User login
Shorter R-CHOP regimen noninferior in certain DLBCL patients
SAN DIEGO—A shortened regimen of four cycles of rituximab (R) plus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy was noninferior in efficacy to the standard six cycles of R-CHOP in younger patients with favorable-risk diffuse large B-cell lymphoma (DLBCL), according to investigators of the FLYER trial.
In addition, the truncated regimen was associated with about a one-third reduction in non-hematologic adverse events.
Viola Poeschel, MD, of Saarland University Medical School in Homburg/Saar, Germany, reported results of this study on behalf of the German High-Grade Non-Hodgkin’s Lymphoma Study Group/German Lymphoma Alliance at the 2018 ASH Annual Meeting (abstract 781).
Among 588 evaluable patients younger than 60 with favorable-prognosis DLBCL, there were no significant differences in progression-free survival (PFS), event-free survival (EFS), or overall survival (OS) between patients who received four cycles of R-CHOP and those who received six cycles, Dr. Poeschel reported.
“Six cycles of R-CHOP led to a higher toxicity with respect to leukocytopenia and anemia, both of any grade and also of grades 3 to 4, compared to four cycles of R-CHOP,” she said.
The findings suggest that, for younger patients with favorable-prognosis DLBCL—defined as an age-adjusted International Prognostic Index score of 0 and low tumor burden (less than 7.5 cm)—four cycles of R-CHOP can be a new standard of care, Dr. Poeschel said.
The investigators were prompted to look at the question of a shorter R-CHOP regimen by results of the MInT trial, in which a subpopulation of favorable-prognosis DLBCL patients had a 3-year PFS rate of 89%.
The FLYER trial (NCT00278421) was designed as a non-inferiority study to see whether, in a similar group of patients, reducing the number of R-CHOP cycles could maintain efficacy while reducing toxicity.
At a median follow-up of 66 months, the PFS rate, the primary endpoint, was 94% in the six-cycle group and 96% for the four-cycle group.
“As the lower limit of the 95% confidence interval of our experimental arm was 94%, it is shown that it is definitely non-inferior to the standard arm, six cycles of R-CHOP,” Dr. Poeschel said.
Similarly, the rate of 3-year OS was 98% in the six-cycle group, compared with 99% in the four-cycle group, and the survival curves were virtually superimposable out to more than 10 years of follow-up.
Treatment with six cycles was associated with more frequent hematologic adverse events than four cycles. Leukopenia of any grade occurred in 237 and 171 patients, respectively. Grade 3-4 leukopenia occurred in 110 and 80 patients, respectively.
Any-grade anemia occurred in 172 patients assigned to six cycles and 107 assigned to four cycles. Rates of grade 3-4 anemia were similar between the groups, as were rates of thrombocytopenia of any grade or grade 3-4.
Non-hematologic adverse events of any grade or grade 3-4 that were more frequent with six cycles included parasthesia, nausea, infection, vomiting, and mucositis.
The total number of non-hematologic adverse events was reduced by about one-third.
“We are certainly always looking for ways to make treatments easier for our patients to reduce adverse effects, and, certainly, for this subgroup of patients, it appears that we can make their treatment shorter and have less burden but equivalent efficacy,” said David Steensma, MD, of the Dana-Farber Cancer Institute/Harvard Cancer Center in Boston, Massachusetts.
Drs. Steensma and Poeschel both cautioned that the results of this study pertain only to those patients with DLBCL who are younger and have favorable-prognosis disease.
“We can’t extend it to other subtypes of large-cell lymphoma, but that’s always a laudable goal, so I think this will immediately influence clinical practice,” Dr. Steensma said.
The study was supported by Deutsche Krebshilfe. Dr. Poeschel disclosed travel grants from Roche and Amgen. Dr. Steensma had no disclosures relevant to the study.
SAN DIEGO—A shortened regimen of four cycles of rituximab (R) plus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy was noninferior in efficacy to the standard six cycles of R-CHOP in younger patients with favorable-risk diffuse large B-cell lymphoma (DLBCL), according to investigators of the FLYER trial.
In addition, the truncated regimen was associated with about a one-third reduction in non-hematologic adverse events.
Viola Poeschel, MD, of Saarland University Medical School in Homburg/Saar, Germany, reported results of this study on behalf of the German High-Grade Non-Hodgkin’s Lymphoma Study Group/German Lymphoma Alliance at the 2018 ASH Annual Meeting (abstract 781).
Among 588 evaluable patients younger than 60 with favorable-prognosis DLBCL, there were no significant differences in progression-free survival (PFS), event-free survival (EFS), or overall survival (OS) between patients who received four cycles of R-CHOP and those who received six cycles, Dr. Poeschel reported.
“Six cycles of R-CHOP led to a higher toxicity with respect to leukocytopenia and anemia, both of any grade and also of grades 3 to 4, compared to four cycles of R-CHOP,” she said.
The findings suggest that, for younger patients with favorable-prognosis DLBCL—defined as an age-adjusted International Prognostic Index score of 0 and low tumor burden (less than 7.5 cm)—four cycles of R-CHOP can be a new standard of care, Dr. Poeschel said.
The investigators were prompted to look at the question of a shorter R-CHOP regimen by results of the MInT trial, in which a subpopulation of favorable-prognosis DLBCL patients had a 3-year PFS rate of 89%.
The FLYER trial (NCT00278421) was designed as a non-inferiority study to see whether, in a similar group of patients, reducing the number of R-CHOP cycles could maintain efficacy while reducing toxicity.
At a median follow-up of 66 months, the PFS rate, the primary endpoint, was 94% in the six-cycle group and 96% for the four-cycle group.
“As the lower limit of the 95% confidence interval of our experimental arm was 94%, it is shown that it is definitely non-inferior to the standard arm, six cycles of R-CHOP,” Dr. Poeschel said.
Similarly, the rate of 3-year OS was 98% in the six-cycle group, compared with 99% in the four-cycle group, and the survival curves were virtually superimposable out to more than 10 years of follow-up.
Treatment with six cycles was associated with more frequent hematologic adverse events than four cycles. Leukopenia of any grade occurred in 237 and 171 patients, respectively. Grade 3-4 leukopenia occurred in 110 and 80 patients, respectively.
Any-grade anemia occurred in 172 patients assigned to six cycles and 107 assigned to four cycles. Rates of grade 3-4 anemia were similar between the groups, as were rates of thrombocytopenia of any grade or grade 3-4.
Non-hematologic adverse events of any grade or grade 3-4 that were more frequent with six cycles included parasthesia, nausea, infection, vomiting, and mucositis.
The total number of non-hematologic adverse events was reduced by about one-third.
“We are certainly always looking for ways to make treatments easier for our patients to reduce adverse effects, and, certainly, for this subgroup of patients, it appears that we can make their treatment shorter and have less burden but equivalent efficacy,” said David Steensma, MD, of the Dana-Farber Cancer Institute/Harvard Cancer Center in Boston, Massachusetts.
Drs. Steensma and Poeschel both cautioned that the results of this study pertain only to those patients with DLBCL who are younger and have favorable-prognosis disease.
“We can’t extend it to other subtypes of large-cell lymphoma, but that’s always a laudable goal, so I think this will immediately influence clinical practice,” Dr. Steensma said.
The study was supported by Deutsche Krebshilfe. Dr. Poeschel disclosed travel grants from Roche and Amgen. Dr. Steensma had no disclosures relevant to the study.
SAN DIEGO—A shortened regimen of four cycles of rituximab (R) plus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy was noninferior in efficacy to the standard six cycles of R-CHOP in younger patients with favorable-risk diffuse large B-cell lymphoma (DLBCL), according to investigators of the FLYER trial.
In addition, the truncated regimen was associated with about a one-third reduction in non-hematologic adverse events.
Viola Poeschel, MD, of Saarland University Medical School in Homburg/Saar, Germany, reported results of this study on behalf of the German High-Grade Non-Hodgkin’s Lymphoma Study Group/German Lymphoma Alliance at the 2018 ASH Annual Meeting (abstract 781).
Among 588 evaluable patients younger than 60 with favorable-prognosis DLBCL, there were no significant differences in progression-free survival (PFS), event-free survival (EFS), or overall survival (OS) between patients who received four cycles of R-CHOP and those who received six cycles, Dr. Poeschel reported.
“Six cycles of R-CHOP led to a higher toxicity with respect to leukocytopenia and anemia, both of any grade and also of grades 3 to 4, compared to four cycles of R-CHOP,” she said.
The findings suggest that, for younger patients with favorable-prognosis DLBCL—defined as an age-adjusted International Prognostic Index score of 0 and low tumor burden (less than 7.5 cm)—four cycles of R-CHOP can be a new standard of care, Dr. Poeschel said.
The investigators were prompted to look at the question of a shorter R-CHOP regimen by results of the MInT trial, in which a subpopulation of favorable-prognosis DLBCL patients had a 3-year PFS rate of 89%.
The FLYER trial (NCT00278421) was designed as a non-inferiority study to see whether, in a similar group of patients, reducing the number of R-CHOP cycles could maintain efficacy while reducing toxicity.
At a median follow-up of 66 months, the PFS rate, the primary endpoint, was 94% in the six-cycle group and 96% for the four-cycle group.
“As the lower limit of the 95% confidence interval of our experimental arm was 94%, it is shown that it is definitely non-inferior to the standard arm, six cycles of R-CHOP,” Dr. Poeschel said.
Similarly, the rate of 3-year OS was 98% in the six-cycle group, compared with 99% in the four-cycle group, and the survival curves were virtually superimposable out to more than 10 years of follow-up.
Treatment with six cycles was associated with more frequent hematologic adverse events than four cycles. Leukopenia of any grade occurred in 237 and 171 patients, respectively. Grade 3-4 leukopenia occurred in 110 and 80 patients, respectively.
Any-grade anemia occurred in 172 patients assigned to six cycles and 107 assigned to four cycles. Rates of grade 3-4 anemia were similar between the groups, as were rates of thrombocytopenia of any grade or grade 3-4.
Non-hematologic adverse events of any grade or grade 3-4 that were more frequent with six cycles included parasthesia, nausea, infection, vomiting, and mucositis.
The total number of non-hematologic adverse events was reduced by about one-third.
“We are certainly always looking for ways to make treatments easier for our patients to reduce adverse effects, and, certainly, for this subgroup of patients, it appears that we can make their treatment shorter and have less burden but equivalent efficacy,” said David Steensma, MD, of the Dana-Farber Cancer Institute/Harvard Cancer Center in Boston, Massachusetts.
Drs. Steensma and Poeschel both cautioned that the results of this study pertain only to those patients with DLBCL who are younger and have favorable-prognosis disease.
“We can’t extend it to other subtypes of large-cell lymphoma, but that’s always a laudable goal, so I think this will immediately influence clinical practice,” Dr. Steensma said.
The study was supported by Deutsche Krebshilfe. Dr. Poeschel disclosed travel grants from Roche and Amgen. Dr. Steensma had no disclosures relevant to the study.
Early caffeine therapy linked to improved neurologic outcomes in premature babies
Premature babies may benefit more if caffeine therapy is given within 2 days of birth, based on a retrospective observational cohort study of more than 2,000 newborns.
When caffeine was given within the first 2 days of birth, neonates had an adjusted odds ratio of significant neurodevelopmental impairment of 0.68, compared with neonates who received caffeine after 2 or more days. Further, the early-caffeine group had a 0.67 adjusted odds ratio for having cognitive scores of less than 85 on the Bayley Scales of Infant and Toddler Development, Third Edition, compared with the late-caffeine group. After researchers corrected for small-for-gestational-age status and other risk factors, however, early-caffeine therapy was associated with lower odds of cerebral palsy and hearing impairment only, according to the study published online in Pediatrics.
Caffeine administration should be a priority once extremely preterm neonates are stabilized, Abhay Lodha, MD, of the University of Calgary (Alta.), and his coauthors wrote. “It is rather easy to organize the administration of caffeine as early as possible for Level 3 nurseries, and many units have already accomplished this. However, certain Level 2 nurseries may not have facilities available for such early administration. We do not have data that indicate the earliest that caffeine would have to be given to get maximum benefit, and thus, it should not be counted as an emergency medication yet,” they wrote.
The study examined data from 2,108 neonates born before 29 weeks of gestational age and given caffeine to treat or prevent apnea; 1,545 received the caffeine within 2 days of birth and the remaining 563 were treated with caffeine after 2 days. Data were adjusted for gestational age, sex, antenatal steroids, and SNAP-II (Score of Neonatal Acute Physiology-II) score.
The early-caffeine group had a significantly reduced odds of hearing impairment and cerebral palsy, bronchopulmonary dysplasia, patent ductus arteriosus, and severe neurologic injury. When the data were further analyzed using propensity-matched groups – which also accounted for small-for-gestational-age status – the difference in outcomes was a nonsignificant trend in favor of early caffeine.
The authors noted that the late-caffeine group contained a higher proportion of infants born at or before 24 weeks’ gestational age, and a lower proportion of infants born at 25-28 weeks’ gestational age, compared with the early-caffeine group. The infants in the early-caffeine group also had higher Apgar scores, higher median birth weight, and lower SNAP-II scores, and received a longer median duration of caffeine treatment.
Dr. Lodha and his coauthors said the reason for the differences between the early- and late-caffeine groups was unclear. “However, it could be attributable to an increased growth of dendrites and spines in neurons that is initiated by the especially prolonged use of caffeine in the early-caffeine group,” they wrote. “The other speculation is that caffeine improves cardiac output and blood pressure in infants who are relatively stable.”
No funding or conflicts of interest were declared.
SOURCE: Lodha A et al. Pediatrics. 2018 Dec. 5. doi. org/10.1542/peds.2018-1348.
Premature babies may benefit more if caffeine therapy is given within 2 days of birth, based on a retrospective observational cohort study of more than 2,000 newborns.
When caffeine was given within the first 2 days of birth, neonates had an adjusted odds ratio of significant neurodevelopmental impairment of 0.68, compared with neonates who received caffeine after 2 or more days. Further, the early-caffeine group had a 0.67 adjusted odds ratio for having cognitive scores of less than 85 on the Bayley Scales of Infant and Toddler Development, Third Edition, compared with the late-caffeine group. After researchers corrected for small-for-gestational-age status and other risk factors, however, early-caffeine therapy was associated with lower odds of cerebral palsy and hearing impairment only, according to the study published online in Pediatrics.
Caffeine administration should be a priority once extremely preterm neonates are stabilized, Abhay Lodha, MD, of the University of Calgary (Alta.), and his coauthors wrote. “It is rather easy to organize the administration of caffeine as early as possible for Level 3 nurseries, and many units have already accomplished this. However, certain Level 2 nurseries may not have facilities available for such early administration. We do not have data that indicate the earliest that caffeine would have to be given to get maximum benefit, and thus, it should not be counted as an emergency medication yet,” they wrote.
The study examined data from 2,108 neonates born before 29 weeks of gestational age and given caffeine to treat or prevent apnea; 1,545 received the caffeine within 2 days of birth and the remaining 563 were treated with caffeine after 2 days. Data were adjusted for gestational age, sex, antenatal steroids, and SNAP-II (Score of Neonatal Acute Physiology-II) score.
The early-caffeine group had a significantly reduced odds of hearing impairment and cerebral palsy, bronchopulmonary dysplasia, patent ductus arteriosus, and severe neurologic injury. When the data were further analyzed using propensity-matched groups – which also accounted for small-for-gestational-age status – the difference in outcomes was a nonsignificant trend in favor of early caffeine.
The authors noted that the late-caffeine group contained a higher proportion of infants born at or before 24 weeks’ gestational age, and a lower proportion of infants born at 25-28 weeks’ gestational age, compared with the early-caffeine group. The infants in the early-caffeine group also had higher Apgar scores, higher median birth weight, and lower SNAP-II scores, and received a longer median duration of caffeine treatment.
Dr. Lodha and his coauthors said the reason for the differences between the early- and late-caffeine groups was unclear. “However, it could be attributable to an increased growth of dendrites and spines in neurons that is initiated by the especially prolonged use of caffeine in the early-caffeine group,” they wrote. “The other speculation is that caffeine improves cardiac output and blood pressure in infants who are relatively stable.”
No funding or conflicts of interest were declared.
SOURCE: Lodha A et al. Pediatrics. 2018 Dec. 5. doi. org/10.1542/peds.2018-1348.
Premature babies may benefit more if caffeine therapy is given within 2 days of birth, based on a retrospective observational cohort study of more than 2,000 newborns.
When caffeine was given within the first 2 days of birth, neonates had an adjusted odds ratio of significant neurodevelopmental impairment of 0.68, compared with neonates who received caffeine after 2 or more days. Further, the early-caffeine group had a 0.67 adjusted odds ratio for having cognitive scores of less than 85 on the Bayley Scales of Infant and Toddler Development, Third Edition, compared with the late-caffeine group. After researchers corrected for small-for-gestational-age status and other risk factors, however, early-caffeine therapy was associated with lower odds of cerebral palsy and hearing impairment only, according to the study published online in Pediatrics.
Caffeine administration should be a priority once extremely preterm neonates are stabilized, Abhay Lodha, MD, of the University of Calgary (Alta.), and his coauthors wrote. “It is rather easy to organize the administration of caffeine as early as possible for Level 3 nurseries, and many units have already accomplished this. However, certain Level 2 nurseries may not have facilities available for such early administration. We do not have data that indicate the earliest that caffeine would have to be given to get maximum benefit, and thus, it should not be counted as an emergency medication yet,” they wrote.
The study examined data from 2,108 neonates born before 29 weeks of gestational age and given caffeine to treat or prevent apnea; 1,545 received the caffeine within 2 days of birth and the remaining 563 were treated with caffeine after 2 days. Data were adjusted for gestational age, sex, antenatal steroids, and SNAP-II (Score of Neonatal Acute Physiology-II) score.
The early-caffeine group had a significantly reduced odds of hearing impairment and cerebral palsy, bronchopulmonary dysplasia, patent ductus arteriosus, and severe neurologic injury. When the data were further analyzed using propensity-matched groups – which also accounted for small-for-gestational-age status – the difference in outcomes was a nonsignificant trend in favor of early caffeine.
The authors noted that the late-caffeine group contained a higher proportion of infants born at or before 24 weeks’ gestational age, and a lower proportion of infants born at 25-28 weeks’ gestational age, compared with the early-caffeine group. The infants in the early-caffeine group also had higher Apgar scores, higher median birth weight, and lower SNAP-II scores, and received a longer median duration of caffeine treatment.
Dr. Lodha and his coauthors said the reason for the differences between the early- and late-caffeine groups was unclear. “However, it could be attributable to an increased growth of dendrites and spines in neurons that is initiated by the especially prolonged use of caffeine in the early-caffeine group,” they wrote. “The other speculation is that caffeine improves cardiac output and blood pressure in infants who are relatively stable.”
No funding or conflicts of interest were declared.
SOURCE: Lodha A et al. Pediatrics. 2018 Dec. 5. doi. org/10.1542/peds.2018-1348.
FROM PEDIATRICS
Key clinical point: Earlier caffeine treatment for premature infants may improve neurologic outcomes.
Major finding: Preterm neonates treated with caffeine within 2 days of birth have a significantly lower risk of hearing impairment and cerebral palsy.
Study details: A retrospective observational cohort study in 2,108 preterm neonates.
Disclosures: No funding or conflicts of interest were declared.
Source: Lodha A et al. Pediatrics. 2018 Dec. 5. doi. org/10.1542/peds.2018-1348.
Job Satisfaction & Burnout
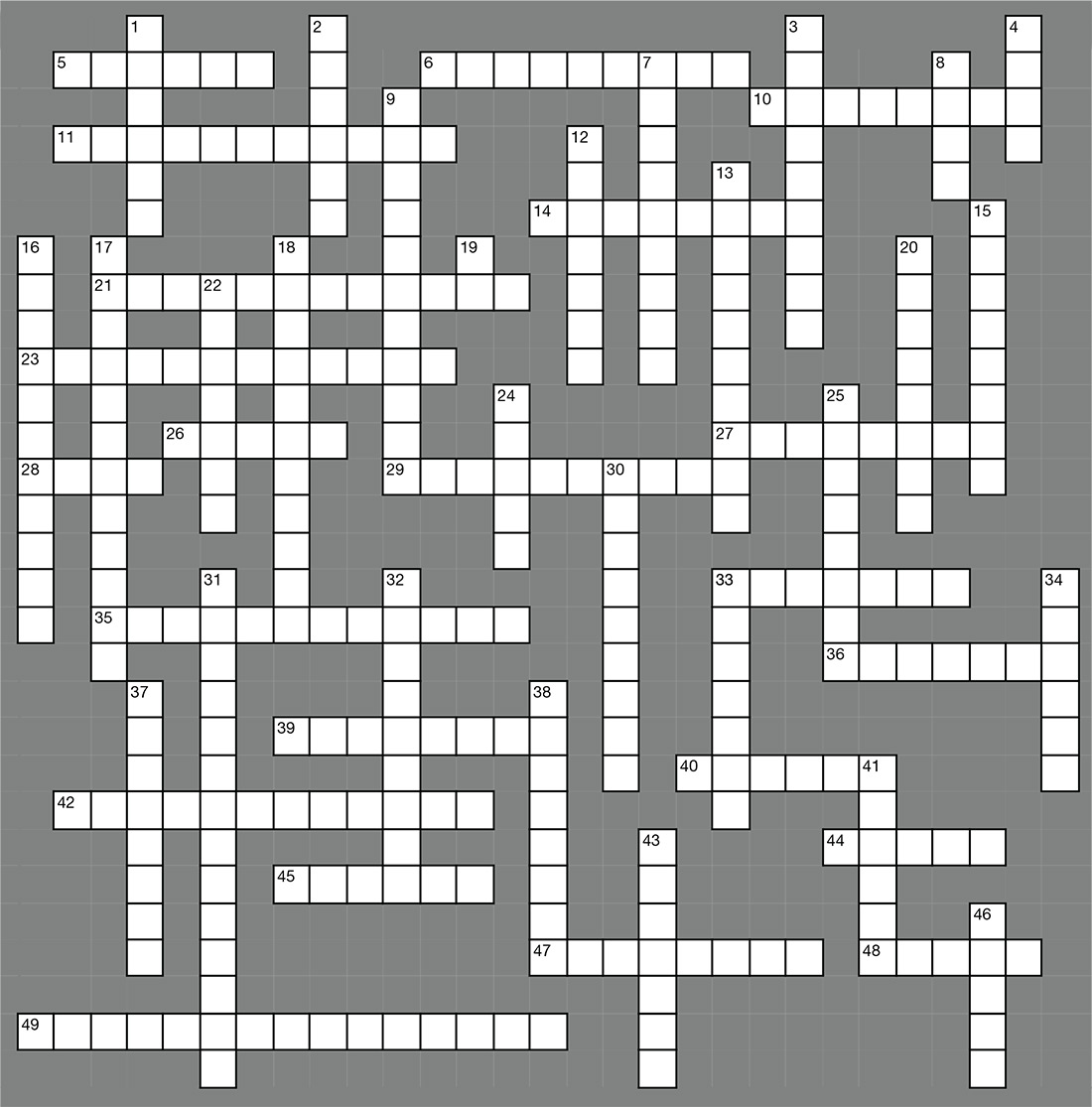
ACROSS
5. Some feel _____ in an unattainable quest.
6. Area of _____.
10. Focus on attributes of an advanced _____ career.
11. Discuss key _____ points with management.
14. High stress nature of _____ environments.
21. Negotiating a ____ package can cause anxiety.
23. Individual _____ compensation.
26. Bureau of _____ Statistics.
27. Inadequate time to _____ with patients.
28. Base pay rates and on-_____ pay.
29. Burn out characterized by emotional _____.
33. Direct _____ care.
35. Poor management with unclear _____.
36. NPs and PAs are more statistically _____ than different.
39. Having no say in ____ making is frustrating.
40. Opportunities for professional _____.
42. Long wait times impede patient _____.
44. Both professions are _____ about their career choice.
45. Feeling you are not a team _____.
47. Bad stress or “___” can cause health problems.
48. Stress is a part of _____ nature.
49. Not satisfied.
DOWN
1. Making decisions about your _____.
2. Health and _____ insurance.
3. Mitigating _____ stress is a challenge.
4. Licensing _____.
7. Good benefits provide _____ to do a better job.
8. NPs and PAs spend their _____ similarly.
9. Low attrition rates and _____ wages.
12. Issues related to work/life _____.
13. Patient _____ can affect job satisfaction.
15. Incentive _____ improve job satisfaction.
16. Threat of _____ lawsuits.
17. Focus on what you have _____.
18. Don’t suppress concerns about the work __.
19. Learn to say “_____.”
20. Employment opportunities are expected to ___.
22. The non-cash portion of a compensation ____.
24. Set realistic _____ for your day.
25. A great _____ package will improve your work climate.
30. ____ stress can be beneficial.
31. A heavy workload or too much _____ causes stress.
32. Professional _____ insurance
33. Stress can motivate people to _____.
34. Increased satisfaction in those who sped more time in _____ patient care.
37. Stress causes _____ release and weight gain.
38. Look for ways to become more _____.
41. Managing stress is key to good _____.
43. Goal _____ provides purposeful direction.
46. The _____ your employer places on you affects attitude.
Answer key on next page...
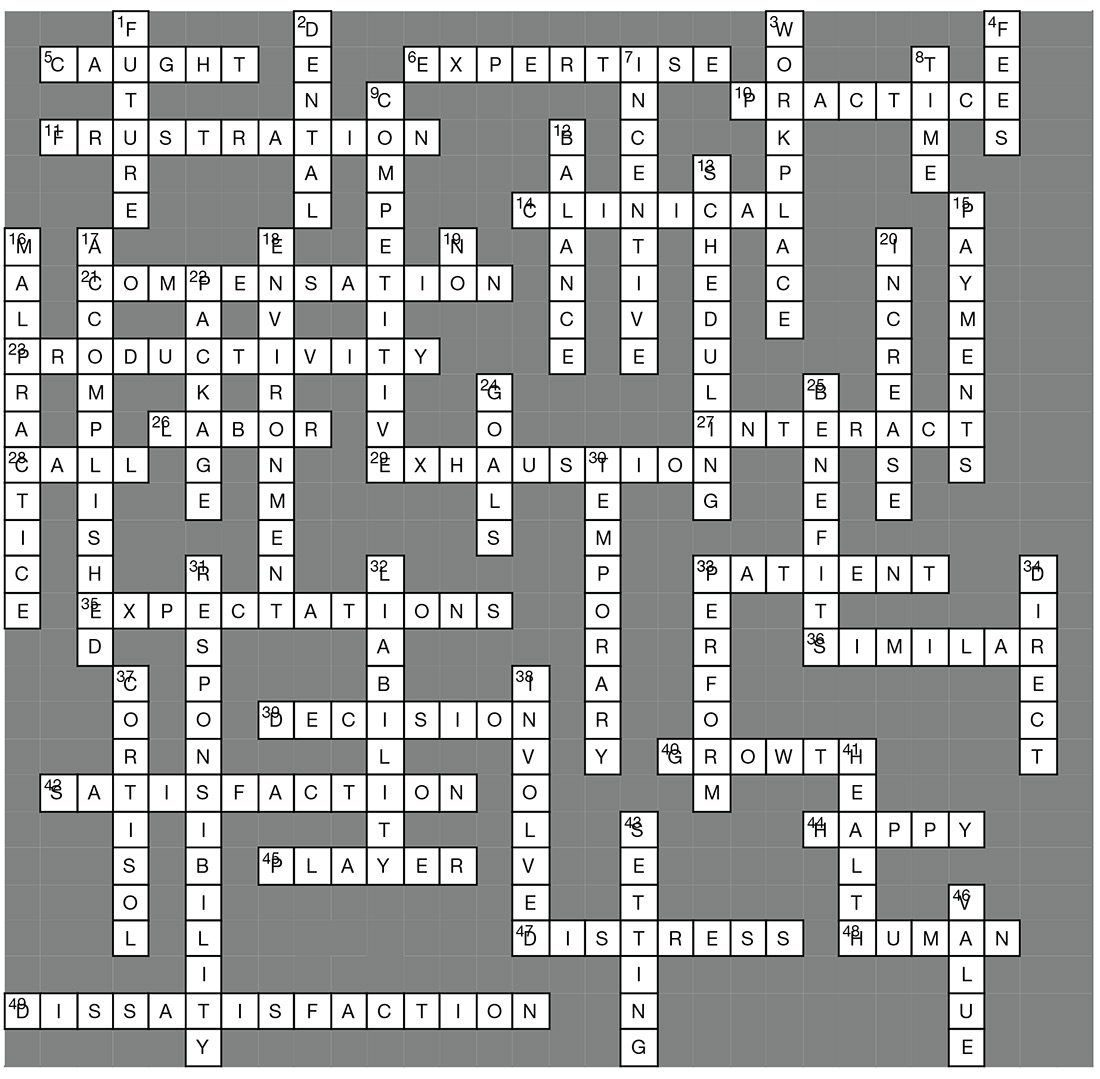

ACROSS
5. Some feel _____ in an unattainable quest.
6. Area of _____.
10. Focus on attributes of an advanced _____ career.
11. Discuss key _____ points with management.
14. High stress nature of _____ environments.
21. Negotiating a ____ package can cause anxiety.
23. Individual _____ compensation.
26. Bureau of _____ Statistics.
27. Inadequate time to _____ with patients.
28. Base pay rates and on-_____ pay.
29. Burn out characterized by emotional _____.
33. Direct _____ care.
35. Poor management with unclear _____.
36. NPs and PAs are more statistically _____ than different.
39. Having no say in ____ making is frustrating.
40. Opportunities for professional _____.
42. Long wait times impede patient _____.
44. Both professions are _____ about their career choice.
45. Feeling you are not a team _____.
47. Bad stress or “___” can cause health problems.
48. Stress is a part of _____ nature.
49. Not satisfied.
DOWN
1. Making decisions about your _____.
2. Health and _____ insurance.
3. Mitigating _____ stress is a challenge.
4. Licensing _____.
7. Good benefits provide _____ to do a better job.
8. NPs and PAs spend their _____ similarly.
9. Low attrition rates and _____ wages.
12. Issues related to work/life _____.
13. Patient _____ can affect job satisfaction.
15. Incentive _____ improve job satisfaction.
16. Threat of _____ lawsuits.
17. Focus on what you have _____.
18. Don’t suppress concerns about the work __.
19. Learn to say “_____.”
20. Employment opportunities are expected to ___.
22. The non-cash portion of a compensation ____.
24. Set realistic _____ for your day.
25. A great _____ package will improve your work climate.
30. ____ stress can be beneficial.
31. A heavy workload or too much _____ causes stress.
32. Professional _____ insurance
33. Stress can motivate people to _____.
34. Increased satisfaction in those who sped more time in _____ patient care.
37. Stress causes _____ release and weight gain.
38. Look for ways to become more _____.
41. Managing stress is key to good _____.
43. Goal _____ provides purposeful direction.
46. The _____ your employer places on you affects attitude.
Answer key on next page...


ACROSS
5. Some feel _____ in an unattainable quest.
6. Area of _____.
10. Focus on attributes of an advanced _____ career.
11. Discuss key _____ points with management.
14. High stress nature of _____ environments.
21. Negotiating a ____ package can cause anxiety.
23. Individual _____ compensation.
26. Bureau of _____ Statistics.
27. Inadequate time to _____ with patients.
28. Base pay rates and on-_____ pay.
29. Burn out characterized by emotional _____.
33. Direct _____ care.
35. Poor management with unclear _____.
36. NPs and PAs are more statistically _____ than different.
39. Having no say in ____ making is frustrating.
40. Opportunities for professional _____.
42. Long wait times impede patient _____.
44. Both professions are _____ about their career choice.
45. Feeling you are not a team _____.
47. Bad stress or “___” can cause health problems.
48. Stress is a part of _____ nature.
49. Not satisfied.
DOWN
1. Making decisions about your _____.
2. Health and _____ insurance.
3. Mitigating _____ stress is a challenge.
4. Licensing _____.
7. Good benefits provide _____ to do a better job.
8. NPs and PAs spend their _____ similarly.
9. Low attrition rates and _____ wages.
12. Issues related to work/life _____.
13. Patient _____ can affect job satisfaction.
15. Incentive _____ improve job satisfaction.
16. Threat of _____ lawsuits.
17. Focus on what you have _____.
18. Don’t suppress concerns about the work __.
19. Learn to say “_____.”
20. Employment opportunities are expected to ___.
22. The non-cash portion of a compensation ____.
24. Set realistic _____ for your day.
25. A great _____ package will improve your work climate.
30. ____ stress can be beneficial.
31. A heavy workload or too much _____ causes stress.
32. Professional _____ insurance
33. Stress can motivate people to _____.
34. Increased satisfaction in those who sped more time in _____ patient care.
37. Stress causes _____ release and weight gain.
38. Look for ways to become more _____.
41. Managing stress is key to good _____.
43. Goal _____ provides purposeful direction.
46. The _____ your employer places on you affects attitude.
Answer key on next page...

Tidying up a motley crew
It probably is buried in a box in your parents’ basement, but try to remember your soccer or football or track team picture from when you were in eighth grade. Tragically but predictably, most of your peers who were chubby in third grade are nowhere to be seen in the photo. But still it was a pretty motley crew. Some of you weren’t even up to the armpits of your taller teammates. Some guys were shaving. Others had little boys’ voices. Half the girls had reached menarche. Another third were still waiting impatiently for a breast bud.
The precocious and the late bloomers, you were all on the team. But it was pretty clear that those who had matured first generally were the more talented and successful athletes. By the time you were juniors in high school, many of those who matured late had quit the sport or been cut from the team, unable to catch up. Others may have been forced to give up the sport by their parents, who were concerned about the risk of injury when bodies of disparate size collide. A few of the early bloomers may have become depressed, older adolescents who had failed to match the hype and expectations that came when they were a head taller than their grade school teammates.
These natural consequences of biological variation are not small potatoes for the fragile egos of adolescents and preadolescents. The lead article in the November 2018 Pediatrics offers a partial solution for the issue of sports participation in a population with widely discrepant states of maturity (“Biobanding: A New Paradigm for Youth Sports and Training,” Pediatrics. 2018 Nov;142[5]:e20180423). The authors describe a system they call biobanding, in which “the percentage of predicted adult stature attained at the time of observation as the indicator of maturity status” is used to create groups or bands of participants with similar levels of maturity. They argue that this method is easy to use and report and that has been used with some success in Great Britain.
At first blush, biobanding sounds appealing, particularly for large communities. However, as someone who grew up in and practiced in a small town, I’m not sure how successfully it could be scaled down. There have been years when I could easily have disqualified a third of the high school football team were I to take into consideration the size and maturity of the competition they would be facing. But I didn’t. The fading interest in football in Maine has prompted some schools to consider moving to less-than-11-player competition or even to flag football. To some extent, the problem is taking care of itself.
How much tinkering should we be doing with something that is arguably a distorted natural selection process? With thoughtfully crafted rules, diligent supervision, and officiating, most of the issues of safety that one might attribute to discrepancies in maturity can be minimized. There always will be children who become discouraged and quit when they see the handwriting on the wall that reads “those who mature early win.” I’m certainly not wild about parents holding their children out of school to give them a jump on their peers. It can spiral out of control.
A more appealing solution is to do a better job of advertising the many successful late bloomers in professional sports ... and making sure that late-blooming children are given an abundance of active and competitive (if they wish) alternatives to sports dominated by their early maturing peers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It probably is buried in a box in your parents’ basement, but try to remember your soccer or football or track team picture from when you were in eighth grade. Tragically but predictably, most of your peers who were chubby in third grade are nowhere to be seen in the photo. But still it was a pretty motley crew. Some of you weren’t even up to the armpits of your taller teammates. Some guys were shaving. Others had little boys’ voices. Half the girls had reached menarche. Another third were still waiting impatiently for a breast bud.
The precocious and the late bloomers, you were all on the team. But it was pretty clear that those who had matured first generally were the more talented and successful athletes. By the time you were juniors in high school, many of those who matured late had quit the sport or been cut from the team, unable to catch up. Others may have been forced to give up the sport by their parents, who were concerned about the risk of injury when bodies of disparate size collide. A few of the early bloomers may have become depressed, older adolescents who had failed to match the hype and expectations that came when they were a head taller than their grade school teammates.
These natural consequences of biological variation are not small potatoes for the fragile egos of adolescents and preadolescents. The lead article in the November 2018 Pediatrics offers a partial solution for the issue of sports participation in a population with widely discrepant states of maturity (“Biobanding: A New Paradigm for Youth Sports and Training,” Pediatrics. 2018 Nov;142[5]:e20180423). The authors describe a system they call biobanding, in which “the percentage of predicted adult stature attained at the time of observation as the indicator of maturity status” is used to create groups or bands of participants with similar levels of maturity. They argue that this method is easy to use and report and that has been used with some success in Great Britain.
At first blush, biobanding sounds appealing, particularly for large communities. However, as someone who grew up in and practiced in a small town, I’m not sure how successfully it could be scaled down. There have been years when I could easily have disqualified a third of the high school football team were I to take into consideration the size and maturity of the competition they would be facing. But I didn’t. The fading interest in football in Maine has prompted some schools to consider moving to less-than-11-player competition or even to flag football. To some extent, the problem is taking care of itself.
How much tinkering should we be doing with something that is arguably a distorted natural selection process? With thoughtfully crafted rules, diligent supervision, and officiating, most of the issues of safety that one might attribute to discrepancies in maturity can be minimized. There always will be children who become discouraged and quit when they see the handwriting on the wall that reads “those who mature early win.” I’m certainly not wild about parents holding their children out of school to give them a jump on their peers. It can spiral out of control.
A more appealing solution is to do a better job of advertising the many successful late bloomers in professional sports ... and making sure that late-blooming children are given an abundance of active and competitive (if they wish) alternatives to sports dominated by their early maturing peers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It probably is buried in a box in your parents’ basement, but try to remember your soccer or football or track team picture from when you were in eighth grade. Tragically but predictably, most of your peers who were chubby in third grade are nowhere to be seen in the photo. But still it was a pretty motley crew. Some of you weren’t even up to the armpits of your taller teammates. Some guys were shaving. Others had little boys’ voices. Half the girls had reached menarche. Another third were still waiting impatiently for a breast bud.
The precocious and the late bloomers, you were all on the team. But it was pretty clear that those who had matured first generally were the more talented and successful athletes. By the time you were juniors in high school, many of those who matured late had quit the sport or been cut from the team, unable to catch up. Others may have been forced to give up the sport by their parents, who were concerned about the risk of injury when bodies of disparate size collide. A few of the early bloomers may have become depressed, older adolescents who had failed to match the hype and expectations that came when they were a head taller than their grade school teammates.
These natural consequences of biological variation are not small potatoes for the fragile egos of adolescents and preadolescents. The lead article in the November 2018 Pediatrics offers a partial solution for the issue of sports participation in a population with widely discrepant states of maturity (“Biobanding: A New Paradigm for Youth Sports and Training,” Pediatrics. 2018 Nov;142[5]:e20180423). The authors describe a system they call biobanding, in which “the percentage of predicted adult stature attained at the time of observation as the indicator of maturity status” is used to create groups or bands of participants with similar levels of maturity. They argue that this method is easy to use and report and that has been used with some success in Great Britain.
At first blush, biobanding sounds appealing, particularly for large communities. However, as someone who grew up in and practiced in a small town, I’m not sure how successfully it could be scaled down. There have been years when I could easily have disqualified a third of the high school football team were I to take into consideration the size and maturity of the competition they would be facing. But I didn’t. The fading interest in football in Maine has prompted some schools to consider moving to less-than-11-player competition or even to flag football. To some extent, the problem is taking care of itself.
How much tinkering should we be doing with something that is arguably a distorted natural selection process? With thoughtfully crafted rules, diligent supervision, and officiating, most of the issues of safety that one might attribute to discrepancies in maturity can be minimized. There always will be children who become discouraged and quit when they see the handwriting on the wall that reads “those who mature early win.” I’m certainly not wild about parents holding their children out of school to give them a jump on their peers. It can spiral out of control.
A more appealing solution is to do a better job of advertising the many successful late bloomers in professional sports ... and making sure that late-blooming children are given an abundance of active and competitive (if they wish) alternatives to sports dominated by their early maturing peers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Phase 3 study of novel pemphigus treatment is initiated
and will enroll about 120 patients with moderate to severe disease, according to Principia Biopharma, which is developing the drug.
In a press release, the company said that the randomized, double-blind PEGASYS study will compare PRN1008 with placebo, in about 120 patients with newly diagnosed or relapsing moderate to severe pemphigus.
The company also reported the results of an open label phase 2 study of patients with newly diagnosed or relapsing mild or moderate pemphigus, including pemphigus vulgaris and pemphigus foliaceus, which found that control of disease activity within 4 weeks of starting treatment – the primary efficacy endpoint – was achieved by more than 50% of patients taking PRN1008. Principia has extended the trial’s active treatment period from 12 to 24 weeks. The results also led the company to initiate the phase 3 trial.
PRN1008 is an inhibitor of BTK, an enzyme that “is present in the signaling pathways of most types of white blood cells except for T cells and plasma cells,” according to the company’s press release.
and will enroll about 120 patients with moderate to severe disease, according to Principia Biopharma, which is developing the drug.
In a press release, the company said that the randomized, double-blind PEGASYS study will compare PRN1008 with placebo, in about 120 patients with newly diagnosed or relapsing moderate to severe pemphigus.
The company also reported the results of an open label phase 2 study of patients with newly diagnosed or relapsing mild or moderate pemphigus, including pemphigus vulgaris and pemphigus foliaceus, which found that control of disease activity within 4 weeks of starting treatment – the primary efficacy endpoint – was achieved by more than 50% of patients taking PRN1008. Principia has extended the trial’s active treatment period from 12 to 24 weeks. The results also led the company to initiate the phase 3 trial.
PRN1008 is an inhibitor of BTK, an enzyme that “is present in the signaling pathways of most types of white blood cells except for T cells and plasma cells,” according to the company’s press release.
and will enroll about 120 patients with moderate to severe disease, according to Principia Biopharma, which is developing the drug.
In a press release, the company said that the randomized, double-blind PEGASYS study will compare PRN1008 with placebo, in about 120 patients with newly diagnosed or relapsing moderate to severe pemphigus.
The company also reported the results of an open label phase 2 study of patients with newly diagnosed or relapsing mild or moderate pemphigus, including pemphigus vulgaris and pemphigus foliaceus, which found that control of disease activity within 4 weeks of starting treatment – the primary efficacy endpoint – was achieved by more than 50% of patients taking PRN1008. Principia has extended the trial’s active treatment period from 12 to 24 weeks. The results also led the company to initiate the phase 3 trial.
PRN1008 is an inhibitor of BTK, an enzyme that “is present in the signaling pathways of most types of white blood cells except for T cells and plasma cells,” according to the company’s press release.
hTERT expression predicts RCC survival, tumor aggressiveness
Human telomerase reverse transcriptase (hTERT) protein expression is associated with clear cell renal carcinoma (ccRCC) tumor aggressiveness and disease-specific survival (DSS), according to investigators.
Associations between hTERT expression and clinicopathologic features and outcomes were less robust or nonexistent in papillary and chromophobe subtypes, reported Leili Saeednejad Zanjani, MD, of the Oncopathology Research Center at Iran University of Medical Sciences in Tehran, and colleagues.
“Evidence shows that telomerase is expressed in 85% of malignancies, and the level of its activity is higher in advanced and metastatic tumors,” the authors wrote in Pathology.
“A number of clinical studies have been performed to evaluate the association between telomerase activity and clinicopathological parameters in renal cancer showing that telomerase activity level correlates with progression of RCC,” Dr. Zanjani and associates wrote. As none of these specifically evaluated hTERT protein expression, the investigators conducted a study to learn more.
The investigators analyzed hTERT expression level in 176 cases of RCC, requiring that each tumor had three core biopsies because of concerns of heterogeneity. The population consisted of 113 clear cell, 12 type I papillary, 20 type II papillary, and 31 chromophobe subtypes. Patient and clinicopathologic features were compared with survival and hTERT expression. Median follow-up time was 42 months.
Correlations between hTERT expression and disease characteristics were pronounced in cases of ccRCC, compared with other subtypes. In ccRCC, hTERT expression was significantly associated with tumor stage, nucleolar grade, tumor size, microvascular invasion, lymph node invasion, renal pelvis involvement, renal sinus fat involvement, Gerota fascia invasion, and distant metastasis. Survival analysis showed that DSS of ccRCC patients with high hTERT expression was 58 months, compared with 68 months for those with low hTERT expression (P =.012). Other parameters associated with survival were nucleolar grade, tumor stage, and tumor size.
For type I and II papillary subtypes, associations were found between hTERT expression and tumor stage and distant metastasis. In contrast, chromophobe RCC revealed no such relationships. No associations were found between hTERT expression and survival in any of these three latter subtypes, for slightly different reasons; no patients with type I disease died of renal cancer, disallowing creation of a Kaplan-Meier survival curve, whereas type II and chromophobe survival curves revealed insignificant relationships with hTERT expression. Along the same lines, no clinicopathologic characteristics of these subtypes were tied with survival.
“From these findings we are able to conclude that hTERT protein expression may be a novel prognostic indicator of worse outcome in tumor biopsies of patients with ccRCC, if follow up time is more prolonged,” the authors wrote. They noted that “telomerase is an attractive and ideal target for therapy due to overexpression in the majority of malignancies and low or nonexpression in most somatic cells.”
The study was funded by the Iran National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Zanjani LS et al. Pathology. 2018 Nov 19. doi: 10.1016/j.pathol.2018.08.019.
Human telomerase reverse transcriptase (hTERT) protein expression is associated with clear cell renal carcinoma (ccRCC) tumor aggressiveness and disease-specific survival (DSS), according to investigators.
Associations between hTERT expression and clinicopathologic features and outcomes were less robust or nonexistent in papillary and chromophobe subtypes, reported Leili Saeednejad Zanjani, MD, of the Oncopathology Research Center at Iran University of Medical Sciences in Tehran, and colleagues.
“Evidence shows that telomerase is expressed in 85% of malignancies, and the level of its activity is higher in advanced and metastatic tumors,” the authors wrote in Pathology.
“A number of clinical studies have been performed to evaluate the association between telomerase activity and clinicopathological parameters in renal cancer showing that telomerase activity level correlates with progression of RCC,” Dr. Zanjani and associates wrote. As none of these specifically evaluated hTERT protein expression, the investigators conducted a study to learn more.
The investigators analyzed hTERT expression level in 176 cases of RCC, requiring that each tumor had three core biopsies because of concerns of heterogeneity. The population consisted of 113 clear cell, 12 type I papillary, 20 type II papillary, and 31 chromophobe subtypes. Patient and clinicopathologic features were compared with survival and hTERT expression. Median follow-up time was 42 months.
Correlations between hTERT expression and disease characteristics were pronounced in cases of ccRCC, compared with other subtypes. In ccRCC, hTERT expression was significantly associated with tumor stage, nucleolar grade, tumor size, microvascular invasion, lymph node invasion, renal pelvis involvement, renal sinus fat involvement, Gerota fascia invasion, and distant metastasis. Survival analysis showed that DSS of ccRCC patients with high hTERT expression was 58 months, compared with 68 months for those with low hTERT expression (P =.012). Other parameters associated with survival were nucleolar grade, tumor stage, and tumor size.
For type I and II papillary subtypes, associations were found between hTERT expression and tumor stage and distant metastasis. In contrast, chromophobe RCC revealed no such relationships. No associations were found between hTERT expression and survival in any of these three latter subtypes, for slightly different reasons; no patients with type I disease died of renal cancer, disallowing creation of a Kaplan-Meier survival curve, whereas type II and chromophobe survival curves revealed insignificant relationships with hTERT expression. Along the same lines, no clinicopathologic characteristics of these subtypes were tied with survival.
“From these findings we are able to conclude that hTERT protein expression may be a novel prognostic indicator of worse outcome in tumor biopsies of patients with ccRCC, if follow up time is more prolonged,” the authors wrote. They noted that “telomerase is an attractive and ideal target for therapy due to overexpression in the majority of malignancies and low or nonexpression in most somatic cells.”
The study was funded by the Iran National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Zanjani LS et al. Pathology. 2018 Nov 19. doi: 10.1016/j.pathol.2018.08.019.
Human telomerase reverse transcriptase (hTERT) protein expression is associated with clear cell renal carcinoma (ccRCC) tumor aggressiveness and disease-specific survival (DSS), according to investigators.
Associations between hTERT expression and clinicopathologic features and outcomes were less robust or nonexistent in papillary and chromophobe subtypes, reported Leili Saeednejad Zanjani, MD, of the Oncopathology Research Center at Iran University of Medical Sciences in Tehran, and colleagues.
“Evidence shows that telomerase is expressed in 85% of malignancies, and the level of its activity is higher in advanced and metastatic tumors,” the authors wrote in Pathology.
“A number of clinical studies have been performed to evaluate the association between telomerase activity and clinicopathological parameters in renal cancer showing that telomerase activity level correlates with progression of RCC,” Dr. Zanjani and associates wrote. As none of these specifically evaluated hTERT protein expression, the investigators conducted a study to learn more.
The investigators analyzed hTERT expression level in 176 cases of RCC, requiring that each tumor had three core biopsies because of concerns of heterogeneity. The population consisted of 113 clear cell, 12 type I papillary, 20 type II papillary, and 31 chromophobe subtypes. Patient and clinicopathologic features were compared with survival and hTERT expression. Median follow-up time was 42 months.
Correlations between hTERT expression and disease characteristics were pronounced in cases of ccRCC, compared with other subtypes. In ccRCC, hTERT expression was significantly associated with tumor stage, nucleolar grade, tumor size, microvascular invasion, lymph node invasion, renal pelvis involvement, renal sinus fat involvement, Gerota fascia invasion, and distant metastasis. Survival analysis showed that DSS of ccRCC patients with high hTERT expression was 58 months, compared with 68 months for those with low hTERT expression (P =.012). Other parameters associated with survival were nucleolar grade, tumor stage, and tumor size.
For type I and II papillary subtypes, associations were found between hTERT expression and tumor stage and distant metastasis. In contrast, chromophobe RCC revealed no such relationships. No associations were found between hTERT expression and survival in any of these three latter subtypes, for slightly different reasons; no patients with type I disease died of renal cancer, disallowing creation of a Kaplan-Meier survival curve, whereas type II and chromophobe survival curves revealed insignificant relationships with hTERT expression. Along the same lines, no clinicopathologic characteristics of these subtypes were tied with survival.
“From these findings we are able to conclude that hTERT protein expression may be a novel prognostic indicator of worse outcome in tumor biopsies of patients with ccRCC, if follow up time is more prolonged,” the authors wrote. They noted that “telomerase is an attractive and ideal target for therapy due to overexpression in the majority of malignancies and low or nonexpression in most somatic cells.”
The study was funded by the Iran National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Zanjani LS et al. Pathology. 2018 Nov 19. doi: 10.1016/j.pathol.2018.08.019.
FROM PATHOLOGY
Key clinical point: Human telomerase reverse transcriptase (hTERT) protein expression is associated with clear cell renal carcinoma (ccRCC) tumor aggressiveness and disease-specific survival (DSS).
Major finding: DSS of ccRCC patients with high hTERT expression was 58 months, compared with 68 months for those with low hTERT expression (P equal to .012).
Study details: An analysis of hTERT protein expression and disease characteristics in 176 patients with RCC. The subtype population consisted of 113 clear cell, 12 type I papillary, 20 type II papillary, and 31 chromophobe cases.
Disclosures: The study was funded by the Iran National Science Foundation. The authors declared no conflicts of interest.
Source: Zanjani LS et al. Pathology. 2018 Nov 19. doi: 10.1016/j.pathol.2018.08.019.
Addressing Mental Health Needs of Patients with Epilepsy
A community-based program that helps patients with epilepsy self-manage their condition and related psychiatric problems has proven effective in reducing the severity of depression according to a study published in Epilepsy and Behavior.
- Community Targeted Self-Management for Epilepsy and Mental Illness (C-TIME), a behavioral program, consisted of ten 60 to 90-minute sessions conducted over 12 weeks.
- The program included outreach and engagement efforts to help patients suffering from both epilepsy and mental health conditions.
- Thirty patients were enrolled in the program; four months after participating in C-TIME, 66% of the enrolled patients were available for outcome evaluation.
- Researchers reported significant reduction in depression severity, and more than 90% of the group said they were satisfied with results.
Sajatovic M, Needham K, Colón-Zimmermann K, et al. The Community-targeted Self-management of Epilepsy and Mental Illness (C-TIME) initiative: A research, community, and healthcare administration partnership to reduce epilepsy burden [published online ahead of print October 29, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.10.004
A community-based program that helps patients with epilepsy self-manage their condition and related psychiatric problems has proven effective in reducing the severity of depression according to a study published in Epilepsy and Behavior.
- Community Targeted Self-Management for Epilepsy and Mental Illness (C-TIME), a behavioral program, consisted of ten 60 to 90-minute sessions conducted over 12 weeks.
- The program included outreach and engagement efforts to help patients suffering from both epilepsy and mental health conditions.
- Thirty patients were enrolled in the program; four months after participating in C-TIME, 66% of the enrolled patients were available for outcome evaluation.
- Researchers reported significant reduction in depression severity, and more than 90% of the group said they were satisfied with results.
Sajatovic M, Needham K, Colón-Zimmermann K, et al. The Community-targeted Self-management of Epilepsy and Mental Illness (C-TIME) initiative: A research, community, and healthcare administration partnership to reduce epilepsy burden [published online ahead of print October 29, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.10.004
A community-based program that helps patients with epilepsy self-manage their condition and related psychiatric problems has proven effective in reducing the severity of depression according to a study published in Epilepsy and Behavior.
- Community Targeted Self-Management for Epilepsy and Mental Illness (C-TIME), a behavioral program, consisted of ten 60 to 90-minute sessions conducted over 12 weeks.
- The program included outreach and engagement efforts to help patients suffering from both epilepsy and mental health conditions.
- Thirty patients were enrolled in the program; four months after participating in C-TIME, 66% of the enrolled patients were available for outcome evaluation.
- Researchers reported significant reduction in depression severity, and more than 90% of the group said they were satisfied with results.
Sajatovic M, Needham K, Colón-Zimmermann K, et al. The Community-targeted Self-management of Epilepsy and Mental Illness (C-TIME) initiative: A research, community, and healthcare administration partnership to reduce epilepsy burden [published online ahead of print October 29, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.10.004
Epilepsy Education That Reaches Underserved Communities
The Epilepsy Foundation, working with a pharmaceutical company, has launched an educational initiative to locate communities most in need of professional and consumer education. The program emphasizes the value of tailored and innovative approaches to reach underserved populations to improve their self-management skills for epilepsy.
- A data analysis conducted by The Connectors Project found 4 states in need of help: Michigan, Oklahoma, Nevada, and West Virginia, all of which have rural and/or underserved communities.
- The Foundation launched outreach and awareness programs in these states, as well as digital and in-person education for clinicians, patients, and families.
- The initiatives were designed to fill critical gaps in patients’ ability to self-manage epilepsy and gaps in their ability to get access to quality professional care.
Owens S, Sirven JI, Shafer PO, et al. Innovative approaches reaching underserved and rural communities to improve epilepsy care: A review of the methodology of the Connectors Project [published online ahead of print October 31, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.09.029
The Epilepsy Foundation, working with a pharmaceutical company, has launched an educational initiative to locate communities most in need of professional and consumer education. The program emphasizes the value of tailored and innovative approaches to reach underserved populations to improve their self-management skills for epilepsy.
- A data analysis conducted by The Connectors Project found 4 states in need of help: Michigan, Oklahoma, Nevada, and West Virginia, all of which have rural and/or underserved communities.
- The Foundation launched outreach and awareness programs in these states, as well as digital and in-person education for clinicians, patients, and families.
- The initiatives were designed to fill critical gaps in patients’ ability to self-manage epilepsy and gaps in their ability to get access to quality professional care.
Owens S, Sirven JI, Shafer PO, et al. Innovative approaches reaching underserved and rural communities to improve epilepsy care: A review of the methodology of the Connectors Project [published online ahead of print October 31, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.09.029
The Epilepsy Foundation, working with a pharmaceutical company, has launched an educational initiative to locate communities most in need of professional and consumer education. The program emphasizes the value of tailored and innovative approaches to reach underserved populations to improve their self-management skills for epilepsy.
- A data analysis conducted by The Connectors Project found 4 states in need of help: Michigan, Oklahoma, Nevada, and West Virginia, all of which have rural and/or underserved communities.
- The Foundation launched outreach and awareness programs in these states, as well as digital and in-person education for clinicians, patients, and families.
- The initiatives were designed to fill critical gaps in patients’ ability to self-manage epilepsy and gaps in their ability to get access to quality professional care.
Owens S, Sirven JI, Shafer PO, et al. Innovative approaches reaching underserved and rural communities to improve epilepsy care: A review of the methodology of the Connectors Project [published online ahead of print October 31, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.09.029
Should Neurologists Treat Psychiatric Problems in Patients with Epilepsy?
Giving neurologists the tools to diagnose and treat common psychiatric disorders can help meet the mental health needs of adults with epilepsy whose psychiatric comorbidities are being ignored, according to investigators from Wake Forest School of Medicine and Johns Hopkins University.
- There is a high prevalence of psychiatric disorders among adults with epilepsy.
- These comorbidities are often overlooked because patients have limited access to mental health services.
- Heidi Munger Clary and Jay Salpekar suggest that letting neurologists diagnose and manage common conditions like mood and anxiety disorders will help address this dilemma.
- The researchers suggest that validated screeners could help neurologists who do not have expertise in psychiatry to manage depression and anxiety.
- With such assistance, adult neurologists could be trained to effectively use selective serotonin reupdate inhibitors (SSRIs).
Munger Clary HM, Salpekar JA. Should adult neurologists play a role in the management of the most common psychiatric comorbidities? Practical considerations [published online ahead of print November 22, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.10.020
Giving neurologists the tools to diagnose and treat common psychiatric disorders can help meet the mental health needs of adults with epilepsy whose psychiatric comorbidities are being ignored, according to investigators from Wake Forest School of Medicine and Johns Hopkins University.
- There is a high prevalence of psychiatric disorders among adults with epilepsy.
- These comorbidities are often overlooked because patients have limited access to mental health services.
- Heidi Munger Clary and Jay Salpekar suggest that letting neurologists diagnose and manage common conditions like mood and anxiety disorders will help address this dilemma.
- The researchers suggest that validated screeners could help neurologists who do not have expertise in psychiatry to manage depression and anxiety.
- With such assistance, adult neurologists could be trained to effectively use selective serotonin reupdate inhibitors (SSRIs).
Munger Clary HM, Salpekar JA. Should adult neurologists play a role in the management of the most common psychiatric comorbidities? Practical considerations [published online ahead of print November 22, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.10.020
Giving neurologists the tools to diagnose and treat common psychiatric disorders can help meet the mental health needs of adults with epilepsy whose psychiatric comorbidities are being ignored, according to investigators from Wake Forest School of Medicine and Johns Hopkins University.
- There is a high prevalence of psychiatric disorders among adults with epilepsy.
- These comorbidities are often overlooked because patients have limited access to mental health services.
- Heidi Munger Clary and Jay Salpekar suggest that letting neurologists diagnose and manage common conditions like mood and anxiety disorders will help address this dilemma.
- The researchers suggest that validated screeners could help neurologists who do not have expertise in psychiatry to manage depression and anxiety.
- With such assistance, adult neurologists could be trained to effectively use selective serotonin reupdate inhibitors (SSRIs).
Munger Clary HM, Salpekar JA. Should adult neurologists play a role in the management of the most common psychiatric comorbidities? Practical considerations [published online ahead of print November 22, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.10.020
Vitamin D–binding protein polymorphisms affect HCV susceptibility
Two specific polymorphisms within the vitamin D–binding protein (VDBP) gene may contribute to susceptibility to hepatitis C virus (HCV) infection in a high-risk Chinese Han population, according to the results of a case-control study published in Gene.
Previous research has indicated that vitamin D deficiency may have an impact on the antiviral response in chronic HCV, and VDBP has been shown to transport vitamin D and its metabolites, thereby influencing vitamin D status. This made VDBP a valid candidate for study as to its effects on HCV infection.
The current study initially recruited around 2,500 Chinese subjects over the period October 2008 to January 2016. The majority were women, and the average age of the subjects was 49-50 years.
The researchers genotyped seven genetic variants in the VDBP gene in 886 patients with HCV persistent infection, 539 subjects with spontaneous clearance, and 1,081 uninfected controls, according to Chao-Nan Xie of the department of epidemiology and biostatistics, Nanjing (China) Medical University, and colleagues.
The researchers found that two variants (rs7041-G and rs3733359-T alleles) were significantly associated with an increased susceptibility of HCV infection. In addition, the combined effect of having the two unfavorable alleles was related to an elevated risk of HCV infection in a locus-dosage manner (P = .000816).
Haplotype analysis suggested that the GT haplotype showed an increased risk effect of HCV infection (odds ratio, 1.464), compared with the most frequent TC haplotype.
“Taken together, polymorphisms within the VDBP gene (rs4588 and rs3733359) may contribute to susceptibility to HCV infection in a high-risk Chinese Han population, which implicates a role of VDR genetic polymorphisms and vitamin D levels in the immune regulation and course of HCV infection,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Xie C-N et al. Gene 2018;679:405-11.
Two specific polymorphisms within the vitamin D–binding protein (VDBP) gene may contribute to susceptibility to hepatitis C virus (HCV) infection in a high-risk Chinese Han population, according to the results of a case-control study published in Gene.
Previous research has indicated that vitamin D deficiency may have an impact on the antiviral response in chronic HCV, and VDBP has been shown to transport vitamin D and its metabolites, thereby influencing vitamin D status. This made VDBP a valid candidate for study as to its effects on HCV infection.
The current study initially recruited around 2,500 Chinese subjects over the period October 2008 to January 2016. The majority were women, and the average age of the subjects was 49-50 years.
The researchers genotyped seven genetic variants in the VDBP gene in 886 patients with HCV persistent infection, 539 subjects with spontaneous clearance, and 1,081 uninfected controls, according to Chao-Nan Xie of the department of epidemiology and biostatistics, Nanjing (China) Medical University, and colleagues.
The researchers found that two variants (rs7041-G and rs3733359-T alleles) were significantly associated with an increased susceptibility of HCV infection. In addition, the combined effect of having the two unfavorable alleles was related to an elevated risk of HCV infection in a locus-dosage manner (P = .000816).
Haplotype analysis suggested that the GT haplotype showed an increased risk effect of HCV infection (odds ratio, 1.464), compared with the most frequent TC haplotype.
“Taken together, polymorphisms within the VDBP gene (rs4588 and rs3733359) may contribute to susceptibility to HCV infection in a high-risk Chinese Han population, which implicates a role of VDR genetic polymorphisms and vitamin D levels in the immune regulation and course of HCV infection,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Xie C-N et al. Gene 2018;679:405-11.
Two specific polymorphisms within the vitamin D–binding protein (VDBP) gene may contribute to susceptibility to hepatitis C virus (HCV) infection in a high-risk Chinese Han population, according to the results of a case-control study published in Gene.
Previous research has indicated that vitamin D deficiency may have an impact on the antiviral response in chronic HCV, and VDBP has been shown to transport vitamin D and its metabolites, thereby influencing vitamin D status. This made VDBP a valid candidate for study as to its effects on HCV infection.
The current study initially recruited around 2,500 Chinese subjects over the period October 2008 to January 2016. The majority were women, and the average age of the subjects was 49-50 years.
The researchers genotyped seven genetic variants in the VDBP gene in 886 patients with HCV persistent infection, 539 subjects with spontaneous clearance, and 1,081 uninfected controls, according to Chao-Nan Xie of the department of epidemiology and biostatistics, Nanjing (China) Medical University, and colleagues.
The researchers found that two variants (rs7041-G and rs3733359-T alleles) were significantly associated with an increased susceptibility of HCV infection. In addition, the combined effect of having the two unfavorable alleles was related to an elevated risk of HCV infection in a locus-dosage manner (P = .000816).
Haplotype analysis suggested that the GT haplotype showed an increased risk effect of HCV infection (odds ratio, 1.464), compared with the most frequent TC haplotype.
“Taken together, polymorphisms within the VDBP gene (rs4588 and rs3733359) may contribute to susceptibility to HCV infection in a high-risk Chinese Han population, which implicates a role of VDR genetic polymorphisms and vitamin D levels in the immune regulation and course of HCV infection,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Xie C-N et al. Gene 2018;679:405-11.
FROM GENE
Key clinical point: VDBP alleles influence susceptibility to HCV infection in a Chinese population.
Major finding: The GT haplotype showed an increased risk effect of HCV infection (odds ratio 1.464) compared to the most frequent TC haplotype.
Study details: Case-control study of 886 HIV-infected, 539 spontaneously cleared, and 1,081 control patients.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Xie C-N et al. Gene. 2018;679:405-11.