User login
CHMP backs expanded approval of tocilizumab
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved use of tocilizumab (RoActemra).
The recommendation is for tocilizumab to treat adults and pediatric patients age 2 and older who have severe or life-threatening cytokine release syndrome (CRS) induced by chimeric antigen receptor (CAR) T-cell therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
Tocilizumab is a humanized interleukin-6 receptor antagonist marketed by Roche Registration GmbH.
The drug is already approved by the European Commission to treat rheumatoid arthritis, active systemic juvenile idiopathic arthritis, and juvenile idiopathic polyarthritis.
The CHMP’s recommendation to expand the approved use of tocilizumab is supported by results from a retrospective analysis of data from clinical trials of CAR T-cell therapies in patients with hematologic malignancies.
For this analysis, researchers assessed 45 pediatric and adult patients treated with tocilizumab, with or without additional high-dose corticosteroids, for severe or life-threatening CRS.
Thirty-one patients (69%) achieved a response, defined as resolution of CRS within 14 days of the first dose of tocilizumab.
No more than 2 doses of tocilizumab were needed, and no drugs other than tocilizumab and corticosteroids were used for treatment.
No adverse reactions related to tocilizumab were reported.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved use of tocilizumab (RoActemra).
The recommendation is for tocilizumab to treat adults and pediatric patients age 2 and older who have severe or life-threatening cytokine release syndrome (CRS) induced by chimeric antigen receptor (CAR) T-cell therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
Tocilizumab is a humanized interleukin-6 receptor antagonist marketed by Roche Registration GmbH.
The drug is already approved by the European Commission to treat rheumatoid arthritis, active systemic juvenile idiopathic arthritis, and juvenile idiopathic polyarthritis.
The CHMP’s recommendation to expand the approved use of tocilizumab is supported by results from a retrospective analysis of data from clinical trials of CAR T-cell therapies in patients with hematologic malignancies.
For this analysis, researchers assessed 45 pediatric and adult patients treated with tocilizumab, with or without additional high-dose corticosteroids, for severe or life-threatening CRS.
Thirty-one patients (69%) achieved a response, defined as resolution of CRS within 14 days of the first dose of tocilizumab.
No more than 2 doses of tocilizumab were needed, and no drugs other than tocilizumab and corticosteroids were used for treatment.
No adverse reactions related to tocilizumab were reported.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved use of tocilizumab (RoActemra).
The recommendation is for tocilizumab to treat adults and pediatric patients age 2 and older who have severe or life-threatening cytokine release syndrome (CRS) induced by chimeric antigen receptor (CAR) T-cell therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
Tocilizumab is a humanized interleukin-6 receptor antagonist marketed by Roche Registration GmbH.
The drug is already approved by the European Commission to treat rheumatoid arthritis, active systemic juvenile idiopathic arthritis, and juvenile idiopathic polyarthritis.
The CHMP’s recommendation to expand the approved use of tocilizumab is supported by results from a retrospective analysis of data from clinical trials of CAR T-cell therapies in patients with hematologic malignancies.
For this analysis, researchers assessed 45 pediatric and adult patients treated with tocilizumab, with or without additional high-dose corticosteroids, for severe or life-threatening CRS.
Thirty-one patients (69%) achieved a response, defined as resolution of CRS within 14 days of the first dose of tocilizumab.
No more than 2 doses of tocilizumab were needed, and no drugs other than tocilizumab and corticosteroids were used for treatment.
No adverse reactions related to tocilizumab were reported.
FDA lifts hold on trial of MYC inhibitor
The US Food and Drug Administration (FDA) has lifted the clinical hold on a phase 1b trial of APTO-253.
APTO-253 is a small molecule that inhibits expression of the c-Myc oncogene without causing general myelosuppression of the bone marrow, according to Aptose Biosciences Inc., the company developing the drug.
Aptose was testing APTO-253 in a phase 1b trial of patients with relapsed or refractory acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS) before the FDA put the trial on hold in November 2015.
The hold was placed after an event that occurred during dosing at a clinical site. The event was stoppage of an intravenous infusion pump that was caused by back pressure resulting from clogging of the in-line filter.
Aptose said no drug-related serious adverse events were reported, and the observed pharmacokinetic levels in patients treated with APTO-253 were within the expected range.
However, a review revealed concerns about the documentation records of the manufacturing procedures associated with APTO-253. So Aptose voluntarily stopped dosing in the phase 1b trial, and the FDA placed the trial on hold.
A root cause investigation revealed that the event with the infusion pump resulted from chemistry and manufacturing-based issues.
Therefore, Aptose developed a new formulation of APTO-253 that did not cause filter clogging or pump stoppage during simulated infusion studies.
Now that the FDA has lifted the hold on the phase 1b trial, Aptose said screening and dosing will resume “as soon as practicable.”
“We are eager to return APTO-253 back into the clinic,” said William G. Rice, PhD, chairman, president and chief executive officer of Aptose.
“Our understanding of this molecule has evolved dramatically, and we are excited to deliver a MYC gene expression inhibitor to patients with debilitating hematologic malignancies.”
The phase 1b trial is designed to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of APTO-253 as a single agent and determine the recommended phase 2 dose of the drug.
APTO-253 will be administered once weekly, over a 28-day cycle. The dose-escalation cohort of the study could potentially enroll up to 20 patients with relapsed or refractory AML or high-risk MDS. The study is designed to then transition, as appropriate, to single-agent expansion cohorts in AML and MDS.
The US Food and Drug Administration (FDA) has lifted the clinical hold on a phase 1b trial of APTO-253.
APTO-253 is a small molecule that inhibits expression of the c-Myc oncogene without causing general myelosuppression of the bone marrow, according to Aptose Biosciences Inc., the company developing the drug.
Aptose was testing APTO-253 in a phase 1b trial of patients with relapsed or refractory acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS) before the FDA put the trial on hold in November 2015.
The hold was placed after an event that occurred during dosing at a clinical site. The event was stoppage of an intravenous infusion pump that was caused by back pressure resulting from clogging of the in-line filter.
Aptose said no drug-related serious adverse events were reported, and the observed pharmacokinetic levels in patients treated with APTO-253 were within the expected range.
However, a review revealed concerns about the documentation records of the manufacturing procedures associated with APTO-253. So Aptose voluntarily stopped dosing in the phase 1b trial, and the FDA placed the trial on hold.
A root cause investigation revealed that the event with the infusion pump resulted from chemistry and manufacturing-based issues.
Therefore, Aptose developed a new formulation of APTO-253 that did not cause filter clogging or pump stoppage during simulated infusion studies.
Now that the FDA has lifted the hold on the phase 1b trial, Aptose said screening and dosing will resume “as soon as practicable.”
“We are eager to return APTO-253 back into the clinic,” said William G. Rice, PhD, chairman, president and chief executive officer of Aptose.
“Our understanding of this molecule has evolved dramatically, and we are excited to deliver a MYC gene expression inhibitor to patients with debilitating hematologic malignancies.”
The phase 1b trial is designed to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of APTO-253 as a single agent and determine the recommended phase 2 dose of the drug.
APTO-253 will be administered once weekly, over a 28-day cycle. The dose-escalation cohort of the study could potentially enroll up to 20 patients with relapsed or refractory AML or high-risk MDS. The study is designed to then transition, as appropriate, to single-agent expansion cohorts in AML and MDS.
The US Food and Drug Administration (FDA) has lifted the clinical hold on a phase 1b trial of APTO-253.
APTO-253 is a small molecule that inhibits expression of the c-Myc oncogene without causing general myelosuppression of the bone marrow, according to Aptose Biosciences Inc., the company developing the drug.
Aptose was testing APTO-253 in a phase 1b trial of patients with relapsed or refractory acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS) before the FDA put the trial on hold in November 2015.
The hold was placed after an event that occurred during dosing at a clinical site. The event was stoppage of an intravenous infusion pump that was caused by back pressure resulting from clogging of the in-line filter.
Aptose said no drug-related serious adverse events were reported, and the observed pharmacokinetic levels in patients treated with APTO-253 were within the expected range.
However, a review revealed concerns about the documentation records of the manufacturing procedures associated with APTO-253. So Aptose voluntarily stopped dosing in the phase 1b trial, and the FDA placed the trial on hold.
A root cause investigation revealed that the event with the infusion pump resulted from chemistry and manufacturing-based issues.
Therefore, Aptose developed a new formulation of APTO-253 that did not cause filter clogging or pump stoppage during simulated infusion studies.
Now that the FDA has lifted the hold on the phase 1b trial, Aptose said screening and dosing will resume “as soon as practicable.”
“We are eager to return APTO-253 back into the clinic,” said William G. Rice, PhD, chairman, president and chief executive officer of Aptose.
“Our understanding of this molecule has evolved dramatically, and we are excited to deliver a MYC gene expression inhibitor to patients with debilitating hematologic malignancies.”
The phase 1b trial is designed to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of APTO-253 as a single agent and determine the recommended phase 2 dose of the drug.
APTO-253 will be administered once weekly, over a 28-day cycle. The dose-escalation cohort of the study could potentially enroll up to 20 patients with relapsed or refractory AML or high-risk MDS. The study is designed to then transition, as appropriate, to single-agent expansion cohorts in AML and MDS.
Long-acting injectable antipsychotics: What to do about missed doses
Antipsychotic agents are the mainstay of treatment for patients with schizophrenia,1-3 and when taken regularly, they can greatly improve patient outcomes. Unfortunately, many studies have documented poor adherence to antipsychotic regimens in patients with schizophrenia, which often leads to an exacerbation of symptoms and preventable hospitalizations.4-8 In order to improve adherence, many clinicians prescribe long-acting injectable antipsychotics (LAIAs).
LAIAs help improve adherence, but these benefits are seen only in patients who receive their injections within a specific time frame.9-11 LAIAs administered outside of this time frame (missed doses) can lead to reoccurrence or exacerbation of symptoms. This article explains how to adequately manage missed LAIA doses.
First-generation long-acting injectable antipsychotics
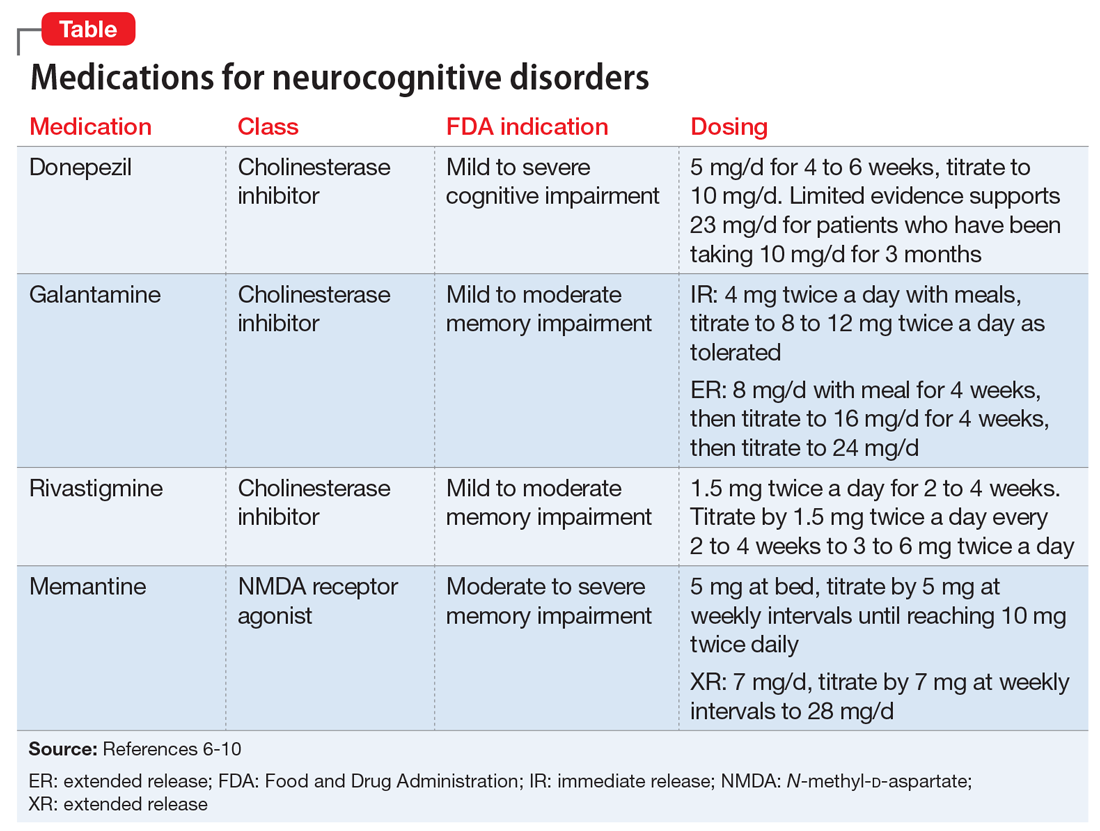
Two first-generation antipsychotics are available as a long-acting injectable formulation: haloperidol decanoate and fluphenazine decanoate. Due to the increased risk of extrapyramidal symptoms, use of these agents have decreased, and they are often less preferred than second-generation LAIAs. Furthermore, unlike many of the newer second-generation LAIAs, first-generation LAIAs lack literature on how to manage missed doses. Therefore, clinicians should analyze the pharmacokinetic properties of these agents (Table 112-28), as well as the patient’s medical history and clinical presentation, in order to determine how best to address missed doses.

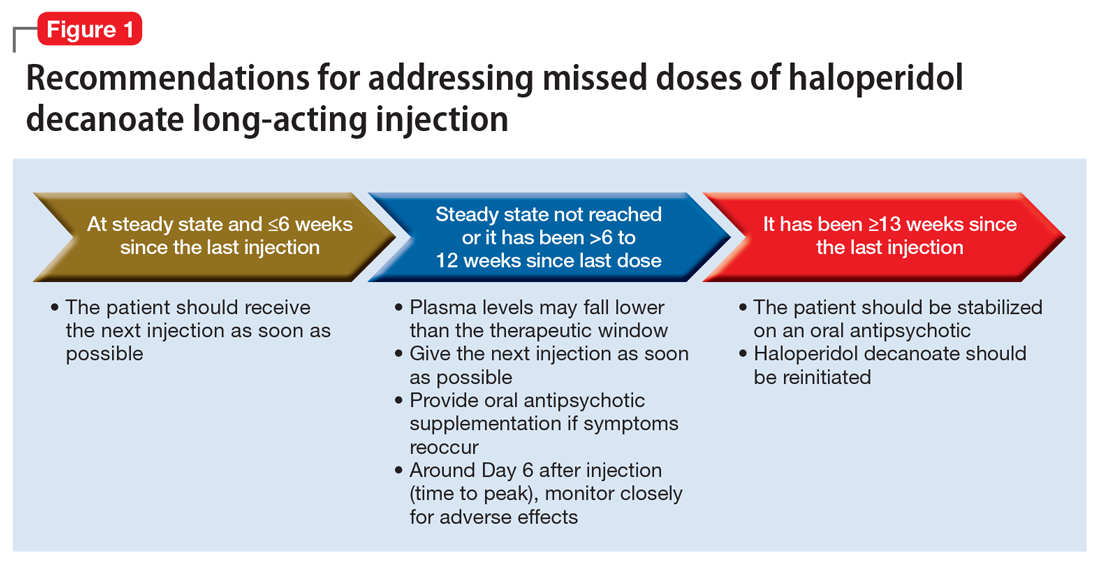
Haloperidol decanoate plasma concentrations peak approximately 6 days after the injection.12 The medication has a half-life of 3 weeks. One study found that haloperidol plasma concentrations were detectable 13 weeks after the discontinuation of haloperidol decanoate.17 This same study also found that the change in plasma levels from 3 to 6 weeks after the last dose was minimal.17 Based on these findings, Figure 1 summarizes our recommendations for addressing missed haloperidol decanoate doses.
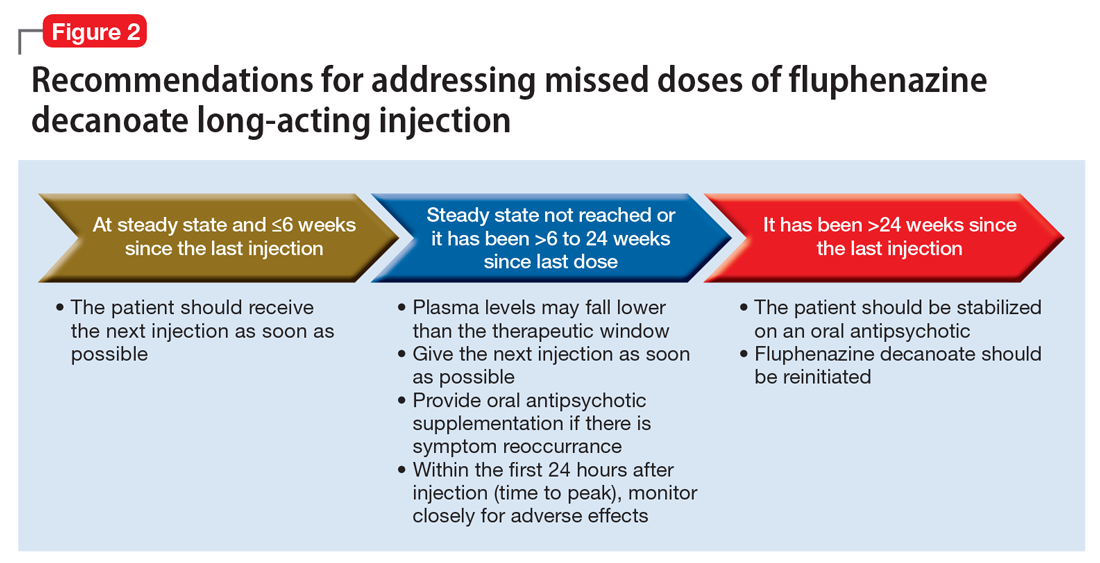
Fluphenazine decanoate levels peak 24 hours after the injection.18 An estimated therapeutic range for fluphenazine is 0.2 to 2 ng/mL.21-25 One study that evaluated fluphenazine decanoate levels following discontinuation after reaching steady state found there was no significant difference in plasma levels 6 weeks after the last dose of fluphenazine, but a significant decrease in levels 8 to 12 weeks after the last dose.26 Other studies found that fluphenazine levels were detectable 21 to 24 weeks following fluphenazine decanoate discontinuation.27,28 Based on these findings, Figure 2 summarizes our recommendations for addressing missed fluphenazine decanoate doses.
Continue to: Second-generation LAIAs
Second-generation LAIAs
Six second-generation LAIAs are available in the United States. Compared with the first-generation LAIAs, second-generation LAIAs have more extensive guidance on how to address missed doses.
Risperidone long-acting injection. When addressing missed doses of risperidone long-acting injection, first determine whether the medication has reached steady state. Steady state occurs approximately after the fourth consecutive injection (approximately 2 months).29
If a patient missed a dose but has not reached steady state, he or she should receive the next dose as well as oral antipsychotic supplementation for 3 weeks.30 If the patient has reached steady state and if it has been ≤6 weeks since the last injection, give the next injection as soon as possible. However, if steady state has been reached and it has been >6 weeks since the last injection, give the next injection, along with 3 weeks of oral antipsychotic supplementation (Figure 3).

Paliperidone palmitate monthly long-acting injection. Once the initiation dosing phase of paliperidone palmitate monthly long-acting injection (PP1M) is completed, the maintenance dose is administered every 4 weeks. When addressing missed doses of PP1M, first determine whether the patient is in the initiation or maintenance dosing phase.31
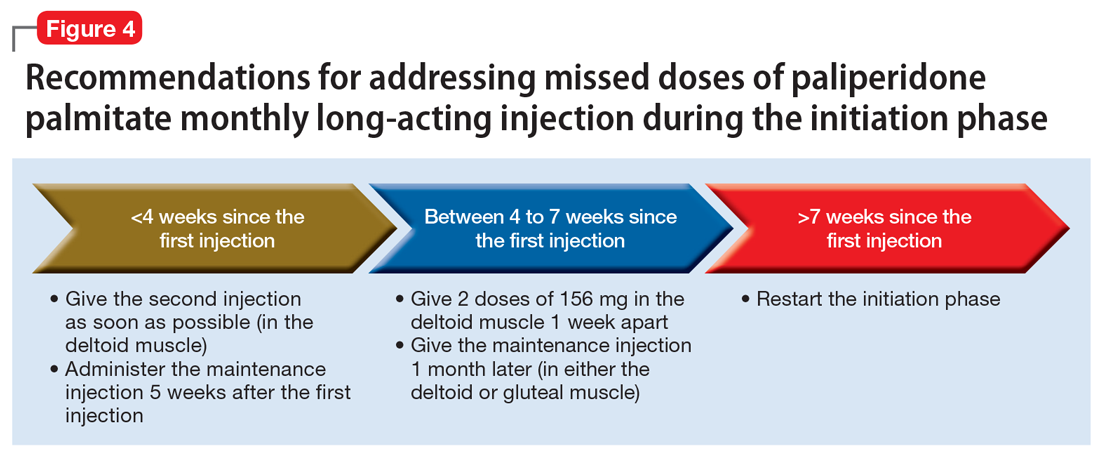
Initiation phase. Patients are in the initiation dosing phase during the first 2 injections of PP1M. During the initiation phase, the patient first receives 234 mg and then 156 mg 1 week later, both in the deltoid muscle. One month later, the patient receives a maintenance dose of PP1M (in the deltoid or gluteal muscle). The second initiation injection may be given 4 days before or after the scheduled administration date. The initiation doses should be adjusted in patients with mild renal function (creatinine clearance 50 to 80 mL/min).31 Figure 4 summarizes the guidance for addressing a missed or delayed second injection during the initiation phase.
Continue to: Maintenance phase
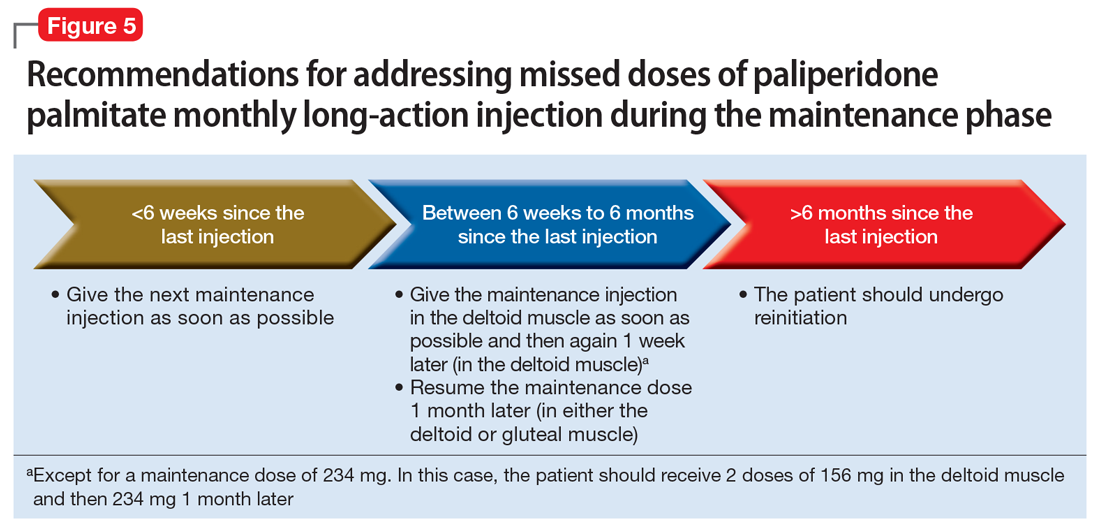
Maintenance phase. During the maintenance phase, PP1M can be administered 7 days before or after the monthly due date. If the patient has missed a maintenance injection and it has been <6 weeks since the last dose, the maintenance injection can be given as soon as possible (Figure 5).31 If it has been 6 weeks to 6 months since the last injection, the patient should receive their prescribed maintenance dose as soon as possible and the same dose 1 week later, with both injections in the deltoid muscle. Following the second dose, the patient can resume their regular monthly maintenance schedule, in either the deltoid or gluteal muscle. For example, if the patient was maintained on 117 mg of PP1M and it had been 8 weeks since the last injection, the patient should receive 117 mg immediately, then 117 mg 1 week later, then 117 mg 1 month later. An exception to this is if a patient’s maintenance dose is 234 mg monthly. In this case, the patient should receive 156 mg of PP1M immediately, then 156 mg 1 week later, and then 234 mg 1 month later.31 If it has been >6 months since the last dose, the patient should start the initiation schedule as if he or she were receiving a new medication.31
Paliperidone palmitate 3-month long-acting injection (PP3M) should be administered every 3 months. This injection can be given 2 weeks before or after the date of the scheduled dose.32
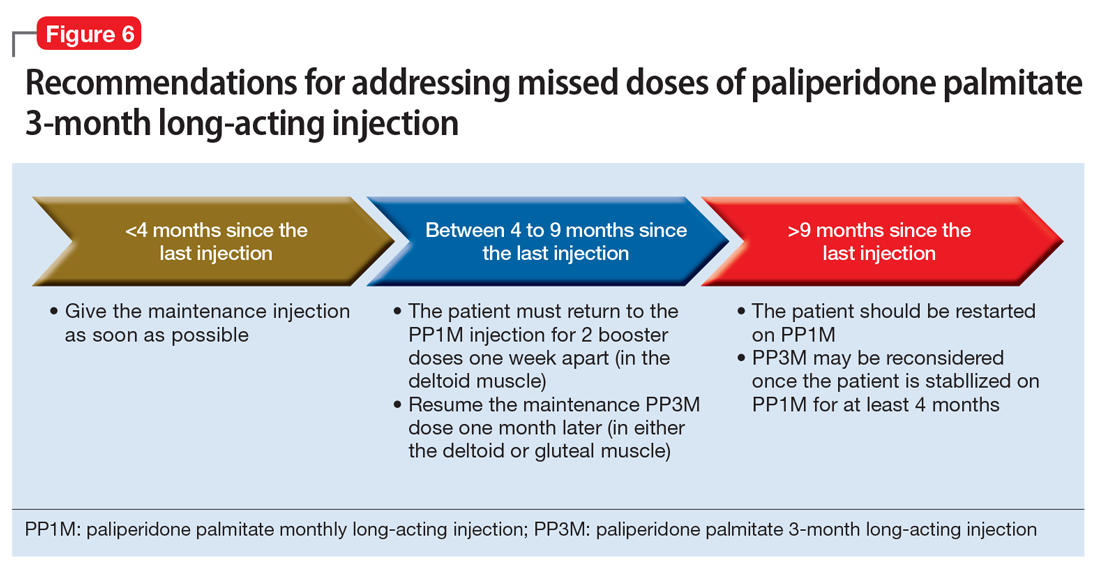
If the patient missed an injection and it has been <4 months since the last dose, the next scheduled dose should be given as soon as possible.32 If it has been 4 to 9 months since the last dose, the patient must return to PP1M for 2 booster injections 1 week apart. The dose of these PP1M booster injections depends on the dose of PP3M that the patient had been stabilized on:
- 78 mg if stabilized on 273 mg
- 117 mg if stabilized on 410 mg
- 156 mg if stabilized on 546 mg or 819 mg.32
After the second booster dose, PP3M can be restarted 1 month later.32 If it has been >9 months since the last PP3M dose, the patient should be restarted on PP1M. PP3M can be reconsidered once the patient has been stabilized on PP1M for ≥4 months (Figure 6).32
Continue to: Aripiprazole long-acting injection
Aripiprazole long-acting injection is administered every 4 weeks. If a patient misses an injection, first determine how many consecutive doses he or she has received.33 If the patient has missed the second or third injection, and it has been <5 weeks since the last dose, give the next injection as soon as possible. If it has been >5 weeks, give the next injection as soon as possible, plus oral aripiprazole supplementation for 2 weeks (Figure 7).
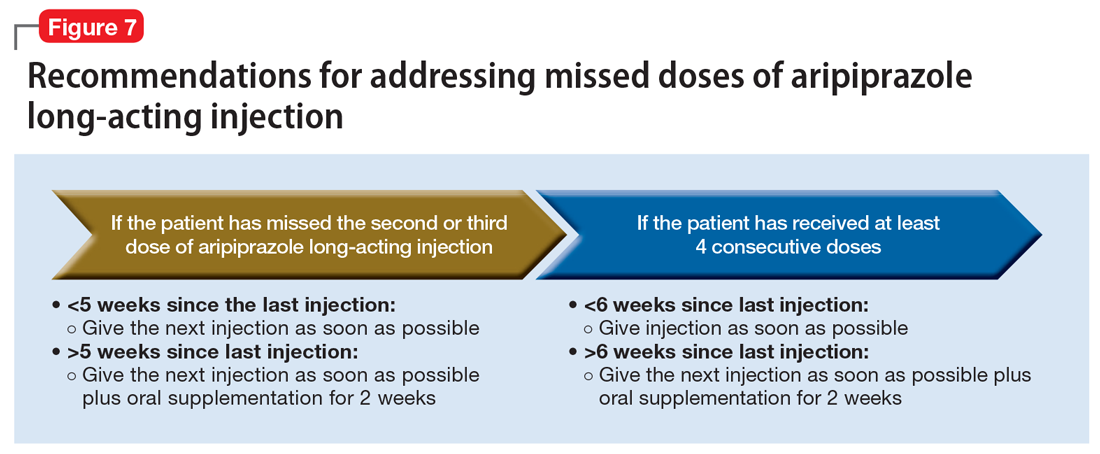
If the patient has received ≥4 consecutive doses and misses a dose and it has been <6 weeks since the last dose, administer an injection as soon as possible. If it has been >6 weeks since the last dose, give the next injection as soon as possible, plus with oral aripiprazole supplementation for 2 weeks.
Aripiprazole lauroxil long-acting injection. Depending on the dose, aripiprazole lauroxil can be administered monthly, every 6 weeks, or every 2 months. Aripiprazole lauroxil can be administered 14 days before or after the scheduled dose.34
The guidance for addressing missed or delayed doses of aripiprazole lauroxil differs depending on the dose the patient is stabilized on, and how long it has been since the last injection. Figure 8 summarizes how missed injections should be managed. When oral aripiprazole supplementation is needed, the following doses should be used:
- 10 mg/d if stabilized on 441 mg every month
- 15 mg/d if stabilized on 662 mg every month, 882 mg every 6 weeks, or 1,064 mg every 2 months
- 20 mg/d if stabilized on 882 mg every month.34

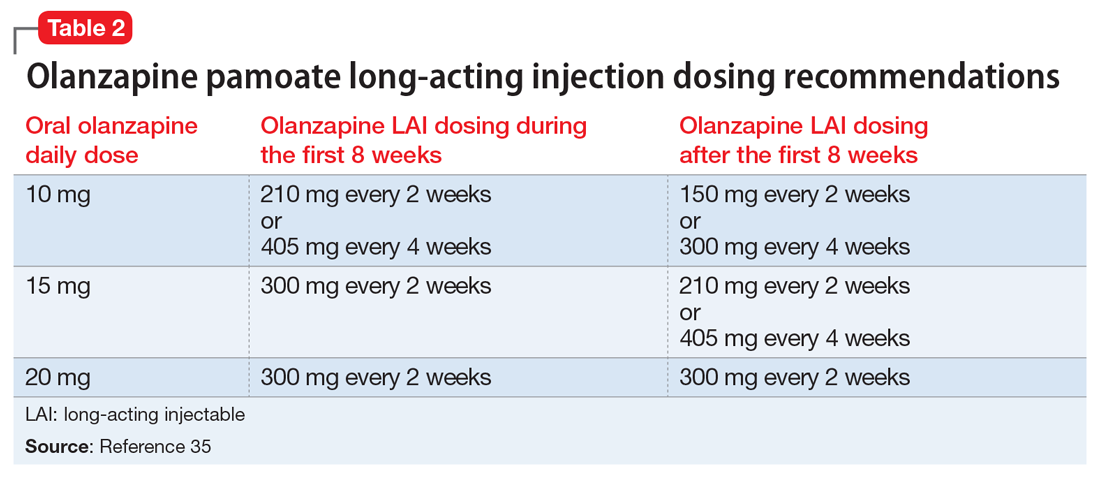
Olanzapine pamoate long-acting injection is a unique LAIA because it requires prescribers and patients to participate in a risk evaluation and mitigation strategies (REMS) program due the risk of post-injection delirium/sedation syndrome. It is administered every 2 to 4 weeks, with loading doses given for the first 2 months of treatment (Table 235). After 2 months, the patient can proceed to the maintenance dosing regimen.
Continue to: Currently, there is no concrete guidance...
Currently, there is no concrete guidance on how to address missed doses of olanzapine long-acting injection; however, the pharmacokinetics of this formulation allow flexibility in dosing intervals. Therapeutic levels are present after the first injection, and the medication reaches steady-state levels in 3 months.35-37 As a result of its specific formulation, olanzapine pamoate long-acting injection provides sustained olanzapine pamoate plasma concentrations between injections, and has a half-life of 30 days.35 Consequently, therapeutic levels of the medication are still present 2 to 4 weeks after an injection.37 Additionally, clinically relevant plasma concentrations may be present 2 to 3 months after the last injection.36
In light of this information, if a patient has not reached steady state and has missed an injection, he or she should receive the recommended loading dose schedule. If the patient has reached steady state and it has been ≤2 months since the last dose, he or she should receive the next dose as soon as possible. If steady state has been reached and it has been >2 months since the last injection, the patient should receive the recommended loading dosing for 2 months (Figure 9). Because of the risk of post-injection delirium/sedation syndrome, and because therapeutic levels are achieved after the first injection, oral olanzapine supplementation is not recommended.

Use a stepwise approach
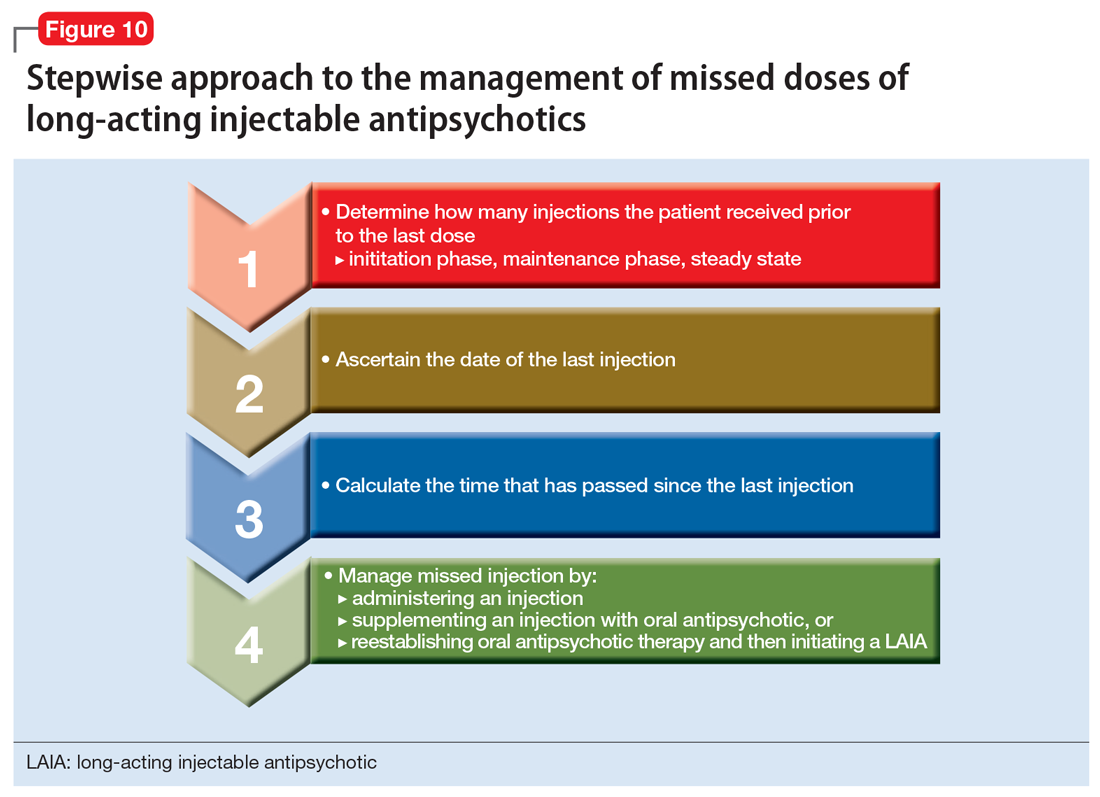
In general, clinicians can use a stepwise approach to managing missed doses of LAIAs (Figure 10). First, establish the number of LAIA doses the patient had received prior to the last dose, and whether these injections were administered on schedule. This will help you determine if the patient is in the initiation or maintenance phase and/or has reached steady state. The second step is to establish the date of the last injection. Use objective tools, such as pharmacy records or the medical chart, to determine the date of the last injection, rather than relying on patient reporting. For the third step, calculate the time that has passed since the last LAIA dose. Once you have completed these steps, use the specific medication recommendations described in this article to address the missed dose.
Continue to: Address barriers to adherence
Address barriers to adherence
When addressing missed LAIA doses, be sure to identify any barriers that may have led to a missed injection. These might include:
- bothersome adverse effects
- transportation difficulties
- issues with insurance/medication coverage
- comorbidities (ie, alcohol/substance use disorders)
- cognitive and functional impairment caused by the patient’s illness
- difficulty with keeping track of appointments.
Clinicians can work closely with patients and/or caregivers to address any barriers to ensure that patients receive their injections in a timely fashion.
The goal: Reducing relapse
LAIAs improve medication adherence. Although nonadherence is less frequent with LAIAs than with oral antipsychotics, when a LAIA dose is missed, it is important to properly follow a stepwise approach based on the unique properties of the specific LAIA prescribed. Proper management of LAIA missed doses can prevent relapse and reoccurrence of schizophrenia symptoms, thus possibly avoiding future hospitalizations.
Acknowledgments
The authors thank Brian Tschosik, JD, Mary Collen O’Rourke, MD, and Amanda Holloway, MD, for their assistance with this article.
Bottom Line
Although long-acting injectable antipsychotics (LAIAs) greatly assist with adherence, these agents are effective only when missed doses are avoided. When addressing missed LAIA doses, use a stepwise approach that takes into consideration the unique properties of the specific LAIA prescribed.
Related Resources
- Haddad P, Lambert T, Lauriello J, eds. Antipsychotic long-acting injections. 2nd ed. Oxford, UK: Oxford University Press; 2016.
- Diefenderfer LA. When should you consider combining 2 long-acting injectable antipsychotics? Current Psychiatry. 2017;16(10):42-46.
Drug Brand Names
Aripiprazole long-acting injection • Abilify Maintena
Aripiprazole lauroxil long-acting injection • Aristada
Fluphenazine decanoate • Prolixin decanoate
Haloperidol decanoate • Haldol decanoate
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month long-acting injection • Invega Trinza
Risperidone long-acting injection • Risperdal Consta
1. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;195(52):S63-S67.
4. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
5. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
6. Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346(8973):477-481.
7. de Sena EP, Santos-Jesus R, Miranda-Scippa Â, et al. Relapse in patients with schizophrenia: a comparison between risperidone and haloperidol. Rev Bras Psiquiatr. 2003;25(4):220-223.
8. Chue P. Long-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychotic. Neuropsychiatr Dis Treat. 2007;3(1):13-39.
9. Lafeuille MH, Frois C, Cloutier M, et al. Factors associated with adherence to the HEDIS Quality Measure in medicaid patients with schizophrenia. Am Health Drug Benefits. 2016;9(7):399-410.
10. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
11. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
12. Haldol Decanoate injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
13. Magliozzi JR, Hollister LE, Arnold KV, et al. Relationship of serum haloperidol levels to clinical response in schizophrenic patients. Am J Psychiatry. 1981;138(3):365-367.
14. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Clinical response and plasma haloperidol levels in schizophrenia. Psychopharmacology (Berl). 1983;81(4):354-356.
15. Reyntigens AJ, Heykants JJ, Woestenborghs RJ, et al. Pharmacokinetics of haloperidol decanoate. A 2-year follow-up. Int Pharmacopsychiatry. 1982;17(4):238-246.
16. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
17. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
18. Ereshefsky L, Saklad SR, Jann MW. Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches. J Clin Psychiatry.1984;45(5 pt 2):50-58.
19. Marder SR, Hawes EM, Van Putten T, et al. Fluphenazine plasma levels in patients receiving low and conventional doses of fluphenazine decanoate. Psychopharmacology (Berl). 1986;88(4):480-483.
20. Marder SR, Hubbard JW, Van Putten T, et al. Pharmacokinetics of long-acting injectable neuroleptic drugs: clinical implications. Psychopharmacology (Berl). 1989;98(4):433-439.
21. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Fluphenazine plasma levels and clinical response. J Clin Psychiatry. 1984;45(9):370-373.
22. Balant-Gorgia AE, Balant LP, Andreoli A. Pharmacokinetic optimisation of the treatment of psychosis. Clin Pharmacokinet. 1993;25(3):217-236.
23. Van Putten T, Marder SR, Wirshing WC, et al. Neuroleptic plasma levels. Schizophr Bull. 1991;17(2):197-216.
24. Dahl SG. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin Pharmacokinet. 1986;11(1):36-61.
25. Miller RS, Peterson GM, McLean S, et al. Monitoring plasma levels of fluphenazine during chronic therapy with fluphenazine decanoate. J Clin Pharm Ther. 1995;20(2):55-62.
26. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
27. Wistedt B, Wiles D, Kolakowska T. Slow decline of plasma drug and prolactin levels after discontinuation of chronic treatment with depot neuroleptics. Lancet. 1981;1(8230):1163.
28. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology (Berl). 1982;78(4):301-304.
29. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
30. Marder SR, Conley R, Ereshefsky L, et al. Clinical guidelines: dosing and switching strategies for long-acting risperidone. J Clin Psychiatry. 2003;64(suppl 16):41-46.
31. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
32. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
33. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; December 2016.
34. Artistada [package insert]. Waltham, MA: Alkermes, Inc.; June 2017.
35. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; February 2017.
36. Heres S, Kraemer S, Bergstrom RF, et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol. 2014;29(6):299-312.
37. Detke HC, Zhao F, Garhyan P, et al. Dose correspondence between olanzapine long-acting injection and oral olanzapine: recommendations for switching. Int Clin Psychopharmacol. 2011;26(1):35-42.
Antipsychotic agents are the mainstay of treatment for patients with schizophrenia,1-3 and when taken regularly, they can greatly improve patient outcomes. Unfortunately, many studies have documented poor adherence to antipsychotic regimens in patients with schizophrenia, which often leads to an exacerbation of symptoms and preventable hospitalizations.4-8 In order to improve adherence, many clinicians prescribe long-acting injectable antipsychotics (LAIAs).
LAIAs help improve adherence, but these benefits are seen only in patients who receive their injections within a specific time frame.9-11 LAIAs administered outside of this time frame (missed doses) can lead to reoccurrence or exacerbation of symptoms. This article explains how to adequately manage missed LAIA doses.
First-generation long-acting injectable antipsychotics
Two first-generation antipsychotics are available as a long-acting injectable formulation: haloperidol decanoate and fluphenazine decanoate. Due to the increased risk of extrapyramidal symptoms, use of these agents have decreased, and they are often less preferred than second-generation LAIAs. Furthermore, unlike many of the newer second-generation LAIAs, first-generation LAIAs lack literature on how to manage missed doses. Therefore, clinicians should analyze the pharmacokinetic properties of these agents (Table 112-28), as well as the patient’s medical history and clinical presentation, in order to determine how best to address missed doses.

Haloperidol decanoate plasma concentrations peak approximately 6 days after the injection.12 The medication has a half-life of 3 weeks. One study found that haloperidol plasma concentrations were detectable 13 weeks after the discontinuation of haloperidol decanoate.17 This same study also found that the change in plasma levels from 3 to 6 weeks after the last dose was minimal.17 Based on these findings, Figure 1 summarizes our recommendations for addressing missed haloperidol decanoate doses.

Fluphenazine decanoate levels peak 24 hours after the injection.18 An estimated therapeutic range for fluphenazine is 0.2 to 2 ng/mL.21-25 One study that evaluated fluphenazine decanoate levels following discontinuation after reaching steady state found there was no significant difference in plasma levels 6 weeks after the last dose of fluphenazine, but a significant decrease in levels 8 to 12 weeks after the last dose.26 Other studies found that fluphenazine levels were detectable 21 to 24 weeks following fluphenazine decanoate discontinuation.27,28 Based on these findings, Figure 2 summarizes our recommendations for addressing missed fluphenazine decanoate doses.

Continue to: Second-generation LAIAs
Second-generation LAIAs
Six second-generation LAIAs are available in the United States. Compared with the first-generation LAIAs, second-generation LAIAs have more extensive guidance on how to address missed doses.
Risperidone long-acting injection. When addressing missed doses of risperidone long-acting injection, first determine whether the medication has reached steady state. Steady state occurs approximately after the fourth consecutive injection (approximately 2 months).29
If a patient missed a dose but has not reached steady state, he or she should receive the next dose as well as oral antipsychotic supplementation for 3 weeks.30 If the patient has reached steady state and if it has been ≤6 weeks since the last injection, give the next injection as soon as possible. However, if steady state has been reached and it has been >6 weeks since the last injection, give the next injection, along with 3 weeks of oral antipsychotic supplementation (Figure 3).

Paliperidone palmitate monthly long-acting injection. Once the initiation dosing phase of paliperidone palmitate monthly long-acting injection (PP1M) is completed, the maintenance dose is administered every 4 weeks. When addressing missed doses of PP1M, first determine whether the patient is in the initiation or maintenance dosing phase.31
Initiation phase. Patients are in the initiation dosing phase during the first 2 injections of PP1M. During the initiation phase, the patient first receives 234 mg and then 156 mg 1 week later, both in the deltoid muscle. One month later, the patient receives a maintenance dose of PP1M (in the deltoid or gluteal muscle). The second initiation injection may be given 4 days before or after the scheduled administration date. The initiation doses should be adjusted in patients with mild renal function (creatinine clearance 50 to 80 mL/min).31 Figure 4 summarizes the guidance for addressing a missed or delayed second injection during the initiation phase.

Continue to: Maintenance phase
Maintenance phase. During the maintenance phase, PP1M can be administered 7 days before or after the monthly due date. If the patient has missed a maintenance injection and it has been <6 weeks since the last dose, the maintenance injection can be given as soon as possible (Figure 5).31 If it has been 6 weeks to 6 months since the last injection, the patient should receive their prescribed maintenance dose as soon as possible and the same dose 1 week later, with both injections in the deltoid muscle. Following the second dose, the patient can resume their regular monthly maintenance schedule, in either the deltoid or gluteal muscle. For example, if the patient was maintained on 117 mg of PP1M and it had been 8 weeks since the last injection, the patient should receive 117 mg immediately, then 117 mg 1 week later, then 117 mg 1 month later. An exception to this is if a patient’s maintenance dose is 234 mg monthly. In this case, the patient should receive 156 mg of PP1M immediately, then 156 mg 1 week later, and then 234 mg 1 month later.31 If it has been >6 months since the last dose, the patient should start the initiation schedule as if he or she were receiving a new medication.31

Paliperidone palmitate 3-month long-acting injection (PP3M) should be administered every 3 months. This injection can be given 2 weeks before or after the date of the scheduled dose.32
If the patient missed an injection and it has been <4 months since the last dose, the next scheduled dose should be given as soon as possible.32 If it has been 4 to 9 months since the last dose, the patient must return to PP1M for 2 booster injections 1 week apart. The dose of these PP1M booster injections depends on the dose of PP3M that the patient had been stabilized on:
- 78 mg if stabilized on 273 mg
- 117 mg if stabilized on 410 mg
- 156 mg if stabilized on 546 mg or 819 mg.32
After the second booster dose, PP3M can be restarted 1 month later.32 If it has been >9 months since the last PP3M dose, the patient should be restarted on PP1M. PP3M can be reconsidered once the patient has been stabilized on PP1M for ≥4 months (Figure 6).32

Continue to: Aripiprazole long-acting injection
Aripiprazole long-acting injection is administered every 4 weeks. If a patient misses an injection, first determine how many consecutive doses he or she has received.33 If the patient has missed the second or third injection, and it has been <5 weeks since the last dose, give the next injection as soon as possible. If it has been >5 weeks, give the next injection as soon as possible, plus oral aripiprazole supplementation for 2 weeks (Figure 7).

If the patient has received ≥4 consecutive doses and misses a dose and it has been <6 weeks since the last dose, administer an injection as soon as possible. If it has been >6 weeks since the last dose, give the next injection as soon as possible, plus with oral aripiprazole supplementation for 2 weeks.
Aripiprazole lauroxil long-acting injection. Depending on the dose, aripiprazole lauroxil can be administered monthly, every 6 weeks, or every 2 months. Aripiprazole lauroxil can be administered 14 days before or after the scheduled dose.34
The guidance for addressing missed or delayed doses of aripiprazole lauroxil differs depending on the dose the patient is stabilized on, and how long it has been since the last injection. Figure 8 summarizes how missed injections should be managed. When oral aripiprazole supplementation is needed, the following doses should be used:
- 10 mg/d if stabilized on 441 mg every month
- 15 mg/d if stabilized on 662 mg every month, 882 mg every 6 weeks, or 1,064 mg every 2 months
- 20 mg/d if stabilized on 882 mg every month.34

Olanzapine pamoate long-acting injection is a unique LAIA because it requires prescribers and patients to participate in a risk evaluation and mitigation strategies (REMS) program due the risk of post-injection delirium/sedation syndrome. It is administered every 2 to 4 weeks, with loading doses given for the first 2 months of treatment (Table 235). After 2 months, the patient can proceed to the maintenance dosing regimen.

Continue to: Currently, there is no concrete guidance...
Currently, there is no concrete guidance on how to address missed doses of olanzapine long-acting injection; however, the pharmacokinetics of this formulation allow flexibility in dosing intervals. Therapeutic levels are present after the first injection, and the medication reaches steady-state levels in 3 months.35-37 As a result of its specific formulation, olanzapine pamoate long-acting injection provides sustained olanzapine pamoate plasma concentrations between injections, and has a half-life of 30 days.35 Consequently, therapeutic levels of the medication are still present 2 to 4 weeks after an injection.37 Additionally, clinically relevant plasma concentrations may be present 2 to 3 months after the last injection.36
In light of this information, if a patient has not reached steady state and has missed an injection, he or she should receive the recommended loading dose schedule. If the patient has reached steady state and it has been ≤2 months since the last dose, he or she should receive the next dose as soon as possible. If steady state has been reached and it has been >2 months since the last injection, the patient should receive the recommended loading dosing for 2 months (Figure 9). Because of the risk of post-injection delirium/sedation syndrome, and because therapeutic levels are achieved after the first injection, oral olanzapine supplementation is not recommended.

Use a stepwise approach
In general, clinicians can use a stepwise approach to managing missed doses of LAIAs (Figure 10). First, establish the number of LAIA doses the patient had received prior to the last dose, and whether these injections were administered on schedule. This will help you determine if the patient is in the initiation or maintenance phase and/or has reached steady state. The second step is to establish the date of the last injection. Use objective tools, such as pharmacy records or the medical chart, to determine the date of the last injection, rather than relying on patient reporting. For the third step, calculate the time that has passed since the last LAIA dose. Once you have completed these steps, use the specific medication recommendations described in this article to address the missed dose.

Continue to: Address barriers to adherence
Address barriers to adherence
When addressing missed LAIA doses, be sure to identify any barriers that may have led to a missed injection. These might include:
- bothersome adverse effects
- transportation difficulties
- issues with insurance/medication coverage
- comorbidities (ie, alcohol/substance use disorders)
- cognitive and functional impairment caused by the patient’s illness
- difficulty with keeping track of appointments.
Clinicians can work closely with patients and/or caregivers to address any barriers to ensure that patients receive their injections in a timely fashion.
The goal: Reducing relapse
LAIAs improve medication adherence. Although nonadherence is less frequent with LAIAs than with oral antipsychotics, when a LAIA dose is missed, it is important to properly follow a stepwise approach based on the unique properties of the specific LAIA prescribed. Proper management of LAIA missed doses can prevent relapse and reoccurrence of schizophrenia symptoms, thus possibly avoiding future hospitalizations.
Acknowledgments
The authors thank Brian Tschosik, JD, Mary Collen O’Rourke, MD, and Amanda Holloway, MD, for their assistance with this article.
Bottom Line
Although long-acting injectable antipsychotics (LAIAs) greatly assist with adherence, these agents are effective only when missed doses are avoided. When addressing missed LAIA doses, use a stepwise approach that takes into consideration the unique properties of the specific LAIA prescribed.
Related Resources
- Haddad P, Lambert T, Lauriello J, eds. Antipsychotic long-acting injections. 2nd ed. Oxford, UK: Oxford University Press; 2016.
- Diefenderfer LA. When should you consider combining 2 long-acting injectable antipsychotics? Current Psychiatry. 2017;16(10):42-46.
Drug Brand Names
Aripiprazole long-acting injection • Abilify Maintena
Aripiprazole lauroxil long-acting injection • Aristada
Fluphenazine decanoate • Prolixin decanoate
Haloperidol decanoate • Haldol decanoate
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month long-acting injection • Invega Trinza
Risperidone long-acting injection • Risperdal Consta
Antipsychotic agents are the mainstay of treatment for patients with schizophrenia,1-3 and when taken regularly, they can greatly improve patient outcomes. Unfortunately, many studies have documented poor adherence to antipsychotic regimens in patients with schizophrenia, which often leads to an exacerbation of symptoms and preventable hospitalizations.4-8 In order to improve adherence, many clinicians prescribe long-acting injectable antipsychotics (LAIAs).
LAIAs help improve adherence, but these benefits are seen only in patients who receive their injections within a specific time frame.9-11 LAIAs administered outside of this time frame (missed doses) can lead to reoccurrence or exacerbation of symptoms. This article explains how to adequately manage missed LAIA doses.
First-generation long-acting injectable antipsychotics
Two first-generation antipsychotics are available as a long-acting injectable formulation: haloperidol decanoate and fluphenazine decanoate. Due to the increased risk of extrapyramidal symptoms, use of these agents have decreased, and they are often less preferred than second-generation LAIAs. Furthermore, unlike many of the newer second-generation LAIAs, first-generation LAIAs lack literature on how to manage missed doses. Therefore, clinicians should analyze the pharmacokinetic properties of these agents (Table 112-28), as well as the patient’s medical history and clinical presentation, in order to determine how best to address missed doses.

Haloperidol decanoate plasma concentrations peak approximately 6 days after the injection.12 The medication has a half-life of 3 weeks. One study found that haloperidol plasma concentrations were detectable 13 weeks after the discontinuation of haloperidol decanoate.17 This same study also found that the change in plasma levels from 3 to 6 weeks after the last dose was minimal.17 Based on these findings, Figure 1 summarizes our recommendations for addressing missed haloperidol decanoate doses.

Fluphenazine decanoate levels peak 24 hours after the injection.18 An estimated therapeutic range for fluphenazine is 0.2 to 2 ng/mL.21-25 One study that evaluated fluphenazine decanoate levels following discontinuation after reaching steady state found there was no significant difference in plasma levels 6 weeks after the last dose of fluphenazine, but a significant decrease in levels 8 to 12 weeks after the last dose.26 Other studies found that fluphenazine levels were detectable 21 to 24 weeks following fluphenazine decanoate discontinuation.27,28 Based on these findings, Figure 2 summarizes our recommendations for addressing missed fluphenazine decanoate doses.

Continue to: Second-generation LAIAs
Second-generation LAIAs
Six second-generation LAIAs are available in the United States. Compared with the first-generation LAIAs, second-generation LAIAs have more extensive guidance on how to address missed doses.
Risperidone long-acting injection. When addressing missed doses of risperidone long-acting injection, first determine whether the medication has reached steady state. Steady state occurs approximately after the fourth consecutive injection (approximately 2 months).29
If a patient missed a dose but has not reached steady state, he or she should receive the next dose as well as oral antipsychotic supplementation for 3 weeks.30 If the patient has reached steady state and if it has been ≤6 weeks since the last injection, give the next injection as soon as possible. However, if steady state has been reached and it has been >6 weeks since the last injection, give the next injection, along with 3 weeks of oral antipsychotic supplementation (Figure 3).

Paliperidone palmitate monthly long-acting injection. Once the initiation dosing phase of paliperidone palmitate monthly long-acting injection (PP1M) is completed, the maintenance dose is administered every 4 weeks. When addressing missed doses of PP1M, first determine whether the patient is in the initiation or maintenance dosing phase.31
Initiation phase. Patients are in the initiation dosing phase during the first 2 injections of PP1M. During the initiation phase, the patient first receives 234 mg and then 156 mg 1 week later, both in the deltoid muscle. One month later, the patient receives a maintenance dose of PP1M (in the deltoid or gluteal muscle). The second initiation injection may be given 4 days before or after the scheduled administration date. The initiation doses should be adjusted in patients with mild renal function (creatinine clearance 50 to 80 mL/min).31 Figure 4 summarizes the guidance for addressing a missed or delayed second injection during the initiation phase.

Continue to: Maintenance phase
Maintenance phase. During the maintenance phase, PP1M can be administered 7 days before or after the monthly due date. If the patient has missed a maintenance injection and it has been <6 weeks since the last dose, the maintenance injection can be given as soon as possible (Figure 5).31 If it has been 6 weeks to 6 months since the last injection, the patient should receive their prescribed maintenance dose as soon as possible and the same dose 1 week later, with both injections in the deltoid muscle. Following the second dose, the patient can resume their regular monthly maintenance schedule, in either the deltoid or gluteal muscle. For example, if the patient was maintained on 117 mg of PP1M and it had been 8 weeks since the last injection, the patient should receive 117 mg immediately, then 117 mg 1 week later, then 117 mg 1 month later. An exception to this is if a patient’s maintenance dose is 234 mg monthly. In this case, the patient should receive 156 mg of PP1M immediately, then 156 mg 1 week later, and then 234 mg 1 month later.31 If it has been >6 months since the last dose, the patient should start the initiation schedule as if he or she were receiving a new medication.31

Paliperidone palmitate 3-month long-acting injection (PP3M) should be administered every 3 months. This injection can be given 2 weeks before or after the date of the scheduled dose.32
If the patient missed an injection and it has been <4 months since the last dose, the next scheduled dose should be given as soon as possible.32 If it has been 4 to 9 months since the last dose, the patient must return to PP1M for 2 booster injections 1 week apart. The dose of these PP1M booster injections depends on the dose of PP3M that the patient had been stabilized on:
- 78 mg if stabilized on 273 mg
- 117 mg if stabilized on 410 mg
- 156 mg if stabilized on 546 mg or 819 mg.32
After the second booster dose, PP3M can be restarted 1 month later.32 If it has been >9 months since the last PP3M dose, the patient should be restarted on PP1M. PP3M can be reconsidered once the patient has been stabilized on PP1M for ≥4 months (Figure 6).32

Continue to: Aripiprazole long-acting injection
Aripiprazole long-acting injection is administered every 4 weeks. If a patient misses an injection, first determine how many consecutive doses he or she has received.33 If the patient has missed the second or third injection, and it has been <5 weeks since the last dose, give the next injection as soon as possible. If it has been >5 weeks, give the next injection as soon as possible, plus oral aripiprazole supplementation for 2 weeks (Figure 7).

If the patient has received ≥4 consecutive doses and misses a dose and it has been <6 weeks since the last dose, administer an injection as soon as possible. If it has been >6 weeks since the last dose, give the next injection as soon as possible, plus with oral aripiprazole supplementation for 2 weeks.
Aripiprazole lauroxil long-acting injection. Depending on the dose, aripiprazole lauroxil can be administered monthly, every 6 weeks, or every 2 months. Aripiprazole lauroxil can be administered 14 days before or after the scheduled dose.34
The guidance for addressing missed or delayed doses of aripiprazole lauroxil differs depending on the dose the patient is stabilized on, and how long it has been since the last injection. Figure 8 summarizes how missed injections should be managed. When oral aripiprazole supplementation is needed, the following doses should be used:
- 10 mg/d if stabilized on 441 mg every month
- 15 mg/d if stabilized on 662 mg every month, 882 mg every 6 weeks, or 1,064 mg every 2 months
- 20 mg/d if stabilized on 882 mg every month.34

Olanzapine pamoate long-acting injection is a unique LAIA because it requires prescribers and patients to participate in a risk evaluation and mitigation strategies (REMS) program due the risk of post-injection delirium/sedation syndrome. It is administered every 2 to 4 weeks, with loading doses given for the first 2 months of treatment (Table 235). After 2 months, the patient can proceed to the maintenance dosing regimen.

Continue to: Currently, there is no concrete guidance...
Currently, there is no concrete guidance on how to address missed doses of olanzapine long-acting injection; however, the pharmacokinetics of this formulation allow flexibility in dosing intervals. Therapeutic levels are present after the first injection, and the medication reaches steady-state levels in 3 months.35-37 As a result of its specific formulation, olanzapine pamoate long-acting injection provides sustained olanzapine pamoate plasma concentrations between injections, and has a half-life of 30 days.35 Consequently, therapeutic levels of the medication are still present 2 to 4 weeks after an injection.37 Additionally, clinically relevant plasma concentrations may be present 2 to 3 months after the last injection.36
In light of this information, if a patient has not reached steady state and has missed an injection, he or she should receive the recommended loading dose schedule. If the patient has reached steady state and it has been ≤2 months since the last dose, he or she should receive the next dose as soon as possible. If steady state has been reached and it has been >2 months since the last injection, the patient should receive the recommended loading dosing for 2 months (Figure 9). Because of the risk of post-injection delirium/sedation syndrome, and because therapeutic levels are achieved after the first injection, oral olanzapine supplementation is not recommended.

Use a stepwise approach
In general, clinicians can use a stepwise approach to managing missed doses of LAIAs (Figure 10). First, establish the number of LAIA doses the patient had received prior to the last dose, and whether these injections were administered on schedule. This will help you determine if the patient is in the initiation or maintenance phase and/or has reached steady state. The second step is to establish the date of the last injection. Use objective tools, such as pharmacy records or the medical chart, to determine the date of the last injection, rather than relying on patient reporting. For the third step, calculate the time that has passed since the last LAIA dose. Once you have completed these steps, use the specific medication recommendations described in this article to address the missed dose.

Continue to: Address barriers to adherence
Address barriers to adherence
When addressing missed LAIA doses, be sure to identify any barriers that may have led to a missed injection. These might include:
- bothersome adverse effects
- transportation difficulties
- issues with insurance/medication coverage
- comorbidities (ie, alcohol/substance use disorders)
- cognitive and functional impairment caused by the patient’s illness
- difficulty with keeping track of appointments.
Clinicians can work closely with patients and/or caregivers to address any barriers to ensure that patients receive their injections in a timely fashion.
The goal: Reducing relapse
LAIAs improve medication adherence. Although nonadherence is less frequent with LAIAs than with oral antipsychotics, when a LAIA dose is missed, it is important to properly follow a stepwise approach based on the unique properties of the specific LAIA prescribed. Proper management of LAIA missed doses can prevent relapse and reoccurrence of schizophrenia symptoms, thus possibly avoiding future hospitalizations.
Acknowledgments
The authors thank Brian Tschosik, JD, Mary Collen O’Rourke, MD, and Amanda Holloway, MD, for their assistance with this article.
Bottom Line
Although long-acting injectable antipsychotics (LAIAs) greatly assist with adherence, these agents are effective only when missed doses are avoided. When addressing missed LAIA doses, use a stepwise approach that takes into consideration the unique properties of the specific LAIA prescribed.
Related Resources
- Haddad P, Lambert T, Lauriello J, eds. Antipsychotic long-acting injections. 2nd ed. Oxford, UK: Oxford University Press; 2016.
- Diefenderfer LA. When should you consider combining 2 long-acting injectable antipsychotics? Current Psychiatry. 2017;16(10):42-46.
Drug Brand Names
Aripiprazole long-acting injection • Abilify Maintena
Aripiprazole lauroxil long-acting injection • Aristada
Fluphenazine decanoate • Prolixin decanoate
Haloperidol decanoate • Haldol decanoate
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month long-acting injection • Invega Trinza
Risperidone long-acting injection • Risperdal Consta
1. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;195(52):S63-S67.
4. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
5. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
6. Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346(8973):477-481.
7. de Sena EP, Santos-Jesus R, Miranda-Scippa Â, et al. Relapse in patients with schizophrenia: a comparison between risperidone and haloperidol. Rev Bras Psiquiatr. 2003;25(4):220-223.
8. Chue P. Long-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychotic. Neuropsychiatr Dis Treat. 2007;3(1):13-39.
9. Lafeuille MH, Frois C, Cloutier M, et al. Factors associated with adherence to the HEDIS Quality Measure in medicaid patients with schizophrenia. Am Health Drug Benefits. 2016;9(7):399-410.
10. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
11. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
12. Haldol Decanoate injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
13. Magliozzi JR, Hollister LE, Arnold KV, et al. Relationship of serum haloperidol levels to clinical response in schizophrenic patients. Am J Psychiatry. 1981;138(3):365-367.
14. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Clinical response and plasma haloperidol levels in schizophrenia. Psychopharmacology (Berl). 1983;81(4):354-356.
15. Reyntigens AJ, Heykants JJ, Woestenborghs RJ, et al. Pharmacokinetics of haloperidol decanoate. A 2-year follow-up. Int Pharmacopsychiatry. 1982;17(4):238-246.
16. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
17. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
18. Ereshefsky L, Saklad SR, Jann MW. Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches. J Clin Psychiatry.1984;45(5 pt 2):50-58.
19. Marder SR, Hawes EM, Van Putten T, et al. Fluphenazine plasma levels in patients receiving low and conventional doses of fluphenazine decanoate. Psychopharmacology (Berl). 1986;88(4):480-483.
20. Marder SR, Hubbard JW, Van Putten T, et al. Pharmacokinetics of long-acting injectable neuroleptic drugs: clinical implications. Psychopharmacology (Berl). 1989;98(4):433-439.
21. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Fluphenazine plasma levels and clinical response. J Clin Psychiatry. 1984;45(9):370-373.
22. Balant-Gorgia AE, Balant LP, Andreoli A. Pharmacokinetic optimisation of the treatment of psychosis. Clin Pharmacokinet. 1993;25(3):217-236.
23. Van Putten T, Marder SR, Wirshing WC, et al. Neuroleptic plasma levels. Schizophr Bull. 1991;17(2):197-216.
24. Dahl SG. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin Pharmacokinet. 1986;11(1):36-61.
25. Miller RS, Peterson GM, McLean S, et al. Monitoring plasma levels of fluphenazine during chronic therapy with fluphenazine decanoate. J Clin Pharm Ther. 1995;20(2):55-62.
26. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
27. Wistedt B, Wiles D, Kolakowska T. Slow decline of plasma drug and prolactin levels after discontinuation of chronic treatment with depot neuroleptics. Lancet. 1981;1(8230):1163.
28. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology (Berl). 1982;78(4):301-304.
29. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
30. Marder SR, Conley R, Ereshefsky L, et al. Clinical guidelines: dosing and switching strategies for long-acting risperidone. J Clin Psychiatry. 2003;64(suppl 16):41-46.
31. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
32. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
33. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; December 2016.
34. Artistada [package insert]. Waltham, MA: Alkermes, Inc.; June 2017.
35. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; February 2017.
36. Heres S, Kraemer S, Bergstrom RF, et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol. 2014;29(6):299-312.
37. Detke HC, Zhao F, Garhyan P, et al. Dose correspondence between olanzapine long-acting injection and oral olanzapine: recommendations for switching. Int Clin Psychopharmacol. 2011;26(1):35-42.
1. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;195(52):S63-S67.
4. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
5. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
6. Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346(8973):477-481.
7. de Sena EP, Santos-Jesus R, Miranda-Scippa Â, et al. Relapse in patients with schizophrenia: a comparison between risperidone and haloperidol. Rev Bras Psiquiatr. 2003;25(4):220-223.
8. Chue P. Long-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychotic. Neuropsychiatr Dis Treat. 2007;3(1):13-39.
9. Lafeuille MH, Frois C, Cloutier M, et al. Factors associated with adherence to the HEDIS Quality Measure in medicaid patients with schizophrenia. Am Health Drug Benefits. 2016;9(7):399-410.
10. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
11. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
12. Haldol Decanoate injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
13. Magliozzi JR, Hollister LE, Arnold KV, et al. Relationship of serum haloperidol levels to clinical response in schizophrenic patients. Am J Psychiatry. 1981;138(3):365-367.
14. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Clinical response and plasma haloperidol levels in schizophrenia. Psychopharmacology (Berl). 1983;81(4):354-356.
15. Reyntigens AJ, Heykants JJ, Woestenborghs RJ, et al. Pharmacokinetics of haloperidol decanoate. A 2-year follow-up. Int Pharmacopsychiatry. 1982;17(4):238-246.
16. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
17. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
18. Ereshefsky L, Saklad SR, Jann MW. Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches. J Clin Psychiatry.1984;45(5 pt 2):50-58.
19. Marder SR, Hawes EM, Van Putten T, et al. Fluphenazine plasma levels in patients receiving low and conventional doses of fluphenazine decanoate. Psychopharmacology (Berl). 1986;88(4):480-483.
20. Marder SR, Hubbard JW, Van Putten T, et al. Pharmacokinetics of long-acting injectable neuroleptic drugs: clinical implications. Psychopharmacology (Berl). 1989;98(4):433-439.
21. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Fluphenazine plasma levels and clinical response. J Clin Psychiatry. 1984;45(9):370-373.
22. Balant-Gorgia AE, Balant LP, Andreoli A. Pharmacokinetic optimisation of the treatment of psychosis. Clin Pharmacokinet. 1993;25(3):217-236.
23. Van Putten T, Marder SR, Wirshing WC, et al. Neuroleptic plasma levels. Schizophr Bull. 1991;17(2):197-216.
24. Dahl SG. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin Pharmacokinet. 1986;11(1):36-61.
25. Miller RS, Peterson GM, McLean S, et al. Monitoring plasma levels of fluphenazine during chronic therapy with fluphenazine decanoate. J Clin Pharm Ther. 1995;20(2):55-62.
26. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
27. Wistedt B, Wiles D, Kolakowska T. Slow decline of plasma drug and prolactin levels after discontinuation of chronic treatment with depot neuroleptics. Lancet. 1981;1(8230):1163.
28. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology (Berl). 1982;78(4):301-304.
29. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
30. Marder SR, Conley R, Ereshefsky L, et al. Clinical guidelines: dosing and switching strategies for long-acting risperidone. J Clin Psychiatry. 2003;64(suppl 16):41-46.
31. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
32. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
33. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; December 2016.
34. Artistada [package insert]. Waltham, MA: Alkermes, Inc.; June 2017.
35. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; February 2017.
36. Heres S, Kraemer S, Bergstrom RF, et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol. 2014;29(6):299-312.
37. Detke HC, Zhao F, Garhyan P, et al. Dose correspondence between olanzapine long-acting injection and oral olanzapine: recommendations for switching. Int Clin Psychopharmacol. 2011;26(1):35-42.
Neuropsychiatric symptoms of dementia: Monotherapy, or combination therapy?
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors
Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors

Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors

Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
Complementary treatments for anxiety: Beyond pharmacotherapy and psychotherapy
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.
Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.

Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.

Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
10 Myths about ECT
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
NEED HAIR: Pinpointing the cause of a patient’s hair loss
Telogen effluvium (TE)—temporary hair loss due to the shedding of telogen (resting phase of hair cycle) hair after exposure to some form of stress—is one of the most common causes of diffuse non-scarring hair loss from the scalp.1 TE is more common than anagen (growing phase of hair cycle) hair loss.2 Hair loss can be triggered by numerous factors, including certain psychotropic medications.3,4 Mood stabilizers, such as valproic acid and lithium, are most commonly implicated. Hair loss also has been associated with the use of the first-generation antipsychotics haloperidol and chlorpromazine and the second-generation antipsychotics olanzapine, quetiapine, and risperidone.5-7 We recently cared for a woman with bipolar I disorder and generalized anxiety disorder who was prescribed lurasidone and later developed TE. Her hair loss completely resolved 4 months after lurasidone was discontinued.
TE can be triggered by events that interrupt the normal hair growth cycle. Typically, it is observed approximately 3 months after a triggering event and is usually self-limiting, lasting approximately 6 months.7 Diagnostic features include a strongly positive hair-pull test, a trichogram showing >25% telogen hair, and a biopsy showing an increase in telogen follicles.8,9 To conduct the hair-pull test, grasp approximately 40 to 60 hairs between the thumb and forefinger while slightly stretching the scalp to allow your fingers to slide along the length of the hair. Usually only 2 to 3 hairs in the telogen phase can be plucked via this method; if >10% of the hairs grasped can be plucked, this indicates a pathologic process.10
Significant hair loss, particularly among women, is a distressing adverse effect that can be an important moderator of compliance, treatment adherence, and relapse. To help clinicians narrow down the wide range of potential causes of a patient’s hair loss, we created the mnemonic NEED HAIR.
Nutritional: Iron deficiency anemia, a “crash” diet, zinc deficiency, vitamin B6 or B12 deficiency, chronic starvation, diarrhea, hypoproteinemia (metabolic or dietary origin), malabsorption, or heavy metal ingestion.1
Endocrine: Thyroid disorders and the early stage of androgenic alopecia.8,11
Environmental: Stress from a severe febrile illness, emotional stress, serious injuries, major surgery, large hemorrhage, and difficult labor.12
Drugs: Oral contraceptives, antithyroid drugs, retinoids (etretinate and acitretin), anticonvulsants, antidepressants, antipsychotics, hypolipidemic drugs, anticoagulants, antihypertensives (beta blockers, angiotensin-converting enzyme inhibitors), and cytotoxic drugs.1,4-7
Continue to: Hormonal fluctuations
Hormonal fluctuations: Polycystic ovarian syndrome and postpartum hormonal changes.11
Autoimmune: Lupus erythematosus, dermatomyositis, scleroderma, Hashimoto’s thyroiditis, Sjögren’s syndrome, inflammatory bowel disease, and autoimmune atrophic gastritis.12,13
Infections and chronic illnesses: Fungal infections (eg, tinea capitis), human immunodeficiency virus, syphilis, typhoid, malaria, tuberculosis, malignancy, renal failure, hepatic failure, and other chronic illnesses.1,9
Radiation: Radiation treatment and excessive UV exposure.12,14,15
Treatment for hair loss depends on the specific caus
1. Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venerol Leprol. 2013;79(5):591-603.
2. Harrison S, Bergfeld W. Diffuse hair loss: its triggers and management. Cleve Clin J Med. 2009;76(6):361-367.
3. Gautam M. Alopecia due to psychotropic medications. Ann Pharmacother. 1999;33(5):631-637.
4. Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12(1):35-42.
5. Kubota T, Ishikura T, Jibiki I. Alopecia areata associated with haloperidol. Jpn J Psychiatry Neurol. 1994;48(3):579-581.
6. McLean RM, Harrison-Woolrych M. Alopecia associated with quetiapine. Int Clin Psychopharmacol 2007;22(2):117-9.
7. Kulolu M, Korkmaz S, Kilic N, et al. Olanzapine induced hair loss: a case report. Bulletin of Clinical Psychopharmacology. 2012;22(4):362-365.
8. Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01-WE03.
9. Shrivastava SB. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venerol Leprol. 2009;75(1):20-28; quiz 27-28.
10. Piérard GE, Piérard-Franchimont C, Marks R, et al; EEMCO group (European Expert Group on Efficacy Measurement of Cosmetics and Other Topical Products). EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004;17(2):98-110.
11. Mirallas O, Grimalt R. The postpartum telogen effluvium fallacy. Skin Appendage Disord. 2016;1(4):198-201.
12. Rebora A. Proposing a simpler classification of telogen effluvium. Skin Appendage Disord. 2016;2(1-2):35-38.
13. Cassano N, Amerio P, D’Ovidio R, et al. Hair disorders associated with autoimmune connective tissue diseases. G Ital Dermatol Venereol. 2014;149(5):555-565.
14. Trüeb RM. Telogen effluvium: is there a need for a new classification? Skin Appendage Disord. 2016;2(1-2):39-44.
15. Ali SY, Singh G. Radiation-induced alopecia. Int J Trichology. 2010;2(2):118-119.
Telogen effluvium (TE)—temporary hair loss due to the shedding of telogen (resting phase of hair cycle) hair after exposure to some form of stress—is one of the most common causes of diffuse non-scarring hair loss from the scalp.1 TE is more common than anagen (growing phase of hair cycle) hair loss.2 Hair loss can be triggered by numerous factors, including certain psychotropic medications.3,4 Mood stabilizers, such as valproic acid and lithium, are most commonly implicated. Hair loss also has been associated with the use of the first-generation antipsychotics haloperidol and chlorpromazine and the second-generation antipsychotics olanzapine, quetiapine, and risperidone.5-7 We recently cared for a woman with bipolar I disorder and generalized anxiety disorder who was prescribed lurasidone and later developed TE. Her hair loss completely resolved 4 months after lurasidone was discontinued.
TE can be triggered by events that interrupt the normal hair growth cycle. Typically, it is observed approximately 3 months after a triggering event and is usually self-limiting, lasting approximately 6 months.7 Diagnostic features include a strongly positive hair-pull test, a trichogram showing >25% telogen hair, and a biopsy showing an increase in telogen follicles.8,9 To conduct the hair-pull test, grasp approximately 40 to 60 hairs between the thumb and forefinger while slightly stretching the scalp to allow your fingers to slide along the length of the hair. Usually only 2 to 3 hairs in the telogen phase can be plucked via this method; if >10% of the hairs grasped can be plucked, this indicates a pathologic process.10
Significant hair loss, particularly among women, is a distressing adverse effect that can be an important moderator of compliance, treatment adherence, and relapse. To help clinicians narrow down the wide range of potential causes of a patient’s hair loss, we created the mnemonic NEED HAIR.
Nutritional: Iron deficiency anemia, a “crash” diet, zinc deficiency, vitamin B6 or B12 deficiency, chronic starvation, diarrhea, hypoproteinemia (metabolic or dietary origin), malabsorption, or heavy metal ingestion.1
Endocrine: Thyroid disorders and the early stage of androgenic alopecia.8,11
Environmental: Stress from a severe febrile illness, emotional stress, serious injuries, major surgery, large hemorrhage, and difficult labor.12
Drugs: Oral contraceptives, antithyroid drugs, retinoids (etretinate and acitretin), anticonvulsants, antidepressants, antipsychotics, hypolipidemic drugs, anticoagulants, antihypertensives (beta blockers, angiotensin-converting enzyme inhibitors), and cytotoxic drugs.1,4-7
Continue to: Hormonal fluctuations
Hormonal fluctuations: Polycystic ovarian syndrome and postpartum hormonal changes.11
Autoimmune: Lupus erythematosus, dermatomyositis, scleroderma, Hashimoto’s thyroiditis, Sjögren’s syndrome, inflammatory bowel disease, and autoimmune atrophic gastritis.12,13
Infections and chronic illnesses: Fungal infections (eg, tinea capitis), human immunodeficiency virus, syphilis, typhoid, malaria, tuberculosis, malignancy, renal failure, hepatic failure, and other chronic illnesses.1,9
Radiation: Radiation treatment and excessive UV exposure.12,14,15
Treatment for hair loss depends on the specific caus
Telogen effluvium (TE)—temporary hair loss due to the shedding of telogen (resting phase of hair cycle) hair after exposure to some form of stress—is one of the most common causes of diffuse non-scarring hair loss from the scalp.1 TE is more common than anagen (growing phase of hair cycle) hair loss.2 Hair loss can be triggered by numerous factors, including certain psychotropic medications.3,4 Mood stabilizers, such as valproic acid and lithium, are most commonly implicated. Hair loss also has been associated with the use of the first-generation antipsychotics haloperidol and chlorpromazine and the second-generation antipsychotics olanzapine, quetiapine, and risperidone.5-7 We recently cared for a woman with bipolar I disorder and generalized anxiety disorder who was prescribed lurasidone and later developed TE. Her hair loss completely resolved 4 months after lurasidone was discontinued.
TE can be triggered by events that interrupt the normal hair growth cycle. Typically, it is observed approximately 3 months after a triggering event and is usually self-limiting, lasting approximately 6 months.7 Diagnostic features include a strongly positive hair-pull test, a trichogram showing >25% telogen hair, and a biopsy showing an increase in telogen follicles.8,9 To conduct the hair-pull test, grasp approximately 40 to 60 hairs between the thumb and forefinger while slightly stretching the scalp to allow your fingers to slide along the length of the hair. Usually only 2 to 3 hairs in the telogen phase can be plucked via this method; if >10% of the hairs grasped can be plucked, this indicates a pathologic process.10
Significant hair loss, particularly among women, is a distressing adverse effect that can be an important moderator of compliance, treatment adherence, and relapse. To help clinicians narrow down the wide range of potential causes of a patient’s hair loss, we created the mnemonic NEED HAIR.
Nutritional: Iron deficiency anemia, a “crash” diet, zinc deficiency, vitamin B6 or B12 deficiency, chronic starvation, diarrhea, hypoproteinemia (metabolic or dietary origin), malabsorption, or heavy metal ingestion.1
Endocrine: Thyroid disorders and the early stage of androgenic alopecia.8,11
Environmental: Stress from a severe febrile illness, emotional stress, serious injuries, major surgery, large hemorrhage, and difficult labor.12
Drugs: Oral contraceptives, antithyroid drugs, retinoids (etretinate and acitretin), anticonvulsants, antidepressants, antipsychotics, hypolipidemic drugs, anticoagulants, antihypertensives (beta blockers, angiotensin-converting enzyme inhibitors), and cytotoxic drugs.1,4-7
Continue to: Hormonal fluctuations
Hormonal fluctuations: Polycystic ovarian syndrome and postpartum hormonal changes.11
Autoimmune: Lupus erythematosus, dermatomyositis, scleroderma, Hashimoto’s thyroiditis, Sjögren’s syndrome, inflammatory bowel disease, and autoimmune atrophic gastritis.12,13
Infections and chronic illnesses: Fungal infections (eg, tinea capitis), human immunodeficiency virus, syphilis, typhoid, malaria, tuberculosis, malignancy, renal failure, hepatic failure, and other chronic illnesses.1,9
Radiation: Radiation treatment and excessive UV exposure.12,14,15
Treatment for hair loss depends on the specific caus
1. Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venerol Leprol. 2013;79(5):591-603.
2. Harrison S, Bergfeld W. Diffuse hair loss: its triggers and management. Cleve Clin J Med. 2009;76(6):361-367.
3. Gautam M. Alopecia due to psychotropic medications. Ann Pharmacother. 1999;33(5):631-637.
4. Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12(1):35-42.
5. Kubota T, Ishikura T, Jibiki I. Alopecia areata associated with haloperidol. Jpn J Psychiatry Neurol. 1994;48(3):579-581.
6. McLean RM, Harrison-Woolrych M. Alopecia associated with quetiapine. Int Clin Psychopharmacol 2007;22(2):117-9.
7. Kulolu M, Korkmaz S, Kilic N, et al. Olanzapine induced hair loss: a case report. Bulletin of Clinical Psychopharmacology. 2012;22(4):362-365.
8. Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01-WE03.
9. Shrivastava SB. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venerol Leprol. 2009;75(1):20-28; quiz 27-28.
10. Piérard GE, Piérard-Franchimont C, Marks R, et al; EEMCO group (European Expert Group on Efficacy Measurement of Cosmetics and Other Topical Products). EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004;17(2):98-110.
11. Mirallas O, Grimalt R. The postpartum telogen effluvium fallacy. Skin Appendage Disord. 2016;1(4):198-201.
12. Rebora A. Proposing a simpler classification of telogen effluvium. Skin Appendage Disord. 2016;2(1-2):35-38.
13. Cassano N, Amerio P, D’Ovidio R, et al. Hair disorders associated with autoimmune connective tissue diseases. G Ital Dermatol Venereol. 2014;149(5):555-565.
14. Trüeb RM. Telogen effluvium: is there a need for a new classification? Skin Appendage Disord. 2016;2(1-2):39-44.
15. Ali SY, Singh G. Radiation-induced alopecia. Int J Trichology. 2010;2(2):118-119.
1. Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venerol Leprol. 2013;79(5):591-603.
2. Harrison S, Bergfeld W. Diffuse hair loss: its triggers and management. Cleve Clin J Med. 2009;76(6):361-367.
3. Gautam M. Alopecia due to psychotropic medications. Ann Pharmacother. 1999;33(5):631-637.
4. Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12(1):35-42.
5. Kubota T, Ishikura T, Jibiki I. Alopecia areata associated with haloperidol. Jpn J Psychiatry Neurol. 1994;48(3):579-581.
6. McLean RM, Harrison-Woolrych M. Alopecia associated with quetiapine. Int Clin Psychopharmacol 2007;22(2):117-9.
7. Kulolu M, Korkmaz S, Kilic N, et al. Olanzapine induced hair loss: a case report. Bulletin of Clinical Psychopharmacology. 2012;22(4):362-365.
8. Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01-WE03.
9. Shrivastava SB. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venerol Leprol. 2009;75(1):20-28; quiz 27-28.
10. Piérard GE, Piérard-Franchimont C, Marks R, et al; EEMCO group (European Expert Group on Efficacy Measurement of Cosmetics and Other Topical Products). EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004;17(2):98-110.
11. Mirallas O, Grimalt R. The postpartum telogen effluvium fallacy. Skin Appendage Disord. 2016;1(4):198-201.
12. Rebora A. Proposing a simpler classification of telogen effluvium. Skin Appendage Disord. 2016;2(1-2):35-38.
13. Cassano N, Amerio P, D’Ovidio R, et al. Hair disorders associated with autoimmune connective tissue diseases. G Ital Dermatol Venereol. 2014;149(5):555-565.
14. Trüeb RM. Telogen effluvium: is there a need for a new classification? Skin Appendage Disord. 2016;2(1-2):39-44.
15. Ali SY, Singh G. Radiation-induced alopecia. Int J Trichology. 2010;2(2):118-119.
Cardiovascular adverse effects of psychotropics: What to look for
Most patients who take psychotropic medications are at low risk for cardiovascular adverse effects from these medications, and require only routine monitoring. However, patients with severe mental illness, those with a personal or family history of cardiovascular disease, or those receiving high doses or multiple medications are considered at high risk for morbidity and mortality from cardiovascular adverse effects. Such patients may need more careful cardiovascular monitoring.
To help identify important cardiovascular-related adverse effects of various psychotropics, we summarize these risks, and offer guidance about what you can do when your patient experiences them (Table).1
Antipsychotics and metabolic syndrome
Patients who take antipsychotics should be monitored for metabolic syndrome. The presence of 3 of the following 5 parameters is considered positive for metabolic syndrome2:
- fasting glucose ≥100 mg/dL or hemoglobin A1c ≥5.6%
- blood pressure ≥130/85 mm Hg
- triglycerides ≥150 mg/dL
- high-density lipoprotein cholesterol
- waist circumference ≥102 cm in men or ≥88 cm in women.
Stimulants and sudden cardiac death
Sudden cardiac death (SCD) in children and adolescents who take stimulants to treat attention-deficit/hyperactivity disorder is rare. For these patients, the risk of SCD is no higher than that of the general population.3 For patients who do not have any known cardiac risk factors, the American Academy of Pediatrics does not recommend performing any cardiac tests before starting stimulants.3
1. Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23(suppl 1):S3-S14.
2. Grundy SM, Brewer HB Jr, Cleeman JI, et al; National Heart, Lung, and Blood Institute; American Heart Association. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24(2):e13-e18.
3. American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874-877.
Most patients who take psychotropic medications are at low risk for cardiovascular adverse effects from these medications, and require only routine monitoring. However, patients with severe mental illness, those with a personal or family history of cardiovascular disease, or those receiving high doses or multiple medications are considered at high risk for morbidity and mortality from cardiovascular adverse effects. Such patients may need more careful cardiovascular monitoring.
To help identify important cardiovascular-related adverse effects of various psychotropics, we summarize these risks, and offer guidance about what you can do when your patient experiences them (Table).1

Antipsychotics and metabolic syndrome
Patients who take antipsychotics should be monitored for metabolic syndrome. The presence of 3 of the following 5 parameters is considered positive for metabolic syndrome2:
- fasting glucose ≥100 mg/dL or hemoglobin A1c ≥5.6%
- blood pressure ≥130/85 mm Hg
- triglycerides ≥150 mg/dL
- high-density lipoprotein cholesterol
- waist circumference ≥102 cm in men or ≥88 cm in women.
Stimulants and sudden cardiac death
Sudden cardiac death (SCD) in children and adolescents who take stimulants to treat attention-deficit/hyperactivity disorder is rare. For these patients, the risk of SCD is no higher than that of the general population.3 For patients who do not have any known cardiac risk factors, the American Academy of Pediatrics does not recommend performing any cardiac tests before starting stimulants.3
Most patients who take psychotropic medications are at low risk for cardiovascular adverse effects from these medications, and require only routine monitoring. However, patients with severe mental illness, those with a personal or family history of cardiovascular disease, or those receiving high doses or multiple medications are considered at high risk for morbidity and mortality from cardiovascular adverse effects. Such patients may need more careful cardiovascular monitoring.
To help identify important cardiovascular-related adverse effects of various psychotropics, we summarize these risks, and offer guidance about what you can do when your patient experiences them (Table).1

Antipsychotics and metabolic syndrome
Patients who take antipsychotics should be monitored for metabolic syndrome. The presence of 3 of the following 5 parameters is considered positive for metabolic syndrome2:
- fasting glucose ≥100 mg/dL or hemoglobin A1c ≥5.6%
- blood pressure ≥130/85 mm Hg
- triglycerides ≥150 mg/dL
- high-density lipoprotein cholesterol
- waist circumference ≥102 cm in men or ≥88 cm in women.
Stimulants and sudden cardiac death
Sudden cardiac death (SCD) in children and adolescents who take stimulants to treat attention-deficit/hyperactivity disorder is rare. For these patients, the risk of SCD is no higher than that of the general population.3 For patients who do not have any known cardiac risk factors, the American Academy of Pediatrics does not recommend performing any cardiac tests before starting stimulants.3
1. Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23(suppl 1):S3-S14.
2. Grundy SM, Brewer HB Jr, Cleeman JI, et al; National Heart, Lung, and Blood Institute; American Heart Association. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24(2):e13-e18.
3. American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874-877.
1. Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23(suppl 1):S3-S14.
2. Grundy SM, Brewer HB Jr, Cleeman JI, et al; National Heart, Lung, and Blood Institute; American Heart Association. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24(2):e13-e18.
3. American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874-877.
Autism spectrum disorder in adults
Psychiatry’s ‘C’ words
Recently, contemporary “culture” has capriciously confounded and confused our national community with a cringeworthy “C” word. Unfortunately, this was followed by only transient public consternation, reflecting the coarsening of our social communication and discourse and the cant of many. But the ripple effects continue.
As a psychiatrist who closely observes the human condition, I contemplated the current confrontational tone in the media, and wondered how corrosive language can confuse and cloud our sensibilities.
Then it occurred to me that there are many commendable clinical “C” words that describe what we psychiatrists do in daily practice. We stay calm while facing crises, and help our patients achieve certainty when stress makes them confused. We compassionately comfort and care for our suffering patients. We strive to engender courage and confidence when fragile patients are confronted with continuous criticism by callous and condescending people. We remain composed when we counsel patients with confrontational and crabby moods and help correct their confusing conflicts. We coordinate their care with courtesy, always aspiring for a cure for their corroded emotional condition.
But then I felt compelled to go further and call on my creativity to consider “C” words that describe severe psychiatric clinical disorders. I came up with the following cogent cascade of common characteristics of serious mental disorders:
- Cerebral pathology
- Circuit disruptions of neural connections
- Chemical imbalance
- Cytokine inflammatory conflagration
- Cognitive impairment
- Chronic course
- Crippled functioning.
My compulsion continued. I decided to contemplate more “C” words that capture our therapeutic course of action to correct a patient whose cortico-limbic circuitry is being threatened with considerable, even calamitous collapse. My consideration led to clarity, and I came up with the following list of what we psychiatrists conform to in our clinical practice:
- Connect with patients
- Correct diagnosis
- Course-specific intervention
- Choose the appropriate medication
- Combine cognitive-behavioral therapy with medication
- Cognitive assessment at baseline
- Compliance/concordance with treatment
- Continuity of care
- Comorbidities, physical and psychiatric
- Collaborative care with other medical specialists
- Comprehensive treatment plan with other mental health professionals.
A book about dirty words written by a psychoanalyst called the “C” expletive the worst of all cuss words.1 Its recent emergence in the national media compromised our civility and created a cesspool of contemptible conversations. Let’s transcend the contemptible “C” word of a caustic contemporary “culture” (which had its 15 minutes of infamy), and consider the many coherent and congenial “C” words of psychiatric practice that bring peace of mind and contentment to those who suffer from psychiatric brain conditions. As for the compulsive or
Editor's note: How many C words are there in this editorial? Email answers to [email protected]
1. Arango AC. Dirty words: psychoanalytic insights. Lanham, MD: Jason Aronson, Inc.; 1989:122.
Recently, contemporary “culture” has capriciously confounded and confused our national community with a cringeworthy “C” word. Unfortunately, this was followed by only transient public consternation, reflecting the coarsening of our social communication and discourse and the cant of many. But the ripple effects continue.
As a psychiatrist who closely observes the human condition, I contemplated the current confrontational tone in the media, and wondered how corrosive language can confuse and cloud our sensibilities.
Then it occurred to me that there are many commendable clinical “C” words that describe what we psychiatrists do in daily practice. We stay calm while facing crises, and help our patients achieve certainty when stress makes them confused. We compassionately comfort and care for our suffering patients. We strive to engender courage and confidence when fragile patients are confronted with continuous criticism by callous and condescending people. We remain composed when we counsel patients with confrontational and crabby moods and help correct their confusing conflicts. We coordinate their care with courtesy, always aspiring for a cure for their corroded emotional condition.
But then I felt compelled to go further and call on my creativity to consider “C” words that describe severe psychiatric clinical disorders. I came up with the following cogent cascade of common characteristics of serious mental disorders:
- Cerebral pathology
- Circuit disruptions of neural connections
- Chemical imbalance
- Cytokine inflammatory conflagration
- Cognitive impairment
- Chronic course
- Crippled functioning.
My compulsion continued. I decided to contemplate more “C” words that capture our therapeutic course of action to correct a patient whose cortico-limbic circuitry is being threatened with considerable, even calamitous collapse. My consideration led to clarity, and I came up with the following list of what we psychiatrists conform to in our clinical practice:
- Connect with patients
- Correct diagnosis
- Course-specific intervention
- Choose the appropriate medication
- Combine cognitive-behavioral therapy with medication
- Cognitive assessment at baseline
- Compliance/concordance with treatment
- Continuity of care
- Comorbidities, physical and psychiatric
- Collaborative care with other medical specialists
- Comprehensive treatment plan with other mental health professionals.
A book about dirty words written by a psychoanalyst called the “C” expletive the worst of all cuss words.1 Its recent emergence in the national media compromised our civility and created a cesspool of contemptible conversations. Let’s transcend the contemptible “C” word of a caustic contemporary “culture” (which had its 15 minutes of infamy), and consider the many coherent and congenial “C” words of psychiatric practice that bring peace of mind and contentment to those who suffer from psychiatric brain conditions. As for the compulsive or
Editor's note: How many C words are there in this editorial? Email answers to [email protected]
Recently, contemporary “culture” has capriciously confounded and confused our national community with a cringeworthy “C” word. Unfortunately, this was followed by only transient public consternation, reflecting the coarsening of our social communication and discourse and the cant of many. But the ripple effects continue.
As a psychiatrist who closely observes the human condition, I contemplated the current confrontational tone in the media, and wondered how corrosive language can confuse and cloud our sensibilities.
Then it occurred to me that there are many commendable clinical “C” words that describe what we psychiatrists do in daily practice. We stay calm while facing crises, and help our patients achieve certainty when stress makes them confused. We compassionately comfort and care for our suffering patients. We strive to engender courage and confidence when fragile patients are confronted with continuous criticism by callous and condescending people. We remain composed when we counsel patients with confrontational and crabby moods and help correct their confusing conflicts. We coordinate their care with courtesy, always aspiring for a cure for their corroded emotional condition.
But then I felt compelled to go further and call on my creativity to consider “C” words that describe severe psychiatric clinical disorders. I came up with the following cogent cascade of common characteristics of serious mental disorders:
- Cerebral pathology
- Circuit disruptions of neural connections
- Chemical imbalance
- Cytokine inflammatory conflagration
- Cognitive impairment
- Chronic course
- Crippled functioning.
My compulsion continued. I decided to contemplate more “C” words that capture our therapeutic course of action to correct a patient whose cortico-limbic circuitry is being threatened with considerable, even calamitous collapse. My consideration led to clarity, and I came up with the following list of what we psychiatrists conform to in our clinical practice:
- Connect with patients
- Correct diagnosis
- Course-specific intervention
- Choose the appropriate medication
- Combine cognitive-behavioral therapy with medication
- Cognitive assessment at baseline
- Compliance/concordance with treatment
- Continuity of care
- Comorbidities, physical and psychiatric
- Collaborative care with other medical specialists
- Comprehensive treatment plan with other mental health professionals.
A book about dirty words written by a psychoanalyst called the “C” expletive the worst of all cuss words.1 Its recent emergence in the national media compromised our civility and created a cesspool of contemptible conversations. Let’s transcend the contemptible “C” word of a caustic contemporary “culture” (which had its 15 minutes of infamy), and consider the many coherent and congenial “C” words of psychiatric practice that bring peace of mind and contentment to those who suffer from psychiatric brain conditions. As for the compulsive or
Editor's note: How many C words are there in this editorial? Email answers to [email protected]
1. Arango AC. Dirty words: psychoanalytic insights. Lanham, MD: Jason Aronson, Inc.; 1989:122.
1. Arango AC. Dirty words: psychoanalytic insights. Lanham, MD: Jason Aronson, Inc.; 1989:122.