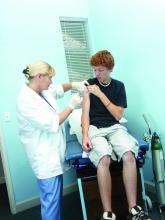
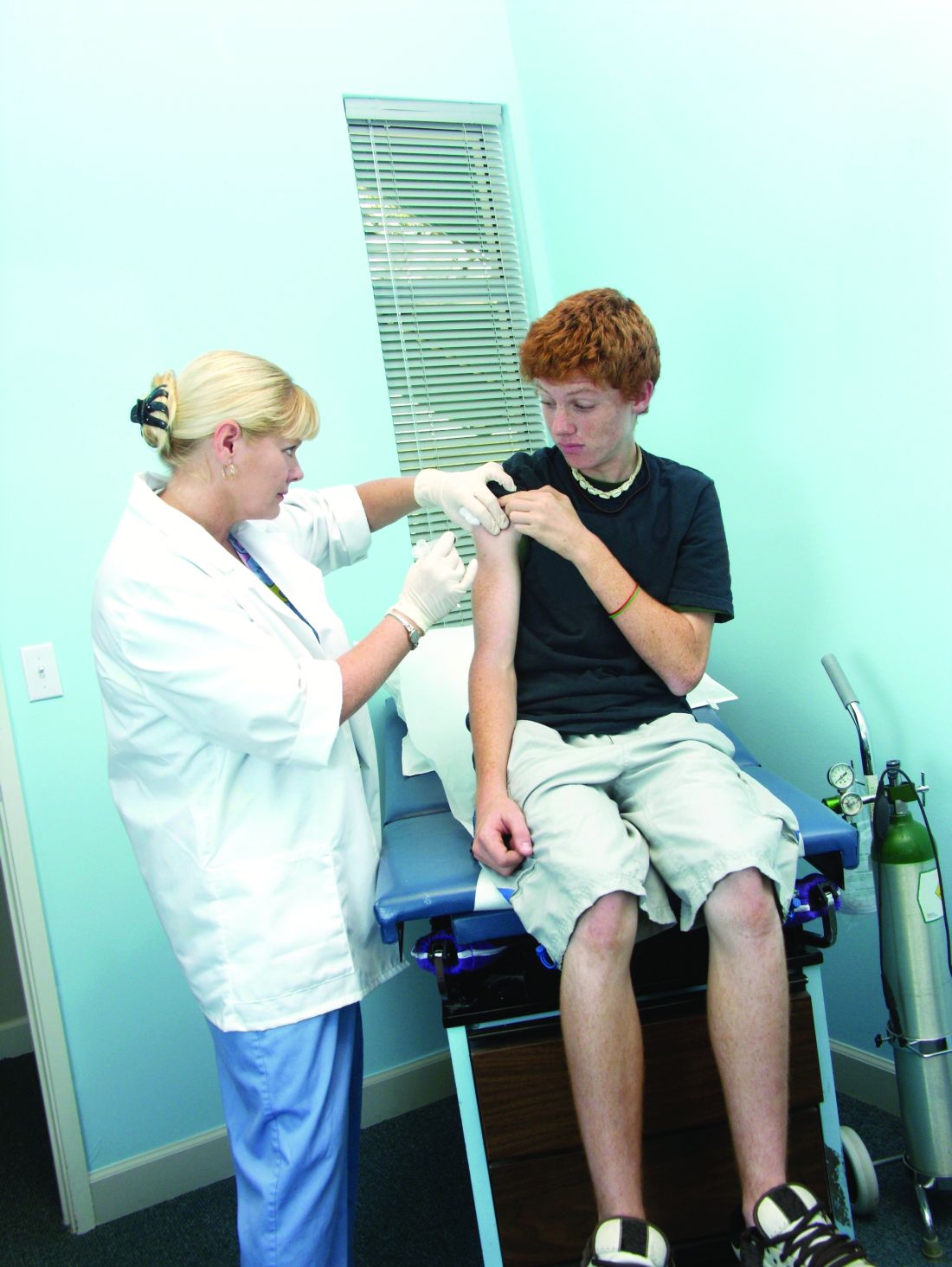
User login
Atypical food allergies common in IBS
, with accompanying changes in epithelial tight junction proteins and eosinophils.
Among 108 patients who completed the study, 61% showed this atypical allergic response to wheat, wrote Annette Fritscher-Ravens, MD, PhD, of University Hospital Schleswig-Holstein in Kiel, Germany, and her associates. Strikingly, almost 70% of patients with atypical food allergies to wheat, yeast, milk, soy, or egg white who eliminated these foods from their diets showed at least an 80% improvement in IBS symptoms after 3 months. These findings were published in Gastroenterology.
Confocal laser endomicroscopy (CLE) “permits real-time detection and quantification of changes in intestinal tissues and cells, including increases in intraepithelial lymphocytes and fluid extravasation through epithelial leaks,” the investigators wrote. This approach helps clinicians objectively detect and measure gastrointestinal pathology in response to specific foods, potentially freeing IBS patients from highly restrictive diets that ease symptoms but are hard to follow, and are not meant for long-term use.
For the study, the researchers enrolled patients meeting Rome III IBS criteria who tested negative for common food antigens on immunoglobulin E serology and skin tests. During endoscopy, each patient underwent sequential duodenal challenges with 20-mL suspensions of wheat, yeast, milk, soy, and egg white, followed by CLE with biopsy.
Among 108 patients who finished the study, 76 (70%) were CLE positive. They and their first-degree relatives were significantly more likely to have atopic disorders than were CLE-negative patients (P = .001). The most common allergen was wheat (61% of patients), followed by yeast (20%), milk (9%), soy (7%), and egg white (4%). Also, nine patients reacted to two of the tested food antigens.
Compared with CLE-negative patients or controls, CLE-positive patients also had significantly more intraepithelial lymphocytes (P = .001) and postchallenge expression of claudin-2 (P = .023), which contributes to tight junction permeability and is known to be upregulated in intestinal barrier dysfunction, IBS, and inflammatory bowel disease. Conversely, levels of the tight junction protein occludin were significantly lower in duodenal biopsies from CLE-positive patients versus controls (P = .022). “Levels of mRNAs encoding inflammatory cytokines were unchanged in duodenal tissues after CLE challenge, but eosinophil degranulation increased,” the researchers wrote.
In a double-blind, randomized, crossover study, patients then excluded from their diet the antigen to which they had tested positive or consumed a sham (placebo) diet that excluded only some foods containing the antigen, with a 2-week washout period in between. The CLE-positive patients showed a 70% average improvement in Francis IBS severity score after 3 months of the intervention diet and a 76% improvement at 6 months. Strikingly, 68% of CLE-positive patients showed at least an 80% improvement in symptoms, while only 4% did not respond at all.
“Since we do not observe a histological mast cell/basophil increase or activation, and [we] do not find increased mast cell mediators (tryptase) in the duodenal fluid after positive challenge, we assume a nonclassical or atypical food allergy as cause of the mucosal reaction observed by CLE,” the researchers wrote. Other immune cell parameters remained unchanged, but additional studies are needed to see if these changes are truly absent or occur later after challenge. The researchers are conducting murine studies of eosinophilic food allergy to shed more light on these nonclassical food allergies.
Funders included the Rashid Hussein Charity Trust, the German Research Foundation, and the Leibniz Foundation. The researchers reported having no conflicts of interest.
SOURCE: Fritscher-Ravens A et al. Gastroenterology. 2019 May 14. doi: 10.1053/j.gastro.2019.03.046.
, with accompanying changes in epithelial tight junction proteins and eosinophils.
Among 108 patients who completed the study, 61% showed this atypical allergic response to wheat, wrote Annette Fritscher-Ravens, MD, PhD, of University Hospital Schleswig-Holstein in Kiel, Germany, and her associates. Strikingly, almost 70% of patients with atypical food allergies to wheat, yeast, milk, soy, or egg white who eliminated these foods from their diets showed at least an 80% improvement in IBS symptoms after 3 months. These findings were published in Gastroenterology.
Confocal laser endomicroscopy (CLE) “permits real-time detection and quantification of changes in intestinal tissues and cells, including increases in intraepithelial lymphocytes and fluid extravasation through epithelial leaks,” the investigators wrote. This approach helps clinicians objectively detect and measure gastrointestinal pathology in response to specific foods, potentially freeing IBS patients from highly restrictive diets that ease symptoms but are hard to follow, and are not meant for long-term use.
For the study, the researchers enrolled patients meeting Rome III IBS criteria who tested negative for common food antigens on immunoglobulin E serology and skin tests. During endoscopy, each patient underwent sequential duodenal challenges with 20-mL suspensions of wheat, yeast, milk, soy, and egg white, followed by CLE with biopsy.
Among 108 patients who finished the study, 76 (70%) were CLE positive. They and their first-degree relatives were significantly more likely to have atopic disorders than were CLE-negative patients (P = .001). The most common allergen was wheat (61% of patients), followed by yeast (20%), milk (9%), soy (7%), and egg white (4%). Also, nine patients reacted to two of the tested food antigens.
Compared with CLE-negative patients or controls, CLE-positive patients also had significantly more intraepithelial lymphocytes (P = .001) and postchallenge expression of claudin-2 (P = .023), which contributes to tight junction permeability and is known to be upregulated in intestinal barrier dysfunction, IBS, and inflammatory bowel disease. Conversely, levels of the tight junction protein occludin were significantly lower in duodenal biopsies from CLE-positive patients versus controls (P = .022). “Levels of mRNAs encoding inflammatory cytokines were unchanged in duodenal tissues after CLE challenge, but eosinophil degranulation increased,” the researchers wrote.
In a double-blind, randomized, crossover study, patients then excluded from their diet the antigen to which they had tested positive or consumed a sham (placebo) diet that excluded only some foods containing the antigen, with a 2-week washout period in between. The CLE-positive patients showed a 70% average improvement in Francis IBS severity score after 3 months of the intervention diet and a 76% improvement at 6 months. Strikingly, 68% of CLE-positive patients showed at least an 80% improvement in symptoms, while only 4% did not respond at all.
“Since we do not observe a histological mast cell/basophil increase or activation, and [we] do not find increased mast cell mediators (tryptase) in the duodenal fluid after positive challenge, we assume a nonclassical or atypical food allergy as cause of the mucosal reaction observed by CLE,” the researchers wrote. Other immune cell parameters remained unchanged, but additional studies are needed to see if these changes are truly absent or occur later after challenge. The researchers are conducting murine studies of eosinophilic food allergy to shed more light on these nonclassical food allergies.
Funders included the Rashid Hussein Charity Trust, the German Research Foundation, and the Leibniz Foundation. The researchers reported having no conflicts of interest.
SOURCE: Fritscher-Ravens A et al. Gastroenterology. 2019 May 14. doi: 10.1053/j.gastro.2019.03.046.
, with accompanying changes in epithelial tight junction proteins and eosinophils.
Among 108 patients who completed the study, 61% showed this atypical allergic response to wheat, wrote Annette Fritscher-Ravens, MD, PhD, of University Hospital Schleswig-Holstein in Kiel, Germany, and her associates. Strikingly, almost 70% of patients with atypical food allergies to wheat, yeast, milk, soy, or egg white who eliminated these foods from their diets showed at least an 80% improvement in IBS symptoms after 3 months. These findings were published in Gastroenterology.
Confocal laser endomicroscopy (CLE) “permits real-time detection and quantification of changes in intestinal tissues and cells, including increases in intraepithelial lymphocytes and fluid extravasation through epithelial leaks,” the investigators wrote. This approach helps clinicians objectively detect and measure gastrointestinal pathology in response to specific foods, potentially freeing IBS patients from highly restrictive diets that ease symptoms but are hard to follow, and are not meant for long-term use.
For the study, the researchers enrolled patients meeting Rome III IBS criteria who tested negative for common food antigens on immunoglobulin E serology and skin tests. During endoscopy, each patient underwent sequential duodenal challenges with 20-mL suspensions of wheat, yeast, milk, soy, and egg white, followed by CLE with biopsy.
Among 108 patients who finished the study, 76 (70%) were CLE positive. They and their first-degree relatives were significantly more likely to have atopic disorders than were CLE-negative patients (P = .001). The most common allergen was wheat (61% of patients), followed by yeast (20%), milk (9%), soy (7%), and egg white (4%). Also, nine patients reacted to two of the tested food antigens.
Compared with CLE-negative patients or controls, CLE-positive patients also had significantly more intraepithelial lymphocytes (P = .001) and postchallenge expression of claudin-2 (P = .023), which contributes to tight junction permeability and is known to be upregulated in intestinal barrier dysfunction, IBS, and inflammatory bowel disease. Conversely, levels of the tight junction protein occludin were significantly lower in duodenal biopsies from CLE-positive patients versus controls (P = .022). “Levels of mRNAs encoding inflammatory cytokines were unchanged in duodenal tissues after CLE challenge, but eosinophil degranulation increased,” the researchers wrote.
In a double-blind, randomized, crossover study, patients then excluded from their diet the antigen to which they had tested positive or consumed a sham (placebo) diet that excluded only some foods containing the antigen, with a 2-week washout period in between. The CLE-positive patients showed a 70% average improvement in Francis IBS severity score after 3 months of the intervention diet and a 76% improvement at 6 months. Strikingly, 68% of CLE-positive patients showed at least an 80% improvement in symptoms, while only 4% did not respond at all.
“Since we do not observe a histological mast cell/basophil increase or activation, and [we] do not find increased mast cell mediators (tryptase) in the duodenal fluid after positive challenge, we assume a nonclassical or atypical food allergy as cause of the mucosal reaction observed by CLE,” the researchers wrote. Other immune cell parameters remained unchanged, but additional studies are needed to see if these changes are truly absent or occur later after challenge. The researchers are conducting murine studies of eosinophilic food allergy to shed more light on these nonclassical food allergies.
Funders included the Rashid Hussein Charity Trust, the German Research Foundation, and the Leibniz Foundation. The researchers reported having no conflicts of interest.
SOURCE: Fritscher-Ravens A et al. Gastroenterology. 2019 May 14. doi: 10.1053/j.gastro.2019.03.046.
FROM GASTROENTEROLOGY
Total margin control surgery warranted for high-risk keratinocyte carcinomas
MILAN – A recent meta-analysis found that total margin control surgery substantially cut the risk of recurrence in patients with high-risk keratinocyte carcinomas, Chrysalyne Schmults, MD, said at the World Congress of Dermatology.
While standard excision will cure the vast majority of low-risk basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), those classified as high risk are best treated with the advanced surgical procedure, also referred to as circumferential peripheral and deep margin assessment (CCPDMA), said Dr. Schmults, director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital in Boston.
For high-risk cases, CCPDMA has superior outcomes, compared with standard excision, according to results of the meta-analysis conducted by Dr. Schmults and colleagues. The meta-analysis has not yet been published.
The in the meta-analysis of medical literature from 1993 to 2017.
“This is a really big drop,” Dr. Schmults said. “If you do total margin control surgery on your perineural invasive cases, you’re going to drop your recurrence rate by two-thirds, and that’s a really big difference.”
For carcinomas without perineural invasion, the recurrence rate is about 4.5% for standard excision and about 2.0% for CCPDMA, the analysis showed. “That’s a difference, but it’s not a big enough difference that we’re going to start doing total margin control surgery on every single basal cell and squamous cell carcinoma that comes to us,” Dr. Schmults commented.
Using techniques such as Mohs surgery that allow for CCPDMA, nearly 100% of the surgical margin can be seen; by contrast, standard histology allows for visualization of only about 1% of the marginal surface, according to Dr. Schmults.
While CCPDMA may have superior outcomes for high-risk keratinocyte carcinomas, defining what constitutes high risk remains a challenge, particularly for patients with BCCs. “We don’t have good literature on which are the rare, bad basal cells,” she said.
But more clarity may be on the way. In a paper under review for publication on Brigham and Women’s Hospital (BWH) staging criteria for BCC, Dr. Schmults and colleagues describe a subset of patients with BCC at higher risk of metastasis and death based on specific high-risk tumor characteristics.
High risk is better defined for SCCs. According to the BWH classification system for cutaneous SCCs, developed by Dr. Schmults and colleagues, high-risk features include larger tumor diameter, poorly differentiated histology, perineural invasion, and tumor invasion beyond subcutaneous fat or to bone.
Patients with SCC classified as high stage by the BWH system have about a 25% risk of metastasis or death. “These patients really need that total margin control surgery,” Dr. Schmults said.
High-stage SCC patients who did not get Mohs surgery in a study (J Clin Oncol. 2014 Feb 1;32[4]:327-34) conducted by Dr. Schmults and her associates, had a quadrupling in risk of death from disease, compared with those who did get the procedure in a more recent study (J Am Acad Dermatol. 2019 Mar;80[3]:633-8).
“These are fairly small studies,” she pointed out. But the large meta-analysis she presented at the meeting showed the same result, “that the higher the stage, the worse your tumor is, and whether it’s basal cell or squamous cell, the greater the advantage for total margin control surgery.”
Dr. Schmults reported that she is the panel chair for the National Comprehensive Cancer Network guidelines on nonmelanoma skin cancer, and that she was a cutaneous SCC committee member for the 8th edition of the American Joint Committee on Cancer cancer staging system. She also participated in the development of the BWH staging system for SCC.
MILAN – A recent meta-analysis found that total margin control surgery substantially cut the risk of recurrence in patients with high-risk keratinocyte carcinomas, Chrysalyne Schmults, MD, said at the World Congress of Dermatology.
While standard excision will cure the vast majority of low-risk basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), those classified as high risk are best treated with the advanced surgical procedure, also referred to as circumferential peripheral and deep margin assessment (CCPDMA), said Dr. Schmults, director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital in Boston.
For high-risk cases, CCPDMA has superior outcomes, compared with standard excision, according to results of the meta-analysis conducted by Dr. Schmults and colleagues. The meta-analysis has not yet been published.
The in the meta-analysis of medical literature from 1993 to 2017.
“This is a really big drop,” Dr. Schmults said. “If you do total margin control surgery on your perineural invasive cases, you’re going to drop your recurrence rate by two-thirds, and that’s a really big difference.”
For carcinomas without perineural invasion, the recurrence rate is about 4.5% for standard excision and about 2.0% for CCPDMA, the analysis showed. “That’s a difference, but it’s not a big enough difference that we’re going to start doing total margin control surgery on every single basal cell and squamous cell carcinoma that comes to us,” Dr. Schmults commented.
Using techniques such as Mohs surgery that allow for CCPDMA, nearly 100% of the surgical margin can be seen; by contrast, standard histology allows for visualization of only about 1% of the marginal surface, according to Dr. Schmults.
While CCPDMA may have superior outcomes for high-risk keratinocyte carcinomas, defining what constitutes high risk remains a challenge, particularly for patients with BCCs. “We don’t have good literature on which are the rare, bad basal cells,” she said.
But more clarity may be on the way. In a paper under review for publication on Brigham and Women’s Hospital (BWH) staging criteria for BCC, Dr. Schmults and colleagues describe a subset of patients with BCC at higher risk of metastasis and death based on specific high-risk tumor characteristics.
High risk is better defined for SCCs. According to the BWH classification system for cutaneous SCCs, developed by Dr. Schmults and colleagues, high-risk features include larger tumor diameter, poorly differentiated histology, perineural invasion, and tumor invasion beyond subcutaneous fat or to bone.
Patients with SCC classified as high stage by the BWH system have about a 25% risk of metastasis or death. “These patients really need that total margin control surgery,” Dr. Schmults said.
High-stage SCC patients who did not get Mohs surgery in a study (J Clin Oncol. 2014 Feb 1;32[4]:327-34) conducted by Dr. Schmults and her associates, had a quadrupling in risk of death from disease, compared with those who did get the procedure in a more recent study (J Am Acad Dermatol. 2019 Mar;80[3]:633-8).
“These are fairly small studies,” she pointed out. But the large meta-analysis she presented at the meeting showed the same result, “that the higher the stage, the worse your tumor is, and whether it’s basal cell or squamous cell, the greater the advantage for total margin control surgery.”
Dr. Schmults reported that she is the panel chair for the National Comprehensive Cancer Network guidelines on nonmelanoma skin cancer, and that she was a cutaneous SCC committee member for the 8th edition of the American Joint Committee on Cancer cancer staging system. She also participated in the development of the BWH staging system for SCC.
MILAN – A recent meta-analysis found that total margin control surgery substantially cut the risk of recurrence in patients with high-risk keratinocyte carcinomas, Chrysalyne Schmults, MD, said at the World Congress of Dermatology.
While standard excision will cure the vast majority of low-risk basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), those classified as high risk are best treated with the advanced surgical procedure, also referred to as circumferential peripheral and deep margin assessment (CCPDMA), said Dr. Schmults, director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital in Boston.
For high-risk cases, CCPDMA has superior outcomes, compared with standard excision, according to results of the meta-analysis conducted by Dr. Schmults and colleagues. The meta-analysis has not yet been published.
The in the meta-analysis of medical literature from 1993 to 2017.
“This is a really big drop,” Dr. Schmults said. “If you do total margin control surgery on your perineural invasive cases, you’re going to drop your recurrence rate by two-thirds, and that’s a really big difference.”
For carcinomas without perineural invasion, the recurrence rate is about 4.5% for standard excision and about 2.0% for CCPDMA, the analysis showed. “That’s a difference, but it’s not a big enough difference that we’re going to start doing total margin control surgery on every single basal cell and squamous cell carcinoma that comes to us,” Dr. Schmults commented.
Using techniques such as Mohs surgery that allow for CCPDMA, nearly 100% of the surgical margin can be seen; by contrast, standard histology allows for visualization of only about 1% of the marginal surface, according to Dr. Schmults.
While CCPDMA may have superior outcomes for high-risk keratinocyte carcinomas, defining what constitutes high risk remains a challenge, particularly for patients with BCCs. “We don’t have good literature on which are the rare, bad basal cells,” she said.
But more clarity may be on the way. In a paper under review for publication on Brigham and Women’s Hospital (BWH) staging criteria for BCC, Dr. Schmults and colleagues describe a subset of patients with BCC at higher risk of metastasis and death based on specific high-risk tumor characteristics.
High risk is better defined for SCCs. According to the BWH classification system for cutaneous SCCs, developed by Dr. Schmults and colleagues, high-risk features include larger tumor diameter, poorly differentiated histology, perineural invasion, and tumor invasion beyond subcutaneous fat or to bone.
Patients with SCC classified as high stage by the BWH system have about a 25% risk of metastasis or death. “These patients really need that total margin control surgery,” Dr. Schmults said.
High-stage SCC patients who did not get Mohs surgery in a study (J Clin Oncol. 2014 Feb 1;32[4]:327-34) conducted by Dr. Schmults and her associates, had a quadrupling in risk of death from disease, compared with those who did get the procedure in a more recent study (J Am Acad Dermatol. 2019 Mar;80[3]:633-8).
“These are fairly small studies,” she pointed out. But the large meta-analysis she presented at the meeting showed the same result, “that the higher the stage, the worse your tumor is, and whether it’s basal cell or squamous cell, the greater the advantage for total margin control surgery.”
Dr. Schmults reported that she is the panel chair for the National Comprehensive Cancer Network guidelines on nonmelanoma skin cancer, and that she was a cutaneous SCC committee member for the 8th edition of the American Joint Committee on Cancer cancer staging system. She also participated in the development of the BWH staging system for SCC.
REPORTING FROM WCD2019
Cryptosporidiosis infections spike during summer swim season
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
Outbreaks of cryptosporidiosis increased in the United States by an average of 13% each year between 2009 and 2017, based on data from the Centers for Disease Control and Prevention.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, researchers reviewed data from 444 reported outbreaks submitted to the CDC’s National Outbreak Reporting System totaling 7,465 cases, including 287 hospitalizations and one death.
The outbreaks during this period were most commonly associated with pools and water parks (35%), exposure to cattle (15%), and child care settings (13%). Another 3% of outbreaks were associated with drinking unpasteurized milk or apple cider. An outbreak was defined as two or more cases linked to a common source.
The profuse, watery diarrhea associated with infection from the cryptosporidium parasite can last for 3 weeks in healthy individuals and can cause life-threatening malnutrition in the immunocompromised, wrote Radhika Gharpure, DVM, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, and colleagues.
The overall number of outbreaks peaked during July and August each year; the number associated with pools and water parks peaked between June and August, the number associated with cattle peaked between March and May, and the number associated with child care settings peaked between July and September.
The results were limited by several factors including likely underestimation of the number of outbreaks, the use of multipathogen testing panels that could have inflated the number of outbreaks, and the variation in the ability of jurisdictions to detect, investigate, and report outbreaks, the researchers noted. CryptoNet, a molecularly-based surveillance system, has shown potential to track disease transmission, they said.
However, primary prevention is important to prevent the spread of disease, and strategies include refraining from swimming when one has diarrhea and for 2 weeks after resolution of diarrhea, not sending children to child care when they have diarrhea, and washing hands thoroughly after contact with animals, the researchers said.
“If a cryptosporidiosis outbreak occurs, substantial decontamination measures are needed, including hyperchlorinating public treated recreational water venues (e.g., swimming pools at a hotel, apartment complex, or water park) and using hydrogen peroxide to disinfect surfaces in child care settings to inactivate Cryptosporidium oocysts,” they emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Gharpure R et al. MMWR. 2019 June 28. 68:568-72.
FROM MMWR
July 2019 - Question 2
Q2. Correct answer: C
Rationale
Oral iron, and not infusions, are associated with peptic ulcer disease. Sumatriptan alone, or tamoxifen, are not known to cause ulcers.
Reference:
Miyake K., Kusunoki M., Shinji Y., et al. Bisphosphonate increases risk of gastroduodenal ulcer in rheumatoid arthritis patients on long-term nonsteroidal anti-inflammatory drug therapy. J Gastroenterol. 2009;44(2):113.
[email protected]
Q2. Correct answer: C
Rationale
Oral iron, and not infusions, are associated with peptic ulcer disease. Sumatriptan alone, or tamoxifen, are not known to cause ulcers.
Reference:
Miyake K., Kusunoki M., Shinji Y., et al. Bisphosphonate increases risk of gastroduodenal ulcer in rheumatoid arthritis patients on long-term nonsteroidal anti-inflammatory drug therapy. J Gastroenterol. 2009;44(2):113.
[email protected]
Q2. Correct answer: C
Rationale
Oral iron, and not infusions, are associated with peptic ulcer disease. Sumatriptan alone, or tamoxifen, are not known to cause ulcers.
Reference:
Miyake K., Kusunoki M., Shinji Y., et al. Bisphosphonate increases risk of gastroduodenal ulcer in rheumatoid arthritis patients on long-term nonsteroidal anti-inflammatory drug therapy. J Gastroenterol. 2009;44(2):113.
[email protected]
Q2. A 63-year-old woman is admitted with abdominal pain and iron deficiency anemia. She reports long-standing anemia and a negative workup in the past year including an upper endoscopy, colonoscopy, and video capsule endoscopy. She was started on iron infusions with a modest improvement in her anemia. Her other medical history includes osteoporosis; osteoarthritis, for which she takes over the counter NSAIDs, breast cancer (20 years ago treated with lumpectomy and local radiation); and migraines for which she takes sumatriptan once or twice a month.
July 2019 - Question 1
Q1. Correct answer: D
Rationale
Achalasia and pseudoachalasia are on the differential. Given the advanced age, progressive course, and significant weight loss, an endoscopy with careful attention to GEJ should be performed to rule out malignancy causing a pseudoachalasia presentation (answer D). Manometry should be done after the endoscopy to confirm and subtype the achalasia. If achalasia is confirmed and malignancy is ruled out, myotomy either with a modified Heller approach or peroral endoscopic myotomy would be appropriate in a surgically fit patient (answer A) and botulinum toxin may be considered in a poor surgical candidate. Medications such as calcium channel blockers and nitrates (answer C) are not definitive treatment options for achalasia and not warranted in malignancy. Additional information is needed on the diagnosis and prognosis prior to committing to a G tube (answer E).
Reference :
Zaninotto G., Bennett C., Boeckxstaens G., et al. The 2018 ISDE achalasia guidelines. Dis Esophagus. 2018 Sep 1;31(9).
Q1. Correct answer: D
Rationale
Achalasia and pseudoachalasia are on the differential. Given the advanced age, progressive course, and significant weight loss, an endoscopy with careful attention to GEJ should be performed to rule out malignancy causing a pseudoachalasia presentation (answer D). Manometry should be done after the endoscopy to confirm and subtype the achalasia. If achalasia is confirmed and malignancy is ruled out, myotomy either with a modified Heller approach or peroral endoscopic myotomy would be appropriate in a surgically fit patient (answer A) and botulinum toxin may be considered in a poor surgical candidate. Medications such as calcium channel blockers and nitrates (answer C) are not definitive treatment options for achalasia and not warranted in malignancy. Additional information is needed on the diagnosis and prognosis prior to committing to a G tube (answer E).
Reference :
Zaninotto G., Bennett C., Boeckxstaens G., et al. The 2018 ISDE achalasia guidelines. Dis Esophagus. 2018 Sep 1;31(9).
Q1. Correct answer: D
Rationale
Achalasia and pseudoachalasia are on the differential. Given the advanced age, progressive course, and significant weight loss, an endoscopy with careful attention to GEJ should be performed to rule out malignancy causing a pseudoachalasia presentation (answer D). Manometry should be done after the endoscopy to confirm and subtype the achalasia. If achalasia is confirmed and malignancy is ruled out, myotomy either with a modified Heller approach or peroral endoscopic myotomy would be appropriate in a surgically fit patient (answer A) and botulinum toxin may be considered in a poor surgical candidate. Medications such as calcium channel blockers and nitrates (answer C) are not definitive treatment options for achalasia and not warranted in malignancy. Additional information is needed on the diagnosis and prognosis prior to committing to a G tube (answer E).
Reference :
Zaninotto G., Bennett C., Boeckxstaens G., et al. The 2018 ISDE achalasia guidelines. Dis Esophagus. 2018 Sep 1;31(9).
Q1. A 70-year-old male presents with progressive dysphagia over the past 4 months and 30-pound weight loss. A barium swallow demonstrates a dilated esophagus with a bird's beak appearance.
Health care gets heated on Night 2 of the Democratic presidential debate

Just as on Wednesday night, moderators asked candidates who would support abolishing private insurance under a single-payer system. Again, only two candidates — this time Sen. Sanders and Sen. Kamala Harris of California— raised their hands.
Former Vice President Joe Biden also jumped on health care, saying Americans “need to have insurance that is covered, and that they can afford.”
But he offered a different view of how to achieve the goal, saying the fastest way would be to “build on Obamacare. To build on what we did.” He also drew a line in the sand, promising to oppose any Democrat or Republican who tried to take down Obamacare.
Candidates including South Bend, Ind., Mayor Pete Buttigieg, New York Sen. Kristen Gillibrand and Colorado Sen. Michael Bennet offered their takes on universal coverage, each underscoring the importance of a transition from the current system and suggesting that a public option approach, something that would allow people to buy into a program like Medicare, would offer a “glide path” to the ultimate goal of universal coverage. Sen. Gillibrand noted that she ran on such a proposal in 2005. (This is true.)
Meanwhile, former Colorado Gov. John Hickenlooper used the issue of Medicare for All to say that it is important to not allow Republicans to paint the Democratic Party as socialist but also to claim his own successes in implementing coverage expansions to reach “near universal coverage” Colorado. PolitiFact examined this claim and found it Mostly True.“You don’t need big government to do big things. I know that because I’m the one person up here who’s actually done the big progressive things everyone else is talking about,” he said.
But still, while candidates were quick to make their differences clear, not all of their claims fully stood up to scrutiny.
We fact-checked some of those remarks.
Sen. Sanders: “President Trump, you’re not standing up for working families when you try to throw 32 million people off the health care that they have.”
This is one of Sanders’ favorite lines, but it falls short of giving the full story of the Republican effort to repeal and replace Obamacare. We rated a similar claim Half True.
Scrapping the Affordable Care Act was a key campaign promise for President Donald Trump. In 2017, as the Republican-led Congress struggled to deliver, Trump tweeted “Republicans should just REPEAL failing Obamacare now and work on a new health care plan that will start from a clean slate.”
The Congressional Budget Office estimated that would lead to 32 million more people without insurance by 2026. But some portion of that 32 million would have chosen not to buy insurance due to the end of the individual mandate, which would happen under repeal. (It happened anyway, when the 2017 tax law repealed the fine for the individual mandate.)
In the end, full repeal didn’t happen. Instead, Trump was only able to zero out the fines for people who didn’t have insurance. Coverage has eroded. The latest survey shows about 1.3 million people have lost insurance since Trump took office.
Sen. Bennet, meanwhile, used his time to attack Medicare for All on a feasibility standpoint.
Sen. Bennet: “Bernie mentioned that the taxes that we would have to pay — because of those taxes, Vermont rejected Medicare for All.”
This is true, although it could use some context.
Vermont’s effort to pass a state-based single-payer health plan — which the state legislature approved in 2011 — officially fell flat in December 2014. Financing the plan ultimately would have required an 11.5% payroll tax on all employers, plus raising the income tax by as much as 9.5%. The governor at the time, Democrat Peter Shumlin, declared this politically untenable.
That said, some analysts suggest other political factors may have played a role, too — for instance, fallout after the state launched its Affordable Care Act health insurance website, which faced technical difficulties.
Nationally, when voters are told Medicare for All could result in higher taxes, support declines.
And a point was made by author Marianne Williamson about the nation’s high burden of chronic disease.
Ms. Williamson: “So many Americans have unnecessary chronic illnesses — so many more compared to other countries.”
There is evidence for this, at least for older Americans.
A November 2014 study by the Commonwealth Fund found that 68% of Americans 65 and older had two or more chronic conditions, and an additional 20% had one chronic condition.
No other country studied — the United Kingdom, New Zealand, Sweden, Norway, France, Switzerland, the Netherlands, Germany, Austria, or Canada — had a higher rate of older residents with at least two chronic conditions. The percentages ranged from 33 percent in the United Kingdom to 56 percent in Canada.
An earlier study published in the journal Health Affairs in 2007 found that “for many of the most costly chronic conditions, diagnosed disease prevalence and treatment rates were higher in the United States than in a sample of European countries in 2004.”
PolitiFact’s Jon Greenberg and Louis Jacobson contributed to this story. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. Politifact is owned by the nonprofit Poynter Institute for Media Studies.

Just as on Wednesday night, moderators asked candidates who would support abolishing private insurance under a single-payer system. Again, only two candidates — this time Sen. Sanders and Sen. Kamala Harris of California— raised their hands.
Former Vice President Joe Biden also jumped on health care, saying Americans “need to have insurance that is covered, and that they can afford.”
But he offered a different view of how to achieve the goal, saying the fastest way would be to “build on Obamacare. To build on what we did.” He also drew a line in the sand, promising to oppose any Democrat or Republican who tried to take down Obamacare.
Candidates including South Bend, Ind., Mayor Pete Buttigieg, New York Sen. Kristen Gillibrand and Colorado Sen. Michael Bennet offered their takes on universal coverage, each underscoring the importance of a transition from the current system and suggesting that a public option approach, something that would allow people to buy into a program like Medicare, would offer a “glide path” to the ultimate goal of universal coverage. Sen. Gillibrand noted that she ran on such a proposal in 2005. (This is true.)
Meanwhile, former Colorado Gov. John Hickenlooper used the issue of Medicare for All to say that it is important to not allow Republicans to paint the Democratic Party as socialist but also to claim his own successes in implementing coverage expansions to reach “near universal coverage” Colorado. PolitiFact examined this claim and found it Mostly True.“You don’t need big government to do big things. I know that because I’m the one person up here who’s actually done the big progressive things everyone else is talking about,” he said.
But still, while candidates were quick to make their differences clear, not all of their claims fully stood up to scrutiny.
We fact-checked some of those remarks.
Sen. Sanders: “President Trump, you’re not standing up for working families when you try to throw 32 million people off the health care that they have.”
This is one of Sanders’ favorite lines, but it falls short of giving the full story of the Republican effort to repeal and replace Obamacare. We rated a similar claim Half True.
Scrapping the Affordable Care Act was a key campaign promise for President Donald Trump. In 2017, as the Republican-led Congress struggled to deliver, Trump tweeted “Republicans should just REPEAL failing Obamacare now and work on a new health care plan that will start from a clean slate.”
The Congressional Budget Office estimated that would lead to 32 million more people without insurance by 2026. But some portion of that 32 million would have chosen not to buy insurance due to the end of the individual mandate, which would happen under repeal. (It happened anyway, when the 2017 tax law repealed the fine for the individual mandate.)
In the end, full repeal didn’t happen. Instead, Trump was only able to zero out the fines for people who didn’t have insurance. Coverage has eroded. The latest survey shows about 1.3 million people have lost insurance since Trump took office.
Sen. Bennet, meanwhile, used his time to attack Medicare for All on a feasibility standpoint.
Sen. Bennet: “Bernie mentioned that the taxes that we would have to pay — because of those taxes, Vermont rejected Medicare for All.”
This is true, although it could use some context.
Vermont’s effort to pass a state-based single-payer health plan — which the state legislature approved in 2011 — officially fell flat in December 2014. Financing the plan ultimately would have required an 11.5% payroll tax on all employers, plus raising the income tax by as much as 9.5%. The governor at the time, Democrat Peter Shumlin, declared this politically untenable.
That said, some analysts suggest other political factors may have played a role, too — for instance, fallout after the state launched its Affordable Care Act health insurance website, which faced technical difficulties.
Nationally, when voters are told Medicare for All could result in higher taxes, support declines.
And a point was made by author Marianne Williamson about the nation’s high burden of chronic disease.
Ms. Williamson: “So many Americans have unnecessary chronic illnesses — so many more compared to other countries.”
There is evidence for this, at least for older Americans.
A November 2014 study by the Commonwealth Fund found that 68% of Americans 65 and older had two or more chronic conditions, and an additional 20% had one chronic condition.
No other country studied — the United Kingdom, New Zealand, Sweden, Norway, France, Switzerland, the Netherlands, Germany, Austria, or Canada — had a higher rate of older residents with at least two chronic conditions. The percentages ranged from 33 percent in the United Kingdom to 56 percent in Canada.
An earlier study published in the journal Health Affairs in 2007 found that “for many of the most costly chronic conditions, diagnosed disease prevalence and treatment rates were higher in the United States than in a sample of European countries in 2004.”
PolitiFact’s Jon Greenberg and Louis Jacobson contributed to this story. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. Politifact is owned by the nonprofit Poynter Institute for Media Studies.

Just as on Wednesday night, moderators asked candidates who would support abolishing private insurance under a single-payer system. Again, only two candidates — this time Sen. Sanders and Sen. Kamala Harris of California— raised their hands.
Former Vice President Joe Biden also jumped on health care, saying Americans “need to have insurance that is covered, and that they can afford.”
But he offered a different view of how to achieve the goal, saying the fastest way would be to “build on Obamacare. To build on what we did.” He also drew a line in the sand, promising to oppose any Democrat or Republican who tried to take down Obamacare.
Candidates including South Bend, Ind., Mayor Pete Buttigieg, New York Sen. Kristen Gillibrand and Colorado Sen. Michael Bennet offered their takes on universal coverage, each underscoring the importance of a transition from the current system and suggesting that a public option approach, something that would allow people to buy into a program like Medicare, would offer a “glide path” to the ultimate goal of universal coverage. Sen. Gillibrand noted that she ran on such a proposal in 2005. (This is true.)
Meanwhile, former Colorado Gov. John Hickenlooper used the issue of Medicare for All to say that it is important to not allow Republicans to paint the Democratic Party as socialist but also to claim his own successes in implementing coverage expansions to reach “near universal coverage” Colorado. PolitiFact examined this claim and found it Mostly True.“You don’t need big government to do big things. I know that because I’m the one person up here who’s actually done the big progressive things everyone else is talking about,” he said.
But still, while candidates were quick to make their differences clear, not all of their claims fully stood up to scrutiny.
We fact-checked some of those remarks.
Sen. Sanders: “President Trump, you’re not standing up for working families when you try to throw 32 million people off the health care that they have.”
This is one of Sanders’ favorite lines, but it falls short of giving the full story of the Republican effort to repeal and replace Obamacare. We rated a similar claim Half True.
Scrapping the Affordable Care Act was a key campaign promise for President Donald Trump. In 2017, as the Republican-led Congress struggled to deliver, Trump tweeted “Republicans should just REPEAL failing Obamacare now and work on a new health care plan that will start from a clean slate.”
The Congressional Budget Office estimated that would lead to 32 million more people without insurance by 2026. But some portion of that 32 million would have chosen not to buy insurance due to the end of the individual mandate, which would happen under repeal. (It happened anyway, when the 2017 tax law repealed the fine for the individual mandate.)
In the end, full repeal didn’t happen. Instead, Trump was only able to zero out the fines for people who didn’t have insurance. Coverage has eroded. The latest survey shows about 1.3 million people have lost insurance since Trump took office.
Sen. Bennet, meanwhile, used his time to attack Medicare for All on a feasibility standpoint.
Sen. Bennet: “Bernie mentioned that the taxes that we would have to pay — because of those taxes, Vermont rejected Medicare for All.”
This is true, although it could use some context.
Vermont’s effort to pass a state-based single-payer health plan — which the state legislature approved in 2011 — officially fell flat in December 2014. Financing the plan ultimately would have required an 11.5% payroll tax on all employers, plus raising the income tax by as much as 9.5%. The governor at the time, Democrat Peter Shumlin, declared this politically untenable.
That said, some analysts suggest other political factors may have played a role, too — for instance, fallout after the state launched its Affordable Care Act health insurance website, which faced technical difficulties.
Nationally, when voters are told Medicare for All could result in higher taxes, support declines.
And a point was made by author Marianne Williamson about the nation’s high burden of chronic disease.
Ms. Williamson: “So many Americans have unnecessary chronic illnesses — so many more compared to other countries.”
There is evidence for this, at least for older Americans.
A November 2014 study by the Commonwealth Fund found that 68% of Americans 65 and older had two or more chronic conditions, and an additional 20% had one chronic condition.
No other country studied — the United Kingdom, New Zealand, Sweden, Norway, France, Switzerland, the Netherlands, Germany, Austria, or Canada — had a higher rate of older residents with at least two chronic conditions. The percentages ranged from 33 percent in the United Kingdom to 56 percent in Canada.
An earlier study published in the journal Health Affairs in 2007 found that “for many of the most costly chronic conditions, diagnosed disease prevalence and treatment rates were higher in the United States than in a sample of European countries in 2004.”
PolitiFact’s Jon Greenberg and Louis Jacobson contributed to this story. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. Politifact is owned by the nonprofit Poynter Institute for Media Studies.
ACIP approves flu vaccine recommendations for 2019-2020 season
All individuals aged 6 months and older should receive the influenza vaccine by the end of October next season, according to the Centers for Disease Control and Prevention’s Committee on Immunization Practices. The committee voted unanimously to accept minor updates to the ACIP flu recommendations for the 2019-2020 season, but no major changes were made from recent years.
The past flu season was moderate overall, but notable for two waves of viral infections of similar magnitude, one with H1N1 and another with H3N2, said Lynette Brewer of the CDC’s National Center for Immunization and Respiratory Diseases, who presented data on last year’s flu activity.
Last year’s vaccine likely prevented between 40,000 and 90,000 hospitalizations, but mostly reduced the burden of H1N1 disease and provided no real protection against H3N2, she said.
The recommended H3N2 component for next season is A/Kansas/14/2017–like virus, which is genetically similar to the H3N2 that circulated last year.
Lisa Grohskopf, MD, of the CDC’s influenza division, presented the minor adjustments that included the changes in vaccine composition for next year, some licensure changes, and a new table summarizing dose volumes. Also, language was changed to advise vaccination for all eligible individuals by the end of October, and individuals who need two doses should have the first one as soon as it becomes available, in July or August if possible. The updated language also clarified that 8 year olds who need two doses should receive the second dose, even if they turn 9 between the two doses.
Additional guidance updates approved by the committee included harmonizing language on groups that should be the focus of vaccination in the event of limited supply to be more consistent with the 2011 ACIP Recommendations for the Immunization of Health Care Personnel.
The committee also voted unanimously to accept the proposed influenza vaccine in the Vaccines for Children program; there were no changes in recommended dosing intervals, dosages, contraindications, or precautions, according to Frank Whitlach of the National Center for Immunization and Respiratory Diseases, who presented the Vaccines for Children information.
The ACIP members had no financial conflicts to disclose.
All individuals aged 6 months and older should receive the influenza vaccine by the end of October next season, according to the Centers for Disease Control and Prevention’s Committee on Immunization Practices. The committee voted unanimously to accept minor updates to the ACIP flu recommendations for the 2019-2020 season, but no major changes were made from recent years.
The past flu season was moderate overall, but notable for two waves of viral infections of similar magnitude, one with H1N1 and another with H3N2, said Lynette Brewer of the CDC’s National Center for Immunization and Respiratory Diseases, who presented data on last year’s flu activity.
Last year’s vaccine likely prevented between 40,000 and 90,000 hospitalizations, but mostly reduced the burden of H1N1 disease and provided no real protection against H3N2, she said.
The recommended H3N2 component for next season is A/Kansas/14/2017–like virus, which is genetically similar to the H3N2 that circulated last year.
Lisa Grohskopf, MD, of the CDC’s influenza division, presented the minor adjustments that included the changes in vaccine composition for next year, some licensure changes, and a new table summarizing dose volumes. Also, language was changed to advise vaccination for all eligible individuals by the end of October, and individuals who need two doses should have the first one as soon as it becomes available, in July or August if possible. The updated language also clarified that 8 year olds who need two doses should receive the second dose, even if they turn 9 between the two doses.
Additional guidance updates approved by the committee included harmonizing language on groups that should be the focus of vaccination in the event of limited supply to be more consistent with the 2011 ACIP Recommendations for the Immunization of Health Care Personnel.
The committee also voted unanimously to accept the proposed influenza vaccine in the Vaccines for Children program; there were no changes in recommended dosing intervals, dosages, contraindications, or precautions, according to Frank Whitlach of the National Center for Immunization and Respiratory Diseases, who presented the Vaccines for Children information.
The ACIP members had no financial conflicts to disclose.
All individuals aged 6 months and older should receive the influenza vaccine by the end of October next season, according to the Centers for Disease Control and Prevention’s Committee on Immunization Practices. The committee voted unanimously to accept minor updates to the ACIP flu recommendations for the 2019-2020 season, but no major changes were made from recent years.
The past flu season was moderate overall, but notable for two waves of viral infections of similar magnitude, one with H1N1 and another with H3N2, said Lynette Brewer of the CDC’s National Center for Immunization and Respiratory Diseases, who presented data on last year’s flu activity.
Last year’s vaccine likely prevented between 40,000 and 90,000 hospitalizations, but mostly reduced the burden of H1N1 disease and provided no real protection against H3N2, she said.
The recommended H3N2 component for next season is A/Kansas/14/2017–like virus, which is genetically similar to the H3N2 that circulated last year.
Lisa Grohskopf, MD, of the CDC’s influenza division, presented the minor adjustments that included the changes in vaccine composition for next year, some licensure changes, and a new table summarizing dose volumes. Also, language was changed to advise vaccination for all eligible individuals by the end of October, and individuals who need two doses should have the first one as soon as it becomes available, in July or August if possible. The updated language also clarified that 8 year olds who need two doses should receive the second dose, even if they turn 9 between the two doses.
Additional guidance updates approved by the committee included harmonizing language on groups that should be the focus of vaccination in the event of limited supply to be more consistent with the 2011 ACIP Recommendations for the Immunization of Health Care Personnel.
The committee also voted unanimously to accept the proposed influenza vaccine in the Vaccines for Children program; there were no changes in recommended dosing intervals, dosages, contraindications, or precautions, according to Frank Whitlach of the National Center for Immunization and Respiratory Diseases, who presented the Vaccines for Children information.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
FDA issues warning on insulin pump cybersecurity weakness
The Food and Drug Administration has issued a warning to patients and health care providers that a pair of Medtronic insulin pumps are being recalled because of potential cybersecurity risks, according to a press release.
The affected devices are the MiniMed 508 and MiniMed Paradigm series insulin pumps, which wirelessly connect to both the patient’s blood glucose meter and continuous glucose monitoring system. A remote controller and CareLink USB – a thumb-sized wireless device that plugs into a computer – are used to operate the devices; the remote controller sends insulin dosing commands to the pump and the CareLink USB can be used to download and share data with the patient’s health care provider.
The potential risk involves the wireless communication between the pumps and related devices such as the blood glucose meter and remote controller. The FDA has identified a cybersecurity vulnerability within the insulin pumps, and is concerned that a third party could connect to the device and change the pump’s settings. Insulin could be given in excess, causing hypoglycemia, or stopped, causing hyperglycemia or diabetic ketoacidosis.
Medtronic has identified 4,000 patients in the United States who are affected by the security weakness. Because the company is unable to adequately update or patch the device to remove the weakness, the FDA is working to ensure that Medtronic addresses the issue in any way possible, including helping patients with affected pumps switch to newer models.
“While we are not aware of patients who may have been harmed by this particular cybersecurity vulnerability, the risk of patient harm if such a vulnerability were left unaddressed is significant. The safety communication issued today contains recommendations for what actions patients and health care providers should take to avoid the risk this vulnerability could pose,” said Suzanne Schwartz, MD, MBA, deputy director of the Office of Strategic Partnerships and Technology Innovation.
Find the full press release on the FDA website.
The Food and Drug Administration has issued a warning to patients and health care providers that a pair of Medtronic insulin pumps are being recalled because of potential cybersecurity risks, according to a press release.
The affected devices are the MiniMed 508 and MiniMed Paradigm series insulin pumps, which wirelessly connect to both the patient’s blood glucose meter and continuous glucose monitoring system. A remote controller and CareLink USB – a thumb-sized wireless device that plugs into a computer – are used to operate the devices; the remote controller sends insulin dosing commands to the pump and the CareLink USB can be used to download and share data with the patient’s health care provider.
The potential risk involves the wireless communication between the pumps and related devices such as the blood glucose meter and remote controller. The FDA has identified a cybersecurity vulnerability within the insulin pumps, and is concerned that a third party could connect to the device and change the pump’s settings. Insulin could be given in excess, causing hypoglycemia, or stopped, causing hyperglycemia or diabetic ketoacidosis.
Medtronic has identified 4,000 patients in the United States who are affected by the security weakness. Because the company is unable to adequately update or patch the device to remove the weakness, the FDA is working to ensure that Medtronic addresses the issue in any way possible, including helping patients with affected pumps switch to newer models.
“While we are not aware of patients who may have been harmed by this particular cybersecurity vulnerability, the risk of patient harm if such a vulnerability were left unaddressed is significant. The safety communication issued today contains recommendations for what actions patients and health care providers should take to avoid the risk this vulnerability could pose,” said Suzanne Schwartz, MD, MBA, deputy director of the Office of Strategic Partnerships and Technology Innovation.
Find the full press release on the FDA website.
The Food and Drug Administration has issued a warning to patients and health care providers that a pair of Medtronic insulin pumps are being recalled because of potential cybersecurity risks, according to a press release.
The affected devices are the MiniMed 508 and MiniMed Paradigm series insulin pumps, which wirelessly connect to both the patient’s blood glucose meter and continuous glucose monitoring system. A remote controller and CareLink USB – a thumb-sized wireless device that plugs into a computer – are used to operate the devices; the remote controller sends insulin dosing commands to the pump and the CareLink USB can be used to download and share data with the patient’s health care provider.
The potential risk involves the wireless communication between the pumps and related devices such as the blood glucose meter and remote controller. The FDA has identified a cybersecurity vulnerability within the insulin pumps, and is concerned that a third party could connect to the device and change the pump’s settings. Insulin could be given in excess, causing hypoglycemia, or stopped, causing hyperglycemia or diabetic ketoacidosis.
Medtronic has identified 4,000 patients in the United States who are affected by the security weakness. Because the company is unable to adequately update or patch the device to remove the weakness, the FDA is working to ensure that Medtronic addresses the issue in any way possible, including helping patients with affected pumps switch to newer models.
“While we are not aware of patients who may have been harmed by this particular cybersecurity vulnerability, the risk of patient harm if such a vulnerability were left unaddressed is significant. The safety communication issued today contains recommendations for what actions patients and health care providers should take to avoid the risk this vulnerability could pose,” said Suzanne Schwartz, MD, MBA, deputy director of the Office of Strategic Partnerships and Technology Innovation.
Find the full press release on the FDA website.
ACIP endorses catch-up hepatitis A vaccinations
The Centers for Disease Control and Prevention’s Committee on Immunization Practices voted unanimously in support of three recommendations for the use of hepatitis A vaccines.
The committee recommended catch-up vaccination at any age for all children aged 2-18 years who had not previously received hepatitis A vaccination, recommended that all persons with HIV aged 1 year and older should be vaccinated with the hepatitis A vaccine, and approved updating the language in the full hepatitis A vaccine statement, “Prevention of Hepatitis A Virus Infection in The United States: Recommendations of The Advisory Committee on Immunization Practices.”
Catch-up vaccination will expand coverage to adolescents who might have missed it, and data show that the vaccine effectiveness is high, and the rates of adverse events are low in the child and adolescent population, said Noele Nelson, MD, of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, who presented the recommendations to the committee. “Recent outbreaks are occurring primarily among adults,” and many cases are among persons who use drugs or are homeless, she added.
Several committee members noted that the specific recommendations for catch-up in children and teens and for vaccination of HIV patients offer more opportunities for protection than risk-based recommendations. Catching up with vaccinating adolescents is “more effective than tracking down high-risk adults later in life,” noted Grace Lee, MD, of Lucile Packard Children’s Hospital at Stanford, Calif.
The committee also recommended that all persons with HIV aged 1 year and older should be vaccinated with the hepatitis A vaccine. Data on persons with HIV show that approximately 60% have at least one risk factor for hepatitis A, such as men who have sex with men or individuals engaged in intravenous drug use, said Dr. Nelson. Data also show that individuals with HIV are at increased risk for complications if they get hepatitis A.
The committee’s approval of the full hepatitis A vaccine statement included one notable change – the removal of clotting factor disorders as a high-risk group. The risk has decreased over time based on improvements such as better screening of source plasma, and this group is now at no greater risk than the general population, according to work group chair Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Committee on Immunization Practices voted unanimously in support of three recommendations for the use of hepatitis A vaccines.
The committee recommended catch-up vaccination at any age for all children aged 2-18 years who had not previously received hepatitis A vaccination, recommended that all persons with HIV aged 1 year and older should be vaccinated with the hepatitis A vaccine, and approved updating the language in the full hepatitis A vaccine statement, “Prevention of Hepatitis A Virus Infection in The United States: Recommendations of The Advisory Committee on Immunization Practices.”
Catch-up vaccination will expand coverage to adolescents who might have missed it, and data show that the vaccine effectiveness is high, and the rates of adverse events are low in the child and adolescent population, said Noele Nelson, MD, of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, who presented the recommendations to the committee. “Recent outbreaks are occurring primarily among adults,” and many cases are among persons who use drugs or are homeless, she added.
Several committee members noted that the specific recommendations for catch-up in children and teens and for vaccination of HIV patients offer more opportunities for protection than risk-based recommendations. Catching up with vaccinating adolescents is “more effective than tracking down high-risk adults later in life,” noted Grace Lee, MD, of Lucile Packard Children’s Hospital at Stanford, Calif.
The committee also recommended that all persons with HIV aged 1 year and older should be vaccinated with the hepatitis A vaccine. Data on persons with HIV show that approximately 60% have at least one risk factor for hepatitis A, such as men who have sex with men or individuals engaged in intravenous drug use, said Dr. Nelson. Data also show that individuals with HIV are at increased risk for complications if they get hepatitis A.
The committee’s approval of the full hepatitis A vaccine statement included one notable change – the removal of clotting factor disorders as a high-risk group. The risk has decreased over time based on improvements such as better screening of source plasma, and this group is now at no greater risk than the general population, according to work group chair Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Committee on Immunization Practices voted unanimously in support of three recommendations for the use of hepatitis A vaccines.
The committee recommended catch-up vaccination at any age for all children aged 2-18 years who had not previously received hepatitis A vaccination, recommended that all persons with HIV aged 1 year and older should be vaccinated with the hepatitis A vaccine, and approved updating the language in the full hepatitis A vaccine statement, “Prevention of Hepatitis A Virus Infection in The United States: Recommendations of The Advisory Committee on Immunization Practices.”
Catch-up vaccination will expand coverage to adolescents who might have missed it, and data show that the vaccine effectiveness is high, and the rates of adverse events are low in the child and adolescent population, said Noele Nelson, MD, of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, who presented the recommendations to the committee. “Recent outbreaks are occurring primarily among adults,” and many cases are among persons who use drugs or are homeless, she added.
Several committee members noted that the specific recommendations for catch-up in children and teens and for vaccination of HIV patients offer more opportunities for protection than risk-based recommendations. Catching up with vaccinating adolescents is “more effective than tracking down high-risk adults later in life,” noted Grace Lee, MD, of Lucile Packard Children’s Hospital at Stanford, Calif.
The committee also recommended that all persons with HIV aged 1 year and older should be vaccinated with the hepatitis A vaccine. Data on persons with HIV show that approximately 60% have at least one risk factor for hepatitis A, such as men who have sex with men or individuals engaged in intravenous drug use, said Dr. Nelson. Data also show that individuals with HIV are at increased risk for complications if they get hepatitis A.
The committee’s approval of the full hepatitis A vaccine statement included one notable change – the removal of clotting factor disorders as a high-risk group. The risk has decreased over time based on improvements such as better screening of source plasma, and this group is now at no greater risk than the general population, according to work group chair Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Severity, itch improvements remain steady with ruxolitinib for atopic dermatitis
MILAN – A in the 4-week open-label period of a 16-week randomized phase 2 study of adults with mild to moderate atopic dermatitis (AD), Leon H. Kircik, MD, said at the World Congress of Dermatology.
Improvements in disease severity and itch in patients receiving 1.5% ruxolitinib cream twice daily were sustained over the open-label period, said Dr. Kircik, a dermatologist in Louisville, Ky., affiliated with Mount Sinai Medical Center, New York.
Patients who switched from vehicle or 0.1% triamcinolone cream to the JAK1/2 selective inhibitor in the open-label period also experienced rapid improvements in disease severity and itch.
“This is a novel treatment that’s a topical JAK inhibitor, which so far we don’t have any in the market for atopic dermatitis, and it does have a very good efficacy and safety profile,” Dr. Kircik said during an oral presentation at the meeting.
Janus kinases modulate inflammatory cytokines implicated in AD, and may also directly modulate itch, Dr. Kircik noted.
The study comprised 307 adults with mild to moderate AD (Investigator’s Global Assessment [IGA] score of 2 or 3) and body surface area involvement of 3%-20%. They were randomized equally to six arms, including vehicle, triamcinolone cream, and ruxolitinib at dosages of 0.15%, 0.5%, 1.5% once daily, or the target dose level of 1.5% twice daily.
After an 8-week double-blind period, there was a 4-week open-label period during which patients randomized to vehicle or triamcinolone were switched to ruxolitinib, and then a 4-week follow-up period during which no treatment was given, Dr. Kircik said.
The mean age of the patients was 35 years, 54% were female, and the median duration of disease was 20.8 years.
In the double-blind period, 1.5% ruxolitinib cream twice daily significantly improved Eczema Area and Severity Index (EASI) score versus vehicle, Dr. Kircik said.
The mean change in EASI scores at weeks 2, 4, and 6 were 52.7%, 71.6%, and 78.5% for ruxolitinib, versus 4.8%, 15.5%, and 26.9% for vehicle (P less than .001 for all comparisons), according to Dr. Kircik.
The patients on the target ruxolitinib dose maintained the improvements in EASI score throughout the open label period, with mean improvement from baseline reaching 81.4% by week 10 and 84.9% by week 12.
Meanwhile, there was a sharp increase in mean EASI score improvement in patients switched to ruxolitinib, according to Dr. Kircik. In the vehicle arm, mean improvement leapt from 26.9% at week 8 to 78.4% by week 12.
Significant reductions in itch scores were seen within 36 hours of starting the 1.5% ruxolitinib cream, with itch Numeric Rating Scale (NRS) scores of –1.8 versus –0.2 for vehicle at that time point (P less than .0001), he added.
Reduction in itch score was similarly maintained in the ruxolitinib target dose group, and rapidly fell to similar levels for patients switched over to that treatment in the open-label period, Dr. Kircik said.
The target ruxolitinib dose was also noninferior to triamcinolone cream, for which mean change in EASI scores at weeks 2 and 4 were 40.0% and 59.8%, respectively.
Recruitment of patients in phase 3 studies of ruxolitinib cream for AD has just started, Dr. Kircik said.
The TRuE AD1 and TRuE AD2 studies are set to enroll 1,200 adolescents and adults with AD who will be randomized to ruxolitinib cream or vehicle, according to listings on ClinicalTrials.gov.
Dr. Kircik disclosed ties to several companies including Incyte, which was the sponsor of the phase 2 study and the phase 3 studies.
MILAN – A in the 4-week open-label period of a 16-week randomized phase 2 study of adults with mild to moderate atopic dermatitis (AD), Leon H. Kircik, MD, said at the World Congress of Dermatology.
Improvements in disease severity and itch in patients receiving 1.5% ruxolitinib cream twice daily were sustained over the open-label period, said Dr. Kircik, a dermatologist in Louisville, Ky., affiliated with Mount Sinai Medical Center, New York.
Patients who switched from vehicle or 0.1% triamcinolone cream to the JAK1/2 selective inhibitor in the open-label period also experienced rapid improvements in disease severity and itch.
“This is a novel treatment that’s a topical JAK inhibitor, which so far we don’t have any in the market for atopic dermatitis, and it does have a very good efficacy and safety profile,” Dr. Kircik said during an oral presentation at the meeting.
Janus kinases modulate inflammatory cytokines implicated in AD, and may also directly modulate itch, Dr. Kircik noted.
The study comprised 307 adults with mild to moderate AD (Investigator’s Global Assessment [IGA] score of 2 or 3) and body surface area involvement of 3%-20%. They were randomized equally to six arms, including vehicle, triamcinolone cream, and ruxolitinib at dosages of 0.15%, 0.5%, 1.5% once daily, or the target dose level of 1.5% twice daily.
After an 8-week double-blind period, there was a 4-week open-label period during which patients randomized to vehicle or triamcinolone were switched to ruxolitinib, and then a 4-week follow-up period during which no treatment was given, Dr. Kircik said.
The mean age of the patients was 35 years, 54% were female, and the median duration of disease was 20.8 years.
In the double-blind period, 1.5% ruxolitinib cream twice daily significantly improved Eczema Area and Severity Index (EASI) score versus vehicle, Dr. Kircik said.
The mean change in EASI scores at weeks 2, 4, and 6 were 52.7%, 71.6%, and 78.5% for ruxolitinib, versus 4.8%, 15.5%, and 26.9% for vehicle (P less than .001 for all comparisons), according to Dr. Kircik.
The patients on the target ruxolitinib dose maintained the improvements in EASI score throughout the open label period, with mean improvement from baseline reaching 81.4% by week 10 and 84.9% by week 12.
Meanwhile, there was a sharp increase in mean EASI score improvement in patients switched to ruxolitinib, according to Dr. Kircik. In the vehicle arm, mean improvement leapt from 26.9% at week 8 to 78.4% by week 12.
Significant reductions in itch scores were seen within 36 hours of starting the 1.5% ruxolitinib cream, with itch Numeric Rating Scale (NRS) scores of –1.8 versus –0.2 for vehicle at that time point (P less than .0001), he added.
Reduction in itch score was similarly maintained in the ruxolitinib target dose group, and rapidly fell to similar levels for patients switched over to that treatment in the open-label period, Dr. Kircik said.
The target ruxolitinib dose was also noninferior to triamcinolone cream, for which mean change in EASI scores at weeks 2 and 4 were 40.0% and 59.8%, respectively.
Recruitment of patients in phase 3 studies of ruxolitinib cream for AD has just started, Dr. Kircik said.
The TRuE AD1 and TRuE AD2 studies are set to enroll 1,200 adolescents and adults with AD who will be randomized to ruxolitinib cream or vehicle, according to listings on ClinicalTrials.gov.
Dr. Kircik disclosed ties to several companies including Incyte, which was the sponsor of the phase 2 study and the phase 3 studies.
MILAN – A in the 4-week open-label period of a 16-week randomized phase 2 study of adults with mild to moderate atopic dermatitis (AD), Leon H. Kircik, MD, said at the World Congress of Dermatology.
Improvements in disease severity and itch in patients receiving 1.5% ruxolitinib cream twice daily were sustained over the open-label period, said Dr. Kircik, a dermatologist in Louisville, Ky., affiliated with Mount Sinai Medical Center, New York.
Patients who switched from vehicle or 0.1% triamcinolone cream to the JAK1/2 selective inhibitor in the open-label period also experienced rapid improvements in disease severity and itch.
“This is a novel treatment that’s a topical JAK inhibitor, which so far we don’t have any in the market for atopic dermatitis, and it does have a very good efficacy and safety profile,” Dr. Kircik said during an oral presentation at the meeting.
Janus kinases modulate inflammatory cytokines implicated in AD, and may also directly modulate itch, Dr. Kircik noted.
The study comprised 307 adults with mild to moderate AD (Investigator’s Global Assessment [IGA] score of 2 or 3) and body surface area involvement of 3%-20%. They were randomized equally to six arms, including vehicle, triamcinolone cream, and ruxolitinib at dosages of 0.15%, 0.5%, 1.5% once daily, or the target dose level of 1.5% twice daily.
After an 8-week double-blind period, there was a 4-week open-label period during which patients randomized to vehicle or triamcinolone were switched to ruxolitinib, and then a 4-week follow-up period during which no treatment was given, Dr. Kircik said.
The mean age of the patients was 35 years, 54% were female, and the median duration of disease was 20.8 years.
In the double-blind period, 1.5% ruxolitinib cream twice daily significantly improved Eczema Area and Severity Index (EASI) score versus vehicle, Dr. Kircik said.
The mean change in EASI scores at weeks 2, 4, and 6 were 52.7%, 71.6%, and 78.5% for ruxolitinib, versus 4.8%, 15.5%, and 26.9% for vehicle (P less than .001 for all comparisons), according to Dr. Kircik.
The patients on the target ruxolitinib dose maintained the improvements in EASI score throughout the open label period, with mean improvement from baseline reaching 81.4% by week 10 and 84.9% by week 12.
Meanwhile, there was a sharp increase in mean EASI score improvement in patients switched to ruxolitinib, according to Dr. Kircik. In the vehicle arm, mean improvement leapt from 26.9% at week 8 to 78.4% by week 12.
Significant reductions in itch scores were seen within 36 hours of starting the 1.5% ruxolitinib cream, with itch Numeric Rating Scale (NRS) scores of –1.8 versus –0.2 for vehicle at that time point (P less than .0001), he added.
Reduction in itch score was similarly maintained in the ruxolitinib target dose group, and rapidly fell to similar levels for patients switched over to that treatment in the open-label period, Dr. Kircik said.
The target ruxolitinib dose was also noninferior to triamcinolone cream, for which mean change in EASI scores at weeks 2 and 4 were 40.0% and 59.8%, respectively.
Recruitment of patients in phase 3 studies of ruxolitinib cream for AD has just started, Dr. Kircik said.
The TRuE AD1 and TRuE AD2 studies are set to enroll 1,200 adolescents and adults with AD who will be randomized to ruxolitinib cream or vehicle, according to listings on ClinicalTrials.gov.
Dr. Kircik disclosed ties to several companies including Incyte, which was the sponsor of the phase 2 study and the phase 3 studies.
REPORTING FROM WCD2019