User login
Patient treatment expectations can outweigh equivocal effectiveness data
I’m getting old and starting to fall apart. Recently, I suffered a lumbar radiculopathy when I injured myself sneezing. (No, really, I did.)
So, as time went by and I didn’t get better, I began looking stuff up. When patients come to me for this, I go through the standard conservative regimen of NSAIDs, physical therapy, steroid tapers ... the standard stuff.
But, when I began looking these things up, I was surprised to find out how much of what we do (at least for lumbar radiculopathy) is taken on faith.
I went through UpToDate, the modern Bible of medicine.
NSAIDs and acetaminophen, to my surprise, have only marginal proof of efficacy for acute lumbosacral radiculopathy pain. Several pooled analyses showed a nonsignificant trend to support their use, and the quality of the data was considered to be low.
Likewise, physical therapy also had “no convincing evidence that such treatments are effective for this indication.” Admittedly, some of the data may be affected by the difficulty in doing sham therapy as part of a placebo controlled-trial.
An oral steroid taper? Again, similar, equivocal data. Marginal improvement in functional capabilities, no improvement in pain, and no improvement in the rate of surgery at 1 year out.
But these are the things that I, and likely most family doctors, physiatrists, and other neurologists recommend on a daily basis. And, in all likelihood, will continue to do so.
Why?
Overall, they are benign when used correctly and in the right patients. That isn’t to say everyone should get them. All drugs have issues, and patients have to be carefully matched to specific treatments.
But, in the grand scheme of “do no harm,” physical therapy, NSAIDs, acetaminophen, or a few days of steroids are reasonably harmless. There certainly are some patients who will benefit, and none of these approaches have clearly been shown to be dangerous.
There’s also patient expectations. They didn’t come to us, or shell out a copay, to be told that “nothing helps, give it time.” We’re the doctors, and they want us to DO SOMETHING. So even if these treatments may be placebos, they still help if for no other reason than (as Voltaire said) to amuse the patient while nature cures the disease.
And getting them better is, after all, a big part of our job.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m getting old and starting to fall apart. Recently, I suffered a lumbar radiculopathy when I injured myself sneezing. (No, really, I did.)
So, as time went by and I didn’t get better, I began looking stuff up. When patients come to me for this, I go through the standard conservative regimen of NSAIDs, physical therapy, steroid tapers ... the standard stuff.
But, when I began looking these things up, I was surprised to find out how much of what we do (at least for lumbar radiculopathy) is taken on faith.
I went through UpToDate, the modern Bible of medicine.
NSAIDs and acetaminophen, to my surprise, have only marginal proof of efficacy for acute lumbosacral radiculopathy pain. Several pooled analyses showed a nonsignificant trend to support their use, and the quality of the data was considered to be low.
Likewise, physical therapy also had “no convincing evidence that such treatments are effective for this indication.” Admittedly, some of the data may be affected by the difficulty in doing sham therapy as part of a placebo controlled-trial.
An oral steroid taper? Again, similar, equivocal data. Marginal improvement in functional capabilities, no improvement in pain, and no improvement in the rate of surgery at 1 year out.
But these are the things that I, and likely most family doctors, physiatrists, and other neurologists recommend on a daily basis. And, in all likelihood, will continue to do so.
Why?
Overall, they are benign when used correctly and in the right patients. That isn’t to say everyone should get them. All drugs have issues, and patients have to be carefully matched to specific treatments.
But, in the grand scheme of “do no harm,” physical therapy, NSAIDs, acetaminophen, or a few days of steroids are reasonably harmless. There certainly are some patients who will benefit, and none of these approaches have clearly been shown to be dangerous.
There’s also patient expectations. They didn’t come to us, or shell out a copay, to be told that “nothing helps, give it time.” We’re the doctors, and they want us to DO SOMETHING. So even if these treatments may be placebos, they still help if for no other reason than (as Voltaire said) to amuse the patient while nature cures the disease.
And getting them better is, after all, a big part of our job.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m getting old and starting to fall apart. Recently, I suffered a lumbar radiculopathy when I injured myself sneezing. (No, really, I did.)
So, as time went by and I didn’t get better, I began looking stuff up. When patients come to me for this, I go through the standard conservative regimen of NSAIDs, physical therapy, steroid tapers ... the standard stuff.
But, when I began looking these things up, I was surprised to find out how much of what we do (at least for lumbar radiculopathy) is taken on faith.
I went through UpToDate, the modern Bible of medicine.
NSAIDs and acetaminophen, to my surprise, have only marginal proof of efficacy for acute lumbosacral radiculopathy pain. Several pooled analyses showed a nonsignificant trend to support their use, and the quality of the data was considered to be low.
Likewise, physical therapy also had “no convincing evidence that such treatments are effective for this indication.” Admittedly, some of the data may be affected by the difficulty in doing sham therapy as part of a placebo controlled-trial.
An oral steroid taper? Again, similar, equivocal data. Marginal improvement in functional capabilities, no improvement in pain, and no improvement in the rate of surgery at 1 year out.
But these are the things that I, and likely most family doctors, physiatrists, and other neurologists recommend on a daily basis. And, in all likelihood, will continue to do so.
Why?
Overall, they are benign when used correctly and in the right patients. That isn’t to say everyone should get them. All drugs have issues, and patients have to be carefully matched to specific treatments.
But, in the grand scheme of “do no harm,” physical therapy, NSAIDs, acetaminophen, or a few days of steroids are reasonably harmless. There certainly are some patients who will benefit, and none of these approaches have clearly been shown to be dangerous.
There’s also patient expectations. They didn’t come to us, or shell out a copay, to be told that “nothing helps, give it time.” We’re the doctors, and they want us to DO SOMETHING. So even if these treatments may be placebos, they still help if for no other reason than (as Voltaire said) to amuse the patient while nature cures the disease.
And getting them better is, after all, a big part of our job.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Dermoscopy in family medicine: A primer
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.
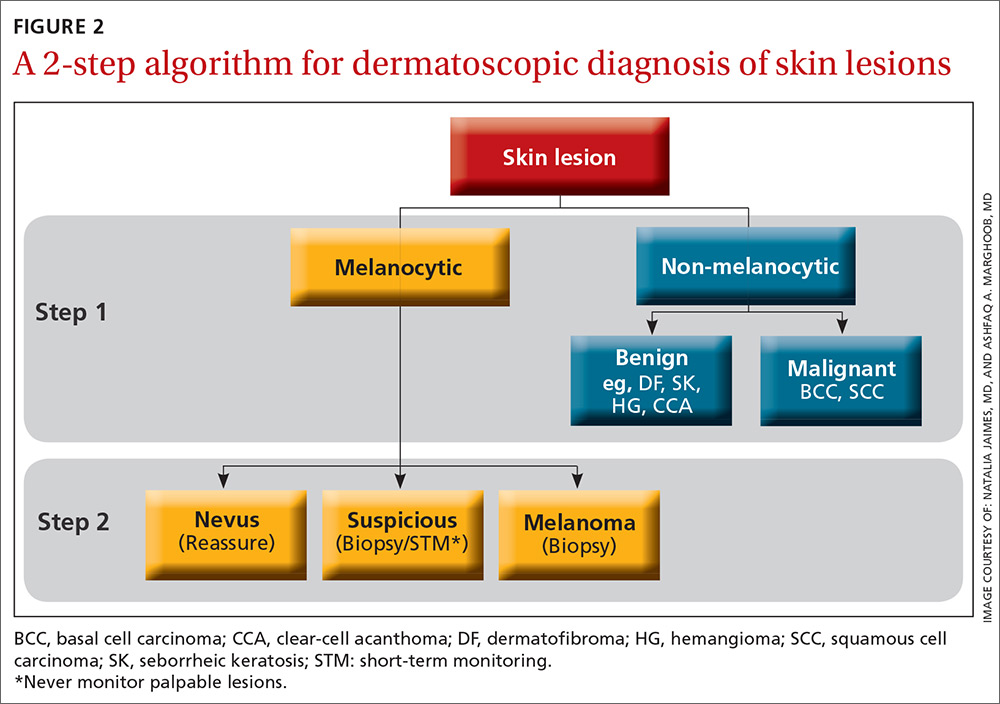
Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
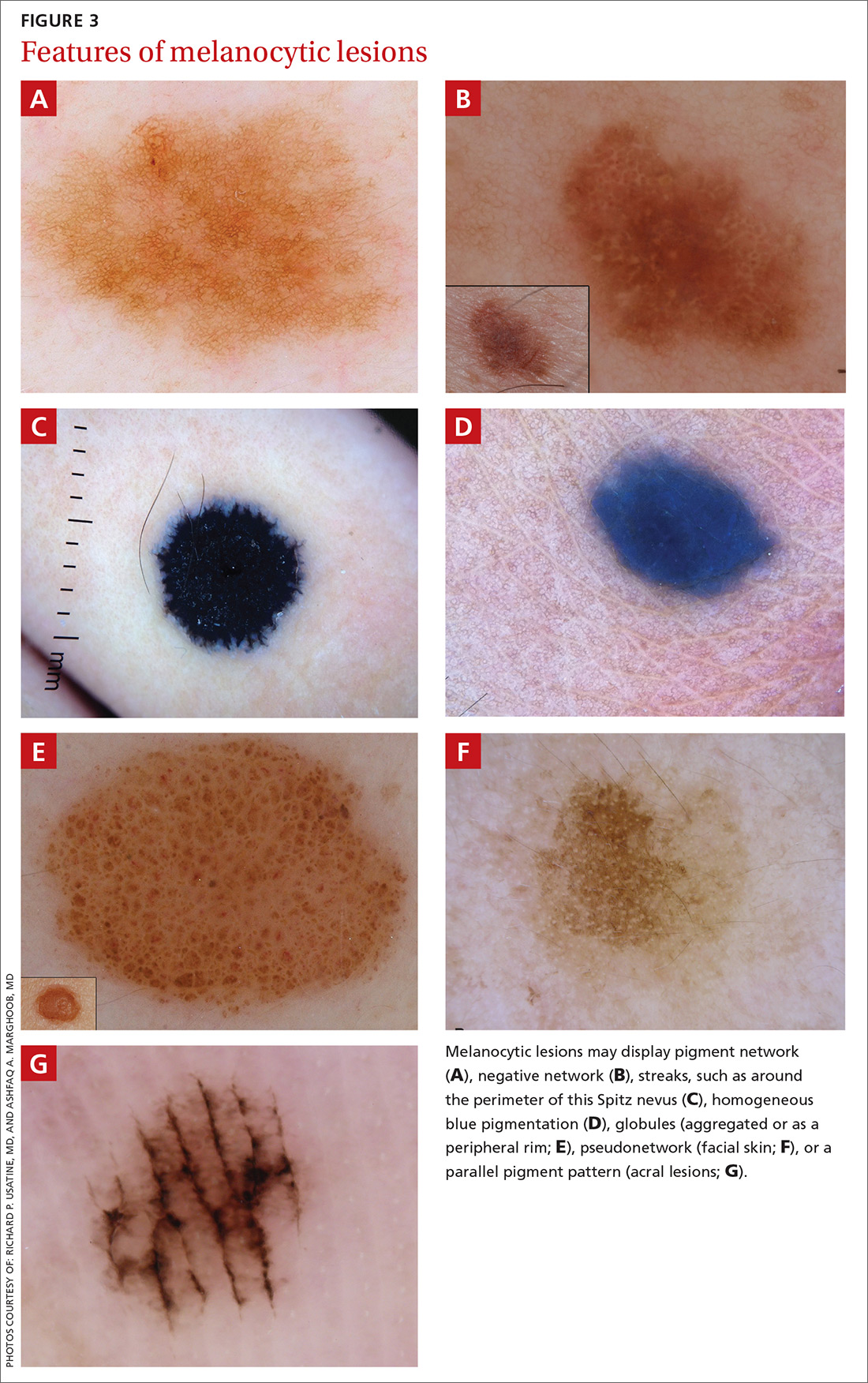
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).
Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
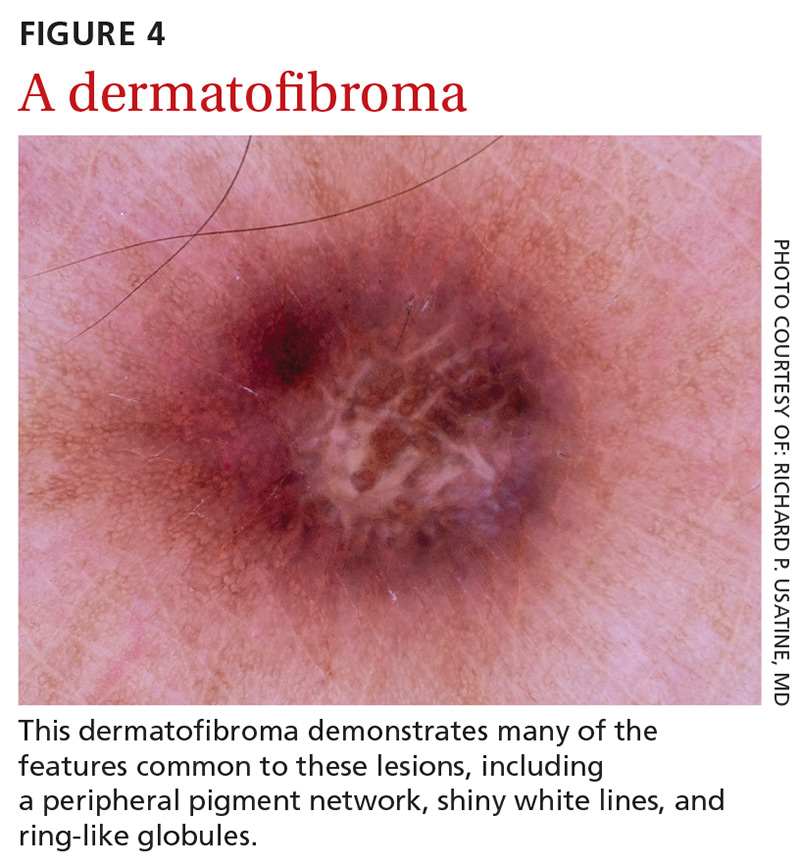
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.
Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
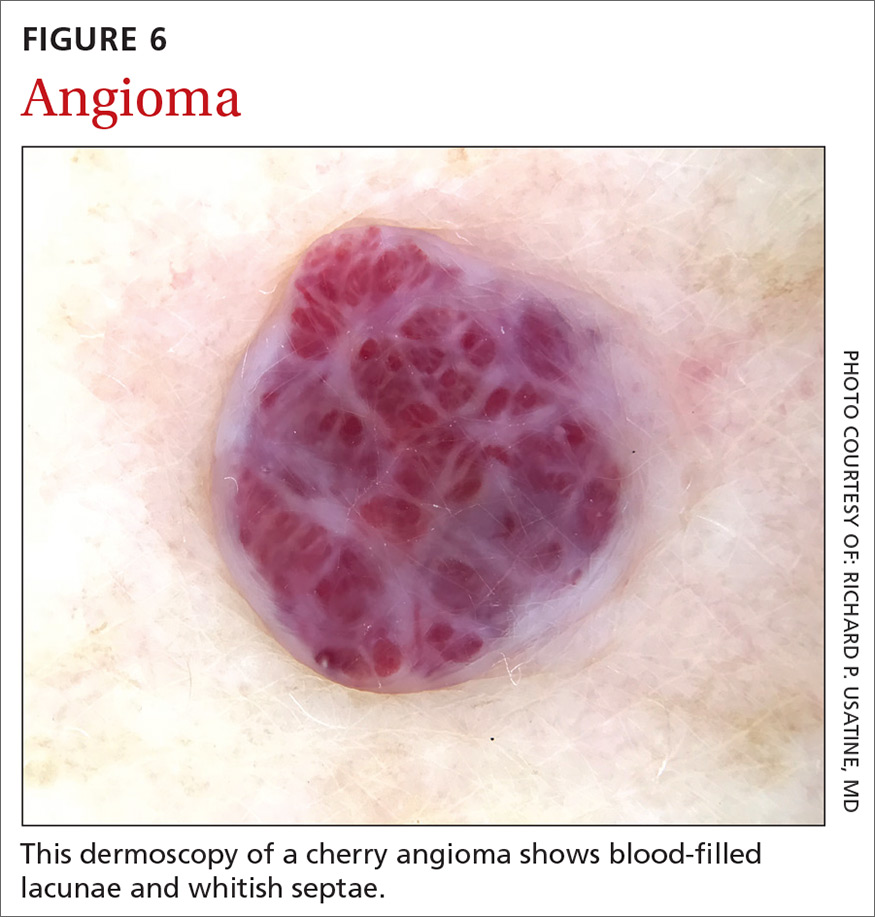
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).
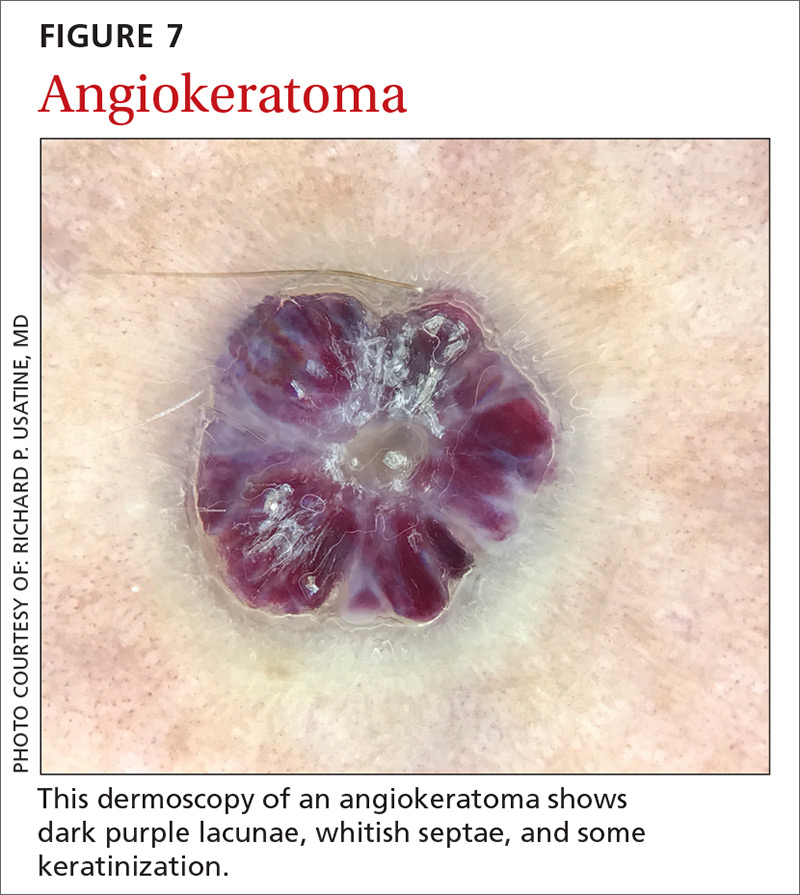
Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.
Continue to: Sebaceous hyperplasia...
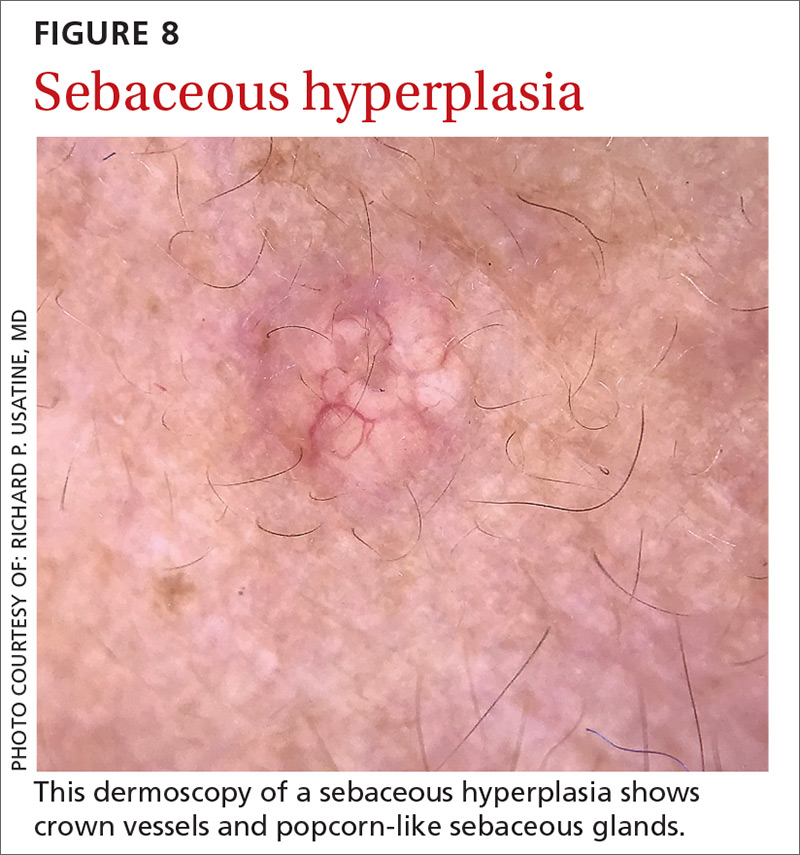
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).
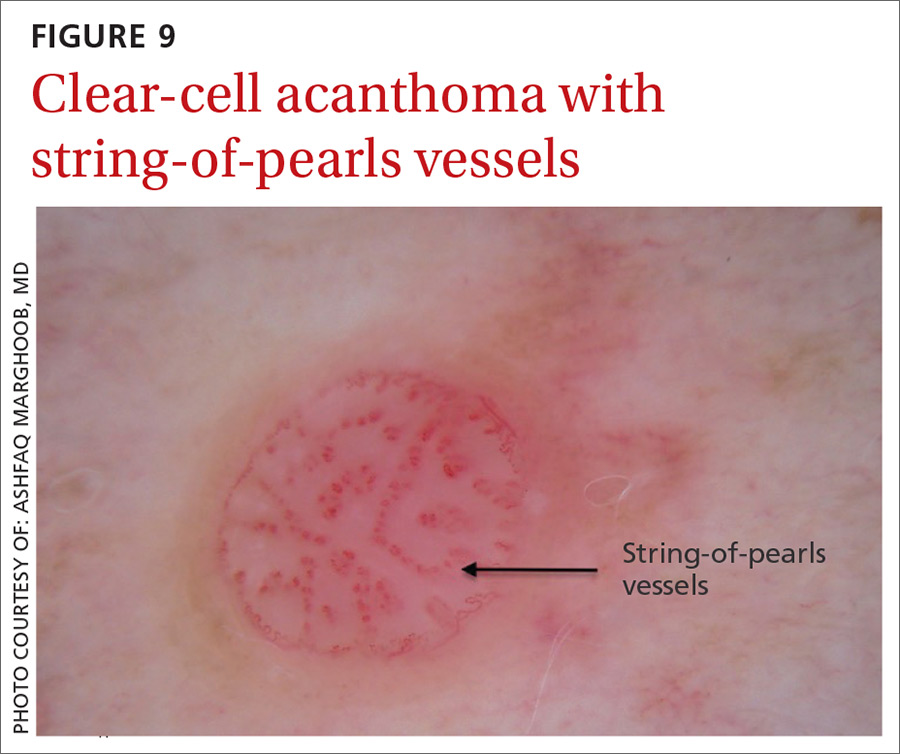
Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).
Malignant nonmelanocytic lesions
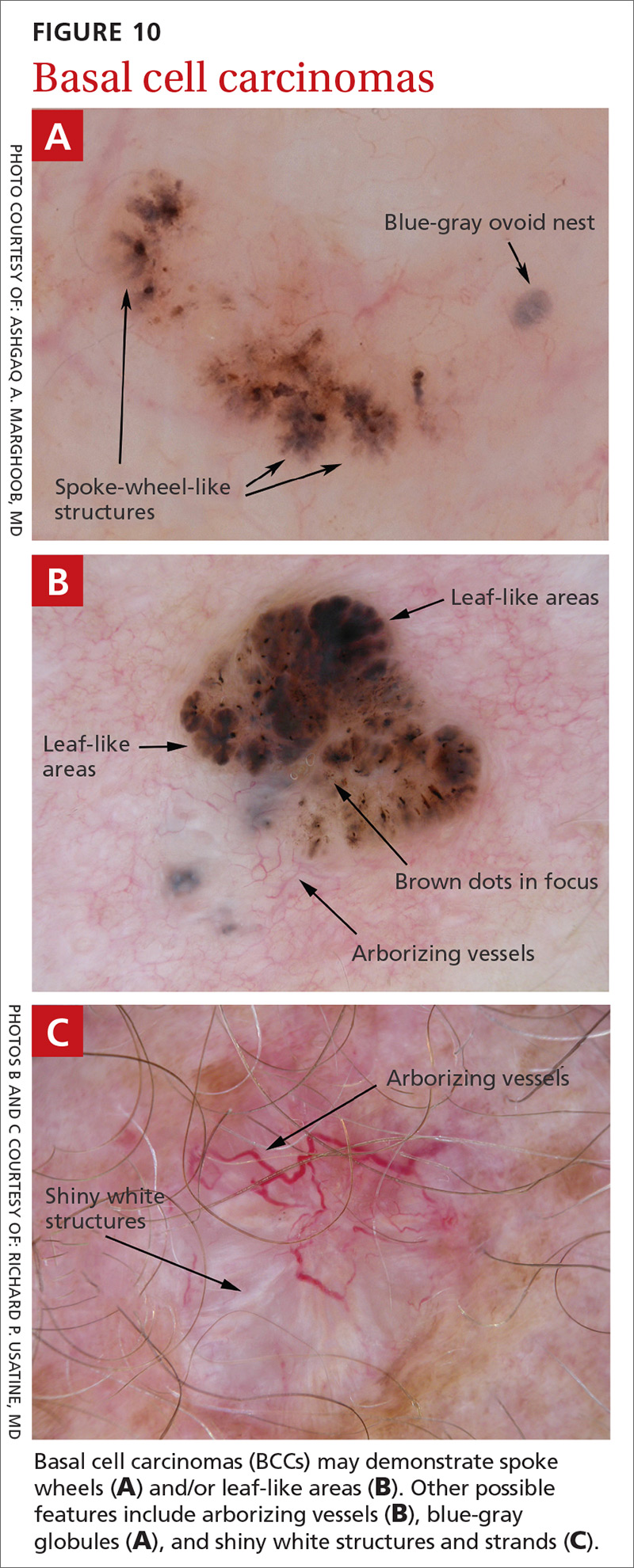
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).
Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
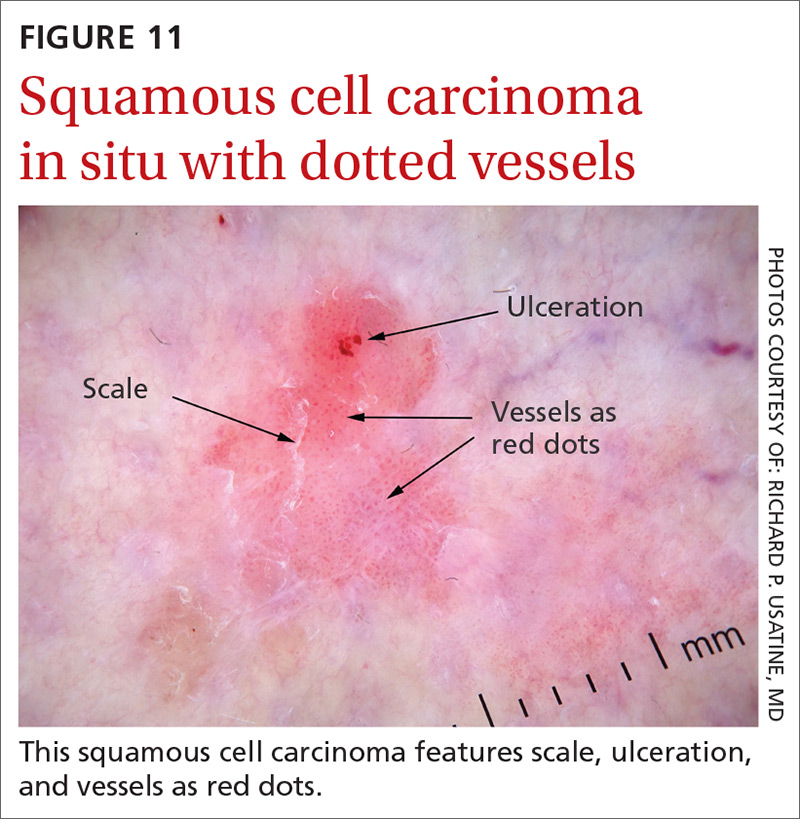
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.
Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17
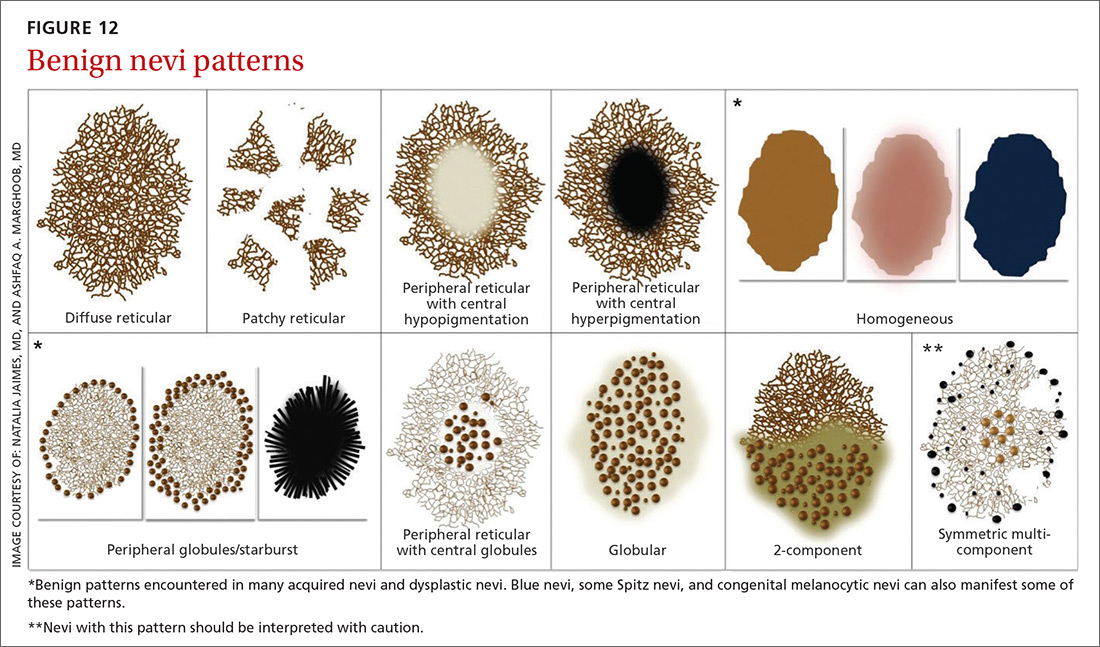
Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).
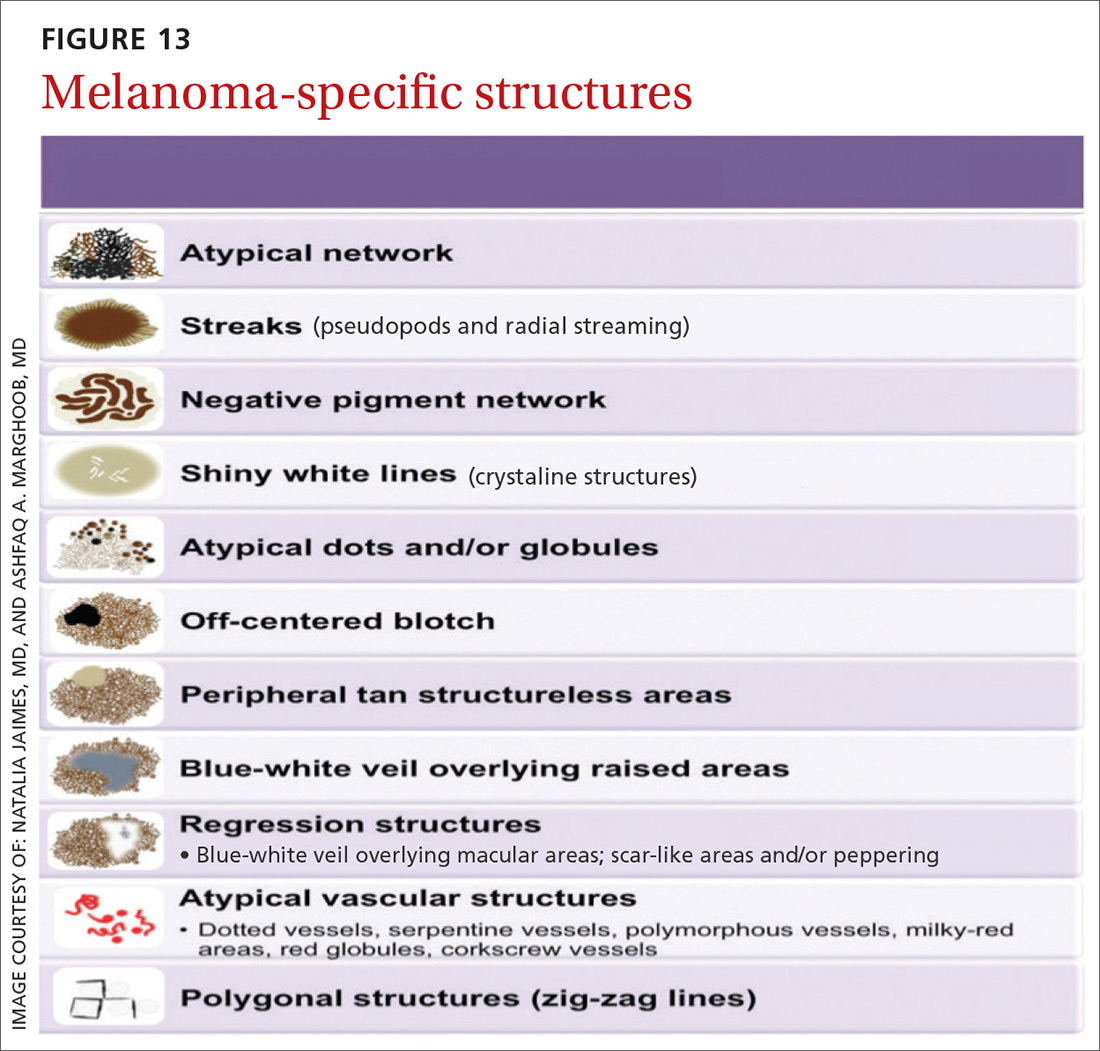
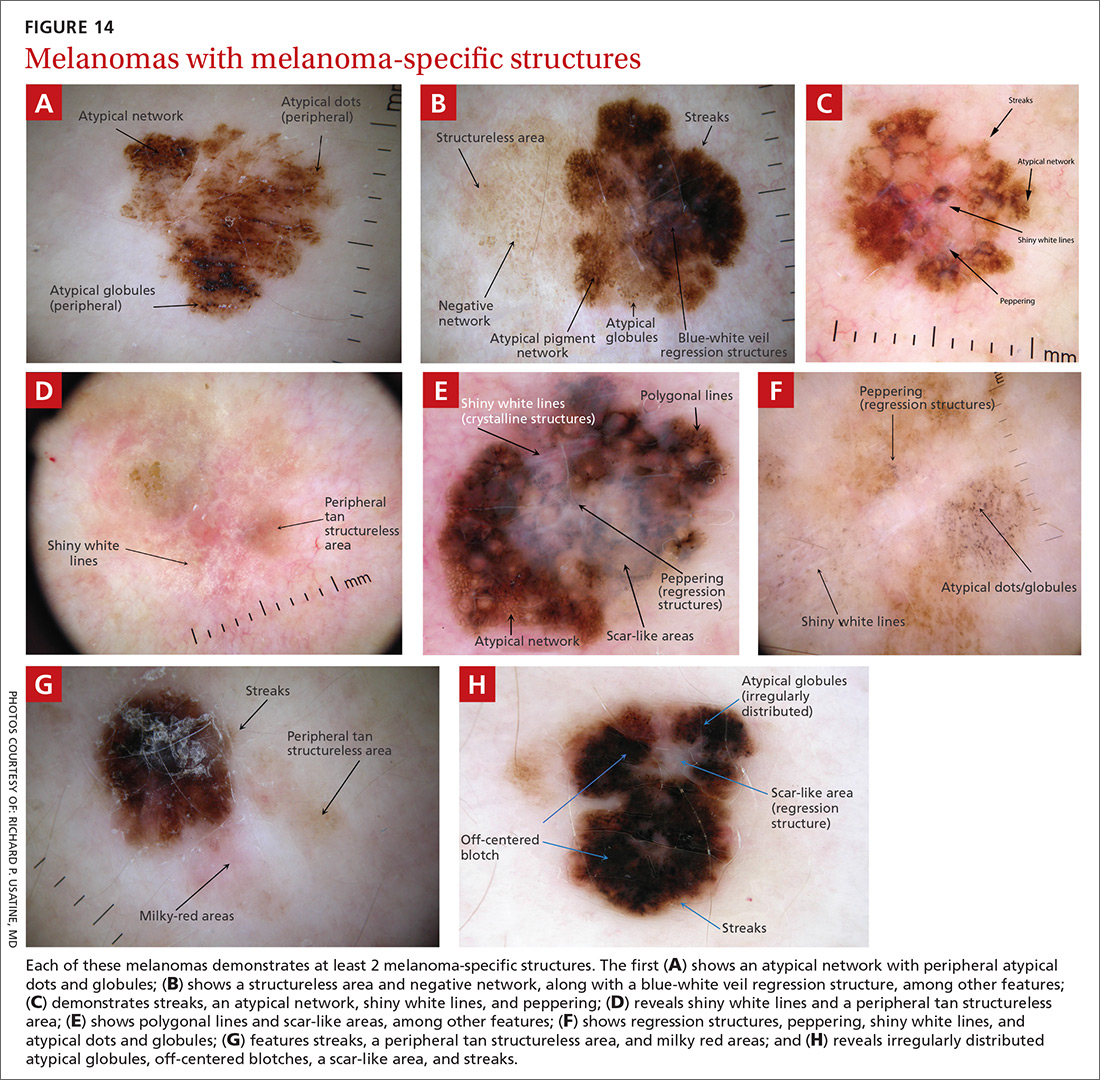
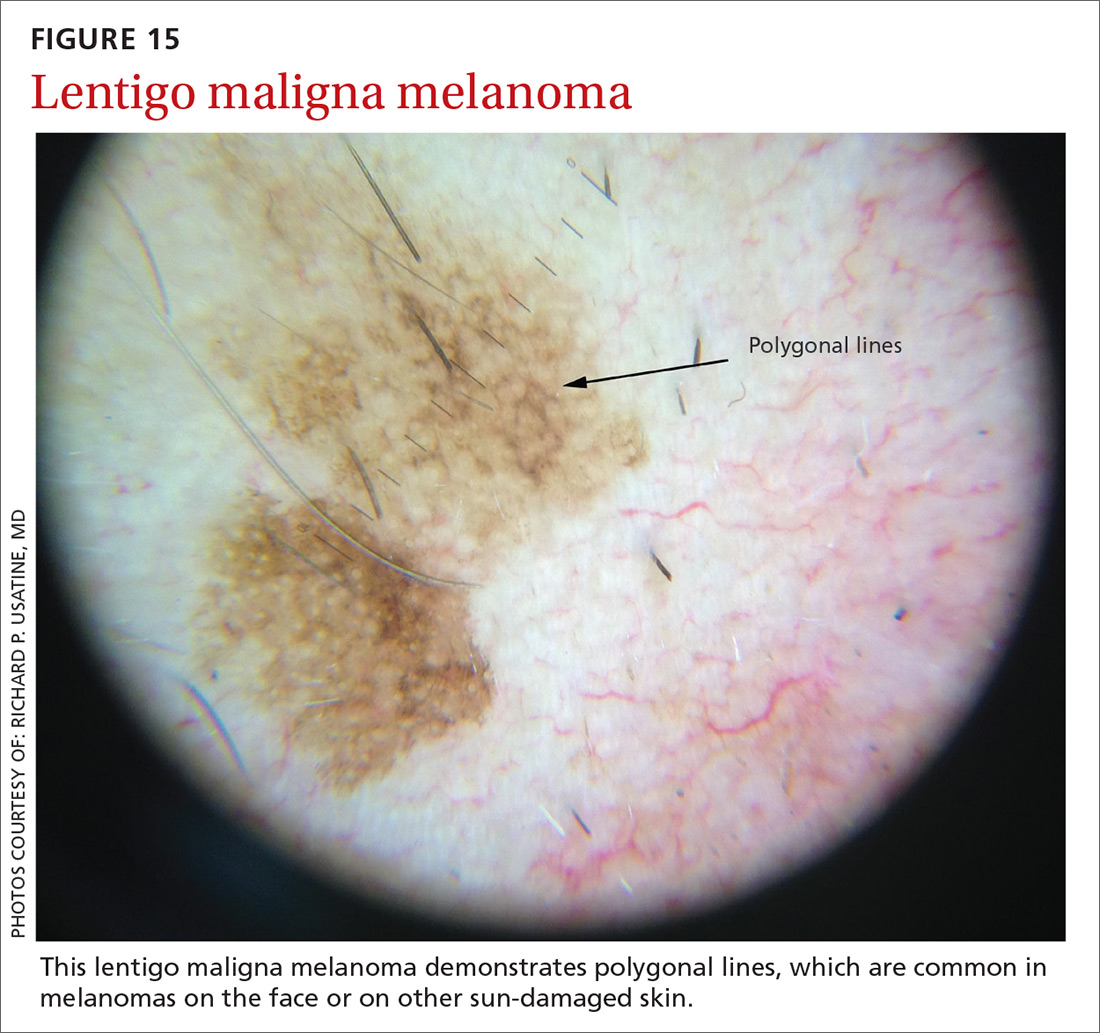
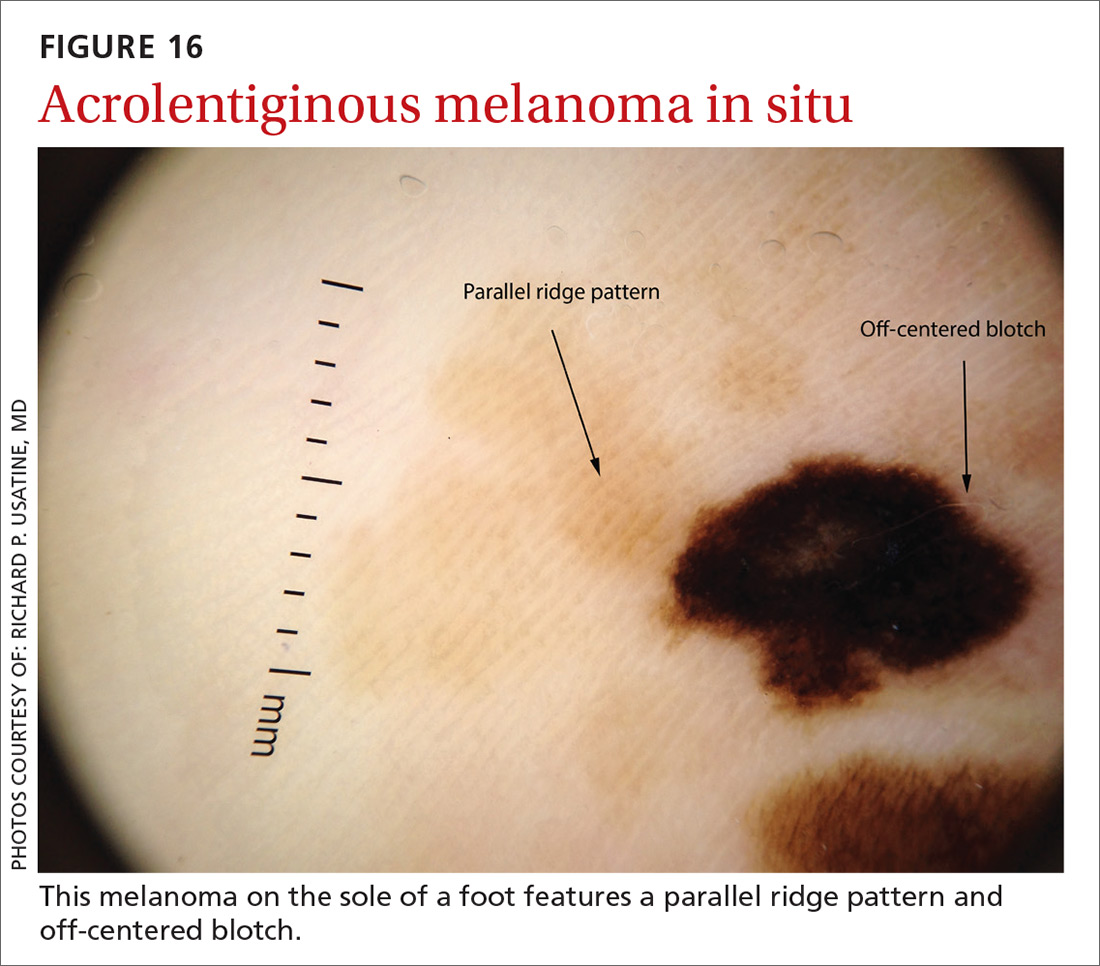
Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).
How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.
This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.
Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.

Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).

Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.

Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).

Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.

Continue to: Sebaceous hyperplasia...
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).

Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).

Malignant nonmelanocytic lesions
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).

Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.

Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17

Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).

Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).

How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.

This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.

Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.

Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).

Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.

Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).

Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.

Continue to: Sebaceous hyperplasia...
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).

Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).

Malignant nonmelanocytic lesions
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).

Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.

Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17

Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).

Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).

How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.

This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.

Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.
Phase 3 study confirms biosimilarity of PF-05280586 with rituximab
SAN DIEGO – The potential rituximab biosimilar drug PF-05280586 showed efficacy, safety, immunogenicity, pharmacokinetics, and pharmacodynamics similar to those of rituximab at up to 26 weeks in a randomized phase 3 study of treatment-naive patients with CD20-positive low tumor burden follicular lymphoma (LTB-FL).
The primary endpoint of overall response rate at 26 weeks was 75.5% in 196 patients randomized to receive PF-05280586, and 70.7% in 198 patients who received a rituximab reference product sourced from the European Union (MabThera; rituximab‑EU), Jeff Sharman, MD, reported at the annual meeting of the American Society of Hematology.
“This resulted in a difference between the two arms of 4.66%,” said Dr. Sharman of Willamette Valley Cancer Institute and Research Center, Springfield, Ore.
The 95% confidence interval for this difference ... was entirely contained within the prespecified equivalence margin, he said.
“Depth of response was a key secondary endpoint, and rates of complete response were 29.3% and 30.4%, respectively,” he said, noting that rates of partial response, stable response, and progressive disease were also similar between the two study arms.
Estimated 1-year progression-free survival (PFS) rates were also highly similar at 76.4% and 81.2% in the PF-05280586 and rituximab-EU arms.
Rapid depletion in CD19-positive B-cell counts was observed in both groups after initial dosing, with recovery by week 39 and a sustained increase until the end of week 52.
Treatment-emergent adverse events (TEAEs) occurred in 78.6% vs. 72.1% of patients in the PF‑05280586 vs. rituximab‑EU arms, respectively, and the rates of serious adverse events and grade 3 events were similar in the groups, as were rates of infusion interruptions or infusion-related reactions (IRRs), Dr. Sharman said.
IRRs occurred in about 25% of patients in each arm, and most were grade 1 or 2. Grade 3 IRRs occurred in 2.6% vs. 0.5% of patients in the groups, respectively, and no grade 4 IRRs occurred.
Rates of anti-drug antibodies were also similar in the two groups, as were serum drug concentrations – regardless of anti-drug antibody status, he noted.
Study subjects were adults with a mean age of 60 years and histologically confirmed CD20-positive grade 1-3a follicular lymphoma with no prior rituximab or system therapy for B-cell non-Hodgkin lymphoma (NHL). They had Ann Arbor disease stages II (26.9%), III (44.2%) or IV (28.9%), ECOG performance status of 0-1, and at least 1 measurable disease lesion identifiable on imaging.
Risk level as assessed by the Follicular Lymphoma International Prognostic Index–2 was low in 28.4%, medium in 66%, and high in 5.6% of patients.
Treatment with each agent was given at intravenous doses of 375 mg/m2 weekly for 4 weeks at days 1, 8, 15, and 22.
PF-05280586 is being developed by Pfizer, and in this 52-week double-blind study – the largest study to date of the early use of the potential rituximab biosimilar in patients with previously untreated CD20-positive LTB-FL – the primary endpoint was met, demonstrating its therapeutic equivalence with rituximab-EU for overall response rate at week 26, Dr. Sharman said.
“These results therefore confirm the biosimilarity of PF-05280586 with rituximab-EU,” he concluded.
Of note, the reporting of these findings comes on the heels of the first Food and Drug Administration approval of a biosimilar rituximab product for the treatment of NHL; Celltrion’s product Truxima (formerly CT-P10), a biosimilar of Genentech’s Rituxan (rituximab), was approved Nov. 28 to treat adults with CD20-positive, B-cell NHL, either as a single agent or in combination with chemotherapy.
The PF-0528056 study was sponsored by Pfizer. Dr. Sharman has been a consultant for, and/or received research funding and honoraria from Acerta, Pharmacyclics (an AbbVie Company), Pfizer, TG Therapeutics, Abbvie, and Genentech.
SOURCE: Sharman J et al. ASH 2018: Abstract 394.
SAN DIEGO – The potential rituximab biosimilar drug PF-05280586 showed efficacy, safety, immunogenicity, pharmacokinetics, and pharmacodynamics similar to those of rituximab at up to 26 weeks in a randomized phase 3 study of treatment-naive patients with CD20-positive low tumor burden follicular lymphoma (LTB-FL).
The primary endpoint of overall response rate at 26 weeks was 75.5% in 196 patients randomized to receive PF-05280586, and 70.7% in 198 patients who received a rituximab reference product sourced from the European Union (MabThera; rituximab‑EU), Jeff Sharman, MD, reported at the annual meeting of the American Society of Hematology.
“This resulted in a difference between the two arms of 4.66%,” said Dr. Sharman of Willamette Valley Cancer Institute and Research Center, Springfield, Ore.
The 95% confidence interval for this difference ... was entirely contained within the prespecified equivalence margin, he said.
“Depth of response was a key secondary endpoint, and rates of complete response were 29.3% and 30.4%, respectively,” he said, noting that rates of partial response, stable response, and progressive disease were also similar between the two study arms.
Estimated 1-year progression-free survival (PFS) rates were also highly similar at 76.4% and 81.2% in the PF-05280586 and rituximab-EU arms.
Rapid depletion in CD19-positive B-cell counts was observed in both groups after initial dosing, with recovery by week 39 and a sustained increase until the end of week 52.
Treatment-emergent adverse events (TEAEs) occurred in 78.6% vs. 72.1% of patients in the PF‑05280586 vs. rituximab‑EU arms, respectively, and the rates of serious adverse events and grade 3 events were similar in the groups, as were rates of infusion interruptions or infusion-related reactions (IRRs), Dr. Sharman said.
IRRs occurred in about 25% of patients in each arm, and most were grade 1 or 2. Grade 3 IRRs occurred in 2.6% vs. 0.5% of patients in the groups, respectively, and no grade 4 IRRs occurred.
Rates of anti-drug antibodies were also similar in the two groups, as were serum drug concentrations – regardless of anti-drug antibody status, he noted.
Study subjects were adults with a mean age of 60 years and histologically confirmed CD20-positive grade 1-3a follicular lymphoma with no prior rituximab or system therapy for B-cell non-Hodgkin lymphoma (NHL). They had Ann Arbor disease stages II (26.9%), III (44.2%) or IV (28.9%), ECOG performance status of 0-1, and at least 1 measurable disease lesion identifiable on imaging.
Risk level as assessed by the Follicular Lymphoma International Prognostic Index–2 was low in 28.4%, medium in 66%, and high in 5.6% of patients.
Treatment with each agent was given at intravenous doses of 375 mg/m2 weekly for 4 weeks at days 1, 8, 15, and 22.
PF-05280586 is being developed by Pfizer, and in this 52-week double-blind study – the largest study to date of the early use of the potential rituximab biosimilar in patients with previously untreated CD20-positive LTB-FL – the primary endpoint was met, demonstrating its therapeutic equivalence with rituximab-EU for overall response rate at week 26, Dr. Sharman said.
“These results therefore confirm the biosimilarity of PF-05280586 with rituximab-EU,” he concluded.
Of note, the reporting of these findings comes on the heels of the first Food and Drug Administration approval of a biosimilar rituximab product for the treatment of NHL; Celltrion’s product Truxima (formerly CT-P10), a biosimilar of Genentech’s Rituxan (rituximab), was approved Nov. 28 to treat adults with CD20-positive, B-cell NHL, either as a single agent or in combination with chemotherapy.
The PF-0528056 study was sponsored by Pfizer. Dr. Sharman has been a consultant for, and/or received research funding and honoraria from Acerta, Pharmacyclics (an AbbVie Company), Pfizer, TG Therapeutics, Abbvie, and Genentech.
SOURCE: Sharman J et al. ASH 2018: Abstract 394.
SAN DIEGO – The potential rituximab biosimilar drug PF-05280586 showed efficacy, safety, immunogenicity, pharmacokinetics, and pharmacodynamics similar to those of rituximab at up to 26 weeks in a randomized phase 3 study of treatment-naive patients with CD20-positive low tumor burden follicular lymphoma (LTB-FL).
The primary endpoint of overall response rate at 26 weeks was 75.5% in 196 patients randomized to receive PF-05280586, and 70.7% in 198 patients who received a rituximab reference product sourced from the European Union (MabThera; rituximab‑EU), Jeff Sharman, MD, reported at the annual meeting of the American Society of Hematology.
“This resulted in a difference between the two arms of 4.66%,” said Dr. Sharman of Willamette Valley Cancer Institute and Research Center, Springfield, Ore.
The 95% confidence interval for this difference ... was entirely contained within the prespecified equivalence margin, he said.
“Depth of response was a key secondary endpoint, and rates of complete response were 29.3% and 30.4%, respectively,” he said, noting that rates of partial response, stable response, and progressive disease were also similar between the two study arms.
Estimated 1-year progression-free survival (PFS) rates were also highly similar at 76.4% and 81.2% in the PF-05280586 and rituximab-EU arms.
Rapid depletion in CD19-positive B-cell counts was observed in both groups after initial dosing, with recovery by week 39 and a sustained increase until the end of week 52.
Treatment-emergent adverse events (TEAEs) occurred in 78.6% vs. 72.1% of patients in the PF‑05280586 vs. rituximab‑EU arms, respectively, and the rates of serious adverse events and grade 3 events were similar in the groups, as were rates of infusion interruptions or infusion-related reactions (IRRs), Dr. Sharman said.
IRRs occurred in about 25% of patients in each arm, and most were grade 1 or 2. Grade 3 IRRs occurred in 2.6% vs. 0.5% of patients in the groups, respectively, and no grade 4 IRRs occurred.
Rates of anti-drug antibodies were also similar in the two groups, as were serum drug concentrations – regardless of anti-drug antibody status, he noted.
Study subjects were adults with a mean age of 60 years and histologically confirmed CD20-positive grade 1-3a follicular lymphoma with no prior rituximab or system therapy for B-cell non-Hodgkin lymphoma (NHL). They had Ann Arbor disease stages II (26.9%), III (44.2%) or IV (28.9%), ECOG performance status of 0-1, and at least 1 measurable disease lesion identifiable on imaging.
Risk level as assessed by the Follicular Lymphoma International Prognostic Index–2 was low in 28.4%, medium in 66%, and high in 5.6% of patients.
Treatment with each agent was given at intravenous doses of 375 mg/m2 weekly for 4 weeks at days 1, 8, 15, and 22.
PF-05280586 is being developed by Pfizer, and in this 52-week double-blind study – the largest study to date of the early use of the potential rituximab biosimilar in patients with previously untreated CD20-positive LTB-FL – the primary endpoint was met, demonstrating its therapeutic equivalence with rituximab-EU for overall response rate at week 26, Dr. Sharman said.
“These results therefore confirm the biosimilarity of PF-05280586 with rituximab-EU,” he concluded.
Of note, the reporting of these findings comes on the heels of the first Food and Drug Administration approval of a biosimilar rituximab product for the treatment of NHL; Celltrion’s product Truxima (formerly CT-P10), a biosimilar of Genentech’s Rituxan (rituximab), was approved Nov. 28 to treat adults with CD20-positive, B-cell NHL, either as a single agent or in combination with chemotherapy.
The PF-0528056 study was sponsored by Pfizer. Dr. Sharman has been a consultant for, and/or received research funding and honoraria from Acerta, Pharmacyclics (an AbbVie Company), Pfizer, TG Therapeutics, Abbvie, and Genentech.
SOURCE: Sharman J et al. ASH 2018: Abstract 394.
REPORTING FROM ASH 2018
Key clinical point: PF-05280586 shows biosimilarity to rituximab at up to 26 weeks.
Major finding: ORR at 26 weeks was 75.5% vs. 70.7% with PF-05280586 vs. rituximab, respectively.
Study details: A phase 3 study of 394 patients.
Disclosures: This study was sponsored by Pfizer. Dr. Sharman has been a consultant for, and/or received research funding and honoraria from Acerta, Pharmacyclics (an AbbVie Company), Pfizer, TG Therapeutics, Abbvie, and Genentech.
Source: Sharman J et al. ASH 2018: Abstract 394.
Factor IX expression stable at up to 8 years with gene therapy
SAN DIEGO – according to interim follow-up data from a phase 1/2 dose-escalation study.
The therapy – a self-complementary adeno-associated virus vector containing a codon-optimized factor IX gene, under control of a synthetic liver specific promoter and pseudotyped with serotype 8 capsid (scAAV2/8-LP1-hFIXco) – was previously shown to result in a dose-dependent increase in plasma factor IX levels in all 10 patients enrolled in the study, and an earlier update showed stable factor IX activity for at least 3 years, Ulrike M. Reiss, MD, reported at the annual meeting of the American Society of Hematology.
However, declining factor IX expression over time remains a concern, because AAV-mediated transgene expression is mediated mainly by episomally retained viral genomes, which may be lost with natural turnover of hepatocytes, noted Dr. Reiss, director of the clinical hematology division and the Hemophilia Treatment Center at St. Jude Children’s Research Hospital in Memphis.
At the “halfway mark,” with a median follow-up of 6.7 years in 10 patients aged 18-64 years who were treated with doses of either 2 x 1011, 6 x 1011, or 2 x 1012 vector genomes per kg (in 2, 2, and 6 patients, respectively), “factor IX expression has been persistent and stable in all participants after vector infusion,” she said.
“Factor IX expression was vector-dose dependent, achieving average levels of 1.9%-2.3% at the lower doses, and 5.1% at the high vector dose. All patients converted from having severe hemophilia to mild-moderate hemophilia,” she added.
The single significant adverse event observed during annual follow-up evaluations in the patients was a vector-related, immune-mediated liver inflammation occurring within 2-3 months of infusion in four of the six high-dose participants.
“There was complete resolution in all cases after a short course of corticosteroids over 8-12 weeks, including the taper. There were no late sequelae or any recurrence of transaminitis over time,” Dr. Reiss said. “We did not observe any new factor IX inhibitor or any late toxicity in any of these participants.”
Additionally, a comparison of average data across 3 years prior to gene therapy with the average data at 6.7 years after gene therapy showed that the annualized bleed rate decreased by 82% in the 10 participants and factor IX use decreased by 66%. In the high-dose group, the bleed rate decreased from 21 bleeds to 2 bleeds per year, and vector consumption was markedly reduced to a mean of 500 IU/kg per year from a mean of more than 2800 IU/kg per year. “Only one of the six patients in the high-dose group currently continues on prophylaxis treatment, whereas three in the low- and mid-dose groups are currently on prophylaxis,” she said. “In all [patients], the interval between prophylactic infusions has lengthened.”
Of note, Dr. Reiss and her colleagues explored the ability of using a modified, empty capsid-reduced vector preparation of the gene therapy to prevent the transaminitis seen in the 2-3 months after infusion. A new clinical preparation of scAAV2/8-LP1-hFIXco was manufactured with most of the empty particles removed by cesium chloride density centrifugation, but this approach provided no benefit in that regard.
“This further supports the observation that the anticapsid immune response is vector-dose dependent,” she said.
Additionally, the pattern of humoral response to AAV8 capsid was consistent with the primary immune response in participants.
“High IgG antibody titers have persisted for over 6 years; this finding is important because it will preclude these patients from any retreatment with the same vector or even potentially alternative AAV vectors of other serotypes with cross-reactive antigenicity,” she said.
Dr. Reiss reported having no relevant disclosures
SOURCE: Reiss UM et al. ASH 2018, Abstract 491.
SAN DIEGO – according to interim follow-up data from a phase 1/2 dose-escalation study.
The therapy – a self-complementary adeno-associated virus vector containing a codon-optimized factor IX gene, under control of a synthetic liver specific promoter and pseudotyped with serotype 8 capsid (scAAV2/8-LP1-hFIXco) – was previously shown to result in a dose-dependent increase in plasma factor IX levels in all 10 patients enrolled in the study, and an earlier update showed stable factor IX activity for at least 3 years, Ulrike M. Reiss, MD, reported at the annual meeting of the American Society of Hematology.
However, declining factor IX expression over time remains a concern, because AAV-mediated transgene expression is mediated mainly by episomally retained viral genomes, which may be lost with natural turnover of hepatocytes, noted Dr. Reiss, director of the clinical hematology division and the Hemophilia Treatment Center at St. Jude Children’s Research Hospital in Memphis.
At the “halfway mark,” with a median follow-up of 6.7 years in 10 patients aged 18-64 years who were treated with doses of either 2 x 1011, 6 x 1011, or 2 x 1012 vector genomes per kg (in 2, 2, and 6 patients, respectively), “factor IX expression has been persistent and stable in all participants after vector infusion,” she said.
“Factor IX expression was vector-dose dependent, achieving average levels of 1.9%-2.3% at the lower doses, and 5.1% at the high vector dose. All patients converted from having severe hemophilia to mild-moderate hemophilia,” she added.
The single significant adverse event observed during annual follow-up evaluations in the patients was a vector-related, immune-mediated liver inflammation occurring within 2-3 months of infusion in four of the six high-dose participants.
“There was complete resolution in all cases after a short course of corticosteroids over 8-12 weeks, including the taper. There were no late sequelae or any recurrence of transaminitis over time,” Dr. Reiss said. “We did not observe any new factor IX inhibitor or any late toxicity in any of these participants.”
Additionally, a comparison of average data across 3 years prior to gene therapy with the average data at 6.7 years after gene therapy showed that the annualized bleed rate decreased by 82% in the 10 participants and factor IX use decreased by 66%. In the high-dose group, the bleed rate decreased from 21 bleeds to 2 bleeds per year, and vector consumption was markedly reduced to a mean of 500 IU/kg per year from a mean of more than 2800 IU/kg per year. “Only one of the six patients in the high-dose group currently continues on prophylaxis treatment, whereas three in the low- and mid-dose groups are currently on prophylaxis,” she said. “In all [patients], the interval between prophylactic infusions has lengthened.”
Of note, Dr. Reiss and her colleagues explored the ability of using a modified, empty capsid-reduced vector preparation of the gene therapy to prevent the transaminitis seen in the 2-3 months after infusion. A new clinical preparation of scAAV2/8-LP1-hFIXco was manufactured with most of the empty particles removed by cesium chloride density centrifugation, but this approach provided no benefit in that regard.
“This further supports the observation that the anticapsid immune response is vector-dose dependent,” she said.
Additionally, the pattern of humoral response to AAV8 capsid was consistent with the primary immune response in participants.
“High IgG antibody titers have persisted for over 6 years; this finding is important because it will preclude these patients from any retreatment with the same vector or even potentially alternative AAV vectors of other serotypes with cross-reactive antigenicity,” she said.
Dr. Reiss reported having no relevant disclosures
SOURCE: Reiss UM et al. ASH 2018, Abstract 491.
SAN DIEGO – according to interim follow-up data from a phase 1/2 dose-escalation study.
The therapy – a self-complementary adeno-associated virus vector containing a codon-optimized factor IX gene, under control of a synthetic liver specific promoter and pseudotyped with serotype 8 capsid (scAAV2/8-LP1-hFIXco) – was previously shown to result in a dose-dependent increase in plasma factor IX levels in all 10 patients enrolled in the study, and an earlier update showed stable factor IX activity for at least 3 years, Ulrike M. Reiss, MD, reported at the annual meeting of the American Society of Hematology.
However, declining factor IX expression over time remains a concern, because AAV-mediated transgene expression is mediated mainly by episomally retained viral genomes, which may be lost with natural turnover of hepatocytes, noted Dr. Reiss, director of the clinical hematology division and the Hemophilia Treatment Center at St. Jude Children’s Research Hospital in Memphis.
At the “halfway mark,” with a median follow-up of 6.7 years in 10 patients aged 18-64 years who were treated with doses of either 2 x 1011, 6 x 1011, or 2 x 1012 vector genomes per kg (in 2, 2, and 6 patients, respectively), “factor IX expression has been persistent and stable in all participants after vector infusion,” she said.
“Factor IX expression was vector-dose dependent, achieving average levels of 1.9%-2.3% at the lower doses, and 5.1% at the high vector dose. All patients converted from having severe hemophilia to mild-moderate hemophilia,” she added.
The single significant adverse event observed during annual follow-up evaluations in the patients was a vector-related, immune-mediated liver inflammation occurring within 2-3 months of infusion in four of the six high-dose participants.
“There was complete resolution in all cases after a short course of corticosteroids over 8-12 weeks, including the taper. There were no late sequelae or any recurrence of transaminitis over time,” Dr. Reiss said. “We did not observe any new factor IX inhibitor or any late toxicity in any of these participants.”
Additionally, a comparison of average data across 3 years prior to gene therapy with the average data at 6.7 years after gene therapy showed that the annualized bleed rate decreased by 82% in the 10 participants and factor IX use decreased by 66%. In the high-dose group, the bleed rate decreased from 21 bleeds to 2 bleeds per year, and vector consumption was markedly reduced to a mean of 500 IU/kg per year from a mean of more than 2800 IU/kg per year. “Only one of the six patients in the high-dose group currently continues on prophylaxis treatment, whereas three in the low- and mid-dose groups are currently on prophylaxis,” she said. “In all [patients], the interval between prophylactic infusions has lengthened.”
Of note, Dr. Reiss and her colleagues explored the ability of using a modified, empty capsid-reduced vector preparation of the gene therapy to prevent the transaminitis seen in the 2-3 months after infusion. A new clinical preparation of scAAV2/8-LP1-hFIXco was manufactured with most of the empty particles removed by cesium chloride density centrifugation, but this approach provided no benefit in that regard.
“This further supports the observation that the anticapsid immune response is vector-dose dependent,” she said.
Additionally, the pattern of humoral response to AAV8 capsid was consistent with the primary immune response in participants.
“High IgG antibody titers have persisted for over 6 years; this finding is important because it will preclude these patients from any retreatment with the same vector or even potentially alternative AAV vectors of other serotypes with cross-reactive antigenicity,” she said.
Dr. Reiss reported having no relevant disclosures
SOURCE: Reiss UM et al. ASH 2018, Abstract 491.
REPORTING FROM ASH 2018
Key clinical point: With a median follow-up of 6.7 years in 10 patients aged 18-64 years who were treated with scAAV2/8-LP1-hFIX–comediated gene therapy, factor IX expression has been persistent and stable.
Major finding: Factor IX expression averaged 1.9%-2.3% at the lower doses, and 5.1% at the high dose at up to 8.6 years.
Study details: An interim follow-up data for 10 patients in a phase 1/2 study.
Disclosures: Dr. Reiss reported having no disclosures.
Source: Reiss UM et al. ASH 2018, Abstract 491.
Similarity of CT-P6 and trastuzumab remain with longer follow-up
SAN ANTONIO –
Similarity in safety and efficacy at 1 year was previously demonstrated in the phase 3 trial, as well as similarity in cardiac toxicity at a median of 19 months. Updated disease-free survival, overall survival, and cardiac toxicity with a median follow-up of 2 years will be presented by Francisco J. Esteva, MD, PhD, of the Laura & Isaac Perlmutter Cancer Center at NYU Langone Health, New York, in a poster presentation at the San Antonio Breast Cancer Symposium.
For the trial, 549 patients with HER2-positive early breast cancer were randomized to receive CT-P6 (n = 271) or trastuzumab (n = 278) in combination with docetaxel (cycles 1-4) and 5-fluorouracil, epirubicin, and cyclophosphamide (cycles 5-8). CT-P6 or trastuzumab was administered at 8 mg/kg (cycle 1 only) followed by 6 mg/kg every 3 weeks. After surgery, patients received CT-P6 or trastuzumab monotherapy and then entered the follow-up period.
A total of 528 patients entered the follow-up period, with a median duration of 27 months. Disease-free and overall survival were similar in the two arms in both the per-protocol set and the intention-to-treat set. In the intention-to-treat set, the 2-year disease-free survival was 86% (95% confidence interval, 80%-90%) in the CT-P6 arm and 90% (95% CI, 85%-93%) in the trastuzumab arm. Two-year overall survival was 97% (95% CI, 93%-98%) in the CT-P6 arm and 98% (95% CI, 96%-99%) in the trastuzumab arm. Median disease-free and overall survival have not been reached, according to the abstract.
No new cases of heart failure were reported during the follow-up period. Left ventricular ejection fraction was similar in both arms. The efficacy and cardiac toxicity profile between CT-P6 and trastuzumab were consistent with published data.
“CT-P6 was consistently well tolerated with a similar cardiotoxicity profile to that of trastuzumab through a long duration of follow-up,” Dr. Esteva and authors said.
The study sponsor is Celltrion, maker of CT-P6. Dr. Esteva disclosed a consulting or advisory role with Celltrion, as well as relationships with various other pharmaceutical companies.
SOURCE: Esteva FJ et al. SABCS 2018, Abstract P6-17-03.
SAN ANTONIO –
Similarity in safety and efficacy at 1 year was previously demonstrated in the phase 3 trial, as well as similarity in cardiac toxicity at a median of 19 months. Updated disease-free survival, overall survival, and cardiac toxicity with a median follow-up of 2 years will be presented by Francisco J. Esteva, MD, PhD, of the Laura & Isaac Perlmutter Cancer Center at NYU Langone Health, New York, in a poster presentation at the San Antonio Breast Cancer Symposium.
For the trial, 549 patients with HER2-positive early breast cancer were randomized to receive CT-P6 (n = 271) or trastuzumab (n = 278) in combination with docetaxel (cycles 1-4) and 5-fluorouracil, epirubicin, and cyclophosphamide (cycles 5-8). CT-P6 or trastuzumab was administered at 8 mg/kg (cycle 1 only) followed by 6 mg/kg every 3 weeks. After surgery, patients received CT-P6 or trastuzumab monotherapy and then entered the follow-up period.
A total of 528 patients entered the follow-up period, with a median duration of 27 months. Disease-free and overall survival were similar in the two arms in both the per-protocol set and the intention-to-treat set. In the intention-to-treat set, the 2-year disease-free survival was 86% (95% confidence interval, 80%-90%) in the CT-P6 arm and 90% (95% CI, 85%-93%) in the trastuzumab arm. Two-year overall survival was 97% (95% CI, 93%-98%) in the CT-P6 arm and 98% (95% CI, 96%-99%) in the trastuzumab arm. Median disease-free and overall survival have not been reached, according to the abstract.
No new cases of heart failure were reported during the follow-up period. Left ventricular ejection fraction was similar in both arms. The efficacy and cardiac toxicity profile between CT-P6 and trastuzumab were consistent with published data.
“CT-P6 was consistently well tolerated with a similar cardiotoxicity profile to that of trastuzumab through a long duration of follow-up,” Dr. Esteva and authors said.
The study sponsor is Celltrion, maker of CT-P6. Dr. Esteva disclosed a consulting or advisory role with Celltrion, as well as relationships with various other pharmaceutical companies.
SOURCE: Esteva FJ et al. SABCS 2018, Abstract P6-17-03.
SAN ANTONIO –
Similarity in safety and efficacy at 1 year was previously demonstrated in the phase 3 trial, as well as similarity in cardiac toxicity at a median of 19 months. Updated disease-free survival, overall survival, and cardiac toxicity with a median follow-up of 2 years will be presented by Francisco J. Esteva, MD, PhD, of the Laura & Isaac Perlmutter Cancer Center at NYU Langone Health, New York, in a poster presentation at the San Antonio Breast Cancer Symposium.
For the trial, 549 patients with HER2-positive early breast cancer were randomized to receive CT-P6 (n = 271) or trastuzumab (n = 278) in combination with docetaxel (cycles 1-4) and 5-fluorouracil, epirubicin, and cyclophosphamide (cycles 5-8). CT-P6 or trastuzumab was administered at 8 mg/kg (cycle 1 only) followed by 6 mg/kg every 3 weeks. After surgery, patients received CT-P6 or trastuzumab monotherapy and then entered the follow-up period.
A total of 528 patients entered the follow-up period, with a median duration of 27 months. Disease-free and overall survival were similar in the two arms in both the per-protocol set and the intention-to-treat set. In the intention-to-treat set, the 2-year disease-free survival was 86% (95% confidence interval, 80%-90%) in the CT-P6 arm and 90% (95% CI, 85%-93%) in the trastuzumab arm. Two-year overall survival was 97% (95% CI, 93%-98%) in the CT-P6 arm and 98% (95% CI, 96%-99%) in the trastuzumab arm. Median disease-free and overall survival have not been reached, according to the abstract.
No new cases of heart failure were reported during the follow-up period. Left ventricular ejection fraction was similar in both arms. The efficacy and cardiac toxicity profile between CT-P6 and trastuzumab were consistent with published data.
“CT-P6 was consistently well tolerated with a similar cardiotoxicity profile to that of trastuzumab through a long duration of follow-up,” Dr. Esteva and authors said.
The study sponsor is Celltrion, maker of CT-P6. Dr. Esteva disclosed a consulting or advisory role with Celltrion, as well as relationships with various other pharmaceutical companies.
SOURCE: Esteva FJ et al. SABCS 2018, Abstract P6-17-03.
REPORTING FROM SABCS 2018
Key clinical point: Trastuzumab biosimilar candidate CT-P6 and trastuzumab, as neoadjuvant and then adjuvant therapy for patients with early HER2-positive breast cancer, have similar efficacy and cardiac toxicity profiles after 2 years.
Major finding: The number of DFS events (32 [12.4%] in CT-P6 and 26 [10.0%] in trastuzumab) and OS events (14 [5.2%] in CT-P6 and 12 [4.3%] in trastuzumab) were comparable in the intention-to-treat group.
Study details: Phase 3 trial of 549 patients with HER2-positive early breast cancer.
Disclosures: The study sponsor is Celltrion, maker of CT-P6. Dr. Esteva disclosed a consulting or advisory role with Celltrion, as well as relationships with various other pharmaceutical companies.
Source: Esteva FJ et al. SABCS 2018, Abstract P6-17-03.
PAD guidelines: Consensus needed between U.S. and Europe
Recent advances in the management of peripheral artery disease (PAD) have resulted in new guideline creation in both the United States and Europe.
While there is considerable consensus between the guidelines, there are multiple differences in emphasis and a differing approach to the types and quality of evidence used to back recommendations, according to a comparative review published in the Journal of the American College of Cardiology. The American Heart Association and American College of Cardiology, together with other organizations, issued an update to their previous guidelines on the management and diagnosis of lower extremity PAD in 2016. In 2017, the European Society of Cardiology in conjunction with the European Society for Vascular Surgery updated their own comprehensive guidelines.
Both the U.S. and the European guidelines stress the importance of lowering risk factors for PAD. This includes stopping smoking, lipid and blood pressure management, and controlling glucose, according to Aaron P. Kithcart, MD, of Brigham and Women’s Hospital, Boston, and Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn.
However, the U.S. guidelines focus more on moderating lifestyle factors, including the pursuit of regular physical activity and the use of supervised exercise, whereas the European guidelines focus considerable attention on recommendations for revascularization in patients with limb-threatening ischemia.
Perhaps the major source of variation between the two sets of guidelines, according to the reviewers, are based upon the intended audiences: “The American document limits its focus to PAD but is applicable to practitioners of every background, whereas the European guideline extends the discussion to all PADs to include carotid and vertebral, upper extremities, mesenteric, and renal arteries in addition to lower-extremity artery disease; but is designed to be a source for cardiologists.”
Accordingly, the ESC/ESVS guidelines approach medical therapy with a more holistic flavor, whereas the ACC/AHA guidelines are specific to the lower-extremity complications of atherosclerosis, according to the reviewers.
Both sets of guidelines come to the conclusion that there is a need for more evidence to identify patients who are at the greatest risk of tissue loss, but overall they differ in their approach to available data. The ACC/AHA is more inclusive of smaller, well-done nonrandomized studies, whereas the ESC/ESVS relegates small studies to Level of Evidence: C. “We believe this difference drives the variation of therapeutic recommendations more than any other factor,” the authors note.
More randomized studies would align recommendations across both organizations, according to Dr. Kithcart and Dr. Beckman (JACC 2018;72:2789-801).
“The management of PAD has progressed a great deal over the last decade. ... Several clinical trials over the coming years should help clarify how revascularization should be approached, and which patients are most likely to benefit. Until then, maintaining good cardiovascular health, including regular physical activity, smoking cessation, lipid-lowering therapy, blood pressure management, and glucose control have the most benefit in patients with PAD,” the researchers concluded.
Dr. Beckman served as a consultant for several pharmaceutical companies, and on the data and safety monitoring board for Bayer and Novartis. Dr. Kithcart reported that he has no relevant conflicts.
SOURCE: Kithcart, AP et al. JACC 2018;72:2789-801.
Recent advances in the management of peripheral artery disease (PAD) have resulted in new guideline creation in both the United States and Europe.
While there is considerable consensus between the guidelines, there are multiple differences in emphasis and a differing approach to the types and quality of evidence used to back recommendations, according to a comparative review published in the Journal of the American College of Cardiology. The American Heart Association and American College of Cardiology, together with other organizations, issued an update to their previous guidelines on the management and diagnosis of lower extremity PAD in 2016. In 2017, the European Society of Cardiology in conjunction with the European Society for Vascular Surgery updated their own comprehensive guidelines.
Both the U.S. and the European guidelines stress the importance of lowering risk factors for PAD. This includes stopping smoking, lipid and blood pressure management, and controlling glucose, according to Aaron P. Kithcart, MD, of Brigham and Women’s Hospital, Boston, and Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn.
However, the U.S. guidelines focus more on moderating lifestyle factors, including the pursuit of regular physical activity and the use of supervised exercise, whereas the European guidelines focus considerable attention on recommendations for revascularization in patients with limb-threatening ischemia.
Perhaps the major source of variation between the two sets of guidelines, according to the reviewers, are based upon the intended audiences: “The American document limits its focus to PAD but is applicable to practitioners of every background, whereas the European guideline extends the discussion to all PADs to include carotid and vertebral, upper extremities, mesenteric, and renal arteries in addition to lower-extremity artery disease; but is designed to be a source for cardiologists.”
Accordingly, the ESC/ESVS guidelines approach medical therapy with a more holistic flavor, whereas the ACC/AHA guidelines are specific to the lower-extremity complications of atherosclerosis, according to the reviewers.
Both sets of guidelines come to the conclusion that there is a need for more evidence to identify patients who are at the greatest risk of tissue loss, but overall they differ in their approach to available data. The ACC/AHA is more inclusive of smaller, well-done nonrandomized studies, whereas the ESC/ESVS relegates small studies to Level of Evidence: C. “We believe this difference drives the variation of therapeutic recommendations more than any other factor,” the authors note.
More randomized studies would align recommendations across both organizations, according to Dr. Kithcart and Dr. Beckman (JACC 2018;72:2789-801).
“The management of PAD has progressed a great deal over the last decade. ... Several clinical trials over the coming years should help clarify how revascularization should be approached, and which patients are most likely to benefit. Until then, maintaining good cardiovascular health, including regular physical activity, smoking cessation, lipid-lowering therapy, blood pressure management, and glucose control have the most benefit in patients with PAD,” the researchers concluded.
Dr. Beckman served as a consultant for several pharmaceutical companies, and on the data and safety monitoring board for Bayer and Novartis. Dr. Kithcart reported that he has no relevant conflicts.
SOURCE: Kithcart, AP et al. JACC 2018;72:2789-801.
Recent advances in the management of peripheral artery disease (PAD) have resulted in new guideline creation in both the United States and Europe.
While there is considerable consensus between the guidelines, there are multiple differences in emphasis and a differing approach to the types and quality of evidence used to back recommendations, according to a comparative review published in the Journal of the American College of Cardiology. The American Heart Association and American College of Cardiology, together with other organizations, issued an update to their previous guidelines on the management and diagnosis of lower extremity PAD in 2016. In 2017, the European Society of Cardiology in conjunction with the European Society for Vascular Surgery updated their own comprehensive guidelines.
Both the U.S. and the European guidelines stress the importance of lowering risk factors for PAD. This includes stopping smoking, lipid and blood pressure management, and controlling glucose, according to Aaron P. Kithcart, MD, of Brigham and Women’s Hospital, Boston, and Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn.
However, the U.S. guidelines focus more on moderating lifestyle factors, including the pursuit of regular physical activity and the use of supervised exercise, whereas the European guidelines focus considerable attention on recommendations for revascularization in patients with limb-threatening ischemia.
Perhaps the major source of variation between the two sets of guidelines, according to the reviewers, are based upon the intended audiences: “The American document limits its focus to PAD but is applicable to practitioners of every background, whereas the European guideline extends the discussion to all PADs to include carotid and vertebral, upper extremities, mesenteric, and renal arteries in addition to lower-extremity artery disease; but is designed to be a source for cardiologists.”
Accordingly, the ESC/ESVS guidelines approach medical therapy with a more holistic flavor, whereas the ACC/AHA guidelines are specific to the lower-extremity complications of atherosclerosis, according to the reviewers.
Both sets of guidelines come to the conclusion that there is a need for more evidence to identify patients who are at the greatest risk of tissue loss, but overall they differ in their approach to available data. The ACC/AHA is more inclusive of smaller, well-done nonrandomized studies, whereas the ESC/ESVS relegates small studies to Level of Evidence: C. “We believe this difference drives the variation of therapeutic recommendations more than any other factor,” the authors note.
More randomized studies would align recommendations across both organizations, according to Dr. Kithcart and Dr. Beckman (JACC 2018;72:2789-801).
“The management of PAD has progressed a great deal over the last decade. ... Several clinical trials over the coming years should help clarify how revascularization should be approached, and which patients are most likely to benefit. Until then, maintaining good cardiovascular health, including regular physical activity, smoking cessation, lipid-lowering therapy, blood pressure management, and glucose control have the most benefit in patients with PAD,” the researchers concluded.
Dr. Beckman served as a consultant for several pharmaceutical companies, and on the data and safety monitoring board for Bayer and Novartis. Dr. Kithcart reported that he has no relevant conflicts.
SOURCE: Kithcart, AP et al. JACC 2018;72:2789-801.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
CLL resistance mechanism to venetoclax identified
SAN DIEGO – A recurrent mutation in BCL2, the therapeutic target of venetoclax (Venclexta), appears to be a major contributor to drug resistance in patients with chronic lymphocytic leukemia (CLL), investigators reported.
The mutation has been detected in some patients with CLL up to 2 years before resistance to venetoclax actually develops, said lead author Piers Blombery, MBBS, from the Peter MacCallum Cancer Center in Melbourne.
“We have identified the first acquired BCL2 mutation developed in patients clinically treated with venetoclax,” he said in a late-breaking oral abstract session at the annual meeting of the American Society of Hematology.
The mutation, which the investigators have labeled BCL2 Gly101Val, “is a recurrent and frequent mediator of resistance and may be detected years before clinical relapse occurs,” he added.
The paper was published online in Cancer Discovery (2018 Dec 4. doi: 10.1158/2159-8290.CD-18-1119) to coincide with the presentation at ASH.
Despite the demonstrated efficacy of venetoclax as continuous therapy in patients with relapsed or refractory CLL, the majority of patients experience disease progression, prompting the investigators to explore molecular mechanisms of secondary resistance.
To do this, they analyzed paired samples from 15 patients with CLL, enrolled in clinical trials of venetoclax, collected both before the start of venetoclax therapy and at the time of disease progression.
In seven of the patients, they identified a novel mutation that showed up at the time of progression, but was absent from the pre-venetoclax samples. The mutation first became detectable from about 19 to 42 months after the start of therapy and preceded clinical progression by as much as 25 months, the investigators found.
They pinned the mutation down to the BH3-binding groove on BCL2, the same molecular site targeted by venetoclax. They found that the mutation was not present in samples from 96 patients with venetoclax-naive CLL nor in any other B-cell malignancies. Searches for references to the mutation in both a cancer database (COSMIC) and a population database (gnomAD) came up empty.
In other experiments, they determined that cell lines overexpressing BCL2 Gly101Val are resistant to venetoclax, and that in the presence of venetoclax in vitro, BCL2 Gly101Val-expressing cells have a growth advantage, compared with wild type cells.
Additionally, they showed that the mutation results in impaired venetoclax binding in vitro.
“BCL2 Gly101Val is observed subclonally, implicating multiple mechanisms of venetoclax resistance in the same patient,” Dr. Blombery said.
In an interview, Dr. Blombery said that the identification of the resistance mutation is a strong rationale for using combination therapy to treat patients with relapsed or refractory CLL to help prevent or attenuate selection pressures that lead to resistance.
The investigators were supported by the Wilson Center for Lymphoma Genomics, Snowdome Foundation, National Health Medical Research Council, Leukemia and Lymphoma Society, Leukemia Foundation, Cancer Council of Victoria, and Australian Cancer Research Foundation. Dr. Blombery reported having no relevant disclosures.
SOURCE: Blombery P et al. ASH 2018, Abstract LBA-7.
SAN DIEGO – A recurrent mutation in BCL2, the therapeutic target of venetoclax (Venclexta), appears to be a major contributor to drug resistance in patients with chronic lymphocytic leukemia (CLL), investigators reported.
The mutation has been detected in some patients with CLL up to 2 years before resistance to venetoclax actually develops, said lead author Piers Blombery, MBBS, from the Peter MacCallum Cancer Center in Melbourne.
“We have identified the first acquired BCL2 mutation developed in patients clinically treated with venetoclax,” he said in a late-breaking oral abstract session at the annual meeting of the American Society of Hematology.
The mutation, which the investigators have labeled BCL2 Gly101Val, “is a recurrent and frequent mediator of resistance and may be detected years before clinical relapse occurs,” he added.
The paper was published online in Cancer Discovery (2018 Dec 4. doi: 10.1158/2159-8290.CD-18-1119) to coincide with the presentation at ASH.
Despite the demonstrated efficacy of venetoclax as continuous therapy in patients with relapsed or refractory CLL, the majority of patients experience disease progression, prompting the investigators to explore molecular mechanisms of secondary resistance.
To do this, they analyzed paired samples from 15 patients with CLL, enrolled in clinical trials of venetoclax, collected both before the start of venetoclax therapy and at the time of disease progression.
In seven of the patients, they identified a novel mutation that showed up at the time of progression, but was absent from the pre-venetoclax samples. The mutation first became detectable from about 19 to 42 months after the start of therapy and preceded clinical progression by as much as 25 months, the investigators found.
They pinned the mutation down to the BH3-binding groove on BCL2, the same molecular site targeted by venetoclax. They found that the mutation was not present in samples from 96 patients with venetoclax-naive CLL nor in any other B-cell malignancies. Searches for references to the mutation in both a cancer database (COSMIC) and a population database (gnomAD) came up empty.
In other experiments, they determined that cell lines overexpressing BCL2 Gly101Val are resistant to venetoclax, and that in the presence of venetoclax in vitro, BCL2 Gly101Val-expressing cells have a growth advantage, compared with wild type cells.
Additionally, they showed that the mutation results in impaired venetoclax binding in vitro.
“BCL2 Gly101Val is observed subclonally, implicating multiple mechanisms of venetoclax resistance in the same patient,” Dr. Blombery said.
In an interview, Dr. Blombery said that the identification of the resistance mutation is a strong rationale for using combination therapy to treat patients with relapsed or refractory CLL to help prevent or attenuate selection pressures that lead to resistance.
The investigators were supported by the Wilson Center for Lymphoma Genomics, Snowdome Foundation, National Health Medical Research Council, Leukemia and Lymphoma Society, Leukemia Foundation, Cancer Council of Victoria, and Australian Cancer Research Foundation. Dr. Blombery reported having no relevant disclosures.
SOURCE: Blombery P et al. ASH 2018, Abstract LBA-7.
SAN DIEGO – A recurrent mutation in BCL2, the therapeutic target of venetoclax (Venclexta), appears to be a major contributor to drug resistance in patients with chronic lymphocytic leukemia (CLL), investigators reported.
The mutation has been detected in some patients with CLL up to 2 years before resistance to venetoclax actually develops, said lead author Piers Blombery, MBBS, from the Peter MacCallum Cancer Center in Melbourne.
“We have identified the first acquired BCL2 mutation developed in patients clinically treated with venetoclax,” he said in a late-breaking oral abstract session at the annual meeting of the American Society of Hematology.
The mutation, which the investigators have labeled BCL2 Gly101Val, “is a recurrent and frequent mediator of resistance and may be detected years before clinical relapse occurs,” he added.
The paper was published online in Cancer Discovery (2018 Dec 4. doi: 10.1158/2159-8290.CD-18-1119) to coincide with the presentation at ASH.
Despite the demonstrated efficacy of venetoclax as continuous therapy in patients with relapsed or refractory CLL, the majority of patients experience disease progression, prompting the investigators to explore molecular mechanisms of secondary resistance.
To do this, they analyzed paired samples from 15 patients with CLL, enrolled in clinical trials of venetoclax, collected both before the start of venetoclax therapy and at the time of disease progression.
In seven of the patients, they identified a novel mutation that showed up at the time of progression, but was absent from the pre-venetoclax samples. The mutation first became detectable from about 19 to 42 months after the start of therapy and preceded clinical progression by as much as 25 months, the investigators found.
They pinned the mutation down to the BH3-binding groove on BCL2, the same molecular site targeted by venetoclax. They found that the mutation was not present in samples from 96 patients with venetoclax-naive CLL nor in any other B-cell malignancies. Searches for references to the mutation in both a cancer database (COSMIC) and a population database (gnomAD) came up empty.
In other experiments, they determined that cell lines overexpressing BCL2 Gly101Val are resistant to venetoclax, and that in the presence of venetoclax in vitro, BCL2 Gly101Val-expressing cells have a growth advantage, compared with wild type cells.
Additionally, they showed that the mutation results in impaired venetoclax binding in vitro.
“BCL2 Gly101Val is observed subclonally, implicating multiple mechanisms of venetoclax resistance in the same patient,” Dr. Blombery said.
In an interview, Dr. Blombery said that the identification of the resistance mutation is a strong rationale for using combination therapy to treat patients with relapsed or refractory CLL to help prevent or attenuate selection pressures that lead to resistance.
The investigators were supported by the Wilson Center for Lymphoma Genomics, Snowdome Foundation, National Health Medical Research Council, Leukemia and Lymphoma Society, Leukemia Foundation, Cancer Council of Victoria, and Australian Cancer Research Foundation. Dr. Blombery reported having no relevant disclosures.
SOURCE: Blombery P et al. ASH 2018, Abstract LBA-7.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The mutation was identified in samples from seven patients after venetoclax therapy, but not in any of the pretherapy samples.
Study details: Genetic analysis of CLL mutations in 15 patients enrolled in clinical trials of venetoclax.
Disclosures: The investigators were supported by the Wilson Center for Lymphoma Genomics, Snowdome Foundation, National Health Medical Research Council, Leukemia and Lymphoma Society, Leukemia Foundation, Cancer Council of Victoria, and Australian Cancer Research Foundation. Dr. Blombery reported having no relevant disclosures.
Source: Blombery P et al. ASH 2018, Abstract LBA-7.
KATHERINE: T-DM1 doubles HER2-positive invasive disease-free survival
SAN ANTONIO – Swapping trastuzumab out for the drug-antibody conjugate trastuzumab emtansine (T-DM1; Kadcyla) as adjuvant therapy resulted in a halving in the risk of invasive disease or death in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy, including trastuzumab.
For the primary endpoint in the KATHERINE trial of invasive disease-free survival – defined as freedom from ipsilateral invasive breast tumor recurrence, ipsilateral locoregional invasive breast cancer recurrence, contralateral invasive breast cancer, distant recurrence, or death from any cause – T-DM1 was associated with a hazard ratio of 0.50 (P less than .001).
The 3-year invasive disease-free survival rate for 743 patients treated with T-DMI 1 was 88.3%, compared with 77% for 743 patients treated with trastuzumab, reported Charles E. Geyer Jr., MD, from Virginia Commonwealth University, Richmond, at the San Antonio Breast Cancer Symposium.
In a video interview, Dr. Geyer discussed results of KATHERINE, which suggest that T-DM1 should be considered as a new standard of care in this patient population.
Dr. Geyer reported travel support from Roche and AstraZeneca, medical writing support from AbbVie and Roche, and honoraria from Celgene.
SAN ANTONIO – Swapping trastuzumab out for the drug-antibody conjugate trastuzumab emtansine (T-DM1; Kadcyla) as adjuvant therapy resulted in a halving in the risk of invasive disease or death in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy, including trastuzumab.
For the primary endpoint in the KATHERINE trial of invasive disease-free survival – defined as freedom from ipsilateral invasive breast tumor recurrence, ipsilateral locoregional invasive breast cancer recurrence, contralateral invasive breast cancer, distant recurrence, or death from any cause – T-DM1 was associated with a hazard ratio of 0.50 (P less than .001).
The 3-year invasive disease-free survival rate for 743 patients treated with T-DMI 1 was 88.3%, compared with 77% for 743 patients treated with trastuzumab, reported Charles E. Geyer Jr., MD, from Virginia Commonwealth University, Richmond, at the San Antonio Breast Cancer Symposium.
In a video interview, Dr. Geyer discussed results of KATHERINE, which suggest that T-DM1 should be considered as a new standard of care in this patient population.
Dr. Geyer reported travel support from Roche and AstraZeneca, medical writing support from AbbVie and Roche, and honoraria from Celgene.
SAN ANTONIO – Swapping trastuzumab out for the drug-antibody conjugate trastuzumab emtansine (T-DM1; Kadcyla) as adjuvant therapy resulted in a halving in the risk of invasive disease or death in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy, including trastuzumab.
For the primary endpoint in the KATHERINE trial of invasive disease-free survival – defined as freedom from ipsilateral invasive breast tumor recurrence, ipsilateral locoregional invasive breast cancer recurrence, contralateral invasive breast cancer, distant recurrence, or death from any cause – T-DM1 was associated with a hazard ratio of 0.50 (P less than .001).
The 3-year invasive disease-free survival rate for 743 patients treated with T-DMI 1 was 88.3%, compared with 77% for 743 patients treated with trastuzumab, reported Charles E. Geyer Jr., MD, from Virginia Commonwealth University, Richmond, at the San Antonio Breast Cancer Symposium.
In a video interview, Dr. Geyer discussed results of KATHERINE, which suggest that T-DM1 should be considered as a new standard of care in this patient population.
Dr. Geyer reported travel support from Roche and AstraZeneca, medical writing support from AbbVie and Roche, and honoraria from Celgene.
REPORTING FROM SABCS 2018
2018 Update on bone health
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
Large cohort study IDs prognostic factors in thromboangiitis obliterans
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Nonwhite ethnicity and limb infection predict poor prognosis in TAO.
Major finding: Ethnicity predicts vascular events (HR, 2.35); limb infection at diagnosis predicts vascular events and amputation (HR, 3.29 and 12.1, respectively).
Study details: A retrospective cohort study of 224 patients.
Disclosures: Dr. Le Joncour reported having no disclosures.
Source: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.