User login
AMA discharge linked to increased readmissions, discontinuity of care
Background: AMA discharges are common (1%-2% of all U.S. discharges) and disproportionately affect vulnerable patient populations, specifically those of lower socioeconomic status and the uninsured. Previous studies have been insufficiently powered to assess the effects of AMA discharge on 30-day readmission rates at a national level.
Study design: Retrospective cohort.
Setting: Community and teaching hospitals in 22 states.
Synopsis: With use of the 2014 Nationwide Readmissions Database of 23,110,641 index hospitalizations of patients 18 years or older, this study found that AMA discharge occurred with 1.3% of admissions. AMA discharge was associated with greater than twice the odds of 30-day readmission, compared with routine discharge. Of patients discharged AMA, 20.2% had an unplanned readmission within 30 days, compared with 10.1% of patients discharged routinely (OR, 2.25; 95% CI, 2.20-2.30; P less than .001).
Patients who were discharged AMA had almost 20 times the odds of undergoing repeat AMA discharge at readmission (OR, 18.41; 95% CI, 17.46-19.41; P less than .001) and twice the odds of presenting to a different hospital (OR, 2.35; 95% CI, 2.22-2.49; P less than .001). The study did not capture readmissions in a different state than that of the index hospital and was limited to the 22 states participating in the 2014 Readmissions Database.
Bottom line: Discharge AMA is associated with significantly higher odds of 30-day readmission, subsequent AMA discharge and presentation to another hospital, compared with routine discharge.
Citation: Kumar N. Burden of 30-day readmissions associated with discharge against medical advice among inpatients in the United States. Am J Med. 2019 Jun;132(6):708-17.
Dr. Webber is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: AMA discharges are common (1%-2% of all U.S. discharges) and disproportionately affect vulnerable patient populations, specifically those of lower socioeconomic status and the uninsured. Previous studies have been insufficiently powered to assess the effects of AMA discharge on 30-day readmission rates at a national level.
Study design: Retrospective cohort.
Setting: Community and teaching hospitals in 22 states.
Synopsis: With use of the 2014 Nationwide Readmissions Database of 23,110,641 index hospitalizations of patients 18 years or older, this study found that AMA discharge occurred with 1.3% of admissions. AMA discharge was associated with greater than twice the odds of 30-day readmission, compared with routine discharge. Of patients discharged AMA, 20.2% had an unplanned readmission within 30 days, compared with 10.1% of patients discharged routinely (OR, 2.25; 95% CI, 2.20-2.30; P less than .001).
Patients who were discharged AMA had almost 20 times the odds of undergoing repeat AMA discharge at readmission (OR, 18.41; 95% CI, 17.46-19.41; P less than .001) and twice the odds of presenting to a different hospital (OR, 2.35; 95% CI, 2.22-2.49; P less than .001). The study did not capture readmissions in a different state than that of the index hospital and was limited to the 22 states participating in the 2014 Readmissions Database.
Bottom line: Discharge AMA is associated with significantly higher odds of 30-day readmission, subsequent AMA discharge and presentation to another hospital, compared with routine discharge.
Citation: Kumar N. Burden of 30-day readmissions associated with discharge against medical advice among inpatients in the United States. Am J Med. 2019 Jun;132(6):708-17.
Dr. Webber is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: AMA discharges are common (1%-2% of all U.S. discharges) and disproportionately affect vulnerable patient populations, specifically those of lower socioeconomic status and the uninsured. Previous studies have been insufficiently powered to assess the effects of AMA discharge on 30-day readmission rates at a national level.
Study design: Retrospective cohort.
Setting: Community and teaching hospitals in 22 states.
Synopsis: With use of the 2014 Nationwide Readmissions Database of 23,110,641 index hospitalizations of patients 18 years or older, this study found that AMA discharge occurred with 1.3% of admissions. AMA discharge was associated with greater than twice the odds of 30-day readmission, compared with routine discharge. Of patients discharged AMA, 20.2% had an unplanned readmission within 30 days, compared with 10.1% of patients discharged routinely (OR, 2.25; 95% CI, 2.20-2.30; P less than .001).
Patients who were discharged AMA had almost 20 times the odds of undergoing repeat AMA discharge at readmission (OR, 18.41; 95% CI, 17.46-19.41; P less than .001) and twice the odds of presenting to a different hospital (OR, 2.35; 95% CI, 2.22-2.49; P less than .001). The study did not capture readmissions in a different state than that of the index hospital and was limited to the 22 states participating in the 2014 Readmissions Database.
Bottom line: Discharge AMA is associated with significantly higher odds of 30-day readmission, subsequent AMA discharge and presentation to another hospital, compared with routine discharge.
Citation: Kumar N. Burden of 30-day readmissions associated with discharge against medical advice among inpatients in the United States. Am J Med. 2019 Jun;132(6):708-17.
Dr. Webber is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Antibiotics fail to improve colon ischemia outcomes
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
Antibiotics may not significantly improve clinical outcomes in patients with colon ischemia (CI), regardless of severity level, based on a retrospective study involving more than 800 patients.
Given these findings, clinicians “should consider not giving antibiotics to patients with CI,” reported lead author Paul Feuerstadt, MD, of Yale University, New Haven , Conn., and colleagues.
“CI is the most common ischemic injury to the GI tract,” the investigators wrote in their abstract, which was presented at the annual meeting of the American College of Gastroenterology. “The clinical utility of antibiotic treatment in CI is unclear and the literature is limited.”
Dr. Feuerstadt and colleagues analyzed data from 838 patients with biopsy-proven CI who were hospitalized between 2005 and 2017, of whom 413 and 425 had moderate and severe disease, respectively.
Across all patients, 67.7% received antibiotics. While there were no significant intergroup differences in age, Charlson Comorbidity Index, or sex, patients who received antibiotics were more likely to have severe CI (54.4% vs. 42.2%; P = .001), small-bowel involvement (12.0% vs. 5.7%; P = .04), and peritonitis (11.3% vs. 4.5%; P = 002), as well as require intensive care (26.1% vs. 19.9%; P = .04).
After adjusting for severity of CI, small-bowel involvement, and comorbidities, analysis revealed no significant associations between antibiotic use and 30-day mortality, 90-day mortality, 30-day colectomy, 90-day recurrence, 90-day readmission, or length of stay. The primary outcome, 30-day mortality, remained insignificant in subgroup analyses based on CI severity and age.
Patients were most frequently prescribed ciprofloxacin-metronidazole (57.1%), followed by piperacillin-tazobactam (13.2%), ceftriaxone-metronidazole (11.1%), and other antibiotics (18.5%).
When each of these antimicrobials was compared with no antibiotic usage, only piperacillin-tazobactam correlated with a higher rate of 30-day mortality, based on an adjusted odds ratio of 3.4 (95% CI, 1.5-8.0; P = .0003). But most patients who received piperacillin-tazobactam underwent colectomy, which prompted independent analyses of patients who underwent colectomy and those who did not undergo colectomy. These findings showed no difference in 30-day mortality based on the type of antibiotic used.
During an oral presentation at the meeting, coauthor Karthik Gnanapandithan, MD, of the Mayo Clinic in Jacksonville, Fla, said, “In practice, it is reasonable to still use antibiotics in patients with small bowel ischemia and those with severe CI with a high risk of poor outcomes pending prospective studies.”
According to John F. Valentine, MD, of the University of Utah, Salt Lake City, the present study “adds to the literature that questions the role of antibiotics in CI.”
Dr. Valentine noted that, even among patients with CI who have severe inflammation, “sepsis rarely occurs without frank perforation.”
Still, like Dr. Gnanapandithan, Dr. Valentine concluded that antibiotics are still a reasonable treatment option for certain patients with CI.
“The risks and potential benefits of antibiotics must be considered,” he said. “Until prospective studies are available, use of antibiotics in colon ischemia is reasonable in the setting of severe disease with peritoneal signs, signs of sepsis, pneumatosis, or portal venous gas.”
Dr. Feuerstadt disclosed relationships with Ferring/Rebiotix, Merck, and Roche. Dr. Valentine reported no relevant conflicts of interest.
FROM ACG 2020
Family planning issues loom large for female radiation oncologists
Results from the survey were reported at the American Society for Radiation Oncology Annual Meeting 2020.
“Female radiation oncologists often spend their childbearing years in training and establishing careers,” commented lead investigator Anna Lee, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Currently, physician fertility and family planning are rarely discussed or taught in medical school or postgraduate training,” Dr. Lee said.
Dr. Lee and colleagues conducted a national anonymous cross-sectional online survey of female oncologists of all types and all career levels (including trainees). The team circulated a 39-item questionnaire exploring attitudes toward and experiences related to family planning and assisted reproductive technology (ART) by email and social media channels.
A total of 351 radiation oncologists participated, representing one-fifth of the specialty’s entire female workforce nationally and making this study the largest to date on family planning among these physicians.
Most respondents were aged 31-40 years (60%) and married (79%), had children (68%), and were in training (26%) or academic practice (48%).
Survey results
Fully 74% of respondents reported that their career plans strongly influenced the timing of when to start a family, and 29% said family planning considerations influenced their decision regarding their choice of academia versus private practice, Dr. Lee reported.
Overall, 24% of respondents indicated that they had difficulty with infertility or required fertility counseling/treatment, 66% said they wished fertility preservation was discussed at some point during their training, and 22% said either that ART would have benefited them if it had been available or that they were planning to or had already used fertility preservation.
On the topic of maternity leave, some respondents reported that their institution either had no formal leave policy during training or provided less than 1 month of leave (23%) and that they felt pressure to take less time off than was policy (15%).
“Of note, 32 women in our survey were not offered non–radiation-exposing assignments during pregnancy, and an additional 57 had to specifically ask for them,” Dr. Lee remarked.
About one-third of respondents each reported that they did not feel supported during training for issues related to fertility and/or pregnancy (33%) and that they experienced discrimination for being pregnant (32%) and taking maternity leave (30%).
“Systemic changes are necessary early in medical education and training to ensure women are supported and able to advance equitably in the field. As less than a third of the current radiation oncology workforce are women, improvement upon these issues will be necessary to draw more women into the field,” Dr. Lee commented. “Education on ART risks, benefits, and success rates can help physicians and those in training in their family planning, while the lack of education and structured policy can exacerbate the emotional, physical, and financial impact of infertility.
“Until recently, there has been a dearth of policy at the programmatic, institutional, and national level allowing time and protection for pregnancy and maternity leave,” she added. “Thankfully, this summer, the American Board of Medical Specialties announced a progressive leave policy for residents and fellows.”
The new policy, which goes into effect July 2021, allows a minimum of 6 weeks away without exhausting time allowed for vacation or sick leave and without requiring an extension in training.
When career and biology collide
“The collision of professional and biological clocks for women in medicine is an important issue highlighted by this study,” Reshma Jagsi, MD, DPhil, of the University of Michigan in Ann Arbor, said in an interview.
“Prior work focused on women in medicine more generally. A deeper dive into the experiences of women in a specific field may be even more compelling to drive change on the part of professional societies and organizations,” Dr. Jagsi added.
The infertility rate observed in the study could have potentially been skewed by the preponderance of younger respondents (resulting in underestimation) or by greater participation of those interested in the subject (resulting in overestimation), she noted. However, it aligns well with the rate in a study Dr. Jagsi and colleagues conducted among female physicians generally using somewhat different methods. That study was published in the Journal of Women’s Health.
Concern about radiation exposure and its potential reproductive health effects should not deter women from choosing radiation oncology as a specialty, according to Dr. Jagsi.
“Radiation exposure is actually very low in radiation oncology, much lower than in specialties like interventional cardiology, where physicians are in the room where fluoroscopy is being used. It is actually an important misconception about this field that merits correction,” she stressed. “Rather, the fertility concerns are related to the expectations of training and demands of work during the prime childbearing years more generally that can lead women to delay pregnancy, which is an issue common to all medical specialties.”
“The investigators’ conclusions are very reasonable,” Dr. Jagsi said. “Although one might quibble whether the exact proportions reflect the experiences of all women in the field perfectly due to the possibility of selection bias, one cannot question whether a substantial number of women are experiencing these challenges and that they merit intervention.”
The study did not receive specific funding. Dr. Lee and Dr. Jagsi disclosed no relevant conflicts of interest.
SOURCE: Lee A et al. ASTRO 2020, Abstract LBA 6.
Results from the survey were reported at the American Society for Radiation Oncology Annual Meeting 2020.
“Female radiation oncologists often spend their childbearing years in training and establishing careers,” commented lead investigator Anna Lee, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Currently, physician fertility and family planning are rarely discussed or taught in medical school or postgraduate training,” Dr. Lee said.
Dr. Lee and colleagues conducted a national anonymous cross-sectional online survey of female oncologists of all types and all career levels (including trainees). The team circulated a 39-item questionnaire exploring attitudes toward and experiences related to family planning and assisted reproductive technology (ART) by email and social media channels.
A total of 351 radiation oncologists participated, representing one-fifth of the specialty’s entire female workforce nationally and making this study the largest to date on family planning among these physicians.
Most respondents were aged 31-40 years (60%) and married (79%), had children (68%), and were in training (26%) or academic practice (48%).
Survey results
Fully 74% of respondents reported that their career plans strongly influenced the timing of when to start a family, and 29% said family planning considerations influenced their decision regarding their choice of academia versus private practice, Dr. Lee reported.
Overall, 24% of respondents indicated that they had difficulty with infertility or required fertility counseling/treatment, 66% said they wished fertility preservation was discussed at some point during their training, and 22% said either that ART would have benefited them if it had been available or that they were planning to or had already used fertility preservation.
On the topic of maternity leave, some respondents reported that their institution either had no formal leave policy during training or provided less than 1 month of leave (23%) and that they felt pressure to take less time off than was policy (15%).
“Of note, 32 women in our survey were not offered non–radiation-exposing assignments during pregnancy, and an additional 57 had to specifically ask for them,” Dr. Lee remarked.
About one-third of respondents each reported that they did not feel supported during training for issues related to fertility and/or pregnancy (33%) and that they experienced discrimination for being pregnant (32%) and taking maternity leave (30%).
“Systemic changes are necessary early in medical education and training to ensure women are supported and able to advance equitably in the field. As less than a third of the current radiation oncology workforce are women, improvement upon these issues will be necessary to draw more women into the field,” Dr. Lee commented. “Education on ART risks, benefits, and success rates can help physicians and those in training in their family planning, while the lack of education and structured policy can exacerbate the emotional, physical, and financial impact of infertility.
“Until recently, there has been a dearth of policy at the programmatic, institutional, and national level allowing time and protection for pregnancy and maternity leave,” she added. “Thankfully, this summer, the American Board of Medical Specialties announced a progressive leave policy for residents and fellows.”
The new policy, which goes into effect July 2021, allows a minimum of 6 weeks away without exhausting time allowed for vacation or sick leave and without requiring an extension in training.
When career and biology collide
“The collision of professional and biological clocks for women in medicine is an important issue highlighted by this study,” Reshma Jagsi, MD, DPhil, of the University of Michigan in Ann Arbor, said in an interview.
“Prior work focused on women in medicine more generally. A deeper dive into the experiences of women in a specific field may be even more compelling to drive change on the part of professional societies and organizations,” Dr. Jagsi added.
The infertility rate observed in the study could have potentially been skewed by the preponderance of younger respondents (resulting in underestimation) or by greater participation of those interested in the subject (resulting in overestimation), she noted. However, it aligns well with the rate in a study Dr. Jagsi and colleagues conducted among female physicians generally using somewhat different methods. That study was published in the Journal of Women’s Health.
Concern about radiation exposure and its potential reproductive health effects should not deter women from choosing radiation oncology as a specialty, according to Dr. Jagsi.
“Radiation exposure is actually very low in radiation oncology, much lower than in specialties like interventional cardiology, where physicians are in the room where fluoroscopy is being used. It is actually an important misconception about this field that merits correction,” she stressed. “Rather, the fertility concerns are related to the expectations of training and demands of work during the prime childbearing years more generally that can lead women to delay pregnancy, which is an issue common to all medical specialties.”
“The investigators’ conclusions are very reasonable,” Dr. Jagsi said. “Although one might quibble whether the exact proportions reflect the experiences of all women in the field perfectly due to the possibility of selection bias, one cannot question whether a substantial number of women are experiencing these challenges and that they merit intervention.”
The study did not receive specific funding. Dr. Lee and Dr. Jagsi disclosed no relevant conflicts of interest.
SOURCE: Lee A et al. ASTRO 2020, Abstract LBA 6.
Results from the survey were reported at the American Society for Radiation Oncology Annual Meeting 2020.
“Female radiation oncologists often spend their childbearing years in training and establishing careers,” commented lead investigator Anna Lee, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Currently, physician fertility and family planning are rarely discussed or taught in medical school or postgraduate training,” Dr. Lee said.
Dr. Lee and colleagues conducted a national anonymous cross-sectional online survey of female oncologists of all types and all career levels (including trainees). The team circulated a 39-item questionnaire exploring attitudes toward and experiences related to family planning and assisted reproductive technology (ART) by email and social media channels.
A total of 351 radiation oncologists participated, representing one-fifth of the specialty’s entire female workforce nationally and making this study the largest to date on family planning among these physicians.
Most respondents were aged 31-40 years (60%) and married (79%), had children (68%), and were in training (26%) or academic practice (48%).
Survey results
Fully 74% of respondents reported that their career plans strongly influenced the timing of when to start a family, and 29% said family planning considerations influenced their decision regarding their choice of academia versus private practice, Dr. Lee reported.
Overall, 24% of respondents indicated that they had difficulty with infertility or required fertility counseling/treatment, 66% said they wished fertility preservation was discussed at some point during their training, and 22% said either that ART would have benefited them if it had been available or that they were planning to or had already used fertility preservation.
On the topic of maternity leave, some respondents reported that their institution either had no formal leave policy during training or provided less than 1 month of leave (23%) and that they felt pressure to take less time off than was policy (15%).
“Of note, 32 women in our survey were not offered non–radiation-exposing assignments during pregnancy, and an additional 57 had to specifically ask for them,” Dr. Lee remarked.
About one-third of respondents each reported that they did not feel supported during training for issues related to fertility and/or pregnancy (33%) and that they experienced discrimination for being pregnant (32%) and taking maternity leave (30%).
“Systemic changes are necessary early in medical education and training to ensure women are supported and able to advance equitably in the field. As less than a third of the current radiation oncology workforce are women, improvement upon these issues will be necessary to draw more women into the field,” Dr. Lee commented. “Education on ART risks, benefits, and success rates can help physicians and those in training in their family planning, while the lack of education and structured policy can exacerbate the emotional, physical, and financial impact of infertility.
“Until recently, there has been a dearth of policy at the programmatic, institutional, and national level allowing time and protection for pregnancy and maternity leave,” she added. “Thankfully, this summer, the American Board of Medical Specialties announced a progressive leave policy for residents and fellows.”
The new policy, which goes into effect July 2021, allows a minimum of 6 weeks away without exhausting time allowed for vacation or sick leave and without requiring an extension in training.
When career and biology collide
“The collision of professional and biological clocks for women in medicine is an important issue highlighted by this study,” Reshma Jagsi, MD, DPhil, of the University of Michigan in Ann Arbor, said in an interview.
“Prior work focused on women in medicine more generally. A deeper dive into the experiences of women in a specific field may be even more compelling to drive change on the part of professional societies and organizations,” Dr. Jagsi added.
The infertility rate observed in the study could have potentially been skewed by the preponderance of younger respondents (resulting in underestimation) or by greater participation of those interested in the subject (resulting in overestimation), she noted. However, it aligns well with the rate in a study Dr. Jagsi and colleagues conducted among female physicians generally using somewhat different methods. That study was published in the Journal of Women’s Health.
Concern about radiation exposure and its potential reproductive health effects should not deter women from choosing radiation oncology as a specialty, according to Dr. Jagsi.
“Radiation exposure is actually very low in radiation oncology, much lower than in specialties like interventional cardiology, where physicians are in the room where fluoroscopy is being used. It is actually an important misconception about this field that merits correction,” she stressed. “Rather, the fertility concerns are related to the expectations of training and demands of work during the prime childbearing years more generally that can lead women to delay pregnancy, which is an issue common to all medical specialties.”
“The investigators’ conclusions are very reasonable,” Dr. Jagsi said. “Although one might quibble whether the exact proportions reflect the experiences of all women in the field perfectly due to the possibility of selection bias, one cannot question whether a substantial number of women are experiencing these challenges and that they merit intervention.”
The study did not receive specific funding. Dr. Lee and Dr. Jagsi disclosed no relevant conflicts of interest.
SOURCE: Lee A et al. ASTRO 2020, Abstract LBA 6.
FROM ASTRO 2020
Let side effects guide treatment choice for refractory OCD
Choosing the most effective treatment for patients with obsessive-compulsive disorder requires flexibility and agility on the part of clinicians, according to Wayne K. Goodman, MD.
“There are no data at this point to suggest that one SSRI is superior to another. It’s really dealer’s choice, and it has to do with really picking medications based upon side effects,” Dr. Goodman said at the Psychopharmacology Update, presented by Current Psychiatry and Global Academy for Medical Education. Clinicians can use family history as a guide, he noted, but pharmacogenetic testing has not been helpful in his experience for selection or dosing of an SSRI.
SSRIs, such as fluvoxamine, are one of two mainstays of treatment for patients with obsessive-compulsive disorder (OCD). The other drug class is serotonin reuptake inhibitors, which include medications such clomipramine. Cognitive-behavioral therapy options, such as Exposure and Response Prevention therapy, also has some, albeit limited, efficacy.
Meanwhile, Dr. Goodman said, antidepressant classes other than SRIs and SSRIs have not been effective in treating obsessive-compulsive symptoms, and some patients do not adhere well to cognitive-behavioral therapy, said Dr. Goodman, who is the D.C. and Irene Ellwood Professor in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston.
Choosing the most effective treatment for patients with obsessive-compulsive disorder requires flexibility and agility on the part of clinicians, according to Wayne K. Goodman, MD.
“There are no data at this point to suggest that one SSRI is superior to another. It’s really dealer’s choice, and it has to do with really picking medications based upon side effects,” Dr. Goodman said at the Psychopharmacology Update, presented by Current Psychiatry and Global Academy for Medical Education. Clinicians can use family history as a guide, he noted, but pharmacogenetic testing has not been helpful in his experience for selection or dosing of an SSRI.
SSRIs, such as fluvoxamine, are one of two mainstays of treatment for patients with obsessive-compulsive disorder (OCD). The other drug class is serotonin reuptake inhibitors, which include medications such clomipramine. Cognitive-behavioral therapy options, such as Exposure and Response Prevention therapy, also has some, albeit limited, efficacy.
Meanwhile, Dr. Goodman said, antidepressant classes other than SRIs and SSRIs have not been effective in treating obsessive-compulsive symptoms, and some patients do not adhere well to cognitive-behavioral therapy, said Dr. Goodman, who is the D.C. and Irene Ellwood Professor in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston.
Choosing the most effective treatment for patients with obsessive-compulsive disorder requires flexibility and agility on the part of clinicians, according to Wayne K. Goodman, MD.
“There are no data at this point to suggest that one SSRI is superior to another. It’s really dealer’s choice, and it has to do with really picking medications based upon side effects,” Dr. Goodman said at the Psychopharmacology Update, presented by Current Psychiatry and Global Academy for Medical Education. Clinicians can use family history as a guide, he noted, but pharmacogenetic testing has not been helpful in his experience for selection or dosing of an SSRI.
SSRIs, such as fluvoxamine, are one of two mainstays of treatment for patients with obsessive-compulsive disorder (OCD). The other drug class is serotonin reuptake inhibitors, which include medications such clomipramine. Cognitive-behavioral therapy options, such as Exposure and Response Prevention therapy, also has some, albeit limited, efficacy.
Meanwhile, Dr. Goodman said, antidepressant classes other than SRIs and SSRIs have not been effective in treating obsessive-compulsive symptoms, and some patients do not adhere well to cognitive-behavioral therapy, said Dr. Goodman, who is the D.C. and Irene Ellwood Professor in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston.
FROM PSYCHOPHARMACOLOGY UPDATE
Maternal oxygen in labor: False reassurance?
False reassurance?
CASE Heart rate tracing suggests fetal distress
Ms. M. presents for elective induction of labor at 39 weeks’ gestation. During the course of her labor, a Category II fetal heart rate (FHR) tracing is noted, and maternal oxygen is administered as part of the intrauterine resuscitative efforts. Her infant ultimately was delivered vaginally with an arterial cord blood pH of 7.1 and Apgar scores of 5 and 7.
Should intrauterine resuscitation include maternal oxygen administration?
It is a common sight on labor and delivery: An FHR monitoring strip is noted to be a Category II tracing. There may be fetal tachycardia, late decelerations, or perhaps decreased variability. The nurse or physician goes to the laboring mother’s room, checks cervical dilation, changes the patient’s position, and puts an oxygen mask over her face.
The American College of Obstetricians and Gynecologists (ACOG) lists maternal oxygen administration, most commonly at 10 L/min via a nonrebreather face mask, as an intrauterine resuscitative measure for Category II or Category III FHR tracings.1 Maternal oxygen is used to treat abnormal FHR tracings in approximately half of all births in the United States.2 Despite these recommendations and the frequency of its use, however, evidence is limited that maternal oxygenation improves neonatal outcome. In fact, there is emerging evidence of potential harm.
Why use oxygen?
The use of maternal oxygen supplementation intuitively makes sense. We know that certain abnormalities in FHR tracings can signal fetal hypoxia. Left untreated, the hypoxia could lead to fetal acidemia and associated neonatal sequelae. Theoretically, the administration of maternal oxygen should lead to improved fetal oxygenation and improved fetal outcome. This is supported by studies from the 1960s that demonstrate improved FHR tracings after maternal oxygen administration.3
This idea was further supported by studies that demonstrated an increase in fetal oxygen levels when maternal oxygen is administered. Haydon and colleagues evaluated the administration of maternal oxygen in women with nonreassuring FHR tracings.4 Their data showed that maternal oxygen administration increased fetal oxygen as measured by fetal pulse oximetry. The lower the initial fetal oxygen levels prior to oxygen administration, the greater the increase.
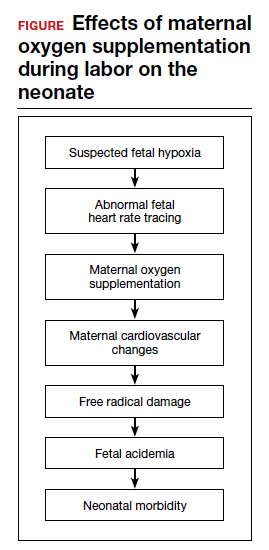
Despite these findings, evidence for improved neonatal outcomes is lacking.5 While heart rate tracings and fetal oxygen saturation may be improved with maternal oxygen supplementation, neonatal morbidity appears to remain unchanged (FIGURE). In fact, newer research suggests potential harm. Although an improved FHR tracing may be comforting to the clinician, the end result may be less so. Given these findings on maternal oxygen supplementation, it is time to break this practice habit.
Maternal cardiovascular effects
Most of the literature on maternal hyperoxygenation focuses on fetal response. Before examining the effects on the fetus, however, we must consider the effect on the mother. Cardiovascular changes occur during and after maternal oxygen administration that should be taken into account.
McHugh and colleagues measured the hemodynamic changes in 46 pregnant and 20 nonpregnant women before, immediately, and 10 minutes after a 30-minute period of high-flow oxygen administration.6 While there were no changes in the nonpregnant women’s parameters, in the pregnant women heart rate and stroke volume were decreased after oxygen administration. Additionally, systemic vascular resistance increased and did not return to baseline by 10 minutes postadministration.
Since the purpose of the maternal oxygen administration is to increase oxygen to the fetus, this decrease in cardiac output and increase in systemic vascular resistance is concerning. These results may negate the intended effect of increased oxygen delivery to the fetus.
Continue to: Maternal and fetal oxidative stress...
Maternal and fetal oxidative stress
Assuming that the abnormal FHR tracing in our case patient is actually due to fetal hypoxia, it would seem prudent to increase fetal oxygenation. However, fetal hyperoxygenation may lead to free radical damage that could worsen neonatal outcomes. Oxidative stress, which can be caused by both hypoxia and hyperoxia, can lead to endothelial and cell receptor damage. This is known to contribute to the cerebral damage of hypoxic-ischemic encephalopathy.
In a randomized trial, Khaw and colleagues measured lipid peroxidases as a “free radical footprint” in women undergoing elective cesarean delivery who were administered oxygen or room air.7 Maternal and fetal oxygen levels were higher in the oxygen-supplementation group, but lipid peroxidases also were elevated. This finding suggests that the excess oxygen results in free radical formation and potentially negative effects on the neonate.
Although maternal oxygen supplementation frequently is viewed as harmless, this research shows that free radical damage may occur in the mother as well.
Additional research shows that longer durations of oxygen administration are correlated with worsening neonatal outcomes. In a study of liberal versus indicated oxygen use, the average time was approximately 90 minutes.8 Use for longer than 176 minutes was associated with lower oxygen levels in fetal blood. A proposed mechanism for this response is placental vasoconstriction thought to protect the fetus from free radical damage.
Again, if the goal is to increase oxygenation, prolonged maternal oxygen supplementation appears to produce the opposite effect.
Fetal acidemia and neonatal morbidity
If a fetus with an abnormal FHR tracing is thought to be hypoxic or acidemic, adding the potentially harmful effects of free radicals could worsen this condition. This is exactly what Raghuraman and colleagues demonstrated in a large prospective cohort analysis.9 While there was no difference in neonatal morbidity between those receiving oxygen and those on room air, there was a significant difference among infants with acidemia and hyperoxia. Composite morbidity (mechanical ventilation, hypothermic therapy, meconium aspiration, and death) was significantly increased in neonates with both hyperoxia and acidemia compared with nonacidemic hyperoxic infants.9 This is further supported by reports of an increased need for neonatal resuscitation and a fourfold increase in umbilical cord pH of less than 7.2.10
While intrauterine and extrauterine life certainly differ, these findings align with the pediatric literature that supports neonatal resuscitation with room air rather than 100% oxygen.11 Additionally, the intrauterine environment is relatively hypoxic, which may make free radical damage more severe.
Continue to: Oxygen use during the COVID-19 pandemic...
Oxygen use during the COVID-19 pandemic
While high-flow oxygen by mask is not considered an aerosol-generating procedure according to the Centers for Disease Control and Prevention, data are limited regarding the cleaning and filtering of oxygen. It is unknown if high-flow oxygen by mask increases the risk of infectious disease transmission to care providers. Therefore, in the midst of the COVID-19 pandemic, ACOG currently recommends against using supplemental oxygen for Category II and Category III tracings, since the benefits are not well established and the possibility of harm to providers may be increased.12 Oxygen supplementation still should be used in mothers with hypoxia.
Other intrauterine resuscitation options
Maternal oxygen administration does not appear beneficial for neonatal outcomes, but other methods can be used. An intravenous fluid bolus and lateral positioning of the mother, for example, are both associated with increased fetal oxygenation. Reducing uterine activity by discontinuing oxytocin or cervical ripening agents or by administering a tocolytic also can improve FHR abnormalities. Oxygen use should be reserved for patients with maternal hypoxia.
The bottom line
The liberal use of maternal oxygenation for the management of abnormal FHR tracings should be stopped. Clear evidence of its benefit is lacking, and the real possibility of fetal and maternal harm remains. This may be especially true during the COVID-19 pandemic. ●
- American College of Obstetricians and Gynecologists. Practice bulletin No. 116. Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116:1232-1240.
- Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. Am J Obstet Gynecol. 2014;211:124-127.
- Althabe O, Schwarcz RL, Pose SV, et al. Effects on fetal heart rate and fetal pO2 of oxygen administration to the mother. Am J Obstet Gynecol. 1967;98:858-870.
- Haydon ML, Gorenberg DM, Nageotte MP, et al. The effect of maternal oxygen administration on fetal pulse oximetry during labor in fetuses with nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2006;195:735-738.
- Fawole B, Hofmeyr GJ. Maternal oxygen administration for fetal distress. Cochrane Database Syst Rev. 2012;12:CD0000136.
- McHugh A, El-Khuffash A, Bussmann N, et al. Hyperoxygenation in pregnancy exerts a more profound effect on cardiovascular hemodynamics than is observed in the nonpregnant state. Am J Obstet Gynecol. 2019;220:397.e1-397.e8.
- Khaw KS, Wang CC, Ngan Kee WD, et al. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18-23.
- Watkins VY, Martin S, Macones GA, et al. The duration of intrapartum supplemental oxygen administration and umbilical cord oxygen content. Am J Obstet Gynecol. 2020;223:440.e1-440.e7.
- Raghuraman N, Temming LA, Stout MJ, et al. Intrauterine hyperoxemia and risk of neonatal morbidity. Obstet Gynecol. 2017;129:676-682.
- Thorp JA, Trobough T, Evans R, et al. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172(2 pt 1):465-474.
- Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72:353-363.
- COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed October 15, 2020.
CASE Heart rate tracing suggests fetal distress
Ms. M. presents for elective induction of labor at 39 weeks’ gestation. During the course of her labor, a Category II fetal heart rate (FHR) tracing is noted, and maternal oxygen is administered as part of the intrauterine resuscitative efforts. Her infant ultimately was delivered vaginally with an arterial cord blood pH of 7.1 and Apgar scores of 5 and 7.
Should intrauterine resuscitation include maternal oxygen administration?
It is a common sight on labor and delivery: An FHR monitoring strip is noted to be a Category II tracing. There may be fetal tachycardia, late decelerations, or perhaps decreased variability. The nurse or physician goes to the laboring mother’s room, checks cervical dilation, changes the patient’s position, and puts an oxygen mask over her face.
The American College of Obstetricians and Gynecologists (ACOG) lists maternal oxygen administration, most commonly at 10 L/min via a nonrebreather face mask, as an intrauterine resuscitative measure for Category II or Category III FHR tracings.1 Maternal oxygen is used to treat abnormal FHR tracings in approximately half of all births in the United States.2 Despite these recommendations and the frequency of its use, however, evidence is limited that maternal oxygenation improves neonatal outcome. In fact, there is emerging evidence of potential harm.
Why use oxygen?
The use of maternal oxygen supplementation intuitively makes sense. We know that certain abnormalities in FHR tracings can signal fetal hypoxia. Left untreated, the hypoxia could lead to fetal acidemia and associated neonatal sequelae. Theoretically, the administration of maternal oxygen should lead to improved fetal oxygenation and improved fetal outcome. This is supported by studies from the 1960s that demonstrate improved FHR tracings after maternal oxygen administration.3
This idea was further supported by studies that demonstrated an increase in fetal oxygen levels when maternal oxygen is administered. Haydon and colleagues evaluated the administration of maternal oxygen in women with nonreassuring FHR tracings.4 Their data showed that maternal oxygen administration increased fetal oxygen as measured by fetal pulse oximetry. The lower the initial fetal oxygen levels prior to oxygen administration, the greater the increase.
Despite these findings, evidence for improved neonatal outcomes is lacking.5 While heart rate tracings and fetal oxygen saturation may be improved with maternal oxygen supplementation, neonatal morbidity appears to remain unchanged (FIGURE). In fact, newer research suggests potential harm. Although an improved FHR tracing may be comforting to the clinician, the end result may be less so. Given these findings on maternal oxygen supplementation, it is time to break this practice habit.

Maternal cardiovascular effects
Most of the literature on maternal hyperoxygenation focuses on fetal response. Before examining the effects on the fetus, however, we must consider the effect on the mother. Cardiovascular changes occur during and after maternal oxygen administration that should be taken into account.
McHugh and colleagues measured the hemodynamic changes in 46 pregnant and 20 nonpregnant women before, immediately, and 10 minutes after a 30-minute period of high-flow oxygen administration.6 While there were no changes in the nonpregnant women’s parameters, in the pregnant women heart rate and stroke volume were decreased after oxygen administration. Additionally, systemic vascular resistance increased and did not return to baseline by 10 minutes postadministration.
Since the purpose of the maternal oxygen administration is to increase oxygen to the fetus, this decrease in cardiac output and increase in systemic vascular resistance is concerning. These results may negate the intended effect of increased oxygen delivery to the fetus.
Continue to: Maternal and fetal oxidative stress...
Maternal and fetal oxidative stress
Assuming that the abnormal FHR tracing in our case patient is actually due to fetal hypoxia, it would seem prudent to increase fetal oxygenation. However, fetal hyperoxygenation may lead to free radical damage that could worsen neonatal outcomes. Oxidative stress, which can be caused by both hypoxia and hyperoxia, can lead to endothelial and cell receptor damage. This is known to contribute to the cerebral damage of hypoxic-ischemic encephalopathy.
In a randomized trial, Khaw and colleagues measured lipid peroxidases as a “free radical footprint” in women undergoing elective cesarean delivery who were administered oxygen or room air.7 Maternal and fetal oxygen levels were higher in the oxygen-supplementation group, but lipid peroxidases also were elevated. This finding suggests that the excess oxygen results in free radical formation and potentially negative effects on the neonate.
Although maternal oxygen supplementation frequently is viewed as harmless, this research shows that free radical damage may occur in the mother as well.
Additional research shows that longer durations of oxygen administration are correlated with worsening neonatal outcomes. In a study of liberal versus indicated oxygen use, the average time was approximately 90 minutes.8 Use for longer than 176 minutes was associated with lower oxygen levels in fetal blood. A proposed mechanism for this response is placental vasoconstriction thought to protect the fetus from free radical damage.
Again, if the goal is to increase oxygenation, prolonged maternal oxygen supplementation appears to produce the opposite effect.
Fetal acidemia and neonatal morbidity
If a fetus with an abnormal FHR tracing is thought to be hypoxic or acidemic, adding the potentially harmful effects of free radicals could worsen this condition. This is exactly what Raghuraman and colleagues demonstrated in a large prospective cohort analysis.9 While there was no difference in neonatal morbidity between those receiving oxygen and those on room air, there was a significant difference among infants with acidemia and hyperoxia. Composite morbidity (mechanical ventilation, hypothermic therapy, meconium aspiration, and death) was significantly increased in neonates with both hyperoxia and acidemia compared with nonacidemic hyperoxic infants.9 This is further supported by reports of an increased need for neonatal resuscitation and a fourfold increase in umbilical cord pH of less than 7.2.10
While intrauterine and extrauterine life certainly differ, these findings align with the pediatric literature that supports neonatal resuscitation with room air rather than 100% oxygen.11 Additionally, the intrauterine environment is relatively hypoxic, which may make free radical damage more severe.
Continue to: Oxygen use during the COVID-19 pandemic...
Oxygen use during the COVID-19 pandemic
While high-flow oxygen by mask is not considered an aerosol-generating procedure according to the Centers for Disease Control and Prevention, data are limited regarding the cleaning and filtering of oxygen. It is unknown if high-flow oxygen by mask increases the risk of infectious disease transmission to care providers. Therefore, in the midst of the COVID-19 pandemic, ACOG currently recommends against using supplemental oxygen for Category II and Category III tracings, since the benefits are not well established and the possibility of harm to providers may be increased.12 Oxygen supplementation still should be used in mothers with hypoxia.
Other intrauterine resuscitation options
Maternal oxygen administration does not appear beneficial for neonatal outcomes, but other methods can be used. An intravenous fluid bolus and lateral positioning of the mother, for example, are both associated with increased fetal oxygenation. Reducing uterine activity by discontinuing oxytocin or cervical ripening agents or by administering a tocolytic also can improve FHR abnormalities. Oxygen use should be reserved for patients with maternal hypoxia.
The bottom line
The liberal use of maternal oxygenation for the management of abnormal FHR tracings should be stopped. Clear evidence of its benefit is lacking, and the real possibility of fetal and maternal harm remains. This may be especially true during the COVID-19 pandemic. ●
CASE Heart rate tracing suggests fetal distress
Ms. M. presents for elective induction of labor at 39 weeks’ gestation. During the course of her labor, a Category II fetal heart rate (FHR) tracing is noted, and maternal oxygen is administered as part of the intrauterine resuscitative efforts. Her infant ultimately was delivered vaginally with an arterial cord blood pH of 7.1 and Apgar scores of 5 and 7.
Should intrauterine resuscitation include maternal oxygen administration?
It is a common sight on labor and delivery: An FHR monitoring strip is noted to be a Category II tracing. There may be fetal tachycardia, late decelerations, or perhaps decreased variability. The nurse or physician goes to the laboring mother’s room, checks cervical dilation, changes the patient’s position, and puts an oxygen mask over her face.
The American College of Obstetricians and Gynecologists (ACOG) lists maternal oxygen administration, most commonly at 10 L/min via a nonrebreather face mask, as an intrauterine resuscitative measure for Category II or Category III FHR tracings.1 Maternal oxygen is used to treat abnormal FHR tracings in approximately half of all births in the United States.2 Despite these recommendations and the frequency of its use, however, evidence is limited that maternal oxygenation improves neonatal outcome. In fact, there is emerging evidence of potential harm.
Why use oxygen?
The use of maternal oxygen supplementation intuitively makes sense. We know that certain abnormalities in FHR tracings can signal fetal hypoxia. Left untreated, the hypoxia could lead to fetal acidemia and associated neonatal sequelae. Theoretically, the administration of maternal oxygen should lead to improved fetal oxygenation and improved fetal outcome. This is supported by studies from the 1960s that demonstrate improved FHR tracings after maternal oxygen administration.3
This idea was further supported by studies that demonstrated an increase in fetal oxygen levels when maternal oxygen is administered. Haydon and colleagues evaluated the administration of maternal oxygen in women with nonreassuring FHR tracings.4 Their data showed that maternal oxygen administration increased fetal oxygen as measured by fetal pulse oximetry. The lower the initial fetal oxygen levels prior to oxygen administration, the greater the increase.
Despite these findings, evidence for improved neonatal outcomes is lacking.5 While heart rate tracings and fetal oxygen saturation may be improved with maternal oxygen supplementation, neonatal morbidity appears to remain unchanged (FIGURE). In fact, newer research suggests potential harm. Although an improved FHR tracing may be comforting to the clinician, the end result may be less so. Given these findings on maternal oxygen supplementation, it is time to break this practice habit.

Maternal cardiovascular effects
Most of the literature on maternal hyperoxygenation focuses on fetal response. Before examining the effects on the fetus, however, we must consider the effect on the mother. Cardiovascular changes occur during and after maternal oxygen administration that should be taken into account.
McHugh and colleagues measured the hemodynamic changes in 46 pregnant and 20 nonpregnant women before, immediately, and 10 minutes after a 30-minute period of high-flow oxygen administration.6 While there were no changes in the nonpregnant women’s parameters, in the pregnant women heart rate and stroke volume were decreased after oxygen administration. Additionally, systemic vascular resistance increased and did not return to baseline by 10 minutes postadministration.
Since the purpose of the maternal oxygen administration is to increase oxygen to the fetus, this decrease in cardiac output and increase in systemic vascular resistance is concerning. These results may negate the intended effect of increased oxygen delivery to the fetus.
Continue to: Maternal and fetal oxidative stress...
Maternal and fetal oxidative stress
Assuming that the abnormal FHR tracing in our case patient is actually due to fetal hypoxia, it would seem prudent to increase fetal oxygenation. However, fetal hyperoxygenation may lead to free radical damage that could worsen neonatal outcomes. Oxidative stress, which can be caused by both hypoxia and hyperoxia, can lead to endothelial and cell receptor damage. This is known to contribute to the cerebral damage of hypoxic-ischemic encephalopathy.
In a randomized trial, Khaw and colleagues measured lipid peroxidases as a “free radical footprint” in women undergoing elective cesarean delivery who were administered oxygen or room air.7 Maternal and fetal oxygen levels were higher in the oxygen-supplementation group, but lipid peroxidases also were elevated. This finding suggests that the excess oxygen results in free radical formation and potentially negative effects on the neonate.
Although maternal oxygen supplementation frequently is viewed as harmless, this research shows that free radical damage may occur in the mother as well.
Additional research shows that longer durations of oxygen administration are correlated with worsening neonatal outcomes. In a study of liberal versus indicated oxygen use, the average time was approximately 90 minutes.8 Use for longer than 176 minutes was associated with lower oxygen levels in fetal blood. A proposed mechanism for this response is placental vasoconstriction thought to protect the fetus from free radical damage.
Again, if the goal is to increase oxygenation, prolonged maternal oxygen supplementation appears to produce the opposite effect.
Fetal acidemia and neonatal morbidity
If a fetus with an abnormal FHR tracing is thought to be hypoxic or acidemic, adding the potentially harmful effects of free radicals could worsen this condition. This is exactly what Raghuraman and colleagues demonstrated in a large prospective cohort analysis.9 While there was no difference in neonatal morbidity between those receiving oxygen and those on room air, there was a significant difference among infants with acidemia and hyperoxia. Composite morbidity (mechanical ventilation, hypothermic therapy, meconium aspiration, and death) was significantly increased in neonates with both hyperoxia and acidemia compared with nonacidemic hyperoxic infants.9 This is further supported by reports of an increased need for neonatal resuscitation and a fourfold increase in umbilical cord pH of less than 7.2.10
While intrauterine and extrauterine life certainly differ, these findings align with the pediatric literature that supports neonatal resuscitation with room air rather than 100% oxygen.11 Additionally, the intrauterine environment is relatively hypoxic, which may make free radical damage more severe.
Continue to: Oxygen use during the COVID-19 pandemic...
Oxygen use during the COVID-19 pandemic
While high-flow oxygen by mask is not considered an aerosol-generating procedure according to the Centers for Disease Control and Prevention, data are limited regarding the cleaning and filtering of oxygen. It is unknown if high-flow oxygen by mask increases the risk of infectious disease transmission to care providers. Therefore, in the midst of the COVID-19 pandemic, ACOG currently recommends against using supplemental oxygen for Category II and Category III tracings, since the benefits are not well established and the possibility of harm to providers may be increased.12 Oxygen supplementation still should be used in mothers with hypoxia.
Other intrauterine resuscitation options
Maternal oxygen administration does not appear beneficial for neonatal outcomes, but other methods can be used. An intravenous fluid bolus and lateral positioning of the mother, for example, are both associated with increased fetal oxygenation. Reducing uterine activity by discontinuing oxytocin or cervical ripening agents or by administering a tocolytic also can improve FHR abnormalities. Oxygen use should be reserved for patients with maternal hypoxia.
The bottom line
The liberal use of maternal oxygenation for the management of abnormal FHR tracings should be stopped. Clear evidence of its benefit is lacking, and the real possibility of fetal and maternal harm remains. This may be especially true during the COVID-19 pandemic. ●
- American College of Obstetricians and Gynecologists. Practice bulletin No. 116. Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116:1232-1240.
- Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. Am J Obstet Gynecol. 2014;211:124-127.
- Althabe O, Schwarcz RL, Pose SV, et al. Effects on fetal heart rate and fetal pO2 of oxygen administration to the mother. Am J Obstet Gynecol. 1967;98:858-870.
- Haydon ML, Gorenberg DM, Nageotte MP, et al. The effect of maternal oxygen administration on fetal pulse oximetry during labor in fetuses with nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2006;195:735-738.
- Fawole B, Hofmeyr GJ. Maternal oxygen administration for fetal distress. Cochrane Database Syst Rev. 2012;12:CD0000136.
- McHugh A, El-Khuffash A, Bussmann N, et al. Hyperoxygenation in pregnancy exerts a more profound effect on cardiovascular hemodynamics than is observed in the nonpregnant state. Am J Obstet Gynecol. 2019;220:397.e1-397.e8.
- Khaw KS, Wang CC, Ngan Kee WD, et al. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18-23.
- Watkins VY, Martin S, Macones GA, et al. The duration of intrapartum supplemental oxygen administration and umbilical cord oxygen content. Am J Obstet Gynecol. 2020;223:440.e1-440.e7.
- Raghuraman N, Temming LA, Stout MJ, et al. Intrauterine hyperoxemia and risk of neonatal morbidity. Obstet Gynecol. 2017;129:676-682.
- Thorp JA, Trobough T, Evans R, et al. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172(2 pt 1):465-474.
- Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72:353-363.
- COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed October 15, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 116. Management of intrapartum fetal heart rate tracings. Obstet Gynecol. 2010;116:1232-1240.
- Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. Am J Obstet Gynecol. 2014;211:124-127.
- Althabe O, Schwarcz RL, Pose SV, et al. Effects on fetal heart rate and fetal pO2 of oxygen administration to the mother. Am J Obstet Gynecol. 1967;98:858-870.
- Haydon ML, Gorenberg DM, Nageotte MP, et al. The effect of maternal oxygen administration on fetal pulse oximetry during labor in fetuses with nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2006;195:735-738.
- Fawole B, Hofmeyr GJ. Maternal oxygen administration for fetal distress. Cochrane Database Syst Rev. 2012;12:CD0000136.
- McHugh A, El-Khuffash A, Bussmann N, et al. Hyperoxygenation in pregnancy exerts a more profound effect on cardiovascular hemodynamics than is observed in the nonpregnant state. Am J Obstet Gynecol. 2019;220:397.e1-397.e8.
- Khaw KS, Wang CC, Ngan Kee WD, et al. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18-23.
- Watkins VY, Martin S, Macones GA, et al. The duration of intrapartum supplemental oxygen administration and umbilical cord oxygen content. Am J Obstet Gynecol. 2020;223:440.e1-440.e7.
- Raghuraman N, Temming LA, Stout MJ, et al. Intrauterine hyperoxemia and risk of neonatal morbidity. Obstet Gynecol. 2017;129:676-682.
- Thorp JA, Trobough T, Evans R, et al. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172(2 pt 1):465-474.
- Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72:353-363.
- COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed October 15, 2020.
False reassurance?
False reassurance?
Early results ‘encouraging’ for CAR NKT cells in neuroblastoma
, according to results of an ongoing phase 1 trial.
In one of three patients treated thus far, the CAR NKT cells induced an objective response with regression of a metastatic bone lesion.
Andras Heczey, MD, of Baylor College of Medicine, Houston, and colleagues reported outcomes for the first three patients in Nature Medicine.
The three boys – two 12-year-olds and one 6-year-old – had relapsed/refractory neuroblastoma.
NKT cells were collected from the patients, then genetically engineered to express a CAR to recognize the GD2-ganglioside expressed in neuroblastomas and also to express interleukin-15, which supports NKT cell survival. The cells were expanded and reinfused back into the patients.
The initial results suggest that CAR NKT cells can be used safely to treat neuroblastomas and perhaps other solid tumors, investigators said.
‘A significant advance’ if confirmed
Treating solid tumors with CAR T cells has been a challenge, in part because of inefficient trafficking into tumors.
However, NKT cells naturally migrate to tumors in response to tumor-derived chemokines, Dr. Heczey and colleagues noted. NKT cells kill macrophages associated with tumor growth and promote NK- and T-cell–mediated antitumor responses.
“We decided to leverage this intrinsic property of NKTs and to arm them with an additional bullet – the so-called CAR – to further potentiate their capacity to destroy the tumor,” investigator Gianpietro Dotti, MD, of the University of North Carolina Lindberger Comprehensive Cancer Center in Chapel Hill, said in a press release.
Overall, the “results are very encouraging and, if confirmed in a larger cohort of patients, present a significant advance in the cell therapy field for solid tumors,” said CAR-T researcher Stephen Gottschalk, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., when asked for comment.
Treatment, safety, and efficacy details
NKT cells are infrequent in human peripheral blood, so the investigators stimulated the NKT cells collected from patients with alpha-galactosylceramide–pulsed irradiated peripheral blood mononuclear cells.
The final products reached a mean NKT cell purity of 95%. The proportion of cells positive for the GD2-CAR ranged from 20% to 70% across the three patients.
After lymphodepletion with cyclophosphamide/fludarabine, the patients were infused with 3 × 106 CAR NKT cells/m2.
The cells were well tolerated, with no dose-limiting toxicities. There were grade 3/4 adverse events, but they occurred before CAR NKT-cell infusion and were thought to be related to lymphodepletion.
NKT-cell frequency and absolute numbers increased in the peripheral blood over baseline and remained elevated at the week 4 assessment.
Two patients had stable disease at 4 weeks, but one had a partial response and a change in Curie score from 2 to 1. The patient’s SPECT- and MIBG-merged scans “revealed a dramatic reduction in the size and MIBG uptake of a bone metastasis. The patient consequently received salvage therapy and achieved a complete response that lasted approximately 6 months,” the investigators noted.
The team found higher percentages of CAR NKT cells in primary tumor and metastatic bone marrow biopsies than in peripheral blood. A high percentage of CAR NKT cells from the tumor specimen, but only a small fraction from the bone metastasis, expressed the GD2-CAR.
This research was funded by Kuur Therapeutics, Alex’s Lemonade Stand Foundation for Childhood Cancer, the American Cancer Society, Cookies for Kids’ Cancer Foundation, and the Cancer Prevention and Research Institute of Texas. Dr. Heczey, Dr. Dotti, and two other researchers are coinventors on pending patent applications for NKT cells in cancer immunotherapy that have been licensed to Kuur Therapeutics for commercial development. Dr. Gottschalk has patent applications in the fields of T-cell and/or gene therapy for cancer. He has relationships with TESSA Therapeutics, Immatics, and Tidal.
SOURCE: Heczey A et al. Nat Med. 2020 Oct 12. doi: 10.1038/s41591-020-1074-2.
, according to results of an ongoing phase 1 trial.
In one of three patients treated thus far, the CAR NKT cells induced an objective response with regression of a metastatic bone lesion.
Andras Heczey, MD, of Baylor College of Medicine, Houston, and colleagues reported outcomes for the first three patients in Nature Medicine.
The three boys – two 12-year-olds and one 6-year-old – had relapsed/refractory neuroblastoma.
NKT cells were collected from the patients, then genetically engineered to express a CAR to recognize the GD2-ganglioside expressed in neuroblastomas and also to express interleukin-15, which supports NKT cell survival. The cells were expanded and reinfused back into the patients.
The initial results suggest that CAR NKT cells can be used safely to treat neuroblastomas and perhaps other solid tumors, investigators said.
‘A significant advance’ if confirmed
Treating solid tumors with CAR T cells has been a challenge, in part because of inefficient trafficking into tumors.
However, NKT cells naturally migrate to tumors in response to tumor-derived chemokines, Dr. Heczey and colleagues noted. NKT cells kill macrophages associated with tumor growth and promote NK- and T-cell–mediated antitumor responses.
“We decided to leverage this intrinsic property of NKTs and to arm them with an additional bullet – the so-called CAR – to further potentiate their capacity to destroy the tumor,” investigator Gianpietro Dotti, MD, of the University of North Carolina Lindberger Comprehensive Cancer Center in Chapel Hill, said in a press release.
Overall, the “results are very encouraging and, if confirmed in a larger cohort of patients, present a significant advance in the cell therapy field for solid tumors,” said CAR-T researcher Stephen Gottschalk, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., when asked for comment.
Treatment, safety, and efficacy details
NKT cells are infrequent in human peripheral blood, so the investigators stimulated the NKT cells collected from patients with alpha-galactosylceramide–pulsed irradiated peripheral blood mononuclear cells.
The final products reached a mean NKT cell purity of 95%. The proportion of cells positive for the GD2-CAR ranged from 20% to 70% across the three patients.
After lymphodepletion with cyclophosphamide/fludarabine, the patients were infused with 3 × 106 CAR NKT cells/m2.
The cells were well tolerated, with no dose-limiting toxicities. There were grade 3/4 adverse events, but they occurred before CAR NKT-cell infusion and were thought to be related to lymphodepletion.
NKT-cell frequency and absolute numbers increased in the peripheral blood over baseline and remained elevated at the week 4 assessment.
Two patients had stable disease at 4 weeks, but one had a partial response and a change in Curie score from 2 to 1. The patient’s SPECT- and MIBG-merged scans “revealed a dramatic reduction in the size and MIBG uptake of a bone metastasis. The patient consequently received salvage therapy and achieved a complete response that lasted approximately 6 months,” the investigators noted.
The team found higher percentages of CAR NKT cells in primary tumor and metastatic bone marrow biopsies than in peripheral blood. A high percentage of CAR NKT cells from the tumor specimen, but only a small fraction from the bone metastasis, expressed the GD2-CAR.
This research was funded by Kuur Therapeutics, Alex’s Lemonade Stand Foundation for Childhood Cancer, the American Cancer Society, Cookies for Kids’ Cancer Foundation, and the Cancer Prevention and Research Institute of Texas. Dr. Heczey, Dr. Dotti, and two other researchers are coinventors on pending patent applications for NKT cells in cancer immunotherapy that have been licensed to Kuur Therapeutics for commercial development. Dr. Gottschalk has patent applications in the fields of T-cell and/or gene therapy for cancer. He has relationships with TESSA Therapeutics, Immatics, and Tidal.
SOURCE: Heczey A et al. Nat Med. 2020 Oct 12. doi: 10.1038/s41591-020-1074-2.
, according to results of an ongoing phase 1 trial.
In one of three patients treated thus far, the CAR NKT cells induced an objective response with regression of a metastatic bone lesion.
Andras Heczey, MD, of Baylor College of Medicine, Houston, and colleagues reported outcomes for the first three patients in Nature Medicine.
The three boys – two 12-year-olds and one 6-year-old – had relapsed/refractory neuroblastoma.
NKT cells were collected from the patients, then genetically engineered to express a CAR to recognize the GD2-ganglioside expressed in neuroblastomas and also to express interleukin-15, which supports NKT cell survival. The cells were expanded and reinfused back into the patients.
The initial results suggest that CAR NKT cells can be used safely to treat neuroblastomas and perhaps other solid tumors, investigators said.
‘A significant advance’ if confirmed
Treating solid tumors with CAR T cells has been a challenge, in part because of inefficient trafficking into tumors.
However, NKT cells naturally migrate to tumors in response to tumor-derived chemokines, Dr. Heczey and colleagues noted. NKT cells kill macrophages associated with tumor growth and promote NK- and T-cell–mediated antitumor responses.
“We decided to leverage this intrinsic property of NKTs and to arm them with an additional bullet – the so-called CAR – to further potentiate their capacity to destroy the tumor,” investigator Gianpietro Dotti, MD, of the University of North Carolina Lindberger Comprehensive Cancer Center in Chapel Hill, said in a press release.
Overall, the “results are very encouraging and, if confirmed in a larger cohort of patients, present a significant advance in the cell therapy field for solid tumors,” said CAR-T researcher Stephen Gottschalk, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., when asked for comment.
Treatment, safety, and efficacy details
NKT cells are infrequent in human peripheral blood, so the investigators stimulated the NKT cells collected from patients with alpha-galactosylceramide–pulsed irradiated peripheral blood mononuclear cells.
The final products reached a mean NKT cell purity of 95%. The proportion of cells positive for the GD2-CAR ranged from 20% to 70% across the three patients.
After lymphodepletion with cyclophosphamide/fludarabine, the patients were infused with 3 × 106 CAR NKT cells/m2.
The cells were well tolerated, with no dose-limiting toxicities. There were grade 3/4 adverse events, but they occurred before CAR NKT-cell infusion and were thought to be related to lymphodepletion.
NKT-cell frequency and absolute numbers increased in the peripheral blood over baseline and remained elevated at the week 4 assessment.
Two patients had stable disease at 4 weeks, but one had a partial response and a change in Curie score from 2 to 1. The patient’s SPECT- and MIBG-merged scans “revealed a dramatic reduction in the size and MIBG uptake of a bone metastasis. The patient consequently received salvage therapy and achieved a complete response that lasted approximately 6 months,” the investigators noted.
The team found higher percentages of CAR NKT cells in primary tumor and metastatic bone marrow biopsies than in peripheral blood. A high percentage of CAR NKT cells from the tumor specimen, but only a small fraction from the bone metastasis, expressed the GD2-CAR.
This research was funded by Kuur Therapeutics, Alex’s Lemonade Stand Foundation for Childhood Cancer, the American Cancer Society, Cookies for Kids’ Cancer Foundation, and the Cancer Prevention and Research Institute of Texas. Dr. Heczey, Dr. Dotti, and two other researchers are coinventors on pending patent applications for NKT cells in cancer immunotherapy that have been licensed to Kuur Therapeutics for commercial development. Dr. Gottschalk has patent applications in the fields of T-cell and/or gene therapy for cancer. He has relationships with TESSA Therapeutics, Immatics, and Tidal.
SOURCE: Heczey A et al. Nat Med. 2020 Oct 12. doi: 10.1038/s41591-020-1074-2.
FROM NATURE MEDICINE
Syphilis: Cutting risk through primary prevention and prenatal screening
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
HF an added risk in COVID-19, regardless of ejection fraction
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
Which hormonal management approach for women with premature ovarian insufficiency is best for bone?
Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
EXPERT COMMENTARY
Premature ovarian insufficiency (POI) refers to a condition in women in whom ovarian function ceases prior to age 40 years. Although hormone therapy (HT) is a mainstay of treatment for women with POI, it is uncertain which approach to HT is most effective in terms of bone mineral density (BMD). Investigators recently published their results of an observational study that aimed to evaluate the use of combined oral contraceptives (COCs) for preserving BMD in women with POI.
Details of the study
At an academic center in Brazil, Carvalho Gazarra and colleagues identified women with POI who had undergone 2 or more BMD assessments performed 2 or more years apart.1 HT regimens (all of which were taken continuously) employed the following: a COC with ethinyl estradiol (EE) 30 µg and levonorgestrel; low-dose estrogen plus progestin therapy (EPT, conjugated equine estrogen [CEE] 0.625 mg with medroxyprogesterone acetate or estradiol 1.0 mg with norethindrone acetate); or high-dose estrogen plus progestin (CEE 1.25 mg or estradiol 2.0 mg combined with the same progestins).
Results. Among 119 evaluable women with POI (mean age, 30.3 years), the use of COC was associated with the most positive BMD trends. For women using COC or high-dose EPT, BMD at the lumbar spine increased. By contrast, BMD of the lumbar spine declined in women who used no treatment or low-dose EPT.1
Other studies’ take on dose, route of administration, and cost considerations
Sequelae of POI include infertility, bothersome hot flashes, vaginal dryness, sexual dysfunction, mood disorders, and an elevated risk of cardiovascular disease, dementia, Parkinson’s disease, and osteoporosis. Importantly, clinicians and patients need to understand that the results from the Women’s Health Initiative studies do not apply to women with POI.2 Physiologic doses of HT (that is, doses higher than those used to treat menopausal symptoms in women with normal/spontaneous menopause) are appropriate for women with POI, at least until they reach the normal age of menopause (51 to 52 years).
A clinical trial conducted in Scotland in women with POI found that high-dose transdermal estrogen (application of one to two 0.1-mg estradiol patches) daily had an impact on BMD that was more positive than that of an oral contraceptive formulated with EE 30 µg.3 Likewise, a trial in the United States found that, among oligo-amenorrheic athletes, a hormone replacement regimen using a 0.1-mg estradiol patch had a more positive impact on BMD than an oral contraceptive formulated with EE 30 µg.4
Although Carvalho Gazarra and colleagues acknowledged awareness of reports suggesting the skeletal health benefits of high-dose estradiol patches, in the Brazilian public health system oral hormone therapy is less expensive and oral contraceptives are available at no charge.1 ●
When replacing estrogen and progestin in young women who lack ovarian function, it is appropriate to use considerably higher doses than those used to treat bothersome vasomotor symptoms in women with normal/spontaneous menopause. From the perspective of venous thromboembolism risk, the transdermal route of administration is safer than the oral route,5 and the Scottish and US studies discussed here indicate that transdermal estradiol is an effective approach to maintaining skeletal health in young women without ovarian function. Accordingly, hormonal management with high-dose transdermal estradiol with a progestin (such as progesterone 200–300 mg at bedtime or medroxyprogesterone 5–10 mg daily) represents an appropriate strategy. In situations where transdermal estradiol plus oral progestin treatment is not covered by health insurance or acceptable to the patient, an oral estrogen-progestin contraceptive formulated with EE 30 or 35 µg will provide protection against bone loss.
- Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
- Jiang XD. Bone health and beyond in women with primary ovarian insufficiency: time to narrow the knowledge-action gap in care. Menopause. 2020;27:1101-1103.
- Crofton PM, Evans N, Bath LE, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf). 2010;73:707-714.
- Ackerman KE, Singhal V, Baskaran C, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53:229-236.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
EXPERT COMMENTARY
Premature ovarian insufficiency (POI) refers to a condition in women in whom ovarian function ceases prior to age 40 years. Although hormone therapy (HT) is a mainstay of treatment for women with POI, it is uncertain which approach to HT is most effective in terms of bone mineral density (BMD). Investigators recently published their results of an observational study that aimed to evaluate the use of combined oral contraceptives (COCs) for preserving BMD in women with POI.
Details of the study
At an academic center in Brazil, Carvalho Gazarra and colleagues identified women with POI who had undergone 2 or more BMD assessments performed 2 or more years apart.1 HT regimens (all of which were taken continuously) employed the following: a COC with ethinyl estradiol (EE) 30 µg and levonorgestrel; low-dose estrogen plus progestin therapy (EPT, conjugated equine estrogen [CEE] 0.625 mg with medroxyprogesterone acetate or estradiol 1.0 mg with norethindrone acetate); or high-dose estrogen plus progestin (CEE 1.25 mg or estradiol 2.0 mg combined with the same progestins).
Results. Among 119 evaluable women with POI (mean age, 30.3 years), the use of COC was associated with the most positive BMD trends. For women using COC or high-dose EPT, BMD at the lumbar spine increased. By contrast, BMD of the lumbar spine declined in women who used no treatment or low-dose EPT.1
Other studies’ take on dose, route of administration, and cost considerations
Sequelae of POI include infertility, bothersome hot flashes, vaginal dryness, sexual dysfunction, mood disorders, and an elevated risk of cardiovascular disease, dementia, Parkinson’s disease, and osteoporosis. Importantly, clinicians and patients need to understand that the results from the Women’s Health Initiative studies do not apply to women with POI.2 Physiologic doses of HT (that is, doses higher than those used to treat menopausal symptoms in women with normal/spontaneous menopause) are appropriate for women with POI, at least until they reach the normal age of menopause (51 to 52 years).
A clinical trial conducted in Scotland in women with POI found that high-dose transdermal estrogen (application of one to two 0.1-mg estradiol patches) daily had an impact on BMD that was more positive than that of an oral contraceptive formulated with EE 30 µg.3 Likewise, a trial in the United States found that, among oligo-amenorrheic athletes, a hormone replacement regimen using a 0.1-mg estradiol patch had a more positive impact on BMD than an oral contraceptive formulated with EE 30 µg.4
Although Carvalho Gazarra and colleagues acknowledged awareness of reports suggesting the skeletal health benefits of high-dose estradiol patches, in the Brazilian public health system oral hormone therapy is less expensive and oral contraceptives are available at no charge.1 ●
When replacing estrogen and progestin in young women who lack ovarian function, it is appropriate to use considerably higher doses than those used to treat bothersome vasomotor symptoms in women with normal/spontaneous menopause. From the perspective of venous thromboembolism risk, the transdermal route of administration is safer than the oral route,5 and the Scottish and US studies discussed here indicate that transdermal estradiol is an effective approach to maintaining skeletal health in young women without ovarian function. Accordingly, hormonal management with high-dose transdermal estradiol with a progestin (such as progesterone 200–300 mg at bedtime or medroxyprogesterone 5–10 mg daily) represents an appropriate strategy. In situations where transdermal estradiol plus oral progestin treatment is not covered by health insurance or acceptable to the patient, an oral estrogen-progestin contraceptive formulated with EE 30 or 35 µg will provide protection against bone loss.
Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
EXPERT COMMENTARY
Premature ovarian insufficiency (POI) refers to a condition in women in whom ovarian function ceases prior to age 40 years. Although hormone therapy (HT) is a mainstay of treatment for women with POI, it is uncertain which approach to HT is most effective in terms of bone mineral density (BMD). Investigators recently published their results of an observational study that aimed to evaluate the use of combined oral contraceptives (COCs) for preserving BMD in women with POI.
Details of the study
At an academic center in Brazil, Carvalho Gazarra and colleagues identified women with POI who had undergone 2 or more BMD assessments performed 2 or more years apart.1 HT regimens (all of which were taken continuously) employed the following: a COC with ethinyl estradiol (EE) 30 µg and levonorgestrel; low-dose estrogen plus progestin therapy (EPT, conjugated equine estrogen [CEE] 0.625 mg with medroxyprogesterone acetate or estradiol 1.0 mg with norethindrone acetate); or high-dose estrogen plus progestin (CEE 1.25 mg or estradiol 2.0 mg combined with the same progestins).
Results. Among 119 evaluable women with POI (mean age, 30.3 years), the use of COC was associated with the most positive BMD trends. For women using COC or high-dose EPT, BMD at the lumbar spine increased. By contrast, BMD of the lumbar spine declined in women who used no treatment or low-dose EPT.1
Other studies’ take on dose, route of administration, and cost considerations
Sequelae of POI include infertility, bothersome hot flashes, vaginal dryness, sexual dysfunction, mood disorders, and an elevated risk of cardiovascular disease, dementia, Parkinson’s disease, and osteoporosis. Importantly, clinicians and patients need to understand that the results from the Women’s Health Initiative studies do not apply to women with POI.2 Physiologic doses of HT (that is, doses higher than those used to treat menopausal symptoms in women with normal/spontaneous menopause) are appropriate for women with POI, at least until they reach the normal age of menopause (51 to 52 years).
A clinical trial conducted in Scotland in women with POI found that high-dose transdermal estrogen (application of one to two 0.1-mg estradiol patches) daily had an impact on BMD that was more positive than that of an oral contraceptive formulated with EE 30 µg.3 Likewise, a trial in the United States found that, among oligo-amenorrheic athletes, a hormone replacement regimen using a 0.1-mg estradiol patch had a more positive impact on BMD than an oral contraceptive formulated with EE 30 µg.4
Although Carvalho Gazarra and colleagues acknowledged awareness of reports suggesting the skeletal health benefits of high-dose estradiol patches, in the Brazilian public health system oral hormone therapy is less expensive and oral contraceptives are available at no charge.1 ●
When replacing estrogen and progestin in young women who lack ovarian function, it is appropriate to use considerably higher doses than those used to treat bothersome vasomotor symptoms in women with normal/spontaneous menopause. From the perspective of venous thromboembolism risk, the transdermal route of administration is safer than the oral route,5 and the Scottish and US studies discussed here indicate that transdermal estradiol is an effective approach to maintaining skeletal health in young women without ovarian function. Accordingly, hormonal management with high-dose transdermal estradiol with a progestin (such as progesterone 200–300 mg at bedtime or medroxyprogesterone 5–10 mg daily) represents an appropriate strategy. In situations where transdermal estradiol plus oral progestin treatment is not covered by health insurance or acceptable to the patient, an oral estrogen-progestin contraceptive formulated with EE 30 or 35 µg will provide protection against bone loss.
- Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
- Jiang XD. Bone health and beyond in women with primary ovarian insufficiency: time to narrow the knowledge-action gap in care. Menopause. 2020;27:1101-1103.
- Crofton PM, Evans N, Bath LE, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf). 2010;73:707-714.
- Ackerman KE, Singhal V, Baskaran C, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53:229-236.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
- Carvalho Gazarra LB, Bonacordi CL, Yela DA, et al. Bone mass in women with premature ovarian insufficiency: a comparative study between hormone therapy and combined oral contraceptives. Menopause. 2020;27:1110-1116.
- Jiang XD. Bone health and beyond in women with primary ovarian insufficiency: time to narrow the knowledge-action gap in care. Menopause. 2020;27:1101-1103.
- Crofton PM, Evans N, Bath LE, et al. Physiological versus standard sex steroid replacement in young women with premature ovarian failure: effects on bone mass acquisition and turnover. Clin Endocrinol (Oxf). 2010;73:707-714.
- Ackerman KE, Singhal V, Baskaran C, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53:229-236.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
Preemptive CMV monitoring beats prophylaxis post liver transplant
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
FROM IDWEEK 2020