User login
‘Beyond a reasonable doubt’: COVID-19 brain health fallout is real, severe
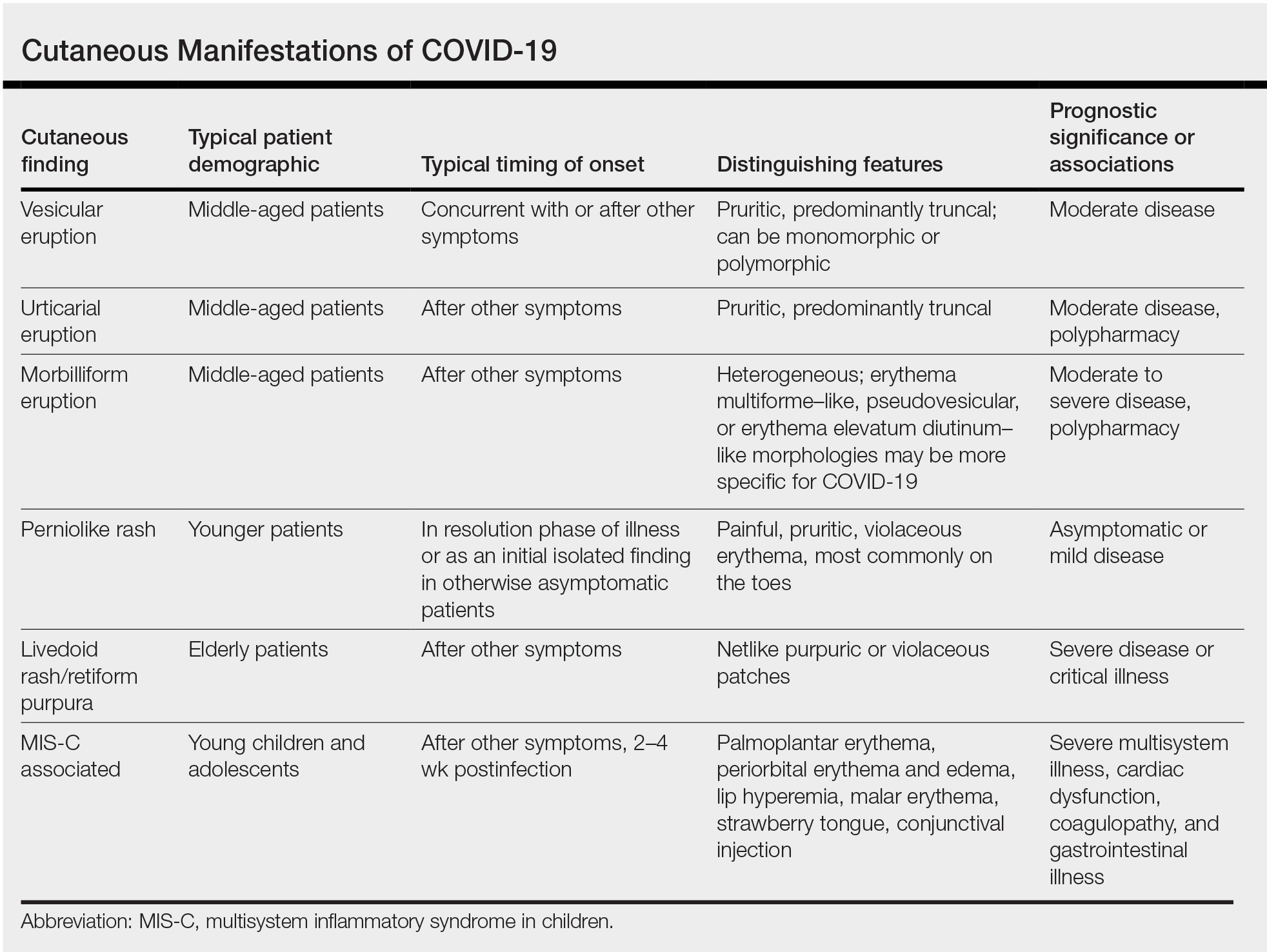
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
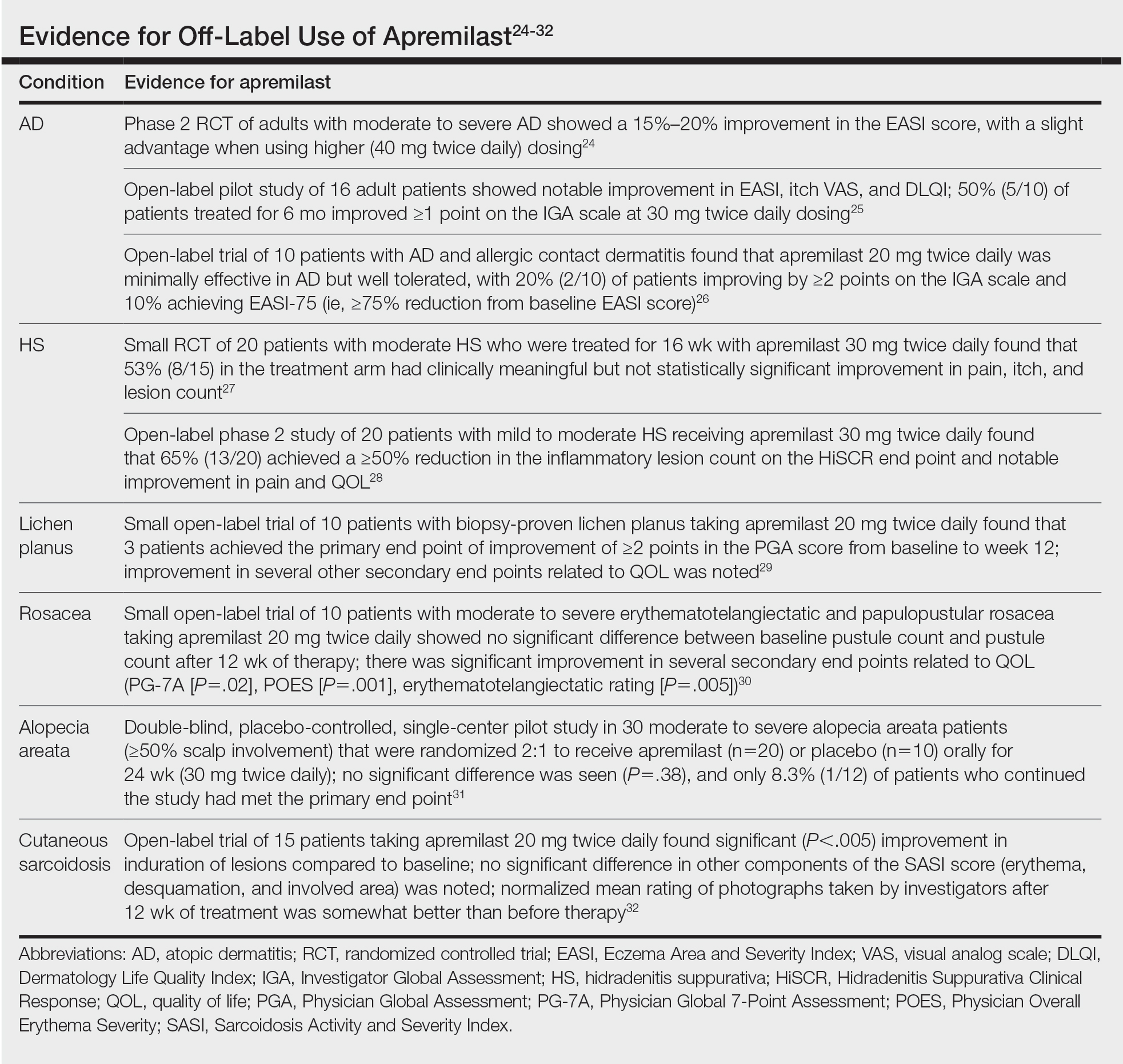
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
About one in five clinicians considers quitting because of pandemic
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
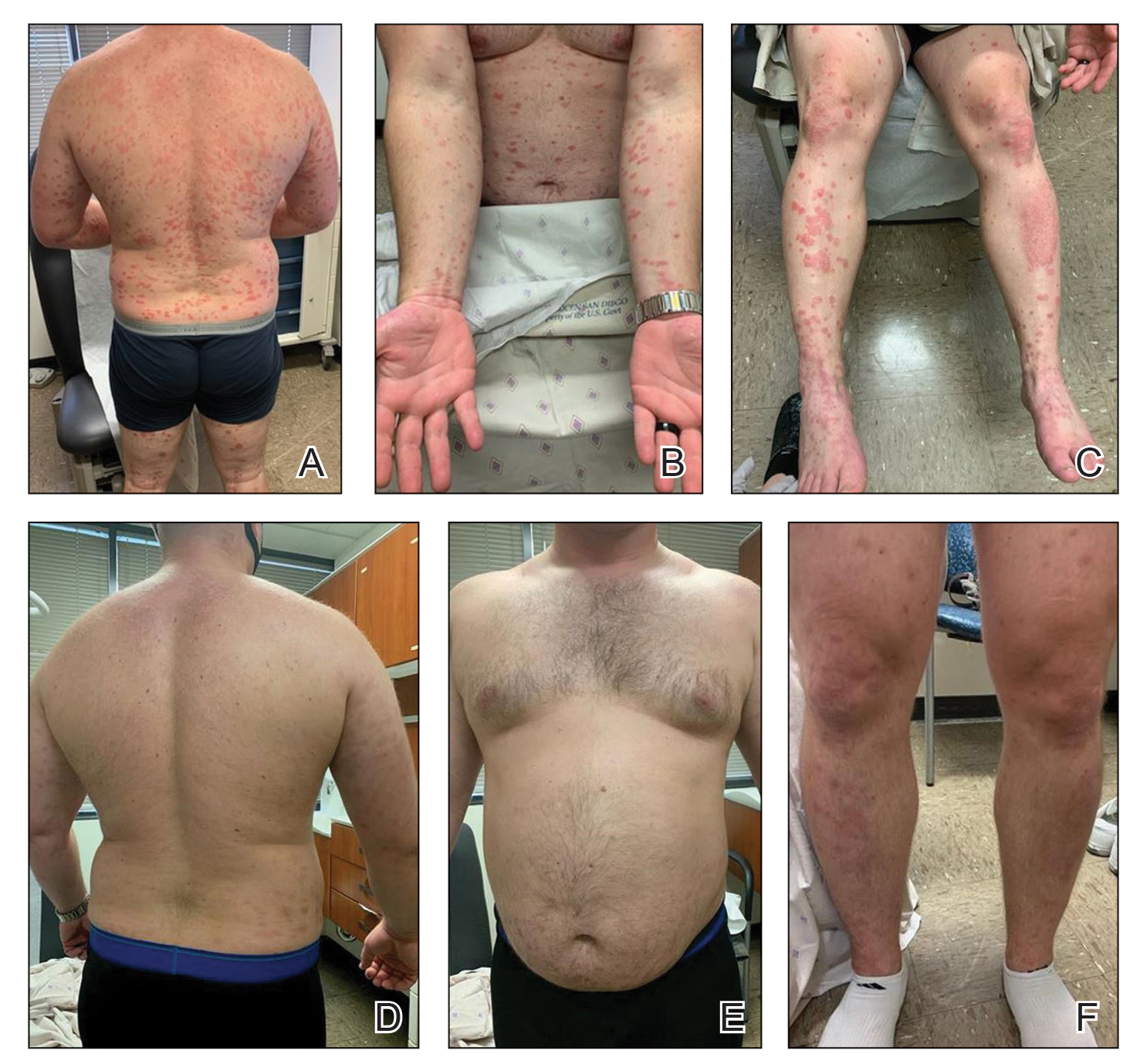
Squamous Cell Carcinoma in Hidradenitis Suppurativa Lesions Following Tumor Necrosis Factor α Inhibitors
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
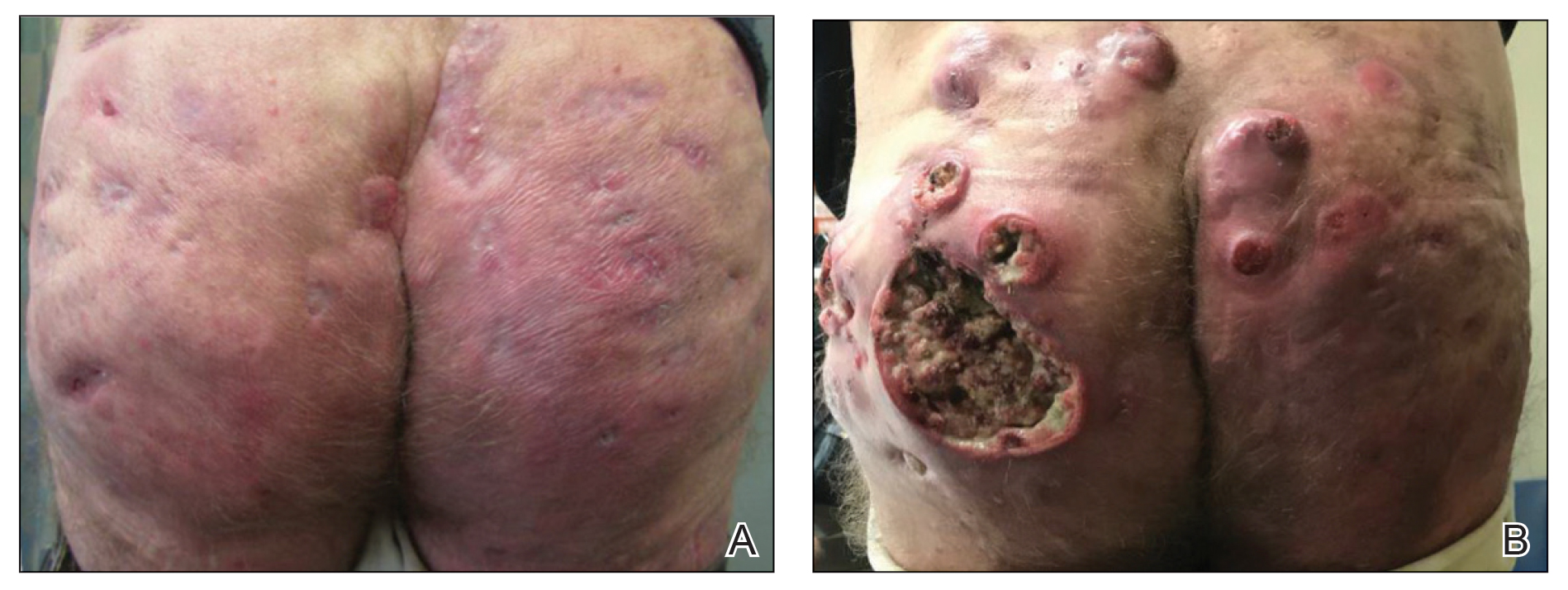
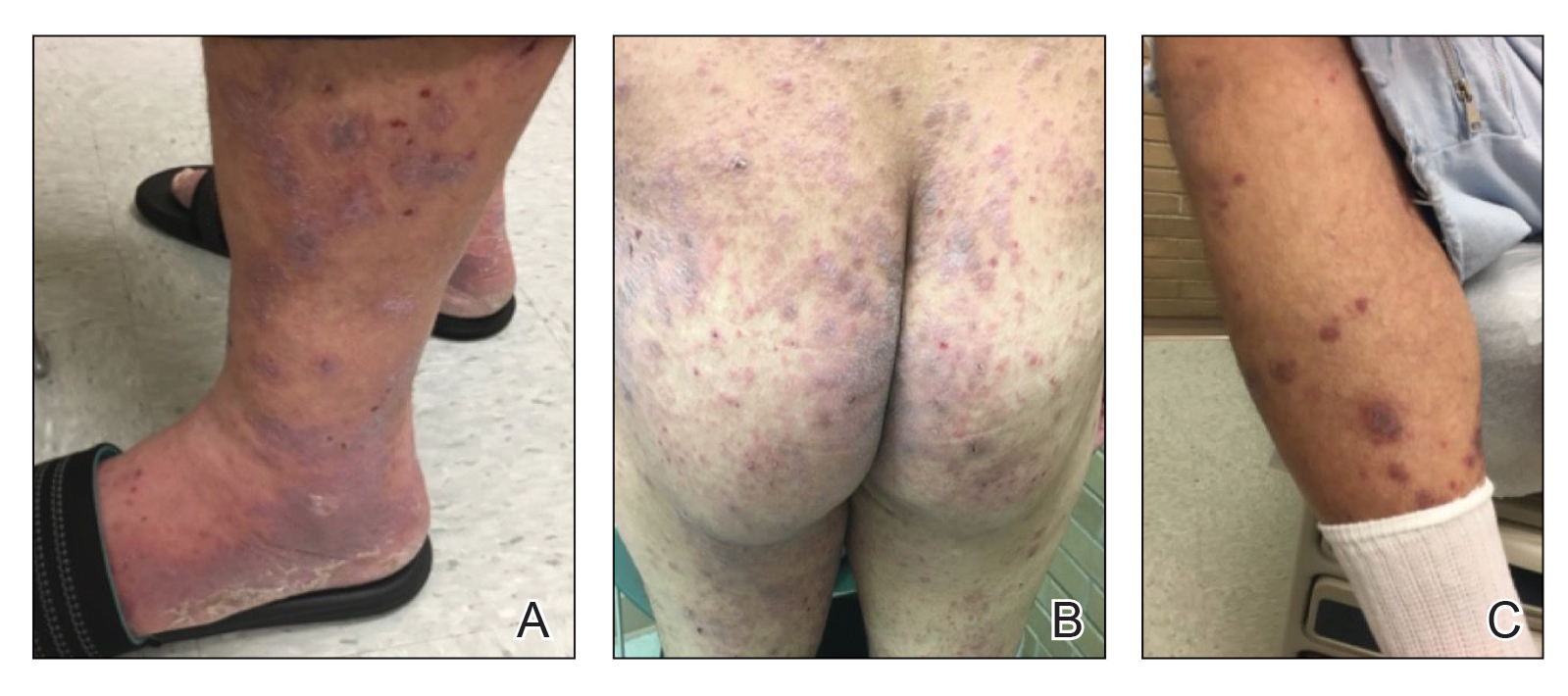
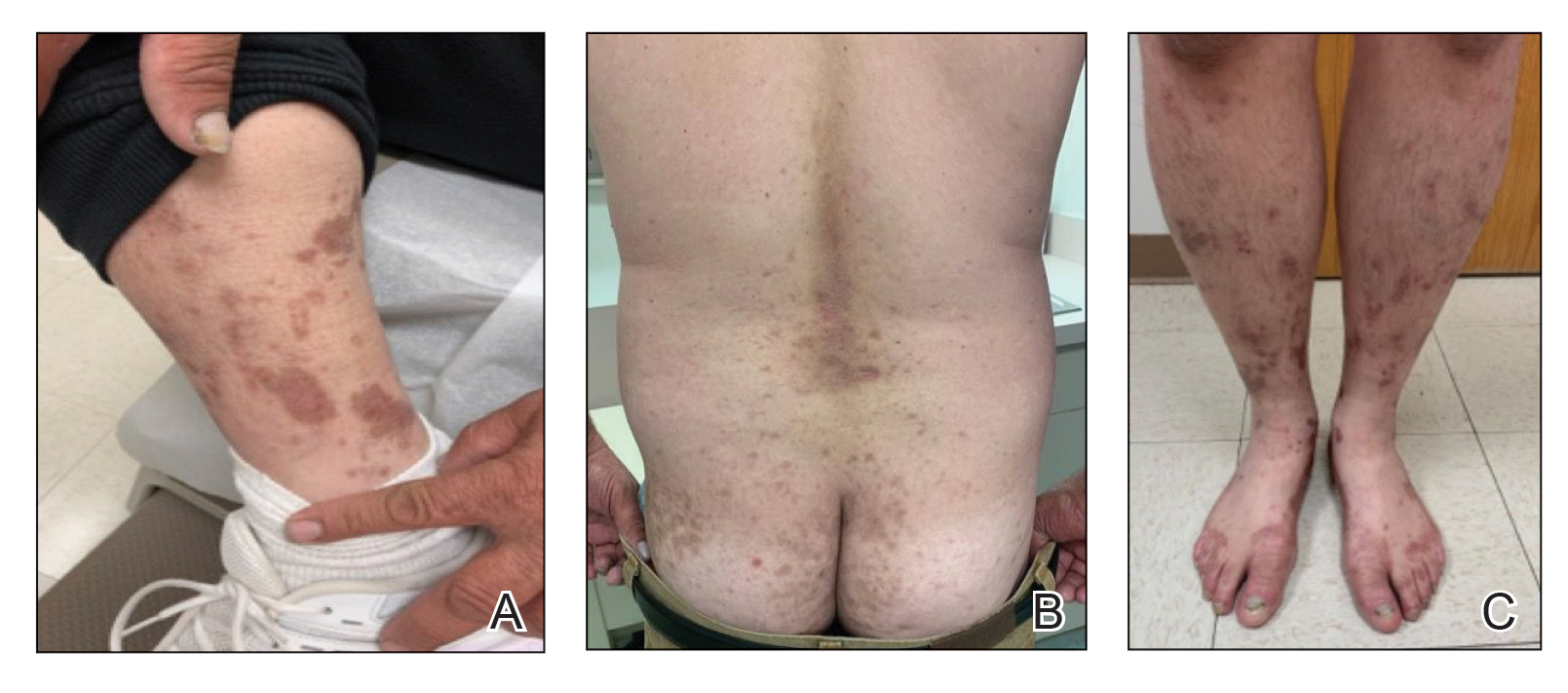
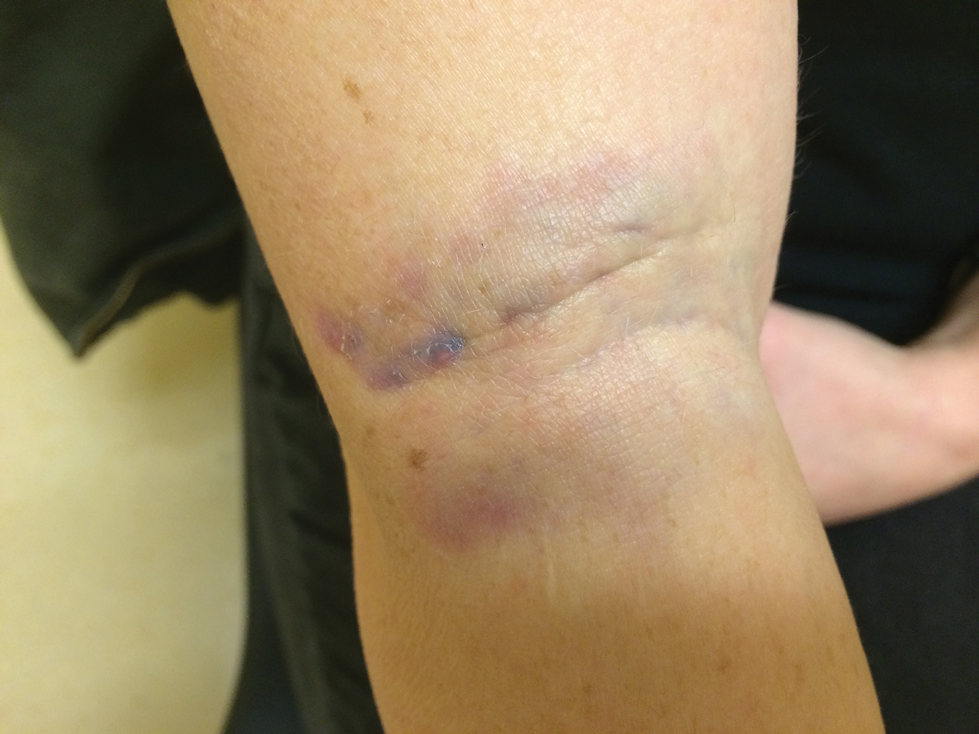
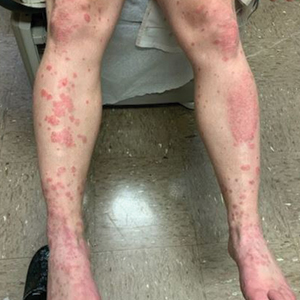
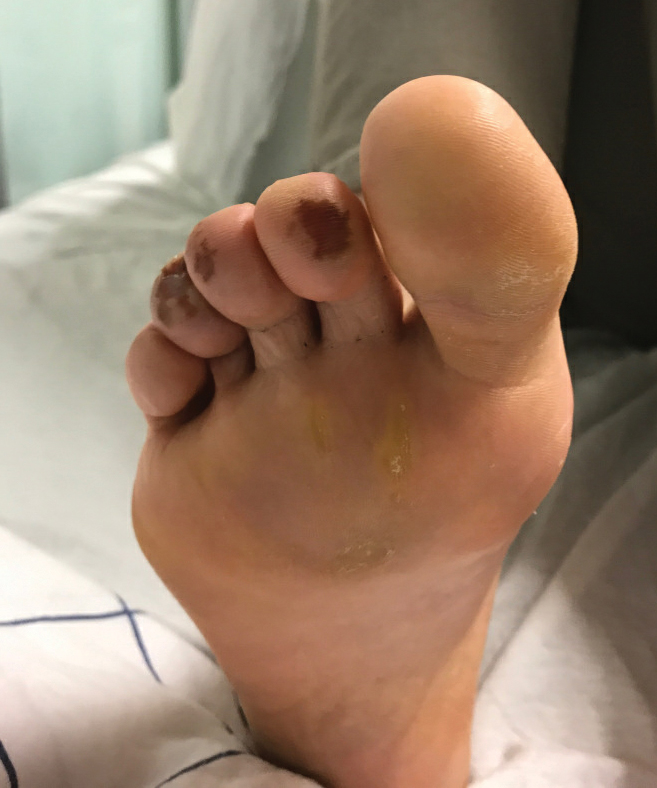
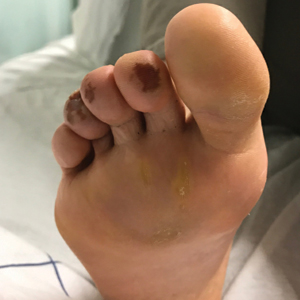
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.
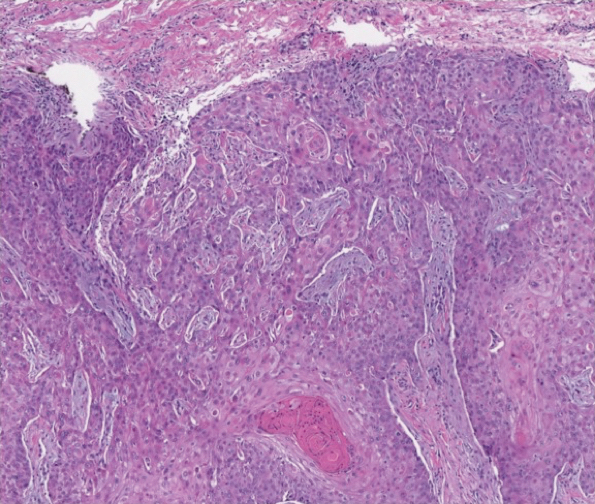
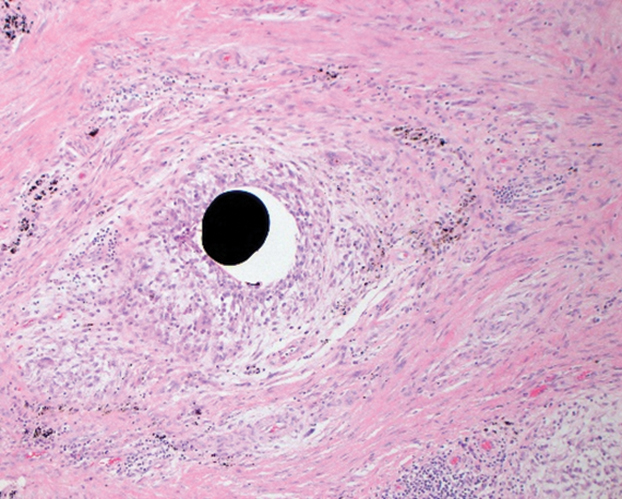
The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.
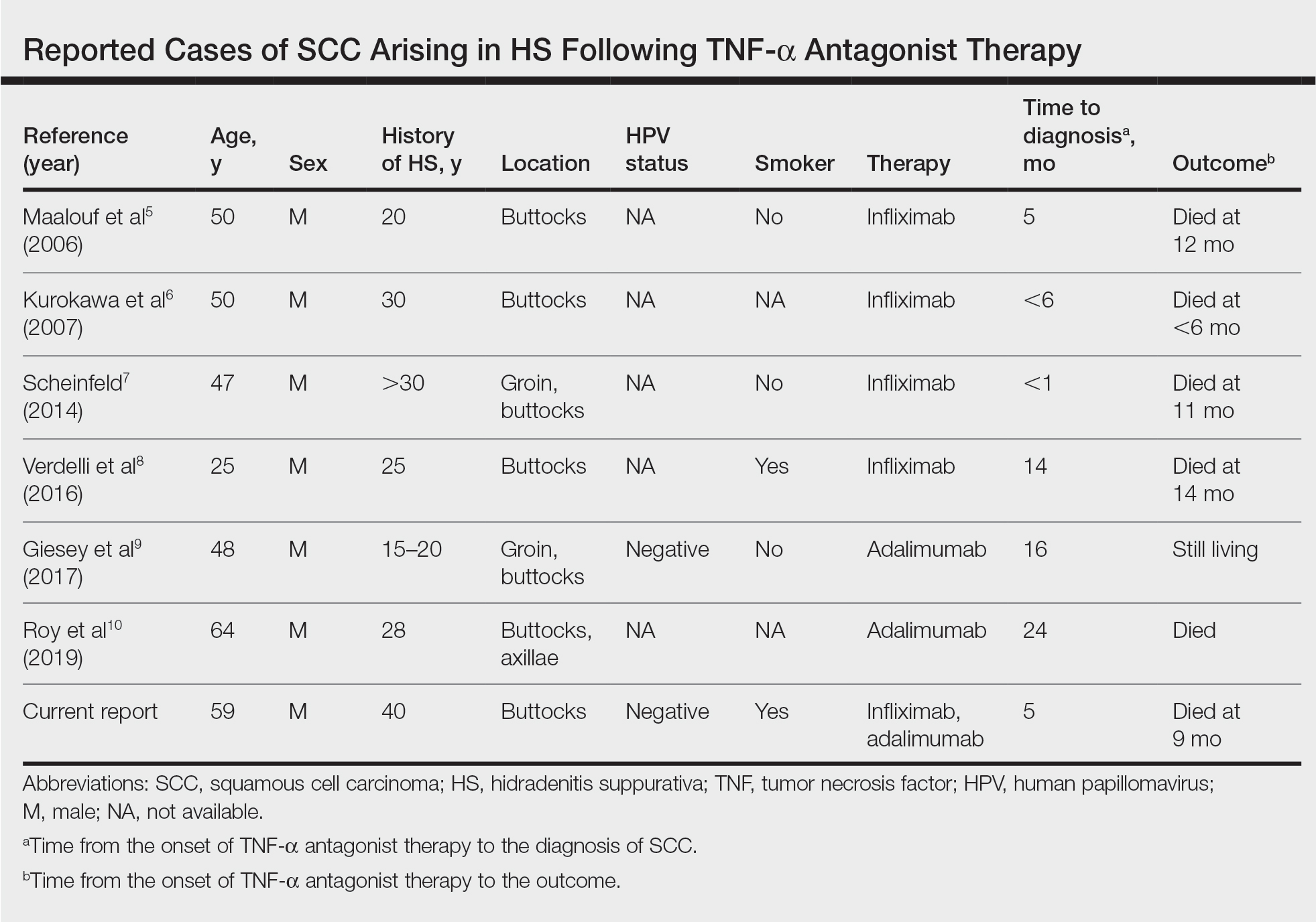
Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.
Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.
The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.
Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.
Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.
The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.
Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.
Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
Practice Points
- Consider biopsy of representative lesions in men older than 20 years with moderate to severe disease of the groin and/or buttocks prior to initiation of tumor necrosis factor inhibitors.
- Consider more frequent clinical monitoring with a decrease in threshold to perform biopsy of any new or ulcerating lesions.
VEXAS: A novel rheumatologic, hematologic syndrome that’s making waves
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
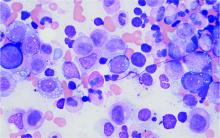
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity.
“As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity.
“As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity.
“As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Dupilumab for the Treatment of Lichen Planus
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).
Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).
Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).
Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
Practice Points
- Lichen planus (LP) is an inflammatory mucocutaneous disorder that can present across various regions of the body with pruritic, purple, polygonal papules or plaques.
- The proposed pathogenesis of LP involves autoimmune destruction of epidermal basal keratinocytes.
- The immunomodulatory properties of dupilumab have been shown to aid various inflammatory cutaneous conditions.
Deaths tied to reprocessed urologic endoscopes, FDA warns
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
Erethism Mercurialis and Reactions to Elemental Mercury
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Practice Points
- Chronic mercury granulomas can present as firm, erythematous to bluish gray plaques.
- Accidental skin contact to elemental mercury may cause urticaria and dermatitis.
- Blood mercury concentrations below 20 11µg/L are considered within reference range; once blood and urine concentrations exceed 100 11µg/L, clinical signs of acute mercury poisoning typically manifest.
- Mercury is toxic to the central and peripheral nervous systems, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory.
Comparison of Dermatologist Ratings on Health Care–Specific and General Consumer Websites
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).
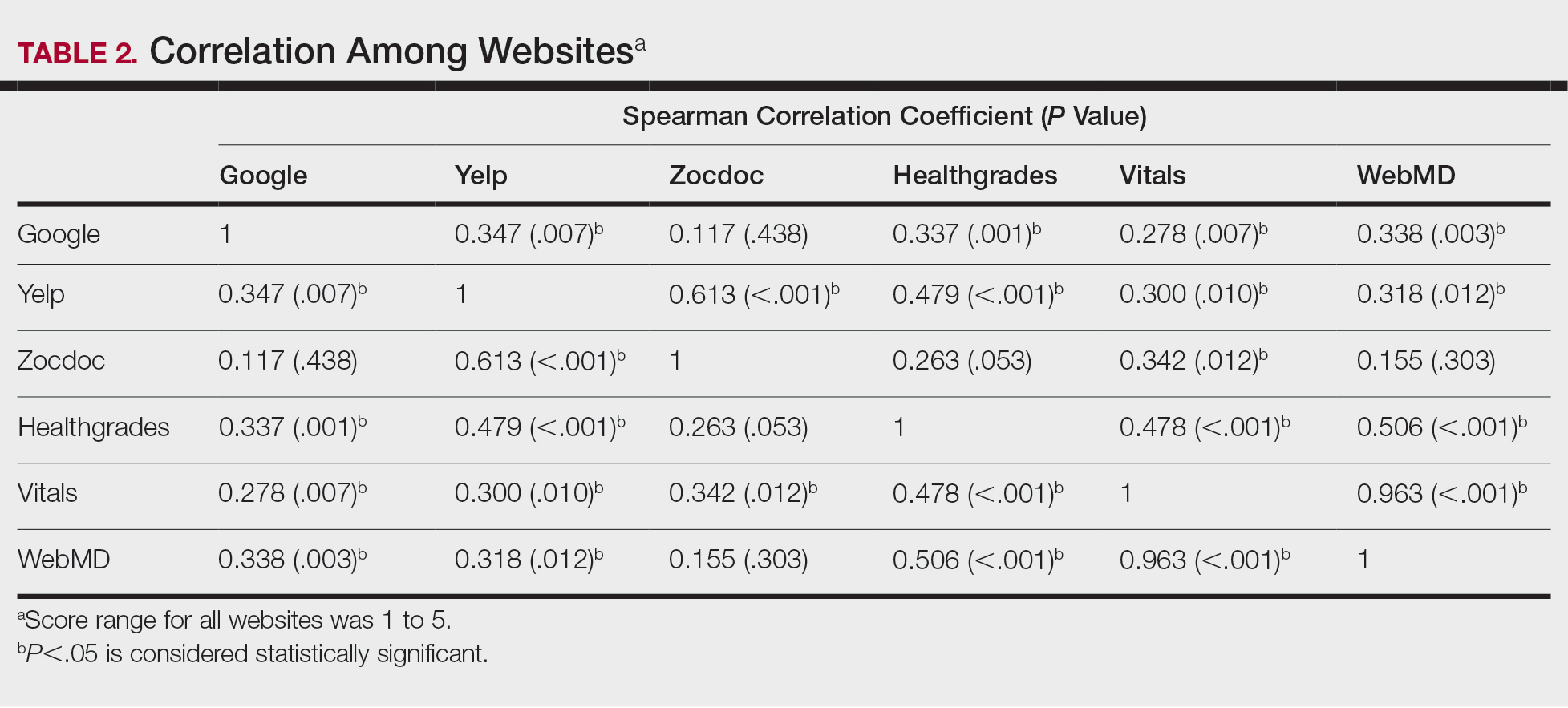
Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).


Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).


Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
Practice Points
- Online physician-rating websites are commonly used by patients to find physicians and review experiences.
- Health care–specific sites may more accurately reflect patient sentiment than general consumer sites.
- Dermatologists can use health care–specific sites to understand patient sentiment and learn how to improve patient experiences.
Apremilast Uses and Relevance to the Military
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Practice Points
- Apremilast is a versatile and easy-to-use therapeutic option for treatment of psoriasis and psoriatic arthritis.
- Ease of transport and storage as well as lack of necessary laboratory monitoring have made apremilast a compelling treatment option for psoriasis and psoriatic arthritis in military populations with high operational tempos.
- Dermatologists should consider apremilast for treatment in populations that work for prolonged periods in austere or resource-limited environments.
Cutaneous Manifestations of COVID-19: Characteristics, Pathogenesis, and the Role of Dermatology in the Pandemic
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
Practice Points
- Cutaneous manifestations of COVID-19 may reflect the range of host immunologic responses to SARS-CoV-2.
- Perniosis appears to be a late manifestation of COVID-19 associated with a comparatively benign disease course, whereas livedoid or other vasculopathic lesions portend poorer outcomes and may warrant further workup for occult thrombotic disease.
- Maculopapular, vesicular, and urticarial eruptions may be seen in association with COVID-19 but are nonspecific and necessitate a broad differential and workup.
- Challenges posed by the COVID-19 pandemic necessitate creative management strategies for immunosuppression and clinical assessment.