User login
EHRA releases new guide for NOAC use
The European Heart Rhythm Association (EHRA) has released a new version of its “practical guide” for the use of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation.
The guide, now in its third edition, gives concrete advice on how to use NOACs in specific clinical situations.
It was published in European Heart Journal and presented at EHRA 2018 in Barcelona, Spain.
The 2018 edition of the guide has several new chapters.
One chapter summarizes the correct dosing of NOACs in conditions other than atrial fibrillation, such as for the prevention of deep vein thrombosis and treatment of venous thromboembolism.
“The dosing for each condition is different, which may lead to confusion, so we have outlined this clearly,” said author Hein Heidbüchel, MD, PhD, of Hasselt University in Hasselt, Belgium.
Another chapter outlines how to use NOACs in particular groups of patients, including those with very low body weight, the very obese, athletes, frail patients in whom there is concern about bleeding, and patients with cognitive impairment who may forget to take their pills.
The guide also includes updated advice on the combined use of antiplatelet agents and NOACs in patients with coronary artery disease, particularly those with an acute coronary syndrome or patients scheduled for percutaneous coronary intervention with stenting.
“We provide guidance around which and how many antiplatelets, for how long, with which NOAC, and at what dose of that NOAC,” Dr Heidbüchel said.
In addition, the guide now includes more scientific evidence on the use of anticoagulants around cardioversion. The document gives detailed advice on what to do in patients on long-term NOAC treatment who need cardioversion, as compared to patients newly diagnosed with atrial fibrillation and started on a NOAC before cardioversion.
Since the previous edition of the guide was published, the first NOAC reversal agent has received market approval. Therefore, the new edition includes advice on how to use this agent, idarucizumab—which reverses the anticoagulant effect of dabigatran—when there is acute bleeding, when urgent surgery is required, or when the patient has a stroke.
The guide also includes advice on andexanet alfa, another reversal agent expected to receive market approval, with the caveat that the instructions on the label should be followed.
Additionally, the guide describes scenarios in which physicians might want to know the NOAC plasma level. One scenario concerns patients undergoing major surgery in whom it is unclear—for example, because of other drugs or renal dysfunction—whether the usual practice of stopping the NOAC 48 hours in advance is sufficient. The plasma level of the NOAC could be measured just before surgery to confirm the anticoagulant effect has waned.
Finally, the chapter on drug-drug interactions has been expanded with anticancer and antiepileptic drugs.
“While this is mostly based on potential pharmacokinetic interactions and case reports, it is the first of its kind,” said author Jan Steffel, MD, of University Heart Center Zurich in Switzerland.
“This is likely to be adapted and become more complete over the years as our experience increases at this new frontier.”
EHRA received unconditional grants from Pfizer/BMS, Daiichi-Sankyo, Boehringer-Ingelheim, and Bayer.
The European Heart Rhythm Association (EHRA) has released a new version of its “practical guide” for the use of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation.
The guide, now in its third edition, gives concrete advice on how to use NOACs in specific clinical situations.
It was published in European Heart Journal and presented at EHRA 2018 in Barcelona, Spain.
The 2018 edition of the guide has several new chapters.
One chapter summarizes the correct dosing of NOACs in conditions other than atrial fibrillation, such as for the prevention of deep vein thrombosis and treatment of venous thromboembolism.
“The dosing for each condition is different, which may lead to confusion, so we have outlined this clearly,” said author Hein Heidbüchel, MD, PhD, of Hasselt University in Hasselt, Belgium.
Another chapter outlines how to use NOACs in particular groups of patients, including those with very low body weight, the very obese, athletes, frail patients in whom there is concern about bleeding, and patients with cognitive impairment who may forget to take their pills.
The guide also includes updated advice on the combined use of antiplatelet agents and NOACs in patients with coronary artery disease, particularly those with an acute coronary syndrome or patients scheduled for percutaneous coronary intervention with stenting.
“We provide guidance around which and how many antiplatelets, for how long, with which NOAC, and at what dose of that NOAC,” Dr Heidbüchel said.
In addition, the guide now includes more scientific evidence on the use of anticoagulants around cardioversion. The document gives detailed advice on what to do in patients on long-term NOAC treatment who need cardioversion, as compared to patients newly diagnosed with atrial fibrillation and started on a NOAC before cardioversion.
Since the previous edition of the guide was published, the first NOAC reversal agent has received market approval. Therefore, the new edition includes advice on how to use this agent, idarucizumab—which reverses the anticoagulant effect of dabigatran—when there is acute bleeding, when urgent surgery is required, or when the patient has a stroke.
The guide also includes advice on andexanet alfa, another reversal agent expected to receive market approval, with the caveat that the instructions on the label should be followed.
Additionally, the guide describes scenarios in which physicians might want to know the NOAC plasma level. One scenario concerns patients undergoing major surgery in whom it is unclear—for example, because of other drugs or renal dysfunction—whether the usual practice of stopping the NOAC 48 hours in advance is sufficient. The plasma level of the NOAC could be measured just before surgery to confirm the anticoagulant effect has waned.
Finally, the chapter on drug-drug interactions has been expanded with anticancer and antiepileptic drugs.
“While this is mostly based on potential pharmacokinetic interactions and case reports, it is the first of its kind,” said author Jan Steffel, MD, of University Heart Center Zurich in Switzerland.
“This is likely to be adapted and become more complete over the years as our experience increases at this new frontier.”
EHRA received unconditional grants from Pfizer/BMS, Daiichi-Sankyo, Boehringer-Ingelheim, and Bayer.
The European Heart Rhythm Association (EHRA) has released a new version of its “practical guide” for the use of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation.
The guide, now in its third edition, gives concrete advice on how to use NOACs in specific clinical situations.
It was published in European Heart Journal and presented at EHRA 2018 in Barcelona, Spain.
The 2018 edition of the guide has several new chapters.
One chapter summarizes the correct dosing of NOACs in conditions other than atrial fibrillation, such as for the prevention of deep vein thrombosis and treatment of venous thromboembolism.
“The dosing for each condition is different, which may lead to confusion, so we have outlined this clearly,” said author Hein Heidbüchel, MD, PhD, of Hasselt University in Hasselt, Belgium.
Another chapter outlines how to use NOACs in particular groups of patients, including those with very low body weight, the very obese, athletes, frail patients in whom there is concern about bleeding, and patients with cognitive impairment who may forget to take their pills.
The guide also includes updated advice on the combined use of antiplatelet agents and NOACs in patients with coronary artery disease, particularly those with an acute coronary syndrome or patients scheduled for percutaneous coronary intervention with stenting.
“We provide guidance around which and how many antiplatelets, for how long, with which NOAC, and at what dose of that NOAC,” Dr Heidbüchel said.
In addition, the guide now includes more scientific evidence on the use of anticoagulants around cardioversion. The document gives detailed advice on what to do in patients on long-term NOAC treatment who need cardioversion, as compared to patients newly diagnosed with atrial fibrillation and started on a NOAC before cardioversion.
Since the previous edition of the guide was published, the first NOAC reversal agent has received market approval. Therefore, the new edition includes advice on how to use this agent, idarucizumab—which reverses the anticoagulant effect of dabigatran—when there is acute bleeding, when urgent surgery is required, or when the patient has a stroke.
The guide also includes advice on andexanet alfa, another reversal agent expected to receive market approval, with the caveat that the instructions on the label should be followed.
Additionally, the guide describes scenarios in which physicians might want to know the NOAC plasma level. One scenario concerns patients undergoing major surgery in whom it is unclear—for example, because of other drugs or renal dysfunction—whether the usual practice of stopping the NOAC 48 hours in advance is sufficient. The plasma level of the NOAC could be measured just before surgery to confirm the anticoagulant effect has waned.
Finally, the chapter on drug-drug interactions has been expanded with anticancer and antiepileptic drugs.
“While this is mostly based on potential pharmacokinetic interactions and case reports, it is the first of its kind,” said author Jan Steffel, MD, of University Heart Center Zurich in Switzerland.
“This is likely to be adapted and become more complete over the years as our experience increases at this new frontier.”
EHRA received unconditional grants from Pfizer/BMS, Daiichi-Sankyo, Boehringer-Ingelheim, and Bayer.
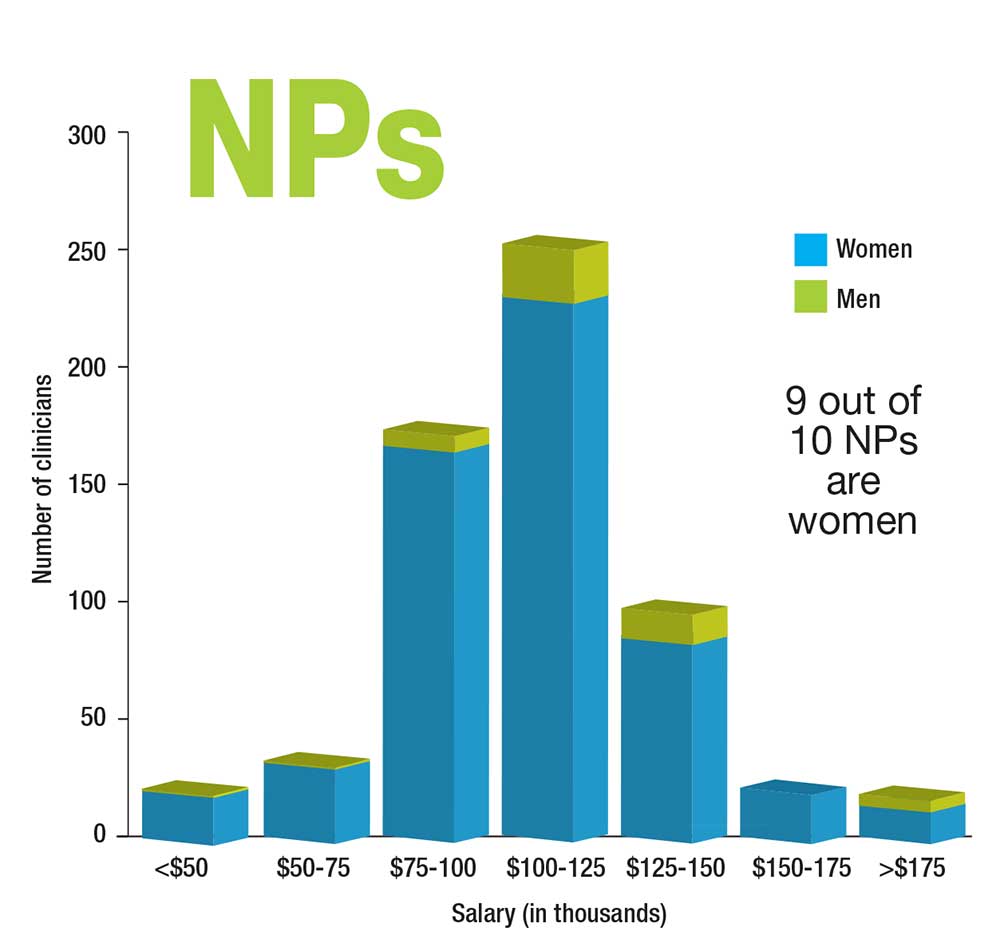
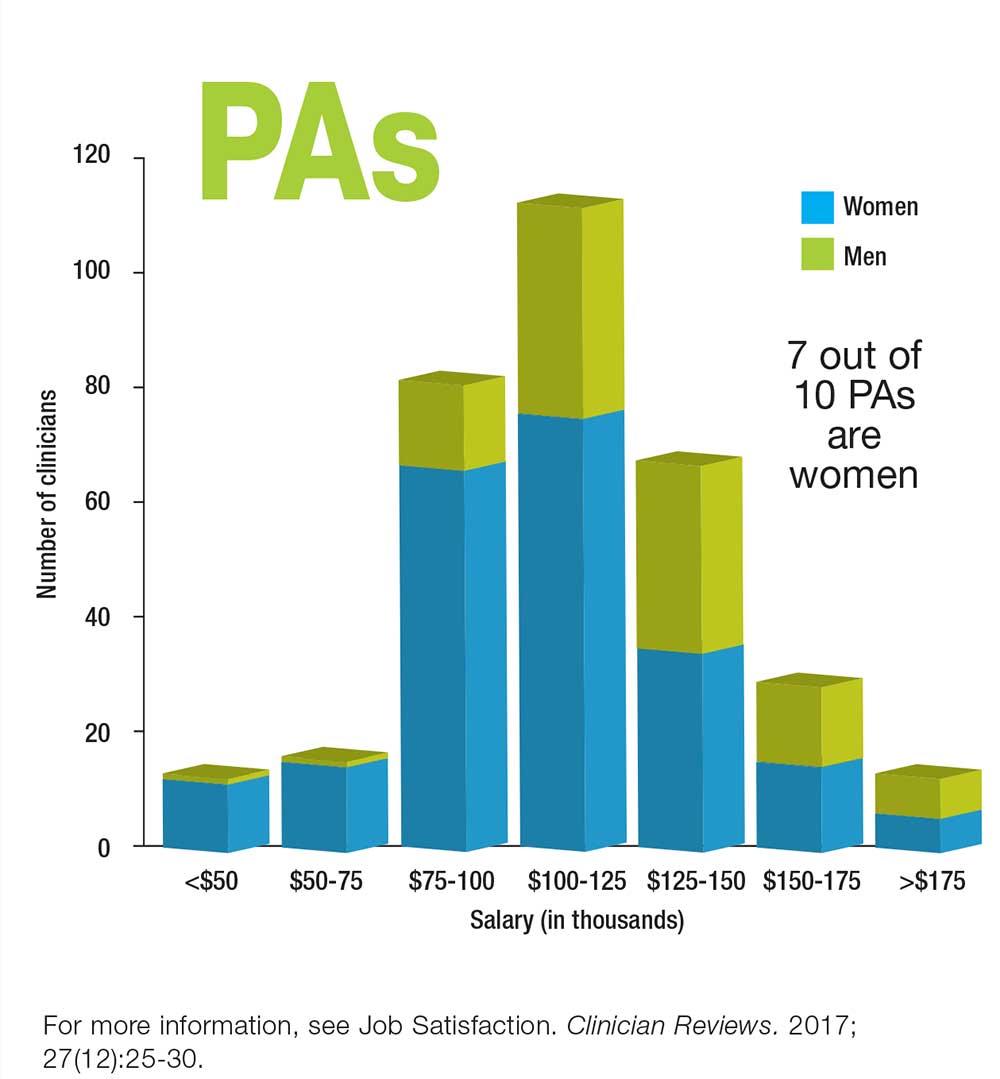
Who Makes More: Male or Female Clinicians?




Fulvestrant plus neratinib reversed treatment-acquired HER2 mutations in metastatic ER+ breast cancer
Dual therapy with fulvestrant and the irreversible HER2 kinase inhibitor neratinib reversed treatment-acquired hormone resistance in metastatic estrogen receptor (ER)–positive breast cancer cells.
Elaine Mardis, PhD, a spokesperson for the American Association of Cancer Research, hailed the research by Utthara Nayar, PhD, and colleagues as “groundbreaking and unexpected” during a briefing held in advance of the annual meeting of the American Association for Cancer Research. The lab experiments were part of a whole-exome sequencing study of metastatic ER-positive tumor biopsies from 168 patients, 12 of whom had acquired the HER2 mutations, said Dr. Nayar of the Dana-Farber Cancer Institute, Boston.
The findings have prompted a phase 2 trial of the combination, which is now recruiting patients, Dr. Nayar said. The 5-year study seeks 152 women with inoperable locally advanced or metastatic ER-positive breast cancer with a confirmed HER2-positive mutation. Patients will be randomized to the combination of neratinib and fulvestrant or to neratinib alone. The primary outcome is progression-free survival.
“We also hope to be able to develop upfront combinations to preempt the resistance and lead to more durable responses,” Dr. Nayar said.
All of the 168 patients who contributed metastatic tumor biopsy samples to the study had developed resistance to estrogen receptor treatments, including aromatase inhibitors, tamoxifen, and fulvestrant. Of these biopsies, 12 had HER2 mutations, 8 of which had been previously characterized as activating.
Dr. Nayar and colleagues examined the untreated primary tumors in five of these patients; there was no mutation in four, suggesting that the mutations were a response to treatment. “In these 80%, the mutations were acquired as tumors were exposed to treatment and not present in the original tumor,” Dr. Nayar said.
These acquired HER2 mutations were mutually exclusive with ER mutations, which suggested a different mechanism of resistance to ER-directed therapies, she noted in her abstract. The mutations conferred resistance to tamoxifen, fulvestrant, and palbociclib.
However, the combination of fulvestrant and neratinib, an irreversible HER2 kinase inhibitor, overcame resistance in these cells.
In addition to pioneering a potentially important therapy for treatment-resistant metastatic breast cancer, the study highlights the importance of gene sequencing metastatic tumors, said Nikhil Wagle, MD, Dr. Nayar’s colleague and deputy director of the Center for Cancer Precision Medicine at Dana-Farber.
“Our study highlights how important it is to profile resistant metastatic tumors since these tumors may harbor targetable mechanisms of resistance that were not present in the original tumor biopsy,” Dr. Wagle noted in a press statement. “Repeated sequencing of tumors can pinpoint new genetic changes that cause resistance to therapies. This in turn can enable physicians to personalize therapy depending on the specific genetic changes in a patient’s tumor over time.”
The study was supported by the Department of Defense, the National Cancer Institute, the Susan G. Komen Foundation, the Dana-Farber Cancer Center, and a number of other private funders. Dr. Wagle is a stockholder in Foundation Medicine. Dr. Nayar had no financial disclosure.
SOURCE: Nayer U et al. AACR 2018, Abstract 4952
Dual therapy with fulvestrant and the irreversible HER2 kinase inhibitor neratinib reversed treatment-acquired hormone resistance in metastatic estrogen receptor (ER)–positive breast cancer cells.
Elaine Mardis, PhD, a spokesperson for the American Association of Cancer Research, hailed the research by Utthara Nayar, PhD, and colleagues as “groundbreaking and unexpected” during a briefing held in advance of the annual meeting of the American Association for Cancer Research. The lab experiments were part of a whole-exome sequencing study of metastatic ER-positive tumor biopsies from 168 patients, 12 of whom had acquired the HER2 mutations, said Dr. Nayar of the Dana-Farber Cancer Institute, Boston.
The findings have prompted a phase 2 trial of the combination, which is now recruiting patients, Dr. Nayar said. The 5-year study seeks 152 women with inoperable locally advanced or metastatic ER-positive breast cancer with a confirmed HER2-positive mutation. Patients will be randomized to the combination of neratinib and fulvestrant or to neratinib alone. The primary outcome is progression-free survival.
“We also hope to be able to develop upfront combinations to preempt the resistance and lead to more durable responses,” Dr. Nayar said.
All of the 168 patients who contributed metastatic tumor biopsy samples to the study had developed resistance to estrogen receptor treatments, including aromatase inhibitors, tamoxifen, and fulvestrant. Of these biopsies, 12 had HER2 mutations, 8 of which had been previously characterized as activating.
Dr. Nayar and colleagues examined the untreated primary tumors in five of these patients; there was no mutation in four, suggesting that the mutations were a response to treatment. “In these 80%, the mutations were acquired as tumors were exposed to treatment and not present in the original tumor,” Dr. Nayar said.
These acquired HER2 mutations were mutually exclusive with ER mutations, which suggested a different mechanism of resistance to ER-directed therapies, she noted in her abstract. The mutations conferred resistance to tamoxifen, fulvestrant, and palbociclib.
However, the combination of fulvestrant and neratinib, an irreversible HER2 kinase inhibitor, overcame resistance in these cells.
In addition to pioneering a potentially important therapy for treatment-resistant metastatic breast cancer, the study highlights the importance of gene sequencing metastatic tumors, said Nikhil Wagle, MD, Dr. Nayar’s colleague and deputy director of the Center for Cancer Precision Medicine at Dana-Farber.
“Our study highlights how important it is to profile resistant metastatic tumors since these tumors may harbor targetable mechanisms of resistance that were not present in the original tumor biopsy,” Dr. Wagle noted in a press statement. “Repeated sequencing of tumors can pinpoint new genetic changes that cause resistance to therapies. This in turn can enable physicians to personalize therapy depending on the specific genetic changes in a patient’s tumor over time.”
The study was supported by the Department of Defense, the National Cancer Institute, the Susan G. Komen Foundation, the Dana-Farber Cancer Center, and a number of other private funders. Dr. Wagle is a stockholder in Foundation Medicine. Dr. Nayar had no financial disclosure.
SOURCE: Nayer U et al. AACR 2018, Abstract 4952
Dual therapy with fulvestrant and the irreversible HER2 kinase inhibitor neratinib reversed treatment-acquired hormone resistance in metastatic estrogen receptor (ER)–positive breast cancer cells.
Elaine Mardis, PhD, a spokesperson for the American Association of Cancer Research, hailed the research by Utthara Nayar, PhD, and colleagues as “groundbreaking and unexpected” during a briefing held in advance of the annual meeting of the American Association for Cancer Research. The lab experiments were part of a whole-exome sequencing study of metastatic ER-positive tumor biopsies from 168 patients, 12 of whom had acquired the HER2 mutations, said Dr. Nayar of the Dana-Farber Cancer Institute, Boston.
The findings have prompted a phase 2 trial of the combination, which is now recruiting patients, Dr. Nayar said. The 5-year study seeks 152 women with inoperable locally advanced or metastatic ER-positive breast cancer with a confirmed HER2-positive mutation. Patients will be randomized to the combination of neratinib and fulvestrant or to neratinib alone. The primary outcome is progression-free survival.
“We also hope to be able to develop upfront combinations to preempt the resistance and lead to more durable responses,” Dr. Nayar said.
All of the 168 patients who contributed metastatic tumor biopsy samples to the study had developed resistance to estrogen receptor treatments, including aromatase inhibitors, tamoxifen, and fulvestrant. Of these biopsies, 12 had HER2 mutations, 8 of which had been previously characterized as activating.
Dr. Nayar and colleagues examined the untreated primary tumors in five of these patients; there was no mutation in four, suggesting that the mutations were a response to treatment. “In these 80%, the mutations were acquired as tumors were exposed to treatment and not present in the original tumor,” Dr. Nayar said.
These acquired HER2 mutations were mutually exclusive with ER mutations, which suggested a different mechanism of resistance to ER-directed therapies, she noted in her abstract. The mutations conferred resistance to tamoxifen, fulvestrant, and palbociclib.
However, the combination of fulvestrant and neratinib, an irreversible HER2 kinase inhibitor, overcame resistance in these cells.
In addition to pioneering a potentially important therapy for treatment-resistant metastatic breast cancer, the study highlights the importance of gene sequencing metastatic tumors, said Nikhil Wagle, MD, Dr. Nayar’s colleague and deputy director of the Center for Cancer Precision Medicine at Dana-Farber.
“Our study highlights how important it is to profile resistant metastatic tumors since these tumors may harbor targetable mechanisms of resistance that were not present in the original tumor biopsy,” Dr. Wagle noted in a press statement. “Repeated sequencing of tumors can pinpoint new genetic changes that cause resistance to therapies. This in turn can enable physicians to personalize therapy depending on the specific genetic changes in a patient’s tumor over time.”
The study was supported by the Department of Defense, the National Cancer Institute, the Susan G. Komen Foundation, the Dana-Farber Cancer Center, and a number of other private funders. Dr. Wagle is a stockholder in Foundation Medicine. Dr. Nayar had no financial disclosure.
SOURCE: Nayer U et al. AACR 2018, Abstract 4952
FROM THE AACR 2018 ANNUAL MEETING
Key clinical point: The combination of fulvestrant and neratinib reversed acquired HER2 mutations in ER+ metastatic breast cancer cells.
Major finding: Of 168 biopsies, 12 had acquired HER2 mutations after hormone treatment; these mutations were reversed with the dual therapy.
Study details: The exome sequencing study comprised 168 biopsies, and the in vitro study comprised 12.
Disclosures: The study was supported by the Department of Defense, the National Cancer Institute, the Susan G. Komen Foundation, the Dana-Farber Cancer Institute, and other private funders. Dr. Wagle is a stockholder in Foundation Medicine. Dr. Nayar had no financial disclosure.
Source: Nayar U et al. AACR 2018, Abstract 4952
Arthritis limits physical activity the most in the South
“Physical activity is a proven strategy for managing arthritis symptoms,” but 35% of Americans with arthritis do not participate in any such activities or exercise, according to investigators who analyzed data from a national survey of more than 440,000 adults.
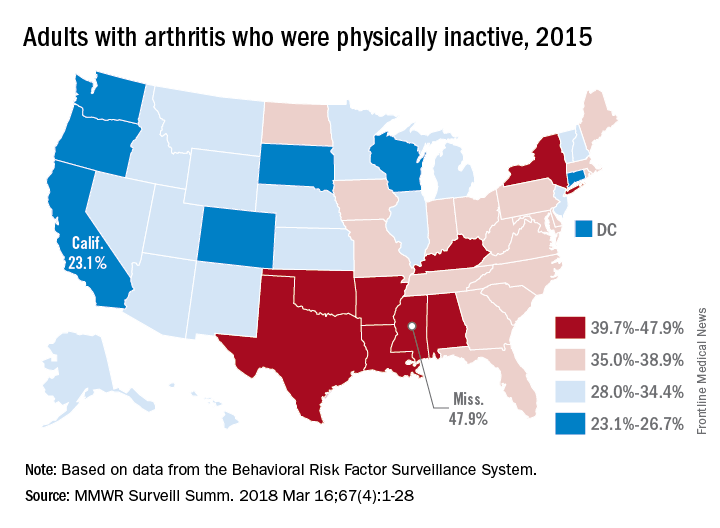
The low rates of inactivity in the western half of the country were topped by California’s 23.1% and South Dakota’s 23.4%, with Oregon (24.0%) and Wisconsin (24.6%) not too far behind, Dr. Barbour and his associates said based on data for 441,456 adults aged 18 years and older who were interviewed for the Behavioral Risk Factor Surveillance System.
Overall prevalence rates show that arthritis has the greatest effect in southern states, which, in addition to high inactivity, had more arthritis-attributable severe joint pain, more arthritis-attributable social participation restriction, and less leisure-time walking among adults with arthritis. This information, the investigators suggested, may help public health professionals “to better understand and target evidence-based nonpharmaceutical interventions, such as arthritis self-management education and physical activity.”
SOURCE: Barbour KE et al. MMWR Surveill Summ. 2018 Mar 16;67(4):1-28.
“Physical activity is a proven strategy for managing arthritis symptoms,” but 35% of Americans with arthritis do not participate in any such activities or exercise, according to investigators who analyzed data from a national survey of more than 440,000 adults.

The low rates of inactivity in the western half of the country were topped by California’s 23.1% and South Dakota’s 23.4%, with Oregon (24.0%) and Wisconsin (24.6%) not too far behind, Dr. Barbour and his associates said based on data for 441,456 adults aged 18 years and older who were interviewed for the Behavioral Risk Factor Surveillance System.
Overall prevalence rates show that arthritis has the greatest effect in southern states, which, in addition to high inactivity, had more arthritis-attributable severe joint pain, more arthritis-attributable social participation restriction, and less leisure-time walking among adults with arthritis. This information, the investigators suggested, may help public health professionals “to better understand and target evidence-based nonpharmaceutical interventions, such as arthritis self-management education and physical activity.”
SOURCE: Barbour KE et al. MMWR Surveill Summ. 2018 Mar 16;67(4):1-28.
“Physical activity is a proven strategy for managing arthritis symptoms,” but 35% of Americans with arthritis do not participate in any such activities or exercise, according to investigators who analyzed data from a national survey of more than 440,000 adults.

The low rates of inactivity in the western half of the country were topped by California’s 23.1% and South Dakota’s 23.4%, with Oregon (24.0%) and Wisconsin (24.6%) not too far behind, Dr. Barbour and his associates said based on data for 441,456 adults aged 18 years and older who were interviewed for the Behavioral Risk Factor Surveillance System.
Overall prevalence rates show that arthritis has the greatest effect in southern states, which, in addition to high inactivity, had more arthritis-attributable severe joint pain, more arthritis-attributable social participation restriction, and less leisure-time walking among adults with arthritis. This information, the investigators suggested, may help public health professionals “to better understand and target evidence-based nonpharmaceutical interventions, such as arthritis self-management education and physical activity.”
SOURCE: Barbour KE et al. MMWR Surveill Summ. 2018 Mar 16;67(4):1-28.
FROM MMWR SURVEILLANCE SUMMARIES
Low vitamin B12 tied to worsening mobility, cognition in early Parkinson’s
suggesting that it may be worthwhile to examine the correction of low levels as a means of slowing disease progression.
Investigators led by Chadwick W. Christine, MD, of the department of neurology at the University of California, San Francisco, sought to understand what contributes to considerable variation in the progression of PD by building on previous research that revealed low serum vitamin B12 levels are common in patients with moderately advanced PD and are associated with neuropathy and cognitive impairment.
They measured serum methylmalonic acid, homocysteine, and holotranscobalamin in addition to B12 because of the limited sensitivity of serum B12 testing alone to detect B12 deficiency. At baseline, 13% of 680 patients had borderline-low B12 levels (less than 184 pmol/L [250 pg/mL]), and 5% had deficient B12 levels (less than 157 pmol/L or 212 pg/mL). Homocysteine was moderately elevated (greater than 15 mmol/L) in 7% of subjects, and 14% of patients with borderline-low B12 also had elevated homocysteine, the investigators reported in Movement Disorders.
Low B12 at baseline predicted greater worsening of mobility in terms of a higher ambulatory capacity score, calculated by adding individual items of the Unified Parkinson’s Disease Rating Scale (UPDRS): falling, freezing when walking, walking, gait, and postural stability. Participants in the low- B12 tertile (less than 234 pmol/L or 317 pg/ mL) developed greater morbidity as assessed by greater annualized worsening of the ambulatory capacity score. For example, those in the low-B12 tertile had annualized change of 1.53, compared with 0.77 in the upper tertile. The worsening score was mostly attributed to poorer gait and postural instability.
To give context to these figures, the researchers pointed to an analysis of the NET-PD LS1 cohort that found a mean ambulatory capacity score of 2.17 among patients who fell and a score of 1.4 for those who did not fall. “Thus, we consider the magnitude of difference to be clinically relevant, particularly given that components of gait dysfunction that develop in PD may not respond to dopaminergic treatments or [deep brain stimulation],” they wrote.
Elevated homocysteine also predicted greater cognitive decline. Baseline elevated homocysteine was associated with lower baseline Mini Mental State Examination (MMSE), as well as greater annualized decline in MMSE (–1.96 vs. 0.06; P = .001).
Of the 456 subjects who continued in the study for 9-24 months and had a second blood sample available, 226 had an increase of more than 20% in B12 levels, 210 stayed within 20% of the original B12 measurement, and 19 had a decrease greater than 20%.
Overall, there was a mean annualized increase in B12 of 52.6 pmol/L, a mean annualized decrease of homocysteine of 0.83 mmol/L, and a mean annualized increase of holotranscobalamin of 14.7 pmol/L.
“These findings are consistent with improved nutritional status during the course of the study, likely attributed to subjects starting the optional [multivitamin] after the baseline visit and/or subjects changing their diets,” the research team said.
While the improvement in B12 status did not lead to statistically significant improvements in UPDRS scores, the researchers said there was a trend toward improvement, which provides “support for a disease-modifying effect of B12,” they wrote.
The researchers speculated that their findings of a link between low B12 levels and worse outcomes could be attributed to an independent (comorbid) effect on the central and peripheral nervous systems, a direct effect on PD pathogenesis, or alternatively, that low B12 may be a marker of an unknown associated factor.
“Given that low B12 status is associated with neurological and other medical morbidities and is readily treated, great care would be needed to design an ethically acceptable randomized, prospective study to evaluate the effect of B12 supplementation on PD progression, given that serum measurements collected as part of a prospective study in unsupplemented patients would likely reveal some subjects with B12 deficiency,” they concluded.
The authors declared no relevant disclosures. The study was supported by a grant from the Michael J. Fox Foundation for Parkinson’s Research, as well as privately donated grants.
SOURCE: Christine C et al. Movement Dis. 2018 Mar 6. doi: 10.1002/mds.27301.
The course of PD can be quite variable, and it is difficult for clinicians to predict what will happen to an individual person with PD, but identifying prognostic factors ought to help practitioners answer their patients’ questions and potentially improve the understanding of mechanisms underlying the disease pathogenesis.
If vitamin B12 is related to the progression of PD as suggested in the study by Christine et al., its replacement may slow the decline of the disease. But there are several issues that need to be addressed in the context of their findings.
First, what constitutes a low vitamin B12 level is not a simple issue. If the evaluation is limited to measurement of vitamin B12 concentration, the diagnosis of genuine deficiency is unreliable. Most experts agree that combined measurement of vitamin B12 with determination of homocysteine levels is necessary and while Christine et al. measured both levels, their statistical analysis and subsequent conclusions are exclusively based on the levels of the vitamin.
Moreover, 34 patients in the study were classified as having “borderline-low” vitamin B12 levels, and 14 had both borderline-low B12 level and high homocysteine concentration. This brings into question whether the researchers identified PD patients who actually had low B12 levels.
The design of the DATATOP trial could also introduce some bias into the findings. At the time of publication, the study was criticized for disregarding the symptomatic effect of selegiline and for a lack of objective definition of criteria for the trial’s primary endpoint – introduction of levodopa.
This could have led to the termination of individuals at different stages of the disease, introducing a potential bias in the sample of patients who remained in the study for enough time to undergo subsequent determination of B12 levels.
The findings in the current study are also in contrast to previous research, and the underlying mechanism of vitamin B12 in PD is also unclear.
Nevertheless, the results of the study by Dr. Christine and his colleagues are intriguing and further investigations to address this hypothesis are warranted.
Francisco Cardoso, MD, PhD, is with the movement disorders unit within the neurology service at the Federal University of Minas Gerais, Belo Horizonte, Brazil. His comments are derived from an editorial accompanying the study by Dr. Christine and colleagues (Movement Dis. 2018 Mar 6. doi: 10.1002/mds.27366). He had no relevant disclosures.
The course of PD can be quite variable, and it is difficult for clinicians to predict what will happen to an individual person with PD, but identifying prognostic factors ought to help practitioners answer their patients’ questions and potentially improve the understanding of mechanisms underlying the disease pathogenesis.
If vitamin B12 is related to the progression of PD as suggested in the study by Christine et al., its replacement may slow the decline of the disease. But there are several issues that need to be addressed in the context of their findings.
First, what constitutes a low vitamin B12 level is not a simple issue. If the evaluation is limited to measurement of vitamin B12 concentration, the diagnosis of genuine deficiency is unreliable. Most experts agree that combined measurement of vitamin B12 with determination of homocysteine levels is necessary and while Christine et al. measured both levels, their statistical analysis and subsequent conclusions are exclusively based on the levels of the vitamin.
Moreover, 34 patients in the study were classified as having “borderline-low” vitamin B12 levels, and 14 had both borderline-low B12 level and high homocysteine concentration. This brings into question whether the researchers identified PD patients who actually had low B12 levels.
The design of the DATATOP trial could also introduce some bias into the findings. At the time of publication, the study was criticized for disregarding the symptomatic effect of selegiline and for a lack of objective definition of criteria for the trial’s primary endpoint – introduction of levodopa.
This could have led to the termination of individuals at different stages of the disease, introducing a potential bias in the sample of patients who remained in the study for enough time to undergo subsequent determination of B12 levels.
The findings in the current study are also in contrast to previous research, and the underlying mechanism of vitamin B12 in PD is also unclear.
Nevertheless, the results of the study by Dr. Christine and his colleagues are intriguing and further investigations to address this hypothesis are warranted.
Francisco Cardoso, MD, PhD, is with the movement disorders unit within the neurology service at the Federal University of Minas Gerais, Belo Horizonte, Brazil. His comments are derived from an editorial accompanying the study by Dr. Christine and colleagues (Movement Dis. 2018 Mar 6. doi: 10.1002/mds.27366). He had no relevant disclosures.
The course of PD can be quite variable, and it is difficult for clinicians to predict what will happen to an individual person with PD, but identifying prognostic factors ought to help practitioners answer their patients’ questions and potentially improve the understanding of mechanisms underlying the disease pathogenesis.
If vitamin B12 is related to the progression of PD as suggested in the study by Christine et al., its replacement may slow the decline of the disease. But there are several issues that need to be addressed in the context of their findings.
First, what constitutes a low vitamin B12 level is not a simple issue. If the evaluation is limited to measurement of vitamin B12 concentration, the diagnosis of genuine deficiency is unreliable. Most experts agree that combined measurement of vitamin B12 with determination of homocysteine levels is necessary and while Christine et al. measured both levels, their statistical analysis and subsequent conclusions are exclusively based on the levels of the vitamin.
Moreover, 34 patients in the study were classified as having “borderline-low” vitamin B12 levels, and 14 had both borderline-low B12 level and high homocysteine concentration. This brings into question whether the researchers identified PD patients who actually had low B12 levels.
The design of the DATATOP trial could also introduce some bias into the findings. At the time of publication, the study was criticized for disregarding the symptomatic effect of selegiline and for a lack of objective definition of criteria for the trial’s primary endpoint – introduction of levodopa.
This could have led to the termination of individuals at different stages of the disease, introducing a potential bias in the sample of patients who remained in the study for enough time to undergo subsequent determination of B12 levels.
The findings in the current study are also in contrast to previous research, and the underlying mechanism of vitamin B12 in PD is also unclear.
Nevertheless, the results of the study by Dr. Christine and his colleagues are intriguing and further investigations to address this hypothesis are warranted.
Francisco Cardoso, MD, PhD, is with the movement disorders unit within the neurology service at the Federal University of Minas Gerais, Belo Horizonte, Brazil. His comments are derived from an editorial accompanying the study by Dr. Christine and colleagues (Movement Dis. 2018 Mar 6. doi: 10.1002/mds.27366). He had no relevant disclosures.
suggesting that it may be worthwhile to examine the correction of low levels as a means of slowing disease progression.
Investigators led by Chadwick W. Christine, MD, of the department of neurology at the University of California, San Francisco, sought to understand what contributes to considerable variation in the progression of PD by building on previous research that revealed low serum vitamin B12 levels are common in patients with moderately advanced PD and are associated with neuropathy and cognitive impairment.
They measured serum methylmalonic acid, homocysteine, and holotranscobalamin in addition to B12 because of the limited sensitivity of serum B12 testing alone to detect B12 deficiency. At baseline, 13% of 680 patients had borderline-low B12 levels (less than 184 pmol/L [250 pg/mL]), and 5% had deficient B12 levels (less than 157 pmol/L or 212 pg/mL). Homocysteine was moderately elevated (greater than 15 mmol/L) in 7% of subjects, and 14% of patients with borderline-low B12 also had elevated homocysteine, the investigators reported in Movement Disorders.
Low B12 at baseline predicted greater worsening of mobility in terms of a higher ambulatory capacity score, calculated by adding individual items of the Unified Parkinson’s Disease Rating Scale (UPDRS): falling, freezing when walking, walking, gait, and postural stability. Participants in the low- B12 tertile (less than 234 pmol/L or 317 pg/ mL) developed greater morbidity as assessed by greater annualized worsening of the ambulatory capacity score. For example, those in the low-B12 tertile had annualized change of 1.53, compared with 0.77 in the upper tertile. The worsening score was mostly attributed to poorer gait and postural instability.
To give context to these figures, the researchers pointed to an analysis of the NET-PD LS1 cohort that found a mean ambulatory capacity score of 2.17 among patients who fell and a score of 1.4 for those who did not fall. “Thus, we consider the magnitude of difference to be clinically relevant, particularly given that components of gait dysfunction that develop in PD may not respond to dopaminergic treatments or [deep brain stimulation],” they wrote.
Elevated homocysteine also predicted greater cognitive decline. Baseline elevated homocysteine was associated with lower baseline Mini Mental State Examination (MMSE), as well as greater annualized decline in MMSE (–1.96 vs. 0.06; P = .001).
Of the 456 subjects who continued in the study for 9-24 months and had a second blood sample available, 226 had an increase of more than 20% in B12 levels, 210 stayed within 20% of the original B12 measurement, and 19 had a decrease greater than 20%.
Overall, there was a mean annualized increase in B12 of 52.6 pmol/L, a mean annualized decrease of homocysteine of 0.83 mmol/L, and a mean annualized increase of holotranscobalamin of 14.7 pmol/L.
“These findings are consistent with improved nutritional status during the course of the study, likely attributed to subjects starting the optional [multivitamin] after the baseline visit and/or subjects changing their diets,” the research team said.
While the improvement in B12 status did not lead to statistically significant improvements in UPDRS scores, the researchers said there was a trend toward improvement, which provides “support for a disease-modifying effect of B12,” they wrote.
The researchers speculated that their findings of a link between low B12 levels and worse outcomes could be attributed to an independent (comorbid) effect on the central and peripheral nervous systems, a direct effect on PD pathogenesis, or alternatively, that low B12 may be a marker of an unknown associated factor.
“Given that low B12 status is associated with neurological and other medical morbidities and is readily treated, great care would be needed to design an ethically acceptable randomized, prospective study to evaluate the effect of B12 supplementation on PD progression, given that serum measurements collected as part of a prospective study in unsupplemented patients would likely reveal some subjects with B12 deficiency,” they concluded.
The authors declared no relevant disclosures. The study was supported by a grant from the Michael J. Fox Foundation for Parkinson’s Research, as well as privately donated grants.
SOURCE: Christine C et al. Movement Dis. 2018 Mar 6. doi: 10.1002/mds.27301.
suggesting that it may be worthwhile to examine the correction of low levels as a means of slowing disease progression.
Investigators led by Chadwick W. Christine, MD, of the department of neurology at the University of California, San Francisco, sought to understand what contributes to considerable variation in the progression of PD by building on previous research that revealed low serum vitamin B12 levels are common in patients with moderately advanced PD and are associated with neuropathy and cognitive impairment.
They measured serum methylmalonic acid, homocysteine, and holotranscobalamin in addition to B12 because of the limited sensitivity of serum B12 testing alone to detect B12 deficiency. At baseline, 13% of 680 patients had borderline-low B12 levels (less than 184 pmol/L [250 pg/mL]), and 5% had deficient B12 levels (less than 157 pmol/L or 212 pg/mL). Homocysteine was moderately elevated (greater than 15 mmol/L) in 7% of subjects, and 14% of patients with borderline-low B12 also had elevated homocysteine, the investigators reported in Movement Disorders.
Low B12 at baseline predicted greater worsening of mobility in terms of a higher ambulatory capacity score, calculated by adding individual items of the Unified Parkinson’s Disease Rating Scale (UPDRS): falling, freezing when walking, walking, gait, and postural stability. Participants in the low- B12 tertile (less than 234 pmol/L or 317 pg/ mL) developed greater morbidity as assessed by greater annualized worsening of the ambulatory capacity score. For example, those in the low-B12 tertile had annualized change of 1.53, compared with 0.77 in the upper tertile. The worsening score was mostly attributed to poorer gait and postural instability.
To give context to these figures, the researchers pointed to an analysis of the NET-PD LS1 cohort that found a mean ambulatory capacity score of 2.17 among patients who fell and a score of 1.4 for those who did not fall. “Thus, we consider the magnitude of difference to be clinically relevant, particularly given that components of gait dysfunction that develop in PD may not respond to dopaminergic treatments or [deep brain stimulation],” they wrote.
Elevated homocysteine also predicted greater cognitive decline. Baseline elevated homocysteine was associated with lower baseline Mini Mental State Examination (MMSE), as well as greater annualized decline in MMSE (–1.96 vs. 0.06; P = .001).
Of the 456 subjects who continued in the study for 9-24 months and had a second blood sample available, 226 had an increase of more than 20% in B12 levels, 210 stayed within 20% of the original B12 measurement, and 19 had a decrease greater than 20%.
Overall, there was a mean annualized increase in B12 of 52.6 pmol/L, a mean annualized decrease of homocysteine of 0.83 mmol/L, and a mean annualized increase of holotranscobalamin of 14.7 pmol/L.
“These findings are consistent with improved nutritional status during the course of the study, likely attributed to subjects starting the optional [multivitamin] after the baseline visit and/or subjects changing their diets,” the research team said.
While the improvement in B12 status did not lead to statistically significant improvements in UPDRS scores, the researchers said there was a trend toward improvement, which provides “support for a disease-modifying effect of B12,” they wrote.
The researchers speculated that their findings of a link between low B12 levels and worse outcomes could be attributed to an independent (comorbid) effect on the central and peripheral nervous systems, a direct effect on PD pathogenesis, or alternatively, that low B12 may be a marker of an unknown associated factor.
“Given that low B12 status is associated with neurological and other medical morbidities and is readily treated, great care would be needed to design an ethically acceptable randomized, prospective study to evaluate the effect of B12 supplementation on PD progression, given that serum measurements collected as part of a prospective study in unsupplemented patients would likely reveal some subjects with B12 deficiency,” they concluded.
The authors declared no relevant disclosures. The study was supported by a grant from the Michael J. Fox Foundation for Parkinson’s Research, as well as privately donated grants.
SOURCE: Christine C et al. Movement Dis. 2018 Mar 6. doi: 10.1002/mds.27301.
FROM MOVEMENT DISORDERS
Key clinical point: Prevention or early correction of low B12 may potentially be a therapeutic target to slow progression in early Parkinson’s disease.
Main finding: Participants in the lowest B12 tertile developed greater morbidity with an annualized worsening of the ambulatory capacity score of 1.53, compared with 0.77 in patients in the upper tertile.
Study details: Analysis of vitamin B12 in 680 baseline and 456 follow-up serum samples of patients with PD participating in the double-blind, randomized DATATOP trial.
Disclosures: The authors declared no relevant disclosures. The study was supported by a grant from the Michael J. Fox Foundation for Parkinson’s Research, as well as privately donated grants.
Source: Christine C et al. Movement Dis. 2018 Mar 6. doi: 10.1002/mds.27301.
Docs worry there’s ‘nowhere to send’ new and expectant moms with depression
Lawmakers in California will begin debate next month on a bill that would require doctors to screen new moms for mental health problems – once while they’re pregnant and again after they give birth.
But many obstetricians and pediatricians bristle at the idea, saying they are afraid to screen new moms for depression and anxiety.
“What are you going to do with those people who screen positive?” asked Laura L. Sirott, MD, an ob.gyn. who practices in Pasadena. “Some providers have nowhere to send them.”
Nationally, depression affects up to one in seven women during or after pregnancy, according to the American Psychological Association.
And, of women who screen positive for the condition, 78% don’t get mental health treatment, according to a 2015 research review published in the journal Obstetrics & Gynecology.
Dr. Sirott said her patients give a range of reasons why they don’t take her up on a referral to a psychologist: “ ‘Oh, they don’t take my insurance.’ Or ‘my insurance pays for three visits.’ ‘I can’t take time off work to go to those visits.’ ‘It’s a 3-month wait to get in to that person.’ ”
She said it’s also hard to find a psychiatrist who is willing to treat them and who is trained in the complexities of prescribing medications to pregnant or breastfeeding women, especially in rural areas.
“So it’s very frustrating,” Dr. Sirott said, “to ask patients about a problem and then not have any way to solve that problem.”
Moms are frustrated, too. After the baby comes, no one asks about the baby’s mother anymore.
Wendy Root Askew struggled for years to get pregnant, and when she finally did, her anxiety got worse. She couldn’t stop worrying that something would go wrong.
“And then, after I had my son, I would have these dreams where someone would come to the door and they would say, ‘Well, you know, we’re just going to wait 2 weeks to see if you get to keep your baby or not,’ ” Ms. Root Askew said. “And it really impacted my ability to bond with him.”
She likes California’s bill, AB 2193, because it goes beyond mandated screening. It would require health insurance companies to set up case management programs to help moms find a therapist, and connect obstetricians or pediatricians to a psychiatric specialist.
“Just like we have case-management programs for patients who have diabetes or sleep issues or back pain, a case-management program requires the insurance company to take some ownership of making sure their patients are getting the treatment they need to be healthy,” said Ms. Root Askew, who is now advocating for the bill on behalf of the group 2020 Mom.
Health insurance companies haven’t taken a position on the legislation. It’s unclear how much it would cost them to comply, because some already have infrastructure in place for case-management programs, and some do not. But there is consensus among insurers and health advocates that such programs save money in the long run.
“The sooner that you can get good treatment for a mom, the less expensive that condition will be to manage over the course of the woman’s life and over the course of that child’s life,” Ms. Root Askew said.
Some doctors still have their objections. Under the bill, they could be disciplined for not screening. Some have said they worry about how much time it would take.
The health care system, and the incentives, aren’t set up for this sort of screening, Dr. Sirott said.
“Currently, I get $6 for screening a patient,” she said. “By the time I put it on a piece of paper and print it, it’s not worth it.”
It’s not clear whether the direct and indirect costs of screening would be worth it to the patients, either. Four other states – Illinois, Massachusetts, New Jersey, and West Virginia – have tried mandated screening, and it did not result in more women getting treatment, according to a 2015 study published in Psychiatric Services.
Even with California’s extra requirement that insurance companies facilitate care, women could still face high copays or limits on the number of therapy sessions. Or, a new mother might be so overwhelmed with care for her newborn that it would be difficult to add anything to her busy schedule.
What does seem to work, according to the study of mandated screening in other states, is when nurses or mental health providers visit new moms at home.
“Despite abundant goodwill, there is no evidence that state policies are addressing this great need,” the study’s authors report.
Supporters of California’s proposed bill, however, say doctors need to start somewhere. Screening is the first step in recognizing the full scope of the problem, said Nirmaljit Dhami, MD, a Mountain View, Calif., psychiatrist. Women should be screened on an ongoing basis throughout pregnancy and for a year after birth, Dr. Dhami said, not just once or twice as the bill requires.
“I often tell doctors that if you don’t know that somebody is suicidal, it doesn’t mean that their suicidality will go away,” she said. “If you don’t ask, the risk is the same.”
This story is part of a partnership that includes KQED, NPR, and Kaiser Health News. KHN’s coverage of women’s health care issues is supported in part by The David and Lucile Packard Foundation. KHN is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Lawmakers in California will begin debate next month on a bill that would require doctors to screen new moms for mental health problems – once while they’re pregnant and again after they give birth.
But many obstetricians and pediatricians bristle at the idea, saying they are afraid to screen new moms for depression and anxiety.
“What are you going to do with those people who screen positive?” asked Laura L. Sirott, MD, an ob.gyn. who practices in Pasadena. “Some providers have nowhere to send them.”
Nationally, depression affects up to one in seven women during or after pregnancy, according to the American Psychological Association.
And, of women who screen positive for the condition, 78% don’t get mental health treatment, according to a 2015 research review published in the journal Obstetrics & Gynecology.
Dr. Sirott said her patients give a range of reasons why they don’t take her up on a referral to a psychologist: “ ‘Oh, they don’t take my insurance.’ Or ‘my insurance pays for three visits.’ ‘I can’t take time off work to go to those visits.’ ‘It’s a 3-month wait to get in to that person.’ ”
She said it’s also hard to find a psychiatrist who is willing to treat them and who is trained in the complexities of prescribing medications to pregnant or breastfeeding women, especially in rural areas.
“So it’s very frustrating,” Dr. Sirott said, “to ask patients about a problem and then not have any way to solve that problem.”
Moms are frustrated, too. After the baby comes, no one asks about the baby’s mother anymore.
Wendy Root Askew struggled for years to get pregnant, and when she finally did, her anxiety got worse. She couldn’t stop worrying that something would go wrong.
“And then, after I had my son, I would have these dreams where someone would come to the door and they would say, ‘Well, you know, we’re just going to wait 2 weeks to see if you get to keep your baby or not,’ ” Ms. Root Askew said. “And it really impacted my ability to bond with him.”
She likes California’s bill, AB 2193, because it goes beyond mandated screening. It would require health insurance companies to set up case management programs to help moms find a therapist, and connect obstetricians or pediatricians to a psychiatric specialist.
“Just like we have case-management programs for patients who have diabetes or sleep issues or back pain, a case-management program requires the insurance company to take some ownership of making sure their patients are getting the treatment they need to be healthy,” said Ms. Root Askew, who is now advocating for the bill on behalf of the group 2020 Mom.
Health insurance companies haven’t taken a position on the legislation. It’s unclear how much it would cost them to comply, because some already have infrastructure in place for case-management programs, and some do not. But there is consensus among insurers and health advocates that such programs save money in the long run.
“The sooner that you can get good treatment for a mom, the less expensive that condition will be to manage over the course of the woman’s life and over the course of that child’s life,” Ms. Root Askew said.
Some doctors still have their objections. Under the bill, they could be disciplined for not screening. Some have said they worry about how much time it would take.
The health care system, and the incentives, aren’t set up for this sort of screening, Dr. Sirott said.
“Currently, I get $6 for screening a patient,” she said. “By the time I put it on a piece of paper and print it, it’s not worth it.”
It’s not clear whether the direct and indirect costs of screening would be worth it to the patients, either. Four other states – Illinois, Massachusetts, New Jersey, and West Virginia – have tried mandated screening, and it did not result in more women getting treatment, according to a 2015 study published in Psychiatric Services.
Even with California’s extra requirement that insurance companies facilitate care, women could still face high copays or limits on the number of therapy sessions. Or, a new mother might be so overwhelmed with care for her newborn that it would be difficult to add anything to her busy schedule.
What does seem to work, according to the study of mandated screening in other states, is when nurses or mental health providers visit new moms at home.
“Despite abundant goodwill, there is no evidence that state policies are addressing this great need,” the study’s authors report.
Supporters of California’s proposed bill, however, say doctors need to start somewhere. Screening is the first step in recognizing the full scope of the problem, said Nirmaljit Dhami, MD, a Mountain View, Calif., psychiatrist. Women should be screened on an ongoing basis throughout pregnancy and for a year after birth, Dr. Dhami said, not just once or twice as the bill requires.
“I often tell doctors that if you don’t know that somebody is suicidal, it doesn’t mean that their suicidality will go away,” she said. “If you don’t ask, the risk is the same.”
This story is part of a partnership that includes KQED, NPR, and Kaiser Health News. KHN’s coverage of women’s health care issues is supported in part by The David and Lucile Packard Foundation. KHN is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Lawmakers in California will begin debate next month on a bill that would require doctors to screen new moms for mental health problems – once while they’re pregnant and again after they give birth.
But many obstetricians and pediatricians bristle at the idea, saying they are afraid to screen new moms for depression and anxiety.
“What are you going to do with those people who screen positive?” asked Laura L. Sirott, MD, an ob.gyn. who practices in Pasadena. “Some providers have nowhere to send them.”
Nationally, depression affects up to one in seven women during or after pregnancy, according to the American Psychological Association.
And, of women who screen positive for the condition, 78% don’t get mental health treatment, according to a 2015 research review published in the journal Obstetrics & Gynecology.
Dr. Sirott said her patients give a range of reasons why they don’t take her up on a referral to a psychologist: “ ‘Oh, they don’t take my insurance.’ Or ‘my insurance pays for three visits.’ ‘I can’t take time off work to go to those visits.’ ‘It’s a 3-month wait to get in to that person.’ ”
She said it’s also hard to find a psychiatrist who is willing to treat them and who is trained in the complexities of prescribing medications to pregnant or breastfeeding women, especially in rural areas.
“So it’s very frustrating,” Dr. Sirott said, “to ask patients about a problem and then not have any way to solve that problem.”
Moms are frustrated, too. After the baby comes, no one asks about the baby’s mother anymore.
Wendy Root Askew struggled for years to get pregnant, and when she finally did, her anxiety got worse. She couldn’t stop worrying that something would go wrong.
“And then, after I had my son, I would have these dreams where someone would come to the door and they would say, ‘Well, you know, we’re just going to wait 2 weeks to see if you get to keep your baby or not,’ ” Ms. Root Askew said. “And it really impacted my ability to bond with him.”
She likes California’s bill, AB 2193, because it goes beyond mandated screening. It would require health insurance companies to set up case management programs to help moms find a therapist, and connect obstetricians or pediatricians to a psychiatric specialist.
“Just like we have case-management programs for patients who have diabetes or sleep issues or back pain, a case-management program requires the insurance company to take some ownership of making sure their patients are getting the treatment they need to be healthy,” said Ms. Root Askew, who is now advocating for the bill on behalf of the group 2020 Mom.
Health insurance companies haven’t taken a position on the legislation. It’s unclear how much it would cost them to comply, because some already have infrastructure in place for case-management programs, and some do not. But there is consensus among insurers and health advocates that such programs save money in the long run.
“The sooner that you can get good treatment for a mom, the less expensive that condition will be to manage over the course of the woman’s life and over the course of that child’s life,” Ms. Root Askew said.
Some doctors still have their objections. Under the bill, they could be disciplined for not screening. Some have said they worry about how much time it would take.
The health care system, and the incentives, aren’t set up for this sort of screening, Dr. Sirott said.
“Currently, I get $6 for screening a patient,” she said. “By the time I put it on a piece of paper and print it, it’s not worth it.”
It’s not clear whether the direct and indirect costs of screening would be worth it to the patients, either. Four other states – Illinois, Massachusetts, New Jersey, and West Virginia – have tried mandated screening, and it did not result in more women getting treatment, according to a 2015 study published in Psychiatric Services.
Even with California’s extra requirement that insurance companies facilitate care, women could still face high copays or limits on the number of therapy sessions. Or, a new mother might be so overwhelmed with care for her newborn that it would be difficult to add anything to her busy schedule.
What does seem to work, according to the study of mandated screening in other states, is when nurses or mental health providers visit new moms at home.
“Despite abundant goodwill, there is no evidence that state policies are addressing this great need,” the study’s authors report.
Supporters of California’s proposed bill, however, say doctors need to start somewhere. Screening is the first step in recognizing the full scope of the problem, said Nirmaljit Dhami, MD, a Mountain View, Calif., psychiatrist. Women should be screened on an ongoing basis throughout pregnancy and for a year after birth, Dr. Dhami said, not just once or twice as the bill requires.
“I often tell doctors that if you don’t know that somebody is suicidal, it doesn’t mean that their suicidality will go away,” she said. “If you don’t ask, the risk is the same.”
This story is part of a partnership that includes KQED, NPR, and Kaiser Health News. KHN’s coverage of women’s health care issues is supported in part by The David and Lucile Packard Foundation. KHN is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
VIDEO: Researchers closing in on the elusive ‘male pill’
CHICAGO –
It blocks gonadotropin signaling and testosterone production in the testes. When capsules ranging in dose from 100 mg to 400 mg were given once daily to 100 men in a randomized, placebo controlled trial, the drop in testosterone was more than sufficient to block sperm production. Testosterone levels jumped back up to normal after the end of the 28-day trial, all without inducing liver toxicity or other serious problems.
“We are very excited [about] the results. It’s a big step forward in the development of the male pill. Our last great advance in male contraception was over 300 years ago with the development of the condom,” said senior investigator Stephanie Page, MD, PhD, head of the division of metabolism, endocrinology, and nutrition at the University of Washington, Seattle.
Some men reported a drop in libido that resolved by the end of the study. There was also mild weight gain and small reductions in HDL cholesterol. A few tweaks to the formulation or dosing might fix those problems. “Overall, we were very encouraged about the safety profile,” Dr. Page said.
She explained the work, its promise, and the next steps in a video interview at the Endocrine Society’s annual meeting. The National Institutes of Health is funding development. Dr. Page didn’t have any relevant disclosures.
SOURCE: Page S et al. ENDO 2018, Abstract OR15-2.
CHICAGO –
It blocks gonadotropin signaling and testosterone production in the testes. When capsules ranging in dose from 100 mg to 400 mg were given once daily to 100 men in a randomized, placebo controlled trial, the drop in testosterone was more than sufficient to block sperm production. Testosterone levels jumped back up to normal after the end of the 28-day trial, all without inducing liver toxicity or other serious problems.
“We are very excited [about] the results. It’s a big step forward in the development of the male pill. Our last great advance in male contraception was over 300 years ago with the development of the condom,” said senior investigator Stephanie Page, MD, PhD, head of the division of metabolism, endocrinology, and nutrition at the University of Washington, Seattle.
Some men reported a drop in libido that resolved by the end of the study. There was also mild weight gain and small reductions in HDL cholesterol. A few tweaks to the formulation or dosing might fix those problems. “Overall, we were very encouraged about the safety profile,” Dr. Page said.
She explained the work, its promise, and the next steps in a video interview at the Endocrine Society’s annual meeting. The National Institutes of Health is funding development. Dr. Page didn’t have any relevant disclosures.
SOURCE: Page S et al. ENDO 2018, Abstract OR15-2.
CHICAGO –
It blocks gonadotropin signaling and testosterone production in the testes. When capsules ranging in dose from 100 mg to 400 mg were given once daily to 100 men in a randomized, placebo controlled trial, the drop in testosterone was more than sufficient to block sperm production. Testosterone levels jumped back up to normal after the end of the 28-day trial, all without inducing liver toxicity or other serious problems.
“We are very excited [about] the results. It’s a big step forward in the development of the male pill. Our last great advance in male contraception was over 300 years ago with the development of the condom,” said senior investigator Stephanie Page, MD, PhD, head of the division of metabolism, endocrinology, and nutrition at the University of Washington, Seattle.
Some men reported a drop in libido that resolved by the end of the study. There was also mild weight gain and small reductions in HDL cholesterol. A few tweaks to the formulation or dosing might fix those problems. “Overall, we were very encouraged about the safety profile,” Dr. Page said.
She explained the work, its promise, and the next steps in a video interview at the Endocrine Society’s annual meeting. The National Institutes of Health is funding development. Dr. Page didn’t have any relevant disclosures.
SOURCE: Page S et al. ENDO 2018, Abstract OR15-2.
REPORTING FROM ENDO 2018
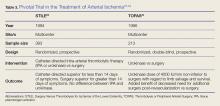
Acute Limb Arterial Ischemia
Acute limb ischemia (ALI) is an emergent medical condition that is characterized by a precipitous decrease in limb perfusion that threatens the viability of the affected limb, and symptoms that have been present for 14 days or less.1-5 The incidence of lower extremity ALI in the US is nine to 16 cases per 100,000 persons per year, and one to three cases per 100,000 persons per year for upper extremity ALI.3 Symptoms suggestive of this condition classically include pain in the effected limb at rest, loss of sensation, impaired motor function, and cyanosis/pallor. Irreparable damage can occur in as quickly as 4 to 6 hours with complete arterial occlusion. A majority of ALI is caused by thrombosis, with the remainder of cases caused by embolism, 85% and 15% respectively.1-6 If not addressed promptly, this condition carries a high degree of morbidity (ie, limb loss) and mortality. Immediate initiation of anticoagulation therapy and vascular surgery consultation are mainstays of ED management. Time-to-treatment is a predictor of success and is an area where the emergency physician (EP) can make a significant difference in patient outcomes.7 Although vascular interruption via traumatic mechanism is not addressed in this review, it should remain a consideration in relation to the historical features of each case.
Presentation
Traditionally, symptoms of ALI can be remembered as the “six Ps”: pain, pallor, paresthesia, paralysis, pulselessness, and poikilothermia. Note that ALI represents a spectrum of disease, and the presenting features depend heavily on the level and degree of obstruction. Pain, however, is most frequently the first presenting symptom of patients with ALI.1-6 Pain will occur in muscle groups distal to the occlusion. Obstruction of the aortoiliac region might produce pain in the buttocks, thigh, and hip, whereas occlusion of the femoral artery may produce pain in the calf. Single-level disease will often produce claudication, but multilevel disease may present as a nonhealing ulcer or with focal gangrene. The differential diagnosis of ALI includes direct arterial injury, vasospasm, compartment syndrome, chronic peripheral artery disease, stroke, spinal cord injury, vasculitis, muscular trauma, and radiculopathy.3,5
Etiology
As stated previously, ALI is a result of either thrombotic or embolic phenomena that either partially or completely occludes a vessel such that adequate perfusion is no longer achieved. Consequently, there is a decrease in the metabolism of the tissue supplied in those territories, which can rapidly progress to necrosis. Thrombosis is the most common cause of ALI, accounting for approximately 85%.2,3Typically, thrombosis presents in the setting of pre-existing peripheral artery disease (PAD).8 As PAD worsens, damage to the arterial endothelium triggers platelet activation, and accumulation in a manner conceptually similar to thrombosis in myocardial infarction.9 Hypercoagulable states increase the risk of thrombi development.10 Symptoms of thrombosis are often more insidious in onset when compared to embolism, and signs/symptoms of prior claudication are almost always present. Consequently, there is a greater chance that collateral circulation has developed over time.2,6,11,12
By contrast, ALI caused by embolism is more acute in onset, and is often more emergent due to complete occlusion without chance of collateral vessel development.1,3,6,12 See Table 1 for differences in risk factors and presentation between thrombotic and embolic occlusion.
Historical Features
Information should be obtained systematically in all presentations and should include time of symptom onset, duration, severity, and location; however, special attention should be paid to the evolution of symptoms over time. One historical pearl that should raise suspicion of embolism vs thrombus is a patient history with a specific time of symptom onset. Specific questioning about history of claudication, including description of typical symptoms, is critical. A full list of other medical comorbidities should be obtained. This may reveal risk factors for thrombotic and embolic disease.1,3-5 This also may alter available treatment options.7,8,12,13 At presentation, the EP should obtain a list of the patient’s current medications, and special attention should be given to prothrombotic medications and medications that may interact with anticoagulants.
Physical Examination
On physical examination, the EP should pay particular attention to both the external appearance and temperature of the skin of the patient’s affected extremity. The presence or absence of peripheral pulses is an important feature when assessing for ALI; however, the presence of peripheral pulses does not exclude ALI from the differential.
Precise evaluation of sensation is important to attempt to quantify the extent of injury, localize a possible obstruction, and differentiate ALI from other possible causes of sensation deficits. Though not referenced in the six Ps, the patient’s limb may also appear mottled or marbled. Embolic phenomena occur at sites of vascular bifurcation. In a patient with aortic embolism, femoral pulses will be absent with bilateral mottling and paralysis of the lower extremities. Iliac artery embolism will produce the same symptoms, except unilateral. Femoral artery embolism will yield coolness and mottling distal to the inguinal ligament. Similarly, popliteal artery embolism will produce those same findings, except distal to the knee with preserved femoral pulses.
Diagnosis and Evaluation
After the history and physical examination are completed, further diagnostic methodologies should be considered, including laboratory evaluation and additional imaging studies.
Imaging Studies
Doppler ultrasound examination to assess for pulses on the affected limb should be performed immediately. Bedside continuous-wave Doppler may be used to differentiate arterial vs venous signal.6,14-16 An ankle brachial index may be calculated through either direct auscultation of pulses or Doppler signal. A ratio of less than 0.9 is suggestive of ischemia, while a ratio of less than 0.5 is considered critical ischemia.2
Laboratory Evaluation
Laboratory evaluation of hematocrit, coagulation studies, renal function, electrolyte levels, lactate, and creatinine kinase should be completed.1,5 An electrocardiogram and bedside/formal echocardiogram may be considered if embolism is suspected.1,6,17
Imaging
Angiography is currently the gold standard imaging modality to diagnose ALI.16 However, other imaging modalities exist that expose the patient to less radiation and are generally preferred by patients.18 Duplex ultrasonography is the least invasive imaging modality, with a reported sensitivity of 88% and specificity of 96% for the detection of greater than 50% stenosis.6,19 Contrast-enhanced magnetic resonance angiography (MRA) of the lower extremity is also relatively noninvasive, but is more limited in its application. It carries a reported sensitivity of 95% and a specificity of 97% for detection of stenosis of greater than 50%.6,19 An MRA without contrast may be considered for patients with an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 and who are not on dialysis, but the diagnostic efficacy is reduced.16 Finally, the sensitivity and specificity of computed tomography angiography (CTA) to detect aortoiliac stenosis of greater than 50% is 96% and 98% respectively.6,18,19 Other studies demonstrated similar results for other arterial locations in the lower extremities.18,19 A principal advantage of CTA is that direct visualization of calcifications, clips, stents, and prior bypasses is possible without the limitations of MRA.16 The American College of Radiology (ACR) lists ultrasound duplex Doppler and noncontrast MRA of the lower extremity as “may be appropriate.”16 In contrast, the ACR classifies MRA with contrast, CTA with contrast, and angiography of the lower extremity as “usually appropriate.”16 Selecting the optimal imaging study for a patient may require involvement of a facility’s radiology department and/or a vascular surgeon.14,15
Management in the ED
Even before the definitive diagnosis of ALI is made, steps can be taken to minimize and/or slow progression of injury. Placing the affected limb in a dependent position and providing intravenous (IV) fluid hydration will maximize perfusion.1,3,5 Even with consideration of the diagnostic modalities previously described, ALI is often considered a clinical diagnosis. Since time is critical, early consultation with vascular surgery services is imperative if ALI is suspected.
Intravenous Fluid Therapy
Suspected ALI should be treated in the ED with an IV heparin bolus, followed by constant IV infusion. Heparin prevents proximal and distal propagation of the thrombus.1,3,5,6,15,17,20 Additionally, it helps maintain the microcirculation surrounding the affected area.6 A heparin bolus dose of 100 U/kg followed by a continuous infusion of heparin 1,000 U/h is the recommended standard.1,3,5,6,15 A goal partial thromboplastin time of 60 to 100 seconds, or an international normalized ratio of 2 to 3 is desirable.8,21
Staging: The Rutherford Classification System
Once immediate therapies are started, the stage of PAD should be determined using the Rutherford classification system in collaboration with vascular surgery services; this will further guide treatment and disposition.
Category I. In category I ALI, the affected limb is considered viable and not immediately threatened.
Category IIa. The limb is considered to be marginally threatened and salvageable.
Category IIb. An ALI classified as a category IIb is the most urgent type in which the limb is immediately threatened.
Category III. The limb is classified as having irreversible damage.2,3,17,22
(See Table 2 for a more detailed summary of the Rutherford classification system.)
Treatment
Definitive treatment decisions should be made in consultation with vascular surgery services. The results of three clinical trials developed the foundation for management of ALI: the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial, Surgery Versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial, and Rutherford trial.22-24 Table 3 provides a summary of the treatment techniques utilized, which include mechanical recanalization with percutaneous aspiration thrombectomy, percutaneous mechanical thrombectomy,6,12,25-28 pharmacological recanalization via catheter-directed thrombolysis,6,8,12,20,23-28 and/or surgical management via thrombectomy or embolectomy.6,11,23,24,27
Complications
The complications of treating ALI can be stratified into three subsets: pharmacologic, mechanical, and reperfusion injury-related. Medical treatment with heparin is not without risk. Intracranial hemorrhage, major bleeding at other sites, and compartment syndrome secondary to bleeding can all occur. Treatment may also cause distal embolization and lead to mechanical occlusion of another site. Finally, reperfusion injury predisposes the patient to a host of new problems. Reperfusion injury occurs when fresh blood enters a previously ischemic zone. When this occurs, oxygen free radicals, inflammatory mediators, and catabolism byproducts mix with the blood. This process can damage surrounding epithelial cells and lead to increased interstitial permeability. As a result, compartment syndrome may also develop via this mechanism.1,3,5,6 The anterior compartment of the lower extremity is most at risk. As such, assessment of peroneal nerve activity via dorsiflexion of the foot and sensation testing is important after the initiation of treatment.6 Systemic distribution of these harmful byproducts can also cause arrhythmias, renal failure, and systemic acadisos.1,3,5,6 It is important for physicians and staff to monitor the patient closely for the development of these complications and be prepared to intervene.
Opportunities for Improvement
In a condition where minutes matter, expedient action is critical. Time-to-recognition, imaging, consultation, and intervention all are potential sources of delay. Two recent studies have investigated the treatment timeline of ALI. The first study showed that the greatest source of delay is time from symptom onset to presentation in the ED; an average of 11.35 hours. The second largest source of delay was time from recognition of ALI to imaging, with an average delay of 4.75 hours. The average ED evaluation time was 40 minutes, and the average total time to intervention was 10.2 hours. While time-to-symptom presentation may represent a failure of public health, time-to-imaging was identified as an area of unacceptable delay by the authors.29 A second study examined the effect of pre-hospital care on the time-to-treatment. Persons who were transported via emergency medical services (EMS) arrived to the hospital at a median time of 5 hours after symptom onset, were seen by a physician at a median time of 51 minutes, and had revascularization at a median time of 23 hours. Those not transported by EMS arrived to the hospital at a median time of 48 hours after symptom onset, were seen by a physician at a median time of 80 minutes, and had revascularization at a median time of 93 hours. As a secondary goal, the study examined the effect of heparinization in the ED and showed that treatment with heparin was associated with a favorable outcome.30 While the total treatment times in each study vary widely, they share several important commonalities and reinforce expedient management as an important goal.
Outcomes
Advances in the treatment of this condition began in the 1970s, with an explosion of new techniques and data in the past several years. Still, ALI is a high morbidity and high mortality condition. Rates of limb loss are approximately 30% for all cases, and it is a fatal condition for as many as one-in-five. As discussed earlier, the patients who are most likely to experience ALI typically have several medical comorbidities. Perhaps unsurprisingly, ALI portends a poor prognosis even if the limb is salvaged. Approximately 15% to 20% of patients with ALI will die within 1 year of diagnosis.3,4,6,12
Conclusion
Acute limb ischemia is a rare condition, but a true medical emergency. Prompt recognition of the signs and symptoms of ALI are critical for optimizing patient outcomes. A high index of clinical suspicion in patients with risk factors may lead to early diagnosis. Early vascular surgery consultation and early IV heparin treatment are important aspects of care. Prompt imaging and staging guide further management. Special care should be paid to possible post-treatment complications and to the investigation of contributing factors. The EP has a great opportunity to positively impact care with expeditious management. Early diagnosis, targeted history and physical examination, prompt treatment, collaboration with specialists, and prevention and treatment of life-threatening complications are all hallmarks of emergency medicine.
1. Braun R, Lin M. Acute limb ischemia: a case report and literature review. J Emerg Med. 2015;49(6):1011-1017. doi:10.1016/j.jemermed.2015.03.008.
2. Callum K, Bradbury A. ABC of arterial and venous disease: acute limb ischaemia. BMJ. 2000;320(7237):764-767.
3. Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366(23):2198-2206. doi:10.1056/NEJMcp1006054.
4. Purushottam B, Gujja K, Zalewski A, Krishnan P. Acute limb ischemia. Interv Cardiol Clin. 2014;3(4):557-572.
5. Santistevan JR. Acute limb ischemia: an emergency medicine approach. Emerg Med Clin North Am. 2017;35(4):889-909. doi:10.1016/j.emc.2017.07.006.
6. Acar RD, Sahin M, Kirma C. One of the most urgent vascular circumstances: acute limb ischemia. SAGE Open Med. 2013;1:2050312113516110. doi:10.1177/2050312113516110.
7. Abou-Zamzam AM Jr, Gomez NR, Molkara A, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21(4):458-463.
8. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465-1508.
9. Rajagopalan S, Mckay I, Ford I, Bachoo P, Greaves M, Brittenden J. Platelet activation increases with the severity of peripheral arterial disease: implications for clinical management. J Vasc Surg. 2007;46(3):485-490.
10. Deitcher SR, Carman TL, Sheikh MA, Gomes M. Hypercoagulable syndromes: evaluation and management strategies for acute limb ischemia. Semin Vasc Surg. 2001;14(2):74-85.
11. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84(6):822-834.
12. Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59(4):988-995.
13. Soden PA, Zettervall SL, Curran T, et al. Regional variation in patient selection and treatment for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg. 2017;65(1):108-118. doi:10.1016/j.jvs.2016.06.105.
14. Aboyans V, Björck M, Brodmann M, et al; ESC Scientific Document Group. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: a companion document of the 2017 ESC Guidelines for the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Endorsed by: the European Stroke Organisation (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2017. doi:10.1093/eurheartj/ehx499. [Epub ahead of print]
15. Authors/Task Force Members, Aboyans V, Ricco JB, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017. doi:10.1016/j.ejvs.2017.07.018. [Epub ahead of print]
16. Expert Panel on Vascular Imaging: Weiss CR, Azene EM, Majdalany BS, et al. ACR Appropriateness Criteria® sudden onset of cold, painful leg. J Am Coll Radiol. 2017;14(5S):S307-S313. doi:10.1016/j.jacr.2017.02.015.
17. Dieter RS, Dieter RA Jr, Dieter RA 3rd, Nanjundappa A. Critical Limb Ischemia: Acute and Chronic. New York, NY: Springer; 2016.
18. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301(4):415-424.
19. Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334(7606):1257.
20. Giannini D, Balbarini A. Thrombolytic therapy in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(3):249-258.
21. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S. doi:10.1378/chest.08-0658.
22. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517-538.
23. Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23(1):64-73; discussion 74-75.
24. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220(3):251-266; discussion 266-268.
25. Kashyap VS, Gilani R, Bena JF, Bannazadeh M, Sarac TP. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53(2):340-346. doi:10.1016/j.jvs.2010.08.064.
26. Patel NH, Krishnamurthy VN, Kim S, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3-15.
27. Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61(1):147-154. doi:10.1016/j.jvs.2014.06.109.
28. Zeller T, Tepe G. Treatment of acute limb ischemia with focus on endovascular techniques. Vasa. 2009;38(2):123-133. doi: 10.1024/0301-1526.38.2.123.
29. Normahani P, Standfield NJ, Jaffer U. Sources of delay in the acute limb ischemia patient pathway. Ann Vasc Surg. 2017;38:279-285. doi:10.1016/j.avsg.2016.05.118.
30. Langenskiöld M, Smidfelt K, Karlsson A, Bohm C, Herlitz J, Nordanstig J. Weak links in the early chain of care of acute lower limb ischaemia in terms of recognition and emergency management. Eur J Vasc Endovasc Surg. 2017;54(2):235-240. doi:10.1016/j.ejvs.2017.04.010.
Acute limb ischemia (ALI) is an emergent medical condition that is characterized by a precipitous decrease in limb perfusion that threatens the viability of the affected limb, and symptoms that have been present for 14 days or less.1-5 The incidence of lower extremity ALI in the US is nine to 16 cases per 100,000 persons per year, and one to three cases per 100,000 persons per year for upper extremity ALI.3 Symptoms suggestive of this condition classically include pain in the effected limb at rest, loss of sensation, impaired motor function, and cyanosis/pallor. Irreparable damage can occur in as quickly as 4 to 6 hours with complete arterial occlusion. A majority of ALI is caused by thrombosis, with the remainder of cases caused by embolism, 85% and 15% respectively.1-6 If not addressed promptly, this condition carries a high degree of morbidity (ie, limb loss) and mortality. Immediate initiation of anticoagulation therapy and vascular surgery consultation are mainstays of ED management. Time-to-treatment is a predictor of success and is an area where the emergency physician (EP) can make a significant difference in patient outcomes.7 Although vascular interruption via traumatic mechanism is not addressed in this review, it should remain a consideration in relation to the historical features of each case.
Presentation
Traditionally, symptoms of ALI can be remembered as the “six Ps”: pain, pallor, paresthesia, paralysis, pulselessness, and poikilothermia. Note that ALI represents a spectrum of disease, and the presenting features depend heavily on the level and degree of obstruction. Pain, however, is most frequently the first presenting symptom of patients with ALI.1-6 Pain will occur in muscle groups distal to the occlusion. Obstruction of the aortoiliac region might produce pain in the buttocks, thigh, and hip, whereas occlusion of the femoral artery may produce pain in the calf. Single-level disease will often produce claudication, but multilevel disease may present as a nonhealing ulcer or with focal gangrene. The differential diagnosis of ALI includes direct arterial injury, vasospasm, compartment syndrome, chronic peripheral artery disease, stroke, spinal cord injury, vasculitis, muscular trauma, and radiculopathy.3,5
Etiology
As stated previously, ALI is a result of either thrombotic or embolic phenomena that either partially or completely occludes a vessel such that adequate perfusion is no longer achieved. Consequently, there is a decrease in the metabolism of the tissue supplied in those territories, which can rapidly progress to necrosis. Thrombosis is the most common cause of ALI, accounting for approximately 85%.2,3Typically, thrombosis presents in the setting of pre-existing peripheral artery disease (PAD).8 As PAD worsens, damage to the arterial endothelium triggers platelet activation, and accumulation in a manner conceptually similar to thrombosis in myocardial infarction.9 Hypercoagulable states increase the risk of thrombi development.10 Symptoms of thrombosis are often more insidious in onset when compared to embolism, and signs/symptoms of prior claudication are almost always present. Consequently, there is a greater chance that collateral circulation has developed over time.2,6,11,12
By contrast, ALI caused by embolism is more acute in onset, and is often more emergent due to complete occlusion without chance of collateral vessel development.1,3,6,12 See Table 1 for differences in risk factors and presentation between thrombotic and embolic occlusion.
Historical Features
Information should be obtained systematically in all presentations and should include time of symptom onset, duration, severity, and location; however, special attention should be paid to the evolution of symptoms over time. One historical pearl that should raise suspicion of embolism vs thrombus is a patient history with a specific time of symptom onset. Specific questioning about history of claudication, including description of typical symptoms, is critical. A full list of other medical comorbidities should be obtained. This may reveal risk factors for thrombotic and embolic disease.1,3-5 This also may alter available treatment options.7,8,12,13 At presentation, the EP should obtain a list of the patient’s current medications, and special attention should be given to prothrombotic medications and medications that may interact with anticoagulants.
Physical Examination
On physical examination, the EP should pay particular attention to both the external appearance and temperature of the skin of the patient’s affected extremity. The presence or absence of peripheral pulses is an important feature when assessing for ALI; however, the presence of peripheral pulses does not exclude ALI from the differential.
Precise evaluation of sensation is important to attempt to quantify the extent of injury, localize a possible obstruction, and differentiate ALI from other possible causes of sensation deficits. Though not referenced in the six Ps, the patient’s limb may also appear mottled or marbled. Embolic phenomena occur at sites of vascular bifurcation. In a patient with aortic embolism, femoral pulses will be absent with bilateral mottling and paralysis of the lower extremities. Iliac artery embolism will produce the same symptoms, except unilateral. Femoral artery embolism will yield coolness and mottling distal to the inguinal ligament. Similarly, popliteal artery embolism will produce those same findings, except distal to the knee with preserved femoral pulses.
Diagnosis and Evaluation
After the history and physical examination are completed, further diagnostic methodologies should be considered, including laboratory evaluation and additional imaging studies.
Imaging Studies
Doppler ultrasound examination to assess for pulses on the affected limb should be performed immediately. Bedside continuous-wave Doppler may be used to differentiate arterial vs venous signal.6,14-16 An ankle brachial index may be calculated through either direct auscultation of pulses or Doppler signal. A ratio of less than 0.9 is suggestive of ischemia, while a ratio of less than 0.5 is considered critical ischemia.2
Laboratory Evaluation
Laboratory evaluation of hematocrit, coagulation studies, renal function, electrolyte levels, lactate, and creatinine kinase should be completed.1,5 An electrocardiogram and bedside/formal echocardiogram may be considered if embolism is suspected.1,6,17
Imaging
Angiography is currently the gold standard imaging modality to diagnose ALI.16 However, other imaging modalities exist that expose the patient to less radiation and are generally preferred by patients.18 Duplex ultrasonography is the least invasive imaging modality, with a reported sensitivity of 88% and specificity of 96% for the detection of greater than 50% stenosis.6,19 Contrast-enhanced magnetic resonance angiography (MRA) of the lower extremity is also relatively noninvasive, but is more limited in its application. It carries a reported sensitivity of 95% and a specificity of 97% for detection of stenosis of greater than 50%.6,19 An MRA without contrast may be considered for patients with an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 and who are not on dialysis, but the diagnostic efficacy is reduced.16 Finally, the sensitivity and specificity of computed tomography angiography (CTA) to detect aortoiliac stenosis of greater than 50% is 96% and 98% respectively.6,18,19 Other studies demonstrated similar results for other arterial locations in the lower extremities.18,19 A principal advantage of CTA is that direct visualization of calcifications, clips, stents, and prior bypasses is possible without the limitations of MRA.16 The American College of Radiology (ACR) lists ultrasound duplex Doppler and noncontrast MRA of the lower extremity as “may be appropriate.”16 In contrast, the ACR classifies MRA with contrast, CTA with contrast, and angiography of the lower extremity as “usually appropriate.”16 Selecting the optimal imaging study for a patient may require involvement of a facility’s radiology department and/or a vascular surgeon.14,15
Management in the ED
Even before the definitive diagnosis of ALI is made, steps can be taken to minimize and/or slow progression of injury. Placing the affected limb in a dependent position and providing intravenous (IV) fluid hydration will maximize perfusion.1,3,5 Even with consideration of the diagnostic modalities previously described, ALI is often considered a clinical diagnosis. Since time is critical, early consultation with vascular surgery services is imperative if ALI is suspected.
Intravenous Fluid Therapy
Suspected ALI should be treated in the ED with an IV heparin bolus, followed by constant IV infusion. Heparin prevents proximal and distal propagation of the thrombus.1,3,5,6,15,17,20 Additionally, it helps maintain the microcirculation surrounding the affected area.6 A heparin bolus dose of 100 U/kg followed by a continuous infusion of heparin 1,000 U/h is the recommended standard.1,3,5,6,15 A goal partial thromboplastin time of 60 to 100 seconds, or an international normalized ratio of 2 to 3 is desirable.8,21
Staging: The Rutherford Classification System
Once immediate therapies are started, the stage of PAD should be determined using the Rutherford classification system in collaboration with vascular surgery services; this will further guide treatment and disposition.
Category I. In category I ALI, the affected limb is considered viable and not immediately threatened.
Category IIa. The limb is considered to be marginally threatened and salvageable.
Category IIb. An ALI classified as a category IIb is the most urgent type in which the limb is immediately threatened.
Category III. The limb is classified as having irreversible damage.2,3,17,22
(See Table 2 for a more detailed summary of the Rutherford classification system.)
Treatment
Definitive treatment decisions should be made in consultation with vascular surgery services. The results of three clinical trials developed the foundation for management of ALI: the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial, Surgery Versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial, and Rutherford trial.22-24 Table 3 provides a summary of the treatment techniques utilized, which include mechanical recanalization with percutaneous aspiration thrombectomy, percutaneous mechanical thrombectomy,6,12,25-28 pharmacological recanalization via catheter-directed thrombolysis,6,8,12,20,23-28 and/or surgical management via thrombectomy or embolectomy.6,11,23,24,27
Complications
The complications of treating ALI can be stratified into three subsets: pharmacologic, mechanical, and reperfusion injury-related. Medical treatment with heparin is not without risk. Intracranial hemorrhage, major bleeding at other sites, and compartment syndrome secondary to bleeding can all occur. Treatment may also cause distal embolization and lead to mechanical occlusion of another site. Finally, reperfusion injury predisposes the patient to a host of new problems. Reperfusion injury occurs when fresh blood enters a previously ischemic zone. When this occurs, oxygen free radicals, inflammatory mediators, and catabolism byproducts mix with the blood. This process can damage surrounding epithelial cells and lead to increased interstitial permeability. As a result, compartment syndrome may also develop via this mechanism.1,3,5,6 The anterior compartment of the lower extremity is most at risk. As such, assessment of peroneal nerve activity via dorsiflexion of the foot and sensation testing is important after the initiation of treatment.6 Systemic distribution of these harmful byproducts can also cause arrhythmias, renal failure, and systemic acadisos.1,3,5,6 It is important for physicians and staff to monitor the patient closely for the development of these complications and be prepared to intervene.
Opportunities for Improvement
In a condition where minutes matter, expedient action is critical. Time-to-recognition, imaging, consultation, and intervention all are potential sources of delay. Two recent studies have investigated the treatment timeline of ALI. The first study showed that the greatest source of delay is time from symptom onset to presentation in the ED; an average of 11.35 hours. The second largest source of delay was time from recognition of ALI to imaging, with an average delay of 4.75 hours. The average ED evaluation time was 40 minutes, and the average total time to intervention was 10.2 hours. While time-to-symptom presentation may represent a failure of public health, time-to-imaging was identified as an area of unacceptable delay by the authors.29 A second study examined the effect of pre-hospital care on the time-to-treatment. Persons who were transported via emergency medical services (EMS) arrived to the hospital at a median time of 5 hours after symptom onset, were seen by a physician at a median time of 51 minutes, and had revascularization at a median time of 23 hours. Those not transported by EMS arrived to the hospital at a median time of 48 hours after symptom onset, were seen by a physician at a median time of 80 minutes, and had revascularization at a median time of 93 hours. As a secondary goal, the study examined the effect of heparinization in the ED and showed that treatment with heparin was associated with a favorable outcome.30 While the total treatment times in each study vary widely, they share several important commonalities and reinforce expedient management as an important goal.
Outcomes
Advances in the treatment of this condition began in the 1970s, with an explosion of new techniques and data in the past several years. Still, ALI is a high morbidity and high mortality condition. Rates of limb loss are approximately 30% for all cases, and it is a fatal condition for as many as one-in-five. As discussed earlier, the patients who are most likely to experience ALI typically have several medical comorbidities. Perhaps unsurprisingly, ALI portends a poor prognosis even if the limb is salvaged. Approximately 15% to 20% of patients with ALI will die within 1 year of diagnosis.3,4,6,12
Conclusion
Acute limb ischemia is a rare condition, but a true medical emergency. Prompt recognition of the signs and symptoms of ALI are critical for optimizing patient outcomes. A high index of clinical suspicion in patients with risk factors may lead to early diagnosis. Early vascular surgery consultation and early IV heparin treatment are important aspects of care. Prompt imaging and staging guide further management. Special care should be paid to possible post-treatment complications and to the investigation of contributing factors. The EP has a great opportunity to positively impact care with expeditious management. Early diagnosis, targeted history and physical examination, prompt treatment, collaboration with specialists, and prevention and treatment of life-threatening complications are all hallmarks of emergency medicine.
Acute limb ischemia (ALI) is an emergent medical condition that is characterized by a precipitous decrease in limb perfusion that threatens the viability of the affected limb, and symptoms that have been present for 14 days or less.1-5 The incidence of lower extremity ALI in the US is nine to 16 cases per 100,000 persons per year, and one to three cases per 100,000 persons per year for upper extremity ALI.3 Symptoms suggestive of this condition classically include pain in the effected limb at rest, loss of sensation, impaired motor function, and cyanosis/pallor. Irreparable damage can occur in as quickly as 4 to 6 hours with complete arterial occlusion. A majority of ALI is caused by thrombosis, with the remainder of cases caused by embolism, 85% and 15% respectively.1-6 If not addressed promptly, this condition carries a high degree of morbidity (ie, limb loss) and mortality. Immediate initiation of anticoagulation therapy and vascular surgery consultation are mainstays of ED management. Time-to-treatment is a predictor of success and is an area where the emergency physician (EP) can make a significant difference in patient outcomes.7 Although vascular interruption via traumatic mechanism is not addressed in this review, it should remain a consideration in relation to the historical features of each case.
Presentation
Traditionally, symptoms of ALI can be remembered as the “six Ps”: pain, pallor, paresthesia, paralysis, pulselessness, and poikilothermia. Note that ALI represents a spectrum of disease, and the presenting features depend heavily on the level and degree of obstruction. Pain, however, is most frequently the first presenting symptom of patients with ALI.1-6 Pain will occur in muscle groups distal to the occlusion. Obstruction of the aortoiliac region might produce pain in the buttocks, thigh, and hip, whereas occlusion of the femoral artery may produce pain in the calf. Single-level disease will often produce claudication, but multilevel disease may present as a nonhealing ulcer or with focal gangrene. The differential diagnosis of ALI includes direct arterial injury, vasospasm, compartment syndrome, chronic peripheral artery disease, stroke, spinal cord injury, vasculitis, muscular trauma, and radiculopathy.3,5
Etiology
As stated previously, ALI is a result of either thrombotic or embolic phenomena that either partially or completely occludes a vessel such that adequate perfusion is no longer achieved. Consequently, there is a decrease in the metabolism of the tissue supplied in those territories, which can rapidly progress to necrosis. Thrombosis is the most common cause of ALI, accounting for approximately 85%.2,3Typically, thrombosis presents in the setting of pre-existing peripheral artery disease (PAD).8 As PAD worsens, damage to the arterial endothelium triggers platelet activation, and accumulation in a manner conceptually similar to thrombosis in myocardial infarction.9 Hypercoagulable states increase the risk of thrombi development.10 Symptoms of thrombosis are often more insidious in onset when compared to embolism, and signs/symptoms of prior claudication are almost always present. Consequently, there is a greater chance that collateral circulation has developed over time.2,6,11,12
By contrast, ALI caused by embolism is more acute in onset, and is often more emergent due to complete occlusion without chance of collateral vessel development.1,3,6,12 See Table 1 for differences in risk factors and presentation between thrombotic and embolic occlusion.
Historical Features
Information should be obtained systematically in all presentations and should include time of symptom onset, duration, severity, and location; however, special attention should be paid to the evolution of symptoms over time. One historical pearl that should raise suspicion of embolism vs thrombus is a patient history with a specific time of symptom onset. Specific questioning about history of claudication, including description of typical symptoms, is critical. A full list of other medical comorbidities should be obtained. This may reveal risk factors for thrombotic and embolic disease.1,3-5 This also may alter available treatment options.7,8,12,13 At presentation, the EP should obtain a list of the patient’s current medications, and special attention should be given to prothrombotic medications and medications that may interact with anticoagulants.
Physical Examination
On physical examination, the EP should pay particular attention to both the external appearance and temperature of the skin of the patient’s affected extremity. The presence or absence of peripheral pulses is an important feature when assessing for ALI; however, the presence of peripheral pulses does not exclude ALI from the differential.
Precise evaluation of sensation is important to attempt to quantify the extent of injury, localize a possible obstruction, and differentiate ALI from other possible causes of sensation deficits. Though not referenced in the six Ps, the patient’s limb may also appear mottled or marbled. Embolic phenomena occur at sites of vascular bifurcation. In a patient with aortic embolism, femoral pulses will be absent with bilateral mottling and paralysis of the lower extremities. Iliac artery embolism will produce the same symptoms, except unilateral. Femoral artery embolism will yield coolness and mottling distal to the inguinal ligament. Similarly, popliteal artery embolism will produce those same findings, except distal to the knee with preserved femoral pulses.
Diagnosis and Evaluation
After the history and physical examination are completed, further diagnostic methodologies should be considered, including laboratory evaluation and additional imaging studies.
Imaging Studies
Doppler ultrasound examination to assess for pulses on the affected limb should be performed immediately. Bedside continuous-wave Doppler may be used to differentiate arterial vs venous signal.6,14-16 An ankle brachial index may be calculated through either direct auscultation of pulses or Doppler signal. A ratio of less than 0.9 is suggestive of ischemia, while a ratio of less than 0.5 is considered critical ischemia.2
Laboratory Evaluation
Laboratory evaluation of hematocrit, coagulation studies, renal function, electrolyte levels, lactate, and creatinine kinase should be completed.1,5 An electrocardiogram and bedside/formal echocardiogram may be considered if embolism is suspected.1,6,17
Imaging
Angiography is currently the gold standard imaging modality to diagnose ALI.16 However, other imaging modalities exist that expose the patient to less radiation and are generally preferred by patients.18 Duplex ultrasonography is the least invasive imaging modality, with a reported sensitivity of 88% and specificity of 96% for the detection of greater than 50% stenosis.6,19 Contrast-enhanced magnetic resonance angiography (MRA) of the lower extremity is also relatively noninvasive, but is more limited in its application. It carries a reported sensitivity of 95% and a specificity of 97% for detection of stenosis of greater than 50%.6,19 An MRA without contrast may be considered for patients with an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 and who are not on dialysis, but the diagnostic efficacy is reduced.16 Finally, the sensitivity and specificity of computed tomography angiography (CTA) to detect aortoiliac stenosis of greater than 50% is 96% and 98% respectively.6,18,19 Other studies demonstrated similar results for other arterial locations in the lower extremities.18,19 A principal advantage of CTA is that direct visualization of calcifications, clips, stents, and prior bypasses is possible without the limitations of MRA.16 The American College of Radiology (ACR) lists ultrasound duplex Doppler and noncontrast MRA of the lower extremity as “may be appropriate.”16 In contrast, the ACR classifies MRA with contrast, CTA with contrast, and angiography of the lower extremity as “usually appropriate.”16 Selecting the optimal imaging study for a patient may require involvement of a facility’s radiology department and/or a vascular surgeon.14,15
Management in the ED
Even before the definitive diagnosis of ALI is made, steps can be taken to minimize and/or slow progression of injury. Placing the affected limb in a dependent position and providing intravenous (IV) fluid hydration will maximize perfusion.1,3,5 Even with consideration of the diagnostic modalities previously described, ALI is often considered a clinical diagnosis. Since time is critical, early consultation with vascular surgery services is imperative if ALI is suspected.
Intravenous Fluid Therapy
Suspected ALI should be treated in the ED with an IV heparin bolus, followed by constant IV infusion. Heparin prevents proximal and distal propagation of the thrombus.1,3,5,6,15,17,20 Additionally, it helps maintain the microcirculation surrounding the affected area.6 A heparin bolus dose of 100 U/kg followed by a continuous infusion of heparin 1,000 U/h is the recommended standard.1,3,5,6,15 A goal partial thromboplastin time of 60 to 100 seconds, or an international normalized ratio of 2 to 3 is desirable.8,21
Staging: The Rutherford Classification System
Once immediate therapies are started, the stage of PAD should be determined using the Rutherford classification system in collaboration with vascular surgery services; this will further guide treatment and disposition.
Category I. In category I ALI, the affected limb is considered viable and not immediately threatened.
Category IIa. The limb is considered to be marginally threatened and salvageable.
Category IIb. An ALI classified as a category IIb is the most urgent type in which the limb is immediately threatened.
Category III. The limb is classified as having irreversible damage.2,3,17,22
(See Table 2 for a more detailed summary of the Rutherford classification system.)
Treatment
Definitive treatment decisions should be made in consultation with vascular surgery services. The results of three clinical trials developed the foundation for management of ALI: the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial, Surgery Versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial, and Rutherford trial.22-24 Table 3 provides a summary of the treatment techniques utilized, which include mechanical recanalization with percutaneous aspiration thrombectomy, percutaneous mechanical thrombectomy,6,12,25-28 pharmacological recanalization via catheter-directed thrombolysis,6,8,12,20,23-28 and/or surgical management via thrombectomy or embolectomy.6,11,23,24,27
Complications
The complications of treating ALI can be stratified into three subsets: pharmacologic, mechanical, and reperfusion injury-related. Medical treatment with heparin is not without risk. Intracranial hemorrhage, major bleeding at other sites, and compartment syndrome secondary to bleeding can all occur. Treatment may also cause distal embolization and lead to mechanical occlusion of another site. Finally, reperfusion injury predisposes the patient to a host of new problems. Reperfusion injury occurs when fresh blood enters a previously ischemic zone. When this occurs, oxygen free radicals, inflammatory mediators, and catabolism byproducts mix with the blood. This process can damage surrounding epithelial cells and lead to increased interstitial permeability. As a result, compartment syndrome may also develop via this mechanism.1,3,5,6 The anterior compartment of the lower extremity is most at risk. As such, assessment of peroneal nerve activity via dorsiflexion of the foot and sensation testing is important after the initiation of treatment.6 Systemic distribution of these harmful byproducts can also cause arrhythmias, renal failure, and systemic acadisos.1,3,5,6 It is important for physicians and staff to monitor the patient closely for the development of these complications and be prepared to intervene.
Opportunities for Improvement
In a condition where minutes matter, expedient action is critical. Time-to-recognition, imaging, consultation, and intervention all are potential sources of delay. Two recent studies have investigated the treatment timeline of ALI. The first study showed that the greatest source of delay is time from symptom onset to presentation in the ED; an average of 11.35 hours. The second largest source of delay was time from recognition of ALI to imaging, with an average delay of 4.75 hours. The average ED evaluation time was 40 minutes, and the average total time to intervention was 10.2 hours. While time-to-symptom presentation may represent a failure of public health, time-to-imaging was identified as an area of unacceptable delay by the authors.29 A second study examined the effect of pre-hospital care on the time-to-treatment. Persons who were transported via emergency medical services (EMS) arrived to the hospital at a median time of 5 hours after symptom onset, were seen by a physician at a median time of 51 minutes, and had revascularization at a median time of 23 hours. Those not transported by EMS arrived to the hospital at a median time of 48 hours after symptom onset, were seen by a physician at a median time of 80 minutes, and had revascularization at a median time of 93 hours. As a secondary goal, the study examined the effect of heparinization in the ED and showed that treatment with heparin was associated with a favorable outcome.30 While the total treatment times in each study vary widely, they share several important commonalities and reinforce expedient management as an important goal.
Outcomes
Advances in the treatment of this condition began in the 1970s, with an explosion of new techniques and data in the past several years. Still, ALI is a high morbidity and high mortality condition. Rates of limb loss are approximately 30% for all cases, and it is a fatal condition for as many as one-in-five. As discussed earlier, the patients who are most likely to experience ALI typically have several medical comorbidities. Perhaps unsurprisingly, ALI portends a poor prognosis even if the limb is salvaged. Approximately 15% to 20% of patients with ALI will die within 1 year of diagnosis.3,4,6,12
Conclusion
Acute limb ischemia is a rare condition, but a true medical emergency. Prompt recognition of the signs and symptoms of ALI are critical for optimizing patient outcomes. A high index of clinical suspicion in patients with risk factors may lead to early diagnosis. Early vascular surgery consultation and early IV heparin treatment are important aspects of care. Prompt imaging and staging guide further management. Special care should be paid to possible post-treatment complications and to the investigation of contributing factors. The EP has a great opportunity to positively impact care with expeditious management. Early diagnosis, targeted history and physical examination, prompt treatment, collaboration with specialists, and prevention and treatment of life-threatening complications are all hallmarks of emergency medicine.
1. Braun R, Lin M. Acute limb ischemia: a case report and literature review. J Emerg Med. 2015;49(6):1011-1017. doi:10.1016/j.jemermed.2015.03.008.
2. Callum K, Bradbury A. ABC of arterial and venous disease: acute limb ischaemia. BMJ. 2000;320(7237):764-767.
3. Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366(23):2198-2206. doi:10.1056/NEJMcp1006054.
4. Purushottam B, Gujja K, Zalewski A, Krishnan P. Acute limb ischemia. Interv Cardiol Clin. 2014;3(4):557-572.
5. Santistevan JR. Acute limb ischemia: an emergency medicine approach. Emerg Med Clin North Am. 2017;35(4):889-909. doi:10.1016/j.emc.2017.07.006.
6. Acar RD, Sahin M, Kirma C. One of the most urgent vascular circumstances: acute limb ischemia. SAGE Open Med. 2013;1:2050312113516110. doi:10.1177/2050312113516110.
7. Abou-Zamzam AM Jr, Gomez NR, Molkara A, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21(4):458-463.
8. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465-1508.
9. Rajagopalan S, Mckay I, Ford I, Bachoo P, Greaves M, Brittenden J. Platelet activation increases with the severity of peripheral arterial disease: implications for clinical management. J Vasc Surg. 2007;46(3):485-490.
10. Deitcher SR, Carman TL, Sheikh MA, Gomes M. Hypercoagulable syndromes: evaluation and management strategies for acute limb ischemia. Semin Vasc Surg. 2001;14(2):74-85.
11. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84(6):822-834.
12. Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59(4):988-995.
13. Soden PA, Zettervall SL, Curran T, et al. Regional variation in patient selection and treatment for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg. 2017;65(1):108-118. doi:10.1016/j.jvs.2016.06.105.
14. Aboyans V, Björck M, Brodmann M, et al; ESC Scientific Document Group. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: a companion document of the 2017 ESC Guidelines for the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Endorsed by: the European Stroke Organisation (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2017. doi:10.1093/eurheartj/ehx499. [Epub ahead of print]
15. Authors/Task Force Members, Aboyans V, Ricco JB, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017. doi:10.1016/j.ejvs.2017.07.018. [Epub ahead of print]
16. Expert Panel on Vascular Imaging: Weiss CR, Azene EM, Majdalany BS, et al. ACR Appropriateness Criteria® sudden onset of cold, painful leg. J Am Coll Radiol. 2017;14(5S):S307-S313. doi:10.1016/j.jacr.2017.02.015.
17. Dieter RS, Dieter RA Jr, Dieter RA 3rd, Nanjundappa A. Critical Limb Ischemia: Acute and Chronic. New York, NY: Springer; 2016.
18. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301(4):415-424.
19. Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334(7606):1257.
20. Giannini D, Balbarini A. Thrombolytic therapy in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(3):249-258.
21. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S. doi:10.1378/chest.08-0658.
22. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517-538.
23. Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23(1):64-73; discussion 74-75.
24. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220(3):251-266; discussion 266-268.
25. Kashyap VS, Gilani R, Bena JF, Bannazadeh M, Sarac TP. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53(2):340-346. doi:10.1016/j.jvs.2010.08.064.
26. Patel NH, Krishnamurthy VN, Kim S, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3-15.
27. Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61(1):147-154. doi:10.1016/j.jvs.2014.06.109.
28. Zeller T, Tepe G. Treatment of acute limb ischemia with focus on endovascular techniques. Vasa. 2009;38(2):123-133. doi: 10.1024/0301-1526.38.2.123.
29. Normahani P, Standfield NJ, Jaffer U. Sources of delay in the acute limb ischemia patient pathway. Ann Vasc Surg. 2017;38:279-285. doi:10.1016/j.avsg.2016.05.118.
30. Langenskiöld M, Smidfelt K, Karlsson A, Bohm C, Herlitz J, Nordanstig J. Weak links in the early chain of care of acute lower limb ischaemia in terms of recognition and emergency management. Eur J Vasc Endovasc Surg. 2017;54(2):235-240. doi:10.1016/j.ejvs.2017.04.010.
1. Braun R, Lin M. Acute limb ischemia: a case report and literature review. J Emerg Med. 2015;49(6):1011-1017. doi:10.1016/j.jemermed.2015.03.008.
2. Callum K, Bradbury A. ABC of arterial and venous disease: acute limb ischaemia. BMJ. 2000;320(7237):764-767.
3. Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366(23):2198-2206. doi:10.1056/NEJMcp1006054.
4. Purushottam B, Gujja K, Zalewski A, Krishnan P. Acute limb ischemia. Interv Cardiol Clin. 2014;3(4):557-572.
5. Santistevan JR. Acute limb ischemia: an emergency medicine approach. Emerg Med Clin North Am. 2017;35(4):889-909. doi:10.1016/j.emc.2017.07.006.
6. Acar RD, Sahin M, Kirma C. One of the most urgent vascular circumstances: acute limb ischemia. SAGE Open Med. 2013;1:2050312113516110. doi:10.1177/2050312113516110.
7. Abou-Zamzam AM Jr, Gomez NR, Molkara A, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg. 2007;21(4):458-463.
8. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465-1508.
9. Rajagopalan S, Mckay I, Ford I, Bachoo P, Greaves M, Brittenden J. Platelet activation increases with the severity of peripheral arterial disease: implications for clinical management. J Vasc Surg. 2007;46(3):485-490.
10. Deitcher SR, Carman TL, Sheikh MA, Gomes M. Hypercoagulable syndromes: evaluation and management strategies for acute limb ischemia. Semin Vasc Surg. 2001;14(2):74-85.
11. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84(6):822-834.
12. Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59(4):988-995.
13. Soden PA, Zettervall SL, Curran T, et al. Regional variation in patient selection and treatment for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg. 2017;65(1):108-118. doi:10.1016/j.jvs.2016.06.105.
14. Aboyans V, Björck M, Brodmann M, et al; ESC Scientific Document Group. Questions and answers on diagnosis and management of patients with Peripheral Arterial Diseases: a companion document of the 2017 ESC Guidelines for the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Endorsed by: the European Stroke Organisation (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2017. doi:10.1093/eurheartj/ehx499. [Epub ahead of print]
15. Authors/Task Force Members, Aboyans V, Ricco JB, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017. doi:10.1016/j.ejvs.2017.07.018. [Epub ahead of print]
16. Expert Panel on Vascular Imaging: Weiss CR, Azene EM, Majdalany BS, et al. ACR Appropriateness Criteria® sudden onset of cold, painful leg. J Am Coll Radiol. 2017;14(5S):S307-S313. doi:10.1016/j.jacr.2017.02.015.
17. Dieter RS, Dieter RA Jr, Dieter RA 3rd, Nanjundappa A. Critical Limb Ischemia: Acute and Chronic. New York, NY: Springer; 2016.
18. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301(4):415-424.
19. Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334(7606):1257.
20. Giannini D, Balbarini A. Thrombolytic therapy in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(3):249-258.
21. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S. doi:10.1378/chest.08-0658.
22. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517-538.
23. Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23(1):64-73; discussion 74-75.
24. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220(3):251-266; discussion 266-268.
25. Kashyap VS, Gilani R, Bena JF, Bannazadeh M, Sarac TP. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53(2):340-346. doi:10.1016/j.jvs.2010.08.064.
26. Patel NH, Krishnamurthy VN, Kim S, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(1):3-15.
27. Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61(1):147-154. doi:10.1016/j.jvs.2014.06.109.
28. Zeller T, Tepe G. Treatment of acute limb ischemia with focus on endovascular techniques. Vasa. 2009;38(2):123-133. doi: 10.1024/0301-1526.38.2.123.
29. Normahani P, Standfield NJ, Jaffer U. Sources of delay in the acute limb ischemia patient pathway. Ann Vasc Surg. 2017;38:279-285. doi:10.1016/j.avsg.2016.05.118.
30. Langenskiöld M, Smidfelt K, Karlsson A, Bohm C, Herlitz J, Nordanstig J. Weak links in the early chain of care of acute lower limb ischaemia in terms of recognition and emergency management. Eur J Vasc Endovasc Surg. 2017;54(2):235-240. doi:10.1016/j.ejvs.2017.04.010.
Early management of patients with acute ischemic stroke
(AIS). Conceptually, early management can be separated into initial triage and decisions about intervention to restore blood flow with thrombolysis or mechanical thrombectomy. If reperfusion therapy is not appropriate, then the focus is on management to minimize further damage from the stroke, decrease the likelihood of recurrence, and lessen secondary problems related to the stroke.
All patients with AIS should receive noncontrast CT to determine if there is evidence of a hemorrhagic stroke and, if such evidence exists, than the patient is not a candidate for thrombolysis. Intravenous alteplase should be considered for patients who present within 3 hours of stroke onset and for selected patients presenting between 3-4.5 hours after stroke onset (for more details, see Table 6 in the guidelines). Selected patients with AIS who present within 6-24 hours of last time they were known to be normal and who have large vessel occlusion in the anterior circulation, may be candidates for mechanical thrombectomy in specialized centers. Patients who are not candidates for acute interventions should then be managed according to early stroke management guidelines.
Early stroke management for patients with AIS admitted to medical floors involves attention to blood pressure, glucose, and antiplatelet therapy. For patients with blood pressure lower than 220/120 mm Hg who did not receive IV alteplase or thrombectomy, treatment of hypertension in the first 48-72 hours after an AIS does not change the outcome. It is reasonable when patients have BP greater than or equal to 220/120 mm Hg, to lower blood pressure by 15% during the first 24 hours after onset of stroke. Starting or restarting antihypertensive therapy during hospitalization in patients with blood pressure higher than 140/90 mm Hg who are neurologically stable improves long-term blood pressure control and is considered a reasonable strategy.
For patients with noncardioembolic AIS, the use of antiplatelet agents rather than oral anticoagulation is recommended. Patients should be treated with aspirin 160 mg-325 mg within 24-48 hours of presentation. In patients unsafe or unable to swallow, rectal or nasogastric administration is recommended. In patients with minor stroke, 21 days of dual-antiplatelet therapy (aspirin and clopidogrel) started within 24 hours can decrease stroke recurrence for the first 90 days after a stroke. This recommendation is based on a single study, the CHANCE trial, in a homogeneous population in China, and its generalizability is not known. If a patient had an AIS while already on aspirin, there is some evidence supporting a decreased risk of major cardiovascular events and recurrent stroke in patients switching to an alternative antiplatelet agent or combination antiplatelet therapy. Because of methodologic issues in the those studies, the guideline concludes that, for those already on aspirin, it is of unclear benefit to increase the dose of aspirin, switch to a different antiplatelet agent, or add a second antiplatelet agent. Switching to warfarin is not beneficial for secondary stroke prevention. High-dose statin therapy should be initiated. For patients with AIS in the setting of atrial fibrillation, oral anticoagulation can be started within 4-14 days after the stroke. One study showed that anticoagulation should not be started before 4 days after the stroke, with a hazard ratio of 0.53 for starting anticoagulation at 4-14 days, compared with less than 4 days.
Hyperglycemia should be controlled to a range of 140-180 mg/dL, because higher values are associated with worse outcomes. Oxygen should be used if needed to maintain oxygen saturation greater than 94%. High-intensity statin therapy should be used, and smoking cessation is strongly encouraged for those who use tobacco, with avoidance of secondhand smoke whenever possible.
Patients should be screened for dysphagia before taking anything per oral, including medications. A nasogastric tube may be considered within the first 7 days, if patients are dysphagic. Oral hygiene protocols may include antibacterial mouth rinse, systematic oral care, and decontamination gel to decrease the risk of pneumonia .
For deep vein thrombosis prophylaxis, intermittent pneumatic compression, in addition to the aspirin that a patient is on is reasonable, and the benefit of prophylactic-dose subcutaneous heparin (unfractionated heparin or low-molecular-weight heparin) in immobile patients with AIS is not well established.
In the poststroke setting, patients should be screened for depression and, if appropriate, treated with antidepressants. Regular skin assessments are recommended with objective scales, and skin friction and pressure should be actively minimized with regular turning, good skin hygiene, and use of specialized mattresses, wheelchair cushions, and seating until mobility returns. Early rehabilitation for hospitalized stroke patients should be provided, but high-dose, very-early mobilization within 24 hours of stroke should not be done because it reduces the odds of a favorable outcome at 3 months.
Completing the diagnostic evaluation for the cause of stroke and decreasing the chance of future strokes should be part of the initial hospitalization. While MRI is more sensitive than is CT for detecting AIS, routine use of MRI in all patients with AIS is not cost effective and therefore is not recommended. For patients with nondisabling AIS in the carotid territory and who are candidates for carotid endarterectomy or stenting, noninvasive imaging of the cervical vessels should be performed within 24 hours of admission, with plans for carotid revascularization between 48 hours and 7 days if indicated. Cardiac monitoring for at least the first 24 hours of admission should be performed, while primarily looking for atrial fibrillation as a cause of stroke. In some patients, prolonged cardiac monitoring may be reasonable. With prolonged cardiac monitoring, atrial fibrillation is newly detected in nearly a quarter of patients with stroke or TIA, but the effect on outcomes is uncertain. Routine use of echocardiography is not recommended but may be done in selected patients. All patients should be screened for diabetes. It is not clear whether screening for thrombophilic states is useful.
All patients should be counseled on stroke, and provided education about it and how it will affect their lives. Following their acute medical stay, all patients will benefit from rehabilitation, with the benefits associated using a program tailored to their needs and outcome goals.
The bottom line
Early management of stroke involves first determining whether someone is a candidate for reperfusion therapy with alteplase or thrombectomy and then, if not, admitting them to a monitored setting to screen for atrial fibrillation and evaluation for carotid stenosis. Patients should be evaluated for both depression and swallowing function, and there should be initiation of deep vein thrombosis prevention, appropriate management of elevated blood pressures, anti-platelet therapy, and statin therapy as well as plans for rehabilitation services.
Reference
Powers WJ et al. on behalf of the American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018 Mar;49(3):e46-e110.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Callahan is an attending physician and preceptor in the family medicine residency program at Abington Jefferson Health.
(AIS). Conceptually, early management can be separated into initial triage and decisions about intervention to restore blood flow with thrombolysis or mechanical thrombectomy. If reperfusion therapy is not appropriate, then the focus is on management to minimize further damage from the stroke, decrease the likelihood of recurrence, and lessen secondary problems related to the stroke.
All patients with AIS should receive noncontrast CT to determine if there is evidence of a hemorrhagic stroke and, if such evidence exists, than the patient is not a candidate for thrombolysis. Intravenous alteplase should be considered for patients who present within 3 hours of stroke onset and for selected patients presenting between 3-4.5 hours after stroke onset (for more details, see Table 6 in the guidelines). Selected patients with AIS who present within 6-24 hours of last time they were known to be normal and who have large vessel occlusion in the anterior circulation, may be candidates for mechanical thrombectomy in specialized centers. Patients who are not candidates for acute interventions should then be managed according to early stroke management guidelines.
Early stroke management for patients with AIS admitted to medical floors involves attention to blood pressure, glucose, and antiplatelet therapy. For patients with blood pressure lower than 220/120 mm Hg who did not receive IV alteplase or thrombectomy, treatment of hypertension in the first 48-72 hours after an AIS does not change the outcome. It is reasonable when patients have BP greater than or equal to 220/120 mm Hg, to lower blood pressure by 15% during the first 24 hours after onset of stroke. Starting or restarting antihypertensive therapy during hospitalization in patients with blood pressure higher than 140/90 mm Hg who are neurologically stable improves long-term blood pressure control and is considered a reasonable strategy.
For patients with noncardioembolic AIS, the use of antiplatelet agents rather than oral anticoagulation is recommended. Patients should be treated with aspirin 160 mg-325 mg within 24-48 hours of presentation. In patients unsafe or unable to swallow, rectal or nasogastric administration is recommended. In patients with minor stroke, 21 days of dual-antiplatelet therapy (aspirin and clopidogrel) started within 24 hours can decrease stroke recurrence for the first 90 days after a stroke. This recommendation is based on a single study, the CHANCE trial, in a homogeneous population in China, and its generalizability is not known. If a patient had an AIS while already on aspirin, there is some evidence supporting a decreased risk of major cardiovascular events and recurrent stroke in patients switching to an alternative antiplatelet agent or combination antiplatelet therapy. Because of methodologic issues in the those studies, the guideline concludes that, for those already on aspirin, it is of unclear benefit to increase the dose of aspirin, switch to a different antiplatelet agent, or add a second antiplatelet agent. Switching to warfarin is not beneficial for secondary stroke prevention. High-dose statin therapy should be initiated. For patients with AIS in the setting of atrial fibrillation, oral anticoagulation can be started within 4-14 days after the stroke. One study showed that anticoagulation should not be started before 4 days after the stroke, with a hazard ratio of 0.53 for starting anticoagulation at 4-14 days, compared with less than 4 days.
Hyperglycemia should be controlled to a range of 140-180 mg/dL, because higher values are associated with worse outcomes. Oxygen should be used if needed to maintain oxygen saturation greater than 94%. High-intensity statin therapy should be used, and smoking cessation is strongly encouraged for those who use tobacco, with avoidance of secondhand smoke whenever possible.
Patients should be screened for dysphagia before taking anything per oral, including medications. A nasogastric tube may be considered within the first 7 days, if patients are dysphagic. Oral hygiene protocols may include antibacterial mouth rinse, systematic oral care, and decontamination gel to decrease the risk of pneumonia .
For deep vein thrombosis prophylaxis, intermittent pneumatic compression, in addition to the aspirin that a patient is on is reasonable, and the benefit of prophylactic-dose subcutaneous heparin (unfractionated heparin or low-molecular-weight heparin) in immobile patients with AIS is not well established.
In the poststroke setting, patients should be screened for depression and, if appropriate, treated with antidepressants. Regular skin assessments are recommended with objective scales, and skin friction and pressure should be actively minimized with regular turning, good skin hygiene, and use of specialized mattresses, wheelchair cushions, and seating until mobility returns. Early rehabilitation for hospitalized stroke patients should be provided, but high-dose, very-early mobilization within 24 hours of stroke should not be done because it reduces the odds of a favorable outcome at 3 months.
Completing the diagnostic evaluation for the cause of stroke and decreasing the chance of future strokes should be part of the initial hospitalization. While MRI is more sensitive than is CT for detecting AIS, routine use of MRI in all patients with AIS is not cost effective and therefore is not recommended. For patients with nondisabling AIS in the carotid territory and who are candidates for carotid endarterectomy or stenting, noninvasive imaging of the cervical vessels should be performed within 24 hours of admission, with plans for carotid revascularization between 48 hours and 7 days if indicated. Cardiac monitoring for at least the first 24 hours of admission should be performed, while primarily looking for atrial fibrillation as a cause of stroke. In some patients, prolonged cardiac monitoring may be reasonable. With prolonged cardiac monitoring, atrial fibrillation is newly detected in nearly a quarter of patients with stroke or TIA, but the effect on outcomes is uncertain. Routine use of echocardiography is not recommended but may be done in selected patients. All patients should be screened for diabetes. It is not clear whether screening for thrombophilic states is useful.
All patients should be counseled on stroke, and provided education about it and how it will affect their lives. Following their acute medical stay, all patients will benefit from rehabilitation, with the benefits associated using a program tailored to their needs and outcome goals.
The bottom line
Early management of stroke involves first determining whether someone is a candidate for reperfusion therapy with alteplase or thrombectomy and then, if not, admitting them to a monitored setting to screen for atrial fibrillation and evaluation for carotid stenosis. Patients should be evaluated for both depression and swallowing function, and there should be initiation of deep vein thrombosis prevention, appropriate management of elevated blood pressures, anti-platelet therapy, and statin therapy as well as plans for rehabilitation services.
Reference
Powers WJ et al. on behalf of the American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018 Mar;49(3):e46-e110.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Callahan is an attending physician and preceptor in the family medicine residency program at Abington Jefferson Health.
(AIS). Conceptually, early management can be separated into initial triage and decisions about intervention to restore blood flow with thrombolysis or mechanical thrombectomy. If reperfusion therapy is not appropriate, then the focus is on management to minimize further damage from the stroke, decrease the likelihood of recurrence, and lessen secondary problems related to the stroke.
All patients with AIS should receive noncontrast CT to determine if there is evidence of a hemorrhagic stroke and, if such evidence exists, than the patient is not a candidate for thrombolysis. Intravenous alteplase should be considered for patients who present within 3 hours of stroke onset and for selected patients presenting between 3-4.5 hours after stroke onset (for more details, see Table 6 in the guidelines). Selected patients with AIS who present within 6-24 hours of last time they were known to be normal and who have large vessel occlusion in the anterior circulation, may be candidates for mechanical thrombectomy in specialized centers. Patients who are not candidates for acute interventions should then be managed according to early stroke management guidelines.
Early stroke management for patients with AIS admitted to medical floors involves attention to blood pressure, glucose, and antiplatelet therapy. For patients with blood pressure lower than 220/120 mm Hg who did not receive IV alteplase or thrombectomy, treatment of hypertension in the first 48-72 hours after an AIS does not change the outcome. It is reasonable when patients have BP greater than or equal to 220/120 mm Hg, to lower blood pressure by 15% during the first 24 hours after onset of stroke. Starting or restarting antihypertensive therapy during hospitalization in patients with blood pressure higher than 140/90 mm Hg who are neurologically stable improves long-term blood pressure control and is considered a reasonable strategy.
For patients with noncardioembolic AIS, the use of antiplatelet agents rather than oral anticoagulation is recommended. Patients should be treated with aspirin 160 mg-325 mg within 24-48 hours of presentation. In patients unsafe or unable to swallow, rectal or nasogastric administration is recommended. In patients with minor stroke, 21 days of dual-antiplatelet therapy (aspirin and clopidogrel) started within 24 hours can decrease stroke recurrence for the first 90 days after a stroke. This recommendation is based on a single study, the CHANCE trial, in a homogeneous population in China, and its generalizability is not known. If a patient had an AIS while already on aspirin, there is some evidence supporting a decreased risk of major cardiovascular events and recurrent stroke in patients switching to an alternative antiplatelet agent or combination antiplatelet therapy. Because of methodologic issues in the those studies, the guideline concludes that, for those already on aspirin, it is of unclear benefit to increase the dose of aspirin, switch to a different antiplatelet agent, or add a second antiplatelet agent. Switching to warfarin is not beneficial for secondary stroke prevention. High-dose statin therapy should be initiated. For patients with AIS in the setting of atrial fibrillation, oral anticoagulation can be started within 4-14 days after the stroke. One study showed that anticoagulation should not be started before 4 days after the stroke, with a hazard ratio of 0.53 for starting anticoagulation at 4-14 days, compared with less than 4 days.
Hyperglycemia should be controlled to a range of 140-180 mg/dL, because higher values are associated with worse outcomes. Oxygen should be used if needed to maintain oxygen saturation greater than 94%. High-intensity statin therapy should be used, and smoking cessation is strongly encouraged for those who use tobacco, with avoidance of secondhand smoke whenever possible.
Patients should be screened for dysphagia before taking anything per oral, including medications. A nasogastric tube may be considered within the first 7 days, if patients are dysphagic. Oral hygiene protocols may include antibacterial mouth rinse, systematic oral care, and decontamination gel to decrease the risk of pneumonia .
For deep vein thrombosis prophylaxis, intermittent pneumatic compression, in addition to the aspirin that a patient is on is reasonable, and the benefit of prophylactic-dose subcutaneous heparin (unfractionated heparin or low-molecular-weight heparin) in immobile patients with AIS is not well established.
In the poststroke setting, patients should be screened for depression and, if appropriate, treated with antidepressants. Regular skin assessments are recommended with objective scales, and skin friction and pressure should be actively minimized with regular turning, good skin hygiene, and use of specialized mattresses, wheelchair cushions, and seating until mobility returns. Early rehabilitation for hospitalized stroke patients should be provided, but high-dose, very-early mobilization within 24 hours of stroke should not be done because it reduces the odds of a favorable outcome at 3 months.
Completing the diagnostic evaluation for the cause of stroke and decreasing the chance of future strokes should be part of the initial hospitalization. While MRI is more sensitive than is CT for detecting AIS, routine use of MRI in all patients with AIS is not cost effective and therefore is not recommended. For patients with nondisabling AIS in the carotid territory and who are candidates for carotid endarterectomy or stenting, noninvasive imaging of the cervical vessels should be performed within 24 hours of admission, with plans for carotid revascularization between 48 hours and 7 days if indicated. Cardiac monitoring for at least the first 24 hours of admission should be performed, while primarily looking for atrial fibrillation as a cause of stroke. In some patients, prolonged cardiac monitoring may be reasonable. With prolonged cardiac monitoring, atrial fibrillation is newly detected in nearly a quarter of patients with stroke or TIA, but the effect on outcomes is uncertain. Routine use of echocardiography is not recommended but may be done in selected patients. All patients should be screened for diabetes. It is not clear whether screening for thrombophilic states is useful.
All patients should be counseled on stroke, and provided education about it and how it will affect their lives. Following their acute medical stay, all patients will benefit from rehabilitation, with the benefits associated using a program tailored to their needs and outcome goals.
The bottom line
Early management of stroke involves first determining whether someone is a candidate for reperfusion therapy with alteplase or thrombectomy and then, if not, admitting them to a monitored setting to screen for atrial fibrillation and evaluation for carotid stenosis. Patients should be evaluated for both depression and swallowing function, and there should be initiation of deep vein thrombosis prevention, appropriate management of elevated blood pressures, anti-platelet therapy, and statin therapy as well as plans for rehabilitation services.
Reference
Powers WJ et al. on behalf of the American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018 Mar;49(3):e46-e110.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Callahan is an attending physician and preceptor in the family medicine residency program at Abington Jefferson Health.
VIDEO: Stem cells may reverse premature menopause, restore fertility
CHICAGO –
Neither of the women had menstruated for about 5 years; both were in premature menopause and wanted desperately to start a family. Estrogen levels began increasing soon after treatment, and night sweats and other symptoms of menopause almost disappeared. After a year, the treated ovary was almost the size of a normal premenopausal ovary. There weren’t any complications or side effects, and both women are now pursuing pregnancy.
The results are “very exciting, very encouraging,” said senior investigator Ayman Al-Hendy, MD, PhD, a gynecology professor at the University of Illinois at Chicago.
“The eggs are there, but they are not active.” Something about the stem cells seems “to activate these eggs to become fertilizable,” he said.
Far more work is planned on the project, and there’s no shortage of volunteers; premature menopause is a devastating diagnosis to young women hoping to start families.
Dr. Al-Hendy explained the procedure and the next steps in a video interview at the Endocrine Society annual meeting.
The work is supported by MD Stem Cells and the National Institutes of Health. Dr. Al-Hendy didn’t have any relevant disclosures.
SOURCE: Al-Hendy A et al. ENDO 2018, Abstract OR33-6.
CHICAGO –
Neither of the women had menstruated for about 5 years; both were in premature menopause and wanted desperately to start a family. Estrogen levels began increasing soon after treatment, and night sweats and other symptoms of menopause almost disappeared. After a year, the treated ovary was almost the size of a normal premenopausal ovary. There weren’t any complications or side effects, and both women are now pursuing pregnancy.
The results are “very exciting, very encouraging,” said senior investigator Ayman Al-Hendy, MD, PhD, a gynecology professor at the University of Illinois at Chicago.
“The eggs are there, but they are not active.” Something about the stem cells seems “to activate these eggs to become fertilizable,” he said.
Far more work is planned on the project, and there’s no shortage of volunteers; premature menopause is a devastating diagnosis to young women hoping to start families.
Dr. Al-Hendy explained the procedure and the next steps in a video interview at the Endocrine Society annual meeting.
The work is supported by MD Stem Cells and the National Institutes of Health. Dr. Al-Hendy didn’t have any relevant disclosures.
SOURCE: Al-Hendy A et al. ENDO 2018, Abstract OR33-6.
CHICAGO –
Neither of the women had menstruated for about 5 years; both were in premature menopause and wanted desperately to start a family. Estrogen levels began increasing soon after treatment, and night sweats and other symptoms of menopause almost disappeared. After a year, the treated ovary was almost the size of a normal premenopausal ovary. There weren’t any complications or side effects, and both women are now pursuing pregnancy.
The results are “very exciting, very encouraging,” said senior investigator Ayman Al-Hendy, MD, PhD, a gynecology professor at the University of Illinois at Chicago.
“The eggs are there, but they are not active.” Something about the stem cells seems “to activate these eggs to become fertilizable,” he said.
Far more work is planned on the project, and there’s no shortage of volunteers; premature menopause is a devastating diagnosis to young women hoping to start families.
Dr. Al-Hendy explained the procedure and the next steps in a video interview at the Endocrine Society annual meeting.
The work is supported by MD Stem Cells and the National Institutes of Health. Dr. Al-Hendy didn’t have any relevant disclosures.
SOURCE: Al-Hendy A et al. ENDO 2018, Abstract OR33-6.
REPORTING FROM ENDO 2018