User login
Critical care medicine: An ongoing journey
My introduction to critical care medicine came about during the summer between my third and fourth years of medical school. During that brief break, I, like most of my classmates, was drawn to the classic medical satire The House of God by Samuel Shem,1 which had become a cult classic in the medical field for its ghoulish medical wisdom and dark humor. In “the house,” the intensive care unit (ICU) is “that mausoleum down the hall,” its patients “perched precariously on the edge of that slick bobsled ride down to death.”1 This sentiment persisted even as I began my critical care medicine fellowship in the mid-1990s.
The science and practice of critical care medicine have changed, evolved, and advanced over the past several decades reflecting newer technology, but also an aging population with higher acuity.2 Critical care medicine has established itself as a specialty in its own right, and the importance of the physician intensivist-led multidisciplinary care teams in optimizing outcome has been demonstrated.3,4 These teams have been associated with improved quality of care, reduced length of stay, improved resource utilization, and reduced rates of complications, morbidity, and death.
While there have been few medical miracles and limited advances in therapeutics over the last 30 years, advances in patient management, adherence to processes of care, better use of technology, and more timely diagnosis and treatment have facilitated improved outcomes.5 Collaboration with nurses, respiratory therapists, pharmacists, and other healthcare personnel is invaluable, as these providers are responsible for executing management protocols such as weaning sedation and mechanical ventilation, nutrition, glucose control, vasopressor and electrolyte titration, positioning, and early ambulation.
Unfortunately, as an increasing number of patients are being discharged from the ICU, evidence is accumulating that ICU survivors may develop persistent organ dysfunction requiring prolonged stays in the ICU and resulting in chronic critical illness. A 2015 study estimated 380,000 cases of chronic critical illness annually, particularly among the elderly population, with attendant hospital costs of up to $26 billion.6 While 70% of these patients may survive their hospitalization, the Society of Critical Care Medicine (SCCM) estimates that the 1-year post-discharge mortality rate may exceed 50%.7
We can take pride and comfort in knowing that the past several decades have seen growth in critical care training, more engaged practice, and heightened communication resulting in lower mortality rates.8 However, a majority of survivors suffer significant morbidities that may be severe and persist for a prolonged period after hospital discharge. These worsening impairments after discharge are termed postintensive care syndrome (PICS), which manifests as a new or worsening mental, cognitive, and physical condition and may affect up to 50% of ICU survivors.6
The impact on daily functioning and quality of life can be devastating, and primary care physicians will be increasingly called on to diagnose and participate in ongoing post-discharge management. Additionally, the impact of critical illness on relatives and informal caregivers can be long-lasting and profound, increasing their own risk of depression, posttraumatic stress disorder, and financial hardship.
In this issue of the Journal, Golovyan and colleagues identify several potential complications and sequelae of critical illness after discharge from the ICU.9 Primary care providers will see these patients in outpatient settings and need to be prepared to triage and treat the new-onset and chronic conditions for which these patients are at high risk.
In addition, as the authors point out, family members and informal caregivers need to be counseled about the proper care of these patients as well as themselves.
The current healthcare system does not appropriately address these survivors and their families. In 2015, the Society of Critical Care Medicine announced the THRIVE initiative, designed to improve support for the patient and family after critical illness. Given the many survivors and caregivers touched by critical illness, the Society has invested in THRIVE with the intent of helping those affected to work together with clinicians to advance recovery. Through peer support groups, post-ICU clinics, and continuing research into quality improvement, THRIVE may help to reduce readmissions and improve quality of life for critical care survivors and their loved ones.
Things have changed since the days of The House of God. Critical care medicine has become a vibrant medical specialty and an integral part of our healthcare system. Dedicated critical care physicians and the multidisciplinary teams they lead have improved outcomes and resource utilization.2–5
The demand for ICU care will continue to increase as our population ages and the need for medical and surgical services increases commensurately. The ratio of ICU beds to hospital beds continues to escalate, and it is feared that the demand for critical care professionals may outstrip the supply.
While we no longer see that mournful shaking of the head when a patient is admitted to the ICU, we need to have the proper vision and use the most up-to-date scientific knowledge and research in treating underlying illness to ensure that once these patients are discharged, communication continues between critical care and primary care providers. This ongoing support will ensure these patients the best possible quality of life.
- Shem S. The House of God: A Novel. New York: R. Marek Publishers, 1978; chapter 18.
- Lilly CM, Swami S, Liu X, Riker RR, Badawi O. Five year trends of critical care practice and outcomes. Chest 2017; 152(4):723–735. doi:10.1016/j.chest.2017.06.050
- Yoo EJ, Edwards JD, Dean ML, Dudley RA. Multidisciplinary critical care and intensivist staffing: results of a statewide survey and association with mortality. J Intensive Care Med 2016; 31(5):325–332. doi:10.1177/0885066614534605
- Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med 2008; 148(11):801–809. pmid:18519926
- Vincent JL, Singer M, Marini JJ, et al. Thirty years of critical care medicine. Crit Care 2010; 14(3):311. doi:10.1186/cc8979
- Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60(6):1070–1077. doi:10.1111/j.1532-5415.2012.03989.x
- Kahn JM, Le T, Angus DC, et al; ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States. Crit Care Med 2015; 43(2):282–287. doi:10.1097/CCM.0000000000000710
- Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long term acute care hospital utilization after critical illness. JAMA 2010; 303(22):2253–2259. doi:10.1001/jama.2010.761
- Golovyan DM, Khan SH, Wang S, Khan BA. What should I address at follow-up of patients who survive critical illness? Cleve Clin J Med 2018; 85(7):523–526. doi:10.3949/ccjm.85a.17104
My introduction to critical care medicine came about during the summer between my third and fourth years of medical school. During that brief break, I, like most of my classmates, was drawn to the classic medical satire The House of God by Samuel Shem,1 which had become a cult classic in the medical field for its ghoulish medical wisdom and dark humor. In “the house,” the intensive care unit (ICU) is “that mausoleum down the hall,” its patients “perched precariously on the edge of that slick bobsled ride down to death.”1 This sentiment persisted even as I began my critical care medicine fellowship in the mid-1990s.
The science and practice of critical care medicine have changed, evolved, and advanced over the past several decades reflecting newer technology, but also an aging population with higher acuity.2 Critical care medicine has established itself as a specialty in its own right, and the importance of the physician intensivist-led multidisciplinary care teams in optimizing outcome has been demonstrated.3,4 These teams have been associated with improved quality of care, reduced length of stay, improved resource utilization, and reduced rates of complications, morbidity, and death.
While there have been few medical miracles and limited advances in therapeutics over the last 30 years, advances in patient management, adherence to processes of care, better use of technology, and more timely diagnosis and treatment have facilitated improved outcomes.5 Collaboration with nurses, respiratory therapists, pharmacists, and other healthcare personnel is invaluable, as these providers are responsible for executing management protocols such as weaning sedation and mechanical ventilation, nutrition, glucose control, vasopressor and electrolyte titration, positioning, and early ambulation.
Unfortunately, as an increasing number of patients are being discharged from the ICU, evidence is accumulating that ICU survivors may develop persistent organ dysfunction requiring prolonged stays in the ICU and resulting in chronic critical illness. A 2015 study estimated 380,000 cases of chronic critical illness annually, particularly among the elderly population, with attendant hospital costs of up to $26 billion.6 While 70% of these patients may survive their hospitalization, the Society of Critical Care Medicine (SCCM) estimates that the 1-year post-discharge mortality rate may exceed 50%.7
We can take pride and comfort in knowing that the past several decades have seen growth in critical care training, more engaged practice, and heightened communication resulting in lower mortality rates.8 However, a majority of survivors suffer significant morbidities that may be severe and persist for a prolonged period after hospital discharge. These worsening impairments after discharge are termed postintensive care syndrome (PICS), which manifests as a new or worsening mental, cognitive, and physical condition and may affect up to 50% of ICU survivors.6
The impact on daily functioning and quality of life can be devastating, and primary care physicians will be increasingly called on to diagnose and participate in ongoing post-discharge management. Additionally, the impact of critical illness on relatives and informal caregivers can be long-lasting and profound, increasing their own risk of depression, posttraumatic stress disorder, and financial hardship.
In this issue of the Journal, Golovyan and colleagues identify several potential complications and sequelae of critical illness after discharge from the ICU.9 Primary care providers will see these patients in outpatient settings and need to be prepared to triage and treat the new-onset and chronic conditions for which these patients are at high risk.
In addition, as the authors point out, family members and informal caregivers need to be counseled about the proper care of these patients as well as themselves.
The current healthcare system does not appropriately address these survivors and their families. In 2015, the Society of Critical Care Medicine announced the THRIVE initiative, designed to improve support for the patient and family after critical illness. Given the many survivors and caregivers touched by critical illness, the Society has invested in THRIVE with the intent of helping those affected to work together with clinicians to advance recovery. Through peer support groups, post-ICU clinics, and continuing research into quality improvement, THRIVE may help to reduce readmissions and improve quality of life for critical care survivors and their loved ones.
Things have changed since the days of The House of God. Critical care medicine has become a vibrant medical specialty and an integral part of our healthcare system. Dedicated critical care physicians and the multidisciplinary teams they lead have improved outcomes and resource utilization.2–5
The demand for ICU care will continue to increase as our population ages and the need for medical and surgical services increases commensurately. The ratio of ICU beds to hospital beds continues to escalate, and it is feared that the demand for critical care professionals may outstrip the supply.
While we no longer see that mournful shaking of the head when a patient is admitted to the ICU, we need to have the proper vision and use the most up-to-date scientific knowledge and research in treating underlying illness to ensure that once these patients are discharged, communication continues between critical care and primary care providers. This ongoing support will ensure these patients the best possible quality of life.
My introduction to critical care medicine came about during the summer between my third and fourth years of medical school. During that brief break, I, like most of my classmates, was drawn to the classic medical satire The House of God by Samuel Shem,1 which had become a cult classic in the medical field for its ghoulish medical wisdom and dark humor. In “the house,” the intensive care unit (ICU) is “that mausoleum down the hall,” its patients “perched precariously on the edge of that slick bobsled ride down to death.”1 This sentiment persisted even as I began my critical care medicine fellowship in the mid-1990s.
The science and practice of critical care medicine have changed, evolved, and advanced over the past several decades reflecting newer technology, but also an aging population with higher acuity.2 Critical care medicine has established itself as a specialty in its own right, and the importance of the physician intensivist-led multidisciplinary care teams in optimizing outcome has been demonstrated.3,4 These teams have been associated with improved quality of care, reduced length of stay, improved resource utilization, and reduced rates of complications, morbidity, and death.
While there have been few medical miracles and limited advances in therapeutics over the last 30 years, advances in patient management, adherence to processes of care, better use of technology, and more timely diagnosis and treatment have facilitated improved outcomes.5 Collaboration with nurses, respiratory therapists, pharmacists, and other healthcare personnel is invaluable, as these providers are responsible for executing management protocols such as weaning sedation and mechanical ventilation, nutrition, glucose control, vasopressor and electrolyte titration, positioning, and early ambulation.
Unfortunately, as an increasing number of patients are being discharged from the ICU, evidence is accumulating that ICU survivors may develop persistent organ dysfunction requiring prolonged stays in the ICU and resulting in chronic critical illness. A 2015 study estimated 380,000 cases of chronic critical illness annually, particularly among the elderly population, with attendant hospital costs of up to $26 billion.6 While 70% of these patients may survive their hospitalization, the Society of Critical Care Medicine (SCCM) estimates that the 1-year post-discharge mortality rate may exceed 50%.7
We can take pride and comfort in knowing that the past several decades have seen growth in critical care training, more engaged practice, and heightened communication resulting in lower mortality rates.8 However, a majority of survivors suffer significant morbidities that may be severe and persist for a prolonged period after hospital discharge. These worsening impairments after discharge are termed postintensive care syndrome (PICS), which manifests as a new or worsening mental, cognitive, and physical condition and may affect up to 50% of ICU survivors.6
The impact on daily functioning and quality of life can be devastating, and primary care physicians will be increasingly called on to diagnose and participate in ongoing post-discharge management. Additionally, the impact of critical illness on relatives and informal caregivers can be long-lasting and profound, increasing their own risk of depression, posttraumatic stress disorder, and financial hardship.
In this issue of the Journal, Golovyan and colleagues identify several potential complications and sequelae of critical illness after discharge from the ICU.9 Primary care providers will see these patients in outpatient settings and need to be prepared to triage and treat the new-onset and chronic conditions for which these patients are at high risk.
In addition, as the authors point out, family members and informal caregivers need to be counseled about the proper care of these patients as well as themselves.
The current healthcare system does not appropriately address these survivors and their families. In 2015, the Society of Critical Care Medicine announced the THRIVE initiative, designed to improve support for the patient and family after critical illness. Given the many survivors and caregivers touched by critical illness, the Society has invested in THRIVE with the intent of helping those affected to work together with clinicians to advance recovery. Through peer support groups, post-ICU clinics, and continuing research into quality improvement, THRIVE may help to reduce readmissions and improve quality of life for critical care survivors and their loved ones.
Things have changed since the days of The House of God. Critical care medicine has become a vibrant medical specialty and an integral part of our healthcare system. Dedicated critical care physicians and the multidisciplinary teams they lead have improved outcomes and resource utilization.2–5
The demand for ICU care will continue to increase as our population ages and the need for medical and surgical services increases commensurately. The ratio of ICU beds to hospital beds continues to escalate, and it is feared that the demand for critical care professionals may outstrip the supply.
While we no longer see that mournful shaking of the head when a patient is admitted to the ICU, we need to have the proper vision and use the most up-to-date scientific knowledge and research in treating underlying illness to ensure that once these patients are discharged, communication continues between critical care and primary care providers. This ongoing support will ensure these patients the best possible quality of life.
- Shem S. The House of God: A Novel. New York: R. Marek Publishers, 1978; chapter 18.
- Lilly CM, Swami S, Liu X, Riker RR, Badawi O. Five year trends of critical care practice and outcomes. Chest 2017; 152(4):723–735. doi:10.1016/j.chest.2017.06.050
- Yoo EJ, Edwards JD, Dean ML, Dudley RA. Multidisciplinary critical care and intensivist staffing: results of a statewide survey and association with mortality. J Intensive Care Med 2016; 31(5):325–332. doi:10.1177/0885066614534605
- Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med 2008; 148(11):801–809. pmid:18519926
- Vincent JL, Singer M, Marini JJ, et al. Thirty years of critical care medicine. Crit Care 2010; 14(3):311. doi:10.1186/cc8979
- Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60(6):1070–1077. doi:10.1111/j.1532-5415.2012.03989.x
- Kahn JM, Le T, Angus DC, et al; ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States. Crit Care Med 2015; 43(2):282–287. doi:10.1097/CCM.0000000000000710
- Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long term acute care hospital utilization after critical illness. JAMA 2010; 303(22):2253–2259. doi:10.1001/jama.2010.761
- Golovyan DM, Khan SH, Wang S, Khan BA. What should I address at follow-up of patients who survive critical illness? Cleve Clin J Med 2018; 85(7):523–526. doi:10.3949/ccjm.85a.17104
- Shem S. The House of God: A Novel. New York: R. Marek Publishers, 1978; chapter 18.
- Lilly CM, Swami S, Liu X, Riker RR, Badawi O. Five year trends of critical care practice and outcomes. Chest 2017; 152(4):723–735. doi:10.1016/j.chest.2017.06.050
- Yoo EJ, Edwards JD, Dean ML, Dudley RA. Multidisciplinary critical care and intensivist staffing: results of a statewide survey and association with mortality. J Intensive Care Med 2016; 31(5):325–332. doi:10.1177/0885066614534605
- Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med 2008; 148(11):801–809. pmid:18519926
- Vincent JL, Singer M, Marini JJ, et al. Thirty years of critical care medicine. Crit Care 2010; 14(3):311. doi:10.1186/cc8979
- Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60(6):1070–1077. doi:10.1111/j.1532-5415.2012.03989.x
- Kahn JM, Le T, Angus DC, et al; ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States. Crit Care Med 2015; 43(2):282–287. doi:10.1097/CCM.0000000000000710
- Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long term acute care hospital utilization after critical illness. JAMA 2010; 303(22):2253–2259. doi:10.1001/jama.2010.761
- Golovyan DM, Khan SH, Wang S, Khan BA. What should I address at follow-up of patients who survive critical illness? Cleve Clin J Med 2018; 85(7):523–526. doi:10.3949/ccjm.85a.17104
Optimizing calcium and vitamin D intake through diet and supplements
Although calcium and vitamin D are often recommended for prevention and treatment of osteoporosis, considerable controversy exists in terms of their safety and efficacy.1 This article highlights the issues, referring readers to reviews and meta-analyses for details and providing some practical advice for patients requiring supplementation.
CALCIUM INTAKE AND BONE DENSITY
Calcium enters the body through diet and supplementation. If intake is low, blood calcium levels fall, resulting in secondary hyperparathyroidism, which has 3 main effects:
- Increased fractional absorption of the calcium that is consumed
- Reduced urinary excretion of calcium
- Increased bone resorption, which releases calcium into the blood,2 which explains the potential for the deleterious effect of deficient intake of calcium on bone.3
Based on the simple physiology outlined above, it seems logical that insufficient intake of calcium over time could lead to mobilization of calcium from bone, lower bone mineral density, and higher fracture risk.3 This topic has been reviewed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and the International Foundation for Osteoporosis.1
Many lines of evidence suggest that low calcium intake adversely affects bone mineral density.1 Low calcium intake has been associated with lower bone density in some cross-sectional studies,4–6 though not all.7 Interventions to increase calcium intake in postmenopausal women have shown beneficial effects on bone density,8–10 though in some studies the benefit was small and nonprogressive.11 The question is whether this improvement in bone mineral density translates into fewer fractures.
Results from individual studies looking at fracture prevention through calcium supplementation have been conflicting,10,12–14 and reviews and meta-analyses have summarized the data.1,3,15 A recent review of these meta-analyses showed a small but significant reduction in some types of fracture.1
Some speculate that the difficulty in demonstrating fracture efficacy might be due to imperfect compliance with calcium intake, and that the participants in the placebo groups often had fairly robust calcium intake from diet and off-study supplemental intake, which could reduce the sensitivity of studies to demonstrate the fracture benefit.1,16
The US Preventive Services Task Force17 recommends that the general public not take supplemental calcium for skeletal health, but emphasizes that this recommendation does not apply to patients with osteoporosis. Most other official guidelines (eg, those of the Endocrine Society,18 American Association of Clinical Endocrinologists,19 Institute of Medicine,20 and National Osteoporosis Foundation21) recommend adequate calcium intake to optimize skeletal health.
CALCIUM INTAKE AND CORONARY ARTERY DISEASE
Patients often wonder if the calcium in their supplements ends up in their coronary arteries rather than their bones. Although we once dismissed such concerns, several studies and meta-analyses have reported higher rates of cardiovascular disease with supplemental calcium use.22–24 A proposed mechanism to explain this increased risk is that taking calcium supplements transiently raises the serum calcium level, resulting in calcium deposition in coronary arteries, accelerating atherosclerosis formation.25
On the other hand, some studies and meta-analyses have not shown any increased risk of cardiovascular disease with calcium and vitamin D supplementation.26,27 This subject has been reviewed by Harvey et al.1
Our conclusions are as follows:
Patients should be told that the National Osteoporosis Foundation and the American Society for Preventive Cardiology released a statement in 2016 adopting the position that calcium intake from food and supplements should be considered safe from a cardiovascular perspective.28
If patients want to avoid the possible increase in risk of cardiovascular disease due to calcium supplementation, they can optimize their calcium intake with dietary calcium. Observational studies that showed increased risk with supplemental calcium found no such increase in cardiovascular disease with a robust dietary intake of calcium.29
This is not to say that patients should be encouraged to boost their dietary calcium intake and avoid heart disease by eating more cheese and ice cream, as these foods are high in saturated fats and cholesterol. Many dairy and nondairy sources of calcium do not contain these undesirable nutrients.
CALCIUM SUPPLEMENTATION AND NEPHROLITHIASIS
High dietary calcium intake has not been shown to increase the risk of kidney stones.
In the Nurses’ Health Study, the multivariate relative risk of stone formation was 0.65 (95% confidence interval [CI] 0.5–0.83) in those in the highest vs the lowest quintiles of dietary calcium intake.30 In contrast, the relative risk of stones in those taking calcium supplements was 1.2 (CI 1.02–1.41),30 although this higher risk was not seen in younger women (ages 27 to 44).31
Similar results were seen in the Women’s Health Initiative, in which calcium carbonate and vitamin D supplements resulted in a relative increased risk of stone formation of 1.17 (95% CI 1.02–1.34) compared with women on placebo.12
Data from male stone-formers also suggests that high dietary calcium intake does not increase the risk of stones.32
A theory to explain the difference between dietary and supplemental calcium with respect to stone formation is that dietary calcium binds to oxalate in the gut and reduces its absorption. The most common type of kidney stones are composed of calcium oxalate, and the oxalate, not the calcium, may be the real culprit. In contrast, calcium supplements are often taken between meals and therefore do not exert this protective effect and may be absorbed more rapidly and raise the serum calcium level more, which could lead to higher urinary calcium excretion.33
CALCIUM INTAKE IN PATIENTS TAKING ANTIRESORPTIVE DRUGS
Patients often mistakenly think that calcium and vitamin D supplements are given for mild cases of bone loss, and that if their bone loss is significant enough to require a medication, then they no longer need calcium and vitamin D supplements. Most clinical trials showing bone mineral density and fracture benefit from antiresorptive therapy were in patients who were taking enough calcium and vitamin D, so the efficacy of antiresorptive therapy is most clear only when taking enough calcium and vitamin D.34,35
Furthermore, patients with inadequate calcium and vitamin D intake essentially maintain their serum calcium levels by mobilizing calcium from bone; the combination of insufficient calcium intake and administration of agents that interfere with the ability to mobilize calcium from bone may put patients at risk of hypocalcemia.36
OPTIMIZING INTAKE OF CALCIUM AND VITAMIN D
Diet is key to calcium intake. People should consume adequate amounts of calcium-rich foods regardless of whether they have a history of kidney stones, since robust dietary intake of calcium does not increase the risk of cardiovascular disease or kidney stones and may actually have a protective effect. We also remain skeptical of the concern that supplemental calcium increases the risk of cardiovascular disease.
We recommend a target total calcium intake from diet, and if necessary, supplements, of 1,000 to 1,200 mg daily, and not to worry about cardiovascular disease or kidney stones. A patient or clinician reluctant to push calcium intake that high with supplements might opt for a more conservative goal of 800 mg of calcium daily. This recommendation is based on data suggesting that in the presence of vitamin D sufficiency, calcium supplementation with 500 mg of calcium citrate does little for patients whose calcium intake is above 400 mg/day.37
Vitamin D: How much do we need?
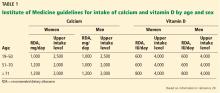
Regarding vitamin D intake, the Institute of Medicine recommends 600 to 800 IU to achieve a 25-hydroxyvitamin D level of 20 to 40 ng/mL.20
The Endocrine Society recommends “at least” 600 to 800 IU, but says that 1,500 to 2,000 IU may be needed to get the 25-hydroxyvitamin D level to 30 to 60 ng/mL.18
The Institute of Medicine based its recommendation on randomized controlled trials that showed fewer fractures with vitamin D intakes of 600 to 800 IU/day.13,14 Also, observational studies show little further reduction in fracture risk when the 25-hydroxyvitamin D levels rise above 20 ng/mL.38 A case-control study found an association between 25-hydroxyvitamin D levels higher than 40 ng/mL and pancreatic cancer.39
The Endocrine Society guidelines recommended higher intakes and levels of vitamin D because there are data suggesting that vitamin D levels higher than 30 ng/mL suppress parathyroid hormone levels further, which should favor less mobilization of bone.40
Levels of 25-hydroxyvitamin D in people exposed to plenty of sunlight rarely go above 60 ng/mL, suggesting 60 ng/mL should be the upper limit of levels to target, and it is unlikely that such levels are harmful.41
Implementing either recommendation—a target 25-hydroxyvitamin D level of 20 to 40 ng/mL or 30 to 60 ng/mL—is reasonable.
CALCULATING A PATIENT’S DIETARY CALCIUM INTAKE
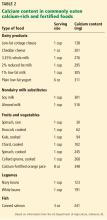
A detailed dietary history can be obtained by a dietitian, or by using the Calcium Calculator app supported by the International Osteoporosis Foundation.43 However, dietary calcium intake can be assessed quickly. To approximate a patient’s total dietary calcium intake (in milligrams), we multiply the number of servings of dietary calcium by 300. A serving of dietary calcium is found in:
- 1 cup of milk, yogurt, calcium-fortified juice, almonds, cooked spinach, or collard greens
- 1.5 ounces of hard cheese
- 2 cups of ice cream, cottage cheese, or beans
- 4 ounces of tofu or canned fish with bones such as salmon or sardines.
Therefore, if a patient consumes 1 cup of milk daily and 1 cup of yogurt 3 times a week, she takes in an estimated 1.5 servings of dietary calcium daily, or 450 mg. What the patient does not receive in the diet should be made up with supplemental calcium.
CALCIUM SUPPLEMENTS
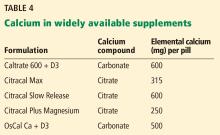
Calcium citrate has certain advantages as a supplement. Calcium carbonate requires gastric acidity to be absorbed and is therefore better absorbed if taken with meals; however, calcium citrate is equally well absorbed in the fasting or fed state and so can be taken without regard for achlorhydria or timing of meals.44
Another potential advantage of calcium citrate is that it has never been shown to increase the risk of kidney stones the way calcium carbonate has.12 Further, potassium citrate is a treatment for certain types of kidney stones,45 and it is possible that when calcium is given as citrate there is less danger of kidney stones.46 For these reasons, we generally recommend calcium citrate over other forms of calcium.
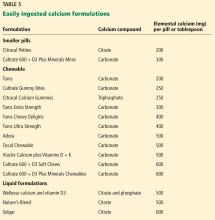
The brand of calcium citrate most readily available is Citracal, but any version of calcium citrate is acceptable.
SOURCES OF CONFUSION
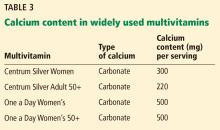
Labels that describe calcium content of supplements are often misleading, and this lack of clarity can interfere with the patient’s ability to correctly identify how much calcium is in each pill.
Serving size. Whereas 1 serving of Caltrate is 1 pill, 1 serving of Tums or Citracal is 2 pills; for other brands a serving may be 3 or 4 pills.
Calcium salt vs elemental calcium. The amount of elemental calcium contained in different calcium salts varies according to the molecular weight of the salt: 1,000 mg of calcium carbonate has 400 mg of elemental calcium, while 1,000 mg of calcium citrate has 200 mg of elemental calcium.
When we recommend 1,000 to 1,200 mg of calcium daily, we mean the amount of elemental calcium. The label on calcium supplements usually indicates the amount of elemental calcium, but some have confusing information about the amount of calcium salt they contain. For instance, Tums lists the amount of calcium carbonate per pill on the top of the label, but elsewhere lists the amount of elemental calcium.
Same brand, different preparation. Some brands of calcium have more than 1 formulation, each with a different amount of calcium. For instance, Citracal has a maximum-strength 315-mg tablet and a “petite” 200-mg tablet. Careful reading of the label is required to make sure that the patient is getting the amount of calcium she thinks she is getting.
OPTIONS FOR THOSE WITH DIFFICULTY SWALLOWING LARGE PILLS
Many calcium pills are large and difficult to swallow. Patients often ask if calcium pills can be crushed, and the answer is that they certainly can, but this approach is cumbersome and usually results in patients eventually stopping calcium in frustration.
CALCIUM SUPPLEMENTS AND CONSTIPATION
Constipation is a common side effect of calcium supplementation.47 Many patients report that they cannot take a calcium supplement because of constipation, or ask if there are calcium preparations that are less constipating than others.
There are ways of overcoming the constipating effects of calcium. Osmotic laxatives and stool softeners such as polyethylene glycol, magnesium citrate, and docusate sodium are safe and effective, although patients are often reluctant to take a medicine to combat the side effects from another medicine.
In such circumstances patients are often amenable to taking a combination product such as calcium with magnesium, since the cathartic effects of magnesium nicely counteract the constipating effects of calcium. This idea is exploited in antacids such as Rolaids, which are combinations of calcium carbonate and magnesium oxide that usually have no net effect on stool consistency.47
Many patients believe that calcium must be combined with magnesium to be absorbed. Although there are no data to support this idea, a patient already harboring this misconception may be more amenable to calcium-magnesium combinations for the purpose of avoiding constipation.
If a patient cannot find a calcium preparation that she can take at the full recommended doses, we often suggest starting with a very small dose for 2 weeks, and then adjusting the dose upward every 2 weeks until reaching the maximum dose that the patient can tolerate. Even if the dose is well below recommended doses, most of the benefit of calcium is obtained by bringing total intake to more than 500 mg daily,37 so continued use should be encouraged even when optimal targets cannot be sustained.
For patients who cannot tolerate enough calcium, we recommend being especially sure to optimize the vitamin D levels, since there are studies that suggest that secondary hyperparathyroidism mostly occurs in states of low calcium intake if vitamin D levels are insufficient.48
If the patient has secondary hyperparathyroidism despite best attempts at supplementation with calcium and vitamin D, consider prescribing calcitriol (activated vitamin D), which stimulates gut absorption of whatever calcium is taken.49 If calcitriol is given, the patient must undergo cumbersome monitoring for hypercalcemia and hypercalciuria. Fortunately, it is unusual to require calcitriol unless the patient has significant structural gastrointestinal abnormalities such as gastric bypass or Crohn disease.
- Harvey NC, Bilver E, Kaufman JM, et al. The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 2017; 28(2):447–462. doi:10.1007/s00198-016-3773-6
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 2005; 115(12):3318–3325. doi.10.1172/JCI27071
- Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med 2013; 369(16):1537–154 doi:10.1056/NEJMcp1210380
- Choi MJ, Park EJ, Jo HJ. Relationship of nutrient intakes and bone mineral density of elderly women in Daegu, Korea. Nutr Res Pract 2007; 1(4):328–33 doi:10.4162/nrp.2007.1.4.328
- Kim KM, Choi SH, Lim S, et al. Interactions between dietary calcium intake and bone mineral density or bone ge6ometry in a low calcium intake population (KNHANES IV 2008–2010). J Clin Endocrinol Metab 2014; 99(7):2409–2417. doi:10.1210/jc.2014-1006
- Joo NS, Dawson-Hughes B, Kim YS, Oh K, Yeum KJ. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J Bone Miner Res 2013; 28(4):764–770. doi:10.1002/jbmr.1790
- Anderson JJ, Roggenkamp KJ, Suchindran CM. Calcium intakes and femoral and lumbar bone density of elderly US men and women: National Health and Nutrition Examination Survey 2005–2006 analysis. J Clin Endocrinol Metab 2012; 97(12):4531–4539. doi:10.1210/jc.2012-1407
- Gui JC, Brašic JR, Liu XD, et al. Bone mineral density in postmenopausal Chinese women treated with calcium fortification in soymilk and cow’s milk. Osteoporos Int 2012; 23(5):1563–1570. doi:10.1007/s00198-012-1895-z
- Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30-month dietary intervention on bone mineral density: the Postmenopausal Health Study. Br J Nutr 2010; 104(1):100–107. doi:10.1017/S000711451000019X
- Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996; 11(12):1961–1966. doi:10.1002/jbmr.5650111218
- Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015; 351:h4183. doi:10.1136/bmj.h4183
- Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006; 354(7):669–683. doi:10.1056/NEJMoa055218
- Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327(23):1637–1642. doi:10.1056/NEJM199212033272305
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337(10):670–676. doi:10.1056/NEJM199709043371003
- Bolland MJ, Leung W, Tai V, et al. Calcium intake and risk of fracture: systematic review. BMJ 2015; 351:h4580. doi:10.1136/bmj.h4580
- Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med 2012; 367(1):77–78. doi:10.1056/NEJMe1206858
- Moyer VA; US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158(9):691–696. doi:10.7326/0003-4819-158-9-201305070-00603
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7):1911–1930. doi:10.1210/jc.2011-0385
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologist and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract 2016; 22(suppl 4):1–42. doi:10.4158/EP161435.ESGL
- Ross AC, Manson JE, Abrams SA et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
- Cosman F, De Beur SJ, Leboff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25(10):2359–2381. doi:10.1007/s00198-014-2794-2
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336(7638):262–266. doi:10.1136/bmj.39440.525752.BE
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011; 342:d2040. doi:10.1136/bmj.d2040
- Mao PJ, Zhang C, Tang L, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 169(2):106–111. doi:10.1016/j.ijcard.2013.08.055
- Reid IR, Bolland MJ. Calcium supplementation and vascular disease. Climacteric 2008; 11(4):280–286. doi:10.1080/13697130802229639
- Lewis JR, Radavelli-Bagatini S, Rejnmark L, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015; 30(1):165–175. doi:10.1002/jbmr.2311
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115(19):846–854. doi:10.1161/CIRCULATIONAHA.106.673491
- Kopecky SL, Bauer DC, Gulati M, et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med 2016; 165(12):867–868. doi:10.7326/M16-1743
- Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc 2016; 5(10):e003815. doi:10.1161/JAHA.116.003815
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126(7):497–504. pmid:9092314
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164(8):885–891. doi:10.1001/archinte.164.8.885
- Borhi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346(2):77–84. doi:10.1056/NEJMoa010369
- Prochaska ML, Taylor EN, Curhan GC. Insights into nephrolithiasis from the Nurses’ Health Studies. Am J Public Health 2016; 106(9):1638–1643. doi:10.2105/AJPH.2016.303319
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Chen J, Smerdely P. Hypocalcaemia after denosumab in older people following fracture. Osteoporos Int 2017; 28(2):517–522. doi:10.1007/s00198-016-3755-8
- Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 1990; 323(13):878–883. doi:10.1056/NEJM199009273231305
- Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab 2010; 95(6):2637–2645. doi:10.1210/jc.2009-2699
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1):81–93. doi:10.1093/aje/kwq120
- Valcour A, Blocki F, Hawkins DM, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab 2012; 97(11):3989–3995. doi:10.1210/jc.2012-2276
- Binkley N, Novotny R, Krueger T, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007; 92(6):2130–2135. doi:10.1210/jc.2006-2250
- United States Department of Agriculture (USDA). Agricultural Research Service. USDA food composition databases. https://ndb.nal.usda.gov/ndb/search/list. Accessed May 7, 2018.
- International Osteoporosis Foundation. IOF calcium calculator version 1.10. Apple App Store. https://itunes.apple.com/us/app/iof-calcium-calculator/id956198268?mt=8. Accessed June 11, 2018.
- Recker RR. Calcium absorption and achlorhydria. N Engl J Med 1985; 313(2):70–73. doi:10.1056/NEJM198507113130202
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115(10):2598–2608. doi:10.1172/JCI26662
- Sakhaee K, Poindexter JR, Griffith CS, Pak CY. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J Urol 2004; 172(3):958–961. doi:10.1097/01.ju.0000136400.14728.cd
- Kitchin B. Nutrition counseling for patients with osteoporosis: a personal approach. J Clin Densitom 2013; 16(4):426–431. doi:10.1016/j.jocd.2013.08.013
- Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005; 294(18):2336–2341. doi:10.1001/jama.294.18.2336
- Need AG, Horowitz M, Philcox JC, Nordin BE. 1,25-dihydroxycalciferol and calcium therapy in osteoporosis with calcium malabsorption. Dose response relationship of calcium absorption and indices of bone turnover. Miner Electrolyte Metab 1985; 11(1):35–40. pmid:3838358
Although calcium and vitamin D are often recommended for prevention and treatment of osteoporosis, considerable controversy exists in terms of their safety and efficacy.1 This article highlights the issues, referring readers to reviews and meta-analyses for details and providing some practical advice for patients requiring supplementation.
CALCIUM INTAKE AND BONE DENSITY
Calcium enters the body through diet and supplementation. If intake is low, blood calcium levels fall, resulting in secondary hyperparathyroidism, which has 3 main effects:
- Increased fractional absorption of the calcium that is consumed
- Reduced urinary excretion of calcium
- Increased bone resorption, which releases calcium into the blood,2 which explains the potential for the deleterious effect of deficient intake of calcium on bone.3
Based on the simple physiology outlined above, it seems logical that insufficient intake of calcium over time could lead to mobilization of calcium from bone, lower bone mineral density, and higher fracture risk.3 This topic has been reviewed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and the International Foundation for Osteoporosis.1
Many lines of evidence suggest that low calcium intake adversely affects bone mineral density.1 Low calcium intake has been associated with lower bone density in some cross-sectional studies,4–6 though not all.7 Interventions to increase calcium intake in postmenopausal women have shown beneficial effects on bone density,8–10 though in some studies the benefit was small and nonprogressive.11 The question is whether this improvement in bone mineral density translates into fewer fractures.
Results from individual studies looking at fracture prevention through calcium supplementation have been conflicting,10,12–14 and reviews and meta-analyses have summarized the data.1,3,15 A recent review of these meta-analyses showed a small but significant reduction in some types of fracture.1
Some speculate that the difficulty in demonstrating fracture efficacy might be due to imperfect compliance with calcium intake, and that the participants in the placebo groups often had fairly robust calcium intake from diet and off-study supplemental intake, which could reduce the sensitivity of studies to demonstrate the fracture benefit.1,16
The US Preventive Services Task Force17 recommends that the general public not take supplemental calcium for skeletal health, but emphasizes that this recommendation does not apply to patients with osteoporosis. Most other official guidelines (eg, those of the Endocrine Society,18 American Association of Clinical Endocrinologists,19 Institute of Medicine,20 and National Osteoporosis Foundation21) recommend adequate calcium intake to optimize skeletal health.
CALCIUM INTAKE AND CORONARY ARTERY DISEASE
Patients often wonder if the calcium in their supplements ends up in their coronary arteries rather than their bones. Although we once dismissed such concerns, several studies and meta-analyses have reported higher rates of cardiovascular disease with supplemental calcium use.22–24 A proposed mechanism to explain this increased risk is that taking calcium supplements transiently raises the serum calcium level, resulting in calcium deposition in coronary arteries, accelerating atherosclerosis formation.25
On the other hand, some studies and meta-analyses have not shown any increased risk of cardiovascular disease with calcium and vitamin D supplementation.26,27 This subject has been reviewed by Harvey et al.1
Our conclusions are as follows:
Patients should be told that the National Osteoporosis Foundation and the American Society for Preventive Cardiology released a statement in 2016 adopting the position that calcium intake from food and supplements should be considered safe from a cardiovascular perspective.28
If patients want to avoid the possible increase in risk of cardiovascular disease due to calcium supplementation, they can optimize their calcium intake with dietary calcium. Observational studies that showed increased risk with supplemental calcium found no such increase in cardiovascular disease with a robust dietary intake of calcium.29
This is not to say that patients should be encouraged to boost their dietary calcium intake and avoid heart disease by eating more cheese and ice cream, as these foods are high in saturated fats and cholesterol. Many dairy and nondairy sources of calcium do not contain these undesirable nutrients.
CALCIUM SUPPLEMENTATION AND NEPHROLITHIASIS
High dietary calcium intake has not been shown to increase the risk of kidney stones.
In the Nurses’ Health Study, the multivariate relative risk of stone formation was 0.65 (95% confidence interval [CI] 0.5–0.83) in those in the highest vs the lowest quintiles of dietary calcium intake.30 In contrast, the relative risk of stones in those taking calcium supplements was 1.2 (CI 1.02–1.41),30 although this higher risk was not seen in younger women (ages 27 to 44).31
Similar results were seen in the Women’s Health Initiative, in which calcium carbonate and vitamin D supplements resulted in a relative increased risk of stone formation of 1.17 (95% CI 1.02–1.34) compared with women on placebo.12
Data from male stone-formers also suggests that high dietary calcium intake does not increase the risk of stones.32
A theory to explain the difference between dietary and supplemental calcium with respect to stone formation is that dietary calcium binds to oxalate in the gut and reduces its absorption. The most common type of kidney stones are composed of calcium oxalate, and the oxalate, not the calcium, may be the real culprit. In contrast, calcium supplements are often taken between meals and therefore do not exert this protective effect and may be absorbed more rapidly and raise the serum calcium level more, which could lead to higher urinary calcium excretion.33
CALCIUM INTAKE IN PATIENTS TAKING ANTIRESORPTIVE DRUGS
Patients often mistakenly think that calcium and vitamin D supplements are given for mild cases of bone loss, and that if their bone loss is significant enough to require a medication, then they no longer need calcium and vitamin D supplements. Most clinical trials showing bone mineral density and fracture benefit from antiresorptive therapy were in patients who were taking enough calcium and vitamin D, so the efficacy of antiresorptive therapy is most clear only when taking enough calcium and vitamin D.34,35
Furthermore, patients with inadequate calcium and vitamin D intake essentially maintain their serum calcium levels by mobilizing calcium from bone; the combination of insufficient calcium intake and administration of agents that interfere with the ability to mobilize calcium from bone may put patients at risk of hypocalcemia.36
OPTIMIZING INTAKE OF CALCIUM AND VITAMIN D
Diet is key to calcium intake. People should consume adequate amounts of calcium-rich foods regardless of whether they have a history of kidney stones, since robust dietary intake of calcium does not increase the risk of cardiovascular disease or kidney stones and may actually have a protective effect. We also remain skeptical of the concern that supplemental calcium increases the risk of cardiovascular disease.
We recommend a target total calcium intake from diet, and if necessary, supplements, of 1,000 to 1,200 mg daily, and not to worry about cardiovascular disease or kidney stones. A patient or clinician reluctant to push calcium intake that high with supplements might opt for a more conservative goal of 800 mg of calcium daily. This recommendation is based on data suggesting that in the presence of vitamin D sufficiency, calcium supplementation with 500 mg of calcium citrate does little for patients whose calcium intake is above 400 mg/day.37
Vitamin D: How much do we need?
Regarding vitamin D intake, the Institute of Medicine recommends 600 to 800 IU to achieve a 25-hydroxyvitamin D level of 20 to 40 ng/mL.20
The Endocrine Society recommends “at least” 600 to 800 IU, but says that 1,500 to 2,000 IU may be needed to get the 25-hydroxyvitamin D level to 30 to 60 ng/mL.18
The Institute of Medicine based its recommendation on randomized controlled trials that showed fewer fractures with vitamin D intakes of 600 to 800 IU/day.13,14 Also, observational studies show little further reduction in fracture risk when the 25-hydroxyvitamin D levels rise above 20 ng/mL.38 A case-control study found an association between 25-hydroxyvitamin D levels higher than 40 ng/mL and pancreatic cancer.39
The Endocrine Society guidelines recommended higher intakes and levels of vitamin D because there are data suggesting that vitamin D levels higher than 30 ng/mL suppress parathyroid hormone levels further, which should favor less mobilization of bone.40
Levels of 25-hydroxyvitamin D in people exposed to plenty of sunlight rarely go above 60 ng/mL, suggesting 60 ng/mL should be the upper limit of levels to target, and it is unlikely that such levels are harmful.41
Implementing either recommendation—a target 25-hydroxyvitamin D level of 20 to 40 ng/mL or 30 to 60 ng/mL—is reasonable.
CALCULATING A PATIENT’S DIETARY CALCIUM INTAKE
A detailed dietary history can be obtained by a dietitian, or by using the Calcium Calculator app supported by the International Osteoporosis Foundation.43 However, dietary calcium intake can be assessed quickly. To approximate a patient’s total dietary calcium intake (in milligrams), we multiply the number of servings of dietary calcium by 300. A serving of dietary calcium is found in:
- 1 cup of milk, yogurt, calcium-fortified juice, almonds, cooked spinach, or collard greens
- 1.5 ounces of hard cheese
- 2 cups of ice cream, cottage cheese, or beans
- 4 ounces of tofu or canned fish with bones such as salmon or sardines.
Therefore, if a patient consumes 1 cup of milk daily and 1 cup of yogurt 3 times a week, she takes in an estimated 1.5 servings of dietary calcium daily, or 450 mg. What the patient does not receive in the diet should be made up with supplemental calcium.
CALCIUM SUPPLEMENTS
Calcium citrate has certain advantages as a supplement. Calcium carbonate requires gastric acidity to be absorbed and is therefore better absorbed if taken with meals; however, calcium citrate is equally well absorbed in the fasting or fed state and so can be taken without regard for achlorhydria or timing of meals.44
Another potential advantage of calcium citrate is that it has never been shown to increase the risk of kidney stones the way calcium carbonate has.12 Further, potassium citrate is a treatment for certain types of kidney stones,45 and it is possible that when calcium is given as citrate there is less danger of kidney stones.46 For these reasons, we generally recommend calcium citrate over other forms of calcium.
The brand of calcium citrate most readily available is Citracal, but any version of calcium citrate is acceptable.
SOURCES OF CONFUSION
Labels that describe calcium content of supplements are often misleading, and this lack of clarity can interfere with the patient’s ability to correctly identify how much calcium is in each pill.
Serving size. Whereas 1 serving of Caltrate is 1 pill, 1 serving of Tums or Citracal is 2 pills; for other brands a serving may be 3 or 4 pills.
Calcium salt vs elemental calcium. The amount of elemental calcium contained in different calcium salts varies according to the molecular weight of the salt: 1,000 mg of calcium carbonate has 400 mg of elemental calcium, while 1,000 mg of calcium citrate has 200 mg of elemental calcium.
When we recommend 1,000 to 1,200 mg of calcium daily, we mean the amount of elemental calcium. The label on calcium supplements usually indicates the amount of elemental calcium, but some have confusing information about the amount of calcium salt they contain. For instance, Tums lists the amount of calcium carbonate per pill on the top of the label, but elsewhere lists the amount of elemental calcium.
Same brand, different preparation. Some brands of calcium have more than 1 formulation, each with a different amount of calcium. For instance, Citracal has a maximum-strength 315-mg tablet and a “petite” 200-mg tablet. Careful reading of the label is required to make sure that the patient is getting the amount of calcium she thinks she is getting.
OPTIONS FOR THOSE WITH DIFFICULTY SWALLOWING LARGE PILLS
Many calcium pills are large and difficult to swallow. Patients often ask if calcium pills can be crushed, and the answer is that they certainly can, but this approach is cumbersome and usually results in patients eventually stopping calcium in frustration.
CALCIUM SUPPLEMENTS AND CONSTIPATION
Constipation is a common side effect of calcium supplementation.47 Many patients report that they cannot take a calcium supplement because of constipation, or ask if there are calcium preparations that are less constipating than others.
There are ways of overcoming the constipating effects of calcium. Osmotic laxatives and stool softeners such as polyethylene glycol, magnesium citrate, and docusate sodium are safe and effective, although patients are often reluctant to take a medicine to combat the side effects from another medicine.
In such circumstances patients are often amenable to taking a combination product such as calcium with magnesium, since the cathartic effects of magnesium nicely counteract the constipating effects of calcium. This idea is exploited in antacids such as Rolaids, which are combinations of calcium carbonate and magnesium oxide that usually have no net effect on stool consistency.47
Many patients believe that calcium must be combined with magnesium to be absorbed. Although there are no data to support this idea, a patient already harboring this misconception may be more amenable to calcium-magnesium combinations for the purpose of avoiding constipation.
If a patient cannot find a calcium preparation that she can take at the full recommended doses, we often suggest starting with a very small dose for 2 weeks, and then adjusting the dose upward every 2 weeks until reaching the maximum dose that the patient can tolerate. Even if the dose is well below recommended doses, most of the benefit of calcium is obtained by bringing total intake to more than 500 mg daily,37 so continued use should be encouraged even when optimal targets cannot be sustained.
For patients who cannot tolerate enough calcium, we recommend being especially sure to optimize the vitamin D levels, since there are studies that suggest that secondary hyperparathyroidism mostly occurs in states of low calcium intake if vitamin D levels are insufficient.48
If the patient has secondary hyperparathyroidism despite best attempts at supplementation with calcium and vitamin D, consider prescribing calcitriol (activated vitamin D), which stimulates gut absorption of whatever calcium is taken.49 If calcitriol is given, the patient must undergo cumbersome monitoring for hypercalcemia and hypercalciuria. Fortunately, it is unusual to require calcitriol unless the patient has significant structural gastrointestinal abnormalities such as gastric bypass or Crohn disease.
Although calcium and vitamin D are often recommended for prevention and treatment of osteoporosis, considerable controversy exists in terms of their safety and efficacy.1 This article highlights the issues, referring readers to reviews and meta-analyses for details and providing some practical advice for patients requiring supplementation.
CALCIUM INTAKE AND BONE DENSITY
Calcium enters the body through diet and supplementation. If intake is low, blood calcium levels fall, resulting in secondary hyperparathyroidism, which has 3 main effects:
- Increased fractional absorption of the calcium that is consumed
- Reduced urinary excretion of calcium
- Increased bone resorption, which releases calcium into the blood,2 which explains the potential for the deleterious effect of deficient intake of calcium on bone.3
Based on the simple physiology outlined above, it seems logical that insufficient intake of calcium over time could lead to mobilization of calcium from bone, lower bone mineral density, and higher fracture risk.3 This topic has been reviewed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and the International Foundation for Osteoporosis.1
Many lines of evidence suggest that low calcium intake adversely affects bone mineral density.1 Low calcium intake has been associated with lower bone density in some cross-sectional studies,4–6 though not all.7 Interventions to increase calcium intake in postmenopausal women have shown beneficial effects on bone density,8–10 though in some studies the benefit was small and nonprogressive.11 The question is whether this improvement in bone mineral density translates into fewer fractures.
Results from individual studies looking at fracture prevention through calcium supplementation have been conflicting,10,12–14 and reviews and meta-analyses have summarized the data.1,3,15 A recent review of these meta-analyses showed a small but significant reduction in some types of fracture.1
Some speculate that the difficulty in demonstrating fracture efficacy might be due to imperfect compliance with calcium intake, and that the participants in the placebo groups often had fairly robust calcium intake from diet and off-study supplemental intake, which could reduce the sensitivity of studies to demonstrate the fracture benefit.1,16
The US Preventive Services Task Force17 recommends that the general public not take supplemental calcium for skeletal health, but emphasizes that this recommendation does not apply to patients with osteoporosis. Most other official guidelines (eg, those of the Endocrine Society,18 American Association of Clinical Endocrinologists,19 Institute of Medicine,20 and National Osteoporosis Foundation21) recommend adequate calcium intake to optimize skeletal health.
CALCIUM INTAKE AND CORONARY ARTERY DISEASE
Patients often wonder if the calcium in their supplements ends up in their coronary arteries rather than their bones. Although we once dismissed such concerns, several studies and meta-analyses have reported higher rates of cardiovascular disease with supplemental calcium use.22–24 A proposed mechanism to explain this increased risk is that taking calcium supplements transiently raises the serum calcium level, resulting in calcium deposition in coronary arteries, accelerating atherosclerosis formation.25
On the other hand, some studies and meta-analyses have not shown any increased risk of cardiovascular disease with calcium and vitamin D supplementation.26,27 This subject has been reviewed by Harvey et al.1
Our conclusions are as follows:
Patients should be told that the National Osteoporosis Foundation and the American Society for Preventive Cardiology released a statement in 2016 adopting the position that calcium intake from food and supplements should be considered safe from a cardiovascular perspective.28
If patients want to avoid the possible increase in risk of cardiovascular disease due to calcium supplementation, they can optimize their calcium intake with dietary calcium. Observational studies that showed increased risk with supplemental calcium found no such increase in cardiovascular disease with a robust dietary intake of calcium.29
This is not to say that patients should be encouraged to boost their dietary calcium intake and avoid heart disease by eating more cheese and ice cream, as these foods are high in saturated fats and cholesterol. Many dairy and nondairy sources of calcium do not contain these undesirable nutrients.
CALCIUM SUPPLEMENTATION AND NEPHROLITHIASIS
High dietary calcium intake has not been shown to increase the risk of kidney stones.
In the Nurses’ Health Study, the multivariate relative risk of stone formation was 0.65 (95% confidence interval [CI] 0.5–0.83) in those in the highest vs the lowest quintiles of dietary calcium intake.30 In contrast, the relative risk of stones in those taking calcium supplements was 1.2 (CI 1.02–1.41),30 although this higher risk was not seen in younger women (ages 27 to 44).31
Similar results were seen in the Women’s Health Initiative, in which calcium carbonate and vitamin D supplements resulted in a relative increased risk of stone formation of 1.17 (95% CI 1.02–1.34) compared with women on placebo.12
Data from male stone-formers also suggests that high dietary calcium intake does not increase the risk of stones.32
A theory to explain the difference between dietary and supplemental calcium with respect to stone formation is that dietary calcium binds to oxalate in the gut and reduces its absorption. The most common type of kidney stones are composed of calcium oxalate, and the oxalate, not the calcium, may be the real culprit. In contrast, calcium supplements are often taken between meals and therefore do not exert this protective effect and may be absorbed more rapidly and raise the serum calcium level more, which could lead to higher urinary calcium excretion.33
CALCIUM INTAKE IN PATIENTS TAKING ANTIRESORPTIVE DRUGS
Patients often mistakenly think that calcium and vitamin D supplements are given for mild cases of bone loss, and that if their bone loss is significant enough to require a medication, then they no longer need calcium and vitamin D supplements. Most clinical trials showing bone mineral density and fracture benefit from antiresorptive therapy were in patients who were taking enough calcium and vitamin D, so the efficacy of antiresorptive therapy is most clear only when taking enough calcium and vitamin D.34,35
Furthermore, patients with inadequate calcium and vitamin D intake essentially maintain their serum calcium levels by mobilizing calcium from bone; the combination of insufficient calcium intake and administration of agents that interfere with the ability to mobilize calcium from bone may put patients at risk of hypocalcemia.36
OPTIMIZING INTAKE OF CALCIUM AND VITAMIN D
Diet is key to calcium intake. People should consume adequate amounts of calcium-rich foods regardless of whether they have a history of kidney stones, since robust dietary intake of calcium does not increase the risk of cardiovascular disease or kidney stones and may actually have a protective effect. We also remain skeptical of the concern that supplemental calcium increases the risk of cardiovascular disease.
We recommend a target total calcium intake from diet, and if necessary, supplements, of 1,000 to 1,200 mg daily, and not to worry about cardiovascular disease or kidney stones. A patient or clinician reluctant to push calcium intake that high with supplements might opt for a more conservative goal of 800 mg of calcium daily. This recommendation is based on data suggesting that in the presence of vitamin D sufficiency, calcium supplementation with 500 mg of calcium citrate does little for patients whose calcium intake is above 400 mg/day.37
Vitamin D: How much do we need?
Regarding vitamin D intake, the Institute of Medicine recommends 600 to 800 IU to achieve a 25-hydroxyvitamin D level of 20 to 40 ng/mL.20
The Endocrine Society recommends “at least” 600 to 800 IU, but says that 1,500 to 2,000 IU may be needed to get the 25-hydroxyvitamin D level to 30 to 60 ng/mL.18
The Institute of Medicine based its recommendation on randomized controlled trials that showed fewer fractures with vitamin D intakes of 600 to 800 IU/day.13,14 Also, observational studies show little further reduction in fracture risk when the 25-hydroxyvitamin D levels rise above 20 ng/mL.38 A case-control study found an association between 25-hydroxyvitamin D levels higher than 40 ng/mL and pancreatic cancer.39
The Endocrine Society guidelines recommended higher intakes and levels of vitamin D because there are data suggesting that vitamin D levels higher than 30 ng/mL suppress parathyroid hormone levels further, which should favor less mobilization of bone.40
Levels of 25-hydroxyvitamin D in people exposed to plenty of sunlight rarely go above 60 ng/mL, suggesting 60 ng/mL should be the upper limit of levels to target, and it is unlikely that such levels are harmful.41
Implementing either recommendation—a target 25-hydroxyvitamin D level of 20 to 40 ng/mL or 30 to 60 ng/mL—is reasonable.
CALCULATING A PATIENT’S DIETARY CALCIUM INTAKE
A detailed dietary history can be obtained by a dietitian, or by using the Calcium Calculator app supported by the International Osteoporosis Foundation.43 However, dietary calcium intake can be assessed quickly. To approximate a patient’s total dietary calcium intake (in milligrams), we multiply the number of servings of dietary calcium by 300. A serving of dietary calcium is found in:
- 1 cup of milk, yogurt, calcium-fortified juice, almonds, cooked spinach, or collard greens
- 1.5 ounces of hard cheese
- 2 cups of ice cream, cottage cheese, or beans
- 4 ounces of tofu or canned fish with bones such as salmon or sardines.
Therefore, if a patient consumes 1 cup of milk daily and 1 cup of yogurt 3 times a week, she takes in an estimated 1.5 servings of dietary calcium daily, or 450 mg. What the patient does not receive in the diet should be made up with supplemental calcium.
CALCIUM SUPPLEMENTS
Calcium citrate has certain advantages as a supplement. Calcium carbonate requires gastric acidity to be absorbed and is therefore better absorbed if taken with meals; however, calcium citrate is equally well absorbed in the fasting or fed state and so can be taken without regard for achlorhydria or timing of meals.44
Another potential advantage of calcium citrate is that it has never been shown to increase the risk of kidney stones the way calcium carbonate has.12 Further, potassium citrate is a treatment for certain types of kidney stones,45 and it is possible that when calcium is given as citrate there is less danger of kidney stones.46 For these reasons, we generally recommend calcium citrate over other forms of calcium.
The brand of calcium citrate most readily available is Citracal, but any version of calcium citrate is acceptable.
SOURCES OF CONFUSION
Labels that describe calcium content of supplements are often misleading, and this lack of clarity can interfere with the patient’s ability to correctly identify how much calcium is in each pill.
Serving size. Whereas 1 serving of Caltrate is 1 pill, 1 serving of Tums or Citracal is 2 pills; for other brands a serving may be 3 or 4 pills.
Calcium salt vs elemental calcium. The amount of elemental calcium contained in different calcium salts varies according to the molecular weight of the salt: 1,000 mg of calcium carbonate has 400 mg of elemental calcium, while 1,000 mg of calcium citrate has 200 mg of elemental calcium.
When we recommend 1,000 to 1,200 mg of calcium daily, we mean the amount of elemental calcium. The label on calcium supplements usually indicates the amount of elemental calcium, but some have confusing information about the amount of calcium salt they contain. For instance, Tums lists the amount of calcium carbonate per pill on the top of the label, but elsewhere lists the amount of elemental calcium.
Same brand, different preparation. Some brands of calcium have more than 1 formulation, each with a different amount of calcium. For instance, Citracal has a maximum-strength 315-mg tablet and a “petite” 200-mg tablet. Careful reading of the label is required to make sure that the patient is getting the amount of calcium she thinks she is getting.
OPTIONS FOR THOSE WITH DIFFICULTY SWALLOWING LARGE PILLS
Many calcium pills are large and difficult to swallow. Patients often ask if calcium pills can be crushed, and the answer is that they certainly can, but this approach is cumbersome and usually results in patients eventually stopping calcium in frustration.
CALCIUM SUPPLEMENTS AND CONSTIPATION
Constipation is a common side effect of calcium supplementation.47 Many patients report that they cannot take a calcium supplement because of constipation, or ask if there are calcium preparations that are less constipating than others.
There are ways of overcoming the constipating effects of calcium. Osmotic laxatives and stool softeners such as polyethylene glycol, magnesium citrate, and docusate sodium are safe and effective, although patients are often reluctant to take a medicine to combat the side effects from another medicine.
In such circumstances patients are often amenable to taking a combination product such as calcium with magnesium, since the cathartic effects of magnesium nicely counteract the constipating effects of calcium. This idea is exploited in antacids such as Rolaids, which are combinations of calcium carbonate and magnesium oxide that usually have no net effect on stool consistency.47
Many patients believe that calcium must be combined with magnesium to be absorbed. Although there are no data to support this idea, a patient already harboring this misconception may be more amenable to calcium-magnesium combinations for the purpose of avoiding constipation.
If a patient cannot find a calcium preparation that she can take at the full recommended doses, we often suggest starting with a very small dose for 2 weeks, and then adjusting the dose upward every 2 weeks until reaching the maximum dose that the patient can tolerate. Even if the dose is well below recommended doses, most of the benefit of calcium is obtained by bringing total intake to more than 500 mg daily,37 so continued use should be encouraged even when optimal targets cannot be sustained.
For patients who cannot tolerate enough calcium, we recommend being especially sure to optimize the vitamin D levels, since there are studies that suggest that secondary hyperparathyroidism mostly occurs in states of low calcium intake if vitamin D levels are insufficient.48
If the patient has secondary hyperparathyroidism despite best attempts at supplementation with calcium and vitamin D, consider prescribing calcitriol (activated vitamin D), which stimulates gut absorption of whatever calcium is taken.49 If calcitriol is given, the patient must undergo cumbersome monitoring for hypercalcemia and hypercalciuria. Fortunately, it is unusual to require calcitriol unless the patient has significant structural gastrointestinal abnormalities such as gastric bypass or Crohn disease.
- Harvey NC, Bilver E, Kaufman JM, et al. The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 2017; 28(2):447–462. doi:10.1007/s00198-016-3773-6
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 2005; 115(12):3318–3325. doi.10.1172/JCI27071
- Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med 2013; 369(16):1537–154 doi:10.1056/NEJMcp1210380
- Choi MJ, Park EJ, Jo HJ. Relationship of nutrient intakes and bone mineral density of elderly women in Daegu, Korea. Nutr Res Pract 2007; 1(4):328–33 doi:10.4162/nrp.2007.1.4.328
- Kim KM, Choi SH, Lim S, et al. Interactions between dietary calcium intake and bone mineral density or bone ge6ometry in a low calcium intake population (KNHANES IV 2008–2010). J Clin Endocrinol Metab 2014; 99(7):2409–2417. doi:10.1210/jc.2014-1006
- Joo NS, Dawson-Hughes B, Kim YS, Oh K, Yeum KJ. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J Bone Miner Res 2013; 28(4):764–770. doi:10.1002/jbmr.1790
- Anderson JJ, Roggenkamp KJ, Suchindran CM. Calcium intakes and femoral and lumbar bone density of elderly US men and women: National Health and Nutrition Examination Survey 2005–2006 analysis. J Clin Endocrinol Metab 2012; 97(12):4531–4539. doi:10.1210/jc.2012-1407
- Gui JC, Brašic JR, Liu XD, et al. Bone mineral density in postmenopausal Chinese women treated with calcium fortification in soymilk and cow’s milk. Osteoporos Int 2012; 23(5):1563–1570. doi:10.1007/s00198-012-1895-z
- Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30-month dietary intervention on bone mineral density: the Postmenopausal Health Study. Br J Nutr 2010; 104(1):100–107. doi:10.1017/S000711451000019X
- Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996; 11(12):1961–1966. doi:10.1002/jbmr.5650111218
- Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015; 351:h4183. doi:10.1136/bmj.h4183
- Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006; 354(7):669–683. doi:10.1056/NEJMoa055218
- Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327(23):1637–1642. doi:10.1056/NEJM199212033272305
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337(10):670–676. doi:10.1056/NEJM199709043371003
- Bolland MJ, Leung W, Tai V, et al. Calcium intake and risk of fracture: systematic review. BMJ 2015; 351:h4580. doi:10.1136/bmj.h4580
- Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med 2012; 367(1):77–78. doi:10.1056/NEJMe1206858
- Moyer VA; US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158(9):691–696. doi:10.7326/0003-4819-158-9-201305070-00603
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7):1911–1930. doi:10.1210/jc.2011-0385
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologist and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract 2016; 22(suppl 4):1–42. doi:10.4158/EP161435.ESGL
- Ross AC, Manson JE, Abrams SA et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
- Cosman F, De Beur SJ, Leboff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25(10):2359–2381. doi:10.1007/s00198-014-2794-2
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336(7638):262–266. doi:10.1136/bmj.39440.525752.BE
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011; 342:d2040. doi:10.1136/bmj.d2040
- Mao PJ, Zhang C, Tang L, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 169(2):106–111. doi:10.1016/j.ijcard.2013.08.055
- Reid IR, Bolland MJ. Calcium supplementation and vascular disease. Climacteric 2008; 11(4):280–286. doi:10.1080/13697130802229639
- Lewis JR, Radavelli-Bagatini S, Rejnmark L, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015; 30(1):165–175. doi:10.1002/jbmr.2311
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115(19):846–854. doi:10.1161/CIRCULATIONAHA.106.673491
- Kopecky SL, Bauer DC, Gulati M, et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med 2016; 165(12):867–868. doi:10.7326/M16-1743
- Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc 2016; 5(10):e003815. doi:10.1161/JAHA.116.003815
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126(7):497–504. pmid:9092314
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164(8):885–891. doi:10.1001/archinte.164.8.885
- Borhi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346(2):77–84. doi:10.1056/NEJMoa010369
- Prochaska ML, Taylor EN, Curhan GC. Insights into nephrolithiasis from the Nurses’ Health Studies. Am J Public Health 2016; 106(9):1638–1643. doi:10.2105/AJPH.2016.303319
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Chen J, Smerdely P. Hypocalcaemia after denosumab in older people following fracture. Osteoporos Int 2017; 28(2):517–522. doi:10.1007/s00198-016-3755-8
- Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 1990; 323(13):878–883. doi:10.1056/NEJM199009273231305
- Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab 2010; 95(6):2637–2645. doi:10.1210/jc.2009-2699
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1):81–93. doi:10.1093/aje/kwq120
- Valcour A, Blocki F, Hawkins DM, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab 2012; 97(11):3989–3995. doi:10.1210/jc.2012-2276
- Binkley N, Novotny R, Krueger T, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007; 92(6):2130–2135. doi:10.1210/jc.2006-2250
- United States Department of Agriculture (USDA). Agricultural Research Service. USDA food composition databases. https://ndb.nal.usda.gov/ndb/search/list. Accessed May 7, 2018.
- International Osteoporosis Foundation. IOF calcium calculator version 1.10. Apple App Store. https://itunes.apple.com/us/app/iof-calcium-calculator/id956198268?mt=8. Accessed June 11, 2018.
- Recker RR. Calcium absorption and achlorhydria. N Engl J Med 1985; 313(2):70–73. doi:10.1056/NEJM198507113130202
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115(10):2598–2608. doi:10.1172/JCI26662
- Sakhaee K, Poindexter JR, Griffith CS, Pak CY. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J Urol 2004; 172(3):958–961. doi:10.1097/01.ju.0000136400.14728.cd
- Kitchin B. Nutrition counseling for patients with osteoporosis: a personal approach. J Clin Densitom 2013; 16(4):426–431. doi:10.1016/j.jocd.2013.08.013
- Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005; 294(18):2336–2341. doi:10.1001/jama.294.18.2336
- Need AG, Horowitz M, Philcox JC, Nordin BE. 1,25-dihydroxycalciferol and calcium therapy in osteoporosis with calcium malabsorption. Dose response relationship of calcium absorption and indices of bone turnover. Miner Electrolyte Metab 1985; 11(1):35–40. pmid:3838358
- Harvey NC, Bilver E, Kaufman JM, et al. The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 2017; 28(2):447–462. doi:10.1007/s00198-016-3773-6
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 2005; 115(12):3318–3325. doi.10.1172/JCI27071
- Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med 2013; 369(16):1537–154 doi:10.1056/NEJMcp1210380
- Choi MJ, Park EJ, Jo HJ. Relationship of nutrient intakes and bone mineral density of elderly women in Daegu, Korea. Nutr Res Pract 2007; 1(4):328–33 doi:10.4162/nrp.2007.1.4.328
- Kim KM, Choi SH, Lim S, et al. Interactions between dietary calcium intake and bone mineral density or bone ge6ometry in a low calcium intake population (KNHANES IV 2008–2010). J Clin Endocrinol Metab 2014; 99(7):2409–2417. doi:10.1210/jc.2014-1006
- Joo NS, Dawson-Hughes B, Kim YS, Oh K, Yeum KJ. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J Bone Miner Res 2013; 28(4):764–770. doi:10.1002/jbmr.1790
- Anderson JJ, Roggenkamp KJ, Suchindran CM. Calcium intakes and femoral and lumbar bone density of elderly US men and women: National Health and Nutrition Examination Survey 2005–2006 analysis. J Clin Endocrinol Metab 2012; 97(12):4531–4539. doi:10.1210/jc.2012-1407
- Gui JC, Brašic JR, Liu XD, et al. Bone mineral density in postmenopausal Chinese women treated with calcium fortification in soymilk and cow’s milk. Osteoporos Int 2012; 23(5):1563–1570. doi:10.1007/s00198-012-1895-z
- Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30-month dietary intervention on bone mineral density: the Postmenopausal Health Study. Br J Nutr 2010; 104(1):100–107. doi:10.1017/S000711451000019X
- Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996; 11(12):1961–1966. doi:10.1002/jbmr.5650111218
- Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015; 351:h4183. doi:10.1136/bmj.h4183
- Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006; 354(7):669–683. doi:10.1056/NEJMoa055218
- Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327(23):1637–1642. doi:10.1056/NEJM199212033272305
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337(10):670–676. doi:10.1056/NEJM199709043371003
- Bolland MJ, Leung W, Tai V, et al. Calcium intake and risk of fracture: systematic review. BMJ 2015; 351:h4580. doi:10.1136/bmj.h4580
- Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med 2012; 367(1):77–78. doi:10.1056/NEJMe1206858
- Moyer VA; US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158(9):691–696. doi:10.7326/0003-4819-158-9-201305070-00603
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7):1911–1930. doi:10.1210/jc.2011-0385
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologist and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract 2016; 22(suppl 4):1–42. doi:10.4158/EP161435.ESGL
- Ross AC, Manson JE, Abrams SA et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
- Cosman F, De Beur SJ, Leboff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25(10):2359–2381. doi:10.1007/s00198-014-2794-2
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336(7638):262–266. doi:10.1136/bmj.39440.525752.BE
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011; 342:d2040. doi:10.1136/bmj.d2040
- Mao PJ, Zhang C, Tang L, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 169(2):106–111. doi:10.1016/j.ijcard.2013.08.055
- Reid IR, Bolland MJ. Calcium supplementation and vascular disease. Climacteric 2008; 11(4):280–286. doi:10.1080/13697130802229639
- Lewis JR, Radavelli-Bagatini S, Rejnmark L, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015; 30(1):165–175. doi:10.1002/jbmr.2311
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115(19):846–854. doi:10.1161/CIRCULATIONAHA.106.673491
- Kopecky SL, Bauer DC, Gulati M, et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med 2016; 165(12):867–868. doi:10.7326/M16-1743
- Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc 2016; 5(10):e003815. doi:10.1161/JAHA.116.003815
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126(7):497–504. pmid:9092314
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164(8):885–891. doi:10.1001/archinte.164.8.885
- Borhi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346(2):77–84. doi:10.1056/NEJMoa010369
- Prochaska ML, Taylor EN, Curhan GC. Insights into nephrolithiasis from the Nurses’ Health Studies. Am J Public Health 2016; 106(9):1638–1643. doi:10.2105/AJPH.2016.303319
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Chen J, Smerdely P. Hypocalcaemia after denosumab in older people following fracture. Osteoporos Int 2017; 28(2):517–522. doi:10.1007/s00198-016-3755-8
- Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 1990; 323(13):878–883. doi:10.1056/NEJM199009273231305
- Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab 2010; 95(6):2637–2645. doi:10.1210/jc.2009-2699
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1):81–93. doi:10.1093/aje/kwq120
- Valcour A, Blocki F, Hawkins DM, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab 2012; 97(11):3989–3995. doi:10.1210/jc.2012-2276
- Binkley N, Novotny R, Krueger T, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007; 92(6):2130–2135. doi:10.1210/jc.2006-2250
- United States Department of Agriculture (USDA). Agricultural Research Service. USDA food composition databases. https://ndb.nal.usda.gov/ndb/search/list. Accessed May 7, 2018.
- International Osteoporosis Foundation. IOF calcium calculator version 1.10. Apple App Store. https://itunes.apple.com/us/app/iof-calcium-calculator/id956198268?mt=8. Accessed June 11, 2018.
- Recker RR. Calcium absorption and achlorhydria. N Engl J Med 1985; 313(2):70–73. doi:10.1056/NEJM198507113130202
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115(10):2598–2608. doi:10.1172/JCI26662
- Sakhaee K, Poindexter JR, Griffith CS, Pak CY. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J Urol 2004; 172(3):958–961. doi:10.1097/01.ju.0000136400.14728.cd
- Kitchin B. Nutrition counseling for patients with osteoporosis: a personal approach. J Clin Densitom 2013; 16(4):426–431. doi:10.1016/j.jocd.2013.08.013
- Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005; 294(18):2336–2341. doi:10.1001/jama.294.18.2336
- Need AG, Horowitz M, Philcox JC, Nordin BE. 1,25-dihydroxycalciferol and calcium therapy in osteoporosis with calcium malabsorption. Dose response relationship of calcium absorption and indices of bone turnover. Miner Electrolyte Metab 1985; 11(1):35–40. pmid:3838358
KEY POINTS
- We advise modest targets for total calcium intake, maximizing dietary calcium intake and making up the deficit with calcium citrate supplements.
- Gastrointestinal complaints are common with calcium supplements and can be mitigated with osmotic cathartics (mixed in the same pill or not) or with dose adjustment.
- Vitamin D levels should be optimized to help prevent secondary hyperparathyroidism.
How well do we understand calcium and vitamin D?
With so much emphasis on clinical trials, evidence-based joint decision-making, and comparative-benefit studies when choosing treatment, the growth of the supplement market is a strong comment on the perceived and often real failings of traditional therapies. It also reflects our apparent failure as a profession to educate ourselves and the public about the difference between anecdote-based belief and clinical trial-based confidence, the difference between evidence and innuendo, and, equally important, the limitations of applying population-based clinical trial data to an individual patient.
Which brings me to the discussion of calcium and vitamin D supplementation by Drs. Kilim and Rosen in this issue of the Journal. We know a lot about calcium homeostasis and the role vitamin D plays in regulating circulating calcium levels. Only a small fraction of the calcium in the body circulates (most is in our skeleton), and likely only about 1% is truly exchangeable. But the circulating free calcium level is tightly controlled, as the function of our neuromuscular system, brain, and heart depend on keeping intra- and extracellular calcium levels within precise limits. If necessary, our bodies maintain stable levels of circulating free calcium at the expense of leaching calcium from our bones, placing us at risk of potentially fatal fractures. Thus was born the concept of guaranteeing adequate calcium stores through calcium supplementation.
Control of the free calcium level is not simple. There are several interrelated sensing and modulatory pathways, eg:
- Gut absorption, which is affected by the total gut load of calcium, intestinal integrity, and the specific ingested foodstuffs, and likely by our microbiome
- The parathyroid hormone (PTH) level, which directly or indirectly affects calcium absorption, the calcium-phosphate ratio, and thus, extraskeletal calcium localization and bone calcium content
- Vitamin D, with its many effects after interorgan multistep activation.
Despite this knowledge of calcium metabolism and the intricate cross-talk between the different pathways, I do not believe we truly understand how to determine the amount of dietary and supplemental calcium or vitamin D that is ideal for a given patient. I also do not believe we know with certainty what is the “normal” or ideal 25-hydroxyvitamin D level in that same patient: note the different ranges of normal proposed by different expert working groups.
How do we know when there is insufficient calcium in our diet? The total or free circulating calcium level is far too insensitive and, as noted above, complex homeostatic mechanisms are always trying to maintain an appropriate physiologic calcium level, whatever the intake. Urinary calcium excretion does not necessarily equal the ingested calcium load; there are too many factors influencing renal calcium excretion. Gastrointestinal excretion is also variable and complex. We know when the vitamin D level is functionally much too low, as the PTH level begins to rise; but the “normal” PTH range is wide, and the slope of the relationship between vitamin D and PTH is affected by many factors.
Additionally, accumulating information suggests that vitamin D metabolites significantly affect immune regulation and the onset and expression of a number of organ-specific and systemic autoimmune disorders. Further, the ideal 25-hydroxyvitamin D level for a healthy immune system is not known. Does the body have to compromise something in reconciling the target for a healthy skeleton and the target for a healthy immune system, which may conceivably be different? But importantly, this new knowledge does not imply that vitamin D supplementation will reduce the pain and symptoms from inflammatory arthritis or noninflammatory fibromyalgia.
Despite these many areas of uncertainty, Kilim and Rosen focus on bone health, summarize the wealth of accumulated data, and provide practical management advice we can use in the clinic. But if we struggle so much to know the correct way to manage calcium and vitamin D supplementation in our patients, it is little surprise that most of us struggle even more when asked about using supplements for which the biology is far less understood. As a medical community, we need to uniformly address this lack of understanding wherever it exists. Believing in a supplement or a treatment is not the same as understanding it or having strong evidence about its efficacy and safety.
With so much emphasis on clinical trials, evidence-based joint decision-making, and comparative-benefit studies when choosing treatment, the growth of the supplement market is a strong comment on the perceived and often real failings of traditional therapies. It also reflects our apparent failure as a profession to educate ourselves and the public about the difference between anecdote-based belief and clinical trial-based confidence, the difference between evidence and innuendo, and, equally important, the limitations of applying population-based clinical trial data to an individual patient.
Which brings me to the discussion of calcium and vitamin D supplementation by Drs. Kilim and Rosen in this issue of the Journal. We know a lot about calcium homeostasis and the role vitamin D plays in regulating circulating calcium levels. Only a small fraction of the calcium in the body circulates (most is in our skeleton), and likely only about 1% is truly exchangeable. But the circulating free calcium level is tightly controlled, as the function of our neuromuscular system, brain, and heart depend on keeping intra- and extracellular calcium levels within precise limits. If necessary, our bodies maintain stable levels of circulating free calcium at the expense of leaching calcium from our bones, placing us at risk of potentially fatal fractures. Thus was born the concept of guaranteeing adequate calcium stores through calcium supplementation.
Control of the free calcium level is not simple. There are several interrelated sensing and modulatory pathways, eg:
- Gut absorption, which is affected by the total gut load of calcium, intestinal integrity, and the specific ingested foodstuffs, and likely by our microbiome
- The parathyroid hormone (PTH) level, which directly or indirectly affects calcium absorption, the calcium-phosphate ratio, and thus, extraskeletal calcium localization and bone calcium content
- Vitamin D, with its many effects after interorgan multistep activation.
Despite this knowledge of calcium metabolism and the intricate cross-talk between the different pathways, I do not believe we truly understand how to determine the amount of dietary and supplemental calcium or vitamin D that is ideal for a given patient. I also do not believe we know with certainty what is the “normal” or ideal 25-hydroxyvitamin D level in that same patient: note the different ranges of normal proposed by different expert working groups.
How do we know when there is insufficient calcium in our diet? The total or free circulating calcium level is far too insensitive and, as noted above, complex homeostatic mechanisms are always trying to maintain an appropriate physiologic calcium level, whatever the intake. Urinary calcium excretion does not necessarily equal the ingested calcium load; there are too many factors influencing renal calcium excretion. Gastrointestinal excretion is also variable and complex. We know when the vitamin D level is functionally much too low, as the PTH level begins to rise; but the “normal” PTH range is wide, and the slope of the relationship between vitamin D and PTH is affected by many factors.
Additionally, accumulating information suggests that vitamin D metabolites significantly affect immune regulation and the onset and expression of a number of organ-specific and systemic autoimmune disorders. Further, the ideal 25-hydroxyvitamin D level for a healthy immune system is not known. Does the body have to compromise something in reconciling the target for a healthy skeleton and the target for a healthy immune system, which may conceivably be different? But importantly, this new knowledge does not imply that vitamin D supplementation will reduce the pain and symptoms from inflammatory arthritis or noninflammatory fibromyalgia.
Despite these many areas of uncertainty, Kilim and Rosen focus on bone health, summarize the wealth of accumulated data, and provide practical management advice we can use in the clinic. But if we struggle so much to know the correct way to manage calcium and vitamin D supplementation in our patients, it is little surprise that most of us struggle even more when asked about using supplements for which the biology is far less understood. As a medical community, we need to uniformly address this lack of understanding wherever it exists. Believing in a supplement or a treatment is not the same as understanding it or having strong evidence about its efficacy and safety.
With so much emphasis on clinical trials, evidence-based joint decision-making, and comparative-benefit studies when choosing treatment, the growth of the supplement market is a strong comment on the perceived and often real failings of traditional therapies. It also reflects our apparent failure as a profession to educate ourselves and the public about the difference between anecdote-based belief and clinical trial-based confidence, the difference between evidence and innuendo, and, equally important, the limitations of applying population-based clinical trial data to an individual patient.
Which brings me to the discussion of calcium and vitamin D supplementation by Drs. Kilim and Rosen in this issue of the Journal. We know a lot about calcium homeostasis and the role vitamin D plays in regulating circulating calcium levels. Only a small fraction of the calcium in the body circulates (most is in our skeleton), and likely only about 1% is truly exchangeable. But the circulating free calcium level is tightly controlled, as the function of our neuromuscular system, brain, and heart depend on keeping intra- and extracellular calcium levels within precise limits. If necessary, our bodies maintain stable levels of circulating free calcium at the expense of leaching calcium from our bones, placing us at risk of potentially fatal fractures. Thus was born the concept of guaranteeing adequate calcium stores through calcium supplementation.
Control of the free calcium level is not simple. There are several interrelated sensing and modulatory pathways, eg:
- Gut absorption, which is affected by the total gut load of calcium, intestinal integrity, and the specific ingested foodstuffs, and likely by our microbiome
- The parathyroid hormone (PTH) level, which directly or indirectly affects calcium absorption, the calcium-phosphate ratio, and thus, extraskeletal calcium localization and bone calcium content
- Vitamin D, with its many effects after interorgan multistep activation.
Despite this knowledge of calcium metabolism and the intricate cross-talk between the different pathways, I do not believe we truly understand how to determine the amount of dietary and supplemental calcium or vitamin D that is ideal for a given patient. I also do not believe we know with certainty what is the “normal” or ideal 25-hydroxyvitamin D level in that same patient: note the different ranges of normal proposed by different expert working groups.
How do we know when there is insufficient calcium in our diet? The total or free circulating calcium level is far too insensitive and, as noted above, complex homeostatic mechanisms are always trying to maintain an appropriate physiologic calcium level, whatever the intake. Urinary calcium excretion does not necessarily equal the ingested calcium load; there are too many factors influencing renal calcium excretion. Gastrointestinal excretion is also variable and complex. We know when the vitamin D level is functionally much too low, as the PTH level begins to rise; but the “normal” PTH range is wide, and the slope of the relationship between vitamin D and PTH is affected by many factors.
Additionally, accumulating information suggests that vitamin D metabolites significantly affect immune regulation and the onset and expression of a number of organ-specific and systemic autoimmune disorders. Further, the ideal 25-hydroxyvitamin D level for a healthy immune system is not known. Does the body have to compromise something in reconciling the target for a healthy skeleton and the target for a healthy immune system, which may conceivably be different? But importantly, this new knowledge does not imply that vitamin D supplementation will reduce the pain and symptoms from inflammatory arthritis or noninflammatory fibromyalgia.
Despite these many areas of uncertainty, Kilim and Rosen focus on bone health, summarize the wealth of accumulated data, and provide practical management advice we can use in the clinic. But if we struggle so much to know the correct way to manage calcium and vitamin D supplementation in our patients, it is little surprise that most of us struggle even more when asked about using supplements for which the biology is far less understood. As a medical community, we need to uniformly address this lack of understanding wherever it exists. Believing in a supplement or a treatment is not the same as understanding it or having strong evidence about its efficacy and safety.
Cardiac rehabilitation: A class 1 recommendation
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
KEY POINTS
- Cardiac rehabilitation should begin in the hospital after heart surgery or myocardial infarction, should continue with a hospital-centered 36-session program, and should be maintained independently by the patient for life.
- Exercise in a cardiac rehabilitation program entails little risk and many proven benefits.
- Cardiac rehabilitation is indicated and covered by the Centers for Medicare and Medicaid Services (CMS) for a number of cardiovascular conditions.
- Utilization of cardiac rehabilitation could be improved through CMS reimbursement incentives, electronic medical record prompts, lower copayments for participation, and home-based programs for patients who live far from medical centers.
Renal disease and the surgical patient: Minimizing the impact
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
KEY POINTS
- Many patients undergoing surgery have CKD—up to 30% in some cardiac surgery populations.
- CKD is a risk factor for perioperative complications including acute kidney injury and death.
- Although challenging, early detection of renal injury is crucial to improving outcomes in this patient population. New biomarkers are being investigated.
- Preoperative assessment and perioperative management of renal dysfunction may reduce the risk of adverse postoperative outcomes.
Federal Health Care Data Trends 2018
Swim at Your Own Risk
Hotel pools and hot tubs are breeding grounds for waterborne bacteria—and they can be deadly. Between 2000 and 2014, germs spread through treated recreational water caused at least 27,219 illnesses and 8 deaths.
According to a CDC study, efforts to prevent outbreaks have had mixed results. The number of Legionella-related respiratory disease outbreaks increased over time, while Pseudomonas-related skin infection outbreaks declined and Cryptosporidium-related diarrheal disease outbreaks leveled off.
Legionella, which can cause severe pneumonia and flulike symptoms, was responsible for 16% of outbreaks. Another 13% was due to Pseudomonas, which can cause “hot tub rash” and swimmer’s ear. When a pool, hot tub, or water playground isn’t cleaned properly, bacteria grow and form “biofilm” on wet surfaces, ideal growing grounds for bacteria like Legionella and Pseudomonas. It’s harder for disinfectants to kill these bacteria when they are protected by biofilm, the CDC says.
The worst offender was Cryptosporidium, which caused 58% of the outbreaks and 89% of the illnesses. “Swallowing just a mouthful of water with Crypto in it can make otherwise healthy kids and adults sick for weeks,” said Michele Hlavsa, RN, MPH, chief of the CDC’s Healthy Swimming Program. Chlorine can’t kill Cryptosporidium quickly, she cautions. The best way to avoid it is to keep it out of the water in the first place. That means keeping anyone (usually young children) with stomach problems or diarrhea out of the pool.
Other CDC tips:
- Check the inspection scores for pools, hot tubs, and water playgrounds.
- Use a test strip from a pool supply store to check the pH and bromine or free chlorine levels.
- Don’t swallow pool water.
- Take kids on regular bathroom breaks; change diapers in the diaper-changing area, away from the water.
Hotel pools and hot tubs are breeding grounds for waterborne bacteria—and they can be deadly. Between 2000 and 2014, germs spread through treated recreational water caused at least 27,219 illnesses and 8 deaths.
According to a CDC study, efforts to prevent outbreaks have had mixed results. The number of Legionella-related respiratory disease outbreaks increased over time, while Pseudomonas-related skin infection outbreaks declined and Cryptosporidium-related diarrheal disease outbreaks leveled off.
Legionella, which can cause severe pneumonia and flulike symptoms, was responsible for 16% of outbreaks. Another 13% was due to Pseudomonas, which can cause “hot tub rash” and swimmer’s ear. When a pool, hot tub, or water playground isn’t cleaned properly, bacteria grow and form “biofilm” on wet surfaces, ideal growing grounds for bacteria like Legionella and Pseudomonas. It’s harder for disinfectants to kill these bacteria when they are protected by biofilm, the CDC says.
The worst offender was Cryptosporidium, which caused 58% of the outbreaks and 89% of the illnesses. “Swallowing just a mouthful of water with Crypto in it can make otherwise healthy kids and adults sick for weeks,” said Michele Hlavsa, RN, MPH, chief of the CDC’s Healthy Swimming Program. Chlorine can’t kill Cryptosporidium quickly, she cautions. The best way to avoid it is to keep it out of the water in the first place. That means keeping anyone (usually young children) with stomach problems or diarrhea out of the pool.
Other CDC tips:
- Check the inspection scores for pools, hot tubs, and water playgrounds.
- Use a test strip from a pool supply store to check the pH and bromine or free chlorine levels.
- Don’t swallow pool water.
- Take kids on regular bathroom breaks; change diapers in the diaper-changing area, away from the water.
Hotel pools and hot tubs are breeding grounds for waterborne bacteria—and they can be deadly. Between 2000 and 2014, germs spread through treated recreational water caused at least 27,219 illnesses and 8 deaths.
According to a CDC study, efforts to prevent outbreaks have had mixed results. The number of Legionella-related respiratory disease outbreaks increased over time, while Pseudomonas-related skin infection outbreaks declined and Cryptosporidium-related diarrheal disease outbreaks leveled off.
Legionella, which can cause severe pneumonia and flulike symptoms, was responsible for 16% of outbreaks. Another 13% was due to Pseudomonas, which can cause “hot tub rash” and swimmer’s ear. When a pool, hot tub, or water playground isn’t cleaned properly, bacteria grow and form “biofilm” on wet surfaces, ideal growing grounds for bacteria like Legionella and Pseudomonas. It’s harder for disinfectants to kill these bacteria when they are protected by biofilm, the CDC says.
The worst offender was Cryptosporidium, which caused 58% of the outbreaks and 89% of the illnesses. “Swallowing just a mouthful of water with Crypto in it can make otherwise healthy kids and adults sick for weeks,” said Michele Hlavsa, RN, MPH, chief of the CDC’s Healthy Swimming Program. Chlorine can’t kill Cryptosporidium quickly, she cautions. The best way to avoid it is to keep it out of the water in the first place. That means keeping anyone (usually young children) with stomach problems or diarrhea out of the pool.
Other CDC tips:
- Check the inspection scores for pools, hot tubs, and water playgrounds.
- Use a test strip from a pool supply store to check the pH and bromine or free chlorine levels.
- Don’t swallow pool water.
- Take kids on regular bathroom breaks; change diapers in the diaper-changing area, away from the water.
Federal Health Care Data Trends: Veteran Demographics
If there is one thing that the VA and DoD have in common—it’s access to high-quality data. With 8.9 million veterans accessing VA care and another 9.4 million utilizing TRICARE, both the Veterans Health Administration and the TRICARE/Military Health System know a tremendous amount about their population. As a result, the VA, DoD, and independent researchers have amassed a great amount of data about health care diagnoses and outcomes. The goal of this supplement to Federal Practitioner is to help federal health care providers synthesize the data by identifying the most significant research and highlighting key findings. We have pulled together the data from a broad cross-section of researchers and developed infographics to clarify and emphasize some of the most important trends in an easy to understand format.
Click here to continue reading.
If there is one thing that the VA and DoD have in common—it’s access to high-quality data. With 8.9 million veterans accessing VA care and another 9.4 million utilizing TRICARE, both the Veterans Health Administration and the TRICARE/Military Health System know a tremendous amount about their population. As a result, the VA, DoD, and independent researchers have amassed a great amount of data about health care diagnoses and outcomes. The goal of this supplement to Federal Practitioner is to help federal health care providers synthesize the data by identifying the most significant research and highlighting key findings. We have pulled together the data from a broad cross-section of researchers and developed infographics to clarify and emphasize some of the most important trends in an easy to understand format.
Click here to continue reading.
If there is one thing that the VA and DoD have in common—it’s access to high-quality data. With 8.9 million veterans accessing VA care and another 9.4 million utilizing TRICARE, both the Veterans Health Administration and the TRICARE/Military Health System know a tremendous amount about their population. As a result, the VA, DoD, and independent researchers have amassed a great amount of data about health care diagnoses and outcomes. The goal of this supplement to Federal Practitioner is to help federal health care providers synthesize the data by identifying the most significant research and highlighting key findings. We have pulled together the data from a broad cross-section of researchers and developed infographics to clarify and emphasize some of the most important trends in an easy to understand format.
Click here to continue reading.
CHMP recommends CAR T for ALL, DLBCL
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
CHMP recommends CAR T for DLBCL, PMBCL
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®, formerly KTE-C19).
The recommendation pertains to axicabtagene ciloleucel as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal large B-cell lymphoma (PMBCL) who have received 2 or more lines of systemic therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome (CRS).
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher CRS occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®, formerly KTE-C19).
The recommendation pertains to axicabtagene ciloleucel as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal large B-cell lymphoma (PMBCL) who have received 2 or more lines of systemic therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome (CRS).
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher CRS occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®, formerly KTE-C19).
The recommendation pertains to axicabtagene ciloleucel as a treatment for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal large B-cell lymphoma (PMBCL) who have received 2 or more lines of systemic therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome (CRS).
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher CRS occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.