User login
Low-risk adenomas may not elevate risk of CRC-related death
Unlike high-risk adenomas (HRAs), low-risk adenomas (LRAs) have a minimal association with risk of metachronous colorectal cancer (CRC), and no relationship with odds of metachronous CRC-related mortality, according to a meta-analysis of more than 500,000 individuals.
These findings should impact surveillance guidelines and make follow-up the same for individuals with LRAs or no adenomas, reported lead author Abhiram Duvvuri, MD, of the division of gastroenterology and hepatology at the University of Kansas, Kansas City, and colleagues. Currently, the United States Multi-Society Task Force on Colorectal Cancer advises colonoscopy intervals of 3 years for individuals with HRAs, 7-10 years for those with LRAs, and 10 years for those without adenomas.
“The evidence supporting these surveillance recommendations for clinically relevant endpoints such as cancer and cancer-related deaths among patients who undergo adenoma removal, particularly LRA, is minimal, because most of the evidence was based on the surrogate risk of metachronous advanced neoplasia,” the investigators wrote in Gastroenterology.
To provide more solid evidence, the investigators performed a systematic review and meta-analysis, ultimately analyzing 12 studies with data from 510,019 individuals at a mean age of 59.2 years. All studies reported rates of LRA, HRA, or no adenoma at baseline colonoscopy, plus incidence of metachronous CRC and/or CRC-related mortality. With these data, the investigators determined incidence of metachronous CRC and CRC-related mortality for each of the adenoma groups and also compared these incidences per 10,000 person-years of follow-up across groups.
After a mean follow-up of 8.5 years, patients with HRAs had a significantly higher rate of CRC compared with patients who had LRAs (13.81 vs. 4.5; odds ratio, 2.35; 95% confidence interval, 1.72-3.20) or no adenomas (13.81 vs. 3.4; OR, 2.92; 95% CI, 2.31-3.69). Similarly, but to a lesser degree, LRAs were associated with significantly greater risk of CRC than that of no adenomas (4.5 vs. 3.4; OR, 1.26; 95% CI, 1.06-1.51).
Data on CRC- related mortality further supported these minimal risk profiles because LRAs did not significantly increase the risk of CRC-related mortality compared with no adenomas (OR, 1.15; 95% CI, 0.76-1.74). In contrast, HRAs were associated with significantly greater risk of CRC-related death than that of both LRAs (OR, 2.48; 95% CI, 1.30-4.75) and no adenomas (OR, 2.69; 95% CI, 1.87-3.87).
The investigators acknowledged certain limitations of their study. For one, there were no randomized controlled trials in the meta-analysis, which can introduce bias. Loss of patients to follow-up is also possible; however, the investigators noted that there was a robust sample of patients available for study outcomes all the same. There is also risk of comparability bias in that HRA and LRA groups underwent more colonoscopies; however, the duration of follow-up and timing of last colonoscopy were similar among groups. Lastly, it’s possible the patient sample wasn’t representative because of healthy screenee bias, but the investigators compared groups against general population to minimize that bias.
The investigators also highlighted several strengths of their study that make their findings more reliable than those of past meta-analyses. For one, their study is the largest of its kind to date, and involved a significantly higher number of patients with LRA and no adenomas. Also, in contrast with previous studies, CRC and CRC-related mortality were evaluated rather than advanced adenomas, they noted.
“Furthermore, we also analyzed CRC incidence and mortality in the LRA group compared with the general population, with the [standardized incidence ratio] being lower and [standardized mortality ratio] being comparable, confirming that it is indeed a low-risk group,” they wrote.
Considering these strengths and the nature of their findings, Dr. Duvvuri and colleagues called for a more conservative approach to CRC surveillance among individuals with LRAs, and more research to investigate extending colonoscopy intervals even further.
“We recommend that the interval for follow-up colonoscopy should be the same in patients with LRAs or no adenomas but that the HRA group should have a more frequent surveillance interval for CRC surveillance compared with these groups,” they concluded. “Future studies should evaluate whether surveillance intervals could be lengthened beyond 10 years in the no-adenoma and LRA groups after an initial high-quality index colonoscopy.”
One author disclosed affiliations with Erbe, Cdx Labs, Aries, and others. Dr. Duvvuri and the remaining authors disclosed no conflicts.
Despite evidence suggesting that colorectal cancer (CRC) incidence and mortality can be decreased through the endoscopic removal of adenomatous polyps, the question remains as to whether further endoscopic surveillance is necessary after polypectomy and, if so, how often. The most recent iteration of the United States Multi-Society Task Force guidelines endorsed a lengthening of the surveillance interval following the removal of low-risk adenomas (LRAs), defined as 1-2 tubular adenomas <10 mm with low-grade dysplasia, while maintaining a shorter interval for high-risk adenomas (HRAs), defined as advanced adenomas (villous histology, high-grade dysplasia, or >10 mm) or >3 adenomas.
Dr. Duvvuri and colleagues present the results of a systematic review and meta-analysis of studies examining metachronous CRC incidence and mortality following index colonoscopy. They found a small but statistically significant increase in the incidence of CRC but no significant difference in CRC mortality when comparing patients with LRAs to those with no adenomas. In contrast, they found both a statistically and clinically significant difference in CRC incidence/mortality when comparing patients with HRAs to both those with no adenomas and those with LRAs. They concluded that these results support a recommendation for no difference in follow-up surveillance between patients with LRAs and no adenomas but do support more frequent surveillance for patients with HRAs at index colonoscopy.
Future studies should better examine the timing of neoplasm incidence/recurrence following adenoma removal and also examine metachronous CRC incidence/mortality in patients with sessile serrated lesions at index colonoscopy.
Reid M. Ness, MD, MPH, AGAF, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center and at the VA Tennessee Valley Healthcare System, Nashville, campus. He is an investigator in the Vanderbilt-Ingram Cancer Center. Dr. Ness has no financial relationships to disclose.
Despite evidence suggesting that colorectal cancer (CRC) incidence and mortality can be decreased through the endoscopic removal of adenomatous polyps, the question remains as to whether further endoscopic surveillance is necessary after polypectomy and, if so, how often. The most recent iteration of the United States Multi-Society Task Force guidelines endorsed a lengthening of the surveillance interval following the removal of low-risk adenomas (LRAs), defined as 1-2 tubular adenomas <10 mm with low-grade dysplasia, while maintaining a shorter interval for high-risk adenomas (HRAs), defined as advanced adenomas (villous histology, high-grade dysplasia, or >10 mm) or >3 adenomas.
Dr. Duvvuri and colleagues present the results of a systematic review and meta-analysis of studies examining metachronous CRC incidence and mortality following index colonoscopy. They found a small but statistically significant increase in the incidence of CRC but no significant difference in CRC mortality when comparing patients with LRAs to those with no adenomas. In contrast, they found both a statistically and clinically significant difference in CRC incidence/mortality when comparing patients with HRAs to both those with no adenomas and those with LRAs. They concluded that these results support a recommendation for no difference in follow-up surveillance between patients with LRAs and no adenomas but do support more frequent surveillance for patients with HRAs at index colonoscopy.
Future studies should better examine the timing of neoplasm incidence/recurrence following adenoma removal and also examine metachronous CRC incidence/mortality in patients with sessile serrated lesions at index colonoscopy.
Reid M. Ness, MD, MPH, AGAF, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center and at the VA Tennessee Valley Healthcare System, Nashville, campus. He is an investigator in the Vanderbilt-Ingram Cancer Center. Dr. Ness has no financial relationships to disclose.
Despite evidence suggesting that colorectal cancer (CRC) incidence and mortality can be decreased through the endoscopic removal of adenomatous polyps, the question remains as to whether further endoscopic surveillance is necessary after polypectomy and, if so, how often. The most recent iteration of the United States Multi-Society Task Force guidelines endorsed a lengthening of the surveillance interval following the removal of low-risk adenomas (LRAs), defined as 1-2 tubular adenomas <10 mm with low-grade dysplasia, while maintaining a shorter interval for high-risk adenomas (HRAs), defined as advanced adenomas (villous histology, high-grade dysplasia, or >10 mm) or >3 adenomas.
Dr. Duvvuri and colleagues present the results of a systematic review and meta-analysis of studies examining metachronous CRC incidence and mortality following index colonoscopy. They found a small but statistically significant increase in the incidence of CRC but no significant difference in CRC mortality when comparing patients with LRAs to those with no adenomas. In contrast, they found both a statistically and clinically significant difference in CRC incidence/mortality when comparing patients with HRAs to both those with no adenomas and those with LRAs. They concluded that these results support a recommendation for no difference in follow-up surveillance between patients with LRAs and no adenomas but do support more frequent surveillance for patients with HRAs at index colonoscopy.
Future studies should better examine the timing of neoplasm incidence/recurrence following adenoma removal and also examine metachronous CRC incidence/mortality in patients with sessile serrated lesions at index colonoscopy.
Reid M. Ness, MD, MPH, AGAF, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center and at the VA Tennessee Valley Healthcare System, Nashville, campus. He is an investigator in the Vanderbilt-Ingram Cancer Center. Dr. Ness has no financial relationships to disclose.
Unlike high-risk adenomas (HRAs), low-risk adenomas (LRAs) have a minimal association with risk of metachronous colorectal cancer (CRC), and no relationship with odds of metachronous CRC-related mortality, according to a meta-analysis of more than 500,000 individuals.
These findings should impact surveillance guidelines and make follow-up the same for individuals with LRAs or no adenomas, reported lead author Abhiram Duvvuri, MD, of the division of gastroenterology and hepatology at the University of Kansas, Kansas City, and colleagues. Currently, the United States Multi-Society Task Force on Colorectal Cancer advises colonoscopy intervals of 3 years for individuals with HRAs, 7-10 years for those with LRAs, and 10 years for those without adenomas.
“The evidence supporting these surveillance recommendations for clinically relevant endpoints such as cancer and cancer-related deaths among patients who undergo adenoma removal, particularly LRA, is minimal, because most of the evidence was based on the surrogate risk of metachronous advanced neoplasia,” the investigators wrote in Gastroenterology.
To provide more solid evidence, the investigators performed a systematic review and meta-analysis, ultimately analyzing 12 studies with data from 510,019 individuals at a mean age of 59.2 years. All studies reported rates of LRA, HRA, or no adenoma at baseline colonoscopy, plus incidence of metachronous CRC and/or CRC-related mortality. With these data, the investigators determined incidence of metachronous CRC and CRC-related mortality for each of the adenoma groups and also compared these incidences per 10,000 person-years of follow-up across groups.
After a mean follow-up of 8.5 years, patients with HRAs had a significantly higher rate of CRC compared with patients who had LRAs (13.81 vs. 4.5; odds ratio, 2.35; 95% confidence interval, 1.72-3.20) or no adenomas (13.81 vs. 3.4; OR, 2.92; 95% CI, 2.31-3.69). Similarly, but to a lesser degree, LRAs were associated with significantly greater risk of CRC than that of no adenomas (4.5 vs. 3.4; OR, 1.26; 95% CI, 1.06-1.51).
Data on CRC- related mortality further supported these minimal risk profiles because LRAs did not significantly increase the risk of CRC-related mortality compared with no adenomas (OR, 1.15; 95% CI, 0.76-1.74). In contrast, HRAs were associated with significantly greater risk of CRC-related death than that of both LRAs (OR, 2.48; 95% CI, 1.30-4.75) and no adenomas (OR, 2.69; 95% CI, 1.87-3.87).
The investigators acknowledged certain limitations of their study. For one, there were no randomized controlled trials in the meta-analysis, which can introduce bias. Loss of patients to follow-up is also possible; however, the investigators noted that there was a robust sample of patients available for study outcomes all the same. There is also risk of comparability bias in that HRA and LRA groups underwent more colonoscopies; however, the duration of follow-up and timing of last colonoscopy were similar among groups. Lastly, it’s possible the patient sample wasn’t representative because of healthy screenee bias, but the investigators compared groups against general population to minimize that bias.
The investigators also highlighted several strengths of their study that make their findings more reliable than those of past meta-analyses. For one, their study is the largest of its kind to date, and involved a significantly higher number of patients with LRA and no adenomas. Also, in contrast with previous studies, CRC and CRC-related mortality were evaluated rather than advanced adenomas, they noted.
“Furthermore, we also analyzed CRC incidence and mortality in the LRA group compared with the general population, with the [standardized incidence ratio] being lower and [standardized mortality ratio] being comparable, confirming that it is indeed a low-risk group,” they wrote.
Considering these strengths and the nature of their findings, Dr. Duvvuri and colleagues called for a more conservative approach to CRC surveillance among individuals with LRAs, and more research to investigate extending colonoscopy intervals even further.
“We recommend that the interval for follow-up colonoscopy should be the same in patients with LRAs or no adenomas but that the HRA group should have a more frequent surveillance interval for CRC surveillance compared with these groups,” they concluded. “Future studies should evaluate whether surveillance intervals could be lengthened beyond 10 years in the no-adenoma and LRA groups after an initial high-quality index colonoscopy.”
One author disclosed affiliations with Erbe, Cdx Labs, Aries, and others. Dr. Duvvuri and the remaining authors disclosed no conflicts.
Unlike high-risk adenomas (HRAs), low-risk adenomas (LRAs) have a minimal association with risk of metachronous colorectal cancer (CRC), and no relationship with odds of metachronous CRC-related mortality, according to a meta-analysis of more than 500,000 individuals.
These findings should impact surveillance guidelines and make follow-up the same for individuals with LRAs or no adenomas, reported lead author Abhiram Duvvuri, MD, of the division of gastroenterology and hepatology at the University of Kansas, Kansas City, and colleagues. Currently, the United States Multi-Society Task Force on Colorectal Cancer advises colonoscopy intervals of 3 years for individuals with HRAs, 7-10 years for those with LRAs, and 10 years for those without adenomas.
“The evidence supporting these surveillance recommendations for clinically relevant endpoints such as cancer and cancer-related deaths among patients who undergo adenoma removal, particularly LRA, is minimal, because most of the evidence was based on the surrogate risk of metachronous advanced neoplasia,” the investigators wrote in Gastroenterology.
To provide more solid evidence, the investigators performed a systematic review and meta-analysis, ultimately analyzing 12 studies with data from 510,019 individuals at a mean age of 59.2 years. All studies reported rates of LRA, HRA, or no adenoma at baseline colonoscopy, plus incidence of metachronous CRC and/or CRC-related mortality. With these data, the investigators determined incidence of metachronous CRC and CRC-related mortality for each of the adenoma groups and also compared these incidences per 10,000 person-years of follow-up across groups.
After a mean follow-up of 8.5 years, patients with HRAs had a significantly higher rate of CRC compared with patients who had LRAs (13.81 vs. 4.5; odds ratio, 2.35; 95% confidence interval, 1.72-3.20) or no adenomas (13.81 vs. 3.4; OR, 2.92; 95% CI, 2.31-3.69). Similarly, but to a lesser degree, LRAs were associated with significantly greater risk of CRC than that of no adenomas (4.5 vs. 3.4; OR, 1.26; 95% CI, 1.06-1.51).
Data on CRC- related mortality further supported these minimal risk profiles because LRAs did not significantly increase the risk of CRC-related mortality compared with no adenomas (OR, 1.15; 95% CI, 0.76-1.74). In contrast, HRAs were associated with significantly greater risk of CRC-related death than that of both LRAs (OR, 2.48; 95% CI, 1.30-4.75) and no adenomas (OR, 2.69; 95% CI, 1.87-3.87).
The investigators acknowledged certain limitations of their study. For one, there were no randomized controlled trials in the meta-analysis, which can introduce bias. Loss of patients to follow-up is also possible; however, the investigators noted that there was a robust sample of patients available for study outcomes all the same. There is also risk of comparability bias in that HRA and LRA groups underwent more colonoscopies; however, the duration of follow-up and timing of last colonoscopy were similar among groups. Lastly, it’s possible the patient sample wasn’t representative because of healthy screenee bias, but the investigators compared groups against general population to minimize that bias.
The investigators also highlighted several strengths of their study that make their findings more reliable than those of past meta-analyses. For one, their study is the largest of its kind to date, and involved a significantly higher number of patients with LRA and no adenomas. Also, in contrast with previous studies, CRC and CRC-related mortality were evaluated rather than advanced adenomas, they noted.
“Furthermore, we also analyzed CRC incidence and mortality in the LRA group compared with the general population, with the [standardized incidence ratio] being lower and [standardized mortality ratio] being comparable, confirming that it is indeed a low-risk group,” they wrote.
Considering these strengths and the nature of their findings, Dr. Duvvuri and colleagues called for a more conservative approach to CRC surveillance among individuals with LRAs, and more research to investigate extending colonoscopy intervals even further.
“We recommend that the interval for follow-up colonoscopy should be the same in patients with LRAs or no adenomas but that the HRA group should have a more frequent surveillance interval for CRC surveillance compared with these groups,” they concluded. “Future studies should evaluate whether surveillance intervals could be lengthened beyond 10 years in the no-adenoma and LRA groups after an initial high-quality index colonoscopy.”
One author disclosed affiliations with Erbe, Cdx Labs, Aries, and others. Dr. Duvvuri and the remaining authors disclosed no conflicts.
FROM GASTROENTEROLOGY
Moderate-to-vigorous physical activity is the answer to childhood obesity
There is no question that none of us, not just pediatricians, is doing a very good job of dealing with the obesity problem this nation faces. We can agree that a more active lifestyle that includes spells of vigorous activity is important for weight management. We know that in general overweight people sleep less than do those whose basal metabolic rate is normal. And, of course, we know that a diet high in calorie-dense foods is associated with unhealthy weight gain.
Not surprisingly, overweight individuals are usually struggling with all three of these challenges. They are less active, get too little sleep, and are ingesting a diet that is too calorie dense. In other words, they would benefit from a total lifestyle reboot. But you know as well as I do a change of that magnitude is much easier said than done. Few families can afford nor would they have the appetite for sending their children to a “fat camp” for 6 months with no guarantee of success.
Instead of throwing up our hands in the face of this monumental task or attacking it at close range, maybe we should aim our efforts at the risk associations that will yield the best results for our efforts. A group of researchers at the University of South Australia has just published a study in Pediatrics in which they provide some data that may help us target our interventions with obese and overweight children. The researchers did not investigate diet, but used accelerometers to determine how much time each child spent sleeping and a variety of activity levels. They then determined what effect changes in the child’s allocation of activity had on their adiposity.
The investigators found on a minute-to-minute basis that an increase in a child’s moderate-to-vigorous physical activity (MVPA) was up to six times more effective at influencing adiposity than was a decrease in sedentary time or an increase in sleep duration. For example, 17 minutes of MVPA had the same beneficial effect as 52 minutes more sleep or 56 minutes less sedentary time. Interestingly and somewhat surprisingly, the researchers found that light activity was positively associated with adiposity.
For those of us in primary care, this study from Australia suggests that our time (and the parents’ time) would be best spent figuring out how to include more MVPA in the child’s day and not focus so much on sleep duration and sedentary intervals.
However, before one can make any recommendation one must first have a clear understanding of how the child and his family spend the day. This process can be done in the office by interviewing the family. I have found that this is not as time consuming as one might think and often yields some valuable additional insight into the family’s dynamics. Sending the family home with an hourly log to be filled in or asking them to use a smartphone to record information will also work.
I must admit that at first I found the results of this study ran counter to my intuition. I have always felt that sleep is the linchpin to the solution of a variety of health style related problems. In my construct, more sleep has always been the first and easy answer and decreasing screen time the second. But, it turns out that increasing MVPA may give us the biggest bang for the buck. Which is fine with me.
The problem facing us is how we can be creative in adding that 20 minutes of vigorous activity. In most communities, we have allowed the school system to drop the ball. We can hope that this study will be confirmed or at least widely publicized. It feels like it is time to guarantee that every child gets a robust gym class every school day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
There is no question that none of us, not just pediatricians, is doing a very good job of dealing with the obesity problem this nation faces. We can agree that a more active lifestyle that includes spells of vigorous activity is important for weight management. We know that in general overweight people sleep less than do those whose basal metabolic rate is normal. And, of course, we know that a diet high in calorie-dense foods is associated with unhealthy weight gain.
Not surprisingly, overweight individuals are usually struggling with all three of these challenges. They are less active, get too little sleep, and are ingesting a diet that is too calorie dense. In other words, they would benefit from a total lifestyle reboot. But you know as well as I do a change of that magnitude is much easier said than done. Few families can afford nor would they have the appetite for sending their children to a “fat camp” for 6 months with no guarantee of success.
Instead of throwing up our hands in the face of this monumental task or attacking it at close range, maybe we should aim our efforts at the risk associations that will yield the best results for our efforts. A group of researchers at the University of South Australia has just published a study in Pediatrics in which they provide some data that may help us target our interventions with obese and overweight children. The researchers did not investigate diet, but used accelerometers to determine how much time each child spent sleeping and a variety of activity levels. They then determined what effect changes in the child’s allocation of activity had on their adiposity.
The investigators found on a minute-to-minute basis that an increase in a child’s moderate-to-vigorous physical activity (MVPA) was up to six times more effective at influencing adiposity than was a decrease in sedentary time or an increase in sleep duration. For example, 17 minutes of MVPA had the same beneficial effect as 52 minutes more sleep or 56 minutes less sedentary time. Interestingly and somewhat surprisingly, the researchers found that light activity was positively associated with adiposity.
For those of us in primary care, this study from Australia suggests that our time (and the parents’ time) would be best spent figuring out how to include more MVPA in the child’s day and not focus so much on sleep duration and sedentary intervals.
However, before one can make any recommendation one must first have a clear understanding of how the child and his family spend the day. This process can be done in the office by interviewing the family. I have found that this is not as time consuming as one might think and often yields some valuable additional insight into the family’s dynamics. Sending the family home with an hourly log to be filled in or asking them to use a smartphone to record information will also work.
I must admit that at first I found the results of this study ran counter to my intuition. I have always felt that sleep is the linchpin to the solution of a variety of health style related problems. In my construct, more sleep has always been the first and easy answer and decreasing screen time the second. But, it turns out that increasing MVPA may give us the biggest bang for the buck. Which is fine with me.
The problem facing us is how we can be creative in adding that 20 minutes of vigorous activity. In most communities, we have allowed the school system to drop the ball. We can hope that this study will be confirmed or at least widely publicized. It feels like it is time to guarantee that every child gets a robust gym class every school day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
There is no question that none of us, not just pediatricians, is doing a very good job of dealing with the obesity problem this nation faces. We can agree that a more active lifestyle that includes spells of vigorous activity is important for weight management. We know that in general overweight people sleep less than do those whose basal metabolic rate is normal. And, of course, we know that a diet high in calorie-dense foods is associated with unhealthy weight gain.
Not surprisingly, overweight individuals are usually struggling with all three of these challenges. They are less active, get too little sleep, and are ingesting a diet that is too calorie dense. In other words, they would benefit from a total lifestyle reboot. But you know as well as I do a change of that magnitude is much easier said than done. Few families can afford nor would they have the appetite for sending their children to a “fat camp” for 6 months with no guarantee of success.
Instead of throwing up our hands in the face of this monumental task or attacking it at close range, maybe we should aim our efforts at the risk associations that will yield the best results for our efforts. A group of researchers at the University of South Australia has just published a study in Pediatrics in which they provide some data that may help us target our interventions with obese and overweight children. The researchers did not investigate diet, but used accelerometers to determine how much time each child spent sleeping and a variety of activity levels. They then determined what effect changes in the child’s allocation of activity had on their adiposity.
The investigators found on a minute-to-minute basis that an increase in a child’s moderate-to-vigorous physical activity (MVPA) was up to six times more effective at influencing adiposity than was a decrease in sedentary time or an increase in sleep duration. For example, 17 minutes of MVPA had the same beneficial effect as 52 minutes more sleep or 56 minutes less sedentary time. Interestingly and somewhat surprisingly, the researchers found that light activity was positively associated with adiposity.
For those of us in primary care, this study from Australia suggests that our time (and the parents’ time) would be best spent figuring out how to include more MVPA in the child’s day and not focus so much on sleep duration and sedentary intervals.
However, before one can make any recommendation one must first have a clear understanding of how the child and his family spend the day. This process can be done in the office by interviewing the family. I have found that this is not as time consuming as one might think and often yields some valuable additional insight into the family’s dynamics. Sending the family home with an hourly log to be filled in or asking them to use a smartphone to record information will also work.
I must admit that at first I found the results of this study ran counter to my intuition. I have always felt that sleep is the linchpin to the solution of a variety of health style related problems. In my construct, more sleep has always been the first and easy answer and decreasing screen time the second. But, it turns out that increasing MVPA may give us the biggest bang for the buck. Which is fine with me.
The problem facing us is how we can be creative in adding that 20 minutes of vigorous activity. In most communities, we have allowed the school system to drop the ball. We can hope that this study will be confirmed or at least widely publicized. It feels like it is time to guarantee that every child gets a robust gym class every school day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Using (dynamic) ultrasound to make an earlier diagnosis of endometriosis
Can you provide some background on endometriosis and the importance of early diagnosis?
Dr. Goldstein: Endometriosis is an inflammatory condition, characterized by endometrial tissue at sites outside the uterus—this definition comes from the World Endometriosis Society.
Endometriosis is said to affect about 10% of women of reproductive age, and if you look at a group, a subset of women with pelvic pain or infertility, the numbers rise to the range of 35% to 50%. It can present in a multitude of locations, mainly in the pelvis, although occasionally even in places like the lung. When it occurs in the uterus, it is known as adenomyosis; when it occurs inside the ovary, it can cause an endometrioma (or what is sometimes referred to as chocolate cyst of the ovary), but you can see endometriotic implants anywhere in the peritoneum—along the urinary tract, rectum, uterosacral ligaments, rectovaginal septum, and even the vaginal wall occasionally.
What I am really interested in is an earlier diagnosis of superficial endometriosis, and it should be apparent to the reader why this is important—the quality of life from pain from endometriosis can be debilitating. It can be a source of infertility, a source of menstrual irregularities, and a source of not only quality of life but also economic consequences. Many women can also undergo as much as a 7-year delay in diagnosis, so the need for a timely diagnosis and initiation of treatment is extremely important.
What is the role of ultrasound in endometriosis diagnostics?
Dr. Goldstein: In an article that I authored 31 years ago, I wrote that there was a difference between an ultrasound examination by referral and examining one’s patients with ultrasound. I coined a phrase: the “ultrasound-enhanced bimanual exam.” I believed that this term should become a routine part of the overall gynecologic exam. I wanted people to think about the bimanual that we had done for at least half a century, which, in my opinion, consists of 2 components:
- An objective component: Is this uterus normal? Is it enlarged or irregular in contour, suggesting maybe fibroids? Is an ovary enlarged? If so, does it feel cystic or solid?
- A subjective component: Does this patient have tenderness through the pelvis. Is there normal mobility of the pelvic organs?
Part of the thesis was that the objective portion could be replaced by an image that could be produced in seconds, dependent on the operator’s training and availability of equipment. The subjective portion, however, depended on the experience and, often, nuance of the examiner. Lately, I have been seeking to expand that thesis by having the imager use examination as part of their overall imaging—this is the concept of dynamic imaging.
Can you expand on the concept of dynamic ultrasound in this setting?
Dr. Goldstein: Presently, most imagers take a multitude of pictures, what I would call 2-dimensional snapshots, to illustrate anatomy. This is usually done by a sonographer, or a technician, who collects the images for viewing by the physician, who then often does so without holding the transducer. Increasing utilization of remote tools like teleradiology only makes this more likely, and for a minority of people who may use video clips instead of still images, they are still simply representations of anatomy. The guidelines for pelvic ultrasound are the underpinning of the expectation of those who are scanning the female pelvis. With dynamic imaging, the operator uses their other hand on the abdomen as well as some motion with the probe to see if they can elicit pain with the vaginal probe, checking for mobility, asking the patient to bear down. Whether you are a sonographer, a radiologist, or an ObGyn, dynamic imaging can bring the examination process into the imager’s hands.
Can you tell us more about the indications for pelvic sonography for endometriosis and what data can you give to support this?
Dr. Goldstein: There is a document titled “Ultrasound Examination of the Female Pelvis,” that was originally developed by the American Institute of Ultrasound in Medicine (AIUM). In this document, there are about 19 different indications for pelvic sonography (in no defined order), and it is interesting that the first indication listed is evaluation of pelvic pain. Well, I would ask you, how do you evaluate pelvic pain with a series of anatomic images? If you have a classic ovarian endometrioma, or you have a classic hydrosalpinx, you can surmise that these are the source of the pain that the patient is reporting. But how do you properly evaluate pain with just an anatomic image? Thus, the need to use dynamic assessment.
There was a concept first introduced by my colleague, Dr. Ilan Timor, known as the sliding organ sign, that was mainly used to determine if 2 structures were adherent or separate. This involved use of the abdominal hand, liberal use of the probe moving in and out, and under real-time vision, examining the patient with the ultrasound transducer; this is the concept of dynamic ultrasound. This practice can be expanded to verify if there is pelvic tenderness and can be a significant part of the nonlaparoscopic, presumptive diagnosis of endometriosis, even when there is no ovarian endometrioma.
To support this theory, I would point you toward a classic article by E Okaro and colleagues in the British Journal of OB-GYN. This study took 120 consecutive women with chronic pelvic pain who were scheduled for laparoscopy, but performed a transvaginal ultrasound prior, and they looked for anatomic abnormalities and divided this into hard markers and soft markers. Hard markers were obvious endometriomas and hydrosalpinges, while soft markers included things like reduced ovarian mobility, site-specific pelvic tenderness, and presence of loculated peritoneal fluid in the pelvis. These were typical of chronic pelvic pain patients that ranged from late teens to almost menopausal, as the average age was about 30 years old.
Patients had experienced pain for anywhere from 6 months to 12 years, but the average was about 4 years. At laparoscopy, 58% of these patients had pelvic pathology, and 42% had a normal pelvis. Of the 58% with pathology, the overwhelming majority—about 51 of 70 women—had endometriosis alone, and another 7 had endometriosis with adhesions. A normal ultrasound, based on the absence of hard markers, was found in 96 of 120 women. Thus, 24 of the 120 women had an abnormal scan based on the presence of these hard markers. At laparoscopy, all 24 women had abnormal laparoscopies. Of those 96 women who would have had a normal ultrasound, based on the anatomic absence of some pathology, 53% had an abnormal scan based on the presence of these soft markers while the remaining women had no soft- or hard-markers suggesting any pelvic pathology. At laparoscopy, 73% of the patients with soft markers had pelvic pathology and 27% had a normal laparoscopy. Of 45 patients who had a normal, transvaginal ultrasound, 9 were found to have small evidence of endometriosis without discrete endometriomas at laparoscopy.
To summarize the study data, 100% of patients with hard markers and chronic pelvic pain had abnormal anatomy at laparoscopy, but 73% of patients who had soft markers but otherwise would have been interpreted as normal anatomic findings had evidence of pelvic pathology. Such an approach, if used, could lead to a reduction in the number of unnecessary laparoscopies.
What it really boils down to is, if you have 100 women with chronic pelvic pain, are you willing to treat 100 patients without laparoscopy, knowing that 73 are going to have a positive laparoscopy and will require treatment anyway? You would treat 27% with a pharmaceutical agent that may provide relief of their pain, or may not, depending on what the true etiology was. I would be willing to do so, as a positive predictive value of 73% makes doing that worthwhile, and I believe a majority of clinicians would agree.
Do you have any other tips or ways to improve the reader’s understanding of transvaginal ultrasound?
Dr. Goldstein: Pelvic organs have mobility. If a premenopausal woman is examined in lithotomy position, if the ovaries are freely mobile, by gravity, they are going to go lateral to the uterus and are seen immediately adjacent to the iliac vessels. But remember, iliac vessels are retroperitoneal as they are outside the peritoneal cavity. If you were to turn that patient onto all fours, so that the ovaries are freely mobile, they are going to move somewhat toward the anterior abdominal wall. When an ovary is seen in a nonanatomic position, it could be normal or it could be held up by a loop of bowel, but it may indicate adhesions. This is where this sliding organ sign and liberal use of the other hand on the lower abdomen can be extremely important. The reader should also understand that our ability to localize ovaries on ultrasound depends on the amount of folliculogenesis. Follicles are black circles that are sonolucent, because they contain fluid, so they make it easy to localize ovaries, but also their anatomic position relative to the iliac vessels. However, there is a caveat—which is, sometimes an ovary might look like it is behind the uterus and not in its normal anatomic location. When dynamic imaging is used, you are able to cajole that ovary to move lateral and sit on top of the iliac vessels, which can enable you make the proper diagnosis.
Can you provide some background on endometriosis and the importance of early diagnosis?
Dr. Goldstein: Endometriosis is an inflammatory condition, characterized by endometrial tissue at sites outside the uterus—this definition comes from the World Endometriosis Society.
Endometriosis is said to affect about 10% of women of reproductive age, and if you look at a group, a subset of women with pelvic pain or infertility, the numbers rise to the range of 35% to 50%. It can present in a multitude of locations, mainly in the pelvis, although occasionally even in places like the lung. When it occurs in the uterus, it is known as adenomyosis; when it occurs inside the ovary, it can cause an endometrioma (or what is sometimes referred to as chocolate cyst of the ovary), but you can see endometriotic implants anywhere in the peritoneum—along the urinary tract, rectum, uterosacral ligaments, rectovaginal septum, and even the vaginal wall occasionally.
What I am really interested in is an earlier diagnosis of superficial endometriosis, and it should be apparent to the reader why this is important—the quality of life from pain from endometriosis can be debilitating. It can be a source of infertility, a source of menstrual irregularities, and a source of not only quality of life but also economic consequences. Many women can also undergo as much as a 7-year delay in diagnosis, so the need for a timely diagnosis and initiation of treatment is extremely important.
What is the role of ultrasound in endometriosis diagnostics?
Dr. Goldstein: In an article that I authored 31 years ago, I wrote that there was a difference between an ultrasound examination by referral and examining one’s patients with ultrasound. I coined a phrase: the “ultrasound-enhanced bimanual exam.” I believed that this term should become a routine part of the overall gynecologic exam. I wanted people to think about the bimanual that we had done for at least half a century, which, in my opinion, consists of 2 components:
- An objective component: Is this uterus normal? Is it enlarged or irregular in contour, suggesting maybe fibroids? Is an ovary enlarged? If so, does it feel cystic or solid?
- A subjective component: Does this patient have tenderness through the pelvis. Is there normal mobility of the pelvic organs?
Part of the thesis was that the objective portion could be replaced by an image that could be produced in seconds, dependent on the operator’s training and availability of equipment. The subjective portion, however, depended on the experience and, often, nuance of the examiner. Lately, I have been seeking to expand that thesis by having the imager use examination as part of their overall imaging—this is the concept of dynamic imaging.
Can you expand on the concept of dynamic ultrasound in this setting?
Dr. Goldstein: Presently, most imagers take a multitude of pictures, what I would call 2-dimensional snapshots, to illustrate anatomy. This is usually done by a sonographer, or a technician, who collects the images for viewing by the physician, who then often does so without holding the transducer. Increasing utilization of remote tools like teleradiology only makes this more likely, and for a minority of people who may use video clips instead of still images, they are still simply representations of anatomy. The guidelines for pelvic ultrasound are the underpinning of the expectation of those who are scanning the female pelvis. With dynamic imaging, the operator uses their other hand on the abdomen as well as some motion with the probe to see if they can elicit pain with the vaginal probe, checking for mobility, asking the patient to bear down. Whether you are a sonographer, a radiologist, or an ObGyn, dynamic imaging can bring the examination process into the imager’s hands.
Can you tell us more about the indications for pelvic sonography for endometriosis and what data can you give to support this?
Dr. Goldstein: There is a document titled “Ultrasound Examination of the Female Pelvis,” that was originally developed by the American Institute of Ultrasound in Medicine (AIUM). In this document, there are about 19 different indications for pelvic sonography (in no defined order), and it is interesting that the first indication listed is evaluation of pelvic pain. Well, I would ask you, how do you evaluate pelvic pain with a series of anatomic images? If you have a classic ovarian endometrioma, or you have a classic hydrosalpinx, you can surmise that these are the source of the pain that the patient is reporting. But how do you properly evaluate pain with just an anatomic image? Thus, the need to use dynamic assessment.
There was a concept first introduced by my colleague, Dr. Ilan Timor, known as the sliding organ sign, that was mainly used to determine if 2 structures were adherent or separate. This involved use of the abdominal hand, liberal use of the probe moving in and out, and under real-time vision, examining the patient with the ultrasound transducer; this is the concept of dynamic ultrasound. This practice can be expanded to verify if there is pelvic tenderness and can be a significant part of the nonlaparoscopic, presumptive diagnosis of endometriosis, even when there is no ovarian endometrioma.
To support this theory, I would point you toward a classic article by E Okaro and colleagues in the British Journal of OB-GYN. This study took 120 consecutive women with chronic pelvic pain who were scheduled for laparoscopy, but performed a transvaginal ultrasound prior, and they looked for anatomic abnormalities and divided this into hard markers and soft markers. Hard markers were obvious endometriomas and hydrosalpinges, while soft markers included things like reduced ovarian mobility, site-specific pelvic tenderness, and presence of loculated peritoneal fluid in the pelvis. These were typical of chronic pelvic pain patients that ranged from late teens to almost menopausal, as the average age was about 30 years old.
Patients had experienced pain for anywhere from 6 months to 12 years, but the average was about 4 years. At laparoscopy, 58% of these patients had pelvic pathology, and 42% had a normal pelvis. Of the 58% with pathology, the overwhelming majority—about 51 of 70 women—had endometriosis alone, and another 7 had endometriosis with adhesions. A normal ultrasound, based on the absence of hard markers, was found in 96 of 120 women. Thus, 24 of the 120 women had an abnormal scan based on the presence of these hard markers. At laparoscopy, all 24 women had abnormal laparoscopies. Of those 96 women who would have had a normal ultrasound, based on the anatomic absence of some pathology, 53% had an abnormal scan based on the presence of these soft markers while the remaining women had no soft- or hard-markers suggesting any pelvic pathology. At laparoscopy, 73% of the patients with soft markers had pelvic pathology and 27% had a normal laparoscopy. Of 45 patients who had a normal, transvaginal ultrasound, 9 were found to have small evidence of endometriosis without discrete endometriomas at laparoscopy.
To summarize the study data, 100% of patients with hard markers and chronic pelvic pain had abnormal anatomy at laparoscopy, but 73% of patients who had soft markers but otherwise would have been interpreted as normal anatomic findings had evidence of pelvic pathology. Such an approach, if used, could lead to a reduction in the number of unnecessary laparoscopies.
What it really boils down to is, if you have 100 women with chronic pelvic pain, are you willing to treat 100 patients without laparoscopy, knowing that 73 are going to have a positive laparoscopy and will require treatment anyway? You would treat 27% with a pharmaceutical agent that may provide relief of their pain, or may not, depending on what the true etiology was. I would be willing to do so, as a positive predictive value of 73% makes doing that worthwhile, and I believe a majority of clinicians would agree.
Do you have any other tips or ways to improve the reader’s understanding of transvaginal ultrasound?
Dr. Goldstein: Pelvic organs have mobility. If a premenopausal woman is examined in lithotomy position, if the ovaries are freely mobile, by gravity, they are going to go lateral to the uterus and are seen immediately adjacent to the iliac vessels. But remember, iliac vessels are retroperitoneal as they are outside the peritoneal cavity. If you were to turn that patient onto all fours, so that the ovaries are freely mobile, they are going to move somewhat toward the anterior abdominal wall. When an ovary is seen in a nonanatomic position, it could be normal or it could be held up by a loop of bowel, but it may indicate adhesions. This is where this sliding organ sign and liberal use of the other hand on the lower abdomen can be extremely important. The reader should also understand that our ability to localize ovaries on ultrasound depends on the amount of folliculogenesis. Follicles are black circles that are sonolucent, because they contain fluid, so they make it easy to localize ovaries, but also their anatomic position relative to the iliac vessels. However, there is a caveat—which is, sometimes an ovary might look like it is behind the uterus and not in its normal anatomic location. When dynamic imaging is used, you are able to cajole that ovary to move lateral and sit on top of the iliac vessels, which can enable you make the proper diagnosis.
Can you provide some background on endometriosis and the importance of early diagnosis?
Dr. Goldstein: Endometriosis is an inflammatory condition, characterized by endometrial tissue at sites outside the uterus—this definition comes from the World Endometriosis Society.
Endometriosis is said to affect about 10% of women of reproductive age, and if you look at a group, a subset of women with pelvic pain or infertility, the numbers rise to the range of 35% to 50%. It can present in a multitude of locations, mainly in the pelvis, although occasionally even in places like the lung. When it occurs in the uterus, it is known as adenomyosis; when it occurs inside the ovary, it can cause an endometrioma (or what is sometimes referred to as chocolate cyst of the ovary), but you can see endometriotic implants anywhere in the peritoneum—along the urinary tract, rectum, uterosacral ligaments, rectovaginal septum, and even the vaginal wall occasionally.
What I am really interested in is an earlier diagnosis of superficial endometriosis, and it should be apparent to the reader why this is important—the quality of life from pain from endometriosis can be debilitating. It can be a source of infertility, a source of menstrual irregularities, and a source of not only quality of life but also economic consequences. Many women can also undergo as much as a 7-year delay in diagnosis, so the need for a timely diagnosis and initiation of treatment is extremely important.
What is the role of ultrasound in endometriosis diagnostics?
Dr. Goldstein: In an article that I authored 31 years ago, I wrote that there was a difference between an ultrasound examination by referral and examining one’s patients with ultrasound. I coined a phrase: the “ultrasound-enhanced bimanual exam.” I believed that this term should become a routine part of the overall gynecologic exam. I wanted people to think about the bimanual that we had done for at least half a century, which, in my opinion, consists of 2 components:
- An objective component: Is this uterus normal? Is it enlarged or irregular in contour, suggesting maybe fibroids? Is an ovary enlarged? If so, does it feel cystic or solid?
- A subjective component: Does this patient have tenderness through the pelvis. Is there normal mobility of the pelvic organs?
Part of the thesis was that the objective portion could be replaced by an image that could be produced in seconds, dependent on the operator’s training and availability of equipment. The subjective portion, however, depended on the experience and, often, nuance of the examiner. Lately, I have been seeking to expand that thesis by having the imager use examination as part of their overall imaging—this is the concept of dynamic imaging.
Can you expand on the concept of dynamic ultrasound in this setting?
Dr. Goldstein: Presently, most imagers take a multitude of pictures, what I would call 2-dimensional snapshots, to illustrate anatomy. This is usually done by a sonographer, or a technician, who collects the images for viewing by the physician, who then often does so without holding the transducer. Increasing utilization of remote tools like teleradiology only makes this more likely, and for a minority of people who may use video clips instead of still images, they are still simply representations of anatomy. The guidelines for pelvic ultrasound are the underpinning of the expectation of those who are scanning the female pelvis. With dynamic imaging, the operator uses their other hand on the abdomen as well as some motion with the probe to see if they can elicit pain with the vaginal probe, checking for mobility, asking the patient to bear down. Whether you are a sonographer, a radiologist, or an ObGyn, dynamic imaging can bring the examination process into the imager’s hands.
Can you tell us more about the indications for pelvic sonography for endometriosis and what data can you give to support this?
Dr. Goldstein: There is a document titled “Ultrasound Examination of the Female Pelvis,” that was originally developed by the American Institute of Ultrasound in Medicine (AIUM). In this document, there are about 19 different indications for pelvic sonography (in no defined order), and it is interesting that the first indication listed is evaluation of pelvic pain. Well, I would ask you, how do you evaluate pelvic pain with a series of anatomic images? If you have a classic ovarian endometrioma, or you have a classic hydrosalpinx, you can surmise that these are the source of the pain that the patient is reporting. But how do you properly evaluate pain with just an anatomic image? Thus, the need to use dynamic assessment.
There was a concept first introduced by my colleague, Dr. Ilan Timor, known as the sliding organ sign, that was mainly used to determine if 2 structures were adherent or separate. This involved use of the abdominal hand, liberal use of the probe moving in and out, and under real-time vision, examining the patient with the ultrasound transducer; this is the concept of dynamic ultrasound. This practice can be expanded to verify if there is pelvic tenderness and can be a significant part of the nonlaparoscopic, presumptive diagnosis of endometriosis, even when there is no ovarian endometrioma.
To support this theory, I would point you toward a classic article by E Okaro and colleagues in the British Journal of OB-GYN. This study took 120 consecutive women with chronic pelvic pain who were scheduled for laparoscopy, but performed a transvaginal ultrasound prior, and they looked for anatomic abnormalities and divided this into hard markers and soft markers. Hard markers were obvious endometriomas and hydrosalpinges, while soft markers included things like reduced ovarian mobility, site-specific pelvic tenderness, and presence of loculated peritoneal fluid in the pelvis. These were typical of chronic pelvic pain patients that ranged from late teens to almost menopausal, as the average age was about 30 years old.
Patients had experienced pain for anywhere from 6 months to 12 years, but the average was about 4 years. At laparoscopy, 58% of these patients had pelvic pathology, and 42% had a normal pelvis. Of the 58% with pathology, the overwhelming majority—about 51 of 70 women—had endometriosis alone, and another 7 had endometriosis with adhesions. A normal ultrasound, based on the absence of hard markers, was found in 96 of 120 women. Thus, 24 of the 120 women had an abnormal scan based on the presence of these hard markers. At laparoscopy, all 24 women had abnormal laparoscopies. Of those 96 women who would have had a normal ultrasound, based on the anatomic absence of some pathology, 53% had an abnormal scan based on the presence of these soft markers while the remaining women had no soft- or hard-markers suggesting any pelvic pathology. At laparoscopy, 73% of the patients with soft markers had pelvic pathology and 27% had a normal laparoscopy. Of 45 patients who had a normal, transvaginal ultrasound, 9 were found to have small evidence of endometriosis without discrete endometriomas at laparoscopy.
To summarize the study data, 100% of patients with hard markers and chronic pelvic pain had abnormal anatomy at laparoscopy, but 73% of patients who had soft markers but otherwise would have been interpreted as normal anatomic findings had evidence of pelvic pathology. Such an approach, if used, could lead to a reduction in the number of unnecessary laparoscopies.
What it really boils down to is, if you have 100 women with chronic pelvic pain, are you willing to treat 100 patients without laparoscopy, knowing that 73 are going to have a positive laparoscopy and will require treatment anyway? You would treat 27% with a pharmaceutical agent that may provide relief of their pain, or may not, depending on what the true etiology was. I would be willing to do so, as a positive predictive value of 73% makes doing that worthwhile, and I believe a majority of clinicians would agree.
Do you have any other tips or ways to improve the reader’s understanding of transvaginal ultrasound?
Dr. Goldstein: Pelvic organs have mobility. If a premenopausal woman is examined in lithotomy position, if the ovaries are freely mobile, by gravity, they are going to go lateral to the uterus and are seen immediately adjacent to the iliac vessels. But remember, iliac vessels are retroperitoneal as they are outside the peritoneal cavity. If you were to turn that patient onto all fours, so that the ovaries are freely mobile, they are going to move somewhat toward the anterior abdominal wall. When an ovary is seen in a nonanatomic position, it could be normal or it could be held up by a loop of bowel, but it may indicate adhesions. This is where this sliding organ sign and liberal use of the other hand on the lower abdomen can be extremely important. The reader should also understand that our ability to localize ovaries on ultrasound depends on the amount of folliculogenesis. Follicles are black circles that are sonolucent, because they contain fluid, so they make it easy to localize ovaries, but also their anatomic position relative to the iliac vessels. However, there is a caveat—which is, sometimes an ovary might look like it is behind the uterus and not in its normal anatomic location. When dynamic imaging is used, you are able to cajole that ovary to move lateral and sit on top of the iliac vessels, which can enable you make the proper diagnosis.
37-year-old man • cough • increasing shortness of breath • pleuritic chest pain • Dx?
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.
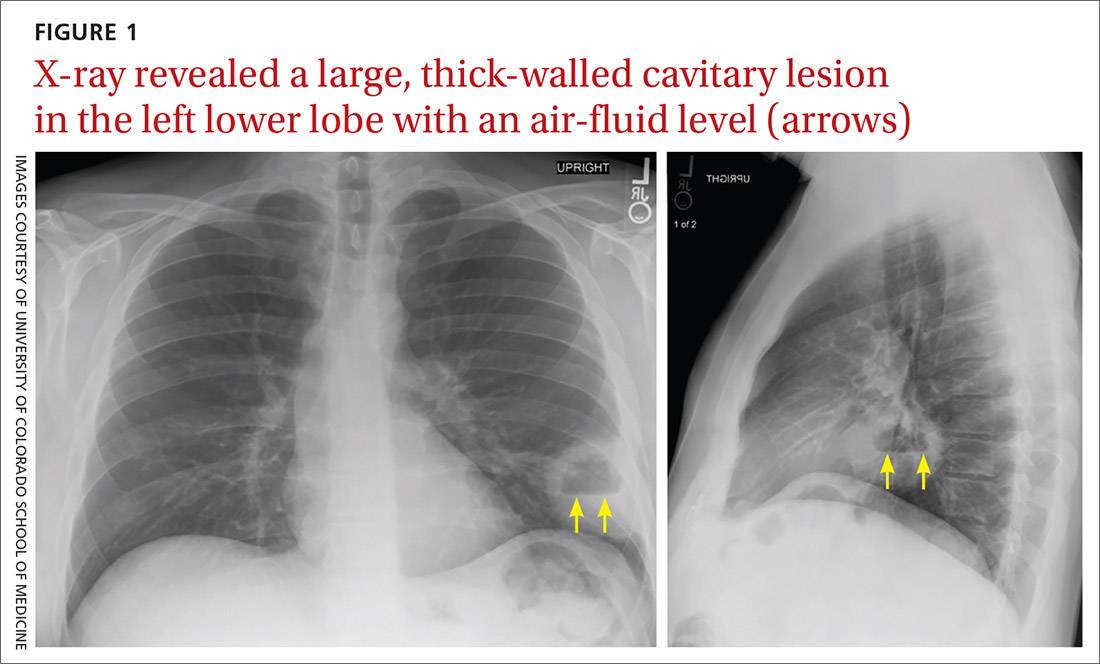
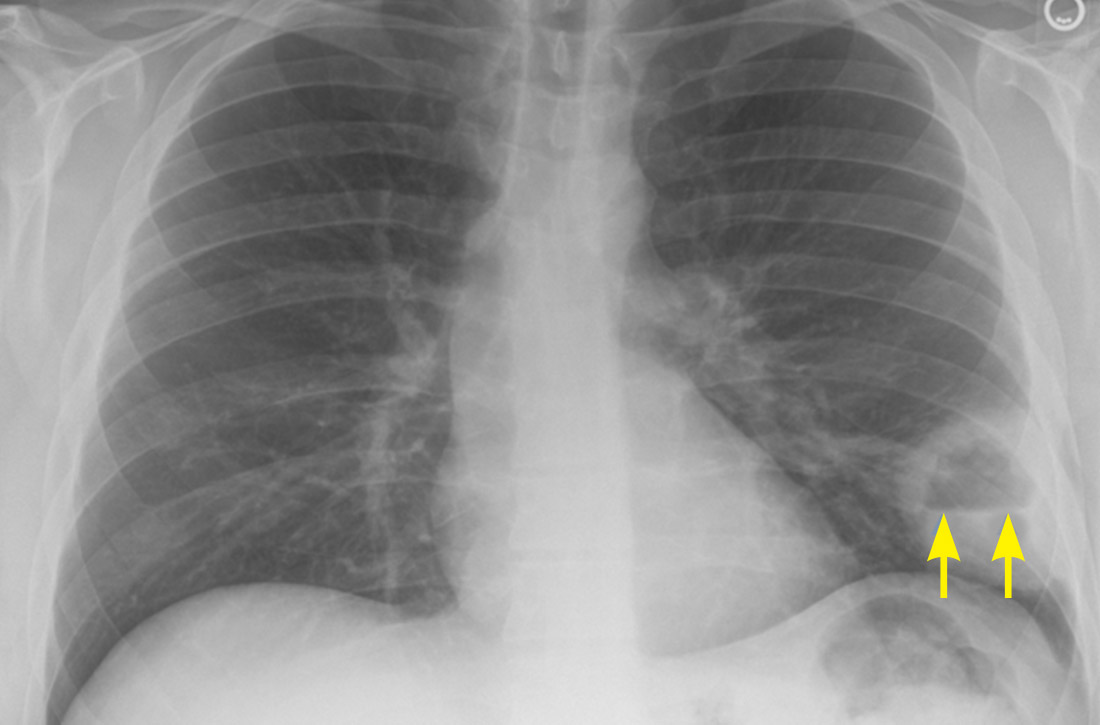
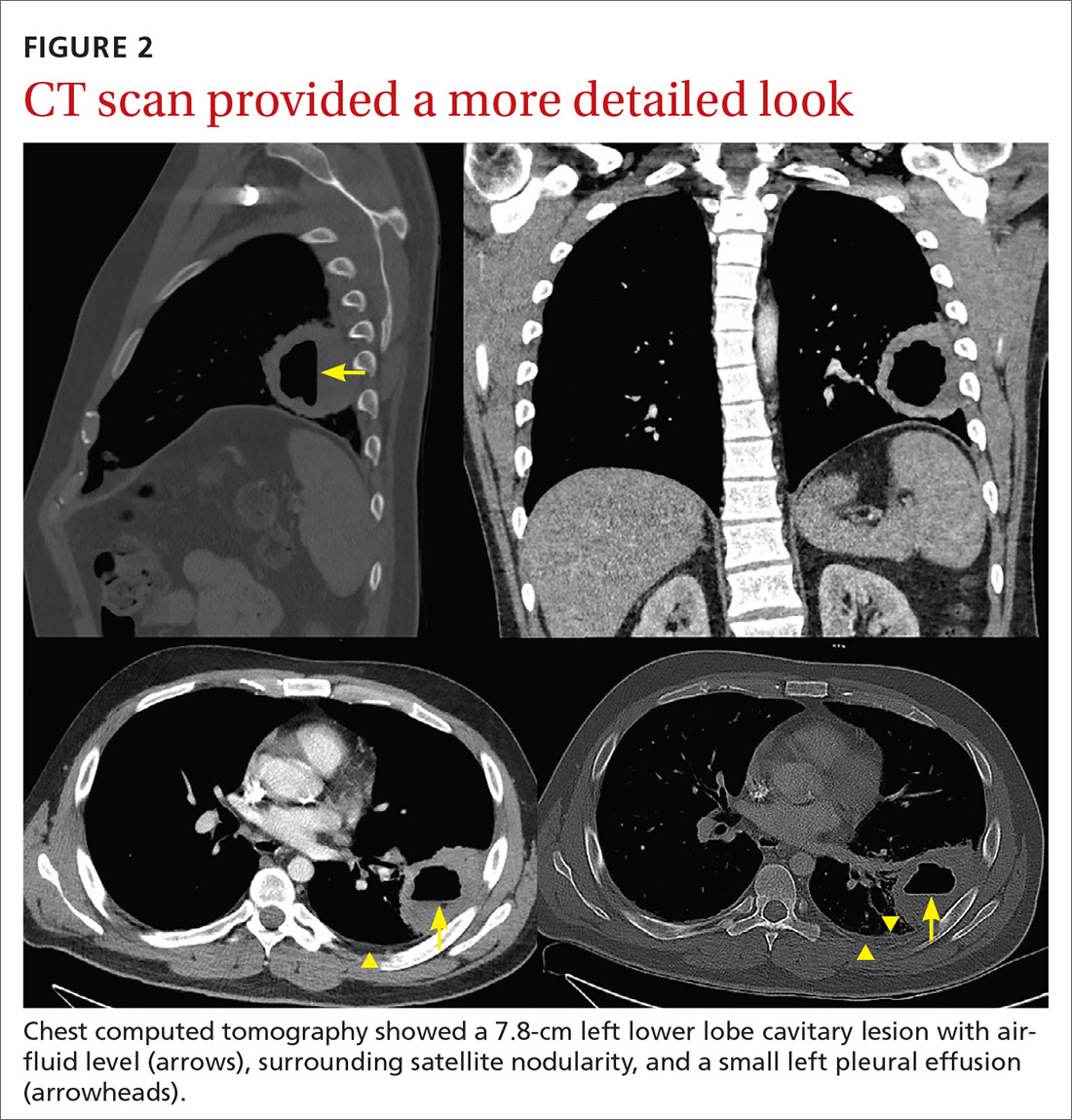
A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.
THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4
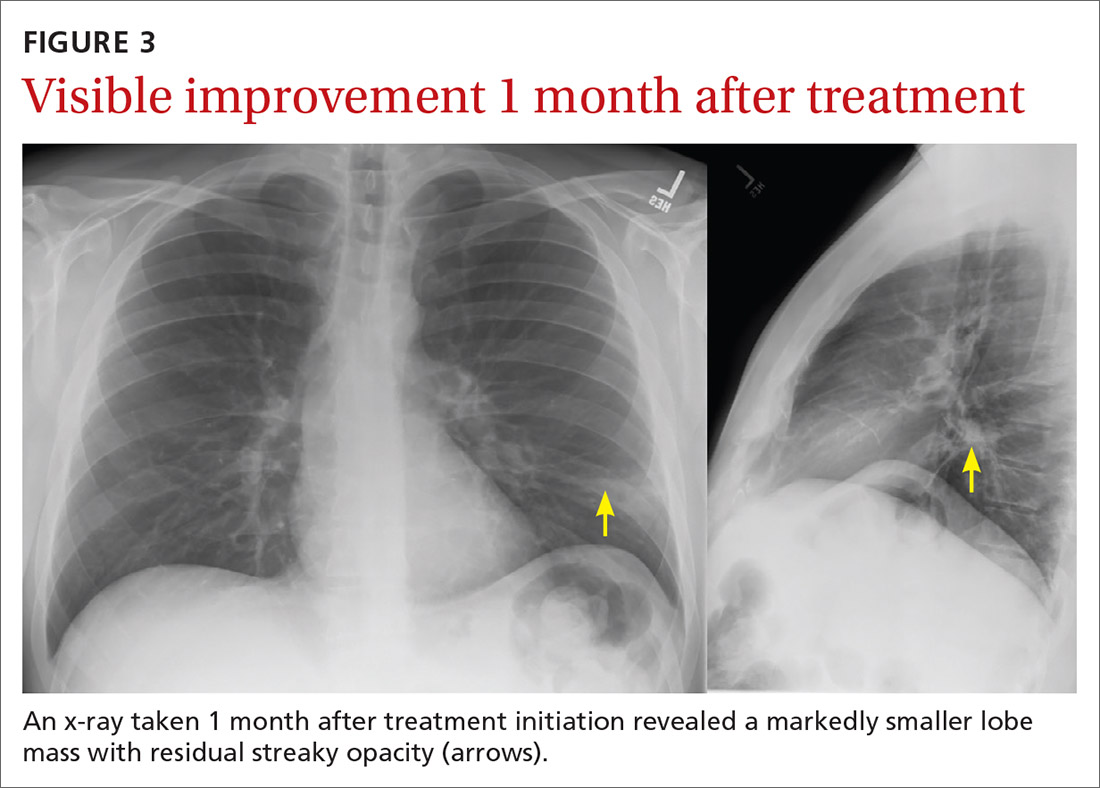
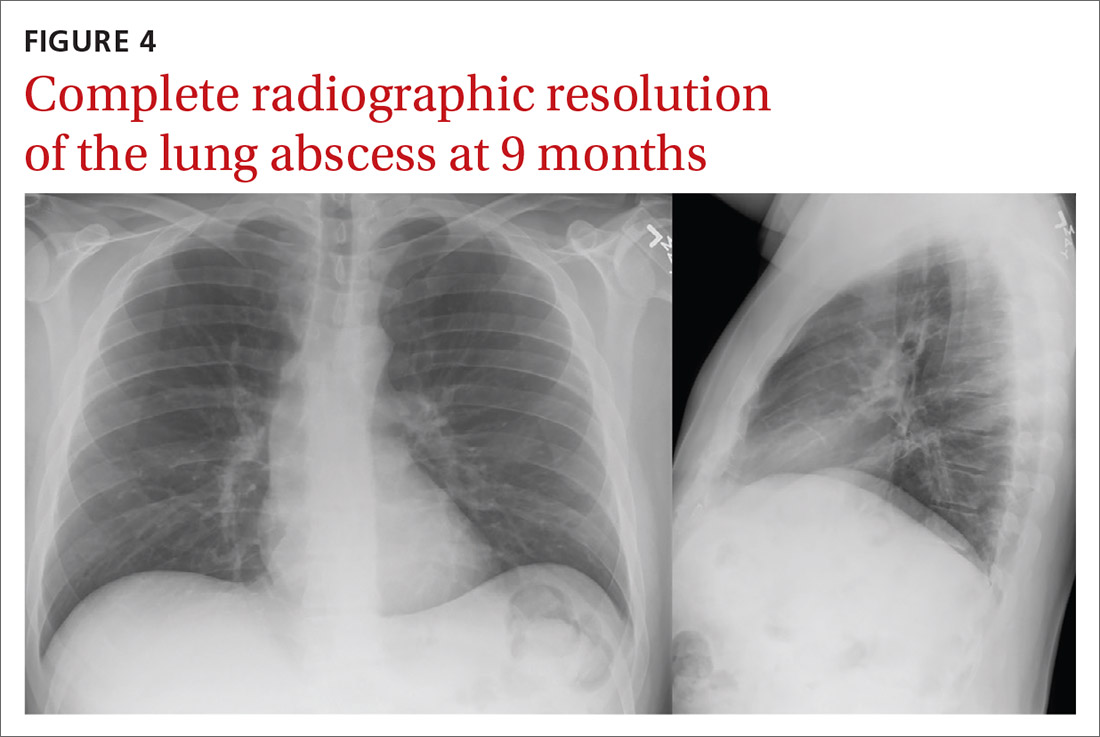
Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).
THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.

A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.

THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4

Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).

THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.

A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.

THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4

Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).

THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
Confidently rule out CAP in the outpatient setting
ILLUSTRATIVE CASE
An otherwise healthy 56-year-old woman presents to the emergency department (ED) with a productive cough of 4 days’ duration. A review of her history is negative for recurrent upper respiratory infections, smoking, or environmental exposures. Her physical exam is unremarkable and, more specifically, her pulmonary exam and vital signs (temperature, respiratory rate, and heart rate) are within normal limits. The patient states that last year her friend had similar symptoms and was given a diagnosis of pneumonia. Is it necessary to order a chest x-ray in this patient to rule out community-acquired pneumonia (CAP)?
CAP is a common pulmonary condition seen in the outpatient setting in the United States, representing more than 4.5 million outpatient visits in the years 2009 to 2010.2 Historically, a diagnosis of CAP has been based on clinical findings in conjunction with infiltrates seen on chest x-ray.
In 2017, more than 5 million visits to the ED were due to a cough.3 The use of radiographic imaging in EDs has been increasing. There were 49 million x-rays and 2.7 million noncardiac chest computed tomography (CT) scans performed in 2016, many of which were for patients with cough.3,4 Although imaging is an extremely useful tool and indicated in many instances, the ability to rule out CAP in an adult who presents with a cough by using a set of simple, clinically based heuristics without requiring imaging would help to increase efficiency, limit cost, and decrease exposure of patients to unnecessary and potentially harmful diagnostic studies.
Clinical decision rules (CDRs) are simple heuristics that can stratify patients as either high risk or low risk for specific diseases. Two older large, prospective cross-sectional studies developed CDRs to determine the probability of CAP based on symptoms (eg, night sweats, myalgias, and sputum production) and clinical findings (eg, temperature > 37.8 °C [100 °F], tachypnea, tachycardia, rales, and decreased breath sounds).5,6 This meta-analysis includes these studies and more recent studies7-9 used to develop a CDR that focuses solely on a few specific signs and symptoms that can reliably rule out CAP without imaging, and so prove highly useful for busy primary care clinicians.
STUDY SUMMARY
This simple approach rules out CAP in outpatients 99.6% of the time
This systematic review and meta-analysis included studies that used 2 or more signs, symptoms, or point-of-care tests to determine the patient’s risk for CAP.1 Twelve studies (N = 10,254) met inclusion criteria by applying a CDR to adults or adolescents presenting with respiratory signs or symptoms potentially suggestive of CAP to either an outpatient setting or an ED. Prospective cohort, cross-sectional, and case-control studies were included when a chest x-ray or CT was utilized as the primary reference standard. Exclusion criteria included studies of military or nursing home populations and studies in which the majority of patients had hospital- or ventilator-associated pneumonia or were immunocompromised.
A simple, highly useful CDR emerged from 3 of the studies (N = 1865).7-9 Two of these studies were described as case-control studies with prospective enrollment of patients older than 17 years in both outpatient and ED settings.7,8 One study was conducted in the United States (mean age, 65 years) and the other in Iran (mean age, 60 years). The third was a Chilean prospective cohort study of ED patients older than 15 years (mean age, 53 years).9 In each of these studies, the outpatient or ED physicians collected all clinical data and documented their physical exam prior to receiving the chest radiograph results. The radiologists were masked to the clinical findings at the time of their interpretation.
Results. From the meta-analysis, a simple CDR emerged for patients with normal vital signs (temperature, respiratory rate, and heart rate) and a normal pulmonary exam that virtually ruled out CAP (sensitivity = 96%; 95% CI, 92%–98%; and negative likelihood ratio = 0.10; 95% CI, 0.07–0.13). In patients presenting to an outpatient clinic with acute cough with a 4% baseline prevalence rate of pneumonia, this CDR ruled out CAP 99.6% of the time.
Continue to: WHAT'S NEW
WHAT’S NEW
A clinical decision rule validated for accuracy
This is the first validated CDR that accurately rules out CAP in the outpatient or ED setting using parameters easily obtainable during a clinical exam.
CAVEATS
Proceed with caution in the young and the very old
Two of the 3 studies in this CDR had an overall moderate risk of bias, whereas the third study was determined to be at low risk of bias, based on appraisal with the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) framework.10
The mean age range in these 3 studies was 53 to 66 years (without further data such as standard deviation), suggesting that application of the CDR to adults who fall at extremes of age should be done with a modicum of caution.
Additionally, although the symptom complex of COVID-19 pneumonia would suggest that this CDR would likely remain accurate today, it has not been validated in patients with COVID-19 infection.
CHALLENGES TO IMPLEMENTATION
Potential reluctance to forgo imaging
Beyond the caveats regarding COVID-19, the use of a simple CDR to reliably exclude pneumonia should have no barrier to implementation in an outpatient primary care setting or ED, although there could be reluctance on the part of both providers and patients to fully embrace this simple tool without a confirmatory chest x-ray.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32:234-247.
2. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88:56-67.
3. CDC. National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2017. Emergency Department Summary Tables. Accessed March 24, 2021. www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf
4. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415-427.
5. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113:664-670.
6. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis. 1984;37:215-225.
7. O’Brien WT Sr, Rohweder DA, Lattin GE Jr, et al. Clinical indicators of radiographic findings in patients with suspected community-acquired pneumonia: who needs a chest x-ray? J Am Coll Radiol. 2006;3:703-706.
8. Ebrahimzadeh A, Mohammadifard M, Naseh G, et al. Clinical and laboratory findings in patients with acute respiratory symptoms that suggest the necessity of chest x-ray for community-acquired pneumonia. Iran J Radiol. 2015;12:e13547.
9. Saldías PF, Cabrera TD, de Solminihac LI, et al. Valor predictivo de la historia clínica y examen físico en el diagnóstico de neumonía del adulto adquirida en la comunidad [Predictive value of history and physical examination for the diagnosis of community-acquired pneumonia in adults]. Abstract in English. Rev Med Chil. 2007;135:143-152.
10. Whiting PF, Rutjes AWS, Westwood ME, et al; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536.
ILLUSTRATIVE CASE
An otherwise healthy 56-year-old woman presents to the emergency department (ED) with a productive cough of 4 days’ duration. A review of her history is negative for recurrent upper respiratory infections, smoking, or environmental exposures. Her physical exam is unremarkable and, more specifically, her pulmonary exam and vital signs (temperature, respiratory rate, and heart rate) are within normal limits. The patient states that last year her friend had similar symptoms and was given a diagnosis of pneumonia. Is it necessary to order a chest x-ray in this patient to rule out community-acquired pneumonia (CAP)?
CAP is a common pulmonary condition seen in the outpatient setting in the United States, representing more than 4.5 million outpatient visits in the years 2009 to 2010.2 Historically, a diagnosis of CAP has been based on clinical findings in conjunction with infiltrates seen on chest x-ray.
In 2017, more than 5 million visits to the ED were due to a cough.3 The use of radiographic imaging in EDs has been increasing. There were 49 million x-rays and 2.7 million noncardiac chest computed tomography (CT) scans performed in 2016, many of which were for patients with cough.3,4 Although imaging is an extremely useful tool and indicated in many instances, the ability to rule out CAP in an adult who presents with a cough by using a set of simple, clinically based heuristics without requiring imaging would help to increase efficiency, limit cost, and decrease exposure of patients to unnecessary and potentially harmful diagnostic studies.
Clinical decision rules (CDRs) are simple heuristics that can stratify patients as either high risk or low risk for specific diseases. Two older large, prospective cross-sectional studies developed CDRs to determine the probability of CAP based on symptoms (eg, night sweats, myalgias, and sputum production) and clinical findings (eg, temperature > 37.8 °C [100 °F], tachypnea, tachycardia, rales, and decreased breath sounds).5,6 This meta-analysis includes these studies and more recent studies7-9 used to develop a CDR that focuses solely on a few specific signs and symptoms that can reliably rule out CAP without imaging, and so prove highly useful for busy primary care clinicians.
STUDY SUMMARY
This simple approach rules out CAP in outpatients 99.6% of the time
This systematic review and meta-analysis included studies that used 2 or more signs, symptoms, or point-of-care tests to determine the patient’s risk for CAP.1 Twelve studies (N = 10,254) met inclusion criteria by applying a CDR to adults or adolescents presenting with respiratory signs or symptoms potentially suggestive of CAP to either an outpatient setting or an ED. Prospective cohort, cross-sectional, and case-control studies were included when a chest x-ray or CT was utilized as the primary reference standard. Exclusion criteria included studies of military or nursing home populations and studies in which the majority of patients had hospital- or ventilator-associated pneumonia or were immunocompromised.
A simple, highly useful CDR emerged from 3 of the studies (N = 1865).7-9 Two of these studies were described as case-control studies with prospective enrollment of patients older than 17 years in both outpatient and ED settings.7,8 One study was conducted in the United States (mean age, 65 years) and the other in Iran (mean age, 60 years). The third was a Chilean prospective cohort study of ED patients older than 15 years (mean age, 53 years).9 In each of these studies, the outpatient or ED physicians collected all clinical data and documented their physical exam prior to receiving the chest radiograph results. The radiologists were masked to the clinical findings at the time of their interpretation.
Results. From the meta-analysis, a simple CDR emerged for patients with normal vital signs (temperature, respiratory rate, and heart rate) and a normal pulmonary exam that virtually ruled out CAP (sensitivity = 96%; 95% CI, 92%–98%; and negative likelihood ratio = 0.10; 95% CI, 0.07–0.13). In patients presenting to an outpatient clinic with acute cough with a 4% baseline prevalence rate of pneumonia, this CDR ruled out CAP 99.6% of the time.
Continue to: WHAT'S NEW
WHAT’S NEW
A clinical decision rule validated for accuracy
This is the first validated CDR that accurately rules out CAP in the outpatient or ED setting using parameters easily obtainable during a clinical exam.
CAVEATS
Proceed with caution in the young and the very old
Two of the 3 studies in this CDR had an overall moderate risk of bias, whereas the third study was determined to be at low risk of bias, based on appraisal with the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) framework.10
The mean age range in these 3 studies was 53 to 66 years (without further data such as standard deviation), suggesting that application of the CDR to adults who fall at extremes of age should be done with a modicum of caution.
Additionally, although the symptom complex of COVID-19 pneumonia would suggest that this CDR would likely remain accurate today, it has not been validated in patients with COVID-19 infection.
CHALLENGES TO IMPLEMENTATION
Potential reluctance to forgo imaging
Beyond the caveats regarding COVID-19, the use of a simple CDR to reliably exclude pneumonia should have no barrier to implementation in an outpatient primary care setting or ED, although there could be reluctance on the part of both providers and patients to fully embrace this simple tool without a confirmatory chest x-ray.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
An otherwise healthy 56-year-old woman presents to the emergency department (ED) with a productive cough of 4 days’ duration. A review of her history is negative for recurrent upper respiratory infections, smoking, or environmental exposures. Her physical exam is unremarkable and, more specifically, her pulmonary exam and vital signs (temperature, respiratory rate, and heart rate) are within normal limits. The patient states that last year her friend had similar symptoms and was given a diagnosis of pneumonia. Is it necessary to order a chest x-ray in this patient to rule out community-acquired pneumonia (CAP)?
CAP is a common pulmonary condition seen in the outpatient setting in the United States, representing more than 4.5 million outpatient visits in the years 2009 to 2010.2 Historically, a diagnosis of CAP has been based on clinical findings in conjunction with infiltrates seen on chest x-ray.
In 2017, more than 5 million visits to the ED were due to a cough.3 The use of radiographic imaging in EDs has been increasing. There were 49 million x-rays and 2.7 million noncardiac chest computed tomography (CT) scans performed in 2016, many of which were for patients with cough.3,4 Although imaging is an extremely useful tool and indicated in many instances, the ability to rule out CAP in an adult who presents with a cough by using a set of simple, clinically based heuristics without requiring imaging would help to increase efficiency, limit cost, and decrease exposure of patients to unnecessary and potentially harmful diagnostic studies.
Clinical decision rules (CDRs) are simple heuristics that can stratify patients as either high risk or low risk for specific diseases. Two older large, prospective cross-sectional studies developed CDRs to determine the probability of CAP based on symptoms (eg, night sweats, myalgias, and sputum production) and clinical findings (eg, temperature > 37.8 °C [100 °F], tachypnea, tachycardia, rales, and decreased breath sounds).5,6 This meta-analysis includes these studies and more recent studies7-9 used to develop a CDR that focuses solely on a few specific signs and symptoms that can reliably rule out CAP without imaging, and so prove highly useful for busy primary care clinicians.
STUDY SUMMARY
This simple approach rules out CAP in outpatients 99.6% of the time
This systematic review and meta-analysis included studies that used 2 or more signs, symptoms, or point-of-care tests to determine the patient’s risk for CAP.1 Twelve studies (N = 10,254) met inclusion criteria by applying a CDR to adults or adolescents presenting with respiratory signs or symptoms potentially suggestive of CAP to either an outpatient setting or an ED. Prospective cohort, cross-sectional, and case-control studies were included when a chest x-ray or CT was utilized as the primary reference standard. Exclusion criteria included studies of military or nursing home populations and studies in which the majority of patients had hospital- or ventilator-associated pneumonia or were immunocompromised.
A simple, highly useful CDR emerged from 3 of the studies (N = 1865).7-9 Two of these studies were described as case-control studies with prospective enrollment of patients older than 17 years in both outpatient and ED settings.7,8 One study was conducted in the United States (mean age, 65 years) and the other in Iran (mean age, 60 years). The third was a Chilean prospective cohort study of ED patients older than 15 years (mean age, 53 years).9 In each of these studies, the outpatient or ED physicians collected all clinical data and documented their physical exam prior to receiving the chest radiograph results. The radiologists were masked to the clinical findings at the time of their interpretation.
Results. From the meta-analysis, a simple CDR emerged for patients with normal vital signs (temperature, respiratory rate, and heart rate) and a normal pulmonary exam that virtually ruled out CAP (sensitivity = 96%; 95% CI, 92%–98%; and negative likelihood ratio = 0.10; 95% CI, 0.07–0.13). In patients presenting to an outpatient clinic with acute cough with a 4% baseline prevalence rate of pneumonia, this CDR ruled out CAP 99.6% of the time.
Continue to: WHAT'S NEW
WHAT’S NEW
A clinical decision rule validated for accuracy
This is the first validated CDR that accurately rules out CAP in the outpatient or ED setting using parameters easily obtainable during a clinical exam.
CAVEATS
Proceed with caution in the young and the very old
Two of the 3 studies in this CDR had an overall moderate risk of bias, whereas the third study was determined to be at low risk of bias, based on appraisal with the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) framework.10
The mean age range in these 3 studies was 53 to 66 years (without further data such as standard deviation), suggesting that application of the CDR to adults who fall at extremes of age should be done with a modicum of caution.
Additionally, although the symptom complex of COVID-19 pneumonia would suggest that this CDR would likely remain accurate today, it has not been validated in patients with COVID-19 infection.
CHALLENGES TO IMPLEMENTATION
Potential reluctance to forgo imaging
Beyond the caveats regarding COVID-19, the use of a simple CDR to reliably exclude pneumonia should have no barrier to implementation in an outpatient primary care setting or ED, although there could be reluctance on the part of both providers and patients to fully embrace this simple tool without a confirmatory chest x-ray.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32:234-247.
2. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88:56-67.
3. CDC. National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2017. Emergency Department Summary Tables. Accessed March 24, 2021. www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf
4. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415-427.
5. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113:664-670.
6. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis. 1984;37:215-225.
7. O’Brien WT Sr, Rohweder DA, Lattin GE Jr, et al. Clinical indicators of radiographic findings in patients with suspected community-acquired pneumonia: who needs a chest x-ray? J Am Coll Radiol. 2006;3:703-706.
8. Ebrahimzadeh A, Mohammadifard M, Naseh G, et al. Clinical and laboratory findings in patients with acute respiratory symptoms that suggest the necessity of chest x-ray for community-acquired pneumonia. Iran J Radiol. 2015;12:e13547.
9. Saldías PF, Cabrera TD, de Solminihac LI, et al. Valor predictivo de la historia clínica y examen físico en el diagnóstico de neumonía del adulto adquirida en la comunidad [Predictive value of history and physical examination for the diagnosis of community-acquired pneumonia in adults]. Abstract in English. Rev Med Chil. 2007;135:143-152.
10. Whiting PF, Rutjes AWS, Westwood ME, et al; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536.
1. Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32:234-247.
2. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88:56-67.
3. CDC. National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2017. Emergency Department Summary Tables. Accessed March 24, 2021. www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf
4. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415-427.
5. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113:664-670.
6. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis. 1984;37:215-225.
7. O’Brien WT Sr, Rohweder DA, Lattin GE Jr, et al. Clinical indicators of radiographic findings in patients with suspected community-acquired pneumonia: who needs a chest x-ray? J Am Coll Radiol. 2006;3:703-706.
8. Ebrahimzadeh A, Mohammadifard M, Naseh G, et al. Clinical and laboratory findings in patients with acute respiratory symptoms that suggest the necessity of chest x-ray for community-acquired pneumonia. Iran J Radiol. 2015;12:e13547.
9. Saldías PF, Cabrera TD, de Solminihac LI, et al. Valor predictivo de la historia clínica y examen físico en el diagnóstico de neumonía del adulto adquirida en la comunidad [Predictive value of history and physical examination for the diagnosis of community-acquired pneumonia in adults]. Abstract in English. Rev Med Chil. 2007;135:143-152.
10. Whiting PF, Rutjes AWS, Westwood ME, et al; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536.
PRACTICE CHANGER
You can safely rule out community-acquired pneumonia (CAP)—without requiring a chest x-ray—in an otherwise healthy adult outpatient who has an acute cough, a normal pulmonary exam, and normal vital signs using this simple clinical decision rule (CDR).1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review of prospective case-control studies and randomized controlled trials in the outpatient setting.1
Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32:234-247.
Integrating primary care into a community mental health center
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
Helping your obese patient achieve a healthier weight
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
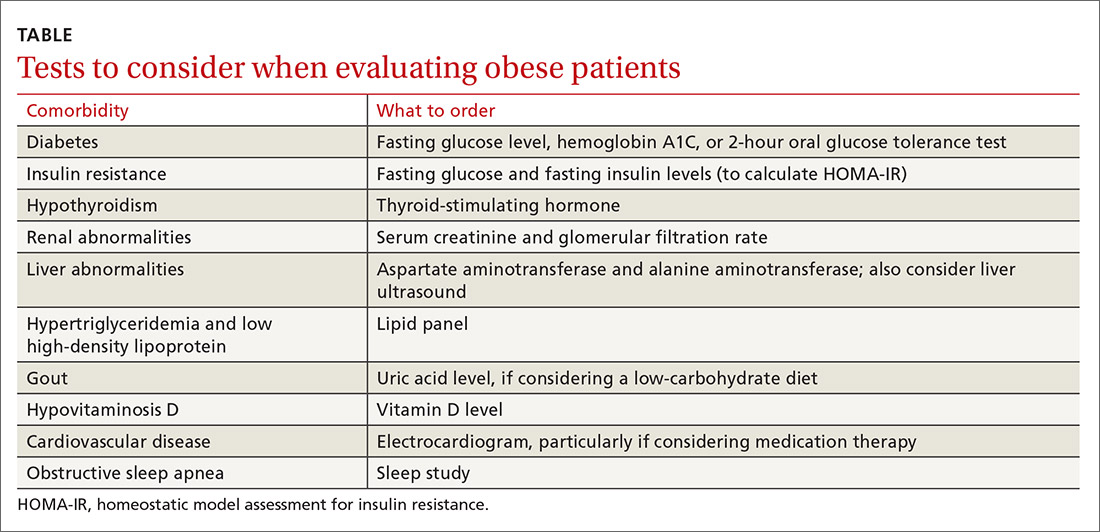
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table

Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table

Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; [email protected]
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
PRACTICE RECOMMENDATIONS
› Create an office environment where patients feel comfortable discussing their weight. C
› Screen overweight and obese patients for comorbidities. B
› Focus on nutritional changes more than exercise when working with patients who want to lose weight. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Surveillance endoscopy in Barrett’s may perform better than expected
For patients with Barrett’s esophagus, surveillance endoscopy detects high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC) more often than previously reported, according to a retrospective analysis of more than 1,000 patients.
Neoplasia detection rate, defined as findings on initial surveillance endoscopy, was also lower than that observed in past studies, according to lead author Lovekirat Dhaliwal, MBBS, of Mayo Clinic, Rochester, Minn., and colleagues.
This study’s findings may help define quality control benchmarks for endoscopic surveillance of Barrett’s esophagus, the investigators wrote in Clinical Gastroenterology and Hepatology. Accurate metrics are needed, they noted, because almost 9 out of 10 patients with Barrett’s esophagus present with EAC outside of a surveillance program, which “may represent missed opportunities at screening.” At the same time, a previous study by the investigators and one from another group, have suggested that 25%-33% of HGD/EAC cases may go undetected by initial surveillance endoscopy.
“Dysplasia detection in [Barrett’s esophagus] is challenging because of its patchy distribution and often subtle appearance,” the investigators noted. “Lack of compliance with recommended biopsy guidelines is also well-documented.”
On the other hand, Dr. Dhaliwal and colleagues suggested that previous studies may not accurately portray community practice and, therefore, have limited value in determining quality control metrics. A 2019 review, for instance, reported a neoplasia detection rate of 7% among patients with Barrett’s esophagus, but this finding “is composed of data from largely referral center cohorts with endoscopy performed by experienced academic gastroenterologists,” they wrote, which may lead to overestimation of such detection.
To better characterize this landscape, the investigators conducted a retrospective analysis involving 1,066 patients with Barrett’s esophagus who underwent initial surveillance endoscopy between 1991 and 2019. Approximately three out of four surveillance endoscopies (77%) were performed by gastroenterologists, while the remaining were performed by nongastroenterologists, such as family practitioners or surgeons. About 60% of patients were adequately biopsied according to the Seattle protocol.
Analysis revealed that the neoplasia detection rate was 4.9% (95% confidence interval, 3.8%-6.4%), which is less than the previously reported rate of 7%. HGD was more common than EAC (33 cases vs. 20 cases). Out of 1,066 patients, 391 without neoplasia on initial endoscopy underwent repeat endoscopy within a year. Among these individuals, HGD or EAC was detected in eight patients, which suggests that 13% of diagnoses were missed on initial endoscopy, a rate well below the previously reported range of 25%-33%.
Technology challenged by technique
The neoplasia detection rate “appeared to increase significantly from 1991 to 2019 on univariate analysis (particularly after 2000), but this was not observed on multivariate analysis,” the investigators wrote. “This was despite the introduction of high definition monitors and high resolution endoscopes in subsequent years.
“This may suggest that in a low dysplasia prevalence setting, basic techniques such as careful white light inspection of the [Barrett’s esophagus] mucosa along with targeted and Seattle protocol biopsies may be more important,” they noted.
The importance of technique may be further supported by another finding: Gastroenterologists detected neoplasia almost four times as often as did nongastroenterologists (odds ratio, 3.6; P = .0154).
“This finding is novel and may be due to additional training in endoscopy, lesion recognition, and familiarity with surveillance guidelines in gastroenterologists,” the investigators wrote. “If this finding is replicated in other cohorts, it may support recommendations for the performance of surveillance by endoscopists trained in gastrointestinal endoscopy and well-versed in surveillance guidelines.
“[U]sing neoplasia detection as a quality metric coupled with outcome measures such as missed dysplasia rates could improve adherence to established biopsy protocols and improve the quality of care to patients,” they wrote. “Ultimately, this can be an opportunity to develop a high-value, evidence-based quality metric in [Barrett’s esophagus] surveillance.”
The authors acknowledged some limitations to their study. Its retrospective design meant no one biopsy protocol could be adopted across the entire study period; however, the results were “unchanged” when restricted to the period after introduction of the Seattle protocol in 2000. The study’s long period could have left results susceptible to changing guidelines, but the neoplasia detection rates remained relatively stable over time.
“Because prior reports consisted largely of tertiary care center cohorts, our findings may reflect the absence of referral bias and be more generalizable,” the investigators wrote.
The study was funded by the National Institute of Aging and the National Cancer Institute. The investigators disclosed relationships with Celgene, Nine Point Medical, Takeda, and others.
The current study by Dr. Dhaliwal and colleagues evaluates the neoplasia detection rate (NDR) for high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) during surveillance endoscopy, which is a proposed novel quality metric for BE. Within a population cohort, the investigators found the NDR was 4.9%, and this did not increase significantly during the study period from 1991 to 2019. Gastroenterologists were more likely to report visible abnormalities during endoscopy and this was a significant predictor of neoplasia detection in a multivariable model. However, the overall rate of missed HGD or EAC was 13%, and this was not associated with procedural specialty. Interestingly, even with only 57% adherence to Seattle protocol in this study, there was no association with missed lesions.
Despite advances in endoscopic imaging and measures establishing quality for biopsy technique, there remains substantial room for improvement in the endoscopic management of patients with BE. While unable to evaluate all factors associated with neoplasia detection, the authors have provided an important real-world benchmark for NDR. Further study is needed to establish the connection between NDR and missed dysplasia, as well as its impact on outcomes such as EAC staging and mortality. Critically, understanding the role of specialized training and other factors such as inspection time to improve NDR is needed.
David A. Leiman, MD, MSHP, is the chair of the AGA Quality Committee. He is an assistant professor of medicine at Duke University, Durham, N.C., where he serves as director of esophageal research and quality. He has no conflicts.
The current study by Dr. Dhaliwal and colleagues evaluates the neoplasia detection rate (NDR) for high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) during surveillance endoscopy, which is a proposed novel quality metric for BE. Within a population cohort, the investigators found the NDR was 4.9%, and this did not increase significantly during the study period from 1991 to 2019. Gastroenterologists were more likely to report visible abnormalities during endoscopy and this was a significant predictor of neoplasia detection in a multivariable model. However, the overall rate of missed HGD or EAC was 13%, and this was not associated with procedural specialty. Interestingly, even with only 57% adherence to Seattle protocol in this study, there was no association with missed lesions.
Despite advances in endoscopic imaging and measures establishing quality for biopsy technique, there remains substantial room for improvement in the endoscopic management of patients with BE. While unable to evaluate all factors associated with neoplasia detection, the authors have provided an important real-world benchmark for NDR. Further study is needed to establish the connection between NDR and missed dysplasia, as well as its impact on outcomes such as EAC staging and mortality. Critically, understanding the role of specialized training and other factors such as inspection time to improve NDR is needed.
David A. Leiman, MD, MSHP, is the chair of the AGA Quality Committee. He is an assistant professor of medicine at Duke University, Durham, N.C., where he serves as director of esophageal research and quality. He has no conflicts.
The current study by Dr. Dhaliwal and colleagues evaluates the neoplasia detection rate (NDR) for high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC) during surveillance endoscopy, which is a proposed novel quality metric for BE. Within a population cohort, the investigators found the NDR was 4.9%, and this did not increase significantly during the study period from 1991 to 2019. Gastroenterologists were more likely to report visible abnormalities during endoscopy and this was a significant predictor of neoplasia detection in a multivariable model. However, the overall rate of missed HGD or EAC was 13%, and this was not associated with procedural specialty. Interestingly, even with only 57% adherence to Seattle protocol in this study, there was no association with missed lesions.
Despite advances in endoscopic imaging and measures establishing quality for biopsy technique, there remains substantial room for improvement in the endoscopic management of patients with BE. While unable to evaluate all factors associated with neoplasia detection, the authors have provided an important real-world benchmark for NDR. Further study is needed to establish the connection between NDR and missed dysplasia, as well as its impact on outcomes such as EAC staging and mortality. Critically, understanding the role of specialized training and other factors such as inspection time to improve NDR is needed.
David A. Leiman, MD, MSHP, is the chair of the AGA Quality Committee. He is an assistant professor of medicine at Duke University, Durham, N.C., where he serves as director of esophageal research and quality. He has no conflicts.
For patients with Barrett’s esophagus, surveillance endoscopy detects high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC) more often than previously reported, according to a retrospective analysis of more than 1,000 patients.
Neoplasia detection rate, defined as findings on initial surveillance endoscopy, was also lower than that observed in past studies, according to lead author Lovekirat Dhaliwal, MBBS, of Mayo Clinic, Rochester, Minn., and colleagues.
This study’s findings may help define quality control benchmarks for endoscopic surveillance of Barrett’s esophagus, the investigators wrote in Clinical Gastroenterology and Hepatology. Accurate metrics are needed, they noted, because almost 9 out of 10 patients with Barrett’s esophagus present with EAC outside of a surveillance program, which “may represent missed opportunities at screening.” At the same time, a previous study by the investigators and one from another group, have suggested that 25%-33% of HGD/EAC cases may go undetected by initial surveillance endoscopy.
“Dysplasia detection in [Barrett’s esophagus] is challenging because of its patchy distribution and often subtle appearance,” the investigators noted. “Lack of compliance with recommended biopsy guidelines is also well-documented.”
On the other hand, Dr. Dhaliwal and colleagues suggested that previous studies may not accurately portray community practice and, therefore, have limited value in determining quality control metrics. A 2019 review, for instance, reported a neoplasia detection rate of 7% among patients with Barrett’s esophagus, but this finding “is composed of data from largely referral center cohorts with endoscopy performed by experienced academic gastroenterologists,” they wrote, which may lead to overestimation of such detection.
To better characterize this landscape, the investigators conducted a retrospective analysis involving 1,066 patients with Barrett’s esophagus who underwent initial surveillance endoscopy between 1991 and 2019. Approximately three out of four surveillance endoscopies (77%) were performed by gastroenterologists, while the remaining were performed by nongastroenterologists, such as family practitioners or surgeons. About 60% of patients were adequately biopsied according to the Seattle protocol.
Analysis revealed that the neoplasia detection rate was 4.9% (95% confidence interval, 3.8%-6.4%), which is less than the previously reported rate of 7%. HGD was more common than EAC (33 cases vs. 20 cases). Out of 1,066 patients, 391 without neoplasia on initial endoscopy underwent repeat endoscopy within a year. Among these individuals, HGD or EAC was detected in eight patients, which suggests that 13% of diagnoses were missed on initial endoscopy, a rate well below the previously reported range of 25%-33%.
Technology challenged by technique
The neoplasia detection rate “appeared to increase significantly from 1991 to 2019 on univariate analysis (particularly after 2000), but this was not observed on multivariate analysis,” the investigators wrote. “This was despite the introduction of high definition monitors and high resolution endoscopes in subsequent years.
“This may suggest that in a low dysplasia prevalence setting, basic techniques such as careful white light inspection of the [Barrett’s esophagus] mucosa along with targeted and Seattle protocol biopsies may be more important,” they noted.
The importance of technique may be further supported by another finding: Gastroenterologists detected neoplasia almost four times as often as did nongastroenterologists (odds ratio, 3.6; P = .0154).
“This finding is novel and may be due to additional training in endoscopy, lesion recognition, and familiarity with surveillance guidelines in gastroenterologists,” the investigators wrote. “If this finding is replicated in other cohorts, it may support recommendations for the performance of surveillance by endoscopists trained in gastrointestinal endoscopy and well-versed in surveillance guidelines.
“[U]sing neoplasia detection as a quality metric coupled with outcome measures such as missed dysplasia rates could improve adherence to established biopsy protocols and improve the quality of care to patients,” they wrote. “Ultimately, this can be an opportunity to develop a high-value, evidence-based quality metric in [Barrett’s esophagus] surveillance.”
The authors acknowledged some limitations to their study. Its retrospective design meant no one biopsy protocol could be adopted across the entire study period; however, the results were “unchanged” when restricted to the period after introduction of the Seattle protocol in 2000. The study’s long period could have left results susceptible to changing guidelines, but the neoplasia detection rates remained relatively stable over time.
“Because prior reports consisted largely of tertiary care center cohorts, our findings may reflect the absence of referral bias and be more generalizable,” the investigators wrote.
The study was funded by the National Institute of Aging and the National Cancer Institute. The investigators disclosed relationships with Celgene, Nine Point Medical, Takeda, and others.
For patients with Barrett’s esophagus, surveillance endoscopy detects high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC) more often than previously reported, according to a retrospective analysis of more than 1,000 patients.
Neoplasia detection rate, defined as findings on initial surveillance endoscopy, was also lower than that observed in past studies, according to lead author Lovekirat Dhaliwal, MBBS, of Mayo Clinic, Rochester, Minn., and colleagues.
This study’s findings may help define quality control benchmarks for endoscopic surveillance of Barrett’s esophagus, the investigators wrote in Clinical Gastroenterology and Hepatology. Accurate metrics are needed, they noted, because almost 9 out of 10 patients with Barrett’s esophagus present with EAC outside of a surveillance program, which “may represent missed opportunities at screening.” At the same time, a previous study by the investigators and one from another group, have suggested that 25%-33% of HGD/EAC cases may go undetected by initial surveillance endoscopy.
“Dysplasia detection in [Barrett’s esophagus] is challenging because of its patchy distribution and often subtle appearance,” the investigators noted. “Lack of compliance with recommended biopsy guidelines is also well-documented.”
On the other hand, Dr. Dhaliwal and colleagues suggested that previous studies may not accurately portray community practice and, therefore, have limited value in determining quality control metrics. A 2019 review, for instance, reported a neoplasia detection rate of 7% among patients with Barrett’s esophagus, but this finding “is composed of data from largely referral center cohorts with endoscopy performed by experienced academic gastroenterologists,” they wrote, which may lead to overestimation of such detection.
To better characterize this landscape, the investigators conducted a retrospective analysis involving 1,066 patients with Barrett’s esophagus who underwent initial surveillance endoscopy between 1991 and 2019. Approximately three out of four surveillance endoscopies (77%) were performed by gastroenterologists, while the remaining were performed by nongastroenterologists, such as family practitioners or surgeons. About 60% of patients were adequately biopsied according to the Seattle protocol.
Analysis revealed that the neoplasia detection rate was 4.9% (95% confidence interval, 3.8%-6.4%), which is less than the previously reported rate of 7%. HGD was more common than EAC (33 cases vs. 20 cases). Out of 1,066 patients, 391 without neoplasia on initial endoscopy underwent repeat endoscopy within a year. Among these individuals, HGD or EAC was detected in eight patients, which suggests that 13% of diagnoses were missed on initial endoscopy, a rate well below the previously reported range of 25%-33%.
Technology challenged by technique
The neoplasia detection rate “appeared to increase significantly from 1991 to 2019 on univariate analysis (particularly after 2000), but this was not observed on multivariate analysis,” the investigators wrote. “This was despite the introduction of high definition monitors and high resolution endoscopes in subsequent years.
“This may suggest that in a low dysplasia prevalence setting, basic techniques such as careful white light inspection of the [Barrett’s esophagus] mucosa along with targeted and Seattle protocol biopsies may be more important,” they noted.
The importance of technique may be further supported by another finding: Gastroenterologists detected neoplasia almost four times as often as did nongastroenterologists (odds ratio, 3.6; P = .0154).
“This finding is novel and may be due to additional training in endoscopy, lesion recognition, and familiarity with surveillance guidelines in gastroenterologists,” the investigators wrote. “If this finding is replicated in other cohorts, it may support recommendations for the performance of surveillance by endoscopists trained in gastrointestinal endoscopy and well-versed in surveillance guidelines.
“[U]sing neoplasia detection as a quality metric coupled with outcome measures such as missed dysplasia rates could improve adherence to established biopsy protocols and improve the quality of care to patients,” they wrote. “Ultimately, this can be an opportunity to develop a high-value, evidence-based quality metric in [Barrett’s esophagus] surveillance.”
The authors acknowledged some limitations to their study. Its retrospective design meant no one biopsy protocol could be adopted across the entire study period; however, the results were “unchanged” when restricted to the period after introduction of the Seattle protocol in 2000. The study’s long period could have left results susceptible to changing guidelines, but the neoplasia detection rates remained relatively stable over time.
“Because prior reports consisted largely of tertiary care center cohorts, our findings may reflect the absence of referral bias and be more generalizable,” the investigators wrote.
The study was funded by the National Institute of Aging and the National Cancer Institute. The investigators disclosed relationships with Celgene, Nine Point Medical, Takeda, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Increased cancer risk from night shift due to gene dysregulation?
Working night shifts has been associated with an increased risk for certain cancers, as well as other health disorders. Indeed, the World Health Organization’s International Agency for Research on Cancer (IARC) has classified night shift work as “probably carcinogenic to humans.”
But why night shift should elevate the risk for cancer has been unclear.
A new study shows that a simulated night shift schedule significantly altered the normal circadian rhythmicity of genes that are involved in cancer hallmark pathways. It also found that this circadian misalignment caused circadian dysregulation of genes involved in key DNA repair pathways.
“Taken together, these findings suggest that night shift schedules throw off the timing of expression of cancer-related genes in a way that reduces the effectiveness of the body’s DNA repair processes when they are most needed,” said co-corresponding author Jason McDermott, a computational scientist with the Pacific Northwest National Laboratory’s biological sciences division in Richland, Wash.
The study was published online in the Journal of Pineal Research.
Study conducted among volunteers
The study was carried out among healthy volunteers who were subjected to simulated night shift or day shift schedules.
The cohort comprised 14 adults between the ages of 22 and 34 years who had normal nighttime sleep schedules. They were randomly assigned (seven in each group) to a simulated day shift schedule that involved 3 days of daytime wakefulness (6 a.m.-10 p.m.), or a simulated night shift schedule involving 3 days of nighttime wakefulness (6 p.m.-10 a.m.).
After the 3 days of simulated shift work, all participants were then kept in a constant routine protocol (used to study humans’ internally generated biological rhythms independent of any external influences). As part of the protocol, they were kept awake for 24 hours in a semi-reclined posture under laboratory conditions with constant light exposure and room temperature and evenly distributed food intake (hourly isocaloric snacks).
Blood samples were collected at 3-hour intervals and used for leukocyte transcriptome analysis and DNA damage assessment.
The authors found that the circadian expression of canonical clock genes was substantially altered by the simulated night shift schedule vs. the day shift schedule. Four genes (CRY1, CRY2, PER2, and NR1D2) lost their normal day-shift rhythmicity following the night shift schedule, and NPAS2 gene expression was not rhythmic during the day shift but exhibited circadian rhythmicity in the simulated night shift condition. Three other genes (NR1D1, PER3, and DBP) were significantly rhythmic during both shifts.
The team also looked at the effect of night shift on circadian rhythmicity in cancer hallmark genes, using a panel of 726 genes. The analysis showed that:
- 257 (35.4%) were rhythmic after at least one of the two simulated shift work conditions.
- 113 (15.6%) were rhythmic in day shift only.
- 96 (13.2%) were rhythmic during night shift only.
- 48 (6.6%) were rhythmic during both shifts.
A subset of 10 (1.4%) genes exhibited a significant phase advance (3.7 to 8.3 hours) or phase delay (2.8 to 7.0 hours) during the night shift vs. the day shift.
Thus, the authors concluded, shift work caused significant disturbances in the rhythmicity of gene expression in cancer hallmark pathways.
Findings also showed that night shift work increases endogenous and exogenous DNA damage. Endogenous DNA damage was generally higher after the night shift compared to the day shift, and across the 24-hour constant routine the percentage of cells with BRCA1 and g H2AX foci was significantly higher for night shift.
Next steps
The team said that the next step is to conduct the same experiment with real-world shift workers who have been consistently on day or night shifts for many years to determine whether in night workers the unrepaired DNA damage builds up over time, which could ultimately increase the risk for cancer.
If what happens in real-world shift workers is consistent with the current findings, this work could eventually be used to develop prevention strategies and drugs that could address the mistiming of DNA repair processes, they suggested.
“Night shift workers face considerable health disparities, ranging from increased risks of metabolic and cardiovascular disease to mental health disorders and cancer,” co-senior author Hans Van Dongen, PhD, a professor at Washington State University in Pullman and director of the WSU Sleep and Performance Research Center, Spokane, said in a statement. “It is high time that we find diagnosis and treatment solutions for this underserved group of essential workers so that the medical community can address their unique health challenges.”
The study was supported by start-up funds from Washington State University and a Center for Human Health and the Environment grant from North Carolina State University, and in part by the United States Army Medical Research and Development Command, the National Institutes of Health, CDMRP (Congressionally Directed Medical Research Programs) Peer Reviewed Cancer Research Program award, and the BRAVE investment.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Working night shifts has been associated with an increased risk for certain cancers, as well as other health disorders. Indeed, the World Health Organization’s International Agency for Research on Cancer (IARC) has classified night shift work as “probably carcinogenic to humans.”
But why night shift should elevate the risk for cancer has been unclear.
A new study shows that a simulated night shift schedule significantly altered the normal circadian rhythmicity of genes that are involved in cancer hallmark pathways. It also found that this circadian misalignment caused circadian dysregulation of genes involved in key DNA repair pathways.
“Taken together, these findings suggest that night shift schedules throw off the timing of expression of cancer-related genes in a way that reduces the effectiveness of the body’s DNA repair processes when they are most needed,” said co-corresponding author Jason McDermott, a computational scientist with the Pacific Northwest National Laboratory’s biological sciences division in Richland, Wash.
The study was published online in the Journal of Pineal Research.
Study conducted among volunteers
The study was carried out among healthy volunteers who were subjected to simulated night shift or day shift schedules.
The cohort comprised 14 adults between the ages of 22 and 34 years who had normal nighttime sleep schedules. They were randomly assigned (seven in each group) to a simulated day shift schedule that involved 3 days of daytime wakefulness (6 a.m.-10 p.m.), or a simulated night shift schedule involving 3 days of nighttime wakefulness (6 p.m.-10 a.m.).
After the 3 days of simulated shift work, all participants were then kept in a constant routine protocol (used to study humans’ internally generated biological rhythms independent of any external influences). As part of the protocol, they were kept awake for 24 hours in a semi-reclined posture under laboratory conditions with constant light exposure and room temperature and evenly distributed food intake (hourly isocaloric snacks).
Blood samples were collected at 3-hour intervals and used for leukocyte transcriptome analysis and DNA damage assessment.
The authors found that the circadian expression of canonical clock genes was substantially altered by the simulated night shift schedule vs. the day shift schedule. Four genes (CRY1, CRY2, PER2, and NR1D2) lost their normal day-shift rhythmicity following the night shift schedule, and NPAS2 gene expression was not rhythmic during the day shift but exhibited circadian rhythmicity in the simulated night shift condition. Three other genes (NR1D1, PER3, and DBP) were significantly rhythmic during both shifts.
The team also looked at the effect of night shift on circadian rhythmicity in cancer hallmark genes, using a panel of 726 genes. The analysis showed that:
- 257 (35.4%) were rhythmic after at least one of the two simulated shift work conditions.
- 113 (15.6%) were rhythmic in day shift only.
- 96 (13.2%) were rhythmic during night shift only.
- 48 (6.6%) were rhythmic during both shifts.
A subset of 10 (1.4%) genes exhibited a significant phase advance (3.7 to 8.3 hours) or phase delay (2.8 to 7.0 hours) during the night shift vs. the day shift.
Thus, the authors concluded, shift work caused significant disturbances in the rhythmicity of gene expression in cancer hallmark pathways.
Findings also showed that night shift work increases endogenous and exogenous DNA damage. Endogenous DNA damage was generally higher after the night shift compared to the day shift, and across the 24-hour constant routine the percentage of cells with BRCA1 and g H2AX foci was significantly higher for night shift.
Next steps
The team said that the next step is to conduct the same experiment with real-world shift workers who have been consistently on day or night shifts for many years to determine whether in night workers the unrepaired DNA damage builds up over time, which could ultimately increase the risk for cancer.
If what happens in real-world shift workers is consistent with the current findings, this work could eventually be used to develop prevention strategies and drugs that could address the mistiming of DNA repair processes, they suggested.
“Night shift workers face considerable health disparities, ranging from increased risks of metabolic and cardiovascular disease to mental health disorders and cancer,” co-senior author Hans Van Dongen, PhD, a professor at Washington State University in Pullman and director of the WSU Sleep and Performance Research Center, Spokane, said in a statement. “It is high time that we find diagnosis and treatment solutions for this underserved group of essential workers so that the medical community can address their unique health challenges.”
The study was supported by start-up funds from Washington State University and a Center for Human Health and the Environment grant from North Carolina State University, and in part by the United States Army Medical Research and Development Command, the National Institutes of Health, CDMRP (Congressionally Directed Medical Research Programs) Peer Reviewed Cancer Research Program award, and the BRAVE investment.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Working night shifts has been associated with an increased risk for certain cancers, as well as other health disorders. Indeed, the World Health Organization’s International Agency for Research on Cancer (IARC) has classified night shift work as “probably carcinogenic to humans.”
But why night shift should elevate the risk for cancer has been unclear.
A new study shows that a simulated night shift schedule significantly altered the normal circadian rhythmicity of genes that are involved in cancer hallmark pathways. It also found that this circadian misalignment caused circadian dysregulation of genes involved in key DNA repair pathways.
“Taken together, these findings suggest that night shift schedules throw off the timing of expression of cancer-related genes in a way that reduces the effectiveness of the body’s DNA repair processes when they are most needed,” said co-corresponding author Jason McDermott, a computational scientist with the Pacific Northwest National Laboratory’s biological sciences division in Richland, Wash.
The study was published online in the Journal of Pineal Research.
Study conducted among volunteers
The study was carried out among healthy volunteers who were subjected to simulated night shift or day shift schedules.
The cohort comprised 14 adults between the ages of 22 and 34 years who had normal nighttime sleep schedules. They were randomly assigned (seven in each group) to a simulated day shift schedule that involved 3 days of daytime wakefulness (6 a.m.-10 p.m.), or a simulated night shift schedule involving 3 days of nighttime wakefulness (6 p.m.-10 a.m.).
After the 3 days of simulated shift work, all participants were then kept in a constant routine protocol (used to study humans’ internally generated biological rhythms independent of any external influences). As part of the protocol, they were kept awake for 24 hours in a semi-reclined posture under laboratory conditions with constant light exposure and room temperature and evenly distributed food intake (hourly isocaloric snacks).
Blood samples were collected at 3-hour intervals and used for leukocyte transcriptome analysis and DNA damage assessment.
The authors found that the circadian expression of canonical clock genes was substantially altered by the simulated night shift schedule vs. the day shift schedule. Four genes (CRY1, CRY2, PER2, and NR1D2) lost their normal day-shift rhythmicity following the night shift schedule, and NPAS2 gene expression was not rhythmic during the day shift but exhibited circadian rhythmicity in the simulated night shift condition. Three other genes (NR1D1, PER3, and DBP) were significantly rhythmic during both shifts.
The team also looked at the effect of night shift on circadian rhythmicity in cancer hallmark genes, using a panel of 726 genes. The analysis showed that:
- 257 (35.4%) were rhythmic after at least one of the two simulated shift work conditions.
- 113 (15.6%) were rhythmic in day shift only.
- 96 (13.2%) were rhythmic during night shift only.
- 48 (6.6%) were rhythmic during both shifts.
A subset of 10 (1.4%) genes exhibited a significant phase advance (3.7 to 8.3 hours) or phase delay (2.8 to 7.0 hours) during the night shift vs. the day shift.
Thus, the authors concluded, shift work caused significant disturbances in the rhythmicity of gene expression in cancer hallmark pathways.
Findings also showed that night shift work increases endogenous and exogenous DNA damage. Endogenous DNA damage was generally higher after the night shift compared to the day shift, and across the 24-hour constant routine the percentage of cells with BRCA1 and g H2AX foci was significantly higher for night shift.
Next steps
The team said that the next step is to conduct the same experiment with real-world shift workers who have been consistently on day or night shifts for many years to determine whether in night workers the unrepaired DNA damage builds up over time, which could ultimately increase the risk for cancer.
If what happens in real-world shift workers is consistent with the current findings, this work could eventually be used to develop prevention strategies and drugs that could address the mistiming of DNA repair processes, they suggested.
“Night shift workers face considerable health disparities, ranging from increased risks of metabolic and cardiovascular disease to mental health disorders and cancer,” co-senior author Hans Van Dongen, PhD, a professor at Washington State University in Pullman and director of the WSU Sleep and Performance Research Center, Spokane, said in a statement. “It is high time that we find diagnosis and treatment solutions for this underserved group of essential workers so that the medical community can address their unique health challenges.”
The study was supported by start-up funds from Washington State University and a Center for Human Health and the Environment grant from North Carolina State University, and in part by the United States Army Medical Research and Development Command, the National Institutes of Health, CDMRP (Congressionally Directed Medical Research Programs) Peer Reviewed Cancer Research Program award, and the BRAVE investment.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can exercise prevent cognitive decline in patients with early Parkinson’s disease?
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY