User login
Interview with Joseph R. Berger, MD, on the Financial Contribution of the MS Specialist
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
2019 Update on fertility
Professional societies, global organizations, and advocacy groups are continually working toward the goal of having the costs of infertility care covered by insurance carriers. Paramount to that effort is obtaining recognition of infertility as a burdensome disease. In this Update, we summarize national and international initiatives and societal trends that are helping to move us closer to that goal, and we encourage ObGyns to lead advocacy efforts.
Next, we detail several notable new features available in the annual report of the Society for Assisted Reproductive Technology (SART), an online interactive document that can be used to assist clinicians and patients in treatment decisions.
We also tackle the complexities of embryo selection for in vitro fertilization (IVF) and describe a potentially promising aneuploidy screening test, and explore its limitations.
Advances in recognizing infertility as a disease that merits insurance coverage
Major reproductive medicine organizations globally have endorsed the definition of infertility as a disease that "generates disability as an impairment of function" (TABLE 1).2 Fortunately, medical, societal, and judicial changes have resulted in progress for the 6.1 million women (and equivalent number of men) affected by infertility in the United States.3

Professional group advocacy efforts, and judicial rulings
The World Health Organization (WHO) has addressed infertility over the past several decades, with the organization's standards on semen analysis being the most recognized outcome. Progress has been limited, however, regarding global or national policy that recognizes the importance of infertility as a medical and public health problem.
In 2009, the glossary published by the WHO with the International Committee for Monitoring Assisted Reproductive Technology (ICMART) defined infertility as a disease.4 This recognition is important because it aids policy making, insurance coverage, and/or other payments for services.
The WHO also has begun the process of developing new infertility guidelines. Recently, the WHO held a summit on safety and access to fertility care, which was attended by many representatives of nation-state governments and international experts. It is hoped that a document from those proceedings will reinforce the public health importance of infertility and support the need to promote equality in access to safe fertility care. WHO initiatives matter because they apply to nation-states.
In the United States, the American Society for Reproductive Medicine (ASRM) for many years has recognized infertility as a disease. Only in 2017, however, did delegates at the American Medical Association's annual meeting vote to support the WHO's designation of infertility as a disease.
Continue to: Judicial views
Judicial views. In 1998, the US Supreme Court held that infertility is a disability under the Americans with Disabilities Act (ADA). The Court subsequently held, however, that a person is not considered disabled under the act if the disability can be overcome by mitigating or corrective measures. In 2000, a lower court held that, while infertility is a disability, an employer's health plan that excludes treatment for it is not discriminatory under the ADA if it applies to all employees.
Societal recognition. Interestingly, improved technology for oocyte cryopreservation has resulted in greater recognition of reproductive issues and the disparity in reproductive health societal norms and rights between men and women.
Media stories and gender issues in employment, especially in such high-profile industries as technology and finance, have highlighted long-standing inequities, many of which concern reproductive issues. These issues have been further disseminated by the #metoo movement. Some employers are beginning to respond by recognizing their employees' reproductive needs and providing improved benefits for reproductive care.
ObGyns must continue to lead advocacy
Not all has been progress. Personhood bills in several states threaten basic reproductive rights of women and men. The ASRM and Resolve (the National Infertility Association) have taken leading roles in opposing these legislative initiatives and supporting reproductive rights.5
Advocacy efforts through events and trends have resulted in gradually improving the recognition of the burden of infertility, inadequate insurance coverage, and continuing gender inequalities in reproduction. Today, patients, professionals, and national and international organizations are coalescing around demands for recognition, access to care, and gender and diversity equality. While much remains to be done, progress is being made in society, government, the workplace, and the health care system.
ObGyns and other women's health care providers can help continue the progress toward equality in reproductive rights, including access to infertility care, by discussing insurance inequities with patients, informing insurance companies that infertility is a disease, and encouraging patients to challenge inadequate and unequal insurance coverage of needed reproductive health care.
The time is now for ObGyns and other women’s health care providers to advocate for insurance coverage of infertility care. When our patients have inadequate coverage, we should encourage them to take action by contacting their insurance company and their employers to explain the reasons and argue for better coverage. Also, contact RESOLVE for additional information.
Latest SART report offers new features to aid in treatment decision making
Knowledge of the prognosis and its various treatment options is an important aspect of infertility treatment. The SART recently updated its annual Clinic Summary Report (CSR), which includes valuable new features for patients and physicians considering assisted reproductive technology (ART) treatment.6
SART compiles complex data and reports outcomes
The SART has been reporting IVF outcomes and other ART outcomes since 1988. The society's annual report is widely read by consumers, patients, physicians, and policy makers, and it has many important uses. However, the report is complicated and difficult to interpret for many reasons. For example, treatments are complex and varied (especially with application of new cryopreservation technology), and there are variations among clinics with respect to patient selection, protocols used, philosophy of practice, and numerous other variables.
Continue to: Because of this...
Because of this, the SART states, "The SART Clinic Summary Report (CSR) allows patients to view national and individual clinic IVF success rates. The data presented in this report should not be used for comparing clinics. Clinics may have differences in patient selection and treatment approaches which may artificially inflate or lower pregnancy rates relative to another clinic. Please discuss this with your doctor."6
Nevertheless, the CSR is extremely useful because it reports outcomes, which can lead to more informed patients and physicians and thus better access to safe and effective use of ART. The SART has redesigned the CSR to make it more useful.
Redesigned CSR focuses on outcomes important to patients
In recent years, new technologies have increased dramatically the use of embryo cryopreservation, genetic testing, and single embryo transfer (SET). The new CSR format is more patient focused and identifies more directly the treatment burden: ovarian stimulation, egg retrieval, intracytoplasmic sperm injection, preimplantation genetic testing (PGT), cryopreservation, frozen embryo transfer, and multiple cycles. It also focuses on the important patient outcomes, including live birth of a healthy child, multiple pregnancy, number of cycles, and chances of success per patient over time (including both fresh and frozen embryo transfers).
Notable changes
A major change in the CSR is that there is a preliminary report for a given year and then a final report the following year. This helps to more accurately report cycles that have been "delayed" because of egg retrieval and embryo freezing performed in the reported year but then transferred in the following reporting year.
Cycle counting. A cycle is counted when a woman has started medications for an ART procedure or, in a "natural" cycle when no medications are used, the first day of menses of the ART cycle. If several cycles are performed to bank eggs or embryos, each will be counted in the denominator when calculating the pregnancy rate. This more accurately reflects the patient treatment burden and costs. A cycle cancelled before egg retrieval is still counted as a cycle.
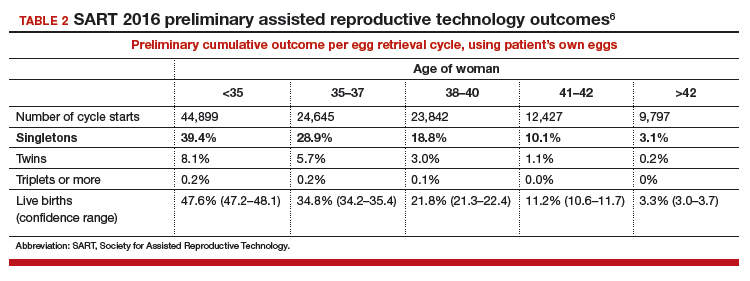
Defining success. Success is characterized as delivery of a child, since this is the outcome patients desire. Singleton deliveries are emphasized, since twin and higher-order multiple pregnancies have a higher risk of prematurity, morbidity, mortality, and cost. The percentages of triplet, twin, and singleton births contributing to the live birth rate are provided for each cycle group, as is prematurity (TABLE 2).6
The end point of a treatment cycle can vary. The new CSR captures the success rate following one or more egg retrievals and the first embryo transfer (primary outcome), the success of subsequent cycles using frozen eggs or embryos not transferred in the first embryo transfer, and the combined contribution of the primary and subsequent cycles to the cumulative live birth rate for a patient both in the preliminary report and the final report for any given year. The live birth rate per patient also is reported and includes the outcomes for patients who are new to an infertility center and starting their first cycle for retrieval of their own eggs during the reporting year.
Continue to: Outcomes and prognostic factors...
Outcomes and prognostic factors. Outcomes are reported by multiple factors, including patient age and source of the eggs. These are important prognostic factors; separating the data allows you to obtain a better idea of both national and individual clinic experience by these factors.
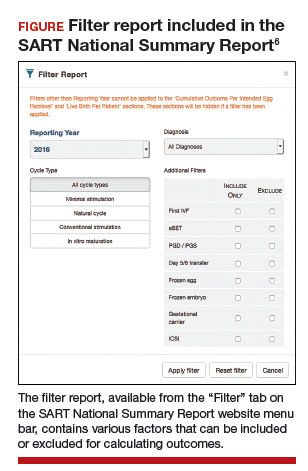
The CSR also contains filters for infertility diagnosis, stimulation type, and other treatment details (FIGURE).6 The filter is a useful feature because multiple types of treatment can be included or excluded. The outcome of different treatment interventions can then be estimated based on outcomes from the entire sample of US patients with similar characteristics and interventions. This powerful tool can help patients and physicians choose the best treatment based on prognosis.
Personalized prognosis. An important new feature is the SART Patient Predictor (https://www.sartcorsonline.com/predictor/patient), a model that permits an individual patient to obtain a more personalized prognosis. While the SART predictor uses only basic patient information, such as age, body mass index, and diagnosis, its estimate is based on the entire US sample of reported ART experience and therefore can help patients in decision making. Furthermore, the predictor calculates percentages for the outcome of one transfer of 2 embryos, and 2 transfers of a single embryo, to demonstrate the advantages of SET that result in a higher live birth rate but a significantly lower multiple pregnancy rate.
Summing up
The SART's new CSR is extremely useful to patients and to any physician who cares for infertility patients. It can help users both understand the expected results from different ART treatments and enable better physician-patient communication and decision making.
The updated annual SART Clinic Summary Report is an exceptionally valuable and easy-to-use online tool for you and your infertility patients.
Embryo selection techniques refined with use of newer technologies
Since the introduction of IVF in 1978, the final cumulative live birth rates per cycle initiated for oocyte retrieval after all resulting embryos have been trasferred continue to rise, currently standing at 54% for women younger than age 35 in the United States.7 A number of achievements have contributed to this remarkable success, namely, improvements in IVF laboratory and embryo culture systems, advances in cryopreservation technology, availability of highly effective gonadotropins and gonadotropin-releasing hormone analogues, improved ultrasound technology, and the introduction of soft catheters for atraumatic embryo transfers.
Treatment now focuses on improved embryo selection
Now that excellent success rates have been attained, the focus of optimizing efforts in fertility treatment has shifted to improving safety by reducing the rates of multiple pregnancy through elective single embryo transfer (eSET), reducing the rates of miscarriage, and shortening the time to live birth. Methods to improve embryo selection lie at the forefront of these initiatives. These vary and include extended culture to blastocyst stage, standard morphologic evaluation as well as morphokinetic assessment of embryonic development via time-lapse imaging, and more recently the reintroduction of preimplantation genetic testing for aneuploidy (PGT-A), formerly known as preimplantation genetic screening (PGS).
Chromosomal abnormalities of the embryo, or embryo aneuploidies, are the most common cause of treatment failure following embryo transfer in IVF. The proportion of embryos affected with aneuploidies significantly increases with advancing maternal age: 40% to 50% of blastocysts in women younger than age 35 and about 90% of blastocysts in women older than age 42.8 The premise with PGT-A is to identify these aneuploid embryos and increase the chances of success per embryo transfer by transferring euploid embryos.
Continue to: That concept was initially applied...
That concept was initially applied to cleavage-stage embryos through the use of fluorescence in situ hybridization (FISH) technology to interrogate a maximum of 5 to 9 chromosomes in a single cell (single blastomere); however, although initial results from observational studies were encouraging, subsequent randomized controlled studies unexpectedly showed a reduction in pregnancy rates.9 This was attributed to several factors, including biopsy-related damage to the cleavage-stage embryo, inability of FISH technology to assess aneuploidies of more than 5 to 9 chromosomes, mosaicism, and technical limitations associated with single-cell analysis.
Second-generation PGT-A testing has promise, and limitations
The newer PGT-A tests the embryos at the blastocyst stage by using biopsy samples from the trophectoderm (which will form the future placenta); this is expected to spare the inner cell mass ([ICM] which will give rise to the embryo proper) from biopsy-related injury.
On the genetics side, newer technologies, such as array comparative genomic hybridization, single nucleotide polymorphism arrays, quantitative polymerase chain reaction, and next-generation sequencing, offer the opportunity to assess all 24 chromosomes in a single biopsy specimen. Although a detailed discussion of these testing platforms is beyond the scope of this Update, certain points are worth mentioning. All these technologies require some form of genetic material amplification (most commonly whole genome amplification or multiplex polymerase chain reaction) to increase the relatively scant amount of DNA obtained from a sample of 4 to 6 cells. These amplification techniques have limitations that can subsequently impact the validity of the test results.
Furthermore, there is no consistency in depth of coverage for various parts of the genome, and subchromosomal (segmental) copy number variations below 3 to 5 Mb may not be detected. The threshold used in bioinformatics algorithms employed to interpret the raw data is subject to several biases and is not consistent among laboratories. As a result, the same sample assessed in different laboratories can potentially yield different results.
In addition to these technical limitations, mosaicism can pose another biologic limitation, as the biopsied trophectoderm cells may not accurately represent the chromosomal makeup of the ICM. Also, an embryo may be able to undergo self-correction during subsequent stages of development, and therefore even a documented trophectoderm abnormality at the blastocyst stage may not necessarily preclude that embryo from developing into a healthy baby.
Standardization is needed. Despite widespread promotion of PGT-A, well-designed randomized clinical trials (RCTs) have not yet consistently shown its benefits in improving pregnancy rates or reducing miscarriage rates. Although the initial small RCTs in a selected group of good prognosis patients suggested a beneficial effect in ongoing pregnancy rates per transfer, the largest multicenter RCT to date did not show any improvement in pregnancy rates or reduction in miscarriage rates.10 In that study, a post hoc subgroup analysis suggested a possible beneficial effect in women aged 35 to 40. However, those results must be validated and reproduced with randomization at the start of stimulation, with the primary outcome being the live birth rate per initiated cycle, instead of per transfer, before PGT-A can be adopted universally in clinical practice.
Continue to: With all the above considerations...
With all the above considerations, the ASRM has appropriately concluded that "the value of preimplantation genetic testing for aneuploidy (PGT-A) as a screening test for IVF patients has yet to be determined."11
Standardization of clinical and laboratory protocols and additional studies to assess the effects of PGT-A on live birth rates per initiated cycles are recommended before this new technology is widely adopted in routine clinical practice. In our practice, we routinely offer and perform extended culture to blastocyst stage and standard morphologic assessment. After a thorough counseling on the current status of PGT-A, about 15% to 20% of our patients opt to undergo PGT-A.
- United Nations website. General Assembly resolution 217A: Declaration of human rights. December 10, 1948. http://www.un.org/en/universal-declara tion-human-rights/. Accessed January 11, 2019.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393-406.
- US Department of Health and Human Services Office on Women's Health website. Infertility. https://www.womenshealth.gov/a-z-topics/infertility. Accessed January 24, 2019.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology, World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
- RESOLVE: The National Infertility Association website. Opposing personhood: Resolve fights to keep fertility medical treatments legal in the US. https://resolve.org/get-involved/advocate-for-access/our-issues/opposing-personhood/. Accessed January 11, 2019.
- Society for Assisted Reproductive Technology website. National summary report. 2016 Preliminary national data. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016 . Accessed January 12, 2019.
- Society for Assisted Reproductive Technology website. National summary report 2015. https://www.sartcorsonline,com/rptCSR_PublicMultYear.aspx ?reportingYear=2015. Accessed January 12, 2019.
- Harton GL, Munne S, Surrey M, et al; PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695-1703.
- Mastenbroek S, Twisk M, van Echten-Arends, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9-17
- Munne S, Kaplan B, Frattarelli JL, et al. Global multicenter randomized controlled trial comparing single embryo transfer with embryo selected by preimplantation genetic screening using next-generation sequencing versus morphologic assessment [abstract O-43]. Fertil Steril. 2017;108(suppl):e19.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
Professional societies, global organizations, and advocacy groups are continually working toward the goal of having the costs of infertility care covered by insurance carriers. Paramount to that effort is obtaining recognition of infertility as a burdensome disease. In this Update, we summarize national and international initiatives and societal trends that are helping to move us closer to that goal, and we encourage ObGyns to lead advocacy efforts.
Next, we detail several notable new features available in the annual report of the Society for Assisted Reproductive Technology (SART), an online interactive document that can be used to assist clinicians and patients in treatment decisions.
We also tackle the complexities of embryo selection for in vitro fertilization (IVF) and describe a potentially promising aneuploidy screening test, and explore its limitations.
Advances in recognizing infertility as a disease that merits insurance coverage
Major reproductive medicine organizations globally have endorsed the definition of infertility as a disease that "generates disability as an impairment of function" (TABLE 1).2 Fortunately, medical, societal, and judicial changes have resulted in progress for the 6.1 million women (and equivalent number of men) affected by infertility in the United States.3

Professional group advocacy efforts, and judicial rulings
The World Health Organization (WHO) has addressed infertility over the past several decades, with the organization's standards on semen analysis being the most recognized outcome. Progress has been limited, however, regarding global or national policy that recognizes the importance of infertility as a medical and public health problem.
In 2009, the glossary published by the WHO with the International Committee for Monitoring Assisted Reproductive Technology (ICMART) defined infertility as a disease.4 This recognition is important because it aids policy making, insurance coverage, and/or other payments for services.
The WHO also has begun the process of developing new infertility guidelines. Recently, the WHO held a summit on safety and access to fertility care, which was attended by many representatives of nation-state governments and international experts. It is hoped that a document from those proceedings will reinforce the public health importance of infertility and support the need to promote equality in access to safe fertility care. WHO initiatives matter because they apply to nation-states.
In the United States, the American Society for Reproductive Medicine (ASRM) for many years has recognized infertility as a disease. Only in 2017, however, did delegates at the American Medical Association's annual meeting vote to support the WHO's designation of infertility as a disease.
Continue to: Judicial views
Judicial views. In 1998, the US Supreme Court held that infertility is a disability under the Americans with Disabilities Act (ADA). The Court subsequently held, however, that a person is not considered disabled under the act if the disability can be overcome by mitigating or corrective measures. In 2000, a lower court held that, while infertility is a disability, an employer's health plan that excludes treatment for it is not discriminatory under the ADA if it applies to all employees.
Societal recognition. Interestingly, improved technology for oocyte cryopreservation has resulted in greater recognition of reproductive issues and the disparity in reproductive health societal norms and rights between men and women.
Media stories and gender issues in employment, especially in such high-profile industries as technology and finance, have highlighted long-standing inequities, many of which concern reproductive issues. These issues have been further disseminated by the #metoo movement. Some employers are beginning to respond by recognizing their employees' reproductive needs and providing improved benefits for reproductive care.
ObGyns must continue to lead advocacy
Not all has been progress. Personhood bills in several states threaten basic reproductive rights of women and men. The ASRM and Resolve (the National Infertility Association) have taken leading roles in opposing these legislative initiatives and supporting reproductive rights.5
Advocacy efforts through events and trends have resulted in gradually improving the recognition of the burden of infertility, inadequate insurance coverage, and continuing gender inequalities in reproduction. Today, patients, professionals, and national and international organizations are coalescing around demands for recognition, access to care, and gender and diversity equality. While much remains to be done, progress is being made in society, government, the workplace, and the health care system.
ObGyns and other women's health care providers can help continue the progress toward equality in reproductive rights, including access to infertility care, by discussing insurance inequities with patients, informing insurance companies that infertility is a disease, and encouraging patients to challenge inadequate and unequal insurance coverage of needed reproductive health care.
The time is now for ObGyns and other women’s health care providers to advocate for insurance coverage of infertility care. When our patients have inadequate coverage, we should encourage them to take action by contacting their insurance company and their employers to explain the reasons and argue for better coverage. Also, contact RESOLVE for additional information.
Latest SART report offers new features to aid in treatment decision making
Knowledge of the prognosis and its various treatment options is an important aspect of infertility treatment. The SART recently updated its annual Clinic Summary Report (CSR), which includes valuable new features for patients and physicians considering assisted reproductive technology (ART) treatment.6
SART compiles complex data and reports outcomes
The SART has been reporting IVF outcomes and other ART outcomes since 1988. The society's annual report is widely read by consumers, patients, physicians, and policy makers, and it has many important uses. However, the report is complicated and difficult to interpret for many reasons. For example, treatments are complex and varied (especially with application of new cryopreservation technology), and there are variations among clinics with respect to patient selection, protocols used, philosophy of practice, and numerous other variables.
Continue to: Because of this...
Because of this, the SART states, "The SART Clinic Summary Report (CSR) allows patients to view national and individual clinic IVF success rates. The data presented in this report should not be used for comparing clinics. Clinics may have differences in patient selection and treatment approaches which may artificially inflate or lower pregnancy rates relative to another clinic. Please discuss this with your doctor."6
Nevertheless, the CSR is extremely useful because it reports outcomes, which can lead to more informed patients and physicians and thus better access to safe and effective use of ART. The SART has redesigned the CSR to make it more useful.
Redesigned CSR focuses on outcomes important to patients
In recent years, new technologies have increased dramatically the use of embryo cryopreservation, genetic testing, and single embryo transfer (SET). The new CSR format is more patient focused and identifies more directly the treatment burden: ovarian stimulation, egg retrieval, intracytoplasmic sperm injection, preimplantation genetic testing (PGT), cryopreservation, frozen embryo transfer, and multiple cycles. It also focuses on the important patient outcomes, including live birth of a healthy child, multiple pregnancy, number of cycles, and chances of success per patient over time (including both fresh and frozen embryo transfers).
Notable changes
A major change in the CSR is that there is a preliminary report for a given year and then a final report the following year. This helps to more accurately report cycles that have been "delayed" because of egg retrieval and embryo freezing performed in the reported year but then transferred in the following reporting year.
Cycle counting. A cycle is counted when a woman has started medications for an ART procedure or, in a "natural" cycle when no medications are used, the first day of menses of the ART cycle. If several cycles are performed to bank eggs or embryos, each will be counted in the denominator when calculating the pregnancy rate. This more accurately reflects the patient treatment burden and costs. A cycle cancelled before egg retrieval is still counted as a cycle.
Defining success. Success is characterized as delivery of a child, since this is the outcome patients desire. Singleton deliveries are emphasized, since twin and higher-order multiple pregnancies have a higher risk of prematurity, morbidity, mortality, and cost. The percentages of triplet, twin, and singleton births contributing to the live birth rate are provided for each cycle group, as is prematurity (TABLE 2).6
The end point of a treatment cycle can vary. The new CSR captures the success rate following one or more egg retrievals and the first embryo transfer (primary outcome), the success of subsequent cycles using frozen eggs or embryos not transferred in the first embryo transfer, and the combined contribution of the primary and subsequent cycles to the cumulative live birth rate for a patient both in the preliminary report and the final report for any given year. The live birth rate per patient also is reported and includes the outcomes for patients who are new to an infertility center and starting their first cycle for retrieval of their own eggs during the reporting year.
Continue to: Outcomes and prognostic factors...
Outcomes and prognostic factors. Outcomes are reported by multiple factors, including patient age and source of the eggs. These are important prognostic factors; separating the data allows you to obtain a better idea of both national and individual clinic experience by these factors.
The CSR also contains filters for infertility diagnosis, stimulation type, and other treatment details (FIGURE).6 The filter is a useful feature because multiple types of treatment can be included or excluded. The outcome of different treatment interventions can then be estimated based on outcomes from the entire sample of US patients with similar characteristics and interventions. This powerful tool can help patients and physicians choose the best treatment based on prognosis.

Personalized prognosis. An important new feature is the SART Patient Predictor (https://www.sartcorsonline.com/predictor/patient), a model that permits an individual patient to obtain a more personalized prognosis. While the SART predictor uses only basic patient information, such as age, body mass index, and diagnosis, its estimate is based on the entire US sample of reported ART experience and therefore can help patients in decision making. Furthermore, the predictor calculates percentages for the outcome of one transfer of 2 embryos, and 2 transfers of a single embryo, to demonstrate the advantages of SET that result in a higher live birth rate but a significantly lower multiple pregnancy rate.

Summing up
The SART's new CSR is extremely useful to patients and to any physician who cares for infertility patients. It can help users both understand the expected results from different ART treatments and enable better physician-patient communication and decision making.
The updated annual SART Clinic Summary Report is an exceptionally valuable and easy-to-use online tool for you and your infertility patients.
Embryo selection techniques refined with use of newer technologies
Since the introduction of IVF in 1978, the final cumulative live birth rates per cycle initiated for oocyte retrieval after all resulting embryos have been trasferred continue to rise, currently standing at 54% for women younger than age 35 in the United States.7 A number of achievements have contributed to this remarkable success, namely, improvements in IVF laboratory and embryo culture systems, advances in cryopreservation technology, availability of highly effective gonadotropins and gonadotropin-releasing hormone analogues, improved ultrasound technology, and the introduction of soft catheters for atraumatic embryo transfers.
Treatment now focuses on improved embryo selection
Now that excellent success rates have been attained, the focus of optimizing efforts in fertility treatment has shifted to improving safety by reducing the rates of multiple pregnancy through elective single embryo transfer (eSET), reducing the rates of miscarriage, and shortening the time to live birth. Methods to improve embryo selection lie at the forefront of these initiatives. These vary and include extended culture to blastocyst stage, standard morphologic evaluation as well as morphokinetic assessment of embryonic development via time-lapse imaging, and more recently the reintroduction of preimplantation genetic testing for aneuploidy (PGT-A), formerly known as preimplantation genetic screening (PGS).
Chromosomal abnormalities of the embryo, or embryo aneuploidies, are the most common cause of treatment failure following embryo transfer in IVF. The proportion of embryos affected with aneuploidies significantly increases with advancing maternal age: 40% to 50% of blastocysts in women younger than age 35 and about 90% of blastocysts in women older than age 42.8 The premise with PGT-A is to identify these aneuploid embryos and increase the chances of success per embryo transfer by transferring euploid embryos.
Continue to: That concept was initially applied...
That concept was initially applied to cleavage-stage embryos through the use of fluorescence in situ hybridization (FISH) technology to interrogate a maximum of 5 to 9 chromosomes in a single cell (single blastomere); however, although initial results from observational studies were encouraging, subsequent randomized controlled studies unexpectedly showed a reduction in pregnancy rates.9 This was attributed to several factors, including biopsy-related damage to the cleavage-stage embryo, inability of FISH technology to assess aneuploidies of more than 5 to 9 chromosomes, mosaicism, and technical limitations associated with single-cell analysis.
Second-generation PGT-A testing has promise, and limitations
The newer PGT-A tests the embryos at the blastocyst stage by using biopsy samples from the trophectoderm (which will form the future placenta); this is expected to spare the inner cell mass ([ICM] which will give rise to the embryo proper) from biopsy-related injury.
On the genetics side, newer technologies, such as array comparative genomic hybridization, single nucleotide polymorphism arrays, quantitative polymerase chain reaction, and next-generation sequencing, offer the opportunity to assess all 24 chromosomes in a single biopsy specimen. Although a detailed discussion of these testing platforms is beyond the scope of this Update, certain points are worth mentioning. All these technologies require some form of genetic material amplification (most commonly whole genome amplification or multiplex polymerase chain reaction) to increase the relatively scant amount of DNA obtained from a sample of 4 to 6 cells. These amplification techniques have limitations that can subsequently impact the validity of the test results.
Furthermore, there is no consistency in depth of coverage for various parts of the genome, and subchromosomal (segmental) copy number variations below 3 to 5 Mb may not be detected. The threshold used in bioinformatics algorithms employed to interpret the raw data is subject to several biases and is not consistent among laboratories. As a result, the same sample assessed in different laboratories can potentially yield different results.
In addition to these technical limitations, mosaicism can pose another biologic limitation, as the biopsied trophectoderm cells may not accurately represent the chromosomal makeup of the ICM. Also, an embryo may be able to undergo self-correction during subsequent stages of development, and therefore even a documented trophectoderm abnormality at the blastocyst stage may not necessarily preclude that embryo from developing into a healthy baby.
Standardization is needed. Despite widespread promotion of PGT-A, well-designed randomized clinical trials (RCTs) have not yet consistently shown its benefits in improving pregnancy rates or reducing miscarriage rates. Although the initial small RCTs in a selected group of good prognosis patients suggested a beneficial effect in ongoing pregnancy rates per transfer, the largest multicenter RCT to date did not show any improvement in pregnancy rates or reduction in miscarriage rates.10 In that study, a post hoc subgroup analysis suggested a possible beneficial effect in women aged 35 to 40. However, those results must be validated and reproduced with randomization at the start of stimulation, with the primary outcome being the live birth rate per initiated cycle, instead of per transfer, before PGT-A can be adopted universally in clinical practice.
Continue to: With all the above considerations...
With all the above considerations, the ASRM has appropriately concluded that "the value of preimplantation genetic testing for aneuploidy (PGT-A) as a screening test for IVF patients has yet to be determined."11
Standardization of clinical and laboratory protocols and additional studies to assess the effects of PGT-A on live birth rates per initiated cycles are recommended before this new technology is widely adopted in routine clinical practice. In our practice, we routinely offer and perform extended culture to blastocyst stage and standard morphologic assessment. After a thorough counseling on the current status of PGT-A, about 15% to 20% of our patients opt to undergo PGT-A.
Professional societies, global organizations, and advocacy groups are continually working toward the goal of having the costs of infertility care covered by insurance carriers. Paramount to that effort is obtaining recognition of infertility as a burdensome disease. In this Update, we summarize national and international initiatives and societal trends that are helping to move us closer to that goal, and we encourage ObGyns to lead advocacy efforts.
Next, we detail several notable new features available in the annual report of the Society for Assisted Reproductive Technology (SART), an online interactive document that can be used to assist clinicians and patients in treatment decisions.
We also tackle the complexities of embryo selection for in vitro fertilization (IVF) and describe a potentially promising aneuploidy screening test, and explore its limitations.
Advances in recognizing infertility as a disease that merits insurance coverage
Major reproductive medicine organizations globally have endorsed the definition of infertility as a disease that "generates disability as an impairment of function" (TABLE 1).2 Fortunately, medical, societal, and judicial changes have resulted in progress for the 6.1 million women (and equivalent number of men) affected by infertility in the United States.3

Professional group advocacy efforts, and judicial rulings
The World Health Organization (WHO) has addressed infertility over the past several decades, with the organization's standards on semen analysis being the most recognized outcome. Progress has been limited, however, regarding global or national policy that recognizes the importance of infertility as a medical and public health problem.
In 2009, the glossary published by the WHO with the International Committee for Monitoring Assisted Reproductive Technology (ICMART) defined infertility as a disease.4 This recognition is important because it aids policy making, insurance coverage, and/or other payments for services.
The WHO also has begun the process of developing new infertility guidelines. Recently, the WHO held a summit on safety and access to fertility care, which was attended by many representatives of nation-state governments and international experts. It is hoped that a document from those proceedings will reinforce the public health importance of infertility and support the need to promote equality in access to safe fertility care. WHO initiatives matter because they apply to nation-states.
In the United States, the American Society for Reproductive Medicine (ASRM) for many years has recognized infertility as a disease. Only in 2017, however, did delegates at the American Medical Association's annual meeting vote to support the WHO's designation of infertility as a disease.
Continue to: Judicial views
Judicial views. In 1998, the US Supreme Court held that infertility is a disability under the Americans with Disabilities Act (ADA). The Court subsequently held, however, that a person is not considered disabled under the act if the disability can be overcome by mitigating or corrective measures. In 2000, a lower court held that, while infertility is a disability, an employer's health plan that excludes treatment for it is not discriminatory under the ADA if it applies to all employees.
Societal recognition. Interestingly, improved technology for oocyte cryopreservation has resulted in greater recognition of reproductive issues and the disparity in reproductive health societal norms and rights between men and women.
Media stories and gender issues in employment, especially in such high-profile industries as technology and finance, have highlighted long-standing inequities, many of which concern reproductive issues. These issues have been further disseminated by the #metoo movement. Some employers are beginning to respond by recognizing their employees' reproductive needs and providing improved benefits for reproductive care.
ObGyns must continue to lead advocacy
Not all has been progress. Personhood bills in several states threaten basic reproductive rights of women and men. The ASRM and Resolve (the National Infertility Association) have taken leading roles in opposing these legislative initiatives and supporting reproductive rights.5
Advocacy efforts through events and trends have resulted in gradually improving the recognition of the burden of infertility, inadequate insurance coverage, and continuing gender inequalities in reproduction. Today, patients, professionals, and national and international organizations are coalescing around demands for recognition, access to care, and gender and diversity equality. While much remains to be done, progress is being made in society, government, the workplace, and the health care system.
ObGyns and other women's health care providers can help continue the progress toward equality in reproductive rights, including access to infertility care, by discussing insurance inequities with patients, informing insurance companies that infertility is a disease, and encouraging patients to challenge inadequate and unequal insurance coverage of needed reproductive health care.
The time is now for ObGyns and other women’s health care providers to advocate for insurance coverage of infertility care. When our patients have inadequate coverage, we should encourage them to take action by contacting their insurance company and their employers to explain the reasons and argue for better coverage. Also, contact RESOLVE for additional information.
Latest SART report offers new features to aid in treatment decision making
Knowledge of the prognosis and its various treatment options is an important aspect of infertility treatment. The SART recently updated its annual Clinic Summary Report (CSR), which includes valuable new features for patients and physicians considering assisted reproductive technology (ART) treatment.6
SART compiles complex data and reports outcomes
The SART has been reporting IVF outcomes and other ART outcomes since 1988. The society's annual report is widely read by consumers, patients, physicians, and policy makers, and it has many important uses. However, the report is complicated and difficult to interpret for many reasons. For example, treatments are complex and varied (especially with application of new cryopreservation technology), and there are variations among clinics with respect to patient selection, protocols used, philosophy of practice, and numerous other variables.
Continue to: Because of this...
Because of this, the SART states, "The SART Clinic Summary Report (CSR) allows patients to view national and individual clinic IVF success rates. The data presented in this report should not be used for comparing clinics. Clinics may have differences in patient selection and treatment approaches which may artificially inflate or lower pregnancy rates relative to another clinic. Please discuss this with your doctor."6
Nevertheless, the CSR is extremely useful because it reports outcomes, which can lead to more informed patients and physicians and thus better access to safe and effective use of ART. The SART has redesigned the CSR to make it more useful.
Redesigned CSR focuses on outcomes important to patients
In recent years, new technologies have increased dramatically the use of embryo cryopreservation, genetic testing, and single embryo transfer (SET). The new CSR format is more patient focused and identifies more directly the treatment burden: ovarian stimulation, egg retrieval, intracytoplasmic sperm injection, preimplantation genetic testing (PGT), cryopreservation, frozen embryo transfer, and multiple cycles. It also focuses on the important patient outcomes, including live birth of a healthy child, multiple pregnancy, number of cycles, and chances of success per patient over time (including both fresh and frozen embryo transfers).
Notable changes
A major change in the CSR is that there is a preliminary report for a given year and then a final report the following year. This helps to more accurately report cycles that have been "delayed" because of egg retrieval and embryo freezing performed in the reported year but then transferred in the following reporting year.
Cycle counting. A cycle is counted when a woman has started medications for an ART procedure or, in a "natural" cycle when no medications are used, the first day of menses of the ART cycle. If several cycles are performed to bank eggs or embryos, each will be counted in the denominator when calculating the pregnancy rate. This more accurately reflects the patient treatment burden and costs. A cycle cancelled before egg retrieval is still counted as a cycle.
Defining success. Success is characterized as delivery of a child, since this is the outcome patients desire. Singleton deliveries are emphasized, since twin and higher-order multiple pregnancies have a higher risk of prematurity, morbidity, mortality, and cost. The percentages of triplet, twin, and singleton births contributing to the live birth rate are provided for each cycle group, as is prematurity (TABLE 2).6
The end point of a treatment cycle can vary. The new CSR captures the success rate following one or more egg retrievals and the first embryo transfer (primary outcome), the success of subsequent cycles using frozen eggs or embryos not transferred in the first embryo transfer, and the combined contribution of the primary and subsequent cycles to the cumulative live birth rate for a patient both in the preliminary report and the final report for any given year. The live birth rate per patient also is reported and includes the outcomes for patients who are new to an infertility center and starting their first cycle for retrieval of their own eggs during the reporting year.
Continue to: Outcomes and prognostic factors...
Outcomes and prognostic factors. Outcomes are reported by multiple factors, including patient age and source of the eggs. These are important prognostic factors; separating the data allows you to obtain a better idea of both national and individual clinic experience by these factors.
The CSR also contains filters for infertility diagnosis, stimulation type, and other treatment details (FIGURE).6 The filter is a useful feature because multiple types of treatment can be included or excluded. The outcome of different treatment interventions can then be estimated based on outcomes from the entire sample of US patients with similar characteristics and interventions. This powerful tool can help patients and physicians choose the best treatment based on prognosis.

Personalized prognosis. An important new feature is the SART Patient Predictor (https://www.sartcorsonline.com/predictor/patient), a model that permits an individual patient to obtain a more personalized prognosis. While the SART predictor uses only basic patient information, such as age, body mass index, and diagnosis, its estimate is based on the entire US sample of reported ART experience and therefore can help patients in decision making. Furthermore, the predictor calculates percentages for the outcome of one transfer of 2 embryos, and 2 transfers of a single embryo, to demonstrate the advantages of SET that result in a higher live birth rate but a significantly lower multiple pregnancy rate.

Summing up
The SART's new CSR is extremely useful to patients and to any physician who cares for infertility patients. It can help users both understand the expected results from different ART treatments and enable better physician-patient communication and decision making.
The updated annual SART Clinic Summary Report is an exceptionally valuable and easy-to-use online tool for you and your infertility patients.
Embryo selection techniques refined with use of newer technologies
Since the introduction of IVF in 1978, the final cumulative live birth rates per cycle initiated for oocyte retrieval after all resulting embryos have been trasferred continue to rise, currently standing at 54% for women younger than age 35 in the United States.7 A number of achievements have contributed to this remarkable success, namely, improvements in IVF laboratory and embryo culture systems, advances in cryopreservation technology, availability of highly effective gonadotropins and gonadotropin-releasing hormone analogues, improved ultrasound technology, and the introduction of soft catheters for atraumatic embryo transfers.
Treatment now focuses on improved embryo selection
Now that excellent success rates have been attained, the focus of optimizing efforts in fertility treatment has shifted to improving safety by reducing the rates of multiple pregnancy through elective single embryo transfer (eSET), reducing the rates of miscarriage, and shortening the time to live birth. Methods to improve embryo selection lie at the forefront of these initiatives. These vary and include extended culture to blastocyst stage, standard morphologic evaluation as well as morphokinetic assessment of embryonic development via time-lapse imaging, and more recently the reintroduction of preimplantation genetic testing for aneuploidy (PGT-A), formerly known as preimplantation genetic screening (PGS).
Chromosomal abnormalities of the embryo, or embryo aneuploidies, are the most common cause of treatment failure following embryo transfer in IVF. The proportion of embryos affected with aneuploidies significantly increases with advancing maternal age: 40% to 50% of blastocysts in women younger than age 35 and about 90% of blastocysts in women older than age 42.8 The premise with PGT-A is to identify these aneuploid embryos and increase the chances of success per embryo transfer by transferring euploid embryos.
Continue to: That concept was initially applied...
That concept was initially applied to cleavage-stage embryos through the use of fluorescence in situ hybridization (FISH) technology to interrogate a maximum of 5 to 9 chromosomes in a single cell (single blastomere); however, although initial results from observational studies were encouraging, subsequent randomized controlled studies unexpectedly showed a reduction in pregnancy rates.9 This was attributed to several factors, including biopsy-related damage to the cleavage-stage embryo, inability of FISH technology to assess aneuploidies of more than 5 to 9 chromosomes, mosaicism, and technical limitations associated with single-cell analysis.
Second-generation PGT-A testing has promise, and limitations
The newer PGT-A tests the embryos at the blastocyst stage by using biopsy samples from the trophectoderm (which will form the future placenta); this is expected to spare the inner cell mass ([ICM] which will give rise to the embryo proper) from biopsy-related injury.
On the genetics side, newer technologies, such as array comparative genomic hybridization, single nucleotide polymorphism arrays, quantitative polymerase chain reaction, and next-generation sequencing, offer the opportunity to assess all 24 chromosomes in a single biopsy specimen. Although a detailed discussion of these testing platforms is beyond the scope of this Update, certain points are worth mentioning. All these technologies require some form of genetic material amplification (most commonly whole genome amplification or multiplex polymerase chain reaction) to increase the relatively scant amount of DNA obtained from a sample of 4 to 6 cells. These amplification techniques have limitations that can subsequently impact the validity of the test results.
Furthermore, there is no consistency in depth of coverage for various parts of the genome, and subchromosomal (segmental) copy number variations below 3 to 5 Mb may not be detected. The threshold used in bioinformatics algorithms employed to interpret the raw data is subject to several biases and is not consistent among laboratories. As a result, the same sample assessed in different laboratories can potentially yield different results.
In addition to these technical limitations, mosaicism can pose another biologic limitation, as the biopsied trophectoderm cells may not accurately represent the chromosomal makeup of the ICM. Also, an embryo may be able to undergo self-correction during subsequent stages of development, and therefore even a documented trophectoderm abnormality at the blastocyst stage may not necessarily preclude that embryo from developing into a healthy baby.
Standardization is needed. Despite widespread promotion of PGT-A, well-designed randomized clinical trials (RCTs) have not yet consistently shown its benefits in improving pregnancy rates or reducing miscarriage rates. Although the initial small RCTs in a selected group of good prognosis patients suggested a beneficial effect in ongoing pregnancy rates per transfer, the largest multicenter RCT to date did not show any improvement in pregnancy rates or reduction in miscarriage rates.10 In that study, a post hoc subgroup analysis suggested a possible beneficial effect in women aged 35 to 40. However, those results must be validated and reproduced with randomization at the start of stimulation, with the primary outcome being the live birth rate per initiated cycle, instead of per transfer, before PGT-A can be adopted universally in clinical practice.
Continue to: With all the above considerations...
With all the above considerations, the ASRM has appropriately concluded that "the value of preimplantation genetic testing for aneuploidy (PGT-A) as a screening test for IVF patients has yet to be determined."11
Standardization of clinical and laboratory protocols and additional studies to assess the effects of PGT-A on live birth rates per initiated cycles are recommended before this new technology is widely adopted in routine clinical practice. In our practice, we routinely offer and perform extended culture to blastocyst stage and standard morphologic assessment. After a thorough counseling on the current status of PGT-A, about 15% to 20% of our patients opt to undergo PGT-A.
- United Nations website. General Assembly resolution 217A: Declaration of human rights. December 10, 1948. http://www.un.org/en/universal-declara tion-human-rights/. Accessed January 11, 2019.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393-406.
- US Department of Health and Human Services Office on Women's Health website. Infertility. https://www.womenshealth.gov/a-z-topics/infertility. Accessed January 24, 2019.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology, World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
- RESOLVE: The National Infertility Association website. Opposing personhood: Resolve fights to keep fertility medical treatments legal in the US. https://resolve.org/get-involved/advocate-for-access/our-issues/opposing-personhood/. Accessed January 11, 2019.
- Society for Assisted Reproductive Technology website. National summary report. 2016 Preliminary national data. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016 . Accessed January 12, 2019.
- Society for Assisted Reproductive Technology website. National summary report 2015. https://www.sartcorsonline,com/rptCSR_PublicMultYear.aspx ?reportingYear=2015. Accessed January 12, 2019.
- Harton GL, Munne S, Surrey M, et al; PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695-1703.
- Mastenbroek S, Twisk M, van Echten-Arends, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9-17
- Munne S, Kaplan B, Frattarelli JL, et al. Global multicenter randomized controlled trial comparing single embryo transfer with embryo selected by preimplantation genetic screening using next-generation sequencing versus morphologic assessment [abstract O-43]. Fertil Steril. 2017;108(suppl):e19.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
- United Nations website. General Assembly resolution 217A: Declaration of human rights. December 10, 1948. http://www.un.org/en/universal-declara tion-human-rights/. Accessed January 11, 2019.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393-406.
- US Department of Health and Human Services Office on Women's Health website. Infertility. https://www.womenshealth.gov/a-z-topics/infertility. Accessed January 24, 2019.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology, World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
- RESOLVE: The National Infertility Association website. Opposing personhood: Resolve fights to keep fertility medical treatments legal in the US. https://resolve.org/get-involved/advocate-for-access/our-issues/opposing-personhood/. Accessed January 11, 2019.
- Society for Assisted Reproductive Technology website. National summary report. 2016 Preliminary national data. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016 . Accessed January 12, 2019.
- Society for Assisted Reproductive Technology website. National summary report 2015. https://www.sartcorsonline,com/rptCSR_PublicMultYear.aspx ?reportingYear=2015. Accessed January 12, 2019.
- Harton GL, Munne S, Surrey M, et al; PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695-1703.
- Mastenbroek S, Twisk M, van Echten-Arends, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9-17
- Munne S, Kaplan B, Frattarelli JL, et al. Global multicenter randomized controlled trial comparing single embryo transfer with embryo selected by preimplantation genetic screening using next-generation sequencing versus morphologic assessment [abstract O-43]. Fertil Steril. 2017;108(suppl):e19.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
Meta-analysis: IVIG bests anti-D on platelet count in pediatric ITP
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: Treatment with IVIG was 15% more likely than anti-D immunoglobulin to raise platelet counts higher than 20 × 109/L within 24-72 hours.
Study details: A systematic review and meta-analysis of 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP.
Disclosures: The meta-analysis did not have outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
Source: Lioger B et al. J Pediatr. 2019; 204:225-33.
Large Hemorrhagic Plaque With Central Crusting
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.
Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.

Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.

Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.

A 54-year-old woman with no notable medical history was referred to dermatology by her primary care provider for evaluation of a hematoma on the posterior neck that had developed gradually over 5 months. The lesion initially was asymptomatic but more recently had started to be painful and bleed intermittently. The patient denied any personal or family history of skin cancer. Physical examination revealed a large hemorrhagic plaque on the left side of the posterior neck with central brown-yellow crusting. There were few smaller, white, thin, sclerotic plaques with crinkling atrophy at the periphery of and inferolateral to the lesion. A punch biopsy specimen was obtained from the hemorrhagic plaque.
Managing Postinflammatory Hyperpigmentation in Pediatric Patients With Skin of Color
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
Practice Points
- The US population of children with skin of color is growing rapidly.
- Treating the underlying inflammatory dermatosis is the most important step in managing postinflammatory hyperpigmentation (PIH); however, many pediatric PIH patients and their parents/guardians presenting with a chief concern of pigmentary changes are unaware of the associated inflammatory condition.
- When appropriate, choose treatments for the underlying inflammatory condition that can simultaneously improve any existing PIH. Gentle skin care, avoidance of rubbing and scrubbing the skin, and photoprotection are essential to halt worsening of PIH.
- Patients’ parents/guardians may consent to more aggressive PIH treatment in select cases (eg, emotional distress in adolescents).
Safety and Efficacy of Halobetasol Propionate Lotion 0.01% in the Treatment of Moderate to Severe Plaque Psoriasis: A Pooled Analysis of 2 Phase 3 Studies
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
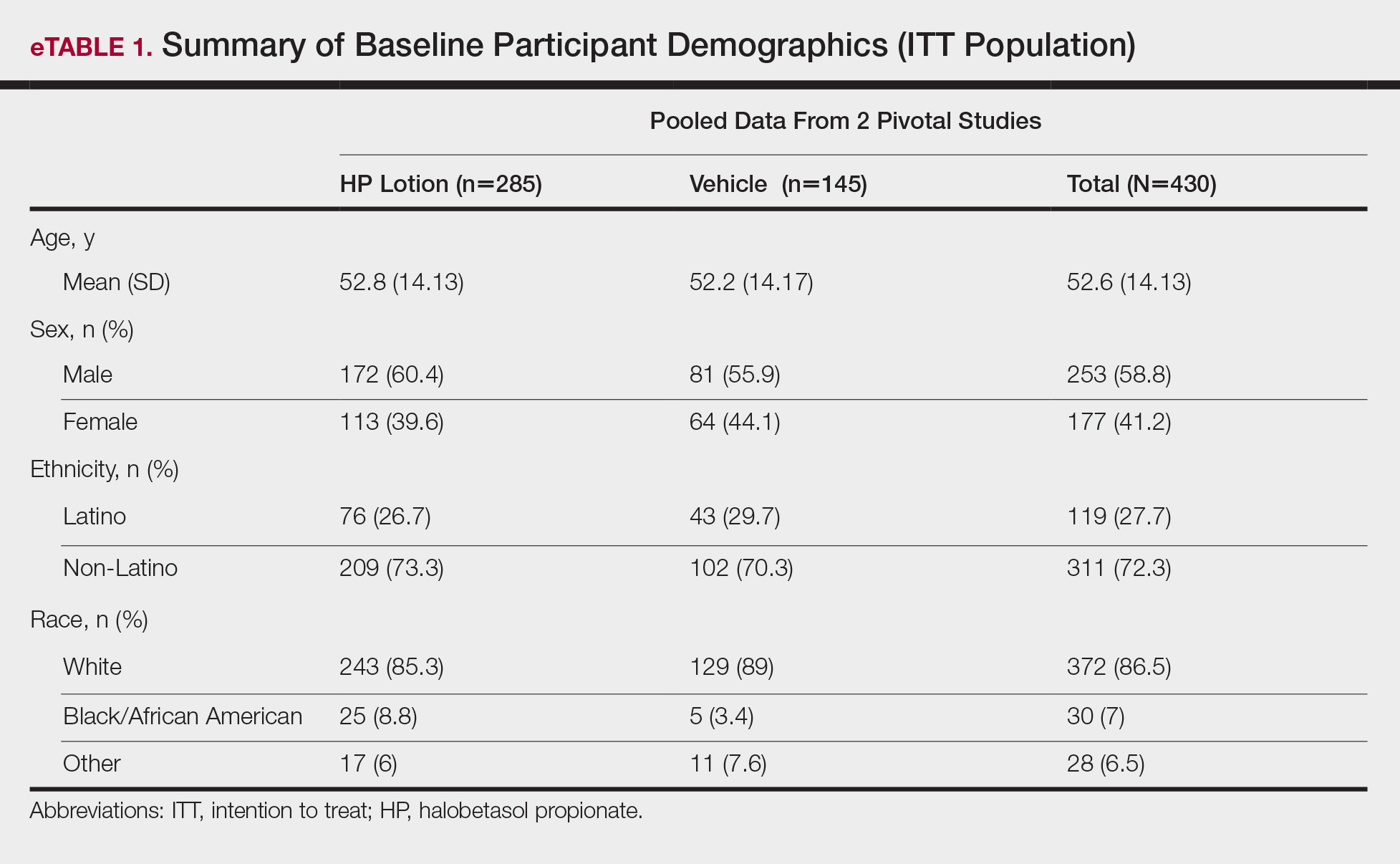
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
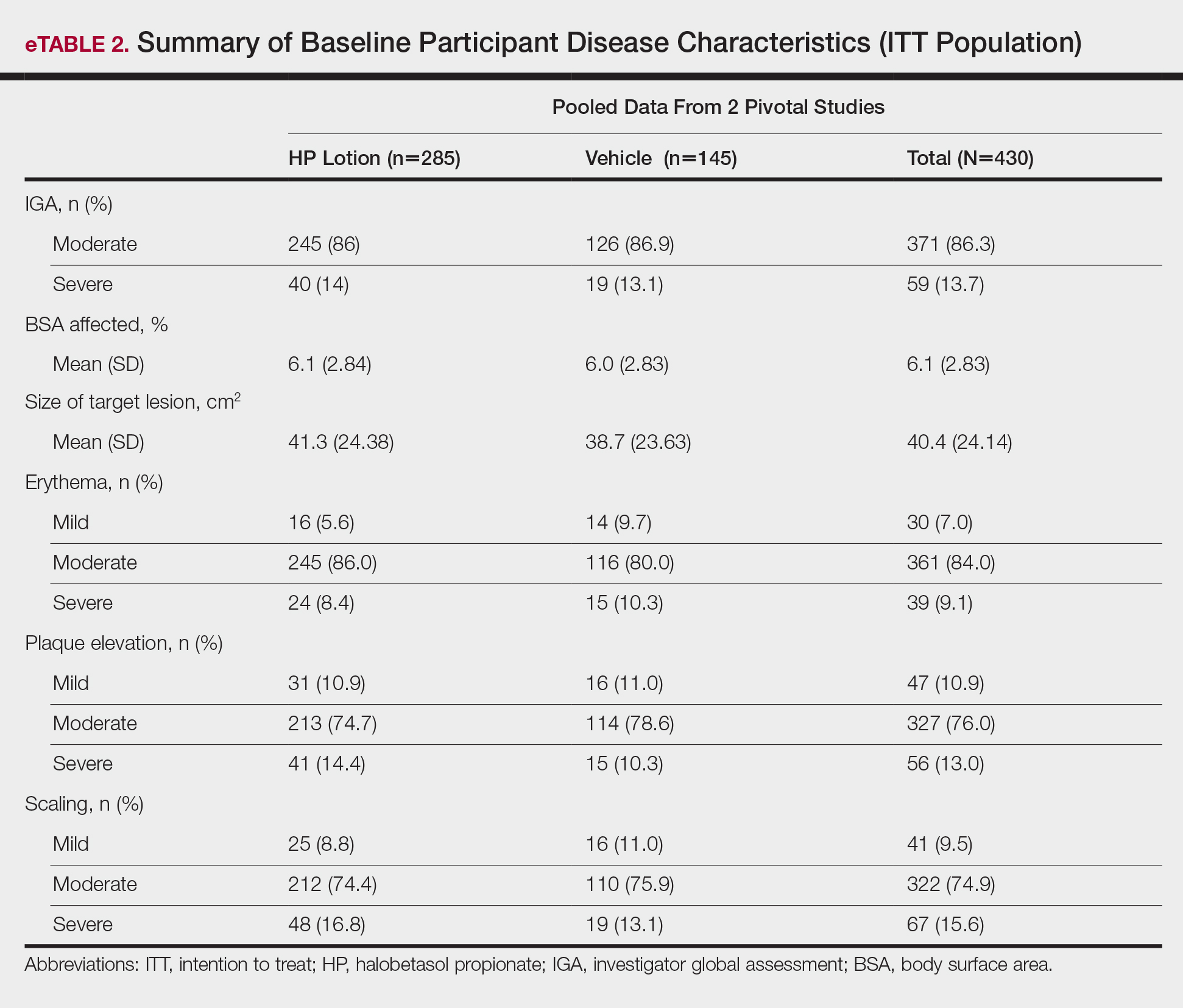
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
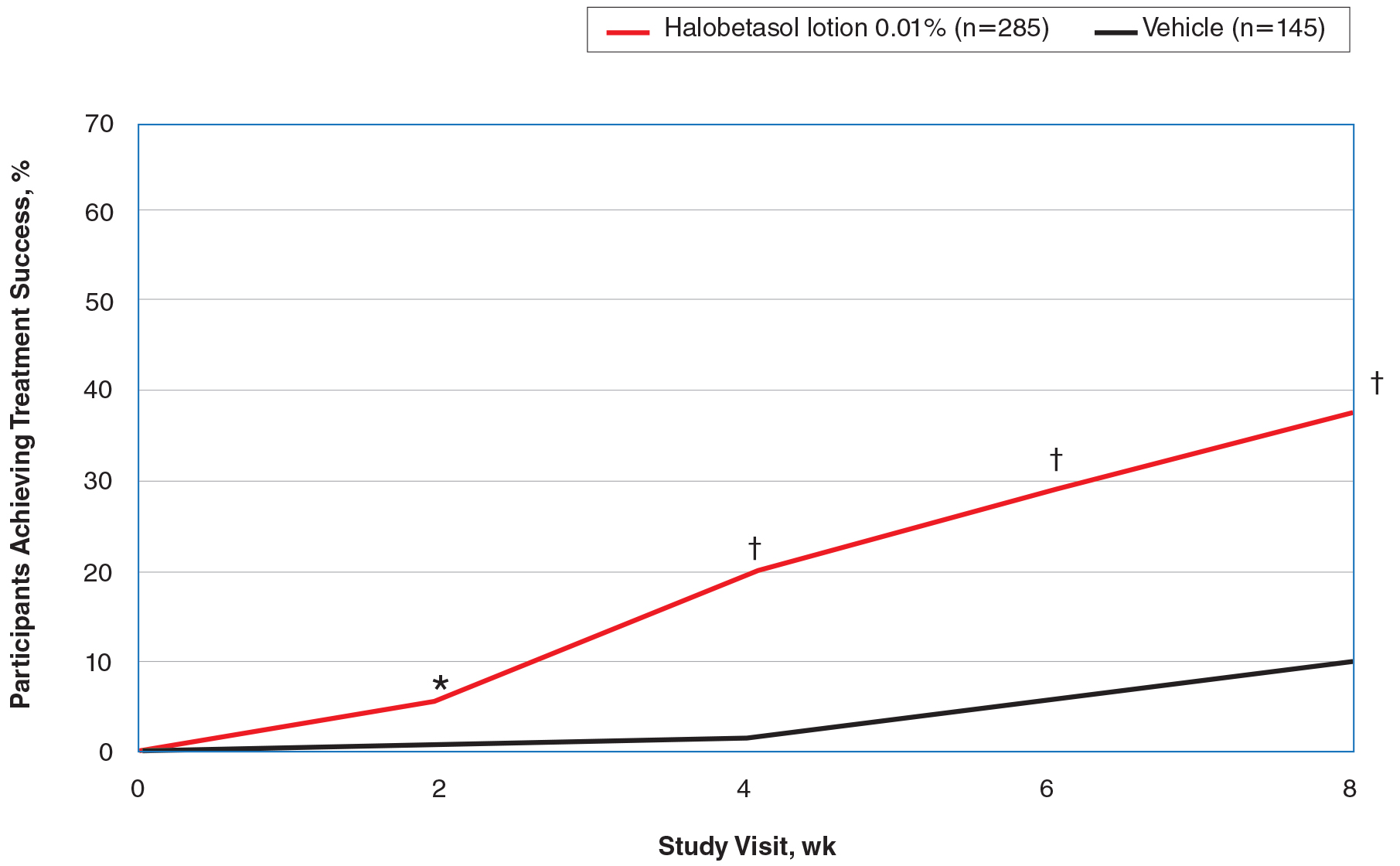
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
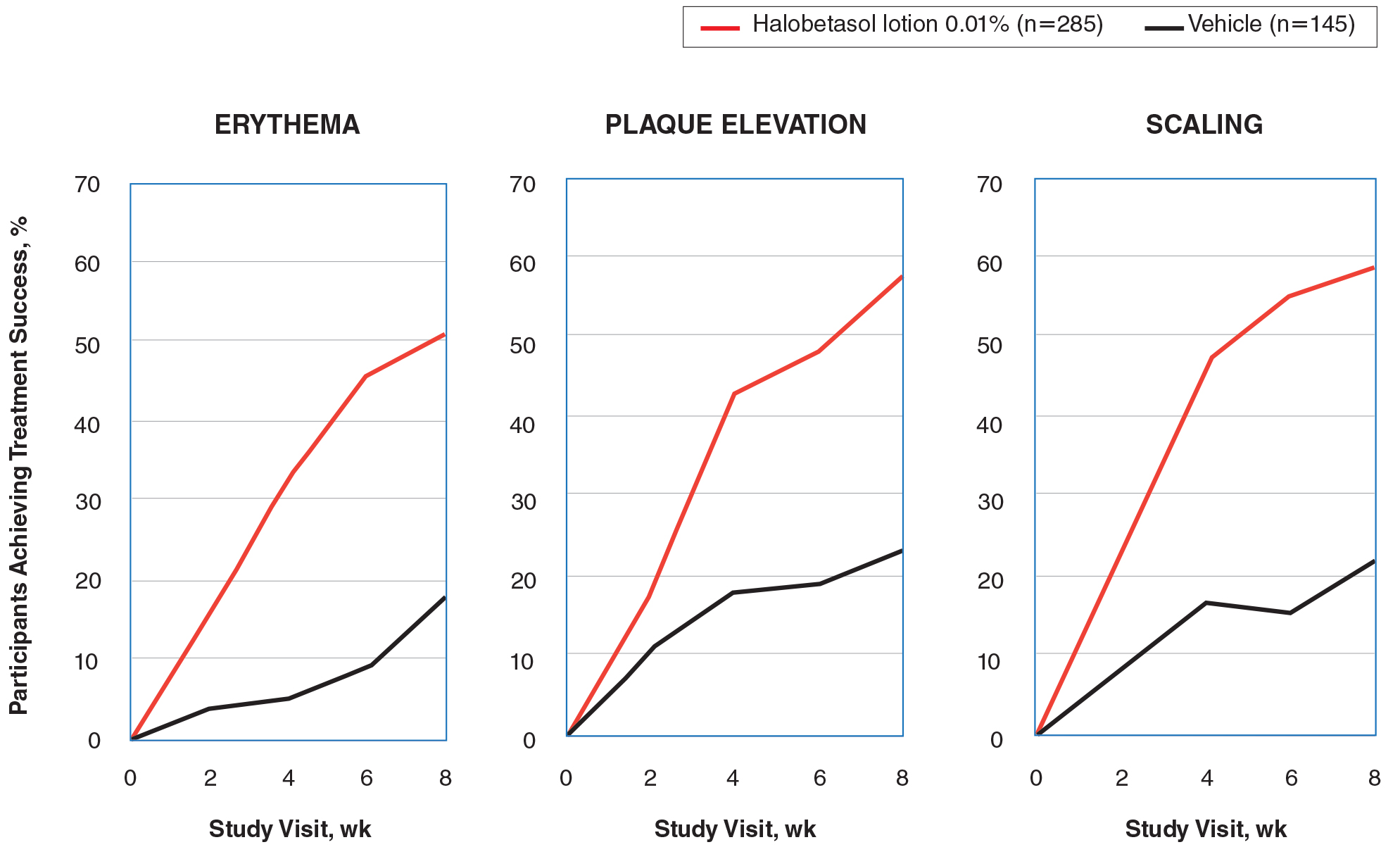
BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
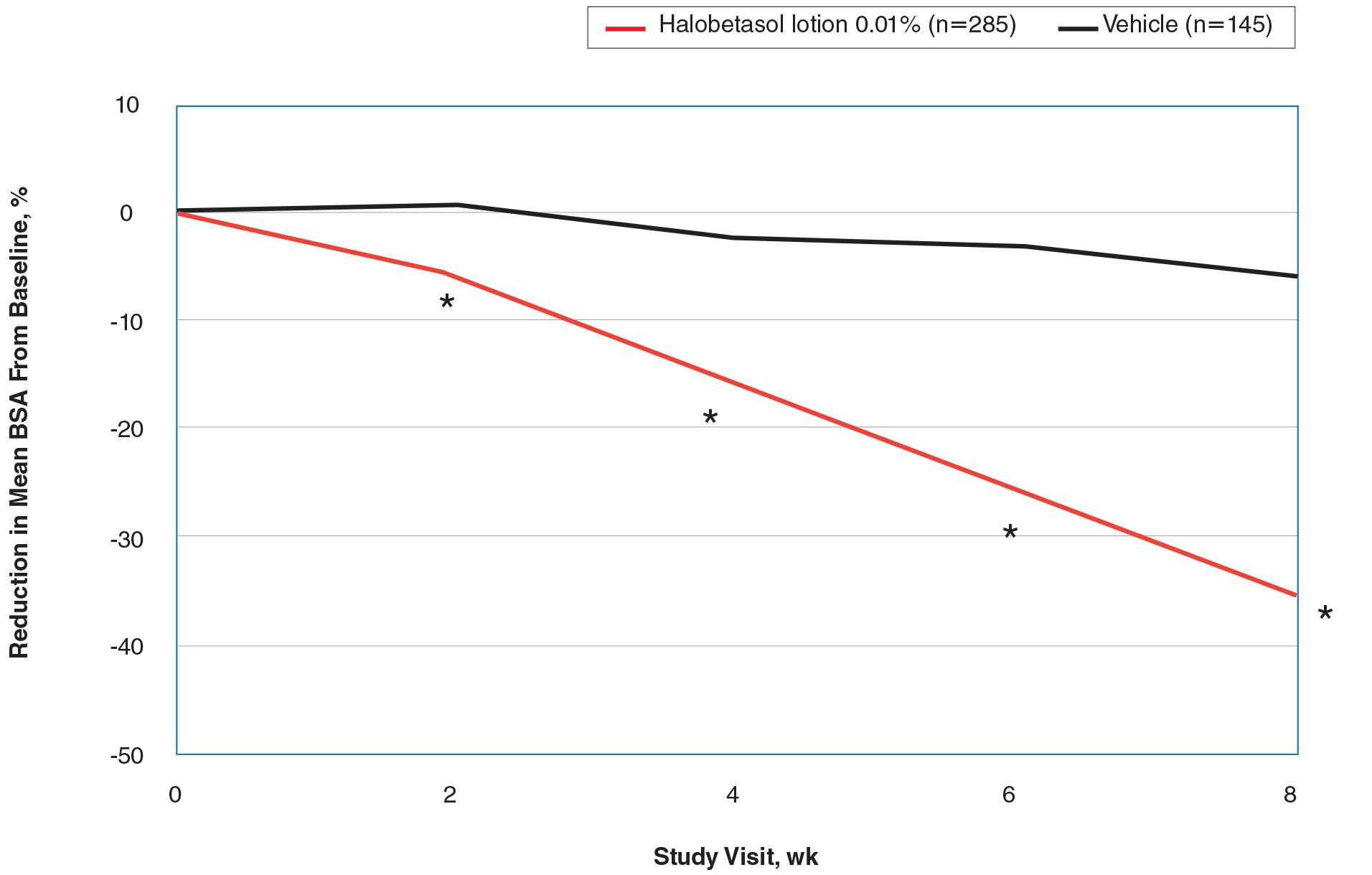
IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
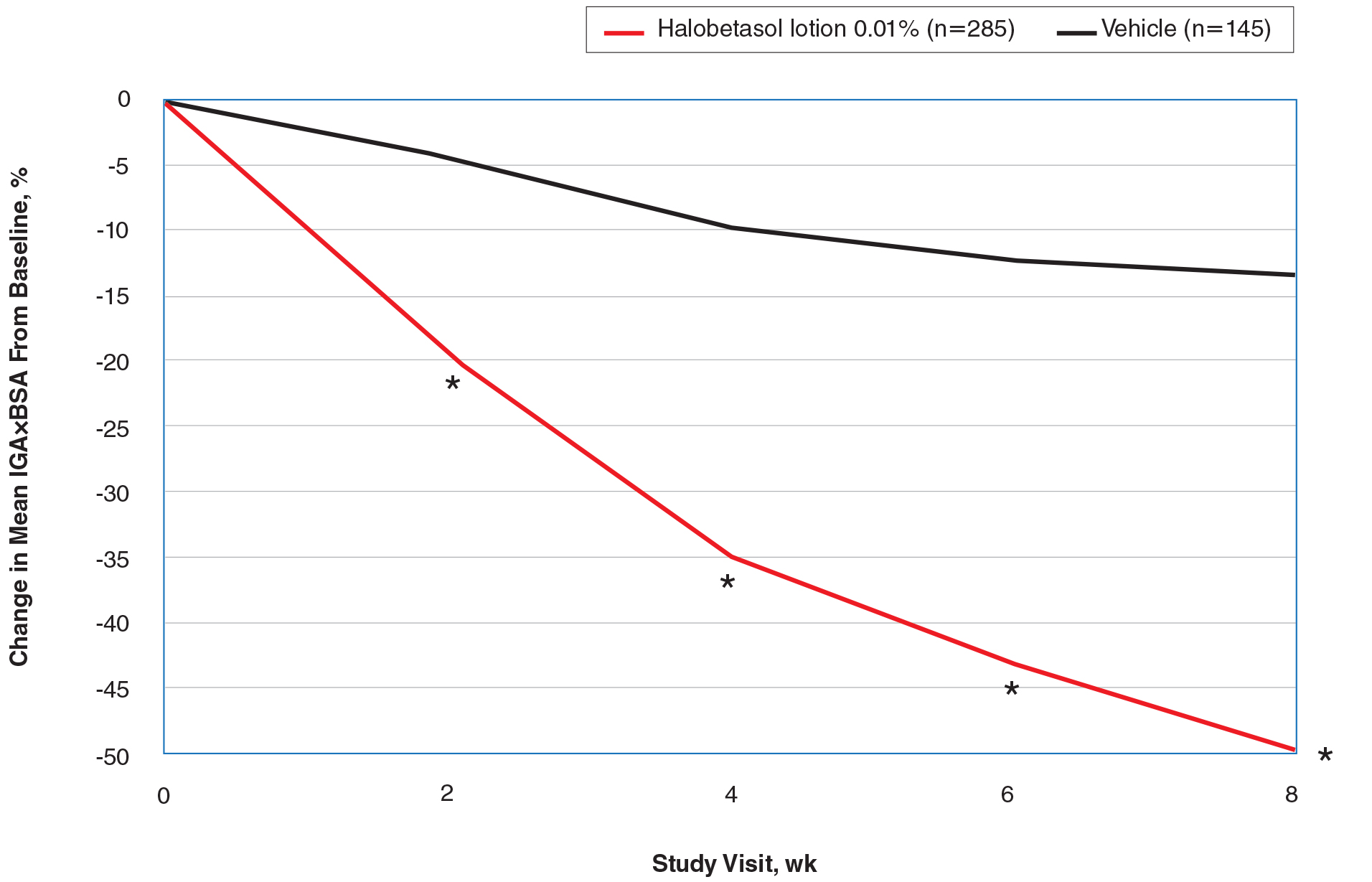
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
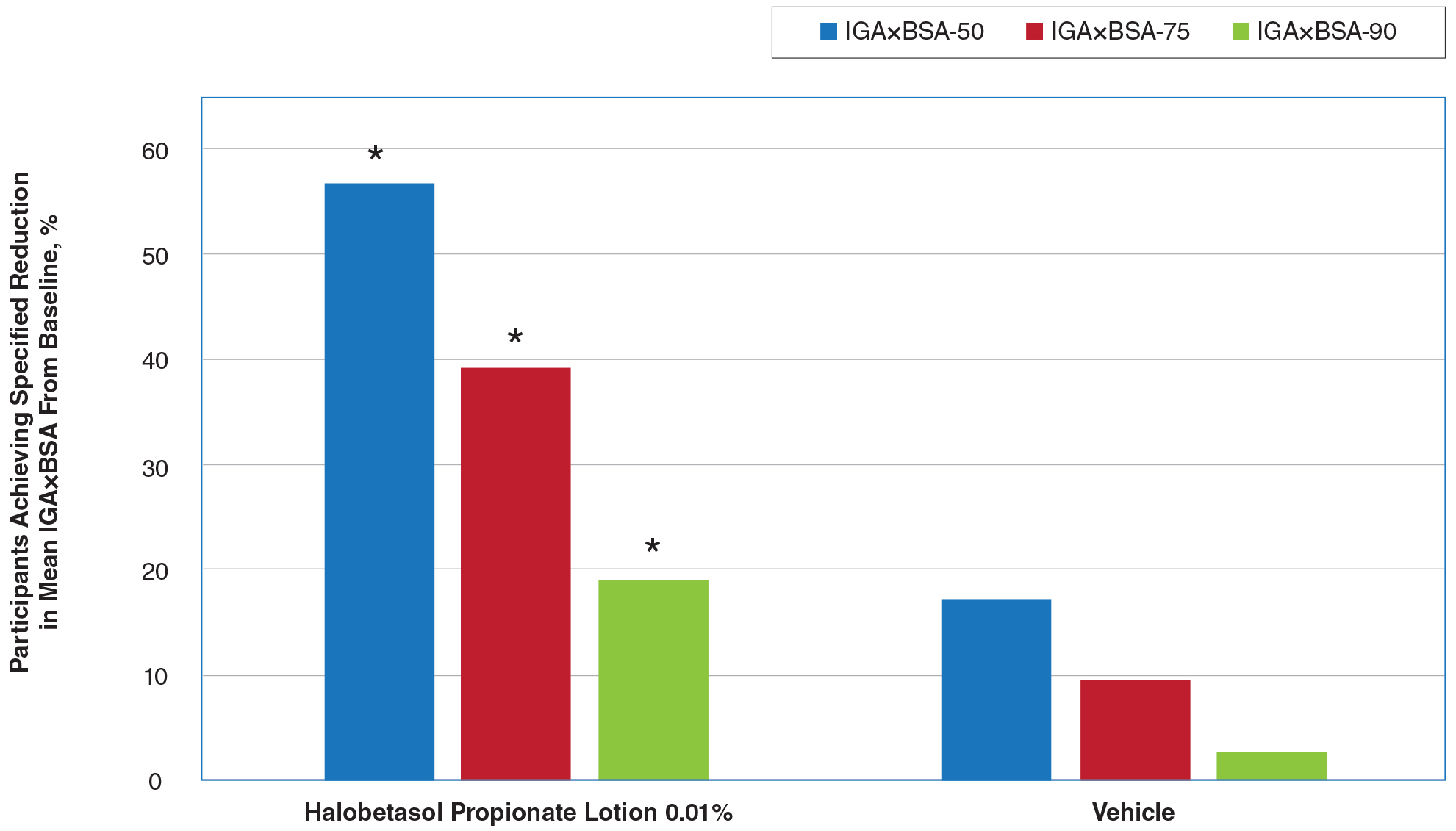
Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
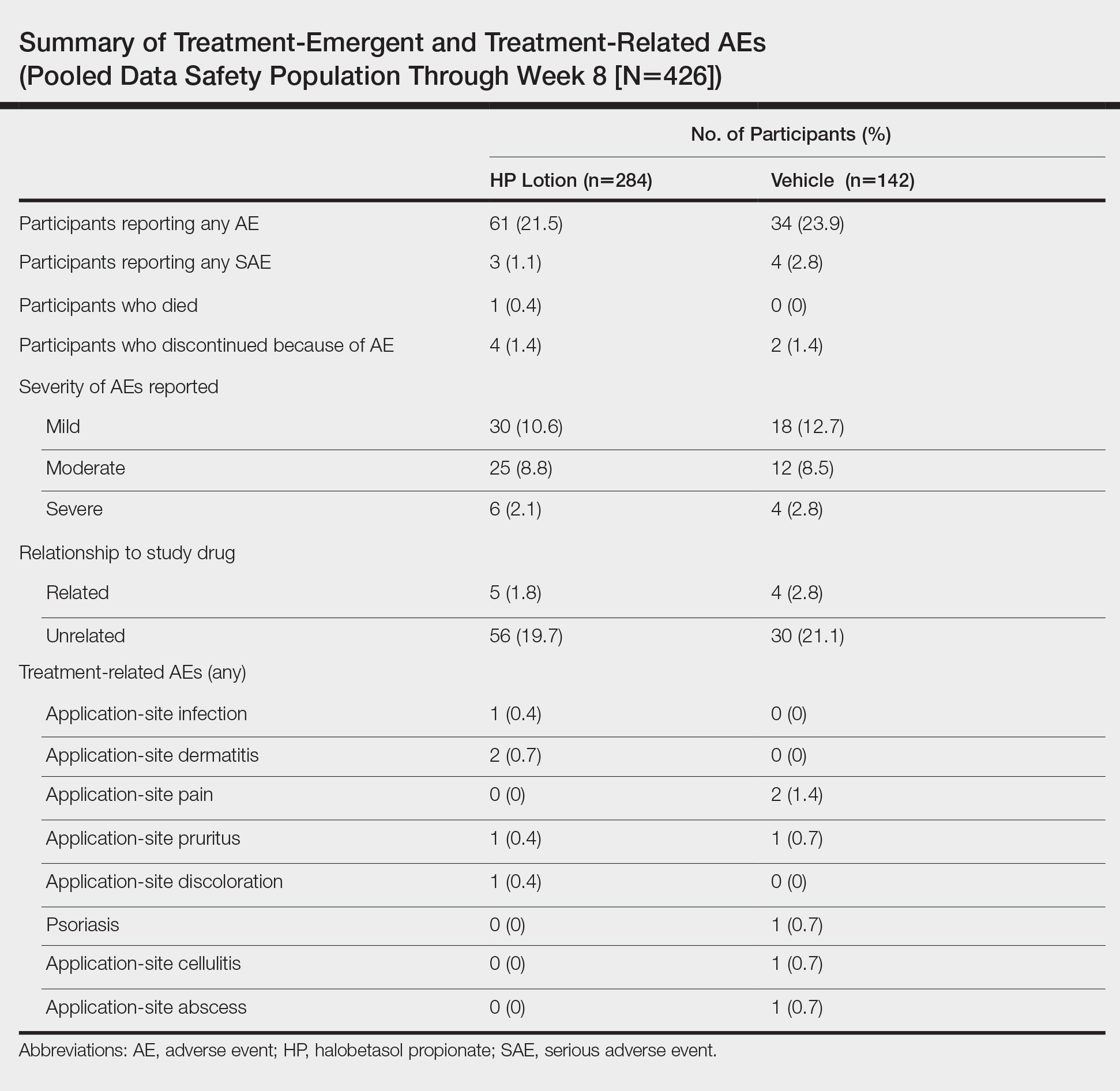
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.

A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).

Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).

Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).

Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).

BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).

IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).

By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).

Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.

Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.

A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).

Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).

Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).

Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).

BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).

IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).

By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).

Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.

Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Life’s Simple 7 tied to lowered PAD risk in long-term study
Adherence to the American Heart Association’s Life’s Simple 7 is associated with lower incidence of peripheral artery disease and less decline in ankle brachial index, according to the results of a retrospective analysis of patients in the Multi-Ethnic Study of Atherosclerosis (MESA) trial.
“These results support the use of LS7 to prevent PAD and decline in ABI in multiple racial/ethnic groups,” according to Jonathan T. Unkart, MD, of the department of medicine and public health, University of California, San Diego, and his colleagues.
MESA recruited 6,814 men and women aged 45-84 years who were free of clinical cardiovascular disease. The cohort comprised 53% women and had the following racial/ethnic composition: 38% non-Hispanic white; 28% African American; 23% Hispanic, and 11% Asian. MESA consisted of six exams, with the baseline exam occurring from 2000 to 2002, including assessment of all LS7 components and PAD assessment using ABI calculated on both left and right sides. The final exam was performed from 2010 to 2012.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for optimum, 1 for average, and 0 for inadequate. The investigators assessed overall LS7 scores on a continuous 0-14 scale, as well as the overall categorical indications of inadequate, average, and optimum. Cox proportional hazard models were used to assess the association of individual LS7 components by overall LS7 with incident PAD, according to the researchers.
Interactions of race/ethnicity by LS7 score were assessed on a multiplicative scale for both incident PAD and decline in ABI outcomes, adjusted for age, sex, education, and income (Am J Prev Med. 2019;56:262-70).
A total of 5,529 participants had complete LS7 information and met inclusion criteria to assess incident PAD. Over a median follow-up of 9.2 years, 251 (4.5%) participants developed incident PAD and 419 (9.8%) participants had a decline of at least 0.15 in ABI. In addition, each point higher on the continuous LS7 scale was associated with 0.94-fold lower odds of decline in ABI (odds ratio, 0.94; 95% confidence interval, 0.87-0.97; P =.003).
Each point higher on the continuous LS7 scale was associated with a 17% lower rate of incident PAD (HR, 0.83; 95% CI, 0.78-0.88; P less than .001), according to the researchers.
The study showed that there was a significant prospective association between LS7 score and incident PAD in African Americans, Hispanics, and non-Hispanic whites. Although the association was not statistically significant for the Chinese Americans in MESA, this was likely because of the low number of incident PAD cases (only 18) in this group, according to the authors.
Analysis by individual LS7 components showed that more optimal levels of smoking, physical activity, glucose, and blood pressure were significantly associated with lower rates of incident PAD. Similarly, after adjustment for age, sex, race/ethnicity, income, education, and baseline ABI, more optimal levels of smoking, and glucose were significantly associated with lower odds of decline. These results for the decline in ABI did not appear to differ across race/ethnicity, according to Dr. Unkart and his colleagues.
In contrast, BMI, diet, and cholesterol were not associated with incident PAD or decline in ABI.
“Higher scores on the AHA LS7 were associated with lower incident PAD and less decline in ABI. Preventive measures targeting LS7 components could assist with reducing PAD-related morbidity and mortality,” the researchers concluded.
The work was supported by the National, Heart, Lung, and Blood Institute. Dr. Unkart and his colleagues reported that they had no disclosures.
SOURCE: Unkart JT et al. Am J Prev Med 2019;56:262-270.
Adherence to the American Heart Association’s Life’s Simple 7 is associated with lower incidence of peripheral artery disease and less decline in ankle brachial index, according to the results of a retrospective analysis of patients in the Multi-Ethnic Study of Atherosclerosis (MESA) trial.
“These results support the use of LS7 to prevent PAD and decline in ABI in multiple racial/ethnic groups,” according to Jonathan T. Unkart, MD, of the department of medicine and public health, University of California, San Diego, and his colleagues.
MESA recruited 6,814 men and women aged 45-84 years who were free of clinical cardiovascular disease. The cohort comprised 53% women and had the following racial/ethnic composition: 38% non-Hispanic white; 28% African American; 23% Hispanic, and 11% Asian. MESA consisted of six exams, with the baseline exam occurring from 2000 to 2002, including assessment of all LS7 components and PAD assessment using ABI calculated on both left and right sides. The final exam was performed from 2010 to 2012.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for optimum, 1 for average, and 0 for inadequate. The investigators assessed overall LS7 scores on a continuous 0-14 scale, as well as the overall categorical indications of inadequate, average, and optimum. Cox proportional hazard models were used to assess the association of individual LS7 components by overall LS7 with incident PAD, according to the researchers.
Interactions of race/ethnicity by LS7 score were assessed on a multiplicative scale for both incident PAD and decline in ABI outcomes, adjusted for age, sex, education, and income (Am J Prev Med. 2019;56:262-70).
A total of 5,529 participants had complete LS7 information and met inclusion criteria to assess incident PAD. Over a median follow-up of 9.2 years, 251 (4.5%) participants developed incident PAD and 419 (9.8%) participants had a decline of at least 0.15 in ABI. In addition, each point higher on the continuous LS7 scale was associated with 0.94-fold lower odds of decline in ABI (odds ratio, 0.94; 95% confidence interval, 0.87-0.97; P =.003).
Each point higher on the continuous LS7 scale was associated with a 17% lower rate of incident PAD (HR, 0.83; 95% CI, 0.78-0.88; P less than .001), according to the researchers.
The study showed that there was a significant prospective association between LS7 score and incident PAD in African Americans, Hispanics, and non-Hispanic whites. Although the association was not statistically significant for the Chinese Americans in MESA, this was likely because of the low number of incident PAD cases (only 18) in this group, according to the authors.
Analysis by individual LS7 components showed that more optimal levels of smoking, physical activity, glucose, and blood pressure were significantly associated with lower rates of incident PAD. Similarly, after adjustment for age, sex, race/ethnicity, income, education, and baseline ABI, more optimal levels of smoking, and glucose were significantly associated with lower odds of decline. These results for the decline in ABI did not appear to differ across race/ethnicity, according to Dr. Unkart and his colleagues.
In contrast, BMI, diet, and cholesterol were not associated with incident PAD or decline in ABI.
“Higher scores on the AHA LS7 were associated with lower incident PAD and less decline in ABI. Preventive measures targeting LS7 components could assist with reducing PAD-related morbidity and mortality,” the researchers concluded.
The work was supported by the National, Heart, Lung, and Blood Institute. Dr. Unkart and his colleagues reported that they had no disclosures.
SOURCE: Unkart JT et al. Am J Prev Med 2019;56:262-270.
Adherence to the American Heart Association’s Life’s Simple 7 is associated with lower incidence of peripheral artery disease and less decline in ankle brachial index, according to the results of a retrospective analysis of patients in the Multi-Ethnic Study of Atherosclerosis (MESA) trial.
“These results support the use of LS7 to prevent PAD and decline in ABI in multiple racial/ethnic groups,” according to Jonathan T. Unkart, MD, of the department of medicine and public health, University of California, San Diego, and his colleagues.
MESA recruited 6,814 men and women aged 45-84 years who were free of clinical cardiovascular disease. The cohort comprised 53% women and had the following racial/ethnic composition: 38% non-Hispanic white; 28% African American; 23% Hispanic, and 11% Asian. MESA consisted of six exams, with the baseline exam occurring from 2000 to 2002, including assessment of all LS7 components and PAD assessment using ABI calculated on both left and right sides. The final exam was performed from 2010 to 2012.
As background, the metrics for Life’s Simple 7 consist of total cholesterol, blood pressure, blood glucose, smoking status, body mass index, physical activity, and adherence to a healthy diet score. Each element can be scored 2 points for optimum, 1 for average, and 0 for inadequate. The investigators assessed overall LS7 scores on a continuous 0-14 scale, as well as the overall categorical indications of inadequate, average, and optimum. Cox proportional hazard models were used to assess the association of individual LS7 components by overall LS7 with incident PAD, according to the researchers.
Interactions of race/ethnicity by LS7 score were assessed on a multiplicative scale for both incident PAD and decline in ABI outcomes, adjusted for age, sex, education, and income (Am J Prev Med. 2019;56:262-70).
A total of 5,529 participants had complete LS7 information and met inclusion criteria to assess incident PAD. Over a median follow-up of 9.2 years, 251 (4.5%) participants developed incident PAD and 419 (9.8%) participants had a decline of at least 0.15 in ABI. In addition, each point higher on the continuous LS7 scale was associated with 0.94-fold lower odds of decline in ABI (odds ratio, 0.94; 95% confidence interval, 0.87-0.97; P =.003).
Each point higher on the continuous LS7 scale was associated with a 17% lower rate of incident PAD (HR, 0.83; 95% CI, 0.78-0.88; P less than .001), according to the researchers.
The study showed that there was a significant prospective association between LS7 score and incident PAD in African Americans, Hispanics, and non-Hispanic whites. Although the association was not statistically significant for the Chinese Americans in MESA, this was likely because of the low number of incident PAD cases (only 18) in this group, according to the authors.
Analysis by individual LS7 components showed that more optimal levels of smoking, physical activity, glucose, and blood pressure were significantly associated with lower rates of incident PAD. Similarly, after adjustment for age, sex, race/ethnicity, income, education, and baseline ABI, more optimal levels of smoking, and glucose were significantly associated with lower odds of decline. These results for the decline in ABI did not appear to differ across race/ethnicity, according to Dr. Unkart and his colleagues.
In contrast, BMI, diet, and cholesterol were not associated with incident PAD or decline in ABI.
“Higher scores on the AHA LS7 were associated with lower incident PAD and less decline in ABI. Preventive measures targeting LS7 components could assist with reducing PAD-related morbidity and mortality,” the researchers concluded.
The work was supported by the National, Heart, Lung, and Blood Institute. Dr. Unkart and his colleagues reported that they had no disclosures.
SOURCE: Unkart JT et al. Am J Prev Med 2019;56:262-270.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Life’s Simple 7 appears to be a valid tool for modifying PAD risk.
Major finding: Each point higher for the overall Life’s Simple 7 score was associated with a 17% lower rate of incident PAD (HR, 0.83; P less than .001).
Study details: Retrospective analysis of 5,529 individuals from the Multi-Ethnic Study of Atherosclerosis who were followed more than 10 years.
Disclosures: The work was supported by the National, Heart, Lung, and Blood Institute. The authors reported that they had no disclosures..
Source: Unkart JT et al. Am J Prev Med. 2019;56:262-70.
Statins cut vascular events in elderly patients
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
FROM THE LANCET
Key clinical point: but patients older than 75 years with occlusive vascular disease have a smaller reduction in major coronary events.
Major finding: Major vascular coronary events were reduced by 24% (risk ratio, 0.76; 95% confidence interval, 0.73-0.79) with a decrease in the reduction of coronary events among patients older than 75 years. Study details: A meta-analysis of 28 trials with 186,854 individuals undergoing statin therapy from the Cholesterol Treatment Trialists’ Collaboration.
Disclosures: This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
Source: Fulcher J et al. Lancet. 2019;393:407-15.
Immunotherapy’s cardiac effects require early monitoring, management
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Monitor for cardiac symptoms and treat or interrupt immunotherapy as needed.
Major finding: Immune checkpoint inhibitors and CAR T-cell therapies are associated with distinct cardiovascular adverse events.
Study details: Review of strategies for managing the cardiovascular consequences of cancer immunotherapies.
Disclosures: Dr. Cornell reported having nothing to disclose.
Psoriasis Treatment in Patients With Sickle Cell Disease
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Practice Points
• Tumor necrosis factor α contributes both to the vascular inflammatory state seen in sickle cell disease as well as the cycle of inflammation seen in the development of psoriasis.
• Tumor necrosis factor α inhibitors may be the drug of choice for patients with both psoriasis and sickle cell disease.