User login
A case is building for personalized, genome-based radiation dosing
A team of researchers from the Cleveland Clinic, the Moffitt Cancer Center in Tampa, and Case Western Reserve University in Cleveland is zeroing in on a way to personalize radiation therapy for cancer patients based on genomic profile, much as genomics is used to tailor oncologic drug therapy.
It’s called “genomic-adjusted radiation dose” (GARD), a dose tailored to a person’s radiosensitivity as determined by the expression of 10 genes, known as the radiosensitivity index (RSI), combined with a linear quadratic model to yield GARD, a prediction of risk and benefit at various radiation doses for a particular patient.
A recent report in The Lancet Oncology validated GARD in 1,615 patients with seven cancer types from 11 study cohorts. If it holds up in clinical trials set to start later this year, GARD should “allow us to predict the benefit of radiation for an individual patient and adjust their treatment strategy,” wrote the authors of an editorial that accompanied the study. “The efforts need to be applauded worldwide, because radiotherapy is considerably lagging, compared with the enormous progress done in the field of personalized medicine,” Orit Kaidar-Person, MD, a radiation oncologist at Sheba Medical Center in Ramat Gan, Israel, and colleagues wrote.
GARD was associated with time to first recurrence and overall survival for patients receiving radiotherapy and predicted radiotherapy benefit, while physical dose did not. The team found a relative 2% reduction in risk of first recurrence for each unit increase of GARD (P = .0017) and a relative 3% increase in overall survival for each unit increase in GARD (P = .0007), among those who got radiotherapy. Values of GARD run from 0 to over 100, with higher scores meaning more radiation benefit.
The radiosensitivity index, which was derived from genomic studies of cancer cell lines exposed to radiation, was previously validated by the team and other groups across several tumor types.
Currently, radiation dosing is generally uniform for a given disease site and stage, based on the assumption that a given dose of radiation results in the same clinical effect across patients. In fact, the biological effect of a given dose varies widely between individual patients. “Patients we treat uniformly do not have a uniform response” which is why a more personalized approach would help, said lead investigator and Cleveland Clinic radiation oncologist Jacob Scott, MD, DPhil.
One patient with a given tumor might benefit from 2 extra fractions, while the next might need an extra 15 for the same benefit. “You need to know about [a patient’s] tumor genomics to know how hard you have to work,” he said.
Dr. Scott and colleagues are working with a genomics company to commercialize the approach. The vision for now is that physicians would ship in biopsy samples to be analyzed; RSI and GARD would be calculated, and then a decision support report would be sent back to the treatment team outlining the risks and benefits of various doses for the patient.
Dr. Scott, who holds proprietary rights on the approach, is bullish. When asked if he anticipates GARD dosing to be standard of care in 10 years, he said that “I can’t imagine another world. Everything else in cancer is personalized. Why aren’t we? It just makes sense. I know there’s a better way” to prescribe radiation, “and I’m excited for the future when I can use it.”
When asked for comment, Brian Marples, PhD, a radiation oncology professor at the University of Rochester (N.Y.), said the data so far for GARD “seem very solid. I’m very excited by the concept.”
It’s been “the holy grail” of radiation researchers to find a biologic marker that predicts what dosages patients need and what can be given safely. “This strategy is a good way of doing that. Other groups are proposing similar strategies, but I think this group is ahead. I can see [GARD] being readily applied to the clinic because patients are [already] getting their tumors genomically characterized as part of care,” Dr. Marples said.
But many questions remain. For instance, the editorial writers questioned how GARD is “affected by tumor heterogeneity, response to systemic therapy, and changes in the tumor microenvironment.” Also, the approach is based on conventional 2 Gy fractions, but other fractionation regimens are becoming more common.
For Dr. Marples, the big caveat is that most cancer patients are treated with both radiation and chemotherapy. He said he would like to see GARD validated in patients who receive both.
They seven tumor types in the study included breast cancer, head and neck cancer, non–small cell lung cancer, pancreatic cancer, endometrial cancer, melanoma, and glioma. The majority of the subjects were treated with radiation, and each had the genomic data needed to calculate GARD.
Dr. Scott, senior author and Moffitt Center radiation oncologist Javier Torres-Roca, MD, and a third author hold intellectual property rights on RSI, GARD, and prescription dose base on RSI, plus equity in Cvergenx, a company that seeks to commercialize the approach. Dr. Torres-Roca and another author are cofounders. The editorial writers and Dr. Marples did not have any relevant disclosures.
A team of researchers from the Cleveland Clinic, the Moffitt Cancer Center in Tampa, and Case Western Reserve University in Cleveland is zeroing in on a way to personalize radiation therapy for cancer patients based on genomic profile, much as genomics is used to tailor oncologic drug therapy.
It’s called “genomic-adjusted radiation dose” (GARD), a dose tailored to a person’s radiosensitivity as determined by the expression of 10 genes, known as the radiosensitivity index (RSI), combined with a linear quadratic model to yield GARD, a prediction of risk and benefit at various radiation doses for a particular patient.
A recent report in The Lancet Oncology validated GARD in 1,615 patients with seven cancer types from 11 study cohorts. If it holds up in clinical trials set to start later this year, GARD should “allow us to predict the benefit of radiation for an individual patient and adjust their treatment strategy,” wrote the authors of an editorial that accompanied the study. “The efforts need to be applauded worldwide, because radiotherapy is considerably lagging, compared with the enormous progress done in the field of personalized medicine,” Orit Kaidar-Person, MD, a radiation oncologist at Sheba Medical Center in Ramat Gan, Israel, and colleagues wrote.
GARD was associated with time to first recurrence and overall survival for patients receiving radiotherapy and predicted radiotherapy benefit, while physical dose did not. The team found a relative 2% reduction in risk of first recurrence for each unit increase of GARD (P = .0017) and a relative 3% increase in overall survival for each unit increase in GARD (P = .0007), among those who got radiotherapy. Values of GARD run from 0 to over 100, with higher scores meaning more radiation benefit.
The radiosensitivity index, which was derived from genomic studies of cancer cell lines exposed to radiation, was previously validated by the team and other groups across several tumor types.
Currently, radiation dosing is generally uniform for a given disease site and stage, based on the assumption that a given dose of radiation results in the same clinical effect across patients. In fact, the biological effect of a given dose varies widely between individual patients. “Patients we treat uniformly do not have a uniform response” which is why a more personalized approach would help, said lead investigator and Cleveland Clinic radiation oncologist Jacob Scott, MD, DPhil.
One patient with a given tumor might benefit from 2 extra fractions, while the next might need an extra 15 for the same benefit. “You need to know about [a patient’s] tumor genomics to know how hard you have to work,” he said.
Dr. Scott and colleagues are working with a genomics company to commercialize the approach. The vision for now is that physicians would ship in biopsy samples to be analyzed; RSI and GARD would be calculated, and then a decision support report would be sent back to the treatment team outlining the risks and benefits of various doses for the patient.
Dr. Scott, who holds proprietary rights on the approach, is bullish. When asked if he anticipates GARD dosing to be standard of care in 10 years, he said that “I can’t imagine another world. Everything else in cancer is personalized. Why aren’t we? It just makes sense. I know there’s a better way” to prescribe radiation, “and I’m excited for the future when I can use it.”
When asked for comment, Brian Marples, PhD, a radiation oncology professor at the University of Rochester (N.Y.), said the data so far for GARD “seem very solid. I’m very excited by the concept.”
It’s been “the holy grail” of radiation researchers to find a biologic marker that predicts what dosages patients need and what can be given safely. “This strategy is a good way of doing that. Other groups are proposing similar strategies, but I think this group is ahead. I can see [GARD] being readily applied to the clinic because patients are [already] getting their tumors genomically characterized as part of care,” Dr. Marples said.
But many questions remain. For instance, the editorial writers questioned how GARD is “affected by tumor heterogeneity, response to systemic therapy, and changes in the tumor microenvironment.” Also, the approach is based on conventional 2 Gy fractions, but other fractionation regimens are becoming more common.
For Dr. Marples, the big caveat is that most cancer patients are treated with both radiation and chemotherapy. He said he would like to see GARD validated in patients who receive both.
They seven tumor types in the study included breast cancer, head and neck cancer, non–small cell lung cancer, pancreatic cancer, endometrial cancer, melanoma, and glioma. The majority of the subjects were treated with radiation, and each had the genomic data needed to calculate GARD.
Dr. Scott, senior author and Moffitt Center radiation oncologist Javier Torres-Roca, MD, and a third author hold intellectual property rights on RSI, GARD, and prescription dose base on RSI, plus equity in Cvergenx, a company that seeks to commercialize the approach. Dr. Torres-Roca and another author are cofounders. The editorial writers and Dr. Marples did not have any relevant disclosures.
A team of researchers from the Cleveland Clinic, the Moffitt Cancer Center in Tampa, and Case Western Reserve University in Cleveland is zeroing in on a way to personalize radiation therapy for cancer patients based on genomic profile, much as genomics is used to tailor oncologic drug therapy.
It’s called “genomic-adjusted radiation dose” (GARD), a dose tailored to a person’s radiosensitivity as determined by the expression of 10 genes, known as the radiosensitivity index (RSI), combined with a linear quadratic model to yield GARD, a prediction of risk and benefit at various radiation doses for a particular patient.
A recent report in The Lancet Oncology validated GARD in 1,615 patients with seven cancer types from 11 study cohorts. If it holds up in clinical trials set to start later this year, GARD should “allow us to predict the benefit of radiation for an individual patient and adjust their treatment strategy,” wrote the authors of an editorial that accompanied the study. “The efforts need to be applauded worldwide, because radiotherapy is considerably lagging, compared with the enormous progress done in the field of personalized medicine,” Orit Kaidar-Person, MD, a radiation oncologist at Sheba Medical Center in Ramat Gan, Israel, and colleagues wrote.
GARD was associated with time to first recurrence and overall survival for patients receiving radiotherapy and predicted radiotherapy benefit, while physical dose did not. The team found a relative 2% reduction in risk of first recurrence for each unit increase of GARD (P = .0017) and a relative 3% increase in overall survival for each unit increase in GARD (P = .0007), among those who got radiotherapy. Values of GARD run from 0 to over 100, with higher scores meaning more radiation benefit.
The radiosensitivity index, which was derived from genomic studies of cancer cell lines exposed to radiation, was previously validated by the team and other groups across several tumor types.
Currently, radiation dosing is generally uniform for a given disease site and stage, based on the assumption that a given dose of radiation results in the same clinical effect across patients. In fact, the biological effect of a given dose varies widely between individual patients. “Patients we treat uniformly do not have a uniform response” which is why a more personalized approach would help, said lead investigator and Cleveland Clinic radiation oncologist Jacob Scott, MD, DPhil.
One patient with a given tumor might benefit from 2 extra fractions, while the next might need an extra 15 for the same benefit. “You need to know about [a patient’s] tumor genomics to know how hard you have to work,” he said.
Dr. Scott and colleagues are working with a genomics company to commercialize the approach. The vision for now is that physicians would ship in biopsy samples to be analyzed; RSI and GARD would be calculated, and then a decision support report would be sent back to the treatment team outlining the risks and benefits of various doses for the patient.
Dr. Scott, who holds proprietary rights on the approach, is bullish. When asked if he anticipates GARD dosing to be standard of care in 10 years, he said that “I can’t imagine another world. Everything else in cancer is personalized. Why aren’t we? It just makes sense. I know there’s a better way” to prescribe radiation, “and I’m excited for the future when I can use it.”
When asked for comment, Brian Marples, PhD, a radiation oncology professor at the University of Rochester (N.Y.), said the data so far for GARD “seem very solid. I’m very excited by the concept.”
It’s been “the holy grail” of radiation researchers to find a biologic marker that predicts what dosages patients need and what can be given safely. “This strategy is a good way of doing that. Other groups are proposing similar strategies, but I think this group is ahead. I can see [GARD] being readily applied to the clinic because patients are [already] getting their tumors genomically characterized as part of care,” Dr. Marples said.
But many questions remain. For instance, the editorial writers questioned how GARD is “affected by tumor heterogeneity, response to systemic therapy, and changes in the tumor microenvironment.” Also, the approach is based on conventional 2 Gy fractions, but other fractionation regimens are becoming more common.
For Dr. Marples, the big caveat is that most cancer patients are treated with both radiation and chemotherapy. He said he would like to see GARD validated in patients who receive both.
They seven tumor types in the study included breast cancer, head and neck cancer, non–small cell lung cancer, pancreatic cancer, endometrial cancer, melanoma, and glioma. The majority of the subjects were treated with radiation, and each had the genomic data needed to calculate GARD.
Dr. Scott, senior author and Moffitt Center radiation oncologist Javier Torres-Roca, MD, and a third author hold intellectual property rights on RSI, GARD, and prescription dose base on RSI, plus equity in Cvergenx, a company that seeks to commercialize the approach. Dr. Torres-Roca and another author are cofounders. The editorial writers and Dr. Marples did not have any relevant disclosures.
FROM LANCET ONCOLOGY
Limited evidence for interventions to reduce post-op pulmonary complications
Background: Despite advances in perioperative care, postoperative pulmonary complications represent a leading cause of morbidity and mortality that are associated with increased risk of admission to critical care and prolonged length of hospital stay. There are multiple interventions that are used, despite there being no consensus guidelines aimed at reducing the risk of PPCs.
Study design: Systemic review and meta-analysis of randomized controlled trials.
Setting: Literature search from Medline, Embase, CINHAL, and the Cochrane Central Register of Controlled Trials from January 1990 to December 2017, including trials investigating short-term, protocolized medical interventions around noncardiac surgeries with clinical diagnostic criteria for PPC outcomes.
Synopsis: The authors reviewed 117 trials that included 21,940 participants. The meta-analysis comprised 95 randomized controlled trials with 18,062 patients. The authors identified 11 categories of perioperative care interventions that were tested to reduce PPCs. None of the interventions evaluated was supported by high-quality evidence. There were seven interventions that showed a probable reduction in PPCs. Goal-directed fluid therapy was the only one that was supported by both moderate quality evidence and trial sequential analysis. Lung protective intraoperative ventilation was supported by moderate quality evidence, but not trial sequential analysis. Five interventions had low-quality evidence of benefit: enhanced recovery pathways, prophylactic mucolytics, postoperative continuous positive airway pressure ventilation, prophylactic respiratory physiotherapy, and epidural analgesia.
Unfortunately, only a minority of the trials reviewed were large, multi-center studies with a low risk of bias. The studies were also heterogeneous, posing a challenge for meta-analysis.
Bottom line: There is limited evidence supporting the efficacy of any intervention preventing postoperative pulmonary complications, with moderate-quality evidence supporting intraoperative lung protective ventilation and goal-directed hemodynamic strategies reducing PPCs.
Citation: Odor PM et al. Perioperative interventions for prevention of postoperative pulmonary complication: Systemic review and meta-analysis. BMJ. 2020 Mar 11. doi: 10.1136/bmj.m540.
Dr. Weaver is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Despite advances in perioperative care, postoperative pulmonary complications represent a leading cause of morbidity and mortality that are associated with increased risk of admission to critical care and prolonged length of hospital stay. There are multiple interventions that are used, despite there being no consensus guidelines aimed at reducing the risk of PPCs.
Study design: Systemic review and meta-analysis of randomized controlled trials.
Setting: Literature search from Medline, Embase, CINHAL, and the Cochrane Central Register of Controlled Trials from January 1990 to December 2017, including trials investigating short-term, protocolized medical interventions around noncardiac surgeries with clinical diagnostic criteria for PPC outcomes.
Synopsis: The authors reviewed 117 trials that included 21,940 participants. The meta-analysis comprised 95 randomized controlled trials with 18,062 patients. The authors identified 11 categories of perioperative care interventions that were tested to reduce PPCs. None of the interventions evaluated was supported by high-quality evidence. There were seven interventions that showed a probable reduction in PPCs. Goal-directed fluid therapy was the only one that was supported by both moderate quality evidence and trial sequential analysis. Lung protective intraoperative ventilation was supported by moderate quality evidence, but not trial sequential analysis. Five interventions had low-quality evidence of benefit: enhanced recovery pathways, prophylactic mucolytics, postoperative continuous positive airway pressure ventilation, prophylactic respiratory physiotherapy, and epidural analgesia.
Unfortunately, only a minority of the trials reviewed were large, multi-center studies with a low risk of bias. The studies were also heterogeneous, posing a challenge for meta-analysis.
Bottom line: There is limited evidence supporting the efficacy of any intervention preventing postoperative pulmonary complications, with moderate-quality evidence supporting intraoperative lung protective ventilation and goal-directed hemodynamic strategies reducing PPCs.
Citation: Odor PM et al. Perioperative interventions for prevention of postoperative pulmonary complication: Systemic review and meta-analysis. BMJ. 2020 Mar 11. doi: 10.1136/bmj.m540.
Dr. Weaver is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Despite advances in perioperative care, postoperative pulmonary complications represent a leading cause of morbidity and mortality that are associated with increased risk of admission to critical care and prolonged length of hospital stay. There are multiple interventions that are used, despite there being no consensus guidelines aimed at reducing the risk of PPCs.
Study design: Systemic review and meta-analysis of randomized controlled trials.
Setting: Literature search from Medline, Embase, CINHAL, and the Cochrane Central Register of Controlled Trials from January 1990 to December 2017, including trials investigating short-term, protocolized medical interventions around noncardiac surgeries with clinical diagnostic criteria for PPC outcomes.
Synopsis: The authors reviewed 117 trials that included 21,940 participants. The meta-analysis comprised 95 randomized controlled trials with 18,062 patients. The authors identified 11 categories of perioperative care interventions that were tested to reduce PPCs. None of the interventions evaluated was supported by high-quality evidence. There were seven interventions that showed a probable reduction in PPCs. Goal-directed fluid therapy was the only one that was supported by both moderate quality evidence and trial sequential analysis. Lung protective intraoperative ventilation was supported by moderate quality evidence, but not trial sequential analysis. Five interventions had low-quality evidence of benefit: enhanced recovery pathways, prophylactic mucolytics, postoperative continuous positive airway pressure ventilation, prophylactic respiratory physiotherapy, and epidural analgesia.
Unfortunately, only a minority of the trials reviewed were large, multi-center studies with a low risk of bias. The studies were also heterogeneous, posing a challenge for meta-analysis.
Bottom line: There is limited evidence supporting the efficacy of any intervention preventing postoperative pulmonary complications, with moderate-quality evidence supporting intraoperative lung protective ventilation and goal-directed hemodynamic strategies reducing PPCs.
Citation: Odor PM et al. Perioperative interventions for prevention of postoperative pulmonary complication: Systemic review and meta-analysis. BMJ. 2020 Mar 11. doi: 10.1136/bmj.m540.
Dr. Weaver is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Type 2 diabetes ‘remission’ is a reality, say major organizations
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
Two swings, two misses with colchicine, Vascepa in COVID-19
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘This food will kill you, that food will save you’
Not sure if you’ve heard the news, but eating a single hot dog will apparently cost you 36 minutes of healthy life. My first thought when hearing this was of course the same as everyone else’s: Poor Joey Chestnut, multiyear winner of Nathan’s annual hot dog–eating contest.
He won this year’s contest with 76 hot dogs, which puts his total number of competition-consumed hot dogs at 1,089 – which cost him, it would seem, 27.2 days of healthy life. Unless, of course, every hot dog he inhaled came with a bun hosting two portions of sesame seeds, which in turn would buy him 50 extra minutes of life (25 minutes per portion, you see) and would consequently have extended his life by 10.6 days.
Clearly, the obvious solution here is to ensure that all hot dog buns have two portions of sesame seeds on them moving forward; that way, hot dogs can transition from being poisonous killers to antiaging medicine.
The other solution, albeit less exciting, perhaps, is for researchers to stop studying single foods’ impacts on health, and/or for journals to stop publishing them, and/or for the media to stop promoting them – because they are all as ridiculously useless as the example above highlighting findings from a newly published study in Nature Food, entitled “Small targeted dietary changes can yield substantial gains for human health and the environment.”
While no doubt we would all love for diet and health to be so well understood that we could choose specific single foods (knowing that they would prolong our lives) while avoiding single foods that would shorten it, there’s this unfortunate truth that the degree of confounding among food alone is staggering. People eat thousands of different foods in thousands of different dietary combinations. Moreover, most (all?) research conducted on dietary impacts of single foods on health don’t actually track consumption of those specific foods over time, let alone their interactions with all other foods consumed, but rather at moments in time.
In the case of the “hot dogs will kill you unless there are sesame seeds on your bun” article, for example, the researchers utilized one solitary dietary recall session upon which to base their ridiculously specific, ridiculous conclusions.
People’s diets also change over time for various reasons, and of course people themselves are very different. You might imagine that people whose diets are rich in chicken wings, sugared soda, and hot dogs will have markedly different lifestyles and demographics than those whose diets are rich in walnuts, sashimi, and avocados.
So why do we keep seeing studies like this being published? Is it because they’re basically clickbait catnip for journals and newspapers, and in our publish-or-perish attention-seeking world, that means they not only get a pass but they get a press release? Is it because peer review is broken and everyone knows it? Is it because as a society, we’re frogs who have been steeping for decades in the ever-heated pot of nutritional nonsense, and consequently don’t think to question it?
I don’t know the answer to any of those questions, but one thing I do know: Studies on single foods’ impact on life length are pointless, impossible, and idiotic, and people who share them noncritically should be forever shunned – or at the very least, forever ignored.
Yoni Freedhoff, MD, is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight-management center.
A version of this article first appeared on Medscape.com.
Not sure if you’ve heard the news, but eating a single hot dog will apparently cost you 36 minutes of healthy life. My first thought when hearing this was of course the same as everyone else’s: Poor Joey Chestnut, multiyear winner of Nathan’s annual hot dog–eating contest.
He won this year’s contest with 76 hot dogs, which puts his total number of competition-consumed hot dogs at 1,089 – which cost him, it would seem, 27.2 days of healthy life. Unless, of course, every hot dog he inhaled came with a bun hosting two portions of sesame seeds, which in turn would buy him 50 extra minutes of life (25 minutes per portion, you see) and would consequently have extended his life by 10.6 days.
Clearly, the obvious solution here is to ensure that all hot dog buns have two portions of sesame seeds on them moving forward; that way, hot dogs can transition from being poisonous killers to antiaging medicine.
The other solution, albeit less exciting, perhaps, is for researchers to stop studying single foods’ impacts on health, and/or for journals to stop publishing them, and/or for the media to stop promoting them – because they are all as ridiculously useless as the example above highlighting findings from a newly published study in Nature Food, entitled “Small targeted dietary changes can yield substantial gains for human health and the environment.”
While no doubt we would all love for diet and health to be so well understood that we could choose specific single foods (knowing that they would prolong our lives) while avoiding single foods that would shorten it, there’s this unfortunate truth that the degree of confounding among food alone is staggering. People eat thousands of different foods in thousands of different dietary combinations. Moreover, most (all?) research conducted on dietary impacts of single foods on health don’t actually track consumption of those specific foods over time, let alone their interactions with all other foods consumed, but rather at moments in time.
In the case of the “hot dogs will kill you unless there are sesame seeds on your bun” article, for example, the researchers utilized one solitary dietary recall session upon which to base their ridiculously specific, ridiculous conclusions.
People’s diets also change over time for various reasons, and of course people themselves are very different. You might imagine that people whose diets are rich in chicken wings, sugared soda, and hot dogs will have markedly different lifestyles and demographics than those whose diets are rich in walnuts, sashimi, and avocados.
So why do we keep seeing studies like this being published? Is it because they’re basically clickbait catnip for journals and newspapers, and in our publish-or-perish attention-seeking world, that means they not only get a pass but they get a press release? Is it because peer review is broken and everyone knows it? Is it because as a society, we’re frogs who have been steeping for decades in the ever-heated pot of nutritional nonsense, and consequently don’t think to question it?
I don’t know the answer to any of those questions, but one thing I do know: Studies on single foods’ impact on life length are pointless, impossible, and idiotic, and people who share them noncritically should be forever shunned – or at the very least, forever ignored.
Yoni Freedhoff, MD, is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight-management center.
A version of this article first appeared on Medscape.com.
Not sure if you’ve heard the news, but eating a single hot dog will apparently cost you 36 minutes of healthy life. My first thought when hearing this was of course the same as everyone else’s: Poor Joey Chestnut, multiyear winner of Nathan’s annual hot dog–eating contest.
He won this year’s contest with 76 hot dogs, which puts his total number of competition-consumed hot dogs at 1,089 – which cost him, it would seem, 27.2 days of healthy life. Unless, of course, every hot dog he inhaled came with a bun hosting two portions of sesame seeds, which in turn would buy him 50 extra minutes of life (25 minutes per portion, you see) and would consequently have extended his life by 10.6 days.
Clearly, the obvious solution here is to ensure that all hot dog buns have two portions of sesame seeds on them moving forward; that way, hot dogs can transition from being poisonous killers to antiaging medicine.
The other solution, albeit less exciting, perhaps, is for researchers to stop studying single foods’ impacts on health, and/or for journals to stop publishing them, and/or for the media to stop promoting them – because they are all as ridiculously useless as the example above highlighting findings from a newly published study in Nature Food, entitled “Small targeted dietary changes can yield substantial gains for human health and the environment.”
While no doubt we would all love for diet and health to be so well understood that we could choose specific single foods (knowing that they would prolong our lives) while avoiding single foods that would shorten it, there’s this unfortunate truth that the degree of confounding among food alone is staggering. People eat thousands of different foods in thousands of different dietary combinations. Moreover, most (all?) research conducted on dietary impacts of single foods on health don’t actually track consumption of those specific foods over time, let alone their interactions with all other foods consumed, but rather at moments in time.
In the case of the “hot dogs will kill you unless there are sesame seeds on your bun” article, for example, the researchers utilized one solitary dietary recall session upon which to base their ridiculously specific, ridiculous conclusions.
People’s diets also change over time for various reasons, and of course people themselves are very different. You might imagine that people whose diets are rich in chicken wings, sugared soda, and hot dogs will have markedly different lifestyles and demographics than those whose diets are rich in walnuts, sashimi, and avocados.
So why do we keep seeing studies like this being published? Is it because they’re basically clickbait catnip for journals and newspapers, and in our publish-or-perish attention-seeking world, that means they not only get a pass but they get a press release? Is it because peer review is broken and everyone knows it? Is it because as a society, we’re frogs who have been steeping for decades in the ever-heated pot of nutritional nonsense, and consequently don’t think to question it?
I don’t know the answer to any of those questions, but one thing I do know: Studies on single foods’ impact on life length are pointless, impossible, and idiotic, and people who share them noncritically should be forever shunned – or at the very least, forever ignored.
Yoni Freedhoff, MD, is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight-management center.
A version of this article first appeared on Medscape.com.
Volunteer Opportunities Within Dermatology: More than Skin Deep
The adage “so much to do, so little time” aptly describes the daily challenges facing dermatologists and dermatology residents. The time and attention required by direct patient care, writing notes, navigating electronic health records, and engaging in education and research as well as family commitments can drain even the most tireless clinician. In addition, dermatologists are expected to play a critical role in clinic and practice management to successfully curate an online presence and adapt their skills to successfully manage a teledermatology practice. Coupled with the time spent socializing with friends or colleagues and time for personal hobbies or exercise, it’s easy to see how sleep deprivation is common in many of our colleagues.
What’s being left out of these jam-packed schedules? Increasingly, it is the time and expertise dedicated to volunteering in our local communities. Two recent research letters highlighted how a dramatic increase in the number of research projects and publications is not mirrored by a similar increase in volunteer experiences as dermatology residency selection becomes more competitive.1,2
Although the rate of volunteerism among practicing dermatologists has yet to be studied, a brief review suggests a component of unmet dermatology need within our communities. It’s estimated that approximately 5% to 10% of all emergency department visits are for dermatologic concerns.3-5 In many cases, the reason for the visit is nonurgent and instead reflects a lack of other options for care. However, the need for dermatologists extends beyond the emergency department setting. A review of the prevalence of patients presenting for care to a group of regional free clinics found that 8% (N=5553) of all visitors sought care for dermatologic concerns.6 The benefit is not just for those seated on the examination table; research has shown that while many of the underlying factors resulting in physician burnout stem from systemic issues, participating in volunteer opportunities helps combat burnout in ourselves and our colleagues.7-9 Herein, opportunities that exist for dermatologists to reconnect with their communities, advocate for causes distinctive to the specialty, and care for neighbors most in need are highlighted.
Camp Wonder
Every year, children from across the United States living with chronic and debilitating skin conditions get the opportunity to join fellow campers and spend a week just being kids without the constant focus on being a patient. Camp Wonder’s founder and director, Francesca Tenconi, describes the camp as a place where kids “can form a community and can feel free to be themselves, without judgment, without stares. They get the chance to forget about their skin disease and be themselves” (oral communication, June 18, 2021). Tenconi and the camp’s cofounders and medical directors, Drs. Jenny Kim and Stefani Takahashi, envisioned the camp as a place for all campers regardless of their skin condition to feel safe and welcome. This overall mission guides camp leadership and staff every year over the course of the camp week where campers participate in a mix of traditional and nontraditional summer activities that are safe and accessible for all, from spending time in the pool to arts and crafts and a ropes course.
Camp Wonder is in its 21st year of hosting children and adolescents from across North America at its camp in Livermore, California. This year, Tenconi expects about 100 campers during the last week in July. Camp Wonder relies on medical staff volunteers to make the camp setting safe, inclusive, and fun. “Our dermatology residents and dermatology volunteers are a huge part of why we’re able to have camp,” said Tenconi. “A lot of our kids require very specific medical care throughout the week. We are able to provide this camp experience for them because we have this medical support system available, this specialized dermatology knowledge.” She also noted the benefit to the volunteers themselves, saying,“The feedback we get a lot from residents and dermatologists is that camp gave them a chance to understand the true-life impact of some of the skin diseases these kids and families are living with. Kids will open up to them and tell them how their disease has impacted them personally” (oral communication, June 18, 2021).
Volunteer medical providers help manage the medical needs of the campers beginning at check-in and work shifts in the infirmary as well as help with dispensing and administering medications, changing dressings, and applying ointments or other topical medications. When not assisting with medical care, medical staff can get to know the campers; help out with arts and crafts, games, sports, and other camp activities; and put on skits and plays for campers at nightly camp hangouts (Figure 1).

How to Get Involved
Visit the website (https://www.csdf.org/camp-wonder) for information on becoming a medical volunteer for 2022. Donations to help keep the camp running also are greatly appreciated, as attendance, including travel costs, is free for families through the Children’s Skin Disease Foundation. Finally, dermatologists can help by keeping their young patients with skin disease in mind as future campers. The camp welcomes kids from across the United States and Canada and invites questions from dermatologists and families on how to become a camper and what the experience is like.
Native American Health Services Rotation
Located in the southwestern United States, the Navajo Nation is North America’s largest Native American tribe by enrollment and resides on the largest reservation in the United States.10 Comprised of 27,000 square miles within portions of Arizona, New Mexico, and Utah, the reservation’s total area is greater than that of Massachusetts, Vermont, and New Hampshire combined.11 The reservation is home to an estimated 180,000 Navajo people, a population roughly the size of Salt Lake City, Utah. Yet, many homes on the reservation are without electricity, running water, telephones, or broadband access, and many roads on the reservation remain unpaved. Prior to the COVID-19 pandemic, 4 dermatology residents were selected each year to travel to this unique and remote location to work with the staff of the Chinle Comprehensive Health Care Facility (Chinle, Arizona), an Indian Health Service facility, as part of the American Academy of Dermatology (AAD)–sponsored Native American Health Services Resident Rotation (NAHSRR).
Dr. Lucinda Kohn, Assistant Professor of Dermatology at the University of Colorado and the director of the NAHSRR program discovered the value of this rotation firsthand as a dermatology resident. In 2017, she traveled to the area to spend 2 weeks serving within the community. “I went because of a personal connection. My husband is Native American, although not Navajo. I wanted to experience what it was like to provide dermatologic care for Native Americans. I found the Navajo people to be so friendly and so grateful for our care. The clinicians we worked with at Chinle were excited to have us share our expertise and to pass on their knowledge to us,” said Dr. Kohn (personal communication, June 24, 2021).
Rotating residents provide dermatologic care for the Navajo people and share their unique medical skill set to local primary care clinicians serving as preceptors. They also may have an opportunity to learn from Native healers about traditional Navajo beliefs and ceremonies used as part of a holistic approach to healing.
The program, similar to volunteer programs across the country, was put on hold during the height of the COVID-19 pandemic. “The Navajo nation witnessed a really tragic surge of COVID cases that required that limited medical resources be diverted to help cope with the pandemic,” says Dr. Kohn. “It really wasn’t safe for residents to travel to the reservation either, so the rotation had to be put on hold.” However, in April 2021, the health care staff of the Chinle Comprehensive Care Facility reached out to revive the program, which is now pending the green light from the AAD. It is unclear if or when AAD leadership will allow this rotation to restart. Dr. Kohn hopes to be able to start accepting new applications soon. “This rotation provides a wealth of benefits to all those involved, from the residents who get the chance to work with a unique population in need to the clinicians who gain a diverse understanding of dermatology treatment techniques. And of course, for the patients, who are so appreciative of the care they receive from our volunteers” (personal communication, June 25, 2021).
How to Get Involved
Dr. Kohn is happy to field questions regarding the rotation and requests for more information via email ([email protected]). Residents interested in this program also may reach out to the AAD’s Education and Volunteers Abroad Committee to express interest in the NAHSRR program’s reinstatement.
Destination Healthy Skin
Since 2017, the Skin Cancer Foundation’s Destination Healthy Skin (DHS) RV has been the setting for more than 3800 free skin cancer screenings provided by volunteers within underserved populations across the United States (Figure 2). After a year hiatus due to the pandemic, DHS hit the road again, starting in New York City on August 1 to 3, 2021. From there, the DHS RV will traverse the country in one large loop, starting with visits to large and small cities in the Midwest and the West Coast. Following a visit to San Diego, California, in early October, the RV will turn east, with stops in Arizona, Texas, and several southern states before ending in Philadelphia, Pennsylvania. Dr. Elizabeth Hale, Senior Vice President of the Skin Cancer Foundation, feels that increasing awareness of the importance of regular skin cancer screening for those at risk is more important than ever. “We know that many people in the past year put routine cancer screening on the back burner, but we’re beginning to appreciate that this has led to significant delays in skin cancer diagnosis and potentially more significant disease when cases are diagnosed.” Dr. Hale noted that as the country continues to return to a degree of normalcy, the backlog of patients now seeking their routine screening has led to longer wait times. She expects DHS may offer some relief. “There are no appointments necessary. If the RV is close to their hometown, patients have an advantage in being able to be seen first come, first served, without having to wait for an appointment or make sure their insurance is accepted. It’s a free screening that can increase access to dermatologists” (personal communication, June 21, 2021).

The program’s organizers acknowledge that DHS is not a long-term solution for improving dermatology access in the United States and recognize that more needs to be done to raise awareness, both of the value that screenings can provide and the importance of sun-protective behavior. “This is an important first step,” says Dr. Hale. “It’s important that we disseminate that no one is immune to skin cancer. It’s about education, and this is a tool to educate patients that everyone should have a skin check once a year, regardless of where you live or what your skin type is” (personal communication, June 21, 2021).
Volunteer dermatologists are needed to assist with screenings when the DHS RV arrives in their community. Providers complete a screening form identifying any concerning lesions and can document specific lesions using the patient’s cell phone. Following the screenings, participating dermatologists are welcome to invite participants to make appointments at their practices or suggest local clinics for follow-up care.
How to Get Involved
The schedule for this year’s screening events can be found online (https://www.skincancer.org/early-detection/destination-healthy-skin/). Consider volunteering (https://www.skincancer.org/early-detection/destination-healthy-skin/physician-volunteers/) or helping to raise awareness by reaching out to local dermatology societies or free clinics in your area. Residents and physician’s assistants are welcome to volunteer as well, as long as they are under the on-site supervision of a board-certified dermatologist.
Final Thoughts
As medical professionals, we all recognize there are valuable contributions we can make to groups and organizations that need our help. The stresses and pressure of work and everyday life can make finding the time to offer that help seem impossible. Although it may seem counterintuitive, volunteering our time to help others can help us better navigate the professional burnout that many medical professionals experience today.
- Ezekor M, Pona A, Cline A, et al. An increasing trend in the number of publications and research projects among dermatology residency applicants. J Am Acad Dermatol. 2020;83:214-216.
- Atluri S, Seivright JR, Shi VY, et al. Volunteer and work experiences among dermatology residency applicants. J Am Acad Dermatol. 2021;84:E97-E98.
- Abokwidir M, Davis SA, Fleischer AB, et al. Use of the emergency department for dermatologic care in the United States by ethnic group. J Dermatolog Treat. 2015;26:392-394.
- Uscher-Pines L, Pines J, Kellermann A, et al. Emergency department visits for nonurgent conditions: systematic literature review. Am J Manag Care. 2013;19:47-59.
- Jack AR, Spence AA, Nichols BJ, et al. Cutaneous conditions leading to dermatology consultations in the emergency department. West J Emerg Med. 2011;12:551-555.
- Ayoubi N, Mirza A-S, Swanson J, et al. Dermatologic care of uninsured patients managed at free clinics. J Am Acad Dermatol. 2019;81:433-437.
- Wright AA, Katz IT. Beyond burnout—redesigning care to restore meaning and sanity for physicians. N Engl J Med. 2018;378:309-311.
- Bull C, Aucoin JB. Voluntary association participation and life satisfaction: a replication note. J Gerontol. 1975;30:73-76.
- Iserson KV. Burnout syndrome: global medicine volunteering as a possible treatment strategy. J Emerg Med. 2018;54:516-521.
- Romero S. Navajo Nation becomes largest tribe in U.S. after pandemic enrollment surge. New York Times. May 21, 2021. Accessed August 19, 2021. https://www.nytimes.com/2021/05/21/us/navajo-cherokee-population.html
- Moore GR, Benally J, Tuttle S. The Navajo Nation: quick facts. University of Arizona website. Accessed August 19, 2021. https://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1471.pdf
The adage “so much to do, so little time” aptly describes the daily challenges facing dermatologists and dermatology residents. The time and attention required by direct patient care, writing notes, navigating electronic health records, and engaging in education and research as well as family commitments can drain even the most tireless clinician. In addition, dermatologists are expected to play a critical role in clinic and practice management to successfully curate an online presence and adapt their skills to successfully manage a teledermatology practice. Coupled with the time spent socializing with friends or colleagues and time for personal hobbies or exercise, it’s easy to see how sleep deprivation is common in many of our colleagues.
What’s being left out of these jam-packed schedules? Increasingly, it is the time and expertise dedicated to volunteering in our local communities. Two recent research letters highlighted how a dramatic increase in the number of research projects and publications is not mirrored by a similar increase in volunteer experiences as dermatology residency selection becomes more competitive.1,2
Although the rate of volunteerism among practicing dermatologists has yet to be studied, a brief review suggests a component of unmet dermatology need within our communities. It’s estimated that approximately 5% to 10% of all emergency department visits are for dermatologic concerns.3-5 In many cases, the reason for the visit is nonurgent and instead reflects a lack of other options for care. However, the need for dermatologists extends beyond the emergency department setting. A review of the prevalence of patients presenting for care to a group of regional free clinics found that 8% (N=5553) of all visitors sought care for dermatologic concerns.6 The benefit is not just for those seated on the examination table; research has shown that while many of the underlying factors resulting in physician burnout stem from systemic issues, participating in volunteer opportunities helps combat burnout in ourselves and our colleagues.7-9 Herein, opportunities that exist for dermatologists to reconnect with their communities, advocate for causes distinctive to the specialty, and care for neighbors most in need are highlighted.
Camp Wonder
Every year, children from across the United States living with chronic and debilitating skin conditions get the opportunity to join fellow campers and spend a week just being kids without the constant focus on being a patient. Camp Wonder’s founder and director, Francesca Tenconi, describes the camp as a place where kids “can form a community and can feel free to be themselves, without judgment, without stares. They get the chance to forget about their skin disease and be themselves” (oral communication, June 18, 2021). Tenconi and the camp’s cofounders and medical directors, Drs. Jenny Kim and Stefani Takahashi, envisioned the camp as a place for all campers regardless of their skin condition to feel safe and welcome. This overall mission guides camp leadership and staff every year over the course of the camp week where campers participate in a mix of traditional and nontraditional summer activities that are safe and accessible for all, from spending time in the pool to arts and crafts and a ropes course.
Camp Wonder is in its 21st year of hosting children and adolescents from across North America at its camp in Livermore, California. This year, Tenconi expects about 100 campers during the last week in July. Camp Wonder relies on medical staff volunteers to make the camp setting safe, inclusive, and fun. “Our dermatology residents and dermatology volunteers are a huge part of why we’re able to have camp,” said Tenconi. “A lot of our kids require very specific medical care throughout the week. We are able to provide this camp experience for them because we have this medical support system available, this specialized dermatology knowledge.” She also noted the benefit to the volunteers themselves, saying,“The feedback we get a lot from residents and dermatologists is that camp gave them a chance to understand the true-life impact of some of the skin diseases these kids and families are living with. Kids will open up to them and tell them how their disease has impacted them personally” (oral communication, June 18, 2021).
Volunteer medical providers help manage the medical needs of the campers beginning at check-in and work shifts in the infirmary as well as help with dispensing and administering medications, changing dressings, and applying ointments or other topical medications. When not assisting with medical care, medical staff can get to know the campers; help out with arts and crafts, games, sports, and other camp activities; and put on skits and plays for campers at nightly camp hangouts (Figure 1).

How to Get Involved
Visit the website (https://www.csdf.org/camp-wonder) for information on becoming a medical volunteer for 2022. Donations to help keep the camp running also are greatly appreciated, as attendance, including travel costs, is free for families through the Children’s Skin Disease Foundation. Finally, dermatologists can help by keeping their young patients with skin disease in mind as future campers. The camp welcomes kids from across the United States and Canada and invites questions from dermatologists and families on how to become a camper and what the experience is like.
Native American Health Services Rotation
Located in the southwestern United States, the Navajo Nation is North America’s largest Native American tribe by enrollment and resides on the largest reservation in the United States.10 Comprised of 27,000 square miles within portions of Arizona, New Mexico, and Utah, the reservation’s total area is greater than that of Massachusetts, Vermont, and New Hampshire combined.11 The reservation is home to an estimated 180,000 Navajo people, a population roughly the size of Salt Lake City, Utah. Yet, many homes on the reservation are without electricity, running water, telephones, or broadband access, and many roads on the reservation remain unpaved. Prior to the COVID-19 pandemic, 4 dermatology residents were selected each year to travel to this unique and remote location to work with the staff of the Chinle Comprehensive Health Care Facility (Chinle, Arizona), an Indian Health Service facility, as part of the American Academy of Dermatology (AAD)–sponsored Native American Health Services Resident Rotation (NAHSRR).
Dr. Lucinda Kohn, Assistant Professor of Dermatology at the University of Colorado and the director of the NAHSRR program discovered the value of this rotation firsthand as a dermatology resident. In 2017, she traveled to the area to spend 2 weeks serving within the community. “I went because of a personal connection. My husband is Native American, although not Navajo. I wanted to experience what it was like to provide dermatologic care for Native Americans. I found the Navajo people to be so friendly and so grateful for our care. The clinicians we worked with at Chinle were excited to have us share our expertise and to pass on their knowledge to us,” said Dr. Kohn (personal communication, June 24, 2021).
Rotating residents provide dermatologic care for the Navajo people and share their unique medical skill set to local primary care clinicians serving as preceptors. They also may have an opportunity to learn from Native healers about traditional Navajo beliefs and ceremonies used as part of a holistic approach to healing.
The program, similar to volunteer programs across the country, was put on hold during the height of the COVID-19 pandemic. “The Navajo nation witnessed a really tragic surge of COVID cases that required that limited medical resources be diverted to help cope with the pandemic,” says Dr. Kohn. “It really wasn’t safe for residents to travel to the reservation either, so the rotation had to be put on hold.” However, in April 2021, the health care staff of the Chinle Comprehensive Care Facility reached out to revive the program, which is now pending the green light from the AAD. It is unclear if or when AAD leadership will allow this rotation to restart. Dr. Kohn hopes to be able to start accepting new applications soon. “This rotation provides a wealth of benefits to all those involved, from the residents who get the chance to work with a unique population in need to the clinicians who gain a diverse understanding of dermatology treatment techniques. And of course, for the patients, who are so appreciative of the care they receive from our volunteers” (personal communication, June 25, 2021).
How to Get Involved
Dr. Kohn is happy to field questions regarding the rotation and requests for more information via email ([email protected]). Residents interested in this program also may reach out to the AAD’s Education and Volunteers Abroad Committee to express interest in the NAHSRR program’s reinstatement.
Destination Healthy Skin
Since 2017, the Skin Cancer Foundation’s Destination Healthy Skin (DHS) RV has been the setting for more than 3800 free skin cancer screenings provided by volunteers within underserved populations across the United States (Figure 2). After a year hiatus due to the pandemic, DHS hit the road again, starting in New York City on August 1 to 3, 2021. From there, the DHS RV will traverse the country in one large loop, starting with visits to large and small cities in the Midwest and the West Coast. Following a visit to San Diego, California, in early October, the RV will turn east, with stops in Arizona, Texas, and several southern states before ending in Philadelphia, Pennsylvania. Dr. Elizabeth Hale, Senior Vice President of the Skin Cancer Foundation, feels that increasing awareness of the importance of regular skin cancer screening for those at risk is more important than ever. “We know that many people in the past year put routine cancer screening on the back burner, but we’re beginning to appreciate that this has led to significant delays in skin cancer diagnosis and potentially more significant disease when cases are diagnosed.” Dr. Hale noted that as the country continues to return to a degree of normalcy, the backlog of patients now seeking their routine screening has led to longer wait times. She expects DHS may offer some relief. “There are no appointments necessary. If the RV is close to their hometown, patients have an advantage in being able to be seen first come, first served, without having to wait for an appointment or make sure their insurance is accepted. It’s a free screening that can increase access to dermatologists” (personal communication, June 21, 2021).

The program’s organizers acknowledge that DHS is not a long-term solution for improving dermatology access in the United States and recognize that more needs to be done to raise awareness, both of the value that screenings can provide and the importance of sun-protective behavior. “This is an important first step,” says Dr. Hale. “It’s important that we disseminate that no one is immune to skin cancer. It’s about education, and this is a tool to educate patients that everyone should have a skin check once a year, regardless of where you live or what your skin type is” (personal communication, June 21, 2021).
Volunteer dermatologists are needed to assist with screenings when the DHS RV arrives in their community. Providers complete a screening form identifying any concerning lesions and can document specific lesions using the patient’s cell phone. Following the screenings, participating dermatologists are welcome to invite participants to make appointments at their practices or suggest local clinics for follow-up care.
How to Get Involved
The schedule for this year’s screening events can be found online (https://www.skincancer.org/early-detection/destination-healthy-skin/). Consider volunteering (https://www.skincancer.org/early-detection/destination-healthy-skin/physician-volunteers/) or helping to raise awareness by reaching out to local dermatology societies or free clinics in your area. Residents and physician’s assistants are welcome to volunteer as well, as long as they are under the on-site supervision of a board-certified dermatologist.
Final Thoughts
As medical professionals, we all recognize there are valuable contributions we can make to groups and organizations that need our help. The stresses and pressure of work and everyday life can make finding the time to offer that help seem impossible. Although it may seem counterintuitive, volunteering our time to help others can help us better navigate the professional burnout that many medical professionals experience today.
The adage “so much to do, so little time” aptly describes the daily challenges facing dermatologists and dermatology residents. The time and attention required by direct patient care, writing notes, navigating electronic health records, and engaging in education and research as well as family commitments can drain even the most tireless clinician. In addition, dermatologists are expected to play a critical role in clinic and practice management to successfully curate an online presence and adapt their skills to successfully manage a teledermatology practice. Coupled with the time spent socializing with friends or colleagues and time for personal hobbies or exercise, it’s easy to see how sleep deprivation is common in many of our colleagues.
What’s being left out of these jam-packed schedules? Increasingly, it is the time and expertise dedicated to volunteering in our local communities. Two recent research letters highlighted how a dramatic increase in the number of research projects and publications is not mirrored by a similar increase in volunteer experiences as dermatology residency selection becomes more competitive.1,2
Although the rate of volunteerism among practicing dermatologists has yet to be studied, a brief review suggests a component of unmet dermatology need within our communities. It’s estimated that approximately 5% to 10% of all emergency department visits are for dermatologic concerns.3-5 In many cases, the reason for the visit is nonurgent and instead reflects a lack of other options for care. However, the need for dermatologists extends beyond the emergency department setting. A review of the prevalence of patients presenting for care to a group of regional free clinics found that 8% (N=5553) of all visitors sought care for dermatologic concerns.6 The benefit is not just for those seated on the examination table; research has shown that while many of the underlying factors resulting in physician burnout stem from systemic issues, participating in volunteer opportunities helps combat burnout in ourselves and our colleagues.7-9 Herein, opportunities that exist for dermatologists to reconnect with their communities, advocate for causes distinctive to the specialty, and care for neighbors most in need are highlighted.
Camp Wonder
Every year, children from across the United States living with chronic and debilitating skin conditions get the opportunity to join fellow campers and spend a week just being kids without the constant focus on being a patient. Camp Wonder’s founder and director, Francesca Tenconi, describes the camp as a place where kids “can form a community and can feel free to be themselves, without judgment, without stares. They get the chance to forget about their skin disease and be themselves” (oral communication, June 18, 2021). Tenconi and the camp’s cofounders and medical directors, Drs. Jenny Kim and Stefani Takahashi, envisioned the camp as a place for all campers regardless of their skin condition to feel safe and welcome. This overall mission guides camp leadership and staff every year over the course of the camp week where campers participate in a mix of traditional and nontraditional summer activities that are safe and accessible for all, from spending time in the pool to arts and crafts and a ropes course.
Camp Wonder is in its 21st year of hosting children and adolescents from across North America at its camp in Livermore, California. This year, Tenconi expects about 100 campers during the last week in July. Camp Wonder relies on medical staff volunteers to make the camp setting safe, inclusive, and fun. “Our dermatology residents and dermatology volunteers are a huge part of why we’re able to have camp,” said Tenconi. “A lot of our kids require very specific medical care throughout the week. We are able to provide this camp experience for them because we have this medical support system available, this specialized dermatology knowledge.” She also noted the benefit to the volunteers themselves, saying,“The feedback we get a lot from residents and dermatologists is that camp gave them a chance to understand the true-life impact of some of the skin diseases these kids and families are living with. Kids will open up to them and tell them how their disease has impacted them personally” (oral communication, June 18, 2021).
Volunteer medical providers help manage the medical needs of the campers beginning at check-in and work shifts in the infirmary as well as help with dispensing and administering medications, changing dressings, and applying ointments or other topical medications. When not assisting with medical care, medical staff can get to know the campers; help out with arts and crafts, games, sports, and other camp activities; and put on skits and plays for campers at nightly camp hangouts (Figure 1).

How to Get Involved
Visit the website (https://www.csdf.org/camp-wonder) for information on becoming a medical volunteer for 2022. Donations to help keep the camp running also are greatly appreciated, as attendance, including travel costs, is free for families through the Children’s Skin Disease Foundation. Finally, dermatologists can help by keeping their young patients with skin disease in mind as future campers. The camp welcomes kids from across the United States and Canada and invites questions from dermatologists and families on how to become a camper and what the experience is like.
Native American Health Services Rotation
Located in the southwestern United States, the Navajo Nation is North America’s largest Native American tribe by enrollment and resides on the largest reservation in the United States.10 Comprised of 27,000 square miles within portions of Arizona, New Mexico, and Utah, the reservation’s total area is greater than that of Massachusetts, Vermont, and New Hampshire combined.11 The reservation is home to an estimated 180,000 Navajo people, a population roughly the size of Salt Lake City, Utah. Yet, many homes on the reservation are without electricity, running water, telephones, or broadband access, and many roads on the reservation remain unpaved. Prior to the COVID-19 pandemic, 4 dermatology residents were selected each year to travel to this unique and remote location to work with the staff of the Chinle Comprehensive Health Care Facility (Chinle, Arizona), an Indian Health Service facility, as part of the American Academy of Dermatology (AAD)–sponsored Native American Health Services Resident Rotation (NAHSRR).
Dr. Lucinda Kohn, Assistant Professor of Dermatology at the University of Colorado and the director of the NAHSRR program discovered the value of this rotation firsthand as a dermatology resident. In 2017, she traveled to the area to spend 2 weeks serving within the community. “I went because of a personal connection. My husband is Native American, although not Navajo. I wanted to experience what it was like to provide dermatologic care for Native Americans. I found the Navajo people to be so friendly and so grateful for our care. The clinicians we worked with at Chinle were excited to have us share our expertise and to pass on their knowledge to us,” said Dr. Kohn (personal communication, June 24, 2021).
Rotating residents provide dermatologic care for the Navajo people and share their unique medical skill set to local primary care clinicians serving as preceptors. They also may have an opportunity to learn from Native healers about traditional Navajo beliefs and ceremonies used as part of a holistic approach to healing.
The program, similar to volunteer programs across the country, was put on hold during the height of the COVID-19 pandemic. “The Navajo nation witnessed a really tragic surge of COVID cases that required that limited medical resources be diverted to help cope with the pandemic,” says Dr. Kohn. “It really wasn’t safe for residents to travel to the reservation either, so the rotation had to be put on hold.” However, in April 2021, the health care staff of the Chinle Comprehensive Care Facility reached out to revive the program, which is now pending the green light from the AAD. It is unclear if or when AAD leadership will allow this rotation to restart. Dr. Kohn hopes to be able to start accepting new applications soon. “This rotation provides a wealth of benefits to all those involved, from the residents who get the chance to work with a unique population in need to the clinicians who gain a diverse understanding of dermatology treatment techniques. And of course, for the patients, who are so appreciative of the care they receive from our volunteers” (personal communication, June 25, 2021).
How to Get Involved
Dr. Kohn is happy to field questions regarding the rotation and requests for more information via email ([email protected]). Residents interested in this program also may reach out to the AAD’s Education and Volunteers Abroad Committee to express interest in the NAHSRR program’s reinstatement.
Destination Healthy Skin
Since 2017, the Skin Cancer Foundation’s Destination Healthy Skin (DHS) RV has been the setting for more than 3800 free skin cancer screenings provided by volunteers within underserved populations across the United States (Figure 2). After a year hiatus due to the pandemic, DHS hit the road again, starting in New York City on August 1 to 3, 2021. From there, the DHS RV will traverse the country in one large loop, starting with visits to large and small cities in the Midwest and the West Coast. Following a visit to San Diego, California, in early October, the RV will turn east, with stops in Arizona, Texas, and several southern states before ending in Philadelphia, Pennsylvania. Dr. Elizabeth Hale, Senior Vice President of the Skin Cancer Foundation, feels that increasing awareness of the importance of regular skin cancer screening for those at risk is more important than ever. “We know that many people in the past year put routine cancer screening on the back burner, but we’re beginning to appreciate that this has led to significant delays in skin cancer diagnosis and potentially more significant disease when cases are diagnosed.” Dr. Hale noted that as the country continues to return to a degree of normalcy, the backlog of patients now seeking their routine screening has led to longer wait times. She expects DHS may offer some relief. “There are no appointments necessary. If the RV is close to their hometown, patients have an advantage in being able to be seen first come, first served, without having to wait for an appointment or make sure their insurance is accepted. It’s a free screening that can increase access to dermatologists” (personal communication, June 21, 2021).

The program’s organizers acknowledge that DHS is not a long-term solution for improving dermatology access in the United States and recognize that more needs to be done to raise awareness, both of the value that screenings can provide and the importance of sun-protective behavior. “This is an important first step,” says Dr. Hale. “It’s important that we disseminate that no one is immune to skin cancer. It’s about education, and this is a tool to educate patients that everyone should have a skin check once a year, regardless of where you live or what your skin type is” (personal communication, June 21, 2021).
Volunteer dermatologists are needed to assist with screenings when the DHS RV arrives in their community. Providers complete a screening form identifying any concerning lesions and can document specific lesions using the patient’s cell phone. Following the screenings, participating dermatologists are welcome to invite participants to make appointments at their practices or suggest local clinics for follow-up care.
How to Get Involved
The schedule for this year’s screening events can be found online (https://www.skincancer.org/early-detection/destination-healthy-skin/). Consider volunteering (https://www.skincancer.org/early-detection/destination-healthy-skin/physician-volunteers/) or helping to raise awareness by reaching out to local dermatology societies or free clinics in your area. Residents and physician’s assistants are welcome to volunteer as well, as long as they are under the on-site supervision of a board-certified dermatologist.
Final Thoughts
As medical professionals, we all recognize there are valuable contributions we can make to groups and organizations that need our help. The stresses and pressure of work and everyday life can make finding the time to offer that help seem impossible. Although it may seem counterintuitive, volunteering our time to help others can help us better navigate the professional burnout that many medical professionals experience today.
- Ezekor M, Pona A, Cline A, et al. An increasing trend in the number of publications and research projects among dermatology residency applicants. J Am Acad Dermatol. 2020;83:214-216.
- Atluri S, Seivright JR, Shi VY, et al. Volunteer and work experiences among dermatology residency applicants. J Am Acad Dermatol. 2021;84:E97-E98.
- Abokwidir M, Davis SA, Fleischer AB, et al. Use of the emergency department for dermatologic care in the United States by ethnic group. J Dermatolog Treat. 2015;26:392-394.
- Uscher-Pines L, Pines J, Kellermann A, et al. Emergency department visits for nonurgent conditions: systematic literature review. Am J Manag Care. 2013;19:47-59.
- Jack AR, Spence AA, Nichols BJ, et al. Cutaneous conditions leading to dermatology consultations in the emergency department. West J Emerg Med. 2011;12:551-555.
- Ayoubi N, Mirza A-S, Swanson J, et al. Dermatologic care of uninsured patients managed at free clinics. J Am Acad Dermatol. 2019;81:433-437.
- Wright AA, Katz IT. Beyond burnout—redesigning care to restore meaning and sanity for physicians. N Engl J Med. 2018;378:309-311.
- Bull C, Aucoin JB. Voluntary association participation and life satisfaction: a replication note. J Gerontol. 1975;30:73-76.
- Iserson KV. Burnout syndrome: global medicine volunteering as a possible treatment strategy. J Emerg Med. 2018;54:516-521.
- Romero S. Navajo Nation becomes largest tribe in U.S. after pandemic enrollment surge. New York Times. May 21, 2021. Accessed August 19, 2021. https://www.nytimes.com/2021/05/21/us/navajo-cherokee-population.html
- Moore GR, Benally J, Tuttle S. The Navajo Nation: quick facts. University of Arizona website. Accessed August 19, 2021. https://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1471.pdf
- Ezekor M, Pona A, Cline A, et al. An increasing trend in the number of publications and research projects among dermatology residency applicants. J Am Acad Dermatol. 2020;83:214-216.
- Atluri S, Seivright JR, Shi VY, et al. Volunteer and work experiences among dermatology residency applicants. J Am Acad Dermatol. 2021;84:E97-E98.
- Abokwidir M, Davis SA, Fleischer AB, et al. Use of the emergency department for dermatologic care in the United States by ethnic group. J Dermatolog Treat. 2015;26:392-394.
- Uscher-Pines L, Pines J, Kellermann A, et al. Emergency department visits for nonurgent conditions: systematic literature review. Am J Manag Care. 2013;19:47-59.
- Jack AR, Spence AA, Nichols BJ, et al. Cutaneous conditions leading to dermatology consultations in the emergency department. West J Emerg Med. 2011;12:551-555.
- Ayoubi N, Mirza A-S, Swanson J, et al. Dermatologic care of uninsured patients managed at free clinics. J Am Acad Dermatol. 2019;81:433-437.
- Wright AA, Katz IT. Beyond burnout—redesigning care to restore meaning and sanity for physicians. N Engl J Med. 2018;378:309-311.
- Bull C, Aucoin JB. Voluntary association participation and life satisfaction: a replication note. J Gerontol. 1975;30:73-76.
- Iserson KV. Burnout syndrome: global medicine volunteering as a possible treatment strategy. J Emerg Med. 2018;54:516-521.
- Romero S. Navajo Nation becomes largest tribe in U.S. after pandemic enrollment surge. New York Times. May 21, 2021. Accessed August 19, 2021. https://www.nytimes.com/2021/05/21/us/navajo-cherokee-population.html
- Moore GR, Benally J, Tuttle S. The Navajo Nation: quick facts. University of Arizona website. Accessed August 19, 2021. https://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1471.pdf
Resident Pearl
- Volunteerism rates among dermatology residents seem to be decreasing. We should work to combat this trend by finding ways to give back to our communities and spur our colleagues to do the same.
‘Deeper dive’ into opioid overdose deaths during COVID pandemic
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Good news is no news
I’ve become kind of a hermit. At least, as much as someone who drives a car, goes to the store, and sees patients 5 days a week can be.
It seemed like the news was always dominated by another senseless mass shooting, an increasingly dysfunctional government, an environmental crisis going to hell (with us along for the ride), and endlessly escalating inflammatory political pundits (who always seem to get far more coverage than they deserve. Personally, I don’t think they deserve any, regardless of which side they’re on).
As things got worse, I became more obsessed with reading about them. I’d read the news on my iPad before bed, and when I first woke up, and several times a day at work.
It was driving me nuts. Perhaps it’s my personality to worry too much about these things. I was losing sleep and wasting valuable time at home and work.
I came to a decision. It was time to stop.
I deleted all my news apps and bookmarks. I’d go to lengths to avoid all news. If in a restaurant where a TV was on, I’d sit with my back to it. I stopped going to the doctor’s lounge (with its TVs constantly on a news network). When I had to wait to pick up my car at the shop, I sat outside and played games on my phone rather than use the waiting room with its blaring TV.
This doesn’t mean I’m completely unplugged. I still read interesting stories about science or history. I check the weather forecast. Family members occasionally send me amusing articles that I look at. I read online medical articles. I use the Internet to look things up. But I make a conscious effort not to look at headlines or other stuff on the periphery.
I’m not stupid or naive enough to believe that the insanity and acrimony won’t continue happening. But the bottom line is that obviously I can’t control or change it.
So I try not to let it upset me any more. If the only way to do that is to completely not read it and not know, I’m fine with that. After almost 50 years of reading news (I started when I was about 7, with my parent’s subscription to Newsweek), I’ve completely stopped.
Instead of reading the day’s events I now mindlessly play Toon Blast or read history books on my iPad before bed. Perhaps a waste of time, but no more so than getting upset, losing sleep, getting ulcers, and going gray over things I can’t control.
I have more time in the morning and my work day, since I’m not spending it scanning headlines.
Now my world is restricted to my family, friends, dogs, and job. Things I enjoy and have control over. Those around me have been told that I wish to discuss nothing about current events, and they respect that.
Now I sleep better, worry less (at least about those things), and have more time to focus on my immediate world. And that’s fine with me. It may be the way of the ostrich, but at this point in my life, that’s what I prefer.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve become kind of a hermit. At least, as much as someone who drives a car, goes to the store, and sees patients 5 days a week can be.
It seemed like the news was always dominated by another senseless mass shooting, an increasingly dysfunctional government, an environmental crisis going to hell (with us along for the ride), and endlessly escalating inflammatory political pundits (who always seem to get far more coverage than they deserve. Personally, I don’t think they deserve any, regardless of which side they’re on).
As things got worse, I became more obsessed with reading about them. I’d read the news on my iPad before bed, and when I first woke up, and several times a day at work.
It was driving me nuts. Perhaps it’s my personality to worry too much about these things. I was losing sleep and wasting valuable time at home and work.
I came to a decision. It was time to stop.
I deleted all my news apps and bookmarks. I’d go to lengths to avoid all news. If in a restaurant where a TV was on, I’d sit with my back to it. I stopped going to the doctor’s lounge (with its TVs constantly on a news network). When I had to wait to pick up my car at the shop, I sat outside and played games on my phone rather than use the waiting room with its blaring TV.
This doesn’t mean I’m completely unplugged. I still read interesting stories about science or history. I check the weather forecast. Family members occasionally send me amusing articles that I look at. I read online medical articles. I use the Internet to look things up. But I make a conscious effort not to look at headlines or other stuff on the periphery.
I’m not stupid or naive enough to believe that the insanity and acrimony won’t continue happening. But the bottom line is that obviously I can’t control or change it.
So I try not to let it upset me any more. If the only way to do that is to completely not read it and not know, I’m fine with that. After almost 50 years of reading news (I started when I was about 7, with my parent’s subscription to Newsweek), I’ve completely stopped.
Instead of reading the day’s events I now mindlessly play Toon Blast or read history books on my iPad before bed. Perhaps a waste of time, but no more so than getting upset, losing sleep, getting ulcers, and going gray over things I can’t control.
I have more time in the morning and my work day, since I’m not spending it scanning headlines.
Now my world is restricted to my family, friends, dogs, and job. Things I enjoy and have control over. Those around me have been told that I wish to discuss nothing about current events, and they respect that.
Now I sleep better, worry less (at least about those things), and have more time to focus on my immediate world. And that’s fine with me. It may be the way of the ostrich, but at this point in my life, that’s what I prefer.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve become kind of a hermit. At least, as much as someone who drives a car, goes to the store, and sees patients 5 days a week can be.
It seemed like the news was always dominated by another senseless mass shooting, an increasingly dysfunctional government, an environmental crisis going to hell (with us along for the ride), and endlessly escalating inflammatory political pundits (who always seem to get far more coverage than they deserve. Personally, I don’t think they deserve any, regardless of which side they’re on).
As things got worse, I became more obsessed with reading about them. I’d read the news on my iPad before bed, and when I first woke up, and several times a day at work.
It was driving me nuts. Perhaps it’s my personality to worry too much about these things. I was losing sleep and wasting valuable time at home and work.
I came to a decision. It was time to stop.
I deleted all my news apps and bookmarks. I’d go to lengths to avoid all news. If in a restaurant where a TV was on, I’d sit with my back to it. I stopped going to the doctor’s lounge (with its TVs constantly on a news network). When I had to wait to pick up my car at the shop, I sat outside and played games on my phone rather than use the waiting room with its blaring TV.
This doesn’t mean I’m completely unplugged. I still read interesting stories about science or history. I check the weather forecast. Family members occasionally send me amusing articles that I look at. I read online medical articles. I use the Internet to look things up. But I make a conscious effort not to look at headlines or other stuff on the periphery.
I’m not stupid or naive enough to believe that the insanity and acrimony won’t continue happening. But the bottom line is that obviously I can’t control or change it.
So I try not to let it upset me any more. If the only way to do that is to completely not read it and not know, I’m fine with that. After almost 50 years of reading news (I started when I was about 7, with my parent’s subscription to Newsweek), I’ve completely stopped.
Instead of reading the day’s events I now mindlessly play Toon Blast or read history books on my iPad before bed. Perhaps a waste of time, but no more so than getting upset, losing sleep, getting ulcers, and going gray over things I can’t control.
I have more time in the morning and my work day, since I’m not spending it scanning headlines.
Now my world is restricted to my family, friends, dogs, and job. Things I enjoy and have control over. Those around me have been told that I wish to discuss nothing about current events, and they respect that.
Now I sleep better, worry less (at least about those things), and have more time to focus on my immediate world. And that’s fine with me. It may be the way of the ostrich, but at this point in my life, that’s what I prefer.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Emerging data point to underlying autoimmunity in ME/CFS
Emerging evidence suggests that autoimmunity plays a role in postinfectious myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and that targeting autoantibodies could be a promising treatment approach.
The same may also apply to many cases of “long COVID,” in which many of the symptoms overlap with those of ME/CFS, Carmen Scheibenbogen, MD, professor of clinical immunology and director of the Institute for Medical Immunology, Charité University Medicine, Berlin, said during the annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis.
Several groups, including Dr. Scheibenbogen’s, have reported finding autoantibodies against neurotransmitter receptor antigens in people with ME/CFS. And, in a paper published in the Journal of Clinical Medicine the day that Dr. Scheibenbogen spoke at the meeting, her team reported significant correlations between autoantibodies to vasoregulative G-protein–coupled receptors and symptom severity, autonomic dysfunction, and disability among 116 patients with infection-triggered ME/CFS who were diagnosed using the symptom-based 2003 Canadian consensus criteria.
People with ME/CFS are also more likely to have genetic risk factors associated with autoimmunity and personal and/or family histories of autoimmune conditions. And, clinical trials have demonstrated early success with various immunomodulatory treatments in subsets of people with ME/CFS, including endoxan, rituximab, and immunoadsorption.
“We have evidence that ME/CFS is an autoantibody-mediated disease, and we have evidence that autoantibody targeting is effective in this disease. So far ... we have few and underfinanced clinical studies, but the good news is we have promising emerging treatment options,” Dr. Scheibenbogen said.
Asked to comment, ME/CFS expert Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said: “There is already strong evidence that there are autoantibodies in ME/CFS. Dr. Scheibenbogen’s work is the latest and employs the latest technology. ... I would bet that autoantibodies to neural targets are likely to cause some of the symptoms of ME/CFS and some of the symptoms of long COVID.”
However, he cautioned, “that has not been proven, and even if it were proven you would have to demonstrate that treatments based on that theory worked.”
Dr. Komaroff said he views autoimmunity as a likely component of the ME/CFS spectrum, but not the only one. “My current view of this illness is that there’s a final common pathway in the brain that leads to the symptoms of the illness. But that final common pathway can be triggered by a variety of different things, one of which could be autoantibodies while another could be infection or inflammation in the brain.”
Emerging evidence points to autoimmunity
Dr. Scheibenbogen summarized the work published in this area over the past few years by her group and others.
In a comparison of ME/CFS patients with 201 healthy controls, significant associations were seen with two specific autoimmunity-related risk alleles only in the ME/CFS patients who reported acute onset of disease with an infection but not in those with ME/CFS without infection-triggered onset or the controls. Both genes play roles in regulating B- and T-cell activation.
Another recent study found associations with ME/CFS and major histocompatibility complex class II molecules, a typical feature of autoimmune diseases, in a comparison between 426 adult Norwegian ME/CFS patients who were diagnosed with the Canadian consensus criteria and 4,511 healthy, ethnically matched controls.
In a 2020 paper, Dr. Scheibenbogen and pharmacologist Klaus Wirth presented a “unifying hypothesis” of ME/CFS pathophysiology based on the finding of elevations in autoantibodies against beta2-adrenergic receptors and muscarinic acetylcholine receptors in some individuals with the condition. Since both of those receptors are important vasodilators, their functional disturbance would be expected to cause vasoconstriction and hypoxemia, which would explain many of the symptoms of ME/CFS. This mechanism would align with other findings of muscular and cerebral hypoperfusion that correlate with fatigue, particularly post exertion, as well as metabolic changes that are in line with the concepts of hypoxemia and ischemia.
Further evidence for vascular dysfunction in ME/CFS came from her group’s study finding evidence of peripheral endothelial dysfunction that was associated with symptom severity in 35 adult patients. “Vasoconstriction, hypovolemia, and release of vasoactive and algesic mediators is probably a key pathomechanism of the disease,” Dr. Scheibenbogen said.
Treatments: Will targeting autoantibodies work?
In the second part of her talk, Dr. Scheibenbogen summarized clinical trials of the following treatment approaches that involve targeting autoantibodies as a way to alleviate ME/CFS symptoms:
Rituximab: Work on infusions of the B-cell depleting agent has been conducted by Norwegian researchers beginning in 2011 with a small randomized trial and an open-label, phase 2 study in 2015, both showing clinical responses in ME/CFS. However, a subsequent phase 3, randomized clinical trial of 151 patients, again diagnosed using the Canadian criteria, was negative.
There are several possible explanations for this, Dr. Scheibenbogen noted. For one, the maintenance dose had to be reduced because of a lack of financial support. “This was probably critical. The lower dose was insufficient to adequately deplete B cells.” Also, there may have been a strong placebo response in the control group since they were being given better care than they normally would receive during the trial. “I think probably nobody will again do a rituximab trial. This was very disappointing for all of us. But, we still have other opportunities to follow this path,” she said.
Dr. Komaroff agreed. “I don’t think the failure of one drug that hits malignant B cells is proof against the autoimmune hypothesis per se. I think the evidence is that rituximab doesn’t work, but that doesn’t invalidate the autoimmunity hypothesis.”
Cyclophosphamide: The same Norwegian group also showed positive findings in an open-label, phase 2 trial of the immune-modifying drug cyclophosphamide in 22 of 40 patients. Interestingly, HLA risk alleles were much more common in responders than nonresponders, Dr. Scheibenbogen noted.
Immunoadsorption: This technique, similar to dialysis, involves separating out the blood plasma by centrifugation and removing IgG autoantibodies by a binding column, then returning the plasma back to the patient. It is used, primarily in Europe, to treat severe autoimmune diseases including dilative cardiomyopathy and refractory systemic lupus erythematosus (SLE).
Dr. Scheibenbogen’s group has conducted two studies of immunoadsorption in ME/CFS. In one, a 5-day procedure led to rapid symptom improvement in 7 of 10 patients, with sustained improvement in 3 patients after 2 years. Autoantibodies decreased rapidly in 9 of the 10 patients. In a follow-up study of five of the responders 2 years later, retreatment with a modified immunoadsorption protocol led to rapid and sustained improvement in four. Further study has been on hold because of the pandemic.
Next-gen IgG-targeting therapies: Another approach that could offer promise for ME/CFS involves therapies that block the Fc receptors of IgG. Several are in phase 1-3 trials for autoimmune conditions. One candidate drug, the Fc fragment efgartigimod, is currently in phase 3 trials for several conditions, including generalized myasthenia gravis, primary immune thrombocytopenia, and chronic inflammatory demyelinating polyneuropathy. Phase 3 trials are planned for the monoclonal antibody rozanolixizumab in those same conditions.
Newer-generation monoclonal antibodies targeting CD19 or CD20 that show benefit in various autoimmune conditions are another possibility for ME/CFS. These include ocrelizumab (Ocrevus), approved in the United States for treating relapsing and progressive multiple sclerosis and in trials for SLE; obinutuzumab (Gazyva), approved for treating lymphoma and also in development for SLE; and ublituximab, in phase 3 trials for multiple sclerosis.
“Most of them are more effective than rituximab,” Dr. Scheibenbogen noted, adding that “currently the data look quite promising. They are effective in different autoimmune diseases and they are quite well tolerated. There’s great hope now with COVID-19 that we can convince some companies to do such trials in ME/CFS as well.”
Dr. Scheibenbogen’s institution, the Charité Fatigue Center, has a patent for beta2-adrenergic receptor antibodies for diagnosing ME/CFS under her name together with Celltrend. Dr. Komaroff has received personal fees from Serimmune.
Emerging evidence suggests that autoimmunity plays a role in postinfectious myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and that targeting autoantibodies could be a promising treatment approach.
The same may also apply to many cases of “long COVID,” in which many of the symptoms overlap with those of ME/CFS, Carmen Scheibenbogen, MD, professor of clinical immunology and director of the Institute for Medical Immunology, Charité University Medicine, Berlin, said during the annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis.
Several groups, including Dr. Scheibenbogen’s, have reported finding autoantibodies against neurotransmitter receptor antigens in people with ME/CFS. And, in a paper published in the Journal of Clinical Medicine the day that Dr. Scheibenbogen spoke at the meeting, her team reported significant correlations between autoantibodies to vasoregulative G-protein–coupled receptors and symptom severity, autonomic dysfunction, and disability among 116 patients with infection-triggered ME/CFS who were diagnosed using the symptom-based 2003 Canadian consensus criteria.
People with ME/CFS are also more likely to have genetic risk factors associated with autoimmunity and personal and/or family histories of autoimmune conditions. And, clinical trials have demonstrated early success with various immunomodulatory treatments in subsets of people with ME/CFS, including endoxan, rituximab, and immunoadsorption.
“We have evidence that ME/CFS is an autoantibody-mediated disease, and we have evidence that autoantibody targeting is effective in this disease. So far ... we have few and underfinanced clinical studies, but the good news is we have promising emerging treatment options,” Dr. Scheibenbogen said.
Asked to comment, ME/CFS expert Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said: “There is already strong evidence that there are autoantibodies in ME/CFS. Dr. Scheibenbogen’s work is the latest and employs the latest technology. ... I would bet that autoantibodies to neural targets are likely to cause some of the symptoms of ME/CFS and some of the symptoms of long COVID.”
However, he cautioned, “that has not been proven, and even if it were proven you would have to demonstrate that treatments based on that theory worked.”
Dr. Komaroff said he views autoimmunity as a likely component of the ME/CFS spectrum, but not the only one. “My current view of this illness is that there’s a final common pathway in the brain that leads to the symptoms of the illness. But that final common pathway can be triggered by a variety of different things, one of which could be autoantibodies while another could be infection or inflammation in the brain.”
Emerging evidence points to autoimmunity
Dr. Scheibenbogen summarized the work published in this area over the past few years by her group and others.
In a comparison of ME/CFS patients with 201 healthy controls, significant associations were seen with two specific autoimmunity-related risk alleles only in the ME/CFS patients who reported acute onset of disease with an infection but not in those with ME/CFS without infection-triggered onset or the controls. Both genes play roles in regulating B- and T-cell activation.
Another recent study found associations with ME/CFS and major histocompatibility complex class II molecules, a typical feature of autoimmune diseases, in a comparison between 426 adult Norwegian ME/CFS patients who were diagnosed with the Canadian consensus criteria and 4,511 healthy, ethnically matched controls.
In a 2020 paper, Dr. Scheibenbogen and pharmacologist Klaus Wirth presented a “unifying hypothesis” of ME/CFS pathophysiology based on the finding of elevations in autoantibodies against beta2-adrenergic receptors and muscarinic acetylcholine receptors in some individuals with the condition. Since both of those receptors are important vasodilators, their functional disturbance would be expected to cause vasoconstriction and hypoxemia, which would explain many of the symptoms of ME/CFS. This mechanism would align with other findings of muscular and cerebral hypoperfusion that correlate with fatigue, particularly post exertion, as well as metabolic changes that are in line with the concepts of hypoxemia and ischemia.
Further evidence for vascular dysfunction in ME/CFS came from her group’s study finding evidence of peripheral endothelial dysfunction that was associated with symptom severity in 35 adult patients. “Vasoconstriction, hypovolemia, and release of vasoactive and algesic mediators is probably a key pathomechanism of the disease,” Dr. Scheibenbogen said.
Treatments: Will targeting autoantibodies work?
In the second part of her talk, Dr. Scheibenbogen summarized clinical trials of the following treatment approaches that involve targeting autoantibodies as a way to alleviate ME/CFS symptoms:
Rituximab: Work on infusions of the B-cell depleting agent has been conducted by Norwegian researchers beginning in 2011 with a small randomized trial and an open-label, phase 2 study in 2015, both showing clinical responses in ME/CFS. However, a subsequent phase 3, randomized clinical trial of 151 patients, again diagnosed using the Canadian criteria, was negative.
There are several possible explanations for this, Dr. Scheibenbogen noted. For one, the maintenance dose had to be reduced because of a lack of financial support. “This was probably critical. The lower dose was insufficient to adequately deplete B cells.” Also, there may have been a strong placebo response in the control group since they were being given better care than they normally would receive during the trial. “I think probably nobody will again do a rituximab trial. This was very disappointing for all of us. But, we still have other opportunities to follow this path,” she said.
Dr. Komaroff agreed. “I don’t think the failure of one drug that hits malignant B cells is proof against the autoimmune hypothesis per se. I think the evidence is that rituximab doesn’t work, but that doesn’t invalidate the autoimmunity hypothesis.”
Cyclophosphamide: The same Norwegian group also showed positive findings in an open-label, phase 2 trial of the immune-modifying drug cyclophosphamide in 22 of 40 patients. Interestingly, HLA risk alleles were much more common in responders than nonresponders, Dr. Scheibenbogen noted.
Immunoadsorption: This technique, similar to dialysis, involves separating out the blood plasma by centrifugation and removing IgG autoantibodies by a binding column, then returning the plasma back to the patient. It is used, primarily in Europe, to treat severe autoimmune diseases including dilative cardiomyopathy and refractory systemic lupus erythematosus (SLE).
Dr. Scheibenbogen’s group has conducted two studies of immunoadsorption in ME/CFS. In one, a 5-day procedure led to rapid symptom improvement in 7 of 10 patients, with sustained improvement in 3 patients after 2 years. Autoantibodies decreased rapidly in 9 of the 10 patients. In a follow-up study of five of the responders 2 years later, retreatment with a modified immunoadsorption protocol led to rapid and sustained improvement in four. Further study has been on hold because of the pandemic.
Next-gen IgG-targeting therapies: Another approach that could offer promise for ME/CFS involves therapies that block the Fc receptors of IgG. Several are in phase 1-3 trials for autoimmune conditions. One candidate drug, the Fc fragment efgartigimod, is currently in phase 3 trials for several conditions, including generalized myasthenia gravis, primary immune thrombocytopenia, and chronic inflammatory demyelinating polyneuropathy. Phase 3 trials are planned for the monoclonal antibody rozanolixizumab in those same conditions.
Newer-generation monoclonal antibodies targeting CD19 or CD20 that show benefit in various autoimmune conditions are another possibility for ME/CFS. These include ocrelizumab (Ocrevus), approved in the United States for treating relapsing and progressive multiple sclerosis and in trials for SLE; obinutuzumab (Gazyva), approved for treating lymphoma and also in development for SLE; and ublituximab, in phase 3 trials for multiple sclerosis.
“Most of them are more effective than rituximab,” Dr. Scheibenbogen noted, adding that “currently the data look quite promising. They are effective in different autoimmune diseases and they are quite well tolerated. There’s great hope now with COVID-19 that we can convince some companies to do such trials in ME/CFS as well.”
Dr. Scheibenbogen’s institution, the Charité Fatigue Center, has a patent for beta2-adrenergic receptor antibodies for diagnosing ME/CFS under her name together with Celltrend. Dr. Komaroff has received personal fees from Serimmune.
Emerging evidence suggests that autoimmunity plays a role in postinfectious myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and that targeting autoantibodies could be a promising treatment approach.
The same may also apply to many cases of “long COVID,” in which many of the symptoms overlap with those of ME/CFS, Carmen Scheibenbogen, MD, professor of clinical immunology and director of the Institute for Medical Immunology, Charité University Medicine, Berlin, said during the annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis.
Several groups, including Dr. Scheibenbogen’s, have reported finding autoantibodies against neurotransmitter receptor antigens in people with ME/CFS. And, in a paper published in the Journal of Clinical Medicine the day that Dr. Scheibenbogen spoke at the meeting, her team reported significant correlations between autoantibodies to vasoregulative G-protein–coupled receptors and symptom severity, autonomic dysfunction, and disability among 116 patients with infection-triggered ME/CFS who were diagnosed using the symptom-based 2003 Canadian consensus criteria.
People with ME/CFS are also more likely to have genetic risk factors associated with autoimmunity and personal and/or family histories of autoimmune conditions. And, clinical trials have demonstrated early success with various immunomodulatory treatments in subsets of people with ME/CFS, including endoxan, rituximab, and immunoadsorption.
“We have evidence that ME/CFS is an autoantibody-mediated disease, and we have evidence that autoantibody targeting is effective in this disease. So far ... we have few and underfinanced clinical studies, but the good news is we have promising emerging treatment options,” Dr. Scheibenbogen said.
Asked to comment, ME/CFS expert Anthony L. Komaroff, MD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said: “There is already strong evidence that there are autoantibodies in ME/CFS. Dr. Scheibenbogen’s work is the latest and employs the latest technology. ... I would bet that autoantibodies to neural targets are likely to cause some of the symptoms of ME/CFS and some of the symptoms of long COVID.”
However, he cautioned, “that has not been proven, and even if it were proven you would have to demonstrate that treatments based on that theory worked.”
Dr. Komaroff said he views autoimmunity as a likely component of the ME/CFS spectrum, but not the only one. “My current view of this illness is that there’s a final common pathway in the brain that leads to the symptoms of the illness. But that final common pathway can be triggered by a variety of different things, one of which could be autoantibodies while another could be infection or inflammation in the brain.”
Emerging evidence points to autoimmunity
Dr. Scheibenbogen summarized the work published in this area over the past few years by her group and others.
In a comparison of ME/CFS patients with 201 healthy controls, significant associations were seen with two specific autoimmunity-related risk alleles only in the ME/CFS patients who reported acute onset of disease with an infection but not in those with ME/CFS without infection-triggered onset or the controls. Both genes play roles in regulating B- and T-cell activation.
Another recent study found associations with ME/CFS and major histocompatibility complex class II molecules, a typical feature of autoimmune diseases, in a comparison between 426 adult Norwegian ME/CFS patients who were diagnosed with the Canadian consensus criteria and 4,511 healthy, ethnically matched controls.
In a 2020 paper, Dr. Scheibenbogen and pharmacologist Klaus Wirth presented a “unifying hypothesis” of ME/CFS pathophysiology based on the finding of elevations in autoantibodies against beta2-adrenergic receptors and muscarinic acetylcholine receptors in some individuals with the condition. Since both of those receptors are important vasodilators, their functional disturbance would be expected to cause vasoconstriction and hypoxemia, which would explain many of the symptoms of ME/CFS. This mechanism would align with other findings of muscular and cerebral hypoperfusion that correlate with fatigue, particularly post exertion, as well as metabolic changes that are in line with the concepts of hypoxemia and ischemia.
Further evidence for vascular dysfunction in ME/CFS came from her group’s study finding evidence of peripheral endothelial dysfunction that was associated with symptom severity in 35 adult patients. “Vasoconstriction, hypovolemia, and release of vasoactive and algesic mediators is probably a key pathomechanism of the disease,” Dr. Scheibenbogen said.
Treatments: Will targeting autoantibodies work?
In the second part of her talk, Dr. Scheibenbogen summarized clinical trials of the following treatment approaches that involve targeting autoantibodies as a way to alleviate ME/CFS symptoms:
Rituximab: Work on infusions of the B-cell depleting agent has been conducted by Norwegian researchers beginning in 2011 with a small randomized trial and an open-label, phase 2 study in 2015, both showing clinical responses in ME/CFS. However, a subsequent phase 3, randomized clinical trial of 151 patients, again diagnosed using the Canadian criteria, was negative.
There are several possible explanations for this, Dr. Scheibenbogen noted. For one, the maintenance dose had to be reduced because of a lack of financial support. “This was probably critical. The lower dose was insufficient to adequately deplete B cells.” Also, there may have been a strong placebo response in the control group since they were being given better care than they normally would receive during the trial. “I think probably nobody will again do a rituximab trial. This was very disappointing for all of us. But, we still have other opportunities to follow this path,” she said.
Dr. Komaroff agreed. “I don’t think the failure of one drug that hits malignant B cells is proof against the autoimmune hypothesis per se. I think the evidence is that rituximab doesn’t work, but that doesn’t invalidate the autoimmunity hypothesis.”
Cyclophosphamide: The same Norwegian group also showed positive findings in an open-label, phase 2 trial of the immune-modifying drug cyclophosphamide in 22 of 40 patients. Interestingly, HLA risk alleles were much more common in responders than nonresponders, Dr. Scheibenbogen noted.
Immunoadsorption: This technique, similar to dialysis, involves separating out the blood plasma by centrifugation and removing IgG autoantibodies by a binding column, then returning the plasma back to the patient. It is used, primarily in Europe, to treat severe autoimmune diseases including dilative cardiomyopathy and refractory systemic lupus erythematosus (SLE).
Dr. Scheibenbogen’s group has conducted two studies of immunoadsorption in ME/CFS. In one, a 5-day procedure led to rapid symptom improvement in 7 of 10 patients, with sustained improvement in 3 patients after 2 years. Autoantibodies decreased rapidly in 9 of the 10 patients. In a follow-up study of five of the responders 2 years later, retreatment with a modified immunoadsorption protocol led to rapid and sustained improvement in four. Further study has been on hold because of the pandemic.
Next-gen IgG-targeting therapies: Another approach that could offer promise for ME/CFS involves therapies that block the Fc receptors of IgG. Several are in phase 1-3 trials for autoimmune conditions. One candidate drug, the Fc fragment efgartigimod, is currently in phase 3 trials for several conditions, including generalized myasthenia gravis, primary immune thrombocytopenia, and chronic inflammatory demyelinating polyneuropathy. Phase 3 trials are planned for the monoclonal antibody rozanolixizumab in those same conditions.
Newer-generation monoclonal antibodies targeting CD19 or CD20 that show benefit in various autoimmune conditions are another possibility for ME/CFS. These include ocrelizumab (Ocrevus), approved in the United States for treating relapsing and progressive multiple sclerosis and in trials for SLE; obinutuzumab (Gazyva), approved for treating lymphoma and also in development for SLE; and ublituximab, in phase 3 trials for multiple sclerosis.
“Most of them are more effective than rituximab,” Dr. Scheibenbogen noted, adding that “currently the data look quite promising. They are effective in different autoimmune diseases and they are quite well tolerated. There’s great hope now with COVID-19 that we can convince some companies to do such trials in ME/CFS as well.”
Dr. Scheibenbogen’s institution, the Charité Fatigue Center, has a patent for beta2-adrenergic receptor antibodies for diagnosing ME/CFS under her name together with Celltrend. Dr. Komaroff has received personal fees from Serimmune.
FROM IACFS/ME 2021
Target lesion
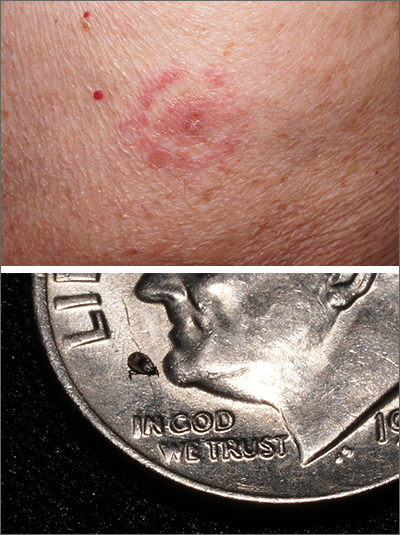
The original diagnosis in this case was correct—Lyme disease (erythema chronicum migrans)—but unfortunately, the treatment was inadequate. Initially, this patient received a single dose of doxycycline 200 mg po, which is the appropriate preventative remedy when a deer tick has been attached for at least 36 hours and treatment can be initiated within 72 hours of tick removal. However, deer ticks, especially nymphs, are very small and their presence can go unnoticed, leading patients to guess (sometimes incorrectly) at the length of time a tick has been attached.
In this case, when the patient and his wife thought about it a bit more, they indicated that the tick may have been attached for several days before they removed it and went to see the PCP. Had the PCP known the tick had been attached longer, she would have advised watchful waiting and observation for signs and symptoms of Lyme disease.
Originally described in cases from Lyme, Connecticut, Lyme disease is now endemic to the northeastern United States from Maine to Virginia, and from the upper midwest to Minnesota. Laboratory diagnosis can be made by a 2-tiered serologic screening and confirmation with an enzyme-linked assay, followed by a western blot for positive or equivocal screening tests.
In 2019, the most recent year of reported Centers for Disease Control and Prevention (CDC) surveillance data, Maine recorded the highest incidence rate of Lyme disease.1 In 70% to 80% of cases, an expanding horizon of infection creates the characteristic targetoid pink patch centered on the site of the tick bite.2
If there is a targetoid rash present in a patient from an endemic area, the CDC recommends empiric therapy with doxycycline 100 mg po bid for 10 to 14 days.3 (Worth noting: Patches may appear rather small in diameter, as was true in this case, or may be many centimeters in size and mimic cellulitis.) Alternative regimens for early localized disease include amoxicillin 500 mg po qid for pregnant patients, and cefuroxime 500 mg bid daily for 14 days for patients who are allergic to doxycycline.
Once treatment has been initiated, it’s important to monitor patients for any worsening symptoms, including fever and weakness, as these may be an indication of treatment failure or co-occurrence of anaplasmosis, ehrlichiosis, or babesiosis. In this case, the patient was prescribed doxycycline 100 mg bid for 14 days without any further signs or symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated April 29, 2021. Accessed August 12, 2021. https://www.cdc.gov/lyme/datasurveillance/maps-recent.html
2. Signs and symptoms of untreated Lyme disease. Centers for Disease Control and Prevention. Updated January 15, 2021. Accessed August 13, 2021. https://www.cdc.gov/lyme/signs_symptoms/index.html
3. Treatment of erythema migrans. Centers for Disease Control and Prevention. Updated November 3, 2020. Accessed August 12, 2021. https://www.cdc.gov/lyme/treatment/index.html

The original diagnosis in this case was correct—Lyme disease (erythema chronicum migrans)—but unfortunately, the treatment was inadequate. Initially, this patient received a single dose of doxycycline 200 mg po, which is the appropriate preventative remedy when a deer tick has been attached for at least 36 hours and treatment can be initiated within 72 hours of tick removal. However, deer ticks, especially nymphs, are very small and their presence can go unnoticed, leading patients to guess (sometimes incorrectly) at the length of time a tick has been attached.
In this case, when the patient and his wife thought about it a bit more, they indicated that the tick may have been attached for several days before they removed it and went to see the PCP. Had the PCP known the tick had been attached longer, she would have advised watchful waiting and observation for signs and symptoms of Lyme disease.
Originally described in cases from Lyme, Connecticut, Lyme disease is now endemic to the northeastern United States from Maine to Virginia, and from the upper midwest to Minnesota. Laboratory diagnosis can be made by a 2-tiered serologic screening and confirmation with an enzyme-linked assay, followed by a western blot for positive or equivocal screening tests.
In 2019, the most recent year of reported Centers for Disease Control and Prevention (CDC) surveillance data, Maine recorded the highest incidence rate of Lyme disease.1 In 70% to 80% of cases, an expanding horizon of infection creates the characteristic targetoid pink patch centered on the site of the tick bite.2
If there is a targetoid rash present in a patient from an endemic area, the CDC recommends empiric therapy with doxycycline 100 mg po bid for 10 to 14 days.3 (Worth noting: Patches may appear rather small in diameter, as was true in this case, or may be many centimeters in size and mimic cellulitis.) Alternative regimens for early localized disease include amoxicillin 500 mg po qid for pregnant patients, and cefuroxime 500 mg bid daily for 14 days for patients who are allergic to doxycycline.
Once treatment has been initiated, it’s important to monitor patients for any worsening symptoms, including fever and weakness, as these may be an indication of treatment failure or co-occurrence of anaplasmosis, ehrlichiosis, or babesiosis. In this case, the patient was prescribed doxycycline 100 mg bid for 14 days without any further signs or symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

The original diagnosis in this case was correct—Lyme disease (erythema chronicum migrans)—but unfortunately, the treatment was inadequate. Initially, this patient received a single dose of doxycycline 200 mg po, which is the appropriate preventative remedy when a deer tick has been attached for at least 36 hours and treatment can be initiated within 72 hours of tick removal. However, deer ticks, especially nymphs, are very small and their presence can go unnoticed, leading patients to guess (sometimes incorrectly) at the length of time a tick has been attached.
In this case, when the patient and his wife thought about it a bit more, they indicated that the tick may have been attached for several days before they removed it and went to see the PCP. Had the PCP known the tick had been attached longer, she would have advised watchful waiting and observation for signs and symptoms of Lyme disease.
Originally described in cases from Lyme, Connecticut, Lyme disease is now endemic to the northeastern United States from Maine to Virginia, and from the upper midwest to Minnesota. Laboratory diagnosis can be made by a 2-tiered serologic screening and confirmation with an enzyme-linked assay, followed by a western blot for positive or equivocal screening tests.
In 2019, the most recent year of reported Centers for Disease Control and Prevention (CDC) surveillance data, Maine recorded the highest incidence rate of Lyme disease.1 In 70% to 80% of cases, an expanding horizon of infection creates the characteristic targetoid pink patch centered on the site of the tick bite.2
If there is a targetoid rash present in a patient from an endemic area, the CDC recommends empiric therapy with doxycycline 100 mg po bid for 10 to 14 days.3 (Worth noting: Patches may appear rather small in diameter, as was true in this case, or may be many centimeters in size and mimic cellulitis.) Alternative regimens for early localized disease include amoxicillin 500 mg po qid for pregnant patients, and cefuroxime 500 mg bid daily for 14 days for patients who are allergic to doxycycline.
Once treatment has been initiated, it’s important to monitor patients for any worsening symptoms, including fever and weakness, as these may be an indication of treatment failure or co-occurrence of anaplasmosis, ehrlichiosis, or babesiosis. In this case, the patient was prescribed doxycycline 100 mg bid for 14 days without any further signs or symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated April 29, 2021. Accessed August 12, 2021. https://www.cdc.gov/lyme/datasurveillance/maps-recent.html
2. Signs and symptoms of untreated Lyme disease. Centers for Disease Control and Prevention. Updated January 15, 2021. Accessed August 13, 2021. https://www.cdc.gov/lyme/signs_symptoms/index.html
3. Treatment of erythema migrans. Centers for Disease Control and Prevention. Updated November 3, 2020. Accessed August 12, 2021. https://www.cdc.gov/lyme/treatment/index.html
1. Lyme disease maps: most recent year. Centers for Disease Control and Prevention. Updated April 29, 2021. Accessed August 12, 2021. https://www.cdc.gov/lyme/datasurveillance/maps-recent.html
2. Signs and symptoms of untreated Lyme disease. Centers for Disease Control and Prevention. Updated January 15, 2021. Accessed August 13, 2021. https://www.cdc.gov/lyme/signs_symptoms/index.html
3. Treatment of erythema migrans. Centers for Disease Control and Prevention. Updated November 3, 2020. Accessed August 12, 2021. https://www.cdc.gov/lyme/treatment/index.html