User login
Mexican Americans With Midlife Stroke May Have Worse Outcomes Than Non-Hispanic Whites
LOS ANGELES—Mexican Americans with midlife stroke may have significantly worse 90-day outcomes, compared with non-Hispanic whites, according to a study presented at the International Stroke Conference 2018. Mexican Americans had lower physical quality of life scores and a high prevalence of depression, the authors noted.
“The growing number of midlife stroke survivors will have unique rehabilitation and health care resource needs,” said Lynda D. Lisabeth, PhD, MPH, Professor of Epidemiology at the University of Michigan Medical School in Ann Arbor. “Particular attention to Mexican American midlife stroke survivors is warranted.”
Midlife stroke can result in many years of stroke-related disability, and midlife stroke rates are stable or increasing. The impact of midlife stroke may be worse in Mexican Americans, who have higher stroke rates, earlier strokes, and appear to have worse outcomes, said the researchers. To compare stroke outcomes in Mexican Americans and non-Hispanic whites younger than 65, Dr. Lisabeth and colleagues analyzed data from the population-based Brain Attack Surveillance in Corpus Christi (BASIC) project in South Texas.
The researchers identified patients younger than 65 with incident ischemic stroke between 2008 and 2015. Strokes were identified using active and passive stroke surveillance and validated by a physician blinded to patients’ ethnicity. Investigators conducted in-person interviews with patients or proxies at baseline and at 90 days. The researchers assessed patients’ functional, neurologic, and cognitive outcomes, quality of life, and depression.
Of 925 patients with a first ischemic stroke between the ages of 45 and 64, 508 patients had outcome data (341 Mexican Americans and 167 non-Hispanic whites). At baseline, Mexican Americans had lower levels of education, higher average BMI, and were more likely to have hypertension and diabetes, compared with non-Hispanic whites. Mexican Americans were less likely to smoke than non-Hispanic whites, however.
After adjusting for confounders (eg, age, sex, education, insurance, marital status, stroke severity, stroke treatment, risk factors, prestroke function, cognition, and comorbidity level), Mexican Americans had significantly worse 90-day outcomes than non-Hispanic whites in every domain except depression. Among Mexican Americans, the median functional outcome score (ie, total Activities of Daily Living/Instrumental Activities of Daily Living score, range 1–4, higher scores worse) was 1.9, compared with 1.5 among non-Hispanic whites (adjusted mean difference, 0.26). The median NIH Stroke Scale score (range 0–42, higher scores worse) was 2 among Mexican Americans, compared with 1 among non-Hispanic whites (adjusted mean difference, 26%).
In addition, the median score on the Modified Mini-Mental State Examination (range 0–100, higher scores better) was 90 for Mexican Americans, compared with 94.5 for non-Hispanic whites. Mexican Americans had a lower median total Stroke-Specific Quality of Life score (range 1–5, higher scores better) and lower median scores on the physical and psychosocial Stroke-Specific Quality of Life subscales, compared with non-Hispanic whites (3.3 vs 3.8, 2.5 vs 3.2, and 4.2 vs 4.5, respectively). In addition, a greater percentage of Mexican Americans had depression, compared with non-Hispanic whites (41.1% vs 34.5%), but this difference was not significant, likely due to the smaller sample size for this outcome.
—Erica Tricarico
LOS ANGELES—Mexican Americans with midlife stroke may have significantly worse 90-day outcomes, compared with non-Hispanic whites, according to a study presented at the International Stroke Conference 2018. Mexican Americans had lower physical quality of life scores and a high prevalence of depression, the authors noted.
“The growing number of midlife stroke survivors will have unique rehabilitation and health care resource needs,” said Lynda D. Lisabeth, PhD, MPH, Professor of Epidemiology at the University of Michigan Medical School in Ann Arbor. “Particular attention to Mexican American midlife stroke survivors is warranted.”
Midlife stroke can result in many years of stroke-related disability, and midlife stroke rates are stable or increasing. The impact of midlife stroke may be worse in Mexican Americans, who have higher stroke rates, earlier strokes, and appear to have worse outcomes, said the researchers. To compare stroke outcomes in Mexican Americans and non-Hispanic whites younger than 65, Dr. Lisabeth and colleagues analyzed data from the population-based Brain Attack Surveillance in Corpus Christi (BASIC) project in South Texas.
The researchers identified patients younger than 65 with incident ischemic stroke between 2008 and 2015. Strokes were identified using active and passive stroke surveillance and validated by a physician blinded to patients’ ethnicity. Investigators conducted in-person interviews with patients or proxies at baseline and at 90 days. The researchers assessed patients’ functional, neurologic, and cognitive outcomes, quality of life, and depression.
Of 925 patients with a first ischemic stroke between the ages of 45 and 64, 508 patients had outcome data (341 Mexican Americans and 167 non-Hispanic whites). At baseline, Mexican Americans had lower levels of education, higher average BMI, and were more likely to have hypertension and diabetes, compared with non-Hispanic whites. Mexican Americans were less likely to smoke than non-Hispanic whites, however.
After adjusting for confounders (eg, age, sex, education, insurance, marital status, stroke severity, stroke treatment, risk factors, prestroke function, cognition, and comorbidity level), Mexican Americans had significantly worse 90-day outcomes than non-Hispanic whites in every domain except depression. Among Mexican Americans, the median functional outcome score (ie, total Activities of Daily Living/Instrumental Activities of Daily Living score, range 1–4, higher scores worse) was 1.9, compared with 1.5 among non-Hispanic whites (adjusted mean difference, 0.26). The median NIH Stroke Scale score (range 0–42, higher scores worse) was 2 among Mexican Americans, compared with 1 among non-Hispanic whites (adjusted mean difference, 26%).
In addition, the median score on the Modified Mini-Mental State Examination (range 0–100, higher scores better) was 90 for Mexican Americans, compared with 94.5 for non-Hispanic whites. Mexican Americans had a lower median total Stroke-Specific Quality of Life score (range 1–5, higher scores better) and lower median scores on the physical and psychosocial Stroke-Specific Quality of Life subscales, compared with non-Hispanic whites (3.3 vs 3.8, 2.5 vs 3.2, and 4.2 vs 4.5, respectively). In addition, a greater percentage of Mexican Americans had depression, compared with non-Hispanic whites (41.1% vs 34.5%), but this difference was not significant, likely due to the smaller sample size for this outcome.
—Erica Tricarico
LOS ANGELES—Mexican Americans with midlife stroke may have significantly worse 90-day outcomes, compared with non-Hispanic whites, according to a study presented at the International Stroke Conference 2018. Mexican Americans had lower physical quality of life scores and a high prevalence of depression, the authors noted.
“The growing number of midlife stroke survivors will have unique rehabilitation and health care resource needs,” said Lynda D. Lisabeth, PhD, MPH, Professor of Epidemiology at the University of Michigan Medical School in Ann Arbor. “Particular attention to Mexican American midlife stroke survivors is warranted.”
Midlife stroke can result in many years of stroke-related disability, and midlife stroke rates are stable or increasing. The impact of midlife stroke may be worse in Mexican Americans, who have higher stroke rates, earlier strokes, and appear to have worse outcomes, said the researchers. To compare stroke outcomes in Mexican Americans and non-Hispanic whites younger than 65, Dr. Lisabeth and colleagues analyzed data from the population-based Brain Attack Surveillance in Corpus Christi (BASIC) project in South Texas.
The researchers identified patients younger than 65 with incident ischemic stroke between 2008 and 2015. Strokes were identified using active and passive stroke surveillance and validated by a physician blinded to patients’ ethnicity. Investigators conducted in-person interviews with patients or proxies at baseline and at 90 days. The researchers assessed patients’ functional, neurologic, and cognitive outcomes, quality of life, and depression.
Of 925 patients with a first ischemic stroke between the ages of 45 and 64, 508 patients had outcome data (341 Mexican Americans and 167 non-Hispanic whites). At baseline, Mexican Americans had lower levels of education, higher average BMI, and were more likely to have hypertension and diabetes, compared with non-Hispanic whites. Mexican Americans were less likely to smoke than non-Hispanic whites, however.
After adjusting for confounders (eg, age, sex, education, insurance, marital status, stroke severity, stroke treatment, risk factors, prestroke function, cognition, and comorbidity level), Mexican Americans had significantly worse 90-day outcomes than non-Hispanic whites in every domain except depression. Among Mexican Americans, the median functional outcome score (ie, total Activities of Daily Living/Instrumental Activities of Daily Living score, range 1–4, higher scores worse) was 1.9, compared with 1.5 among non-Hispanic whites (adjusted mean difference, 0.26). The median NIH Stroke Scale score (range 0–42, higher scores worse) was 2 among Mexican Americans, compared with 1 among non-Hispanic whites (adjusted mean difference, 26%).
In addition, the median score on the Modified Mini-Mental State Examination (range 0–100, higher scores better) was 90 for Mexican Americans, compared with 94.5 for non-Hispanic whites. Mexican Americans had a lower median total Stroke-Specific Quality of Life score (range 1–5, higher scores better) and lower median scores on the physical and psychosocial Stroke-Specific Quality of Life subscales, compared with non-Hispanic whites (3.3 vs 3.8, 2.5 vs 3.2, and 4.2 vs 4.5, respectively). In addition, a greater percentage of Mexican Americans had depression, compared with non-Hispanic whites (41.1% vs 34.5%), but this difference was not significant, likely due to the smaller sample size for this outcome.
—Erica Tricarico
What Is the Impact of Poststroke Cognitive Impairment in Patients With Mild Stroke?
LOS ANGELES—Poststroke cognitive impairment is associated with inability to return to work and to drive, according to research presented at the International Stroke Conference 2018. Patients who are able to return to work appear to have higher Montreal Cognitive Assessment (MOCA) scores. In addition, factors such as being older than 65, having a history of stroke and diabetes, being unemployed, and living in a facility may suggest a higher risk of poststroke cognitive impairment, the authors noted.
“Since cognitive impairment can impact life quality, screening, even in mild stroke, could be beneficial,” said Ilavarasy Maran, MD, a resident at the University of Connecticut Health/Hartford Hospital.
Two of the main causes of dependency in stroke survivors are poststroke cognitive impairment and poststroke dementia. Approximately two-thirds of patients with stroke develop cognitive decline. This prevalence is expected to rise because of the aging population, said the researchers.
To evaluate the burden and risk factors associated with cognitive impairment after mild stroke, Dr. Maran and colleagues conducted a retrospective observational cohort study of 56 patients (51 with ischemic stroke, five with hemorrhagic stroke) evaluated between July 2016 and June 2017. Patients completed a questionnaire that elicited demographic information and history, as well as previously known cognitive impairment. Researchers used MOCA to evaluate cognition.
Median age was 61.5, 66.1% of patients were men, 76.8% were Caucasian, and the median discharge NIH Stroke Scale score was 1. Four percent of patients presented to the clinic at fewer than six weeks, 71.4% presented to the clinic at six to 12 weeks, 17.9% presented to the clinic at 12 to 24 weeks, and 7.1% of patients presented to the clinic at more than 24 weeks after discharge for stroke. None of the patients had a previous history of dementia, said the researchers.
In all, 50% of patients had no cognitive impairment, 19.6% had mild impairment, 16.1% had moderate impairment, and 14.3% had dementia. In addition, 50% of patients who had been working previously were able to return to work. This group had higher MOCA scores than did participants who did not return to work. Approximately 19.6% of patients who stopped driving did so due to cognitive impairment. No significant difference in MOCA scores based on the location of the stroke was reported.
Researchers also observed that patients with cognitive impairment had a previous history of hypertension and that history of depression did not appear to influence poststroke cognitive impairment.
—Erica Tricarico
LOS ANGELES—Poststroke cognitive impairment is associated with inability to return to work and to drive, according to research presented at the International Stroke Conference 2018. Patients who are able to return to work appear to have higher Montreal Cognitive Assessment (MOCA) scores. In addition, factors such as being older than 65, having a history of stroke and diabetes, being unemployed, and living in a facility may suggest a higher risk of poststroke cognitive impairment, the authors noted.
“Since cognitive impairment can impact life quality, screening, even in mild stroke, could be beneficial,” said Ilavarasy Maran, MD, a resident at the University of Connecticut Health/Hartford Hospital.
Two of the main causes of dependency in stroke survivors are poststroke cognitive impairment and poststroke dementia. Approximately two-thirds of patients with stroke develop cognitive decline. This prevalence is expected to rise because of the aging population, said the researchers.
To evaluate the burden and risk factors associated with cognitive impairment after mild stroke, Dr. Maran and colleagues conducted a retrospective observational cohort study of 56 patients (51 with ischemic stroke, five with hemorrhagic stroke) evaluated between July 2016 and June 2017. Patients completed a questionnaire that elicited demographic information and history, as well as previously known cognitive impairment. Researchers used MOCA to evaluate cognition.
Median age was 61.5, 66.1% of patients were men, 76.8% were Caucasian, and the median discharge NIH Stroke Scale score was 1. Four percent of patients presented to the clinic at fewer than six weeks, 71.4% presented to the clinic at six to 12 weeks, 17.9% presented to the clinic at 12 to 24 weeks, and 7.1% of patients presented to the clinic at more than 24 weeks after discharge for stroke. None of the patients had a previous history of dementia, said the researchers.
In all, 50% of patients had no cognitive impairment, 19.6% had mild impairment, 16.1% had moderate impairment, and 14.3% had dementia. In addition, 50% of patients who had been working previously were able to return to work. This group had higher MOCA scores than did participants who did not return to work. Approximately 19.6% of patients who stopped driving did so due to cognitive impairment. No significant difference in MOCA scores based on the location of the stroke was reported.
Researchers also observed that patients with cognitive impairment had a previous history of hypertension and that history of depression did not appear to influence poststroke cognitive impairment.
—Erica Tricarico
LOS ANGELES—Poststroke cognitive impairment is associated with inability to return to work and to drive, according to research presented at the International Stroke Conference 2018. Patients who are able to return to work appear to have higher Montreal Cognitive Assessment (MOCA) scores. In addition, factors such as being older than 65, having a history of stroke and diabetes, being unemployed, and living in a facility may suggest a higher risk of poststroke cognitive impairment, the authors noted.
“Since cognitive impairment can impact life quality, screening, even in mild stroke, could be beneficial,” said Ilavarasy Maran, MD, a resident at the University of Connecticut Health/Hartford Hospital.
Two of the main causes of dependency in stroke survivors are poststroke cognitive impairment and poststroke dementia. Approximately two-thirds of patients with stroke develop cognitive decline. This prevalence is expected to rise because of the aging population, said the researchers.
To evaluate the burden and risk factors associated with cognitive impairment after mild stroke, Dr. Maran and colleagues conducted a retrospective observational cohort study of 56 patients (51 with ischemic stroke, five with hemorrhagic stroke) evaluated between July 2016 and June 2017. Patients completed a questionnaire that elicited demographic information and history, as well as previously known cognitive impairment. Researchers used MOCA to evaluate cognition.
Median age was 61.5, 66.1% of patients were men, 76.8% were Caucasian, and the median discharge NIH Stroke Scale score was 1. Four percent of patients presented to the clinic at fewer than six weeks, 71.4% presented to the clinic at six to 12 weeks, 17.9% presented to the clinic at 12 to 24 weeks, and 7.1% of patients presented to the clinic at more than 24 weeks after discharge for stroke. None of the patients had a previous history of dementia, said the researchers.
In all, 50% of patients had no cognitive impairment, 19.6% had mild impairment, 16.1% had moderate impairment, and 14.3% had dementia. In addition, 50% of patients who had been working previously were able to return to work. This group had higher MOCA scores than did participants who did not return to work. Approximately 19.6% of patients who stopped driving did so due to cognitive impairment. No significant difference in MOCA scores based on the location of the stroke was reported.
Researchers also observed that patients with cognitive impairment had a previous history of hypertension and that history of depression did not appear to influence poststroke cognitive impairment.
—Erica Tricarico
EMS Stroke Field Triage Improves Outcomes
LOS ANGELES—An emergency medical services (EMS) protocol to identify large-vessel occlusions and deliver patients to a comprehensive stroke center (CSC) within 30 minutes reduced the time to recanalization, when compared with a protocol that optimized the transfer of such patients from primary stroke centers (PSCs) to CSCs. The study was presented at the International Stroke Conference 2018.
The findings, which come from a sequential study conducted in an urban Rhode Island region, offer evidence to resolve the controversy over whether field triage in EMS units will improve outcomes. The controversy arose from concerns that stroke severity scores measured in the field are not always accurate, and that longer travel to a CSC could delay treatment for a patient who does not need thrombectomy.
“A lot of people have done mathematical modeling, but nobody has done the work to change the system so we can see what happens. This is the first study that has shown a real-world example of what it means for patients,” said Ryan McTaggart, MD, Director of Interventional Neuroradiology at Brown University Rhode Island Hospital in Providence.
Instituting a Transfer Protocol
The region where the study was carried out has one CSC and eight PSCs. The large-vessel occlusions transfer protocol instructed PSCs to contact the CSC when a patient scored 4 or 5 on the Los Angeles Motor Scale (LAMS) and to conduct CT and CT angiography. The PSC was instructed to share the images with the CSC, which decided whether to transfer the patient.
The field-based protocol relied on a LAMS score assessment by EMS personnel. Patients scoring 4 or 5 would be delivered to the CSC if it was within 30 minutes of their current location. Patients scoring less than 4 would be brought to the nearest facility. When the field LAMS score was 4 or greater and the nearest CSC was more than 30 minutes away, EMS personnel were instructed to travel to the closest PSC, but immediately send word of an inbound patient that might need a transfer to a CSC. In those cases, the PSC’s goal was to get images to the CSC for review within 45 minutes. The protocol was executed out to 24 hours after the patient was last known well.
Even in patients who were closer to a PSC than the CSC, process outcomes were better with the field triage protocol. “Despite eight additional minutes of transport time, IV t-PA was given 17 minutes earlier, and recanalization occurred almost an hour earlier,” said Dr. McTaggart. “That would indicate that perhaps even a 30-minute window is too conservative of a protocol, because the number needed to treat for mechanical thrombectomy is two or three, so you have this tremendously powerful treatment effect for these patients. If you can get it to them an hour earlier, it is a no-brainer to me that they need to go to the right place the first time,” he said.
Instituting the changes was difficult. Dr. McTaggart spent thousands of hours working with EMS personnel and emergency department physicians at PSCs. “It is a lot of work, but the downstream gains are huge, not only from a disability standpoint for patients, but for the economics of the health care system. We are potentially saving patients from disability health care costs,” he said.
Travel Time Increased
The study population included consecutive stroke patients in the region whose first contact was with EMS personnel during the following three time periods: before PSC–CSC transfer optimization and before field triage (ie, July 2015 to January 2016), after transfer optimization and with voluntary field triage (ie, January 2016 to January 2017), and when transfer optimization and field triage were mandatory (ie, January 2017 to January 2018).
The patients had an anterior large-vessel occlusion and mild-to-moderate early ischemic change. Outcomes included time from hospital arrival (ie, PSC or CSC) to alteplase treatment, arterial puncture, and recanalization. Clinical measures included favorable outcomes (ie, modified Rankin scale score 0–2) at 90 days, or discharge with an NIH Stroke Scale score of 4 or less, in cases where 90-day follow-up did not occur.
A total of 38 patients were seen before any procedural change occurred, 100 after transfer optimization, and 94 after transfer optimization and field triage were implemented. A Google Maps analysis showed that the median additional time required to travel to the CSC instead of a PSC was eight minutes.
The time to first use of IV alteplase decreased from 54 minutes before any procedural change to 49 minutes after transfer optimization and to 36 minutes after transfer optimization and field triage. Similar decreases were seen in time to arterial puncture (105 minutes, 101 minutes, and 88 minutes, respectively) and time to recanalization (156 minutes, 132 minutes, and 116 minutes, respectively). These differences did not reach statistical significance.
The clinical outcomes also became more favorable. Approximately 58% of patients had a favorable outcome at 90 days with both protocols in place, compared with 51% with only transfer optimization and 31% before any procedural changes.
The researchers conducted a subanalysis of 150 patients for whom a PSC was closer than the CSC. Of these patients, 94 went to the CSC and 56 went to a PSC. The elapsed time between EMS leaving the scene with the patient aboard and IV t-PA treatment was an average of 51 minutes in patients taken to the CSC, compared with 68 minutes in patients taken to PSCs. The time to arterial puncture was also shorter (98 minutes vs 155 minutes), as was time to recanalization (131 minutes vs 174 minutes).
Patients taken to the CSC were more likely to have a favorable outcome (65% vs 42%).
The study received no external funding. Dr. McTaggart reported no financial disclosures.
—Jim Kling
LOS ANGELES—An emergency medical services (EMS) protocol to identify large-vessel occlusions and deliver patients to a comprehensive stroke center (CSC) within 30 minutes reduced the time to recanalization, when compared with a protocol that optimized the transfer of such patients from primary stroke centers (PSCs) to CSCs. The study was presented at the International Stroke Conference 2018.
The findings, which come from a sequential study conducted in an urban Rhode Island region, offer evidence to resolve the controversy over whether field triage in EMS units will improve outcomes. The controversy arose from concerns that stroke severity scores measured in the field are not always accurate, and that longer travel to a CSC could delay treatment for a patient who does not need thrombectomy.
“A lot of people have done mathematical modeling, but nobody has done the work to change the system so we can see what happens. This is the first study that has shown a real-world example of what it means for patients,” said Ryan McTaggart, MD, Director of Interventional Neuroradiology at Brown University Rhode Island Hospital in Providence.
Instituting a Transfer Protocol
The region where the study was carried out has one CSC and eight PSCs. The large-vessel occlusions transfer protocol instructed PSCs to contact the CSC when a patient scored 4 or 5 on the Los Angeles Motor Scale (LAMS) and to conduct CT and CT angiography. The PSC was instructed to share the images with the CSC, which decided whether to transfer the patient.
The field-based protocol relied on a LAMS score assessment by EMS personnel. Patients scoring 4 or 5 would be delivered to the CSC if it was within 30 minutes of their current location. Patients scoring less than 4 would be brought to the nearest facility. When the field LAMS score was 4 or greater and the nearest CSC was more than 30 minutes away, EMS personnel were instructed to travel to the closest PSC, but immediately send word of an inbound patient that might need a transfer to a CSC. In those cases, the PSC’s goal was to get images to the CSC for review within 45 minutes. The protocol was executed out to 24 hours after the patient was last known well.
Even in patients who were closer to a PSC than the CSC, process outcomes were better with the field triage protocol. “Despite eight additional minutes of transport time, IV t-PA was given 17 minutes earlier, and recanalization occurred almost an hour earlier,” said Dr. McTaggart. “That would indicate that perhaps even a 30-minute window is too conservative of a protocol, because the number needed to treat for mechanical thrombectomy is two or three, so you have this tremendously powerful treatment effect for these patients. If you can get it to them an hour earlier, it is a no-brainer to me that they need to go to the right place the first time,” he said.
Instituting the changes was difficult. Dr. McTaggart spent thousands of hours working with EMS personnel and emergency department physicians at PSCs. “It is a lot of work, but the downstream gains are huge, not only from a disability standpoint for patients, but for the economics of the health care system. We are potentially saving patients from disability health care costs,” he said.
Travel Time Increased
The study population included consecutive stroke patients in the region whose first contact was with EMS personnel during the following three time periods: before PSC–CSC transfer optimization and before field triage (ie, July 2015 to January 2016), after transfer optimization and with voluntary field triage (ie, January 2016 to January 2017), and when transfer optimization and field triage were mandatory (ie, January 2017 to January 2018).
The patients had an anterior large-vessel occlusion and mild-to-moderate early ischemic change. Outcomes included time from hospital arrival (ie, PSC or CSC) to alteplase treatment, arterial puncture, and recanalization. Clinical measures included favorable outcomes (ie, modified Rankin scale score 0–2) at 90 days, or discharge with an NIH Stroke Scale score of 4 or less, in cases where 90-day follow-up did not occur.
A total of 38 patients were seen before any procedural change occurred, 100 after transfer optimization, and 94 after transfer optimization and field triage were implemented. A Google Maps analysis showed that the median additional time required to travel to the CSC instead of a PSC was eight minutes.
The time to first use of IV alteplase decreased from 54 minutes before any procedural change to 49 minutes after transfer optimization and to 36 minutes after transfer optimization and field triage. Similar decreases were seen in time to arterial puncture (105 minutes, 101 minutes, and 88 minutes, respectively) and time to recanalization (156 minutes, 132 minutes, and 116 minutes, respectively). These differences did not reach statistical significance.
The clinical outcomes also became more favorable. Approximately 58% of patients had a favorable outcome at 90 days with both protocols in place, compared with 51% with only transfer optimization and 31% before any procedural changes.
The researchers conducted a subanalysis of 150 patients for whom a PSC was closer than the CSC. Of these patients, 94 went to the CSC and 56 went to a PSC. The elapsed time between EMS leaving the scene with the patient aboard and IV t-PA treatment was an average of 51 minutes in patients taken to the CSC, compared with 68 minutes in patients taken to PSCs. The time to arterial puncture was also shorter (98 minutes vs 155 minutes), as was time to recanalization (131 minutes vs 174 minutes).
Patients taken to the CSC were more likely to have a favorable outcome (65% vs 42%).
The study received no external funding. Dr. McTaggart reported no financial disclosures.
—Jim Kling
LOS ANGELES—An emergency medical services (EMS) protocol to identify large-vessel occlusions and deliver patients to a comprehensive stroke center (CSC) within 30 minutes reduced the time to recanalization, when compared with a protocol that optimized the transfer of such patients from primary stroke centers (PSCs) to CSCs. The study was presented at the International Stroke Conference 2018.
The findings, which come from a sequential study conducted in an urban Rhode Island region, offer evidence to resolve the controversy over whether field triage in EMS units will improve outcomes. The controversy arose from concerns that stroke severity scores measured in the field are not always accurate, and that longer travel to a CSC could delay treatment for a patient who does not need thrombectomy.
“A lot of people have done mathematical modeling, but nobody has done the work to change the system so we can see what happens. This is the first study that has shown a real-world example of what it means for patients,” said Ryan McTaggart, MD, Director of Interventional Neuroradiology at Brown University Rhode Island Hospital in Providence.
Instituting a Transfer Protocol
The region where the study was carried out has one CSC and eight PSCs. The large-vessel occlusions transfer protocol instructed PSCs to contact the CSC when a patient scored 4 or 5 on the Los Angeles Motor Scale (LAMS) and to conduct CT and CT angiography. The PSC was instructed to share the images with the CSC, which decided whether to transfer the patient.
The field-based protocol relied on a LAMS score assessment by EMS personnel. Patients scoring 4 or 5 would be delivered to the CSC if it was within 30 minutes of their current location. Patients scoring less than 4 would be brought to the nearest facility. When the field LAMS score was 4 or greater and the nearest CSC was more than 30 minutes away, EMS personnel were instructed to travel to the closest PSC, but immediately send word of an inbound patient that might need a transfer to a CSC. In those cases, the PSC’s goal was to get images to the CSC for review within 45 minutes. The protocol was executed out to 24 hours after the patient was last known well.
Even in patients who were closer to a PSC than the CSC, process outcomes were better with the field triage protocol. “Despite eight additional minutes of transport time, IV t-PA was given 17 minutes earlier, and recanalization occurred almost an hour earlier,” said Dr. McTaggart. “That would indicate that perhaps even a 30-minute window is too conservative of a protocol, because the number needed to treat for mechanical thrombectomy is two or three, so you have this tremendously powerful treatment effect for these patients. If you can get it to them an hour earlier, it is a no-brainer to me that they need to go to the right place the first time,” he said.
Instituting the changes was difficult. Dr. McTaggart spent thousands of hours working with EMS personnel and emergency department physicians at PSCs. “It is a lot of work, but the downstream gains are huge, not only from a disability standpoint for patients, but for the economics of the health care system. We are potentially saving patients from disability health care costs,” he said.
Travel Time Increased
The study population included consecutive stroke patients in the region whose first contact was with EMS personnel during the following three time periods: before PSC–CSC transfer optimization and before field triage (ie, July 2015 to January 2016), after transfer optimization and with voluntary field triage (ie, January 2016 to January 2017), and when transfer optimization and field triage were mandatory (ie, January 2017 to January 2018).
The patients had an anterior large-vessel occlusion and mild-to-moderate early ischemic change. Outcomes included time from hospital arrival (ie, PSC or CSC) to alteplase treatment, arterial puncture, and recanalization. Clinical measures included favorable outcomes (ie, modified Rankin scale score 0–2) at 90 days, or discharge with an NIH Stroke Scale score of 4 or less, in cases where 90-day follow-up did not occur.
A total of 38 patients were seen before any procedural change occurred, 100 after transfer optimization, and 94 after transfer optimization and field triage were implemented. A Google Maps analysis showed that the median additional time required to travel to the CSC instead of a PSC was eight minutes.
The time to first use of IV alteplase decreased from 54 minutes before any procedural change to 49 minutes after transfer optimization and to 36 minutes after transfer optimization and field triage. Similar decreases were seen in time to arterial puncture (105 minutes, 101 minutes, and 88 minutes, respectively) and time to recanalization (156 minutes, 132 minutes, and 116 minutes, respectively). These differences did not reach statistical significance.
The clinical outcomes also became more favorable. Approximately 58% of patients had a favorable outcome at 90 days with both protocols in place, compared with 51% with only transfer optimization and 31% before any procedural changes.
The researchers conducted a subanalysis of 150 patients for whom a PSC was closer than the CSC. Of these patients, 94 went to the CSC and 56 went to a PSC. The elapsed time between EMS leaving the scene with the patient aboard and IV t-PA treatment was an average of 51 minutes in patients taken to the CSC, compared with 68 minutes in patients taken to PSCs. The time to arterial puncture was also shorter (98 minutes vs 155 minutes), as was time to recanalization (131 minutes vs 174 minutes).
Patients taken to the CSC were more likely to have a favorable outcome (65% vs 42%).
The study received no external funding. Dr. McTaggart reported no financial disclosures.
—Jim Kling
Endometriosis: Expert perspectives on medical and surgical management
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
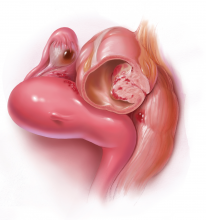
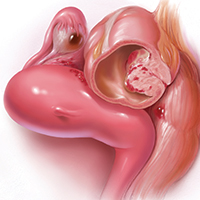
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.

Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.

Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Take-home points
- Endometriosis management involves fluidity of care. Treatment approaches will change throughout a patient's reproductive life, depending on the patient's presenting symptoms and reproductive goals.
- Inform the patient of the disease process and how it may affect her menstrual pain symptoms and family planning.
- Educate patients so they may effectively participate in the management discussion. Hear the voice of the patient to make a tailored plan of care for each individual.
- Endometriosis can be a complex medical problem. Use a comprehensive multidisciplinary approach when appropriate.
Watch: Video roundtable–Endometriosis: Expert perspectives on medical and surgical management
New and Noteworthy Information—March 2018
Short Stature in Children Linked to Future Stroke
Short stature at ages 7 to 13 is associated with increased risk of stroke in adulthood, according to a study published online ahead of print February 15 in Stroke. Data were examined for 311,009 schoolchildren born between 1930 and 1989. Cox proportional hazards regressions were performed to estimate hazard ratios. Among the participants, 10,412 were diagnosed with ischemic stroke, and 2,546 were diagnosed with intracerebral hemorrhage. Height at age 7 was inversely and significantly associated with ischemic stroke in both sexes and with intracerebral hemorrhage in men, but not in women. Associations were similar at older childhood ages and were stable throughout the study period. No statistically significant associations for growth from ages 7 to 13 were observed for ischemic stroke or intracerebral hemorrhage.
Gjærde LK, Truelsen TC, Baker JL. Childhood stature and growth in relation to first ischemic stroke or intracerebral hemorrhage. Stroke. 2018 Feb 15 [Epub ahead of print].
FDA Expands Treatment Window for Trevo Device
The FDA has cleared the use of the Trevo clot-retrieval device to treat certain patients with stroke as long as 24 hours after symptom onset, thus expanding its indications. The device is cleared as an initial therapy for acute ischemic stroke to reduce paralysis, speech difficulties, and other disabilities. It is to be used in addition to t-PA. Trevo was previously cleared for use in patients six hours after symptom onset. The agency evaluated data from a clinical trial comparing 107 patients treated with Trevo and medical management with 99 patients receiving medical management alone. About 48% of patients treated with Trevo were functionally independent three months after stroke, compared with 13% of patients receiving medical management. Stryker, headquartered in Kalamazoo, Michigan, markets the device.
Positive Views on Aging May Reduce Dementia Risk
Older adults who gain positive beliefs about old age from their culture are less likely to develop dementia, according to a study published February 7 in PLoS One. The cohort included 4,765 Health and Retirement Study participants age 60 or older who were dementia-free at baseline. In the total sample, people with positive age beliefs at baseline were significantly less likely to develop dementia, after adjusting for relevant covariates. Among people with APOE ε4, participants with positive age beliefs were 49.8% less likely to develop dementia than people with negative age beliefs. The results of this study suggest that positive age beliefs, which are modifiable and reduce stress, can be protective, even for older individuals at high risk of dementia, said the investigators.
Levy BR, Slade MD, Pietrzak RH, Ferrucci L. Positive age beliefs protect against dementia even among elders with high-risk gene. PLoS One. 2018 Feb 7;13(2):e0191004.
Should Women Stop MS Treatment During Pregnancy?
Natalizumab exposure for as long as 12 weeks of gestation increases the risk of spontaneous abortion, compared with exposure to injectable treatments or no treatment, in women with multiple sclerosis (MS), according to a study published online ahead of print February 7 in Neurology. Data for all pregnancies occurring between 2009 and 2015 in patients with MS treated with natalizumab were collected and compared with data for pregnancies in untreated patients and patients treated with injectable immunomodulatory agents. A total of 92 pregnancies were tracked in 83 women. In the multivariable analysis, natalizumab exposure was associated with spontaneous abortion. The rate of spontaneous abortion was within the estimates for the general population, however, as was the rate of major congenital anomalies.
Portaccio E, Annovazzi P, Ghezzi A, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: Fetal risks. Neurology. 2018 Feb 7 [Epub ahead of print].
Walking Ability After Stroke Improves With Arm Exercise
Arm exercise may improve walking ability after stroke, according to a study published online ahead of print December 6, 2017, in the Journal of Neurophysiology. Researchers worked with a group of older adults who had had a stroke between seven months and 17 years before the study. Participants underwent three 30-minute, moderate-intensity arm cycling training sessions each week for five weeks. Bilateral soleus stretch reflexes were elicited at rest and during 1-Hz arm cycling. Investigators measured physical abilities before and after arm training using several standardized scales and tests of physical function. Performance significantly improved on all walking tests and improved as much as 28% on the Timed Up and Go test. Several subjects had less tightness in their muscles after completing the arm cycling trial.
Kaupp C, Pearcey GE, Klarner T, et al. Rhythmic arm cycling training improves walking and neurophysiological integrity in chronic stroke-the arms can give legs a helping hand in rehabilitation. J Neurophysiol. 2017 Dec 6 [Epub ahead of print].
CSF May Indicate Parkinson’s Disease Phenotype
Lower CSF alpha-synuclein level is associated with diagnosis and motor phenotype in moderate and advanced Parkinson’s disease, according to a study published in the February issue of Movement Disorders. Researchers analyzed data from BioFIND, a cross-sectional, observational study that examines clinical and biomarker characteristics in moderate and advanced Parkinson’s disease and matched healthy controls. Investigators compared alpha-synuclein concentrations across diagnosis, biofluids, and CSF biomarkers. Correlations of CSF biomarkers and Movement Disorders Society Unified Parkinson’s Disease Rating Scale, motor phenotype, Montreal Cognitive Assessment, and REM sleep behavior disorder questionnaire scores in Parkinson’s disease were examined. CSF alpha-synuclein level was lower in Parkinson’s disease versus controls. Plasma and saliva alpha-synuclein levels did not differ between Parkinson’s disease and controls, and alpha-synuclein did not significantly correlate among biofluids.
Goldman JG, Andrews H, Amara A, et al. Cerebrospinal fluid, plasma, and saliva in the BioFIND study: relationships among biomarkers and Parkinson’s disease features. Mov Disord. 2018;33(2):282-288.
Social Interaction May Improve Quality of Life in Dementia
Increasing the amount of social interaction for people with dementia living in care homes to one hour a week improves quality of life when combined with personalized care, according to a study published February 6 in PLoS Med. In all, 847 people with dementia in 69 care homes were included in this study, which compared a psychosocial intervention plus antipsychotic treatment review with standard treatment using an intention-to-treat analysis. The primary outcome was quality of life. Staff were trained in person-centered care, social interaction, and education in antipsychotic medications. A total of 553 participants completed the nine-month randomized controlled trial. The intervention significantly improved quality of life, agitation, and overall neuropsychiatric symptoms. Benefits were greatest in people with moderately severe dementia.
Ballard C, Corbett A, Orrell M, et al. Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLoS Med. 2018 Feb 6;15(2):e1002500.
Adjunctive Cetirizine Reduces Relapses in Neuromyelitis Optica
In patients with neuromyelitis optica, adding cetirizine to standard therapy is safe and well tolerated and may reduce relapses, according to a study published online ahead of print February 2 in Neurology Neuroimmunology & Neuroinflammation. This pilot, open-label, add-on trial of cetirizine followed 16 patients with neuromyelitis optica taking 10 mg/day of oral cetirizine for one year in addition to their usual treatment. The primary end point was the annualized relapse rate while on the same disease-modifying therapy before starting cetirizine, compared with that while taking cetirizine. Participants were monitored for new neurologic episodes and potential adverse events related to the study drug. Annualized relapse rate was 0.4 before cetirizine treatment and 0.1 afterward. Cetirizine did not affect participants’ Expanded Disability Status Scale scores.
Katz Sand I, Fabian MT, Telford R, et al. Open-label, add-on trial of cetirizine for neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2018 Feb 2 [Epub ahead of print].
Stroke Risk Factors Specific to Women Identified
Investigators have identified stroke risk factors specific to women, according to a study published online ahead of print February 8 in Stroke. A literature review found risk factors in the three main categories of endogenous hormones, exogenous hormones, and pregnancy-related exposures. These factors include early age of menarche, early age at menopause, low levels of dehydroepiandrosterone, and taking oral estrogen or combined oral contraceptives. The risk factors are common, and not all women who have one or more of them will have a stroke. Clinicians should consider history of pregnancy complications, including gestational diabetes, pre-eclampsia, or hypertension during or immediately following pregnancy, when evaluating a patient for stroke risk, said the investigators. These women should be monitored carefully and told that they are at higher risk, they added.
Demel SL, Kittner S, Ley SH, et al. Stroke risk factors unique to women. Stroke. 2018 Feb 8 [Epub ahead of print].
FDA Clears Embrace for Monitoring Seizure Activity
The FDA has cleared the Embrace smart watch for detecting generalized tonic-clonic seizures. The device uses advanced machine learning and measures multiple indicators, including electrodermal activity. It also sends alerts to summon caregivers when it detects seizures. In a multisite clinical study, 135 patients with epilepsy were admitted to epilepsy monitoring units for continuous monitoring with video-EEG while they wore the device. A total of 6,530 hours of data were recorded over 272 days, including data for 40 generalized tonic-clonic seizures. The device’s algorithm detected 100% of the seizures. The trial used the gold standard of defining seizures as those clinically labelled by at least two out of three independent epileptologists, who examined the video-EEG data without seeing any data used by Embrace. The Embrace watch was approved in Europe in April 2017 as a medical device for seizure monitoring and alert. Empatica, which markets the watch, is located in Cambridge, Massachusetts.
FDA Approves Glatopa for Relapsing Forms of MS
The FDA has approved Glatopa (glatiramer acetate injection) 40 mg/mL, as a thrice-weekly generic treatment option for relapsing forms of multiple sclerosis (MS). Doses should be administered at 48-hour intervals. The treatment is intended to be fully substitutable for Copaxone. A 20-mg/mL formulation of the drug has been available since June 2015. Glatopa is contraindicated in patients with known hypersensitivity to glatiramer acetate or mannitol. The most common adverse reactions with glatiramer acetate injection 40 mg/mL versus placebo are injection-site reactions such as erythema (22% vs 2%). The most common side effects of glatiramer acetate injection 20 mg/mL versus placebo are erythema, vasodilatation, rash, dyspnea, and chest pain. Sandoz, which markets Glatopa, is headquartered in Holzkirchen, Germany.
—Kimberly Williams
Short Stature in Children Linked to Future Stroke
Short stature at ages 7 to 13 is associated with increased risk of stroke in adulthood, according to a study published online ahead of print February 15 in Stroke. Data were examined for 311,009 schoolchildren born between 1930 and 1989. Cox proportional hazards regressions were performed to estimate hazard ratios. Among the participants, 10,412 were diagnosed with ischemic stroke, and 2,546 were diagnosed with intracerebral hemorrhage. Height at age 7 was inversely and significantly associated with ischemic stroke in both sexes and with intracerebral hemorrhage in men, but not in women. Associations were similar at older childhood ages and were stable throughout the study period. No statistically significant associations for growth from ages 7 to 13 were observed for ischemic stroke or intracerebral hemorrhage.
Gjærde LK, Truelsen TC, Baker JL. Childhood stature and growth in relation to first ischemic stroke or intracerebral hemorrhage. Stroke. 2018 Feb 15 [Epub ahead of print].
FDA Expands Treatment Window for Trevo Device
The FDA has cleared the use of the Trevo clot-retrieval device to treat certain patients with stroke as long as 24 hours after symptom onset, thus expanding its indications. The device is cleared as an initial therapy for acute ischemic stroke to reduce paralysis, speech difficulties, and other disabilities. It is to be used in addition to t-PA. Trevo was previously cleared for use in patients six hours after symptom onset. The agency evaluated data from a clinical trial comparing 107 patients treated with Trevo and medical management with 99 patients receiving medical management alone. About 48% of patients treated with Trevo were functionally independent three months after stroke, compared with 13% of patients receiving medical management. Stryker, headquartered in Kalamazoo, Michigan, markets the device.
Positive Views on Aging May Reduce Dementia Risk
Older adults who gain positive beliefs about old age from their culture are less likely to develop dementia, according to a study published February 7 in PLoS One. The cohort included 4,765 Health and Retirement Study participants age 60 or older who were dementia-free at baseline. In the total sample, people with positive age beliefs at baseline were significantly less likely to develop dementia, after adjusting for relevant covariates. Among people with APOE ε4, participants with positive age beliefs were 49.8% less likely to develop dementia than people with negative age beliefs. The results of this study suggest that positive age beliefs, which are modifiable and reduce stress, can be protective, even for older individuals at high risk of dementia, said the investigators.
Levy BR, Slade MD, Pietrzak RH, Ferrucci L. Positive age beliefs protect against dementia even among elders with high-risk gene. PLoS One. 2018 Feb 7;13(2):e0191004.
Should Women Stop MS Treatment During Pregnancy?
Natalizumab exposure for as long as 12 weeks of gestation increases the risk of spontaneous abortion, compared with exposure to injectable treatments or no treatment, in women with multiple sclerosis (MS), according to a study published online ahead of print February 7 in Neurology. Data for all pregnancies occurring between 2009 and 2015 in patients with MS treated with natalizumab were collected and compared with data for pregnancies in untreated patients and patients treated with injectable immunomodulatory agents. A total of 92 pregnancies were tracked in 83 women. In the multivariable analysis, natalizumab exposure was associated with spontaneous abortion. The rate of spontaneous abortion was within the estimates for the general population, however, as was the rate of major congenital anomalies.
Portaccio E, Annovazzi P, Ghezzi A, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: Fetal risks. Neurology. 2018 Feb 7 [Epub ahead of print].
Walking Ability After Stroke Improves With Arm Exercise
Arm exercise may improve walking ability after stroke, according to a study published online ahead of print December 6, 2017, in the Journal of Neurophysiology. Researchers worked with a group of older adults who had had a stroke between seven months and 17 years before the study. Participants underwent three 30-minute, moderate-intensity arm cycling training sessions each week for five weeks. Bilateral soleus stretch reflexes were elicited at rest and during 1-Hz arm cycling. Investigators measured physical abilities before and after arm training using several standardized scales and tests of physical function. Performance significantly improved on all walking tests and improved as much as 28% on the Timed Up and Go test. Several subjects had less tightness in their muscles after completing the arm cycling trial.
Kaupp C, Pearcey GE, Klarner T, et al. Rhythmic arm cycling training improves walking and neurophysiological integrity in chronic stroke-the arms can give legs a helping hand in rehabilitation. J Neurophysiol. 2017 Dec 6 [Epub ahead of print].
CSF May Indicate Parkinson’s Disease Phenotype
Lower CSF alpha-synuclein level is associated with diagnosis and motor phenotype in moderate and advanced Parkinson’s disease, according to a study published in the February issue of Movement Disorders. Researchers analyzed data from BioFIND, a cross-sectional, observational study that examines clinical and biomarker characteristics in moderate and advanced Parkinson’s disease and matched healthy controls. Investigators compared alpha-synuclein concentrations across diagnosis, biofluids, and CSF biomarkers. Correlations of CSF biomarkers and Movement Disorders Society Unified Parkinson’s Disease Rating Scale, motor phenotype, Montreal Cognitive Assessment, and REM sleep behavior disorder questionnaire scores in Parkinson’s disease were examined. CSF alpha-synuclein level was lower in Parkinson’s disease versus controls. Plasma and saliva alpha-synuclein levels did not differ between Parkinson’s disease and controls, and alpha-synuclein did not significantly correlate among biofluids.
Goldman JG, Andrews H, Amara A, et al. Cerebrospinal fluid, plasma, and saliva in the BioFIND study: relationships among biomarkers and Parkinson’s disease features. Mov Disord. 2018;33(2):282-288.
Social Interaction May Improve Quality of Life in Dementia
Increasing the amount of social interaction for people with dementia living in care homes to one hour a week improves quality of life when combined with personalized care, according to a study published February 6 in PLoS Med. In all, 847 people with dementia in 69 care homes were included in this study, which compared a psychosocial intervention plus antipsychotic treatment review with standard treatment using an intention-to-treat analysis. The primary outcome was quality of life. Staff were trained in person-centered care, social interaction, and education in antipsychotic medications. A total of 553 participants completed the nine-month randomized controlled trial. The intervention significantly improved quality of life, agitation, and overall neuropsychiatric symptoms. Benefits were greatest in people with moderately severe dementia.
Ballard C, Corbett A, Orrell M, et al. Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLoS Med. 2018 Feb 6;15(2):e1002500.
Adjunctive Cetirizine Reduces Relapses in Neuromyelitis Optica
In patients with neuromyelitis optica, adding cetirizine to standard therapy is safe and well tolerated and may reduce relapses, according to a study published online ahead of print February 2 in Neurology Neuroimmunology & Neuroinflammation. This pilot, open-label, add-on trial of cetirizine followed 16 patients with neuromyelitis optica taking 10 mg/day of oral cetirizine for one year in addition to their usual treatment. The primary end point was the annualized relapse rate while on the same disease-modifying therapy before starting cetirizine, compared with that while taking cetirizine. Participants were monitored for new neurologic episodes and potential adverse events related to the study drug. Annualized relapse rate was 0.4 before cetirizine treatment and 0.1 afterward. Cetirizine did not affect participants’ Expanded Disability Status Scale scores.
Katz Sand I, Fabian MT, Telford R, et al. Open-label, add-on trial of cetirizine for neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2018 Feb 2 [Epub ahead of print].
Stroke Risk Factors Specific to Women Identified
Investigators have identified stroke risk factors specific to women, according to a study published online ahead of print February 8 in Stroke. A literature review found risk factors in the three main categories of endogenous hormones, exogenous hormones, and pregnancy-related exposures. These factors include early age of menarche, early age at menopause, low levels of dehydroepiandrosterone, and taking oral estrogen or combined oral contraceptives. The risk factors are common, and not all women who have one or more of them will have a stroke. Clinicians should consider history of pregnancy complications, including gestational diabetes, pre-eclampsia, or hypertension during or immediately following pregnancy, when evaluating a patient for stroke risk, said the investigators. These women should be monitored carefully and told that they are at higher risk, they added.
Demel SL, Kittner S, Ley SH, et al. Stroke risk factors unique to women. Stroke. 2018 Feb 8 [Epub ahead of print].
FDA Clears Embrace for Monitoring Seizure Activity
The FDA has cleared the Embrace smart watch for detecting generalized tonic-clonic seizures. The device uses advanced machine learning and measures multiple indicators, including electrodermal activity. It also sends alerts to summon caregivers when it detects seizures. In a multisite clinical study, 135 patients with epilepsy were admitted to epilepsy monitoring units for continuous monitoring with video-EEG while they wore the device. A total of 6,530 hours of data were recorded over 272 days, including data for 40 generalized tonic-clonic seizures. The device’s algorithm detected 100% of the seizures. The trial used the gold standard of defining seizures as those clinically labelled by at least two out of three independent epileptologists, who examined the video-EEG data without seeing any data used by Embrace. The Embrace watch was approved in Europe in April 2017 as a medical device for seizure monitoring and alert. Empatica, which markets the watch, is located in Cambridge, Massachusetts.
FDA Approves Glatopa for Relapsing Forms of MS
The FDA has approved Glatopa (glatiramer acetate injection) 40 mg/mL, as a thrice-weekly generic treatment option for relapsing forms of multiple sclerosis (MS). Doses should be administered at 48-hour intervals. The treatment is intended to be fully substitutable for Copaxone. A 20-mg/mL formulation of the drug has been available since June 2015. Glatopa is contraindicated in patients with known hypersensitivity to glatiramer acetate or mannitol. The most common adverse reactions with glatiramer acetate injection 40 mg/mL versus placebo are injection-site reactions such as erythema (22% vs 2%). The most common side effects of glatiramer acetate injection 20 mg/mL versus placebo are erythema, vasodilatation, rash, dyspnea, and chest pain. Sandoz, which markets Glatopa, is headquartered in Holzkirchen, Germany.
—Kimberly Williams
Short Stature in Children Linked to Future Stroke
Short stature at ages 7 to 13 is associated with increased risk of stroke in adulthood, according to a study published online ahead of print February 15 in Stroke. Data were examined for 311,009 schoolchildren born between 1930 and 1989. Cox proportional hazards regressions were performed to estimate hazard ratios. Among the participants, 10,412 were diagnosed with ischemic stroke, and 2,546 were diagnosed with intracerebral hemorrhage. Height at age 7 was inversely and significantly associated with ischemic stroke in both sexes and with intracerebral hemorrhage in men, but not in women. Associations were similar at older childhood ages and were stable throughout the study period. No statistically significant associations for growth from ages 7 to 13 were observed for ischemic stroke or intracerebral hemorrhage.
Gjærde LK, Truelsen TC, Baker JL. Childhood stature and growth in relation to first ischemic stroke or intracerebral hemorrhage. Stroke. 2018 Feb 15 [Epub ahead of print].
FDA Expands Treatment Window for Trevo Device
The FDA has cleared the use of the Trevo clot-retrieval device to treat certain patients with stroke as long as 24 hours after symptom onset, thus expanding its indications. The device is cleared as an initial therapy for acute ischemic stroke to reduce paralysis, speech difficulties, and other disabilities. It is to be used in addition to t-PA. Trevo was previously cleared for use in patients six hours after symptom onset. The agency evaluated data from a clinical trial comparing 107 patients treated with Trevo and medical management with 99 patients receiving medical management alone. About 48% of patients treated with Trevo were functionally independent three months after stroke, compared with 13% of patients receiving medical management. Stryker, headquartered in Kalamazoo, Michigan, markets the device.
Positive Views on Aging May Reduce Dementia Risk
Older adults who gain positive beliefs about old age from their culture are less likely to develop dementia, according to a study published February 7 in PLoS One. The cohort included 4,765 Health and Retirement Study participants age 60 or older who were dementia-free at baseline. In the total sample, people with positive age beliefs at baseline were significantly less likely to develop dementia, after adjusting for relevant covariates. Among people with APOE ε4, participants with positive age beliefs were 49.8% less likely to develop dementia than people with negative age beliefs. The results of this study suggest that positive age beliefs, which are modifiable and reduce stress, can be protective, even for older individuals at high risk of dementia, said the investigators.
Levy BR, Slade MD, Pietrzak RH, Ferrucci L. Positive age beliefs protect against dementia even among elders with high-risk gene. PLoS One. 2018 Feb 7;13(2):e0191004.
Should Women Stop MS Treatment During Pregnancy?
Natalizumab exposure for as long as 12 weeks of gestation increases the risk of spontaneous abortion, compared with exposure to injectable treatments or no treatment, in women with multiple sclerosis (MS), according to a study published online ahead of print February 7 in Neurology. Data for all pregnancies occurring between 2009 and 2015 in patients with MS treated with natalizumab were collected and compared with data for pregnancies in untreated patients and patients treated with injectable immunomodulatory agents. A total of 92 pregnancies were tracked in 83 women. In the multivariable analysis, natalizumab exposure was associated with spontaneous abortion. The rate of spontaneous abortion was within the estimates for the general population, however, as was the rate of major congenital anomalies.
Portaccio E, Annovazzi P, Ghezzi A, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: Fetal risks. Neurology. 2018 Feb 7 [Epub ahead of print].
Walking Ability After Stroke Improves With Arm Exercise
Arm exercise may improve walking ability after stroke, according to a study published online ahead of print December 6, 2017, in the Journal of Neurophysiology. Researchers worked with a group of older adults who had had a stroke between seven months and 17 years before the study. Participants underwent three 30-minute, moderate-intensity arm cycling training sessions each week for five weeks. Bilateral soleus stretch reflexes were elicited at rest and during 1-Hz arm cycling. Investigators measured physical abilities before and after arm training using several standardized scales and tests of physical function. Performance significantly improved on all walking tests and improved as much as 28% on the Timed Up and Go test. Several subjects had less tightness in their muscles after completing the arm cycling trial.
Kaupp C, Pearcey GE, Klarner T, et al. Rhythmic arm cycling training improves walking and neurophysiological integrity in chronic stroke-the arms can give legs a helping hand in rehabilitation. J Neurophysiol. 2017 Dec 6 [Epub ahead of print].
CSF May Indicate Parkinson’s Disease Phenotype
Lower CSF alpha-synuclein level is associated with diagnosis and motor phenotype in moderate and advanced Parkinson’s disease, according to a study published in the February issue of Movement Disorders. Researchers analyzed data from BioFIND, a cross-sectional, observational study that examines clinical and biomarker characteristics in moderate and advanced Parkinson’s disease and matched healthy controls. Investigators compared alpha-synuclein concentrations across diagnosis, biofluids, and CSF biomarkers. Correlations of CSF biomarkers and Movement Disorders Society Unified Parkinson’s Disease Rating Scale, motor phenotype, Montreal Cognitive Assessment, and REM sleep behavior disorder questionnaire scores in Parkinson’s disease were examined. CSF alpha-synuclein level was lower in Parkinson’s disease versus controls. Plasma and saliva alpha-synuclein levels did not differ between Parkinson’s disease and controls, and alpha-synuclein did not significantly correlate among biofluids.
Goldman JG, Andrews H, Amara A, et al. Cerebrospinal fluid, plasma, and saliva in the BioFIND study: relationships among biomarkers and Parkinson’s disease features. Mov Disord. 2018;33(2):282-288.
Social Interaction May Improve Quality of Life in Dementia
Increasing the amount of social interaction for people with dementia living in care homes to one hour a week improves quality of life when combined with personalized care, according to a study published February 6 in PLoS Med. In all, 847 people with dementia in 69 care homes were included in this study, which compared a psychosocial intervention plus antipsychotic treatment review with standard treatment using an intention-to-treat analysis. The primary outcome was quality of life. Staff were trained in person-centered care, social interaction, and education in antipsychotic medications. A total of 553 participants completed the nine-month randomized controlled trial. The intervention significantly improved quality of life, agitation, and overall neuropsychiatric symptoms. Benefits were greatest in people with moderately severe dementia.
Ballard C, Corbett A, Orrell M, et al. Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLoS Med. 2018 Feb 6;15(2):e1002500.
Adjunctive Cetirizine Reduces Relapses in Neuromyelitis Optica
In patients with neuromyelitis optica, adding cetirizine to standard therapy is safe and well tolerated and may reduce relapses, according to a study published online ahead of print February 2 in Neurology Neuroimmunology & Neuroinflammation. This pilot, open-label, add-on trial of cetirizine followed 16 patients with neuromyelitis optica taking 10 mg/day of oral cetirizine for one year in addition to their usual treatment. The primary end point was the annualized relapse rate while on the same disease-modifying therapy before starting cetirizine, compared with that while taking cetirizine. Participants were monitored for new neurologic episodes and potential adverse events related to the study drug. Annualized relapse rate was 0.4 before cetirizine treatment and 0.1 afterward. Cetirizine did not affect participants’ Expanded Disability Status Scale scores.
Katz Sand I, Fabian MT, Telford R, et al. Open-label, add-on trial of cetirizine for neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2018 Feb 2 [Epub ahead of print].
Stroke Risk Factors Specific to Women Identified
Investigators have identified stroke risk factors specific to women, according to a study published online ahead of print February 8 in Stroke. A literature review found risk factors in the three main categories of endogenous hormones, exogenous hormones, and pregnancy-related exposures. These factors include early age of menarche, early age at menopause, low levels of dehydroepiandrosterone, and taking oral estrogen or combined oral contraceptives. The risk factors are common, and not all women who have one or more of them will have a stroke. Clinicians should consider history of pregnancy complications, including gestational diabetes, pre-eclampsia, or hypertension during or immediately following pregnancy, when evaluating a patient for stroke risk, said the investigators. These women should be monitored carefully and told that they are at higher risk, they added.
Demel SL, Kittner S, Ley SH, et al. Stroke risk factors unique to women. Stroke. 2018 Feb 8 [Epub ahead of print].
FDA Clears Embrace for Monitoring Seizure Activity
The FDA has cleared the Embrace smart watch for detecting generalized tonic-clonic seizures. The device uses advanced machine learning and measures multiple indicators, including electrodermal activity. It also sends alerts to summon caregivers when it detects seizures. In a multisite clinical study, 135 patients with epilepsy were admitted to epilepsy monitoring units for continuous monitoring with video-EEG while they wore the device. A total of 6,530 hours of data were recorded over 272 days, including data for 40 generalized tonic-clonic seizures. The device’s algorithm detected 100% of the seizures. The trial used the gold standard of defining seizures as those clinically labelled by at least two out of three independent epileptologists, who examined the video-EEG data without seeing any data used by Embrace. The Embrace watch was approved in Europe in April 2017 as a medical device for seizure monitoring and alert. Empatica, which markets the watch, is located in Cambridge, Massachusetts.
FDA Approves Glatopa for Relapsing Forms of MS
The FDA has approved Glatopa (glatiramer acetate injection) 40 mg/mL, as a thrice-weekly generic treatment option for relapsing forms of multiple sclerosis (MS). Doses should be administered at 48-hour intervals. The treatment is intended to be fully substitutable for Copaxone. A 20-mg/mL formulation of the drug has been available since June 2015. Glatopa is contraindicated in patients with known hypersensitivity to glatiramer acetate or mannitol. The most common adverse reactions with glatiramer acetate injection 40 mg/mL versus placebo are injection-site reactions such as erythema (22% vs 2%). The most common side effects of glatiramer acetate injection 20 mg/mL versus placebo are erythema, vasodilatation, rash, dyspnea, and chest pain. Sandoz, which markets Glatopa, is headquartered in Holzkirchen, Germany.
—Kimberly Williams
Thrombectomy’s success treating strokes prompts rethinking of selection criteria
LOS ANGELES – CT or magnetic resonance brain imaging of acute ischemic stroke patients was the key triage tool in two groundbreaking thrombectomy trials, DAWN and DEFUSE; results from both showed that patients found by imaging to have limited infarcted cores could safely benefit from endovascular thrombectomy, even when they are more than 6 hours out from their stroke onset, breaking the 6-hour barrier created 3 years ago by the first wave of thrombectomy trials.
But some stroke neurologists studying thrombectomy are now convinced that imaging is not needed and may actually harm acute ischemic stroke patients early on by introducing an unnecessary time delay when they present within the first 6 hours after stroke onset.
This new thinking on how to best use brain imaging in acute ischemic stroke patients is part of the rapid evolution of acute stroke management as experts process new data and refine their approach both within 6 hours of stroke onset and during the new treatment window of 6-24 hours post onset. The dramatic success achieved with thrombectomy in highly selected late-window patients prompted researchers to promote pushing the boundaries further to find less-selected late patients who could also potentially benefit from thrombectomy.
The downside of early imaging
“Early on, we have a mix of fast and slow progressors. Slow progressors are about a third to half the patients, so there is a lot of potential for [late] treatment, but the majority of patients during the first 6 hours after onset are fast progressors,” patients who won’t benefit from thrombectomy delivery beyond 6 hours, said Dr. Jovin, an interventional neurologist and director of the Stroke Institute of the University of Pittsburgh Medical Center.
“Time is very precious in the 0- to 6-hour window. When we’re dealing with a lot of fast progressors, we pay a price [in added time to treatment] for any imaging we do. We need to understand that this is a real price we pay, even when CT takes perhaps 24 minutes, and MRI adds about 12 minutes. It’s not the case in all patients that doing CT angiography just adds 5 minutes. It can take 15, 20 minutes,” especially at centers that don’t treat these types of stroke patients day in and day out. “There is no question that imaging slows us down,” Dr. Jovin said.
He highlighted that “the main role of imaging is to exclude patients from treatment, a treatment that has unbelievable effects.” Imaging can rule out patients who have a hemorrhage, lack an occlusion, have a large infarcted core, or have none of the brain at risk or just a small amount, he noted. “Excluding hemorrhage is reasonable, but we can do that in the angiography suite, when the patient is on the table. The main benefit from advanced imaging is to more precisely define the core,” but for most patients the size of their core is not important because the vast majority of acute ischemic stroke patients seen within 6 hours of onset have cores smaller than 70 mL.
“Is this much ado about nothing – especially because we punish all the other patients [with smaller cores] who need to be treated [quickly] when we do additional imaging?” asked Dr. Jovin, who was one of the two lead investigators for the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) trial (N Engl J Med. 2018 Jan 4;378[1]:11-21). Another factor undercutting the value of imaging and determining core size is registry results that show patients who undergo thrombectomy with a large infarcted core are not harmed by treatment.
Current practice often uses imaging “to exclude the 20% of patients who may not have a large vessel occlusion or may not get benefit but whom we are unlikely to harm. But we harm the other 80% by delaying treatment by 30 minutes because of imaging. That’s why we need to rethink imaging and minimize its use.”
Dr. Jovin noted that at his center in Pittsburgh, the stroke institute staff began in 2013 to take patients transported from other stroke facilities and already diagnosed with a large vessel occlusion directly to the angiography suite, bypassing further brain imaging. By doing this, they cut their average door-to-groin puncture time down to 22 minutes from what had been an average of 81 minutes with imaging (Stroke. 2017 July;48[7]:1884-9). Right now the door-to-groin time is even lower, he added.
“Don’t waste time imaging,” said Dr. Nogueira, a stroke neurologist who is director of the neuroendovascular division of the Marcus Stroke and Neuroscience Center and professor of neurology, neurosurgery, and radiology at Emory University, both in Atlanta, as well as the second lead investigator for DAWN. “Time is crucial and trumps patient selection. Most selection criteria are informative rather than truly selective. It is important to understand that in every time window, we do not yet know who doesn’t benefit from treatment. Select faster, select less, and treat more” during the 0- to 6-hour window, he told his colleagues.
Expanding thrombectomy 6-24 hours after stroke onset
While Dr. Jovin and Dr. Nogueira called for more aggressive and less selective use of thrombectomy in patients who present within 6 hours of their stroke onset, they acknowledged that for patients who present during the 6- to 24-hour window, selection for thrombectomy should follow the rules applied in DAWN and in the more inclusive Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trial (N Engl J Med. 2018 Feb 22;378:708-18). That means using imaging to confirm that the patient’s infarcted core is within the enrollment ceiling, but both neurologists also downplayed the need for the more sophisticated imaging approaches that were often used in both trials as well as in current routine practice at comprehensive stroke centers. They agreed that noncontrast CT, a widely available method, seems adequate for patient selection, based on the admittedly limited data available right now.
Dr. Nogueira cited data from the Trevo stent retriever registry, collected from nearly 1,000 U.S. acute ischemic stroke patients who underwent thrombectomy with this device. (Researchers at the conference reported updated registry data with nearly 2,000 patients with findings similar to what Dr. Nogueira referenced.) Although these patients underwent treatment before results of DAWN and DEFUSE 3 came out and before release of the new U.S. acute stroke management guidelines (Stroke. 2018 Jan 24; doi: 10.1161/STR.0000000000000158) that endorsed targeted thrombectomy for patients 6-24 hours out from their stroke onset, 278 (28%) of the registry patients underwent thrombectomy treatment during the 6- to 24-hour time window. In this subgroup, 34 patients underwent noncontrast CT to assess their infarcted core prior to thrombectomy, while the other patients underwent perfusion CT, MRI, or both. The noncontrast CT patients had recanalization rates, adverse event rates, and 90-day modified Rankin Scale (mRS) scores comparable with those of patients assessed with more advanced imaging.
Based on this, “just looking at CT only seems reasonable. Noncontrast CT is a pretty valid way to select patients,” Dr. Nogueira said.
“This is the direction we should follow to simplify the paradigm for treating beyond 6 hours,” agreed Dr. Jovin, who also called for further advances in patient selection to expand the pool of patients who qualify for thrombectomy during the 6- to 24-hour postonset period.
“We want the DAWN results to be generalizable, to be simpler. We are exploring some more easily generalizable inclusion criteria that would allow us to treat more patients in more parts of the world,” Dr. Jovin added.
Both clinicians cited the remarkably low number needed to treat found in both DAWN and DEFUSE 3 of roughly three patients to produce one additional patient with a statistically significant and clinically meaningful improvement in their 90-day mRS score, compared with controls, as an unmistakable sign that the treatment in both trials was too targeted.
“When we planned the DAWN and DEFUSE 3 trials we didn’t expect how powerful the treatment effect would be. There are probably other patients who could also benefit, so how low can we go? How liberal can we be in our inclusion criteria and still get benefit?” Dr. Jovin asked.
“When intravenous TPA [tissue plasminogen activator] first came out, we went by the book [for patient selection], but as we got to know the treatment and became more comfortable with it, we began to bend the rules. Now we’re at the point of getting comfortable with endovascular treatment, and we need to figure out where to bend the rules by building the database. There is no doubt that the rules need bending because of the treatment effect that we’ve seen. We need to get our patients to endovascular treatment,” she said in her presentation at the conference.
But these physicians realize that for the time being, standard of care will follow the imaging and data processing primarily used in DAWN and DEFUSE 3, which not only involved perfusion CT or MRI but also a proprietary, automated image processing software, RAPID, that takes imaging data and calculates the amount and ratio of infarcted core and hypoperfused, ischemic brain tissue.
“I asked our imaging experts [at the University of Cincinnati] what should my threshold be [for mismatch between the infarcted core and ischemic tissue], and they said, ‘Use the automated software,’ ” Dr. Khatri said. If centers managing acute ischemic stroke patients don’t already have this software, “they need it. I think there is no way around that. It’s the only way we’ll be able to do this,” she commented. Most U.S. community hospitals that admit stroke patients currently lack this software, largely because of its high cost, she added.
“We’re struggling because it is very difficult to get some community hospitals – primary stroke centers – to invest in the software, but that’s really the only way we’ll be able to do this. There are issues of cost, and of getting technicians trained,” she noted.
That’s because DEFUSE 3 enrolled patients with a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 6, while patients in DAWN required a score of at least 10, a loosening that allowed inclusion of 31 patients with scores of 6-9 in DEFUSE 3. Another, less restrictive criterion was the patient’s prestroke mRS score, which could have been 0-2 in DEFUSE 3 but could be only 0-1 in DAWN. Thirteen of the DEFUSE 3 patients had prestroke mRS scores of 2. DEFUSE 3 also had somewhat more liberal criteria for the size of a patient’s infarcted core at enrollment, with 41 patients who would have been excluded from DAWN because of an overly large infarcted core, Dr. Albers said in his presentation at the conference.
Currently, for patients presenting more than 16 hours following their stroke onset but within 24 hours, the DAWN enrollment criteria exclusively determine which patients should get thrombectomy.
One area where these rules could be bent is by a more thoughtful approach to the prestroke mRS score rule out, such as patients with orthopedic or rheumatic complications that limit mobility and give them an mRS score of 3. “We don’t have data for patients with back pain who can’t walk. I currently take these patients to endovascular therapy, and I’m sure many others do, too,” Dr. Khatri said.
Another potential way to grow the inclusion criteria is to investigate thrombectomy in patients with larger infarcted cores than were enrolled in DAWN and DEFUSE 3, but assessing this will require a new prospective study, Dr. Albers said.
Running the 6- to 24-hour numbers
Adoption of the 6- to 24-hour time window for endovascular intervention in selected patients means that suddenly the U.S. acute stroke infrastructure needs to accommodate a significantly increased number of patients. Just how many added patients this means is uncertain for the time being, and will vary from region to region and center to center. Dr. Albers roughly guessed that the new late time window might double the number of stroke patients undergoing thrombectomy at his center in Stanford. Dr. Khatri put together a more data-driven but still very speculative estimate that at the University of Cincinnati, it will mean about 40% more stroke patients going to thrombectomy. She shared the numbers behind this estimate in a report she gave at the conference.
To calculate the incremental change produced by the late time window, she used data collected on 2,297 acute ischemic stroke patients from the Greater Cincinnati/Northern Kentucky region who were seen at the University of Cincinnati during 2010. Prior analysis by Dr. Khatri and her associates showed that 159 of these patients presented quickly enough and with an appropriate stroke to qualify for thrombolytic therapy, and that 29 patients would have qualified for thrombectomy performed during the 0- to 6-hour time window.
In the new analysis Dr. Khatri calculated that 791 patients presented at 5-23 hours, and of these 34 had other features that would have made them eligible for enrollment in DAWN. Because no imaging data existed for these 2010 patients, she applied an estimate that 22% of these patients would qualify by the size of their infarcted core and ischemic penumbra, resulting in seven additional thrombectomy-eligible patients. Accounting for patients who would qualify by the more liberal DEFUSE 3 criteria added another 5 patients for a total increment of 12 patients during 2010 who would have been eligible for thrombectomy, about 40% of the number from the 0- to 6-hour window.
She noted that “this is likely an underestimate,” and “too small a sample to project to national estimates,” but concluded that “resources must be adapted to account for this increased volume in endovascular treatment.”
Dr. Khatri acknowledged that the new 6- to 24-hour window for endovascular therapy, and concerns about imaging delays in 0- to 6-hour patients, raise challenging issues regarding the message to give U.S. clinicians about treating acute ischemic stroke patients.
“We have a mandate to figure it out in every region. There is no doubt that stroke patients need access to this care. We need to become a lot more aggressive with endovascular treatment. It’s so gratifying to see the outcomes that we’re seeing,” Dr. Khatri said. “A lot of work is needed to accommodate endovascular therapy–eligible patients in an extended time window. We need more refined prehospital triage tools, we need to adequately implement imaging software, and we need increased capacity to perform endovascular treatment with additional procedure suites, operators, and ICU beds.”
Dr. Jovin has been a consultant to Anaconda Biomed, Blockade Medical, Cerenovus, FreeOx Biotech, and Silk Road Medical. Dr. Nogueira has received travel expense reimbursement from Stryker. Dr. Khatri has been a consultant to Biogen, Medpace/Novartis, and St. Jude; has received travel support from Neuravi and EmstoPA; and has received research support from Genentech, Lumosa, and Neurospring. Dr. Albers has an ownership interest in iSchemaView, the company that markets the RAPID imaging software, and is a consultant to iSchemaView and Medtronic.
LOS ANGELES – CT or magnetic resonance brain imaging of acute ischemic stroke patients was the key triage tool in two groundbreaking thrombectomy trials, DAWN and DEFUSE; results from both showed that patients found by imaging to have limited infarcted cores could safely benefit from endovascular thrombectomy, even when they are more than 6 hours out from their stroke onset, breaking the 6-hour barrier created 3 years ago by the first wave of thrombectomy trials.
But some stroke neurologists studying thrombectomy are now convinced that imaging is not needed and may actually harm acute ischemic stroke patients early on by introducing an unnecessary time delay when they present within the first 6 hours after stroke onset.
This new thinking on how to best use brain imaging in acute ischemic stroke patients is part of the rapid evolution of acute stroke management as experts process new data and refine their approach both within 6 hours of stroke onset and during the new treatment window of 6-24 hours post onset. The dramatic success achieved with thrombectomy in highly selected late-window patients prompted researchers to promote pushing the boundaries further to find less-selected late patients who could also potentially benefit from thrombectomy.
The downside of early imaging
“Early on, we have a mix of fast and slow progressors. Slow progressors are about a third to half the patients, so there is a lot of potential for [late] treatment, but the majority of patients during the first 6 hours after onset are fast progressors,” patients who won’t benefit from thrombectomy delivery beyond 6 hours, said Dr. Jovin, an interventional neurologist and director of the Stroke Institute of the University of Pittsburgh Medical Center.
“Time is very precious in the 0- to 6-hour window. When we’re dealing with a lot of fast progressors, we pay a price [in added time to treatment] for any imaging we do. We need to understand that this is a real price we pay, even when CT takes perhaps 24 minutes, and MRI adds about 12 minutes. It’s not the case in all patients that doing CT angiography just adds 5 minutes. It can take 15, 20 minutes,” especially at centers that don’t treat these types of stroke patients day in and day out. “There is no question that imaging slows us down,” Dr. Jovin said.
He highlighted that “the main role of imaging is to exclude patients from treatment, a treatment that has unbelievable effects.” Imaging can rule out patients who have a hemorrhage, lack an occlusion, have a large infarcted core, or have none of the brain at risk or just a small amount, he noted. “Excluding hemorrhage is reasonable, but we can do that in the angiography suite, when the patient is on the table. The main benefit from advanced imaging is to more precisely define the core,” but for most patients the size of their core is not important because the vast majority of acute ischemic stroke patients seen within 6 hours of onset have cores smaller than 70 mL.
“Is this much ado about nothing – especially because we punish all the other patients [with smaller cores] who need to be treated [quickly] when we do additional imaging?” asked Dr. Jovin, who was one of the two lead investigators for the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) trial (N Engl J Med. 2018 Jan 4;378[1]:11-21). Another factor undercutting the value of imaging and determining core size is registry results that show patients who undergo thrombectomy with a large infarcted core are not harmed by treatment.
Current practice often uses imaging “to exclude the 20% of patients who may not have a large vessel occlusion or may not get benefit but whom we are unlikely to harm. But we harm the other 80% by delaying treatment by 30 minutes because of imaging. That’s why we need to rethink imaging and minimize its use.”
Dr. Jovin noted that at his center in Pittsburgh, the stroke institute staff began in 2013 to take patients transported from other stroke facilities and already diagnosed with a large vessel occlusion directly to the angiography suite, bypassing further brain imaging. By doing this, they cut their average door-to-groin puncture time down to 22 minutes from what had been an average of 81 minutes with imaging (Stroke. 2017 July;48[7]:1884-9). Right now the door-to-groin time is even lower, he added.
“Don’t waste time imaging,” said Dr. Nogueira, a stroke neurologist who is director of the neuroendovascular division of the Marcus Stroke and Neuroscience Center and professor of neurology, neurosurgery, and radiology at Emory University, both in Atlanta, as well as the second lead investigator for DAWN. “Time is crucial and trumps patient selection. Most selection criteria are informative rather than truly selective. It is important to understand that in every time window, we do not yet know who doesn’t benefit from treatment. Select faster, select less, and treat more” during the 0- to 6-hour window, he told his colleagues.
Expanding thrombectomy 6-24 hours after stroke onset
While Dr. Jovin and Dr. Nogueira called for more aggressive and less selective use of thrombectomy in patients who present within 6 hours of their stroke onset, they acknowledged that for patients who present during the 6- to 24-hour window, selection for thrombectomy should follow the rules applied in DAWN and in the more inclusive Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trial (N Engl J Med. 2018 Feb 22;378:708-18). That means using imaging to confirm that the patient’s infarcted core is within the enrollment ceiling, but both neurologists also downplayed the need for the more sophisticated imaging approaches that were often used in both trials as well as in current routine practice at comprehensive stroke centers. They agreed that noncontrast CT, a widely available method, seems adequate for patient selection, based on the admittedly limited data available right now.
Dr. Nogueira cited data from the Trevo stent retriever registry, collected from nearly 1,000 U.S. acute ischemic stroke patients who underwent thrombectomy with this device. (Researchers at the conference reported updated registry data with nearly 2,000 patients with findings similar to what Dr. Nogueira referenced.) Although these patients underwent treatment before results of DAWN and DEFUSE 3 came out and before release of the new U.S. acute stroke management guidelines (Stroke. 2018 Jan 24; doi: 10.1161/STR.0000000000000158) that endorsed targeted thrombectomy for patients 6-24 hours out from their stroke onset, 278 (28%) of the registry patients underwent thrombectomy treatment during the 6- to 24-hour time window. In this subgroup, 34 patients underwent noncontrast CT to assess their infarcted core prior to thrombectomy, while the other patients underwent perfusion CT, MRI, or both. The noncontrast CT patients had recanalization rates, adverse event rates, and 90-day modified Rankin Scale (mRS) scores comparable with those of patients assessed with more advanced imaging.
Based on this, “just looking at CT only seems reasonable. Noncontrast CT is a pretty valid way to select patients,” Dr. Nogueira said.
“This is the direction we should follow to simplify the paradigm for treating beyond 6 hours,” agreed Dr. Jovin, who also called for further advances in patient selection to expand the pool of patients who qualify for thrombectomy during the 6- to 24-hour postonset period.
“We want the DAWN results to be generalizable, to be simpler. We are exploring some more easily generalizable inclusion criteria that would allow us to treat more patients in more parts of the world,” Dr. Jovin added.
Both clinicians cited the remarkably low number needed to treat found in both DAWN and DEFUSE 3 of roughly three patients to produce one additional patient with a statistically significant and clinically meaningful improvement in their 90-day mRS score, compared with controls, as an unmistakable sign that the treatment in both trials was too targeted.
“When we planned the DAWN and DEFUSE 3 trials we didn’t expect how powerful the treatment effect would be. There are probably other patients who could also benefit, so how low can we go? How liberal can we be in our inclusion criteria and still get benefit?” Dr. Jovin asked.
“When intravenous TPA [tissue plasminogen activator] first came out, we went by the book [for patient selection], but as we got to know the treatment and became more comfortable with it, we began to bend the rules. Now we’re at the point of getting comfortable with endovascular treatment, and we need to figure out where to bend the rules by building the database. There is no doubt that the rules need bending because of the treatment effect that we’ve seen. We need to get our patients to endovascular treatment,” she said in her presentation at the conference.
But these physicians realize that for the time being, standard of care will follow the imaging and data processing primarily used in DAWN and DEFUSE 3, which not only involved perfusion CT or MRI but also a proprietary, automated image processing software, RAPID, that takes imaging data and calculates the amount and ratio of infarcted core and hypoperfused, ischemic brain tissue.
“I asked our imaging experts [at the University of Cincinnati] what should my threshold be [for mismatch between the infarcted core and ischemic tissue], and they said, ‘Use the automated software,’ ” Dr. Khatri said. If centers managing acute ischemic stroke patients don’t already have this software, “they need it. I think there is no way around that. It’s the only way we’ll be able to do this,” she commented. Most U.S. community hospitals that admit stroke patients currently lack this software, largely because of its high cost, she added.
“We’re struggling because it is very difficult to get some community hospitals – primary stroke centers – to invest in the software, but that’s really the only way we’ll be able to do this. There are issues of cost, and of getting technicians trained,” she noted.
That’s because DEFUSE 3 enrolled patients with a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 6, while patients in DAWN required a score of at least 10, a loosening that allowed inclusion of 31 patients with scores of 6-9 in DEFUSE 3. Another, less restrictive criterion was the patient’s prestroke mRS score, which could have been 0-2 in DEFUSE 3 but could be only 0-1 in DAWN. Thirteen of the DEFUSE 3 patients had prestroke mRS scores of 2. DEFUSE 3 also had somewhat more liberal criteria for the size of a patient’s infarcted core at enrollment, with 41 patients who would have been excluded from DAWN because of an overly large infarcted core, Dr. Albers said in his presentation at the conference.
Currently, for patients presenting more than 16 hours following their stroke onset but within 24 hours, the DAWN enrollment criteria exclusively determine which patients should get thrombectomy.
One area where these rules could be bent is by a more thoughtful approach to the prestroke mRS score rule out, such as patients with orthopedic or rheumatic complications that limit mobility and give them an mRS score of 3. “We don’t have data for patients with back pain who can’t walk. I currently take these patients to endovascular therapy, and I’m sure many others do, too,” Dr. Khatri said.
Another potential way to grow the inclusion criteria is to investigate thrombectomy in patients with larger infarcted cores than were enrolled in DAWN and DEFUSE 3, but assessing this will require a new prospective study, Dr. Albers said.
Running the 6- to 24-hour numbers
Adoption of the 6- to 24-hour time window for endovascular intervention in selected patients means that suddenly the U.S. acute stroke infrastructure needs to accommodate a significantly increased number of patients. Just how many added patients this means is uncertain for the time being, and will vary from region to region and center to center. Dr. Albers roughly guessed that the new late time window might double the number of stroke patients undergoing thrombectomy at his center in Stanford. Dr. Khatri put together a more data-driven but still very speculative estimate that at the University of Cincinnati, it will mean about 40% more stroke patients going to thrombectomy. She shared the numbers behind this estimate in a report she gave at the conference.
To calculate the incremental change produced by the late time window, she used data collected on 2,297 acute ischemic stroke patients from the Greater Cincinnati/Northern Kentucky region who were seen at the University of Cincinnati during 2010. Prior analysis by Dr. Khatri and her associates showed that 159 of these patients presented quickly enough and with an appropriate stroke to qualify for thrombolytic therapy, and that 29 patients would have qualified for thrombectomy performed during the 0- to 6-hour time window.
In the new analysis Dr. Khatri calculated that 791 patients presented at 5-23 hours, and of these 34 had other features that would have made them eligible for enrollment in DAWN. Because no imaging data existed for these 2010 patients, she applied an estimate that 22% of these patients would qualify by the size of their infarcted core and ischemic penumbra, resulting in seven additional thrombectomy-eligible patients. Accounting for patients who would qualify by the more liberal DEFUSE 3 criteria added another 5 patients for a total increment of 12 patients during 2010 who would have been eligible for thrombectomy, about 40% of the number from the 0- to 6-hour window.
She noted that “this is likely an underestimate,” and “too small a sample to project to national estimates,” but concluded that “resources must be adapted to account for this increased volume in endovascular treatment.”
Dr. Khatri acknowledged that the new 6- to 24-hour window for endovascular therapy, and concerns about imaging delays in 0- to 6-hour patients, raise challenging issues regarding the message to give U.S. clinicians about treating acute ischemic stroke patients.
“We have a mandate to figure it out in every region. There is no doubt that stroke patients need access to this care. We need to become a lot more aggressive with endovascular treatment. It’s so gratifying to see the outcomes that we’re seeing,” Dr. Khatri said. “A lot of work is needed to accommodate endovascular therapy–eligible patients in an extended time window. We need more refined prehospital triage tools, we need to adequately implement imaging software, and we need increased capacity to perform endovascular treatment with additional procedure suites, operators, and ICU beds.”
Dr. Jovin has been a consultant to Anaconda Biomed, Blockade Medical, Cerenovus, FreeOx Biotech, and Silk Road Medical. Dr. Nogueira has received travel expense reimbursement from Stryker. Dr. Khatri has been a consultant to Biogen, Medpace/Novartis, and St. Jude; has received travel support from Neuravi and EmstoPA; and has received research support from Genentech, Lumosa, and Neurospring. Dr. Albers has an ownership interest in iSchemaView, the company that markets the RAPID imaging software, and is a consultant to iSchemaView and Medtronic.
LOS ANGELES – CT or magnetic resonance brain imaging of acute ischemic stroke patients was the key triage tool in two groundbreaking thrombectomy trials, DAWN and DEFUSE; results from both showed that patients found by imaging to have limited infarcted cores could safely benefit from endovascular thrombectomy, even when they are more than 6 hours out from their stroke onset, breaking the 6-hour barrier created 3 years ago by the first wave of thrombectomy trials.
But some stroke neurologists studying thrombectomy are now convinced that imaging is not needed and may actually harm acute ischemic stroke patients early on by introducing an unnecessary time delay when they present within the first 6 hours after stroke onset.
This new thinking on how to best use brain imaging in acute ischemic stroke patients is part of the rapid evolution of acute stroke management as experts process new data and refine their approach both within 6 hours of stroke onset and during the new treatment window of 6-24 hours post onset. The dramatic success achieved with thrombectomy in highly selected late-window patients prompted researchers to promote pushing the boundaries further to find less-selected late patients who could also potentially benefit from thrombectomy.
The downside of early imaging
“Early on, we have a mix of fast and slow progressors. Slow progressors are about a third to half the patients, so there is a lot of potential for [late] treatment, but the majority of patients during the first 6 hours after onset are fast progressors,” patients who won’t benefit from thrombectomy delivery beyond 6 hours, said Dr. Jovin, an interventional neurologist and director of the Stroke Institute of the University of Pittsburgh Medical Center.
“Time is very precious in the 0- to 6-hour window. When we’re dealing with a lot of fast progressors, we pay a price [in added time to treatment] for any imaging we do. We need to understand that this is a real price we pay, even when CT takes perhaps 24 minutes, and MRI adds about 12 minutes. It’s not the case in all patients that doing CT angiography just adds 5 minutes. It can take 15, 20 minutes,” especially at centers that don’t treat these types of stroke patients day in and day out. “There is no question that imaging slows us down,” Dr. Jovin said.
He highlighted that “the main role of imaging is to exclude patients from treatment, a treatment that has unbelievable effects.” Imaging can rule out patients who have a hemorrhage, lack an occlusion, have a large infarcted core, or have none of the brain at risk or just a small amount, he noted. “Excluding hemorrhage is reasonable, but we can do that in the angiography suite, when the patient is on the table. The main benefit from advanced imaging is to more precisely define the core,” but for most patients the size of their core is not important because the vast majority of acute ischemic stroke patients seen within 6 hours of onset have cores smaller than 70 mL.
“Is this much ado about nothing – especially because we punish all the other patients [with smaller cores] who need to be treated [quickly] when we do additional imaging?” asked Dr. Jovin, who was one of the two lead investigators for the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) trial (N Engl J Med. 2018 Jan 4;378[1]:11-21). Another factor undercutting the value of imaging and determining core size is registry results that show patients who undergo thrombectomy with a large infarcted core are not harmed by treatment.
Current practice often uses imaging “to exclude the 20% of patients who may not have a large vessel occlusion or may not get benefit but whom we are unlikely to harm. But we harm the other 80% by delaying treatment by 30 minutes because of imaging. That’s why we need to rethink imaging and minimize its use.”
Dr. Jovin noted that at his center in Pittsburgh, the stroke institute staff began in 2013 to take patients transported from other stroke facilities and already diagnosed with a large vessel occlusion directly to the angiography suite, bypassing further brain imaging. By doing this, they cut their average door-to-groin puncture time down to 22 minutes from what had been an average of 81 minutes with imaging (Stroke. 2017 July;48[7]:1884-9). Right now the door-to-groin time is even lower, he added.
“Don’t waste time imaging,” said Dr. Nogueira, a stroke neurologist who is director of the neuroendovascular division of the Marcus Stroke and Neuroscience Center and professor of neurology, neurosurgery, and radiology at Emory University, both in Atlanta, as well as the second lead investigator for DAWN. “Time is crucial and trumps patient selection. Most selection criteria are informative rather than truly selective. It is important to understand that in every time window, we do not yet know who doesn’t benefit from treatment. Select faster, select less, and treat more” during the 0- to 6-hour window, he told his colleagues.
Expanding thrombectomy 6-24 hours after stroke onset
While Dr. Jovin and Dr. Nogueira called for more aggressive and less selective use of thrombectomy in patients who present within 6 hours of their stroke onset, they acknowledged that for patients who present during the 6- to 24-hour window, selection for thrombectomy should follow the rules applied in DAWN and in the more inclusive Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trial (N Engl J Med. 2018 Feb 22;378:708-18). That means using imaging to confirm that the patient’s infarcted core is within the enrollment ceiling, but both neurologists also downplayed the need for the more sophisticated imaging approaches that were often used in both trials as well as in current routine practice at comprehensive stroke centers. They agreed that noncontrast CT, a widely available method, seems adequate for patient selection, based on the admittedly limited data available right now.
Dr. Nogueira cited data from the Trevo stent retriever registry, collected from nearly 1,000 U.S. acute ischemic stroke patients who underwent thrombectomy with this device. (Researchers at the conference reported updated registry data with nearly 2,000 patients with findings similar to what Dr. Nogueira referenced.) Although these patients underwent treatment before results of DAWN and DEFUSE 3 came out and before release of the new U.S. acute stroke management guidelines (Stroke. 2018 Jan 24; doi: 10.1161/STR.0000000000000158) that endorsed targeted thrombectomy for patients 6-24 hours out from their stroke onset, 278 (28%) of the registry patients underwent thrombectomy treatment during the 6- to 24-hour time window. In this subgroup, 34 patients underwent noncontrast CT to assess their infarcted core prior to thrombectomy, while the other patients underwent perfusion CT, MRI, or both. The noncontrast CT patients had recanalization rates, adverse event rates, and 90-day modified Rankin Scale (mRS) scores comparable with those of patients assessed with more advanced imaging.
Based on this, “just looking at CT only seems reasonable. Noncontrast CT is a pretty valid way to select patients,” Dr. Nogueira said.
“This is the direction we should follow to simplify the paradigm for treating beyond 6 hours,” agreed Dr. Jovin, who also called for further advances in patient selection to expand the pool of patients who qualify for thrombectomy during the 6- to 24-hour postonset period.
“We want the DAWN results to be generalizable, to be simpler. We are exploring some more easily generalizable inclusion criteria that would allow us to treat more patients in more parts of the world,” Dr. Jovin added.
Both clinicians cited the remarkably low number needed to treat found in both DAWN and DEFUSE 3 of roughly three patients to produce one additional patient with a statistically significant and clinically meaningful improvement in their 90-day mRS score, compared with controls, as an unmistakable sign that the treatment in both trials was too targeted.
“When we planned the DAWN and DEFUSE 3 trials we didn’t expect how powerful the treatment effect would be. There are probably other patients who could also benefit, so how low can we go? How liberal can we be in our inclusion criteria and still get benefit?” Dr. Jovin asked.
“When intravenous TPA [tissue plasminogen activator] first came out, we went by the book [for patient selection], but as we got to know the treatment and became more comfortable with it, we began to bend the rules. Now we’re at the point of getting comfortable with endovascular treatment, and we need to figure out where to bend the rules by building the database. There is no doubt that the rules need bending because of the treatment effect that we’ve seen. We need to get our patients to endovascular treatment,” she said in her presentation at the conference.
But these physicians realize that for the time being, standard of care will follow the imaging and data processing primarily used in DAWN and DEFUSE 3, which not only involved perfusion CT or MRI but also a proprietary, automated image processing software, RAPID, that takes imaging data and calculates the amount and ratio of infarcted core and hypoperfused, ischemic brain tissue.
“I asked our imaging experts [at the University of Cincinnati] what should my threshold be [for mismatch between the infarcted core and ischemic tissue], and they said, ‘Use the automated software,’ ” Dr. Khatri said. If centers managing acute ischemic stroke patients don’t already have this software, “they need it. I think there is no way around that. It’s the only way we’ll be able to do this,” she commented. Most U.S. community hospitals that admit stroke patients currently lack this software, largely because of its high cost, she added.
“We’re struggling because it is very difficult to get some community hospitals – primary stroke centers – to invest in the software, but that’s really the only way we’ll be able to do this. There are issues of cost, and of getting technicians trained,” she noted.
That’s because DEFUSE 3 enrolled patients with a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 6, while patients in DAWN required a score of at least 10, a loosening that allowed inclusion of 31 patients with scores of 6-9 in DEFUSE 3. Another, less restrictive criterion was the patient’s prestroke mRS score, which could have been 0-2 in DEFUSE 3 but could be only 0-1 in DAWN. Thirteen of the DEFUSE 3 patients had prestroke mRS scores of 2. DEFUSE 3 also had somewhat more liberal criteria for the size of a patient’s infarcted core at enrollment, with 41 patients who would have been excluded from DAWN because of an overly large infarcted core, Dr. Albers said in his presentation at the conference.
Currently, for patients presenting more than 16 hours following their stroke onset but within 24 hours, the DAWN enrollment criteria exclusively determine which patients should get thrombectomy.
One area where these rules could be bent is by a more thoughtful approach to the prestroke mRS score rule out, such as patients with orthopedic or rheumatic complications that limit mobility and give them an mRS score of 3. “We don’t have data for patients with back pain who can’t walk. I currently take these patients to endovascular therapy, and I’m sure many others do, too,” Dr. Khatri said.
Another potential way to grow the inclusion criteria is to investigate thrombectomy in patients with larger infarcted cores than were enrolled in DAWN and DEFUSE 3, but assessing this will require a new prospective study, Dr. Albers said.
Running the 6- to 24-hour numbers
Adoption of the 6- to 24-hour time window for endovascular intervention in selected patients means that suddenly the U.S. acute stroke infrastructure needs to accommodate a significantly increased number of patients. Just how many added patients this means is uncertain for the time being, and will vary from region to region and center to center. Dr. Albers roughly guessed that the new late time window might double the number of stroke patients undergoing thrombectomy at his center in Stanford. Dr. Khatri put together a more data-driven but still very speculative estimate that at the University of Cincinnati, it will mean about 40% more stroke patients going to thrombectomy. She shared the numbers behind this estimate in a report she gave at the conference.
To calculate the incremental change produced by the late time window, she used data collected on 2,297 acute ischemic stroke patients from the Greater Cincinnati/Northern Kentucky region who were seen at the University of Cincinnati during 2010. Prior analysis by Dr. Khatri and her associates showed that 159 of these patients presented quickly enough and with an appropriate stroke to qualify for thrombolytic therapy, and that 29 patients would have qualified for thrombectomy performed during the 0- to 6-hour time window.
In the new analysis Dr. Khatri calculated that 791 patients presented at 5-23 hours, and of these 34 had other features that would have made them eligible for enrollment in DAWN. Because no imaging data existed for these 2010 patients, she applied an estimate that 22% of these patients would qualify by the size of their infarcted core and ischemic penumbra, resulting in seven additional thrombectomy-eligible patients. Accounting for patients who would qualify by the more liberal DEFUSE 3 criteria added another 5 patients for a total increment of 12 patients during 2010 who would have been eligible for thrombectomy, about 40% of the number from the 0- to 6-hour window.
She noted that “this is likely an underestimate,” and “too small a sample to project to national estimates,” but concluded that “resources must be adapted to account for this increased volume in endovascular treatment.”
Dr. Khatri acknowledged that the new 6- to 24-hour window for endovascular therapy, and concerns about imaging delays in 0- to 6-hour patients, raise challenging issues regarding the message to give U.S. clinicians about treating acute ischemic stroke patients.
“We have a mandate to figure it out in every region. There is no doubt that stroke patients need access to this care. We need to become a lot more aggressive with endovascular treatment. It’s so gratifying to see the outcomes that we’re seeing,” Dr. Khatri said. “A lot of work is needed to accommodate endovascular therapy–eligible patients in an extended time window. We need more refined prehospital triage tools, we need to adequately implement imaging software, and we need increased capacity to perform endovascular treatment with additional procedure suites, operators, and ICU beds.”
Dr. Jovin has been a consultant to Anaconda Biomed, Blockade Medical, Cerenovus, FreeOx Biotech, and Silk Road Medical. Dr. Nogueira has received travel expense reimbursement from Stryker. Dr. Khatri has been a consultant to Biogen, Medpace/Novartis, and St. Jude; has received travel support from Neuravi and EmstoPA; and has received research support from Genentech, Lumosa, and Neurospring. Dr. Albers has an ownership interest in iSchemaView, the company that markets the RAPID imaging software, and is a consultant to iSchemaView and Medtronic.
EXPERT ANALYSIS FROM ISC 2018
DDSEP® 8 Quick Quiz - March 2018 Question 2
Correct Answer: B
Rationale
The patient has a favorable anatomy for a surgical drainage procedure such as a lateral pancreaticojejunostomy (Peustow procedure). Surgery has been noted to provide superior pain relief over 5 years compared with endoscopy. Hospital costs and length of stay were similar between the groups. Continued medical therapy is unlikely to add further benefit on top of what she has already achieved. EUS-guided celiac plexus block will only provide temporary pain relief. There are limited long-term data on the effectiveness of total pancreatectomy with islet autotransplantation in alleviating pain.
References
1. Cahen D.L., Gouma D.J., Laramée P., et al. Gastroenterology. 2011;141(5):1690-5.
2. Conwell D.L., Lee L.S., Yadav D., et al. American pancreatic association practice guidelines in chronic pancreatitis. Pancreas. 2014;43:1143-62.
Correct Answer: B
Rationale
The patient has a favorable anatomy for a surgical drainage procedure such as a lateral pancreaticojejunostomy (Peustow procedure). Surgery has been noted to provide superior pain relief over 5 years compared with endoscopy. Hospital costs and length of stay were similar between the groups. Continued medical therapy is unlikely to add further benefit on top of what she has already achieved. EUS-guided celiac plexus block will only provide temporary pain relief. There are limited long-term data on the effectiveness of total pancreatectomy with islet autotransplantation in alleviating pain.
References
1. Cahen D.L., Gouma D.J., Laramée P., et al. Gastroenterology. 2011;141(5):1690-5.
2. Conwell D.L., Lee L.S., Yadav D., et al. American pancreatic association practice guidelines in chronic pancreatitis. Pancreas. 2014;43:1143-62.
Correct Answer: B
Rationale
The patient has a favorable anatomy for a surgical drainage procedure such as a lateral pancreaticojejunostomy (Peustow procedure). Surgery has been noted to provide superior pain relief over 5 years compared with endoscopy. Hospital costs and length of stay were similar between the groups. Continued medical therapy is unlikely to add further benefit on top of what she has already achieved. EUS-guided celiac plexus block will only provide temporary pain relief. There are limited long-term data on the effectiveness of total pancreatectomy with islet autotransplantation in alleviating pain.
References
1. Cahen D.L., Gouma D.J., Laramée P., et al. Gastroenterology. 2011;141(5):1690-5.
2. Conwell D.L., Lee L.S., Yadav D., et al. American pancreatic association practice guidelines in chronic pancreatitis. Pancreas. 2014;43:1143-62.
A 68-year-old woman with alcoholic chronic pancreatitis has constant, disabling pain. She has previously tried gabapentin, celecoxib, and antioxidants with partial improvement. She currently takes nonenteric coated pancrealipase (90,000 IU per meal) and controlled-release oxycontin. CT of the abdomen demonstrates a few small punctate calcifications in the head of the pancreas, a 1-cm calculus in the genu with a markedly dilated pancreatic duct in the body and tail, and moderate distal atrophy. There are no pseudocysts. She discusses further options to treat her pain.
Which intervention will most likely improve her pain and quality of life over the next 5 years?
Gender affirmation surgery has become more common
, and private insurers and Medicare and Medicaid are covering more of them, according to a review of the National Inpatient Sample from 2000 to 2014.
“As coverage for these procedures increases, likely so will demand for qualified surgeons to perform them,” said investigators led by Joseph Canner, MHS, of Johns Hopkins University, Baltimore (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231).
The team sought to correct a glaring lack of demographic, hospital, and surgical data on transgender people, a problem due mainly to “the absence of routine, standardized collection and reporting of gender identity in health care settings,” the investigators said.
The Affordable Care Act banned gender identity discrimination, and state and federal governments – as well as private insurers – are expanding coverage of gender affirmation surgery and care. Given the trend, it’s essential that quality improvement agencies begin to “focus on adopting a new set of patient-centered measures to better monitor transgender care and identify opportunities for advancing transition-related services,” the researchers said.
The team identified 37,827 hospital encounters in the national database that had ICD-9 diagnosis codes of transsexualism or gender identity disorder that were listed in the National Inpatient Sample. The rate of those codes increased from 3.87/100,000 patients in 2000 to 14.22 /100,000 patients in 2014.
Mental health was the leading cause of hospitalization, accounting for 40.5% of admissions, a finding that “is consistent with the high prevalence of depression, anxiety, and suicidal ideation in this population,” the investigators said.
Patients were a median age of 38 years old; 57.1% identified as white; and 54.3% were categorized as male, 38.3% as female, and most of the rest as “inconsistent,” meaning their sex in the medical record did not match their procedures.
Almost 11% were hospitalized for gender affirmation surgery, including penile or vaginal construction. From 2000 to 2005, 72% of patients who underwent affirmation procedures had genital surgery; the number increased to 83.9% from 2006 to 2011. More than half of the patients weren’t covered by insurance, but the number covered by Medicare or Medicaid increased from 25 patients in 2012-2013 to 70 in 2014. Meanwhile, no one died in the hospital from gender reassignment surgery, and the median stay was 4 days.
“Our data suggest that genital surgery is the most common type of inpatient gender-affirming surgery; however, these data do not include gender-affirming surgical procedures performed in outpatient settings, which likely include most chest, breast, and facial surgery,” the investigators wrote.
Surgery patients in high-volume centers (performing more than 50 gender-affirming procedures per year) were mostly self-pay, while those admitted to low-volume centers were not. “It is possible that self-paying patients may be getting higher-quality care at high-volume centers, as has been observed in other types of surgery,” according to the investigators. There is a need for national clinical and patient-reported outcomes data to assess and improve the quality of gender-affirming surgery. Gender identity information should be a part of all electronic health records and reported back to national data repositories, they said.
The investigators are supported by the Patient-Centered Outcomes Research Institute, the Agency for Healthcare Research and Quality, and the National Institutes of Health, among others. They had no industry disclosures.
SOURCE: Canner JK et al. JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231.
The study is thought provoking and suggests many areas for future study.
Gender-affirming surgery is the final step in a spectrum of treatments for gender identity disorders or transsexualism, including psychological counseling, hormonal therapies, and pubertal hormone blockers. Referrals for these treatments are increasing, and likely the demand for surgical treatment will also continue to increase.
A comprehensive database or other prospective tool to assess the comparative efficacy of these treatments, create quality metrics, and address long-term health or psychiatric outcomes should be pursued.
Future research must address cost effectiveness and cost burdens, given increased public funding for gender affirmation surgeries. Most longitudinal studies of patients who have undergone gender affirmation procedures have found high satisfaction rates with low rates of regret (less than 5%). However, when regret occurs, it can be surgically challenging and costly to reverse these procedures.
Marie Crandall , MD, is a professor of surgery at the University of Florida, Jacksonville. She made her comments in an editorial and had no industry disclosures (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6232).
The study is thought provoking and suggests many areas for future study.
Gender-affirming surgery is the final step in a spectrum of treatments for gender identity disorders or transsexualism, including psychological counseling, hormonal therapies, and pubertal hormone blockers. Referrals for these treatments are increasing, and likely the demand for surgical treatment will also continue to increase.
A comprehensive database or other prospective tool to assess the comparative efficacy of these treatments, create quality metrics, and address long-term health or psychiatric outcomes should be pursued.
Future research must address cost effectiveness and cost burdens, given increased public funding for gender affirmation surgeries. Most longitudinal studies of patients who have undergone gender affirmation procedures have found high satisfaction rates with low rates of regret (less than 5%). However, when regret occurs, it can be surgically challenging and costly to reverse these procedures.
Marie Crandall , MD, is a professor of surgery at the University of Florida, Jacksonville. She made her comments in an editorial and had no industry disclosures (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6232).
The study is thought provoking and suggests many areas for future study.
Gender-affirming surgery is the final step in a spectrum of treatments for gender identity disorders or transsexualism, including psychological counseling, hormonal therapies, and pubertal hormone blockers. Referrals for these treatments are increasing, and likely the demand for surgical treatment will also continue to increase.
A comprehensive database or other prospective tool to assess the comparative efficacy of these treatments, create quality metrics, and address long-term health or psychiatric outcomes should be pursued.
Future research must address cost effectiveness and cost burdens, given increased public funding for gender affirmation surgeries. Most longitudinal studies of patients who have undergone gender affirmation procedures have found high satisfaction rates with low rates of regret (less than 5%). However, when regret occurs, it can be surgically challenging and costly to reverse these procedures.
Marie Crandall , MD, is a professor of surgery at the University of Florida, Jacksonville. She made her comments in an editorial and had no industry disclosures (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6232).
, and private insurers and Medicare and Medicaid are covering more of them, according to a review of the National Inpatient Sample from 2000 to 2014.
“As coverage for these procedures increases, likely so will demand for qualified surgeons to perform them,” said investigators led by Joseph Canner, MHS, of Johns Hopkins University, Baltimore (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231).
The team sought to correct a glaring lack of demographic, hospital, and surgical data on transgender people, a problem due mainly to “the absence of routine, standardized collection and reporting of gender identity in health care settings,” the investigators said.
The Affordable Care Act banned gender identity discrimination, and state and federal governments – as well as private insurers – are expanding coverage of gender affirmation surgery and care. Given the trend, it’s essential that quality improvement agencies begin to “focus on adopting a new set of patient-centered measures to better monitor transgender care and identify opportunities for advancing transition-related services,” the researchers said.
The team identified 37,827 hospital encounters in the national database that had ICD-9 diagnosis codes of transsexualism or gender identity disorder that were listed in the National Inpatient Sample. The rate of those codes increased from 3.87/100,000 patients in 2000 to 14.22 /100,000 patients in 2014.
Mental health was the leading cause of hospitalization, accounting for 40.5% of admissions, a finding that “is consistent with the high prevalence of depression, anxiety, and suicidal ideation in this population,” the investigators said.
Patients were a median age of 38 years old; 57.1% identified as white; and 54.3% were categorized as male, 38.3% as female, and most of the rest as “inconsistent,” meaning their sex in the medical record did not match their procedures.
Almost 11% were hospitalized for gender affirmation surgery, including penile or vaginal construction. From 2000 to 2005, 72% of patients who underwent affirmation procedures had genital surgery; the number increased to 83.9% from 2006 to 2011. More than half of the patients weren’t covered by insurance, but the number covered by Medicare or Medicaid increased from 25 patients in 2012-2013 to 70 in 2014. Meanwhile, no one died in the hospital from gender reassignment surgery, and the median stay was 4 days.
“Our data suggest that genital surgery is the most common type of inpatient gender-affirming surgery; however, these data do not include gender-affirming surgical procedures performed in outpatient settings, which likely include most chest, breast, and facial surgery,” the investigators wrote.
Surgery patients in high-volume centers (performing more than 50 gender-affirming procedures per year) were mostly self-pay, while those admitted to low-volume centers were not. “It is possible that self-paying patients may be getting higher-quality care at high-volume centers, as has been observed in other types of surgery,” according to the investigators. There is a need for national clinical and patient-reported outcomes data to assess and improve the quality of gender-affirming surgery. Gender identity information should be a part of all electronic health records and reported back to national data repositories, they said.
The investigators are supported by the Patient-Centered Outcomes Research Institute, the Agency for Healthcare Research and Quality, and the National Institutes of Health, among others. They had no industry disclosures.
SOURCE: Canner JK et al. JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231.
, and private insurers and Medicare and Medicaid are covering more of them, according to a review of the National Inpatient Sample from 2000 to 2014.
“As coverage for these procedures increases, likely so will demand for qualified surgeons to perform them,” said investigators led by Joseph Canner, MHS, of Johns Hopkins University, Baltimore (JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231).
The team sought to correct a glaring lack of demographic, hospital, and surgical data on transgender people, a problem due mainly to “the absence of routine, standardized collection and reporting of gender identity in health care settings,” the investigators said.
The Affordable Care Act banned gender identity discrimination, and state and federal governments – as well as private insurers – are expanding coverage of gender affirmation surgery and care. Given the trend, it’s essential that quality improvement agencies begin to “focus on adopting a new set of patient-centered measures to better monitor transgender care and identify opportunities for advancing transition-related services,” the researchers said.
The team identified 37,827 hospital encounters in the national database that had ICD-9 diagnosis codes of transsexualism or gender identity disorder that were listed in the National Inpatient Sample. The rate of those codes increased from 3.87/100,000 patients in 2000 to 14.22 /100,000 patients in 2014.
Mental health was the leading cause of hospitalization, accounting for 40.5% of admissions, a finding that “is consistent with the high prevalence of depression, anxiety, and suicidal ideation in this population,” the investigators said.
Patients were a median age of 38 years old; 57.1% identified as white; and 54.3% were categorized as male, 38.3% as female, and most of the rest as “inconsistent,” meaning their sex in the medical record did not match their procedures.
Almost 11% were hospitalized for gender affirmation surgery, including penile or vaginal construction. From 2000 to 2005, 72% of patients who underwent affirmation procedures had genital surgery; the number increased to 83.9% from 2006 to 2011. More than half of the patients weren’t covered by insurance, but the number covered by Medicare or Medicaid increased from 25 patients in 2012-2013 to 70 in 2014. Meanwhile, no one died in the hospital from gender reassignment surgery, and the median stay was 4 days.
“Our data suggest that genital surgery is the most common type of inpatient gender-affirming surgery; however, these data do not include gender-affirming surgical procedures performed in outpatient settings, which likely include most chest, breast, and facial surgery,” the investigators wrote.
Surgery patients in high-volume centers (performing more than 50 gender-affirming procedures per year) were mostly self-pay, while those admitted to low-volume centers were not. “It is possible that self-paying patients may be getting higher-quality care at high-volume centers, as has been observed in other types of surgery,” according to the investigators. There is a need for national clinical and patient-reported outcomes data to assess and improve the quality of gender-affirming surgery. Gender identity information should be a part of all electronic health records and reported back to national data repositories, they said.
The investigators are supported by the Patient-Centered Outcomes Research Institute, the Agency for Healthcare Research and Quality, and the National Institutes of Health, among others. They had no industry disclosures.
SOURCE: Canner JK et al. JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231.
FROM JAMA SURGERY
Key clinical point: With gender affirmation surgeries on the rise in the United States, it’s time for a more rigorous system to track outcomes.
Major finding: The rate of transsexual and gender identity disorder codes in the National Inpatient Sample increased from 3.87/100,000 patients in 2000 to 14.22/100,000 patients in 2014.
Study details: A review of 37,827 hospital encounters reported in the National Inpatient Sample.
Disclosures: The investigators are supported by the Patient-Centered Outcomes Research Institute, the Agency for Healthcare Research and Quality, and the National Institutes of Health, among others. They had no industry disclosures.
Source: Canner JK et al. JAMA Surg. 2018 Feb 28. doi: 10.1001/jamasurg.2017.6231.
DDSEP® 8 Quick Quiz - March 2018 Question 1
Q1. Correct answer: C
Rationale
The initial management of dyspepsia depends on symptoms and presence of any “alarm features.” In patients without “alarm features” presenting with symptoms suggestive of hepatobiliary or pancreatic causes, the initial diagnostic tests should include liver/pancreatic blood tests and abdominal imaging. For other dyspeptic patients without alarm features, initial management would include H. pylori testing (breath, stool antigen, or antibody) and/or empiric antisecretory (PPI) therapy. However, for patients who present with “alarm features” such as dysphagia, anemia, GI bleeding, anorexia, significant weight loss, etc., an upper endoscopy should be performed to evaluate for the presence of any upper GI tract malignancy. In this patient, the presence of microcytic anemia is an alarm feature. Tricyclic antidepressants such as amitriptyline may be used as treatment for functional dyspepsia, after organic causes have been ruled out.
Reference
1. Talley N.J., Vakil N.B., Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology 2005;129:1756-80.
Q1. Correct answer: C
Rationale
The initial management of dyspepsia depends on symptoms and presence of any “alarm features.” In patients without “alarm features” presenting with symptoms suggestive of hepatobiliary or pancreatic causes, the initial diagnostic tests should include liver/pancreatic blood tests and abdominal imaging. For other dyspeptic patients without alarm features, initial management would include H. pylori testing (breath, stool antigen, or antibody) and/or empiric antisecretory (PPI) therapy. However, for patients who present with “alarm features” such as dysphagia, anemia, GI bleeding, anorexia, significant weight loss, etc., an upper endoscopy should be performed to evaluate for the presence of any upper GI tract malignancy. In this patient, the presence of microcytic anemia is an alarm feature. Tricyclic antidepressants such as amitriptyline may be used as treatment for functional dyspepsia, after organic causes have been ruled out.
Reference
1. Talley N.J., Vakil N.B., Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology 2005;129:1756-80.
Q1. Correct answer: C
Rationale
The initial management of dyspepsia depends on symptoms and presence of any “alarm features.” In patients without “alarm features” presenting with symptoms suggestive of hepatobiliary or pancreatic causes, the initial diagnostic tests should include liver/pancreatic blood tests and abdominal imaging. For other dyspeptic patients without alarm features, initial management would include H. pylori testing (breath, stool antigen, or antibody) and/or empiric antisecretory (PPI) therapy. However, for patients who present with “alarm features” such as dysphagia, anemia, GI bleeding, anorexia, significant weight loss, etc., an upper endoscopy should be performed to evaluate for the presence of any upper GI tract malignancy. In this patient, the presence of microcytic anemia is an alarm feature. Tricyclic antidepressants such as amitriptyline may be used as treatment for functional dyspepsia, after organic causes have been ruled out.
Reference
1. Talley N.J., Vakil N.B., Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology 2005;129:1756-80.
A 37-year-old man with no significant past medical history presents with a dull, nonradiating epigastric pain for 3 months. The pain is not associated with eating or positional changes. He denies any heartburn, regurgitation, chest pain, nausea, vomiting, dysphagia, odynophagia, or weight loss. He currently does not take any medications or supplements. Social and family history are not significant. Physical examination reveals minimal tenderness to deep palpation in the epigastrum, but otherwise it is unremarkable. A complete blood count reveals a white blood cell count of 6, hemoglobin 10 g/dL, MCV 72 fL, and platelet count of 200 x 103/mcL. What is the most important next step of management?
Survey Highlights Challenges in Asian Americans With Stroke
LOS ANGELES—A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive IV t-PA than white patients, according to research presented at the International Stroke Conference 2018. Investigators also found that stroke severity declined for whites between 2004 and 2016, but not for Asian Americans.
The research encompassed all self-identified Asian Americans in the Get With the Guidelines stroke database, which is a voluntary quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program from 2004 to 2016.
Largest Analysis of Asian Americans With Stroke
“There have been some limited studies, and studies in Asia, but this is the largest analysis of Asian-American stroke patients,” said Sarah Song, MD, Assistant Professor of Neurology at Rush University Medical Center in Chicago. “I think the most important finding is that they are not getting as much t-PA and [are] having more t-PA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins (LDL). Asian Americans “did not know that they had high cholesterol, but they had a higher LDL [cholesterol level] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in white patients, which was a statistically significant difference, said the researchers.
In addition, white patients had higher rates of atrial fibrillation (21.2% vs 16.0%), coronary artery disease (27.8% vs 17.5%), and stenosis (4.7% vs 2.0%), while Asian Americans were more likely to have diabetes (38.0% vs 29.2%). Severe strokes (defined as an NIH Stroke Scale score of 16 or greater) were more common among Asian Americans (odds ratio [OR], 1.35) than whites. After adjusting for stroke severity, the researchers found that Asian Americans were less likely to receive t-PA (OR, 0.90) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving t-PA (OR, 1.23). “I think that may have something to do with the pathophysiology … that we do not quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality initially appeared to be higher among Asian Americans, this trend was reversed after researchers corrected the analysis for stroke severity, which suggested better outcome for Asian Americans (OR, 0.95). Some quality-of-care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08), receipt of IV t-PA within 60 minutes of arrival (OR, 1.14), LDL cholesterol documentation (OR, 1.19), and receipt of intensive statin therapy (OR, 1.15). Asian Americans were less likely to receive a CT scan within 25 minutes of arrival, however (OR, 0.92).
Between 2004 and 2016, both groups benefited from similar improvements, but not in the same ways. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97), while there was no change in Asian Americans (OR, 1.00).
Study Limitations
One study limitation was that participation in the database was voluntary, which could lead to selection bias. Another limitation was that all Asian Americans were combined into one group. Stroke pathophysiology and outcomes may vary between Asian countries.
Still, the study suggests challenges that must be addressed. “I think it highlights the problem that Asian ischemic stroke patients do not do as well, they bleed more, and they receive less t-PA,” said Dr. Song.
—Jim Kling
LOS ANGELES—A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive IV t-PA than white patients, according to research presented at the International Stroke Conference 2018. Investigators also found that stroke severity declined for whites between 2004 and 2016, but not for Asian Americans.
The research encompassed all self-identified Asian Americans in the Get With the Guidelines stroke database, which is a voluntary quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program from 2004 to 2016.
Largest Analysis of Asian Americans With Stroke
“There have been some limited studies, and studies in Asia, but this is the largest analysis of Asian-American stroke patients,” said Sarah Song, MD, Assistant Professor of Neurology at Rush University Medical Center in Chicago. “I think the most important finding is that they are not getting as much t-PA and [are] having more t-PA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins (LDL). Asian Americans “did not know that they had high cholesterol, but they had a higher LDL [cholesterol level] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in white patients, which was a statistically significant difference, said the researchers.
In addition, white patients had higher rates of atrial fibrillation (21.2% vs 16.0%), coronary artery disease (27.8% vs 17.5%), and stenosis (4.7% vs 2.0%), while Asian Americans were more likely to have diabetes (38.0% vs 29.2%). Severe strokes (defined as an NIH Stroke Scale score of 16 or greater) were more common among Asian Americans (odds ratio [OR], 1.35) than whites. After adjusting for stroke severity, the researchers found that Asian Americans were less likely to receive t-PA (OR, 0.90) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving t-PA (OR, 1.23). “I think that may have something to do with the pathophysiology … that we do not quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality initially appeared to be higher among Asian Americans, this trend was reversed after researchers corrected the analysis for stroke severity, which suggested better outcome for Asian Americans (OR, 0.95). Some quality-of-care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08), receipt of IV t-PA within 60 minutes of arrival (OR, 1.14), LDL cholesterol documentation (OR, 1.19), and receipt of intensive statin therapy (OR, 1.15). Asian Americans were less likely to receive a CT scan within 25 minutes of arrival, however (OR, 0.92).
Between 2004 and 2016, both groups benefited from similar improvements, but not in the same ways. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97), while there was no change in Asian Americans (OR, 1.00).
Study Limitations
One study limitation was that participation in the database was voluntary, which could lead to selection bias. Another limitation was that all Asian Americans were combined into one group. Stroke pathophysiology and outcomes may vary between Asian countries.
Still, the study suggests challenges that must be addressed. “I think it highlights the problem that Asian ischemic stroke patients do not do as well, they bleed more, and they receive less t-PA,” said Dr. Song.
—Jim Kling
LOS ANGELES—A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive IV t-PA than white patients, according to research presented at the International Stroke Conference 2018. Investigators also found that stroke severity declined for whites between 2004 and 2016, but not for Asian Americans.
The research encompassed all self-identified Asian Americans in the Get With the Guidelines stroke database, which is a voluntary quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program from 2004 to 2016.
Largest Analysis of Asian Americans With Stroke
“There have been some limited studies, and studies in Asia, but this is the largest analysis of Asian-American stroke patients,” said Sarah Song, MD, Assistant Professor of Neurology at Rush University Medical Center in Chicago. “I think the most important finding is that they are not getting as much t-PA and [are] having more t-PA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins (LDL). Asian Americans “did not know that they had high cholesterol, but they had a higher LDL [cholesterol level] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in white patients, which was a statistically significant difference, said the researchers.
In addition, white patients had higher rates of atrial fibrillation (21.2% vs 16.0%), coronary artery disease (27.8% vs 17.5%), and stenosis (4.7% vs 2.0%), while Asian Americans were more likely to have diabetes (38.0% vs 29.2%). Severe strokes (defined as an NIH Stroke Scale score of 16 or greater) were more common among Asian Americans (odds ratio [OR], 1.35) than whites. After adjusting for stroke severity, the researchers found that Asian Americans were less likely to receive t-PA (OR, 0.90) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving t-PA (OR, 1.23). “I think that may have something to do with the pathophysiology … that we do not quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality initially appeared to be higher among Asian Americans, this trend was reversed after researchers corrected the analysis for stroke severity, which suggested better outcome for Asian Americans (OR, 0.95). Some quality-of-care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08), receipt of IV t-PA within 60 minutes of arrival (OR, 1.14), LDL cholesterol documentation (OR, 1.19), and receipt of intensive statin therapy (OR, 1.15). Asian Americans were less likely to receive a CT scan within 25 minutes of arrival, however (OR, 0.92).
Between 2004 and 2016, both groups benefited from similar improvements, but not in the same ways. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97), while there was no change in Asian Americans (OR, 1.00).
Study Limitations
One study limitation was that participation in the database was voluntary, which could lead to selection bias. Another limitation was that all Asian Americans were combined into one group. Stroke pathophysiology and outcomes may vary between Asian countries.
Still, the study suggests challenges that must be addressed. “I think it highlights the problem that Asian ischemic stroke patients do not do as well, they bleed more, and they receive less t-PA,” said Dr. Song.
—Jim Kling