User login
FDA approves Brukinsa for relapsed, refractory MCL
The Food and Drug Administration has approved zanubrutinib (Brukinsa) for the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy.
The approval is based on results from two separate studies; in a global phase 1/2 trial, patients with relapsed or refractory MCL who received zanubrutinib had an overall response rate of 84%, with 22% experiencing a complete response and 62% experiencing partial response. Median duration of response was 18.5 months. The ORR in the second study – a multicenter phase 2 trial – was also 84%, but with 59% experiencing a complete response and 24% experiencing partial response; duration of response was 19.5 months.
The most common adverse events reported during the trials were decreased neutrophil count, decreased platelet count, upper respiratory tract infection, decreased white blood cell count, decreased hemoglobin, rash, bruising, diarrhea, cough, musculoskeletal pain, pneumonia, urinary tract infection, hematuria, fatigue, constipation, and hemorrhage. The most common serious adverse events were pneumonia and hemorrhage.
Of the 118 patients with MCL treated with zanubrutinib over the two trials, 8 had to be discontinued because of adverse events.
The recommended dose of zanubrutinib is 320 mg, taken orally 160 mg twice daily or 320 mg once daily, with or without food.
“BTK [Bruton kinase] inhibition is an established mode of treatment for patients with MCL, but many patients treated with previously approved BTK inhibitors do not fully respond to BTK therapy or are forced to discontinue treatment early due to side effects. Today we have a new option for our adult patients who have received one prior systemic or targeted therapy and are living with MCL,” Luhua (Michael) Wang, MD, clinical trial investigator and professor in the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, said in a statement.
The Food and Drug Administration has approved zanubrutinib (Brukinsa) for the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy.
The approval is based on results from two separate studies; in a global phase 1/2 trial, patients with relapsed or refractory MCL who received zanubrutinib had an overall response rate of 84%, with 22% experiencing a complete response and 62% experiencing partial response. Median duration of response was 18.5 months. The ORR in the second study – a multicenter phase 2 trial – was also 84%, but with 59% experiencing a complete response and 24% experiencing partial response; duration of response was 19.5 months.
The most common adverse events reported during the trials were decreased neutrophil count, decreased platelet count, upper respiratory tract infection, decreased white blood cell count, decreased hemoglobin, rash, bruising, diarrhea, cough, musculoskeletal pain, pneumonia, urinary tract infection, hematuria, fatigue, constipation, and hemorrhage. The most common serious adverse events were pneumonia and hemorrhage.
Of the 118 patients with MCL treated with zanubrutinib over the two trials, 8 had to be discontinued because of adverse events.
The recommended dose of zanubrutinib is 320 mg, taken orally 160 mg twice daily or 320 mg once daily, with or without food.
“BTK [Bruton kinase] inhibition is an established mode of treatment for patients with MCL, but many patients treated with previously approved BTK inhibitors do not fully respond to BTK therapy or are forced to discontinue treatment early due to side effects. Today we have a new option for our adult patients who have received one prior systemic or targeted therapy and are living with MCL,” Luhua (Michael) Wang, MD, clinical trial investigator and professor in the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, said in a statement.
The Food and Drug Administration has approved zanubrutinib (Brukinsa) for the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy.
The approval is based on results from two separate studies; in a global phase 1/2 trial, patients with relapsed or refractory MCL who received zanubrutinib had an overall response rate of 84%, with 22% experiencing a complete response and 62% experiencing partial response. Median duration of response was 18.5 months. The ORR in the second study – a multicenter phase 2 trial – was also 84%, but with 59% experiencing a complete response and 24% experiencing partial response; duration of response was 19.5 months.
The most common adverse events reported during the trials were decreased neutrophil count, decreased platelet count, upper respiratory tract infection, decreased white blood cell count, decreased hemoglobin, rash, bruising, diarrhea, cough, musculoskeletal pain, pneumonia, urinary tract infection, hematuria, fatigue, constipation, and hemorrhage. The most common serious adverse events were pneumonia and hemorrhage.
Of the 118 patients with MCL treated with zanubrutinib over the two trials, 8 had to be discontinued because of adverse events.
The recommended dose of zanubrutinib is 320 mg, taken orally 160 mg twice daily or 320 mg once daily, with or without food.
“BTK [Bruton kinase] inhibition is an established mode of treatment for patients with MCL, but many patients treated with previously approved BTK inhibitors do not fully respond to BTK therapy or are forced to discontinue treatment early due to side effects. Today we have a new option for our adult patients who have received one prior systemic or targeted therapy and are living with MCL,” Luhua (Michael) Wang, MD, clinical trial investigator and professor in the department of lymphoma and myeloma at the University of Texas MD Anderson Cancer Center, Houston, said in a statement.
PREVENT trial shows benefits of secukinumab for nonradiographic axSpA
ATLANTA – Patients with nonradiographic axial spondyloarthritis who received secukinumab with or without loading doses showed improvements in physical function, quality of life, inflammation, and other disease signs and symptoms, according to results from a phase 3 study presented at the annual meeting of the American College of Rheumatology.
For patients with nonradiographic axial spondyloarthritis in the double-blind, randomized, placebo-controlled PREVENT trial, these benefits persisted up to 52 weeks, Atul A. Deodhar, MD, professor of medicine in the division of arthritis and rheumatic diseases at Oregon Health & Science University, Portland, said in his presentation.
“This is the largest study done for a biologic agent in nonradiographic axial spondyloarthritis,” Dr. Deodhar said. The trial enrolled 185 patients who received subcutaneous secukinumab (Cosentyx) at a dose of 150 mg, 184 patients who received the medication without a loading dose, and 186 patients who received placebo.
Patients were included if they were aged at least 18 years with 6 months or more of inflammatory back pain, had objective signs of inflammation (sacroiliitis on MRI and/or C-reactive protein [CRP] at 5.0 mg/dL or higher), had active disease and spinal pain according to the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), had total back pain with a visual analog scale of 40 mm or greater, and had not received a tumor necrosis factor inhibitor (TNFi) or had an inadequate response to no more than one TNFi. Patients were also stratified by inflammation measured on MRI and CRP. A little more than half of the patients in each group were women, and at baseline their mean age was 39 years, with a mean symptom duration of more than 8 years and mean Ankylosing Spondylitis Disease Activity Score of 3.5-3.7.
The primary endpoint was at least 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks to meet European Union regulatory requirements and at 52 weeks for the Food and Drug Administration. Escape to open-label secukinumab or standard of care was permitted any time after week 20 for patients deemed to have inadequate response based on clinical judgment of disease activity by the investigator and patient; at 52 weeks, the trial became open label and patients in the placebo group could begin secukinumab or standard of care. The U.S. and European Union analyses were performed independently, with the European analysis including only secukinumab with loading doses and the U.S. analysis including secukinumab without loading.
At 16 weeks, an analysis of the overall population showed that 40.8% of patients in the secukinumab nonloading group had an ASAS40 response, compared with 40.0% in those who got a loading dose and 28.0% with placebo (P less than .05 for both). Among the 90% of patients who were TNFi naive, ASAS40 responses occurred in 42.2% of patients in the nonloading group, 41.5% who received a loading dose, and 29.2% with placebo (P less than .05 for both). ASAS40 response rates persisted at 52 weeks for patients in the nonloading (39.8%), loading (35.4%), and placebo (19.9%) groups (P less than .05).
Over the same time period, the least-square mean changes in total BASDAI score improved from baseline by 2.43 in the nonloading group, 2.35 in the loading group, and 1.46 in the placebo group (P less than .05). The percentage of patients who had 50% or greater improvement in BASDAI was 37% in both treatment groups, compared with 21% with placebo (P less than .05).
Function score as measured by the Bath Ankylosing Spondylitis Functional Index also showed significantly greater improvements at 16 weeks for both loading and nonloading patients versus placebo (–1.75 and –1.64 vs. –1.01; P less than .05). Treatment with or without a loading dose led to significant reductions in sacroiliac joint edema on MRI and high-sensitivity CRP. The percentage patients who met ASAS partial remission criteria were significantly higher in the loading (21.6%) and nonloading (21.2%) groups, compared with placebo (7.0%; P less than .05).
Physical function and quality of life assessments at 16 weeks using the 36-item Short Form Health Survey physical component score and the Ankylosing Spondylitis Quality of Life questionnaire showed significant improvements both with and without a loading dose.
There were no new safety concerns with secukinumab that arose in the trial, Dr. Deodhar said.
Dr. Deodhar admitted the placebo effect was high in the PREVENT study, but noted that this was a reoccurring problem in other areas of rheumatology. “The rates are going up in several studies, including in RA, so [in terms of] axial spondyloarthritis and why that happens, we really don’t know.”
When asked about the effect of the loading dose, Dr. Deodhar said that “the load is not really going to take over or have different response by 52 weeks; the load would have a response by 8 weeks or maybe 12 weeks, but then beyond that, I don’t think the load would have any response at all.
“In my clinical experience, speaking outside this trial, load obviously helps the patient quickly to feel better, and so that’s the way I practice my medicine,” he added.
The PREVENT study was sponsored by Novartis, which markets secukinumab. Some of the authors reported relationships with Novartis and many other pharmaceutical companies. Four authors were employees of Novartis.
SOURCE: Deodhar AA et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L21.
ATLANTA – Patients with nonradiographic axial spondyloarthritis who received secukinumab with or without loading doses showed improvements in physical function, quality of life, inflammation, and other disease signs and symptoms, according to results from a phase 3 study presented at the annual meeting of the American College of Rheumatology.
For patients with nonradiographic axial spondyloarthritis in the double-blind, randomized, placebo-controlled PREVENT trial, these benefits persisted up to 52 weeks, Atul A. Deodhar, MD, professor of medicine in the division of arthritis and rheumatic diseases at Oregon Health & Science University, Portland, said in his presentation.
“This is the largest study done for a biologic agent in nonradiographic axial spondyloarthritis,” Dr. Deodhar said. The trial enrolled 185 patients who received subcutaneous secukinumab (Cosentyx) at a dose of 150 mg, 184 patients who received the medication without a loading dose, and 186 patients who received placebo.
Patients were included if they were aged at least 18 years with 6 months or more of inflammatory back pain, had objective signs of inflammation (sacroiliitis on MRI and/or C-reactive protein [CRP] at 5.0 mg/dL or higher), had active disease and spinal pain according to the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), had total back pain with a visual analog scale of 40 mm or greater, and had not received a tumor necrosis factor inhibitor (TNFi) or had an inadequate response to no more than one TNFi. Patients were also stratified by inflammation measured on MRI and CRP. A little more than half of the patients in each group were women, and at baseline their mean age was 39 years, with a mean symptom duration of more than 8 years and mean Ankylosing Spondylitis Disease Activity Score of 3.5-3.7.
The primary endpoint was at least 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks to meet European Union regulatory requirements and at 52 weeks for the Food and Drug Administration. Escape to open-label secukinumab or standard of care was permitted any time after week 20 for patients deemed to have inadequate response based on clinical judgment of disease activity by the investigator and patient; at 52 weeks, the trial became open label and patients in the placebo group could begin secukinumab or standard of care. The U.S. and European Union analyses were performed independently, with the European analysis including only secukinumab with loading doses and the U.S. analysis including secukinumab without loading.
At 16 weeks, an analysis of the overall population showed that 40.8% of patients in the secukinumab nonloading group had an ASAS40 response, compared with 40.0% in those who got a loading dose and 28.0% with placebo (P less than .05 for both). Among the 90% of patients who were TNFi naive, ASAS40 responses occurred in 42.2% of patients in the nonloading group, 41.5% who received a loading dose, and 29.2% with placebo (P less than .05 for both). ASAS40 response rates persisted at 52 weeks for patients in the nonloading (39.8%), loading (35.4%), and placebo (19.9%) groups (P less than .05).
Over the same time period, the least-square mean changes in total BASDAI score improved from baseline by 2.43 in the nonloading group, 2.35 in the loading group, and 1.46 in the placebo group (P less than .05). The percentage of patients who had 50% or greater improvement in BASDAI was 37% in both treatment groups, compared with 21% with placebo (P less than .05).
Function score as measured by the Bath Ankylosing Spondylitis Functional Index also showed significantly greater improvements at 16 weeks for both loading and nonloading patients versus placebo (–1.75 and –1.64 vs. –1.01; P less than .05). Treatment with or without a loading dose led to significant reductions in sacroiliac joint edema on MRI and high-sensitivity CRP. The percentage patients who met ASAS partial remission criteria were significantly higher in the loading (21.6%) and nonloading (21.2%) groups, compared with placebo (7.0%; P less than .05).
Physical function and quality of life assessments at 16 weeks using the 36-item Short Form Health Survey physical component score and the Ankylosing Spondylitis Quality of Life questionnaire showed significant improvements both with and without a loading dose.
There were no new safety concerns with secukinumab that arose in the trial, Dr. Deodhar said.
Dr. Deodhar admitted the placebo effect was high in the PREVENT study, but noted that this was a reoccurring problem in other areas of rheumatology. “The rates are going up in several studies, including in RA, so [in terms of] axial spondyloarthritis and why that happens, we really don’t know.”
When asked about the effect of the loading dose, Dr. Deodhar said that “the load is not really going to take over or have different response by 52 weeks; the load would have a response by 8 weeks or maybe 12 weeks, but then beyond that, I don’t think the load would have any response at all.
“In my clinical experience, speaking outside this trial, load obviously helps the patient quickly to feel better, and so that’s the way I practice my medicine,” he added.
The PREVENT study was sponsored by Novartis, which markets secukinumab. Some of the authors reported relationships with Novartis and many other pharmaceutical companies. Four authors were employees of Novartis.
SOURCE: Deodhar AA et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L21.
ATLANTA – Patients with nonradiographic axial spondyloarthritis who received secukinumab with or without loading doses showed improvements in physical function, quality of life, inflammation, and other disease signs and symptoms, according to results from a phase 3 study presented at the annual meeting of the American College of Rheumatology.
For patients with nonradiographic axial spondyloarthritis in the double-blind, randomized, placebo-controlled PREVENT trial, these benefits persisted up to 52 weeks, Atul A. Deodhar, MD, professor of medicine in the division of arthritis and rheumatic diseases at Oregon Health & Science University, Portland, said in his presentation.
“This is the largest study done for a biologic agent in nonradiographic axial spondyloarthritis,” Dr. Deodhar said. The trial enrolled 185 patients who received subcutaneous secukinumab (Cosentyx) at a dose of 150 mg, 184 patients who received the medication without a loading dose, and 186 patients who received placebo.
Patients were included if they were aged at least 18 years with 6 months or more of inflammatory back pain, had objective signs of inflammation (sacroiliitis on MRI and/or C-reactive protein [CRP] at 5.0 mg/dL or higher), had active disease and spinal pain according to the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), had total back pain with a visual analog scale of 40 mm or greater, and had not received a tumor necrosis factor inhibitor (TNFi) or had an inadequate response to no more than one TNFi. Patients were also stratified by inflammation measured on MRI and CRP. A little more than half of the patients in each group were women, and at baseline their mean age was 39 years, with a mean symptom duration of more than 8 years and mean Ankylosing Spondylitis Disease Activity Score of 3.5-3.7.
The primary endpoint was at least 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks to meet European Union regulatory requirements and at 52 weeks for the Food and Drug Administration. Escape to open-label secukinumab or standard of care was permitted any time after week 20 for patients deemed to have inadequate response based on clinical judgment of disease activity by the investigator and patient; at 52 weeks, the trial became open label and patients in the placebo group could begin secukinumab or standard of care. The U.S. and European Union analyses were performed independently, with the European analysis including only secukinumab with loading doses and the U.S. analysis including secukinumab without loading.
At 16 weeks, an analysis of the overall population showed that 40.8% of patients in the secukinumab nonloading group had an ASAS40 response, compared with 40.0% in those who got a loading dose and 28.0% with placebo (P less than .05 for both). Among the 90% of patients who were TNFi naive, ASAS40 responses occurred in 42.2% of patients in the nonloading group, 41.5% who received a loading dose, and 29.2% with placebo (P less than .05 for both). ASAS40 response rates persisted at 52 weeks for patients in the nonloading (39.8%), loading (35.4%), and placebo (19.9%) groups (P less than .05).
Over the same time period, the least-square mean changes in total BASDAI score improved from baseline by 2.43 in the nonloading group, 2.35 in the loading group, and 1.46 in the placebo group (P less than .05). The percentage of patients who had 50% or greater improvement in BASDAI was 37% in both treatment groups, compared with 21% with placebo (P less than .05).
Function score as measured by the Bath Ankylosing Spondylitis Functional Index also showed significantly greater improvements at 16 weeks for both loading and nonloading patients versus placebo (–1.75 and –1.64 vs. –1.01; P less than .05). Treatment with or without a loading dose led to significant reductions in sacroiliac joint edema on MRI and high-sensitivity CRP. The percentage patients who met ASAS partial remission criteria were significantly higher in the loading (21.6%) and nonloading (21.2%) groups, compared with placebo (7.0%; P less than .05).
Physical function and quality of life assessments at 16 weeks using the 36-item Short Form Health Survey physical component score and the Ankylosing Spondylitis Quality of Life questionnaire showed significant improvements both with and without a loading dose.
There were no new safety concerns with secukinumab that arose in the trial, Dr. Deodhar said.
Dr. Deodhar admitted the placebo effect was high in the PREVENT study, but noted that this was a reoccurring problem in other areas of rheumatology. “The rates are going up in several studies, including in RA, so [in terms of] axial spondyloarthritis and why that happens, we really don’t know.”
When asked about the effect of the loading dose, Dr. Deodhar said that “the load is not really going to take over or have different response by 52 weeks; the load would have a response by 8 weeks or maybe 12 weeks, but then beyond that, I don’t think the load would have any response at all.
“In my clinical experience, speaking outside this trial, load obviously helps the patient quickly to feel better, and so that’s the way I practice my medicine,” he added.
The PREVENT study was sponsored by Novartis, which markets secukinumab. Some of the authors reported relationships with Novartis and many other pharmaceutical companies. Four authors were employees of Novartis.
SOURCE: Deodhar AA et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L21.
REPORTING FROM ACR 2019
PHM19: Mitigating the harm we cause learners in medical education
PHM19 session
Mitigating the harm we cause learners in medical education
Presenters
Benjamin Kinnear, MD, MEd
Andrew Olson, MD
Matthew Kelleher, MD, MEd
Session summary
Dr. Kinnear, Dr. Olson, and Dr. Kelleher expertly led this TED-Talk style session at Pediatric Hospital Medicine 2019, convincing the audience that medical educators persistently harm the learners under their supervision.
Dr. Kinnear, of Cincinnati Children’s Hospital, opened the session noting that the path through medical school presently has a perverse focus on grades as a necessary achievement. As an expert in competency-based assessment, he asserted that the current learner assessment strategy is neither valid nor robust enough to indicate actual competence. Summary assessments presented throughout medical school are lacking continuous constructive feedback, leaving early residents in a state of shock when receiving corrective or negative assessments. He also noted that structurally many rotations create both team and patient discontinuity, leaving the learner with a feeling of detachment and limited ownership of the human patient under his/her/their care.
Dr. Olson of the University of Minnesota next described the need for the USMLE STEP 1 exam to be transitioned to a pass/fail endeavor. He cited the error of measurement of 24 points (i.e., the same test taker could have a 220 one day and a 244 the next) and the potential loss of valuable rotation experiences during the several-month period of intense study. He challenged audience members to complete an esoteric exam question to prove his point and asserted that many learners are lacking in humility, communication skills, and professionalism, and seek only the honors designation on rotations. He likened the experience of medical students on rotation and residents on service weeks to a series of first dates and affirmed the value of longitudinal learner-educator relationships.
Further, he outlined the detachment of learners from patient outcomes, demonstrated by frequent hand-offs and rotation transitions. Dr Olson also cited medical pedagogy as failing to meet the known needs of adult learners to engage in deliberate progressive practice, reflective practice, or to use concepts such as spacing or interleaving to reinforce knowledge.
Dr. Kelleher, also of Cincinnati Children’s Hospital, ended the session by taking those in attendance on an imagined “what-if” journey where each of the wrongs currently done to early learners in medical education were corrected. This included engagement in daily reflection (5 minutes at a time), reporting system issues on rounds that had failed the patient, presenting learners with a CV of attending failures to reinforce the imperfection that is a reality in medicine, praising learners when they admit “they don’t know the answer” to a question posed on rounds, completing assessments in real time in the learner’s presence, rounding until specific feedback can be identified for each learner on the team, having a kiosk on each floor where ANY team member could provide feedback to learners, using cognitive science on rounds for teaching (i.e., Socratic) rather than pimping, modeling interprofessional teamwork daily using a culture of vulnerability rather than infallibility (i.e., airline culture), and by encouraging the attending to care for patients or complete tasks independently, showing the value of education over service and model ideal family-centered communication with the team.
One might wonder, if all of the above were accomplished at the request of our talented presenters, would a pass/fail USMLE world where medical education was learner centered and filled with longitudinal relationships with teams and patients, and outcomes were connected to education produce more engaged, knowledgeable, and holistic physicians? According to this team of presenters, yes.
Key takeaways
• Current processes in medical education are harming today’s adult learner.
• Harms include reliance on numerical rather than competency-based assessment, fragmented learning environments, focus on perfection rather than improvement, ignorance of updates in cognitive science for instructional methodology, and individualist rather than team-based learning.
• Reforms are needed to remedy harms in health professional education, including making USMLE pass/fail, creating a learning-centered rather than service-centered residency environment, encouraging longitudinal relationships between teacher and learner, and connecting education to clinical outcomes.
Dr. King is associate program director, University of Minnesota Pediatric Residency Program, Minneapolis.
PHM19 session
Mitigating the harm we cause learners in medical education
Presenters
Benjamin Kinnear, MD, MEd
Andrew Olson, MD
Matthew Kelleher, MD, MEd
Session summary
Dr. Kinnear, Dr. Olson, and Dr. Kelleher expertly led this TED-Talk style session at Pediatric Hospital Medicine 2019, convincing the audience that medical educators persistently harm the learners under their supervision.
Dr. Kinnear, of Cincinnati Children’s Hospital, opened the session noting that the path through medical school presently has a perverse focus on grades as a necessary achievement. As an expert in competency-based assessment, he asserted that the current learner assessment strategy is neither valid nor robust enough to indicate actual competence. Summary assessments presented throughout medical school are lacking continuous constructive feedback, leaving early residents in a state of shock when receiving corrective or negative assessments. He also noted that structurally many rotations create both team and patient discontinuity, leaving the learner with a feeling of detachment and limited ownership of the human patient under his/her/their care.
Dr. Olson of the University of Minnesota next described the need for the USMLE STEP 1 exam to be transitioned to a pass/fail endeavor. He cited the error of measurement of 24 points (i.e., the same test taker could have a 220 one day and a 244 the next) and the potential loss of valuable rotation experiences during the several-month period of intense study. He challenged audience members to complete an esoteric exam question to prove his point and asserted that many learners are lacking in humility, communication skills, and professionalism, and seek only the honors designation on rotations. He likened the experience of medical students on rotation and residents on service weeks to a series of first dates and affirmed the value of longitudinal learner-educator relationships.
Further, he outlined the detachment of learners from patient outcomes, demonstrated by frequent hand-offs and rotation transitions. Dr Olson also cited medical pedagogy as failing to meet the known needs of adult learners to engage in deliberate progressive practice, reflective practice, or to use concepts such as spacing or interleaving to reinforce knowledge.
Dr. Kelleher, also of Cincinnati Children’s Hospital, ended the session by taking those in attendance on an imagined “what-if” journey where each of the wrongs currently done to early learners in medical education were corrected. This included engagement in daily reflection (5 minutes at a time), reporting system issues on rounds that had failed the patient, presenting learners with a CV of attending failures to reinforce the imperfection that is a reality in medicine, praising learners when they admit “they don’t know the answer” to a question posed on rounds, completing assessments in real time in the learner’s presence, rounding until specific feedback can be identified for each learner on the team, having a kiosk on each floor where ANY team member could provide feedback to learners, using cognitive science on rounds for teaching (i.e., Socratic) rather than pimping, modeling interprofessional teamwork daily using a culture of vulnerability rather than infallibility (i.e., airline culture), and by encouraging the attending to care for patients or complete tasks independently, showing the value of education over service and model ideal family-centered communication with the team.
One might wonder, if all of the above were accomplished at the request of our talented presenters, would a pass/fail USMLE world where medical education was learner centered and filled with longitudinal relationships with teams and patients, and outcomes were connected to education produce more engaged, knowledgeable, and holistic physicians? According to this team of presenters, yes.
Key takeaways
• Current processes in medical education are harming today’s adult learner.
• Harms include reliance on numerical rather than competency-based assessment, fragmented learning environments, focus on perfection rather than improvement, ignorance of updates in cognitive science for instructional methodology, and individualist rather than team-based learning.
• Reforms are needed to remedy harms in health professional education, including making USMLE pass/fail, creating a learning-centered rather than service-centered residency environment, encouraging longitudinal relationships between teacher and learner, and connecting education to clinical outcomes.
Dr. King is associate program director, University of Minnesota Pediatric Residency Program, Minneapolis.
PHM19 session
Mitigating the harm we cause learners in medical education
Presenters
Benjamin Kinnear, MD, MEd
Andrew Olson, MD
Matthew Kelleher, MD, MEd
Session summary
Dr. Kinnear, Dr. Olson, and Dr. Kelleher expertly led this TED-Talk style session at Pediatric Hospital Medicine 2019, convincing the audience that medical educators persistently harm the learners under their supervision.
Dr. Kinnear, of Cincinnati Children’s Hospital, opened the session noting that the path through medical school presently has a perverse focus on grades as a necessary achievement. As an expert in competency-based assessment, he asserted that the current learner assessment strategy is neither valid nor robust enough to indicate actual competence. Summary assessments presented throughout medical school are lacking continuous constructive feedback, leaving early residents in a state of shock when receiving corrective or negative assessments. He also noted that structurally many rotations create both team and patient discontinuity, leaving the learner with a feeling of detachment and limited ownership of the human patient under his/her/their care.
Dr. Olson of the University of Minnesota next described the need for the USMLE STEP 1 exam to be transitioned to a pass/fail endeavor. He cited the error of measurement of 24 points (i.e., the same test taker could have a 220 one day and a 244 the next) and the potential loss of valuable rotation experiences during the several-month period of intense study. He challenged audience members to complete an esoteric exam question to prove his point and asserted that many learners are lacking in humility, communication skills, and professionalism, and seek only the honors designation on rotations. He likened the experience of medical students on rotation and residents on service weeks to a series of first dates and affirmed the value of longitudinal learner-educator relationships.
Further, he outlined the detachment of learners from patient outcomes, demonstrated by frequent hand-offs and rotation transitions. Dr Olson also cited medical pedagogy as failing to meet the known needs of adult learners to engage in deliberate progressive practice, reflective practice, or to use concepts such as spacing or interleaving to reinforce knowledge.
Dr. Kelleher, also of Cincinnati Children’s Hospital, ended the session by taking those in attendance on an imagined “what-if” journey where each of the wrongs currently done to early learners in medical education were corrected. This included engagement in daily reflection (5 minutes at a time), reporting system issues on rounds that had failed the patient, presenting learners with a CV of attending failures to reinforce the imperfection that is a reality in medicine, praising learners when they admit “they don’t know the answer” to a question posed on rounds, completing assessments in real time in the learner’s presence, rounding until specific feedback can be identified for each learner on the team, having a kiosk on each floor where ANY team member could provide feedback to learners, using cognitive science on rounds for teaching (i.e., Socratic) rather than pimping, modeling interprofessional teamwork daily using a culture of vulnerability rather than infallibility (i.e., airline culture), and by encouraging the attending to care for patients or complete tasks independently, showing the value of education over service and model ideal family-centered communication with the team.
One might wonder, if all of the above were accomplished at the request of our talented presenters, would a pass/fail USMLE world where medical education was learner centered and filled with longitudinal relationships with teams and patients, and outcomes were connected to education produce more engaged, knowledgeable, and holistic physicians? According to this team of presenters, yes.
Key takeaways
• Current processes in medical education are harming today’s adult learner.
• Harms include reliance on numerical rather than competency-based assessment, fragmented learning environments, focus on perfection rather than improvement, ignorance of updates in cognitive science for instructional methodology, and individualist rather than team-based learning.
• Reforms are needed to remedy harms in health professional education, including making USMLE pass/fail, creating a learning-centered rather than service-centered residency environment, encouraging longitudinal relationships between teacher and learner, and connecting education to clinical outcomes.
Dr. King is associate program director, University of Minnesota Pediatric Residency Program, Minneapolis.
Support for medical marijuana transcends political affiliation
There is not much common ground between Republicans and Democrats these days, but both sides strongly supported the use of medical marijuana in a recent survey by the American Society of Clinical Oncology.
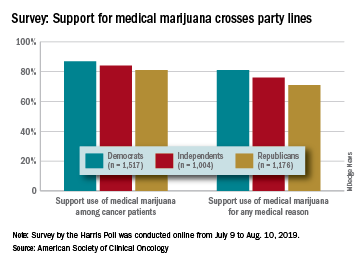
Overall support of medical marijuana among all 4,001 respondents was higher (84%) for use among cancer patients, but 76% also supported its use for any medical reason, according to data from the survey conducted for ASCO by the Harris Poll.
The differences in support between Republicans and Democrats were significant, but both parties were over 80% for marijuana use by cancer patients and over 70% for use for any medical reason. In both cases, the independents in between mirrored the overall population, with support at 84% and 76%, respectively, ASCO said.
Support for medical marijuana also was consistent based on the respondents’ cancer experience. For use by cancer patients, those who were current or previous patients were at 84%, caregivers (those providing unpaid care to an immediate family member or loved one with cancer) and other family members/loved ones were both at 87%, and those with no cancer experiences were at 82%, the survey results showed.
Use of marijuana for any medical reason was supported by 72% of current/previous patients, 79% of family members and loved ones, 80% of caregivers, and 74% of those with no cancer experience, ASCO reported.
In a question asked only of current or previous patients, 62% said that they are/were open to use of marijuana to alleviate cancer-related pain, nausea, or other symptoms, and 60% said that they wished they had more information about the benefits of medical marijuana use, according to the results of the survey, which was conducted online from July 9 to Aug. 10, 2019.
There is not much common ground between Republicans and Democrats these days, but both sides strongly supported the use of medical marijuana in a recent survey by the American Society of Clinical Oncology.

Overall support of medical marijuana among all 4,001 respondents was higher (84%) for use among cancer patients, but 76% also supported its use for any medical reason, according to data from the survey conducted for ASCO by the Harris Poll.
The differences in support between Republicans and Democrats were significant, but both parties were over 80% for marijuana use by cancer patients and over 70% for use for any medical reason. In both cases, the independents in between mirrored the overall population, with support at 84% and 76%, respectively, ASCO said.
Support for medical marijuana also was consistent based on the respondents’ cancer experience. For use by cancer patients, those who were current or previous patients were at 84%, caregivers (those providing unpaid care to an immediate family member or loved one with cancer) and other family members/loved ones were both at 87%, and those with no cancer experiences were at 82%, the survey results showed.
Use of marijuana for any medical reason was supported by 72% of current/previous patients, 79% of family members and loved ones, 80% of caregivers, and 74% of those with no cancer experience, ASCO reported.
In a question asked only of current or previous patients, 62% said that they are/were open to use of marijuana to alleviate cancer-related pain, nausea, or other symptoms, and 60% said that they wished they had more information about the benefits of medical marijuana use, according to the results of the survey, which was conducted online from July 9 to Aug. 10, 2019.
There is not much common ground between Republicans and Democrats these days, but both sides strongly supported the use of medical marijuana in a recent survey by the American Society of Clinical Oncology.

Overall support of medical marijuana among all 4,001 respondents was higher (84%) for use among cancer patients, but 76% also supported its use for any medical reason, according to data from the survey conducted for ASCO by the Harris Poll.
The differences in support between Republicans and Democrats were significant, but both parties were over 80% for marijuana use by cancer patients and over 70% for use for any medical reason. In both cases, the independents in between mirrored the overall population, with support at 84% and 76%, respectively, ASCO said.
Support for medical marijuana also was consistent based on the respondents’ cancer experience. For use by cancer patients, those who were current or previous patients were at 84%, caregivers (those providing unpaid care to an immediate family member or loved one with cancer) and other family members/loved ones were both at 87%, and those with no cancer experiences were at 82%, the survey results showed.
Use of marijuana for any medical reason was supported by 72% of current/previous patients, 79% of family members and loved ones, 80% of caregivers, and 74% of those with no cancer experience, ASCO reported.
In a question asked only of current or previous patients, 62% said that they are/were open to use of marijuana to alleviate cancer-related pain, nausea, or other symptoms, and 60% said that they wished they had more information about the benefits of medical marijuana use, according to the results of the survey, which was conducted online from July 9 to Aug. 10, 2019.
Researchers seek a way to predict cognitive deficits in children treated for ALL
Researchers are attempting to determine, early in the treatment process, which children with acute lymphoblastic leukemia (ALL) have an increased risk of neurocognitive deficits after chemotherapy.
The goal of the researchers’ project (5R01CA220568-02) is to determine if gene variants and biomarkers associated with oxidative stress, neuroinflammation, and folate physiology correlate with cognitive decline during and after chemotherapy. Ideally, certain variants and biomarkers will reveal patients who might benefit from interventions to prevent or even reverse cognitive deficits.
Peter D. Cole, MD, of Rutgers Cancer Institute, New Brunswick, N.J., and colleagues are conducting this research in patients from the DFCI-16-001 trial (NCT03020030). This multicenter, phase 3 study is enrolling patients (aged 1-21 years) with B- or T-cell ALL who then receive a multidrug chemotherapy regimen.
Dr. Cole and colleagues are analyzing a subset of patients from the trial, looking for relationships between chemotherapy-induced neurocognitive changes, gene variants, and changes in biomarkers detected in cerebrospinal fluid (CSF).
“We’re looking at a broad panel of target gene variants that are associated with either drug metabolism, defenses against oxidative stress, neuroinflammation, or folate physiology,” Dr. Cole said in an interview.
This includes variants Dr. Cole and colleagues identified in a previous, retrospective study of ALL survivors. The researchers found that survivors who were homozygous for NOS3 894T, had a variant SLCO2A1 G allele, or had at least one GSTP1 T allele were more likely to exhibit cognitive deficits (J Clin Oncol. 2015 Jul 1;33[19]:2205-11).
The researchers are also analyzing CSF samples, looking for changes in tau protein, homocysteine, homocysteic acid, the adenosylmethionine to adenosylhomocysteine ratio, and other biomarkers of oxidative stress, neuroinflammation, and folate physiology. The CSF is collected at five time points: the start of chemotherapy, day 18, the start of first consolidation, the end of first consolidation, and 7 weeks later in second consolidation.
Cognitive testing
While Dr. Cole is leading the genetic and biomarker analyses, Stephen A. Sands, PsyD, of Memorial Sloan Kettering Cancer Center in New York, is leading the cognitive testing.
The researchers are evaluating patients for cognitive decline using computerized tests from a company called Cogstate. The tests are designed to assess functions such as processing speed, attention, visual learning, and working memory. The tests are administered on an iPad and involve tasks like identifying features of playing cards and finding the correct way through a maze.
The patients – aged 3 years and older – undergo cognitive testing at six time points: baseline, which is any time between days 8 and 32 of induction (except within 72 hours after sedation or anesthesia); at first consolidation; the end of central nervous system therapy; 1 year into chemotherapy; the end of chemotherapy; and 1 year after chemotherapy ends.
In a prior study, Cogstate testing proved reliable for detecting neurocognitive changes in patients undergoing treatment for ALL (Support Care Cancer. 2017;25[2]:449-57). In the current study, the researchers are supplementing Cogstate test results with Wechsler IQ tests administered 1 year after patients complete chemotherapy.
Dr. Sands noted that Cogstate tests provide benefits over the Wechsler “paper-and-pencil” tests. One benefit is that Cogstate tests can be given more often without inducing practice effects (J Clin Exp Neuropsychol. 2006 Oct;28[7]:1095-112). Another is that Cogstate tests can be administered by anyone with a bachelor’s degree who has undergone the appropriate training, while Wechsler IQ tests must be given by psychologists.
Preliminary results
This research is ongoing, so it’s too early to announce any discoveries, but the study is moving along as planned.
“The preliminary data we have so far are demonstrating the validity of the study,” Dr. Cole said. “Things are going well. We’re able to do the cognitive testing and collect the samples that we need and ship them without losing the integrity of the samples.”
Dr. Sands noted that enrollment has been encouraging. As this is a substudy of DFCI-16-001, the researchers must obtain consent separately from the main study. Dr. Sands said about 89% of parents involved in the main study have agreed to enroll their children in the substudy.
Dr. Sands also said that early results from Cogstate testing have revealed patients who are experiencing cognitive decline during treatment. The researchers still have to determine if these results correlate with any biomarkers or gene variants.
Potential interventions
If the researchers can pinpoint patients at risk for cognitive deficits, the next step will be to investigate pharmacologic and behavioral interventions.
Dr. Cole said he is particularly interested in treatments that reduce oxidative stress, such as dextromethorphan and memantine. Dextromethorphan has been shown to resolve symptoms of methotrexate-induced neurotoxicity in patients (Pediatr Hematol Oncol. 2002 Jul-Aug;19[5]:319-27), and memantine reduced memory deficits in animals treated with methotrexate (Clin Cancer Res. 2013 Aug 15;19[16]:4446-54).
“Memantine hasn’t been used in kids with leukemia yet, but it’s something that I’d like to see brought to a clinical trial,” Dr. Cole said.
Dr. Sands pointed to other potential pharmacologic interventions, including the stimulants methylphenidate and modafinil. Both drugs have been shown to improve cognitive deficits in cancer survivors (J Clin Oncol. 2001 Mar 15;19[6]:1802-8; Cancer. 2009 Jun 15; 115[12]: 2605-16).
Computer-based cognitive training tools may be another option. One such tool, Lumosity, improved executive functions in a study of breast cancer survivors (Clin Breast Cancer. 2013 Aug;13[4]:299-306). Another tool, CogMed, improved working memory in survivors of brain tumors and ALL (Psychooncology. 2013 Aug; 22[8]: 1856-65).
Other behavioral interventions might include sleep hygiene and exercise. Sleep hygiene has been shown to improve cognitive function in childhood cancer survivors (Cancer. 2011 Jun 1;117[11]:2559-68), and a recent study revealed an association between exercise intolerance and negative neurocognitive outcomes in ALL survivors (Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510).
“What we need to figure out is which children will respond to which interventions,” Dr. Sands said, adding that interventions will likely need to be combined.
“It’s not going to be one thing that will work for everybody,” he said. “It’s going to be: What packages of things will work for different people?”
Dr. Sands and Dr. Cole reported having no relevant financial disclosures.
Researchers are attempting to determine, early in the treatment process, which children with acute lymphoblastic leukemia (ALL) have an increased risk of neurocognitive deficits after chemotherapy.
The goal of the researchers’ project (5R01CA220568-02) is to determine if gene variants and biomarkers associated with oxidative stress, neuroinflammation, and folate physiology correlate with cognitive decline during and after chemotherapy. Ideally, certain variants and biomarkers will reveal patients who might benefit from interventions to prevent or even reverse cognitive deficits.
Peter D. Cole, MD, of Rutgers Cancer Institute, New Brunswick, N.J., and colleagues are conducting this research in patients from the DFCI-16-001 trial (NCT03020030). This multicenter, phase 3 study is enrolling patients (aged 1-21 years) with B- or T-cell ALL who then receive a multidrug chemotherapy regimen.
Dr. Cole and colleagues are analyzing a subset of patients from the trial, looking for relationships between chemotherapy-induced neurocognitive changes, gene variants, and changes in biomarkers detected in cerebrospinal fluid (CSF).
“We’re looking at a broad panel of target gene variants that are associated with either drug metabolism, defenses against oxidative stress, neuroinflammation, or folate physiology,” Dr. Cole said in an interview.
This includes variants Dr. Cole and colleagues identified in a previous, retrospective study of ALL survivors. The researchers found that survivors who were homozygous for NOS3 894T, had a variant SLCO2A1 G allele, or had at least one GSTP1 T allele were more likely to exhibit cognitive deficits (J Clin Oncol. 2015 Jul 1;33[19]:2205-11).
The researchers are also analyzing CSF samples, looking for changes in tau protein, homocysteine, homocysteic acid, the adenosylmethionine to adenosylhomocysteine ratio, and other biomarkers of oxidative stress, neuroinflammation, and folate physiology. The CSF is collected at five time points: the start of chemotherapy, day 18, the start of first consolidation, the end of first consolidation, and 7 weeks later in second consolidation.
Cognitive testing
While Dr. Cole is leading the genetic and biomarker analyses, Stephen A. Sands, PsyD, of Memorial Sloan Kettering Cancer Center in New York, is leading the cognitive testing.
The researchers are evaluating patients for cognitive decline using computerized tests from a company called Cogstate. The tests are designed to assess functions such as processing speed, attention, visual learning, and working memory. The tests are administered on an iPad and involve tasks like identifying features of playing cards and finding the correct way through a maze.
The patients – aged 3 years and older – undergo cognitive testing at six time points: baseline, which is any time between days 8 and 32 of induction (except within 72 hours after sedation or anesthesia); at first consolidation; the end of central nervous system therapy; 1 year into chemotherapy; the end of chemotherapy; and 1 year after chemotherapy ends.
In a prior study, Cogstate testing proved reliable for detecting neurocognitive changes in patients undergoing treatment for ALL (Support Care Cancer. 2017;25[2]:449-57). In the current study, the researchers are supplementing Cogstate test results with Wechsler IQ tests administered 1 year after patients complete chemotherapy.
Dr. Sands noted that Cogstate tests provide benefits over the Wechsler “paper-and-pencil” tests. One benefit is that Cogstate tests can be given more often without inducing practice effects (J Clin Exp Neuropsychol. 2006 Oct;28[7]:1095-112). Another is that Cogstate tests can be administered by anyone with a bachelor’s degree who has undergone the appropriate training, while Wechsler IQ tests must be given by psychologists.
Preliminary results
This research is ongoing, so it’s too early to announce any discoveries, but the study is moving along as planned.
“The preliminary data we have so far are demonstrating the validity of the study,” Dr. Cole said. “Things are going well. We’re able to do the cognitive testing and collect the samples that we need and ship them without losing the integrity of the samples.”
Dr. Sands noted that enrollment has been encouraging. As this is a substudy of DFCI-16-001, the researchers must obtain consent separately from the main study. Dr. Sands said about 89% of parents involved in the main study have agreed to enroll their children in the substudy.
Dr. Sands also said that early results from Cogstate testing have revealed patients who are experiencing cognitive decline during treatment. The researchers still have to determine if these results correlate with any biomarkers or gene variants.
Potential interventions
If the researchers can pinpoint patients at risk for cognitive deficits, the next step will be to investigate pharmacologic and behavioral interventions.
Dr. Cole said he is particularly interested in treatments that reduce oxidative stress, such as dextromethorphan and memantine. Dextromethorphan has been shown to resolve symptoms of methotrexate-induced neurotoxicity in patients (Pediatr Hematol Oncol. 2002 Jul-Aug;19[5]:319-27), and memantine reduced memory deficits in animals treated with methotrexate (Clin Cancer Res. 2013 Aug 15;19[16]:4446-54).
“Memantine hasn’t been used in kids with leukemia yet, but it’s something that I’d like to see brought to a clinical trial,” Dr. Cole said.
Dr. Sands pointed to other potential pharmacologic interventions, including the stimulants methylphenidate and modafinil. Both drugs have been shown to improve cognitive deficits in cancer survivors (J Clin Oncol. 2001 Mar 15;19[6]:1802-8; Cancer. 2009 Jun 15; 115[12]: 2605-16).
Computer-based cognitive training tools may be another option. One such tool, Lumosity, improved executive functions in a study of breast cancer survivors (Clin Breast Cancer. 2013 Aug;13[4]:299-306). Another tool, CogMed, improved working memory in survivors of brain tumors and ALL (Psychooncology. 2013 Aug; 22[8]: 1856-65).
Other behavioral interventions might include sleep hygiene and exercise. Sleep hygiene has been shown to improve cognitive function in childhood cancer survivors (Cancer. 2011 Jun 1;117[11]:2559-68), and a recent study revealed an association between exercise intolerance and negative neurocognitive outcomes in ALL survivors (Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510).
“What we need to figure out is which children will respond to which interventions,” Dr. Sands said, adding that interventions will likely need to be combined.
“It’s not going to be one thing that will work for everybody,” he said. “It’s going to be: What packages of things will work for different people?”
Dr. Sands and Dr. Cole reported having no relevant financial disclosures.
Researchers are attempting to determine, early in the treatment process, which children with acute lymphoblastic leukemia (ALL) have an increased risk of neurocognitive deficits after chemotherapy.
The goal of the researchers’ project (5R01CA220568-02) is to determine if gene variants and biomarkers associated with oxidative stress, neuroinflammation, and folate physiology correlate with cognitive decline during and after chemotherapy. Ideally, certain variants and biomarkers will reveal patients who might benefit from interventions to prevent or even reverse cognitive deficits.
Peter D. Cole, MD, of Rutgers Cancer Institute, New Brunswick, N.J., and colleagues are conducting this research in patients from the DFCI-16-001 trial (NCT03020030). This multicenter, phase 3 study is enrolling patients (aged 1-21 years) with B- or T-cell ALL who then receive a multidrug chemotherapy regimen.
Dr. Cole and colleagues are analyzing a subset of patients from the trial, looking for relationships between chemotherapy-induced neurocognitive changes, gene variants, and changes in biomarkers detected in cerebrospinal fluid (CSF).
“We’re looking at a broad panel of target gene variants that are associated with either drug metabolism, defenses against oxidative stress, neuroinflammation, or folate physiology,” Dr. Cole said in an interview.
This includes variants Dr. Cole and colleagues identified in a previous, retrospective study of ALL survivors. The researchers found that survivors who were homozygous for NOS3 894T, had a variant SLCO2A1 G allele, or had at least one GSTP1 T allele were more likely to exhibit cognitive deficits (J Clin Oncol. 2015 Jul 1;33[19]:2205-11).
The researchers are also analyzing CSF samples, looking for changes in tau protein, homocysteine, homocysteic acid, the adenosylmethionine to adenosylhomocysteine ratio, and other biomarkers of oxidative stress, neuroinflammation, and folate physiology. The CSF is collected at five time points: the start of chemotherapy, day 18, the start of first consolidation, the end of first consolidation, and 7 weeks later in second consolidation.
Cognitive testing
While Dr. Cole is leading the genetic and biomarker analyses, Stephen A. Sands, PsyD, of Memorial Sloan Kettering Cancer Center in New York, is leading the cognitive testing.
The researchers are evaluating patients for cognitive decline using computerized tests from a company called Cogstate. The tests are designed to assess functions such as processing speed, attention, visual learning, and working memory. The tests are administered on an iPad and involve tasks like identifying features of playing cards and finding the correct way through a maze.
The patients – aged 3 years and older – undergo cognitive testing at six time points: baseline, which is any time between days 8 and 32 of induction (except within 72 hours after sedation or anesthesia); at first consolidation; the end of central nervous system therapy; 1 year into chemotherapy; the end of chemotherapy; and 1 year after chemotherapy ends.
In a prior study, Cogstate testing proved reliable for detecting neurocognitive changes in patients undergoing treatment for ALL (Support Care Cancer. 2017;25[2]:449-57). In the current study, the researchers are supplementing Cogstate test results with Wechsler IQ tests administered 1 year after patients complete chemotherapy.
Dr. Sands noted that Cogstate tests provide benefits over the Wechsler “paper-and-pencil” tests. One benefit is that Cogstate tests can be given more often without inducing practice effects (J Clin Exp Neuropsychol. 2006 Oct;28[7]:1095-112). Another is that Cogstate tests can be administered by anyone with a bachelor’s degree who has undergone the appropriate training, while Wechsler IQ tests must be given by psychologists.
Preliminary results
This research is ongoing, so it’s too early to announce any discoveries, but the study is moving along as planned.
“The preliminary data we have so far are demonstrating the validity of the study,” Dr. Cole said. “Things are going well. We’re able to do the cognitive testing and collect the samples that we need and ship them without losing the integrity of the samples.”
Dr. Sands noted that enrollment has been encouraging. As this is a substudy of DFCI-16-001, the researchers must obtain consent separately from the main study. Dr. Sands said about 89% of parents involved in the main study have agreed to enroll their children in the substudy.
Dr. Sands also said that early results from Cogstate testing have revealed patients who are experiencing cognitive decline during treatment. The researchers still have to determine if these results correlate with any biomarkers or gene variants.
Potential interventions
If the researchers can pinpoint patients at risk for cognitive deficits, the next step will be to investigate pharmacologic and behavioral interventions.
Dr. Cole said he is particularly interested in treatments that reduce oxidative stress, such as dextromethorphan and memantine. Dextromethorphan has been shown to resolve symptoms of methotrexate-induced neurotoxicity in patients (Pediatr Hematol Oncol. 2002 Jul-Aug;19[5]:319-27), and memantine reduced memory deficits in animals treated with methotrexate (Clin Cancer Res. 2013 Aug 15;19[16]:4446-54).
“Memantine hasn’t been used in kids with leukemia yet, but it’s something that I’d like to see brought to a clinical trial,” Dr. Cole said.
Dr. Sands pointed to other potential pharmacologic interventions, including the stimulants methylphenidate and modafinil. Both drugs have been shown to improve cognitive deficits in cancer survivors (J Clin Oncol. 2001 Mar 15;19[6]:1802-8; Cancer. 2009 Jun 15; 115[12]: 2605-16).
Computer-based cognitive training tools may be another option. One such tool, Lumosity, improved executive functions in a study of breast cancer survivors (Clin Breast Cancer. 2013 Aug;13[4]:299-306). Another tool, CogMed, improved working memory in survivors of brain tumors and ALL (Psychooncology. 2013 Aug; 22[8]: 1856-65).
Other behavioral interventions might include sleep hygiene and exercise. Sleep hygiene has been shown to improve cognitive function in childhood cancer survivors (Cancer. 2011 Jun 1;117[11]:2559-68), and a recent study revealed an association between exercise intolerance and negative neurocognitive outcomes in ALL survivors (Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510).
“What we need to figure out is which children will respond to which interventions,” Dr. Sands said, adding that interventions will likely need to be combined.
“It’s not going to be one thing that will work for everybody,” he said. “It’s going to be: What packages of things will work for different people?”
Dr. Sands and Dr. Cole reported having no relevant financial disclosures.
Football for the young
A few weeks ago I was at a Friday-night football game, but not to watch the game. I’ve been there and done that too many times when I used to be the team physician. I was there to listen to my granddaughter drumming in the pep band. And there was a lot of drumming because her high school’s team is having a hot year and outscoring opponents by three and four touchdowns every week.
At half time, the field was swarmed by 45-50 early grade schoolers looking like bobblehead dolls in their oversize helmets and surprisingly professional-appearing miniature football outfits. Under the lights, on the local college’s turf field, they were in football heaven. The pep band got into it and there was more drumming as the few kids who had a clue what football was about were scampering over and around their teammates and opponents who were roughhousing with each other, rolling around on the turf having a grand time, blissfully unimpressed by such trivial concepts as the line of scrimmage or the difference between blocking and tackling or even offense and defense.
Despite all the alarming articles both lay and professional that you and I see, this was an evening on which no one seemed particularly concerned about sports-related concussions. This is class B football in Maine, not a state well known as an incubator of Division I college football players. While there were a few scrawny kids with some speed,
Watching 4- and 5-year-olds in their football uniforms seemed to me to be a rather harmless exercise and certainly a more positive investment in their time on a Friday night than sitting on the couch with an electronic device clutched in their little hands. A recent report in JAMA Pediatrics suggests that my lack of concern has some validity (“Consensus statement on sports-related concussions in youth sports using a modified delphi approach.” JAMA Pediatr. 2019 Nov 11. doi: 10.1001/jamapediatrics.2019.4006). Eleven experts in sports-related injuries were surveyed with multiple rounds of questionnaires. Their anonymous responses were aggregated and shared with the group after each round until a consensus could be arrived on for each of seven broad questions about sports-related concussions. It is a paper worth reading and like most good literature surveys determined that in many situations more study needs to be done.
Among the many findings that impressed me was the group’s failure to find an “association between repetitive head impact exposure in youth and long-term neurocognitive outcomes.” In addition, “there is little evidence that age at first exposure repetitive head impacts in sports is independently associated with neurodegenerative changes.” The experts also could find “no evidence that growth or development affect the risk of sports-related concussions.”
The problem with youth football is that it is the portal that can lead to college and professional football, in which large bodies are allowed to collide after accelerating at speeds we mortals only can achieve behind the wheel of our motor vehicles. Rules to minimize those collisions do exist, but lax enforcement has failed to prevent their cumulative damage.
Whether the culture of big-time football is going to change to a point at which a conscientious parent could encourage his or her child to play after adolescence remains to be seen. However, the evidence seems to suggest that allowing young children to bang themselves around imitating the big guys seems to be reasonably safe. At least as safe as what kids used to do to each other before we adults invented television and video games.
When my son was 3 or 4 years old, he played on a hockey team he thought was called the Toronto Make-Believes (Maple Leafs). Maybe we should be telling parents it’s safe for their children to play make-believe contact sports. The challenge comes after those kids reach puberty and want to start playing the real thing.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A few weeks ago I was at a Friday-night football game, but not to watch the game. I’ve been there and done that too many times when I used to be the team physician. I was there to listen to my granddaughter drumming in the pep band. And there was a lot of drumming because her high school’s team is having a hot year and outscoring opponents by three and four touchdowns every week.
At half time, the field was swarmed by 45-50 early grade schoolers looking like bobblehead dolls in their oversize helmets and surprisingly professional-appearing miniature football outfits. Under the lights, on the local college’s turf field, they were in football heaven. The pep band got into it and there was more drumming as the few kids who had a clue what football was about were scampering over and around their teammates and opponents who were roughhousing with each other, rolling around on the turf having a grand time, blissfully unimpressed by such trivial concepts as the line of scrimmage or the difference between blocking and tackling or even offense and defense.
Despite all the alarming articles both lay and professional that you and I see, this was an evening on which no one seemed particularly concerned about sports-related concussions. This is class B football in Maine, not a state well known as an incubator of Division I college football players. While there were a few scrawny kids with some speed,
Watching 4- and 5-year-olds in their football uniforms seemed to me to be a rather harmless exercise and certainly a more positive investment in their time on a Friday night than sitting on the couch with an electronic device clutched in their little hands. A recent report in JAMA Pediatrics suggests that my lack of concern has some validity (“Consensus statement on sports-related concussions in youth sports using a modified delphi approach.” JAMA Pediatr. 2019 Nov 11. doi: 10.1001/jamapediatrics.2019.4006). Eleven experts in sports-related injuries were surveyed with multiple rounds of questionnaires. Their anonymous responses were aggregated and shared with the group after each round until a consensus could be arrived on for each of seven broad questions about sports-related concussions. It is a paper worth reading and like most good literature surveys determined that in many situations more study needs to be done.
Among the many findings that impressed me was the group’s failure to find an “association between repetitive head impact exposure in youth and long-term neurocognitive outcomes.” In addition, “there is little evidence that age at first exposure repetitive head impacts in sports is independently associated with neurodegenerative changes.” The experts also could find “no evidence that growth or development affect the risk of sports-related concussions.”
The problem with youth football is that it is the portal that can lead to college and professional football, in which large bodies are allowed to collide after accelerating at speeds we mortals only can achieve behind the wheel of our motor vehicles. Rules to minimize those collisions do exist, but lax enforcement has failed to prevent their cumulative damage.
Whether the culture of big-time football is going to change to a point at which a conscientious parent could encourage his or her child to play after adolescence remains to be seen. However, the evidence seems to suggest that allowing young children to bang themselves around imitating the big guys seems to be reasonably safe. At least as safe as what kids used to do to each other before we adults invented television and video games.
When my son was 3 or 4 years old, he played on a hockey team he thought was called the Toronto Make-Believes (Maple Leafs). Maybe we should be telling parents it’s safe for their children to play make-believe contact sports. The challenge comes after those kids reach puberty and want to start playing the real thing.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A few weeks ago I was at a Friday-night football game, but not to watch the game. I’ve been there and done that too many times when I used to be the team physician. I was there to listen to my granddaughter drumming in the pep band. And there was a lot of drumming because her high school’s team is having a hot year and outscoring opponents by three and four touchdowns every week.
At half time, the field was swarmed by 45-50 early grade schoolers looking like bobblehead dolls in their oversize helmets and surprisingly professional-appearing miniature football outfits. Under the lights, on the local college’s turf field, they were in football heaven. The pep band got into it and there was more drumming as the few kids who had a clue what football was about were scampering over and around their teammates and opponents who were roughhousing with each other, rolling around on the turf having a grand time, blissfully unimpressed by such trivial concepts as the line of scrimmage or the difference between blocking and tackling or even offense and defense.
Despite all the alarming articles both lay and professional that you and I see, this was an evening on which no one seemed particularly concerned about sports-related concussions. This is class B football in Maine, not a state well known as an incubator of Division I college football players. While there were a few scrawny kids with some speed,
Watching 4- and 5-year-olds in their football uniforms seemed to me to be a rather harmless exercise and certainly a more positive investment in their time on a Friday night than sitting on the couch with an electronic device clutched in their little hands. A recent report in JAMA Pediatrics suggests that my lack of concern has some validity (“Consensus statement on sports-related concussions in youth sports using a modified delphi approach.” JAMA Pediatr. 2019 Nov 11. doi: 10.1001/jamapediatrics.2019.4006). Eleven experts in sports-related injuries were surveyed with multiple rounds of questionnaires. Their anonymous responses were aggregated and shared with the group after each round until a consensus could be arrived on for each of seven broad questions about sports-related concussions. It is a paper worth reading and like most good literature surveys determined that in many situations more study needs to be done.
Among the many findings that impressed me was the group’s failure to find an “association between repetitive head impact exposure in youth and long-term neurocognitive outcomes.” In addition, “there is little evidence that age at first exposure repetitive head impacts in sports is independently associated with neurodegenerative changes.” The experts also could find “no evidence that growth or development affect the risk of sports-related concussions.”
The problem with youth football is that it is the portal that can lead to college and professional football, in which large bodies are allowed to collide after accelerating at speeds we mortals only can achieve behind the wheel of our motor vehicles. Rules to minimize those collisions do exist, but lax enforcement has failed to prevent their cumulative damage.
Whether the culture of big-time football is going to change to a point at which a conscientious parent could encourage his or her child to play after adolescence remains to be seen. However, the evidence seems to suggest that allowing young children to bang themselves around imitating the big guys seems to be reasonably safe. At least as safe as what kids used to do to each other before we adults invented television and video games.
When my son was 3 or 4 years old, he played on a hockey team he thought was called the Toronto Make-Believes (Maple Leafs). Maybe we should be telling parents it’s safe for their children to play make-believe contact sports. The challenge comes after those kids reach puberty and want to start playing the real thing.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
FDA noncommittal on e-cigarette action
on when the agency would act and what actions it was planning on taking.
“I was actually shocked that, in a hearing that is focused in part on the youth vaping epidemic [that] your testimony, both written and oral here, made no mention of the administration’s Sept. 11 announcement that it intended to clear the market of all unauthorized non–tobacco-flavored vaping products,” said Patty Murray (D-Wash.), ranking member of the Senate Health, Education, Labor and Pensions Committee, during a Nov. 13 hearing to Mitchell Zeller, director of the FDA’s Center for Tobacco Products. “Why is that not included in your testimony?”
Director Zeller would only offer a vague response, testifying that the agency is “committed to doing everything that we can to prevent kids from using any tobacco product, including e-cigarettes, and that we are continuing to develop a policy approach that aligns with that concern.”
When Sen. Murray pressed further, Director Zeller deflected: “I think that any questions that the committee has about the announcement that the White House and anything related to what remains a deliberative process on policy is best referred to the White House itself.”
He would not even offer any perspective on when the FDA might take actual regulatory action when asked about it by Sen. Murray.
“I can’t give you a specific timeline, Senator, other than to say that the deliberative process continues,” Director Zeller responded, telling her that “I really would refer you and the committee to the White House to ask specific questions about where we are.”
The hearing, called to examine the response to lung illnesses and rising youth e-cigarette usage, shed no new light on the issue. And while Director Zeller outlined the numerous educational campaigns being aimed at convincing youth to not use e-cigarettes, Committee Chairman Lamar Alexander (R-Tenn.) questioned whether the FDA was doing an adequate job.
The FDA, from late 2017 to the end of 2020, “will wind up investing about $150 million in a massive, multimedia public education campaign to get the word out to kids” on the dangers of vaping, Director Zeller said, adding that the agency is “aggressively enforcing” youth access restrictions in targeting sellers of e-cigarette products to minors.
“Well, obviously we are not making much progress with youth use ... if one in four of American high schoolers, according to your statistics, are using e-cigarettes,” Sen. Alexander said.
While most on the committee were focused on the rising numbers of youth vaping and e-cigarette usage, Sen. Rand Paul (R-Ky.) cautioned that any regulatory action, particularly a ban on all flavored e-cigarette products, would adversely affect adults, particularly those who are turning to e-cigarettes as a smoking cessation tool.
His solution, noting that it is already illegal for kids to be purchasing vaping and e-cigarette products, was to increase the penalties for those found selling to minors, adding that “most adults are using the flavors as well” and it could lead them back to combustible tobacco products if they are prevented from accessing flavored e-cigarettes.
on when the agency would act and what actions it was planning on taking.
“I was actually shocked that, in a hearing that is focused in part on the youth vaping epidemic [that] your testimony, both written and oral here, made no mention of the administration’s Sept. 11 announcement that it intended to clear the market of all unauthorized non–tobacco-flavored vaping products,” said Patty Murray (D-Wash.), ranking member of the Senate Health, Education, Labor and Pensions Committee, during a Nov. 13 hearing to Mitchell Zeller, director of the FDA’s Center for Tobacco Products. “Why is that not included in your testimony?”
Director Zeller would only offer a vague response, testifying that the agency is “committed to doing everything that we can to prevent kids from using any tobacco product, including e-cigarettes, and that we are continuing to develop a policy approach that aligns with that concern.”
When Sen. Murray pressed further, Director Zeller deflected: “I think that any questions that the committee has about the announcement that the White House and anything related to what remains a deliberative process on policy is best referred to the White House itself.”
He would not even offer any perspective on when the FDA might take actual regulatory action when asked about it by Sen. Murray.
“I can’t give you a specific timeline, Senator, other than to say that the deliberative process continues,” Director Zeller responded, telling her that “I really would refer you and the committee to the White House to ask specific questions about where we are.”
The hearing, called to examine the response to lung illnesses and rising youth e-cigarette usage, shed no new light on the issue. And while Director Zeller outlined the numerous educational campaigns being aimed at convincing youth to not use e-cigarettes, Committee Chairman Lamar Alexander (R-Tenn.) questioned whether the FDA was doing an adequate job.
The FDA, from late 2017 to the end of 2020, “will wind up investing about $150 million in a massive, multimedia public education campaign to get the word out to kids” on the dangers of vaping, Director Zeller said, adding that the agency is “aggressively enforcing” youth access restrictions in targeting sellers of e-cigarette products to minors.
“Well, obviously we are not making much progress with youth use ... if one in four of American high schoolers, according to your statistics, are using e-cigarettes,” Sen. Alexander said.
While most on the committee were focused on the rising numbers of youth vaping and e-cigarette usage, Sen. Rand Paul (R-Ky.) cautioned that any regulatory action, particularly a ban on all flavored e-cigarette products, would adversely affect adults, particularly those who are turning to e-cigarettes as a smoking cessation tool.
His solution, noting that it is already illegal for kids to be purchasing vaping and e-cigarette products, was to increase the penalties for those found selling to minors, adding that “most adults are using the flavors as well” and it could lead them back to combustible tobacco products if they are prevented from accessing flavored e-cigarettes.
on when the agency would act and what actions it was planning on taking.
“I was actually shocked that, in a hearing that is focused in part on the youth vaping epidemic [that] your testimony, both written and oral here, made no mention of the administration’s Sept. 11 announcement that it intended to clear the market of all unauthorized non–tobacco-flavored vaping products,” said Patty Murray (D-Wash.), ranking member of the Senate Health, Education, Labor and Pensions Committee, during a Nov. 13 hearing to Mitchell Zeller, director of the FDA’s Center for Tobacco Products. “Why is that not included in your testimony?”
Director Zeller would only offer a vague response, testifying that the agency is “committed to doing everything that we can to prevent kids from using any tobacco product, including e-cigarettes, and that we are continuing to develop a policy approach that aligns with that concern.”
When Sen. Murray pressed further, Director Zeller deflected: “I think that any questions that the committee has about the announcement that the White House and anything related to what remains a deliberative process on policy is best referred to the White House itself.”
He would not even offer any perspective on when the FDA might take actual regulatory action when asked about it by Sen. Murray.
“I can’t give you a specific timeline, Senator, other than to say that the deliberative process continues,” Director Zeller responded, telling her that “I really would refer you and the committee to the White House to ask specific questions about where we are.”
The hearing, called to examine the response to lung illnesses and rising youth e-cigarette usage, shed no new light on the issue. And while Director Zeller outlined the numerous educational campaigns being aimed at convincing youth to not use e-cigarettes, Committee Chairman Lamar Alexander (R-Tenn.) questioned whether the FDA was doing an adequate job.
The FDA, from late 2017 to the end of 2020, “will wind up investing about $150 million in a massive, multimedia public education campaign to get the word out to kids” on the dangers of vaping, Director Zeller said, adding that the agency is “aggressively enforcing” youth access restrictions in targeting sellers of e-cigarette products to minors.
“Well, obviously we are not making much progress with youth use ... if one in four of American high schoolers, according to your statistics, are using e-cigarettes,” Sen. Alexander said.
While most on the committee were focused on the rising numbers of youth vaping and e-cigarette usage, Sen. Rand Paul (R-Ky.) cautioned that any regulatory action, particularly a ban on all flavored e-cigarette products, would adversely affect adults, particularly those who are turning to e-cigarettes as a smoking cessation tool.
His solution, noting that it is already illegal for kids to be purchasing vaping and e-cigarette products, was to increase the penalties for those found selling to minors, adding that “most adults are using the flavors as well” and it could lead them back to combustible tobacco products if they are prevented from accessing flavored e-cigarettes.
REPORTING FROM A SENATE HELP COMMITTEE HEARING
Halal nail polish
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Piceatannol: The other potent antioxidant in grapes and wine
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
FDA panel supports Vascepa expanded indication for CVD reduction
Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid, received unanimous backing from a Food and Drug Administration advisory panel for a new indication for reducing cardiovascular event risk.
Icosapent ethyl (Vascepa) received initial agency approval in 2012 for the indication of cutting triglyceride levels once they reached at least 500 mg/dL.
The target patient population for this new, cardiovascular-event protection role will reflect some or all of the types of patients enrolled in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), which tested icosapent ethyl in 8,179 patients with either established cardiovascular disease or diabetes and at least one additional cardiovascular disease risk factor. This study provided the bulk of the data considered by the FDA panel.
REDUCE-IT showed that, during a median of 4.9 years, patients who received icosapent ethyl had a statistically significant 25% relative risk reduction in the trial’s primary, composite endpoint (New Engl J Med. 2019 Jan 3;380[1]:11-22).
Icosapent ethyl “appeared effective and safe,” and would be a “useful, new, added agent for treating patients” like those enrolled in the trial, said Kenneth D. Burman, MD, professor and chief of endocrinology at Medstar Washington (D.C.) Hospital Center and chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
The advisory panel members appeared uniformly comfortable with recommending that the FDA add a cardiovascular disease indication based on the REDUCE-IT findings.
But while they agreed that icosapent ethyl should receive some type of indication for cardiovascular event reduction, the committee split over which patients the indication should include. Specifically, they diverged on the issue of primary prevention.
Some said that the patient enrollment that produced a positive result in REDUCE-IT should not be retrospectively subdivided, while others said that combining secondary- and primary-prevention patients in a single large trial inappropriately lumped together patients who would be better considered separately.
Committee members also expressed uncertainty over the appropriate triglyceride level to warrant treatment. The REDUCE-IT trial was designed to enroll patients with triglycerides of 135 mg/dL or greater, but several panel members suggested that, for labeling, the threshold should be at least 150 mg/dL, or even 200 mg/dL.
Safety was another aspect that generated a lot of panel discussion throughout their day-long discussion, with particular focus on a signal of a small but concerning increased rate of incident atrial fibrillation among patients who received icosapent ethyl, as well as a small but nearly significant increase in the rate of serious bleeds.
Further analyses presented during the meeting showed that an increased bleeding rate linked with icosapent ethyl was focused in patients who concurrently received one or more antiplatelet drugs or an anticoagulant.
However, panel members rejected the notion that these safety concerns warranted a boxed warning, agreeing that it could be managed with appropriate labeling information.
Clinician reaction
Clinicians who manage these types of patients viewed the prospect of an expanded indication for icosapent ethyl as an important advance.
The REDUCE-IT results by themselves “were convincing” for patients with established cardiovascular disease without need for a confirmatory trial, Robert H. Eckel, MD, an endocrinologist and professor of medicine at the University of Colorado at Denver, Aurora, said in an interview. But he remained unconvinced about efficacy for primary-prevention patients, or even for secondary-prevention patients with a triglyceride level below 150 mg/dL.
“Icosapent ethyl will clearly be a mainstay for managing high-risk patients. It gives us another treatment option,” Yehuda Handelsman, MD, an endocrinologist and medical director and principal investigator of the Metabolic Institute of America in Tarzana, Calif., said in an interview. “I do not see the atrial fibrillation or bleeding effects as reasons not to approve this drug. It should be a precaution. Overall, icosapent ethyl is one of the easier drugs for patients to take.”
Dr. Handelsman said it would be unethical to run a confirmatory trial and randomize patients to placebo. “Another trial makes no sense,” he said.
But the data from REDUCE-IT were “not as convincing” for primary-prevention patients, suggesting a need for caution about using icosapent ethyl for patients without established cardiovascular disease, Paul S. Jellinger, MD, an endocrinologist in Fort Lauderdale, Fla., said in an interview.
Cost-effectiveness
An analysis of the cost-effectiveness of icosapent ethyl as used in REDUCE-IT showed that the drug fell into the rare category of being a “dominant” treatment, meaning that it both improved patient outcomes and reduced medical costs. William S. Weintraub, MD, will report findings from this analysis on Nov. 16, 2019, at the annual scientific sessions of the American Heart Association.
The analysis used a wholesale acquisition cost for a 1-day dosage of icosapent ethyl of $4.16, derived from a commercial source for prescription-drug pricing and actual hospitalization costs for the patients in the trial.
Based on the REDUCE-IT outcomes, treatment with icosapent ethyl was linked with a boost in quality-adjusted life-years that extrapolated to an average 0.26 increase during the full lifetime of REDUCE-IT participants, at a cost that averaged $1,284 less per treated patient over their lifetime, according to Dr. Weintraub, director of Outcomes Research at Medstar Washington Hospital Center, Washington.
Although the 0.26 lifetime increase in quality-adjusted life-years may sound modest, “in the cost-effectiveness world, 0.26 is actually significant,” Dr. Weintraub said. He also highlighted how unusual it is to find a patented drug that improves quality of life and longevity while also saving money.
“I know of no other on-patent, branded pharmaceutical that is dominant,” he said.
Off-patent pharmaceuticals, like statins, can be quite inexpensive and may also be dominant, he noted. Being dominant for cost-effectiveness means that icosapent ethyl “provides good value and is worth what we pay for it, well within social thresholds of willingness to pay,” Dr. Weintraub said.
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Burman has received research funding from AstraZeneca, Eisai, and IBSA. Dr. Eckel has received personal fees from Kowa Pharmaceuticals, Merck, Novartis, and Sanofi/Regeneron, as well as research funding from Endece, Ionis Pharmaceuticals, and UniQure. Dr. Handelsman has been a consultant to and received research funding from Amarin and several other companies. Dr. Jellinger has been a speaker on behalf of Amarin, Amgen, and Regeneron. Dr. Weintraub has received honoraria and research support from Amarin, and honoraria from AstraZeneca.
Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid, received unanimous backing from a Food and Drug Administration advisory panel for a new indication for reducing cardiovascular event risk.
Icosapent ethyl (Vascepa) received initial agency approval in 2012 for the indication of cutting triglyceride levels once they reached at least 500 mg/dL.
The target patient population for this new, cardiovascular-event protection role will reflect some or all of the types of patients enrolled in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), which tested icosapent ethyl in 8,179 patients with either established cardiovascular disease or diabetes and at least one additional cardiovascular disease risk factor. This study provided the bulk of the data considered by the FDA panel.
REDUCE-IT showed that, during a median of 4.9 years, patients who received icosapent ethyl had a statistically significant 25% relative risk reduction in the trial’s primary, composite endpoint (New Engl J Med. 2019 Jan 3;380[1]:11-22).
Icosapent ethyl “appeared effective and safe,” and would be a “useful, new, added agent for treating patients” like those enrolled in the trial, said Kenneth D. Burman, MD, professor and chief of endocrinology at Medstar Washington (D.C.) Hospital Center and chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
The advisory panel members appeared uniformly comfortable with recommending that the FDA add a cardiovascular disease indication based on the REDUCE-IT findings.
But while they agreed that icosapent ethyl should receive some type of indication for cardiovascular event reduction, the committee split over which patients the indication should include. Specifically, they diverged on the issue of primary prevention.
Some said that the patient enrollment that produced a positive result in REDUCE-IT should not be retrospectively subdivided, while others said that combining secondary- and primary-prevention patients in a single large trial inappropriately lumped together patients who would be better considered separately.
Committee members also expressed uncertainty over the appropriate triglyceride level to warrant treatment. The REDUCE-IT trial was designed to enroll patients with triglycerides of 135 mg/dL or greater, but several panel members suggested that, for labeling, the threshold should be at least 150 mg/dL, or even 200 mg/dL.
Safety was another aspect that generated a lot of panel discussion throughout their day-long discussion, with particular focus on a signal of a small but concerning increased rate of incident atrial fibrillation among patients who received icosapent ethyl, as well as a small but nearly significant increase in the rate of serious bleeds.
Further analyses presented during the meeting showed that an increased bleeding rate linked with icosapent ethyl was focused in patients who concurrently received one or more antiplatelet drugs or an anticoagulant.
However, panel members rejected the notion that these safety concerns warranted a boxed warning, agreeing that it could be managed with appropriate labeling information.
Clinician reaction
Clinicians who manage these types of patients viewed the prospect of an expanded indication for icosapent ethyl as an important advance.
The REDUCE-IT results by themselves “were convincing” for patients with established cardiovascular disease without need for a confirmatory trial, Robert H. Eckel, MD, an endocrinologist and professor of medicine at the University of Colorado at Denver, Aurora, said in an interview. But he remained unconvinced about efficacy for primary-prevention patients, or even for secondary-prevention patients with a triglyceride level below 150 mg/dL.
“Icosapent ethyl will clearly be a mainstay for managing high-risk patients. It gives us another treatment option,” Yehuda Handelsman, MD, an endocrinologist and medical director and principal investigator of the Metabolic Institute of America in Tarzana, Calif., said in an interview. “I do not see the atrial fibrillation or bleeding effects as reasons not to approve this drug. It should be a precaution. Overall, icosapent ethyl is one of the easier drugs for patients to take.”
Dr. Handelsman said it would be unethical to run a confirmatory trial and randomize patients to placebo. “Another trial makes no sense,” he said.
But the data from REDUCE-IT were “not as convincing” for primary-prevention patients, suggesting a need for caution about using icosapent ethyl for patients without established cardiovascular disease, Paul S. Jellinger, MD, an endocrinologist in Fort Lauderdale, Fla., said in an interview.
Cost-effectiveness
An analysis of the cost-effectiveness of icosapent ethyl as used in REDUCE-IT showed that the drug fell into the rare category of being a “dominant” treatment, meaning that it both improved patient outcomes and reduced medical costs. William S. Weintraub, MD, will report findings from this analysis on Nov. 16, 2019, at the annual scientific sessions of the American Heart Association.
The analysis used a wholesale acquisition cost for a 1-day dosage of icosapent ethyl of $4.16, derived from a commercial source for prescription-drug pricing and actual hospitalization costs for the patients in the trial.
Based on the REDUCE-IT outcomes, treatment with icosapent ethyl was linked with a boost in quality-adjusted life-years that extrapolated to an average 0.26 increase during the full lifetime of REDUCE-IT participants, at a cost that averaged $1,284 less per treated patient over their lifetime, according to Dr. Weintraub, director of Outcomes Research at Medstar Washington Hospital Center, Washington.
Although the 0.26 lifetime increase in quality-adjusted life-years may sound modest, “in the cost-effectiveness world, 0.26 is actually significant,” Dr. Weintraub said. He also highlighted how unusual it is to find a patented drug that improves quality of life and longevity while also saving money.
“I know of no other on-patent, branded pharmaceutical that is dominant,” he said.
Off-patent pharmaceuticals, like statins, can be quite inexpensive and may also be dominant, he noted. Being dominant for cost-effectiveness means that icosapent ethyl “provides good value and is worth what we pay for it, well within social thresholds of willingness to pay,” Dr. Weintraub said.
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Burman has received research funding from AstraZeneca, Eisai, and IBSA. Dr. Eckel has received personal fees from Kowa Pharmaceuticals, Merck, Novartis, and Sanofi/Regeneron, as well as research funding from Endece, Ionis Pharmaceuticals, and UniQure. Dr. Handelsman has been a consultant to and received research funding from Amarin and several other companies. Dr. Jellinger has been a speaker on behalf of Amarin, Amgen, and Regeneron. Dr. Weintraub has received honoraria and research support from Amarin, and honoraria from AstraZeneca.
Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid, received unanimous backing from a Food and Drug Administration advisory panel for a new indication for reducing cardiovascular event risk.
Icosapent ethyl (Vascepa) received initial agency approval in 2012 for the indication of cutting triglyceride levels once they reached at least 500 mg/dL.
The target patient population for this new, cardiovascular-event protection role will reflect some or all of the types of patients enrolled in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), which tested icosapent ethyl in 8,179 patients with either established cardiovascular disease or diabetes and at least one additional cardiovascular disease risk factor. This study provided the bulk of the data considered by the FDA panel.
REDUCE-IT showed that, during a median of 4.9 years, patients who received icosapent ethyl had a statistically significant 25% relative risk reduction in the trial’s primary, composite endpoint (New Engl J Med. 2019 Jan 3;380[1]:11-22).
Icosapent ethyl “appeared effective and safe,” and would be a “useful, new, added agent for treating patients” like those enrolled in the trial, said Kenneth D. Burman, MD, professor and chief of endocrinology at Medstar Washington (D.C.) Hospital Center and chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
The advisory panel members appeared uniformly comfortable with recommending that the FDA add a cardiovascular disease indication based on the REDUCE-IT findings.
But while they agreed that icosapent ethyl should receive some type of indication for cardiovascular event reduction, the committee split over which patients the indication should include. Specifically, they diverged on the issue of primary prevention.
Some said that the patient enrollment that produced a positive result in REDUCE-IT should not be retrospectively subdivided, while others said that combining secondary- and primary-prevention patients in a single large trial inappropriately lumped together patients who would be better considered separately.
Committee members also expressed uncertainty over the appropriate triglyceride level to warrant treatment. The REDUCE-IT trial was designed to enroll patients with triglycerides of 135 mg/dL or greater, but several panel members suggested that, for labeling, the threshold should be at least 150 mg/dL, or even 200 mg/dL.
Safety was another aspect that generated a lot of panel discussion throughout their day-long discussion, with particular focus on a signal of a small but concerning increased rate of incident atrial fibrillation among patients who received icosapent ethyl, as well as a small but nearly significant increase in the rate of serious bleeds.
Further analyses presented during the meeting showed that an increased bleeding rate linked with icosapent ethyl was focused in patients who concurrently received one or more antiplatelet drugs or an anticoagulant.
However, panel members rejected the notion that these safety concerns warranted a boxed warning, agreeing that it could be managed with appropriate labeling information.
Clinician reaction
Clinicians who manage these types of patients viewed the prospect of an expanded indication for icosapent ethyl as an important advance.
The REDUCE-IT results by themselves “were convincing” for patients with established cardiovascular disease without need for a confirmatory trial, Robert H. Eckel, MD, an endocrinologist and professor of medicine at the University of Colorado at Denver, Aurora, said in an interview. But he remained unconvinced about efficacy for primary-prevention patients, or even for secondary-prevention patients with a triglyceride level below 150 mg/dL.
“Icosapent ethyl will clearly be a mainstay for managing high-risk patients. It gives us another treatment option,” Yehuda Handelsman, MD, an endocrinologist and medical director and principal investigator of the Metabolic Institute of America in Tarzana, Calif., said in an interview. “I do not see the atrial fibrillation or bleeding effects as reasons not to approve this drug. It should be a precaution. Overall, icosapent ethyl is one of the easier drugs for patients to take.”
Dr. Handelsman said it would be unethical to run a confirmatory trial and randomize patients to placebo. “Another trial makes no sense,” he said.
But the data from REDUCE-IT were “not as convincing” for primary-prevention patients, suggesting a need for caution about using icosapent ethyl for patients without established cardiovascular disease, Paul S. Jellinger, MD, an endocrinologist in Fort Lauderdale, Fla., said in an interview.
Cost-effectiveness
An analysis of the cost-effectiveness of icosapent ethyl as used in REDUCE-IT showed that the drug fell into the rare category of being a “dominant” treatment, meaning that it both improved patient outcomes and reduced medical costs. William S. Weintraub, MD, will report findings from this analysis on Nov. 16, 2019, at the annual scientific sessions of the American Heart Association.
The analysis used a wholesale acquisition cost for a 1-day dosage of icosapent ethyl of $4.16, derived from a commercial source for prescription-drug pricing and actual hospitalization costs for the patients in the trial.
Based on the REDUCE-IT outcomes, treatment with icosapent ethyl was linked with a boost in quality-adjusted life-years that extrapolated to an average 0.26 increase during the full lifetime of REDUCE-IT participants, at a cost that averaged $1,284 less per treated patient over their lifetime, according to Dr. Weintraub, director of Outcomes Research at Medstar Washington Hospital Center, Washington.
Although the 0.26 lifetime increase in quality-adjusted life-years may sound modest, “in the cost-effectiveness world, 0.26 is actually significant,” Dr. Weintraub said. He also highlighted how unusual it is to find a patented drug that improves quality of life and longevity while also saving money.
“I know of no other on-patent, branded pharmaceutical that is dominant,” he said.
Off-patent pharmaceuticals, like statins, can be quite inexpensive and may also be dominant, he noted. Being dominant for cost-effectiveness means that icosapent ethyl “provides good value and is worth what we pay for it, well within social thresholds of willingness to pay,” Dr. Weintraub said.
REDUCE-IT was sponsored by Amarin, the company that markets icosapent ethyl (Vascepa). Dr. Burman has received research funding from AstraZeneca, Eisai, and IBSA. Dr. Eckel has received personal fees from Kowa Pharmaceuticals, Merck, Novartis, and Sanofi/Regeneron, as well as research funding from Endece, Ionis Pharmaceuticals, and UniQure. Dr. Handelsman has been a consultant to and received research funding from Amarin and several other companies. Dr. Jellinger has been a speaker on behalf of Amarin, Amgen, and Regeneron. Dr. Weintraub has received honoraria and research support from Amarin, and honoraria from AstraZeneca.




















