Development Act of 1974. 42 USC § 300 (1975).
8. American Academy of Family Physicians. Provider, use of term (Position paper). Published 2018. Accessed September 22, 2021. https://www.aafp.org/about/policies/all/provider.html
9. Sanniec K, Gellis M. I am not a medical provider; I am a doctor. Aesthet Surg J. 2013;33(5):749-750. doi:10.1177/1090820X13487017
10. Goroll AH. Eliminating the term primary care “provider”: consequences of language for the future of primary care. JAMA. 2016;315(17):1833-1834. doi:10.1001/jama.2016.2329
11. Lee BY. Time to stop labeling physicians as providers. Published May 5, 2019. Accessed September 22, 2021. https://www.forbes.com/sites/brucelee/2019/05/05/time-to-stop-labeling-physicians-as-providers/?sh=7edfc865118e
12. Dhand S, Carbone WJ. Physicians are not providers: An open letter to the AMA and medical boards. Published November 30, 2015. Accessed September 22, 2021. https://www.kevinmd.com/blog/2015/11/physicians-are-not-providers-an-open-letter-to-the-ama-and-medical-boards.html
13. Al-Agba N. If you call me a provider, I will assume you are a Nazi. Published February 8, 2019. Accessed September 22, 2021. https://thedeductible.com/2019/02/08/if-you-call-me-a-provider-i-will-assume-you-are-a-nazi
14. Weiss JM. Physician or surgeon, but not “provider.” Published February 5, 2020. Accessed September 22, 2021. https://www.psychologytoday.com/us/blog/women-in-medicine/202002/physician-or-surgeon-not-provider
15. Liao L. Providers or professionals: how our conceptions of physician as machine or person lead to burnout. Med Teach. 2021;43(2):234-236. doi:10.1080/0142159X.2020.1769049
16. Weiss J. ‘Physician’ not ‘provider’ is better for doctor and patient. Published December 16, 2019. Accessed September 22, 2021. https://permanente.org/physician-not-provider-is-better-for-doctor-and-patient
17. Definition of Health Care Provider. 29 CFR § 825.125 (1993).
A Facility-Wide Plan to Increase Access to Medication for Opioid Use Disorder in Primary Care and General Mental Health Settings
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
Operational Curriculum and Research Initiatives: Shaping the Future of Military Medicine
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
It is a time of significant change as the Military Health System (MHS) transitions to the purview of the Defense Health Agency (DHA). Additionally, the landscape of combat is ever changing, and military medicine needs to evolve to ensure that the lessons learned are utilized to optimize care of the war fighters. The purpose of this review is to evaluate the available literature on existing operational medicine curriculums and make recommendations to restructure current military medicine training to produce operationally prepared clinicians who are informed in operationally focused research principles.
Operational Medicine
Before diving into the importance of creating a curriculum and investing in training for scholarly activity proficiency, operational medicine needs to be defined. It can be defined as medical care provided in an austere environment with limited resources and possibly under hostile conditions. Another way to look at operational medicine is as the evaluation of normal human physiology and pathology under abnormal conditions. The mission set of each of the services is unique. The Marines and Army may operate forward past the wire vulnerable to the environment, gunfire, and improvised explosive devices, remote from fixed medical facilities. The Navy has divers exposed to the risks of decompression sickness. The Air Force has pilots exposed to altitude changes and strains of G-forces during flight. Locations vary from cold high-altitude mountainous regions to high-temperature desolate deserts. Many times, medical practitioners may be remotely stationed, far from specialty or immediate definitive care. Patient care may consist of low-acuity management of individual patients in sick call to mass casualty events where patient numbers and morbidity may outstrip available resources, making the difficult task of triage necessary.
Despite the challenges of being a uniformed physician, the benefits of being embedded is a better understanding of the roles and capability of the unit. Military physicians need to have the unique knowledge of the type of injuries sustained in that particular theater of war, such as differentiating between the trauma pattern and care required for blast injuries vs high-velocity missiles. There are also chemical, biologic, radiologic, and nuclear threats that military physicians need to recognize. Much of what disables a military fighting force is not a direct relationship to combat-related injuries; however, entire units have been taken down by infectious diarrhea or trench foot. There is also a need for familiarity of the infections and parasitology endemic to the particular theater with the aim of implementation of prevention whenever possible.
Military medicine does not fit in any box. Military physicians need to know the job requirements of various specialties, including elements of occupational medicine, such as aircrew piloting high-performance fighters or ground troops fully loaded with body armor and 80-lb backpacks. There are musculoskeletal injuries from the stressors of various military occupations. Working around weaponry and contact with hostile forces will create scenarios requiring emergent and critical care. In addition to physical injuries, there is the mental strain of combat with the risk of imminent personal injury, the guilt of survivorship, dealing with the scars and permanent physical damage of combat, and prolonged separation from family and other support systems.
The National Defense Authorization Act 2017 mandated the establishment of a standardized process to oversee all military graduate medical education (GME) programs with the goal of ensuring medical operational readiness.1 This is no small task with > 3000 residents in more than 70 specialties, comprising approximately 12% of US residents.1,2 Presently, 26 to 32% of the medical corps is enrolled in full-time training compared with 12% of the total force.2 With significant time and resources expended during this period, it is vital to maximize the potential of the training.
Literature Review
A literature review was performed, evaluating historical precedence of specialized military medical training and research as well as current operational curriculums. Literature search was conducted in the PubMed and Uniformed Services University (USU) Learning Resource databases using the terms “operational medicine curriculum,” “military medicine curriculum,” “operational medicine training,” “military medicine training,” “operational medicine research,” and “military medicine research,” and included all articles from 1997 to 2020. Inclusion criteria included studies that detailed military medicine training programs and/or outcomes. The source types used in this research project included peer-reviewed journal publications—both review articles and original research—from medical and military journals. The citations of these articles were also reviewed for additional usable publications. Secondary sources included official reports and studies by the RAND Corporation, the US Government Accountability Office, and the Institute for Defense Analysis (IDA). Due to lack of literature on the topic, other sources such as talking papers, letters, and formal presentations from subject matter experts were included to showcase the current state and gaps on this topic. Key findings from peer-reviewed publications are presented in Table 1.
Overall, the literature review showed that longitudinal deliberately mapped out curriculums can be well integrated into the existing medical curriculum.3 The military medicine course topics include environmental medicine, applied field medicine, combat casualty care, medical support planning, mass casualty incident preparation, and military-focused problem solving, decision making, and leadership.4
One 1997 study looked at the degree of implementation of military unique curriculum in 18 family medicine residencies. Only 30% of residents stated that their program had a specific operational medicine curriculum.5 Salerno and colleagues surveyed current residents and recently graduated internal medicine physicians at 14 facilities in the Army, Air Force, and Navy to determine confidence level with military medicine. More than half did not feel ready to practice deployment medicine; just 19% felt comfortable treating nuclear, biologic, and chemical warfare injuries; and 32% felt unfamiliar with the command and administrative duties. A subgroup analysis showed that USU graduates felt more prepared in these areas compared with civilian program graduates.6 Additional studies showed perceived smoother transition in the first active-duty tour after participation in an operational curriculum.7
Didactics can provide a foundation. However, just as the practice of medicine is learned in the clinic, the art of military medicine is learned in the field. Hands-on training in one study was accomplished through the Combat Casualty Care Course (C4), the USU Bushmaster exercise, and a field training exercise. The field exercise included components of mission planning, medical threat assessments, triage of a mass casualty situation, management of disease and nonbattle injuries, combat stress casualties, resource management, and patient evacuation.8
Another publication described a similar longitudinal curriculum with C4 after the first year of training and the Medical Management of Chemical and Biological Casualty Course during the second year. The operational curriculum 3-day capstone occurred at the end of medical training utilizing mannequins to realistically simulate combat casualty care, including emergency airways, chest tube, and tourniquets.9 Due to the current deployment tempo, just in time refresher courses like this could be valuable preparation.
While most of the operational curriculums evaluated assessed efficiency over a short time interval, one study looked at 1189 graduates from the military medical school from the past 20 years. Preparedness was perceived to be high for military-unique practice and leadership.10 The operational curriculum at USU had been purposefully structured to provide continuity. Didactics and casework were reinforced with hands-on training whether through realistic simulator training or field exercises. The authors note a weakness of many operational curriculums is inconsistency and fragmented training without deliberate longitudinal planning.
One of the more recent military GME curriculums include the creation of the operational medicine residency in 2013, which created a standardized longitudinal operational curriculum integrated along with the existing family medicine, emergency medicine, or internal medicine curriculum to create mission-ready military physicians upon graduation. Scheduled rotations include global medicine, aeromedical evacuation, occupational medicine, and tropical medicine. Completing military officer professional development and an operationally relevant research project is an expectation (Table 2).11
In addition to in-program training, other options include operational rotations offsite and military courses conducted outside the GME program.12 Some of these courses may include just-in-time training such as expeditionary medical support system training prior to scheduled deployments. Examples of experiential training are listed in Table 3.
Critical Analysis
Current gaps were identified in the military medicine training pipeline’s operational medicine curriculum and research programs. The analysis looked at specific components that make the operational medicine curriculum and research unique as well as current readiness goals, to determine how to best align both to meet the mission requirements. Some factors considered included efficiency, cost, program portability, duplication minimization, retention, and sustainability.
Efficiency
A well-created curriculum that meets objectives will require more than an assigned rotation and a few lectures. The most successful ones in the literature review were the ones that were deliberately planned and longitudinal, such as the ones at USU that combined a mixture of classroom and field exercises over the course of 4 years.4,8 In that way, the curriculum may not be considered time efficient, but if integrated well into the already existing medical training, the production of military physicians who are mission ready upon graduation—ready to serve as military medical leaders and deploy—will be invaluable.
Cost Comparison
Due to the associated overhead of running a training platform and the additional hours of operational training, military GME is more expensive initially compared with civilian outsourcing. In USU, for example, there is an additional 700 hours of operational curriculum alone. This cost difference more than doubles the cost of a USU education vs a Health Professional Scholarship Program (HPSP) scholarship at a civilian medical school. However, a causal analysis performed by the IDA to determine value basis noted that USU graduates deploy almost 3 times as much and serve 6 years longer on active duty.3
After graduating medical school through either accession source, physicians complete specialization training in a GME program. The IDA study noted an average $12,000 increased cost of military GME compared with civilian programs. The analysis included resident compensation and overhead costs of running the program as well as the net cost, which also accounted for resident productivity and workload by training in a military facility.3 Calculations due to mandated budget cuts estimated cost savings of closing the military medical school at < $100 million while significantly impacting the military physician pipeline and operational research output.3
Duplication of Effort
There are already established training programs such as Tactical Combat Casualty Care (TCCC) that could be incorporated into the curriculum to avoid expending additional resources to recreate the wheel. USU has a validated operational training curriculum and may be able to make opportunities available for outside trainees to participate in some of its military-unique training and leadership exercises. Other ways to decrease duplication of effort and improve cost efficiency include focusing on the creation of an academic health system (AHS) and consolidating similar programs to conserve resources. Increasing existing military program sizes will not only ensure the continuation of the military medicine pipeline, but will spread overhead costs over a larger cohort, decrease costs of civilian outsourcing, and ensure the less tangible benefits of military cultural exposure early in trainees’ careers. For example, increasing the class size of USU by 30 students actually reduces the cost per student to $239,000 per year from $253,000, while decreasing the need for HPSP accessions training in civilian programs, making the endeavor overall cost neutral.3
Program Portability
The operational medicine residency has proved that an operational curriculum can be remotely managed and reproduced at a variety of residency specialties.12 Remote education could be developed and distributed throughout the MHS, such as the proposed USU course Military Medicine and Leadership course.3 Centralized training programs like Global Medicine and C-STARS could be scheduled TDYs during the medical training calendar.
Retention
The military medical school, USU, is the largest military medicine accession source. An IDA report notes that retention of USU graduates is 15.2 years compared with 9.2 years served by civilian trainees. Due to the longevity in service, USU graduates also make up more than 25% of military medical leadership.4 The long-term outcome study that looked at the past 40 years of USU graduates observed that over 70% of graduates served until retirement eligibility and are overrepresented in special operations units.3,13 While some of this longevity may be attributed to the longer USU service contracts, military GME graduates were still noted to be 4 times more likely to commit to a multiyear service contract.14 A RAND study on the retention of military physicians in the Army, Air Force, and Navy noted that overall retention increased throughout all the services for physicians who went through the military GME pipeline.15 Conversely, civilian GME training was associated with a 45% chance in leaving active duty.16
It is theorized that early military acculturation during training increases the likelihood of instilling a sense of mission. Being involved in military GME on the teaching side also showed increased retention rates for 63% of survey respondents.17 Reduced burnout and increased work satisfaction for those involved in military GME was noted on another faculty satisfaction survey.17
Sustainability
Programs like USU, which have been around for decades, and the newer operational residency program evolving since 2013 have shown sustainability.4,11 Dissemination of proven curriculums as well as centralization of already validated training programs can help standardize operational medical training throughout the MHS. In order to flourish at individual programs, the faculty need to be well versed in a train the trainer model and have institutional support. The ability to engage with the line at individual locations may be a factor as well.18 In regard to research, once residents are taught the principles of scholarly activity, they will have the tools to continue operational medicine research advancements and mentoring students.
Discussion
The 2020 NDAA recommends the establishment of an AHS.3 This step will create a culture of military medical readiness from the top down as congressional mandates push reorganization of the MHS, including military GME programs. An overall restructuring of military medicine will require prioritization of resources toward operational requirements vs the historic significant division of attention to beneficiary care that has caused a lack of unity of effort and additional strain on an already heavily tasked medical force. The changes in military GME are just one aspect of that. It is vital to look at the restructuring with a comprehension of the unique challenges of combat health rather than only from an in-garrison, hospital-based aspect.19 Benefits of having a military medicine AHS include opportunities to share resources and successful business models as well as foster interdisciplinary teamwork and partnerships with civilian health care facilities and research institutions as a force multiplier.19
There has been recent discussion about budget cuts, including shutting down USU and military GME and transitioning all training to civilian programs to be cost-effective.4 If this were to happen, it would be a step backward from the goal of operational readiness. Maintaining US Department of Defense (DoD) control of the military medicine pipeline has innumerable benefits, including built-in mentorship from operationally-seasoned faculty, military leadership development, proficiency in MHS systems, open communication between GME programs and DoD, and curriculum control to ensure focus on readiness.20 Military GME programs are also a significant production source of military-related scholarly activity. Over fiscal year 2017/2018, 63% of the publications out of the San Antonio Uniformed Services Health Education Consortium—the largest Air Force GME platform and second largest multiservice GME platform—involved military relevant medical topics.17 Much of the volume of operational research as well as the relevant skills learned and future innovations secondary to conducting this research would be lost if military GME did not exist.17,21
Practically speaking, military GME provides the majority of the military medicine accessions. For example, a presentation by the Air Force Chief of Physician Education noted that the total military GME pipeline included 2875 students, but direct physician access averaged only 20 physicians a year.22 Even if the decision was made to defer to civilian education, capacity does not exist in civilian GME programs. This is worsened by the increased competitiveness of the GME match with the proliferation of medical schools without concurrent increase in residency spots. The 2018 National Resident Matching Program noted that there were more than 37,103 US and foreign applicants for only 33,000 residency positions, leaving many US applicants unmatched.17 It is doubtful that the civilian GME programs would be able to absorb the influx of military residents, affecting both the military and civilian medicine pipelines. As a secondary effect, the military treatment centers that house the military GME programs would have to close, with surrounding civilian medical facilities also likely unable to absorb the sudden influx of patients and residents losing the intangible benefits of caring for a military population.15 This was even recognized by the civilian president of the Accreditation Council for Graduate Medical Education:
Military physicians must be trained in the systems of care that are operative in military medicine, which is significantly unlike civilian medicine in many ways. It is often practiced in circumstances that are not seen in civilian medicine, within care structures that are not encountered in American medical practice… Military medicine has advanced research into the care of individuals suffering traumatic injury, critical care, rehabilitation medicine, prosthetics, psychiatric care of those traumatized, and closed head injury, to name a just a few. The sacrifices of our active military demand these advances, and the American Public benefit from these advances.21
Where deficiencies exist in military GME, it is possible to use the growing military-civilian training institution partnerships. Two prime examples are the just-in-time deployment training done with civilian trauma facilities by the Air Force Center for the Sustainment of Trauma Readiness Skills and the Air Force Special Operations Surgical Team-Special Operations Critical Care Evacuation Team being embedded in civilian facilities to maintain trauma, surgical, and emergency care skills. While military physicians can maintain competencies, at the same time, the civilian sector can benefit from the lessons learned in the military in regard to mass casualty and disaster responses. Fostering military and civilian training agreements can also enhance research opportunities.1
Just as the realities of operational medicine frequently require the military physician to think outside the box, the most successful methods of instruction of military medicine tend to be nontraditional. Classroom education should be involved beyond lectures and can include other methods, such as case-based, role-playing, small group discussion, and computer-based teaching. Maintaining flexibility in live vs distance learning as well as synchronous vs asynchronous learning can expand the capacity of available instructors and standardize material over several sites.23 Asking learners to consider operational concerns, such as whether certain medical conditions would be compatible with military duty in addition to the routine investigation is an easy way to incorporate military training in preexisting medical training.12 The advancement of technology has made simulation one of the best ways to engage in hands-on learning, whether through computer simulations, animal models, standardized or moulaged patients, or mannequins that can realistically mimic medical or trauma-related conditions.24 Many times, simulation can be combined with exercises in the field to create a realistic operational environment.23
There are 3 pillars of an operational curriculum that should be integrated into the existing residency curriculum—operational medicine, leadership, and research principles (Appendix).
Conclusions
Judging by the continuing operational tempo and evolution of warfare, maintaining enhanced military medical readiness will remain a priority. Operational medicine is a unique field that requires specialized preparation. Studies have shown that longitudinal deliberately mapped out curriculums are able to be integrated well into the existing medical curriculum. The recommendation moving forward is increasing the access of existing operational training structures that have well established programs and modeling individual GME program curriculums after those that have shown proven success with a focus on the 3 pillars of operational training, leadership, and research.
Acknowledgments
Previously submitted in April 2020 in expanded form as part of graduation requirements for the Masters of Military Arts and Science degree program at Air University, Maxwell Air Force Base in Alabama.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
1. US Government Accountability Office. Defense Health Care: DoD’s proposed plan for oversight of graduate medical education program. Published March 2019. Accessed September 24, 2021. https://www.gao.gov/assets/700/698075.pdf
2. De Lorenzo RA. Accreditation status of U.S. military graduate medical education programs. Mil Med. 2008;173(7):635-640. doi:10.7205/milmed.173.7.635
3. John SK, Bishop JM, Hidreth LA, et al; Institute for Defense Analysis. Analysis of DoD accession alternatives for military physicians: readiness value and cost. Published October 2019. Accessed September 24, 2021. https://www.ida.org/-/media/feature/publications/a/an/analysis-of-dod-accession-alternatives-for-military-physicians-readiness-value-and-cost/p-10815.ashx.
4. O’Connor FG, Grunberg N, Kellermann AL, Schoomaker E. Leadership education and development at the Uniformed Services University. Mil Med. 2015;180(suppl 4):147-152. doi:10.7205/MILMED-D-14-00563
5. Suls H, Karnei K, Gardner JW, Fogarty JP, Llewellyn CH. The extent of military medicine topics taught in military family practice residency programs: Part II, a survey of residency graduates from 1987-1990. Mil Med. 1997;162(6):428-434. doi:10.1093/milmed/162.6.428
6. Salerno S, Cash B, Cranston M, Schoomaker E. Perceptions of current and recent military internal medicine residents on operational medicine, managed care, graduate medical education, and continued military service. Mil Med. 1998;163(6):392-397. doi:10.1093/milmed/163.6.392
7. Roop SA, Murray CK, Pugh AM, Phillips YY, Bolan CD. Operational medicine experience integrated into a military internal medicine residency curriculum. Mil Med. 2001;166(1):34-39. doi:10.1093/milmed/166.1.34
8. Perkins JG, Roy MJ, Bolan CD, Phillips YY. Operational experiences during medical residency: perspectives from the Walter Reed Army Medical Center Department of Medicine. Mil Med. 2001;166(12):1038-1045. doi:10.1093/milmed/166.12.1038
9. Murray CK, Reynolds JC, Boyer DA, et al. Development of a deployment course for graduating military internal medicine residents. Mil Med. 2006;171(10):933-936. doi:10.7205/milmed.171.10.933. doi:10.7205/milmed.171.10.933
10. Picho K, Gilliland WR, Artino AR Jr, et al. Assessing curriculum effectiveness: a survey of Uniformed Services University medical school graduates. Mil Med. 2015;180(suppl 4):113-128. doi:10.7205/MILMED-D-14-00570
11. Jacobson MD: Operational Aerospace medicine collaborative programs: past, present, and future. US Air Force School of Aerospace Medicine Presentation. November 1, 2018.
12. Roy MJ, Brietzke S, Hemmer P, Pangaro L, Goldstein R. Teaching military medicine: enhancing military relevance within the fabric of current medical training. Mil Med. 2002;167(4):277-280. doi:10.1093/miled.milmed.167.4.277
13. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170. doi:10.7205/MILMED-D-14-00574
14. Keating EG, Brauner MK, Galway LA, Mele JD, Burks JJ, Saloner B. The Air Force Medical Corps’ status and how its physicians respond to multiyear special pay. Mil Med. 2009;174(11):1155-1162. doi:10.7205/milmed-d-01-4309
15. Mundell BF. Retention of military physicians: the differential effects of practice opportunities across the three services. RAND Corporation; 2010:74-77. Accessed September 24, 2021. https://www.rand.org/pubs/rgs_dissertations/RGSD275.html
16. Nagy CJ. The importance of a military-unique curriculum in active duty graduate medical education. Mil Med. 2012;177(3):243-244. doi:10.7205/milmed-d-11-00280
17. True M: The value of military graduate medical education. SAUSHEC interim dean talking paper. November 2, 2018.
18. Hatzfeld JJ, Khalili RA, Hendrickson TL, Reilly PA. Publishing military medical research: appreciating the process. Mil Med. 2016;181(suppl 5):5-6. doi:10.7205/MILMED-D-15-00517
19. Sauer SW, Robinson JB, Smith MP, et al. Lessons learned: saving lives on the battlefield. J Spec Oper Med. 2016;15(2). 25-41.
20. Tankersley MS: Air Force Physician Education Branch response to GME questions. Talking Paper. Feb 23, 2015.
21. Nasca TJ. [Letter] Published October 26, 2019. Accessed September 24, 2021. https://www.moaa.org/uploadedfiles/nasca-to-kellerman-a--cordts-p-2019-10-26.pdf
22. Forgione MA: USAF-SAM GME Brief. Air Force Personnel Center. October 2018.
23. Turner M, Wilson C, Gausman K, Roy MJ. Optimal methods of learning for military medical education. Mil Med. 2003;168(suppl 9):46-50. doi:10.1093/milmed/168.suppl_1.46
24. Goolsby C, Deering S. Hybrid simulation during military medical student field training--a novel curriculum. Mil Med. 2013;178(7):742-745. doi:10.7205/MILMED-D-12-00541
25. Hartzell JD, Yu CE, Cohee BM, Nelson MR, Wilson RL. Moving beyond accidental leadership: a graduate medical education leadership curriculum needs assessment. Mil Med. 2017;182(7):e1815-e1822. doi:10.7205/MILMED-D-16-00365
26. Barry ES, Dong T, Durning SJ, Schreiber-Gregory D, Torre D, Grunberg NE. Medical Student Leader Performance in an Applied Medical Field Practicum. Mil Med. 2019;184(11-12):653-660. doi:10.1093/milmed/usz121
27. Air Force Medical Corps Development Team: Medical corps integrated OPS career path. MC Pyramids 2019 Presentation. January 18, 2019. https://kx.health.mil [Nonpublic source, not verified]
28. Polski MM: Back to basics—research design for the operational level of war. Naval War College Rev. 2019;72(3):1-23. https://digital-commons.usnwc.edu/nwc-review/vol72/iss3/6.
VA Firearm Policy Got It Half Right
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
To the Editor: September is National Suicide Prevention and Awareness month. In 2021, the US Department of Veterans Affairs (VA) Office of Mental Health and Suicide Prevention marked the month by demonstrating why it is the national visionary when it comes to preventing suicide. The office rolled out several public service announcements (PSAs) about creating “space between thought and trigger.”1 These incredibly sensitive spots, the first of their kind, encourage safer storage and reduced access to firearms at points of heightened crises. The PSAs are timely, especially given the just released annual report showing that 69.2% of veteran suicide deaths are by firearm.2 Wide PSA dissemination is vital.
But concerningly, the PSAs completely missed the importance of critical partnerships. As described in Federal Practitioner 2 years ago, VA forged a groundbreaking collaboration with the National Shooting Sports Foundation (NSSF), the firearms industry trade association, and the American Foundation for Suicide Prevention (AFSP).3 Having NSSF as a partner advanced VA’s effort to ensure that lethal means safety counseling is culturally relevant, comes from a trusted source, and contains no antifirearm bias. Since then, VA and NSSF cobranded billboards in 8 states, encouraging storing firearms responsibly to prevent suicide. They collectively developed an educational, training, and resource toolkit that guides communities through the process of building coalitions to raise awareness about securely storing firearms when not in use.4 VA and NSSF have cross-listed safe storage websites. In May 2020, the VA cosponsored a COVID-19 suicide prevention video with the NSSF, AFSP, and the US Concealed Carry Association, including ways that the firearm industry, gun owners, and their families can help.5
Yet when the VA launched its PSA campaign last month, NSSF’s name was conspicuously absent. That must be corrected going forward. Reaching vulnerable veterans who own firearms requires partnerships with individuals and groups who own firearms. Going it alone undercuts the essence of what VA has worked so hard to achieve in the past few years.
Russell B. Lemle, PhD
Veterans Healthcare
Policy Institute
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
1. US Department of Veterans Affairs. Firearm suicide and lethal means safety, space between thought and trigger. Updated September 22, 2021. Accessed October 1, 2021. https://www.va.gov/reach/lethal-means
2. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 National veteran suicide prevention annual report. Published September 8, 2021. Accessed October 1, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
3. Lemle, RB. VA forges a historic partnership with the national shooting sports foundation and the American foundation for suicide prevention to prevent veteran suicide. Published February 15, 2019. Accessed October 1, 2021. https://www.mdedge.com/fedprac/article/194610/mental-health/va-forges-historic-partnership-national-shooting-sports
4. US Department of Veterans Affairs, National Shooting Sports Foundation, American Foundation for Suicide Prevention. Suicide prevention is everyone’s business: a toolkit for safe firearm storage in your community. Published February 24, 2020. Accessed October 1, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Toolkit_Safe_Firearm_Storage_CLEARED_508_2-24-20.pdf
5. US Concealed Carry Association. Protecting mental health and preventing suicide during COVID 19. Published May 14, 2020. Accessed October 1, 2021. https://www.youtube.com/watch?app=desktop&v=Rp48Pnl5fUA&feature=youtube
Pacemakers after TAVR: No long-term survival differences
A comparison of long-term survival between patients who either did or did not undergo permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) revealed no differences, according to results of the SWEDEHEART observational study.
The nationwide population-based cohort study included all patients who underwent transfemoral TAVR in Sweden from 2008 to 2018.
Most frequent complications
While newer-generation aortic valve prostheses are less likely to necessitate PPI, the need for PPI is higher after TAVR than after surgical aortic valve replacement (SAVR), and the need for PPI remains the most frequent complication after TAVR, the study authors noted. Use of self-expandable valves, deep prosthetic valve implantation, preprocedural conduction disturbances, older age, and a high number of comorbidities are among the risk factors for PPI following TAVR.
With prior studies producing conflicting results, the authors stated, the impact of PPI after TAVR remains unknown. Expanding use of TAVR to include younger and low-risk patients with a long life expectancy underscores the importance of gaining greater understand of the impact of PPI after TAVR. Accordingly, the study was conducted to investigate long-term, clinically important outcomes in this post-TAVR population.
Out of 4,750 patients who underwent TAVR in the study period, 3,420 patients in SWEDEHEART met study criteria, with 481 (14.1%) undergoing PPI within 30 days after TAVR, and 2,939 not receiving a pacemaker. PPI exposure was defined as implantation of a permanent pacemaker or implantable cardioverter-defibrillator. The study primary outcome was all-cause mortality, with cardiovascular death, heart failure, and endocarditis as secondary outcomes. It was reported in JACC: Cardiovascular Interventions.
Similar survival
Mean patient age was 81.3 years (50.4% female). The rate for all-cause mortality in those with no pacemaker was 11.4 per 100 patient years and 13.1 for those with a pacemaker (hazard ratio, 1.04; 95% confidence interval, 0.89-1.23). The cardiovascular death rate in the no-pacemaker group was 6.0 per 100 patient years and 7.1 per 100 patient years in the pacemaker group (HR, 0.96; 95% CI, 0.75-1.23). For heart failure the rates were 4.5 per 100 patient years in the no pacemaker group and 6.3 in the pacemaker group (HR, 1.22; 95% CI, 0.93-1.672). Endocarditis rates were 1.2 and 1.1 per 100 patient years in the no pacemaker and pacemaker groups, respectively (HR, 0.93; 95% CI, 0.51-1.71).
The authors pointed out that their prior study had found PPI after SAVR in almost 25,000 patients to be associated with increased all-cause mortality and heart failure rates. Patients who undergo TAVR, however, are older and have more comorbidities than patients who undergo SAVR.
It is thus likely that patients who undergo TAVR die of other causes before the negative effects of their pacemaker become clinically evident.
Also, the incidence of conduction abnormalities increases with age, making it more likely that beneficial effects of pacemakers occur in older patients rather than younger ones, counterbalancing the detrimental effects to a larger extent.
Reduce PPI rates after TAVR
The study authors also observed that, although they did not find increased mortality or heart failure in patients who underwent PPI, PPI is associated with risks, including lead- and pocket-related complications, other traumatic complications, longer hospital stays and higher societal costs. These factors justify the search for strategies to reduce PPI rates after TAVR.
“Our study adds important information about the prognosis in patients who received a permanent pacemaker implantation following TAVR,” study author Natalie Glaser, MD, PhD, said in an interview. “Increased knowledge about prognosis after TAVR in different patient populations has important implications for preoperative risk stratification and can help to optimize postoperative follow-up and treatment for these patients.” Future studies, Dr. Glaser added, should include younger and low-risk patients with longer follow-up to confirm the present findings.
Balancing factors
In an accompanying editorial, Antonio J. Muñoz-García, MD, PhD, and Erika Muñoz-García, MD also noted factors potentially counterbalancing and masking adverse effects of PPI, echoing some mentioned by the study authors. Among those without PPI, 10%-50% develop new-onset left bundle branch block (LBBB) after TAVR. LBBB is a known marker of low long-term survival in TAVR populations, producing intraventricular dyssynchrony leading potentially to left ventricular dysfunction or development of complete atrioventricular block with higher mortality risk in those without pacemakers. PPI, as well, can be protective against unexpected death in those with advanced conduction disorders. Still, they point out, PPI can entail lead dysfunction, need for generator replacement, infection, and tricuspid valve regurgitation.
Commenting in an interview that an observed trend of a greater increase in events in the group of patients with pacemakers for the first 4 years is consistent with prior studies, Dr. Antonio Muñoz-García said: “This can be explained because long-term survival in the TAVI population is conditioned by comorbidities. It is true that the presence of a pacemaker can cause left ventricular ejection fraction to deteriorate and therefore condition heart failure and increased mortality. But the involvement of the pacemaker in left ventricular function in patients with TAVI is multifactorial and depends on the indication of the pacemaker, whether prophylactic or absolute, on the time-dependent pacing, whether or not the patients prior to TAVI present with alterations in atrioventricular conduction [and therefore could benefit from the implantation of pacemakers], as well as the forms of pacing optimization [resynchronization, hisian pacing, etc]. All of these are issues to be considered in clinical practice.”
The editorialists concluded: “To date, the impact of PPI on late clinical outcomes after TAVR remains controversial; however, this study to some extent helps clarify this controversy.” In accord with the study authors, they called for reductions in PPI rates and long-term clinical follow-up.
The study was funded by several Swedish research organizations. The study investigators and editorial authors declared having no disclosures.
A comparison of long-term survival between patients who either did or did not undergo permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) revealed no differences, according to results of the SWEDEHEART observational study.
The nationwide population-based cohort study included all patients who underwent transfemoral TAVR in Sweden from 2008 to 2018.
Most frequent complications
While newer-generation aortic valve prostheses are less likely to necessitate PPI, the need for PPI is higher after TAVR than after surgical aortic valve replacement (SAVR), and the need for PPI remains the most frequent complication after TAVR, the study authors noted. Use of self-expandable valves, deep prosthetic valve implantation, preprocedural conduction disturbances, older age, and a high number of comorbidities are among the risk factors for PPI following TAVR.
With prior studies producing conflicting results, the authors stated, the impact of PPI after TAVR remains unknown. Expanding use of TAVR to include younger and low-risk patients with a long life expectancy underscores the importance of gaining greater understand of the impact of PPI after TAVR. Accordingly, the study was conducted to investigate long-term, clinically important outcomes in this post-TAVR population.
Out of 4,750 patients who underwent TAVR in the study period, 3,420 patients in SWEDEHEART met study criteria, with 481 (14.1%) undergoing PPI within 30 days after TAVR, and 2,939 not receiving a pacemaker. PPI exposure was defined as implantation of a permanent pacemaker or implantable cardioverter-defibrillator. The study primary outcome was all-cause mortality, with cardiovascular death, heart failure, and endocarditis as secondary outcomes. It was reported in JACC: Cardiovascular Interventions.
Similar survival
Mean patient age was 81.3 years (50.4% female). The rate for all-cause mortality in those with no pacemaker was 11.4 per 100 patient years and 13.1 for those with a pacemaker (hazard ratio, 1.04; 95% confidence interval, 0.89-1.23). The cardiovascular death rate in the no-pacemaker group was 6.0 per 100 patient years and 7.1 per 100 patient years in the pacemaker group (HR, 0.96; 95% CI, 0.75-1.23). For heart failure the rates were 4.5 per 100 patient years in the no pacemaker group and 6.3 in the pacemaker group (HR, 1.22; 95% CI, 0.93-1.672). Endocarditis rates were 1.2 and 1.1 per 100 patient years in the no pacemaker and pacemaker groups, respectively (HR, 0.93; 95% CI, 0.51-1.71).
The authors pointed out that their prior study had found PPI after SAVR in almost 25,000 patients to be associated with increased all-cause mortality and heart failure rates. Patients who undergo TAVR, however, are older and have more comorbidities than patients who undergo SAVR.
It is thus likely that patients who undergo TAVR die of other causes before the negative effects of their pacemaker become clinically evident.
Also, the incidence of conduction abnormalities increases with age, making it more likely that beneficial effects of pacemakers occur in older patients rather than younger ones, counterbalancing the detrimental effects to a larger extent.
Reduce PPI rates after TAVR
The study authors also observed that, although they did not find increased mortality or heart failure in patients who underwent PPI, PPI is associated with risks, including lead- and pocket-related complications, other traumatic complications, longer hospital stays and higher societal costs. These factors justify the search for strategies to reduce PPI rates after TAVR.
“Our study adds important information about the prognosis in patients who received a permanent pacemaker implantation following TAVR,” study author Natalie Glaser, MD, PhD, said in an interview. “Increased knowledge about prognosis after TAVR in different patient populations has important implications for preoperative risk stratification and can help to optimize postoperative follow-up and treatment for these patients.” Future studies, Dr. Glaser added, should include younger and low-risk patients with longer follow-up to confirm the present findings.
Balancing factors
In an accompanying editorial, Antonio J. Muñoz-García, MD, PhD, and Erika Muñoz-García, MD also noted factors potentially counterbalancing and masking adverse effects of PPI, echoing some mentioned by the study authors. Among those without PPI, 10%-50% develop new-onset left bundle branch block (LBBB) after TAVR. LBBB is a known marker of low long-term survival in TAVR populations, producing intraventricular dyssynchrony leading potentially to left ventricular dysfunction or development of complete atrioventricular block with higher mortality risk in those without pacemakers. PPI, as well, can be protective against unexpected death in those with advanced conduction disorders. Still, they point out, PPI can entail lead dysfunction, need for generator replacement, infection, and tricuspid valve regurgitation.
Commenting in an interview that an observed trend of a greater increase in events in the group of patients with pacemakers for the first 4 years is consistent with prior studies, Dr. Antonio Muñoz-García said: “This can be explained because long-term survival in the TAVI population is conditioned by comorbidities. It is true that the presence of a pacemaker can cause left ventricular ejection fraction to deteriorate and therefore condition heart failure and increased mortality. But the involvement of the pacemaker in left ventricular function in patients with TAVI is multifactorial and depends on the indication of the pacemaker, whether prophylactic or absolute, on the time-dependent pacing, whether or not the patients prior to TAVI present with alterations in atrioventricular conduction [and therefore could benefit from the implantation of pacemakers], as well as the forms of pacing optimization [resynchronization, hisian pacing, etc]. All of these are issues to be considered in clinical practice.”
The editorialists concluded: “To date, the impact of PPI on late clinical outcomes after TAVR remains controversial; however, this study to some extent helps clarify this controversy.” In accord with the study authors, they called for reductions in PPI rates and long-term clinical follow-up.
The study was funded by several Swedish research organizations. The study investigators and editorial authors declared having no disclosures.
A comparison of long-term survival between patients who either did or did not undergo permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) revealed no differences, according to results of the SWEDEHEART observational study.
The nationwide population-based cohort study included all patients who underwent transfemoral TAVR in Sweden from 2008 to 2018.
Most frequent complications
While newer-generation aortic valve prostheses are less likely to necessitate PPI, the need for PPI is higher after TAVR than after surgical aortic valve replacement (SAVR), and the need for PPI remains the most frequent complication after TAVR, the study authors noted. Use of self-expandable valves, deep prosthetic valve implantation, preprocedural conduction disturbances, older age, and a high number of comorbidities are among the risk factors for PPI following TAVR.
With prior studies producing conflicting results, the authors stated, the impact of PPI after TAVR remains unknown. Expanding use of TAVR to include younger and low-risk patients with a long life expectancy underscores the importance of gaining greater understand of the impact of PPI after TAVR. Accordingly, the study was conducted to investigate long-term, clinically important outcomes in this post-TAVR population.
Out of 4,750 patients who underwent TAVR in the study period, 3,420 patients in SWEDEHEART met study criteria, with 481 (14.1%) undergoing PPI within 30 days after TAVR, and 2,939 not receiving a pacemaker. PPI exposure was defined as implantation of a permanent pacemaker or implantable cardioverter-defibrillator. The study primary outcome was all-cause mortality, with cardiovascular death, heart failure, and endocarditis as secondary outcomes. It was reported in JACC: Cardiovascular Interventions.
Similar survival
Mean patient age was 81.3 years (50.4% female). The rate for all-cause mortality in those with no pacemaker was 11.4 per 100 patient years and 13.1 for those with a pacemaker (hazard ratio, 1.04; 95% confidence interval, 0.89-1.23). The cardiovascular death rate in the no-pacemaker group was 6.0 per 100 patient years and 7.1 per 100 patient years in the pacemaker group (HR, 0.96; 95% CI, 0.75-1.23). For heart failure the rates were 4.5 per 100 patient years in the no pacemaker group and 6.3 in the pacemaker group (HR, 1.22; 95% CI, 0.93-1.672). Endocarditis rates were 1.2 and 1.1 per 100 patient years in the no pacemaker and pacemaker groups, respectively (HR, 0.93; 95% CI, 0.51-1.71).
The authors pointed out that their prior study had found PPI after SAVR in almost 25,000 patients to be associated with increased all-cause mortality and heart failure rates. Patients who undergo TAVR, however, are older and have more comorbidities than patients who undergo SAVR.
It is thus likely that patients who undergo TAVR die of other causes before the negative effects of their pacemaker become clinically evident.
Also, the incidence of conduction abnormalities increases with age, making it more likely that beneficial effects of pacemakers occur in older patients rather than younger ones, counterbalancing the detrimental effects to a larger extent.
Reduce PPI rates after TAVR
The study authors also observed that, although they did not find increased mortality or heart failure in patients who underwent PPI, PPI is associated with risks, including lead- and pocket-related complications, other traumatic complications, longer hospital stays and higher societal costs. These factors justify the search for strategies to reduce PPI rates after TAVR.
“Our study adds important information about the prognosis in patients who received a permanent pacemaker implantation following TAVR,” study author Natalie Glaser, MD, PhD, said in an interview. “Increased knowledge about prognosis after TAVR in different patient populations has important implications for preoperative risk stratification and can help to optimize postoperative follow-up and treatment for these patients.” Future studies, Dr. Glaser added, should include younger and low-risk patients with longer follow-up to confirm the present findings.
Balancing factors
In an accompanying editorial, Antonio J. Muñoz-García, MD, PhD, and Erika Muñoz-García, MD also noted factors potentially counterbalancing and masking adverse effects of PPI, echoing some mentioned by the study authors. Among those without PPI, 10%-50% develop new-onset left bundle branch block (LBBB) after TAVR. LBBB is a known marker of low long-term survival in TAVR populations, producing intraventricular dyssynchrony leading potentially to left ventricular dysfunction or development of complete atrioventricular block with higher mortality risk in those without pacemakers. PPI, as well, can be protective against unexpected death in those with advanced conduction disorders. Still, they point out, PPI can entail lead dysfunction, need for generator replacement, infection, and tricuspid valve regurgitation.
Commenting in an interview that an observed trend of a greater increase in events in the group of patients with pacemakers for the first 4 years is consistent with prior studies, Dr. Antonio Muñoz-García said: “This can be explained because long-term survival in the TAVI population is conditioned by comorbidities. It is true that the presence of a pacemaker can cause left ventricular ejection fraction to deteriorate and therefore condition heart failure and increased mortality. But the involvement of the pacemaker in left ventricular function in patients with TAVI is multifactorial and depends on the indication of the pacemaker, whether prophylactic or absolute, on the time-dependent pacing, whether or not the patients prior to TAVI present with alterations in atrioventricular conduction [and therefore could benefit from the implantation of pacemakers], as well as the forms of pacing optimization [resynchronization, hisian pacing, etc]. All of these are issues to be considered in clinical practice.”
The editorialists concluded: “To date, the impact of PPI on late clinical outcomes after TAVR remains controversial; however, this study to some extent helps clarify this controversy.” In accord with the study authors, they called for reductions in PPI rates and long-term clinical follow-up.
The study was funded by several Swedish research organizations. The study investigators and editorial authors declared having no disclosures.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
New nonhormonal therapies for hot flashes on the horizon
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
FROM NAMS 2021
Depression rates up threefold since start of COVID-19
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
FROM LANCET REGIONAL HEALTH – AMERICAS