User login
Nonoperative Treatment of Closed Extra-Articular Distal Humeral Shaft Fractures in Adults: A Comparison of Functional Bracing and Above-Elbow Casting
ABSTRACT
Diaphyseal fractures of the distal humerus have a high rate of union when treated with a functional brace or an above-elbow cast (AEC). This study compares alignment of the humerus and motion of the elbow after functional brace or AEC treatment.
One-hundred and five consecutive patients with a closed, extra-articular fracture of the distal humeral diaphysis were identified in the orthopedic trauma databases of 3 hospitals between 2003 and 2012. Seventy-five patients with a follow-up of at least 6 months or with radiographic and clinical evidence of fracture union were included (51 treated with functional bracing and 24 treated with an AEC).
All of the fractures healed. The average arc of elbow flexion was 130° ± 9° in braced patients vs 127° ± 12° in casted patients. Four patients (8%) in the bracing group and 4 (17%) in the casting group lost >20° of elbow motion. The average varus angulation on radiographs was 17° ± 8° in braced and 13° ± 8° in casted patients, while the average posterior angulation was 9° ± 6° vs 7° ± 7°, respectively.
Closed extra-articular distal diaphyseal humerus fractures heal with both bracing and casting and there are no differences in average elbow motion or radiographic alignment.
Nonoperative treatment of closed fractures of the humeral shaft (AO/OTA [Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association] type 12) with a functional brace or above-elbow cast (AEC) is associated with a high union rate, good motion, and good function. Advocates of casting believe that a brace cannot control fracture alignment as well as a cast that allows for immobilization and molding. Advocates of brace treatment are concerned that immobilization in a cast will cause elbow stiffness.1-11
Continue to: In our differing institutions...
In our differing institutions, there are advocates of each type of treatment, providing the opportunity for a comparison. This retrospective study compares brace and cast treatment. The working hypothesis was that there is no difference in elbow motion 6 months or more after fracture. We also compared radiographic alignment after union.
MATERIALS AND METHODS
Between 2003 and 2012, consecutive adult patients treated for a nonpathological fracture of the diaphysis of the distal humerus at the orthopedic trauma service of 3 level 1 academic trauma centers were identified from prospectively collected trauma injury databases. Patients with vascular injury, ipsilateral upper extremity fracture, and periprosthetic fractures were excluded. The attending orthopedic surgeon chose the treatment method and evaluated the range of motion (ROM) of the elbow and radiographic union at the final ambulatory visit. We included patients followed to clinical and radiographic union with a minimum of 6 months of follow-up. We also included patients with <6 months’ follow-up who demonstrated union and had elbow ROM within 10° of the uninjured arm.
We identified 105 consecutive adult patients with a closed nonpathological extra-articular distal humeral shaft fracture (fracture of the distal humeral shaft with an AO/OTA type-12.A, 12.B, or 12.C pattern) treated with an AEC or a brace in our databases.12 Two patients in the brace group chose surgery to improve alignment within 3 weeks of injury and were excluded from the analysis. Twenty-eight patients had inadequate follow-up.
A total of 75 patients were included in the study. At the first and second institutions, 51 patients were treated with functional bracing with an average follow-up of 7 months. At the third institution, 24 patients were treated with an AEC with an average follow-up of 4 months. Seventeen out of 24 patients in the long arm casting group and 19 out of 51 patients in the bracing group, who were included since they had <6 months of follow-up, demonstrated union and had elbow ROM within 10° of the uninjured arm. Differing methods of closed immobilization were the result of differing treatment algorithms at each institution.
The patients who were treated with a functional brace averaged 34 years of age (range, 18-90 years) and included 27 men and 24 women. The brace was removed at an average of 11.5 weeks (range, 8-18 weeks) after initial injury. Six patients had an injury-associated radial nerve palsy, all of which fully recovered within an average of 4 months (range, 0.5-7 months). Sixteen patients were injured due to a fall from standing height, 2 due to a fall from a greater height than standing, 16 in a motor-vehicle accident, 15 during a sport activity, and 2 were not specifically documented.
Continue to: Four patients had concomitant...
Four patients had concomitant injuries: one patient had a mid-shaft humeral fracture on the contralateral arm; a second had an ankle fracture; a third had an ankle fracture, acetabular fracture, a rib fracture, and pneumothorax; and the fourth had 2 rib fractures.
The patients who were treated with an AEC had an average age of 32 years (range,18-82 years) and included 14 men and 10 women. The cast was removed at an average of 4.2 weeks (range, 3-7 weeks) after the initial injury. Two patients had an injury-associated radial nerve palsy, both of which fully recovered. Five patients were injured due to a fall from standing height, 1 due to a fall from a height greater than standing, 7 during a motor-vehicle accident, 5 during a sport activity, and 6 were not documented. Two patients sustained concomitant injuries: one patient sustained a tibia-fibula fracture, and another patient sustained facial trauma.
The 2 groups were comparable in age and gender, as well as the injury mechanism (Table).
Table. Patient Demographics and Outcome Data
| Functional Bracing (n = 51) | Long Arm Casting (n = 24) | Significance (P < .05) |
Sex |
|
|
|
Male | 27 (54%) | 14 (58%) |
|
Female | 24 (46%) | 10 (42%) |
|
Average age (y) | 34 (range, 18-90) | 32 (range, 18-82) |
|
Mechanism of injury |
|
|
|
Standing height | 16 (31%) | 5 (20%) |
|
Greater height | 2 (4%) | 1 (4%) |
|
Motor vehicle collision | 16 (31%) | 7 (29%) |
|
Sports activity | 15 (29 %) | 5 (21%) |
|
Other | 2 (4%) | 6 (25%) |
|
Follow-up (months) | 7 (range, 2-25) | 4 (range, 2-15) |
|
Elbow range of motion (degrees) | 130 ± 9.4 | 127 ± 11.9 | P = .26 |
Varus/valgus angulation (degrees) | 17 ± 7.8 varus | 13 ± 8.4 varus | P = .11 |
Anterior/posterior angulation (degrees) | 9 ± 6.2 posterior | 7 ± 7.5 posterior | P = .54 |
FUNCTIONAL BRACING TECHNIQUE
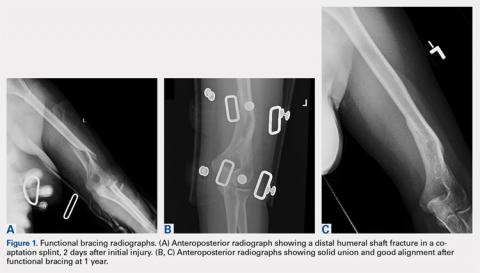
Upon presentation after injury, patients were immobilized in a coaptation splint (Figure 1A). Within 10 days, the arm was placed in a pre-manufactured polyethylene functional brace (Corflex) and the arm was supported with a simple sling. Patients were allowed to use the hand for light tasks and move the elbow, but most patients were not capable of active elbow flexion exercises until early healing was established 4 to 6 weeks after injury. Shoulder motion was discouraged until radiographic union. Patients started active, self-assisted elbow and shoulder stretching exercises, and weaned from the brace once radiographic union was confirmed between 6 and 10 weeks after injury (Figures 1B, 1C).
ABOVE-ELBOW CASE
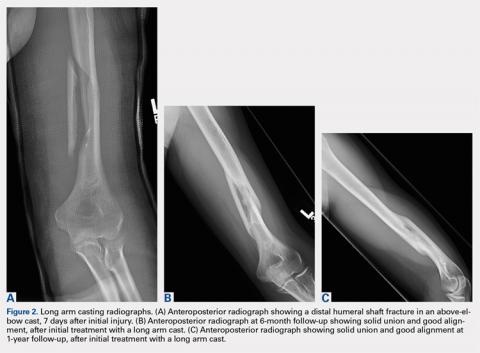
Patients were also initially immobilized in a coaptation splint upon initial presentation. Within 7 days, an above-elbow fiberglass cast with neutral forearm rotation and 90° of elbow flexion was applied with a supracondylar mold, followed by radiographic imaging (Figure 2A). With the fractured arm dependent, a valgus mold was applied as the material hardened in order to align the fracture site and limit varus angulation.
Continue to: There were no shoulder...
There were no shoulder ROM restrictions. Casts were removed, skin checked, and replaced every week for 4 to 6 weeks. Casts were removed when callus was noted on radiographs. After cast removal, physician-taught active and active-assisted elbow stretching exercises were given to patients to be performed on a daily basis at home. Patients were followed until clinical and radiographic union and elbow ROM to within 10° of the injured arm (Figures 2B, 2C).
STATISTICAL ANALYSIS
Alignment of the humerus (including varus-valgus alignment and apex anterior-posterior alignment) was measured on anteroposterior and lateral radiographs as the angle between lines bisecting the humeral diaphysis proximal and distal to the fracture. The normality of the data was tested using the Kolmogorov-Smirnov test. To statistically compare continuous variables with a normal distribution, t-tests were used; otherwise the Wilcoxon t-test was applied. The Pearson’s Chi-Square test was used to statistically compare dichotomous variables, except when expected cell frequency was <5, in which case the Fisher exact test was used. The level of significance was set at P < .05.
RESULTS
RANGE OF MOTION AND RADIOGRAPHIC ALIGNMENT
The average range of elbow motion was 130° ± 9° after brace treatment and 127° ± 12° after cast treatment (P = .26). Four patients (8%) treated with a brace and 3 (12%) treated with a cast lost >20° of elbow motion.
All the fractures healed. The average varus angulation on the anteroposterior radiograph was 17° (range, 2°-26°) in braced patients and 13 (range, 5°-31°) in casted patients (P = .11). The average posterior angulation on the lateral radiograph was 9° (range, 0°-28°) in braced patients vs 7° (range, 2°-33°) in casted patients (P = .54).
Continue to: Two weeks after initiating brace...
COMPLICATIONS
Two weeks after initiating brace treatment, an obese patient suffered a rash with desquamation that necessitated discontinuation of the brace. However, the skin and fracture ultimately healed with a coaptation splint and sling support without additional complications. In the casting cohort, 2 patients returned to the emergency department after AEC placement because of swelling of the hand and pain in the cast. Both casts were removed and reapplied.
DISCUSSION
Fractures of the distal third of the humeral diaphysis heal without surgery. Fracture angulation and elbow stiffness are the concerns that lead to variations in nonoperative treatment.1-3 Advocates of casting believe they can get better alignment without losing elbow motion, and advocates of bracing feel that the brace is less cumbersome.1-3,5-8 We compared these treatments retrospectively and found them comparable.
This study should be considered in light of its limitations. Many patients were lost to follow-up in our urban trauma centers. We do not know if these patients did better, worse, or the same as the patients we were able to evaluate, but our opinion is that patients having problems were more likely to return. The evaluation time was relatively short, but motion can only improve in the longer-term. Two patients that were initially braced chose surgery, probably because either they or their surgeon were nervous about the radiographic appearance of the fracture. In our opinion, continued nonoperative treatment of these patients would not affect the findings.
Cast treatment of distal diaphyseal humerus fractures does not cause permanent elbow stiffness. This is confirmed by our results; as casted patients did not lose final ROM compared to the bracing cohort. These injuries are extra-articular and casted patients are transitioned to bracing once humeri have significant union demonstrated by the arm moving as a unit. To our knowledge, there is no other study that has evaluated casting for these fractures, but it may be that evidence of permanent stiffness with nonoperative treatment of distal metaphyseal fractures of the humerus [AO/OTA type 13] is misapplied to distal humeral shaft fractures [AO/OTA type 12].3,9,10,12 For brace treatment, Sarmiento and colleagues9 showed no significant elbow stiffness in a consecutive cohort of 69 patients, while Jawa and colleagues5 showed no increased elbow stiffness compared to plate fixation. Given the accumulated data,3,5,6,8,13 advocates of operative treatment for distal third diaphyseal humerus fractures12 can no longer site elbow stiffness as a disadvantage of nonoperative treatment, whether with cast or brace.
As shown in this study, patients that choose nonoperative treatment can expect their fracture to heal with an average of approximately 15° of varus angulation, as well as 2 others evaluating brace treatment.5,9 Some will heal with as much as 30° of varus angulation.5,9 The arm may look a little different, particularly in thin patients, but there is no evidence that this angulation affects function. The risks, discomforts, and inconveniences of surgery can be balanced with the ability of surgery to improve alignment and allow elbow motion a few weeks earlier. The aesthetics of the scar after surgery may not be better than the deformity after nonoperative treatment. Patients should be involved in these decisions.
Continue to: No cost comparison...
No cost comparison was done between these 2 treatment modalities. However, both casting and bracing offer substantially lower costs comparted to surgical treatment with high efficacy and less risk for the patient. In some billing environments, closed treatments of fractures are captured as “surgical interventions” with global periods included in the reimbursement. Both casting and bracing are relatively inexpensive with materials that are readily accessible in nearly any general or subspecialty orthopedic practice.
There is a passive implication that operative treatment of distal third diaphyseal humerus fractures affords better results and union for patients in the discussed literature. Our results demonstrate that the distal diaphyseal humerus has a natural anatomic and biologic propensity to heal with closed immobilization. Patients should be made aware that while operative treatments exist for this fracture pattern, nonoperative treatment modalities have proven to be efficacious using a variety of immobilization methods. Thus, patients that prefer nonoperative treatment of a distal third diaphyseal humerus fracture can choose between a cast or a brace with confidence of the efficacy of the nonoperative treatment.
1. McKee MD. Fractures of the shaft of the humerus. In: Bucholz R, Heckman JD, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia: Lippencott Williams & Wilkins; 2006:1117-1159.
2. Schemitsch E, Bhandari M, Talbot M. Fractures of the humeral shaft. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma. 4th ed. Philadelphia: Saunders-Elsevier Company; 2009:1593-1622.
3. Walker M, Palumbo B, Badman B, Brooks J, Van Gelderen J, Mighell M. Humeral shaft fractures: a review. J Shoulder Elbow Surg. 2011;20(5):833-844. doi:10.1016/j.jse.2010.11.030.
4. Balfour GW, Mooney V, Ashby ME. Diaphyseal fractures of the humerus treated with a ready-made fracture brace. J Bone Joint Surg Am. 1982;64(1):11-13. doi:10.2106/00004623-198264010-00002.
5. Jawa A, McCarty P, Doornberg J, Harris M, Ring D. Extra-articular distal-third diaphyseal fractures of the humerus. A comparison of functional bracing and plate fixation. J Bone Joint Surg Am. 2006;88(11):2343-2347. doi:10.2106/JBJS.F.00334.
6. Pehlivan O. Functional treatment of the distal third humeral shaft fractures. Arch Orthop Trauma Surg. 2002;122(7):390-395. doi:10.1007/s00402-002-0403-x.
7. Ring D, Chin K, Taghinia AH, Jupiter JB. Nonunion after functional brace treatment of diaphyseal humerus fractures. J Trauma. 2007;62(5):1157-1158. doi:10.1097/01.ta.0000222719.52619.2c.
8. Sarmiento A, Horowitch A, Aboulafia A, Vangsness CT Jr. Functional bracing for comminuted extra-articular fractures of the distal third of the humerus. J Bone Joint Surg Br. 1990;72(4):283-287.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
10. Toivanen JA, Nieminen J, Laine HJ, Honkonen SE, Jarvinen MJ. Functional treatment of closed humeral shaft fractures. Int Orthop. 2005;29(1):10-13. doi:10.1007/s00264-004-0612-8.
11. Wallny T, Westermann K, Sagebiel C, Reimer M, Wagner UA. Functional treatment of humeral shaft fractures: indications and results. J Orthop Trauma. 1997;11(4):283-287.
12. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 Suppl):S1-S133.
13. Paris H, Tropiano P, Clouet D'orval B, Chaudet H, Poitout DG. Fractures of the shaft of the humerus: systematic plate fixation. Anatomic and functional results in 156 cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2000;86(4):346-359.
ABSTRACT
Diaphyseal fractures of the distal humerus have a high rate of union when treated with a functional brace or an above-elbow cast (AEC). This study compares alignment of the humerus and motion of the elbow after functional brace or AEC treatment.
One-hundred and five consecutive patients with a closed, extra-articular fracture of the distal humeral diaphysis were identified in the orthopedic trauma databases of 3 hospitals between 2003 and 2012. Seventy-five patients with a follow-up of at least 6 months or with radiographic and clinical evidence of fracture union were included (51 treated with functional bracing and 24 treated with an AEC).
All of the fractures healed. The average arc of elbow flexion was 130° ± 9° in braced patients vs 127° ± 12° in casted patients. Four patients (8%) in the bracing group and 4 (17%) in the casting group lost >20° of elbow motion. The average varus angulation on radiographs was 17° ± 8° in braced and 13° ± 8° in casted patients, while the average posterior angulation was 9° ± 6° vs 7° ± 7°, respectively.
Closed extra-articular distal diaphyseal humerus fractures heal with both bracing and casting and there are no differences in average elbow motion or radiographic alignment.
Nonoperative treatment of closed fractures of the humeral shaft (AO/OTA [Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association] type 12) with a functional brace or above-elbow cast (AEC) is associated with a high union rate, good motion, and good function. Advocates of casting believe that a brace cannot control fracture alignment as well as a cast that allows for immobilization and molding. Advocates of brace treatment are concerned that immobilization in a cast will cause elbow stiffness.1-11
Continue to: In our differing institutions...
In our differing institutions, there are advocates of each type of treatment, providing the opportunity for a comparison. This retrospective study compares brace and cast treatment. The working hypothesis was that there is no difference in elbow motion 6 months or more after fracture. We also compared radiographic alignment after union.
MATERIALS AND METHODS
Between 2003 and 2012, consecutive adult patients treated for a nonpathological fracture of the diaphysis of the distal humerus at the orthopedic trauma service of 3 level 1 academic trauma centers were identified from prospectively collected trauma injury databases. Patients with vascular injury, ipsilateral upper extremity fracture, and periprosthetic fractures were excluded. The attending orthopedic surgeon chose the treatment method and evaluated the range of motion (ROM) of the elbow and radiographic union at the final ambulatory visit. We included patients followed to clinical and radiographic union with a minimum of 6 months of follow-up. We also included patients with <6 months’ follow-up who demonstrated union and had elbow ROM within 10° of the uninjured arm.
We identified 105 consecutive adult patients with a closed nonpathological extra-articular distal humeral shaft fracture (fracture of the distal humeral shaft with an AO/OTA type-12.A, 12.B, or 12.C pattern) treated with an AEC or a brace in our databases.12 Two patients in the brace group chose surgery to improve alignment within 3 weeks of injury and were excluded from the analysis. Twenty-eight patients had inadequate follow-up.
A total of 75 patients were included in the study. At the first and second institutions, 51 patients were treated with functional bracing with an average follow-up of 7 months. At the third institution, 24 patients were treated with an AEC with an average follow-up of 4 months. Seventeen out of 24 patients in the long arm casting group and 19 out of 51 patients in the bracing group, who were included since they had <6 months of follow-up, demonstrated union and had elbow ROM within 10° of the uninjured arm. Differing methods of closed immobilization were the result of differing treatment algorithms at each institution.
The patients who were treated with a functional brace averaged 34 years of age (range, 18-90 years) and included 27 men and 24 women. The brace was removed at an average of 11.5 weeks (range, 8-18 weeks) after initial injury. Six patients had an injury-associated radial nerve palsy, all of which fully recovered within an average of 4 months (range, 0.5-7 months). Sixteen patients were injured due to a fall from standing height, 2 due to a fall from a greater height than standing, 16 in a motor-vehicle accident, 15 during a sport activity, and 2 were not specifically documented.
Continue to: Four patients had concomitant...
Four patients had concomitant injuries: one patient had a mid-shaft humeral fracture on the contralateral arm; a second had an ankle fracture; a third had an ankle fracture, acetabular fracture, a rib fracture, and pneumothorax; and the fourth had 2 rib fractures.
The patients who were treated with an AEC had an average age of 32 years (range,18-82 years) and included 14 men and 10 women. The cast was removed at an average of 4.2 weeks (range, 3-7 weeks) after the initial injury. Two patients had an injury-associated radial nerve palsy, both of which fully recovered. Five patients were injured due to a fall from standing height, 1 due to a fall from a height greater than standing, 7 during a motor-vehicle accident, 5 during a sport activity, and 6 were not documented. Two patients sustained concomitant injuries: one patient sustained a tibia-fibula fracture, and another patient sustained facial trauma.
The 2 groups were comparable in age and gender, as well as the injury mechanism (Table).
Table. Patient Demographics and Outcome Data
| Functional Bracing (n = 51) | Long Arm Casting (n = 24) | Significance (P < .05) |
Sex |
|
|
|
Male | 27 (54%) | 14 (58%) |
|
Female | 24 (46%) | 10 (42%) |
|
Average age (y) | 34 (range, 18-90) | 32 (range, 18-82) |
|
Mechanism of injury |
|
|
|
Standing height | 16 (31%) | 5 (20%) |
|
Greater height | 2 (4%) | 1 (4%) |
|
Motor vehicle collision | 16 (31%) | 7 (29%) |
|
Sports activity | 15 (29 %) | 5 (21%) |
|
Other | 2 (4%) | 6 (25%) |
|
Follow-up (months) | 7 (range, 2-25) | 4 (range, 2-15) |
|
Elbow range of motion (degrees) | 130 ± 9.4 | 127 ± 11.9 | P = .26 |
Varus/valgus angulation (degrees) | 17 ± 7.8 varus | 13 ± 8.4 varus | P = .11 |
Anterior/posterior angulation (degrees) | 9 ± 6.2 posterior | 7 ± 7.5 posterior | P = .54 |
FUNCTIONAL BRACING TECHNIQUE
Upon presentation after injury, patients were immobilized in a coaptation splint (Figure 1A). Within 10 days, the arm was placed in a pre-manufactured polyethylene functional brace (Corflex) and the arm was supported with a simple sling. Patients were allowed to use the hand for light tasks and move the elbow, but most patients were not capable of active elbow flexion exercises until early healing was established 4 to 6 weeks after injury. Shoulder motion was discouraged until radiographic union. Patients started active, self-assisted elbow and shoulder stretching exercises, and weaned from the brace once radiographic union was confirmed between 6 and 10 weeks after injury (Figures 1B, 1C).
ABOVE-ELBOW CASE
Patients were also initially immobilized in a coaptation splint upon initial presentation. Within 7 days, an above-elbow fiberglass cast with neutral forearm rotation and 90° of elbow flexion was applied with a supracondylar mold, followed by radiographic imaging (Figure 2A). With the fractured arm dependent, a valgus mold was applied as the material hardened in order to align the fracture site and limit varus angulation.
Continue to: There were no shoulder...
There were no shoulder ROM restrictions. Casts were removed, skin checked, and replaced every week for 4 to 6 weeks. Casts were removed when callus was noted on radiographs. After cast removal, physician-taught active and active-assisted elbow stretching exercises were given to patients to be performed on a daily basis at home. Patients were followed until clinical and radiographic union and elbow ROM to within 10° of the injured arm (Figures 2B, 2C).
STATISTICAL ANALYSIS
Alignment of the humerus (including varus-valgus alignment and apex anterior-posterior alignment) was measured on anteroposterior and lateral radiographs as the angle between lines bisecting the humeral diaphysis proximal and distal to the fracture. The normality of the data was tested using the Kolmogorov-Smirnov test. To statistically compare continuous variables with a normal distribution, t-tests were used; otherwise the Wilcoxon t-test was applied. The Pearson’s Chi-Square test was used to statistically compare dichotomous variables, except when expected cell frequency was <5, in which case the Fisher exact test was used. The level of significance was set at P < .05.
RESULTS
RANGE OF MOTION AND RADIOGRAPHIC ALIGNMENT
The average range of elbow motion was 130° ± 9° after brace treatment and 127° ± 12° after cast treatment (P = .26). Four patients (8%) treated with a brace and 3 (12%) treated with a cast lost >20° of elbow motion.
All the fractures healed. The average varus angulation on the anteroposterior radiograph was 17° (range, 2°-26°) in braced patients and 13 (range, 5°-31°) in casted patients (P = .11). The average posterior angulation on the lateral radiograph was 9° (range, 0°-28°) in braced patients vs 7° (range, 2°-33°) in casted patients (P = .54).
Continue to: Two weeks after initiating brace...
COMPLICATIONS
Two weeks after initiating brace treatment, an obese patient suffered a rash with desquamation that necessitated discontinuation of the brace. However, the skin and fracture ultimately healed with a coaptation splint and sling support without additional complications. In the casting cohort, 2 patients returned to the emergency department after AEC placement because of swelling of the hand and pain in the cast. Both casts were removed and reapplied.
DISCUSSION
Fractures of the distal third of the humeral diaphysis heal without surgery. Fracture angulation and elbow stiffness are the concerns that lead to variations in nonoperative treatment.1-3 Advocates of casting believe they can get better alignment without losing elbow motion, and advocates of bracing feel that the brace is less cumbersome.1-3,5-8 We compared these treatments retrospectively and found them comparable.
This study should be considered in light of its limitations. Many patients were lost to follow-up in our urban trauma centers. We do not know if these patients did better, worse, or the same as the patients we were able to evaluate, but our opinion is that patients having problems were more likely to return. The evaluation time was relatively short, but motion can only improve in the longer-term. Two patients that were initially braced chose surgery, probably because either they or their surgeon were nervous about the radiographic appearance of the fracture. In our opinion, continued nonoperative treatment of these patients would not affect the findings.
Cast treatment of distal diaphyseal humerus fractures does not cause permanent elbow stiffness. This is confirmed by our results; as casted patients did not lose final ROM compared to the bracing cohort. These injuries are extra-articular and casted patients are transitioned to bracing once humeri have significant union demonstrated by the arm moving as a unit. To our knowledge, there is no other study that has evaluated casting for these fractures, but it may be that evidence of permanent stiffness with nonoperative treatment of distal metaphyseal fractures of the humerus [AO/OTA type 13] is misapplied to distal humeral shaft fractures [AO/OTA type 12].3,9,10,12 For brace treatment, Sarmiento and colleagues9 showed no significant elbow stiffness in a consecutive cohort of 69 patients, while Jawa and colleagues5 showed no increased elbow stiffness compared to plate fixation. Given the accumulated data,3,5,6,8,13 advocates of operative treatment for distal third diaphyseal humerus fractures12 can no longer site elbow stiffness as a disadvantage of nonoperative treatment, whether with cast or brace.
As shown in this study, patients that choose nonoperative treatment can expect their fracture to heal with an average of approximately 15° of varus angulation, as well as 2 others evaluating brace treatment.5,9 Some will heal with as much as 30° of varus angulation.5,9 The arm may look a little different, particularly in thin patients, but there is no evidence that this angulation affects function. The risks, discomforts, and inconveniences of surgery can be balanced with the ability of surgery to improve alignment and allow elbow motion a few weeks earlier. The aesthetics of the scar after surgery may not be better than the deformity after nonoperative treatment. Patients should be involved in these decisions.
Continue to: No cost comparison...
No cost comparison was done between these 2 treatment modalities. However, both casting and bracing offer substantially lower costs comparted to surgical treatment with high efficacy and less risk for the patient. In some billing environments, closed treatments of fractures are captured as “surgical interventions” with global periods included in the reimbursement. Both casting and bracing are relatively inexpensive with materials that are readily accessible in nearly any general or subspecialty orthopedic practice.
There is a passive implication that operative treatment of distal third diaphyseal humerus fractures affords better results and union for patients in the discussed literature. Our results demonstrate that the distal diaphyseal humerus has a natural anatomic and biologic propensity to heal with closed immobilization. Patients should be made aware that while operative treatments exist for this fracture pattern, nonoperative treatment modalities have proven to be efficacious using a variety of immobilization methods. Thus, patients that prefer nonoperative treatment of a distal third diaphyseal humerus fracture can choose between a cast or a brace with confidence of the efficacy of the nonoperative treatment.
ABSTRACT
Diaphyseal fractures of the distal humerus have a high rate of union when treated with a functional brace or an above-elbow cast (AEC). This study compares alignment of the humerus and motion of the elbow after functional brace or AEC treatment.
One-hundred and five consecutive patients with a closed, extra-articular fracture of the distal humeral diaphysis were identified in the orthopedic trauma databases of 3 hospitals between 2003 and 2012. Seventy-five patients with a follow-up of at least 6 months or with radiographic and clinical evidence of fracture union were included (51 treated with functional bracing and 24 treated with an AEC).
All of the fractures healed. The average arc of elbow flexion was 130° ± 9° in braced patients vs 127° ± 12° in casted patients. Four patients (8%) in the bracing group and 4 (17%) in the casting group lost >20° of elbow motion. The average varus angulation on radiographs was 17° ± 8° in braced and 13° ± 8° in casted patients, while the average posterior angulation was 9° ± 6° vs 7° ± 7°, respectively.
Closed extra-articular distal diaphyseal humerus fractures heal with both bracing and casting and there are no differences in average elbow motion or radiographic alignment.
Nonoperative treatment of closed fractures of the humeral shaft (AO/OTA [Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association] type 12) with a functional brace or above-elbow cast (AEC) is associated with a high union rate, good motion, and good function. Advocates of casting believe that a brace cannot control fracture alignment as well as a cast that allows for immobilization and molding. Advocates of brace treatment are concerned that immobilization in a cast will cause elbow stiffness.1-11
Continue to: In our differing institutions...
In our differing institutions, there are advocates of each type of treatment, providing the opportunity for a comparison. This retrospective study compares brace and cast treatment. The working hypothesis was that there is no difference in elbow motion 6 months or more after fracture. We also compared radiographic alignment after union.
MATERIALS AND METHODS
Between 2003 and 2012, consecutive adult patients treated for a nonpathological fracture of the diaphysis of the distal humerus at the orthopedic trauma service of 3 level 1 academic trauma centers were identified from prospectively collected trauma injury databases. Patients with vascular injury, ipsilateral upper extremity fracture, and periprosthetic fractures were excluded. The attending orthopedic surgeon chose the treatment method and evaluated the range of motion (ROM) of the elbow and radiographic union at the final ambulatory visit. We included patients followed to clinical and radiographic union with a minimum of 6 months of follow-up. We also included patients with <6 months’ follow-up who demonstrated union and had elbow ROM within 10° of the uninjured arm.
We identified 105 consecutive adult patients with a closed nonpathological extra-articular distal humeral shaft fracture (fracture of the distal humeral shaft with an AO/OTA type-12.A, 12.B, or 12.C pattern) treated with an AEC or a brace in our databases.12 Two patients in the brace group chose surgery to improve alignment within 3 weeks of injury and were excluded from the analysis. Twenty-eight patients had inadequate follow-up.
A total of 75 patients were included in the study. At the first and second institutions, 51 patients were treated with functional bracing with an average follow-up of 7 months. At the third institution, 24 patients were treated with an AEC with an average follow-up of 4 months. Seventeen out of 24 patients in the long arm casting group and 19 out of 51 patients in the bracing group, who were included since they had <6 months of follow-up, demonstrated union and had elbow ROM within 10° of the uninjured arm. Differing methods of closed immobilization were the result of differing treatment algorithms at each institution.
The patients who were treated with a functional brace averaged 34 years of age (range, 18-90 years) and included 27 men and 24 women. The brace was removed at an average of 11.5 weeks (range, 8-18 weeks) after initial injury. Six patients had an injury-associated radial nerve palsy, all of which fully recovered within an average of 4 months (range, 0.5-7 months). Sixteen patients were injured due to a fall from standing height, 2 due to a fall from a greater height than standing, 16 in a motor-vehicle accident, 15 during a sport activity, and 2 were not specifically documented.
Continue to: Four patients had concomitant...
Four patients had concomitant injuries: one patient had a mid-shaft humeral fracture on the contralateral arm; a second had an ankle fracture; a third had an ankle fracture, acetabular fracture, a rib fracture, and pneumothorax; and the fourth had 2 rib fractures.
The patients who were treated with an AEC had an average age of 32 years (range,18-82 years) and included 14 men and 10 women. The cast was removed at an average of 4.2 weeks (range, 3-7 weeks) after the initial injury. Two patients had an injury-associated radial nerve palsy, both of which fully recovered. Five patients were injured due to a fall from standing height, 1 due to a fall from a height greater than standing, 7 during a motor-vehicle accident, 5 during a sport activity, and 6 were not documented. Two patients sustained concomitant injuries: one patient sustained a tibia-fibula fracture, and another patient sustained facial trauma.
The 2 groups were comparable in age and gender, as well as the injury mechanism (Table).
Table. Patient Demographics and Outcome Data
| Functional Bracing (n = 51) | Long Arm Casting (n = 24) | Significance (P < .05) |
Sex |
|
|
|
Male | 27 (54%) | 14 (58%) |
|
Female | 24 (46%) | 10 (42%) |
|
Average age (y) | 34 (range, 18-90) | 32 (range, 18-82) |
|
Mechanism of injury |
|
|
|
Standing height | 16 (31%) | 5 (20%) |
|
Greater height | 2 (4%) | 1 (4%) |
|
Motor vehicle collision | 16 (31%) | 7 (29%) |
|
Sports activity | 15 (29 %) | 5 (21%) |
|
Other | 2 (4%) | 6 (25%) |
|
Follow-up (months) | 7 (range, 2-25) | 4 (range, 2-15) |
|
Elbow range of motion (degrees) | 130 ± 9.4 | 127 ± 11.9 | P = .26 |
Varus/valgus angulation (degrees) | 17 ± 7.8 varus | 13 ± 8.4 varus | P = .11 |
Anterior/posterior angulation (degrees) | 9 ± 6.2 posterior | 7 ± 7.5 posterior | P = .54 |
FUNCTIONAL BRACING TECHNIQUE
Upon presentation after injury, patients were immobilized in a coaptation splint (Figure 1A). Within 10 days, the arm was placed in a pre-manufactured polyethylene functional brace (Corflex) and the arm was supported with a simple sling. Patients were allowed to use the hand for light tasks and move the elbow, but most patients were not capable of active elbow flexion exercises until early healing was established 4 to 6 weeks after injury. Shoulder motion was discouraged until radiographic union. Patients started active, self-assisted elbow and shoulder stretching exercises, and weaned from the brace once radiographic union was confirmed between 6 and 10 weeks after injury (Figures 1B, 1C).
ABOVE-ELBOW CASE
Patients were also initially immobilized in a coaptation splint upon initial presentation. Within 7 days, an above-elbow fiberglass cast with neutral forearm rotation and 90° of elbow flexion was applied with a supracondylar mold, followed by radiographic imaging (Figure 2A). With the fractured arm dependent, a valgus mold was applied as the material hardened in order to align the fracture site and limit varus angulation.
Continue to: There were no shoulder...
There were no shoulder ROM restrictions. Casts were removed, skin checked, and replaced every week for 4 to 6 weeks. Casts were removed when callus was noted on radiographs. After cast removal, physician-taught active and active-assisted elbow stretching exercises were given to patients to be performed on a daily basis at home. Patients were followed until clinical and radiographic union and elbow ROM to within 10° of the injured arm (Figures 2B, 2C).
STATISTICAL ANALYSIS
Alignment of the humerus (including varus-valgus alignment and apex anterior-posterior alignment) was measured on anteroposterior and lateral radiographs as the angle between lines bisecting the humeral diaphysis proximal and distal to the fracture. The normality of the data was tested using the Kolmogorov-Smirnov test. To statistically compare continuous variables with a normal distribution, t-tests were used; otherwise the Wilcoxon t-test was applied. The Pearson’s Chi-Square test was used to statistically compare dichotomous variables, except when expected cell frequency was <5, in which case the Fisher exact test was used. The level of significance was set at P < .05.
RESULTS
RANGE OF MOTION AND RADIOGRAPHIC ALIGNMENT
The average range of elbow motion was 130° ± 9° after brace treatment and 127° ± 12° after cast treatment (P = .26). Four patients (8%) treated with a brace and 3 (12%) treated with a cast lost >20° of elbow motion.
All the fractures healed. The average varus angulation on the anteroposterior radiograph was 17° (range, 2°-26°) in braced patients and 13 (range, 5°-31°) in casted patients (P = .11). The average posterior angulation on the lateral radiograph was 9° (range, 0°-28°) in braced patients vs 7° (range, 2°-33°) in casted patients (P = .54).
Continue to: Two weeks after initiating brace...
COMPLICATIONS
Two weeks after initiating brace treatment, an obese patient suffered a rash with desquamation that necessitated discontinuation of the brace. However, the skin and fracture ultimately healed with a coaptation splint and sling support without additional complications. In the casting cohort, 2 patients returned to the emergency department after AEC placement because of swelling of the hand and pain in the cast. Both casts were removed and reapplied.
DISCUSSION
Fractures of the distal third of the humeral diaphysis heal without surgery. Fracture angulation and elbow stiffness are the concerns that lead to variations in nonoperative treatment.1-3 Advocates of casting believe they can get better alignment without losing elbow motion, and advocates of bracing feel that the brace is less cumbersome.1-3,5-8 We compared these treatments retrospectively and found them comparable.
This study should be considered in light of its limitations. Many patients were lost to follow-up in our urban trauma centers. We do not know if these patients did better, worse, or the same as the patients we were able to evaluate, but our opinion is that patients having problems were more likely to return. The evaluation time was relatively short, but motion can only improve in the longer-term. Two patients that were initially braced chose surgery, probably because either they or their surgeon were nervous about the radiographic appearance of the fracture. In our opinion, continued nonoperative treatment of these patients would not affect the findings.
Cast treatment of distal diaphyseal humerus fractures does not cause permanent elbow stiffness. This is confirmed by our results; as casted patients did not lose final ROM compared to the bracing cohort. These injuries are extra-articular and casted patients are transitioned to bracing once humeri have significant union demonstrated by the arm moving as a unit. To our knowledge, there is no other study that has evaluated casting for these fractures, but it may be that evidence of permanent stiffness with nonoperative treatment of distal metaphyseal fractures of the humerus [AO/OTA type 13] is misapplied to distal humeral shaft fractures [AO/OTA type 12].3,9,10,12 For brace treatment, Sarmiento and colleagues9 showed no significant elbow stiffness in a consecutive cohort of 69 patients, while Jawa and colleagues5 showed no increased elbow stiffness compared to plate fixation. Given the accumulated data,3,5,6,8,13 advocates of operative treatment for distal third diaphyseal humerus fractures12 can no longer site elbow stiffness as a disadvantage of nonoperative treatment, whether with cast or brace.
As shown in this study, patients that choose nonoperative treatment can expect their fracture to heal with an average of approximately 15° of varus angulation, as well as 2 others evaluating brace treatment.5,9 Some will heal with as much as 30° of varus angulation.5,9 The arm may look a little different, particularly in thin patients, but there is no evidence that this angulation affects function. The risks, discomforts, and inconveniences of surgery can be balanced with the ability of surgery to improve alignment and allow elbow motion a few weeks earlier. The aesthetics of the scar after surgery may not be better than the deformity after nonoperative treatment. Patients should be involved in these decisions.
Continue to: No cost comparison...
No cost comparison was done between these 2 treatment modalities. However, both casting and bracing offer substantially lower costs comparted to surgical treatment with high efficacy and less risk for the patient. In some billing environments, closed treatments of fractures are captured as “surgical interventions” with global periods included in the reimbursement. Both casting and bracing are relatively inexpensive with materials that are readily accessible in nearly any general or subspecialty orthopedic practice.
There is a passive implication that operative treatment of distal third diaphyseal humerus fractures affords better results and union for patients in the discussed literature. Our results demonstrate that the distal diaphyseal humerus has a natural anatomic and biologic propensity to heal with closed immobilization. Patients should be made aware that while operative treatments exist for this fracture pattern, nonoperative treatment modalities have proven to be efficacious using a variety of immobilization methods. Thus, patients that prefer nonoperative treatment of a distal third diaphyseal humerus fracture can choose between a cast or a brace with confidence of the efficacy of the nonoperative treatment.
1. McKee MD. Fractures of the shaft of the humerus. In: Bucholz R, Heckman JD, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia: Lippencott Williams & Wilkins; 2006:1117-1159.
2. Schemitsch E, Bhandari M, Talbot M. Fractures of the humeral shaft. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma. 4th ed. Philadelphia: Saunders-Elsevier Company; 2009:1593-1622.
3. Walker M, Palumbo B, Badman B, Brooks J, Van Gelderen J, Mighell M. Humeral shaft fractures: a review. J Shoulder Elbow Surg. 2011;20(5):833-844. doi:10.1016/j.jse.2010.11.030.
4. Balfour GW, Mooney V, Ashby ME. Diaphyseal fractures of the humerus treated with a ready-made fracture brace. J Bone Joint Surg Am. 1982;64(1):11-13. doi:10.2106/00004623-198264010-00002.
5. Jawa A, McCarty P, Doornberg J, Harris M, Ring D. Extra-articular distal-third diaphyseal fractures of the humerus. A comparison of functional bracing and plate fixation. J Bone Joint Surg Am. 2006;88(11):2343-2347. doi:10.2106/JBJS.F.00334.
6. Pehlivan O. Functional treatment of the distal third humeral shaft fractures. Arch Orthop Trauma Surg. 2002;122(7):390-395. doi:10.1007/s00402-002-0403-x.
7. Ring D, Chin K, Taghinia AH, Jupiter JB. Nonunion after functional brace treatment of diaphyseal humerus fractures. J Trauma. 2007;62(5):1157-1158. doi:10.1097/01.ta.0000222719.52619.2c.
8. Sarmiento A, Horowitch A, Aboulafia A, Vangsness CT Jr. Functional bracing for comminuted extra-articular fractures of the distal third of the humerus. J Bone Joint Surg Br. 1990;72(4):283-287.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
10. Toivanen JA, Nieminen J, Laine HJ, Honkonen SE, Jarvinen MJ. Functional treatment of closed humeral shaft fractures. Int Orthop. 2005;29(1):10-13. doi:10.1007/s00264-004-0612-8.
11. Wallny T, Westermann K, Sagebiel C, Reimer M, Wagner UA. Functional treatment of humeral shaft fractures: indications and results. J Orthop Trauma. 1997;11(4):283-287.
12. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 Suppl):S1-S133.
13. Paris H, Tropiano P, Clouet D'orval B, Chaudet H, Poitout DG. Fractures of the shaft of the humerus: systematic plate fixation. Anatomic and functional results in 156 cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2000;86(4):346-359.
1. McKee MD. Fractures of the shaft of the humerus. In: Bucholz R, Heckman JD, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia: Lippencott Williams & Wilkins; 2006:1117-1159.
2. Schemitsch E, Bhandari M, Talbot M. Fractures of the humeral shaft. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma. 4th ed. Philadelphia: Saunders-Elsevier Company; 2009:1593-1622.
3. Walker M, Palumbo B, Badman B, Brooks J, Van Gelderen J, Mighell M. Humeral shaft fractures: a review. J Shoulder Elbow Surg. 2011;20(5):833-844. doi:10.1016/j.jse.2010.11.030.
4. Balfour GW, Mooney V, Ashby ME. Diaphyseal fractures of the humerus treated with a ready-made fracture brace. J Bone Joint Surg Am. 1982;64(1):11-13. doi:10.2106/00004623-198264010-00002.
5. Jawa A, McCarty P, Doornberg J, Harris M, Ring D. Extra-articular distal-third diaphyseal fractures of the humerus. A comparison of functional bracing and plate fixation. J Bone Joint Surg Am. 2006;88(11):2343-2347. doi:10.2106/JBJS.F.00334.
6. Pehlivan O. Functional treatment of the distal third humeral shaft fractures. Arch Orthop Trauma Surg. 2002;122(7):390-395. doi:10.1007/s00402-002-0403-x.
7. Ring D, Chin K, Taghinia AH, Jupiter JB. Nonunion after functional brace treatment of diaphyseal humerus fractures. J Trauma. 2007;62(5):1157-1158. doi:10.1097/01.ta.0000222719.52619.2c.
8. Sarmiento A, Horowitch A, Aboulafia A, Vangsness CT Jr. Functional bracing for comminuted extra-articular fractures of the distal third of the humerus. J Bone Joint Surg Br. 1990;72(4):283-287.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
10. Toivanen JA, Nieminen J, Laine HJ, Honkonen SE, Jarvinen MJ. Functional treatment of closed humeral shaft fractures. Int Orthop. 2005;29(1):10-13. doi:10.1007/s00264-004-0612-8.
11. Wallny T, Westermann K, Sagebiel C, Reimer M, Wagner UA. Functional treatment of humeral shaft fractures: indications and results. J Orthop Trauma. 1997;11(4):283-287.
12. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 Suppl):S1-S133.
13. Paris H, Tropiano P, Clouet D'orval B, Chaudet H, Poitout DG. Fractures of the shaft of the humerus: systematic plate fixation. Anatomic and functional results in 156 cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2000;86(4):346-359.
TAKE-HOME POINTS
- Closed extra-articular distal diaphyseal humerus fractures heal predictably with both bracing and casting.
- There are no differences in average elbow motion between bracing and casting of these fractures.
- There are no differences in radiographic alignment between bracing and casting of these fractures.
- The distal diaphyseal humerus has a natural anatomic and biologic propensity to heal with closed immobilization.
- Patients preferring nonoperative treatment can choose between a cast or a brace with confidence of the efficacy of either treatment.
Sprifermin moves FORWARD with sustained effects in osteoarthritis
LIVERPOOL, ENGLAND – At 3 years of follow-up, the cartilage-building effects of sprifermin appear to be sustained, according to further data to be released from the phase 2 FORWARD trial.
The difference from placebo in mean cartilage thickness at the tibiofemoral joint (TFJ) at 3 years was 0.05 mm for a 100-mcg dose of sprifermin given every 6 months (P less than .0001), 0.02 mm for a 100-mcg dose given every 12 months (P = .193), 0.01 mm for a 50-mcg dose given every 6 months (P = .530), and –0.02 mm for a 50-mcg dose given every 12 months (P = .160).
“These data, 18 months after the last active injection, extend the results which we previously presented and assessed at 6 months after the last injection,” study investigator Marc Hochberg, MD, said at the World Congress on Osteoarthritis.
The prior 2-year findings, which were reported at the annual meeting of the American College of Rheumatology in November 2017, showed statistically significant dose-dependent structural modification in TFJ cartilage. Furthermore, the increase in cartilage thickness was seen in both medial and lateral compartments of the TFJ, and in the central medial subregion of the TFJ.
Sprifermin is one of several drugs currently being investigated as a potential disease-modifying osteoarthritis drug, none of which are currently licensed for use. It is a novel human recombinant version of fibroblast growth factor 18 that has been shown to increase chondrocyte proliferation that results in overall extracellular matrix production and subsequent hyaline-line cartilage formation.
The study comprised 549 patients with symptomatic radiographic primary femorotibial knee OA who were aged 40-85 years. For inclusion, patients had to have Kellgren-Lawrence Grade 2 or 3, with a medial minimum joint space width of 2.5 mm or more. In addition, patients had to have a history of OA pain for at least 6 months and either symptoms requiring pain medication or a pain score of 4-9 on the 10-point question 1 of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
Dr. Hochberg, professor of medicine, epidemiology, and public health and head of the division of rheumatology and clinical immunology at the University of Maryland, Baltimore, noted that the current analysis at 3 years’ follow up had been prespecified and that the plan was to continue follow-up out to 5 years.
There was a statistically significant treatment effect and dose response effect seen in TFJ cartilage thickness.
“Although cartilage thickness declined in all treatment groups between years 2 and 3, the difference in cartilage thickness observed in year 2 with sprifermin at the highest dose [100 mcg every 6 months] versus placebo persisted through year 3,” Dr. Hochberg said at the congress, which was sponsored by the Osteoarthritis Research Society International.
As for secondary endpoints of thickness in the medial and lateral tibiofemoral cartilage, “there are significant differences between the higher-dose sprifermin group and the placebo group,” he said.
“Based on imaging, sprifermin appears to be effective at modifying structural changes in articular cartilage in a dose-dependent manner in patients with knee osteoarthritis, with an acceptable safety profile.”
Dr. Hochberg added that there was “marked symptomatic improvement” as shown by changes in WOMAC scores in all treatment groups including placebo. The improvement in total WOMAC scores by approximately 50% in all treatment groups by the second year was continued to the third year.
Adverse effects occurred with a similar frequency in the active treatment groups and the placebo group. They were also of a similar nature. The most commonly reported side effects involved the musculoskeletal system or were connective tissue disorders (e.g. arthralgia). Importantly, there was no difference in the frequency, severity, or nature of serious adverse events, treatment-related adverse events, or discontinuation due to adverse events with active versus placebo therapy, Dr. Hochberg said.
The percentages of patients completing the study to the second and third years were a respective 87.8% and 81.6% in the sprifermin groups and 80.6% and 75.9% in the placebo group.
Merck KGaA and EMD Serono Research Institute funded the study. Dr. Hochberg has received consulting fees from EMD Serono and multiple other companies developing treatments for OA.
SOURCE: Hochberg M et al. Osteoarthritis Cartilage. 2018:26(1):S26-27. Abstract 32
LIVERPOOL, ENGLAND – At 3 years of follow-up, the cartilage-building effects of sprifermin appear to be sustained, according to further data to be released from the phase 2 FORWARD trial.
The difference from placebo in mean cartilage thickness at the tibiofemoral joint (TFJ) at 3 years was 0.05 mm for a 100-mcg dose of sprifermin given every 6 months (P less than .0001), 0.02 mm for a 100-mcg dose given every 12 months (P = .193), 0.01 mm for a 50-mcg dose given every 6 months (P = .530), and –0.02 mm for a 50-mcg dose given every 12 months (P = .160).
“These data, 18 months after the last active injection, extend the results which we previously presented and assessed at 6 months after the last injection,” study investigator Marc Hochberg, MD, said at the World Congress on Osteoarthritis.
The prior 2-year findings, which were reported at the annual meeting of the American College of Rheumatology in November 2017, showed statistically significant dose-dependent structural modification in TFJ cartilage. Furthermore, the increase in cartilage thickness was seen in both medial and lateral compartments of the TFJ, and in the central medial subregion of the TFJ.
Sprifermin is one of several drugs currently being investigated as a potential disease-modifying osteoarthritis drug, none of which are currently licensed for use. It is a novel human recombinant version of fibroblast growth factor 18 that has been shown to increase chondrocyte proliferation that results in overall extracellular matrix production and subsequent hyaline-line cartilage formation.
The study comprised 549 patients with symptomatic radiographic primary femorotibial knee OA who were aged 40-85 years. For inclusion, patients had to have Kellgren-Lawrence Grade 2 or 3, with a medial minimum joint space width of 2.5 mm or more. In addition, patients had to have a history of OA pain for at least 6 months and either symptoms requiring pain medication or a pain score of 4-9 on the 10-point question 1 of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
Dr. Hochberg, professor of medicine, epidemiology, and public health and head of the division of rheumatology and clinical immunology at the University of Maryland, Baltimore, noted that the current analysis at 3 years’ follow up had been prespecified and that the plan was to continue follow-up out to 5 years.
There was a statistically significant treatment effect and dose response effect seen in TFJ cartilage thickness.
“Although cartilage thickness declined in all treatment groups between years 2 and 3, the difference in cartilage thickness observed in year 2 with sprifermin at the highest dose [100 mcg every 6 months] versus placebo persisted through year 3,” Dr. Hochberg said at the congress, which was sponsored by the Osteoarthritis Research Society International.
As for secondary endpoints of thickness in the medial and lateral tibiofemoral cartilage, “there are significant differences between the higher-dose sprifermin group and the placebo group,” he said.
“Based on imaging, sprifermin appears to be effective at modifying structural changes in articular cartilage in a dose-dependent manner in patients with knee osteoarthritis, with an acceptable safety profile.”
Dr. Hochberg added that there was “marked symptomatic improvement” as shown by changes in WOMAC scores in all treatment groups including placebo. The improvement in total WOMAC scores by approximately 50% in all treatment groups by the second year was continued to the third year.
Adverse effects occurred with a similar frequency in the active treatment groups and the placebo group. They were also of a similar nature. The most commonly reported side effects involved the musculoskeletal system or were connective tissue disorders (e.g. arthralgia). Importantly, there was no difference in the frequency, severity, or nature of serious adverse events, treatment-related adverse events, or discontinuation due to adverse events with active versus placebo therapy, Dr. Hochberg said.
The percentages of patients completing the study to the second and third years were a respective 87.8% and 81.6% in the sprifermin groups and 80.6% and 75.9% in the placebo group.
Merck KGaA and EMD Serono Research Institute funded the study. Dr. Hochberg has received consulting fees from EMD Serono and multiple other companies developing treatments for OA.
SOURCE: Hochberg M et al. Osteoarthritis Cartilage. 2018:26(1):S26-27. Abstract 32
LIVERPOOL, ENGLAND – At 3 years of follow-up, the cartilage-building effects of sprifermin appear to be sustained, according to further data to be released from the phase 2 FORWARD trial.
The difference from placebo in mean cartilage thickness at the tibiofemoral joint (TFJ) at 3 years was 0.05 mm for a 100-mcg dose of sprifermin given every 6 months (P less than .0001), 0.02 mm for a 100-mcg dose given every 12 months (P = .193), 0.01 mm for a 50-mcg dose given every 6 months (P = .530), and –0.02 mm for a 50-mcg dose given every 12 months (P = .160).
“These data, 18 months after the last active injection, extend the results which we previously presented and assessed at 6 months after the last injection,” study investigator Marc Hochberg, MD, said at the World Congress on Osteoarthritis.
The prior 2-year findings, which were reported at the annual meeting of the American College of Rheumatology in November 2017, showed statistically significant dose-dependent structural modification in TFJ cartilage. Furthermore, the increase in cartilage thickness was seen in both medial and lateral compartments of the TFJ, and in the central medial subregion of the TFJ.
Sprifermin is one of several drugs currently being investigated as a potential disease-modifying osteoarthritis drug, none of which are currently licensed for use. It is a novel human recombinant version of fibroblast growth factor 18 that has been shown to increase chondrocyte proliferation that results in overall extracellular matrix production and subsequent hyaline-line cartilage formation.
The study comprised 549 patients with symptomatic radiographic primary femorotibial knee OA who were aged 40-85 years. For inclusion, patients had to have Kellgren-Lawrence Grade 2 or 3, with a medial minimum joint space width of 2.5 mm or more. In addition, patients had to have a history of OA pain for at least 6 months and either symptoms requiring pain medication or a pain score of 4-9 on the 10-point question 1 of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
Dr. Hochberg, professor of medicine, epidemiology, and public health and head of the division of rheumatology and clinical immunology at the University of Maryland, Baltimore, noted that the current analysis at 3 years’ follow up had been prespecified and that the plan was to continue follow-up out to 5 years.
There was a statistically significant treatment effect and dose response effect seen in TFJ cartilage thickness.
“Although cartilage thickness declined in all treatment groups between years 2 and 3, the difference in cartilage thickness observed in year 2 with sprifermin at the highest dose [100 mcg every 6 months] versus placebo persisted through year 3,” Dr. Hochberg said at the congress, which was sponsored by the Osteoarthritis Research Society International.
As for secondary endpoints of thickness in the medial and lateral tibiofemoral cartilage, “there are significant differences between the higher-dose sprifermin group and the placebo group,” he said.
“Based on imaging, sprifermin appears to be effective at modifying structural changes in articular cartilage in a dose-dependent manner in patients with knee osteoarthritis, with an acceptable safety profile.”
Dr. Hochberg added that there was “marked symptomatic improvement” as shown by changes in WOMAC scores in all treatment groups including placebo. The improvement in total WOMAC scores by approximately 50% in all treatment groups by the second year was continued to the third year.
Adverse effects occurred with a similar frequency in the active treatment groups and the placebo group. They were also of a similar nature. The most commonly reported side effects involved the musculoskeletal system or were connective tissue disorders (e.g. arthralgia). Importantly, there was no difference in the frequency, severity, or nature of serious adverse events, treatment-related adverse events, or discontinuation due to adverse events with active versus placebo therapy, Dr. Hochberg said.
The percentages of patients completing the study to the second and third years were a respective 87.8% and 81.6% in the sprifermin groups and 80.6% and 75.9% in the placebo group.
Merck KGaA and EMD Serono Research Institute funded the study. Dr. Hochberg has received consulting fees from EMD Serono and multiple other companies developing treatments for OA.
SOURCE: Hochberg M et al. Osteoarthritis Cartilage. 2018:26(1):S26-27. Abstract 32
REPORTING FROM OARSI 2018
Key clinical point:
Major finding: The difference from placebo in mean cartilage thickness at the tibiofemoral joint at 3 years was 0.05 mm for the 100-mcg dose of sprifermin given every 6 months (P less than .0001).
Study details: Phase 2 study of 549 patients with knee osteoarthritis treated with one of four intra-articular doses of sprifermin or placebo.
Disclosures: Merck KGaA and EMD Serono Research Institute funded the study. Dr. Hochberg has received consulting fees from EMD Serono and multiple other companies developing treatments for OA.
Source: Hochberg M et al. Osteoarthritis Cartilage. 2018:26(1):S26-27. Abstract 32.
ECT cost effective in treatment-resistant depression
Electroconvulsive therapy might be a cost-effective treatment option for patients with treatment-resistant depression, results of a mathematical modeling analysis suggest.
The health-economic value of electroconvulsive therapy is most likely maximized when the intervention is tried after two failed lines of pharmacotherapy/psychotherapy, authors of the analysis said in JAMA Psychiatry.
“Increasing use of ECT by offering it earlier in the course of treatment-resistant depression could greatly improve outcomes for this difficult-to-treat patient population,” wrote Eric L. Ross, a medical student at the University of Michigan, Ann Arbor.
In clinical practice, patients with uncontrolled depression might not be offered electroconvulsive therapy for months or years, despite evidence from multiple studies that it is significantly more effective than pharmacotherapy in that setting, Mr. Ross and his coauthors in the university’s department of psychiatry wrote in their report.
One barrier to use of electroconvulsive therapy might be its cost, which they said can run in excess of $10,000 for initial therapy and maintenance treatments, compared with several hundred dollars for an antidepressant prescription.
However, data are limited showing the efficacy of electroconvulsive therapy in relation to that higher price tag. Accordingly, the investigators used a decision analytic model to simulate the clinical and economic effects of seven different electroconvulsive therapy treatment strategies, and calculated the incremental cost-effectiveness ratio of each.
, depending on the treatment strategy, the investigators found.
It was 74%-78% likely that electroconvulsive therapy would be cost effective, they added, based on commonly accepted cost-effectiveness thresholds, they said.
They found that third-line electroconvulsive therapy had an incremental cost-effectiveness ratio of $54,000 per quality-adjusted life-year. Based on that, investigators projected that the third-line strategy would be cost effective, considering a willingness-to-pay threshold of $100,000 per quality-adjusted life-year.
Although offering electroconvulsive therapy after two lines of pharmacotherapy/psychotherapy maximized its health-economic value in this analysis, the treatment still would be cost effective in patients with three or more previous treatments, authors said.
Based on those findings, Mr. Ross and his coauthors said they would recommend that patients with major depressive disorder be offered ECT when two or more trials of pharmacotherapy or psychotherapy have failed. That aligns with other recent recommendations, including 2017 Florida best practice guidelines that classify ECT as a level 3 treatment option, they said.
Mr. Ross and his coauthors cited several limitations. One is that much of the input data used in the mathematical model is more than 10 years old. In addition, other novel interventions for treatment-resistant depression – such as repetitive transcranial magnetic stimulation – were not evaluated.
The study was supported by the Department of Veterans Affairs Health Services Research & Development Services. The study authors had no conflicts of interest to report.
SOURCE: Ross EL et al. JAMA Psychiatry. 2018 May 9. doi: 10.1001/jamapsychiatry.2018.0768..
Electroconvulsive therapy might be a cost-effective treatment option for patients with treatment-resistant depression, results of a mathematical modeling analysis suggest.
The health-economic value of electroconvulsive therapy is most likely maximized when the intervention is tried after two failed lines of pharmacotherapy/psychotherapy, authors of the analysis said in JAMA Psychiatry.
“Increasing use of ECT by offering it earlier in the course of treatment-resistant depression could greatly improve outcomes for this difficult-to-treat patient population,” wrote Eric L. Ross, a medical student at the University of Michigan, Ann Arbor.
In clinical practice, patients with uncontrolled depression might not be offered electroconvulsive therapy for months or years, despite evidence from multiple studies that it is significantly more effective than pharmacotherapy in that setting, Mr. Ross and his coauthors in the university’s department of psychiatry wrote in their report.
One barrier to use of electroconvulsive therapy might be its cost, which they said can run in excess of $10,000 for initial therapy and maintenance treatments, compared with several hundred dollars for an antidepressant prescription.
However, data are limited showing the efficacy of electroconvulsive therapy in relation to that higher price tag. Accordingly, the investigators used a decision analytic model to simulate the clinical and economic effects of seven different electroconvulsive therapy treatment strategies, and calculated the incremental cost-effectiveness ratio of each.
, depending on the treatment strategy, the investigators found.
It was 74%-78% likely that electroconvulsive therapy would be cost effective, they added, based on commonly accepted cost-effectiveness thresholds, they said.
They found that third-line electroconvulsive therapy had an incremental cost-effectiveness ratio of $54,000 per quality-adjusted life-year. Based on that, investigators projected that the third-line strategy would be cost effective, considering a willingness-to-pay threshold of $100,000 per quality-adjusted life-year.
Although offering electroconvulsive therapy after two lines of pharmacotherapy/psychotherapy maximized its health-economic value in this analysis, the treatment still would be cost effective in patients with three or more previous treatments, authors said.
Based on those findings, Mr. Ross and his coauthors said they would recommend that patients with major depressive disorder be offered ECT when two or more trials of pharmacotherapy or psychotherapy have failed. That aligns with other recent recommendations, including 2017 Florida best practice guidelines that classify ECT as a level 3 treatment option, they said.
Mr. Ross and his coauthors cited several limitations. One is that much of the input data used in the mathematical model is more than 10 years old. In addition, other novel interventions for treatment-resistant depression – such as repetitive transcranial magnetic stimulation – were not evaluated.
The study was supported by the Department of Veterans Affairs Health Services Research & Development Services. The study authors had no conflicts of interest to report.
SOURCE: Ross EL et al. JAMA Psychiatry. 2018 May 9. doi: 10.1001/jamapsychiatry.2018.0768..
Electroconvulsive therapy might be a cost-effective treatment option for patients with treatment-resistant depression, results of a mathematical modeling analysis suggest.
The health-economic value of electroconvulsive therapy is most likely maximized when the intervention is tried after two failed lines of pharmacotherapy/psychotherapy, authors of the analysis said in JAMA Psychiatry.
“Increasing use of ECT by offering it earlier in the course of treatment-resistant depression could greatly improve outcomes for this difficult-to-treat patient population,” wrote Eric L. Ross, a medical student at the University of Michigan, Ann Arbor.
In clinical practice, patients with uncontrolled depression might not be offered electroconvulsive therapy for months or years, despite evidence from multiple studies that it is significantly more effective than pharmacotherapy in that setting, Mr. Ross and his coauthors in the university’s department of psychiatry wrote in their report.
One barrier to use of electroconvulsive therapy might be its cost, which they said can run in excess of $10,000 for initial therapy and maintenance treatments, compared with several hundred dollars for an antidepressant prescription.
However, data are limited showing the efficacy of electroconvulsive therapy in relation to that higher price tag. Accordingly, the investigators used a decision analytic model to simulate the clinical and economic effects of seven different electroconvulsive therapy treatment strategies, and calculated the incremental cost-effectiveness ratio of each.
, depending on the treatment strategy, the investigators found.
It was 74%-78% likely that electroconvulsive therapy would be cost effective, they added, based on commonly accepted cost-effectiveness thresholds, they said.
They found that third-line electroconvulsive therapy had an incremental cost-effectiveness ratio of $54,000 per quality-adjusted life-year. Based on that, investigators projected that the third-line strategy would be cost effective, considering a willingness-to-pay threshold of $100,000 per quality-adjusted life-year.
Although offering electroconvulsive therapy after two lines of pharmacotherapy/psychotherapy maximized its health-economic value in this analysis, the treatment still would be cost effective in patients with three or more previous treatments, authors said.
Based on those findings, Mr. Ross and his coauthors said they would recommend that patients with major depressive disorder be offered ECT when two or more trials of pharmacotherapy or psychotherapy have failed. That aligns with other recent recommendations, including 2017 Florida best practice guidelines that classify ECT as a level 3 treatment option, they said.
Mr. Ross and his coauthors cited several limitations. One is that much of the input data used in the mathematical model is more than 10 years old. In addition, other novel interventions for treatment-resistant depression – such as repetitive transcranial magnetic stimulation – were not evaluated.
The study was supported by the Department of Veterans Affairs Health Services Research & Development Services. The study authors had no conflicts of interest to report.
SOURCE: Ross EL et al. JAMA Psychiatry. 2018 May 9. doi: 10.1001/jamapsychiatry.2018.0768..
FROM JAMA Psychiatry
Key clinical point: Electroconvulsive therapy (ECT) should be considered as a third-line treatment after two or more lines of pharmacotherapy and/or psychotherapy have failed.
Major finding: Third-line ECT had an incremental cost-effectiveness ratio of $54,000/quality-adjusted life-year.
Study details: A simulation of depression treatment using a decision analytic model taking into account the efficacy, cost, and quality of life impact of ECT based on meta-analyses, randomized trials, and observational studies.
Disclosures: The study was supported by the Department of Veterans Affairs Health Services Research & Development Services. Authors had no conflicts of interest to report.
Source: Ross EL et al. JAMA Psychiatry. 2018 May 9. doi: 10.1001/jamapsychiatry.2018.0768.
Persistent providers sway parents to accept HPV vaccination
according to data from 43 pediatrician clinic visits at six clinics in Texas.
Vaccine hesitancy is on the rise among parents in the United States, wrote Laura A. Shay, PhD, of the University of Texas School of Public Health, San Antonio, and her colleagues. “Although vaccine hesitancy is subject to influence, to our knowledge, no authors of previous studies have analyzed actual provider discussions with undecided parents to explore how parents express hesitancy about the HPV vaccine and how providers respond.”
Overall, 37 parents expressed hesitancy one or more times in different ways, including assertive responses (such as “No, not right now” or “We need to think about that”) at 27 visits, questions (such as “Is it safe?”) at 16 visits, and concerns (such as “I’m just nervous about it”) at 12 visits.
In responding to these parents, pediatricians used only persistence to promote vaccination in 18 cases, a combination of acquiescence and persistence in 13 cases, and only acquiescence in 6 cases. The teens were vaccinated the same day in 17 of the 18 cases of persistence, compared with only 2 of 13 cases of combined acquiescence and persistence, and none of the 6 cases in which health care providers acquiesced to parents’ concerns without further discussion.
The findings were limited by several factors, including a relatively homogeneous population of low socioeconomic families, too small a sample to determine significance, and possible influence of the audio recorder on behavior, Dr. Shay and her researchers noted. However, the results provide a framework for studies of parental hesitancy in larger and more diverse groups. “Parental hesitancy is an opportunity to practice patient-centered communication. Without understanding the source of parental hesitancy, a provider’s response may not be suitably tailored to counter hesitation”
“With our exploratory examination of the relationship between parent-provider communication about HPV vaccine hesitancy and vaccination behavior, we suggest that persistently engaging parents who express hesitancy can lead to same-day vaccinations and that these conversations are short (approximately 2-3 minutes),” they added.
The study was funded by the National Institutes of Health. Additional support was provided by the Simmons Comprehensive Cancer Center, University of Texas Southwestern Center for Translational Medicine, through the NIH and National Center for Advancing Translational Sciences, and University of Texas Southwestern Center of Patient-Centered Outcomes Research. Dr. Shay and her associates had no financial conflicts.
SOURCE: Shay LA et al. Pediatrics. 2018 May 15. doi: 10. 1542/ peds. 2017- 2312.
according to data from 43 pediatrician clinic visits at six clinics in Texas.
Vaccine hesitancy is on the rise among parents in the United States, wrote Laura A. Shay, PhD, of the University of Texas School of Public Health, San Antonio, and her colleagues. “Although vaccine hesitancy is subject to influence, to our knowledge, no authors of previous studies have analyzed actual provider discussions with undecided parents to explore how parents express hesitancy about the HPV vaccine and how providers respond.”
Overall, 37 parents expressed hesitancy one or more times in different ways, including assertive responses (such as “No, not right now” or “We need to think about that”) at 27 visits, questions (such as “Is it safe?”) at 16 visits, and concerns (such as “I’m just nervous about it”) at 12 visits.
In responding to these parents, pediatricians used only persistence to promote vaccination in 18 cases, a combination of acquiescence and persistence in 13 cases, and only acquiescence in 6 cases. The teens were vaccinated the same day in 17 of the 18 cases of persistence, compared with only 2 of 13 cases of combined acquiescence and persistence, and none of the 6 cases in which health care providers acquiesced to parents’ concerns without further discussion.
The findings were limited by several factors, including a relatively homogeneous population of low socioeconomic families, too small a sample to determine significance, and possible influence of the audio recorder on behavior, Dr. Shay and her researchers noted. However, the results provide a framework for studies of parental hesitancy in larger and more diverse groups. “Parental hesitancy is an opportunity to practice patient-centered communication. Without understanding the source of parental hesitancy, a provider’s response may not be suitably tailored to counter hesitation”
“With our exploratory examination of the relationship between parent-provider communication about HPV vaccine hesitancy and vaccination behavior, we suggest that persistently engaging parents who express hesitancy can lead to same-day vaccinations and that these conversations are short (approximately 2-3 minutes),” they added.
The study was funded by the National Institutes of Health. Additional support was provided by the Simmons Comprehensive Cancer Center, University of Texas Southwestern Center for Translational Medicine, through the NIH and National Center for Advancing Translational Sciences, and University of Texas Southwestern Center of Patient-Centered Outcomes Research. Dr. Shay and her associates had no financial conflicts.
SOURCE: Shay LA et al. Pediatrics. 2018 May 15. doi: 10. 1542/ peds. 2017- 2312.
according to data from 43 pediatrician clinic visits at six clinics in Texas.
Vaccine hesitancy is on the rise among parents in the United States, wrote Laura A. Shay, PhD, of the University of Texas School of Public Health, San Antonio, and her colleagues. “Although vaccine hesitancy is subject to influence, to our knowledge, no authors of previous studies have analyzed actual provider discussions with undecided parents to explore how parents express hesitancy about the HPV vaccine and how providers respond.”
Overall, 37 parents expressed hesitancy one or more times in different ways, including assertive responses (such as “No, not right now” or “We need to think about that”) at 27 visits, questions (such as “Is it safe?”) at 16 visits, and concerns (such as “I’m just nervous about it”) at 12 visits.
In responding to these parents, pediatricians used only persistence to promote vaccination in 18 cases, a combination of acquiescence and persistence in 13 cases, and only acquiescence in 6 cases. The teens were vaccinated the same day in 17 of the 18 cases of persistence, compared with only 2 of 13 cases of combined acquiescence and persistence, and none of the 6 cases in which health care providers acquiesced to parents’ concerns without further discussion.
The findings were limited by several factors, including a relatively homogeneous population of low socioeconomic families, too small a sample to determine significance, and possible influence of the audio recorder on behavior, Dr. Shay and her researchers noted. However, the results provide a framework for studies of parental hesitancy in larger and more diverse groups. “Parental hesitancy is an opportunity to practice patient-centered communication. Without understanding the source of parental hesitancy, a provider’s response may not be suitably tailored to counter hesitation”
“With our exploratory examination of the relationship between parent-provider communication about HPV vaccine hesitancy and vaccination behavior, we suggest that persistently engaging parents who express hesitancy can lead to same-day vaccinations and that these conversations are short (approximately 2-3 minutes),” they added.
The study was funded by the National Institutes of Health. Additional support was provided by the Simmons Comprehensive Cancer Center, University of Texas Southwestern Center for Translational Medicine, through the NIH and National Center for Advancing Translational Sciences, and University of Texas Southwestern Center of Patient-Centered Outcomes Research. Dr. Shay and her associates had no financial conflicts.
SOURCE: Shay LA et al. Pediatrics. 2018 May 15. doi: 10. 1542/ peds. 2017- 2312.
FROM PEDIATRICS
Key clinical point: A majority of unvaccinated adolescents received the HPV vaccine when clinicians engaged with hesitant parents.
Major finding: At 18 office visits when parents hesitated but doctors persisted, 17 adolescents received the HPV vaccine
Study details: The data come from audio recordings of 43 visits to six pediatric clinics in Dallas at which parents were unsure about HPV vaccination for their teens.
Disclosures: The study was funded by the National Institutes of Health. Additional support was provided by the Simmons Comprehensive Cancer Center, University of Texas Southwestern Center for Translational Medicine, through the NIH and National Center for Advancing Translational Sciences, and University of Texas Southwestern Center of Patient-Centered Outcomes Research. Dr. Shay and her associates had no financial conflicts.
Source: Shay LA et al. Pediatrics. 2018 May 15. doi: 10. 1542/ peds. 2017- 2312.
Study: No link between non-Hodgkin lymphoma and Q fever
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
FROM LANCET HAEMATOLOGY
‘Bright future’ for growth factor therapy in osteoarthritis
LIVERPOOL, ENGLAND – (OA), according to David Hunter, MBBS, PhD.
Dr. Hunter, who is the Florance and Cope Chair of Rheumatology and professor of medicine at the University of Sydney and the Royal North Shore Hospital, Sydney, Australia, outlined some of the recent research with these agents in OA.
In 2013, a U.S. study (Arthritis Care Res. 2013;65[5]:703-11) showed that the 2007-2008 incidence of symptomatic knee OA was highest between the ages of 54 and 64 years, with the estimated median age at diagnosis of 55.8 years. Patients were substantially younger than were those who had been diagnosed at a median age of 68.5 years more than a decade earlier in 1991-1992.
“So, we’re developing [OA] at a much earlier age, but we’re obviously living longer and living with that morbidity for a substantial period of time,” Dr. Hunter said at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
“It’s within that context that we really need to think about both the individual and societal burden of osteoarthritis, and why we’re developing agents such as growth factors to help retard symptoms related to disease,” continued Dr. Hunter, chair of the Institute of Bone and Joint Research, deputy dean of the Northern Clinical School, and consultant rheumatologist at North Sydney Orthopaedic and Sports Medicine Centre, Sydney.
OA is characterized by articular cartilage damage, low-grade synovial inflammation, and hypertrophic bone changes that lead to pain and functional deterioration (Expert Opin Emerg Drugs. 2015;20[3]:361-78). Attempts to modify the disease process have thus focused on these three areas, aiming to regulate cartilage catabolism and anabolism, control inflammation, and remodel subchondral bone (Rheumatology [Oxford]. 2018;57[suppl. 4]:iv108-iv123).
Growth factors fall under the category of regulating cartilage catabolism and anabolism, playing a “significant role in musculoskeletal tissues’ homeostasis and repair,” Dr. Hunter explained. “They influence chemotaxis, differentiation, proliferation, and synthetic activity of cartilage and bone cells, and can regulate physiological remodeling and cartilage healing.”
Examples of growth factors of interest in OA include transforming growth factor-beta superfamily and bone morphogenic proteins 2 and 7, fibroblast growth factors 2 and 18, and insulin-like growth factor-1.
Two currently unproven and somewhat controversial ways of regulating cartilage catabolism and anabolism is the use of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP). Neither are ready for widespread use, Dr. Hunter observed, and more data on these approaches still need to be gathered.
The rationale behind the use of MSCs is that all joint tissues contain these cells, and they can potentially differentiate into cartilage, bone, or other tissues. There are changes in the quantity, phenotype, and differentiation potential of these cells in patients with OA, however, leading researchers to see if intra-articular injection of MSCs could be a possible therapeutic strategy. MSCs can be isolated from a number of different tissues and differentiate into multiple cells types. Animal model data suggest that their transplantation into an affected joint can stimulate articular repair.
“From a clinical perspective, there have been a vast array of clinical trials looking at different types of MSCs and different types of administration,” Dr. Hunter noted. Pooled data suggest that stem cell therapy does help to improve pain and function. However, the quality of trials has been called into question, with a positive publication bias suggested. There’s no clear evidence of any structural benefits, Dr. Hunter stated. “There’s a lot of stem cells being used for clinical purposes without the evidence to support their efficacy,” he cautioned. “We need much better regulation.”
There are several PRP agents, which deliver growth factors, cytokines, adhesive proteins, and other plasma proteins, such as clotting factors. It’s unknown how PRP therapy works in OA, whether it is just pain relieving or could affect the progression of joint damage. So far, studies have been done only in knee OA, so data in other joints need to be obtained.
“Whilst they have great potential, the literature on the use of PRP is conflicting,” Dr. Hunter said. “There are a lot of positive trials out there, but their quality in general is quite low.”
Better data are perhaps available for the recombinant human fibroblast growth factor–18 sprifermin, with recent 3-year data from an ongoing 5-year randomized, placebo-controlled, phase 2 study showing increased total cartilage thickness in the tibiofemoral joint relative to placebo. “There does appear to be a structure benefit,” Dr. Hunter said of sprifermin’s effects in the study versus intra-articular saline.
Although improvement in total Western Ontario and McMaster Universities Osteoarthritis Index scores were seen in the patients with knee OA, there was no real differentiation by dose or by placebo. The rationale for having a longer trial follow-up – the last dose of sprifermin was given at 18 months – is to see if there is a later effect.
In the later stages of development is Invossa TissueGene-C; this is drug that consists of patients’ normal and genetically-modified chondrocyte cells that express transforming growth factor-beta 1. Phase 2 findings have shown that it can improve pain and function scores in patients with moderate to advanced knee OA (BMC Musculoskelet Disord. 2017;18[1]:461). Based on those findings, two phase 3 trials are currently underway, with some further findings presented elsewhere at the OARSI meeting.
Even though there is still no disease-modifying osteoarthritis drug currently licensed for use, “I’m really positive about all of what we’ve learned, particularly with the application to future trials and ongoing trials in this space,” Dr. Hunter observed.
“Future strategies should differentiate between those agents aiming to reduce pain, and those targeting structural evolution, incorporating a combination of anabolic and anti-catabolic therapies,” Dr. Hunter suggested. One of the challenges, however, is to address the placebo effect, which can be exaggerated by the context of administration or surgery.
Furthermore, he proposed that new treatments should “fit into the natural progression of OA” by focusing on early disease where changes might still be reversible. “We’re getting more nuanced about where to intervene,” Dr. Hunter said.
Emerging treatments should also take the location of the disease and its heterogenic nature into account.
Dr. Hunter has consulted for Zynerba, TLC, Kolon TissueGene, and Merck Serono, and he disclosed receiving royalties from DJO for a patellofemoral brace patent.
SOURCE: Lohmander S et al. Osteoarthritis Cartilage. 2018:26(1):S6. Abstract I-18
LIVERPOOL, ENGLAND – (OA), according to David Hunter, MBBS, PhD.
Dr. Hunter, who is the Florance and Cope Chair of Rheumatology and professor of medicine at the University of Sydney and the Royal North Shore Hospital, Sydney, Australia, outlined some of the recent research with these agents in OA.
In 2013, a U.S. study (Arthritis Care Res. 2013;65[5]:703-11) showed that the 2007-2008 incidence of symptomatic knee OA was highest between the ages of 54 and 64 years, with the estimated median age at diagnosis of 55.8 years. Patients were substantially younger than were those who had been diagnosed at a median age of 68.5 years more than a decade earlier in 1991-1992.
“So, we’re developing [OA] at a much earlier age, but we’re obviously living longer and living with that morbidity for a substantial period of time,” Dr. Hunter said at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
“It’s within that context that we really need to think about both the individual and societal burden of osteoarthritis, and why we’re developing agents such as growth factors to help retard symptoms related to disease,” continued Dr. Hunter, chair of the Institute of Bone and Joint Research, deputy dean of the Northern Clinical School, and consultant rheumatologist at North Sydney Orthopaedic and Sports Medicine Centre, Sydney.
OA is characterized by articular cartilage damage, low-grade synovial inflammation, and hypertrophic bone changes that lead to pain and functional deterioration (Expert Opin Emerg Drugs. 2015;20[3]:361-78). Attempts to modify the disease process have thus focused on these three areas, aiming to regulate cartilage catabolism and anabolism, control inflammation, and remodel subchondral bone (Rheumatology [Oxford]. 2018;57[suppl. 4]:iv108-iv123).
Growth factors fall under the category of regulating cartilage catabolism and anabolism, playing a “significant role in musculoskeletal tissues’ homeostasis and repair,” Dr. Hunter explained. “They influence chemotaxis, differentiation, proliferation, and synthetic activity of cartilage and bone cells, and can regulate physiological remodeling and cartilage healing.”
Examples of growth factors of interest in OA include transforming growth factor-beta superfamily and bone morphogenic proteins 2 and 7, fibroblast growth factors 2 and 18, and insulin-like growth factor-1.
Two currently unproven and somewhat controversial ways of regulating cartilage catabolism and anabolism is the use of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP). Neither are ready for widespread use, Dr. Hunter observed, and more data on these approaches still need to be gathered.
The rationale behind the use of MSCs is that all joint tissues contain these cells, and they can potentially differentiate into cartilage, bone, or other tissues. There are changes in the quantity, phenotype, and differentiation potential of these cells in patients with OA, however, leading researchers to see if intra-articular injection of MSCs could be a possible therapeutic strategy. MSCs can be isolated from a number of different tissues and differentiate into multiple cells types. Animal model data suggest that their transplantation into an affected joint can stimulate articular repair.
“From a clinical perspective, there have been a vast array of clinical trials looking at different types of MSCs and different types of administration,” Dr. Hunter noted. Pooled data suggest that stem cell therapy does help to improve pain and function. However, the quality of trials has been called into question, with a positive publication bias suggested. There’s no clear evidence of any structural benefits, Dr. Hunter stated. “There’s a lot of stem cells being used for clinical purposes without the evidence to support their efficacy,” he cautioned. “We need much better regulation.”
There are several PRP agents, which deliver growth factors, cytokines, adhesive proteins, and other plasma proteins, such as clotting factors. It’s unknown how PRP therapy works in OA, whether it is just pain relieving or could affect the progression of joint damage. So far, studies have been done only in knee OA, so data in other joints need to be obtained.
“Whilst they have great potential, the literature on the use of PRP is conflicting,” Dr. Hunter said. “There are a lot of positive trials out there, but their quality in general is quite low.”
Better data are perhaps available for the recombinant human fibroblast growth factor–18 sprifermin, with recent 3-year data from an ongoing 5-year randomized, placebo-controlled, phase 2 study showing increased total cartilage thickness in the tibiofemoral joint relative to placebo. “There does appear to be a structure benefit,” Dr. Hunter said of sprifermin’s effects in the study versus intra-articular saline.
Although improvement in total Western Ontario and McMaster Universities Osteoarthritis Index scores were seen in the patients with knee OA, there was no real differentiation by dose or by placebo. The rationale for having a longer trial follow-up – the last dose of sprifermin was given at 18 months – is to see if there is a later effect.
In the later stages of development is Invossa TissueGene-C; this is drug that consists of patients’ normal and genetically-modified chondrocyte cells that express transforming growth factor-beta 1. Phase 2 findings have shown that it can improve pain and function scores in patients with moderate to advanced knee OA (BMC Musculoskelet Disord. 2017;18[1]:461). Based on those findings, two phase 3 trials are currently underway, with some further findings presented elsewhere at the OARSI meeting.
Even though there is still no disease-modifying osteoarthritis drug currently licensed for use, “I’m really positive about all of what we’ve learned, particularly with the application to future trials and ongoing trials in this space,” Dr. Hunter observed.
“Future strategies should differentiate between those agents aiming to reduce pain, and those targeting structural evolution, incorporating a combination of anabolic and anti-catabolic therapies,” Dr. Hunter suggested. One of the challenges, however, is to address the placebo effect, which can be exaggerated by the context of administration or surgery.
Furthermore, he proposed that new treatments should “fit into the natural progression of OA” by focusing on early disease where changes might still be reversible. “We’re getting more nuanced about where to intervene,” Dr. Hunter said.
Emerging treatments should also take the location of the disease and its heterogenic nature into account.
Dr. Hunter has consulted for Zynerba, TLC, Kolon TissueGene, and Merck Serono, and he disclosed receiving royalties from DJO for a patellofemoral brace patent.
SOURCE: Lohmander S et al. Osteoarthritis Cartilage. 2018:26(1):S6. Abstract I-18
LIVERPOOL, ENGLAND – (OA), according to David Hunter, MBBS, PhD.
Dr. Hunter, who is the Florance and Cope Chair of Rheumatology and professor of medicine at the University of Sydney and the Royal North Shore Hospital, Sydney, Australia, outlined some of the recent research with these agents in OA.
In 2013, a U.S. study (Arthritis Care Res. 2013;65[5]:703-11) showed that the 2007-2008 incidence of symptomatic knee OA was highest between the ages of 54 and 64 years, with the estimated median age at diagnosis of 55.8 years. Patients were substantially younger than were those who had been diagnosed at a median age of 68.5 years more than a decade earlier in 1991-1992.
“So, we’re developing [OA] at a much earlier age, but we’re obviously living longer and living with that morbidity for a substantial period of time,” Dr. Hunter said at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
“It’s within that context that we really need to think about both the individual and societal burden of osteoarthritis, and why we’re developing agents such as growth factors to help retard symptoms related to disease,” continued Dr. Hunter, chair of the Institute of Bone and Joint Research, deputy dean of the Northern Clinical School, and consultant rheumatologist at North Sydney Orthopaedic and Sports Medicine Centre, Sydney.
OA is characterized by articular cartilage damage, low-grade synovial inflammation, and hypertrophic bone changes that lead to pain and functional deterioration (Expert Opin Emerg Drugs. 2015;20[3]:361-78). Attempts to modify the disease process have thus focused on these three areas, aiming to regulate cartilage catabolism and anabolism, control inflammation, and remodel subchondral bone (Rheumatology [Oxford]. 2018;57[suppl. 4]:iv108-iv123).
Growth factors fall under the category of regulating cartilage catabolism and anabolism, playing a “significant role in musculoskeletal tissues’ homeostasis and repair,” Dr. Hunter explained. “They influence chemotaxis, differentiation, proliferation, and synthetic activity of cartilage and bone cells, and can regulate physiological remodeling and cartilage healing.”
Examples of growth factors of interest in OA include transforming growth factor-beta superfamily and bone morphogenic proteins 2 and 7, fibroblast growth factors 2 and 18, and insulin-like growth factor-1.
Two currently unproven and somewhat controversial ways of regulating cartilage catabolism and anabolism is the use of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP). Neither are ready for widespread use, Dr. Hunter observed, and more data on these approaches still need to be gathered.
The rationale behind the use of MSCs is that all joint tissues contain these cells, and they can potentially differentiate into cartilage, bone, or other tissues. There are changes in the quantity, phenotype, and differentiation potential of these cells in patients with OA, however, leading researchers to see if intra-articular injection of MSCs could be a possible therapeutic strategy. MSCs can be isolated from a number of different tissues and differentiate into multiple cells types. Animal model data suggest that their transplantation into an affected joint can stimulate articular repair.
“From a clinical perspective, there have been a vast array of clinical trials looking at different types of MSCs and different types of administration,” Dr. Hunter noted. Pooled data suggest that stem cell therapy does help to improve pain and function. However, the quality of trials has been called into question, with a positive publication bias suggested. There’s no clear evidence of any structural benefits, Dr. Hunter stated. “There’s a lot of stem cells being used for clinical purposes without the evidence to support their efficacy,” he cautioned. “We need much better regulation.”
There are several PRP agents, which deliver growth factors, cytokines, adhesive proteins, and other plasma proteins, such as clotting factors. It’s unknown how PRP therapy works in OA, whether it is just pain relieving or could affect the progression of joint damage. So far, studies have been done only in knee OA, so data in other joints need to be obtained.
“Whilst they have great potential, the literature on the use of PRP is conflicting,” Dr. Hunter said. “There are a lot of positive trials out there, but their quality in general is quite low.”
Better data are perhaps available for the recombinant human fibroblast growth factor–18 sprifermin, with recent 3-year data from an ongoing 5-year randomized, placebo-controlled, phase 2 study showing increased total cartilage thickness in the tibiofemoral joint relative to placebo. “There does appear to be a structure benefit,” Dr. Hunter said of sprifermin’s effects in the study versus intra-articular saline.
Although improvement in total Western Ontario and McMaster Universities Osteoarthritis Index scores were seen in the patients with knee OA, there was no real differentiation by dose or by placebo. The rationale for having a longer trial follow-up – the last dose of sprifermin was given at 18 months – is to see if there is a later effect.
In the later stages of development is Invossa TissueGene-C; this is drug that consists of patients’ normal and genetically-modified chondrocyte cells that express transforming growth factor-beta 1. Phase 2 findings have shown that it can improve pain and function scores in patients with moderate to advanced knee OA (BMC Musculoskelet Disord. 2017;18[1]:461). Based on those findings, two phase 3 trials are currently underway, with some further findings presented elsewhere at the OARSI meeting.
Even though there is still no disease-modifying osteoarthritis drug currently licensed for use, “I’m really positive about all of what we’ve learned, particularly with the application to future trials and ongoing trials in this space,” Dr. Hunter observed.
“Future strategies should differentiate between those agents aiming to reduce pain, and those targeting structural evolution, incorporating a combination of anabolic and anti-catabolic therapies,” Dr. Hunter suggested. One of the challenges, however, is to address the placebo effect, which can be exaggerated by the context of administration or surgery.
Furthermore, he proposed that new treatments should “fit into the natural progression of OA” by focusing on early disease where changes might still be reversible. “We’re getting more nuanced about where to intervene,” Dr. Hunter said.
Emerging treatments should also take the location of the disease and its heterogenic nature into account.
Dr. Hunter has consulted for Zynerba, TLC, Kolon TissueGene, and Merck Serono, and he disclosed receiving royalties from DJO for a patellofemoral brace patent.
SOURCE: Lohmander S et al. Osteoarthritis Cartilage. 2018:26(1):S6. Abstract I-18
EXPERT ANALYSIS FROM OARSI 2018
Female cancer researchers receive less funding than male counterparts
Female cancer researchers receive significantly less funding than their male counterparts in terms of total investment, number of awards, and mean and median funding, according to an analysis of data on public and philanthropic cancer research funding awarded to U.K. institutions between 2000 and 2013.
In an analysis of 4,186 awards totaling 2.33 billion pounds, 2,890 grants (69%) with a total value of 1.82 billion pounds (78%) were awarded to male primary investigators (PIs), compared with just 1,296 grants (31%) with a total value of 512 million pounds(22%) for female PIs, investigators reported in BMJ Open.
Investigators studied openly accessible information on funding awards from public and philanthropic sources including the Medical Research Council, Department of Health, Biotechnology and Biological Sciences Research Council, Engineering and Physical Science Research Council, Wellcome Trust, European Commission, and nine members of the Association of Medical Research Charities. Awards were excluded if they were not relevant to oncology, led by a non-U.K. institution, and/or not considered a research and development activity, wrote Charlie D. Zhou, MD, of the Royal Free NHS Foundation Trust Department of Nuclear Medicine in London, and coauthors.
Median grant value was greater for men (252,647 pounds; interquartile range, 127,343-553,560 pounds) than for women (198,485 pounds; IQR, 99,317-382,650 pounds) (P less than .001). Mean grant value was also greater for men (630,324 pounds; standard deviation, 1,662,559 pounds) than for women (394,730 pounds; SD, 666,574 pounds), Dr. Zhou and colleagues reported.
Large funding discrepancies were seen for sex-specific cancer research. For instance, males received 13.8, 3.5, and 2.0 times the investment of their female counterparts in total, mean, and median prostate cancer funding, respectively. Likewise, men received 9.9, 6.6, and 2.9 times the funding of women PIs in total, mean, and median funding, respectively, for cervical cancer research. This pattern was true for ovarian cancer and breast cancer research, as well.
Men also received significantly greater median funding at all points of the research and development pipeline. For preclinical, phase 1, 2, or 3 clinical trials; and public health, men received 20%, 90%, and 50% more, respectively (P less than .001); for product development and cross-disciplinary research, the difference was 50% and 20%, respectively (P less than .01).
The results of the analysis demonstrate that “female PIs clearly and consistently receive less funding than their male counterparts,” the authors wrote. Although the study results are descriptive in nature and do not identify the underlying mechanisms for these discrepancies, they “demonstrate substantial gender imbalances in cancer research investment.
“We would strongly urge policy makers, funders and the academic and scientific community to investigate the factors leading to our observed differences and seek to ensure that women are appropriately supported in scientific endeavor,” they concluded.
No disclosures or conflicts of interest were reported.
SOURCE: Zhou CD et al. BMJ Open. 2018 Apr 30. doi: 10.1136/bmjopen-2017-018625.
Female cancer researchers receive significantly less funding than their male counterparts in terms of total investment, number of awards, and mean and median funding, according to an analysis of data on public and philanthropic cancer research funding awarded to U.K. institutions between 2000 and 2013.
In an analysis of 4,186 awards totaling 2.33 billion pounds, 2,890 grants (69%) with a total value of 1.82 billion pounds (78%) were awarded to male primary investigators (PIs), compared with just 1,296 grants (31%) with a total value of 512 million pounds(22%) for female PIs, investigators reported in BMJ Open.
Investigators studied openly accessible information on funding awards from public and philanthropic sources including the Medical Research Council, Department of Health, Biotechnology and Biological Sciences Research Council, Engineering and Physical Science Research Council, Wellcome Trust, European Commission, and nine members of the Association of Medical Research Charities. Awards were excluded if they were not relevant to oncology, led by a non-U.K. institution, and/or not considered a research and development activity, wrote Charlie D. Zhou, MD, of the Royal Free NHS Foundation Trust Department of Nuclear Medicine in London, and coauthors.
Median grant value was greater for men (252,647 pounds; interquartile range, 127,343-553,560 pounds) than for women (198,485 pounds; IQR, 99,317-382,650 pounds) (P less than .001). Mean grant value was also greater for men (630,324 pounds; standard deviation, 1,662,559 pounds) than for women (394,730 pounds; SD, 666,574 pounds), Dr. Zhou and colleagues reported.
Large funding discrepancies were seen for sex-specific cancer research. For instance, males received 13.8, 3.5, and 2.0 times the investment of their female counterparts in total, mean, and median prostate cancer funding, respectively. Likewise, men received 9.9, 6.6, and 2.9 times the funding of women PIs in total, mean, and median funding, respectively, for cervical cancer research. This pattern was true for ovarian cancer and breast cancer research, as well.
Men also received significantly greater median funding at all points of the research and development pipeline. For preclinical, phase 1, 2, or 3 clinical trials; and public health, men received 20%, 90%, and 50% more, respectively (P less than .001); for product development and cross-disciplinary research, the difference was 50% and 20%, respectively (P less than .01).
The results of the analysis demonstrate that “female PIs clearly and consistently receive less funding than their male counterparts,” the authors wrote. Although the study results are descriptive in nature and do not identify the underlying mechanisms for these discrepancies, they “demonstrate substantial gender imbalances in cancer research investment.
“We would strongly urge policy makers, funders and the academic and scientific community to investigate the factors leading to our observed differences and seek to ensure that women are appropriately supported in scientific endeavor,” they concluded.
No disclosures or conflicts of interest were reported.
SOURCE: Zhou CD et al. BMJ Open. 2018 Apr 30. doi: 10.1136/bmjopen-2017-018625.
Female cancer researchers receive significantly less funding than their male counterparts in terms of total investment, number of awards, and mean and median funding, according to an analysis of data on public and philanthropic cancer research funding awarded to U.K. institutions between 2000 and 2013.
In an analysis of 4,186 awards totaling 2.33 billion pounds, 2,890 grants (69%) with a total value of 1.82 billion pounds (78%) were awarded to male primary investigators (PIs), compared with just 1,296 grants (31%) with a total value of 512 million pounds(22%) for female PIs, investigators reported in BMJ Open.
Investigators studied openly accessible information on funding awards from public and philanthropic sources including the Medical Research Council, Department of Health, Biotechnology and Biological Sciences Research Council, Engineering and Physical Science Research Council, Wellcome Trust, European Commission, and nine members of the Association of Medical Research Charities. Awards were excluded if they were not relevant to oncology, led by a non-U.K. institution, and/or not considered a research and development activity, wrote Charlie D. Zhou, MD, of the Royal Free NHS Foundation Trust Department of Nuclear Medicine in London, and coauthors.
Median grant value was greater for men (252,647 pounds; interquartile range, 127,343-553,560 pounds) than for women (198,485 pounds; IQR, 99,317-382,650 pounds) (P less than .001). Mean grant value was also greater for men (630,324 pounds; standard deviation, 1,662,559 pounds) than for women (394,730 pounds; SD, 666,574 pounds), Dr. Zhou and colleagues reported.
Large funding discrepancies were seen for sex-specific cancer research. For instance, males received 13.8, 3.5, and 2.0 times the investment of their female counterparts in total, mean, and median prostate cancer funding, respectively. Likewise, men received 9.9, 6.6, and 2.9 times the funding of women PIs in total, mean, and median funding, respectively, for cervical cancer research. This pattern was true for ovarian cancer and breast cancer research, as well.
Men also received significantly greater median funding at all points of the research and development pipeline. For preclinical, phase 1, 2, or 3 clinical trials; and public health, men received 20%, 90%, and 50% more, respectively (P less than .001); for product development and cross-disciplinary research, the difference was 50% and 20%, respectively (P less than .01).
The results of the analysis demonstrate that “female PIs clearly and consistently receive less funding than their male counterparts,” the authors wrote. Although the study results are descriptive in nature and do not identify the underlying mechanisms for these discrepancies, they “demonstrate substantial gender imbalances in cancer research investment.
“We would strongly urge policy makers, funders and the academic and scientific community to investigate the factors leading to our observed differences and seek to ensure that women are appropriately supported in scientific endeavor,” they concluded.
No disclosures or conflicts of interest were reported.
SOURCE: Zhou CD et al. BMJ Open. 2018 Apr 30. doi: 10.1136/bmjopen-2017-018625.
FROM BMJ OPEN
Key clinical point: Female cancer researchers receive significantly less funding than their male counterparts.
Major finding: Of 4,186 awards, 2,890 grants (69%) were awarded to male primary investigators (PIs), compared with 1,296 grants (31%) for female PIs.
Study details: An analysis of data on public and philanthropic cancer research funding awarded to U.K. institutions between 2000 and 2013.
Disclosures: No disclosures or conflicts of interest were reported.
Source: Zhou CD et al. BMJ Open. 2018 Apr 30. doi: 10.1136/bmjopen-2017-018625.
Oncology postmarketing requirements mostly on schedule
Food and Drug Administration postmarketing requirement (PMR) studies for cancer drugs tend to be completed on schedule, but the drugs can remain on the market even if primary endpoints in those studies are not met, according to a research letter in JAMA Oncology.
“These examples underscore the importance of collecting the additional clinical safety and efficacy data outlined in the PMRs and the need for collaborative efforts between the FDA, sponsors, and investigators,” wrote Chadi Nabhan, MD, MBA, chief medical officer at Cardinal Health in Dublin, Ohio, and Marjorie Zettler, PhD, MPH, senior scientist at Cardinal Health.
The researchers reviewed the FDA’s Novel Drug Summary and compared the requirements listed there with information in the FDA’s Postmarket Requirements and Commitments database and on the Clinicaltrials.gov website.
The FDA has relied on its accelerated approval program, using surrogate or intermediate endpoints deemed to reasonably predict clinical benefit, in order to balance expeditious approval with patient safety. And it has used postmarketing requirements to try to mitigate the risk of this program.
From January 2011 to December 2016, 49 new drugs were approved in oncology, 23 of which were granted an accelerated approval. Of those 23, 17 had postmarketing requirements to complete, including 34 clinical trials. Of the 34 trials, researchers found, 15 have been completed, 14 are ongoing, 2 have been terminated, and 3 are pending.
Out of the 15 clinical studies that have been completed, 3 failed to meet their primary efficacy end points – for atezolizumab, nivolumab and pembrolizumab. None of the drugs have been pulled from the market, researchers noted.
The two terminated studies, both for idelalisib, were stopped because of safety concerns. One product, ponatinib, was temporarily pulled, and the FDA required further studies, with a risk evaluation and mitigation study. That postmarketing study was eventually resumed and completed.
“Recently, the FDA has been criticized for its oversight of PMR clinical studies. The agency has come under fire for failure to penalize sponsors for PMR clinical studies completed late,” the authors wrote. “Our own review of PMR clinical studies for novel oncology drug products granted AA [accelerated approval] within the last 6 years found that no studies were behind their original schedules.”
“However,” they went on to note, “PMR studies identified serious safety concerns in two incidents that resulted in changes to the labeling for both products [idelalisib and ponatinib]. In addition, our analysis identified three instances [20%] where confirmatory PMR clinical studies for drugs granted AA failed to meet their primary efficacy end points.”
No disclosures were reported
SOURCE: Nabhan C et al. JAMA Oncol. 2018 May 10. doi: 10.1001/jamaoncol.2018.0610.
Food and Drug Administration postmarketing requirement (PMR) studies for cancer drugs tend to be completed on schedule, but the drugs can remain on the market even if primary endpoints in those studies are not met, according to a research letter in JAMA Oncology.
“These examples underscore the importance of collecting the additional clinical safety and efficacy data outlined in the PMRs and the need for collaborative efforts between the FDA, sponsors, and investigators,” wrote Chadi Nabhan, MD, MBA, chief medical officer at Cardinal Health in Dublin, Ohio, and Marjorie Zettler, PhD, MPH, senior scientist at Cardinal Health.
The researchers reviewed the FDA’s Novel Drug Summary and compared the requirements listed there with information in the FDA’s Postmarket Requirements and Commitments database and on the Clinicaltrials.gov website.
The FDA has relied on its accelerated approval program, using surrogate or intermediate endpoints deemed to reasonably predict clinical benefit, in order to balance expeditious approval with patient safety. And it has used postmarketing requirements to try to mitigate the risk of this program.
From January 2011 to December 2016, 49 new drugs were approved in oncology, 23 of which were granted an accelerated approval. Of those 23, 17 had postmarketing requirements to complete, including 34 clinical trials. Of the 34 trials, researchers found, 15 have been completed, 14 are ongoing, 2 have been terminated, and 3 are pending.
Out of the 15 clinical studies that have been completed, 3 failed to meet their primary efficacy end points – for atezolizumab, nivolumab and pembrolizumab. None of the drugs have been pulled from the market, researchers noted.
The two terminated studies, both for idelalisib, were stopped because of safety concerns. One product, ponatinib, was temporarily pulled, and the FDA required further studies, with a risk evaluation and mitigation study. That postmarketing study was eventually resumed and completed.
“Recently, the FDA has been criticized for its oversight of PMR clinical studies. The agency has come under fire for failure to penalize sponsors for PMR clinical studies completed late,” the authors wrote. “Our own review of PMR clinical studies for novel oncology drug products granted AA [accelerated approval] within the last 6 years found that no studies were behind their original schedules.”
“However,” they went on to note, “PMR studies identified serious safety concerns in two incidents that resulted in changes to the labeling for both products [idelalisib and ponatinib]. In addition, our analysis identified three instances [20%] where confirmatory PMR clinical studies for drugs granted AA failed to meet their primary efficacy end points.”
No disclosures were reported
SOURCE: Nabhan C et al. JAMA Oncol. 2018 May 10. doi: 10.1001/jamaoncol.2018.0610.
Food and Drug Administration postmarketing requirement (PMR) studies for cancer drugs tend to be completed on schedule, but the drugs can remain on the market even if primary endpoints in those studies are not met, according to a research letter in JAMA Oncology.
“These examples underscore the importance of collecting the additional clinical safety and efficacy data outlined in the PMRs and the need for collaborative efforts between the FDA, sponsors, and investigators,” wrote Chadi Nabhan, MD, MBA, chief medical officer at Cardinal Health in Dublin, Ohio, and Marjorie Zettler, PhD, MPH, senior scientist at Cardinal Health.
The researchers reviewed the FDA’s Novel Drug Summary and compared the requirements listed there with information in the FDA’s Postmarket Requirements and Commitments database and on the Clinicaltrials.gov website.
The FDA has relied on its accelerated approval program, using surrogate or intermediate endpoints deemed to reasonably predict clinical benefit, in order to balance expeditious approval with patient safety. And it has used postmarketing requirements to try to mitigate the risk of this program.
From January 2011 to December 2016, 49 new drugs were approved in oncology, 23 of which were granted an accelerated approval. Of those 23, 17 had postmarketing requirements to complete, including 34 clinical trials. Of the 34 trials, researchers found, 15 have been completed, 14 are ongoing, 2 have been terminated, and 3 are pending.
Out of the 15 clinical studies that have been completed, 3 failed to meet their primary efficacy end points – for atezolizumab, nivolumab and pembrolizumab. None of the drugs have been pulled from the market, researchers noted.
The two terminated studies, both for idelalisib, were stopped because of safety concerns. One product, ponatinib, was temporarily pulled, and the FDA required further studies, with a risk evaluation and mitigation study. That postmarketing study was eventually resumed and completed.
“Recently, the FDA has been criticized for its oversight of PMR clinical studies. The agency has come under fire for failure to penalize sponsors for PMR clinical studies completed late,” the authors wrote. “Our own review of PMR clinical studies for novel oncology drug products granted AA [accelerated approval] within the last 6 years found that no studies were behind their original schedules.”
“However,” they went on to note, “PMR studies identified serious safety concerns in two incidents that resulted in changes to the labeling for both products [idelalisib and ponatinib]. In addition, our analysis identified three instances [20%] where confirmatory PMR clinical studies for drugs granted AA failed to meet their primary efficacy end points.”
No disclosures were reported
SOURCE: Nabhan C et al. JAMA Oncol. 2018 May 10. doi: 10.1001/jamaoncol.2018.0610.
FROM JAMA ONCOLOGY
Key clinical point: Of 49 oncology drugs approved by the FDA from January 2011 to December 2016, 23 were given accelerated approval, with 17 needing postmarketing research.
Major finding: None of the pending or ongoing studies are behind their original schedules.
Study details: A review of the FDA’s Novel Drugs Summary, the FDA’s Postmarket Requirements and Commitments database, and Clinicaltrials.gov information.
Disclosures: No disclosures were reported.
Source: Nabhan C et al. JAMA Oncol. 2018 May 10. doi:10.1001/jamaoncol.2018.0610.
What is the microbiology of liver abscess?
Case
A 29-year-old woman with chronic urticaria, previously on omalizumab, presented with 2 weeks of abdominal pain and fever. She had traveled to Nicaragua within the past 10 months. CT showed a 6 x 5 cm liver abscess. Entamoeba histolytica was detected by stool polymerase chain reaction, and E. histolytica antibody was positive. The abscess was drained, and she completed a 10-day course of metronidazole followed by a 7-day course of paromomycin.
Brief overview
Bacterial, parasitic, and fungal organisms can cause liver abscess. Worldwide, bacteria are the most common cause of liver abscess. Infection is usually polymicrobial, though Klebsiella and the Streptococcus milleri group are the most common organisms identified.
Entamoeba histolytica is the most frequent cause of amoebic liver abscess and should be strongly considered in a returning traveler, visitor from another country, or those on monoclonal antibody therapy directed against IgE such as omalizumab. Candida species are the most common fungal etiology; an example being hepatosplenic candidiasis in hematologic malignancies.
Overview of the data
Pyogenic liver abscess. A variety of bacteria have been isolated from pyogenic liver abscesses (PLA) because of differences in mechanism of infection (such as biliary tract interventions, postoperative complications, and hematogenous spread), immunocompromised states, and geographical variation. The literature is not robust for pyogenic liver abscesses, and the microbiology isolated via epidemiologic studies are confounded by the mechanism of infection and thus difficult to generalize.
Initially, the Enterobacteriaceae including Klebsiella pneumoniae and Escherichia coli were the most common cause of PLA in the United States. The emergence of improved culturing techniques, which has improved the yield of facultative anaerobes such as the Streptococcus milleri (also known as Streptococcus anginosus) group, has led to an increased incidence and wider assortment of bacteria, with a more recent study of 38 patients in the Cleveland Clinic system showing that about half of the PLA were polymicrobial with the predominant organism when monomicrobial being of the Strep milleri group in 9/22 patients (Chemaly et al.).
Biliary tract disease, whether from choledocholithiasis, stricture, or malignant obstruction is the most common etiology of PLA. Much of the PLA-focused literature is from Asia, where Klebsiella is more commonly a cause of liver abscess. In a study of 248 Taiwanese patients with PLA, Klebsiella was responsible for 69% of PLA (Yang et al.). In a study of 79 patients hospitalized in New York, Klebsiella was the most common bacteria isolated, although more than half of the patients studied were Asian, and Klebsiella was more common among Asian patients than the other groups studied. An 8-year analysis of patients admitted to a University Hospital in Taiwan with cryptogenic PLA showed that the etiology was Klebsiella in 46/52 patients (Chen et al.). Most patients with Klebsiella liver abscess have documented bacteremia.
The second most common mechanism is bacterial translocation through the portal venous system. E. coli is commonly isolated and is frequently spread from intra-abdominal infections such as appendicitis leading to pylephlebitis. As the diagnosis and management of appendicitis has improved, the incidence of appendicitis causing a PLA has decreased.
PLA should be cultured to guide therapy and catheter drainage may be required. However, common organisms causing liver abscess should also be considered when selecting initial antibiotic therapy as cultures are frequently affected by previous antibiotic exposure or imprecise culturing techniques. Blood cultures should be obtained, and empiric therapy with a beta-lactam/beta-lactamase inhibitor or third-generation cephalosporin plus metronidazole should be started thereafter.
Entamoeba histolytica. E. histolytica, an anaerobic parasite that can lead to amoebic dysentery and liver abscess, affects upwards of 50 million people worldwide, predominantly in India and sub-Saharan Africa. Travel to an endemic area for longer than 1 month carries a high risk of transmission, though cases have been described with less than a week of exposure.
Infection occurs following consumption of affected food or water and can lead to dysentery within 3 weeks. Fever and right upper quadrant pain develop later, anywhere from 3 months to years following initial exposure. To diagnose, both serologic and stool testing for E. histolytica are recommended owing to the high sensitivity and low specificity of the serologic antibody test and the low sensitivity and high specificity of the stool antigen test. Imaging may reveal a single cyst with surrounding edema, which is characteristic.
Effective treatment is a two-step process. Metronidazole targets trophozoites that cause liver abscesses followed by paromomycin or diloxanide furoate to eradicate luminal oocysts and prevent reinfection. Aspiration and catheter drainage is necessary if the microbiology or etiology of the liver abscess remains uncertain, patients are not responding to antibiotics, or there is concern for impending rupture with cyst size greater than 6 cm (Jun et al.).
Hydatid cysts. Serologic testing via enzyme-linked immunoassay and radiographic characteristics are used to diagnose cysts caused by Echinococcus, of which there are many species. Imaging typically shows a well-defined cyst with calcifications and budding daughter cysts. Aspiration of an echinococcal cyst carries a risk of anaphylaxis and spread of infection and should only be undertaken if there is serologic and radiographic uncertainty.
Fungal abscesses. Fungal abscesses are most commonly caused by Candida species. The typical patient presentation includes high fever and elevated alkaline phosphatase, usually during the count recovery phase of patients with hematologic malignancies undergoing chemotherapy.
Fungal abscesses are frequently too small to aspirate. Fortunately, serum and radiographic results, as well as the clinical setting, make diagnosis more straightforward. Serum fungal markers can be checked and empiric treatment with amphotericin B or an echinocandin is recommended, followed by narrowing to oral fluconazole. Treatment should continue until abscesses resolve.
Interestingly, if patients become neutropenic during their antifungal course, the microabscesses may disappear on CT or MRI, only to reappear once neutrophils return. Once patients have a stable neutrophil count and imaging shows no abscesses, antifungal treatment can be discontinued, but must be restarted if patients are to undergo additional chemotherapy with expected neutropenia.
Back to the case
While impossible to state with certainty, infection with E. histolytica while in Nicaragua was thought most likely in this case. This patient was on omalizumab for chronic urticaria immediately prior to acquiring the infection and this anti-IgE monoclonal antibody likely predisposed her to a parasitic infection. Knowing this epidemiology, she may not have required catheter drainage, however, the cyst was causing pain and drainage provided decompression. She was treated with antibiotics followed by paromomycin.
Bottom line
Entamoeba histolytica is the most common cause of liver abscess worldwide, but identifying risk factors and mechanism of infection can lead to the most likely infecting organism.
Dr. Mehra is assistant professor of medicine in the Section of Hospital Medicine at the University of Virginia Medical Center and School of Medicine, Charlottesville. Dr. Parsons is also assistant professor of medicine in the Section of Hospital Medicine at the University of Virginia Medical Center and School of Medicine.
References
Chemaly RF. Microbiology of liver abscesses and the predictive value of abscess gram stain and associated blood cultures. Diagn Microbiol Infect Dis. 2003;46(4):245-8.
Yang CC. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37(3):176.
Chen SC. Comparison of pyogenic liver abscesses of biliary and cryptogenic origin. An eight-year analysis in a University Hospital. Swiss Med Wkly. 2005;135(23-24):344-51.
Yang CC. Pyogenic liver abscess in Taiwan: emphasis on gas-forming liver abscess in diabetics. Am J Gastroenterol. 1993;88:1911-15.
Jun CH. Risk factors and clinical outcomes for spontaneous rupture of pyogenic liver abscess. J Dig Dis. 2015;16(1):31-6.
Smego RA Jr. Treatment options for hepatic cystic echinococcosis. Int J Infect Dis. 2005;9(2):69-76.
Additional reading
Huang CJ et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223(5):600-7.
Meddings L et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105(1):117-24..
Petri WA, Singh U. Diagnosis and management of amoebiasis. Clin Infect Dis. 1999 Nov;29(5):1117-25.
Accompanying Photo caption: CT scan showing E. histolytica liver abscess. RELATED PHOTO IS PHOTOMICROGRAPH, NOT CT SCAN. G
Quiz
Microbiology of liver abscesses
Bacterial, parasitic, and fungal organisms can all cause liver abscesses. History, including travel history, is very important in deciphering which organisms are present.
Question 1: What is the most common cause of an amoebic liver abscess worldwide?
A. Echinococcus
B. Klebsiella pneumoniae
C. Entamoeba histolytica
D. Escherichia coli
The best answer is choice C. E. histolytica causes amoebic dysentery and liver abscess. This anaerobic parasite affects 50 million people worldwide, with the highest prevalence in India, sub-Saharan Africa, Mexico, and parts of Central and South America. Travelers spending a month or more in endemic areas are at highest risk, but cases have been reported with less than one week of exposure.
Question 2: Which type of parasitic cyst should not be aspirated?
A. Echinococcus
B. Entamoeba histolytica
C. Schistosomiasis
D. Paramoeba
The best answer is choice A. Aspiration of an echinococcal cyst carries a risk of anaphylaxis and spread of infection and should only be done if there is serologic and radiographic uncertainty. Imaging typically shows a well-defined cyst with calcifications and budding daughter cysts.
Key Points
- Risk factors and mechanism of action will suggest the most likely organisms and guide antibiotic choice.
- Entamoeba histolytica is the most common cause of liver abscess worldwide.
- Stool PCR and antibody testing for E. histolytica should both be ordered in the work-up of a liver abscess.
- Calcifications and daughter cysts budding off the main cyst can distinguish echinococcal cyst from E. histolytica abscess radiographically.
Case
A 29-year-old woman with chronic urticaria, previously on omalizumab, presented with 2 weeks of abdominal pain and fever. She had traveled to Nicaragua within the past 10 months. CT showed a 6 x 5 cm liver abscess. Entamoeba histolytica was detected by stool polymerase chain reaction, and E. histolytica antibody was positive. The abscess was drained, and she completed a 10-day course of metronidazole followed by a 7-day course of paromomycin.
Brief overview
Bacterial, parasitic, and fungal organisms can cause liver abscess. Worldwide, bacteria are the most common cause of liver abscess. Infection is usually polymicrobial, though Klebsiella and the Streptococcus milleri group are the most common organisms identified.
Entamoeba histolytica is the most frequent cause of amoebic liver abscess and should be strongly considered in a returning traveler, visitor from another country, or those on monoclonal antibody therapy directed against IgE such as omalizumab. Candida species are the most common fungal etiology; an example being hepatosplenic candidiasis in hematologic malignancies.
Overview of the data
Pyogenic liver abscess. A variety of bacteria have been isolated from pyogenic liver abscesses (PLA) because of differences in mechanism of infection (such as biliary tract interventions, postoperative complications, and hematogenous spread), immunocompromised states, and geographical variation. The literature is not robust for pyogenic liver abscesses, and the microbiology isolated via epidemiologic studies are confounded by the mechanism of infection and thus difficult to generalize.
Initially, the Enterobacteriaceae including Klebsiella pneumoniae and Escherichia coli were the most common cause of PLA in the United States. The emergence of improved culturing techniques, which has improved the yield of facultative anaerobes such as the Streptococcus milleri (also known as Streptococcus anginosus) group, has led to an increased incidence and wider assortment of bacteria, with a more recent study of 38 patients in the Cleveland Clinic system showing that about half of the PLA were polymicrobial with the predominant organism when monomicrobial being of the Strep milleri group in 9/22 patients (Chemaly et al.).
Biliary tract disease, whether from choledocholithiasis, stricture, or malignant obstruction is the most common etiology of PLA. Much of the PLA-focused literature is from Asia, where Klebsiella is more commonly a cause of liver abscess. In a study of 248 Taiwanese patients with PLA, Klebsiella was responsible for 69% of PLA (Yang et al.). In a study of 79 patients hospitalized in New York, Klebsiella was the most common bacteria isolated, although more than half of the patients studied were Asian, and Klebsiella was more common among Asian patients than the other groups studied. An 8-year analysis of patients admitted to a University Hospital in Taiwan with cryptogenic PLA showed that the etiology was Klebsiella in 46/52 patients (Chen et al.). Most patients with Klebsiella liver abscess have documented bacteremia.
The second most common mechanism is bacterial translocation through the portal venous system. E. coli is commonly isolated and is frequently spread from intra-abdominal infections such as appendicitis leading to pylephlebitis. As the diagnosis and management of appendicitis has improved, the incidence of appendicitis causing a PLA has decreased.
PLA should be cultured to guide therapy and catheter drainage may be required. However, common organisms causing liver abscess should also be considered when selecting initial antibiotic therapy as cultures are frequently affected by previous antibiotic exposure or imprecise culturing techniques. Blood cultures should be obtained, and empiric therapy with a beta-lactam/beta-lactamase inhibitor or third-generation cephalosporin plus metronidazole should be started thereafter.
Entamoeba histolytica. E. histolytica, an anaerobic parasite that can lead to amoebic dysentery and liver abscess, affects upwards of 50 million people worldwide, predominantly in India and sub-Saharan Africa. Travel to an endemic area for longer than 1 month carries a high risk of transmission, though cases have been described with less than a week of exposure.
Infection occurs following consumption of affected food or water and can lead to dysentery within 3 weeks. Fever and right upper quadrant pain develop later, anywhere from 3 months to years following initial exposure. To diagnose, both serologic and stool testing for E. histolytica are recommended owing to the high sensitivity and low specificity of the serologic antibody test and the low sensitivity and high specificity of the stool antigen test. Imaging may reveal a single cyst with surrounding edema, which is characteristic.
Effective treatment is a two-step process. Metronidazole targets trophozoites that cause liver abscesses followed by paromomycin or diloxanide furoate to eradicate luminal oocysts and prevent reinfection. Aspiration and catheter drainage is necessary if the microbiology or etiology of the liver abscess remains uncertain, patients are not responding to antibiotics, or there is concern for impending rupture with cyst size greater than 6 cm (Jun et al.).
Hydatid cysts. Serologic testing via enzyme-linked immunoassay and radiographic characteristics are used to diagnose cysts caused by Echinococcus, of which there are many species. Imaging typically shows a well-defined cyst with calcifications and budding daughter cysts. Aspiration of an echinococcal cyst carries a risk of anaphylaxis and spread of infection and should only be undertaken if there is serologic and radiographic uncertainty.
Fungal abscesses. Fungal abscesses are most commonly caused by Candida species. The typical patient presentation includes high fever and elevated alkaline phosphatase, usually during the count recovery phase of patients with hematologic malignancies undergoing chemotherapy.
Fungal abscesses are frequently too small to aspirate. Fortunately, serum and radiographic results, as well as the clinical setting, make diagnosis more straightforward. Serum fungal markers can be checked and empiric treatment with amphotericin B or an echinocandin is recommended, followed by narrowing to oral fluconazole. Treatment should continue until abscesses resolve.
Interestingly, if patients become neutropenic during their antifungal course, the microabscesses may disappear on CT or MRI, only to reappear once neutrophils return. Once patients have a stable neutrophil count and imaging shows no abscesses, antifungal treatment can be discontinued, but must be restarted if patients are to undergo additional chemotherapy with expected neutropenia.
Back to the case
While impossible to state with certainty, infection with E. histolytica while in Nicaragua was thought most likely in this case. This patient was on omalizumab for chronic urticaria immediately prior to acquiring the infection and this anti-IgE monoclonal antibody likely predisposed her to a parasitic infection. Knowing this epidemiology, she may not have required catheter drainage, however, the cyst was causing pain and drainage provided decompression. She was treated with antibiotics followed by paromomycin.
Bottom line
Entamoeba histolytica is the most common cause of liver abscess worldwide, but identifying risk factors and mechanism of infection can lead to the most likely infecting organism.
Dr. Mehra is assistant professor of medicine in the Section of Hospital Medicine at the University of Virginia Medical Center and School of Medicine, Charlottesville. Dr. Parsons is also assistant professor of medicine in the Section of Hospital Medicine at the University of Virginia Medical Center and School of Medicine.
References
Chemaly RF. Microbiology of liver abscesses and the predictive value of abscess gram stain and associated blood cultures. Diagn Microbiol Infect Dis. 2003;46(4):245-8.
Yang CC. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37(3):176.
Chen SC. Comparison of pyogenic liver abscesses of biliary and cryptogenic origin. An eight-year analysis in a University Hospital. Swiss Med Wkly. 2005;135(23-24):344-51.
Yang CC. Pyogenic liver abscess in Taiwan: emphasis on gas-forming liver abscess in diabetics. Am J Gastroenterol. 1993;88:1911-15.
Jun CH. Risk factors and clinical outcomes for spontaneous rupture of pyogenic liver abscess. J Dig Dis. 2015;16(1):31-6.
Smego RA Jr. Treatment options for hepatic cystic echinococcosis. Int J Infect Dis. 2005;9(2):69-76.
Additional reading
Huang CJ et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223(5):600-7.
Meddings L et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105(1):117-24..
Petri WA, Singh U. Diagnosis and management of amoebiasis. Clin Infect Dis. 1999 Nov;29(5):1117-25.
Accompanying Photo caption: CT scan showing E. histolytica liver abscess. RELATED PHOTO IS PHOTOMICROGRAPH, NOT CT SCAN. G
Quiz
Microbiology of liver abscesses
Bacterial, parasitic, and fungal organisms can all cause liver abscesses. History, including travel history, is very important in deciphering which organisms are present.
Question 1: What is the most common cause of an amoebic liver abscess worldwide?
A. Echinococcus
B. Klebsiella pneumoniae
C. Entamoeba histolytica
D. Escherichia coli
The best answer is choice C. E. histolytica causes amoebic dysentery and liver abscess. This anaerobic parasite affects 50 million people worldwide, with the highest prevalence in India, sub-Saharan Africa, Mexico, and parts of Central and South America. Travelers spending a month or more in endemic areas are at highest risk, but cases have been reported with less than one week of exposure.
Question 2: Which type of parasitic cyst should not be aspirated?
A. Echinococcus
B. Entamoeba histolytica
C. Schistosomiasis
D. Paramoeba
The best answer is choice A. Aspiration of an echinococcal cyst carries a risk of anaphylaxis and spread of infection and should only be done if there is serologic and radiographic uncertainty. Imaging typically shows a well-defined cyst with calcifications and budding daughter cysts.
Key Points
- Risk factors and mechanism of action will suggest the most likely organisms and guide antibiotic choice.
- Entamoeba histolytica is the most common cause of liver abscess worldwide.
- Stool PCR and antibody testing for E. histolytica should both be ordered in the work-up of a liver abscess.
- Calcifications and daughter cysts budding off the main cyst can distinguish echinococcal cyst from E. histolytica abscess radiographically.
Case
A 29-year-old woman with chronic urticaria, previously on omalizumab, presented with 2 weeks of abdominal pain and fever. She had traveled to Nicaragua within the past 10 months. CT showed a 6 x 5 cm liver abscess. Entamoeba histolytica was detected by stool polymerase chain reaction, and E. histolytica antibody was positive. The abscess was drained, and she completed a 10-day course of metronidazole followed by a 7-day course of paromomycin.
Brief overview
Bacterial, parasitic, and fungal organisms can cause liver abscess. Worldwide, bacteria are the most common cause of liver abscess. Infection is usually polymicrobial, though Klebsiella and the Streptococcus milleri group are the most common organisms identified.
Entamoeba histolytica is the most frequent cause of amoebic liver abscess and should be strongly considered in a returning traveler, visitor from another country, or those on monoclonal antibody therapy directed against IgE such as omalizumab. Candida species are the most common fungal etiology; an example being hepatosplenic candidiasis in hematologic malignancies.
Overview of the data
Pyogenic liver abscess. A variety of bacteria have been isolated from pyogenic liver abscesses (PLA) because of differences in mechanism of infection (such as biliary tract interventions, postoperative complications, and hematogenous spread), immunocompromised states, and geographical variation. The literature is not robust for pyogenic liver abscesses, and the microbiology isolated via epidemiologic studies are confounded by the mechanism of infection and thus difficult to generalize.
Initially, the Enterobacteriaceae including Klebsiella pneumoniae and Escherichia coli were the most common cause of PLA in the United States. The emergence of improved culturing techniques, which has improved the yield of facultative anaerobes such as the Streptococcus milleri (also known as Streptococcus anginosus) group, has led to an increased incidence and wider assortment of bacteria, with a more recent study of 38 patients in the Cleveland Clinic system showing that about half of the PLA were polymicrobial with the predominant organism when monomicrobial being of the Strep milleri group in 9/22 patients (Chemaly et al.).
Biliary tract disease, whether from choledocholithiasis, stricture, or malignant obstruction is the most common etiology of PLA. Much of the PLA-focused literature is from Asia, where Klebsiella is more commonly a cause of liver abscess. In a study of 248 Taiwanese patients with PLA, Klebsiella was responsible for 69% of PLA (Yang et al.). In a study of 79 patients hospitalized in New York, Klebsiella was the most common bacteria isolated, although more than half of the patients studied were Asian, and Klebsiella was more common among Asian patients than the other groups studied. An 8-year analysis of patients admitted to a University Hospital in Taiwan with cryptogenic PLA showed that the etiology was Klebsiella in 46/52 patients (Chen et al.). Most patients with Klebsiella liver abscess have documented bacteremia.
The second most common mechanism is bacterial translocation through the portal venous system. E. coli is commonly isolated and is frequently spread from intra-abdominal infections such as appendicitis leading to pylephlebitis. As the diagnosis and management of appendicitis has improved, the incidence of appendicitis causing a PLA has decreased.
PLA should be cultured to guide therapy and catheter drainage may be required. However, common organisms causing liver abscess should also be considered when selecting initial antibiotic therapy as cultures are frequently affected by previous antibiotic exposure or imprecise culturing techniques. Blood cultures should be obtained, and empiric therapy with a beta-lactam/beta-lactamase inhibitor or third-generation cephalosporin plus metronidazole should be started thereafter.
Entamoeba histolytica. E. histolytica, an anaerobic parasite that can lead to amoebic dysentery and liver abscess, affects upwards of 50 million people worldwide, predominantly in India and sub-Saharan Africa. Travel to an endemic area for longer than 1 month carries a high risk of transmission, though cases have been described with less than a week of exposure.
Infection occurs following consumption of affected food or water and can lead to dysentery within 3 weeks. Fever and right upper quadrant pain develop later, anywhere from 3 months to years following initial exposure. To diagnose, both serologic and stool testing for E. histolytica are recommended owing to the high sensitivity and low specificity of the serologic antibody test and the low sensitivity and high specificity of the stool antigen test. Imaging may reveal a single cyst with surrounding edema, which is characteristic.
Effective treatment is a two-step process. Metronidazole targets trophozoites that cause liver abscesses followed by paromomycin or diloxanide furoate to eradicate luminal oocysts and prevent reinfection. Aspiration and catheter drainage is necessary if the microbiology or etiology of the liver abscess remains uncertain, patients are not responding to antibiotics, or there is concern for impending rupture with cyst size greater than 6 cm (Jun et al.).
Hydatid cysts. Serologic testing via enzyme-linked immunoassay and radiographic characteristics are used to diagnose cysts caused by Echinococcus, of which there are many species. Imaging typically shows a well-defined cyst with calcifications and budding daughter cysts. Aspiration of an echinococcal cyst carries a risk of anaphylaxis and spread of infection and should only be undertaken if there is serologic and radiographic uncertainty.
Fungal abscesses. Fungal abscesses are most commonly caused by Candida species. The typical patient presentation includes high fever and elevated alkaline phosphatase, usually during the count recovery phase of patients with hematologic malignancies undergoing chemotherapy.
Fungal abscesses are frequently too small to aspirate. Fortunately, serum and radiographic results, as well as the clinical setting, make diagnosis more straightforward. Serum fungal markers can be checked and empiric treatment with amphotericin B or an echinocandin is recommended, followed by narrowing to oral fluconazole. Treatment should continue until abscesses resolve.
Interestingly, if patients become neutropenic during their antifungal course, the microabscesses may disappear on CT or MRI, only to reappear once neutrophils return. Once patients have a stable neutrophil count and imaging shows no abscesses, antifungal treatment can be discontinued, but must be restarted if patients are to undergo additional chemotherapy with expected neutropenia.
Back to the case
While impossible to state with certainty, infection with E. histolytica while in Nicaragua was thought most likely in this case. This patient was on omalizumab for chronic urticaria immediately prior to acquiring the infection and this anti-IgE monoclonal antibody likely predisposed her to a parasitic infection. Knowing this epidemiology, she may not have required catheter drainage, however, the cyst was causing pain and drainage provided decompression. She was treated with antibiotics followed by paromomycin.
Bottom line
Entamoeba histolytica is the most common cause of liver abscess worldwide, but identifying risk factors and mechanism of infection can lead to the most likely infecting organism.
Dr. Mehra is assistant professor of medicine in the Section of Hospital Medicine at the University of Virginia Medical Center and School of Medicine, Charlottesville. Dr. Parsons is also assistant professor of medicine in the Section of Hospital Medicine at the University of Virginia Medical Center and School of Medicine.
References
Chemaly RF. Microbiology of liver abscesses and the predictive value of abscess gram stain and associated blood cultures. Diagn Microbiol Infect Dis. 2003;46(4):245-8.
Yang CC. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37(3):176.
Chen SC. Comparison of pyogenic liver abscesses of biliary and cryptogenic origin. An eight-year analysis in a University Hospital. Swiss Med Wkly. 2005;135(23-24):344-51.
Yang CC. Pyogenic liver abscess in Taiwan: emphasis on gas-forming liver abscess in diabetics. Am J Gastroenterol. 1993;88:1911-15.
Jun CH. Risk factors and clinical outcomes for spontaneous rupture of pyogenic liver abscess. J Dig Dis. 2015;16(1):31-6.
Smego RA Jr. Treatment options for hepatic cystic echinococcosis. Int J Infect Dis. 2005;9(2):69-76.
Additional reading
Huang CJ et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223(5):600-7.
Meddings L et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105(1):117-24..
Petri WA, Singh U. Diagnosis and management of amoebiasis. Clin Infect Dis. 1999 Nov;29(5):1117-25.
Accompanying Photo caption: CT scan showing E. histolytica liver abscess. RELATED PHOTO IS PHOTOMICROGRAPH, NOT CT SCAN. G
Quiz
Microbiology of liver abscesses
Bacterial, parasitic, and fungal organisms can all cause liver abscesses. History, including travel history, is very important in deciphering which organisms are present.
Question 1: What is the most common cause of an amoebic liver abscess worldwide?
A. Echinococcus
B. Klebsiella pneumoniae
C. Entamoeba histolytica
D. Escherichia coli
The best answer is choice C. E. histolytica causes amoebic dysentery and liver abscess. This anaerobic parasite affects 50 million people worldwide, with the highest prevalence in India, sub-Saharan Africa, Mexico, and parts of Central and South America. Travelers spending a month or more in endemic areas are at highest risk, but cases have been reported with less than one week of exposure.
Question 2: Which type of parasitic cyst should not be aspirated?
A. Echinococcus
B. Entamoeba histolytica
C. Schistosomiasis
D. Paramoeba
The best answer is choice A. Aspiration of an echinococcal cyst carries a risk of anaphylaxis and spread of infection and should only be done if there is serologic and radiographic uncertainty. Imaging typically shows a well-defined cyst with calcifications and budding daughter cysts.
Key Points
- Risk factors and mechanism of action will suggest the most likely organisms and guide antibiotic choice.
- Entamoeba histolytica is the most common cause of liver abscess worldwide.
- Stool PCR and antibody testing for E. histolytica should both be ordered in the work-up of a liver abscess.
- Calcifications and daughter cysts budding off the main cyst can distinguish echinococcal cyst from E. histolytica abscess radiographically.
Patients who record office visits
Question: During an office visit, the patient used a smartphone to record his conversation with the doctor. Which of the following statements is best?
A. This is an intrusion into a private and confidential physician-patient encounter and violates laws against eavesdropping and wiretapping.
B. Recordings are rarely made in the doctor’s office.
C. Both parties must consent before the patient or doctor can legally make such a recording.
D. Surreptitious recording by one party is always illegal.
E. All are incorrect.
Answer: E.
Scholars from Dartmouth recently published their viewpoint on this topic in the Aug. 7, 2017, issue of JAMA.1 Many individuals believe that taping or recording a private conversation is per se illegal.
This is a misconception. Although it is a serious felony to violate wiretapping laws, in fact every jurisdiction permits the taping or recording of doctor-patient conversations where there is all-party consent. A majority of states actually allow the recording even if one party has not given his/her consent. This one-party consent rule is the law in 39 states, including Hawaii and New York. On the other hand, 11 states, such as California, Florida, Massachusetts, and Washington, deem such recordings illegal. A listing of the law in the various states can be found in the JAMA article, in which the authors call for “clear policies that facilitate the positive use of digital recordings.”
In a 2011 case against the Cleveland Clinic, a patient died of a cardiac arrest from hyperkalemia 3 days after elective knee surgery.2 The patient’s children had made a covert recording of a meeting with the chief medical officer when discussing the incident. The hospital attempted to bar the use of the recording, claiming that the information was nondiscoverable under the “peer review” privilege.
Both the trial court and the court of appeals disagreed, being unconvinced that such discussions fell within peer review protection. That the recording was made surreptitiously was not raised as an issue, as Ohio is a one-party consent state, i.e., the law permits a patient to legally tape his/her conversations without obtaining prior approval from the doctor.3
There are clear advantages to having a permanent record of a doctor’s professional opinion. The patient can review the information after the visit for a better understanding or for recall purposes, even sharing the information with family members, caregivers, or others, especially where there is a lack of clarity on instructions.4 In the area of informed consent, this is particularly useful for a reminder of medication side effects and potential complications of proposed surgery.
However, many doctors believe that recordings may be disruptive or prove inhibitory to free and open discussions, and they are concerned about their potential use should litigation arises.
Risk managers and malpractice carriers are divided in their views. For example, it has been stated that, “at the Barrow Neurological Institute, in Phoenix, Arizona, where patients are routinely offered video recordings of their visits, clinicians who participate in these recordings receive a 10% reduction in the cost of their medical defense and $1 million extra liability coverage” (P.J. Barr, unpublished data, 2017, as cited in reference 1). Other carriers are not as supportive, discouraging their insureds from allowing recordings to be made.
In the majority of jurisdictions, recordings are legal if consented to by one of the parties. This means that recordings by the patient with/without consent from or with/without knowledge of the doctor are fully legitimate. It also means that the recordings will be admissible into evidence in a courtroom, unless the information is privileged (protected from discovery) or is otherwise irrelevant or unreliable.
On the other hand, in states requiring all-party consent, such recordings are illegal absent across-the-board consent, and they will be inadmissible into evidence. This cardinal difference in state law raises vital implications for both plaintiff and defendant in litigation, because the recordings may contain incriminating or exculpatory information.
Recordings of conversations in the doctor’s office are by no means rare. A survey in the United Kingdom revealed that 15% of the public had secretly recorded a clinic visit, and 11% were aware of someone else doing the same.5 The concerned physician could proactively prohibit all office recordings by posting a “no recording” sign in the waiting room in the name of confidentiality and privacy. And should a physician discover that a patient is covertly recording, risk managers have suggested terminating the visit with a warning that a repeat attempt will result in discharge.
Like it or not, recordings are here to stay, and the omnipresence of modern communications devices such as smartphones, tablets, etc., is likely to increase the prevalence of recordings. A practical approach for practicing physicians is to familiarize themselves with the law in the individual state in which they practice and to improve their communication skills irrespective of whether or not there is a recording.
They may wish to consider the view attributed to Richard Boothman, JD, chief risk officer at the University of Michigan Health System: “Recording should cause any caregiver to mind their professionalism and be disciplined in their remarks to their patients. … I believe it can be a very powerful tool to cement the patient/physician relationship and the patient’s understanding of the clinical messages and information. Physicians are significantly benefited by an informed patient.”6
References
1. JAMA. 2017 Aug 8;318(6):513-4.
2. Smith v. Cleveland Clinic, 197 Ohio App.3d 524, 2011.
3. Ohio Revised Code 2933.52.
4. JAMA. 2015 Apr 28;313(16):1615-6.
5. BMJ Open. 2015 Aug 11;5(8):e008566.
6. “Your office is being recorded.” Medscape, April 3, 2018.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Question: During an office visit, the patient used a smartphone to record his conversation with the doctor. Which of the following statements is best?
A. This is an intrusion into a private and confidential physician-patient encounter and violates laws against eavesdropping and wiretapping.
B. Recordings are rarely made in the doctor’s office.
C. Both parties must consent before the patient or doctor can legally make such a recording.
D. Surreptitious recording by one party is always illegal.
E. All are incorrect.
Answer: E.
Scholars from Dartmouth recently published their viewpoint on this topic in the Aug. 7, 2017, issue of JAMA.1 Many individuals believe that taping or recording a private conversation is per se illegal.
This is a misconception. Although it is a serious felony to violate wiretapping laws, in fact every jurisdiction permits the taping or recording of doctor-patient conversations where there is all-party consent. A majority of states actually allow the recording even if one party has not given his/her consent. This one-party consent rule is the law in 39 states, including Hawaii and New York. On the other hand, 11 states, such as California, Florida, Massachusetts, and Washington, deem such recordings illegal. A listing of the law in the various states can be found in the JAMA article, in which the authors call for “clear policies that facilitate the positive use of digital recordings.”
In a 2011 case against the Cleveland Clinic, a patient died of a cardiac arrest from hyperkalemia 3 days after elective knee surgery.2 The patient’s children had made a covert recording of a meeting with the chief medical officer when discussing the incident. The hospital attempted to bar the use of the recording, claiming that the information was nondiscoverable under the “peer review” privilege.
Both the trial court and the court of appeals disagreed, being unconvinced that such discussions fell within peer review protection. That the recording was made surreptitiously was not raised as an issue, as Ohio is a one-party consent state, i.e., the law permits a patient to legally tape his/her conversations without obtaining prior approval from the doctor.3
There are clear advantages to having a permanent record of a doctor’s professional opinion. The patient can review the information after the visit for a better understanding or for recall purposes, even sharing the information with family members, caregivers, or others, especially where there is a lack of clarity on instructions.4 In the area of informed consent, this is particularly useful for a reminder of medication side effects and potential complications of proposed surgery.
However, many doctors believe that recordings may be disruptive or prove inhibitory to free and open discussions, and they are concerned about their potential use should litigation arises.
Risk managers and malpractice carriers are divided in their views. For example, it has been stated that, “at the Barrow Neurological Institute, in Phoenix, Arizona, where patients are routinely offered video recordings of their visits, clinicians who participate in these recordings receive a 10% reduction in the cost of their medical defense and $1 million extra liability coverage” (P.J. Barr, unpublished data, 2017, as cited in reference 1). Other carriers are not as supportive, discouraging their insureds from allowing recordings to be made.
In the majority of jurisdictions, recordings are legal if consented to by one of the parties. This means that recordings by the patient with/without consent from or with/without knowledge of the doctor are fully legitimate. It also means that the recordings will be admissible into evidence in a courtroom, unless the information is privileged (protected from discovery) or is otherwise irrelevant or unreliable.
On the other hand, in states requiring all-party consent, such recordings are illegal absent across-the-board consent, and they will be inadmissible into evidence. This cardinal difference in state law raises vital implications for both plaintiff and defendant in litigation, because the recordings may contain incriminating or exculpatory information.
Recordings of conversations in the doctor’s office are by no means rare. A survey in the United Kingdom revealed that 15% of the public had secretly recorded a clinic visit, and 11% were aware of someone else doing the same.5 The concerned physician could proactively prohibit all office recordings by posting a “no recording” sign in the waiting room in the name of confidentiality and privacy. And should a physician discover that a patient is covertly recording, risk managers have suggested terminating the visit with a warning that a repeat attempt will result in discharge.
Like it or not, recordings are here to stay, and the omnipresence of modern communications devices such as smartphones, tablets, etc., is likely to increase the prevalence of recordings. A practical approach for practicing physicians is to familiarize themselves with the law in the individual state in which they practice and to improve their communication skills irrespective of whether or not there is a recording.
They may wish to consider the view attributed to Richard Boothman, JD, chief risk officer at the University of Michigan Health System: “Recording should cause any caregiver to mind their professionalism and be disciplined in their remarks to their patients. … I believe it can be a very powerful tool to cement the patient/physician relationship and the patient’s understanding of the clinical messages and information. Physicians are significantly benefited by an informed patient.”6
References
1. JAMA. 2017 Aug 8;318(6):513-4.
2. Smith v. Cleveland Clinic, 197 Ohio App.3d 524, 2011.
3. Ohio Revised Code 2933.52.
4. JAMA. 2015 Apr 28;313(16):1615-6.
5. BMJ Open. 2015 Aug 11;5(8):e008566.
6. “Your office is being recorded.” Medscape, April 3, 2018.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Question: During an office visit, the patient used a smartphone to record his conversation with the doctor. Which of the following statements is best?
A. This is an intrusion into a private and confidential physician-patient encounter and violates laws against eavesdropping and wiretapping.
B. Recordings are rarely made in the doctor’s office.
C. Both parties must consent before the patient or doctor can legally make such a recording.
D. Surreptitious recording by one party is always illegal.
E. All are incorrect.
Answer: E.
Scholars from Dartmouth recently published their viewpoint on this topic in the Aug. 7, 2017, issue of JAMA.1 Many individuals believe that taping or recording a private conversation is per se illegal.
This is a misconception. Although it is a serious felony to violate wiretapping laws, in fact every jurisdiction permits the taping or recording of doctor-patient conversations where there is all-party consent. A majority of states actually allow the recording even if one party has not given his/her consent. This one-party consent rule is the law in 39 states, including Hawaii and New York. On the other hand, 11 states, such as California, Florida, Massachusetts, and Washington, deem such recordings illegal. A listing of the law in the various states can be found in the JAMA article, in which the authors call for “clear policies that facilitate the positive use of digital recordings.”
In a 2011 case against the Cleveland Clinic, a patient died of a cardiac arrest from hyperkalemia 3 days after elective knee surgery.2 The patient’s children had made a covert recording of a meeting with the chief medical officer when discussing the incident. The hospital attempted to bar the use of the recording, claiming that the information was nondiscoverable under the “peer review” privilege.
Both the trial court and the court of appeals disagreed, being unconvinced that such discussions fell within peer review protection. That the recording was made surreptitiously was not raised as an issue, as Ohio is a one-party consent state, i.e., the law permits a patient to legally tape his/her conversations without obtaining prior approval from the doctor.3
There are clear advantages to having a permanent record of a doctor’s professional opinion. The patient can review the information after the visit for a better understanding or for recall purposes, even sharing the information with family members, caregivers, or others, especially where there is a lack of clarity on instructions.4 In the area of informed consent, this is particularly useful for a reminder of medication side effects and potential complications of proposed surgery.
However, many doctors believe that recordings may be disruptive or prove inhibitory to free and open discussions, and they are concerned about their potential use should litigation arises.
Risk managers and malpractice carriers are divided in their views. For example, it has been stated that, “at the Barrow Neurological Institute, in Phoenix, Arizona, where patients are routinely offered video recordings of their visits, clinicians who participate in these recordings receive a 10% reduction in the cost of their medical defense and $1 million extra liability coverage” (P.J. Barr, unpublished data, 2017, as cited in reference 1). Other carriers are not as supportive, discouraging their insureds from allowing recordings to be made.
In the majority of jurisdictions, recordings are legal if consented to by one of the parties. This means that recordings by the patient with/without consent from or with/without knowledge of the doctor are fully legitimate. It also means that the recordings will be admissible into evidence in a courtroom, unless the information is privileged (protected from discovery) or is otherwise irrelevant or unreliable.
On the other hand, in states requiring all-party consent, such recordings are illegal absent across-the-board consent, and they will be inadmissible into evidence. This cardinal difference in state law raises vital implications for both plaintiff and defendant in litigation, because the recordings may contain incriminating or exculpatory information.
Recordings of conversations in the doctor’s office are by no means rare. A survey in the United Kingdom revealed that 15% of the public had secretly recorded a clinic visit, and 11% were aware of someone else doing the same.5 The concerned physician could proactively prohibit all office recordings by posting a “no recording” sign in the waiting room in the name of confidentiality and privacy. And should a physician discover that a patient is covertly recording, risk managers have suggested terminating the visit with a warning that a repeat attempt will result in discharge.
Like it or not, recordings are here to stay, and the omnipresence of modern communications devices such as smartphones, tablets, etc., is likely to increase the prevalence of recordings. A practical approach for practicing physicians is to familiarize themselves with the law in the individual state in which they practice and to improve their communication skills irrespective of whether or not there is a recording.
They may wish to consider the view attributed to Richard Boothman, JD, chief risk officer at the University of Michigan Health System: “Recording should cause any caregiver to mind their professionalism and be disciplined in their remarks to their patients. … I believe it can be a very powerful tool to cement the patient/physician relationship and the patient’s understanding of the clinical messages and information. Physicians are significantly benefited by an informed patient.”6
References
1. JAMA. 2017 Aug 8;318(6):513-4.
2. Smith v. Cleveland Clinic, 197 Ohio App.3d 524, 2011.
3. Ohio Revised Code 2933.52.
4. JAMA. 2015 Apr 28;313(16):1615-6.
5. BMJ Open. 2015 Aug 11;5(8):e008566.
6. “Your office is being recorded.” Medscape, April 3, 2018.
Dr. Tan is emeritus professor of medicine and a former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].