User login
‘Soak-and-smear’ AD protocol backed by evidence
The most effective initial step for clearing atopic dermatitis in infants and young children involves daily bathing, followed by immediate application of a moisturizer, topical steroid, or both, according to an expert speaking at the virtual annual Coastal Dermatology Symposium.
“If they are really severe, you can do it twice-daily, but there are several studies that show there is not a huge benefit of twice-daily over once-daily,” said Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland.
He called this technique “soak-and-smear.” The “smear” is performed immediately after the bath when the skin is still damp, he said. When clearing is the goal, and the child has moderate to severe atopic dermatitis (AD), 0.1% triamcinolone or a similar medium potency topical steroid can be applied, and after clearing, the steroid can be switched for a moisturizer, according to Dr. Simpson.
Rather than restricting application to areas of greatest skin involvement, “put it all over,” he advised.
The clearing regimen should be continued “for a couple of more days” after the lesions have resolved, with a return visit in about a week to confirm clearing and reinforce the next steps for keeping patients clear, he added.
The next steps depend on severity. According to Dr. Simpson, severity is defined less by the extent of skin involvement at the baseline examination than the speed at which symptoms return.
For those with only mild symptoms after an extended period of clearing, moisturizer might be sufficient to prevent a significant relapse. For children with a more rapid relapse, it will be necessary to reintroduce topical steroid either every day, every other day, or twice per week.
Whether with moisturizer or with topical steroids, the soak-and-smear technique has now been validated in a recently published crossover randomized trial.
In the trial, children aged 6 months to 11 years, with moderate to severe AD, were randomized to a twice-daily bath, called the “wet method,” versus a twice-weekly bath, called the “dry method.” Both groups received a cleanser and moisturizer along with a low-potency topical steroid as needed.
After 2 weeks, the 40 evaluable patients were crossed over to the opposite bathing technique. The wet, or soak-and-smear approach, was associated with a highly significant reduction in the primary endpoint of SCORing Atopic Dermatitis (SCORAD) index, compared with the dry method (95% confidence interval, 14.9-27.6; P less than .0001). In a secondary analysis, this translated into a 30% relative reduction in favor of the wet method.
In addition, there was improvement in a caregiver assessment of the Atopic Dermatitis Quickscore (ADQ). These data show that “twice-daily baths with topical steroids and moisturizer can help in more moderate to severe population,” said Dr. Simpson, who noted that he has participated in open-label studies with the same soak-and-smear technique that have produced similar results.
Once children are clear, Dr. Simpson recommends a maintenance strategy individualized for severity. In many cases, this will involve moisturizers applied after the bath, supplemented intermittently, such as once or twice per week, with topical steroids. However, if parents find themselves resorting to daily steroids to maintain control, “that’s when you incorporate the TCIs [topical calcineurin inhibitors].”
TCIs “can help you stay at twice-per-week topical steroids,” Dr. Simpson said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
TCIs also help patients avoid steroid withdrawal, a particularly common phenomenon when topical steroids are applied repeatedly to the face. He recommended a proactive approach. By applying TCIs to areas where skin lesions frequently recur, such as the eyelids, flares can often be prevented.
Repeated applications of TCIs “is perfectly safe and effective, and there are many studies that show proactive treatment is very effective and can prevent you from having to use too much topical steroids” or move to a systemic steroid, Dr. Simpson said.
These steps have been highly effective for sustained control even in challenging cases of AD, but he emphasized the importance of explaining the rationale to parents and eliciting their adherence to these treatment steps. Writing out the instructions will reduce confusion and help parents keep their children clear, he added.
Lawrence F. Eichenfield, MD, professor of pediatrics and dermatology at the University of California, San Diego, agreed that this recently published crossover trial has been helpful in counseling parents about how to manage AD in their children.
“Many times, pediatricians tell parents to avoid bathing because they feel that bathing will dry out the skin,” he said. The crossover study, by showing better control of AD with frequent bathing, dispels that notion, although he is not convinced that bathing at this frequency is necessary.
“I have not advised anyone to do twice-daily bathing, with rare exceptions, on the basis on this study, but, basically, I think that whether people do daily bathing or every other day bathing, it is pretty reasonable that bathing might help as long as they are applying moisturizer immediately afterward,” he said.
Dr. Simpson reports financial relationships with AbbVie, Celgene Dermira, Genentech, GlaxoSmithKline, Incyte, Lilly, Medimmune, Pfizer, Regeneron/Sanofi, and Tioga.
This publication and Global Academy for Medical Education are owned by the same parent company.
The most effective initial step for clearing atopic dermatitis in infants and young children involves daily bathing, followed by immediate application of a moisturizer, topical steroid, or both, according to an expert speaking at the virtual annual Coastal Dermatology Symposium.
“If they are really severe, you can do it twice-daily, but there are several studies that show there is not a huge benefit of twice-daily over once-daily,” said Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland.
He called this technique “soak-and-smear.” The “smear” is performed immediately after the bath when the skin is still damp, he said. When clearing is the goal, and the child has moderate to severe atopic dermatitis (AD), 0.1% triamcinolone or a similar medium potency topical steroid can be applied, and after clearing, the steroid can be switched for a moisturizer, according to Dr. Simpson.
Rather than restricting application to areas of greatest skin involvement, “put it all over,” he advised.
The clearing regimen should be continued “for a couple of more days” after the lesions have resolved, with a return visit in about a week to confirm clearing and reinforce the next steps for keeping patients clear, he added.
The next steps depend on severity. According to Dr. Simpson, severity is defined less by the extent of skin involvement at the baseline examination than the speed at which symptoms return.
For those with only mild symptoms after an extended period of clearing, moisturizer might be sufficient to prevent a significant relapse. For children with a more rapid relapse, it will be necessary to reintroduce topical steroid either every day, every other day, or twice per week.
Whether with moisturizer or with topical steroids, the soak-and-smear technique has now been validated in a recently published crossover randomized trial.
In the trial, children aged 6 months to 11 years, with moderate to severe AD, were randomized to a twice-daily bath, called the “wet method,” versus a twice-weekly bath, called the “dry method.” Both groups received a cleanser and moisturizer along with a low-potency topical steroid as needed.
After 2 weeks, the 40 evaluable patients were crossed over to the opposite bathing technique. The wet, or soak-and-smear approach, was associated with a highly significant reduction in the primary endpoint of SCORing Atopic Dermatitis (SCORAD) index, compared with the dry method (95% confidence interval, 14.9-27.6; P less than .0001). In a secondary analysis, this translated into a 30% relative reduction in favor of the wet method.
In addition, there was improvement in a caregiver assessment of the Atopic Dermatitis Quickscore (ADQ). These data show that “twice-daily baths with topical steroids and moisturizer can help in more moderate to severe population,” said Dr. Simpson, who noted that he has participated in open-label studies with the same soak-and-smear technique that have produced similar results.
Once children are clear, Dr. Simpson recommends a maintenance strategy individualized for severity. In many cases, this will involve moisturizers applied after the bath, supplemented intermittently, such as once or twice per week, with topical steroids. However, if parents find themselves resorting to daily steroids to maintain control, “that’s when you incorporate the TCIs [topical calcineurin inhibitors].”
TCIs “can help you stay at twice-per-week topical steroids,” Dr. Simpson said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
TCIs also help patients avoid steroid withdrawal, a particularly common phenomenon when topical steroids are applied repeatedly to the face. He recommended a proactive approach. By applying TCIs to areas where skin lesions frequently recur, such as the eyelids, flares can often be prevented.
Repeated applications of TCIs “is perfectly safe and effective, and there are many studies that show proactive treatment is very effective and can prevent you from having to use too much topical steroids” or move to a systemic steroid, Dr. Simpson said.
These steps have been highly effective for sustained control even in challenging cases of AD, but he emphasized the importance of explaining the rationale to parents and eliciting their adherence to these treatment steps. Writing out the instructions will reduce confusion and help parents keep their children clear, he added.
Lawrence F. Eichenfield, MD, professor of pediatrics and dermatology at the University of California, San Diego, agreed that this recently published crossover trial has been helpful in counseling parents about how to manage AD in their children.
“Many times, pediatricians tell parents to avoid bathing because they feel that bathing will dry out the skin,” he said. The crossover study, by showing better control of AD with frequent bathing, dispels that notion, although he is not convinced that bathing at this frequency is necessary.
“I have not advised anyone to do twice-daily bathing, with rare exceptions, on the basis on this study, but, basically, I think that whether people do daily bathing or every other day bathing, it is pretty reasonable that bathing might help as long as they are applying moisturizer immediately afterward,” he said.
Dr. Simpson reports financial relationships with AbbVie, Celgene Dermira, Genentech, GlaxoSmithKline, Incyte, Lilly, Medimmune, Pfizer, Regeneron/Sanofi, and Tioga.
This publication and Global Academy for Medical Education are owned by the same parent company.
The most effective initial step for clearing atopic dermatitis in infants and young children involves daily bathing, followed by immediate application of a moisturizer, topical steroid, or both, according to an expert speaking at the virtual annual Coastal Dermatology Symposium.
“If they are really severe, you can do it twice-daily, but there are several studies that show there is not a huge benefit of twice-daily over once-daily,” said Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland.
He called this technique “soak-and-smear.” The “smear” is performed immediately after the bath when the skin is still damp, he said. When clearing is the goal, and the child has moderate to severe atopic dermatitis (AD), 0.1% triamcinolone or a similar medium potency topical steroid can be applied, and after clearing, the steroid can be switched for a moisturizer, according to Dr. Simpson.
Rather than restricting application to areas of greatest skin involvement, “put it all over,” he advised.
The clearing regimen should be continued “for a couple of more days” after the lesions have resolved, with a return visit in about a week to confirm clearing and reinforce the next steps for keeping patients clear, he added.
The next steps depend on severity. According to Dr. Simpson, severity is defined less by the extent of skin involvement at the baseline examination than the speed at which symptoms return.
For those with only mild symptoms after an extended period of clearing, moisturizer might be sufficient to prevent a significant relapse. For children with a more rapid relapse, it will be necessary to reintroduce topical steroid either every day, every other day, or twice per week.
Whether with moisturizer or with topical steroids, the soak-and-smear technique has now been validated in a recently published crossover randomized trial.
In the trial, children aged 6 months to 11 years, with moderate to severe AD, were randomized to a twice-daily bath, called the “wet method,” versus a twice-weekly bath, called the “dry method.” Both groups received a cleanser and moisturizer along with a low-potency topical steroid as needed.
After 2 weeks, the 40 evaluable patients were crossed over to the opposite bathing technique. The wet, or soak-and-smear approach, was associated with a highly significant reduction in the primary endpoint of SCORing Atopic Dermatitis (SCORAD) index, compared with the dry method (95% confidence interval, 14.9-27.6; P less than .0001). In a secondary analysis, this translated into a 30% relative reduction in favor of the wet method.
In addition, there was improvement in a caregiver assessment of the Atopic Dermatitis Quickscore (ADQ). These data show that “twice-daily baths with topical steroids and moisturizer can help in more moderate to severe population,” said Dr. Simpson, who noted that he has participated in open-label studies with the same soak-and-smear technique that have produced similar results.
Once children are clear, Dr. Simpson recommends a maintenance strategy individualized for severity. In many cases, this will involve moisturizers applied after the bath, supplemented intermittently, such as once or twice per week, with topical steroids. However, if parents find themselves resorting to daily steroids to maintain control, “that’s when you incorporate the TCIs [topical calcineurin inhibitors].”
TCIs “can help you stay at twice-per-week topical steroids,” Dr. Simpson said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
TCIs also help patients avoid steroid withdrawal, a particularly common phenomenon when topical steroids are applied repeatedly to the face. He recommended a proactive approach. By applying TCIs to areas where skin lesions frequently recur, such as the eyelids, flares can often be prevented.
Repeated applications of TCIs “is perfectly safe and effective, and there are many studies that show proactive treatment is very effective and can prevent you from having to use too much topical steroids” or move to a systemic steroid, Dr. Simpson said.
These steps have been highly effective for sustained control even in challenging cases of AD, but he emphasized the importance of explaining the rationale to parents and eliciting their adherence to these treatment steps. Writing out the instructions will reduce confusion and help parents keep their children clear, he added.
Lawrence F. Eichenfield, MD, professor of pediatrics and dermatology at the University of California, San Diego, agreed that this recently published crossover trial has been helpful in counseling parents about how to manage AD in their children.
“Many times, pediatricians tell parents to avoid bathing because they feel that bathing will dry out the skin,” he said. The crossover study, by showing better control of AD with frequent bathing, dispels that notion, although he is not convinced that bathing at this frequency is necessary.
“I have not advised anyone to do twice-daily bathing, with rare exceptions, on the basis on this study, but, basically, I think that whether people do daily bathing or every other day bathing, it is pretty reasonable that bathing might help as long as they are applying moisturizer immediately afterward,” he said.
Dr. Simpson reports financial relationships with AbbVie, Celgene Dermira, Genentech, GlaxoSmithKline, Incyte, Lilly, Medimmune, Pfizer, Regeneron/Sanofi, and Tioga.
This publication and Global Academy for Medical Education are owned by the same parent company.
FROM COASTAL DERM
Orbital Granuloma Formation Following Autoinjection of Paraffin Oil: Management Considerations
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.
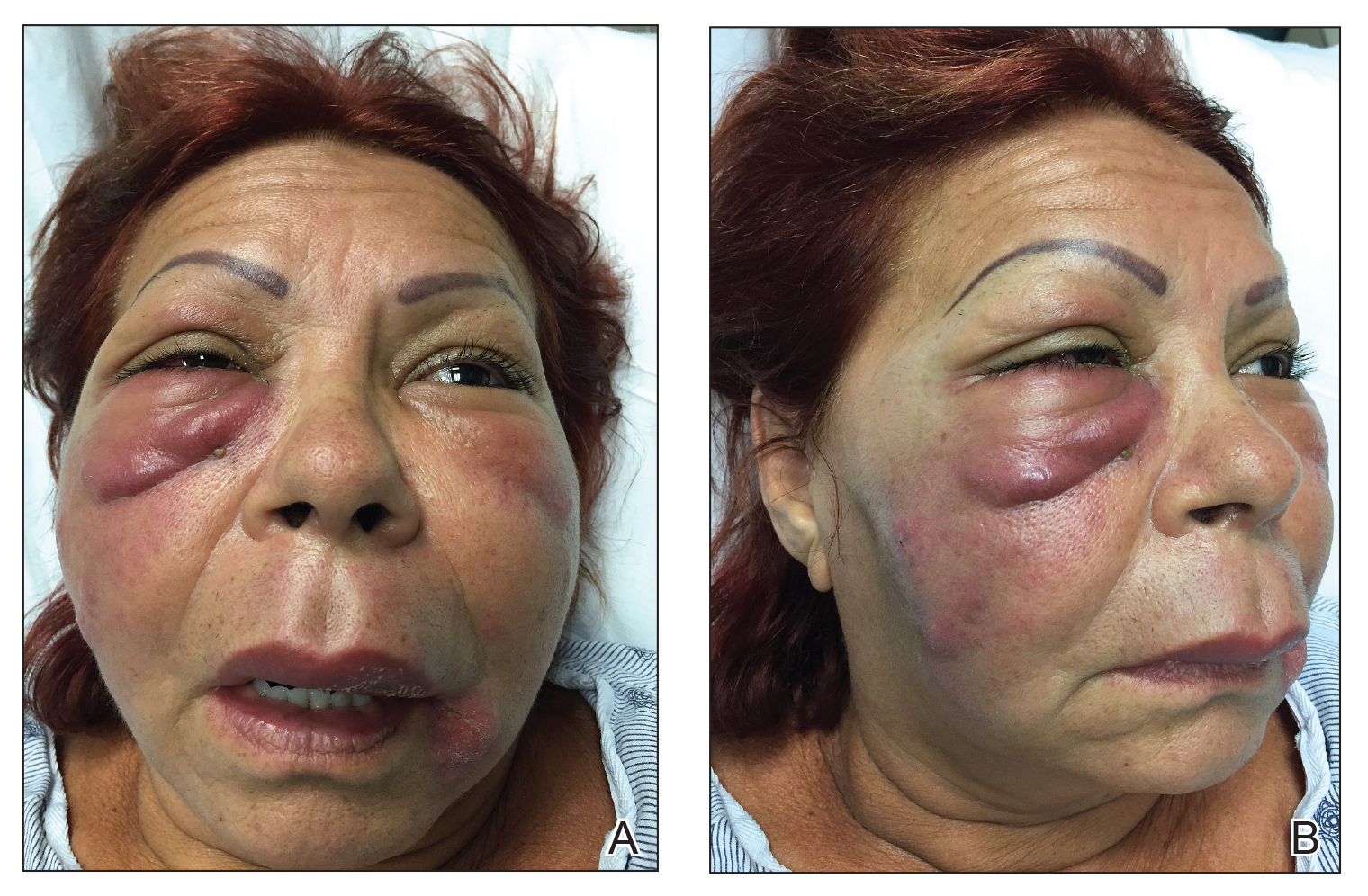
Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
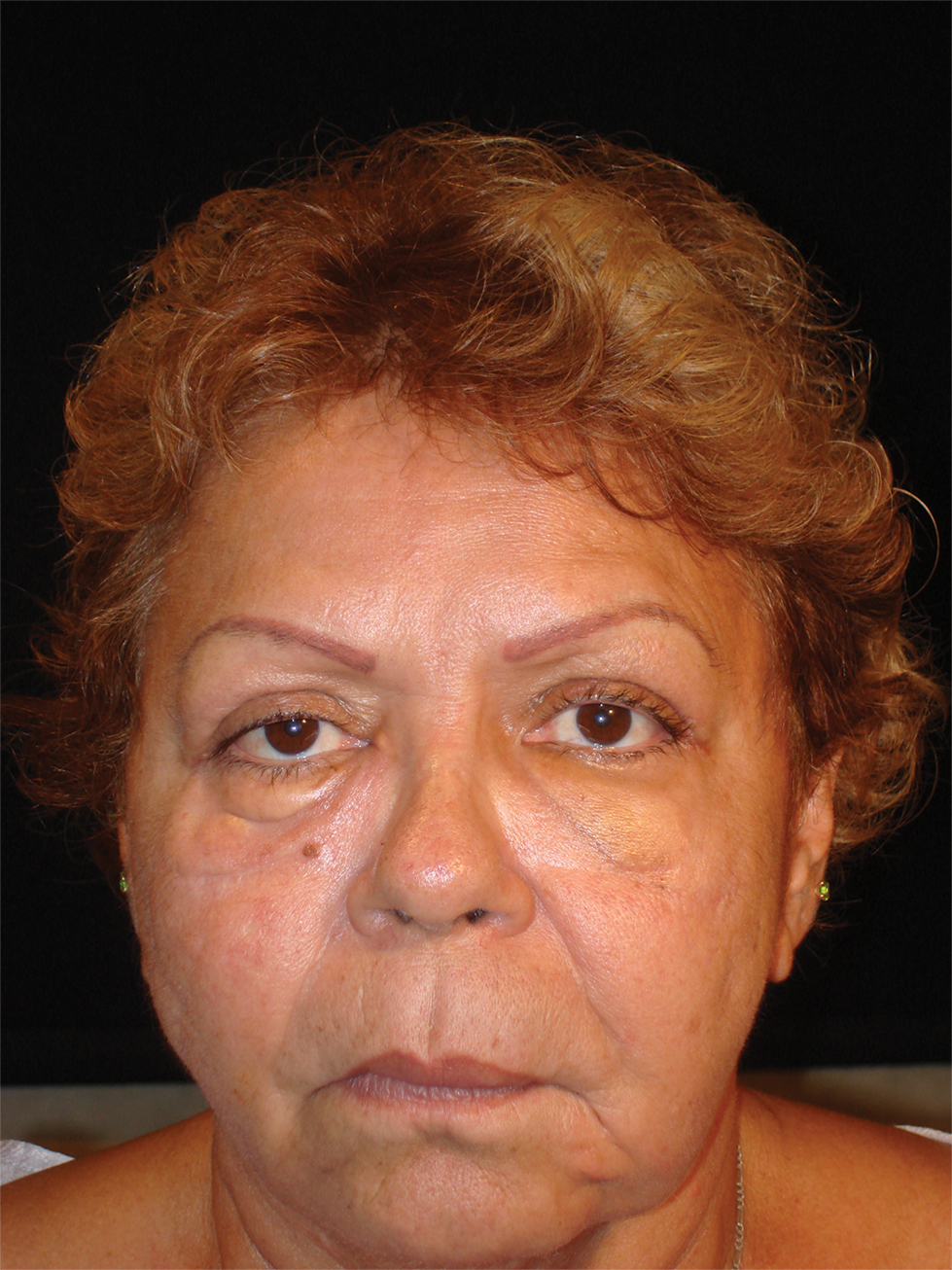
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.
Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.

Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.

Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.

Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.

Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
Practice Points
- The initial presentation of a foreign-body granulomatous process in a patient with surreptitious use of nonmedical filler can mimic infection; thus, careful history and diagnostic measures are paramount.
- Treatment of paraffin oil granuloma can be multifactorial and involves supportive care, systemic anti-inflammatory medications, time, and surgery.
- When a paraffin granuloma involves the orbital region, particular care is required to avoid long-term complications including cicatricial lagophthalmos, ectropion, or retractions, which can be mitigated with the help of oculoplastic surgery.
Tylosis in a Patient With Howel-Evans Syndrome: Management With Acitretin
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
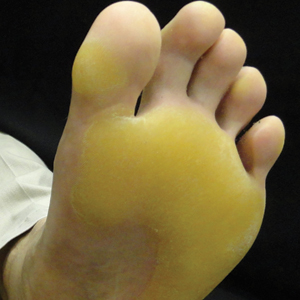
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.
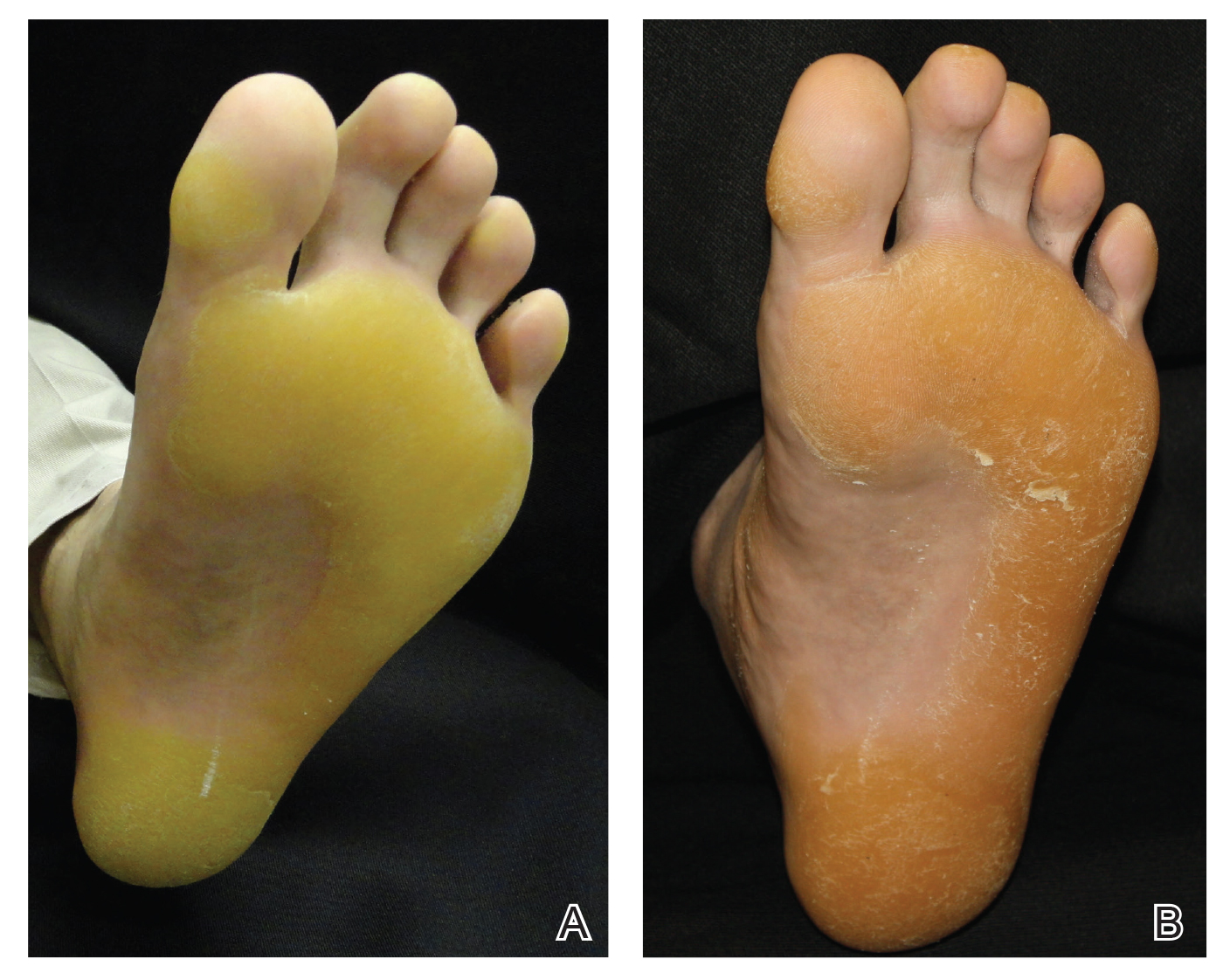
There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.

There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.

There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
Practice Points
- Keratoderma can be especially painful for patients and can have a great impact on their quality of life. For these patients, acitretin should be considered when topical therapies have failed.
- Howel-Evans syndrome is an autosomal-dominant condition that predominantly presents with plantar keratoderma and has a high risk for esophageal cancer.
Medicare fines half of hospitals for readmitting too many patients
Nearly half the nation’s hospitals, many of which are still wrestling with the financial fallout of the unexpected coronavirus, will get lower payments for all Medicare patients because of their history of readmitting patients, federal records show.
The penalties are the ninth annual round of the Hospital Readmissions Reduction Program created as part of the Affordable Care Act’s broader effort to improve quality and lower costs. The latest penalties are calculated using each hospital case history between July 2016 and June 2019, so the flood of coronavirus patients that have swamped hospitals this year were not included.
The Centers for Medicare & Medicaid Services announced in September it may suspend the penalty program in the future if the chaos surrounding the pandemic, including the spring’s moratorium on elective surgeries, makes it too difficult to assess hospital performance.
For this year, the penalties remain in effect. Retroactive to the federal fiscal year that began Oct. 1, Medicare will lower a year’s worth of payments to 2,545 hospitals, the data show. The average reduction is 0.69%, with 613 hospitals receiving a penalty of 1% or more.
Out of 5,267 hospitals in the country, Congress has exempted 2,176 from the threat of penalties, either because they are critical access hospitals – defined as the only inpatient facility in an area – or hospitals that specialize in psychiatric patients, children, veterans, rehabilitation or long-term care. Of the 3,080 hospitals CMS evaluated, 83% received a penalty.
The number and severity of penalties were comparable to those of recent years, although the number of hospitals receiving the maximum penalty of 3% dropped from 56 to 39. Because the penalties are applied to new admission payments, the total dollar amount each hospital will lose will not be known until after the fiscal year ends on July 30.
“It’s unfortunate that hospitals will face readmission penalties in fiscal year 2021,” said Akin Demehin, director of policy at the American Hospital Association. “Given the financial strain that hospitals are under, every dollar counts, and the impact of any penalty is significant.”
The penalties are based on readmissions of Medicare patients who initially came to the hospital with diagnoses of congestive heart failure, heart attack, pneumonia, chronic obstructive pulmonary disease, hip or knee replacement, or coronary artery bypass graft surgery. Medicare counts as a readmission any of those patients who ended up back in any hospital within 30 days of discharge, except for planned returns like a second phase of surgery.
A hospital will be penalized if its readmission rate is higher than expected given the national trends in any one of those categories.
The industry has disapproved of the program since its inception, complaining the measures aren’t precise and it unfairly punishes hospitals that treat low-income patients, who often don’t have the resources to ensure their recoveries are successful.
Michael Millenson, a health quality consultant who focuses on patient safety, said the penalties are a useful but imperfect mechanism to push hospitals to improve their care. The designers of the penalty system envisioned it as a way to neutralize the economic benefit hospitals get from readmitted patients under Medicare’s fee-for-service payment model, as they are otherwise paid for two stays instead of just one.
“Every industry complains the penalties are too harsh,” he said. “if you’re going to tell me we don’t need any economic incentives to do the right thing because we’re always doing the right thing – that’s not true.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Nearly half the nation’s hospitals, many of which are still wrestling with the financial fallout of the unexpected coronavirus, will get lower payments for all Medicare patients because of their history of readmitting patients, federal records show.
The penalties are the ninth annual round of the Hospital Readmissions Reduction Program created as part of the Affordable Care Act’s broader effort to improve quality and lower costs. The latest penalties are calculated using each hospital case history between July 2016 and June 2019, so the flood of coronavirus patients that have swamped hospitals this year were not included.
The Centers for Medicare & Medicaid Services announced in September it may suspend the penalty program in the future if the chaos surrounding the pandemic, including the spring’s moratorium on elective surgeries, makes it too difficult to assess hospital performance.
For this year, the penalties remain in effect. Retroactive to the federal fiscal year that began Oct. 1, Medicare will lower a year’s worth of payments to 2,545 hospitals, the data show. The average reduction is 0.69%, with 613 hospitals receiving a penalty of 1% or more.
Out of 5,267 hospitals in the country, Congress has exempted 2,176 from the threat of penalties, either because they are critical access hospitals – defined as the only inpatient facility in an area – or hospitals that specialize in psychiatric patients, children, veterans, rehabilitation or long-term care. Of the 3,080 hospitals CMS evaluated, 83% received a penalty.
The number and severity of penalties were comparable to those of recent years, although the number of hospitals receiving the maximum penalty of 3% dropped from 56 to 39. Because the penalties are applied to new admission payments, the total dollar amount each hospital will lose will not be known until after the fiscal year ends on July 30.
“It’s unfortunate that hospitals will face readmission penalties in fiscal year 2021,” said Akin Demehin, director of policy at the American Hospital Association. “Given the financial strain that hospitals are under, every dollar counts, and the impact of any penalty is significant.”
The penalties are based on readmissions of Medicare patients who initially came to the hospital with diagnoses of congestive heart failure, heart attack, pneumonia, chronic obstructive pulmonary disease, hip or knee replacement, or coronary artery bypass graft surgery. Medicare counts as a readmission any of those patients who ended up back in any hospital within 30 days of discharge, except for planned returns like a second phase of surgery.
A hospital will be penalized if its readmission rate is higher than expected given the national trends in any one of those categories.
The industry has disapproved of the program since its inception, complaining the measures aren’t precise and it unfairly punishes hospitals that treat low-income patients, who often don’t have the resources to ensure their recoveries are successful.
Michael Millenson, a health quality consultant who focuses on patient safety, said the penalties are a useful but imperfect mechanism to push hospitals to improve their care. The designers of the penalty system envisioned it as a way to neutralize the economic benefit hospitals get from readmitted patients under Medicare’s fee-for-service payment model, as they are otherwise paid for two stays instead of just one.
“Every industry complains the penalties are too harsh,” he said. “if you’re going to tell me we don’t need any economic incentives to do the right thing because we’re always doing the right thing – that’s not true.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Nearly half the nation’s hospitals, many of which are still wrestling with the financial fallout of the unexpected coronavirus, will get lower payments for all Medicare patients because of their history of readmitting patients, federal records show.
The penalties are the ninth annual round of the Hospital Readmissions Reduction Program created as part of the Affordable Care Act’s broader effort to improve quality and lower costs. The latest penalties are calculated using each hospital case history between July 2016 and June 2019, so the flood of coronavirus patients that have swamped hospitals this year were not included.
The Centers for Medicare & Medicaid Services announced in September it may suspend the penalty program in the future if the chaos surrounding the pandemic, including the spring’s moratorium on elective surgeries, makes it too difficult to assess hospital performance.
For this year, the penalties remain in effect. Retroactive to the federal fiscal year that began Oct. 1, Medicare will lower a year’s worth of payments to 2,545 hospitals, the data show. The average reduction is 0.69%, with 613 hospitals receiving a penalty of 1% or more.
Out of 5,267 hospitals in the country, Congress has exempted 2,176 from the threat of penalties, either because they are critical access hospitals – defined as the only inpatient facility in an area – or hospitals that specialize in psychiatric patients, children, veterans, rehabilitation or long-term care. Of the 3,080 hospitals CMS evaluated, 83% received a penalty.
The number and severity of penalties were comparable to those of recent years, although the number of hospitals receiving the maximum penalty of 3% dropped from 56 to 39. Because the penalties are applied to new admission payments, the total dollar amount each hospital will lose will not be known until after the fiscal year ends on July 30.
“It’s unfortunate that hospitals will face readmission penalties in fiscal year 2021,” said Akin Demehin, director of policy at the American Hospital Association. “Given the financial strain that hospitals are under, every dollar counts, and the impact of any penalty is significant.”
The penalties are based on readmissions of Medicare patients who initially came to the hospital with diagnoses of congestive heart failure, heart attack, pneumonia, chronic obstructive pulmonary disease, hip or knee replacement, or coronary artery bypass graft surgery. Medicare counts as a readmission any of those patients who ended up back in any hospital within 30 days of discharge, except for planned returns like a second phase of surgery.
A hospital will be penalized if its readmission rate is higher than expected given the national trends in any one of those categories.
The industry has disapproved of the program since its inception, complaining the measures aren’t precise and it unfairly punishes hospitals that treat low-income patients, who often don’t have the resources to ensure their recoveries are successful.
Michael Millenson, a health quality consultant who focuses on patient safety, said the penalties are a useful but imperfect mechanism to push hospitals to improve their care. The designers of the penalty system envisioned it as a way to neutralize the economic benefit hospitals get from readmitted patients under Medicare’s fee-for-service payment model, as they are otherwise paid for two stays instead of just one.
“Every industry complains the penalties are too harsh,” he said. “if you’re going to tell me we don’t need any economic incentives to do the right thing because we’re always doing the right thing – that’s not true.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Blood test for Alzheimer’s disease comes to the clinic
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.
How cannabis-based therapeutics could help fight COVID inflammation
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Sublingual apomorphine alleviates off episodes in Parkinson’s disease
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
FROM MOVEMENT DISORDERS SOCIETY 2020
A skin test for Parkinson’s disease diagnosis?
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM MOVEMENT DISORDERS
Twelve medical groups pen letter opposing UHC copay accumulator program
ACR leads outcry against the insurer’s proposed move
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
ACR leads outcry against the insurer’s proposed move
ACR leads outcry against the insurer’s proposed move
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
PARTNER registry valve-in-valve outcomes reassuring at 5 years
Transcatheter replacement of a failing surgical bioprosthetic valve showed durably favorable valve hemodynamics coupled with markedly improved patient functional status and excellent quality of life benefits at 5 years of follow-up in the prospective multicenter PARTNER 2 ViV Registry, Rebecca T. Hahn, MD, reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
She provided an update on previously reported 3-year outcomes in 365 patients at high to extreme surgical risk who underwent transcatheter aortic valve replacement (TAVR) with a 23-mm or 26-mm Sapien XT valve to address a failing surgical aortic bioprosthesis. The ViV (valve-in-valve) results are quite encouraging, she said at the meeting sponsored by the Cardiovascular Research Foundation.
“I think that this information is changing our algorithm for how we initially make treatment decisions in our patients,” according to the cardiologist.
“We now know that we can salvage a surgical bioprosthetic valve failure with a transcatheter procedure that is relatively safe and has good outcomes out to 5 years – and that’s with a second-generation TAVR valve, not even the third-generation valve,” observed Dr. Hahn, director of interventional echocardiography at New York–Presbyterian/Columbia University Medical Center and professor of clinical medicine at Columbia University, both in New York.
Interventionalists consider the third-generation valve, the Sapien 3, a superior platform compared to the Sapien 2 in use when the PARTNERS 2 ViV Registry started, she added.
At 5 years of follow-up since TAVR valve implantation, the all-cause mortality rate was 50.6%, up significantly from 32.7% at 3 years. However, this high mortality comes as no surprise given that registry participants had a profound comorbidity burden, as reflected in their mean Society of Thoracic Surgeons risk score of 9.1% at the time of TAVR. Of note, the 5-year mortality in surgically high- to extreme-risk patients in the ViV registry was comparable with the 45.9% rate at 5 years following TAVR of a native valve in intermediate-risk patients in the PARTNER 2b trial and superior to the 73% rate with TAVR of a native aortic valve in inoperable patients in PARTNER 2a, the cardiologist said.
The 5-year stroke rate in the ViV registry was 10.1%, up from 6.2% at 3 years. The cumulative incidence of death or stroke through 5 years was 53.8%.
Mortality was significantly lower in recipients of a 26-mm Sapien 2 valve than with the 23-mm version, at 40% at 5 years versus 53%. Recipients of the smaller valve were more often male, had a higher prevalence of coronary artery disease, a higher surgical risk score, a significantly smaller baseline aortic valve area, and a higher mean gradient. Dr. Hahn and her coinvestigators are now examining their data to determine if surgical valve size/patient mismatch was a major driver of adverse outcomes, as has been reported in some other datasets.
At 5 years, the rate of structural valve deterioration–related hemodynamic valve deterioration (SVD-HVD) or bioprosthetic valve failure (BVF) using the soon-to-be-published Valve Academic Research Consortium–3 definitions was 6.6%. The rates of each class of valve deterioration at 5 years in this high- to extreme-risk population were 1.2 per 100 patient-years for SVD-HVD, 0.88 per 100 patient-years for all BVF, and 0.4 per 100 patient-years for SVD-related BVF.
Fully 51% of 5-year survivors were NYHA functional class I, whereas more than 90% of patients were class III or IV at baseline. The mean gradient was 16.8 mm Hg at 5 years, the Doppler velocity index was 0.35, and the mean Kansas City Cardiomyopathy Questionnaire overall summary score was 74.2, all closely similar to the values at 3 years. That dramatic and sustained improvement in the Kansas City Cardiomyopathy Questionnaire from a baseline of 43.1 points is larger than ever seen in any clinical trial of native valve TAVR, Dr. Hahn noted.
For discussant Vinayak N. Bapat, MD a cardiothoracic surgeon at the Minneapolis Heart Institute Foundation, the 5-year PARTNER 2 follow-up data contains a clear take-home message: “These data show that, when we as surgeons are putting in small valves, we ought to put in valves that are expandable.”
Discussant Jeroen J. Bax, MD, had one major caveat regarding the PARTNER 2 ViV Registry findings: They focused on high-surgical-risk patients.
“I think we would all agree that in high-risk patients, valve-in-valve is a better option than redo surgery. But in young, low-risk patients who are getting a bioprosthetic valve – and we’re going to be seeing more and more of them because over 90% of patients in Europe getting aortic valve surgery now are getting a bioprosthetic valve – we really don’t know what the best option is,” said Dr. Bax, professor of cardiology at the University of Leiden (the Netherlands).
He suggested a randomized trial of TAVR versus redo surgery in low-risk patients with failing bioprosthetic valves is in order, particularly in light of concerns raised by a recent report from a French national patient registry. These were “high-quality, real-world data,” Dr. Bax said, and while they showed better early outcomes for TAVR ViV than with redo surgery, there was a crossing of the curves for heart failure hospitalization already by 2 years.
“We need to look closely at younger, low-risk patients,” he concluded.
The PARTNER 2 ViV Registry is funded by Edwards Lifesciences. Dr. Hahn reported receiving research support from Philips Healthcare and 3Mensio and honoraria from Boston Scientific, Edwards Lifesciences, and Philips Healthcare.
SOURCE: Hahn RT. TCT 2020, Late breaker.
Transcatheter replacement of a failing surgical bioprosthetic valve showed durably favorable valve hemodynamics coupled with markedly improved patient functional status and excellent quality of life benefits at 5 years of follow-up in the prospective multicenter PARTNER 2 ViV Registry, Rebecca T. Hahn, MD, reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
She provided an update on previously reported 3-year outcomes in 365 patients at high to extreme surgical risk who underwent transcatheter aortic valve replacement (TAVR) with a 23-mm or 26-mm Sapien XT valve to address a failing surgical aortic bioprosthesis. The ViV (valve-in-valve) results are quite encouraging, she said at the meeting sponsored by the Cardiovascular Research Foundation.
“I think that this information is changing our algorithm for how we initially make treatment decisions in our patients,” according to the cardiologist.
“We now know that we can salvage a surgical bioprosthetic valve failure with a transcatheter procedure that is relatively safe and has good outcomes out to 5 years – and that’s with a second-generation TAVR valve, not even the third-generation valve,” observed Dr. Hahn, director of interventional echocardiography at New York–Presbyterian/Columbia University Medical Center and professor of clinical medicine at Columbia University, both in New York.
Interventionalists consider the third-generation valve, the Sapien 3, a superior platform compared to the Sapien 2 in use when the PARTNERS 2 ViV Registry started, she added.
At 5 years of follow-up since TAVR valve implantation, the all-cause mortality rate was 50.6%, up significantly from 32.7% at 3 years. However, this high mortality comes as no surprise given that registry participants had a profound comorbidity burden, as reflected in their mean Society of Thoracic Surgeons risk score of 9.1% at the time of TAVR. Of note, the 5-year mortality in surgically high- to extreme-risk patients in the ViV registry was comparable with the 45.9% rate at 5 years following TAVR of a native valve in intermediate-risk patients in the PARTNER 2b trial and superior to the 73% rate with TAVR of a native aortic valve in inoperable patients in PARTNER 2a, the cardiologist said.
The 5-year stroke rate in the ViV registry was 10.1%, up from 6.2% at 3 years. The cumulative incidence of death or stroke through 5 years was 53.8%.
Mortality was significantly lower in recipients of a 26-mm Sapien 2 valve than with the 23-mm version, at 40% at 5 years versus 53%. Recipients of the smaller valve were more often male, had a higher prevalence of coronary artery disease, a higher surgical risk score, a significantly smaller baseline aortic valve area, and a higher mean gradient. Dr. Hahn and her coinvestigators are now examining their data to determine if surgical valve size/patient mismatch was a major driver of adverse outcomes, as has been reported in some other datasets.
At 5 years, the rate of structural valve deterioration–related hemodynamic valve deterioration (SVD-HVD) or bioprosthetic valve failure (BVF) using the soon-to-be-published Valve Academic Research Consortium–3 definitions was 6.6%. The rates of each class of valve deterioration at 5 years in this high- to extreme-risk population were 1.2 per 100 patient-years for SVD-HVD, 0.88 per 100 patient-years for all BVF, and 0.4 per 100 patient-years for SVD-related BVF.
Fully 51% of 5-year survivors were NYHA functional class I, whereas more than 90% of patients were class III or IV at baseline. The mean gradient was 16.8 mm Hg at 5 years, the Doppler velocity index was 0.35, and the mean Kansas City Cardiomyopathy Questionnaire overall summary score was 74.2, all closely similar to the values at 3 years. That dramatic and sustained improvement in the Kansas City Cardiomyopathy Questionnaire from a baseline of 43.1 points is larger than ever seen in any clinical trial of native valve TAVR, Dr. Hahn noted.
For discussant Vinayak N. Bapat, MD a cardiothoracic surgeon at the Minneapolis Heart Institute Foundation, the 5-year PARTNER 2 follow-up data contains a clear take-home message: “These data show that, when we as surgeons are putting in small valves, we ought to put in valves that are expandable.”
Discussant Jeroen J. Bax, MD, had one major caveat regarding the PARTNER 2 ViV Registry findings: They focused on high-surgical-risk patients.
“I think we would all agree that in high-risk patients, valve-in-valve is a better option than redo surgery. But in young, low-risk patients who are getting a bioprosthetic valve – and we’re going to be seeing more and more of them because over 90% of patients in Europe getting aortic valve surgery now are getting a bioprosthetic valve – we really don’t know what the best option is,” said Dr. Bax, professor of cardiology at the University of Leiden (the Netherlands).
He suggested a randomized trial of TAVR versus redo surgery in low-risk patients with failing bioprosthetic valves is in order, particularly in light of concerns raised by a recent report from a French national patient registry. These were “high-quality, real-world data,” Dr. Bax said, and while they showed better early outcomes for TAVR ViV than with redo surgery, there was a crossing of the curves for heart failure hospitalization already by 2 years.
“We need to look closely at younger, low-risk patients,” he concluded.
The PARTNER 2 ViV Registry is funded by Edwards Lifesciences. Dr. Hahn reported receiving research support from Philips Healthcare and 3Mensio and honoraria from Boston Scientific, Edwards Lifesciences, and Philips Healthcare.
SOURCE: Hahn RT. TCT 2020, Late breaker.
Transcatheter replacement of a failing surgical bioprosthetic valve showed durably favorable valve hemodynamics coupled with markedly improved patient functional status and excellent quality of life benefits at 5 years of follow-up in the prospective multicenter PARTNER 2 ViV Registry, Rebecca T. Hahn, MD, reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
She provided an update on previously reported 3-year outcomes in 365 patients at high to extreme surgical risk who underwent transcatheter aortic valve replacement (TAVR) with a 23-mm or 26-mm Sapien XT valve to address a failing surgical aortic bioprosthesis. The ViV (valve-in-valve) results are quite encouraging, she said at the meeting sponsored by the Cardiovascular Research Foundation.
“I think that this information is changing our algorithm for how we initially make treatment decisions in our patients,” according to the cardiologist.
“We now know that we can salvage a surgical bioprosthetic valve failure with a transcatheter procedure that is relatively safe and has good outcomes out to 5 years – and that’s with a second-generation TAVR valve, not even the third-generation valve,” observed Dr. Hahn, director of interventional echocardiography at New York–Presbyterian/Columbia University Medical Center and professor of clinical medicine at Columbia University, both in New York.
Interventionalists consider the third-generation valve, the Sapien 3, a superior platform compared to the Sapien 2 in use when the PARTNERS 2 ViV Registry started, she added.
At 5 years of follow-up since TAVR valve implantation, the all-cause mortality rate was 50.6%, up significantly from 32.7% at 3 years. However, this high mortality comes as no surprise given that registry participants had a profound comorbidity burden, as reflected in their mean Society of Thoracic Surgeons risk score of 9.1% at the time of TAVR. Of note, the 5-year mortality in surgically high- to extreme-risk patients in the ViV registry was comparable with the 45.9% rate at 5 years following TAVR of a native valve in intermediate-risk patients in the PARTNER 2b trial and superior to the 73% rate with TAVR of a native aortic valve in inoperable patients in PARTNER 2a, the cardiologist said.
The 5-year stroke rate in the ViV registry was 10.1%, up from 6.2% at 3 years. The cumulative incidence of death or stroke through 5 years was 53.8%.
Mortality was significantly lower in recipients of a 26-mm Sapien 2 valve than with the 23-mm version, at 40% at 5 years versus 53%. Recipients of the smaller valve were more often male, had a higher prevalence of coronary artery disease, a higher surgical risk score, a significantly smaller baseline aortic valve area, and a higher mean gradient. Dr. Hahn and her coinvestigators are now examining their data to determine if surgical valve size/patient mismatch was a major driver of adverse outcomes, as has been reported in some other datasets.
At 5 years, the rate of structural valve deterioration–related hemodynamic valve deterioration (SVD-HVD) or bioprosthetic valve failure (BVF) using the soon-to-be-published Valve Academic Research Consortium–3 definitions was 6.6%. The rates of each class of valve deterioration at 5 years in this high- to extreme-risk population were 1.2 per 100 patient-years for SVD-HVD, 0.88 per 100 patient-years for all BVF, and 0.4 per 100 patient-years for SVD-related BVF.
Fully 51% of 5-year survivors were NYHA functional class I, whereas more than 90% of patients were class III or IV at baseline. The mean gradient was 16.8 mm Hg at 5 years, the Doppler velocity index was 0.35, and the mean Kansas City Cardiomyopathy Questionnaire overall summary score was 74.2, all closely similar to the values at 3 years. That dramatic and sustained improvement in the Kansas City Cardiomyopathy Questionnaire from a baseline of 43.1 points is larger than ever seen in any clinical trial of native valve TAVR, Dr. Hahn noted.
For discussant Vinayak N. Bapat, MD a cardiothoracic surgeon at the Minneapolis Heart Institute Foundation, the 5-year PARTNER 2 follow-up data contains a clear take-home message: “These data show that, when we as surgeons are putting in small valves, we ought to put in valves that are expandable.”
Discussant Jeroen J. Bax, MD, had one major caveat regarding the PARTNER 2 ViV Registry findings: They focused on high-surgical-risk patients.
“I think we would all agree that in high-risk patients, valve-in-valve is a better option than redo surgery. But in young, low-risk patients who are getting a bioprosthetic valve – and we’re going to be seeing more and more of them because over 90% of patients in Europe getting aortic valve surgery now are getting a bioprosthetic valve – we really don’t know what the best option is,” said Dr. Bax, professor of cardiology at the University of Leiden (the Netherlands).
He suggested a randomized trial of TAVR versus redo surgery in low-risk patients with failing bioprosthetic valves is in order, particularly in light of concerns raised by a recent report from a French national patient registry. These were “high-quality, real-world data,” Dr. Bax said, and while they showed better early outcomes for TAVR ViV than with redo surgery, there was a crossing of the curves for heart failure hospitalization already by 2 years.
“We need to look closely at younger, low-risk patients,” he concluded.
The PARTNER 2 ViV Registry is funded by Edwards Lifesciences. Dr. Hahn reported receiving research support from Philips Healthcare and 3Mensio and honoraria from Boston Scientific, Edwards Lifesciences, and Philips Healthcare.
SOURCE: Hahn RT. TCT 2020, Late breaker.
FROM TCT 2020