User login
Disparity in endometrial cancer outcomes: What can we do?
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
Could the biosimilar market stall before it ever really started?
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett continued: “It’s not even acknowledged as a basic truth in the United States, which again suggests the business model may not be there for biosimilars.”
Ms. Woollett disclosed no conflicts of interest relevant to her presentation.
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett continued: “It’s not even acknowledged as a basic truth in the United States, which again suggests the business model may not be there for biosimilars.”
Ms. Woollett disclosed no conflicts of interest relevant to her presentation.
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett continued: “It’s not even acknowledged as a basic truth in the United States, which again suggests the business model may not be there for biosimilars.”
Ms. Woollett disclosed no conflicts of interest relevant to her presentation.
REPORTING FROM AMCP NEXUS 2019
Premiums down slightly for 2020 plans on HealthCare.gov
The average family of four will see a 4% drop in its premium for a benchmark plan on the HealthCare.gov Marketplace in 2020, compared with 2019, according to the Centers for Medicare & Medicaid Services.
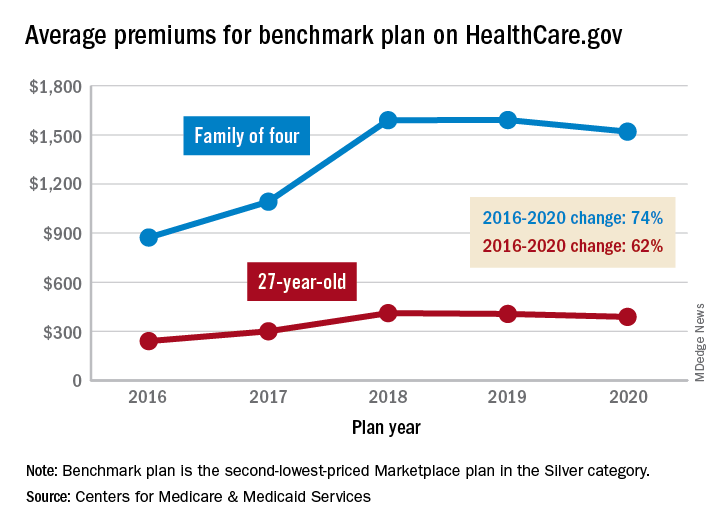
That family’s $1,520 premium for coverage next year will, however, be 74% higher than the average of $873 for the same plan in 2016. The situation is similar for a single person with a benchmark plan – defined as the second-lowest-priced Marketplace health insurance plan in the Silver category. The average premium for a 27-year-old, $388, for coverage in 2020 also will be 4% lower than in 2019, but that’s still 62% higher than in 2016, CMS said in a recent report.
The 4% average reductions for 2020 follow a 1% decrease for a 27-year-old on the benchmark plan and a $1 increase for a family of four between 2018 and 2019. “We have been committed to taking every step possible to lower premiums and provide more choices. A second year of declining premiums and expanding choice is proof that our actions to promote more stability are working. But premiums are still too high for people without subsidies,” CMS Administrator Seema Verma said in a statement.
Consumers looking for health care coverage on 1 of the 38 state exchanges during the upcoming open enrollment period (Nov. 1 to Dec. 15) also will have more choices. The average number of qualified health plans available to enrollees will be 37.9 for the 2020 plan year, compared with 25.9 for 2019 and 24.8 in 2018. The number of companies issuing those plans also rose for the second consecutive year, going from 132 for the 2018 plan year to 155 in 2019 and 175 in 2020, CMS reported.
The average family of four will see a 4% drop in its premium for a benchmark plan on the HealthCare.gov Marketplace in 2020, compared with 2019, according to the Centers for Medicare & Medicaid Services.

That family’s $1,520 premium for coverage next year will, however, be 74% higher than the average of $873 for the same plan in 2016. The situation is similar for a single person with a benchmark plan – defined as the second-lowest-priced Marketplace health insurance plan in the Silver category. The average premium for a 27-year-old, $388, for coverage in 2020 also will be 4% lower than in 2019, but that’s still 62% higher than in 2016, CMS said in a recent report.
The 4% average reductions for 2020 follow a 1% decrease for a 27-year-old on the benchmark plan and a $1 increase for a family of four between 2018 and 2019. “We have been committed to taking every step possible to lower premiums and provide more choices. A second year of declining premiums and expanding choice is proof that our actions to promote more stability are working. But premiums are still too high for people without subsidies,” CMS Administrator Seema Verma said in a statement.
Consumers looking for health care coverage on 1 of the 38 state exchanges during the upcoming open enrollment period (Nov. 1 to Dec. 15) also will have more choices. The average number of qualified health plans available to enrollees will be 37.9 for the 2020 plan year, compared with 25.9 for 2019 and 24.8 in 2018. The number of companies issuing those plans also rose for the second consecutive year, going from 132 for the 2018 plan year to 155 in 2019 and 175 in 2020, CMS reported.
The average family of four will see a 4% drop in its premium for a benchmark plan on the HealthCare.gov Marketplace in 2020, compared with 2019, according to the Centers for Medicare & Medicaid Services.

That family’s $1,520 premium for coverage next year will, however, be 74% higher than the average of $873 for the same plan in 2016. The situation is similar for a single person with a benchmark plan – defined as the second-lowest-priced Marketplace health insurance plan in the Silver category. The average premium for a 27-year-old, $388, for coverage in 2020 also will be 4% lower than in 2019, but that’s still 62% higher than in 2016, CMS said in a recent report.
The 4% average reductions for 2020 follow a 1% decrease for a 27-year-old on the benchmark plan and a $1 increase for a family of four between 2018 and 2019. “We have been committed to taking every step possible to lower premiums and provide more choices. A second year of declining premiums and expanding choice is proof that our actions to promote more stability are working. But premiums are still too high for people without subsidies,” CMS Administrator Seema Verma said in a statement.
Consumers looking for health care coverage on 1 of the 38 state exchanges during the upcoming open enrollment period (Nov. 1 to Dec. 15) also will have more choices. The average number of qualified health plans available to enrollees will be 37.9 for the 2020 plan year, compared with 25.9 for 2019 and 24.8 in 2018. The number of companies issuing those plans also rose for the second consecutive year, going from 132 for the 2018 plan year to 155 in 2019 and 175 in 2020, CMS reported.
Telemedicine migraine consults effective for management
Key clinical point: Follow-up consultations with migraine patients via telemedicine are effective and can increase physician productivity and patient convenience.
Major finding: Migraine disability assessment improved by an average of 24 points in the telemedicine patients and by 19 points in the control group.
Study details: A single-center, randomized study with 40 migraine patients.
Disclosures: The study received partial funding from Merck.
Citation: Friedman DI. Headache. 2019 June;59(S1):1-208, LBOR01
Key clinical point: Follow-up consultations with migraine patients via telemedicine are effective and can increase physician productivity and patient convenience.
Major finding: Migraine disability assessment improved by an average of 24 points in the telemedicine patients and by 19 points in the control group.
Study details: A single-center, randomized study with 40 migraine patients.
Disclosures: The study received partial funding from Merck.
Citation: Friedman DI. Headache. 2019 June;59(S1):1-208, LBOR01
Key clinical point: Follow-up consultations with migraine patients via telemedicine are effective and can increase physician productivity and patient convenience.
Major finding: Migraine disability assessment improved by an average of 24 points in the telemedicine patients and by 19 points in the control group.
Study details: A single-center, randomized study with 40 migraine patients.
Disclosures: The study received partial funding from Merck.
Citation: Friedman DI. Headache. 2019 June;59(S1):1-208, LBOR01
Vestibular migraine: New study may help in diagnosis
Key clinical point: Loudness discomfort levels and temporal auditory processing may help diagnosis patients with vestibular migraine (VM).
Major finding: VM patients experienced response latencies with longer frequencies and a lower noise tolerance, compared with patients without migraine. The statistically significant findings showed that “the frequency following response latencies were significantly longer in the patients with vestibular migraine than in the control group, suggesting altered pure tone temporal processing, which may also affect the processing of complex sounds,” noted the investigators. “The lower discomfort thresholds suggest the presence of mild hyperacusis, in concordance with other previous studies.”
Study details: Fifty-four women were split up into two groups: 29 women with VM and 25 healthy women without migraine.
Disclosures: The authors reported having no conflicts of interest.
Citation: Takeuti AA, et al. BMC Neurol. 2019 Jun 27;19(1):144. doi: 10.1186/s12883-019-1368-5.
Key clinical point: Loudness discomfort levels and temporal auditory processing may help diagnosis patients with vestibular migraine (VM).
Major finding: VM patients experienced response latencies with longer frequencies and a lower noise tolerance, compared with patients without migraine. The statistically significant findings showed that “the frequency following response latencies were significantly longer in the patients with vestibular migraine than in the control group, suggesting altered pure tone temporal processing, which may also affect the processing of complex sounds,” noted the investigators. “The lower discomfort thresholds suggest the presence of mild hyperacusis, in concordance with other previous studies.”
Study details: Fifty-four women were split up into two groups: 29 women with VM and 25 healthy women without migraine.
Disclosures: The authors reported having no conflicts of interest.
Citation: Takeuti AA, et al. BMC Neurol. 2019 Jun 27;19(1):144. doi: 10.1186/s12883-019-1368-5.
Key clinical point: Loudness discomfort levels and temporal auditory processing may help diagnosis patients with vestibular migraine (VM).
Major finding: VM patients experienced response latencies with longer frequencies and a lower noise tolerance, compared with patients without migraine. The statistically significant findings showed that “the frequency following response latencies were significantly longer in the patients with vestibular migraine than in the control group, suggesting altered pure tone temporal processing, which may also affect the processing of complex sounds,” noted the investigators. “The lower discomfort thresholds suggest the presence of mild hyperacusis, in concordance with other previous studies.”
Study details: Fifty-four women were split up into two groups: 29 women with VM and 25 healthy women without migraine.
Disclosures: The authors reported having no conflicts of interest.
Citation: Takeuti AA, et al. BMC Neurol. 2019 Jun 27;19(1):144. doi: 10.1186/s12883-019-1368-5.
Economic burden of cycling through migraine preventive medications
Key clinical point: Patients that cycle through migraine preventive medications have a higher economic burden.
Major finding: Migraine patients cycling through more than two preventive migraine medications (PMMs) had an increased financial burden, compared with patients that received their initial medication class. “Mean all-cause total direct costs, including prescription costs, were significantly higher in PMM2 ($13,429) and PMM3 ($18,394) subgroups versus the persistent subgroup ($11,941),” noted the investigators
Study details: This was a retrospective observational study of 55,402 adult patients with migraine beginning their first PMM: antidepressants, antiepileptics, beta blockers, or neurotoxins. Patients had to have continuous medical and prescription enrollment for 12 months to be included in the study.
Disclosures: Eli Lilly and Company funded and designed the study. All study investigators reported being employees and stockholders of Eli Lily and Company.
Citation: Ford JH, et al. J Manag Care Spec Pharm. 2019 Jan;25(1):46-59. doi: 10.18553/jmcp.2018.18058.
Key clinical point: Patients that cycle through migraine preventive medications have a higher economic burden.
Major finding: Migraine patients cycling through more than two preventive migraine medications (PMMs) had an increased financial burden, compared with patients that received their initial medication class. “Mean all-cause total direct costs, including prescription costs, were significantly higher in PMM2 ($13,429) and PMM3 ($18,394) subgroups versus the persistent subgroup ($11,941),” noted the investigators
Study details: This was a retrospective observational study of 55,402 adult patients with migraine beginning their first PMM: antidepressants, antiepileptics, beta blockers, or neurotoxins. Patients had to have continuous medical and prescription enrollment for 12 months to be included in the study.
Disclosures: Eli Lilly and Company funded and designed the study. All study investigators reported being employees and stockholders of Eli Lily and Company.
Citation: Ford JH, et al. J Manag Care Spec Pharm. 2019 Jan;25(1):46-59. doi: 10.18553/jmcp.2018.18058.
Key clinical point: Patients that cycle through migraine preventive medications have a higher economic burden.
Major finding: Migraine patients cycling through more than two preventive migraine medications (PMMs) had an increased financial burden, compared with patients that received their initial medication class. “Mean all-cause total direct costs, including prescription costs, were significantly higher in PMM2 ($13,429) and PMM3 ($18,394) subgroups versus the persistent subgroup ($11,941),” noted the investigators
Study details: This was a retrospective observational study of 55,402 adult patients with migraine beginning their first PMM: antidepressants, antiepileptics, beta blockers, or neurotoxins. Patients had to have continuous medical and prescription enrollment for 12 months to be included in the study.
Disclosures: Eli Lilly and Company funded and designed the study. All study investigators reported being employees and stockholders of Eli Lily and Company.
Citation: Ford JH, et al. J Manag Care Spec Pharm. 2019 Jan;25(1):46-59. doi: 10.18553/jmcp.2018.18058.
COGS update shows viability of endophenotypes in schizophrenia research
Lead author Tiffany A. Greenwood, PhD, noted that clinically diverse schizophrenia patients often are grouped together to get large sample sizes for genome-wide association studies, which can miss specific features of the heterogeneous disorder. Instead, in phase 2 of the Consortium on the Genetics of Schizophrenia (COGS) study, Dr. Greenwood and associates sought to connect features clustered into endophenotypes with certain genetic regions of interest.
“As stable biomarkers of the underlying brain dysfunctions, endophenotypes hold promise for parsing clinical heterogeneity of schizophrenia and refining the genetic signal,” they wrote. The study was published in JAMA Psychiatry.
Using this approach among 1,533 participants, they found seven regions exceeding the conventional genome-wide association significance of P less than 5 x 10–8, including regions associated with the endophenotypes of face memory (chromosome 3p21; effect size, –0.72; P = 4.2 x 10–8), antisaccade task (chromosome 9q31; effect size, –0.24; P = 3.5 x 10–8), and abstraction and mental flexibility (chromosome 10q23; effect size, –0.56; P = 1.5 x 10–8).
Those endophenotypes and genes intersect theoretical molecular and biological processes that have been identified in other research and could explain underlying mechanisms of schizophrenia. For example, research has suggested that NRG3, which is near the region associated with abstraction and mental flexibility, and affects certain cellular signaling pathways, could be a locus of susceptibility; in particular, some variants of NRG3 have been associated with cognitive and psychotic symptom severity in previous research.
“Although shared genetic substrates appear likely, this is not a study of schizophrenia but rather a study of neurophysiological and neurocognitive deficits that occur in the general population but are more pronounced in the context of schizophrenia and have implications for treatment,” wrote Dr. Greenwood, of the University of California, San Diego, and associates.
One limitation of the study is that, as investigators have demonstrated elsewhere, P values can prove highly variable over the course of replication studies, so the significance thresholds shown in this study still could run the risk of hiding false positives or negatives.
“As many of the 11 endophenotypes have been endorsed as targets for the development of novel treatments for schizophrenia, a better understanding of the corresponding cellular and molecular processes may pave the way for precision-based medicine in schizophrenia and perhaps other psychiatric illnesses with a shared genetic liability,” Dr. Greenwood and associates concluded.
SOURCE: Greenwood TA et al. JAMA Psychiatry. 2019 Oct 9. doi: 10.1001/jamapsychiatry.2019.2850.
Lead author Tiffany A. Greenwood, PhD, noted that clinically diverse schizophrenia patients often are grouped together to get large sample sizes for genome-wide association studies, which can miss specific features of the heterogeneous disorder. Instead, in phase 2 of the Consortium on the Genetics of Schizophrenia (COGS) study, Dr. Greenwood and associates sought to connect features clustered into endophenotypes with certain genetic regions of interest.
“As stable biomarkers of the underlying brain dysfunctions, endophenotypes hold promise for parsing clinical heterogeneity of schizophrenia and refining the genetic signal,” they wrote. The study was published in JAMA Psychiatry.
Using this approach among 1,533 participants, they found seven regions exceeding the conventional genome-wide association significance of P less than 5 x 10–8, including regions associated with the endophenotypes of face memory (chromosome 3p21; effect size, –0.72; P = 4.2 x 10–8), antisaccade task (chromosome 9q31; effect size, –0.24; P = 3.5 x 10–8), and abstraction and mental flexibility (chromosome 10q23; effect size, –0.56; P = 1.5 x 10–8).
Those endophenotypes and genes intersect theoretical molecular and biological processes that have been identified in other research and could explain underlying mechanisms of schizophrenia. For example, research has suggested that NRG3, which is near the region associated with abstraction and mental flexibility, and affects certain cellular signaling pathways, could be a locus of susceptibility; in particular, some variants of NRG3 have been associated with cognitive and psychotic symptom severity in previous research.
“Although shared genetic substrates appear likely, this is not a study of schizophrenia but rather a study of neurophysiological and neurocognitive deficits that occur in the general population but are more pronounced in the context of schizophrenia and have implications for treatment,” wrote Dr. Greenwood, of the University of California, San Diego, and associates.
One limitation of the study is that, as investigators have demonstrated elsewhere, P values can prove highly variable over the course of replication studies, so the significance thresholds shown in this study still could run the risk of hiding false positives or negatives.
“As many of the 11 endophenotypes have been endorsed as targets for the development of novel treatments for schizophrenia, a better understanding of the corresponding cellular and molecular processes may pave the way for precision-based medicine in schizophrenia and perhaps other psychiatric illnesses with a shared genetic liability,” Dr. Greenwood and associates concluded.
SOURCE: Greenwood TA et al. JAMA Psychiatry. 2019 Oct 9. doi: 10.1001/jamapsychiatry.2019.2850.
Lead author Tiffany A. Greenwood, PhD, noted that clinically diverse schizophrenia patients often are grouped together to get large sample sizes for genome-wide association studies, which can miss specific features of the heterogeneous disorder. Instead, in phase 2 of the Consortium on the Genetics of Schizophrenia (COGS) study, Dr. Greenwood and associates sought to connect features clustered into endophenotypes with certain genetic regions of interest.
“As stable biomarkers of the underlying brain dysfunctions, endophenotypes hold promise for parsing clinical heterogeneity of schizophrenia and refining the genetic signal,” they wrote. The study was published in JAMA Psychiatry.
Using this approach among 1,533 participants, they found seven regions exceeding the conventional genome-wide association significance of P less than 5 x 10–8, including regions associated with the endophenotypes of face memory (chromosome 3p21; effect size, –0.72; P = 4.2 x 10–8), antisaccade task (chromosome 9q31; effect size, –0.24; P = 3.5 x 10–8), and abstraction and mental flexibility (chromosome 10q23; effect size, –0.56; P = 1.5 x 10–8).
Those endophenotypes and genes intersect theoretical molecular and biological processes that have been identified in other research and could explain underlying mechanisms of schizophrenia. For example, research has suggested that NRG3, which is near the region associated with abstraction and mental flexibility, and affects certain cellular signaling pathways, could be a locus of susceptibility; in particular, some variants of NRG3 have been associated with cognitive and psychotic symptom severity in previous research.
“Although shared genetic substrates appear likely, this is not a study of schizophrenia but rather a study of neurophysiological and neurocognitive deficits that occur in the general population but are more pronounced in the context of schizophrenia and have implications for treatment,” wrote Dr. Greenwood, of the University of California, San Diego, and associates.
One limitation of the study is that, as investigators have demonstrated elsewhere, P values can prove highly variable over the course of replication studies, so the significance thresholds shown in this study still could run the risk of hiding false positives or negatives.
“As many of the 11 endophenotypes have been endorsed as targets for the development of novel treatments for schizophrenia, a better understanding of the corresponding cellular and molecular processes may pave the way for precision-based medicine in schizophrenia and perhaps other psychiatric illnesses with a shared genetic liability,” Dr. Greenwood and associates concluded.
SOURCE: Greenwood TA et al. JAMA Psychiatry. 2019 Oct 9. doi: 10.1001/jamapsychiatry.2019.2850.
FROM JAMA PSYCHIATRY
‘How did I get cancer?’
We are 20 minutes into the visit. My patient is 77 years old, a retired school administrator. She was sent to the oncology clinic for a new diagnosis of lung cancer with metastases to the liver and bones.
I was asking my usual questions – how did this all begin? – and I was hearing the usual answers. The cough that didn’t get better with antibiotics. The unintentional weight loss. The chest x-ray that looked “fuzzy.”
I continue: How many packs of cigarettes a day, and for how many years? Any family history of cancer?
These were my standard questions. They were met by hers: “How did I get this?”
I recently hosted a podcast on common, difficult questions we hear in hematology and oncology. How long do I have to live? What would you do if this were your family member?
This was another. There are variations to be sure. How, why, why me, what did I do, what didn’t I do, did my doctor miss it, if I had this or that test would they have caught it sooner?
When I was an internist, I talked about prevention. Meeting a new patient meant sizing them up for risk factors. In their habits I saw opportunities for healthier choices. In their family histories I gathered warning signs.
Now, I ask the same probing questions, but the purpose is not the same. Smoking, alcohol, family history, I ask these of everyone, I reassure them. It’s no longer about assessing risk. It’s not to place blame. But they read into the fact that I am asking, because they have asked themselves the same.
They ask why.
I try not to overdo the pity. I say that I’m sorry this is happening, but I don’t dwell. What I want to convey is the opposite – it’s normalcy. What I want to convey is: I’ve seen this a million times. This is where we are, and here is where we go. We don’t dwell or regret or wonder what if. My patients don’t want sympathy – at least, not from their doctor. They want a plan.
They ask: How did I get this?
It’s bad luck, I say. It’s a genetic mutation causing a cell to replicate.
My answers do not always satisfy their questions. Because it’s not a question seeking an informational answer. The truth is, medically and existentially, I don’t know. None of us do. The question is an existential itch no medical jargon can scratch.
I have a modern Hippocratic oath tacked to a wall in my room. “I will prevent disease whenever I can, because prevention is preferable to cure,” it says. True, but that offers little solace to those who already have the illness. Yes, we need prevention. And we need a path forward when tragedy has already struck.
I am humbled when I meet a new cancer patient because the visit is a metaphor for a nonjudgmental life. There’s something beautiful about meeting someone exactly where they are, where decisions made in the past are as irrelevant to me now as they were to the cancer.
When they inevitably ask “how did I get this?” and I answer, what I’m really saying is this: I don’t care what you did, or didn’t do, or how we got here. But we are here, and so I am here with you, and from now on the only place we care about is here and now, the only direction forward.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
We are 20 minutes into the visit. My patient is 77 years old, a retired school administrator. She was sent to the oncology clinic for a new diagnosis of lung cancer with metastases to the liver and bones.
I was asking my usual questions – how did this all begin? – and I was hearing the usual answers. The cough that didn’t get better with antibiotics. The unintentional weight loss. The chest x-ray that looked “fuzzy.”
I continue: How many packs of cigarettes a day, and for how many years? Any family history of cancer?
These were my standard questions. They were met by hers: “How did I get this?”
I recently hosted a podcast on common, difficult questions we hear in hematology and oncology. How long do I have to live? What would you do if this were your family member?
This was another. There are variations to be sure. How, why, why me, what did I do, what didn’t I do, did my doctor miss it, if I had this or that test would they have caught it sooner?
When I was an internist, I talked about prevention. Meeting a new patient meant sizing them up for risk factors. In their habits I saw opportunities for healthier choices. In their family histories I gathered warning signs.
Now, I ask the same probing questions, but the purpose is not the same. Smoking, alcohol, family history, I ask these of everyone, I reassure them. It’s no longer about assessing risk. It’s not to place blame. But they read into the fact that I am asking, because they have asked themselves the same.
They ask why.
I try not to overdo the pity. I say that I’m sorry this is happening, but I don’t dwell. What I want to convey is the opposite – it’s normalcy. What I want to convey is: I’ve seen this a million times. This is where we are, and here is where we go. We don’t dwell or regret or wonder what if. My patients don’t want sympathy – at least, not from their doctor. They want a plan.
They ask: How did I get this?
It’s bad luck, I say. It’s a genetic mutation causing a cell to replicate.
My answers do not always satisfy their questions. Because it’s not a question seeking an informational answer. The truth is, medically and existentially, I don’t know. None of us do. The question is an existential itch no medical jargon can scratch.
I have a modern Hippocratic oath tacked to a wall in my room. “I will prevent disease whenever I can, because prevention is preferable to cure,” it says. True, but that offers little solace to those who already have the illness. Yes, we need prevention. And we need a path forward when tragedy has already struck.
I am humbled when I meet a new cancer patient because the visit is a metaphor for a nonjudgmental life. There’s something beautiful about meeting someone exactly where they are, where decisions made in the past are as irrelevant to me now as they were to the cancer.
When they inevitably ask “how did I get this?” and I answer, what I’m really saying is this: I don’t care what you did, or didn’t do, or how we got here. But we are here, and so I am here with you, and from now on the only place we care about is here and now, the only direction forward.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
We are 20 minutes into the visit. My patient is 77 years old, a retired school administrator. She was sent to the oncology clinic for a new diagnosis of lung cancer with metastases to the liver and bones.
I was asking my usual questions – how did this all begin? – and I was hearing the usual answers. The cough that didn’t get better with antibiotics. The unintentional weight loss. The chest x-ray that looked “fuzzy.”
I continue: How many packs of cigarettes a day, and for how many years? Any family history of cancer?
These were my standard questions. They were met by hers: “How did I get this?”
I recently hosted a podcast on common, difficult questions we hear in hematology and oncology. How long do I have to live? What would you do if this were your family member?
This was another. There are variations to be sure. How, why, why me, what did I do, what didn’t I do, did my doctor miss it, if I had this or that test would they have caught it sooner?
When I was an internist, I talked about prevention. Meeting a new patient meant sizing them up for risk factors. In their habits I saw opportunities for healthier choices. In their family histories I gathered warning signs.
Now, I ask the same probing questions, but the purpose is not the same. Smoking, alcohol, family history, I ask these of everyone, I reassure them. It’s no longer about assessing risk. It’s not to place blame. But they read into the fact that I am asking, because they have asked themselves the same.
They ask why.
I try not to overdo the pity. I say that I’m sorry this is happening, but I don’t dwell. What I want to convey is the opposite – it’s normalcy. What I want to convey is: I’ve seen this a million times. This is where we are, and here is where we go. We don’t dwell or regret or wonder what if. My patients don’t want sympathy – at least, not from their doctor. They want a plan.
They ask: How did I get this?
It’s bad luck, I say. It’s a genetic mutation causing a cell to replicate.
My answers do not always satisfy their questions. Because it’s not a question seeking an informational answer. The truth is, medically and existentially, I don’t know. None of us do. The question is an existential itch no medical jargon can scratch.
I have a modern Hippocratic oath tacked to a wall in my room. “I will prevent disease whenever I can, because prevention is preferable to cure,” it says. True, but that offers little solace to those who already have the illness. Yes, we need prevention. And we need a path forward when tragedy has already struck.
I am humbled when I meet a new cancer patient because the visit is a metaphor for a nonjudgmental life. There’s something beautiful about meeting someone exactly where they are, where decisions made in the past are as irrelevant to me now as they were to the cancer.
When they inevitably ask “how did I get this?” and I answer, what I’m really saying is this: I don’t care what you did, or didn’t do, or how we got here. But we are here, and so I am here with you, and from now on the only place we care about is here and now, the only direction forward.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
STI update: Testing, treatment, and emerging threats
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
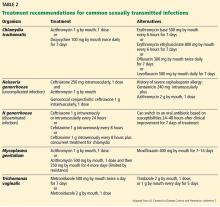
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
KEY POINTS
- Screen for gonorrhea and chlamydia annually—and more frequently for those at highest risk—in sexually active women age 25 and younger and in men who have sex with men, who should also be screened at the same time for human immunodeficiency virus (HIV) and syphilis.
- Test for Trichomonas vaginalis in women who have symptoms suggesting it, and routinely screen for this pathogen in women who are HIV-positive.
- Nucleic acid amplification is the preferred test for gonorrhea, chlamydia, trichomoniasis, and M genitalium infection; the use of urine specimens is acceptable.
- Consider M genitalium if therapy for gonorrhea and chlamydia fails or tests for those diseases are negative.
- Single-dose antibiotic therapy is preferred for chlamydia and uncomplicated gonorrhea. It is also available for trichomoniasis, although metronidazole 500 mg twice a day for 7 days has a higher cure rate.
Current management of Barrett esophagus and esophageal adenocarcinoma
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
KEY POINTS
- Screening is recommended for patients with long-standing reflux symptoms (> 5 years) and 1 or more key risk factors: male sex, age over 50, white race, central obesity, and history of smoking.
- In Barrett esophagus without dysplasia, surveillance endoscopy is recommended every 3 to 5 years to detect dysplasia and early esophageal adenocarcinoma.
- The recommended treatment of dysplasia is endoscopic eradication followed by surveillance endoscopy.