User login
Haplo-HSCT regimen can cure SCD, team says
A haploidentical transplant regimen has led to long-term engraftment in adults with sickle cell disease (SCD), according to researchers.
Seven of 8 patients treated with this regimen were still alive at last follow-up, and 6 of them maintained engraftment.
Two patients developed graft-versus-host disease (GVHD). One of them had acute and chronic GVHD and died about 400 days after transplant. The other had acute GVHD that resolved with treatment.
Damiano Rondelli, MD, of the University of Illinois at Chicago, and his colleagues reported these results in Biology of Blood and Marrow Transplantation.
The researchers initially screened 50 adult SCD patients as candidates for haploidentical hematopoietic stem cell transplant (haplo-HSCT) between January 2014 and March 2017. Most patients were ineligible or declined the procedure.
Ultimately, 10 patients received a haplo-HSCT. Unfortunately, the first 2 patients failed to engraft. These patients had received conditioning with alemtuzumab and 3 Gy total body irradiation (TBI) as well as post-transplant cyclophosphamide.
Because of this failure, the researchers used the following regimen for the remaining 8 patients. It’s a modified version of the regimen described in Blood in 2012.
“We modified the transplant protocol by increasing the dose of radiation used before the transplant and by infusing growth factor-mobilized peripheral blood stem cells instead of bone marrow cells,” Dr Rondelli said. “These two modifications helped ensure the patient’s body could accept the healthy donor cells.”
Modified regimen
Patients received growth-factor-mobilized peripheral blood stem cells after conditioning with rabbit antithymocyte globulin (0.5 mg/kg on day -9, 2 mg/kg on day -8 and -7), cyclophosphamide (14.5 mg/kg on day -6 and -5), fludarabine (30 mg/m2 on day -6 to -2), and single-dose TBI (3 Gy on day -1).
For GVHD prophylaxis, patients received intravenous cyclophosphamide (50 mg/kg on day 3 and 4), oral mycophenolate mofetil (15 mg/kg 3 times daily from day 5 to 35), and sirolimus (from day 5 dosed for a target trough of 5 to 15 ng/mL). In patients who had T-cell chimerism greater than 50% at 1 year after HSCT and did not have signs of GVHD, sirolimus was tapered off over 3 months.
Patients stopped taking hydroxyurea on day -9. They received red blood cell exchange transfusion on day -10 (with the goal of getting hemoglobin S below 30%) and received platelet transfusions to maintain platelet counts greater than 50 x 109 cells/L.
Patients also received penicillin V (250 mg twice daily) in addition to standard antimicrobial prophylaxis.
Results
All 8 patients on the modified regimen engrafted. The median time to neutrophil engraftment was 22 days (range, 18 to 23 days). One patient experienced secondary graft failure on day 90.
Seven neutropenic patients with hemoglobin S less than 30% received G-CSF after transplant. They received a median of 7 doses (range, 3 to 14) at 5 μg/kg, starting at day 12 post-HSCT. One of these patients experienced mild bone pain in the lower extremities.
Two patients developed GVHD. At day 83, one patient developed acute or chronic GVHD involving the skin, liver, and eyes. Steroids and sirolimus improved eye and liver symptoms, but the patient died at home on day 407.
The other patient had grade 2, gastrointestinal, acute GVHD that resolved with steroid therapy.
Three patients had grade 2 or higher mucositis, and 2 had cytomegalovirus (CMV) reactivation without CMV infection.
Two patients had small subarachnoid hemorrhages. One of these patients had a history of multiple red blood cell antibodies, became refractory to platelet transfusions, and developed small subarachnoid hemorrhages day 10. The patient’s symptoms and brain imaging resolved after platelet counts were maintained above 50 x 109 cells/L with cross-matched platelets.
The second patient had a history of stroke, experienced a seizure when the platelet count was 68 x 109 cells/L, and was found to have a subarachnoid hemorrhage on day 12. Symptoms and imaging results improved once the patient began levetiracetam and platelet levels were maintained above 100 x 109 cells/L.
At a median follow-up of 17 months (range, 12 to 30), 7 of the 8 patients were still alive.
Six patients had maintained greater than 95% stable donor engraftment with improvements in their hemoglobin concentrations. Three of these patients have stopped immunosuppression, and 3 are being tapered off it.
“These patients are cured of sickle cell disease,” Dr Rondelli said. “The takeaway message is two-fold. First, this transplant protocol may cure many more adult patients with advanced sickle cell disease.”
“Second, despite the increasing safety of the transplant protocols and new compatibility of HLA half-matched donors, many sickle cell patients still face barriers to care. Of the patients we screened, only 20% underwent a transplant.”
Dr Rondelli noted that 20% of the patients screened could not undergo transplant because of insurance denial. Other patients were ineligible because they had high rates of donor-specific antigens, and still others declined transplant.
A haploidentical transplant regimen has led to long-term engraftment in adults with sickle cell disease (SCD), according to researchers.
Seven of 8 patients treated with this regimen were still alive at last follow-up, and 6 of them maintained engraftment.
Two patients developed graft-versus-host disease (GVHD). One of them had acute and chronic GVHD and died about 400 days after transplant. The other had acute GVHD that resolved with treatment.
Damiano Rondelli, MD, of the University of Illinois at Chicago, and his colleagues reported these results in Biology of Blood and Marrow Transplantation.
The researchers initially screened 50 adult SCD patients as candidates for haploidentical hematopoietic stem cell transplant (haplo-HSCT) between January 2014 and March 2017. Most patients were ineligible or declined the procedure.
Ultimately, 10 patients received a haplo-HSCT. Unfortunately, the first 2 patients failed to engraft. These patients had received conditioning with alemtuzumab and 3 Gy total body irradiation (TBI) as well as post-transplant cyclophosphamide.
Because of this failure, the researchers used the following regimen for the remaining 8 patients. It’s a modified version of the regimen described in Blood in 2012.
“We modified the transplant protocol by increasing the dose of radiation used before the transplant and by infusing growth factor-mobilized peripheral blood stem cells instead of bone marrow cells,” Dr Rondelli said. “These two modifications helped ensure the patient’s body could accept the healthy donor cells.”
Modified regimen
Patients received growth-factor-mobilized peripheral blood stem cells after conditioning with rabbit antithymocyte globulin (0.5 mg/kg on day -9, 2 mg/kg on day -8 and -7), cyclophosphamide (14.5 mg/kg on day -6 and -5), fludarabine (30 mg/m2 on day -6 to -2), and single-dose TBI (3 Gy on day -1).
For GVHD prophylaxis, patients received intravenous cyclophosphamide (50 mg/kg on day 3 and 4), oral mycophenolate mofetil (15 mg/kg 3 times daily from day 5 to 35), and sirolimus (from day 5 dosed for a target trough of 5 to 15 ng/mL). In patients who had T-cell chimerism greater than 50% at 1 year after HSCT and did not have signs of GVHD, sirolimus was tapered off over 3 months.
Patients stopped taking hydroxyurea on day -9. They received red blood cell exchange transfusion on day -10 (with the goal of getting hemoglobin S below 30%) and received platelet transfusions to maintain platelet counts greater than 50 x 109 cells/L.
Patients also received penicillin V (250 mg twice daily) in addition to standard antimicrobial prophylaxis.
Results
All 8 patients on the modified regimen engrafted. The median time to neutrophil engraftment was 22 days (range, 18 to 23 days). One patient experienced secondary graft failure on day 90.
Seven neutropenic patients with hemoglobin S less than 30% received G-CSF after transplant. They received a median of 7 doses (range, 3 to 14) at 5 μg/kg, starting at day 12 post-HSCT. One of these patients experienced mild bone pain in the lower extremities.
Two patients developed GVHD. At day 83, one patient developed acute or chronic GVHD involving the skin, liver, and eyes. Steroids and sirolimus improved eye and liver symptoms, but the patient died at home on day 407.
The other patient had grade 2, gastrointestinal, acute GVHD that resolved with steroid therapy.
Three patients had grade 2 or higher mucositis, and 2 had cytomegalovirus (CMV) reactivation without CMV infection.
Two patients had small subarachnoid hemorrhages. One of these patients had a history of multiple red blood cell antibodies, became refractory to platelet transfusions, and developed small subarachnoid hemorrhages day 10. The patient’s symptoms and brain imaging resolved after platelet counts were maintained above 50 x 109 cells/L with cross-matched platelets.
The second patient had a history of stroke, experienced a seizure when the platelet count was 68 x 109 cells/L, and was found to have a subarachnoid hemorrhage on day 12. Symptoms and imaging results improved once the patient began levetiracetam and platelet levels were maintained above 100 x 109 cells/L.
At a median follow-up of 17 months (range, 12 to 30), 7 of the 8 patients were still alive.
Six patients had maintained greater than 95% stable donor engraftment with improvements in their hemoglobin concentrations. Three of these patients have stopped immunosuppression, and 3 are being tapered off it.
“These patients are cured of sickle cell disease,” Dr Rondelli said. “The takeaway message is two-fold. First, this transplant protocol may cure many more adult patients with advanced sickle cell disease.”
“Second, despite the increasing safety of the transplant protocols and new compatibility of HLA half-matched donors, many sickle cell patients still face barriers to care. Of the patients we screened, only 20% underwent a transplant.”
Dr Rondelli noted that 20% of the patients screened could not undergo transplant because of insurance denial. Other patients were ineligible because they had high rates of donor-specific antigens, and still others declined transplant.
A haploidentical transplant regimen has led to long-term engraftment in adults with sickle cell disease (SCD), according to researchers.
Seven of 8 patients treated with this regimen were still alive at last follow-up, and 6 of them maintained engraftment.
Two patients developed graft-versus-host disease (GVHD). One of them had acute and chronic GVHD and died about 400 days after transplant. The other had acute GVHD that resolved with treatment.
Damiano Rondelli, MD, of the University of Illinois at Chicago, and his colleagues reported these results in Biology of Blood and Marrow Transplantation.
The researchers initially screened 50 adult SCD patients as candidates for haploidentical hematopoietic stem cell transplant (haplo-HSCT) between January 2014 and March 2017. Most patients were ineligible or declined the procedure.
Ultimately, 10 patients received a haplo-HSCT. Unfortunately, the first 2 patients failed to engraft. These patients had received conditioning with alemtuzumab and 3 Gy total body irradiation (TBI) as well as post-transplant cyclophosphamide.
Because of this failure, the researchers used the following regimen for the remaining 8 patients. It’s a modified version of the regimen described in Blood in 2012.
“We modified the transplant protocol by increasing the dose of radiation used before the transplant and by infusing growth factor-mobilized peripheral blood stem cells instead of bone marrow cells,” Dr Rondelli said. “These two modifications helped ensure the patient’s body could accept the healthy donor cells.”
Modified regimen
Patients received growth-factor-mobilized peripheral blood stem cells after conditioning with rabbit antithymocyte globulin (0.5 mg/kg on day -9, 2 mg/kg on day -8 and -7), cyclophosphamide (14.5 mg/kg on day -6 and -5), fludarabine (30 mg/m2 on day -6 to -2), and single-dose TBI (3 Gy on day -1).
For GVHD prophylaxis, patients received intravenous cyclophosphamide (50 mg/kg on day 3 and 4), oral mycophenolate mofetil (15 mg/kg 3 times daily from day 5 to 35), and sirolimus (from day 5 dosed for a target trough of 5 to 15 ng/mL). In patients who had T-cell chimerism greater than 50% at 1 year after HSCT and did not have signs of GVHD, sirolimus was tapered off over 3 months.
Patients stopped taking hydroxyurea on day -9. They received red blood cell exchange transfusion on day -10 (with the goal of getting hemoglobin S below 30%) and received platelet transfusions to maintain platelet counts greater than 50 x 109 cells/L.
Patients also received penicillin V (250 mg twice daily) in addition to standard antimicrobial prophylaxis.
Results
All 8 patients on the modified regimen engrafted. The median time to neutrophil engraftment was 22 days (range, 18 to 23 days). One patient experienced secondary graft failure on day 90.
Seven neutropenic patients with hemoglobin S less than 30% received G-CSF after transplant. They received a median of 7 doses (range, 3 to 14) at 5 μg/kg, starting at day 12 post-HSCT. One of these patients experienced mild bone pain in the lower extremities.
Two patients developed GVHD. At day 83, one patient developed acute or chronic GVHD involving the skin, liver, and eyes. Steroids and sirolimus improved eye and liver symptoms, but the patient died at home on day 407.
The other patient had grade 2, gastrointestinal, acute GVHD that resolved with steroid therapy.
Three patients had grade 2 or higher mucositis, and 2 had cytomegalovirus (CMV) reactivation without CMV infection.
Two patients had small subarachnoid hemorrhages. One of these patients had a history of multiple red blood cell antibodies, became refractory to platelet transfusions, and developed small subarachnoid hemorrhages day 10. The patient’s symptoms and brain imaging resolved after platelet counts were maintained above 50 x 109 cells/L with cross-matched platelets.
The second patient had a history of stroke, experienced a seizure when the platelet count was 68 x 109 cells/L, and was found to have a subarachnoid hemorrhage on day 12. Symptoms and imaging results improved once the patient began levetiracetam and platelet levels were maintained above 100 x 109 cells/L.
At a median follow-up of 17 months (range, 12 to 30), 7 of the 8 patients were still alive.
Six patients had maintained greater than 95% stable donor engraftment with improvements in their hemoglobin concentrations. Three of these patients have stopped immunosuppression, and 3 are being tapered off it.
“These patients are cured of sickle cell disease,” Dr Rondelli said. “The takeaway message is two-fold. First, this transplant protocol may cure many more adult patients with advanced sickle cell disease.”
“Second, despite the increasing safety of the transplant protocols and new compatibility of HLA half-matched donors, many sickle cell patients still face barriers to care. Of the patients we screened, only 20% underwent a transplant.”
Dr Rondelli noted that 20% of the patients screened could not undergo transplant because of insurance denial. Other patients were ineligible because they had high rates of donor-specific antigens, and still others declined transplant.
Health Canada approves blood system for platelets
Health Canada has approved commercialization of the INTERCEPT Blood System for platelets, which is used for the ex vivo treatment and storage of platelet components.
The INTERCEPT Blood System for platelets can inactivate a range of pathogens—including viruses, bacteria, and protozoan parasites—to reduce the risk of transfusion-transmitted infections.
The system also inactivates contaminating donor leukocytes to reduce the risk of transfusion-associated graft-versus-host disease.
“[The INTERCEPT Blood System for platelets] provides an important, proactive safety measure to reduce the risk of transfusion-transmitted infections from known and emerging pathogens,” said Carol Moore, of Cerus Corporation, the company marketing the system.
“The approval provides blood centers the flexibility to pathogen-inactivate platelets derived from whole blood or apheresis collections, and stored in either platelet additive solution or plasma. In addition, Canadian blood centers will be able to create larger platelet pools from whole blood collections and use our double-dose INTERCEPT kits to yield 2 therapeutic platelet doses to realize improved operational efficiencies and economics.”
The INTERCEPT Blood System for platelets is Cerus Corporation’s second pathogen-inactivated system to be approved in Canada. The INTERCEPT Blood System for plasma was approved in May 2016.
The platelet and plasma systems use the same illumination device, the same active compound (amotosalen), and very similar production steps.
The INTERCEPT systems target a basic biological difference between the therapeutic components of blood. Platelets, plasma, and red blood cells do not require functional DNA or RNA for therapeutic efficacy. But pathogens and white blood cells do, in order to transmit infection.
The INTERCEPT systems use a proprietary molecule (amotosalen) that, when activated by UVA light, binds to and blocks the replication of DNA and RNA, preventing nucleic acid replication and rendering the pathogen inactive.
The INTERCEPT Blood System for platelets has been approved in Europe since 2002 and in the US since 2014.
Health Canada has approved commercialization of the INTERCEPT Blood System for platelets, which is used for the ex vivo treatment and storage of platelet components.
The INTERCEPT Blood System for platelets can inactivate a range of pathogens—including viruses, bacteria, and protozoan parasites—to reduce the risk of transfusion-transmitted infections.
The system also inactivates contaminating donor leukocytes to reduce the risk of transfusion-associated graft-versus-host disease.
“[The INTERCEPT Blood System for platelets] provides an important, proactive safety measure to reduce the risk of transfusion-transmitted infections from known and emerging pathogens,” said Carol Moore, of Cerus Corporation, the company marketing the system.
“The approval provides blood centers the flexibility to pathogen-inactivate platelets derived from whole blood or apheresis collections, and stored in either platelet additive solution or plasma. In addition, Canadian blood centers will be able to create larger platelet pools from whole blood collections and use our double-dose INTERCEPT kits to yield 2 therapeutic platelet doses to realize improved operational efficiencies and economics.”
The INTERCEPT Blood System for platelets is Cerus Corporation’s second pathogen-inactivated system to be approved in Canada. The INTERCEPT Blood System for plasma was approved in May 2016.
The platelet and plasma systems use the same illumination device, the same active compound (amotosalen), and very similar production steps.
The INTERCEPT systems target a basic biological difference between the therapeutic components of blood. Platelets, plasma, and red blood cells do not require functional DNA or RNA for therapeutic efficacy. But pathogens and white blood cells do, in order to transmit infection.
The INTERCEPT systems use a proprietary molecule (amotosalen) that, when activated by UVA light, binds to and blocks the replication of DNA and RNA, preventing nucleic acid replication and rendering the pathogen inactive.
The INTERCEPT Blood System for platelets has been approved in Europe since 2002 and in the US since 2014.
Health Canada has approved commercialization of the INTERCEPT Blood System for platelets, which is used for the ex vivo treatment and storage of platelet components.
The INTERCEPT Blood System for platelets can inactivate a range of pathogens—including viruses, bacteria, and protozoan parasites—to reduce the risk of transfusion-transmitted infections.
The system also inactivates contaminating donor leukocytes to reduce the risk of transfusion-associated graft-versus-host disease.
“[The INTERCEPT Blood System for platelets] provides an important, proactive safety measure to reduce the risk of transfusion-transmitted infections from known and emerging pathogens,” said Carol Moore, of Cerus Corporation, the company marketing the system.
“The approval provides blood centers the flexibility to pathogen-inactivate platelets derived from whole blood or apheresis collections, and stored in either platelet additive solution or plasma. In addition, Canadian blood centers will be able to create larger platelet pools from whole blood collections and use our double-dose INTERCEPT kits to yield 2 therapeutic platelet doses to realize improved operational efficiencies and economics.”
The INTERCEPT Blood System for platelets is Cerus Corporation’s second pathogen-inactivated system to be approved in Canada. The INTERCEPT Blood System for plasma was approved in May 2016.
The platelet and plasma systems use the same illumination device, the same active compound (amotosalen), and very similar production steps.
The INTERCEPT systems target a basic biological difference between the therapeutic components of blood. Platelets, plasma, and red blood cells do not require functional DNA or RNA for therapeutic efficacy. But pathogens and white blood cells do, in order to transmit infection.
The INTERCEPT systems use a proprietary molecule (amotosalen) that, when activated by UVA light, binds to and blocks the replication of DNA and RNA, preventing nucleic acid replication and rendering the pathogen inactive.
The INTERCEPT Blood System for platelets has been approved in Europe since 2002 and in the US since 2014.
CDK inhibitor synergizes with venetoclax in CLL
CHICAGO—Researchers have reported “strong synergy” between the CDK2/9 inhibitor CYC065 and the Bcl-2 inhibitor venetoclax in chronic lymphocytic leukemia (CLL).
Experiments indicated that CYC065 and venetoclax target parallel mechanisms that promote survival in CLL cells, working together to induce apoptosis.
The drugs demonstrated synergy even in CLL samples that are inherently resistant to each drug alone.
William Plunkett, PhD, of The University of Texas MD Anderson Cancer Center in Houston, Texas, and his colleagues reported these findings at the AACR Annual Meeting 2018 (abstract 3905).
This research was supported by Cyclacel Pharmaceuticals, Inc., the company developing CYC065.
The researchers explained that CYC065 depletes Mcl-1 to induce apoptosis in CLL cells, while venetoclax induces apoptosis via inhibition of Bcl-2. However, upregulation of Mcl-1 is associated with resistance to venetoclax.
Therefore, the researchers theorized that combining CYC065 and venetoclax would serve to target 2 mechanisms that promote survival in CLL cells.
Experiments showed that CYC065 and venetoclax combined synergistically in CLL samples with or without 17p deletion. However, the researchers observed heterogeneity in response across samples.
The team said both drugs appeared to be less potent in some del(17p) samples. However, they also observed “great synergy” in del(17p) samples that were resistant to CYC065 or venetoclax alone.
The researchers noted differences in the kinetics of cell death in response to each drug and said this is consistent with the drugs’ different mechanisms of action.
Maximal cell death was reached at 6 to 8 hours with venetoclax but took at least 24 hours with CYC065.
The researchers also assessed the reversibility of CYC065 and venetoclax. They incubated CLL cells with each drug alone and in combination, then washed and incubated cells in drug-free media.
The team observed no additional cell death after the removal of CYC065, venetoclax, or the combination. They said this suggests an “adequate exposure time” is needed to maximize the induction of apoptosis with these drugs.
“[T]he combination of CYC065 and venetoclax is strongly synergistic in primary CLL cells from patients, including those with 17p deletions,” said Spiro Rombotis, president and chief executive officer of Cyclacel.
“In addition, the combination was active in 2 CLL samples which were resistant to either agent alone. These findings support the hypothesis that dual targeting of the Mcl-1- and Bcl-2-dependent mechanisms could induce synergistic cell death by apoptosis.”
Based on these results, Cyclacel is planning a trial of CYC065 and venetoclax in patients with relapsed/refractory CLL.
CHICAGO—Researchers have reported “strong synergy” between the CDK2/9 inhibitor CYC065 and the Bcl-2 inhibitor venetoclax in chronic lymphocytic leukemia (CLL).
Experiments indicated that CYC065 and venetoclax target parallel mechanisms that promote survival in CLL cells, working together to induce apoptosis.
The drugs demonstrated synergy even in CLL samples that are inherently resistant to each drug alone.
William Plunkett, PhD, of The University of Texas MD Anderson Cancer Center in Houston, Texas, and his colleagues reported these findings at the AACR Annual Meeting 2018 (abstract 3905).
This research was supported by Cyclacel Pharmaceuticals, Inc., the company developing CYC065.
The researchers explained that CYC065 depletes Mcl-1 to induce apoptosis in CLL cells, while venetoclax induces apoptosis via inhibition of Bcl-2. However, upregulation of Mcl-1 is associated with resistance to venetoclax.
Therefore, the researchers theorized that combining CYC065 and venetoclax would serve to target 2 mechanisms that promote survival in CLL cells.
Experiments showed that CYC065 and venetoclax combined synergistically in CLL samples with or without 17p deletion. However, the researchers observed heterogeneity in response across samples.
The team said both drugs appeared to be less potent in some del(17p) samples. However, they also observed “great synergy” in del(17p) samples that were resistant to CYC065 or venetoclax alone.
The researchers noted differences in the kinetics of cell death in response to each drug and said this is consistent with the drugs’ different mechanisms of action.
Maximal cell death was reached at 6 to 8 hours with venetoclax but took at least 24 hours with CYC065.
The researchers also assessed the reversibility of CYC065 and venetoclax. They incubated CLL cells with each drug alone and in combination, then washed and incubated cells in drug-free media.
The team observed no additional cell death after the removal of CYC065, venetoclax, or the combination. They said this suggests an “adequate exposure time” is needed to maximize the induction of apoptosis with these drugs.
“[T]he combination of CYC065 and venetoclax is strongly synergistic in primary CLL cells from patients, including those with 17p deletions,” said Spiro Rombotis, president and chief executive officer of Cyclacel.
“In addition, the combination was active in 2 CLL samples which were resistant to either agent alone. These findings support the hypothesis that dual targeting of the Mcl-1- and Bcl-2-dependent mechanisms could induce synergistic cell death by apoptosis.”
Based on these results, Cyclacel is planning a trial of CYC065 and venetoclax in patients with relapsed/refractory CLL.
CHICAGO—Researchers have reported “strong synergy” between the CDK2/9 inhibitor CYC065 and the Bcl-2 inhibitor venetoclax in chronic lymphocytic leukemia (CLL).
Experiments indicated that CYC065 and venetoclax target parallel mechanisms that promote survival in CLL cells, working together to induce apoptosis.
The drugs demonstrated synergy even in CLL samples that are inherently resistant to each drug alone.
William Plunkett, PhD, of The University of Texas MD Anderson Cancer Center in Houston, Texas, and his colleagues reported these findings at the AACR Annual Meeting 2018 (abstract 3905).
This research was supported by Cyclacel Pharmaceuticals, Inc., the company developing CYC065.
The researchers explained that CYC065 depletes Mcl-1 to induce apoptosis in CLL cells, while venetoclax induces apoptosis via inhibition of Bcl-2. However, upregulation of Mcl-1 is associated with resistance to venetoclax.
Therefore, the researchers theorized that combining CYC065 and venetoclax would serve to target 2 mechanisms that promote survival in CLL cells.
Experiments showed that CYC065 and venetoclax combined synergistically in CLL samples with or without 17p deletion. However, the researchers observed heterogeneity in response across samples.
The team said both drugs appeared to be less potent in some del(17p) samples. However, they also observed “great synergy” in del(17p) samples that were resistant to CYC065 or venetoclax alone.
The researchers noted differences in the kinetics of cell death in response to each drug and said this is consistent with the drugs’ different mechanisms of action.
Maximal cell death was reached at 6 to 8 hours with venetoclax but took at least 24 hours with CYC065.
The researchers also assessed the reversibility of CYC065 and venetoclax. They incubated CLL cells with each drug alone and in combination, then washed and incubated cells in drug-free media.
The team observed no additional cell death after the removal of CYC065, venetoclax, or the combination. They said this suggests an “adequate exposure time” is needed to maximize the induction of apoptosis with these drugs.
“[T]he combination of CYC065 and venetoclax is strongly synergistic in primary CLL cells from patients, including those with 17p deletions,” said Spiro Rombotis, president and chief executive officer of Cyclacel.
“In addition, the combination was active in 2 CLL samples which were resistant to either agent alone. These findings support the hypothesis that dual targeting of the Mcl-1- and Bcl-2-dependent mechanisms could induce synergistic cell death by apoptosis.”
Based on these results, Cyclacel is planning a trial of CYC065 and venetoclax in patients with relapsed/refractory CLL.
Unusual patch of skin
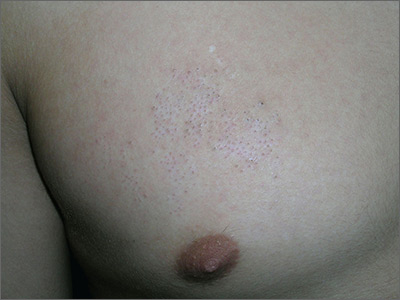
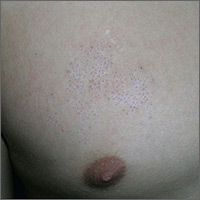
The physician diagnosed nevus comedonicus on the chest of this 15-year-old boy.
This is a congenital hamartoma with open comedones; it is not acne. Nevus comedonicus (comedonal nevus) is a rare congenital hamartoma characterized by an aggregation of comedones in one region of the skin. Comedonal nevi do not become melanoma because they lack melanocytes. Therefore, there is no reason to excise them other than for cosmetic reasons. However, comedonal nevi are large and the risks of excision outweigh the benefits.
In this case, the patient and his mother were reassured that no treatment was needed and they opted to leave it alone.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The physician diagnosed nevus comedonicus on the chest of this 15-year-old boy.
This is a congenital hamartoma with open comedones; it is not acne. Nevus comedonicus (comedonal nevus) is a rare congenital hamartoma characterized by an aggregation of comedones in one region of the skin. Comedonal nevi do not become melanoma because they lack melanocytes. Therefore, there is no reason to excise them other than for cosmetic reasons. However, comedonal nevi are large and the risks of excision outweigh the benefits.
In this case, the patient and his mother were reassured that no treatment was needed and they opted to leave it alone.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The physician diagnosed nevus comedonicus on the chest of this 15-year-old boy.
This is a congenital hamartoma with open comedones; it is not acne. Nevus comedonicus (comedonal nevus) is a rare congenital hamartoma characterized by an aggregation of comedones in one region of the skin. Comedonal nevi do not become melanoma because they lack melanocytes. Therefore, there is no reason to excise them other than for cosmetic reasons. However, comedonal nevi are large and the risks of excision outweigh the benefits.
In this case, the patient and his mother were reassured that no treatment was needed and they opted to leave it alone.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Walking the walk
In March 2018, the Human Rights Campaign (HRC), an advocacy organization dedicated to improving the lives of LGBTQ people, released its 11th Annual Healthcare Equality Index. The HEI is an indicator of how inclusive and equitable health care facilities are in providing care for their LGBTQ patients. My own institution, Children’s Hospital of Pittsburgh, scored very high on this index and received the “Leader in LGBTQ Healthcare Equality” designation. The process of receiving this designation is very rigorous, and I am proud of my institution for making great strides in expanding health care access for LGBTQ patients, especially transgender patients. However, this is no time to rest on one’s laurels, as many transgender people still experience challenges and barriers in navigating the health care system.
Insurance access continues to be a problem. I wrote a column in June 2017 about obtaining health care insurance for transgender patients. Preauthorization is common for obtaining cross-sex hormones or pubertal blockers even for insurance companies that are willing to pay for them – a process that can take weeks, even months, to complete. This creates delays in obtaining necessary care for transgender patients. This is just one of the many barriers transgender people face in navigating the health care system.
Increasing access to health care services for transgender patients is more about improving health outcomes than patient satisfaction. Even the smallest policy change may have a meaningful impact on the lives of transgender individuals. A study by Russell et al., in the April 2018 issue of Journal of Adolescent Health found that transgender youth allowed to use their chosen name (instead of the name assigned to them by their parents at birth) were more likely to have fewer depressive symptoms and lower rates of suicidal ideation and suicidal behavior.3 These findings highlight that even a small change can have a huge impact on the health and well-being of this patient population.
What can you do to expand access? First, you must educate yourself and teach others. Many providers report never having received education on LGBTQ health during their training,4 and most barriers for transgender patients stem from this lack of training. Second, work with the transgender community – it is very tempting to see your institution’s name on the HEI and think all the work is done, but the lived experiences of transgender patients sometimes are different than what is seen on paper (or online). Team up with local organizations such as PFLAG (formerly known as Parents and Families of Lesbians and Gays) that can create support groups for both transgender youth and their families. Help create a network of referral systems for your transgender patients – the community is often small enough that they know which providers or establishments are safe for transgender individuals. Many transgender patients find this extremely helpful.5 You still wield significant influence in the community, so work with the health care and insurance systems to improve access and coverage for gender-related services. The HRC HEI is becoming coveted by health care institutions. This is a prime opportunity to be involved in committees seeking to improve health care access for transgender individuals. Finally, as there are champions for transgender health in your clinic, there also are champions for transgender health in insurance companies. They often are well known in the community, so find that individual for counseling on how to navigate the insurance system for your transgender patients.
Although an increasing number of health care institutions and clinics are recognizing the health care needs of transgender patients and providing appropriate care, the health care system remains challenging for transgender individuals to navigate. Small policy changes may have a substantial impact on the health and well-being of transgender individuals. Although creating change within an institution may seem like a monumental task, you do have the agency to help create this type change within the system to expand health care access for transgender patients.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resources
- HRC HEI: If you’re interested in learning what policies are inclusive and equitable for LGBT patients, check out the HRC HEI scoring criteria. It’s a good place to start if you want to expand health care access for transgender individuals.
- To find out more about the health care legal protections transgender individuals are entitled to, check out the National Center for Transgender Equality.
References
1. Psychol Bull. 2003 Sep;129(5):674-97.
2. “Injustice at every turn: A report of the National Transgender Discrimination Survey.” (Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.)
3. J. Adolesc Health. 2018 Apr. doi: 10.1016/j.jadohealth.2018.02.003.
4. Int J Transgenderism. 2008. doi: 10.1300/J485v08n02_08.
5. Transgend Health. 2016 Nov 1;1(1):238-49.
In March 2018, the Human Rights Campaign (HRC), an advocacy organization dedicated to improving the lives of LGBTQ people, released its 11th Annual Healthcare Equality Index. The HEI is an indicator of how inclusive and equitable health care facilities are in providing care for their LGBTQ patients. My own institution, Children’s Hospital of Pittsburgh, scored very high on this index and received the “Leader in LGBTQ Healthcare Equality” designation. The process of receiving this designation is very rigorous, and I am proud of my institution for making great strides in expanding health care access for LGBTQ patients, especially transgender patients. However, this is no time to rest on one’s laurels, as many transgender people still experience challenges and barriers in navigating the health care system.
Insurance access continues to be a problem. I wrote a column in June 2017 about obtaining health care insurance for transgender patients. Preauthorization is common for obtaining cross-sex hormones or pubertal blockers even for insurance companies that are willing to pay for them – a process that can take weeks, even months, to complete. This creates delays in obtaining necessary care for transgender patients. This is just one of the many barriers transgender people face in navigating the health care system.
Increasing access to health care services for transgender patients is more about improving health outcomes than patient satisfaction. Even the smallest policy change may have a meaningful impact on the lives of transgender individuals. A study by Russell et al., in the April 2018 issue of Journal of Adolescent Health found that transgender youth allowed to use their chosen name (instead of the name assigned to them by their parents at birth) were more likely to have fewer depressive symptoms and lower rates of suicidal ideation and suicidal behavior.3 These findings highlight that even a small change can have a huge impact on the health and well-being of this patient population.
What can you do to expand access? First, you must educate yourself and teach others. Many providers report never having received education on LGBTQ health during their training,4 and most barriers for transgender patients stem from this lack of training. Second, work with the transgender community – it is very tempting to see your institution’s name on the HEI and think all the work is done, but the lived experiences of transgender patients sometimes are different than what is seen on paper (or online). Team up with local organizations such as PFLAG (formerly known as Parents and Families of Lesbians and Gays) that can create support groups for both transgender youth and their families. Help create a network of referral systems for your transgender patients – the community is often small enough that they know which providers or establishments are safe for transgender individuals. Many transgender patients find this extremely helpful.5 You still wield significant influence in the community, so work with the health care and insurance systems to improve access and coverage for gender-related services. The HRC HEI is becoming coveted by health care institutions. This is a prime opportunity to be involved in committees seeking to improve health care access for transgender individuals. Finally, as there are champions for transgender health in your clinic, there also are champions for transgender health in insurance companies. They often are well known in the community, so find that individual for counseling on how to navigate the insurance system for your transgender patients.
Although an increasing number of health care institutions and clinics are recognizing the health care needs of transgender patients and providing appropriate care, the health care system remains challenging for transgender individuals to navigate. Small policy changes may have a substantial impact on the health and well-being of transgender individuals. Although creating change within an institution may seem like a monumental task, you do have the agency to help create this type change within the system to expand health care access for transgender patients.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resources
- HRC HEI: If you’re interested in learning what policies are inclusive and equitable for LGBT patients, check out the HRC HEI scoring criteria. It’s a good place to start if you want to expand health care access for transgender individuals.
- To find out more about the health care legal protections transgender individuals are entitled to, check out the National Center for Transgender Equality.
References
1. Psychol Bull. 2003 Sep;129(5):674-97.
2. “Injustice at every turn: A report of the National Transgender Discrimination Survey.” (Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.)
3. J. Adolesc Health. 2018 Apr. doi: 10.1016/j.jadohealth.2018.02.003.
4. Int J Transgenderism. 2008. doi: 10.1300/J485v08n02_08.
5. Transgend Health. 2016 Nov 1;1(1):238-49.
In March 2018, the Human Rights Campaign (HRC), an advocacy organization dedicated to improving the lives of LGBTQ people, released its 11th Annual Healthcare Equality Index. The HEI is an indicator of how inclusive and equitable health care facilities are in providing care for their LGBTQ patients. My own institution, Children’s Hospital of Pittsburgh, scored very high on this index and received the “Leader in LGBTQ Healthcare Equality” designation. The process of receiving this designation is very rigorous, and I am proud of my institution for making great strides in expanding health care access for LGBTQ patients, especially transgender patients. However, this is no time to rest on one’s laurels, as many transgender people still experience challenges and barriers in navigating the health care system.
Insurance access continues to be a problem. I wrote a column in June 2017 about obtaining health care insurance for transgender patients. Preauthorization is common for obtaining cross-sex hormones or pubertal blockers even for insurance companies that are willing to pay for them – a process that can take weeks, even months, to complete. This creates delays in obtaining necessary care for transgender patients. This is just one of the many barriers transgender people face in navigating the health care system.
Increasing access to health care services for transgender patients is more about improving health outcomes than patient satisfaction. Even the smallest policy change may have a meaningful impact on the lives of transgender individuals. A study by Russell et al., in the April 2018 issue of Journal of Adolescent Health found that transgender youth allowed to use their chosen name (instead of the name assigned to them by their parents at birth) were more likely to have fewer depressive symptoms and lower rates of suicidal ideation and suicidal behavior.3 These findings highlight that even a small change can have a huge impact on the health and well-being of this patient population.
What can you do to expand access? First, you must educate yourself and teach others. Many providers report never having received education on LGBTQ health during their training,4 and most barriers for transgender patients stem from this lack of training. Second, work with the transgender community – it is very tempting to see your institution’s name on the HEI and think all the work is done, but the lived experiences of transgender patients sometimes are different than what is seen on paper (or online). Team up with local organizations such as PFLAG (formerly known as Parents and Families of Lesbians and Gays) that can create support groups for both transgender youth and their families. Help create a network of referral systems for your transgender patients – the community is often small enough that they know which providers or establishments are safe for transgender individuals. Many transgender patients find this extremely helpful.5 You still wield significant influence in the community, so work with the health care and insurance systems to improve access and coverage for gender-related services. The HRC HEI is becoming coveted by health care institutions. This is a prime opportunity to be involved in committees seeking to improve health care access for transgender individuals. Finally, as there are champions for transgender health in your clinic, there also are champions for transgender health in insurance companies. They often are well known in the community, so find that individual for counseling on how to navigate the insurance system for your transgender patients.
Although an increasing number of health care institutions and clinics are recognizing the health care needs of transgender patients and providing appropriate care, the health care system remains challenging for transgender individuals to navigate. Small policy changes may have a substantial impact on the health and well-being of transgender individuals. Although creating change within an institution may seem like a monumental task, you do have the agency to help create this type change within the system to expand health care access for transgender patients.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resources
- HRC HEI: If you’re interested in learning what policies are inclusive and equitable for LGBT patients, check out the HRC HEI scoring criteria. It’s a good place to start if you want to expand health care access for transgender individuals.
- To find out more about the health care legal protections transgender individuals are entitled to, check out the National Center for Transgender Equality.
References
1. Psychol Bull. 2003 Sep;129(5):674-97.
2. “Injustice at every turn: A report of the National Transgender Discrimination Survey.” (Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.)
3. J. Adolesc Health. 2018 Apr. doi: 10.1016/j.jadohealth.2018.02.003.
4. Int J Transgenderism. 2008. doi: 10.1300/J485v08n02_08.
5. Transgend Health. 2016 Nov 1;1(1):238-49.
What’s in a name? Get ready for the feds’ Promoting Interoperability program
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.
The federal EHR Incentive Program – a program most doctors love to hate – is getting a new name to better reflect a focus on interoperability and improved patient access to their health care data, the Centers for Medicare & Medicaid Services announced.
For clinicians who participate in fee-for-service Medicare, eligible hospitals, and critical access hospitals, the new name of the program will be the Promoting Interoperability Program. For those participating in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program (QPP), the Advancing Care Information performance category will be rebranded the Promoting Interoperability performance category.
The first steps of these changes were announced April 25 in the 2019 proposed rule for the inpatient prospective payment system and the Long-Term Care Hospital Prospective Payment System.
CMS notes in the proposed rule that the name EHR Incentive Program “does not adequately reflect the current status of the programs, as the incentive payments under Medicare generally have ended.” Eligible medical professionals and hospitals have received Medicare bonuses for adopting and demonstrating meaningful use of EHRs. Penalties for not doing so are still in place.
Intrauterine therapy showed promise in XLHED
Intra-amniotic therapy with a novel recombinant protein enabled three patients with X-linked hypohidrotic ectodermal dysplasia (XLHED) to sweat normally, researchers reported.
For up to 22 months of postnatal follow-up, patients had no hyperthermia and were not hospitalized for respiratory reasons, reported Holm Schneider, MD, of the University of Erlangen-Nürnberg, Erlangen, Germany, and his associates. Treatment may have induced premature delivery at 33 weeks of a pair of twins, although most twins are born preterm, they noted. “Combined with the ability to identify affected fetuses through noninvasive sonographic prenatal screening, the approach we describe here represents a new means of protein-replacement therapy to correct XLHED,” they wrote online April 25 in the New England Journal of Medicine.
XLHED is caused by loss-of-function variants of the gene encoding ectodysplasin A (EDA). The investigational recombinant fusion protein Fc-EDA (EDI200) developed by Edimer Pharmaceuticals, which contains the receptor-binding domain of EDA and the Fc domain of human immunoglobulin G1, has shown no signs of toxicity in nonhuman primates. In prior studies, its intra-amniotic infusion prevented XLHED in EDA-deficient murine fetuses, while human postnatal Fc-EDA therapy was ineffective (NCT01775462).
Based on these data, University Hospital Erlangan approved a parental request for compassionate use of Fc-EDA in male twin fetuses with genetic deficiency of EDA. Treatment (100 mg/kg estimated fetal body weight) occurred at gestational weeks 26 and 31. Despite premature delivery at 33 weeks, 5-minute Apgar scores were 9 for one twin and 10 for the other. Cord blood testing detected Fc-EDA, suggesting its continuous uptake into fetal blood. The twins both had normal sweat-duct density, sweated as much as healthy controls, salivated normally, and had 8-10 tooth germs; their 5-year-old brother with XLHED had only three teeth and one tooth germ.
Parents of another EDA-deficient fetus also requested compassionate use of Fc-EDA, which was administrated as a single dose (because of limited supply) at gestational week 26. Birth occurred at week 39 and Apgar scores all were 10s. Sweat pore density was slightly low, compared with healthy controls, and by age 4 months, the patient had developed moderate urticaria pigmentosa.
In all cases, maternal circulation showed no trace of Fc-EDA within 24 hours of treatment.
Funders included Edimer Pharmaceuticals, Deutsche Forschungsgemeinschaft, Swiss National Science Foundation, the German-Swiss-Austrian ectodermal dysplasia patient organization, and the National Foundation for Ectodermal Dysplasias. Three of the investigators have either patents issued or patents pending related to the treatment, one is an employee of Edimer Pharmaceuticals, and two have grants from some of the abovementioned companies or organizations.
SOURCE: Schneider H et al. N Engl J Med. 2018 Apr 25. doi: 10.1056/NEJMoa1714322.
These early findings are “remarkable and encouraging,” especially because routine ultrasonography can identify fetuses with X-linked hypohidrotic ectodermal dysplasia (XLHED), wrote Marja L. Mikkola, PhD.
Although the study was small, intranatal therapy with recombinant ectodysplasin A produced “sustained sweating ability,” normalized saliva production, and substantially corrected tooth bud count, Dr. Mikkola wrote in an editorial accompanying the study.
Treatment might have induced early delivery of the set of twins in this study, who were born at week 33, she noted. However, twins often are born early, and the study uncovered no other safety concerns. Taken together, the findings justify a larger trial of this new approach.
Dr. Mikkola is with the University of Helsinki (Finland). She reported having no conflicts of interest. These comments paraphrase her editorial (N Engl J Med. 2018 Apr 25. doi: 10.1056/NEJMe1803224).
These early findings are “remarkable and encouraging,” especially because routine ultrasonography can identify fetuses with X-linked hypohidrotic ectodermal dysplasia (XLHED), wrote Marja L. Mikkola, PhD.
Although the study was small, intranatal therapy with recombinant ectodysplasin A produced “sustained sweating ability,” normalized saliva production, and substantially corrected tooth bud count, Dr. Mikkola wrote in an editorial accompanying the study.
Treatment might have induced early delivery of the set of twins in this study, who were born at week 33, she noted. However, twins often are born early, and the study uncovered no other safety concerns. Taken together, the findings justify a larger trial of this new approach.
Dr. Mikkola is with the University of Helsinki (Finland). She reported having no conflicts of interest. These comments paraphrase her editorial (N Engl J Med. 2018 Apr 25. doi: 10.1056/NEJMe1803224).
These early findings are “remarkable and encouraging,” especially because routine ultrasonography can identify fetuses with X-linked hypohidrotic ectodermal dysplasia (XLHED), wrote Marja L. Mikkola, PhD.
Although the study was small, intranatal therapy with recombinant ectodysplasin A produced “sustained sweating ability,” normalized saliva production, and substantially corrected tooth bud count, Dr. Mikkola wrote in an editorial accompanying the study.
Treatment might have induced early delivery of the set of twins in this study, who were born at week 33, she noted. However, twins often are born early, and the study uncovered no other safety concerns. Taken together, the findings justify a larger trial of this new approach.
Dr. Mikkola is with the University of Helsinki (Finland). She reported having no conflicts of interest. These comments paraphrase her editorial (N Engl J Med. 2018 Apr 25. doi: 10.1056/NEJMe1803224).
Intra-amniotic therapy with a novel recombinant protein enabled three patients with X-linked hypohidrotic ectodermal dysplasia (XLHED) to sweat normally, researchers reported.
For up to 22 months of postnatal follow-up, patients had no hyperthermia and were not hospitalized for respiratory reasons, reported Holm Schneider, MD, of the University of Erlangen-Nürnberg, Erlangen, Germany, and his associates. Treatment may have induced premature delivery at 33 weeks of a pair of twins, although most twins are born preterm, they noted. “Combined with the ability to identify affected fetuses through noninvasive sonographic prenatal screening, the approach we describe here represents a new means of protein-replacement therapy to correct XLHED,” they wrote online April 25 in the New England Journal of Medicine.
XLHED is caused by loss-of-function variants of the gene encoding ectodysplasin A (EDA). The investigational recombinant fusion protein Fc-EDA (EDI200) developed by Edimer Pharmaceuticals, which contains the receptor-binding domain of EDA and the Fc domain of human immunoglobulin G1, has shown no signs of toxicity in nonhuman primates. In prior studies, its intra-amniotic infusion prevented XLHED in EDA-deficient murine fetuses, while human postnatal Fc-EDA therapy was ineffective (NCT01775462).
Based on these data, University Hospital Erlangan approved a parental request for compassionate use of Fc-EDA in male twin fetuses with genetic deficiency of EDA. Treatment (100 mg/kg estimated fetal body weight) occurred at gestational weeks 26 and 31. Despite premature delivery at 33 weeks, 5-minute Apgar scores were 9 for one twin and 10 for the other. Cord blood testing detected Fc-EDA, suggesting its continuous uptake into fetal blood. The twins both had normal sweat-duct density, sweated as much as healthy controls, salivated normally, and had 8-10 tooth germs; their 5-year-old brother with XLHED had only three teeth and one tooth germ.
Parents of another EDA-deficient fetus also requested compassionate use of Fc-EDA, which was administrated as a single dose (because of limited supply) at gestational week 26. Birth occurred at week 39 and Apgar scores all were 10s. Sweat pore density was slightly low, compared with healthy controls, and by age 4 months, the patient had developed moderate urticaria pigmentosa.
In all cases, maternal circulation showed no trace of Fc-EDA within 24 hours of treatment.
Funders included Edimer Pharmaceuticals, Deutsche Forschungsgemeinschaft, Swiss National Science Foundation, the German-Swiss-Austrian ectodermal dysplasia patient organization, and the National Foundation for Ectodermal Dysplasias. Three of the investigators have either patents issued or patents pending related to the treatment, one is an employee of Edimer Pharmaceuticals, and two have grants from some of the abovementioned companies or organizations.
SOURCE: Schneider H et al. N Engl J Med. 2018 Apr 25. doi: 10.1056/NEJMoa1714322.
Intra-amniotic therapy with a novel recombinant protein enabled three patients with X-linked hypohidrotic ectodermal dysplasia (XLHED) to sweat normally, researchers reported.
For up to 22 months of postnatal follow-up, patients had no hyperthermia and were not hospitalized for respiratory reasons, reported Holm Schneider, MD, of the University of Erlangen-Nürnberg, Erlangen, Germany, and his associates. Treatment may have induced premature delivery at 33 weeks of a pair of twins, although most twins are born preterm, they noted. “Combined with the ability to identify affected fetuses through noninvasive sonographic prenatal screening, the approach we describe here represents a new means of protein-replacement therapy to correct XLHED,” they wrote online April 25 in the New England Journal of Medicine.
XLHED is caused by loss-of-function variants of the gene encoding ectodysplasin A (EDA). The investigational recombinant fusion protein Fc-EDA (EDI200) developed by Edimer Pharmaceuticals, which contains the receptor-binding domain of EDA and the Fc domain of human immunoglobulin G1, has shown no signs of toxicity in nonhuman primates. In prior studies, its intra-amniotic infusion prevented XLHED in EDA-deficient murine fetuses, while human postnatal Fc-EDA therapy was ineffective (NCT01775462).
Based on these data, University Hospital Erlangan approved a parental request for compassionate use of Fc-EDA in male twin fetuses with genetic deficiency of EDA. Treatment (100 mg/kg estimated fetal body weight) occurred at gestational weeks 26 and 31. Despite premature delivery at 33 weeks, 5-minute Apgar scores were 9 for one twin and 10 for the other. Cord blood testing detected Fc-EDA, suggesting its continuous uptake into fetal blood. The twins both had normal sweat-duct density, sweated as much as healthy controls, salivated normally, and had 8-10 tooth germs; their 5-year-old brother with XLHED had only three teeth and one tooth germ.
Parents of another EDA-deficient fetus also requested compassionate use of Fc-EDA, which was administrated as a single dose (because of limited supply) at gestational week 26. Birth occurred at week 39 and Apgar scores all were 10s. Sweat pore density was slightly low, compared with healthy controls, and by age 4 months, the patient had developed moderate urticaria pigmentosa.
In all cases, maternal circulation showed no trace of Fc-EDA within 24 hours of treatment.
Funders included Edimer Pharmaceuticals, Deutsche Forschungsgemeinschaft, Swiss National Science Foundation, the German-Swiss-Austrian ectodermal dysplasia patient organization, and the National Foundation for Ectodermal Dysplasias. Three of the investigators have either patents issued or patents pending related to the treatment, one is an employee of Edimer Pharmaceuticals, and two have grants from some of the abovementioned companies or organizations.
SOURCE: Schneider H et al. N Engl J Med. 2018 Apr 25. doi: 10.1056/NEJMoa1714322.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Patients could sweat normally through up to 22 months of follow-up.
Study details: Intra-amniotic treatment of three fetuses with confirmed XLHED.
Disclosures: Funders included Edimer Pharmaceuticals, Deutsche Forschungsgemeinschaft, Swiss National Science Foundation, the German-Swiss-Austrian ectodermal dysplasia patient organization, and the National Foundation for Ectodermal Dysplasias. Three of the investigators have either patents issued or patents pending related to the treatment, one is an employee of Edimer Pharmaceuticals, and two have grants from some of the abovementioned companies or organizations.
Source: Schneider H et al. N Engl J Med. 2018 Apr 25. doi: 10.1056/NEJMoa1714322.
Antiretroviral choice for pregnant women with HIV does not appear to impact birth outcomes
Three different regimens of antiretroviral therapy did not produce significantly different adverse birth outcomes in women with HIV despite previous research showing an increased risk in premature birth or death in infants after a regimen of tenofovir, emtricitabine, and ritonavir-boosted lopinavir therapy, according to a recent analysis of two multicenter cohort studies published in the New England Journal of Medicine.
Kathryn Rough, ScD, of the Brigham and Women’s Hospital and Harvard Medical School in Boston and colleagues analyzed 4,646 birth outcomes in the SMARTT (NCT01310023) and P1025 (NCT00028145) trials from 3,847 unique women who received tenofovir, emtricitabine, and ritonavir-boosted lopinavir (TDF–FTC–LPV/r), zidovudine, lamivudine, and ritonavir-boosted lopinavir (ZDV–3TC–LPV/r), or TDF–FTC with ritonavir-boosted atazanavir (ATV/r) during gestation. There were 954 infants or fetuses exposed to ZDV–3TC–LPV/r (20.5%), 539 infants or fetuses exposed to TDF–FTC–ATV/r (11.6%), and 128 infants or fetuses exposed to TDF–FTC–LPV/r (2.8%), with 4,480 singleton, 80 twin, and 2 triplet pregnancies.
“Concerns regarding the use of TDF–FTC–LPV/r during pregnancy remain; further investigation is warranted to understand why women who initiated TDF–FTC– LPV/r before conception had higher risks of preterm birth, low birth weight, and any adverse outcome than women who initiated ZDV–3TC– LPV/r or TDF–FTC–ATV/r before conception in subgroup analyses.”
Overall, there was a premature birth risk between 16.1% and 21.4% and a low birth weight risk between 16.2% and 23.8% across all antiretroviral therapy regimens, with an overall adverse outcome rate between 23.7% and 28.1%. For women who received TDF–FTC–LPV/r, there was a risk ratio of 0.90 (95% confidence interval, 0.60-1.33) for preterm births, 1.13 for low birth weight (95% CI, 0.78-1.64) and 0.92 for any adverse outcome (95% CI, 0.67-1.28) compared with women who received ZDV–3TC–LPV/r.
“For the outcomes of preterm birth, low birth weight, and any adverse outcome, TDF–FTC–ATV/r appeared to have lower risks than the LPV/r-based regimens; however, many of these associations were not significant,” the authors wrote.
Women who received TDF–FTC–LPV/r had a risk ratio of 1.14 (95% CI, 0.75-1.72) and a low-birth-weight risk ratio of 1.45 (95% CI, 0.96-2.17), compared with women who received TDF-FTC-ATV/r. Regarding very-low-birth-weight and very-preterm birth, the researchers noted no significant differences among regimen groups.
One author reported stock from Abbott, AbbVie, Novartis, and Roche outside the submitted work; one author reported personal fees from Boehringer-Ingelheim; and five authors reported grants from pharmaceutical companies and Google outside the submitted work.
SOURCE: Rough K et al. N Engl J Med 2018;378:1593-603.
Three different regimens of antiretroviral therapy did not produce significantly different adverse birth outcomes in women with HIV despite previous research showing an increased risk in premature birth or death in infants after a regimen of tenofovir, emtricitabine, and ritonavir-boosted lopinavir therapy, according to a recent analysis of two multicenter cohort studies published in the New England Journal of Medicine.
Kathryn Rough, ScD, of the Brigham and Women’s Hospital and Harvard Medical School in Boston and colleagues analyzed 4,646 birth outcomes in the SMARTT (NCT01310023) and P1025 (NCT00028145) trials from 3,847 unique women who received tenofovir, emtricitabine, and ritonavir-boosted lopinavir (TDF–FTC–LPV/r), zidovudine, lamivudine, and ritonavir-boosted lopinavir (ZDV–3TC–LPV/r), or TDF–FTC with ritonavir-boosted atazanavir (ATV/r) during gestation. There were 954 infants or fetuses exposed to ZDV–3TC–LPV/r (20.5%), 539 infants or fetuses exposed to TDF–FTC–ATV/r (11.6%), and 128 infants or fetuses exposed to TDF–FTC–LPV/r (2.8%), with 4,480 singleton, 80 twin, and 2 triplet pregnancies.
“Concerns regarding the use of TDF–FTC–LPV/r during pregnancy remain; further investigation is warranted to understand why women who initiated TDF–FTC– LPV/r before conception had higher risks of preterm birth, low birth weight, and any adverse outcome than women who initiated ZDV–3TC– LPV/r or TDF–FTC–ATV/r before conception in subgroup analyses.”
Overall, there was a premature birth risk between 16.1% and 21.4% and a low birth weight risk between 16.2% and 23.8% across all antiretroviral therapy regimens, with an overall adverse outcome rate between 23.7% and 28.1%. For women who received TDF–FTC–LPV/r, there was a risk ratio of 0.90 (95% confidence interval, 0.60-1.33) for preterm births, 1.13 for low birth weight (95% CI, 0.78-1.64) and 0.92 for any adverse outcome (95% CI, 0.67-1.28) compared with women who received ZDV–3TC–LPV/r.
“For the outcomes of preterm birth, low birth weight, and any adverse outcome, TDF–FTC–ATV/r appeared to have lower risks than the LPV/r-based regimens; however, many of these associations were not significant,” the authors wrote.
Women who received TDF–FTC–LPV/r had a risk ratio of 1.14 (95% CI, 0.75-1.72) and a low-birth-weight risk ratio of 1.45 (95% CI, 0.96-2.17), compared with women who received TDF-FTC-ATV/r. Regarding very-low-birth-weight and very-preterm birth, the researchers noted no significant differences among regimen groups.
One author reported stock from Abbott, AbbVie, Novartis, and Roche outside the submitted work; one author reported personal fees from Boehringer-Ingelheim; and five authors reported grants from pharmaceutical companies and Google outside the submitted work.
SOURCE: Rough K et al. N Engl J Med 2018;378:1593-603.
Three different regimens of antiretroviral therapy did not produce significantly different adverse birth outcomes in women with HIV despite previous research showing an increased risk in premature birth or death in infants after a regimen of tenofovir, emtricitabine, and ritonavir-boosted lopinavir therapy, according to a recent analysis of two multicenter cohort studies published in the New England Journal of Medicine.
Kathryn Rough, ScD, of the Brigham and Women’s Hospital and Harvard Medical School in Boston and colleagues analyzed 4,646 birth outcomes in the SMARTT (NCT01310023) and P1025 (NCT00028145) trials from 3,847 unique women who received tenofovir, emtricitabine, and ritonavir-boosted lopinavir (TDF–FTC–LPV/r), zidovudine, lamivudine, and ritonavir-boosted lopinavir (ZDV–3TC–LPV/r), or TDF–FTC with ritonavir-boosted atazanavir (ATV/r) during gestation. There were 954 infants or fetuses exposed to ZDV–3TC–LPV/r (20.5%), 539 infants or fetuses exposed to TDF–FTC–ATV/r (11.6%), and 128 infants or fetuses exposed to TDF–FTC–LPV/r (2.8%), with 4,480 singleton, 80 twin, and 2 triplet pregnancies.
“Concerns regarding the use of TDF–FTC–LPV/r during pregnancy remain; further investigation is warranted to understand why women who initiated TDF–FTC– LPV/r before conception had higher risks of preterm birth, low birth weight, and any adverse outcome than women who initiated ZDV–3TC– LPV/r or TDF–FTC–ATV/r before conception in subgroup analyses.”
Overall, there was a premature birth risk between 16.1% and 21.4% and a low birth weight risk between 16.2% and 23.8% across all antiretroviral therapy regimens, with an overall adverse outcome rate between 23.7% and 28.1%. For women who received TDF–FTC–LPV/r, there was a risk ratio of 0.90 (95% confidence interval, 0.60-1.33) for preterm births, 1.13 for low birth weight (95% CI, 0.78-1.64) and 0.92 for any adverse outcome (95% CI, 0.67-1.28) compared with women who received ZDV–3TC–LPV/r.
“For the outcomes of preterm birth, low birth weight, and any adverse outcome, TDF–FTC–ATV/r appeared to have lower risks than the LPV/r-based regimens; however, many of these associations were not significant,” the authors wrote.
Women who received TDF–FTC–LPV/r had a risk ratio of 1.14 (95% CI, 0.75-1.72) and a low-birth-weight risk ratio of 1.45 (95% CI, 0.96-2.17), compared with women who received TDF-FTC-ATV/r. Regarding very-low-birth-weight and very-preterm birth, the researchers noted no significant differences among regimen groups.
One author reported stock from Abbott, AbbVie, Novartis, and Roche outside the submitted work; one author reported personal fees from Boehringer-Ingelheim; and five authors reported grants from pharmaceutical companies and Google outside the submitted work.
SOURCE: Rough K et al. N Engl J Med 2018;378:1593-603.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: .
Major finding: Women receiving TDF–FTC–LPV/r had a preterm birth risk ratio of 0.90 and a low-birth-weight risk ratio of 1.13 when compared with women receiving ZDV–3TC–LPV/r, and risk ratios of 1.14 and 1.45 for preterm birth and low birth weight, compared with TDF–FTC–ATV/r, respectively.
Study details: An analysis of two multisite cohort studies of 3,847 women and 4,646 birth outcomes between 2002 and 2016.
Disclosures: The study was funded by NIH, NIAID and NICHD. One author reported stock from Abbott, AbbVie, Novartis, and Roche; one author reported personal fees from Boehringer-Ingelheim; and five authors reported grants from pharmaceutical companies and Google outside the submitted work.
Source: Rough K. et al. N Engl J Med 2018;378:1593-603.
On cardiology training
I recently received a letter from a former fellow who completed his training almost 25 years ago, thanking me for guiding him through his education and to his successful medical career. It’s one of those letters that we all have received and that makes us feel that it is all worth it. When he went through his requisite 2 years of training, it seemed to me that my responsibility was to provide a model of how to provide excellent care at the bedside and clinic with expertise and compassion.
Percutaneous coronary angiography, echocardiography, radioisotope imaging, and new dramatically effective lifesaving drugs such as beta-blockers, ACE inhibitors, and thrombolytic therapies were developed almost overnight. Their application to the patient became a challenge, and an exciting period of clinical research ensued. As a training director, it seemed that our responsibility was not only to continue to provide a model of competent care but also to create an environment in which our new tools of diagnosis and therapy could be applied at the bedside. At the time, it became apparent that there was a need for staff members to develop expertise in all of these areas in order to provide an adequate teaching environment. These new developments also provided a unique opportunity to conduct clinical research in order develop the full range of the new therapeutic and diagnostic potentials.
Within a few years, we changed from being the bedside cardiologists who could do everything in a very limited way, to a staff focused on special areas of expertise in order to provide an optimal teaching environment. This led to the development of subspecialty areas of cardiac care, which became the future framework of the contemporary cardiology unit. It resulted in a decreased time on bedside care and a greater emphasis on pursuing specialty care. Many of the aspects of interpersonal relationships at the bedside became less important in order to provide trainees with the sufficient experience in the newly developing subspecialty areas. The competence of a cardiology fellow was no longer judged by his commitment to patient care but rather, by the achievement of sufficient number of procedures to meet certification exams. Both students and teachers became focused on the numbers game.
It is clear that the body of cardiology knowledge has expanded to a point where most of us cannot handle it all, and we need to turn to our colleagues with special expertise for help. This transition, which is not unique to cardiology, removed the teacher-practitioners from their role as the model of ethical and compassionate caregiver to that of the provider of procedures. Now, more than ever, there is a need to return to the model of the compassionate and concerned doctor. The need for expertise is undeniable, but in the process of achieving that, we cannot forget that we are doctors to patients and not just procedure readers and number crunchers. There is still time in our day to do that. If there isn’t, we need to find it.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
I recently received a letter from a former fellow who completed his training almost 25 years ago, thanking me for guiding him through his education and to his successful medical career. It’s one of those letters that we all have received and that makes us feel that it is all worth it. When he went through his requisite 2 years of training, it seemed to me that my responsibility was to provide a model of how to provide excellent care at the bedside and clinic with expertise and compassion.
Percutaneous coronary angiography, echocardiography, radioisotope imaging, and new dramatically effective lifesaving drugs such as beta-blockers, ACE inhibitors, and thrombolytic therapies were developed almost overnight. Their application to the patient became a challenge, and an exciting period of clinical research ensued. As a training director, it seemed that our responsibility was not only to continue to provide a model of competent care but also to create an environment in which our new tools of diagnosis and therapy could be applied at the bedside. At the time, it became apparent that there was a need for staff members to develop expertise in all of these areas in order to provide an adequate teaching environment. These new developments also provided a unique opportunity to conduct clinical research in order develop the full range of the new therapeutic and diagnostic potentials.
Within a few years, we changed from being the bedside cardiologists who could do everything in a very limited way, to a staff focused on special areas of expertise in order to provide an optimal teaching environment. This led to the development of subspecialty areas of cardiac care, which became the future framework of the contemporary cardiology unit. It resulted in a decreased time on bedside care and a greater emphasis on pursuing specialty care. Many of the aspects of interpersonal relationships at the bedside became less important in order to provide trainees with the sufficient experience in the newly developing subspecialty areas. The competence of a cardiology fellow was no longer judged by his commitment to patient care but rather, by the achievement of sufficient number of procedures to meet certification exams. Both students and teachers became focused on the numbers game.
It is clear that the body of cardiology knowledge has expanded to a point where most of us cannot handle it all, and we need to turn to our colleagues with special expertise for help. This transition, which is not unique to cardiology, removed the teacher-practitioners from their role as the model of ethical and compassionate caregiver to that of the provider of procedures. Now, more than ever, there is a need to return to the model of the compassionate and concerned doctor. The need for expertise is undeniable, but in the process of achieving that, we cannot forget that we are doctors to patients and not just procedure readers and number crunchers. There is still time in our day to do that. If there isn’t, we need to find it.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
I recently received a letter from a former fellow who completed his training almost 25 years ago, thanking me for guiding him through his education and to his successful medical career. It’s one of those letters that we all have received and that makes us feel that it is all worth it. When he went through his requisite 2 years of training, it seemed to me that my responsibility was to provide a model of how to provide excellent care at the bedside and clinic with expertise and compassion.
Percutaneous coronary angiography, echocardiography, radioisotope imaging, and new dramatically effective lifesaving drugs such as beta-blockers, ACE inhibitors, and thrombolytic therapies were developed almost overnight. Their application to the patient became a challenge, and an exciting period of clinical research ensued. As a training director, it seemed that our responsibility was not only to continue to provide a model of competent care but also to create an environment in which our new tools of diagnosis and therapy could be applied at the bedside. At the time, it became apparent that there was a need for staff members to develop expertise in all of these areas in order to provide an adequate teaching environment. These new developments also provided a unique opportunity to conduct clinical research in order develop the full range of the new therapeutic and diagnostic potentials.
Within a few years, we changed from being the bedside cardiologists who could do everything in a very limited way, to a staff focused on special areas of expertise in order to provide an optimal teaching environment. This led to the development of subspecialty areas of cardiac care, which became the future framework of the contemporary cardiology unit. It resulted in a decreased time on bedside care and a greater emphasis on pursuing specialty care. Many of the aspects of interpersonal relationships at the bedside became less important in order to provide trainees with the sufficient experience in the newly developing subspecialty areas. The competence of a cardiology fellow was no longer judged by his commitment to patient care but rather, by the achievement of sufficient number of procedures to meet certification exams. Both students and teachers became focused on the numbers game.
It is clear that the body of cardiology knowledge has expanded to a point where most of us cannot handle it all, and we need to turn to our colleagues with special expertise for help. This transition, which is not unique to cardiology, removed the teacher-practitioners from their role as the model of ethical and compassionate caregiver to that of the provider of procedures. Now, more than ever, there is a need to return to the model of the compassionate and concerned doctor. The need for expertise is undeniable, but in the process of achieving that, we cannot forget that we are doctors to patients and not just procedure readers and number crunchers. There is still time in our day to do that. If there isn’t, we need to find it.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Probe linked to smartphone found effective in diagnosing oral cancer
DALLAS – A low-cost , in a clinical study of 92 people.
“Oral cancer is the sixth most common cancer in the world, but it’s the only major cancer whose outcome has not improved in the last 50 years,” study author Petra Wilder-Smith DDS, PhD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery Inc. “The main challenge is that over two-thirds of oral cancers are detected after they’ve metastasized. When you get spread like that, your survival is about 20% at 5 years, whereas if you detect it before spread, your survival is about 80% at 5 years.”
At the meeting, Vania Firmalino, an undergraduate student at the University of California, Irvine, discussed efforts by Dr. Wilder-Smith, Rongguang Liang, PhD, of the College of Optical Sciences at the University of Arizona, Tucson, and their colleagues to develop and evaluate the screening performance of a novel, low-cost smartphone-based mini probe for oral cancer screening and oral potentially premalignant lesions (OPMLs). The device provides high-resolution polarized white light images in combination with autofluorescence (AF) imaging capability.
The researchers found that inter-subject variation at each location was small, but inter-site differences were considerable. For example, optical data from OPMLs and oral cancer sites differed from normal with regard to white-light reflectance intensities, vascular homogeneity, and standard deviation. The AF signal in OPMLs and oral cancers shifted progressively to the red, together with a diminished green fluorescence signal. The cloud-based diagnostic algorithm based on these properties performed well, with an agreement with standard-of-care diagnosis of 80.6%.
“Artificial intelligence improves with data,” said Dr. Wilder-Smith, who is also a senior fellow at the university’s Chao Family Comprehensive Cancer Center. “We trained this system on about 200 images. When you’re up to 1,000 images per condition, that’s when you really start to get the benefits of artificial intelligence and machine learning. There’s huge potential here, especially when you think that 40% of the world’s risk for oral cancer is in India, which has good cell phone coverage. India also has a government-financed public health program whereby they already send health care workers to the remote areas of India to screen for basic diseases.”
The study won an award for best overall clinical abstract at the meeting. Dr. Wilder-Smith reported having no financial disclosures. The project was supported with funding from the National Institute of Biomedical Imaging and Bioengineering and the Beckman Foundation.
DALLAS – A low-cost , in a clinical study of 92 people.
“Oral cancer is the sixth most common cancer in the world, but it’s the only major cancer whose outcome has not improved in the last 50 years,” study author Petra Wilder-Smith DDS, PhD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery Inc. “The main challenge is that over two-thirds of oral cancers are detected after they’ve metastasized. When you get spread like that, your survival is about 20% at 5 years, whereas if you detect it before spread, your survival is about 80% at 5 years.”
At the meeting, Vania Firmalino, an undergraduate student at the University of California, Irvine, discussed efforts by Dr. Wilder-Smith, Rongguang Liang, PhD, of the College of Optical Sciences at the University of Arizona, Tucson, and their colleagues to develop and evaluate the screening performance of a novel, low-cost smartphone-based mini probe for oral cancer screening and oral potentially premalignant lesions (OPMLs). The device provides high-resolution polarized white light images in combination with autofluorescence (AF) imaging capability.
The researchers found that inter-subject variation at each location was small, but inter-site differences were considerable. For example, optical data from OPMLs and oral cancer sites differed from normal with regard to white-light reflectance intensities, vascular homogeneity, and standard deviation. The AF signal in OPMLs and oral cancers shifted progressively to the red, together with a diminished green fluorescence signal. The cloud-based diagnostic algorithm based on these properties performed well, with an agreement with standard-of-care diagnosis of 80.6%.
“Artificial intelligence improves with data,” said Dr. Wilder-Smith, who is also a senior fellow at the university’s Chao Family Comprehensive Cancer Center. “We trained this system on about 200 images. When you’re up to 1,000 images per condition, that’s when you really start to get the benefits of artificial intelligence and machine learning. There’s huge potential here, especially when you think that 40% of the world’s risk for oral cancer is in India, which has good cell phone coverage. India also has a government-financed public health program whereby they already send health care workers to the remote areas of India to screen for basic diseases.”
The study won an award for best overall clinical abstract at the meeting. Dr. Wilder-Smith reported having no financial disclosures. The project was supported with funding from the National Institute of Biomedical Imaging and Bioengineering and the Beckman Foundation.
DALLAS – A low-cost , in a clinical study of 92 people.
“Oral cancer is the sixth most common cancer in the world, but it’s the only major cancer whose outcome has not improved in the last 50 years,” study author Petra Wilder-Smith DDS, PhD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery Inc. “The main challenge is that over two-thirds of oral cancers are detected after they’ve metastasized. When you get spread like that, your survival is about 20% at 5 years, whereas if you detect it before spread, your survival is about 80% at 5 years.”
At the meeting, Vania Firmalino, an undergraduate student at the University of California, Irvine, discussed efforts by Dr. Wilder-Smith, Rongguang Liang, PhD, of the College of Optical Sciences at the University of Arizona, Tucson, and their colleagues to develop and evaluate the screening performance of a novel, low-cost smartphone-based mini probe for oral cancer screening and oral potentially premalignant lesions (OPMLs). The device provides high-resolution polarized white light images in combination with autofluorescence (AF) imaging capability.
The researchers found that inter-subject variation at each location was small, but inter-site differences were considerable. For example, optical data from OPMLs and oral cancer sites differed from normal with regard to white-light reflectance intensities, vascular homogeneity, and standard deviation. The AF signal in OPMLs and oral cancers shifted progressively to the red, together with a diminished green fluorescence signal. The cloud-based diagnostic algorithm based on these properties performed well, with an agreement with standard-of-care diagnosis of 80.6%.
“Artificial intelligence improves with data,” said Dr. Wilder-Smith, who is also a senior fellow at the university’s Chao Family Comprehensive Cancer Center. “We trained this system on about 200 images. When you’re up to 1,000 images per condition, that’s when you really start to get the benefits of artificial intelligence and machine learning. There’s huge potential here, especially when you think that 40% of the world’s risk for oral cancer is in India, which has good cell phone coverage. India also has a government-financed public health program whereby they already send health care workers to the remote areas of India to screen for basic diseases.”
The study won an award for best overall clinical abstract at the meeting. Dr. Wilder-Smith reported having no financial disclosures. The project was supported with funding from the National Institute of Biomedical Imaging and Bioengineering and the Beckman Foundation.
REPORTING FROM ASLMS 2018
Key clinical point: A compact oral probe that links to a smartphone was able to detect oral cancer.
Major finding: The optical diagnostic probe had a high rate of agreement (80.6%) with standard-of-care diagnosis.
Study details: A clinical analysis of 92 people with visually healthy oral mucosa or oral leukoplakia, erythroplakia, or ulceration.
Disclosures: Dr. Wilder-Smith reported having no financial disclosures. The National Institute of Biomedical Imaging and Bioengineering and the Beckman Foundation funded the project.