User login
Palliative care helpful but underutilized for blood cancer patients
Specialty palliative care interventions improve outcomes in patients with hematologic malignancies but are underutilized, according to findings from a systematic literature review.
Outcomes that were improved, as demonstrated by 16 studies that met inclusion criteria for the review, included symptom management, inpatient mortality, health care utilization, health care costs, and caregiver-reported outcomes, Elizabeth Elliott, DO, a hematology and oncology fellow at the Cardinal Bernardin Cancer Center, Loyola University, Maywood, Ill., and colleagues reported.
The findings were published online in the Journal of Pain and Symptom Management.
Palliative care needs
Patients with hematologic malignancies, including leukemia, myeloma, and lymphoma, have a high need for supportive care, the authors noted, adding that, although its use has increased over time, palliative care (PC) is often provided late in the disease course – sometimes only in the final days of life.
“Compared with their solid tumor counterparts, patients with hematologic malignancies experience higher symptom burdens, have higher rates of cancer-directed care near death, and are more likely to die while hospitalized than at home or in hospice,” they wrote. “Despite this need, specialist palliative care is less commonly utilized in patients with hematologic malignancies than other cancer types.”
Given the high health care utilization among patients with hematologic malignancies, earlier and more widespread utilization of PC in this population may significantly reduce health care costs, they added.
Palliative care benefits
Of 5,345 studies published between 2005 and 2020 and screened for the current review, 16 met inclusion criteria, including 10 retrospective cohort studies; 4 prospective cohort studies; and 2 randomized, controlled studies.
Nine studies included only patients with hematologic malignancies and seven included both patients with solid tumors and patients with hematologic malignancies. Each study assessed as being of moderate quality.
Benefits of PC as demonstrated in the studies included:
Symptom management: One study, for example, showed that an integrated psychological and PC intervention improved traumatic stress levels, degree and number of physical symptoms, pain intensity, depressive symptoms, and quality of life, compared with no intervention. Another showed that the percentage of patients reporting moderate to severe pain improved from 57% to 18% with a PC intervention, and the number reporting depressive episodes improved from 13% to 5%.
Reduced in-patient death: Findings from eight studies showed that 21.9%-83% of those receiving PC died at home, compared with 6.0%-8.9% of controls. Two studies showed that PC provided at least 20 days prior to death decreased the likelihood of inpatient death and death in an ICU, compared with controls, and one showed that the rate of in-hospital deaths was 30% for those with home PC or hospice, compared with 80% of controls.
Health care utilization: The studies showed that hospitalization occurred in 45%-76.3% of hematologic malignancy patients who received PC, compared with 98% of controls. The odds ratio for hospitalization among acute leukemia patients receiving PC was 0.64, compared with 2.53 among those in a historical control group.
Caregiver-reported outcomes: One randomized, controlled study showed that PC was associated with smaller increases in depression scores, improved coping, and improved scores in multiple quality of life domains in caregivers versus controls.
Survival: One study showed that a larger percentage of hematologic malignancy patients who died 1-6 months after diagnosis had not received PC (28% vs. 23%), whereas more of those who died 6-12 months or 12 or more months after diagnosis had received PC (23.9 vs. 14.9% and 42.5% vs. 22.0%).
Health care costs: Two studies showed a decrease in inpatient costs after a palliative care consultation. Decreases in hospitalization costs were $2,321 and $1,506 for less medically complex patients and $3,515 and $5,617 for more medically complex patient.
Improving PC utilization
One potential strategy to promote earlier referrals to PC is improved education for hematologists, the authors said, citing a study showing that 98% of oncology fellows at one center reported improvement in their ability to assess and manage patient symptoms after completion of a 4-week mandatory PC rotation.
“Another strategy to improve referrals to PC of hematologic malignancies patients could be the creation of programs which facilitate collaboration between PC providers and hematologists, such as the palliative and supportive care special interest group within the American Society for Transplantation and Cellular Therapy,” they wrote.
A third strategy “could be to provide a concurrent care model, in which cancer directed therapy (such as transfusions) is provided at the same time as hospice care,” they added, explaining that such an approach was shown in a study of patients with advanced non–small cell lung cancer to be associated with less aggressive medical treatment and lower costs.
The authors also stressed that patient with solid tumors and those with hematologic malignancies have differing supportive care needs and health care utilization, but several studies included in the current review included both types of cancer.
“Further studies investigating PC use exclusively in patients with hematologic malignancies are needed. Our results demonstrate a strong argument for hematologists to refer their patients early and often for specialized PC,” they concluded.
Indeed, when PC is integrated within hematologic malignancies, impacts occur that are similar to those seen in a variety of other diseases and include improved symptom control, enhanced caregiver experience, and reduced burdens on the health care system, Toby C Campbell, MD, said in an interview.
“The benefits of providing palliative care concurrent with standard cancer care is felt by all the major stakeholders in this care: the patients, their caregivers, and the health care system around them,” said Dr. Campbell, a thoracic medical oncologist and professor in the division of hematology, medical oncology, and palliative care at the University of Wisconsin–Madison.
Overcoming challenges
However, this is “new territory” for most programs, added Dr. Campbell, who also is the University of Wisconsin health chief of palliative care and holds the Ellen and Peter O. Johnson Chair in Palliative Care .
“The palliative care clinicians have a lot of learning to do if they’re going to enter this space and provide expert care,” he said, adding that expert care is what is needed and what was studied in this review. “Providing palliative care to patients with hematologic malignancies has a unique pace and a number of subspecialized therapeutic options with which the palliative care clinician must become familiar.”
Examples include bone marrow transplantation with prolonged hospitalizations and transfusion support, he said.
“Palliative care programs, in order to provide high quality care, will need to familiarize themselves with these therapies and develop close partnership with hematologists to integrate seamlessly into the patient’s care,” he added. “At some centers, culture changes will be necessary concurrent with the clinical practice change of integrating palliative care and it is the responsibility of the palliative care clinicians to bring their very best to these new relationships and patient populations.”
The authors reported having no disclosures. Dr. Campbell also reported having no disclosures.
Specialty palliative care interventions improve outcomes in patients with hematologic malignancies but are underutilized, according to findings from a systematic literature review.
Outcomes that were improved, as demonstrated by 16 studies that met inclusion criteria for the review, included symptom management, inpatient mortality, health care utilization, health care costs, and caregiver-reported outcomes, Elizabeth Elliott, DO, a hematology and oncology fellow at the Cardinal Bernardin Cancer Center, Loyola University, Maywood, Ill., and colleagues reported.
The findings were published online in the Journal of Pain and Symptom Management.
Palliative care needs
Patients with hematologic malignancies, including leukemia, myeloma, and lymphoma, have a high need for supportive care, the authors noted, adding that, although its use has increased over time, palliative care (PC) is often provided late in the disease course – sometimes only in the final days of life.
“Compared with their solid tumor counterparts, patients with hematologic malignancies experience higher symptom burdens, have higher rates of cancer-directed care near death, and are more likely to die while hospitalized than at home or in hospice,” they wrote. “Despite this need, specialist palliative care is less commonly utilized in patients with hematologic malignancies than other cancer types.”
Given the high health care utilization among patients with hematologic malignancies, earlier and more widespread utilization of PC in this population may significantly reduce health care costs, they added.
Palliative care benefits
Of 5,345 studies published between 2005 and 2020 and screened for the current review, 16 met inclusion criteria, including 10 retrospective cohort studies; 4 prospective cohort studies; and 2 randomized, controlled studies.
Nine studies included only patients with hematologic malignancies and seven included both patients with solid tumors and patients with hematologic malignancies. Each study assessed as being of moderate quality.
Benefits of PC as demonstrated in the studies included:
Symptom management: One study, for example, showed that an integrated psychological and PC intervention improved traumatic stress levels, degree and number of physical symptoms, pain intensity, depressive symptoms, and quality of life, compared with no intervention. Another showed that the percentage of patients reporting moderate to severe pain improved from 57% to 18% with a PC intervention, and the number reporting depressive episodes improved from 13% to 5%.
Reduced in-patient death: Findings from eight studies showed that 21.9%-83% of those receiving PC died at home, compared with 6.0%-8.9% of controls. Two studies showed that PC provided at least 20 days prior to death decreased the likelihood of inpatient death and death in an ICU, compared with controls, and one showed that the rate of in-hospital deaths was 30% for those with home PC or hospice, compared with 80% of controls.
Health care utilization: The studies showed that hospitalization occurred in 45%-76.3% of hematologic malignancy patients who received PC, compared with 98% of controls. The odds ratio for hospitalization among acute leukemia patients receiving PC was 0.64, compared with 2.53 among those in a historical control group.
Caregiver-reported outcomes: One randomized, controlled study showed that PC was associated with smaller increases in depression scores, improved coping, and improved scores in multiple quality of life domains in caregivers versus controls.
Survival: One study showed that a larger percentage of hematologic malignancy patients who died 1-6 months after diagnosis had not received PC (28% vs. 23%), whereas more of those who died 6-12 months or 12 or more months after diagnosis had received PC (23.9 vs. 14.9% and 42.5% vs. 22.0%).
Health care costs: Two studies showed a decrease in inpatient costs after a palliative care consultation. Decreases in hospitalization costs were $2,321 and $1,506 for less medically complex patients and $3,515 and $5,617 for more medically complex patient.
Improving PC utilization
One potential strategy to promote earlier referrals to PC is improved education for hematologists, the authors said, citing a study showing that 98% of oncology fellows at one center reported improvement in their ability to assess and manage patient symptoms after completion of a 4-week mandatory PC rotation.
“Another strategy to improve referrals to PC of hematologic malignancies patients could be the creation of programs which facilitate collaboration between PC providers and hematologists, such as the palliative and supportive care special interest group within the American Society for Transplantation and Cellular Therapy,” they wrote.
A third strategy “could be to provide a concurrent care model, in which cancer directed therapy (such as transfusions) is provided at the same time as hospice care,” they added, explaining that such an approach was shown in a study of patients with advanced non–small cell lung cancer to be associated with less aggressive medical treatment and lower costs.
The authors also stressed that patient with solid tumors and those with hematologic malignancies have differing supportive care needs and health care utilization, but several studies included in the current review included both types of cancer.
“Further studies investigating PC use exclusively in patients with hematologic malignancies are needed. Our results demonstrate a strong argument for hematologists to refer their patients early and often for specialized PC,” they concluded.
Indeed, when PC is integrated within hematologic malignancies, impacts occur that are similar to those seen in a variety of other diseases and include improved symptom control, enhanced caregiver experience, and reduced burdens on the health care system, Toby C Campbell, MD, said in an interview.
“The benefits of providing palliative care concurrent with standard cancer care is felt by all the major stakeholders in this care: the patients, their caregivers, and the health care system around them,” said Dr. Campbell, a thoracic medical oncologist and professor in the division of hematology, medical oncology, and palliative care at the University of Wisconsin–Madison.
Overcoming challenges
However, this is “new territory” for most programs, added Dr. Campbell, who also is the University of Wisconsin health chief of palliative care and holds the Ellen and Peter O. Johnson Chair in Palliative Care .
“The palliative care clinicians have a lot of learning to do if they’re going to enter this space and provide expert care,” he said, adding that expert care is what is needed and what was studied in this review. “Providing palliative care to patients with hematologic malignancies has a unique pace and a number of subspecialized therapeutic options with which the palliative care clinician must become familiar.”
Examples include bone marrow transplantation with prolonged hospitalizations and transfusion support, he said.
“Palliative care programs, in order to provide high quality care, will need to familiarize themselves with these therapies and develop close partnership with hematologists to integrate seamlessly into the patient’s care,” he added. “At some centers, culture changes will be necessary concurrent with the clinical practice change of integrating palliative care and it is the responsibility of the palliative care clinicians to bring their very best to these new relationships and patient populations.”
The authors reported having no disclosures. Dr. Campbell also reported having no disclosures.
Specialty palliative care interventions improve outcomes in patients with hematologic malignancies but are underutilized, according to findings from a systematic literature review.
Outcomes that were improved, as demonstrated by 16 studies that met inclusion criteria for the review, included symptom management, inpatient mortality, health care utilization, health care costs, and caregiver-reported outcomes, Elizabeth Elliott, DO, a hematology and oncology fellow at the Cardinal Bernardin Cancer Center, Loyola University, Maywood, Ill., and colleagues reported.
The findings were published online in the Journal of Pain and Symptom Management.
Palliative care needs
Patients with hematologic malignancies, including leukemia, myeloma, and lymphoma, have a high need for supportive care, the authors noted, adding that, although its use has increased over time, palliative care (PC) is often provided late in the disease course – sometimes only in the final days of life.
“Compared with their solid tumor counterparts, patients with hematologic malignancies experience higher symptom burdens, have higher rates of cancer-directed care near death, and are more likely to die while hospitalized than at home or in hospice,” they wrote. “Despite this need, specialist palliative care is less commonly utilized in patients with hematologic malignancies than other cancer types.”
Given the high health care utilization among patients with hematologic malignancies, earlier and more widespread utilization of PC in this population may significantly reduce health care costs, they added.
Palliative care benefits
Of 5,345 studies published between 2005 and 2020 and screened for the current review, 16 met inclusion criteria, including 10 retrospective cohort studies; 4 prospective cohort studies; and 2 randomized, controlled studies.
Nine studies included only patients with hematologic malignancies and seven included both patients with solid tumors and patients with hematologic malignancies. Each study assessed as being of moderate quality.
Benefits of PC as demonstrated in the studies included:
Symptom management: One study, for example, showed that an integrated psychological and PC intervention improved traumatic stress levels, degree and number of physical symptoms, pain intensity, depressive symptoms, and quality of life, compared with no intervention. Another showed that the percentage of patients reporting moderate to severe pain improved from 57% to 18% with a PC intervention, and the number reporting depressive episodes improved from 13% to 5%.
Reduced in-patient death: Findings from eight studies showed that 21.9%-83% of those receiving PC died at home, compared with 6.0%-8.9% of controls. Two studies showed that PC provided at least 20 days prior to death decreased the likelihood of inpatient death and death in an ICU, compared with controls, and one showed that the rate of in-hospital deaths was 30% for those with home PC or hospice, compared with 80% of controls.
Health care utilization: The studies showed that hospitalization occurred in 45%-76.3% of hematologic malignancy patients who received PC, compared with 98% of controls. The odds ratio for hospitalization among acute leukemia patients receiving PC was 0.64, compared with 2.53 among those in a historical control group.
Caregiver-reported outcomes: One randomized, controlled study showed that PC was associated with smaller increases in depression scores, improved coping, and improved scores in multiple quality of life domains in caregivers versus controls.
Survival: One study showed that a larger percentage of hematologic malignancy patients who died 1-6 months after diagnosis had not received PC (28% vs. 23%), whereas more of those who died 6-12 months or 12 or more months after diagnosis had received PC (23.9 vs. 14.9% and 42.5% vs. 22.0%).
Health care costs: Two studies showed a decrease in inpatient costs after a palliative care consultation. Decreases in hospitalization costs were $2,321 and $1,506 for less medically complex patients and $3,515 and $5,617 for more medically complex patient.
Improving PC utilization
One potential strategy to promote earlier referrals to PC is improved education for hematologists, the authors said, citing a study showing that 98% of oncology fellows at one center reported improvement in their ability to assess and manage patient symptoms after completion of a 4-week mandatory PC rotation.
“Another strategy to improve referrals to PC of hematologic malignancies patients could be the creation of programs which facilitate collaboration between PC providers and hematologists, such as the palliative and supportive care special interest group within the American Society for Transplantation and Cellular Therapy,” they wrote.
A third strategy “could be to provide a concurrent care model, in which cancer directed therapy (such as transfusions) is provided at the same time as hospice care,” they added, explaining that such an approach was shown in a study of patients with advanced non–small cell lung cancer to be associated with less aggressive medical treatment and lower costs.
The authors also stressed that patient with solid tumors and those with hematologic malignancies have differing supportive care needs and health care utilization, but several studies included in the current review included both types of cancer.
“Further studies investigating PC use exclusively in patients with hematologic malignancies are needed. Our results demonstrate a strong argument for hematologists to refer their patients early and often for specialized PC,” they concluded.
Indeed, when PC is integrated within hematologic malignancies, impacts occur that are similar to those seen in a variety of other diseases and include improved symptom control, enhanced caregiver experience, and reduced burdens on the health care system, Toby C Campbell, MD, said in an interview.
“The benefits of providing palliative care concurrent with standard cancer care is felt by all the major stakeholders in this care: the patients, their caregivers, and the health care system around them,” said Dr. Campbell, a thoracic medical oncologist and professor in the division of hematology, medical oncology, and palliative care at the University of Wisconsin–Madison.
Overcoming challenges
However, this is “new territory” for most programs, added Dr. Campbell, who also is the University of Wisconsin health chief of palliative care and holds the Ellen and Peter O. Johnson Chair in Palliative Care .
“The palliative care clinicians have a lot of learning to do if they’re going to enter this space and provide expert care,” he said, adding that expert care is what is needed and what was studied in this review. “Providing palliative care to patients with hematologic malignancies has a unique pace and a number of subspecialized therapeutic options with which the palliative care clinician must become familiar.”
Examples include bone marrow transplantation with prolonged hospitalizations and transfusion support, he said.
“Palliative care programs, in order to provide high quality care, will need to familiarize themselves with these therapies and develop close partnership with hematologists to integrate seamlessly into the patient’s care,” he added. “At some centers, culture changes will be necessary concurrent with the clinical practice change of integrating palliative care and it is the responsibility of the palliative care clinicians to bring their very best to these new relationships and patient populations.”
The authors reported having no disclosures. Dr. Campbell also reported having no disclosures.
FROM THE JOURNAL OF PAIN AND SYMPTOM MANAGEMENT
Lasting norovirus immunity may depend on T cells
Protection against norovirus gastroenteritis is supported in part by norovirus-specific CD8+ T cells that reside in peripheral, intestinal, and lymphoid tissues, according to investigators.
These findings, and the molecular tools used to discover them, could guide development of a norovirus vaccine and novel cellular therapies, according to lead author Ajinkya Pattekar, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“Currently, there are no approved pharmacologic therapies against norovirus, and despite several promising clinical trials, an effective vaccine is not available,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, which may stem from an incomplete understanding of norovirus immunity, according to Dr. Pattekar and colleagues.
They noted that most previous research has focused on humoral immunity, which appears variable between individuals, with some people exhibiting a strong humoral response, while others mount only partial humoral protection. The investigators also noted that, depending on which studies were examined, this type of defense could last years or fade within weeks to months and that “immune mechanisms other than antibodies may be important for protection against noroviruses.”
Specifically, cellular immunity may be at work. A 2020 study involving volunteers showed that T cells were cross-reactive to a type of norovirus the participants had never been exposed to.
“These findings suggest that T cells may target conserved epitopes and could offer cross-protection against a broad range of noroviruses,” Dr. Pattekar and colleagues wrote.
To test this hypothesis, they first collected peripheral blood mononuclear cells (PBMCs) from three healthy volunteers with unknown norovirus exposure history. Then serum samples were screened for norovirus functional antibodies via the binding between virus-like particles (VLPs) and histo–blood group antigens (HBGAs). This revealed disparate profiles of blocking antibodies against various norovirus strains. While donor 1 and donor 2 had antibodies against multiple strains, donor 3 lacked norovirus antibodies. Further testing showed that this latter individual was a nonsecretor with limited exposure history.
Next, the investigators tested donor PBMCs for norovirus-specific T-cell responses with use of overlapping libraries of peptides for each of the three norovirus open reading frames (ORF1, ORF2, and ORF3). T-cell responses, predominantly involving CD8+ T cells, were observed in all donors. While donor 1 had the greatest response to ORF1, donors 2 and 3 had responses that focused on ORF2.
“Thus, norovirus-specific T cells targeting ORF1 and ORF2 epitopes are present in peripheral blood from healthy donors regardless of secretor status,” the investigators wrote.
To better characterize T-cell epitopes, the investigators subdivided the overlapping peptide libraries into groups of shorter peptides, then exposed serum to these smaller component pools. This revealed eight HLA class I restricted epitopes that were derived from a genogroup II.4 pandemic norovirus strain; this group of variants has been responsible for all six of the norovirus pandemics since 1996.
Closer examination of the epitopes showed that they were “broadly conserved beyond GII.4.” Only one epitope exhibited variation in the C-terminal aromatic anchor, and it was nondominant. The investigators therefore identified seven immunodominant CD8+ epitopes, which they considered “valuable targets for vaccine and cell-based therapies.
“These data further confirm that epitope-specific CD8+ T cells are a universal feature of the overall norovirus immune response and could be an attractive target for future vaccines,” the investigators wrote.
Additional testing involving samples of spleen, mesenteric lymph nodes, and duodenum from deceased individuals showed presence of norovirus-specific CD8+ T cells, with particular abundance in intestinal tissue, and distinct phenotypes and functional properties in different tissue types.
“Future studies using tetramers and intestinal samples should build on these observations and fully define the location and microenvironment of norovirus-specific T cells,” the investigators wrote. “If carried out in the context of a vaccine trial, such studies could be highly valuable in elucidating tissue-resident memory correlates of norovirus immunity.”
The study was funded by the National Institutes of Health, the Wellcome Trust, and Deutsche Forschungsgemeinschaft. The investigators reported no conflicts of interest.
Protection against norovirus gastroenteritis is supported in part by norovirus-specific CD8+ T cells that reside in peripheral, intestinal, and lymphoid tissues, according to investigators.
These findings, and the molecular tools used to discover them, could guide development of a norovirus vaccine and novel cellular therapies, according to lead author Ajinkya Pattekar, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“Currently, there are no approved pharmacologic therapies against norovirus, and despite several promising clinical trials, an effective vaccine is not available,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, which may stem from an incomplete understanding of norovirus immunity, according to Dr. Pattekar and colleagues.
They noted that most previous research has focused on humoral immunity, which appears variable between individuals, with some people exhibiting a strong humoral response, while others mount only partial humoral protection. The investigators also noted that, depending on which studies were examined, this type of defense could last years or fade within weeks to months and that “immune mechanisms other than antibodies may be important for protection against noroviruses.”
Specifically, cellular immunity may be at work. A 2020 study involving volunteers showed that T cells were cross-reactive to a type of norovirus the participants had never been exposed to.
“These findings suggest that T cells may target conserved epitopes and could offer cross-protection against a broad range of noroviruses,” Dr. Pattekar and colleagues wrote.
To test this hypothesis, they first collected peripheral blood mononuclear cells (PBMCs) from three healthy volunteers with unknown norovirus exposure history. Then serum samples were screened for norovirus functional antibodies via the binding between virus-like particles (VLPs) and histo–blood group antigens (HBGAs). This revealed disparate profiles of blocking antibodies against various norovirus strains. While donor 1 and donor 2 had antibodies against multiple strains, donor 3 lacked norovirus antibodies. Further testing showed that this latter individual was a nonsecretor with limited exposure history.
Next, the investigators tested donor PBMCs for norovirus-specific T-cell responses with use of overlapping libraries of peptides for each of the three norovirus open reading frames (ORF1, ORF2, and ORF3). T-cell responses, predominantly involving CD8+ T cells, were observed in all donors. While donor 1 had the greatest response to ORF1, donors 2 and 3 had responses that focused on ORF2.
“Thus, norovirus-specific T cells targeting ORF1 and ORF2 epitopes are present in peripheral blood from healthy donors regardless of secretor status,” the investigators wrote.
To better characterize T-cell epitopes, the investigators subdivided the overlapping peptide libraries into groups of shorter peptides, then exposed serum to these smaller component pools. This revealed eight HLA class I restricted epitopes that were derived from a genogroup II.4 pandemic norovirus strain; this group of variants has been responsible for all six of the norovirus pandemics since 1996.
Closer examination of the epitopes showed that they were “broadly conserved beyond GII.4.” Only one epitope exhibited variation in the C-terminal aromatic anchor, and it was nondominant. The investigators therefore identified seven immunodominant CD8+ epitopes, which they considered “valuable targets for vaccine and cell-based therapies.
“These data further confirm that epitope-specific CD8+ T cells are a universal feature of the overall norovirus immune response and could be an attractive target for future vaccines,” the investigators wrote.
Additional testing involving samples of spleen, mesenteric lymph nodes, and duodenum from deceased individuals showed presence of norovirus-specific CD8+ T cells, with particular abundance in intestinal tissue, and distinct phenotypes and functional properties in different tissue types.
“Future studies using tetramers and intestinal samples should build on these observations and fully define the location and microenvironment of norovirus-specific T cells,” the investigators wrote. “If carried out in the context of a vaccine trial, such studies could be highly valuable in elucidating tissue-resident memory correlates of norovirus immunity.”
The study was funded by the National Institutes of Health, the Wellcome Trust, and Deutsche Forschungsgemeinschaft. The investigators reported no conflicts of interest.
Protection against norovirus gastroenteritis is supported in part by norovirus-specific CD8+ T cells that reside in peripheral, intestinal, and lymphoid tissues, according to investigators.
These findings, and the molecular tools used to discover them, could guide development of a norovirus vaccine and novel cellular therapies, according to lead author Ajinkya Pattekar, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“Currently, there are no approved pharmacologic therapies against norovirus, and despite several promising clinical trials, an effective vaccine is not available,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, which may stem from an incomplete understanding of norovirus immunity, according to Dr. Pattekar and colleagues.
They noted that most previous research has focused on humoral immunity, which appears variable between individuals, with some people exhibiting a strong humoral response, while others mount only partial humoral protection. The investigators also noted that, depending on which studies were examined, this type of defense could last years or fade within weeks to months and that “immune mechanisms other than antibodies may be important for protection against noroviruses.”
Specifically, cellular immunity may be at work. A 2020 study involving volunteers showed that T cells were cross-reactive to a type of norovirus the participants had never been exposed to.
“These findings suggest that T cells may target conserved epitopes and could offer cross-protection against a broad range of noroviruses,” Dr. Pattekar and colleagues wrote.
To test this hypothesis, they first collected peripheral blood mononuclear cells (PBMCs) from three healthy volunteers with unknown norovirus exposure history. Then serum samples were screened for norovirus functional antibodies via the binding between virus-like particles (VLPs) and histo–blood group antigens (HBGAs). This revealed disparate profiles of blocking antibodies against various norovirus strains. While donor 1 and donor 2 had antibodies against multiple strains, donor 3 lacked norovirus antibodies. Further testing showed that this latter individual was a nonsecretor with limited exposure history.
Next, the investigators tested donor PBMCs for norovirus-specific T-cell responses with use of overlapping libraries of peptides for each of the three norovirus open reading frames (ORF1, ORF2, and ORF3). T-cell responses, predominantly involving CD8+ T cells, were observed in all donors. While donor 1 had the greatest response to ORF1, donors 2 and 3 had responses that focused on ORF2.
“Thus, norovirus-specific T cells targeting ORF1 and ORF2 epitopes are present in peripheral blood from healthy donors regardless of secretor status,” the investigators wrote.
To better characterize T-cell epitopes, the investigators subdivided the overlapping peptide libraries into groups of shorter peptides, then exposed serum to these smaller component pools. This revealed eight HLA class I restricted epitopes that were derived from a genogroup II.4 pandemic norovirus strain; this group of variants has been responsible for all six of the norovirus pandemics since 1996.
Closer examination of the epitopes showed that they were “broadly conserved beyond GII.4.” Only one epitope exhibited variation in the C-terminal aromatic anchor, and it was nondominant. The investigators therefore identified seven immunodominant CD8+ epitopes, which they considered “valuable targets for vaccine and cell-based therapies.
“These data further confirm that epitope-specific CD8+ T cells are a universal feature of the overall norovirus immune response and could be an attractive target for future vaccines,” the investigators wrote.
Additional testing involving samples of spleen, mesenteric lymph nodes, and duodenum from deceased individuals showed presence of norovirus-specific CD8+ T cells, with particular abundance in intestinal tissue, and distinct phenotypes and functional properties in different tissue types.
“Future studies using tetramers and intestinal samples should build on these observations and fully define the location and microenvironment of norovirus-specific T cells,” the investigators wrote. “If carried out in the context of a vaccine trial, such studies could be highly valuable in elucidating tissue-resident memory correlates of norovirus immunity.”
The study was funded by the National Institutes of Health, the Wellcome Trust, and Deutsche Forschungsgemeinschaft. The investigators reported no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Fit-for-Fertility program boosts births, is cost effective
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rheumatology clinics find success with smoking cessation referral program
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
A new protocol designed to help patients in rheumatology clinics quit smoking proved both efficient and effective in referring willing participants to free tobacco quit lines.
“Rheumatology visits provide a unique opportunity to address smoking as a chronic modifiable risk factor in populations at high risk for cardiovascular disease, pulmonary disease, and rheumatic disease progression,” wrote Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, and colleagues. The study was published in Arthritis Care & Research.
To assess the effectiveness of implementing a smoking cessation protocol for patients with rheumatic diseases, the researchers launched a quasi-experimental cohort study in which their Quit Connect protocol was tested at three rheumatology clinics. Adapting the Ask, Advise, Connect primary care protocol to a new setting, nurses and medical assistants were trained to use electronic health record (EHR) prompts that would check if patients who smoked were ready to quit within 30 days, advise them to do so, and then use electronic referrals to connect them to state-run tobacco quit lines. An extended baseline period – October 2012 to March 2016 – was compared to a 6-month intervention period from April to October 2016.
Across 54,090 pre- and postimplementation rheumatology clinic visits, 4,601 were with current smokers. Demographics were similar across both periods: The mean age of the patients was 51 years, about two-thirds were female, and 85% were White.
Clinicians’ assessment of tobacco use before and after implementation of the program stayed steady at 96% of patient visits, but the percentage of tobacco users’ visits that included checking for readiness to quit within the next 30 days rose from 3% (135 of 4,078) to 80% (421 of 523).
Before the implementation of the program, 0.6% of eligible visits with current smokers included a quit-line referral offer. After implementation, 93 (18%) of the 523 smokers who visited – 122 of whom said they were ready to quit – were offered referrals, a 26-fold increase. Of the 93 offered referrals, 66 (71%) accepted and 16 set a quit date or reported having quit; 11 accepted counseling services and nicotine replacement.
Although clinic staff reported encountering several obstacles, such as the need to craft nonthreatening language for challenging patients, they also contributed their own talking points that were included in the EHR tools and desktop brochures. On average, the protocol took less than 90 seconds to perform.
Rheumatologists can make headway on patients quitting smoking
“While smoking cessation programs require time and resources to implement, this study suggests a role for evidence-based protocols within rheumatology centers,” Medha Barbhaiya, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. “Given that current smokers are at an increased risk of developing more severe rheumatic disease and cardiovascular disease, and patients often visit their rheumatologist multiple times yearly, rheumatologists may be well-positioned to address smoking cessation with patients.”
In regard to next steps, she noted that “while future large studies in diverse cohorts are needed to confirm these findings, implementing a formal smoking cessation protocol within rheumatology centers may provide a unique opportunity for rheumatologists to directly help patients modify their disease risk, leading to improved health outcomes.”
The authors acknowledged their study’s limitations, including the fact that it was a prepost design and not a randomized trial. They also recognized that many tobacco users require 8-10 attempts before permanently quitting, likely lessening the lasting impact of the short-term study. They did cite expert analysis, however, that says “connecting patients to evidence-based resources makes them more likely to permanently quit.”
The study was supported in part by Pfizer’s office of Independent Grants for Learning and Change and by a grant collaboration from the University of Wisconsin Clinical and Translational Science Award and the University of Wisconsin School of Medicine and Public Health’s Wisconsin Partnership Program, through the NIH National Center for Advancing Translational Sciences.
FROM ARTHRITIS CARE & RESEARCH
Cancer screening stopped by pandemic: Repercussions to come?
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Treating metastatic TNBC: Where are we now?
Treating triple-negative breast cancer (TNBC), one of the more lethal breast cancer subtypes, remains a challenge. By definition, TNBC lacks the three telltale molecular signatures known to spur tumor growth: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). A growing amount of literature shows that these frequently aggressive tumors harbor a rich array of molecular characteristics but no clear oncogenic driver.
“TNBC is incredibly heterogeneous, which makes it challenging to treat,” said Rita Nanda, MD, director of the breast oncology program and associate professor of medicine at the University of Chicago. “We have subsets of TNBC that don’t respond to currently available therapies and, as of yet, have no identifiable therapeutic targets.”
Overall, about 40% of patients with TNBC show a pathologic complete response after first-line neoadjuvant chemotherapy – typically anthracycline and taxane-based agents. But for 50% of patients, chemotherapy leaves behind substantial residual cancer tissue. These patients subsequently face a 40%-80% risk for recurrence and progression to advanced disease.
When triple-negative disease metastasizes, survival rates plummet. The most recent data from the National Cancer Institute, which tracked patients by stage of diagnosis between 2010 and 2016, showed steep declines in 5-year survival as TNBC progressed from local (91.2%) to regional (65%) to advanced-stage disease (11.5%).
Experts have started to make headway identifying and targeting different molecular features of advanced TNBC. These approaches often focus on three key areas: targeting cell surface proteins or oncogenes, stimulating an anticancer immune response, or inhibiting an overactive signaling pathway.
“For a patient with metastatic breast cancer, finding a molecular target or an oncogenic driver is essential,” said Kelly McCann, MD, PhD, a hematologist/oncologist in the department of medicine at the University of California, Los Angeles. “Because TNBC encompasses many different molecular subsets of breast cancer, the development of effective new therapeutics is going to depend on subdividing TNBC into categories with more clear targets.”
A targeted strategy
The Food and Drug Administration’s approval of sacituzumab govitecan, the first antibody-drug conjugate to treat metastatic TNBC, marked an important addition to the TNBC drug armamentarium. “Sacituzumab govitecan is one of the most exciting drugs available for the treatment of metastatic disease,” Dr. Nanda said.
Sacituzumab govitecan, approved as third-line therapy for metastatic TNBC, works by targeting the cell surface protein TROP2, expressed in about 88% of TNBC tumors but rarely in healthy cells.
In the phase 1/2 ASCENT trial, the median progression-free survival was 5.5 months and overall survival was 13.0 months in 108 patients with metastatic TNBC who had received at least two therapies prior to sacituzumab govitecan.
A subsequent phase 3 trial showed progression-free survival of 5.6 months with sacituzumab govitecan and 1.7 months with physician’s choice of chemotherapy. The median overall survival was 12.1 months and 6.7 months, respectively.
But, according to the analysis, TROP2 expression did not necessarily predict who would benefit from sacituzumab govitecan. A biomarker study revealed that although patients with moderate to high TROP2 expression exhibited the strongest treatment response, those with low TROP2 expression also survived longer when given sacituzumab govitecan, compared with chemotherapy alone.
In other words, “patients did better on sacituzumab govitecan regardless of TROP2 expression, which suggests we do not have a good biomarker for identifying who will benefit,” Dr. Nanda said.
Two other investigational antibody-drug conjugates, trastuzumab deruxtecan and ladiratuzumab vedotin, show promise in the metastatic space as well. For instance, the recent phase 2 trial evaluating trastuzumab deruxtecan in patients with HER2-positive breast cancer reported treatment response in 44% of patients with HER2-low tumors.
Given that about 36.6% of TNBC tumors exhibit low levels of HER2 expression, “trastuzumab deruxtecan represents potential in treating HER2-low TNBC,” said Yuan Yuan, MD, PhD, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
Early results from a phase 1b study showed that trastuzumab deruxtecan produced a response rate of 37% in patients with HER2-low breast cancer.
Investigators are now recruiting for an open-label phase 3 trial to determine whether trastuzumab deruxtecan extends survival in patients with HER2-low metastatic breast cancers.
Immunotherapy advances
Immune checkpoint inhibitors represent another promising treatment avenue for metastatic TNBC. Pembrolizumab and atezolizumab, recently approved by the FDA, show moderate progression-free and overall survival benefits in patients with metastatic TNBC expressing PD-L1. Estimates of PD-L1 immune cells present in TNBC tumors vary widely, from about 20% to 65%.
Yet, data on which patients will benefit are not so clear-cut. “These drugs give us more choices and represent the fast-evolving therapeutic landscape in TNBC, but they also leave a lot of unanswered questions about PD-L1 as a biomarker,” Dr. Yuan said.
Take two recent phase 3 trials evaluating atezolizumab: IMpassion130 and IMpassion131. In IMpassion130, patients with PDL1–positive tumors exhibited significantly longer median overall survival on atezolizumab plus nab-paclitaxel (25.0 months) compared with nab-paclitaxel alone (15.5 months). As with the trend observed in the TROP2 data for sacituzumab govitecan, all patients survived longer on atezolizumab plus nab-paclitaxel regardless of PD-L1 status: 21.3 months vs. 17.6 months with nab-paclitaxel alone.
However, in IMpassion131, neither progression-free survival nor overall survival significantly improved in the PD-L1–positive group receiving atezolizumab plus paclitaxel compared with paclitaxel alone: Progression-free survival was 5.7 months vs. 6 months, respectively, and overall survival was 28.3 months vs. 22.1 months.
“It is unclear why this study failed to demonstrate a significant improvement in progression-free survival with the addition of atezolizumab to paclitaxel,” Dr. Nanda said. “Perhaps the negative finding has to do with how the trial was conducted, or perhaps the PD-L1 assay used is an unreliable biomarker of immunotherapy benefit.”
Continued efforts to understand TNBC
Given the diversity of metastatic TNBC and the absence of clear molecular targets, researchers are exploring a host of therapeutic strategies in addition to antibody-drug conjugates and immunotherapies.
On the oncogene front, researchers are investigating common mutations in TNBC. About 11% of TNBC tumors, for instance, carry germline mutations in BRCA1 and BRCA2. These tumors may be more likely to respond to platinum agents and PARP inhibitors, such as FDA-approved olaparib. In a phase 3 trial, patients with metastatic HER2-negative breast cancer and a germline BRCA mutation who received olaparib exhibited a 2.8-month longer median progression-free survival and a 42% reduced risk for disease progression or death compared with those on standard chemotherapy.
When considering signaling pathways, the PI3K/AKT/mTOR pathway has been the target of numerous clinical trials. Dysregulation of signaling through the PI3K and AKT signaling pathway occurs in 25%-30% of patients with advanced TNBC, and AKT inhibitors have been shown to extend survival in these patients. Data show, for instance, that adding capivasertib to first-line paclitaxel therapy in patients with metastatic TNBC led to longer overall survival – 19.1 months vs. 12.6 with placebo plus paclitaxel – with better survival results in patients with PIK3CA/AKT1/PTEN altered tumors.
But there’s more to learn about treating metastatic TNBC. “Relapses tend to occur early in TNBC, and some tumors are inherently resistant to chemotherapy from the get-go,” said Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai, New York. “Understanding the causes of drug response and resistance in patients with metastatic TNBC represents the holy grail.”
Dr. Nanda agreed, noting that advancing treatments for TNBC will hinge on identifying the key factors driving metastasis. “For TNBC, we are still trying to elucidate the best molecular targets, while at the same time trying to identify robust biomarkers to predict benefit from therapies we already have available,” she said.
A version of this article first appeared on Medscape.com.
Treating triple-negative breast cancer (TNBC), one of the more lethal breast cancer subtypes, remains a challenge. By definition, TNBC lacks the three telltale molecular signatures known to spur tumor growth: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). A growing amount of literature shows that these frequently aggressive tumors harbor a rich array of molecular characteristics but no clear oncogenic driver.
“TNBC is incredibly heterogeneous, which makes it challenging to treat,” said Rita Nanda, MD, director of the breast oncology program and associate professor of medicine at the University of Chicago. “We have subsets of TNBC that don’t respond to currently available therapies and, as of yet, have no identifiable therapeutic targets.”
Overall, about 40% of patients with TNBC show a pathologic complete response after first-line neoadjuvant chemotherapy – typically anthracycline and taxane-based agents. But for 50% of patients, chemotherapy leaves behind substantial residual cancer tissue. These patients subsequently face a 40%-80% risk for recurrence and progression to advanced disease.
When triple-negative disease metastasizes, survival rates plummet. The most recent data from the National Cancer Institute, which tracked patients by stage of diagnosis between 2010 and 2016, showed steep declines in 5-year survival as TNBC progressed from local (91.2%) to regional (65%) to advanced-stage disease (11.5%).
Experts have started to make headway identifying and targeting different molecular features of advanced TNBC. These approaches often focus on three key areas: targeting cell surface proteins or oncogenes, stimulating an anticancer immune response, or inhibiting an overactive signaling pathway.
“For a patient with metastatic breast cancer, finding a molecular target or an oncogenic driver is essential,” said Kelly McCann, MD, PhD, a hematologist/oncologist in the department of medicine at the University of California, Los Angeles. “Because TNBC encompasses many different molecular subsets of breast cancer, the development of effective new therapeutics is going to depend on subdividing TNBC into categories with more clear targets.”
A targeted strategy
The Food and Drug Administration’s approval of sacituzumab govitecan, the first antibody-drug conjugate to treat metastatic TNBC, marked an important addition to the TNBC drug armamentarium. “Sacituzumab govitecan is one of the most exciting drugs available for the treatment of metastatic disease,” Dr. Nanda said.
Sacituzumab govitecan, approved as third-line therapy for metastatic TNBC, works by targeting the cell surface protein TROP2, expressed in about 88% of TNBC tumors but rarely in healthy cells.
In the phase 1/2 ASCENT trial, the median progression-free survival was 5.5 months and overall survival was 13.0 months in 108 patients with metastatic TNBC who had received at least two therapies prior to sacituzumab govitecan.
A subsequent phase 3 trial showed progression-free survival of 5.6 months with sacituzumab govitecan and 1.7 months with physician’s choice of chemotherapy. The median overall survival was 12.1 months and 6.7 months, respectively.
But, according to the analysis, TROP2 expression did not necessarily predict who would benefit from sacituzumab govitecan. A biomarker study revealed that although patients with moderate to high TROP2 expression exhibited the strongest treatment response, those with low TROP2 expression also survived longer when given sacituzumab govitecan, compared with chemotherapy alone.
In other words, “patients did better on sacituzumab govitecan regardless of TROP2 expression, which suggests we do not have a good biomarker for identifying who will benefit,” Dr. Nanda said.
Two other investigational antibody-drug conjugates, trastuzumab deruxtecan and ladiratuzumab vedotin, show promise in the metastatic space as well. For instance, the recent phase 2 trial evaluating trastuzumab deruxtecan in patients with HER2-positive breast cancer reported treatment response in 44% of patients with HER2-low tumors.
Given that about 36.6% of TNBC tumors exhibit low levels of HER2 expression, “trastuzumab deruxtecan represents potential in treating HER2-low TNBC,” said Yuan Yuan, MD, PhD, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
Early results from a phase 1b study showed that trastuzumab deruxtecan produced a response rate of 37% in patients with HER2-low breast cancer.
Investigators are now recruiting for an open-label phase 3 trial to determine whether trastuzumab deruxtecan extends survival in patients with HER2-low metastatic breast cancers.
Immunotherapy advances
Immune checkpoint inhibitors represent another promising treatment avenue for metastatic TNBC. Pembrolizumab and atezolizumab, recently approved by the FDA, show moderate progression-free and overall survival benefits in patients with metastatic TNBC expressing PD-L1. Estimates of PD-L1 immune cells present in TNBC tumors vary widely, from about 20% to 65%.
Yet, data on which patients will benefit are not so clear-cut. “These drugs give us more choices and represent the fast-evolving therapeutic landscape in TNBC, but they also leave a lot of unanswered questions about PD-L1 as a biomarker,” Dr. Yuan said.
Take two recent phase 3 trials evaluating atezolizumab: IMpassion130 and IMpassion131. In IMpassion130, patients with PDL1–positive tumors exhibited significantly longer median overall survival on atezolizumab plus nab-paclitaxel (25.0 months) compared with nab-paclitaxel alone (15.5 months). As with the trend observed in the TROP2 data for sacituzumab govitecan, all patients survived longer on atezolizumab plus nab-paclitaxel regardless of PD-L1 status: 21.3 months vs. 17.6 months with nab-paclitaxel alone.
However, in IMpassion131, neither progression-free survival nor overall survival significantly improved in the PD-L1–positive group receiving atezolizumab plus paclitaxel compared with paclitaxel alone: Progression-free survival was 5.7 months vs. 6 months, respectively, and overall survival was 28.3 months vs. 22.1 months.
“It is unclear why this study failed to demonstrate a significant improvement in progression-free survival with the addition of atezolizumab to paclitaxel,” Dr. Nanda said. “Perhaps the negative finding has to do with how the trial was conducted, or perhaps the PD-L1 assay used is an unreliable biomarker of immunotherapy benefit.”
Continued efforts to understand TNBC
Given the diversity of metastatic TNBC and the absence of clear molecular targets, researchers are exploring a host of therapeutic strategies in addition to antibody-drug conjugates and immunotherapies.
On the oncogene front, researchers are investigating common mutations in TNBC. About 11% of TNBC tumors, for instance, carry germline mutations in BRCA1 and BRCA2. These tumors may be more likely to respond to platinum agents and PARP inhibitors, such as FDA-approved olaparib. In a phase 3 trial, patients with metastatic HER2-negative breast cancer and a germline BRCA mutation who received olaparib exhibited a 2.8-month longer median progression-free survival and a 42% reduced risk for disease progression or death compared with those on standard chemotherapy.
When considering signaling pathways, the PI3K/AKT/mTOR pathway has been the target of numerous clinical trials. Dysregulation of signaling through the PI3K and AKT signaling pathway occurs in 25%-30% of patients with advanced TNBC, and AKT inhibitors have been shown to extend survival in these patients. Data show, for instance, that adding capivasertib to first-line paclitaxel therapy in patients with metastatic TNBC led to longer overall survival – 19.1 months vs. 12.6 with placebo plus paclitaxel – with better survival results in patients with PIK3CA/AKT1/PTEN altered tumors.
But there’s more to learn about treating metastatic TNBC. “Relapses tend to occur early in TNBC, and some tumors are inherently resistant to chemotherapy from the get-go,” said Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai, New York. “Understanding the causes of drug response and resistance in patients with metastatic TNBC represents the holy grail.”
Dr. Nanda agreed, noting that advancing treatments for TNBC will hinge on identifying the key factors driving metastasis. “For TNBC, we are still trying to elucidate the best molecular targets, while at the same time trying to identify robust biomarkers to predict benefit from therapies we already have available,” she said.
A version of this article first appeared on Medscape.com.
Treating triple-negative breast cancer (TNBC), one of the more lethal breast cancer subtypes, remains a challenge. By definition, TNBC lacks the three telltale molecular signatures known to spur tumor growth: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). A growing amount of literature shows that these frequently aggressive tumors harbor a rich array of molecular characteristics but no clear oncogenic driver.
“TNBC is incredibly heterogeneous, which makes it challenging to treat,” said Rita Nanda, MD, director of the breast oncology program and associate professor of medicine at the University of Chicago. “We have subsets of TNBC that don’t respond to currently available therapies and, as of yet, have no identifiable therapeutic targets.”
Overall, about 40% of patients with TNBC show a pathologic complete response after first-line neoadjuvant chemotherapy – typically anthracycline and taxane-based agents. But for 50% of patients, chemotherapy leaves behind substantial residual cancer tissue. These patients subsequently face a 40%-80% risk for recurrence and progression to advanced disease.
When triple-negative disease metastasizes, survival rates plummet. The most recent data from the National Cancer Institute, which tracked patients by stage of diagnosis between 2010 and 2016, showed steep declines in 5-year survival as TNBC progressed from local (91.2%) to regional (65%) to advanced-stage disease (11.5%).
Experts have started to make headway identifying and targeting different molecular features of advanced TNBC. These approaches often focus on three key areas: targeting cell surface proteins or oncogenes, stimulating an anticancer immune response, or inhibiting an overactive signaling pathway.
“For a patient with metastatic breast cancer, finding a molecular target or an oncogenic driver is essential,” said Kelly McCann, MD, PhD, a hematologist/oncologist in the department of medicine at the University of California, Los Angeles. “Because TNBC encompasses many different molecular subsets of breast cancer, the development of effective new therapeutics is going to depend on subdividing TNBC into categories with more clear targets.”
A targeted strategy
The Food and Drug Administration’s approval of sacituzumab govitecan, the first antibody-drug conjugate to treat metastatic TNBC, marked an important addition to the TNBC drug armamentarium. “Sacituzumab govitecan is one of the most exciting drugs available for the treatment of metastatic disease,” Dr. Nanda said.
Sacituzumab govitecan, approved as third-line therapy for metastatic TNBC, works by targeting the cell surface protein TROP2, expressed in about 88% of TNBC tumors but rarely in healthy cells.
In the phase 1/2 ASCENT trial, the median progression-free survival was 5.5 months and overall survival was 13.0 months in 108 patients with metastatic TNBC who had received at least two therapies prior to sacituzumab govitecan.
A subsequent phase 3 trial showed progression-free survival of 5.6 months with sacituzumab govitecan and 1.7 months with physician’s choice of chemotherapy. The median overall survival was 12.1 months and 6.7 months, respectively.
But, according to the analysis, TROP2 expression did not necessarily predict who would benefit from sacituzumab govitecan. A biomarker study revealed that although patients with moderate to high TROP2 expression exhibited the strongest treatment response, those with low TROP2 expression also survived longer when given sacituzumab govitecan, compared with chemotherapy alone.
In other words, “patients did better on sacituzumab govitecan regardless of TROP2 expression, which suggests we do not have a good biomarker for identifying who will benefit,” Dr. Nanda said.
Two other investigational antibody-drug conjugates, trastuzumab deruxtecan and ladiratuzumab vedotin, show promise in the metastatic space as well. For instance, the recent phase 2 trial evaluating trastuzumab deruxtecan in patients with HER2-positive breast cancer reported treatment response in 44% of patients with HER2-low tumors.
Given that about 36.6% of TNBC tumors exhibit low levels of HER2 expression, “trastuzumab deruxtecan represents potential in treating HER2-low TNBC,” said Yuan Yuan, MD, PhD, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
Early results from a phase 1b study showed that trastuzumab deruxtecan produced a response rate of 37% in patients with HER2-low breast cancer.
Investigators are now recruiting for an open-label phase 3 trial to determine whether trastuzumab deruxtecan extends survival in patients with HER2-low metastatic breast cancers.
Immunotherapy advances
Immune checkpoint inhibitors represent another promising treatment avenue for metastatic TNBC. Pembrolizumab and atezolizumab, recently approved by the FDA, show moderate progression-free and overall survival benefits in patients with metastatic TNBC expressing PD-L1. Estimates of PD-L1 immune cells present in TNBC tumors vary widely, from about 20% to 65%.
Yet, data on which patients will benefit are not so clear-cut. “These drugs give us more choices and represent the fast-evolving therapeutic landscape in TNBC, but they also leave a lot of unanswered questions about PD-L1 as a biomarker,” Dr. Yuan said.
Take two recent phase 3 trials evaluating atezolizumab: IMpassion130 and IMpassion131. In IMpassion130, patients with PDL1–positive tumors exhibited significantly longer median overall survival on atezolizumab plus nab-paclitaxel (25.0 months) compared with nab-paclitaxel alone (15.5 months). As with the trend observed in the TROP2 data for sacituzumab govitecan, all patients survived longer on atezolizumab plus nab-paclitaxel regardless of PD-L1 status: 21.3 months vs. 17.6 months with nab-paclitaxel alone.
However, in IMpassion131, neither progression-free survival nor overall survival significantly improved in the PD-L1–positive group receiving atezolizumab plus paclitaxel compared with paclitaxel alone: Progression-free survival was 5.7 months vs. 6 months, respectively, and overall survival was 28.3 months vs. 22.1 months.
“It is unclear why this study failed to demonstrate a significant improvement in progression-free survival with the addition of atezolizumab to paclitaxel,” Dr. Nanda said. “Perhaps the negative finding has to do with how the trial was conducted, or perhaps the PD-L1 assay used is an unreliable biomarker of immunotherapy benefit.”
Continued efforts to understand TNBC
Given the diversity of metastatic TNBC and the absence of clear molecular targets, researchers are exploring a host of therapeutic strategies in addition to antibody-drug conjugates and immunotherapies.
On the oncogene front, researchers are investigating common mutations in TNBC. About 11% of TNBC tumors, for instance, carry germline mutations in BRCA1 and BRCA2. These tumors may be more likely to respond to platinum agents and PARP inhibitors, such as FDA-approved olaparib. In a phase 3 trial, patients with metastatic HER2-negative breast cancer and a germline BRCA mutation who received olaparib exhibited a 2.8-month longer median progression-free survival and a 42% reduced risk for disease progression or death compared with those on standard chemotherapy.
When considering signaling pathways, the PI3K/AKT/mTOR pathway has been the target of numerous clinical trials. Dysregulation of signaling through the PI3K and AKT signaling pathway occurs in 25%-30% of patients with advanced TNBC, and AKT inhibitors have been shown to extend survival in these patients. Data show, for instance, that adding capivasertib to first-line paclitaxel therapy in patients with metastatic TNBC led to longer overall survival – 19.1 months vs. 12.6 with placebo plus paclitaxel – with better survival results in patients with PIK3CA/AKT1/PTEN altered tumors.
But there’s more to learn about treating metastatic TNBC. “Relapses tend to occur early in TNBC, and some tumors are inherently resistant to chemotherapy from the get-go,” said Charles Shapiro, MD, medical oncologist, Icahn School of Medicine at Mount Sinai, New York. “Understanding the causes of drug response and resistance in patients with metastatic TNBC represents the holy grail.”
Dr. Nanda agreed, noting that advancing treatments for TNBC will hinge on identifying the key factors driving metastasis. “For TNBC, we are still trying to elucidate the best molecular targets, while at the same time trying to identify robust biomarkers to predict benefit from therapies we already have available,” she said.
A version of this article first appeared on Medscape.com.
COVID-19 Vaccine in Veterans with Multiple Sclerosis: Protect the Vulnerable
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19 and we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
This article has been updated to reflect new US Food and Drug Administration and Centers for Disease Control and Prevention recommendations to pause administration of the Johnson and Johnson Jansen (JNJ-78436735) COVID-19 vaccine.1
Since the outbreak of the pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),a plethora of studies have been performed to increase our knowledge of its associated illness COVID-19.2 There is no cure for COVID-19, which can be lethal. In the absence of a cure, preventive measures are of vital importance. In order to help prevent the spread of the virus, the Centers for Diseases Control and Prevention (CDC) advocates for: (1) the use of a face mask over the mouth and nose; (2) a minimum of 6-foot distance between individuals; and (3) avoidance of gatherings.As of March 2021, the US Food and Drug Administration (FDA) approved 3 vaccines for the prevention of COVID-19, under an emergency use authorization (EUA).3-5
COVID-19 and Multiple Sclerosis
Since the beginning of the pandemic, neurologists have faced a new challenge—determining whether persons with multiple sclerosis (pwMS) were more at risk than others of becoming ill from COVID-19 or were destined for a worse outcome. The National MS Society has advised a personalized approach in relation to particularly vulnerable persons when needed and has also initiated worldwide registries to collect information regarding incidence and outcome of COVID-19 in pwMS. Accordingly, through the MS Center of Excellence (MSCoE), the Veterans Health Administration (VHA) has established a national registry assembling data regarding COVID-19 in veterans with MS.
A recent descriptive literature review summarized the outcomes of 873 persons with both MS and COVID-19 and reported that about 36% of COVID-19 cases were treated with B-cell depleting therapies (ocrelizumab or rituximab).6 This proportion was relatively higher when compared with other disease modifying agents. Of those who became infected with SARS-CoV-2, death from COVID-19 occurred in about 4%, and an additional 3% required assisted invasive or noninvasive ventilation. Persons reported to have passed away from COVID-19 generally were older; had progressive MS; or had associated comorbidities such as obesity, hypertension, heart or lung conditions, or cancers. Of these, 50% were not on any disease modifying agent, 25% were on B-cell depleting therapies (ocrelizumab or rituximab), and the remaining 25% were on various medications for MS. It is important to highlight that no formal statistical analyses were performed in this review. On the contrary, in the recently published Italian report on 844 pwMS who had suspected or confirmed COVID-19, the authors used univariate and multivariate models to analyze their findings and noted that the use of ocrelizumab was significantly associated with a worse clinical outcome.7 These authors also identified age, sex, disability score, and recent (within 1 month) use of steroids as risk factors for a severe COVID-19 outcome. The incidence of death from COVID-19 in this cohort was 1.54%.
The recently published data from the North American Registry of the National MS Society based on 1,626 patients reported a 3.3% incidence of death from COVID-19.8 The following factors were identified as risks for worse outcome: male sex, nonambulatory status, age, Black race, and cardiovascular disease. The use of rituximab, ocrelizumab, and steroids (the latter medication over the preceding 2 months) increased the risks of hospitalization for COVID-19.
COVID-19 Vaccines
Of the 3 available vaccines, the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is approved for individuals aged ≥ 16 years, while the Moderna COVID-19 (mRNA-1273) and the Johnson and Johnson/Jannsen COVID-19 (JNJ-78436735) vaccines are approved for individuals aged ≥ 18 years, though the latter vaccine has been temporarily suspended.1,3-5 The EUAs were released following the disclosure of the results of 3 phase 3 clinical trials and several phase 1 and 2 clinical trials.9-16
The BNT162b2 vaccine from Pfizer-BioNTech encodes the SARS-CoV-2 full-length spike protein (S) in prefusion conformation locked by the mutation in 2 prolines.9 Differently from the BNT162b2 vaccine, the BNT162b1 vaccine encodes a secreted trimerized SARS-CoV-2 receptor–binding domain. The S-glycoprotein is required for viral entry, as implicated in host cell attachment, and is the target of the neutralizing antibodies. In a phase 1 clinical study on 195 volunteers treated with BNT162b1 (10 mg, 20 mg, 30 mg, or 100 mg doses) or BNT162b2 (10 mg, 20 mg, or 30 mg doses) vaccines or placebo 21 days apart, both the binding and neutralizing antibody response was found to be age and “somewhat” dose dependent.9
Higher neutralization titers were measured at day 28 and 35 (7 and 14 days after the second dose, respectively) and compared with titers of persons who recovered from a COVID-19 infection.9 Serum neutralization was measured using a fluorescence-based high-throughput neutralization assay, while binding activity was assessed using the receptor-binding domain (RBD)–binding or S1-binding IgG direct Luminex immunoassays.
The overall reactogenicity/immunogenicity profile of BNT162b2 administered twice (30 mg each time) led to its selection for the phase 3 clinical trial.9,10 In a large phase 3 clinical trial on 43,458 participants, the BNT162b2 vaccine given at 30 mg doses 21 days apart conferred 95% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.10 No safety concerns to stop the trial were identified, though related severe and life-threatening events were reported in 0.3% and 0.1% of the volunteers, respectively. We note that these incidence rates were the same for the treated and the placebo group.
The mRNA-1273 vaccine from Moderna also encodes the SARS-CoV-2 S-glycoprotein. In a dose escalation phase 1 trial of 45 participants aged between 18 and 55 years (25 mg, 100 mg or 250 mg, given at days 1 and 29) and 40 participants aged ≥ 57 years (25 mg and 100 mg, given at days 1 and 29), a dose-dependent effect was observed for both binding (receptor-binding domain and S-2p IgG on enzyme-linked immunosorbent assay [ELISA])and neutralizing antibodies (SARS-CoV-2 nanoluciferase high-throughput neutralization assay, focus reduction neutralization test mNeonGreen and SARS-CoV-2 plaque-reduction neutralization testing assay) development.11,12 The geometric mean of both binding and neutralizing antibodies declined over time but persisted high as late as 119 days after the first burst of 100 mg dose.13 The same dose of the vaccine also elicited a strong T helper-1 response with little T helper-2 response across all ages.11 The strength of the memory cellular response remains to be defined and is the subject of ongoing investigations. In a large phase 3 clinical trial with 30,420 participants, the Moderna COVID-19 mRNA-1273 vaccine, given 28 days apart at the dose of 100 mg, met 94.1% clinical efficacy in reducing the likelihood of being affected by symptomatic COVID-19.14
Less than 0.1% of volunteers in both groups withdrew from the trial due to adverse effects (AEs); 0.5% in the placebo group and 0.3% in the treated group had AEs after the first dose, which precluded receiving the second dose.14
The Johnson and Johnson/Jannsen JNJ-78436735 vaccine is based upon a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector, which encodes the full-length, stabilized S-glycoprotein of SARS-CoV-2. The currently reported results of the phase 1 and 2 clinical study indicated that 805 volunteers (402 participants between ages 18 and 55 years and 403 individuals aged ≥ 65 years) were randomized to receive a single or double dose of either 5 x 1010 viral particles per 0.5 mL (low dose) or 1 x 1011 viral particles per 0.5 mL (high dose), each compared with a placebo group. Incidence of seroconversion to binding antibodies against the full-length stabilized S-glycoprotein, as measured by ELISA, showed ≥ 96% seroconversion by day 29 after the first dose. The incidence of seroconversion to neutralizing antibodies was ≥ 90% as early as early as 29 days after the first of either dose. In this study, neutralization activity was measured using the wild-type virus microneutralization assay based on the Victoria/1/2020/ SARS-CoV-2 strain.15 We note that the data related to this study have been partially reported and additional information will be available when each participant will have received the second dose.
In a large phase 3 clinical trial with 40,000 participants aged between 18 and 100 years, the Johnson and Johnson/Jannsen JNJ-78436735 vaccine, given as single dose of 5 x 1010 viral particles per 0.5 mL, met 65.5% clinical efficacy in the likelihood of being affected by symptomatic COVID-19 ≥ 28 days postimmunization.16 In this study, the vaccine efficacy was found to have a geographic distribution with highest efficacy in the US (74.4%), followed by Latin America (64.7%) where Brazil showed a predominance of the P2 COVID-19 lineage (64.7%), and Africa (52%) where the B.1.351 lineage was most frequent (94.5%). The vaccine also proved to be effective in reducing the likelihood of asymptomatic seroconversion, as measured by the level of a non-S protein, eg, 0.7% of positive cases in the vaccine group vs 2.8% in the placebo group. Immunological data indicated that the vaccine response was mainly driven by T-helper 1 lymphocytes. As of April 13, 2021 the FDA has recommend suspending the administration of the Johnson and Johnson/Janssen vaccine due to the occurrence of severe blood clots reported in a 6 subjects out of ~6.8 millions administered doses.1
It is noteworthy to highlight that all vaccines reduced the likelihood of hospitalizations and deaths due to COVID-19.
As of April 17, 2021, the CDC reports that more than 130 million (40%) Americans, nearly 1/3 of the population, have received at least 1 dose of any of the 3 available vaccines, including 4.6 million at the VHA.17 Using the Vaccine Adverse Event Reporting System and v-safe, the US is conducting what has been defined the most “intense and comprehensive safety monitoring in the US history.”18 Thus far, data affirm the overall safety of the available vaccines against COVID-19. Individuals should not receive the COVID-19 vaccines if they have had a severe allergic reaction to any ingredient in the vaccine or a severe allergic reaction to a prior dose of the vaccine. Additionally, individuals who have received convalescent plasma should wait 90 days before getting the COVID-19 vaccine.
Vaccination for Persons with MS
PwMS or those on immunosuppressive medications were excluded from the clinical trial led by Pfizer-BioNTech. There is no mention of MS as comorbidity in the study from Moderna, although this condition is not listed as an exclusion criterion either. The results of the phase 3 clinical trial for the Johnson and Johnson/Janssen vaccine are not fully public yet, thus this information is not known as well. As a result, the use of this vaccine in pwMS under immunomodulatory agents is based on previous knowledge of other vaccines. Evidence is growing for the safety of the BNT162b2 COVID-19 vaccination in pwMS.19 Data regarding COVID-19 efficacy and safety are still largely based on previous knowledge on other vaccines.20,21
Immunization of pwMS is considered safe and should proceed with confidence in those persons who have no other contraindication to receive a vaccine. A fundamental problem for pwMS treated with immunomodulatory or immunosuppressive medications is whether the vaccine will remain safe or be able to solicit an adequate immune response.20,21 As of the time of publication 2021, there is consensus that mRNA based or inactivated vaccines are also considered safe in pwMS undergoing immunomodulatory or immunosuppressive treatments.20-23 We advise a one-on-one conversation between each veteran with MS and their primary neurologist to understand the importance of the vaccination, the minimal risks associated with it and if any specific treatment modification should be made.
To provide guidance, the National MS Society released a position statement that is regularly updated.22 Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. In addition, on the basis of available literature and the American Academy of Neurology recommendations on the use of vaccines in general, the following recommendations are proposed.20-23
Recommendation 1: injections, orals, and natalizumab. Given the risks associated with discontinuation of disease modifying agents, pwMS opting to receive a COVID-19 vaccine should continue taking their medications unless recommended otherwise by their primary neurologist. Neither delay in start nor adjustments in dosing or timing of administration are advised for pwMS taking currently available either generic or brand formulations of β interferons, glatiramer acetate, teriflunomide, dimethyl or monomethyl fumarate, or natalizumab.22
Recommendation 2: anti-CD20 monoclonal infusions. As an attenuated humoral response is predicted in pwMS treated with anti-CD20 monoclonal infusions, coordinating the timing of vaccination with treatment schedule may maximize efficacy of the vaccine. Whenever possible, it is advised to be vaccinated ≥ 12 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting anti-CD20 monoclonal infusions are advised to be fully vaccinated first and start these medications ≥ 2 to 4 weeks later.22
Recommendation 3: alemtuzumab infusion. Given its effect on CD52+ cells, it is advised to be vaccinated ≥ 24 weeks after the last infusion and to resume infusion 4 weeks after the last dose of the vaccine. PwMS starting alemtuzumab infusions are advised to get fully vaccinated first and start this medication 4 weeks or more after completing the vaccine.22
Recommendation 4: sphingosine 1 phosphate receptor modulators, oral cladribine, and ofatumumab. PwMS starting any of these medications are advised to be fully vaccinated first and start these medications 2 to 4 weeks after completing the vaccine. PwMS already on those medications are not advised to change the schedule of administration. When possible, though, one should resume the dose of cladribine or ofatumumab 2 to 4 weeks after the last dose of the vaccine. 20
Notably, all these recommendations hold true when there is enough disease stability to allow delaying treatment. We also add that it remains unclear if persons with an overall very low number of lymphocytes will be able to elicit a strong reaction to the vaccine. Blood collection and analysis of white blood cell count and lymphocyte subset estimates should be obtained in those persons with a markedly suppressed immune system. Whenever possible, to maximize outcome, timing the vaccination with treatment should be considered in those persons with a markedly reduced number of T-helper 1 cells.
Vaccination for Veterans
Currently the VHA is offering to veterans the Pfizer and Moderna COVID-19 vaccines with FDA EUAs. In accordance with FDA regulations, the VHA has paused administration of the Johnson and Johnson/Janssen vaccine. The VHA has launched its vaccination program in December 2020 by first providing the vaccine to health care personnel, nursing home patients, spinal cord injury patients, chemotherapy patients, dialysis and transplant patients, as well as homeless veterans. Most VA health care systems have passed this phase and are now able to provide vaccines to veterans with MS.
In December 2020, the MSCoE released a position statement regarding the importance and safety of the COVID-19 vaccine for veterans with MS.24 This statement will be updated on a regular basis as new information becomes available from major organizations like the National MS Society, FDA, CDC, and World Health Organization (WHO) or relevant literature.
Conclusions
Older veterans with progressive MS and associated comorbidities are at higher risk of death should they be infected by COVID-19. Fortunately, we live in a time where vaccines are recognized as a critical tool to prevent this infection and to significantly reduce its morbidity and mortality. Yet, hesitancy to vaccinate has been identified as one of the most important threats to public health by the WHO in 2019.25 Understandably such hesitancy is even more profound for the COVID-19 vaccine, which is being administered under an EUA. In light of this indecision, and given the current state of the pandemic, we urge health care providers to educate every veteran about the benefits of being vaccinated against COVID-19. Within the VHA, a solid campaign of vaccination has been put in place at an unprecedented speed.
Health care providers interacting with veterans with MS are encouraged to use the MSCoE website (www.va.gov/ms) for any questions or concerns, or to reach out to MSCoE staff. It is vitally important that our community of veterans receives appropriate education on the importance of this vaccination for their own safety, for that of their household and society.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
1. Centers for Disease Control and Prevention. Recommendation to pause use of Johnson & Johnson’s Janssen COVID-19 vaccine. Updated April 16, 2021. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed March 9, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
3. US Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
4. US Food and Drug Administration. Moderna COVID-19 vaccine. Updated February 3, 2021. Accessed March 22, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
5. US Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine [press release]. Published February 27, 2021. Accessed March 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine
6. Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9(12):4067. Published 2020 Dec 16. doi:10.3390/jcm9124067
7. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780-789. doi:10.1002/ana.26028
8. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis [published online ahead of print, 2021 Mar 19]. JAMA Neurol. 2021;10.1001/jamaneurol.2021.0688. doi:10.1001/jamaneurol.2021.0688
9. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. doi:10.1056/NEJMoa2027906
10. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
11. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report. N Engl J Med. 2020;383(20):1920-1931. doi:10.1056/NEJMoa2022483
12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
13. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi:10.1056/NEJMc2032195
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
15. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine [published online ahead of print, 2021 Jan 13]. N Engl J Med. 2021;NEJMoa2034201. doi:10.1056/NEJMoa2034201
16. Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329-332. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009e4
17. US Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Updated March 21, 2021. Accessed March 22, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations
18. Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283-288. Published 2021 Feb 26. doi:10.15585/mmwr.mm7008e3
19. Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021 [published online ahead of print, 2021 Apr 15]. Mult Scler. 2021;13524585211003476. doi:10.1177/13524585211003476
20. Righi E, Gallo T, Azzini AM, et al. A review of vaccinations in adult patients with secondary immunodeficiency [published online ahead of print, 2021 Mar 9]. Infect Dis Ther. 2021;1-25. doi:10.1007/s40121-021-00404-y
21. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord. 2020;45:102439. doi:10.1016/j.msard.2020.102439
22. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Accessed March 22, 2021. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance
23. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi:10.1212/WNL.0000000000008157
24. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Coronavirus (COVID-19) and vaccine information. Updated February 25. 2021. Accessed March 9, 2021. https://www.va.gov/ms
25. World Health Organization. Ten threats to global health in 2019. Accessed March 18, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
University taking aim at racial disparities in COVID vaccine trials
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Guarding against nonmelanoma skin cancer in solid organ transplant recipients
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
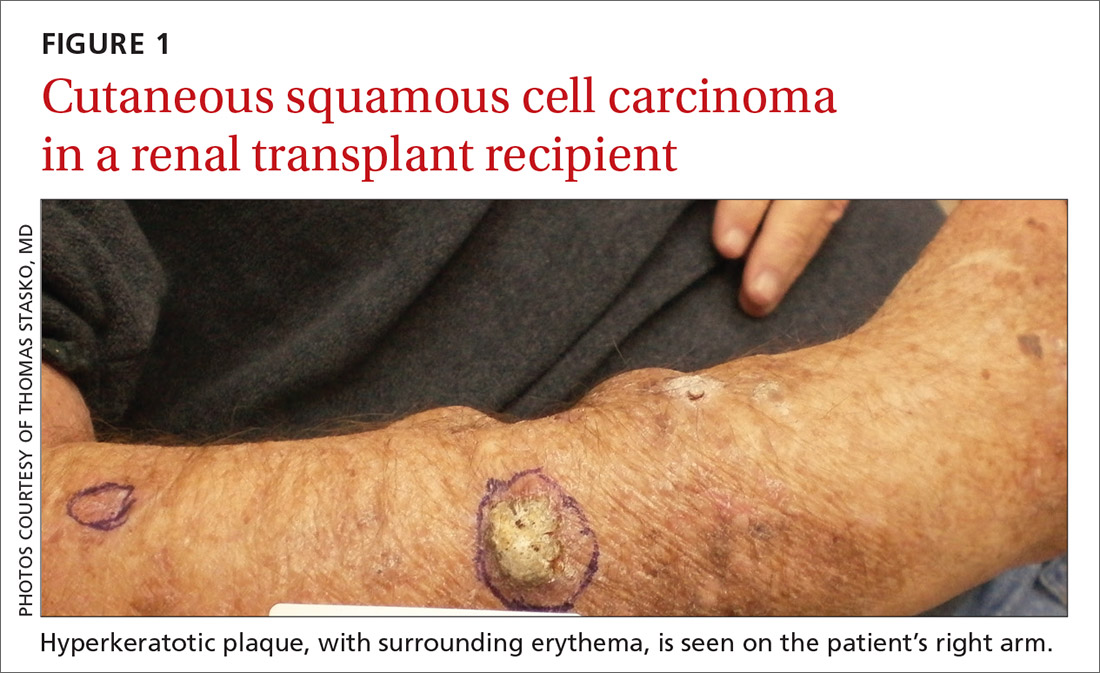
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
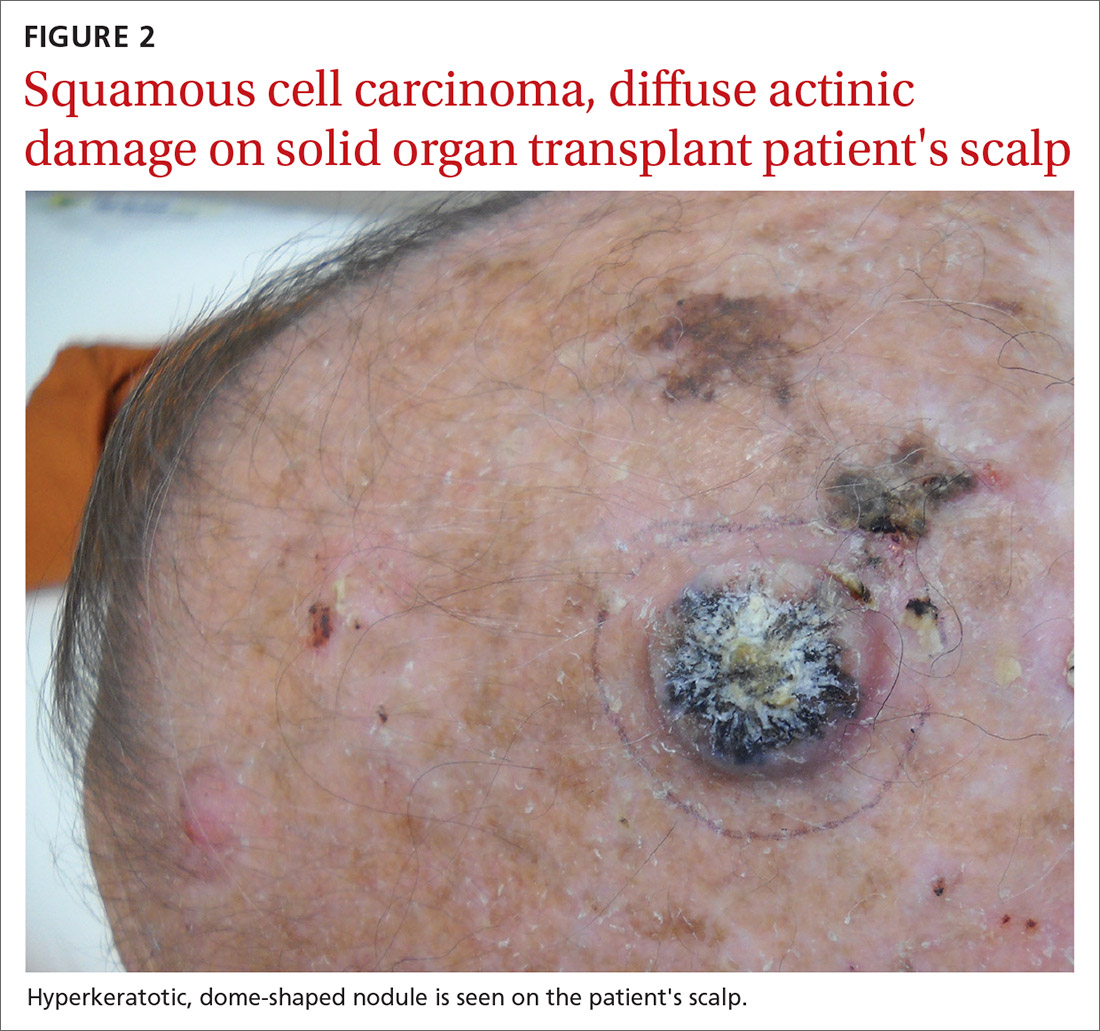
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
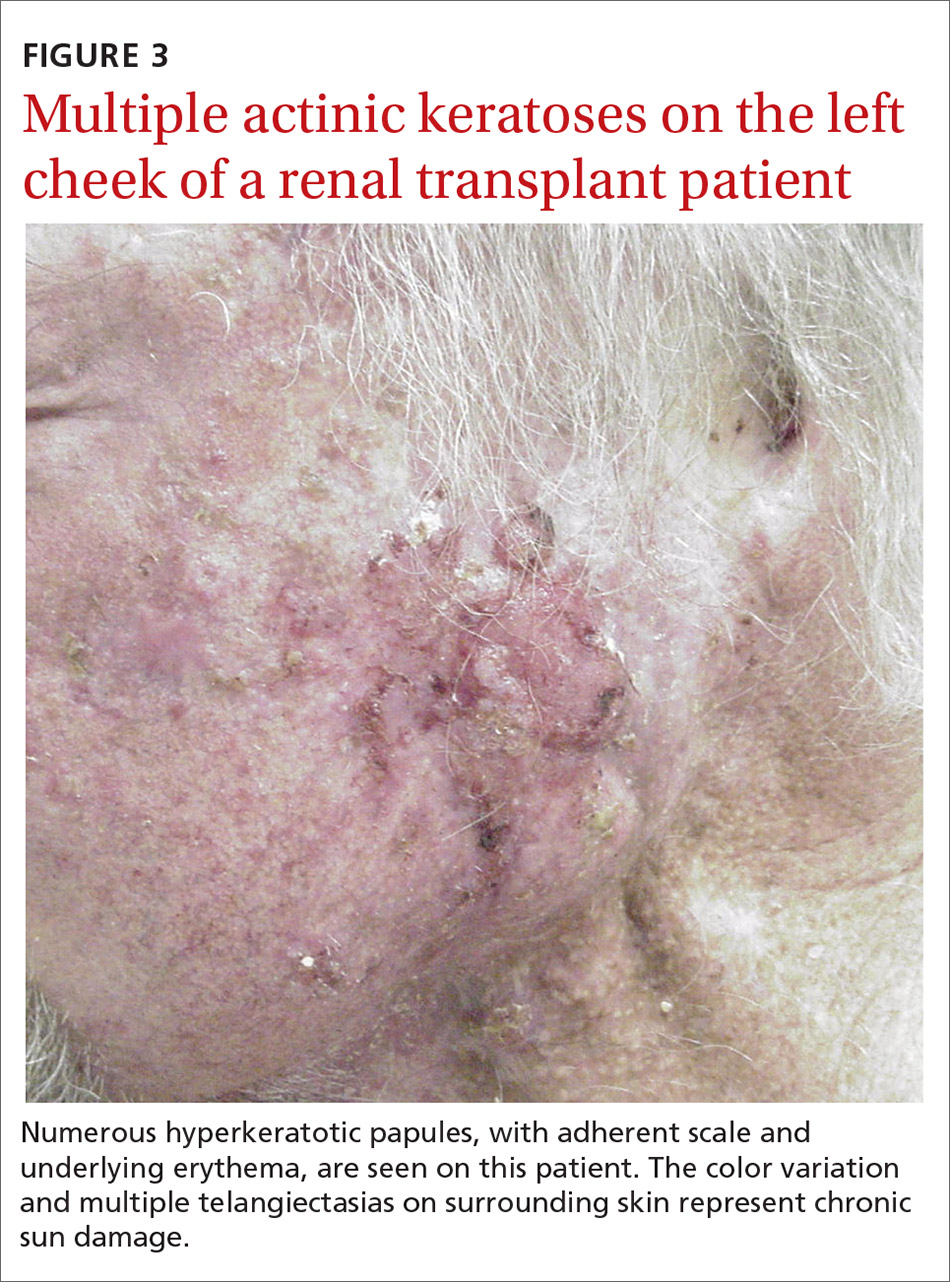
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
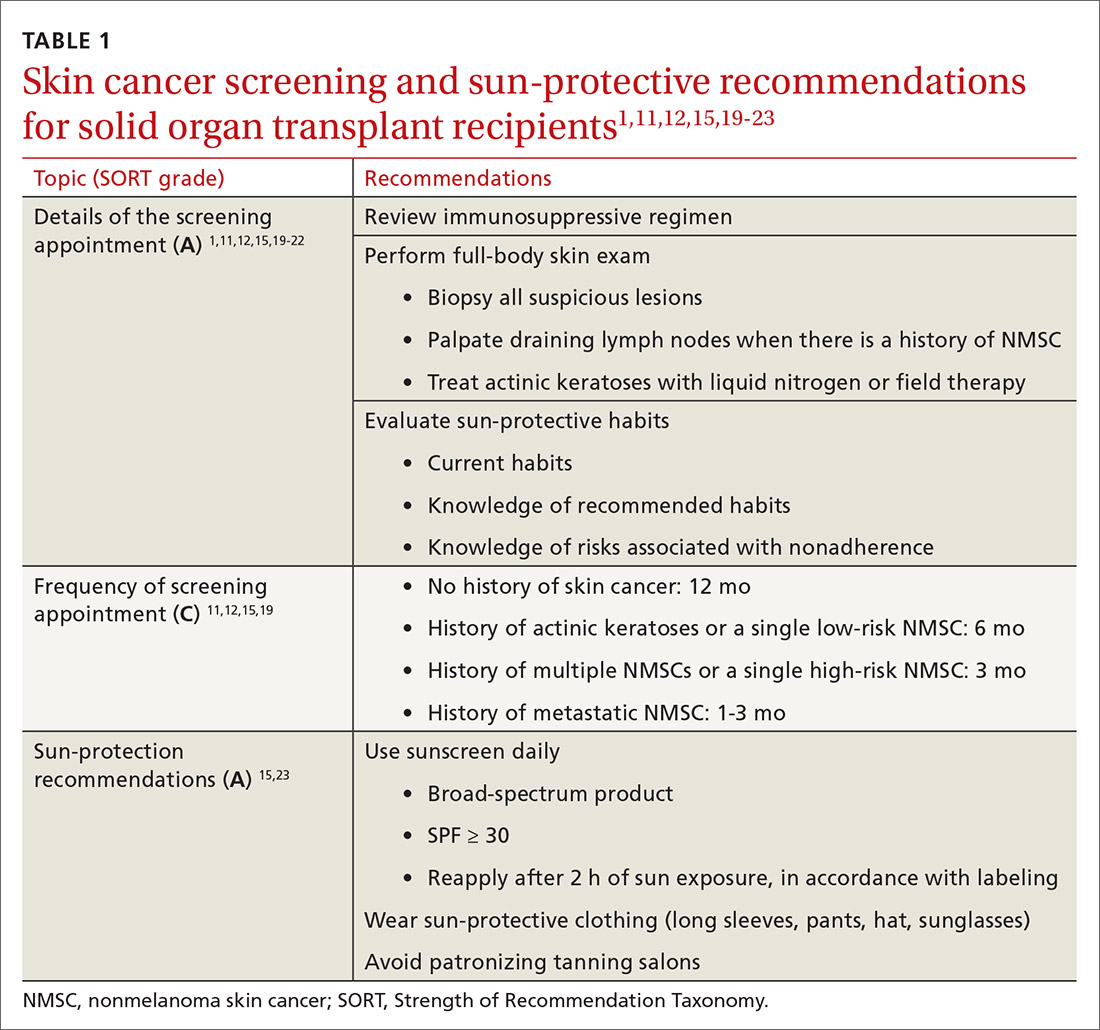
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
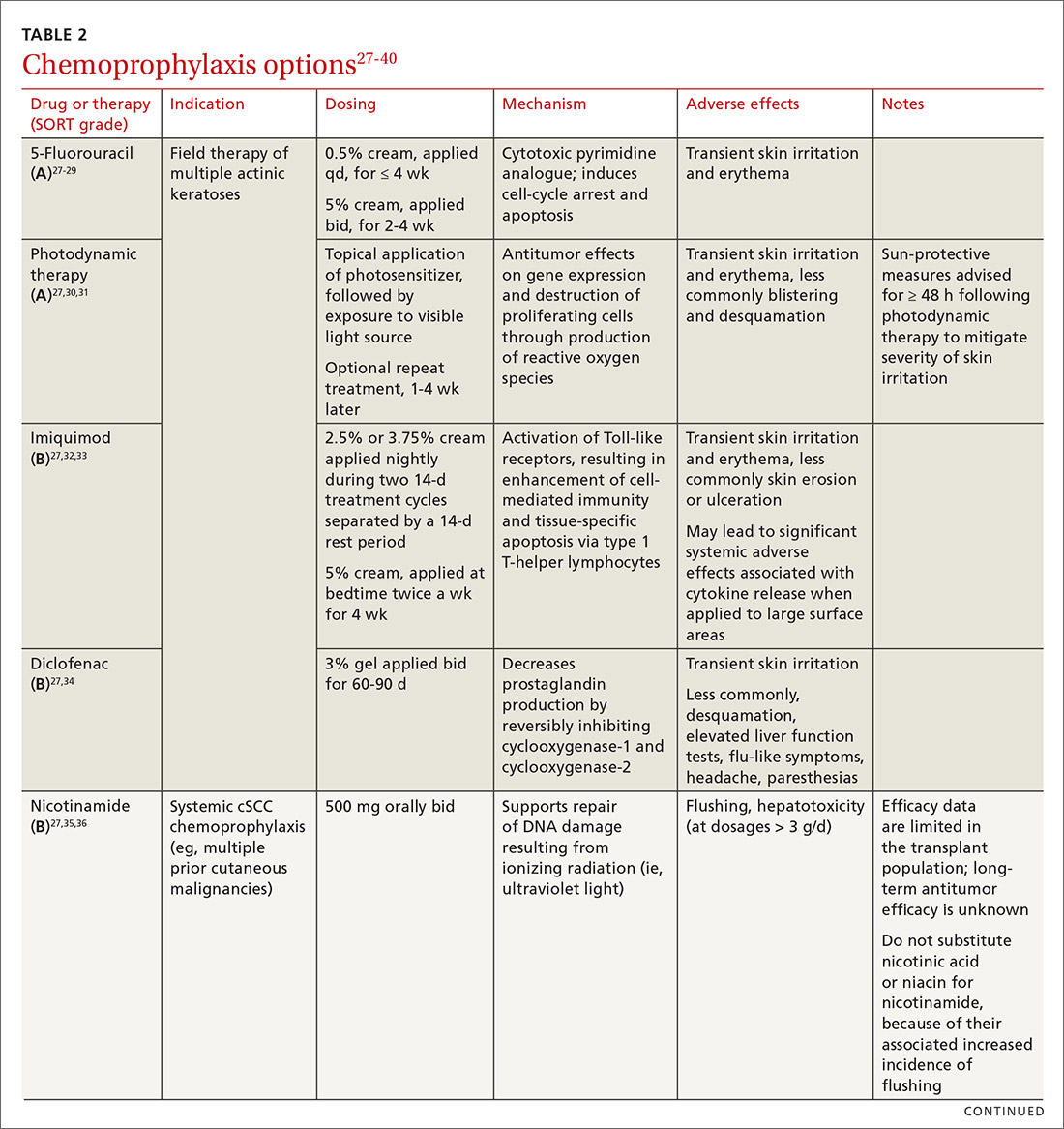
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
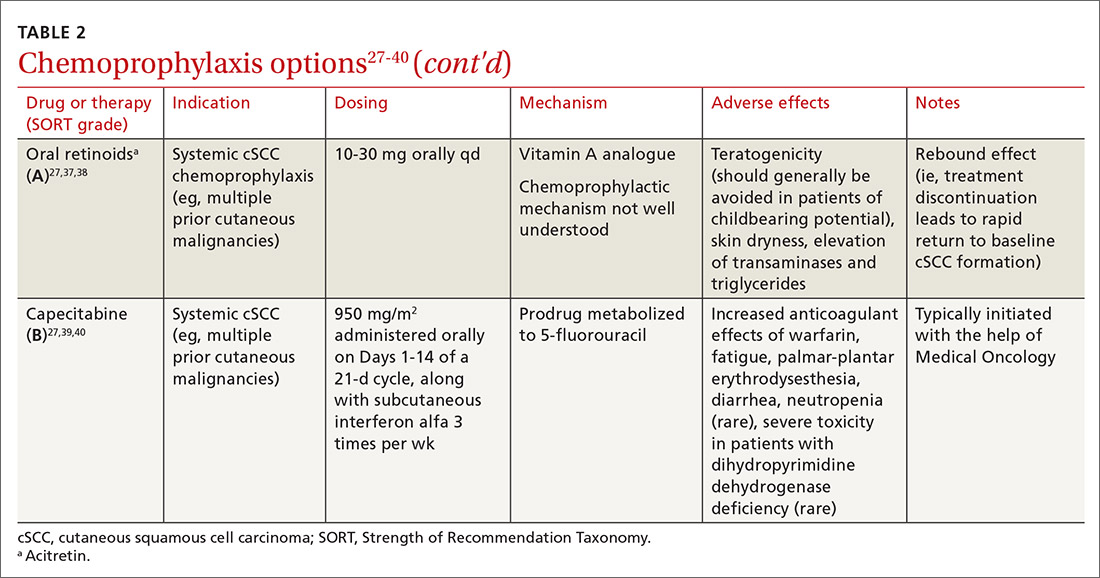
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
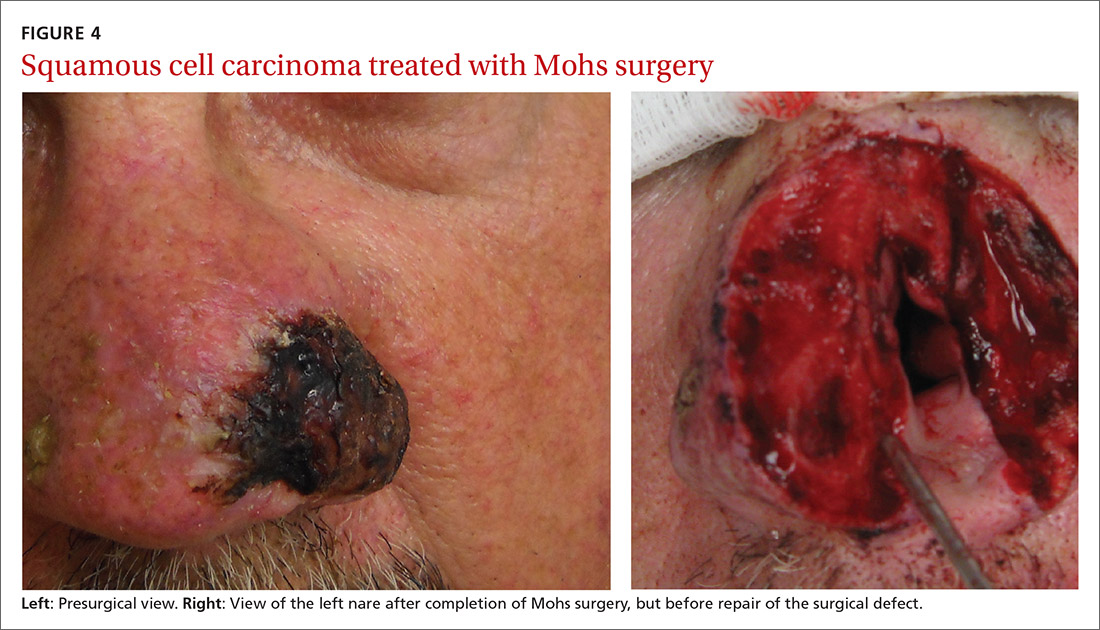
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
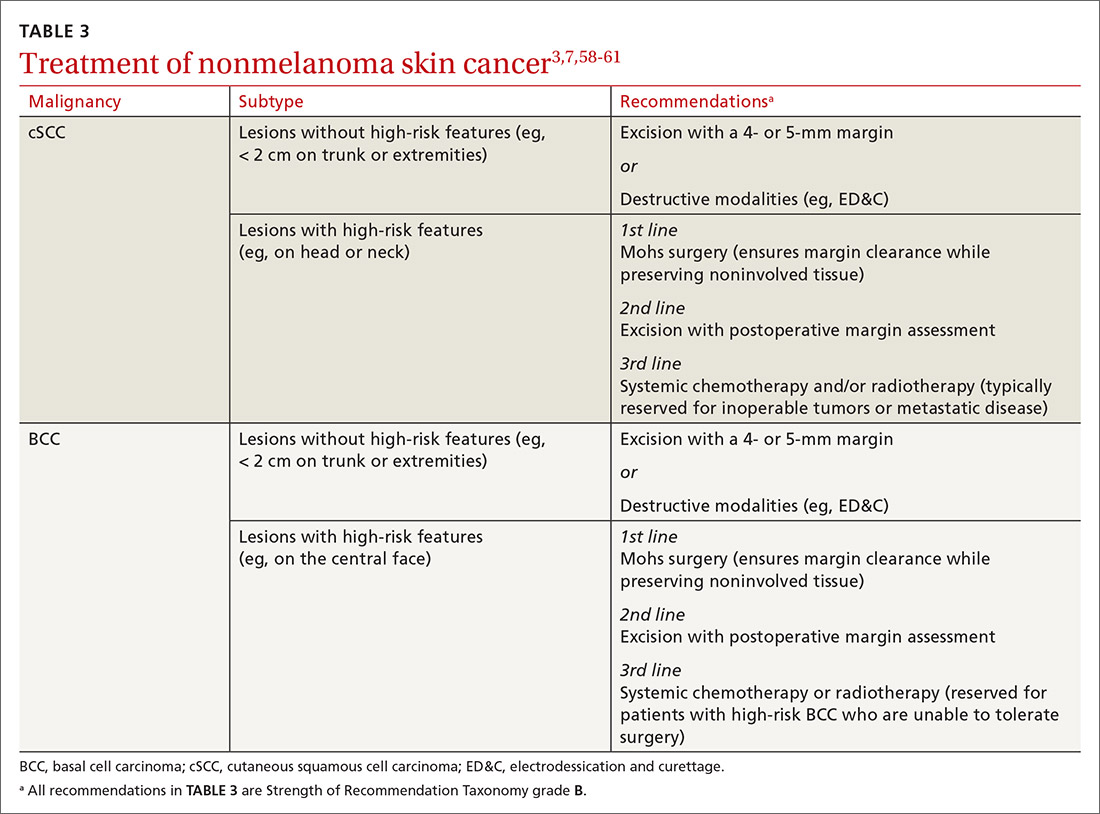
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.

NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8

The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22

Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15

SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27

5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27

PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3

Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)

Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.

NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8

The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22

Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15

SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27

5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27

PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3

Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)

Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
PRACTICE RECOMMENDATIONS
› Conduct a full-body skin examination at least once annually for solid organ transplant recipients. C
› Encourage daily use of broad-spectrum SPF ≥ 30 sunscreen and sun-protective clothing (long sleeves, pants, wide-brimmed hats) for these patients. A
› Consider chemoprophylactic agents for patients at especially high risk of nonmelanoma skin cancer. A
› Treat nonmelanoma skin cancer in a solid organ transplant recipient aggressively because of their increased risk of recurrence, local invasion, and metastasis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Urine drug screening: A guide to monitoring Tx with controlled substances
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4
A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15
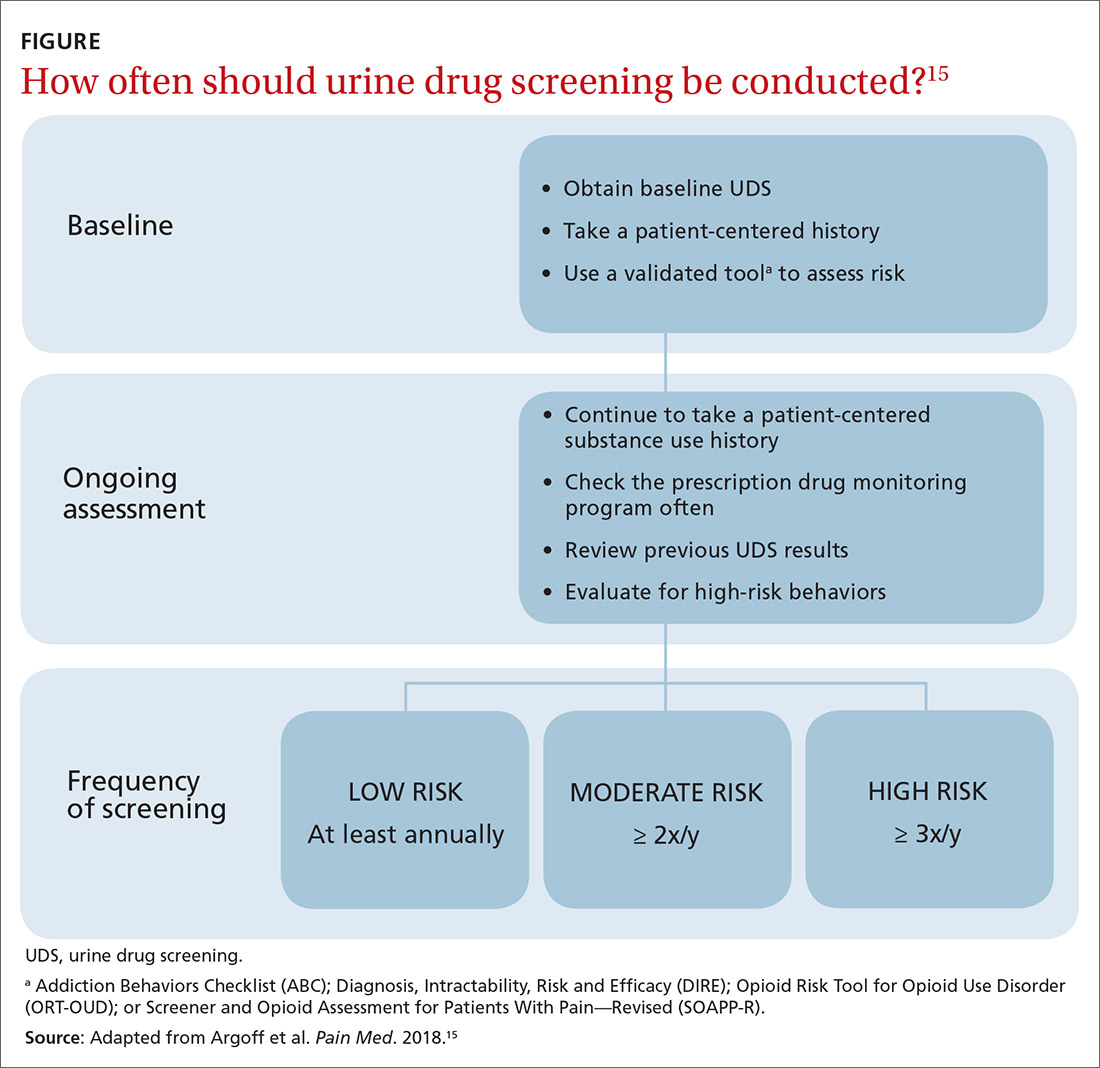
Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19
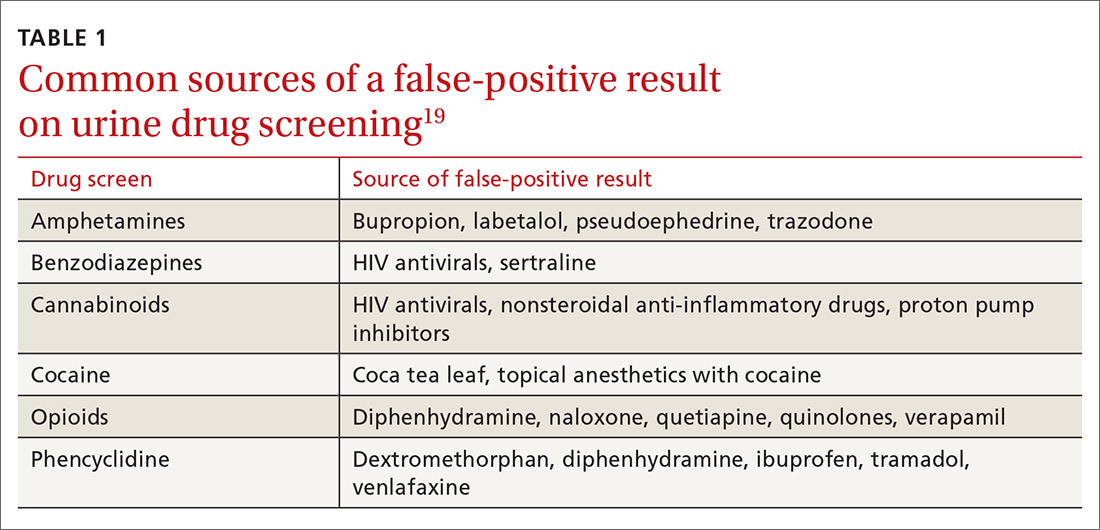
Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20
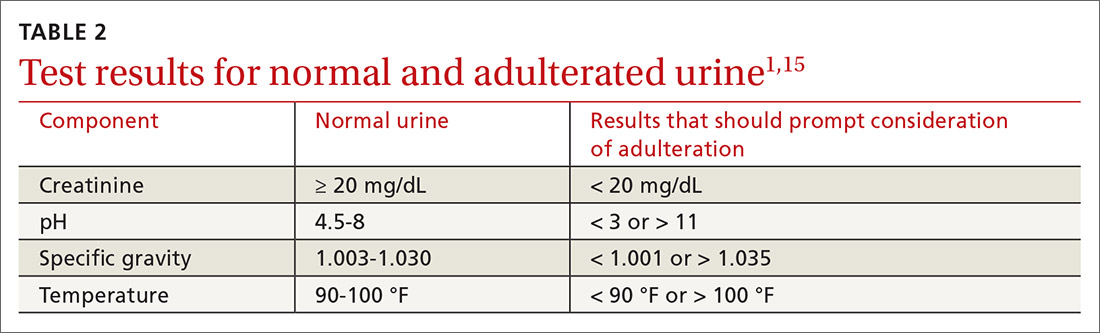
Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).
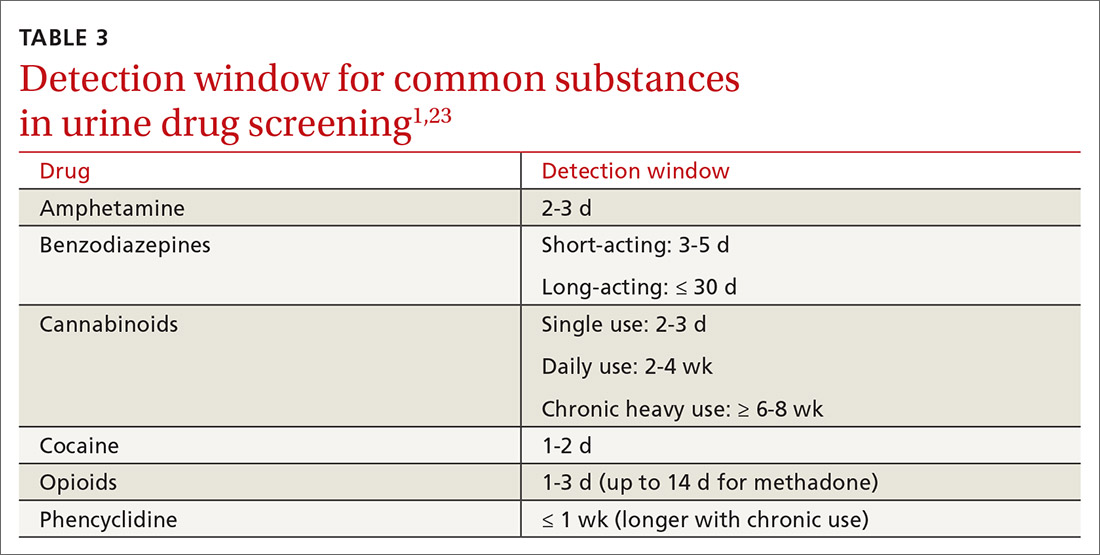
Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4

A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15

Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19

Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20

Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).

Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4

A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15

Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19

Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20

Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).

Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
PRACTICE RECOMMENDATIONS
› Consider developing a risk-based urine drug testing protocol for all patients who are on chronic opioid therapy. C
› Consider urine drug testing to augment a thorough history when identifying and offering treatment to patients with a substance use disorder. A
› Do not change your management plan based on results of a single screening urine test. Revisit unexpected positive or negative results with a thorough history or confirmatory testing. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series