User login
Peficitinib safe and effective for long-term management of RA
Key clinical point: Peficitinib (ASP015K) showed improvement in clinical outcomes, which was maintained throughout the treatment duration of 32 months along with a generally tolerable safety profile in patients with rheumatoid arthritis (RA).
Major finding: The American College of Rheumatology (ACR)20, ACR50, and ACR70 response rates were maintained throughout from baseline (71.6%, 52.1%, and 34.7%, respectively) to the end of treatment (78.7%, 63.3%, and 44.1%, respectively). Treatment-emergent adverse events, mostly grade 1 or 2 in severity, were experienced by 94.4% of patients, leading to drug discontinuation in 16.6% of patients.
Study details: Findings are from the final analysis of an open-label long-term extension study involving 843 Asian patients with RA who previously completed phase 2b and phase 3 studies of peficitinib.
Disclosures: This work was funded by the Astellas Pharma, Inc. The authors including the lead author reported receiving grants, speaker’s fees, consultancy fees, personal fees, and/or honoraria from various sources including Astellas Pharma, Inc. Four of the authors reported being employees of Astellas Pharma, Inc.
Source: Takeuchi T et al. Rheumatol Ther. 2021 Mar 3. doi: 10.1007/s40744-021-00280-5.
Key clinical point: Peficitinib (ASP015K) showed improvement in clinical outcomes, which was maintained throughout the treatment duration of 32 months along with a generally tolerable safety profile in patients with rheumatoid arthritis (RA).
Major finding: The American College of Rheumatology (ACR)20, ACR50, and ACR70 response rates were maintained throughout from baseline (71.6%, 52.1%, and 34.7%, respectively) to the end of treatment (78.7%, 63.3%, and 44.1%, respectively). Treatment-emergent adverse events, mostly grade 1 or 2 in severity, were experienced by 94.4% of patients, leading to drug discontinuation in 16.6% of patients.
Study details: Findings are from the final analysis of an open-label long-term extension study involving 843 Asian patients with RA who previously completed phase 2b and phase 3 studies of peficitinib.
Disclosures: This work was funded by the Astellas Pharma, Inc. The authors including the lead author reported receiving grants, speaker’s fees, consultancy fees, personal fees, and/or honoraria from various sources including Astellas Pharma, Inc. Four of the authors reported being employees of Astellas Pharma, Inc.
Source: Takeuchi T et al. Rheumatol Ther. 2021 Mar 3. doi: 10.1007/s40744-021-00280-5.
Key clinical point: Peficitinib (ASP015K) showed improvement in clinical outcomes, which was maintained throughout the treatment duration of 32 months along with a generally tolerable safety profile in patients with rheumatoid arthritis (RA).
Major finding: The American College of Rheumatology (ACR)20, ACR50, and ACR70 response rates were maintained throughout from baseline (71.6%, 52.1%, and 34.7%, respectively) to the end of treatment (78.7%, 63.3%, and 44.1%, respectively). Treatment-emergent adverse events, mostly grade 1 or 2 in severity, were experienced by 94.4% of patients, leading to drug discontinuation in 16.6% of patients.
Study details: Findings are from the final analysis of an open-label long-term extension study involving 843 Asian patients with RA who previously completed phase 2b and phase 3 studies of peficitinib.
Disclosures: This work was funded by the Astellas Pharma, Inc. The authors including the lead author reported receiving grants, speaker’s fees, consultancy fees, personal fees, and/or honoraria from various sources including Astellas Pharma, Inc. Four of the authors reported being employees of Astellas Pharma, Inc.
Source: Takeuchi T et al. Rheumatol Ther. 2021 Mar 3. doi: 10.1007/s40744-021-00280-5.
Sustained remission more likely with biological vs. triple therapy after inadequate response to MTX
Key clinical point: Patients with early rheumatoid arthritis (RA) who initiated biological vs. triple therapy after inadequate response to methotrexate (MTX) were more likely to achieve sustained remission (SR).
Major finding: Patients initiating biological vs. triple therapy were more likely to achieve short-term and long-term SR at 1 year (adjusted odds ratio [aOR], 1.79; 95% confidence interval [CI], 1.18-2.71 and aOR, 1.86; 95% CI, 1.00-3.48, respectively) and 2 years (aOR, 1.92; 95% CI, 1.21-3.06 and aOR, 1.62; 95% CI, 0.94-2.79, respectively) from treatment initiation.
Study details: Findings are from an analysis of 1,502 patients with relatively early RA who initiated biological (biological disease-modifying antirheumatic drugs+MTX; n=1,155) or triple (MTX+sulfasalazine+hydroxychloroquine/chloroquine; n=347) therapy as the first treatment strategy after inadequate response to MTX monotherapy.
Disclosures: This work funded by the Swedish Rheumatism Association, the Medical Faculty of Lund University, Alfred Österlund ́s Foundation, Greta and Johan Kock ́s foundation, the King Gustaf V Foundation, Lund University Hospital, Professor Nanna Svartz Foundation, and Anna-Greta Crafoord Foundation. The authors declared no conflicts of interest.
Source: Källmark H et al. Arthritis Rheumatol. 2021 Mar 7. doi: 10.1002/art.41720.
Key clinical point: Patients with early rheumatoid arthritis (RA) who initiated biological vs. triple therapy after inadequate response to methotrexate (MTX) were more likely to achieve sustained remission (SR).
Major finding: Patients initiating biological vs. triple therapy were more likely to achieve short-term and long-term SR at 1 year (adjusted odds ratio [aOR], 1.79; 95% confidence interval [CI], 1.18-2.71 and aOR, 1.86; 95% CI, 1.00-3.48, respectively) and 2 years (aOR, 1.92; 95% CI, 1.21-3.06 and aOR, 1.62; 95% CI, 0.94-2.79, respectively) from treatment initiation.
Study details: Findings are from an analysis of 1,502 patients with relatively early RA who initiated biological (biological disease-modifying antirheumatic drugs+MTX; n=1,155) or triple (MTX+sulfasalazine+hydroxychloroquine/chloroquine; n=347) therapy as the first treatment strategy after inadequate response to MTX monotherapy.
Disclosures: This work funded by the Swedish Rheumatism Association, the Medical Faculty of Lund University, Alfred Österlund ́s Foundation, Greta and Johan Kock ́s foundation, the King Gustaf V Foundation, Lund University Hospital, Professor Nanna Svartz Foundation, and Anna-Greta Crafoord Foundation. The authors declared no conflicts of interest.
Source: Källmark H et al. Arthritis Rheumatol. 2021 Mar 7. doi: 10.1002/art.41720.
Key clinical point: Patients with early rheumatoid arthritis (RA) who initiated biological vs. triple therapy after inadequate response to methotrexate (MTX) were more likely to achieve sustained remission (SR).
Major finding: Patients initiating biological vs. triple therapy were more likely to achieve short-term and long-term SR at 1 year (adjusted odds ratio [aOR], 1.79; 95% confidence interval [CI], 1.18-2.71 and aOR, 1.86; 95% CI, 1.00-3.48, respectively) and 2 years (aOR, 1.92; 95% CI, 1.21-3.06 and aOR, 1.62; 95% CI, 0.94-2.79, respectively) from treatment initiation.
Study details: Findings are from an analysis of 1,502 patients with relatively early RA who initiated biological (biological disease-modifying antirheumatic drugs+MTX; n=1,155) or triple (MTX+sulfasalazine+hydroxychloroquine/chloroquine; n=347) therapy as the first treatment strategy after inadequate response to MTX monotherapy.
Disclosures: This work funded by the Swedish Rheumatism Association, the Medical Faculty of Lund University, Alfred Österlund ́s Foundation, Greta and Johan Kock ́s foundation, the King Gustaf V Foundation, Lund University Hospital, Professor Nanna Svartz Foundation, and Anna-Greta Crafoord Foundation. The authors declared no conflicts of interest.
Source: Källmark H et al. Arthritis Rheumatol. 2021 Mar 7. doi: 10.1002/art.41720.
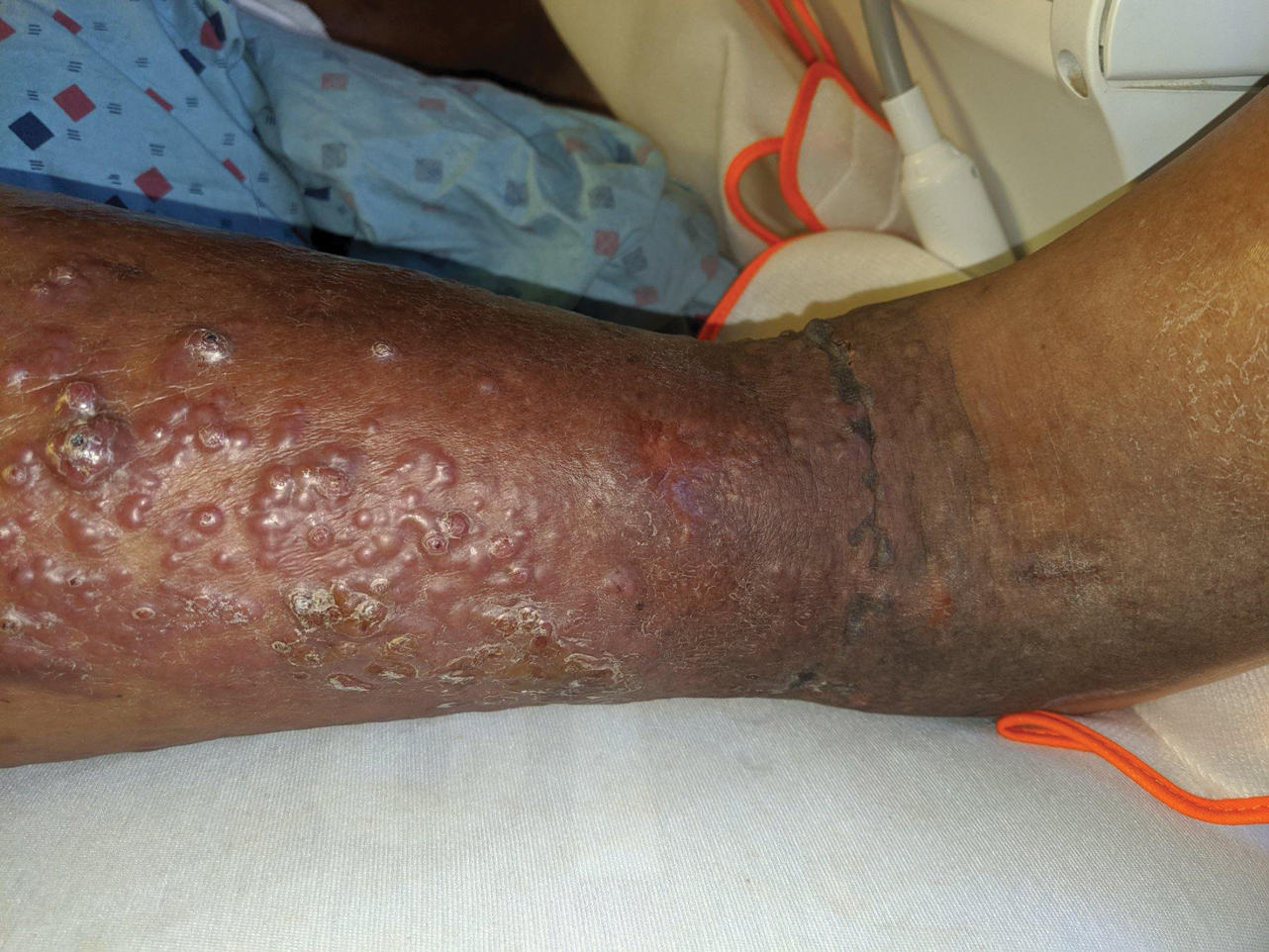
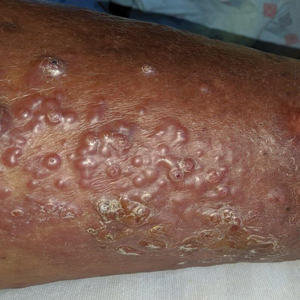
Vesicles and Bullae on the Leg
The Diagnosis: Cutaneous B-cell Lymphoma
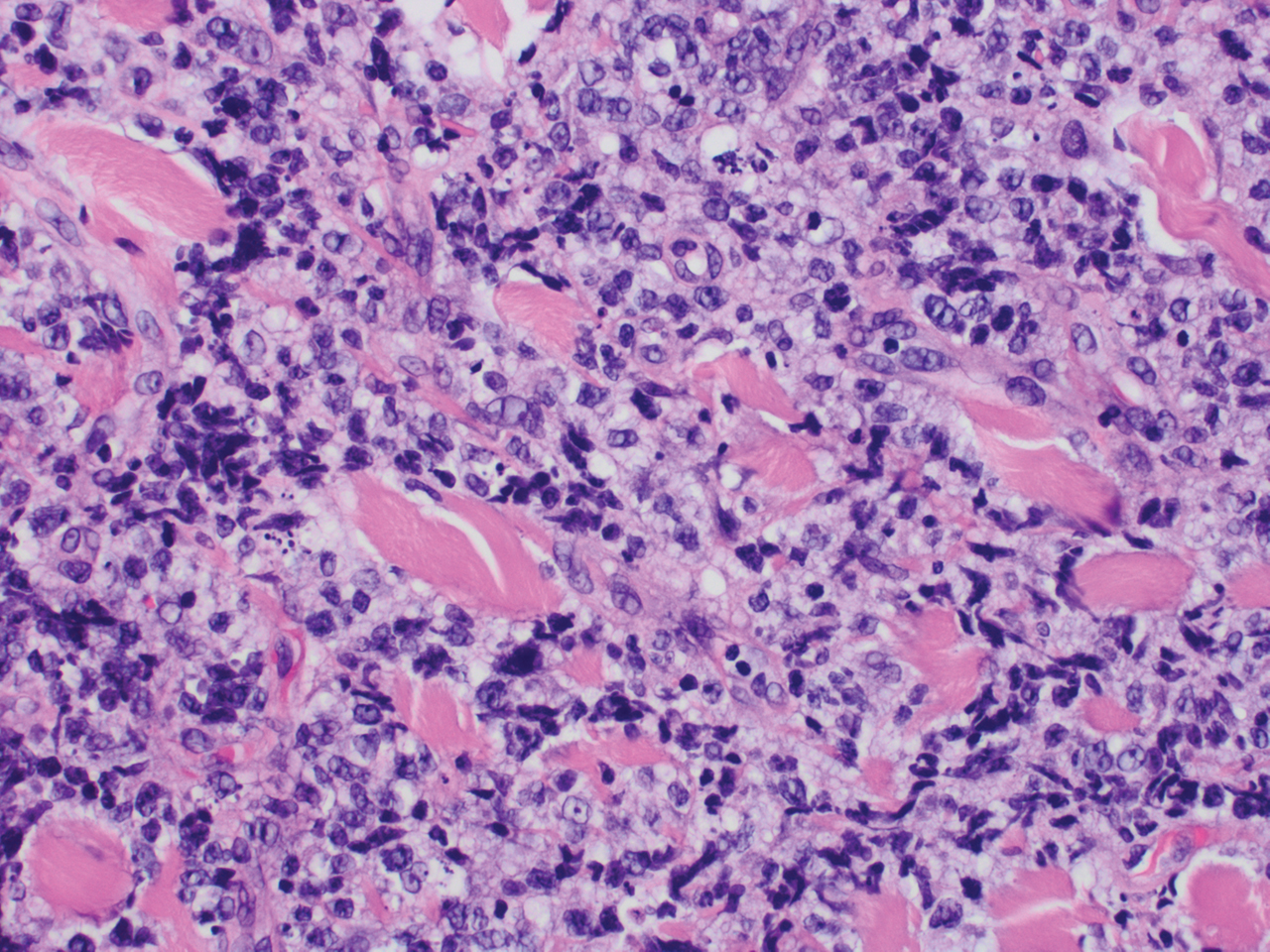
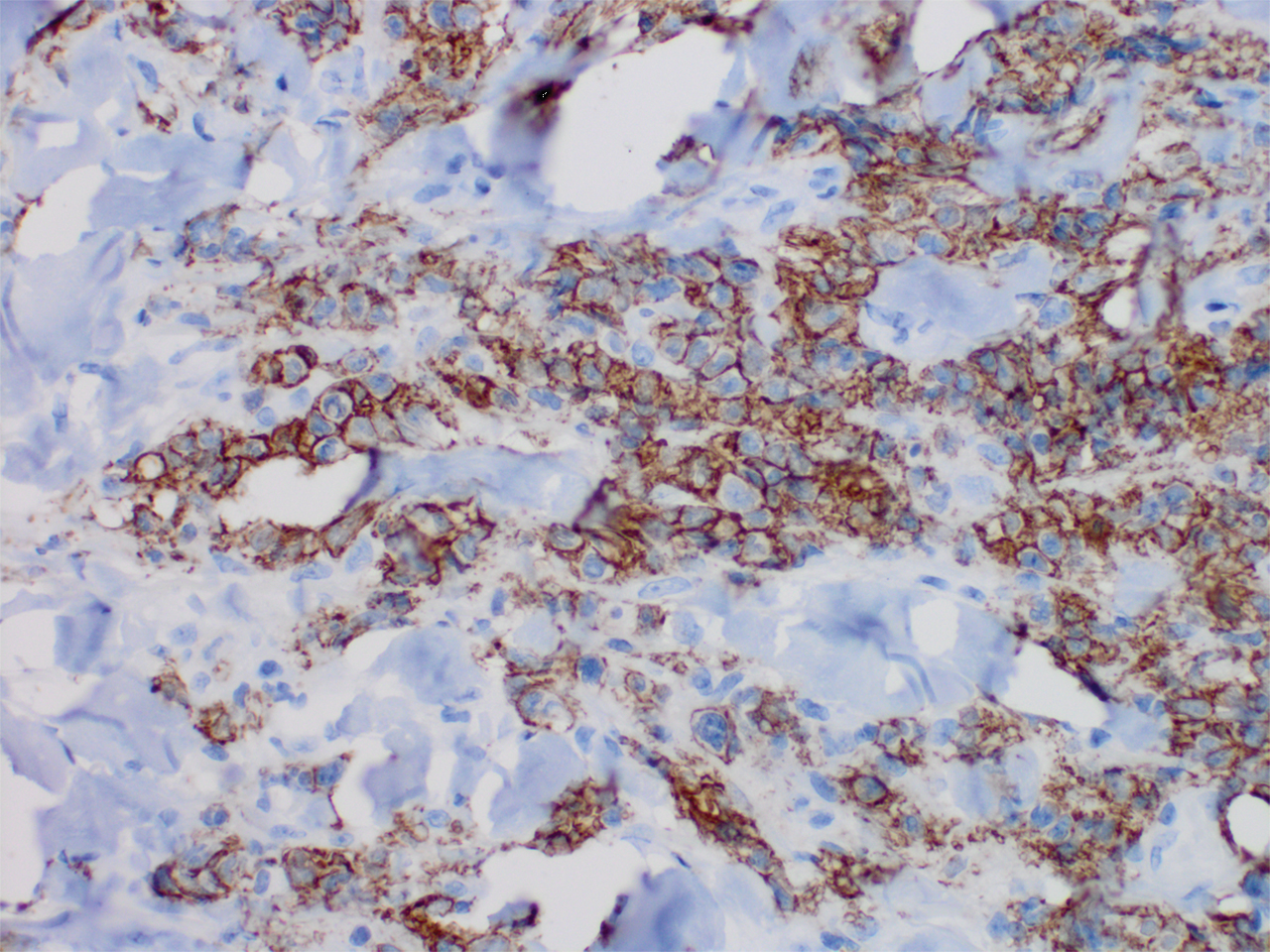
Histopathology revealed a dense and diffuse lymphocytic infiltrate throughout the dermis with occasional individual cell necrosis. On closer inspection, the infiltrate consisted of intermediate-sized lymphocytes, some with a vesiculated nucleus and ample amount of cytoplasm, while others contained hyperchromatic nuclei (Figure 1). These cells stained strongly positive for B-cell marker (CD20), while only a few mature lymphocytes demonstrated T-cell phenotype (CD3)(Figure 2).
Although the patient recounted a 3-month history of lower leg edema, he also reported that the rash began a few weeks after his diagnosis of systemic B-cell follicular lymphoma. Throughout this time, he was seen by various physicians who attributed the edema and skin changes to chronic stasis, peripheral venous insufficiency, and diabetic peripheral neuropathy. His primary care physician prescribed an antifungal lotion, which he discontinued on his own due to lack of improvement. Upon arrival to the emergency department, he was started on intravenous cefazolin and subcutaneous heparin. Doppler ultrasonography of the legs was ordered to rule out a deep venous thrombosis. Dermatology was consulted and proceeded with a punch biopsy to investigate for cutaneous B-cell lymphoma (BCL) with a plan to follow up as an outpatient for results upon discharge. He also was prescribed triamcinolone ointment 0.1% twice daily for symptomatic relief.
The patient's left axillary lymph node was biopsied for pathologic evaluation. Immunohistochemical staining revealed expression of B-cell markers CD20, CD79a, and PAX5, along with the antiapoptotic markers BCL-2 and BCL-6. Fluorescence in situ hybridization displayed gene rearrangements of BCL-2, BCL-6, and t(14;18)/IgH-BCL2 in the majority of cells. CD3 and CD5 immunostains were negative, indicating that T cells were not involved in this process. Flow cytometry identified a monoclonal κ B-cell population in 40% to 50% of the total cells, which co-expressed CD10, CD19, CD22, and CD38; the cells were negative for CD5, CD20, and CD23. Cell size was variably enlarged and CD71 positive, otherwise known as transferrin receptor 1, indicating the mediation of iron transport into cells of erythroid lineage that is necessary for proliferation.1 Bone marrow core biopsy did not identify features of bone marrow involvement by the lymphoma. Based on these results, the patient was diagnosed with systemic B-cell follicular lymphoma grade 3b stage IIIA. Oncology initiated a systemic chemotherapy regimen with obinutuzumab, cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate, and prednisone.
Skin involvement in B-cell follicular lymphoma can be primary or secondary. Although all subtypes of BCL can have secondary cutaneous involvement, it is most common in advanced-stage disease (stages III or IV).2 Cutaneous manifestations of primary cutaneous follicle-center lymphoma (PCFCL) and systemic/nodal follicular lymphoma secondarily involving the skin can be difficult to distinguish clinically and histopathologically; both appear as solitary or grouped plaques and nodules most commonly on the head, neck, or trunk, and rarely on the legs.3 Although the pathologic features of these two diagnoses can seem almost identical, it is important to differentiate them due to their differing prognosis and management. Patients with follicular lymphoma involving the skin are more likely than those with PCFCL to develop lymphadenopathy and B symptoms.3 Primary cutaneous follicle-center lymphoma also generally runs an indolent course and requires local therapy, while secondary involvement of the skin due to systemic/nodal follicular lymphoma has a worse prognosis and requires systemic chemotherapy treatment.4
Immunohistochemical markers are the most helpful tool used to distinguish PCFCL from systemic/nodal follicular lymphoma involving the skin. Tumors of B-cell origin are expected to express associated B-cell markers such as CD20, CD79a, and PAX52; BCL-6, a marker of germinal center cells, also is expected to stain positive.2 CD10 is positive in a majority of cases with a follicular growth pattern, while those with a diffuse pattern of growth may have a negative stain.2 The most valuable histopathologic indicator differentiating primary and secondary skin involvement is the intensity of BCL-2 expression.5 The prognostic significance of the t(14;18)/IgH-BCL2 rearrangement is controversial, with rearrangement identified in more than 75% of systemic/nodal follicular lymphoma cases and less commonly found in PCFCL, with one report arguing an incidence ranging from 1% to 40%.5
A comprehensive history and physical examination are necessary to develop a differential diagnosis. Our patient's lower leg edema and extensive medical history made the diagnosis more complicated. Pitting edema was present on physical examination, making elephantiasis nostras verrucosa less likely, as it would instead present with nonpitting edema and a woody feel.6 Our patient did not have epidemiologic exposure to filariasis through foreign travel and did not present with any classic signs or symptoms of lymphatic filariasis, such as fever, eosinophilia, chyluria, or hydrocele.7 Although a negative history of HIV makes Kaposi sarcoma and bacillary angiomatosis less likely diagnoses, a biopsy would be useful to rule out these conditions. Positive inguinal lymphadenopathy present on physical examination may have contributed to lymphatic flow obstruction leading to the leg lymphedema in our patient.
- Marsee DK, Pinkus GS, Yu H. CD71 (transferrin receptor): an effective marker for erythroid precursors in bone marrow biopsy specimens. Am J Clin Pathol. 2010;134:429-435.
- Jaffe ES. Navigating the cutaneous B-cell lymphomas: avoiding the rocky shoals. Mod Pathol. 2020;33(suppl 1):96-106.
- Skala SL, Hristov B, Hristov AC. Primary cutaneous follicle center lymphoma. Arch Pathol Lab Med. 2018;142:1313-1321.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Servitje O, Climent F, Colomo L, et al. Primary cutaneous vs secondary cutaneous follicular lymphomas: a comparative study focused on BCL2, CD10, and t(14;18) expression. J Cutan Pathol. 2018;46:182-189.
- Fredman R, Tenenhaus M. Elephantiasis nostras verrucose [published online October 12, 2012]. Eplasty. 2012;12:ic14.
- Lourens GB, Ferrell DK. Lymphatic filariasis. Nurs Clin of North Am. 2019;54:181-192.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology revealed a dense and diffuse lymphocytic infiltrate throughout the dermis with occasional individual cell necrosis. On closer inspection, the infiltrate consisted of intermediate-sized lymphocytes, some with a vesiculated nucleus and ample amount of cytoplasm, while others contained hyperchromatic nuclei (Figure 1). These cells stained strongly positive for B-cell marker (CD20), while only a few mature lymphocytes demonstrated T-cell phenotype (CD3)(Figure 2).

Although the patient recounted a 3-month history of lower leg edema, he also reported that the rash began a few weeks after his diagnosis of systemic B-cell follicular lymphoma. Throughout this time, he was seen by various physicians who attributed the edema and skin changes to chronic stasis, peripheral venous insufficiency, and diabetic peripheral neuropathy. His primary care physician prescribed an antifungal lotion, which he discontinued on his own due to lack of improvement. Upon arrival to the emergency department, he was started on intravenous cefazolin and subcutaneous heparin. Doppler ultrasonography of the legs was ordered to rule out a deep venous thrombosis. Dermatology was consulted and proceeded with a punch biopsy to investigate for cutaneous B-cell lymphoma (BCL) with a plan to follow up as an outpatient for results upon discharge. He also was prescribed triamcinolone ointment 0.1% twice daily for symptomatic relief.
The patient's left axillary lymph node was biopsied for pathologic evaluation. Immunohistochemical staining revealed expression of B-cell markers CD20, CD79a, and PAX5, along with the antiapoptotic markers BCL-2 and BCL-6. Fluorescence in situ hybridization displayed gene rearrangements of BCL-2, BCL-6, and t(14;18)/IgH-BCL2 in the majority of cells. CD3 and CD5 immunostains were negative, indicating that T cells were not involved in this process. Flow cytometry identified a monoclonal κ B-cell population in 40% to 50% of the total cells, which co-expressed CD10, CD19, CD22, and CD38; the cells were negative for CD5, CD20, and CD23. Cell size was variably enlarged and CD71 positive, otherwise known as transferrin receptor 1, indicating the mediation of iron transport into cells of erythroid lineage that is necessary for proliferation.1 Bone marrow core biopsy did not identify features of bone marrow involvement by the lymphoma. Based on these results, the patient was diagnosed with systemic B-cell follicular lymphoma grade 3b stage IIIA. Oncology initiated a systemic chemotherapy regimen with obinutuzumab, cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate, and prednisone.
Skin involvement in B-cell follicular lymphoma can be primary or secondary. Although all subtypes of BCL can have secondary cutaneous involvement, it is most common in advanced-stage disease (stages III or IV).2 Cutaneous manifestations of primary cutaneous follicle-center lymphoma (PCFCL) and systemic/nodal follicular lymphoma secondarily involving the skin can be difficult to distinguish clinically and histopathologically; both appear as solitary or grouped plaques and nodules most commonly on the head, neck, or trunk, and rarely on the legs.3 Although the pathologic features of these two diagnoses can seem almost identical, it is important to differentiate them due to their differing prognosis and management. Patients with follicular lymphoma involving the skin are more likely than those with PCFCL to develop lymphadenopathy and B symptoms.3 Primary cutaneous follicle-center lymphoma also generally runs an indolent course and requires local therapy, while secondary involvement of the skin due to systemic/nodal follicular lymphoma has a worse prognosis and requires systemic chemotherapy treatment.4
Immunohistochemical markers are the most helpful tool used to distinguish PCFCL from systemic/nodal follicular lymphoma involving the skin. Tumors of B-cell origin are expected to express associated B-cell markers such as CD20, CD79a, and PAX52; BCL-6, a marker of germinal center cells, also is expected to stain positive.2 CD10 is positive in a majority of cases with a follicular growth pattern, while those with a diffuse pattern of growth may have a negative stain.2 The most valuable histopathologic indicator differentiating primary and secondary skin involvement is the intensity of BCL-2 expression.5 The prognostic significance of the t(14;18)/IgH-BCL2 rearrangement is controversial, with rearrangement identified in more than 75% of systemic/nodal follicular lymphoma cases and less commonly found in PCFCL, with one report arguing an incidence ranging from 1% to 40%.5
A comprehensive history and physical examination are necessary to develop a differential diagnosis. Our patient's lower leg edema and extensive medical history made the diagnosis more complicated. Pitting edema was present on physical examination, making elephantiasis nostras verrucosa less likely, as it would instead present with nonpitting edema and a woody feel.6 Our patient did not have epidemiologic exposure to filariasis through foreign travel and did not present with any classic signs or symptoms of lymphatic filariasis, such as fever, eosinophilia, chyluria, or hydrocele.7 Although a negative history of HIV makes Kaposi sarcoma and bacillary angiomatosis less likely diagnoses, a biopsy would be useful to rule out these conditions. Positive inguinal lymphadenopathy present on physical examination may have contributed to lymphatic flow obstruction leading to the leg lymphedema in our patient.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology revealed a dense and diffuse lymphocytic infiltrate throughout the dermis with occasional individual cell necrosis. On closer inspection, the infiltrate consisted of intermediate-sized lymphocytes, some with a vesiculated nucleus and ample amount of cytoplasm, while others contained hyperchromatic nuclei (Figure 1). These cells stained strongly positive for B-cell marker (CD20), while only a few mature lymphocytes demonstrated T-cell phenotype (CD3)(Figure 2).

Although the patient recounted a 3-month history of lower leg edema, he also reported that the rash began a few weeks after his diagnosis of systemic B-cell follicular lymphoma. Throughout this time, he was seen by various physicians who attributed the edema and skin changes to chronic stasis, peripheral venous insufficiency, and diabetic peripheral neuropathy. His primary care physician prescribed an antifungal lotion, which he discontinued on his own due to lack of improvement. Upon arrival to the emergency department, he was started on intravenous cefazolin and subcutaneous heparin. Doppler ultrasonography of the legs was ordered to rule out a deep venous thrombosis. Dermatology was consulted and proceeded with a punch biopsy to investigate for cutaneous B-cell lymphoma (BCL) with a plan to follow up as an outpatient for results upon discharge. He also was prescribed triamcinolone ointment 0.1% twice daily for symptomatic relief.
The patient's left axillary lymph node was biopsied for pathologic evaluation. Immunohistochemical staining revealed expression of B-cell markers CD20, CD79a, and PAX5, along with the antiapoptotic markers BCL-2 and BCL-6. Fluorescence in situ hybridization displayed gene rearrangements of BCL-2, BCL-6, and t(14;18)/IgH-BCL2 in the majority of cells. CD3 and CD5 immunostains were negative, indicating that T cells were not involved in this process. Flow cytometry identified a monoclonal κ B-cell population in 40% to 50% of the total cells, which co-expressed CD10, CD19, CD22, and CD38; the cells were negative for CD5, CD20, and CD23. Cell size was variably enlarged and CD71 positive, otherwise known as transferrin receptor 1, indicating the mediation of iron transport into cells of erythroid lineage that is necessary for proliferation.1 Bone marrow core biopsy did not identify features of bone marrow involvement by the lymphoma. Based on these results, the patient was diagnosed with systemic B-cell follicular lymphoma grade 3b stage IIIA. Oncology initiated a systemic chemotherapy regimen with obinutuzumab, cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate, and prednisone.
Skin involvement in B-cell follicular lymphoma can be primary or secondary. Although all subtypes of BCL can have secondary cutaneous involvement, it is most common in advanced-stage disease (stages III or IV).2 Cutaneous manifestations of primary cutaneous follicle-center lymphoma (PCFCL) and systemic/nodal follicular lymphoma secondarily involving the skin can be difficult to distinguish clinically and histopathologically; both appear as solitary or grouped plaques and nodules most commonly on the head, neck, or trunk, and rarely on the legs.3 Although the pathologic features of these two diagnoses can seem almost identical, it is important to differentiate them due to their differing prognosis and management. Patients with follicular lymphoma involving the skin are more likely than those with PCFCL to develop lymphadenopathy and B symptoms.3 Primary cutaneous follicle-center lymphoma also generally runs an indolent course and requires local therapy, while secondary involvement of the skin due to systemic/nodal follicular lymphoma has a worse prognosis and requires systemic chemotherapy treatment.4
Immunohistochemical markers are the most helpful tool used to distinguish PCFCL from systemic/nodal follicular lymphoma involving the skin. Tumors of B-cell origin are expected to express associated B-cell markers such as CD20, CD79a, and PAX52; BCL-6, a marker of germinal center cells, also is expected to stain positive.2 CD10 is positive in a majority of cases with a follicular growth pattern, while those with a diffuse pattern of growth may have a negative stain.2 The most valuable histopathologic indicator differentiating primary and secondary skin involvement is the intensity of BCL-2 expression.5 The prognostic significance of the t(14;18)/IgH-BCL2 rearrangement is controversial, with rearrangement identified in more than 75% of systemic/nodal follicular lymphoma cases and less commonly found in PCFCL, with one report arguing an incidence ranging from 1% to 40%.5
A comprehensive history and physical examination are necessary to develop a differential diagnosis. Our patient's lower leg edema and extensive medical history made the diagnosis more complicated. Pitting edema was present on physical examination, making elephantiasis nostras verrucosa less likely, as it would instead present with nonpitting edema and a woody feel.6 Our patient did not have epidemiologic exposure to filariasis through foreign travel and did not present with any classic signs or symptoms of lymphatic filariasis, such as fever, eosinophilia, chyluria, or hydrocele.7 Although a negative history of HIV makes Kaposi sarcoma and bacillary angiomatosis less likely diagnoses, a biopsy would be useful to rule out these conditions. Positive inguinal lymphadenopathy present on physical examination may have contributed to lymphatic flow obstruction leading to the leg lymphedema in our patient.
- Marsee DK, Pinkus GS, Yu H. CD71 (transferrin receptor): an effective marker for erythroid precursors in bone marrow biopsy specimens. Am J Clin Pathol. 2010;134:429-435.
- Jaffe ES. Navigating the cutaneous B-cell lymphomas: avoiding the rocky shoals. Mod Pathol. 2020;33(suppl 1):96-106.
- Skala SL, Hristov B, Hristov AC. Primary cutaneous follicle center lymphoma. Arch Pathol Lab Med. 2018;142:1313-1321.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Servitje O, Climent F, Colomo L, et al. Primary cutaneous vs secondary cutaneous follicular lymphomas: a comparative study focused on BCL2, CD10, and t(14;18) expression. J Cutan Pathol. 2018;46:182-189.
- Fredman R, Tenenhaus M. Elephantiasis nostras verrucose [published online October 12, 2012]. Eplasty. 2012;12:ic14.
- Lourens GB, Ferrell DK. Lymphatic filariasis. Nurs Clin of North Am. 2019;54:181-192.
- Marsee DK, Pinkus GS, Yu H. CD71 (transferrin receptor): an effective marker for erythroid precursors in bone marrow biopsy specimens. Am J Clin Pathol. 2010;134:429-435.
- Jaffe ES. Navigating the cutaneous B-cell lymphomas: avoiding the rocky shoals. Mod Pathol. 2020;33(suppl 1):96-106.
- Skala SL, Hristov B, Hristov AC. Primary cutaneous follicle center lymphoma. Arch Pathol Lab Med. 2018;142:1313-1321.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Servitje O, Climent F, Colomo L, et al. Primary cutaneous vs secondary cutaneous follicular lymphomas: a comparative study focused on BCL2, CD10, and t(14;18) expression. J Cutan Pathol. 2018;46:182-189.
- Fredman R, Tenenhaus M. Elephantiasis nostras verrucose [published online October 12, 2012]. Eplasty. 2012;12:ic14.
- Lourens GB, Ferrell DK. Lymphatic filariasis. Nurs Clin of North Am. 2019;54:181-192.
A 60-year-old man presented to the emergency department with slowly progressing edema of the lower legs of 3 months’ duration. In the week prior to presentation to the emergency department, he noticed a sudden eruption of vesicles and bullae on the right leg that drained clear fluid and healed with brown crust. The lesions were associated with mild burning, pruritus, and pain. He denied fever, chills, recent travel, or injury. His medical history was notable for poorly controlled diabetes mellitus, congestive heart failure, hypertension, chronic kidney disease, hyperlipidemia, and chronic anemia. Physical examination revealed multiple scattered erythematous vesicles and bullae on the right leg on a background of hyperpigmentation. Bilateral 2+ pitting edema of the legs also was present. A punch biopsy of a lesion was performed.
Bimekizumab superior to adalimumab in head-to-head psoriasis study
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Clinical Edge Commentary: CML April 2021
ENESTfreedom is an important study that set the current guidelines for TFR. The trial included 190 patients treated with front line nilotinib at 300mg twice daily for two years that had achieved an MR4.5, followed by one year of consolidation maintaining deep molecular response of at least MR4 were allowed to discontinued therapy. A recent 5 year update of the trial showed that 81/190 patients (42.6%) still remained on TFR (MR4) with 76 (40.0%) in MR4.5. From the patients who lost major molecular response (MMR) and were re-treated, 90/91 patients entering this phase (98.9%) regained MMR and 84/91 patients (92.3%) regained MR4.5. More important, no disease progression or CML-related deaths were observed, and the adverse event profile was consistent with that reported previously, including a declined incidence of AEs at 96 weeks of the TFR follow up (with low incidence CV AEs), while an expected increase on those in the treatment re-initiation phase. As previously has been described in other trials, Low Sokal risk score, BCR-ABL1 IS levels at 48 weeks of TFR and stable MR4.5 response for the first year of TFR were associated with higher TFR rates.
When looking at patients who can be candidates for TFR, it is important to take in consideration the likelihood of successful discontinuation and educate patients on certain factors that may contribute to good outcomes. The Australian CML group had previously reported the importance of the initial decline rate of BCR-ABL1 measured as halving time as an important factor associated to deep molecular response. The same group, in a very interesting publication, reported the impact of halving time as a strong predictor of sustained TFR post-tyrosine kinase inhibitor (TKI) cessation in CML patients treated with front line TKI therapies.
Out of 115 patients who attempted TFR and had ≥12 months of follow-up, 55% sustained TFR, defined as remaining in major molecular response off TKI therapy for 12 months, similar percentage seen in other studies. However, when the time taken for the BCR-ABL1 value to halve was applied, it became the strongest independent predictor of sustained TFR with 80% in patients with a halving time of <9.35 days (first quartile) compared with only 4% if the halving time was >21.85 days (last quartile) (P < .001). The e14a2 BCR-ABL1 transcript type and duration of TKI exposure before attempting TFR were also independent predictors of sustained TFR. However, the BCR-ABL1 value measured at 3 months of TKI was not an independent predictor of sustained TFR.
ENESTfreedom is an important study that set the current guidelines for TFR. The trial included 190 patients treated with front line nilotinib at 300mg twice daily for two years that had achieved an MR4.5, followed by one year of consolidation maintaining deep molecular response of at least MR4 were allowed to discontinued therapy. A recent 5 year update of the trial showed that 81/190 patients (42.6%) still remained on TFR (MR4) with 76 (40.0%) in MR4.5. From the patients who lost major molecular response (MMR) and were re-treated, 90/91 patients entering this phase (98.9%) regained MMR and 84/91 patients (92.3%) regained MR4.5. More important, no disease progression or CML-related deaths were observed, and the adverse event profile was consistent with that reported previously, including a declined incidence of AEs at 96 weeks of the TFR follow up (with low incidence CV AEs), while an expected increase on those in the treatment re-initiation phase. As previously has been described in other trials, Low Sokal risk score, BCR-ABL1 IS levels at 48 weeks of TFR and stable MR4.5 response for the first year of TFR were associated with higher TFR rates.
When looking at patients who can be candidates for TFR, it is important to take in consideration the likelihood of successful discontinuation and educate patients on certain factors that may contribute to good outcomes. The Australian CML group had previously reported the importance of the initial decline rate of BCR-ABL1 measured as halving time as an important factor associated to deep molecular response. The same group, in a very interesting publication, reported the impact of halving time as a strong predictor of sustained TFR post-tyrosine kinase inhibitor (TKI) cessation in CML patients treated with front line TKI therapies.
Out of 115 patients who attempted TFR and had ≥12 months of follow-up, 55% sustained TFR, defined as remaining in major molecular response off TKI therapy for 12 months, similar percentage seen in other studies. However, when the time taken for the BCR-ABL1 value to halve was applied, it became the strongest independent predictor of sustained TFR with 80% in patients with a halving time of <9.35 days (first quartile) compared with only 4% if the halving time was >21.85 days (last quartile) (P < .001). The e14a2 BCR-ABL1 transcript type and duration of TKI exposure before attempting TFR were also independent predictors of sustained TFR. However, the BCR-ABL1 value measured at 3 months of TKI was not an independent predictor of sustained TFR.
ENESTfreedom is an important study that set the current guidelines for TFR. The trial included 190 patients treated with front line nilotinib at 300mg twice daily for two years that had achieved an MR4.5, followed by one year of consolidation maintaining deep molecular response of at least MR4 were allowed to discontinued therapy. A recent 5 year update of the trial showed that 81/190 patients (42.6%) still remained on TFR (MR4) with 76 (40.0%) in MR4.5. From the patients who lost major molecular response (MMR) and were re-treated, 90/91 patients entering this phase (98.9%) regained MMR and 84/91 patients (92.3%) regained MR4.5. More important, no disease progression or CML-related deaths were observed, and the adverse event profile was consistent with that reported previously, including a declined incidence of AEs at 96 weeks of the TFR follow up (with low incidence CV AEs), while an expected increase on those in the treatment re-initiation phase. As previously has been described in other trials, Low Sokal risk score, BCR-ABL1 IS levels at 48 weeks of TFR and stable MR4.5 response for the first year of TFR were associated with higher TFR rates.
When looking at patients who can be candidates for TFR, it is important to take in consideration the likelihood of successful discontinuation and educate patients on certain factors that may contribute to good outcomes. The Australian CML group had previously reported the importance of the initial decline rate of BCR-ABL1 measured as halving time as an important factor associated to deep molecular response. The same group, in a very interesting publication, reported the impact of halving time as a strong predictor of sustained TFR post-tyrosine kinase inhibitor (TKI) cessation in CML patients treated with front line TKI therapies.
Out of 115 patients who attempted TFR and had ≥12 months of follow-up, 55% sustained TFR, defined as remaining in major molecular response off TKI therapy for 12 months, similar percentage seen in other studies. However, when the time taken for the BCR-ABL1 value to halve was applied, it became the strongest independent predictor of sustained TFR with 80% in patients with a halving time of <9.35 days (first quartile) compared with only 4% if the halving time was >21.85 days (last quartile) (P < .001). The e14a2 BCR-ABL1 transcript type and duration of TKI exposure before attempting TFR were also independent predictors of sustained TFR. However, the BCR-ABL1 value measured at 3 months of TKI was not an independent predictor of sustained TFR.
Bosutinib is effective, relative safe in elderly CML patients resistant/intolerant to prior TKIs
Key clinical point: Bosutinib was effective and safe as a second or subsequent line of treatment in a real-life cohort of elderly patients with chronic-phase chronic myeloid leukemia (CML-CP) and multiple baseline comorbidities who were resistant or intolerant to previous tyrosine kinase inhibitors (TKIs).
Major finding: Overall, rates of cytogenic response, molecular response, 3-year event-free survival, and 3-year overall survival were 81.2%, 66.6%, 60.9%, and 86.4%, respectively. Grade 3/4 hematological and extra-hematological toxicities were reported in 6.9% and 18.8% of patients, respectively, with 11.9% of patients permanently discontinuing bosutinib because of toxicity.
Study details: Findings are from a retrospective analysis of 101 elderly (age, older than 65 years) patients with CML-CP treated with bosutinib in second or subsequent line. Patients switched to bosutinib because of intolerance (n=46) or resistance (n=55) to previous TKI therapies.
Disclosures: This study did not receive any type of funding. Some investigators including the lead author reported consulting for or receiving honoraria from various pharmaceutical companies.
Source: Latagliata R et al. Hematol Oncol. 2021 Feb 22. doi: 10.1002/hon.2851.
Key clinical point: Bosutinib was effective and safe as a second or subsequent line of treatment in a real-life cohort of elderly patients with chronic-phase chronic myeloid leukemia (CML-CP) and multiple baseline comorbidities who were resistant or intolerant to previous tyrosine kinase inhibitors (TKIs).
Major finding: Overall, rates of cytogenic response, molecular response, 3-year event-free survival, and 3-year overall survival were 81.2%, 66.6%, 60.9%, and 86.4%, respectively. Grade 3/4 hematological and extra-hematological toxicities were reported in 6.9% and 18.8% of patients, respectively, with 11.9% of patients permanently discontinuing bosutinib because of toxicity.
Study details: Findings are from a retrospective analysis of 101 elderly (age, older than 65 years) patients with CML-CP treated with bosutinib in second or subsequent line. Patients switched to bosutinib because of intolerance (n=46) or resistance (n=55) to previous TKI therapies.
Disclosures: This study did not receive any type of funding. Some investigators including the lead author reported consulting for or receiving honoraria from various pharmaceutical companies.
Source: Latagliata R et al. Hematol Oncol. 2021 Feb 22. doi: 10.1002/hon.2851.
Key clinical point: Bosutinib was effective and safe as a second or subsequent line of treatment in a real-life cohort of elderly patients with chronic-phase chronic myeloid leukemia (CML-CP) and multiple baseline comorbidities who were resistant or intolerant to previous tyrosine kinase inhibitors (TKIs).
Major finding: Overall, rates of cytogenic response, molecular response, 3-year event-free survival, and 3-year overall survival were 81.2%, 66.6%, 60.9%, and 86.4%, respectively. Grade 3/4 hematological and extra-hematological toxicities were reported in 6.9% and 18.8% of patients, respectively, with 11.9% of patients permanently discontinuing bosutinib because of toxicity.
Study details: Findings are from a retrospective analysis of 101 elderly (age, older than 65 years) patients with CML-CP treated with bosutinib in second or subsequent line. Patients switched to bosutinib because of intolerance (n=46) or resistance (n=55) to previous TKI therapies.
Disclosures: This study did not receive any type of funding. Some investigators including the lead author reported consulting for or receiving honoraria from various pharmaceutical companies.
Source: Latagliata R et al. Hematol Oncol. 2021 Feb 22. doi: 10.1002/hon.2851.
Hedgehog inhibitor alternative dosing advantageous for BCC
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Quality of life and health state utility in patients with CML in real-life setting
Key clinical point: Even after effective treatment with tyrosine kinase inhibitors, patients with chronic myeloid leukemia (CML) had notably altered global quality of life (QoL) and health utility scores.
Major finding: Compared with the general population, patients with CML had notably affected QoL in terms of social, role, and cognitive functioning with a mean difference of −16.0, −13.1, and −11.7, respectively. The utility score had a deviation from the reference norm of −0.15 on an average (standard deviation, 0.25) compared with the general population.
Study details: Findings are from a prospective web-based survey that assessed QoL and health utility scores in 383 patients with CML (92% in chronic-phase) in France.
Disclosures: This study was funded by the French National Agency for Research and the French Institute for Public Health Research. The authors declared no conflicts of interest.
Source: Foulon S et al. Qual Life Res. 2021 Mar 2. doi: 10.1007/s11136-021-02794-5.
Key clinical point: Even after effective treatment with tyrosine kinase inhibitors, patients with chronic myeloid leukemia (CML) had notably altered global quality of life (QoL) and health utility scores.
Major finding: Compared with the general population, patients with CML had notably affected QoL in terms of social, role, and cognitive functioning with a mean difference of −16.0, −13.1, and −11.7, respectively. The utility score had a deviation from the reference norm of −0.15 on an average (standard deviation, 0.25) compared with the general population.
Study details: Findings are from a prospective web-based survey that assessed QoL and health utility scores in 383 patients with CML (92% in chronic-phase) in France.
Disclosures: This study was funded by the French National Agency for Research and the French Institute for Public Health Research. The authors declared no conflicts of interest.
Source: Foulon S et al. Qual Life Res. 2021 Mar 2. doi: 10.1007/s11136-021-02794-5.
Key clinical point: Even after effective treatment with tyrosine kinase inhibitors, patients with chronic myeloid leukemia (CML) had notably altered global quality of life (QoL) and health utility scores.
Major finding: Compared with the general population, patients with CML had notably affected QoL in terms of social, role, and cognitive functioning with a mean difference of −16.0, −13.1, and −11.7, respectively. The utility score had a deviation from the reference norm of −0.15 on an average (standard deviation, 0.25) compared with the general population.
Study details: Findings are from a prospective web-based survey that assessed QoL and health utility scores in 383 patients with CML (92% in chronic-phase) in France.
Disclosures: This study was funded by the French National Agency for Research and the French Institute for Public Health Research. The authors declared no conflicts of interest.
Source: Foulon S et al. Qual Life Res. 2021 Mar 2. doi: 10.1007/s11136-021-02794-5.
Dasatinib/nivolumab combo safe but shows no meaningful activity in previously treated CML
Key clinical point: Combination of dasatinib and nivolumab was safe but did not show any clinical activity in patients with difficult-to-treat chronic myeloid leukemia (CML) in chronic phase (CP) or accelerated phase (AP) who received at least 2 prior tyrosine kinase inhibitors (TKIs).
Major finding: No drug limiting toxicities were observed even with the maximum dose of both drugs. The most common grade 3 or higher adverse event (AE) of any cause was diarrhea (13%), of which only 3% were considered drug related. Serious AEs occurred in 48% of patients; however, only 2 were considered drug related. The overall response rate was low with none being durable.
Study details: Findings are from a phase 1b dose-escalation study involving 31 patients with CML in CP (n=24) or AP (n=7) who received 2 or more prior TKIs and showed progression, resistance, suboptimal response, or intolerance to the most recent therapy.
Disclosures: This trial was funded by Bristol Myers Squibb, Princeton, NJ, USA. The lead author along with other authors declared receiving grants, advisory boards, honoraria, and/or research funding from various pharmaceutical companies including Bristol Myers Squibb. R Swanink and PM Regueira were employees of Bristol Myers Squibb.
Source: Martínez-López J et al. Leuk Lymphoma. 2021 Mar 2. doi: 10.1080/10428194.2021.1889536.
Key clinical point: Combination of dasatinib and nivolumab was safe but did not show any clinical activity in patients with difficult-to-treat chronic myeloid leukemia (CML) in chronic phase (CP) or accelerated phase (AP) who received at least 2 prior tyrosine kinase inhibitors (TKIs).
Major finding: No drug limiting toxicities were observed even with the maximum dose of both drugs. The most common grade 3 or higher adverse event (AE) of any cause was diarrhea (13%), of which only 3% were considered drug related. Serious AEs occurred in 48% of patients; however, only 2 were considered drug related. The overall response rate was low with none being durable.
Study details: Findings are from a phase 1b dose-escalation study involving 31 patients with CML in CP (n=24) or AP (n=7) who received 2 or more prior TKIs and showed progression, resistance, suboptimal response, or intolerance to the most recent therapy.
Disclosures: This trial was funded by Bristol Myers Squibb, Princeton, NJ, USA. The lead author along with other authors declared receiving grants, advisory boards, honoraria, and/or research funding from various pharmaceutical companies including Bristol Myers Squibb. R Swanink and PM Regueira were employees of Bristol Myers Squibb.
Source: Martínez-López J et al. Leuk Lymphoma. 2021 Mar 2. doi: 10.1080/10428194.2021.1889536.
Key clinical point: Combination of dasatinib and nivolumab was safe but did not show any clinical activity in patients with difficult-to-treat chronic myeloid leukemia (CML) in chronic phase (CP) or accelerated phase (AP) who received at least 2 prior tyrosine kinase inhibitors (TKIs).
Major finding: No drug limiting toxicities were observed even with the maximum dose of both drugs. The most common grade 3 or higher adverse event (AE) of any cause was diarrhea (13%), of which only 3% were considered drug related. Serious AEs occurred in 48% of patients; however, only 2 were considered drug related. The overall response rate was low with none being durable.
Study details: Findings are from a phase 1b dose-escalation study involving 31 patients with CML in CP (n=24) or AP (n=7) who received 2 or more prior TKIs and showed progression, resistance, suboptimal response, or intolerance to the most recent therapy.
Disclosures: This trial was funded by Bristol Myers Squibb, Princeton, NJ, USA. The lead author along with other authors declared receiving grants, advisory boards, honoraria, and/or research funding from various pharmaceutical companies including Bristol Myers Squibb. R Swanink and PM Regueira were employees of Bristol Myers Squibb.
Source: Martínez-López J et al. Leuk Lymphoma. 2021 Mar 2. doi: 10.1080/10428194.2021.1889536.
CML-CP: Imatinib shows long-term efficacy after interferon therapy failure
Key clinical point: Imatinib treatment following interferon-α failure showed long-term efficacy in a real-life setting of patients with late chronic-phase chronic myeloid leukemia (CML-CP).
Major finding: After a median of 4.5 months of therapy initiation, 84% of patients achieved complete cytogenic response as their best cytogenetic response. The estimated 18-year overall survival, event-free survival, and progression-free survival were 64.8%, 69%, and 64.4%, respectively. Imatinib discontinuation and CML-related deaths occurred in 36% and 34% of patients, respectively, and 86% of patients were in treatment-free remission at the last follow-up.
Study details: Findings are from a retrospective analysis of 139 patients with late CML-CP treated with imatinib following interferon-α therapy failure followed up for a median of 16.6 years.
Disclosures: No specific funding source was identified. The authors declared no conflicts of interest.
Source: Pepe S et al. Leuk Lymphoma. 2021 Mar 16. doi: 10.1080/10428194.2021.1901094.
Key clinical point: Imatinib treatment following interferon-α failure showed long-term efficacy in a real-life setting of patients with late chronic-phase chronic myeloid leukemia (CML-CP).
Major finding: After a median of 4.5 months of therapy initiation, 84% of patients achieved complete cytogenic response as their best cytogenetic response. The estimated 18-year overall survival, event-free survival, and progression-free survival were 64.8%, 69%, and 64.4%, respectively. Imatinib discontinuation and CML-related deaths occurred in 36% and 34% of patients, respectively, and 86% of patients were in treatment-free remission at the last follow-up.
Study details: Findings are from a retrospective analysis of 139 patients with late CML-CP treated with imatinib following interferon-α therapy failure followed up for a median of 16.6 years.
Disclosures: No specific funding source was identified. The authors declared no conflicts of interest.
Source: Pepe S et al. Leuk Lymphoma. 2021 Mar 16. doi: 10.1080/10428194.2021.1901094.
Key clinical point: Imatinib treatment following interferon-α failure showed long-term efficacy in a real-life setting of patients with late chronic-phase chronic myeloid leukemia (CML-CP).
Major finding: After a median of 4.5 months of therapy initiation, 84% of patients achieved complete cytogenic response as their best cytogenetic response. The estimated 18-year overall survival, event-free survival, and progression-free survival were 64.8%, 69%, and 64.4%, respectively. Imatinib discontinuation and CML-related deaths occurred in 36% and 34% of patients, respectively, and 86% of patients were in treatment-free remission at the last follow-up.
Study details: Findings are from a retrospective analysis of 139 patients with late CML-CP treated with imatinib following interferon-α therapy failure followed up for a median of 16.6 years.
Disclosures: No specific funding source was identified. The authors declared no conflicts of interest.
Source: Pepe S et al. Leuk Lymphoma. 2021 Mar 16. doi: 10.1080/10428194.2021.1901094.