User login
Help parents manage screen time thoughtfully
It has been 2 years since we last wrote about the potential risks to children and adolescents of spending too much time on screens. While there have been studies in the interval that offer us more information about the effects of heavy screen use and the developing brain, there is little certainty about what is optimal for children and adolescents, and less still on how parents might effectively equip their children to make good use of screens without suffering ill effects.
You might recall that back in October of 2016, the American Academy of Pediatrics published screen time guidelines: recommending no screen time for infants and children up to 18 months old, limiting all screen time to 1 hour per day for children up to 5 years old, and 2 hours daily for older children (up to 11 years old), so that it would not interfere with homework, social time, exercise, and sleep. At the time, data suggested that children from 2 to 11 years old were spending an average of 4.5 hours per day on screens (TV, computer, tablets, or smartphones, not counting homework).
The Adolescent Brain Cognitive Development Study began in September 2016 to evaluate the effects of Canadian recommendations for 8- to 11-year-olds (9-11 hours sleep nightly, 1 hour of exercise daily, and 2 hours or less of screen time daily; the study subjects are in the United States). This fall they published their initial results, demonstrating that only 51% get the recommended amount of sleep, only 37% kept their daily screen time to under 2 hours, and only 18% were getting the recommended amount of exercise. Only 5% of children consistently met all three recommendations while 29% of children didn’t meet any of the recommendations.
The researchers assessed the children’s cognitive development and found that after 1 year, those children who met the screen time recommendations, both screen time and sleep, or all three recommendations demonstrated “superior global cognition.” Children were spending an average of 3.7 hours daily on screens, and those children who were spending 2 hours or less on screens performed 4% better on tests of cognitive function than did children spending the average amount of time. Sleep and exercise differences alone did not contribute to significant differences in cognitive function. This study will continue for another 10 years.1
In a much smaller study out of Cincinnati Children’s Hospital, researchers asked parents to describe the amount of time a child spent on reading and in screen-based media activities, then completed MRI scans of the children’s brains.2 They found a strong association between reading time and higher functional connectivity between the parts of the brain responsible for visual word formation and those responsible for language and cognitive control, with a negative correlation between functional connectivity and time spent in screen-based media activities.
While these studies are important pieces of data as we build a deeper understanding about the effects of screen-based media use on children’s cognitive and behavioral development, they do not offer certainty about causality. These studies do not yet clarify whether certain children are especially vulnerable to the untoward effects of heavy screen-based media use. Perhaps the research will someday offer guidelines with certainty, but families need guidance now. Without doubt, digital devices are here to stay, are important to homework, and can facilitate independence, long-distance connections, important technical work-skills, and even senseless fun and relaxation. So we will focus on offering some principles to help you guide young people (or their parents) in approaching screen time thoughtfully.
While recommending no more than 2 hours of daily screen time seems reasonable, it may be more useful to focus on what young people are doing with the rest of their time. Are they getting adequate, restful sleep? Are they able to exercise most days? Do they have enough time for homework? Do they have time for friends (time actually together, not just texting)? What about time for hobbies? When parents focus on the precious resource of time and all of the activities their children both need and want to do, it sets the frame for them to say that their children are allowed to have time to relax with screen-based media as long as it does not take away from these other priorities. Ensuring that the child has at least 8 hours of sleep, after homework and sports, also will set natural limits on screen time.
Parents also can use the frame of development to guide their rules about screen time. If use of an electronic device serves a developmental task, then it is reasonable. If it interferes with a developmental task, then it should be limited. Adolescents (ages 12-20) should be exploring their own identities, establishing independence, deepening social relationships, and learning to manage their impulses. Some interests can be most easily explored with the aid of a computer (such as with programming, art history, or astronomy). Use of cellphones can facilitate teenagers’ being more independent with plans or transportation. Social connections can be supported by texting or FaceTime. Some close friends may be in a different sport or live far away, and it is possible to stay connected only virtually. However, when use of electronic devices keeps the child from engaging with new friends and new interests or from getting into the world to establish real independence (i.e., a job), then there should be limits. In all of these cases, it is critical that adults explain to teenagers what is guiding their thinking about limits on screen time. Open discussions about the great utility and fun that screens can provide, as well as the challenge of keeping those activities in balance with other important activities, helps adolescents set the frame for that rapidly approaching time when they will be making those choices without adult supervision.
Younger children (ages 8-11) should be sampling a wide array of activities and interests and experiencing challenges and eventual mastery across domains. Video games can be very compelling for this age group because they appeal to exactly this drive to master a challenge. Parents want to ensure that their children can have senseless fun, and still have enough time to explore actual activities: social, athletic, creative, and academic. They can be ready to explain the why of rules, but consistent rules, enforced for everyone at home, are most helpful for this age group.
You also can help parents to consider the child’s temperament when thinking about which rules will be appropriate. Anxious children and teenagers may be especially prone to immersive virtual activities that allow them to avoid the stress of real undertakings or interactions. But anxious children may be able to prepare for something anxiety provoking by exploring it virtually first. Youth with ADHD are going to struggle with shifting away from video games or other electronic activities they enjoy that don’t have a natural ending, and will need strict rules and patient support around balanced screen time use. Screen time may play to a child’s strengths, enabling creative children to take in a wide range of art or music and even create their own when other resources are limited.
Finally, all parents should consider what their own screen use is teaching their children. Adolescents are unlikely to listen to their parents’ recommendations if the parents spend hours online after work. Younger children need their parents’ engaged attention: being coaches and cheerleaders for all of their efforts at mastery. You can help parents to imagine rules that the whole family can follow. They can consider how screen time helps them connect with their children, such as watching a favorite program or sport together. They can explore shared interests online together. They can even relax with ridiculous cat videos together! Screen time together is valuable if it supports parents’ connections with their children, while their rules ensure adequate time for sleep, physical activity, and developmental priorities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Lancet Child Adolesc Health. 2018 Nov 1;2(11):783-91.
2. Acta Paediatra. 2018 Apr;107(4):685-93
It has been 2 years since we last wrote about the potential risks to children and adolescents of spending too much time on screens. While there have been studies in the interval that offer us more information about the effects of heavy screen use and the developing brain, there is little certainty about what is optimal for children and adolescents, and less still on how parents might effectively equip their children to make good use of screens without suffering ill effects.
You might recall that back in October of 2016, the American Academy of Pediatrics published screen time guidelines: recommending no screen time for infants and children up to 18 months old, limiting all screen time to 1 hour per day for children up to 5 years old, and 2 hours daily for older children (up to 11 years old), so that it would not interfere with homework, social time, exercise, and sleep. At the time, data suggested that children from 2 to 11 years old were spending an average of 4.5 hours per day on screens (TV, computer, tablets, or smartphones, not counting homework).
The Adolescent Brain Cognitive Development Study began in September 2016 to evaluate the effects of Canadian recommendations for 8- to 11-year-olds (9-11 hours sleep nightly, 1 hour of exercise daily, and 2 hours or less of screen time daily; the study subjects are in the United States). This fall they published their initial results, demonstrating that only 51% get the recommended amount of sleep, only 37% kept their daily screen time to under 2 hours, and only 18% were getting the recommended amount of exercise. Only 5% of children consistently met all three recommendations while 29% of children didn’t meet any of the recommendations.
The researchers assessed the children’s cognitive development and found that after 1 year, those children who met the screen time recommendations, both screen time and sleep, or all three recommendations demonstrated “superior global cognition.” Children were spending an average of 3.7 hours daily on screens, and those children who were spending 2 hours or less on screens performed 4% better on tests of cognitive function than did children spending the average amount of time. Sleep and exercise differences alone did not contribute to significant differences in cognitive function. This study will continue for another 10 years.1
In a much smaller study out of Cincinnati Children’s Hospital, researchers asked parents to describe the amount of time a child spent on reading and in screen-based media activities, then completed MRI scans of the children’s brains.2 They found a strong association between reading time and higher functional connectivity between the parts of the brain responsible for visual word formation and those responsible for language and cognitive control, with a negative correlation between functional connectivity and time spent in screen-based media activities.
While these studies are important pieces of data as we build a deeper understanding about the effects of screen-based media use on children’s cognitive and behavioral development, they do not offer certainty about causality. These studies do not yet clarify whether certain children are especially vulnerable to the untoward effects of heavy screen-based media use. Perhaps the research will someday offer guidelines with certainty, but families need guidance now. Without doubt, digital devices are here to stay, are important to homework, and can facilitate independence, long-distance connections, important technical work-skills, and even senseless fun and relaxation. So we will focus on offering some principles to help you guide young people (or their parents) in approaching screen time thoughtfully.
While recommending no more than 2 hours of daily screen time seems reasonable, it may be more useful to focus on what young people are doing with the rest of their time. Are they getting adequate, restful sleep? Are they able to exercise most days? Do they have enough time for homework? Do they have time for friends (time actually together, not just texting)? What about time for hobbies? When parents focus on the precious resource of time and all of the activities their children both need and want to do, it sets the frame for them to say that their children are allowed to have time to relax with screen-based media as long as it does not take away from these other priorities. Ensuring that the child has at least 8 hours of sleep, after homework and sports, also will set natural limits on screen time.
Parents also can use the frame of development to guide their rules about screen time. If use of an electronic device serves a developmental task, then it is reasonable. If it interferes with a developmental task, then it should be limited. Adolescents (ages 12-20) should be exploring their own identities, establishing independence, deepening social relationships, and learning to manage their impulses. Some interests can be most easily explored with the aid of a computer (such as with programming, art history, or astronomy). Use of cellphones can facilitate teenagers’ being more independent with plans or transportation. Social connections can be supported by texting or FaceTime. Some close friends may be in a different sport or live far away, and it is possible to stay connected only virtually. However, when use of electronic devices keeps the child from engaging with new friends and new interests or from getting into the world to establish real independence (i.e., a job), then there should be limits. In all of these cases, it is critical that adults explain to teenagers what is guiding their thinking about limits on screen time. Open discussions about the great utility and fun that screens can provide, as well as the challenge of keeping those activities in balance with other important activities, helps adolescents set the frame for that rapidly approaching time when they will be making those choices without adult supervision.
Younger children (ages 8-11) should be sampling a wide array of activities and interests and experiencing challenges and eventual mastery across domains. Video games can be very compelling for this age group because they appeal to exactly this drive to master a challenge. Parents want to ensure that their children can have senseless fun, and still have enough time to explore actual activities: social, athletic, creative, and academic. They can be ready to explain the why of rules, but consistent rules, enforced for everyone at home, are most helpful for this age group.
You also can help parents to consider the child’s temperament when thinking about which rules will be appropriate. Anxious children and teenagers may be especially prone to immersive virtual activities that allow them to avoid the stress of real undertakings or interactions. But anxious children may be able to prepare for something anxiety provoking by exploring it virtually first. Youth with ADHD are going to struggle with shifting away from video games or other electronic activities they enjoy that don’t have a natural ending, and will need strict rules and patient support around balanced screen time use. Screen time may play to a child’s strengths, enabling creative children to take in a wide range of art or music and even create their own when other resources are limited.
Finally, all parents should consider what their own screen use is teaching their children. Adolescents are unlikely to listen to their parents’ recommendations if the parents spend hours online after work. Younger children need their parents’ engaged attention: being coaches and cheerleaders for all of their efforts at mastery. You can help parents to imagine rules that the whole family can follow. They can consider how screen time helps them connect with their children, such as watching a favorite program or sport together. They can explore shared interests online together. They can even relax with ridiculous cat videos together! Screen time together is valuable if it supports parents’ connections with their children, while their rules ensure adequate time for sleep, physical activity, and developmental priorities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Lancet Child Adolesc Health. 2018 Nov 1;2(11):783-91.
2. Acta Paediatra. 2018 Apr;107(4):685-93
It has been 2 years since we last wrote about the potential risks to children and adolescents of spending too much time on screens. While there have been studies in the interval that offer us more information about the effects of heavy screen use and the developing brain, there is little certainty about what is optimal for children and adolescents, and less still on how parents might effectively equip their children to make good use of screens without suffering ill effects.
You might recall that back in October of 2016, the American Academy of Pediatrics published screen time guidelines: recommending no screen time for infants and children up to 18 months old, limiting all screen time to 1 hour per day for children up to 5 years old, and 2 hours daily for older children (up to 11 years old), so that it would not interfere with homework, social time, exercise, and sleep. At the time, data suggested that children from 2 to 11 years old were spending an average of 4.5 hours per day on screens (TV, computer, tablets, or smartphones, not counting homework).
The Adolescent Brain Cognitive Development Study began in September 2016 to evaluate the effects of Canadian recommendations for 8- to 11-year-olds (9-11 hours sleep nightly, 1 hour of exercise daily, and 2 hours or less of screen time daily; the study subjects are in the United States). This fall they published their initial results, demonstrating that only 51% get the recommended amount of sleep, only 37% kept their daily screen time to under 2 hours, and only 18% were getting the recommended amount of exercise. Only 5% of children consistently met all three recommendations while 29% of children didn’t meet any of the recommendations.
The researchers assessed the children’s cognitive development and found that after 1 year, those children who met the screen time recommendations, both screen time and sleep, or all three recommendations demonstrated “superior global cognition.” Children were spending an average of 3.7 hours daily on screens, and those children who were spending 2 hours or less on screens performed 4% better on tests of cognitive function than did children spending the average amount of time. Sleep and exercise differences alone did not contribute to significant differences in cognitive function. This study will continue for another 10 years.1
In a much smaller study out of Cincinnati Children’s Hospital, researchers asked parents to describe the amount of time a child spent on reading and in screen-based media activities, then completed MRI scans of the children’s brains.2 They found a strong association between reading time and higher functional connectivity between the parts of the brain responsible for visual word formation and those responsible for language and cognitive control, with a negative correlation between functional connectivity and time spent in screen-based media activities.
While these studies are important pieces of data as we build a deeper understanding about the effects of screen-based media use on children’s cognitive and behavioral development, they do not offer certainty about causality. These studies do not yet clarify whether certain children are especially vulnerable to the untoward effects of heavy screen-based media use. Perhaps the research will someday offer guidelines with certainty, but families need guidance now. Without doubt, digital devices are here to stay, are important to homework, and can facilitate independence, long-distance connections, important technical work-skills, and even senseless fun and relaxation. So we will focus on offering some principles to help you guide young people (or their parents) in approaching screen time thoughtfully.
While recommending no more than 2 hours of daily screen time seems reasonable, it may be more useful to focus on what young people are doing with the rest of their time. Are they getting adequate, restful sleep? Are they able to exercise most days? Do they have enough time for homework? Do they have time for friends (time actually together, not just texting)? What about time for hobbies? When parents focus on the precious resource of time and all of the activities their children both need and want to do, it sets the frame for them to say that their children are allowed to have time to relax with screen-based media as long as it does not take away from these other priorities. Ensuring that the child has at least 8 hours of sleep, after homework and sports, also will set natural limits on screen time.
Parents also can use the frame of development to guide their rules about screen time. If use of an electronic device serves a developmental task, then it is reasonable. If it interferes with a developmental task, then it should be limited. Adolescents (ages 12-20) should be exploring their own identities, establishing independence, deepening social relationships, and learning to manage their impulses. Some interests can be most easily explored with the aid of a computer (such as with programming, art history, or astronomy). Use of cellphones can facilitate teenagers’ being more independent with plans or transportation. Social connections can be supported by texting or FaceTime. Some close friends may be in a different sport or live far away, and it is possible to stay connected only virtually. However, when use of electronic devices keeps the child from engaging with new friends and new interests or from getting into the world to establish real independence (i.e., a job), then there should be limits. In all of these cases, it is critical that adults explain to teenagers what is guiding their thinking about limits on screen time. Open discussions about the great utility and fun that screens can provide, as well as the challenge of keeping those activities in balance with other important activities, helps adolescents set the frame for that rapidly approaching time when they will be making those choices without adult supervision.
Younger children (ages 8-11) should be sampling a wide array of activities and interests and experiencing challenges and eventual mastery across domains. Video games can be very compelling for this age group because they appeal to exactly this drive to master a challenge. Parents want to ensure that their children can have senseless fun, and still have enough time to explore actual activities: social, athletic, creative, and academic. They can be ready to explain the why of rules, but consistent rules, enforced for everyone at home, are most helpful for this age group.
You also can help parents to consider the child’s temperament when thinking about which rules will be appropriate. Anxious children and teenagers may be especially prone to immersive virtual activities that allow them to avoid the stress of real undertakings or interactions. But anxious children may be able to prepare for something anxiety provoking by exploring it virtually first. Youth with ADHD are going to struggle with shifting away from video games or other electronic activities they enjoy that don’t have a natural ending, and will need strict rules and patient support around balanced screen time use. Screen time may play to a child’s strengths, enabling creative children to take in a wide range of art or music and even create their own when other resources are limited.
Finally, all parents should consider what their own screen use is teaching their children. Adolescents are unlikely to listen to their parents’ recommendations if the parents spend hours online after work. Younger children need their parents’ engaged attention: being coaches and cheerleaders for all of their efforts at mastery. You can help parents to imagine rules that the whole family can follow. They can consider how screen time helps them connect with their children, such as watching a favorite program or sport together. They can explore shared interests online together. They can even relax with ridiculous cat videos together! Screen time together is valuable if it supports parents’ connections with their children, while their rules ensure adequate time for sleep, physical activity, and developmental priorities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Lancet Child Adolesc Health. 2018 Nov 1;2(11):783-91.
2. Acta Paediatra. 2018 Apr;107(4):685-93
Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
Women who have a healthier lifestyle during the menopausal transition could significantly reduce their risk of cardiovascular disease, new research suggests.
Because women experience a steeper increase in CVD risk during and after the menopausal transition, researchers analyzed data from the Study of Women’s Health Across the Nation (SWAN), a prospective longitudinal cohort study of 1,143 women aged 42-52 years. The report is in JAHA: Journal of the American Heart Association.
The analysis revealed that women with the highest average Healthy Lifestyle Score – a composite score of dietary quality, levels of physical activity, and smoking – over 10 years of follow-up had a 0.024-mm smaller common carotid artery intima-media thickness and 0.16-mm smaller adventitial diameter, compared to those with the lowest average score. This was after adjustment for confounders and physiological risk factors such as ethnicity, age, menopausal status, body mass index, and cholesterol levels.
“Smoking, unhealthy diet, and lack of physical activity are three well-known modifiable behavioral risk factors for CVD,” wrote Dongqing Wang of the University of Michigan, Ann Arbor, and his coauthors. “Even after adjusting for the lifestyle-related physiological risk factors, the adherence to a healthy lifestyle composed of abstinence from smoking, healthy diet, and regular engagement in physical activity is inversely associated with atherosclerosis in midlife women.”
Women with higher average health lifestyle score also had lower levels of carotid plaque after adjustment for confounding factors, but this was no longer significant after adjustment for physiological risk factors.
The authors analyzed the three components of the healthy lifestyle score separately, and found that not smoking was strongly and significantly associated with lower scores for all three measures of subclinical atherosclerosis. Women who never smoked across the duration of the study had a 49% lower odds of having a high carotid plaque index compared with women who smoked at some point during the follow-up period.
The analysis showed an inverse association between average Alternate Healthy Eating Index score – a measure of diet quality – and smaller common carotid artery adventitial diameter, although after adjustment for BMI this association was no longer statistically significant. Likewise, the association between dietary quality and intima-media thickness was only marginally significant and lost that significance after adjustment for BMI.
Long-term physical activity was only marginally significantly associated with common carotid artery intima-media thickness, but this was not significant after adjustment for physiological risk factors. No association was found between physical activity and common carotid artery adventitial diameter or carotid plaque.
The authors said that 1.7% of the study population managed to stay in the top category for all three components of healthy lifestyle at all three follow-up time points in the study.
“The low prevalence of a healthy lifestyle in midlife women highlights the potential for lifestyle interventions aimed at this vulnerable population,” they wrote.
In particular, they highlighted abstinence from smoking as having the strongest impact on all three measures of subclinical atherosclerosis, which is known to affect women more than men. However, the outcomes from diet and physical activity weren’t so strong: The authors suggested that BMI could partly mediate the effects of healthier diet and greater levels of physical activity.
One strength of the study was its ethnically diverse population, which included African American, Chinese, and Hispanic women in addition to non-Hispanic white women. However, the study was not powered to examine the impacts ethnicity may have had on outcomes, the researchers wrote.
The Study of Women’s Health Across the Nation is supported by the National Institutes of Health. No conflicts of interest were declared.
SOURCE: Wang D et al. JAHA 2018 Nov. 28.
Women who have a healthier lifestyle during the menopausal transition could significantly reduce their risk of cardiovascular disease, new research suggests.
Because women experience a steeper increase in CVD risk during and after the menopausal transition, researchers analyzed data from the Study of Women’s Health Across the Nation (SWAN), a prospective longitudinal cohort study of 1,143 women aged 42-52 years. The report is in JAHA: Journal of the American Heart Association.
The analysis revealed that women with the highest average Healthy Lifestyle Score – a composite score of dietary quality, levels of physical activity, and smoking – over 10 years of follow-up had a 0.024-mm smaller common carotid artery intima-media thickness and 0.16-mm smaller adventitial diameter, compared to those with the lowest average score. This was after adjustment for confounders and physiological risk factors such as ethnicity, age, menopausal status, body mass index, and cholesterol levels.
“Smoking, unhealthy diet, and lack of physical activity are three well-known modifiable behavioral risk factors for CVD,” wrote Dongqing Wang of the University of Michigan, Ann Arbor, and his coauthors. “Even after adjusting for the lifestyle-related physiological risk factors, the adherence to a healthy lifestyle composed of abstinence from smoking, healthy diet, and regular engagement in physical activity is inversely associated with atherosclerosis in midlife women.”
Women with higher average health lifestyle score also had lower levels of carotid plaque after adjustment for confounding factors, but this was no longer significant after adjustment for physiological risk factors.
The authors analyzed the three components of the healthy lifestyle score separately, and found that not smoking was strongly and significantly associated with lower scores for all three measures of subclinical atherosclerosis. Women who never smoked across the duration of the study had a 49% lower odds of having a high carotid plaque index compared with women who smoked at some point during the follow-up period.
The analysis showed an inverse association between average Alternate Healthy Eating Index score – a measure of diet quality – and smaller common carotid artery adventitial diameter, although after adjustment for BMI this association was no longer statistically significant. Likewise, the association between dietary quality and intima-media thickness was only marginally significant and lost that significance after adjustment for BMI.
Long-term physical activity was only marginally significantly associated with common carotid artery intima-media thickness, but this was not significant after adjustment for physiological risk factors. No association was found between physical activity and common carotid artery adventitial diameter or carotid plaque.
The authors said that 1.7% of the study population managed to stay in the top category for all three components of healthy lifestyle at all three follow-up time points in the study.
“The low prevalence of a healthy lifestyle in midlife women highlights the potential for lifestyle interventions aimed at this vulnerable population,” they wrote.
In particular, they highlighted abstinence from smoking as having the strongest impact on all three measures of subclinical atherosclerosis, which is known to affect women more than men. However, the outcomes from diet and physical activity weren’t so strong: The authors suggested that BMI could partly mediate the effects of healthier diet and greater levels of physical activity.
One strength of the study was its ethnically diverse population, which included African American, Chinese, and Hispanic women in addition to non-Hispanic white women. However, the study was not powered to examine the impacts ethnicity may have had on outcomes, the researchers wrote.
The Study of Women’s Health Across the Nation is supported by the National Institutes of Health. No conflicts of interest were declared.
SOURCE: Wang D et al. JAHA 2018 Nov. 28.
Women who have a healthier lifestyle during the menopausal transition could significantly reduce their risk of cardiovascular disease, new research suggests.
Because women experience a steeper increase in CVD risk during and after the menopausal transition, researchers analyzed data from the Study of Women’s Health Across the Nation (SWAN), a prospective longitudinal cohort study of 1,143 women aged 42-52 years. The report is in JAHA: Journal of the American Heart Association.
The analysis revealed that women with the highest average Healthy Lifestyle Score – a composite score of dietary quality, levels of physical activity, and smoking – over 10 years of follow-up had a 0.024-mm smaller common carotid artery intima-media thickness and 0.16-mm smaller adventitial diameter, compared to those with the lowest average score. This was after adjustment for confounders and physiological risk factors such as ethnicity, age, menopausal status, body mass index, and cholesterol levels.
“Smoking, unhealthy diet, and lack of physical activity are three well-known modifiable behavioral risk factors for CVD,” wrote Dongqing Wang of the University of Michigan, Ann Arbor, and his coauthors. “Even after adjusting for the lifestyle-related physiological risk factors, the adherence to a healthy lifestyle composed of abstinence from smoking, healthy diet, and regular engagement in physical activity is inversely associated with atherosclerosis in midlife women.”
Women with higher average health lifestyle score also had lower levels of carotid plaque after adjustment for confounding factors, but this was no longer significant after adjustment for physiological risk factors.
The authors analyzed the three components of the healthy lifestyle score separately, and found that not smoking was strongly and significantly associated with lower scores for all three measures of subclinical atherosclerosis. Women who never smoked across the duration of the study had a 49% lower odds of having a high carotid plaque index compared with women who smoked at some point during the follow-up period.
The analysis showed an inverse association between average Alternate Healthy Eating Index score – a measure of diet quality – and smaller common carotid artery adventitial diameter, although after adjustment for BMI this association was no longer statistically significant. Likewise, the association between dietary quality and intima-media thickness was only marginally significant and lost that significance after adjustment for BMI.
Long-term physical activity was only marginally significantly associated with common carotid artery intima-media thickness, but this was not significant after adjustment for physiological risk factors. No association was found between physical activity and common carotid artery adventitial diameter or carotid plaque.
The authors said that 1.7% of the study population managed to stay in the top category for all three components of healthy lifestyle at all three follow-up time points in the study.
“The low prevalence of a healthy lifestyle in midlife women highlights the potential for lifestyle interventions aimed at this vulnerable population,” they wrote.
In particular, they highlighted abstinence from smoking as having the strongest impact on all three measures of subclinical atherosclerosis, which is known to affect women more than men. However, the outcomes from diet and physical activity weren’t so strong: The authors suggested that BMI could partly mediate the effects of healthier diet and greater levels of physical activity.
One strength of the study was its ethnically diverse population, which included African American, Chinese, and Hispanic women in addition to non-Hispanic white women. However, the study was not powered to examine the impacts ethnicity may have had on outcomes, the researchers wrote.
The Study of Women’s Health Across the Nation is supported by the National Institutes of Health. No conflicts of interest were declared.
SOURCE: Wang D et al. JAHA 2018 Nov. 28.
FROM JAHA: JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point: .
Major finding: Following a healthier diet and not smoking were significantly linked with lower subclinical carotid atherosclerosis in menopausal women.
Study details: A prospective, longitudinal cohort study of 1,143 women.
Disclosures: The Study of Women’s Health Across the Nation is supported by the National Institutes of Health. No conflicts of interest were declared.
Source: Wang D et al. JAHA 2018 Nov. 28.
Teva recalls valsartan-containing combo pills
Teva Pharmaceuticals has issued a voluntary recall of all unexpired lots of amlodipine/valsartan and amlodipine/valsartan /hydrochlorothiazide combination tablets because of contamination with N-nitrosodiethylamine (NDEA), according to a company announcement posted on the website of the Food and Drug Administration.
The contaminant was detected at above acceptable limits in the valsartan component of the pills, which were manufactured by Mylan India. Mylan has already recalled its own valsartan-containing products. Teva has recalled other valsartan-containing products in recent months because of the presence of N-nitrosodimethylamine (NDMA).
Teva has now recalled all of its unexpired valsartan-containing products from the U.S. market. The Teva recall affects almost 50 lots, all distributed nationwide to Teva’s direct accounts, which include wholesalers, retailers, repackagers, Veterans Affairs pharmacies, and others. The lot and National Drug Code numbers of the affected products, as well as the company’s announcement, are available on the Food and Drug Administration website.
Teva is notifying its U.S. customers by certified mail and arranging for returns and reimbursements. Distribution of recalled products should stop immediately, the company said.
Patients, however, should contact their pharmacists or physicians for alternative options before stopping treatment. “The risk of harm to a patient’s health may be higher if the treatment is stopped immediately without any comparable alternative treatment,” Teva said in its announcement.
The company plans regular updates as it works through the problem. NDEA is typically found in very small amounts in certain foods, drinking water, air pollution, and certain industrial processes.
Questions and other concerns can be directed to Teva directly at 888-838-2872 or via email to [email protected].
Teva Pharmaceuticals has issued a voluntary recall of all unexpired lots of amlodipine/valsartan and amlodipine/valsartan /hydrochlorothiazide combination tablets because of contamination with N-nitrosodiethylamine (NDEA), according to a company announcement posted on the website of the Food and Drug Administration.
The contaminant was detected at above acceptable limits in the valsartan component of the pills, which were manufactured by Mylan India. Mylan has already recalled its own valsartan-containing products. Teva has recalled other valsartan-containing products in recent months because of the presence of N-nitrosodimethylamine (NDMA).
Teva has now recalled all of its unexpired valsartan-containing products from the U.S. market. The Teva recall affects almost 50 lots, all distributed nationwide to Teva’s direct accounts, which include wholesalers, retailers, repackagers, Veterans Affairs pharmacies, and others. The lot and National Drug Code numbers of the affected products, as well as the company’s announcement, are available on the Food and Drug Administration website.
Teva is notifying its U.S. customers by certified mail and arranging for returns and reimbursements. Distribution of recalled products should stop immediately, the company said.
Patients, however, should contact their pharmacists or physicians for alternative options before stopping treatment. “The risk of harm to a patient’s health may be higher if the treatment is stopped immediately without any comparable alternative treatment,” Teva said in its announcement.
The company plans regular updates as it works through the problem. NDEA is typically found in very small amounts in certain foods, drinking water, air pollution, and certain industrial processes.
Questions and other concerns can be directed to Teva directly at 888-838-2872 or via email to [email protected].
Teva Pharmaceuticals has issued a voluntary recall of all unexpired lots of amlodipine/valsartan and amlodipine/valsartan /hydrochlorothiazide combination tablets because of contamination with N-nitrosodiethylamine (NDEA), according to a company announcement posted on the website of the Food and Drug Administration.
The contaminant was detected at above acceptable limits in the valsartan component of the pills, which were manufactured by Mylan India. Mylan has already recalled its own valsartan-containing products. Teva has recalled other valsartan-containing products in recent months because of the presence of N-nitrosodimethylamine (NDMA).
Teva has now recalled all of its unexpired valsartan-containing products from the U.S. market. The Teva recall affects almost 50 lots, all distributed nationwide to Teva’s direct accounts, which include wholesalers, retailers, repackagers, Veterans Affairs pharmacies, and others. The lot and National Drug Code numbers of the affected products, as well as the company’s announcement, are available on the Food and Drug Administration website.
Teva is notifying its U.S. customers by certified mail and arranging for returns and reimbursements. Distribution of recalled products should stop immediately, the company said.
Patients, however, should contact their pharmacists or physicians for alternative options before stopping treatment. “The risk of harm to a patient’s health may be higher if the treatment is stopped immediately without any comparable alternative treatment,” Teva said in its announcement.
The company plans regular updates as it works through the problem. NDEA is typically found in very small amounts in certain foods, drinking water, air pollution, and certain industrial processes.
Questions and other concerns can be directed to Teva directly at 888-838-2872 or via email to [email protected].
Ashwin Patkar: Opioid Epidemic
And later, Dr. RK knows that some of your patients are blue this time of year, she joins us to talk about what that is and what you can do about it.
In an evidence-based review, Dr. Patkar overviews the issue and includes dosages and references for FDA-approved buprenophine and burprenorphine/naloxone formulation. Click here to read that review.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
And later, Dr. RK knows that some of your patients are blue this time of year, she joins us to talk about what that is and what you can do about it.
In an evidence-based review, Dr. Patkar overviews the issue and includes dosages and references for FDA-approved buprenophine and burprenorphine/naloxone formulation. Click here to read that review.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
And later, Dr. RK knows that some of your patients are blue this time of year, she joins us to talk about what that is and what you can do about it.
In an evidence-based review, Dr. Patkar overviews the issue and includes dosages and references for FDA-approved buprenophine and burprenorphine/naloxone formulation. Click here to read that review.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Pot and Pain? We’ll see
Also today, comorbid depression and anxiety are linked to double the risk of diabetes, abortion rates continue to fall, and staying up-to-date on screening decreases risk for colorectal cancer death.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, comorbid depression and anxiety are linked to double the risk of diabetes, abortion rates continue to fall, and staying up-to-date on screening decreases risk for colorectal cancer death.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, comorbid depression and anxiety are linked to double the risk of diabetes, abortion rates continue to fall, and staying up-to-date on screening decreases risk for colorectal cancer death.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
EC approves mogamulizumab for MF, SS
The European Commission (EC) has granted marketing authorization for mogamulizumab (Poteligeo), a humanized monoclonal antibody directed against CCR4.
This means mogamulizumab is approved for use in adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
This approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
Kyowa Kirin plans to launch mogamulizumab in various European markets starting next year.
The EC’s decision to approve mogamulizumab was supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to an independent review committee, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
The European Commission (EC) has granted marketing authorization for mogamulizumab (Poteligeo), a humanized monoclonal antibody directed against CCR4.
This means mogamulizumab is approved for use in adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
This approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
Kyowa Kirin plans to launch mogamulizumab in various European markets starting next year.
The EC’s decision to approve mogamulizumab was supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to an independent review committee, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
The European Commission (EC) has granted marketing authorization for mogamulizumab (Poteligeo), a humanized monoclonal antibody directed against CCR4.
This means mogamulizumab is approved for use in adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
This approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
Kyowa Kirin plans to launch mogamulizumab in various European markets starting next year.
The EC’s decision to approve mogamulizumab was supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to an independent review committee, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
Canada expands approval for antihemophilic factor
Health Canada has extended the approved indication for Adynovate, a recombinant pegylated factor VIII (FVIII) product, in patients with hemophilia A.
The product is now approved for use in hemophilia A patients under the age of 12 for the control and prevention of bleeding episodes, as prophylaxis to prevent or reduce the frequency of bleeding, and for perioperative management of bleeding.
Adynovate (formerly BAX 855) was first approved in Canada in November 2016. At that time, it was authorized for use in hemophilia A patients age 12 and older as on-demand treatment, as prophylaxis, and for perioperative management.
Adynovate is built on the full-length Advate molecule, but Adynovate leverages pegylation technology designed to extend the amount of FVIII available for use in the body.
The technology was selected because it maintains the integrity of the parent molecule (Advate) while reducing the time at which the body clears Adynovate, resulting in an extended circulating half-life.
Health Canada’s decision to expand the indication for Adynovate is supported by results from a phase 3 trial of pediatric patients as well as a phase 3 trial of patients undergoing surgery.
Pediatric trial
The pediatric trial enrolled 73 patients, ages 1 to 11, with previously treated hemophilia A.
Sixty-six patients received twice-weekly prophylaxis with Adynovate (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median annualized bleeding rate was 2.0 for all bleeds and 0 for both joint and spontaneous bleeds.
Thirty-eight percent of patients did not have any bleeding episodes, 67% had no spontaneous bleeds, and 73% had no joint bleeds.
One patient developed inhibitors, but there were no other treatment-related adverse events.
Results from this trial were published in Haemophilia in November 2016 and are available in the Canadian product monograph for Adynovate.
Perioperative study
The perioperative study included 15 patients, ages 19 to 52, with severe hemophilia A who were undergoing surgical procedures (11 of them major and four minor).
The patients received Adynovate at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or Adynovate incremental recovery for minor procedures.
Intraoperative and perioperative hemostatic efficacy of Adynovate was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Postoperatively (day 1 after the procedure), hemostatic efficacy was rated “good” for one procedure and “excellent” for the rest. The “good” rating meant that postoperative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016 and are available in the Canadian product monograph for Adynovate.
Health Canada has extended the approved indication for Adynovate, a recombinant pegylated factor VIII (FVIII) product, in patients with hemophilia A.
The product is now approved for use in hemophilia A patients under the age of 12 for the control and prevention of bleeding episodes, as prophylaxis to prevent or reduce the frequency of bleeding, and for perioperative management of bleeding.
Adynovate (formerly BAX 855) was first approved in Canada in November 2016. At that time, it was authorized for use in hemophilia A patients age 12 and older as on-demand treatment, as prophylaxis, and for perioperative management.
Adynovate is built on the full-length Advate molecule, but Adynovate leverages pegylation technology designed to extend the amount of FVIII available for use in the body.
The technology was selected because it maintains the integrity of the parent molecule (Advate) while reducing the time at which the body clears Adynovate, resulting in an extended circulating half-life.
Health Canada’s decision to expand the indication for Adynovate is supported by results from a phase 3 trial of pediatric patients as well as a phase 3 trial of patients undergoing surgery.
Pediatric trial
The pediatric trial enrolled 73 patients, ages 1 to 11, with previously treated hemophilia A.
Sixty-six patients received twice-weekly prophylaxis with Adynovate (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median annualized bleeding rate was 2.0 for all bleeds and 0 for both joint and spontaneous bleeds.
Thirty-eight percent of patients did not have any bleeding episodes, 67% had no spontaneous bleeds, and 73% had no joint bleeds.
One patient developed inhibitors, but there were no other treatment-related adverse events.
Results from this trial were published in Haemophilia in November 2016 and are available in the Canadian product monograph for Adynovate.
Perioperative study
The perioperative study included 15 patients, ages 19 to 52, with severe hemophilia A who were undergoing surgical procedures (11 of them major and four minor).
The patients received Adynovate at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or Adynovate incremental recovery for minor procedures.
Intraoperative and perioperative hemostatic efficacy of Adynovate was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Postoperatively (day 1 after the procedure), hemostatic efficacy was rated “good” for one procedure and “excellent” for the rest. The “good” rating meant that postoperative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016 and are available in the Canadian product monograph for Adynovate.
Health Canada has extended the approved indication for Adynovate, a recombinant pegylated factor VIII (FVIII) product, in patients with hemophilia A.
The product is now approved for use in hemophilia A patients under the age of 12 for the control and prevention of bleeding episodes, as prophylaxis to prevent or reduce the frequency of bleeding, and for perioperative management of bleeding.
Adynovate (formerly BAX 855) was first approved in Canada in November 2016. At that time, it was authorized for use in hemophilia A patients age 12 and older as on-demand treatment, as prophylaxis, and for perioperative management.
Adynovate is built on the full-length Advate molecule, but Adynovate leverages pegylation technology designed to extend the amount of FVIII available for use in the body.
The technology was selected because it maintains the integrity of the parent molecule (Advate) while reducing the time at which the body clears Adynovate, resulting in an extended circulating half-life.
Health Canada’s decision to expand the indication for Adynovate is supported by results from a phase 3 trial of pediatric patients as well as a phase 3 trial of patients undergoing surgery.
Pediatric trial
The pediatric trial enrolled 73 patients, ages 1 to 11, with previously treated hemophilia A.
Sixty-six patients received twice-weekly prophylaxis with Adynovate (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median annualized bleeding rate was 2.0 for all bleeds and 0 for both joint and spontaneous bleeds.
Thirty-eight percent of patients did not have any bleeding episodes, 67% had no spontaneous bleeds, and 73% had no joint bleeds.
One patient developed inhibitors, but there were no other treatment-related adverse events.
Results from this trial were published in Haemophilia in November 2016 and are available in the Canadian product monograph for Adynovate.
Perioperative study
The perioperative study included 15 patients, ages 19 to 52, with severe hemophilia A who were undergoing surgical procedures (11 of them major and four minor).
The patients received Adynovate at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or Adynovate incremental recovery for minor procedures.
Intraoperative and perioperative hemostatic efficacy of Adynovate was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Postoperatively (day 1 after the procedure), hemostatic efficacy was rated “good” for one procedure and “excellent” for the rest. The “good” rating meant that postoperative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016 and are available in the Canadian product monograph for Adynovate.
Pegfilgrastim biosimilar approved by EC
The European Commission (EC) has granted marketing authorization for Sandoz’s pegfilgrastim product Ziextenzo®, a biosimilar of Amgen’s Neulasta.
Ziextenzo is approved for the same use as the reference medicine—to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval was based on research suggesting Ziextenzo is comparable to Neulasta in terms of safety, efficacy, pharmacokinetics, and pharmacodynamics.1,2,3,4
1. Blackwell K. et al. Pooled analysis of two randomized, double-blind trials comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Ann Oncol 28, 2272-2277 (2017).
2. Nakov R. et al. Proposed biosimilar pegfilgrastim LA-EP2006 shows similarity in pharmacokinetics and pharmacodynamics to reference pegfilgrastim in healthy subjects. 2017 San Antonio Breast Cancer Symposium, abstract P3-14-10.
3. Blackwell K. et al. A Comparison of Proposed Biosimilar LA-EP2006 and Reference Pegfilgrastim for the Prevention of Neutropenia in Patients With Early-Stage Breast Cancer Receiving Myelosuppressive Adjuvant or Neoadjuvant Chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a Phase III, Randomized, Double-Blind Trial. Oncologist 21, 789-794 (2016).
4. Harbeck N. et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol 12, 1359-1367 (2016).
The European Commission (EC) has granted marketing authorization for Sandoz’s pegfilgrastim product Ziextenzo®, a biosimilar of Amgen’s Neulasta.
Ziextenzo is approved for the same use as the reference medicine—to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval was based on research suggesting Ziextenzo is comparable to Neulasta in terms of safety, efficacy, pharmacokinetics, and pharmacodynamics.1,2,3,4
1. Blackwell K. et al. Pooled analysis of two randomized, double-blind trials comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Ann Oncol 28, 2272-2277 (2017).
2. Nakov R. et al. Proposed biosimilar pegfilgrastim LA-EP2006 shows similarity in pharmacokinetics and pharmacodynamics to reference pegfilgrastim in healthy subjects. 2017 San Antonio Breast Cancer Symposium, abstract P3-14-10.
3. Blackwell K. et al. A Comparison of Proposed Biosimilar LA-EP2006 and Reference Pegfilgrastim for the Prevention of Neutropenia in Patients With Early-Stage Breast Cancer Receiving Myelosuppressive Adjuvant or Neoadjuvant Chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a Phase III, Randomized, Double-Blind Trial. Oncologist 21, 789-794 (2016).
4. Harbeck N. et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol 12, 1359-1367 (2016).
The European Commission (EC) has granted marketing authorization for Sandoz’s pegfilgrastim product Ziextenzo®, a biosimilar of Amgen’s Neulasta.
Ziextenzo is approved for the same use as the reference medicine—to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
The approval is valid in all countries of the European Union as well as Norway, Iceland, and Liechtenstein.
The EC’s approval was based on research suggesting Ziextenzo is comparable to Neulasta in terms of safety, efficacy, pharmacokinetics, and pharmacodynamics.1,2,3,4
1. Blackwell K. et al. Pooled analysis of two randomized, double-blind trials comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Ann Oncol 28, 2272-2277 (2017).
2. Nakov R. et al. Proposed biosimilar pegfilgrastim LA-EP2006 shows similarity in pharmacokinetics and pharmacodynamics to reference pegfilgrastim in healthy subjects. 2017 San Antonio Breast Cancer Symposium, abstract P3-14-10.
3. Blackwell K. et al. A Comparison of Proposed Biosimilar LA-EP2006 and Reference Pegfilgrastim for the Prevention of Neutropenia in Patients With Early-Stage Breast Cancer Receiving Myelosuppressive Adjuvant or Neoadjuvant Chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a Phase III, Randomized, Double-Blind Trial. Oncologist 21, 789-794 (2016).
4. Harbeck N. et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol 12, 1359-1367 (2016).
Lower Extremity Injuries in Ice Hockey: Current Concepts
ABSTRACT
Ice hockey is a fast-paced, collision sport requiring tremendous skill and finesse, yet ice hockey can be a harsh and violent game. It has one of the highest musculoskeletal injury rates in all of competitive sports. Razor sharp skates, aluminum sticks and boards made from high density polyethylene (HDPE), all contribute to the intrinsic hazards of the game. The objective of this article is to review evaluation, management, and return-to-the-rink guidelines after common lower extremity ice hockey injuries.
“Hockey is a fast body-contact game played by men with clubs in their hands and knives laced to their feet, since the skates are razor sharp, and before the evening is over it is almost a certainty that someone will be hurt and will fleck the ice with a generous contribution of gore before he is led away to be hemstitched together again.” —Paul Gallico in Farewell to Sport (1938)
Ice hockey is a collision sport with player speeds in excess of 30 miles/hour, on a sheet of ice surrounded by unforgiving boards, with a vulcanized rubber puck moving at speeds approaching 100 miles/hour.1-3 Understanding injuries specific to this fast-paced sport is an essential part of being a team physician at any level of competitive ice hockey. We are continuing to improve our ability to correctly identify and treat injuries in ice hockey players.2,4 On the prevention side, rule changes in hockey have been implemented, such as raising the age to allow checking and penalties for deliberate hits to the head and checking from behind, to make the game safer to play.3 Additionally, advancements in biomechanical research and 3D modeling are providing new insights into the pathoanatomy of the hip joint, which can be utilized for surgical planning in hockey players and goalies suffering from symptomatic femoroacetabular impingement (FAI) of the hip.5
During the 2010 Winter Olympics, more than 30% of ice hockey players were injured, which was the highest percentage amongst all competing sports.6 They also tallied the highest percentage of player-to-player injuries during the Olympics of any sport. Consequently, the team physician covering ice hockey should be prepared to manage upper and lower extremity musculoskeletal injuries, but also concussions, cervical spine injuries, and ocular and dental trauma.2
One of the earliest epidemiological studies of ice hockey injuries looked at elite Danish hockey players over 2 seasons and found that head trauma accounted for 28% of all injuries, followed by lower extremity injuries at 27% with upper extremity injuries accounting for 19%.7 More recent epidemiological studies have shown similar rates based on body region while further defining individual diagnoses and their incidence. This should help clinicians and researchers develop prevention strategies, as well as improve treatments to optimize player outcomes and return to sport.8,9 Our group recently reviewed the evaluation and management of common head, neck, and shoulder injuries at all competitive levels of ice hockey, and this article serves to complement the former by focusing on lower extremity injuries (Table).2
Continue to: Hip and groin...
EVALUATION AND MANAGEMENT OF COMMON LOWER EXTREMITY HOCKEY INJURIES
HIP INJURIES
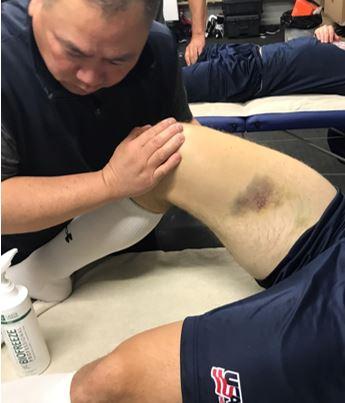
Hip and groin injuries are very common amongst this group of athletes and account for approximately 9% of all ice hockey injuries.1 Unfortunately, they are also known for their high recurrence rates, which may be in part due to delayed diagnosis, inadequate rest and rehabilitation, as well as the extreme loads that are placed on the hip during competition.10,11 In hockey, the most commonly reported hip injuries include goaltender’s hip, FAI, sports hernia/hockey groin syndrome, adductor strains, hip pointer, and quadriceps contusions. Dalton and colleagues12 performed the largest epidemiological study to date on hip and groin injuries amongst National Collegiate Athletic Association ice hockey players and reported that the most common injury mechanism was noncontact in nature. Contact injuries accounted for 13% (55 of 421) in men’s ice hockey players while less than 4% (4 of 114) injuries in female ice hockey players, which is likely attributed to a no checking rule in the women’s division. Some of these hip and groin injuries are difficult to diagnose so it is important for the team physician to perform a thorough history and physical examination. Advanced imaging (magnetic resonance imaging [MRI] or a computed tomography (CT) scan with 3D reconstructions) may be necessary to make the correct diagnosis. This is important for providing proper treatment as well as setting player expectations for return to sport.12
Table 1. Return-to-Play Guidelines for Common Lower Extremity Ice Hockey Injuries | ||
Lower Extremity Injury | Treatment Options | Return-to-the-Rink Goal |
FAI | In-season: injection, physical therapy program, NSAIDS. Off-season or unable to play: requires arthroscopic surgery | Nonoperative can take up to 6 weeks. Surgical depends on what is fixed but goal is 4 months to return to ice24,26
|
Sports hernia/athletic pubalgia
| In-season: physical therapy program, NSAIDS Off-season or unable to play requiring surgery. Essential to make sure no other pathology (eg, FAI, osteitis pubis, adductor strain) to maximize success
| Nonoperative 6-8 wk trial of physical therapy Operative: depends if concomitant FAI but in isolation goal is 3-4 mo33,54
|
Adductor strains | Ice, NSAIDS, physical therapy, use of Hypervolt Hyperice | Depends on position (goalie vs skater) and severity; can take up to 4-8 wk to return to ice. Want 70% strength and painless ROM to skate successfully;55 in chronic cases, may take up to 6 mo35
|
Quadriceps contusion
| Hinged knee brace to maintain 120° of flexion, ice, compression wrap.
| When player regains motion and strength, return to ice can be as fast a couple of days or as long as 3 wk8,46
|
MCL | Hinged knee brace, shin pad modification, ice, NSAIDs | Depends on Grade; if Grade I, 1-2 wk; Grade II, 2-4 wk; Grade III, 4-6 wk8
|
ACL | Surgery autograft BTB autograft soft tissue
| 9-10 mo41 |
Meniscus tear | Depends on type of tear and seasonal timing (in-season or off-season) | If surgical, 3-4 mo; if repair, 4-6 wk if partial menisectomy
|
High ankle sprain
| Cam boot, NSAIDS, ice and physical therapy
| 6 wk49 |
Boot top laceration | Repair of cut structures, depends on depth and what is injured; best treatment is prevention with Kevlar socks | If laceration is deep and severs any medial tendons/vascular structures, return to ice can be ≥6 mo
|
Lace bite
| Bunga pad, ice, diclofenac gel
| Couple of days to up to 2 wk in recalcitrant cases3 |
Abbreviations: ACL, anterior cruciate ligament; BTB, bone-patellar tendon-bone; Cam, controlled ankle motion boot; MCL, medial collateral ligament; FAI, femoroacetabular impingement; NSAIDS, nonsteroidal anti-inflammatory drugs; ROM, range of motion.
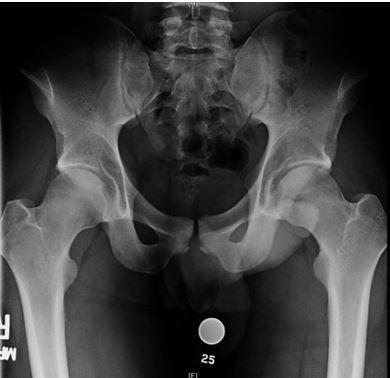
Throughout the hockey community, FAI is being examined as a possible source of symptomatic hip pain amongst players at all levels. A recent study, which utilized the National Hockey League (NHL) injury surveillance database, reported that FAI accounted for 5.3% of all hip and groin injuries.13 The etiology of FAI is thought to arise from a combination of genetic predisposition coupled with repetitive axial loading/hip flexion. This causes a bony overgrowth of the proximal femoral physes resulting in a cam deformity (Figure 1).5,14 The abnormal bony anatomy allows for impingement between the acetabulum and proximal femur, which can injure the labrum and articular cartilage of the hip joint.
In the recent study by Ross and colleagues,15 the authors focused on symptomatic hip impingement in ice hockey goalies.15 Goaltender’s hip may be the result of the “butterfly style,” which is a technique of goaltending that emphasizes guarding the lower part of the goal. The goalie drops to his/her knees and internally rotates the hips to allow the leg pads to be parallel to the ice. This style acquired the name butterfly because of the resemblance of the spread goalie pads to a butterfly’s wings. Bedi and associates16 have evaluated hip biomechanics using 3D-generated bone models and showed in their study that arthroscopic treatment can improve hip kinematics and range of motion.
Plain radiographs showed that 90% (61 of 68) of hockey goalies had an elevated alpha angle signifying a femoral cam-type deformity.15 Goalies had a significantly lower mean lateral center-edge angle (27.3° vs 29.6°; P = .03) and 13.2% of them were found to have acetabular dysplasia (lateral center-edge angle<20°) compared to only 3% of positional players. The CT scan measurements demonstrated that hockey goalies have a unique cam-type deformity that is located more lateral (1:00 o’clock vs 1:45 o’clock; P < .0001) along the proximal femur, an elevated maximum alpha angle (80.9° vs 68.6°; P < .0001) and loss of offset, when compared to positional players. These findings provide an anatomical basis in support of reports that goaltenders are more likely to experience intra-articular hip injuries compared to other positional players.13
Regardless of position, symptomatic FAI in a hockey player is generally a problem that slowly builds and is made worse with activity.17 On examination, the player may have limited hip flexion and internal rotation, as well as weakness compared to the contralateral side when testing hip flexion and abduction.18,19 Plain radiographs plus MRI or CT allow for proper characterization and diagnosis (to include underlying chondrolabral pathology).20,21
In the young athlete, initial management includes physical therapy, which focuses on core strengthening. Emphasis is placed on hip flexion and extension, as well as abduction and external rotation with the goal of reducing symptoms and avoiding injuries.22 A similar approach may be applied to the elite athlete, but failure of nonoperative management may necessitate surgical intervention. Hip arthroscopy continues to grow in popularity over open surgical dislocation with low complication rate and high return-to-play rate.23-25
For the in-season athlete, attempts to continue to play can be assisted with the role of an intra-articular corticosteroid injection, which can help calm inflammation within the hip joint and mitigate pain, while rehabilitation focuses on core stabilization, postural retraining and focusing on any muscle imbalances that might be present. For positional players, ice time and shift duration can be adjusted to give the player’s hip a period of rest; meanwhile, for goaltenders, shot volumes in practice can be decreased.
Continue to: For athletes who...
For athletes who fail nonoperative care, surgical treatment varies depending on underlying hip pathology and may include femoroplasty, acetabuloplasty, and microfracture as well as labral repair or debridement. Though data are limited, Philippon and colleagues26 have published promising results in a case series of 28 NHL players after surgical intervention for FAI. All players returned to sport at an average of 3.8 months and players who had surgery within 1 year of injury returned on average 1.1 months sooner than those who waited more than 1 year. Rehabilitation protocol varies between goaltenders compared to defensemen and offensive players due to the different demands required for blocking shots on goal.27
One of the most challenging injuries to correctly identify in the hip area is athletic pubalgia (also referred to as sports hernia or core muscle injury) because pain in the groin may be referred from the lumbar spine, hip joint, urologic, or perineal etiologies.28 Sports hernias involve dilatation of the external ring of the inguinal canal and thinning of the posterior wall. Players may report to the athletic trainer or team physician with a complaint of groin pain that is worse when pushing off with their skate or taking a slap shot.29 On exam, pain can be reproduced by hip extension, contralateral torso rotation, or with a resisted sit-up with palpation of the inferolateral edge of the distal rectus abdominus.30 An MRI with specific sequences centered over the pubic symphysis is usually warranted to aid in the workup of sports hernia. An MRI in these cases may also demonstrate avulsions of the rectus abdominus.31
Most of these injuries are managed conservatively but can warrant surgical intervention if the symptoms persist. In the study by Jakoi and colleagues,32 they identified 43 ice hockey players over an 8-year period (2001-2008) who had repairs of their sports hernias and assessed the statistics during the 2 years prior and 2 years after surgery. The authors found that 80% of these players were able to return to the ice for 2 or more full seasons. The return-to-sport rate was comparable to other sports after sports hernia repair, but players who had played in ≥7 seasons demonstrated a greater decrease in number of games played, goals, assists and time on ice compared to those who had played in ≤6 seasons prior to the time of injury. Between 1989 and 2000, 22 NHL players who failed to respond to nonoperative management of their groin injuries underwent surgical exploration.29 At the time of surgical exploration, their hockey groin syndrome, consisted of small tears in the external oblique aponeurosis through which branches of the ilioinguinal or iliohypogastric could be identified. These surgical procedures were all through a standard inguinal approach and the perforating neurovascular structures were excised, while the main trunk of the ilioinguinal nerve was ablated and the external oblique aponeurosis was repaired and reinforced with Goretex (W.L. Gore & Associates Inc, Flagstaff, AZ). At follow-up, 18 of the 22 players (82%) had no pain and 19 (86%) were able to resume their careers in the NHL.29 Ice hockey players with sports hernias or hockey groin syndrome often return to the sport, but it is important to identify these problems early so that surgical options can be discussed if the player fails conservative management. It is also critical to make sure that all pathology is identified, because in players with mixed sports hernia and FAI, return-to-play results improve when both issues are addressed. In a study of athletes (some of whom were ice hockey players), who had both FAI and sports hernia, and only hernia/pubalgia surgery was performed, 25% of these athletes returned to sport. If only FAI was addressed, 50% of the athletes returned to sport; however, when hernia and FAI were treated, 89% returned to play.33
Adductor strains includes injury to the adductor muscles, pectineus, obturator externus and gracilis, and are prevalent in ice hockey players. A study of elite Swedish ice hockey players published in 1988 reported that adductor strains accounted for 10% (10 of 95) of all injuries.34 Given the prevalence of these injuries, considerable research has been dedicated to understanding their mechanism and prevention.35 Adductor strains within the ice hockey population have been attributed to the eccentric forces on the adductors when players attempt to decelerate the leg during a stride.36 A study of NHL players revealed that a ratio <80% of adductor-to-abductor muscle is the best predictor of a groin strain.37
These injuries are also well known for their recurrence rates, as was the case in an NHL study where 4 of the 9 adductor strains (44%) were recurrent injuries.37 The authors attributed the recurrence to an incomplete rehabilitation program and an accelerated return to sport. This was followed by an NHL prevention program that spanned 2 seasons and analyzed 58 players whose adductor-to-abductor ratio was <80% and placed them into a 6-week intervention program during the preseason.37 Only 3 players sustained an adductor strain in the 2 subsequent seasons after the intervention, compared to 11 strains in the previous 2 seasons. Thus, early identification of muscle strength imbalance coupled with an appropriate intervention program has proven to be an effective means of reducing adductor strains in this at-risk population.
Continue to: Contact injuries may...
Contact injuries may vary with checking into the boards being unique to men’s ice hockey. Hip pointers occur as a result of a direct compression injury to the iliac crest, which causes trauma to the bone but also to the overlying hip abductor musculature, and represent roughly 2.4% of ice hockey injuries.23 The resulting contusion may cause a local hematoma formation. Early identification of the injury plus treatment with RICE (rest, ice, compression, elevation) coupled with crutches to limit weight-bearing status may minimize soft tissue trauma and swelling, and ultimately aid in pain control and return to sport.38 Hip abductor strengthening, added padding over the injured area, as well as a compressive hip spica wrapping, have all been suggested to expedite return to play and help prevent recurrence of the hip pointer.8
KNEE INJURIES
Injury to the medial collateral ligament (MCL) is the most commonly reported knee injury (Figure 2) and second only to concussion amongst all injuries in National Collegiate Athletic Association ice hockey players.8,39 The mechanism of injury typically involves a valgus force on the knee, which is often caused by collision into another player.39 Valgus stress testing with the knee in 30° of flexion is used to grade the severity of injury (Grade I: 0-5 mm of medial opening; Grade II: 5-10 mm of medial opening; Grade III: >10 mm of medial opening).39 One study that followed a single college hockey team for 8 seasons reported that 77% of injuries (10 of 13) occurred during player-to-player collision,39 with 5 being Grade 1 injuries, 6 Grade 2 injuries, 1 Grade 3; information was missing for 1 player. Nonoperative management of incomplete injuries, grade 1 and 2 sprains, with RICE and early physical therapy intervention to work on knee range of motion and quadriceps strengthening typically helps the player return to sport within days for grade 1 and 2 injuries to 3 weeks for grade 2 injuries. Complete tears have been managed both operatively and nonoperatively with evidence to suggest better outcomes after surgical intervention if there is a concomitant ACL injury requiring reconstruction.8,9
Anterior cruciate ligament (ACL) tears occur less frequently in hockey players compared to the players in other sports such as football and basketball.38,40 Between 2006 and 2010, 47 players were identified by the NHL Injury Surveillance System as having sustained an ACL injury, which equates to an incidence of 9.4 ACL injuries per NHL season over this time span.41 The mechanism of ACL tears in ice hockey players appears to be different from other sports players based on a recent MRI study that evaluated players for concomitant injuries following ACL tear and noted significantly fewer bone bruises on the lateral femoral condyle compared to players in other sports.42 Early evaluation after injury with Lachman and/or pivot shift tests aids the diagnosis. Data from the NHL study identified 32 players (68%) with concomitant meniscal injuries and 32 (68%) had MCL injuries in conjunction with their ACL tears.41 Average length in the league prior to injury was 5.65 seasons. Twenty-nine of the injured players (61.7%) underwent reconstruction with a patellar tendon autograft, 13 (27.7%) had a hamstring autograft, and 5 (10.6%) had either a patellar tendon or hamstring allograft.41 Meniscus and ACL injuries were associated with a decreased length of career compared to age-matched controls and, notably, players >30 years at the time of injury had only a 67% rate of return to sport whereas those <30 years had a return-to-sport rate of 80%. Players who were able to return did so at an average of 9.8 months (range, 6-21 months) and had a significant reduction in total number of goals, assists, and points scored compared to controls. Decline in performance was typically associated with forwards and wings, while defensemen did not demonstrate the same decrease in performance following return to ice hockey.41
Meniscal tears are a well-documented concomitant injury with ruptures of the ACL, and the combination is a known pattern associated with shorter careers compared to isolated ACL tears in ice hockey players.41 The lateral meniscus is known for increased mobility compared to the medial meniscus and is more commonly injured (39% vs 8.5%) in ACL tears that occur in contact sports and downhill skiing.42 Ice hockey presents a scenario that is different from other contact sports because of the near frictionless interaction between the player’s ice skates and playing surface. This likely equates to a different injury mechanism and dissipation of energy after contact as well as non-contact injuries.38 A recent study reviewed knee MRI findings associated with ACL tears in collegiate ice hockey players and compared to other sports known for their high rates of concomitant meniscal pathology. The authors reported a statistically significant decrease in lateral meniscus tears and bone-bruising patterns in ice hockey players with ACL injuries compared to athletes with ACL tears in other sports.43 In contrast, an NHL study of ACL tears in professional ice hockey players found that 68% of players had concomitant meniscal tears (32 out of 47 players).41
Continue to: The presence of...
The presence of a meniscal tear on MRI is typically a surgical problem, especially if it occurred with an ACL injury. Meniscal repair is preferable, if possible, because there is a known association of increased cartilage contact pressures associated with meniscal debridement. Return to sport following meniscus injury hinges upon whether it is an isolated injury and how it is treated. If the meniscus injury occurs in isolation and can be treated with a debridement and partial resection alone, there is obviously a quicker return to sport as the player can be weight-bearing immediately following surgery. Return to skating after meniscal debridement and partial resection is usually 4 to 6 weeks, whereas meniscal repair protocols vary depending on surgeon; players may need 3 months to 4 months to return to the ice.
Quadriceps contusions are contact injuries that are not unique to ice hockey (Figure 3). They may result from player collision but also from direct blows from a hockey puck. A high velocity puck is known to cause immense trauma to the quadriceps muscles, which may result in localized bleeding and hematoma formation. If the player is able to anticipate the event, active contraction of the quadriceps muscle has been shown to absorb some of the energy and result in a less traumatic injury, but in a fast paced ice hockey game, the player’s anticipation is less likely than in other sports such as baseball.44Interestingly, the degree of knee flexion after injury is predictive of injury severity with milder injuries associated with angles >90 and more severe injuries resulting in knee flexion angles <45° and typically an antalgic gait.45 It is important to treat these injuries during the first 24 hours with the knee maintained in 120°of flexion, plus ice and compression, which can be achieved using a locked knee brace or elastic compression wrap. Quadriceps stretching and isometric strengthening should immediately follow the period of immobilization. The addition of NSAIDs may help prevent the formation of myositis ossificans. A study from West Point suggests that the average return to sport or activity ranges from 13 days (mild contusion) to 21 days (severe contusions), while others8 have indicated that if the injury is treated acutely and a player is able to regain motion and strength, return to ice hockey within a few days is possible.
FOOT AND ANKLE
Ice hockey has some unique injuries that can be attributed to the use of ice skates for play. One such injury is boot-top lacerations, which are fortunately rare as they can be a career-ending injury.47 The spectrum of injury ranges from superficial abrasions to more severe soft tissue disruption, including the extensor tendons and neurovascular structures. The actual mechanism of injury involves an opponent’s skate blade cutting across the anterior ankle. One early case report described a protective method of having players place their skate tongues deep to their protective shin pads, instead of turning the tongues down.47 Kevlar socks have also been shown to help prevent or minimize the damage from a skate blade.48
Injury to the lateral ankle ligaments, anterior talofibular ligament or calcaneofibular ligament, are usually more common than the higher ankle sprains involving the syndesmosis. However, this is not the case in ice hockey. The rigidity of the ice skate at the level of the lateral ligaments seems to impart a protective mechanism to the lower ligaments, but this results in a higher incidence of syndesmotic injuries. These high ankle injuries are unfortunately more debilitating and often require a longer recovery period. In a study of these injuries in NHL players, syndesmotic sprains made up 74% of all ankle sprains, whereas only 18.4% of ankle sprains involved the syndesmosis in American football players..49,50 The average number of days between injury and return to play is 45 days, and some authors believe that defensemen may have a harder time recovering because of the demands on their ankles by having to switch continuously between forward and backward skating.49
Most patients are treated conservatively when their ankle plain radiographs show a congruent mortise and no evidence of syndesmotic widening. If the player expresses pain when squeezing the syndesmosis, it is helpful to obtain stress radiographs to further evaluate for syndesmotic injury. Nonoperative management includes RICE, immobilization in a rigid boot with crutches to protect weight-bearing with gradual advancements and eventually physical therapy to address any ankle stiffness, followed by dynamic functional activities. Treatment options for syndesmotic widening and failed conservative management includes both screw and plate options as well as suture buttons.49,51,52
Ankle and foot fractures were historically a rare injury in ice hockey players based on radiograph evaluation; however, the recent study by Baker and colleagues4 demonstrated that MRI can be helpful in detecting subradiographic fractures. Most of the injuries detected after MRI were from being hit by a hockey puck; this was a novel mechanism that had not been previously reported in the literature.4 Of the injuries that resulted from a direct blow, 14 of 17 occurred on the medial aspect of the foot and ankle, which is believed to result another word? from a defender skating towards an offensive player and attempting to block shots on goal. In this study, all occult fractures involving the medial malleolus were eventually treated with open reduction and internal fixation and underwent routine healing.4 The navicular bone and base of the first metatarsal accounted for the remaining medial-sided fractures. In a recent analysis of risk factors for reoperation following operative fixation of foot fractures across the National Basketball Association, the National Football Leagues, Major League Baseball, and the National Hockey League only a total of 3 fractures involving the foot (1 navicular and 2 first metatarsal) were identified in NHL players over a 30-year period.53 The study acknowledged a major limitation being a public source for identifying players with fractures.
Lace bite is another common ice hockey injury. It typically occurs at the beginning of a season or whenever a player is breaking in a new pair of skates. The cause of the lace bite is the rigid tongue in the skate that rubs against the anterior ankle. Skating causes inflammation in the area of the tibialis anterior tendon, and the player will complain of significant anterior ankle pain. First line treatment for lace bite is ice (Figure 4A), NSAID gel (eg, diclofenac 1%), and a Bunga lace-bite pad (Absolute Athletics). (Figure 4B).
SUMMARY
Lower extremity injuries are common in ice hockey players, and a covering physician should be comfortable managing these injuries from breezers to skate. Proper evaluation and work-up is critical for early diagnosis and identification of pathology, which can minimize the impact of the injury and expedite a treatment plan to return the player safely to the ice and in the game.
1. Flik K, Lyman S, Marx RG. American collegiate men's ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
2. Popkin CA, Nelson BJ, Park CN, et al. Head, neck, and shoulder injuries in ice hockey: current concepts. Am J Orthop (Belle Mead NJ). 2017;46(3):123-134.
3. Popkin CA, Schulz BM, Park CN, Bottiglieri TS, Lynch TS. Evaluation, management and prevention of lower extremity youth ice hockey injuries. Open Access J Sports Med. 534 2016;7:167-176.
4. Baker JC, Hoover EG, Hillen TJ, Smith MV, Wright RW, Rubin DA. Subradiographic foot and ankle fractures and bone contusions detected by MRI in elite ice hockey players. Am J Sports Med. 2016;44(5):1317-1323.
5. Philippon MJ, Ho CP, Briggs KK, Stull J, LaPrade RF. Prevalence of increased alpha angles as a measure of cam-type femoroacetabular impingement in youth ice hockey players. Am J Sports Med. 2013;41(6):1357-1362.
6. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
7. Jorgensen U, Schmidt-Olsen S. The epidemiology of ice hockey injuries. Br J Sports Med. 1986;20(1):7-9.
8. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. BrJ Sports Med. 2014;48(1):4-10.
9. Mosenthal W, Kim M, Holzshu R, Hanypsiak B, Athiviraham A. Common ice hockey injuries and treatment: a current concepts review. Curr Sports Med Rep. 2017;16(5):357-362.
10. Tyler TF, Silvers HJ, Gerhardt MB, Nicholas SJ. Groin injuries in sports medicine. Sports Health. 2010;2(3):231-236.
11. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
12. Dalton SL, Zupon AB, Gardner EC, Djoko A, Dompier TP, Kerr ZY. The epidemiology of hip/groin injuries in National Collegiate Athletic Association men's and women's ice hockey: 2009-2010 through 2014-2015 academic years. Orthop J Sports Med. 2016;4(3):2325967116632692.
13. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Ross JR, Bedi A, Stone RM, Sibilsky Enselman E, Kelly BT, Larson CM. Characterization of symptomatic hip impingement in butterfly ice hockey goalies. Arthroscopy. 2015;31(4):635-642.
16. Bedi A, Dolan M, Hetsroni I, et al. Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med. 2011;39(Suppl):43S-49S.
17. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
18. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral ears. Arthroscopy. 2015;31(11):2106-2111.
19. Audenaert EA, Peeters I, Vigneron L, Baelde N, Pattyn C. Hip morphological characteristics and range of internal rotation in femoroacetabular impingement. Am J Sports Med. 2012;40(6):1329-1336.
20. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
21. Kuhn AW, Ross JR, Bedi A. Three-dimensional imaging and computer navigation in planning for hip preservation surgery. Sports Med Arthrosc Rev. 2015;23(4):e31-e38.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Kuhn AW, Noonan BC, Kelly BT, Larson CM, Bedi A. The hip in ice hockey: a current concepts review. Arthroscopy. 2016;32(9):1928-1938.
24. O'Connor M, Minkara AA, Westermann RW, Rosneck J, Lynch TS. Return to play after hip arthroscopy: a systematic review and meta-analysis. Am J Sports Med. 2018:46(11):2780-2788.
25. Minkara AA, Westermann RW, Rosneck J, Lynch TS. Systematic review and meta-analysis of outcomes after hip arthroscopy in femoroacetabular impingement. Am J Sports Med. 2018:363546517749475.
26. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
27. Pierce CM, Laprade RF, Wahoff M, O'Brien L, Philippon MJ. Ice hockey goaltender rehabilitation, including on-ice progression, after arthroscopic hip surgery for femoroacetabular impingement. J Orthop Sports Phys Ther. 2013;43(3):129-141.
28. MacLeod DA, Gibbon WW. The sportsman's groin. Br J Surg. 1999;86(7):849-850.
29. Irshad K, Feldman LS, Lavoie C, Lacroix VJ, Mulder DS, Brown RA. Operative management of "hockey groin syndrome": 12 years of experience in National Hockey League players. Surgery. 2001;130(4):759-764; discussion 764-756.
30. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
31. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the "sports hernia": MR imaging findings. Radiology. 2008;247(3):797-807.
32. Jakoi A, O'Neill C, Damsgaard C, Fehring K, Tom J. Sports hernia in National Hockey League players: does surgery affect performance? Am J Sports Med. 2013;41(1):107-110.
33. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775.
34. Lorentzon R, Wedren H, Pietila T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
35. Holmich P, Uhrskou P, Ulnits L, et al. Effectiveness of active physical training as treatment for long-standing adductor-related groin pain in athletes: randomised trial. Lancet. 1999;353(9151):439-443.
36. Sim FH, Chao EY. Injury potential in modern ice hockey. Am J Sports Med. 1978;6(6):378-384.
37. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
38. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. BrJ Sports Med. 2009;43(13):1000-1005.
39. Grant JA, Bedi A, Kurz J, Bancroft R, Miller BS. Incidence and injury characteristics of medial collateral ligament injuries in male collegiate ice hockey players. Sports Health. 2013;5(3):270-272.
40. Erickson BJ, Harris JD, Cole BJ, et al. Performance and return to sport after anterior cruciate ligament reconstruction in National Hockey League players. Orthop J Sports Med. 2014;2(9):2325967114548831.
41. Sikka R, Kurtenbach C, Steubs JT, Boyd JL, Nelson BJ. Anterior Cruciate Ligament Injuries in Professional Hockey Players. Am J Sports Med. 2016;44(2):378-383.
42. Friden T, Erlandsson T, Zatterstrom R, Lindstrand A, Moritz U. Compression or distraction of the anterior cruciate injured knee: variations in injury pattern in contact sports and downhill skiing. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):144-147.
43. Kluczynski MA, Kang JV, Marzo JM, Bisson LJ. Magnetic resonance imaging and intra-articular findings after anterior cruciate ligament injuries in ice hockey versus other sports. Orthop J Sports Med. 2016;4(5):2325967116646534. 44. Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Orthop Surg. 2001;9(4):227-237.
45. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
46. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
47. Johnson PN, Mark; Green, Eric. Boot-top lacerations in ice hockey players: a new injury. Clin J Sports Med. 1991:205-208.
48. Nauth A, Aziz M, Tsuji M, Whalen DB, Theodoropoulos JS, Zdero R. The protective effect of Kevlar socks against hockey skate blade injuries: a biomechanical study. Orthop J Sports Med. 2014;2(Suppl 2):7.
49. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
50. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
51. Marymont JV, Lynch MA, Henning CE. Acute ligamentous diastasis of the ankle without fracture. Evaluation by radionuclide imaging. Am J Sports Med. 1986;14(5):407-409.
52. Miller CD, Shelton WR, Barrett GR, Savoie FH, Dukes AD. Deltoid and syndesmosis ligament injury of the ankle without fracture. Am J Sports Med. 1995;23(6):746-750.
53. Singh SK, Larkin KE, Kadakia AR, Hsu WK. Risk factors for reoperation and performance-based outcomes after operative fixation of foot fractures in the professional athlete: a cross-sport analysis. Sports Health. 2018;10(1):70-74.
54. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
55. Elattar O, Choi HR, Dills VD, Busconi B. Groin injuries (athletic pubalgia) and return to play. Sports Health. 2016;8(4):313-323.
ABSTRACT
Ice hockey is a fast-paced, collision sport requiring tremendous skill and finesse, yet ice hockey can be a harsh and violent game. It has one of the highest musculoskeletal injury rates in all of competitive sports. Razor sharp skates, aluminum sticks and boards made from high density polyethylene (HDPE), all contribute to the intrinsic hazards of the game. The objective of this article is to review evaluation, management, and return-to-the-rink guidelines after common lower extremity ice hockey injuries.
“Hockey is a fast body-contact game played by men with clubs in their hands and knives laced to their feet, since the skates are razor sharp, and before the evening is over it is almost a certainty that someone will be hurt and will fleck the ice with a generous contribution of gore before he is led away to be hemstitched together again.” —Paul Gallico in Farewell to Sport (1938)
Ice hockey is a collision sport with player speeds in excess of 30 miles/hour, on a sheet of ice surrounded by unforgiving boards, with a vulcanized rubber puck moving at speeds approaching 100 miles/hour.1-3 Understanding injuries specific to this fast-paced sport is an essential part of being a team physician at any level of competitive ice hockey. We are continuing to improve our ability to correctly identify and treat injuries in ice hockey players.2,4 On the prevention side, rule changes in hockey have been implemented, such as raising the age to allow checking and penalties for deliberate hits to the head and checking from behind, to make the game safer to play.3 Additionally, advancements in biomechanical research and 3D modeling are providing new insights into the pathoanatomy of the hip joint, which can be utilized for surgical planning in hockey players and goalies suffering from symptomatic femoroacetabular impingement (FAI) of the hip.5
During the 2010 Winter Olympics, more than 30% of ice hockey players were injured, which was the highest percentage amongst all competing sports.6 They also tallied the highest percentage of player-to-player injuries during the Olympics of any sport. Consequently, the team physician covering ice hockey should be prepared to manage upper and lower extremity musculoskeletal injuries, but also concussions, cervical spine injuries, and ocular and dental trauma.2
One of the earliest epidemiological studies of ice hockey injuries looked at elite Danish hockey players over 2 seasons and found that head trauma accounted for 28% of all injuries, followed by lower extremity injuries at 27% with upper extremity injuries accounting for 19%.7 More recent epidemiological studies have shown similar rates based on body region while further defining individual diagnoses and their incidence. This should help clinicians and researchers develop prevention strategies, as well as improve treatments to optimize player outcomes and return to sport.8,9 Our group recently reviewed the evaluation and management of common head, neck, and shoulder injuries at all competitive levels of ice hockey, and this article serves to complement the former by focusing on lower extremity injuries (Table).2
Continue to: Hip and groin...
EVALUATION AND MANAGEMENT OF COMMON LOWER EXTREMITY HOCKEY INJURIES
HIP INJURIES
Hip and groin injuries are very common amongst this group of athletes and account for approximately 9% of all ice hockey injuries.1 Unfortunately, they are also known for their high recurrence rates, which may be in part due to delayed diagnosis, inadequate rest and rehabilitation, as well as the extreme loads that are placed on the hip during competition.10,11 In hockey, the most commonly reported hip injuries include goaltender’s hip, FAI, sports hernia/hockey groin syndrome, adductor strains, hip pointer, and quadriceps contusions. Dalton and colleagues12 performed the largest epidemiological study to date on hip and groin injuries amongst National Collegiate Athletic Association ice hockey players and reported that the most common injury mechanism was noncontact in nature. Contact injuries accounted for 13% (55 of 421) in men’s ice hockey players while less than 4% (4 of 114) injuries in female ice hockey players, which is likely attributed to a no checking rule in the women’s division. Some of these hip and groin injuries are difficult to diagnose so it is important for the team physician to perform a thorough history and physical examination. Advanced imaging (magnetic resonance imaging [MRI] or a computed tomography (CT) scan with 3D reconstructions) may be necessary to make the correct diagnosis. This is important for providing proper treatment as well as setting player expectations for return to sport.12
Table 1. Return-to-Play Guidelines for Common Lower Extremity Ice Hockey Injuries | ||
Lower Extremity Injury | Treatment Options | Return-to-the-Rink Goal |
FAI | In-season: injection, physical therapy program, NSAIDS. Off-season or unable to play: requires arthroscopic surgery | Nonoperative can take up to 6 weeks. Surgical depends on what is fixed but goal is 4 months to return to ice24,26
|
Sports hernia/athletic pubalgia
| In-season: physical therapy program, NSAIDS Off-season or unable to play requiring surgery. Essential to make sure no other pathology (eg, FAI, osteitis pubis, adductor strain) to maximize success
| Nonoperative 6-8 wk trial of physical therapy Operative: depends if concomitant FAI but in isolation goal is 3-4 mo33,54
|
Adductor strains | Ice, NSAIDS, physical therapy, use of Hypervolt Hyperice | Depends on position (goalie vs skater) and severity; can take up to 4-8 wk to return to ice. Want 70% strength and painless ROM to skate successfully;55 in chronic cases, may take up to 6 mo35
|
Quadriceps contusion
| Hinged knee brace to maintain 120° of flexion, ice, compression wrap.
| When player regains motion and strength, return to ice can be as fast a couple of days or as long as 3 wk8,46
|
MCL | Hinged knee brace, shin pad modification, ice, NSAIDs | Depends on Grade; if Grade I, 1-2 wk; Grade II, 2-4 wk; Grade III, 4-6 wk8
|
ACL | Surgery autograft BTB autograft soft tissue
| 9-10 mo41 |
Meniscus tear | Depends on type of tear and seasonal timing (in-season or off-season) | If surgical, 3-4 mo; if repair, 4-6 wk if partial menisectomy
|
High ankle sprain
| Cam boot, NSAIDS, ice and physical therapy
| 6 wk49 |
Boot top laceration | Repair of cut structures, depends on depth and what is injured; best treatment is prevention with Kevlar socks | If laceration is deep and severs any medial tendons/vascular structures, return to ice can be ≥6 mo
|
Lace bite
| Bunga pad, ice, diclofenac gel
| Couple of days to up to 2 wk in recalcitrant cases3 |
Abbreviations: ACL, anterior cruciate ligament; BTB, bone-patellar tendon-bone; Cam, controlled ankle motion boot; MCL, medial collateral ligament; FAI, femoroacetabular impingement; NSAIDS, nonsteroidal anti-inflammatory drugs; ROM, range of motion.
Throughout the hockey community, FAI is being examined as a possible source of symptomatic hip pain amongst players at all levels. A recent study, which utilized the National Hockey League (NHL) injury surveillance database, reported that FAI accounted for 5.3% of all hip and groin injuries.13 The etiology of FAI is thought to arise from a combination of genetic predisposition coupled with repetitive axial loading/hip flexion. This causes a bony overgrowth of the proximal femoral physes resulting in a cam deformity (Figure 1).5,14 The abnormal bony anatomy allows for impingement between the acetabulum and proximal femur, which can injure the labrum and articular cartilage of the hip joint.
In the recent study by Ross and colleagues,15 the authors focused on symptomatic hip impingement in ice hockey goalies.15 Goaltender’s hip may be the result of the “butterfly style,” which is a technique of goaltending that emphasizes guarding the lower part of the goal. The goalie drops to his/her knees and internally rotates the hips to allow the leg pads to be parallel to the ice. This style acquired the name butterfly because of the resemblance of the spread goalie pads to a butterfly’s wings. Bedi and associates16 have evaluated hip biomechanics using 3D-generated bone models and showed in their study that arthroscopic treatment can improve hip kinematics and range of motion.
Plain radiographs showed that 90% (61 of 68) of hockey goalies had an elevated alpha angle signifying a femoral cam-type deformity.15 Goalies had a significantly lower mean lateral center-edge angle (27.3° vs 29.6°; P = .03) and 13.2% of them were found to have acetabular dysplasia (lateral center-edge angle<20°) compared to only 3% of positional players. The CT scan measurements demonstrated that hockey goalies have a unique cam-type deformity that is located more lateral (1:00 o’clock vs 1:45 o’clock; P < .0001) along the proximal femur, an elevated maximum alpha angle (80.9° vs 68.6°; P < .0001) and loss of offset, when compared to positional players. These findings provide an anatomical basis in support of reports that goaltenders are more likely to experience intra-articular hip injuries compared to other positional players.13
Regardless of position, symptomatic FAI in a hockey player is generally a problem that slowly builds and is made worse with activity.17 On examination, the player may have limited hip flexion and internal rotation, as well as weakness compared to the contralateral side when testing hip flexion and abduction.18,19 Plain radiographs plus MRI or CT allow for proper characterization and diagnosis (to include underlying chondrolabral pathology).20,21
In the young athlete, initial management includes physical therapy, which focuses on core strengthening. Emphasis is placed on hip flexion and extension, as well as abduction and external rotation with the goal of reducing symptoms and avoiding injuries.22 A similar approach may be applied to the elite athlete, but failure of nonoperative management may necessitate surgical intervention. Hip arthroscopy continues to grow in popularity over open surgical dislocation with low complication rate and high return-to-play rate.23-25
For the in-season athlete, attempts to continue to play can be assisted with the role of an intra-articular corticosteroid injection, which can help calm inflammation within the hip joint and mitigate pain, while rehabilitation focuses on core stabilization, postural retraining and focusing on any muscle imbalances that might be present. For positional players, ice time and shift duration can be adjusted to give the player’s hip a period of rest; meanwhile, for goaltenders, shot volumes in practice can be decreased.
Continue to: For athletes who...
For athletes who fail nonoperative care, surgical treatment varies depending on underlying hip pathology and may include femoroplasty, acetabuloplasty, and microfracture as well as labral repair or debridement. Though data are limited, Philippon and colleagues26 have published promising results in a case series of 28 NHL players after surgical intervention for FAI. All players returned to sport at an average of 3.8 months and players who had surgery within 1 year of injury returned on average 1.1 months sooner than those who waited more than 1 year. Rehabilitation protocol varies between goaltenders compared to defensemen and offensive players due to the different demands required for blocking shots on goal.27
One of the most challenging injuries to correctly identify in the hip area is athletic pubalgia (also referred to as sports hernia or core muscle injury) because pain in the groin may be referred from the lumbar spine, hip joint, urologic, or perineal etiologies.28 Sports hernias involve dilatation of the external ring of the inguinal canal and thinning of the posterior wall. Players may report to the athletic trainer or team physician with a complaint of groin pain that is worse when pushing off with their skate or taking a slap shot.29 On exam, pain can be reproduced by hip extension, contralateral torso rotation, or with a resisted sit-up with palpation of the inferolateral edge of the distal rectus abdominus.30 An MRI with specific sequences centered over the pubic symphysis is usually warranted to aid in the workup of sports hernia. An MRI in these cases may also demonstrate avulsions of the rectus abdominus.31
Most of these injuries are managed conservatively but can warrant surgical intervention if the symptoms persist. In the study by Jakoi and colleagues,32 they identified 43 ice hockey players over an 8-year period (2001-2008) who had repairs of their sports hernias and assessed the statistics during the 2 years prior and 2 years after surgery. The authors found that 80% of these players were able to return to the ice for 2 or more full seasons. The return-to-sport rate was comparable to other sports after sports hernia repair, but players who had played in ≥7 seasons demonstrated a greater decrease in number of games played, goals, assists and time on ice compared to those who had played in ≤6 seasons prior to the time of injury. Between 1989 and 2000, 22 NHL players who failed to respond to nonoperative management of their groin injuries underwent surgical exploration.29 At the time of surgical exploration, their hockey groin syndrome, consisted of small tears in the external oblique aponeurosis through which branches of the ilioinguinal or iliohypogastric could be identified. These surgical procedures were all through a standard inguinal approach and the perforating neurovascular structures were excised, while the main trunk of the ilioinguinal nerve was ablated and the external oblique aponeurosis was repaired and reinforced with Goretex (W.L. Gore & Associates Inc, Flagstaff, AZ). At follow-up, 18 of the 22 players (82%) had no pain and 19 (86%) were able to resume their careers in the NHL.29 Ice hockey players with sports hernias or hockey groin syndrome often return to the sport, but it is important to identify these problems early so that surgical options can be discussed if the player fails conservative management. It is also critical to make sure that all pathology is identified, because in players with mixed sports hernia and FAI, return-to-play results improve when both issues are addressed. In a study of athletes (some of whom were ice hockey players), who had both FAI and sports hernia, and only hernia/pubalgia surgery was performed, 25% of these athletes returned to sport. If only FAI was addressed, 50% of the athletes returned to sport; however, when hernia and FAI were treated, 89% returned to play.33
Adductor strains includes injury to the adductor muscles, pectineus, obturator externus and gracilis, and are prevalent in ice hockey players. A study of elite Swedish ice hockey players published in 1988 reported that adductor strains accounted for 10% (10 of 95) of all injuries.34 Given the prevalence of these injuries, considerable research has been dedicated to understanding their mechanism and prevention.35 Adductor strains within the ice hockey population have been attributed to the eccentric forces on the adductors when players attempt to decelerate the leg during a stride.36 A study of NHL players revealed that a ratio <80% of adductor-to-abductor muscle is the best predictor of a groin strain.37
These injuries are also well known for their recurrence rates, as was the case in an NHL study where 4 of the 9 adductor strains (44%) were recurrent injuries.37 The authors attributed the recurrence to an incomplete rehabilitation program and an accelerated return to sport. This was followed by an NHL prevention program that spanned 2 seasons and analyzed 58 players whose adductor-to-abductor ratio was <80% and placed them into a 6-week intervention program during the preseason.37 Only 3 players sustained an adductor strain in the 2 subsequent seasons after the intervention, compared to 11 strains in the previous 2 seasons. Thus, early identification of muscle strength imbalance coupled with an appropriate intervention program has proven to be an effective means of reducing adductor strains in this at-risk population.
Continue to: Contact injuries may...
Contact injuries may vary with checking into the boards being unique to men’s ice hockey. Hip pointers occur as a result of a direct compression injury to the iliac crest, which causes trauma to the bone but also to the overlying hip abductor musculature, and represent roughly 2.4% of ice hockey injuries.23 The resulting contusion may cause a local hematoma formation. Early identification of the injury plus treatment with RICE (rest, ice, compression, elevation) coupled with crutches to limit weight-bearing status may minimize soft tissue trauma and swelling, and ultimately aid in pain control and return to sport.38 Hip abductor strengthening, added padding over the injured area, as well as a compressive hip spica wrapping, have all been suggested to expedite return to play and help prevent recurrence of the hip pointer.8
KNEE INJURIES
Injury to the medial collateral ligament (MCL) is the most commonly reported knee injury (Figure 2) and second only to concussion amongst all injuries in National Collegiate Athletic Association ice hockey players.8,39 The mechanism of injury typically involves a valgus force on the knee, which is often caused by collision into another player.39 Valgus stress testing with the knee in 30° of flexion is used to grade the severity of injury (Grade I: 0-5 mm of medial opening; Grade II: 5-10 mm of medial opening; Grade III: >10 mm of medial opening).39 One study that followed a single college hockey team for 8 seasons reported that 77% of injuries (10 of 13) occurred during player-to-player collision,39 with 5 being Grade 1 injuries, 6 Grade 2 injuries, 1 Grade 3; information was missing for 1 player. Nonoperative management of incomplete injuries, grade 1 and 2 sprains, with RICE and early physical therapy intervention to work on knee range of motion and quadriceps strengthening typically helps the player return to sport within days for grade 1 and 2 injuries to 3 weeks for grade 2 injuries. Complete tears have been managed both operatively and nonoperatively with evidence to suggest better outcomes after surgical intervention if there is a concomitant ACL injury requiring reconstruction.8,9
Anterior cruciate ligament (ACL) tears occur less frequently in hockey players compared to the players in other sports such as football and basketball.38,40 Between 2006 and 2010, 47 players were identified by the NHL Injury Surveillance System as having sustained an ACL injury, which equates to an incidence of 9.4 ACL injuries per NHL season over this time span.41 The mechanism of ACL tears in ice hockey players appears to be different from other sports players based on a recent MRI study that evaluated players for concomitant injuries following ACL tear and noted significantly fewer bone bruises on the lateral femoral condyle compared to players in other sports.42 Early evaluation after injury with Lachman and/or pivot shift tests aids the diagnosis. Data from the NHL study identified 32 players (68%) with concomitant meniscal injuries and 32 (68%) had MCL injuries in conjunction with their ACL tears.41 Average length in the league prior to injury was 5.65 seasons. Twenty-nine of the injured players (61.7%) underwent reconstruction with a patellar tendon autograft, 13 (27.7%) had a hamstring autograft, and 5 (10.6%) had either a patellar tendon or hamstring allograft.41 Meniscus and ACL injuries were associated with a decreased length of career compared to age-matched controls and, notably, players >30 years at the time of injury had only a 67% rate of return to sport whereas those <30 years had a return-to-sport rate of 80%. Players who were able to return did so at an average of 9.8 months (range, 6-21 months) and had a significant reduction in total number of goals, assists, and points scored compared to controls. Decline in performance was typically associated with forwards and wings, while defensemen did not demonstrate the same decrease in performance following return to ice hockey.41
Meniscal tears are a well-documented concomitant injury with ruptures of the ACL, and the combination is a known pattern associated with shorter careers compared to isolated ACL tears in ice hockey players.41 The lateral meniscus is known for increased mobility compared to the medial meniscus and is more commonly injured (39% vs 8.5%) in ACL tears that occur in contact sports and downhill skiing.42 Ice hockey presents a scenario that is different from other contact sports because of the near frictionless interaction between the player’s ice skates and playing surface. This likely equates to a different injury mechanism and dissipation of energy after contact as well as non-contact injuries.38 A recent study reviewed knee MRI findings associated with ACL tears in collegiate ice hockey players and compared to other sports known for their high rates of concomitant meniscal pathology. The authors reported a statistically significant decrease in lateral meniscus tears and bone-bruising patterns in ice hockey players with ACL injuries compared to athletes with ACL tears in other sports.43 In contrast, an NHL study of ACL tears in professional ice hockey players found that 68% of players had concomitant meniscal tears (32 out of 47 players).41
Continue to: The presence of...
The presence of a meniscal tear on MRI is typically a surgical problem, especially if it occurred with an ACL injury. Meniscal repair is preferable, if possible, because there is a known association of increased cartilage contact pressures associated with meniscal debridement. Return to sport following meniscus injury hinges upon whether it is an isolated injury and how it is treated. If the meniscus injury occurs in isolation and can be treated with a debridement and partial resection alone, there is obviously a quicker return to sport as the player can be weight-bearing immediately following surgery. Return to skating after meniscal debridement and partial resection is usually 4 to 6 weeks, whereas meniscal repair protocols vary depending on surgeon; players may need 3 months to 4 months to return to the ice.
Quadriceps contusions are contact injuries that are not unique to ice hockey (Figure 3). They may result from player collision but also from direct blows from a hockey puck. A high velocity puck is known to cause immense trauma to the quadriceps muscles, which may result in localized bleeding and hematoma formation. If the player is able to anticipate the event, active contraction of the quadriceps muscle has been shown to absorb some of the energy and result in a less traumatic injury, but in a fast paced ice hockey game, the player’s anticipation is less likely than in other sports such as baseball.44Interestingly, the degree of knee flexion after injury is predictive of injury severity with milder injuries associated with angles >90 and more severe injuries resulting in knee flexion angles <45° and typically an antalgic gait.45 It is important to treat these injuries during the first 24 hours with the knee maintained in 120°of flexion, plus ice and compression, which can be achieved using a locked knee brace or elastic compression wrap. Quadriceps stretching and isometric strengthening should immediately follow the period of immobilization. The addition of NSAIDs may help prevent the formation of myositis ossificans. A study from West Point suggests that the average return to sport or activity ranges from 13 days (mild contusion) to 21 days (severe contusions), while others8 have indicated that if the injury is treated acutely and a player is able to regain motion and strength, return to ice hockey within a few days is possible.
FOOT AND ANKLE
Ice hockey has some unique injuries that can be attributed to the use of ice skates for play. One such injury is boot-top lacerations, which are fortunately rare as they can be a career-ending injury.47 The spectrum of injury ranges from superficial abrasions to more severe soft tissue disruption, including the extensor tendons and neurovascular structures. The actual mechanism of injury involves an opponent’s skate blade cutting across the anterior ankle. One early case report described a protective method of having players place their skate tongues deep to their protective shin pads, instead of turning the tongues down.47 Kevlar socks have also been shown to help prevent or minimize the damage from a skate blade.48
Injury to the lateral ankle ligaments, anterior talofibular ligament or calcaneofibular ligament, are usually more common than the higher ankle sprains involving the syndesmosis. However, this is not the case in ice hockey. The rigidity of the ice skate at the level of the lateral ligaments seems to impart a protective mechanism to the lower ligaments, but this results in a higher incidence of syndesmotic injuries. These high ankle injuries are unfortunately more debilitating and often require a longer recovery period. In a study of these injuries in NHL players, syndesmotic sprains made up 74% of all ankle sprains, whereas only 18.4% of ankle sprains involved the syndesmosis in American football players..49,50 The average number of days between injury and return to play is 45 days, and some authors believe that defensemen may have a harder time recovering because of the demands on their ankles by having to switch continuously between forward and backward skating.49
Most patients are treated conservatively when their ankle plain radiographs show a congruent mortise and no evidence of syndesmotic widening. If the player expresses pain when squeezing the syndesmosis, it is helpful to obtain stress radiographs to further evaluate for syndesmotic injury. Nonoperative management includes RICE, immobilization in a rigid boot with crutches to protect weight-bearing with gradual advancements and eventually physical therapy to address any ankle stiffness, followed by dynamic functional activities. Treatment options for syndesmotic widening and failed conservative management includes both screw and plate options as well as suture buttons.49,51,52
Ankle and foot fractures were historically a rare injury in ice hockey players based on radiograph evaluation; however, the recent study by Baker and colleagues4 demonstrated that MRI can be helpful in detecting subradiographic fractures. Most of the injuries detected after MRI were from being hit by a hockey puck; this was a novel mechanism that had not been previously reported in the literature.4 Of the injuries that resulted from a direct blow, 14 of 17 occurred on the medial aspect of the foot and ankle, which is believed to result another word? from a defender skating towards an offensive player and attempting to block shots on goal. In this study, all occult fractures involving the medial malleolus were eventually treated with open reduction and internal fixation and underwent routine healing.4 The navicular bone and base of the first metatarsal accounted for the remaining medial-sided fractures. In a recent analysis of risk factors for reoperation following operative fixation of foot fractures across the National Basketball Association, the National Football Leagues, Major League Baseball, and the National Hockey League only a total of 3 fractures involving the foot (1 navicular and 2 first metatarsal) were identified in NHL players over a 30-year period.53 The study acknowledged a major limitation being a public source for identifying players with fractures.
Lace bite is another common ice hockey injury. It typically occurs at the beginning of a season or whenever a player is breaking in a new pair of skates. The cause of the lace bite is the rigid tongue in the skate that rubs against the anterior ankle. Skating causes inflammation in the area of the tibialis anterior tendon, and the player will complain of significant anterior ankle pain. First line treatment for lace bite is ice (Figure 4A), NSAID gel (eg, diclofenac 1%), and a Bunga lace-bite pad (Absolute Athletics). (Figure 4B).
SUMMARY
Lower extremity injuries are common in ice hockey players, and a covering physician should be comfortable managing these injuries from breezers to skate. Proper evaluation and work-up is critical for early diagnosis and identification of pathology, which can minimize the impact of the injury and expedite a treatment plan to return the player safely to the ice and in the game.
ABSTRACT
Ice hockey is a fast-paced, collision sport requiring tremendous skill and finesse, yet ice hockey can be a harsh and violent game. It has one of the highest musculoskeletal injury rates in all of competitive sports. Razor sharp skates, aluminum sticks and boards made from high density polyethylene (HDPE), all contribute to the intrinsic hazards of the game. The objective of this article is to review evaluation, management, and return-to-the-rink guidelines after common lower extremity ice hockey injuries.
“Hockey is a fast body-contact game played by men with clubs in their hands and knives laced to their feet, since the skates are razor sharp, and before the evening is over it is almost a certainty that someone will be hurt and will fleck the ice with a generous contribution of gore before he is led away to be hemstitched together again.” —Paul Gallico in Farewell to Sport (1938)
Ice hockey is a collision sport with player speeds in excess of 30 miles/hour, on a sheet of ice surrounded by unforgiving boards, with a vulcanized rubber puck moving at speeds approaching 100 miles/hour.1-3 Understanding injuries specific to this fast-paced sport is an essential part of being a team physician at any level of competitive ice hockey. We are continuing to improve our ability to correctly identify and treat injuries in ice hockey players.2,4 On the prevention side, rule changes in hockey have been implemented, such as raising the age to allow checking and penalties for deliberate hits to the head and checking from behind, to make the game safer to play.3 Additionally, advancements in biomechanical research and 3D modeling are providing new insights into the pathoanatomy of the hip joint, which can be utilized for surgical planning in hockey players and goalies suffering from symptomatic femoroacetabular impingement (FAI) of the hip.5
During the 2010 Winter Olympics, more than 30% of ice hockey players were injured, which was the highest percentage amongst all competing sports.6 They also tallied the highest percentage of player-to-player injuries during the Olympics of any sport. Consequently, the team physician covering ice hockey should be prepared to manage upper and lower extremity musculoskeletal injuries, but also concussions, cervical spine injuries, and ocular and dental trauma.2
One of the earliest epidemiological studies of ice hockey injuries looked at elite Danish hockey players over 2 seasons and found that head trauma accounted for 28% of all injuries, followed by lower extremity injuries at 27% with upper extremity injuries accounting for 19%.7 More recent epidemiological studies have shown similar rates based on body region while further defining individual diagnoses and their incidence. This should help clinicians and researchers develop prevention strategies, as well as improve treatments to optimize player outcomes and return to sport.8,9 Our group recently reviewed the evaluation and management of common head, neck, and shoulder injuries at all competitive levels of ice hockey, and this article serves to complement the former by focusing on lower extremity injuries (Table).2
Continue to: Hip and groin...
EVALUATION AND MANAGEMENT OF COMMON LOWER EXTREMITY HOCKEY INJURIES
HIP INJURIES
Hip and groin injuries are very common amongst this group of athletes and account for approximately 9% of all ice hockey injuries.1 Unfortunately, they are also known for their high recurrence rates, which may be in part due to delayed diagnosis, inadequate rest and rehabilitation, as well as the extreme loads that are placed on the hip during competition.10,11 In hockey, the most commonly reported hip injuries include goaltender’s hip, FAI, sports hernia/hockey groin syndrome, adductor strains, hip pointer, and quadriceps contusions. Dalton and colleagues12 performed the largest epidemiological study to date on hip and groin injuries amongst National Collegiate Athletic Association ice hockey players and reported that the most common injury mechanism was noncontact in nature. Contact injuries accounted for 13% (55 of 421) in men’s ice hockey players while less than 4% (4 of 114) injuries in female ice hockey players, which is likely attributed to a no checking rule in the women’s division. Some of these hip and groin injuries are difficult to diagnose so it is important for the team physician to perform a thorough history and physical examination. Advanced imaging (magnetic resonance imaging [MRI] or a computed tomography (CT) scan with 3D reconstructions) may be necessary to make the correct diagnosis. This is important for providing proper treatment as well as setting player expectations for return to sport.12
Table 1. Return-to-Play Guidelines for Common Lower Extremity Ice Hockey Injuries | ||
Lower Extremity Injury | Treatment Options | Return-to-the-Rink Goal |
FAI | In-season: injection, physical therapy program, NSAIDS. Off-season or unable to play: requires arthroscopic surgery | Nonoperative can take up to 6 weeks. Surgical depends on what is fixed but goal is 4 months to return to ice24,26
|
Sports hernia/athletic pubalgia
| In-season: physical therapy program, NSAIDS Off-season or unable to play requiring surgery. Essential to make sure no other pathology (eg, FAI, osteitis pubis, adductor strain) to maximize success
| Nonoperative 6-8 wk trial of physical therapy Operative: depends if concomitant FAI but in isolation goal is 3-4 mo33,54
|
Adductor strains | Ice, NSAIDS, physical therapy, use of Hypervolt Hyperice | Depends on position (goalie vs skater) and severity; can take up to 4-8 wk to return to ice. Want 70% strength and painless ROM to skate successfully;55 in chronic cases, may take up to 6 mo35
|
Quadriceps contusion
| Hinged knee brace to maintain 120° of flexion, ice, compression wrap.
| When player regains motion and strength, return to ice can be as fast a couple of days or as long as 3 wk8,46
|
MCL | Hinged knee brace, shin pad modification, ice, NSAIDs | Depends on Grade; if Grade I, 1-2 wk; Grade II, 2-4 wk; Grade III, 4-6 wk8
|
ACL | Surgery autograft BTB autograft soft tissue
| 9-10 mo41 |
Meniscus tear | Depends on type of tear and seasonal timing (in-season or off-season) | If surgical, 3-4 mo; if repair, 4-6 wk if partial menisectomy
|
High ankle sprain
| Cam boot, NSAIDS, ice and physical therapy
| 6 wk49 |
Boot top laceration | Repair of cut structures, depends on depth and what is injured; best treatment is prevention with Kevlar socks | If laceration is deep and severs any medial tendons/vascular structures, return to ice can be ≥6 mo
|
Lace bite
| Bunga pad, ice, diclofenac gel
| Couple of days to up to 2 wk in recalcitrant cases3 |
Abbreviations: ACL, anterior cruciate ligament; BTB, bone-patellar tendon-bone; Cam, controlled ankle motion boot; MCL, medial collateral ligament; FAI, femoroacetabular impingement; NSAIDS, nonsteroidal anti-inflammatory drugs; ROM, range of motion.
Throughout the hockey community, FAI is being examined as a possible source of symptomatic hip pain amongst players at all levels. A recent study, which utilized the National Hockey League (NHL) injury surveillance database, reported that FAI accounted for 5.3% of all hip and groin injuries.13 The etiology of FAI is thought to arise from a combination of genetic predisposition coupled with repetitive axial loading/hip flexion. This causes a bony overgrowth of the proximal femoral physes resulting in a cam deformity (Figure 1).5,14 The abnormal bony anatomy allows for impingement between the acetabulum and proximal femur, which can injure the labrum and articular cartilage of the hip joint.
In the recent study by Ross and colleagues,15 the authors focused on symptomatic hip impingement in ice hockey goalies.15 Goaltender’s hip may be the result of the “butterfly style,” which is a technique of goaltending that emphasizes guarding the lower part of the goal. The goalie drops to his/her knees and internally rotates the hips to allow the leg pads to be parallel to the ice. This style acquired the name butterfly because of the resemblance of the spread goalie pads to a butterfly’s wings. Bedi and associates16 have evaluated hip biomechanics using 3D-generated bone models and showed in their study that arthroscopic treatment can improve hip kinematics and range of motion.
Plain radiographs showed that 90% (61 of 68) of hockey goalies had an elevated alpha angle signifying a femoral cam-type deformity.15 Goalies had a significantly lower mean lateral center-edge angle (27.3° vs 29.6°; P = .03) and 13.2% of them were found to have acetabular dysplasia (lateral center-edge angle<20°) compared to only 3% of positional players. The CT scan measurements demonstrated that hockey goalies have a unique cam-type deformity that is located more lateral (1:00 o’clock vs 1:45 o’clock; P < .0001) along the proximal femur, an elevated maximum alpha angle (80.9° vs 68.6°; P < .0001) and loss of offset, when compared to positional players. These findings provide an anatomical basis in support of reports that goaltenders are more likely to experience intra-articular hip injuries compared to other positional players.13
Regardless of position, symptomatic FAI in a hockey player is generally a problem that slowly builds and is made worse with activity.17 On examination, the player may have limited hip flexion and internal rotation, as well as weakness compared to the contralateral side when testing hip flexion and abduction.18,19 Plain radiographs plus MRI or CT allow for proper characterization and diagnosis (to include underlying chondrolabral pathology).20,21
In the young athlete, initial management includes physical therapy, which focuses on core strengthening. Emphasis is placed on hip flexion and extension, as well as abduction and external rotation with the goal of reducing symptoms and avoiding injuries.22 A similar approach may be applied to the elite athlete, but failure of nonoperative management may necessitate surgical intervention. Hip arthroscopy continues to grow in popularity over open surgical dislocation with low complication rate and high return-to-play rate.23-25
For the in-season athlete, attempts to continue to play can be assisted with the role of an intra-articular corticosteroid injection, which can help calm inflammation within the hip joint and mitigate pain, while rehabilitation focuses on core stabilization, postural retraining and focusing on any muscle imbalances that might be present. For positional players, ice time and shift duration can be adjusted to give the player’s hip a period of rest; meanwhile, for goaltenders, shot volumes in practice can be decreased.
Continue to: For athletes who...
For athletes who fail nonoperative care, surgical treatment varies depending on underlying hip pathology and may include femoroplasty, acetabuloplasty, and microfracture as well as labral repair or debridement. Though data are limited, Philippon and colleagues26 have published promising results in a case series of 28 NHL players after surgical intervention for FAI. All players returned to sport at an average of 3.8 months and players who had surgery within 1 year of injury returned on average 1.1 months sooner than those who waited more than 1 year. Rehabilitation protocol varies between goaltenders compared to defensemen and offensive players due to the different demands required for blocking shots on goal.27
One of the most challenging injuries to correctly identify in the hip area is athletic pubalgia (also referred to as sports hernia or core muscle injury) because pain in the groin may be referred from the lumbar spine, hip joint, urologic, or perineal etiologies.28 Sports hernias involve dilatation of the external ring of the inguinal canal and thinning of the posterior wall. Players may report to the athletic trainer or team physician with a complaint of groin pain that is worse when pushing off with their skate or taking a slap shot.29 On exam, pain can be reproduced by hip extension, contralateral torso rotation, or with a resisted sit-up with palpation of the inferolateral edge of the distal rectus abdominus.30 An MRI with specific sequences centered over the pubic symphysis is usually warranted to aid in the workup of sports hernia. An MRI in these cases may also demonstrate avulsions of the rectus abdominus.31
Most of these injuries are managed conservatively but can warrant surgical intervention if the symptoms persist. In the study by Jakoi and colleagues,32 they identified 43 ice hockey players over an 8-year period (2001-2008) who had repairs of their sports hernias and assessed the statistics during the 2 years prior and 2 years after surgery. The authors found that 80% of these players were able to return to the ice for 2 or more full seasons. The return-to-sport rate was comparable to other sports after sports hernia repair, but players who had played in ≥7 seasons demonstrated a greater decrease in number of games played, goals, assists and time on ice compared to those who had played in ≤6 seasons prior to the time of injury. Between 1989 and 2000, 22 NHL players who failed to respond to nonoperative management of their groin injuries underwent surgical exploration.29 At the time of surgical exploration, their hockey groin syndrome, consisted of small tears in the external oblique aponeurosis through which branches of the ilioinguinal or iliohypogastric could be identified. These surgical procedures were all through a standard inguinal approach and the perforating neurovascular structures were excised, while the main trunk of the ilioinguinal nerve was ablated and the external oblique aponeurosis was repaired and reinforced with Goretex (W.L. Gore & Associates Inc, Flagstaff, AZ). At follow-up, 18 of the 22 players (82%) had no pain and 19 (86%) were able to resume their careers in the NHL.29 Ice hockey players with sports hernias or hockey groin syndrome often return to the sport, but it is important to identify these problems early so that surgical options can be discussed if the player fails conservative management. It is also critical to make sure that all pathology is identified, because in players with mixed sports hernia and FAI, return-to-play results improve when both issues are addressed. In a study of athletes (some of whom were ice hockey players), who had both FAI and sports hernia, and only hernia/pubalgia surgery was performed, 25% of these athletes returned to sport. If only FAI was addressed, 50% of the athletes returned to sport; however, when hernia and FAI were treated, 89% returned to play.33
Adductor strains includes injury to the adductor muscles, pectineus, obturator externus and gracilis, and are prevalent in ice hockey players. A study of elite Swedish ice hockey players published in 1988 reported that adductor strains accounted for 10% (10 of 95) of all injuries.34 Given the prevalence of these injuries, considerable research has been dedicated to understanding their mechanism and prevention.35 Adductor strains within the ice hockey population have been attributed to the eccentric forces on the adductors when players attempt to decelerate the leg during a stride.36 A study of NHL players revealed that a ratio <80% of adductor-to-abductor muscle is the best predictor of a groin strain.37
These injuries are also well known for their recurrence rates, as was the case in an NHL study where 4 of the 9 adductor strains (44%) were recurrent injuries.37 The authors attributed the recurrence to an incomplete rehabilitation program and an accelerated return to sport. This was followed by an NHL prevention program that spanned 2 seasons and analyzed 58 players whose adductor-to-abductor ratio was <80% and placed them into a 6-week intervention program during the preseason.37 Only 3 players sustained an adductor strain in the 2 subsequent seasons after the intervention, compared to 11 strains in the previous 2 seasons. Thus, early identification of muscle strength imbalance coupled with an appropriate intervention program has proven to be an effective means of reducing adductor strains in this at-risk population.
Continue to: Contact injuries may...
Contact injuries may vary with checking into the boards being unique to men’s ice hockey. Hip pointers occur as a result of a direct compression injury to the iliac crest, which causes trauma to the bone but also to the overlying hip abductor musculature, and represent roughly 2.4% of ice hockey injuries.23 The resulting contusion may cause a local hematoma formation. Early identification of the injury plus treatment with RICE (rest, ice, compression, elevation) coupled with crutches to limit weight-bearing status may minimize soft tissue trauma and swelling, and ultimately aid in pain control and return to sport.38 Hip abductor strengthening, added padding over the injured area, as well as a compressive hip spica wrapping, have all been suggested to expedite return to play and help prevent recurrence of the hip pointer.8
KNEE INJURIES
Injury to the medial collateral ligament (MCL) is the most commonly reported knee injury (Figure 2) and second only to concussion amongst all injuries in National Collegiate Athletic Association ice hockey players.8,39 The mechanism of injury typically involves a valgus force on the knee, which is often caused by collision into another player.39 Valgus stress testing with the knee in 30° of flexion is used to grade the severity of injury (Grade I: 0-5 mm of medial opening; Grade II: 5-10 mm of medial opening; Grade III: >10 mm of medial opening).39 One study that followed a single college hockey team for 8 seasons reported that 77% of injuries (10 of 13) occurred during player-to-player collision,39 with 5 being Grade 1 injuries, 6 Grade 2 injuries, 1 Grade 3; information was missing for 1 player. Nonoperative management of incomplete injuries, grade 1 and 2 sprains, with RICE and early physical therapy intervention to work on knee range of motion and quadriceps strengthening typically helps the player return to sport within days for grade 1 and 2 injuries to 3 weeks for grade 2 injuries. Complete tears have been managed both operatively and nonoperatively with evidence to suggest better outcomes after surgical intervention if there is a concomitant ACL injury requiring reconstruction.8,9
Anterior cruciate ligament (ACL) tears occur less frequently in hockey players compared to the players in other sports such as football and basketball.38,40 Between 2006 and 2010, 47 players were identified by the NHL Injury Surveillance System as having sustained an ACL injury, which equates to an incidence of 9.4 ACL injuries per NHL season over this time span.41 The mechanism of ACL tears in ice hockey players appears to be different from other sports players based on a recent MRI study that evaluated players for concomitant injuries following ACL tear and noted significantly fewer bone bruises on the lateral femoral condyle compared to players in other sports.42 Early evaluation after injury with Lachman and/or pivot shift tests aids the diagnosis. Data from the NHL study identified 32 players (68%) with concomitant meniscal injuries and 32 (68%) had MCL injuries in conjunction with their ACL tears.41 Average length in the league prior to injury was 5.65 seasons. Twenty-nine of the injured players (61.7%) underwent reconstruction with a patellar tendon autograft, 13 (27.7%) had a hamstring autograft, and 5 (10.6%) had either a patellar tendon or hamstring allograft.41 Meniscus and ACL injuries were associated with a decreased length of career compared to age-matched controls and, notably, players >30 years at the time of injury had only a 67% rate of return to sport whereas those <30 years had a return-to-sport rate of 80%. Players who were able to return did so at an average of 9.8 months (range, 6-21 months) and had a significant reduction in total number of goals, assists, and points scored compared to controls. Decline in performance was typically associated with forwards and wings, while defensemen did not demonstrate the same decrease in performance following return to ice hockey.41
Meniscal tears are a well-documented concomitant injury with ruptures of the ACL, and the combination is a known pattern associated with shorter careers compared to isolated ACL tears in ice hockey players.41 The lateral meniscus is known for increased mobility compared to the medial meniscus and is more commonly injured (39% vs 8.5%) in ACL tears that occur in contact sports and downhill skiing.42 Ice hockey presents a scenario that is different from other contact sports because of the near frictionless interaction between the player’s ice skates and playing surface. This likely equates to a different injury mechanism and dissipation of energy after contact as well as non-contact injuries.38 A recent study reviewed knee MRI findings associated with ACL tears in collegiate ice hockey players and compared to other sports known for their high rates of concomitant meniscal pathology. The authors reported a statistically significant decrease in lateral meniscus tears and bone-bruising patterns in ice hockey players with ACL injuries compared to athletes with ACL tears in other sports.43 In contrast, an NHL study of ACL tears in professional ice hockey players found that 68% of players had concomitant meniscal tears (32 out of 47 players).41
Continue to: The presence of...
The presence of a meniscal tear on MRI is typically a surgical problem, especially if it occurred with an ACL injury. Meniscal repair is preferable, if possible, because there is a known association of increased cartilage contact pressures associated with meniscal debridement. Return to sport following meniscus injury hinges upon whether it is an isolated injury and how it is treated. If the meniscus injury occurs in isolation and can be treated with a debridement and partial resection alone, there is obviously a quicker return to sport as the player can be weight-bearing immediately following surgery. Return to skating after meniscal debridement and partial resection is usually 4 to 6 weeks, whereas meniscal repair protocols vary depending on surgeon; players may need 3 months to 4 months to return to the ice.
Quadriceps contusions are contact injuries that are not unique to ice hockey (Figure 3). They may result from player collision but also from direct blows from a hockey puck. A high velocity puck is known to cause immense trauma to the quadriceps muscles, which may result in localized bleeding and hematoma formation. If the player is able to anticipate the event, active contraction of the quadriceps muscle has been shown to absorb some of the energy and result in a less traumatic injury, but in a fast paced ice hockey game, the player’s anticipation is less likely than in other sports such as baseball.44Interestingly, the degree of knee flexion after injury is predictive of injury severity with milder injuries associated with angles >90 and more severe injuries resulting in knee flexion angles <45° and typically an antalgic gait.45 It is important to treat these injuries during the first 24 hours with the knee maintained in 120°of flexion, plus ice and compression, which can be achieved using a locked knee brace or elastic compression wrap. Quadriceps stretching and isometric strengthening should immediately follow the period of immobilization. The addition of NSAIDs may help prevent the formation of myositis ossificans. A study from West Point suggests that the average return to sport or activity ranges from 13 days (mild contusion) to 21 days (severe contusions), while others8 have indicated that if the injury is treated acutely and a player is able to regain motion and strength, return to ice hockey within a few days is possible.
FOOT AND ANKLE
Ice hockey has some unique injuries that can be attributed to the use of ice skates for play. One such injury is boot-top lacerations, which are fortunately rare as they can be a career-ending injury.47 The spectrum of injury ranges from superficial abrasions to more severe soft tissue disruption, including the extensor tendons and neurovascular structures. The actual mechanism of injury involves an opponent’s skate blade cutting across the anterior ankle. One early case report described a protective method of having players place their skate tongues deep to their protective shin pads, instead of turning the tongues down.47 Kevlar socks have also been shown to help prevent or minimize the damage from a skate blade.48
Injury to the lateral ankle ligaments, anterior talofibular ligament or calcaneofibular ligament, are usually more common than the higher ankle sprains involving the syndesmosis. However, this is not the case in ice hockey. The rigidity of the ice skate at the level of the lateral ligaments seems to impart a protective mechanism to the lower ligaments, but this results in a higher incidence of syndesmotic injuries. These high ankle injuries are unfortunately more debilitating and often require a longer recovery period. In a study of these injuries in NHL players, syndesmotic sprains made up 74% of all ankle sprains, whereas only 18.4% of ankle sprains involved the syndesmosis in American football players..49,50 The average number of days between injury and return to play is 45 days, and some authors believe that defensemen may have a harder time recovering because of the demands on their ankles by having to switch continuously between forward and backward skating.49
Most patients are treated conservatively when their ankle plain radiographs show a congruent mortise and no evidence of syndesmotic widening. If the player expresses pain when squeezing the syndesmosis, it is helpful to obtain stress radiographs to further evaluate for syndesmotic injury. Nonoperative management includes RICE, immobilization in a rigid boot with crutches to protect weight-bearing with gradual advancements and eventually physical therapy to address any ankle stiffness, followed by dynamic functional activities. Treatment options for syndesmotic widening and failed conservative management includes both screw and plate options as well as suture buttons.49,51,52
Ankle and foot fractures were historically a rare injury in ice hockey players based on radiograph evaluation; however, the recent study by Baker and colleagues4 demonstrated that MRI can be helpful in detecting subradiographic fractures. Most of the injuries detected after MRI were from being hit by a hockey puck; this was a novel mechanism that had not been previously reported in the literature.4 Of the injuries that resulted from a direct blow, 14 of 17 occurred on the medial aspect of the foot and ankle, which is believed to result another word? from a defender skating towards an offensive player and attempting to block shots on goal. In this study, all occult fractures involving the medial malleolus were eventually treated with open reduction and internal fixation and underwent routine healing.4 The navicular bone and base of the first metatarsal accounted for the remaining medial-sided fractures. In a recent analysis of risk factors for reoperation following operative fixation of foot fractures across the National Basketball Association, the National Football Leagues, Major League Baseball, and the National Hockey League only a total of 3 fractures involving the foot (1 navicular and 2 first metatarsal) were identified in NHL players over a 30-year period.53 The study acknowledged a major limitation being a public source for identifying players with fractures.
Lace bite is another common ice hockey injury. It typically occurs at the beginning of a season or whenever a player is breaking in a new pair of skates. The cause of the lace bite is the rigid tongue in the skate that rubs against the anterior ankle. Skating causes inflammation in the area of the tibialis anterior tendon, and the player will complain of significant anterior ankle pain. First line treatment for lace bite is ice (Figure 4A), NSAID gel (eg, diclofenac 1%), and a Bunga lace-bite pad (Absolute Athletics). (Figure 4B).
SUMMARY
Lower extremity injuries are common in ice hockey players, and a covering physician should be comfortable managing these injuries from breezers to skate. Proper evaluation and work-up is critical for early diagnosis and identification of pathology, which can minimize the impact of the injury and expedite a treatment plan to return the player safely to the ice and in the game.
1. Flik K, Lyman S, Marx RG. American collegiate men's ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
2. Popkin CA, Nelson BJ, Park CN, et al. Head, neck, and shoulder injuries in ice hockey: current concepts. Am J Orthop (Belle Mead NJ). 2017;46(3):123-134.
3. Popkin CA, Schulz BM, Park CN, Bottiglieri TS, Lynch TS. Evaluation, management and prevention of lower extremity youth ice hockey injuries. Open Access J Sports Med. 534 2016;7:167-176.
4. Baker JC, Hoover EG, Hillen TJ, Smith MV, Wright RW, Rubin DA. Subradiographic foot and ankle fractures and bone contusions detected by MRI in elite ice hockey players. Am J Sports Med. 2016;44(5):1317-1323.
5. Philippon MJ, Ho CP, Briggs KK, Stull J, LaPrade RF. Prevalence of increased alpha angles as a measure of cam-type femoroacetabular impingement in youth ice hockey players. Am J Sports Med. 2013;41(6):1357-1362.
6. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
7. Jorgensen U, Schmidt-Olsen S. The epidemiology of ice hockey injuries. Br J Sports Med. 1986;20(1):7-9.
8. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. BrJ Sports Med. 2014;48(1):4-10.
9. Mosenthal W, Kim M, Holzshu R, Hanypsiak B, Athiviraham A. Common ice hockey injuries and treatment: a current concepts review. Curr Sports Med Rep. 2017;16(5):357-362.
10. Tyler TF, Silvers HJ, Gerhardt MB, Nicholas SJ. Groin injuries in sports medicine. Sports Health. 2010;2(3):231-236.
11. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
12. Dalton SL, Zupon AB, Gardner EC, Djoko A, Dompier TP, Kerr ZY. The epidemiology of hip/groin injuries in National Collegiate Athletic Association men's and women's ice hockey: 2009-2010 through 2014-2015 academic years. Orthop J Sports Med. 2016;4(3):2325967116632692.
13. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Ross JR, Bedi A, Stone RM, Sibilsky Enselman E, Kelly BT, Larson CM. Characterization of symptomatic hip impingement in butterfly ice hockey goalies. Arthroscopy. 2015;31(4):635-642.
16. Bedi A, Dolan M, Hetsroni I, et al. Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med. 2011;39(Suppl):43S-49S.
17. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
18. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral ears. Arthroscopy. 2015;31(11):2106-2111.
19. Audenaert EA, Peeters I, Vigneron L, Baelde N, Pattyn C. Hip morphological characteristics and range of internal rotation in femoroacetabular impingement. Am J Sports Med. 2012;40(6):1329-1336.
20. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
21. Kuhn AW, Ross JR, Bedi A. Three-dimensional imaging and computer navigation in planning for hip preservation surgery. Sports Med Arthrosc Rev. 2015;23(4):e31-e38.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Kuhn AW, Noonan BC, Kelly BT, Larson CM, Bedi A. The hip in ice hockey: a current concepts review. Arthroscopy. 2016;32(9):1928-1938.
24. O'Connor M, Minkara AA, Westermann RW, Rosneck J, Lynch TS. Return to play after hip arthroscopy: a systematic review and meta-analysis. Am J Sports Med. 2018:46(11):2780-2788.
25. Minkara AA, Westermann RW, Rosneck J, Lynch TS. Systematic review and meta-analysis of outcomes after hip arthroscopy in femoroacetabular impingement. Am J Sports Med. 2018:363546517749475.
26. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
27. Pierce CM, Laprade RF, Wahoff M, O'Brien L, Philippon MJ. Ice hockey goaltender rehabilitation, including on-ice progression, after arthroscopic hip surgery for femoroacetabular impingement. J Orthop Sports Phys Ther. 2013;43(3):129-141.
28. MacLeod DA, Gibbon WW. The sportsman's groin. Br J Surg. 1999;86(7):849-850.
29. Irshad K, Feldman LS, Lavoie C, Lacroix VJ, Mulder DS, Brown RA. Operative management of "hockey groin syndrome": 12 years of experience in National Hockey League players. Surgery. 2001;130(4):759-764; discussion 764-756.
30. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
31. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the "sports hernia": MR imaging findings. Radiology. 2008;247(3):797-807.
32. Jakoi A, O'Neill C, Damsgaard C, Fehring K, Tom J. Sports hernia in National Hockey League players: does surgery affect performance? Am J Sports Med. 2013;41(1):107-110.
33. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775.
34. Lorentzon R, Wedren H, Pietila T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
35. Holmich P, Uhrskou P, Ulnits L, et al. Effectiveness of active physical training as treatment for long-standing adductor-related groin pain in athletes: randomised trial. Lancet. 1999;353(9151):439-443.
36. Sim FH, Chao EY. Injury potential in modern ice hockey. Am J Sports Med. 1978;6(6):378-384.
37. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
38. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. BrJ Sports Med. 2009;43(13):1000-1005.
39. Grant JA, Bedi A, Kurz J, Bancroft R, Miller BS. Incidence and injury characteristics of medial collateral ligament injuries in male collegiate ice hockey players. Sports Health. 2013;5(3):270-272.
40. Erickson BJ, Harris JD, Cole BJ, et al. Performance and return to sport after anterior cruciate ligament reconstruction in National Hockey League players. Orthop J Sports Med. 2014;2(9):2325967114548831.
41. Sikka R, Kurtenbach C, Steubs JT, Boyd JL, Nelson BJ. Anterior Cruciate Ligament Injuries in Professional Hockey Players. Am J Sports Med. 2016;44(2):378-383.
42. Friden T, Erlandsson T, Zatterstrom R, Lindstrand A, Moritz U. Compression or distraction of the anterior cruciate injured knee: variations in injury pattern in contact sports and downhill skiing. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):144-147.
43. Kluczynski MA, Kang JV, Marzo JM, Bisson LJ. Magnetic resonance imaging and intra-articular findings after anterior cruciate ligament injuries in ice hockey versus other sports. Orthop J Sports Med. 2016;4(5):2325967116646534. 44. Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Orthop Surg. 2001;9(4):227-237.
45. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
46. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
47. Johnson PN, Mark; Green, Eric. Boot-top lacerations in ice hockey players: a new injury. Clin J Sports Med. 1991:205-208.
48. Nauth A, Aziz M, Tsuji M, Whalen DB, Theodoropoulos JS, Zdero R. The protective effect of Kevlar socks against hockey skate blade injuries: a biomechanical study. Orthop J Sports Med. 2014;2(Suppl 2):7.
49. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
50. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
51. Marymont JV, Lynch MA, Henning CE. Acute ligamentous diastasis of the ankle without fracture. Evaluation by radionuclide imaging. Am J Sports Med. 1986;14(5):407-409.
52. Miller CD, Shelton WR, Barrett GR, Savoie FH, Dukes AD. Deltoid and syndesmosis ligament injury of the ankle without fracture. Am J Sports Med. 1995;23(6):746-750.
53. Singh SK, Larkin KE, Kadakia AR, Hsu WK. Risk factors for reoperation and performance-based outcomes after operative fixation of foot fractures in the professional athlete: a cross-sport analysis. Sports Health. 2018;10(1):70-74.
54. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
55. Elattar O, Choi HR, Dills VD, Busconi B. Groin injuries (athletic pubalgia) and return to play. Sports Health. 2016;8(4):313-323.
1. Flik K, Lyman S, Marx RG. American collegiate men's ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
2. Popkin CA, Nelson BJ, Park CN, et al. Head, neck, and shoulder injuries in ice hockey: current concepts. Am J Orthop (Belle Mead NJ). 2017;46(3):123-134.
3. Popkin CA, Schulz BM, Park CN, Bottiglieri TS, Lynch TS. Evaluation, management and prevention of lower extremity youth ice hockey injuries. Open Access J Sports Med. 534 2016;7:167-176.
4. Baker JC, Hoover EG, Hillen TJ, Smith MV, Wright RW, Rubin DA. Subradiographic foot and ankle fractures and bone contusions detected by MRI in elite ice hockey players. Am J Sports Med. 2016;44(5):1317-1323.
5. Philippon MJ, Ho CP, Briggs KK, Stull J, LaPrade RF. Prevalence of increased alpha angles as a measure of cam-type femoroacetabular impingement in youth ice hockey players. Am J Sports Med. 2013;41(6):1357-1362.
6. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
7. Jorgensen U, Schmidt-Olsen S. The epidemiology of ice hockey injuries. Br J Sports Med. 1986;20(1):7-9.
8. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. BrJ Sports Med. 2014;48(1):4-10.
9. Mosenthal W, Kim M, Holzshu R, Hanypsiak B, Athiviraham A. Common ice hockey injuries and treatment: a current concepts review. Curr Sports Med Rep. 2017;16(5):357-362.
10. Tyler TF, Silvers HJ, Gerhardt MB, Nicholas SJ. Groin injuries in sports medicine. Sports Health. 2010;2(3):231-236.
11. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
12. Dalton SL, Zupon AB, Gardner EC, Djoko A, Dompier TP, Kerr ZY. The epidemiology of hip/groin injuries in National Collegiate Athletic Association men's and women's ice hockey: 2009-2010 through 2014-2015 academic years. Orthop J Sports Med. 2016;4(3):2325967116632692.
13. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Ross JR, Bedi A, Stone RM, Sibilsky Enselman E, Kelly BT, Larson CM. Characterization of symptomatic hip impingement in butterfly ice hockey goalies. Arthroscopy. 2015;31(4):635-642.
16. Bedi A, Dolan M, Hetsroni I, et al. Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med. 2011;39(Suppl):43S-49S.
17. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
18. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral ears. Arthroscopy. 2015;31(11):2106-2111.
19. Audenaert EA, Peeters I, Vigneron L, Baelde N, Pattyn C. Hip morphological characteristics and range of internal rotation in femoroacetabular impingement. Am J Sports Med. 2012;40(6):1329-1336.
20. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
21. Kuhn AW, Ross JR, Bedi A. Three-dimensional imaging and computer navigation in planning for hip preservation surgery. Sports Med Arthrosc Rev. 2015;23(4):e31-e38.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Kuhn AW, Noonan BC, Kelly BT, Larson CM, Bedi A. The hip in ice hockey: a current concepts review. Arthroscopy. 2016;32(9):1928-1938.
24. O'Connor M, Minkara AA, Westermann RW, Rosneck J, Lynch TS. Return to play after hip arthroscopy: a systematic review and meta-analysis. Am J Sports Med. 2018:46(11):2780-2788.
25. Minkara AA, Westermann RW, Rosneck J, Lynch TS. Systematic review and meta-analysis of outcomes after hip arthroscopy in femoroacetabular impingement. Am J Sports Med. 2018:363546517749475.
26. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
27. Pierce CM, Laprade RF, Wahoff M, O'Brien L, Philippon MJ. Ice hockey goaltender rehabilitation, including on-ice progression, after arthroscopic hip surgery for femoroacetabular impingement. J Orthop Sports Phys Ther. 2013;43(3):129-141.
28. MacLeod DA, Gibbon WW. The sportsman's groin. Br J Surg. 1999;86(7):849-850.
29. Irshad K, Feldman LS, Lavoie C, Lacroix VJ, Mulder DS, Brown RA. Operative management of "hockey groin syndrome": 12 years of experience in National Hockey League players. Surgery. 2001;130(4):759-764; discussion 764-756.
30. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
31. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the "sports hernia": MR imaging findings. Radiology. 2008;247(3):797-807.
32. Jakoi A, O'Neill C, Damsgaard C, Fehring K, Tom J. Sports hernia in National Hockey League players: does surgery affect performance? Am J Sports Med. 2013;41(1):107-110.
33. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775.
34. Lorentzon R, Wedren H, Pietila T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
35. Holmich P, Uhrskou P, Ulnits L, et al. Effectiveness of active physical training as treatment for long-standing adductor-related groin pain in athletes: randomised trial. Lancet. 1999;353(9151):439-443.
36. Sim FH, Chao EY. Injury potential in modern ice hockey. Am J Sports Med. 1978;6(6):378-384.
37. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
38. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. BrJ Sports Med. 2009;43(13):1000-1005.
39. Grant JA, Bedi A, Kurz J, Bancroft R, Miller BS. Incidence and injury characteristics of medial collateral ligament injuries in male collegiate ice hockey players. Sports Health. 2013;5(3):270-272.
40. Erickson BJ, Harris JD, Cole BJ, et al. Performance and return to sport after anterior cruciate ligament reconstruction in National Hockey League players. Orthop J Sports Med. 2014;2(9):2325967114548831.
41. Sikka R, Kurtenbach C, Steubs JT, Boyd JL, Nelson BJ. Anterior Cruciate Ligament Injuries in Professional Hockey Players. Am J Sports Med. 2016;44(2):378-383.
42. Friden T, Erlandsson T, Zatterstrom R, Lindstrand A, Moritz U. Compression or distraction of the anterior cruciate injured knee: variations in injury pattern in contact sports and downhill skiing. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):144-147.
43. Kluczynski MA, Kang JV, Marzo JM, Bisson LJ. Magnetic resonance imaging and intra-articular findings after anterior cruciate ligament injuries in ice hockey versus other sports. Orthop J Sports Med. 2016;4(5):2325967116646534. 44. Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Orthop Surg. 2001;9(4):227-237.
45. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
46. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
47. Johnson PN, Mark; Green, Eric. Boot-top lacerations in ice hockey players: a new injury. Clin J Sports Med. 1991:205-208.
48. Nauth A, Aziz M, Tsuji M, Whalen DB, Theodoropoulos JS, Zdero R. The protective effect of Kevlar socks against hockey skate blade injuries: a biomechanical study. Orthop J Sports Med. 2014;2(Suppl 2):7.
49. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
50. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
51. Marymont JV, Lynch MA, Henning CE. Acute ligamentous diastasis of the ankle without fracture. Evaluation by radionuclide imaging. Am J Sports Med. 1986;14(5):407-409.
52. Miller CD, Shelton WR, Barrett GR, Savoie FH, Dukes AD. Deltoid and syndesmosis ligament injury of the ankle without fracture. Am J Sports Med. 1995;23(6):746-750.
53. Singh SK, Larkin KE, Kadakia AR, Hsu WK. Risk factors for reoperation and performance-based outcomes after operative fixation of foot fractures in the professional athlete: a cross-sport analysis. Sports Health. 2018;10(1):70-74.
54. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
55. Elattar O, Choi HR, Dills VD, Busconi B. Groin injuries (athletic pubalgia) and return to play. Sports Health. 2016;8(4):313-323.
TAKE-HOME POINTS:
Ice hockey is a high-speed, collision sport with one of the highest injury rates in all of sports.
Femoroacetabular impingement is a cause of hip pain at all levels of ice hockey; studies indicate goaltenders are at high risk—particularly those who utilize the butterfly, as opposed to hybrid or stand-up, goaltending style.
Medial collateral ligament (MCL) tears are common in ice hockey and are usually the result of a collision with another player.
Use of Kevlar socks and placement of skate tongues deep to the shin pads can help reduce the chance of a boot-top laceration.
High-ankle sprains are more prevalent in ice hockey because of the rigidity of hockey skates and can be a cause of significant loss of time away from the rink.
Patterns of malignancies in patients with HIV-AIDS: a single institution observational study
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.