User login
Severe Acne Fulminans Following Low-Dose Isotretinoin and Testosterone Use
To the Editor:
Acne fulminans (AF), the most severe form of acne, is a rare condition with an incidence of less than 1% of total acne cases.1 Adolescent boys are the most susceptible group of patients.2 Painful inflammatory pustules that transform into deep ulcerations covered by abundant hemorrhagic crust are typical of AF. Commonly affected areas include the face, back, neck, and chest. Additionally, fever and polyarthralgia may be present, and there often is myopathy due to rapid weight loss.3,4 Less often, erythema nodosum and splenomegaly may be observed.5 Laboratory testing also may reveal markers of systemic inflammation such as leukocytosis with neutrophilia, elevated C-reactive protein levels, increased erythrocyte sedimentation rate, and thrombocytosis. Anemia and elevated hepatic enzyme levels also may be present in AF.2 It is suspected that AF may be induced by low doses of isotretinoin therapy with concomitant inherited susceptibility.6
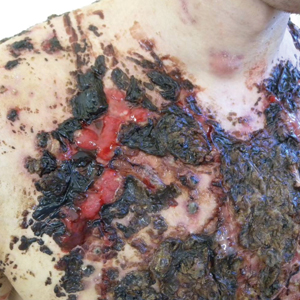
We report the case of a 21-year-old man who was referred to the Department of Dermatology by his primary care physician for evaluation of severe hemorrhagic lesions on the trunk following use of oral isotretinoin (Figure 1). Prior to development of the lesions, the patient had started weekly intramuscular injections of testosterone 500 mg, which he purchased online without consulting a physician, to address muscle mass reduction associated with sudden weight loss from intense physical training. After 8 months of testosterone supplementation along with continued physical training, the patient presented to his primary care physician for treatment of acne vulgaris on the back and trunk of 2 months’ duration. Oral isotretinoin 20 mg once daily was initiated; however, the patient reported that the acne lesions showed progression after 1 month of treatment. Isotretinoin was increased to a more weight-appropriate dosage of 60 mg once daily 2 weeks before admission to our dermatology clinic.
At the current presentation, dermatologic examination revealed numerous inflamed ulcerations covered by a hemorrhagic crust on the back and trunk. The patient also reported knee, elbow, and inguinal pain, especially at night. No fever or loss of appetite was reported. The patient was otherwise healthy and had no remarkable family history of acne or other dermatologic diseases.
Laboratory testing showed leukocytosis (11,000/µL [reference range, 4500–11,000/µL]), an elevated C-reactive protein level (66 mg/L [reference range, 0.08–3.1 mg/L]), and an elevated erythrocyte sedimentation rate (46 mm/h [reference range, 0–20 mm/h]). There were laboratory and clinical signs of a secondary bacterial infection in the affected areas, and a culture of secretions collected from lesions on the back grew Staphylococcus aureus with sensitivity to erythromycin, clindamycin, doxycycline, and trimethoprim-sulfamethoxazole and resistance to penicillin. A diagnosis of AF was made based on the clinical presentation and systemic symptoms, and anabolic-androgenic steroids and low-dose isotretinoin were identified as etiologic factors.
Treatment initially included cessation of isotretinoin and administration of prednisone, omeprazole, clindamycin, and doxycycline. Prednisone was given at a dosage of 40 mg once daily for 1 week, then decreased by 5 mg every 7 days. Omeprazole was given concurrently as prophylaxis for the gastrointestinal tract side effects of long-term prednisone use. Clindamycin was given at a dosage of 300 mg 3 times daily. Doxycycline was given for 6 weeks at a dosage of 100 mg twice daily. Topical octenidine dihydrochloride also was given.
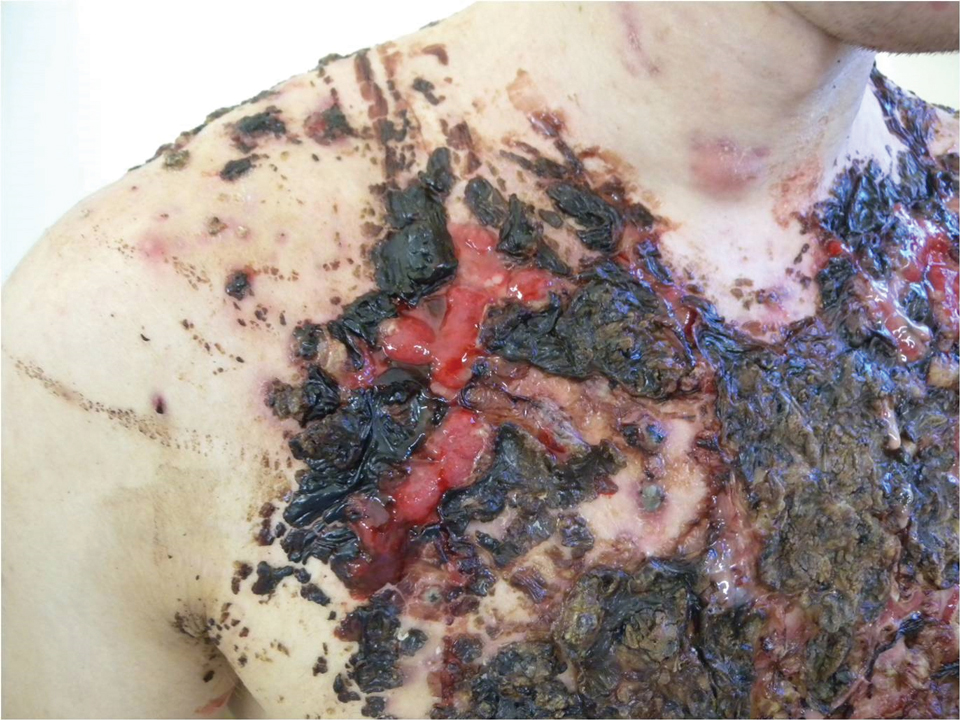
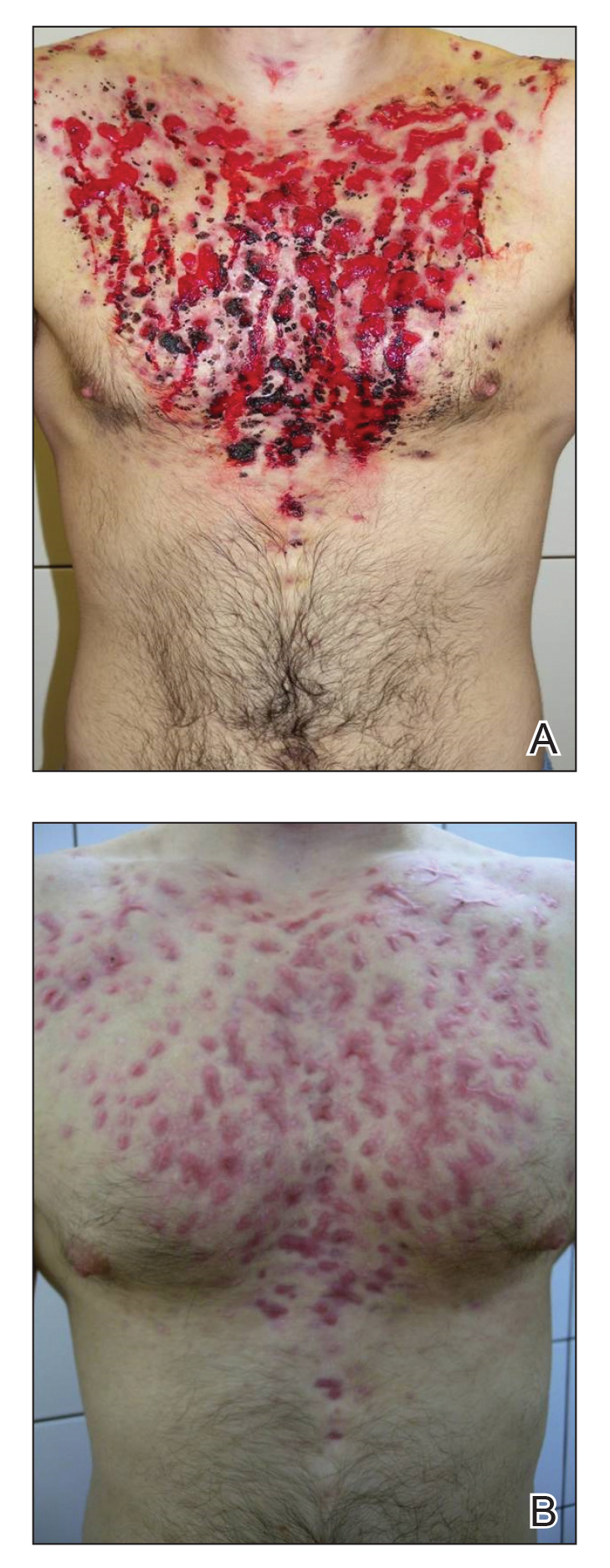
Marked improvement was noted after 24 hours (Figure 2) as well as on the third day of treatment (Figure 3A). After 6 weeks, only disfiguring scars were visible (Figure 3B). Oral isotretinoin was reincorporated after 8 weeks and was subsequently discontinued after 5 months of therapy with a cumulative dose of 150 mg/kg.

It is important to differentiate AF from exacerbation of acne vulgaris because patients typically have mild or moderate acne vulgaris before the onset of acute symptoms.1 Acne fulminans is characterized by systemic symptoms such as myalgia, polyarthralgia, fatigue, and osteolytic bone lesions.1,7 Additionally, hematologic symptoms such as fever, leukocytosis, anemia, splenomegaly, and hepatomegaly may be present.1,5,7 Our patient demonstrated the polysymptomatic form of AF. The patient had severe acne with a tendency to scar. There also were some systemic manifestations such as polyarthralgia, weight loss, leukocytosis, an elevated erythrocyte sedimentation rate, and an elevated C-reactive protein level.
The clinical diagnosis in our patient also was supported by the hypothesis that heredity, overactive immune reactions, bacterial infections, and use of some drugs (eg, isotretinoin, tetracycline, testosterone) can trigger AF.8 The most well-known theory is that low doses of isotretinoin induce AF.6 The majority of cases are caused by doses of less than 20 mg/kg once daily, but there have been reports of patients using full doses and developing this condition.9 The fact that the use of low- and high-dose isotretinoin can provoke AF suggests an idiosyncratic reaction that is not clearly dose related. The most dangerous triggering factor of AF is concomitant usage of testosterone and isotretinoin.10 Our patient used testosterone injections to increase muscle mass and underwent treatment with isotretinoin for acne.
Treatment of AF is controversial, as there is no standard therapy. Currently, steroids and isotretinoin are the treatments of choice. Antibiotic use is controversial because of a lack of randomized trials.11
In the first stage of treatment, prednisone 0.5 to 1 mg/kg once daily is recommended as an initial anti-inflammatory therapy, with gradual dose reduction. According to evidence-based recommendations, a low dose of isotretinoin can be introduced after crusted lesions have healed. The overlapping therapy with steroids and isotretinoin should be provided for at least 4 weeks. High-potency topical corticosteroids can be used on granulation tissue, which can shorten the systemic treatment with prednisone or can be an alternative treatment for patients with contraindications to systemic corticosteroids.11
Additionally, local care of the lesions including compresses and topical emollients is crucial. There are some case reports in which there is introduction of high doses of isotretinoin, subsequently with systemic steroids.7,8,12 Seukeran and Cunliffe5 proved that it is beneficial to give acne prophylaxis to prevent further episodes. Our patient was similarly treated with systemic steroids and isotretinoin. Treatment guidelines for AF do not recommend oral antibiotics,11 but data are limited in the case of isotretinoin-induced AF. Our patient was given doxycycline concomitant with systemic steroids, which was necessary due to signs of secondary infection from a lesion culture. Doxycycline was stopped when isotretinoin treatment was initiated to prevent pseudotumor cerebri. The patient achieved good clinical improvement with no relapse.
Using isotretinoin to treat acne vulgaris has many benefits, despite the possibility of developing AF as an extremely rare complication. Clinicians should be aware of the risk of this complication to make the diagnosis and provide appropriate care, especially in young men. It is particularly important to consider the possibility of concomitant testosterone and isotretinoin when documenting the patient’s medical history.
- Romiti R, Jansen T, Plewig G. Acne fulminans. An Bras Dermatol. 2000;75:611-617.
- Karvonen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol. 1993;28:572-579.
- Kelly AP, Burns RE. Acute febrile ulcerative conglobate acne with polyarthralgia. Arch Dermatol. 1971;104:182-187.
- Plewig G, Kligman AM. Vitamin A acid in acneiform dermatoses. Acta Derm Venereol Suppl. 1975;74:119-127.
- Seukeran DC, Cunliffe WJ. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol. 1999;141:307-309.
- Kraus SL, Emmert S, Schön MP, et al. The dark side of beauty: acne fulminans induced by anabolic steroids in a male bodybuilder. Arch Dermatol. 2012;148:1210-1212.
- Jansen T, Plewig G. Acne fulminans. Int J Dermatol. 1998;37:254-257.
- Zanelato TP, Gontijo GM, Alves CA, et al. Disabling acne fulminans. An Bras Dermatol. 2011;86:9-12.
- Azulay DR, Abulafia LA, Costa JAN, et al. Tecido de granulação exuberante. efeito colateral da terapêutica com isotretinoína. An Bras Dermatol. 1985;60:179-182.
- Traupe H, von Mühlendahl KE, Brämswig J, et al. Acne of the fulminans type following testosterone therapy in three excessively tall boys. Arch Dermatol. 1988;124:414-417.
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117.
- Honma M, Murakami M, Iinuma S, et al. Acne fulminans following measles infection. J Dermatol. 2009;36:471-473.
To the Editor:
Acne fulminans (AF), the most severe form of acne, is a rare condition with an incidence of less than 1% of total acne cases.1 Adolescent boys are the most susceptible group of patients.2 Painful inflammatory pustules that transform into deep ulcerations covered by abundant hemorrhagic crust are typical of AF. Commonly affected areas include the face, back, neck, and chest. Additionally, fever and polyarthralgia may be present, and there often is myopathy due to rapid weight loss.3,4 Less often, erythema nodosum and splenomegaly may be observed.5 Laboratory testing also may reveal markers of systemic inflammation such as leukocytosis with neutrophilia, elevated C-reactive protein levels, increased erythrocyte sedimentation rate, and thrombocytosis. Anemia and elevated hepatic enzyme levels also may be present in AF.2 It is suspected that AF may be induced by low doses of isotretinoin therapy with concomitant inherited susceptibility.6
We report the case of a 21-year-old man who was referred to the Department of Dermatology by his primary care physician for evaluation of severe hemorrhagic lesions on the trunk following use of oral isotretinoin (Figure 1). Prior to development of the lesions, the patient had started weekly intramuscular injections of testosterone 500 mg, which he purchased online without consulting a physician, to address muscle mass reduction associated with sudden weight loss from intense physical training. After 8 months of testosterone supplementation along with continued physical training, the patient presented to his primary care physician for treatment of acne vulgaris on the back and trunk of 2 months’ duration. Oral isotretinoin 20 mg once daily was initiated; however, the patient reported that the acne lesions showed progression after 1 month of treatment. Isotretinoin was increased to a more weight-appropriate dosage of 60 mg once daily 2 weeks before admission to our dermatology clinic.

At the current presentation, dermatologic examination revealed numerous inflamed ulcerations covered by a hemorrhagic crust on the back and trunk. The patient also reported knee, elbow, and inguinal pain, especially at night. No fever or loss of appetite was reported. The patient was otherwise healthy and had no remarkable family history of acne or other dermatologic diseases.
Laboratory testing showed leukocytosis (11,000/µL [reference range, 4500–11,000/µL]), an elevated C-reactive protein level (66 mg/L [reference range, 0.08–3.1 mg/L]), and an elevated erythrocyte sedimentation rate (46 mm/h [reference range, 0–20 mm/h]). There were laboratory and clinical signs of a secondary bacterial infection in the affected areas, and a culture of secretions collected from lesions on the back grew Staphylococcus aureus with sensitivity to erythromycin, clindamycin, doxycycline, and trimethoprim-sulfamethoxazole and resistance to penicillin. A diagnosis of AF was made based on the clinical presentation and systemic symptoms, and anabolic-androgenic steroids and low-dose isotretinoin were identified as etiologic factors.
Treatment initially included cessation of isotretinoin and administration of prednisone, omeprazole, clindamycin, and doxycycline. Prednisone was given at a dosage of 40 mg once daily for 1 week, then decreased by 5 mg every 7 days. Omeprazole was given concurrently as prophylaxis for the gastrointestinal tract side effects of long-term prednisone use. Clindamycin was given at a dosage of 300 mg 3 times daily. Doxycycline was given for 6 weeks at a dosage of 100 mg twice daily. Topical octenidine dihydrochloride also was given.
Marked improvement was noted after 24 hours (Figure 2) as well as on the third day of treatment (Figure 3A). After 6 weeks, only disfiguring scars were visible (Figure 3B). Oral isotretinoin was reincorporated after 8 weeks and was subsequently discontinued after 5 months of therapy with a cumulative dose of 150 mg/kg.


It is important to differentiate AF from exacerbation of acne vulgaris because patients typically have mild or moderate acne vulgaris before the onset of acute symptoms.1 Acne fulminans is characterized by systemic symptoms such as myalgia, polyarthralgia, fatigue, and osteolytic bone lesions.1,7 Additionally, hematologic symptoms such as fever, leukocytosis, anemia, splenomegaly, and hepatomegaly may be present.1,5,7 Our patient demonstrated the polysymptomatic form of AF. The patient had severe acne with a tendency to scar. There also were some systemic manifestations such as polyarthralgia, weight loss, leukocytosis, an elevated erythrocyte sedimentation rate, and an elevated C-reactive protein level.
The clinical diagnosis in our patient also was supported by the hypothesis that heredity, overactive immune reactions, bacterial infections, and use of some drugs (eg, isotretinoin, tetracycline, testosterone) can trigger AF.8 The most well-known theory is that low doses of isotretinoin induce AF.6 The majority of cases are caused by doses of less than 20 mg/kg once daily, but there have been reports of patients using full doses and developing this condition.9 The fact that the use of low- and high-dose isotretinoin can provoke AF suggests an idiosyncratic reaction that is not clearly dose related. The most dangerous triggering factor of AF is concomitant usage of testosterone and isotretinoin.10 Our patient used testosterone injections to increase muscle mass and underwent treatment with isotretinoin for acne.
Treatment of AF is controversial, as there is no standard therapy. Currently, steroids and isotretinoin are the treatments of choice. Antibiotic use is controversial because of a lack of randomized trials.11
In the first stage of treatment, prednisone 0.5 to 1 mg/kg once daily is recommended as an initial anti-inflammatory therapy, with gradual dose reduction. According to evidence-based recommendations, a low dose of isotretinoin can be introduced after crusted lesions have healed. The overlapping therapy with steroids and isotretinoin should be provided for at least 4 weeks. High-potency topical corticosteroids can be used on granulation tissue, which can shorten the systemic treatment with prednisone or can be an alternative treatment for patients with contraindications to systemic corticosteroids.11
Additionally, local care of the lesions including compresses and topical emollients is crucial. There are some case reports in which there is introduction of high doses of isotretinoin, subsequently with systemic steroids.7,8,12 Seukeran and Cunliffe5 proved that it is beneficial to give acne prophylaxis to prevent further episodes. Our patient was similarly treated with systemic steroids and isotretinoin. Treatment guidelines for AF do not recommend oral antibiotics,11 but data are limited in the case of isotretinoin-induced AF. Our patient was given doxycycline concomitant with systemic steroids, which was necessary due to signs of secondary infection from a lesion culture. Doxycycline was stopped when isotretinoin treatment was initiated to prevent pseudotumor cerebri. The patient achieved good clinical improvement with no relapse.
Using isotretinoin to treat acne vulgaris has many benefits, despite the possibility of developing AF as an extremely rare complication. Clinicians should be aware of the risk of this complication to make the diagnosis and provide appropriate care, especially in young men. It is particularly important to consider the possibility of concomitant testosterone and isotretinoin when documenting the patient’s medical history.
To the Editor:
Acne fulminans (AF), the most severe form of acne, is a rare condition with an incidence of less than 1% of total acne cases.1 Adolescent boys are the most susceptible group of patients.2 Painful inflammatory pustules that transform into deep ulcerations covered by abundant hemorrhagic crust are typical of AF. Commonly affected areas include the face, back, neck, and chest. Additionally, fever and polyarthralgia may be present, and there often is myopathy due to rapid weight loss.3,4 Less often, erythema nodosum and splenomegaly may be observed.5 Laboratory testing also may reveal markers of systemic inflammation such as leukocytosis with neutrophilia, elevated C-reactive protein levels, increased erythrocyte sedimentation rate, and thrombocytosis. Anemia and elevated hepatic enzyme levels also may be present in AF.2 It is suspected that AF may be induced by low doses of isotretinoin therapy with concomitant inherited susceptibility.6
We report the case of a 21-year-old man who was referred to the Department of Dermatology by his primary care physician for evaluation of severe hemorrhagic lesions on the trunk following use of oral isotretinoin (Figure 1). Prior to development of the lesions, the patient had started weekly intramuscular injections of testosterone 500 mg, which he purchased online without consulting a physician, to address muscle mass reduction associated with sudden weight loss from intense physical training. After 8 months of testosterone supplementation along with continued physical training, the patient presented to his primary care physician for treatment of acne vulgaris on the back and trunk of 2 months’ duration. Oral isotretinoin 20 mg once daily was initiated; however, the patient reported that the acne lesions showed progression after 1 month of treatment. Isotretinoin was increased to a more weight-appropriate dosage of 60 mg once daily 2 weeks before admission to our dermatology clinic.

At the current presentation, dermatologic examination revealed numerous inflamed ulcerations covered by a hemorrhagic crust on the back and trunk. The patient also reported knee, elbow, and inguinal pain, especially at night. No fever or loss of appetite was reported. The patient was otherwise healthy and had no remarkable family history of acne or other dermatologic diseases.
Laboratory testing showed leukocytosis (11,000/µL [reference range, 4500–11,000/µL]), an elevated C-reactive protein level (66 mg/L [reference range, 0.08–3.1 mg/L]), and an elevated erythrocyte sedimentation rate (46 mm/h [reference range, 0–20 mm/h]). There were laboratory and clinical signs of a secondary bacterial infection in the affected areas, and a culture of secretions collected from lesions on the back grew Staphylococcus aureus with sensitivity to erythromycin, clindamycin, doxycycline, and trimethoprim-sulfamethoxazole and resistance to penicillin. A diagnosis of AF was made based on the clinical presentation and systemic symptoms, and anabolic-androgenic steroids and low-dose isotretinoin were identified as etiologic factors.
Treatment initially included cessation of isotretinoin and administration of prednisone, omeprazole, clindamycin, and doxycycline. Prednisone was given at a dosage of 40 mg once daily for 1 week, then decreased by 5 mg every 7 days. Omeprazole was given concurrently as prophylaxis for the gastrointestinal tract side effects of long-term prednisone use. Clindamycin was given at a dosage of 300 mg 3 times daily. Doxycycline was given for 6 weeks at a dosage of 100 mg twice daily. Topical octenidine dihydrochloride also was given.
Marked improvement was noted after 24 hours (Figure 2) as well as on the third day of treatment (Figure 3A). After 6 weeks, only disfiguring scars were visible (Figure 3B). Oral isotretinoin was reincorporated after 8 weeks and was subsequently discontinued after 5 months of therapy with a cumulative dose of 150 mg/kg.


It is important to differentiate AF from exacerbation of acne vulgaris because patients typically have mild or moderate acne vulgaris before the onset of acute symptoms.1 Acne fulminans is characterized by systemic symptoms such as myalgia, polyarthralgia, fatigue, and osteolytic bone lesions.1,7 Additionally, hematologic symptoms such as fever, leukocytosis, anemia, splenomegaly, and hepatomegaly may be present.1,5,7 Our patient demonstrated the polysymptomatic form of AF. The patient had severe acne with a tendency to scar. There also were some systemic manifestations such as polyarthralgia, weight loss, leukocytosis, an elevated erythrocyte sedimentation rate, and an elevated C-reactive protein level.
The clinical diagnosis in our patient also was supported by the hypothesis that heredity, overactive immune reactions, bacterial infections, and use of some drugs (eg, isotretinoin, tetracycline, testosterone) can trigger AF.8 The most well-known theory is that low doses of isotretinoin induce AF.6 The majority of cases are caused by doses of less than 20 mg/kg once daily, but there have been reports of patients using full doses and developing this condition.9 The fact that the use of low- and high-dose isotretinoin can provoke AF suggests an idiosyncratic reaction that is not clearly dose related. The most dangerous triggering factor of AF is concomitant usage of testosterone and isotretinoin.10 Our patient used testosterone injections to increase muscle mass and underwent treatment with isotretinoin for acne.
Treatment of AF is controversial, as there is no standard therapy. Currently, steroids and isotretinoin are the treatments of choice. Antibiotic use is controversial because of a lack of randomized trials.11
In the first stage of treatment, prednisone 0.5 to 1 mg/kg once daily is recommended as an initial anti-inflammatory therapy, with gradual dose reduction. According to evidence-based recommendations, a low dose of isotretinoin can be introduced after crusted lesions have healed. The overlapping therapy with steroids and isotretinoin should be provided for at least 4 weeks. High-potency topical corticosteroids can be used on granulation tissue, which can shorten the systemic treatment with prednisone or can be an alternative treatment for patients with contraindications to systemic corticosteroids.11
Additionally, local care of the lesions including compresses and topical emollients is crucial. There are some case reports in which there is introduction of high doses of isotretinoin, subsequently with systemic steroids.7,8,12 Seukeran and Cunliffe5 proved that it is beneficial to give acne prophylaxis to prevent further episodes. Our patient was similarly treated with systemic steroids and isotretinoin. Treatment guidelines for AF do not recommend oral antibiotics,11 but data are limited in the case of isotretinoin-induced AF. Our patient was given doxycycline concomitant with systemic steroids, which was necessary due to signs of secondary infection from a lesion culture. Doxycycline was stopped when isotretinoin treatment was initiated to prevent pseudotumor cerebri. The patient achieved good clinical improvement with no relapse.
Using isotretinoin to treat acne vulgaris has many benefits, despite the possibility of developing AF as an extremely rare complication. Clinicians should be aware of the risk of this complication to make the diagnosis and provide appropriate care, especially in young men. It is particularly important to consider the possibility of concomitant testosterone and isotretinoin when documenting the patient’s medical history.
- Romiti R, Jansen T, Plewig G. Acne fulminans. An Bras Dermatol. 2000;75:611-617.
- Karvonen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol. 1993;28:572-579.
- Kelly AP, Burns RE. Acute febrile ulcerative conglobate acne with polyarthralgia. Arch Dermatol. 1971;104:182-187.
- Plewig G, Kligman AM. Vitamin A acid in acneiform dermatoses. Acta Derm Venereol Suppl. 1975;74:119-127.
- Seukeran DC, Cunliffe WJ. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol. 1999;141:307-309.
- Kraus SL, Emmert S, Schön MP, et al. The dark side of beauty: acne fulminans induced by anabolic steroids in a male bodybuilder. Arch Dermatol. 2012;148:1210-1212.
- Jansen T, Plewig G. Acne fulminans. Int J Dermatol. 1998;37:254-257.
- Zanelato TP, Gontijo GM, Alves CA, et al. Disabling acne fulminans. An Bras Dermatol. 2011;86:9-12.
- Azulay DR, Abulafia LA, Costa JAN, et al. Tecido de granulação exuberante. efeito colateral da terapêutica com isotretinoína. An Bras Dermatol. 1985;60:179-182.
- Traupe H, von Mühlendahl KE, Brämswig J, et al. Acne of the fulminans type following testosterone therapy in three excessively tall boys. Arch Dermatol. 1988;124:414-417.
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117.
- Honma M, Murakami M, Iinuma S, et al. Acne fulminans following measles infection. J Dermatol. 2009;36:471-473.
- Romiti R, Jansen T, Plewig G. Acne fulminans. An Bras Dermatol. 2000;75:611-617.
- Karvonen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol. 1993;28:572-579.
- Kelly AP, Burns RE. Acute febrile ulcerative conglobate acne with polyarthralgia. Arch Dermatol. 1971;104:182-187.
- Plewig G, Kligman AM. Vitamin A acid in acneiform dermatoses. Acta Derm Venereol Suppl. 1975;74:119-127.
- Seukeran DC, Cunliffe WJ. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol. 1999;141:307-309.
- Kraus SL, Emmert S, Schön MP, et al. The dark side of beauty: acne fulminans induced by anabolic steroids in a male bodybuilder. Arch Dermatol. 2012;148:1210-1212.
- Jansen T, Plewig G. Acne fulminans. Int J Dermatol. 1998;37:254-257.
- Zanelato TP, Gontijo GM, Alves CA, et al. Disabling acne fulminans. An Bras Dermatol. 2011;86:9-12.
- Azulay DR, Abulafia LA, Costa JAN, et al. Tecido de granulação exuberante. efeito colateral da terapêutica com isotretinoína. An Bras Dermatol. 1985;60:179-182.
- Traupe H, von Mühlendahl KE, Brämswig J, et al. Acne of the fulminans type following testosterone therapy in three excessively tall boys. Arch Dermatol. 1988;124:414-417.
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117.
- Honma M, Murakami M, Iinuma S, et al. Acne fulminans following measles infection. J Dermatol. 2009;36:471-473.
Practice Points
- Acne fulminans, the most severe form of acne, is characterized by deep ulcerations covered by a hemorrhagic crust. It is commonly associated with fever, polyarthralgia, and myopathy caused by rapid weight loss.
- This rare condition is recognized as a potential complication of oral isotretinoin therapy.
Genetic variant could dictate rituximab response in lupus
MADRID – Response to rituximab in patients with systemic lupus erythematosus (SLE) might be dictated by the presence of a genetic variant that encodes the Fc gamma receptors (FcGRs), expressed on natural killer (NK) cells, according to findings from a single-center, longitudinal cohort study.

It is well known that not everyone with SLE will respond well to rituximab, but that some will, first author Md Yuzaiful Md Yusof, MBChB, PhD, explained in an interview at the European Congress of Rheumatology.
Although data from clinical trials with rituximab in this patient setting have been essentially negative, the methodology of those trials has since been disputed, he observed. Indeed, subsequent data (Ann Rheum Dis. 2017;76:1829-36) have suggested that as many as 80% of patients could achieve a response with rituximab, particularly if there is complete B-cell depletion.
Previous researchers (Ann Rheum Dis. 2012;71:875-7) have shown that a polymorphism (158V) in the Fc gamma receptor IIIA (FCGR3A) gene is associated with the response to rituximab-based therapy in patients with rheumatoid arthritis (RA). This gene is important for antibody-dependent cellular-mediated cytotoxicity (ADCC).
The objective of the current study – an observational, prospective, longitudinal cohort study conducted in Leeds (England) – was therefore to see if the FCGR3A-158V polymorphism might influence response in patients with SLE.
“We were trying to find pretreatment biomarkers that could predict response to rituximab in SLE,” Dr. Md Yusof explained.
For the study, 85 patients who were treated with rituximab were assessed. The cohort was predominantly female (96%), with a mean age of 40 years. All of the patients had antinuclear antibodies, with just over half having anti–double-stranded DNA antibodies, and two-thirds having extractable nuclear antigens. One-third had low complement (C3/C4) levels.
Complete B-cell depletion occurred in 63% of patients with the FCGR3A-158V allele, a significantly higher rate than the 40% observed among those with 158 FF genotype (odds ratio, 2.73; P = .041). A significantly higher percentage of patients with the FCGR3A-158V allele also achieved a major BILAG (British Isles Lupus Assessment Group) response when compared against patients with the 158 FF variant (48% vs. 23%), with an odds ratio of 3.06 (P = .033).
Rituximab’s effect on NK cell-mediated B-cell killing may have played a key role in treatment response. Carrying the FCGR3A-158V allele was associated with greater degranulation activity versus the 158 FF variant.
Lastly, patients were more likely to remain on treatment with rituximab over a 10-year period if they had the FCGR3A-158V allele, compared with the 158 FF variant.
“These data suggest one mechanism by which patients with SLE might become resistant to the effects of rituximab, and could be used to guide therapy in the future,” Dr. Md Yusof suggested.
“Once this finding is validated, the clinical implication is that this genetic testing could be done prior to rituximab to identify those who will respond to therapy,” he postulated. “People with SLE who have this genetic variant with high affinity for rituximab are the ones that are better suited for rituximab therapy,” he added, otherwise a different CD20-directed antibody or alternative B-cell blockade therapies should be used.
The U.K. National Institute for Health Research funded the study. Dr. Md Yusof had no conflicts of interest to disclose; some coauthors disclosed ties to Roche, GlaxoSmithKline, and AstraZeneca, among other companies.
SOURCE: Md Yusof MY et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):1069-70. Abstract SAT0009, doi: 10.1136/annrheumdis-2019-eular.6919.
MADRID – Response to rituximab in patients with systemic lupus erythematosus (SLE) might be dictated by the presence of a genetic variant that encodes the Fc gamma receptors (FcGRs), expressed on natural killer (NK) cells, according to findings from a single-center, longitudinal cohort study.

It is well known that not everyone with SLE will respond well to rituximab, but that some will, first author Md Yuzaiful Md Yusof, MBChB, PhD, explained in an interview at the European Congress of Rheumatology.
Although data from clinical trials with rituximab in this patient setting have been essentially negative, the methodology of those trials has since been disputed, he observed. Indeed, subsequent data (Ann Rheum Dis. 2017;76:1829-36) have suggested that as many as 80% of patients could achieve a response with rituximab, particularly if there is complete B-cell depletion.
Previous researchers (Ann Rheum Dis. 2012;71:875-7) have shown that a polymorphism (158V) in the Fc gamma receptor IIIA (FCGR3A) gene is associated with the response to rituximab-based therapy in patients with rheumatoid arthritis (RA). This gene is important for antibody-dependent cellular-mediated cytotoxicity (ADCC).
The objective of the current study – an observational, prospective, longitudinal cohort study conducted in Leeds (England) – was therefore to see if the FCGR3A-158V polymorphism might influence response in patients with SLE.
“We were trying to find pretreatment biomarkers that could predict response to rituximab in SLE,” Dr. Md Yusof explained.
For the study, 85 patients who were treated with rituximab were assessed. The cohort was predominantly female (96%), with a mean age of 40 years. All of the patients had antinuclear antibodies, with just over half having anti–double-stranded DNA antibodies, and two-thirds having extractable nuclear antigens. One-third had low complement (C3/C4) levels.
Complete B-cell depletion occurred in 63% of patients with the FCGR3A-158V allele, a significantly higher rate than the 40% observed among those with 158 FF genotype (odds ratio, 2.73; P = .041). A significantly higher percentage of patients with the FCGR3A-158V allele also achieved a major BILAG (British Isles Lupus Assessment Group) response when compared against patients with the 158 FF variant (48% vs. 23%), with an odds ratio of 3.06 (P = .033).
Rituximab’s effect on NK cell-mediated B-cell killing may have played a key role in treatment response. Carrying the FCGR3A-158V allele was associated with greater degranulation activity versus the 158 FF variant.
Lastly, patients were more likely to remain on treatment with rituximab over a 10-year period if they had the FCGR3A-158V allele, compared with the 158 FF variant.
“These data suggest one mechanism by which patients with SLE might become resistant to the effects of rituximab, and could be used to guide therapy in the future,” Dr. Md Yusof suggested.
“Once this finding is validated, the clinical implication is that this genetic testing could be done prior to rituximab to identify those who will respond to therapy,” he postulated. “People with SLE who have this genetic variant with high affinity for rituximab are the ones that are better suited for rituximab therapy,” he added, otherwise a different CD20-directed antibody or alternative B-cell blockade therapies should be used.
The U.K. National Institute for Health Research funded the study. Dr. Md Yusof had no conflicts of interest to disclose; some coauthors disclosed ties to Roche, GlaxoSmithKline, and AstraZeneca, among other companies.
SOURCE: Md Yusof MY et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):1069-70. Abstract SAT0009, doi: 10.1136/annrheumdis-2019-eular.6919.
MADRID – Response to rituximab in patients with systemic lupus erythematosus (SLE) might be dictated by the presence of a genetic variant that encodes the Fc gamma receptors (FcGRs), expressed on natural killer (NK) cells, according to findings from a single-center, longitudinal cohort study.

It is well known that not everyone with SLE will respond well to rituximab, but that some will, first author Md Yuzaiful Md Yusof, MBChB, PhD, explained in an interview at the European Congress of Rheumatology.
Although data from clinical trials with rituximab in this patient setting have been essentially negative, the methodology of those trials has since been disputed, he observed. Indeed, subsequent data (Ann Rheum Dis. 2017;76:1829-36) have suggested that as many as 80% of patients could achieve a response with rituximab, particularly if there is complete B-cell depletion.
Previous researchers (Ann Rheum Dis. 2012;71:875-7) have shown that a polymorphism (158V) in the Fc gamma receptor IIIA (FCGR3A) gene is associated with the response to rituximab-based therapy in patients with rheumatoid arthritis (RA). This gene is important for antibody-dependent cellular-mediated cytotoxicity (ADCC).
The objective of the current study – an observational, prospective, longitudinal cohort study conducted in Leeds (England) – was therefore to see if the FCGR3A-158V polymorphism might influence response in patients with SLE.
“We were trying to find pretreatment biomarkers that could predict response to rituximab in SLE,” Dr. Md Yusof explained.
For the study, 85 patients who were treated with rituximab were assessed. The cohort was predominantly female (96%), with a mean age of 40 years. All of the patients had antinuclear antibodies, with just over half having anti–double-stranded DNA antibodies, and two-thirds having extractable nuclear antigens. One-third had low complement (C3/C4) levels.
Complete B-cell depletion occurred in 63% of patients with the FCGR3A-158V allele, a significantly higher rate than the 40% observed among those with 158 FF genotype (odds ratio, 2.73; P = .041). A significantly higher percentage of patients with the FCGR3A-158V allele also achieved a major BILAG (British Isles Lupus Assessment Group) response when compared against patients with the 158 FF variant (48% vs. 23%), with an odds ratio of 3.06 (P = .033).
Rituximab’s effect on NK cell-mediated B-cell killing may have played a key role in treatment response. Carrying the FCGR3A-158V allele was associated with greater degranulation activity versus the 158 FF variant.
Lastly, patients were more likely to remain on treatment with rituximab over a 10-year period if they had the FCGR3A-158V allele, compared with the 158 FF variant.
“These data suggest one mechanism by which patients with SLE might become resistant to the effects of rituximab, and could be used to guide therapy in the future,” Dr. Md Yusof suggested.
“Once this finding is validated, the clinical implication is that this genetic testing could be done prior to rituximab to identify those who will respond to therapy,” he postulated. “People with SLE who have this genetic variant with high affinity for rituximab are the ones that are better suited for rituximab therapy,” he added, otherwise a different CD20-directed antibody or alternative B-cell blockade therapies should be used.
The U.K. National Institute for Health Research funded the study. Dr. Md Yusof had no conflicts of interest to disclose; some coauthors disclosed ties to Roche, GlaxoSmithKline, and AstraZeneca, among other companies.
SOURCE: Md Yusof MY et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):1069-70. Abstract SAT0009, doi: 10.1136/annrheumdis-2019-eular.6919.
REPORTING FROM EULAR 2019 CONGRESS
PsA Fast Facts: PsA and psoriasis links



Minimally invasive cosmetic surgery: Steady growth in 2018
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.
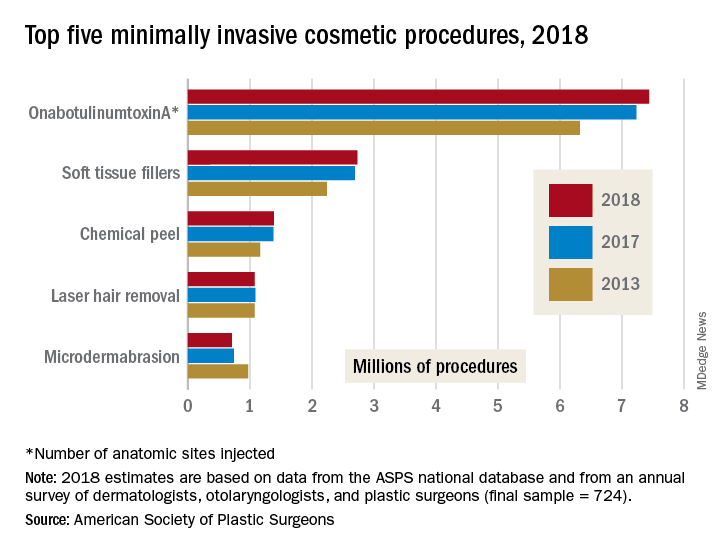
The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.

The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.

The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
ACIP approves meningococcal booster for persons at increased risk
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Physician innovator working to bring new tech to patients, thanks to AGA funding
The AGA Research Foundation’s career development awards are invaluable tools for early career investigators to advance their careers in gastroenterology and hepatology research. When Ashish Nimgaonkar, MD, MTech, MS, received the AGA-Boston Scientific Career Development Technology and Innovation Award in 2014, he was able to step up his research and develop a new technological approach for managing patients with chronic liver disease-related complications. We are delighted to introduce you to the work of Dr. Nimgaonkar, medical director in the Johns Hopkins Center for Bioengineering Innovation and Design, department of biomedical engineering, and an assistant professor of medicine and business at Johns Hopkins University.
When Dr. Nimgaonkar received his funding from the AGA Research Foundation in 2014, he was able to focus on developing a technology that would enable patients with refractory ascites to manage their condition at home. This is a condition in which a large volume of fluid accumulates in the abdomen, causes difficulty breathing and affects patients’ quality of life. Patients visit a hospital or clinic several times a month to drain the fluid, which could weigh as much as 10 pounds or more. Refractory ascites is stubbornly resistant to standard medical therapy. The only definitive treatment is liver transplantation.
Dr. Nimgaonkar was able to combine his dual training in gastroenterology and in medical technology innovation through the biodesign program at Stanford University, along with the breadth of engineering and research expertise at Johns Hopkins University, to develop a bio-powered shunt that moves a patient’s fluid buildup out of the peritoneal cavity to the urinary bladder, where it can be eliminated naturally. His shunt has another major advantage for patients who are on liver transplant lists and are required to undergo MRI and other diagnostics: it contains no metal components.
Read more and get to know Ashish Nimgaonkar, MD, MTech, MS by visiting:
https://www.gastro.org/news/physician-innovator-working-to-bring-new-tech-to-patients-thanks-to-aga-funding
Help AGA build a community of investigators through the AGA Research Foundation.
Your donation to the AGA Research Foundation can fund future success stories by keeping young scientists working to advance our understanding of digestive diseases. Donate today at www.gastro.org/donateonline.
The AGA Research Foundation’s career development awards are invaluable tools for early career investigators to advance their careers in gastroenterology and hepatology research. When Ashish Nimgaonkar, MD, MTech, MS, received the AGA-Boston Scientific Career Development Technology and Innovation Award in 2014, he was able to step up his research and develop a new technological approach for managing patients with chronic liver disease-related complications. We are delighted to introduce you to the work of Dr. Nimgaonkar, medical director in the Johns Hopkins Center for Bioengineering Innovation and Design, department of biomedical engineering, and an assistant professor of medicine and business at Johns Hopkins University.
When Dr. Nimgaonkar received his funding from the AGA Research Foundation in 2014, he was able to focus on developing a technology that would enable patients with refractory ascites to manage their condition at home. This is a condition in which a large volume of fluid accumulates in the abdomen, causes difficulty breathing and affects patients’ quality of life. Patients visit a hospital or clinic several times a month to drain the fluid, which could weigh as much as 10 pounds or more. Refractory ascites is stubbornly resistant to standard medical therapy. The only definitive treatment is liver transplantation.
Dr. Nimgaonkar was able to combine his dual training in gastroenterology and in medical technology innovation through the biodesign program at Stanford University, along with the breadth of engineering and research expertise at Johns Hopkins University, to develop a bio-powered shunt that moves a patient’s fluid buildup out of the peritoneal cavity to the urinary bladder, where it can be eliminated naturally. His shunt has another major advantage for patients who are on liver transplant lists and are required to undergo MRI and other diagnostics: it contains no metal components.
Read more and get to know Ashish Nimgaonkar, MD, MTech, MS by visiting:
https://www.gastro.org/news/physician-innovator-working-to-bring-new-tech-to-patients-thanks-to-aga-funding
Help AGA build a community of investigators through the AGA Research Foundation.
Your donation to the AGA Research Foundation can fund future success stories by keeping young scientists working to advance our understanding of digestive diseases. Donate today at www.gastro.org/donateonline.
The AGA Research Foundation’s career development awards are invaluable tools for early career investigators to advance their careers in gastroenterology and hepatology research. When Ashish Nimgaonkar, MD, MTech, MS, received the AGA-Boston Scientific Career Development Technology and Innovation Award in 2014, he was able to step up his research and develop a new technological approach for managing patients with chronic liver disease-related complications. We are delighted to introduce you to the work of Dr. Nimgaonkar, medical director in the Johns Hopkins Center for Bioengineering Innovation and Design, department of biomedical engineering, and an assistant professor of medicine and business at Johns Hopkins University.
When Dr. Nimgaonkar received his funding from the AGA Research Foundation in 2014, he was able to focus on developing a technology that would enable patients with refractory ascites to manage their condition at home. This is a condition in which a large volume of fluid accumulates in the abdomen, causes difficulty breathing and affects patients’ quality of life. Patients visit a hospital or clinic several times a month to drain the fluid, which could weigh as much as 10 pounds or more. Refractory ascites is stubbornly resistant to standard medical therapy. The only definitive treatment is liver transplantation.
Dr. Nimgaonkar was able to combine his dual training in gastroenterology and in medical technology innovation through the biodesign program at Stanford University, along with the breadth of engineering and research expertise at Johns Hopkins University, to develop a bio-powered shunt that moves a patient’s fluid buildup out of the peritoneal cavity to the urinary bladder, where it can be eliminated naturally. His shunt has another major advantage for patients who are on liver transplant lists and are required to undergo MRI and other diagnostics: it contains no metal components.
Read more and get to know Ashish Nimgaonkar, MD, MTech, MS by visiting:
https://www.gastro.org/news/physician-innovator-working-to-bring-new-tech-to-patients-thanks-to-aga-funding
Help AGA build a community of investigators through the AGA Research Foundation.
Your donation to the AGA Research Foundation can fund future success stories by keeping young scientists working to advance our understanding of digestive diseases. Donate today at www.gastro.org/donateonline.
Washington makes low drug prices a priority
The House of Representatives passed two bills aimed at speeding up the development of generics and biosimilars while the Trump administration finalized a rule to require drug companies to list the price of their products in their television ads.
The House passed two bills to address drug pricing. The House passed H.R. 1503, the Orange Book Transparency Act of 2019, legislation that would make changes to the FDA’s “orange” book to provide better information on brand drug and patent exclusivity. The orange book is used by doctors and pharmacists for information on generic drug approvals and availability. It is also used by generic drug manufacturers to make decisions on where to invest in research and development as it provides information on the exclusivity period for brand name drugs. Similarly, the House passed H.R. 1520, the Purple Book Continuity Act, legislation that would update FDA’s “purple” book on patents and exclusivity for biologics. These are the first bills of the 116th Congress to pass that address the costs of drugs.
The Administration finalizes rule on drug costs in advertising. The Trump administration finalized a rule that would require drug manufacturers to disclose prices on their products in television advertisements. Manufacturers must list a product’s monthly wholesale price or the cost of a typical treatment if it is greater than $35 for 30 days. The information must appear in text large enough for people to read it and should also include a statement that people with insurance may pay a different amount for the product. The rule takes effect in 60 days and the drug industry opposes the rule, which they say could sway patients away from certain medications and lead to more misinformation on the actual costs.
House Appropriations Committee approves $2 billion NIH increase. The House Appropriations Committee approved their fiscal year 2020 Labor, HHS, and Education Appropriations bill that includes a $2 billion increase in NIH funding. The Committee also includes critical report language on several GI research areas including inflammatory bowel disease, colorectal cancer screenings, early onset colorectal cancer, and the role of food as medicine in treating diseases. The bill also includes important language directing CMS to require Medicare Advantage plans to exclude from prior authorization requirements those services that align with evidence-based guidelines and have a high prior authorization approval rate. The language also calls for more transparency for MA plans with prior authorization so physicians are aware of what services require it.
Medical Nutrition Equity Act introduced in House. Rep. Jim McGovern, D-Mass., introduced H.R. 2501, the Medical Nutrition Equity Act, legislation that would mandate coverage of medically necessary foods for individuals with digestive and inherited metabolic disorders. AGA is supportive of this legislation that is critical for patients with digestive diseases and ensures their access to these lifesaving products.
The House of Representatives passed two bills aimed at speeding up the development of generics and biosimilars while the Trump administration finalized a rule to require drug companies to list the price of their products in their television ads.
The House passed two bills to address drug pricing. The House passed H.R. 1503, the Orange Book Transparency Act of 2019, legislation that would make changes to the FDA’s “orange” book to provide better information on brand drug and patent exclusivity. The orange book is used by doctors and pharmacists for information on generic drug approvals and availability. It is also used by generic drug manufacturers to make decisions on where to invest in research and development as it provides information on the exclusivity period for brand name drugs. Similarly, the House passed H.R. 1520, the Purple Book Continuity Act, legislation that would update FDA’s “purple” book on patents and exclusivity for biologics. These are the first bills of the 116th Congress to pass that address the costs of drugs.
The Administration finalizes rule on drug costs in advertising. The Trump administration finalized a rule that would require drug manufacturers to disclose prices on their products in television advertisements. Manufacturers must list a product’s monthly wholesale price or the cost of a typical treatment if it is greater than $35 for 30 days. The information must appear in text large enough for people to read it and should also include a statement that people with insurance may pay a different amount for the product. The rule takes effect in 60 days and the drug industry opposes the rule, which they say could sway patients away from certain medications and lead to more misinformation on the actual costs.
House Appropriations Committee approves $2 billion NIH increase. The House Appropriations Committee approved their fiscal year 2020 Labor, HHS, and Education Appropriations bill that includes a $2 billion increase in NIH funding. The Committee also includes critical report language on several GI research areas including inflammatory bowel disease, colorectal cancer screenings, early onset colorectal cancer, and the role of food as medicine in treating diseases. The bill also includes important language directing CMS to require Medicare Advantage plans to exclude from prior authorization requirements those services that align with evidence-based guidelines and have a high prior authorization approval rate. The language also calls for more transparency for MA plans with prior authorization so physicians are aware of what services require it.
Medical Nutrition Equity Act introduced in House. Rep. Jim McGovern, D-Mass., introduced H.R. 2501, the Medical Nutrition Equity Act, legislation that would mandate coverage of medically necessary foods for individuals with digestive and inherited metabolic disorders. AGA is supportive of this legislation that is critical for patients with digestive diseases and ensures their access to these lifesaving products.
The House of Representatives passed two bills aimed at speeding up the development of generics and biosimilars while the Trump administration finalized a rule to require drug companies to list the price of their products in their television ads.
The House passed two bills to address drug pricing. The House passed H.R. 1503, the Orange Book Transparency Act of 2019, legislation that would make changes to the FDA’s “orange” book to provide better information on brand drug and patent exclusivity. The orange book is used by doctors and pharmacists for information on generic drug approvals and availability. It is also used by generic drug manufacturers to make decisions on where to invest in research and development as it provides information on the exclusivity period for brand name drugs. Similarly, the House passed H.R. 1520, the Purple Book Continuity Act, legislation that would update FDA’s “purple” book on patents and exclusivity for biologics. These are the first bills of the 116th Congress to pass that address the costs of drugs.
The Administration finalizes rule on drug costs in advertising. The Trump administration finalized a rule that would require drug manufacturers to disclose prices on their products in television advertisements. Manufacturers must list a product’s monthly wholesale price or the cost of a typical treatment if it is greater than $35 for 30 days. The information must appear in text large enough for people to read it and should also include a statement that people with insurance may pay a different amount for the product. The rule takes effect in 60 days and the drug industry opposes the rule, which they say could sway patients away from certain medications and lead to more misinformation on the actual costs.
House Appropriations Committee approves $2 billion NIH increase. The House Appropriations Committee approved their fiscal year 2020 Labor, HHS, and Education Appropriations bill that includes a $2 billion increase in NIH funding. The Committee also includes critical report language on several GI research areas including inflammatory bowel disease, colorectal cancer screenings, early onset colorectal cancer, and the role of food as medicine in treating diseases. The bill also includes important language directing CMS to require Medicare Advantage plans to exclude from prior authorization requirements those services that align with evidence-based guidelines and have a high prior authorization approval rate. The language also calls for more transparency for MA plans with prior authorization so physicians are aware of what services require it.
Medical Nutrition Equity Act introduced in House. Rep. Jim McGovern, D-Mass., introduced H.R. 2501, the Medical Nutrition Equity Act, legislation that would mandate coverage of medically necessary foods for individuals with digestive and inherited metabolic disorders. AGA is supportive of this legislation that is critical for patients with digestive diseases and ensures their access to these lifesaving products.
Top AGA Community patient cases
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Crohn’s disease, infliximab and liver abscess (http://ow.ly/mTod50uyXCQ)
A 22-year-old Crohn’s patient presented to the hospital in septic shock with acute renal failure due to pyogenic liver abscess, which had ruptured into the peritoneal cavity. Member seeks consult from the AGA Community on treatment options given this serious infection.
2. EUS-guided cholecystoenterostomy with LAMS (http://ow.ly/IqLP50uyXLg)
A member poses the question: how long should the stent stay in?
3. Colorectal cancer surveillance in Crohn’s colitis and small duct PSC (http://ow.ly/tbe650uyXQh)
A member asks if you would continue yearly CRC surveillance on a patient with Crohn’s colitis with very limited colonic involvement in the ascending colon, who is currently in clinical remission. The patient also has small duct PSC with early cirrhosis.
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Crohn’s disease, infliximab and liver abscess (http://ow.ly/mTod50uyXCQ)
A 22-year-old Crohn’s patient presented to the hospital in septic shock with acute renal failure due to pyogenic liver abscess, which had ruptured into the peritoneal cavity. Member seeks consult from the AGA Community on treatment options given this serious infection.
2. EUS-guided cholecystoenterostomy with LAMS (http://ow.ly/IqLP50uyXLg)
A member poses the question: how long should the stent stay in?
3. Colorectal cancer surveillance in Crohn’s colitis and small duct PSC (http://ow.ly/tbe650uyXQh)
A member asks if you would continue yearly CRC surveillance on a patient with Crohn’s colitis with very limited colonic involvement in the ascending colon, who is currently in clinical remission. The patient also has small duct PSC with early cirrhosis.
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Crohn’s disease, infliximab and liver abscess (http://ow.ly/mTod50uyXCQ)
A 22-year-old Crohn’s patient presented to the hospital in septic shock with acute renal failure due to pyogenic liver abscess, which had ruptured into the peritoneal cavity. Member seeks consult from the AGA Community on treatment options given this serious infection.
2. EUS-guided cholecystoenterostomy with LAMS (http://ow.ly/IqLP50uyXLg)
A member poses the question: how long should the stent stay in?
3. Colorectal cancer surveillance in Crohn’s colitis and small duct PSC (http://ow.ly/tbe650uyXQh)
A member asks if you would continue yearly CRC surveillance on a patient with Crohn’s colitis with very limited colonic involvement in the ascending colon, who is currently in clinical remission. The patient also has small duct PSC with early cirrhosis.
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
FDA approves bevacizumab-bvzr for several cancers
The Food and Drug Administration has approved bevacizumab-bvzr (Zirabev) – a biosimilar to bevacizumab (Avastin) – for the treatment of five cancers: metastatic colorectal cancer (mCRC); unresectable, locally advanced, recurrent or metastatic non-squamous non–small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma (RCC); and persistent, recurrent or metastatic cervical cancer.
Approval was based on “review of a comprehensive data package which demonstrated biosimilarity of [bevacizumab-bvzr] to the reference product,” Pfizer said in a statement announcing the approval.
Bevacizumab-bvzr is the second bevacizumab biosimilar to be approved, following approval of Amgen’s bevacizumab-awwb (Mvasi) in 2017.
Warnings and precautions with the biosimilars, as with bevacizumab, include serious and sometimes fatal gastrointestinal perforation, surgery and wound healing complications, and sometimes serious and fatal hemorrhage.
The most common adverse events observed in bevacizumab patients are epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, rectal hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Specific indications for the biosimilar are as follows:
Metastatic colorectal cancer
Bevacizumab-bvzr, in combination with intravenous fluorouracil-based chemotherapy, is indicated for the first- or second-line treatment of patients with mCRC.
Bevacizumab-bvzr, in combination with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin–based chemotherapy, is indicated for the second-line treatment of patients with mCRC who have progressed on a first-line bevacizumab product–containing regimen.
Bevacizumab-bvzr is not indicated for adjuvant treatment of colon cancer.
First-line nonsquamous non–small cell lung cancer
Bevacizumab-bvzr, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic NSCLC.
Recurrent glioblastoma
Bevacizumab-bvzr is indicated for the treatment of recurrent glioblastoma in adults.
Metastatic renal cell carcinoma
Bevacizumab-bvzr, in combination with interferon alfa, is indicated for the treatment of metastatic RCC.
Persistent, recurrent, or metastatic cervical cancer
Bevacizumab-bvzr, in combination with paclitaxel and cisplatin or paclitaxel and topotecan, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer.
Complete prescribing information can be found on the FDA website.
The Food and Drug Administration has approved bevacizumab-bvzr (Zirabev) – a biosimilar to bevacizumab (Avastin) – for the treatment of five cancers: metastatic colorectal cancer (mCRC); unresectable, locally advanced, recurrent or metastatic non-squamous non–small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma (RCC); and persistent, recurrent or metastatic cervical cancer.
Approval was based on “review of a comprehensive data package which demonstrated biosimilarity of [bevacizumab-bvzr] to the reference product,” Pfizer said in a statement announcing the approval.
Bevacizumab-bvzr is the second bevacizumab biosimilar to be approved, following approval of Amgen’s bevacizumab-awwb (Mvasi) in 2017.
Warnings and precautions with the biosimilars, as with bevacizumab, include serious and sometimes fatal gastrointestinal perforation, surgery and wound healing complications, and sometimes serious and fatal hemorrhage.
The most common adverse events observed in bevacizumab patients are epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, rectal hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Specific indications for the biosimilar are as follows:
Metastatic colorectal cancer
Bevacizumab-bvzr, in combination with intravenous fluorouracil-based chemotherapy, is indicated for the first- or second-line treatment of patients with mCRC.
Bevacizumab-bvzr, in combination with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin–based chemotherapy, is indicated for the second-line treatment of patients with mCRC who have progressed on a first-line bevacizumab product–containing regimen.
Bevacizumab-bvzr is not indicated for adjuvant treatment of colon cancer.
First-line nonsquamous non–small cell lung cancer
Bevacizumab-bvzr, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic NSCLC.
Recurrent glioblastoma
Bevacizumab-bvzr is indicated for the treatment of recurrent glioblastoma in adults.
Metastatic renal cell carcinoma
Bevacizumab-bvzr, in combination with interferon alfa, is indicated for the treatment of metastatic RCC.
Persistent, recurrent, or metastatic cervical cancer
Bevacizumab-bvzr, in combination with paclitaxel and cisplatin or paclitaxel and topotecan, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer.
Complete prescribing information can be found on the FDA website.
The Food and Drug Administration has approved bevacizumab-bvzr (Zirabev) – a biosimilar to bevacizumab (Avastin) – for the treatment of five cancers: metastatic colorectal cancer (mCRC); unresectable, locally advanced, recurrent or metastatic non-squamous non–small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma (RCC); and persistent, recurrent or metastatic cervical cancer.
Approval was based on “review of a comprehensive data package which demonstrated biosimilarity of [bevacizumab-bvzr] to the reference product,” Pfizer said in a statement announcing the approval.
Bevacizumab-bvzr is the second bevacizumab biosimilar to be approved, following approval of Amgen’s bevacizumab-awwb (Mvasi) in 2017.
Warnings and precautions with the biosimilars, as with bevacizumab, include serious and sometimes fatal gastrointestinal perforation, surgery and wound healing complications, and sometimes serious and fatal hemorrhage.
The most common adverse events observed in bevacizumab patients are epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, rectal hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Specific indications for the biosimilar are as follows:
Metastatic colorectal cancer
Bevacizumab-bvzr, in combination with intravenous fluorouracil-based chemotherapy, is indicated for the first- or second-line treatment of patients with mCRC.
Bevacizumab-bvzr, in combination with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin–based chemotherapy, is indicated for the second-line treatment of patients with mCRC who have progressed on a first-line bevacizumab product–containing regimen.
Bevacizumab-bvzr is not indicated for adjuvant treatment of colon cancer.
First-line nonsquamous non–small cell lung cancer
Bevacizumab-bvzr, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic NSCLC.
Recurrent glioblastoma
Bevacizumab-bvzr is indicated for the treatment of recurrent glioblastoma in adults.
Metastatic renal cell carcinoma
Bevacizumab-bvzr, in combination with interferon alfa, is indicated for the treatment of metastatic RCC.
Persistent, recurrent, or metastatic cervical cancer
Bevacizumab-bvzr, in combination with paclitaxel and cisplatin or paclitaxel and topotecan, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer.
Complete prescribing information can be found on the FDA website.
Prior authorization – a call to action
Have you noticed that you and your staff are spending more time on prior authorization than in the past? Insurance companies are increasing the number of Current Procedural Terminology (CPT®) codes for services and procedures included in their prior authorization programs. More importantly, they are doing so without providing evidence that this approach improves patient safety or decreases unindicated medical procedures. There is also no transparency about how these prior authorization processes are developed, evaluated, or adjusted over time. Physicians and their staff are pushing back on social media, calling prior authorization programs a hassle and citing lengthy waits to speak to a physician reviewer who is often not even in their specialty.
Historically, insurers have used prior authorization to control costs, particularly those related to procedures and tests that may be inappropriately overutilized or no longer the standard of care; however, current activity suggests a much broader, indiscriminate approach. For example, insurers are requiring prior authorization for whole families of services and procedures. Anthem, the second largest insurance company in the United States, recently added the entire family of esophagogastroduodenoscopy (EGD) codes to its list of procedures requiring prior authorization in 10 states including Calif., Conn., Ind, Ohio, Ky., Mo., Nev., N.H., Va., and Wisc. A conversation earlier this year with the Anthem national prior authorization team revealed that they intend to keep adding codes for all specialties to their prior authorization program, portraying the process conducted by AIM Specialty Health® (a wholly-owned subsidiary of Anthem, Inc.), as fast, simple, and easy. However, many physicians and their office staff find the prior authorization process complex, time consuming, and frustrating.
Social media is rife with accounts from physicians who were forced to cancel planned procedures because the prior authorization process took weeks instead of days, received denials, and later found out that procedures were actually approved, or found themselves in peer-to-peer review with nonphysicians. Gastroenterologists have also reported cases of patients having flares of inflammatory bowel disease because of medication delays related to a cumbersome preauthorization process.
Because prior authorization impacts gastroenterologists’ ability to provide timely care to patients, AGA and the entire physician community have been calling for regulatory change related to prior authorization in Medicare Advantage (MA) plans to reduce physician burden and enhance patient safety and care.
Last year, AGA worked with our congressional champions Reps. Phil Roe, MD, (R-Tenn.) and Ami Bera, MD, (D-Calif.) to secure 150 signatures on a letter to the CMS Administrator requesting the agency provide guidance to MA plans to ensure that prior authorization requirements do not create barriers to care.
One in every three people with Medicare is enrolled in a Medicare Advantage (MA) plan. Under current law, MA plans may not create inappropriate barriers to care that do not already exist within the original Medicare program. A recent survey by the American Medical Association found that over 90% of physician respondents felt that the prior authorization process led to delays in care for patients that could negatively impact clinical outcomes. AGA and other physician organizations are advocating for regulatory changes related to how MA plans use prior authorization.
In addition to our regulatory efforts, the AGA is working with members of Congress on legislative solutions to require the MA plans to increase transparency, streamline the prior authorization process, and minimize the impact on Medicare beneficiaries. Reps. Susan DelBene, D-Wash., Mike Kelly, R-Penna., Ami Bera, D-Calif., and Roger Marshall, R-Kans. introduced the Improving Seniors Timely Access to Care Act of 2019, legislation that would streamline the prior authorization process in the Medicare Advantage program to relieve the administrative burdens this poses for physicians and help patients receive quicker access to the medical care they need. Although this legislation only addresses MA plans, we are hopeful that this will be the first step in requiring health plans to streamline this process and ease administrative burden. Please help us increase support for this bill by contacting your legislators and asking that they cosponsor. It will take less than 5 minutes of your time and will have a significant effect, given the opposition we face from insurers. The AGA is working on your behalf to address prior authorization hassles with private payors, but to be effective we need to hear your experiences. We know private payors continue to develop more and more restrictive prior authorization policies covering an increasing number of services and procedures without evidence that these actions provide benefit to patients. Frequently, these policies are put into action without advance warning and your reports are the first signs we have that a change has been made. Reach out to the AGA via the AGA Community or Twitter to let us know what’s happening. We will take your stories directly to the insurance companies and demand that they work with us to reduce physician burden and improve transparency.
You may also consider filing a complaint with the State Insurance Commissioner. State Insurance Commissioners are responsible for regulating the insurance industry in their state and can investigate to make sure the laws in their state are being followed and providers and patients are being treated fairly. While insurance law and regulation are established at the state level, the insurance commissioners are members of the National Association of Insurance Commissioners (NAIC), which allows them to coordinate insurance regulation among the states and territories.
If you decide to file a complaint with your State Insurance Commissioner, first familiarize yourself with your state’s complaint process. Many state insurance commissioners have a standard complaint form you can download or fill out online. Be sure to keep records of all conversations and interactions with the insurance company to document the steps you’ve taken to attempt to resolve the issue. Consider creating a log of the dates, times, and nature of your contact with the insurance company.
Once you have filed a complaint, the commissioner may send a copy to the insurance company and give them a date by which they must respond. If the commissioner believes the response is sufficient, she or he will send a copy of the insurance company’s response to you. If the commissioner feels the insurance company’s response is inadequate, staff from the commissioner’s office will work with you and the insurer to resolve the issue.
While a report of one negative experience with an insurer may not be enough to elicit action, a pattern of delays and difficulties with an insurer’s prior authorization process noted by many physicians is likely to catch an Insurance Commissioner’s attention. The NAIC cannot tell a problem is widespread if providers and patients don’t report it to the State Insurance Commissioners.
Please reach out to AGA with your stories about prior authorization problems, consider reporting insurance companies that employ systems that cause undue burden and delay to your State Insurance Commissioner and help us increase support for the Improving Seniors Timely Access to Care Act of 2019 by contacting your legislators and asking that they cosponsor using this link https://app.govpredict.com/portal/grassroots/campaigns/io77ozaa/take_action. Together, we can pressure insurers, Congress, and Medicare to relieve physician burden and help our patients receive the timely care they need.
Dr. Garcia is a member of the AGA Practice Management and Economics Committee’s Coverage And Reimbursement Subcommittee and clinical assistant professor of medicine, gastroenterology & hepatology, Stanford Medicine, Stanford, California. Dr. Mathews is a member of the AGA Government Affairs Committee and leads efforts in clinical innovation at the Johns Hopkins Armstrong Institute for Patient Safety and Quality, Baltimore.
Tihs story was updated on July 29, 2019.
Have you noticed that you and your staff are spending more time on prior authorization than in the past? Insurance companies are increasing the number of Current Procedural Terminology (CPT®) codes for services and procedures included in their prior authorization programs. More importantly, they are doing so without providing evidence that this approach improves patient safety or decreases unindicated medical procedures. There is also no transparency about how these prior authorization processes are developed, evaluated, or adjusted over time. Physicians and their staff are pushing back on social media, calling prior authorization programs a hassle and citing lengthy waits to speak to a physician reviewer who is often not even in their specialty.
Historically, insurers have used prior authorization to control costs, particularly those related to procedures and tests that may be inappropriately overutilized or no longer the standard of care; however, current activity suggests a much broader, indiscriminate approach. For example, insurers are requiring prior authorization for whole families of services and procedures. Anthem, the second largest insurance company in the United States, recently added the entire family of esophagogastroduodenoscopy (EGD) codes to its list of procedures requiring prior authorization in 10 states including Calif., Conn., Ind, Ohio, Ky., Mo., Nev., N.H., Va., and Wisc. A conversation earlier this year with the Anthem national prior authorization team revealed that they intend to keep adding codes for all specialties to their prior authorization program, portraying the process conducted by AIM Specialty Health® (a wholly-owned subsidiary of Anthem, Inc.), as fast, simple, and easy. However, many physicians and their office staff find the prior authorization process complex, time consuming, and frustrating.
Social media is rife with accounts from physicians who were forced to cancel planned procedures because the prior authorization process took weeks instead of days, received denials, and later found out that procedures were actually approved, or found themselves in peer-to-peer review with nonphysicians. Gastroenterologists have also reported cases of patients having flares of inflammatory bowel disease because of medication delays related to a cumbersome preauthorization process.
Because prior authorization impacts gastroenterologists’ ability to provide timely care to patients, AGA and the entire physician community have been calling for regulatory change related to prior authorization in Medicare Advantage (MA) plans to reduce physician burden and enhance patient safety and care.
Last year, AGA worked with our congressional champions Reps. Phil Roe, MD, (R-Tenn.) and Ami Bera, MD, (D-Calif.) to secure 150 signatures on a letter to the CMS Administrator requesting the agency provide guidance to MA plans to ensure that prior authorization requirements do not create barriers to care.
One in every three people with Medicare is enrolled in a Medicare Advantage (MA) plan. Under current law, MA plans may not create inappropriate barriers to care that do not already exist within the original Medicare program. A recent survey by the American Medical Association found that over 90% of physician respondents felt that the prior authorization process led to delays in care for patients that could negatively impact clinical outcomes. AGA and other physician organizations are advocating for regulatory changes related to how MA plans use prior authorization.
In addition to our regulatory efforts, the AGA is working with members of Congress on legislative solutions to require the MA plans to increase transparency, streamline the prior authorization process, and minimize the impact on Medicare beneficiaries. Reps. Susan DelBene, D-Wash., Mike Kelly, R-Penna., Ami Bera, D-Calif., and Roger Marshall, R-Kans. introduced the Improving Seniors Timely Access to Care Act of 2019, legislation that would streamline the prior authorization process in the Medicare Advantage program to relieve the administrative burdens this poses for physicians and help patients receive quicker access to the medical care they need. Although this legislation only addresses MA plans, we are hopeful that this will be the first step in requiring health plans to streamline this process and ease administrative burden. Please help us increase support for this bill by contacting your legislators and asking that they cosponsor. It will take less than 5 minutes of your time and will have a significant effect, given the opposition we face from insurers. The AGA is working on your behalf to address prior authorization hassles with private payors, but to be effective we need to hear your experiences. We know private payors continue to develop more and more restrictive prior authorization policies covering an increasing number of services and procedures without evidence that these actions provide benefit to patients. Frequently, these policies are put into action without advance warning and your reports are the first signs we have that a change has been made. Reach out to the AGA via the AGA Community or Twitter to let us know what’s happening. We will take your stories directly to the insurance companies and demand that they work with us to reduce physician burden and improve transparency.
You may also consider filing a complaint with the State Insurance Commissioner. State Insurance Commissioners are responsible for regulating the insurance industry in their state and can investigate to make sure the laws in their state are being followed and providers and patients are being treated fairly. While insurance law and regulation are established at the state level, the insurance commissioners are members of the National Association of Insurance Commissioners (NAIC), which allows them to coordinate insurance regulation among the states and territories.
If you decide to file a complaint with your State Insurance Commissioner, first familiarize yourself with your state’s complaint process. Many state insurance commissioners have a standard complaint form you can download or fill out online. Be sure to keep records of all conversations and interactions with the insurance company to document the steps you’ve taken to attempt to resolve the issue. Consider creating a log of the dates, times, and nature of your contact with the insurance company.
Once you have filed a complaint, the commissioner may send a copy to the insurance company and give them a date by which they must respond. If the commissioner believes the response is sufficient, she or he will send a copy of the insurance company’s response to you. If the commissioner feels the insurance company’s response is inadequate, staff from the commissioner’s office will work with you and the insurer to resolve the issue.
While a report of one negative experience with an insurer may not be enough to elicit action, a pattern of delays and difficulties with an insurer’s prior authorization process noted by many physicians is likely to catch an Insurance Commissioner’s attention. The NAIC cannot tell a problem is widespread if providers and patients don’t report it to the State Insurance Commissioners.
Please reach out to AGA with your stories about prior authorization problems, consider reporting insurance companies that employ systems that cause undue burden and delay to your State Insurance Commissioner and help us increase support for the Improving Seniors Timely Access to Care Act of 2019 by contacting your legislators and asking that they cosponsor using this link https://app.govpredict.com/portal/grassroots/campaigns/io77ozaa/take_action. Together, we can pressure insurers, Congress, and Medicare to relieve physician burden and help our patients receive the timely care they need.
Dr. Garcia is a member of the AGA Practice Management and Economics Committee’s Coverage And Reimbursement Subcommittee and clinical assistant professor of medicine, gastroenterology & hepatology, Stanford Medicine, Stanford, California. Dr. Mathews is a member of the AGA Government Affairs Committee and leads efforts in clinical innovation at the Johns Hopkins Armstrong Institute for Patient Safety and Quality, Baltimore.
Tihs story was updated on July 29, 2019.
Have you noticed that you and your staff are spending more time on prior authorization than in the past? Insurance companies are increasing the number of Current Procedural Terminology (CPT®) codes for services and procedures included in their prior authorization programs. More importantly, they are doing so without providing evidence that this approach improves patient safety or decreases unindicated medical procedures. There is also no transparency about how these prior authorization processes are developed, evaluated, or adjusted over time. Physicians and their staff are pushing back on social media, calling prior authorization programs a hassle and citing lengthy waits to speak to a physician reviewer who is often not even in their specialty.
Historically, insurers have used prior authorization to control costs, particularly those related to procedures and tests that may be inappropriately overutilized or no longer the standard of care; however, current activity suggests a much broader, indiscriminate approach. For example, insurers are requiring prior authorization for whole families of services and procedures. Anthem, the second largest insurance company in the United States, recently added the entire family of esophagogastroduodenoscopy (EGD) codes to its list of procedures requiring prior authorization in 10 states including Calif., Conn., Ind, Ohio, Ky., Mo., Nev., N.H., Va., and Wisc. A conversation earlier this year with the Anthem national prior authorization team revealed that they intend to keep adding codes for all specialties to their prior authorization program, portraying the process conducted by AIM Specialty Health® (a wholly-owned subsidiary of Anthem, Inc.), as fast, simple, and easy. However, many physicians and their office staff find the prior authorization process complex, time consuming, and frustrating.
Social media is rife with accounts from physicians who were forced to cancel planned procedures because the prior authorization process took weeks instead of days, received denials, and later found out that procedures were actually approved, or found themselves in peer-to-peer review with nonphysicians. Gastroenterologists have also reported cases of patients having flares of inflammatory bowel disease because of medication delays related to a cumbersome preauthorization process.
Because prior authorization impacts gastroenterologists’ ability to provide timely care to patients, AGA and the entire physician community have been calling for regulatory change related to prior authorization in Medicare Advantage (MA) plans to reduce physician burden and enhance patient safety and care.
Last year, AGA worked with our congressional champions Reps. Phil Roe, MD, (R-Tenn.) and Ami Bera, MD, (D-Calif.) to secure 150 signatures on a letter to the CMS Administrator requesting the agency provide guidance to MA plans to ensure that prior authorization requirements do not create barriers to care.
One in every three people with Medicare is enrolled in a Medicare Advantage (MA) plan. Under current law, MA plans may not create inappropriate barriers to care that do not already exist within the original Medicare program. A recent survey by the American Medical Association found that over 90% of physician respondents felt that the prior authorization process led to delays in care for patients that could negatively impact clinical outcomes. AGA and other physician organizations are advocating for regulatory changes related to how MA plans use prior authorization.
In addition to our regulatory efforts, the AGA is working with members of Congress on legislative solutions to require the MA plans to increase transparency, streamline the prior authorization process, and minimize the impact on Medicare beneficiaries. Reps. Susan DelBene, D-Wash., Mike Kelly, R-Penna., Ami Bera, D-Calif., and Roger Marshall, R-Kans. introduced the Improving Seniors Timely Access to Care Act of 2019, legislation that would streamline the prior authorization process in the Medicare Advantage program to relieve the administrative burdens this poses for physicians and help patients receive quicker access to the medical care they need. Although this legislation only addresses MA plans, we are hopeful that this will be the first step in requiring health plans to streamline this process and ease administrative burden. Please help us increase support for this bill by contacting your legislators and asking that they cosponsor. It will take less than 5 minutes of your time and will have a significant effect, given the opposition we face from insurers. The AGA is working on your behalf to address prior authorization hassles with private payors, but to be effective we need to hear your experiences. We know private payors continue to develop more and more restrictive prior authorization policies covering an increasing number of services and procedures without evidence that these actions provide benefit to patients. Frequently, these policies are put into action without advance warning and your reports are the first signs we have that a change has been made. Reach out to the AGA via the AGA Community or Twitter to let us know what’s happening. We will take your stories directly to the insurance companies and demand that they work with us to reduce physician burden and improve transparency.
You may also consider filing a complaint with the State Insurance Commissioner. State Insurance Commissioners are responsible for regulating the insurance industry in their state and can investigate to make sure the laws in their state are being followed and providers and patients are being treated fairly. While insurance law and regulation are established at the state level, the insurance commissioners are members of the National Association of Insurance Commissioners (NAIC), which allows them to coordinate insurance regulation among the states and territories.
If you decide to file a complaint with your State Insurance Commissioner, first familiarize yourself with your state’s complaint process. Many state insurance commissioners have a standard complaint form you can download or fill out online. Be sure to keep records of all conversations and interactions with the insurance company to document the steps you’ve taken to attempt to resolve the issue. Consider creating a log of the dates, times, and nature of your contact with the insurance company.
Once you have filed a complaint, the commissioner may send a copy to the insurance company and give them a date by which they must respond. If the commissioner believes the response is sufficient, she or he will send a copy of the insurance company’s response to you. If the commissioner feels the insurance company’s response is inadequate, staff from the commissioner’s office will work with you and the insurer to resolve the issue.
While a report of one negative experience with an insurer may not be enough to elicit action, a pattern of delays and difficulties with an insurer’s prior authorization process noted by many physicians is likely to catch an Insurance Commissioner’s attention. The NAIC cannot tell a problem is widespread if providers and patients don’t report it to the State Insurance Commissioners.
Please reach out to AGA with your stories about prior authorization problems, consider reporting insurance companies that employ systems that cause undue burden and delay to your State Insurance Commissioner and help us increase support for the Improving Seniors Timely Access to Care Act of 2019 by contacting your legislators and asking that they cosponsor using this link https://app.govpredict.com/portal/grassroots/campaigns/io77ozaa/take_action. Together, we can pressure insurers, Congress, and Medicare to relieve physician burden and help our patients receive the timely care they need.
Dr. Garcia is a member of the AGA Practice Management and Economics Committee’s Coverage And Reimbursement Subcommittee and clinical assistant professor of medicine, gastroenterology & hepatology, Stanford Medicine, Stanford, California. Dr. Mathews is a member of the AGA Government Affairs Committee and leads efforts in clinical innovation at the Johns Hopkins Armstrong Institute for Patient Safety and Quality, Baltimore.
Tihs story was updated on July 29, 2019.