User login
Higher AML, MDS risk linked to solid tumor chemotherapy
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Treatment with chemotherapy was linked with a 1.5-fold to more than 10-fold increased risk for tMDS/AML.
Study details: A retrospective analysis of 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Disclosures: The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
Source: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
Immediate acting inhibitors complicate hemophilia A diagnosis
A small but substantial proportion of patients with hemophilia A develop immediate acting factor VIII inhibitors, the diversity and complexity of which create a diagnostic challenge in the laboratory, according to authors of a recent observational study.
The great majority of the inhibitor-positive patients in the 4,900-patient study had classical FVIII inhibitors, which are typically time- and temperature-dependent and react more slowly in mixing studies, the researchers reported.
By contrast, about 1 in 10 patients demonstrated immediate acting inhibitors, and of those, some had lupus anticoagulants, some had factor VIII inhibitors, and some had both, according to Shrimati Shetty, PhD, of the National Institute of Immunohaematology in Mumbai, India, and her colleagues.
“There is a possibility of misdiagnosis of the patient when they present for the first time,” the researchers wrote. The report is in Thrombosis Research.
In the case of immediate-acting inhibitors, use of ELISA or chromogenic assays alongside lupus anticoagulant testing may help clarify the diagnosis; however, those tests are costly and may not be routinely available.
In the study by Dr. Shetty and her colleagues, patients in India with congenital hemophilia were initially screened for inhibitors. A total of 451 were found to be positive, and of those, 398 were observed to have classical factor VIII inhibitors, while the remaining 53 had immediate-acting inhibitors.
Looking specifically at hemophilia A patients with immediate-acting inhibitors, which comprised 48 of those 53 patients, the majority, or 42 patients, were positive for lupus anticoagulants, and of those, 38 were positive for both lupus anticoagulants and factor VIII inhibitors, while 4 patients were positive for lupus anticoagulants only.
“These are a heterogeneous group of antibodies interfering with all phospholipid dependent reactions,” the researchers wrote.
Properly interpreting factor inhibitor assays is an important step that helps guide later management of inhibitor-positive patients, according to Dr. Shetty and her coauthors.
“Once the patients become positive for inhibitors, they have to opt for alternate modalities of treatment, i.e. bypassing agents like activated prothrombin complex concentrate and activated recombinant factor VII, which are much more expensive,” they wrote.
In light of the diagnostic difficulties they highlighted, Dr. Shetty and her coauthors recommended a “systematic approach” to testing. Both factor VIII and factor IX assays need to be conducted, along with a lupus anticoagulant test. For inhibitor titer, either chromogenic assays or ELISA tests are recommended, they wrote.
Dr. Shetty and her coauthors reported that they had no conflicts of interest.
SOURCE: Patil R et al. Thromb Res. 2018 Dec;172:29-35.
A small but substantial proportion of patients with hemophilia A develop immediate acting factor VIII inhibitors, the diversity and complexity of which create a diagnostic challenge in the laboratory, according to authors of a recent observational study.
The great majority of the inhibitor-positive patients in the 4,900-patient study had classical FVIII inhibitors, which are typically time- and temperature-dependent and react more slowly in mixing studies, the researchers reported.
By contrast, about 1 in 10 patients demonstrated immediate acting inhibitors, and of those, some had lupus anticoagulants, some had factor VIII inhibitors, and some had both, according to Shrimati Shetty, PhD, of the National Institute of Immunohaematology in Mumbai, India, and her colleagues.
“There is a possibility of misdiagnosis of the patient when they present for the first time,” the researchers wrote. The report is in Thrombosis Research.
In the case of immediate-acting inhibitors, use of ELISA or chromogenic assays alongside lupus anticoagulant testing may help clarify the diagnosis; however, those tests are costly and may not be routinely available.
In the study by Dr. Shetty and her colleagues, patients in India with congenital hemophilia were initially screened for inhibitors. A total of 451 were found to be positive, and of those, 398 were observed to have classical factor VIII inhibitors, while the remaining 53 had immediate-acting inhibitors.
Looking specifically at hemophilia A patients with immediate-acting inhibitors, which comprised 48 of those 53 patients, the majority, or 42 patients, were positive for lupus anticoagulants, and of those, 38 were positive for both lupus anticoagulants and factor VIII inhibitors, while 4 patients were positive for lupus anticoagulants only.
“These are a heterogeneous group of antibodies interfering with all phospholipid dependent reactions,” the researchers wrote.
Properly interpreting factor inhibitor assays is an important step that helps guide later management of inhibitor-positive patients, according to Dr. Shetty and her coauthors.
“Once the patients become positive for inhibitors, they have to opt for alternate modalities of treatment, i.e. bypassing agents like activated prothrombin complex concentrate and activated recombinant factor VII, which are much more expensive,” they wrote.
In light of the diagnostic difficulties they highlighted, Dr. Shetty and her coauthors recommended a “systematic approach” to testing. Both factor VIII and factor IX assays need to be conducted, along with a lupus anticoagulant test. For inhibitor titer, either chromogenic assays or ELISA tests are recommended, they wrote.
Dr. Shetty and her coauthors reported that they had no conflicts of interest.
SOURCE: Patil R et al. Thromb Res. 2018 Dec;172:29-35.
A small but substantial proportion of patients with hemophilia A develop immediate acting factor VIII inhibitors, the diversity and complexity of which create a diagnostic challenge in the laboratory, according to authors of a recent observational study.
The great majority of the inhibitor-positive patients in the 4,900-patient study had classical FVIII inhibitors, which are typically time- and temperature-dependent and react more slowly in mixing studies, the researchers reported.
By contrast, about 1 in 10 patients demonstrated immediate acting inhibitors, and of those, some had lupus anticoagulants, some had factor VIII inhibitors, and some had both, according to Shrimati Shetty, PhD, of the National Institute of Immunohaematology in Mumbai, India, and her colleagues.
“There is a possibility of misdiagnosis of the patient when they present for the first time,” the researchers wrote. The report is in Thrombosis Research.
In the case of immediate-acting inhibitors, use of ELISA or chromogenic assays alongside lupus anticoagulant testing may help clarify the diagnosis; however, those tests are costly and may not be routinely available.
In the study by Dr. Shetty and her colleagues, patients in India with congenital hemophilia were initially screened for inhibitors. A total of 451 were found to be positive, and of those, 398 were observed to have classical factor VIII inhibitors, while the remaining 53 had immediate-acting inhibitors.
Looking specifically at hemophilia A patients with immediate-acting inhibitors, which comprised 48 of those 53 patients, the majority, or 42 patients, were positive for lupus anticoagulants, and of those, 38 were positive for both lupus anticoagulants and factor VIII inhibitors, while 4 patients were positive for lupus anticoagulants only.
“These are a heterogeneous group of antibodies interfering with all phospholipid dependent reactions,” the researchers wrote.
Properly interpreting factor inhibitor assays is an important step that helps guide later management of inhibitor-positive patients, according to Dr. Shetty and her coauthors.
“Once the patients become positive for inhibitors, they have to opt for alternate modalities of treatment, i.e. bypassing agents like activated prothrombin complex concentrate and activated recombinant factor VII, which are much more expensive,” they wrote.
In light of the diagnostic difficulties they highlighted, Dr. Shetty and her coauthors recommended a “systematic approach” to testing. Both factor VIII and factor IX assays need to be conducted, along with a lupus anticoagulant test. For inhibitor titer, either chromogenic assays or ELISA tests are recommended, they wrote.
Dr. Shetty and her coauthors reported that they had no conflicts of interest.
SOURCE: Patil R et al. Thromb Res. 2018 Dec;172:29-35.
FROM THROMBOSIS RESEARCH
Key clinical point:
Major finding: Of 48 inhibitor-positive hemophilia A patients with immediate acting inhibitors, 42 were positive for lupus anticoagulants.
Study details: An analysis of 4,900 patients in India with confirmed or suspected congenital hemophilia.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Patil R et al. Thromb Res. 2018 Dec;172:29-35.
Patient-reported outcomes for patients with chronic liver disease
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).
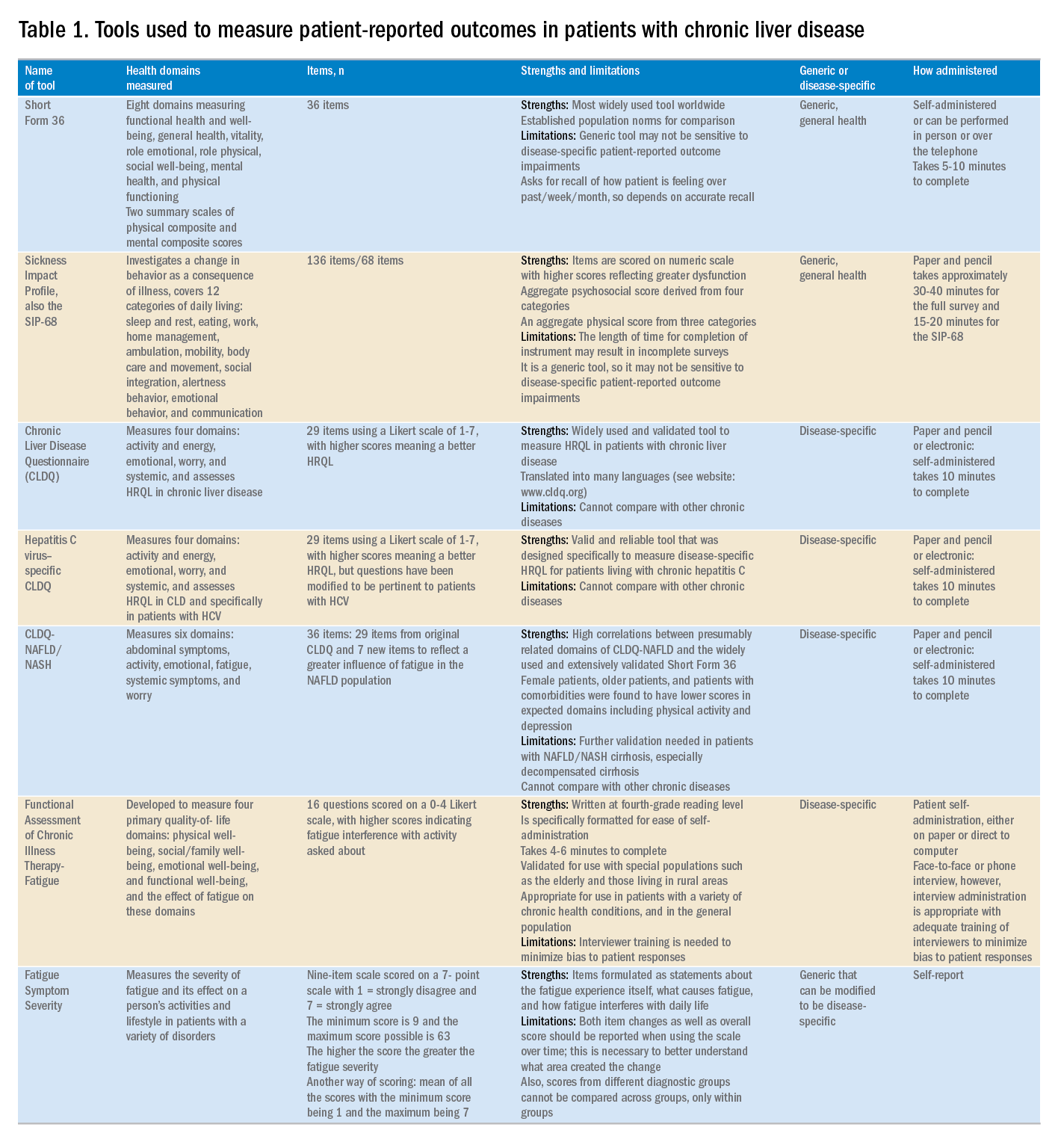
Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).

Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).

Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Pityriasis Amiantacea Following Bone Marrow Transplant
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
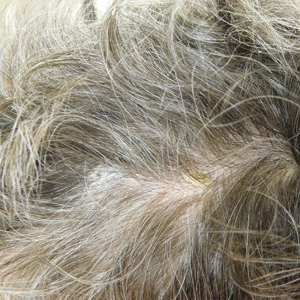
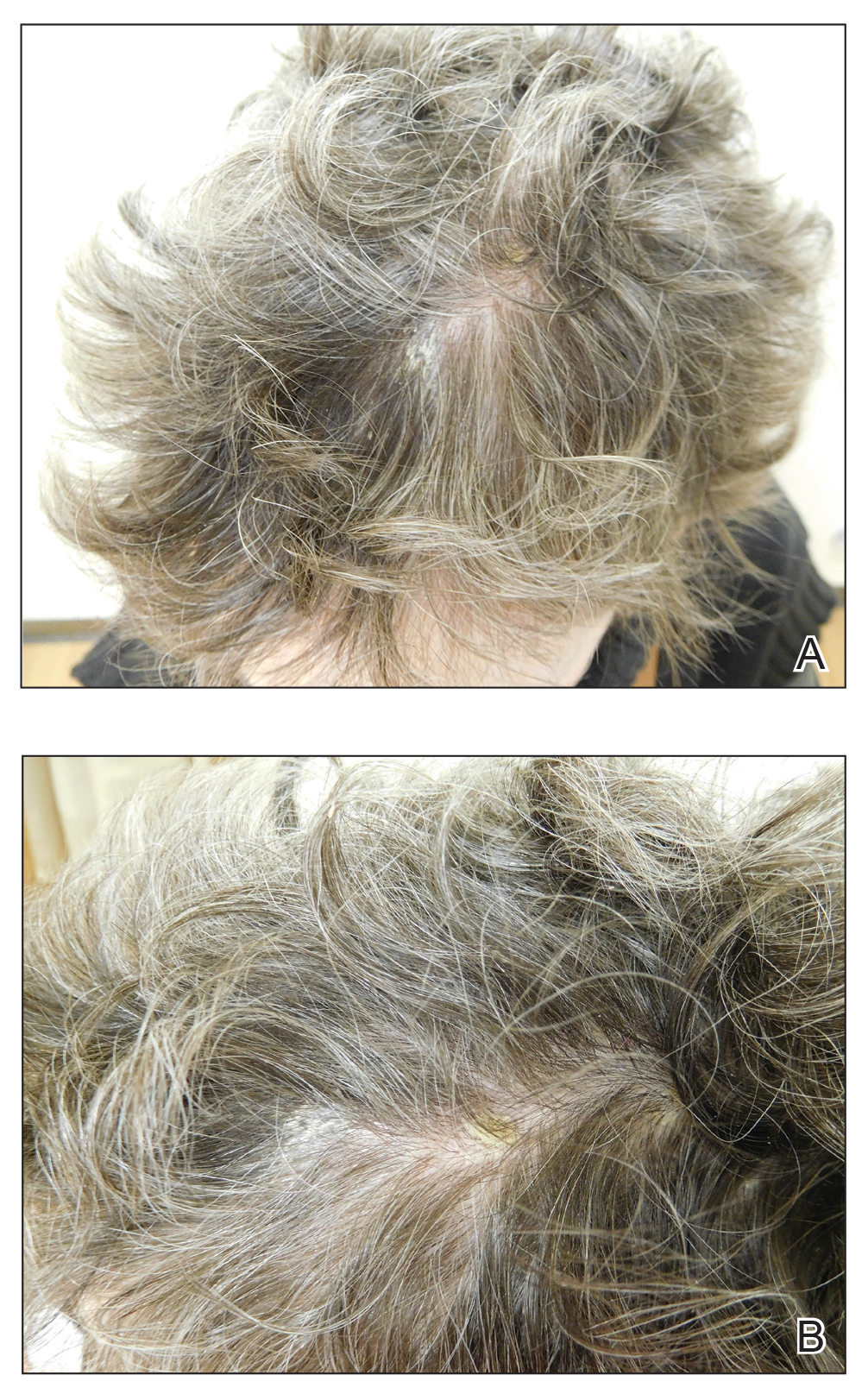
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
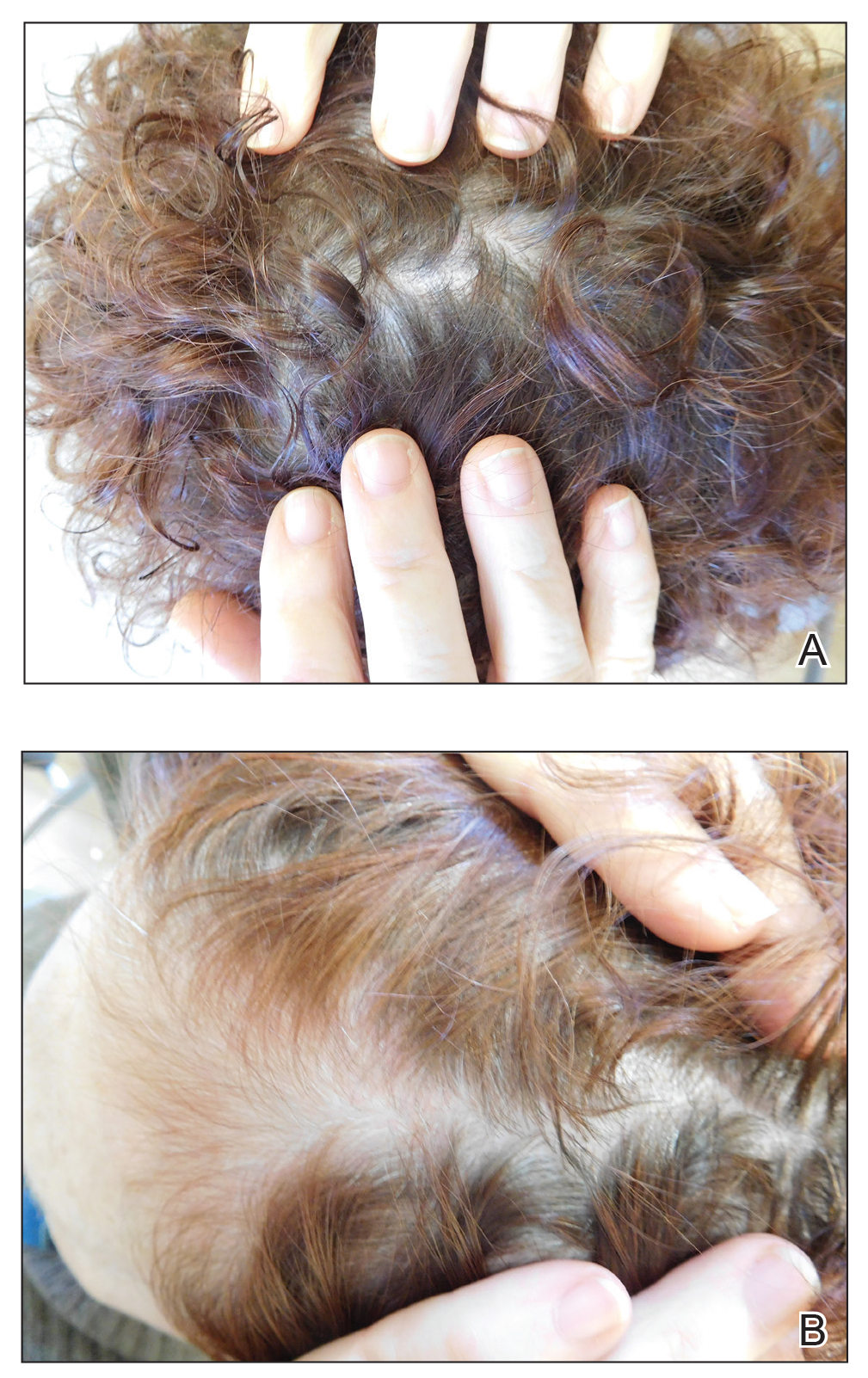
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).
Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25
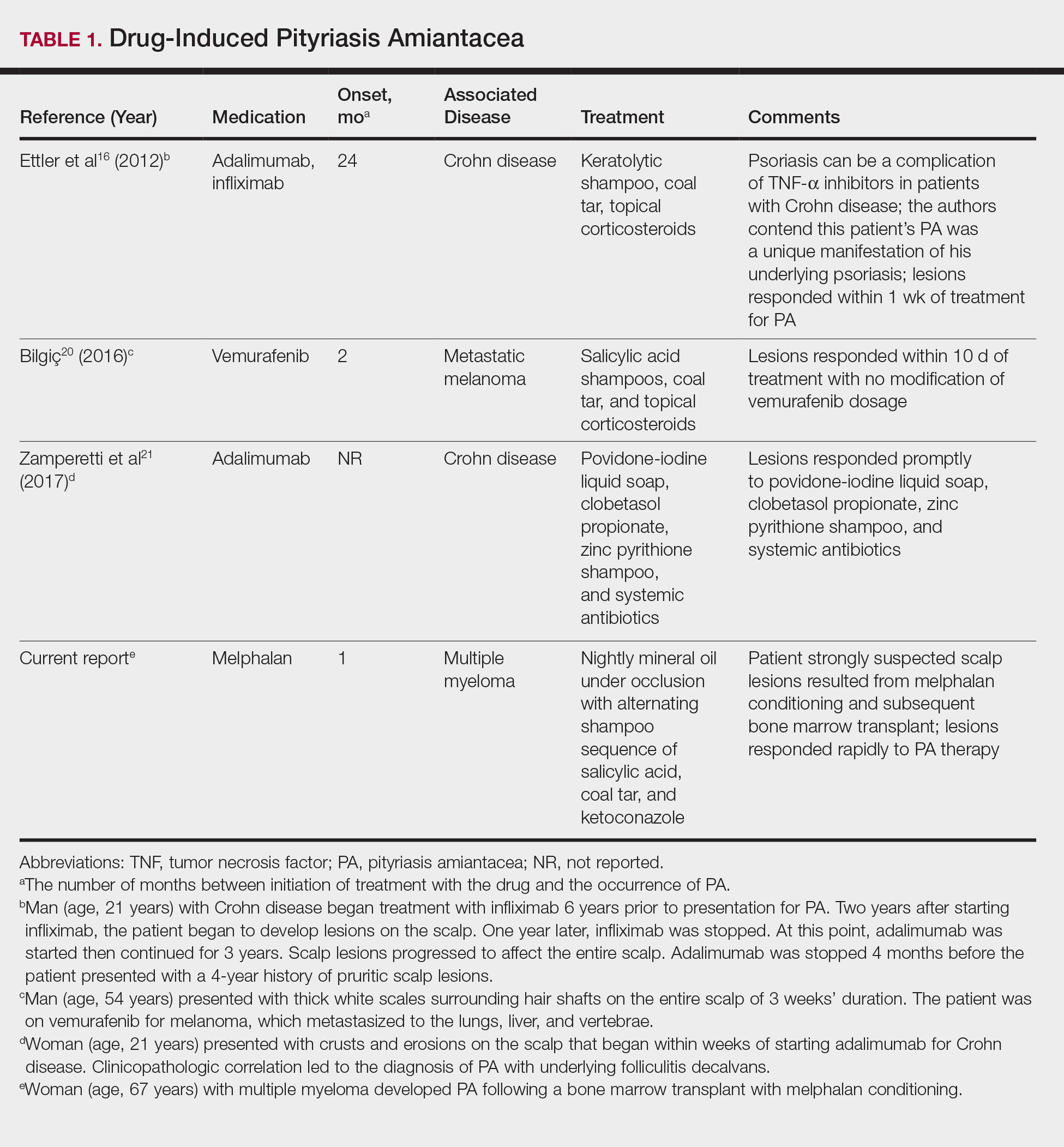
A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.
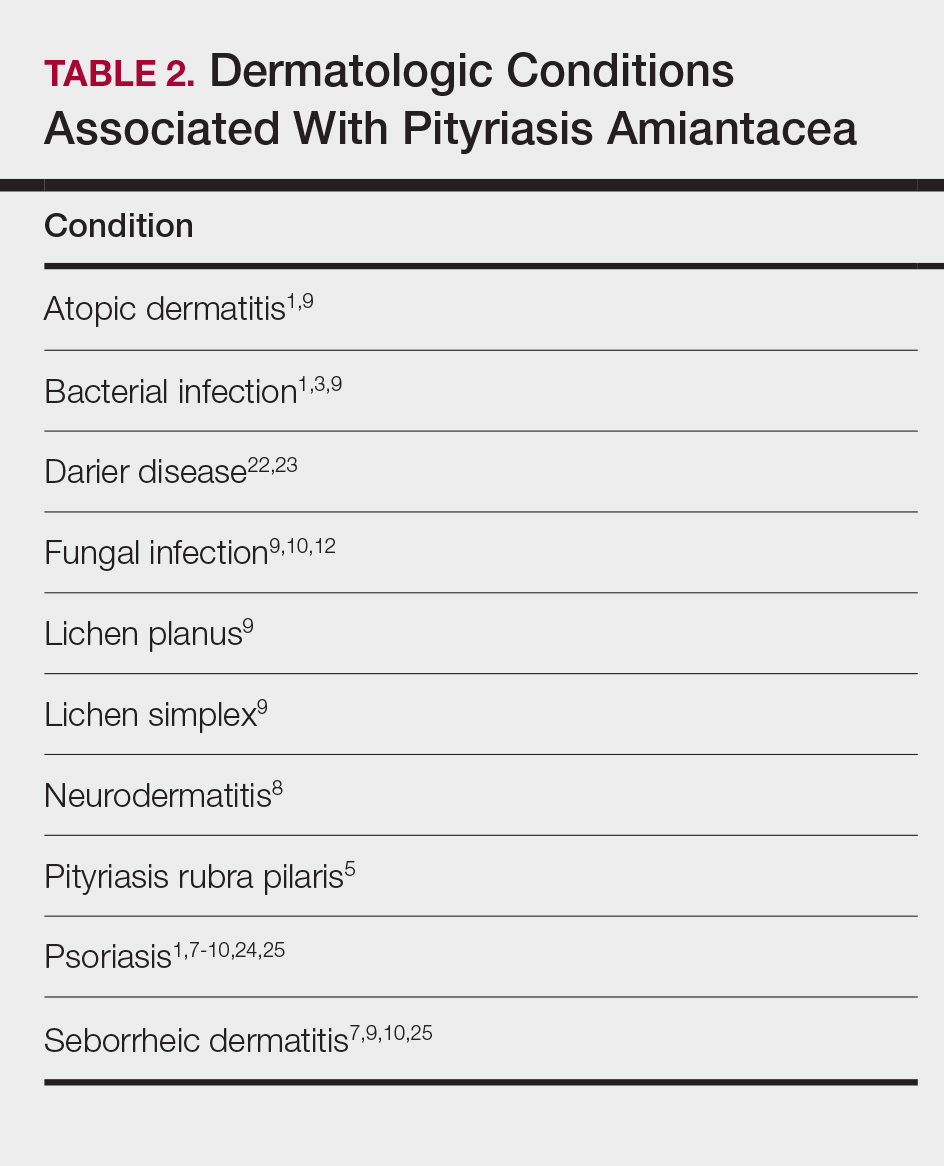
Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).


Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25

A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.

Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).


Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25

A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.

Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
Practice Points
- Pityriasis amiantacea (PA) is associated with several dermatologic conditions, including atopic dermatitis, bacterial and fungal infections, psoriasis, and seborrheic dermatitis.
- Drug-induced PA is rare, but the condition has been reported in the context of treatment with tumor necrosis factor Symbol Stdα inhibitors and vemurafenib.
- Our report suggests that PA may be associated with either melphalan conditioning, bone marrow transplant, or both.
Topical antibiotic decolonizes S. aureus in NICU infants
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
FROM PEDIATRICS
Too high to drive: States grapple with setting limits on weed use behind wheel
It used to be the stuff of stoner comedies and “Just Say No” campaigns. Today, marijuana is becoming mainstream as voters across the country approve ballot questions for legalization or medical use.
In response, state governments are testing ways to ensure that the integration of this once-illicit substance into everyday life doesn’t create new public health risks. These efforts are sparking a difficult question: At what point is someone too high to get behind the wheel?
The answer is complicated. Brain scientists and pharmacologists don’t know how to measure if and to what extent marijuana causes impairment.
The reason: Existing blood and urine tests can detect marijuana use, but, because traces of the drug stay in the human body for a long time, those tests can’t specify whether the use occurred earlier that day or that month. They also don’t indicate the level at which a driver would be considered “under the influence.”
“It’s a really hard problem,” said Keith Humphreys, a psychiatry professor and drug policy expert at Stanford University (Calif.), the first state to legalize medical marijuana and where recreational pot use among adults became legal in 2016. “We don’t really have good evidence – even if we know someone has been using – [to gauge] what their level of impairment is.”
Marijuana is now legal for recreational use in 10 states and the District of Columbia – including Michigan, where a ballot initiative passed in November 2018 took effect Dec. 6. In New York, the governor said Dec. 17 that legalization would be a top priority for 2019. And nearly three dozen states have cleared the use of medical cannabis.
For alcohol, there is a clear, national standard. If your blood alcohol content is 0.08% or higher, you’re considered cognitively impaired at a level that is unsafe to drive. Extensive research supports this determination, and the clarity makes enforcement of drunken-driving laws easier.
Setting a marijuana-related impairment level is a much murkier proposition. But states that have legalized pot have to figure it out, experts said.
“You can’t legalize a substance and not have a coherent policy for controlling driving under the influence of that substance,” said Steven Davenport, an assistant policy researcher at the nonprofit Rand Corporation, who specializes in marijuana research.
Marijuana, after all, weakens a driver’s ability to maintain focus, and it slows reflexes. But regulators are “playing catch-up,” suggested Thomas Marcotte, a psychiatry professor at the University of California, San Diego, and one of a number of academics around the country who is researching driving while high.
States have put forth a bevy of approaches. At least five have what’s called a “per se” law, which outlaws driving if someone’s blood level of tetrahydrocannabinol, or THC, exceeds a set amount. THC is marijuana’s main intoxicant.
Colorado, where voters approved legalization of recreational marijuana in 2012, has this type of driving law on the books. It took 3 years to pass amid fiery debate and deems “intoxicated” any driver who tests higher than 5 ng of THC per milliliter of blood.
Indiana, Pennsylvania and Rhode Island are among states that forbid driving at any THC level. Still others say drivers should be penalized only if they are impaired by the chemical – a standard that sounds reasonable but quickly gets difficult to measure or even define.
None of these approaches offers an ideal solution, experts said.
“We’re still definitely evaluating which policies are the most effective,” said Ann Kitch, who tracks the marijuana and driving issue for the National Conference of State Legislatures.
States that set a THC-level standard confront weak technology and limited science. THC testing is imprecise at best, since the chemical can stay in someone’s bloodstream for weeks after it was ingested. Someone could legally smoke a joint and still have THC appear in blood or urine samples long after the high passes.
There’s general agreement that driving while high is bad, but there’s no linear relationship between THC levels and degree of impairment. States that have picked a number to reflect when THC in the bloodstream becomes a hazard have “made it up,” argued Dr. Humphreys.
“The ones who wrote [a number] into legislation felt they had to say something,” he said. But “we don’t know what would be the analogy. Is the legal amount [of THC] equal to a beer? Is that how impaired you are? Is it a six-pack?”
Roadside testing for THC is also logistically difficult. Blood, for instance, needs to be analyzed in a lab, and collecting urine gets ... complicated.
In Canada, which legalized recreational pot just this year, law enforcement will test drivers with a saliva test called the Dräger DrugTest 5000, but that isn’t perfect, either.
Some private companies are trying to develop a sort of breathalyzer for marijuana. But Jonathan Caulkins, a drug policy researcher at Carnegie Mellon University, Pittsburgh, said, “There are fundamental issues with the chemistry and pharmacokinetics. It’s really hard to have an objective, easy-to-administer roadside test.”
Some states rely on law enforcement to assess whether someone’s driving appears impaired and ascertain after the fact if marijuana was involved.
In California, every highway patrol member learns to administer “field sobriety tests” – undergoing an extra 16 hours of training to recognize the influence of different drugs, including marijuana. Because medical marijuana has been legal there since 1996, officers are “very used” to recognizing its influence, said Glenn Glazer, the state’s coordinator for its drug recognition expert training program.
That kind of training is taking off in other states, too, Ms. Kitch said. Lobbying groups such as Mothers Against Drunk Driving are pushing to bump up law enforcement training and rely on officers to assess whether a driver is impaired.
These tests, though, risk their own kind of error.
“They are subjective,” Mr. Davenport warned.
For one thing, officer-administered tests can be influenced by racial bias. Someone who has previously had poor experiences with law enforcement may also perform worse, not because of greater impairment but because of nervousness.
Indeed, relying on more subjective testing is in some ways the direct opposite of conventional wisdom.
“A general pattern of the last ... 40 years is to try to take human judgment out of decision making processes when possible. Because we fear exactly these issues,” Mr. Caulkins said. “The idea that you could come up with a completely objective test of performance ... is ambitious.”
Researchers like Dr. Marcotte are trying to devise some kind of test that can, in fact, gauge whether someone is showing signs of marijuana impairment. But that could take years.
In the meantime, the public health threat is real. States with legalized pot do appear to experience more car crashes, though the relationship is muddled. “This is going to be a headache of an issue for a decade,” Mr. Caulkins said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
It used to be the stuff of stoner comedies and “Just Say No” campaigns. Today, marijuana is becoming mainstream as voters across the country approve ballot questions for legalization or medical use.
In response, state governments are testing ways to ensure that the integration of this once-illicit substance into everyday life doesn’t create new public health risks. These efforts are sparking a difficult question: At what point is someone too high to get behind the wheel?
The answer is complicated. Brain scientists and pharmacologists don’t know how to measure if and to what extent marijuana causes impairment.
The reason: Existing blood and urine tests can detect marijuana use, but, because traces of the drug stay in the human body for a long time, those tests can’t specify whether the use occurred earlier that day or that month. They also don’t indicate the level at which a driver would be considered “under the influence.”
“It’s a really hard problem,” said Keith Humphreys, a psychiatry professor and drug policy expert at Stanford University (Calif.), the first state to legalize medical marijuana and where recreational pot use among adults became legal in 2016. “We don’t really have good evidence – even if we know someone has been using – [to gauge] what their level of impairment is.”
Marijuana is now legal for recreational use in 10 states and the District of Columbia – including Michigan, where a ballot initiative passed in November 2018 took effect Dec. 6. In New York, the governor said Dec. 17 that legalization would be a top priority for 2019. And nearly three dozen states have cleared the use of medical cannabis.
For alcohol, there is a clear, national standard. If your blood alcohol content is 0.08% or higher, you’re considered cognitively impaired at a level that is unsafe to drive. Extensive research supports this determination, and the clarity makes enforcement of drunken-driving laws easier.
Setting a marijuana-related impairment level is a much murkier proposition. But states that have legalized pot have to figure it out, experts said.
“You can’t legalize a substance and not have a coherent policy for controlling driving under the influence of that substance,” said Steven Davenport, an assistant policy researcher at the nonprofit Rand Corporation, who specializes in marijuana research.
Marijuana, after all, weakens a driver’s ability to maintain focus, and it slows reflexes. But regulators are “playing catch-up,” suggested Thomas Marcotte, a psychiatry professor at the University of California, San Diego, and one of a number of academics around the country who is researching driving while high.
States have put forth a bevy of approaches. At least five have what’s called a “per se” law, which outlaws driving if someone’s blood level of tetrahydrocannabinol, or THC, exceeds a set amount. THC is marijuana’s main intoxicant.
Colorado, where voters approved legalization of recreational marijuana in 2012, has this type of driving law on the books. It took 3 years to pass amid fiery debate and deems “intoxicated” any driver who tests higher than 5 ng of THC per milliliter of blood.
Indiana, Pennsylvania and Rhode Island are among states that forbid driving at any THC level. Still others say drivers should be penalized only if they are impaired by the chemical – a standard that sounds reasonable but quickly gets difficult to measure or even define.
None of these approaches offers an ideal solution, experts said.
“We’re still definitely evaluating which policies are the most effective,” said Ann Kitch, who tracks the marijuana and driving issue for the National Conference of State Legislatures.
States that set a THC-level standard confront weak technology and limited science. THC testing is imprecise at best, since the chemical can stay in someone’s bloodstream for weeks after it was ingested. Someone could legally smoke a joint and still have THC appear in blood or urine samples long after the high passes.
There’s general agreement that driving while high is bad, but there’s no linear relationship between THC levels and degree of impairment. States that have picked a number to reflect when THC in the bloodstream becomes a hazard have “made it up,” argued Dr. Humphreys.
“The ones who wrote [a number] into legislation felt they had to say something,” he said. But “we don’t know what would be the analogy. Is the legal amount [of THC] equal to a beer? Is that how impaired you are? Is it a six-pack?”
Roadside testing for THC is also logistically difficult. Blood, for instance, needs to be analyzed in a lab, and collecting urine gets ... complicated.
In Canada, which legalized recreational pot just this year, law enforcement will test drivers with a saliva test called the Dräger DrugTest 5000, but that isn’t perfect, either.
Some private companies are trying to develop a sort of breathalyzer for marijuana. But Jonathan Caulkins, a drug policy researcher at Carnegie Mellon University, Pittsburgh, said, “There are fundamental issues with the chemistry and pharmacokinetics. It’s really hard to have an objective, easy-to-administer roadside test.”
Some states rely on law enforcement to assess whether someone’s driving appears impaired and ascertain after the fact if marijuana was involved.
In California, every highway patrol member learns to administer “field sobriety tests” – undergoing an extra 16 hours of training to recognize the influence of different drugs, including marijuana. Because medical marijuana has been legal there since 1996, officers are “very used” to recognizing its influence, said Glenn Glazer, the state’s coordinator for its drug recognition expert training program.
That kind of training is taking off in other states, too, Ms. Kitch said. Lobbying groups such as Mothers Against Drunk Driving are pushing to bump up law enforcement training and rely on officers to assess whether a driver is impaired.
These tests, though, risk their own kind of error.
“They are subjective,” Mr. Davenport warned.
For one thing, officer-administered tests can be influenced by racial bias. Someone who has previously had poor experiences with law enforcement may also perform worse, not because of greater impairment but because of nervousness.
Indeed, relying on more subjective testing is in some ways the direct opposite of conventional wisdom.
“A general pattern of the last ... 40 years is to try to take human judgment out of decision making processes when possible. Because we fear exactly these issues,” Mr. Caulkins said. “The idea that you could come up with a completely objective test of performance ... is ambitious.”
Researchers like Dr. Marcotte are trying to devise some kind of test that can, in fact, gauge whether someone is showing signs of marijuana impairment. But that could take years.
In the meantime, the public health threat is real. States with legalized pot do appear to experience more car crashes, though the relationship is muddled. “This is going to be a headache of an issue for a decade,” Mr. Caulkins said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
It used to be the stuff of stoner comedies and “Just Say No” campaigns. Today, marijuana is becoming mainstream as voters across the country approve ballot questions for legalization or medical use.
In response, state governments are testing ways to ensure that the integration of this once-illicit substance into everyday life doesn’t create new public health risks. These efforts are sparking a difficult question: At what point is someone too high to get behind the wheel?
The answer is complicated. Brain scientists and pharmacologists don’t know how to measure if and to what extent marijuana causes impairment.
The reason: Existing blood and urine tests can detect marijuana use, but, because traces of the drug stay in the human body for a long time, those tests can’t specify whether the use occurred earlier that day or that month. They also don’t indicate the level at which a driver would be considered “under the influence.”
“It’s a really hard problem,” said Keith Humphreys, a psychiatry professor and drug policy expert at Stanford University (Calif.), the first state to legalize medical marijuana and where recreational pot use among adults became legal in 2016. “We don’t really have good evidence – even if we know someone has been using – [to gauge] what their level of impairment is.”
Marijuana is now legal for recreational use in 10 states and the District of Columbia – including Michigan, where a ballot initiative passed in November 2018 took effect Dec. 6. In New York, the governor said Dec. 17 that legalization would be a top priority for 2019. And nearly three dozen states have cleared the use of medical cannabis.
For alcohol, there is a clear, national standard. If your blood alcohol content is 0.08% or higher, you’re considered cognitively impaired at a level that is unsafe to drive. Extensive research supports this determination, and the clarity makes enforcement of drunken-driving laws easier.
Setting a marijuana-related impairment level is a much murkier proposition. But states that have legalized pot have to figure it out, experts said.
“You can’t legalize a substance and not have a coherent policy for controlling driving under the influence of that substance,” said Steven Davenport, an assistant policy researcher at the nonprofit Rand Corporation, who specializes in marijuana research.
Marijuana, after all, weakens a driver’s ability to maintain focus, and it slows reflexes. But regulators are “playing catch-up,” suggested Thomas Marcotte, a psychiatry professor at the University of California, San Diego, and one of a number of academics around the country who is researching driving while high.
States have put forth a bevy of approaches. At least five have what’s called a “per se” law, which outlaws driving if someone’s blood level of tetrahydrocannabinol, or THC, exceeds a set amount. THC is marijuana’s main intoxicant.
Colorado, where voters approved legalization of recreational marijuana in 2012, has this type of driving law on the books. It took 3 years to pass amid fiery debate and deems “intoxicated” any driver who tests higher than 5 ng of THC per milliliter of blood.
Indiana, Pennsylvania and Rhode Island are among states that forbid driving at any THC level. Still others say drivers should be penalized only if they are impaired by the chemical – a standard that sounds reasonable but quickly gets difficult to measure or even define.
None of these approaches offers an ideal solution, experts said.
“We’re still definitely evaluating which policies are the most effective,” said Ann Kitch, who tracks the marijuana and driving issue for the National Conference of State Legislatures.
States that set a THC-level standard confront weak technology and limited science. THC testing is imprecise at best, since the chemical can stay in someone’s bloodstream for weeks after it was ingested. Someone could legally smoke a joint and still have THC appear in blood or urine samples long after the high passes.
There’s general agreement that driving while high is bad, but there’s no linear relationship between THC levels and degree of impairment. States that have picked a number to reflect when THC in the bloodstream becomes a hazard have “made it up,” argued Dr. Humphreys.
“The ones who wrote [a number] into legislation felt they had to say something,” he said. But “we don’t know what would be the analogy. Is the legal amount [of THC] equal to a beer? Is that how impaired you are? Is it a six-pack?”
Roadside testing for THC is also logistically difficult. Blood, for instance, needs to be analyzed in a lab, and collecting urine gets ... complicated.
In Canada, which legalized recreational pot just this year, law enforcement will test drivers with a saliva test called the Dräger DrugTest 5000, but that isn’t perfect, either.
Some private companies are trying to develop a sort of breathalyzer for marijuana. But Jonathan Caulkins, a drug policy researcher at Carnegie Mellon University, Pittsburgh, said, “There are fundamental issues with the chemistry and pharmacokinetics. It’s really hard to have an objective, easy-to-administer roadside test.”
Some states rely on law enforcement to assess whether someone’s driving appears impaired and ascertain after the fact if marijuana was involved.
In California, every highway patrol member learns to administer “field sobriety tests” – undergoing an extra 16 hours of training to recognize the influence of different drugs, including marijuana. Because medical marijuana has been legal there since 1996, officers are “very used” to recognizing its influence, said Glenn Glazer, the state’s coordinator for its drug recognition expert training program.
That kind of training is taking off in other states, too, Ms. Kitch said. Lobbying groups such as Mothers Against Drunk Driving are pushing to bump up law enforcement training and rely on officers to assess whether a driver is impaired.
These tests, though, risk their own kind of error.
“They are subjective,” Mr. Davenport warned.
For one thing, officer-administered tests can be influenced by racial bias. Someone who has previously had poor experiences with law enforcement may also perform worse, not because of greater impairment but because of nervousness.
Indeed, relying on more subjective testing is in some ways the direct opposite of conventional wisdom.
“A general pattern of the last ... 40 years is to try to take human judgment out of decision making processes when possible. Because we fear exactly these issues,” Mr. Caulkins said. “The idea that you could come up with a completely objective test of performance ... is ambitious.”
Researchers like Dr. Marcotte are trying to devise some kind of test that can, in fact, gauge whether someone is showing signs of marijuana impairment. But that could take years.
In the meantime, the public health threat is real. States with legalized pot do appear to experience more car crashes, though the relationship is muddled. “This is going to be a headache of an issue for a decade,” Mr. Caulkins said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
FDA expands dasatinib indication to children with Ph+ ALL
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
All the Little Lesions in a Row
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.
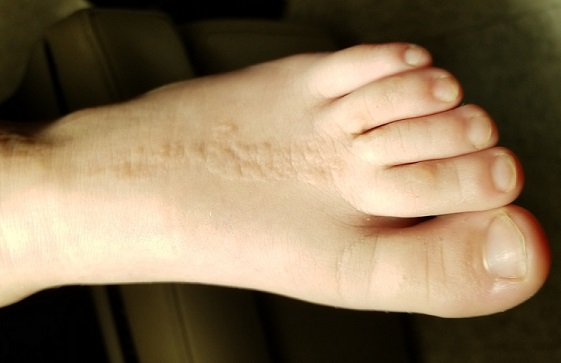
EXAMINATION
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.

EXAMINATION
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.

EXAMINATION
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.
Single-dose propranolol tied to ‘selective erasure’ of anxiety disorders
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: A single 40-mg dose of oral propranolol, judiciously timed, is a highly promising novel treatment for anxiety disorders.
Major finding: The beta-blocker must be given within an hour before to an hour after therapist-facilitated reactivation of the fear memory.
Study details: This study included 30 healthy volunteers who underwent a cued Pavlovian fear-conditioning program.
Disclosures: Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
Endometriosis surgery: Women can expect years-long benefits
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Key clinical point: Endometriosis excision improves quality of life for at least 7 years, even when women have conservative, fertility-sparing surgery.
Major finding: The overall score on the Endometriosis Health Profile-30 fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at the 7-year follow-up.
Study details: A review of 61 cases
Disclosures: The investigators didn’t report any disclosures.
Source: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.